Abstract
The fungal pathogen Pyricularia oryzae causes blast, a severe disease of rice (Oryza sativa L.). Improving blast resistance is important in rice breeding programs. Inoculation tests have been used to select for resistance genotypes, with DNA marker-based selection becoming an efficient alternative. No comprehensive DNA marker system for race-specific resistance alleles in the Japanese rice breeding program has been developed because some loci contain multiple resistance alleles. Here, we used the Fluidigm SNP genotyping platform to determine a set of 96 single nucleotide polymorphism (SNP) markers for 10 loci with race-specific resistance. The markers were then used to evaluate the presence or absence of 24 resistance alleles in 369 cultivars; results were 93.5% consistent with reported inoculation test-based genotypes in japonica varieties. The evaluation system was successfully applied to high-yield varieties with indica genetic backgrounds. The system includes polymorphisms that distinguish the resistant alleles at the tightly linked Pita and Pita-2 loci, thereby confirming that all the tested cultivars with Pita-2 allele carry Pita allele. We also developed and validated insertion/deletion (InDel) markers for ten resistance loci. Combining SNP and InDel markers is an accurate and efficient strategy for selection for race-specific resistance to blast in breeding programs.
Keywords: blast resistance, race-specific resistance, DNA marker, SNP, marker-assisted selection, Oryza sativa L.
Introduction
Rice blast caused by the fungal pathogen Pyricularia oryzae (syn. Magnaporthe oryzae) is a devastating disease of rice (Oryza sativa L.). Use of cultivars that carry resistance to this disease is a cost-effective and environmentally friendly means to control the pathogen. Therefore, breeders and researchers worldwide have made extensive efforts to explore genes for blast resistance and incorporate them in breeding programs. Since more than 100 loci for blast resistance have been reported (Ashkani et al. 2016, Koide et al. 2009), their characterization and the establishment of systems for selecting desirable resistance alleles are indispensable for enhancing breeding programs.
Blast resistance genotypes are conventionally determined by inoculating with differential pathogen strains to test sample rice varieties against reference varieties for respective resistance alleles (Hayashi 2015). Such conventional differential systems used in the Japanese rice breeding program have discriminated 12 resistance alleles (Pik-s, Pia, Pii, Pik, Pik-m, Piz, Pita, Pita-2, Piz-t, Pik-p, Pib, and Pit) (Kiyosawa 1984, Yamada et al. 1976). Recent breeding efforts to introduce beneficial agricultural traits from exotic genetic resources have increased the diversity in allelic combinations for blast resistance (Yonemaru et al. 2014); this has meant that determination of blast resistance genotypes has become difficult, especially in varieties with the indica genetic background (Hayashi et al. 2014, Hirabayashi et al. 2010, Kato 2008). To overcome this problem, new differential systems have been proposed (Hayashi and Fukuta 2009, Hayashi et al. 2014, Kobayashi et al. 2007, Telebanco-Yanoria et al. 2010, Tsunematsu et al. 2000). Such new systems are readily acceptable when (a) results are compatible with those obtained by conventional systems, and (b) the pathogen strains used give distinctive resistant or susceptible phenotypes against a wide range of rice varieties. The differential system reported by Hayashi and Fukuta (2009) meets these requirements and has successfully determined the presence or absence of 23 resistance alleles (Pia, Pish, Pib, Pit, Pii, Pi3, Pi5, Pik-s, Pik, Pik-p, Pi7, Pik-m, Pi1, Pik-h, Piz, Piz-5, Piz-t, Pi9, Pi19, Pi20, Pita, Pita-2, and Pi12) in 10 varieties with the indica genetic background. Such improved differential systems require more labor and expertise compared with conventional systems, which restricts their use. Another limitation of inoculation-based differential systems is the difficulty in updating the system, because the numbers of differential fungus strains and reference varieties increases proportionally to the number of alleles to be discriminated.
Genetic mapping of genes for blast resistance has contributed to the development of DNA markers for several resistance alleles (Hayashi et al. 2004, 2006, 2010a, Koide et al. 2009, Nonoue et al. 2018, Tian et al. 2016, Wu et al. 2015). Since DNA markers allow us to readily and reproducibly determine sample genotypes (i.e., without influence of environmental factors), they have been used to efficiently introduce resistance in the Japanese rice breeding program (Ashkani et al. 2015, Hasan et al. 2015, Miah et al. 2013). The utility of a DNA marker depends on the degree of association between its genotype and the resistant/susceptible phenotype that is conditioned by that locus when it is tested against diverse cross combinations. If a certain polymorphism is distant from the target resistance locus or is in the resistance gene but does not determine the resistant/susceptible phenotype, that marker would work in limited cross combinations and/or its reliability would be low (Hayashi et al. 2010a). In addition, for multiple resistance alleles, researchers need to select multiple polymorphisms carefully to identify respective alleles (Wu et al. 2015, Yadav et al. 2017, Zhai et al. 2011). Finding polymorphisms that condition phenotypic differences, i.e., “functional polymorphisms” is one solution for the development of reliable markers (Hayashi et al. 2010a); however, design of such markers can be difficult in cases where (a) combinations of multiple polymorphisms determine phenotype (Fukuoka et al. 2014, Su et al. 2015, Xu et al. 2014b); or (b) the resistance gene contains highly conserved DNA sequences or resides in a genome region that has undergone frequent rearrangement events (Hayashi et al. 2010b, Takahashi et al. 2010, Wu et al. 2012, Zhou et al. 2007).
Associating phenotypic variation with haplotypes defined by a small number of marker loci is a practical approach for developing DNA markers for breeding in cases where marker loci are tightly linked with each other and predict the presence or absence of the resistance gene. This approach, termed haplotype-based association, is widely used for detecting genes associated with agricultural traits in crop plants (Contreras-Soto et al. 2017, Lorenz et al. 2010). In this approach, markers do not need to be based on functional polymorphisms when association between haplotypes and phenotype is validated in diverse genetic resources. Hence, researchers can find DNA markers without identifying functional polymorphisms and can use DNA markers in genomic regions where amplification and allelic discrimination are stable. However, to conduct genotyping at a comparable high-throughput level to that of the differential system developed by Hayashi et al. (2014), which targets 23 alleles, a set of 46 markers or more would need to be analyzed. Since the haplotype-based approach uses multiple markers per locus, reliable discrimination of blast resistance alleles requires a large number of markers. Use of a 96.96 Dynamic Array IFC (96.96 IFC) chip provided by Fluidigm Inc. (South San Francisco, CA, USA) solves this issue, because genotypes at 96 SNP marker loci for 96 samples can be obtained in one or two days (excluding DNA preparation time) (Thomson 2014, Wang et al. 2009). Under these circumstances, SNP haplotype-based genotyping is a promising alternative to determine blast resistance alleles in the Japanese rice breeding program.
Here, we undertook to use the Fluidigm SNP genotyping platform to develop a rapid instant DNA genotyping system for 10 race-specific blast resistance loci that include all the known resistance alleles used in the Japanese breeding program. The system was developed to include insertion/deletion (InDel) markers to select for certain resistance alleles at these loci, which could be useful in breeding programs. To assess the utility of the system, the genotypes of varieties with the indica genetic background, including those undetermined by inoculation-based differential systems, were determined.
Materials and Methods
Plant materials
To design and validate DNA markers for 24 blast resistance alleles, we used the rice varieties and lines listed in Table 1. They include donor varieties used for cloning of resistance genes (Ashkani et al. 2016, Koide et al. 2009), differential varieties for blast resistance (Kiyosawa 1984, Kobayashi et al. 2007, Telebanco-Yanoria et al. 2010, Tsunematsu et al. 2000, Yamada et al. 1976), and blast-resistant modern cultivars. To determine SNP and InDel marker haplotypes in the vicinity of resistance loci and to associate them with allelic variations at those loci, we used a set of rice varieties representing the wide range of accessions used in the Japanese rice breeding program (i.e., varieties used for staple food, rice cake, sake rice, and animal feed, including those categorized as upland rice, landraces, and cultivars of foreign origin) (Supplemental Table 1). To determine the resistance allele at the Piz locus in an indica cultivar ‘Nona Bokra’, two chromosome segment substitution lines (CSSLs), SL519 and SL521, were used. Both lines have a chromosome segment containing the Piz locus introduced from ‘Nona Bokra’ in the ‘Koshihikari’ genetic background (Takai et al. 2007). As a control for Piz-5 and Piz-t alleles (at the Piz locus), two monogenic lines (‘IRBLz5-CA’ and ‘IRBLzt-T-19F’) (Tsunematsu et al. 2000) and their recurrent variety ‘Lijiangxintuanheigu’ were used. Similarly, to determine resistance or susceptibility alleles for several SNP haplotypes at Pish, Pib, Piz, Pii, Pia, Pik, Pita, or Pita-2 loci, we used 6 to 14 CSSLs that each carry a target chromosomal region from diverse donors in a well-characterized genetic background (‘Koshihikari’) (Abe et al. 2013, Ebitani et al. 2005, Hori et al. 2015, Nagata et al. 2015a, 2015b, Takai et al. 2007, 2014). To discriminate alleles at the Pik locus, further inoculation tests were conducted by using 17 lines for Pik alleles and 19 blast isolates. For the direct sequencing of the Pita-2 locus, we used six varieties, which included ‘Ikuhikari’ carrying the Pita-2 allele; ‘Nipponbare’ carrying the Pi19 allele, which is allelic to Pita-2; and two cultivars (‘Yashiromochi’ and ‘Sasanishiki BL6’) and two monogenic lines (‘IRBLta-CP1’ and ‘IRBLta-CT2’) for the resistance allele at the Pita locus, which is located close to Pita-2.
Table 1.
