Summary
Clostridium difficile infection (CDI) is the number one hospital-acquired infection in the United States. CDI is more common and severe in inflammatory bowel disease patients. Here we studied the mechanism by which prior colitis exacerbates CDI. Mice were given Dextran Sulfate Sodium (DSS)-colitis, recovered for two weeks and infected with C. difficile. Mortality and CDI severity are increased in DSS-treated mice compared to controls. Severe CDI is dependent on CD4+ T cells, which persist after colitis-associated inflammation subsides. Adoptive transfer of Th17 cells to naïve mice is sufficient to increase CDI- associated mortality, through elevated IL-17 production. Finally, in humans, the Th17 cytokines IL-6 and IL-23 associate with severe CDI and patients with high serum IL-6 are 7.6 times more likely to die post-infection. These findings establish a central role for Th17 cells in CDI pathogenesis following colitis and identify them as a potential target for preventing severe disease.
eTOC Blurb
C. difficile infection is more common and severe in patients with inflammatory bowel disease. Saleh et al. demonstrate that colitis induces a population of Th17 cells that persists beyond the resolution of inflammation. These cells lead to increased disease severity and host mortality after infection with C. difficile.
Graphical Abstract

Introduction
Clostridium difficile is a spore-forming, Gram-positive, anaerobic bacterium that was identified as the cause of antibiotic-associated pseudomembranous colitis in 1978 (Bartlett et al., 1978). Despite an overall decline in infectious disease mortalities over the last 3.5 decades, mortality due to diarrheal diseases in the U.S. continues to rise (Bcheraoui et al., 2018). This trend is attributed to the spread of hypervirulent strains of C. difficile over the last decade (Bacci et al., 2011; Shuman and Malani, 2018), including the epidemic ribotype 027 strain used in our studies. The Center for Disease Control estimates that C. difficile causes almost 500,000 infections and 29,000 deaths annually in the United States alone (Lessa et al., 2015). These reports and others highlight the importance of studying C. difficile infection (CDI) and identifying therapeutic targets for reducing disease severity.
Inflammatory Bowel Disease (IBD) is a set of conditions, including Crohn’s disease and ulcerative colitis, characterized by gastrointestinal tract inflammation due to dysregulation of the immune response to commensal bacteria (Baumgart and Carding, 2007). IBD is an independent risk factor for CDI (Issa, et al., 2007; Nguyen et al., 2008; Cojocariu et al., 2015). Studies from the National Inpatient Sample, the largest short-stay hospital discharge database in the U.S., as well as single-center studies have reported a higher incidence of CDI in IBD patients compared to non-IBD patients (Rodemann et al., 2007; Issa, et al., 2007; Nguyen et al., 2008). Moreover, CDI in IBD patients is more severe as characterized by reduced times from admission to CDI, longer hospital stays, increased surgery and in-hospital mortality rates (Rodemann et al., 2007; Nguyen et al., 2008; Ananthakrishnan et al., 2008). However, the cause of increased incidence and severity of CDI in IBD patients remains poorly understood.
We hypothesized that in IBD patients, aberrant immune responses resulting in gut inflammation are the cause of increased CDI severity. Indeed, studies in mouse models as well as human patients have shown that the type of immune response mounted against CDI by the host can determine the severity and outcome of infection (Feghaly et al., 2013; Buonomo et al., 2016; Cowardin et al., 2016). In this study, we used Dextran Sulfate Sodium (DSS) colitis to model how prior gut inflammation due to IBD can influence the outcome of subsequent CDI by altering the host immune response. Our results show that colitis induces Th17 cells that are still present in the mesenteric lymph nodes after full recovery from colitis. We demonstrated by adoptive transfer that these Th17 cells are sufficient to increase mortality after challenge with C. difficile. Prior to this study, previous reports showed similar CDI-associated mortality in WT and Rag1−/− mice and so a role for T cells during primary CDI has been overlooked (Abt et al., 2015). Our findings describe an important role for CD4 T cells in general, and Th17 cells in particular, during CDI and identify them as a potential therapeutic target for patients with IBD who are at risk for severe disease.
Results
Increased CDI-associated mortality in mice with prior DSS colitis
To determine whether prior gut inflammation can predispose the host to severe subsequent CDI, we designed a mouse model where we treated mice with 2% DSS for 6 days to induce gut inflammation. After a two-week recovery period, mice were challenged with C. difficile (Figure 1 A). The recovery period was sufficient to reverse the DSS-induced weight loss, as well as restore gut barrier integrity as measured by permeability to FITC dextran (Figure 1, B-C). Examination of hematoxylin and eosin (H&E) stained histopathological sections of the caeca revealed that although DSS treatment caused an acute disruption of the epithelial barrier, the recovery period was sufficient to restore epithelial integrity (Figure S1 A). Furthermore, several studies have shown that DSS treatment causes the upregulation of several inflammatory cytokines, including TNFα, IFNγ, KC, IL-6, IL-1α and IL-1β (Alex et al., 2009; Arai et al., 1998 and Jeengar et al., 2017). Our data showed that although these cytokines were elevated during acute DSS colitis, the levels of these inflammatory cytokines were comparable between the untreated and DSS-treated groups after the recovery period (Figure S1 B). Despite the resolution of acute colitis, mice previously treated with DSS had higher CDI-associated mortality when compared to previously untreated controls (Figure 1 D). Mice with prior DSS colitis also had more severe CDI when scored for: weight loss, coat appearance, eyes/nose discharge, activity, posture and diarrhea (Figure 1, E-F). We observed that DSS mice developed severe disease early starting at day 2 of infection. Interestingly, when we measured C. difficile burden at the peak of infection on day 2, no differences were found between DSS mice and untreated mice (Figure 1 G). Additionally, C. difficile toxin A/B levels during infection were comparable between the two groups (Figure 1 H). Next, we tested whether increased severity of disease in the DSS-treated mice was due to DSS-induced changes in the gut microbiota. To characterize changes in the bacterial communities in the gut, we utilized 16S rRNA gene sequencing of the V4 region. We found that after two weeks of recovery, the DSS-treated mice had a significantly different fecal microbiota from the untreated mice (Figure S2 A). However, when we analyzed the caecal communities after antibiotic treatment, we found that the two groups were no longer significantly different suggesting that antibiotic treatment normalizes the changes in the gut microbiota caused by DSS (Figure S2 C-D). Finally, to test the role of the microbiota in this model, we co-housed DSS-treated mice with untreated mice before C. difficile infection. We found that co-housing was not sufficient to protect DSS mice from severe CDI or to worsen disease severity for untreated mice (Figure S2 B). Given the similarities in C. difficile colonization and toxin production and the lack of protection after co-housing, we hypothesized that increased severity of CDI in DSS mice is due to differences in the host immune response to infection.
Figure 1: Increased CDI-associated mortality in mice with prior DSS colitis.
