Abstract
Objective:
We sought evidence for altered adolescent brain growth trajectory associated with moderate and heavy alcohol use in a national, multi-site, prospective study of hundreds of participants studied before and after they initiated harmful levels of alcohol use.
Method:
This study examined 483 adolescents (12-21 years) before initiation of drinking and one and two years later. At the two-year session, 356 participants continued to meet study entry no/low alcohol consumption criteria, 65 initiated moderate drinking, and 62 initiated heavy drinking. MRI quantified regional cortical and white matter volumes. Percent change/year (slopes) of adolescents who continued to meet no/low criteria served as developmental control trajectories against which to compare youth who initiated moderate or heavy drinking.
Results:
In no/low drinkers, gray matter volume declined throughout adolescence and slowed in many regions in later adolescence. Complementing gray matter declines, white matter regions grew at faster rates at younger ages and slowed toward young adulthood. Youth who initiated heavy drinking exhibited frontal cortical gray matter trajectory acceleration divergent from the norm. Although significant effects on trajectories were not forthcoming in moderate drinkers, their intermediate position between no/low and heavy drinkers suggested a dose effect. Neither marijuana co-use nor baseline volumes contributed significantly to the alcohol effect.
Conclusions:
Initiation of drinking during adolescence—with or without marijuana co-use—disordered normal brain growth trajectories. Factors possibly contributing to abnormal cortical volume trajectories include peak consumption in the past year and family history of alcohol misuse.
Keywords: adolescence, drinking, brain, growth trajectories, gray matter, white matter
INTRODUCTION
Normal brain maturation determined from cross-sectional studies (e.g.,1, 2, 3) with longitudinal confirmation (e.g.,4, 5-10) is characterized as increasing in cortical gray matter volume through the first decade followed by continuous decline thereafter. Concurrent with the gray matter decline, supratentorial white matter volume continues growing throughout adolescence with a slowing of the growth trajectory in the third decade (e.g., 11). Although there are local regional differences in rates of change (5, 11-14), these complementary tissue changes define the nature of brain structural maturation. These significant and predictable changes in normal neurodevelopment have led to the speculation that the evolution of the adolescent brain is especially vulnerable to environmental insult. Given that adolescence is also a time of risk-taking and experimentation with "adult behaviors," such as alcohol drinking, a likely prediction is that excesses of such potentially deleterious agents might result in accelerated gray matter loss, attenuated white matter growth, or both (cf., 15, 16).
Abnormal growth patterns were observed in two recent longitudinal studies of youth who initiated and continued heavy drinking. The first study examined 55 youth, age 14-19 years at baseline, none of whom had ever imbibed in any alcohol or drugs (15). At that study's two-year follow-up, structural MRI revealed “greater than expected decreased cortical thickness in the right middle frontal gyrus” and "blunted development" in some regional white matter volumes in the 30 youth who initiated “regular” but also “subclinical” alcohol use (1 to 10 drinks per occasion several times weekly) compared with the 25 youth who remained alcohol and drug free. It is notable, however, that the youth who refrained from drinking showed increasing rather than the typically-observed decreasing cortical gray matter thickness, which has implications for interpreting the relative decreasing thickness reported in the drinking group. The second, larger study examined youth over 1- to 8-year intervals and found accelerated gray matter volume reductions in the lateral frontal and temporal cortices and attenuated white matter volume growth of the corpus callosum and pons in 75 heavy drinkers relative to 59 light to nondrinking youth, a pattern common to both sexes (16). Critically, moderate drinkers were excluded from these reports, leaving unaddressed whether highly prevalent, moderate drinking levels (17) could interfere with normal developmental trajectories.
To address this and related questions, we conducted a longitudinal analysis of structural MRI data collected at baseline (18), one year, and two years later on 483 youth participating in the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA) study (19). At baseline, all of these participants had met study entry criteria for no-to-low drinking and drug consumption. As anticipated, however, a proportion of these youth (N=127) initiated drinking to levels that exceeded, to varying degrees, study entry criteria, thereby enabling pursuit of a naturalistic study on the effects of drinking on the adolescent brain. Accordingly, we pursued two primary aims: 1) to establish normal growth trajectories, which would be predicted to show declines in regional gray matter volume concurrent with growth of white matter volumes in the youth who continued to meet the no-to-low age-dependent criteria (“no/low” participants); and 2) to test whether the developmental trajectories of youth who transitioned to exceeding the initial alcohol consumption criteria would conform to patterns identified in previous work in heavy-drinking youth showing accelerated gray matter declines with attenuated white matter growth. In light of previous reports, expected group differences would be greatest in frontal and temporal cortices and detectable in white matter volumes (15, 16). We speculated that the trajectories of moderate drinkers would show accelerated volume loss at a level intermediate between normal decline and accelerated decline of the heavy-drinkers. Consideration of these different drinking levels enabled testing for a dose effect based on reported quantities of drinking and seeking correlations between consumption quantities and cortical volume slopes showing group differences by drinking levels. Exploratory analyses examined potential differences related to family history of alcoholism (e.g.,20, 21) and compounding effects of marijuana and alcohol co-use on brain volume trajectories.
METHODS
Participants
The participants were 483 adolescents (age 12 to 21 years at entry) with useable baseline and 2-year follow-up data. Of these 483 participants, 457 had 1-year and 2-year follow-up data, and 26 had 2-year follow-up data having missed their 1-year visit. At baseline, these 483 youth met our double alcohol inclusion criteria, described in the next section. These youth were drawn from the 674 no/low alcohol-consuming adolescents tested at baseline and recruited across the five NCANDA sites: University of California at San Diego (UCSD), SRI International, Duke University Medical Center, University of Pittsburgh Medical Center, and Oregon Health & Science University (OHSU) (19). The study followed an accelerated longitudinal design, which specified a three-age band recruitment strategy at baseline with annual follow-up examinations: 12-14.9 years, 15-17.9 years, and 18-21.9 years. The Institutional Review Boards (IRB) of each site approved this study (see Supplement).
Alcohol history determination.
Participants completed the Customary Drinking and Drug use Record (CDDR) (22) to characterize past and current alcohol and substance use (see Supplement). At entry, all participants selected for this analysis met two sets of drinking criteria. First, the initial NCANDA inclusion criteria for "no/low" drinking, which were based on National Institute on Alcohol Abuse and Alcoholism guidelines for risky drinking and determined in terms of maximum drinking days and maximum drinks on an occasion as follows: maximum drinking days for male and female participants varied by age, where the maximum days drinking for 12-15.9 year olds was ≤ 5, for 16-16.9 year olds it was ≤ 11; for 17-17.9 year olds it was ≤ 23; for 18 and older it was ≤ 51. The maximum allowable drinks per occasion was ≤ 3 for female participants at any age but varied by age for male participants, for whom the maximum drinks per occasion was ≤ 3 for 12-13.9 year olds, ≤ 4 for 14-19.9 year olds, and ≤ 5 for 20 year olds and older.
