Abstract
Fungal infections remain a burden worldwide, thus underpinning the need for effective new therapeutic approaches. In the present study, the antifungal effect of the essential oils of two thyme species, Thymus camphoratus and Thymus carnosus, used in traditional medicine in Portugal, as well as their major compounds was assessed. A special focus was placed on their effect on Candida albicans virulence factors. Also, the safety profile of the essential oils was assessed on keratinocytes. The essential oils were analyzed by gas chromatography (GC) and gas chromatography/mass spectroscopy (GC/MS). The minimal inhibitory and minimal fungicidal concentrations of the essential oils and their main compounds were assessed on reference and clinical strains. Also, their effect on C. albicans germ tube formation, metabolism, and biofilm disruption were considered. T. camphoratus oil was rich in 1,8-cineole and α-pinene whereas T. carnosus oil showed high amounts of borneol and camphene. Regarding the antifungal effect, both oils were more active against Cryptococcus neoformans and dermatophytes and very effective in inhibiting C. albicans germ tube formation, at doses well below their MIC and in a higher extend than the isolated compounds and fluconazole, an antifungal drug widely used in the clinic. The oils also disrupted preformed C. albicans biofilms. Furthermore, no toxicity was observed at pharmacological relevant concentrations towards keratinocytes. Our study validates the traditional uses ascribed to these Iberian species. Furthermore, it brings new insights on the antifungal potential and mechanism of action of these thyme species, thus paving the way for the development of novel effective antifungal drugs.
Keywords: Thymus camphoratus, Thymus carnosus, essential oil, germ tube, biofilm, cytotoxicity
Introduction
In the last years, the prevalence of fungal infections has increased significantly, especially in immunocompromised individuals, with concomitant life-threatening increments (Richardson and Lass-Flörl, 2008). Additionally, the epidemiology behind systemic fungal infections has changed. Indeed, bloodstream systemic infections (BSI) are mainly caused by Candida and Aspergillus species being C. albicans, C. glabrata, C. parapsilosis. C. krusei and C. tropicalis the most common infectiousagents for Candida-associated BSI, while A. fumigatus, A. niger, and A. flavus are responsible for the majority of Aspergillus-associated BSI (Pfaller et al., 2006). Regarding superficial mycoses, the major causal agents are dermatophytes that remain a health concern worldwide, in both immunocompromised and healthy individuals. This is mainly attributable to the resistance of fungi to conventional antifungal therapies as well as to high relapse rates (Gupta and Cooper, 2008).
For these reasons, fungal infections are still considered a serious problem worldwide that require effective therapeutic strategies. The most common antifungal agents used nowadays include fluconazole, amphotericin B and terbinafine. Nevertheless, these drugs have several reported adverse effects, such as nephrotoxicity and hepatotoxicity. Furthermore, they show a slow response in immunocompromised individuals (Khan and Ahmad, 2011) and have several therapeutic limitations, such as drug interactions, insufficient bioavailability and a fungistatic mechanism of action. In addition, development of resistance as well as innate resistance by emerging species have also been reported (Pfaller, 2012). Therefore, actual antifungal therapies are far from acceptable reinforcing the urgent need for the development of effective antifungal drugs.
Aromatic and medicinal plants, especially those belonging to Lamiaceae and Apiaceae families, have been used for centuries in traditional medicine for the treatment of several pathologies with many therapeutic effects being associated with the presence of volatile compounds, i.e., essential oils. In fact, these compounds have been described as bioactive agents, primarily due to their antifungal, antibacterial and anti-inflammatory properties (Cowan, 1999; Zuzarte et al., 2013; Alves-Silva et al., 2016). In many cases, the use of these plants/compounds is based on empiric knowledge. Thus, the spectrum of action as well as the underlying mechanisms of action are lacking and should be deeply explored.
The genus Thymus, one of the most relevant in the Lamiaceae family, includes ca. 215 species, many of them endemic to the Mediterranean region (Pirbalouti et al., 2015). Several thyme species are widely used in culinary as flavoring agents and show a pharmacological relevance in traditional medicine being used as spasmolytic, antiseptic, and expectorant agents (Stahl-Biskup, 2003; Pirbalouti et al., 2015). This genus is highly polymorphic regarding the chemical composition of its essential oils, which means that plants morphologically identical can chemically differ, allowing the identification of chemotypes. Indeed, several chemotypes have been identified, for example, in Thymus vulgaris (Thompson et al., 2003), Thymus zygis subsp. sylvestris and subsp. zygis, Thymus mastichina subsp. mastichina, and Thymus pulegioides (Figueiredo et al., 2008). The species T. vulgaris is one of the most polymorphic species within the genus (Thompson et al., 2003).
Plants from the genus Thymus are known for their antifungal properties (Figueiredo et al., 2008). Indeed T. vulgaris and T. zygis have a European Medicines Agency (EMA) monograph highlighting several effects including their antifungal ones (HMPC/EMA, 2010). Moreover, several thyme species are used in traditional medicine, namely T. zygis, T. mastichina, T. camphoratus, Thymus carnosus, and Thymus caespititius. These species are often employed for the treatment of infections and inflammation of the mouth, pharynx, skin, and genital tract as well as for the relief of rheumatism (Rivera and Obón, 1995; Carapeto, 2006; Proença da Cunha et al., 2007; Silva et al., 2011). Regarding T. camphoratus and T. carnosus, both species are traditionally used in Portugal for the treatment of infections of the respiratory tract (dos Santos, 2004; Camejo Rodrigues, 2007). Although some studies on their essential oil composition (Salgueiro et al., 1995, 1997) and biological effects have been reported (Faleiro et al., 2003; Dandlen et al., 2011; Zuzarte et al., 2018), the knowledge regarding the antifungal potential of T. camphoratus and T. carnosus remains limited. Therefore, the present study was designed to unveil the antifungal activity of these essential oils and their major compounds against yeasts and filamentous fungi and concomitantly to elucidate the mechanisms of action underlying their potential antifungal effect. Importantly, the putative cytotoxicity of the essential oils to human keratinocytes was also assessed since this is a crucial step for further incorporation of these compounds in topical pharmaceutical formulations.
Materials and Methods
Plant Material
Flowering aerial parts of T. camphoratus Hoffmanns. & Link (Lamiaceae) were collected in Lagos, Algarve, Portugal (N 37° 5′ 53″, W 8° 40′ 4″) whereas those of T. carnosus Boiss (Lamiaceae) were collected in Praia da Manta Rota, Algarve, Portugal (N 37° 9′ 49″, W 7° 31′ 7″), during Spring (June). Several plants were obtained to gather a representative sample. Voucher specimens were added to the Herbarium of the University of Coimbra (COI), with accession numbers L. Salgueiro 55 and L. Salgueiro 333 for T. camphoratus and T. carnosus, respectively. Species authenticity were confirmed by Dr. Jorge Paiva, a taxonomist at the University of Coimbra.
Essential Oil Isolation and Analysis
The essential oils were obtained by hydrodistillation in a Clevenger-type apparatus and analyzed by gas chromatography (GC) and gas chromatography/mass spectrometry (GC/MS) using fused silica capillary columns with two different stationary phases: SPB-1 and SupelcoWax-10, as described by Cavaleiro et al. (2004). Volatile compounds were identified according to their GC retention indices on both SPB-1 and SupelcoWax-10 columns as well as mass spectra. Retention indices, calculated by linear interpolation relative to retention times of C8–C23 of n-alkanes, were compared with those of authentic products included in the Faculty of Pharmacy, University of Coimbra laboratory database (Faculty of Pharmacy, University of Coimbra) and/or literature data (Stein, 2017). Acquired mass spectra were compared with reference spectra from the laboratory database, Wiley/NIST library and literature data (Joulain and König, 1998; Adams, 2007). Relative amounts of individual components were calculated based on GC raw data areas without FID response factor correction.
