Abstract
Aims:
To evaluate the clinical significance of bronchiolocentric fibrosis in patients with a histopathologic pattern of usual interstitial pneumonia.
Methods and results:
Two hundred fifty-two patients with pathological usual interstitial pneumonia pattern were identified. Two hundred fifteen of these patients (215/252) had the multidisciplinary diagnosis of idiopathic pulmonary fibrosis. Prospectively defined clinical, radiologic and pathological features (including bronchiolocentric fibrosis) were recorded, and peripheral blood MUC5B genotype and telomere length were measured. Bronchiolocentric fibrosis was observed in 38% (96/252) of all patients and 33% (72/215) of idiopathic pulmonary fibrosis patients; its presence was associated with a non-IPF diagnosis on multivariate analysis (odds ratio 3.71, [95% confidence interval 1.68–8.19]). Bronchiolocentric fibrosis was not significantly associated with environmental exposures, gastroesophageal reflux, cigarette smoking, or radiologic patterns. There was no significant association of bronchiolocentric fibrosis with MUC5B genotype or telomere length. Bronchiolocentric fibrosis has no significant impact on survival time.
Conclusions:
Most patients with bronchiolocentric fibrosis and a histopathologic pattern of usual interstitial pneumonia have idiopathic pulmonary fibrosis. However, this combined fibrotic pattern is associated with a non-idiopathic pulmonary fibrosis multidisciplinary diagnosis with approximately one-quarter of these patients being diagnosed as chronic hypersensitivity pneumonia or unclassifiable interstitial fibrosis. The presence of bronchiolocentric fibrosis in these patients is not significantly associated with presumed clinical risk factors for bronchiolocentric involvement, radiologic findings, MUC5B genotype, telomere length, or survival time.
Introduction
Usual interstitial pneumonia (UIP) is a histopathologic pattern most commonly associated with the clinical condition idiopathic pulmonary fibrosis (IPF) [1]. Features of histopathologic UIP pattern include a patchy, subpleural distribution of fibrosis, cystic spaces lined by bronchiolar epithelial cells (so called microscopic honeycombing), and foci of loosely formed collagen and fibroblasts (so called fibroblastic foci) [1, 2]. Importantly, a UIP pattern also requires the absence of atypical features such as extensive lymphoplasmocytic inflammation (e.g. lymphoid follicles with germinal centers) and interstitial poorly formed granulomas. Bronchiolocentric fibrosis has also been proposed as a pertinent negative finding for the diagnosis of usual interstitial pneumonia.
Bronchiolocentric fibrosis is a term used to describe histopathological fibroinflammatory changes centered on small airways [3]. Its postulated etiologies include inhalational antigen exposure (causing the clinical condition hypersensitivity pneumonitis (HP)) [4–9], gastroesophageal reflux and chronic aspiration [9, 10], and cigarette smoking [4]. Some case series have described bronchiolocentric fibrosis as an occasional histopathological finding in UIP pattern [9, 11]. However, the frequency and clinical relevance of bronchiolocentric fibrosis in the setting of UIP pattern is unknown.
Small airways have been recently addressed in the pathogenesis of IPF [12]. A common genetic variant in the MUC5B gene (rs35705950) is strongly associated with disease susceptibility to IPF [13], preclinical pulmonary fibrosis [14] and survival in IPF [15], and overexpression of MUC5B proteins in the bronchiolar and alveolar epithelia [16]. Dysfunction of small airways caused by the MUC5B polymorphism may play a role in IPF [12] and also be associated with bronchiolocentric fibrosis seen with UIP pattern.
In addition, short telomere length is associated with disease susceptibility to familial pulmonary fibrosis and sporadic IPF [17], and survival in IPF [18]. Rare variants in the telomere-related genes are implicated in IPF. In addition, short telomeres and telomere-related gene mutations are found in patients with pulmonary fibrosis other than IPF, such as chronic HP, rheumatoid arthritis [19], and unclassifiable lung fibrosis [20, 21]. Given the wide range of manifestations with short telomeres and telomere-related gene mutations in pulmonary fibrosis, bronchiolocentric fibrosis may be associated with telomere length.
The objective of this study was to evaluate the epidemiology and clinical relevance of bronchiolocentric fibrosis in patients with pathological UIP pattern. We hypothesized that bronchiolocentric fibrosis would be associated with a non-IPF diagnosis, findings inconsistent with UIP on HRCT, the presence of a MUC5B genetic polymorphism, longer telomere length, and improved survival.
Materials and Methods
Study population
Patients with pathological UIP pattern were identified from a prospectively developed registry of patients evaluated at the University of California, San Francisco (UCSF) between January 1, 2000 and December 31, 2016. Clinical diagnoses for all patients were established at the time of evaluation through multidisciplinary consensus. Patients with ILDs associated with connective-tissue disease were excluded because connective tissue diseases are defined independently from pathological features of ILDs. The UCSF institutional review board (IRB) approved the database protocols (IRB no. 10‐00198, first approved 5/10/2000), and patients provided written informed consent prior to enrolment in accordance with the Declaration of Helsinki.