Second, heavy, moderate, and no/low drinkers were categorized using the modified Cahalan et al. (23) inventory, comprising quantity (average and maximum consumption) and frequency combinations to classify drinking levels based on past year patterns (details are in Supplement). At the 2-year follow-up, the final data set, after applying MRI exclusion criteria (noted later), comprised 356 youth who remained in the no/low double criterion group, and 127 youth who transitioned from the no/low to one of two drinking groups: 65 moderate drinkers and 62 heavy drinkers (Table 1).
Table 1.
National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA) demographics for baseline and MRI 2-year follow-up subgroups defined by interim drinking
| Baseline |
Longitudinal |
|||||||
|---|---|---|---|---|---|---|---|---|
| Full Group | Maintained | Transitioned to Drinking |
post hoc differences |
|||||
| No/low | No/low | Moderate | Heavy | |||||
| Age at baseline (years) | mean= | 15.55 | 15.08 | 16.69 | 17.07 | F= | 34.474 | N<M=H |
| SD= | 2.25 | 2.19 | 1.95 | 1.75 | p= | 0.0000 | ||
| N= | 483 | 356 | 65 | 62 | ||||
| Male | mean= | 15.43 | 15.03 | 16.04 | 16.97 | F= | 15.143 | N<M=H |
| SD= | 2.15 | 2.14 | 1.85 | 1.56 | p= | 0.0000 | ||
| N= | 241 | 180 | 24 | 37 | ||||
| Female | mean= | 15.67 | 15.13 | 17.07 | 17.21 | F= | 20.794 | N<M=H |
| SD= | 2.34 | 2.24 | 1.92 | 2.03 | p= | 0.0000 | ||
| N= | 242 | 176 | 41 | 25 | ||||
| Socioeconomic status† | mean= | 16.71 | 16.63 | 16.98 | 16.85 | F= | 0.703 | |
| SD= | 2.46 | 2.53 | 2.04 | 2.45 | p= | 0.4956 | n.s. | |
| Internalizing symptoms T-score | mean= | 44.91 | 44.97 | 44.80 | 44.66 | F= | 0.034 | |
| SD= | 9.449 | 9.54 | 8.98 | 9.58 | p= | 0.9665 | n.s. | |
| Externalizing symptoms T-score | mean= | 43.38 | 42.92 | 44.26 | 45.08 | F= | 2.281 | |
| SD= | 8.18 | 8.34 | 7.30 | 7.93 | p= | 0.1033 | n.s. | |
| Lifetime drinking days | mean= | – | 0.60 | 14.83 | 42.79 | F= | 179.621 | |
| SD= | 1.81 | 15.07 | 42.73 | p= | 0.0000 | N<M<H | ||
| N= | 347 | 63 | 61 | |||||
| Lifetime drinks | mean= | – | 0.70 | 35.77 | 157.39 | F= | 227.74 | |
| SD= | 2.21 | 33.69 | 144.02 | p= | 0.0000 | N<M<H | ||
| N= | 347 | 63 | 59 | |||||
| Lifetime binges | mean= | – | 0 | 3.71 | 15.81 | F= | 136.321 | |
| SD= | 0 | 5.12 | 18.88 | p= | 0.0000 | N<M<H | ||
| N= | 356 | 65 | 62 | |||||
| Maximum drinks/episode in past year | mean= | – | 0.2 | 5.2 | 8.4 | F= | 734.292 | |
| SD= | 0.6 | 2.8 | 3.6 | p= | 0.0000 | N<M<H | ||
| N= | 355 | 65 | 62 | |||||
| χ2 | p | |||||||
| Lifetime marijuana days | mean= | – | 1.15 | 25.78 | 70.34 | |||
| median= | 0†† | 2†† | 10†† | |||||
| N reporting more than 50 uses= | 1††† | 10††† | 17††† | 83.855 | 0.0000 | N<M<H | ||
| Family history of alcoholism | ||||||||
| negative, positive= | 442, 41 | 329, 27 | 60, 5 | 53, 9 | 3.328 | 0.1890 | n.s. | |
| Self-declared ethnicity | ||||||||
| Caucasian | N= | 355 | 248 | 54 | 53 | 10.309 | 0.0355 | N vs. M vs. H§ |
| African-American | N= | 70 | 60 | 4 | 6 | 5.449 | 0.0656 | N vs. M |
| Asian | N= | 51 | 41 | 7 | 3 | 5.694 | 0.0580 | N vs. H |
| Other | N= | 7 | 7 | 0 | 0 | 1.940 | 0.3790 | M v. H |
| Site (scanner manufacturer) | 5.629 | 0.6890 | n.s. | |||||
| UPitt (Siemens) | N= | 60 | 45 | 6 | 9 | – | – | |
| SRI (GE) | N= | 81 | 57 | 14 | 10 | – | – | |
| Duke (GE) | N= | 100 | 81 | 10 | 9 | – | – | |
| OHSU (Siemens) | N= | 104 | 75 | 16 | 13 | – | – | |
| UCSD (GE) | N= | 138 | 98 | 19 | 21 | – | – | |
N=no/low; M=moderate drinkers; H=heavy drinkers
Highest education of a parent;
median;
number reporting more than 50 uses of marijuana
χ2 to test for 2-group or 3-group differences in distributions of Caucasian, African-American, and Asian ethnicities
Subject demographics.
As described (18, 24), participants were characterized by age, sex, self-identified ethnicity, and socioeconomic status (SES) determined as the highest level of education achieved by either parent (25) (Table 1).
To examine potential additive effects of alcohol and marijuana consumption on developmental trajectories, a marijuana-use criterion applied at the two-year follow-up determined that greater than 50 lifetime uses served as a dichotomous variable of marijuana use in the 127 heavy and moderate alcohol users. This produced 27 alcohol plus marijuana users (17 heavy and 10 moderate drinkers) and 100 alcohol non-marijuana users. A step-wise difference in use occasions emerged between drinking groups: no/low < moderate (t(58.7)=3.417, p=.0012), moderate < heavy (t(76.2)=2.523, p=.0137) (Table 1). All participants also submitted samples to a 14-panel urine toxicology screen (Supplement for details).