Blast resistance alleles targeted in this study
| Locus | Allele | Cloned/Mapped | Chr. | Map position (bp) | RAPd (Os ID) | MSUe (LOC_Os ID) | Donor and cultivar or line carrying the allele | Reference | Notef |
|---|---|---|---|---|---|---|---|---|---|
| Pit | Pit | Cloned | 1 | 2682019–2684988 | Os01g0149500 | LOC_Os01g05620.1 | K59, Deng Pao Zhai | Hayashi and Yoshida 2009 | * |
|
| |||||||||
| Pish | Pish | Cloned | 1 | 33141127–33144999 | Os01g0782100 | LOC_Os01g57340.1 | Nipponbare, Koshihikari | Takahashi et al. 2010 | – |
| Pi35a | Cloned | As above | As above | Hokkai 188 | Fukuoka et al. 2014 | – | |||
|
| |||||||||
| Pib | Pib | Cloned | 2 | 35108842–35115834 | Os02g0818450 | LOC_Os02g57305.1 | BL1 | Wang et al. 1999 | * |
|
| |||||||||
| Piz | Piz | Mapped | 6 | 10372676–10471201 | Not available | Not available | Fukunishiki, Hanaetizen | Hayashi et al. 2006 | * |
| Piz-t | Cloned | Os06g0287000 | LOC_Os06g17920.1 | Toride 1, IRBLzt-T | Zhou et al. 2006 | * | |||
| Piz-5(Pi2) | Cloned | As above | As above | IRBLz5-CA[LT], IRBLz5-TA[LT] | Zhou et al. 2006 | – | |||
| Pi9 | Cloned | As above | As above | IRBL9-W[LT], IRBL9-W[US], Koshihikari Kanto BL1 | Qu et al. 2006 | * | |||
|
| |||||||||
| Pi13 | Pi13 | Mapped | 6 | 11677578–19614430b | Not available | Not available | Kasalath, Koshihikari Toyama BL7 | Ebitani et al. 2011 | * |
|
| |||||||||
| Pii | Pii | Cloned | 9 | 9667216–9674617 | Os09g0327600 | LOC_Os09g15840.1 | Fujisaka 5, Ishikarishiroke | Takagi et al. 2013 | * |
| Pi5 | Cloned | As above | As above | IRBL5-M[LT], IRBL5-M | Lee et al. 2009 | – | |||
| Pi3 | Mapped | Not available | Not available | IRBL3-CP4[LT], IRBL3-CP4 | Jeon et al. 2003 | – | |||
|
| |||||||||
| Pia | Pia | Cloned | 11 | 6542301–6561363 | Os11g0225100 | LOC_Os11g11790.1 | Sasanishiki, Aichiasahi | Okuyama et al. 2011 | * |
| Os11g0225300 | LOC_Os11g11810.1 | ||||||||
|
| |||||||||
| Pik | Pik | Cloned | 11 | 27978523–27988874 | no hit | LOC_Os11g46200.1 | Kusabue, Kanto 51 | Zhai et al. 2011, | * |
| Os11g0689100 | LOC_Os11g46210.1 | Ashikawa et al. 2012 | |||||||
| Pik-m | Cloned | As above | As above | Tsuyuake, Hinohikari Kanto BL2 | Ashikawa et al. 2008 | * | |||
| Pik-p | Cloned | As above | As above | K60, IRBLkp-K60 | Yuan et al. 2011 | * | |||
| Pik-s | Mapped | Not available | Not available | Shin 2, IRBLks-B40[LT], IRBLks-Zh[LT] | Fjellstrom et al. 2004 | * | |||
| Pik-h | Cloned | As above | As above | IRBLkh-K3, IRBLkh-K3[LT] | Zhai et al. 2014 | * | |||
| Pi7 | Mapped | Not available | Not available | IRBL7-M[LT] | Campbell et al. 2004 | – | |||
| Pi1 | Cloned | As above | As above | IRBL1-CL[LT] | Hua et al. 2012 | – | |||
|
| |||||||||
| Pita | Pita | Cloned | 12 | 10607519–10611770 | Os12g0281300 | LOC_Os12g18360.2 | Yashiromochi, IRBLta-CP1 | Bryan et al. 2000 | * |
|
| |||||||||
| Pita-2 | Pita-2 | Cloned | 12 | 10824087–10833494 | Os12g0285100 | LOC_Os12g18729.2 | PiNo.4, Ikuhikari | Takahashi et al. 2017 | * |
| Pi19 | Cloned | As above | As above | Nipponbare, Koshihikari | Takahashi et al. 2017 | – | |||
|
| |||||||||
| Pi20 | Pi20 | Mapped | 12 | 10081105–14181731c | Not available | Not available | IR 24 | Li et al. 2008 | – |
Chr., chromosome number.
Quantitative resistance allele at the Pish locus.
The interval delimited by marker loci R2123 and RM20155 in rough mapping.
The interval delimited by marker loci OSR32 and RM28050 in rough mapping.
Rice Annotation Project: http://rapdb.dna.affrc.go.jp/.
Michigan State University Rice Genome Annotation Project: http://rice.plantbiology.msu.edu/.
The alleles with asterisks (*) are required in the application for variety registration in Japan.
Detection and extraction of SNPs for design of genotyping assay
To detect candidates for the SNP genotyping assay for 10 loci with race-specific resistance to blast (Fig. 1), we used two methods in silico. In the first method, genomic or cDNA sequences of the resistance genes in the resistant or susceptible cultivars in the public databases, DDBJ (http://www.ddbj.nig.ac.jp/index-j.html) and NCBI GenBank (https://www.ncbi.nlm.nih.gov/genbank/), were aligned against the corresponding sequences in the japonica reference ‘Nipponbare’ IRGSP-1.0 reference genome sequence (Kawahara et al. 2013) and indica reference ‘IR64’ de novo assembly sequence (Schatz et al. 2014) by using ClustalW version 2.1 (http://clustalw.ddbj.nig.ac.jp/index.php?lang=ja) or Kalign (https://www.ebi.ac.uk/Tools/msa/kalign/) software. In the second method, whole genome re-sequencing data (mainly from NCBI SRA database; https://www.ncbi.nlm.nih.gov/sra/, but also our unpublished data) were mapped to the ‘Nipponbare’ IRGSP-1.0 sequence by using CLC Genomics Workbench v8.0 software (Qiagen, Hilden, Germany) with default parameter settings; only uniquely mapped reads with a mapping quality score of ≥20 were used in mapping. SNPs that were informative (i.e., could discriminate resistant and susceptible varieties) were extracted.
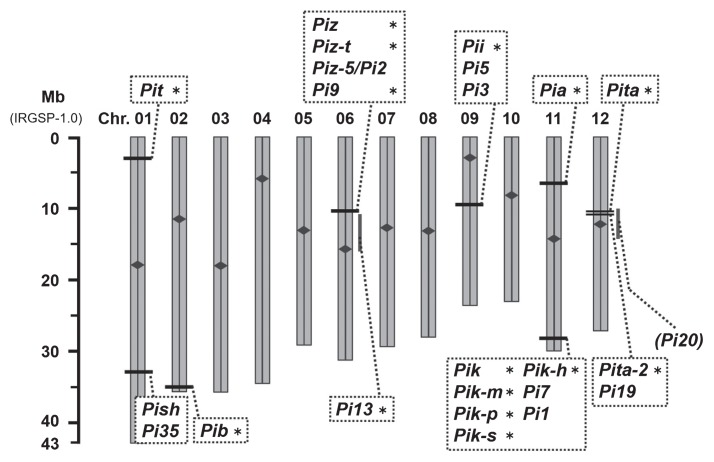
Fig. 1.
Positions of the 24 blast resistance alleles used for SNP genotyping assays and PCR-based markers on the rice chromosomes. Scale in Mb (‘Nipponbare’ IRGSP-1.0.) is indicated on the left. Positions of the resistance loci are indicated by horizontal bars; ranges for roughly mapped loci are indicated by vertical gray bars. For each chromosome (Chr.), the centromere is indicated by a rhombus. Alleles with asterisks (*) are required in the application for variety registration in Japan.
To distinguish Piz-t and Piz-5 (also known as Pi2), we used three SNPs in the Pi2 locus (Zhou et al. 2006) and SNPs between re-sequencing data of ‘Nona Bokra’ (Yonemaru et al. 2015), which had a similar haplotype to that of Piz-t–carrying ‘Toride 1’ variety at the Piz locus (Supplemental Table 1) and was confirmed to carry Piz-t by an inoculation test using CSSLs (Supplemental Table 2), and the corresponding sequences of Piz-5–carrying ‘C101A51’ (DQ352453).
Recently Pi19, which is allelic to Pita-2, was cloned (Takahashi et al. 2017). Here, we screened for polymorphisms in cDNA sequences of Pi19 among six varieties, a subset of which harbor alleles at the Pita-2 locus, by direct sequencing using a BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Waltham, MA, USA) and ABI 3730xl DNA analyzer (Thermo Fisher Scientific). Primers are listed in Supplemental Table 3. Sequences were assembled by using Sequencher 5.0.1 software (Gene Codes Corp., Ann Arbor, MI, USA) and aligned by using ClustalW 2.1.
Finally, 338 SNP genotyping assays based on allele-specific polymerase chain reaction (PCR) on the Fluidigm dynamic array platform were designed and oligonucleotides were synthesized by Fluidigm Inc. (Table 2).
Table 2.
Summary of the SNP genotyping assays designed in this study
| Locusa | Chr. | Number of alleles | Number of extracted SNPs | Number of qualified SNPs | Selected (Tested) assays | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Number | Distribution range (kb) | Distance (kb) | ||||||
|
| ||||||||
| from 5′ end of CDS | from 3′ end of CDS | |||||||
| Pit | 1 | 1 | 88 | 46 | 6 (34) | 8.8 (26.5) | 2.0 (12.1) | 3.8 (11.4) |
| Pish | 1 | 2 | 179 | 153 | 11 (42) | 189.1 (196.3) | 155.4 (155.4) | 29.8 (37.0) |
| Pib | 2 | 1 | 62 | 47 | 9 (26) | 49.0 (49.7) | 19.2 (20.0) | 22.7 (22.7) |
| Piz | 6 | 4 | 160 | 74 | 12 (38) | 66.5 (76.6) | 11.5 (11.5) | −43.4 (−33.4)c |
| Pi13 | 6 | 1 | 80 | 58 | 9 (37) | 144.7 (165.8) | 64.4 (85.5) | 77.8 (77.8) |
| Pii | 9 | 3 | 81 | 74 | 9 (29) | 202.6 (208.0) | 9.8 (15.3) | 185.3 (185.3) |
| Pia | 11 | 1 | 51 | 44 | 8 (24) | 185.7 (196.8) | 20.9 (32.0) | 145.7 (145.7) |
| Pik | 11 | 7 | 119 | 106 | 20 (67) | 188.4 (314.2) | 160.6 (281.3) | 17.4 (22.5) |
| Pita | 12 | 1 | 75 | 44 | 6 (24) | 28.0 (62.9) | 0.0 (34.9) | 23.7 (23.7) |
| Pita-2 | 12 | 2 | 87b | 79 | 6 (17) | 51.8 (54.5) | 22.8 (22.8) | 19.6 (22.3) |
|
| ||||||||
| Total | 23 | 982 | 725 | 96 (338) | ||||
Chr., chromosome number; CDS, coding sequence.
Genotyping assays for Pi20 are included in those for Pita and Pita-2 loci.
Includes one insertion–deletion polymorphism in the Pita-2 gene.
Negative values indicate that the assay at the 3′ end of the target region is located upstream of the CDS (see Supplemental Fig. 4).
