Abstract
Acetylation of lysine residues occurs in lens proteins. Previous studies have shown an improvement in the chaperone activity of αA-crystallin upon acetylation. Sirtuins are NAD+-dependent enzymes that can deacylate proteins. The roles of sirtuins in regulating the acetylation of lens proteins and their impacts on the function of α-crystallin are not known. Here, we detected sirtuin activity in mouse lenses, and SIRT3 and SIRT5 were present primarily in the mitochondria of cultured primary mouse lens epithelial cells. Western blotting showed higher levels of protein acetylation in the lenses of SIRT3 KO and SIRT5 KO mice than in lenses of WT mice. Mass spectrometry analyses revealed a greater number of acetylated lysine residues in α-crystallin isolated from the SIRT3 and SIRT5 KO lenses than from WT lenses. α-Crystallin isolated from SIRT3 and SIRT5 KO lenses displayed a higher surface hydrophobicity and higher chaperone activity than the protein isolated from WT lenses. Thus, SIRTs regulate the acetylation levels of crystallins in mouse lenses, and acetylation in lenses enhances the chaperone activity of α-crystallin.
Keywords: Lens, α-crystallin, sirtuins, protein acetylation, molecular chaperone, mass spectrometry
1. INTRODUCTION
Posttranslational modifications (PTMs) of lens proteins have been implicated in lens aging and cataract formation, which include glycation, deamidation, carbamylation, oxidation, and acetylation (Ahmed, 2005; Lin et al., 1998; Nagaraj et al., 1991; Nagaraj et al., 1996; Wilmarth et al., 2006). As lens proteins exhibit negligible protein turnover, the PTMs accumulate with age and cataract formation (Harrington et al., 2007; Nagaraj et al., 1991; Wilmarth et al., 2006).
Lysine acylation is a widespread phenomenon that is a well-known regulator of cellular metabolism (Fritz, 2013; Hirschey and Zhao, 2015; Weinert et al., 2013) and gene expression (Choudhary et al., 2009; Zhang et al., 2009). Lysine acylation involves the addition of an acyl group, such as acetyl, succinyl, propionyl and malonyl groups, to the ε-amino group of lysine residues through the catalytic activity of lysine acyl transferases (Allis et al., 2007). The precursors for these acylation reactions are acetyl CoA, succinyl CoA, propionyl CoA and malonyl CoA, respectively (Weinert et al., 2013). While acetylation and propionylation neutralize the positive charge of the ε-amino group of lysine residues, succinylation and malonylation transform it to a negatively charged carboxylic group. This change in the charge can affect protein structure and function. In addition to enzymatic acylation, non-enzymatic acylation is also known to occur under the slightly basic pH conditions in the mitochondria (Verdin and Ott, 2015). Acylation of lysine residues is not mutually exclusive; for example, a lysine residue in a given protein may be either acetylated or succinylated (Pan et al., 2015), which possibly depends on the availability of the respective CoAs.
Protein acylation is a reversible phenomenon. For example, histone deacetylases (HDACs) primarily deacetylate histones, but can also modify non-histone proteins (Haberland et al., 2009). Sirtuins are NAD+-dependent class III HDACs that have been implicated in longevity and age-related diseases (Longo and Kennedy, 2006; Marmorstein, 2004; Michan and Sinclair, 2007). In mammals, 7 different SIRTs (SIRT 1-7) have been identified. These proteins are responsible for deacetylation (SIRT1-7), demalonylation (SIRT5) and desuccinylation (SIRT5) of histones and other non-histone proteins (Du et al., 2011; Laurent et al., 2013; Mimura et al., 2013). While SIRT1 and SIRT2 are predominantly located in the cytosol, SIRT3, SIRT4 and SIRT5 are found in mitochondria, and SIRT3, SIRT6 and SIRT7 are located in the nucleus (Turkmen et al., 2014).
In the human lens, lysine acetylation in αA- and αB-crystallin was first reported by the Smith laboratory (Lapko et al., 2001; Lin et al., 1998). Our group previously identified the acetylation of K70 and K99 in αA-crystallin and K92 and K166 in αB-crystallin in the human lens (Nagaraj et al., 2012; Nahomi et al., 2013a). Furthermore, acetylation improves the chaperone activity of α-crystallin (Nagaraj et al., 2012; Nahomi et al., 2013b; Nandi et al., 2017). More interestingly, the introduction of an acetyllysine (AcK) mimic at K92 by a chemical method substantially improved the chaperone and anti-apoptotic activities of αB-crystallin (Nahomi et al., 2013a). In addition to acetylation, we have recently observed succinylation in human lens proteins and have identified succinylated lysine residues in αA- and αB-crystallins (Nandi et al., 2017). Moreover, K2 acetylation in γD-crystallin alters the protein structure (DiMauro et al., 2014). Based on our observations, acylation occurs in human lenses, and acylation of α-crystallin improves its activities.
In the lens, SIRT1 is the most studied protein among all SIRTs (Mimura et al., 2013). As shown in the study by Zheng et al. (Zheng and Lu, 2011), SIRT1 expression in lens epithelial cells decreases with age. In contrast, increased expression of SIRT1 was observed in lenses from donors aged >50 years with senile cataracts compared to non-cataractous lenses (Zheng and Lu, 2011). Kang et al. (Kang et al., 2015) observed increased expression of SIRT1 in the epithelial cells of cataractous lenses compared to cells from age-matched non-cataractous lenses. Another study suggested that SIRT1 protects human lens epithelial cells from oxidative damage by reducing p53 acetylation (Zheng and Lu, 2016). Thus, the human lens employs mechanisms to deacetylate proteins. It has not been determined whether deacylation has any role in regulating crystallin functions. More importantly, the mechanism by which SIRT-mediated deacylation affects the chaperone activity of α-crystallin has not been investigated.
In this study, we investigated the effects of the absence of SIRT3 and SIRT5 on crystallin acetylation and subsequent chaperone activity of α-crystallin.
2. MATERIALS AND METHODS
2.1. Materials
Insulin, a protease inhibitor cocktail, dithiothreitol and 4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid, dipotassium salt were obtained from Sigma-Aldrich (St. Louis, MO). Antibodies against AcK (Cat# 9681S), SIRT3 (Cat# 5490S), SIRT5 (Cat# 8782S), β-actin (Cat# 4970L), histone H3 (Cat# 4499S), and SOD2 (Cat# 1314S), as well as horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (Cat# 7074S) were obtained from Cell Signaling Technology (Danvers, MA). Recombinant SIRT3 (Cat# 10011194) and SIRT5 (Cat# 10318) were obtained from Cayman Chemical (Ann Arbor, MI). The αA-crystallin antibody was purchased from Enzo Life Sciences (Farmingdale, NY, Cat# ADI-SPA-221-F). The αB-crystallin antibody was obtained from Millipore Sigma (Burlington, MA, Cat# ABN185). All other chemicals were of analytical grade.