MRI Acquisition
The longitudinal data for the current analysis comprised MR images collected on 483 of the initial 674 no-to-low baseline participants (18) who had 2-year (and in most cases, 1 year) follow-up MRI and CDDR data, met the double alcohol inclusion criteria, met FreeSurfer (26, 27) SNR criteria, and had adequate quality imaging data. Details appear in the Supplement.
Statistical Analysis
Normal Developmental Brain Structural Trajectories.
Developmental effects were determined by quantifying regional brain volume trajectories in the participants who remained in the no/low drinking group. Accordingly, we computed the slope of six major bilateral lobar gray matter regions-of-interest (ROIs) (28) and the three white matter ROIs as change in native values per year divided by the native value at baseline and multiplied by 100 to yield a slope expressed as percent change per year, thus placing all ROIs on the same scale. The effects of site, ethnicity, and supratentorial volume (svol) were removed by regression analysis from the slopes. Effects due to MRI scanner manufacturer differences were removed by controlling for site (18). Effects of age, sex, and their interaction were tested with general linear models (GLMs). The extent to which gray matter slopes were negative and white matter slopes positive was tested with one-sample t-tests against the population 0 (Figure 1; see Supplement).
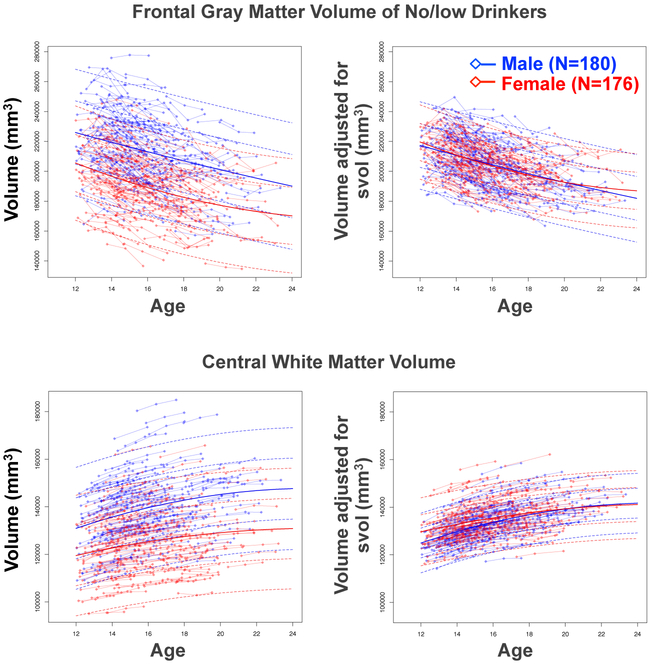
Figure 1.
Left: Plots of 356 adolescents who continued to meet no/low criteria with connected baseline, 1-year, and 2-year follow-up values (boys in blue, girls in red) plotted as a function of their baseline age for frontal gray matter volume (top) and the central white matter sample volume (bottom). The lmer fits with +/− 1 and 2 SD separately computed for boys (blue) and girls (red) are also plotted. Note that the frontal lobe values decrease fairly linearly, whereas the growth in the white matter volume slows over age. For both regions, boys had larger volumes than girls.
Right: The same data as above but adjusted for variation in supratentorial volume (svol), which attenuated sex differences in regional volumes.
Effect of Alcohol Drinking on Developmental Trajectories.
The analysis tested group differences separately between the 356 who remained in the no/low drinking group and all 127 youth who transitioned to drinking by dividing the transition group into moderate (N=65) and heavy (N=62) drinkers based on Cahalan et al. (23) scores. The effects of alcohol consumption on each ROI were tested with a series of GLMs of slope as a function of alcohol use group plus age, sex, site, ethnicity, and svol as well as an extended model testing for group by age and group by sex interactions. For display and effect size calculations (Cohen's d) of the differences between no/low and the two drinking transition groups over age, the effects of sex, site, ethnicity, and svol were removed from the native values by regression analysis prior to application of LMER of the regressed values as a function of age and age2. Analyses were tested unidirectionally, where steeper cortical volume declines and attenuated white matter volume growth were expected in the drinking youth; thus, using a one-tailed test with 10 ROI comparisons, results were considered statistically significant if p≤0.01.
These analyses were repeated for the 34 individual Desikan-Killiany bilateral cortical ROI FreeSurfer parcellations (28) for finer-grained examination. Exploratory correlational analyses used Pearson tests and Spearman Rank Order confirmatory tests of slopes corrected for age, sex, site, ethnicity, and svol as functions of drinking variables at follow-up MRI 2 (lifetime drinking days, number of drinks, number of binges, and maximum number of drinks per occasion in the past year).
RESULTS
Slopes of each ROI and tissue type were expressed as percent change per year for each individual. Trajectories of the no/low-drinking group are presented first and provide the context for identifying where the drinking youth deviated from the normal developmental change patterns.
Trajectories of Normal Brain Structural Maturation
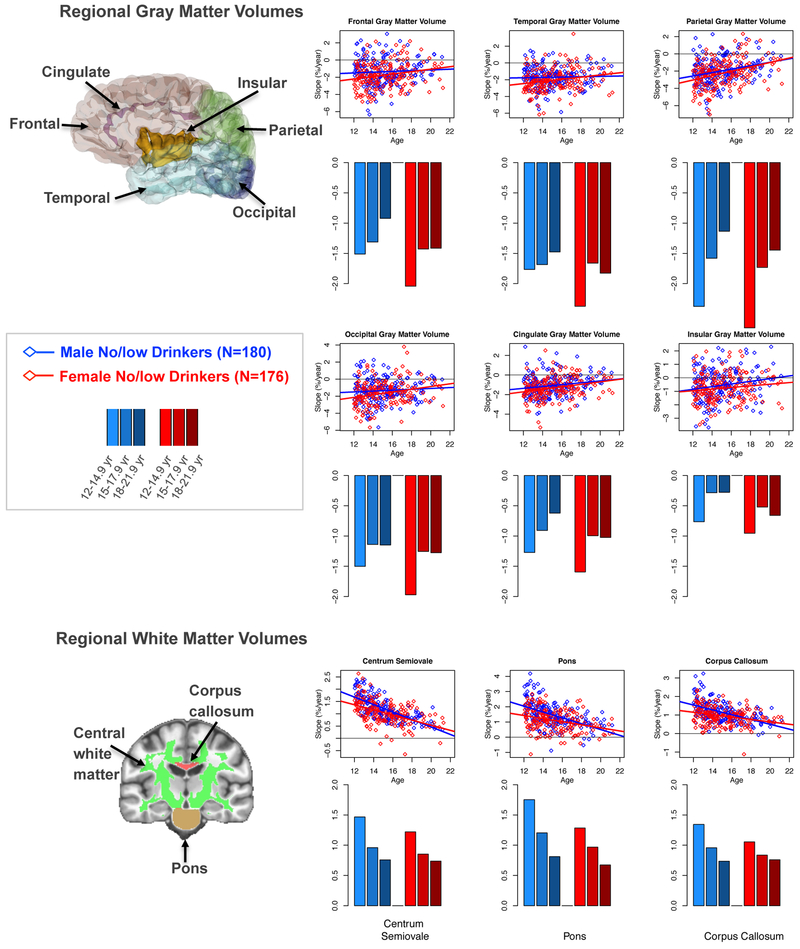
On average all six regional neocortical and cortical gray matter regions exhibited negative slopes when tested against 0 (t-values ranged from −11.466 to −27.158, p< 10−8 to 10−87, Table 2). All ROIs showed monotonic decelerating trajectories with older age (Figure 2; Table 2). The annual rate of decline was greater for the female than male participants, but no age-by-sex interaction was significant (Table 2).