Pathological evaluation
All surgical lung biopsies were reviewed by a pulmonary pathologist (K.D.J.) using a standardized case evaluation form (see Table e1): most (212/252, 84%) were reviewed and scored at the time of initial clinical evaluation and others (40/252, 16%) were reviewed retrospectively for this study. Pathological UIP pattern was diagnosed based on the following features: accentuation of fibrosis at the pleural surface, heterogenous distribution of abnormal areas with/without areas of normal lung, temporal heterogeneity, the presence of fibroblastic foci, dense collagen fibrosis with/without microscopic honeycombing, inflammation confined to areas of fibrosis; no features inconsistent with UIP [1].
In addition to the overall pathological pattern, individual pathological features (fibroblastic foci; organizing pneumonia; lymphocytic interstitial infiltration in areas on non-fibrotic lung; alveolar macrophages; dense collagen fibrosis; granuloma/giant cells; germinal centers; inorganic dust deposits; hemosiderin laden macrophages and intra-alveolar blood; eosinophilia/eosinophilic abscess; airway-centered inflammation; small airways disease; emphysema; acute lung injury) were recorded, either as dichotomous variables or using a four-point grading scale: absent, mild, moderate or marked. To simplify the analysis, the absent and mild categories and the moderate and marked categories were consolidated.
Cases with bronchiolocentric fibrosis were noted on the case report form with a check box for “abnormalities airway centered”, “moderate/marked airway-centered inflammation”, or “moderate/marked small airways disease”. Bronchiolocentric fibrosis was subsequently graded as none if absent, 1 if there was peribronchiolar metaplasia with minimal to mild septal thickening, 2 if there was peribronchiolar metaplasia with prominent alveolar septal thickening, and 3 if there was bronchiolocentric septal thickening and architectural distortion (Figure 1). Score 2 and 3 bronchiolocentric fibrosis was consolidated as present, and score 0 and 1 as absent. Severe bronchiolocentric fibrosis may be difficult to differentiate from typical microscopic honeycombing of usual interstitial pneumonia, but differs by its central location (adjacent to pulmonary artery) and presence of normal non-fibrotic alveolar septa between the fibrotic region and the pleura. Patients with a pathological pattern showing only microscopic honeycombing were excluded as it was difficult to differentiate peripheral from central scarring in these cases.
Figure 1.
Grading of bronchiolocentric fibrosis. A. No bronchiolocentric fibrosis, score = 0. The peribronchiolar alveolar septa are thin and without significant fibrosis. B. Mild bronchiolocentric fibrosis, score = 1. There is peribronchiolar metaplasia with slight alveolar septal fibrosis. C. Moderate bronchiolocentric fibrosis, score = 2. There is peribronchiolar metaplasia with prominent alveolar septal thickening extending across half of the pulmonary lobule. D. Severe bronchiolocentric fibrosis, score = 3. There is peribronchiolar metaplasia and bronchiolocentric fibrosis with loss of normal alveolar architecture. Scale bars = 500 μm).
Clinical variables
Baseline clinical variables prospectively obtained on all patients included in this analysis were age, sex, ethnicity/race, smoking status (ever/never smoking, pack-years), selected comorbidities (asthma; congestive heart failure; gastroesophageal reflux; sleep apnea; diabetes), exposure history (yes/no for each of bird and feather, environmental organic antigens, asbestos, other dusts, chemical substances, farm worker, and occupational organic antigens), baseline (closest to the date of biopsy) pulmonary function test values (forced vital capacity; forced expiratory volume in one second; total lung capacity; diffusion capacity for carbon monoxide), and high-resolution computed tomography (HRCT) scan of the chest (closest to the date of biopsy).
All HRCT scans were reviewed for this study by two expert chest radiologists (B.M.E. and T.S.H.) and categorized as definite UIP, possible UIP or inconsistent with UIP pattern according to published criteria [1]. For HRCT scans categorized as inconsistent with UIP pattern, the inconsistent features were recorded. These included upper/mid-lung predominance, peribronchovascular predominance, extensive ground glass opacities, profuse micronodules, discrete cysts, diffuse mosaic attenuation/air trapping in three or more lobes, and consolidations in bronchopulmonary segment(s)/lobe(s).
MUC5B genotyping and telomere length measurement
Peripheral blood leukocyte DNA was available in a subgroup of patients (MUC5B genotyping: 84/215, 39%; telomere length measurement: 72/215, 33%). In this subgroup, the MUC5B rs35705950 polymorphism was genotyped using TaqMan Predesigned SNP Genotyping Assays (Applied Biosystems, Carlsbad, CA) according to the manufacturer’s instructions [21]. Telomere length was measured by quantitative polymerase chain reaction as described previously [21, 22].
Statistical analysis
Clinical and pathological features are summarized using median (interquartile range) and number (percentage) as appropriate. Logistic regression was used to evaluate the association of bronchiolocentric fibrosis with clinical and pathologic features, clinical diagnosis (IPF or non-IPF) and radiologic pattern (UIP or non-UIP). Candidate co-variates identified a priori as potential confounders were age, sex, ever-smoking, history of any inhaled exposure, GERD, and definite UIP on HRCT.
