Background
Uterine corpus cancer is the most common invasive gynecologic cancer among United States (U.S.) women. Studies of endometrial cancers, which comprise approximately 90% of all uterine cancers, have identified numerous risk factors, many of which appear to reflect high levels of estrogens in the absence of sufficient progesterone. Recent advances have indicated that the disease is etiologically heterogeneous, consisting of at least two major subgroups. This heterogeneity extends to important racial differences in both incidence and survival, possibly partially attributable to genetic factors.
Descriptive Epidemiology
Uterine cancer incidence is highest in North America and Northern Europe, intermediate in Southern Europe and temperate South America, and lowest in Southern and Eastern Asia and most of Africa (Figure 1) (1). This likely reflects prevalence differences in risk factors, including obesity and reproductive patterns. In the U.S., uterine cancer is the fourth most frequently diagnosed cancer, with estimates of 63,230 diagnoses in 2018 (lifetime risk of 1 out of every 40 women) (2). The average annual age-adjusted incidence of uterine cancer from the Surveillance, Epidemiology and End Results Program (SEER) was 25.7 per 100,000 women between 2010–2014 (3). The disease is rare before the age of 45 years, but risk rises sharply among women of all races in their late 40s to middle 60s (Figure 2). Worldwide, uterine cancer ranked in 2012 as the sixth most common cancer, with 319,600 estimated cases (4).
Figure 1 –
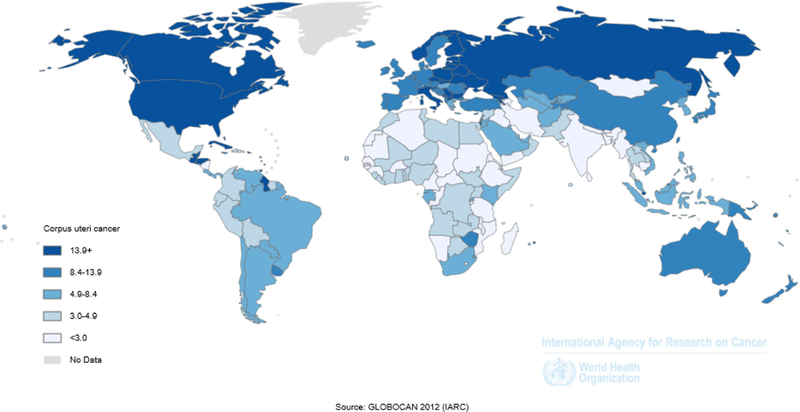
Age-standardized incidence rates for corpus uteri cancer, GLOBOCAN, 2012 shows the age-standardized incidence rates for corpus uteri cancer using data from GLOBOCAN 2012.
Figure 2 –

Age-specific uterine cancer incidence rates by race among U.S. women, SEER-18, 2003–2014 shows age-specific uterine cancer incidence rates among non-Hispanic White, Hispanic White, Black, American Indian/Alaskan Native, and Asian/Pacific Islander U.S. women using data from the SEER Program.
Dramatic changes in the incidence of uterine cancers have occurred over time. A marked increase in U.S. incidence peaked around 1975, a trend later linked with the widespread use of menopausal estrogens in the late 1960s and early 1970s (Figure 3). After a subsequent period of steady or declining incidence rates in many countries, endometrial cancer is again on the rise, mirroring increases in obesity prevalence (4, 5).
Figure 3 –
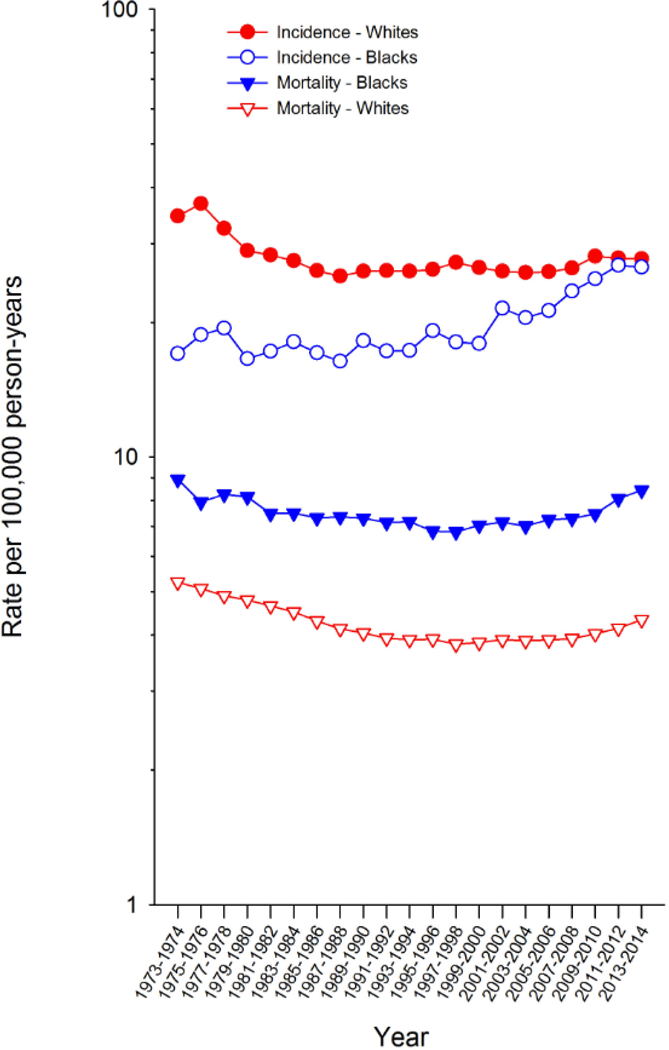
Trends in uterine cancer incidence and mortality among U.S. women, SEER-9, 1973–2014 shows uterine cancer incidence among White and Black U.S. women using data from the SEER Program.
In the U.S., age-adjusted mortality is 4.6 per 100,00 women, while in Europe mortality ranges between 2–4 per 100,000 (3, 6) (Figure 4). Similar to recent incidence increases, endometrial cancer mortality rates are also on the rise (4, 7). Overall, five-year survival is approximately 82%, which represents a marked increase since the 1960’s when it was 60% (8, 9). The distribution of uterine cancer stage, a strong prognostic factor, has remained stable (8, 10–12). Five-year survival is 95.3% for localized, 67.5% for regional, and 16.9% for distant-stage diseases (9).