Antifungal Activity
Fungal Strains
The antifungal activity of the essential oils was evaluated against both reference and clinically isolated strains of several yeasts and filamentous fungi. Yeast strains included Candida albicans ATCC 10231, C. guilliermondii MAT23, C. krusei H9, C. parapsilosis ATCC 90018, C. tropicalis ATCC 13803, and Cryptococcus neoformans CECT 1078; Filamentous fungi namely dermatophyte and Aspergilllus strains were selected. Dermatophyte strains included Epidermophyton floccosum FF9, Microsporum canis FF1, M. gypseum CECT 2908, Trichophyton mentagrophytes FF7, T. mentagrophytes var. interdigitale CECT 2958, T. rubrum CECT 2794 and T. verrucosum CECT 2992 whereas Aspergillus strains were A. flavus F44, A. fumigatus ATCC 46645 and A. niger ATCC 16404.
The fungal isolates identified by standard microbiological methods were stored at –80°C on Sabouraud broth with 20% glycerol. Prior to testing, each isolate was inoculated on Sabouraud agar (SDA) to ensure optimal growth and purity.
Fungal Growth (MIC and MFC)
Broth macrodilution methods based on the Clinical and Laboratory Standards Institute (CLSI) reference protocols M27-A3 (CLSI, 2008b) and M38-A2 (CLSI, 2008a) for yeasts and filamentous fungi, respectively, were used to determine the minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC) of the essential oils and their major compounds (borneol, camphene, 1,8-cineole, α-pinene), as described previously (Zuzarte et al., 2013). MICs were recorded as the lowest concentration of the essential oil/compound that inhibited fungal growth and MFCs were determined as the lowest concentration of the essential oil/compound able to kill the fungal strain. Two reference antifungal compounds, amphotericin B (Fluka, Buchs, Switzerland) and fluconazole (Pfizer, New York, United States), were used to control the sensitivity of the tested microorganisms. The experiments were performed at least in triplicates and a range of values presented whenever the results were not in agreement between replicates.
Candida albicans Germ Tube Formation
To determine the effect of the essential oils and their major compounds on the yeast-mycelium transition, cell suspensions of C. albicans ATCC 10231, from overnight cultures on SDA, were prepared in NYP medium [N-acetylglucosamine (Sigma; 10−3 mol/L), Yeast Nitrogen Base (Difco; 3.35 g/L), proline (Fluka; 10−3 mol/L), NaCl (4.5 g/L), and pH 6.7 ± 0.1 (Marichal et al., 1986)]. The assay was then carried out as previously described by Pinto et al. (2013). Briefly, C. albicans suspension was adjusted to a density of 1.0 ± 0.2 × 106 CFU/mL and 990 μL of this suspension was distributed to test tubes. To each tube, 10 μL of sub-inhibitory concentrations of each essential oil and their major compounds were added and the cells were incubated without agitation for 3 h. Then, germ tube formation was registered under a light microscope (40×). Germ tubes were considered when the germinating protuberance was at least as long as the diameter of the blastopore. The conventional antifungal drug, fluconazole, was also tested and DMSO in a maximum concentration of 1% (v/v) was used as a control. The results are presented as the mean ± standard deviation (SD) of three independent experiments.
Fungal Viability (MTT Assay)
The effect of the essential oils on C. albicans ATCC 10231 viability was determined using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] reduction assay (Pinto et al., 2013) with slight modifications. Briefly, cell suspensions were prepared in RPMI and adjusted to a final density of 0.5–2.5 × 103 CFU/Ml. The suspensions were then distributed into 12-well plates (1 mL/well) and incubated, without agitation, at 37°C. After 18–24 h of incubation, the cells were carefully homogenized, collected and centrifuged at 10,000 rpm for 5 min. Essential oils (0.07–0.57 mg/mL) were added to the pellets in a final volume of 1 mL and then the solutions were transferred to the microwell plate. Following an incubation at 37°C for 1 h, cells were harvested by centrifugation at 10,000 rpm for 5 min and 500 μL of 0.5 mg/mL of MTT (Sigma–Aldrich, St. Louis, MO, United States) in RPMI was added. After an incubation at 37°C for 30 min, the water-insoluble blue formazan crystals (viable cells) were solubilized with 300 μL of DMSO and measured spectrophotometrically at 510 nm using an ELISA microplate reader (SLT, Austria). An additional trypan blue exclusion assay was also performed to confirm the number of living cells.
Biofilm Disruption
Candida albicans biofilm disruption assays were performed as previously reported (Taweechaisupapong et al., 2012). Briefly, a loop from a C. albicans culture in SDA grown for 24 h at 37°C was resuspended in Yeast Peptone Dextrose (YPD) broth (1% yeast extract, 2% peptone, and 2% dextrose) and further incubated for 24 h at 37°C. Then, cells were washed with PBS (pH 7.4) (0.8% NaCl, 0.02% KH2PO4, 0.31% Na2HPO4⋅12H2O, and 0.02% KCl). Cell density was adjusted to 1 × 106 CFU/mL, and then 100 μL of this suspension was added to 96-well microtiter plates and incubated for 24 h at 37°C to allow biofilm adhesion and formation. Then, the wells were washed 3 times with PBS and the essential oils (0.28–2.2 mg/mL, prepared in RPMI) were added and incubated for 24 h, at 37°C. Essential oil-free wells, with DMSO in a maximum concentration of 1% (v/v), and biofilm-free wells were also included as positive and negative controls, respectively. Biofilm mass was quantified using crystal violet according to Raut et al. (2013). Briefly, after treatments and medium removal, biofilms were fixed with 99% methanol for 15 min. The supernatant was removed, and the wells air-dried. Then, a solution of crystal violet (0.02%) was added to each well and allowed to stain the biofilm for 15 min. Wells were washed 2–3 times with sterile distilled water. Absorbed stain was released by addition of 150 μL of acetic acid (33%) and transferred to fresh wells. The optical density (OD) was read at 620 nm using a microplate reader. The percentage of biofilm formation was calculated by comparing the OD of treated biofilm with that of control biofilm. Biofilm viability was assessed according to the method described by Saharkhiz et al. (2012) with some modifications. Briefly, after the treatments, the medium was removed and 100 μL of a XTT (1 mg/mL in PBS) solution containing 4 μM of menadione (10 mM in acetone) was added to biofilms prewashed with PBS. After 2 h of incubation at 37°C in the dark, the absorbance was read at 490 nm. Biofilm viability was calculated by comparing the absorbance of treated samples with that of positive controls. Results are presented as mean ± SEM of three independent experiments performed in duplicate.
In vitro Cytotoxicity to Keratinocytes (MTT Assay)
The human keratinocytes cell line (HaCaT) was obtained from DKFZ (Heidelberg). Keratinocytes were cultured in Dulbecco’s Modified Eagle Medium (high glucose) supplemented with 100 μg/mL streptomycin and 100 U/mL penicillin, 3.02 g/L sodium bicarbonate and 10% (v/v) inactivated fetal bovine serum, at 37°C in a humidified atmosphere of 95% air and 5% CO2. Evaluation of cell viability was performed by the colorimetric MTT assay, as previously reported. Quantification of formazan crystals was performed using an ELISA microplate reader at 570 nm with a reference wavelength of 620 nm. The results were expressed as the percentage of MTT reduction relatively to control cells.
Statistical Analysis
Data are expressed as mean ± standard error (SEM) of the mean. Statistical significance was determined using one-way analysis of variance (ANOVA), followed by Dunnett’s post hoc test. The statistical analysis was performed using Prism 5.0 Software (GraphPad Software). Differences were considered significant for p < 0.05.
