Abstract
Despite significant progress in our understanding of the etiology, biology and genetics of colorectal cancer, as well as important clinical advances, it remains the third most frequently diagnosed cancer worldwide and is the second leading cause of cancer death. Based on demographic projections, the global burden of colorectal cancer would be expected to rise by 72% from 1.8 million new cases in 2018 to over 3 million in 2040 with substantial increases anticipated in low- and middle-income countries. In this meeting report, we summarize the content of a joint workshop led by the National Cancer Institute and the International Agency for Research on Cancer, which was held to summarize the important achievements that have been made in our understanding of colorectal cancer etiology, genetics, early detection and treatment and to identify key research questions that remain to be addressed.
Keywords: colorectal cancer, prevention, genetics, etiology, screening, therapy
Key Message
In this meeting report, we summarize the content of a joint workshop led by the National Cancer Institute (NCI) and the International Agency for Research on Cancer (IARC) which was held to summarize the important achievements that have been made in our understanding of colorectal cancer etiology, genetics, early detection and treatment and to identify key research questions that remain to be addressed.
Introduction
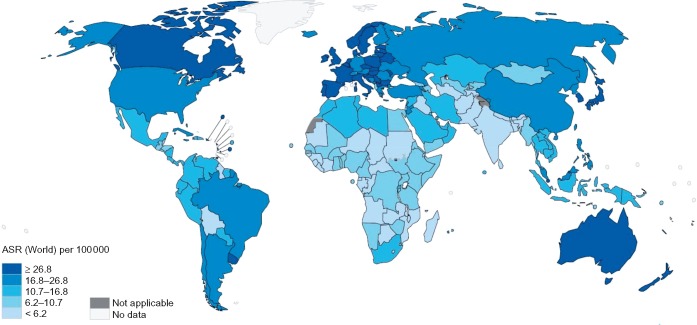
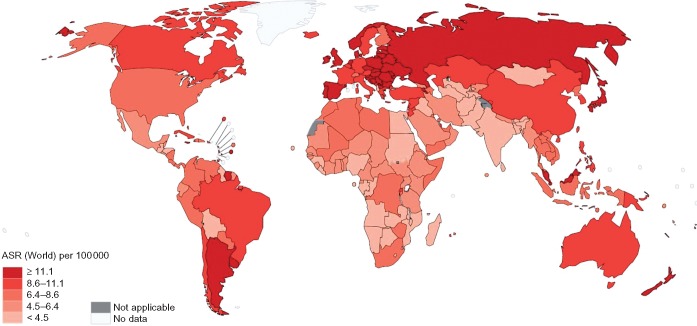
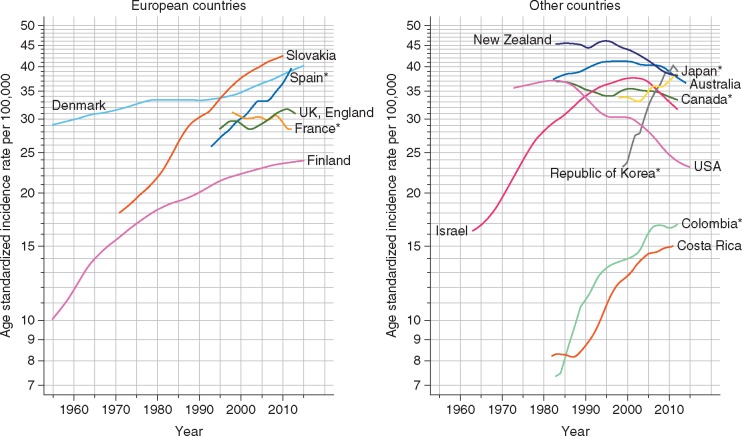
Colorectal cancer is the third most commonly diagnosed malignancy and the second leading cause of cancer death in the world, accounting for around 1.8 million new cases and 860 000 deaths in 2018 [1]. Given current demographic projections, the global burden of colorectal cancer is anticipated to increase by 72% to over 3 million new cases and by 82% to 1.6 million cancer deaths annually by 2040 [1]. The geographic distribution of the colorectal cancer burden varies widely, with more than 50% of all cases and 42% of all deaths occurring in countries with a very high human development index (HDI; see Figures 1 and 2). Being one of the clearest examples of disease transition related to human development, rapid increases in both colorectal cancer incidence and mortality are now being seen in many medium to high HDI countries, particularly in Eastern Europe, Asia and South America [1, 2] (see Figure 3). In contrast, colorectal cancer incidence and mortality rates have been plateauing or declining in many very high HDI countries including the United States, Australia, and several Western European countries [1, 2]. The reasons for the recent declining trends in overall incidence in these countries are not fully understood but may be attributable to a large extent by early detection and removal of colorectal cancer precursors through population-based screening programs. Concomitant improvements in chemotherapy and radiotherapy for colorectal cancer are also likely to have contributed to the declining mortality in these countries [3, 4]. While the incidence of colorectal cancer in many very high-HDI countries has decreased among those older than 50 years, rates in younger individuals appear to be rising, for reasons that are not clear [5, 6]. In addition, age-specific incidence of colorectal cancer is higher in men than in women, and men have poorer survival rates than women [7]. Although the reasons for this disparity by sex are not clear, it may reflect differences in exposure to colorectal cancer risk factors, as well as underlying biology.
Figure 1.
Worldwide incidence of colorectal cancer (age standardized rates per 100 000; GLOBOCAN 2018).
Figure 2.
Age-standardized rates for colorectal cancer mortality per 100 000 (GLOBOCAN 2018).
Figure 3.
Trends in colorectal cancer incidence in selected countries. *Regional registries. Source: GLOBOCAN 2018 [1].
Survival following colorectal cancer varies considerably between countries. While 5-year relative survival is now ∼65% in high-income countries settings, it is <50% in low-income countries [8, 9]. Stage at diagnosis remains the most important prognostic factor for colorectal cancer. In the United States, 5-year relative survival is currently 89.8% for patients with localized stage, 71.1% for patients with regional spread, and 13.8% for patients with metastatic disease at diagnosis [5]. However, differences can be observed in stage-specific survival even across high-income countries [10]. Ancestry may also represent an important factor for both disease incidence and survival—in the United States, for example, the burden of colorectal cancer appears to be greatest among African-Americans and is lowest in Hispanics [7].
