Abstract
The basal ganglia and the cerebellum are considered to be distinct subcortical systems that perform unique functional operations. The outputs of the basal ganglia and the cerebellum influence many of the same cortical areas but do so by projecting to distinct thalamic nuclei. As a consequence, the two subcortical systems were thought to be independent and to communicate only at the level of the cerebral cortex. Here, we review recent data showing that the basal ganglia and the cerebellum are interconnected at the subcortical level. The subthalamic nucleus in the basal ganglia is the source of a dense disynaptic projection to the cerebellar cortex. Similarly, the dentate nucleus in the cerebellum is the source of a dense disynaptic projection to the striatum. These observations lead to a new functional perspective that the basal ganglia, the cerebellum and the cerebral cortex form an integrated network. This network is topographically organized so that the motor, cognitive and affective territories of each node in the network are interconnected. This perspective explains how synaptic modifications or abnormal activity at one node can have network-wide effects. A future challenge is to define how the unique learning mechanisms at each network node interact to improve performance.
The essential functional architectures of basal ganglia and cerebellar circuits with the cerebral cortex have several elements in common. The input stages of these circuits are targets of extensive projections from widespread regions of the cerebral cortex1,2, and the output stages send efferents to regions of the thalamus that innervate multiple motor and non-motor areas of the cerebral cortex3–5. An important element of cortico-basal ganglia and cortico-cerebellar circuits is their ‘closed-loop’ architecture: the regions of the cerebral cortex that are the major sources of input to a circuit are also major targets of output from the circuit6,7. Although the thalamic targets of basal ganglia output are different from those of cerebellar output8, the two systems influence many of the same areas of the cerebral cortex (FIG. 1). This structural arrangement has led to proposals that these subcortical circuits with the cerebral cortex have independent but complementary functions9.
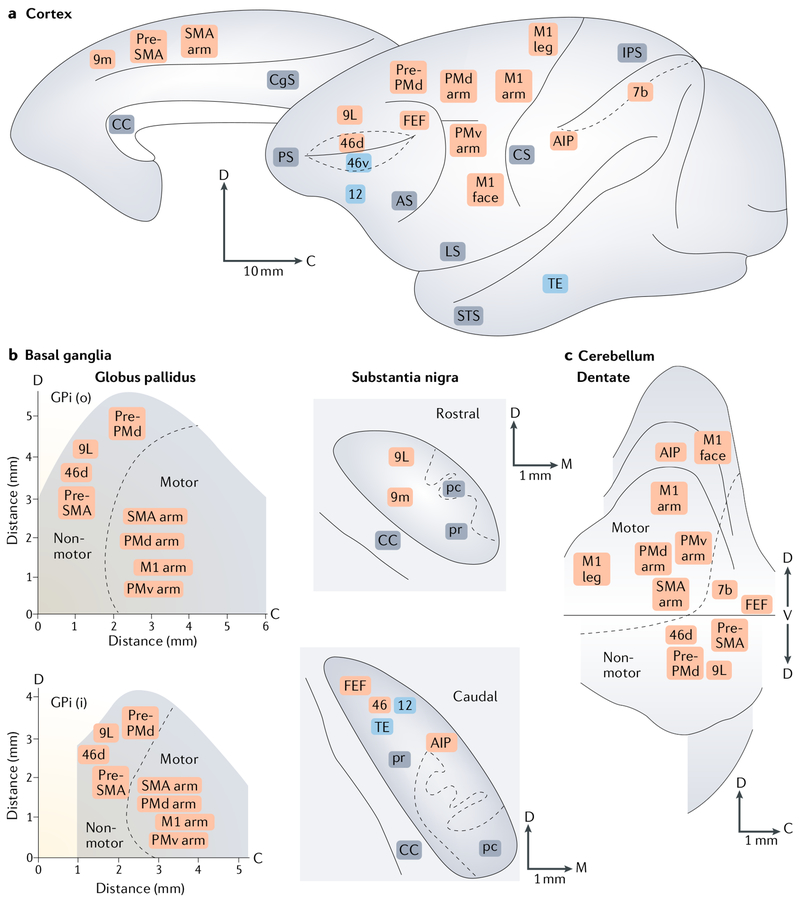
Fig. 1 |. Organization of basal ganglia and cerebellar outputs to the cerebral cortex.
a | The cortical targets of basal ganglia and cerebellar outputs are indicated on medial and lateral views of the Cebus monkey brain. b,c | These panels show summary maps of topography in the basal ganglia (part b) and cerebellar (part c) output nuclei on the basis of their cortical targets. The division between motor and non-motor areas of the internal segment of the globus pallidus (GPi) and the dentate nucleus is indicated by the dashed lines. In all panels, orange labels indicate areas of the cerebral cortex that are the targets of both basal ganglia and cerebellar outputs, whereas blue labels indicate areas of the cerebral cortex that are the targets of basal ganglia, but not cerebellar, output. The numbers refer to cytoarchitectonic areas. AIP, anterior intraparietal area; AS, arcuate sulcus; C, caudal; CC, corpus callosum; CgS, cingulate sulcus; CS, central sulcus; D and d, dorsal; FEF, frontal eye field; i, the inner portion of the internal segment of the globus pallidus; IPS, intraparietal sulcus; LS, lateral sulcus; M and m, medial; M1, primary motor cortex; M1 arm, arm area of M1; M1 face, face area of M1; M1 leg; leg area of M1; o, the outer portion of the internal segment of the globus pallidus; pc, pars compacta; PMd arm, arm area of the dorsal premotor area; PMv arm, arm area of the ventral premotor area; pr, pars reticulata; Pre-PMd, predorsal premotor area; Pre-SMA, presupplementary motor area; PS, principal sulcus; SMA arm, arm area of the supplementary motor area; STS, superior temporal sulcus; TE, area of inferotemporal cortex. Based on data from REFS4,5,154–156.
Interactions between basal ganglia and cerebellar circuits were thought to occur mainly at the level of the cerebral cortex. Over the past decade, results from neuroanatomical studies in nonhuman primates have inspired a dramatic shift in this perspective. These results have provided evidence that the basal ganglia and the cerebellum are not independent subcortical systems but, instead, form a densely interconnected network10. Thus, changes at one node can percolate throughout the entire network to influence operations at other nodes. This integrated network perspective provides a new way of conceptualizing the functional organization of basal ganglia and cerebellar circuits with the cerebral cortex.
In this Review, we first present the evidence from studies in non-human primates that output from motor and non-motor regions of the cerebellum can influence the input stage of basal ganglia processing through a disynaptic subcortical pathway11. Second, we present the evidence that output from motor and non-motor regions of a nucleus in the basal ganglia, the subthalamic nucleus (STN), can influence the input stage of cerebellar processing through a disynaptic subcortical pathway12. For each of these connections, we discuss the presence of corresponding pathways in rodents. Last, we highlight evidence, largely from human neuroimaging studies, that these pathways may mediate meaningful interactions between cortico-basal ganglia and cortico-cerebellar circuits in health and disease. These observations provide support for the concept that the basal ganglia, the cerebellum and the cerebral cortex are nodes in an interconnected network that operates over multiple functional domains.
Anatomical evidence
Cerebellar output to the basal ganglia
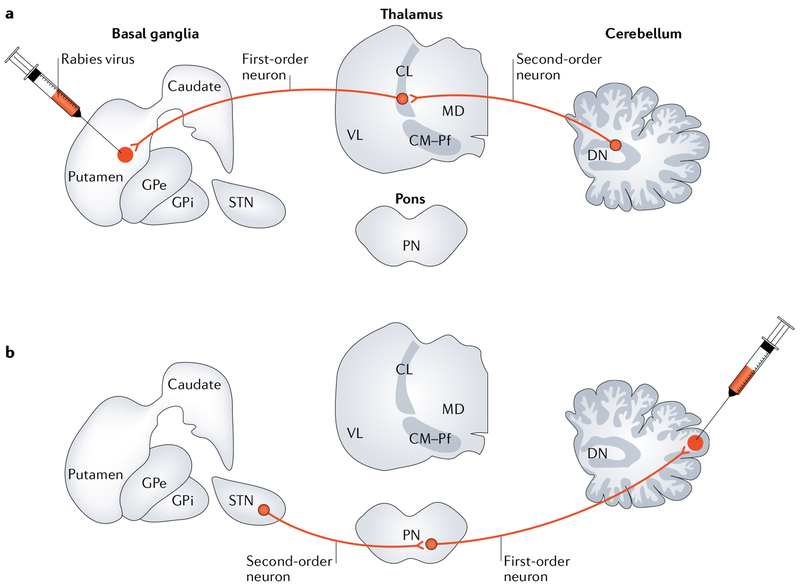
It is now clear that neurons in the cerebellar output nuclei, especially in the dentate nucleus, are the origin of a disynaptic pathway to an input stage of basal ganglia processing, the striatum (FIG. 2a). Specifically, rabies virus injections into the sensorimotor territory of the putamen of macaque monkeys labelled substantial numbers of neurons in the cerebellar nuclei11. In these experiments, rabies virus underwent retrograde transport to first-order neurons that project to the striatum (for example, neurons in the cerebral cortex and thalamus) and then retrograde transneuronal transport to second-order neurons that innervate the first-order neurons. Second-order neurons in the cerebellum were labelled primarily in the dentate nucleus. A few second-order neurons were labelled in the interposed and fastigial nuclei, suggesting that these nuclei are an additional source of cerebellar influence over basal ganglia function.