Survival time was calculated from the date of baseline pulmonary function testing (or if not present, date of initial evaluation) until the patient’s death, with patients right-censored at the time of lung transplantation or time of last contact. Kaplan-Meier curves and the log-rank test were used to evaluate overall survival time between the patients with and without bronchiolocentric fibrosis, both in the full cohort and in the subgroup of patients with a diagnosis of IPF. Cox proportional hazards regression was performed used to adjust survival analysis for age, sex, ever-smoking, GERD, baseline forced vital capacity percent predicted value (%FVC), and baseline diffusion capacity of carbon monoxide percent predicted value (%DLCO). These variables were identified a priori as potential confounders between bronchiolocentric fibrosis and survival. All data analyses were performed using STATA version 14.2 (Stata Corp., Lakeway, TX, USA). For all analyses, a p-value < 0.05 was considered as statistically significant.
Results
Patients characteristics
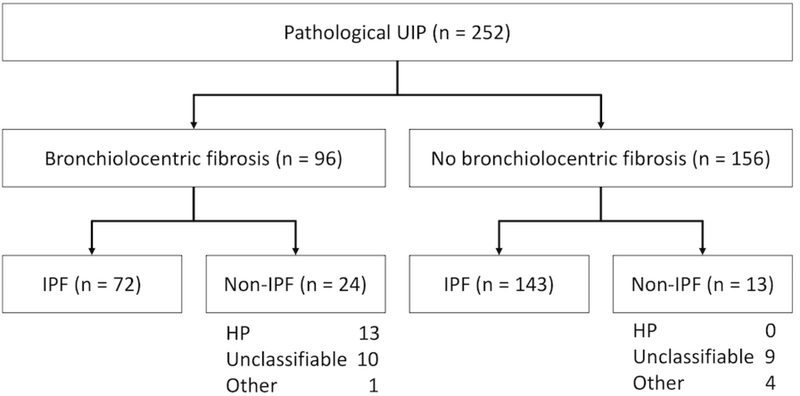
Two hundred and fifty-two patients were identified from the UCSF registry with histopathologic UIP pattern (Figure 2). Of these, 215 had IPF, with unclassifiable ILD (n=19) and hypersensitivity pneumonitis (n=13) as the most common alternative diagnoses. 96 of 252 (38%) in the total cohort and 72 of 215 (33%) in the IPF cohort had bronchiolocentric fibrosis. Baseline clinical characteristics are compared between patients with and without bronchiolocentric fibrosis in Tables 1 and 2, and pathologic features are compared in Table e2.
Figure 2.
Cohort inclusion diagram.
Abbreviation: UIP, usual interstitial pneumonia; IPF, idiopathic pulmonary fibrosis; CTD, connective-tissue disease; HP, hypersensitivity pneumonitis; BCF, bronchiolocentric fibrosis
Table 1.
Cohort characteristics, grouped by presence or absence of bronchiolocentric fibrosis, in the total cohort and in patients diagnosed with idiopathic pulmonary fibrosis.
| Total cohort (n=252) |
IPF cohort (n=215) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| With BCF | Without BCF | P value | With BCF | Without BCF | P value | |||||
| Number, n(%) | 96 | 156 | 72 | 143 | ||||||
| Age in years, median (IQR) | 68.1 | (62.1, 72.6) | 67.7 | (62.5, 73.8) | 0.65 | 68.9 | (63.8, 73.6) | 67.5 | (62.6, 73.0) | 0.26 |
| Male, n (%) | 64 | (66.7) | 113 | (72.4) | 0.39 | 54 | (75.0) | 105 | (73.4) | 0.87 |
| Hispanic/Latino, n (%) | 35 | (36.5) | 33 | (21.2) | 0.01 | 13 | (18.1) | 21 | (14.7) | 0.56 |
| Race/ethnicity, n (%) | 0.61 | 0.71 | ||||||||
| White | 85 | (88.5) | 130 | (83.3) | 64 | (88.9) | 120 | (83.9) | ||
| Asian | 4 | (4.1) | 9 | (5.8) | 3 | (4.2) | 9 | (6.3) | ||
| Others | 7 | (7.3) | 17 | (10.9) | 5 | (6.9) | 14 | (9.8) | ||
