Abstract
Peripheral sensory ganglia contain the somata of neurons mediating mechanical, thermal and painful sensations from somatic, visceral and oro-facial organs. Each neuronal cell body is closely surrounded by satellite glial cells (SGCs) that have properties and functions similar to those of central astrocytes, including expression of gap junction proteins and functional dye coupling. As shown in other pain models, after systemic pain induction by intra-peritoneal injection of lipopolysaccharide (LPS), dye coupling among SGCs in intact trigeminal ganglion was enhanced. Moreover, neuron-neuron and neuron-SGC coupling was also detected. To verify the presence of gap junction-mediated coupling between SGCs and sensory neurons, we performed dual whole cell patch clamp recordings from both freshly isolated and short term cultured cell pairs dissociated from mouse trigeminal ganglia. Bidirectional gap junction mediated electrical responses were frequently recorded between SGCs, between neurons and between neurons and SGCs. Polarization of SGC altered neuronal excitability, providing evidence that gap junction-mediated interactions between neurons and glia within sensory ganglia may contribute to integration of peripheral sensory responses, modulation and coordination of neuronal activity.
Keywords: dye coupling, electrical coupling, glia-neuron communication, dual whole cell recording, connexin
Graphical Abstract
Introduction
Sensory ganglia contain somata of primary neurons whose nerve endings transduce cutaneous sensations of touch, pain, and temperature. Each neuronal cell body is closely ensheathed by satellite glial cells (SGCs) with properties and functions similar to those of astrocytes in the central nervous system (CNS), including expression of the gap junction protein connexin43 (Cx43), glutamine synthetase, and purinergic P2 receptors and regulation of the neuronal microenvironment by buffering K+ and actively removing extracellular glutamate and other transmitters and metabolites (Hanani and Spray 2013). Although there are no synapses within sensory ganglia, activity in one sensory neuron has been reported to depolarize others nearby, a phenomenon attributed to chemical interactions and termed “cross-excitation” (Amir and Devor 2000).
Gap junction mediated intercellular communication among SGCs connects these glial cells into functional networks (Hanani and Spray 2013). The extent of coupling among SGCs in rodent dorsal root and trigeminal ganglia (DRG and TG) increases in response to various treatments leading to chronic pain, including nerve section, local inflammation and other insults (Hanani 2012; Huang et al. 2010; Jasmin et al. 2010; Kim et al. 2016; Ledda et al. 2009; Ohara et al. 2008; Spray and Hanani 2017). We have hypothesized that peripheral pain sensitization initiated by injury-evoked activity in neurons is sustained and enhanced by coupled SGCs, being amplified over time to form, remodel and maintain aberrant activity in sensory ganglia (Spray and Hanani 2017).
Bidirectional chemical signaling between neurons and SGCs has been reported (Gu et al. 2010; Magni et al. 2015; Rozanski et al. 2012; Suadicani et al. 2010), and intercellular transfer of a gap junction permeant dye from sensory neurons to SGCs in trigeminal ganglion (TG) was reported after capsaicin injection into the temporo-mandibular joint (Thalakoti et al. 2007), which raised the possibility that direct gap junction mediated neuron-glial signaling may be induced under painful conditions. Our previous Ca2+ imaging study on dissociated TG cultures revealed bidirectional neuron-SGC (N-G) interactions that were partially inhibited by gap junction blockers and also partially inhibited by P2 purinergic receptor blockade, indicating that both gap junctions and nonjunctional channels contribute (Suadicani et al. 2010). Further evidence for N-G coupling was provided by imaging and electrophysiological studies of DRG in hindlimb pain studies (Kim et al. 2016) and by studies that have revealed dye spread between the cell types in both DRG and TG from several pain models (Spray and Hanani 2017). Additional evidence for nonjunctional pathways includes the finding that the Pannexin 1 (Panx1) channels in SGCs release ATP and that allodynia is absent in Panx1 deficient mice (Hanstein et al. 2016; Weaver et al. 2017).
In the present study, we have quantified dye coupling in intact TG from mice following systemic lipopolysaccharide (LPS) injection, a treatment that causes generalized mechanical hypersensitivity (Blum et al. 2014; Feldman-Goriachnik et al. 2015). This finding uniquely demonstrated enhanced dye coupling among SGCs and induction of dye coupling between SGCs and neurons and between neurons in intact TG in the LPS model of chronic pain. We then followed up our finding of gap junction mediated neuron-glial communication in sensory ganglia using dye injections and paired cell recordings to characterize electrical and dye coupling in dissociated N-G co-cultures from TG. Strength of coupling was highest between SGCs, lowest between neurons, and intermediate between N-G pairs. Neuron-SGC coupling was sufficiently strong that in current clamp recordings polarization of SGCs could directly modulate excitability of neurons to which they are coupled. We conclude that functional coupling between neurons and SGCs provides a potentially important mechanism whereby glia may modulate neuronal excitability under conditions of neuropathic pain.
Materials and Methods
Trigeminal ganglion isolation and preparation of dissociated cell cultures.
C57BL/6 male and female mice (2–3 months old; Charles River, Boston, MA or Jackson Laboratories, Bar Harbor, ME, USA) were anesthetized with isoflurane (Abbott Laboratories, IL, USA) and euthanized by decapitation, as approved by the Hadassah Medical School and Einstein College of Medicine Animal Use and Care Committees. The skull was then opened and the brain removed, exposing both trigeminal ganglia (TG); the ganglia were then removed and transferred into cold DPBS (Dulbecco’s Phosphate-Buffered Saline, pH 7.4; Mediatech Cellgro, Herndon, VA, USA). For dye injection into cells in isolated ganglia, TG were attached to Sylgard (Dow Corning) covered dishes in cold DPBS. The connective tissue capsule around the ganglion was removed by fine dissection; no enzymes were used.
For tissue culture, ganglia were cleaned from connective tissue and transferred to 1.5 mL vials containing DPBS and collagenase (1 mg/ml, Type 1A, with 3 mg/ml dispase; Sigma, St. Louis, MO, USA). After digestion for 45 min at 37°C, the enzyme solution was substituted by fresh DPBS; the ganglia were then repeatedly pipetted up and down until tissue was completely dissociated, Then, tissue was spun down (1,000 RPM for 5 min), re-suspended and placed in glass-bottomed MatTek dishes (MatTek, Ashland, MA, USA) or on 1 cm diameter coverslips or Ibidi (Martinsried, Germany, product #80136) coated dishes in Dulbecco’s Modified Eagle Medium (DMEM, GIBCO, Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (GIBCO) and 1% penicillin-streptomycin (Mediatech Cellgro) and transferred to a humidified 5% CO2 incubator at 37°C. Cultures were generally used at 1–3 days after their preparation, although recordings of coupling between N-N and N-G pairs were also obtained at 2–18 hr after dissociation (termed “freshly dissociated cells”) plated onto Ibidi 80136 coated dishes. Within 3 days in culture, neurons remained closely apposed by SGCs (Belzer et al. 2010), whose identity could be confirmed by their positive immunoreactivity for the glial marker glutamine synthetase, shape and size and absence of inward currents upon depolarization. Viability of neurons in the cultures was confirmed by their ability to fire action potentials in current clamp and inducibility of inward currents in voltage clamp.
Electrophysiology.