DNA preparation
Genomic DNA was prepared from fresh leaves by one of two methods. In the first method, 1- to 3-cm leaf sections of 2- to 3-week-old seedlings were disrupted in 30 μL 0.5 M NaOH by using a Multi-Beads Shocker (Yasuikikai, Osaka, Japan), and then added to 120 μL 1 M Tris-HCl (pH 8.0) for neutralization. After centrifugation (1500 rpm for 1 min), the supernatant was directly used as a template. In the second method, a minor modification of the method reported by Monna et al. (2002), 3- to 4-cm leaf sections of a 2- to 3-week-old seedling were disrupted in 300 μL TPS buffer (100 mM Tris-HCl, 1 M KCl, 10 mM EDTA, pH 8.0) by using a Malti-Beads Shocker, and then centrifuged. From the resulting supernatant, DNA was precipitated by addition of 2-propanol, washed with 70% ethanol, and dissolved in 50 μL 0.1× TE buffer (1 mM Tris-HCl, 0.1 mM EDTA, pH 8.0). DNA samples prepared by the first method were used for InDel markers, and those prepared by the second method were used for SNP genotyping.
SNP genotyping
SNP genotypes were determined by using the 96.96 Dynamic Array IFC (96.96 IFC) chip according to the ‘SNPtype 96×96 v1’ protocol except that the number of additional cycles after touchdown PCR was reduced from 34 to 30. Scanned data obtained with an EP1 reader (Fluidigm Inc.) were analyzed with SNP genotype analysis software (Fluidigm Inc.) and converted to scatter plot diagrams and allele data. SNP genotyping assays that gave clear and stable plot diagrams were selected to produce the set of 96 SNPs used in further experiments (Table 2).
Assignment of resistance alleles in tested varieties
Resistance alleles at respective resistance loci in tested varieties were determined based on the identity between their SNP haplotypes and those of differential varieties. Public information on resistance alleles at race-specific resistance loci in Japanese commercial cultivars, mostly in a japonica genetic background (Plant variety protection database of the Ministry of Agriculture, Forestry and Fisheries, http://www.hinshu2.maff.go.jp/; Rice variety database of the Institute of Crop Science, National Agriculture and Food Research Organization, http://ineweb.narcc.affrc.go.jp/), was used to confirm the validity of the respective SNP haplotypes. The resistance alleles of the SNP haplotypes that were not found in the differential varieties were assigned by using public information pertaining to blast resistance of the varieties or by conducting inoculation tests. The resistance alleles for SNP haplotypes that lack information on resistance phenotypes, i.e., 5 SNP haplotypes at Pish locus; 10 at Pib locus; 8 at Piz locus; 6 at Pii locus; 10 at Pia locus; 9 at Pik locus; 3 at Pita locus; and 4 at Pita-2 locus were determined by inoculation tests using CSSLs carrying respective SNP haplotypes and reference varieties (Supplemental Table 4). Presence or absence of resistance alleles at the various loci listed below were determined from the results of the challenge by differential blast isolates: Kyu77-07A for Pish; Ina86-137 for Pib; IW81-04, Ai74-134, Ken54-20, Ina86-137, Ken54-04, 24-22-1-1, Ina91-10, K59, and H97-227-1 for Piz; Ken54-20 for Pii; Mu-95 for Pia; V850196, P-2b, H05-99-1, and the other 17 isolates for Pik (Supplemental Table 5); and CHNOS58-3-1, C08, CHNOS121-2-4, and PH77-111-1 for Pita-2, Pita, and Pi20.
Design of InDel markers and genotyping
To facilitate discrimination by electrophoresis using a 4% (w/v) agarose gel, InDel variations larger than 10 bp and less than 500 bp were selected by the procedures outlined above for SNP detection. Primer pairs for each InDel were designed using Primer 3 software (Rozen and Skaletsky 2000) with the default settings except for the following: optimum product size, 85 to120 bp (range, 85 to 500); optimum primer melting temperature, 59°C or 63°C (range, 50°C to 70°C); optimum primer size, 22-mer (range, 18- to 28-mer); and optimum primer GC%, 50 (range, 20 to 80). To distinguish Pita from other alleles, two SNPs in the Pita gene (Bryan et al. 2000) were used as SNP markers. PCR with confronting two-pair primers (PCR-CTPP) was used to develop markers for Pita. All marker information is listed in Supplemental Table 6.
Each PCR was conducted in a 10 μL reaction mixture containing 0.5 μL DNA, 0.4 μM primers, 1× GoTaq Green Master Mix (Promega, Madison, WI, USA) with initial denaturation at 94°C for 1 min, followed by 35 cycles of 94°C for 15 s, 55°C or 60°C for 30 s, and 68°C for 1 min, and a final extension at 68°C for 5 min, using a thermal cycler ProFlex PCR system with 2× 384-well blocks (Applied Biosystems, Foster City, CA, USA). The PCR products were separated on 4% agarose gels with a 3:1 ratio of agarose Type I-A (Sigma-Aldrich, St. Louis, MO, USA): Metaphor agarose (Lonza, Basel, Switzerland) in 0.5× TBE buffer; the gels were stained with ethidium bromide and photographed under a transilluminator with a UV lamp.
Results
Development of SNP genotyping assays
To design SNP genotyping assays for 10 blast resistance gene loci, 982 polymorphisms were extracted in silico (Table 2); these included one InDel to discriminate between Pita-2 and Pi19 at the Pita-2 locus. Of these, we excluded 198 SNPs (20.2% of the total) that were located in multi-hit sequences in a BLASTN search against the ‘Nipponbare’ genome, and 59 SNPs (6.0%) that were judged unsuitable on the basis of proximal sequences (e.g., presence of other polymorphisms or lack of sequence information in some reference varieties). From the resultant 725 SNPs (73.8%), we selected representative SNPs that were closest to the resistance loci and gave identical allelic distribution patterns in blast differential varieties. Finally, we obtained 338 SNPs for SNP genotyping assays. The distribution of the SNP markers for resistance loci ranged from 26.5 kb (Pit) to 314.2 kb (Pik) (mean, 135.1 kb) (Table 2, Supplemental Figs. 1–10).
Validation and selection of representative genotyping assays by using blast differential varieties
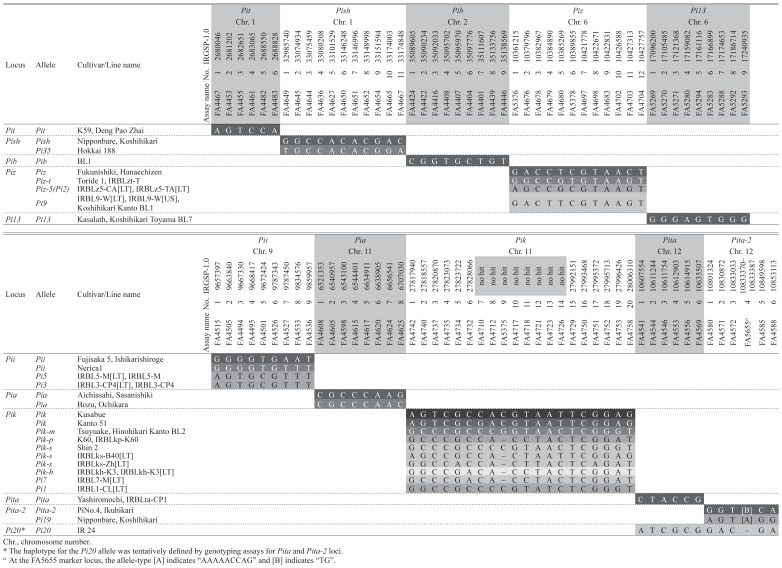
To confirm the discriminating ability of SNP genotyping performed using the Fluidigm 96.96 IFC chip platform, the 338 assays were tested on DNA samples from blast differential varieties. On the basis of signal intensity, allelic signal balance of respective assays, and the discriminating ability of the SNP haplotypes, we selected a set of 96 genotyping assays designated as ‘Blast resistance gene-assays version 1 (BRA1)’. These assays distinguished the 23 resistance alleles, except for Pi5 vs. Pi3 at the Pii locus (Supplemental Table 7). At the Pik locus, two genome types (“N-type” and “K-type”) had extremely low sequence similarity to each other (Ashikawa et al. 2008 and Supplemental Fig. 8C). To discriminate seven resistance alleles in K-type genomes, the set of 96 assays included 8 assays (FA4710, FA4712, FA5375, FA4717, FA4718, FA4721, FA4723, and FA4726) that produced signals for K-type genomes but not N-type genomes. In these 8 assays, genotypes in about half of the tested varieties were judged as “No Call” (labeled “-” in allele type). The number of the assays per locus in BRA1 ranged from 6 (Pit, Pita, and Pita-2) to 20 (Pik) (Table 2, Supplemental Figs. 1B–10B). The distribution range of the assays for resistance loci in BRA1ranged from 8.8 kb (Pit) to 202.6 kb (Pii) (mean, 111.4 kb; Table 2, Supplemental Figs. 1B, 1C–10B, 10C). The distribution of the SNP markers differed among loci in terms of distance from the coding sequences of the resistance loci; in particular, at the Pish, Pii, Pia, and Pik loci, some SNPs were located more than 140-kb upstream or downstream of the resistance gene, owing to unsuitable genome structure (lower sequence similarity among varieties or abundance of multi-copy sequences) close to the locus (Table 2, Supplemental Figs. 2, 6, 7, 8). The haplotypes for resistance alleles obtained by using BRA1 are listed for each locus in Table 3. At the Pii locus, two SNP haplotypes were detected among the varieties that carry Pii allele (‘Fujisaka 5’ and ‘Ishikarishiroge’ vs. ‘Nerica 1’); these were discriminated by two assays, FA4527 and FA4533. Similarly, we identified seven SNP haplotypes for the Pia allele by assay FA4598, two SNP haplotypes for the Pik allele (‘Kusabue’ vs. ‘Kanto 51’) by assay FA4710, and seven SNP haplotypes for Pik-s allele by a combination of SNP assays. Further inoculation tests using additional differential rice lines and pathogen isolates to characterize SNP haplotypes at the Pik locus showed that the lines with the SNP haplotypes Pik_H12 and Pik_H04-3 had different blast response patterns from those of known resistance alleles, despite variations in response among certain differential lines that shared the same SNP haplotype (Supplemental Table 5).
Table 3.
Haplotypes of each resistance locus in the donor varieties
The genotypes of the marker locus FA4541, which targets the functional polymorphism at the Pita locus, were identical between varieties carrying the Pita-2 allele and those carrying the Pita allele. At the Pita and Pita-2 loci, three SNP haplotypes were detected; the genotypes detected by FA5655 assay distinguished between the presence of the Pita-2 allele and its absence (including presence of allelic Pi19) (Fig. 2). Another SNP haplotype (Pita_H02 and Pita-2_H04) was observed in a rice variety (‘IR24’) carrying the Pi20 resistance allele whose locus has been roughly mapped to near the Pita-2 locus (Li et al. 2008).
