Abstract
The Fmr1 knockout (KO) mouse has commonly been used to investigate communication impairments, one of the key diagnostic symptoms, observed in Fragile X syndrome (FXS) and Autism spectrum disorder (ASD). Many studies have found alterations in ultrasonic vocalizations (USVs) in neonatal Fmr1 KO mice, however, there is limited research investigating whether these deficits continue into adulthood. In the present study, we examine differences in female urine-induced ultrasonic vocalizations, scent marking behavior, odor discrimination, and open field activity in adult male Fmr1 KO and WT mice. Overall, we found extensive alterations between genotypes in both spectral and temporal properties of ultrasonic vocalizations. There was no difference in the average number of calls emitted by both genotypes, however, Fmr1 KO mice emitted calls of a higher frequency, decreased amplitude, and shorter duration than WT mice. Spectrographic analyses revealed statistically significant differences between genotypes in the types of calls emitted. Contrastingly, we found no differences in scent marking behavior, a form of social communication, or in odor discrimination and activity levels of the mice. The results corroborate previous studies emphasizing the importance of qualitative differences observed in vocalization behavior of Fmr1 KO mice, rather than quantitative measurements such as number of calls emitted. Overall, the study confirms the presence of abnormalities in vocalization behavior in adult Fmr1 KO mice that we believe are consistent with communication deficits seen in the syndrome.
Keywords: Fragile X syndrome, FXS, ultrasonic vocalizations, USV, communication deficits, scent marking
1. Introduction
Fragile X syndrome (FXS), an X-linked neurodevelopmental disorder, is the most common heritable cause of intellectual disability (Hawkins et al., 2011). It is characterized by expansion of a CGG repeat in the 5’ untranslated region of the fragile X mental retardation gene (FMR1) (Fatemi & Folsom, 2011; Laggerbauer, Ostareck, Keidel, Ostareck-Lederer, & Fischer, 2001). This expansion ultimately results in silencing of the gene leading to an absence of fragile X mental retardation protein (FMRP) (Levenga, de Vrij, Oostra, & Willemsen, 2010; Roberts, Mirrett, Anderson, Burchinal, & Neebe, 2002). Deficiency of FMRP can lead to intellectual disability, behavioral and social deficits, as well as altered communicative abilities exhibited by FXS patients (Brady, Skinner, Roberts, & Hennon, 2006; Roberts et al., 2002).
Previous research has found those with FXS to have substantial impairments in conversational speech, including poor topic maintenance, run-on sentences, and disorganized speech (Belser & Sudhalter, 2001; Dykens, Hodapp, & Leckman, 1994). In addition, conversations are often affected by impairments in sociability and anxiety, such as inappropriate eye contact with others and gaze aversion (Cohen, Vietze, Sudhalter, Jenkins, & Brown, 1991; Mirrett, Roberts, & Price, 2003). Individuals with FXS also demonstrate difficulty with pragmatic skills, such as perseveration of words, sentences, or topics, which may be reflective of a type of stereotypic behavior commonly seen in the syndrome (Ferrier, Bashir, Meryash, Johnston, & Wolff, 1991; Hanson, Jackson, & Hagerman, 1986). Furthermore, individuals with FXS have a tendency to speak in short bursts, produce stuttering-like repetition of sounds, and have variability in rate of speech (Abbeduto & Hagerman, 1997; Largo & Schinzel, 1985). Overall, those affected by FXS exhibit altered communicative abilities that could potentially have a negative effect on their cognition, sociability, and quality of life.
The investigation of ultrasonic vocalization (USV) deficits has been previously explored in various mouse models of neurological diseases, both in early development from postnatal day (PD) 2 to 14 (Lai et al., 2014; Roy, Watkins, & Heck, 2012; Scattoni, Gandhy, Ricceri, & Crawley, 2008; Wohr, Roullet, Hung, Sheng, & Crawley, 2011; Wohr et al., 2013) and in adulthood ranging from PD60 to 120 (Belagodu, Johnson, & Galvez, 2016; Rotschafer, Trujillo, Dansie, Ethell, & Razak, 2012; Wohr, Roullet, Hung, et al., 2011). Mouse pups begin emitting ultrasonic vocalizations (USVs) shortly after birth and continue, although at a reduced rate, throughout adulthood in response to specific situations. In neonatal mice, they are known to occur between frequencies of 30 to 90 kHz and are elicited from mice upon separation from the nest, dam, or littermates (Branchi, Santucci, & Alleva, 2001; Holy & Guo, 2005). Numerous studies have found examinations of USVs in FXS and ASD mouse models to be an important parameter when investigating social communication in mouse pups, however, there have been few studies exploring USVs in adult mouse models (Lai et al., 2014; Roy et al., 2012; Scattoni et al., 2008; Scearce-Levie et al., 2008; Wohr, Roullet, & Crawley, 2011). USVs in adult mice have been investigated in various mating and courtship paradigms, social encounters and intruder settings, and in response to urine of female mice (Holy & Guo, 2005; Scattoni, Ricceri, & Crawley, 2011).
The few studies examining adult USVs in FXS focus mainly on courtship behavior (Belagodu et al., 2016; Rotschafer et al., 2012). Rotschafer et al. (2012) found the number of USVs in adult male Fmr1 KO mice to be reduced compared to wild type (WT) controls in a mating paradigm (Rotschafer et al., 2012). However, this study recorded USVs with both male and female mice present in the chamber. This makes it difficult to determine whether analyzed USVs consisted of solely male vocalization patterns and that the presence of a female did not confound results. In a separate study, investigators used a different courtship paradigm to induce USVs and found no difference in the number of USVs emitted by adult male Fmr1 KO mice, but found an increase in “u” syllables, while WT littermates vocalized “h” syllables more often (Belagodu et al., 2016). While the functional importance of specific syllables based on frequency and complex patterns is currently unknown, they are organized into phrases that are thought to resemble repetitive speech patterns typically found in those with the syndrome. This study removed females prior to recording, however, one caveat to both of these studies is that USVs are induced via direct exposure to the female mice. Call production has shown to be at its peak during initial exploratory activity in response to the female, thus a more ideal method would have female urine present for the entire testing period (Wohr, Roullet, & Crawley, 2011). Urine from female mice has found to be sufficient to elicit USVs in male mice without the presence of the female (Whitney, Alpern, Dizinno, & Horowitz, 1974).