Cells were plated on MatTek or Ibidi dishes or on glass coverslips 2 hr to 3 days prior to recordings. Neuron-neuron, G-G and N-G pairs were used for dual whole cell patch clamp recordings (del Corsso et al. 2006) performed at room temperature on cells bathed in external solution containing (mM): NaCl 140, MgCl2 1, HEPES 5, KCl, Glucose 5, Sodium pyruvate 2 and BaCl2 1. Patch pipettes (4–6 MOhms) were filled with solution containing (mM): CsCl (voltage clamp) or KCl (current clamp) 130, EGTA 10, HEPES 10, CaCl2 2 and connected to an Axopatch 1D amplifier (Molecular Devices); in experiments on freshly dissociated N-N and N-G pairs, 130 mM KGluconate replaced CsCl or KCl in internal solution. Data were acquired with Clampex 6.0, 8.2 or 10 software, digitized using an Axon Instruments Digitizer; and analyzed with Clampfit 9.0 or later software (Molecular Devices).
Dye coupling.
For whole ganglion preparations, individual neurons and SGCs were injected with the fluorescent dye Lucifer yellow (LY, Sigma, St. Louis, MO, USA), 3% in 0.5 M LiCl solution from sharp glass microelectrodes connected to a preamplifier (Neuro Data Instrument Corp., New York, NY, USA). LY Injections began about 30 min after ganglion removal in both controls and LPS-treated mice; dye was iontophoresed by 100 msec duration, 0.5 nA, 10 Hz hyperpolarizing current pulses for 3–5 min. Neuron-neuron and neuron-SGC coupling was evaluated at 8 days after LPS injection.
For injections in culture, neuron or SGC was voltage clamped using patch electrodes containing 0.1% LY in CsCl patch solution above or in current clamp (WPI SYS-705 electrometer) with high resistance electrodes containing 1–3% LY in 150 mM LiCl. Negative 0.5–1 nA current pulses were passed through the electrode until cells glowed brightly. At 5–10 min after injection, cells were photographed and images were quantified with regard to incidence of dye spread (fractions of injections resulting in dye transfer) and efficacy (fraction of adjacent cells to which dye spread). Some dye injections were performed in the presence of the live nuclear stain Hoechst 33342 (Invitrogen H1399; 0.1 mg/ml in DPBS for 10 min).
Gap junction inhibitors.
Drugs were prepared fresh or from frozen stocks at 100× final concentration in DMSO or PBS. Final concentrations used for gap junction blockade were 0.1–0.2 mM carbenoxolone, 2 mM heptanol, 1 mM probenecid, 0.1 mM 2-APB, 0.1 mM flufenamic acid and, to achieve intracellular acidification, bathing in solution equilibrated with 100% CO2.
Lipopolysaccharide (LPS) injection.
To induce systemic inflammation, we used a single intra-peritoneal E. coli LPS injection of 2.5 mg/kg (Sigma St. Louis, MO, USA), dissolved in saline. Uninjected mice served as controls. These LPS experiments were performed in Jerusalem and methods were approved by the Hebrew University-Hadassah Medical School Animal Care and Use Committee.
Experimental Design and Statistical Analysis
To analyze statistical significance of dye injections, in each culture dish we performed up to 10 separate injections (first control injections, then treatment). For every injection into neuron or SGC, we counted fluorescent cells at 5–10 min after injection, and calculated the fraction of cells to which LY spread with respect to the total number of cells in contact with the injected cell. Results are presented as n(N), where n indicates number of cells injected and N indicates numbers of independent cell cultures in which fractions of cells filled were calculated. To assess significance between each treatment and controls in the same experiments, we used Student two-tailed t or Fisher exact test with significant differences considered when p<0.05. In cases where multiple treatments were compared, we used one way ANOVA.
Results
Sensory neuron-SGC coupling in intact TG in a mouse orofacial pain model
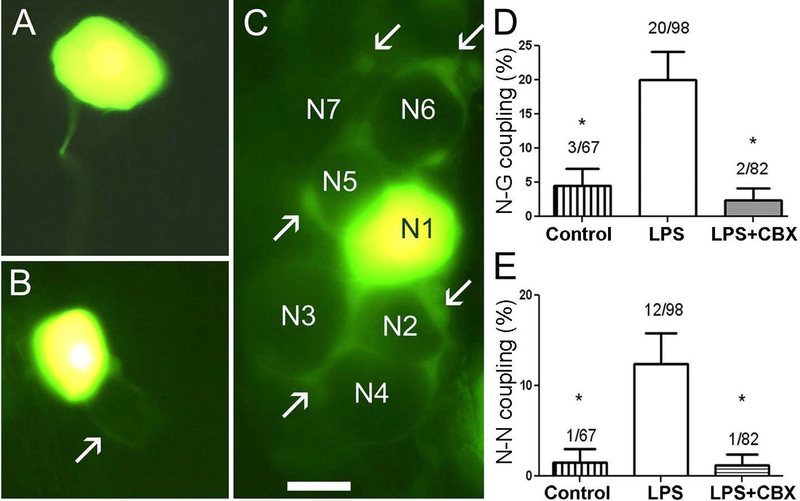
To evaluate the extent to which dye coupling was altered in TG in the setting of chronic pain, we compared spread of the gap junction permeant dye LY, which was injected into neurons in acutely isolated intact ganglia from control mice and at eight days after LPS treatment. In control ganglia, N-G coupling was generally not detectable (Fig. 1A) or was rarely observed (Fig. 1B). By contrast, in TG from LPS treated mice, N-G dye coupling was more common and more extensive (Fig. 1C). Figure 1D, E show the results of dye spread to SGCs or to neurons from a total of 247 injections into neurons. In TG from control mice, incidence of N-G spread was 4.4% (Fig. 1B, D), whereas after LPS injection the N-G coupling incidence was much higher (21.5%; Fig. 1C,D). Treatment of the TG from LPS injected mice with the gap junction blocker carbenoxolone (CBX) reduced N-G dye coupling to 2.4% (Fig. 1D). These results indicate that a low level of N-G coupling is present in the intact TG, that it is upregulated by systemic inflammation, and that it is gap junction mediated. We previously reported that LPS injection also evoked neuron to neuron (N-N) dye coupling in DRG (Blum et al. 2014). Similarly, injections into TG neurons revealed that that LPS treatment increased dye coupling incidence among neurons from 1.5 to 13%, whereas CBX exposure of ganglia from LPS treated mice reduced the coupling to 1.2% (Fig. 1E).
Figure 1. Lucifer yellow (LY) injection into neurons in intact TG from LPS-treated mice reveals homocellular and heterocellular gap junction-mediated coupling.
A. Injected neuron in TG isolated from control animal is typically not dye-coupled to any cell. B. In a very small number of cases an injected neuron in a control TG was found to be coupled to a surrounding SGC and to the SGC sheath surrounding a neighboring neuron (arrow indicates the SGC envelope coupled to the injected neuron). C. TG from a mouse that had been injected with LPS (2.5 mg/kg) eight days earlier. This is an example of the larger proportion of cases in which N-G coupling was present. The injected neuron (N1) was dye-coupled to SGCs around at least six other neurons (N2–N7). Some of the coupled SGCs are indicated by arrows. Calibration, 20 μm. D. Summary of frequency of observing LY dye transfer to SGC (G) when a neuron was injected. In control conditions such coupling was rare (about 5%), but after LPS injection, N-G coupling increased four-fold. The addition of CBX (100 μM) to the medium during LY injection into cells in ganglia from LPS injected mice reduced coupling incidence to about 3% (* p<0.01 between control and LPS treatment and between LPS treatment and LPS + CBX). E. Effects of LPS injection on coupling between neurons in intact TG. In controls the prevalence of such coupling was low (<2%); however, at eight days after LPS injection we observed neuron-neuron coupling in about 13% of the cases. The gap junction blocker CBX blocked the intercellular spread of LY, confirming that gap junctions were involved. (* p<0.05: Fisher’s exact test).
