Abstract
Obligately intracytosolic rickettsiae that cycle between arthropod and vertebrate hosts cause a spectrum of severity of human diseases by primarily targeting microvascular endothelial cells resulting in endothelial dysfunction. Endothelium and mononuclear phagocytes play important roles in intracellular killing of rickettsiae upon activation by the effector molecules in innate and adaptive immunity. In overwhelming infection, immunosuppressive effects contribute to the severity of illness. Rickettsia- host cell interactions involve host cell receptors for rickettsial ligands that mediate cell adhesion and, in some instances, trigger induced phagocytosis. Rickettsiae interact with host cell actin to effect both cellular entry and intracellular actin-based mobility. The interaction of rickettsiae with the host cell also involves rickettsial evasion of host defenses and exploitation of the intracellular environment. Signal transduction events exemplify these effects. An intriguing frontier is the array of rickettsial noncoding RNA molecules and their potential effects on the pathogenesis and transmission of rickettsial diseases.
Keywords: Rickettsia – host cell interaction, endothelium, vascular permeability, innate immune signaling, immunosuppression, non-coding RNA
INTRODUCTION
What Are Rickettsiae?
Rickettsiae are commonly defined as genetically related, obligately intracellular bacteria that reside in an arthropod host during a part of their zoonotic cycle. By this definition, rickettsiae are members of at least seven genera: Rickettsia, Orientia, Ehrlichia, Anaplasma, Neorickettsia, Neoerhrlichia, and Wolbachia. This review will address the diseases caused by Rickettsia. Pathogenic Rickettsia are associated with hematophagous arthropods: ticks, mites, fleas, and lice. However, organisms belonging to the genus are found in a vast array of hosts including herbivorous arthropods, leeches, and amoebae (1). Rickettsia that are established pathogens have evolved into the obligately intracellular lifestyle with an evolutionarily reduced genome. Residing free in the cytosol of the host cell, they acquire many necessary components via transport mechanisms instead of maintaining genes for synthesizing sugars, lipids, nucleotides, and amino acids. Indeed, Rickettsia can parasitize host ATP via exchange of ADP through translocation systems, yet when the host cell ATP is depleted rickettsiae can also synthesize ATP.
Contemporary phylogeny of Rickettsia recognizes three pathogenic clades, the classic spotted fever and typhus groups and a transitional group in addition to numerous other clades of organisms of undetermined pathogenicity that are ancestral (2) to the pathogens. The spotted fever group contains many closely related organisms, such as R. rickettsii and R. conorii that reside in a limited number of tick species. The typhus group contains R. prowazekii and R. typhi, the etiologic agents of louse-borne epidemic typhus and murine typhus, respectively. The transitional group contains R. akari, R. australis, and R. felis, a ubiquitous organism found throughout the world in cat fleas, other fleas, ill persons and in some reports also in healthy persons and mosquitoes (3–6)].
The Spectrum of Rickettsial Diseases
The organisms and diseases of importance in North America are R. rickettsii (Rocky Mountain spotted fever) (7), R. parkeri (maculatum disease) (8), R. akari (rickettsialpox), Candidatus R. philippi (an eschar-associated illness in California) (9), R. typhi (10), R. prowazekii (in the US a zoonosis spread to humans from infected flying squirrels by their ectoparasites) (11), and R. africae (a disease frequently associated with travel to sub-Saharan Africa) (12). Candidatus refers to the taxonomic status of a proposed species designation that has yet to be officially adjudicated.
In general, rickettsioses comprise diseases with a continuous spectrum of severity of illness and overlapping clinical manifestations. R.rickettsii, R. prowazekii, R. conorii, and R. typhi can cause life-threatening diseases. The case fatality rate of Rocky Mountain spotted fever in the preantibiotic era was 20–25% and remains 3–4% (13; 14). In Latin America, the current case fatality rate is an amazing 30–40%, apparently owing to more virulent strains of R. rickettsii. The case fatality rate of R. prowazekii infection is 15% with much higher rates in the settings of extreme poverty and lack of adequate supportive care, a common condition under circumstances of natural disasters, war, and famine. There have been no fatalities in flying squirrel-associated R. prowazekii infections in the United States, and whether or not this can be attributed to reduced virulence or better general health of infected persons remains undetermined. Human rickettsioses progress from chills, fever, headaches, myalgia, nausea, and vomiting in the first few days to the appearance of a rash after 3 to 5 days and progression to respiratory failure, hypotensive shock, oliguric acute kidney injury, jaundice, hemorrhagic lesions, coma, and seizures in the most severe cases. Patients with non-life-threatening rickettsioses due to R.parkeri and R. africae usually develop a focus of epidermal and dermal necrosis (eschar) at the site of tick feeding and inoculation of rickettsiae prior to the onset of fever, headache, myalgias, and draining lymphadenopathy (8; 12). Rash occurs in a smaller percentage of patients, tends to be sparser, and is sometimes vesicular or pustular. Tickborne lymphadenopathy, a disease in Europe caused by R. slovaca, is characterized by an eschar usually at the site of a tick bite on the scalp followed in 7–9 days by painful draining cervical lymphadenopathy, but rarely manifests as fever or rash (15; 16). Strong circumstantial evidence suggests that R. amblyommatis, which is carried by approximately 50% of Amblyomma americanum (lone star ticks), the most prevalent human biting ticks in the southeastern and south-central US, is spreading steadily northward and causes subclinical infection in the majority of persons who asymptomatically develop anti-spotted fever group antibodies (17).
Vector Biology of Rickettsial Diseases
The vector biology of rickettsial diseases is only partially known. The human body louse vector of R. prowazekii is the least successful rickettsial host; 100% of infected lice are killed by the rickettsiae. Similarly the most pathogenic organism, R. rickettsii, is found in fewer than 0.1% of its vector ticks (Dermacentor variabilis in the US) compared with high carriage rates of much less pathogenic R. africae and R. amblyommatis in their tick vectors. Adult and nymphal ticks feed for several days, often more than a week, while usually remaining unnoticed. Studies of the salivary gland secretions of Ixodes scapularis that transmits Lyme borreliosis, anaplasmosis, babesiosis, Powasson virus encephalitis, and Ehrlichia muris eauclairensis infection have identified a veritable salivary pharmacy of anticoagulants to maintain blood flow in the tick bite lesion, immunomodulators to suppress inflammation and rejection, and pain suppressors to prevent detection (18). Studies of the salivary secretions of other evolutionarily divergent tick vectors of rickettsioses are not as advanced, but similar phenomena likely mediated by different molecules would seem likely. Vector biology of Dermacentor, Amblyomma, and Rhipicephalus ticks represents a gap in knowledge that is relevant to the transmission of Rickettsia species, including phenomena such as reactivation of rickettsial virulence from organisms in unfed ticks that do not cause disease, but are reactivated to virulence during tick feeding (19).
Pathology and Pathophysiology of Rickettsial Diseases
The pathogenic sequence of events that occur in rickettsial infection begins with the entry of organisms inoculated by the feeding tick or mite or scratched into the skin from infected louse or flea feces deposited on the skin. The initial target cells of infection are CD68+ cells (macrophages and/or dendritic cells) (20). The rickettsiae then spread via lymphatic vessels to the regional lymph nodes as has been observed vividly in the lymphangitis associated with R. sibirica mongolitimoniae infection (21). Rickettsiae then spread hematogenously throughout the body and infect mainly endothelial cells, but also to a lesser extent, macrophages, in the skin, lungs, brain, liver, gastrointestinal tract, kidneys, heart, and other organs.
The fundamental lesion of rickettsial diseases is vasculitis (22–24), which comprises the events that follow rickettsial infection and activation of endothelium and subsequent spread to involve focal continuous networks of endothelial cells mainly of the microcirculation. Rickettsial infection of endothelium stimulates cell signaling cascades leading to the secretion of cytokines and chemokines and other events as described in the later section on host innate immune signaling. What pathologists visualize in the foci of vascular infection by rickettsiae is, in fact, the cellular host defense comprising CD8 and CD4 T lymphocytes and macrophages that infiltrate both the vascular wall and perivascular space (25; 26). A small minority of vascular lesions contain non-occlusive hemostatic plugs, and the pathophysiologically important interstitial edema owing to increased vascular permeability is usually obscured by the artifact of formalin-induced tissue shrinkage (27–29).
These lesions form the basis for the rash, which progresses from macules to maculopapules with the accumulation of interstitial edema, and subsequently to petechial lesions with extravasation of blood around the most intensely infected networks of microcirculation in the center of the maculopapules. In the lungs, this sequence of events leads in the most severe cases to interstitial pneumonia, non-cardiogenic pulmonary edema, and diffuse alveolar damage (30). In the central nervous system, rickettsial meningoencephalitis consists of perivascular infiltrates of CD8 and CD4 T cells and macrophages, classically termed “glial nodules” in the neuropil and also present in the subarachnoid space (25; 31). Microinfarcts and perivascular hemorrhages occur in some severe cases, particularly in white matter. The liver shows vasculitis in portal triads and multiple randomly distributed small foci of hepatocellular death and lymphocytic inflammation associated with mildly elevated hepatic enzymes and, in some cases, hyperbilirubinemia, but massive hepatic necrosis and hepatic failure do not occur (32; 33). Focal lesions in the wall of the gastrointestinal tract, gallbladder, and pancreas, although not extensive, are observed not only in fatal cases at autopsy, but also in resected tissues from patients in whom abdominal pain and tenderness lead to exploratory laparotomy with appendectomy or cholecystectomy (34–36). Acute kidney injury is usually a result of prerenal azotemia associated with hypovolemia and rarely acute tubular necrosis. The multifocal vasculitis observed typically in the renal cortical medullary junction is unlikely to contribute significantly to the renal pathophysiology (37). The heart manifests interstitial lymphohistiocytic infiltrates without necrosis of cardiac myocytes consistent with an appropriate cellular host defense against the rickettsiae infecting the cardiac microcirculation (27). Echocardiography usually demonstrates adequate cardiac function. In most fatal cases, death is associated with respiratory failure, encephalitis, and hypotensive shock.
The pathophysiologic events can be attributed principally to rickettsia infection-associated increased vascular permeability leading to edema, hypovolemia, hypotension, reduced perfusion of organs, acute respiratory distress syndrome, and CNS abnormalities. Increased permeability of endothelial cell monolayers depends on the quantity and virulence of the rickettsiae and duration of intracellular rickettsial growth and is enhanced by the presence of proinflammatory cytokines such as interleukin 1-beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) (38–40). Infection of endothelial monolayers in the presence of proinflammatory cytokines is associated with the disruption of intercellular adherens junctions and redistribution of P120 and β-catenin proteins, which attach endothelial cells to the extracellular matrix and regulate interaction of vascular endothelial (VE) cadherin with the actin cytoskeleton. Rickettsial activation of endothelial cells causes phosphorylation of the VE-cadherin resulting in decreased VE-cadherin homotypic interactions at adherens junctions. A further mechanism related to increased vascular permeability is mediated by the RNase activity of cytoplasmic angiogenin, which yields 5’tRNA fragments associated with tyrosine phosphorylation and internalization of the VE-cadherin and decreased endothelial cell barrier function (41). The functional contributions of vasoactive prostaglandins, VEGF, thrombin, and other potential mediators remain unknown.
Host factors often play an important role in the severity of rickettsial infections. Greater risk of a fatal outcome is associated with older patients, males, and patients with diabetes, alcoholism, or glucose-6-phosphate dehydrogenase deficiency, which predisposes to fulminant Rocky Mountain spotted fever resulting in death within five days of onset of illness (42–45).