2.2. Extraction of water-soluble (WS) proteins from the mouse lens and heart for measurements of sirtuin activity
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of Colorado and were performed in accordance with published National Institutes of Health guidelines and ARVO guidelines. Lenses and heart tissues from C57BL/6J mice (2-3 months old) were homogenized in buffer A [50 mM Tris-HCl (pH 8), 1.5 mM KCl, 1 mM MgCl2, and 0.5 mM DTT] and WS proteins were extracted by centrifuging at 20,000 g for 30 min at 4°C. Isolated WS protein fractions were dialyzed against buffer A overnight at 4°C. The deacetylase activity was tested against acetylated lysozyme. Lysozyme (1 mg/ml) was acetylated in vitro by incubating it with acetic anhydride (500 μM) for 1 h at room temperature (RT) in PBS followed by an overnight dialysis in buffer A at 4°C. The acetylation of lysozyme was confirmed by Western blotting with an AcK antibody. A 100 μl assay mixture containing lens proteins (400 μg) or heart proteins (200 μg), NAD+ (1 mM), and acetylated lysozyme (20 μg) was incubated for 1.5 h at 37°C in buffer A to assess the deacetylation activity of sirtuins in mouse lens/heart proteins. Western blotting was performed using an AcK antibody to determine the deacetylation of lysozyme.
2.3. Isolation of cytosolic, mitochondrial and nuclear fractions from mouse lens epithelial cells
Lens epithelial cells were isolated from mouse lenses (C57BL/6J) as previously described (Mailankot et al., 2008). Cells were cultured in minimum essential medium containing 20% FBS and gentamicin/L-glutamate (1:100). Cytosolic, mitochondrial, and nuclear fractions were isolated using the ProteoExtract subcellular protein extraction kit (Millipore, Cat# 539790) according to the manufacturer’s protocol. Western blotting was performed with the extracted proteins (10 μg), as described below. Membranes were incubated with one of the following primary antibodies: β-actin (diluted 1:5,000), SOD2 (diluted 1:2,000), or histone H3 (diluted 1:2,500). SIRT3 and SIRT5 antibodies were both used at 1:1000 dilutions. The secondary antibody was horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (diluted 1:5,000).
2.4. Detection of SIRT3 and SIRT5 in mouse lenses
Lenses from WT, SIRT3 KO, and SIRT5 KO mice on the C57BL/6J background were extracted from animals at 16 weeks of age. SIRT3 KO and SIRT5 KO mice were a kind gift from Dr. Fred Alt at Boston Children’s Hospital and Dr. Eric Verdin at The Buck Institute for Research on Aging. Knockout 129 mice were backcrossed 10 generations onto the C57BL/6J background. Genotyping of SIRT3 KO and SIRT5 KO mice was performed by PCR using mouse tail snips. SIRT3 was amplified using primers 5’ TGCAACAAGGCTTTATCTTCC 3’ (WT reverse), 5’ CTTCTGCGGCTCTATACACAG 3’ (common forward), and 5’ TACTGAATATCAGTGGGAACG 3’ (mutant forward). SIRT5 was amplified using primers 5’ AGGAGGTGGCAAAGGTCTTGC 3’ (WT forward), 5’ CTGAGGTAGAGTCTCTCATTG 3’ (common reverse), and 5’ TCATTCTCAGTATTGTTTTGCC 3’ (mutant forward). Water-soluble (WS) protein fractions were prepared from mouse lenses as described previously (Nagaraj et al., 2012). For Western blotting, proteins (15 μg) were separated on 12% SDS-PAGE and then electrophoretically transferred to a nitrocellulose membrane. The membrane was then blocked with 5% blocking grade nonfat dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBST). Coomassie staining of the gel was performed after transfer to Western blots to show equal loading of protein samples. The membrane was incubated overnight at 4°C with a primary antibody against one of the following proteins overnight at 4°C: SIRT3 (diluted 1:1,000) or SIRT5 (diluted 1:1000). Subsequently, the membrane was incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody. A SuperSignal West Pico or Femto Kit (Pierce Chemicals, Rockford, IL) was used to detect protein bands.
2.5. Immunohistochemistry for AcK in mouse lenses
Mouse eyes from WT, SIRT3 KO and SIRT5 KO were fixed first in Davidson’s fixative overnight and then in 10% neutral buffered formalin for 4 h. After this, eyes were switched to 70% ethanol and stored at 4°C until they embedded in paraffin blocks. Sections were deparaffinized and blocked with 5% goat serum (Cat# PCN5000, Life Technologies, Grand Island, NY) for 1 h at RT. Sections were next incubated with the AcK antibody (mouse monoclonal, 1:50 dilution in 5% goat serum) overnight at 4°C. Sections were then incubated with a Texas Red-conjugated goat anti-mouse secondary antibody (Cat# T6390, Life Technologies, 1:250 dilution in 5% goat serum) for 1 h at 37°C. Sections incubated with the Texas Red-conjugated secondary antibody alone served as negative controls. DAPI was used to visualize nuclei and images were captured at 20X magnification using a Nikon confocal microscope (Nikon instruments, Inc., Melville, NY).
2.6. Detection of AcK in mouse lens
WS lens proteins (15 μg) from WT, SIRT3 KO and SIRT5 KO mice were separated by SDS-PAGE (on a 12% gel), electrophoretically transferred to a nitrocellulose membrane and then probed with AcK antibody (diluted 1:2,500). Other details of the Western blotting procedure are described above.
2.7. Isolation of α-crystallin from mouse lenses
α-Crystallin was isolated from mouse lens proteins by gel filtration chromatography in an FPLC (NGC Chromatography System, Bio-Rad, Hercules, CA) instrument equipped with an Enrich SEC-650 analytical gel filtration column (10 mm × 300 mm, 24 ml). Four to six lenses in three batches from the WT, SIRT3 KO and SIRT5 KO mice were pooled and homogenized. Five hundred microliters of WS protein (10 mg/ml) were injected onto the column after equilibration with PBS. The absorbance of the column effluent was monitored at 280 nm. A flow rate of 0.5 ml/min was used in all separations. α-Crystallin was detected in all peak fractions by Western blotting using an αB-crystallin antibody or an αA-crystallin antibody. Acetylation levels in isolated α-crystallin proteins were measured by Western blotting using AcK antibody, as described above.