Figure 4 –
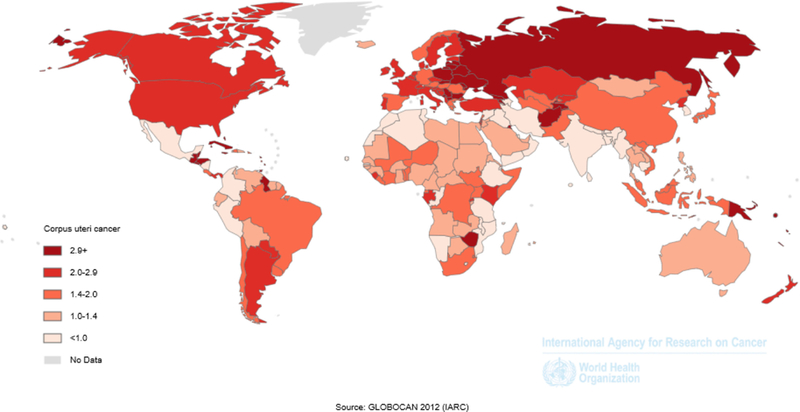
Age-standardized mortality rates for corpus uteri cancer, GLOBOCAN, 2012 shows the age-standardized mortality rates for corpus uteri cancer using data from GLOBOCAN 2012.
Disparities
Historically, endometrial cancer incidence was lower among black compared to white women; however, that gap has narrowed significantly over time (13–17). However, once hysterectomy rates are taken into account, incidence in blacks surpasses that of whites (18). Although the associations for established endometrial cancer risk factors among black and white women are similar (19), prevalence differences may partially explain the markedly higher incidence increases among blacks. Endometrial cancer mortality is twice as high among black compared to white women (8.1 vs. 4.2 per 100,000 women) and has been attributed to aggressive clinical characteristics, lower socioeconomic status, higher prevalence of comorbid conditions, poor patient-provider interactions, and inferior treatment (20). Although less frequently studied, Asian and Hispanic women have lower risks of endometrial cancer compared with white women; however, five-year survival is the same or better (17, 21).
Risk Factors (see Table 1 and Figure 5)
Table 1:
Summary of endometrial cancer risk factors, genetics, and biomarkers
| Domain | Factor | Estimated Relative Riska |
Heterogeneity of risk | Comments | Highest level of evidence |
Refs |
|---|---|---|---|---|---|---|
| Metabolic factors | Obesity | 2.0–5.0 | Association stronger for type I than II cancers | Each 5 kg/m2 increase in body mass index (BMI) is associated with a 62% increased risk | Cohort study | (22, 83) |
| Diabetes | 2.0 | No heterogeneity observed | Uncertain extent to which relations are confounded by obesity | Meta-analysis of cohort studies | (26, 83) | |
| Hypertension | 1.1–1.3 | Not examined | Association between hypertension and endometrial cancer was weaker, but still significant, among studies with adjustment for BMI | Meta-analysis of case-control and cohort studies | (27) | |
| Metabolic syndrome | 1.4–2.0 | No heterogeneity observed | Adjustment for overweight/obesity does not eliminate increased risks associated with metabolic syndrome factors | Meta-analysis of case-control and cohort studies | (29, 114) | |
| Polycystic ovary syndrome | 2.8 | Not examined | Uncertain extent to which relations are confounded by obesity | Meta-analysis of case-control studies | (28) | |
| Reproductive factors | Nulliparity | 3.0 | Association restricted to type I cancers | Further reductions for multi-parous women | Meta-analysis of case-control and cohort studies | (31, 115) |
| Infertility | 1.8 | No heterogeneity observed | Even after adjusting for nulliparity, infertile women had increased risk | Pooled analysis of case-control and cohort studies | (32) | |
| Early age at menarche | 1.5–2.0 | No heterogeneity observed | 4% reduction in risk per 2 years delay in menarcheal age | Meta-analysis of cohort studies | (33, 86) | |
| Late age at natural menopause | 1.5–2.2 | No heterogeneity observed | Pronounced risks among nonusers of menopausal hormones | Cohort studies | (86, 115, 116) | |
| Breastfeeding | 0.9 | No heterogeneity observed | Greatest reductions for long-term breastfeeding | Pooled analysis of case-control and cohort studies | (36) | |
| Contraceptives | Combination oral contraceptives | 0.3–0.5 | No heterogeneity observed | Risk reduction persists for > 30 years | Pooled analysis of case-control and cohort studies | (83, 86) |
| Intrauterine device use | 0.5–0.8 | Association stronger for type I than II cancers | More studies needed on the effects of progestin-releasing devices | Pooled analysis of case-control and cohort studies | (37, 38) | |
| Menopausal hormone therapy | Menopausal estrogens | 10.0–20.0 | Not examined | Highest risks for long-term and high dose users of unopposed estrogens | Cohort study | (39) |
| Menopausal estrogen plus progestins | 0.7 | Association stronger for type I than II cancers | Risk reduction is greatest for obese women | Randomized trial | (39, 42, 43) | |
| Tamoxifen use | High cumulative doses of tamoxifen | 2.2 | Non-endometrioid histology subtypes appear to be especially affected by tamoxifen | Endometrial cancer risks highest shortly after exposure | Randomized trial | (44, 117) |
| Lifestyle factors | Cigarette smoking | 0.5 | No heterogeneity observed | Effects of cigarette smoking are particularly strong among postmenopausal women and menopausal hormone users | Meta-analysis of case-control and cohort studies | (46, 83) |
| Moderate-to-vigorous physical activity | 0.8 | No heterogeneity observed | Inverse relation with physical activity restricted to overweight or obese women | Meta-analysis of case-control and cohort studies | (48, 86, 118) | |
| Family history | Family history | 1.8 | No heterogeneity observed | Association is independent of Lynch syndrome status | Meta-analysis of case-control and cohort studies | (73, 119) |
| High penetrance gene mutations | MLH1 | 18–54% lifetime risk | Not examined | (74–76) | ||
| MSH2 | 21–49% lifetime risk | Not examined | (74–76) | |||
| MSH6 | 16–61% lifetime risk | Not examined | (75, 76) | |||
| PMS2 | 12% lifetime risk | Not examined | (77) | |||
| EPCAM | 12% lifetime risk | Not examined | (78) | |||
