Abstract
Pseudomonas aeruginosa and Aspergillus fumigatus are pathogens frequently co-inhabiting immunocompromised patient airways, particularly in people with cystic fibrosis. Both microbes depend on the availability of iron, and compete for iron in their microenvironment. We showed previously that the P. aeruginosa siderophore pyoverdine is the main instrument in battling A. fumigatus biofilms, by iron chelation and denial of iron to the fungus. Here we show that A. fumigatus siderophores defend against anti-fungal P. aeruginosa effects. P. aeruginosa supernatants produced in the presence of wildtype A. fumigatus planktonic supernatants (Afsup) showed less activity against A. fumigatus biofilms than P. aeruginosa supernatants without Afsup, despite higher production of pyoverdine by P. aeruginosa. Supernatants of A. fumigatus cultures lacking the sidA gene (AfΔsidA), unable to produce hydroxamate siderophores, were less capable of protecting A. fumigatus biofilms from P. aeruginosa supernatants and pyoverdine. AfΔsidA biofilm was more sensitive towards inhibitory effects of pyoverdine, the iron chelator deferiprone (DFP), or amphothericin B than wildtype A. fumigatus biofilm. Supplementation of sidA-deficient A. fumigatus biofilm with A. fumigatus siderophores restored resistance to pyoverdine. The A. fumigatus siderophore production inhibitor celastrol sensitized wildtype A. fumigatus biofilms towards the anti-fungal activity of DFP. In conclusion, A. fumigatus hydroxamate siderophores play a pivotal role in A. fumigatus competition for iron against P. aeruginosa.
Introduction
Ecosystems of pathogens have been described with regard to a multitude of diseases [1–3]. The bacterium Pseudomonas aeruginosa and the fungus Aspergillus fumigatus form such an ecosystem, e.g. when chronically colonizing the lungs of cystic fibrosis (CF) individuals [4–7]. Both pathogens have been associated with deterioration of lung function [4–17], and their combined presence in airways of CF patients seems to aggravate disease progression [18,19]. P. aeruginosa and A. fumigatus also are prominent opportunistic pathogens in immune-compromised patients, particularly in those with neutropenia [20,21].
Previous studies have focused on A. fumigatus inhibition caused by P. aeruginosa products such as pyocyanin (5-N-methyl-1-hydroxyphenazine) [22–25], 1-hydroxyphenazine [22,24,25], phenazine-1-carboxamide and phenazine-1-carboxylic acid [25]. We recently reported that the P. aeruginosa product pyoverdine is the major mediator of P. aeruginosa inhibitory function towards A. fumigatus biofilms [26]. Pyoverdine, the major siderophore of P. aeruginosa [27,28], strongly binds to iron, which is an essential co-factor for both P. aeruginosa and A. fumigatus [29–31]. Pyoverdine-bound iron is no longer available for A. fumigatus, starving A. fumigatus of iron, and resulting in fungistasis [26]. The question arose whether A. fumigatus could counteract P. aeruginosa inhibition. Here we provide evidence that A. fumigatus hydroxamate siderophores in times of iron shortage, created by a competing microbe, ensure availability of the essential co-factor iron exclusively to the fungus. Concomitantly, interference with A. fumigatus siderophore production renders the fungus more sensitive to anti-fungal effects of iron chelators, and possibly more sensitive even to effects of anti-fungal drugs not involved in iron chelation, like amphotericin B.
Materials and methods
Materials
Pyoverdine (PYOV), 3-hydroxy-1,2-dimethyl-4(1H)pyridine (deferiprone, DFP), celastrol, 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide inner salt (XTT), and menadione were purchased from Sigma-Aldrich (St. Louis, MO). Amphotericin B (AmB) was derived from X-Gen Pharmaceuticals Inc. (Horseheads, NY). Chrome Azurol S (CAS) was purchased from MP Biomedicals (Solon, OH). Ferri- and desferri-triacetylfusarinine C (TAFC, DF-TAFC) were purified as described previously [32].
Isolates
All isolates used in this study are summarized in Table 1.
Table 1. Isolates used in this study.
| Organism | Isolate | Description | ATCC | Reference |
|---|---|---|---|---|
| A. fumigatus | 10AF | Virulent patient isolate | 90240 | [33,34] |
| A. fumigatus | AF13073 | Parental strain for AfΔsidA | 13073 | |
| A. fumigatus | AfΔsidA | l-ornithine-N 5-mono-oxygenase deficient A. fumigatus mutant strain | [35] | |
| A. fumigatus | AF46645 | Parental strain for AfΔsidC and AfΔsidF | 46645 | |
| A. fumigatus | AfΔsidC | Deficient for the hydroxamate siderophores ferricrocin (FC) and hydroxy-FC (HFC) | [36] | |
| A. fumigatus | AfΔsidF | Deficient for the hydroxamate siderophores fusarinine C (FsC) and triacetylfusarinine C (TAFC). | [36] | |
| A. fumigatus | AfS77 | Derivate of ATCC 46645 | [37] | |
| P. aeruginosa | PA14 | Parental strain for pvdD- and pvdD-pchE- | [38] | |
| P. aeruginosa | pvdD- | Pyoverdine deficient mutant | [39] | |
| P. aeruginosa | pvdD-pchE- | Pyoverdine/pyochelin deficient mutant | [26] |
The work flow for the following procedures is summarized in S1 Fig.A. fumigatus supernatant production
A. fumigatus conidia were inoculated into RPMI 1640 medium (RPMI, Lonza, Walkersville, MD) at 2.5x104 conidia/ml. A. fumigatus suspensions were incubated at 37°C for 48h (S1 Fig). A. fumigatus supernatants (Afsup) were filtered (0.22 μm) for sterility after the growth period.
Pseudomonas supernatant production and pyoverdine measurement
PA14 supernatants were prepared as detailed previously [40]. Briefly, P. aeruginosa [5 x 107 cells/ml] was inoculated into RPMI 1640 medium, or mixtures of RPMI and Afsup, and incubated at 37°C for 24h. Bacterial growth was measured at 600 nm with a spectrophotometer (Genesys 20, Thermo Fisher Scientific Inc., Waltham, MA). Bacterial cultures were centrifuged at 200 x g for 30 min at room temperature, and filtered (0.22 μm). Pyoverdine production in the supernatant was measured as described previously [41] at 405 nm. Pyoverdine measurements were normalized to bacterial growth using the formula: Relative PYOV expression = OD405 / OD600. At the concentrations used in this study, pyoverdine, a colored substance, did not interfere with the colorimetric XTT assay used for determination of fungal metabolism. PYOV concentrations in undiluted P. aeruginosa supernatants are about 30 μM. Pyoverdine concentrations in sputum have been shown to be between 0.3 and 51 μM [42].
Assay for the measurement of metabolism of A. fumigatus forming (BCAM assay, Bioassay-Conidia-Agar-Metabolic) or preformed (BHAM assay, Bioassay-Hyphae-Agar-Metabolic) biofilms
BCAM and BHAM assays were performed as described previously [26]. In these assays, A. fumigatus grows out into biofilms covering the agar surface. Briefly, RPMI agar containing 2.5x104 to 105 A. fumigatus conidia/ml agar (as specified for different experiments in the Results section) was distributed into sterile flat-bottom 96 well cell culture plates (COSTAR, Corning, NY) at 100 μl/well. Upon agar solidification, wells were either incubated at 37°C for 24 hours before loading (= BHAM assays), or immediately loaded with 100 μl of test substances (= BCAM assays). Control wells on each test plate contained 100 μl of RPMI 1640 medium, allowing the conversion of test results to % of the RPMI control (= 100%). Loaded plates were incubated at 37°C for 24 hours. Fungal metabolism was determined by XTT metabolic assay at 490 nm [40,43]. Menadione (vitamin K3) was used as an ingredient in the XTT metabolic assay, boosting the reduction of tetrazolium salts to formazans. XTT assays were evaluated using a plate reader (Opsys MR, DYNEX Technologies, Chantilly, VA). Although XTT is a measure of metabolic activity of cells, previous studies of A. fumigatus have indicated that XTT results are linear with mass, and equated XTT result with dry weight [44–46].
Aspergillus growth assays
AfΔsidA (104conidia) was point-inoculated on 2 ml solid minimal medium [47] in the presence of 50–600 μl PA14 wildtype or PA14 PaΔpvdD bacterial supernatant with or without supplementation of FeSO4 [1 μM]. Radial fungal growth was scored after incubation of the plates for 48 hours at 37°C.
Chrome azurol S (CAS) assay
For measurement of siderophore production 10x CAS assay reagent was prepared as described previously [48]. One part 10x CAS reagent was combined with 9 parts Afsups in RPMI, and incubated at 37°C for 24 hours. Mixtures were measured using the plate reader, and compared to RPMI not containing CAS reagent or RPMI/1x CAS reagent as reference points.
Statistical analysis
Results were analyzed using Student’s t test, if two groups were compared, and by 1-way ANOVA, combined with a Tukey’s post-test for multiple comparisons. Data reported as percentages of the control value were compared after arcsin transformation of the proportions [26]. All data in this study are expressed as a mean ± SD.The number of replicates in each assay is four or higher. Assays were repeated at least twice, and a representative experiment is shown. Supporting information on data sets used in this study is provided in S1 Table.
