Abstract
BACKGROUND
The BRAF mutation has been identified as a potent target for the treatment of metastatic melanoma and BRAF inhibitors (BRAFi) have demonstrated promising results against melanoma brain metastases (BM).
OBJECTIVE
To further investigate the effectiveness of this combined treatment regimen.
METHODS
In this multicenter retrospective cohort study, 198 patients with known BRAF mutation status and treated with stereotactic radiosurgery (SRS) between 2011 and 2015 were identified. Kaplan–Meier methodology and multivariate regression analysis was then used to compare survival based on each parameter.
RESULTS
The median survival after the diagnosis of BM in patients with BRAF mutation who received BRAFi was increased compared to survival in patients with wild-type BRAF (BRAF wt). In multivariate analysis, the BRAF mutation was an independent, positive prognostic factor with a hazard ratio of 0.59. BRAF mutated Patients who received BRAFi following SRS had improved survival compared to patients who received it before (P < .001) or concurrently (P = .007). PD-1 inhibitors improved survival, with more pronounced effect in patients not carrying the BRAF mutation. Among the patients who were treated with BRAFi, 10.4% developed intracerebral hematoma (ICH), in comparison to 3% of patients who were not treated with BRAFi (P = .03).
CONCLUSION
In the setting of widespread use of BRAFi, the presence of a BRAF mutation is an independent predictor of better prognosis in patients with melanoma BM that underwent SRS. The effect of BRAFi is optimal when treatment is initiated at least 1 wk following SRS. BRAFi may increase the frequency of asymptomatic ICH.
Keywords: BRAF, Melanoma, Brain metastasis, Stereotactic radiosurgery, BRAF inhibitors
ABBREVIATIONS
- BM
brain metastases
- BRAFi
BRAF inhibitors
- CI
confidence interval
- CH
intracerebral hematoma
- KPS
karnofsky performance status
- OS
overall survival
- SRS
stereotactic radiosurgery
- WBRT
whole brain radiation therapy
Malignant melanoma is responsible for 75% of all skin cancer-related deaths.1 The incidence of brain metastases (BM) in patients with malignant melanoma ranges from 10% to 73% based on clinical and autopsy series and the median survival of patients following diagnosis of BM is 6.74 mo (3.38-13.2 mo) depending on Karnofsky Performance Status (KPS) score and number of BM.2-5 Treatment modalities include surgical resection, radiation therapy, chemotherapy, immunotherapy (IT), targeted therapies, and stereotactic radiosurgery (SRS).2,6 Historically, whole brain radiation therapy (WBRT) has had limited utility due to its relative radioresistance.7 SRS, however, overcomes this radioresistance safely by delivering higher radiation doses to the tumor with sharp radiation dose falloff.3,8
BRAF protein (a serine/threonine kinase involved in the mitogen-activated protein (MAP) kinase pathway) mutation is seen in approximately 50% of melanoma patients.9 Somatic point mutations in BRAF results in upregulation of signaling pathways that lead to neoplastic cell proliferation.9 Several studies demonstrate improved survival of patients with metastatic melanoma without brain metastasis following use of BRAF inhibitors (BRAFi).10-13 Use of BRAFi has demonstrated meaningful tumor regression in clinical trials for patients with unresectable BM who did not receive SRS.3,14,15 A number of retrospective studies have also reported intracranial response following treatment with vemurafenib16,17 Also, Wolf et al18 demonstrated in a prospective study that patients with melanoma who underwent SRS for BM had increased survival if they had a BRAF mutation and were treated with a BRAFi.
We recently completed a retrospective study to evaluate the use of BRAFi and SRS in the treatment of patients with melanoma metastasis to the brain. We concluded that BRAF mutation status appears to be a potent prognostic factor in patients with melanoma BM. Moreover, we reported that BRAFi in conjunction with SRS may benefit this group of patients in terms of improving survival following diagnosis of BM.19 In this study, we aimed to confirm our previous findings in a larger multicentered cohort and further investigate the effectiveness of this combined treatment regimen.
METHODS
Patient data were gathered from four institutions from patients who developed melanoma BM between 2011 and 2015. A population of at least 150 was calculated to be necessary in order to achieve power of 0.9 with P value of .05 in the survival analysis among groups. More specifically we also calculated the power offered for survival analysis between each 2 groups. Using the IBM SPSS sample software (SPSS Inc, Armonk, New York) and taking account total subjects per group, hazard ratio, attrition, and mean follow-up we calculated the power for survival analysis amongst the different groups. For the comparison between group A and B, the power is 0.72, for comparison between B, and C the power is 0.9, and for comparison between A and C the power is 0.94. After gathering the relevant data, the patient name, medical record number, and any additional identifiable information were removed to deidentify the data. The institutional review board of each participating center approved this retrospective cohort study and patient consent was obtained when required. The STROBE statement guidelines were implemented. Inclusion criteria included all patients who underwent SRS treatment of a melanoma BM and whose BRAFV600 mutation status was determined. Patients were excluded if BRAF status was not known or if they were treated with partial dose of BRAFi after diagnosis of BM. In this way, 198 patients with a total of 710 cerebral metastases at presentation were available for analysis. Data from patient follow-up were included up to February 2016 and no patients were lost to follow-up. Average follow-up was 25.6 mo from diagnosis of BM.
Patients were then stratified based on BRAFV600 mutation status and use of BRAFi such as dabrafenib or vemurafenib (Table 1). Group A patients had confirmed BRAFV600 mutation but did not receive BRAFi after diagnosis of BM. Group B patients had confirmed BRAFV600 mutation and were treated with therapeutic doses of BRAFi. All patients who received dabrafenib received adjuvant MEK inhibitor. Group C patients were those with wild type BRAF protein status. All patients in this study were treated with SRS. For part of the analysis the patients were also divided into a group with wild-type BRAF melanoma (BRAF wt) and a group including patients with the BRAF V600 mutation (BRAF mut). The patients from group A may have received BRAFi prior to diagnosis of BM and this was not repeated either due to development of adverse reactions, contraindications, or failed therapy. Survival was measured from (1) the diagnosis of BM, (2) the day of first SRS treatment and (3) the day of primary diagnosis (overall survival; OS).
TABLE 1.