6-week old C57Bl/6J mice were treated with 2% DSS or no treatment in the drinking water for 6 days, then allowed to recover for 2 weeks. Both groups were treated with antibiotics and infected with 1×106-1×107 CFU of C. difficile strain R20291. (A) Model for CDI after recovery from DSS colitis. (B) DSS-induced weight loss and recovery before C. difficile infection (n=18–19 per group). (C) FITC dextran detection assay in the serum was used to determine gut permeability in untreated mice, during acute DSS colitis, and after recovery from DSS colitis (n=8–10 per group). After infection, survival (D), clinical scores: weight loss, coat appearance, eyes/nose discharge, activity, posture and diarrhea (E) and weight loss (F) of the two groups were assessed twice a day for 7 days (n=16 per group). Day 0 post infection in (D-F) refers to Day 27 in the timeline in (A). (G) Caecal contents were collected from mice with/without prior DSS colitis on day 2. The samples were homogenized and plated anaerobically on C. difficile- selective agar plates (n=10–14 per group). (H) Toxins were detected within caecal contents using the C. difficile TOXA/B enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (n=18 per group). Data represent mean ± SEM.*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 by two-tailed t-test (B, F-H), one-way analysis of variance (C), Logrank statistical test (D) and Mann-Whitney test (E). These data are combined from two independent experiments. See also Figures S1 and S2.
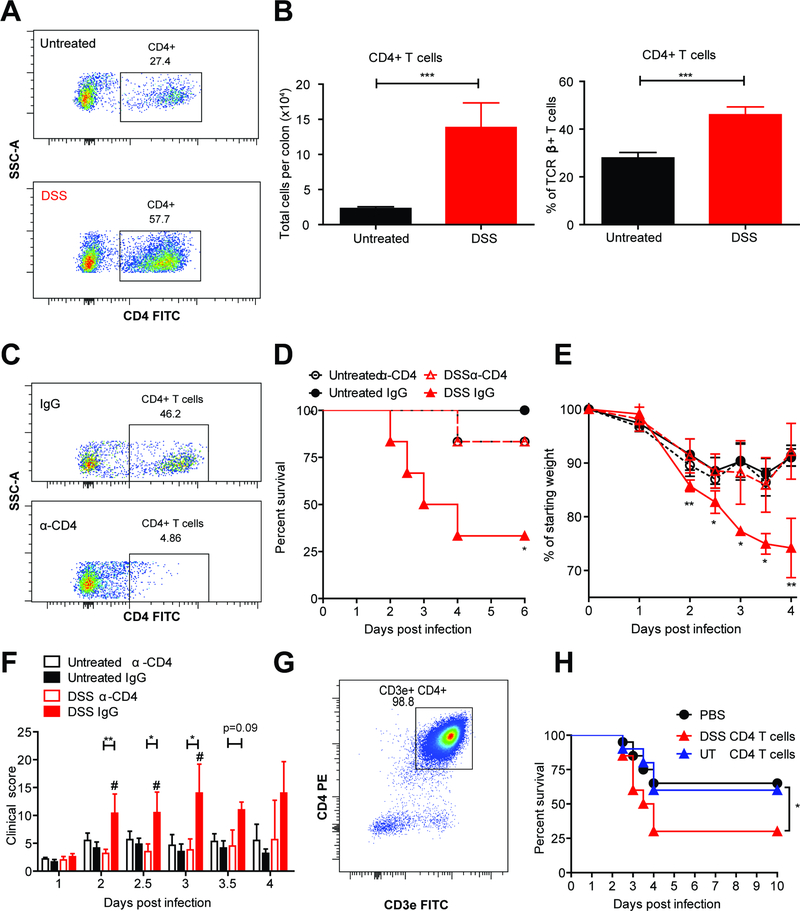
CD4+ T cells increase CDI severity in mice with prior DSS colitis
To understand the differences in the host immune response to CDI between DSS and untreated mice that might influence disease severity, we used flow cytometry to characterize immune cell recruitment to the colon on day 2, the peak of infection. We found no significant difference in the numbers of neutrophils, eosinophils, Ly6Chi and Ly6Clo monocytes between the two groups (Figure S3 A). Surprisingly, CD4+ T cell numbers were consistently and significantly higher in DSS mice compared to untreated mice during CDI, both as a total number and as a percentage of TCRβ+ T cells (Figure 2, A-B). Given the robust increase in CD4+ T cells, we hypothesized that these cells are the cause of increased severity of CDI in DSS mice. To test this hypothesis, first we depleted CD4-expressing cells using an anti-CD4 depleting antibody. We used flow cytometry to confirm successful depletion of CD4+ T cells from the colon lamina propria using the anti-CD4 antibody (Figure 2 C). Interestingly, depletion of CD4-expressing cells from DSS mice prior to CDI protected them from increased severity of disease as measured by reduced mortality, weight loss and clinical scores (Figure 2, D-F). Since CD4 can also be expressed by NK T cells, splenic DCs and lymphoid tissue inducer (LTi) cells in mice, we tested directly whether DSS-induced CD4+ T cells are sufficient to exacerbate CDI severity. To do this, we isolated CD4+ T cells from the colon lamina propria and mesenteric lymph nodes of both DSS-treated and untreated mice using a negative selection microbeads isolation kit. We used flow cytometry to confirm that the isolated cells were CD3+ CD4+ T cells (with a purity of 98.8%) (Figure 2 G) and transferred them into naïve recipients that were subsequently infected with C. difficile. Mice receiving CD4+ T cells from DSS-treated donors had significantly lower survival rates post-infection compared to mice receiving CD4+ T cells from untreated donors and mice that did not receive a T cell transfer (Figure 2 H). Taken together, these studies show that in mice with prior gut inflammation, CD4+ T cells are necessary and sufficient to increase CDI-associated mortality.
Figure 2: CD4+ T cells increase C. difficile disease severity in mice with prior DSS colitis.
Mice were given DSS or regular water (as described before), recovered for 2 weeks and infected with C. difficile. Colon tissue was isolated, processed into single-cell suspension and stained for flow cytometry. (A) Representative flow plots depicting colonic CD4+ T cells (CD45+ TCRβ+). (B) Quantification of colonic CD4+ T cells (n=10–12 per group). (C-F) Mice were injected i.p. with 400μg α-CD4 or IgG isotype control on days −6, −3 and on the day of infection with C. difficile. (C) Representative flow plots showing depletion of colonic CD4 T cells. (D-F) Survival, weight loss and clinical scores were assessed twice daily for 6 days (n=6 per group). (G) Donor mice were given 2% DSS or regular water for 6 days. On day 7, CD4+ T cells were isolated from the colon and mesenteric lymph nodes using negative selection magnetic beads and the purity was checked by flow cytometry. (H) 1×106 CD4+ T cells were transferred into naïve, antibiotic-treated recipients. The following day, recipient mice were infected with C. difficile. Survival curves were compared using a Logrank statistical test. Weight loss and clinical scores were compared using a one-way analysis of variance and Kruskal Wallis test, respectively (n=10 per group per experiment). Data represent mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. # p<0.05: DSS IgG compared to Untreated IgG. Data are combined from two independent experiments. See also Figure S3.
