Abstract
Objective:
The importance of PI3K/Akt signaling in the vasculature has been demonstrated in several models, as global loss of Akt1 results in impaired postnatal ischemia- and VEGF-induced angiogenesis. The ubiquitous expression of Akt1, however, raises the possibility of cell-type dependent Akt1-driven actions, thereby necessitating tissue-specific characterization.
Approach & Results:
Herein, we utilized an inducible, endothelial-specific Akt1-deleted adult mouse model (Akt1iECKO) to characterize the endothelial cell autonomous functions of Akt1 in the vascular system. Endothelial targeted ablation of Akt1 reduces eNOS phosphorylation and promotes both increased vascular contractility in isolated vessels and elevated diastolic blood pressures throughout the diurnal cycle in vivo. Furthermore, Akt1iECKO mice subject to the hindlimb ischemia model display impaired blood flow and decreased arteriogenesis.
Conclusions:
Endothelial Akt1 signaling is necessary for ischemic resolution post-injury and likely reflects the consequence of NO insufficiency critical for vascular repair.
Keywords: Vascular, Endothelial
Subject Codes: Endothelium/Vascular Type/Nitric Oxide, Vascular Biology
INTRODUCTION
Nitric oxide (NO) is an important mediator of cardiovascular homeostasis through its ability to exert physiological effects on numerous cells types of the vasculature. NO coordinates several biological functions, including blood flow, proportional vascular remodeling, and angiogenesis. In the endothelium, NO is primarily generated through endothelial nitric oxide synthase (eNOS) in response to local agonists (i.e. bradykinin, acetylcholine) and alterations in hemodynamics. NO can subsequently diffuse through the plasma membrane to the underlying vascular smooth muscle layer to regulate cGMP-dependent vascular relaxation and systemic blood pressure. Enzyme activity is also essential for NO diffusion into the lumen, where binding to hemoglobin in erythrocytes provides circulating NO bioequivalents.
The in vivo regulation of eNOS is a complex process that involves multiple interdependent control mechanisms such as lipidation, subcellular localization, intracellular calcium, substrate availability, and phosphorylation at key activation sites, namely serine 1177 (in human eNOS; mouse: S1176; bovine: S1179)1,2. Phosphorylation of eNOS at S1177 has previously been shown to occur at low levels of intracellular calcium (i.e. with shear stress, insulin)3,4, highlighting the various avenues of eNOS activation. While several kinases have been identified for eNOS-S1177 phosphorylation (i.e. PKA, AMPK)5, S1177 phosphorylation occurs in vivo predominantly through Akt6. The protein kinase Akt is a major hub for several signal transduction pathways through its ability to phosphorylate numerous downstream targets directly involved in various cellular processes including proliferation, cell survival, and metabolism – all critical for vascular remodeling7,8. Akt has also been shown to preferentially phosphorylate the eNOS isoform over other NOS isoforms, as co-transfection of Akt with nNOS and iNOS does not induce NO release4. Biochemical approaches validate S1176 phosphorylation as the main activation site in intact cells, as a ‘loss-of-function’ mutation to alanine (S1176A) abolishes Akt-dependent eNOS phosphorylation and reduces NO release. Similarly, a ‘gain-of-function’ mutation to mimic phosphorylation (S1176D) results in significantly enhanced in vitro eNOS enzyme activity3,4. We have additionally shown the functional importance of S1176 phosphorylation in vasomotor tone regulation, as S1179D adenoviral delivery restores EC-dependent vasodilatory responses in pressurized arteries from eNOS knockout (KO) mice9. Furthermore, genetic knock-in approaches indicate that Akt1 is the primary kinase for eNOS in vivo, as introduction of the S1176D phospho-mimic mutation in global Akt1 KO mice rescues the profound defects in revascularization after hindlimb ischemia (HLI) and wounding these mice6.
Of the three Akt isoforms (Akt1/Akt2/Akt3), endothelial cells (EC) express predominantly Akt1, the major isoform involved in regulation of cardiovascular function10–13. We have previously shown the isoform-dependent role of Akt in vascular remodeling, as ischemic limb repair is severely impaired in global Akt1 KO mice10. Unlike the pronounced defects seen in Akt1 KO mice upon injury, global Akt2 KO mice display normal hindlimb flow recovery, paralleling the in vitro observation of preferential eNOS-S1177 phosphorylation through the Akt1 isoform when compared to the Akt2 isoform10,11. On a whole organism level, however, global loss of Akt1 results in severe growth defects both in utero and postnatal life, as Akt1KO mice remain smaller than average throughout adulthood14,15. The obvious growth retardation together with the increased propensity to apoptosis in global Akt1 KO mice thus limits the interpretation of how the Akt1 isoform regulates adult vascular remodeling. Furthermore, the protein kinase Akt1 is positioned as a key signaling intermediate in all cell types, thereby confounding theoretical predictions as to the tissue-specific role of Akt1.
Here, we use an inducible, endothelial-specific Akt1-null adult mouse model (Akt1iECKO) to address the EC-specific, post-natal role of Akt1 in vivo. Akt1iECKO mice display phenotypes indicative of impaired eNOS function and consequent endothelial dysfunction. We show that endothelial Akt1 ablation promotes a spontaneous hypertensive phenotype associated with EC dysfunction. Furthermore, Akt1iECKO mice subject to HLI display diminished blood flow recovery where microCT imaging indicates an associated impairment of arteriogenesis. Collectively, the cardiovascular phenotypes that arise due to the postnatal deletion of Akt1 in EC supports the critical role of the Akt-eNOS signaling axis in the regulation of vascular function in vivo.
MATERIALS & METHODS
Inducible endothelial-specific Akt1 conditional mice
The Akt1flox/flox16 were bred to the Cdh5-Cre-ERT217 mice to obtain inducible, endothelial-targeted Akt1 mice, as previously described11. Young adult, male mice (~4–5wks of age) were injected with tamoxifen (100ug/g total body weight) via intraperitoneal delivery for 7 consecutive days. Phenotyping assessments were performed 6 weeks post-tamoxifen administration (~10–12 wks of age).
