Abstract
Fundamental for understanding cerebellar function is determining the representations in Purkinje cells activity, the sole output of the cerebellar cortex. Up to the present, the most accurate descriptions of the information encoded by Purkinje cells were obtained in the context of motor behavior and reveal a high degree of heterogeneity of kinematic and performance error signals encoded. The most productive framework for organizing Purkinje cell firing representations is provided by the forward internal model hypothesis. Direct tests of this hypothesis show that individual Purkinje cells encode at two different forward models simultaneously, one for effector kinematics and one for task performance. Newer results demonstrate that the timing of simple spike encoding of motor parameters span an extend interval of up to ± 2 seconds. Further, complex spike discharge is not limited to signaling errors, can be predictive and dynamically controls the information in the simple spike firing to meet the demands of upcoming behavior. These rich, diverse and changing representations highlight the integrative aspects of cerebellar function and offer the opportunity to generalize the cerebellar computational framework over both motor and non-motor domains.
Keywords: Purkinje cell, simple spike, complex spike, kinematics, performance error, sensory prediction error, forward internal model
Introduction
The cerebellum is essential for the production of smooth, continuous movements. More recently, the cerebellum’s role in non-motor functions has emerged including cognitive processes and executive control. With its remarkably stereotypic circuitry, it is widely held that the cerebellum provides a uniform computation (Ramnani 2006; Thach 2007; Ito 2008; Schmahmann 2010). One of the main challenges in cerebellar neurobiology is to define the uniform computation and determine how it is used across all functional domains. Solving this challenge requires understanding how information is encoded and processed during behaviors throughout the cerebellar circuitry. At present, most of the available information centers on Purkinje cell encoding of motor behavior. As the only output neurons of the cerebellar cortex, Purkinje cells are a key node in the network and, therefore, are integral to understanding cerebellar function. This review focuses on the signals represented in the discharge of Purkinje cells and what those signals tell us about cerebellar function.
Cerebellar Cortical Circuitry
Purkinje cells receive a massive number of inputs from a wide spectrum of structures in the central nervous system (CNS). Purkinje cells have an expansive dendritic tree in the molecular layer that spans 200–250 µm in the sagittal plane. The dendritic tree receives parallel fiber input from one of the two canonical cerebellar circuits, the highly divergent mossy-fiber-granule cell- parallel fiber network. Mossy fibers originate from a large number of sites including the spinal cord, brainstem nuclei and a large projection from the cerebral cortex via the pons and to a lesser extent the reticular formation(Eccles and others 1967; Ito 1984; Lena 2016). These different inputs provide a spectrum of information to the cerebellar cortex, including fast exteroceptive and proprioceptive feedback, state of the spinal cord circuits and higher order signals from most of the cerebral cortex (Bloedel and Courville 1981; Apps and Garwicz 2005; Lena 2016). Mossy fibers from several structures send collaterals in both the transverse and sagittal planes suggesting that a given input is heavily redundant in the cerebellar cortex (for reviews see (Apps and Garwicz 2005; Lena 2016)). Also, integration of information from multiple pathways can occur at the level of individual granule cells. The mossy fiber to granule cell glutamatergic synapse is particularly powerful with individual excitatory post-synaptic potentials of 4 – 8 mv (Jorntell and Ekerot 2006). As most of the intrinsic synaptic connections in the cerebellar cortex have much weaker unitary amplitudes, mossy fiber input may have a large influence on the activity of cerebellar cortical neurons (Jorntell 2016).
As the bifurcated axons of the granule cells, parallel fibers run transversely along a folium for several millimeters. An individual parallel fiber synapses on several hundred Purkinje neurons but makes only a few en-passant synapses on an individual Purkinje cell (Eccles and others 1967; Ito 1984). A Purkinje cell receives excitatory input from between 100,000 to 200,000 parallel fibers, which modulate the intrinsically driven high frequency simple spike (SS) discharge of 50 to 150 spikes/sec (Eccles and others 1967; Raman and Bean 1997). Given that fewer than 200 active parallel fiber synapses are needed to generate a SS (Isope and Barbour 2002), Purkinje cells have a high bandwidth and the capacity to carry a large number of signals. The information carrying capacity is shaped by synaptic plasticity at the parallel fiber-Purkinje cell synapses as well as other synapses in the cerebellar cortex (for review see (Gao and others 2012)). Many theoretical studies have emphasized the combinatorial potential of Purkinje cell discharge. Therefore, the properties of the mossy-fiber-granule cell-parallel fiber-Purkinje cell circuit suggest Purkinje cells integrate and represent information about a remarkably diverse set of inputs.
The second canonical circuit in the cerebellar cortex consists of the climbing fiber projection to Purkinje cells. A striking feature of these two circuits are their vastly different properties. In contrast with mossy fibers, climbing fiber afferents originate solely from the contralateral inferior olive, a group of nuclei in the lower medulla. Inputs to the inferior olive include excitatory and inhibitory inputs from the spinal cord, nuclei near the mesodiencephalic junction (including the red nucleus), cerebellar nuclei, and cerebral cortex (for reviews see (Oscarsson 1980; De Zeeuw and others 1998; Apps and Garwicz 2005)). Climbing fibers monosynaptically innervate Purkinje cells through hundreds of glutamatergic synaptic contacts that are distributed on the lower two thirds of the dendritic tree. In an adult animal, a Purkinje cell receives input from a single climbing fiber. Typically, a climbing fiber synapses on 5–10 Purkinje cells located a parasagittal plane. A prominent feature of the olivocerebellar projection is a parasagittal architecture that matches the overall longitudinal zonation of the cerebellum. In this organization, a parasagittal zone or strip of Purkinje cells receives climbing fiber input from a circumscribed region of the inferior olive, and the same zone of Purkinje cells project to a specific region of the cerebellar nuclei (for review see (Voogd and Ruigrok 2004; Sugihara and Shinoda 2004; Najac and Raman 2015)).
In contrast with parallel fiber synapses, the climbing fiber-Purkinje cell synapse is one of the most powerful in the CNS (Simpson and others 1995; Schmolesky and others 2002; Llinas 2013). Firing at low rates (~0.5–2.0/sec), a climbing fiber produces a massive depolarization of the entire Purkinje cell resulting in complex spike (CS) that consists of a large Na+ somatic spike and burst of smaller spikelets generated in the initial axon segment. Also, opening voltage- gated Ca2+ channels, the strong depolarization generates Ca2+ spikes throughout the entire dendritic tree (Llinas and Sugimori 1980; Davie and others 2008). Although traditionally considered an all-or-none response (Eccles and others 1967), both CSs and the dendritic Ca2+ responses can be graded via pre- and post-synaptic modulation (for review see (Najafi and Medina 2013)).
To complete the circuitry, Purkinje cells project to and inhibit the cerebellar and vestibular nuclei. In turn, a population of excitatory neurons in the cerebellar and vestibular nuclei project to the spinal cord, brainstem and thalamic nuclei, modulating downstream structures including the cerebral cortex via the cerebello-thalamo-cortical pathway. (Allen and Tsukahara 1974; Lena 2016). A separate population of inhibitory neurons in the cerebellar nuclei project to the inferior olive, completing a closed-loop circuit of the cerebellar cortex, cerebellar nuclei and inferior olive (Chan-Palay 1977; Bakay and others 1988; Teune and others 1998). Using this nucleo-olivary circuit, the cerebellar cortex can modulate climbing fiber input to Purkinje cells (Marshall and Lang 2009; Witter and others 2013; Chaumont and others 2013; Yang and Lisberger 2013).
Elements of motor behavior represented in Purkinje cell discharge
As discussed above, inputs to the cerebellar cortex via mossy fibers are highly diverse, raising the question of how this input heterogeneity is reflected in Purkinje cell output. The most detailed descriptions of Purkinje cell discharge in relation to movements comes from experiments that manipulate the behavioral parameters and rigorously monitor behavior.
A universal observation is that Purkinje cell firing modulates with kinematics, irrespective of effector or task. Numerous studies documented the correlation of the SS activity with arm/hand kinematic parameters including position, direction, speed, and movement distance (Mano and Yamamoto 1980; Fortier and others 1989; Fu and others 1997a; Coltz and others 1999; Roitman and others 2005; Pasalar and others 2006; Hewitt and others 2015). For example, during a visually guided reaching task, Purkinje cell SS discharge covaries with arm velocity (Fig. 1A) (Marple-Horvat and Stein 1987). Eye movement kinematics, including position, velocity and acceleration, are also encoded in the SS discharge across a large spectrum of other behaviors including smooth pursuit, ocular following, saccades, and vestibulo-ocular reflex (Miles and others 1980a; Miles and others 1980b; Stone and Lisberger 1990; Lisberger and others 1994; Gomi and others 1998; Medina and Lisberger 2009; Dash and others 2012). The fidelity of eye movement kinematic representations is highlighted as the exact SS firing can be reconstructed from eye position, velocity and acceleration (Fig. 1B) (Shidara and others 1993).
Figure 1.