The most valuable animal models that represent the target cells, organ involvement, and pathologic lesions of human rickettsioses with dose-dependent lethality are C3H/HeN mice infected intravenously with R. conorii for spotted fever group rickettsioses or R. typhi for typhus group rickettsioses (46; 47). Intravenous infection of C57BL/6 mice with R. australis has allowed studies of specific immune mechanisms in gene knockout mice (48; 49). Guinea pigs and nonhuman primates are susceptible to R. rickettsii and R. prowazekii, providing models for preclinical testing of vaccines and novel therapeutics.
The Pathogenesis of Severe Rickettsioses
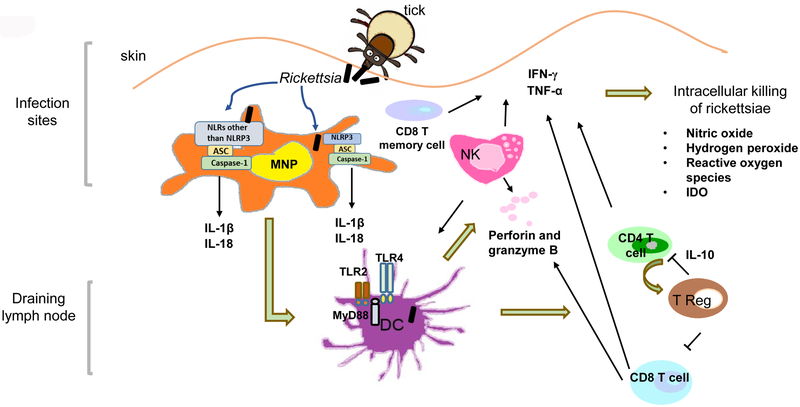
The pathogenesis of rickettsioses is incompletely understood although the immune system is known to play an important role. Both human and animal studies have demonstrated that numerous elements of the immune system, including dendritic cells, innate immune signaling, macrophages, NK cells, CD8 T cells, CD4 T cells, endothelial cells, , antibodies, and inflammatory cytokines and chemokines, have a high impact on controlling rickettsial infection, predominantly via mechanisms of facilitating intracellular killing of rickettsiae (Figure 1). Apparently, if these components of protective immunity ineffectively engage in the host response, or rickettsiae modify/manipulate the response of these immune elements, the disease will progress with increased severity, even leading to a fatal outcome. Moreover, other immune components such as inducible T regulatory cells (TR) contribute to the progression and pathogenesis of rickettsial diseases. Here, we address how rickettsiae subvert or evade host immune mechanisms to promote the disease.
Figure 1.
A schematic diagram of interactions of spotted fever group rickettsiae with different components of the host immune system. After tick bite, the inoculated rickettsiae initially target mononuclear phagocytes (MNPs) and then activate dendritic cells (DCs). Rickettsial antigen is presented by DCs and subsequently activates natural killer (NK) cells, CD4 T cells and CD8 T cells. Simultaneously, IL-10-producing inducible CD4+CD25+ T regulatory (T Reg) cells are generated, leading to immunosuppressive T cell response.
Mechanisms involved in intracellular killing of rickettsiae
Upon activation by immune stimuli such as TNF-α, IFN-γ, and RANTES, or through an Fc-dependent antibody effect, human endothelial cells and macrophages elicit intracellular killing of rickettsiae (50; 51). Mouse endothelial cells in a model of spotted fever rickettsiosis also exhibit anti-rickettsial activity under cytokine stimulation. The mechanisms of rickettsial killing in these cells involve one or a combination of three possibilities: inducible nitric oxide (NO) synthesis, hydrogen peroxide and reactive oxygen species production, and limited availability of tryptophan via its degradation by indoleamine 2,3‑dioxygenase (IDO), (52). Although in vivo rickettsicidal activity is not completely understood in humans, increased mRNA levels of IDO and inducible nitric oxide synthase have been detected in the infection sites of patients with mild-to moderate boutonneuse fever due to R. conorii (53). However, further experimental studies using iNOS- knockout mice suggest that NO is partially involved in CpG type B (CpG-B)-induced death of R. australis-infected mice (54). Interestingly, IDO and NO may not be involved in protection conferred by pre-treatment with CpG-B against a lethal dose infection with R. australis as demonstrated in a study using IDO-knockout and iNOS- knockout mice. Thus, IDO is not the exclusive mechanism of protection conferred by treatment with CpGs before infection (54).
Another mechanism related to the degradation of cytosolic rickettsiae is autophagy. By electron microscopy, R. conorii are destroyed in structures resembling autophagolysosomes in mouse brain endothelial cells activated by IFN-γ and TNF-α (55). However, the question whether autophagy really plays a role in clearance of rickettsiae in immune cells other than endothelial cells, particularly before being activated by immune effector cytokines produced by the major players in adaptive immunity will require further studies using both in vitro and in vivo approaches.
Recently, our studies have demonstrated that endothelial cell responses to rickettsiae, including a proinflammatory cytokine profile and endothelial permeability, play a role in the pathogenesis of rickettsial diseases. Secretion of MCP-1 by human endothelial cells upon infection with rickettsiae accompanied by a minimum level of cell death prognosticates a mild rickettsial disease as demonstrated by R. massiliae (40). In contrast, human endothelial cells infected with Israeli spotted fever strain (ISF) of R. conorii manifest a pro-inflammatory profile characterized by IL-8 and IL-6 with a significant level of cell death that predicts a severe infection outcome. The endothelial dysfunction caused by R. conorii ISF leads to increased permeability at least in part mediated by caspase-1.
Although endothelial cells are the primary target cells for rickettsial infection, pathogenic rickettsiae also infect monocytes, macrophages and hepatocytes as illustrated in both humans and established animal models. Upon infection, perivascular infiltration of lymphocytes and macrophages comprises what is called rickettsial vasculitis. In response to IFN-γ and TNF-α, macrophages are activated and serve as crucial effector cells mediating clearance of pathogens within these macrophages. As discussed earlier, macrophages are the initial targets of rickettsiae in the inoculation site of infection in the skin. Mononuclear cells are one of the major inflammatory cells in the cutaneous inoculation sites in patients infected with R. parkeri (56). CD68+ macrophages are the predominant infected cells in the skin lesions of patients with rickettsialpox (20). Recent findings have demonstrated that the severity of rickettsial diseases clearly correlates with whether these rickettsiae can survive and proliferate in human macrophage-like cells (57). Thus, further studies are required to determine how macrophages contribute to the pathogenesis of these diseases.
Innate immune effectors in host defense
Animal models of rickettsial infection provide very useful tools to investigate host immune effectors against and pathogenesis of severe rickettsioses. Currently, mouse models of R. australis, R. conorii, and R. typhi infection have been well described and exploited (46; 47; 49). The mouse models mimic human pathology; R. conorii-infected C3H/HeN mice provide one of the best models because endothelial cells are the major targets for rickettsiae in vivo. It is worth noting, however, that C57BL/6 mice, the background of most knockout mice, are only susceptible to infection with R. australis, and are highly resistant to R. conorii and other rickettsiae Although R. australis is not the most virulent rickettsial species in humans, R. australis-infected B6 mice allow us to mechanistically study the critical components of the host immune system by employing a variety of knockout mice on the B6 background.
Understanding the mechanisms of host resistance against rickettsial infection advances our knowledge of the innate effectors of host defense. Rag−/− mice, lacking acquired immunity, develop significantly greater rickettsial loads in tissues compared to WT controls during the very early stage of infection, which may be attributed to an expanded population of IFN-γ-producing memory CD8 T cells (58). Although a deficiency of CD8 T cells does not alter the innate resistance to R. conorii infection in B6 mice, the contribution of CD8 T cells to innate resistance has been demonstrated with a variety of other pathogens, such as murine cytomegalovirus and Listeria monocytogenes. (59–61). Depletion of NK cells enhances the susceptibility of mice to R. conorii infection in a background in which acquired immunity is intact (62). Further investigations using Rag−/− mice reveal that NK cells, as the major effector cells of innate immunity, mediate host resistance against rickettsial infection independent of B-and T-cells via modulation of IFN-γ (58). Furthermore, NK cells contribute to the prevention of vascular damage by secreting perforin.
Dendritic cells
As the most potent professional antigen presenting cells, dendritic cells play a crucial role in bridging innate and adaptive immune responses against rickettsial diseases. In contrast with other intracellular pathogens such as Leishmania, rickettsiae efficiently infect bone marrow derived-dendritic cells (BMDCs) (63). After undergoing phagocytosis, rickettsiae are localized in both phagosomes and the cytosol of BMDCs compared to residing free in the cytosol of endothelial cells. The distinct intracellular location of rickettsiae in both cytosol and vacuoles in BMDCs may be the basis of processing and presenting rickettsial antigen to both CD4 and CD8 T cells through MHC-II and MHC-I pathways, respectively. Indeed, R. conorii drives the maturation of BMDCs, which prime both naïve CD4 and CD8 T cells though TCR stimulation (Signal 1), costimulatory molecules (Signal 2), and cytokines (Signal 3). Rickettsiae-infected dendritic cells activate naïve CD8 T cells in vitro. Rickettsiae activate dendritic cells through mechanisms involving TLR4 and MyD88 (64; 65), both of which play a critical role in mediating host protection in vivo. Adoptive transfer of rickettsiae-stimulated DCs provides host protection against an ordinarily lethal challenge through increased activity of CD4+, CD8+ and NK cells (64; 66; 67).
Innate immune signaling
The first line of host immune cells to recognize the invasion of pathogens is pattern recognition receptors (PRRs), which perceive molecular signatures characteristic of a whole class of microbes, termed pathogen-associated (or microbe-associated) molecular patterns (PAMPs) (68). PRRs are germline encoded receptors, including transmembrane Toll-like receptors (TLRs), cytosolic nucleotide-binding oligomerization-domain-(NOD-) like receptor (NLR) family proteins, and RIG-I (69). Toll-like receptor 4 signaling is one of the molecular mechanisms by which rickettsiae activate the host immune system. Among the important components of pattern-recognition receptors (PRRs), TLRs trigger signaling through adaptor molecules, including MyD88 and toll-receptor associated activator of interferon (TRIF), mediating production of inflammatory cytokines as well as host cellular immunity (70; 71). Lipopolysaccharide is one of the TLR4 ligands. Indeed, TLR4 activation has been reported to mediate host protection against other Gram-negative bacteria such as Salmonella typhimurium (72). Mice naturally defective in TLR4 signaling (C3H/HeJ) succumb to a dose of R. conorii that is sublethal for TLR4-competent mice (63). The immune response attributed to TLR4 signaling during rickettsial infection in vivo is associated with greater concentrations of inflammatory cytokines (IFN-γ, IL-6, IL-12 and TNF-α) and increased expansion of activated NK cells, CD4 and CD8 T lymphocytes. Further studies investigating DC maturation upon rickettsial infection in mice deficient in TLRs will reveal the molecular mechanisms by which DCs polarize and activate NK cells and T lymphocytes (66; 67). In addition, R. akari triggers cell activation via TLR2 or TLR4 (73). Live and heat-killed R. akari cause phosphorylation of IRAK1 and p38 MAPK in 293/TLR4/MD-2 or 293/TLR2 stable cell lines, whereas only live bacteria elicit responses in TLR2/4-negative HEK293T cells. These results suggest that live R. akari are recognized by additional TLRs and/or NLRs in addition to TLR2 and TLR4.