| Low and moderate penetrance genes | 1.1–1.4 | Some SNP associations differ according to histology | (120) | |||
| Serum biomarkers | Estradiol and other endogenous estrogens | 2.0–6.2 | Some support for stronger relations with type I than II cancers | Associations persist after adjustment for body mass and show slightly stronger relations for type I than II cancers | (91) | |
| Insulin | Significant mean difference between endometrial cancer cases and controls: 33.94 | Not examined | This meta-analysis did not detect an association among studies restricted to postmenopausal women, possibly due to small numbers | (94) | ||
| C-peptide | Significant mean difference between endometrial cancer cases and controls: 0.14 | Not examined | A lack of information on fasting time since the last meal may have led to misclassification of C-peptide levels | (94) | ||
| Androgen | Postmenopausal: 1.7 Premenopausal: 0.9 |
Similar associations observed when restricted to women with type I | Higher circulating levels of androgens are associated with endometrial cancer among postmenopausal women | (88–90, 92, 93) | ||
| Inflammatory markers | SERPINE1: 2.4 VEGF-A: 2.6 Anti-inflammatory cytokines (IL13, IL21): 0.5–0.6 Pro-inflammatory cytokines (CCL3, IL1B, IL23): 0.5–0.6 |
No heterogeneity observed although the number of women with type II was small | Endometrial cancer risk was most pronounced among obese women with the highest inflammation score | (97) | ||
| Adiponectin | 0.5 | Not examined | Inverse associations were strongest among postmenopausal women, nulliparous women, and non-hormone users | (98) | ||
| Leptin | 2.2 | Not examined | Associations were strongest among non-hormone users, diabetic women, and in prospective studies | (98) |
Figure 5 –
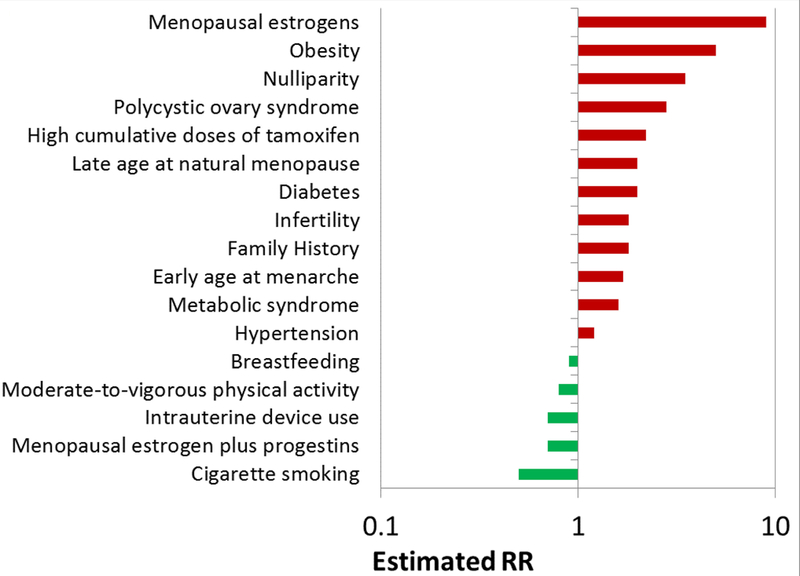
Summary of the magnitude of association for established endometrial cancer risk factors summarizes the magnitude of associations for established endometrial cancer risk factors. Risks are approximate and can vary depending on the extent of exposure.
Metabolic factors.
A strong risk factor for endometrial cancer is obesity, accounting for 40–50% of all U.S. cases (22, 23). Overall body size appears to be more important than body fat distribution (24). Women with obesity-associated diseases such as diabetes (25, 26), hypertension (27), and polycystic ovary syndrome (28) are also at elevated risk, although obesity may contribute to these relationships. Metabolic syndrome has also been associated with significant risk elevations, although to a lesser extent than obesity (29).
Reproductive factors.
Nulliparous women are at substantially higher risks than parous women (30, 31), with infertility additionally contributing to risk (32). Other established reproductive risk factors include young ages at menarche and/or late ages at menopause (30, 33), potentially reflecting increased numbers of lifetime ovulatory cycles (34). Breastfeeding has also recently emerged as a possible protective factor (35, 36).
Contraceptives.
The use of combination oral contraceptives has been linked with marked risk reductions which persist for more than 30 years after discontinuation. Intrauterine devices also appear to reduce endometrial cancer risk (37, 38).
Menopausal hormone therapy.
Menopausal hormones have been strongly linked with risk increases, particularly for extended usage of high-dose unopposed estrogens (39). Progestins cause regression of estrogen-induced endometrial hyperplasia, the presumed precursor of most endometrial cancers (40), leading to estrogens commonly being prescribed with a progestin (particularly among non-hysterectomized women). Sequential progestin use, i.e., <10 days per month, is associated with only slight risk reductions compared to unopposed estrogen use (41). However, continuous combination therapy reduces risk compared to non-hormone usage (39, 42). Associations of hormone usage are strongly modified by body mass index (BMI) (39, 43).
Tamoxifen use.
Clinical trials have demonstrated increased endometrial cancer risk among tamoxifen-treated breast cancer patients, with risks highest shortly after exposure, among those receiving high cumulative doses, and for histologies usually associated with a poor prognosis (44).
Lifestyle factors.
Cigarette smoking (45, 46) and moderate to active physical activity levels (47, 48) have been associated with reduced risks, relations that are independent of other risk factors, including obesity.
Other factors.
It remains less clear whether risk reductions associated with high levels of fruit and vegetable consumption and/or of micronutrients are independent risk factors (49–52). Higher dairy product intake (53), coffee consumption (54), and consumption of green, but not black, tea (55) may lead to risk reductions. High-fat diets (51, 52) and alcohol consumption (56) have not generally been associated with risk. Use of the anti-diabetic drug metformin (57) or aspirin (58) appear to slightly reduce risk.
Controversial risk factors.
Less accepted as potential risk factors are several occupational exposures (59, 60); talcum powder use (61–63); thyroid diseases, cholecystectomy and endometriosis (64–66); antidepressants, statins, and acetaminophen (67–69); endocrine disruptors (70); tubal ligation (71); and electromagnetic radiation (72).
Familial and genetic factors.
