Abstract
Ovarian theca androgen production is regulated by the pituitary LH and intrafollicular factors. Enhanced androgen biosynthesis by theca cells contributes to polycystic ovary syndrome (PCOS) in women, but the ovarian consequences of elevated androgens are not completely understood. Our study documents the molecular events that are altered in the theca and stromal cells of mice exposed to high androgen levels, using the nonaromatizable androgen DHT. Changes in ovarian morphology and function were observed not only in follicles, but also in the stromal compartment. Genome-wide microarray analyses revealed marked changes in the ovarian transcriptome of DHT-treated females within 1 week. Particularly striking was the increased expression of vascular cell adhesion molecule 1 (Vcam1) specifically in the NR2F2/COUPTF-II lineage theca cells, not granulosa cells, of growing follicles and throughout the stroma of the androgen-treated mice. This response was mediated by androgen receptors (ARs) present in theca and stromal cells. Human theca-derived cultures expressed both ARs and NR2F2 that were nuclear. VCAM1 mRNA and protein were higher in PCOS-derived theca cells compared with control theca and reduced markedly by the AR antagonist flutamide. In the DHT-treated mice, VCAM1 was transiently induced by equine chorionic gonadotropin, when androgen and estrogen biosynthesis peak in preovulatory follicles, and was potently suppressed by a superovulatory dose of human chorionic gonadotropin. High levels of VCAM1 in the theca and interstitial cells of DHT-treated mice and in adult Leydig cells indicate that there may be novel functions for VCAM1 in reproductive tissues, including the gonads.
Ovarian theca cells are essential for normal follicular development and fertility but they also contribute to ovarian dysfunction in polycystic ovary syndrome (PCOS), ovarian hyperthecosis, and premature ovarian failure (1–4). Theca cells are critical, in part, for providing (i) a protective shield around the granulosa cells and the oocyte and (ii) nutrients, growth regulatory factors, and steroid hormones, principally androgens, for granulosa cell survival, growth, and differentiation (1, 2). In mice, theca cells (endocrine and mesenchymal) are derived from the embryonic mesonephros and the embryonic gonadal mesenchyme, respectively. Murine theca cells are recruited to primary follicles by paracrine hedgehog (HH) signaling in which the oocyte-derived factor GDF9 stimulates granulosa cells to produce the ligands Indian HH (IHH) and desert HH (DHH). These ligands bind the patched receptor on progenitor theca cells, facilitating the development of the endocrine and fibroblastic theca layers (2, 5). Following recruitment and during early follicular development, theca endocrine cells (derived from the embryonic mesonephros) differentiate and, as folliculogenesis progresses, become the primary source of ovarian androgen biosynthesis (2, 5).
Theca androgen production is promoted by pituitary LH as well as by insulin, IGF-1, and insulin-like 3 that regulate the expression and activity of steroidogenic enzymes, including cholesterol side-chain cleavage (i.e., CYP11A1) and 17α-hydroxylase/17,20-lyase (i.e., CYP17A1) (1). Theca androgen production is also tightly regulated by paracrine factors produced primarily by granulosa cells, including bone morphogenic proteins, activin, and estradiol (1, 6). Women with PCOS are characterized, in part, by ovarian hyperandrogenism (7, 8). Furthermore, theca endocrine functions in women with PCOS are intrinsically altered, leading to elevated expression and activity of CYP17A1, increased production of ovarian-derived androgens, and anovulation (9–15).
Mechanistic studies of the role of androgens and the androgen receptor (AR) in ovarian follicular development have primarily focused on their roles in regulating granulosa cell functions (16–19), whereas the potential impact of androgens and the role of AR in theca and stromal/interstitial cells have been largely overlooked. The oversight is especially evident at the molecular level, despite the fact that mouse and human theca cells express AR (2), as well as a modulator of AR action, the corepressor NR2F2/COUPTF-II (14, 15, 20, 21), which is present as a marker of presumptive theca progenitor cells in the XX mouse embryonic gonad at embryonic day 12.5 (22). A recent theca cell–specific AR knockout (ThARKO) mouse model (23) documented that loss of AR selectively in theca cells prevents excess androgens from promoting a PCOS-like phenotype. These observations link theca/stromal-specific AR and NR2F2, in addition to elevated androgens, to changes in steroidogenic stromal cell functions within the ovary, possibly contributing to PCOS phenotypes.
The following studies were designed to identify the molecular and cellular events that are altered in ovarian theca and stromal cells compared with granulosa cells in mice exposed to excess androgen. Based on previous studies (24), we used a DHT-treated mouse model and showed that this nonaromatizable androgen dramatically altered the gene expression profiles in ovarian stromal cells at 1 week and 2 months posttreatment, leading to changes in theca and stromal differentiation and function. Most notable was the intense nucleation of ovarian stromal AR, colocalization (in the nucleus) of AR and the ovarian stromal marker NR2F2, and the marked induction of vascular cell adhesion molecule 1 (VCAM1) expression in ovarian theca and interstitial cells.
Material and Methods
Animal studies
All mice were housed in the Baylor College of Medicine (BCM) Center for Comparative Medicine in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experimental studies were approved by the BCM Animal Care and Use Committee. C57BL/6 mice were provided with food ad libitum and kept under a 16-hour light/8-hour dark cycle. Sixty-day release DHT pellets (12.5 mg per pellet; Innovative Research of America, Sarasota, FL) or sham pellets were surgically implanted subcutaneously into prepubertal females. Ovarian RNA derived from theca-specific (CYP17A1iCRE) AR knockout mice was generated as previously described (23). The CX3CR1-GFP knock-in mice were a gift from Dr. Mary Dickinson (BCM) (25). In these studies, CX3CR1 mice were maintained in a heterozygous state; DHT-mediated androgenization of these female mice was carried out as described above. Superovulation of mice exposed to excess androgen was carried out using pharmaceutical grade injections of 5 IU of equine chorionic gonadotropin (eCG; for 48 hours) followed by 5 IU of human chorionic gonadotropin (hCG; for an additional 24 hours after eCG administration).
Human studies
Normal and PCOS theca cells
Human theca interna tissue was obtained from follicles of women undergoing hysterectomy, following informed consent under a protocol approved by the Institutional Review Board of the Pennsylvania State University College of Medicine. As a standard of care, oophorectomies were performed during the luteal phase of the cycle. Theca cells from normal cycling and PCOS follicles were isolated and grown as previously reported in detail (11, 26, 27). The theca cell preparations used in these studies have been described and characterized previously. The steroidogenic phenotypes of the normal and PCOS theca cells have been reported to result from the inherent properties of the cells, rather than the cycle phase at the time when they were isolated (9, 10, 13, 28). PCOS and normal ovarian tissue came from age-matched women, 38 to 40 years old. The diagnosis of PCOS was made according to National Institutes of Health consensus guidelines, which include hyperandrogenemia, oligoovulation, polycystic ovaries, and the exclusion of 21-hydroxylase deficiency, Cushing syndrome, and hyperprolactinemia, as we have described previously (29). All of the PCOS theca cell preparations studied came from ovaries of women with fewer than six menses per year and elevated serum total testosterone or bioavailable testosterone levels (9, 13, 28, 29). The control (normal) theca cell preparations came from ovaries of fertile women with normal menstrual histories, menstrual cycles of 21 to 35 days, and no clinical signs of hyperandrogenism. Neither subjects with PCOS nor normal subjects were receiving hormonal medications at the time of surgery. Indications for surgery were dysfunctional uterine bleeding, endometrial cancer, and/or pelvic pain. Experiments comparing PCOS and normal theca were performed using fourth-passage (31 to 38 population doublings) theca cells isolated from individual size-matched follicles (4 to 6 mm in diameter) obtained from age-matched subjects, in the absence of in vivo stimulation as previously described (29). The fourth-passage cells allowed us to perform multiple experiments from the same patient population and were propagated from frozen stocks of second-passage cells in the media described above. The passage conditions and split ratios for all normal and PCOS cells were identical.
Microarray, gene expression, and Western blot analyses
Microarray experimental procedures
Total RNA from whole ovary and ovarian theca/stromal and granulosa cell–enriched fractions was isolated with RNAeasy columns (Qiagen, Germantown, MD) using the standard manufacturer’s protocol. Stromal and granulosa fractions were isolated by gentle puncturing of the ovaries with a 26-gauge needle (Becton Dickinson, Franklin Lakes, NJ). Granulosa cells were readily released into the media and collected by centrifugation; the remaining residual ovarian tissue containing mostly stroma as well as preantral and primordial follicles was collected separately by centrifugation. Microarray analyses were done at the Genomic and RNA Profiling Core at BCM. Specifically, total RNA was converted into cRNA using a Low Input Quick Amp Labeling Kit (Agilent Technologies, Santa Clara, CA) and hybridized to a Mouse GE 8×60K v2 Array (Agilent Technologies) for single-color analysis according to the manufacturer’s protocol. An Agilent C microarray scanner was used to image the arrays. Signal intensity values from individual probes were corrected for local background variation, and data across arrays were normalized (quantile) using the lumi package (https://www.bioconductor.org). Heat maps and bioinformatic analyses were generated in the Morpheus application (Broad Institute, Cambridge, MA) according to parameters described in the text.
Quantification of critical individual genes from mouse studies was validated by quantitative PCR.
Total RNA was reverse transcribed using the M-MLV reverse transcription system (Invitrogen, Carlsbad, CA). Quantitative PCR (qPCR) reactions were amplified in the Rotor-Gene 6000 thermocycler using RT2 SYBR Green Mastermix (Qiagen) and 0.5 μM of each individual primer (30), according to the manufacturer’s protocol. Cycle values from independently generated samples were normalized to the housekeeping gene Rpl19. A Student t test was used to grade statistical significance in this section as follows: *P < 0.05, **P < 0.025, and ***P < 0.01.
Quantification of VCAM1, NR2F2, AR, and CYP17A1 in human theca cells was validated using TaqMan quantitative RT-PCR.
Quantification of VCAM1, NR2F2, AR, and CYP17A1 mRNA abundance was determined in control and PCOS theca samples using single-step Brilliant III Ultra Fast qRT-PCR Reagents (Agilent Technologies) mixed with 50 to 100 ng of total RNA per tube, 200 nM final concentration of each forward and reverse primer, and 100 nM probe. The gene-specific one-step PCR was carried out in duplicate for each mRNA sample and for a series of dilutions in an AriaMx real-time PCR system (Agilent Technologies) according to the manufacturer’s instructions for this instrument as previously described. The TaqMan primer and probe sets for VCAM1, NR2F2, and AR were commercially obtained from Thermo Fisher Scientific (Waltham, MA). The primer and probe sets for TBP were used as we have previously reported (29). The same process was carried out for TBP to use TBP values for normalization of each reaction. The mean target value for each unknown was divided by the mean TBP value to normalize each sample. A Student t test was used to grade statistical significance in this section is as follows: *P < 0.05, **P < 0.025, and ***P < 0.01.
