Abstract
Women’s physical functioning declines with age and the rate of decline increases with age, but substantial disparities exist in trajectories over time. To inform development of interventions to optimise physical functioning across the adult life span, the aim is to explore which lifestyle and socio-economic position (SEP) factors contribute to disparities in physical functioning across the adult life span in women. Younger (born 1973–1978, n = 14,247), middle-aged (born 1946–1951, n = 13,715) and older (born 1921–1926, n = 12,432) participants from the Australian Longitudinal Study on Women’s Health completed six questionnaires between 1996 and 2012 at approximate 3-year intervals. Physical functioning was measured with a 10-item subscale of the Short-Form Health Survey (score 1–100). Relationships between age and physical functioning were modelled using spline regression, stratified by baseline categories of physical activity, alcohol intake, smoking status, level of education, managing on income and index of neighbourhood socio-economic disadvantage for area. Multivariable models excluding one of the six factors were compared with models including all six factors to examine the relative importance of each factor. Women with unhealthy lifestyles (inactive, smokers or risky alcohol intake) and lower SEP had lower levels of physical functioning and more rapid declines across the adult life span. The variables with the greatest relative contribution to the models for physical functioning differed by age cohort: i.e. education and physical activity in younger women, managing on income and physical activity in middle-aged women and physical activity in older women. For optimal physical functioning, socio-economic factors seemed particularly important in younger and middle-aged women, while physical activity seemed important at all ages.
Electronic supplementary material
The online version of this article (10.1007/s10433-018-0484-1) contains supplementary material, which is available to authorized users.
Keywords: Physical activity, Alcohol use, Smoking, Education, Income, Life course
Introduction
Physical functioning is defined as the individual’s capacity to undertake the physical tasks of everyday living (Cooper et al. 2011a). By enabling people to do the things that matter to them, physical functioning importantly contributes to people’s wellbeing as evidenced by its prominent role in various quality of life instruments (Ware and Sherbourne 1992; Brooks 1996). Women’s physical functioning declines with age and the rate of decline increases with age (Peeters et al. 2013; Long and Pavalko 2004). With each year of increase in age, the decline is minimal in younger adults, somewhat greater in middle-aged adults and more rapid in older adults (Peeters et al. 2013; Long and Pavalko 2004). Higher functioning at younger ages may delay the onset of the need for assistance with daily activities at older ages (Peeters et al. 2013).
In 1997, Kalache and Kickbusch suggested that different interventions are required at each life stage to optimise functional capacities and delay care dependence (Kalache and Kickbusch 1997). Early life interventions are required to ensure the highest possible functional capacity; adult life interventions are required to slow down declines; later life interventions should be adapted to the circumstances of older adults; and for those at older ages with substantial disability, interventions should aim at improving quality of life. The authors provide examples of interventions, but evidence for what type of intervention is likely to be most effective at each life stage is lacking.
Previous observational studies have found associations between lifestyle and socio-economic position (SEP) factors and physical functioning at specific life stages (Cooper et al. 2011b; Peel et al. 2005; Broese van Groenou et al. 2003; Montez 2013; Stuck et al. 1999). Many lifestyle and socio-economic factors cluster together, with adults with lower SEP being less likely to engage in healthy lifestyle behaviours (Meader et al. 2016; Noble et al. 2015). It is, however, unclear which of these factors are the most dominant drivers of disparities in physical functioning and whether this may be different at specific life stages. To date, no studies have compared the relative influence of each of these factors at different stages of the adult life span in one study. Understanding the modifiable determinants of physical functioning at different life stages would help to target health promotion practice and policy.
The aim in this paper is to explore which lifestyle and SEP factors contribute to disparities in physical functioning across the adult life span in women. Lifestyle factors include physical activity, smoking and alcohol intake; SEP factors include level of education, the ability to manage on available income, and a geographically based index of socio-economic disadvantage. These factors were selected based on known associations with physical functioning (Cooper et al. 2011b; Peel et al. 2005; Broese van Groenou et al. 2003; Montez 2013; Stuck et al. 1999), being modifiable, and availability in the dataset. We were unable to include diet, as no dietary data were available at baseline. We considered including body mass index, but decided against it as it is not a behaviour, but a consequence of physical activity and diet.
Methods
Participants
Data were from the Australian Longitudinal Study on Women’s Health (ALSWH), a large population-based study of factors affecting the health and well being of three generations of women (Lee et al. 2005). The study methods were approved by the Human Research Ethics Committees of the Universities of Newcastle (H0760795) and Queensland (2004000224) in Australia, and all participants signed informed consent. Detailed information on design, recruitment, and attrition can be found elsewhere (Brown et al. 1998; Lee et al. 2005; Dobson et al. 2015). In brief, participants were randomly selected from all women aged 18–23, 45–50 and 70–75 years in 1996 listed in the Australian national health insurance database, which includes all citizens and permanent residents. There was intentional oversampling of women from rural and remote areas. The sample was largely representative of the wider Australian female population in these age groups, with somewhat higher representation of women who were married or partnered and of women with post-school education in all three cohorts (Brown et al. 1998). This study used data from the younger (born in 1973–1978), middle-aged (1946–1951) and older (1921–1926) cohorts. The first postal survey in 1996 included 14,247, 13,715 and 12,432 women in each cohort, respectively. Between 1998 and 2012, five follow-up surveys have been completed at approximate 3-year intervals. Postal surveys are used, but in recent years the option is offered to complete the surveys online for the younger and middle-aged cohorts. For the current analyses, data were included from participants with complete data on physical functioning over all six surveys.
Physical functioning
Physical functioning was measured with the physical functioning subscale of the well validated Medical Outcomes Survey 36-item Short-Form health survey (SF-36) (McHorney et al. 1993; Bohannon and DePasquale 2010; Ware and Sherbourne 1992). This subscale consists of ten items about whether health limits the participant in doing a range of activities, including vigorous and moderate activities, stair climbing, mobility and self-care. The score ranges from 0 to 100 with higher scores indicating better physical functioning. Baseline physical functioning was categorised using cohort-specific quintiles, to represent the continuum of physical functioning while also capturing the most extreme groups.
Lifestyle factors
All lifestyle factors were measured at baseline and based on self-report. Level of physical activity was assessed from responses to questions about frequency of vigorous (e.g. sports, aerobics) and moderate (e.g. walking, swimming) exercise in a usual week (Brown et al. 2000). Participants were classified as reporting none (moderate activity 0–1 times per week), low (moderate activity 2–4 times or vigorous activity 1–2 times per week), medium (moderate activity 5–8 times or vigorous activity 3–5 times per week) or high (moderate activity > 8 times or vigorous activity > 5 times per week, or equivalent combination) level of moderate-to-vigorous physical activity. These levels of physical activity were based on the 1996 US National Physical Activity Guideline, which recommended 5 bouts of moderate activity per week or 3 bouts of vigorous activity or any combination of the two (U.S. Department of Health and Human Services 1996).
Baseline smoking and alcohol intake were based on questions asking about the history, frequency and quantity of use. Smoking status was defined as never, ex- or current smoker. Alcohol intake was categorised in accordance with the Australian National Health and Medical Research Council recommendations as: none/rarely, low risk (≤ 14 drinks/week), risky (15–28 drinks/week) and high risk (> 28 drinks/week) (National Health and Medical Research Council 2001).