Fig. 2 |. Anatomical connections.
a | A study using retrograde transneuronai transport of rabies virus in monkeys revealed a disynaptic pathway from the dentate nucleus (DN) to the putamen11. Rabies virus was injected into the putamen and underwent retrograde transport to first-order neurons that project to the striatum (for example, neurons in the intralaminar thalamic nuclei) and then retrograde transneuronal transport to second-order neurons that innervate the first-order neurons. The second-order neurons in the cerebellum were located primarily in the DN. b | A study using retrograde transneuronal transport of rabies virus in monkeys revealed a disynaptic pathway from the subthalamic nucleus (STN) to the cerebellar cortex12. Rabies virus was injected into the lateral cerebellar cortex and underwent retrograde transport to first-order neurons that project to the cerebellar cortex (for example, neurons in the pontine nuclei (PN)) and then retrograde transneuronal transport to second-order neurons that innervate the first-order neurons. The study revealed second-order neurons labelled in the basal ganglia, primarily in the STN. CL, central lateral thalamic nucleus; CM, central medial thalamic nucleus; GPe, external segment of the globus pallidus; GPi, internal segment of the globus pallidus; MD, medial dorsal thalamic nucleus; Pf, parafascicular thalamic nucleus; VL, ventral lateral thalamic nucleus.
At survival times that enabled transneuronal transport of rabies virus to third-order neurons, injections into the external segment of the globus pallidus (GPe) led to the labelling of large numbers of neurons in the dentate nucleus11. Different regions of the dentate contained labelled neurons after injections into spatially separate regions of GPe. This finding suggests that the projection from the cerebellum to the basal ganglia is topographically organized. Furthermore, labelled neurons were located in both the motor and non-motor domains of the dentate nucleus5. Thus, both motor and non-motor outputs from the cerebellum may affect the motor and non-motor functions of the basal ganglia.
The basal ganglia include at least two internal routes of information processing: the ‘direct’ and ‘indirect’ path-ways (reviewed in REF.13). The direct pathway refers to the monosynaptic projection from specific medium spiny neurons (MSNs) in the striatum, which express substance P and dopamine D1 receptors, to neurons in the internal segment of the globus pallidus (GPi) and substantia nigra pars reticulata (SNpr). The indirect pathway refers to the projection from a different set of MSNs in the striatum, which express enkephalin and dopamine D2 receptors, to neurons in the GPe. Neurons in the GPe then project to the GPi and SNpr monosynaptically, as well as disynaptically via the STN. As noted above, at survival times long enough to allow transport across three synapses, rabies virus injections into the GPe resulted in transneuronal transport to the cerebellar nuclei of non-human primates11. Thus, cerebellar output targets MSNs in the indirect pathway. By contrast, at the same survival time, rabies virus injections into the GPi did not lead to substantial transneuronal labelling of neurons in the cerebellar nuclei11. Thus, cerebellar output may not target MSNs in the direct pathway in non-human primates. This negative result needs further exploration, but it suggests that cerebellar output preferentially influences the indirect pathway through the basal ganglia.
Studies in rodents have also provided support for the notion that cerebellar output may be biased towards the indirect pathway. The presence of the disynaptic projection from the cerebellar nuclei to the striatum in rodents was first confirmed in a study that used a combination of double labelling with conventional tracers and electron microscopy14. This study demonstrated that the circuit from the cerebellar nuclei to the striatum is mediated by several thalamic nuclei, in particular the intralaminar nuclei14. A recent study in awake, freely moving mice explored the physiology of the disynaptic pathway from the cerebellar nuclei to the striatum via the central lateral nucleus15. In this study, cerebellar stimulation elicited short-latency (9 ms) changes in the activity of about half of the examined striatal cells and could modulate cortico–striatal plasticity. Specifically, high-frequency stimulation of the motor cortex induced depression of the cortico–striatal response; however, concurrent stimulation of the motor cortex and the cerebellar nuclei potentiated the cortico–striatal response15. This study did not specifically explore whether stimulation of the cerebellar nuclei had differential effects on direct-pathway and indirect-pathway MSNs. However, stimulation of the cerebellar nuclei altered activity of striatal neurons that also received cortico–striatal inputs from the motor cortex. In both rodents and non-human primates, there is evidence that the motor cortex primarily targets indirect-pathway MSNs16,17. Thus, cerebellar output in both rodents and non-human primates may preferentially target the indirect pathway through the basal ganglia and influence the plasticity of the cortico–striatal synapse.
As mentioned above, the cerebellar influence over the basal ganglia is primarily mediated through the intralaminar thalamic nuclei11,14,15. In non-human primates, the intralaminar nuclei send topographically organized projections to the entire striatum18–20. Thus, the potential exists for cerebellar output to influence all the functional territories of the striatum.
Basal ganglia output to the cerebellum
The presence of substantial outputs from the cerebellum to the basal ganglia raised the question of whether a reciprocal pathway from the basal ganglia to the cerebellum exists. Early studies provided suggestive evidence for such a connection on the basis of the observation that electrical stimulation in basal ganglia nuclei evoked activity in the cerebellum (reviewed in REF.21). However, the origin and efficacy of any basal ganglia outputs to the cerebellum were unclear. To address this issue, we injected rabies virus into different regions of the cerebellar cortex in monkeys. We set the survival time to allow for retro-grade transport of rabies virus to first-order neurons that project to the injection sites in the cerebellar cortex (for example, neurons in the pontine nuclei) and then retrograde transneuronal transport to second-order neurons that innervate the first-order neurons12. Using this approach, we showed that neurons in the STN are the origin of a disynaptic pathway to the cerebellar cortex (FIG. 2b). Rabies virus injections into a motor region (lobule HVIIB) and into a non-motor region (crus II) of the lateral cerebellar cortex labelled substantial numbers of second-order neurons in motor and non-motor (associative) regions of STN, respectively12. These findings indicate that the disynaptic pathway from the STN to the cerebellar cortex in non-human primates is topographically organized and influences both motor and non-motor aspects of cerebellar function.
The STN is the only excitatory nucleus in the basal ganglia and has been considered to be a driver of basal ganglia output22. Tracing studies in non-human primates have provided evidence that the STN also targets regions outside the basal ganglia, including the pontine nuclei and the pedunculopontine tegmental nucleus (PPTg)23,24. As the pontine nuclei are the main source of projections to the cerebellar cortex25, we proposed that these nuclei mediate the STN projection to the cerebellar cortex12 (BOX 1).
Box 1 |. Potential routes from the subthalamic nucleus to the cerebellum.
There are two potential mediators of the subthalamic nucleus (STN) input to the cerebellar cortex — namely, the pontine nuclei (PN) and the pedunculopontine nucleus (PPTg). In non-human primates, the STN sends projections to the PN24 (see the figure), and these nuclei are a key source of mossy fibre input to the cerebellar cortex and to the cerebellar nuclei25. By contrast, tracing studies in rats have shown that the STN projects to the PPTg150. The rat PPTg is a source of cholinergic inputs to the cerebellar nuclei and, possibly, to the cerebellar cortex151–153. In a recent study in rats, PPTg microstimulation resulted in short-latency activation of the cerebellar nuclei153. This activation was reduced in the presence of cholinergic antagonists, indicating that cholinergic fibres from the PPTg to the cerebellum are responsible for this activation153. A recent report26 raised the possibility that the PPTg may mediate STN input to the cerebellar cortex (see the figure). However, conventional tracer injections into the cerebellar cortex of rats result in limited retrograde labelling of PPTg cells26,151. Thus, the extent to which the PPTg may mediate STN input to the cerebellar cortex remains to be determined in both rats and monkeys. GC, granule cell; PC, Purkinje cell.
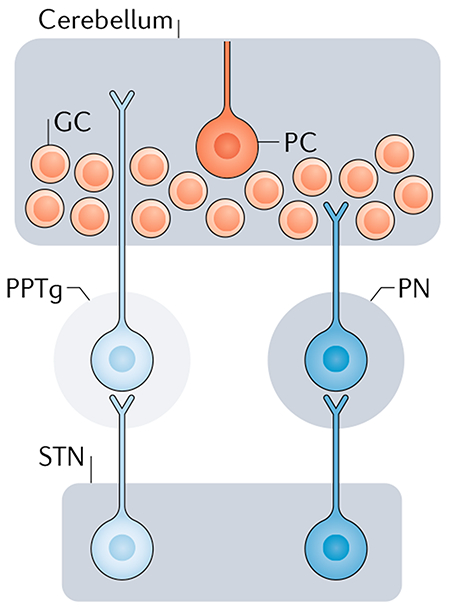
A recent study using the transneuronal transport of rabies virus in rats confirmed the presence of a disynaptic projection from the STN to the cerebellar cortex26. However, the authors concluded that the disynaptic pathway from the STN to the cerebellum in this species is largely limited to a portion of the cerebellar vermis. In fact, unlike the results in non-human primates, injections into the lateral cerebellar cortex of rats did not label substantial numbers of second-order neurons in the STN. The authors also noted that they did not observe a clear topographical organization of STN inputs to different sites within the cerebellar cortex. These findings suggest that, although a disynaptic pathway from the STN to the cerebellar cortex exists in rats, this pathway is less prominent than it is in non-human primates.
Although the extent of the disynaptic projection from the STN to the cerebellar cortex in rats remains to be determined, there is evidence for the physiological relevance of this connection in this species. Specifically, two studies examined the effects of STN deep brain stimulation (DBS) on cerebellar activity in rats27,28. One study observed an increase in cFOS expression in the cerebellar nuclei following STN-DBS27. This finding is consistent with the notion that DBS reduces STN activity (reviewed in REF.29): reduced STN activity may lead to a reduction in the activity of cerebellar Purkinje cells and, as a consequence, disinhibition of cerebellar nuclei neurons and increased cFOS expression. This perspective was confirmed in another study that used STN-DBS in rats that had been rendered hemi-parkinsonian by unilateral injection of 6-hydroxydopamine into the median fore-brain bundle28. In these animals, STN-DBS decreased the activity of STN neurons (by 55%) and Purkinje cells (by 28%) and increased the activity of cerebellar nuclei neurons (by 45%). Additional studies are needed to test whether the effects of STN-DBS on cerebellar activity are mediated in whole, or in part, by the disynaptic pathway from the STN to the cerebellar cortex.
