Abstract
The high-altitude environment is a challenge for human settlement. Low oxygen concentrations, extreme cold, and a harsh arid climate are doubtlessly challenges for the colonization of the Tibetan plateau. I am delighted to comment on the article of Pan et al. (2018) on mutations in endothelial PAS domain-containing protein 1 (EPAS1) in congenital heart disease in Tibetans. In humans, the EPAS1 gene is responsible for coding EPAS1 protein, an alias of which is HIF2α, an acronym for hypoxia-inducible factor 2 alpha. EPAS1 is a type of hypoxia-inducible factors, which are collected as a group of transcription factors involved in body response to oxygen level. EPAS1 gene is active under hypoxic conditions and plays an essential role in the development of the heart and in the management of the catecholamine balance, mutations of which have been identified in neuroendocrine tumors. In this article, Pan et al. investigated Tibetan patients with and without non-syndromic congenital heart disease. They identified two novel EPAS1 gene mutations, of which N203H mutation significantly affected the transcription activity of the vascular endothelial growth factor (VEGF) promoter, particularly in situations of hypoxia. VEGF is a downstream target of HIF-2 (other than HIF-1), and the expression levels of either HIF-1α or HIF-2α correlate positively to VEGF expression. Pan et al.’s data may be of incitement to further evaluate protein–protein interaction and using experimental animal models. Moreover, it may also be a stimulus for setting up genetic epidemiologic studies for other populations living at high altitudes.
Keywords: congenital, EPAS1, heart
Congenital heart disease (CHD) affects millions of individuals worldwide, including over one million children in the United States with about one-fourth of children born with CHD requiring intensive surgical intervention within the first year of life. Despite improved cardiac surgical procedures and rates of survival into adulthood, incidences that surpass 90% of children remain at risk for neurological injury and neurobehavioral challenges that pose a threat to the quality of life across the lifespan [1]. The high-altitude environment is a challenge for human settlement. Low oxygen concentrations, extreme cold, and a harsh arid climate are doubtlessly daily challenges for the colonization of the Tibetan plateau [2]. Hypoxia is a central key of several widespread human diseases, such as ischemic heart disease, pulmonary arterial hypertension (PAH), and stroke. CHD is an ongoing topic for populations living in high altitudes. The incidence of CHD in newborns at high altitude is about 20 times higher than neonates born at a low height, comprising left to right shunt defects and rarely complex CHD. Infants, aged 12–18 months, living in high altitude have an incidence of CHD about 10 times higher than infants living at low altitude and most importantly, about 8% of these patients develop PAH or death [3]. The Tibetan autonomous prefecture of Yushu (average elevation over 4000 m) and the independent Mongolian county of Henan (average height over 3600 m) in Huangnan show the highest prevalence of CHD [4]. Chun et al. investigated 84302 students from Nagqu, Tibet and found a prevalence rate of CHD of 0.5%, i.e., 1 case every 200 [5]. The most common defects were patent ductus arteriosus (PDA; about 2/3), atrial septal defects (ASDs; about 1/5), and ventricular septal defects (VSD; about 1/10) with the prevalence of CHD in girls being higher than in boys. The condition of high altitudes may also have some effect on two routine cardiac surgery operations, including Fontan and bidirectional Glenn’s anastomosis, although a sum of factors should probably be taken into account (e.g., age) [6–9]. The Fontan procedure comprises any surgical operation that results in the blood flow of systemic venous blood to the lungs without passing through a ventricle, while the bidirectional Glenn is a common surgical fashion of the second stage of the total cavopulmonary connection (TCPC) surgery where the end of the superior vena cava (SVC) is connected to the side of the pulmonary artery (PA).