Table 2.
No/low group: General linear model (GLM)† t and p for age, sex, and age × sex and for each MRI region of interest (ROI) (df=1/353) and one-sample t-test results testing the mean slope against 0 (df=355)
| GLM | t | p |
|---|---|---|
| Gray Matter ROIs | ||
| Frontal | ||
| age | 2.610 | 0.0094 |
| sex | −2.494 | 0.0131 |
| age × sex | 1.356 | 0.1761 |
| t-test | −19.4147 | 0.0000 |
| Temporal | ||
| age | 2.4378 | 0.0153 |
| sex | −2.6914 | 0.0075 |
| age × sex | 1.7281 | 0.0848 |
| t-test | −27.1579 | 0.0000 |
| Parietal | ||
| age | 6.2510 | 0.0000 |
| sex | −1.8733 | 0.0619 |
| age × sex | 0.7591 | 0.4483 |
| t-test | −25.1887 | 0.0000 |
| Occipital | ||
| age | 3.0873 | 0.0022 |
| sex | −2.0508 | 0.0410 |
| age × sex | 1.5664 | 0.1181 |
| t-test | −19.4511 | 0.0000 |
| Cingulate | ||
| age | 4.3569 | 0.0000 |
| sex | −2.2973 | 0.0222 |
| age × sex | 0.6297 | 0.5293 |
| t-test | −20.7309 | 0.0000 |
| Insular | ||
| age | 3.0430 | 0.0025 |
| sex | −1.9836 | 0.0481 |
| age × sex | −0.7437 | 0.4575 |
| t-test | −11.4663 | 0.0000 |
| Total cortex | ||
| age | 3.9778 | 0.0001 |
| sex | −2.5317 | 0.0118 |
| age × sex | 1.3426 | 0.1803 |
| t-test | −24.4961 | 0.0000 |
| White Matter ROIs | ||
| Central white matter | ||
| age | −13.6165 | 0.0000 |
| sex | −4.0492 | 0.0001 |
| age × sex | 2.5857 | 0.0101 |
| t-test | 44.1339 | 0.0000 |
| Pons | ||
| age | −8.4879 | 0.0000 |
| sex | −4.4966 | 0.0000 |
| age × sex | 2.6598 | 0.0082 |
| t-test | 30.6557 | 0.0000 |
| Corpus callosum | ||
| age | −8.1726 | 0.0000 |
| sex | −3.6623 | 0.0003 |
| age × sex | 2.8009 | 0.0054 |
| t-test | 36.7118 | 0.0000 |
All GLM analyses include age, sex, site, ethnicity, and svol
Figure 2.
The figures of the brain note the regions-of-interest (ROI) measured. The slope of regional gray matter volumes (top 6 scatter plots) and white matter volumes (bottom 3 scatter plots) for each of the 356 participants in the continuing no/low drinking group (boys in blue, girls in red) with slope over age linear regression lines for each sex plotted separately. Below each scatter plot is a bar graph displaying the average slope for each of the three initial recruitment age groups (12-14.9, 15-17.9, 18-21.9 years old).
In contrast with cortical regions, the 3 white matter volumes exhibited growth (t-values ranged from +30.656 to +44.134, p< 10−100, Table 2). This growth slowed with advancing age (simple age effects ranged from t=−8.173 to −13.617, p<.0001; Table 2). Significant age-by-sex interactions for all 3 white matter ROIs indicated more rapid declines in growth over age in the boys than the girls (Figure 2; Table 2).
Trajectory Deviations from Normal Developmental Patterns Observed in Drinkers
All analyses seeking group differences controlled for age, sex, site, ethnicity, and svol.
Paired Analyses: 65 Moderate vs. 356 No/Low and 62 Heavy vs. 356 No/Low Group Differences.