Evidence from disease states
The evidence we have just reviewed provides a framework for a new perspective about interactions between basal ganglia and cerebellar circuits. Specifically, basal ganglia and cerebellar circuits are densely interconnected and form an integrated network. A major prediction of this perspective is that in disease states, abnormalities at one node in the network could percolate throughout the entire system and alter activity at other nodes in the network. Such changes in activity could be compensatory or could exacerbate dysfunction. Thus, symptoms of a ‘basal ganglia disorder’ may be the consequence of abnormal activity in cerebellar circuits that are remote from the initial site of the disorder. Likewise, symptoms of a ‘cerebellar disorder’ may be the consequence of abnormal activity in basal ganglia circuits. In the following sections, we present some of the critical evidence supporting this perspective for two disorders generally considered to originate in the basal ganglia — namely, Parkinson disease (PD) and dystonia. This subject has been comprehensively reviewed in several recent publications (see REFS30–34); thus, we summarize only the major findings here. In addition, we present similar supporting evidence from observations in other disorders, including Tourette syndrome (TS), obsessive–compulsive disorder (OCD) and Huntington disease (HD).
Parkinson disease
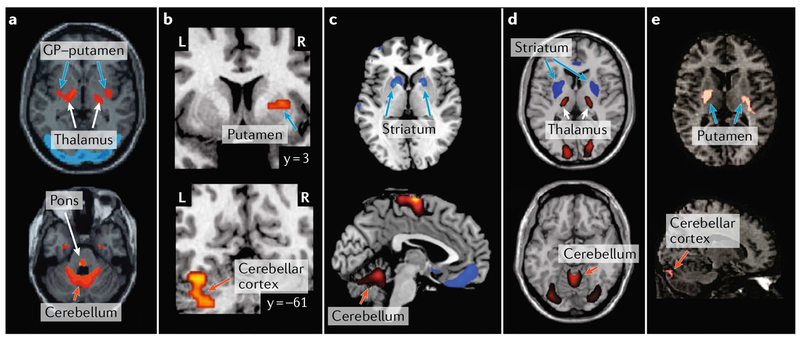
PD provides a prime example of how abnormal activity in basal ganglia circuits may propagate to cerebellar circuits and cause dysfunction. From studies of individuals with PD and animal models of the disease, it is well known that the initial relevant insult is the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc). The resultant depletion of dopamine from the sensorimotor territory of the striatum leads to changes in basal ganglia activity and the characteristic motor deficits of PD (reviewed in REF35). Numerous findings suggest that the changes in basal ganglia activity are accompanied by striking changes in cerebellar activity. For example, an analysis of 18F-fluorodeoxyglucose (FDG) using positron emission tomography (PET) indicated that metabolic brain activity in individuals with PD, relative to age-matched controls, is characterized by co-varying increases in the basal ganglia (putamen-GP) and the cerebellar cortex36 (FIG. 3a). The increased metabolic activity in the cerebellum may reflect a pathological drive from the STN, although other pathways may also contribute to the observed changes in the cerebellum (see also REF30). In essence, the disynaptic pathway from the STN to the cerebellar cortex may enable the abnormally high STN activity, which is observed in individuals with PD and in animal models of the disorder37,38, to drive the hyperactivity in the cerebellar cortex39–47. This mechanism is further supported by the finding that STN-DBS — which disrupts abnormal output from this nucleus — reduces cerebellar hyperactivity and improves motor function in individuals with PD48–50.
Fig. 3 |. Neuroimaging evidence for basal ganglia and cerebellar interactions in disease.
Findings from neuroimaging studies of several disorders provide evidence for interactions between the basal ganglia and cerebellum. Indeed, abnormal structure, connectivity and activity in both the basal ganglia and the cerebellum have been reported in several debilitating disorders. Note that in this figure, orange arrows point to sites in the cerebellum, blue arrows point to sites in the basal ganglia and white arrows point to sites in the thalamus and/or the cerebral cortex. a | Analysis of 18F-fluorodeoxyglucose (FDC) positron emission tomography (PET) scans indicated that brain metabolism in individuals with Parkinson disease (PD), relative to age-matched controls, is characterized by co-varying pallido-thalamic, pontine and cerebellar hypermetabolism36. The metabolic pattern associated with PD is illustrated on two axial sections in which marked metabolic increases and decreases are shown on red to yellow and blue to purple colour scales, respectively. b | In individuals with obsessive-compulsive disorder (OCD), relative to healthy control subjects, regions within both the basal ganglia and the cerebellum demonstrated increased whole-brain connectivity69. Clusters in which individuals with OCD showed markedly increased whole-brain connectivity in the putamen and cerebellar cortex are illustrated on two coronal sections on a red to yellow colour scale. c | Analysis of FDC-PET scans indicated that brain metabolism in individuals with Tourette syndrome (TS), relative to healthy controls, is characterized by hypometabolism in the striatum that co-varies with hypermetabolism in the cerebellum70. The metabolic pattern associated withTS is illustrated on axial and sagittal sections in which notable metabolic increases and decreases are shown in red and blue, respectively. d | A similar metabolic pattern was identified in Huntington disease (HD) gene carriers, relative to healthy controls71. The metabolic pattern associated with HD is illustrated on axial sections, and marked metabolic increases and decreases are shown on red to yellow and blue to purple colour scales, respectively. e | Finally, analysis of FDC-PET scans in non-manifesting carriers of dystonia-associated mutations in torsin 1A (TOR1A; also known as DYT1), relative to healthy controls, revealed co-varying hypermetabolism (red) in the striatum and the cerebellar cortex80. GP, globus pallidus; L, left; R, right. Part a is adapted with permission from REF36., John Wiley and Sons. Part b is adapted with permission from REF.69, Elsevier. Parte c is adapted with permission from REF.70, American Academy of Neurology. Part d is adapted from Feigin, A et al. Thalamic metabolism and sympton onset in preclinical Huntington’s disease. Brain (2007) 130, 2858–2867, by permission of Oxford University Press, REF.71. Part e is adapted with permission from REF.80, John Wiley and Sons.
Some of the changes in cerebellar activity in individuals with PD are thought to reflect a compensatory mechanism whereas others have been associated with dysfunction51,52. In fact, resting tremor, one of the cardinal symptoms of PD, is optimally treated with surgical interventions (lesions or DBS) in the thalamic region that receives inputs from the cerebellum (that is, the ventral intermediate nucleus (VIM) of the thalamus), whereas interventions in the thalamic region that receives input from the basal ganglia are not as effective53–55. This result suggests that the cerebellum is a key source of the abnormal signals that contribute to the generation of resting tremor in PD. The beneficial effects of surgical STN interventions on resting tremor56 may be mediated, in part, through the connection between the STN and the cere-bellum30. Indeed, alleviation of resting tremor in individuals with PD following both STN-DBS and VIM-DBS is associated with normalization of cerebellar metabolism57.
Tourette syndrome and obsessive–compulsive disorder
Several neuropsychiatric disorders, including TS and OCD, have been associated with dysfunction in cortico–basal ganglia circuits. For example, although the precise origin of tics in individuals with TS remains unclear, reduced GABA function in the striatum has been considered a primary cause of tic production (reviewed in REFS58,59). In line with this perspective, microinjections of the GABA antagonist bicuculline into the striatum60,61 or into the GPe62 of non-human primates can result in tics and stereotyped behaviours. Furthermore, there is evidence that abnormal basal ganglia activity drives activity changes in both the cerebellum and the primary motor cortex (M1) that predict the onset of tics63. Additional evidence for cerebellar involvement in TS comes from imaging studies in humans, which revealed tic-related activation not only in the basal ganglia but also in the cerebellum64,65. In fact, some of these studies report that tic severity correlated with cerebellar activity66.
Alterations in cerebellar activity have been observed in imaging studies of both TS and OCD (reviewed in REFS67,68). For example, a study using resting-state functional MRI (fMRI) demonstrated that individuals with OCD, relative to healthy controls, exhibit increased whole-brain connectivity in regions of both the putamen and the cerebellar cortex69 (FIG. 3b). Brain metabolism in individuals with TS, relative to healthy controls, is characterized by hypometabolism in the striatum covarying with hypermetabolism in the cerebellar cortex70 (FIG. 3c). A similar metabolic pattern has also been observed in carriers of genetic mutations associated with HD, another prototypical basal ganglia disorder71 (FIG. 3d). Thus, there is growing evidence that alterations in the function of the basal ganglia are associated with changes in cerebellar activity and metabolism. We propose that the disynaptic pathway from the STN to the cerebellar cortex provides a key link for such effects, although other pathways or independent structural changes in the cerebellum may also be involved72,73. In line with our proposal, a recent computational model of basal ganglia–cerebellar–cerebral cortical network function in TS indicated that hyperactivity in the STN may propagate to the cerebellar cortex and contribute to tic generation74. Thus, similar to observations in PD, the reduction in OCD and TS symptoms75,76 in patients undergoing STN-DBS may be mediated, in part, through the STN influence on the cerebellar cortex.
Dystonia
Although it is traditionally considered to be a disorder of the basal ganglia (reviewed in REF.77), dystonia provides a compelling example of how abnormal activity in the cerebellum may propagate to the basal ganglia and cause dysfunction. Abnormalities in the cerebello–thalamo–cortical tracts78,79, cerebellar activity and functional connectivity have been observed in many forms of dystonia (reviewed in REF.31). For example, manifesting and non-manifesting carriers of genetic mutations that are associated with dystonia show co-varying increases in metabolic activity in the putamen–GP and in the cerebellar cortex. This pattern of metabolic activity is not present in controls80 (FIG. 3e).
Mouse models of dystonia also display structural and functional abnormalities in cerebello-thalamo-cortical pathways81,82. In such models, abnormal (for example, bursting) cerebellar output evoked dystonic postures15,83–85. Silencing, lesioning or normalizing cerebellar output in these animal models abolished the dystonic postures86,87. In line with these data, bursting cerebellar output has been recorded during surgery in a patient with dystonia88. Currently, DBS and transcranial magnetic stimulation of the cerebellum are being explored as potential treatments for some forms of dystonia89–91.