Western blotting
Lysates from whole ovary and testis tissues were prepared according to the conditions listed in the manuscript using RIPA lysis buffer (Boston BioProducts, Ashland, MA). Electrophoresis was carried out using the XCell SureLock mini-cell electrophoresis system and NuPAGE Bis-Tris gels (Invitrogen). Proteins were transferred to Immobilon-P membranes (MilliporeSigma, Burlington, MA) using the NuPAGE MOPS SDS buffer kit (Invitrogen) at 15 mAmps for 90 minutes in a Bio-Rad Mini-PROTEAN II cell box. VCAM1 [Abcam, Cambridge, UK (31)] and β-actin [Sigma-Aldrich, St. Louis, MO (32)] protein bands were detected using horseradish peroxidase (HRP)–conjugated secondary antibodies (GE Healthcare, Chicago, IL). Detection of HRP was carried out using Pierce ECL 2 Western blotting substrate (Thermo Fisher Scientific) followed by exposure to HyBlot ES autoradiography film (Denville Scientific, Holliston MA).
Immunohistochemistry, immunofluorescence, and in situ hybridization
Immunohistochemistry and immunofluorescence
Whole ovary and testis tissues were collected and fixed in either Bouin solution for histology or in 4% paraformaldehyde-PBS overnight for immunohistochemical (IHC) and immunoflurorescent (IF) staining procedures. Tissues were subsequently embedded in paraffin, sectioned at a thickness of 5 µm, and collected on charged Superfrost Plus micro slides (VWR, Radnor, PA). For IHC stains, tissue sections were cleared in xylene with subsequent alcohol-based washes; endogenous peroxidase activity was quenched using 3% hydrogen peroxide in methanol; antigen retrieval was carried out in sodium citrate buffer (pH 6.5), followed by gradual hydration in PBS. Blocking was performed in 5% to 10% normal goat serum (Invitrogen) in 0.1% Tween 20 in PBS. Primary antibodies used are as follows: cleaved CASP3 [Cell Signaling Technology, Danvers, MA (33)], α-smooth muscle actin [αSMA; Abcam (34)], AR [used for mouse AR; Santa Cruz Biotechnology (35)], CYP17A1 [gift from Dr. Alan J. Conley (36)], COLIVA1 [Abcam (37)], NR2F2 [Perseus Proteomics, Meguro, Tokyo, Japan (38)], VCAM1 [Abcam (31)], F4/80 [Abcam (39)], GFP [Cell Signaling Technology (40)], vimentin [Cell Signaling Technology (41)], and AR [AR441, stain for human AR; gift from Nancy L. Weigel (Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX) (42)]. Tissue sections were then incubated with goat-derived biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA) and then coupled to a streptavidin-HRP–based ABC kit for signal amplification (Vector Laboratories). Staining was developed using a diaminobenzidine substrate peroxidase kit (Vector Laboratories). For IF labeling, tissue sections were subjected to a similar protocol described for IHC stains, but without quenching endogenous peroxidase or signal amplification. For better preservation of native tissue morphology, Bouin solution (saturated aqueous picric acid, 10% formaldehyde, glacial acetic acid; all Sigma-Aldrich) was used to fix ovaries for ∼4 hours. Ovaries fixed using Bouin solution were subjected to Picrosirius staining for collagen I/III [Sirius Red F3B (Sigma-Aldrich) dissolved in picric acid]. For lipid visualization, Oil Red O stains (Abcam) were carried out on frozen sections (5 µm). Tissue histology was imaged on a Nikon CiL upright microscope with a color camera using 4×, 10×, 20×, 40×, and 100× objectives. Cultured human control and PCOS theca cells were fixed and stained for NR2F2, AR, and VCAM1 using specific antibodies and IF imaging or were prepared for Western analyses as above.
In situ hybridization
For detection of mRNA, in situ hybridization (ISH) (43) was performed on 25-µm-thick sections cut from fresh-frozen postnatal day 25 (D25) ovaries isolated from mice before and after DHT treatment of 1 week. We generated digoxigenin (DIG)-labeled mRNA antisense probes against Vcam1 using reverse-transcribed mouse cDNA as a template. The DIG-labeled probe was made using an RNA labeling kit from Roche (Sigma-Aldrich). Primer and probe sequences for the Vcam1 probe are published in the Allen Brain Atlas (www.mouse.brain-map.org). ISH was performed by the RNA In Situ Hybridization Core at BCM using an automated robotic platform as previously described with modifications of the protocol for fluorescent ISH (43). Modifications, in brief, were as follows: after the described washes and blocking steps, the DIG-labeled probe was visualized using tyramide-Cy3 Plus (Perkin Elmer Kit #NEL744001KT; 1:50 dilution, 15-minute incubation; PerkinElmer, Waltham, MA). Following washes in TNT [Tris-HCl (pH 7.5), 0.15 M NaCl, 0.05% Tween 20], the slides were stained with 4′,6-diamidino-2-phenylindole (Molecular Probes, Eugene, OR), washed again, removed from the machine, and mounted in ProLong Diamond (Molecular Probes).
Microscopy
Imaging was performed at the Integrated Microscopy Core at BCM on the GE Healthcare DVLive epifluorescence image restoration microscope using Olympus objectives (UPlanSApo 20×/0.75 numerical aperture, PlanApo 40×/0.95 numerical aperture, and UPlanSApo 100×/1.4 numerical aperture) and an sCMOS camera. Z-stacks (0.25 µm for the 100× objective) covering the entire nucleus (∼10 µm) were acquired before applying a conservative restorative algorithm for quantitative image deconvolution. A single in-focus z-stack then was used to pseudo-color image channels, which were then layered using Adobe Photoshop Creative Cloud 2018 software.
Results
Characterization of a mouse model of androgen excess
DHT pellets were implanted subcutaneously into prepubertal female mice on D25. DHT was selected over other AR ligands to prevent the conversion of androgens to estradiol by aromatase present in granulosa cells, thereby prompting a more mechanistically specific activation of AR. Serum levels of FSH and LH in the DHT-treated mice were detectable at low basal concentrations observed in control mice at D25. No statistically significant difference was observed with either DHT treatment at 1 week or 2 months compared with control mice at D25 (44). FSH levels measured 2.555 ± 2.02 ng/mL in D25 females, 0.739 ± 0.300 ng/mL after 1 week of DHT treatment, and 0.326 ± 0.121 ng/mL after 2 months of DHT exposure. At D25, LH levels were measured at 12.01 ± 5.00 ng/mL, 4.51 ± 0.530 ng/mL after 1 week of DHT treatment, and 7.154 ± 0.854 ng/mL after 2 months of DHT exposure. In any case, FSH and LH levels in the DHT-treated mice were not statistically different from levels observed in control mice at D25 (N = 5) (Student t test).
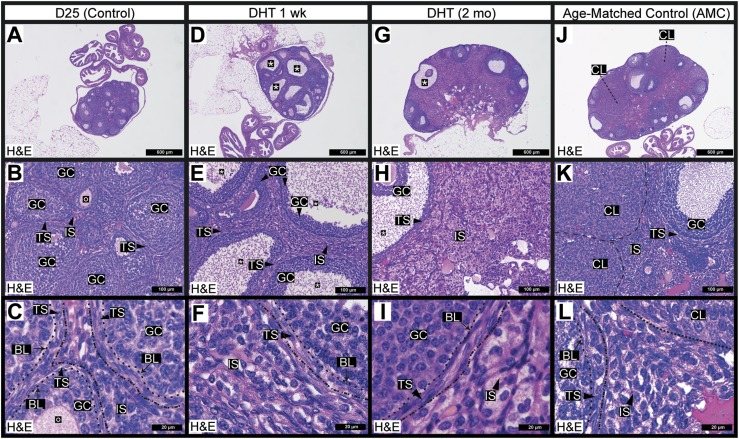
Prepubertal mouse ovaries contained numerous growing follicles but lacked corpora lutea (Fig. 1A). As mice age, theca from atretic follicles generate an interstitial stroma, which was minimal in the D25 mouse ovary (Fig. 1B) (45). Theca within growing follicles are comprised of two to three cell layers of mesenchymal spindle-shaped cells, whereas interstitial stromal cells external to the theca are round and found in large clusters (Fig. 1C).
Figure 1.
Ovarian follicular and stromal morphology was markedly altered in a mouse model of androgen excess. (A) Histological [hematoxylin and eosin (H&E) stain] examination of the D25 mouse ovary revealed healthy growing follicles and served as a nonluteinized, noncycling control. (B, C) Many follicles in the D25 ovaries contained oocytes surrounded by granulosa cells and theca cells. Theca cells are polarized spindle-shaped mesenchymal cells (generally two to three layers thick) that were found outside the follicle-associated basal lamina. Outside the theca layer was an immature interstitial compartment, the cells of which were derived from steroidogenic theca cells remaining from atretic follicles. This compartment was sparse in D25 control mice. (D–F) DHT treatment of 1 wk led to the formation of many cystic follicles (annotated with asterisks). (E) DHT-treated animals developed an abnormal thickening of the ovarian interstitial layer. (G–I) DHT treatment of 2 mo prevented the generation of corpora lutea and promoted the formation of cystic follicles but did not prevent the formation of theca stroma or the expansion of the interstitial stroma. (I) The expanded interstitial stroma exhibited an abnormal, hyperplastic, and fatty appearance. (J–L) The ovary of a representative AMC mouse contained multiple corpora lutea, which were absent in D25 mouse ovary. (K) Healthy luteal and granulosa cells in the ovary were evident on morphologic comparison. (L) Theca cells were well polarized in the AMC control ovaries, and the interstitial compartment bore a distinct, presumably healthy appearance. N ≥ 3 for all conditions. Dashed lines outline specific ovarian structures that are labeled individually. BL, basal lamina; CL, corpora lutea; GC, granulosa cell; IS, interstitial stroma; O, oocyte; TS, theca stroma.