Upon activation by microbes, TLRs transduce signals mostly via an adaptor molecule, MyD88. MyD88 controls downstream signaling of the IL-1R family and most TLRs, except for TLR3 and in part TLR4 (74). Our recent studies demonstrate that MyD88 is essential for host resistance to both R. australis and R. conorii (65). During rickettsial infection, MyD88 is required for inducing a protective inflammatory cytokine response consisting of IFN-γ, IL-6, and IL-1β, accompanied by inflammatory infiltrates of macrophages and neutrophils in vivo. In dendritic cells infected with R. australis, MyD88 is responsible for increased expression of MHC-IIhigh and production of IL-12p40, which are the two major signals that play a critical role in priming and educating CD4 T cells to differentiate into type 1 effector T cells.
It has been enigmatic how rickettsiae interact with the immune events occurring in host cytosol in order to replicate free in this location. The inflammasome is a large multi-protein complex consisting of NLRs and the protease, caspase-1 (75). Inflammasome activation by pathogens hinges upon violation of the host cell cytosol by activities such as those of pore-forming toxins, specialized microbial secretion systems, or the cytosolic presence of the pathogen itself (75). R. australis activates inflammasome in mouse macrophages via time- and dose-dependent mechanisms (76). ASC-dependent inflammasomes are responsible for recognition of R. australis in host cytosol, and NLRP3 inflammasome contributes significantly to this process. The role of NLRP3 inflammasome in host immune responses to R. australis in vivo is tissue-specific as evidenced by significantly increased bacterial loads in the spleen, but not in the liver and lungs, of NLRP3−/− mice compared to WT mice. Mouse macrophages secrete IL-1β and IL-18 in response to Listeria at 5 h post infection (p.i.) (77), Shigella at 6 h p.i. (78), Burkholderia at 4 h p.i. (79), and Francisella at 5 h p.i. (80). Our data suggest that the kinetics and possibly the mechanisms of inflammasome activation by R. australis are distinct from other cytosolic bacteria. In response to R. australis, mouse macrophages secrete IL-1β and IL-18 as late as 8 h after a high dose of infection and at 12 h after a low dose of infection. The levels of these inflammasome-derived cytokines increase progressively as the infection progresses and reach a peak at 24 h p.i. regardless of the dose. The delayed activation of inflammasome by R. australis in mouse macrophages compared with several facultative cytosolic bacteria mentioned above suggests that rickettsiae may employ an evasion strategy to delay inflammasome assembly at the early stage of infection. The dose-independent secretion of IL-1β at 24 h post infection by infected mouse macrophages suggests that inflammasomes responsible for recognizing these intracellular bacteria at the late stage of infection are very sensitive to the activation of ligand(s) generated during rickettsial infection, and could be an ideal candidate for future vaccine development targeting inflammasome activation. Furthermore, the secretion levels of IL-1β by Rickettsia-infected BMDCs and in the sera of infected mice were significantly lower in MyD88-knockout mice compared to WT controls, suggesting that in vitro and in vivo production of IL-1β is MyD88-dependent. Taken together, these results suggest that MyD88 signaling mediates instructive signals in DCs and secretion of IL-1β and type 1 immune cytokines, which may account for the protective inflammatory response during rickettsial infection.
Acquired immunity against rickettsiae and CD8 T cells
MHC-I molecules, IFN-γ and CD8 T cells are well-described as the major effectors of an effective adaptive immune response to rickettsial infection (48). Mice deficient in class I MHC are 50,000-fold more susceptible to a lethal infection with R. australis than wild type B6 mice. Rollwagen et al showed that MHC class I-, but not MHC class II–matched R. typhi-infected fibroblasts are targets of immune T lymphocyte-mediated cytotoxicity (81). In line with these findings, splenocytes from mice immune to R. conorii elicit cytotoxicity on specific MHC class I-matched R. conorii-infected SVEC-1 endothelial cells, while splenocytes from mice immune to R. australis exert cytotoxic activity against an MHC class I-matched R. australis-infected macrophage-like cell line (48). These results suggest that MHC-class I matched cytotoxicity of CD8 T lymphocytes plays an important role in host immunity against rickettsiae. The critical role for CD8 T lymphocytes in the clearance of rickettsial infections has also been demonstrated by CD8 T cell depletion, immune CD8 T cell adoptive transfer, and experiments in mice with knockout of selected immune response genes (48; 82). Furthermore, following infection with R. australis, IFN-γ-knockout mice are more than 100-fold more susceptible than wild type mice, while perforin gene knockout mice are 1,000-fold more susceptible than that of wild type mice (48). Thus, IFN-γ does not account for all of the anti-rickettsial activity mediated by CD8 T cells. Indeed, adoptive transfer of immune CD8 T lymphocytes from IFN-γ-knockout mice into R. australis-infected IFN-γ-knockout mice dramatically reduced the infectious rickettsial content in the organs, indicating that cytotoxic activity is an effective mechanism of rickettsial elimination. Thus, in addition to perforin-mediated cytotoxicity, other mechanisms such as granulysin or Fas/Fas ligand interactions could also be actively involved in CD8 T cell mediated cytotoxicity in clearing rickettsiae. Clearance of R. australis by CD8 T cells is mediated at least in part by cytotoxic apoptotic elimination of R. australis-infected endothelial cells (48).
Immunosuppression and CD4 T cells
CD8 cell-depletion enhances the susceptibility of the murine host to R. conorii infection while depletion of CD4 cells has no effect on the outcome of infection. Upon infection with 0.07 ordinarily median lethal doses (LD50) of rickettsiae, both CD4 T-cell-depleted and sham-depleted mice become ill, but clear infection by day 10 (82). Recent studies demonstrated that IFN-γ produced by CD4 T cells is important in host protection as evidenced by the observation that a greater percentage of IFN-γ-producing CD4 T cells correlates with protective immunity in a sublethal infection compared to mice infected with a lethal dose of R. conorii (F41). Furthermore, we employed susceptible and resistant mouse models of rickettsial infection to investigate the immune mechanisms involved in the pathogenesis of severe rickettsioses. In dendritic cells (DCs) from both resistant B6 mice and susceptible C3H mice, R. conorii infection in vitro upregulates their expression levels of maturation markers including MHC class II, MHC class I, CD40, CD86, and CD80 (63). However, DCs from susceptible mice express a significantly lower level of MHC-II and a significantly higher level of CD40 compared to resistant mice. The distinct expression pattern of DC maturation markers is likely associated with host resistance vs. susceptibility to severe rickettsial disease. R. conorii-infected susceptible DCs prime CD4 T cells with delayed activation kinetics as well as significantly impaired IFN-γ production. Strikingly, these in vitro findings are further confirmed in vivo by the experimental observation that an immunosuppressed CD4 T cell response is a component of the immune pathogenesis of severe rickettsioses (83). The impaired host immune response in a lethal model of rickettsial infection was characterized by suppressed CD4 T cell proliferation, significantly reduced levels of systemic and splenic CD4 T cell-specific IFN-γ as well as high levels of IL-10. CD4 T cells from mice infected with a sublethal dose of R. conorii undergo proliferation in response to in vitro polyclonal and Rickettsia-specific stimulation. In contrast, lethal rickettsial infection results in suppressed CD4 T-cell proliferation or even a lack of responsiveness to T cell-receptor and rickettsial stimuli compared to the proliferation of CD4 T cells from uninfected and sublethally infected mice. The suppressed CD4+ T-cell proliferation in lethal infection is linked to the failure to produce IL-2. Our studies suggest that CD4 T cell anergy and suppressed CD4 T cell response play a critical role in severe or even fatal rickettsial diseases. Interestingly, human rickettsial infection has been characterized as having a dysregulated immune response including reduction in peripheral CD4 T lymphocytes (84). Our studies further demonstrated that CD4+CD25+Foxp3- inducible T regulatory cells are most likely responsible for induction of immunosuppression during fatal rickettsioses.
Rickettsia-host cell interactions
Pathogenic rickettsiae replicate in many different cell types, including monocytes, macrophages, and hepatocytes, but preferentially target the vascular endothelial cell lining of small- and medium-sized vessels to multiply and establish infection in their mammalian hosts. Consequently, interactions of pathogenic rickettsiae with vascular endothelium constitute the major determinants of the mechanisms underlying rickettsial pathogenesis, host defense and immunity, and the manifestations as well as complications of disease. As such, pathogenesis of rickettsial infections primarily involves a series of events leading to vascular damage and dysfunction, characterized by significant changes in the expression of hemostatic proteins (29), altered vascular permeability causing pulmonary and cerebral edema (85), and in severe cases, abnormalities of the coagulation system (86).
Adhesion to and entry into host cells
As obligately intracellular bacteria, rickettsiae need to adhere to and invade target cells to gain access to a nutrient-rich cytoplasmic niche, which facilitates their proliferation, replication, and dissemination leading to successful establishment of the infection in the host. Analysis of rickettsial genomes utilizing bioinformatics-based approaches has identified multiple surface cell antigens (Sca), belonging to the family of rickettsial autotransporters involved in adhesion to host cell receptors. Although 17 Sca proteins have so far been identified, only five [Sca0 (outer membrane protein A {OmpA}), Sca1, Sca2, Sca4, and Sca5 (OmpB)] are active and functional in the spotted fever group (SFG). The Sca proteins are localized within the surface layer of the rickettsial membrane and aid in adhesion to the host cell. Among these, OmpA and OmpB are conserved throughout the SFG and are thought to be fundamental to pathogenesis, whereas OmpB, which is conserved in all Rickettsia species with the exception of R. canadensis plays a major role in the typhus group species (87–89).
OmpA of R. rickettsii is expressed as a 247 kDa protein, while OmpA of R. conorii is approximately 224 kDa (90)and is further processed to a 190 kDa protein. The OmpB protein is composed of three different domains, namely signal peptide, passenger domain, and a β-barrel transmembrane domain. OmpB, expressed as a 168 kDa protein, is also proteolytically processed into a 135 kDa passenger domain and a 32 kDa β-barrel domain. Of these, the passenger domain remains attached to the outer membrane (91), while the signal peptide translocates the protein across the inner membrane and deposits it into the periplasmic space. Following this event, the β-barrel transmembrane domain implants into the bacterial outer membrane to form a pore, the mechanism for which is not yet fully elucidated. The passenger domain is then fed through the β-barrel pore and subsequently cleaved (90). The first identified host cell-specific receptor Ku70 (a subunit of a nuclear DNA-dependent protein kinase with subcellular localization in the cytoplasm and plasma membrane) interacts with rickettsial OmpB (92). Ubiquitination of the cholesterol-rich micro-domains containing Ku70, which is mediated by the recruitment of ubiquitin ligase c-Cbl, is required for internalization of rickettsiae into host cells (92). Ku70-OmpB interactions and clathrin and caveolin-2 contribute to endocytosis and rickettsial internalization (92). OmpA was initially shown to be critical for R. rickettsii adhesion to host cells (93), and later it was suggested that rickettsial OmpA interacts with α2β1 integrin to promote invasion of the bacteria into the host cells (94). Recent work has further demonstrated that OmpA is not critical for the virulence in the guinea pig model, but may play a role in the survival or transmission from the tick vector (95).