Investigation of USV deficits, along with social behavior, by examination of male USVs in response to female urine allows calls to be directly attributed to the male and for consistent pheromone exposure throughout the paradigm. In addition, urine-induced vocalization behavior is highly dependent on previous social interaction and social status in mice (D’Amato, 1991; Dizinno, Whitney, & Nyby, 1978; Guo & Holy, 2007; Holy & Guo, 2005). Wohr et al. (2011) found that adult BTBR T+tf/J mice, another mouse model of autism, emitted minimal USVs and scent markings in response to female urine compared to WT mice (Wohr, Roullet, & Crawley, 2011). Scent markings are urinary pheromone traces deposited in strategic areas that act as an additional mode of communication. They can be produced by mice in a variety of situations, including to attract partners, demarcate territories, and recognize the reproductive state of potential mates (Hurst, 1990a, 1990b). This additional social communicative behavior has not yet been examined in Fmr1 KO mice.
Further investigation of the communicative behavior of adult male Fmr1 KO mice is critical in order to gain a comprehensive understanding of specific call types emitted in the context of mating. While there is evidence of alterations of USVs in postnatal Fmr1 KO mice, few studies have examined vocalization behavior in FXS adult mice. In the present study, we investigate differences in female urine-induced USVs of male Fmr1 KO mice compared to WT mice. Spectrographic analyses were conducted on USVs to examine both spectral and temporal variability between the calls of Fmr1 KO and WT mice. We also examined scent marking behavior to determine whether Fmr1 KO mice differ in how they use olfactory communicative signaling to attract mates and assert their dominance (Roullet, Wohr, & Crawley, 2011). In addition, we tested overall activity levels of the mice during USV recording to identify whether hyperactivity effected vocalization behavior, as well as tested odor discrimination abilities to confirm the absence of any underlying olfactory differences. We hypothesized that Fmr1 KO mice will exhibit altered vocalization patterns compared to WT mice, including specific call types that may be reflective of repetitive behavioral tendencies characteristic of Fragile X syndrome. We postulate that both groups of mice will produce a similar amount of scent markings. We also hypothesize Fmr1 KO mice to exhibit hyperactivity in the open field and similar odor discriminatory abilities as WT mice.
2. Materials and Methods
2.1. Animals and housing
Subject mice included adult male FVB/NJ littermates in which 19 were Fmr1 knockout (KO) and 18 were wild type (WT) mice. All mice were generated and group housed at Baylor University in standard laboratory conditions (22°C, 14-hour light/10-hour dark diurnal cycles) with food and water provided ad libitum. Experimental testing was performed between 12:00 and 16:30 each day during the 14-hour light period. All procedures were conducted in compliance with the Baylor University Institutional Animal Care and Use Committee and the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
2.2. Experimental design
2.2.1. Previous female exposure
Prior to vocalization recording, male subject mice were introduced to female mice of the same FVB/NJ strain in order to control for history of social experience. One male Fmr1 KO or WT mouse was placed with a female Fmr1 WT mouse of similar age in a clean polycarbonate cage with bedding for 5 minutes, one week prior to testing. The female mice were not littermates with the male subject mice.
2.2.2. Urine collection
Female urine was obtained from the Fmr1 WT mice in estrous that testing mice were previously exposed to. In order to coordinate estrous cycles of 2 to 4 female mice housed together, bedding from a male FVB/NJ cage was placed in a cage of females. Estrous cycles of female mice were then visually inspected and tracked daily. A female was considered in estrous when the vaginal area was red, inflamed, and open (Byers, Wiles, Dunn, & Taft, 2012). To elicit urine dissemination from the donor female, the mouse was removed from its cage and gently stroked in an anterior to posterior direction on its abdomen. Urine was collected in a 1.7ml Eppendorf tube. Immediately prior to testing, we used a pipette to transfer 20ul of fresh female urine onto the center of a piece of Strathmore paper (Strathmore Drawing Paper Premium, recycled, microperforated, 400 series; Strathmore Artist Papers, Neenah, WI, USA) able to effectively absorb drops of mouse urine which lined the bottom of the testing chamber.
2.2.3. Test procedure
Before testing, mice were weighed and allowed to habituate in the testing room for 30 minutes. Ultrasonic vocalization recordings, scent markings, and open field activity were simultaneously recorded for each subject mouse for 5 minutes. Mice were individually placed in an acrylic, sound-attenuating chamber (40cm × 40cm × 30cm) in an isolated room controlled for temperature, light levels, and background noise. Urine-induced USVs were recorded for 5 minutes using a condenser ultrasonic microphone (CM16/CMPA, Avisoft Bioacoustics, Germany, part #40011) connected to an ultrasound-recording interface (UltraSoundGate 116Hb, Avisoft Bioacoustics, part #41161/1162) suspended within the testing chamber. To measure locomotion and repetitive behavior during the testing period, locomotor activity was measured with automatic optical animal detection software (Fusion by Omnitech Electronics, Inc., Columbus, OH). Total distance and time spent in the center and surround regions, stereotypy time and episode count, vertical episode count, and clockwise and counterclockwise rotations were recorded. A map consisting of a center region (20cm by 20cm) was imposed, and any activity beyond this area is considered to be time spent in the surround region. Stereotypy is typically observed during grooming and consists of an animal breaking the same beam (or set of beams) repeatedly in the open field apparatus. A break in stereotypy of 1 or more seconds distinguishes one stereotypic episode from the next. A vertical episode is considered anytime the mouse rears on their hind legs. Rotations are calculated anytime the mouse travels in a clockwise or counter-clockwise revolution in the open field. To prevent contamination of olfactory cues, between each trial any feces deposited by the mouse were removed and the chamber was cleaned with 30% isopropyl alcohol. Following each testing session, the paper was treated with Ninhydrin spray (Sigma-Aldrich, St. Louis, MO, USA) and left to develop for approximately 24h before visual inspection of urine traces demarcated in purple. Scent marks were counted and analyzed as done previously by (Arakawa, Arakawa, Blanchard, & Blanchard, 2008; Arakawa, Blanchard, Arakawa, Dunlap, & Blanchard, 2008). All scent marks were outlined with blue pen and total scent marks were counted. In addition, number of scent marks within the 20cm by 20cm inner region surrounding the center female urine spot and in the area beyond this designated region were counted and totaled. The same center and surround parameters were utilized for open field activity and quantifying scent marks.
2.3. Odor discrimination task
Methods for this task were previously described in Arbuckle et al., 2015 (Arbuckle, Smith, Gomez, & Lugo, 2015). A separate cohort of similarly aged adult male Fmr1 KO and WT mice were used in this task to assess basic olfactory sensory abilities. The first tube contained water, a neutral odor. Two non-social odors, almond and banana extract (100 μ1 each) were prepared fresh each day in a 1:100 dilution. The social odors were prepared on the morning of testing by swabbing the floor of a cage of mice that had not been cleaned for three days. Two different cages were used to create the two social odors. These odors were obtained from members of the same sex as the test subject (male). The test mouse was acclimated to a room other than the test room for 30 minutes. Each odor was presented for 1 minute and was repeatedly presented 3 times to each test mouse. Sniffing behaviors were recorded on a stopwatch by a live observer. The inter-trial interval was approximately 1 minute.
