Abstract
Low-income and middle-income countries are struggling with a growing epidemic of non-communicable diseases. To achieve the Sustainable Development Goals, their healthcare systems need to be strengthened and redesigned. The Starfield 4Cs of primary care—first-contact access, care coordination, comprehensiveness and continuity—offer practical, high-quality design options for non-communicable disease care in low-income and middle-income countries. We describe an integrated non-communicable disease intervention in rural Nepal using the 4C principles. We present 18 months of retrospective assessment of implementation for patients with type II diabetes, hypertension and chronic obstructive pulmonary disease. We assessed feasibility using facility and community follow-up as proxy measures, and assessed effectiveness using singular ‘at-goal’ metrics for each condition. The median follow-up for diabetes, hypertension and chronic obstructive pulmonary disease was 6, 6 and 7 facility visits, and 10, 10 and 11 community visits, respectively (0.9 monthly patient touch-points). Loss-to-follow-up rates were 16%, 19% and 22%, respectively. The median time between visits was approximately 2 months for facility visits and 1 month for community visits. ‘At-goal’ status for patients with chronic obstructive pulmonary disease improved from baseline to endline (p=0.01), but not for diabetes or hypertension. This is the first integrated non-communicable disease intervention, based on the 4C principles, in Nepal. Our experience demonstrates high rates of facility and community follow-up, with comparatively low lost-to-follow-up rates. The mixed effectiveness results suggest that while this intervention may be valuable, it may not be sufficient to impact outcomes. To achieve the Sustainable Development Goals, further implementation research is urgently needed to determine how to optimise non-communicable disease interventions.
Keywords: non-communicable diseases; global health; nepal; delivery of health care, integrated; implementation research
7
Summary box.
The Starfield 4C principles of high-quality primary care—first-contact access, care coordination, continuity and comprehensiveness—offer insights for non-communicable disease management in low-income and middle-income countries.
We present our experience designing and implementing the first integrated non-communicable disease intervention in rural Nepal based on the 4C principles.
Our experience suggests that high rates of follow-up are feasible with comparatively low lost-to-follow-up rates, but our mixed effectiveness results suggest that our intervention may not be sufficient to impact outcomes.
Further implementation research is urgently needed to determine how to best optimise non-communicable disease interventions to achieve the Sustainable Development Goals.
Introduction
As low-income and middle-income countries strive towards the Sustainable Development Goals to provide universal health coverage,1 the burden of non-communicable diseases (NCDs) continues to grow,2 raising urgent questions about the healthcare systems needed to achieve these targets. Four major NCD classes—cardiovascular disease, chronic respiratory disease, diabetes and cancer—lead to more deaths globally than all other diseases combined,3 while depression and other mental and behavioural disorders account for the highest proportion of global burden of disease by disability.4 Low-income and middle-income countries are disproportionately struggling as their primary healthcare systems are insufficiently resourced and poorly designed to address this changing epidemiology.5 6
For low-income and middle-income countries to better address NCDs, their healthcare systems will not only need more resources, they will need to be redesigned. Historically, in addition to significant financial and workforce shortages, these healthcare systems have focused on providing acute, episodic care with little capacity for the longitudinal follow-up and care coordination needed for NCD management.7 In many settings, NCD interventions have been disease-specific programmes, without integration across programmes, constituting an unsustainable, fragmented approach from the patient perspective.8 9 The result is that individuals with NCDs in low-income and middle-income countries often receive suboptimal care, on a visit-by-visit basis, focused on only one condition at a time. These systems become overburdened with late-stage complications due to weak prevention strategies and ineffective early disease management.9
In juxtaposition to these challenges, successful primary healthcare systems are characterised by the Starfield ‘4C’ functions10: first-contact access, continuity, coordination of care and comprehensiveness. Originally described in 199410 for high-income settings, the 4Cs have recently been highlighted as critical functions of primary healthcare in low-income and middle-income countries such as Nepal.11 12 The WHO features the 4Cs as key priorities in its Operational Framework for the Declaration of Astana,13 which has been endorsed by Nepal’s Ministry of Health and Population as core to its national health strategy.14 15 Given that the overwhelming burden of NCDs can be effectively managed by high-quality primary healthcare systems, building NCD care programmes based on these same principles is a promising option for low-income and middle-income countries that are grappling with these challenges.11
A key barrier to high-quality primary healthcare in low-income and middle-income countries, and specifically to the longitudinal follow-up required for NCD management, are workforce shortages. In order to move away from physician-focused care, employing mid-level practitioners (MLPs) is a proven workforce strategy for managing NCDs in many global settings.16 When coupled with digital tools enabling clinical decision support and algorithmic care, MLPs can reliably and effectively deploy NCD care, such as the WHO’s Package of Essential Noncommunicable Disease Interventions.17–19
Integrating NCD care into the community will be a critical next step, shifting from the historical facility-based healthcare models that have struggled to engage and continually follow patients across their lifespan. Recent years have seen a renewed recognition of community health workers (CHWs) who augment facility-based care models by providing follow-up, counselling, diagnostics, treatment and care coordination in the communities where patients live.1 20 Trials in multiple countries have demonstrated clear benefits of CHWs engaging in the longitudinal process of NCD care management.20 21 However, these initiatives have yet to be integrated at scale into most primary healthcare systems.