Overall, these observations suggest that there is a need to re-conceptualize the pathophysiology of several debilitating disorders. What were formerly considered to be basal ganglia or cerebellar disorders may be better understood as network disorders. Even if the initial damage is confined to one node in the basal ganglia or cerebellar circuits, some aspects of the symptoms may be caused by abnormal signals transmitted through the interconnecting pathways.
Evidence from normal function
Given current concepts about basal ganglia and cerebellar function (detailed below), it is commonly considered that specific behavioural tasks rely exclusively on the operations of one of these subcortical structures. However, the integrated network perspective implies that, in many instances, the two structures are likely to cooperate in generating normal behaviours. In support of this perspective, we provide several striking examples of co-activation of the basal ganglia and the cerebellum from imaging studies in humans (FIG. 4). These examples include evidence that the cerebellum is involved in functions that are usually associated with the basal ganglia. We also provide an example of basal ganglia activation in a task that is often considered to rely exclusively on the cerebellum.
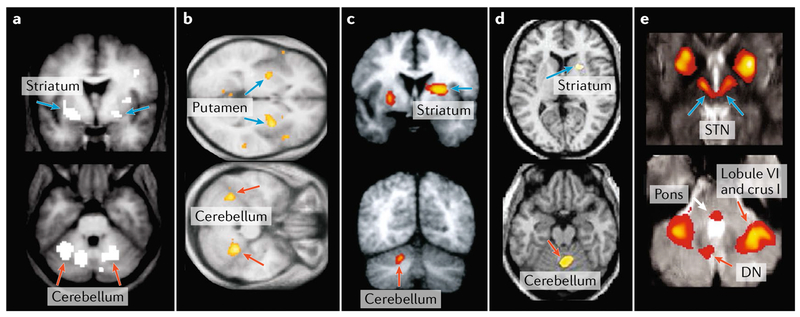
Fig. 4 |. Neuroimaging evidence for basal ganglia and cerebellar interactions in normal function.
Findings from neuroimaging studies in heaithy individuals have shown co-activations in the basal ganglia and cerebellum in various tasks. Event-related functional MRI (fMRI) studies revealed that activity in both regions of the basal ganglia and the cerebellum is correlated with temporal difference prediction error during appetitive conditioning with a pleasant taste reward97 (part a) and during higher-order aversive conditioning98 (part b). A positron emission tomography (PET) study demonstrated that movement vigour (that is, extent and speed) is associated with regional cerebral blood flow increases in regions of both the basal ganglia (putamen and globus pallidus) and the cerebellum105 (part c). An fMRI study of healthy subjects adapting to visuomotor rotations, in the context of a joystick aiming task, indicated that sensorimotor adaptation is associated with increased activity in both the basal ganglia and the cerebellum108 (part d). Finally, early stages of sequence learning are associated with activation of both the subthalamic nucleus (STN) and the cerebellum119 (part e). Note that orange arrows in this figure point to activation sites in the cerebellum and blue arrows point to activation sites in the basal ganglia. DN, dentate nucleus. Part a is adapted with permission from REF.97, Elsevier. Part b is adapted from REF.98, Macmillan Publishers Limited. Part c is adapted with permission from REF.105, American Physiological Society. Part d is adapted from REF.108, Macmillan Publishers Limited. Part e is adapted with permission from REF.119, Proceedings of the National Academy of Sciences.
Reward and motivation
The basal ganglia are considered to be essential for reward-based (reinforcement) learning, which is guided by reward prediction errors (reviewed in REF.92). Midbrain dopamine neurons in rodents, non-human primates and humans have been associated with reward processing and coding of reward prediction errors (reviewed in REF.93). Recently, a study using two-photon calcium imaging in behaving mice provided evidence for reward-related activity in granule cells of the cerebellar cortex94. In this study, some granule cells responded preferentially to reward or reward omission whereas others selectively encoded reward anticipation. In line with these findings, a recent overview of imaging studies in humans indicated that brain regions outside the basal ganglia, including the cerebellum, are associated with rewards and reward prediction errors95. For example, imaging studies in humans have shown that activation in the anterior cerebellar cortex correlates with reward and reward prediction error in studies of both appetitive and aversive conditioning96–101 (FIG. 4a,b). Furthermore, humans with cerebellar lesions showed impairments in reward-based reversal learning102. These observations suggest that the cerebellum functions along with the basal ganglia to provide the neural substrate for reward coding and reward-based learning.
The cerebellum has also been involved in motivation-based adjustment of motor output, a behaviour that is usually associated with the basal ganglia. Basal ganglia activity and reward-based learning mechanisms have been used to explain how individuals adjust action choices and modulate the kinematic parameters of movement on the basis of motivation103,104. Even so, the results of imaging studies in humans demonstrated that cerebellar activation also correlates with movement parameters (such as movement extent and speed105) that are modulated with changes in motivation (FIG. 4c). The integrated network perspective suggests that motivation-related signals from the basal ganglia drive the cerebellum to optimize movement parameters. This perspective may help explain how interactions between the basal ganglia and the cerebellum mediate the motivational effects of verbal encouragement on movement force106.
Sensorimotor adaptation
The cerebellum is considered to be a major component of the neural substrate for adapting motor output on the basis of sensory feedback from movement (reviewed in REF.107). Even so, a recent imaging study in human subjects also provided evidence for striatal activation in a task involving (error-based) sensorimotor adaptation108 (FIG. 4d). The disynaptic pathway from the cerebellar nuclei to the input stage of basal ganglia processing may underlie this activation, although alternative pathways could also be involved.
Overall, communication between the cerebellum and basal ganglia could be essential to linking sensorimotor adaptation to reinforcement mechanisms for motor learning. Human behavioural studies have shown that combining error-based and reward-based feedback accelerates sensorimotor adaptation109 and that reward and punishment have differential effects on sensorimotor adaptation110. Thus, motor learning in any number of specific situations can be a complex interplay between the learning mechanisms supported by the basal ganglia and the cerebellum111.
Integrated networks for learning
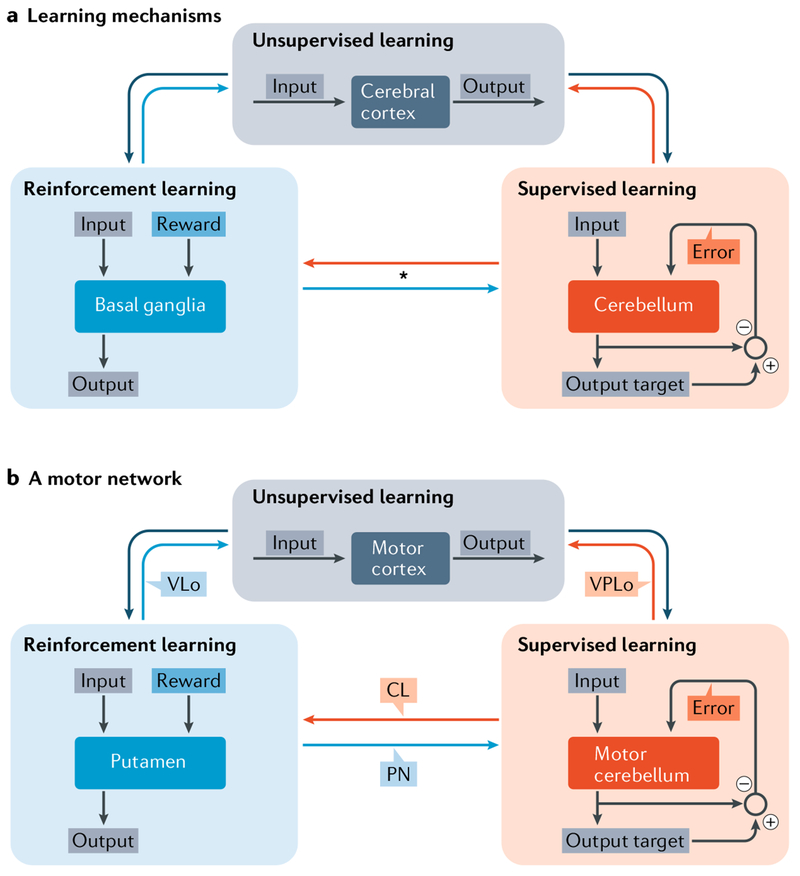
Computationally, the cerebral cortex, the basal ganglia and the cerebellum have been hypothesized to implement distinct learning processes9,112–115. Learning within the cerebral cortex is considered to be primarily unsupervised and driven by Hebbian processes (use-dependent; synaptic efficacy strengthens with co-activation). As mentioned above, the basal ganglia are usually associated with reward-based (reinforcement) learning, and the cerebellum is associated with error-based learning. However, in light of the anatomical and functional data reviewed here, the view that basal ganglia and cerebellar circuits with the cerebral cortex are independent needs to be modified (FIG. 5). This perspective is in line with previous proposals that use-dependent, reward-based and error-based learning require operation of an integrated mechanism that gradually guides performance116.
Fig. 5 |. Learning specializations of cortico–basal ganglia and cortico–cerebellar loops.
a | The cerebral cortex, basal ganglia and cerebellum are thought to implement distinct learning algorithms9. According to this view, the cerebral cortex is specialized for unsupervised learning based on Hebbian plasticity The basal ganglia are specialized for reinforcement (reward-based) learning, guided by the reward signals from midbrain dopaminergic neurons. The cerebellum is specialized for supervised (error-based) learning, guided by error signals from the inferior olive. The interconnections between these structures are illustrated by large arrows. Outputs from the cerebral cortex are depicted as large dark grey arrows. Outputs from the basal ganglia are depicted as large blue arrows. Outputs from the cerebellum are depicted as large orange arrows. The asterisk emphasizes the newly discovered connections between the basal ganglia and cerebellum that are the subject of this Review11,12. b | An example of learning in the motor domain. Within the basal ganglia, only the input stage of basal ganglia processing (putamen) is included for simplicity. Labelling next to the interconnection arrows identifies the intermediary structure for the connection. CL, central lateral nucleus; PN, pontine nuclei; VLo, ventral lateral nucleus, pars oralis; VPLo, ventral posterior lateral nucleus, pars oralis. Part a is adapted with permission from REF.9, Elsevier.