Ovaries from DHT-treated mice at both 1 week and 2 months of treatment lacked corpora lutea, providing evidence that ovulation had not occurred (Fig. 1D and 1G). In agreement with other androgen excess models (24), DHT enhanced apoptosis of centrally located granulosa cells as shown by activated (cleaved) caspase-3 immunostaining (33) and induced follicle cyst formation (44). Ovaries of DHT-treated mice exhibited an unusually divergent morphological appearance evidenced by: (i) more pronounced oocyte and cumulus cell loss in what appeared to be periovulatory follicles (Fig. 1D and 1G); (ii) expansion of the ovarian stroma marked by a poor delineation between theca and interstitial layers (Fig. 1E and 1H); and (iii) formation of a stroma that was diffuse, hyperplastic, and lipid-filled (Fig. 1F and 1I), as reported in previous PCOS mouse models (46, 47). Mice aged past their first ovulation generated corpora lutea that were present in ovaries of the age-matched control (AMC; 3 months) mice (Fig. 1J–1L). Relative to the D25 controls, the AMC mice also exhibited an expanded interstitial compartment (Fig. 1K) where stromal cells were densely packed and interconnected (Fig. 1L). Taken together, the D25 mice and the AMC mice (3 months) represented controls for the two DHT treatment groups at 1 week and 2 months, respectively.
Changes in the theca/stromal compartment of DHT-treated mice
To better understand the phenotypes observed in the ovarian stroma in response to DHT, biochemical (Picrosirius Red and Oil Red O) and IHC stains for stromal markers were carried out: Picrosirius Red for fibrillar collagens I and III; immunostaining of the basal lamina (COLIVA1) (37) that outlined both the hyperplastic interstitial “packets” and “open, foamy spaces”; Oil Red O that stained lipid accumulation (48); and vimentin that stained (41) vascular elements associated with interstitial cells and corpora lutea and was broadly disrupted in the stroma and theca cells of DHT-treated ovaries. Collectively, these changes in the overall structure and composition of the stroma suggest a fundamental shift in stromal differentiation in response to androgens (49).
CYP17A1 and AR expression and localization in the mouse ovary in response to DHT
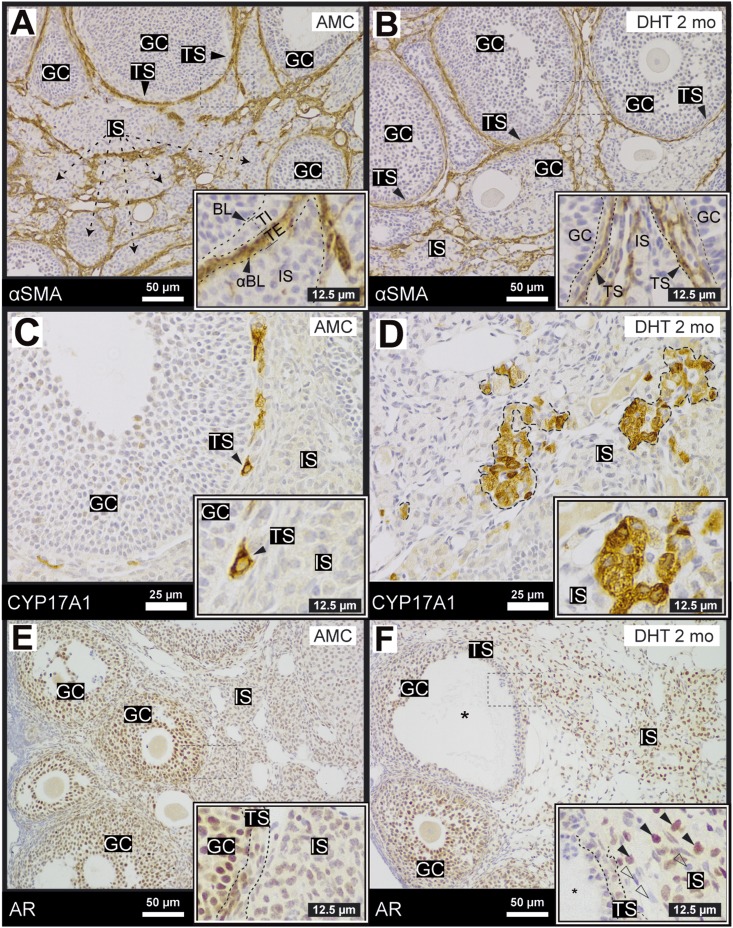
In the ovary, αSMA immunostaining (34) marks the theca externa (or the vascular/fibroblastic theca) (Fig. 2A) (2, 5, 50). DHT treatment did not impact αSMA localization relative to the internal theca layer and granulosa cells (Fig. 2B) and provided an effective demarcator between theca externa and the ovarian stroma. To analyze the basic endocrine functions of the theca and interstitial cells, IHC for the key steroidogenic enzyme CYP17A1 (36) (Fig. 2C) was carried out (51, 52). Generally, small populations of CYP17A1 immunoreactive cells were observed closely associated with the theca interna of large follicles in AMC mice (Fig. 2C). In contrast, ovaries of DHT-treated mice exhibited severe dislocation of CYP17A1+ cells to patches within the stroma (Fig. 2D). To determine the link between androgens and AR in the theca and interstitial ovarian compartments, and whether the classical ligand-induced nuclear translocation of AR in response to androgens was observable in DHT-treated mice, AR immunolabeling (35) was carried out. AR was present and nuclear in granulosa cells (2E) as previously described (2, 19). AR was also present but mostly cytoplasmic in the theca and stroma cells of AMC mice (Fig. 2E). In response to DHT treatment, AR nucleated in the stromal compartment specifically, with no distinct differences noted in AR localization in the granulosa cells of DHT-treated ovaries (Fig. 2F). These data suggest that AR activation by DHT drives phenotypic changes in the stroma that may contribute to abnormal ovarian function in this particular model (Figs. 1 and 2).
Figure 2.
Ovaries of DHT-treated females exhibit changes in AR localization in the theca and interstitial compartments. (A, B) The vascular cell marker, αSMA (ACTA2) (34) was used to demarcate the external theca because it did not exhibit major changes on DHT treatment (B). (C, D) CYP17A1 was expressed (36) exclusively in specific cells within the theca layer but was not present in granulosa cells or the stromal compartment. After DHT treatment, clusters of CYP17A1+ cells were randomly dispersed in the stroma. (E, F) AR, stained by IHC (35), was nuclear and expressed at high levels in most granulosa cells in control ovaries. AR was nuclear in some theca cells but appeared diffuse and cytoplasmic in the interstitial compartment. On treatment with DHT, AR was nucleated in a subpopulation of interstitial cells (black arrow, inset); an AR− population of cells also expanded (open arrow, inset). N > 3 for all conditions. BL, basal lamina; GC, granulosa cell; IS, interstitial stroma; TE, theca externa; TI, theca interna; TS, theca stroma (TI and TE); αBL, α-smooth muscle actin boundary.
DHT impacts gene expression profiles in both the ovarian stromal and granulosa compartments
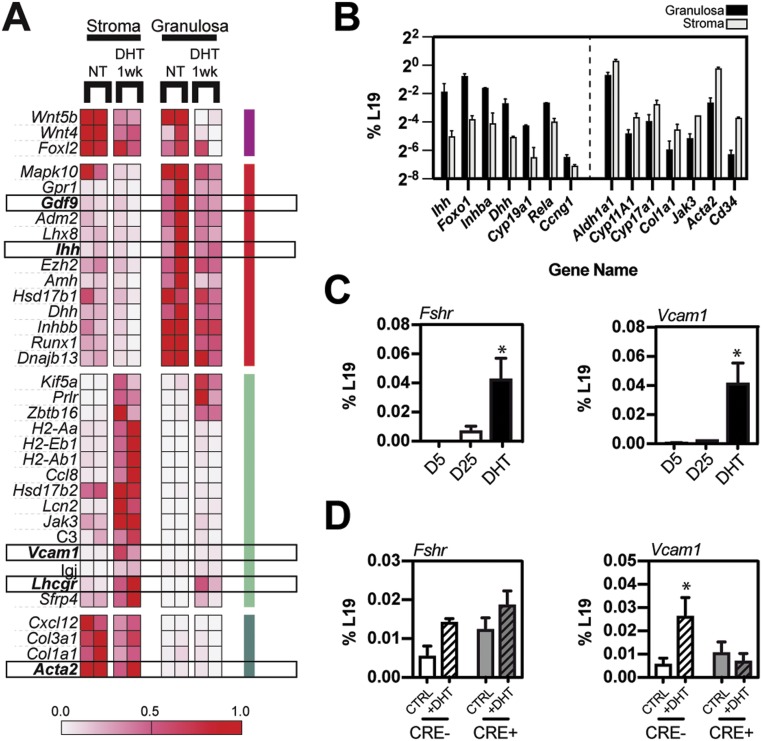
To determine the impact of androgens on the molecular transcriptional landscape within the ovary, microarray analyses were carried out on RNA prepared from mechanically separated stroma (theca, interstitial, vascular, and immune cells and primordial follicles) and granulosa cells of control mice at D25, as well as from mice at 1 week after DHT treatment. Tissues from D25 controls were preferable to AMC control ovaries where corpora lutea were abundant and added complexity to the stromal tissue compartment. Analysis of the microarray data identified specific genes regulated by androgens in the ovarian stroma and granulosa tissues (Fig. 3A). As shown, DHT treatment (i) selectively and markedly induced Vcam1 in stromal cells (Fig. 3A, 3C, and 3D), (ii) increased Lhcgr expression in theca and granulosa cells (Fig. 3A), and (iii) downregulated genes that are selectively expressed in the oocyte (Gdf9) and granulosa cells (Ihh, Dhh) and are factors known to regulate theca cell recruitment and steroidogenesis (Fig. 3A and 3B) (2, 5). Arrays carried out on isolated stromal and granulosa compartments were validated by qPCR to assess the purity of mechanically separated compartments (Fig. 3B). Genes encoding known granulosa markers Ihh, Dhh, Inhba, and Foxo1 were enriched in the granulosa compartment, whereas genes encoding theca-associated steroidogenic and connective tissue markers were enriched in the stromal fraction. FSH receptor (Fshr) is a known AR target gene (53) and was upregulated in response to DHT treatment, but only within the granulosa compartment (Fig. 3C and 3D) (data not shown). In contrast, Wnt4, a critical upstream regulator of female sex differentiation and regulator of granulosa cell proliferation and differentiation (54, 55), was persistently suppressed by DHT (Fig. 3A). Within the stromal compartment, DHT induced genes associated with immune function (C3, MHII molecules, Jak3) and triggered the broad suppression of genes involved in stromal reactions and biogenesis (Acta2, Col3a1, Col1a1, Cxcl12) (Fig. 3A and 3B).
Figure 3.