Intercellular communication between sensory neurons and SGCs in dissociated TG cultures
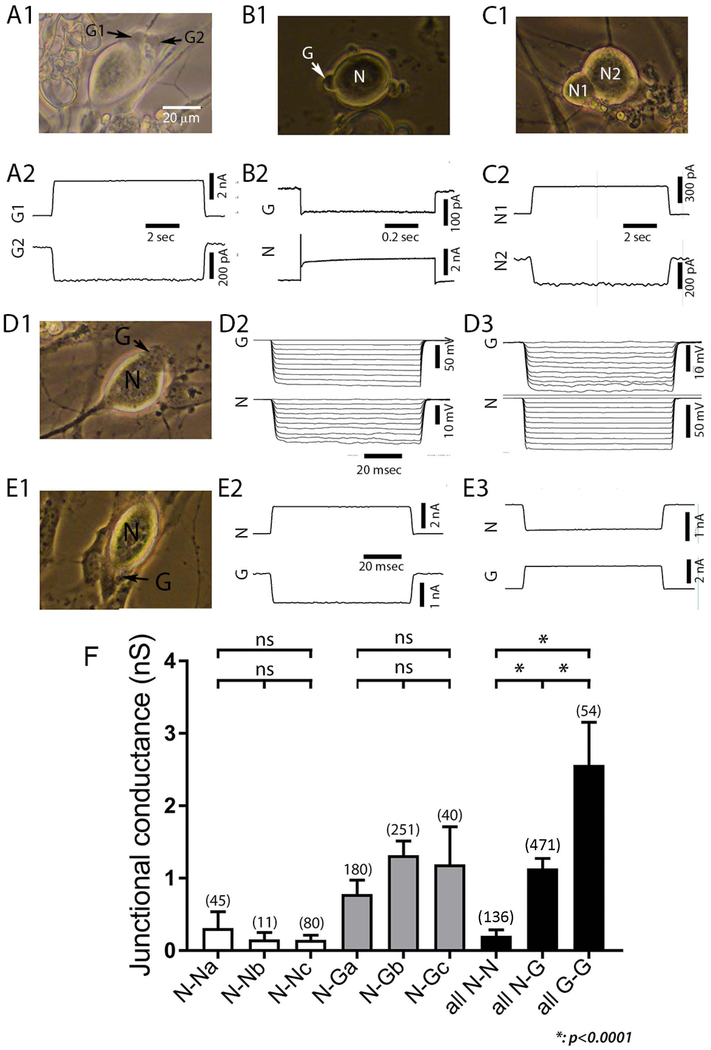
Neurons in culture are readily distinguished from SGCs by their large size (generally 20–30 μm diameter), round shape and bright birefringence in phase microscopy (see example micrographs in Figs. 2,3,5,6). To determine whether SGCs and neurons were electrically connected to cells of the same or different type, we recorded from G-G, N-G and N-N cell pairs using dual whole cell voltage clamp (representative recordings in Fig. 2A1–2, B1–2, C1–2). Coupled pairs were recorded in each configuration, and coupling was found to be bi-directional, as evidenced for N-G pairs by both current clamp (Fig. 2D1–3) and voltage clamp protocols (Fig. 2E1–3).
Figure 2. Neurons and SGCs are coupled to themselves and each other in dissociated cultures from trigeminal ganglion.
A-C: Strength of gap junction mediated coupling between representative pairs of satellite glial cells (SGCs, labeled as G1, G2: A1, A2), SGC-neuron pairs (N and G in B1, B2, D1–3, E1–3) and pairs of sensory neurons (N1 and N2 in C1, C2). Accompanying photographs were taken immediately prior to the electrophysiological recordings. Recordings were selected from series of depolarizing or hyperpolarizing steps applied to each cell of the pair. In A2, +40 mV step was applied to G1, current recorded in G2 (IG2) is termed junctional current, and represents the current passed by the voltage clamp circuit on that cell to hold its voltage constant; junctional conductance is calculated as –IG2/V, in this case about 2.5 nS. In B2, +40 mV step was applied to N, in C2, +40 mV step was applied to N2. D2–3 illustrate current clamp recording of bidirectional coupling between SGC and neuron; in D2 10 nA hyperpolarizing steps are applied to G; whereas the same stimulation paradigm is applied to the neuron in D3. E2–3 illustrate bidirectional coupling under voltage clamp conditions, with the neuron depolarized in E2 and the SGC depolarized in E3. F. Summary of results obtained for cell pairs of each type. NN, N-G and GG designate neuron-neuron, N-G and G-G cell pairs respectively. Subgroups a and b represent recordings made of cultures 2–3 days old during two time periods. Group c represent cells pairs recorded within 18 hr after dissociation. There were no significant differences in coupling strength between recording epochs or with regard to whether cells were freshly isolated or maintained in culture and thus groups were combined to compare coupling strength between homocellular and heterocellular pairs (all N-N, all N-G, all G-G), each of which differed significantly from the other groups (p<0.0001).
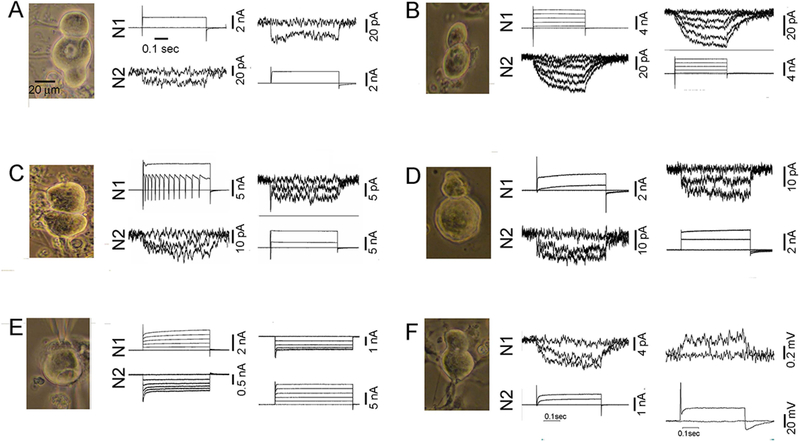
Figure 3. In freshly dissociated (<18 hours) TG cultures neuron pairs are bidirectionally electrically coupled, and junctional conductance is generally weak.