Clinicopathological Characteristics of Patient Population in Relation to the BRAF Mutation Status and Use of BRAFi
| Variable | Group A | Group B | Group C |
|---|---|---|---|
| No. of patients | 23 | 67 | 108 |
| Sex (%) | |||
| Males | 12 (52.2%) | 38 (56.7%) | 84 (77.8%) |
| Females | 11 (47.8%) | 29 (43.1%) | 24 (22.2%) |
| Age at diagnosis of primary melanoma (yr) | |||
| Median | 53 | 47 | 61 |
| Mean ± SD | 53 ± 17 | 47 ± 13.8 | 61 ± 13.4 |
| Range | 20-80 | 16-74 | 13-84 |
| Site of primary melanoma (%) | |||
| Extremity | 12 (52%) | 19 (28%) | 31 (29%) |
| Trunk | 7 (30%) | 22 (33%) | 28 (26%) |
| Head and neck | 1 (4%) | 16 (24%) | 29 (27%) |
| Occult | 3 (13%) | 11 (16%) | 20 (19%) |
| Ulceration (%) | |||
| Present | 4 (17%) | 15 (22%) | 22 (22.4%) |
| Absent | 6 (26%) | 15 (22%) | 34 (31.5%) |
| Unknown | 13 (57%) | 37 (55%) | 51 (47.2%) |
| Clark's level (%) | |||
| II | 1 (4%) | 2 (3%) | 3 (2.8%) |
| III | 2 (8%) | 4 (6%) | 7 (6.5%) |
| IV | 4 (17%) | 15 (22%) | 25 (23.1%) |
| V | 0 | 1 (1%) | 6 (5.6%) |
| Unknown | 16 (70%) | 44 (66%) | 64 (59.3%) |
| Breslow thickness in mm (%) | |||
| 0.01-1.0 | 2 (9%) | 5 (7%) | 7 (6%) |
| 1.01-2.0 | 3 (13%) | 10 (15%) | 20 (19%) |
| 2.01-4.0 | 6 (26%) | 14 (21%) | 18 (17%) |
| >4.0 | 3 (13%) | 4 (5%) | 17 (16%) |
| Unknown | 8 (35%) | 22 (33%) | 38 (35%) |
| Extracranial metastatic disease | |||
| Active extracranial metastatic disease (%) | 15 (65%) | 58 (86%) | 90 (83%) |
| Mean number of organs with active metastasis (range) | 1.2 (0-3) | 1.8 (0-4) | 2 (0-5) |
Percentages may not equal 100% because of rounding.
SRS was delivered in a single fraction using a common radiosurgical platform, the Gamma Knife (Elekta AB, Stockholm, Sweden). The technique is described previously19 and dosing followed the RTOG 95-08 guidelines. After SRS, patients were followed clinically and radiographically. Imaging was evaluated by a treating neurosurgeon or a neuroradiologist at the treating site. Failure of SRS was considered when the tumor volume increased by more than 15% compared to the time of SRS.20
Parametric data are presented as mean ± SD. Student t-test was performed for parametric data and X2-test was used for comparison of nonparametric data. Kaplan–Meier methodology was used in the survival analysis as well as the analysis of local tumor progression and remote failure. The log-rank test was used to detect the survival difference in different groups of patients. Multivariate Cox proportional hazards regression analysis was used to investigate the effect of the different parameters on survival. IBM SPSS statistics data editor (SPSS Inc) was used for all statistical analyses. All statistical studies were 2-sided, and a P value < .05 was deemed statistically significant.
RESULTS
Of a total of 198 patients included in this analysis, 90 (45.5%) exhibited a BRAF mutation and 108 (54.5%) were wild-type (Figure, Supplemental Digital Content 1). Group A included 23 patients (11.6%), Group B, 67 patients (33.8%), and Group C, 108 patients (54.5%). Clinicopathological characteristics of the patient population are recorded in Tables 1 and 2. The clinical characteristics of our patient population divided by institution are described in Table, Supplemental Digital Content 2.
TABLE 2.
Clinicopathological Characteristics of Metastatic Melanoma in Relation to BRAF Mutation Status and Use of BRAFi
| Variable | Entire cohort | Group A | Group B | Group C |
|---|---|---|---|---|
| No. of patients | 198 | 23 | 67 | 108 |
| Age at BM (yr) | ||||
| Median | 62 | 58 | 53 | 66 |
| Mean ± SD | 62 ± 13.8 | 58 ± 15.9 | 53 ± 13.1 | 66 ± 11.9 |
| Range | 20-86 | 20-83 | 21-81 | 25-86 |
| KPS score median (range) | 90 (40-100) | 90 (70-100) | 100 (50-100) | 90 (40-100) |
| Mean number of cerebral metastases ± SD | 3.7 ± 4.2 | 4 ± 3.6 | 4 ± 5.2 | 3.4 ± 3.7 |
| Average metastasis volume ± SD | 1.1 ± 2.6 | 1.4 ± 2.6 | 1 ± 2.6 | 1.1 ± 2.6 |
| Diagnosis interval (mo) | ||||
| Median | 34 | 32 | 42 | 31 |
| Range | 0-451 | 0-368 | 0-451 | 0-271 |
| SRS | ||||
| Mean isodose line (range; %) | 63.2 (16-97) | 58.6 (16-97) | 63.8 (32-97) | 64.1 (40-97) |
| Mean margin dose (Gy) | 19.1 ± 2.3 | 19.7 ± 1.7 | 19.2 ± 2.0 | 18.9 ± 2.6 |
| Mean repeat SRS local (range) | 0.47 (0-3) | 0.35 (0-2) | 0.57 (0-2) | 0.43 (0-3) |
| Mean repeat SRS remote (range) | 0.47 (0-3) | 0.35 (0-2) | 0.55 (0-3) | 0.45 (0-3) |
| WBRT status (%) | ||||
| None | 149 (75%) | 20 (87%) | 45 (67%) | 85 (79%) |
| Prior to SRS | 29 (15%) | 3 (13%) | 14 (21%) | 12 (11%) |
| Salvage after SRS | 19 (10%) | 0 | 8 (12%) | 11 (10%) |
| Craniotomy and resection prior to SRS (%) | 44 (22%) | 7 (30%) | 12 (18%) | 25 (23%) |
The median age at diagnosis of primary melanoma was 53 among Group A patients, 47 among Group B patients, and 61 among Group C patients (Table 1). The median age at diagnosis of BM was 58, 53, and 66 years for groups A, B, and C, respectively (Table 2). The age of diagnosis of BM was 58 and 66 yr in the BRAF mut and BRAF wt group, respectively, which was statistically significant (P < .01). Similarly, the age at diagnosis of primary melanoma was 49 and 61 yr in the BRAF mut and BRAF wt group, respectively, which was statistically significant (P < .01). There was no difference in the time to diagnosis of intracranial metastases from initial diagnosis between groups (P = .09). Interestingly in our cohort, BRAF wt group (group C) comprised of significantly more males (77.8%) as opposed to 52.2% and 56.7% male in groups A and B, respectively (P < .01). There was no statistically significant difference in extracranial disease burden between groups (Table 1). The KPS score at the time of diagnosis ranged from 40 to 100 with a median of 90; 9 patients had KPS score less than 70. The mean number of BM on presentation was 3.7 with an average volume of 1.1 cm3, there was no statistically significant difference among the different groups. The mean margin dose used was 19.1 Gy with isodose lines ranging from 16% to 97%. Among our cohort, 37% of patients underwent 1 to 3 repeat sessions of SRS for local or remote tumor control. There was no difference in the use of repeat SRS amongst the different groups (Table 2).