2.4. Ultrasonic vocalization analysis
Spectral analyses of USV recordings were conducted using Avisoft SASLab Pro software. Avisoft SASLab Pro automatically detects ultrasonic calls via a threshold-based algorithm and programmed hold time mechanisms. To create spectrograms, fast Fourier transformations (FFT) were performed on all recording files with an FFT-length of 1024 points, time window overlap of 75% (100% Frame, Hamming Window), frequency resolution of 488 Hz, and a time resolution of 1ms, as previously defined (Scattoni et al., 2008). Each call was visually and manually identified by the experimenter using 1 of 10 distinct categories outlined in a call type classification scheme created by Scattoni et al., 2008. Categories of call types include 10 waveform patterns that are based on internal pitch changes, lengths, and shapes of individual calls. The call types are denoted as complex, harmonics, two-syllable, upward, downward, flat, chevron, short, composite, and frequency steps. Complex calls include those that contain one syllable with two or more directional changes in pitch that are each greater than 6.25 kHz. Harmonics are a single call that is similar to the complex type, with additional calls of varying frequencies surrounding the main call. Two-syllable calls contain one call that is either of flat or downward type and another punctuated call near the end. Upward- and downward- modulated calls have a continuous increase or decrease in pitch that is greater than 12.5 kHz and end with a frequency at least 6.25 kHz more or less than the pitch in which the call began at. Flat calls have a consistent frequency across the duration of the call and short calls are punctuated and less than 5ms in length. Chevron calls resemble an “inverted-U” shape and have a continuous increase in pitch that is greater than 12.5 kHz, followed by a decrease that is greater than 6.25 kHz. Composite calls contain two harmonically independent components that are emitted simultaneously. Lastly, frequency step calls are rapid frequency changes that appear as discontinuous “steps” on the spectrogram with no interruptions of time between calls.
2.5. Statistical analysis
Data was analyzed using GraphPad Prism 7 software (La Jolla, CA) or SPSS 21.0 (IBM, USA). For ultrasonic vocalization analysis, each individual call was treated as a statistical unit. Differences in acoustic and temporal parameters of USVs between groups were analyzed using a Pillai’s Trace MANOVA and post hoc analyses included independent t-tests to detect differences in the duration, mean peak frequency, mean entire fundamental frequency, and mean peak amplitude of specific call types. In cases in which the homogeneity of variance assumption was violated, nonparametric Mann-Whitney U tests were conducted. Spectral properties of call types were analyzed with a Pearson Chi-Square, along with z-tests to compare call type proportions between genotypes. Independent t-tests and nonparametric Mann-Whitney U tests were performed to examine differences in scent marks and activity levels between Fmr1 KO and WT mice in the open field task. A repeated measures ANOVA was used to examine the impact of genotype on the frequency and duration for each trial of the odor discrimination task. For all analyses a value of p < .05 was considered significant and data are expressed as the mean ± standard error of the mean (SEM).
3. Results
3.1. Ultrasonic vocalization (USV) recordings in response to female urine
3.1.1. Average number of USVs emitted
There was no difference between the mean number of calls emitted from the WT (n=19) and Fmr1 KO (n=18) mice (t[35] = 0.51, p = 0.61). As a result of 5 WT and 8 Fmr1 KO mice failing to produce ultrasonic vocalizations, they were taken out for all further USV analyses. Once removed, still no difference was detected in mean number of calls emitted by WT (n=14) and Fmr1 KO (n=10) mice (t[22] = 1.44, p = 0.16) (Fig. 1a). Overall, the total number of calls for WT mice was 2,208 and Fmr1 KO mice was 2,625.
Figure 1. Ultrasonic vocalizations (USVs) in adult male Fmr1 KO and WT mice in response to female urine.
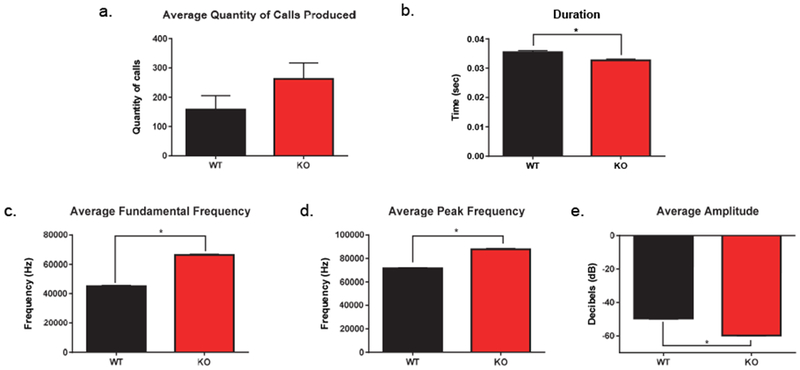
There was no difference in average quantity of calls produced by Fmr1 KO (n = 10) and WT (n = 14) mice (a). Ultrasonic vocalizations emitted by KO mice were of significantly shorter duration (b) and had a higher fundamental frequency (c) and peak frequency (d). The average amplitude of vocalizations was significantly decreased in KO mice compared to WT controls (e). Data are presented as mean ± standard error of the mean (SEM), * p < .05.
3.1.2. Acoustic and temporal characteristics of USVs
Analyses of acoustic and temporal characteristics of ultrasonic vocalizations revealed a significant MANOVA with genotype and call type as fixed factors and duration, mean peak frequency, mean fundamental frequency, and mean peak amplitude as dependent factors (F[4,4810] = 51.37, p < 0.001). There was a main effect of call type (F[36,19252] = 101.38, p < 0.001). There was an interaction between genotype and call type (F[36,19252] = 6.16, p < 0.001). The subsequent individual between-subjects statistics revealed several main effects. There was evidence of a significant main effect of genotype when examining duration (F[1,4813] = 17.46, p < 0.001), mean peak frequency (F[1,4813] = 111.62, p < 0.001), mean fundamental frequency (F[1,4813] = 203.17, p < 0.001), and mean amplitude (F[1,4813] = 11.54, p = 0.001) (Fig. 1b–e). There were also main effects of call types when examining duration (F[9,4813] = 401.62, p < 0.001), mean peak frequency (F[9,4813] = 19.30, p < 0.001), mean fundamental frequency (F[9,4813] = 38.26, p < 0.001), and mean amplitude (F[9,4813] = 134.29, p < 0.001). Overall, Fmr1 KO mice emitted calls of significantly shorter duration, higher peak and fundamental frequency, and at a lower amplitude than WT counterparts. Significant interactions between genotype and call type were also detected for duration (F[9,4813] = 11.17, p < 0.001), mean peak frequency (F[9,4813] = 6.90, p < 0.001), mean fundamental frequency (F[9,4813] = 8.84, p < 0.001), and mean amplitude (F[9,4813] = 6.51, p < 0.001).