In each case illustrated, neuronal identity was validated by large inward currents in response to depolarizing pulses in addition to appearance of the cells. In frames A-E, top and bottom traces are recordings from two neurons, generally depolarizing pulses applied first to N1 (middle traces) and then to N2 (right-hand traces). Current recorded in the non-pulsed cells reflects junctional current, from which junctional conductance is calculated. A. Traces shown for each cell correspond to no pulse and depolarization from −60 to 0 mV. B. Traces shown correspond to steps from −60 to −60, −40,−20, 0, 20, 40 mV. C. Traces shown correspond to steps from −60 to 0 and +40 mV. D. Traces shown correspond to steps from −60 to −60, 0 and +40 mV. E. Traces shown correspond to steps from 0 to −60, −40, −20, 0 and +20 mV. F. In these recordings, N2 was depolarized from −60 to −60, −20 and 0 mV in voltage clamp mode (middle traces) and 0 or 0.1 nA pulse was applied to N2 under current clamp conditions in the right-hand panel. Scale bar in A (20 μm) applies to all photographs.
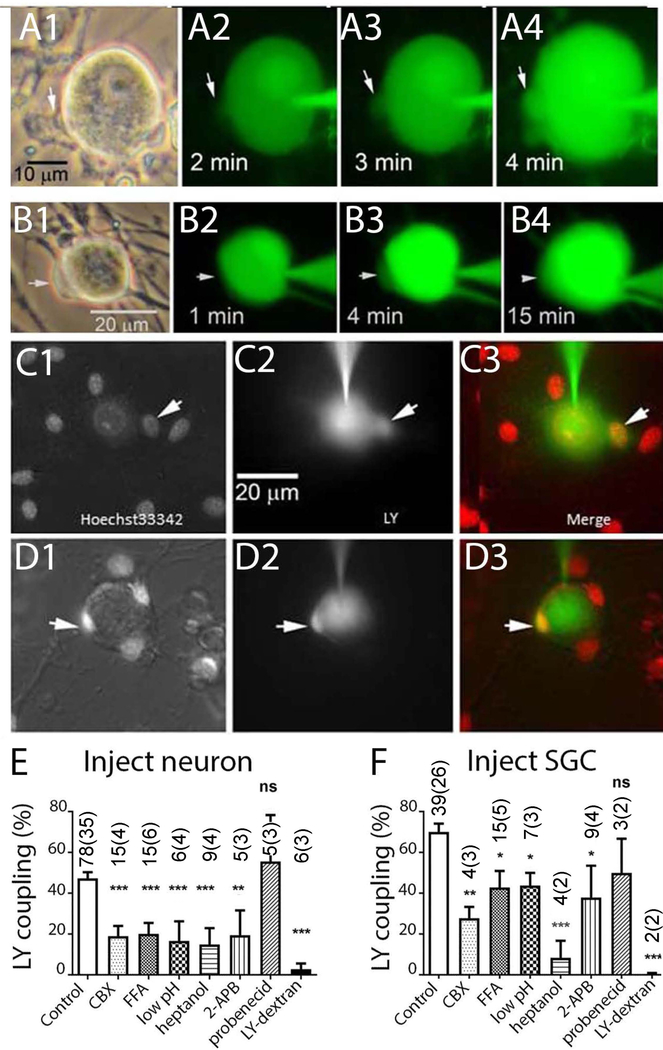
Figure 5. Lucifer Yellow (LY) injections performed in dissociated cultures of trigeminal ganglion demonstrate gap junction mediated coupling between neurons and SGCs.
A1–4 and B1–4 illustrate time course of dye spread into SGCs (arrows) during LY injection into neurons. C1–D3 illustrate two experiments in which LY was injected into neurons in cultures which were acutely treated with the nuclear indicator Hoechst 33342. Overlays in C3, D3 demonstrate LY presence in small SGCs that are closely apposed to the neuron surface. E, F. Summary of experiments in which incidence of LY dye coupling was quantified after injection into neurons (E) or SGC (F). Drugs applied were 200 μM CBX, 100 μM flufenamic acid (FFA), 2 mM heptanol, 100 μM 2-APB, 1 mM probenecid. Intracellular acidification (low pH) was obtained through application of solution bubbled with 100% CO2, LY Dextran (MW 10 Da), a large fluorescent gap junction impermeant molecule, was completely retained in the injected cell. * indicates p<0.05, ** p< 0.01 and *** p< 0.005 (Fisher’s exact test). The numbers in the bars indicate the number of cells injected. In the case of both SGC and neuron injections, most recipients of the dye injections were SGCs.
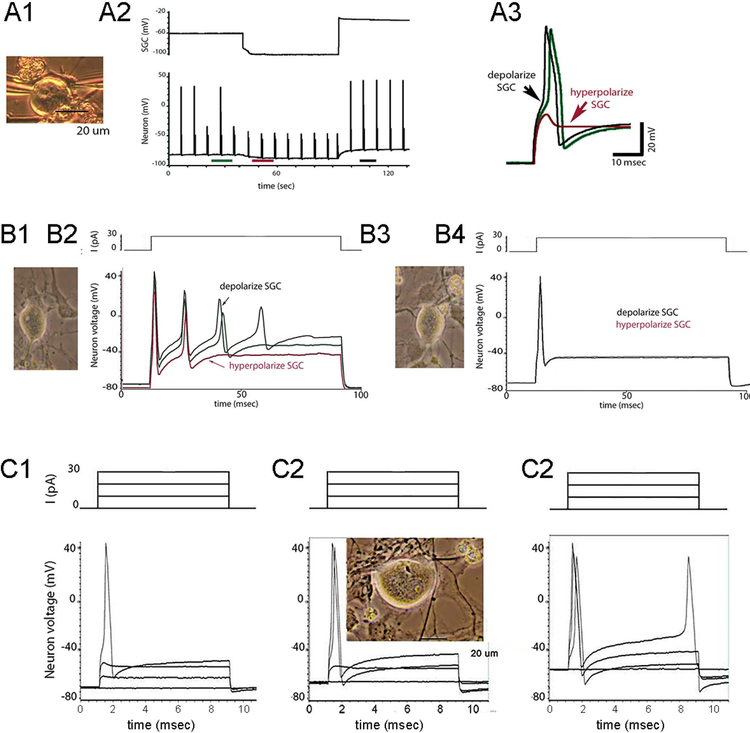
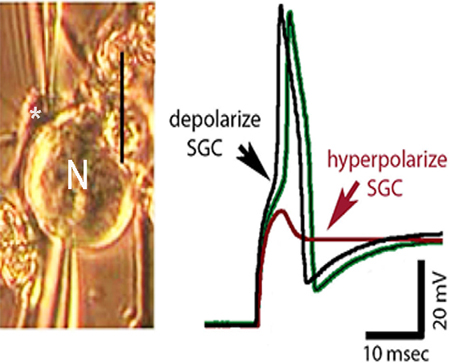
Figure 6. SGC-neuron coupling strength is sufficiently high that glial polarization can modify neuron excitability.