Elevated endometrial cancer risks have been noted among women with a first-degree family history of endometrial cancer (73). This could reflect familial obesity (genetic or environment) or inherited risk, such as Lynch syndrome, an autosomal dominant cancer predisposition syndrome attributed to germline mutations in one of several mismatch repair (MMR) genes. Specific mutations have been estimated to result in cumulative lifetime endometrial cancer risks ranging between 12–61% (74–78), with MSH6 showing the highest risks (79) (Table 1). However, the higher range estimates may reflect reliance on data from clinical cancer genetic cohorts that are biased to include patients with family histories of cancer. Although Lynch syndrome is associated with a high cumulative lifetime risk of endometrial cancer, the relative rarity of the condition translates to an attributable fraction of only 5%.
The genome-wide association study (GWAS) approach has identified 18 risk loci for endometrial cancer, which are modestly associated with risk (odds ratios 0.8–1.4) (80). Some risk loci are significant only for endometrioid cancers. Few rare variants have been identified through exome-wide association studies (81), but candidate gene studies (82) have identified a number of single nucleotide polymorphisms (SNPs) in genes that may possibly impact risk.
Etiologic Heterogeneity (see Tables 1 and 2)
Table 2:
Summary of associations of established risk factors with type and histology of endometrial cancer
| Risk factor | Type I | Type II | Endometrioid | Serous | Carcinosarcoma | Clear cell |
|---|---|---|---|---|---|---|
| Obesity | +++ | ++ | +++ | ++ | ++ | ++ |
| Diabetes | + | + | + | + | NA | NA |
| Metabolic syndrome | + | + | + | + | + | + |
| Nulliparity | ++ | + | ++ | + | + | NA |
| Infertility | + | + | ||||
| Early age at menarche | + | ++ | + | ++ | NA | NA |
| Late age at natural menopause | -- | NA | ||||
| Breastfeeding | - | NA | ||||
| Combination oral contraceptives | -- | -- | --- | NA | NA | -- |
| Intrauterine device use | - | NA | ||||
| Menopausal estrogen plus progestins | -- | NA | ||||
| High cumulative doses of tamoxifen | ++ | ++ | ||||
| Cigarette smoking | -- | -- | -- | -- | NA | |
| Moderate-to-vigorous physical activity | -- | -- | ||||
| Family history | + | ++ |
Red indicates that the factor is positively associated with risk of the particular subtype.
indicates a strong association (RR/OR ≥5.0),
a moderate association (RR/OR, 2–5), and
, a modest association (RR/OR <2).
Green indicates that the factor is negatively associated with risk of the particular subtype.
, a strong association (RR/OR ≤0.6);
, a moderate association (RR/OR, 0.6–0.8); and
, a modest association (RR/OR ≥0.8).
Blue (N/A) indicates the factor is not associated with risk of the particular subtype
Important heterogeneity has been noted between type I (predominantly endometrioid adenocarcinomas with a hormonally driven etiology) and type II (mainly non-endometrioid malignancies that occur frequently among older and non-white women) cancers. Several epidemiological studies have found that type II cancers are less strongly linked to classic risk factors, such as obesity, nulliparity and hormones (44, 83).
Stronger relationships of hormonal, reproductive, and anthropometric risk factors have been found for endometrioid endometrial cancers compared with serous, clear cell, mucinous, or mixed tumors (44, 83–86). Furthermore, the Cancer Genome Atlas (TCGA) study has identified four molecular subtypes of endometrial cancer: polymerase Ɛ (POLE) ultramutated, microsatellite instability hypermutated, copy-number low, and copy-number high clusters (87). A comprehensive evaluation of endometrial cancer risk factors according to TCGA subtype has not yet been conducted.
Biologic Underpinnings of Identified Risk Factors
Estrogens are strongly related to risk (Table 1) (88–90), with one study showing generalized uterotropic activity of both parent estrogens and metabolites (91). Circulating androgens, the main source of estrogens in postmenopausal women, have also been linked with increased risk (88–90, 92, 93). Consistent with an association between diabetes and endometrial cancer risk, insulin and c-peptide have been demonstrated to be elevated among women with endometrial cancer (94). Insulin-like growth factor 1 (IGF)-1 and the IGF binding proteins are less consistently linked with risk (95, 96). Risk has also been related to circulating levels of inflammatory biomarkers (97) and with several obesity-related hormones (98).
Risk Prediction Models
Two risk prediction models, one developed in U.S.-based cohorts (99) and the other in a European cohort (100), demonstrated moderate discriminatory ability for established endometrial cancer risk factors (respective discrimination assessed by the area under the curve of 0.68 and 0.77). In the latter model, the addition of pre-diagnostic serum biomarkers only modestly (1.7%) increased discrimination (101).
Future Trends
Projection models indicate that endometrial cancer incidence will continue to rise, mainly as a consequence of rising obesity prevalence (7, 102). Changes in the distribution of other endometrial cancer risk factors also contribute to the projected growth in incidence, including increases in diabetes and metabolic syndrome (103, 104), declines in use of combination hormone therapy (5), and decreases in childbearing and smoking (105, 106). Moreover, hysterectomy for benign conditions has declined in recent decades, particularly among whites, contributing to more at-risk women (18, 107). In the next decade, mortality rates are also projected to increase (108).
Prevention
Primary prevention efforts focused on weight loss or use of medications are attractive prevention strategies. For high-risk patients, bariatric surgery is associated with a 44% reduced risk of developing endometrial cancer (109). Among Lynch syndrome patients, there is some evidence that oral contraceptive use may reduce risk (110).
Screening
Endometrial cancer screening is not recommended for women in the general population (111). Studies evaluating the use of endometrial biopsy and/or transvaginal ultrasound have generally shown low detection specificity (112). Nonetheless, the American Cancer Society Cancer recommends annual screening for Lynch syndrome patients with endometrial biopsy beginning at age 35 years. Development of early detection blood-based biomarkers are being explored (113).
Future Directions
Although considered an indolent tumor, the rapid increase in both endometrial cancer incidence and mortality warrants additional etiologic and prevention research. While progress has been made in identifying risk factors for the most common endometrial cancer subtype, this has not translated into effective primary prevention strategies. Future efforts should be directed at reducing the prevalence of modifiable risk factors (e.g., obesity). Additional research is needed to identify risk factors for aggressive endometrial cancer subtypes, particularly among black women.
To favorably impact survival, research on screening modalities to identify endometrial cancer at early stages is needed. Currently, screening in the general population is not recommended, but efforts to identify high-risk women could be beneficial.
Abbreviations list:
- U.S.