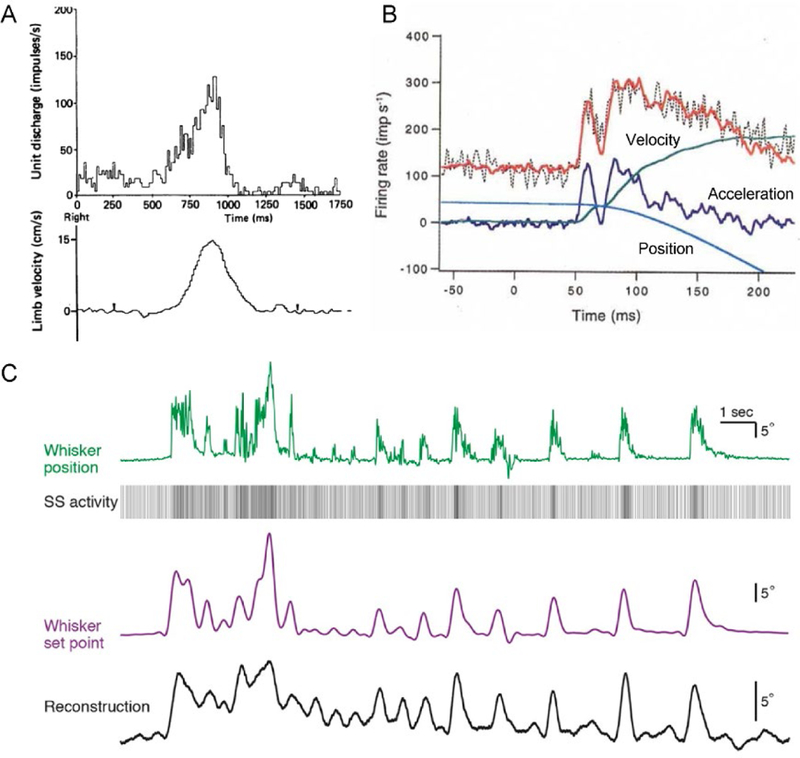
Purkinje cell SS discharge encodes movement kinematics. A) Simple spike firing rate during an arm movement task in monkeys is well correlated to arm velocity. Note that in this example, SS discharge leads movement. Adapted with permission from (Marple-Horvat and Stein 1987). B) Activity of Purkinje cell in ventral paraflocculus can be accurately reconstructed using the kinematic parameters of the eye movements during ocular following. Raw SS firing rate – black dotted line, reconstructed firing rate – red line, acceleration – dark blue line, velocity – green line, position – green line. Adapted with permission from (Shidara and others 1993). C) Simple spike encoding of whisker position in the mouse allows accurate reconstruction of the set point trajectories. Whisker position – green line, whisker set point trajectory – purple line, reconstructed set point – black line, SS activity – vertical lines sequence. Correlation coefficient between reconstruction and set point is 0.78. Adapted from (Chen and others 2016).
Whisker kinematics are also encoded as SS activity encodes set point (Fig. 1C), a slowly changing component of whisker position (Chen and others 2016) (Chen and others 2017). Similarly, SS firing modulates with limb kinematics during cat locomotion (Bosco and Poppele 1997; Valle and others 2008). In addition, SS kinematic representations are preserved across behaviors including for tracking and reaching arm movements and for saccades and smooth pursuit (Hewitt and others 2011; Sun and others 2017).
Purkinje cells also receive information about head movement kinematics from the semicircular canals and otoliths (for review see (Laurens and Angelaki 2018)). An interesting aspect of these inputs is that they cannot distinguish between head tilt and translational acceleration. It has been shown that Purkinje cells in the vermis disambiguate the head movement using an internal representation of the gravitational field (Yakusheva and others 2007; Laurens and others 2013; Dugue and others 2017).
Several hypotheses of cerebellar function require that Purkinje cells represent movement dynamics, i.e. the forces and torques or the muscle activation command necessary to execute a body part movement (Wolpert and others 1998; Kawato and Wolpert 1998). However, support for encoding of dynamics is limited or ambiguous. Only a small percentage of Purkinje cells signal grasp force and the extent of the modulation is small (Smith and Bourbonnais 1981; Espinoza and Smith 1990; Mason and others 2006). In a reach and button push task, the SS modulation with muscle activation is rather weak (Holdefer and Miller 2009). For monkeys producing invariant movement kinematics while performing elbow rotation under assistive or resistive force fields (Yamamoto and others 2007), brief changes in SS discharge occur when switching between resistive and assistive forces. The modulation of the Purkinje cell activity with eye position, velocity, and acceleration has been interpreted as evidence for the encoding of elastic, viscous and inertial forces, respectively (Shidara and others 1993; Gomi and others 1998; Kobayashi and others 1998), however, this interpretation is controversial (Ostry and Feldman 2003; Ebner and Pasalar 2008). To test for dynamic SS encoding, monkeys were trained to track a target moving on a circular trajectory under elastic and viscous force fields to disambiguate the kinematics and dynamics of arm movements (Fig. 2A). As expected, the forces required to track the target varied markedly with the type of force field and the load (Fig. 2B). However, Purkinje cell SS discharge was remarkably unresponsive to these task dynamics, instead modulating with invariant movement kinematics (Fig. 2C) (Pasalar and others 2006).
Figure 2.
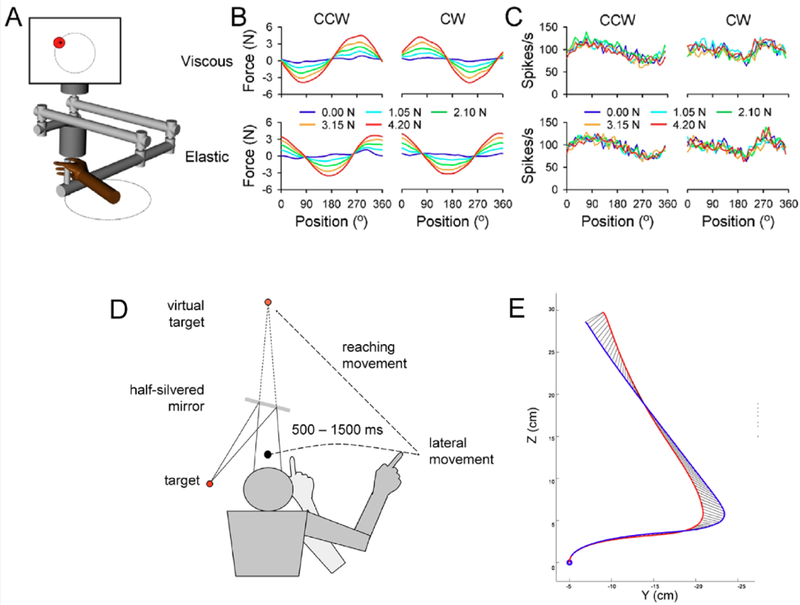
Cerebellum provides a kinematic forward internal model. A-C) Force fields effects on monkey Purkinje cell SS discharge during circular tracking. A) Monkeys use a robotic manipulandum to track a target moving circularly in viscous or elastic force fields. B) Forces at the manipulandum handle change dramatically with force field type and load. C) Simple spike activity correlates with the movement kinematics and not with the forces applied to the manipulandum. A – C adapted with permission from (Pasalar and others 2006). D-E) Effects of disrupting cerebellar function on arm state estimation. D) Subjects moving their arm left to right without visual feedback are instructed to reach for a known, fixed target by an auditory cue. D) Average arm trajectories for control trials (blue trace) and with transcranial magnetic stimulation over the ipsilateral arm region of the cerebellum (red trace). C and D adapted from (Miall and others 2007).
One of the earliest and most prominent hypotheses is that the cerebellum processes motor errors for both online control and motor learning and that CSs are the unique conduits of error information (Marr 1969; Albus 1971; Oscarsson 1980; Ito and Kano 1982). Numerous studies support this view, as CS discharge modulates with retinal slip and other error measured during vestibulo-ocular reflexes, ocular following, smooth pursuit, and saccades (Graf and others 1988; Barmack and Shojaku 1995; Kobayashi and others 1998; Soetedjo and Fuchs 2006; Herzfeld and others 2018). During limb movements, CSs modulate with reach end point errors (Kitazawa and others 1998), unexpected loads (Gilbert and Thach 1977), redirection of a reach (Wang and others 1987), adaptation to visuomotor transformations (Ojakangas and Ebner 1994) and with perturbations applied during locomotion (Andersson and Armstrong 1987; Kim and others 1987; Lou and Bloedel 1992).
However, the hypothesis that CS discharge is the primary or sole conveyer of error information does not cover accurately the spectrum of observations. Error-related CS modulation does not occur in many experimental paradigms (for reviews see (Catz and others 2005; Llinas 2013; Popa and others 2016b)). For example, during saccadic and smooth pursuit adaptation, in the oculomotor vermis CS discharge increases late in adaptation when the errors are minimal and persists after learning has stabilized (Catz and others 2005; Dash and others 2010; Prsa and Thier 2011). Also, during reaching movements in the monkey, CS were not associated with learning a mechanical perturbation (Hewitt and others 2015). Cerebellar dependent VOR learning occurs in the absence of climbing fiber input (Boyden and others 2004; Ke and others 2009) and can be driven by optogenetic increases in SS discharge (Nguyen-Vu and others 2013), arguing for the presence of error signals in the SS activity. A few early studies hinted at SS error signaling, for example with retinal slip during visual tracking (Kase and others 1979) or in relation to trial success or failure during reaching (Greger and Norris 2005). However, the nature and extent of error encoding in the SS discharge has only recently come to light.
In contrast to the error signaling hypothesis, climbing fibers also carry parametric information about movements. In the flocculus, CS firing modulates in relation to head and eye movements during VOR rotation in the dark when retinal slip is absent (Winkelman and others 2014). In the ventral paraflocculus, CSs carry eye movement kinematics during ocular pursuit (Kobayashi and others 1998) and in the nodulus, CSs exhibit directional tuning during three-dimensional vestibular stimulation (Fig. 3A) (Yakusheva and others 2010). Also, CS firing modulates with the direction, amplitude and end point of reaching movements (Fu and others 1997b; Kitazawa and others 1998).
Figure 3.
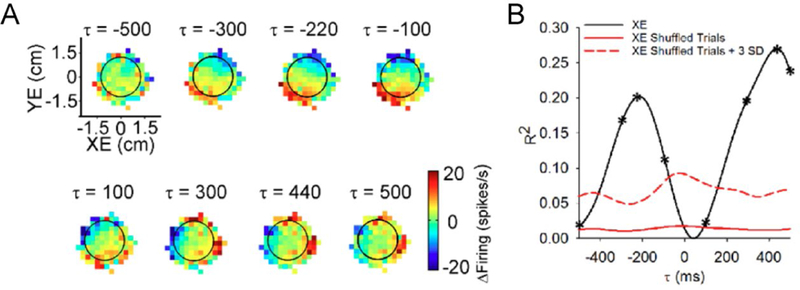
Dual encoding of position error by the Purkinje cell SS discharge. A) Sequence of firing maps showing SS modulation with position error in 200 msec steps. B) R2 temporal profile showing the strength of XE encoding as a function of time (τ). Negative τ-values signify SS firing leads behavior. Adapted from (Popa and others 2016a).