In humans, the Cbp/p300-interacting transactivator 2 is a protein that is encoded by the CITED2 gene located on chromosome 6. CITED2 gene is a cardiac transcription factor that plays a crucial role in the development of the embryonic cardiovascular system. Knock-out experiments of CITED2 in mice may result in several cardiac defects. In a study involving 187 unrelated Tibetans with CHD, Liu et al. found a novel mutation of CITED2 that enhanced the expression of vascular endothelial growth factor (VEGF) under the role of co-receptor hypoxia-inducible factor 1 alpha (HIF-1α) [10]. In this journal, in a recent issue, Pan et al. studied the endothelial PAS domain-containing gene 1 (EPAS1) in CHD in Tibetans [11]. A group of 286 Tibetan patients with non-syndromic CHD and 250 separate Tibetan healthy controls were engaged from Qinghai, China using Sanger DNA sequencing and confirming the novelty of identified variants by the examination of 1000G and ExAC databases of the human genome. Moreover, Pan et al. investigated the effect of EPAS1 mutations on the transcription of its target gene, VEGF, by dual-luciferase reporter assay. The authors of the Qinghai High Altitude Medical Research Institute together with colleagues from the Center for Genetics, National Research Institute for Family Planning, and Graduate School of Peking Union Medical College, Beijing, China identified two novel EPAS1 gene mutations (N203H and G724W) in two patients. Although the G724W is silent, the N203H mutation significantly affects the transcription activity of the VEGF promoter, specifically in the setting of hypoxia. There is an enhanced protein–protein interaction between EPAS1 and the proteins arising from endoglin 1 (EGLN1) or Von-Hippel–Lindau (VHL) genes. The endoglin gene (EGLN1), often known as PHD2, encodes an enzyme called prolyl hydroxylase domain 2 (PHD2). The VHL gene encodes a protein (pVHL) that functions as part of a complex called the VCB–CuL2 complex, which is formed by pVHL and the gene products of elongin C, elongin B, Cul-2, and Rbx1, which functions as a ubiquitin-protein ligase [12]. The alpha-subunits of the HIFs have been identified as targets for the VCB–CuL2 ubiquitin ligase. The authors suggest that EPAS1 gene mutations may play an etiologic role in the development of Tibetan non-syndromic CHD.
However, genetic defects should not be considered as merely deterministic factors for a pathological condition [13,14]. The twin-reversed arterial perfusion (TRAP) sequence, or acardia, is the most severe lethal condition in monozygotic twinning. It is part of the twin–twin transfusion syndrome (TTTS), a subtype of monochorionic twin pregnancy, showing an extremely high pre- and perinatal morbidity and mortality [15,16]. In TTTS there is a net transfusion of whole blood from the umbilical artery of the donor twin to the umbilical vein of the recipient twin in a villous zone of overlapping perfusion. The transfusion of blood from the donor to the recipient via placental arterio-arterial anastomoses in monochorionic gestations is the supposed mechanism, resulting in the formation of a TRAP sequence [17]. TRAP sequence is mainly linked to hypoxia [18]. The lack of oxygen during early embryogenesis can induce severe disruptions of head–brain and heart formation. An oxygen deficiency due to TRAP may be responsible not only for the encephaloclastic (destructive) changes but also for the developmental arrest of the brain in the receiving twin. Developmental abnormalities may result either from genetic events (e.g., point mutations, aneuploidy) or from exogenous factors disrupting the healthy development of the embryo [19]. About exogenous factors, abnormalities are primarily dependent on the time of interference of the teratogenic injuries (‘noxa’) with typical development, which means that their effects are highly phase-specific [20–24]. Hypoxia has been demonstrated to be a very useful teratogen, causing disruption, particularly of neurulation, if it interferes with early stages of embryonic development [21,23–26]. Experimental studies performed in amphibian and chick embryos showed that hypobaric-mediated hypoxia determines disruptions of the head, brain, and heart predominantly. The most severe brain and head changes resulted if the hypoxia was induced at the beginning of gastrulation, i.e., before the onset of neurulation, when oxygen consumption is known to be exceptionally