Key Points
Question
Is implementation of prehospital TBI treatment guidelines in demographically diverse emergency medical services systems associated with survival in patients with major traumatic brain injury (TBI)?
Findings
In this cohort study, among 21 852 patients with moderate, severe, or critical TBI (15 228 preimplementation and 6624 postimplementation), guideline implementation was not associated with improved adjusted survival. However, it was associated with improved outcome in the severe and severe, intubated subgroups.
Meaning
Statewide implementation of the prehospital TBI guidelines was independently associated with improvement in survival among patients with severe TBI and in the severe, intubated group; these findings support the widespread implementation of the prehospital TBI treatment guidelines.
Abstract
Importance
Traumatic brain injury (TBI) is a massive public health problem. While evidence-based guidelines directing the prehospital treatment of TBI have been promulgated, to our knowledge, no studies have assessed their association with survival.
Objective
To evaluate the association of implementing the nationally vetted, evidence-based, prehospital treatment guidelines with outcomes in moderate, severe, and critical TBI.
Design, Setting, and Participants
The Excellence in Prehospital Injury Care (EPIC) Study included more than 130 emergency medical services systems/agencies throughout Arizona. This was a statewide, multisystem, intention-to-treat study using a before/after controlled design with patients with moderate to critically severe TBI (US Centers for Disease Control and Prevention Barell Matrix-Type 1 and/or Abbreviated Injury Scale Head region severity ≥3) transported to trauma centers between January 1, 2007, and June 30, 2015. Data were analyzed between October 25, 2017, and February 22, 2019.
Interventions
Implementation of the prehospital TBI guidelines emphasizing avoidance/treatment of hypoxia, prevention/correction of hyperventilation, and avoidance/treatment of hypotension.
Main Outcomes and Measures
Primary: survival to hospital discharge; secondary: survival to hospital admission.
Results
Of the included patients, the median age was 45 years, 14 666 (67.1%) were men, 7181 (32.9%) were women; 16 408 (75.1% ) were white, 1400 (6.4%) were Native American, 743 (3.4% ) were Black, 237 (1.1%) were Asian, and 2791 (12.8%) were other race/ethnicity. Of the included patients, 21 852 met inclusion criteria for analysis (preimplementation phase [P1]: 15 228; postimplementation [P3]: 6624). The primary analysis (P3 vs P1) revealed an adjusted odds ratio (aOR) of 1.06 (95% CI, 0.93-1.21; P = .40) for survival to hospital discharge. The aOR was 1.70 (95% CI, 1.38-2.09; P < .001) for survival to hospital admission. Among the severe injury cohorts (but not moderate or critical), guideline implementation was significantly associated with survival to discharge (Regional Severity Score–Head 3-4: aOR, 2.03; 95% CI, 1.52-2.72; P < .001; Injury Severity Score 16-24: aOR, 1.61; 95% CI, 1.07-2.48; P = .02). This was also true for survival to discharge among the severe, intubated subgroups (Regional Severity Score–Head 3-4: aOR, 3.14; 95% CI, 1.65-5.98; P < .001; Injury Severity Score 16-24: aOR, 3.28; 95% CI, 1.19-11.34; P = .02).
Conclusions and Relevance
Statewide implementation of the prehospital TBI guidelines was not associated with significant improvement in overall survival to hospital discharge (across the entire, combined moderate to critical injury spectrum). However, adjusted survival doubled among patients with severe TBI and tripled in the severe, intubated cohort. Furthermore, guideline implementation was significantly associated with survival to hospital admission. These findings support the widespread implementation of the prehospital TBI treatment guidelines.
Trial Registration
ClinicalTrials.gov identifier: NCT01339702
This study evaluates the association of implementing the nationally vetted, evidence-based, prehospital treatment guidelines with outcomes in moderate, severe, and critical traumatic brain injury.
Introduction
The burden of traumatic brain injury (TBI) on US society is enormous: annually it leads to 2.2 million emergency department visits, 280 000 hospitalizations, 52 000 deaths, and more than $60 billion in economic costs.1,2 While improving outcomes has been difficult,3,4,5,6,7,8,9 early treatment may help mitigate secondary brain injury,10,11,12,13,14,15 and this has led to promulgation of official evidence-based TBI treatment guidelines.10,11,12,15 There is limited in-hospital evidence supporting the effectiveness of guideline-based treatment.16,17,18,19,20,21 However, to our knowledge, association of implementation by prehospital emergency medical services systems (EMS) with overall survival has not been evaluated.11,12 The objective of this study was to implement the nationally vetted TBI guidelines11 among the EMS agencies of Arizona and to evaluate the association with outcomes in moderate, severe, and critical TBI.
Methods
Study Design, Setting, and Oversight
The Excellence in Prehospital Injury Care (EPIC) study was conducted throughout Arizona using a controlled, before-after, multisystem, intention-to-treat design.22,23,24,25,26 The methods have been reported in detail.27,28,29,30 The study phases were based on each EMS agency’s training schedule: phase 1 (P1), preimplementation; phase 2, training (initiation to completion); and phase 3 (P3), postimplementation (eFigure in the Supplement).27
Regulatory approvals were obtained from the state of Arizona. The University of Arizona institutional review board and Arizona Department of Health Services human subjects review board approved the project and the publication of deidentified data.27,28,29,30 The institutional review board exempted this project from informed consent, by virtue of being an official, state-vetted public health initiative. While not a clinical trial, EPIC is registered at ClinicalTrials.gov (NCT01339702).
Data Collection
The Arizona State Trauma Registry contains extensive data on patients taken to level I trauma centers (TCs). Information from included patients (January 1, 2007, to June 30, 2015) was linked to EMS data by accessing paper-based or electronic records from participating agencies, creating a comprehensive prehospital/TC database27 (eTables 1 and 2 in the Supplement).
Participants
Inclusion criteria were adults/children with physical trauma who (1) were transported directly or transferred to a TC by participating agencies, (2) had hospital diagnoses consistent with TBI (isolated or multisystem), and (3) met at least 1 of the following definitions for major TBI: US Centers for Disease Control and Prevention Barell Matrix–Type 1 (eTable 3 in the Supplement)31,32,33 and/or Abbreviated Injury Scale–Head of at least 3. To prevent selection bias, all patients meeting criteria were included regardless of whether EMS data were obtained.34,35
Interventions
All Arizona EMS agencies were invited to participate. Program requirements included: EMS TBI guideline training (train-the-trainer strategy27); implementing systemwide, guideline-based treatment (eTables 4-7 in the Supplement)27; and providing prehospital data. Training emphasized guideline use in patients with physical trauma, reported/apparent loss of consciousness, and injury sufficient to warrant transport to a hospital.27
The guideline-based clinical protocols and algorithms (eTables 4-7 in the Supplement)27 focused on 4 interventions: (1) prevention/treatment of hypoxia through early oxygen administration; (2) airway interventions to optimize oxygenation/ventilation (bag-valve-mask [BVM] for airway/ventilatory compromise and endotracheal intubation [ETI] and extraglottic/supraglottic airways reserved for patients with Glasgow Coma Scale score <9 when basic airway interventions were inadequate); (3) prevention of hyperventilation by using age-appropriate ventilation rates and ventilation adjuncts27; and (4) avoidance and treatment of hypotension by infusing isotonic fluids.27 Primary outcome was survival to hospital discharge (survival), and the secondary outcome was survival to hospital admission (SHA).
Statistical Analysis
Continuous variables were summarized by median and interquartile range (IQR) and were compared between 2 groups using the Wilcoxon rank sum test. Categorical variables were summarized by frequency and proportion (with 95% Clopper-Pearson confidence intervals) and were compared between 2 groups by χ2 or Fisher exact test.
The primary analysis of risk-adjusted association between survival and intervention was examined by logistic regression, adjusting for important risk factors and potential confounders (see eTable 8 in the Supplement for rationale for choosing the covariates).28,29,30 Sensitivity analysis was performed incorporating the physiologic measures in the Trauma Injury and Injury Severity Score methodology. The effect of age (continuous variable) was fitted nonparametrically using penalized thin plate regression splines through the generalized additive model.36 The same procedure used for survival was used to model SHA. Fitted models were assessed by deviance residual plots, and area under the receiver operating characteristic curve, with 95% confidence intervals, was obtained by the DeLong method.
In the secondary analyses (ie, moderate/severe/critical severity-based cohorts and intubated subgroup), standard logistic regression was used when there were at least 200 patients with the outcome and 200 without. Otherwise, the Firth penalized-likelihood logistic regression was used.37,38 Collinearity was checked using variance inflation factors for the parametric terms and concurvity for the nonparametric term. Mixed-effect models for survival/SHA were used to assess the effect of potential correlation of patients treated by the same EMS agency.
In a sensitivity analysis, a propensity score (the probability of being in P3 vs P1) was estimated by logistic regression using all covariates from the final models for outcomes. The propensity score was then included as either linear or smooth function predictors to evaluate the change in adjusted odds ratio (aOR) estimates of intervention for survival and SHA. In secondary analyses, the absolute change in event rates for the same patient at 2 different times (eg, prehospital vs emergency department hypoxia rates) was compared between P1 and P3 by fitting generalized estimating equation models for binary outcome with identity link and exchangeable working correlation matrix and testing an interaction term between time and study phase.