3.1.3. Spectral properties of USVs
Since there were no differences in the number of calls emitted by Fmr1 KO and WT mice, we examined the spectral properties of ultrasonic vocalizations to determine specific call type differences. We first categorized the calls by assigning them to 10 different types of calls (complex, harmonics, two-syllable, upward, downward, flat, chevron, short, composite, frequency steps) (Scattoni et al., 2008). We then performed a Pearson Chi-Square analysis to determine whether there were differences between the groups in terms of the types of calls that were produced by the WT and Fmr1 KO mice. The Pearson Chi-Square revealed a significant population difference between types of calls emitted (X2[9, N = 4833] = 63.72, p < 0.001). Percentages of each call type emitted is represented in Figure 2a. Separate call type x genotype analyses were used after the main Chi-square test by performing separate z-tests to analyze the number of ultrasonic vocalizations emitted from Fmr1 KO and WT mice in each vocalization category. Knockout animals emitted a significantly greater amount of complex (p < 0.05), chevron (p < 0.05), and flat calls (p < 0.05) compared to WT mice. Wild type mice produced a significantly greater amount of composite (p < 0.05) and frequency step calls (p < 0.05). No other differences in number of call types were detected between groups (Fig. 2b).
Figure 2. Call types emitted by Fmr1 KO and WT mice.

The types of calls vocalized by Fmr1 KO (n = 10) and WT (n = 14) mice differed significantly (X2[9, N = 4833] = 63.72, p < .001) (a). On average, KO mice produced a significantly increased number of complex, chevron, and flat calls and a decreased number of composite and frequency step call types (b). Data are presented as mean ± standard error of the mean (SEM), * p < .05.
3.1.4. USV call type characteristics
To further analyze differences in specific call types emitted by Fmr1 KO and WT mice, independent t-tests and when necessary, non-parametric Mann-Whitney U tests, were used to examine duration, peak and fundamental frequency, and amplitude of each call type. The following calls were emitted by Fmr1 KO mice for a significantly shorter duration than WT mice: complex (U = 62722, p < 0.001); downward (U = 2177, p < 0.001); chevron (t[390] = 2.19, p = 0.03); short (U = 51210, p < 0.001); and flat calls (t[145] = 3.78, p < 0.001). The only call type emitted by Fmr1 KO mice for a significantly longer duration were harmonic calls (t[158] = 2.41, p = 0.02) (Fig. 3a).
Figure 3. Temporal and acoustic characteristics of USV call types.

The average duration (a), peak frequency (b), fundamental frequency (c), and amplitude (d) were significantly different for many of the call types emitted by Fmr1 KO (n = 10) and WT (n = 14) mice. Data are presented as mean ± standard error of the mean (SEM), * p < .05.
The mean peak frequency, measuring the frequency of the highest peak of each vocalization, was significantly higher in Fmr1 KO mice for the following call types: complex (U = 39086, p < 0.001); harmonics (U = 2291, p < .01); two-syllable (U = 22, p < 0.001); upward (U = 223869, p < 0.001); downward (U = 1835, p < 0.001); chevron (U = 9119, p < 0.001); short (U = 40609, p < 0.001); frequency steps (U = 23527, p < 0.001); and flat calls (U = 1743,p < 0.01) (Fig. 3b). The fundamental frequency, measuring the average frequency of each individual vocalization, was also higher in Fmr1 KO mice for the majority of call types: complex (U = 29152, p < 0.001); harmonics (t[158] = 5.43, p < 0.001); two-syllable (U = 47, p = 0.04); upward (t[1756] = 15.29, p < 0.001); downward (U = 1225, p < 0.001); chevron (U = 5666, p < 0.001); short (U = 41380, p < 0.001); frequency steps (U = 14494, p < 0.001); flat (U = 1356, p < 0.001) (Fig. 3c). Compared to WT, Fmr1 KO mice vocalized at an overall lower amplitude when emitting complex (U = 60941, p < 0.001); downward (U = 2206, p < 0.001); chevron (t[390] = 4.18, p < 0.001; composite (U = 236, p = 0.01; frequency steps (U = 28047, p < 0.001); and flat calls (t[145] = 2.19, p < 0.01). However, the amplitude of short calls (U = 63204, p < 0.01) were significantly higher in KO mice (Fig. 3d).
3.2. Scent marking in response to female urine
In response to female urine, we found no differences in scent marking between Fmr1 KO (n=16) and WT (n=19) mice during the 5 min testing period. Due to Ninhydrin spraying error, scent marking results from two Fmr1 KO mice were not included. Unpaired t-tests and nonparametric Mann-Whitney U tests revealed no differences in total scent marks (U = 109.5, p = 0.16), total scent marks in the surround region of the chamber (U = 107.5, p = 0.14), or center region (t[33] = 0.08, p = 0.93). There was also no difference in percent of scent marks in the surround area (t[33] = 1.53, p = 0.14).
To determine whether mice that did not emit any ultrasonic vocalizations affected scent marking behavior, we conducted the same analyses excluding these mice. We still found there to be no difference in total scent marks (U = 48, p = 0.60), scent marks in the surround region (U = 46.50, p = 0.54), or center region (t[20] = 1.27, p = 0.22) between Fmr1 KO (n=8) and WT (n=14) mice. There was also no difference in percent of scent marks in the surround region when excluding these mice (U = 29, p > 0.05). The mean and SEM for scent marking behavior of Fmr1 KO and WT mice are shown in Table 1.
Table 1.
Scent marking results. No significant differences were detected between Fmr1 KO and WT for all analyses.
| Analyses including all mice (WT: n = 19, KO: n = 16) | Excluding mice that did not vocalize (WT: n = 14, KO: n = 8) | |||||
|---|---|---|---|---|---|---|
| WT | KO | P value | WT | KO | P value | |
| Total scent marks | 29.11 ± 3.96 | 26.88 ± 8.17 | 0.16 | 31.79 ± 4.78 | 38.63 ± 15.21 | 0.60 |
| Scent marks in surround region | 20.58 ± 3.44 | 18.50 ± 7.05 | 0.14 | 23.36 ± 4.28 | 27.13 ± 13.49 | 0.54 |
| Scent marks in center region | 8.53 ± 1.13 | 8.38 ± 1.49 | 0.93 | 8.43 ± 1.24 | 11.50 ± 2.36 | 0.22 |
| Percent of surround scent marks | 66.11 ± 4.51 | 54.77 ± 6.08 | 0.14 | 73.27 ± 2.84 | 56.43 ± 8.57 | 0.07 |
Data shown as mean ± standard error of the mean (SEM).