| Ever-smoking, n (%) | 69 | (71.9) | 102 | (65.4) | 0.33 | 55 | (76.4) | 94 | (65.7) | 0.12 |
| Pack-year, median (IQR) | 20 | (9, 40) | 20 | (9, 40) | 0.79 | 20 | (9, 40) | 20 | (9.4, 40) | 0.99 |
| Family history, n (%) | 18 | (18.8) | 25 | (16.0) | 0.61 | 13 | (18.1) | 24 | (16.8) | 0.85 |
| Diagnosis, n (%) | <0.001 | |||||||||
| IPF | 72 | (75.0) | 143 | (91.7) | ||||||
| HP | 13 | (13.5) | 0 | (0.0) | ||||||
| Unclassifiable | 10 | (10.4) | 9 | (5.8) | ||||||
| Others | 1 | (1.0) | 4 | (2.6) | ||||||
| Asthma, n (%) | 14 | (14.7) | 27 | (17.3) | 0.73 | 10 | (14.1) | 24 | (16.8) | 0.69 |
| COPD, n (%) | 22 | (22.9) | 37 | (23.9) | 0.88 | 17 | (23.6) | 33 | (23.2) | 1.00 |
| CHF, n (%) | 6 | (6.2) | 13 | (8.4) | 0.63 | 5 | (6.9) | 10 | (7.0) | 1.00 |
| GERD, n (%) | 39 | (40.6) | 56 | (35.9) | 0.50 | 26 | (36.1) | 51 | (35.7) | 1.00 |
| Sleep apnea, n (%) | 19 | (19.8) | 34 | (21.8) | 0.75 | 13 | (18.1) | 31 | (21.7) | 0.59 |
| Diabetes, n (%) | 17 | (17.7) | 28 | (17.9) | 1.00 | 13 | (18.1) | 24 | (16.8) | 0.85 |
| Any inhaled exposure, n (%) | 93 | (96.9) | 143 | (91.7) | 0.12 | 70 | (97.2) | 132 | (92.3) | 0.23 |
| Bird, n (%) | 46 | (47.9) | 80 | (51.3) | 0.70 | 31 | (43.1) | 74 | (51.7) | 0.25 |
| Organic, n (%) | 53 | (55.2) | 85 | (54.5) | 1.00 | 38 | (52.8) | 77 | (53.8) | 0.89 |
| Asbestos, n (%) | 16 | (16.7) | 12 | (7.7) | 0.04 | 12 | (16.7) | 8 | (5.6) | 0.01 |
| Dust, n (%) | 53 | (55.2) | 90 | (57.7) | 0.79 | 39 | (54.2) | 82 | (57.3) | 0.67 |
| Chemical, n (%) | 34 | (34.5) | 84 | (53.8) | 0.01 | 33 | (45.8) | 80 | (55.9) | 0.19 |
| Farmer, n (%) | 10 | (10.4) | 18 | (11.5) | 0.84 | 7 | (9.7) | 17 | (11.9) | 0.82 |
| Occupational organic, n (%) | 14 | (14.6) | 21 | (13.5) | 0.85 | 10 | (13.9) | 20 | (14.0) | 1.00 |
| %FVC, median (IQR) | 72 | (58, 82.5) | 66 | (57, 77) | 0.07 | 72 | (58.5, 84) | 66 | (57, 77.5) | 0.09 |
| %FEV1, median (IQR) | 80.5 | (67, 92.5) | 74.5 | (63, 85) | 0.046 | 81.5 | (67, 94) | 75 | (64, 85) | 0.048 |
| FEV1/FVC, median (IQR) | 83 | (80, 88) | 84 | (78, 87) | 0.39 | 82 | (80, 87) | 84 | (78, 87) | 0.74 |
| %TLC, median (IQR) | 70 | (56, 79) | 66 | (56, 74) | 0.056 | 70 | (56, 78) | 66 | (55, 74) | 0.13 |
| %DLCO, median (IQR) | 50 | (39, 62) | 48 | (38, 57) | 0.09 | 53 | (40, 62) | 47.5 | (38, 57) | 0.09 |
Abbreviation: IPF, idiopathic pulmonary fibrosis; BCF, bronchiolocentric fibrosis; IQR, interquartile range; HP, hypersensitivity pneumonitis; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; GERD, gastroesophageal reflux disease; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; TLC, total lung capacity; DLCO, diffusion capacity for carbon monoxide
Table 2.
Radiological findings on HRCT, grouped by presence or absence of bronchiolocentric fibrosis, in the total cohort and in patients diagnosed with idiopathic pulmonary fibrosis.