Multiplicity of representations in Purkinje cell firing
As individual Purkinje cells have the capacity to convey a multitude of different signals, it is not unexpected that several studies reveal Purkinje cell multiplexing. In the paraflocculus, the SS firing of each Purkinje cell represents a mixture of kinematic parameters during vestibulo-ocular reflexes, smooth pursuit and ocular following (Shidara and others 1993; Medina and Lisberger 2009; Sun and others 2017). In the occulomotor vermis, the ongoing SS activity encodes saccade kinematics, while the beginning or ending of SS firing pauses encode movement onset (Hong and others 2016). During reaching and manual tracking tasks, the SS and CS firing of individual Purkinje cells modulate with multiple kinematic parameters (Marple-Horvat and Stein 1987; Fu and others 1997a; Coltz and others 1999; Roitman and others 2005). During reaching movements, early CSs represent kinematic aspects of the behavior such as reach destination while late CSs encode performance errors (Kitazawa and others 1998). However, many of these findings were obtained in low dimensional tasks, such as reaching or saccadic eye movements, that impose a high degree of covariance between the kinematic parameters and could mask the true extent of complexity present in Purkinje cell firing.
For a better understanding of the behavioral representations in Purkinje cell discharge requires studying complex, higher dimensional movements allowing higher independence of the kinematics and disambiguating the Purkinje cell representations. Therefore, Purkinje cells were recorded in nonhuman primates during a manual, pseudo-random tracking task (Hewitt and others 2011; Popa and others 2012). Pseudo-random tracking has several advantages including providing excellent coverage of the kinematic workspaces and eliminating the confound of predictability (Paninski and others 2004; Hewitt and others 2011; Popa and others 2012; Popa and others 2017). Pseudo-random tracking also reduces the statistical dependencies among behavioral parameters and requires continuous control of the movement throughout the task. Purkinje cells recorded during pseudo-random tracking, confirm that the SS firing of individual Purkinje cells signal multiple kinematic parameters including position, velocity and acceleration (Hewitt and others 2011; Popa and others 2012; Popa and others 2017; Streng and others 2017b). Furthermore, using linear regressions based on residual SS firing show that these representations are fully independent (Hewitt and others 2011; Popa and others 2017), establishing that the SS firing simultaneously encodes multiple streams of kinematic information.
In addition to a more robust evaluation of kinematic representations, during pseudo-random tracking the monkeys strive to maintain the cursor close to the target center that provides for several natural and continuous performance errors. These include position error (cursor position relative to the target center), radial error (distance between cursor and target center) and direction error (angular direction necessary to move from the current position to the target center) (Hewitt and others 2011; Popa and others 2012). In a large majority of Purkinje cells, the SS discharge encodes one or more of these error measures. The modulation of SS discharge with position error, illustrated by the sequence of firing maps (Fig. 3A) and quantified using linear regressions of the firing residuals (Fig. 3B) shows significant encoding of XE by this example Purkinje cell. Fitting SS firing to multi-linear models including only kinematics or error measures, respectively, results in similar distributions of R2 values showing that overall error encoding in the SS firing is robust, being approximately as strong as the encoding of kinematics. Moreover, the SS firing of Purkinje cells simultaneously and independently encodes predictive and feedback representations of the same parameter as shown for the example in Figure 3 with position error modulation at negative and positive lags. Therefore, the SS firing of an individual Purkinje cell carries a rich representation of motor behavior, combining detailed predictive and feedback information about effector kinematics with task performance.
Pseudo-random tracking also highlights both the parametric and non-error encoding properties of climbing fiber input as CSs have rather robust modulation with hand position, velocity and acceleration as well as with position error (Streng and others 2017b). These results show the need to move beyond CSs serving solely as an error feedback signal and acknowledge that climbing fiber input has spatially rich information about movement kinematics. Therefore, the alluring functional segregation between SS and CS discharge cannot withstand closer scrutiny. It is more likely that these two modalities of Purkinje cells activity represent two different computational stages in the processing of the same motor information to fine tune cerebellar output.
For both SS and CS discharge, linear models of the firing provide accurate characterizations of the encoding of kinematics and performance errors across effectors and tasks (Shidara and others 1993; Medina and Lisberger 2009; Popa and others 2012; Sun and others 2017).
Moreover, during pseudo-random tracking, the encoding of single parameters sum to a multi- linear encoding of all parameters, further suggesting linear representation of motor behavior (Hewitt and others 2011; Popa and others 2012). The mapping of both the SS and CS discharge in relation to behavioral parameters consistently show planar modulation (see Figs. 3–6) and supports the view that linear encoding is not an artifact of convenient analytical choices but a fundamental feature of Purkinje cell signal processing. This functional linearity is consistent with the observations that Purkinje cells linearly integrate parallel fiber input (Walter and Khodakhah 2006; Walter and Khodakhah 2009) and that of the linearity of the mossy fiber – granule cell – Purkinje cell circuit (Jorntell 2016; Chen and others 2017). The multi-linear encoding of different motor parameters presents important advantages such as scaling, either to expand individual workspaces or to acquire new signals without affecting existing representations. Linearity also allows for robust readout downstream and reliable decoding.
Figure 6.
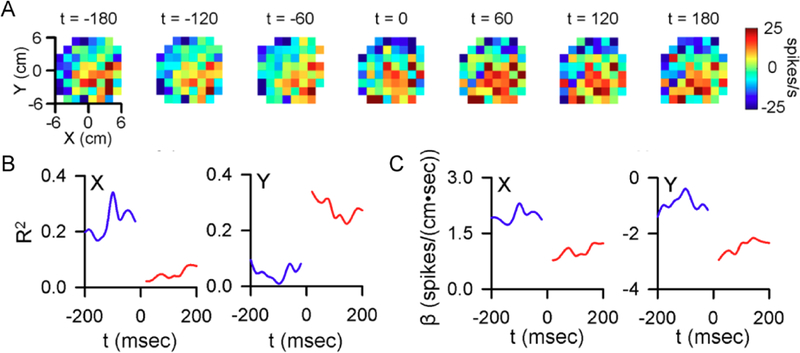
Climbing fiber input changes the representations encoded in the SS discharge. A) Firing maps illustrating example SS modulation with position relative to CS occurrence (t = 0). B) Pre- and post-CS encoding strength of X and Y. C) Pre-and post-CS SS firing sensitivity for this cell to X (left) and Y (right). B-C) Blue traces denote pre-CS, red traces denote post-CS. A- C adapted from (Streng and others 2017a).
Forward internal model hypothesis
The broad range of signals observed in the discharge of Purkinje cells makes constructing a unified theory of the cerebellar cortical function elusive. But the diversity of signals should not be surprising given the cerebellar circuitry and that effective motor control requires the continuous monitoring of and acting on multiple streams of information including correcting for errors in an ever-changing environment (Wolpert and Ghahramani 2000; Todorov and Jordan 2002; Berniker and Kording 2008; Shadmehr and others 2010). Early views stressed classical closed-loop schemes, in which motor commands are updated by sensory feedback. However, motor control theorists pointed out that the inherent delays and low gains of sensory feedback loops render closed-loop control unstable generating discontinuous and under/over corrective movements (Miall and Wolpert 1996; Kawato 1999; Wolpert and Ghahramani 2000; Shadmehr and others 2010). As exemplified for saccadic eye movements, error correction occurs more rapidly than and even in the absence of sensory feedback (Flanagan and Wing 1997; Wagner and Smith 2008; Golla and others 2008; Xu-Wilson and others 2009; Shadmehr and others 2010).
One solution is that the CNS predicts the consequences of motor commands using a forward internal model (Robinson 1975; Imamizu and others 2000; Flanagan and others 2003; Maschke and others 2004; Diedrichsen and others 2005; Morton and Bastian 2006; Xu-Wilson and others 2009; Shadmehr and others 2010). Receiving an efferent copy of motor command, a forward internal model predicts the sensory consequences of that command and computes a sensory prediction error by integrating it with the sensory feedback conveying information about the current state (Held and Freedman 1963; Jordan and Rumelhart 1992; Wolpert and others 1995; Miall and Wolpert 1996; Shadmehr and others 2010). The sensory prediction error is used both to improve subsequent predictions through learning and to guide future actions (Wallman and Fuchs 1998; Noto and Robinson 2001; Morton and Bastian 2006; Mazzoni and Krakauer 2006; Xu-Wilson and others 2009). Historically, as the forward internal model theory was tested, the sensory consequences of the motor commands were strongly assumed to be manifest in the kinematic domain.
A notable demonstration of the forward internal model hypothesis was provided by disrupting cerebellar activity using transcranial magnetic stimulation (TMS). In the absence of visual feedback, subjects moved their hand from left to right until an auditory cue instructed them to reach to a known target (Fig. 2D). Applying TMS to the cerebellum between cue and reach onset resulted in end-point and initial direction errors. The errors were consistent with subjects executing the reach from an earlier position conveyed by delayed sensory feedback (Fig. 2E). These results are consistent with the cerebellum providing an internal prediction of the effector state that is used to plan and execute upcoming motor commands (Miall and others 2007).
Functional imaging in healthy subjects reveal activation changes with motor learning and sensory prediction errors, consistent with the cerebellum acquiring and storing forward internal model (Shadmehr and Holcomb 1997; Imamizu and others 2000; Diedrichsen and others 2005; Tseng and others 2007; Grafton and others 2008; Schlerf and others 2012). Conversely, predictive, feedforward control and sensory prediction error-dependent adaptation are defective during a wide variety of motor behaviors in patients with cerebellar pathology (Horak and Diener 1994; Lang and Bastian 1999; Nowak and others 2004; Smith and Shadmehr 2005; Bastian 2006; Morton and Bastian 2006; Tseng and others 2007; Golla and others 2008; Xu-Wilson and others 2009; Taylor and others 2010). While the imaging and behavioral data are compelling, critical to testing the forward model hypothesis requires determining whether the discharge of cerebellar neurons have the expected representations.