3.3. Open field activity in response to female urine
There was no difference in total activity levels between Fmr1 KO (n=18) and WT (n=19) mice (t[35] = 0.28, p = 0.78). We also found no differences between Fmr1 KO and WT mice in measures examining repetitive behavior, including stereotypy time (t[35] = 0.45, p = 0.66), stereotypy episode count (t[35] = 0.13, p = 0.90), vertical episode count (t[35] = 0.36, p = 0.72), clockwise rotations (t[35] = 0.71, p = 0.48), or counter-clockwise rotations (t[35] = 0.33, p = 0.74). To examine differences in duration and total distance in the center of the testing chamber compared to the surround area, separate independent t-tests were conducted for each location. Increased time in the surround area is often an indicator of increased anxiety (Prut & Belzung, 2003). For duration, we found no differences between Fmr1 KO and WT in the center area (t[35] = 0.57, p = 0.57) or surround region (t[35] = 0.96, p = 0.34). We also found no differences between genotypes when examining total distance in the center (t[35] = 1.06, p = 0.30) or surround area (t[35] = 0.34, p = 0.74).
To determine whether mice that did produce any ultrasonic vocalizations affected activity levels in the open field task, we performed the same analyses excluding these mice. We found there to still be no difference in total activity levels between Fmr1 KO (n=10) and WT (n=14) mice (t[22] = 1.73, p = 0.10). We also found there to be no difference in stereotypy time (t[22] = 0.62, p = 0.54), stereotypy episode count (t[22] = 0.24, p = 0.81), vertical episode count (t[22] = 1.19, p = 0.25), or counter-clockwise rotations (t[22] = 0.72, p = 0.48). With these mice excluded, Fmr1 KO mice had a significantly increased amount of clockwise rotations compared to WT mice (t[22] = 2.05, p = 0.05). When examining differences in the center versus surround area of the chamber excluding mice that did not vocalize, there was no difference between Fmr1 KO and WT mice in duration spent in the center (t[22] = 1.28, p = 0.21) or surround areas (t[22] = 1.11, p = 0.28). There was also no difference in the distance spent in the surround area (t[22] = 0.73, p = 0.47), however, Fmr1 KO mice had significantly increased activity in the center (t[22] = 2.06, p = 0.05). The mean and SEM of Fmr1 KO and WT mice for all open field parameters are shown in Table 2.
Table 2.
Results for open field activity.
| Analyses including all mice (WT: n = 19, KO: n = 18) | Excluding mice that did not vocalize (WT: n = 14, KO: n = 10) | |||||
|---|---|---|---|---|---|---|
| WT | KO | P value | WT | KO | P value | |
| Total distance (cm) | 1373 ± 68.97 | 1407 ± 100.90 | 0.78 | 1334 ± 89.68 | 1578 ± 111 | 0.10 |
| Distance in surround region (cm) | 1163 ± 77.35 | 1118 ± 108 | 0.74 | 1119 ± 102.50 | 1242 ± 138.90 | 0.47 |
| Distance in center region (cm) | 168.40 ± 19.02 | 195.90 ± 17.80 | 0.30 | 160.40 ± 20.59 | 222.40 ± 20.82 | 0.05* |
| Total duration in surround region (s) | 241.30 ± 6.05 | 231.60 ± 8.11 | 0.34 | 238 ± 6.67 | 226.20 ± 8.43 | 0.28 |
| Total duration in center region (s) | 49.94 ± 4.67 | 53.79 ± 4.83 | 0.57 | 50.14 ± 4.47 | 59.46 ± 5.95 | 0.21 |
| Stereotypy time (s) | 4.33 ± 0.88 | 4.96 ± 1.10 | 0.66 | 4.87 ± 1.13 | 6.07 ± 1.66 | 0.54 |
| Stereotypy episode count | 23.21 ± 5.47 | 24.22 ± 5.66 | 0.90 | 27.43 ± 7.12 | 30.10 ± 8.43 | 0.81 |
| Vertical episode count | 36.05 ± 3.23 | 37.83 ± 3.84 | 0.72 | 38.50 ± 3.88 | 45.80 ± 4.77 | 0.25 |
| Clockwise rotations | 4.26 ± 0.56 | 4.94 ± 0.78 | 0.48 | 4.36 ± 0.72 | 6.80 ± 0.99 | 0.05* |
| Counter-clockwise rotations | 4.05 ± 0.46 | 4.28 ± 0.49 | 0.74 | 3.50 ± 0.51 | 4.10 ± 0.67 | 0.48 |
Data shown as mean ± standard error of the mean (SEM)
p < .05.
3.4. Odor discrimination task
To examine potential changes in habituation to social and non-social odors, a separate cohort of similarly aged Fmr1 WT (n=12) and KO (n=16) mice were tested in the odor discrimination task. Results indicated no impact of genotype on the duration spent sniffing all odors, (F[1,26] = 0.46, p = 0.50) (Fig. 4). There was a main effect of trial presentation (F[14,364] = 28.7, p < 0.001), but no interaction of genotype and trial was detected (F[14,364] = 0.41, p = 0.97). There was also no difference between Fmr1 KO and WT in the frequency of sniffing all odors, (F[1,26] = 0.03, p = 0.86) (Data not shown). A main effect of trial presentation for frequency was detected (F[14,364] = 7.67, p < 0.001), but no interaction of genotype and trial (F[14,364] = 0.89, p = 0.57) (Data not shown).
Figure 4. Odor discrimination task.

There was no effect of genotype on the duration spent sniffing all odors for Fmr1 KO (n = 16) and WT mice (n = 12), suggesting they habituate and dishabituate similarly to social and non-social odors. Data are presented as mean ± standard error of the mean (SEM).
4. Discussion
In the present study we compared the number of ultrasonic vocalizations emitted per group, the spectral properties of the calls emitted, the impact of scent marking, and measured whether the mice had olfactory deficits. While there are many studies examining vocalization behavior in pups, there are limited studies exploring USVs in adult Fmr1 KO mice and it is not understood whether deficits present in early development continue into adulthood. We found Fmr1 KO mice to emit ultrasonic vocalizations of a higher frequency, decreased amplitude, and shorter duration than WT mice. Further, the repertoire of calls emitted by Fmr1 KO mice differed substantially from call types vocalized by WT mice, with Fmr1 KO mice having an increased number of complex, chevron, and flat calls, but a reduced amount of composite and frequency step calls. However, we did not find any differences in scent marking activity between Fmr1 KO and WT mice or in the ability of the subjects to habituate or dishabituate to social and non-social odors. Similarly, the average number of calls emitted was not different, suggesting that both groups displayed a similar amount of communicative behavior during the testing period.