Patient Survival
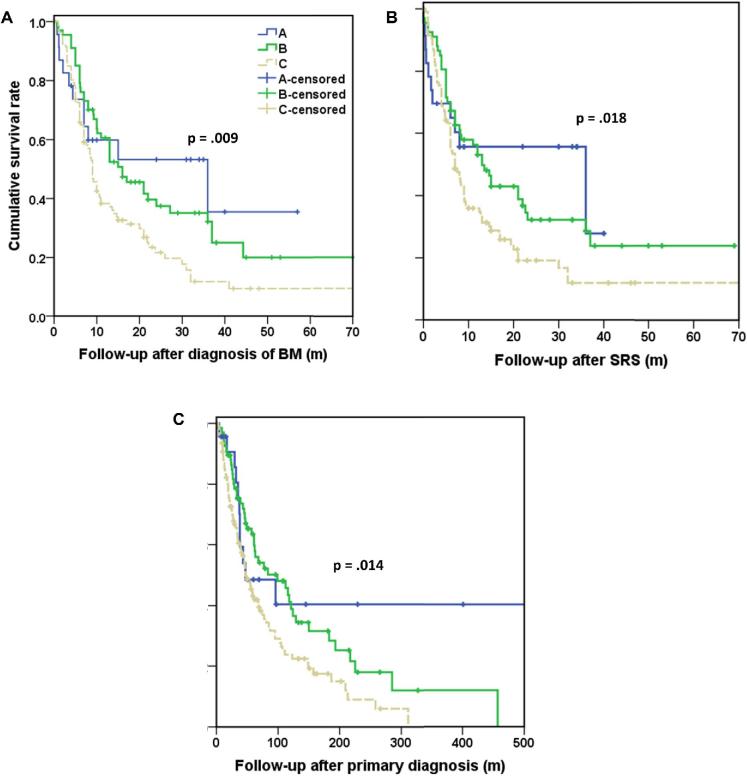
At the time of analysis, 136 patients (68.7%) had died and 62 patients (31.3%) maintained their clinical follow-up. The median OS, BM survival, and SRS survival were 61 mo, 10.9 mo, and 8.1 mo, respectively (Table 3). Median survival times and actuarial survival rates after the diagnosis of primary melanoma, after the diagnosis of BM, and after SRS are detailed in Table 3. The median survival after diagnosis of BM and after SRS was not statistically different between patients with mutant BRAF and treated with SRS in conjunction with BRAFi (group B) and patients with mutant BRAF and not treated with BRAFi (group A); however, as mentioned previously this comparison is underpowered. The median survival after the diagnosis of BM and after SRS in group B patients was increased compared with survival in patients with wild-type BRAF (group C). Also, the median survival after the diagnosis of BM in patients in group A was increased compared to group C. Results of the P-values calculated by log-rank test for comparison of survival times between different groups and the Kaplan–Meier plots that show patient survival in the 3 groups regarding BM survival, SRS survival and OS are included in Figure 1 and Table 4.
TABLE 3.
Patient Survival Times in Relation to BRAF Mutation Status and Administration of BRAFi
| Variable | Entire Cohort | Group A | Group B | Group C |
|---|---|---|---|---|
| No. of patients | 198 | 23 | 67 | 108 |
| Survival after diagnosis of primary melanoma | ||||
| MST in mo (95% CI) | 61 (46-76) | 47 (0-116) | 99 (43.7-154.3) | 47 (35.3-58.7) |
| 1-yr survival % | 89.9 | 87.0 | 97.0 | 86.1 |
| 3-yr survival % | 59.6 | 60.9 | 67.2 | 54.6 |
| 5-yr survival % | 39.9 | 34.8 | 50.7 | 34.3 |
| Survival after diagnosis of BM | ||||
| MST in mo (95% CI) | 10.9 (8.2-13.6) | 36 (0-72.6) | 16 (9.5-22.5) | 9 (7.7-10.3) |
| 0.5-yr survival % | 75.3 | 69.6 | 85.1 | 70.4 |
| 1-yr survival % | 40.9 | 39.1 | 56.7 | 31.5 |
| 2-yr survival % | 19.2 | 34.8 | 25.4 | 11.1 |
| Survival after SRS | ||||
| MST in mo (95% CI) | 8.4 (5.9-10.9) | 36 (0-76.1) | 13 (9.4-16.7) | 7 (5.6-8.4) |
| 0.5-yr survival % | 64.1 | 65.2 | 70.1 | 60.2 |
| 1-yr survival % | 37.4 | 34.8 | 52.2 | 28.7 |
| 2-yr survival % | 15.7 | 30.4 | 20.9 | 8.3 |
CI = confidence interval; MST = median survival time.
FIGURE 1.
Kaplan–Meier curves demonstrating survival times, including A, survival after the diagnosis of BM, B, survival after SRS, and C, survival after primary diagnosis. Group A = mutant BRAF without BRAFi, Group B = mutant BRAF with BRAFi, Group C = wild-type BRAF (n: 198).
TABLE 4.
Statistical Significance of Difference in the Cumulative Survival Rate
| Groups | BM | SRS | Primary melanoma |
|---|---|---|---|
| A-B | .698 | .621 | .625 |
| B-C | .006a | .013a | .009a |
| A-C | .036a | .05 | .064 |
SRS denotes follow-up after initial SRS; primary melanoma denotes follow-up after diagnosis of primary disease, P values are calculated using the log-rank test, aP < .05.
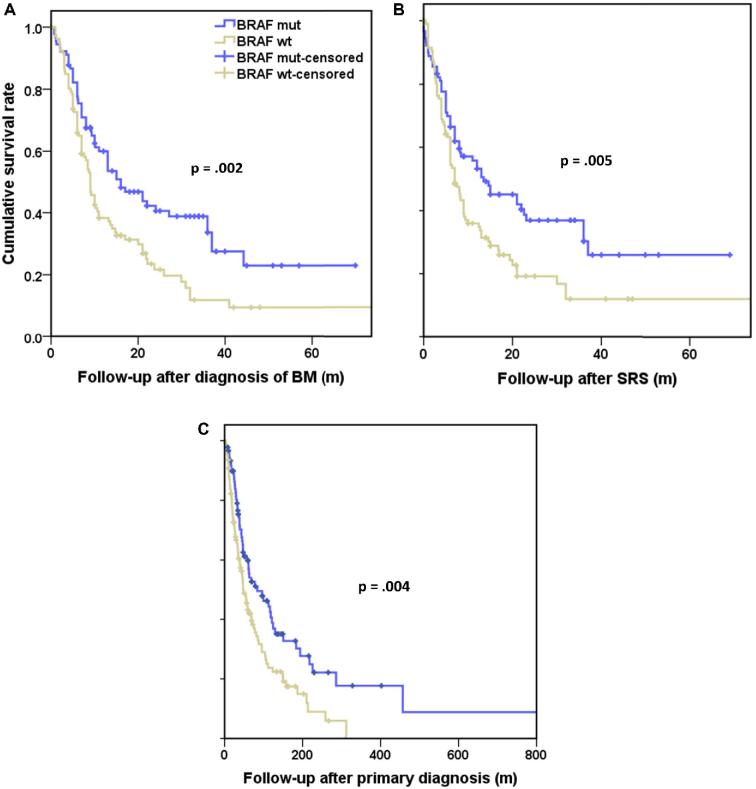
Given no difference in survival between Group A and Group B, we directly compared the survival after diagnosis of BM, survival after SRS and OS for the BRAF wt (group C) and BRAF mut (group A and group B). The medians for BM survival, SRS survival and OS were 16 (confidence interval (CI) 9.3-22.7) mo, 13.5 (CI 5.8-21.2) mo, and 83.6 (CI 33-134.2) mo for the BRAF mut group, respectively, and 9 (CI 7.7-10.3) mo, 7 (CI 5.6-8.4) mo, and 47 (CI 35.3-58.7) mo for wild type, respectively. There was statistically significant increased survival in BRAF mutated patients compared to wild-type patients (Figure 2). Following correction for age, the statistically significant increase in survival in BRAF mutated patients compared to wild-type patients remained (Figure, Supplemental Digital Content 3).