En face immunostaining
Thoracic aortas were isolated from control and Akt1iECKO immediately after sacrifice. Aortas (or indicated arteries) were isolated and cut longitudinally for en face immunostaining. The isolated tissues were incubated at 37°C in serum-free base media for ~4hrs prior to ex vivo treatment with Angiopoetin1 (500ng/mL) or PDGF-BB (50 ng/mL) for the indicated times. Samples were then fixed in 4% PFA/PBS and permeabilized overnight in TNB blocking buffer (0.1M Tris pH7.4, 150mM NaCl, 0.2% Triton-X-100, 0.5% blocking reagent) for whole-mount analysis. Aortas were stained with rat anti-VECD (BD555289, 1:200), mouse anti-GM130 (BD610822, 1:100), rabbit anti-panAkt (Cell Sig #9272, 1:200), and rabbit anti-p-eNOS-S1176 (Cell Sig #9571, 1:200). Images were acquired using a Leica SP5 confocal microscope with the Leica Application Suite (LAS) software. Images reflect a z-stack compression of the endothelial layer using the sequential scan mode with a HCX PL APO lambda blue 63x/1.40 oil objective.
NO bioavailability (Electric Paramagnetic Resonance)
Electric paramagnetic resonance was applied to measure hemoglobin-bound NO in whole blood, as previously described18. In brief, mice were euthanized by CO2 inhalation for subsequent blood isolation from the inferior vena cava. Samples were immediately flash-frozen in liquid N2 for later spectrometer analysis. Mice harboring germline eNOS knock-in mutations (S1176D+/+ and S1176A+/+)6 and lacking eNOS 19 were used as gating controls for Hb-NO signal detection. All mice were given standard chow to ensure fixed levels of nitrate/nitrite content to prevent dietary influences on NO bioavailability.
Wire myography
Wire myography was performed, as previously described20. Thoracic aortas were isolated and cleaned under a dissecting scope to remove residual fat and connective tissues. Aortic rings of uniform length were suspended using two tungsten wires through the lumen and subsequently mounted onto myograph chambers containing Krebs buffer solution (95% O2/5% CO2, 37°C). Aortic rings were set to a resting tension of 1.5g under isometric conditions and allowed to equilibrate. Following equilibration, aortic rings were subject to several rounds of KCl contraction followed by contractile-induced dose response curves. For relaxation studies, aortic rings were pre-constricted to ~80% maximal constriction prior to acetylcholine dose-curve generation. Increasing concentrations of the desired agents were administered at half-log increments where real-time responses were recorded using Chart5 (AD Instruments). Control and Akti1ECKO aortic rings were studied simultaneously, isolating at least 3–4 aortic rings per mouse. Tension-response curve data reflect a nonlinear regression curve (log [agonist] vs. response) using Prism 6.0 (GraphPad Software).
Blood pressure telemetry
Radiotelemetric blood pressure catheters were implanted in the right carotid artery of adult male (10–12 wks old) mice and allowed to recover 1 week from surgery, as previously described21,22. Mice were housed in individually controlled environments with a 12-hr light/dark cycle with free access to food and water. Parameters of interest were measured at 1-minute intervals for 7 consecutive days. Data reflect averaged values over a 3-day reading period.
Hindlimb ischemia
The hindlimb ischemia (HLI) model was performed, as previously described23,24. In brief, the left femoral artery and the proximal portion of the saphenous artery is exposed, ligated, and excised. Ischemic and non-ischemic limb perfusion is measured pre-, post-surgery, 1-, 2-, 3-, and 4-weeks after surgery using Laser Doppler scanning (LDI, Moor Instruments Ltd). Perfusion data is analyzed and reported as a ratio of flow in the injured relative to contralateral leg values. S-Nitroco-N-acetyl-DL penicillamine (SNAP) was injected locally (1mg/mL, 30uL injection) where indicated prior to Laser Doppler scanning and flow measurements.
MicroCT imaging
2D microCT scans were obtained using a GE eXplore MS Micro-CT System, as previously described23. Mice were administered 2% adenosine prior to delivery of the contrast agent to dilate the vascular bed and definitively assess the effects on anatomy and vascular remodeling rather than vascular tone. Vessel density analyses was performed on captured images, as previously described23.
Statistical Analysis
All data are shown as mean ± standard error of mean (SEM). Statistical significance was determined based on a p-value calculation of p<0.05 where all comparisons reflect a 2-WAY ANOVA, Bonferroni analyses.
RESULTS
Inducible deletion of adult endothelial Akt1 expression
Although viable, global Akt1-null mice exhibit significant neonatal mortality and are substantially smaller than WT littermate controls14,15. Therefore, we utilized an inducible, tissue-specific approach to bypass potential retardation effects during gestation/growth as a result of global Akt1 loss. Inducible, endothelial targeted (Cdh5-Cre-ERT2) Akt1 mice were generated using Cre-loxP technology, as described previously 11. Young adult mice (~4–5 weeks of age) were administered tamoxifen for 7 consecutive days via intraperitoneal injection for adult phenotyping at ~10–12weeks of age (Supp. Fig. 1A). While global Akt1 deletion yields significantly smaller mice, inducible deletion does not affect total body weight (Supp. Fig. 1B), thereby eliminating body mass as a variable. En face whole-mount immunofluorescent imaging of the endothelial layer from various arterial vessels indicate near complete ablation of total Akt1 protein expression (Supp. Fig. 2), thus validating the inducible deletion strategy. The Akt antibody was determined to be specific, as immunofluorescent labeling is undetectable in the endothelium of global Akt1 KO mice (Supp. Fig. 3).