An important property that many animal and human studies examining communication in autism has focused on is the frequency or pitch of ultrasonic vocalizations (Esposito & Venuti, 2008, 2010; Roy et al., 2012). Our study found Fmr1 KO mice to vocalize at an overall higher average peak frequency and fundamental frequency than WT mice. While this acoustic parameter is often examined in mouse pups as well as in infant cries, our findings suggest it may also be a key indicator of altered ultrasonic vocalizations observed in adulthood. As a result of the high degree of conservation observed in neonatal crying among non-human species, many have used rodent pup USVs to further explore aspects of infant crying (Lummaa, Vuorisalo, Barr, & Lehtonen, 1998; Zeskind et al., 2011). Higher frequency cries in human infants are found to be more aversive, evoke states of uneasiness in caregivers, and negatively affect parental responses (Esposito & Venuti, 2008; LaGasse, Neal, & Lester, 2005). Further, one study found the cries of normally developing children to decrease in pitch over the first two years of life, while infants with ASD continued to emit cries at a higher frequency that could negatively affect the infant-caregiver relationship that is critical to normal development (Esposito & Venuti, 2010). Research investigating communication in those with FXS has focused mainly on conversational and pragmatic aspects of language development (Abbeduto & Hagerman, 1997; Finestack, Richmond, & Abbeduto, 2009). Acoustic dimensions of speech, including pitch, have not been documented in adults with the syndrome. While the Fmr1 KO mouse exhibits face validity by paralleling many of the behavioral phenotypes of humans with FXS, the model would further demonstrate strong predictive validity if acoustic properties of speech early in life could predict communication deficits in adulthood (Kazdoba, Leach, Silverman, & Crawley, 2014). Further investigation is necessary to determine whether higher frequency vocalizations observed in adult Fmr1 KO mice may represent a continuation of this deficit beyond early stages of development.
Interestingly, even though Fmr1 KO mice produced a higher number of complex, chevron, and flat calls, the overall duration of these call types was significantly lower in Fmr1 KO mice. A complex call contains only one syllable with two or more directional changes in pitch (Scattoni et al., 2008). This overall increase in number, but decrease in duration of complex calls along with a significantly shorter duration of many other call types may be representative of the repetitive-like speech sounds often seen in FXS patients (Belser & Sudhalter, 2001; Levy, Gottesman, Borochowitz, Frydman, & Sagi, 2006).
It is important to note that the shorter duration of calls in Fmr1 KO mice cannot be attributed to a difference in average number of calls emitted by both groups, as Fmr1 KO and WT mice produced an overall similar number of calls. A previous study that used a different mating paradigm to elicit calls from the males also found that Fmr1 KO mice emitted the same number of calls compared to the WT mice. In contrast to our findings they did not detect any differences in the average duration of USVs (Belagodu et al., 2016). One other adult FXS USV study reported a significantly decreased amount of calls in Fmr1 KO mice (Rotschafer et al., 2012). One important difference in this study is that the USVs were recorded from the Fmr1 KO and WT mice during mating behavior. While it is possible that direct interaction with a female mouse may be more effective in eliciting vocalizations compared to female urine, presence of the female mouse may contribute to the total number of calls emitted. The use of female urine to elicit USVs in males ensures that the testing mouse is entirely responsible for the number of vocalizations detected (Wohr, Roullet, & Crawley, 2011).
Discrepancies in USV measures could be due to multiple factors, such as type of USV induction (i.e. urine-, mating-, isolation-induced), as well as type of mouse model, age, and background strain. The number of USVs may not be a key determinant in detecting communication deficits in FXS and other autism mouse models. Our results support recent findings suggesting call types and acoustic properties to be more representative of the atypical communication patterns observed in the syndrome (Belagodu et al., 2016; Holy & Guo, 2005; Scattoni et al., 2008).
The neurobiological mechanism mediating USV production in rodents and how this relates to structural abnormalities seen in FXS patients is currently unknown. Furthermore, the underlying causes of perseveration in those with FXS has not been elucidated. One hypothesis suggests the tendency could be related to frontal lobe and executive dysfunction deficits, including a lack of inhibition (Mazzocco, Pennington, & Hagerman, 1993; Sobesky et al., 1996). Without appropriate inhibition of responses, it can lead to repetition of words, thoughts, and utterances. Evidence of enlargement of the caudate nucleus, involved in executive function and inhibitory control, in FXS may reflect a lack of synaptic pruning leading to excessive neuronal activity without appropriate frontal inhibition, ultimately leading to perseveration (Abrams & Reiss, 1995; Comery et al., 1997; Hazlett et al., 2012). Other neuroanatomical regions known to be altered in FXS include a decrease in size of the amygdala, posterior cerebellar vermis, and superior temporal gyrus (Gothelf et al., 2008; Reiss, Lee, & Freund, 1994). Similar neuroanatomical differences have been found in Fmr1 KO mice, however, findings have not been consistent in C57BL/6 and FVB background strains suggesting underlying gene-strain interactions that effect structural manifestations (Lai, Lerch, Doering, Foster, & Ellegood, 2016; Mineur, Sluyter, de Wit, Oostra, & Crusio, 2002). More profound anatomical abnormalities that mimic those in FXS patients have been shown in the FVB strain that we used in our study, such as increases in white matter volume and changes in frontostriatal circuitry (Dennis & Thompson, 2013; Hallahan et al., 2011). Interestingly, the enlarged caudate nucleus often noted in FXS patients is contrasted in both C57BL/6 and FVB strains which have shown smaller striatum volumes in mice (Ellegood, Pacey, Hampson, Lerch, & Henkelman, 2010; Lai et al., 2016). Additional research is critical to understand how structural brain differences contribute to the communication deficits seen in FXS.
While we found numerous alterations in vocalization behavior in Fmr1 KO mice, we did not detect any significant differences in scent marking behavior, a form of social communication. Males may deposit scent marks in response to female urine for a variety of reasons, including to mark their territory, prevent potential mating competition, and to attract females (Hurst, 1990a, 1990b). An important control behavioral test that we included was the habitation/dishabituation odor test (Arbuckle et al., 2015; Yang & Crawley, 2009). The purpose of this test was to determine whether the Fmr1 KO and WT mice had impairments in odor discrimination. The ability to detect social and non-social odors is an important control when examining scent marking. We found that the mice habituated to each odor after repeated presentation and displayed dishabituation when presented with a novel odor or social odor. This pattern is important as it provides support that the mice can detect social odors. Inability to detect social odors would introduce a confounding variable into the ability of the mice to elicit scent markings.
We found no differences in overall activity levels and repetitive behavior in the open field task. This contradicts previous studies that have found Fmr1 KO mice to display hyperactivity and stereotypy behaviors similar to humans with the syndrome (Ding, Sethna, & Wang, 2014; Tsiouris & Brown, 2004). Although we measured activity in the open field apparatus, the setting was comprised of Strathmore paper lining the bottom of the chamber and female urine in the center, thus creating an enriched, novel setting for the mice. We believe this novelty may alter the typical findings in hyperactivity commonly found in the Fmr1 KO mouse. It should be noted that locomotor and repetitive behaviors were not measured in previous vocalization studies in adult Fmr1 KO and WT mice (Belagodu et al., 2016; Rotschafer et al., 2012). Further, with no detected differences in activity we can confirm that locomotion does not account for any of the observed alterations in vocalization behavior.