Results
Essential Oil Composition
Thymus camphoratus and T. carnosus essential oils were obtained with yields of 1.4 and 1.8%, respectively (v/w). The identified volatile compounds accounted for a total amount of 92.5 and 90.1% of the oil for T. camphoratus and T. carnosus, respectively (Table 1). Both essential oils were characterized by high amounts of oxygen-containing monoterpenes being T. carnosus also rich in monoterpene hydrocarbons. The essential oil from T. camphoratus was characterized by high amounts of 1,8-cineole (15.5%), α-pinene (12.7%), borneol (8.5%), and camphene (6.6%; Table 1 and Supplementary Figure 1A). T. carnosus essential oil was mostly rich in borneol (29.0%), camphene (19.5%), and α-pinene (8.9%; Table 1 and Supplementary Figure 1B).
Table 1.
Essential oil composition of two Iberian Thymus spp.
| RI† | RI‡ | Ref RIx | Ref RI# | Compound | Essential oils |
|
|---|---|---|---|---|---|---|
| Thymus camphoratus | Thymus carnosus | |||||
| 922 | 1030 | 922 | 1030 | α-Thujene | 0.5 | 1.7 |
| 928 | 1025 | 930 | 1028 | α-Pinene | 12.7 | 8.9 |
| 941 | 1072 | 943 | 1073 | Camphene | 6.6 | 19.5 |
| 959 | 1447 | 959 | 1447 | Oct-1-en-3-ol | – | t |
| 962 | 1253 | 962 | 1253 | Octan-3-one | – | t |
| 963 | 1124 | 964 | 1126 | Sabinene | 1.9 | 1.4 |
| 968 | 1115 | 969 | 1116 | β-Pinene | 0.7 | 2.1 |
| 980 | 1159 | 980 | 1161 | Myrcene | 0.2 | 0.1 |
| 997 | 1167 | 997 | 1168 | α-Phellandrene | 0.3 | 0.1 |
| 1008 | 1187 | 1010 | 1187 | α-Terpinene | 0.6 | 0.2 |
| 1011 | 1275 | 1011 | 1273 | p-Cymene | 0.3 | 3.5 |
| 1019 | 1204 | 1020 | 1205 | Limonene | 1.5 | 1.3 |
| 1020 | 1215 | 1020 | 1214 | β-Phellandrene | – | 0.1 |
| 1020 | 1212 | 1020 | 1214 | 1,8-Cineole | 15.5 | 0.4 |
| 1025 | 1235 | 1025 | 1235 | Z-Ocimene | 0.1 | 0.1 |
| 1035 | 1253 | 1035 | 1253 | E-Ocimene | 0.9 | 0.1 |
| 1046 | 1246 | 1046 | 1248 | γ-Terpinene | 0.3 | 0.9 |
| 1050 | 1459 | 1051 | 1459 | E-Sabinene hydrate | 0.2 | 0.3 |
| 1055 | 1438 | 1055 | 1439 | cis-Linalool oxide | 0.2 | – |
| 1070 | 1466 | 1070 | 1466 | trans-Linalool oxide | 0.2 | – |
| 1077 | 1284 | 1077 | 1288 | Terpinolene | 0.2 | 0.1 |
| 1082 | 1542 | 1080 | 1544 | Z-Sabinene hydrate | 0.6 | 2.1 |
| 1084 | 1539 | 1083 | 1542 | Linalool | 5.0 | 0.2 |
| 1106 | 1488 | 1104 | 1487 | α-Campholenal | 0.4 | 0.2 |
| 1109 | n.d. | 1106 | – | Z-p-Menth-2-en-1-ol | t | – |
| 1119 | 1514 | 1118 | 1515 | Camphor | 4.9 | 3.5 |
| 1122 | 1645 | 1119 | 1649 | E-Pinocarveol | 0.6 | 0.8 |
| 1123 | 1645 | 1122 | 1648 | Z-Verbenol | 0.1 | – |
| 1129 | 1668 | 1126 | 1672 | E-Verbenol | 1.1 | 1.1 |
| 1136 | 1564 | 1135 | 1562 | Pinocarvone | 0.3 | – |
| 1145 | 1691 | 1146 | 1695 | Borneol | 8.5 | 29.0 |
| 1158 | 1845 | 1157 | 1845 | p-Cymene-8-ol | 0.3 | 0.2 |
| 1160 | 1594 | 1158 | 1595 | Terpinene-4-ol | 4.3 | 4.9 |
| 1164 | 1622 | 1165 | 1621 | Myrtenal | 0.3 | – |
| 1167 | 1602 | 1167 | 1602 | Dihydrocarvone | – | 0.5 |
| 1171 | 1687 | 1169 | 1692 | α-Terpineol | 0.9 | 0.5 |
| 1177 | 1698 | 1177 | 1698 | Verbenone | t | 0.1 |
| 1178 | 1780 | 1177 | 1786 | Myrtenol | 0.3 | – |
| 1191 | 1830 | 1192 | 1830 | E-Carveol | 0.4 | t |
| 1211 | 1728 | 1711 | 1727 | Carvone | 0.2 | – |
| 1214 | 1679 | 1214 | 1679 | Neral | – | 0.1 |
| 1233 | 1842 | 1233 | 1842 | Geraniol | 0.3 | t |
| 1264 | 1580 | 1264 | 1578 | Bornyl acetate | 3.5 | 5.2 |
| 1268 | 2183 | 1267 | 2183 | Thymol | – | 0.2 |
| 1275 | 2212 | 1275 | 2212 | Carvacrol | – | 0.1 |
| 1324 | 2159 | 1325 | 2163 | Eugenol | t | – |
| 1329 | 1688 | 1328 | 1688 | α-Terpinyl acetate | 0.1 | 0.2 |
| 1342 | 1455 | 1342 | 1455 | α-Cubebene | 0.2 | – |
| 1359 | 1755 | 1359 | 1755 | Geranyl acetate | 0.8 | – |
| 1376 | 1517 | 1375 | 1517 | β-Bourbonene | 0.1 | t |
| 1380 | 1536 | 1380 | 1536 | β-Cubebene | 0.1 | – |
| 1401 | 1526 | 1401 | 1524 | α-Gurjunene | – | 0.1 |
| 1407 | 1591 | 1407 | 1590 | E-caryophyllene | 1.0 | 0.8 |
| 1427 | 1160 | 1428 | 1602 | Aromadendrene | 0.4 | t |
| 1442 | 1665 | 1442 | 1664 | α-Humulene | – | t |
| 1447 | 1636 | 1447 | 1637 | Alloaromadendrene | – | 0.1 |
| 1466 | 1699 | 1466 | 1699 | Germacrene D | 3.6 | 0.1 |
| 1482 | 1726 | 1482 | 1726 | Bicyclogermacrene | 0.2 | – |
| 1495 | 1723 | 1495 | 1723 | β-Bisabolene | 0.1 | – |
| 1502 | 1752 | 1498 | 1752 | γ-Cadinene | 1.4 | 0.1 |
| 1508 | 1751 | 1508 | 1752 | δ-Cadinene | 1.1 | – |
| 1529 | 1768 | 1531 | 1767 | α-Bisabolene | 0.5 | – |
| 1553 | 2113 | 1552 | 2113 | Spathulenol | – | t |
| 1559 | 1971 | 1558 | 1968 | Caryophyllene oxide | 1.0 | 0.6 |
| 1569 | 2072 | 1598 | 2073 | Viridiflorol | – | 0.2 |
| 1579 | 2025 | 1582 | 2024 | Ledol | – | 0.1 |
| 1594 | 2089 | 2596 | 2091 | 10-epi-γ-Eudesmol | 0.5 | t |
| 1607 | 2158 | 1606 | 2158 | γ-Eudesmol | – | 0.1 |
| 1615 | 2153 | 1615 | 2160 | τ-Cadinol | 2.7 | – |
| 1618 | 2188 | 1615 | 2176 | α-Muurolol | 0.2 | – |
| 1618 | 2043 | 1620 | 2052 | Cubenol | 0.3 | – |
| 1622 | 2215 | 1622 | 2215 | β-Eudesmol | t | t |
| 1628 | 2208 | 1622 | 2208 | α-Eudesmol | – | 0.1 |
| 1628 | 2218 | 1628 | 2221 | α-Cadinol | t | – |
| 1659 | 2210 | 1659 | 2209 | α-Bisabolol | t | – |
| Monoterpene hydrocarbons | 26.8 | 40.1 | ||||
| Oxygen containing monoterpenes | 49.1 | 49.7 | ||||
| Sesquiterpene hydrocarbons | 8.7 | 1.4 | ||||
| Oxygen containing sesquiterpenes | 5.4 | 1.2 | ||||
| Others | 0.1 | 0.1 | ||||
| Total identified | 92.5 | 90.1 | ||||
Compounds listed in order to their elution on the SPB-1 column; t = traces ≤0.05%. †Experimental retention indices on the SPB-1 column relative to C8–C24 n-alkanes. ‡Experimental retention indices on the SupelcoWax-10 column relative to C8–C24 n-alkanes. xReference retention indices for a polydimethylsiloxane column (Stein, 2017). #Reference retention indices for a polyethylene glycol column (Stein, 2017).