Increased Th17 responses in mice with prior DSS colitis during CDI
Given our discovery that CD4+ T cells play an essential role during CDI in mice with prior gut inflammation, we aimed to further characterize the T cell subset that might exacerbate CDI severity. Using flow cytometry, we found a significant increase in Th17 cells both in absolute numbers and as a percentage of CD4+ T cells in DSS mice during CDI (Figure 3 A). Similarly, using IL-17A-GFP reporter mice we found higher numbers of IL-17A-producing CD4+ T cells during CDI in DSS mice compared to untreated controls (Figure 3 B). Furthermore, when we quantified colonic Th1, Th2, and Treg cells, we did not find a compensatory increase in these cell subsets. Instead, our data showed a disruption in the homeostatic Th17/Treg balance and a significant skew towards Th17 responses in DSS mice (Figure S3 B). Therefore, we focused our studies on understanding the role of Th17 cells during CDI.
Figure 3: Increased Th17 responses in mice with prior DSS colitis cause severe subsequent CDI.
(A-D) Mice were given DSS or regular water (as described before), recovered for 2 weeks and infected with C. difficile. On day 2 of infection, colon and mesenteric lymph node tissue was isolated, processed into single-cell suspension and stained for flow cytometry. (A) Representative flow plots and quantification of Th17 cells (CD45+ TCRβ+ CD4+ RORγt+ FOXP3-) (n=5–6 per group per experiment). (B) IL-17-GFP reporter mice were used to determine the number and frequency of IL-17A+ CD4 T cells during infection by flow cytometry (n=5 per group per experiment). (C) For protein data, caecal tissue was homogenized using a bead beater, data is normalized to total protein quantified using a Pierce BCA Assay (n=8–14 per group). (D) caecal IL-23 protein levels plotted against clinical scores. (E) Mice were injected i.p. with 125μg α-IL-17RA or IgG isotype control on days −1, 1 and 2 of infection (n=16 per group). (F-I) Naïve CD4+ T cells were isolated from the spleen of IL17A-GFP reporter mice and differentiated into Th17 cells ex vivo in the presence of TGFβ and IL-6. (F) CD3e+CD4+IL-17A+ and CD3e+CD4+IL-17A- T cells were sorted and 1×106 cells were transferred i.p. to antibiotic-treated recipients. (G-I) One day following the T cell transfer, recipients were infected with C. difficile. Survival, weight loss and clinical scores were assessed twice daily (n= 10 per group). Data represent mean ± SEM.*p<0.05, **p<0.01, ***p<0.001 by a two-tailed student t-test (A-C), Pearson correlation statistical test (D), Logrank statistical test (E,G), Kruskal-Wallis test (H) and one-way analysis of variance (I). Data are representative of two independent experiments. See also Figures S3 and S4.
Th17 cells express the IL-23 receptor, and the presence of IL-23 during Th17 differentiation has been shown to lead to a pathogenic Th17 phenotype that has been implicated in autoimmunity, inflammation and infection (Langrish et al., 2005; Lee et al. 2012). Furthermore, the IL-23 axis upstream of Th17 cells has long been thought to play a role in the pathogenesis of IBD (McGovern and Powrie, 2007; Ahern et al., 2008). Finally, studies in our lab and others have shown that blocking IL-23 signaling during CDI protects from severe disease (Buonomo et al., 2013). Therefore, we investigated whether mice with DSS colitis have higher IL-23 levels before and after CDI. Indeed, we found higher levels of IL-23 protein in the caeca of DSS mice on days 0 and 2 of infection. Additionally, we found higher levels of the Th17 cytokine IL-17A in the caeca of DSS mice during infection (Figure 3 C). Finally, our data showed a direct correlation between IL-23 levels in the cecum during infection and CDI severity (Figure 3 D). Taken together, these findings indicated that a colitis-induced skew towards Th17 responses was associated with worse disease outcome during CDI.
In addition to the production of IL-17A, Th17 cells can produce other cytokines including IL-17F, IL-22, IFNγ and GMCSF. In order to determine which of these cytokines are important for the Th17-mediated exacerbation of CDI severity, we used flow cytometry and ELISA assays to compare the levels of these cytokines between DSS and untreated mice. We found that the majority of IL-17A+ T cells do not co-express IFNγ or IL-22. Our analysis showed that on Day 0 before infection, DSS mice had elevated levels of IL-17A+ IFNγ- and IL-17A+ IL-22- T cells in the mesenteric lymph nodes but not in the colon. On Day 2 during infection, however, we found significantly increased numbers of these cells in the mesenteric lymph nodes as well as the colon (Figure S4 A). Additionally, we found an increase in the levels of IL-17F and IL-22 caecal proteins in DSS mice during infection and no difference in GMCSF and IFNγ (Figure S4 B).
Because of the significant increase in IL-17A and IL-17F protein levels in the tissue and because TH17 cells in DSS mice were predominantly negative for IFNγ and IL-22, we hypothesized that IL-17 signaling is the mechanism by which Th17 cells cause increased severity of disease. To test this hypothesis, we used a blocking antibody against the IL-17RA, which binds to IL-17A homodimers and with less affinity to IL-17A IL-17F heterodimers and IL-17F homodimers. Our data showed that blocking IL-17RA protected DSS mice from severe disease early during infection (Figure 3 E). Similarly, neutralizing IL-17A provided significant protection early during infection (Figure S4 C). Together, these data suggest that IL-17 signaling is important for Th17-mediated exacerbation of CDI severity in DSS mice.
Adoptive transfer of Th17 cells is sufficient to increase CDI severity
Given the significant Th17 skew in DSS mice and our discovery that CD4+ T cells are necessary and sufficient to cause severe CDI in these mice, we wanted to directly test whether Th17 cells alone can exacerbate CDI severity. To do this, we isolated naïve CD4+ T cells from the spleens of IL-17-GFP reporter mice and differentiated them ex vivo into Th17 cells. On the day before infection, we sorted CD3+ CD4+ T cells into IL-17A+ and IL-17A- cells (Figure 3 F). The sorted cells were transferred into naïve recipient mice that were subsequently infected with C. difficile. After infection, mice that received IL-17A+ CD4+ T cell transfer had higher CDI-associated mortality rates, weight loss and clinical scores compared to mice that either received IL-17A- CD4+ T cells or no T cell transfer (Figure 3, G-I). It is important to note that while recipients of the IL-17A- CD4+ T cells had similar survival and clinical scores to the PBS controls, they lost more weight post infection. This modest effect is likely due to some IL-17A- CD4 T cells gaining IL-17A expression post-transfer. Indeed, when we looked by flow cytometry after infection, we found some GFP+ cells in the colons of these mice. Taken together, these results establish a central role for Th17 cells in determining the severity and outcome of CDI following colitis.