high [25]. In these experiments, the underlying developmental mechanisms responsible for the malformations occurring with hypoxia were multiple including (1) disturbance of the migration of blastema (altered ‘topogenesis’ of the German Embryological School according to Lehmann [27,28] and ‘integrated cell and tissue movements’ according to Gilbert [19]), (2) decreased inductive capacity of the altered blastema, and (3) disturbance of further differentiation of the organ anlage [25]. These investigations that continued the Spemann–Mangold experiments on organizers [29] also used experimental animals and revealed that hypoxia must occur before the onset of the formation of the organ anlage to induce severe developmental deviation. These findings found by hypoxia in amphibians and chicks are also fundamentally valid for mammals [30] and can be extrapolated to humans as well [25]. The effect of chronic hypoxia on some cardiac parameters was compared in rodents (rats) acclimatized either from the 4th day or the 12th week of postnatal life. PAH and right ventricular enlargement were found in both age groups [31]. The young hypoxic animals showed an increase in the weight of the right ventricle with a linear tendency with the pressure values of the right ventricle, while adult high-altitude exposed rats did not demonstrate such a relationship. Moreover, high altitude induces a significant increase in collagenous proteins with collagen I and III in young animals and collagen III only in the adult ones [31]. In comparing with the cardiovascular defects encountered at high altitudes, e.g., PAH, PDA, ASD, and VSD, it is impressive that these abnormalities can also be seen in experimental animals which have been used in a hypoxia-related environment. PAH is a vasculopathy of the pulmonary circulation characterized by arterial obliteration secondary to unchecked pathologic angiogenic processes. PAH is characterized by high circulating CD34 positive, CD133 positive proangiogenic progenitors, and endothelial cells that have a pathologic expression of HIF-1α [32]. Human urotensin II (U-II) is a cyclic vasoactive peptide composed of 11 amino acids with a structure similar to somatostatin [33,34]. The human form of U-II (hU-II) has been identified as an endogenous ligand for the G-protein-coupled receptor GPR14, also re-labeled as U-II receptor. Both hU-II and its receptor are intriguingly expressed in different cardiac and extracardiac tissues, including the brain, kidney, smooth muscle, and endothelium. It is known that hU-II is among the most potent vasoconstrictor peptides identified, with a potency greater than that of endothelin-1. The hU-II is a potent activator of reactive oxygen species (ROS) generation by reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in PA smooth muscle cells (PASMCs), leading to redox-sensitive activation of mitogen-activated protein kinases (MAPK) and Akt and subsequently to enhanced PAI-1 expression and increased proliferation. The hU-II may play an important role in pulmonary hypertension by promoting remodeling processes via activation of NADPH oxidases [33,34].
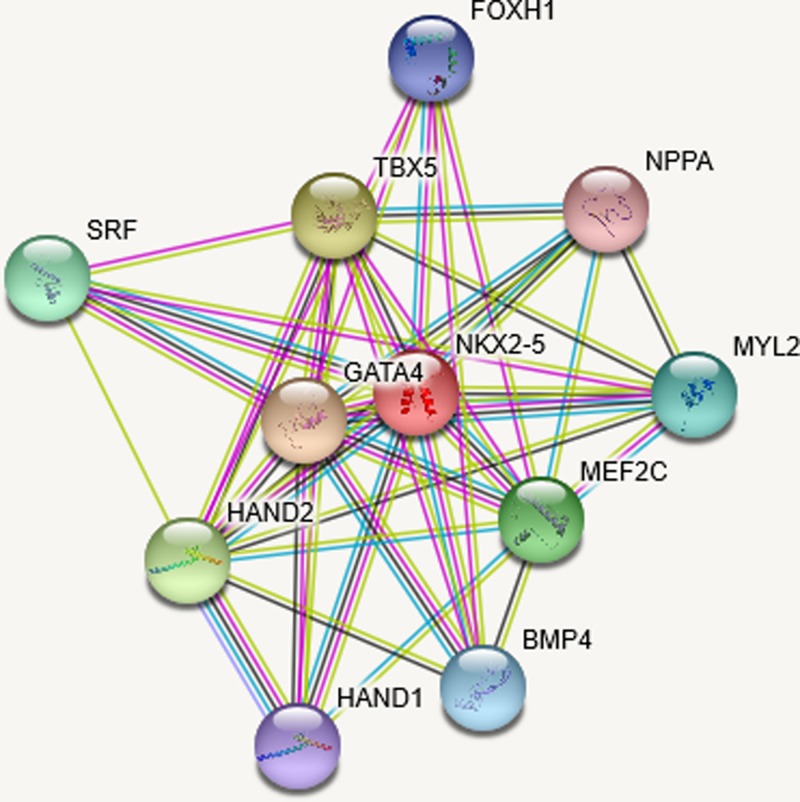
There is a higher risk for structural CHD in twin pregnancies, and the prevalence of CHD is 2% in otherwise uncomplicated monochorionic diamniotic (MCDA) gestations and 5% in cases of the twin–twin-transfusion syndrome (TTTS), particularly among recipient twins. Some hypotheses have been formulated, and theories have been based on abnormal placentation that occurs in monochorionic twins, particularly in cases that develop TTTS contributing to abnormal fetal heart formation [35,36]. The twinning process itself could lead to cardiac defects, but also the division of the fertilized ovum could be teratogenic. Moreover, early hypoxia, damage of the inner cell mass and the zona pellucida, delayed fertilization time, and slow tubal transport are possible causes of monozygotic twinning, which may also affect the development of the embryos. After segmentation, at least one major body axis in each embryo needs to rearrange, and this event is prone to errors, which might explain the higher prevalence of midline and laterality defects in monozygotic twins (e.g., cloacal exstrophy, anal atresia, anencephaly, spine defects, and CHD). Springer et al. [37] evaluated the prevalence of CHD in a large unselected cohort of monochorionic twin pregnancies combining