A1–3. Supra-threshold current pulses were applied repeatedly to the neuron (lower trace in A2) while glial resting membrane potential (upper trace) was changed by injecting hyperpolarizing and then depolarizing current. Note that at resting potential (A2) responses are variable in amplitude, reflecting intermittent action potential failure. When SGC was hyperpolarized (middle portion of recording epoch) firing was blocked, whereas depolarizing the SGC resulted in 1:1 firing of neuron. A3. Neuronal responses to current pulses in each of the recording epochs are enlarged to show impact of glial depolarization on neuron responses to the same amplitude current step. B1–4. Comparison of effects of SGC polarization on coupled (B1–2) and uncoupled (B3–4) SGC-neuron pair. Note that same current pulse applied to neuron results in markedly different firing patterns when SGC is depolarized or hyperpolarized in the coupled pair but has no effect when coupling is absent (red, green and black traces are coincident). C1–3. Series of current steps applied to neuron under conditions where SGC was hyperpolarized (C1), not polarized (C2) and depolarized (C3). Note profound effects on firing threshold and activity patterns in response to this 4-pulse paradigm depending on SGC polarization. Photographs of cells from which recordings were made were taken immediately before dual whole cell recordings.(Magni et al. 2015)
In order to determine whether N-N or N-G coupling strength changed within a few days after TG dissociation and culture, we compared recordings obtained in freshly dissociated cells (2–18 hr after isolation) with those at 2–3 days in culture. Moreover, to confirm reproducibility in cell culture techniques and electrophysiology recordings, we compared junctional conductance values for N-N and N-G pairs recorded by different investigators (labeled a and b in Fig. 2F). For N-N pairs, junctional conductance (gj) measured by different investigators after 2–3 days in culture was 0.31±0.22 nS (n=45; N-Na in Fig. 2F) and 0.15±0.09 nS (n=11; N-Nb) during these recording sessions and was not significantly different from values recorded in freshly dissociated cell pairs (0.21±0.06 nS, n=80; N-Nc); mean gj measured in all 136 N-N pairs in all conditions (all N-N) was 0.20+0.08 nS. For N-G pairs at 2–3 days in culture, gj values obtained by the two investigators were 0.78+0.2 (n=180; N-Ga) and 1.19+0.2 nS (n=251; N-Gb), not significantly different from that measured in freshly dissociated N-G pairs (1.19±0.51 nS, n=40; N-Gc); overall gj measured in 471 N-G pairs (all N-G) was 1.14±0.14 nS. Recordings from 54 G-G pairs, only obtained from cells in culture for 24 hr or longer, revealed average gj values of 2.56±0.6 nS (all G-G). As indicated in the right-most bars in Fig. 2F, although each cell type combination showed coupling, strength of coupling in each configuration differed from one another, with G-G coupling the strongest, N-G of intermediate strength and N-N coupling the weakest (p<0.0001 for each intergroup comparison).
As noted above, pairs of freshly dissociated sensory neurons were often found to be electrically coupled, although mean gj was quite low (about 0.2 nS). Examples of dual voltage clamp recordings from pairs of freshly dissociated neurons selected to illustrate a range of properties are shown in Fig. 3. In coupled N-N pairs, voltage pulses usually evoked rapid and sustained junctional currents (Fig. 3A,D). Variants included very slowly developing junctional currents (as in Fig. 3B,F) and cases in which depolarizing voltage pulses resulted in apparent inward currents in both the pulsed cell and its coupled partner (Fig. 3C,E). Both the slow time course of junctional current and the unclamped inward currents indicate that site of interaction is electrically distant from the cell body. In some cases, as in Fig. 3C, rise time is slow in one direction and inward current is seen in the coupled cell when assessed in the other direction (Fig. 3C), possibly indicating that there is asymmetry in access resistance from the soma.
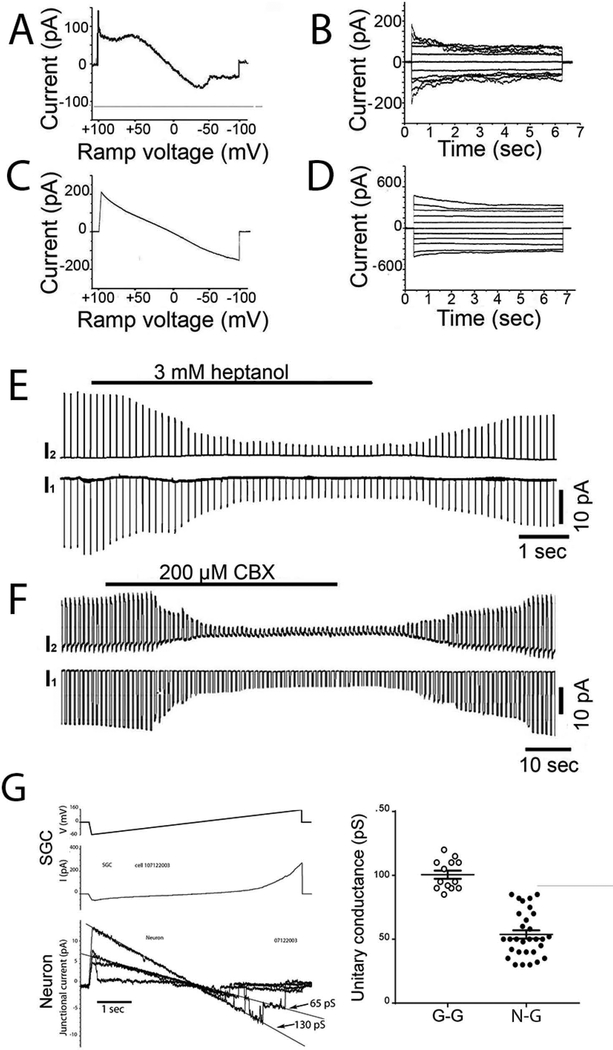
The presence of electrical coupling between neurons and SGCs in TG raised the issue of whether the properties of gap junction channels in sensory ganglia were similar to those in other tissues and whether properties varied when comparing different cell combinations. Because coupling strength between neurons was quite low, and because whole cell recordings (Fig. 3) suggested that the location of gap junctions might not be isopotential with respect to the soma, we focused these studies on G-G and N-G pairs. To evaluate voltage sensitivity, we applied slow voltage ramps ±100 mV and long voltage command steps ±100 mV in 20 mV increments to one cell while recording current in the other cell (Fig. 4A–D). For G-G pairs, currents showed marked sensitivity to transjunctional voltage (Vj), with steady-state junctional currents being lower at Vj >50 mV than at smaller voltages (Fig. 4A, B). For N-G pairs, voltage sensitivity was lower, as indicated by less deviation from linearity in response to ramps (Fig. 4C) and less decay of the currents during long Vj steps (Fig. 4D). This comparison between G-G and N-G properties suggests that gap junctions that connect these cells are likely formed of different connexin proteins.
Figure 4. Properties of gap junctions recorded in pairs of SGCs and in SGC-neuron pairs.
A,B. Illustrated examples of voltage dependence of gap junction-mediated coupling in pairs of SGCs and C, D in SGC-neuron pairs; in A, C voltage ramps from −100 mV to +100 mV were applied to one cell (that cell’s current is not shown), and illustrated currents were recorded in the coupled cell. In B, D pulses from 0 to ±100 mV were applied in 20 mV increments to estimate steady state voltage dependence. Note the moderate and symmetric bidirectional rectification in SGC pairs, which is much less in SGC-neuron pairs. E,F illustrate experiments in which SGC-neurons pairs were treated with gap junction uncoupling agents heptanol (2 mM) and CBX (0.2 mM) while hyperpolarizing pulses were delivered repeatedly to cell 2. Note that uncoupling is virtually complete within a short time in these recordings, and is quickly reversible. G. Illustrates junctional currents recorded in an SGC-neuron pair after substantial reduction of junctional conductance by heptanol. The uppermost trace represents the transjunctional voltage applied to cell 1, the middle trace is the input current evoked in cell 1 and the lower four traces are selected from the junctional current recordings in which stepwise fluctuations reveal individual gap junction channels opening and closing. From the slopes of the current / trans-junctional voltage relations, junctional conductance for a single opening is about 65 pS and for two channels open is about 130 pA. H. From recordings such as those illustrated in G performed on SGC cell pairs (N=7) and SGC-neuron pairs (N=7), we measured unitary currents and calculated single channel conductance. Results are plotted in the graphs, demonstrating that in SGC pairs (G-G) gj was 100.5±3 pS and in G-N pairs it was substantially lower (56±4 pS).