| Total cohort (n=252) |
IPF cohort (n=215) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| With BCF | Without BCF | P value | With BCF | Without BCF | P value | |||||
| Number with HRCT, n (%) | 88 | (91.7) | 138 | (88.5) | 65 | (90.3) | 126 | (88.1) | ||
| Pattern, n (%) | 0.33 | 0.48 | ||||||||
| UIP | 18 | (20.5) | 40 | (29.0) | 15 | (23.1) | 39 | (31.0) | ||
| Possible UIP | 27 | (30.7) | 36 | (26.1) | 22 | (33.8) | 35 | (27.8) | ||
| Inconsistent UIP | 43 | (48.9) | 62 | (44.9) | 28 | (43.1) | 52 | (41.3) | ||
| Inconsistent features, n (%) | ||||||||||
| Upper or mid-lung predominance | 5 | (5.7) | 14 | (10.1) | 0.33 | 3 | (4.6) | 10 | (7.9) | 0.55 |
| Peribronchovascular predominance | 20 | (22.7) | 26 | (18.8) | 0.50 | 13 | (20.0) | 20 | (15.9) | 0.55 |
| Extensive ground glass abnormality | 18 | (20.5) | 20 | (14.5) | 0.28 | 15 | (23.1) | 17 | (13.5) | 0.10 |
| Profuse micronodules | 2 | (2.3) | 1 | (0.7) | 0.56 | 1 | (1.5) | 1 | (0.8) | 1.00 |
| Discrete cysts | 4 | (4.5) | 9 | (6.5) | 0.77 | 3 | (4.6) | 9 | (7.1) | 0.75 |
| Diffuse mosaic attenuation/air-trapping | 18 | (20.5) | 23 | (16.7) | 0.48 | 11 | (16.9) | 19 | (15.1) | 0.83 |
| Consolidation in bronchopulmonary segment(s)/lobe(s) | 2 | (2.3) | 3 | (2.2) | 1.00 | 2 | (3.1) | 3 | (2.4) | 1.00 |
Abbreviation: HRCT, high-resolution computed tomography; UIP, usual interstitial pneumonia
Clinical and biological predictors of bronchiolocentric fibrosis
The presence of bronchiolocentric fibrosis was significantly associated with a non-IPF diagnosis (OR 3.71, 95%CI 1.68–8.19) (Table 3). However, none of the clinical features commonly considered as potential causes of bronchiolocentic fibrosis was significantly associated with bronchiolocentric fibrosis in the total cohort: exposure history (OR 2.82, 95% CI 0.78–10.2)); GERD (OR 1.22, 95%CI 0.72–2.06); and cigarette smoking (OR 1.35, 95%CI, 0.78–2.35) [4–6, 9–11, 23]. Bronchiolocentric fibrosis was similarly not associated with CT pattern in the total cohort, with an unadjusted OR for non-definite UIP HRCT pattern of 1.59 (95%CI 0.91–2.78).
Table 3.
Multivariable logistic regression model for the independent association of bronchiolocentric fibrosis with a diagnosis of non-idiopathic pulmonary fibrosis versus idiopathic pulmonary fibrosis in total cohort.
| Odds ratio | 95%CI | P value | |
|---|---|---|---|
| Bronchiolocentric fibrosis | 3.71 | ( 1.68, 8.19 ) | 0.001 |
| Age | 1.00 | ( 0.95, 1.05 ) | 0.94 |
| Male | 0.38 | ( 0.17, 0.83 ) | 0.02 |
| Ever-smoking | 0.73 | ( 0.33, 1.60 ) | 0.43 |
| Any exposure | 0.41 | ( 0.09, 1.88 ) | 0.25 |
| GERD | 1.63 | ( 0.75, 3.55 ) | 0.21 |
| Radiological UIP | 0.44 | ( 0.14, 1.39 ) | 0.16 |
Abbreviation: CI, confidence interval
MUC5B genotype and telomere length measurement were available for 84 of the 215 patients with a diagnosis of IPF (39%) and for 72 of 215 (33%), respectively. Clinical, radiological, and pathological features were similar in the IPF cohort, regardless of the presence/absence of MUC5B genotyping and telomere length measurement, except for higher %DLCO in patients with MUC5B genotyping (median, 51.5% vs. 46%, p=0.029) and telomere length measurement (median, 51.5% vs. 46%, p=0.029). The MUC5B minor T allele was present in 58% of patients and was not associated with the presence of bronchiolocentric fibrosis (unadjusted OR 1.64, 95%CI 0.61–4.40). The median telomere length was 5,689 base pairs, and telomere length was likewise not associated with the presence of bronchiolocentric fibrosis (unadjusted OR per kilobase pair increase = 1.93, 95%CI 0.67–5.54; age-adjusted OR 2.04, 95%CI 0.70–5.93).