United States
- SEER
Surveillance, Epidemiology and End Results Program
- GWAS
genome-wide association study
- TCGA
The Cancer Genome Atlas
- POLE
polymerase Ɛ
- SNPs
single nucleotide polymorphisms
- IGF
insulin like growth factor
Footnotes
Conflict of interest: The authors have nothing to disclose.
References:
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. [ [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 4.Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol Biomarkers Prev. 2017;26(4):444–57. [DOI] [PubMed] [Google Scholar]
- 5.Wartko P, Sherman ME, Yang HP, Felix AS, Brinton LA, Trabert B. Recent changes in endometrial cancer trends among menopausal-age U.S. women. Cancer epidemiology. 2013;37(4):374–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiderpass E, Antoine J, Bray FI, Oh JK, Arbyn M. Trends in corpus uteri cancer mortality in member states of the European Union. Eur J Cancer. 2014;50(9):1675–84. [DOI] [PubMed] [Google Scholar]
- 7.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–21. [DOI] [PubMed] [Google Scholar]
- 8.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2006;95 Suppl 1:S105–43. [DOI] [PubMed] [Google Scholar]
- 9.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA: a cancer journal for clinicians. 2014;64(4):252–71. [DOI] [PubMed] [Google Scholar]
- 10.Ueda SM, Kapp DS, Cheung MK, Shin JY, Osann K, Husain A, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obstet Gynecol. 2008;198(2):218 e1–6. [DOI] [PubMed] [Google Scholar]
- 11.Creasman WT, Ali S, Mutch DG, Zaino RJ, Powell MA, Mannel RS, et al. Surgical-pathological findings in type 1 and 2 endometrial cancer: An NRG Oncology/Gynecologic Oncology Group study on GOG-210 protocol. Gynecol Oncol. 2017;145(3):519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright JD, Fiorelli J, Schiff PB, Burke WM, Kansler AL, Cohen CJ, et al. Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time. Cancer. 2009;115(6):1276–85. [DOI] [PubMed] [Google Scholar]
- 13.Farley J, Risinger JI, Rose GS, Maxwell GL. Racial disparities in blacks with gynecologic cancers. Cancer. 2007;110(2):234–43. [DOI] [PubMed] [Google Scholar]
- 14.Allard JE, Maxwell GL. Race disparities between black and white women in the incidence, treatment, and prognosis of endometrial cancer. Cancer Control. 2009;16(1):53–6. [DOI] [PubMed] [Google Scholar]
- 15.Long B, Liu FW, Bristow RE. Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States. Gynecol Oncol. 2013;130(3):652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: a report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol. 2014;133(2):353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R. The Growing Burden of Endometrial Cancer: A Major Racial Disparity Affecting Black Women. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1407–15. [DOI] [PubMed] [Google Scholar]
- 18.Jamison PM, Noone AM, Ries LA, Lee NC, Edwards BK. Trends in endometrial cancer incidence by race and histology with a correction for the prevalence of hysterectomy, SEER 1992 to 2008. Cancer Epidemiol Biomarkers Prev. 2013;22(2):233–41. [DOI] [PubMed] [Google Scholar]
- 19.Cote ML, Alhajj T, Ruterbusch JJ, Bernstein L, Brinton LA, Blot WJ, et al. Risk factors for endometrial cancer in black and white women: a pooled analysis from the Epidemiology of Endometrial Cancer Consortium (E2C2). Cancer causes & control : CCC. 2015;26(2):287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. Journal of the American College of Surgeons. 2010;211(1):105–13. [DOI] [PubMed] [Google Scholar]
- 21.Setiawan VW, Pike MC, Kolonel LN, Nomura AM, Goodman MT, Henderson BE. Racial/ethnic differences in endometrial cancer risk: the multiethnic cohort study. Am J Epidemiol. 2007;165(3):262–70. [DOI] [PubMed] [Google Scholar]
- 22.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onstad MA, Schmandt RE, Lu KH. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J Clin Oncol. 2016;34(35):4225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ju W, Kim HJ, Hankinson SE, De Vivo I, Cho E. Prospective study of body fat distribution and the risk of endometrial cancer. Cancer epidemiology. 2015;39(4):567–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. Bmj. 2015;350:g7607. [DOI] [PubMed] [Google Scholar]
- 26.Liao C, Zhang D, Mungo C, Andrew Tompkins D, Zeidan AM. Is diabetes mellitus associated with increased incidence and disease-specific mortality in endometrial cancer? A systematic review and meta-analysis of cohort studies. Gynecol Oncol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aune D, Sen A, Vatten LJ. Hypertension and the risk of endometrial cancer: a systematic review and meta-analysis of case-control and cohort studies. Scientific reports. 2017;7:44808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Human reproduction update. 2014;20(5):748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Giugliano D. Metabolic syndrome and endometrial cancer: a meta-analysis. Endocrine. 2014;45(1):28–36. [DOI] [PubMed] [Google Scholar]
- 30.Dossus L, Allen N, Kaaks R, Bakken K, Lund E, Tjonneland A, et al. Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2010;127(2):442–51. [DOI] [PubMed] [Google Scholar]
- 31.Wu QJ, Li YY, Tu C, Zhu J, Qian KQ, Feng TB, et al. Parity and endometrial cancer risk: a meta-analysis of epidemiological studies. Scientific reports. 2015;5:14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang HP, Cook LS, Weiderpass E, Adami HO, Anderson KE, Cai H, et al. Infertility and incident endometrial cancer risk: a pooled analysis from the epidemiology of endometrial cancer consortium (E2C2). Br J Cancer. 2015;112(5):925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong TT, Wang YL, Ma XX. Age at menarche and endometrial cancer risk: a dose-response meta-analysis of prospective studies. Scientific reports. 2015;5:14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang HP, Murphy KR, Pfeiffer RM, George N, Garcia-Closas M, Lissowska J, et al. Lifetime Number of Ovulatory Cycles and Risks of Ovarian and Endometrial Cancer Among Postmenopausal Women. Am J Epidemiol. 2016;183(9):800–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan SJ, Na R, Johnatty SE, Wise LA, Adami HO, Brinton LA, et al. Breastfeeding and Endometrial Cancer Risk: An Analysis From the Epidemiology of Endometrial Cancer Consortium. Obstet Gynecol. 2017;129(6):1059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhan B, Liu X, Li F, Zhang D. Breastfeeding and the incidence of endometrial cancer: A meta-analysis. Oncotarget. 2015;6(35):38398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Felix AS, Gaudet MM, La Vecchia C, Nagle CM, Shu XO, Weiderpass E, et al. Intrauterine devices and endometrial cancer risk: a pooled analysis of the Epidemiology of Endometrial Cancer Consortium. Int J Cancer. 2015;136(5):E410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beining RM, Dennis LK, Smith EM, Dokras A. Meta-analysis of intrauterine device use and risk of endometrial cancer. Ann Epidemiol. 2008;18(6):492–9. [DOI] [PubMed] [Google Scholar]