Gap junctions are blocked by exposure to several pharmacological agents, including heptanol and carbenoxolone (CBX). Recordings from N-G pairs also revealed that junctional conductance was reversibly reduced by exposure to heptanol (Fig. 4E) and to CBX (Fig. 4F). Long voltage ramps applied when overall conductance was reduced by heptanol or CBX revealed currents corresponding to individual gap junction channel opening and closing events. Such recordings from N-G pairs (see Fig. 4G) revealed a main state unitary conductance of about 55 pS (mean from 31 measured events in seven cell pairs: 53.7±3.2 pS: Fig. 4H); apparent subconductance state is detectable as a non-zero current at the highest voltages, with conductance about 10 pS (Fig. 4G). Measurements of unitary conductance of G-G pairs measured under similar manipulations revealed substantially higher values (100.5 ±3.5 pS, Fig. 4H). The distinct unitary conductances of G-G and N-G gap junctions provides further evidence that different connexin ensembles mediate coupling in the G-G and N-G pairs.
Dye coupling between cultured TG cells
As another test for the presence of functional gap junctions between SGCs and sensory neurons in TG cell cultures, we performed intracellular injections of the gap junction permeant dye LY. As illustrated in Fig. 5, neuron injections commonly resulted in dye spread to adjacent SGCs. Two examples of the time course of such spread are shown in Fig. 5A1–4, B1–4. Additional evidence for N-G spread was provided by LY dye injections performed after live cell staining of nuclei with the fluorescent dye Hoechst 33342. As shown in Figs. 5C–3, D1–3, neuron injection filled some but not all of the neighboring SGCs, indicating the presence of direct gap junction connections between neurons and some adjacent SGCs. The incidence of dye spread (fraction of injections in which dye spread to at least one other cell, either neuron or glia), was about 45% for neuron and about 65% for SGC injections (Fig. 5E,F). To ensure that dye spread was through gap junction channels, we treated TG cell cultures with the gap junction channel blockers CBX, flufenamic acid, low intracellular pH (induced by equilibrating bathing solution with 100% CO2), heptanol or 2-aminoethoxydiphenyl borate (2-APB). Incidence of dye spread was quantified following injection into neuron (Fig. 5E) or SGC (Fig. 5F); N-N dye coupling was rarely detected and was not quantified. Both for neuron and for SGC injections, each of the gap junction blockers significantly inhibited LY spread. As a control for possible leakage and uptake through other channels, we tested the Panx1 channel blocker probenecid (1 mM) and injection of a large fluorescent gap junction impermeant molecule (LY dextran, MW 10 kDa). Reinforcing the conclusion that gap junctions mediated the transfer, probenecid did not affect dye spread and LY dextran was completely retained in the injected cell (Fig. 5E,F). Although other channel types are also affected by each of the drugs tested, their combined effectiveness is generally accepted as evidence for gap junction involvement (Spray et al. 2006), and we conclude that the N-G spread is through gap junctions.
Coupling between SGCs and neurons can modulate neuronal excitability
The average coupling strength measured in N-G pairs is about 1 nS (Fig. 1F). In order to determine whether such low junctional conductance can affect neuron excitability, we performed experiments under current clamp conditions where brief depolarizing currents were applied to neurons while depolarizing or hyperpolarizing an SGC to which it was coupled. Representative examples are presented in Fig. 6. One protocol involved continuously applying suprathreshold current pulses to the neuron with the SGC initially at its normal resting potential and then hyperpolarizing and depolarizing the SGC while applying the same current step to the neuron (Fig. 6A2). When the neuron recordings are displayed at faster sweep (time indicated in Fig. 6A2), it is apparent that the threshold for activation is increased during SGC hyperpolarization, whereas firing occurs with shorter latency during SGC depolarization (Fig. 6A3). In a second protocol, longer current pulses were applied to the neuron with an amplitude sufficient to generate firing. When N-G pairs were coupled, SGC hyperpolarization reduced, and depolarization increased the number of action potentials evoked by the neuronal current pulse (Fig. 6B1,2). However, when N-G coupling was absent, SGC polarization did not influence neuronal activity (Fig. 6B3,4). In a third protocol, a series of current pulses were applied to the neuron while hyperpolarizing (Fig, 6C1), not affecting (Fig. 6C2) or depolarizing the SGC (Fig, 6C3), demonstrating additive effects over a range of applied currents. To rule out field effects, we also performed experiments on N-G pairs that were not coupled (Fig. 6B4); in those cases, polarization of the SGC did not affect baseline holding current or change neuronal firing threshold.
Discussion
The studies reported here reveal that systemic LPS injection leads to enhanced dye coupling between neighboring sensory neurons and between neurons and SGCs in intact trigeminal ganglion. As summarized in a recent review (Spray and Hanani 2017), previous studies have detected increased coupling among glial cells in both peripheral ganglia and in the CNS in response to nerve injury and other painful conditions. Dye coupling among neurons and between neurons and glia is reportedly rare in normal sensory ganglia but emerges following local or systemic inflammation (Blum et al. 2014; Huang et al. 2010; Ledda et al. 2009), perhaps reflecting the emergence of neuron-neuron coupling that occurs as a consequence of nerve damage both in invertebrate ganglia (Murphy et al. 1983) and in the spinal cord (Chang et al. 2000).
In the studies on intact ganglion, dye coupling was used as a readout for functional gap junction-mediated interaction between cells, and gap junction involvement was confirmed through pharmacological blockade of the coupling. Definitive evidence of gap junction involvement and characterization of the properties of such intercellular channels requires electrophysiological evaluation, which is most readily performed in dissociated cell culture due to technical challenges of recording in intact ganglia. When we measured junctional conductance between cell pairs, coupling strength was highest for SGC pairs, weakest for pairs of neurons and intermediate for N-G pairs, and both electrical and dye coupling was blocked by a range of gap junction inhibitors. G-G and N-G pairs were shown to exhibit distinctive voltage sensitivity and single channel properties, implying that the gap junction proteins (connexins) forming the channels are likely different (heterotypic). Several connexin types have been reported in TG and DRG including Cx26, Cx32, Cx36, Cx43 (Garrett and Durham 2008; Ohara et al. 2008; Perez Armendariz et al. 2018; Procacci et al. 2008). In a study quantifying expression levels in sensory ganglia (Manteniotis et al. 2013), genes encoding Cx32 and Cx43 were most abundant, followed by Cx30.2, Cx37, Cx26, Cx30, Cx45 and Cx36. Based on voltage sensitivity of the gap junctions between SGCs and their unitary conductances, the main gap junction protein expressed in SGCs is likely to be Cx43, which is the major gap junction protein expressed in CNS astrocytes (Scemes and Spray 2008). By contrast, the low and asymmetric voltage dependence of junctional conductance in N-G pairs and the small unitary conductance of junctional channels between them (Fig. 3) suggest that Cx43 or other connexin proteins expressed in the SGCs may pair with a less voltage sensitive connexon with lower unitary conductance contributed by the neurons. One such possibility is Cx36, the major gap junction protein of CNS (Srinivas et al. 1999) and sensory ganglion neurons (Perez Armendariz et al. 2018), which is less voltage sensitive than Cx43 and would be expected to yield channels of about 60 pS conductance if their hemichannels were paired end to end with Cx43 hemichannels. Cx45 is another candidate for the neuron side; it has been found in a subtype of retinal ganglion cells (Schubert et al. 2005) and in the brain it is widely expressed early in development, but becomes restricted to thalamus postnatally (Maxeiner et al. 2003). Cx26, Cx30 and Cx30.2 are additional candidates, based on gene expression analysis cited above and presence in CNS glia and/or neurons (Garrett and Durham 2008; Kreuzberg et al. 2008; Muller et al. 2010; Nagy et al. 2004). Although Cx26 was proposed as the gap junction protein induced between SGCs and neurons in TG under painful conditions (Garrett and Durham 2008), the unitary conductance and asymmetric voltage sensitivity reported here indicate that other connexins likely participate.