Association of bronchiolocentric fibrosis with survival time
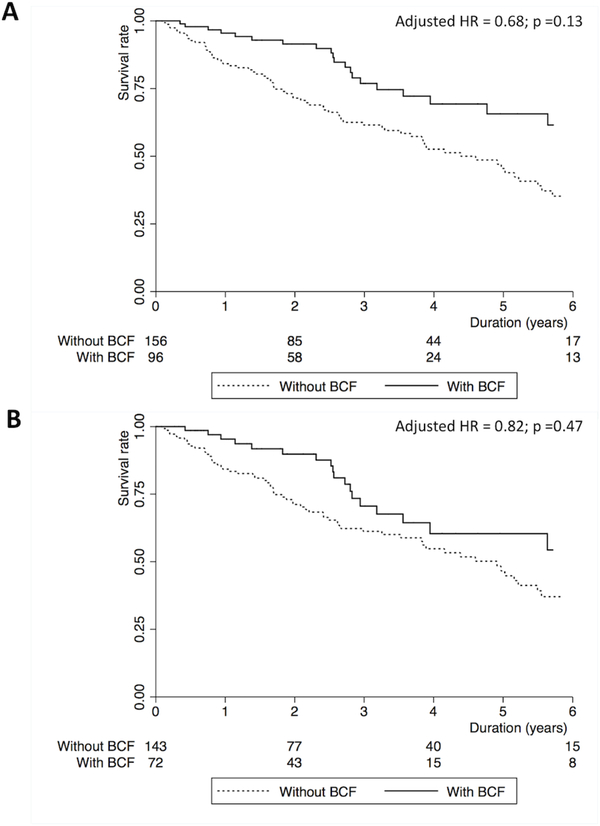
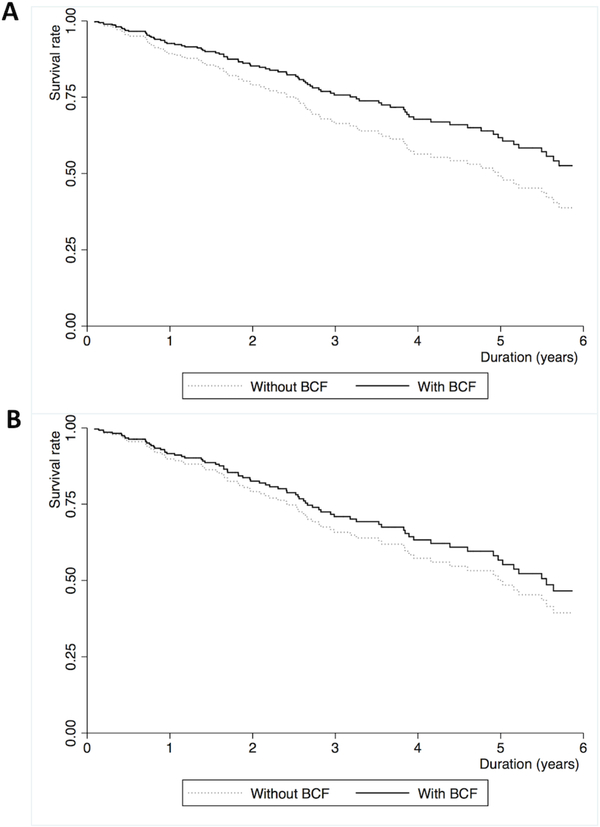
The median survival time for the cohort was 2.54 years (interquartile range: 1.11, 4.14) in the total cohort. Ninety-three patients (37%) died and 37 patients (15%) underwent lung transplantation. Unadjusted Kaplan-Meier plots and log-rank tests demonstrate significantly improved survival for patients with bronchiolocentric fibrosis, compared to those without it, in the full cohort (Figure 3A, p=0.002) and the IPF subgroup (Figure 3B, p=0.047). However, this association was no longer statistically significant in multivariate models accounting for potential confounders and known predictors of survival in fibrotic ILDs (Table 4 and Figure 4). Any small airway abnormality (bronchiolocentric fibrosis score 1–3) was not associated with survival in the full cohort (adjusted OR 0.97, 95%CI 0.62–1.52) and the IPF subgroup (adjusted OR 1.11, 95%CI 0.69–1.78).
Figure 3.
Kaplan-Meier plots demonstrating overall survival in patients with histopathologic usual interstitial pneumonia with and without bronchiolocentric fibrosis in the (A) total cohort (n=252) and (B) the cohort with idiopathic pulmonary fibrosis (n=215).
Table 4.
Multivariable Cox proportional hazards model for the independent association of bronchiolocentric fibrosis with overall survival in the total cohort and the cohort diagnosed with idiopathic pulmonary fibrosis.
| Total cohort (n=252) | Hazard ratio | 95%CI | P value |
|---|---|---|---|
| Bronchiolocentric fibrosis | 0.68 | ( 0.41, 1.13 ) | 0.13 |
| Age | 1.03 | ( 1.00, 1.06 ) | 0.06 |
| Male | 0.92 | ( 0.55, 1.55 ) | 0.75 |
| Ever-smoking | 1.40 | ( 0.87, 2.25 ) | 0.17 |
| IPF | 1.40 | ( 0.72, 2.74 ) | 0.32 |
| GERD | 0.59 | ( 0.36, 0.95 ) | 0.03 |
| %FVC | 0.98 | ( 0.96, 0.99 ) | 0.004 |
| %DLCO | 0.99 | ( 0.98, 1.01 ) | 0.22 |
| IPF cohort (n=215) | |||
| Bronchiolocentric fibrosis | 0.82 | ( 0.48, 1.40 ) | 0.47 |
| Age | 1.03 | ( 1.00, 1.06 ) | 0.08 |
| Male | 1.01 | ( 0.56, 1.81 ) | 0.97 |
| Ever-smoking | 1.12 | ( 0.68, 1.86 ) | 0.65 |
| GERD | 0.60 | ( 0.36, 1.01 ) | 0.06 |
| %FVC | 0.98 | ( 0.96, 0.99 ) | 0.01 |
| %DLCO | 0.98 | ( 0.97, 1.00 ) | 0.11 |
Figure 4.
Estimated Cox survival curves adjusted for confounding factors in patients with histopathologic usual interstitial pneumonia with and without bronchiolocentric fibrosis in the (A) total cohort (n=252) and (B) the cohort with idiopathic pulmonary fibrosis (IPF) (n=215). Adjusted confounding factors are age, male, ever-smoking, IPF diagnosis, gastroesophageal reflux disease (GERD), baseline forced vital capacity percent predicted value (%FVC), and baseline diffusion capacity of carbon monoxide percent predicted value (%DLCO) for the total cohort, and age, male, ever-smoking, GERD, %FVC, and %DLCO for the IPF cohort.