Endothelial Akt1 deletion attenuates angiopoietin-1 (Ang1)- and platelet-derived growth factor (PDGF)-stimulated eNOS phosphorylation
Akt1 is the major isoform in the endothelium necessary for mediating pro-angiogenic signaling events13 and has been demonstrated by several groups as a major kinase for eNOS phosphorylation and subsequent eNOS activation3,4,10,25. Therefore, we sought to assess eNOS function using an ex vivo approach to validate the endothelial-targeted Akt1 KO mouse model11. In brief, segments of the thoracic aorta were isolated from adult Akt1iECKO mice along with respective littermate controls and incubated with Ang1, a prototypical PI3K/Akt agonist to induce eNOS phosphorylation on S117626-29. The phospho-eNOS antibody was determined to be specific for S1176, as immunofluorescence labeling detected phospho-eNOS-S1176 signal in control vessels but not in vessels isolated from mice harboring a ‘loss-of-function’ mutation in the Akt phosphorylation site (eNOS-S1176A mice6; Supp. Fig. 4). As seen in Figure 1A and 1B, en face staining for phospho-eNOS-S1176 demonstrates enhanced perinuclear phospho-eNOS levels30 in response to acute Ang1 treatment, where this effect is significantly reduced in Akt1iECKO mice. We similarly stimulated vessels with PDGF-BB, a potent growth factor that activates Akt in the endothelium and induces endothelium-dependent relaxation in intact vessels31,32. PDGF-BB treatment of control aortas induces eNOS phosphorylation, thus corroborating previous observations where PDGF-BB treatment produced NO-mediated vessel relaxation32, as endothelial-specific Akt1 deletion reduces eNOS-S1176 phosphorylation levels (Supp. Fig. 5A, quantified in 5B).
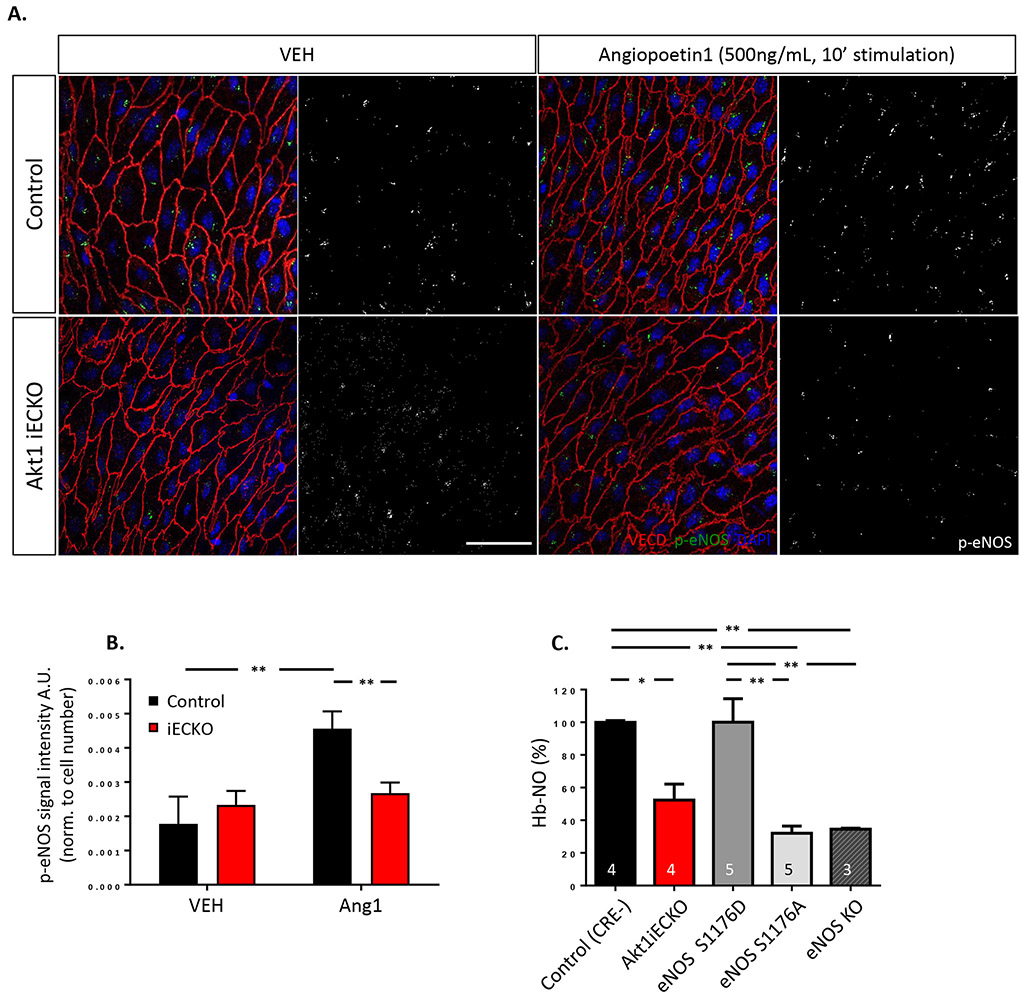
Figure 1. Endothelial loss of Akt1 impairs stimuli-induced eNOS phosphorylation and decreases NO bioavailability.
(A) Isolated thoracic aorta segments from Akt1iECKO mice display decreased phospho-eNOS S1176 when treated with angiopoetin-1 ex vivo, quantified in (B). (C) Less hemoglobin-bound NO (NO-Hb) levels were detected in peripheral blood of Akt1iECKO adult mice when assessed by EPR techniques. eNOS mutant (S1176D and S1176A) and global eNOS KO mice were used as gating controls for NO-Hb levels. The number of mice for each genotype shown as indicated. All data are mean+SEM, n=3-6 mice per group, 2-6 images per mouse. * p<0.05, **p<0.01.