FIGURE 2.
Kaplan–Meier curves demonstrating survival times, including A, survival after the diagnosis of BM, B, survival after SRS, and C, survival after primary diagnosis (n: 198).
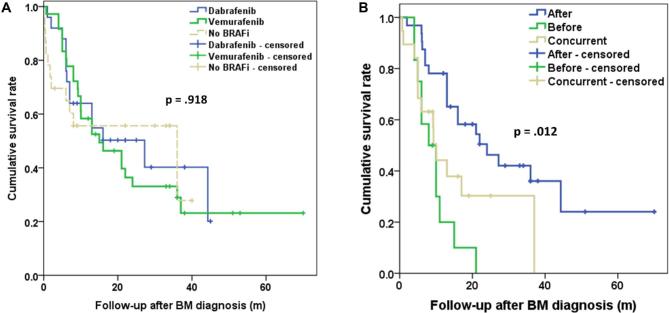
Multivariate analysis of survival after diagnosis of BM, survival after SRS, and OS is demonstrated in Table 5. The BRAF mutation was an independent, positive prognostic factor with a hazard ratio of 0.58, 0.64, and 0.55 for survival after BM diagnosis, after SRS, and primary diagnosis, respectively. Age at BM diagnosis had a statistically significant effect on survival from BM diagnosis and from SRS treatment (hazard ratio: 1.02). The use of WBRT was associated with worse outcome. Inclusion of surgical resection in the treatment regimen was associated with improved survival after BM diagnosis (Table 5). The use of PD-1 inhibitors provided improved survival from BM diagnosis, from SRS, and from primary diagnosis (hazard ratio: 0.4, 0.4, and 0.49, respectively). The type of BRAFi used Vemurafenib or Dabrafenib did not affect survival using Kaplan–Meier methodology (P = .9; Figure 3A).
TABLE 5.
Multivariate Analysis of BM Survival, SRS Survival and OS
| Survival after diagnosis of BM | Survival after SRS | Survival after primary diagnosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95.0% CI | Sig. (P) | Hazard ratio | 95.0% CI | Sig. (P) | Hazard ratio | 95.0% CI | Sig. (P) | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||
| BRAF mut. | 0.58 | 0.39 | 0.84 | .004a | 0.64 | 0.44 | 0.94 | .021a | 0.55 | 0.38 | 0.81 | .002a |
| Age | 1.02 | 1.07 | 1.04 | .007a | 1.02 | 1.01 | 1.04 | .009a | 1 | 0.99 | 1.02 | .995 |
| WBRT | 1.68 | 1.12 | 2.52 | .012a | 1.91 | 1.28 | 2.86 | .002a | 1.64 | 1.09 | 2.45 | .017a |
| Resection | 0.64 | 0.41 | 0.99 | .044a | 0.66 | 0.42 | 1.02 | .063 | 0.81 | 0.52 | 1.27 | .357 |
| anti-PD1 | 0.4 | 0.25 | 0.66 | .000b | 0.4 | 0.24 | 0.65 | .000b | 0.49 | 0.3 | 0.81 | .005a |
| anti-CTLA4 | 0.9 | 0.63 | 1.28 | .511 | 0.89 | 0.63 | 1.27 | .524 | 0.97 | 0.67 | 1.37 | .843 |
a P < .05
b P < .001
FIGURE 3.
A, Kaplan–Meier curves demonstrating survival after the diagnosis of BM in relation to use of BRAF inhibitor and type of BRAF inhibitor used. B, Kaplan–Meier curves demonstrating survival after the diagnosis of BM depending on timing of BRAF inhibitor administration in relation to SRS treatment.
We also evaluated the effect of timing of BRAFi administration on survival following BM diagnosis. We divided group B into patients who completed BRAFi treatment at least a week prior to first SRS, patients who received BRAFi concurrently to SRS and patients who started BRAFi treatment following SRS. Of note, for patient who received BRAFi concurrently to SRS this was held on the day of SRS. Among patients from Group B 36 patients received BRAFi following SRS, 12 patients received BRAFi prior to SRS, and 19 received BRAFi concurrently to SRS. The medians for BM survival were 24 (CI 11.2-36.7) mo, 8 (CI 4.0-11.9) mo, and 10.1 (8.4-11.7) mo (Figure 3B). Patients who received BRAFi following SRS demonstrated improved survival compared to patient who prior to SRS (P < .001) or concurrently to SRS (P = .007).
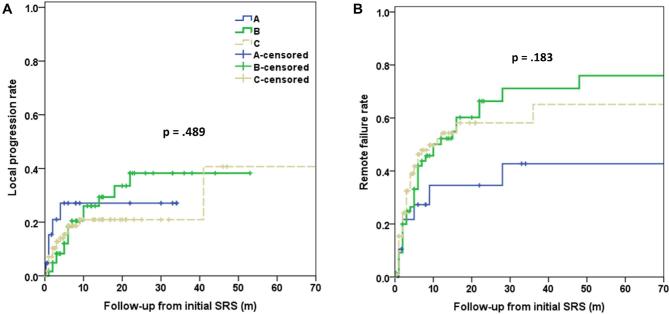
Local tumor control (<15% increase in size of treated lesions) as well as remote failure (development of new lesions) were evaluated using Kaplan–Meier analysis. Twenty-eight patients did not have available follow-up imaging, leaving 170/198 patients (85%) available for analysis (Figure 4). No statistically significant difference was identified between the different groups.
FIGURE 4.
Kaplan–Meier curves demonstrating rate of A, local tumor progression following SRS and B, remote failure following SRS. Group A = mutant BRAF without BRAFi, Group B = mutant BRAF with BRAFi, Group C = wild-type BRAF (n: 198).
Additional Treatment for Melanoma BM
Systemic treatments for melanoma are outlined in Table 6. Of the 23 group A patients, 30% received CTLA-4 inhibitors and 21% received PD-1 inhibitors. Of the 67 group B patients, 32% received CTLA-4 inhibitors and 26% received PD-1 inhibitors. Of the 108 BRAF wt patients, 47% received CTLA-4 inhibitors and 29% received PD-1 inhibitors. There was no statistically significant difference in either the use of CTLA-4 or PD-1 inhibitors between the different groups. Based on our multivariate analysis there was no correlation between use of CTLA-4 inhibitors and survival (Table 6).
TABLE 6.