We evaluated secular trends in TBI outcomes in 2 groups of patients brought to the TCs that met study diagnostic criteria but were not eligible for study inclusion: those cared for solely by EMS agencies not participating in EPIC training and those brought to TCs by privately owned vehicle (ie, not affected by EMS care).
We used software environment R for the analysis39 (The R Foundation; packages mgcv,36 gamm4,40 gee,41 and logistf42 for regression models). All tests were 2-sided, with α = .05, except for the primary analysis, which was .04 (1 interim analysis was conducted with α = .01).
Results
Enrollment
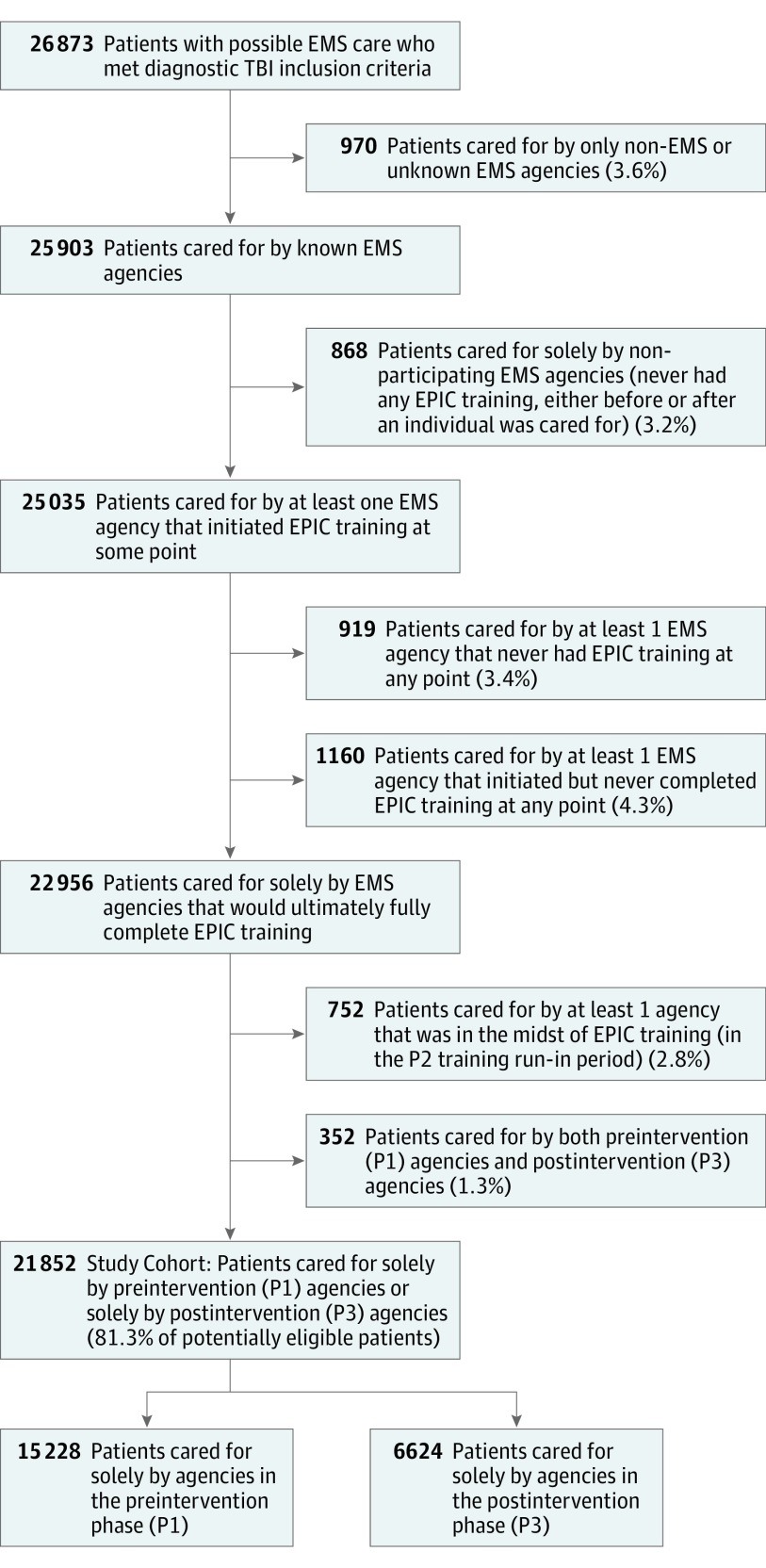
Phase 1 began for all agencies on January 1, 2007. Phase 2 began and ended at different times for each agency (the first agency began training on February 22, 2012, and the last agency completed training on January 23, 2015). Phase 3 ended for all agencies on June 30, 2015. Total enrollment was 26 873, and 5021 were excluded, leaving 21 852 (88% of estimated sample size) for analysis (15 228 patients in the preintervention phase [P1 control group] and 6624 patients in the postintervention phase [P3 intervention group]; Figure 1).
Figure 1. Enrollment Tree.
EMS indicates emergency medical services; EPIC, Excellence In Prehospital Injury Care study; P1, study phase 1 (preimplementation phase); P2, study phase 2 (training run-in phase; for each EMS agency, time from initiation to completion of training); P3, study phase 3 (postimplementation phase); TBI, traumatic brain injury.
Treatment and Treatment-Related Physiologic Changes
Training was associated with changes in treatment and treatment-related physiology. The rate of patients having at least 1 EMS oxygen saturation value of 100% increased significantly after implementation (P1%, 4823 of 13 552 [35.6%]; P3%, 2513 of 6141 [40.9%]; P < .001; intubated cohort: P1%, 985 of 2226 [44.2%]; P3%, 482 of 885 [54.6%]; P < .001). Among intubated patients, hypoxia decreased after EMS care in both P1 and P3. However, the decrease was significantly greater after guideline implementation, and this association was seen at multiple levels of hypoxia (eTable 9 in the Supplement).
The rates of administering intravenous fluid boluses (eTable 10 in the Supplement) and the volume infused (eTable 11 in the Supplement) increased in P3. In addition, after implementation, patients with hypotension were more likely to arrive at the TC with a higher systolic blood pressure (SBP) compared with their prehospital SBP.
The intubation rate decreased significantly after implementation, and the BVM-only rate increased (eTable 12 in the Supplement). Among patients receiving positive-pressure ventilation (PPV), the rate of basic airway use only (BVM) increased (P1, 534 of 3531 [15.1%]; P3, 325 of 1491 [21.8%]; P < .001). Among intubated patients, the rate of hypocapnia (end-tidal carbon dioxide <35 mm Hg, reflecting hyperventilation) decreased significantly after implementation (P1, 899 of 1486 [60.5%]; P3, 372 of 712 [52.2%]; P < .001).
Analysis and Outcome
Table 1 shows patient demographics and clinical characteristics. Median age was higher in P3 (50.5 years; IQR, 27-70 years) than P1 (median, 43 years; IQR, 23-63.5 years; P < .001). The proportion of older patients (by multiple definitions) was much higher in P3 (eTable 13 in the Supplement).
Table 1. Patient Characteristics and Outcomesa.