More specifically, a number of observations suggest that learning is implemented gradually at the level of topographically organized circuits that link the basal ganglia, the cerebellum and the cerebral cortex. Support for this proposal comes from several imaging studies of skill learning in humans. These studies have revealed the engagement of a distributed network involving topographically organized sites in the basal ganglia, the cerebellum and the cerebral cortex that display linked changes in activation over the course of learning108,114.
For example, in the early stages of sequence learning, activations have been observed in the associative territory of the striatum, the STN, as well as in the cerebellar hemispheres112,117–119 (FIG. 4e). The sites of activation in the basal ganglia and in the cerebellum are likely to be anatomically interconnected, and these interconnections probably provide the substrate for the observation that the ‘functional connectivity’ between the basal ganglia and the cerebellum is enhanced early in learning120. Additionally, transcranial magnetic stimulation over the cerebellar cortex during early stages of sequence learning causes complex changes in basal ganglia activity and can alter the speed of sequence acquisition121. Later in the learning process, activity in associative basal ganglia and cerebellar regions declines and the foci of activation shift to the sensorimotor regions within these structures119. Moreover, enhanced effective connectivity from the cerebellum to the sensorimotor putamen is associated with improved learning in the late-stage phase of motor sequence acquisition122. These data suggest that improvements in cognitive and motor performance during different stages of sequence acquisition are implemented in distinct networks of functionally related regions of the basal ganglia, the cerebellum and the cerebral cortex.
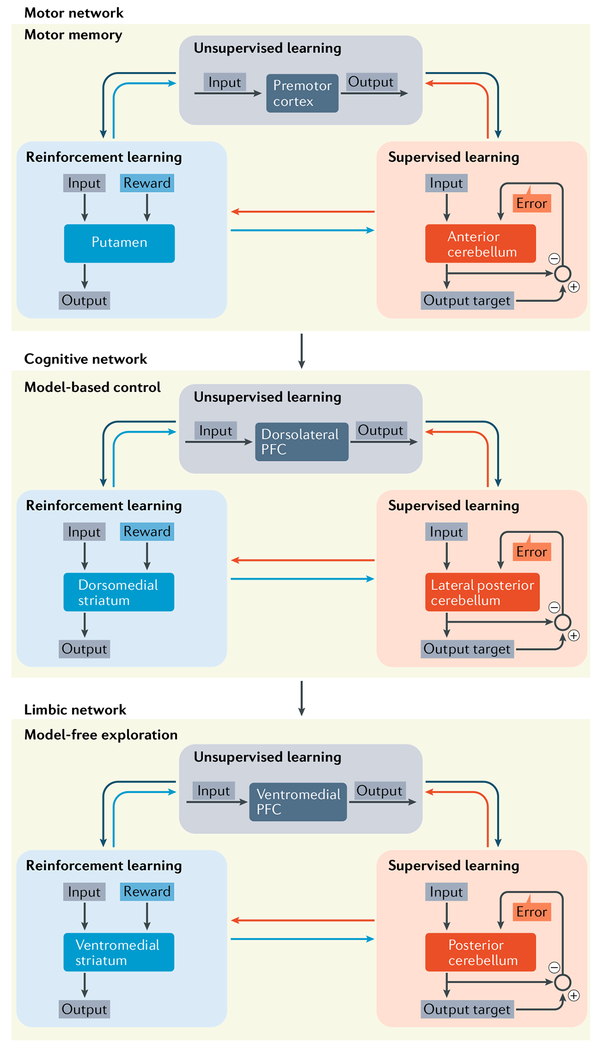
In support of this view, a recent imaging study in humans provided evidence that distinct basal ganglia–cerebellar–cerebral cortical networks implement different strategies to improve performance on an action selection task123 (FIG. 6). Performance during initial stages of the task was primarily based on trial and error-type exploration (described by model-free reinforcement learning) and involved a limbic network, including the ventromedial prefrontal cortex (PFC), ventral striatum and posterior cerebellum. As learning progressed and subjects acquired a model of the environment (described by model-based reinforcement learning), the site of activation shifted to an associative (cognitive) network, including the dorsolateral PFC, dorsomedial striatum and lateral posterior cerebellum. Finally, with extensive experience, performance relied on motor memory, and the site of activation shifted to a motor network, including the supplementary motor area, putamen and anterior cerebellum. Overall, these data indicate that different learning strategies recruit distinct and topographically organized nodes within an interconnected network that includes the basal ganglia, the cerebellum and the cerebral cortex. This perspective predicts that shifts in learning strategy, skill level or context will be associated with topographic shifts in the sites of activation within the interconnected network.
Fig. 6 |. Action planning.
Functionally related corticai, basal ganglia and cerebellar sites within interconnected networks participate in progressive stages of action planning123. On the basis of these results, learning through exploration involves a limbic network, including the ventromedial prefrontal cortex (PFC), ventromedial striatum and posterior cerebellum. Model-based learning involves an associative (cognitive) network, including the dorsolateral PFC, dorsomedial striatum and lateral posterior cerebellum. Performance based on motor memory involves a motor network , including the supplementary motor area, putamen and anterior cerebellum. The authors’ imaging data suggest that as learning progresses, the sites of activation shift in a topographically organized fashion. Our interpretation of these data is that each stage of the learning progress involves a different set of interconnected basal ganglia, cerebellar and cerebral cortical regions.
Future directions
Functions of the basal ganglia–cerebellar–cerebral cortical network
The anatomical findings reviewed here have provided compelling evidence in non-human primates that the sensorimotor and the associative domains of the basal ganglia are interconnected with functionally related regions of both the cerebellum and the cerebral cortex. One important issue that remains to be investigated is whether the limbic domain of the basal ganglia is also interconnected with the cerebellum. Specifically, do regions of the ventral striatum (including the nucleus accumbens) receive disynaptic projections from the cerebellar nuclei? If so, it would be important to determine which regions of the cerebellar nuclei are involved. Furthermore, do limbic regions of the basal ganglia (for example, the limbic domain of the STN) send disynaptic projections to the cerebellar cortex? If so, it would be important to determine the regions of basal ganglia at the origin of this output and the regions of the cerebellar cortex that are its target. In short, basal ganglia–cerebellar–cerebral cortical networks that operate in the limbic domain remain to be identified using anatomical and physiological techniques in non-human primates. However, neuroimaging studies in humans support the idea that there exist functional interactions between limbic regions of the basal ganglia, the cerebellum and the cerebral cortex124–126.
Basal ganglia–cerebellar–cerebral cortical networks could provide an important component of the substrate for multiple intrinsic resting-state networks124. For example, interconnections between cognitive regions of the basal ganglia, the cerebellum and the cerebral cortex could provide part of the foundation for the executive control network. This network includes specific regions of the caudate (associative territory of the striatum), lateral cerebellum (hemispheric crus I and II) and the dorsolateral prefrontal cortex and has been associated with executive function, working memory and verbal fluency124. Abnormal activity in this network has been associated with cognitive dysfunction in Alzheimer disease127, anxiety128, Friedreich ataxia129 and spinocerebellar ataxia type 6 (REF.130). Similarly, interconnections between limbic regions of the basal ganglia, the cerebellum and the cerebral cortex could provide part of the foundation for the salience network. This network includes specific regions of the striatum, cerebellum (hemispheric lobule VI and crus I) and the dorsal anterior cingulate cortex and has been associated with processing of interoceptive, autonomic and emotional information124. Abnormal activity in this network has been associated with symptoms in major depression, schizophrenia, substance use, anxiety and eating disorders (reviewed in REF.131). Thus, further studies of basal ganglia–cerebellar–cerebral cortical networks may suggest new approaches for intervening in a wide range of neuropsychiatric disorders.
Other interconnections between the basal ganglia and cerebellum
An additional pathway for interactions between the basal ganglia and the cerebellum has been proposed in rodents132–134. Specifically, early studies suggested that dentate output influences the substantia nigra (SN) and other catecholamine centres132,134,135. A recent study confirmed the presence of a direct pathway from the dentate nucleus to dopaminergic and GABAergic neurons in the mouse SN and ventral tegmental area133. There currently is no compelling evidence that this pathway exists in monkeys, although diffusion tensor imaging studies provide some support for the pathway in humans136–139. If this connection exists, it will be important to define the specific cells of origin in the dentate and the physiological consequences of this input on target neurons in the SN and the ventral tegmental area.
Computational roles
To date, there have been limited proposals regarding the computational roles of interconnections between the basal ganglia and the cerebellum140. According to one computational framework141, cortico-cerebellar processing provides a means to evaluate the sensory consequences of an action whereas cortico–basal ganglia processing provides a means to evaluate the value of outcomes from an action. As discussed above, optogenetic stimulation of the pathway from the cerebellum to the striatum alters cortico–striatal plasticity15. Further studies are needed to confirm the evidence that cerebellar output primarily influences the indirect pathway. However, if this finding proves to be correct, then cerebellar output could influence the balance of activity in the direct versus indirect pathways through the basal ganglia. In doing so, cerebellar outputs may provide the basal ganglia with information that is necessary to evaluate competing action candidates140. However, it is noteworthy that cerebellar activation has been associated with action timing as opposed to action selection142,143. Thus, an alternative contribution of cerebellar outputs may be to control the timing of actions selected by basal ganglia processing.
The computational role of the disynaptic pathway from the STN to the cerebellar cortex is also unclear. Some have viewed the role of the STN as mediating a ‘stop signal’ to inhibit action144–146. Thus, one potential role for STN projections to the cerebellar cortex is to convey information relevant to the suppression or termination of action140. However, a recent study in non-human primates found that STN neurons capable of providing a signal for action stopping or switching are restricted to a small, ventromedial portion of the nucleus. Neurons in other regions of the STN encoded other task dimensions147. Our data indicate that the STN projection to the cerebellar cortex originates from a broad region of the nucleus12. Thus, any further speculation about the computational role of STN output to the cerebellum must await a firmer understanding of the signals encoded by the activity of STN neurons.