Etiologic risk factors
Colorectal cancer is a complex disease with a significant number of recognized risk factors. Advancing age, male sex, family history of colorectal cancer, inflammatory bowel disease, smoking, excessive alcohol drinking, overweight and obesity, low levels of physical activity and sedentary lifestyle, diabetes and high consumption of red and processed meat are established risk factors (Table 1) [11–14]. Individuals with inflammatory bowel disease or those with first-degree relatives with colorectal cancer have an approximately twofold higher risk of developing the disease [15, 16]. Use of menopausal hormone therapy has been associated with lower risk of colorectal cancer in observational studies but findings from subsequent clinical trials have not shown consistent results [17–19]. Conversely, both observational studies and trials have demonstrated that nonsteroidal anti-inflammatory drugs (NSAID) and aspirin specifically can reduce the risk of colorectal cancer and pre-malignant colorectal adenomas [20–23]. Given the relative safety and high prevalence of NSAID/aspirin use worldwide, there has been anticipation that the use of these drugs could offer an effective preventive strategy for colorectal cancer. In particular, such pharmacologic interventions could be directed toward those at higher risk for this disease. Currently, trials are underway to examine the effect of aspirin administration on colorectal cancer development in both average-risk populations and among individuals with hereditary colorectal cancer (e.g. Lynch syndrome) and such a strategy could be extended to individuals at higher risk because of other risk factors [24].
Table 1.
Major risk factors for colorectal cancer and potential biological mechanisms
| Risk factor | Association with colorectal cancer | Potential mechanisms |
|---|---|---|
| Obesity | Raises risk: RR per 5 kg/m2 in BMI=1.08 (95% CI 1.04–1.11) | Hyperinsulinemia, elevated bioavailable IGF-I; elevated inflammation |
| Physical activity | Reduction in risk: RR comparing highest with lowest levels=0.81 (95% CI 0.69–0.95) | Reduction in insulin and inflammation (long-term); improved immune function |
| Adult height | Raises risk: RR per 5 cm=1.05 (95% CI 1.04–1.07) | Higher IGF-I levels; longer intestines/greater number of cells—greater opportunity for mutation acquisition |
| Alcohol | Raises risk: RR per 10 g/day=1.07 (95% CI 1.05–1.09) | Elevated acetaldehyde leading to oxidative stress, lipid peroxidation; pro-inflammatory effect; folate deficiency—interference with one carbon metabolism |
| Red and processed meat | Red meat: increases risk: RR per 100 g/day=1.12 (95% CI 1.00–1.25); processed meat—increases risk: RR per 50 g/day=1.16 (95% CI 1.08–1.26) | Elevated exposure to nitrites; endogenous N-nitroso compound formation; heme iron exposure; heterocyclic amine (HCA) and polycyclic aromatic hydrocarbon (PAH) exposure for meats cooked at high temperature |
| Fruit and vegetable intake | Reduction in risk: RR per 100 g/day=0.91 (95% CI 0.84–1.00) | Source of vitamins A, C, E; folate as well as other phytochemicals with potential antitumorigenic properties; fiber |
| Fiber | Reduction in risk: RR per 10 g/day=0.91 (95% CI 0.84–1.00) | Butyrate and other fermentation products; improved insulin sensitivity; reduced transit time |
| Dairy foods | Reduction in risk: RR per 400 g/day=0.84 (95% CI 0.80–0.89) | Elevated calcium; vitamin D; changes to gut microbiota (short chain fatty acids) |
| Aspirin/NSAIDs | Reduction in risk: RR comparing regular versus nonuse=0.79 (95% CI 0.74–0.85) | Inhibition of COX leads to reduction in inflammation; Inhibition of NF-kB activation (COX2-independent pathway)—anti-inflammatory and antiangiogenic effect |
| HRT | Reduction in risk for ‘current/recent use’ RR=0.67 (95% CI 0.59–0.77) | Possible antitumorigenic effect of estrogen in colorectal tissue mediated through ER-β |
Decades of research have focused on dietary factors: some studies have suggested a protective effect of diets rich in fruit, vegetables, fish, fiber and whole grains, calcium and dairy products against colorectal cancer [11] (Table 1). Epidemiological studies have also consistently shown an inverse association between circulating vitamin D concentrations and risk of this malignancy [25], although findings from recent genetic analyses do not support a causal relationship [26].
Overweight, obesity and type 2 diabetes (T2D) are established risk factors for colorectal cancer and it has been estimated that they may account for more than 10% of colorectal cancer cases worldwide [27–29]. Given the global rising prevalence of obesity and T2D, these conditions are likely to impact significantly on colorectal cancer incidence in the coming decades [30, 31]. Important questions remain regarding the underlying mechanisms linking colorectal cancer risk with many of its risk factors. The application of novel technologies such as metabolomics and proteomics that can simultaneously identify thousands of biological features in a single bio-sample hold promise and some novel insights have been provided by recent analyses [32, 33].
There is a growing body of experimental and observational evidence implicating the gut microbiome in colorectal cancer development and progression [34–36]. However, studies linking variation in the gut microbiome with colorectal cancer in human studies are still in their infancy. A small case–control study with available fecal samples demonstrated differences in the relative distribution of bacterial taxa between colorectal cancer cases and controls with enrichment of Bacteroidetes and depletion of Firmicutes [34]. In addition, increased carriage of genera Fusobacterium, Atopobium and Porphyromonas has also been associated with colorectal cancer [34, 37]. Fusobacterium are prevalent in colon tissue and can be observed in distal metastases, suggesting a possible role in the latter [38]. Atopobium, a Gram-positive anaerobic bacterium, has been associated with Crohn’s disease and has been reported to inhibit colonocyte apoptosis in vitro [37, 39]. These studies are consistent with the concept of ‘dysbiosis’, or microbiotic imbalance, leading to a pro-inflammatory microenvironment that is conducive to colorectal tumorigenesis. However, caution is required in the interpretation of case–control and cross-sectional studies due to the potential of reverse causality [40]. The prospective, systematic, and standardized collection of fecal samples as well as colorectal tissue specimens within the framework of well-characterized, population-based cohort studies is required to advance our knowledge on the etiologic role of the microbiota and possible effects through immunity on colorectal tumorigenesis [41].