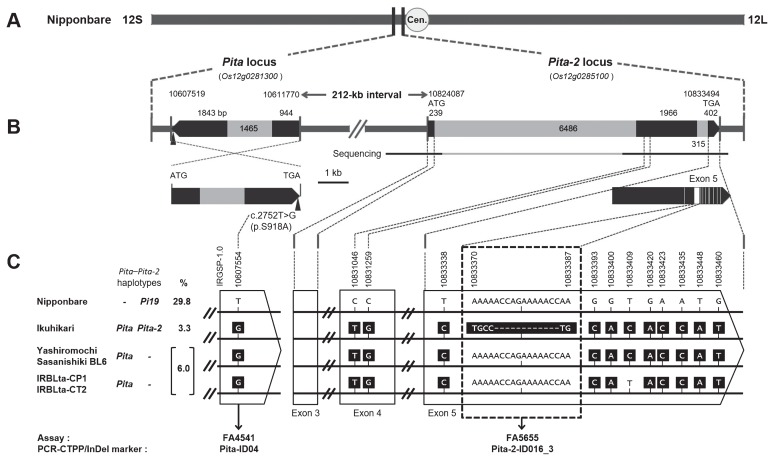
Fig. 2.
Identification of polymorphisms to discriminate Pita-2 from Pi19 and frequency of various Pita–Pita-2 haplotypes among Asian cultivated rice. (A) Positions of the Pita (Os12g0281300) and Pita-2 (Os12g0285100) loci on chromosome 12 in ‘Nipponbare’ IRGSP-1.0. 12S, short arm side of chromosome 12; 12L, long arm side of chromosome 12; Cen., centromere. (B) Gene structures of Pita and Pita-2 in ‘Nipponbare’. Black, coding regions; gray, introns. The upward arrowhead on Pita represents the position of the functional nucleotide polymorphism described previously (Bryan et al. 2000). For Pita-2, the horizontal black lines labeled “Sequencing” indicate regions where the DNA sequences of reference varieties were determined by direct sequencing, and the regions in white within exon 5 represent sites containing polymorphisms between ‘Nipponbare’ (Pi19) and ‘Ikuhikari’ (Pita-2). (C) Pita–Pita-2 haplotypes in the six cultivars, functional nucleotide polymorphisms of Pita (detected by allele-specific markers FA4541 and Pita-ID04) and polymorphisms that discriminate between Pita-2 and Pi19 (detected by allele-specific markers FA5655 and Pita-2-ID016_3). Frequency of each of the three DNA haplotypes among tested samples (Supplemental Table 1) is indicated as a percentage. The sequences within the dotted line box show variations that specifically discriminate the Pita-2 allele (‘Ikuhikari’) from the other alleles (Pi19 and susceptibility allele).
Application of BRA1 to diverse rice varieties
To test the selected genotyping assays in diverse rice samples, genotypes of a total of 369 varieties were determined with BRA1 (Supplemental Table 1). After preparation of sample DNA, we successfully determined genotypes of 192 varieties within 2 days; SNP haplotypes at 10 blast resistance loci were determined (Table 3, Supplemental Table 4); SNP haplotypes that are absent in Table 3 were found mostly in cultivars of foreign origin, Japanese upland rice, landraces, or some indica varieties. The number of SNP haplotypes at each locus ranged from 7 (Pit) to 29 (Pik). Most SNP haplotypes were judged as a susceptibility allele based on previously available information on resistance gene genotypes of varieties; this was confirmed here by inoculation test (Supplemental Table 4). The rate of concordance of the genotypes for blast resistance between the present study and publicly available data in japonica varieties was 93.5% (Supplemental Table 1). To increase the high-throughput capabilities of the system for future studies, we selected a “core set” consisting of 35 assays from BRA1 (Supplemental Table 4, Supplemental Figs. 1B–10B). Although this core set was unable to discriminate all the SNP haplotypes detected in the present study, it was sufficient to determine the presence or absence of all the 24 resistance alleles used in the present study.
Application of BRA1 to Japanese high-yielding varieties
To comprehensively summarize the resistance genotypes of Japanese high-yielding varieties with mainly indica genetic backgrounds, we used BRA1 to genotype 23 varieties selected across the country from north to south (Table 4). Our data include information on four alleles (Pish, Pik-s, Pi19, and a new allele represented by the SNP haplotype Pik_H04-3) that are absent from public databases for variety registration (http://www.hinshu2.maff.go.jp/, http://ineweb.narcc.affrc.go.jp/). The number of estimated resistance alleles at 10 loci among these varieties ranged from 2 (e.g., ‘Kitaaoba’ and ‘Kusahonami’) to 5 (‘Iwaidawara’) (mean, 3.9). The resistance genotypes estimated in our study were compared with those determined by inoculation tests (Table 4). When we compared our data to those in the public databases, in which the genotypes of three varieties were undetermined, the rate of concordance in resistance loci ranged from 45% (Pia) to 100% (Pit, Pib, and Piz) (mean, 88%; Table 4). When we compared our data to an advanced differential system that allows estimation of the genotypes for Pish, Pik-s, Pi19, and Pi20 (Hayashi et al. 2014), the rate of concordance in resistance loci ranged from 44% (Pia) to 100% (Pit, Pish, Pib, Piz, Pii, and Pita), (mean, 88%; Table 4); the second highest number of mismatches was at the Pita-2 locus.
Table 4.
Estimation of race-specific resistance alleles in Japanese high-yielding varieties
| Cultivar name | Alleles estimated by SNP genotyping assays | Discrepancy in genotype estimate betweeen present study and public databasesa | Discrepancy in genotype estimate betweeen present study and Hayashi et al. (2014) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||||||
| Pit | Pib | Piz | Pii | Pia | Pik | Pita | Pita-2 | Pit | Pish | Pib | Piz | Pii | Pia | Pik | Pita | Pita-2 | |||
| Kitaaoba | Pish Pi19 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||||||||
| Kitamizuho | Pish Pia Pik Pi19 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||||||||
| Tachijobu | Pii Pia Pi19 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||||||||
| Bekogonomi | Pish Pib Pia Pik Pi19 | pia | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||||||||
| Natsuaoba | Pish Pib Pia Pik-s Pi19 | pia | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||||||||
| Iwaidawara | Pish Pib Pia Pik Pi19 | pia | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||||||||
| Fukuhibiki | Pish Pib Pia Pi19 | ||||||||||||||||||
| Bekoaoba | Pia Pik-s Pita Pita-2 | pia | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||||||||
| Yumeaoba | Pish Pib Pia Pik-s Pi20 | pia | Pita-2 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||||||
| Kusayutaka | Pish Pia Pik-m Pi19 | Pik | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||||||||
| Tachisugata | Pib Pii Pia Pik-s Pi19 | pii | pia | ||||||||||||||||
| Takanari | Pib Pia Pik-s Pi20 | ND | ND | ND | ND | ND | ND | ND | ND | ||||||||||
| Hoshiaoba | Pish Pib Pia Pik-m Pi20 | pia | pik-m | Pita-2 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||||||
| Mochidawara | Pib Pi5/Pi3 Pia New gene (Pik_H04-3) Pi20 | ND | ND | ND | ND | ND | ND | ND | ND | pia | |||||||||
| Hokuriku 193 | Pi5/Pi3 Pia Pik-s Pi20 | Pii | pia | pia | Pi19 | ||||||||||||||
| Momiroman | Pish Pib Pia Pik-s Pi20 | pia | Pita | pia | pik-s | Pi19 | |||||||||||||
| Nishiaoba | Pish Pia Pik-m Pi19 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||||||||
| Kusahonami | Pik-s Pi20 | ND | ND | ND | ND | ND | ND | ND | ND | Pik or Pk-p | |||||||||
| Kusanohoshi | Pib Pia Pi20 | pia | Pita | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||||||
| Hamasari | Pish Pia Pi19 | pia | pi19 | ||||||||||||||||
| Leafstar | Pish Pi19 | Pia | Pia | ||||||||||||||||
| Tachisuzuka | Pib Pi20 | Pita | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||||||||
| Tachiaoba | Pish Pii Pia Pi19 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||||||||
|
| |||||||||||||||||||
| Match rate (%) | 100 | 100 | 100 | 90 | 45 | 90 | 85 | 90 | 100 | 100 | 100 | 100 | 100 | 44 | 78 | 100 | 67 | ||
ND, not determined through lack of information in the public databases.
Alleles in public databases that differ from those in the present study; genotypes beginning with a lowercase letter indicate susceptibility alleles.
Development of InDel markers for genotyping 10 resistance loci
To establish a genotyping workflow for practical breeding programs, we developed a total of 172 InDel markers for 10 loci, containing two PCR-CTPP markers (Pita-ID03 and Pita-ID04) targeting the two SNPs in the Pita gene. The number of the designed markers per locus ranged from 6 (Pia) to 38 (Piz) (mean, 17.2). Based on the SNP haplotypes obtained by BRA1 (Supplemental Table 4), a total of 22 or 23 varieties were genotyped for each locus. Finally, 155 out of 172 markers that gave stable amplification were selected (Supplemental Table 6). The number of the selected markers per locus ranged from 6 (Pia) to 27 (Piz) (mean, 15.5). In the case of Pit, the markers were located in the target gene and adjacent regions. In other loci, markers were located in chromosomal regions that ranged in length from 121.9 kb (Piz) to 385.8 kb (Pi13) (mean, 231.5 kb). The product size of the InDel markers in the ‘Nipponbare’ reference genome IRGSP-1.0 ranged from 80 bp to 779 bp (mean, 133.7 bp) The variation in amplicon size for different alleles at the same locus ranged from 6 bp to 430 bp (mean, 33.7 bp). These variations were readily distinguishable by 4% agarose gel electrophoresis. The number of alleles per marker locus ranged from 2 to 5, including alleles that were not amplified by PCR (Supplemental Table 6). Genotypes obtained by using the markers in the selected varieties are shown together with the SNP haplotypes obtained by SNP genotyping assays in Supplemental Fig. 11-1A–11-10A.