Antifungal Activity
Fungal Growth (MIC and MFC)
The antifungal activity (MICs and MFCs) of both essential oils and their main isolated compounds is presented in Table 2. Overall, both oils were more effective against C. neoformans with MIC values of 0.14 mg/mL and showed poor antifungal activity against the tested Aspergillus strains. For Candida spp., the essential oils of both species showed a slight fungicidal effect for most of the strains. For dermatophytes, both oils showed a fungicidal effect, but T. camphoratus was more effective than T. carnosus, presenting lower MIC values for most of the tested strains (Table 2).
Table 2.
Antifungal activity of Thymus camphoratus and Thymus carnosus essential oils and their major compounds against pathogenic yeasts, dermatophytes and Aspergillus strains.
|
T. camphoratus |
T. carnosus |
Borneol |
Camphene |
α-Pinene |
1,8-Cineole |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | MIC† | MFC† | MIC† | MFC† | MIC† | MFC† | MIC† | MFC† | MIC† | MFC† | MIC† | MFC† |
| Candida albicans ATCC 10231 | 1.11–2.23 | 1.11–2.23 | 1.11 | 1.11 | 2.23 | >17.8 | >17.8 | >17.8 | 0.57–1.11 | 0.57–1.11 | 8.9 | 8.9 |
| Candida guilliermondii MAT23 | 2.23 | 2.23 | 1.11 | 1.11 | 2.23 | >17.8 | >17.8 | >17.8 | 1.11 | 1.11–2.23 | 17.8 | 17.8 |
| Candida krusei H9 | 2.23 | 2.23 | 2.23 | 2.23 | 4.5 | >17.8 | >8.9 | >17.8 | 0.14–0.28 | 0.28 | 8.9 | 8.9 |
| Candida parapsilosis ATCC 90018 | 1.11 | 2.23 | 2.23 | 4.5 | 2.23 | 2.23 | >8.9 | >8.9 | 0.57 | 0.57 | 8.9 | 8.9 |
| Candida tropicalis ATCC 13803 | 4.5 | 4.5 | 2.23 | 2.23 | 4.5 | >17.8 | >17.8 | >17.8 | 0.28 | 0.28 | 8.9 | 8.9 |
| Cryptococcus neoformans CECT 1078 | 0.14 | 0.28 | 0.14 | 0.28 | 1.11 | 11.1 | 4.5 | 4.5 | 0.07 | 0.28 | 4.5–8.9 | 8.9 |
| Epidermophyton floccosum FF9 | 0.57 | 0.57 | 1.11 | 1.11 | 2.23 | 2.23 | 4.5 | 4.5 | 0.14 | 0.14 | 4.5 | 4.5 |
| Microsporum canis FF1 | 0.57 | 0.57 | 1.11 | 1.11 | 2.23 | 2.23 | 4.5 | 4.5 | 0.14 | 0.14–0.28 | 4.5 | 4.5 |
| Microsporum gypseum CECT 2908 | 0.57 | 0.57–1.11 | 2.23 | 2.23 | 2.23 | 2.23 | 8.9 | 8.9 | 0.14 | 0.14 | 4.5–8.9 | 4.5–8.9 |
| Trichophyton mentagrophytes FF7 | 0.57 | 0.57 | 1.11 | 1.11 | 2.23 | 4.5 | 4.5 | 4.5–8.9 | 0.28 | 0.28–0.57 | 4.5 | 4.5 |
| Trichophyton mentagrophytes var. interdigitale CECT 2958 | 1.11 | 1.11 | 1.11 | 2.23 | 2.23 | 4.5 | 8.9–17.8 | 8.9–17.8 | 0.28 | 0.28 | 8.9 | 8.9 |
| Trichophyton verrucosum CECT 2992 | 1.11 | 1.11 | 2.23 | 2.23 | 2.23 | 2.23 | 17.8 | 17.8 | 1.11 | 1.11 | 8.9 | 8.9–17.8 |
| Trichophyton rubrum CECT 2794 | 0.57 | 0.57 | 0.57 | 1.11 | 2.23 | 2.23 | 2.23–4.5 | 4.5 | 0.07 | 0.07 | 2.23–4.5 | 4.5 |
| Aspergillus flavus F44 | 4.5 | >8.9 | 4.5 | >8.9 | 4.5 | >17.8 | >17.8 | >17.8 | 1.11 | 1.11 | 17.8 | 17.8 |
| Aspergillus fumigatus ATCC 46645 | 2.23 | >8.9 | 2.23 | 8.9 | 2.23 | >17.8 | >17.8 | >17.8 | 1.1 | 1.11–223 | 8.9 | 8.9–17.8 |
| Aspergillus niger ATCC 16404 | 2.23 | >8.9 | 2.23 | >17.8 | 4.5 | >17.8 | >17.8 | >17.8 | 2.23 | 4.5 | 8.9 | >17.8 |
†MIC and MFC were determined by a macrodilution method and expressed in mg/mL. Results were obtained from 3 independent experiments performed in duplicate.
Regarding the antifungal activity of the isolated compounds, α-pinene was, by far, the most effective compound (Table 2). Indeed, α-pinene showed a fungicidal effect for most of the tested strains, being very effective against C. neoformans and T. rubrum (MIC = 0.07 mg/mL). Contrarily, 1,8-cineole and camphene were ineffective against many of the tested strains, while borneol showed a slight antifungal effect (MIC ranging from 1.11 to 4.5 mg/mL; Table 2).
Germ Tube Inhibition in Candida albicans
Since the yeast-to-hypha transition is an important virulence factor associated with the pathogenesis of C. albicans infections, the effect of the oils on the germ tube formation in C. albicans ATCC 10231 was assessed. As shown in Table 3, both essential oils inhibited the germ tube formation at concentrations well below their respective MIC, with T. camphoratus being more effective and attaining ca. 40% inhibition at 0.07 mg/mL (MIC/16). Fluconazole (MIC = 0.001 mg/mL), the antifungal drug of choice in the clinic for the management of candidiasis, failed to inhibit this feature even at concentrations much higher than its MIC. Indeed, even at 0.2 mg/mL, that corresponds to fluconazole’s MIC×200 (Table 4), the antifungal drug was completely ineffective.
Table 3.