Direct evidence that sensory neurons and SGCs in TG form gap junctions complements and extends previous evidence for dye transfer between these cell types in sensory ganglia (Spray and Hanani 2017; Thalakoti et al. 2007). The finding of coupling among each of these cell types is remarkable on several levels, including the novelty of direct N-G gap junctions and the potential for glial cells to directly modulate neuronal excitability.
Several previous reports have shown N-G dye and electrical coupling in the CNS (Alvarez-Maubecin et al. 2000; Bittman et al. 2002; Froes et al. 1999; Ledda et al. 2009; Nedergaard 1994; Pakhotin and Verkhratsky 2005; Rozental et al. 2001; Walker and Hild 1969), although it is generally believed that maintenance of separate communication compartments is a critical organizational feature that is required for their independent functions of these cell types in CNS.
The functional impact of coupling between SGCs, between neurons and between SGCs and neurons is likely in directly modulating neuronal excitability, as speculated in studies of radial glial-Purkinje cell coupling in cerebellum (Pakhotin and Verkhratsky 2005). Our experiments provide evidence for such a physiological consequence of N-G coupling, such that glial depolarization increased neuronal excitability, and glial hyperpolarization decreased it. SGCs undergo considerable changes under pathological conditions, such as nerve section or inflammation (Hanani 2005; Ohara et al. 2009). Such injury likely depolarizes glia due to damage and activation of the purinergic P2X7 and other receptors, which can increase excitability in the neurons to which the SGC are coupled. Our recordings of coupling between sensory neurons revealed that coupling strength is low, which is consistent with our recent report of electrical coupling in acutely dissociated DRG neurons in a model of inflammatory pain (Kim et al. 2016).
The importance of intercellular coupling in generation and modulation of pain likely lies in providing pathways for spread of excitation within the sensory ganglia. Similar spread, attributed to chemical interactions, has previously been described as “cross-excitation”, in which stimulation of axons of sensory neurons can depolarize other neurons within the sensory ganglia (Amir and Devor 2000). These authors proposed an “ignition” model to explain trigeminal neuralgia, in which triggering paroxysmal activity could involve both “ephaptic” and chemical elements (Devor et al. 2002). Our present results do not exclude chemical communication that involves both neurons and SGCs. Indeed, we have shown that calcium waves in sensory ganglia are mediated by both these elements (Suadicani et al. 2010) and that Panx1 deletion or blockade provides pain relief (Hanstein et al. 2016; Weaver et al. 2017). Although the strength of coupling we have recorded between neurons and between SGCs and neurons is low, it could provide an important pathway for diffusional exchange of metabolites and signaling molecules. Together with extracellular chemical signaling, this mode of intercellular communication likely endows the SGCs with the capacity to modulate neuronal activity and behavioral hypersensitivity.
In conclusion, we suggest that the process of peripheral sensitization, which is important for the emergence of pain involves generation of hyperexcitability in sensory neurons that is associated with increased gap junction-mediated interactions between neurons, between glia and between neurons and glia. Together with facilitated neuron-glial paracrine signaling through chemokine, purinergic and other pathways, we propose that this coupling serves to sustain endogenous activity within the ganglia. We also note that SGC hyperpolarization reduced neuronal excitability, perhaps offering a novel target for intervention in chronic pain.
Main Points:
Voltage clamp recordings revealed that satellite glial cells in trigeminal ganglia are mutually coupled, sensory neurons are also mutually coupled, and are coupled to glia. Glial electrical polarization can depress or enhance neuronal excitability.
Acknowledgement:
Supported by the Israel Science Foundation ISF 508/13 to MH, U.S.-Israel Binational Science Foundation BSF-2011044 to MH and DCS, German Research Foundation DFG)-HA5860/1–1 to RH and National Institutes of Health R01NS092466 to DCS. The late Yonathan Zuckerman contributed enormously to initial aspects of these studies both in NY and in Jerusalem; we gratefully acknowledge his participation and friendship.
Footnotes
Conflict of Interest Statement: The authors declare no competing financial interests
References
- Alvarez-Maubecin V, Garcia-Hernandez F, Williams JT, Van Bockstaele EJ. 2000. Functional coupling between neurons and glia. Journal of Neuroscience 20:4091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Devor M. 2000. Functional cross-excitation between afferent A- and C-neurons in dorsal root ganglia. Neuroscience 95:189–95. [DOI] [PubMed] [Google Scholar]
- Belzer V, Shraer N, Hanani M. 2010. Phenotypic changes in satellite glial cells in cultured trigeminal ganglia. Neuron Glia Biol 6:237–43. [DOI] [PubMed] [Google Scholar]
- Bittman K, Becker DL, Cicirata F, Parnavelas JG. 2002. Connexin expression in homotypic and heterotypic cell coupling in the developing cerebral cortex. J Comp Neurol 443:201–12. [DOI] [PubMed] [Google Scholar]
- Blum E, Procacci P, Conte V, Hanani M. 2014. Systemic inflammation alters satellite glial cell function and structure. A possible contribution to pain. Neuroscience 274:209–17. [DOI] [PubMed] [Google Scholar]
- Chang Q, Pereda A, Pinter MJ, Balice-Gordon RJ. 2000. Nerve injury induces gap junctional coupling among axotomized adult motor neurons. J Neurosci 20:674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Corsso C, Srinivas M, Urban-Maldonado M, Moreno AP, Fort AG, Fishman GI, Spray DC. 2006. Transfection of mammalian cells with connexins and measurement of voltage sensitivity of their gap junctions. Nat Protoc 1:1799–809. [DOI] [PubMed] [Google Scholar]
- Devor M, Amir R, Rappaport ZH. 2002. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain 18:4–13. [DOI] [PubMed] [Google Scholar]
- Feldman-Goriachnik R, Belzer V, Hanani M. 2015. Systemic inflammation activates satellite glial cells in the mouse nodose ganglion and alters their functions. Glia. [DOI] [PubMed] [Google Scholar]