Socio-economic factors
All socio-economic factors were measured at baseline. Level of education was assessed as the highest educational qualification completed, with the response options collapsed into: no formal/school, high school, trade/apprenticeship/certificate/diploma and university/higher degree. Managing on income was assessed by asking: “How do you manage on the income you have available?” Response options were collapsed into ‘impossible/always difficult’, ‘sometimes difficult’, ‘not too bad’ and ‘easy’. Locations identified from participants’ addresses were geo-coded and mapped to statistical local areas and corresponding index of relative socio-economic disadvantage, referred to as neighbourhood socio-economic disadvantage. These values are based on the economic and social characteristics of families and households, and education level and occupation of individuals living in each location (Australian Bureau of Statistics 1998). These values were categorised into sample specific quintiles.
Other variables
Other demographic and health variables were included for descriptive purposes. Age and marital status (married or partnered, widowed/divorced/never married) were based on self-report. Area of residence was based on addresses and categorised as urban, rural or remote. Body mass index (BMI) was calculated from self-reported weight and height (kg/m2). General health and mental health were measured with the respective subscales of the SF-36 (range 0–100). Further details can be found at www.alswh.org.au.
Statistical analysis
Baseline characteristics were summarised for the three cohorts separately. Women who provided data on physical functioning on all six surveys were compared with women with missing data on physical functioning on one or more surveys, using t tests for approximately normally distributed continuous variables, rank sum tests for non-normally distributed continuous variables and chi-squared tests for categorical variables.
To depict the associations between baseline lifestyle and SEP factors with the temporal course of physical functioning, spline regression was used, assuming linear associations between knots (Harrell 2001). Only baseline measures of lifestyle and SEP were included, to mimic the targeting at risk groups for health promotion interventions based on their characteristics at a given point in time. The same knots were used as previously described, based on a structured selection procedure whereby knots were placed at 3-year intervals and systematically removed if no statistically significant difference in slope was found before and after the knot (Peeters et al. 2013). Exploratory analyses showed that linear associations between knots fitted the data as well as higher order polynomial functions. Separate models were fitted for each of the lifestyle and SEP factors. In each model, separate lines were fitted for each of the strata of the factor of interest. This approach meant that we were unable to test whether differences in trajectories were statistically significant. In addition, in a separate model, the trajectory of physical functioning was modelled for the highest and lowest baseline quintiles of physical functioning. The trajectories for the highest and lowest baseline quintiles are shown in the figures for each of the lifestyle and SEP factors to indicate variability of physical functioning in the sample. The main analyses used data from all participants who provided any data at any time point. Sensitivity analyses included data only from participants with complete data.
To quantify how each factor explains the model fit of the trajectory of physical functioning with age, a random effects linear model was fitted, with physical functioning as the outcome and with a squared term for age to accommodate for the non-linearity in the slope. The full model included all lifestyle and SEP factors plus interaction terms for each of these factors with age. For this purpose, age was standardised and divided by 5 to reflect 5-year increments. Subsequently, the models were rerun after excluding each of the factors in turn, plus their interaction with age. These models were compared with the full model using the likelihood ratio test. This procedure was repeated for each of the cohorts separately to examine the relative contributions of each factor to the model fit of the physical functioning trajectories at the different life stages. To check for multicollinearity between the lifestyle and SEP factors, correlations (r < 0.25) and variance inflation factors (VIF < 1.2) were estimated and did not indicate multicollinearity. No adjustment was made for chronic conditions and BMI, as these were believed to, at least in part, mediate the associations of lifestyle and SEP with physical functioning. All analyses were performed using STATA version 13.0 (StataCorp LP, College Station, TX).
Results
Of the 14,247, 13,715 and 12,432 younger, middle-aged and older women who returned the first survey in 1996, 5087, 8092 and 3001 participants provided complete data on physical functioning over all six surveys. The number of respondents and reasons for non-response at each survey is shown in Supplementary Figure 1. The women with complete data were on average 20.8 ± 1.5, 47.6 ± 1.5 and 72.3 ± 1.4 years old at baseline, respectively (Table 1). Compared with women with incomplete data, women with complete data were the same age, but scored higher on physical, mental and general health, had higher levels of education, lived in less disadvantaged neighbourhoods, were less likely to report difficulty managing on income, and were less likely to report being inactive, smoking or risky levels of alcohol intake (p < 0.001). In addition, middle-aged and older women with complete data were more likely to be married or partnered, whereas younger women with complete data were less likely to be married or partnered (p < 0.001). Middle-aged women with complete data were also more likely to live in rural or remote areas (p < 0.001).