Molecular pathology
Approximately 95% of presumed colorectal cancers are adenocarcinomas, invariably developing over more than 10 years, with dysplastic adenomas the most common form of premalignant precursor lesions. A number of different genomic alterations have been shown to be important for the development of colorectal cancer. Mutations in APC are an early event in the development of this cancer, followed by activating mutations of the KRAS oncogene and inactivating mutations of the TP53 tumor suppressor gene [42–44]. Molecular characterization of tumors, including somatic mutations in BRAF and KRAS, microsatellite instability (MSI), and CpG island methylator phenotype (CIMP), has provided evidence of multiple tumor subtypes that develop through activation of diverse neoplastic pathways [42, 43]. More recently, new landscape-style technologies such as extensive next-generation sequencing as applied in The Cancer Genome Atlas (TCGA) Project have enabled further characterization of the mutational repertoire of colorectal tumors, highlighting mutations in genes such as APC, TP53, SMAD4, PTEN, RNF43, FBXW7, and PIK3CA as well as in genes that are less well known, e.g. SOX9, B2M, or ACVR1B; such results indicate the importance of a complex number of key pathways, including those of MAPK, Wnt, or TGFβ signaling and more recently immune-modulating pathways [45, 46]. In addition, transcriptomic analyses have permitted the classification of colorectal tumors into four consensus molecular subtypes (CMS) with distinct features, namely; CMS1, which accounts for around 14% of colorectal tumors, exhibit hypermutated status, MSI and strong immune activation; CMS2 (37%), or the canonical epithelial subtype, shows marked Wnt and MYC signaling activation; CMS3 (13%) is characterized by substantial metabolic dysregulation; and CMS4 (23%) is a mesenchymal subtype that exhibits prominent transforming growth factor-β activation, stromal invasion and angiogenesis [47]. The integration of data on tumor molecular features such as genomic alterations and gene expression into epidemiological studies—a rapidly evolving field known as molecular pathological epidemiology—offers an opportunity to better map the importance of risk factors to specific subtypes of colorectal cancer [48, 49]. Already some important observations have been made—e.g. showing that smoking appears to be a specific risk factor for MSI-high, CIMP-positive and BRAF mutation positive tumors [50], while aspirin use is associated with lower mortality in COX2 positive, but not negative, colorectal cancer [22, 51, 52]. Such an approach may also inform the pathways linking diet and colorectal cancer development. For example, in a recent analysis it was demonstrated that the association between ω-3 polyunsaturated fatty acids was restricted to colorectal cancers characterized by a specific pattern of tumor-infiltrating T cells [53]. The integration of tumor molecular pathological data into large-scale investigations of germline genetic, lifestyle and environmental risk factors and colorectal cancer risk will likely yield new insights into the mechanisms of colorectal cancer development.
Another important consideration in the molecular pathology of colorectal cancer is the role of the immune system. Within the tumor microenvironment, both innate and adaptive immune cells are present and interact with the tumor via direct contact or through cytokine signaling that shapes the behavior of the tumor and its response to therapy. In particular, the presence of T cells in colorectal tumor tissue suggests that the adaptive immune system may be activated in colorectal tumorigenesis [54, 55]. Recent studies have demonstrated that the presence of a lymphocytic immune response in colorectal cancer is associated with more favorable patient outcomes [56–58]. However, while the prognostic implications of such an immune response are increasingly evident, the factors that modulate that reaction remains unclear. It is likely that the immune infiltrate is influenced by many factors, including the tumor microenvironment, tumor genomic alterations and the genetic background of the patient. The gut microbiota as well as physical activity, dietary and other exogenous environmental factors also likely play a role [59–62]. Future research that characterizes interactions between the immune system, tumor biology, and environmental factors may uncover important pathophysiological pathways for colorectal cancer.
Genetic factors
Colorectal cancer has a substantial heritable component, with large studies of twins suggesting up to 35% of colorectal cancer risk could be attributable to heritable factors [63]. The two most common forms of hereditary colorectal cancer are hereditary nonpolyposis colon cancer (Lynch syndrome) and familial adenomatous polyposis (FAP) coli which account for <5% of all colorectal cancer [64, 65]. Both syndromes are autosomal dominant disorders and follow the molecular pathogenesis typical of colorectal cancer: Lynch syndrome-associated cancers show signs of mismatch repair deficiency and are consequently MSI-high, whereas FAP-associated cancers follow the classic adenoma–carcinoma sequence. Additional rare, but high-penetrance, genetic variants have recently been implicated in colorectal cancer susceptibility including those in POLE, POLD1, and GREM1 associated with an autosomal dominant pattern of inheritance and MUTYH, MSH3, and NTHL1 with autosomal recessive inheritance [66–69]. However, together these genes account for <1% of colorectal cancers.
Despite intensive research efforts, the genetic factors that determine susceptibility to colorectal cancer beyond these hereditary forms are still incompletely understood. Genome-wide association studies (GWAS) have identified an increasing number of single nucleotide polymorphisms (SNPs) showing statistically significant but typically very small associations with risk of colorectal cancer. To date, more than 90 common SNPs associated with colorectal cancer susceptibility have been identified using genome-wide scans [70–79]. While the observed effect sizes are small, these discoveries have substantially strengthened and expanded our understanding of the biological processes underlying the development of this cancer. Genome-wide scans have implicated biological pathways anticipated to be involved in colorectal tumorigenesis (e.g. TGF-β, Wnt signaling, p53, PI3K, MAPK), as well as unexpected pathways (e.g. extracellular matrix maintenance, laminin gene family, Krüppel-like factors, HLA genes, Hedgehog signaling genes). These findings can point to new drug targets both for treating colorectal cancer as well as chemoprevention in high-risk groups. The identified loci explain <10% of the relative familial risk, suggesting that, as with other cancers, a significant number of genetic susceptibility loci remain to be identified for colorectal cancer. There is now a focus on the discovery of rare variants and on insertion/deletion polymorphisms using exome and whole-genome sequencing approaches. To date, the majority of GWAS analyses for colorectal cancer have been conducted in populations of European and Asian descent and there is a paucity of data in African, Hispanic, and other non-European populations.