| Characteristic | No. (%) | P Valuec | ||
|---|---|---|---|---|
| All (N = 21 852)b | P1 (n = 15 228)b | P3 (n = 6624)b | ||
| Age, median (IQR), y | 45 (24-66) | 43 (23-63.5) | 50.5 (27-70) | <.001 |
| Male | ||||
| No | 7181 (32.9) | 4940 (32.4) | 2241 (33.8) | .048 |
| Yes | 14 666 (67.1) | 10 283 (67.5) | 4383 (66.2) | |
| Unknown | 5 | 5 | 0 | |
| Race/ethnicity | ||||
| Black | 743 (3.4) | 506 (3.3) | 237 (3.6) | .16 |
| Asian | 237 (1.1) | 151 (1) | 86 (1.3) | |
| American Indian/Alaska native | 1400 (6.4) | 973 (6.4) | 427 (6.4) | |
| White | 16 408 (75.1) | 11 454 (75.2) | 4954 (74.8) | |
| Other | 2791 (12.8) | 1976 (13) | 815 (12.3) | |
| Unknown | 273 (1.2) | 168 (1.1) | 105 (1.6) | |
| Hispanic | ||||
| No | 16 488 (75.5) | 11 276 (74) | 5212 (78.7) | <.001 |
| Yes | 4719 (21.6) | 3405 (22.4) | 1314 (19.8) | |
| Unknown | 645 (3) | 547 (3.6) | 98 (1.5) | |
| Payer | ||||
| Private | 7109 (32.5) | 5035 (33.1) | 2074 (31.3) | <.001 |
| AHCCCS/Medicaid | 5378 (24.6) | 3920 (25.7) | 1458 (22) | |
| Medicare | 4901 (22.4) | 3081 (20.2) | 1820 (27.5) | |
| Self pay | 3119 (14.3) | 2144 (14.1) | 975 (14.7) | |
| Other | 910 (4.2) | 648 (4.3) | 262 (4) | |
| Unknown | 435 (2) | 400 (2.6) | 35 (0.5) | |
| Trauma type | ||||
| Blunt | 20 794 (95.2) | 14 504 (95.2) | 6290 (95) | .01 |
| Penetrating | 1053 (4.8) | 723 (4.7) | 330 (5) | |
| Burn | 4 (0) | 0 | 4 (0.1) | |
| Unknown | 1 (0) | 1 (0) | 0 | |
| Regional Severity Score–Head (ICD-9) | ||||
| 1 to 3 | 10 233 (46.8) | 7563 (49.7) | 2670 (40.3) | <.001 |
| 4 | 7004 (32.1) | 4585 (30.1) | 2419 (36.5) | |
| 5 to 6 | 4444 (20.3) | 2952 (19.4) | 1492 (22.5) | |
| Unknown | 171 (0.8) | 128 (0.8) | 43 (0.6) | |
| Injury Severity score (ICD-9) | ||||
| 1 to 14 | 7826 (35.8) | 5765 (37.9) | 2061 (31.1) | <.001 |
| 16 to 24 | 7274 (33.3) | 4863 (31.9) | 2411 (36.4) | |
| ≥25 | 6745 (30.9) | 4597 (30.2) | 2148 (32.4) | |
| Unknown | 7 (0) | 3 (0) | 4 (0) | |
| Body region | ||||
| Isolated TBI | 16 663 (76.3) | 11 602 (76.2) | 5061 (76.4) | .74 |
| Multisystem TBI | 5189 (23.7) | 3626 (23.8) | 1563 (23.6) | |
| Transfer | ||||
| No | 14 671 (67.1) | 10 310 (67.7) | 4361 (65.8) | <.001 |
| Yes | 6646 (30.4) | 4383 (28.8) | 2263 (34.2) | |
| Unknown | 535 (2.4) | 535 (3.5) | 0 | |
| CPR | ||||
| No | 20 988 (96) | 14 658 (96.3) | 6330 (95.6) | .02 |
| Yes | 864 (4) | 570 (3.7) | 294 (4.4) | |
| Airway management | ||||
| No PPV | 16 830 (77) | 11 697 (76.8) | 5133 (77.5) | <.001 |
| BVM | 859 (3.9) | 534 (3.5) | 325 (4.9) | |
| SGA | 149 (0.7) | 81 (0.5) | 68 (1) | |
| Intubation | 4014 (18.4) | 2916 (19.1) | 1098 (16.6) | |
| Survival to discharge | ||||
| No | 3036 (13.9) | 2036 (13.4) | 1000 (15.1) | <.001 |
| Yes | 18 816 (86.1) | 13 192 (86.6) | 5624 (84.9) | |
| Survival to hospital admission | ||||
| No | 1018 (4.7) | 723 (4.7) | 295 (4.5) | .36 |
| Yes | 20 834 (95.3) | 14 505 (95.3) | 6329 (95.5) | |
Abbreviations: AHCCCS, Arizona Health Care Cost Containment System; BVM, bag-valve mask (basic airway providing positive-pressure ventilation); CPR, cardiopulmonary resuscitation; ICD-9, International Classification of Diseases, Ninth Revision; IQR, interquartile range; isolated TBI, patients who met TBI inclusion criteria but had no injury with Regional Severity Score of at least 3 in any other (nonhead) body region; multisystem TBI, patients who met TBI inclusion criteria and also had at least 1 nonhead region injury with Regional Severity Score of at least 3; PPV, positive-pressure ventilation (patients received active ventilation regardless of basic or advanced airway type); P1, study phase 1 (preimplementation); P3, study phase 3 (postimplementation); SGA, supraglottic airway (eg, Laryngeal Mask Airway or King Airway); TBI, traumatic brain injury.
Treating trauma center was also highly significant. To protect the mandated anonymity of the participating hospitals, the numbers are not shown (preventing any possible identification or inference of facility-specific outcome differences).
Median (IQR) for numerical variables and count (percentage) for categorical variables.
Fisher exact test or χ2 test, as appropriate, for categorical variables and Wilcoxon rank sum test for continuous variables; the unknown category, if present, is excluded from the testing procedure.
Brain injury severity was greater in P3 (Regional Severity Score–Head [RSS-H] of 4: 2419 of 6624 [36.5%; P3] vs 4585 of 15 228 [30.1%; P1]; RSS-H of 5-6, 1492 of 6624 [22.5%; P3] vs 2952 of 15 228 [19.4%; P1]; P < .001). This was also true of overall injury severity (Injury Severity Score [ISS] of 16-24, 2411 of 6624 [36.4%; P3] vs 4863 of 15 228 [31.9%; P1]; P < .001; ISS ≥25, 2148 of 6624 [32.4%; P3] vs 4597 of 15 228 [30.2%; P1]; P < .001). Patients in P3 were also more likely to receive prehospital cardiopulmonary resuscitation (4.4%; 294 of 6624) than those in P1 (3.7%; 570 of 15 228; P = .02; Table 1).
The overall preimplementation/postimplementation analysis revealed an aOR of 1.06 (95% CI, 0.93-1.21; P = .40; Table 2) for survival and 1.70 (95% CI, 1.38-2.09; P < .001) for SHA (eTable 14 in the Supplement). Sensitivity analyses incorporating random EMS agency effects in the models for survival and SHA yielded only minimal changes in the estimated intervention effects (aOR, 1.04; 95% CI, 0.91-1.19 and aOR, 1.73; 95% CI, 1.40-2.13, respectively; eTables 15 and 16 in the Supplement). Sensitivity analyses incorporating the physiologic components of Trauma Injury and Injury Severity Score methodology in the models for survival and SHA also resulted in inconsequential changes (SBP: aOR, 1.06; 95% CI, 0.93-1.21 and aOR, 1.76; 95% CI, 1.39-2.21; GCS: aOR, 1.01; 95% CI, 0.87-1.16 and aOR, 1.63; 95% CI, 1.32-2.01; respiratory rate: aOR, 1.04 95% CI, 0.91-1.18 and aOR, 1.79; 95% CI, 1.43-2.22; eTables 17-22 in the Supplement). Sensitivity analyses that included the propensity score in the models yielded minimal changes (linear predictor: change in aOR survival, 0.47%; SHA, 0.42%; smooth function survival, 0.89%; SHA, 0.42%).
Table 2. Primary Analysis of Adjusted Survival in Phase 3 Vs Phase 1.
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Intervention | 1.06 (0.929-1.21) | .40 |
| Male | ||
| No | 1 [Reference] | .74 |
| Yes | 1.02 (0.896-1.17) | |
| Race/ethnicity | ||
| Black | 1 [Reference] | .005 |
| Asian | 0.787 (0.417-1.49) | |
| American Indian/Alaska native | 0.719 (0.476-1.08) | |
| White | 0.850 (0.611-1.18) | |
| Other | 0.678 (0.462-0.994) | |
| Unknown | 0.382 (0.214-0.679) | |
| Hispanic | ||
| No | 1 [Reference] | .02 |
| Yes | 1.14 (0.959-1.36) | |
| Unknown | 0.686 (0.483-0.974) | |
| Payer | ||
| Private | 1 [Reference] | <.001 |
| AHCCCS/Medicaid | 1.12 (0.949-1.33) | |
| Medicare | 0.942 (0.762-1.17) | |
| Self pay | 0.464 (0.385-0.559) | |
| Other | 0.829 (0.619-1.11) | |
| Unknown | 0.309 (0.210-0.454) | |
| Trauma type | ||
| Blunt | 1 [Reference] | <.001 |
| Penetrating | 0.159 (0.130-0.196) | |
| Head Injury Severity score (ICD-9) | ||
| 1 to 3 | 1 [Reference] | <.001 |
| 4 | 0.835 (0.649-1.07) | |
| 5 to 6 | 0.047 (0.036-0.061) | |
| Injury Severity score (ICD-9) | ||
| 1 to 14 | 1 [Reference] | <.001 |
| 16 to 24 | 0.444 (0.303-0.649) | |
| ≥25 | 0.181 (0.122-0.269) | |
| Body region | ||
| Isolated TBI | 1 [Reference] | <.001 |
| Multisystem TBI | 0.488 (0.423-0.563) | |
| Transfer | ||
| No | 1 [Reference] | <.001 |
| Yes | 2.12 (1.79-2.51) | |
| Unknown | 1.25 (0.798-1.96) | |
| CPR | ||
| No | 1 [Reference] | <.001 |
| Yes | 0.029 (0.021-0.040) | |
| Hospitala | Not shown | <.001 |
| Age, y | Nonparametric function | <.001 |
Abbreviations, AHCCCS, Arizona Health Care Cost Containment System; CPR, cardiopulmonary resuscitation; ICD-9, International Classification of Diseases, Ninth Revision; isolated TBI, patients who met TBI inclusion criteria but had no injury with Regional Severity Score ≥3 in any other (nonhead) body region; multisystem TBI, patients who met TBI inclusion criteria and also had at least 1 nonhead region injury with Regional Severity Score ≥3; OR, odds ratio.