Results
A. fumigatus supernatants induce pyoverdine production by P. aeruginosa
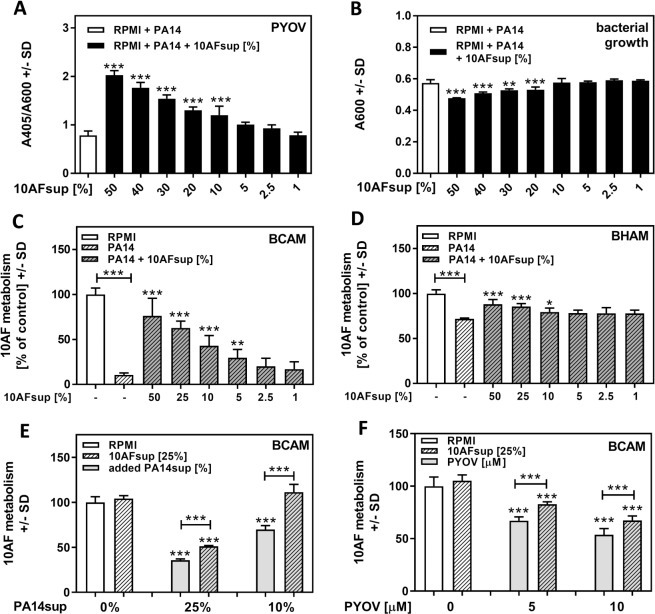
Fungal supernatants (Afsup), produced by planktonic growth of A. fumigatus strain 10AF in RPMI (experimental setup described in S1 Fig), induced pyoverdine production by P. aeruginosa in a dose-dependent manner, with 10% Afsup still significantly inducing pyoverdine production. As pyoverdine is induced in response to iron shortage, increased pyoverdine production here suggests sequestration of iron from the growth medium by Afsup (Fig 1A).
Fig 1. A. fumigatus supernatant effects on P. aeruginosa growth and pyoverdine production.
Planktonic 10AF supernatant (10AFsup) was diluted in RPMI from 50% to 0% 10AFsup, and incubated with P. aeruginosa cells (5x107/ml) at 37°C for 24h. Relative pyoverdine (PYOV) concentrations (A) were calculated using the quotient A405 (PYOV)/A600 (bacterial growth: B). Supernatants shown in A and B, as well as PA14 supernatant not containing 10Afsup, were compared with respect to their activities on 10AF forming biofilm (C: BCAM) or preformed biofilm (D: BHAM) metabolism. E: 10AF forming biofilm was incubated with 10 or 25% PA14 wildtype supernatant with or without the addition of 25% 10AFsup for 24 hours. Effects on 10AF forming biofilm metabolism were evaluated by XTT assay. F: 10AF forming biofilm was incubated with 5 or 10 μM pyoverdine (PYOV) with or without the addition of 25% 10AFsup for 24 hours. Effects on 10AF forming biofilm metabolism were evaluated by XTT assay. Statistics for A and B: 1way ANOVA, RPMI (white bar) vs. all groups containing Afsup (black bars). Statistics for C and D: 1way ANOVA, PA14 supernatant (white striped bar) vs. PA14 supernatant containing 10AFsup (grey striped bars). Other comparisons by t-Test as indicated by the ends of the brackets. Statistics for E and F: t-Test, RPMI (white bar) vs. all other bars. Other comparisons as indicated by the ends of the brackets. One, two or three asterisks = p ≤ 0.05, p ≤ 0.01 or p ≤ 0.001, respectively.
Concentrations of Afsup higher than 10% interfered with bacterial growth in a concentration-dependent manner (Fig 1B). As iron is a major co-factor for microbial growth, the reason for inhibitory effects of Afsup on P. aeruginosa growth might be a reaction to iron denial. Although gliotoxin has been suggested as an anti-microbial factor [49], in our hands supernatants produced by an A. fumigatus mutant unable to produce gliotoxin [50] affected P. aeruginosa growth to a similar degree as supernatants produced by its parent (S2 Fig). Distilled water (25%), instead of Afsup (25%) during P. aeruginosa supernatant preparation did not result in interference with P. aeruginosa effects on A. fumigatus biofilm metabolism, indicating that P. aeruginosa supernatant dilution by Afsups was not the reason for the protective effects of Afsups (S3 Fig).
In order to verify that Afsup indeed induced production of pyoverdine, we used a PA14 mutant not able to produce pyoverdine (PaΔpvdD) [39]. With or without the presence of Afsup, PaΔpvdD supernatant did not absorb at 405 nm, confirming that Afsup did not induce production of an unknown P. aeruginosa product detectable at 405 nm (S4A Fig).
Afsup protects A. fumigatus forming biofilm from P. aeruginosa anti-fungal activity and pure pyoverdine
Pyoverdine has detrimental effects on Af biofilm metabolism [26]. Surprisingly, although containing high concentrations of pyoverdine (Fig 1A), P. aeruginosa supernatants produced in the presence of Afsup were less inhibitory for forming (Fig 1C) or preformed (Fig 1D) A. fumigatus biofilms than P. aeruginosa supernatants produced without Afsups,whereas Afsups up to 50% did not affect A. fumigatus biofilms when administered alone. Similarly, when P. aeruginosa supernatants and Afsups were prepared separately, their combination was less inhibitory to A. fumigatus biofilms than P. aeruginosa supernatant alone (Fig 1E).
Presumably owing to their lack of pyoverdine production, supernatants of PaΔpvdD were less inhibitory to 10AF biofilms than PA14 supernatants. The presence of Afsup further decreased the inhibitory activity of PaΔpvdD supernatants to RPMI control levels (S4B Fig). Protective Afsup effects were also observed when Afsups were combined with pure pyoverdine (Fig 1F).
Taken together, these data indicate that despite its ability to induce pyoverdine production by P. aeruginosa, Afsup protects A. fumigatus biofilms.
Stability of protective Af supernatant effects
In order to determine the reason for protection of A. fumigatus biofilms by Afsup, we first tested stability of 10AFsup to heat, and long-term storage. 10AFsup was heated to 56°C or 90°C for 30 minutes, or subjected to three freeze-thaw cycles. Treated or untreated 10AFsups were diluted to 25%, combined with P. aeruginosa supernatants, and tested for effects on A. fumigatus biofilm metabolism. Our results show that heat does not destroy the protective compound in Afsup (Fig 2A). Repeated freeze-thaw cycles diminished, but did not abolish protection (Fig 2A). We also kept 10AFsup at 4°C for 12 months, and measured protection from pyoverdine every 3 months. The protective potential of Afsup was almost constant over the 12 months period (Fig 2B). When 10AFsup was kept at room temperature for 12 months, protection was marginally lower, but still significant (Fig 2B). Taken together, our data suggest that the protective compound in 10AFsup is stable.
Fig 2. Stability of Afsup.
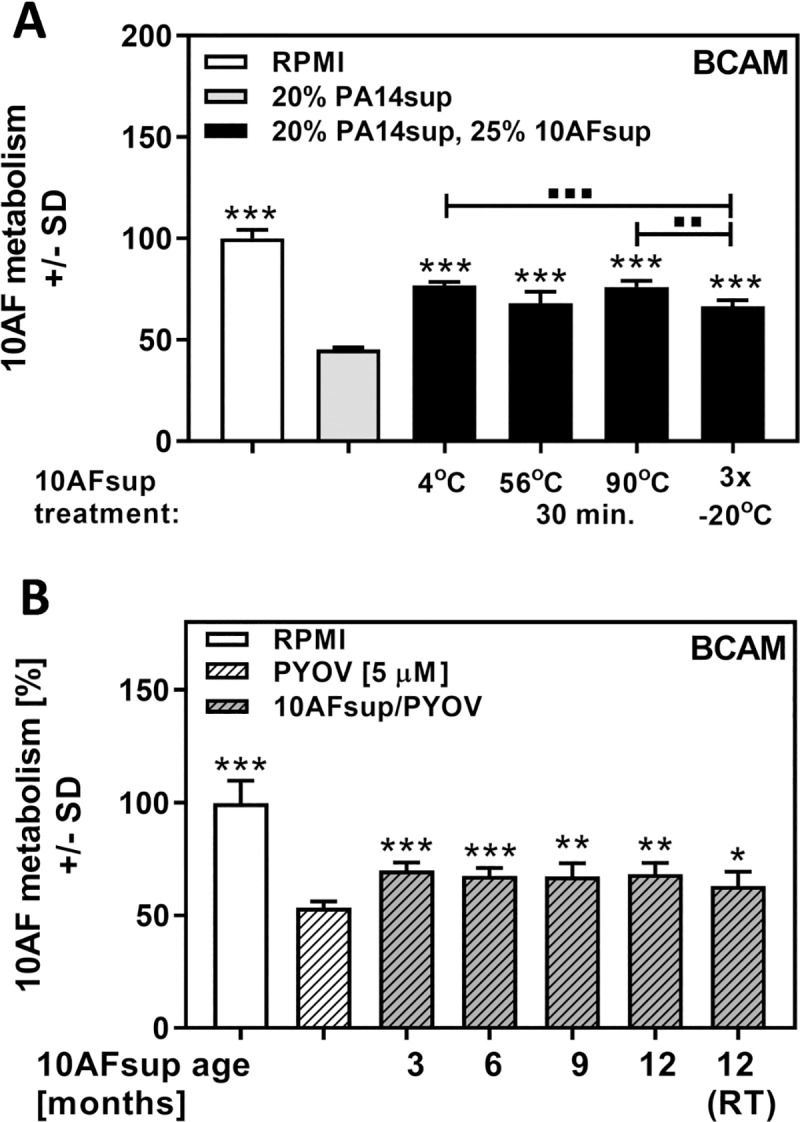
A: Mixtures (25%) of freshly prepared 10AFsup in RPMI were kept at 4°C, or heated to 56°C or 90°C for 30 minutes, or subjected to 3 freeze-thaw cycles. Treated 10AFsups (25% in RPMI), were combined with 20% P. aeruginosa supernatants, and tested for effects on A. fumigatus forming biofilm metabolism. B: 10AFsup was stored at 4°C for up to 12 months, and tested for protective activity against 5 μM pyoverdine (PYOV) every 3 months. A portion of the 10AFsup was stored at room temperature (RT), and tested after 12 months of storage. Protective activity was tested using a BCAM assay. Statistics for A: t-Test, PA14 supernatant (grey bar) vs. all other bars. Other comparisons as indicated by the ends of the brackets. * indicate significant increases, ▪ indicate significant decreases. Statistics for B: t-Test, pyoverdine (white striped bar) vs. all other bars. One, two or three asterisks or squares = p ≤ 0.05, p ≤ 0.01 or p ≤ 0.001, respectively.