Selection of an InDel marker set to discriminate the resistance alleles
To select a set of InDel markers that efficiently discriminate resistance alleles, haplotypes at resistance gene loci obtained by using InDel markers in 22 or 23 varieties were compared with SNP haplotypes at the corresponding resistance loci (Supplemental Fig. 11-1A–11-10A). At the Pit locus, all 7 SNP haplotypes (Pit_H01 to H07) were discriminated by 16 InDel markers (Supplemental Fig. 11-1A). At the Pish locus, 7 out of 16 SNP haplotypes (exceptions being Pish_H05, H08, and H10 to H16) were discriminated by 14 InDel markers (Supplemental Fig. 11-2A). Similarly, at the other resistance loci, SNP haplotypes for resistance alleles were discriminated from other resistance alleles at the same locus or from major SNP haplotypes that represent susceptibility alleles. Importantly, the haplotypes for a certain resistance allele were distinguished from those for the susceptibility allele or other resistance alleles at the same locus by using a single marker or a combination of 2 to 5 markers at each of the 10 loci: e.g., at the Pib locus (Supplemental Fig. 11-3A), the resistance allele represented by haplotype Pib_H01 was distinguished from the others by the marker Pib-ID11 alone. With respect to the Pita and Pita-2 resistance alleles, which lie at a distance of 212 kb from each other, the markers for both alleles (Pita-ID04 and Pita-2-ID016_3) are required to identify varieties having Pita allele but not Pita-2 allele (Fig. 2). Finally, from the 155 markers described above, we selected 23 markers, including 22 InDel and 1 PCR-CTPP, that clearly and efficiently discriminated the resistance alleles by electrophoresis in 4% agarose gel (Table 5, Supplemental Fig. 11-1B–11-10B). The number of the selected markers per locus ranged from 1 (Pit, Pib, Pi13, and Pia) to 5 (Pik). We note that these 23 markers could not distinguish between the following resistance alleles: Piz-t vs. Piz-5, Pik-m vs. Pik-s, and Pik-h vs. Pi7.
Table 5.
Selected markers for 21 allele types at the 10 blast resistance loci
| Locus | Marker name | Primer sequences (5′ to 3′) | Marker type | Temp. (°C)b | Gel (%)b | Genotype A (‘Nipponbare’ type)c | Genotype Bc | Genotype Cc | Allele types distinguishable by marker combinations | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Forward | Reverse | Size (bp) | Allele type | Size (bp) | Allele type | Size (bp) | Allele type | ||||||
| Pit | Pit-ID003 | CCTGAAACACATTATCTATGTTG | AGAAAGAAGCATAAGTTTAAAATAGA | InDel | 55 | 4 | 160 | 190 | Pit | 1) Pit | |||
|
| |||||||||||||
| Pish | Pish-ID007 | TACACCGCTCGGCTTTTCACC | ATGCCCCTCGTTGCAGCC | InDel | ↓ | ↓ | 100 | Pish or Pi35 | 87 | 1) Pish 2) Pi35 |
|||
| Pish-ID010 | TGCTACATATATATGATAATTGTCGAGG | TCAATCTACACCGTTAGATCAT | InDel | 50 | ↓ | 94 | Pish | 81 | Pi35 | ||||
| Pish-ID011 | AGCACTTGACACTCCACAGCAG | GGCAAAACCCGTGTTTCTGACG | InDel | 55 | ↓ | 102 | Pish | 116 | Pi35 | ||||
|
| |||||||||||||
| Pib | Pib-ID11 | AGAGTGGTTGGTTGGAGGTG | GCCCATATTGCTTTGCTCCAAA | InDel | ↓ | ↓ | 103 | 92 | Pib | 1) Pib | |||
|
| |||||||||||||
| Piz | Piz-ID18 | CTGCTGCTACCGTTTGGAAGTCA | CTCCTGGCCCCACGCGTC | InDel | ↓ | ↓ | 98 | Piz-t, Piz-5, or Pi9 | 84 | Piz | 1) Piz 2) Piz-t or Piz-5 3) Pi9 |
||
| Piz-ID22 | ATGTGGGGTTTCTGATTCCAAT | CTTGATTAGTGAGATCCATTGTTCC | InDel | ↓ | ↓ | 127 | Piz | 100 | Piz-t or Piz-5 | 118 | Pi9 | ||
| Piz-ID31 | CCAATTTCAGGCTTAGTTTGAT | AGCTATTTATTAAGCTGATTTCTCA | InDel | ↓ | ↓ | 107 | Piz or Pi9 | 84 | Piz-t or Piz-5 | ||||
|
| |||||||||||||
| Pi13 | Pi13-ID008 | GTGAGCTGGAATACTAGATCGA | GTCAAAGTTCCTGCAATTTTGTGA | InDel | ↓ | ↓ | 114 | 88 | Pi13 | 1) Pi13 | |||
|
| |||||||||||||
| Pii | Pii-ID07 | TTCGGTCATTAGCCGGTGCT | GGCGGCAGGTATGGTACTTCA | InDel | ↓ | 3 or 4 | 450 | 289 | Pii, Pii*, Pi5, or Pi3 | 493 | 1) Pii 2) Pii* 3) Pi5 or Pi3 |
||
| Pii-ID21 | AAGCGAACGACTCTAGCTAGAA | TCTCCATATGTATGTATAACTGGCTT | InDel | ↓ | 4 | 84 | Pii* | 96 | Pii, Pi5, or Pi3 | ||||
| Pii-ID24 | ATGAGGAGATGACAACGAGGAG | GAAGAGGGGAACGCCGAG | InDel | ↓ | ↓ | 100 | Pii*, Pi5, or Pi3 | 88 | Pii | ||||
|
| |||||||||||||
| Pia | Pia-ID01_2 | ACGGTAGAGCAATTTAGAAGCAGTGA | AGTGCGACTGACACTTTCAATAGCA | InDel | 55 | ↓ | 195 | 152 | Pia | 1) Pia | |||
|
| |||||||||||||
| Pik | Pik-ID001 | CTTCTTAGCCTCCAGATTTGCA | TCATGTGCATCAAAATGGGCTA | InDel | ↓ | ↓ | 100 | Pik, Pik-h, Pi7, or Pi1 | 88 | Pik-m, Pik-p, or Pik-s | 1) Pik 2) Pik-m or Pik-s 3) Pik-p 4) Pi1 5) Pik-h or Pi7 |
||
| Pik-ID007 | AACGAATATTTATGACTAAAGAAAGT | AGAAGCTTGACTCCGTTAG | InDel | ↓ | ↓ | 120 | Pik | 406 | Pik-m, Pik-p, Pik-s, Pik-h, Pi7, or Pi1 | ||||
| Pik-ID011 | GGTTAAATAGGACTCCCTCTA | GCATCCAATAGAATCAGAGA | InDel | ↓ | ↓ | 169 | Pik, Pik-m, Pik-s, or Pi1 | 150 | Pik-p, Pik-h, or Pi7 | ||||
| Pik-ID014 | TTCTTCTTTATCCCGTCTTCTT | ATGAGGAAAACGAAGATGAGAG | InDel | ↓ | ↓ | 344 | Pik, Pik-p, Pik-h, or Pi7 | 149, 150 | Pik-m, Pik-s, or Pi1 | ||||
| Pik-ID018 | ATCCTCTGTGTCTGAAGCCAT | AGGCTTCTCTGCTCCTATAACA | InDel | ↓ | ↓ | 110 | Pik, Pik-m, Pik-p, Pik-s, Pik-h, Pi7, or Pi1 | 100 | |||||
|
| |||||||||||||
| Pita | Pita-ID13 | AGGCAAGAGTACAATGGAAAC | TGCCCTCTGAAAATAAAGTTT | InDel | ↓ | ↓ | 100 | Pita | 112 | (Pi20) | 1) Pita 2) (Pi20) |
||
| Pita-ID04a | CGTGAAGAGGATTCCGGTAGCA | TGCCGTGGCTTCTATCTTTACgTT | PCR-CTPP | ↓ | ↓ | 137 | (Pi20) | 216 | Pita | ||||
| CAAGTCAGGTTGAAGATGCATtGC | CCGACGCCGAGCACTCTTAT | ||||||||||||
|
| |||||||||||||
| Pita-2 | Pita-2-ID009 | CACATAGCATTAGAGCACTAAACT | AACCATCATACCGGCCTATTTA | InDel | ↓ | ↓ | 102 | Pi19 or Pita-2 | 114 | (Pi20) | 1) Pita-2 2) Pi19 3) (Pi20) |
||
| Pita-2-ID016_3 | AAAGTTCATCGCATCGAATTTA | ACACGCGTTGGGATCTTCCTC | InDel | 50 | ↓ | 135 | Pi19 or (Pi20) | 123 | Pita-2 | ||||
| Pita-2-ID011 | TGCAATTAGTCTGGTGTTGTTGA | ACATGAATCAGTCTGACGTCAT | InDel | 55 | ↓ | 115 | Pi19 | 85 | Pita-2 | 104 | (Pi20) | ||
Pii allele type with an asterisk (‘Nerica 1’) differs in marker genotypes from that in the reference varieties for Pii (‘Fujisaka 5’ and ‘Ishikarishiroke’) despite its typical reaction against differential pathogen races.
Lowercase letters in primer sequences indicate mismatch bases against target complementary sequences. PCR-CTPP, polymerase chain reaction with confronting two-pair primers to distinguish co-dominant markers (Hamajima et al. 2000). PCR conditions for this marker: 95°C for 2 min, followed by 35 cycles of 94°C for 30 s, 55°C for 1 min 30 s, 72°C for 1 min, and finally 68°C for 5 min.
The condition with a downward arrow is the same as the one above. Temp., annealing temperature; Gel, agarose gel.
Genotype A represents the ‘Nipponbare’-type, and B and C represent genotypes that differ from each other and from genotype A in terms of product size.
The genotype A obtained by the three markers (Pik-ID007, Pik-ID011, and Pik-ID014) represents the genotype of ‘Kusabue’ (HM048900) or ‘Kanto 51’ (AB616659), whose product sizes are indicated in gray boxes.
The genotype B obtained by the marker Pik-ID014 includes amplicons of two sizes that could not be clearly discriminated in an 4% agarose gel.
Discussion
Here we demonstrate the use of SNP haplotypes of regions containing race-specific blast resistance loci to estimate the presence or absence of resistance alleles among diverse rice varieties. Assays for a set of 96 SNPs (BRA1) selected for analysis by using Fluidigm 96.96 IFC chip could efficiently determine 24 resistance alleles at 10 loci in one round of experiment. Additionally, we developed InDel markers for 10 resistance loci that distinguish 20 resistance alleles from respective susceptibility alleles. These InDel marker sets would be useful for selecting for resistance alleles in a wide range of breeding populations after the resistance alleles of the parents were determined by using BRA1. Thus, our DNA-based marker system could be used to comprehensively and rapidly identify genotypes of blast resistance loci, and provides a cost-effective genotyping workflow for practical breeding programs.