Table 1.
Baseline (1996) characteristics of the younger, middle-aged and older women in the Australian Longitudinal Study on Women’s Health
| Younger cohort | Middle-aged cohort | Older cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Complete data | Incomplete data | Complete data | Incomplete data | Complete data | Incomplete data | ||||
| N | 5087 | 9160 | 8092 | 5623 | 3001 | 9431 | |||
| Age (range) | 18–23 | 18–23 | 45–50 | 45–50 | 70–75 | 70–75 | |||
| Age [mean (SD)] | 20.8 (1.5) | 20.7 (1.5) | <0.001 | 47.6 (1.5) | 47.6 (1.5) | 0.27 | 72.3 (1.4) | 72.6 (1.5) | < 0.001 |
| Living in rural/remote areas (%) | 44.0 | 45.2 | 0.16 | 64.8 | 61.9 | < 0.001 | 58.7 | 59.7 | 0.30 |
| Married or partnered (%) | 19.9 | 24.0 | < 0.001 | 85.2 | 79.5 | < 0.001 | 60.9 | 55.5 | < 0.001 |
| Body mass index [mean (SD)] | 22.7 (0.1) | 22.8 (0.1) | 0.11 | 25.8 (0.1) | 26.1 (0.1) | < 0.001 | 25.2 (0.1) | 25.4 (0.1) | 0.16 |
| General health (median [IQR]) | 72 [57–87] | 72 [52–82] | < 0.001 | 77 [67–87] | 72 [55–87] | < 0.001 | 77 [62–87] | 67 [47–82] | < 0.001 |
| Mental health (median [IQR]) | 72 [60–84] | 72 [56–80] | < 0.001 | 80 [64–88] | 72 [56–84] | < 0.001 | 84 [72–92] | 80 [64–88] | < 0.001 |
| Physical functioning (median [IQR]) | 95 [90–100] | 95 [85–100] | < 0.001 | 95 [85–100] | 90 [75–100] | < 0.001 | 75 [60–90] | 65 [40–83] | < 0.001 |
| Level of education (%) | < 0.001 | < 0.001 | < 0.001 | ||||||
| No formal/school | 9.7 | 21.2 | 46.4 | 54.5 | 66.9 | 74.9 | |||
| High school | 57.7 | 51.4 | 16.5 | 17.4 | 14.4 | 11.9 | |||
| Trade/apprentice/diploma/certificate | 17.1 | 18.7 | 21.0 | 16.4 | 13.9 | 10.3 | |||
| University/higher degree | 15.5 | 8.7 | 16.2 | 10.7 | 5.8 | 3.0 | |||
| Managing on income (%) | < 0.001 | < 0.001 | < 0.001 | ||||||
| Impossible/always difficult | 15.0 | 20.4 | 12.2 | 18.8 | 5.3 | 7.2 | |||
| Sometimes difficult | 31.0 | 34.4 | 27.6 | 30.5 | 17.1 | 20.7 | |||
| Not too bad | 38.7 | 34.1 | 43.3 | 38.7 | 50.9 | 51.2 | |||
| It is easy | 15.3 | 11.1 | 17.0 | 12.0 | 26.7 | 20.9 | |||
| Neighbourhood socio-economic disadvantage (%) | < 0.001 | < 0.001 | < 0.001 | ||||||
| Lowest quintile (most disadvantaged) | 17.3 | 21.6 | 19.1 | 21.8 | 17.3 | 20.7 | |||
| 2nd quintile | 18.8 | 20.3 | 20.0 | 19.5 | 20.7 | 20.4 | |||
| 3rd quintile | 20.3 | 19.8 | 20.0 | 20.6 | 18.2 | 20.1 | |||
| 4th quintile | 21.3 | 19.6 | 19.9 | 19.2 | 20.3 | 19.7 | |||
| Highest quintile (least disadvantaged) | 22.3 | 18.7 | 21.1 | 19.0 | 23.5 | 19.1 | |||
| Physical activity (%) | 0.001 | < 0.001 | < 0.001 | ||||||
| Inactive | 13.6 | 15.9 | 25.3 | 30.9 | 18.9 | 33.0 | |||
| Low | 29.2 | 28.6 | 31.7 | 29.0 | 32.0 | 27.5 | |||
| Medium | 26.9 | 25.1 | 27.2 | 22.9 | 34.7 | 28.0 | |||
| High | 30.3 | 30.3 | 15.8 | 17.2 | 14.4 | 11.6 | |||
| Smoking status (%) | < 0.001 | < 0.001 | < 0.001 | ||||||
| Never smoked | 60.0 | 47.9 | 56.5 | 48.2 | 68.8 | 60.5 | |||
| Ex-smoker | 14.5 | 15.8 | 29.2 | 27.4 | 27.2 | 30.8 | |||
| Current smoker | 25.5 | 36.4 | 14.3 | 24.5 | 4.0 | 8.7 | |||
| Alcohol intake (%) | < 0.001 | < 0.001 | < 0.001 | ||||||
| None/rarely | 40.3 | 45.1 | 43.5 | 51.3 | 56.6 | 66.1 | |||
| Low risk | 54.6 | 49.1 | 51.5 | 43.1 | 39.9 | 30.6 | |||
| Risky/High risk | 5.1 | 5.8 | 5.0 | 5.6 | 3.6 | 3.4 | |||
Participants with complete data had valid data on physical functioning at all surveys, whereas participants with incomplete data had missing values for physical functioning on at least one of the surveys
SD standard deviation, IQR interquartile range
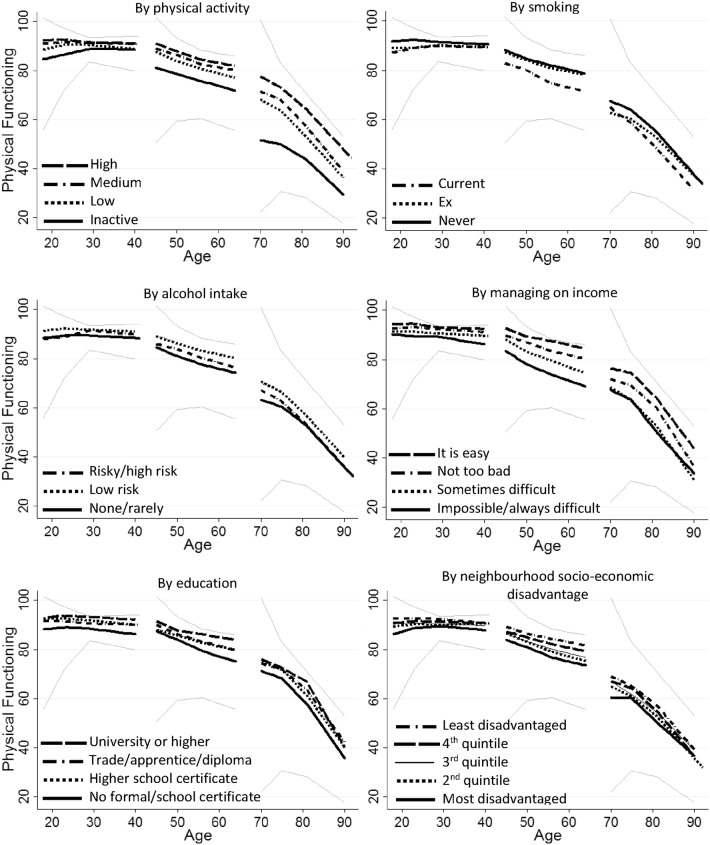
At all ages, a clear gradient in level of physical functioning was observed across the levels of baseline physical activity, with less active participants having lower physical functioning scores across the next 14–16 years (p < 0.001) (Fig. 1, Table 2). However, in the younger and older cohorts, the decline in physical functioning was less rapid in the inactive group than in the highly active group, as indicated by the statistically significant and positive regression coefficient of the interaction term with age (B = 0.3, p < 0.001, Table 2). In the younger and middle-aged cohorts, but not in the older cohort, current and ex-smokers had lower physical functioning scores than the never smokers (Fig. 1, Table 2). In the middle-aged and older cohorts, the decline in physical functioning was also more rapid in the current smokers than in the never smokers (Table 2). However, in the younger cohort, less decline in physical functioning was observed in the current and ex-smokers than in the never smokers (Fig. 1, Table 2). In all three cohorts, the low-risk drinkers had higher scores for physical functioning than none/rarely drinkers (Fig. 1, Table 2). In the older cohort, the decline in physical functioning was more rapid in the risky drinkers than in the none/rarely drinkers (Table 2). These patterns were similar in the complete case analysis (Supplementary Figure 2).
Fig. 1.
Course of physical functioning with age and by level of six lifestyle and socio-economic factors. The figures are based on the results from the spline regression. The black solid, dotted and dashed lines indicate the course of physical functioning for each of the categories of lifestyle and SEP factors. The grey lines indicate the course of physical functioning for the highest and lowest quintiles of physical functioning at baseline for each of the three cohorts. The narrowing of the gap between these lines over the first years of follow-up reflects regression to the mean
Table 2.