Patient Treatment After Diagnosis of BM in Relation to BRAF Mutation Status and Use of BRAFi
| Variable | Group A | Group B | Group C |
|---|---|---|---|
| No. of patients | 23 | 67 | 108 |
| IL-2 | 2 | 6 | 10 |
| Ipilimumab only | 7 | 10 | 51 |
| Investigational agentsa | 2 | 4 | 5 |
| Noneb | 10 | 0 | 22 |
| BRAFi only | 0 | 24 | 0 |
| BRAFi + ipilimumab | 0 | 12 | 0 |
| BRAFi + MEK inhibitor | 0 | 31 | 0 |
| Temozolomide | 3 | 4 | 18 |
| Taxol | 2 | 0 | 7 |
| PD1 inhibitors | 5 | 18 | 32 |
IL-2 = interleukin-2.
aInvestigational agents include MEL58, MEL51, MEL44, MK3475, and CDX-1127.
bAll patients received at least SRS for brain metastases.
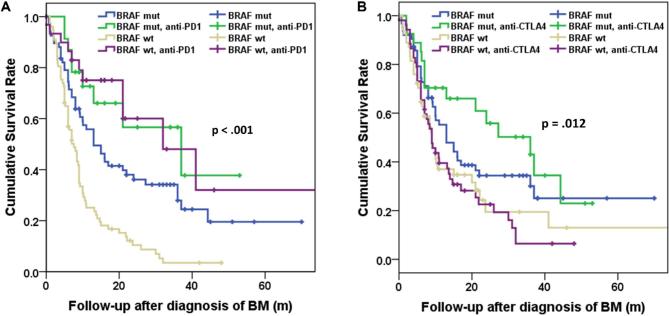
We proceeded to evaluate the use of IT in correlation with BRAF mutation status (Figure 5; Table 7). For patients carrying the BRAF mutation, the median survival time following the diagnosis of BM was 13 (CI 7.5-18.5) mo and 37 (CI 7.8-66.2) mo for the population that did not receive or received PD-1 inhibitors, respectively (P = .087). For the BRAF wt group the survival time following the diagnosis of BM was 7.6 (CI 5.7-9.5) mo and 32 (CI 12.2-51.8) mo for the population that did not receive or received PD-1 inhibitors, respectively (P < .001). The survival from BM diagnosis between the BRAF wt and the BRAF mut group remained statistically significantly different amongst the population that was not treated with PD-1 inhibitors (P < .001). However, there was no statistically significant difference in survival between the BRAF wt and the BRAF mut group among the population that was treated with PD-1 inhibitors (P = .85). The use of CTLA-4 inhibitors did not appear to have an effect in survival of the BRAF mut or BRAF wt group (P = .221 and P = .796, respectively).
FIGURE 5.
Kaplan–Meier curves demonstrating survival after the diagnosis of BM in relation to BRAF mutation status. A, use of PD-1 inhibitors. B, Use of CTLA-4 inhibitors (n: 198).
TABLE 7.
Statistical Significance of Difference in Cumulative Survival Rate in Correlation With Check Point Inhibitor Use
| Groups | PD-1 inhibitors | CTLA-4 inhibitors |
|---|---|---|
| BRAFmut-BRAFmutIT | .087 | .221 |
| BRAFmutIT-BRAFwt | < .001a | .016a |
| BRAFwt-BRAFwtIT | < .001a | .796 |
| BRAFmut-BRAFwt | < .001a | .13 |
| BRAFmut-BRAFwtIT | .055 | .031a |
| BRAFmutIT-BRAFwtIT | .85 | .002a |
aStatistically significant difference, IT = immunotherapy.
Adverse Events in Response to BRAFi
Adverse effects of BRAFi are demonstrated in Table 8. Of note 4.3% of patients in group A, 10.4% of patients in group B, and 2.7% of patients in group C developed intracerebral hematoma (ICH) (P = .09). Among the patients who were treated with BRAFi and SRS 10.4% developed ICH in comparison to 3% of patients who were not treated with BRAFi, this difference was statistically significant (P = .03).
TABLE 8.
Adverse Events in Patients Treated With BRAFi (Group B) After the Development of BM
| Variable | Dabrafenib | Vemurafenib | Both |
|---|---|---|---|
| No. of patients | 25 | 36 | 6 |
| Elevation of alanine transaminase | 3 | 1 | |
| Arthralgia | 4 | ||
| Squamous cell carcinoma of the skin | 1 | ||
| Rash | 4 | 8 | 1 |
| Myalgia | 1 | 3 | |
| Diminished appetite | 3 | 2 | |
| Hyperkeratotic reaction | 4 | ||
| Fatigue | 1 | 5 | |
| Abdominal pain | 1 | 1 | |
| Intracranial hemorrhage | 3 | 3 | 1 |
DISCUSSION
Intracranial Response to BRAFi and SRS
The combination of BRAFi and SRS is considered a promising approach to treatment of melanoma BM given the fact that BRAFi act as radiosensitizers.21-23 A number of case reports and retrospective studies have reported evidence of intracranial response following the combination of vemurafenib with radiation therapy.16,17,24 We previously found that the group treated with BRAFi and SRS was associated with significantly longer survival times and better local tumor control.18,19 However, the effect of BRAFi in combination with SRS for the treatment in melanoma BM remains unclear. A number of retrospective studies demonstrated no effect of BRAFi use on survival of patients with melanoma BM who also underwent SRS.25,26 These studies were underpowered with only a small number of patients treated with BRAFi and radiosurgery. Chowdhary et al27 in a recent review concluded that there may be a benefit to combining BRAFi with radiation therapy. Here, we demonstrate that among patients who received SRS the patients carrying the BRAF mutation and received BRAFi had improved survival compared to patients who did not carry the mutation. This finding is in accordance with previous reports in the literature.18 The clinical effectiveness of BRAFi may be limited by the development of resistance to the BRAFi over time.28,29 Also, the physiochemical properties of vemurafenib allow only limited distribution in the brain. Currently active clinical trials that are expected to shed further light on the effectiveness of BRAFi.27 Of note, the statistically significant age imbalance between groups is in accordance with previous findings30 and did not seem to affect the survival outcomes. This is in accordance with the DS-GPA prognostication system.5
In this cohort, there were a limited number of patients with the presence of a BRAF mutation who did not undergo BRAF treatment. This is representative of the population as in clinical practice given the aforementioned evidence most patients with metastatic melanoma carrying the BRAF mutation receive BRAFi if possible.18 Patients from group A demonstrated high variability in survival rates and we were unable to identify a difference in survival depending on BRAFi use among BRAF mut. Nevertheless, we found that the presence of the BRAF mutation was an independent positive prognostic factor in patients with melanoma brain metastasis. Previous studies have demonstrated that BRAF mutation is associated with unfavorable prognosis in patients with melanoma.31,32 El-Osta et al33 have previously demonstrated that BRAF mutation in advanced melanoma results in an insignificant trend to longer median survival from diagnosis. Rutter et al34 showed no difference in survival or recurrence following SRS for brain metastasis depending on BRAF status. Other reports have suggested that the presence of a BRAF mutation correlates with worse local metastasis control and survival.19,35 These variable results underline that the effect of the BRAF mutation on the natural history of BM is multifactorial. While we demonstrate that it acts as an independent prognostic factor not affected by the use of BRAFi, SRS, WBRT or the use of checkpoint inhibitors, it is likely affected by the genomic profile of the melanoma BM.36,37
Timing of BRAFi Administration
Wolf et al18 previously reported that BRAF administration prior to SRS yielded a worse result compared to administration concurrently or following SRS. Here, we confirm that the administration of BRAFi has optimal effect when treatment is initiated at least 1 wk following SRS. This phenomenon may be attributed to the development of resistance to BRAFi over time.28,29 Additionally, as we noted the physiochemical properties of vemurafenib allow only limited distribution in the brain.38-40 The improved effect of BRAFi following SRS may be attributed in part to increased permeability of the blood brain barrier by SRS. However, there is no current evidence that SRS is altering the blood brain barrier to allow better penetration of BRAFi.