Hospital (treating trauma center) was also highly significant. To protect the mandated anonymity of the participating hospitals, the numbers are not shown (preventing any possible identification or inference of facility-specific outcome differences).
The results of the severity-based cohort analyses (moderate: RSS-H 1-2 or ISS 1-14; severe: RSS-H 3-4 or ISS 16-24; and critical: RSS-H 5-6 or ISS 25-75) are shown in Figure 2. The severe subgroups (both TBI-specific and overall injury) showed positive and significant improvement in survival after guideline implementation, while the moderate and critical groups did not. Increases in SHA were most pronounced in the severe TBI group but also occurred in the critical cohort (Figure 2).
Figure 2. Primary Analysis: Adjusted Survival.
Postintervention adjusted odds of survival to hospital discharge or admission for the moderate (Injury Severity Score [ISS] of 1-14), severe (Regional Severity Score–Head of 3 or 4; ISS of 16-24), and critical (Regional Severity Score-Head of 5 or 6; ISS of 25-75) injury cohorts. Logistic regression was used when there were at least 200 patients with the event (eg, survived to discharge) and 200 without (eg, did not survive to discharge). Number of events/number of subjects in each subgroup: Head Injury Severity 1-2: survival to discharge 2072 of 2090; survival to hospital admission (SHA) 2084 of 2090; Head Injury Severity 3-4: survival to discharge 14 754 of 15 147; SHA 15 038 of 15 147; Head Injury Severity 5-6: survival to discharge 1885 of 4444; SHA, 3587 of 4444; ISS 1 to 14: survival to discharge, 7757 of 7826; SHA, 7801/7826; ISS 16 to 24: survival to discharge, 7115 of 7274; SHA, 7241/7274; ISS ≥25: survival to discharge, 3937/6745; SHA, 5785/6745. aOR indicates adjusted odds ratio; NA, not applicable owing to numbers being too small for adjusted analysis.
aIn comparisons that did not meet the criteria of at least 200 patients with the event and 200 without, Firth penalized-likelihood logistic regression was used.
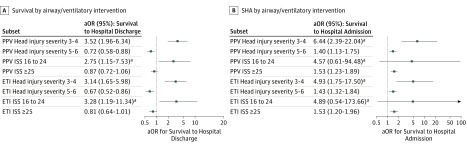
The outcomes associated with airway interventions are shown in Figure 3. Patients with severe injury who received any method of PPV (BVM, supraglottic/extraglottic airway, or intubation) and the severe, intubated subgroup showed marked survival improvement after implementation. The critical-injury subgroups yielded conflicting results, showing either no significant change (overall injury, ISS ≥25) or a small negative change in survival (brain injury, RSS-H 5/6).
Figure 3. Adjusted Analysis of Survival and Survival to Hospital Admission by Severity Cohorts in Patients with Positive-Pressure Ventilation (PPV)/Intubation.
Postintervention adjusted odds of survival to hospital discharge or admission, by airway intervention category, for the severe (Regional Severity Score–Head of 3 or 4; Injury Severity Score [ISS] of 16-24) and critical injury cohorts (Regional Severity Score–Head of 5 or 6; Injury Severity Score of 25-75). The moderate severity category analyses (Regional Severity Score-Head of 1 or 2; ISS of 1-14) are not shown owing to the very small number of deaths in these cohorts, preventing meaningful/stable regression model results. Logistic regression was used when there were at least 200 patients with the event (eg, survived to discharge) and 200 without (eg, did not survive to discharge). For PPV, inclusion criteria were all patients with active ventilation whether basic (bag-valve mask) or advanced airway (supraglottic/extraglottic airway or endotracheal intubation). Number of events/number of subjects in each subgroup: PPV Head Injury Severity 3-4: survival to discharge, 1618 of 1842; SHA 1741 of 1842; PPV Head Injury Severity 5-6: survival to discharge, 771 of 2,992; SHA, 2149 of 2992; PPV ISS 16 to 24: survival to discharge, 751 of 822; SHA, 793 of 822; PPV ISS ≥25: survival to discharge, 1359/3770; SHA, 2829/3770; endotracheal intubation (ETI) Head Injury Severity 3-4: survival to discharge 1257/1457; SHA 1364/1457; ETI Head Injury Severity 5-6: survival to discharge 603 of 2402; SHA 1689 of 2402; ETI ISS 16 to 24: survival to discharge, 586 of 647; SHA, 620 of 647; ETI ISS ≥25: survival to discharge, 1055 of 3024; SHA, 2224/3024. aOR indicates adjusted odds ratio.
aIn comparisons that did not meet the criteria of at least 200 patients with the event and 200 without, Firth penalized-likelihood logistic regression was used.
Discussion
The EMS TBI guidelines emphasize prevention and treatment of hypoxia, hypotension, and hyperventilation.11 These recommendations are based on observational studies demonstrating increased TBI mortality from these insults (hypoxia,14,43,44,45,46,47,48,49,50 hypotension,13,14,44,45,47,49,50,51,52,53,54,55,56,57 and hyperventilation11,14,46,58,59,60,61,62,63,64,65,66). However, the supporting evidence remains weak because to our knowledge, no controlled prehospital studies have directly evaluated the association of guideline-based care with survival.11,12
The EPIC study is a statewide public health initiative implementing the prehospital TBI guidelines10,11,12,15 among patients who experienced injury-associated loss of consciousness. This inclusive approach to guideline implementation at the individual patient level was taken because TBI can be difficult to identify in the field, and its severity may not be immediately apparent.10,11,12,15,67,68,69,70,71,72
We used an intention-to-treat design because we expected participation from more than 100 EMS agencies and could not guarantee access to prehospital records.35,73 Nonetheless, we achieved a 98.7% linkage rate (ie, EMS data linked to TC data), and eTables 9 to 12 in the Supplement provide evidence that guideline-based treatment increased significantly. As expected in a large implementation effort, there was incomplete application of the guidelines at the individual patient/personnel level (eTables 9-12 in the Supplement). However, there is reason for optimism if future innovations in training and technology can improve guideline compliance (eg, real-time physiologic audiovisual feedback).
The primary analysis (across the entire moderate-to-critical severity spectrum) revealed an aOR of 1.70 (95% CI, 1.38-2.09; P < .001) for survival to admission. However, the overall aOR of 1.06 (95% CI, 0.93-1.21) for survival to discharge was nonsignificant (P = .40). The increase in SHA is important because this outcome is proximate to the intervention and likely reflects changes in EMS care. Early outcomes have been recognized to have value for evaluating the effect of prehospital interventions in other serious, time-sensitive conditions.74,75,76,77,78 In addition, improved early survival creates the potential for patients to benefit from subsequent specialized care.76,77,79,80,81
We chose broad inclusion criteria because it is not known which severity subgroups benefit from treatment.3,4,6,7,8,9,10,11,67,71,72,82 This approach prevented unknowingly excluding patients who might benefit (if we made the criteria too narrow). However, it had the risk of diluting the treatment effect (by including nonresponding cohorts). Thus, we planned (a priori) to evaluate the moderate, severe, and critical cohorts separately to prevent some subgroups from potentially hiding the effectiveness of others. Indeed, this approach identified that implementation was strongly associated with improved survival in the severe subgroups (RSS-H 3-4: aOR, 2.03; 95% CI, 1.52-2.72; P < .001; ISS 16-24: aOR, 1.61; 95% CI, 1.07-2.48; P = .02; Figure 2).
These findings support a concept of an interventional sweet spot between the extremes of TBI severity. At the moderate end of the spectrum, detecting differences in mortality is unlikely owing to the low death rate. But in critical TBI, even optimal treatment may have little effect on survival. In contrast, the severe group may benefit far more from the prevention of secondary insults than patients who have devastating primary injury and whose mortality risk is high regardless of treatment. Indeed, the effect of hypoxia and hypotension on survival was significantly greater in patients with severe injury than those with critical injury. Both of these secondary insults were associated with lower odds of survival among the patients with RSS-H of 3 or 4 (hypoxia OR, 0.181; 95% CI, 0.137-0.243; hypotension OR, 0.193; 95% CI, 0.146-0.258) than in those with RSS-H of 5-6 (hypoxia OR, 0.262; 95% CI, 0.220-0.313; P = .03 for the difference; hypotension OR, 0.280; 95% CI, 0.229-0.342; P = .03). Hence, the relative association of primary vs secondary injury with survival may vary with severity, with primary injury being the main determiner of outcome in critical patients and secondary injury taking on more significance in patients with less severe injuries, where the initial insult leaves greater potential for improvement or deterioration. It is notable that other TBI studies have focused on the middle-severity cohort as well.3,9,72,83,84,85
The lack of treatment effect in critical patients may not be solely owing to irreversible pathoanatomic injury. Rather, variations in severity-associated physiologic response may also play a role. For instance, while the rate of intubated patients with at least 1 oxygen saturation of 100% increased significantly after implementation (P1, 985 of 2226 [44.2%] vs P3%, 482 of 885 [54.6%]; P < .001). This increase varied dramatically with severity. In the severe group (RSS-H 3-4), the rate increased from 50.7% (491 of 968; P1) to 66.9% (216 of 323; P3) (16.2% absolute increase). However, in the critical cohort (RSS-H 5-6), the increase was only 7.1% (39.4% [463 of 1176] to 46.5% [252 of 542]; P = .02 for the difference between the severe and critical subgroups ). Furthermore, the patients with hypotension and severe injury in P3 were less than half as likely to arrive at the TC with a further drop in SBP (2.0%) than those in P1 (5.1%, P = .048). In contrast, the hypotensive, critical cohorts in P3 and P1 had a similar likelihood of experiencing an additional SBP drop on arrival (P3 = 8.5%; P1 = 10.7%; P = .49). These findings support the concept that physiologic improvement may be more difficult to achieve in critical patients.