Purkinje cell representation of a forward internal model
At single cell level, Purkinje cell SS discharge contains many properties consistent with the predictive and feedback components of a forward internal model. Consistent with predictive encoding, SS discharge tends to lead effector kinematics (Marple-Horvat and Stein 1987; Stone and Lisberger 1990; Shidara and others 1993; Shidara and Kawano 1993; Fu and others 1997a; Gomi and others 1998; Roitman and others 2005; Hewitt and others 2011; Dash and others 2013; Hewitt and others 2015). In the same behaviors SS discharge provides feedback, as the firing also lags kinematics. Also, SS firing modulates with the passive movement kinematics (Rubia and Kolb 1978; Kolb and others 1987; Valle and others 2000; Giaquinta and others 2000). Therefore, at the population level, SS and CS Purkinje cell firing contains both predictions about upcoming movements and sensory feedback of the movement consequences.
Although the prediction-feedback dichotomy was documented at population level, historically it was assumed that individual Purkinje cells would show a clear preference for encoding either the future or the past. Pseudo-random tracking offers a closer investigation of individual Purkinje cell representations by providing extended timing trials and uncoupling the past and future states. In a significant majority of the SS discharge representing errors, the modulation includes both prediction and feedback of the same parameter (Fig. 3). This dual encoding has opposing effects on the SS firing, consistent with the predictive and feedback signals necessary to compute SPEs (Popa and others 2012; Popa and others 2014).
The timing of CS discharge modulation has a similar prediction-feedback dichotomy. Although historically thought to be feedback driven, CSs of individual Purkinje cells predict both kinematics and performance errors during pseudo-random tracking (Streng and others 2017b). Highlighting the predictive nature of the climbing fiber input, feedforward CS firing is much more common than feedback modulation. Feedforward CS responses occur during eye blink conditioning, with CS increases prior to and predicting the conditioned response (Ohmae and Medina 2015; Ten Brinke and others 2015).
We sought to directly test the hypothesis that the SS lead and lag representations represent the output of a forward internal model (Streng and others 2018). To do this, we leveraged two manipulations of visual feedback during pseudorandom tracking. In the first manipulation, visual feedback delay, a lag was introduced between the movement of the hand and the movement of the cursor. The expectation was that the visual feedback delay would alter predictive encoding of position error, as a forward internal model makes predictions with respect to the timing of the hand movement, not the delayed movement of the cursor (Fig. 4A–B). Consistent with this hypothesis, the leading SS modulation with position errors occurs earlier, while the timing of lagging modulation is not affected (Fig. 4C–D). At the population level, delaying the visual feedback shifts the timing of the predictive position error modulation to earlier leads by an interval matching the experimental delay, while preserving the timing of the feedback modulation (Fig. 4E). These results are consistent with a forward internal model that predicts the upcoming position errors based on motor commands and not on the current position error provided by visual feedback. Conversely, the invariant timing of the SS feedback modulation shows its dependence on visual feedback.
Figure 4.
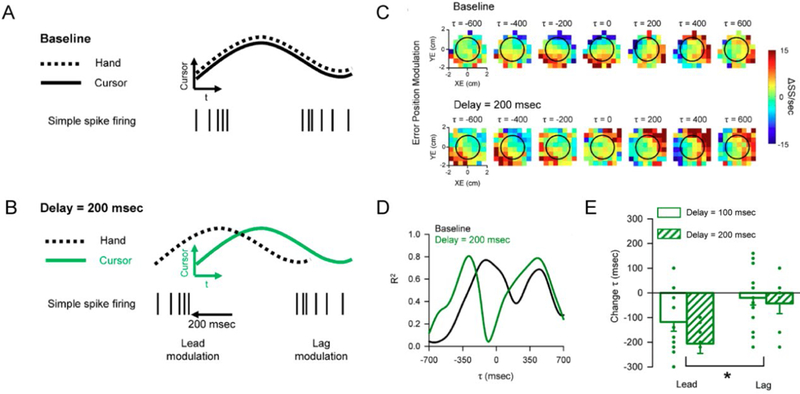
Simple spike representations of position error reflect the components of a forward internal model. A) In the baseline condition the cursor (continuous trace) and hand (dashed trace) movements are indistinguishable. Simple spike firing both leads (left spike train) and lags (right spike train) the cursor movement. B) In the delay condition, hand movement occurs before the cursor movement by the delay imposed. Based on the forward model hypothesis, the lead SS modulation is time-locked to hand movement and will shift earlier relative to cursor movement. The lag modulation should be time-locked to the cursor movement. C) Firing maps for an example Purkinje cell with lead and lag SS position error modulation in both baseline (top row) and 200 msec delay (bottom row) conditions. Each map shows SS modulation at a specific lead (negative τ) or lag (positive τ). Black circle indicates target edge. D) Quantifying the SS encoding of position error for the cell shown in C, R2 temporal profiles determined by linear regression in both the baseline (black line) and 200 msec delay (green line) conditions. E) Average peak timing of encoding across the population illustrates the significant shift in timing of lead encoding for both 100 msec (solid green) and 200 msec (checkered green) delays. The time of lag encoding was not significantly affected for either delay. A-E adapted from (Streng and others 2018).
For the second manipulation, visual feedback was reduced by hiding the cursor from view when within the target. Reducing the visual feedback tests if the lagged SS modulation with position error is driven by the visual feedback and the reduction in visual feedback should decrease the feedback encoding of position error. However, the predictive encoding should remain unaffected as the predictions are based on the motor commands. As expected, the hidden cursor condition reduces the lagging SS modulation with position error inside the target. Lagging modulation is restricted to outside the target edge, where visual feedback is available. Importantly, the predictive modulation is not affected. Therefore, visual input drives the feedback SS modulation with position error. Finally, neither feedback manipulation affects lead nor lag encoding of hand kinematics. We interpret the differential effects of the feedback manipulations on the SS encoding of kinematics and position error as the predictive and feedback components of multiple forward internal models operating independently to achieve optimal task performance. The outputs of these forward internal models are encoded simultaneously in the SS firing of individual Purkinje cells.
Timing of predictive and feedback representations
Purkinje cells carry comprehensive information about the current behavior, from effector implementation of motor commands, reflected in the kinematic representations, to the status of achieving self-directed goals, manifest in performance error encoding. The diversity of signals raises the question whether the encoding heterogeneity extends over extended time periods in which current movements can be integrated into behavioral sequences and planning processes.
During pseudo-random tracking, both kinematic and error parameters are encoded at multiple times ranging from 2 s predictions of upcoming behavior to 2 s working memories of past behavior (Popa and others 2017). The SS firing maps of the velocity workspace for an example PC demonstrate this long-range encoding with significant predictive and feedback modulation with velocity from −2000 msec to 2000 msec (Fig. 5A–C). Decoding these long-term signals demonstrates there is remarkably accurate and rich behavioral information in the SS firing across the +/− 2 s time span. The quality and accuracy of the long range information is indicated by the slope of decoded versus observed velocity (Fig. 5D). Surprisingly, Purkinje cells uniformly cover the various combinations of parameters and timing, without obvious preferences or clustering.
Figure 5.
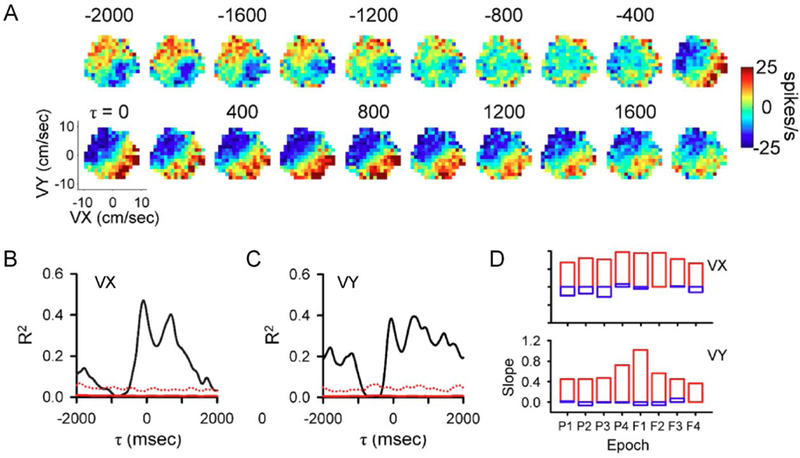
Long-range kinematic representations in the SS discharge. A) Firing maps for an example Purkinje cell with both lead and lag SS modulation relative to the velocity workspace (VX and VY). Each map shows SS modulation at a specific lead (negative τ) or lag (positive τ) ranging from −2000 to 2000 msec. B-C) R2 temporal profiles show the strength of VX (B) and VY (C) encoding as function of lead or lag. D) Decoding performance across all epochs during track period illustrated by decoding slope for VX and VY. Red columns illustrate population based decoding and blue column illustrate chance decoding. A-D adapted from (Popa and others 2017).
The long-term SS modulation has important implications for understanding the implementation of forward internal models, specifically how performance from past actions informs subsequent actions. The long-range feedback SS modulation provides a mechanism by which the motor system retains information about past performance to both evaluate the consequences of previous motor commands as well as update subsequent commands. That both kinematic and task error information persists over several seconds suggests the cerebellum has access to multiple classes of information about past performance in making these computations. The presence of long-range representations of both upcoming and past behavior in Purkinje cell discharge provides a neural substrate for movement corrections, anticipatory signals, working memory, and temporal integration across multiple classes of behaviors.
The source of these long-term signals in not clear. One possible mechanism is the information held in temporary storage as described for working memory in the cerebral cortex (Gazzaley and Nobre 2012; Nyberg and Eriksson 2015; D’Esposito and Postle 2015). The cerebellum has strong closed-loop connections with the cerebral cortex, including the motor, prefrontal and parietal cortices (for reviews see (Schmahmann and Pandya 1997; Strick and others 2009; Bostan and others 2013)). Neurons in these cortical regions have feedforward and/or working memory discharge consistent with the time courses found for Purkinje cells (Shima and Tanji 2000; Lu and Ashe 2005; Averbeck and Lee 2007) (Tanji and others 1980; Kurata and Wise 1988; Thach 2007). Together these observations suggest a likely source of the long-range signaling in SS firing involves recursive network interactions between the cerebellum and cerebral cortex.