Reliability and efficiency of identifying plants carrying desirable resistance alleles determines the efficiency of breeding. Notably, five (Pish, Piz, Pii, Pik, and Pita-2) of the ten loci tested in our study have multiple resistance alleles; discrimination of such alleles from each other by using inoculation testing or by DNA markers requires more labor than for loci with single resistance alleles (Ashikawa et al. 2008, 2012, Fukuoka et al. 2014, Hua et al. 2012, Lee et al. 2009, Qu et al. 2006, Su et al. 2015, Takagi et al. 2013, Takahashi et al. 2010, 2017, Yuan et al. 2011, Zhai et al. 2011, 2014, Zhou et al. 2006). The PCR-based (SNP or InDel) markers previously developed for Piz and Pik resistance loci discriminated two to six resistance alleles (Hayashi et al. 2006, Wu et al. 2015, Yadav et al. 2017, Zhai et al. 2011). Those studies selected markers that discriminate respective resistance alleles in pairs of donor varieties, but did not confirm that the marker loci selected were suitable for discrimination of alleles among diverse genetic resources. This potential lack of reliability would limit the use of these markers in a wide range of breeding programs. A recent study has validated the use of four markers (i.e., for alleles Pia, Pii, Pik, and Pik-m) among diverse varieties or breeding lines (Nonoue et al. 2018); however, this limited number of markers would not be sufficient to comprehensively determine required resistance alleles for variety registration. Here, we successfully developed SNP markers for 24 resistance alleles at 10 loci and validated their ability to discriminate all the multiple resistance alleles from each other, except for Pi3 vs. Pi5 (Table 3). Since Pi3 and Pi5 have not been used in the Japanese rice breeding program, our system covers all the resistance alleles that are required for cultivar registration in Japan. In addition, we developed 155 PCR-based markers (153 InDel and 2 PCR-CTPP markers); a subset of 23 of these PCR-based markers was sufficient to discriminate almost all resistance alleles from the counterpart susceptibility alleles. These markers are useful for selecting plants based on single resistance alleles even in large-scale breeding populations (Table 5, Supplemental Table 6, Supplemental Fig. 11-1–11-10). After analysis of parental lines by BRA1, loci that are segregating in respective populations can be selected by PCR-based markers. Therefore, these two estimation systems are a precise and efficient means for selecting genes for blast resistance from early generations onwards in breeding programs.
Haplotype-based association is a widely used approach for gene detection among crop plant varieties (Contreras-Soto et al. 2017, Gawenda et al. 2015, Lorenz et al. 2010). We applied this approach to resistance alleles by using multiple SNP markers around respective resistance gene loci and confirmed its applicability among the varieties tested (Supplemental Table 1). Our study successfully discriminated even among resistance alleles with highly similar DNA sequences that were found in the “K-type” genome at the Pik locus (Supplemental Fig. 8D). We carefully selected SNPs to discriminate resistance alleles located in a region containing multiple NBS-LRR (nucleotide-binding site–leucine-rich repeat) protein encoding genes, which were highly variable in number owing to genome rearrangement events in that region among diverse rice varieties. Key to the effective discrimination of the alleles at the Piz locus was the identification of combinations of allele-specific SNPs in single copy sequences that were conserved enough to among varieties to enable the design of genotyping assays or primers (Supplemental Fig. 4C). As mentioned above, DNA markers previously reported for the Piz and Pik loci have not been validated among diverse varieties; so, the user would need to confirm the utility of respective markers in their own cross combinations. We extended our evaluation system from the discrimination of reference varieties to the validation of haplotypes found in diverse rice varieties. Users can readily select suitable marker combinations from these haplotypes, which are listed in Supplemental Table 4. An important observation from our study is the identification of varieties that show the same response as reference varieties for certain alleles against a set of differential pathogen strains but have different SNP haplotypes at the Pii, Pia, and Pik loci. Since varieties that differ in SNP haplotypes are considered to have distinctive origins, the resistance alleles therein might have functional variations that have not been identified by conventional differential strain systems. Hence, the systematic identification of haplotypes suggests candidate varieties that require further pathogenic characterization (e.g., ‘Nerica 1’ for the Pii locus and ‘Kanto 51’ for the Pik locus); such trials would contribute to further improvement of differential systems. We validated the resistance genotypes by inoculation testing using experimental lines (here, CSSLs) that each carry a chromosomal region harboring certain resistance allele(s) in a well-characterized genetic background. This procedure was even effective for characterization of SNP haplotypes that lack information on resistance. These examples reinforce the idea that the haplotype-based approach, in combination with use of experimental lines, can enhance the development of selective markers for a wide range of varieties.
DNA markers designed around the Pita and Pita-2 loci in previous studies (Hayashi et al. 2006, Jia et al. 2002, 2004) and our preliminary survey were unable to discriminate reference varieties for Pita and Pita-2, even though they differed in their responses to differential isolates. Since all the tested varieties carrying Pita-2 also have Pita, researchers speculated that the broader resistance spectrum of Pita-2 compared with Pita is due to the combined effect of Pita and Pita-2 (Bryan et al. 2000, Jia et al. 2003). Thus, we assumed that donor varieties for these two resistance alleles are closely related and a loss-of-function or gain-of-function mutation at the Pita-2 locus in one of the donors resulted in the allelic difference at this locus. Recently, Pi19 was identified as an allele of the Pita-2 locus, which is located 212 kb from Pita (Takahashi et al. 2017). Accordingly, we sequenced the Pita-2 coding region in reference varieties for Pita and Pita-2 to identify polymorphisms that could distinguish the presence or absence of Pita-2. We successfully developed two allele-specific markers (FA5655 and Pita-2-ID016_3) that target polymorphisms in Pita-2 locus (Fig. 2C). This observation reminds us that rapid evolution of disease resistance genes should be taken into account when using the haplotype-based approach. Accumulating whole genome sequence data in multiple rice varieties will allow researchers to search for DNA variations in resistance genes to increase the number of DNA markers for blast resistance.
The recent increase in the use of high-yield varieties with an indica genetic background in Japan (Kato 2008, Yonemaru et al. 2014) has made it difficult to determine the genotypes for blast resistance by using conventional differential systems because such varieties harbor multiple resistance alleles and there is a lack of pathogen isolates to discriminate such alleles. The genotypes for some of these varieties are undetermined, as shown in the variety registration databases (http://www.hinshu2.maff.go.jp/, http://ineweb.narcc.affrc.go.jp/). From the viewpoint of reducing the use of agricultural chemicals and environmental burden when using such varieties, enhancement of blast resistance is a high-priority breeding objective. Thus, determining unknown resistance genotypes and setting up marker-assisted selection systems for these varieties is of paramount importance. For example, the presence or absence of Pi20, which has a wide blast resistance spectrum (Li et al. 2008), cannot be determined by a conventional evaluation system in these varieties. To overcome this issue, an improved inoculation-based differential system surveying 23 alleles including Pi20 was developed to characterize 10 varieties, mostly in indica genetic background (Hayashi et al. 2014). Concordance between the two systems was high except for Pia and Pita-2 loci, confirming the discriminatory ability of our system (Table 4). Among the 23 alleles, only Pia (at Pia locus) and Pi19 (at Pita-2 locus) have extremely narrow resistance spectra and lack fungus strains that selectively identify them. This might be the reason for the difficulty in estimating these alleles by inoculation testing, and thus we believe that the DNA-based estimation of this locus in the current study is more reliable than that provided by inoculation test-based estimation in the previous study (Hayashi et al. 2014). Furthermore, the phenotypes of some differential strains previously used to discriminate between Pita-2 and Pi20 are unstable, being influenced by environmental factors or unidentified resistance alleles in the genetic background. By contrast, DNA genotyping in our study clearly discriminated these alleles based on SNP haplotype. We were unable to discriminate between Pi3 and Pi5 (at Pii locus), as was the case for the inoculation-based system (Hayashi et al. 2014); we need to confirm whether the reference lines that we used for Pi3 and Pi5 are identical or not. Collectively, the results indicate that our DNA marker-based genotyping system is an efficient and reliable alternative for determining the resistance genotypes of varieties or breeding lines with indica genetic background.
Continual updating of the evaluation system is important to meet breeders’ demands for new resistance alleles and to improve discriminability. The differential system by Hayashi et al. (2014) uses many differential experimental lines and pathogenic races obtained from large-scale screening and establishment of lines, whose improvement requires much effort and expertise. By contrast, the DNA-based system is readily updated by addition or replacement of markers based on accumulated SNP-haplotype information including newly-identified resistance loci. Thus, we propose that our system can be used as a standard procedure for selection of blast resistance alleles in the Japanese rice breeding program. As discussed above for the case of Pita-2, recent mutation events that cause loss or change of resistance spectrum might be undetectable in the SNP haplotype-based approach; mutant alleles with extremely low frequency may also be undetectable by this approach. In case of the Pik locus, where more than eight resistance alleles have been reported, we found that some monogenic lines for a resistance allele differed in response type according to the pathogen isolate used (Supplemental Table 5). Unidentified resistance alleles in the genetic background and/or recent variation at the Pik locus could explain the observations; the former is more likely when the lines carry the same SNP haplotype. To distinguish between these possibilities and to precisely determine resistance genotype for variety registration, it is desirable to determine the entire coding sequence of each sample with reference resistance alleles. Alternatively, the resistance alleles identified by BRA1 could be confirmed by inoculation testing. Breeders or pathologists would be able to reduce the number of inoculation tests because they could select appropriate differential strains according to the BRA1 results. Trials to clarify the reasons for any disagreements between the two methods would contribute to the identification of new resistance alleles at known or unidentified loci, thus improving the system.
Our method of developing a genotyping system for blast resistance could also be applied to the development of markers for genes involved in quantitative resistance rather than race-specific resistance (e.g., pi21, Pb1, Pi39, Pi34, Pi63; Fukuoka et al. 2009, Hayashi et al. 2010b, Terashima et al. 2008, Xu et al. 2014a, Zenbayashi-Sawata et al. 2007). Furthermore, based on breeders’ requests, our procedures could be expanded to set up genotyping systems for several agronomic traits, such as days to heading, yield-related traits, grain quality, and resistance to other diseases and pests in breeding programs. Establishment of such systems will further enhance marker-assisted breeding in rice.
Supplementary Information
Acknowledgments
This work was partly supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics-based Technology for Agricultural Improvement, RBS3001). We thank two editors from ELSS, Inc. (http://elss.co.jp/en/) for editing our manuscript before submission.