Endothelial loss of Akt1 decreases NO bioavailability
The transfer of eNOS-derived NO to hemoglobin in circulating erythrocytes relies on the diffusional distance and serves as a critical determinant of plasma NO bioavailability33. Thus, the formation of hemoglobin-bound NO (Hb-NO) is directly proportional to the amount of bioavailable NO34. Due to the stable nature of Hb-NO under anaerobic conditions, Hb-NO levels measured by electron paramagnetic spin resonance (EPR) techniques in isolated mouse blood35 reflects eNOS-derived NO levels in blood. Therefore, we measured Hb-NO levels in Akt1iECKO adult mice to determine whether the loss of endothelial Akt1 affected NO bioavailability (Figure 1C). Venous whole blood was collected from adult mice and Hb-NO was measured using EPR, as previously described18. Genetically modified eNOS mutant mice either mimicking (S1176D) or lacking (S1176A) eNOS phosphorylation6 were additionally assessed together with eNOS KO as thresholding controls to determine the window of detection. All mice were given standard chow and food restricted overnight to ensure fixed levels of nitrate/nitrite content to prevent dietary influences on NO bioavailability measurements. Although higher than the ‘loss-of-function’ eNOS-S1176A mutant, Akt1iECKO mice show significantly less Hb-NO in blood compared to WT littermate controls, highlighting the importance of the Akt1-eNOS axis in regulating NO bioactivity.
Endothelial loss of Akt1 decreases basal NO levels and impairs vascular function
Ex vivo examination of adult Akt1iECKO aortas clearly demonstrate blunted levels of eNOS phosphorylation. To test the significance of these findings in intact vessels, myography was used to examine vascular function in aortic rings from Akt1iECKO mice. Aortic rings isolated from both control and Akt1iECKO adult mice were subjected to various agonists, and tension-response curves were generated. Typical contraction and relaxation responses were observed in control mice, with an EC50 for phenylephrine (PE) and acetylcholine (Ach) matching previously reported values36 (Figure 2A and 2B). However, aortic rings from Akt1iECKO mice had increased sensitivity to the contractile actions of PE and KCl (Figure 2A and 2C), where pre-incubation of vessels with the NOS inhibitor, L-NAME, normalized the PE response in Akt1iECKO mice (Figure 2D). This response suggests that tension-induced release of NO, or basal NO production, was impaired in vessels from Akt1iECKO mice. Ach-dependent vessel relaxation in conduit vessels occurs primarily through the release of endothelial-derived NO37. Interestingly, Ach-mediated reductions in vascular tone were virtually indistinguishable between the groups (Figure 2B), demonstrating that endothelial Akt1 is not critical for Ach-mediated NO production, which occurs primarily through Ca2+/CaM-dependent mechanisms2,38.
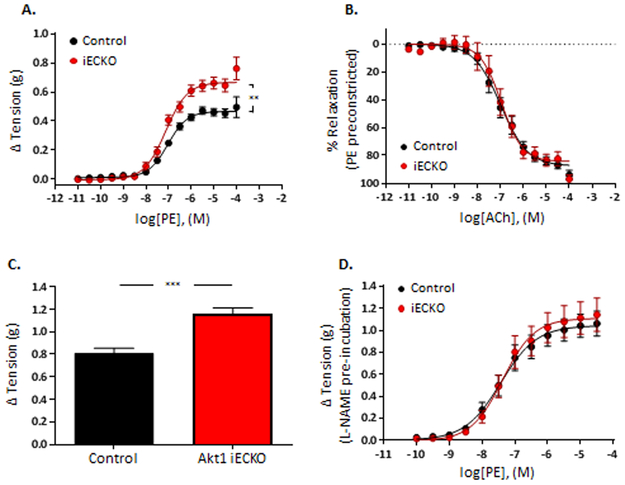
Figure 2. Basal NO production in vessels requires endothelial Akt1.
(A) Aortic rings from Akt1iECKO display increased sensitivity to phenylephrine (PE)-induced constriction, whereas (B) Ach-induced vascular relaxation is maintained, suggesting basal impaired NO production in Akt1iECKO mice. Aortic rings from Akt1iECKO mice display enhanced (C) KCl-induced (60mM) constriction, where (D) pre-incubation of vessels with L-NAME normalizes the differences in PE sensitivity. Data are mean± SEM, n=4-9 mice per group, 3-4 aortic rings/mouse. **p<0.01 ***p<0.001.
Endothelial-specific loss of Akt1 significantly increases diastolic blood pressure
Arterial pressure is of particular importance in maintaining vascular function and response, relying heavily on shear stress-mediated endothelial NO production39. The observed decrease in eNOS phosphorylation and circulating Hb-NO together with the increased sensitivity to PE suggests that overall systemic blood pressure may be affected in Akt1iECKO mice. Therefore, arterial blood pressure and heart rate was monitored in intact, conscious mice for over 72 hours using implanted telemetry devices40. Akt1iECKO mice display elevated diastolic pressures independent of the time of day (Figures 3A and 3B) and an associated inclination toward increased mean arterial pressures (Supp. Fig. 6A). Previous studies using similar telemetry-based blood pressure measurements in global Akt1KO mice report non-significant trends toward increased arterial pressure41, thus denoting the Akt1iECKO phenotype as the first report of hypertension resulting from Akt1 impairment. Other parameters, such as systolic blood pressure, heart rate, and activity levels, were comparable between control and adult Akt1iECKO mice (Figure 3C and 3D, Supp. Fig. 6B).
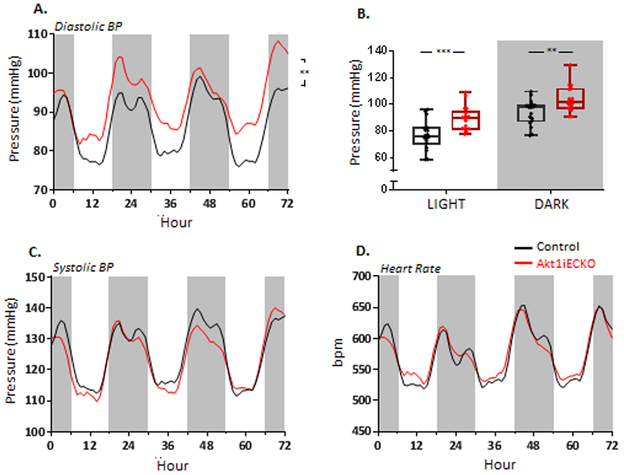
Figure 3. Adult, endothelial-specific loss of Akt1 significantly increases diastolic BP.