A. fumigatus siderophores protect A. fumigatus biofilm from P. aeruginosa anti-fungal activity
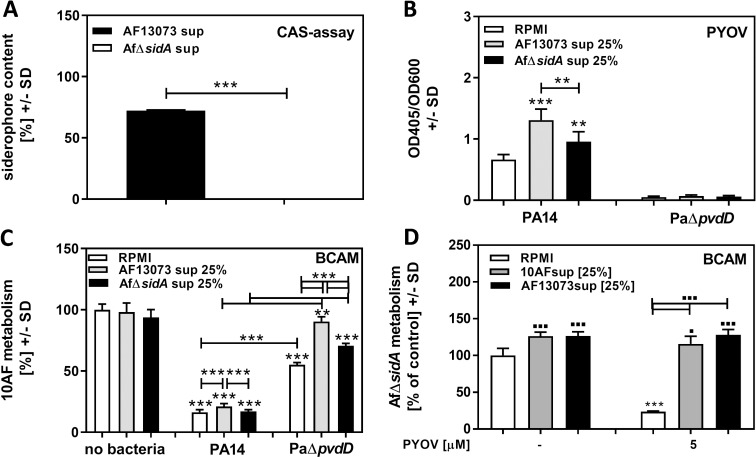
Knowing that iron is a crucial factor for A. fumigatus biofilm, that pyoverdine inhibitory activity is owing to withholding iron from the fungus, and that the protective compound in Afsup is stable (Fig 2), we investigated the hypothesis that the protective compound might be an A. fumigatus siderophore. We produced supernatant of an A. fumigatus mutant lacking sidA, a gene crucial for the production of all four hydroxamate siderophores (AfΔsidAsup), and compared to wildtype Afsup, produced by the AfΔsidA parent AF13073 (AF13073sup). A CAS assay confirmed the lack of siderophores in AfΔsidAsup (Fig 3A). Dilutions (25%) of AF13073sup and AfΔsidAsup were incubated with PA14 or PaΔpvdD. AF13073sup stimulated pyoverdine production by PA14 significantly more than AfΔsidA sup (Fig 3B). AfΔsidAsup also showed less protection for A. fumigatus biofilm against P. aeruginosa anti-fungal activity than AF13073sup (Fig 3C). When siderophore-deficient fungus was treated with pyoverdine, significant damage was induced (Fig 3D), whereas Afsup derived from either 10AF or AF13073 wildtype strains protected AfΔsidA from pyoverdine-induced damage (Fig 3D). It has to be noted that neither the absence of pyoverdine nor the presence of Afsup from siderophore-deficient fungus prevented P. aeruginosa anti-fungal activity completely.
Fig 3. A. fumigatus siderophores protect A. fumigatus biofilm from P. aeruginosa anti-fungal activity.
A: Planktonic supernatants produced by an A. fumigatus mutant lacking hydroxamate siderophore production (AfΔsidA) or its parental strain (AF13073) were subjected to siderophore production measurement by CAS assay. B: RPMI, or 25% AfΔsidA or AF13073 supernatant in RPMI, were inoculated with PA14 wildtype or the PA14 mutant PaΔpvdD [5x107 cells/ml], and incubated at 37°C for 24h. Pyoverdine production was measured. C: Supernatants obtained in B (middle and right sets of 3 bars), as well as 25% AfΔsidA or AF13073 supernatants in RPMI (left 3 bars) were tested for activity against A. fumigatus biofilm formation. D: AfΔsidA forming biofilm was incubated with 5 μM pyoverdine (PYOV) with or without the addition of 25% 10AFsup or AF13073sup for 24 hours. Effects on forming biofilm metabolism were evaluated by XTT assay. Statistics: t-Test. Comparisons without brackets: B: RPMI vs. A. fumigatus supernatants for each bacterial strain. C: RPMI vs. all other bars. D: RPMI (leftmost white bar) vs. all other bars. Other comparisons as indicated by the ends of the brackets. * indicate significant decreases, ▪ indicate significant increases. One, two, or three asterisks or squares = p ≤ 0.05, or p ≤ 0.01, or p ≤ 0.001, respectively.
In comparison to wildtype A. fumigatus, AfΔsidA has a growth disadvantage due to missing Fe3+ uptake, which requires siderophores. 10AF or AF13073 wildtype supernatants, containing siderophores and iron, partially compensated AfΔsidA disadvantages, as indicated by higher XTT values for AfΔsidA in the presence of Afsups (Fig 3D). In conclusion, A. fumigatus siderophores are able to protect A. fumigatus biofilms against P. aeruginosa anti-fungal activity. Fig 3C also shows that AfΔsidA sup was able to provide protection for A. fumigatus biofilm from PaΔpvdD supernatant, whereas there was no protection against PA14 wildtype sup by either Afsup. This finding indicates that A. fumigatus hydroxamate siderophores are crucial for protection from detrimental pyoverdine effects, but that Afsup seems to contain other compounds which are able to protect A. fumigatus biofilm when the Pasup challenge lacks the powerful inhibitor pyoverdine.
AfΔsidA is more sensitive to P. aeruginosa anti-fungal activity and pyoverdine than its wildtype
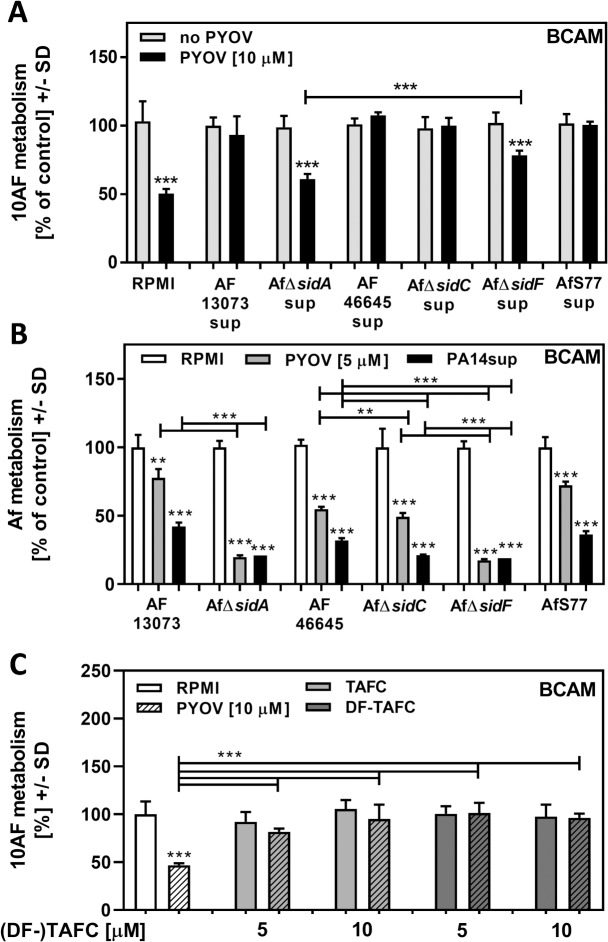
AfΔsidA-derived supernatants were significantly less protective against pyoverdine than wildtype supernatants (Fig 4A). AfΔsidA is lacking the intracellular hydroxamate siderophores ferricrocin (FC) and hydroxy-ferricrocin (HFC), as well as the extracellular hydroxamate siderophores fusarinin C (FsC) and triacetylfusarinine C (TAFC). Using A. fumigatus mutants with specific mutations in intracellular (AfΔsidC), or extracellular hydroxamate siderophores (AfΔsidF), we found that a lack of extracellular siderophores significantly interfered with protection from pyoverdine by A. fumigatus supernatants (Fig 4A). Protective effects of AfΔsidF sup were significantly higher than protective effects of AfΔsidA sup (Fig 4A), indicating that there might be some contribution to protection by other molecules missing in AfΔsidA sup. Fig 4A also shows that supernatants, derived from three different A. fumigatus wildtypes (AF13073, AF46645, AfS77) protected forming biofilm of a fourth A. fumigatus wildtype (10AF), indicating that protection is not strain specific.