Effect of sub-inhibitory concentrations of the essential oil of Thymus camphoratus, Thymus carnosus and their major compounds on the germ tube formation of Candida albicans ATCC 10231.
| T. camphoratus | T. carnosus | α-Pinene | Borneol | 1,8-Cineole | Camphene | |
|---|---|---|---|---|---|---|
| Control† | 100 | 100 | 100 | 100 | 100 | 100 |
| 0.02‡ | 91.7 ± 3.0∗∗ | 98.1 ± 1.7 | – | – | – | – |
| 0.04 | 81.9 ± 1.2∗∗∗∗ | 90.3 ± 2.2 | 93.2 ± 0.8 | 93.1 ± 2.3 | – | – |
| 0.07 | 62.0 ± 2.1∗∗∗∗ | 79.7 ± 2.8∗∗∗ | 86.3 ± 0.7∗ | 89.2 ± 2.5 | – | – |
| 0.14 | 43.1 ± 5.3∗∗∗∗ | 47.1 ± 4.9∗∗∗∗ | 55.7 ± 7.0∗∗∗∗ | 76.6 ± 4.3∗∗∗∗ | – | – |
| 0.28 | 11.3 ± 2.2∗∗∗∗ | 7.3 ± 2.6∗∗∗∗ | 4.9 ± 1.8∗∗∗∗ | 58.7 ± 4.0∗∗∗∗ | 85.7 ± 7.1 | 91.8 ± 0.5 |
| 0.57 | 0.0 ± 0.0∗∗∗∗ | 0.0 ± 0.0∗∗∗∗ | 0.0 ± 0.0∗∗∗∗ | 29.6 ± 2.1∗∗∗∗ | 66.7 ± 1.2∗∗ | 74.5 ± 7.3 |
| 1.11 | – | – | – | 4.5 ± 0.9∗∗∗∗ | 29.4 ± 3.2∗∗∗ | 48.4 ± 12.9∗ |
| 2.23 | – | – | – | – | 1.2 ± 0.1∗∗∗∗ | 10.0 ± 6.6∗∗∗∗ |
| 4.5 | – | – | – | – | – | 1.9 ± 1.9∗∗∗∗ |
| 8.9 | – | – | – | – | – | 0.0 ± 0.0∗∗∗∗ |
†Samples without essential oil, with 1% (v/v) DMSO. ‡Absolute concentration of essential oil in mg/mL. Results are means ± SEM of a minimum of three independent experiments; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, compared to control (100%).
Table 4.
Effect of sub-inhibitory concentrations of fluconazole on the germ tube formation of Candida albicans ATCC 10231.
| Fluconazole | |
|---|---|
| Control† | 100 |
| 0.200‡ | 89.10 ± 5.09 |
| 0.128 | 90.04 ± 9.94 |
| 0.004 | 98.86 ± 6.07 |
| 0.002 | 100.53 ± 7.81 |
| 0.001 | 101.00 ± 5.27 |
†Sample without fluconazole, with 1% (v/v) DMSO. ‡Absolute concentration of fluconazole in mg/mL. Results are means ± SEM of a minimum of three independent experiments.
The effect of the isolated major compounds, namely borneol, camphene, 1,8-cineole and α-pinene was also considered to assess which of these compounds could contribute to the activity of the oils. Our results show that both essential oils have a stronger effect on the inhibition of the germ tube formation in comparison to the isolated major compounds. Remarkably, although α-pinene showed lower MIC and MFC values than the essential oil (Table 2), our results demonstrate that both essential oils affect C. albicans germ tube formation at lower concentrations than α-pinene (Table 3).
Candida albicans Viability
The effect of the essential oils on C. albicans viability was also assessed (Figure 1). Once again, our results show that both oils inhibited C. albicans mitochondrial activity at concentrations lower than their respective MIC. Nevertheless, T. carnosus essential oil showed better results since it significantly reduced C. albicans mitochondrial activity at concentrations ranging from 0.07 to 0.57 mg/mL. Indeed, a viability decrease of more than 70% was observed at 0.28 mg/mL for T. carnosus essential oil whereas T. camphoratus oil decreased cell viability of the yeast only ca. 44% at 0.57 mg/mL and at lower concentrations (≤0.28 mg/mL) was ineffective (Figure 1). A trypan blue exclusion assay, that assesses the integrity of the cell wall, showed 100% of living fungi cells in all the tested concentrations (data not shown), thus confirming the adverse effect on the essential oils directly on mitochondrial activity.
FIGURE 1.
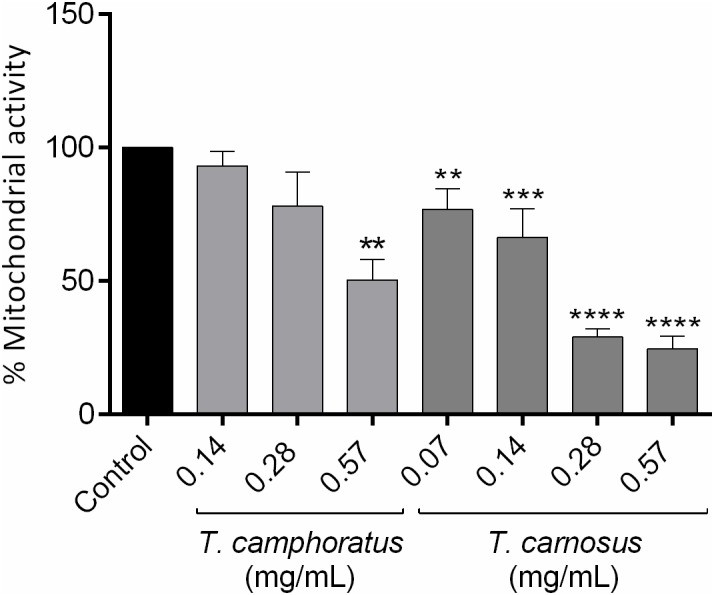
Viability of C. albicans ATCC 10231 cells treated with different concentrations of Thymus camphoratus and Thymus carnosus essential oils. One-way ANOVA was performed separately for each species and values are expressed as % of mitochondrial activity relative to control (mean ± SEM of three independent assays). ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, compared to control.
Disruption of Preformed Biofilms in Candida albicans
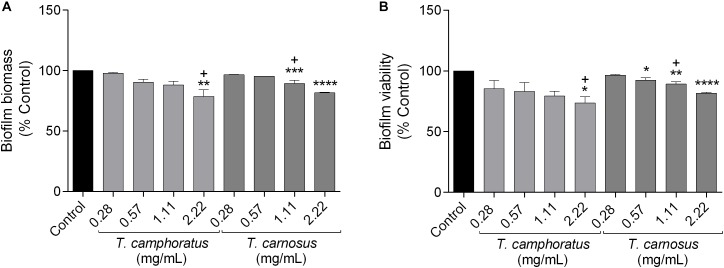
Bearing in mind that C. albicans biofilms are also important virulence factors, the capacity of the essential oils to decrease both biofilm mass (Figure 2A) and biofilm viability (Figure 2B) were also assessed. Overall, both oils were effective at concentrations close to their respective MIC (Figure 2), with T. carnosus essential oil being slightly more active in reducing biofilm viability since it decreased this feature at MIC/2 (0.57 mg/mL; Figure 2B). Interestingly, fluconazole was ineffective in decreasing biofilm biomass showing a slight effect in biofilm viability reduction at concentrations much higher than its MIC (MIC×128; data not shown).
FIGURE 2.
Candida albicans ATCC 10231 biofilm biomass (A) and biofilm viability (B). Cells were treated with different concentrations of Thymus camphoratus and Thymus carnosus essential oil. Biofilm mass was quantified by the crystal violet assay and biofilm viability assessed using the XTT assay. One-way ANOVA was performed separately for each species and values are expressed as a percentage of biofilm biomass or viability relative to control (mean ± SEM of three independent assays). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, compared to control. “+” indicates MIC values of the essential oils.