Estimates from the random effects linear regression model including all six lifestyle and socio-economic factors
| Variable | Category | Younger cohort | Middle-aged cohort | Older cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | p value | B | 95% CI | p value | B | 95% CI | p value | |||||
| Constant | 97.2 | 95.9 | 98.5 | < 0.001 | 95.1 | 93.6 | 96.6 | < 0.001 | 86.0 | 82.9 | 89.1 | < 0.001 | |
| Age | − 0.6 | − 1.2 | 0.0 | 0.04 | − 2.0 | − 2.6 | − 1.4 | < 0.001 | − 3.0 | − 4.2 | − 1.8 | < 0.001 | |
| Age2 | − 0.2 | − 0.2 | − 0.1 | 0.001 | 0.3 | 0.2 | 0.4 | < 0.001 | − 1.4 | − 1.6 | − 1.3 | < 0.001 | |
| Physical activity | High | ref | ref | ref | |||||||||
| Medium | − 1.4 | − 2.1 | − 0.7 | < 0.001 | − 1.8 | − 2.8 | − 0.8 | < 0.001 | − 5.0 | − 6.7 | − 3.3 | < 0.001 | |
| Low | − 2.7 | − 3.4 | − 2.0 | < 0.001 | − 3.5 | − 4.5 | − 2.5 | < 0.001 | − 9.5 | − 11.2 | − 7.8 | < 0.001 | |
| Inactive | − 6.4 | − 7.3 | − 5.6 | < 0.001 | − 8.5 | − 9.4 | − 7.5 | < 0.001 | − 24.6 | − 26.4 | − 22.9 | < 0.001 | |
| Physical activity × age | High | ref | ref | ref | |||||||||
| Medium | 0.4 | 0.2 | 0.7 | 0.001 | 0.0 | − 0.3 | 0.3 | 0.87 | − 1.0 | − 1.6 | − 0.4 | 0.001 | |
| Low | 0.5 | 0.3 | 0.8 | < 0.001 | 0.0 | − 0.4 | 0.3 | 0.78 | − 0.3 | − 0.9 | 0.3 | 0.29 | |
| Inactive | 1.4 | 1.0 | 1.7 | < 0.001 | 0.2 | − 0.1 | 0.5 | 0.18 | 1.3 | 0.7 | 2.0 | < 0.001 | |
| Smoking status | Never smoked | ref | ref | ref | |||||||||
| Ex-smoker | − 2.3 | − 3.1 | − 1.5 | < 0.001 | − 0.9 | − 1.7 | − 0.2 | 0.02 | − 4.8 | − 6.0 | − 3.6 | < 0.001 | |
| Current smoker | − 3.0 | − 3.7 | − 2.4 | < 0.001 | − 2.5 | − 3.4 | − 1.6 | < 0.001 | − 0.4 | − 2.5 | 1.6 | 0.68 | |
| Smoking × age | Never smoked | ref | ref | ref | |||||||||
| Ex-smoker | 0.4 | 0.2 | 0.7 | 0.002 | − 0.1 | − 0.3 | 0.1 | 0.47 | 0.3 | − 0.1 | 0.8 | 0.11 | |
| Current smoker | 0.6 | 0.4 | 0.9 | < 0.001 | − 0.9 | − 1.2 | − 0.6 | < 0.001 | − 1.9 | − 2.7 | − 1.1 | < 0.001 | |
| Alcohol intake | Non drinker | ref | ref | ref | |||||||||
| Low risk | 2.5 | 1.9 | 3.0 | < 0.001 | 3.5 | 2.9 | 4.2 | < 0.001 | 4.7 | 3.6 | 5.8 | < 0.001 | |
| Risky/high risk | 0.9 | − 0.3 | 2.1 | 0.13 | 2.9 | 1.4 | 4.4 | < 0.001 | 4.0 | 1.1 | 6.8 | 0.006 | |
| Alcohol × age | Non drinker | ref | ref | ref | |||||||||
| Low risk | − 0.1 | − 0.3 | 0.1 | 0.57 | 0.2 | − 0.1 | 0.4 | 0.14 | − 0.3 | − 0.7 | 0.1 | 0.12 | |
| Risky/high risk | 0.4 | 0.0 | 0.9 | 0.08 | − 0.2 | − 0.7 | 0.3 | 0.44 | − 1.3 | − 2.3 | − 0.3 | 0.01 | |
| Managing on income | Easy | ref | ref | ref | |||||||||
| Not too bad | − 0.8 | − 1.7 | 0.0 | 0.06 | − 1.8 | − 2.8 | − 0.9 | < 0.001 | − 3.4 | − 4.7 | − 2.1 | < 0.001 | |
| Sometimes difficult | − 2.2 | − 3.0 | − 1.3 | < 0.001 | − 4.7 | − 5.7 | − 3.7 | < 0.001 | − 9.8 | − 11.3 | − 8.2 | < 0.001 | |
| Impossible/always difficult | − 3.9 | − 4.9 | − 3.0 | < 0.001 | − 10.2 | − 11.4 | − 9.0 | < 0.001 | − 9.7 | − 12.0 | − 7.4 | < 0.001 | |
| Income × age | Easy | ref | ref | ref | |||||||||
| Not too bad | 0.0 | − 0.3 | 0.3 | 0.99 | − 0.3 | − 0.6 | 0.0 | 0.07 | 0.0 | − 0.5 | 0.5 | 0.99 | |
| Sometimes difficult | 0.0 | − 0.3 | 0.4 | 0.78 | − 0.6 | − 1.0 | − 0.3 | < 0.001 | 0.2 | − 0.4 | 0.7 | 0.61 | |
| Impossible/always difficult | − 0.2 | − 0.5 | 0.2 | 0.40 | − 0.9 | − 1.3 | − 0.5 | < 0.001 | 0.7 | − 0.2 | 1.5 | 0.14 | |
| Level of education | University or higher | ref | ref | ref | |||||||||
| Trade/Apprentice/Cert. | − 2.2 | − 3.2 | − 1.1 | < 0.001 | − 0.2 | − 0.2 | − 0.1 | < 0.001 | − 3.5 | − 6.4 | − 0.6 | 0.02 | |
| Higher school cert. | − 0.9 | − 1.8 | 0.0 | 0.05 | − 0.2 | − 0.2 | − 0.1 | < 0.001 | − 2.8 | − 5.6 | 0.1 | 0.06 | |
| No formal/school | − 5.8 | − 6.9 | − 4.7 | < 0.001 | − 0.3 | − 0.4 | − 0.3 | < 0.001 | − 3.7 | − 6.2 | − 1.1 | 0.005 | |
| Education × age | University or higher | ref | ref | ref | |||||||||
| Trade/Apprentice/Cert. | 0.1 | − 0.3 | 0.5 | 0.57 | − 0.8 | − 1.2 | − 0.5 | < 0.001 | 0.1 | − 0.8 | 1.1 | 0.77 | |
| Higher school cert. | 0.0 | − 0.3 | 0.3 | 0.83 | − 0.8 | − 1.1 | − 0.4 | < 0.001 | − 0.4 | − 1.3 | 0.6 | 0.43 | |
| No formal/school | 0.4 | 0.0 | 0.8 | 0.08 | − 1.6 | − 1.9 | − 1.3 | < 0.001 | − 0.6 | − 1.5 | 0.2 | 0.15 | |
| Neighbourhood socio-economic disadvantage | Least disadvantaged | ref | ref | ref | |||||||||
| Quintile 2 | − 0.8 | − 1.6 | 0.1 | 0.07 | − 0.6 | − 1.6 | 0.4 | 0.24 | 0.9 | − 0.6 | 2.5 | 0.25 | |
| Quintile 3 | − 1.1 | − 2.0 | − 0.3 | 0.008 | − 1.3 | − 2.3 | − 0.2 | 0.02 | − 1.0 | − 2.6 | 0.6 | 0.23 | |
| Quintile 4 | − 1.9 | − 2.8 | − 1.1 | < 0.001 | − 0.5 | − 1.5 | 0.5 | 0.33 | − 1.2 | − 2.8 | 0.4 | 0.15 | |
| Most disadvantaged | − 2.9 | − 3.8 | − 2.1 | < 0.001 | − 2.3 | − 3.4 | − 1.3 | < 0.001 | − 2.3 | − 3.9 | − 0.7 | 0.006 | |
| Neighbourhood socio-economic disadvantage × age | Least disadvantaged | ref | ref | ref | |||||||||
| Quintile 2 | 0.1 | − 0.2 | 0.4 | 0.39 | 0.0 | − 0.3 | 0.4 | 0.78 | − 0.9 | − 1.5 | − 0.4 | 0.001 | |
| Quintile 3 | 0.1 | − 0.2 | 0.4 | 0.73 | − 0.3 | − 0.6 | 0.0 | 0.07 | − 0.2 | − 0.8 | 0.4 | 0.45 | |
| Quintile 4 | 0.3 | 0.0 | 0.6 | 0.03 | − 0.8 | − 1.1 | − 0.5 | < 0.001 | − 1.0 | − 1.5 | − 0.4 | 0.001 | |
| Most disadvantaged | 0.3 | 0.0 | 0.6 | 0.05 | − 0.6 | − 1.0 | − 0.3 | < 0.001 | − 0.6 | − 1.2 | 0.0 | 0.06 | |
B regression coefficient
Interaction terms for each of the factors with age were added to model the non-linearity in slope with age. A statistically significant regression coefficient for the interaction term with age indicates that there is either a more (negative values) or less (positive values) rapid decline in physical functioning with age in the respective category compared with the reference category for that factor