Fig 4. AfΔsidA is more sensitive towards PA14 or pure pyoverdine than its wildtype.
A: Mixtures (25%) of freshly prepared AF13073, AfΔsidA, AF46645, AfΔsidC, AfΔsidF, or AfS77 supernatants were combined with pyoverdine [10 μM], and tested for effects on 10AF forming biofilm metabolism. Fungal metabolism was measured by XTT assay. Measurements for controls (no pyoverdine) in each group were regarded as 100%. Statistics: t-Test, for each group: no pyoverdine (grey bar) vs. pyoverdine (black bar). Other comparison as indicated by the ends of the bracket. B: AF13073, AfΔsidA, AF46645, AfΔsidC, AfΔsidF or AfS77 BCAM assays were incubated with either RPMI, PA14 supernatant, or 5 μM pyoverdine. Fungal metabolism was measured by XTT assay. For each fungus RPMI control measurements were regarded as 100%. Statistics: t-Test, comparison: RPMI (white bars) vs. PA14 supernatant (grey bars), or pyoverdine (black bars) for each fungus. Other comparisons as indicated by the ends of the brackets. C: A 10AF BCAM assay was incubated with either RPMI, pyoverdine [10 μM], TAFC [5 or 10 μM], DF-TAFC [5 or 10 μM], or combinations of pyoverdine and TAFC or DF-TAFC. Fungal metabolism was measured by XTT assay. RPMI control measurements were regarded as 100%. Statistics: t-Test, comparison: RPMI (white bar) vs. all other bars. Other comparisons as indicated by the ends of the brackets. One, two or three asterisks = p ≤ 0.05, p ≤ 0.01 or p ≤ 0.001, respectively.
PA14 supernatants, prepared in RPMI, as well as pure pyoverdine, were significantly more inhibitory during the formation of A. fumigatus biofilms derived from AfΔsidA conidia than they were for biofilms derived from AF13073 conidia (Fig 4B). A. fumigatus mutants lacking either intracellular (AfΔsidC), or extracellular hydroxamate siderophores (AfΔsidF) showed increased sensitivity to PA14 supernatants or pure pyoverdine, compared to their wildtype AF46645 (Fig 4B). The loss of extracellular hydroxamate siderophores was more important for sensitivity than the loss of intracellular hydroxamate siderophores (Fig 4B). Using pure TAFC, or desferri-TAFC (DF-TAFC) we found complete protection from pyoverdine anti-fungal activity (Fig 4C), confirming the importance for A. fumigatus siderophores for protection from P. aeruginosa anti-fungal activity.
As observed in Fig 2, the protective compound in Afsup was stable to prolonged heat treatment (90°C, 30 min.). After being subject to the same treatment pure TAFC and DF-TAFC still significantly protected from pyoverdine toxicity (S5 Fig), further supporting the assumption that A. fumigatus siderophores are the protective compound in Afsup. It was also noted that pyoverdine was heat stable (S5 Fig).
The absence of A. fumigatus hydroxamate siderophores might have therapeutic relevance
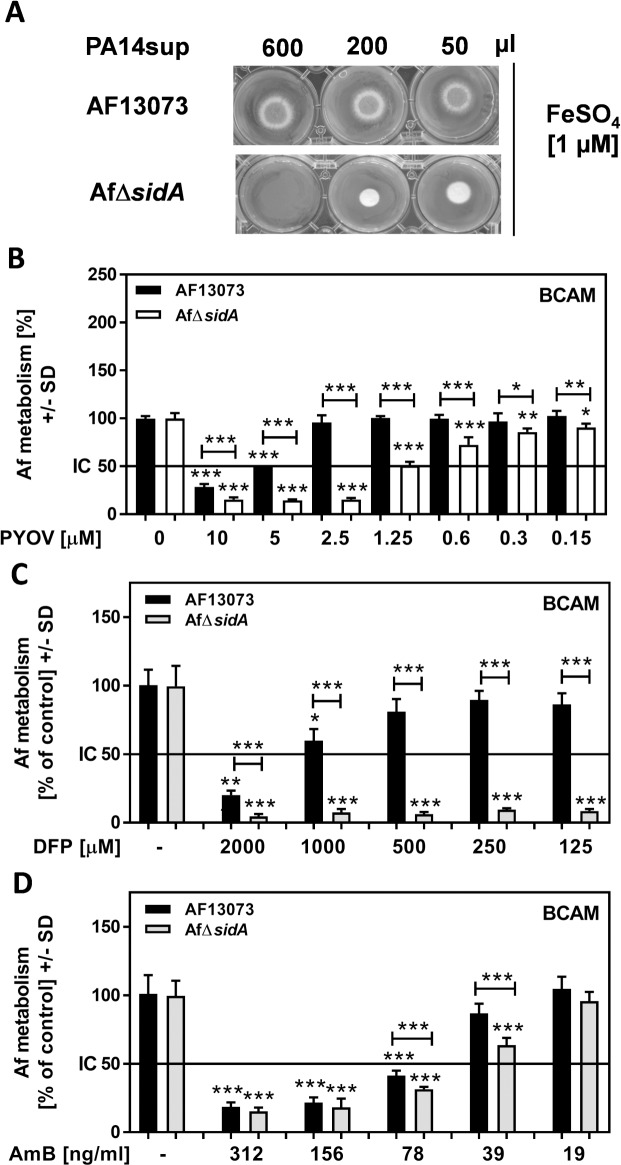
Compared to wildtype A. fumigatus (AF13073), AfΔsidA growth on plate was more affected with the highest concentration (600 μl) of PA14 supernatant blocking growth (Fig 5A). Likewise, the IC50 of pyoverdine for AF13073-derived forming biofilm was about 4 times higher than the IC50 for AfΔsidA-derived forming biofilm (Fig 5B). Recently, the iron chelator deferiprone (DFP), which similar to pyoverdine, exerts anti-fungal activity by denying iron from A. fumigatus biofilms, has been proposed to be useful in anti-fungal therapy [51,52]. We tested effects of DFP on AfΔsidA, or its wildtype, and found significantly higher sensitivity of A. fumigatus biofilms to DFP when siderophore production was missing (Fig 5C). Genetic inhibition of siderophore production also increased anti-fungal effects of amphotericin B (AmB), an anti-fungal agent used against serious fungal infections, not only by Aspergillus, but also by other fungi [53], on A. fumigatus forming biofilm metabolism (Fig 5D).
Fig 5. Absence of hydroxamate siderophores sensitizes A. fumigatus.
A: RPMI was inoculated with PA14 [5x107 cells/ml], incubated for 24 hours, and the culture supernatant was sterile filtered. Growth of point inoculated AF13073 or AfΔsidA (104conidia) on 3 ml solid minimal medium in the presence of 1 μM FeSO4 plus 50–600 μl of the sterile filtered supernatants was compared after incubation for 48 h at 37°C. B: AfΔsidA (white bars) or AF13073 (black bars) BCAM assays were incubated with either RPMI or different concentrations of pyoverdine. Fungal metabolism was measured by XTT assay. Statistics: t-Test. For each fungus RPMI controls were regarded as 100%. RPMI controls for each fungus vs. all pyoverdine concentration. Other comparisons as indicated by the ends of the brackets. C: Wildtype (AF13073) or AfΔsidA forming biofilms were incubated with DFP [0.125–2 mM] at 37°C for 24 hour. Fungal metabolism was measured by XTT assay. Statistics: t-Test, RPMI vs. all other bars of the same group. Other comparisons as indicated by the ends of the brackets. D: Wildtype (AF13073) or AfΔsidA forming biofilms were incubated with AmB [19–312 ng/ml] at 37°C for 24 hour. Fungal metabolism was measured by XTT assay. Statistics: t-Test, RPMI vs. all other bars of the same group. Other comparisons as indicated by the ends of the brackets. One, two or three asterisks = p ≤ 0.05, p ≤ 0.01 or p ≤ 0.001, respectively.
As a pharmacological complementation of our data obtained using AfΔsidA, we investigated effects of the SidA-biosynthesis inhibitor celastrol [54]. Celastrol showed anti-fungal activity when used alone at concentrations above 5 μM (Fig 6). When combined with DFP, celastrol significant enhanced anti-fungal effects by DFP (Fig 6).
Fig 6. Celastrol sensitizes A. fumigatus for anti-fungal activity of DFP.