Understanding the complex interplay between genetic (G) and environmental (E) factors in cancer development poses an important challenge. Increasingly larger GWAS datasets and novel statistical methods allow for comprehensive ‘agnostic’ genome-wide GxE interaction scans for colorectal cancer and these have started to yield novel statistically significant interactions [80–84]. Despite these successes and methodological developments, limited statistical power due to sample sizes remains a primary challenge for GxE analyses. Furthermore, the biology underlying the association of many of the identified genetic variants with colorectal cancer risk remains to be discovered. New technological approaches, e.g. using normal 3D epithelial colon organoids as models for testing the interaction between gene function and environmental factors are now being employed and are expected to enhance understanding of GxE interaction in colorectal cancer [85, 86].
Screening and early detection
The majority of colorectal cancers develop from normal epithelium through sequentially worsening degrees of adenomatous dysplasia. This, together with the strong correlation between stage at diagnosis and survival, provides the rationale for colorectal cancer screening programs [87]. However, questions remain regarding the optimal modality of colorectal cancer screening in terms of specificity, sensitivity, uptake, and economic impact. The guaiac-based fecal occult blood test (gFOBT) that has been widely implemented has excellent specificity but poor sensitivity, particularly for detection of colorectal adenomas. Nevertheless, screening-based randomized trials using gFOBT have reported significant reductions in colorectal cancer mortality [87–89]. Fecal immunochemical tests (FIT) for human hemoglobin in stool have been subsequently developed and are increasingly used. FIT has higher sensitivity for the detection of colorectal cancers and its precursors compared with gFOBT [90, 91]. However, no randomized trials of FIT as a screening tool to reduce colorectal cancer incidence or mortality have been reported thus far. Findings from observational studies are highly consistent with one incidence-based mortality study showing relative risks of death from colorectal cancer 10%–40% lower among those screened by FIT [92–94].
Results from a number of randomized trials from the United States and European countries on the effects of screening by flexible sigmoidoscopy have been published [95–99]. In all of the trials, flexible sigmoidoscopy was associated with a significant reduction in colorectal cancer incidence and colorectal cancer-related mortality. Long-term follow-up of the trial from the UK showed 26% lower colorectal cancer incidence and 30% lower colorectal cancer mortality after 17 years in those assigned to flexible sigmoidoscopy screening compared with those in the control arm [99]. In addition, observational data on the association of endoscopy-based screening with colorectal cancer incidence and mortality have generally shown consistent risk reductions. A meta-analysis of observational studies estimated risk reductions in both incidence and mortality of almost 70% with colonoscopy and almost 50% with sigmoidoscopy with a consistently stronger effect in the distal compared with the proximal colon [100]. Randomized trials of colonoscopy are currently ongoing but data on the effect on colorectal cancer incidence or mortality are not yet available. On the basis of current evidence, national, and international screening guidelines mostly recommend colorectal cancer screening starting between 50 and 55 years of age for individuals at average risk, with use of either annual or biennial gFOBT or FIT, flexible sigmoidoscopy every 5 years, or colonoscopy every 10 years [101–105]. For individuals at increased risk, such as first-degree relatives of individuals diagnosed with colorectal cancer at a younger age, initiation of screening at younger ages is recommended (e.g. starting at age 40 years). For high-risk groups (FAP, Lynch syndrome, or those with inflammatory bowel disease) specialized and much more rigorous prevention programs starting in early life are recommended. Organized colorectal cancer screening programs are yet to be developed and offered for most countries, however, in many countries with rising rates of colorectal cancer, screening programs are currently being evaluated [101]. Finally, recent changes in the guidelines in some countries, including the United States, now promote the initiation of screening at a younger age (45 years) for an average risk population based on modeling studies that support a benefit in view of the recently observed increase in the incidence rates of colorectal cancer among individuals younger than 50 years [106, 107].
Major research efforts are ongoing toward the development of alternative noninvasive blood or stool-based screening tests, such as blood-based DNA methylation or protein markers, circulating colorectal tumor cells (CTCs) and circulating tumor DNA (ctDNA), as well as stool-based DNA assays [108]. Thus far, these methods are in preliminary testing stages but it is plausible that the application of high throughput molecular and cellular methodology may lead to the discovery of highly sensitive biomarkers with clinical efficacy. Alternative imaging technologies, such as CT colonography for colorectal cancer screening is also an area of active exploration [87, 109]. In addition, due to economic constraints and low rates of uptake, there is a growing need for stratified or targeted screening. The development of risk prediction models for colorectal cancer that incorporate demographic, epidemiologic as well as genetic data has thus far yielded discriminative estimates (C-statistics) of 0.6–0.7 [110–113]. It is anticipated that the incorporation of additional genetic information as well as newly discovered biomarkers may eventually improve the efficacy of such models and render them useful for population-based risk stratification.
Therapy
Until recently, front-line therapy for colorectal cancer has relied on combination chemotherapy. For example, in high-risk stage II and III colorectal cancer, a combination of therapies such as 5-fluorouracil (5-FU), leucovorin and oxaliplatin or capecitabine with oxaliplatin are administered [114]. For metastatic colorectal cancer, oxaliplatin or 5-FU/leucovorin/irinotecan are standard treatment [115]. Profiling of colorectal tumors for RAS mutational status as a prognostic marker and indicator of therapeutic response has been common practice for some time, however, a number of biomarkers beyond RAS mutational status are now emerging which may impact on the response to all classes of new targeted agents, and specifically for EGFR-antibody therapies. The era of targeted therapy has widened the horizons for treatment of newly diagnosed and relapsed colorectal cancer. Tests focused on known recurrent genetic aberrations in colorectal cancer include HER2, MET and KRAS gene amplification, ligands such as transforming growth factor-α, amphiregulin and epiregulin, EGFR mutations and alterations/mutations in HER3, PI3KCA and PTEN [114]. PIK3CA and PTEN alterations, which often co-occur with KRAS or BRAF mutations [116], are also under investigation but there is currently inadequate evidence for their use as biomarkers of resistance to EGFR-antibody therapy.