The lack of improvement in the highest-severity cohort is not surprising and may be due to the Stocchetti effect.83,86 Improvements in prehospital trauma care may lead to a paradoxical effect of improved prehospital survival but decreased hospital survival because critical patients who previously died in the field may survive to hospital admission but die in hospital from extremely severe injury.83,86 We believe that the postimplementation increases in SHA in the critical cohorts (RSS-H 5/6: 1.42; 95% CI, 1.15-1.76; ISS ≥25: 1.63; 95% CI, 1.32-2.00; Figures 2 and 3), the larger proportion of older patients (eTable 13 in the Supplement), and the higher rate of patients receiving prehospital CPR (Table 1) all support the interpretation that the Stocchetti Effect explained at least part of the lack of improvement in the critical cohort.
Prehospital intubation for TBI has been controversial for decades.10,11,12,15,23,59,64,66,67,87,88,89,90,91 However, in studies associating intubation with negative outcomes,23,66,67,88,91 it is unclear whether the primary issue was the procedure itself or the high rate of inadvertent hyperventilation following intubation.43,64,65,85,87,92 In EPIC, training emphasized the guideline-based approach of reserving intubation for those with markedly depressed level of consciousness and in whom basic interventions were inadequate for airway protection and oxygenation.10,11,12,15 Several findings provide evidence that this approach was implemented. Despite increased severity of both brain and overall injury in P3, the intubation rate decreased and the BVM-only rate increased (eTable 12 in the Supplement). Furthermore, among PPV cases, the rate of BVM-only use increased markedly (relative increase = 44.1%, P < .001).
Postimplementation adjusted survival tripled in the PPV and ETI severe cohorts (both TBI-specific and overall injury; Figure 3). This may be owing to the focus on oxygenation/preoxygenation and preventing hyperventilation via (1) intentional emphasis on achieving target end-tidal carbon dioxide (35-45 mm Hg), (2) the use of ventilation rate timers as real-time visual cues for manual ventilation, and (3) use of flow-controlled ventilation bags. These findings imply that intubation, combined with proper ventilation, may be the optimal approach to prehospital airway management in patients with major TBI who meet the criteria recommended in the guidelines. Clearly, many questions related to this issue require further study.
Two challenges of EPIC were its length (the typical agency participated for 3 years following implementation) and our inability to enforce retraining after initial education. Thus, there was a potential for decreased emphasis on guideline adherence over time. To evaluate this, we assessed temporal changes in outcome by comparing P1 to early P3 (months 1-18) and late P3 (≥19 months). There was initial improvement, but the effect faded (aOR for survival: early P3, 1.16; 95% CI, 0.99-1.37; “late” P3, 0.95; 0.80-1.13). This was also reflected by the interim analysis (accrual: March 31, 2014), which was positive for survival (aOR, 1.16; 95% CI, 1.00-1.34; P = .048). However, the final analysis reverted (aOR, 1.06; 95% CI, 0.93-1.21; P = .40). The reason we did not report a positive study at the interim analysis was because the P value required for early termination was P less than .01. Interestingly, in retrospect, if we had planned the study to only last until the interim analysis, we would have reported a positive study at that time (ie, P < .05). We believe these findings reflect the need for focused, recurrent training to help prevent deterioration of guideline adherence over time.
Limitations
This study has limitations. First, it was not randomized. Although a randomized clinical trial might definitively identify optimal treatment, such a trial was not feasible. Because existing studies overwhelmingly report detrimental effects of hypoxia, hypotension, and hyperventilation, randomization (to treat/not treat) would be unacceptable to most EMS systems. Use of a pragmatic trial design (eg, stepped-wedge or cluster-randomized)93 was also nonfeasible because the timing of EPIC training had to be determined primarily by agency-specific operational factors.
Because the guidelines were implemented as a “bundle,” we cannot identify the relative effect of specific interventions (eg, oxygenation/preoxygenation). This would have required stepwise, intervention-specific implementation, and this was not feasible.
To evaluate potential influence of secular trends, we assessed concurrent outcomes in 2 cohorts taken to the TCs that met diagnostic inclusion criteria but were unaffected by EPIC. First, we assessed patients transported by nonparticipating EMS agencies. However, the agency recruitment was so successful that this cohort was much smaller than anticipated (233 total; mean, 27.4 per year). Second, we evaluated patients brought to TCs by privately owned vehicle (n = 1486). The before/after analysis (early patients [January 1, 2007, to December 31, 2012] vs late [January 1, 2013, to June 30, 2015]) yielded no evidence of outcome improvement over time. Indeed, there was a trend toward somewhat worse outcomes in the late group (eTable 23 in the Supplement). It is noteworthy that the trauma system in Arizona developed during the 1980s and was very stable during EPIC. The TCs accounting for 98.1% of patients (21 432 of 21 852) were established more than a decade before the study began.
Finally, we could not control for the effects of inpatient care. Thus, we cannot know conclusively that the improvements were directly caused by EMS guideline implementation. However, the concurrent increase in survival to hospital admission (aOR, 1.70; 95% CI, 1.38-2.09; P < .001) is supportive of the conclusion that EMS implementation was associated with the improvements in outcome.
Conclusions
Statewide implementation of the prehospital TBI guidelines was not associated with improved overall survival (across the entire, combined moderate to critical spectrum). However, survival doubled among patients with severe TBI and tripled in the patients with severe TBI who received PPV and/or intubation. Implementation was also independently associated with significant improvement in survival to hospital admission. These findings support the widespread implementation of the prehospital TBI treatment guidelines.
eFigure. EPIC Study Design: Before-After System Evaluation
eTable 1. EPIC EMS Dataset
eTable 2. ASTR—Examples of Data Elements for Trauma Center Reporting
eTable 3. The Barell Injury Diagnosis Matrix for Traumatic Brain Injury
eTable 4. EMS Care of moderate and severe TBI Treatment and Monitoring Guidelines/Protocols—ADULTS
eTable 5. EPIC TBI Algorithm for Adults
eTable 6. EMS Care of moderate and severe TBI Treatment and Monitoring Guidelines/Protocols—Infants and Children
eTable 7. EPIC4Kids TBI Algorithm for Children
eTable 8. Important Risk Factors and Potential Confounders Used in the Final Models
eTable 9. Changes in Rates of Hypoxia in the Field Versus on Arrival at the Trauma Center Among Prehospital-Intubated Patients
eTable 10. Rates of Patients Receiving Intravenous Fluid Boluses in P1 Versus P3
eTable 11. Rates of Patients Receiving Various Total Prehospital Volumes of Isotonic Intravenous Fluid in P1 Versus P3
eTable 12. Rates of Intubation and Bag-Valve-Mask Airways Pre/Post-implementation
eTable 13. Proportions of Older Patients in P1 Versus P3 by Various Age Definitions
eTable 14. Analysis of Adjusted Survival to Hospital Admission in Phase 3 Versus Phase 1
eTable 15. Logistic Regression Model for Survival with Random Agency Effects
eTable 16. Logistic Regression Model for Survival to Hospital Admission with Random Agency Effects
eTable 17. Logistic Regression Model for Survival with Systolic Blood Pressure Included by TRISS Category
eTable 18. Logistic Regression Model for Survival with Glasgow Coma Scale Included by TRISS Category
eTable 19. Logistic Regression Model for Survival with Respiratory Rate Included by TRISS Category
eTable 20. Logistic Regression Model for Survival to Hospital Admission with Systolic Blood Pressure Included by TRISS Category
eTable 21. Logistic Regression Model for Survival to Hospital Admission with Glasgow Coma Scale Included by TRISS Category
eTable 22. Logistic Regression Model for Survival to Hospital Admission with Respiratory Rate Included by TRISS Category
eTable 23. Temporal Analysis of Survival Among Patients Arriving at Trauma Centers By Privately-Owned Vehicle
eReferences.