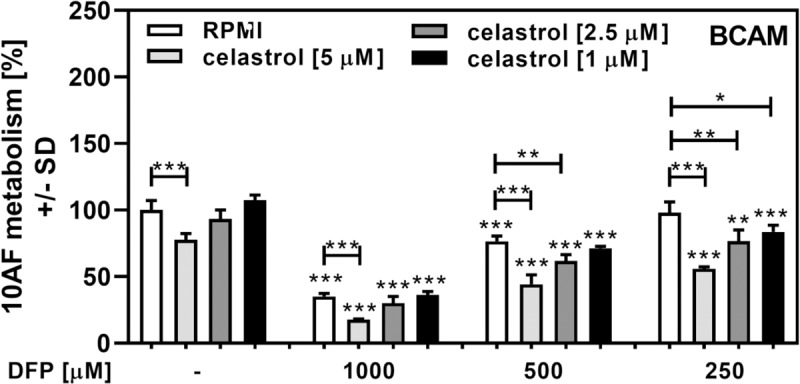
Forming wildtype A. fumigatus biofilm (10AF) was incubated with 1, 2.5, or 5 μM celastrol, 0.25–1 mM of DFP, or combinations of these two substances at 37°C for 24 hour. Fungal metabolism was measured by XTT assay. Statistics: t-Test. Bars without DFP (leftmost group) vs. all other bars with the same celastrol concentration. Other comparisons as indicated by the ends of the brackets. One, two or three asterisks = p ≤ 0.05, p ≤ 0.01 or p ≤ 0.001, respectively.
Discussion
Fungal and bacterial biofilms e.g. frequently found co-inhabiting lungs of persons suffering from cystic fibrosis, represent a potentially severe pathogenicity factor. The present study mainly focuses on events during formation of A. fumigatus biofilm. In previous studies [40] and in studies by many others, it has been shown that biofilm formation by A. fumigatus is substantial within the first 16 hours of incubation. We have also performed many of the studies described in the present communication against fully formed A. fumigatus biofilms that develop over the subsequent 24 hours of incubation, and found the same phenomena, although to a lesser degree than in the earlier phase of A. fumigatus biofilm formation, as illustrated in Fig 1C vs. 1D. This may suggest that iron is more important for the initial development of A. fumigatus biofilms.
The human body contains free iron levels of 10−24 M [55]. Free iron levels are decreased during infections due to increased levels of ferritin and the release of lactoferrin from neutrophils [56]. In the lungs of cystic fibrosis patients, P. aeruginosa and A. fumigatus, which both are crucially dependent on the availability of free iron for metabolism and growth, aggravate disease pathology [4–7]. Under low iron conditions, these organisms are forced to compete for resources in the same environment [29,30].
As summarized in Fig 7, for P. aeruginosa as well as A. fumigatus a lack of iron is the signal to increase production of siderophores [27,28]. Siderophores specifically chelate ferric iron with a high affinity [57]. Siderophores are of different types, based on the way the iron is complexed: phenolate-, catecholate-, hydroxamate-, carboxylate-, or mixed type of siderophores have been described [58].
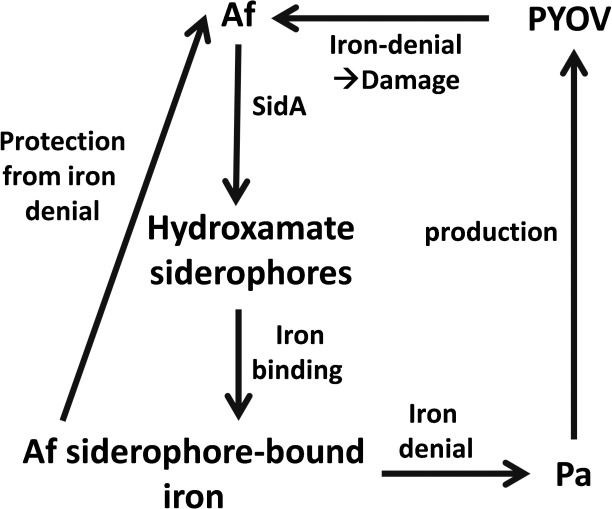
Fig 7. Summary.
In need for iron P. aeruginosa (Pa) produces its siderophore pyoverdine (PYOV). PYOV-chelated iron is not available to A. fumigatus (Af), resulting in iron deficiency and damage to the fungus. Anti-fungal activity in part is counter-balanced by SidA-dependent A. fumigatus hydroxamate siderophores, providing iron to the fungus, further denying iron from P. aeruginosa.
The P. aeruginosa siderophore pyoverdine is a composite (mixed) siderophore comprising a peptide chain and a chromophore [59]. Pyoverdines bind iron with very high affinity, are able to acquire iron from transferrin, and their production is absolutely needed in mouse pulmonary infections [60–62]. We have described pyoverdine to be the Pseudomonas-derived key inhibitor of A. fumigatus in their intermicrobial competition via iron sequestration under low iron conditions [26]. We note that the loss of pyoverdine did not prevent P. aeruginosa anti-fungal activity completely, however. Pyochelin, the second siderophore of P. aeruginosa, is produced by all P. aeruginosa isolates, but its affinity for iron is much lower compared to pyoverdine [63,64]. Pyoverdine does not act as a xenosiderophore for A. fumigatus [26], thus withholding iron from the fungus, and inducing anti-fungal effects [26]. P. aeruginosa does not seem to be able to use A. fumigatus siderophores either. Our results show that Afsups provoke increased pyoverdine production by P. aeruginosa, indicating that there is a paucity of iron in the medium. If P. aeruginosa could use iron bound to A. fumigatus siderophores, there would be an abundancy of iron available to the bacterium, and hence no increase in pyoverdine production. We here for the first time provide evidence that A. fumigatus is able to use iron bound to its hydroxamate siderophores as the main defense against P. aeruginosa competition for iron. These findings are summarized in Fig 7. Our results using A. fumigatus mutants defective in hydroxamate siderophore production also indicate that additional defense mechanisms might be in place, since supernatants derived from these mutants still partially protected from P. aeruginosa toxicity. Other microorganisms have developed defense mechanisms against P. aeruginosa not based on protective siderophore production. Candida albicans appears to defend itself against P. aeruginosa in part by down-regulating P. aeruginosa siderophore production [65].
Anti-bacterial A. fumigatus supernatant effects as a reason for protective effects against P. aeruginosa anti-fungal activity are highly unlikely. In the presence of Afsup, P. aeruginosa is able to even produce more pyoverdine, which requires functional bacterial metabolism. Also, Afsup protects from P. aeruginosa supernatants produced without Afsup being present, and Afsup, as well as pure A. fumigatus siderophores, protect from pure pyoverdine. Additionally, Afsups derived from a giotoxin mutant affected bacterial growth To the same degree as wildtype Afsups. The most plausible explanation for anti-bacterial effects of Afsups is depletion of essential factors in the medium, especially that of iron.
To overcome iron starvation, A. fumigatus produces its own siderophores [35]. A. fumigatus is able to produce four hydroxamate-containing siderophores: ferricrocin (FC) as well as hydroxyferricrocin (HFC) for intracellular iron trafficking, and fusarinine C (FsC) as well as its derivative triacetylfusarinine C (TAFC) for extracellular iron scavenging [36,66,67]. The first step in the biosynthesis of all four hydroxamate-containing siderophores is catalyzed by the enzyme L-ornithine N5-monooxygenase, termed SidA [30,67]. SidA catalyzes oxygen and NADPH-dependent hydroxylation of L-ornithine to N5-L-hydroxyornithine, a crucial step for the biosynthesis of hydroxamate-containing siderophores [35]. In a similar fashion to P. aeruginosa siderophores, A. fumigatus siderophores are essential for pathogenesis, as the AfΔsidA strain is unable to establish invasive aspergillosis in a mouse model [35,56]. We show that in contrast to wildtype A. fumigatus supernatants, supernatants derived from AfΔsidA were unable to protect A. fumigatus biofilms from detrimental effects of P. aeruginosa supernatants, or pyoverdine. This finding indicates the relevance of A. fumigatus siderophores for protection of A. fumigatus from P. aeruginosa-induced iron denial, and was supported by our finding that A. fumigatus siderophores (FC as well as TAFC) each could partially protect A. fumigatus from detrimental P. aeruginosa effects (Fig 4A and 4B). Pure preparations of the sideropore TAFC protected A. fumigatus from pyoverdine, even after heat treatment (Fig 4C, and S5 Fig). Protection by TAFC and its desferri form DF-TAFC was about equal, indicating that TAFC very efficiently binds free iron in medium, before pyoverdine can do the same. TAFC-bound iron does not seem to be transferable to pyoverdine, and exclusively is available to A. fumigatus.
A. fumigatus lacking hydroxamate siderophores, especially of the extracellular type, was more susceptible to pyoverdine (Fig 4B). The most pronounced detrimental effects of pyoverdine were observed when all four siderophores were missing.
A lack of siderophores, especially owing to a loss in SidA, renders the fungus more sensitive to iron denial by either pyoverdine (Fig 5B), or the clinically used iron chelator deferiprone (DFP, Fig 5B). Siderophore deficiency even sensitized the fungus to effects of amphotericin B (AmB, Fig 5C). While sensitization to DFP might be expected knowing that iron chelation by pyoverdine powerfully inhibits the fungus, sensitization to AmB is more surprising. It might be that a struggle for iron takes away energy from the fungus, and dampens intrinsic defense mechanisms, or that the membrane action of AmB [53] may adversely affect iron flux in the fungus.