Microarray analyses of DHT-treated ovaries identified specific changes in gene expression in granulosa and stromal cells, with the notable induction of Vcam1 in the stroma. (A) Microarray analyses were done on total RNA derived from mechanically separated stromal and granulosa cells from ovaries of control mice and mice treated with DHT for 1 wk. A curated list, presented as a heat map, identified ovarian genes regulated selectively by DHT in the ovarian stroma and granulosa fractions. (B) A comparison of the expression levels of ovarian marker genes was carried out on isolated stromal and granulosa compartments to validate the purity (∼90%) of mechanically separated stromal and granulosa compartments. (C) Fshr, a granulosa marker and androgen target, was induced by androgens but only in the granulosa compartment, whereas Vcam1 was potently induced specifically in the stromal compartment. (D) Theca-specific AR deletion (in CYP17A1-iCRE, ARf/f mice) did not prevent the induction of Fshr expression in response to DHT (a granulosa-specific response), but it did inhibit Vcam1 induction in the theca, demonstrating that the signal to upregulate stromal Vcam1 mRNA levels is transmitted through theca-specific AR. (A) Microarrays were carried out on two biological replicates. (B) Microarray validation and (C, D) other results were carried out on N > 3 for all conditions. *P < 0.05 by Student t test.
To determine whether theca cell AR impacts Vcam1 expression selectively in theca cells, we analyzed the expression of Vcam1 and Fshr mRNA in ovaries obtained from theca cell–specific (Cyp17a1-iCre) ARf/f knockout (ThARKO) mice untreated or treated with DHT where theca cell depletion of AR prevented a PCOS-like phenotype in mice exposed to excess androgens (23). DHT treatment of control mice increased ovarian Vcam1 mRNA expression; this response was blocked in the ThARKO mouse ovaries (Fig. 3D), demonstrating that the effect of DHT on Vcam1 induction was dependent on theca-specific AR expression and activation. The expression of Fshr mRNA remained relatively steady under these same treatment conditions, indicating that granulosa cell AR activation was not altered when AR is lost in theca cells (Fig. 3D). These experiments further confirmed that Vcam1 expression was induced by AR specifically in cells derived from the CYP17A1-expressing lineage, which likely includes both active steroidogenic theca cells and derivatives of those cells populating the interstitial stroma (Fig. 3C and 3D).
Vcam1 is a DHT-responsive gene in ovarian theca and stromal cells
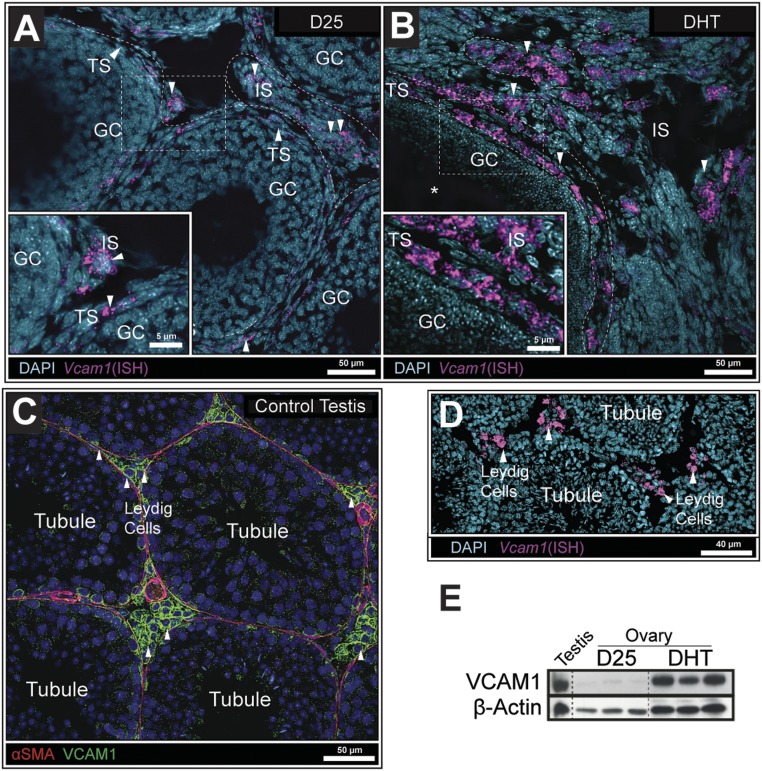
To further confirm that Vcam1 was selectively expressed and induced by DHT in the ovarian stromal compartment, we performed mRNA ISH. These experiments were critical for determining the cell type origin of Vcam1 mRNA because VCAM1 protein is known to be proteolytically cleaved and solubilized externally (56). In situ analyses documented that Vcam1 mRNA was expressed in a few theca cells of small growing follicles in ovaries of D25 control mice (Fig. 4A), but it was highly and selectively induced in theca and interstitial cells of DHT-treated mice (Fig. 4B). Vcam1 mRNA was clearly not detected in granulosa cells. Because VCAM1 is a known marker of fetal and adult Leydig cells (57–59), the expression of Vcam1 mRNA was also analyzed in testis tissue. As expected, Vcam1 mRNA was distinctly and highly expressed in Leydig cells as indicated by the intense in situ and IF staining (31, 34) in the stromal compartment of the testis (Fig. 4C and 4D). The induction of VCAM1 protein was confirmed by Western analyses (31) of tissue lysates prepared from (i) whole ovaries of D25 control mice and 1 week DHT-treated mice, a time point sufficient to observe substantial VCAM1 induction (Fig. 4E), and (ii) from mouse testes as a comparison and positive control. As shown, VCAM1 protein was high in testis samples and in ovarian samples from DHT-treated mice, but low in ovaries from D25 control mice (Fig. 4E). These observations provide intriguing evidence of a functional link between VCAM1 and the steroidogenic cells that produce androgens in both the male and female gonads.
Figure 4.
Vcam1 transcripts were detected in ovarian theca and interstitial cells but not in granulosa cells. (A) ISH in D25 mouse ovaries detected low Vcam1 expression in a few theca cells within the ovary. (B) In contrast, Vcam1 expression was highly elevated in both the theca and stroma of DHT-treated mice. (C) IF images show that VCAM1+ Leydig cells were in contact with VCAM1−/αSMA+ cells lining the seminiferous tubule (31, 34). (D) Vcam1 mRNA was localized exclusively to Leydig cells as determined by ISH. (E) Western analyses of tissue lysates showed that VCAM1 was low in ovaries of D25 control mice but highly induced in ovaries of DHT-treated mice to levels observed in testes from adult mice, as a control. β-Actin was used as a loading control (32). N > 3 for all conditions.
Localization of AR, NR2F2, and VCAM1 in the mouse ovary
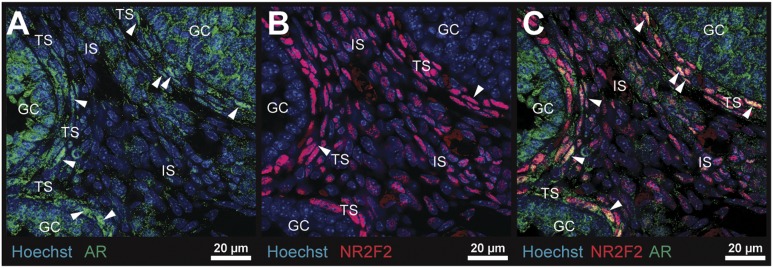
To more precisely document the localization of AR and the AR comodulatory factor NR2F2 in specific ovarian cell types, IF analyses (35, 38) were carried out on a D25 control ovary. As shown, AR was nucleated in granulosa cells and theca cells of growing follicles (Fig. 5A) in association with active androgen biosynthesis, a phenomenon absent in the ovarian interstitial stroma of D25 control mice (Fig. 5A). In contrast, NR2F2 immunostaining (38) was localized exclusively and intensely to nuclei in theca cells and some stromal cells but was not present in any granulosa cells (Fig. 5B). NR2F2 colocalized with nuclear AR+ theca cells, providing strong evidence for possible functional interactions of AR and NR2F2 in theca cells but not in granulosa cells (Fig. 5C).
Figure 5.
Expression and localization of AR and NR2F2 in the immature mouse ovary. (A) AR (green) was localized (35) to granulosa and theca cell nuclei in D25 control ovaries: theca ARs are denoted by the white arrowheads. (B) NR2F2 (red) was localized (38) to theca and stromal cell nuclei but was not expressed in granulosa cells. (C) AR and NR2F2 (yellow) colocalized to many but not all theca cells and stromal cells. GC, granulosa cell; IS, interstitial stroma; TS, theca stroma.
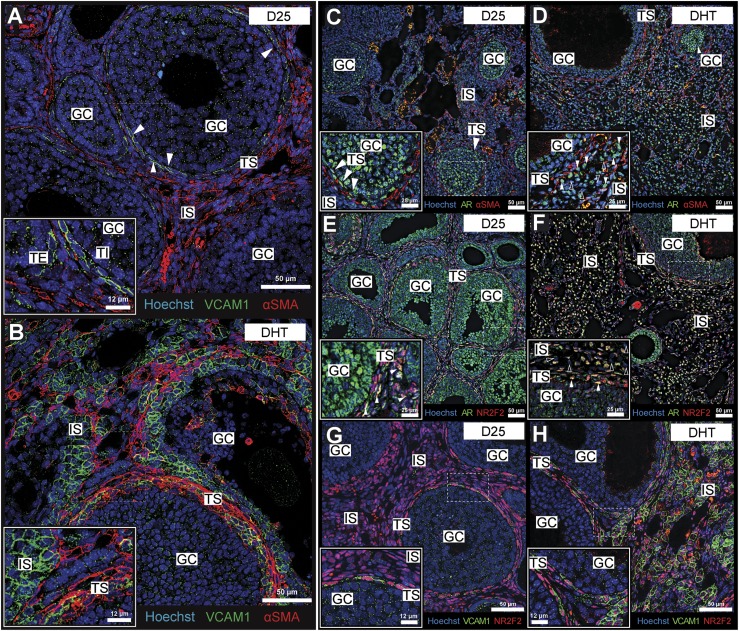
To determine the impact of DHT on the cellular and spatial localization of VCAM1 relative to AR and NR2F2, additional IF images were obtained from ovaries of control and DHT-treated mice. Considering that VCAM1 is best known for its expression in the vascular endothelium in response to an inflammatory challenge and is shown to participate in the recruitment of immune cells from circulation (60, 61), we originally hypothesized that VCAM1 might be expressed in the vascular endothelium. This is clearly not the case, as VCAM1 did not colocalize (31, 34) with the αSMA+ fibroblastic layer (Fig. 6A). Rather, VCAM1 was present on cells adjacent to the basal lamina, possibly associated with the steroidogenic theca cells (Fig. 6A). Furthermore, with DHT treatment, VCAM1+ cells expanded into the stroma and became disorganized relative to the αSMA boundary, suggesting a general loss of theca integrity in ovaries of the DHT-treated females (Fig. 6B). The localization of AR exhibited a similar pattern of expression (34, 35) as VCAM1 (Fig. 6C and 6D): it was not associated with αSMA+ cells in the theca layer but was highly expressed in theca and stromal cells that expanded in the ovaries of the DHT-treated mice. Furthermore, AR colocalized (35, 38) with NR2F2 not only in the theca layer of control mice but also with NR2F2+ cells in the stroma of the DHT-treated mice (Fig. 6E and 6F). Lastly, VCAM1+ cells were also NR2F2+ in the theca and stroma of control and DHT-treated mice (31, 38) (Fig. 6G and 6H). These data provide compelling evidence that the expanded populations of VCAM1+ cells are derived from AR+/NR2F2+ theca and stromal cells, supporting the evidence that they are likely to be functionally linked to AR activation (Fig. 3D).