2.8. Mass spectrometry detection of acetylated lysine in α-crystallin
Following isolation, the α-crystallin protein (100 μg) was digested using the 4X trifluoroethanol (TFE) method (Reisdorph et al., 2018). Briefly, the purified protein was denatured and reduced with 50% TFE in 100 mM ammonium bicarbonate (ABC) and 4 mM DTT, and then alkylated using 4 mM iodoacetamide (IAA). The denatured, reduced, and alkylated sample was then diluted to a final concentration of 5% TFE with 25 mM ABC. One hundred micrograms of protein was digested with 2.5 μg of trypsin overnight. The digestion was stopped with 2 μl of formic acid and digests were evaporated to dryness in a speedvac at 45°C. Samples were resuspended in water and desalted using Pierce C18 spin column (Thermo Fisher Scientific Inc., Waltham, MA) according to the manufacturer’s instructions before being dried in a speedvac concentrator. AcK enrichment was accomplished according to the manufacturer’s recommended protocol, except Pierce C18 spin columns were used to purify immunoprecipitated peptides. Briefly, peptides were immunoprecipitated using the PTMScan Acetyl-lysine Motif (Ac-K) Kit (Cell Signaling Technology, Inc., Cat # 13416). The beads were washed with PBS four times before incubation on a Labquake shaker for 2 h at 4°C. After incubation, the supernatant was removed, and the beads were sequentially washed twice with ice-cold 1X IAP buffer and three times with ice cold water (Honeywell Inc. Burdick and Jackson, Morris Plains, NJ). Peptides were eluted twice with 0.15% TFA, pooled, and desalted with Pierce C18 spin columns before being evaporated to dryness in a speedvac at 25°C. Peptides were resuspended in 3% ACN in 0.1% formic acid for the MS analysis.
Enriched peptide samples were loaded onto a 0.07 X 2 0 mm PepMap 100, nanoViper trapping column and chromatographically resolved on-line using a 0.075 × 250 mm, 3 μm Acclaim PepMap RSLC reverse phase nano column (Thermo Scientific) and an UltiMate 3000 RSLCnano System (Thermo Scientific). Mobile phases consisted of water + 0.1% formic acid (A) and acetonitrile + 0.1% formic acid (B). Peptides were chromatographically separated at a flow rate of 400 nl/min using a gradient of 2-40% B over 30 min. Data were collected on an Impact HD Q-TOF instrument equipped with a Captive Spray source (Bruker Daltonics, Inc., Billerica, MA) operated using intensity-dependent CID MS/MS. Proteinscape software (Bruker) was used to submit data to Mascot v2.4 for the database searches. Peptides were searched against the Mus musculus SwissProt database, allowing up to 4 missed tryptic cleavages with variable carbamidomethylation (C), deamidation (NQ), oxidation (M), and acetylation (K) modifications. Peptides with a minimum ion score of 13 were accepted.
2.9. Chaperone activity assays
The chaperone activity of the isolated α-crystallin protein was measured against two client proteins.
i). DTT-induced aggregation of insulin:
Insulin (0.25 mg/ml) was incubated in the presence or absence of α-crystallin (0.05 mg/ml or 2.5 μM) isolated from WT, SIRT3 KO or SIRT5 KO mouse lenses in 50 mM phosphate buffer, pH 7.4, at 25°C. Insulin aggregation was initiated by adding 20 mM DTT. Light scattering was measured in a UV spectrophotometer (Spectra max 190, Molecular Probes, Sunnyvale, CA) at 400 nm in kinetic mode for 60 min.
ii). Thermal aggregation of βL-crystallin:
α-Crystallin (0.002 mg/ml or 0.1 μM) isolated from WT, SIRT3 KO and SIRT5 KO lenses was incubated with βL-crystallin (purified in our laboratory from the WS mouse lens proteins, as described for α-crystallin above) in 50 mM phosphate buffer, pH 7.4. βL-Crystallin aggregation was initiated by inducing thermal stress at 55°C. Light scattering was measured at 400 nm in a UV spectrophotometer (V630 Bio-spectrophotometer, Easton, MD) for 30 min.
2.10. Surface hydrophobicity measurement
The surface hydrophobicity of α-crystallin (0.05 mg/ml) was measured using bis-ANS (10 μM) in 50 mM phosphate buffer, pH 7.4. Emission spectra (excitation at 390 nm) were recorded using a spectrofluorimeter (Fluoromax 4P, Horiba Jobin Mayer, Edison, NJ), as previously described (Nahomi et al., 2013a).
2.11. Statistical analysis
The data are presented as the means ± SD from the number of experimental replicates indicated in the figure legends. Statistically significant differences between the groups were analyzed by one-way ANOVA with Dunnett’s multiple comparison test using GraphPad Prism 7 software. A p-value ≤ 0.05 was considered statistically significant.
3. RESULTS
3.1. Detection of sirtuin activity in mouse lens protein
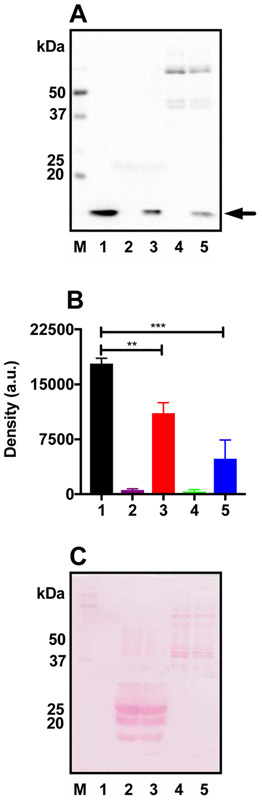
As shown in Fig. 1A, acetylated lysozyme underwent deacetylation upon incubation with WS mouse lens proteins. A densitometric scan of the acetyllysine content in lysozyme (at approximately the 14 kDa region) shown in Fig. 1B revealed that sirtuins in the WS lens fraction deacetylated lysozyme by 37% (Fig. 1B). WS proteins from heart tissues were used as a positive control and deacetylated lysozyme by 72% (Fig. 1B). Ponceau staining of the membrane (Fig. 1C) is also shown.
Figure 1: Sirtuin activity in lens homogenates.
(A) A Western blot of acetylated lysozyme in the presence or absence of WS lens or heart proteins using the AcK antibody. (B) Densitometric plot of the acetylated lysozyme shown in A. (C) Ponceau staining of the membrane to show equal protein loading. The bar graphs depict the means ± SD of triplicate measurements. M, molecular weight markers; 1, Acetylated lysozyme alone; 2, WS mouse lens proteins alone; 3, Acetylated lysozyme + WS mouse lens proteins; 4, WS mouse heart proteins alone; 5, Acetylated lysozyme + WS mouse heart proteins. **p<0.01-; ***p<0.001.