As discussed, the immune system plays a critical role in colorectal cancer development and progression. Immunotherapies targeting immune checkpoints such as CTLA4, PDCD1 (PD-1), and CD274 (PD-L1) have led to important advances that have revolutionized the treatment of many solid tumors [117]. For colorectal cancer, promising activity has been documented for immune checkpoint inhibitors in patients with metastatic MSI high colorectal cancer [118]. Additional trials to determine the role of immune check point inhibitors in earlier stages of MSI colorectal cancer or according to other molecular markers are currently underway. In situ immune cell infiltrate in tumors has been consistently associated with a favorable prognosis and an ‘immunoscore’ which is derived from a measure of CD3-positive and CD8-positive density in the tumor is predictive of tumor recurrence [119]. In a recent multi-national investigation of ∼2650 patients with stage I–III stage colorectal cancer, patients with a high immunoscore had a statistically significant 60% reduction in risk of recurrence over the follow-up period and the score was shown to have greater prognostic value than TMN stage, lymphovascular invasion and MSI status [56]. Such a score could prove invaluable for identifying patients who would benefit from adjuvant therapies. Future studies should investigate whether the immunoscore has utility for therapeutic response, as well as go more deeply in the precise interactions between the tumor and immune system. New technologies including single-cell transcriptome analysis, and in vivo pathology, will likely improve the characterization of tumour–immune interactions.
Multiple tumor-associated antigens have been identified and utilized for vaccination with varying degrees of success, e.g. carcinoembryonic antigen, mucin-1, squamous cell carcinoma antigen recognized by T cells 3, as well as p53, all of which have been employed as targets for immunotherapy in colorectal cancer, as well as for other tumors [117]. Finally, although CTC number correlates with prognosis in patients with metastatic colorectal cancer, the clinical utility of CTC assessments is not yet clear and therefore cannot be recommended [114, 120]. Similarly, the utility of liquid ctDNA biopsies to guide treatment decisions is currently under investigation in clinical trials, but cannot yet be proposed in routine practice.
Discussion
Conclusion
Colorectal cancer is one of the most commonly diagnosed malignancies worldwide and its incidence is rising in many countries. There are a number of potentially modifiable risk factors for colorectal cancer and measures to alter the prevalence of those risk factors and promote healthy lifestyles could provide strategies for primary prevention. Several risk factors, including smoking, excessive alcohol consumption, and obesity, are shared with other common cancers and noncommunicable diseases such as diabetes and cardiovascular disease, and could be included in comprehensive primary prevention strategies. Improved understanding of the molecular pathogenesis of colorectal cancer including its etiologic pathways, genetic determinants and causes of somatic changes, as well as interactions with the immune system and the microbiome, could enable more targeted prevention and therapeutic strategies. As has been shown for cardiovascular disease, substantial progress has been made in prevention through understanding of etiology (e.g. dietary and lifestyle changes, statins), rather than treating advanced disease. Accordingly, it is important to extend our efforts to gaining mechanistic insight into how certain risk factors affect colorectal cancer development. The pathogenesis of colorectal cancer and the relative accessibility of the colorectum render this malignancy amenable to screening and secondary prevention. Future efforts should be directed toward identifying population strata that would benefit most from screening and chemopreventive strategies that could be better tailored to individual risk assessment, as a realization of precision prevention in oncology.
Important questions to be prioritized:
What factors explain the geographical, racial/ethnic and sex differences in the incidence of colorectal cancer and what are the factors driving distinct trends in different populations?
What are the underlying factors driving recent increases in colorectal cancer incidence in younger adults observed in several high-income countries? More broadly, what is the influence of age on risk factors and preventive interventions for colorectal cancer?
What are the specific metabolic pathways that underlie the association of obesity and diabetes with colorectal cancer? Can the application of ‘omics’ technologies such as metabolomics and proteomics help uncover relevant pathophysiological mechanisms and identify novel biomarkers of risk and progression?
Can aspirin/NSAIDs or novel chemopreventive agents be used to prevent colorectal cancer in high risk groups including those defined by high penetrance mutations (e.g. Lynch syndrome patients) and those with specific risk factors (e.g. obesity)?
What is the contribution of the gut microbiota to colorectal cancer development and can we evaluate this in population-based studies with pre-diagnostic measurements of the microbiota?
Can we develop a more comprehensive portrait of the colorectal tumor somatic profile and its etiologic origins and continue to incorporate molecular pathological data into large-scale epidemiological studies?
What is the precise contribution of the immune system to colorectal tumorigenesis and what factors modulate this response?
Can we continue to define a comprehensive catalog of germline susceptibility alleles assessed at the population and family level and explore their interactions with environmental factors?
Can we advance genomic functional analysis to understand individual susceptibility alleles and pathways in order to give new insights into biology (early developmental changes, mechanism of carcinogenesis)?
Can we better define the penetrance of high-risk colorectal cancer alleles by investigating their association with colorectal cancer in different populations and in family and clinic-based settings?
Will it be possible to move toward an integrative model for colorectal cancer risk in different populations based on epidemiologic factors, genetics and biomarkers (including microbiome) and can this model be of clinical/translational utility?
What are the optimal screening modalities for colorectal cancer in different countries? How can we best promote uptake and adherence to high quality screening programs and can we make screening more efficacious and cost-effective by targeting to population strata at greater risk?
As we acquire greater understanding of colorectal cancer biology can such insights translate into new therapies and can we more precisely identify patient strata who would receive greater benefit from adjuvant therapy?
Funding
This seminar was funded jointly by the International Agency for Research on Cancer (IARC) and the Division of Cancer Epidemiology and Genetics (DCEG) of the US National Cancer Institute Intramural Research Program (no grant number applicable).
Disclosure
The authors have declared no conflicts of interest.
References
- 1. Bray F, Ferlay J, Soerjomataram I. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6): 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Arnold M, Sierra MS, Laversanne M. et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017; 66(4): 683–691. [DOI] [PubMed] [Google Scholar]
- 3. Murphy CC, Harlan LC, Lund JL. et al. Patterns of colorectal cancer care in the United States: 1990–2010. J Natl Cancer Inst 2015; 107(10). doi: 10.1093/jnci/djv198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenner H, Schrotz-King P, Holleczek B. et al. Declining bowel cancer incidence and mortality in Germany: an analysis of time trends in the first ten years after the introduction of screening colonoscopy. Deutsch Ärztebl Int 2016; 113(7): 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siegel R, Miller KD, Fedewa SA. et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017; 67(3): 177–193. [DOI] [PubMed] [Google Scholar]
- 6. Troeung L, Sodhi-Berry N, Martini A. et al. Increasing incidence of colorectal cancer in adolescents and young adults aged 15–39 years in Western Australia 1982–2007: examination of colonoscopy history. Front Public Health 2017; 5: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy G, Devesa SS, Cross AJ. et al. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer 2011; 128(7): 1668–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allemani C, Matsuda T, Di Carlo V. et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391(10125): 1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sankaranarayanan R, Swaminathan R, Brenner H. et al. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol 2010; 11(2): 165–173. [DOI] [PubMed] [Google Scholar]
- 10. Maringe C, Walters S, Rachet B. et al. Stage at diagnosis and colorectal cancer survival in six high-income countries: a population-based study of patients diagnosed during 2000-2007. Acta Oncol 2013; 52(5): 919–932. [DOI] [PubMed] [Google Scholar]
- 11. https://www.wcrf.org/dietandcancer/colorectal-cancer (18 December 2018, date last accessed).