Literature Cited
- Abe, T., Nonoue, Y., Ono, N., Omoteno, M., Kuramata, M., Fukuoka, S., Yamamoto, T., Yano, M. and Ishikawa, S. (2013) Detection of QTLs to reduce cadmium content in rice grains using LAC23/Koshihikari chromosome segment substitution lines. Breed. Sci. 63: 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikawa, I., Hayashi, N., Yamane, H., Kanamori, H., Wu, J., Matsumoto, T., Ono, K. and Yano, M. (2008) Two adjacent nucleotide-binding site–leucine-rich repeat class genes are required to confer Pikm-specific rice blast resistance. Genetics 180: 2267–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikawa, I., Hayashi, N., Abe, F., Wu, J. and Matsumoto, T. (2012) Characterization of the rice blast resistance gene Pik cloned from Kanto51. Mol. Breed. 30: 485–494. [Google Scholar]
- Ashkani, S., Rafii, M.Y., Shabanimofrad, M., Miah, G., Sahebi, M., Azizi, P., Tanweer, F.A., Akhtar, M.S. and Nasehi, A. (2015) Molecular breeding strategy and challenges towards improvement of blast disease resistance in rice crop. Front. Plant Sci. 6: 886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkani, S., Rafii, M.Y., Shabanimofrad, M., Ghasemzadeh, A., Ravanfar, S.A. and Latif, M.A. (2016) Molecular progress on the mapping and cloning of functional genes for blast disease in rice (Oryza sativa L.): current status and future considerations. Crit. Rev. Biotechnol. 36: 353–367. [DOI] [PubMed] [Google Scholar]
- Bryan, G.T., Wu, K.S., Farrall, L., Jia, Y., Hershey, H.P., McAdams, S.A., Faulk, K.N., Donaldson, G.K., Tarchini, R. and Valent, B. (2000) A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 12: 2033–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, M.A., Chen, D. and Ronald, P.C. (2004) Development of co-dominant amplified polymorphic sequence markers in rice that flank the Magnaporthe grisea resistance gene Pi7(t) in recombinant inbred line 29. Phytopathology 94: 302–307. [DOI] [PubMed] [Google Scholar]
- Contreras-Soto, R.I., Mora, F., de Oliveira, M.A.R., Higashi, W., Scapim, C.A. and Schuster, I. (2017) A genome-wide association study for agronomic traits in soybean using SNP markers and SNP-based haplotype analysis. PLoS ONE 12: e0171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebitani, T., Takeuchi, Y., Nonoue, Y., Yamamoto, T., Takeuchi, K. and Yano, M. (2005) Construction and evaluation of chromosome segment substitution lines carrying overlapping chromosome segments of indica rice cultivar ‘Kasalath’ in a genetic background of japonica elite cultivar ‘Koshihikari’. Breed. Sci. 55: 65–73. [Google Scholar]
- Ebitani, T., Hayashi, N., Omoteno, M., Ozaki, H., Yano, M., Morikawa, M. and Fukuta, Y. (2011) Characterization of Pi13, a blast resistance gene that maps to chromosome 6 in indica rice (Oryza sativa L. variety, Kasalath). Breed. Sci. 61: 251–259. [Google Scholar]
- Fjellstrom, R., Conaway-Bormans, C.A., McClung, A.M., Marchetti, M.A., Shank, A.R. and Park, W.D. (2004) Development of DNA markers suitable for marker assisted selection of three Pi genes conferring resistance to multiple Pyricularia grisea pathotypes. Crop Sci. 44: 1790–1798. [Google Scholar]
- Fukuoka, S., Saka, N., Koga, H., Ono, K., Shimizu, T., Ebana, K., Hayashi, N., Takahashi, A., Hirochika, H., Okuno, K.et al. (2009) Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 325: 998–1001. [DOI] [PubMed] [Google Scholar]
- Fukuoka, S., Yamamoto, S., Mizobuchi, R., Yamanouchi, U., Ono, K., Kitazawa, N., Yasuda, N., Fujita, Y., Nguyen, T.T.T., Koizumi, S.et al. (2014) Multiple functional polymorphisms in a single disease resistance gene in rice enhance durable resistance to blast. Sci. Rep. 4: 1–7. [Google Scholar]
- Gawenda, I., Thorwarth, P., Günther, T., Ordon, F. and Schmid, K.J. (2015) Genome-wide association studies in elite varieties of German winter barley using single-marker and haplotype-based methods. Plant Breed. 134: 28–39. [Google Scholar]
- Hamajima, N., Saito, T., Matsuo, K., Kozaki, K., Takahashi, T. and Tajima, K. (2000) Polymerase chain reaction with confronting two-pair primers for polymorphism genotyping. Jpn. J. Cancer Res. 91: 865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan, M.M., Rafii, M.Y., Ismail, M.R., Mahmood, M., Rahim, H.A., Alam, M.A., Ashkani, S., Malek, M.A. and Latif, M.A. (2015) Marker-assisted backcrossing: a useful method for rice improvement. Biotechnol. Biotechnol. Equip. 29: 237–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, K., Hashimoto, N., Daigen, M. and Ashikawa, I. (2004) Development of PCR-based SNP markers for rice blast resistance genes at the Piz locus. Theor. Appl. Genet. 108: 1212–1220. [DOI] [PubMed] [Google Scholar]
- Hayashi, K., Yoshida, H. and Ashikawa, I. (2006) Development of PCR-based allele-specific and InDel marker sets for nine rice blast resistance genes. Theor. Appl. Genet. 113: 251–260. [DOI] [PubMed] [Google Scholar]
- Hayashi, K. and Yoshida, H. (2009) Refunctionalization of the ancient rice blast disease resistance gene Pit by the recruitment of a retrotransposon as a promoter. Plant J. 57: 413–425. [DOI] [PubMed] [Google Scholar]
- Hayashi, K., Yasuda, N., Fujita, Y., Koizumi, S. and Yoshida, H. (2010a) Identification of the blast resistance gene Pit in rice cultivars using functional markers. Theor. Appl. Genet. 121: 1357–1367. [DOI] [PubMed] [Google Scholar]
- Hayashi, N. and Fukuta, Y. (2009) Proposal for a new international system of differentiating races of blast (Pyricularia oryzae Cavara) by using LTH monogenic lines in rice (Oryza sativa L.). JIRCAS Working report No. 63: 11–15. [Google Scholar]
- Hayashi, N., Inoue, H., Kato, T., Funao, T., Shirota, M., Shimizu, T., Kanamori, H., Yamane, H., Hayano-Saito, Y., Matsumoto, T.et al. (2010b) Durable panicle blast-resistance gene Pb1 encodes an atypical CC-NBS-LRR protein and was generated by acquiring a promoter through local genome duplication. Plant J. 64: 498–510. [DOI] [PubMed] [Google Scholar]
- Hayashi, N., Ishii, T., Kato, H. and Fukuta, Y. (2014) Estimation of true blast resistance genes in feed rice varieties by using international differential fungal isolates. Breed. Res. 16 (Suppl. 2): 60. [Google Scholar]
- Hayashi, N. (2015) Estimations of true blast resistance genes in rice varieties, MAFF microorganism genetic resources manual No. 38. National Institute of Agrobiological Sciences, Tsukuba, Ibaraki. [Google Scholar]
- Hirabayashi, H., Nemoto, H., Ando, I., Kato, H., Oota, H., Satou, H., Takeuchi, Y., Ishii, T., Maeda, H., Imbe, T.et al. (2010) “Momiroman”, a new rice cultivar for feed use. Bull. Natl. Inst. Crop Sci. 11: 31–47. [Google Scholar]
- Hori, K., Nonoue, Y., Ono, N., Shibaya, T., Ebana, K., Matsubara, K., Ogiso-Tanaka, E., Tanabata, T., Sugimoto, K., Taguchi-Shiobara, F.et al. (2015) Genetic architecture of variation in heading date among Asian rice accessions. BMC Plant Biol. 15: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, L., Wu, J., Chen, C., Wu, W., He, X., Lin, F., Wang, L., Ashikawa, I., Matsumoto, T. and Pan, Q. (2012) The isolation of Pi1, an allele at the Pik locus which confers broad spectrum resistance to rice blast. Theor. Appl. Genet. 125: 1047–1055. [DOI] [PubMed] [Google Scholar]
- Jeon, J.S., Chen, D., Yi, G.H., Wang, G.L. and Ronald, P.C. (2003) Genetic and physical mapping of Pi5(t), a locus associated with broad-spectrum resistance to rice blast. Mol. Genet. Genomics 269: 280–289. [DOI] [PubMed] [Google Scholar]
- Jia, Y., Wang, Z. and Singh, P. (2002) Development of dominant rice blast Pi-ta resistance gene markers. Crop Sci. 42: 2145–2149. [Google Scholar]
- Jia, Y., Bryan, G.T., Farrall, L. and Valent, B. (2003) Natural variation at the Pi-ta rice blast resistance locus. Phytopathology 93: 1452–1459. [DOI] [PubMed] [Google Scholar]
- Jia, Y., Wang, Z., Fjellstrom, R.G., Moldenhauer, K.A., Azam, M.A., Correll, J., Lee, F.N., Xia, Y. and Rutger, J.N. (2004) Rice Pi-ta gene confers resistance to the major pathotypes of the rice blast fungus in the United States. Phytopathology 94: 296–301. [DOI] [PubMed] [Google Scholar]
- Kato, H. (2008) Development of rice varieties for whole crop silage (WCS) in Japan. Jpn. Agric. Res. Q. 42: 231–236. [Google Scholar]
- Kawahara, Y., de la Bastide, M., Hamilton, J.P., Kanamori, H., McCombie, W.R., Ouyang, S., Schwartz, D.C., Tanaka, T., Wu, J., Zhou, S.et al. (2013) Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice (N Y) 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosawa, S. (1984) Establishment of differential varieties for pathogenicity test of rice blast fungus. Rice Genet. Newsl. 1: 95–97. [Google Scholar]
- Kobayashi, N., Telebanco-Yanoria, M.J., Tsunematsu, H., Kato, H., Imbe, T. and Fukuta, Y. (2007) Development of new sets of international standard differential varieties for blast resistance in rice (Oryza sativa L.). Jpn. Agric. Res. Q. 41: 31–37. [Google Scholar]
- Koide, Y., Kobayashi, N., Xu, D. and Fukuta, Y. (2009) Resistance genes and selection DNA markers for blast disease in rice (Oryza sativa L.). Jpn. Agric. Res. Q. 43: 255–280. [Google Scholar]
- Lee, S.K., Song, M.Y., Seo, Y.S., Kim, H.K., Ko, S., Cao, P.J., Suh, J.P., Yi, G., Roh, J.H., Lee, S.et al. (2009) Rice Pi5-mediated resistance to Magnaporthe oryzae requires the presence of two coiled-coil-nucleotide-binding-leucine-rich repeat genes. Genetics 181: 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Lei, C., Cheng, Z., Jia, Y., Huang, D., Wang, J., Wang, J., Zhang, X., Su, N., Guo, X.et al. (2008) Identification of SSR markers for a broad-spectrum blast resistance gene Pi20(t) for marker-assisted breeding. Mol. Breed. 22: 141–149. [Google Scholar]