In all three cohorts, difficulty with managing on income was associated with lower scores on physical functioning (Fig. 1, Table 2). In the middle-aged women, difficulty with managing on income was also associated with a more rapid decline in physical functioning (Table 2). In all three cohorts, those with no formal education/school certificate had lower scores for physical functioning than those with university degrees. In the middle-aged women, those with lower levels of education also had more rapid decline in physical functioning than those with a university degree (Table 2). In all three cohorts, participants living in the most disadvantaged neighbourhoods had lower scores for physical functioning than those in the least disadvantaged neighbourhoods (Fig. 1, Table 2). Participants in more disadvantaged neighbourhoods also tended to have a more rapid decline in physical functioning than those in the least disadvantaged neighbourhoods (Table 2). These patterns were similar in the complete case analysis (Supplementary Figure 2).
The difference in model fit (expressed as the − 2 log likelihood) between the full model and models in which factors were excluded one at the time was greatest for level of education in younger women, for income in middle-aged women and for physical activity in older women (Table 3).
Table 3.
Model fit of associations between lifestyle and socio-economic factors and physical functioning at three life stages
| Younger cohort | Middle-aged cohort | Older cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| − 2LL | Difference | p | − 2LL | Difference | p | − 2LL | Difference | p | |
| Full model | − 222,204 | − 267,404 | − 173,350 | ||||||
| Full model excluding | |||||||||
| Physical activity | − 222,331 | 127 | < 0.001 | − 267,625 | 221 | < 0.001 | − 173,964 | 614 | < 0.001 |
| Smoking | − 222,254 | 50 | < 0.001 | − 267,473 | 69 | < 0.001 | − 173,401 | 51 | < 0.001 |
| Alcohol intake | − 222,272 | 68 | < 0.001 | − 267,492 | 88 | < 0.001 | − 173,392 | 42 | < 0.001 |
| Managing on income | − 222,299 | 95 | < 0.001 | − 267,743 | 339 | < 0.001 | − 173,464 | 114 | < 0.001 |
| Level of education | − 222,334 | 130 | < 0.001 | − 267,516 | 112 | < 0.001 | − 173,365 | 15 | < 0.001 |
| Neighbourhood socio-economic disadvantage | − 222,240 | 36 | < 0.001 | − 267,460 | 56 | < 0.001 | − 173,373 | 23 | < 0.001 |
The full model included interaction terms of each of the lifestyle and socio-economic factors with age. For detailed results of the full model, please refer to Table 2. Subsequently, each of the factors, plus their interaction with age, were excluded from the model one by one and these models were compared with the full model in terms of model fit using the likelihood ratio test. A higher − 2 log likelihood (− 2LL) indicates better model fit. Results for the factors that contribute most to the model fit in each of the cohorts are highlighted in bold
Discussion
We explored lifestyle and SEP factors that may contribute to disparities in physical functioning across the adult life span in this longitudinal study of Australian women. The findings suggest that all studied factors contribute, but that some factors are more important at certain ages, i.e. level of education and physical activity in younger women, managing on income in middle-aged women and physical activity in older women.
Cross-sectional and longitudinal associations between physical activity and physical functioning are well established and were confirmed in this study (Cooper et al. 2011b; Yorston et al. 2012; Tikkanen et al. 2012; Michael et al. 1999; Peeters et al. 2014a; Pluijm et al. 2007). Our measure of physical activity predominantly measured frequency of moderate and vigorous sports/exercise and leisure activities. Other types of activities may also affect physical functioning, such as occupational and domestic activities. As occupational and domestic activities may affect physical functioning in ways different from exercise or leisure activities (Peeters et al. 2014b), the current findings may not be generalised to other forms of physical activity. In older women, the decline in physical functioning was less rapid in those who were physically inactive than in those who were physically active. This is likely explained by the fact that the inactive group already had low scores at baseline and therefore had less potential to further decline (floor effect). Healthy survivor bias may also play a role.
In line with previous studies, the current results suggest that smokers have poorer physical functioning than never smokers in younger and middle-aged adults (Strand et al. 2011b; van den Borst et al. 2011; Rapuri et al. 2007; Michael et al. 1999; Inzitari et al. 2006), while in the older adults, ex-smokers (but not current smokers) had poorer physical functioning than never smokers. It should be noted that there were few current smokers in the older cohort and those who did smoke were likely to die sooner than non-smokers, or withdraw from the study (Brilleman et al. 2010) and contribute only to the earlier years of follow-up. Older women who continue to smoke may be healthier than those who quit, because adults who quit smoking may do so because they are ill (van Gool et al. 2007). However, physical function of smokers declines more rapidly than in those who never smoked. Our findings suggest that the physical function of quitters remains poor, but the rate of decline is similar in quitters and in women who never smoked.