BRAF Mutation and Prognosis
Following diagnosis of BM the BRAF mut group had a median survival of 16 mo, compared to the BRAF wt group that had a median survival of 9 mo. Previous studies have demonstrated that BRAF mutation in primary melanomas either have no effect on survival41-43 or is associated with a trend toward unfavorable prognosis31 as well as the presence of multiple cutaneous melanomas.30 In patients with melanoma spread to regional lymph nodes presence of BRAF mutation resulted in shorter interval to disease recurrence following lymphadenectomy as well as worse prognosis.32 El-Osta et al33 demonstrated that BRAF mutation in advanced metastatic melanoma results in an insignificant trend to longer median survival from diagnosis. As far as the effect of the BRAF mutation on patients with cerebral metastasis, Rutter et al34 showed no difference in survival or recurrence following SRS for brain metastasis depending on BRAF status. Other reports have suggested that the presence of a BRAF mutation correlates with worse local metastasis control and survival.19,35 Our findings demonstrate that in the setting of widespread use of BRAFi the presence of a BRAF mutation offers better prognosis and this observation appears to be a shift compared to previous reports when BRAFi were not used with such frequency. Studies of gene expression in melanoma support the hypothesis that other factors likely act together with the BRAF mutation to determine the prognostic phenotype. Namely, the gene expression profile associated with BRAF mutations may contribute to multiple pathways including to enhanced immune responsiveness, cell motility, and melanosome processing.44-46
BRAFi Safety
Several case studies have questioned the safety of the use of BRAFi concurrently with radiotherapy, due to the occurrence of liver and skin toxicity as well as radiation necrosis and intracranial hemorrhage. The most common adverse events include arthralgia, seizures, alopecia, diarrhea, dizziness, muscular weakness, and maculopapular rash.47-51 Intratumoral hemorrhage and radiation necrosis25 have been associated with the use of BRAFi.26 In this study, we found a small percentage of reversible liver toxicity and skin reactions among the patients treated with BRAFi. The patients treated with BRAFi had an increased risk of ICH compared to those not treated with BRAFi. However, ICH in patients following BRAFi use did not result in clinically significant deterioration or lead to hospitalization. This result is in accordance with previous reports,26 and a similar effect has been reported with the combination of ipilimumab and SRS.52
Other Treatment Modalities
Use of checkpoint inhibitors has demonstrated promising result in patients with melanoma. Nivolumab has proven to be effective in the treatment of BM.53 We found that the use of PD-1 inhibitors had a strong correlation with increased survival. Interestingly, PD-1 inhibitors were associated with a more robust improvement in survival compared among the population with BRAF wt compared to their effect on the BRAF mut patients. The BRAF protein has been associated with tumor-induced immune escape mechanisms through increased expression of immunosuppressive cytokines such as IL6, IL10, and VEGF, which can promote T regulatory cells.54 Also, BRAF V600E expression results in increased expression of IL1 by melanoma cells that can lead to upregulation of the checkpoint ligand molecules COX-2, PD-L1, and PD-L2.55 Moreover, overexpression of V600E in melanoma cells results in MHCI down regulation.56 These mechanisms may contribute to the relatively more pronounced effect of PD-1 inhibitors on BRAF wt patient as compared to BRAF mut patients.
Treatment with ipilimumab results in improved survival of patients with advanced melanoma.1 Treatment with ipilimumab followed by BRAFi may improve survival in select patients with metastatic melanoma.57 However, the low life expectancy of patients with BM drastically decreases its benefit.2 Indeed, in our study, we demonstrated no effect in survival with the use of ipilimumab.
Other interventions were found to impact survival. Surgery was associated with improved survival after BM diagnosis. The use of WBRT was associated with worse outcome, though this result is likely to be due to selection bias since only 15% of the patients received WBRT prior to SRS. Use of WBRT is rare for melanoma, is often performed without uniform indication criteria, and usually reflects large burden of symptomatic disease.
Study Limitations
This multicenter, retrospective cohort study has some intrinsic limitations, including unavoidable selection and referral bias. Moreover, our cohort had been treated with heterogeneous treatment modalities and follow-up was variable depending on the institution. Given the increasing use of BRAFi and despite the total number of patients included in this study, for the comparison between group A-B the power is 0.72, which is underpowered. This study is not designed to provide insight into whether progression in the central nervous system, progression outside the central nervous system, or progression in both compartments led to patient deaths. There remains considerable variability in melanoma cell responsiveness to treatment as well as sensitivity to radiation.
CONCLUSION
Melanoma BM has a high dissemination rate and poor prognosis. The rise of new therapies including SRS, checkpoint, and BRAFi has offered the potential to improve survival in patients with melanoma BM. In this multicenter retrospective study, we found that in the setting of widespread use of BRAFi the presence of a BRAF mutation is an independent predictor of better prognosis in patients with melanoma BM that underwent SRS. Importantly, the effect of BRAFi was significantly affected by the timing of administration and appears to have optimal effect when treatment is initiated at least 1 wk following SRS. We also found that the use of BRAFi may increase the risk of ICH. Finally, we confirmed the effectiveness of PD-1 inhibitors in patients with melanoma BM who undergo SRS and found that their effect is more pronounced in BRAF wt patients.
Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Supplemental Digital Content 1. Figure. Flow chart of the study participants.
Supplemental Digital Content 2. Table. Clinical characteristics of patient population regarding primary and metastatic disease in relation to institution.
Supplemental Digital Content 3. Figure. Age-adjusted survival curves demonstrating survival times, including A, survival after the diagnosis of BM, B, survival after SRS, and C, survival after primary diagnosis, adjusted for age using Cox regression analysis (n: 198).
COMMENTS
Despite the limitations inherent in retrospective studies, this multicenter study adds to the current literature in this rapidly developing field. What is unexpected is the lack of survival benefit associated with the use of BRAFi in patients with BRAF mutated melanoma. The authors alluded to the fact that the comparison was underpowered. This study confirms that BRAFi can increase the risk of intratumoral hemorrhage. It also sheds some light with regard to the optimal timing of treatment with BRAFi relative to SRS although more research is needed to validate these findings.