diagnoses of prenatal and postnatal echocardiography and autopsy results. The authors confirmed a high prevalence of structural CHD (5.5%) showing that structural CHD, as well as ventricular hypertrophy and cardiomegaly, occurred significantly more often in monochorionic twin pregnancies complicated by TTTS compared with fetuses without TTTS [37]. Single gene defects associated with isolated or non-syndromic CHD have been delineated. Mutations in NKX2.5 lead to isolated ASDs with atrioventricular conduction delay, while mutations in GATA4 (a family of transcription factors characterized by their ability to bind to the DNA sequence GATA), a zinc finger transcription factor known to interact with the NK2 homeobox 5 (NKX2.5), which have been linked to isolated ASDs without conduction system abnormalities. Moreover, a mutation in GATA4 specifically disrupted an interaction with T-box transcription factor (TBX5) suggesting that mutations in any of these interacting transcription factors can lead to CHD [38]. In Figure 1 is shown an interaction panel of several proteins linked to CHD using version 11 of STRING, an online bioinformatic tool using several databases [39]. It may be postulated that HIF-1α is linked to bone morphogenetic protein 4 (BMP4) in this early hypoxic insult causing not acardiac fetuses, but fetuses with structural defects such as those identified in high-altitude pregnancies. HIF-1α-dependent up-regulation of BMP4 mediates hypoxia-induced increase in TRPC expression in PASMCs, and BMP4 interaction is key in determining CHD. In the panel of Figure 1, there is also FOXH1, which is Forkhead box protein H1 with the ability to bind SMAD2 (Mothers against decapentaplegic homolog 2). It activates an activin response element via binding the DNA motif TGT(G/T)(T/G)ATT. FOXH1 is essential for the development of the growth of the embryo and its anterior heart field [40,41].
Figure 1. Splice isoforms or post-translational modifications are collapsed, while each node represents all the proteins produced by a single, protein-coding gene locus.
The edges represent protein–protein interactions with different color according to the interaction type. A red line indicates the presence of fusion evidence, green line a neighborhood evidence, blue line a cooccurrence evidence, purple line an experimental evidence, yellow line a textmining evidence, light blue line a database evidence, and black line a coexpression evidence.
Finally, it must be precisely known that children with CHD may have an intellectual impairment [42]. Neurodevelopmental outcomes are weakened in survivors of critical CHD in several developmental cerebral domains including motor, cognitive, and sensory outcomes. The cause of these neurodevelopmental deficits is multi-factorial and includes individual risk factors, cardiac anatomy, and cardiovascular physiology, brain development (e.g., myelination) as seen on magnetic resonance imaging (MRI). Despite early surgery, these shortfalls can extend into the adolescent and early adulthood years [42–45]. MRI studies have shown decreased total brain volume and white matter (WM) injury, which could be because of hypoxia [46]. The cellular/molecular mechanisms linked to brain immaturity and preoperative WM injury in newborns affected with CHD remain mostly unexplored. There is a rodent model of diffuse WM-injury exposing mice at neonatal age to chronic hypoxia, which showed alterations in oligodendrocyte development resulting in hypomyelination, including oligodendrocyte death, delayed differentiation of the oligodendrocytes, and, even, abnormal patterns of myelination [47,48]. Although the present study does not show encephaloclastic changes of early hypoxic damage, it is a well-established model to mimic hypoxic brain injury in premature infants [47–50]. In the future, it may be essential to evaluate whether the residents of high altitude also have such brain lesions or other micro- and macrostructural brain abnormalities [51]. There is a possibility that hypoxia could be a common factor responsible for intellectual impairment in children with CHD and high-altitude residents, and whether they might have similar cerebral lesions on high-quality MRI would be useful to know. The interaction between placenta and heart is just starting to be explored adequately and will deliver unconfutable data for the future development of the embryonic heart in the next decade [52].