3.2. Subcellular localization of SIRT3 and SIRT5 in mouse lens epithelial cells
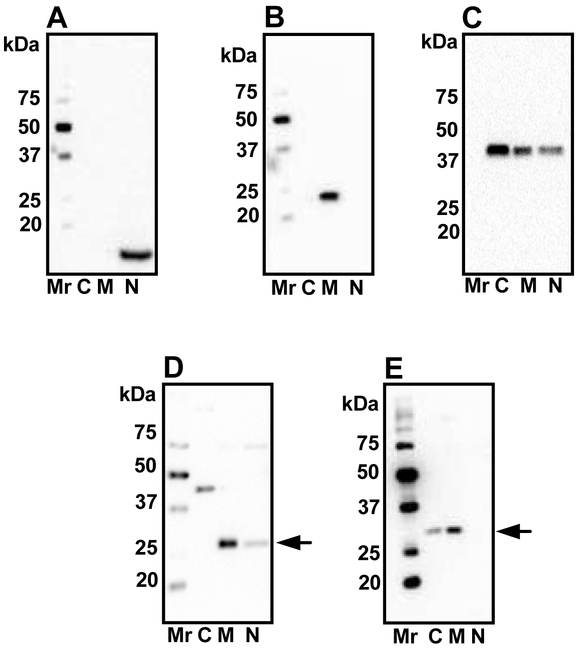
Sequential separation of cytosolic, mitochondrial and nuclear fractions of mouse lens epithelial cells was performed to determine the subcellular localization of SIRT3 and SIRT5. The efficiency of fractionation was verified by Western blotting of fractions for β-actin, SOD2 and histone H3. In Fig. 2A, while histone H3 and SOD2 were present exclusively in the nuclear and mitochondrial fractions, β-actin was primarily present in the cytosol and to a lesser extent in the nuclear and mitochondrial fractions (Fig. 2B and 2C). Western blotting of these fractions revealed that the mitochondrial fraction was enriched in SIRT3 and SIRT5 (Fig. 2D and 2E), and the nuclear fraction had traces of SIRT3 (Fig. 2D). SIRT5 was also detected in the cytosolic fraction (Fig. 2E). Unexpectedly, we also observed a band in the cytosolic fraction at approximately 44 kDa that immunoreacted with the SIRT3 antibody (Fig. 2D).
Figure 2: Subcellular localization of SIRT3 and SIRT5.
Mouse lens epithelial cells were cultured, fractionated as described in Material and Methods and immunoblotted using histone H3 antibodies (A), SOD2 (B) and β-actin (C) antibodies. The isolated fractions were Western blotted using SIRT3 (D) and SIRT5 (E) antibodies. Arrows in panels D and E indicate SIRT3 and SIRT5, respectively. C, cytosolic; M, mitochondrial; N, nuclear. Mr, molecular weight markers.
3.3. SIRT3 and SIRT5 are absent in KO mouse lenses
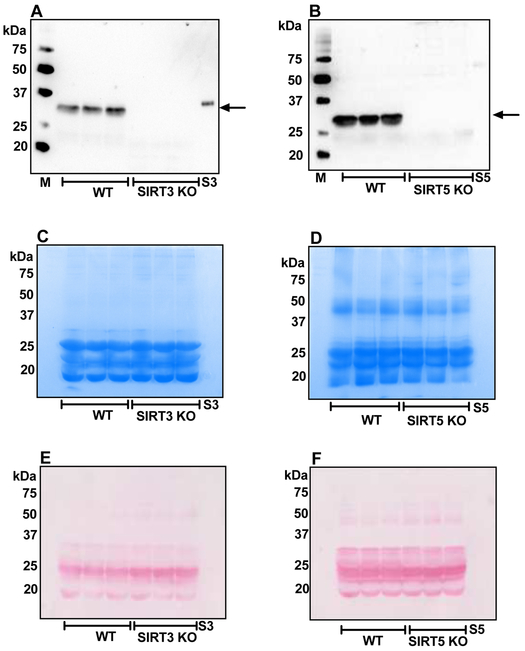
WS fractions from mouse lenses (WT) subjected to Western blotting showed strong immunoreactivity for SIRT3 at 35 kDa (Fig. 3A) and for SIRT5 at 31 kDa (Fig. 3B). Fig. 3A and 3B confirm the absence of SIRT3 and SIRT5 in the respective KO mouse lenses. Coomassie staining of the gels (after transfer to the membrane) and Ponceau staining are shown in Figs. 3C, 3D, 3E and 3F.
Figure 3: SIRT3 and SIRT5 are absent in knockout mouse lenses.
Western blots of WS fractions from WT, SIRT3 KO and SIRT5 KO mice using monoclonal antibodies against SIRT3 (A) and SIRT5 (B). Arrows in panels A and B point to SIRT3 and SIRT5, respectively. Equal protein loading in panels A and B was confirmed by Coomassie staining of the gel after transfer to Western blots (panels C and D) and Ponceau staining (panels E and F). M, molecular weight markers, S3, recombinant SIRT3; S5, recombinant SIRT5.
3.4. Higher levels of acetylated proteins are detected in SIRT3 and SIRT5 KO lenses
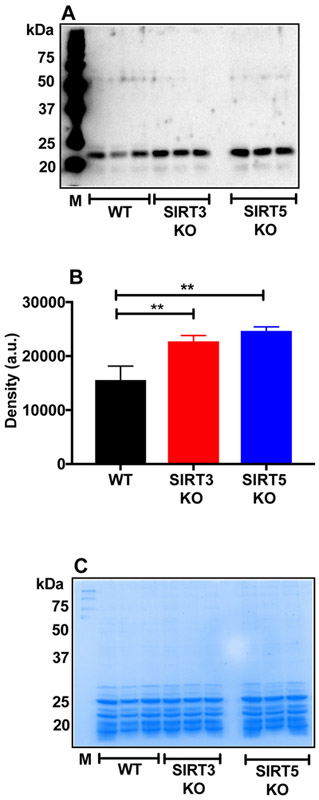
WS fractions were Western blotted using an AcK antibody. Fig. 4A shows the Western blot for AcK. Stronger immunoreactivity was observed at ~20 kDa for AcK in the KO lenses than in WT lenses, suggesting higher AcK levels in crystallins. The densitometry analysis of whole lanes revealed higher AcK levels in the KO lenses than WT lenses (Fig. 4B). Coomassie staining of gel after transfer to membrane showed equal protein loading in samples (Fig. 4C). To determine whether acetylation of proteins occurred during lens homogenization, we homogenized lenses in the presence of a detergent (0.01% triton X or 1 % sodium deoxycholate, W/V). The protein preparations were then dialyzed against the homogenization buffer overnight and subjected to Western blotting for AcK modified proteins. The results show that AcK modification does not occur during homogenization of lenses and dialysis (Fig. S1). We also measured acetylation in the WT, SIRT3 KO and SIRT5 KO lenses by immunohistochemistry. Immunohistochemistry of the WT lens mainly showed acetylation in epithelial cells and in the outer cortical fiber cells (Fig. S2A). SIRT3 KO and SIRT5 KO lenses showed more intense staining in the same regions. Negative controls treated with only the secondary antibody showed no staining. Phase contrast images are shown in Fig. S2B.