As a pharmacological analog to sidA knockout we used celastrol treatment [54]. Celastrol, a pentacyclic triterpenoid that belongs to the family of quinone methides, exerts potent anti-cancer and anti-metastatic [68,69], anti-inflammatory [70,71], and antioxidant [72] activities. Recently celastrol was identified as a noncompetitive inhibitor of SidA production [54]. Inhibition of SidA production by celastrol is detrimental to A. fumigatus growth [54]. We observed inhibitory effects of celastrol on A. fumigatus metabolism as well (Fig 6). Since celastrol has numerous effects [68–71] it can’t be excluded that effects on A. fumigatus are not solely owed to inhibition of siderophores production.
SidA-deficiency or addition of celastrol to A. fumigatus wildtype cultures resulted not only in reduced fungal growth [54], and reduced A. fumigatus biofilm metabolism (Fig 6), but also in increased sensitivity towards the iron chelator DFP. DFP is clinically used to treat iron overload, as in thalassemia major [73], but also interferes with iron needs of bacteria [74], and A. fumigatus biofilms [51]. Given that celastrol does not have unwanted effects on the host it might be quite useful in supporting anti-fungal therapy.
Previous studies have focused on P. aeruginosa products and their inhibition of A. fumigatus, at high P. aeruginosa product concentrations. Such studies have not considered the possible response of A. fumigatus at the onset of P. aeruginosa competition. We here show that A. fumigatus uses its siderophores to counter-balance iron denial by P. aeruginosa. In vivo, the winning microbe in this competition might be the one which unleashes its products first, and in the greatest quantity. A. fumigatus siderophores seem to also strengthen the fungus against certain types of therapy. Therefore, interference with siderophore production might boost existing therapy against A. fumigatus.
Supporting information
Af: Aspergillus fumigatus; Afsup: planktonic A. fumigatus supernatant, Pa: Pseudomonas; Pasup: planktonic P. aeruginosa supernatant, PYOV: pyoverdine;
(TIF)
P. aeruginosa cells (5 x 107 /ml) were incubated with planktonic supernatants (25%) derived from AF5322 wildtype, AFgliΔP (gliotoxin mutant), or AFgliPR (reversion of the gliotoxin mutant) at 37°C for 24h. Bacterial growth (A600: A), and pyoverdine (PYOV; A405) were measured, and relative pyoverdine concentration (B) was calculated using the quotient A405/A600. Statistics by t-Test: PA14 supernatant, not containing Afsup (white bar) vs. PA14 supernatants containing Afsup. Two or three asterisks = p ≤ 0.01 or p ≤ 0.001, respectively.
(TIF)
P. aeruginosa cells (5 x 107 /ml) were incubated in RPMI 1640 medium containing 25% 10AFsup, or 25% sterile water, at 37°C for 24h. A: Bacterial growth (A600) was measured. Supernatants derived from A were tested for toxicity against A. fumigatus biofilm formation (XTT assay: B). Statistics by t-Test: A: PA14 supernatant prepared without Afsup or water addition (white bar) vs all other bars. B: RPMI (while bar) vs. all other bars. Other comparisons as indicated by the ends of the brackets. Two or three asterisks = p ≤ 0.01 or p ≤ 0.001, respectively.
(TIF)
A: RPMI was inoculated with PA14 wildtype or the PA14 mutant PaΔpvdD (5x107 cells/ml), with (black bars) or without (white bars) the presence of 25% 10AFsup, and incubated at 37°C for 24h. Pyoverdine production was measured. B: Samples produced in A were used in a BCAM assay, and compared to metabolism of 10AF forming biofilm in the presence of RPMI or 25% 10AFsup, incubated without bacteria. Statistics: t-Test, as indicated by the ends of the brackets. Two or three asterisks = p ≤ 0.01 or p ≤ 0.001, respectively.
(TIF)
A 10AF BCAM assay was incubated with RPMI, TAFC [10 μM], DF-TAFC [10 μM], either fresh or heat treated (90°C for 30 min), and combined with pyoverdine (not heated) [PYOV, 10 μM]. Fungal metabolism was measured by XTT assay. Control (RPMI incubation without heat treatment) was regarded as 100%. Statistics: t-Test, comparison: PYOV without heat treatment vs. all other PYOV-containing bars. Other comparisons as indicated by the ends of the brackets. One, two or three asterisks = p ≤ 0.05, p ≤ 0.01 or p ≤ 0.001, respectively. Comparison of heat treatment of PYOV to unheated PYOV is also shown.
(TIF)
(PDF)
Acknowledgments
The authors thank Marife Martinez for excellent technical support. The A. fumigatus mutant strain AfΔsidA was kindly provided by M. M. Moore, Department of Biological Sciences, Simon Fraser University, Burnaby, Canada.
Abbreviations
- Af
Aspergillus fumigatus
- Pa
P. Aeruginosa
- PS
planktonic P. aeruginosa culture filtrate
- PYOV
pyoverdine
- BCAM
metabolic assay of A. fumigatus biofilm formation on agar
- DFP
deferiprone
- AmB
amphotericin B
- FC
ferricrocin
- HFC
hydroxy-ferricrocin
- FsC
fusarinin C
- TAFC
triacetylfusarinine C
- DF-TAFC
desferri-triacetylfusarinine C
- CAS
chrome azurol S
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was funded by John Flatley (CIMR no. 3770) to D.A.S., Child Health Research Institute, Stanford Transdisciplinary Initiatives Program (CIMR no. 3777) to D.A.S., Austrian Science Fund/Infect-ERA program (FWF grant I1616/Infect-ERA project AspMetNet) to H.H., and HOROS program (W1253) to A.-M.D. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lerner A, Arleevskaya M, Schmiedl A, Matthias T. Microbes and viruses are bugging the gut in celiac disease. Are they friends or foes? Front Microbiol. 2017;8: 1392 eCollection 2017. Review. 10.3389/fmicb.2017.01392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Souza HSP, Fiocchi C, Iliopoulos D. The IBD interactome: an integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14: 739–749. 10.1038/nrgastro.2017.110 [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Li F, Tian Z. Role of microbiota on lung homeostasis and diseases. Sci China Life Sci. 2017;60: 1407–1415. Review. 10.1007/s11427-017-9151-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams HD, Davies JC. Basic science for the chest physician: Pseudomonas aeruginosa and the cystic fibrosis airway. Thorax. 2012;67: 465–467. 10.1136/thoraxjnl-2011-201498 [DOI] [PubMed] [Google Scholar]
- 5.Smyth AR, Hurley MN. Targeting the Pseudomonas aeruginosa biofilm to combat infections in patients with cystic fibrosis. Drugs Fut. 2010;35: 1007–1014. [Google Scholar]
- 6.Folkesson A, Jelsbak L, Yang L, Johansen HK, Ciofu O, Høiby N, et al. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol. 2012;10: 841–851. Review. 10.1038/nrmicro2907 [DOI] [PubMed] [Google Scholar]
- 7.Sabino R, Ferreira JA, Moss RB, Valente J, Veríssimo C, Carolino E, et al. Molecular epidemiology of Aspergillus collected from cystic fibrosis patients. J Cyst Fibros. 2015;14: 474–481. 10.1016/j.jcf.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 8.Fillaux J, Brémont F, Murris M, Cassaing S, Rittié JL, Tétu L, et al. Assessment of Aspergillus sensitization or persistent carriage as a factor in lung function impairment in cystic fibrosis patients. Scand J Infect Dis. 2012;44: 842–847. 10.3109/00365548.2012.695454 [DOI] [PubMed] [Google Scholar]
- 9.Speirs JJ, van der Ent CK, Beekman JM. Effects of Aspergillus fumigatus colonization on lung function in cystic fibrosis. Curr Opin Pulm Med. 2012;18: 632–638. 10.1097/MCP.0b013e328358d50b [DOI] [PubMed] [Google Scholar]
- 10.Ramsey KA, Ranganathan S, Park J, Skoric B, Adams AM, Simpson SJ, et al. Early respiratory infection is associated with reduced spirometry in children with cystic fibrosis. Am J Respir Crit Care Med. 2014;190: 1111–1116. 10.1164/rccm.201407-1277OC [DOI] [PubMed] [Google Scholar]
- 11.de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax. 2011;66: 680–685. 10.1136/thx.2011.161117 [DOI] [PubMed] [Google Scholar]
- 12.Nicolai T, Arleth S, Spaeth A, Bertele-Harms RM, Harms HK. Correlation of IgE antibody titer to Af with decreased lung function in cystic fibrosis. Pediatr Pulmonol. 1990;8: 12–15. [DOI] [PubMed] [Google Scholar]
- 13.Forsyth KD, Hohmann AW, Martin AJ, Bradley J. IgG antibodies to Aspergillus fumigatus in cystic fibrosis: a laboratory correlate of disease activity. Arch Dis Child. 1988;63: 953–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schønheyder H, Jensen T, Høiby N, Andersen P, Koch C. Frequency of Aspergillus fumigatus isolates and antibodies to Aspergillus antigens in cystic fibrosis. Acta Pathol Microbiol Immunol Scand B. 1985;93: 105–112. [DOI] [PubMed] [Google Scholar]