Serum IL-6 and IL-23 in C. difficile patients correlate with severe disease
In light of our data establishing a role for Th17 cells during CDI in a mouse model, we wished to explore whether Th17 responses are also important during human CDI. Serum samples from C. difficile patients were analyzed for IL-6, IL-23, IL-17A and IL-4 protein levels. Severe CDI was defined with a white blood cell (WBC) count ≥15,000 per microliter as previously described (Shivashankar et al. 2013; Yu et al. 2017). We used a Kaplan-Meier survival analysis to confirm that the severe CDI group had significantly lower survival probability (Figure 4 A). When patients were categorized based on disease severity, those with severe CDI had significantly higher serum IL-6 and IL-23 levels compared to those with non-severe CDI. We found no difference in serum IL-17A levels between the two groups (Figure 4 B). In addition to its role in Th17 differentiation, IL-6 can promote IL-4 production by Th2 cells and lead to enhanced Th2 differentiation through an IL-4 feedback loop (Dienz and Rincon, 2009). However, when we measured the levels of IL-4 in the human serum samples, we actually found a decrease in IL-4 levels in patients with severe CDI suggesting no enhanced Th2 responses downstream of IL-6 (Figure 4 B). Strikingly, when we divided the patients into four quartiles based on IL-6 levels in the serum, patients with higher IL-6 (second, third and fourth quartiles) were significantly less likely to survive post-infection than patients in the lowest quartile (Figure 4 C). After adjustment for age, sex, race, comorbidities and ICU admission, patients in the highest quartile for serum IL-6 were 7.6 times less likely to survive than those in the lowest quartile (p=0.0009) (Table S1). A survival analysis based on serum IL-23 levels could not be completed due to the high number of samples under the assay’s limit of detection, which limited the statistical power of the survival studies for this cytokine. Taken together, these data suggest that serum IL-6 and IL-23, two cytokines upstream of pathogenic Th17 cells, likely play a role in increasing CDI-associated mortality in human patients.
Figure 4: The Th17 cytokines IL-6 and IL-23 in the serum of C. difficile patients correlate with severe disease.
IL-23 and IL-17A in the serum of C. difficile patients were quantified using high sensitivity ELISAs from R&D. IL-6 and IL-4 were quantified using a Luminex bead-based multiplex assay. (A) Kaplan-Meier survival curve post CDI diagnosis for patients with non-severe CDI (WBC<15,000 per microliter, n=226) and severe CDI (WBC≥15,000 per microliter, n=100). (B) serum IL-17A, IL-23, IL-6 and IL-4 for patients with non-severe and severe CDI, n=323, 323 and 362, 379 respectively. (C) Kaplan-Meier survival curve post CDI diagnosis for patients categorized into quartiles based on IL-6 serum levels, n=92–94 per quartile. Data represent mean ± SEM.*p<0.05, **p<0.01, ***p<0.001 using Logrank (A,C) and Mann-Whitney (B) statistical tests. See also Table S1.
Discussion
Altogether, our study demonstrates that in a colitis setting, Th17 cells are an important source of IL-17A and these cells alone can increase the risk for severe CDI. Our findings are particularly important in light of recent evidence showing that IBD patients have higher numbers of Th17 cells in the colon and circulation (Hegazy et al., 2017), which might explain their increased risk for severe C. difficile colitis. In addition to C. difficile infection, Salmonella infections have also been linked to IBD (Gradel et al., 2009); Hegazy et al. found more S. typhimurium-specific Th17 cells in PBMCs from patients with IBD compared to controls. There is also evidence that suppressing T cell responses during S. typhimurium infection can protect the host from severe disease by preventing the downstream neutrophil recruitment and off-target tissue pathology (Godinez, 2008). This suggests that aberrant Th17 responses in IBD patients may generally put them at risk for severe gastrointestinal infections.
Previous studies have found that targeting IL-23 and IL-17A can protect mice from C. difficile-associated epithelial damage and mortality (Buonomo et al., 2013; McDermott et al., 2016; Tateda et al., 2016). In our study, we found that neutralizing IL-17A and blocking the IL-17RA receptor protected DSS mice from severe disease early on during infection. Blocking the receptor was more robust at providing protection suggesting that IL-17F, also elevated in DSS mice, might play a role in exacerbating CDI severity. Blocking IL-17 signaling did not provide protection later during infection, suggesting that perhaps some IL-17 signaling is required to control the infection. Indeed, other studies have shown that although increased neutrophilia downstream of IL-17A is associated with severe disease, complete depletion of neutrophils does not protect mice from disease because of their essential role in clearing the infection (Jarchum et al., 2012 and Feghaly et al., 2013). The lack of complete protection by IL-17 signaling blockade might suggest that other products of Th17 cells perhaps play a role in the exacerbation of CDI severity. Although we did not see striking differences in the production of IFNγ or IL-22 by Th17 cells in DSS mice, further studies are needed to determine whether any other Th17 cytokines work synergistically with IL-17A to cause severe disease.
Interestingly, in a T cell transfer model of colitis, Wedebye et al. (2013) found that blocking IL-17A and IL-17F simultaneously was more successful at ameliorating colitis than blocking either cytokine individually. Furthermore, targeting Th17 cells with pharmacologic inhibitors of RORγt was successful in reducing gut inflammation in two different murine models of IBD (Withers et al., 2016). The findings of our study suggest that specifically targeting Th17 cells and their effector cytokines in IBD patients may also protect them from acquiring severe CDI.
The limitations of this study include the use of DSS colitis as a way to induce gut inflammation prior to CDI. DSS colitis does not perfectly model human IBD because it is chemically-induced colitis. However, for the purposes of this study, DSS colitis did cause a skew towards Th17 responses that persisted beyond the resolution of acute colitis and allowed us to study the effect of the Th17 axis on subsequent CDI. Therefore, the model we developed can be used for future studies to identify the downstream mechanisms that are required for the Th17-mediated exacerbation of CDI. Another strength of the DSS model for our studies is that it is an acute form of colitis, which allows for full recovery before CDI. Recently, Zhou et al. (2018) showed that mice with acute DSS colitis are more susceptible to C. difficile infection. However, the cause of this increased susceptibility is unclear and could be attributed to the physical damage to the epithelial barrier during acute DSS colitis. In patients, time-to-CDI diagnosis data suggest that a high percentage of IBD patients acquire CDI during remission and not during an IBD flare (Rodemann et al., 2007). For this reason, we designed a model where mice are recovered from acute colitis before CDI and we suggest that this model might better reflect what happens in IBD patients.
Consistent with a previous report by Yu et al. (2017), we found no difference in serum IL-17A between the patients with severe vs. non-severe disease. Yu and colleagues, however, found no difference in serum IL-6, but the two studies were done on two different cohorts and our study included 362 patients, compared to 36 in the Yu et al. report. While we found differences in IL-6 and IL-23 systemically in the serum, perhaps the effects on IL-17A production are localized to colonic Th17 cells and evaluation of tissue IL-17A levels might reveal differences between the two patient groups.
Future experimentation is needed to understand how Th17 cells exacerbate CDI severity. Th17 cells produce IL-17A, F and IL-22 that can act on epithelial cells, which in turn produce several chemokines that ultimately lead to neutrophil recruitment (Ouyang et al., 2008). While neutrophils are important for defense against bacterial infections, they often can have off-target damaging effects on host tissue. Our studies on day 2 did not show enhanced neutrophil recruitment in DSS mice, but future studies can further characterize the effects of Th17 cells on neutrophil activation and function during CDI.