In conclusion, Pan’s findings and my interactome analysis have important implications for signaling in CHD. New options may be accurately explored using current bioinformatic tools. Small groups may also be investigated to find mutations which could have an impact on finding pathways which have broader implications. The identification of mutations in multiple interacting cardiac developmental genes will be part of a routine screening of the nearest future that may be applied to our cardiac patients with CHD. This procedure may notably improve our understanding of the pathogenesis, promote surgical options, and ultimately improve the quality of healthcare of the 21st century.
Abbreviations
- ASD
atrial septal defect
- BMP4
bone morphogenetic protein 4
- CHD
congenital heart disease
- EGLN1
endoglin 1
- EPAS1
endothelial PAS domain-containing protein 1
- HIF-1α
hypoxia-inducible factor 1 alpha
- MRI
magnetic resonance imaging
- NADPH
nicotinamide adenine dinucleotide phosphate
- PA
pulmonary artery
- PAH
pulmonary arterial hypertension
- PASMC
pulmonary artery smooth muscle cell
- PDA
patent ductus arteriosus
- PHD2
prolyl hydroxylase domain 2
- TRAP
twin-reversed arterial perfusion
- TTTS
twin–twin transfusion syndrome
- U-II
urotensin II
- VEGF
vascular endothelial growth factor
- VHL
Von-Hippel–Lindau
- VSD
ventricular septal defect
- WM
white matter
Competing Interests
The author declares that there are no competing interests associated with the manuscript.
Funding
This research has been funded by the generosity of the Stollery Children’s Hospital Foundation and supporters of the Lois Hole Hospital for Women through the Women and Children’s Health Research Institute (WCHRI, Grant ID #: 2096), Hubei Province Natural Science Funding for Hubei University of Technology (100-Talent Grant for Recruitment Program of Foreign Experts Total Funding: Digital PCR and NGS-based diagnosis for infection and oncology, 2017-2022), Österreichische Krebshilfe Tyrol (Krebsgesellschaft Tirol, Austrian Tyrolean Cancer Research Institute, 2008), Austrian Research Fund (Fonds zur Förderung der wissenschaftlichen Forschung, FWF, Grant ID L313-B13), Canadian Foundation for Women’s Health, and the Saudi Cultural Bureau, Ottawa, Canada. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cassidy A.R., Ilardi D., Bowen S.R., Hampton L.E., Heinrich K.P., Loman M.M.. et al. (2018) Congenital heart disease: a primer for the pediatric neuropsychologist. Child Neuropsychol. 24, 859–902 10.1080/09297049.2017.1373758 [DOI] [PubMed] [Google Scholar]
- 2.Huerta-Sanchez E. and Casey F.P. (2015) Archaic inheritance: supporting high-altitude life in Tibet. J. Appl. Physiol. (1985) 119, 1129–1134 10.1152/japplphysiol.00322.2015 [DOI] [PubMed] [Google Scholar]
- 3.Li J.J., Liu Y., Xie S.Y., Zhao G.D., Dai T., Chen H.. et al. (2019) Newborn screening for congenital heart disease using echocardiography and follow-up at high altitude in China. Int. J. Cardiol. 274, 106–112 10.1016/j.ijcard.2018.08.102 [DOI] [PubMed] [Google Scholar]
- 4.Ma L.G., Chen Q.H., Wang Y.Y., Wang J., Ren Z.P., Cao Z.F.. et al. (2018) Spatial pattern and variations in the prevalence of congenital heart disease in children aged 4-18 years in the Qinghai-Tibetan Plateau. Sci. Total Environ. 627, 158–165 10.1016/j.scitotenv.2018.01.194 [DOI] [PubMed] [Google Scholar]
- 5.Chun H., Yue Y., Wang Y., Dawa Z., Zhen P., La Q.. et al. (2018) High prevalence of congenital heart disease at high altitudes in Tibet. Eur. J. Prev. Cardiol. 26, 756–759 2047487318812502 [DOI] [PubMed] [Google Scholar]
- 6.Brida M. and Diller G.P. (2016) Impact of short-term high altitude exposure on exercise capacity and symptoms in Fontan patients. Heart 102, 1255–1256 10.1136/heartjnl-2016-309903 [DOI] [PubMed] [Google Scholar]