- 12. Johnson CM, Wei C, Ensor JE. et al. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013; 24(6): 1207–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moore SC, Lee IM, Weiderpass E. et al. Association of Leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med 2016; 176(6): 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morris JS, Bradbury KE, Cross AJ. et al. Physical activity, sedentary behaviour and colorectal cancer risk in the UK Biobank. Br J Cancer 2018; 118(6): 920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taylor DP, Burt RW, Williams MS. et al. Population-based family history-specific risks for colorectal cancer: a constellation approach. Gastroenterology 2010; 138(3): 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jess T, Rungoe C, Peyrin-Biroulet L.. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol 2012; 10(6): 639–645. [DOI] [PubMed] [Google Scholar]
- 17. Green J, Czanner G, Reeves G. et al. Menopausal hormone therapy and risk of gastrointestinal cancer: nested case–control study within a prospective cohort, and meta-analysis. Int J Cancer 2012; 130(10): 2387–2396. [DOI] [PubMed] [Google Scholar]
- 18. Chlebowski RT, Wactawski-Wende J, Ritenbaugh C. et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med 2004; 350(10): 991–1004. [DOI] [PubMed] [Google Scholar]
- 19. Simon MS, Chlebowski RT, Wactawski-Wende J. et al. Estrogen plus progestin and colorectal cancer incidence and mortality. J Clin Oncol 2012; 30 (32): 3983–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baron JA, Cole BF, Sandler RS. et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 2003; 348(10): 891–899. [DOI] [PubMed] [Google Scholar]
- 21. Sandler RS, Halabi S, Baron JA. et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 2003; 348(10): 883–890. [DOI] [PubMed] [Google Scholar]
- 22. Chan AT, Ogino S, Fuchs CS.. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 2007; 356(21): 2131–2142. [DOI] [PubMed] [Google Scholar]
- 23. Cook NR, Lee IM, Zhang SM. et al. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med 2013; 159(2): 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burn J, Gerdes AM, Macrae F. et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011; 378(9809): 2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCullough ML, Zoltick ES, Weinstein SJ. et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J Natl Cancer Inst 2019; 111(2): 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dimitrakopoulou VI, Tsilidis KK, Haycock PC. et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ 2017; 359: j4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lauby-Secretan B, Scoccianti C, Loomis D. et al. International agency for research on cancer handbook working group. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med 2016; 375(8): 794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thrift AP, Gong J, Peters U. et al. Mendelian randomization study of body mass index and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 2015; 24(7): 1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsilidis KK, Kasimis JC, Lopez DS. et al. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 2015; 350: g7607.. [DOI] [PubMed] [Google Scholar]
- 30. Pearson-Stuttard J, Zhou B, Kontis V. et al. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol 2018; 6(2): 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017; 390: 2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murphy N, Jenab M, Gunter MJ.. Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nat Rev Gastroenterol Hepatol 2018; 15(11): 659–670. [DOI] [PubMed] [Google Scholar]
- 33. Shu X, Xiang YB, Rothman N. et al. Prospective study of blood metabolites associated with colorectal cancer risk. Int J Cancer 2018; 143(3): 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahn J, Sinha R, Pei Z. et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst 2013; 105(24): 1907–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zeller G, Tap J, Voigt AY. et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol Syst Biol 2014; 10(11): 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zackular JP, Baxter NT, Iverson KD. et al. The gut microbiome modulates colon tumorigenesis. mBio 2013; 4: e00692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen W, Liu F, Ling Z. et al. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One 2012; 7(6): e39743.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bullman S, Pedamallu CS, Sicinska E. et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017; 358(6369): 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manichanh C, Rigottier-Gois L, Bonnaud E. et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 2006; 55(2): 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Amitay EL, Werner S, Vital M. et al. Fusobacterium and colorectal cancer: causal factor or passenger? Results from a large colorectal cancer screening study. Carcinogenesis 2017; 38(8): 781–788. [DOI] [PubMed] [Google Scholar]
- 41. Sinha R, Abu-Ali G, Vogtmann E. et al. Assessment of variation in microbial community amplicon sequencing by the Microbiome Quality Control (MBQC) project consortium. Nat Biotech 2017; 35: 1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vogelstein B, Fearon ER, Hamilton SR. et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988; 319(9): 525–532. [DOI] [PubMed] [Google Scholar]
- 43. Armaghany T, Wilson JD, Chu Q, Mills G.. Genetic alterations in colorectal cancer. Gastrointest Cancer Res 2012; 5(1): 19–27. [PMC free article] [PubMed] [Google Scholar]
- 44. Vogelstein B, Papadopoulos N, Velculescu VE. et al. Cancer genome landscapes. Science 2013; 339(6127): 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grasso CS, Giannakis M, Wells DK. et al. Genetic mechanisms of immune evasion in colorectal cancer. Cancer Discov 2018; 8(6): 730–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guinney J, Dienstmann R, Wang X. et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015; 21(11): 1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ogino S, Stampfer M.. Colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst 2010; 102(6): 365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ogino S, Chan AT, Fuchs CS, Giovannucci E.. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut 2011; 60(3): 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Limsui D, Vierkant RA, Tillmans LS. et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst 2010; 102(14): 1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chan AT, Ogino S, Fuchs CS.. Aspirin use and survival after diagnosis of colorectal cancer. JAMA 2009; 302(6): 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gray RT, Cantwell MM, Coleman HG. et al. Evaluation of PTGS2 expression, PIK3CA mutation, aspirin use and colon cancer survival in a population-based cohort study. Clin Transl Gastroenterol 2017; 8(4): e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Song M, Nishihara R, Cao Y. et al. Marine ω-3 polyunsaturated fatty acid intake and risk of colorectal cancer characterized by tumor-infiltrating T cells. JAMA Oncol 2016; 2(9): 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Le DT, Hubbard-Lucey VM, Morse MA. et al. A blueprint to advance colorectal cancer immunotherapies. Cancer Immunol Res 2017; 5(11): 942–949. [DOI] [PubMed] [Google Scholar]