Autotransporter Sca1 is present in all rickettsial species; full length Sca1 is expressed in R. conorii and R. typhi, while it is split in R. prowazekii and R. canadensis and present as a pseudogene (88; 89; 96). Sca1 is localized on the bacterial membrane and contains a β-barrel transmembrane domain similar to OmpA and OmpB (87). Sca1 has also been implicated in rickettsial attachment to mammalian host cells, with no demonstrated role in invasion (96).
Another autotransporter Sca2, highly conserved in SFG Rickettsia (97), is fragmented in R. prowazekii (87–89). Initially, R. typhi was reported to have a fragmented sca2, but later data reported that a complete sca2 is expressed in R. typhi (88; 98). Sca2 in R. conorii is expressed as a 150 kDa protein consisting of a short N-terminal signal sequence, a passenger domain, and an autotransporter domain. Sca2 of R. parkeri, R. typhi, R. prowazekii, and R. bellii contain five WH2 domains, and their location is different than in most SFG rickettsiae. Sca2 mediates adherence to and invasion of target host cells (97). Mammalian receptors interacting with Sca1 and Sca2 currently remain unknown.
Sca4, another surface antigen, is present in all rickettsial groups; initial sequencing data demonstrated Sca4 to be split in two orfs, but later independent sequencing of this gene in R. prowazekii MadridE reveals a complete sequence; UniProt# Q9ZD49.2 (87). Sca4 is complete in the virulent strain of R. prowazekii Rp22 (88). Sca4 is expressed R. bellii, but appears to be absent in R. canadensis (87). Sca4 is active in L929 cells infected with R. typhi in vitro and in cat fleas in vivo (88). Sca4 lacks an autotransporter domain, but is still transported to the rickettsial surface by an unknown mechanism (87; 99). Surface antigen Sca4 co-localizes with and activates host vinculin at focal adhesion sites, suggesting a potential role in host cell invasion (100).
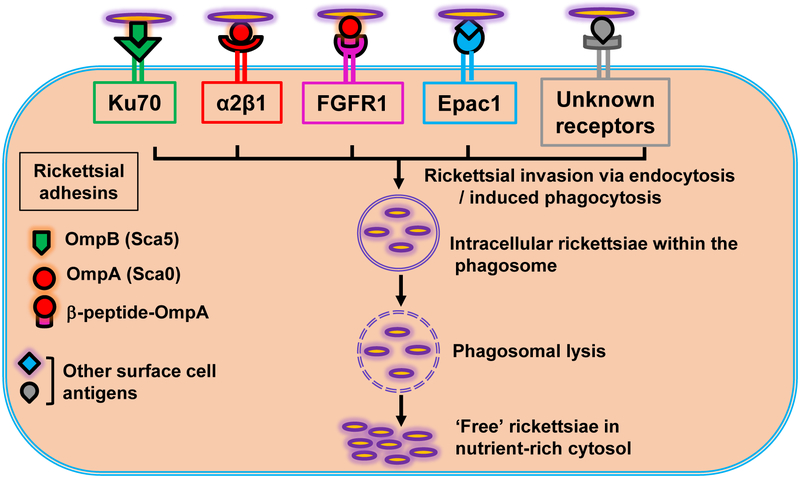
Another host protein, Exchange protein directly activated by cAMP (Epac), is also involved in rickettsial entry into host endothelial cells, and inhibition of Epac using a small molecule inhibitor significantly diminishes the morbidity and mortality in a murine model of infection with R. australis, the causative agent of Queensland tick typhus (101). More recently, yet another host cell receptor FGFR1 (fibroblast growth factor receptor-1) has been implicated in the internalization of SFG rickettsiae and shown to interact with rickettsial OmpA in a caveolin 1-dependent manner (102). Mass spectrometry and biochemical techniques reveal that a 32 kDa fragment of OmpA interacts with FGFR1, mediating the internalization of SFG rickettsiae into host endothelium (102). Previously, OmpB was shown to be proteolytically processed at the carboxy-terminal end of the protein, cleaving the 168 kDa precursor into 135 kDa and 32 kDa fragments implying that the 32 kDa fragment functions as the membrane anchor domain (91). Similarly, a recent study has provided evidence for post-translational processing of OmpA, revealing a 32 kDa fragment at the carboxyl-terminal of the protein during surface proteome analysis (103). Once intracellular, rickettsiae escape from the phagosomes prior to their fusion with lysosomes to free themselves in the cytosol. This is accomplished through a number of membranolytic activities, including phospholipase A2 (PLA2), phospholipase D (PLD), and hemolysin C (86). Due to their absolute dependence on the host intracellular machinery for replication and spread, it is not surprising that Rickettsia species exploit redundant mechanisms for host cell adhesion and invasion (Figure 2).
Figure 2:
A model of rickettsial invasion into host cells. The mammalian receptors interact with rickettsial outer membrane proteins, resulting in the internalization of rickettsiae into the cells via endocytosis and/or induced phagocytosis. Internalized rickettsiae quickly escape from the phagosomes using membranolytic activities to establish free, i.e., non-vacuole bound parasites in the cytosol.
Actin-based motility:
After invasion, rapid microbial dissemination between host cells is a critical step for the spread of intracellular bacterial pathogens and involves a phenomenon called actin-based motility. Bacterial motility between the cells requires the polymerization of actin filaments derived from the host’s cytoskeleton to form the typical F-actin “comet tail”. Host mediated motility allows Rickettsia to move from cell-to-cell while avoiding the immune system of the host. For spotted fever group rickettsiae, polar actin tail formation was first observed within 30 minutes post-infection through dual fluorescence staining (104). Double fluorescence labeling of F-actin revealed comet-like structures extending from a polar end of R. conorii. In contrast, formation of actin tails was observed very infrequently in R. typhi. Also, it was shown that R. conorii were randomly dispersed throughout the cell, whereas R. typhi remained confined to the same location (105). R. prowazekii does not produce actin tails unlike the short tails of R. typhi. Actin-based motility has been documented in all spotted fever group rickettsiae except R. peacockii. Host cell actin polymerization also plays an important role in rickettsial internalization, as indicated by potential contributions of upstream signaling mechanisms involving Cdc42 (a GTPase), phosphoinositide 3-kinase, c-Src and possibly other protein tyrosine kinases, resulting in the activation of Arp2/3 complex (106). A Wiskott–Aldrich syndrome protein (WASP) RickA is sufficient to direct actin-based motility via activation of actin nucleation and recruitment of Arp2/3 complex (107; 108). RickA (RC0909) is found in R. conorii, but is not present in R. prowazekii (107). An ancestral group member R. bellii encodes an ortholog of RickA and forms actin tails for movement into the host cell nucleus. R. peacockii, a SFG species, lacks actin based motility due to the disruption of rickA gene (109). Upon entering into the nucleus, the bacterium becomes trapped due to the lack of Arp2/3 complex required for actin tail formation. Transitional group members, R. australis (104)and R. felis (110), both form actin tails, and RickA expression in R. raoultii has been demonstrated by Western blotting (108). These findings demonstrate the presence of actin-based motility in Rickettsia belonging to different groups (SFG, TRG, and AG) with the exception of R. prowazekii and illustrate that actin-based motility represents an important lifestyle feature for the genus Rickettsia, which may have been lost during the course of evolution in R. prowazekii while being retained in the other rickettsiae. Interestingly, further research on this topic has revealed biphasic rickettsial motility with distinct movement parameters (111). Motility is slow and meandering right after invasion (within 15–30 min) due, in part, to the short, curved actin tails, which consist of Arp2/3 complex and cofilin requiring RickA and Arp2/3 complex (111). In contrast, motility is faster and directionally persistent later in the infection (within 24–48 hours), resulting in longer straight actin tails. In the late phase, motility is independent of Arp2/3 complex and RickA, and requires rickettsial Sca2 protein. The amino-terminal domain of Sca2 is predicted to share secondary structure similarity with the FH2 domains of formins (112), and a central region in Sca2 has amino acid similarity to FH1 domains (113). Sca2 also has three WASP homology 2 (WH2) domains, that may bind to actin monomers (112; 113). Sca2 nucleates the assembly of linear actin filaments and promotes filament elongation by using profilin and inhibiting the activity of capping proteins (114). A number of SFG and TRG species, including R. africae, R. akari, R. australis, R. conorii, and R. rickettsii, encode full-length Sca2 containing an N-terminal Sec-dependent secretion signal, four putative WASP homology (WH2) domains, proline-rich regions (similar to formins), and autotransporter domain in the C-terminus. In contrast, Sca2 of R. prowazekii is truncated lacking the first three WH2 domains, proline-rich segments, and secretory signal sequence. Sca2 of R. typhi is shortened with overlapping autotransporter and WH2 domains, but divergent formin homology 1 (FH1) domain and secretion signal (113). R. prowazekii effector, RalF, also contains a proline-rich region which can serve as a formin modulator to regulate actin polymerization (115). These findings suggest that SFG rickettsiae utilize eukaryotic formin-like properties of Sca2 as a primary mechanism for moving intracellularly and spreading from cell to cell.
Signal transduction events:
An in vitro model of infection of cultured endothelial cells with R. rickettsii demonstrated early cell-to-cell spread with no noticeable host cell injury, accompanied by membrane damage and leading to cell death [44]. R. rickettsii and R. conorii infection of endothelial cells results in increased expression of tissue factor, IL-1α, intercellular and vascular cell-adhesion molecules, thrombomodulin, and E-selectin, increased synthesis of plasminogen activator inhibitor-1, von Willebrand factor release from Weibel-Palade bodies, and altered adhesiveness of platelets to the endothelial cell surface (86). Infection of cultured cells with R. prowazekii leads to increased secretion of prostaglandins PGE2 and PGI2 (116), enhanced synthesis of platelet activating factor (117), and transmigration of leukocytes (118). R. rickettsii and R. conorii infection in vitro also activates the fibrinolytic system leading to elevated levels of plasma fibrin(ogen)/degradation products (119).
The mechanisms underlying early host cell responses to rickettsial infection are mediated by the activation of nuclear transcription factor NF-κB, MAP kinase pathway(s), and phosphorylation-dephosphorylation mechanisms leading to the activation of protein kinase pathways. Infection of vascular endothelial cells with R. rickettsii or R. conorii results in the activation of NF-κB and p38 mitogen-activated protein kinase/activating transcription factor-2, as well as expression and secretion of several cytokines and chemokines (120; 121). Infection of endothelial cells with R. rickettsii or R. conorii stimulates the secretion of high levels of IL-6 and IL-8, increases the expression of adhesion molecules on the cell surface and adhesion of leukocytes in an IL-1α pathway-dependent manner (122). R. conorii infection of endothelium induces the expression of chemokines, namely CCL2, CCL3, CCL4, CCL5, CCL12, CCL19, CCL21, CX3CL1, CXCL1, CXCL9, and CXCL10 (123). The genes expressed in response to early rickettsial infection include IL-8 and monocyte chemoattractant protein, both of which contain NF-κB binding sites in their promoter regions, indicating that infection-induced alterations in gene expression may be regulated, in part, by activation of NF-κB (124). Apparently, R. rickettsii is also capable of directly interacting with NF-κB in the cytoplasm by an unidentified bacterial protease activity (125). Rickettsiae also manipulate host cell death mechanisms, as illustrated by apoptosis of infected cells consequent to the inhibition of infection-induced activation of NF-κB (124). Follow up studies to identify upstream signaling mechanisms further reveal an essential role for NF-κB activation in the maintenance of host cell mitochondrial integrity, maintenance of the balance between pro- and anti-apoptotic proteins, and prevention of activation of caspase cascade during R. rickettsii infection of cultured human endothelial cells (126; 127).