- 7.Jonas R.A. (1995) Advances in surgical care of infants and children with congenital heart disease. Curr. Opin. Pediatr. 7, 572–579 10.1097/00008480-199510000-00014 [DOI] [PubMed] [Google Scholar]
- 8.Ramirez-Marroquin S., Calderon-Colmenero J., Curi-Curi P., Garcia-Montes J.A., Patino-Bahena E., Buendia A.. et al. (2012) Fontan procedure at 2,240 m above sea level. World J. Pediatr. Congenit. Heart Surg. 3, 206–213 10.1177/2150135111425065 [DOI] [PubMed] [Google Scholar]
- 9.Vallecilla C., Khiabani R.H., Sandoval N., Fogel M., Briceno J.C. and Yoganathan A.P. (2014) Effect of high altitude exposure on the hemodynamics of the bidirectional Glenn physiology: modeling incremented pulmonary vascular resistance and heart rate. J. Biomech. 47, 1846–1852 10.1016/j.jbiomech.2014.03.021 [DOI] [PubMed] [Google Scholar]
- 10.Liu S., Su Z., Tan S., Ni B., Pan H., Liu B.. et al. (2017) Functional analyses of a novel CITED2 nonsynonymous mutation in Chinese Tibetan patients with congenital heart disease. Pediatr. Cardiol. 38, 1226–1231 10.1007/s00246-017-1649-y [DOI] [PubMed] [Google Scholar]
- 11.Pan H., Chen Q., Qi S., Li T., Liu B., Liu S.. et al. (2018) Mutations in EPAS1 in congenital heart disease in Tibetans. Biosci. Rep. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuda H., Saitoh K., Hirai S., Iwai K., Takaki Y., Baba M.. et al. (2001) The von Hippel-Lindau tumor suppressor protein mediates ubiquitination of activated atypical protein kinase C. J. Biol. Chem. 276, 43611–43617 10.1074/jbc.M107880200 [DOI] [PubMed] [Google Scholar]
- 13.Carver R.B., Castera J., Gericke N., Evangelista N.A. and El-Hani C.N. (2017) Young adults’ belief in genetic determinism, and knowledge and attitudes towards modern genetics and genomics: the PUGGS questionnaire. PLoS One 12, e0169808 10.1371/journal.pone.0169808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gericke N., Carver R., Castéra J., Menezes Evangelista N.A., Coiffard Marre C. and El-Hani C.N. (2017) Exploring relationships among belief in genetic determinism, genetics knowledge, and social factors. Sci. Educ. 26, 1223–1259 [Google Scholar]
- 15.Tasha I. and Lazebnik N. (2017) Clinical management of twin reversed arterial perfusion cases: insights into a complex and challenging twinning. Clin. Exp. Obstet. Gynecol. 44, 319–325 [PubMed] [Google Scholar]
- 16.Yang X.H., Xu Y.Q., Chen X.L., Zhao S., Zhang L. and Pugash D. (2016) Cardiac failure of the twin reversed arterial perfusion sequence pump twin during the first-trimester: a case report. Clin. Exp. Obstet. Gynecol. 43, 448–452 [PubMed] [Google Scholar]
- 17.Lewi L., Valencia C., Gonzalez E., Deprest J. and Nicolaides K.H. (2010) The outcome of twin reversed arterial perfusion sequence diagnosed in the first trimester. Am. J. Obstet. Gynecol. 203, e211–e214, 213 10.1016/j.ajog.2010.04.018 [DOI] [PubMed] [Google Scholar]
- 18.Sergi C. and Schmitt H.P. (2000) Central nervous system in twin reversed arterial perfusion sequence with special reference to examination of the brain in acardius anceps. Teratology 61, 284–290 [DOI] [PubMed] [Google Scholar]
- 19.Gilbert S.F. (1997) Developmental Biology, Sinauer, Sunderland, MA [Google Scholar]
- 20.Töndury G. (1955) Entwicklungsstörungen durch chemische Faktoren und Viren. Naturwissenschaften 42, 312–319 10.1007/BF00589645 [DOI] [Google Scholar]
- 21.Starck D. (1965) Embryologie. Ein Lehrbuch auf allgemein biologischer Grundlage, Thieme, Stuttgart, Germany [Google Scholar]
- 22.Zamorano L. and Chuaqui B. (1979) Teratogenetic periods for the principal malformations of the central nervous system. Virchows Arch. A Pathol. Anat. Histol. 384, 1–18 10.1007/BF00427147 [DOI] [PubMed] [Google Scholar]