Figure 4: Higher acetylation of lens protein in SIRT3 and SIRT5 KO mouse lenses.
Western blots of the WS fractions from WT, SIRT3 and SIRT5 KO mouse lenses using monoclonal antibodies against AcK (A). Densitometric plot of the Western blot is shown in panel B. Protein loading is shown in panels C. The bar graphs represent the means ± SD of three samples. M, molecular weight markers. **p< 0.01..
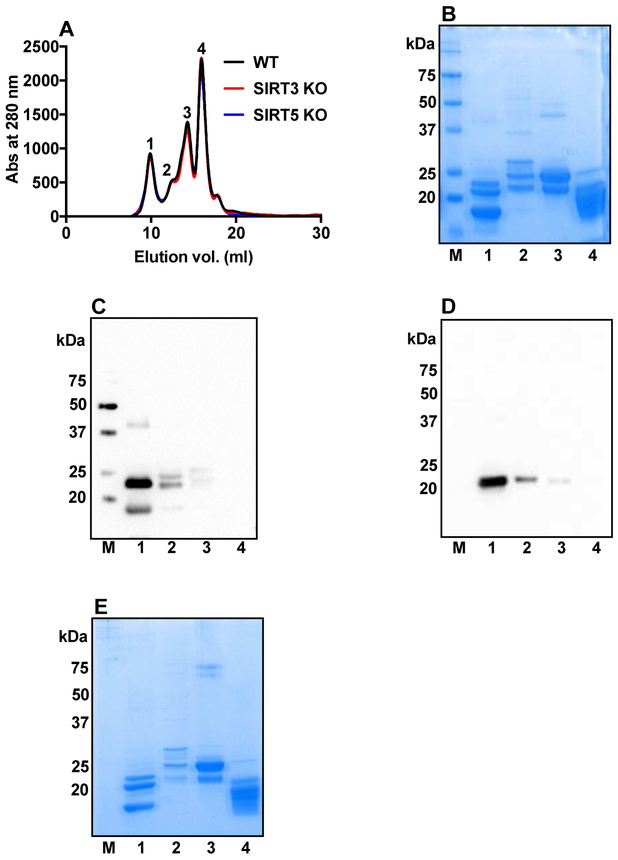
3.5. Isolation of α-crystallin from the mouse lens proteins
Figure 5A shows the FPLC elution profile for lens proteins. As the elution profile of WT, SIRT3 KO and SIRT5 KO mouse lens WS protein were similar, only WT protein was further analyzed in SDS-PAGE and Western blotting in Fig. 5B-E. Equal volume of each peak fraction after FPLC elution of WS protein from WT mouse lenses was run on SDS-PAGE (12% gel) (Fig. 5B). Proteins in each peak from the WS fractions of WT mouse lenses were analyzed by Western blotting for αA-crystallin (Fig. 5C). Two protein bands for αA-crystallin were identified: αA(ins) [~22 kDa] and αA [~20 kDa]. The same membrane was stripped with stripping buffer (Cat# 21059, Thermo Scientific, Rockford, IL), blocked with 5% nonfat dry milk (Cat# 170-6404, Bio-Rad, Hercules, CA) and re-probed for αB-crystallin (Fig. 5D). A band at ~20 kDa was observed for αB-crystallin. Thus, we concluded that peak 1 was enriched in α-crystallin. Coomassie staining of the gel after transfer to membrane is shown in Fig. 5E.
Figure 5: Isolation of α-crystallin from mouse lenses.
FPLC elution profiles of WS proteins from WT, SIRT3 and SIRT5 KO mouse lenses. Four fractions were collected for further analysis (A). The SDS-PAGE analysis of the fractions from WT marked as 1-4 in panel A is shown in B. Western blots of fractions 1 to 4 in panel A using a polyclonal antibody against αA-crystallin (C) and αB-crystallin (D). Protein loading is shown in SDS-PAGE (E). M, molecular weight markers.
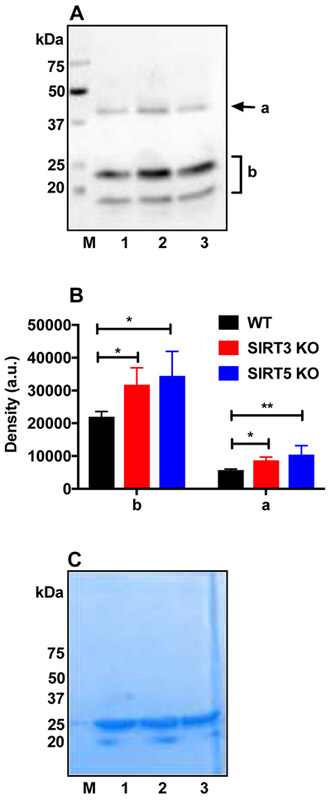
3.6. AcK levels in α-crystallin from SIRT3 and SIRT5 KO lenses
The isolated α-crystallin proteins from mouse lenses were Western blotted using AcK antibody. Fig. 6A shows a Western blot for AcK. Strong immunoreactivity for AcK was observed at approximately 20 kDa and 40 kDa. Densitometry analyses of individual lanes (Fig. 6B) showed higher AcK levels in SIRT3 and SIRT5 KO lenses than in WT lenses. Coomassie staining of the gel after transfer to membrane showed equal protein loading (Fig. 6C).
Figure 6: AcK levels in α-crystallin purified from mouse lenses.
Western blots of α-crystallin isolated from WT (lane 1), SIRT3 KO (lane 2) and SIRT5 KO (lane 3) lenses using AcK (A) antibody. Densitometric plots of regions a and b of panel A are shown in panel B. Equal protein loading in panels A was confirmed by Coomassie staining of the gel after transfer as depicted in panel C. The bar graphs represent the means ± SD of triplicate measurements. *p< 0.05, **p< 0.01.M, molecular weight markers.