(A) Telemetry readings indicate an elevated diastolic pressure in free roaming, conscious Akt1iECKO mice. The increase occurred during the diurnal cycle (B). The loss of Akt had no effect on systolic pressure, (C) or heart rate (D), Data are mean ± SEM with n=6 mice per group. **p<0.01, ***p<0.001. Graphs illustrate a 3-day reading period where white and gray regions indicate light and dark cycles, respectively.
Endothelial loss of Akt1 results in impaired blood flow recovery and decreased arteriogenesis upon hindlimb ischemia (HLI)
In a model of HLI, the global loss of PI3Kγ42 or Akt110 results in critical limb ischemia and limb loss concomitant with marked impairment in arteriogenesis and angiogenesis. Nevertheless, the observed defects in global Akt1 deficient mice likely reflects a complex response, involving multiple cells types (e.g. EC, SMC, macrophages), all of which express the Akt1 isoform. We first assessed baseline arterial density in the EC-inducible Akt1 deletion model using a phase contrast agent together with microCT imaging. Arterial vessels were maximally dilated to definitively assess the vascular network rather than vascular tone, where quantification did not indicate any underlying deficits in artery number prior to hind-limb injury between both groups (data not shown). We subsequently performed the HLI model in Akt1iECKO and littermate controls to assess the role of EC-specific Akt1 expression in this injury response. In brief, the left femoral artery was ligated and removed, upon which blood flow is measured over time using Laser Doppler imaging. Perfusion rates in the surgically-manipulated leg were normalized to the uninjured contralateral limb for blood flow quantification. In control mice, an acute decrease in blood flow was followed by progressive recovery of flow over 28 days post-injury. In contrast, flow recovery in Akt1iECKO was significantly impaired by 14 days and persisted to 28 days post-ischemia when compared to littermate controls (Figure 4A and 4B). To assess arterial density after injury, microCT imaging was conducted 14 days post-HLI43. MicroCT analyses indicate a significant decline in the number of smaller arteries both above and below the knee in Akt1iECKO mice post-ischemia (Figure 4C-E), suggesting that collateral remodeling and/or the development of new arterial branches was impaired by the loss of the Akt1 isoform in ECs.
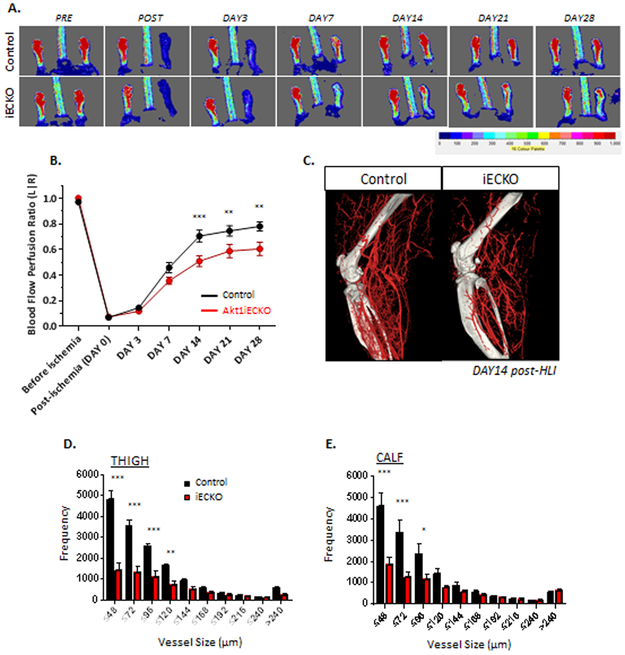
Figure 4. Endothelial loss of Akt1 results in impaired blood flow recovery upon femoral artery ligation and reduced arteriogenesis.
(A) Endothelial-specific loss of Akt1 results in impaired blood flow recovery in response to HLI 14 days post-ischemia. Relative blood flow perfusion ratio shown in (B). (C) Arterial phase microCT analyses at 14 days post-injury indicates significant reduction in small arteries/arterioles in thigh (D) and calf (E) regions in Akt1iECKO mice. Data are mean ± SEM, with n=5-6 mice/group. *p<0.05, **p<0.01, ***p<0.001.
In order to reinforce the importance of Akt1-mediated eNOS activation for vascular recovery, a rescue experiment was performed using S-Nitroso-N-acetyl-DL-penicillamine (SNAP)44, as an NO donor. Global Akt1 KO mice were used for rescue experiments since the deficit in blood flow after HLI is much larger compared to reduced flow recovery in the Akt1iECKO mice10. As seen in Supp Fig. 7, administration of SNAP with a single-injection downstream of the injury site resulted in near immediate flow recovery in the injured limb of control mice, with even greater flow increases in global Akt1 KO mice. The timely increase in blood flow suggests the presence of a remodeled, yet dysfunctional and constricted vasculature in global Akt1 KO mice, where eNOS activation may be critical for injury resolution through both neovessel formation and vasomotor tone regulation.
DISCUSSION
Akt1 controls many signaling pathways that regulate cell survival, morphogenesis and growth7. While global Akt1-null mice have been instrumental in elucidating the in vivo biological role of Akt1, the contribution of Akt1 signaling from a cell-specific perspective has only recently begun to be explored11,16,45–47. Here, we show that endothelial loss of Akt1 using an inducible, postnatal EC-specific strategy results in spontaneous hypertension, decreased NO bioavailability, and impaired recovery to tissue limb ischemia – all indicative of impaired NO production. These data are surprising given that eNOS can be phosphorylated on S1176 in vitro by several kinases including other AGC kinases (protein kinase A, G, C) and AMPK48. The Akt1iECKO phenotypes indicative of impaired NO production suggest that physiologically, eNOS-S1176 phosphorylation in vivo through Akt1 is crucial for ‘NO tone’ and blood pressure control.