Simon S. Lo
Seattle, Washington
In this paper, the author present a multi-centered retrospective cohort study of 198 patients with melanoma with known brain metastases. The patients were broken down into groups, 1 of known BRAFV600 mutation and the other with wild type BRAF. This study sought to assess the efficacy of using a BRAFi regimen and stereotactic radiosurgery in improving patient survival among the 2 patient populations. The authors proved the BRAFV600 mutation to be an independent positive prognostic factor. During their analysis they found that there was no significant difference in survival time when comparing BRAFV600 patients that received or did not receive BRAFi treatment. However, when comparing patients with the BRAFV600 mutation to patients with wild-type BRAF it was shown that treatment with the BRAFi regimen and SRS increased survival time for patients with the BRAFV600 mutation. Interestingly, the only patients with a survival advantage received the drug after finishing SRS as compared to those receiving the drug prior to and during radiation treatment. As the authors only provide limited mechanism to understand this result, a more detailed pre-clinical study would be of value. Despite the underpowered comparison group as well as the heterogeneous BRAFi treatment regimen, this paper provides valuable insight into a select group of patients that may benefit from a BRAFi regimen. An appropriately powered prospective trial should be conducted to validate these results.
Trevor Hebenstreit
Jonathan H. Sherman
Washington, District of Columbia
This retrospective study with data on 198 patients from 4 institutions reveals the complexity of thinking related to the current management of melanoma brain metastasis and the absence of a clear treatment paradigm. The highlights of the study seem to indicate that when patients got SRS as part of their management the BRAF mutant patients who got BRAF inhibitors did better than patients who did not get BRAFi. It is clear that these 4 institutions had wildly different management paradigms (eg, institution 1 had fewer craniotomies, institution 2 gave wbrt to 70% of their patients most of whom presumably also had craniotomies). Selection bias is therefore likely to be large. In addition to the BRAFi, which was only given to some, most patients got many other systemic modalities. The use of BRAFi also was associated with a higher risk of intratumoral bleeding. Because of the variability in options by institution it is difficult to conclude what is the best treatment paradigm. The oncologic concept that targeted therapies reduce the risk of new brain metastases or can be used to treat existing metastases remains to be validated. In the mean time, radiosurgery for brain disease remains the best option and avoids the leukoencephalopathy associated with whole brain radiation therapy. Additional studies are needed to validate BRAFi role as well as other targeted immunotherapies once melanoma has spread to the brain.
L. Dade Lunsford
Pittsburgh, Pennsylvania
The authors report on 198 patient with metastatic melanoma who developed brain metastasis. All of these patient were treated with Gamma Knife (Elekta AB). Patients were divided into those with BRAF mutations, either treated or not treated with a BRAF inhibitor, and those without the BRAF mutation. Also, patients were analyzed by the use of a PD-1 one inhibitor and a CTLA-4 inhibitor. There are a number of conclusion reported in the paper which may be of help to the clinician responsible for treating these patients. The median survival after the treatment of the brain metastasis was superior in BRAF positive patients treated with a BRAF inhibitor. Of these patients, those receiving the agents after Gamma Knife had improved survival compared to those receiving it prior to or concomitant with Gamma Knife. The reason for this remains unexplained. Patients receiving the BRAF inhibitors had an increase in the incidence of intracranial hemorrhage compared to those not receiving them. The authors also note that patients receiving a PD-1 inhibitors had improved survival. Overall the paper addresses some important questions but the nature of a retrospective study leaves many questions unanswered.
Robert A. Lustig
Philadelphia, Pennsylvania
REFERENCES
- 1. Burgeiro A, Mollinedo F, Oliveira PJ. Ipilimumab and vemurafenib: two different routes for targeting melanoma. Curr Cancer Drug Targets. 2013;13(8):879-894. [DOI] [PubMed] [Google Scholar]
- 2. Konstantinou MP, Dutriaux C, Gaudy-Marqueste C, et al. Ipilimumab in melanoma patients with brain metastasis: a retrospective multicentre evaluation of thirty-eight patients. Acta Derm Venerol. 2014;94(1):45-49. [DOI] [PubMed] [Google Scholar]
- 3. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): A multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13(11):1087-1095. [DOI] [PubMed] [Google Scholar]
- 4. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48-54. [DOI] [PubMed] [Google Scholar]
- 5. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mingione V, Oliveira M, Prasad D, Steiner M, Steiner L. Gamma surgery for melanoma metastases in the brain. J Neurosurg. 2002;96(3):544-551. [DOI] [PubMed] [Google Scholar]
- 7. Goyal S, Silk AW, Tian S, et al. Clinical management of multiple melanoma brain metastases. JAMA Oncol. 2015;1(5):668-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012;117(2):227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949-954. [DOI] [PubMed] [Google Scholar]
- 10. Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26(35):5748-5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hauschild A, Grob J-J, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet North Am Ed. 2012;380(9839):358-365. [DOI] [PubMed] [Google Scholar]
- 13. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dummer R, Goldinger SM, Turtschi CP, et al. Vemurafenib in patients with BRAFV600 mutation-positive melanoma with symptomatic brain metastases: Final results of an open-label pilot study. Eur J Cancer. 2014;50(3):611-621. [DOI] [PubMed] [Google Scholar]
- 15. Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: A phase 1 dose-escalation trial. Lancet North Am Ed. 2012;379(9829):1893-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dzienis MR, Atkinson VG. Response rate to vemurafenib in patients with B-RAF-positive melanoma brain metastases. Melanoma Res. 2014;24(4):349-353. [DOI] [PubMed] [Google Scholar]
- 17. Harding JJ, Catalanotti F, Munhoz RR, et al. A Retrospective evaluation of vemurafenib as treatment for BRAF-Mutant melanoma brain metastases. Oncologist. 2015;20(7):789-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolf A, Zia S, Verma R, et al. Impact on overall survival of the combination of BRAF inhibitors and stereotactic radiosurgery in patients with melanoma brain metastases. J Neurooncol. 2016;127(3):607-615. [DOI] [PubMed] [Google Scholar]
- 19. Xu Z, Lee CC, Ramesh A, et al. BRAF V600E mutation and BRAF kinase inhibitors in conjunction with stereotactic radiosurgery for intracranial melanoma metastases. J Neurosurg. 2017;126(3):726–734. [DOI] [PubMed] [Google Scholar]
- 20. Snell JW, Sheehan J, Stroila M, Steiner L. Assessment of imaging studies used with radiosurgery: a volumetric algorithm and an estimation of its error. J Neurosurg. 2006;104(1):157-162. [DOI] [PubMed] [Google Scholar]
- 21. Chung EJ, Brown AP, Asano H, et al. In vitro and in vivo radiosensitization with AZD6244 (ARRY-142886), an inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 kinase. Clin Cancer Res. 2009;15(9):3050-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sambade MJ, Peters EC, Thomas NE, Kaufmann WK, Kimple RJ, Shields JM. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98(3):394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hecht M, Zimmer L, Loquai C, et al. Radiosensitization by BRAF inhibitor therapy–mechanism and frequency of toxicity in melanoma patients. Ann Oncol. 2015;26(6):1238-1244. [DOI] [PubMed] [Google Scholar]