Cytotoxicity of the Essential Oils
Thymus carnosus essential oil was slightly more toxic (IC50 = 0.705 mg/mL) than T. camphoratus oil (IC50 = 0.848 mg/mL). Nevertheless, at bioactive concentrations, against the majority of the dermatophyte strains, as well as at concentrations that inhibited C. albicans germ tube formation, no toxicity was observed, thus pointing out a safety profile for a further development of topical formulations (Table 5). Additionally, at lower concentrations (below 0.28 mg/mL) both essential oils increased cell viability (Table 5). This could be due to an increase in keratinocytes proliferation or due to the stimulation of their mitochondrial activity and therefore, additional studies should be conducted to further understand this event.
Table 5.
Effect of Thymus camphoratus and Thymus carnosus essential oils on keratinocytes viability.
| Essential oil | Concentration (mg/mL) | Keratinocytes HaCaT (% of control) |
|---|---|---|
| Thymus camphoratus (IC50 = 0.953 mg/mL) | 1.11 | 54.03 ± 8.42 |
| 0.57 | 96.81 ± 4.91 | |
| 0.28 | 127.2 ± 25.62 | |
| 0.14 | 146.8 ± 29.70 | |
| 0.07 | 134.6 ± 20.33 | |
| Thymus carnosus (IC50 = 0.793 mg/mL) | 1.11 | 44.05 ± 4.48∗ |
| 0.57 | 82.56 ± 1.31 | |
| 0.28 | 103.2 ± 18.94 | |
| 0.14 | 107.4 ± 13.71 | |
| 0.07 | 122.6 ± 21.88 |
Results are expressed as percentage of MTT reduction relatively to control cells maintained in culture medium. Each value represents mean ± SEM of at least three independent experiments performed in duplicate. Statistical differences compared to control cells (100%). ∗p < 0.05, using a one-way ANOVA followed by a Dunnett’s multiple comparison test.
Discussion
Previous studies on the chemical composition of T. camphoratus and T. carnosus essential oils reported some chemical variability,as frequently shown for other thyme species. For example, Salgueiro et al. (1997) studied different populations of T. camphoratus collected in Algarve and Baixo Alentejo (south of Portugal) and showed a high chemical variability, particularly among individual samples or small populations, being α-pinene (7.0–11.0%), borneol (1.0–24.0%), and 1,8-cineole (3.9–20.0%) the major compounds identified. In another study, the essential oils from both flowers and leaves of T. camphoratus collected in Algarve, Portugal, showed very high amounts of 1,8-cineole, 56.7 and 62.7% for flowers and leaves, respectively (Faleiro et al., 2003). Similarly, for T. carnosus of different regions of Algarve and Baixo Alentejo, Portugal, a chemical variability was observed between the samples. Nevertheless, most of the essential oils were characterized by high amounts of borneol (23.0–32.0%), camphene (6.0–13.0%), terpinen-4-ol (4.0–12.2%), and cis-sabinene hydrate (2.5–10.7%) (Salgueiro et al., 1995). In our study, plants growing in large populations of the Algarve region (Portugal) were collected for essential oil extraction, therefore, the chemical profile described is the most prevalent in T. carnosus and T. camphoratus growing in the south of Portugal. Furthermore, Stahl-Biskup (Stahl-Biskup, 2003) described that thymol and carvacrol, both terpene phenols, as well as their biogenetic derivatives are important compounds in the genus Thymus, followed by linalool, borneol, terpinen-4-ol, and 1,8-cineole. Indeed, thymes rich in 1,8-cineole, such as T. mastichina have a very high industrial interest, having an ISO monograph (ISO 4728:2003, 2015), thus highlighting the potential industrial interest for the species herein described.
Studies on the antifungal activity of T. camphoratus and T. carnosus essential oils are scarce and based upon different methodologies. Indeed, previous studies using the disk diffusion method, showed a weak or even no inhibitory effect of both oils on C. albicans (Faleiro et al., 2003; Dandlen et al., 2011). Regarding the antifungal potential of other thyme species with high amounts of 1,8-cineole, some studies have been reported. For example, the essential oils of T. mastichina and T. capitellatus (Pina-Vaz et al., 2004; Salgueiro et al., 2006) showed a weaker antifungal effect against several yeasts, dermatophytes and Aspergillus strains compared with the oils tested in the present study. In opposition, the essential oil from T. herba-barona (Zuzarte et al., 2013), T. zygis subsp. sylvestris (Pina-Vaz et al., 2004), T. zygis subsp. zygis (Gonçalves et al., 2010), and T. vulgaris (Pina-Vaz et al., 2004) showed a more preeminent activity due to their high content in phenolic terpenoids, namely thymol and/or carvacrol. In the present study, the antifungal effect of the essential oils could be mainly attributed to α-pinene since it was found in considerable amounts in both oils. Nevertheless, active minor compounds may also contribute to the activity of the essential oil. For example, linalool, cadinol, and terpinen-4-ol, all minor compounds of both T. carnosus and T. camphoratus essential oils, have also been widely described as antifungal agents (Pinto et al., 2013; Su and Ho, 2016) and an anti-yeast addictive effect has been reported for α-pinene and limonene (Tserennadmid et al., 2011).
To the best of our knowledge, the present study is the first report on the effect of T. camphoratus and T. carnosus essential oils on C. albicans germ tube formation. Assessing this feature is of great relevance since the yeast-to-hypha transition represents the main virulence factor associated with candidosis. Also, filamentation is essential for the development of robust biofilms, another major virulence factor associated with the pathogenesis of C. albicans (Romo et al., 2017). Indeed, fluconazole, the main antifungal used in the clinic, is ineffective on germ tube inhibition. Our findings show that very low doses of the essential oils are able to inhibit filamentation in C. albicans, being more effective than the isolated compounds tested. The stronger effect of the essential oils may be explained by the presence of active minor compounds. Indeed, linalool, a minor compound in both oils has been described as an effective natural compound for inhibiting germ tube formation in C. albicans (Hsu et al., 2013). Regarding other thyme species, few studies have demonstrated inhibitory effects, but with variable results between species. Interestingly, although phenolic thyme essential oils, such as those from T. vulgaris and T. zygis, show potent antifungal activities they are much less effective in inhibiting C. albicans germ tube formation than T. camphoratus and T. carnosus oils. Indeed T. vulgaris and T. zygis essential oils were only able to achieve significant inhibitions of filamentation (over 50%) at MIC values (0.16–0.32 μL/mL) (Pina-Vaz et al., 2004), whereas T. camphoratus essential oil attained about 40% of inhibition at much lower concentrations (0.07 mg/mL) and at MIC/4, ca. 90% of inhibition was observed (Table 3).
Regarding C. albicans viability, once again, as far as it is known, the present study is the first to report the effect of T. camphoratus and T. carnosus oils on C. albicans mitochondrial activity. Previously, other thyme essential oils were tested, namely T. villosus subsp. lusitanicus. Nevertheless, contrarily to our results, only detrimental effects were observed at concentrations close to the respective MIC (Pinto et al., 2013). Moreover, both T. camphoratus and T. carnosus essential oils were effective in disrupting C. albicans biofilms, being T. carnosus oil slightly more active in reducing yeast viability. It is well known that, similarly to bacteria, fungi in biofilms show a higher resistance toward antifungals than planktonic fungi, due to the expression of several resistance genes and phenotypic modifications. Indeed, C. albicans biofilms have been reported as highly resistant to fluconazole (Uppuluri et al., 2011). Therefore, the disruption of this highly organized system is a very attractive target for the development of effective antifungals (Alves-Silva et al., 2016). It is important to emphasize that studies evaluating the effect of thyme essential oils on preformed biofilms are lacking. Nevertheless, regarding isolated compounds, several terpenes found in T. carnosus and T. camphoratus essential oil have been previously tested. Some compounds such as 1,8-cineole showed an inhibitory effect at high concentrations, 8 mg/mL (Hendry et al., 2009). Moreover, several other compounds showed an inhibitory effect on the biofilm formation, namely camphor, camphene, and borneol (Manoharan et al., 2017) as well as α-pinene (Silva et al., 2012). Furthermore, linalool, a minor compound found in both oils, was able to disrupt preformed C. albicans biofilms (Hsu et al., 2013; Raut et al., 2013) and terpinen-4-ol was able to disrupt preformed single and dual-species Candida biofilms in a dose-dependent manner (Francisconi et al., 2015).