A common feature of endothelial dysfunction is diminished NO bioavailability, where eNOS KO mice are an extreme state of NO deficiency.1 Regardless of the methodology used for blood pressure measurements, eNOS KO mice are consistently hypertensive19,49–52 and display an associated increase in cardiac mass and function52. Akt1 has been well-established as a major kinase for eNOS phosphorylation3,4,6 and manipulation of Akt activity in vivo through use of viral transfection methods in combination with arterial flow measurements indicate that short-term hyperactivation of Akt1 results in augmented NO release, enlarged vessel diameter and enhanced blood flow53. Additionally, EC-specific transgenic expression of Akt1 increases NO production and reduces injury-induced neointima formation54. While global Akt1 KO mice present phenotypes similar to eNOS KO, such as decreased homozygote offspring number and reduced adult body weight14,19, global Akt1 KO mice are not hypertensive11,41. Normal blood pressures in global Akt1 KO mice suggests that other kinases may regulate eNOS, or that the global loss of Akt1 promotes compensation by other expressed Akt isoforms given the significant overlap in substrate specificity for Akt1 versus Akt2 in the endothelium11. Thus, the hypertensive phenotype in adult Akt1iECKO mice emphasizes the importance of the endothelial Akt1-eNOS axis in vivo6 and suggests that adaptive changes likely occur during development that preclude an obvious blood pressure phenotype in global Akt1 KO mice.
Moreover, cardiac output rates derived from echocardiography measurements did not indicate any differences between Akt1iECKO and littermate controls (data not shown). Given the direct relationship between blood pressure and peripheral resistance, the observed pressure elevation together with the lack of change in cardiac output suggests that the Akt1iECKO mice likely exhibit increased systemic vascular resistance. While smaller resistance arteries have been conventionally reliant on endothelium-derived hyperpolarizing factor (EDHF) as the predominant mediator of vascular tone regulation, the recent discovery of hemoglobin alpha (Hbα) in the myoendothelial junctions of resistance arteries suggest a role for NO scavenging55. The localized nature of Hbα at the myoendothelial junction (MEJ) may serve as a chelator of eNOS-derived NO to favor EDHF activity while maintaining control over NO levels. Previous groups have also shown the importance of eNOS expression on peripheral vascular tone, as partial deletion of eNOS increases coronary vascular tone56 and pulmonary resistance57, hence complementing the impaired vascular tone seen in isolated vessels from Akt1iECKO mice (Figure 2).
Vascular remodeling occurs in both development and under adult pathological conditions, requiring various adaptations in response to the physiological environment and/or pathological stimuli. Angiogenesis denotes the de novo formation of capillaries from pre-existing post-capillary venules and is largely initiated by hypoxic events, whereas arteriogenesis denotes the remodeling of pre-existing collaterals or arterioles/capillaries, which is generally ischemia-independent and driven through hemodynamic and inflammatory factors (i.e. shear stress, wall tension)58,59. In experimental models of hindlimb ischemia, arteriogenesis is stimulated through a marked increase in fluid shear stress in arterial conduit vessels, providing a driving force to promote collateralization60. In the distal lower limb, vascular remodeling is primarily mediated through tissue ischemia, resulting in the upregulation of several growth factors critical for vascularization. eNOS-derived NO serves as a naturally occurring, compensatory mechanism to regulate hemodynamic changes in both normal and ischemic tissue61. eNOS function is also essential for native collateral formation, immediate flow recovery post-ligation, and cellular recruitment in the remodeling vasculature – all of which are critical for efficient arteriogenesis62,63. The defective flow recovery in adult Akt1iECKO mice herein likely reflects the combinatorial effects of impaired eNOS-derived NO generation given the established role of the Akt1 kinase in eNOS-S1179 phosphorylation6. However, the impairment in blood flow and arteriogenesis in Akt1iECKO mice is markedly less than that observed in global Akt1 KO 10 or eNOS KO mice64, implying that other kinases may contribute to eNOS activation under these conditions or that expression of Akt1 is also critical in SMCs and macrophages for proper ischemic injury repair.
Recent work using conditional EC-specific Akt1 deletion mice has shown that the Akt1 isoform is necessary for developmental retinal angiogenesis through regulation of EC proliferation and survival11, two well-described functions of Akt and downstream substrate phosphorylation. Furthermore, the global deletion of Akt2 in combination with EC-specific deletion of Akt1 did not further impair the developing retinal vasculature. In contrast, deletion of endothelial Akt1 in adult mice had a mild effect on cardiac arteriogenesis (quantified via microCT), and this effect was markedly exacerbated by the global deletion of Akt245, implying functional redundancy of these isoforms in the adult coronary vasculature. Mechanistically, the loss of both isoforms in EC, and Akt2 in smooth muscle, reduce endothelial expression of the Notch ligand, Jagged1, thereby impairing Notch-Jagged regulation of vascular stability. The means by which Akt as a kinase regulates this pathway are not clear, as an Akt substrate has not yet been identified to explain the reduced Jagged1 levels. Prior work has shown that Akt2 exerts a more prominent role in determining vascular SMC phenotype and function65,66. Global Akt2 KO mice are additionally more susceptible to aortic aneurysms67, further demonstrating a role for the Akt2 isoform in vascular tone regulation and/or vessel compliance through an NO-independent pathway. Nevertheless, our studies indicate that in vivo eNOS activity is mediated primarily through the endothelial expression of Akt1 where postnatal deletion results in defects consistent with decreased NO bioavailability.
Collectively, our data supports the importance of the Akt1-eNOS signaling axis in intact endothelium given its primary role in NO production and vasomotor tone regulation. Akt1iECKO mice display phenotypes indicative of NO insufficiency as seen through impaired agonist-induced eNOS phosphorylation, blunted endothelial NO production in vivo, diastolic hypertension, and defects in post-ischemic arteriogenesis. Hence, PI3K-Akt1-NO signaling in EC is critical for vascular tone regulation and adaptive vessel remodeling.
Supplementary Material
HIGHLIGHTS.