- 24. Narayana A, Mathew M, Tam M, et al. Vemurafenib and radiation therapy in melanoma brain metastases. J Neurooncol. 2013;113(3):411-416. [DOI] [PubMed] [Google Scholar]
- 25. Patel KR, Chowdhary M, Switchenko JM, et al. BRAF inhibitor and stereotactic radiosurgery is associated with an increased risk of radiation necrosis. Melanoma Res. 2016;26(4):387-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ly D, Bagshaw HP, Anker CJ, et al. Local control after stereotactic radiosurgery for brain metastases in patients with melanoma with and without BRAF mutation and treatment. J Neurosurg. 2015;123(2):395-401. [DOI] [PubMed] [Google Scholar]
- 27. Chowdhary M, Patel KR, Danish HH, Lawson DH, Khan MK. BRAF inhibitors and radiotherapy for melanoma brain metastases: potential advantages and disadvantages of combination therapy. OTT. 2016;9:7149-7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puzanov I, Burnett P, Flaherty KT. Biological challenges of BRAF inhibitor therapy. Mol Oncol. 2011;5(2):116-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sullivan RJ, Flaherty KT. Resistance to BRAF-targeted therapy in melanoma. Eur J Cancer. 2013;49(6):1297-1304. [DOI] [PubMed] [Google Scholar]
- 30. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29(10):1239-1246. [DOI] [PubMed] [Google Scholar]
- 31. Wu S, Kuo H, Li WQ, Canales AL, Han J, Qureshi AA. Association between BRAF V600E and NRAS Q61R mutations and clinicopathologic characteristics, risk factors and clinical outcome of primary invasive cutaneous melanoma. Cancer Causes Control. 2014;25(10):1379-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barbour AP, Tang YH, Armour N, et al. BRAF mutation status is an independent prognostic factor for resected stage IIIB and IIIC melanoma: implications for melanoma staging and adjuvant therapy. Eur J Cancer. 2014;50(15):2668-2676. [DOI] [PubMed] [Google Scholar]
- 33. El-Osta H, Falchook G, Tsimberidou A, et al. BRAF mutations in advanced cancers: clinical characteristics and outcomes. PLoS One. 2011;6(10):e25806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rutter CE GE, Yu JB, Bindra RS, Kluger HM, Chiang VL. Influence of BRAF and NRAS mutations on distant intracranial recurrence and survival in metastatic melanoma following radiosurgery. Int J Rad Oncol Biol Phys. 2013;872(2):S275. [Google Scholar]
- 35. Eugene Jon Koay ADB, John Andrew Jakob, Eric D, et al. Correlation of BRAF and NRAS mutation status with tumor characteristics and treatment outcomes in melanoma patients with brain metastasis. J Clin Oncol. 2012;30(15_suppl):8584. [Google Scholar]
- 36. Maertens O, Johnson B, Hollstein P, et al. Elucidating distinct roles for NF1 in melanomagenesis. Cancer Discov. 2013;3(3):338-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nissan MH, Pratilas CA, Jones AM, et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Res. 2014;74(8):2340-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mittapalli RK, Vaidhyanathan S, Sane R, Elmquist WF. Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on the brain distribution of a novel BRAF inhibitor: vemurafenib (PLX4032). J Pharmacol Exp Ther. 2012;342(1):33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mittapalli RK, Vaidhyanathan S, Dudek AZ, Elmquist WF. Mechanisms limiting distribution of the threonine-protein kinase B-RaFV600E inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther. 2013;344(3):655-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vaidhyanathan S, Mittapalli RK, Sarkaria JN, Elmquist WF. Factors influencing the CNS distribution of a novel MEK-1/2 Inhibitor: Implications for combination therapy for melanoma brain metastases. Drug Metab Dispos. 2014;42(8):1292-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Edlundh-Rose E, Egyhazi S, Omholt K, et al. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res. 2006;16(6):471-478. [DOI] [PubMed] [Google Scholar]
- 42. Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. JNCI J Nat Cancer Inst. 2003;95(24):1878-1890. [DOI] [PubMed] [Google Scholar]
- 43. Akslen LA, Angelini S, Straume O, et al. BRAF and NRAS mutations are frequent in nodular melanoma but are not associated with tumor cell proliferation or patient survival. J Invest Dermatol. 2005;125(2):312-317. [DOI] [PubMed] [Google Scholar]
- 44. Kannengiesser C, Spatz A, Michiels S, et al. Gene expression signature associated with BRAF mutations in human primary cutaneous melanomas. Mol Oncol. 2008;1(4):425-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jonsson G, Busch C, Knappskog S, et al. Gene expression profiling-based identification of molecular subtypes in stage IV melanomas with different clinical outcome. Clin Cancer Res. 2010;16(13):3356-3367. [DOI] [PubMed] [Google Scholar]
- 46. Pavey S, Johansson P, Packer L, et al. Microarray expression profiling in melanoma reveals a BRAF mutation signature. Oncogene. 2004;23(23):4060-4067. [DOI] [PubMed] [Google Scholar]
- 47. Liebner DA, Walston SA, Cavaliere R, et al. Radiation necrosis mimicking rapid intracranial progression of melanoma metastasis in two patients treated with vemurafenib. Melanoma Res. 2014;24(2):172-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Anker CJ, Ribas A, Grossmann AH, et al. Severe liver and skin toxicity after radiation and vemurafenib in metastatic melanoma. J Clin Oncol. 2013;31(17):e283-e287. [DOI] [PubMed] [Google Scholar]
- 49. Schulze B, Meissner M, Wolter M, Rodel C, Weiss C. Unusual acute and delayed skin reactions during and after whole-brain radiotherapy in combination with the BRAF inhibitor vemurafenib. Strahlenther Onkol. 2014;190(2):229-232. [DOI] [PubMed] [Google Scholar]
- 50. Lang N, Sterzing F, Enk AH, Hassel JC. Cutis verticis gyrata-like skin toxicity during treatment of melanoma patients with the BRAF inhibitor vemurafenib after whole-brain radiotherapy is a consequence of the development of multiple follicular cysts and milia. Strahlenther Onkol. 2014;190(11):1080-1081. [DOI] [PubMed] [Google Scholar]
- 51. Harding JJ, Barker CA, Carvajal RD, Wolchok JD, Chapman PB, Lacouture ME. Cutis verticis gyrata in association with vemurafenib and whole-brain radiotherapy. J Clin Oncol. 2014;32(14):e54-e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Diao K, Bian SX, Routman DM, et al. Combination ipilimumab and radiosurgery for brain metastases: tumor, edema, and adverse radiation effects. J Neurosurg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ahmed KA, Stallworth DG, Ki Y, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol. 2016;27(3):434-441. [DOI] [PubMed] [Google Scholar]
- 54. Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF–MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203(7):1651-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Khalili JS, Liu S, Rodriguez-Cruz TG, et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin Cancer Res. 2012;18(19):5329-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sapkota B, Hill CE, Pollack BP. Vemurafenib enhances MHC induction in BRAF(V600E) homozygous melanoma cells. OncoImmunology. 2013;2(1):e22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Balakan O, Suner A, Yigiter R, Balakan T, Sirikci A, Sevinc A. Long-term survival in metastatic malignant melanoma: ipilimumab followed by vemurafenib in a patient with brain metastasis. Intern Med. 2012;51(19):2819-2823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.