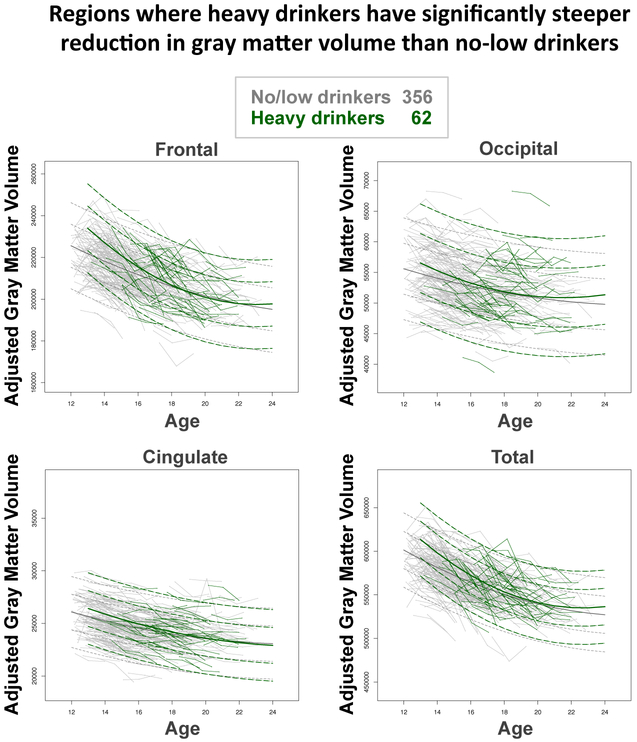
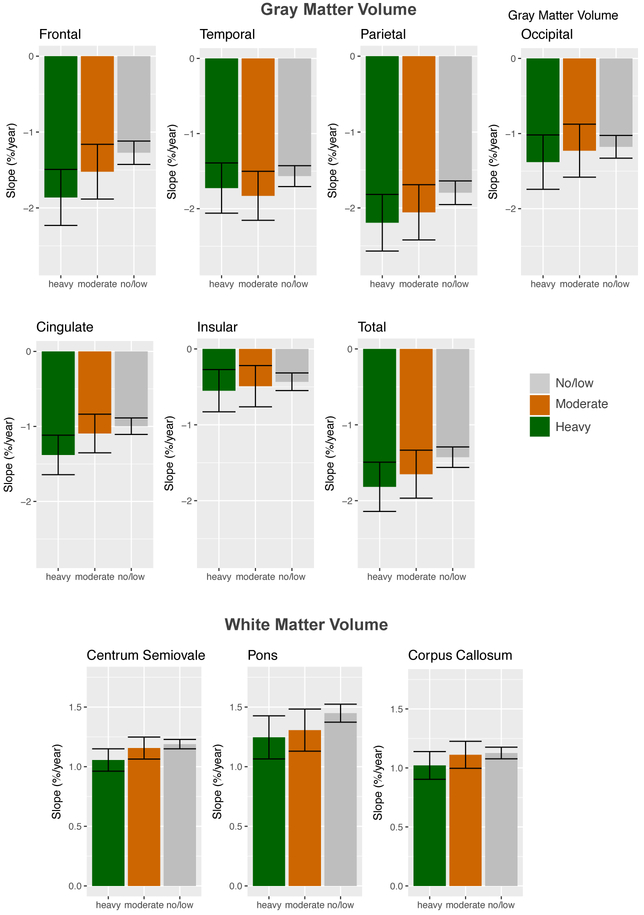
Significant group effects were limited to the 62 heavy vs. the 356 no/low drinker comparisons (Table 3). Volumes of frontal, cingulate, and total gray matter declined faster and central white matter expanded slower in the heavy drinkers than the no/low drinking group (Figures 3-4); these differences were significant with Bonferroni p-value adjustment for the frontal region. Although the moderate drinking group generally showed the heavy-drinking pattern of steeper than expected slopes, none of those group differences was significant (Table 3; Figure 4).
Table 3.
General linear model (GLM) t, p, and Cohen's d effect size estimate for each MRI region of interest
| Group GLM† | t | p | Cohen's d |
|---|---|---|---|
| Gray Matter Volume | |||
| Frontal | |||
| 65 moderate vs. 356 no/low drinkers | −1.0487 | 0.2949 | −0.1510 |
| 62 heavy vs. 356 no/low drinkers | −2.6263 | 0.0090* | −0.3937 |
| Temporal | |||
| 65 moderate vs. 356 no/low drinkers | −1.2941 | −0.1864 | −0.1864 |
| 62 heavy vs. 356 no/low drinkers | −0.8096 | 0.4186 | −0.1214 |
| Parietal | |||
| 65 moderate vs. 356 no/low drinkers | −1.1400 | 0.2550 | −0.1642 |
| 62 heavy vs. 356 no/low drinkers | −1.8372 | 0.0669 | −0.2754 |
| Occipital | |||
| 65 moderate vs. 356 no/low drinkers | −0.1510 | 0.8800 | −0.0217 |
| 62 heavy vs. 356 no/low drinkers | −1.1056 | 0.2696 | −0.1657 |
| Cingulate | |||
| 65 moderate vs. 356 no/low drinkers | −0.5395 | 0.5898 | −0.0777 |
| 62 heavy vs. 356 no/low drinkers | −2.3195 | 0.0209 | −0.3477 |
| Insular | |||
| 65 moderate vs. 356 no/low drinkers | −0.3872 | 0.4352 | −0.1171 |
| 62 heavy vs. 356 no/low drinkers | −0.7810 | 0.4352 | −0.1171 |
| Total cortex | |||
| 65 moderate vs. 356 no/low drinkers | −1.0977 | 0.2730 | −0.1581 |
| 62 heavy vs. 356 no/low drinkers | −2.0456 | 0.0414 | −0.3067 |
| White Matter Volume | |||
| Central white matter | |||
| 65 moderate vs. 356 no/low drinkers | −0.4140 | 0.6791 | −0.0596 |
| 62 heavy vs. 356 no/low drinkers | −2.1225 | 0.0344 | −0.3182 |
| Pons | |||
| 65 moderate vs. 356 no/low drinkers | −1.1274 | 0.2602 | −0.1624 |
| 62 heavy vs. 356 no/low drinkers | −1.7969 | 0.0731 | −0.2694 |
| Corpus callosum | |||
| 65 moderate vs.356 no/low drikers | −0.0528 | 0.9578 | −0.0076 |
| 62 heavy vs. 356 no/low drinkers | −1.4456 | 0.1490 | −0.2167 |
GLM controlled for age, sex, site, ethnicity, and svol
Bonferroni-corrected, 1-tailed
Differences were not significant between moderate and no/low drinkers.
Figure 3.
Plots of connected baseline, 1-year, and 2-year follow-up values for 356 adolescents who continued to meet no/low criteria (gray) and 62 heavy drinkers (green) as a function of baseline age with lmer fits with +/− 1 and 2 SD computed separately for no/low (gray) and heavy drinkers (green) superimposed for frontal, occipital, cingulate, and total gray matter volume.
Figure 4.
Bar graphs of mean slope and 95% confidence interval for heavy (green), moderate (orange), and continuing no/low (gray) drinking groups for each regional volume measured.
Because the heavy drinkers were in the older age range, we conducted a follow-up test on a subset of 62 controls matched to the heavy drinkers in age, sex, and ethnicity. Frontal gray matter volume continued to show a steeper decline in the heavy compared with no/low drinking groups (t=−2.004, p=.0474), although the p-value did not meet Bonferroni correction. Additional GLMs entered family history status and SES to seek group differences by region and found none.