The activation of various STAT proteins is required for the antiviral activities of interferons [65]. Infection of endothelial cells by R. rickettsii and R. conorii triggers differential activation of STAT proteins (128; 129). Notably, STAT1 activation is mainly attributed to the secretion of IFN-β by infected endothelium, and IFN- β -mediated inhibition of rickettsial replication in host cells is dependent on STAT 1 (129). Because the transcription enhancer of IFN- β promoter binds to NF-κB, ATF-2, and interferon regulatory factors IRF3 and IRF7 (128), Rickettsia-induced IFN- β could be due to the outcome of one specific mechanism or possibly involve a combination of multiple mechanisms. In this regard, although there is no evidence for Ser396 phosphorylation as an indicator of IRF3 activation (129), there is increased expression of IRF7 and IRF9, suggesting a possible role for either one or potentially both of these IRFs during R. conorii infection. Analysis of downstream targets regulated by the JAK-STAT pathway further reveals that R. conorii infection induces the expression of interferon-regulated genes such as interferon stimulated gene 15 (ISG15), oligoadenylate synthase 1 (OAS1), and guanylate-binding protein 1 (GBP1) (130). Among these, ISG15 is mainly induced by type I interferons, whereas GBP1 and OAS1 are stimulated by both type I and type II IFNs. Intriguingly, there is unequivocal evidence for the robust expression of ISG15 mRNA and protein in R. conorii-infected endothelial cells, providing solid and novel evidence for its participation in post-translational modification of other cellular proteins via ISGylation (130; 131). Since ISG15 has been implicated in the regulation of anti-viral immune responses, it will be highly informative to determine its role(s) in bacterial infections of vascular endothelial cells.
R. rickettsii infection of endothelial cells causing extensive membrane damage leading to changes in the cytoskeleton suggests the involvement of oxidative stress in the pathogenesis of rickettsioses. Infected endothelial cells undergo oxidative stress with accumulation of intracellular superoxide anion (O2-) and extracellular H2O2, and marked reduction in the levels of both the protective intracellular thiols and activities of antioxidant enzymes (132). Further, in patients the genetic deficiency of enzyme glucose-6-phosphate dehydrogenase (G6PDH) results in greater severity of rickettsioses, suggesting the involvement of redox systems in combating the disease (43; 133). In this regard, higher expression of heme oxygenase (HO)-1, a rate-limiting enzyme in heme catabolism with established anti-oxidant functions, is an imoprtant cellular response to oxidative stress caused by the infection (134; 135). Bilirubin and carbon monoxide, generated as end products of HO-1 activity, decrease oxidative stress, reduce vascular constriction, attenuate inflammation, and inhibit apoptosis. Another important function of the heme-HO system is to influence the synthesis and secretion of prostaglandins (PGs) by regulating vascular cyclooxygenase (COX) activity. Marked induction in the levels of COX-2 and increased secretion of PGE2 and PGI2 occur in rickettsial infection of endothelial cells, and the biphasic increase in COX-2 expression is similar to the activation of NF-κB (116; 136). Induction in the levels of COX-2 and prostaglandin release in the vasculature may also contribute to increased vascular permeability during Rickettsia infection.
Noncoding RNAs
The term non-coding RNAs refers to those that do not encode a protein, and it has been assumed that most genetic information is transacted by proteins. However, recent evidence suggests that a significant portion of the genomes of prokaryotes and eukaryotes is transcribed into non-coding RNAs, many of which are alternatively spliced and/or processed into smaller products. Non-coding RNAs include microRNAs, small-RNAs (sRNAs), long non-coding RNAs, siRNAs, and snoRNAs. Non-coding RNAs are generally involved in the regulation of gene expression at the transcriptional and post-transcriptional levels. sRNAs types include true antisense RNAs, which are synthesized from the strand complementary to the mRNA they regulate, sRNAs that have limited complementarity with their targets, and sRNAs that bind to proteins and regulate protein activity. The sRNAs with limited complementarity are similar to eukaryotic microRNAs in their ability to modulate the activity and stability of multiple mRNAs. Recently, differentially expressed miRNAs have been identified in Rickettsia-infected endothelial cells (137).
MicroRNAs
MicroRNAs (miRNAs) are single stranded, typically 21–23 nucleotides long, non-coding RNAs expressed by eukaryotic cells, that function by degradation and/or suppression of protein translation based on sequence complementarity between the miRNA and targeted mRNA. As novel post-transcriptional regulators of gene expression, miRNAs have also been implicated as important determinants of immune responses during normal and pathogenic immunological responses (138). Therapeutic applications of miRNAs include a novel anti-miRNA treatment miravirsen currently in phase 2b clinical trials and viral vaccines attenuated through incorporation of miRNA target sequences in the preclinical testing, while miRNAs as biomarkers of infection also hold substantial promise. It is now well established that modulation of miRNAs associated with biological processes is one of the strategies adopted by bacterial pathogens to survive inside host cells. Pathogens alter the expression pattern of cellular miRNAs during infection, and it is now experimentally validated that intracellular bacterial pathogens, such as Listeria monocytogenes, Salmonella enterica serovar Typhimurium, Mycobacterium tuberculosis, and others have the ability to alter the host cellular miRNAs for their own survival (139). A recently published study has documented differential expression of several miRNAs in endothelial cells infected with R. rickettsii (137). Specifically, miRNA 200a-3p is highly upregulated both in infected endothelial cells in vitro and in vivo in lungs of R. conorii-infected mice. The findings further suggest inhibitory cross-talk between miR-200a-3p and NOTCH1 expression and indicate a potentially important role for this interrelationship in rickettsial biology, because NOTCH1 signaling along with other signaling mechanisms regulates endothelial proliferation and angiogenesis. Ongoing studies of mechanisms of altered endothelial miRNA expression are expected to provide new insight into regulatory elements governing host responses and pathogenesis during human rickettsial infections.
Small RNAs
Bacterial small RNAs (sRNAs) are typically 50–500 bp long noncoding RNAs, and comprise three different types, namely riboswitches which are located upstream of mRNAs, cis-acting sRNAs synthesized from the complementary strand of an open reading frame (ORF), and trans-acting sRNAs transcribed from the intergenic regions with only partial complementarity to their target genes. Bacterial sRNAs can activate or inhibit translation by either stabilizing the mRNA and opening the ribosome binding site or by degrading the target mRNA, respectively (140). Rickettsia prowazekii carries a high portion (approximately 24%) of non-coding DNA and an AT-rich genome with a GC content of 29.1% (141). A number of sRNAs were predicted in rickettsial genomes using complementary computational approaches (142). Analysis utilizing the next generation sequencing approach identified 35 novel trans-acting and 23 cis-acting sRNAs, and the expression of four novel sRNAs was confirmed by Northern blotting (143). The transcription start sites of five novel rickettsial sRNAs were also determined. These were further characterized using computational approaches to determine the location of −10 and −35 promoter motifs, secondary structure, and potential targets (143). These non-coding RNAs are hypothesized to be potentially critical post-transcriptional regulators involved in bacterial virulence, survival, stress response, metabolism, and other pathways. A recent report has further demonstrated the sRNA expression profile of R. prowazekii during infection of arthropod host cells in vitro (144). Analysis of the R. prowazekii transcriptome by strand-specific RNA sequencing has identified 67 cis-acting (antisense) and 26 trans-acting (intergenic) sRNAs expressed during the infection of Amblyomma americanum (AAE2) tick cells. Comparative expression during R. prowazekii infection of endothelial cells and AAE2 cells demonstrated significantly higher expression of four selected sRNAs in tick cells. Examination of the coding transcriptome revealed differential up-regulation of >150 rickettsial genes in either endothelial cells or AAE2 cells and yielded evidence for host cell-dependent utilization of alternative transcription start sites by 18 rickettsial genes (144). These results suggest differences in the expression of sRNAs as well as the coding transcriptome during the infection of human endothelial cells and arthropod cells and yield new insights into rickettsial virulence and transmission mechanisms. Investigations are underway to further elucidate the importance of these novel sRNAs in regulating rickettsial pathogenesis.
Strand-specific RNA sequencing of infected endothelial cells has also identified four riboswitches, 13 trans-acting (intergenic), and 22 cis-acting (antisense) small RNAs in R. conorii (145). The expression of four selected novel trans-acting sRNAs (Rc_sR31, Rc_sR33, Rc_sR35, and Rc_sR42) was further confirmed by Northern hybridization. Comparative analysis during infection of endothelial cells and arthropod AAE2 cells revealed significantly higher expression of Rc_sR35 and Rc_sR42 in endothelial cells, whereas Rc_sR31 and Rc_sR33 were expressed at similar levels in both cell types. Further, bioinformatics analysis predicted that several genes are involved in important biological processes as potential targets of Rc_sRs, and interaction of Rc_sR42 with cydA (cytochrome d ubiquinol oxidase subunit I) was validated (145). Rc_sR42 could potentially be involved either in stabilizing the cydA transcript as a result of the cleavage of polycistronic cydAB transcript or in the degradation of cydAB transcript by forming double stranded RNA. Rickettsiae are capable of synthesizing ATP during later stages of infection when the ATP supply in the host cytosol is exhausted. Significantly higher expression of Rc_sR42 at 24 h post-infection likely suggests the regulation of cydA mRNA at later stages of infection, which may facilitate survival of the bacteria in the intracellular niche. Differentially expressed sRNAs during vertebrate host-pathogen and vector-pathogen interactions suggests their role in survival and transovarial/transstadial transmission in arthropod vectors and in regulation of virulence in the human host. Comprehensive molecular studies employing appropriate heterologous model systems to generate knock-out mutants may reveal functional implications of these regulatory sRNAs during rickettsial pathogenesis. Identification of novel immune evasion strategies employed by bacterial sRNAs will significantly contribute to understanding of pathogenesis and designing of novel effective therapeutic approaches to combat diseases caused by intracellular pathogens.
CONCLUSION
Despite the small number of scientists pursuing knowledge of these extremely recalcitrant bacteria, the knowledge coming forth continues to surprise and emphasizes the rich opportunities for scientific discovery that the field offers.
Summary and Future Directions.
Arthropod-borne pathogenic Rickettsia species capable of causing significant morbidity and mortality in humans remain a major health issue across the globe.
Major rickettsial surface proteins OmpA, OmpB, and the beta peptide of OmpA interact with the host receptors α2β1, Ku70, and FGFR1, respectively, for internalization, indicating the strategic importance of redundant entry mechanisms to achieve intracytosolic niche in the host cells.
SFG rickettsiae form polar actin tails and exploit biphasic actin-based motility for intracellular movements and intercellular spread. The representative typhus group rickettsiae, either display erratic motility (R. typhi) or lack this activity (R. prowazekii).
As the preferred target cells in humans and in vivo animal models, Rickettsia-infected endothelial cells display pro-adhesive, pro-coagulant, and pro-inflammatory properties and anti-apoptotic functions of infection-induced NF-κB either prevent or delay host cell death to maintain their ‘intracellular niche’. R. rickettsii and R. conorii infection of endothelial cells also trigger IFN-β-mediated activation of JAK-STAT signaling and downstream interferon-stimulated genes as antiviral-like host responses.