Dynamic encoding hypothesis
Error and motor learning roles for climbing fiber action in the cerebellum have dominated the literature since their introduction but this view is undergoing revision (Catz and others 2005; Llinas 2013; Popa and others 2016b). However, as reviewed above CS modulate linearly with movement parameters, can predict behavior and do not simply signal errors. Two additional features of CS discharge emphasize the need to re-consider climbing fiber function. First, spontaneous CS firing is essential for cerebellar function, suggesting a role for climbing fiber input beyond error signaling (Llinas and others 1975; Mountcastle and others 1975; Montarolo and others 1982; Horn and others 2013). Second, the unique and massive depolarization of the Purkinje cell due to climbing fiber input likely resets the residual effects of prior inputs as well as change how subsequent inputs act on the Purkinje cell (for review see (Kitamura and Kano 2013)). These considerations led us to hypothesize that climbing fiber input changes the information encoded by the SS firing.
We recently quantified SS modulation with kinematics and performance errors before and after CS discharge (Streng and others 2017b). During tracking, CSs trigger robust and rapid changes in the SS modulation with limb kinematics and position error. An example of CS- coupled encoding changes in position (X and Y) encoding is shown for a Purkinje cell in Figure 6. Prior to CS occurrence, the SS firing contains a strong representation of X position, while only weakly modulated with Y position. This representation switches following a CS, with the SS modulation occurring predominately with Y position. Another important observation is that the CS-coupled increases in SS encoding of position error are followed by and scale with decreases in error. Stated differently, the increases in SS encoding of performance errors lead to improved task performance, indicating that CS control of SS information functions to optimize behavior. Also, increases in SS encoding of a kinematic parameter are associated with larger changes in that parameter than are decreases in SS encoding. Intriguingly, the CS-coupled changes in SS encoding for a given Purkinje cell tend to oppose the encoding drift occurring independent of CS firing. For example, a Purkinje cell with a CS-coupled increase in SS encoding of velocity tends to decrease in velocity encoding in the absence of CSs.
Together, these observations indicate that climbing fiber discharge fine-tunes the computational state of a Purkinje cell, either to compensate for drifts in encoding or to select for the most salient representations of behavior to optimize performance. This hypothesis accommodates previously documented error signaling as well as newer observations that CSs are predictive and not restricted to error modulation. This hypothesis also helps explain the stochastic nature of CS discharge, occurring even in the absence of obvious behavioral triggers to maintain encoding homeostasis.
Conclusions
Modulation of the primary output neuron of the cerebellum has been described during a wealth of behavioral tasks involving multiple effectors, from eye movements to whisking and arm movements to locomotion. This review outlines three fundamental properties of how Purkinje cells represent behavior (Figure 7). First, rather than encoding one ‘preferred’ parameter, individual Purkinje cells linearly encode high dimensional representations of behavior. A second property is that SS firing conveys a wide array of temporal information about behavior, ranging from short term predictive and feedback representations to longer time scales before and after movements. While the short range representations are consistent with the output of multiple independent forward internal models, the long range signals likely reflect a form of working memory. A final property is the ability of Purkinje cells to toggle between representations via CS discharge. The powerful excitation triggered by climbing fiber discharge serves to both maintain the large computational bandwidth of SS firing by correcting for drifts in encoding (illustrated in Figure 7 by the changes triggered by spontaneous CS discharge), as well as dynamically reallocate the information in advance of a change in behavior (illustrated by the changes triggered by a CS predicting behavior change). The high behavioral dimensionality combined with timing multiplexing argues for a more general cerebellar computational framework in which current action can be integrated into a much wider behavioral context.
Figure 7.
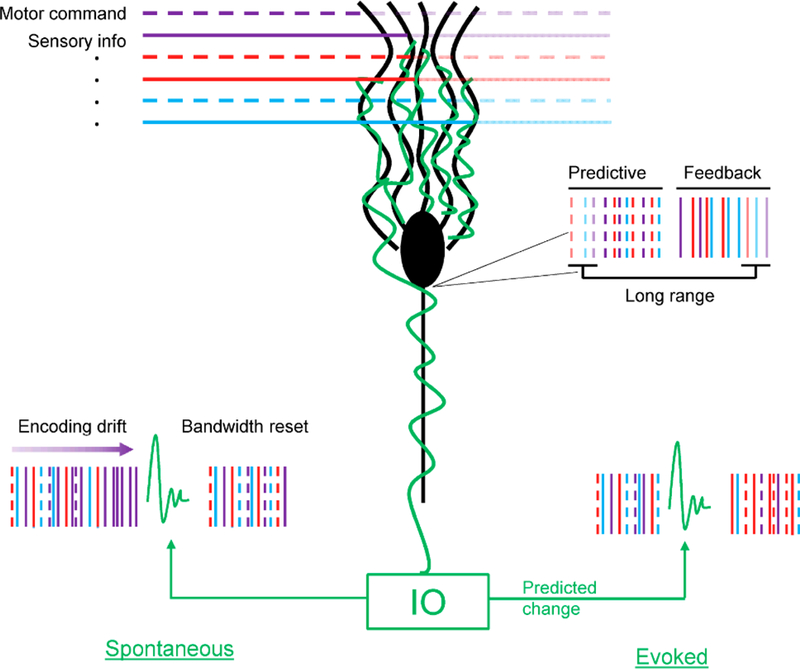
Purkinje cell representations. Purkinje cell (black) receives two classes of inputs: parallel fibers (multi-colored horizontal traces), climbing fibers (green trace). Parallel fibers convey diverse information including but not limited to sensory (continuous traces) and cerebral cortical signals inputs (dash traces). Purkinje cell output encodes multiple motor parameters over multiple timings. The climbing fiber input selects the signals present in the Purkinje cell output. Vertical lines represent spike trains. Continuous lines reflect feedback information, while dashed lines reflect predictive information. The transparency reflects the timing of the information conveyed (no transparency – short range, high transparency – long range). In the diagram, different colors represent different streams of information.
Box Figure.
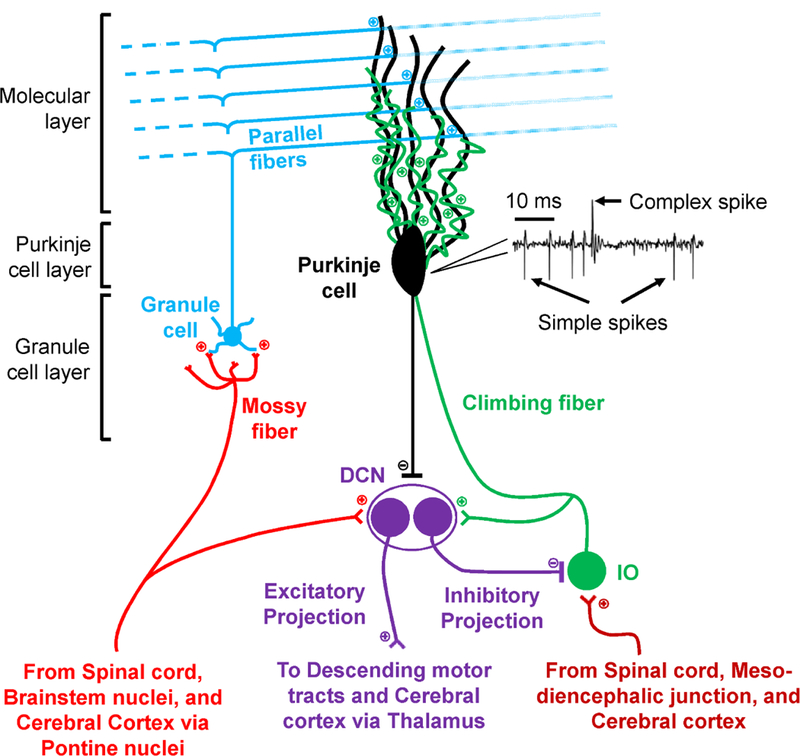
Architecture of the cerebellum. Cerebellar cortex is structured in three distinct layers (Molecular layer, Purkinje cell layer and Granule cell layer), receives two distinct inputs (mossy fibers and climbing fibers) and provides a single output (the axons of the Purkinje cells). The interneurons present in the cerebellar cortex layers are not shown. Mossy fibers excite the granule cells that form the deepest of the cerebellar cortex layers. Granule cells axons ascend into the molecular layer, at the surface of the cerebellar cortex, forming the parallel fibers that excite the extensive dendritic tree of the Purkinje cells. The parallel fiber input is relatively weak and modulates the high frequency intrinsic Purkinje cell simple spike discharge. The mossy fibers collaterals also provide excitatory input to the deep cerebellar nuclei (DCN), the target of the cerebellar cortex output. The second input to the cerebellar cortex is provided by the climbing fibers originating from the inferior olive (IO). A Purkinje cell is innervated by a single climbing fiber that synapses extensively throughout the Purkinje cell dendritic tree and generates the low frequency complex spike discharge. Climbing fiber collaterals also excite DCN. Purkinje cells provide the sole cerebellar cortex output, inhibiting the two distinct populations of DCN neurons. The inhibitory neurons project to IO and the excitatory neurons provide the cerebellar output. The Purkinje cell activity includes the high frequency simple spikes and the low frequency complex spikes, illustrated by an extracellular recording example. Each complex spike is followed by a simple spike pause lasting an average of 10 ms.
Acknowledgements:
We would like to thank Kathleen Beterams for her help with the manuscript.
Supported in part by NIH grants R01 NS18338 and T32 GM008471 and NSF grant IGERT DGE-1069104.