The results for alcohol intake were also in line with those found in previous studies, with poorer physical functioning in non-drinkers and risky drinkers than in low risk drinkers (Maraldi et al. 2009; Michael et al. 1999). The poorer physical functioning among non-drinkers could either be explained by abstinence due to illness and/or medication use or the potential beneficial effects of low levels of alcohol use on cardiovascular health (O’Keefe et al. 2014). For example, one important symptom of cardiovascular disease, shortness of breath, may result in inability to climb a flight of stairs or walk longer distances. Thus, if low alcohol intake contributes to better cardiovascular health, it may also contribute to maintaining physical functioning. The current findings add that throughout adulthood physical activity is more strongly associated with physical functioning than smoking and alcohol intake. One of the explanations for this may be that physical activity affects physical functioning directly by increasing or maintaining physical capacities, including musculoskeletal strength and neuromuscular coordination, whereas the influence of smoking and alcohol on physical functioning are indirect via their detrimental effects on health.
Previous studies have shown that higher education, higher income and living in more affluent areas are associated with better physical functioning in middle-aged and older adulthood (Hurst et al. 2013; Strand et al. 2011a; Ramlagan et al. 2014; Feng and Astell-Burt 2013; Broese van Groenou et al. 2003). One study of 55–85 year old adults found that the 3-year risk of developing care dependence in low-income groups was nearly double that of high income groups (Broese van Groenou et al. 2003). In the current study, associations with physical functioning were stronger for the person-level factors ‘level of education’ and ‘managing on income’ than the area-level factor ‘neighbourhood socio-economic disadvantage’. This likely reflects that education and income are better descriptors of the participant than aggregate measures of everyone who lives in the same area. Previous studies have also shown that lifestyle factors and SEP factors cluster together (Meader et al. 2016; Noble et al. 2015) and that lifestyle may mediate the association between SEP and health outcomes (Cheval et al. 2018; Elhakeem et al. 2017). By including lifestyle and SEP factors in one model, we may have underestimated the true contribution of each factor. We, therefore, ran a post hoc analysis including the SEP factors only in the first model and adding the lifestyle factors in the second model (Supplementary Table 1). Indeed, associations between the SEP factors and physical functioning attenuate somewhat after adding in the lifestyle factors. The current findings suggest that SEP factors seem more important in the younger and middle-aged cohorts than in the older cohort (Table 2). These findings may also mean that the association between SEP and physical functioning is less mediated by lifestyle in younger and middle-aged women than in older women. In middle-aged women, those with lower SEP not only started at lower levels of physical functioning, they also had more rapid declines with age than the women with higher SEP. This emphasises the need for interventions to improve physical functioning in lower SEP groups in middle-aged women. Interventions that may be considered include broader interventions to reduce socio-economic inequalities, such as encouraging life-long learning, tax benefits for low-income households, educational programs to improve occupational and life skills, improving affordable access to appropriate health care, and improving opportunities for being physically active.
Although younger women who were smokers or risky alcohol drinkers, or who had the lowest level of education, initially had lower levels of physical functioning, these groups also showed an improvement in physical functioning over the first few years of follow-up. The findings for smoking are in contrast with findings from previous studies, which indicate no difference in rate of decline between smokers and non-smokers (Leyk et al. 2012; Bernaards et al. 2003). This apparent improvement in the groups with initially the poorest physical functioning may be explained by regression to the mean. In middle-aged women, smoking made a relatively smaller contribution to the models for physical functioning than physical activity. Nonetheless, smokers had substantially lower scores than never- and ex-smokers (Fig. 1). Hence, middle-aged smokers may benefit from combined smoking cessation and physical activity promotion interventions.
BMI may confound and/or mediate the associations of lifestyle and SEP factors with physical functioning. Post hoc analyses in which BMI was included as a covariate in the full model shows that the associations attenuate somewhat for most factors, but the overall patterns remain the same (Supplemental Table 2).
Kalache and Kickbusch proposed that different interventions are required at each life stage to improve functional capacities and delay care dependence (Kalache and Kickbusch 1997). As all six factors significantly contributed to explaining the differences in physical functioning at all ages, interventions targeting one or more of these have the potential to improve physical functioning. Interventions that aim to maximise the early life peak in physical functioning may have lasting benefits, and later interventions to slow declines may also be effective. Differences between SEP groups in psychosocial needs and perceived barriers to adopting healthy lifestyle behaviours have been well documented (Janssen et al. 2010; Bukman et al. 2014; Gray et al. 2016). Unless interventions to foster physical functioning at all stages in life pay particular attention to the needs of people of lower SEP, the health inequities represented by these findings may be exacerbated rather than reduced.
The current results suggest that across the adult life span physical activity is an important target for interventions to optimise physical functioning. An observational study among 407 adults aged 25–80 years found that income modified the association between physical activity and physical functioning, with effect sizes being 3–4 times greater in the low-income group (Peterson et al. 2006). These results suggested that physical activity may help reduce SEP-related disparities in physical functioning. However, certain individual physical activity interventions may only be effective if facilitated by the social and physical environment. For example, interventions that promote walking are effective only if safe footpaths are available. Less educated younger women and low-income middle-aged women form priority target groups, but also present particular challenges for public health interventions. A qualitative study showed that low SEP participants may be more motivated to change their lifestyle when they experience health complaints than for preventive purposes (Bukman et al. 2014). Low physical functioning in middle-aged and older women has also been associated with low health literacy that may limit uptake of lifestyle interventions (Smith et al. 2015). Interventions in lower SEP groups require specific tailoring, such as simplifying and reducing reading materials and using interactive approaches (e.g. games, competitions and discussion) (Khare et al. 2009).
Strengths of this study include the large and broadly nationally representative samples of women in three age groups who were followed over 14–16 years with low attrition rates in the middle-aged women. However, the following limitations need to be considered. The associations may partly be explained by reverse causality, as those who had health problems at baseline may have been less able, for example, to engage in physical activity or participate in the workforce. Analysis of lifestyle and SEP factors was limited to the six factors for which data were available at baseline. While it would have been interesting to include other factors, such as diet or wealth, these data were unavailable. The main reason for drop-out was withdrawal in the younger women and death in the older women. Women with complete or incomplete data differed in terms of SEP, lifestyle and health (Table 1). However, repeating the analyses with data from women with complete data only (Supplementary Figure 2) showed no differences with results from women who provided data at any time in the study. The approximately parallel trajectories across the strata could partly be explained by a healthy survivor bias, but may also result from using fixed knots in the spline modelling, which were based on the total sample. The marked increase and decrease in physical functioning from baseline to the first follow-up in the lowest and highest quintiles of baseline functioning, respectively, are likely to be explained by regression to the mean. The study design allowed us to track changes in PF at three life stages which are characterised by markedly different life-stage events such as starting work, having a family, retiring from paid work, and a progressively increasing need for care in older age. Some of the differences between the three cohorts may be explained by a combination of age, period and cohort effects. Age and period and cohort effects cannot be disentangled. The emphasis of this study is on ageing, but period changes (which affect all participants) happened during follow-up. For example, the SEP-health gap is widening. In addition, differences exist between the cohorts in distribution of SEP and lifestyle factors. Physical functioning was based on self-report, but the SF-36 is well validated (McHorney et al. 1993; Bohannon and DePasquale 2010; Ware and Sherbourne 1992). The physical functioning subscale has a ceiling effect in younger adults, which may have limited the sensitivity to variations in physical functioning in the youngest cohort. However, consistency in measures used across the cohorts enabled comparisons of the findings between the cohorts. Moreover, the associations with physical functioning are similar to those from studies that used performance tests and self-reported limitations. For the current analyses, only the baseline measures of lifestyle and SEP factors were used. Changes in these factors over time were ignored, which may have resulted in less accurate estimation of their associations with physical functioning. However, the aim was to inform interventions and policy. Interventions are designed to help people with specific lifestyle or SEP circumstances at a given time. Our analyses mirror this situation. Apart from oversampling of the women living in rural and remote areas, the sample was broadly nationally representative at baseline. The included women were more likely to have better health, higher SEP and better health-related behaviour. It is therefore likely that the current results underestimate the lifestyle and SEP-related disparities in physical functioning in the whole population. The sample included women only. As previous studies have shown gender differences in associations between lifestyle, SES and physical functioning (Bernaards et al. 2003; Leyk et al. 2012; Ramlagan et al. 2014), the results cannot be generalised to men.