The incidence of C. difficile over the last two decades has continued to rise and the CDC identifies C. difficile as an urgent threat. Although antibiotic treatment is the first line of defense against CDI, the antibiotic-induced disruption of the gut microbiota contributes to disease relapse in 1 of 5 patients. Therefore, discovering new therapies is essential for treating this life-threatening infection. Previous studies have reported a role for CD4 T cells in protection against CDI via a TLR4-dependent recognition of C. difficile surface layer proteins (Ryan et al., 2011). However, these cells’ role during infection remains underexplored. Using adoptive transfer studies, we have directly shown that Th17 cells are sufficient to increase CDI severity. Furthermore, by analyzing serum samples from C. difficile patients, we found that IL-23 and IL-6 correlate with disease severity and that patients with high IL-6 levels are significantly more likely to die after infection. This is supported by a previous study that described higher levels of IL-6 in 8 patients with severe CDI (Rao et al. 2014). These mouse studies and their human correlate suggest than in patients with increased pathogenic Th17 responses, such as IBD, targeting these cells may not only ameliorate IBD symptoms but also reduce their risk of developing severe CDI.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, William A. Petri, Jr. (wap3g@virginia.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
C57BL6 and IL17-GFP reporter mice (strain: C57BL/6-Il17atm1Bcgen/J, Cat# JAX: 018472, RRID:IMSR_JAX:018472) were ordered from Jackson Laboratories. All mice were housed under specific pathogen-free conditions at the University of Virginia’s animal facility. All experiments were performed according to provisions of the Animal Welfare Act of 1996 and were approved by the University of Virginia Institutional Animal Care and Use Committee. Littermates were randomly assigned to experimental groups. Male mice were used that were 6 weeks old at the start of each experiment. During the infection, mice were weighed and scored daily and euthanized if they lost more than 25% of the starting body weight, or had a clinical score of 14 or above.
Human serum samples
Information on the sex and sample size of the human subjects in each experimental group is listed in Table S1. Ages ranged from 12–97 years, with a mean of 60 years and a median of 63 years. Cox proportional hazards model was used to adjust for age among other variables including sex, race, Charlson comorbidity index and ICU admission.
Data collection and analysis were approved by the UVA Institutional Review Board (IRB-HSR #16926). The Institutional Review Board for Health Sciences Research confirms that this project meets the criteria of research involving coded private information or biological specimens. According to the Office for Human Research Protections (OHRP) guidance, this project is considered to not involve human subjects.
METHOD DETAILS
DSS treatment and C. difficile infection
For DSS treatment, mice were given 2% Dextran Sulfate Sodium (DSS, Thermo Fisher Scientific, Cat# AAJ1448922) in the drinking water for 6 days, then switched to regular drinking water and allowed to recover. During DSS colitis, weight loss was monitored and mice were euthanized in the rare instance that they developed severe DSS colitis. For C. difficile infections, age and sex-matched mice were given 45 mg/L vancomycin (Mylan), 35 mg/L colistin (Sigma), 35 mg/L gentamicin (Sigma) and 215 mg/L metronidazole (Hospira) ad libitum for 3 days. Then, mice were switched to regular drinking water for two days. On the day before infection, mice were injected intraperitoneally with 0.016 mg/g of clindamycin (Hospira). To prepare the inoculum, C. difficile strain R20291 was grown from a frozen glycerol stock onto Brain Heart Infusion (BHI) agar plates supplemented with cycloserine and cefoxitin (C. difficile supplement, Sigma) overnight at 37°C in an anaerobic chamber (Shel Labs). A single colony was then transferred into a tube containing BHI liquid media and grown overnight at 37°C anaerobically. The next day, the liquid culture was centrifuged and the pellet was washed twice with PBS, the optical density determined using a spectrophotometer and the inoculum was diluted to 5×107 CFU/ml using sterile, anaerobic PBS. The inoculum was loaded into sterile syringes and transported in sealed biohazard bags to the animal facility. The concentration of each inoculum was confirmed by counting CFUs after plating on BHI plates supplemented with 1% sodium taurocholate and grown overnight at 37°C anaerobically. Mice were orally gavaged with 5×106 CFU/mouse in randomized order. During the infection, mice were weighed and scored daily and euthanized if they developed severe disease based on the scoring criteria. Clinical scores are based on weight loss, coat appearance, eyes/nose discharge, activity, posture and diarrhea. To quantify C. difficile burden during infection, caecal contents were suspended using sterile, anaerobic PBS, serially diluted and plated on BHI plates supplemented with cycloserine and cefoxitin (C. difficile supplement, Sigma). After an overnight incubation at 37°C in an anaerobic chamber, CFUs of C. difficile were counted and normalized to stool weight. Toxins A/B were quantified using the ELISA C. difficile TOX A/B II kit from Techlab according to the manufacturer’s instructions and normalized to stool weight.
FITC dextran Assay
Mice were gavaged with 40mg/100g body weight Fluorescein isothiocyanate (FITC)–dextran solution (Sigma, Cat# 46944–500MG-F). 4 hours later, mice were sacrificed and a spectrophotometer was used to detect FITC in the serum at 485/530 nm.
LP Isolation and Flow Cytometry
To isolate the lamina propria, colons were removed, cut longitudinally and rinsed thoroughly in Hank’s balanced salt solution (HBSS) supplemented with 5% FBS and 25 mM HEPES. Epithelial cells were removed with two 20-minute incubations in pre-warmed HBSS with 15 mM HEPES, 5 mM EDTA, 10% FBS and 1 mM dithiothreitol in a 37°C shaking incubator. The colons were then cut into small sections and incubated in pre-warmed RPMI media containing 0.17 mg/ml liberase TL (Sigma) and 30 mg/ml DNase (Sigma) in a 37°C shaking incubator. Following the digestion step, the tissue was passed through 40 and 100 μM cell strainers, respectively, counted and resuspended to a concentration of 1×107 cells/ml in fluorescence-activated cell sorting (FACS) buffer (PBS with 2% FBS). 1×106 cells were plated in a 96-well plate and stained for flow cytometry. After an Fc blocking step (anti-mouse CD16/32 TruStain, BioLegend Cat #101320, RRID:AB_1574975) and staining with a fixable viability dye (Zombie Aqua, Biolegend Cat# 423102), the following antibodies were used for staining: CD11b-APC (M1/70, Biolegend Cat# 101212, RRID:AB_312795), CD45-APC-Cy7 (30-F11, Biolegend Cat# 103116, RRID:AB_312981), CD11c-BV421 (N418, Biolegend Cat# 117330, RRID:AB_11219593), CD3e-APC (500A2, Biolegend Cat# 152306, RRID:AB_2632669), CD4-PE (RM4–4, Biolegend Cat# 116006, RRID:AB_313691), CD45-PE/Cy7 (30-F11, Biolegend Cat# 103114, RRID:AB_312979), T-bet-BV421 (4B10, Biolegend 644815, RRID:AB_10896427), TCRβ-PerCp/Cy5.5 (H57–597, Biolegend Cat# 109228, RRID:AB_1575173), CD4-FITC (GK1.5, Cat# 100406, RRID:AB_312691), CD3e-FITC (500A2, Biolegend Cat# 152304, RRID:AB_2632667),CD45-BV421 (30-F11, Biolegend Cat# 103134, RRID:AB_2562559), CD4-PE/Cy7 (GK1.5, Biolegend Cat# 100422, RRID:AB_312707), GATA3-AF647 (16E10A23, Biolegend Cat# 653810, RRID:AB_2563217), TCRβ-FITC (H57–597, Biolegend Cat# 109206, RRID:AB_313429), IL-17A-PE (TC11–18H10.1, Biolegend Cat# 506904, RRID:AB_315464), IFNγ-APC (XMG1.2, Biolegend Cat# 505810, RRID:AB_315404), Ly6G-PeCy7 (1A8, Biolegend Cat# 127618, RRID:AB_1877261), Ly6C-FITC (HK1.4, Biolegend Cat# 128006, RRID:AB_1186135), SiglecF-PE (E50–2440) (BD Biosciences Cat# 552126, RRID:AB_394341), FOXP3-APC (FJK-16s Thermo Fisher Scientific Cat# 17–5773-82, RRID:AB_469457), RORγt-PE (B2D, Thermo Fisher Scientific Cat# 12–6981-82, RRID:AB_10807092), IL-22-PerCP-eFluor 710 (IL22JOP, Thermo Fisher Scientific Cat# 46–7222-82, RRID:AB_2573839).