The isolated α-crystallin protein from mouse lenses was also subjected to LC-MS analysis to determine whether the absence of SIRT3 and SIRT5 affected AcK levels in α-crystallin. While a number of common AcK residues were observed in αA-crystallin proteins isolated from WT and KO lenses, acetylation at K189 was only detected in SIRT3 and SIRT5 KO lenses (Table 1) Annotated mass spectra for peptides identified by Mascot database search are provided in Fig. S3A-H. Acetylation at K166 in αB-crystallin was observed in only one peptide covering that amino acid from the WT lenses, but all detected peptides from SIRT3 and SIRT5 KO lenses contained acetylation at K166, suggesting greater acetylation at K166. Based on these data, acetylation of α-crystallin occurs at higher levels in the absence of SIRT3 or SIRT5.
Table 1:
Identification of AcK residues in α-crystallin proteins from WT, SIRT3 KO and SIRT5 KO mouse lenses (bold acetylated K)
| Protein | Peptide Sequence | Modification Site |
WT | SIRT 3 KO |
SIRT 5 KO |
|---|---|---|---|---|---|
| αA-Crystallin | K.HFSPEDLTVKVLEDFVEIHGK.H | K111 | √ | √ | √ |
| K.VLEDFVEIHGKHNER.Q | K122 | √ | √ | √ | |
| R.SDRDKFVIFLDVK.H | K93 | √ | √ | √ | |
| R.AIPVSREEKPSSAPSS- | K189 | Not Found | √ | √ | |
| αB-Crystallin | R.EEKPAVAAAPKK- | K166 | Not Found | √ | √ |
| R.EEKPAVAAAPK.K | K166 | Not Found | √ | √ | |
| R.TIPITREEKPAVAAAPK.K | K166 | √ | √ | √ | |
| K.HFSPEELKVK.V | K90 | √ | √ | √ |
The site of trypsin cleavage is denoted by a period (e.g., K.H). Protein C-terminal residue is denoted by a dash (e.g., S-)
3.7. Surface hydrophobicity of α-crystallin proteins isolated from SIRT3 and SIRT5 KO lenses
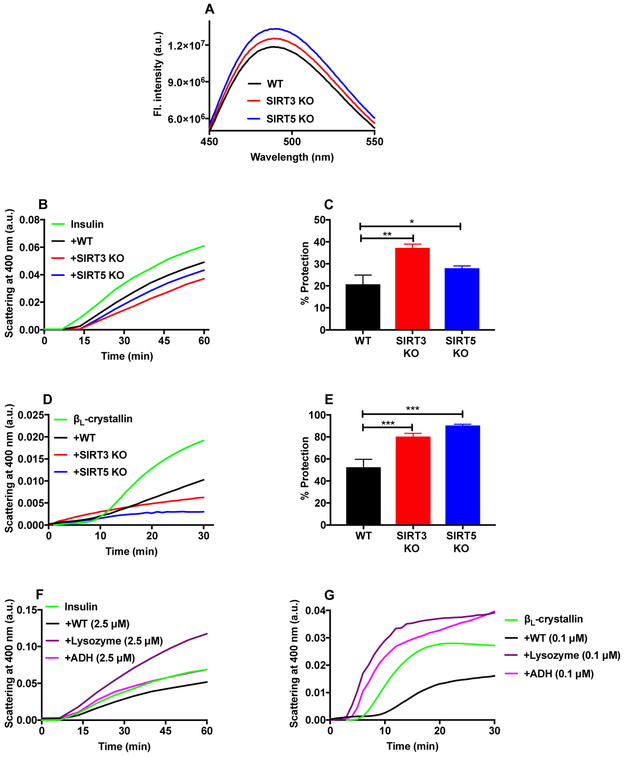
The surface hydrophobicity of α-crystallin was measured using the hydrophobic probe bis-ANS. Bis-ANS bound to α-crystallin from SIRT3 and SIRT5 KO lenses showed 7% and 13% higher fluorescence compared to α-crystallin from WT lenses (Fig. 7A).
Figure 7: Higher surface hydrophobicity and chaperone activity of α-crystallin from SIRT3 and SIRT5 KO mice.
The surface hydrophobicity of α-crystallin was measured using bis-ANS (A). An excitation wavelength of 390 nm was used. The chaperone activity of α-crystallin was assayed by measuring DTT-induced insulin aggregation at 25°C (B) and the thermal aggregation of βL-crystallin at 55°C (D). Percentages by which α-crystallin protected against the aggregation of insulin and βL-crystallin are shown in panels C and E. The bar graphs represent the means ± SD of triplicate measurements. DTT-induced aggregation of insulin (F) and the thermal aggregation of βL-crystallin (G) were performed in the presence or absence of equimolar concentration of α-crystallin, ADH or lysozyme, as above. *p<0.05, **p< 0.01, ***p<0.001.
3.8. Chaperone activity of α-crystallin from SIRT3 and SIRT5 KO lenses
In the chemically induced insulin aggregation assay, α-crystallin from WT lenses prevented 21% of insulin aggregation. However, α-crystallin from SIRT3 KO and SIRT5 KO lenses prevented up to 37% and 28% of aggregation, respectively (Fig. 7B and 7C). Similar observations were obtained from the thermally induced βL-crystallin aggregation assay. α-Crystallin from WT, SIRT3 and SIRT5 lenses prevented 52%, 80% and 90%, respectively, of the thermally induced βL-crystallin aggregation (Fig. 7D and 7E). Thus, a higher acetylation level increases the chaperone activity of α-crystallin in both SIRT3 and SIRT5 KO mouse lenses. To determine the specificity of α-crystallin’s chaperone activity, we conducted chaperone assays using lysozyme or alcohol dehydrogenase (ADH) against the same client proteins. Fig. 7F and 7G show that while α-crystallin (from WT mouse lenses) inhibited the DTT-induced insulin aggregation and thermal aggregation of βL-crystallin, neither lysozyme nor ADH was able to inhibit aggregation of the two client proteins (Fig. 7F and 7G). These results confirmed that the chaperone activity α-crystallin is specific and not due to an effect from protein addition.
4. DISCUSSION
The purpose of this study was to investigate: 1) whether the absence of SIRT3 and SIRT5 leads to greater acetylation of lens proteins and 2) whether higher acetylation of α-crystallin improves the chaperone activity.
We detected sirtuin activity in WT mouse lenses. The activity was detected using acetylated lysozyme. This assay precluded us from identifying which of the sirtuins was responsible for the activity. However, both SIRT3 and SIRT5 are protein deacetylases and, therefore, based the Western blotting data, we reasonably hypothesized that both sirtuins are metabolically active in the lens. Furthermore, subcellular localization assays showed that SIRT3 and SIRT5 are enriched in the mitochondria, along with low levels of SIRT3 detected in the nucleus and SIRT5 in the cytosol, providing further evidence for the activity of the two sirtuins in lenses. Using SIRT3 KO mice, the absence of SIRT3 enhanced the acetylation of lens proteins with a molecular weight of approximately 20 kDa, suggesting that acetylation mainly occurs in crystallins. In addition, the absence of SIRT5 enhanced acetylation of lens proteins. Thus, SIRT3 and SIRT5 regulate the acetylation of proteins in the mouse lens.