- 23.Sulik K.K., Cook C.S. and Webster W.S. (1988) Teratogens and craniofacial malformations: relationships to cell death. Development 103, 213–231 [DOI] [PubMed] [Google Scholar]
- 24.Webster W.S., Lipson A.H. and Sulik K.K. (1988) Interference with gastrulation during the third week of pregnancy as a cause of some facial abnormalities and CNS defects. Am. J. Med. Genet. 31, 505–512 10.1002/ajmg.1320310304 [DOI] [PubMed] [Google Scholar]
- 25.Rübsaamen H. (1955) Über die teratogenetische Wirkung des Sauerstoffmangels in der Frühentwicklung: Ein Beitrag zur Kausalgenese der Missbildungen bei Mensch und Tier. Naturwissenschaften 42, 319–325 10.1007/BF00589646 [DOI] [PubMed] [Google Scholar]
- 26.Werthemann A. (1955) Allgemeine Teratologie mit besonderer Berücksichtigung der Verhältnisse beim Menschen. 1. Zwillinge, Mehrlinge und Doppelbildungen in ihrer Beziehung zum Organisationsfeld. In Handbuch der allgemeinen Pathologie (Büchner F., Letterer E. and Roulet F., eds), Springer, Berlin, Germany [Google Scholar]
- 27.Lehmann F.E. (1955) Die embryonale Entwicklung. Entwicklungsphysiologie und experimentelle Teratologie. In Handbuch der allgemeinen Pathologie (Büchner F., Letterer E. and Roulet F., eds), pp. 1–53, Springer, Berlin, Germany [Google Scholar]
- 28.Lehmann F.E. (1963) Zellbiologische und biochemische Probleme der Morphogenese. 13. Colloquium der Gesellschaft für Physiologische Chemie ed, pp. 1–20, Springer, Berlin, Germany [Google Scholar]
- 29.Spemann H. and Mangold H. (1924) Über Induktion von embryonalanlagen durch Implantation artfremder Organisatoren. Archiv für Mikroskopische Anatomie und Entwicklungsmechanik 100, 599–638 10.1007/BF02108133 [DOI] [Google Scholar]
- 30.Murakami U. and Kameyama Y. (1963) Vertebral malformation in the mouse foetus caused by maternal hypoxia during early stages of pregnancy. J. Embryol. Exp. Morphol. 11, 107–118 [Google Scholar]
- 31.Ostadal B., Kolar F., Pelouch V., Bass A., Samanek M. and Prochazka J. (1989) The effect of chronic hypoxia on the developing cardiopulmonary system. Biomed. Biochim. Acta 48, S58–62 [PubMed] [Google Scholar]
- 32.Farha S., Asosingh K., Xu W., Sharp J., George D., Comhair S.. et al. (2011) Hypoxia-inducible factors in human pulmonary arterial hypertension: a link to the intrinsic myeloid abnormalities. Blood 117, 3485–3493 10.1182/blood-2010-09-306357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Djordjevic T., BelAiba R.S., Bonello S., Pfeilschifter J., Hess J. and Gorlach A. (2005) Human urotensin II is a novel activator of NADPH oxidase in human pulmonary artery smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 25, 519–525 10.1161/01.ATV.0000154279.98244.eb [DOI] [PubMed] [Google Scholar]
- 34.Diebold I., Petry A., Sabrane K., Djordjevic T., Hess J. and Gorlach A. (2012) The HIF1 target gene NOX2 promotes angiogenesis through urotensin-II. J. Cell. Sci. 125, 956–964 10.1242/jcs.094060 [DOI] [PubMed] [Google Scholar]
- 35.Bahtiyar M.O. and Copel J.A. (2015) Screening for congenital heart disease during anatomical survey ultrasonography. Obstet. Gynecol. Clin. North Am. 42, 209–223 10.1016/j.ogc.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 36.Bahtiyar M.O., Dulay A.T., Weeks B.P., Friedman A.H. and Copel J.A. (2007) Prevalence of congenital heart defects in monochorionic/diamniotic twin gestations: a systematic literature review. J. Ultrasound. Med. 26, 1491–1498 10.7863/jum.2007.26.11.1491 [DOI] [PubMed] [Google Scholar]
- 37.Springer S., Mlczoch E., Krampl-Bettelheim E., Mailath-Pokorny M., Ulm B., Worda C.. et al. (2014) Congenital heart disease in monochorionic twins with and without twin-to-twin transfusion syndrome. Prenat. Diagn. 34, 994–999 10.1002/pd.4411 [DOI] [PubMed] [Google Scholar]