References
- 1.Bell JMBM, Jenkins EL, Haarbauer-Krupa J. Traumatic Brain Injury In the United States: Epidemiology and Rehabilitation. Washington, DC: National Center for Injury Prevention and Control, Division of Unintentional Injury Prevention, Centers for Disease Control; 2014. [Google Scholar]
- 2.Finkelstein E, Corso PS, Miller TR. The Incidence and Economic Burden of Injuries in the United States. New York, NY: Oxford University Press; 2006. doi: 10.1093/acprof:oso/9780195179484.001.0001 [DOI] [Google Scholar]
- 3.Wright DW, Yeatts SD, Silbergleit R, et al. ; NETT Investigators . Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014;371(26):-. doi: 10.1056/NEJMoa1404304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maas AI, Steyerberg EW, Marmarou A, et al. IMPACT recommendations for improving the design and analysis of clinical trials in moderate to severe traumatic brain injury. Neurotherapeutics. 2010;7(1):127-134. doi: 10.1016/j.nurt.2009.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maas AI, Marmarou A, Murray GD, Teasdale SG, Steyerberg EW. Prognosis and clinical trial design in traumatic brain injury: the IMPACT study. J Neurotrauma. 2007;24(2):232-238. doi: 10.1089/neu.2006.0024 [DOI] [PubMed] [Google Scholar]
- 6.Doppenberg EM, Choi SC, Bullock R. Clinical trials in traumatic brain injury: lessons for the future. J Neurosurg Anesthesiol. 2004;16(1):87-94. doi: 10.1097/00008506-200401000-00019 [DOI] [PubMed] [Google Scholar]
- 7.Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31(12):596-604. doi: 10.1016/j.tips.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT; Workshop Scientific Team and Advisory Panel Members . Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25(7):719-738. doi: 10.1089/neu.2008.0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall LF. Head injury: recent past, present, and future. Neurosurgery. 2000;47(3):546-561. [DOI] [PubMed] [Google Scholar]
- 10.Brain Trauma F; American Association of Neurological S; Congress of Neurological S. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(suppl 1):S1-S106. [DOI] [PubMed] [Google Scholar]
- 11.Badjatia N, Carney N, Crocco TJ, et al. ; Brain Trauma Foundation; BTF Center for Guidelines Management . Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehosp Emerg Care. 2008;12(suppl 1):S1-S52. doi: 10.1080/10903120701732052 [DOI] [PubMed] [Google Scholar]
- 12.Kochanek PM, Carney N, Adelson PD, et al. ; American Academy of Pediatrics-Section on Neurological Surgery; American Association of Neurological Surgeons/Congress of Neurological Surgeons; Child Neurology Society; European Society of Pediatric and Neonatal Intensive Care; Neurocritical Care Society; Pediatric Neurocritical Care Research Group; Society of Critical Care Medicine; Paediatric Intensive Care Society UK; Society for Neuroscience in Anesthesiology and Critical Care; World Federation of Pediatric Intensive and Critical Care Societies . Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents: second edition. Pediatr Crit Care Med. 2012;13(suppl 1):S1-S82. doi: 10.1097/PCC.0b013e318259ee85 [DOI] [PubMed] [Google Scholar]
- 13.Jankowitz BT, Adelson PD. Pediatric traumatic brain injury: past, present and future. Dev Neurosci. 2006;28(4-5):264-275. doi: 10.1159/000094153 [DOI] [PubMed] [Google Scholar]
- 14.Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34(2):216-222. doi: 10.1097/00005373-199302000-00006 [DOI] [PubMed] [Google Scholar]
- 15.Adelson PD, Bratton SL, Carney NA, et al. ; American Association for Surgery of Trauma; Child Neurology Society; International Society for Pediatric Neurosurgery; International Trauma Anesthesia and Critical Care Society; Society of Critical Care Medicine; World Federation of Pediatric Intensive and Critical Care Societies . Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents, chapter 1: introduction. Pediatr Crit Care Med. 2003;4(3)(suppl):S2-S4. doi: 10.1097/01.CCM.0000066600.71233.01 [DOI] [PubMed] [Google Scholar]
- 16.Faul M, Wald MM, Rutland-Brown W, Sullivent EE, Sattin RW. Using a cost-benefit analysis to estimate outcomes of a clinical treatment guideline: testing the Brain Trauma Foundation guidelines for the treatment of severe traumatic brain injury. J Trauma. 2007;63(6):1271-1278. doi: 10.1097/TA.0b013e3181493080 [DOI] [PubMed] [Google Scholar]
- 17.Fakhry SM, Trask AL, Waller MA, Watts DD, Force INT; IRTC Neurotrauma Task Force . Management of brain-injured patients by an evidence-based medicine protocol improves outcomes and decreases hospital charges. J Trauma. 2004;56(3):492-499. doi: 10.1097/01.TA.0000115650.07193.66 [DOI] [PubMed] [Google Scholar]
- 18.Palmer S, Bader MK, Qureshi A, et al. ; Americans Associations for Neurologic Surgeons . The impact on outcomes in a community hospital setting of using the AANS traumatic brain injury guidelines. J Trauma. 2001;50(4):657-664. doi: 10.1097/00005373-200104000-00010 [DOI] [PubMed] [Google Scholar]
- 19.Kreutzer JS, Kolakowsky-Hayner SA, Ripley D, et al. Charges and lengths of stay for acute and inpatient rehabilitation treatment of traumatic brain injury 1990-1996. Brain Inj. 2001;15(9):763-774. doi: 10.1080/02699050010025786 [DOI] [PubMed] [Google Scholar]
- 20.Vavilala MS, Kernic MA, Wang J, et al. ; Pediatric Guideline Adherence and Outcomes Study . Acute care clinical indicators associated with discharge outcomes in children with severe traumatic brain injury. Crit Care Med. 2014;42(10):2258-2266. doi: 10.1097/CCM.0000000000000507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Lynnger TM, Shannon CN, Le TM, et al. Standardizing ICU management of pediatric traumatic brain injury is associated with improved outcomes at discharge. J Neurosurg Pediatr. 2016;17(1):19-26. doi: 10.3171/2015.5.PEDS1544 [DOI] [PubMed] [Google Scholar]
- 22.Stiell IG, Spaite DW, Field B, et al. ; OPALS Study Group . Advanced life support for out-of-hospital respiratory distress. N Engl J Med. 2007;356(21):2156-2164. doi: 10.1056/NEJMoa060334 [DOI] [PubMed] [Google Scholar]
- 23.Stiell IG, Nesbitt LP, Pickett W, et al. ; OPALS Study Group . The OPALS Major Trauma Study: impact of advanced life-support on survival and morbidity. CMAJ. 2008;178(9):1141-1152. doi: 10.1503/cmaj.071154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stiell IG, Wells GA, Field B, et al. ; Ontario Prehospital Advanced Life Support Study Group . Advanced cardiac life support in out-of-hospital cardiac arrest. N Engl J Med. 2004;351(7):647-656. doi: 10.1056/NEJMoa040325 [DOI] [PubMed] [Google Scholar]
- 25.Stiell IG, Wells GA, Spaite DW, et al. ; OPALS Study Group . The Ontario Prehospital Advanced Life Support (OPALS) study part II: rationale and methodology for trauma and respiratory distress patients. Ann Emerg Med. 1999;34(2):256-262. doi: 10.1016/S0196-0644(99)70241-6 [DOI] [PubMed] [Google Scholar]
- 26.Spaite DW, Beskind DL, Garrison HG Evaluating the effectiveness of EMS systems: utilizing outcomes research methods to identify the impact of prehospital care. In: Cone DC, O'Conner RE, Fowler R, eds. Emergency Medical Services: Clinical Practice and Systems Oversight Overland Park, KS: National Association of EMS Physicians; 2009. [Google Scholar]
- 27.Spaite DW, Bobrow BJ, Stolz U, et al. Evaluation of the impact of implementing the emergency medical services traumatic brain injury guidelines in Arizona: the Excellence in Prehospital Injury Care (EPIC) study methodology. Acad Emerg Med. 2014;21(7):818-830. doi: 10.1111/acem.12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spaite DW, Hu C, Bobrow BJ, et al. Mortality and prehospital blood pressure in patients with major traumatic brain injury: implications for the hypotension threshold. JAMA Surg. 2017;152(4):360-368. doi: 10.1001/jamasurg.2016.4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaite DW, Hu C, Bobrow BJ, et al. Association of out-of-hospital hypotension depth and duration with traumatic brain injury mortality. Ann Emerg Med. 2017;70(4):522-530.e1. doi: 10.1016/j.annemergmed.2017.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spaite DW, Hu C, Bobrow BJ, et al. The effect of combined out-of-hospital hypotension and hypoxia on mortality in major traumatic brain injury. Ann Emerg Med. 2017;69(1):62-72. doi: 10.1016/j.annemergmed.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barell V, Aharonson-Daniel L, Fingerhut LA, et al. An introduction to the Barell body region by nature of injury diagnosis matrix. Inj Prev. 2002;8(2):91-96. doi: 10.1136/ip.8.2.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark DE, Ahmad S. Estimating injury severity using the Barell matrix. Inj Prev. 2006;12(2):111-116. doi: 10.1136/ip.2005.010058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Centers for Disease Control and Prevention Barell matrix CDC website. https://www.cdc.gov/nchs/injury/injury_matrices.htm. Accessed April 22, 2010.