Interestingly, the absence of SIRT5 enhanced acetylation modifications investigated here. Thus, in mouse lenses SIRT5 deacetylates AcK, which was not surprising since SIRT5 is known to remove this modification (Du et al., 2011). Moreover, as expected, the absence of SIRT3 increased protein acetylation. Both SIRT3 and SIRT5 are primarily located in the mitochondria, where they are implicated in regulating the activities of metabolic enzymes. How do these proteins regulate the acetylation of lens proteins that are mainly cytosolic? One potential explanation is that the contents of mitochondria could “spill out” during organelle degradation, which occurs in mature fiber cells of the lens, to release SIRTs that in turn deacetylate lens proteins. For this scenario to occur, sufficient NAD+ must be present. Since the majority of the lens is metabolically inactive, this observation poses another conundrum regarding how sufficient NAD+ was present to support the activities of SIRTs. These issues must be investigated in future studies. According to some reports, SIRT3 and SIRT5 deacetylate cytosolic proteins (Nishida et al., 2015; Sundaresan et al., 2008), which, if occurring in the lens, would indicate that crystallins could be deacetylated.
While we detected α-crystallin dimers containing AcK in the purified preparation of α-crystallin (Fig. 6A), dimers containing AcK were not detected when we analyzed WS proteins (Fig. 4A). This discrepancy could be due to the differences in the concentration of α-crystallin in WS proteins. While we used 20 μg of WS proteins, which is likely to contain 6-8 μg of α-crystallin, in Fig. 4A, we used 45 μg of purified α-crystallin in Fig. 6A. One of the important findings in the current work was that the absence of SIRT3 and SIRT5 enhanced AcK levels in α-crystallin. Mass spectrometry studies revealed acetylation at K189 in α-crystallin isolated from SIRT3 and SIRT5 KO mice; this modification was absent in α-crystallin from WT lenses. In addition, higher levels of acetylation at K166 were observed in αB-crystallin from KO animal lenses. K166 in αB-crystallin is the dominant acetylation site when acetylated with aspirin (Hasan et al., 1993). Thus, the absence of SIRT3 and SIRT5 altered the lysine acetylation pattern in α-crystallin. These changes, although subtle, might affect the chaperone activity of α-crystallin. In fact, the chaperone activity of α-crystallin isolated from SIRT3 KO and SIRT5 KO lenses was higher than the protein isolated from WT lenses. Thus, higher acetylation, even at a single lysine residue, is likely to increase the chaperone activity of α-crystallin. This observation is consistent with our previous study showing that acetylation at K92 in human aB-crystallin improves the chaperone activity compared to its absence (Nahomi et al., 2013a). Furthermore, acetylation at a single lysine residue affects the activity of cytosolic and mitochondrial enzymes (Baeza et al., 2016; Min et al., 2015; Starai et al., 2002). Thus, a subtle increase in acetylation might have significant consequences for proteins, and in the case of the lens, it likely alters the chaperone activity of α-crystallin. In addition, aspirin has been shown to reduce the risk of cataract formation (Cotlier and Sharma, 1981; Rao et al., 1985). A possible mechanism underlying this effect might be mediated by the improved chaperone activity of α-crystallin.
In summary, the absence of SIRT3 and SIRT5 promoted the acetylation of mouse lens proteins. Using SIRT3 KO and SIRT5 KO mice, we showed that acetylation improves the chaperone activity of α-crystallin. Thus, SIRTs potentially play a role in regulating the acetylation of α-crystallin and thereby its function.
Supplementary Material
Figure S1. Effect of detergent on AcK levels in mouse lens proteins. WT mouse lenses were homogenized and dialyzed overnight in the presence or absence of the indicated detergent and Western blotted against AcK (A). Densitometric analysis of the Western blot is shown in Panel B. The bar graphs represent the means ± SD of triplicate measurements. Coomassie staining of the gel after transfer is shown in panel C. SDC, sodium deoxycholate; M, molecular weight markers. NS, not significant.
Figure S2. Immunofluorescence staining for AcK in the WT, SIRT3 KO and SIRT5 KO lenses. AcK in lenses was detected by immunofluorescence staining using an AcK monoclonal antibody followed by a Texas Red-conjugated goat anti-mouse secondary antibody (A, top panels). In negative controls (A, bottom panels), sections were incubated with the secondary antibody alone. Phase-contrast images are shown in B. Scale bar = 100 μm.
Figure S3. MS/MS spectra for AcK bearing peptides of αA- and αB-crystallin present in peak 1 of FPLC separation, as shown in Table 1.
HIGHLIGHTS.
SIRT3/5 KO mouse lenses contain higher levels of AcK- modified proteins than WT lenses
α-Crystallin contains more AcK in SIRT3/5 KO mouse lenses than in WT lenses
α-Crystallin from SIRT3/5 KO mouse lenses is a better chaperone than the protein from WT lenses
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grants EY022061 and EY023286 (R.H.N.) and AA022146 (K.S.F.), and a challenge grant from the RPB to the Department of Ophthalmology, University of Colorado. The authors acknowledge the Skaggs School of Pharmacy and Pharmaceutical Sciences Mass Spectrometry Core Facility, which is supported by the Colorado Clinical and Translational Sciences Institute UL-1-RRO25780, for assistance with sample analysis. We thank Drs. Johanna Rankenberg and Mi-hyun Nam for critical reading of the manuscript and Steven Droho for help with FPLC.