Another source of insight about the computational role of the interconnections between the basal ganglia and the cerebellum may come from a comparative approach. The basal ganglia and cerebellum are phylogenetically old structures that have undergone extensive expansion with the evolution of the cerebral cortex. This expansion is especially striking in primates and it appears to support the increased complexity of the motor, cognitive and affective functions in these animals148,149. However, it is possible that the subcortical connections between the basal ganglia and the cerebellum precede the appearance of cortico–basal ganglia and cortico–cerebellar connections. Clearly, there are animals that lack a cerebral cortex but have a well-developed cerebellum and basal ganglia (for example, song birds). We speculate that the two subcortical systems may be linked to form an integrated network even in these animals. These interconnections would enable each system to utilize the learning mechanisms implemented by the other. If this is the case, then studies in animals that lack a cerebral cortex might provide some unique insights into the role of the connections that link the basal ganglia and the cerebellum.
Summary
In summary, further experimental and modelling studies are needed to determine the full extent of the interconnections between the basal ganglia and the cerebellum as well as their computational role. However, the evidence reviewed here makes it clear that the basal ganglia and the cerebellum are densely interconnected to form an integrated network with the cerebral cortex. We discussed evidence that the interconnections between the basal ganglia, the cerebellum and the cerebral cortex are topographically organized and create functional distinct networks that operate over the domains of movement, cognition and perhaps affect. We illustrated results showing that abnormal activity at one node can percolate throughout the entire network to cause dysfunction at other nodes in the network. We also showed evidence that during a task, linked nodes are activated and there is an orderly shift in activation as performance changes. Ultimately, we think that these observations suggest that questions about basal ganglia or cerebellar function should be reframed to consider the entire basal ganglia–cerebellar–cerebral cortical network.
Acknowledgements
The preparation of this manuscript was supported in part by US National Institutes of Health grants R01 NS24328, P40 OD010996 and P30 NS076405 (all to PL.S.).
Glossary
- Rabies virus
An RNA virus that is highly neurotropic and can be used as a retrograde transneuronal tracer. Rabies virus is transported retrogradely to neurons that project to an injection site (that is, first-order neurons). The virus replicates in the first-order neurons and is transmitted transneuronally to neurons that project to the first-order neurons. The virus continues to replicate and move transneuronally through chains of synaptically connected neurons in a time-dependent fashion.
- Direct pathway
A monosynaptic pathway that connects one type of MSN in the striatum with neurons in the CPi and the SNpr.
- Indirect pathway
A polysynaptic pathway that connects another type of MSN in the striatum to neurons in the CPi and the SNpr.
- Reward-based (reinforcement) learning
Learning process (algorithm) that allows reward signals to optimize performance.
- Error-based learning
Learning process (algorithm) that allows error signals to improve performance in a gradual manner.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Kemp JM & Powell TP The connexions of the striatum and globus pallidus: synthesis and speculation. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 262, 441–457 (1971). [DOI] [PubMed] [Google Scholar]
- 2.Glickstein M, May JG 3rd & Mercier BE Corticopontine projection in the macaque: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J. Comp. Neurol. 235, 343–359 (1985). [DOI] [PubMed] [Google Scholar]
- 3.Alexander GE, DeLong MR & Strick PL Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381 (1986). [DOI] [PubMed] [Google Scholar]
- 4.Middleton FA & Strick PL Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 42, 183–200 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Strick PL, Dum RP & Fiez JA Cerebellum and nonmotor function. Annu. Rev. Neurosci. 32, 413–434 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Kelly RM & Strick PL Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog. Brain Res. 143, 449–459 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Kelly RM & Strick PL Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci. 23, 8432–8444 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Percheron G, Francois C, Talbi B, Yelnik J & Fenelon G The primate motor thalamus. Brain Res. Brain Res. Rev. 22, 93–181 (1996). [PubMed] [Google Scholar]
- 9.Doya K Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr. Opin. Neurobiol. 10, 732–739 (2000). [DOI] [PubMed] [Google Scholar]; This opinion paper provides a perspective on the learning-oriented specializations of the basal ganglia and the cerebellum.
- 10.Bostan AC, Dum RP & Strick PL Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn. Sci. 17, 241–254 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshi E, Tremblay L, Feger J, Carras PL & Strick PL The cerebellum communicates with the basal ganglia. Nat. Neurosci. 8, 1491–1493 (2005). [DOI] [PubMed] [Google Scholar]; This study provides evidence for the disynaptic pathway from the cerebellar nuclei to the striatum in non-human primates.
- 12.Bostan AC, Dum RP & Strick PL The basal ganglia communicate with the cerebellum. Proc. Natl Acad. Sci. USA 107, 8452–8456 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLong M & Wichmann T Update on models of basal ganglia function and dysfunction. Parkinsonism Relat. Disord. 15 (Suppl. 3), S237–S240 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides evidence for the disynaptic pathway from the STN to the lateral cerebellar cortex in non-human primates.
- 14.Ichinohe N, Mori F & Shoumura K A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res. 880, 191–197 (2000). [DOI] [PubMed] [Google Scholar]; This study provides evidence for the disynaptic pathway from the deep cerebellar nuclei to the striatum in rats.
- 15.Chen CH, Fremont R, Arteaga-Bracho EE & Khodakhah K Short latency cerebellar modulation of the basal ganglia. Nat. Neurosci. 17, 1767–1775 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides physiological evidence that the cerebellum modulates the striatum through the disynaptic pathway to the striatum in mice.
- 16.Zemanick MC, Strick PL & Dix RD Direction of transneuronal transport of herpes-simplex virus-1 in the primate motor system is strain-dependent. Proc. Natl Acad. Sci. USA 88, 8048–8051 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parthasarathy HB & Graybiel AM Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J. Neurosci. 17, 2477–2491 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith Y & Parent A Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus). Neuroscience 18, 347–371 (1986). [DOI] [PubMed] [Google Scholar]
- 19.McFarland NR & Haber SN Organization of thalamostriatal terminals from the ventral motor nuclei in the macaque. J. Comp. Neurol. 429, 321–336 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Smith Y, Raju DV, Pare JF & Sidibe M The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 27, 520–527 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Calderon D & Khodakhah K in The Basal Ganglia. Innovations in Cognitive Neuroscience (ed. Soghomonian JJ) 135–153 (Springer, Cham, Switzerland, 2016). [Google Scholar]
- 22.Kitai ST & Kita H in The Basal Ganglia II. Advances in Behavioral Biology Vol. 32 (eds Carpenter MB & Jayaraman A) 357–373 (Springer, Boston, 1987). [Google Scholar]
- 23.Carpenter MB, Carleton SC, Keller JT & Conte P Connections of the subthalamic nucleus in the monkey. Brain Res. 224, 1–29 (1981). [DOI] [PubMed] [Google Scholar]
- 24.Giolli RA et al. Cortical and subcortical afferents to the nucleus reticularis tegmenti pontis and basal pontine nuclei in the macaque monkey. Vis. Neurosci. 18, 725–740 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Brodal P The pontocerebellar projection in the rhesus monkey: an experimental study with retrograde axonal transport of horseradish peroxidase. Neuroscience 4, 193–208 (1979). [DOI] [PubMed] [Google Scholar]
- 26.Jwair S, Coulon P & Ruigrok TJ Disynaptic subthalamic input to the posterior cerebellum in rat. Front. Neuroanat. 11, 13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moers-Hornikx VM et al. Cerebellar nuclei are activated by high-frequency stimulation of the subthalamic nucleus. Neurosci. Lett. 496, 111–115 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Sutton AC, O’Connor KA, Pilitsis JG & Shin DS Stimulation of the subthalamic nucleus engages the cerebellum for motor function in parkinsonian rats. Brain Struct. Funct. 220, 3595–3609 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Wichmann T, Bergman H & DeLong MR Basal ganglia, movement disorders and deep brain stimulation: advances made through non-human primate research. J. Neural Transm. (Vienna) 125, 419–430 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu T & Hallett M The cerebellum in Parkinson’s disease. Brain 136, 696–709 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article offers a comprehensive review of the role of the cerebellum in PD.