Communication impairments are one of the core behavioral deficits observed in Fragile X syndrome. Many areas of language development are known to be effected, including receptive, expressive, conversational, and repetitive language abilities (Finestack et al., 2009). Our results provide evidence that the adult Fmr1 KO mouse demonstrates significant abnormalities in vocalization behavior that are consistent with deficits seen in the syndrome, particularly in the domain of repetitive language. Fmr1 KO mice produced a similar amount of USVs compared to WT mice, however, many specific call types were of reduced duration. In addition, our results demonstrate the tendency of adult Fmr1 KO mice to emit calls of increased pitch, possibly inferring that acoustic alterations observed in early development also exist later in life. Taken together, these results corroborate previous studies using the Fmr1 KO mouse model to further characterize and examine USV deficits present in FXS. While many studies have examined this core behavioral impairment early in development, further research is needed to elucidate the neurobiological cause of the deficit and how these changes may persist into adulthood. Deficits in communication can negatively affect the lives of those with FXS in many ways, including socially and cognitively, thus gaining a better understanding of these abnormalities is critical for the development of future therapeutic interventions.
Highlights.
No difference in average quantity of calls produced between Fmr1 KO and WT mice
Fmr1 KO mice emit vocalizations at a higher frequency and decreased amplitude
Fmr1 KO vocalizations were of significantly shorter duration
Spectral analysis reveal Fmr1 KO mice to produce different types of calls
Results emphasize qualitative differences in Fmr1 KO mice vocalizations
Acknowledgments
Funding sources
This research was funded by NIH NS088776 to JNL.
References
- Abbeduto L, & Hagerman RJ (1997). Language and communication in fragile X syndrome. Ment Retard Dev Disabil Res Rev, 3(4), 313–322. [Google Scholar]
- Abrams MT, & Reiss AL (1995). The neurobiology of fragile X syndrome. Ment Retard Dev Disabil Res Rev. [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, & Blanchard RJ (2008). A new test paradigm for social recognition evidenced by urinary scent marking behavior in C57BL/6J mice. Behav Brain Res, 190(1), 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Blanchard DC, Arakawa K, Dunlap C, & Blanchard RJ (2008). Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev, 32(7), 1236–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle EP, Smith GD, Gomez MC, & Lugo JN (2015). Testing for odor discrimination and habituation in mice. J Vis Exp(99), e52615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belagodu AP, Johnson AM, & Galvez R (2016). Characterization of ultrasonic vocalizations of Fragile X mice. Behav Brain Res, 310, 76–83. [DOI] [PubMed] [Google Scholar]
- Belser RC, & Sudhalter V (2001). Conversational Characteristics of Children With Fragile X Syndrome: Repetitive Speech. American Journal on Mental Retardation, 106(1), 28–38. [DOI] [PubMed] [Google Scholar]
- Brady N, Skinner D, Roberts J, & Hennon E (2006). Communication in young children with fragile X syndrome: a qualitative study of mothers’ perspectives. Am J Speech Lang Pathol, 15(4), 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, Santucci D, & Alleva E (2001). Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res, 125(1-2), 49–56. [DOI] [PubMed] [Google Scholar]
- Byers SL, Wiles MV, Dunn SL, & Taft RA (2012). Mouse estrous cycle identification tool and images. PLoS One, 7(4), e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, & Brown WT (1991). Effects of age and communication level on eye contact in fragile X males and non-fragile X autistic males. Am J Med Genet, 38(2-3), 498–502. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, & Greenough WT (1997). Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A, 94(10), 5401–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato FR (1991). Courtship ultrasonic vocalizations and social status in mice. Animal Behaviour, 41(5), 875–885. [Google Scholar]
- Dennis EL, & Thompson PM (2013). Typical and atypical brain development: a review of neuroimaging studies. Dialogues Clin Neurosci, 15(3), 359–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Sethna F, & Wang H (2014). Behavioral analysis of male and female Fmr1 knockout mice on C57BL/6 background. Behav Brain Res, 271, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizinno G, Whitney G, & Nyby J (1978). Ultrasonic vocalizations by male mice (Mus musculus) to female sex pheromone: Experiential determinants. Behavioral Biology, 22(1), 104–113. [Google Scholar]
- Dykens EM, Hodapp RM, & Leckman JF (1994). Behavior and development in fragile X syndrome: Sage Publications, Inc. [Google Scholar]
- Ellegood J, Pacey LK, Hampson DR, Lerch JP, & Henkelman RM (2010). Anatomical phenotyping in a mouse model of fragile X syndrome with magnetic resonance imaging. Neuroimage, 53(3), 1023–1029. [DOI] [PubMed] [Google Scholar]
- Esposito G, & Venuti P (2008). How Is Crying Perceived in Children with Autistic Spectrum Disorder. Research in Autism Spectrum Disorders, 2(2), 371–384. [Google Scholar]
- Esposito G, & Venuti P (2010). Developmental changes in the fundamental frequency (f0) of infants’ cries: A study of children with Autism Spectrum Disorder. Early Child Development and Care, 180(8), 1093–1102. [Google Scholar]
- Fatemi SH, & Folsom TD (2011). The role of fragile X mental retardation protein in major mental disorders. Neuropharmacology, 60(7-8), 1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier LJ, Bashir AS, Meryash DL, Johnston J, & Wolff P (1991). Conversational skills of individuals with fragile-X syndrome: a comparison with autism and Down syndrome. Dev Med Child Neurol, 33(9), 776–788. [DOI] [PubMed] [Google Scholar]
- Finestack LH, Richmond EK, & Abbeduto L (2009). Language Development in Individuals with Fragile X Syndrome. Topics in language disorders, 29(2), 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Furfaro JA, Hoeft F, Eckert MA, Hall SS, O’Hara R, … Reiss AL (2008). Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP). Ann Neurol, 63(1), 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, & Holy TE (2007). Sex selectivity of mouse ultrasonic songs. Chem Senses, 32(5), 463–473. [DOI] [PubMed] [Google Scholar]
- Hallahan BP, Craig MC, Toal F, Daly EM, Moore CJ, Ambikapathy A, … Murphy DG (2011). In vivo brain anatomy of adult males with Fragile X syndrome: an MRI study. Neuroimage, 54(1), 16–24. [DOI] [PubMed] [Google Scholar]
- Hanson DM, Jackson AW 3rd, & Hagerman RJ (1986). Speech disturbances (cluttering) in mildly impaired males with the Martin-Bell/fragile X syndrome. Am J Med Genet, 23(1-2), 195–206. [DOI] [PubMed] [Google Scholar]