Figure 6.
VCAM1 was expressed in the NR2F2+ ovarian stromal lineage and coexpressed with AR+ stromal cells. (A) VCAM1 was not expressed (31) on fibroblastic (αSMA+) theca cells (34), but it was present on steroidogenic theca cells in the D25 mouse ovary. (B) DHT treatment triggers a broad thickening of the VCAM1+ theca layer, especially in abnormal follicles, as well as an abundance of VCAM1+ (αSMA−) cells in the ovarian interstitial compartment. (C) In the D25 control ovary, AR was nucleated (35) in the ovarian theca cells, but it was rarely nucleated in stromal interstitial cells. (D) Stains for AR in the DHT-treated ovaries revealed an abundance of nucleated AR+ cells, especially in the interstitial compartment. (E) The theca/interstitial marker NR2F2, costained (38) with nuclear AR (35) in the theca of D25 ovaries. (F) Similarly, nucleated AR and NR2F2 colocalized in both the theca and interstitial layer upon treatment with DHT. (G) In the D25 ovary, VCAM1 was expressed in NR2F2+ theca cells (31, 38), particularly those adjacent to the collagen boundary with granulosa cells (inner theca). (H) DHT induced the expression of VCAM1 in NR2F2+ theca and interstitial cells that expanded in the stromal compartment. N ≥ 3 for all conditions. GC, granulosa cell; IS, interstitial stroma; TS, theca stroma.
Using CX3CR1-GFP knock-in mice and costaining GFP (antibody) (40) with the immune cell marker F4/80 (39), we observed distinct ovarian-associated immune cell populations [(62); see also (25, 63)]. Although immune cells increased within the stroma of DHT-treated CX3CR1-GFP reporter mice, they appeared to be recruited independently of VCAM1 and primarily to atretic follicles that are more abundant in DHT-treated mice (62).
Regulation of VCAM1 in human PCOS theca cells
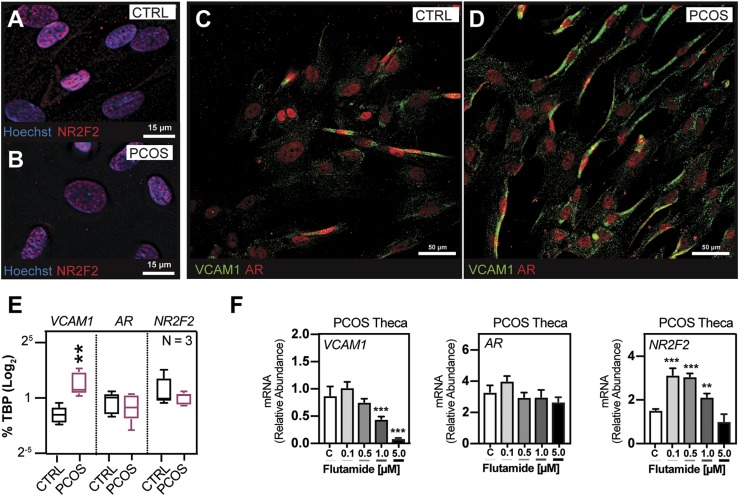
To further unravel the molecular mechanisms underlying AR-driven induction of Vcam1 mRNA, we used a serum-free defined medium to culture human theca cells derived from normal and age-matched patients with PCOS. Human PCOS theca interna cells that have been passaged in culture for numerous population doublings display intrinsically augmented CYP17A1 transcript levels, which are associated with elevated DHEA and androgen biosynthesis (15). The cause of increased androgen production in PCOS theca cells is currently unknown, but it is thought to result from altered intracellular signaling, as well as altered genetic and epigenetic regulation (15, 64). A comparison of passaged human theca cells isolated from normal cycling and women with PCOS demonstrated that both AR and NR2F2 immunostaining (38, 42) was nuclear in these cells. These results indicate that endogenous production of androgens is sufficient to nucleate AR and induce expression of VCAM1 that was localized to the theca cell membrane in control and PCOS samples (Fig. 7A–7D). However, IF staining (31, 42) of VCAM1 protein was more intense and levels of VCAM1 mRNA were higher in the PCOS theca cells than in control theca cells (Fig. 7C–7E; Western data not shown), whereas the levels of AR and NR2F2 mRNAs were similar in normal and PCOS theca cells (Fig. 7E). Of critical relevance, exposure of PCOS theca cells to DHT did not alter the expression of VCAM1 mRNA (65), whereas exposure of PCOS theca to the potent AR antagonist flutamide significantly reduced the expression of VCAM1 mRNA without altering the expression of AR mRNA (Fig. 7F). Levels of NR2F2 mRNA increased at low doses of flutamide (Fig. 7F). These results provide strong evidence that AR activation in human theca cells impacts the expression of Vcam1 mRNA, as was observed in the ThARKO mouse ovary, and that this may involve changes in the expression and/or activation status of NR2F2 (Fig. 3D and Fig. 7F).
Figure 7.
Nuclear NR2F2 and AR were present in VCAM1+ PCOS theca cells; the AR antagonist flutamide markedly and selectively reduced VCAM1 mRNA. (A, B) IF showed that NR2F2 was expressed and nuclear (38) in control and PCOS theca cells in culture. (C, D) IF also showed that AR was nuclear (35) and was colocalized with VCAM1+ theca cells where VCAM1 appears membrane bound (31). (E) PCOS patients expressed higher levels of VCAM1 mRNA than did control patients, whereas AR and NR2F2 mRNA levels were similar in control and PCOS samples. (F) Exposure of PCOS theca cells to a dose-dependent increase of the AR antagonist flutamide markedly reduced expression of VCAM1 mRNA and increased NR2F2 without changes in AR transcript level. N = 3 for all conditions. **P < 0.025, ***P < 0.01 by Student t test.
Because LH regulates theca cell androgen biosynthesis and differentiation, we tested the response of the human theca cells in culture to forskolin, an agonist that potently mimics LH signaling by increasing cAMP, the expression of CYP17A1, and androgen biosynthesis (15). Surprisingly, forskolin potently reduced VCAM1 expression in human theca cells, indicating that theca expression of VCAM1 might also be regulated by gonadotropins in vivo (65). To assess this, ovaries were isolated from immature DHT-treated mice after a superovulatory regimen of eCG to induce preovulatory follicle growth and hCG to induce ovulation and luteinization (66, 67). DHT-treated mice ovulated in response to gonadotropin stimulation, but the number of cumulus cell–oocyte complexes released was reduced compared with control mice (68). D25 control females ovulated an average of 24.4 ± 3.20 oocytes, whereas DHT-treated females ovulated significantly fewer (12.0 ± 4.03) oocytes (P = 0.031).
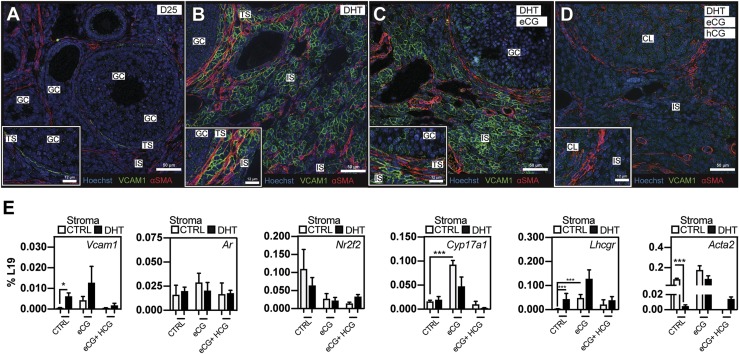
IHC analyses of ovaries isolated from the superovulated mice revealed dramatic changes in stroma and theca cell morphology. Specifically, eCG administration triggered a partial reduction of interstitial hyperplasia, whereas a complete superovulatory regimen with a luteinizing dose of hCG fully reverted interstitial pathology associated with the DHT-only condition to a more normal phenotype (68). Key observations from these studies are as follows: (i) females, even in the presence of high androgen levels, were capable of ovulating; (ii) superovulation rescued normal stromal morphology from that associated with the DHT-treated condition; and (iii) forskolin treatment of human theca cells suppressed VCAM1 transcription of both normal patients and patients with PCOS. With these findings in mind, we sought to determine the impact of gonadotropins on VCAM1 expression in the ovary. IF costaining (31, 34) revealed that VCAM1 was (i) expressed in selected theca cells in the ovaries of control D25 mice (Fig. 8A), as shown in Fig. 6; (ii) induced by DHT in the stroma as expected (Fig. 8B); (iii) further induced by eCG in theca of large antral follicles (Fig. 8C); and (iv) suppressed completely following exposure to an ovulatory and luteinizing dose of hCG (Fig. 8D), providing evidence for remarkable plasticity of VCAM1 expression in cells within the ovarian stroma.
Figure 8.
Markers of theca cell function, including VCAM1, are increased by eCG and inhibited by a luteinizing dose of hCG, regardless of DHT treatment. (A) In D25 controls, VCAM1 was expressed (31) at low levels in theca cells of a developing follicle internal to the fibroblastic theca (αSMA+) (34). (B) Ovaries of DHT-treated females exhibited abnormal Vcam1 localization in the theca and interstitial compartment of the ovary. (C) When eCG was administered to DHT-treated females, a small enhancement/compaction of VCAM1+ cells occurred in both the theca and interstitial stroma, whereas (D) a subsequent luteinizing dose of hCG potently abrogated VCAM1 staining in the stromal compartment as well as in theca cells surrounding small growing follicles. (E) To determine the impact of cotreatment with DHT and gonadotropins, and to more precisely document the general physiological timing of VCAM1 expression, qPCR analyses on ovarian RNA prepared from mice on single and combination treatments with DHT and/or eCG and hCG were carried out. Under these conditions, stromal Acta2 expression is inhibited by DHT treatment, but it is reexpressed on treatment with eCG, suggesting a link to stromal remodeling. Whereas Nr2f2 levels decreased in response to eCG/hCG treatments, Ar mRNA remain unchanged. Vcam1, Lhcgr, and Cyp17a1 theca markers were induced on treatment with eCG and conversely suppressed when treated with ovulatory hCG. N = 3 for all conditions. *P < 0.05, ***P < 0.01 by Student t test. GC, granulosa cell; IS, interstitial stroma; TS, theca stroma.
qPCR analyses confirmed the IF data indicating that eCG can upregulate Vcam1 in the theca of growing follicles but hCG potently suppressed expression of Vcam1 mRNA upon ovulation and luteinization (Fig. 8E). The effects of eCG and hCG were not related to major changes in expression of either stromal Ar or Nr2f2 (Fig. 8E). As shown, eCG also triggered the upregulation of Cyp17a1 and Lhcgr in theca/stromal cells, recapitulating the pattern but not the magnitude of the response to DHT. Lastly, Cyp17a1 was substantially induced by eCG, but it was reduced to basal levels upon treatment with hCG, a pattern that mirrors the regulation of Vcam1 by eCG and hCG. Collectively, these results indicate that Vcam1 and other genes appear to be tightly regulated by the gonadotropins and that this may involve specific changes in the intrafollicular levels of androgens, as well as AR activation in theca/stromal cells.