- 15.Coughlan CA, Chotirmall SH, Renwick J, Hassan T, Low TB, Bergsson G, et al. The effect of Aspergillus fumigatus infection on vitamin D receptor expression in cystic fibrosis. Am J Respir Crit Care Med. 2012;186: 999–1007. 10.1164/rccm.201203-0478OC [DOI] [PubMed] [Google Scholar]
- 16.Mirković B, Lavelle GM, Azim AA, Helma K, Gargoum FS, Molloy K, et al. The basophil surface marker CD203c identifies Aspergillus species sensitization in patients with cystic fibrosis. J Allergy Clin Immunol. 2016;137: 436–443. 10.1016/j.jaci.2015.07.045 [DOI] [PubMed] [Google Scholar]
- 17.Baxter CG, Moore CB, Jones AM, Webb AK, Denning DW. IgE-mediated immune responses and airway detection of Aspergillus and Candida in adult cystic fibrosis. Chest. 2013;143: 1351–1357. 10.1378/chest.12-1363 [DOI] [PubMed] [Google Scholar]
- 18.Shoseyov D, Brownlee KG, Conway SP, Kerem E. Aspergillus bronchitis in cystic fibrosis. Chest. 2006;130: 222–226. 10.1378/chest.130.1.222 [DOI] [PubMed] [Google Scholar]
- 19.Amin R, Dupuis A, Aaron SD, Ratjen F. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest. 2010;137: 171–176. 10.1378/chest.09-1103 [DOI] [PubMed] [Google Scholar]
- 20.de Bentzmann S, Plésiat P. The Pseudomonas aeruginosa opportunistic pathogen and human infections. Environ Microbiol. 2011;13: 1655–1665. 10.1111/j.1462-2920.2011.02469.x [DOI] [PubMed] [Google Scholar]
- 21.Walsh TJ, Stevens DA. Aspergillosis Chapter 347, Cecil Textbook of Medicine, 24th ed. Goldman L, Schafer A, eds.; Elsevier; 2011. [Google Scholar]
- 22.Mangan A. Interactions between some aural Aspergillus species and bacteria. J Gen Microbiol. 1969;58: 261–266. 10.1099/00221287-58-2-261 [DOI] [PubMed] [Google Scholar]
- 23.Blyth W, Forey A. The influence of respiratory bacteria and their biochemical fractions on Aspergillus fumigatus. Sabouraudia. 1971;9: 273–282. [DOI] [PubMed] [Google Scholar]
- 24.Kerr JR, Taylor GW, Rutman A, Høiby N, Cole PJ, Wilson R. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J Clin Pathol. 1999;52: 385–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briard B, Bomme P, Lechner BE, Mislin GL, Lair V, Prévost MC, et al. Pseudomonas aeruginosa manipulates redox and iron homeostasis of its microbiota partner Aspergillus fumigatus via phenazines. Sci Rep. 2015;5: 8220 10.1038/srep08220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sass G, Nazik H, Penner J, Shah H, Ansari SR, Clemons KV, et al. Studies of Pseudomonas aeruginosa mutants indicate pyoverdine as the central factor in inhibition of Aspergillus fumigatus biofilm. J Bacteriol. 2017. pii: JB.00345-17. 10.1128/JB.00345-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cornelis P, Dingemans J. Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front Cell Infect Microbiol. 2013;3: 75 Review. 10.3389/fcimb.2013.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schalk IJ, Guillon L. Pyoverdine biosynthesis and secretion in Pseudomonas aeruginosa: implications for metal homeostasis. Environ Microbiol. 2013;15: 1661–73. Review. 10.1111/1462-2920.12013 [DOI] [PubMed] [Google Scholar]
- 29.Minandri F, Imperi F, Frangipani E, Bonchi C, Visaggio D, Facchini M, et al. Role of iron uptake systems in Pseudomonas aeruginosa virulence and airway infection. Infect Immun. 2016;84: 2324–2335. 10.1128/IAI.00098-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haas H. Iron—a key nexus in the virulence of Aspergillus fumigatus. Front Microbiol. 2012;3: 28 eCollection 2012. 10.3389/fmicb.2012.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthaiou EI, Sass G, Stevens DA, Hsu JL. Iron: an essential nutrient for Aspergillus fumigatus and a fulcrum for pathogenesis. Current Opinion Infect Dis. 2018;31: 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberegger H, Schoeser M, Zadra I, Abt B, Haas H. SREA is involved in regulation of siderophore biosynthesis, utilization and uptake in Aspergillus nidulans. Mol Microbiol. 2001;41: 1077–89. [DOI] [PubMed] [Google Scholar]
- 33.Denning DW, Clemons KV, Hanson LH, Stevens DA. Restriction endonuclease analysis of total cellular DNA of Aspergillus fumigatus isolates of geographically and epidemiologically diverse origin. J Infect Dis. 1990;162: 1151–1158. [DOI] [PubMed] [Google Scholar]
- 34.Denning DW, Stevens DA. Efficacy of cilofungin alone and in combination with amphotericin B in a murine model of disseminated aspergillosis. Antimicrob Agents Chemother. 1991;35: 1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schrettl M, Bignell E, Kragl C, Joechl C, Rogers T, Arst HN Jr, et al. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J Exp Med. 2004;200: 1213–1219. 10.1084/jem.20041242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrettl M, Bignell E, Kragl C, Sabiha Y, Loss O, Eisendle M, et al. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 2007;3:e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartmann T, Dümig M, Jaber BM, Szewczyk E, Olbermann P, Morschhäuser J, et al. Validation of a self-excising marker in the human pathogen Aspergillus fumigatus by employing the beta-rec/six site-specific recombination system. Appl Environ Microbiol. 2010;76: 6313–6317. 10.1128/AEM.00882-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM. Common virulence factors for bacterial pathogenicity in plants and animals. Science 1995;268: 1899–1902. [DOI] [PubMed] [Google Scholar]
- 39.Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, Wu G, et al. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci U S A. 2006;103: 2833–2838. 10.1073/pnas.0511100103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira JA, Penner JC, Moss RB, Haagensen JA, Clemons KV, Spormann AM, et al. Inhibition of Aspergillus fumigatus and its biofilm by Pseudomonas aeruginosa is dependent on the source, phenotype and growth conditions of the bacterium. PLoS One. 2015;10: e0134692 10.1371/journal.pone.0134692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilderman PJ, Vasil AI, Johnson Z, Wilson MJ, Cunliffe HE, Lamont IL, et al. Characterization of an endoprotease (PrpL) encoded by a PvdS-regulated gene in Pseudomonas aeruginosa. Infect Immun. 2001;69: 5385–5394. 10.1128/IAI.69.9.5385-5394.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin LW, Reid DW, Sharples KJ, Lamont IL. Pseudomonas siderophores in the sputum of patients with cystic fibrosis. Biometals. 2011;24: 1059–67. 10.1007/s10534-011-9464-z [DOI] [PubMed] [Google Scholar]
- 43.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48: 4827–4833. [PubMed] [Google Scholar]
- 44.Meletiadis J, Mouton JW, Meis JFGM, Bouman BA, Donnelly JP, Verweij PE, et al. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J Clin Microbiol. 2001;39: 3402–3408. 10.1128/JCM.39.9.3402-3408.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moss BJ, Kim Y, Nandakumar MP, Marten MR. Quantifying metabolic activity of filamentous fungi using a colorimetric XTT assay. Biotechnol Prog. 2008;24: 780–783. 10.1021/bp070334t [DOI] [PubMed] [Google Scholar]
- 46.Pierce CG, Uppuluri P, Tristan AR, Wormley FL, Mowat E, Ramage G, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nature Protocols. 2008;3: 1494–1500. 10.1038/nport.2008.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pontecorvo G, Roper JA, Hemmons LM, Macdonald KD, Bufton AWJ. The genetics of Aspergillus nidulans. Adv Genet. 1953;5: 141–238. [DOI] [PubMed] [Google Scholar]
- 48.Andrews MY, Santelli CM, Duckworth OW. Layer plate CAS assay for the quantitation of siderophore production and determination of exudation patterns for fungi. J Microbiol Methods. 2016;121: 41–43. 10.1016/j.mimet.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 49.Reece E, Doyle S, Greally P, Renwick J, McClean S. Aspergillus fumigatus inhibits Pseudomonas aeruginosa in co-culture: Implications of a mutually antagonistic relationship on virulence and inflammation in the CF airway. Front Microbiol. 2018;9: 1205 eCollection 2018. 10.3389/fmicb.2018.01205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugui JA, Pardo J, Chang YC, Zarember KA, Nardone G, Galvez EM, Müllbacher A, Gallin JI, Simon MM, Kwon-Chung KJ. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot Cell. 2007;6: 1562–1569. 10.1128/EC.00141-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nazik H, Penner JC, Ferreira JA, Haagensen JA, Cohen K, Spormann AM, et al. Effects of iron chelators on the formation and development of Aspergillus fumigatus biofilm. Antimicrob Agents Chemother. 2015;59: 6514–6520. 10.1128/AAC.01684-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu JL, Clemons KV, Manouvakhova O, Inayathulla M, Tu AB, Sobel RA, et al. Microhemorrhage-associated tissue iron enhances the risk for Aspergillus fumigatus invasion in murine airway transplantation. Sci Transl Med. 2018;10(429). pii: eaag2616. 10.1126/scitranslmed.aag2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stevens DA. Systemic antifungal agents Cecil Textbook of Medicine, 25th ed. Chapter 331; Goldman L Schafer A, eds; Elsevier; 2015. [Google Scholar]