In conclusion, all six lifestyle and SEP factors considered here contributed to explaining the disparities in physical functioning across the adult life span in women. To achieve the best possible level of physical functioning, SEP factors may be particularly important in younger and middle-aged women, while physical activity is important at all ages.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The research on which this paper is based was conducted as part of the Australian Longitudinal Study on Women’s Health by The University of Newcastle and The University of Queensland. We are grateful to the Australian Government Department of Health for funding and to the women who provided the survey data.
Funding
This work was supported by the Australian Government Department of Health; and Australian National Health and Medical Research Council Centre of Research Excellence [Grant number APP1000986]. The funding source had no role in the data collection, analyses, interpretation and decision to publish the manuscript. GP is supported by a fellowship from the Global Brain Health Institute.
Conflict of interests
The authors declare that they have no conflict of interest.
Data sharing statement
ALSWH data may be made available to collaborating researchers where there is a formal request to make use of the material. Permission to use the data must be obtained from the Publications, Analyses and Substudies (PSA) Committee of ALSWH. Further details can be found at http://alswh.org.au/for-researchers.
References
- Australian Bureau of Statistics (1998) 1996 Census of Population and housing. Socio-economic indexes for areas. Canberra: ABS. http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/2033.0.55.0011996?OpenDocument. Accessed 18 Dec 2003
- Bernaards CM, Twisk JW, Van Mechelen W, Snel J, Kemper HC. A longitudinal study on smoking in relationship to fitness and heart rate response. Med Sci Sports Exerc. 2003;35(5):793–800. doi: 10.1249/01.MSS.0000064955.31005.E0. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, DePasquale L. Physical functioning scale of the short-form (SF) 36: internal consistency and validity with older adults. J Geriatr Phys Ther. 2010;33(1):16–18. [PubMed] [Google Scholar]
- Brilleman SL, Pachana NA, Dobson AJ. The impact of attrition on the representativeness of cohort studies of older people. BMC Med Res Methodol. 2010;10:71. doi: 10.1186/1471-2288-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broese van Groenou MI, Deeg DJ, Penninx BW. Income differentials in functional disability in old age: relative risks of onset, recovery, decline, attrition and mortality. Aging Clin Exp Res. 2003;15(2):174–183. doi: 10.1007/BF03324497. [DOI] [PubMed] [Google Scholar]
- Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Bryson L, Byles JE, Dobson AJ, Lee C, Mishra G, et al. Women’s Health Australia: recruitment for a national longitudinal cohort study. Women Health. 1998;28(1):23–40. doi: 10.1300/J013v28n01_03. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Mishra G, Lee C, Bauman A. Leisure time physical activity in Australian women: relationship with well being and symptoms. Res Q Exerc Sport. 2000;71(3):206–216. doi: 10.1080/02701367.2000.10608901. [DOI] [PubMed] [Google Scholar]
- Bukman AJ, Teuscher D, Feskens EJ, van Baak MA, Meershoek A, Renes RJ. Perceptions on healthy eating, physical activity and lifestyle advice: opportunities for adapting lifestyle interventions to individuals with low socioeconomic status. BMC Public Health. 2014;14:1036. doi: 10.1186/1471-2458-14-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheval B, Sieber S, Guessous I, Orsholits D, Courvoisier DS, Kliegel M, et al. Effect of early- and adult-life socioeconomic circumstances on physical inactivity. Med Sci Sports Exerc. 2018;50(3):476–485. doi: 10.1249/MSS.0000000000001472. [DOI] [PubMed] [Google Scholar]
- Cooper R, Kuh D, Cooper C, Gale CR, Lawlor DA, Matthews F, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40(1):14–23. doi: 10.1093/ageing/afq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Mishra GD, Kuh D. Physical activity across adulthood and physical performance in midlife: findings from a British birth cohort. Am J Prev Med. 2011;41(4):376–384. doi: 10.1016/j.amepre.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson AJ, Hockey R, Brown WJ, Byles JE, Loxton DJ, McLaughlin D, et al. Cohort profile update: Australian longitudinal study on women’s health. Int J Epidemiol. 2015;44(5):1547, 1547a–1547f. doi: 10.1093/ije/dyv110. [DOI] [PubMed] [Google Scholar]
- Elhakeem A, Hardy R, Bann D, Caleyachetty R, Cosco TD, Hayhoe RP, et al. Intergenerational social mobility and leisure-time physical activity in adulthood: a systematic review. J Epidemiol Community Health. 2017;71(7):673–680. doi: 10.1136/jech-2016-208052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Astell-Burt T. Neighborhood socioeconomic circumstances and the co-occurrence of unhealthy lifestyles: evidence from 206,457 Australians in the 45 and up study. PLoS ONE. 2013;8(8):e72643. doi: 10.1371/journal.pone.0072643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PM, Murphy MH, Gallagher AM, Simpson EE. Motives and barriers to physical activity among older adults of different socioeconomic status. J Aging Phys Act. 2016;24(3):419–429. doi: 10.1123/japa.2015-0045. [DOI] [PubMed] [Google Scholar]
- Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- Hurst L, Stafford M, Cooper R, Hardy R, Richards M, Kuh D. Lifetime socioeconomic inequalities in physical and cognitive aging. Am J Public Health. 2013;103(9):1641–1648. doi: 10.2105/AJPH.2013.301240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzitari M, Carlo A, Baldereschi M, Pracucci G, Maggi S, Gandolfo C, et al. Risk and predictors of motor-performance decline in a normally functioning population-based sample of elderly subjects: the Italian longitudinal study on aging. J Am Geriatr Soc. 2006;54(2):318–324. doi: 10.1111/j.1532-5415.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- Janssen E, Sugiyama T, Winkler E, de Vries H, te Poel F, Owen N. Psychosocial correlates of leisure-time walking among Australian adults of lower and higher socio-economic status. Health Educ Res. 2010;25(2):316–324. doi: 10.1093/her/cyp012. [DOI] [PubMed] [Google Scholar]
- Kalache A, Kickbusch I. A global strategy for healthy ageing. World Health. 1997;50(4):2. [Google Scholar]
- Khare MM, Huber R, Carpenter RA, Balmer PW, Bates NJ, Nolen KN, et al. A lifestyle approach to reducing cardiovascular risk factors in underserved women: design and methods of the Illinois WISEWOMAN Program. J Womens Health (Larchmt) 2009;18(3):409–419. doi: 10.1089/jwh.2008.0911. [DOI] [PubMed] [Google Scholar]