Taken together these results show that T. camphoratus and T. carnosus essential oils have anti-virulent potential, thus preventing disseminative candidosis.
Bearing in mind a future topical application that is more amenable and shows fewer side effects than oral or intranasal applications, the effect of the essential oil on keratinocytes was assessed, with no toxicity observed at pharmacological relevant concentrations.
Conclusion
This study highlights the antifungal potential of two Iberian endemic thyme species: T. camphoratus and T. carnosus. Of relevance, the results demonstrated that both oils were able to inhibit the growth of Cryptococcus neoformans and several dermatophyte strains. Moreover, the oils were very effective in inhibiting C. albicans germ tube formation at concentrations well below the MIC and in a much higher extend than fluconazole, an antifungal drug widely used in the clinic. Finally, T. carnosus oil was more effective in decreasing yeast mitochondrial activity and disrupting preformed biofilms in C. albicans. Overall, our results point out promising anti-virulent effects for these oils, foreseeing a potential application in the management of disseminative candidiasis.
Our findings add relevant information to the pharmacological activity of these species concomitantly providing new insights to their mechanism of action and reinforcing the use of thyme plants as topical antiseptics and disinfectants, since toxicity towards keratinocytes was absent at most of the bioactive concentrations. In addition to the relevant antifungal activity, both thymes have a very good essential oil yield, which increases their commercial interest and industrial potential. Overall, our results raise awareness on species poorly recognized and valued, thus paving the way for a better industrial exploitation of these plants namely in the pharmaceutical field.
Author Contributions
MA and MG performed the experiments that originated this work and analyzed the data. MZ and JA-S made the literature review and wrote the manuscript. CC carried out the chemical characterization of the essential oils. CC and MC reviewed the manuscript. LS supervised the work and reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Dr. Eugenia Carvalho (Centre for Neuroscience and Cell Biology, University of Coimbra, Portugal) for kindly providing the human keratinocytes cell line (HaCaT).
Footnotes
Funding. This work was supported by Fundação para a Ciência e a Tecnologia (FCT) UID/EQU/00102/2019.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00446/full#supplementary-material
References
- Adams R. P. (2007). Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. Illinois, IL: Allured Publishing Corporation, Carol Stream. [Google Scholar]
- Alves-Silva J. M., Zuzarte M., Gonçalves M. J., Cavaleiro C., Cruz M. T., Cardoso S. M., et al. (2016). New claims for wild carrot (Daucus carota subsp. carota) essential oil. Evid. Based Complement. Altern. Med. 2016 1–10. 10.1155/2016/9045196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camejo Rodrigues J. S. (2007). Plantas e Usos Medicinais Populares: Concelhos de Aljezur, Lagos e Vila do Bispo. Bordeira: Associação Aflosul. [Google Scholar]
- Carapeto A. (2006). “Levantamento etnobotânico na reserva natural do sapal de castro marim e vila real de santo antónio,” in Proceedings of the Relatório final do Projeto AGRO n.o 800 “Rede Nacional para a Conservação e Utilização das Plantas Aromáticas e Medicinais, (Castro Marim: ). [Google Scholar]
- Cavaleiro C., Salgueiro L. R., Miguel M. G., da Cunha A. P. (2004). Analysis by gas chromatography-mass spectrometry of the volatile components of Teucrium lusitanicum and Teucrium algarbiensis. J. Chromatogr. A 1033 187–190. 10.1016/j.chroma.2004.01.005 [DOI] [PubMed] [Google Scholar]
- CLSI (2008a). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard M38-A2, 2nd Edn. Wayne, PA: CLSI. [Google Scholar]
- CLSI (2008b). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard M27-A3, 3rd Edn. Wayne, PA: CLSI. [Google Scholar]
- Cowan M. M. (1999). Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12 564–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandlen S. A., Lima A. S., Mendes M. D., Miguel M. G., Faleiro M. L., Sousa M. J., et al. (2011). Antimicrobial activity, cytotoxicity and intracellular growth inhibition of portuguese Thymus essential oils. Rev. Bras. Farmacogn. 21 1012–1024. 10.1590/S0102-695X2011005000155 [DOI] [Google Scholar]
- dos Santos S. I. A. (2004). Plantas Medicinais da Península de Setúbal. Contribuição Para o Conhecimento da Sua Relevância Etnobotânica. [Google Scholar]
- Faleiro M. L., Miguel M. G., Ladeiro F., Venancio F., Tavares R., Brito J. C., et al. (2003). Antimicrobial activity of essential oils isolated from portuguese endemic species of Thymus. Lett. Appl. Microbiol. 36 35–40. 10.1046/j.1472-765X.2003.01259.x [DOI] [PubMed] [Google Scholar]
- Figueiredo A. C., Barroso J., Pedro L., Salgueiro L., Miguel M., Faleiro M. (2008). Portuguese thymbra and Thymus species volatiles: chemical composition and biological activities. Curr. Pharm. Des. 14 3120–3140. 10.2174/138161208786404218 [DOI] [PubMed] [Google Scholar]
- Francisconi R. S., Bordini E. A. F., Nogueira M. N. M., Fontana A., Bedran T. B. L., Correia M. F., et al. (2015). Effect of melaleuca alternifolia and its components on candida albicans and candida tropicalis. J. U.S.China Med. Sci. 12 91–98. 10.17265/1548-6648/2015.03.001 [DOI] [Google Scholar]
- Gonçalves M. J., Cruz M. T., Cavaleiro C., Lopes M. C., Salgueiro L. (2010). Chemical, antifungal and cytotoxic evaluation of the essential oil of Thymus zygis subsp. sylvestris. Ind. Crops Prod. 32 70–75. 10.1016/j.indcrop.2010.03.005 [DOI] [Google Scholar]
- Gupta A. K., Cooper E. A. (2008). Update in antifungal therapy of dermatophytosis. Mycopathologia 166 353–367. 10.1007/s11046-008-9109-0 [DOI] [PubMed] [Google Scholar]
- Hendry E. R., Worthington T., Conway B. R., Lambert P. A. (2009). Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 64 1219–1225. 10.1093/jac/dkp362 [DOI] [PubMed] [Google Scholar]
- HMPC/EMA (2010). Thymi Aetheroleum - Monograph. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2010/12/WC500100055.pdf (accessed March 8, 2018) [Google Scholar]
- Hsu C.-C., Lai W.-L., Chuang K.-C., Lee M.-H., Tsai Y.-C. (2013). The inhibitory activity of linalool against the filamentous growth and biofilm formation in Candida albicans. Med. Mycol. 51 473–482. 10.3109/13693786.2012.743051 [DOI] [PubMed] [Google Scholar]
- ISO 4728:2003. (2015). Oil of Spanish Wild Marjoram (Thymus mastichina L.). Geneva: International Organization for Standardization. [Google Scholar]
- Joulain D., König W. A. (1998). The Atlas of Spectral Data of Sesquiterpene Hydrocarbons. Hamburg: E.B.-Verlag. [Google Scholar]