- 54.Martín Del Campo JS, Vogelaar N, Tolani K, Kizjakina K, Harich K, Sobrado P. Inhibition of the flavin-dependent monooxygenase siderophore A (SidA) blocks siderophore biosynthesis and Aspergillus fumigatus growth. ACS Chem Biol. 2016;11: 3035–3042. 10.1021/acschembio.6b00666 [DOI] [PubMed] [Google Scholar]
- 55.Potrykus J, Ballou ER, Childers DS, Brown AJP. Conflicting interests in the pathogen−host tug of war: Fungal micronutrient scavenging versus mammalian nutritional immunity. PLoS Pathog. 2014;10: e1003910 10.1371/journal.ppat.1003910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hissen AHT, Chow JMT, Pinto LJ, Moore MM. Survival of Aspergillus fumigatus in serum involves removal of iron from transferrin: the role of siderophores. Infect Immun. 2004;72: 1402−1408. 10.1128/IAI.72.3.1402-1408.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braun V, Killmann H. Bacterial solutions to the iron-supply problem. Trends Biochem Sci. 1999;24: 104–109.doi:10.1016/S0968-0004(99) 01359–6 [DOI] [PubMed] [Google Scholar]
- 58.Khan A, Singh P, Srivastava A. Synthesis, nature and utility of universal iron chelator—Siderophore: A review. Microbiol Res. 2017. pii: S0944-5013(17)30673-0. Review. [DOI] [PubMed] [Google Scholar]
- 59.Visca P, Imperi F, Lamont IL. Pyoverdine siderophores: from biogenesis to biosignificance. Trends Microbiol. 2007;15: 22–30. Review. 10.1016/j.tim.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 60.Albrecht-Gary AM, Blanc S, Rochel N, Ocacktan AZ, Abdallah MA. Bacterial iron transport: coordination properties of pyoverdin PaA, a peptidic siderophore of Pseudomonas aeruginosa. Inorg Chem. 1994;33: 6391–6402. 10.1021/ic00104a059 [DOI] [Google Scholar]
- 61.Meyer JM. Pyoverdines: pigments, siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch Microbiol. 2000;174: 135–142. 10.1007/s0020300001 [DOI] [PubMed] [Google Scholar]
- 62.Imperi F, Massai F, Facchini M, Frangipani E, Visaggio D, Leoni L, et al. Repurposing the antimycotic drug flucytosine for suppression of Pseudomonas aeruginosa pathogenicity. Proc Natl Acad Sci USA. 2013;110: 7458–7463. 10.1073/pnas.1222706110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ankenbauer RG, Toyokuni T, Staley A, Rinehart KL Jr, Cox CD. Synthesis and biological activity of pyochelin, a siderophore of Pseudomonas aeruginosa. J Bacteriol. 1988;170: 5344–5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brandel J, Humbert N, Elhabiri M, Schalk IJ, Mislin GL, Albrecht-Gary AM. Pyochelin, a siderophore of Pseudomonas aeruginosa: physicochemical characterization of the iron(III), copper(II) and zinc(II) complexes. Dalton Trans. 2012;41: 2820–2834. 10.1039/c1dt11804h [DOI] [PubMed] [Google Scholar]
- 65.Lopez-Medina E, Fan D, Coughlin LA, Ho EX, Lamont IL, Reimmann C, et al. Candida albicans inhibits Pseudomonas aeruginosa virulence through suppression of pyochelin and pyoverdine biosynthesis. PLoS Pathog. 2015;11: e1005129 eCollection 2015 Aug. 10.1371/journal.ppat.1005129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haas H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat Prod Rep. 2014;31: 1266–1276. 10.1039/c4np00071d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blatzer M, Schrettl M, Sarg B, Lindner HH, Pfaller K, Haas H. SidL, an Aspergillus fumigatus transacetylase involved in biosynthesis of the siderophores ferricrocin and hydroxyferricrocin. Appl Environ Microbiol. 2011;77: 4959−4966. 10.1128/AEM.00182-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tiedemann RE, Schmidt J, Keats JJ, Shi C-X, Zhu YX, Palmer SE, et al. Identification of a potent natural triterpenoid inhibitor of proteosome chymotrypsin-like activity and NF- B with antimyeloma activity in vitro and in vivo. Blood. 2008;113: 4027–4037. 10.1182/blood-2008-09-179796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu H, Liu XW, Cai TY, Cao J, Tu CX, Lu W, et al. Celastrol acts as a potent antimetastatic agent targeting beta1 integrin and inhibiting cell-extracellular matrix adhesion, in part via the p38 mitogen-activated protein kinase pathway. J Pharmacol Exp Ther. 2010;334: 489–499. 10.1124/jpet.110.165654 [DOI] [PubMed] [Google Scholar]
- 70.Kim DH, Shin EK, Kim YH, Lee BW, Jun JG, Park JH, et al. Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii. European Journal of Clinical Investigation. 2010;39: 819–827. [DOI] [PubMed] [Google Scholar]
- 71.Venkatesha SH, Yu H, Rajaiah R, Tong L, Moudgil KD. Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. Journal of Biological Chemistry. 2011;286: 15138–15146. 10.1074/jbc.M111.226365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Allison AC, Cacabelos R, Lombardi VRM, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer's disease. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2001;25: 1341–1357. [DOI] [PubMed] [Google Scholar]
- 73.Savulescu J. Thalassaemia major: The murky story of deferiprone. BMJ. 2004;328: 358–359. 10.1136/bmj.328.7436.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paradkar PN, De Domenico I, Durchfort N, Zohn I, Kaplan J, Ward DM. Iron depletion limits intracellular bacterial growth in macrophages. Blood. 2008;112: 866–874. 10.1182/blood-2007-12-126854 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Af: Aspergillus fumigatus; Afsup: planktonic A. fumigatus supernatant, Pa: Pseudomonas; Pasup: planktonic P. aeruginosa supernatant, PYOV: pyoverdine;
(TIF)
P. aeruginosa cells (5 x 107 /ml) were incubated with planktonic supernatants (25%) derived from AF5322 wildtype, AFgliΔP (gliotoxin mutant), or AFgliPR (reversion of the gliotoxin mutant) at 37°C for 24h. Bacterial growth (A600: A), and pyoverdine (PYOV; A405) were measured, and relative pyoverdine concentration (B) was calculated using the quotient A405/A600. Statistics by t-Test: PA14 supernatant, not containing Afsup (white bar) vs. PA14 supernatants containing Afsup. Two or three asterisks = p ≤ 0.01 or p ≤ 0.001, respectively.
(TIF)
P. aeruginosa cells (5 x 107 /ml) were incubated in RPMI 1640 medium containing 25% 10AFsup, or 25% sterile water, at 37°C for 24h. A: Bacterial growth (A600) was measured. Supernatants derived from A were tested for toxicity against A. fumigatus biofilm formation (XTT assay: B). Statistics by t-Test: A: PA14 supernatant prepared without Afsup or water addition (white bar) vs all other bars. B: RPMI (while bar) vs. all other bars. Other comparisons as indicated by the ends of the brackets. Two or three asterisks = p ≤ 0.01 or p ≤ 0.001, respectively.
(TIF)
A: RPMI was inoculated with PA14 wildtype or the PA14 mutant PaΔpvdD (5x107 cells/ml), with (black bars) or without (white bars) the presence of 25% 10AFsup, and incubated at 37°C for 24h. Pyoverdine production was measured. B: Samples produced in A were used in a BCAM assay, and compared to metabolism of 10AF forming biofilm in the presence of RPMI or 25% 10AFsup, incubated without bacteria. Statistics: t-Test, as indicated by the ends of the brackets. Two or three asterisks = p ≤ 0.01 or p ≤ 0.001, respectively.
(TIF)
A 10AF BCAM assay was incubated with RPMI, TAFC [10 μM], DF-TAFC [10 μM], either fresh or heat treated (90°C for 30 min), and combined with pyoverdine (not heated) [PYOV, 10 μM]. Fungal metabolism was measured by XTT assay. Control (RPMI incubation without heat treatment) was regarded as 100%. Statistics: t-Test, comparison: PYOV without heat treatment vs. all other PYOV-containing bars. Other comparisons as indicated by the ends of the brackets. One, two or three asterisks = p ≤ 0.05, p ≤ 0.01 or p ≤ 0.001, respectively. Comparison of heat treatment of PYOV to unheated PYOV is also shown.
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.