Endothelial-specific Akt1 ablation in adult mice results in spontaneous hypertension
Adult, inducible, endothelial-targeted Akt1 deletion results in decreased NO bioavailability
Endothelial loss of Akt1 signaling impairs injury-induced arteriogenesis, indicative of impaired eNOS function
ACKNOWLEDGMENTS/SOURCES OF FUNDING
This work was supported by Grants R01 HL64793, R01 HL61371, R01 HL081190, R35 HL139945 (to WCS), the Leducq Fondation (MIRVAD network; to WCS), RO1 HL053793 (to MS), P01 HL70295 (to MS and WCS), HL 1K99HL130581–01 to MYL, RO1 GM072194 to TRK and the George M O’Brian Kidney Center at Yale, NIH grant P30DK079310 to HV from the National Institutes of Health.
Footnotes
DISCLOSURES
None
References
- 1.Huang PL Endothelial nitric oxide synthase and endothelial dysfunction. Curr Hypertens Rep 5, 473–480 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Qian J & Fulton D Post-translational regulation of endothelial nitric oxide synthase in vascular endothelium. Front Physiol 4, 347, doi: 10.3389/fphys.2013.00347 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimmeler S et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399, 601–605, doi: 10.1038/21224 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Fulton D et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399, 597–601, doi: 10.1038/21218 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sessa WC eNOS at a glance. Journal of Cell Science 117, 2427–2429, doi: 10.1242/jcs.01165 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Schleicher M et al. The Akt1-eNOS axis illustrates the specificity of kinase-substrate relationships in vivo. Sci Signal 2, ra41, doi:2/82/ra41 [pii] 10.1126/scisignal.2000343 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manning BD & Cantley LC AKT/PKB signaling: Navigating downstream. Cell 129, 1261–1274, doi: 10.1016/j.cell.2007.06.009 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu HX, Littlewood T & Bennett M Akt isoforms in vascular disease. Vascular Pharmacology 71, 57–64, doi: 10.1016/j.vph.2015.03.003 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scotland RS et al. Functional reconstitution of endothelial nitric oxide synthase reveals the importance of serine 1179 in endothelium-dependent vasomotion. Circulation Research 90, 904–910, doi: 10.1161/01.Res.0000016506.04193.96 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Ackah E et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest 115, 2119–2127, doi: 10.1172/JCI24726 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee MY et al. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc Natl Acad Sci U S A 111, 12865–12870, doi: 10.1073/pnas.1408472111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med 11, 1188–1196, doi: 10.1038/nm1307 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiojima I & Walsh K Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res 90, 1243–1250 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Cho H, Thorvaldsen JL, Chu Q, Feng F & Birnbaum MJ Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 276, 38349–38352, doi: 10.1074/jbc.C100462200 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Chen WS et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev 15, 2203–2208, doi: 10.1101/gad.913901 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan M et al. Loss of Akt1 in mice increases energy expenditure and protects against diet-induced obesity. Mol Cell Biol 32, 96–106, doi: 10.1128/MCB.05806-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benedito R et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137, 1124–1135, doi: 10.1016/j.cell.2009.03.025 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Kraehling JR et al. Uncoupling Caveolae From Intracellular Signaling In Vivo. Circulation Research 118, 48–55, doi: 10.1161/Circresaha.115.307767 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shesely EG et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 93, 13176–13181 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucci M et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nature Medicine 6, 1362–1367 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Goodwin JE, Feng Y, Velazquez H & Sessa WC Endothelial glucocorticoid receptor is required for protection against sepsis. Proc Natl Acad Sci U S A 110, 306–311, doi: 10.1073/pnas.1210200110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y et al. Renalase deficiency aggravates ischemic myocardial damage. Kidney Int 79, 853–860, doi: 10.1038/ki.2010.488 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Lanahan AA et al. PTP1b is a physiologic regulator of vascular endothelial growth factor signaling in endothelial cells. Circulation 130, 902–909, doi: 10.1161/CIRCULATIONAHA.114.009683 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chittenden TW et al. Selective regulation of arterial branching morphogenesis by synectin. Developmental Cell 10, 783–795, doi: 10.1016/j.devcel.2006.03.012 (2006). [DOI] [PubMed] [Google Scholar]
- 25.McCabe TJ, Fulton D, Roman LJ & Sessa WC Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem 275, 6123–6128 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Alfieri A et al. Angiopoietin-1 regulates microvascular reactivity and protects the microcirculation during acute endothelial dysfunction: role of eNOS and VE-cadherin. Pharmacol Res 80, 43–51, doi: 10.1016/j.phrs.2013.12.008 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Urano T et al. Angiopoietin-related growth factor enhances blood flow via activation of the ERK½-eNOS-NO pathway in a mouse hind-limb ischemia model. Arterioscler Thromb Vasc Biol 28, 827–834, doi: 10.1161/ATVBAHA.107.149674 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Kim I et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3’-Kinase/Akt signal transduction pathway. Circ Res 86, 24–29 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Papapetropoulos A et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem 275, 9102–9105 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Fulton D et al. Targeting of endothelial nitric-oxide synthase to the cytoplasmic face of the Golgi complex or plasma membrane regulates Akt- versus calcium-dependent mechanisms for nitric oxide release. J Biol Chem 279, 30349–30357, doi: 10.1074/jbc.M402155200 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Cunningham LD, Brecher P & Cohen RA Platelet-derived growth factor receptors on macrovascular endothelial cells mediate relaxation via nitric oxide in rat aorta. J Clin Invest 89, 878–882, doi: 10.1172/JCI115667 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maejima D, Kawai Y, Ajima K & Ohhashi T Platelet-derived growth factor (PDGF)-BB produces NO-mediated relaxation and PDGF receptor beta-dependent tonic contraction in murine iliac lymph vessels. Microcirculation 18, 474–486, doi: 10.1111/j.1549-8719.2011.00108.x (2011). [DOI] [PubMed] [Google Scholar]