References
- 1.Albus JS. 1971. A theory of cerebellar function. Math Biosci 10:25–61. [Google Scholar]
- 2.Allen GI, Tsukahara N. 1974. Cerebro-cerebellar communication systems. Physiol Rev 54(4):957–1006. [DOI] [PubMed] [Google Scholar]
- 3.Andersson G, Armstrong DM. 1987. Complex spikes in Purkinje cells in the lateral vermis (b zone) of the cat cerebellum during locomotion. J Physiol 385:107–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apps R, Garwicz M. 2005. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci 6(4):297–311. [DOI] [PubMed] [Google Scholar]
- 5.Averbeck BB, Lee D. 2007. Prefrontal neural correlates of memory for sequences. J Neurosci 27(9):2204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakay RA, Fiandaca MS, Sweeney KM, Colbassani HJ, Collins DC. 1988. Delayed stereotactic transplantation technique in non-human primates. In: Gash DM, Sladek JR, editors. Progress in Brain Research Elsevier Science Publishers B.V.; p 463–71. [DOI] [PubMed] [Google Scholar]
- 7.Barmack NH, Shojaku H. 1995. Vestibular and visual climbing fiber signals evoked in the uvula-nodulus of the rabbit cerebellum by natural stimulation. J Neurophysiol 74(6):2573–89. [DOI] [PubMed] [Google Scholar]
- 8.Bastian AJ. 2006. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol 16(6):645–9. [DOI] [PubMed] [Google Scholar]
- 9.Berniker M, Kording K. 2008. Estimating the sources of motor errors for adaptation and generalization. Nat Neurosci 11(12):1454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloedel JR, Courville J. 1981. A review of cerebellar afferent systems. In: Brooks VB, Geiger SR, editors. Handbook of Physiology, Sect. 1, The Nervous System, Vol. II. Motor Control, Part 2 1 ed. Baltimore: Williams and Wilkins; p 735–830. [Google Scholar]
- 11.Bosco G, Poppele RE. 1997. Representation of multiple kinematic parameters of the cat hindlimb in spinocerebellar activity. J Neurophysiol 78(3):1421–32. [DOI] [PubMed] [Google Scholar]
- 12.Bostan AC, Dum RP, Strick PL. 2013. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci 17(5):241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyden ES, Katoh A, Raymond JL. 2004. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci 27:581–609. [DOI] [PubMed] [Google Scholar]
- 14.Catz N, Dicke PW, Thier P. 2005. Cerebellar complex spike firing is suitable to induce as well as to stabilize motor learning. Curr Biol 15(24):2179–89. [DOI] [PubMed] [Google Scholar]
- 15.Chan-Palay V 1977. Cerebellar Dentate Nucleus New York: Springer; −548 p. [Google Scholar]
- 16.Chaumont J, Guyon N, Valera AM, Dugue GP, Popa D, Marcaggi P, Gautheron V, Reibel-Foisset S, Dieudonne S, Stephan A, Barrot M, Cassel JC, Dupont JL, Doussau F, Poulain B, Selimi F, Lena C, Isope P. 2013. Clusters of cerebellar Purkinje cells control their afferent climbing fiber discharge. Proc Natl Acad Sci U S A 110(40):16223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Augustine GJ, Chadderton P. 2016. The cerebellum linearly encodes whisker position during voluntary movement. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Augustine GJ, Chadderton P. 2017. Serial processing of kinematic signals by cerebellar circuitry during voluntary whisking. Nat Commun 8(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coltz JD, Johnson MT, Ebner TJ. 1999. Cerebellar Purkinje cell simple spike discharge encodes movement velocity in primates during visuomotor arm tracking. J Neurosci 19(5):1782–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Esposito M, Postle BR. 2015. The cognitive neuroscience of working memory. Annu Rev Psychol 66:115–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dash S, Catz N, Dicke PW, Thier P. 2010. Specific vermal complex spike responses build up during the course of smooth-pursuit adaptation, paralleling the decrease of performance error. Exp Brain Res 205(1):41–55. [DOI] [PubMed] [Google Scholar]
- 22.Dash S, Catz N, Dicke PW, Thier P. 2012. Encoding of smooth-pursuit eye movement initiation by a population of vermal Purkinje cells. Cereb Cortex 22(4):877–91. [DOI] [PubMed] [Google Scholar]
- 23.Dash S, Dicke PW, Thier P. 2013. A vermal Purkinje cell simple spike population response encodes the changes in eye movement kinematics due to smooth pursuit adaptation. Front Syst Neurosci 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davie JT, Clark BA, Hausser M. 2008. The origin of the complex spike in cerebellar Purkinje cells. J Neurosci 28(30):7599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Zeeuw CI, Simpson JI, Hoogenraad CC, Galjart N, Koekkoek SK, Ruigrok TJ. 1998. Microcircuitry and function of the inferior olive. Trends Neurosci 21(9):391–400. [DOI] [PubMed] [Google Scholar]
- 26.Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. 2005. Neural correlates of reach errors. J Neurosci 25(43):9919–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dugue GP, Tihy M, Gourevitch B, Lena C. 2017. Cerebellar re-encoding of self- generated head movements. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebner TJ, Pasalar S. 2008. Cerebellum predicts the future motor state. Cerebellum 7(4):583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eccles JC, Ito M, Szentagothai J. 1967. The Cerebellum as a Neuronal Machine Berlin: Springer-Verlag. [Google Scholar]
- 30.Espinoza E, Smith AM. 1990. Purkinje cell simple spike activity during grasping and lifting objects of different textures and weights. J Neurophysiol 64(3):698–714. [DOI] [PubMed] [Google Scholar]
- 31.Flanagan JR, Vetter P, Johansson RS, Wolpert DM. 2003. Prediction precedes control in motor learning. Curr Biol 13(2):146–50. [DOI] [PubMed] [Google Scholar]
- 32.Flanagan JR, Wing AM. 1997. The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci 17(4):1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fortier PA, Kalaska JF, Smith AM. 1989. Cerebellar neuronal activity related to whole- arm reaching movements in the monkey. J Neurophysiol 62(1):198–211. [DOI] [PubMed] [Google Scholar]
- 34.Fu QG, Flament D, Coltz JD, Ebner TJ. 1997a. Relationship of cerebellar Purkinje cell simple spike discharge to movement kinematics in the monkey. J Neurophysiol 78(1):478–91. [DOI] [PubMed] [Google Scholar]
- 35.Fu QG, Mason CR, Flament D, Coltz JD, Ebner TJ. 1997b. Movement kinematics encoded in complex spike discharge of primate cerebellar Purkinje cells. NeuroReport 8(2):523–9. [DOI] [PubMed] [Google Scholar]
- 36.Gao Z, van Beugen BJ, De Zeeuw CI. 2012. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci 13(9):619–35. [DOI] [PubMed] [Google Scholar]
- 37.Gazzaley A, Nobre AC. 2012. Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci 16(2):129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giaquinta G, Valle MS, Caserta C, Casabona A, Bosco G, Perciavalle V. 2000. Sensory representation of passive movement kinematics by rat’s spinocerebellar Purkinje cells. Neurosci Lett 285(1):41–4. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert PF, Thach WT. 1977. Purkinje cell activity during motor learning. Brain Res 128(2):309–28. [DOI] [PubMed] [Google Scholar]
- 40.Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Thier P. 2008. Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosci 27(1):132–44. [DOI] [PubMed] [Google Scholar]
- 41.Gomi H, Shidara M, Takemura A, Inoue Y, Kawano K, Kawato M. 1998. Temporal firing patterns of Purkinje cells in the cerebellar ventral paraflocculus during ocular following responses in monkeys I. Simple spikes. J Neurophysiol 80(2):818–31. [DOI] [PubMed] [Google Scholar]
- 42.Graf W, Simpson JI, Leonard CS. 1988. Spatial organization of visual messages of the rabbit’s cerebellar flocculus. II. Complex and simple spike responses of Purkinje cells. J Neurophysiol 60(6):2091–121. [DOI] [PubMed] [Google Scholar]
- 43.Grafton ST, Schmitt P, Van HJ, Diedrichsen J. 2008. Neural substrates of visuomotor learning based on improved feedback control and prediction. Neuroimage 39(3):1383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greger B, Norris S. 2005. Simple spike firing in the posterior lateral cerebellar cortex of Macaque Mulatta was correlated with success-failure during a visually guided reaching task. Exp Brain Res 167(4):660–5. [DOI] [PubMed] [Google Scholar]
- 45.Held R, Freedman SJ. 1963. Plasticity in human sensorimotor control. Science 142(3591):455–62. [DOI] [PubMed] [Google Scholar]
- 46.Herzfeld DJ, Kojima Y, Soetedjo R, Shadmehr R. 2018. Encoding of error and learning to correct that error by the Purkinje cells of the cerebellum. Nat Neurosci 21(5):736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hewitt A, Popa LS, Pasalar S, Hendrix CM, Ebner TJ. 2011. Representation of limb kinematics in Purkinje cell simple spike discharge is conserved across multiple tasks. J Neurophysiol 106(5):2232–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hewitt AL, Popa LS, Ebner TJ. 2015. Changes in Purkinje cell simple spike encoding of reach kinematics during adaptation to a mechanical perturbation. J Neurosci 35(3):1106–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holdefer RN, Miller LE. 2009. Dynamic correspondence between Purkinje cell discharge and forelimb muscle activity during reaching. Brain Res 1295:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong S, Negrello M, Junker M, Smilgin A, Thier P, De SE. 2016. Multiplexed coding by cerebellar Purkinje neurons. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horak FB, Diener HC. 1994. Cerebellar control of postural scaling and central set in stance. J Neurophysiol 72(2):479–93. [DOI] [PubMed] [Google Scholar]
- 52.Horn KM, Deep A, Gibson AR. 2013. Progressive limb ataxia following inferior olive lesions. J Physiol 591(Pt 22):5475–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M. 2000. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403(6766):192–5. [DOI] [PubMed] [Google Scholar]
- 54.Isope P, Barbour B. 2002. Properties of unitary granule cell-->Purkinje cell synapses in adult rat cerebellar slices. J Neurosci 22(22):9668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ito M 1984. The Cerebellum and Neural Control New York: Raven Press; 1 p. [Google Scholar]