- Froes MM, Correia AH, Garcia-Abreu J, Spray DC, Campos de Carvalho AC, Neto MV. 1999. Gap-junctional coupling between neurons and astrocytes in primary central nervous system cultures. Proceedings of the National Academy of Sciences of the United States of America 96:7541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett FG, Durham PL. 2008. Differential expression of connexins in trigeminal ganglion neurons and satellite glial cells in response to chronic or acute joint inflammation. Neuron GLIA Biology 4:295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Chen Y, Zhang X, Li GW, Wang C, Huang LY. 2010. Neuronal soma-satellite glial cell interactions in sensory ganglia and the participation of purinergic receptors. Neuron GLIA Biology 6:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanani M 2005. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev 48:457–76. [DOI] [PubMed] [Google Scholar]
- Hanani M 2012. Intercellular communication in sensory ganglia by purinergic receptors and gap junctions: implications for chronic pain. Brain Res 1487:183–91. [DOI] [PubMed] [Google Scholar]
- Hanani M, Spray DC. 2013. Glial Cells in Autonomic and Sensory Ganglia In: Kettenmann H RB, editor. Neuroglia: Oxford University Press. p 122–133. [Google Scholar]
- Hanstein R, Hanani M, Scemes E, Spray DC. 2016. Glial pannexin1 contributes to tactile hypersensitivity in a mouse model of orofacial pain. Sci Rep 6:38266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TY, Belzer V, Hanani M. 2010. Gap junctions in dorsal root ganglia: possible contribution to visceral pain. Eur J Pain 14:49 e1–11. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Vit JP, Bhargava A, Ohara PT. 2010. Can satellite glial cells be therapeutic targets for pain control? Neuron Glia Biol 6:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Anderson M, Park K, Zheng Q, Agarwal A, Gong C, Saijilafu, Young L, He S, LaVinka PC and et al. 2016. Coupled Activation of Primary Sensory Neurons Contributes to Chronic Pain. Neuron 91:1085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzberg MM, Deuchars J, Weiss E, Schober A, Sonntag S, Wellershaus K, Draguhn A, Willecke K. 2008. Expression of connexin30.2 in interneurons of the central nervous system in the mouse. Mol Cell Neurosci 37:119–34. [DOI] [PubMed] [Google Scholar]
- Ledda M, Blum E, De Palo S, Hanani M. 2009. Augmentation in gap junction-mediated cell coupling in dorsal root ganglia following sciatic nerve neuritis in the mouse. Neuroscience 164:1538–45. [DOI] [PubMed] [Google Scholar]
- Magni G, Merli D, Verderio C, Abbracchio MP, Ceruti S. 2015. P2Y2 receptor antagonists as anti-allodynic agents in acute and sub-chronic trigeminal sensitization: role of satellite glial cells. Glia 63:1256–69. [DOI] [PubMed] [Google Scholar]
- Manteniotis S, Lehmann R, Flegel C, Vogel F, Hofreuter A, Schreiner BS, Altmuller J, Becker C, Schobel N, Hatt H and et al. 2013. Comprehensive RNA-Seq expression analysis of sensory ganglia with a focus on ion channels and GPCRs in Trigeminal ganglia. PLoS One 8:e79523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxeiner S, Kruger O, Schilling K, Traub O, Urschel S, Willecke K. 2003. Spatiotemporal transcription of connexin45 during brain development results in neuronal expression in adult mice. Neuroscience 119:689–700. [DOI] [PubMed] [Google Scholar]
- Muller LP, Dedek K, Janssen-Bienhold U, Meyer A, Kreuzberg MM, Lorenz S, Willecke K, Weiler R. 2010. Expression and modulation of connexin 30.2, a novel gap junction protein in the mouse retina. Vis Neurosci 27:91–101. [DOI] [PubMed] [Google Scholar]
- Murphy AD, Hadley RD, Kater SB. 1983. Axotomy-induced parallel increases in electrical and dye coupling between identified neurons of Helisoma. J Neurosci 3:1422–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Dudek FE, Rash JE. 2004. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Brain Res Rev 47:191–215. [DOI] [PubMed] [Google Scholar]
- Nedergaard M 1994. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263:1768–71. [DOI] [PubMed] [Google Scholar]
- Ohara PT, Vit JP, Bhargava A, Jasmin L. 2008. Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo. Journal of Neurophysiology 100:3064–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara PT, Vit JP, Bhargava A, Romero M, Sundberg C, Charles AC, Jasmin L. 2009. Gliopathic pain: when satellite glial cells go bad. Neuroscientist 15:450–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakhotin P, Verkhratsky A. 2005. Electrical synapses between Bergmann glial cells and Purkinje neurones in rat cerebellar slices. Molecular & Cellular Neurosciences 28:79–84. [DOI] [PubMed] [Google Scholar]
- Perez Armendariz EM, Norcini M, Hernandez-Tellez B, Castell-Rodriguez A, Coronel-Cruz C, Alquicira RG, Sideris A, Recio-Pinto E. 2018. Neurons and satellite glial cells in adult rat lumbar dorsal root ganglia express connexin 36. Acta Histochem 120:168–178. [DOI] [PubMed] [Google Scholar]
- Procacci P, Magnaghi V, Pannese E. 2008. Perineuronal satellite cells in mouse spinal ganglia express the gap junction protein connexin43 throughout life with decline in old age. Brain Research Bulletin 75:562–9. [DOI] [PubMed] [Google Scholar]
- Rozanski GM, Kim H, Li Q, Wong FK, Stanley EF. 2012. Slow chemical transmission between dorsal root ganglion neuron somata. European Journal of Neuroscience 36:3314–21. [DOI] [PubMed] [Google Scholar]
- Rozental R, Andrade-Rozental AF, Zheng X, Urban M, Spray DC, Chiu FC. 2001. Gap junction-mediated bidirectional signaling between human fetal hippocampal neurons and astrocytes. Developmental Neuroscience 23:420–31. [DOI] [PubMed] [Google Scholar]
- Scemes E, Spray DC. 2008. Connexin expression (gap junction and hemichannels) in astrocytes. New York: Springer Verlag. p 108–150. [Google Scholar]
- Schubert T, Maxeiner S, Kruger O, Willecke K, Weiler R. 2005. Connexin45 mediates gap junctional coupling of bistratified ganglion cells in the mouse retina. J Comp Neurol 490:29–39. [DOI] [PubMed] [Google Scholar]
- Spray DC, Hanani M. 2017. Gap junctions, pannexins and pain. Neurosci Lett. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Ye ZC, Ransom BR. 2006. Functional connexin “hemichannels”: a critical appraisal. GLIA 54:758–73. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC. 1999. Functional properties of channels formed by the neuronal gap junction protein connexin36. J Neurosci 19:9848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suadicani SO, Cherkas PS, Zuckerman J, Smith DN, Spray DC, Hanani M. 2010. Bidirectional calcium signaling between satellite glial cells and neurons in cultured mouse trigeminal ganglia. Neuron GLIA Biology 6:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. 2007. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache 47:1008–23; discussion 24–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker FD, Hild WJ. 1969. Neuroglia electrically coupled to neurons. Science 165:602–3. [DOI] [PubMed] [Google Scholar]
- Weaver JL, Arandjelovic S, Brown G, S KM, M SS, Buckley MW, Chiu YH, Shu S, Kim JK, Chung J and et al. 2017. Hematopoietic pannexin 1 function is critical for neuropathic pain. Sci Rep 7:42550. [DOI] [PMC free article] [PubMed] [Google Scholar]