- Hawkins M, Boyle J, Wright KE, Elles R, Ramsden SC, O’Grady A, … Hawkins JR (2011). Preparation and validation of the first WHO international genetic reference panel for Fragile X syndrome. Eur J Hum Genet, 19(1), 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Lightbody AA, Styner M, MacFall JR, Reiss AL, & Piven J (2012). Trajectories of early brain volume development in fragile X syndrome and autism. J Am Acad Child Adolesc Psychiatry, 51(9), 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holy TE, & Guo Z (2005). Ultrasonic songs of male mice. PLoS Biol, 3(12), e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst JL (1990a). Urine marking in populations of wild house mice Mus domesticus rutty. I. Communication between males. Animal Behaviour, 40(2), 209–222. [Google Scholar]
- Hurst JL (1990b). Urine marking in populations of wild house mice Mus domesticus rutty. II. Communication between females. Animal Behaviour, 40(2), 223–232. [Google Scholar]
- Kazdoba TM, Leach PT, Silverman JL, & Crawley JN (2014). Modeling fragile X syndrome in the Fmr1 knockout mouse. Intractable Rare Dis Res, 3(4), 118–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGasse LL, Neal AR, & Lester BM (2005). Assessment of infant cry: acoustic cry analysis and parental perception. Ment Retard Dev Disabil Res Rev, 11(1), 83–93. [DOI] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, & Fischer U (2001). Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet, 10(4), 329–338. [DOI] [PubMed] [Google Scholar]
- Lai JK, Sobala-Drozdowski M, Zhou L, Doering LC, Faure PA, & Foster JA (2014). Temporal and spectral differences in the ultrasonic vocalizations of fragile X knock out mice during postnatal development. Behav Brain Res, 259, 119–130. [DOI] [PubMed] [Google Scholar]
- Lai JKY, Lerch JP, Doering LC, Foster JA, & Ellegood J (2016). Regional brain volumes changes in adult male FMR1-KO mouse on the FVB strain. Neuroscience, 318, 12–21. [DOI] [PubMed] [Google Scholar]
- Largo RH, & Schinzel A (1985). Developmental and behavioural disturbances in 13 boys with fragile X syndrome. Eur J Pediatr, 143(4), 269–275. [DOI] [PubMed] [Google Scholar]
- Levenga J, de Vrij FM, Oostra BA, & Willemsen R (2010). Potential therapeutic interventions for fragile X syndrome. Trends Mol Med, 16(11), 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y, Gottesman R, Borochowitz ZVI, Frydman M, & Sagi M (2006). Language in boys with fragile X syndrome. Journal of Child Language, 33(1), 125–144. [DOI] [PubMed] [Google Scholar]
- Lummaa V, Vuorisalo T, Barr RG, & Lehtonen L (1998). Why Cry? Adaptive Significance of Intensive Crying in Human Infants. Evolution and Human Behavior, 19(3), 193–202. [Google Scholar]
- Mazzocco MM, Pennington BF, & Hagerman RJ (1993). The neurocognitive phenotype of female carriers of fragile X: additional evidence for specificity. J Dev Behav Pediatr, 14(5), 328–335. [PubMed] [Google Scholar]
- Mineur YS, Sluyter F, de Wit S, Oostra BA, & Crusio WE (2002). Behavioral and neuroanatomical characterization of the Fmr1 knockout mouse. Hippocampus, 12(1), 39–46. [DOI] [PubMed] [Google Scholar]
- Mirrett PL, Roberts JE, & Price J (2003). Early Intervention Practices and Communication Intervention Strategies for Young Males With Fragile X Syndrome. Language, Speech, and Hearing Services in Schools, 34(4), 320–331. [DOI] [PubMed] [Google Scholar]
- Prut L, & Belzung C (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol, 463(1-3), 3–33. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Lee J, & Freund L (1994). Neuroanatomy of fragile X syndrome: the temporal lobe. Neurology, 44(7), 1317–1324. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Mirrett P, Anderson K, Burchinal M, & Neebe E (2002). Early Communication, Symbolic Behavior, and Social Profiles of Young Males With Fragile X Syndrome. American Journal of Speech-Language Pathology, 11(3), 295–304. [Google Scholar]
- Rotschafer SE, Trujillo MS, Dansie LE, Ethell IM, & Razak KA (2012). Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of Fragile X Syndrome. Brain Res, 1439, 7–14. [DOI] [PubMed] [Google Scholar]
- Roullet FI, Wohr M, & Crawley JN (2011). Female urine-induced male mice ultrasonic vocalizations, but not scent-marking, is modulated by social experience. Behav Brain Res, 216(1), 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Watkins N, & Heck D (2012). Comprehensive analysis of ultrasonic vocalizations in a mouse model of fragile X syndrome reveals limited, call type specific deficits. PLoS One, 7(9), e44816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, & Crawley JN (2008). Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One, 3(8), e3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, & Crawley JN (2011). Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav, 10(1), 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM, … Mucke L (2008). Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav, 7(3), 344–354. [DOI] [PubMed] [Google Scholar]
- Sobesky WE, Taylor AK, Pennington BF, Bennetto L, Porter D, Riddle J, & Hagerman RJ (1996). Molecular/clinical correlations in females with fragile X. Am J Med Genet, 64(2), 340–345. [DOI] [PubMed] [Google Scholar]
- Tsiouris JA, & Brown WT (2004). Neuropsychiatric symptoms of fragile X syndrome: pathophysiology and pharmacotherapy. CNS Drugs, 18(11), 687–703. [DOI] [PubMed] [Google Scholar]
- Whitney G, Alpern M, Dizinno G, & Horowitz G (1974). Female odors evoke ultrasounds from male mice. Anim Learn Behav, 2(1), 13–18. [DOI] [PubMed] [Google Scholar]
- Wohr M, Roullet FI, & Crawley JN (2011). Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav, 10(1), 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Roullet FI, Hung AY, Sheng M, & Crawley JN (2011). Communication impairments in mice lacking Shank1: reduced levels of ultrasonic vocalizations and scent marking behavior. PLoS One, 6(6), e20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Silverman JL, Scattoni ML, Turner SM, Harris MJ, Saxena R, & Crawley JN (2013). Developmental delays and reduced pup ultrasonic vocalizations but normal sociability in mice lacking the postsynaptic cell adhesion protein neuroligin2. Behav Brain Res, 251, 50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, & Crawley JN (2009). Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci, Chapter 8, Unit 8 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeskind PS, McMurray MS, Garber KA, Neuspiel JM, Cox ET, Grewen KM, … Johns JM (2011). Development of Translational Methods in Spectral Analysis of Human Infant Crying and Rat Pup Ultrasonic Vocalizations for Early Neurobehavioral Assessment. Frontiers in Psychiatry, 2, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]