- 39.Beral V, Bull D, Reeves G, Million Women Study C. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365(9470):1543–51. [DOI] [PubMed] [Google Scholar]
- 40.Lacey JV Jr., Sherman ME, Rush BB, Ronnett BM, Ioffe OB, Duggan MA, et al. Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. J Clin Oncol. 2010;28(5):788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Razavi P, Pike MC, Horn-Ross PL, Templeman C, Bernstein L, Ursin G. Long-term postmenopausal hormone therapy and endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(2):475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chlebowski RT, Anderson GL, Sarto GE, Haque R, Runowicz CD, Aragaki AK, et al. Continuous Combined Estrogen Plus Progestin and Endometrial Cancer: The Women’s Health Initiative Randomized Trial. J Natl Cancer Inst. 2016;108(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trabert B, Wentzensen N, Yang HP, Sherman ME, Hollenbeck AR, Park Y, et al. Is estrogen plus progestin menopausal hormone therapy safe with respect to endometrial cancer risk? Int J Cancer. 2013;132(2):417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brinton LA, Felix AS, McMeekin DS, Creasman WT, Sherman ME, Mutch D, et al. Etiologic heterogeneity in endometrial cancer: Evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013;129(2):277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang HP, Brinton LA, Platz EA, Lissowska J, Lacey JV Jr., Sherman ME, et al. Active and passive cigarette smoking and the risk of endometrial cancer in Poland. Eur J Cancer. 2010;46(4):690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou B, Yang L, Sun Q, Cong R, Gu H, Tang N, et al. Cigarette smoking and the risk of endometrial cancer: a meta-analysis. The American journal of medicine. 2008;121(6):501–8 e3. [DOI] [PubMed] [Google Scholar]
- 47.Moore SC, Gierach GL, Schatzkin A, Matthews CE. Physical activity, sedentary behaviours, and the prevention of endometrial cancer. Br J Cancer. 2010;103(7):933–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmid D, Behrens G, Keimling M, Jochem C, Ricci C, Leitzmann M. A systematic review and meta-analysis of physical activity and endometrial cancer risk. European journal of epidemiology. 2015;30(5):397–412. [DOI] [PubMed] [Google Scholar]
- 49.Biel RK, Csizmadi I, Cook LS, Courneya KS, Magliocco AM, Friedenreich CM. Risk of endometrial cancer in relation to individual nutrients from diet and supplements. Public health nutrition. 2011;14(11):1948–60. [DOI] [PubMed] [Google Scholar]
- 50.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Fruits and vegetables and endometrial cancer risk: a systematic literature review and meta-analysis. Nutrition and cancer. 2007;58(1):6–21. [DOI] [PubMed] [Google Scholar]
- 51.Prentice RL, Thomson CA, Caan B, Hubbell FA, Anderson GL, Beresford SA, et al. Low-fat dietary pattern and cancer incidence in the Women’s Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst. 2007;99(20):1534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao J, Lyu C, Gao J, Du L, Shan B, Zhang H, et al. Dietary fat intake and endometrial cancer risk: A dose response meta-analysis. Medicine. 2016;95(27):e4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X, Zhao J, Li P, Gao Y. Dairy Products Intake and Endometrial Cancer Risk: A Meta-Analysis of Observational Studies. Nutrients. 2017;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lafranconi A, Micek A, Galvano F, Rossetti S, Del Pup L, Berretta M, et al. Coffee Decreases the Risk of Endometrial Cancer: A Dose-Response Meta-Analysis of Prospective Cohort Studies. Nutrients. 2017;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Q, Li H, Zhou JG, Ma Y, Wu T, Ma H. Green tea, black tea consumption and risk of endometrial cancer: a systematic review and meta-analysis. Archives of gynecology and obstetrics. 2016;293(1):143–55. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Q, Guo P, Li H, Chen XD. Does alcohol consumption modify the risk of endometrial cancer? A dose-response meta-analysis of prospective studies. Archives of gynecology and obstetrics. 2017;295(2):467–79. [DOI] [PubMed] [Google Scholar]
- 57.Tang YL, Zhu LY, Li Y, Yu J, Wang J, Zeng XX, et al. Metformin Use Is Associated with Reduced Incidence and Improved Survival of Endometrial Cancer: A Meta-Analysis. BioMed research international. 2017;2017:5905384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verdoodt F, Friis S, Dehlendorff C, Albieri V, Kjaer SK. Non-steroidal anti-inflammatory drug use and risk of endometrial cancer: A systematic review and meta-analysis of observational studies. Gynecol Oncol. 2016;140(2):352–8. [DOI] [PubMed] [Google Scholar]
- 59.Weiderpass E, Pukkala E, Vasama-Neuvonen K, Kauppinen T, Vainio H, Paakkulainen H, et al. Occupational exposures and cancers of the endometrium and cervix uteri in Finland. American journal of industrial medicine. 2001;39(6):572–80. [DOI] [PubMed] [Google Scholar]
- 60.Wernli KJ, Ray RM, Gao DL, Fitzgibbons ED, Camp JE, Astrakianakis G, et al. Occupational risk factors for endometrial cancer among textile workers in Shanghai, China. American journal of industrial medicine. 2008;51(9):673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karageorgi S, Gates MA, Hankinson SE, De Vivo I. Perineal use of talcum powder and endometrial cancer risk. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neill AS, Nagle CM, Spurdle AB, Webb PM. Use of talcum powder and endometrial cancer risk. Cancer causes & control : CCC. 2012;23(3):513–9. [DOI] [PubMed] [Google Scholar]