Infection-induced regulatory effects of certain host microRNAs and expression of bacterial small RNAs (sRNAs) has recently been demonstrated in Rickettsia-infected endothelial cells. Further in-depth investigations of the mechanisms of miRNA and sRNA expression in the host and invading pathogens and their downstream regulatory effects are expected to reveal insights into host-pathogen interactions and determinants of host responses during human rickettsioses. In concert with established fundamentals of rickettsial pathogenesis, this new knowledge will facilitate the development of new and improved diagnostics, additional therapeutic remedies, and preventative vaccines.
As cytosolic sensors, ASC/caspase-1-dependent inflammasome contributes to detection of intracellular rickettsiae and overall host protection in vivo. Compared to other cytosolic bacteria, rickettsiae activate inflammasome with delayed kinetics. Identifying NLRs other than NLRP3 responsible for recognition of rickettsiae and understanding of the delayed kinetic mechanisms modified by rickettsiae could contribute to the design of an inflammasome-target vaccine or therapeutic approach.
TLR2/TLR4/MyD88 signaling plays a critical role in host protection against rickettsiae through mediating efficient antigen presentation by dendritic cells to immune effector cells of both innate and adaptive immunity.
In an overwhelming rickettsial infection, rickettsiae activate and differentiate CD4 T cells into both protective IFN-γ producing Th1 cells and CD4+CD25+IL-10-producing T regulatory cells. These inducible T regulatory cells suppress the protective response and, at least in part, contribute to the pathogenesis of fatal rickettsioses.
Contributor Information
Abha Sahni, The University of Texas Medical Branch at Galveston; absahni@utmb.edu.
Rong Fang, The University of Texas Medical Branch at Galveston; rofang@utmb.edu.
Sanjeev Sahni, The University of Texas Medical Branch at Galveston; sksahni@utmb.edu.
David H. Walker, The University of Texas Medical Branch at Galveston; dwalker@utmb.edu
References:
- 1.Weisburg WG, Dobson ME, Samuel JE, Dasch GA, Mallavia LP, et al. 1989. Phylogenetic diversity of the Rickettsiae. J Bacteriol 171:4202–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillespie JJ, Williams K, Shukla M, Snyder EE, Nordberg EK, et al. 2008. Rickettsia phylogenomics: unwinding the intricacies of obligate intracellular life. PLoS One 3:e2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouyer DH, Stenos J, Crocquet-Valdes P, Moron CG, Popov VL, et al. 2001. Rickettsia felis: molecular characterization of a new member of the spotted fever group. Int J Syst Evol Microbiol 51:339–47 [DOI] [PubMed] [Google Scholar]
- 4.Labruna MB, Walker DH. 2014. Rickettsia felis and changing paradigms about pathogenic rickettsiae. Emerg Infect Dis 20:1768–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parola P, Musso D, Raoult D. 2016. Rickettsia felis: the next mosquito-borne outbreak? Lancet Infect Dis 16:1112–3 [DOI] [PubMed] [Google Scholar]
- 6.Maina AN, Knobel DL, Jiang J, Halliday J, Feikin DR, et al. 2012. Rickettsia felis infection in febrile patients, western Kenya, 2007–2010. Emerg Infect Dis 18:328–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricketts HT. 1991. Some aspects of Rocky Mountain spotted fever as shown by recent investigations. 1909. Rev Infect Dis 13:1227–40 [DOI] [PubMed] [Google Scholar]
- 8.Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, et al. 2008. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis 47:1188–96 [DOI] [PubMed] [Google Scholar]
- 9.Shapiro MR, Fritz CL, Tait K, Paddock CD, Nicholson WL, et al. 2010. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis 50:541–8 [DOI] [PubMed] [Google Scholar]
- 10.Blanton LS, Walker DH. 2017. Flea-Borne Rickettsioses and Rickettsiae. Am J Trop Med Hyg 96:53–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman AS, Swerdlow DL, Dato VM, Anderson AD, Moodie CE, et al. 2009. Cluster of sylvatic epidemic typhus cases associated with flying squirrels, 2004–2006. Emerg Infect Dis 15:1005–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raoult D, Fournier PE, Fenollar F, Jensenius M, Prioe T, et al. 2001. Rickettsia africae, a tick-borne pathogen in travelers to sub-Saharan Africa. N Engl J Med 344:1504–10 [DOI] [PubMed] [Google Scholar]
- 13.Dumler JS, Walker DH. 2005. Rocky Mountain spotted fever--changing ecology and persisting virulence. N Engl J Med 353:551–3 [DOI] [PubMed] [Google Scholar]
- 14.Walker DH. 1989. Rocky Mountain spotted fever: a disease in need of microbiological concern. Clin Microbiol Rev 2:227–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oteo JA, Ibarra V, Blanco JR, Martinez de Artola V, Marquez FJ, et al. 2004. Dermacentor-borne necrosis erythema and lymphadenopathy: clinical and epidemiological features of a new tick-borne disease. Clin Microbiol Infect 10:327–31 [DOI] [PubMed] [Google Scholar]
- 16.Silva-Pinto A, Santos Mde L, Sarmento A. 2014. Tick-borne lymphadenopathy, an emerging disease. Ticks Tick Borne Dis 5:656–9 [DOI] [PubMed] [Google Scholar]
- 17.Walker DH, Paddock CD, Dumler JS. 2008. Emerging and re-emerging tick-transmitted rickettsial and ehrlichial infections. Med Clin North Am 92:1345–61, x [DOI] [PubMed] [Google Scholar]
- 18.Kazimirova M, Stibraniova I. 2013. Tick salivary compounds: their role in modulation of host defences and pathogen transmission. Front Cell Infect Microbiol 3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spencer RR, Parker RR. 1923. Rocky Mountain spotted fever: infectivity of fasting and recently fed ticks. Public Health Rep 38:333–919314866 [Google Scholar]
- 20.Walker DH, Hudnall SD, Szaniawski WK, Feng HM. 1999. Monoclonal antibody-based immunohistochemical diagnosis of rickettsialpox: the macrophage is the principal target. Mod Pathol 12:529–33 [PubMed] [Google Scholar]
- 21.Fournier PE, Gouriet F, Brouqui P, Lucht F, Raoult D. 2005. Lymphangitis-associated rickettsiosis, a new rickettsiosis caused by Rickettsia sibirica mongolotimonae: seven new cases and review of the literature. Clin Infect Dis 40:1435–44 [DOI] [PubMed] [Google Scholar]
- 22.Walker DH, Gear JH. 1985. Correlation of the distribution of Rickettsia conorii, microscopic lesions, and clinical features in South African tick bite fever. Am J Trop Med Hyg 34:361–71 [DOI] [PubMed] [Google Scholar]
- 23.Walker DH, Parks FM, Betz TG, Taylor JP, Muehlberger JW. 1989. Histopathology and immunohistologic demonstration of the distribution of Rickettsia typhi in fatal murine typhus. Am J Clin Pathol 91:720–4 [DOI] [PubMed] [Google Scholar]
- 24.Walker DH, Herrero-Herrero JI, Ruiz-Beltran R, Bullon-Sopelana A, Ramos-Hidalgo A. 1987. The pathology of fatal Mediterranean spotted fever. Am J Clin Pathol 87:669–72 [DOI] [PubMed] [Google Scholar]
- 25.Valbuena G, Bradford W, Walker DH. 2003. Expression analysis of the T-cell-targeting chemokines CXCL9 and CXCL10 in mice and humans with endothelial infections caused by rickettsiae of the spotted fever group. Am J Pathol 163:1357–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrero-Herrero JI, Walker DH, Ruiz-Beltran R. 1987. Immunohistochemical evaluation of the cellular immune response to Rickettsia conorii in taches noires. J Infect Dis 155:802–5 [DOI] [PubMed] [Google Scholar]
- 27.Walker DH, Paletta CE, Cain BG. 1980. Pathogenesis of myocarditis in Rocky Mountain spotted fever. Arch Pathol Lab Med 104:171–4 [PubMed] [Google Scholar]
- 28.Schmaier AH, Srikanth S, Elghetany MT, Normolle D, Gokhale S, et al. 2001. Hemostatic/fibrinolytic protein changes in C3H/HeN mice infected with Rickettsia conorii--a model for Rocky Mountain spotted fever. Thromb Haemost 86:871–9 [PubMed] [Google Scholar]
- 29.Elghetany MT, Walker DH. 1999. Hemostatic changes in Rocky Mountain spotted fever and Mediterranean spotted fever. Am J Clin Pathol 112:159–68 [DOI] [PubMed] [Google Scholar]
- 30.Walker DH, Crawford CG, Cain BG. 1980. Rickettsial infection of the pulmonary microcirculation: the basis for interstitial pneumonitis in Rocky Mountain spotted fever. Hum Pathol 11:263–72 [DOI] [PubMed] [Google Scholar]
- 31.Horney LF, Walker DH. 1988. Meningoencephalitis as a major manifestation of Rocky Mountain spotted fever. South Med J 81:915–8 [DOI] [PubMed] [Google Scholar]
- 32.Adams JS, Walker DH. 1981. The liver in Rocky Mountain spotted fever. Am J Clin Pathol 75:156–61 [DOI] [PubMed] [Google Scholar]
- 33.Walker DH, Staiti A, Mansueto S, Tringali G. 1986. Frequent occurrence of hepatic lesions in boutonneuse fever. Acta Trop 43:175–81 [PubMed] [Google Scholar]
- 34.Randall MB, Walker DH. 1984. Rocky Mountain spotted fever. Gastrointestinal and pancreatic lesions and rickettsial infection. Arch Pathol Lab Med 108:963–7 [PubMed] [Google Scholar]
- 35.Ruiz-Beltran R, Herrero-Herrero JI, Walker DH, Cunado-Rodriguez A. 1990. Mechanism of upper gastrointestinal hemorrhage in Mediterranean spotted fever. Trop Geogr Med 42:78–82 [PubMed] [Google Scholar]
- 36.Walker DH, Henderson FW, Hutchins GM. 1986. Rocky Mountain spotted fever: mimicry of appendicitis or acute surgical abdomen? Am J Dis Child 140:742–4 [DOI] [PubMed] [Google Scholar]
- 37.Walker DH, Mattern WD. 1979. Acute renal failure in Rocky Mountain spotted fever. Arch Intern Med 139:443–8 [PubMed] [Google Scholar]
- 38.Valbuena G, Walker DH. 2005. Changes in the adherens junctions of human endothelial cells infected with spotted fever group rickettsiae. Virchows Arch 446:379–82 [DOI] [PubMed] [Google Scholar]
- 39.Woods ME, Olano JP. 2008. Host defenses to Rickettsia rickettsii infection contribute to increased microvascular permeability in human cerebral endothelial cells. J Clin Immunol 28:174–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bechelli J, Smalley C, Milhano N, Walker DH, Fang R. 2015. Rickettsia massiliae and Rickettsia conorii Israeli Spotted Fever Strain Differentially Regulate Endothelial Cell Responses. PLoS One 10:e0138830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong B, Lee YS, Lee I, Shelite TR, Kunkeaw N, et al. 2013. Compartmentalized, functional role of angiogenin during spotted fever group rickettsia-induced endothelial barrier dysfunction: evidence of possible mediation by host tRNA-derived small noncoding RNAs. BMC Infect Dis 13:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sousa R, Franca A, Doria Nobrega S, Belo A, Amaro M, et al. 2008. Host- and microbe-related risk factors for and pathophysiology of fatal Rickettsia conorii infection in Portuguese patients. J Infect Dis 198:576–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker DH, Kirkman HN. 1980. Rocky Mountain spotted fever and deficiency in glucose-6-phosphate dehydrogenase. J Infect Dis 142:771. [DOI] [PubMed] [Google Scholar]
- 44.Walker DH, Hawkins HK, Hudson P. 1983. Fulminant Rocky Mountain spotted fever. Its pathologic characteristics associated with glucose-6-phosphate dehydrogenase deficiency. Arch Pathol Lab Med 107:121–5 [PubMed] [Google Scholar]
- 45.Walker DH, Radisch DL, Kirkman HN. 1983. Haemolysis with rickettsiosis and glucose-6-phosphate dehydrogenase deficiency. Lancet 2:217. [DOI] [PubMed] [Google Scholar]