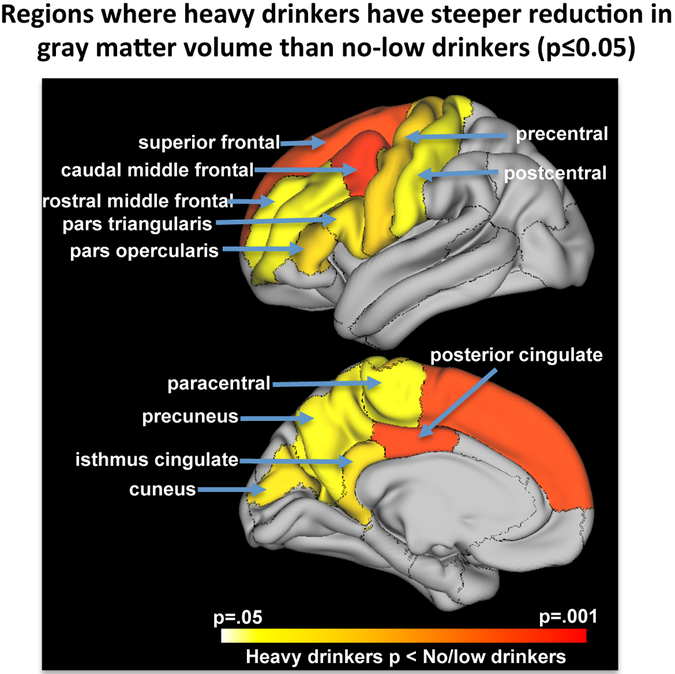
Having identified accelerated regional volume declines in several lobar regions of cortex in heavy drinkers, we expanded our analysis to seek potential local effects in group differences in 34 more finely parcellated cortical regions. This analysis identified 12 cortical regions showing faster volume declines in the heavy drinkers than the no/low drinkers, 3 of which met false discovery rate p-value correction of p≤.025: caudal middle frontal, superior frontal, and posterior cingulate cortices (Figure 5, Supplemental Table).
Figure 5.
Lateral (top) and medial (bottom) view of the left hemisphere with color scale showing regions where heavy drinkers have significantly steeper reduction in gray matter volumes than no/low drinkers. Regions showing faster cortical volume declines in the heavy drinkers relative to the no/low drinkers displayed in bright orange are FDR-corrected (p<.025).
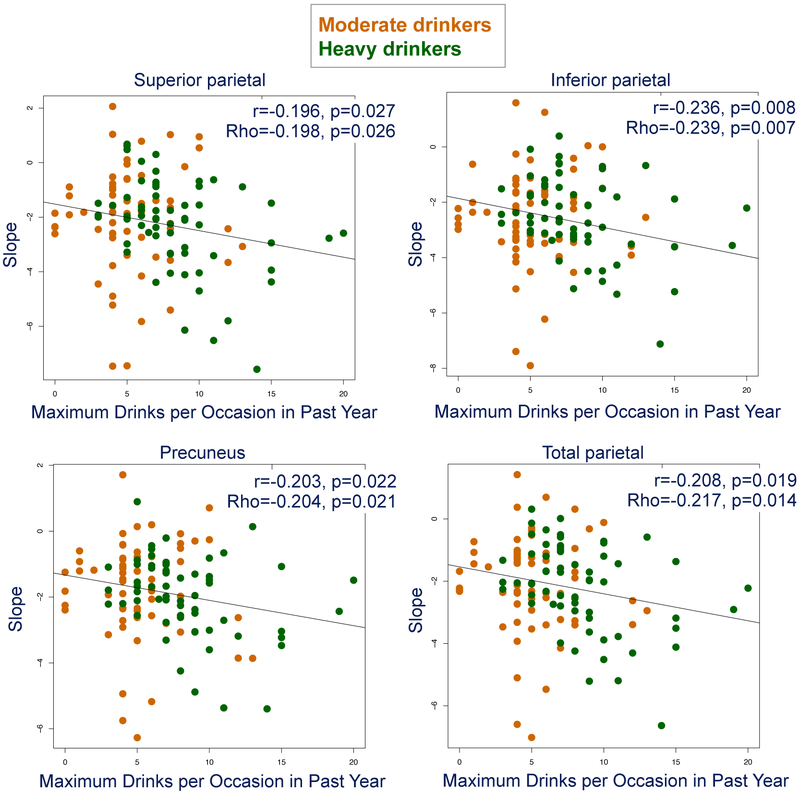
Exploratory tests yielded four correlations meeting our double criteria based on parametric and nonparametric analyses, where faster tissue loss correlated with more maximum drinks per occasion reported in the past year (Figure 6).
Figure 6.
Scatter plots, Pearson r, and Spearman Rho of slopes vs. maximum drinks per year for parietal regions of interest with heavy drinkers in green and moderate drinkers in orange.
Test of baseline group differences.
We tested whether the drinking youth had smaller regional brain volumes at baseline (cf., 29, 30). Analysis using a GLM, accounting for age, sex, site, ethnicity, and svol, yielded no differences (p≤.05) between baseline volumes of any ROIs for either the heavy or the moderate drinking groups compared with the no/low group. Examination of baseline volumes of the 34 ROIs identified one region as smaller (parahippocampal, p=.0459) and one as larger (middle temporal, p=.0454), but no group differences for the isthmus cingulate and pars triangularis, as found in (29); none of these differences was significant with correction for multiple comparisons.
Family history of alcoholism.
To examine the contribution of a positive family history of alcoholism to the observed cortical volume results, family history positive was defined as having at least one first-degree relative with a history of Alcohol Use Disorder (AUD). For each participant group (i.e., heavy, moderate, and no/low drinker), the slopes of family history positive vs. negative were compared with nonparametric (Mann-Whitney-Wilcoxon) tests. There were no significant differences based on family history within the no/low or moderate groups, but within the heavy-use group, the 21 family history positive participants had steeper slopes than the 41 family history negative participants for parietal (W=231, p=.003), occipital (W=255, p= .008), and total gray matter volumes (W=274, p=.019). Frontal (W=304, p=.061) and cingulate (W=303, p=.058) gray matter volumes showed the same pattern at trend levels.
Marijuana and Alcohol Co-Use.
Slopes of the 10 moderate plus 17 heavy drinkers with >50 marijuana occasions were compared with slopes of the remaining 100 drinkers who did not meet this marijuana criterion. Analysis of the primary ROIs yielded one group difference in the direction opposite to expectations: insular gray matter volume slopes were less negative in the alcohol+marijuana group than the alcohol only group (t=2.551, p=0.0141).