Abbreviations used:
- AcK
Nε-acetyllysine
- WS
water-soluble lens protein
- sHSPs
small heat shock proteins
- PTMs
posttranslational modifications
- DTT
dithiothreitol
- bis-ANS
4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid, dipotassium salt
- SIRTs
sirtuins
- HDACs
histone deacetylases
- WT
wild type
- ADH
alcohol dehydrogenase
- SDC
sodium deoxycholate
Footnotes
The authors declare that they have no conflicts of interest with the contents of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmed N, 2005. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res. Clin. Pract 67, 3–21. [DOI] [PubMed] [Google Scholar]
- Allis CD, et al. , 2007. New nomenclature for chromatin-modifying enzymes. Cell 131, 633–636. [DOI] [PubMed] [Google Scholar]
- Baeza J, et al. , 2016. Mechanisms and Dynamics of Protein Acetylation in Mitochondria. Trends Biochem. Sci 41, 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C, et al. , 2009. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840. [DOI] [PubMed] [Google Scholar]
- Cotlier E, Sharma YR, 1981. Aspirin and senile cataracts in rheumatoid arthritis. Lancet 1, 338–339. [DOI] [PubMed] [Google Scholar]
- DiMauro MA, et al. , 2014. Acetylation of Gly1 and Lys2 promotes aggregation of human gammaD-crystallin. Biochemistry 53, 7269–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, et al. , 2011. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334, 806–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz KS, 2013. Chemical acetylation and deacetylation. Methods Mol. Biol 1077, 191–201. [DOI] [PubMed] [Google Scholar]
- Haberland M, et al. , 2009. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington V, et al. , 2007. Proteomic analysis of water insoluble proteins from normal and cataractous human lenses. Mol. Vis 13, 1680–1694. [PubMed] [Google Scholar]
- Hasan A, et al. , 1993. The reaction of bovine lens alpha A-crystallin with aspirin. Exp. Eye Res 57, 29–35. [DOI] [PubMed] [Google Scholar]
- Hirschey MD, Zhao Y, 2015. Metabolic Regulation by Lysine Malonylation, Succinylation, and Glutarylation. Mol. Cell. Proteomics 14, 2308–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, et al. , 2015. Acetylated 8-oxoguanine DNA glycosylase 1 and its relationship with p300 and SIRT1 in lens epithelium cells from age-related cataract. Exp. Eye Res 135, 102–108. [DOI] [PubMed] [Google Scholar]
- Lapko VN, et al. , 2001. In vivo carbamylation and acetylation of water-soluble human lens alphaB-crystallin lysine 92. Protein Sci. 10, 1130–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, et al. , 2013. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol. Cell 50, 686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PP, et al. , 1998. In vivo acetylation identified at lysine 70 of human lens alphaA-crystallin. Protein Sci. 7, 1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Kennedy BK, 2006. Sirtuins in aging and age-related disease. Cell 126, 257–268. [DOI] [PubMed] [Google Scholar]
- Mailankot M, et al. , 2008. Cell cycle arrest by kynurenine in lens epithelial cells. Invest. Ophthalmol. Vis. Sci 49, 5466–5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein R, 2004. Structure and chemistry of the Sir2 family of NAD+-dependent histone/protein deactylases. Biochem. Soc. Trans 32, 904–909. [DOI] [PubMed] [Google Scholar]
- Michan S, Sinclair D, 2007. Sirtuins in mammals: insights into their biological function. Biochem. J 404, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura T, et al. , 2013. The role of SIRT1 in ocular aging. Exp. Eye Res 116, 17–26. [DOI] [PubMed] [Google Scholar]
- Min SW, et al. , 2015. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med 21, 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj RH, et al. , 2012. Acetylation of alphaA-crystallin in the human lens: effects on structure and chaperone function. Biochim Biophys Acta 1822, 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj RH, et al. , 1991. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc. Natl. Acad. Sci. U. S. A 88, 10257–10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj RH, et al. , 1996. Protein cross-linking by the Maillard reaction. Isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal. J. Biol. Chem 271, 19338–19345. [DOI] [PubMed] [Google Scholar]
- Nahomi RB, et al. , 2013a. Acetylation of lysine 92 improves the chaperone and anti-apoptotic activities of human alphaB-crystallin. Biochemistry 52, 8126–8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahomi RB, et al. , 2013b. The combined effect of acetylation and glycation on the chaperone and anti-apoptotic functions of human alpha-crystallin. Biochim Biophys Acta 1832, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi SK, et al. , 2017. αB-Crystallin is the Major Succinylated Protein in Human Lenses, ARVO Annual Meeting Abstract. [Google Scholar]
- Nishida Y, et al. , 2015. SIRT5 Regulates both Cytosolic and Mitochondrial Protein Malonylation with Glycolysis as a Major Target. Mol. Cell 59, 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, et al. , 2015. Global Analysis of Protein Lysine Succinylation Profiles and Their Overlap with Lysine Acetylation in the Marine Bacterium Vibrio parahemolyticus. J Proteome Res 14, 4309–4318. [DOI] [PubMed] [Google Scholar]
- Rao GN, et al. , 1985. Acetylation of lens crystallins: a possible mechanism by which aspirin could prevent cataract formation. Biochem. Biophys. Res. Commun 128, 1125–1132. [DOI] [PubMed] [Google Scholar]
- Reisdorph N, et al. , 2018. Quantitation of peptides from non-invasive skin tapings using isotope dilution and tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 1084, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starai VJ, et al. , 2002. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science 298, 2390–2392. [DOI] [PubMed] [Google Scholar]
- Sundaresan NR, et al. , 2008. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol. Cell. Biol 28, 6384–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkmen K, et al. , 2014. Sirtuins as novel players in the pathogenesis of diabetes mellitus. World J. Diabetes 5, 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E, Ott M, 2015. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol 16, 258–264. [DOI] [PubMed] [Google Scholar]
- Weinert BT, et al. , 2013. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep 4, 842–851. [DOI] [PubMed] [Google Scholar]
- Wilmarth PA, et al. , 2006. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J. Proteome Res 5, 2554–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. , 2009. Lysine acetylation is a highly abundant and evolutionarily conserved modification in Escherichia coli. Mol Cell Proteomics 8, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T, Lu Y, 2011. Changes in SIRT1 expression and its downstream pathways in age-related cataract in humans. Curr. Eye Res 36, 449–455. [DOI] [PubMed] [Google Scholar]
- Zheng T, Lu Y, 2016. SIRT1 Protects Human Lens Epithelial Cells Against Oxidative Stress by Inhibiting p53-Dependent Apoptosis. Curr. Eye Res 41, 1068–1075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of detergent on AcK levels in mouse lens proteins. WT mouse lenses were homogenized and dialyzed overnight in the presence or absence of the indicated detergent and Western blotted against AcK (A). Densitometric analysis of the Western blot is shown in Panel B. The bar graphs represent the means ± SD of triplicate measurements. Coomassie staining of the gel after transfer is shown in panel C. SDC, sodium deoxycholate; M, molecular weight markers. NS, not significant.
Figure S2. Immunofluorescence staining for AcK in the WT, SIRT3 KO and SIRT5 KO lenses. AcK in lenses was detected by immunofluorescence staining using an AcK monoclonal antibody followed by a Texas Red-conjugated goat anti-mouse secondary antibody (A, top panels). In negative controls (A, bottom panels), sections were incubated with the secondary antibody alone. Phase-contrast images are shown in B. Scale bar = 100 μm.
Figure S3. MS/MS spectra for AcK bearing peptides of αA- and αB-crystallin present in peak 1 of FPLC separation, as shown in Table 1.