- 38.Richards A.A. and Garg V. (2010) Genetics of congenital heart disease. Curr. Cardiol. Rev. 6, 91–97 10.2174/157340310791162703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J.. et al. (2019) STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Both I., Silvestri C., Erdemir T., Lickert H., Walls J.R., Henkelman R.M.. et al. (2004) Foxh1 is essential for development of the anterior heart field. Dev. Cell 7, 331–345 10.1016/j.devcel.2004.07.023 [DOI] [PubMed] [Google Scholar]
- 41.Sergi C., Shen F. and Liu S.-M. (2019) Insulin/IGF-1R, SIRT1, and FOXOs pathways—an intriguing interaction platform for bone and osteosarcoma. Front. Endocrinol. 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peyvandi S., Latal B., Miller S.P. and McQuillen P.S. (2019) The neonatal brain in critical congenital heart disease: insights and future directions. Neuroimage 185, 776–782 10.1016/j.neuroimage.2018.05.045 [DOI] [PubMed] [Google Scholar]
- 43.Peyvandi S., Chau V., Guo T., Xu D., Glass H.C., Synnes A.. et al. (2018) Neonatal brain injury and timing of neurodevelopmental assessment in patients with congenital heart disease. J. Am. Coll. Cardiol. 71, 1986–1996 10.1016/j.jacc.2018.02.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peyvandi S. and Donofrio M.T. (2018) Circulatory changes and cerebral blood flow and oxygenation during transition in newborns with congenital heart disease. Semin. Pediatr. Neurol. 28, 38–47 10.1016/j.spen.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 45.Peyvandi S., Kim H., Lau J., Barkovich A.J., Campbell A., Miller S.. et al. (2018) The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. J. Thorac. Cardiovasc. Surg. 155, e293, 291-300 10.1016/j.jtcvs.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morton P.D., Ishibashi N. and Jonas R.A. (2017) Neurodevelopmental abnormalities and congenital heart disease: insights into altered brain maturation. Circ. Res. 120, 960–977 10.1161/CIRCRESAHA.116.309048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scafidi J., Fagel D.M., Ment L.R. and Vaccarino F.M. (2009) Modeling premature brain injury and recovery. Int. J. Dev. Neurosci. 27, 863–871 10.1016/j.ijdevneu.2009.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agematsu K., Korotcova L., Scafidi J., Gallo V., Jonas R.A. and Ishibashi N. (2014) Effects of preoperative hypoxia on white matter injury associated with cardiopulmonary bypass in a rodent hypoxic and brain slice model. Pediatr. Res. 75, 618–625 10.1038/pr.2014.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jablonska B., Scafidi J., Aguirre A., Vaccarino F., Nguyen V., Borok E.. et al. (2012) Oligodendrocyte regeneration after neonatal hypoxia requires FoxO1-mediated p27Kip1 expression. J. Neurosci. 32, 14775–14793 10.1523/JNEUROSCI.2060-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raymond M., Li P., Mangin J.M., Huntsman M. and Gallo V. (2011) Chronic perinatal hypoxia reduces glutamate-aspartate transporter function in astrocytes through the Janus kinase/signal transducer and activator of transcription pathway. J. Neurosci. 31, 17864–17871 10.1523/JNEUROSCI.3179-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghimire L.V. (2017) Congenital heart disease and high altitude: is chronic hypoxia a common factor in intellectual impairment? High Alt. Med. Biol. 18, 299–300 10.1089/ham.2017.0043 [DOI] [PubMed] [Google Scholar]
- 52.Camm E.J., Botting K.J. and Sferruzzi-Perri A.N. (2018) Near to one’s heart: the intimate relationship between the placenta and fetal heart. Front. Physiol. 9, 629 10.3389/fphys.2018.00629 [DOI] [PMC free article] [PubMed] [Google Scholar]