Discussion
In a cohort of well-defined patients with UIP pattern on surgical lung biopsy, bronchiolocentric fibrosis is associated with a non-IPF multidisciplinary diagnosis. This association is independent of clinical, genetic, and radiologic features that were hypothesized to be related to bronchiolocentric fibrosis. On the other hand, bronchiolocentric fibrosis is also commonly seen in the context of IPF diagnosis, suggesting its limited impact on the diagnostic reasoning process despite statistical significance; the confidence interval was wide. Although bronchiolocentric fibrosis may be a diagnostically informative finding, confirmation bias should be also considered when interpreting our results.
Confirmation bias is the tendency to interpret an independent finding as confirmation of one’s existing beliefs or theories – in this case the belief that IPF is a peripheral, subpleural disease that spares the airways from fibrosis. It is possible that a clinician evaluating a patient with bronchiolocentric fibrosis would be influenced by this belief and arrive at a non-IPF diagnosis incorrectly. There is no way for us to exclude this possibility from our analyses, although at least 9 of 24 diagnoses of non-IPF among patients with bronchiolocentric fibrosis would need to be mistaken for the unadjusted association to become non-significant. The ultimate confirmation would require a prospective study to diagnose patients with pathologic UIP absent knowledge of whether bronchiolocentric fibrosis is present, and then analyze the impact of bronchiolocentric fibrosis on the diagnostic classification.
After adjustment for potential confounders of outcome, bronchiolocentric fibrosis had no statistically significant impact on survival in the overall cohort or in the subgroup with IPF; while the HR estimate was well below 1.0, the confidence interval was wide. It remains unclear why some patients with IPF demonstrate this pathological finding. MUC5B rs35705950 polymorphism or telomere length cannot account for bronchiolocentric fibrosis although the number of patients with these measurements is small.
Bronchiolocentric fibrosis was similarly not associated with non-UIP pattern on HRCT, or any radiological features inconsistent with UIP. This finding suggests that HRCT images cannot predict the presence of bronchiolocentric fibrosis correctly, without surgical lung biopsy. It should be also taken into account that lung biopsy specimens can represent a limited amount of lesions, compared to HRCT images.
It is important to note that patients with bronchiolocentric fibrosis that were included in this study demonstrated a predominant pathologic pattern of UIP. Patients with predominantly airway-centered changes on pathology were given other pathological diagnoses than UIP, including the recently described “bronchiolocentric fibrosis” pattern, and were not included [3, 24]. Patients with UIP pattern and subtler airway findings of peribronchiolar metaplasia (defined as a peribronchiolar proliferation of bronchial epithelium along thickened peribronchiolar alveolar walls, that is a bronchiolocentric fibrosis score = 1) were considered to not have bronchiolocentric fibrosis in order to increase specificity of this finding in this study. Peribronchiolar metaplasia has been reported in 59% of UIP biopsy samples in a previous small study [11]. In this study, adding score 1 to bronchiolocentric fibrosis did not change the results.
The strength of this study is that it utilizes one of the largest cohorts of patients with UIP pattern on surgical biopsy in the published literature. In a well-documented, relatively large cohort, bronchiolocentric fibrosis was seen in 38% of patients with pathological UIP and 33% of patients with IPF diagnosis, respectively. Bronchiolocentric fibrosis was not associated with survival, clinical features suggestive of potential causes, non-UIP features on HRCT, or genetic traits. On the other hand, confidence intervals were wide and did not exclude the null, although point estimates were generally in the expected direction. Analyses in larger multi-center cohorts may be more definitive. Due to the retrospective design, symptoms at the initial visit, changes in pulmonary physiology over time, treatment after the initial visit, and cause of mortality could not be evaluated. Re-review of surgical lung biopsies by another pathologist was also unavailable. Cause of death is particularly relevant, as respiratory-related death may be a more specific outcome in evaluating the impact of bronchiolocentric fibrosis than all-cause death.
In conclusion, although most patients with a histologic UIP pattern and bronchiolocentric fibrosis have idiopathic pulmonary fibrosis, approximately one-quarter have an alternative multidisciplinary diagnosis including hypersensitivity pneumonia or unclassifiable interstitial fibrosis. The presence of bronchiolocentric fibrosis was not shown to be associated with specific clinical, genetic, or radiographic features or survival time. If prospectively validated in a larger cohort to protect against confirmation bias, these findings suggest that bronchiolocentric fibrosis should be noted and considered by multidisciplinary teams in their diagnostic process as a clue to a possible non-IPF diagnosis. However, its common presence in idiopathic pulmonary fibrosis suggests that it should not be used as an absolute pertinent negative to rule out a multidisciplinary diagnosis of idiopathic pulmonary fibrosis.
Supplementary Material
Acknowledgements
The authors thank the patients who participated in this clinical study and Jane Berkeley, BS for running the UCSF ILD database. This work was supported by NIH/NHLBI grant K24HL127131 and the Nina Ireland Program for Lung Health. Author contributions as follows: drafting the manuscript: Tanizawa; critical revision and final approval of the manuscript: all authors; data collection, analysis, and interpretation: all authors; conception / design: Collard and Jones.