- 56.Ito M 2008. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci 9(4):304–13. [DOI] [PubMed] [Google Scholar]
- 57.Ito M, Kano M. 1982. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett 33(3):253–8. [DOI] [PubMed] [Google Scholar]
- 58.Jordan MI, Rumelhart DE. 1992. Forward models: Supervised learning with a distal teacher. Cognitive Science 16(3):307–54. [Google Scholar]
- 59.Jorntell H 2016. Cerebellar Neuronal Codes - Perspectives from Intracellular Analysis In Vivo. In: Heck DH, editor. Neuronal Codes of the Cerebellum New York, NY: Elsevier; p 155–72. [Google Scholar]
- 60.Jorntell H, Ekerot CF. 2006. Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. J Neurosci 26(45):11786–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kase M, Noda H, Suzuki DA, Miller DC. 1979. Target velocity signals of visual tracking in vermal Purkinje cells of the monkey. Science 205(4407):717–20. [DOI] [PubMed] [Google Scholar]
- 62.Kawato M 1999. Internal models for motor control and trajectory planning. Curr Opin Neurobiol 9(6):718–27. [DOI] [PubMed] [Google Scholar]
- 63.Kawato M, Wolpert D. 1998. Internal models for motor control. Novartis Found Symp 218:291–304. [DOI] [PubMed] [Google Scholar]
- 64.Ke MC, Guo CC, Raymond JL. 2009. Elimination of climbing fiber instructive signals during motor learning. Nat Neurosci 12(9):1171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JH, Wang JJ, Ebner TJ. 1987. Climbing fiber afferent modulation during treadmill locomotion in the cat. J Neurophysiol 57(3):787–802. [DOI] [PubMed] [Google Scholar]
- 66.Kitamura K, Kano M. 2013. Dendritic calcium signaling in cerebellar Purkinje cell. Neural Netw 47:11–7. [DOI] [PubMed] [Google Scholar]
- 67.Kitazawa S, Kimura T, Yin PB. 1998. Cerebellar complex spikes encode both destinations and errors in arm movements. Nature 392(6675):494–7. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi Y, Kawano K, Takemura A, Inoue Y, Kitama T, Gomi H, Kawato M. 1998. Temporal firing patterns of Purkinje cells in the cerebellar ventral paraflocculus during ocular following responses in monkeys II. Complex spikes. J Neurophysiol 80(2):832–48. [DOI] [PubMed] [Google Scholar]
- 69.Kolb FP, Rubia FJ, Bauswein E. 1987. Cerebellar unit responses of the mossy fibre system to passive movements in the decerebrate cat. I. Responses to static parameters. Exp Brain Res 68(2):234–48. [DOI] [PubMed] [Google Scholar]
- 70.Kurata K, Wise SP. 1988. Premotor cortex of rhesus monkeys: set-related activity during two conditional motor tasks. Exp Brain Res 69(2):327–43. [DOI] [PubMed] [Google Scholar]
- 71.Lang CE, Bastian AJ. 1999. Cerebellar subjects show impaired adaptation of anticipatory EMG during catching. J Neurophysiol 82(5):2108–19. [DOI] [PubMed] [Google Scholar]
- 72.Laurens J, Angelaki DE. 2018. How the Vestibulocerebellum Builds an Internal Model of Self-motion. In: Heck DH, editor. Neuronal Codes of the Cerebellum New York, NY: Elsevier; p 97–115. [Google Scholar]
- 73.Laurens J, Meng H, Angelaki DE. 2013. Neural representation of orientation relative to gravity in the macaque cerebellum. Neuron 80(6):1508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lena C 2016. Cerebrocerebellar Loops in the Rodent Brain. In: Heck DH, editor. The Neuronal Codes of the Cerebellum New York, NY: Elsevier; p 135–53. [Google Scholar]
- 75.Lisberger SG, Pavelko TA, Bronte-Stewart HM, Stone LS. 1994. Neural basis for motor learning in the vestibuloocular reflex of primates. II. Changes in the responses of horizontal gaze velocity Purkinje cells in the cerebellar flocculus and ventral paraflocculus. J Neurophysiol 72(2):954–73. [DOI] [PubMed] [Google Scholar]
- 76.Llinas R, Sugimori M. 1980. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol 305:197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Llinas R, Walton K, Hillman DE, Sotelo C. 1975. Inferior olive: its role in motor learing. Science 190(4220):1230–1. [DOI] [PubMed] [Google Scholar]
- 78.Llinas RR. 2013. The olivo-cerebellar system: a key to understanding the functional significance of intrinsic oscillatory brain properties. Front Neural Circuits 7:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lou JS, Bloedel JR. 1992. Responses of sagittally aligned Purkinje cells during perturbed locomotion: synchronous activation of climbing fiber inputs. J Neurophysiol 68(2):570–80. [DOI] [PubMed] [Google Scholar]
- 80.Lu X, Ashe J. 2005. Anticipatory activity in primary motor cortex codes memorized movement sequences. Neuron 45(6):967–73. [DOI] [PubMed] [Google Scholar]
- 81.Mano N, Yamamoto K. 1980. Simple-spike activity of cerebellar Purkinje cells related to visually guided wrist tracking movement in the monkey. J Neurophysiol 43(3):713–28. [DOI] [PubMed] [Google Scholar]
- 82.Marple-Horvat DE, Stein JF. 1987. Cerebellar neuronal activity related to arm movements in trained rhesus monkeys. J Physiol 394:351–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marr D 1969. A theory of cerebellar cortex. J Physiol 202(2):437–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marshall SP, Lang EJ. 2009. Local changes in the excitability of the cerebellar cortex produce spatially restricted changes in complex spike synchrony. J Neurosci 29(45):14352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maschke M, Gomez CM, Ebner TJ, Konczak J. 2004. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol 91(1):230–8. [DOI] [PubMed] [Google Scholar]
- 86.Mason CR, Hendrix CM, Ebner TJ. 2006. Purkinje cells signal hand shape and grasp force during reach-to-grasp in the monkey. J Neurophysiol 95(1):144–58. [DOI] [PubMed] [Google Scholar]
- 87.Mazzoni P, Krakauer JW. 2006. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26(14):3642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Medina JF, Lisberger SG. 2009. Encoding and decoding of learned smooth pursuit eye movements in the floccular complex of the monkey cerebellum. J Neurophysiol 102(4):2039–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miall RC, Christensen LO, Cain O, Stanley J. 2007. Disruption of state estimation in the human lateral cerebellum. PLoS Biol 5(11):e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miall RC, Wolpert DM. 1996. Forward models for physiological motor control. Neural Netw 9(8):1265–79. [DOI] [PubMed] [Google Scholar]
- 91.Miles FA, Braitman DJ, Dow BM. 1980a. Long-term adaptive changes in primate vestibuloocular reflex. IV. Electrophysiological observations in flocculus of adapted monkeys. J Neurophysiol 43(5):1477–93. [DOI] [PubMed] [Google Scholar]
- 92.Miles FA, Fuller JH, Braitman DJ, Dow BM. 1980b. Long-term adaptive changes in primate vestibuloocular reflex. III. Electrophysiological observations in flocculus of normal monkeys. J Neurophysiol 43(5):1437–76. [DOI] [PubMed] [Google Scholar]
- 93.Montarolo PG, Palestini M, Strata P. 1982. The inhibitory effect of the olivocerebellar input on the cerebellar Purkinje cells in the rat. J Physiol 332:187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morton SM, Bastian AJ. 2006. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26(36):9107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. 1975. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol 38(4):871–908. [DOI] [PubMed] [Google Scholar]
- 96.Najac M, Raman IM. 2015. Integration of Purkinje cell inhibition by cerebellar nucleo- olivary neurons. J Neurosci 35(2):544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Najafi F, Medina JF. 2013. Beyond “all-or-nothing” climbing fibers: graded representation of teaching signals in Purkinje cells. Front Neural Circuits 7:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nguyen-Vu TD, Kimpo RR, Rinaldi JM, Kohli A, Zeng H, Deisseroth K, Raymond JL. 2013. Cerebellar Purkinje cell activity drives motor learning. Nat Neurosci 16(12):1734–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Noto CT, Robinson FR. 2001. Visual error is the stimulus for saccade gain adaptation. Brain Res Cogn Brain Res 12(2):301–5. [DOI] [PubMed] [Google Scholar]
- 100.Nowak DA, Hermsdorfer J, Rost K, Timmann D, Topka H. 2004. Predictive and reactive finger force control during catching in cerebellar degeneration. Cerebellum 3(4):227–35. [DOI] [PubMed] [Google Scholar]
- 101.Nyberg L, Eriksson J. 2015. Working Memory: Maintenance, Updating, and the Realization of Intentions. Cold Spring Harb Perspect Biol 8(2):a021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ohmae S, Medina JF. 2015. Climbing fibers encode a temporal-difference prediction error during cerebellar learning in mice. Nat Neurosci 18(12):1798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ojakangas CL, Ebner TJ. 1994. Purkinje cell complex spike activity during voluntary motor learning: relationship to kinematics. J Neurophysiol 72(6):2617–30. [DOI] [PubMed] [Google Scholar]
- 104.Oscarsson O 1980. Functional organization of olivary projection to the cerebellar anterior lobe. In: Courville J, editor. The Inferior Olivary Nucleus: Anatomy and Physiology New York: Raven; p 279–90. [Google Scholar]
- 105.Ostry DJ, Feldman AG. 2003. A critical evaluation of the force control hypothesis in motor control. Exp Brain Res 153(3):275–88. [DOI] [PubMed] [Google Scholar]
- 106.Paninski L, Fellows MR, Hatsopoulos NG, Donoghue JP. 2004. Spatiotemporal tuning of motor cortical neurons for hand position and velocity. J Neurophysiol 91(1):515–32. [DOI] [PubMed] [Google Scholar]
- 107.Pasalar S, Roitman AV, Durfee WK, Ebner TJ. 2006. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci 9:1404–11. [DOI] [PubMed] [Google Scholar]
- 108.Popa LS, Hewitt AL, Ebner TJ. 2014. The cerebellum for jocks and nerds alike. Front Syst Neurosci 8(113):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Popa LS, Hewitt AL, Ebner TJ. 2012. Predictive and feedback performance errors are signaled in the simple spike discharge of individual Purkinje cells. J Neurosci 32(44):15345–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Popa LS, Streng ML, Ebner TJ. 2016a. Signaling of predictive and feedback information in Purkinje cell simple spike activity. In: Heck DH, editor. Neuronal Codes of the Cerebellum New York,NY: Elsevier; p 1–25. [Google Scholar]