Discussion
These studies show that AR was present in multiple cell types—granulosa, theca, and stroma—within the mouse ovary, and that exposure of mice to excess androgen (DHT) led to marked changes in the structure and function of the theca and stromal compartments, as well as the granulosa cells. Specifically, these studies document that excess androgens profoundly altered the molecular, cellular, and matrix landscapes in the ovarian stroma, shown by marked changes in the content and distribution of AR, NR2F2, VCAM1, and CYP17A1.
Most dramatic was the induction of Vcam1 mRNA in the theca and stroma compartments and the elevated presence of VCAM1 protein in cells throughout the stroma in DHT-treated mice. Whether these VCAM1+ cells in the stroma represent an expansion and displacement of theca stem cells to the stroma or the differentiation/expansion of stromal stem cells to VCAM1-expressing cells remains to be determined. However, VCAM1+ cells expressed both AR and NR2F2, the latter of which is a known marker of mesenchymal cells that in the embryonic ovary (22) and testis (69) are presumed to be progenitors of theca/stromal and Leydig cells, respectively (22). Recent studies showed that VCAM1 is also a marker of interstitial progenitor cells in the testis (58) and is present at high levels in adult Leydig cells (57, 59). Thus, excess androgens in the female may steer theca/stromal cell differentiation toward a Leydig cell phenotype. In support of this, recent studies reported that damaging mutations in NR2F2 in human 46,XX embryos can lead to the appearance of testis tissue and excess androgen production by the fetal ovary, suggesting that NR2F2 plays a key role in suppressing AR signaling in the human fetal female embryonic gonad (70). That theca cell disruption of AR gene expression blocked the induction of Vcam1 in the ovaries of DHT-treated mice compared with controls provided additional, more direct evidence that theca levels of AR and its activation by excess androgens are critical for the induction of VCAM1 in these cells. The marked reduction of VCAM1 expression by the potent AR antagonist flutamide in androgen-producing PCOS theca cells in culture further supports the hypothesis that AR activation in theca cells impacts VCAM1 expression, possibly by also altering the expression and/or activity of NR2F2. Thus, we propose that AR and NR2F2 comprise a regulatory feedback loop within theca cells to prevent excessive androgenization of the ovary in female mice (Fig. 9).
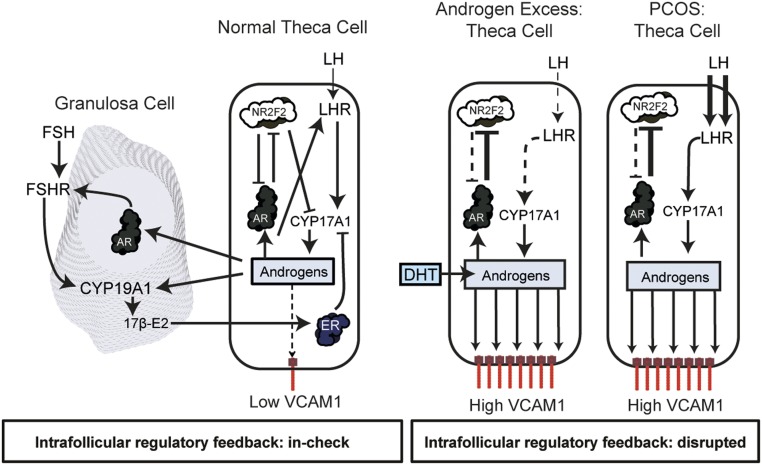
Figure 9.
Elevated androgens alter intrafollicular regulatory mechanisms that impact the expression of VCAM1 and the expansion of VCAM1+ cells in the stroma. In the normal follicle, LH induction of androgen biosynthesis in theca cells is critical for androgen action via AR in granulosa cells, leading to the induction of FSHR and CYP19A1, the enzyme that converts androgens to estrogens. Estrogens, in turn, bind estrogen receptors (ESR1) in theca cells to suppress the activity of CYP17A1. Similar to granulosa cells, theca cells express AR. However, unlike granulosa cells, theca cells also express NR2F2, a coregulatory molecule that can modulate/suppress AR functional activity and the activation of AR target genes such as VCAM1. We hypothesize that in the presence of excess androgens (such as in the DHT mouse model) or in theca of patients with PCOS who express elevated endogenous androgens, chronic activation of AR by androgens disrupts the AR–NR2F2 regulatory feedback mechanism, leading to marked induction of AR target genes such as VCAM1. When this regulatory pathway is disrupted, elevated androgens promote the expansion of Leydig-like VCAM1+ theca and stromal cells within the ovary.
Although VCAM1 is most often associated with endothelial cells and the attachment of immune cells at sites of inflammation (71), its high level in the stroma of DHT-treated mice and in adult Leydig cells indicated that there are additional functions for VCAM1 in reproductive tissues, including the gonads. Importantly, VCAM1 did not appear to be associated or localized selectively with αSMA+ fibroblastic cells or immune cells in the DHT-treated ovaries, suggesting that VCAM1 in the expanded theca/stromal and Leydig cells likely exerts other functions, such as mediating cell–cell interactions. For example, the embryonic allantoic membrane expresses VCAM1 and this is essential for embryo viability and attachment to luminal endometrial cells of the uterus (72, 73). Although heart defects were thought to mediate embryonic mortality in the Vcam1-null mice (74), recent analyses of the Foxo1-null mice, which also die early in embryogenesis, indicated that embryo attachment to the luminal cells via VCAM1 was the primary critical role for this protein in preventing embryo lethality at this stage (73). VCAM1 has also been shown to be associated with cancer cells (75, 76) and stem cells (77–80), indicating complex roles for this protein in many tissues.
Excess androgens also altered the number and spatial arrangement of endocrine cells expressing CYP11A1 and CYP17A1 in the theca/stromal landscape. In control mice, CYP17A1 was expressed in specific cells within the theca layer but appeared displaced in random patches in the stroma of DHT-treated mice. These patches of endocrine cells were distinct but also overlapping with some VCAM1+ cells, indicating that the steroidogenic cells may represent a distinct population of VCAM1 theca/stromal cells. These changes in theca endocrine cells and mesenchymal cells may also be related to changes in oocyte and granulosa cell functions that impact theca cell functions. Specifically, the levels Gdf9 mRNA were reduced in the DHT-treated mice, reflecting either the loss of oocytes and/or reduced expression of this transcript in oocytes. Strikingly, reduced expression of Gdf9 was associated with markedly reduced expression of Dhh and Ihh in granulosa cells of the DHT-treated mice. Because these two HH signaling molecules are essential for theca cell recruitment, organization, and function at early stages of follicular growth (5), it is possible that downregulation of HH signaling impaired the development of theca endocrine cells as well as theca mesenchymal-like cells and that in the absence of HH signaling these cells distributed differently in the stroma during early stages of follicle growth. Altered localization of CYP17A1+ cells to the stroma has also been observed in the theca cell–specific Esr1 knockout mice that exhibited elevated levels of androgens and a PCOS-like phenotype (6). Lastly, ovaries of aging mice in which androgen levels were elevated exhibited dislocation of CYP17A1+ clusters of cells to the stroma (81) that are also VCAM1+ (data not shown).
Excess androgens altered the expression of other genes that impact granulosa cell specification and differentiation. Genes that were markedly downregulated include Cyp19a1, Amh, Foxl2, Wnt4, and Wnt5a (82, 83). The reduced expression of these genes likely relates to granulosa cell apoptosis and granulosa cell specification, as well as the lack of luteinization (Wnt4). Genes that were upregulated include Ar, Foxo1, Fshr, and Lhcgr. The increase in Fshr and Lhcgr represent known targets of AR action in granulosa cells and possibly theca cells (16, 19, 84, 85). Markers of immune cells were also elevated in the DHT-treated mice compared with controls and likely represent the immune cell infiltration related to the hemorrhagic follicles and apoptotic granulosa cells in follicles undergoing atresia and may be involved in suppressing autoimmune responses.
In summary, these studies provide novel evidence that androgens regulate theca and stroma cell functions and promote molecular characteristics of Leydig cells, namely increased expression of VCAM1, a marker of adult Leydig cells. These studies also highlight the presence and potential impact of NR2F2 in regulating androgen and AR activation and function in theca and stromal cells. Importantly, NR2F2 was not coexpressed with AR in granulosa cells, indicating that this may reflect key differences in androgen action in these two cell types in growing follicles (Fig. 9). VCAM1 mRNA was elevated in human theca cells of patients with PCOS relative to control patients and was markedly reduced by an AR antagonist. In human theca cells treated with forskolin to mimic LH action, VCAM1 mRNA levels were unexpectedly reduced. This response may represent (i) a differentiation phenomenon that coincides with VCAM1 suppression or (ii) activation of the PKA and other pathways that antagonize AR and/or NR2F2 actions, providing another regulatory loop in theca cells (85). In either case, to more precisely understand the effects of gonadotropins on the regulation of VCAM1 in the ovary, superovulation studies were carried out in mice. Whereas eCG increased theca cell expression of VCAM1, hCG suppressed VCAM1 expression in ovaries of both eCG- and eCG/DHT-treated mice. These observations provide novel evidence for both the normal physiological expression of VCAM1 in theca cells, as well as the abnormal expression of VCAM1 in response to elevated androgens, and that a superovulatory regimen of gonadotropins was capable of reversing this striking phenotype. Thus, tight intraovarian regulatory mechanisms involving androgen biosynthesis and AR activation control VCAM1 expression and normal follicular development. Disruption of these regulatory mechanisms may contribute to the etiology of PCOS and other androgenizing disorders in women (Fig. 9).