- 31.Filip P, Lungu OV & Bares M Dystonia and the cerebellum: a new field of interest in movement disorders? Clin. Neurophysiol 124, 1269–1276 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Caligiore D et al. Parkinson’s disease as a system-level disorder. NPJ Parkinsons Dis. 2, 16025 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shakkottai VG Physiologic changes associated with cerebellar dystonia. Cerebellum 13, 637–644 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shakkottai VG et al. Current opinions and areas of consensus on the role of the cerebellum in dystonia. Cerebellum 16, 577–594 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeLong M & Wichmann T Changing views of basal ganglia circuits and circuit disorders. Clin. EEG Neurosci. 41, 61–67 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asanuma K et al. The metabolic pathology of dopa-responsive dystonia. Ann. Neurol. 57, 596–600 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Wichmann T, Bergman H & DeLong MR The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J. Neurophysiol. 72, 521–530 (1994). [DOI] [PubMed] [Google Scholar]
- 38.Schrock LE, Ostrem JL, Turner RS, Shimamoto SA & Starr PA The subthalamic nucleus in primary dystonia: single-unit discharge characteristics. J. Neurophysiol. 102, 3740–3752 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mentis MJ et al. Early stage Parkinson’s disease patients and normal volunteers: comparative mechanisms of sequence learning. Hum. Brain Mapp. 20, 246–258 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J et al. Akinetic-rigid and tremor-dominant Parkinson’s disease patients show different patterns of intrinsic brain activity. Parkinsonism Relat. Disord. 21, 23–30 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Lewis MM et al. Task specific influences of Parkinson’s disease on the striato-thalamo-cortical and cerebello-thalamo-cortical motor circuitries. Neuroscience 147, 224–235 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ballanger B, Jahanshahi M, Broussolle E & Thobois S PET functional imaging of deep brain stimulation in movement disorders and psychiatry. J. Cereb. Blood Flow Metab. 29, 1743–1754 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Palmer SJ, Li J, Wang ZJ & McKeown MJ Joint amplitude and connectivity compensatory mechanisms in Parkinson’s disease. Neuroscience 166, 1110–1118 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Palmer SJ, Ng B, Abugharbieh R, Eigenraam L & McKeown MJ Motor reserve and novel area recruitment: amplitude and spatial characteristics of compensation in Parkinson’s disease. Eur. J. Neurosci. 29, 2187–2196 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Wu T et al. Changes of functional connectivity of the motor network in the resting state in Parkinson’s disease. Neurosci. Lett. 460, 6–10 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Wu T et al. Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. Neuroimage 55, 204–215 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Festini SB et al. Altered cerebellar connectivity in Parkinson’s patients ON and OFF L-DOPA medication. Front. Hum. Neurosci. 9, 214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Payoux P et al. Subthalamic nucleus stimulation reduces abnormal motor cortical overactivity in Parkinson disease. Arch. Neurol. 61, 1307–1313 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Asanuma K et al. Network modulation in the treatment of Parkinson’s disease. Brain 129, 2667–2678 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geday J, Ostergaard K, Johnsen E & Gjedde A STN-stimulation in Parkinson’s disease restores striatal inhibition of thalamocortical projection. Hum. Brain Mapp. 30, 112–121 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinu K & Monchi O Cortico-basal ganglia and cortico-cerebellar circuits in Parkinson’s disease: pathophysiology or compensation? Behav. Neurosci. 127, 222–236 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Mirdamadi JL Cerebellar role in Parkinson’s disease. J. Neurophysiol. 116, 917–919 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papavassiliou E et al. Thalamic deep brain stimulation for essential tremor: relation of lead location to outcome. Neurosurgery 54, 1120–1129; discussion 1129–1130 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Narabayashi H, Maeda T & Yokochi F Long-term follow-up study of nucleus ventralis intermedius and ventrolateralis thalamotomy using a microelectrode technique in Parkinsonism. Stereotact. Funct. Neurosurg. 50, 330–337 (1988). [DOI] [PubMed] [Google Scholar]
- 55.Lenz FA et al. Single unit analysis of the human ventral thalamic nuclear group. Tremor-related activity in functionally identified cells. Brain 117, 531–543 (1994). [DOI] [PubMed] [Google Scholar]
- 56.Krack P, Pollak P, Limousin P, Benazzouz A & Benabid AL Stimulation of subthalamic nucleus alleviates tremor in Parkinson’s disease. Lancet 350, 1675 (1997). [DOI] [PubMed] [Google Scholar]
- 57.Mure H et al. Parkinson’s disease tremor-related metabolic network: characterization, progression, and treatment effects. Neuroimage 54, 1244–1253 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jackson GM, Draper A, Dyke K, Pepes SE & Jackson SR Inhibition, disinhibition, and the control of action in Tourette syndrome. Trends Cogn. Sci. 19, 655–665 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Worbe Y, Lehericy S & Hartmann A Neuroimaging of tic genesis: Present status and future perspectives. Mov. Disord. 30, 1179–1183 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Worbe Y et al. Towards a primate model of Gilles de la Tourette syndrome: anatomo-behavioural correlation of disorders induced by striatal dysfunction. Cortex 49, 1126–1140 (2013). [DOI] [PubMed] [Google Scholar]
- 61.McCairn KW, Bronfeld M, Belelovsky K & Bar-Gad I The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain 132, 2125–2138 (2009). [DOI] [PubMed] [Google Scholar]
- 62.Grabli D et al. Behavioural disorders induced by external globus pallidus dysfunction in primates:I. Behavioural study. Brain 127, 2039–2054 (2004). [DOI] [PubMed] [Google Scholar]
- 63.McCairn KW, Iriki A & Isoda M Global dysrhythmia of cerebro-basal ganglia-cerebellar networks underlies motor tics following striatal disinhibition. J. Neurosci. 33, 697–708 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lerner A et al. Widespread abnormality of the gamma-aminobutyric acid-ergic system in Tourette syndrome. Brain 135, 1926–1936 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neuner I et al. Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Front. Hum. Neurosci. 8, 362 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z et al. The neural circuits that generate tics in Tourette’s syndrome. Am. J. Psychiatry 168, 1326–1337 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Menzies L et al. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci. Biobehav Rev. 32, 525–549 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pedroarena-Leal N & Ruge D Cerebellar neurophysiology in Gilles de la Tourette syndrome and its role as a target for therapeutic intervention. J. Neuropsychol 11, 327–346 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Anticevic A et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol. Psychiatry 75, 595–605 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pourfar M et al. Abnormal metabolic brain networks in Tourette syndrome. Neurology 76, 944–952 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feigin A et al. Thalamic metabolism and symptom onset in preclinical Huntington’s disease. Brain 130, 2858–2867 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rub U et al. Degeneration of the cerebellum in Huntington’s disease (HD): possible relevance for the clinical picture and potential gateway to pathological mechanisms of the disease process. Brain Pathol. 23, 165–177 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Samson M & Claassen DO Neurodegeneration and the cerebellum. Neurodegener Dis. 17, 155–165 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Caligiore D, Mannella F, Arbib MA & Baldassarre G Dysfunctions of the basal ganglia-cerebellar-thalamo-cortical system produce motor tics in Tourette syndrome. PLOS Comput. Biol 13, e1005395 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article proposes a computational model of how activity in the basal ganglia-cerebellum-cortical network contributes to the generation of motor tics.
- 75.Mallet L et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N. Engl. J. Med. 359, 2121–2134 (2008). [DOI] [PubMed] [Google Scholar]
- 76.Martinez-Torres I, Hariz MI, Zrinzo L, Foltynie T & Limousin P Improvement of tics after subthalamic nucleus deep brain stimulation. Neurology 72, 1787–1789 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Breakefield XO et al. The pathophysiological basis of dystonias. Nat. Rev. Neurosci. 9, 222–234 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Argyelan M et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J. Neurosci. 29, 9740–9747 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vo A et al. Thalamocortical connectivity correlates with phenotypic variability in dystonia. Cereb. Cortex 25, 3086–3094 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trost M et al. Primary dystonia: is abnormal functional brain architecture linked to genotype? Ann. Neurol. 52, 853–856 (2002). [DOI] [PubMed] [Google Scholar]
- 81.Ulug AM et al. Cerebellothalamocortical pathway abnormalities in torsinA DYT1 knock-in mice. Proc. Natl Acad. Sci. USA 108, 6638–6643 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DeSimone JC et al. In vivo imaging reveals impaired connectivity across cortical and subcortical networks in a mouse model of DYT1 dystonia. Neurobiol. Dis. 95, 35–45 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Calderon DP, Fremont R, Kraenzlin F & Khodakhah K The neural substrates of rapid-onset Dystonia-Parkinsonism. Nat. Neurosci. 14, 357–365 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fremont R, Tewari A, Angueyra C & Khodakhah K A role for cerebellum in the hereditary dystonia DYT1. Elite 6, e22775 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fremont R, Calderon DP, Maleki S & Khodakhah K Abnormal high-frequency burst firing of cerebellar neurons in rapid-onset dystonia-parkinsonism. J. Neurosci. 34, 11723–11732 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.LeDoux MS, Lorden JF & Ervin JM Cerebellectomy eliminates the motor syndrome of the genetically dystonic rat. Exp. Neurol. 120, 302–310 (1993). [DOI] [PubMed] [Google Scholar]
- 87.Neychev VK, Fan X, Mitev VI, Hess EJ & Jinnah HA The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain 131, 2499–2509 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Slaughter DG, Nashold BS Jr & Somjen GG Electrical recording with micro- and macroelectrodes from the cerebellum of man. J. Neurosurg. 33, 524–528 (1970). [DOI] [PubMed] [Google Scholar]
- 89.Koch G et al. Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimul. 7, 564–572 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Teixeira MJ, Schroeder HK & Lepski G Evaluating cerebellar dentatotomy for the treatment of spasticity with or without dystonia. Br. J. Neurosurg. 29, 772–777 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Tewari A, Fremont R & Khodakhah K It’s not just the basal ganglia: cerebellum as a target for dystonia therapeutics. Mov. Disord. 32, 1537–1545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee D, Seo H & Jung MW Neural basis of reinforcement learning and decision making. Annu. Rev. Neurosci. 35, 287–308 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watabe-Uchida M, Eshel N & Uchida N Neural circuitry of reward prediction error. Annu. Rev. Neurosci. 40, 373–394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wagner MJ, Kim TH, Savall J, Schnitzer MJ & Luo L Cerebellar granule cells encode the expectation of reward. Nature 544, 96–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garrison J, Erdeniz B & Done J Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neurosci. Biobehav Rev. 37, 1297–1310 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Ploghaus A et al. Learning about pain: the neural substrate of the prediction error for aversive events. Proc. Natl Acad. Sci. USA 97, 9281–9286 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’Doherty JP, Dayan P, Friston K, Critchley H & Dolan RJ Temporal difference models and reward-related learning in the human brain. Neuron 38, 329–337 (2003). [DOI] [PubMed] [Google Scholar]
- 98.Seymour B et al. Temporal difference models describe higher-order learning in humans. Nature 429, 664–667 (2004). [DOI] [PubMed] [Google Scholar]
- 99.Ramnani N, Elliott R, Athwal BS & Passingham RE Prediction error for free monetary reward in the human prefrontal cortex. Neuroimage 23, 777–786 (2004). [DOI] [PubMed] [Google Scholar]
- 100.Rodriguez PF, Aron AR & Poldrack RA Ventral-striatal/nucleus-accumbens sensitivity to prediction errors during classification learning. Hum. Brain Mapp. 27, 306–313 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tobler PN, O’Doherty JP, Dolan RJ & Schultz W Human neural learning depends on reward prediction errors in the blocking paradigm. J. Neurophysiol. 95, 301–310 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thoma P, Bellebaum C, Koch B, Schwarz M & Daum I The cerebellum is involved in reward-based reversal learning. Cerebellum 7, 433–443 (2008). [DOI] [PubMed] [Google Scholar]
- 103.Turner RS & Desmurget M Basal ganglia contributions to motor control: a vigorous tutor. Curr. Opin. Neurobiol. 20, 704–716 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dudman JT & Krakauer JW The basal ganglia: from motor commands to the control of vigor. Curr. Opin. Neurobiol. 37, 158–166 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Turner RS, Desmurget M, Grethe J, Crutcher MD & Grafton ST Motor subcircuits mediating the control of movement extent and speed. J. Neurophysiol. 90, 3958–3566 (2003). [DOI] [PubMed] [Google Scholar]
- 106.Belkhiria C et al. Exploration and identification of cortico-cerebellar-brainstem closed loop during a motivational-motor task: an fMRI study. Cerebellum 16, 326–339 (2017). [DOI] [PubMed] [Google Scholar]
- 107.Wolpert DM, Diedrichsen J & Flanagan JR Principles of sensorimotor learning. Nat. Rev. Neurosci. 12, 739–751 (2011). [DOI] [PubMed] [Google Scholar]
- 108.Seidler RD, Noll DC & Chintalapati P Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp. Brain Res. 175, 544–555 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Nikooyan AA & Ahmed AA Reward feedback accelerates motor learning. J. Neurophysiol. 113, 633–646 (2015). [DOI] [PubMed] [Google Scholar]
- 110.Galea JM, Mallia E, Rothwell J & Diedrichsen J The dissociable effects of punishment and reward on motor learning. Nat. Neurosci. 18, 597–602 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Izawa J & Shadmehr R Learning from sensory and reward prediction errors during motor adaptation. PLOS Comput. Biol. 7, e1002012 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hikosaka O, Nakamura K, Sakai K & Nakahara H Central mechanisms of motor skill learning. Curr. Opin. Neurobiol. 12, 217–222 (2002). [DOI] [PubMed] [Google Scholar]
- 113.Doyon J, Penhune V & Ungerleider LG Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 41, 252–262 (2003). [DOI] [PubMed] [Google Scholar]
- 114.Doyon J & Benali H Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 15, 161–167 (2005). [DOI] [PubMed] [Google Scholar]
- 115.Houk JC Agents of the mind. Biol. Cybern. 92, 427–437 (2005). [DOI] [PubMed] [Google Scholar]
- 116.Taylor JA & Ivry RB Cerebellar and prefrontal cortex contributions to adaptation, strategies, and reinforcement learning. Prog. Brain Res. 210, 217–253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article offers a comprehensive review of the contributions of the cerebellum to error-based and reward-based learning.