For intracellular transcription factor staining, a FOXP3/Transcription Factor Staining Buffer Set (eBioscience, catalog# 00–5523-00) was used according to the manufacturer’s instructions. For intracellular cytokine staining, isolated colonic cells were re-suspended in 5 ml 10% percoll (Sigma, catalog# P1644) and layered over 80% percoll, centrifuged at 1000×g for 15 minutes at RT with maximum acceleration and no brakes. Lymphocytes were collected at the interphase, washed with RPMI, plated at 1×105−6 cells per well. Cells were stimulated in T cell culture media (RPMI Meida 1640, 10% FBS, 2mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 5mM 2-β-mercaptoethanol, BD GolgiPlug containing Brefeldin A (BD Cytofix/Cytoperm kit cat#555028), 50ng/ml PMA, 750ng/ml ionomycin) for 4 hours at 37°C with 5% CO2. Following the stimulation, cells were stained using Cytofix/Cytoperm™ Plus kit (BD, catalog# 555028) according to the manufacturer’s protocol. Flow cytometry data was acquired using an LSR Fortessa cytometer (BD Biosciences) and data analysis was performed via FlowJo.
Tissue protein and cytokine analysis
Caecal tissue was isolated and rinsed gently with PBS, then homogenized by beadbeating in 400 μl of a buffer containing 1× HALT protease inhibitor (Pierce) and 5 mM HEPES. The homogenate was incubated on ice for 30 minutes after the addition of 400ul of another buffer containing 1× HALT protease inhibitor (Pierce), 5 mM HEPES and 2% Triton X-100. Finally, the lysates were spun at 13,000g for 5 minutes and the supernatants were transferred to a new tube and used for protein analysis. Mouse tissue IL-23, IL-17A, IL-17F and IL-22 ELISAs were done according to the manufacturer’s instructions (DuoSet, R&D). Protein levels were normalized to the total protein concentration determined using a Pierce BCA Protein Assay (Thermo Fisher Scientific). For TNFα, IFNγ, KC, IL-6, IL-1α, IL-1β, GMCSF and IFNγ caecal tissue was processed using the same protocol and total protein was quantified using a Pierce BCA Protein Assay. Cytokine levels were determined using a Luminex MAGPIX bead-based multiplex analyzer through the University of Virginia’s Flow Cytometry Core Facility. Human serum IL-23 and IL-17A were measured using the Human IL-23 Quantikine ELISA Kit and Human IL-17 Quantikine HS ELISA Kit from R&D, respectively, according to the manufacturer’s instructions. Human serum IL-6 and IL-4 were measured using a Luminex bead-based multiplex assay from R&D according to the manufacturer’s instructions.
Cell depletion and antibody neutralization
For CD4 cell depletion: DSS-treated and untreated mice were injected i.p. with 400μg α-CD4 (clone GK1.5, BioXcell Cat# BE0003–1, RRID:AB_1107636) or IgG isotype control (BE0090, BioXcell Cat# BE0090, RRID:AB_1107780) on days −6, −3 and on the day of infection with C. difficile as previously reported (Moynihan et al., 2016). Flow cytometry was used to confirm depletion of CD4-expressing T cells from the colon with 90% efficiency.
For IL-17RA blockade and IL-17A neutralization: DSS-treated mice were injected i.p. with 125μg α-IL-17RA (R&D Systems, Cat# MAB4481, RRID:AB_10891109), 125μg αIL-17A (R&D Systems, Cat# MAB421, RRID:AB_2125018) or IgG isotype control (R&D Systems, Cat# MAB006, RRID:AB_357349) on days −1, 1 and 2 of C. difficile infection.
T cell transfers
For the adoptive transfer of CD4+ T cells, mice were either treated with DSS or regular drinking water. On day 7 after DSS treatment, colons and mesenteric lymph nodes were removed and processed into single-cell suspensions as described above. Then, CD4+ T cells were isolated using a negative selection CD4+ T cell isolation kit from Miltenyi Biotec (Cat# 130–104-454). The purity of the cells isolated was checked by flow cytometry. 1×106 cells were transferred i.p. into naïve recipients on the day before infection with C. difficile.
For the adoptive transfer of Th17 cells, splenocytes were isolated from IL17A-GFP reporter mice and cultured ex vivo using a CellXVivo Mouse Th17 Cell Differentiation Kit (R&D Systems, Cat# CDK017) according to the manufacturer’s instructions. After differentiation, the cells were stained for CD3e and CD4 expression and viability was determined using 7-AAD Viability Staining Solution (BioLegend, Cat# 420404). IL-17A+ and IL-17A- CD4+ T cells were sorted using an Influx Cell Sorter (BD Biosciences) and 1×106 cells were transferred into naïve recipients on the day before C. difficile infection.
16S rRNA Sequencing
Fecal pellets were collected from mice given untreated or DSS-treated water after a two-week recovery period and before antibiotics treatment, on day 21 in Figure 1A. Caecal contents were collected from untreated or DSS-treated mice after antibiotics treatment, on day 27 in Figure 1A, and diluted 1:2 with sterile PBS. DNA was extracted using Qiagen MagAttract PowerMicrobiome kit DNA/RNA kit (Qiagen, catalog no. 27500–4-EP). The V4 region of the 16S rRNA gene was amplified from each sample using the dual indexing sequencing strategy as described previously (Kozich et al., 2013). Sequencing was done on the Illumina MiSeq platform, using a MiSeq Reagent Kit V2 500 cycles (Illumina cat# MS102–2003), according to the manufacturer’s instructions with modifications found in the Schloss SOP: https://github.com/SchlossLab/MiSeq_WetLab_SOP. The mock community produced ZymoBIOMICS Microbial Community DNA Standard (Zymo Research cat# D6306) was sequenced to monitor sequencing error. The overall error rate was 0.019%.
Sequence Curation and Analysis
Raw sequences were curated using the software package mothur version 1.39.5 (Schloss et al., 2009) following the Illumina MiSeq standard operating procedure. Briefly, paired end reads were assembled into contigs and aligned to the V4 region using the SLIVA 16S rRNA sequence database (Quast et al., 2013), sequences that failed to align or were flagged as possible chimeras were removed. A naïve Bayesian classifier using the Ribosomal Database Project (Wang et al., 2007) classified sequences. Operational Taxonomic Units (OTUs) using a 97% similarity cutoff were generated using the Opticlust clustering algorithm (Westcott et al., 2017).