Acknowledgments
Advanced imaging was supported by the Integrated Microscopy Core directed by Drs. M. Mancini and Fabio Stossi. This project was also supported by the RNA In Situ Hybridization Core facility with the expert assistance of Dr. Cecilia Ljungberg. Microarray analyses were conducted by the Genomic and RNA Profiling Core, directed by Lisa White. Extensive sample preparation support was provided by the Pathology and Histology Core (M. Sayeeduddin, Shahida Salar, and Zahida Sayeeduddin) under the direction of Dr. Patricia Castro. All Cores are at the Baylor College of Medicine.
Financial Support: This work was supported by National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants NIH-HD076980 (to J.S.R.), R00-HD068130 04 (to S.W.), 5R01-HD083323-03 (to J.M.M.), and U54 HD083092 (RNA In Situ Hybridization Core); NIH/National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK056338 (Genomic and RNA Profiling Core); NIH/National Cancer Institute Grant CA125123 (Genomic and RNA Profiling Core and Pathology and Histology Core); NIH Office of the Director Grant S10 OD016167; Cancer Prevention and Research Institute of Texas Grant RP150578; the Dan L. Duncan Comprehensive Cancer Center; and the John S. Dunn Gulf Coast Consortium for Chemical Genomics (Integrated Microscopy; to F.S.).
Author Contributions: N.R.C. was responsible for experimental design, executing major experiments, and the drafting of the manuscript. A.P. was responsible for experimental design and manuscript writing. F.S. provided advanced microscopy expertise and participated in the drafting of the manuscript. M.C.L. carried out in situ hybridization experiments and was consulted during manuscript preparation. K.E.S. generated samples in use throughout the manuscript. B.K.P. supported the bioinformatics analysis in this manuscript. M.S. carried out staining and qPCR experiments used in this study. A.M.R. provided technical support and data analysis. J.M.M. carried out key human theca culture experiments and participated in the preparation of the manuscript. S.W. provided RNA from the ovarian theca-specific knockouts of AR and assisted in the drafting of the manuscript. J.S.R. directed this project, provided expert assistance in experimental planning, coordinated extensive collaborative efforts with other research groups, enabled sufficient resources to fund the project, and drafted the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AMC
age-matched control
- AR
androgen receptor
- BCM
Baylor College of Medicine
- D25
postnatal day 25
- DHH
desert hedgehog
- DIG
digoxigenin
- eCG
equine chorionic gonadotropin
- FSHR
FSH receptor
- hCG
human chorionic gonadotropin
- HH
hedgehog
- HRP
horseradish peroxidase
- IF
immunofluorescent
- IHC
immunohistochemical
- IHH
Indian hedgehog
- ISH
in situ hybridization
- PCOS
polycystic ovary sydrome
- qPCR
quantitative PCR
- ThARCO
theca cell–specific androgen receptor knockout
- VCAM1
vascular cell adhesion molecule 1
- αSMA
α-smooth muscle actin
References
- 1. Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140(4):489–504. [DOI] [PubMed] [Google Scholar]
- 2. Richards JS, Ren YA, Candelaria N, Adams JE, Rajkovic A. Ovarian follicular theca cell recruitment, differentiation, and impact on fertility: 2017 update. Endocr Rev. 2018;39(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Beek AP, Cantineau AE. Ovarian hyperthecosis. Available at: https://www.uptodate.com/contents/ovarian-hyperthecosis. Accessed 14 February 2018.
- 4. Dumesic DA, Richards JS. Ontogeny of the ovary in polycystic ovary syndrome. Fertil Steril. 2013;100(1):23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu C, Peng J, Matzuk MM, Yao HH. Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells. Nat Commun. 2015;6(1):6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee S, Kang DW, Hudgins-Spivey S, Krust A, Lee EY, Koo Y, Cheon Y, Gye MC, Chambon P, Ko C. Theca-specific estrogen receptor-α knockout mice lose fertility prematurely. Endocrinology. 2009;150(8):3855–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dunaif A. Perspectives in polycystic ovary syndrome: from hair to eternity. J Clin Endocrinol Metab. 2016;101(3):759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunaif A, Thomas A. Current concepts in the polycystic ovary syndrome. Annu Rev Med. 2001;52(1):401–419. [DOI] [PubMed] [Google Scholar]
- 9. Nelson VL, Legro RS, Strauss JF III, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13(6):946–957. [DOI] [PubMed] [Google Scholar]
- 10. Nelson VL, Qin KN, Rosenfield RL, Wood JR, Penning TM, Legro RS, Strauss JF III, McAllister JM. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86(12):5925–5933. [DOI] [PubMed] [Google Scholar]
- 11. Wickenheisser JK, Biegler JM, Nelson-Degrave VL, Legro RS, Strauss JF III, McAllister JM. Cholesterol side-chain cleavage gene expression in theca cells: augmented transcriptional regulation and mRNA stability in polycystic ovary syndrome. PLoS One. 2012;7(11):e48963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wickenheisser JK, Nelson-DeGrave VL, Hendricks KL, Legro RS, Strauss JF III, McAllister JM. Retinoids and retinol differentially regulate steroid biosynthesis in ovarian theca cells isolated from normal cycling women and women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(8):4858–4865. [DOI] [PubMed] [Google Scholar]
- 13. Wickenheisser JK, Quinn PG, Nelson VL, Legro RS, Strauss JF III, McAllister JM. Differential activity of the cytochrome P450 17α-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. J Clin Endocrinol Metab. 2000;85(6):2304–2311. [DOI] [PubMed] [Google Scholar]
- 14. Wood JR, Ho CK, Nelson-Degrave VL, McAllister JM, Strauss JF III. The molecular signature of polycystic ovary syndrome (PCOS) theca cells defined by gene expression profiling. J Reprod Immunol. 2004;63(1):51–60. [DOI] [PubMed] [Google Scholar]
- 15. Wood JR, Nelson VL, Ho C, Jansen E, Wang CY, Urbanek M, McAllister JM, Mosselman S, Strauss JF III. The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. J Biol Chem. 2003;278(29):26380–26390. [DOI] [PubMed] [Google Scholar]
- 16. Sen A, Prizant H, Light A, Biswas A, Hayes E, Lee HJ, Barad D, Gleicher N, Hammes SR. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci USA. 2014;111(8):3008–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walters KA, Allan CM, Jimenez M, Lim PR, Davey RA, Zajac JD, Illingworth P, Handelsman DJ. Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology. 2007;148(8):3674–3684. [DOI] [PubMed] [Google Scholar]
- 18. Wang F, Pan J, Liu Y, Meng Q, Lv P, Qu F, Ding GL, Klausen C, Leung PC, Chan HC, Yao W, Zhou CY, Shi B, Zhang J, Sheng J, Huang H. Alternative splicing of the androgen receptor in polycystic ovary syndrome. Proc Natl Acad Sci USA. 2015;112(15):4743–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24(7):1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang LH, Ing NH, Tsai SY, O’Malley BW, Tsai MJ. The COUP-TFs compose a family of functionally related transcription factors. Gene Expr. 1991;1(3):207–216. [PMC free article] [PubMed] [Google Scholar]
- 21. Petit FG, Jamin SP, Kurihara I, Behringer RR, DeMayo FJ, Tsai MJ, Tsai SY. Deletion of the orphan nuclear receptor COUP-TFII in uterus leads to placental deficiency (published correction appears in Proc Natl Acad Sci USA. 2007;104(23):9911). Proc Natl Acad Sci USA. 2007;104(15):6293–6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rastetter RH, Bernard P, Palmer JS, Chassot AA, Chen H, Western PS, Ramsay RG, Chaboissier MC, Wilhelm D. Marker genes identify three somatic cell types in the fetal mouse ovary. Dev Biol. 2014;394(2):242–252. [DOI] [PubMed] [Google Scholar]
- 23. Ma Y, Andrisse S, Chen Y, Childress S, Xue P, Wang Z, Jones D, Ko C, Divall S, Wu S. Androgen receptor in the ovary theca cells plays a critical role in androgen-induced reproductive dysfunction. Endocrinology. 2017;158(1):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walters KA, Allan CM, Handelsman DJ. Rodent models for human polycystic ovary syndrome. Biol Reprod. 2012;86(5):149, 1–12. [DOI] [PubMed] [Google Scholar]
- 25. Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20(11):4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McAllister J, Simpson E. Human Theca Interna Cells in Culture. San Diego, CA: Academic Press; 1993. [Google Scholar]
- 27. Nelson-Degrave VL, Wickenheisser JK, Hendricks KL, Asano T, Fujishiro M, Legro RS, Kimball SR, Strauss JF III, McAllister JM. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol. 2005;19(2):379–390. [DOI] [PubMed] [Google Scholar]
- 28. Nelson-DeGrave VL, Wickenheisser JK, Cockrell JE, Wood JR, Legro RS, Strauss JF III, McAllister JM. Valproate potentiates androgen biosynthesis in human ovarian theca cells. Endocrinology. 2004;145(2):799–808. [DOI] [PubMed] [Google Scholar]
- 29. McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, Legro RS, Strauss JF III. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci USA. 2014;111(15):E1519–E1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Candelaria NR, Padmanabhan A, Stossi F, Ljungberg MC, Shelly KE, Pew BK, Solis M, Rossano AM, McAllister JM, Wu S, Richards JS. Data from: VCAM1 is induced in ovarian theca and stromal cells in a mouse model of androgen excess. figshare 2019. Accessed 22 February 2019 https://figshare.com/s/868ca6e631f4c78be4eb.
- 31.RRID:AB_2721053, https://scicrunch.org/resolver/AB_2721053.
- 32.RRID:AB_476697, https://scicrunch.org/resolver/AB_476697.
- 33.RRID:AB_2070042, https://scicrunch.org/resolver/AB_2070042.
- 34.RRID:AB_444285, https://scicrunch.org/resolver/AB_444285.
- 35.RRID:AB_1563391, https://scicrunch.org/resolver/AB_1563391.
- 36.RRID:AB_2491005, https://scicrunch.org/resolver/AB_2491005.
- 37.RRID:AB_445160, https://scicrunch.org/resolver/AB_445160.
- 38.RRID:AB_2155627, https://scicrunch.org/resolver/AB_2155627.
- 39.RRID:AB_1140040, https://scicrunch.org/resolver/AB_1140040.
- 40.RRID:AB_10692764, https://scicrunch.org/resolver/AB_10692764.
- 41.RRID:AB_10695459, https://scicrunch.org/resolver/AB_10695459.
- 42.RRID:AB_367516, https://scicrunch.org/resolver/AB_367516.