Footnotes
Conflicts of interest
None of the authors report conflicts of interest relevant to this study.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collard HR, Cool CD, Leslie KO, Curran-Everett D, Groshong S, Brown KK. Organizing pneumonia and lymphoplasmacytic inflammation predict treatment response in idiopathic pulmonary fibrosis. Histopathology. 2007;50:258–65. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Costabel U, Hansell DM, King TE Jr., Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yousem SA, Dacic S. Idiopathic bronchiolocentric interstitial pneumonia. Mod Pathol. 2002;15:1148–53. [DOI] [PubMed] [Google Scholar]
- 5.Churg A, Myers J, Suarez T, Gaxiola M, Estrada A, Mejia M, et al. Airway-centered interstitial fibrosis: a distinct form of aggressive diffuse lung disease. Am J Surg Pathol. 2004;28:62–8. [DOI] [PubMed] [Google Scholar]
- 6.Fenton ME, Cockcroft DW, Wright JL, Churg A. Hypersensitivity pneumonitis as a cause of airway-centered interstitial fibrosis. Ann Allergy Asthma Immunol. 2007;99:465–6. [DOI] [PubMed] [Google Scholar]
- 7.Mark EJ, Ruangchira-urai R . Bronchiolitis interstitial pneumonitis: a pathologic study of 31 lung biopsies with features intermediate between bronchiolitis obliterans organizing pneumonia and usual interstitial pneumonitis, with clinical correlation. Ann Diagn Pathol. 2008;12:171–80. [DOI] [PubMed] [Google Scholar]
- 8.Colombat M, Groussard O, Taille C, Marrash-Chahla R, Brugiere O, Mal H, et al. Lung transplantation in a patient with airway-centered fibrosis. Am J Surg Pathol. 2004;28:1540–2. [DOI] [PubMed] [Google Scholar]
- 9.Kuranishi LT, Leslie KO, Ferreira RG, Coletta EA, Storrer KM, Soares MR, et al. Airway-centered interstitial fibrosis: etiology, clinical findings and prognosis. Respir Res. 2015;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Carvalho ME, Kairalla RA, Capelozzi VL, Deheinzelin D, do Nascimento Saldiva PH, de Carvalho CR. Centrilobular fibrosis: a novel histological pattern of idiopathic interstitial pneumonia. Pathol Res Pract. 2002;198:577–83. [DOI] [PubMed] [Google Scholar]
- 11.Fukuoka J, Franks TJ, Colby TV, Flaherty KR, Galvin JR, Hayden D, et al. Peribronchiolar metaplasia: a common histologic lesion in diffuse lung disease and a rare cause of interstitial lung disease: clinicopathologic features of 15 cases. Am J Surg Pathol. 2005;29:948–54. [DOI] [PubMed] [Google Scholar]
- 12.Evans CM, Fingerlin TE, Schwarz MI, Lynch D, Kurche J, Warg L, et al. Idiopathic Pulmonary Fibrosis: A Genetic Disease That Involves Mucociliary Dysfunction of the Peripheral Airways. Physiol Rev. 2016;96:1567–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368:2192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peljto AL, Zhang Y, Fingerlin TE, Ma SF, Garcia JG, Richards TJ, et al. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA. 2013;309:2232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano Y, Yang IV, Walts AD, Watson AM, Helling BA, Fletcher AA, et al. MUC5B Promoter Variant rs35705950 Affects MUC5B Expression in the Distal Airways in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2016;193:464–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuart BD, Lee JS, Kozlitina J, Noth I, Devine MS, Glazer CS, et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir Med. 2014;2:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juge PA, Borie R, Kannengiesser C, Gazal S, Revy P, Wemeau-Stervinou L, et al. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur Respir J. 2017;49. [DOI] [PubMed] [Google Scholar]
- 20.Newton CA, Batra K, Torrealba J, Kozlitina J, Glazer CS, Aravena C, et al. Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. Eur Respir J. 2016;48:1710–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ley B, Newton CA, Arnould I, Elicker BM, Henry TS, Vittinghoff E, et al. The MUC5B promoter polymorphism and telomere length in patients with chronic hypersensitivity pneumonitis: an observational cohort-control study. Lancet Respir Med. 2017;5:639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faust HE, Golden JA, Rajalingam R, Wang AS, Green G, Hays SR, et al. Short lung transplant donor telomere length is associated with decreased CLAD-free survival. Thorax. 2017;72:1052–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokosi MA, Nicholson AG, Hansell DM, Wells AU. Rare idiopathic interstitial pneumonias: LIP and PPFE and rare histologic patterns of interstitial pneumonias: AFOP and BPIP. Respirology. 2016;21:600–14. [DOI] [PubMed] [Google Scholar]
- 24.Wang P, Jones KD, Urisman A, Elicker BM, Urbania T, Johannson KA, et al. Pathological Findings and Prognosis in a Large Prospective Cohort of Chronic Hypersensitivity Pneumonitis. Chest. 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.