- 117.Doyon J et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 199, 61–75 (2009). [DOI] [PubMed] [Google Scholar]
- 118.Doyon J et al. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc. Natl Acad. Sci. USA 99, 1017–1022 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lehericy S et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc. Natl Acad. Sci. USA 102, 12566–12571 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]; This fMRI study shows a similar time course of activation in the STN and the lateral cerebellum during sequence learning.
- 120.Sami S, Robertson EM & Miall RC The time course of task-specific memory consolidation effects in resting state networks. J. Neurosci. 34, 3982–3992 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gheysen F et al. Taking the brakes off the learning curve. Hum. Brain Mapp. 38, 1676–1691 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tzvi E, Stoldt A, Witt K & Kramer UM Striatal-cerebellar networks mediate consolidation in a motor sequence learning task: An fMRI study using dynamic causal modelling. Neuroimage 122, 52–64 (2015). [DOI] [PubMed] [Google Scholar]
- 123.Fermin AS et al. Model-based action planning involves cortico-cerebellar and basal ganglia networks. Sci. Rep. 6, 31378 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This fMRI study provides evidence that different learning strategies recruit distinct basal ganglia–cerebellum–cortical networks.
- 124.Habas C et al. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 29, 8586–8594 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cauda F et al. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J. Cogn. Neurosci 23, 2864–2877 (2011). [DOI] [PubMed] [Google Scholar]
- 126.Li CS et al. Resting state functional connectivity of the basal nucleus of Meynert in humans: in comparison to the ventral striatum and the effects of age. Neuroimage 97, 321–332 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zheng W, Liu X, Song H, Li K & Wang Z Altered functional connectivity of cognitive-related cerebellar subregions in Alzheimer’s Disease. Front. Aging Neurosci. 9, 143 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Caulfield MD, Zhu DC, McAuley JD & Servatius RJ Individual differences in resting-state functional connectivity with the executive network: support for a cerebellar role in anxiety vulnerability. Brain Struct. Funct. 221,3081–3093 (2016). [DOI] [PubMed] [Google Scholar]
- 129.Harding IH et al. Fronto-cerebellar dysfunction and dysconnectivity underlying cognition in friedreich ataxia: The IMAGE-FRDA study. Hum. Brain Mapp. 37, 338–350 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pereira L et al. Resting-state functional connectivity and cognitive dysfunction correlations in spinocerebelellar ataxia type 6 (SCA6). Hum. Brain Mapp. 38, 3001–3010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peters SK, Dunlop K & Downar J Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front. Syst. Neurosci. 10, 104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Snider RS, Maiti A & Snider SR Cerebellar pathways to ventral midbrain and nigra. Exp. Neurol. 53, 714–728 (1976). [DOI] [PubMed] [Google Scholar]
- 133.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A & Uchida N Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012). [DOI] [PubMed] [Google Scholar]
- 134.Snider RS, Maiti A & Snider SR Cerebellar connections to catecholamine systems: anatomical and biochemical studies. Trans. Am. Neurol. Assoc. 101, 295–297 (1976). [PubMed] [Google Scholar]
- 135.Nieoullon A, Cheramy A & Glowinski J Release of dopamine in both caudate nuclei and both substantia nigrae in response to unilateral stimulation of cerebellar nuclei in the cat. Brain Res. 148, 143–152 (1978). [DOI] [PubMed] [Google Scholar]
- 136.Pelzer EA et al. Cerebellar networks with basal ganglia: feasibility for tracking cerebello-pallidal and subthalamo-cerebellar projections in the human brain. Eur. J. Neurosci. 38, 3106–3114 (2013). [DOI] [PubMed] [Google Scholar]
- 137.Milardi D et al. Extensive direct subcortical cerebellum-basal ganglia connections in human brain as revealed by constrained spherical deconvolution tractography. Front. Neuroanat. 10, 29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cacciola A et al. The known and missing links between the cerebellum, basal ganglia, and cerebral cortex. Cerebellum 16, 753–755 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Cacciola A et al. A connectomic analysis of the human basal ganglia network. Front. Neuroanat. 11, 85 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Caligiore D et al. Consensus paper: towards a systems-level view of cerebellar function: the interplay between cerebellum, basal ganglia, and cortex. Cerebellum 16, 203–229 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Doya K What are the computations of the cerebellum, the basal ganglia and the cerebral cortex? Neural Netw. 12, 961–974 (1999). [DOI] [PubMed] [Google Scholar]
- 142.Sakai K et al. Neural representation of a rhythm depends on its interval ratio. J. Neurosci. 19, 10074–10081 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sakai K et al. What and when: parallel and convergent processing in motor control. J. Neurosci. 20, 2691–2700 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Isoda M & Hikosaka O Switching from automatic to controlled action by monkey medial frontal cortex. Nat. Neurosci. 10, 240–248 (2007). [DOI] [PubMed] [Google Scholar]
- 145.Isoda M & Hikosaka O Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J. Neurosci. 28, 7209–7218 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hikosaka O & Isoda M Switching from automatic to controlled behavior: cortico-basal ganglia mechanisms. Trends Cogn. Sci. 14, 154–161 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pasquereau B & Turner RS A selective role for ventromedial subthalamic nucleus in inhibitory control. Elife 6, e31627 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Barton RA & Venditti C Rapid evolution of the cerebellum in humans and other great apes. Curr. Biol. 24, 2440–2444 (2014). [DOI] [PubMed] [Google Scholar]
- 149.Stephenson-Jones M, Ericsson J, Robertson B & Grillner S Evolution of the basal ganglia: dual-output pathways conserved throughout vertebrate phylogeny. J. Comp. Neurol. 520, 2957–2973 (2012). [DOI] [PubMed] [Google Scholar]
- 150.Takada M, Nishihama MS, Nishihama CC & Hattori T Two separate neuronal populations of the rat subthalamic nucleus project to the basal ganglia and pedunculopontine tegmental region. Brain Res. 442, 72–80 (1988). [DOI] [PubMed] [Google Scholar]
- 151.Woolf NJ & Butcher LL Cholinergic systems in the rat brain: IV. Descending projections of the pontomesencephalic tegmentum. Brain Res. Bull. 23, 519–540 (1989). [DOI] [PubMed] [Google Scholar]
- 152.Ruggiero DA, Anwar M, Golanov EV & Reis DJ The pedunculopontine tegmental nucleus issues collaterals to the fastigial nucleus and rostral ventrolateral reticular nucleus in the rat. Brain Res. 760, 272–276 (1997). [DOI] [PubMed] [Google Scholar]
- 153.Vitale F et al. Cholinergic excitation from the pedunculopontine tegmental nucleus to the dentate nucleus in the rat. Neuroscience 317, 12–22 (2016). [DOI] [PubMed] [Google Scholar]
- 154.Dum RP & Strick PL An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J. Neurophysiol. 89, 634–639 (2003). [DOI] [PubMed] [Google Scholar]
- 155.Clower DM, Dum RP & Strick PL Basal ganglia and cerebellar inputs to ‘AIP’. Cereb. Cortex 15, 913–920 (2005). [DOI] [PubMed] [Google Scholar]
- 156.Akkal D, Dum RP & Strick PL Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J. Neurosci. 27, 10659–10673 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]