- 43. Yaylaoglu MB, Titmus A, Visel A, Alvarez-Bolado G, Thaller C, Eichele G. Comprehensive expression atlas of fibroblast growth factors and their receptors generated by a novel robotic in situ hybridization platform. Dev Dyn. 2005;234(2):371–386. [DOI] [PubMed] [Google Scholar]
- 44. Candelaria NR, Padmanabhan A, Stossi F, Ljungberg MC, Shelly KE, Pew BK, Solis M, Rossano AM, McAllister JM, Wu S, Richards JS. Data from: VCAM1 is induced in ovarian theca and stromal cells in a mouse model of androgen excess. figshare 2019. Accessed 22 February 2019 https://figshare.com/s/b8315026b023673f3ae8.
- 45. Lombardi LA, Simões RS, Maganhin CC, Baracat MC, Silva-Sasso GR, Florencio-Silva R, Soares JM Jr, Baracat EC. Immunohistochemical evaluation of proliferation, apoptosis and steroidogenic enzymes in the ovary of rats with polycystic ovary. Rev Assoc Med Bras (1992). 2014;60(4):349–356. [DOI] [PubMed] [Google Scholar]
- 46. Solano ME, Sander VA, Ho H, Motta AB, Arck PC. Systemic inflammation, cellular influx and up-regulation of ovarian VCAM-1 expression in a mouse model of polycystic ovary syndrome (PCOS). J Reprod Immunol. 2011;92(1–2):33–44. [DOI] [PubMed] [Google Scholar]
- 47. van Houten EL, Kramer P, McLuskey A, Karels B, Themmen AP, Visser JA. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology. 2012;153(6):2861–2869. [DOI] [PubMed] [Google Scholar]
- 48. Ramírez-Zacarías JL, Castro-Muñozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97(6):493–497. [DOI] [PubMed] [Google Scholar]
- 49. Candelaria NR, Padmanabhan A, Stossi F, Ljungberg MC, Shelly KE, Pew BK, Solis M, Rossano AM, McAllister JM, Wu S, Richards JS. Data from: VCAM1 is induced in ovarian theca and stromal cells in a mouse model of androgen excess. figshare 2019. Accessed 22 February 2019 https://figshare.com/s/b36f6d3d760d27f9f70d.
- 50. Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12(9):2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hu MC, Hsu N-C, El Hadj NB, Pai CI, Chu HP, Wang CK, Chung BC. Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1. Mol Endocrinol. 2002;16(8):1943–1950. [DOI] [PubMed] [Google Scholar]
- 52. Borgeest C, Greenfeld C, Tomic D, Flaws JA. The effects of endocrine disrupting chemicals on the ovary. Front Biosci. 2002;7(1–3):d1941–d1948. [DOI] [PubMed] [Google Scholar]
- 53. Prizant H, Gleicher N, Sen A. Androgen actions in the ovary: balance is key. J Endocrinol. 2014;222(3):R141–R151. [DOI] [PubMed] [Google Scholar]
- 54. Boyer A, Lapointe E, Zheng X, Cowan RG, Li H, Quirk SM, DeMayo FJ, Richards JS, Boerboom D. WNT4 is required for normal ovarian follicle development and female fertility. FASEB J. 2010;24(8):3010–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ottolenghi C, Pelosi E, Tran J, Colombino M, Douglass E, Nedorezov T, Cao A, Forabosco A, Schlessinger D. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet. 2007;16(23):2795–2804. [DOI] [PubMed] [Google Scholar]
- 56. Garton KJ, Gough PJ, Philalay J, Wille PT, Blobel CP, Whitehead RH, Dempsey PJ, Raines EW. Stimulated shedding of vascular cell adhesion molecule 1 (VCAM-1) is mediated by tumor necrosis factor-α-converting enzyme (ADAM 17). J Biol Chem. 2003;278(39):37459–37464. [DOI] [PubMed] [Google Scholar]
- 57. Wen Q, Zheng QS, Li XX, Hu ZY, Gao F, Cheng CY, Liu YX. Wt1 dictates the fate of fetal and adult Leydig cells during development in the mouse testis. Am J Physiol Endocrinol Metab. 2014;307(12):E1131–E1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wen Q, Wang Y, Tang J, Cheng CY, Liu YX. Sertoli cell Wt1 regulates peritubular myoid cell and fetal leydig cell differentiation during fetal testis development. PLoS One. 2016;11(12):e0167920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Karpova T, Ravichandiran K, Insisienmay L, Rice D, Agbor V, Heckert LL. Steroidogenic factor 1 differentially regulates fetal and adult Leydig cell development in male mice. Biol Reprod. 2015;93(4):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aziz KE, Wakefield D. Modulation of endothelial cell expression of ICAM-1, E-selectin, and VCAM-1 by β-estradiol, progesterone, and dexamethasone. Cell Immunol. 1996;167(1):79–85. [DOI] [PubMed] [Google Scholar]
- 61. Li H, Cybulsky MI, Gimbrone MA Jr, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993;13(2):197–204. [DOI] [PubMed] [Google Scholar]
- 62. Candelaria NR, Padmanabhan A, Stossi F, Ljungberg MC, Shelly KE, Pew BK, Solis M, Rossano AM, McAllister JM, Wu S, Richards JS. Data from: VCAM1 is induced in ovarian theca and stromal cells in a mouse model of androgen excess. figshare 2019. Accessed 22 February 2019 https://figshare.com/s/b4bf95fa44c114daddcc.
- 63. DeFalco T, Potter SJ, Williams AV, Waller B, Kan MJ, Capel B. Macrophages contribute to the spermatogonial niche in the adult testis. Cell Reports. 2015;12(7):1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tata B, Mimouni NEH, Barbotin AL, Malone SA, Loyens A, Pigny P, Dewailly D, Catteau-Jonard S, Sundström-Poromaa I, Piltonen TT, Dal Bello F, Medana C, Prevot V, Clasadonte J, Giacobini P. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24(6):834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Candelaria NR, Padmanabhan A, Stossi F, Ljungberg MC, Shelly KE, Pew BK, Solis M, Rossano AM, McAllister JM, Wu S, Richards JS. Data from: VCAM1 is induced in ovarian theca and stromal cells in a mouse model of androgen excess. figshare 2019. Accessed 22 February 2019 https://figshare.com/s/0377274849e581a25e6b.
- 66. Papkoff H, Bewley TA, Ramachandran J. Physicochemical and biological characterizations of pregnant mare serum gonadotropin and its subunits. Biochim Biophys Acta. 1978;532(1):185–194. [DOI] [PubMed] [Google Scholar]
- 67. Papkoff H. Variations in the properties of equine chorionic gonadotropin. Theriogenology. 1981;15(1):1–11. [DOI] [PubMed] [Google Scholar]
- 68. Candelaria NR, Padmanabhan A, Stossi F, Ljungberg MC, Shelly KE, Pew BK, Solis M, Rossano AM, McAllister JM, Wu S, Richards JS. Data from: VCAM1 is induced in ovarian theca and stromal cells in a mouse model of androgen excess. figshare 2019. Accessed 22 February 2019 https://figshare.com/s/c3bc242e4b126942e9a9.
- 69. Qin J, Tsai MJ, Tsai SY. Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS One. 2008;3(9):e3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bashamboo A, Eozenou C, Jorgensen A, Bignon-Topalovic J, Siffroi JP, Hyon C, Tar A, Nagy P, Sólyom J, Halász Z, Paye-Jaouen A, Lambert S, Rodriguez-Buritica D, Bertalan R, Martinerie L, Rajpert-De Meyts E, Achermann JC, McElreavey K. Loss of function of the nuclear receptor NR2F2, encoding COUP-TF2, causes testis development and cardiac defects in 46,XX children. Am J Hum Genet. 2018;102(3):487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59(6):1203–1211. [DOI] [PubMed] [Google Scholar]
- 72. Gurtner GC, Davis V, Li H, McCoy MJ, Sharpe A, Cybulsky MI. Targeted disruption of the murine VCAM1 gene: essential role of VCAM-1 in chorioallantoic fusion and placentation. Genes Dev. 1995;9(1):1–14. [DOI] [PubMed] [Google Scholar]
- 73. Ferdous A, Morris J, Abedin MJ, Collins S, Richardson JA, Hill JA. Forkhead factor FoxO1 is essential for placental morphogenesis in the developing embryo. Proc Natl Acad Sci USA. 2011;108(39):16307–16312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, Buck CA, Labow MA. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121(2):489–503. [DOI] [PubMed] [Google Scholar]
- 75. Chen Q, Massagué J. Molecular pathways: VCAM-1 as a potential therapeutic target in metastasis. Clin Cancer Res. 2012;18(20):5520–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schlesinger M, Bendas G. Vascular cell adhesion molecule-1 (VCAM-1)–an increasing insight into its role in tumorigenicity and metastasis. Int J Cancer. 2015;136(11):2504–2514. [DOI] [PubMed] [Google Scholar]
- 77. Hu XL, Chen G, Zhang S, Zheng J, Wu J, Bai QR, Wang Y, Li J, Wang H, Feng H, Li J, Sun X, Xia Q, Yang F, Hang J, Qi C, Phoenix TN, Temple S, Shen Q. Persistent expression of VCAM1 in radial glial cells is required for the embryonic origin of postnatal neural stem cells. Neuron. 2017;95(2):309–325.e6. [DOI] [PubMed] [Google Scholar]
- 78. Lai AY, Lin SM, Kondo M. Heterogeneity of Flt3-expressing multipotent progenitors in mouse bone marrow. J Immunol. 2005;175(8):5016–5023. [DOI] [PubMed] [Google Scholar]
- 79. Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20(13):1692–1708. [DOI] [PubMed] [Google Scholar]
- 80. Dutta P, Hoyer FF, Grigoryeva LS, Sager HB, Leuschner F, Courties G, Borodovsky A, Novobrantseva T, Ruda VM, Fitzgerald K, Iwamoto Y, Wojtkiewicz G, Sun Y, Da Silva N, Libby P, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med. 2015;212(4):497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Umehara T, Kawai T, Kawashima I, Tanaka K, Okuda S, Kitasaka H, Richards JS, Shimada M. The acceleration of reproductive aging in Nrg1flox/flox;Cyp19-Cre female mice. Aging Cell. 2017;16(6):1288–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30(6):624–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Boyer A, Goff AK, Boerboom D. WNT signaling in ovarian follicle biology and tumorigenesis. Trends Endocrinol Metab. 2010;21(1):25–32. [DOI] [PubMed] [Google Scholar]
- 84. Comim FV, Teerds K, Hardy K, Franks S. Increased protein expression of LHCG receptor and 17α-hydroxylase/17-20-lyase in human polycystic ovaries. Hum Reprod. 2013;28(11):3086–3092. [DOI] [PubMed] [Google Scholar]
- 85. Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120(4):963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]