- 63.Crawford L, Reeves KW, Luisi N, Balasubramanian R, Sturgeon SR. Perineal powder use and risk of endometrial cancer in postmenopausal women. Cancer causes & control : CCC. 2012;23(10):1673–80. [DOI] [PubMed] [Google Scholar]
- 64.Fortuny J, Sima C, Bayuga S, Wilcox H, Pulick K, Faulkner S, et al. Risk of endometrial cancer in relation to medical conditions and medication use. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poole EM, Lin WT, Kvaskoff M, De Vivo I, Terry KL, Missmer SA. Endometriosis and risk of ovarian and endometrial cancers in a large prospective cohort of U.S. nurses. Cancer causes & control : CCC. 2017;28(5):437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morimoto LM, Newcomb PA, Hampton JM, Trentham-Dietz A. Cholecystectomy and endometrial cancer: a marker of long-term elevated estrogen exposure? Int J Gynecol Cancer. 2006;16(3):1348–53. [DOI] [PubMed] [Google Scholar]
- 67.Lin CF, Chan HL, Hsieh YH, Liang HY, Chiu WC, Huang KY, et al. Endometrial cancer and antidepressants: A nationwide population-based study. Medicine. 2016;95(29):e4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sperling CD, Verdoodt F, Friis S, Dehlendorff C, Kjaer SK. Statin use and risk of endometrial cancer: a nationwide registry-based case-control study. Acta obstetricia et gynecologica Scandinavica. 2017;96(2):144–9. [DOI] [PubMed] [Google Scholar]
- 69.Ding YY, Yao P, Verma S, Han ZK, Hong T, Zhu YQ, et al. Use of acetaminophen and risk of endometrial cancer: evidence from observational studies. Oncotarget. 2017;8(21):34643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weiderpass E, Adami HO, Baron JA, Wicklund-Glynn A, Aune M, Atuma S, et al. Organochlorines and endometrial cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9(5):487–93. [PubMed] [Google Scholar]
- 71.Winer I, Lehman A, Wactawski-Wende J, Robinson R, Simon M, Cote M. Tubal Ligation and Risk of Endometrial Cancer: Findings From the Women’s Health Initiative. Int J Gynecol Cancer. 2016;26(3):464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abel EL, Hendrix SL, McNeeley GS, O’Leary ES, Mossavar-Rahmani Y, Johnson SR, et al. Use of electric blankets and association with prevalence of endometrial cancer. Eur J Cancer Prev. 2007;16(3):243–50. [DOI] [PubMed] [Google Scholar]
- 73.Win AK, Reece JC, Ryan S. Family history and risk of endometrial cancer: a systematic review and meta-analysis. Obstet Gynecol. 2015;125(1):89–98. [DOI] [PubMed] [Google Scholar]
- 74.Dowty JG, Win AK, Buchanan DD, Lindor NM, Macrae FA, Clendenning M, et al. Cancer risks for MLH1 and MSH2 mutation carriers. Human mutation. 2013;34(3):490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonadona V, Bonaiti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA : the journal of the American Medical Association. 2011;305(22):2304–10. [DOI] [PubMed] [Google Scholar]
- 76.Ramsoekh D, Wagner A, van Leerdam ME, Dooijes D, Tops CM, Steyerberg EW, et al. Cancer risk in MLH1, MSH2 and MSH6 mutation carriers; different risk profiles may influence clinical management. Hereditary cancer in clinical practice. 2009;7(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.ten Broeke SW, Brohet RM, Tops CM, van der Klift HM, Velthuizen ME, Bernstein I, et al. Lynch syndrome caused by germline PMS2 mutations: delineating the cancer risk. J Clin Oncol. 2015;33(4):319–25. [DOI] [PubMed] [Google Scholar]
- 78.Kempers MJ, Kuiper RP, Ockeloen CW, Chappuis PO, Hutter P, Rahner N, et al. Risk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: a cohort study. Lancet Oncol. 2011;12(1):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sijmons RH, Hofstra RM. Review: Clinical aspects of hereditary DNA Mismatch repair gene mutations. DNA repair. 2016;38:155–62. [DOI] [PubMed] [Google Scholar]
- 80.O’Mara TA, Glubb DM, Amant F, Annibali D, Ashton K, Attia J, et al. Identification of ten new susceptibility loci for endometrial cancer. Nature genetics. 2018;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen MM, Crous-Bou M, Setiawan VW, Prescott J, Olson SH, Wentzensen N, et al. Exome-wide association study of endometrial cancer in a multiethnic population. PloS one. 2014;9(5):e97045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meyer LA, Westin SN, Lu KH, Milam MR. Genetic polymorphisms and endometrial cancer risk. Expert review of anticancer therapy. 2008;8(7):1159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31(20):2607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sherman ME, Sturgeon S, Brinton LA, Potischman N, Kurman RJ, Berman ML, et al. Risk factors and hormone levels in patients with serous and endometrioid uterine carcinomas. Mod Pathol. 1997;10(10):963–8. [PubMed] [Google Scholar]
- 85.Felix AS, Weissfeld JL, Stone RA, Bowser R, Chivukula M, Edwards RP, et al. Factors associated with Type I and Type II endometrial cancer. Cancer causes & control : CCC. 2010;21(11):1851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang HP, Wentzensen N, Trabert B, Gierach GL, Felix AS, Gunter MJ, et al. Endometrial cancer risk factors by 2 main histologic subtypes: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2013;177(2):142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Allen NE, Key TJ, Dossus L, Rinaldi S, Cust A, Lukanova A, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer. 2008;15(2):485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lukanova A, Lundin E, Micheli A, Arslan A, Ferrari P, Rinaldi S, et al. Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. Int J Cancer. 2004;108(3):425–32. [DOI] [PubMed] [Google Scholar]
- 90.Zeleniuch-Jacquotte A, Akhmedkhanov A, Kato I, Koenig KL, Shore RE, Kim MY, et al. Postmenopausal endogenous oestrogens and risk of endometrial cancer: results of a prospective study. Br J Cancer. 2001;84(7):975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brinton LA, Trabert B, Anderson GL, Falk RT, Felix AS, Fuhrman BJ, et al. Serum Estrogens and Estrogen Metabolites and Endometrial Cancer Risk among Postmenopausal Women. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Potischman N, Hoover RN, Brinton LA, Siiteri P, Dorgan JF, Swanson CA, et al. Case-control study of endogenous steroid hormones and endometrial cancer. J Natl Cancer Inst. 1996;88(16):1127–35. [DOI] [PubMed] [Google Scholar]