- 46.Walker DH, Popov VL, Wen J, Feng HM. 1994. Rickettsia conorii infection of C3H/HeN mice. A model of endothelial-target rickettsiosis. Lab Invest 70:358–68 [PubMed] [Google Scholar]
- 47.Walker DH, Popov VL, Feng HM. 2000. Establishment of a novel endothelial target mouse model of a typhus group rickettsiosis: evidence for critical roles for gamma interferon and CD8 T lymphocytes. Lab Invest 80:1361–72 [DOI] [PubMed] [Google Scholar]
- 48.Walker DH, Olano JP, Feng HM. 2001. Critical role of cytotoxic T lymphocytes in immune clearance of rickettsial infection. Infect Immun 69:1841–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng HM, Wen J, Walker DH. 1993. Rickettsia australis infection: a murine model of a highly invasive vasculopathic rickettsiosis. Am J Pathol 142:1471–82 [PMC free article] [PubMed] [Google Scholar]
- 50.Feng HM, Popov VL, Walker DH. 1994. Depletion of gamma interferon and tumor necrosis factor alpha in mice with Rickettsia conorii-infected endothelium: impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect Immun 62:1952–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng HM, Whitworth T, Popov V, Walker DH. 2004. Effect of antibody on the rickettsia-host cell interaction. Infect Immun 72:3524–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng HM, Walker DH. 2000. Mechanisms of intracellular killing of Rickettsia conorii in infected human endothelial cells, hepatocytes, and macrophages. Infect Immun 68:6729–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Sousa R, Ismail N, Nobrega SD, Franca A, Amaro M, et al. 2007. Intralesional expression of mRNA of interferon- gamma, tumor necrosis factor- alpha, interleukin-10, nitric oxide synthase, indoleamine-2,3-dioxygenase, and RANTES is a major immune effector in Mediterranean spotted fever rickettsiosis. J Infect Dis 196:770–81 [DOI] [PubMed] [Google Scholar]
- 54.Xin L, Shelite TR, Gong B, Mendell NL, Soong L, et al. 2012. Systemic treatment with CpG-B after sublethal rickettsial infection induces mouse death through indoleamine 2,3-dioxygenase (IDO). PLoS One 7:e34062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker DH, Popov VL, Crocquet-Valdes PA, Welsh CJ, Feng HM. 1997. Cytokine-induced, nitric oxide-dependent, intracellular antirickettsial activity of mouse endothelial cells. Lab Invest 76:129–38 [PubMed] [Google Scholar]
- 56.Cragun WC, Bartlett BL, Ellis MW, Hoover AZ, Tyring SK, et al. 2010. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol 146:641–8 [DOI] [PubMed] [Google Scholar]
- 57.Curto P, Simoes I, Riley SP, Martinez JJ. 2016. Differences in Intracellular Fate of Two Spotted Fever Group Rickettsia in Macrophage-Like Cells. Front Cell Infect Microbiol 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fang R, Ismail N, Walker DH. 2012. Contribution of NK cells to the innate phase of host protection against an intracellular bacterium targeting systemic endothelium. Am J Pathol 181:185–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berg RE, Crossley E, Murray S, Forman J. 2005. Relative contributions of NK and CD8 T cells to IFN-gamma mediated innate immune protection against Listeria monocytogenes. J Immunol 175:1751–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D’Orazio SE, Troese MJ, Starnbach MN. 2006. Cytosolic localization of Listeria monocytogenes triggers an early IFN-gamma response by CD8+ T cells that correlates with innate resistance to infection. J Immunol 177:7146–54 [DOI] [PubMed] [Google Scholar]
- 61.Sumaria N, van Dommelen SL, Andoniou CE, Smyth MJ, Scalzo AA, Degli-Esposti MA. 2009. The roles of interferon-gamma and perforin in antiviral immunity in mice that differ in genetically determined NK-cell-mediated antiviral activity. Immunol Cell Biol 87:559–66 [DOI] [PubMed] [Google Scholar]
- 62.Billings AN, Feng HM, Olano JP, Walker DH. 2001. Rickettsial infection in murine models activates an early anti-rickettsial effect mediated by NK cells and associated with production of gamma interferon. Am J Trop Med Hyg 65:52–6 [DOI] [PubMed] [Google Scholar]
- 63.Fang R, Ismail N, Soong L, Popov VL, Whitworth T, et al. 2007. Differential interaction of dendritic cells with Rickettsia conorii: impact on host susceptibility to murine spotted fever rickettsiosis. Infect Immun 75:3112–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jordan JM, Woods ME, Olano J, Walker DH. 2008. The absence of Toll-like receptor 4 signaling in C3H/HeJ mice predisposes them to overwhelming rickettsial infection and decreased protective Th1 responses. Infect Immun 76:3717–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bechelli J, Smalley C, Zhao X, Judy B, Valdes P, et al. 2016. MyD88 Mediates Instructive Signaling in Dendritic Cells and Protective Inflammatory Response during Rickettsial Infection. Infect Immun 84:883–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jordan JM, Woods ME, Soong L, Walker DH. 2009. Rickettsiae stimulate dendritic cells through toll-like receptor 4, leading to enhanced NK cell activation in vivo. J Infect Dis 199:236–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jordan JM, Woods ME, Feng HM, Soong L, Walker DH. 2007. Rickettsiae-stimulated dendritic cells mediate protection against lethal rickettsial challenge in an animal model of spotted fever rickettsiosis. J Infect Dis 196:629–38 [DOI] [PubMed] [Google Scholar]
- 68.Fukata M, Vamadevan AS, Abreu MT. 2009. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin Immunol 21:242–53 [DOI] [PubMed] [Google Scholar]
- 69.Abdullah Z, Knolle PA. 2014. Scaling of immune responses against intracellular bacterial infection. Embo j 33:2283–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, et al. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 2:253–8 [DOI] [PubMed] [Google Scholar]
- 71.Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, et al. 2003. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med 198:1043–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arpaia N, Godec J, Lau L, Sivick KE, McLaughlin LM, et al. 2011. TLR signaling is required for Salmonella typhimurium virulence. Cell 144:675–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quevedo-Diaz MA, Song C, Xiong Y, Chen H, Wahl LM, et al. 2010. Involvement of TLR2 and TLR4 in cell responses to Rickettsia akari. J Leukoc Biol 88:675–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akira S, Takeda K. 2004. Toll-like receptor signalling. Nat Rev Immunol 4:499–511 [DOI] [PubMed] [Google Scholar]
- 75.Schroder K, Tschopp J. 2010. The inflammasomes. Cell 140:821–32 [DOI] [PubMed] [Google Scholar]
- 76.Smalley C, Bechelli J, Rockx-Brouwer D, Saito T, Azar SR, et al. 2016. Rickettsia australis Activates Inflammasome in Human and Murine Macrophages. PLoS One 11:e0157231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu J, Fernandes-Alnemri T, Alnemri ES. 2010. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J Clin Immunol 30:693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cai S, Batra S, Wakamatsu N, Pacher P, Jeyaseelan S. 2012. NLRC4 inflammasome-mediated production of IL-1beta modulates mucosal immunity in the lung against gram-negative bacterial infection. J Immunol 188:5623–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ceballos-Olvera I, Sahoo M, Miller MA, Del Barrio L, Re F. 2011. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1beta is deleterious. PLoS Pathog 7:e1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones JW, Kayagaki N, Broz P, Henry T, Newton K, et al. 2010. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A 107:9771–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rollwagen FM, Bakun AJ, Dorsey CH, Dasch GA. 1985. Mechanisms of immunity to infection with typhus rickettsiae: infected fibroblasts bear rickettsial antigens on their surfaces. Infect Immun 50:911–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feng H, Popov VL, Yuoh G, Walker DH. 1997. Role of T lymphocyte subsets in immunity to spotted fever group Rickettsiae. J Immunol 158:5314–20 [PubMed] [Google Scholar]
- 83.Fang R, Ismail N, Shelite T, Walker DH. 2009. CD4+ CD25+ Foxp3- T-regulatory cells produce both gamma interferon and interleukin-10 during acute severe murine spotted fever rickettsiosis. Infect Immun 77:3838–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mansueto P, Vitale G, Di Lorenzo G, Arcoleo F, Mansueto S, Cillari E. 2008. Immunology of human rickettsial diseases. J Biol Regul Homeost Agents 22:131–9 [PubMed] [Google Scholar]
- 85.Valbuena G, Walker DH. 2006. The endothelium as a target for infections. Annu Rev Pathol 1:171–98 [DOI] [PubMed] [Google Scholar]
- 86.Sahni A, Narra HP, Walker DH, Sahni SK. Endothelial activation and injury: the mechanisms of rickettsial vasculitis In Vascular Responses to Pathogens, Edited: Stokes K and Gavins F 2016:111–22: Elsevier Press [Google Scholar]
- 87.Blanc G, Ngwamidiba M, Ogata H, Fournier PE, Claverie JM, Raoult D. 2005. Molecular evolution of rickettsia surface antigens: evidence of positive selection. Mol Biol Evol 22:2073–83 [DOI] [PubMed] [Google Scholar]
- 88.Sears KT, Ceraul SM, Gillespie JJ, Allen ED Jr., Popov VL, et al. 2012. Surface proteome analysis and characterization of surface cell antigen (Sca) or autotransporter family of Rickettsia typhi. PLoS Pathog 8:e1002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ngwamidiba M, Blanc G, Raoult D, Fournier PE. 2006. Sca1, a previously undescribed paralog from autotransporter protein-encoding genes in Rickettsia species. BMC Microbiol 6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chan YG, Riley SP, Martinez JJ. 2010. Adherence to and invasion of host cells by spotted fever group rickettsia species. Front Microbiol 1:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hackstadt T, Messer R, Cieplak W, Peacock MG. 1992. Evidence for proteolytic cleavage of the 120-kilodalton outer membrane protein of rickettsiae: identification of an avirulent mutant deficient in processing. Infect Immun 60:159–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinez JJ, Seveau S, Veiga E, Matsuyama S, Cossart P. 2005. Ku70, a component of DNA-dependent protein kinase, is a mammalian receptor for Rickettsia conorii. Cell 123:1013–23 [DOI] [PubMed] [Google Scholar]
- 93.Li H, Walker DH. 1998. rOmpA is a critical protein for the adhesion of Rickettsia rickettsii to host cells. Microb Pathog 24:289–98 [DOI] [PubMed] [Google Scholar]