DISCUSSION
This longitudinal study revealed tissue-specific neuromaturational age-linked rates and patterns of regional volume regression and expansion over two years that occur in normally developing adolescents. For cortical gray matter, the general pattern followed a steeper trajectory of volume decline in early adolescence followed by a deceleration in this rate as youth approached young adulthood. Trajectories for both sexes were relatively constant (monotonic) although steeper in girls than boys. The parietal cortex exhibited the greatest change per year, with an average decline of about 2.5% per year in the youngest boys and about 3% per year in the youngest girls. Complementing the gray matter volume decline was evidence for growth of white matter volume, which grew at faster rates in the younger ages, especially in boys, and slowed in later adolescence and young adulthood. For example, central white matter volume expanded by about 1.5% annually in the younger adolescents but fell to less than 0.75% annual increases in the older adolescents. This dynamic growth pattern formed the context for addressing whether and how initiation of moderate or heavy drinking altered components of these developmental trajectories.
In search of evidence for a dose effect of amount drunk on volume trajectories, we combined two sets of quantitative criteria based on drinking patterns over the past year to distinguish moderate from heavy drinkers. The heavy drinking initiators showed significantly steeper slopes than the no/low drinking youth. In no case did the volume trajectories of the moderate drinkers differ statistically from the no/low drinkers even though their arithmetic means were intermediate between the no/low and heavy drinking groups for frontal cortical slopes. Modest support for a dose effect derives from correlations between steeper regional parietal slopes indicative of faster volume loss and the maximum number of drinks consumed per occasion in the past year. One speculative interpretation of this apparent acceleration of the pruning trend notable in young adolescent drinkers is an over-exuberance of the typical synaptic refinements, suggesting an alteration of progression into the later stages of neurodevelopment.
Although the heavy drinking youth met strict drinking criteria that significantly exceeded drinking reported in the no/low drinkers, we gathered data from the published tables that, on average, our heavy drinkers consumed alcohol at lower levels and less frequently than youth in two other longitudinal MRI studies of heavy drinking initiation. We estimated that the number of days drinking per year was 47 by the Luciana et al. study (15), 115 by the Squeglia et al. (16), and 43 for the heavy drinkers but only 15 for the moderate drinkers in the present study; also estimated were the number of drinks consumed per year: 268 by Luciana et al. and 157 for the heavy drinkers and 36 for the moderate drinkers herein. Taken together, this study and the two earlier longitudinal studies of more heavily drinking youth reported greater than normal age-related cortical thinning or volume reduction in frontal and cingulate cortices in two studies (Luciana et al. and the current study) and frontal cortex in all three studies. Developmentally, the frontal cortex is the last region to mature and may retain the plasticity for modification from environmental factors throughout late adolescence and into early adulthood.
The more finely grained parcellation analysis of cortex revealed substantially faster declines in the heavy than no/low drinkers involving a constellation of superior and caudal middle frontal and posterior cingulate cortices volumes. The posterior cingulate cortex has been found to have the highest cerebral blood perfusion to acute alcohol infusion measured with arterial spin labeling imaging (31). Given that this region has been observed as affected in heavily drinking youth (15, 16), it is tempting to speculate a relation between repeated acute regional perfusion experiences with high doses of alcohol and ultimate effects on local tissue volumes.
To the extent that alcohol histories documented retrospectively in interviews reflects accurate assessment of consumption, quantity/frequency metrics of alcohol consumption provide estimates of amount drunk. Exploration of available drinking variables (lifetime drinking days, lifetime drinks, lifetime binges, and maximum drinks per occasion in the past year) identified greater maximum drinks as a correlate of accelerating brain structural change slopes but only in the total and regional parietal volumes. These relations present novel support for an alcohol dose effect on volume declines in recently initiated, adolescent drinkers. The step-wise steeper slopes in no/low to moderate to heavy drinking youth indicating volume declines also suggest a dose-response effect, with the moderate drinkers falling statistically insignificantly between the other drinking groups, most apparent for frontal and total gray matter volumes (Figure 4). Other studies, too, have found maximum drinks per occasion of utility in understanding drinking patterns and their consequences. For example, a trajectory analysis of young adults' alcohol consumption in the Collaborative Study on the Genetics of Alcoholism (COGA) revealed the utility of maximum drinks per occasion in predicting drinking patterns (32). An escalation of maximum drinks has been found to be predictive of greater impulsivity/compusivity scores and disturbance of fronto-parietal control mechanisms (33).
The developmental trajectories of the no/low consuming participants are consistent with the general literature. The accelerated longitudinal study design and multi-site geographically widespread recruitment provided some assurance for broad age sampling over just a few years and a measure of generalizability. Nonetheless, even this controlled, longitudinal study design has limitations. The results remain limited in age sampling, demographic representation, especially with respect to family history of alcoholism, and finely-grained alcohol consumption quantification. Despite the prospective nature of this study, factors unmeasured at baseline could present pre-existing conditions underlying the ostensibly alcohol-related effects on brain structure. Indeed, having a positive family history of alcoholism may have compounded the abnormal slope accelerations observed in the youth who transitioned into heavy drinking.
An earlier study reported smaller brain volumes at baseline in youth who initiated drinking at hazardous levels compared with their low drinking controls (29), whereas we found no volume difference at baseline between our no/low and heavy drinkers. The former study had smaller samples, recruited participants at high risk for substance use disorders, under-represented female participants, and had a higher proportion of family history positive drinkers than our NCANDA sample. Recognizing these sampling differences, we believe that the lack of regional differences at baseline in the NCANDA sample is a strength, supporting the possibility that the greater decline in volumes over age in the drinkers was attributable to drinking rather than to pre-existing conditions.
In conclusion, these results provide evidence that initiation of heavy alcohol drinking during adolescence can disrupt normal, differential growth trajectories of selective cortical gray matter regions. Although significant effects on brain volumetric trajectories were not forthcoming in moderate drinkers, their intermediate position between no/low and heavy drinkers on many measures suggests a dose or threshold effect. A factor potentially contributing to the abnormally smaller cortical volumes, notably in frontal regions, may be alcohol-related acceleration of normal pruning mechanisms (34) in regions with high cerebral blood perfusion during acute alcohol (31, 35). Whether reduction in drinking reinstates the normal trajectories or acceleration in alcohol consumption to dependent levels results in further acceleration of gray matter volume declines and attenuation of white matter growth awaits continued longitudinal study of these NCANDA participants.
Supplementary Material
Disclosures and acknowledgments:
None of the authors have conflicts of interest with the reported data or their interpretation. This work was supported by the U.S. National Institute on Alcohol Abuse and Alcoholism with co-funding from the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Child Health and Human Development [NCANDA grant numbers: AA021697 (Multiple Principal Investigators (MPI), Drs. Pfefferbaum and Pohl), AA021695 (MPI, Drs. Brown and Tapert), AA021692 (MPI, Drs. Tapert and Brown), AA021696 (MPI, Drs. Colrain and Baker), AA021681 (PI, Dr. De Bellis), AA021690 (PI, Dr. Clark), AA021691 (PI, Dr. Nagel); K05 AA017168 and the Moldow Women's Hope and Healing Fund. (PI, Dr. Sullivan)].