- 55. Grizzi F, Basso G, Borroni EM. et al. Evolving notions on immune response in colorectal cancer and their implications for biomarker development. Inflamm Res 2018; 67(5): 375–389. [DOI] [PubMed] [Google Scholar]
- 56. Pages F, Mlecnik B, Marliot F. et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 2018; 391(10135): 2128–2139. [DOI] [PubMed] [Google Scholar]
- 57. Ogino S, Nosho K, Irahara N. et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res 2009; 15(20): 6412–6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Galon J, Costes A, Sanchez CF. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313(5795): 1960–1964. [DOI] [PubMed] [Google Scholar]
- 59. Mima K, Sukawa Y, Nishihara R. et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol 2015; 1(5): 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kalra R, Singh SP, Savage SM. et al. Effects of cigarette smoke on immune response: chronic exposure to cigarette smoke impairs antigen-mediated signaling in T cells and depletes IP3-sensitive Ca(2+) stores. J Pharmacol Exp Ther 2000; 293: 166–171. [PubMed] [Google Scholar]
- 61. Hussain M, Javeed A, Ashraf M. et al. Aspirin and immune system. Int Immunopharmacol 2012; 12(1): 10–20. [DOI] [PubMed] [Google Scholar]
- 62. Huh JY, Park YJ, Ham M. et al. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells 2014; 37(5): 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lichtenstein P, Holm NV, Verkasalo PK. et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 2000; 343(2): 78–85. [DOI] [PubMed] [Google Scholar]
- 64. Lynch HT, de la Chapelle A.. Hereditary colorectal cancer. N Engl J Med 2003; 348(10): 919–932. [DOI] [PubMed] [Google Scholar]
- 65. Jasperson KW, Tuohy TM, Neklason DW, Burt RW.. Hereditary and familial colon cancer. Gastroenterology 2010; 138(6): 2044–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Palles C, Cazier J-B, Howarth KM. et al. Germline mutations in the proof-reading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet 2013; 45(2): 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jaeger E, Leedham S, Lewis A. et al. Hereditary mixed polyposis syndrome is caused by a 40kb upstream duplication that leads to increased and ectopic expression of the BMP antagonist GREM1. Nat Genet 2012; 44(6): 699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weren RD, Ligtenberg MJ, Kets CM. et al. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat Genet 2015; 47(6): 668–671. [DOI] [PubMed] [Google Scholar]
- 69. Adam R, Spier I, Zhao B. et al. Exome sequencing identifies biallelic MSH3 germline mutations as a recessive subtype of colorectal adenomatous polyposis. Am J Hum Genet 2016; 99(2): 337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tomlinson I, Webb E, Carvajal-Carmona L. et al. A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat Genet 2007; 39(8): 984–988. [DOI] [PubMed] [Google Scholar]
- 71. Broderick P, Carvajal-Carmona L, Pittman AM. et al. A genome-wide association study shows that common alleles of SMAD7 influence colorectal cancer risk. Nat Genet 2007; 39(11): 1315–1317. [DOI] [PubMed] [Google Scholar]
- 72. Tenesa A, Farrington SM, Prendergast JGD. et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on 11q23 and replicates risk loci at 8q24 and 18q21. Nat Genet 2008; 40(5): 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Houlston RS, Webb E, Broderick P. et al. Meta-analysis of genome-wide association data identifies four new susceptibility loci for colorectal cancer. Nat Genet 2008; 40(12): 1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Houlston RS, Cheadle J, Dobbins SE. et al. Meta-analysis of three genome-wide association studies identifies susceptibility loci for colorectal cancer at 1q41, 3q26.2, 12q13.13 and 20q13.33. Nat Genet 2010; 42(11): 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jia W-H, Zhang B, Matsuo K. et al. Genome-wide association analyses in East Asians identify new susceptibility loci for colorectal cancer. Nat Genet 2013; 45(2): 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Peters U, Hutter CM, Hsu L. et al. Meta-analysis of new genome-wide association studies of colorectal cancer risk. Hum Genet 2012; 131(2): 217–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Peters U, Jiao S, Schumacher FR. et al. Identification of genetic susceptibility loci for colorectal tumors in a genome-wide meta-analysis. Gastroenterology 2013; 144(4): 799–807.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schmit SL, Edlund CK, Schumacher FR. et al. Novel common genetic susceptibility loci for colorectal cancer. J Natl Cancer Inst 2019; 111(2): 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Huyghe JR, Bien SA, Harrison TA. et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat Genet 2019; 51(1): 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gauderman WJ, Zhang P, Morrison JL, Lewinger JP.. Finding novel genes by testing G × E interactions in a genome-wide association study. Genet Epidemiol 2013; 37(6): 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Siegert S, Hampe J, Schafmayer C. et al. Genome-wide investigation of gene-environment interactions in colorectal cancer. Hum Genet 2013; 132(2): 219–231. [DOI] [PubMed] [Google Scholar]
- 82. Hutter CM, Slattery ML, Duggan DJ. et al. Characterization of the association between 8q24 and colon cancer: gene-environment exploration and meta-analysis. BMC Cancer 2010; 10: 670.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hutter CM, Chang-Claude J, Slattery ML. et al. Characterization of gene-environment interactions for colorectal cancer susceptibility loci. Cancer Res 2012; 72: 2036–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Figueiredo JC, Hsu L, Hutter CM. et al. Genome-wide diet-gene interaction analyses for risk of colorectal cancer. PLoS Genet 10(4): e1004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Crespo M, Vilar E, Tsai S-Y. et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat Med 2017; 23(7): 878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sato T, Clevers H.. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 2013; 340(6137): 1190–1194. [DOI] [PubMed] [Google Scholar]