- 111.Popa LS, Streng ML, Ebner TJ. 2017. Long-term predictive and feedback encoding of motor signals in the simple spike discharge of Purkinje cells. eNeuro 4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Popa LS, Streng ML, Hewitt AL, Ebner TJ. 2016b. The errors of our ways: Understanding error representations in cerebellar-dependent motor learning. Cerebellum 15(2):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prsa M, Thier P. 2011. The role of the cerebellum in saccadic adaptation as a window into neural mechanisms of motor learning. Eur J Neurosci 33(11):2114–28. [DOI] [PubMed] [Google Scholar]
- 114.Raman IM, Bean BP. 1997. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci 17(12):4517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ramnani N 2006. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci 7(7):511–22. [DOI] [PubMed] [Google Scholar]
- 116.Robinson DA. 1975. Oculomotor control signals. In: Bachyrita P, Lennerstrand G, editors. Basic Mechanisms of Ocular Motility and Their Clinical Implications Oxford, UK: Pergamon; p 337–74. [Google Scholar]
- 117.Roitman AV, Pasalar S, Johnson MT, Ebner TJ. 2005. Position, direction of movement, and speed tuning of cerebellar Purkinje cells during circular manual tracking in monkey. J Neurosci 25(40):9244–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rubia FJ, Kolb FP. 1978. Responses of cerebellar units to a passive movement in the decerebrate cat. Exp Brain Res 31(3):387–401. [DOI] [PubMed] [Google Scholar]
- 119.Schlerf JE, Ivry RB, Diedrichsen J. 2012. Encoding of sensory prediction errors in the human cerebellum. J Neurosci 32(14):4913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schmahmann JD. 2010. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev 20(3):236–60. [DOI] [PubMed] [Google Scholar]
- 121.Schmahmann JD, Pandya DN. 1997. The cerebrocerebellar system. Int Rev Neurobiol 41:31–60. [DOI] [PubMed] [Google Scholar]
- 122.Schmolesky MT, Weber JT, De Zeeuw CI, Hansel C. 2002. The making of a complex spike: ionic composition and plasticity. Ann N Y Acad Sci 978:359–90. [DOI] [PubMed] [Google Scholar]
- 123.Shadmehr R, Holcomb HH. 1997. Neural correlates of motor memory consolidation. Science 277(5327):821–5. [DOI] [PubMed] [Google Scholar]
- 124.Shadmehr R, Smith MA, Krakauer JW. 2010. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33:89–108. [DOI] [PubMed] [Google Scholar]
- 125.Shidara M, Kawano K. 1993. Role of Purkinje cells in the ventral paraflocculus in short- latency ocular following responses. Exp Brain Res 93(2):185–95. [DOI] [PubMed] [Google Scholar]
- 126.Shidara M, Kawano K, Gomi H, Kawato M. 1993. Inverse-dynamics model eye movement control by Purkinje cells in the cerebellum. Nature 365(6441):50–2. [DOI] [PubMed] [Google Scholar]
- 127.Shima K, Tanji J. 2000. Neuronal activity in the supplementary and presupplementary motor areas for temporal organization of multiple movements. J Neurophysiol 84(4):2148–60. [DOI] [PubMed] [Google Scholar]
- 128.Simpson JI, Wylie DR, DeZeeuw CI. 1995. On climbing fiber signals and their consequence(s). Behav Brain Sci 19(3):385–98. [Google Scholar]
- 129.Smith AM, Bourbonnais D. 1981. Neuronal activity in cerebellar cortex related to control of prehensile force. J Neurophysiol 45(2):286–303. [DOI] [PubMed] [Google Scholar]
- 130.Smith MA, Shadmehr R. 2005. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol 93(5):2809–21. [DOI] [PubMed] [Google Scholar]
- 131.Soetedjo R, Fuchs AF. 2006. Complex spike activity of Purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades. J Neurosci 26(29):7741–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stone LS, Lisberger SG. 1990. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. I. Simple spikes. J Neurophysiol 63(5):1241–61. [DOI] [PubMed] [Google Scholar]
- 133.Streng ML, Popa LS, Ebner TJ. 2017a. Climbing fibers control Purkinje cell representations of behavior. J Neurosci 37(8):1997–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Streng ML, Popa LS, Ebner TJ. 2017b. Climbing fibers predict movement kinematics and performance errors. J Neurophysiol 118(3):1888–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Streng ML, Popa LS, Ebner TJ. 2018. Modulation of sensory prediction error in Purkinje cells during visual feedback manipulations. Nat Commun 9(1):1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Strick PL, Dum RP, Fiez JA. 2009. Cerebellum and nonmotor function. Annu Rev Neurosci 32:413–34. [DOI] [PubMed] [Google Scholar]
- 137.Sugihara I, Shinoda Y. 2004. Molecular, topographic, and functional organization of the cerebellar cortex: a study with combined aldolase C and olivocerebellar labeling. J Neurosci 24(40):8771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sun Z, Smilgin A, Junker M, Dicke PW, Thier P. 2017. The same oculomotor vermal Purkinje cells encode the different kinematics of saccades and of smooth pursuit eye movements. Sci Rep 7:40613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tanji J, Taniguchi K, Saga T. 1980. Supplementary motor area: neuronal response to motor instructions. J Neurophysiol 43(1):60–8. [DOI] [PubMed] [Google Scholar]
- 140.Taylor JA, Klemfuss NM, Ivry RB. 2010. An explicit strategy prevails when the cerebellum fails to compute movement errors. Cerebellum 9(4):580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ten Brinke MM, Boele HJ, Spanke JK, Potters JW, Kornysheva K, Wulff P, IJpelaar AC, Koekkoek SK, De Zeeuw CI. 2015. Evolving models of Pavlovian conditioning: cerebellar cortical dynamics in awake behaving mice. Cell Rep 13(9):1977–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Teune TM, Van der BJ, De Zeeuw CI, Voogd J, Ruigrok TJ. 1998. Single Purkinje cell can innervate multiple classes of projection neurons in the cerebellar nuclei of the rat: a light microscopic and ultrastructural triple-tracer study in the rat. J Comp Neurol 392(2):164–78. [DOI] [PubMed] [Google Scholar]
- 143.Thach WT. 2007. On the mechanism of cerebellar contributions to cognition. Cerebellum 6(3):163–7. [DOI] [PubMed] [Google Scholar]
- 144.Todorov E, Jordan MI. 2002. Optimal feedback control as a theory of motor coordination. Nature Neuroscience 5(11):1226–35. [DOI] [PubMed] [Google Scholar]
- 145.Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. 2007. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98(1):54–62. [DOI] [PubMed] [Google Scholar]
- 146.Valle MS, Bosco G, Poppele R. 2000. Information processing in the spinocerebellar system. NeuroReport 11(18):4075–9. [DOI] [PubMed] [Google Scholar]
- 147.Valle MS, Eian J, Bosco G, Poppele RE. 2008. Cerebellar cortical activity in the cat anterior lobe during hindlimb stepping. Exp Brain Res 187(3):359–72. [DOI] [PubMed] [Google Scholar]
- 148.Voogd J, Ruigrok TJ. 2004. The organization of the corticonuclear and olivocerebellar climbing fiber projections to the rat cerebellar vermis: the congruence of projection zones and the zebrin pattern. J Neurocytol 33(1):5–21. [DOI] [PubMed] [Google Scholar]
- 149.Wagner MJ, Smith MA. 2008. Shared internal models for feedforward and feedback control. J Neurosci 28(42):10663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wallman J, Fuchs AF. 1998. Saccadic gain modification: visual error drives motor adaptation. J Neurophysiol 80(5):2405–16. [DOI] [PubMed] [Google Scholar]
- 151.Walter JT, Khodakhah K. 2006. The linear computational algorithm of cerebellar Purkinje cells. J Neurosci 26(50):12861–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Walter JT, Khodakhah K. 2009. The advantages of linear information processing for cerebellar computation. Proc Natl Acad Sci USA 106(11):4471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang JJ, Kim JH, Ebner TJ. 1987. Climbing fiber afferent modulation during a visually guided, multi-joint arm movement in the monkey. Brain Res 410(2):323–9. [DOI] [PubMed] [Google Scholar]
- 154.Winkelman BH, Belton T, Suh M, Coesmans M, Morpurgo MM, Simpson JI. 2014. Nonvisual complex spike signals in the rabbit cerebellar flocculus. J Neurosci 34(9):3218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Witter L, Canto CB, Hoogland TM, de G Jr., De Zeeuw CI. 2013. Strength and timing of motor responses mediated by rebound firing in the cerebellar nuclei after Purkinje cell activation. Front Neural Circuits 7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wolpert DM, Ghahramani Z. 2000. Computational principles of movement neuroscience. Nat Neurosci 3 Suppl:1212–7. [DOI] [PubMed] [Google Scholar]
- 157.Wolpert DM, Ghahramani Z, Jordan MI. 1995. An internal model for sensorimotor integration. Science 269(5232):1880–2. [DOI] [PubMed] [Google Scholar]
- 158.Wolpert DM, Miall RC, Kawato M. 1998. Internal models in the cerebellum. Trends in Cognitive Sciences 2:338–47. [DOI] [PubMed] [Google Scholar]
- 159.Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R. 2009. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci 29(41):12930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yakusheva T, Blazquez PM, Angelaki DE. 2010. Relationship between complex and simple spike activity in macaque caudal vermis during three-dimensional vestibular stimulation. J Neurosci 30(24):8111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yakusheva TA, Shaikh AG, Green AM, Blazquez PM, Dickman JD, Angelaki DE. 2007. Purkinje cells in posterior cerebellar vermis encode motion in an inertial reference frame. Neuron 54(6):973–85. [DOI] [PubMed] [Google Scholar]
- 162.Yamamoto K, Kawato M, Kotosaka S, Kitazawa S. 2007. Encoding of movement dynamics by Purkinje cell simple spike activity during fast arm movements under resistive and assistive force fields. J Neurophysiol 97(2):1588–99. [DOI] [PubMed] [Google Scholar]
- 163.Yang Y, Lisberger SG. 2013. Interaction of plasticity and circuit organization during the acquisition of cerebellum-dependent motor learning. Elife 2:e01574. [DOI] [PMC free article] [PubMed] [Google Scholar]