- 93.Clendenen TV, Hertzmark K, Koenig KL, Lundin E, Rinaldi S, Johnson T, et al. Premenopausal Circulating Androgens and Risk of Endometrial Cancer: results of a Prospective Study. Hormones & cancer. 2016;7(3):178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hernandez AV, Pasupuleti V, Benites-Zapata VA, Thota P, Deshpande A, Perez-Lopez FR. Insulin resistance and endometrial cancer risk: A systematic review and meta-analysis. Eur J Cancer. 2015;51(18):2747–58. [DOI] [PubMed] [Google Scholar]
- 95.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Manson JE, Li J, et al. A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(4):921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhan Y, Wang J, Ma Y, Liu Z, Xu H, Lu S, et al. Serum insulin-like, growth factor binding protein-related protein 1 (IGFBP-rP1) and endometrial cancer risk in Chinese women. Int J Cancer. 2013;132(2):411–6. [DOI] [PubMed] [Google Scholar]
- 97.Trabert B, Eldridge RC, Pfeiffer RM, Shiels MS, Kemp TJ, Guillemette C, et al. Pre-diagnostic circulating inflammation markers and endometrial cancer risk in the Prostate, Lung, Colorectal and Ovarian Cancer (PLCO) Screening Trial. Int J Cancer. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gong TT, Wu QJ, Wang YL, Ma XX. Circulating adiponectin, leptin and adiponectin-leptin ratio and endometrial cancer risk: Evidence from a meta-analysis of epidemiologic studies. Int J Cancer. 2015;137(8):1967–78. [DOI] [PubMed] [Google Scholar]
- 99.Pfeiffer RM, Park Y, Kreimer AR, Lacey JV Jr., Pee D, Greenlee RT, et al. Risk prediction for breast, endometrial, and ovarian cancer in white women aged 50 y or older: derivation and validation from population-based cohort studies. PLoS medicine. 2013;10(7):e1001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Husing A, Dossus L, Ferrari P, Tjonneland A, Hansen L, Fagherazzi G, et al. An epidemiological model for prediction of endometrial cancer risk in Europe. European journal of epidemiology. 2016;31(1):51–60. [DOI] [PubMed] [Google Scholar]
- 101.Fortner RT, Husing A, Kuhn T, Konar M, Overvad K, Tjonneland A, et al. Endometrial cancer risk prediction including serum-based biomarkers: results from the EPIC cohort. Int J Cancer. 2017;140(6):1317–23. [DOI] [PubMed] [Google Scholar]
- 102.Sheikh MA, Althouse AD, Freese KE, Soisson S, Edwards RP, Welburn S, et al. USA endometrial cancer projections to 2030: should we be concerned? Future oncology. 2014;10(16):2561–8. [DOI] [PubMed] [Google Scholar]
- 103.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA : the journal of the American Medical Association. 2015;314(10):1021–9. [DOI] [PubMed] [Google Scholar]
- 104.Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999–2006. Diabetes care. 2011;34(1):216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Curtin SC, Abma JC, Ventura SJ, Henshaw SK. Pregnancy rates for U.S. women continue to drop. NCHS data brief. 2013(136):1–8. [PubMed] [Google Scholar]
- 106.Jamal A, Agaku I, O’Connor E, King B, Kenemer J, Neff L. Current Cigarette Smoking Among Adults — United States, 2005–2013. Morbidity and Mortality Weekly Report. 2014;63(47):4. [PMC free article] [PubMed] [Google Scholar]
- 107.Wright JD, Herzog TJ, Tsui J, Ananth CV, Lewin SN, Lu YS, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 Pt 1):233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Anveden A, Taube M, Peltonen M, Jacobson P, Andersson-Assarsson JC, Sjoholm K, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145(2):224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu KH, Loose DS, Yates MS, Nogueras-Gonzalez GM, Munsell MF, Chen LM, et al. Prospective multicenter randomized intermediate biomarker study of oral contraceptive versus depo-provera for prevention of endometrial cancer in women with Lynch syndrome. Cancer prevention research. 2013;6(8):774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jacobs I, Gentry-Maharaj A, Burnell M, Manchanda R, Singh N, Sharma A, et al. Sensitivity of transvaginal ultrasound screening for endometrial cancer in postmenopausal women: a case-control study within the UKCTOCS cohort. Lancet Oncol. 2011;12(1):38–48. [DOI] [PubMed] [Google Scholar]
- 112.Sonoda Y, Barakat RR. Screening and the prevention of gynecologic cancer: endometrial cancer. Best practice & research Clinical obstetrics & gynaecology. 2006;20(2):363–77. [DOI] [PubMed] [Google Scholar]
- 113.Martinez-Garcia E, Lopez-Gil C, Campoy I, Vallve J, Coll E, Cabrera S, et al. Advances in endometrial cancer protein biomarkers for use in the clinic. Expert review of proteomics. 2018;15(1):81–99. [DOI] [PubMed] [Google Scholar]
- 114.Trabert B, Wentzensen N, Felix AS, Yang HP, Sherman ME, Brinton LA. Metabolic syndrome and risk of endometrial cancer in the united states: a study in the SEER-medicare linked database. Cancer Epidemiol Biomarkers Prev. 2015;24(1):261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dossus L, Allen N, Kaaks R, Bakken K, Lund E, Tjonneland A, et al. Reproductive risk factors and endometrial cancer: The European prospective investigation into cancer and nutrition. Int J Cancer. 2009. [DOI] [PubMed] [Google Scholar]
- 116.Karageorgi S, Hankinson SE, Kraft P, De Vivo I. Reproductive factors and postmenopausal hormone use in relation to endometrial cancer risk in the Nurses’ Health Study cohort 1976–2004. Int J Cancer. 2010;126(1):208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Keum N, Ju W, Lee DH, Ding EL, Hsieh CC, Goodman JE, et al. Leisure-time physical activity and endometrial cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer. 2014;135(3):682–94. [DOI] [PubMed] [Google Scholar]
- 119.Johnatty SE, Tan YY, Buchanan DD, Bowman M, Walters RJ, Obermair A, et al. Family history of cancer predicts endometrial cancer risk independently of Lynch Syndrome: Implications for genetic counselling. Gynecol Oncol. 2017;147(2):381–7. [DOI] [PubMed] [Google Scholar]
- 120.Spurdle AB, Thompson DJ, Ahmed S, Ferguson K, Healey CS, O’Mara T, et al. Genome-wide association study identifies a common variant associated with risk of endometrial cancer. Nature genetics. 2011;43(5):451–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