The number of sequences in each sample was then rarefied to 25,000 sequences to minimize bias due to uneven sampling. Following curation in mothur, further data analysis and figure generation was carried out in R (v 3.4.1) using the package vegan (Oksanen et al, 2018). This includes determining the axes for the multidimensional scaling (MDS) plots using Bray-Curtis dissimilarity calculated from sequence abundance. Additionally, vegan was used to determine significance between groups using PERMANOVA. The sequences associated with analysis were deposited to the SRA under the bioproject PRJNA475161. Final figures were modified and arranged in Adobe Illustrator CC.
Histology
Caecal tissue was isolated and fixed in Bouin’s solution for 24 hours then transferred to 70% ethanol, paraffin embedded and stained with haematoxylin and eosin (H&E). The tissue was sectioned onto slides and scored by two independent, blinded observers. Each sample was given a score of 0–3 for each of the following parameters: epithelial disruption, submucosal edema, inflammatory infiltrate, mucosal thickening and luminal exudates as described previously (Pawlowski et al., 2010).
QUANTIFICATION AND STATISTICAL ANALYSIS
For mouse data, survival curves were compared using a Log-Rank (Mantel-Cox) statistical test. Comparisons between two groups were assessed using a student t-test or Mann-Whitney test depending on whether the data were normally distributed. Statistical significance between multiple groups was tested using a one-way multiple analysis of variance (ANOVA) or Kruskal-Wallis. Data points were only excluded if a statistically significant outlier was identified using Grubbs’ test for normally-distributed data with a p<0.05. For human data, patients were categorized into quartiles based on IL-6 serum levels. Survival curves were compared using a Kaplan-Meyer test, then further evaluated by a Cox proportional hazards model to adjust for age, sex, race, Charlson comorbidity index and ICU admission. Statistical significance between severe and non-severe patients was assessed using a Mann-Whitney test. Statistical analysis was performed using GraphPad Prism (GraphPad Software Inc., La Jolla, CA) and SAS 9.4 (SAS Institute, Cary, NC). All subjects with available survival time and WBC count data were included in the study. No data points were excluded from the analysis. Information on the statistical test used, number of subjects “n”, definition of center and dispersion and precision measures are included in each figure legend.
Data are presented as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
DATA AND SOFTWARE AVAILABILITY
The sequences associated with the 16S microbiota analysis were deposited to the SRA under the bioproject PRJNA475161.
Supplementary Material
Highlights.
C. difficile infection is more severe in mice with prior DSS-induced colitis
Severe disease depends on CD4+ T cells that persist beyond recovery from colitis
Transfer of Th17 cells to naïve mice worsens C. difficile severity via IL-17 production
In C. difficile patients, high IL-23 and IL-6 levels correlate with disease severity
Acknowledgements
The authors thank the flow cytometry and research histology cores at the University of Virginia for providing their expertise. The authors thank A. Criss, J. Lukens, M. Rutkowski, L. Erickson, E. Frost and the Mann and Ramakrishnan laboratories for helpful discussions. This work was supported by NIH grant T32GM008715 to M.M.S., the UVA Wagner Fellowship and T32AI007496 to A.L.F., T32AI007496 to J.L.L. and M.E.S., T32AI07496 and 5F31AI114203 to E.L.B. and 1R21AI114734 and 1R01AI124214 to W.A.P. The 16S sequencing analysis was supported by work performed by The University of Michigan Microbial Systems Molecular Biology Laboratory.
Footnotes
Declaration of Interests
W.A.P. is a consultant for TechLab, a company that makes diagnostics for CDI. Some of the data in this manuscript was used to file U.S. Provisional Patent Application Serial No. 62/630,370.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abt MC, Lewis BB, Caballero S, Xiong H, Carter RA, Sušac B, Ling L, Leiner I, and Pamer EG (2015). Innate Immune Defenses Mediated by Two ILC Subsets Are Critical for Protection against Acute Clostridium difficile Infection. Cell Host & Microbe 18, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern PP, Izcue A, Maloy KJ, and Powrie F (2008). The interleukin-23 axis in intestinal inflammation. Immunological Reviews 226, 147–159. [DOI] [PubMed] [Google Scholar]
- Alex P, Zachos NC, Nguyen T, Gonzales L, Chen T-E, Conklin LS, Centola M, and Li X (2009). Distinct Cytokine Patterns Identified from Multiplex Profiles of Murine DSS and TNBS-Induced Colitis: Inflammatory Bowel Diseases 15, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthakrishnan AN, McGinley EL, and Binion DG (2008). Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut 57, 205–210. [DOI] [PubMed] [Google Scholar]
- Arai Y, Takanashi H, Kitagawa H, and Okayasu I (1998). Involvement of interleukin-1 in the development of ulcerative colitis induced by dextran sulfate sodium in mice. Cytokine 10, 890–896. [DOI] [PubMed] [Google Scholar]
- Bacci S, Mølbak K, Kjeldsen MK, and Olsen KEP (2011). Binary toxin and death after Clostridium difficile infection. Emerging Infect. Dis 17, 976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JG, Chang TW, Gurwith M, Gorbach SL, and Onderdonk AB (1978). Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N. Engl. J. Med 298, 531–534. [DOI] [PubMed] [Google Scholar]
- Baumgart DC, and Carding SR (2007). Inflammatory bowel disease: cause and immunobiology. The Lancet 369, 1627–1640. [DOI] [PubMed] [Google Scholar]
- Bcheraoui C. El, Mokdad AH, Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Shirude S, Naghavi M, and Murray CJL (2018). Trends and Patterns of Differences in Infectious Disease Mortality Among US Counties, 1980–2014. JAMA 319, 1248–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomo EL, Madan R, Pramoonjago P, Li L, Okusa MD, and Petri WA (2013). Role of Interleukin 23 Signaling in Clostridium difficile Colitis. J Infect Dis 208, 917–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomo EL, Cowardin CA, Wilson MG, Saleh MM, Pramoonjago P, and Petri WA (2016). Microbiota-Regulated IL-25 Increases Eosinophil Number to Provide Protection during Clostridium difficile Infection. Cell Reports 16, 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cojocariu C, Stanciu C, Stoica O, Singeap AM, Sfarti C, Girleanu I, and Trifan A (2015). Clostridium difficile infection and inflammatory bowel disease. The Turkish Journal of Gastroenterology 25, 603–610. [DOI] [PubMed] [Google Scholar]
- Cowardin CA, Buonomo EL, Saleh MM, Wilson MG, Burgess SL, Kuehne SA, Schwan C, Eichhoff AM, Koch-Nolte F, Lyras D, et al. (2016). The binary toxin CDT enhances Clostridium difficile virulence by suppressing protective colonic eosinophilia. Nat Microbiol 1, 16108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienz O, and Rincon M (2009). The effects of IL-6 on CD4 T cell responses. Clinical Immunology 130, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feghaly REE, Stauber JL, Tarr PI, and Haslam DB (2013). Intestinal Inflammatory Biomarkers and Outcome in Pediatric Clostridium difficile Infections. The Journal of Pediatrics 163, 1697–1704.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinez I, Haneda T, Raffatellu M, George MD, Paixao TA, Rolan HG, Santos RL, Dandekar S, Tsolis RM, and Baumler AJ (2008). T Cells Help To Amplify Inflammatory Responses Induced by Salmonella enterica Serotype Typhimurium in the Intestinal Mucosa. Infection and Immunity 76, 2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradel KO, Nielsen HL, Schønheyder HC, Ejlertsen T, Kristensen B, and Nielsen H (2009). Increased Short- and Long-Term Risk of Inflammatory Bowel Disease After Salmonella or Campylobacter Gastroenteritis. Gastroenterology 137, 495–501. [DOI] [PubMed] [Google Scholar]