- 34.Spaite DW, Criss EA, Valenzuela TD, Guisto J. Emergency medical service systems research: problems of the past, challenges of the future. Ann Emerg Med. 1995;26(2):146-152. doi: 10.1016/S0196-0644(95)70144-3 [DOI] [PubMed] [Google Scholar]
- 35.Spaite DW, Valenzuela TD, Meislin HW. Barriers to EMS system evaluation: problems associated with field data collection. Prehosp Disaster Med. 1993;8:S35-S40. [DOI] [PubMed] [Google Scholar]
- 36.Wood SN. Generalized Additive Models: An Introduction with R. 2nd ed Boca Raton, FL: Chapman and Hall/CRC; 2017. doi: 10.1201/9781315370279 [DOI] [Google Scholar]
- 37.Firth D. Bias reduction of maximum-likelihood-estimates. Biometrika. 1993;80:27-38. doi: 10.1093/biomet/80.1.27 [DOI] [Google Scholar]
- 38.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med. 2002;21(16):2409-2419. doi: 10.1002/sim.1047 [DOI] [PubMed] [Google Scholar]
- 39.R: a language and environment for statistical computing: Version 3.4.4. R Foundation for Statistical Computing. https://www.R-project.org/. Accessed April 26, 2018.
- 40.gamm4: Generalized Additive Mixed Models using 'mgcv' and 'lme4' (R package version 0.2-5). https://CRAN.R-project.org/package=gamm4. Published 2017. Accessed July 30, 2018.
- 41.gee: Generalized Estimation Equation Solver (R package version 4.13-19). https://CRAN.R-project.org/package=gee. Accessed May 10, 2018.
- 42.logistf: Firth's Bias-Reduced Logistic Regression (R package version 1.22). https://CRAN.R-project.org/package=logistf. Accessed May 10, 2018.
- 43.Davis DP, Idris AH, Sise MJ, et al. Early ventilation and outcome in patients with moderate to severe traumatic brain injury. Crit Care Med. 2006;34(4):1202-1208. doi: 10.1097/01.CCM.0000208359.74623.1C [DOI] [PubMed] [Google Scholar]
- 44.Stocchetti N, Furlan A, Volta F. Hypoxemia and arterial hypotension at the accident scene in head injury. J Trauma. 1996;40(5):764-767. doi: 10.1097/00005373-199605000-00014 [DOI] [PubMed] [Google Scholar]
- 45.Cooke RS, McNicholl BP, Byrnes DP. Early management of severe head injury in Northern Ireland. Injury. 1995;26(6):395-397. doi: 10.1016/0020-1383(95)00003-R [DOI] [PubMed] [Google Scholar]
- 46.Davis DP, Dunford JV, Poste JC, et al. The impact of hypoxia and hyperventilation on outcome after paramedic rapid sequence intubation of severely head-injured patients. J Trauma. 2004;57(1):1-8. doi: 10.1097/01.TA.0000135503.71684.C8 [DOI] [PubMed] [Google Scholar]
- 47.Fearnside MR, Cook RJ, McDougall P, McNeil RJ. The Westmead Head Injury Project outcome in severe head injury: a comparative analysis of pre-hospital, clinical and CT variables. Br J Neurosurg. 1993;7(3):267-279. doi: 10.3109/02688699309023809 [DOI] [PubMed] [Google Scholar]
- 48.Marmarou A, Anderson RL, Ward JD, et al. Impact of Icp instability and hypotension on outcome in patients with severe head trauma. J Neurosurg. 1991;75:S59-S66. doi: 10.3171/sup.1991.75.1s.0s59 [DOI] [Google Scholar]
- 49.Mayer TA, Walker ML. Pediatric head injury: the critical role of the emergency physician. Ann Emerg Med. 1985;14(12):1178-1184. doi: 10.1016/S0196-0644(85)81025-8 [DOI] [PubMed] [Google Scholar]
- 50.Ong L, Selladurai BM, Dhillon MK, Atan M, Lye MS. The prognostic value of the Glasgow Coma Scale, hypoxia and computerised tomography in outcome prediction of pediatric head injury. Pediatr Neurosurg. 1996;24(6):285-291. doi: 10.1159/000121057 [DOI] [PubMed] [Google Scholar]
- 51.Shutter LA, Narayan RK. Blood pressure management in traumatic brain injury. Ann Emerg Med. 2008;51(3)(suppl):S37-S38. doi: 10.1016/j.annemergmed.2007.11.013 [DOI] [PubMed] [Google Scholar]
- 52.Gentleman D. Causes and effects of systemic complications among severely head injured patients transferred to a neurosurgical unit. Int Surg. 1992;77(4):297-302. [PubMed] [Google Scholar]
- 53.Pigula FA, Wald SL, Shackford SR, Vane DW. The effect of hypotension and hypoxia on children with severe head injuries. J Pediatr Surg. 1993;28(3):310-314. doi: 10.1016/0022-3468(93)90223-8 [DOI] [PubMed] [Google Scholar]
- 54.Manley G, Knudson MM, Morabito D, Damron S, Erickson V, Pitts L. Hypotension, hypoxia, and head injury: frequency, duration, and consequences. Arch Surg. 2001;136(10):1118-1123. doi: 10.1001/archsurg.136.10.1118 [DOI] [PubMed] [Google Scholar]
- 55.Price DJ, Murray A. The influence of hypoxia and hypotension on recovery from head injury. Injury. 1972;3(4):218-224. doi: 10.1016/0020-1383(72)90104-0 [DOI] [PubMed] [Google Scholar]
- 56.Miller JD, Sweet RC, Narayan R, Becker DP. Early insults to the injured brain. JAMA. 1978;240(5):439-442. doi: 10.1001/jama.1978.03290050029011 [DOI] [PubMed] [Google Scholar]
- 57.McHugh GS, Engel DC, Butcher I, et al. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):287-293. doi: 10.1089/neu.2006.0031 [DOI] [PubMed] [Google Scholar]
- 58.Davis DP, Dunford JV, Ochs M, Park K, Hoyt DB. The use of quantitative end-tidal capnometry to avoid inadvertent severe hyperventilation in patients with head injury after paramedic rapid sequence intubation. J Trauma. 2004;56(4):808-814. doi: 10.1097/01.TA.0000100217.05066.87 [DOI] [PubMed] [Google Scholar]
- 59.Davis DP. Early ventilation in traumatic brain injury. Resuscitation. 2008;76(3):333-340. doi: 10.1016/j.resuscitation.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 60.Davis DP, Heister R, Poste JC, Hoyt DB, Ochs M, Dunford JV. Ventilation patterns in patients with severe traumatic brain injury following paramedic rapid sequence intubation. Neurocrit Care. 2005;2(2):165-171. doi: 10.1385/NCC:2:2:165 [DOI] [PubMed] [Google Scholar]
- 61.Manley GT, Hemphill JC, Morabito D, et al. Cerebral oxygenation during hemorrhagic shock: perils of hyperventilation and the therapeutic potential of hypoventilation. J Trauma. 2000;48(6):1025-1032. doi: 10.1097/00005373-200006000-00005 [DOI] [PubMed] [Google Scholar]
- 62.Zornow MH, Prough DS. Does acute hyperventilation cause cerebral ischemia in severely head-injured patients? Crit Care Med. 2002;30(12):2774-2775. doi: 10.1097/00003246-200212000-00026 [DOI] [PubMed] [Google Scholar]
- 63.Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75(5):731-739. doi: 10.3171/jns.1991.75.5.0731 [DOI] [PubMed] [Google Scholar]
- 64.Gaither JB, Spaite DW, Bobrow BJ, et al. Balancing the potential risks and benefits of out-of-hospital intubation in traumatic brain injury: the intubation/hyperventilation effect. Ann Emerg Med. 2012;60(6):732-736. doi: 10.1016/j.annemergmed.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 65.Denninghoff KR, Griffin MJ, Bartolucci AA, Lobello SG, Fine PR. Emergent endotracheal intubation and mortality in traumatic brain injury. West J Emerg Med. 2008;9(4):184-189. [PMC free article] [PubMed] [Google Scholar]
- 66.Wang HE, Peitzman AB, Cassidy LD, Adelson PD, Yealy DM. Out-of-hospital endotracheal intubation and outcome after traumatic brain injury. Ann Emerg Med. 2004;44(5):439-450. doi: 10.1016/j.annemergmed.2004.04.008 [DOI] [PubMed] [Google Scholar]
- 67.Davis DP, Hoyt DB, Ochs M, et al. The effect of paramedic rapid sequence intubation on outcome in patients with severe traumatic brain injury. J Trauma. 2003;54(3):444-453. doi: 10.1097/01.TA.0000053396.02126.CD [DOI] [PubMed] [Google Scholar]
- 68.Davis DP, Vadeboncoeur TF, Ochs M, Poste JC, Vilke GM, Hoyt DB. The association between field Glasgow Coma Scale score and outcome in patients undergoing paramedic rapid sequence intubation. J Emerg Med. 2005;29(4):391-397. doi: 10.1016/j.jemermed.2005.04.012 [DOI] [PubMed] [Google Scholar]
- 69.Feldman A, Hart KW, Lindsell CJ, McMullan JT. Randomized controlled trial of a scoring aid to improve Glasgow Coma Scale scoring by emergency medical services providers. Ann Emerg Med. 2015;65(3):325-329.e2. doi: 10.1016/j.annemergmed.2014.07.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bledsoe BE, Casey MJ, Feldman J, et al. Glasgow Coma Scale Scoring is often inaccurate. Prehosp Disaster Med. 2015;30(1):46-53. doi: 10.1017/S1049023X14001289 [DOI] [PubMed] [Google Scholar]