- 87. Lauby-Secretan B, Vilahur N, Bianchini F. et al. ; International Agency for Research on Cancer Handbook Working Group. The IARC Perspective on Colorectal Cancer Screening. N Engl J Med 2018; 378(18): 1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shaukat A, Mongin SJ, Geisser MS. et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013; 369(12): 1106–1114. [DOI] [PubMed] [Google Scholar]
- 89. Scholefield JH, Moss SM, Mangham CM. et al. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut 2012; 61(7): 1036–1040. [DOI] [PubMed] [Google Scholar]
- 90. Lee JK, Liles EG, Bent S. et al. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 2014; 160: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brenner H, Tao S.. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer 2013; 49(14): 3049–3054. [DOI] [PubMed] [Google Scholar]
- 92. Chiu HM, Chen SL, Yen AM. et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer 2015; 121(18): 3221–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Giorgi Rossi P, Vicentini M, Sacchettini C. et al. Impact of screening program on incidence of colorectal cancer: a cohort study in Italy. Am J Gastroenterol 2015; 110(9): 1359–1366. [DOI] [PubMed] [Google Scholar]
- 94. Ventura L, Mantellini P, Grazzini G. et al. The impact of immunochemical faecal occult blood testing on colorectal cancer incidence. Dig Liver Dis 2014; 46(1): 82–86. [DOI] [PubMed] [Google Scholar]
- 95. Schoen RE, Pinsky PF, Weissfeld JL. et al. Colorectal cancers not detected by screening flexible sigmoidoscopy in the prostate, lung, colorectal, and ovarian cancer screening trial. Gastrointest Endosc 2012; 75(3): 612–620. [DOI] [PubMed] [Google Scholar]
- 96. Atkin WS, Edwards R, Kralj-Hans I. et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010; 375(9726): 1624–1633. [DOI] [PubMed] [Google Scholar]
- 97. Holme Ø, Løberg M, Kalager M. et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA 2014; 312(6): 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Segnan N, Senore C, Andreoni B. et al. Baseline findings of the Italian multicenter randomized controlled trial of “once-only sigmoidoscopy”—SCORE. J Natl Cancer Inst 2002; 94(23): 1763–1772. [DOI] [PubMed] [Google Scholar]
- 99. Atkin W, Wooldrage K, Parkin DM. et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet 2017; 389(10076): 1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Brenner H, Stock C, Hoffmeister M.. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. The BMJ 2014; 348(apr09 1): g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schreuders EH, Ruco A, Rabeneck L. et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015; 64(10): 1637–1649. [DOI] [PubMed] [Google Scholar]
- 102. Koo S, Neilson LJ, Von Wagner C, Rees CJ.. The NHS Bowel Cancer Screening Program: current perspectives on strategies for improvement. Risk Manag Health Policy 2017; 10: 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.European Colorectal Cancer Screening Guidelines Working Group, von Karsa L, Patnick J. et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy 2013; 45(1): 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Alsanea N, Almadi MA, Abduljabbar AS. et al. National guidelines for colorectal cancer screening in Saudi Arabia with strength of recommendations and quality of evidence. Ann Saudi Med 2015; 35(3): 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Siew C, Ng Sunny H.. Wong Colorectal cancer screening in Asia. Br Med Bull 2013; 105(1): 29–42. [DOI] [PubMed] [Google Scholar]
- 106. https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/acs-recommendations.html (18 December 2018, date last accessed).
- 107. Wolf AMD, Fontham ETH, Church TR. et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018; 68(4): 250–281. [DOI] [PubMed] [Google Scholar]
- 108. Shah R, Jones E, Vidart V. et al. Biomarkers for early detection of colorectal cancer and polyps: systematic review. Cancer Epidemiol Biomarkers Prev 2014; 23(9): 1712–1728. [DOI] [PubMed] [Google Scholar]
- 109. Gandon Y. Screening for colorectal cancer: the role of CT colonography. Diagn Interv Imaging 2014; 95(5): 467–474. [DOI] [PubMed] [Google Scholar]
- 110. Smith T, Muller DC, Moons KGM. et al. Comparison of prognostic models to predict the occurrence of colorectal cancer in asymptomatic individuals: a systematic literature review and external validation in the EPIC and UK Biobank prospective cohort studies. Gut 2018. Apr 3 [Epub ahead of print], doi: 10.1136/gutjnl-2017-315730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hsu L, Jeon J, Brenner H. et al. A model to determine colorectal cancer risk using common genetic susceptibility loci. Gastroenterology 2015; 148(7): 1330–1339.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dunlop MG, Tenesa A, Farrington SM. et al. Cumulative impact of common genetic variants and other risk factors on colorectal cancer risk in 42, 103 individuals. Gut 2013; 62(6): 871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jeon J, Du M, Schoen RE. et al. Determining risk of colorectal cancer and starting age of screening based on lifestyle, environmental and genetic factors. Gastroenterology 2018; 154(8): 2152–2164.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Labianca R, Nordlinger B, Beretta GD. et al. Primary colon cancer: ESMO clinical practice guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol 2013; 24(Suppl 6): vi64–vi72. [DOI] [PubMed] [Google Scholar]
- 115. Grothey A. Optimizing systemic therapy selection in metastatic colorectal cancer. J Natl Compr Canc Netw 2015; 13(Suppl 5): 682–685. [DOI] [PubMed] [Google Scholar]
- 116. Yang ZY, Wu XY, Huang YF. et al. Promising biomarkers for predicting the outcomes of patients with KRAS wild-type metastatic colorectal cancer treated with anti-epidermal growth factor receptor monoclonal antibodies: a systematic review with meta-analysis. Int J Cancer 2013; 133(8): 1914–1925. [DOI] [PubMed] [Google Scholar]
- 117. Basile D, Garattini SK, Bonotto M. et al. Immunotherapy for colorectal cancer: where are we heading? Expert Opin Biol Ther 2017; 17(6): 709–721. [DOI] [PubMed] [Google Scholar]
- 118. Le DT, Uram JN, Wang H. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372(26): 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wirta EV, Seppala T, Friman M. et al. Immunoscore in mismatch repair-proficient and –deficient colon cancer. J Pathol Clin Res 2017; 3(3): 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cohen SJ, Punt CJA, Iannotti N. et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26(19): 3212–3221. [DOI] [PubMed] [Google Scholar]