- 33.Huang KT et al. Modulation of nitric oxide bioavailability by erythrocytes. Proc Natl Acad Sci U S A 98, 11771–11776, doi: 10.1073/pnas.201276698 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ongini E et al. Nitric oxide (NO)-releasing statin derivatives, a class of drugs showing enhanced antiproliferative and antiinflammatory properties. Proc Natl Acad Sci U S A 101, 8497–8502, doi: 10.1073/pnas.0401996101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogg N Detection of nitric oxide by electron paramagnetic resonance spectroscopy. Free Radic Biol Med 49, 122–129, doi: 10.1016/j.freeradbiomed.2010.03.009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell A & Watts S Vascular reactivity of isolated thoracic aorta of the C57BL/6J mouse. J Pharmacol Exp Ther 294, 598–604 (2000). [PubMed] [Google Scholar]
- 37.Chataigneau T et al. Acetylcholine-induced relaxation in blood vessels from endothelial nitric oxide synthase knockout mice. Br J Pharmacol 126, 219–226, doi: 10.1038/sj.bjp.0702300 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleming I & Busse R Signal transduction of eNOS activation. Cardiovasc Res 43, 532–541 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Umans JG & Levi R Nitric oxide in the regulation of blood flow and arterial pressure. Annu Rev Physiol 57, 771–790, doi: 10.1146/annurev.ph.57.030195.004011 (1995). [DOI] [PubMed] [Google Scholar]
- 40.Huetteman DA & Bogie H Direct blood pressure monitoring in laboratory rodents via implantable radio telemetry. Methods Mol Biol 573, 57–73, doi: 10.1007/978-1-60761-247-6_4 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Symons JD et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res 104, 1085–1094, doi: 10.1161/CIRCRESAHA.108.189316 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madeddu P et al. Phosphoinositide 3-kinase gamma gene knockout impairs postischemic neovascularization and endothelial progenitor cell functions. Arterioscler Thromb Vasc Biol 28, 68–76, doi: 10.1161/ATVBAHA.107.145573 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren B et al. ERK½-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish. The Journal of clinical investigation 120, 1217–1228, doi: 10.1172/JCI39837 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mees B et al. Endothelial nitric oxide synthase activity is essential for vasodilation during blood flow recovery but not for arteriogenesis. Arteriosclerosis, thrombosis, and vascular biology 27, 1926–1933, doi: 10.1161/ATVBAHA.107.145375 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Kerr BA et al. Stability and function of adult vasculature is sustained by Akt/Jagged1 signalling axis in endothelium. Nat Commun 7, 10960, doi: 10.1038/ncomms10960 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rotllan N et al. Genetic Evidence Supports a Major Role for Akt1 in VSMCs During Atherogenesis. Circ Res 116, 1744–1752, doi: 10.1161/CIRCRESAHA.116.305895 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu WN et al. Systemic Akt1 Deletion after Tumor Onset in p53(−/−) Mice Increases Lifespan and Regresses Thymic Lymphoma Emulating p53 Restoration. Cell Rep 12, 610–621, doi: 10.1016/j.celrep.2015.06.057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forstermann U & Sessa WC Nitric oxide synthases: regulation and function. Eur Heart J, doi:ehr304 [pii] 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang PL et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377, 239–242, doi: 10.1038/377239a0 (1995). [DOI] [PubMed] [Google Scholar]
- 50.Stauss HM, Godecke A, Mrowka R, Schrader J & Persson PB Enhanced blood pressure variability in eNOS knockout mice. Hypertension 33, 1359–1363 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Van Vliet BN, Chafe LL & Montani JP Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. J Physiol 549, 313–325, doi: 10.1113/jphysiol.2003.041897 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang XP et al. Endothelial nitric oxide gene knockout mice: cardiac phenotypes and the effect of angiotensin-converting enzyme inhibitor on myocardial ischemia/reperfusion injury. Hypertension 34, 24–30 (1999). [DOI] [PubMed] [Google Scholar]
- 53.Luo Z et al. Acute modulation of endothelial Akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. J Clin Invest 106, 493–499, doi: 10.1172/JCI9419 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukai Y et al. Decreased vascular lesion formation in mice with inducible endothelial-specific expression of protein kinase Akt. J Clin Invest 116, 334–343, doi: 10.1172/JCI26223 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Straub AC et al. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature 491, 473–477, doi: 10.1038/nature11626 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vecoli C et al. Partial deletion of eNOS gene causes hyperinsulinemic state, unbalance of cardiac insulin signaling pathways and coronary dysfunction independently of high fat diet. PLoS One 9, e104156, doi: 10.1371/journal.pone.0104156 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steudel W et al. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res 81, 34–41 (1997). [DOI] [PubMed] [Google Scholar]
- 58.Carmeliet P Mechanisms of angiogenesis and arteriogenesis. Nat Med 6, 389–395, doi: 10.1038/74651 (2000). [DOI] [PubMed] [Google Scholar]
- 59.Persson AB & Buschmann IR Vascular growth in health and disease. Front Mol Neurosci 4, 14, doi: 10.3389/fnmol.2011.00014 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Limbourg A et al. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc 4, 1737–1746, doi: 10.1038/nprot.2009.185 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Murohara T et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest 101, 2567–2578, doi: 10.1172/JCI1560 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cai WJ, Kocsis E, Luo X, Schaper W & Schaper J Expression of endothelial nitric oxide synthase in the vascular wall during arteriogenesis. Mol Cell Biochem 264, 193–200 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Dai X & Faber JE Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res 106, 1870–1881, doi: 10.1161/CIRCRESAHA.109.212746 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu J et al. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A 102, 10999–11004, doi: 10.1073/pnas.0501444102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin KA et al. Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling. J Biol Chem 282, 36112–36120, doi: 10.1074/jbc.M703914200 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Xie Y et al. Phosphorylation of GATA-6 is required for vascular smooth muscle cell differentiation after mTORC1 inhibition. Sci Signal 8, ra44, doi: 10.1126/scisignal.2005482 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen YH et al. AKT2 confers protection against aortic aneurysms and dissections. Circ Res 112, 618–632, doi: 10.1161/CIRCRESAHA.112.300735 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.