- 94.Hillman RD Jr., Baktash YM, Martinez JJ 2013. OmpA-mediated rickettsial adherence to and invasion of human endothelial cells is dependent upon interaction with alpha2beta1 integrin. Cell Microbiol 15:727–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Noriea NF, Clark TR, Hackstadt T. 2015. Targeted knockout of the Rickettsia rickettsii OmpA surface antigen does not diminish virulence in a mammalian model system. MBio 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Riley SP, Goh KC, Hermanas TM, Cardwell MM, Chan YG, Martinez JJ. 2010. The Rickettsia conorii autotransporter protein Sca1 promotes adherence to nonphagocytic mammalian cells. Infect Immun 78:1895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cardwell MM, Martinez JJ. 2009. The Sca2 autotransporter protein from Rickettsia conorii is sufficient to mediate adherence to and invasion of cultured mammalian cells. Infect Immun 77:5272–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dreher-Lesnick SM, Ceraul SM, Rahman MS, Azad AF. 2008. Genome-wide screen for temperature-regulated genes of the obligate intracellular bacterium, Rickettsia typhi. BMC Microbiol 8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gillespie JJ, Kaur SJ, Rahman MS, Rennoll-Bankert K, Sears KT, et al. 2015. Secretome of obligate intracellular Rickettsia. FEMS Microbiol Rev 39:47–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park H, Lee JH, Gouin E, Cossart P, Izard T. 2011. The rickettsia surface cell antigen 4 applies mimicry to bind to and activate vinculin. J Biol Chem 286:35096–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gong B, Shelite T, Mei FC, Ha T, Hu Y, et al. 2013. Exchange protein directly activated by cAMP plays a critical role in bacterial invasion during fatal rickettsioses. Proc Natl Acad Sci U S A 110:19615–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sahni A, Patel J, Narra HP, Schroeder CLC, Walker DH, Sahni SK. 2017. Fibroblast growth factor receptor-1 mediates internalization of pathogenic spotted fever rickettsiae into host endothelium. PLoS One 12:e0183181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Noriea NF, Clark TR, Mead D, Hackstadt T. 2017. Proteolytic Cleavage of the Immunodominant Outer Membrane Protein rOmpA in Rickettsia rickettsii. J Bacteriol 199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heinzen RA, Hayes SF, Peacock MG, Hackstadt T. 1993. Directional actin polymerization associated with spotted fever group Rickettsia infection of Vero cells. Infect Immun 61:1926–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Teysseire N, Chiche-Portiche C, Raoult D. 1992. Intracellular movements of Rickettsia conorii and R. typhi based on actin polymerization. Res Microbiol 143:821–9 [DOI] [PubMed] [Google Scholar]
- 106.Martinez JJ, Cossart P. 2004. Early signaling events involved in the entry of Rickettsia conorii into mammalian cells. J Cell Sci 117:5097–106 [DOI] [PubMed] [Google Scholar]
- 107.Gouin E, Egile C, Dehoux P, Villiers V, Adams J, et al. 2004. The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature 427:457–61 [DOI] [PubMed] [Google Scholar]
- 108.Balraj P, Nappez C, Raoult D, Renesto P. 2008. Western-blot detection of RickA within spotted fever group rickettsiae using a specific monoclonal antibody. FEMS Microbiol Lett 286:257–62 [DOI] [PubMed] [Google Scholar]
- 109.Simser JA, Rahman MS, Dreher-Lesnick SM, Azad AF. 2005. A novel and naturally occurring transposon, ISRpe1 in the Rickettsia peacockii genome disrupting the rickA gene involved in actin-based motility. Mol Microbiol 58:71–9 [DOI] [PubMed] [Google Scholar]
- 110.Ogata H, Renesto P, Audic S, Robert C, Blanc G, et al. 2005. The genome sequence of Rickettsia felis identifies the first putative conjugative plasmid in an obligate intracellular parasite. PLoS Biol 3:e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reed SC, Lamason RL, Risca VI, Abernathy E, Welch MD. 2014. Rickettsia actin-based motility occurs in distinct phases mediated by different actin nucleators. Curr Biol 24:98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haglund CM, Choe JE, Skau CT, Kovar DR, Welch MD. 2010. Rickettsia Sca2 is a bacterial formin-like mediator of actin-based motility. Nat Cell Biol 12:1057–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kleba B, Clark TR, Lutter EI, Ellison DW, Hackstadt T. 2010. Disruption of the Rickettsia rickettsii Sca2 autotransporter inhibits actin-based motility. Infect Immun 78:2240–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lamason RL, Welch MD. 2017. Actin-based motility and cell-to-cell spread of bacterial pathogens. Curr Opin Microbiol 35:48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alix E, Chesnel L, Bowzard BJ, Tucker AM, Delprato A, et al. 2012. The capping domain in RalF regulates effector functions. PLoS Pathog 8:e1003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rydkina E, Sahni A, Baggs RB, Silverman DJ, Sahni SK. 2006. Infection of human endothelial cells with spotted fever group rickettsiae stimulates cyclooxygenase 2 expression and release of vasoactive prostaglandins. Infect Immun 74:5067–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Walker TS, Mellott GE. 1993. Rickettsial stimulation of endothelial platelet-activating factor synthesis. Infect Immun 61:2024–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bechah Y, Capo C, Raoult D, Mege JL. 2008. Infection of endothelial cells with virulent Rickettsia prowazekii increases the transmigration of leukocytes. J Infect Dis 197:142–7 [DOI] [PubMed] [Google Scholar]
- 119.Rao AK, Schapira M, Clements ML, Niewiarowski S, Budzynski AZ, et al. 1988. A prospective study of platelets and plasma proteolytic systems during the early stages of Rocky Mountain spotted fever. N Engl J Med 318:1021–8 [DOI] [PubMed] [Google Scholar]
- 120.Clifton DR, Rydkina E, Huyck H, Pryhuber G, Freeman RS, et al. 2005. Expression and secretion of chemotactic cytokines IL-8 and MCP-1 by human endothelial cells after Rickettsia rickettsii infection: regulation by nuclear transcription factor NF-kappaB. Int J Med Microbiol 295:267–78 [DOI] [PubMed] [Google Scholar]
- 121.Rydkina E, Silverman DJ, Sahni SK. 2005. Activation of p38 stress-activated protein kinase during Rickettsia rickettsii infection of human endothelial cells: role in the induction of chemokine response. Cell Microbiol 7:1519–30 [DOI] [PubMed] [Google Scholar]
- 122.Kaplanski G, Teysseire N, Farnarier C, Kaplanski S, Lissitzky JC, et al. 1995. IL-6 and IL-8 production from cultured human endothelial cells stimulated by infection with Rickettsia conorii via a cell-associated IL-1 alpha-dependent pathway. J Clin Invest 96:2839–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mansueto P, Vitale G, Cascio A, Seidita A, Pepe I, et al. 2012. New insight into immunity and immunopathology of Rickettsial diseases. Clin Dev Immunol 2012:967852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Clifton DR, Rydkina E, Freeman RS, Sahni SK. 2005. NF-kappaB activation during Rickettsia rickettsii infection of endothelial cells involves the activation of catalytic IkappaB kinases IKKalpha and IKKbeta and phosphorylation-proteolysis of the inhibitor protein IkappaBalpha. Infect Immun 73:155–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sporn LA, Sahni SK, Lerner NB, Marder VJ, Silverman DJ, et al. 1997. Rickettsia rickettsii infection of cultured human endothelial cells induces NF-kappaB activation. Infect Immun 65:2786–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Clifton DR, Goss RA, Sahni SK, van Antwerp D, Baggs RB, et al. 1998. NF-κB-dependent inhibition of apoptosis is essential for host cellsurvival during Rickettsia rickettsii infection. Proc Natl Acad Sci U S A 95:4646–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sahni SK, Van Antwerp DJ, Eremeeva ME, Silverman DJ, Marder VJ, Sporn LA. 1998. Proteasome-independent activation of nuclear factor kappaB in cytoplasmic extracts from human endothelial cells by Rickettsia rickettsii. Infect Immun 66:1827–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sahni SK, Kiriakidi S, Colonne MP, Sahni A, Silverman DJ. 2009. Selective activation of signal transducer and activator of transcription (STAT) proteins STAT1 and STAT3 in human endothelial cells infected with Rickettsia rickettsii. Clin Microbiol Infect 15 Suppl 2:303–4 [DOI] [PubMed] [Google Scholar]
- 129.Colonne PM, Eremeeva ME, Sahni SK. 2011. Beta interferon-mediated activation of signal transducer and activator of transcription protein 1 interferes with Rickettsia conorii replication in human endothelial cells. Infect Immun 79:3733–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Colonne PM, Sahni A, Sahni SK. 2013. Suppressor of cytokine signalling protein SOCS1 and UBP43 regulate the expression of type I interferon-stimulated genes in human microvascular endothelial cells infected with Rickettsia conorii. J Med Microbiol 62:968–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Colonne PM, Sahni A, Sahni SK. 2011. Rickettsia conorii infection stimulates the expression of ISG15 and ISG15 protease UBP43 in human microvascular endothelial cells. Biochem Biophys Res Commun 416:153–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sahni SK, Rydkina E. 2009. Host-cell interactions with pathogenic Rickettsia species. Future Microbiol 4:323–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Whelton A, Donadio JV Jr., Elisberg BL 1968. Acute renal failure complicating rickettsial infections in glucose-6-phosphate dehydrogenase-deficient individuals. Ann Intern Med 69:323–8 [DOI] [PubMed] [Google Scholar]
- 134.Rydkina E, Sahni A, Silverman DJ, Sahni SK. 2002. Rickettsia rickettsii infection of cultured human endothelial cells induces heme oxygenase 1 expression. Infect Immun 70:4045–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chung SW, Hall SR, Perrella MA. 2009. Role of haem oxygenase-1 in microbial host defence. Cell Microbiol 11:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rydkina E, Turpin LC, Silverman DJ, Sahni SK. 2009. Rickettsia rickettsii infection of human pulmonary microvascular endothelial cells: modulation of cyclooxygenase-2 expression. Clin Microbiol Infect 15 Suppl 2:300–2 [DOI] [PubMed] [Google Scholar]
- 137.Sahni A, Narra HP, Patel J, Sahni SK. 2017. MicroRNA Signature of Human Microvascular Endothelium Infected with Rickettsia rickettsii. Int J Mol Sci 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. 2010. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol 10:111–22 [DOI] [PubMed] [Google Scholar]
- 139.Das K, Garnica O, Dhandayuthapani S. 2016. Modulation of Host miRNAs by Intracellular Bacterial Pathogens. Front Cell Infect Microbiol 6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Storz G, Vogel J, Wassarman KM. 2011. Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell 43:880–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Holste D, Weiss O, Grosse I, Herzel H. 2000. Are noncoding sequences of Rickettsia prowazekii remnants of “neutralized” genes? J Mol Evol 51:353–62 [DOI] [PubMed] [Google Scholar]
- 142.Schroeder CL, Narra HP, Rojas M, Sahni A, Patel J, et al. 2015. Bacterial small RNAs in the Genus Rickettsia. BMC Genomics 16:1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schroeder CL, Narra HP, Sahni A, Rojas M, Khanipov K, et al. 2016. Identification and characterization of novel small RNAs in Rickettsia prowazekii. Front Microbiol 7:859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schroeder CLC, Narra HP, Sahni A, Khanipov K, Patel J, et al. 2017. Transcriptional profiling of Rickettsia prowazekii coding and non-coding transcripts during in vitro host-pathogen and vector-pathogen interactions. Ticks Tick Borne Dis 8:827–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Narra HP, Schroeder CLC, Sahni A, Rojas M, Khanipov K, et al. 2016. Small regulatory RNAs of Rickettsia conorii. Sci Rep 6:36728. [DOI] [PMC free article] [PubMed] [Google Scholar]