- Hegazy AN, West NR, Stubbington MJT, Wendt E, Suijker KIM, Datsi A, This S, Danne C, Campion S, Duncan SH, et al. (2017). Circulating and Tissue-Resident CD4+ T Cells With Reactivity to Intestinal Microbiota Are Abundant in Healthy Individuals and Function Is Altered During Inflammation. Gastroenterology 153, 1320–1337.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa M, Vijayapal A, Graham MB, Beaulieu DB, Otterson MF, Lundeen S, Skaros S, Weber LR, Komorowski RA, Knox JF, et al. (2007). Impact of Clostridium difficile on Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology 5, 345–351. [DOI] [PubMed] [Google Scholar]
- Jarchum I, Liu M, Shi C, Equinda M, and Pamer EG (2012). Critical Role for MyD88-Mediated Neutrophil Recruitment during Clostridium difficile Colitis. Infection and Immunity 80, 2989–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeengar MK, Thummuri D, Magnusson M, Naidu VGM, and Uppugunduri S (2017). Uridine Ameliorates Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Scientific Reports 7. doi: 10.1038/s41598-017-04041-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozich JJ, Westcott SL, Baxter NT, Highlander SK, and Schloss PD (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol 79, 5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, and Cua DJ (2005). IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med 201, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, et al. (2012). Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol 13, 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessa FC, Winston LG, McDonald LC, and Emerging Infections Program C. difficile Surveillance Team (2015). Burden of Clostridium difficile infection in the United States. N. Engl. J. Med 372, 2369–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AJ, Falkowski NR, McDonald RA, Pandit CR, Young VB, and Huffnagle GB (2016). Interleukin-23 (IL-23), independent of IL-17 and IL-22, drives neutrophil recruitment and innate inflammation during Clostridium difficile colitis in mice. Immunology 147, 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern D, and Powrie F (2007). The IL23 axis plays a key role in the pathogenesis of IBD. Gut 56, 1333–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, Williams RT, Rakhra K, Zhang MH, Rothschilds AM, et al. (2016). Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nature Medicine 22, 1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen GC, Kaplan GG, Harris ML, and Brant SR (2008). A National Survey of the Prevalence and Impact of Clostridium difficile Infection Among Hospitalized Inflammatory Bowel Disease Patients. The American Journal of Gastroenterology 103, 1443–1450. [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, et al. (2018). vegan: Community Ecology Package, vR package version 2.4–6. CRAN. https://CRAN.R-project.org/package=vegan [Google Scholar]
- Ouyang W, Kolls JK, and Zheng Y (2008). The Biological Functions of T Helper 17 Cell Effector Cytokines in Inflammation. Immunity 28, 454–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski SW, Calabrese G, Kolling GL, Freire R, AlcantaraWarren C, Liu B, Sartor RB, and Guerrant RL (2010). Murine Model of Clostridium difficile Infection with Aged Gnotobiotic C57BL/6 Mice and a BI/NAP1 Strain. J Infect Dis 202, 1708–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, and Glöckner FO (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K, Erb-Downward JR, Walk ST, Micic D, Falkowski N, Santhosh K, Mogle JA, Ring C, Young VB, Huffnagle GB, et al. (2014). The Systemic Inflammatory Response to Clostridium difficile Infection. PLoS ONE 9, e92578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodemann JF, Dubberke ER, Reske KA, Seo DH, and Stone CD (2007). Incidence of Clostridium difficile Infection in Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology 5, 339–344. [DOI] [PubMed] [Google Scholar]
- Ryan A, Lynch M, Smith SM, Amu S, Nel HJ, McCoy CE, Dowling JK, Draper E, O’Reilly V, McCarthy C, et al. (2011). A Role for TLR4 in Clostridium difficile Infection and the Recognition of Surface Layer Proteins. PLoS Pathogens 7, e1002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol 75, 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivashankar R, Khanna S, Kammer PP, Harmsen WS, Zinsmeister AR, Baddour LM, and Pardi DS (2013). Clinical Factors Associated With Development of Severe-Complicated Clostridium difficile Infection. Clinical Gastroenterology and Hepatology 11, 1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman EK, and Malani PN (2018). Infectious Diseases Mortality in the United States: Ongoing Investment Needed for Continued Progress. JAMA 319, 1205–1206. [DOI] [PubMed] [Google Scholar]
- Tateda K, Saji T, Ishii Y, Akasaka Y, Kimura S, Mori N, Kajiwara C, and Nakagawa T (2016). Endogenous IL-17 as a factor determining the severity of Clostridium difficile infection in mice. Journal of Medical Microbiology 65, 821–827. [DOI] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, and Cole JR (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol 73, 5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedebye Schmidt EG, Larsen HL, Kristensen NN, Poulsen SS, Lynge Pedersen AM, Claesson MH, and Pedersen AE (2013). TH17 Cell Induction and Effects of IL-17A and IL-17F Blockade in Experimental Colitis: Inflammatory Bowel Diseases 19, 1567–1576. [DOI] [PubMed] [Google Scholar]
- Westcott SL, and Schloss PD (2017). OptiClust, an Improved Method for Assigning Amplicon-Based Sequence Data to Operational Taxonomic Units. mSphere 2, e00073–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers DR, Hepworth MR, Wang X, Mackley EC, Halford EE, Dutton EE, Marriott CL, Brucklacher-Waldert V, Veldhoen M, Kelsen J, et al. (2016). Transient inhibition of ROR-γt therapeutically limits intestinal inflammation by reducing TH17 cells and preserving group 3 innate lymphoid cells. Nature Medicine 22, 319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Chen K, Sun Y, Carter M, Garey KW, Savidge TC, Devaraj S, Tessier ME, von Rosenvinge EC, and Kelly CP (2017). Cytokines are markers of the Clostridium difficile-induced inflammatory response and predict disease severity. Clinical and Vaccine Immunology 24, e00037–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Hamza T, Fleur AS, Zhang Y, Yu H, Chen K, Heath JE, Chen Y, Huang H, and Feng H (2018). Mice with Inflammatory Bowel Disease are Susceptible to Clostridium difficile Infection With Severe Disease Outcomes. Inflammatory Bowel Diseases 24, 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequences associated with the 16S microbiota analysis were deposited to the SRA under the bioproject PRJNA475161.