- Lorenz, A.J., Hamblin, M.T. and Jannink, J.-L. (2010) Performance of single nucleotide polymorphisms versus haplotypes for genome-wide association analysis in barley. PLoS ONE 5: e14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miah, G., Rafii, M.Y., Ismail, M.R., Puteh, A.B., Rahim, H.A., Islam, K.N. and Latif, M.A. (2013) A review of microsatellite markers and their applications in rice breeding programs to improve blast disease resistance. Int. J. Mol. Sci. 14: 22499–22528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monna, L., Kitazawa, N., Yoshino, R., Suzuki, J., Masuda, H., Maehara, Y., Tanji, M., Sato, M., Nasu, S. and Minobe, Y. (2002) Positional cloning of rice semidwarfing gene, sd-1: rice “green revolution gene” encodes a mutant enzyme involved in gibberellin synthesis. DNA Res. 9: 11–17. [DOI] [PubMed] [Google Scholar]
- Nagata, K., Ando, T., Nonoue, Y., Mizubayashi, T., Kitazawa, N., Shomura, A., Matsubara, K., Ono, N., Mizobuchi, R., Shibaya, T.et al. (2015a) Advanced backcross QTL analysis reveals complicated genetic control of rice grain shape in a japonica × indica cross. Breed. Sci. 65: 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata, K., Nonoue, Y., Mizobuchi, R., Ono, N., Shibaya, T., Ebana, K., Matsubara, K., Ogiso-Tanaka, E., Tanabata, T., Sugimoto, K.et al. (2015b) Development of twelve sets of chromosomal segment substitution lines that enhance allele mining in cultivated rice. Breed. Res. 17: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoue, Y., Sato, H. and Ishii, T. (2018) Verification of DNA markers to estimate genotype at blast resistance loci Pia, Pii and Pik in rice. Breed. Res. 20: 16–22. [Google Scholar]
- Okuyama, Y., Kanzaki, H., Abe, A., Yoshida, K., Tamiru, M., Saitoh, H., Fujibe, T., Matsumura, H., Shenton, M., Galam, D.C.et al. (2011) A multifaceted genomics approach allows the isolation of the rice Pia-blast resistance gene consisting of two adjacent NBS-LRR protein genes. Plant J. 66: 467–479. [DOI] [PubMed] [Google Scholar]
- Qu, S., Liu, G., Zhou, B., Bellizzi, M., Zeng, L., Dai, L., Han, B. and Wang, G.L. (2006) The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 172: 1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen, S. and Skaletsky, H. (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132: 365–386. [DOI] [PubMed] [Google Scholar]
- Schatz, M.C., Maron, L.G., Stein, J.C., Hernandez Wences, A., Gurtowski, J., Biggers, E., Lee, H., Kramer, M., Antoniou, E., Ghiban, E.et al. (2014) Whole genome de novo assemblies of three divergent strains of rice, Oryza sativa, document novel gene space of aus and indica. Genome Biol. 15: 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, J., Wang, W., Han, J., Chen, S., Wang, C., Zeng, L., Feng, A., Yang, J., Zhou, B. and Zhu, X. (2015) Functional divergence of duplicated genes results in a novel blast resistance gene Pi50 at the Pi2/9 locus. Theor. Appl. Genet. 128: 2213–2225. [DOI] [PubMed] [Google Scholar]
- Takagi, H., Uemura, A., Yaegashi, H., Tamiru, M., Abe, A., Mitsuoka, C., Utsushi, H., Natsume, S., Kanzaki, H., Matsumura, H.et al. (2013) MutMap-Gap: whole-genome resequencing of mutant F2 progeny bulk combined with de novo assembly of gap regions identifies the rice blast resistance gene Pii. New Phytol. 200: 276–283. [DOI] [PubMed] [Google Scholar]
- Takahashi, A., Hayashi, N., Miyao, A. and Hirochika, H. (2010) Unique features of the rice blast resistance Pish locus revealed by large scale retrotransposon-tagging. BMC Plant Biol. 10: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, A., Hayashi, N. and Hirochika, H. (2017) Isolation of blast resistance genes, Pita-2 and Pi19, which encode non-NBS-LRR proteins. Jpn. J. Phytopathol. 83: 171. [Google Scholar]
- Takai, T., Nonoue, Y., Yamamoto, S.-i., Yamanouchi, U., Matsubara, K., Liang, Z.-W., Lin, H.-X., Ono, N., Uga, Y. and Yano, M. (2007) Development of chromosome segment substitution lines derived from backcross between indica donor rice cultivar ‘Nona Bokra’ and japonica recipient cultivar ‘Koshihikari’. Breed. Sci. 57: 257–261. [Google Scholar]
- Takai, T., Ikka, T., Kondo, K., Nonoue, Y., Ono, N., Arai-Sanoh, Y., Yoshinaga, S., Nakano, H., Yano, M., Kondo, M.et al. (2014) Genetic mechanisms underlying yield potential in the rice high-yielding cultivar Takanari, based on reciprocal chromosome segment substitution lines. BMC Plant Biol. 14: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telebanco-Yanoria, M.J., Koide, Y., Fukuta, Y., Imbe, T., Kato, H., Tsunematsu, H. and Kobayashi, N. (2010) Development of near-isogenic lines of Japonica-type rice variety Lijiangxintuanheigu as differentials for blast resistance. Breed. Sci. 60: 629–638. [Google Scholar]
- Terashima, T., Fukuoka, S., Saka, N. and Kudo, S. (2008) Mapping of a blast field resistance gene Pi39 (t) of elite rice strain Chubu 111. Plant Breed. 127: 485–489. [Google Scholar]
- Thomson, M.J. (2014) High-throughput SNP genotyping to accelerate crop improvement. Plant Breed. Biotechnol. 2: 195–212. [Google Scholar]
- Tian, D., Chen, Z., Chen, Z., Zhou, Y., Wang, Z., Wang, F. and Chen, S. (2016) Allele-specific marker-based assessment revealed that the rice blast resistance genes Pi2 and Pi9 have not been widely deployed in Chinese indica rice cultivars. Rice (N Y) 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunematsu, H., Yanoria, M.J.T., Ebron, L.A., Hayashi, N., Ando, I., Kato, H., Imbe, T. and Khush, G.S. (2000) Development of monogenic lines of rice for blast resistance. Breed. Sci. 50: 229–234. [Google Scholar]
- Wang, J., Lin, M., Crenshaw, A., Hutchinson, A., Hicks, B., Yeager, M., Berndt, S., Huang, W.-Y., Hayes, R.B., Chanock, S.J.et al. (2009) High-throughput single nucleotide polymorphism genotyping using nanofluidic Dynamic Arrays. BMC genomics 10: 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z.X., Yano, M., Yamanouchi, U., Iwamoto, M., Monna, L., Hayasaka, H., Katayose, Y. and Sasaki, T. (1999) The Pib gene for rice blast resistance belongs to the nucleotide binding and leucine-rich repeat class of plant disease resistance genes. Plant J. 19: 55–64. [DOI] [PubMed] [Google Scholar]
- Wu, K., Xu, T., Guo, C., Zhang, X. and Yang, S. (2012) Heterogeneous evolutionary rates of Pi2/9 homologs in rice. BMC Genet. 13: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y., Xiao, N., Yu, L., Pan, C., Li, Y., Zhang, X., Liu, G., Dai, Z., Pan, X. and Li, A. (2015) Combination patterns of major R genes determine the level of resistance to the M. oryzae in rice (Oryza sativa L.). PLoS ONE 10: e0126130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X., Hayashi, N., Wang, C.-T., Fukuoka, S., Kawasaki, S., Takatsuji, H. and Jiang, C.-J. (2014a) Rice blast resistance gene Pikahei-1 (t), a member of a resistance gene cluster on chromosome 4, encodes a nucleotide-binding site and leucine-rich repeat protein. Mol. Breed. 34: 691–700. [Google Scholar]
- Xu, X., Lv, Q., Shang, J., Pang, Z., Zhou, Z., Wang, J., Jiang, G., Tao, Y., Xu, Q., Li, X.et al. (2014b) Excavation of Pid3 orthologs with differential resistance spectra to Magnaporthe oryzae in rice resource. PLoS ONE 9: e93275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, M.K., Aravindan, S., Ngangkham, U., Shubudhi, H., Bag, M.K., Adak, T., Munda, S., Samantaray, S. and Jena, M. (2017) Use of molecular markers in identification and characterization of resistance to rice blast in India. PLoS ONE 12: e0176236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, M., Kiyosawa, S., Yamaguchi, T., Hirano, T., Kobayashi, T., Kushibuchi, K. and Watanabe, S. (1976) Proposal of a new method for differentiating races of Pyricularia oryzae Cavara in Japan. Ann. Phytopathol. Soc. Jpn. 42: 216–219. [Google Scholar]
- Yonemaru, J., Mizobuchi, R., Kato, H., Yamamoto, T., Yamamoto, E., Matsubara, K., Hirabayashi, H., Takeuchi, Y., Tsunematsu, H., Ishii, T.et al. (2014) Genomic regions involved in yield potential detected by genome-wide association analysis in Japanese high-yielding rice cultivars. BMC Genomics 15: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemaru, J., Choi, S.H., Sakai, H., Ando, T., Shomura, A., Yano, M., Wu, J. and Fukuoka, S. (2015) Genome-wide indel markers shared by diverse Asian rice cultivars compared to Japanese rice cultivar ‘Koshihikari’. Breed. Sci. 65: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, B., Zhai, C., Wang, W., Zeng, X., Xu, X., Hu, H., Lin, F., Wang, L. and Pan, Q. (2011) The Pik-p resistance to Magnaporthe oryzae in rice is mediated by a pair of closely linked CC-NBS-LRR genes. Theor. Appl. Genet. 122: 1017–1028. [DOI] [PubMed] [Google Scholar]
- Zenbayashi-Sawata, K., Fukuoka, S., Katagiri, S., Fujisawa, M., Matsumoto, T., Ashizawa, T. and Koizumi, S. (2007) Genetic and physical mapping of the partial resistance gene, Pi34, to blast in rice. Phytopathology 97: 598–602. [DOI] [PubMed] [Google Scholar]
- Zhai, C., Lin, F., Dong, Z., He, X., Yuan, B., Zeng, X., Wang, L. and Pan, Q. (2011) The isolation and characterization of Pik, a rice blast resistance gene which emerged after rice domestication. New Phytol. 189: 321–334. [DOI] [PubMed] [Google Scholar]
- Zhai, C., Zhang, Y., Yao, N., Lin, F., Liu, Z., Dong, Z., Wang, L. and Pan, Q. (2014) Function and interaction of the coupled genes responsible for Pik-h encoded rice blast resistance. PLoS ONE 9: e98067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B., Qu, S., Liu, G., Dolan, M., Sakai, H., Lu, G., Bellizzi, M. and Wang, G.L. (2006) The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol. Plant Microbe Interact. 19: 1216–1228. [DOI] [PubMed] [Google Scholar]
- Zhou, B., Dolan, M., Sakai, H. and Wang, G.L. (2007) The genomic dynamics and evolutionary mechanism of the Pi2/9 locus in rice. Mol. Plant Microbe Interact. 20: 63–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.