REFERENCES
- 1.Jernigan TL, Brown TT, Hagler DJ Jr., Akshoomoff N, Bartsch H, Newman E, Thompson WK, Bloss CS, Murray SS, Schork N, Kennedy DN, Kuperman JM, McCabe C, Chung Y, Libiger O, Maddox M, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Sowell ER, Kenet T, Kaufmann WE, Mostofsky S, Amaral DG, Dale AM, Pediatric Imaging N, Genetics S. The Pediatric Imaging, Neurocognition, and Genetics (PING) Data Repository. Neuroimage. 2016;124:1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. [DOI] [PubMed] [Google Scholar]
- 3.Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wierenga LM, Langen M, Oranje B, Durston S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 2014;87:120–126. [DOI] [PubMed] [Google Scholar]
- 8.Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. NeuroImage. 2013;65:176–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan EV, Pfefferbaum A, Rohlfing T, Baker FC, Padilla ML, Colrain IM. Developmental change in regional brain structure over 7 months in early adolescence: Comparison of approaches for longitudinal atlas-based parcellation. Neuroimage. 2011;57:214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ducharme S, Albaugh MD, Nguyen TV, Hudziak JJ, Mateos-Perez JM, Labbe A, Evans AC, Karama S, Brain Development Cooperative. Trajectories of cortical thickness maturation in normal brain development--The importance of quality control procedures. Neuroimage. 2016;125:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, Rapoport JL. Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology. 2015;40:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raznahan A, Lerch JP, Lee N, Greenstein D, Wallace GL, Stockman M, Clasen L, Shaw PW, Giedd JN. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72:873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, Pipitone J, Chakravarty MM, Giedd JN. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc Natl Acad Sci U S A. 2014;111:1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luciana M, Collins PF, Muetzel RL, Lim KO. Effects of alcohol use initiation on brain structure in typically developing adolescents. Am J Drug Alcohol Abuse. 2013;39:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A. Brain development in heavy drinking adolescents. American Journal of Psychiatry. 2015;172:531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(SAMHSA) SAaMHSA: 2015 National Survey on Drug Use and Health (NSDUH). Substance Abuse and Mental Health Services Administration (SAMHSA); 2015. [Google Scholar]
- 18.Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, Nichols BN, Brown SA, Tapert SF, Cummins K, Thompson WK, Brumback T, Meloy MJ, Jernigan TL, Dale A, Colrain IM, Baker FC, Prouty D, De Bellis MD, Voyvodic JT, Clark DB, Luna B, Chung T, Nagel BJ, Sullivan EV. Adolescent Development of Cortical and White Matter Structure in the NCANDA Sample: Role of Sex, Ethnicity, Puberty, and Alcohol Drinking. Cereb Cortex. 2016;26:4101–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, De Bellis MD, Hooper SR, Clark DB, Chung T, Hasler BP, Colrain IM, Baker FC, Prouty D, Pfefferbaum A, Sullivan EV, Pohl KM, Rohlfing T, Nichols BN, Chu W, Tapert SF. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A multi-site study of adolescent development and substance use. Journal of Studies on Alcohol and Drugs. 2015;76:895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. NeuroImage. 2011;54:2582–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biological psychiatry. 2001;49:894–905. [DOI] [PubMed] [Google Scholar]
- 22.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. [DOI] [PubMed] [Google Scholar]
- 23.Cahalan D, Cisin IH, Crossley HM: American Drinking Practices: A National Study of Drinking Behavior and Attitudes. New Brunswick, N.J., Journal of Studies on Alcohol, Incorporated; 1969. [Google Scholar]
- 24.Sullivan EV, Brumback T, Tapert SF, Fama R, Prouty D, Brown SA, Cummins K, Thompson WK, Colrain IM, Baker FC, De Bellis MD, Hooper SR, Clark DB, Chung T, Nagel BJ, Nichols BN, Rohlfing T, Chu W, Pohl KM, Pfefferbaum A. Cognitive, emotion control, and motor performance of adolescents in the NCANDA study: Contributions from alcohol consumption, age, sex, ethnicity, and family history of addiction. Neuropsychology. 2016;30:449–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akshoomoff N, Newman E, Thompson WK, McCabe C, Bloss CS, Chang L, Amaral DG, Casey BJ, Ernst TM, Frazier JA, Gruen JR, Kaufmann WE, Kenet T, Kennedy DN, Libiger O, Mostofsky S, Murray SS, Sowell ER, Schork N, Dale AM, Jernigan TL. The NIH Toolbox Cognition Battery: results from a large normative developmental sample (PING). Neuropsychology. 2014;28:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. [DOI] [PubMed] [Google Scholar]
- 27.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. [DOI] [PubMed] [Google Scholar]
- 28.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 29.Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, Jernigan TL, Tapert SF. Brain volume reductions in adolescent heavy drinkers. Dev Cogn Neurosci. 2014;9:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brumback TY, Worley M, Nguyen-Louie TT, Squeglia LM, Jacobus J, Tapert SF. Neural predictors of alcohol use and psychopathology symptoms in adolescents. Dev Psychopathol. 2016;28:1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marxen M, Gan G, Schwarz D, Mennigen E, Pilhatsch M, Zimmermann US, Guenther M, Smolka MN. Acute effects of alcohol on brain perfusion monitored with arterial spin labeling magnetic resonance imaging in young adults. J Cereb Blood Flow Metab. 2014;34:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuckit MA, Smith TL, Danko GP, Bucholz KK, Agrawal A, Dick DM, Nurnberger JI, Kramer J, Hesselbrock M, Saunders G, Hesselbrock V. Predictors of subgroups based on maximum drinks per occasion over six years for 833 adolescents and young adults in COGA. J Stud Alcohol Drugs. 2014;75:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Worhunsky PD, Dager AD, Meda SA, Khadka S, Stevens MC, Austad CS, Raskin SA, Tennen H, Wood RM, Fallahi CR, Potenza MN, Pearlson GD. A Preliminary Prospective Study of an Escalation in 'Maximum Daily Drinks', Fronto-Parietal Circuitry and Impulsivity-Related Domains in Young Adult Drinkers. Neuropsychopharmacology. 2016;41:1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry. 2013;3:e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, Dewey S. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res. 1988;24:201–209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.