- 71.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728-741. doi: 10.1016/S1474-4422(08)70164-9 [DOI] [PubMed] [Google Scholar]
- 72.Narayan RK, Michel ME, Ansell B, et al. Clinical trials in head injury. J Neurotrauma. 2002;19(5):503-557. doi: 10.1089/089771502753754037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spaite DW, Hanlon T, Criss EA, Valenzuela TD, Meislin HW, Ross J. Prehospital data entry compliance by paramedics after institution of a comprehensive EMS data collection tool. Ann Emerg Med. 1990;19(11):1270-1273. doi: 10.1016/S0196-0644(05)82286-3 [DOI] [PubMed] [Google Scholar]
- 74.Cummins RO, Chamberlain DA, Abramson NS, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the Utstein Style: a statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Circulation. 1991;84(2):960-975. doi: 10.1161/01.CIR.84.2.960 [DOI] [PubMed] [Google Scholar]
- 75.Zaritsky A, Nadkarni V, Hazinski MF, et al. Recommended guidelines for uniform reporting of pediatric advanced life support: the Pediatric Utstein Style: a statement for healthcare professionals from a task force of the American Academy of Pediatrics, the American Heart Association, and the European Resuscitation Council. Resuscitation. 1995;30(2):95-115. doi: 10.1016/0300-9572(95)00884-V [DOI] [PubMed] [Google Scholar]
- 76.Wang CH, Chou NK, Becker LB, et al. Improved outcome of extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: a comparison with that for extracorporeal rescue for in-hospital cardiac arrest. Resuscitation. 2014;85(9):1219-1224. doi: 10.1016/j.resuscitation.2014.06.022 [DOI] [PubMed] [Google Scholar]
- 77.Callaway CW, Donnino MW, Fink EL, et al. Part 8: post-cardiac arrest care: 2015 american heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18)(suppl 2):S465-S482. doi: 10.1161/CIR.0000000000000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perkins GD, Jacobs IG, Nadkarni VM, et al. ; Utstein Collaborators . Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation. 2015;132(13):1286-1300. doi: 10.1161/CIR.0000000000000144 [DOI] [PubMed] [Google Scholar]
- 79.MacKenzie EJ, Rivara FP, Jurkovich GJ, et al. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med. 2006;354(4):366-378. doi: 10.1056/NEJMsa052049 [DOI] [PubMed] [Google Scholar]
- 80.MacKenzie EJ, Weir S, Rivara FP, et al. The value of trauma center care. J Trauma. 2010;69(1):1-10. doi: 10.1097/TA.0b013e3181e03a21 [DOI] [PubMed] [Google Scholar]
- 81.Spaite DW, Bobrow BJ, Stolz U, et al. ; Arizona Cardiac Receiving Center Consortium . Statewide regionalization of postarrest care for out-of-hospital cardiac arrest: association with survival and neurologic outcome. Ann Emerg Med. 2014;64(5):496-506.e1. doi: 10.1016/j.annemergmed.2014.05.028 [DOI] [PubMed] [Google Scholar]
- 82.Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57(6):1173-1182. doi: 10.1227/01.NEU.0000186013.63046.6B [DOI] [PubMed] [Google Scholar]
- 83.Klemen P, Grmec S. Effect of pre-hospital advanced life support with rapid sequence intubation on outcome of severe traumatic brain injury. Acta Anaesthesiol Scand. 2006;50(10):1250-1254. doi: 10.1111/j.1399-6576.2006.01039.x [DOI] [PubMed] [Google Scholar]
- 84.Schüttler J, Schmitz B, Bartsch AC, Fischer M. The efficiency of emergency therapy in patients with head-brain, multiple injury. Quality assurance in emergency medicine. Anaesthesist. 1995;44(12):850-858. [DOI] [PubMed] [Google Scholar]
- 85.Denninghoff KR, Nuño T, Pauls Q, et al. Prehospital intubation is associated with favorable outcomes and lower mortality in ProTECT III. Prehosp Emerg Care. 2017;21(5):539-544. doi: 10.1080/10903127.2017.1315201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stocchetti N. Risk prevention, avoidable deaths and mortality-morbidity reduction in head injury. Eur J Emerg Med. 2001;8(3):215-219. doi: 10.1097/00063110-200109000-00009 [DOI] [PubMed] [Google Scholar]
- 87.Bernard SA, Nguyen V, Cameron P, et al. Prehospital rapid sequence intubation improves functional outcome for patients with severe traumatic brain injury: a randomized controlled trial. Ann Surg. 2010;252(6):959-965. doi: 10.1097/SLA.0b013e3181efc15f [DOI] [PubMed] [Google Scholar]
- 88.Davis DP, Peay J, Sise MJ, et al. The impact of prehospital endotracheal intubation on outcome in moderate to severe traumatic brain injury. J Trauma. 2005;58(5):933-939. doi: 10.1097/01.TA.0000162731.53812.58 [DOI] [PubMed] [Google Scholar]
- 89.Winchell RJ, Hoyt DB; Trauma Research and Education Foundation of San Diego . Endotracheal intubation in the field improves survival in patients with severe head injury. Arch Surg. 1997;132(6):592-597. doi: 10.1001/archsurg.1997.01430300034007 [DOI] [PubMed] [Google Scholar]
- 90.Bulger EM, Copass MK, Sabath DR, Maier RV, Jurkovich GJ. The use of neuromuscular blocking agents to facilitate prehospital intubation does not impair outcome after traumatic brain injury. J Trauma. 2005;58(4):718-723. doi: 10.1097/01.TA.0000159239.14181.BC [DOI] [PubMed] [Google Scholar]
- 91.Stockinger ZT, McSwain NE Jr. Prehospital endotracheal intubation for trauma does not improve survival over bag-valve-mask ventilation. J Trauma. 2004;56(3):531-536. doi: 10.1097/01.TA.0000111755.94642.29 [DOI] [PubMed] [Google Scholar]
- 92.Davis DP, Stern J, Sise MJ, Hoyt DB. A follow-up analysis of factors associated with head-injury mortality after paramedic rapid sequence intubation. J Trauma. 2005;59(2):486-490. doi: 10.1097/00005373-200508000-00037 [DOI] [PubMed] [Google Scholar]
- 93.Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375(5):454-463. doi: 10.1056/NEJMra1510059 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. EPIC Study Design: Before-After System Evaluation
eTable 1. EPIC EMS Dataset
eTable 2. ASTR—Examples of Data Elements for Trauma Center Reporting
eTable 3. The Barell Injury Diagnosis Matrix for Traumatic Brain Injury
eTable 4. EMS Care of moderate and severe TBI Treatment and Monitoring Guidelines/Protocols—ADULTS
eTable 5. EPIC TBI Algorithm for Adults
eTable 6. EMS Care of moderate and severe TBI Treatment and Monitoring Guidelines/Protocols—Infants and Children
eTable 7. EPIC4Kids TBI Algorithm for Children
eTable 8. Important Risk Factors and Potential Confounders Used in the Final Models
eTable 9. Changes in Rates of Hypoxia in the Field Versus on Arrival at the Trauma Center Among Prehospital-Intubated Patients
eTable 10. Rates of Patients Receiving Intravenous Fluid Boluses in P1 Versus P3
eTable 11. Rates of Patients Receiving Various Total Prehospital Volumes of Isotonic Intravenous Fluid in P1 Versus P3
eTable 12. Rates of Intubation and Bag-Valve-Mask Airways Pre/Post-implementation
eTable 13. Proportions of Older Patients in P1 Versus P3 by Various Age Definitions
eTable 14. Analysis of Adjusted Survival to Hospital Admission in Phase 3 Versus Phase 1
eTable 15. Logistic Regression Model for Survival with Random Agency Effects
eTable 16. Logistic Regression Model for Survival to Hospital Admission with Random Agency Effects
eTable 17. Logistic Regression Model for Survival with Systolic Blood Pressure Included by TRISS Category
eTable 18. Logistic Regression Model for Survival with Glasgow Coma Scale Included by TRISS Category
eTable 19. Logistic Regression Model for Survival with Respiratory Rate Included by TRISS Category
eTable 20. Logistic Regression Model for Survival to Hospital Admission with Systolic Blood Pressure Included by TRISS Category
eTable 21. Logistic Regression Model for Survival to Hospital Admission with Glasgow Coma Scale Included by TRISS Category
eTable 22. Logistic Regression Model for Survival to Hospital Admission with Respiratory Rate Included by TRISS Category
eTable 23. Temporal Analysis of Survival Among Patients Arriving at Trauma Centers By Privately-Owned Vehicle
eReferences.