- Khan M. S. A., Ahmad I. (2011). Antifungal activity of essential oils and their synergy with fluconazole against drug-resistant strains of Aspergillus fumigatus and Trichophyton rubrum. Appl. Microbiol. Biotechnol. 90 1083–1094. 10.1007/s00253-011-3152-3 [DOI] [PubMed] [Google Scholar]
- Manoharan R. K., Lee J.-H., Kim Y.-G., Kim S.-I., Lee J. (2017). Inhibitory effects of the essential oils α-longipinene and linalool on biofilm formation and hyphal growth of Candida albicans. Biofouling 7014 1–13. 10.1080/08927014.2017.1280731 [DOI] [PubMed] [Google Scholar]
- Marichal P., Gorrens J., Van Cutsem J., Vanden Bossche H. (1986). Culture media for the study of the effects of azole derivatives on germ tube formation and hyphal growth of C. albicans/nährböden zur untersuchung der wirkungen von azolderivaten auf die keimschlauchbildung und das hyphenwachstum bei C. albicans. Mycoses 29 76–81. 10.1111/j.1439-0507.1986.tb03753.x [DOI] [PubMed] [Google Scholar]
- Pfaller M. A. (2012). Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 125 S3–S13. 10.1016/j.amjmed.2011.11.001 [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Pappas P. G., Wingard J. R. (2006). Invasive fungal pathogens: current epidemiological trends. Clin. Infect. Dis. 43 S3–S14. 10.1086/504490 [DOI] [Google Scholar]
- Pina-Vaz C., Goncalves Rodrigues A., Pinto E., Costa-de-Oliveira S., Tavares C., Salgueiro L., et al. (2004). Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 18 73–78. 10.1111/j.1468-3083.2004.00886.x [DOI] [PubMed] [Google Scholar]
- Pinto E., Gonçalves M. J., Hrimpeng K., Pinto J., Vaz S., Vale-Silva L. A., et al. (2013). Antifungal activity of the essential oil of Thymus villosus subsp. lusitanicus against candida, cryptococcus, aspergillus and dermatophyte species. Ind. Crops Prod. 51 93–99. 10.1016/j.indcrop.2013.08.033 [DOI] [Google Scholar]
- Pirbalouti A. G., Bistghani E. Z., Malekpoor F. (2015). An overview on genus Thymus. J. Herb. Drugs 6 93–100. [Google Scholar]
- Proença da Cunha A., Ribeiro J. A., Roque O. R. (2007). Plantas Aromáticas em Portugal- Caraterização e Utilizações. Lisboa: Fundação Calouste Gulbenkian. [Google Scholar]
- Raut J. S., Shinde R. B., Chauhan N. M., Karuppayil S. M. (2013). Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling 29 87–96. 10.1080/08927014.2012.749398 [DOI] [PubMed] [Google Scholar]
- Richardson M., Lass-Flörl C. (2008). Changing epidemiology of systemic fungal infections. Clin. Microbiol. Infect. 14 5–24. 10.1111/j.1469-0691.2008.01978.x [DOI] [PubMed] [Google Scholar]
- Rivera D., Obón C. (1995). The ethnopharmacology of madeira and porto santo islands, a review. J. Ethnopharmacol. 4673–93. [DOI] [PubMed] [Google Scholar]
- Romo J. A., Pierce C. G., Chaturvedi A. K., Lazzell A. L., McHardy S. F., Saville S. P., et al. (2017). Development of anti-virulence approaches for candidiasis via a novel series of small-molecule inhibitors of candida albicans filamentation. MBio 8 e1991-17. 10.1128/mBio.01991-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharkhiz M. J., Motamedi M., Zomorodian K., Pakshir K., Miri R., Hemyari K. (2012). Chemical composition, antifungal and antibiofilm activities of the essential oil of Mentha piperita L. ISRN Pharm. 2012 718645. 10.5402/2012/718645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgueiro L., Vila R., Tomas X., Tomi F., Cañigueral S., Casanova J., et al. (1995). Chemical polymorphism of the essential oil of Thymus carnosus from portugal. Phytochemistry 38 391–396. 10.1016/0031-9422(94)00657-F [DOI] [Google Scholar]
- Salgueiro L. R., Pinto E., Gonçalves M. J., Costa I., Palmeira A., Cavaleiro C., et al. (2006). Antifungal activity of the essential oil of Thymus capitellatus against Candida, Aspergillus and dermatophyte strains. Flavour Fragr. J. 21 749–753. 10.1002/ffj.1610 [DOI] [Google Scholar]
- Salgueiro L. R., Vila R., Tomi F., Tomas X., Cañigueral S., Casanova J., et al. (1997). Composition and infraspecific variability of essential oil from Thymus camphoratus. Phytochemistry 45 1177–1183. 10.1016/S0031-9422(97)00117-9 [DOI] [Google Scholar]
- Silva A., Meireles C., Dias C., Sales F., Conde J., Salgueiro L., et al. (2011). Plantas Aromáticas e Medicinais do Parque Natural da Serra da Estrela. Seia: CISE. [Google Scholar]
- Silva A. C. R., Da, Lopes P. M., Azevedo M. M. B., De Costa D. C. M., Alviano C. S., et al. (2012). Biological activities of a-Pinene and β-Pinene enantiomers. Molecules 17 6305–6316. 10.3390/molecules17066305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl-Biskup E. (2003). “Essential oil chemistry of the genus Thymus - a global view,” in Thyme, The genus Thymus, eds Stahl-Biskup E., Saez F. (London: CRC Press/Taylor & Francis Group; ), 75–125. [Google Scholar]
- Stein S. E. (2017). “Retention Indices’ by NIST Mass Spec Data Center,” in NIST Chemistry WebBook, NIST Standard Reference Database Number 69, eds Linstrom P. J., Mallard W. J. (Gaithersburg MD: National Institute of Standards and Technology; ). [Google Scholar]
- Su Y.-C., Ho C.-L. (2016). Composition of the leaf essential oil of phoebe formosana from taiwan and its in vitro cytotoxic, antibacterial, and antifungal activities. Nat. Prod. Commun. 11 845–848. [PubMed] [Google Scholar]
- Taweechaisupapong S., Ngaonee P., Patsuk P., Pitiphat W., Khunkitti W. (2012). Antibiofilm activity and post antifungal effect of lemongrass oil on clinical Candida dubliniensis isolate. South Afr. J. Bot. 78 37–43. 10.1016/j.sajb.2011.04.003 [DOI] [Google Scholar]
- Thompson J. D., Chalchat J.-C., Michet A., Linhart Y. B., Ehlers B. (2003). Qualitative and quantitative variation in monoterpene co-occurrence and composition in the essential oil of Thymus vulgaris chemotypes. J. Chem. Ecol. 29 859–880. [DOI] [PubMed] [Google Scholar]
- Tserennadmid R., Takó M., Galgóczy L., Papp T., Pesti M., Vágvölgyi C., et al. (2011). Anti yeast activities of some essential oils in growth medium, fruit juices and milk. Int. J. Food Microbiol. 144 480–486. 10.1016/j.ijfoodmicro.2010.11.004 [DOI] [PubMed] [Google Scholar]
- Uppuluri P., Srinivasan A., Ramasubramanian A., Lopez-Ribot J. L. (2011). Effects of fluconazole, amphotericin b, and caspofungin on Candida albicans biofilms under conditions of flow and on biofilm dispersion. Antimicrob. Agents Chemother. 55 3591–3593. 10.1128/AAC.01701-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuzarte M., Alves-Silva J. M., Alves M., Cavaleiro C., Salgueiro L., Cruz M. T. (2018). New insights on the anti-inflammatory potential and safety profile of Thymus carnosus and Thymus camphoratus essential oils and their main compounds. J. Ethnopharmacol. 225 10–17. 10.1016/j.jep.2018.06.025 [DOI] [PubMed] [Google Scholar]
- Zuzarte M., Gonçalves M. J., Cavaleiro C., Cruz M. T., Benzarti A., Marongiu B., et al. (2013). Antifungal and anti-inflammatory potential of Lavandula stoechas and Thymus herba-barona essential oils. Ind. Crops Prod. 44 97–103. 10.1016/j.indcrop.2012.11.002 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.