- Lee C, Dobson AJ, Brown WJ, Bryson L, Byles J, Warner-Smith P, et al. Cohort profile: the Australian longitudinal study on women’s health. Int J Epidemiol. 2005;34(5):987–991. doi: 10.1093/ije/dyi098. [DOI] [PubMed] [Google Scholar]
- Leyk D, Ruther T, Witzki A, Sievert A, Moedl A, Blettner M, et al. Physical fitness, weight, smoking, and exercise patterns in young adults. Dtsch Arztebl Int. 2012;109(44):737–745. doi: 10.3238/arztebl.2012.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JS, Pavalko EK. The life course of activity limitations: exploring indicators of functional limitations over time. J Aging Health. 2004;16(4):490–516. doi: 10.1177/0898264304265776. [DOI] [PubMed] [Google Scholar]
- Maraldi C, Harris TB, Newman AB, Kritchevsky SB, Pahor M, Koster A, et al. Moderate alcohol intake and risk of functional decline: the health, aging, and body composition study. J Am Geriatr Soc. 2009;57(10):1767–1775. doi: 10.1111/j.1532-5415.2009.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Meader N, King K, Moe-Byrne T, Wright K, Graham H, Petticrew M, et al. A systematic review on the clustering and co-occurrence of multiple risk behaviours. BMC Public Health. 2016;16:657. doi: 10.1186/s12889-016-3373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael YL, Colditz GA, Coakley E, Kawachi I. Health behaviors, social networks, and healthy aging: cross-sectional evidence from the Nurses’ health study. Qual Life Res. 1999;8(8):711–722. doi: 10.1023/A:1008949428041. [DOI] [PubMed] [Google Scholar]
- Montez JK. The socioeconomic origins of physical functioning among older U.S. adults. Adv Life Course Res. 2013;18(4):244–256. doi: 10.1016/j.alcr.2013.08.001. [DOI] [PubMed] [Google Scholar]
- National Health and Medical Research Council (2001) Australian Institute of Health and Welfare. Australian alcohol guidelines: health risks and benefits. Canberra (ACT): Commonwealth of Australia: National Health and Medical Research Council
- Noble N, Paul C, Turon H, Oldmeadow C. Which modifiable health risk behaviours are related? A systematic review of the clustering of Smoking, Nutrition, Alcohol and Physical activity (‘SNAP’) health risk factors. Prev Med. 2015;81:16–41. doi: 10.1016/j.ypmed.2015.07.003. [DOI] [PubMed] [Google Scholar]
- O’Keefe JH, Bhatti SK, Bajwa A, DiNicolantonio JJ, Lavie CJ. Alcohol and cardiovascular health: the dose makes the poison…or the remedy. Mayo Clin Proc. 2014;89(3):382–393. doi: 10.1016/j.mayocp.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Peel NM, McClure RJ, Bartlett HP. Behavioral determinants of healthy aging. Am J Prev Med. 2005;28(3):298–304. doi: 10.1016/j.amepre.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Peeters G, Dobson AJ, Deeg DJ, Brown WJ. A life-course perspective on physical functioning in women. Bull World Health Organ. 2013;91(9):661–670. doi: 10.2471/BLT.13.123075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters G, Lips P, Brown WJ. Changes in physical functioning over 6 years in older women: effects of sitting time and physical activity. Eur J Ageing. 2014;11(3):205–212. doi: 10.1007/s10433-013-0300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters G, van Gellecum YR, van Uffelen JG, Burton NW, Brown WJ. Contribution of house and garden work to the association between physical activity and well-being in young, mid-aged and older women. Br J Sports Med. 2014;48:996–1001. doi: 10.1136/bjsports-2012-091103. [DOI] [PubMed] [Google Scholar]
- Peterson JJ, Lowe JB, Peterson NA, Janz KF. The relationship between active living and health-related quality of life: income as a moderator. Health Educ Res. 2006;21(1):146–156. doi: 10.1093/her/cyh050. [DOI] [PubMed] [Google Scholar]
- Pluijm SM, Visser M, Puts MT, Dik MG, Schalk BW, van Schoor NM, et al. Unhealthy lifestyles during the life course: association with physical decline in late life. Aging Clin Exp Res. 2007;19(1):75–83. doi: 10.1007/BF03325214. [DOI] [PubMed] [Google Scholar]
- Ramlagan S, Peltzer K, Phaswana-Mafuya N. Hand grip strength and associated factors in non-institutionalised men and women 50 years and older in South Africa. BMC Res Notes. 2014;7:8. doi: 10.1186/1756-0500-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapuri PB, Gallagher JC, Smith LM. Smoking is a risk factor for decreased physical performance in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62(1):93–100. doi: 10.1093/gerona/62.1.93. [DOI] [PubMed] [Google Scholar]
- Smith SG, O’Conor R, Curtis LM, Waite K, Deary IJ, Paasche-Orlow M, et al. Low health literacy predicts decline in physical function among older adults: findings from the LitCog cohort study. J Epidemiol Community Health. 2015;69(5):474–480. doi: 10.1136/jech-2014-204915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand BH, Cooper R, Hardy R, Kuh D, Guralnik J. Lifelong socioeconomic position and physical performance in midlife: results from the British 1946 birth cohort. Eur J Epidemiol. 2011;26(6):475–483. doi: 10.1007/s10654-011-9562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand BH, Mishra G, Kuh D, Guralnik JM, Patel KV. Smoking history and physical performance in midlife: results from the British 1946 birth cohort. J Gerontol A Biol Sci Med Sci. 2011;66(1):142–149. doi: 10.1093/gerona/glq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuck AE, Walthert JM, Nikolaus T, Bula CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48(4):445–469. doi: 10.1016/S0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- Tikkanen P, Nykanen I, Lonnroos E, Sipila S, Sulkava R, Hartikainen S. Physical activity at age of 20-64 years and mobility and muscle strength in old age: a community-based study. J Gerontol A Biol Sci Med Sci. 2012;67(8):905–910. doi: 10.1093/gerona/gls005. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (1996) Physical activity and health: a report of the Surgeon General. Atlanta, GA. http://www.cdc.gov/nccdphp/sgr/pdf/sgrfull.pdf. Accessed 18 Dec 2003
- van den Borst B, Koster A, Yu B, Gosker HR, Meibohm B, Bauer DC, et al. Is age-related decline in lean mass and physical function accelerated by obstructive lung disease or smoking? Thorax. 2011;66(11):961–969. doi: 10.1136/thoraxjnl-2011-200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool CH, Kempen GI, Penninx BW, Deeg DJ, van Eijk JT. Chronic disease and lifestyle transitions: results from the longitudinal aging study Amsterdam. J Aging Health. 2007;19(3):416–438. doi: 10.1177/0898264307300189. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- Yorston LC, Kolt GS, Rosenkranz RR. Physical activity and physical function in older adults: the 45 and up study. J Am Geriatr Soc. 2012;60(4):719–725. doi: 10.1111/j.1532-5415.2012.03906.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.