In Nepal, where the annual healthcare investment is approximately US$16 per capita and 51% of disability-adjusted life years are attributed to NCDs, the problem of NCD management is increasingly acute.22 Healthcare spending has focused on vertical programmes including vaccine-preventable diseases, maternal and child health, and infectious diseases. While these have been integral in improving health outcomes, the healthcare system itself continues to struggle with the provision of the basic Starfield functions, thereby limiting its ability to respond to the growing NCD burden. In forward-looking steps, the Ministry of Health and Population launched the Multisectoral NCD Action Plan in 2014,23 and in 2016 established an NCD and Injuries Poverty Commission.22 A nationwide roll-out of the WHO’s Package of Essential Noncommunicable Disease Interventions is ongoing. Despite this recent progress, available data show that NCD services fall short of the population’s needs.22
Here, we discuss the design and implementation of an integrated NCD care management intervention, built around the Starfield 4C principles of primary care,10 in rural Nepal. This intervention focuses on type II diabetes, hypertension, chronic obstructive pulmonary disease (COPD) and depression, four conditions prioritised based on national and local disease burden. Due to the lack of prior mental healthcare capacity at the primary care level,24 and thus the need for a larger capacity-building initiative,25 we will describe our depression intervention separately. Here, we describe the intervention for diabetes, hypertension and COPD, presenting early data from a patient cohort, and the lessons learnt from our implementation experience.
The intervention
Setting
This intervention was implemented in Achham, Nepal, a remote, impoverished district of 260 000 people with large migrant populations and a history of social disruption during the Nepali civil conflict.26 27 Achham has one of Nepal’s highest district-level, under-5 mortality rates28 and one of the lowest human development indices.29 The population of Achham is 66% Bahun and Chhetri (socioculturally and politically advantaged castes) and 34% non-Bahun/Chhetri and other castes.30 The intervention was based out of Bayalpata Hospital, a district-level hospital in Achham, managed via a public–private partnership between the Ministry of Health and Population and the non-profit organisation Nyaya Health Nepal. Bayalpata Hospital serves approximately 90 000 outpatients per year, of which 72% are Bahun/Chhetri and 28% are other castes. The intervention was designed and initially implemented to serve a local catchment population of 60 000, who receive their care at Bayalpata Hospital and are served by a cadre of CHWs.31 All services provided at Bayalpata Hospital and by the CHWs are free of charge, without any point-of-care user fees.
Integrated non-communicable disease management intervention
In line with the Starfield 4C principles of primary care, we designed an intervention for NCDs to prioritise first-contact access, continuity, care coordination and comprehensiveness. Our intervention included the following:
Workforce strengthening with MLPs and CHWs to optimise first-contact access at the facility and community levels, continuity of care and care coordination.
Digital tools and shared online electronic health records for MLPs and CHWs using algorithmic care with clinical decision support to optimise continuity and quality.
Individual-level risk modification and counselling to provide comprehensive preventative and curative NCD services.
Each component of the intervention and the supportive staffing structure are described more extensively in online supplementary files 1 and 2, respectively.
Studying the intervention
Study inclusion
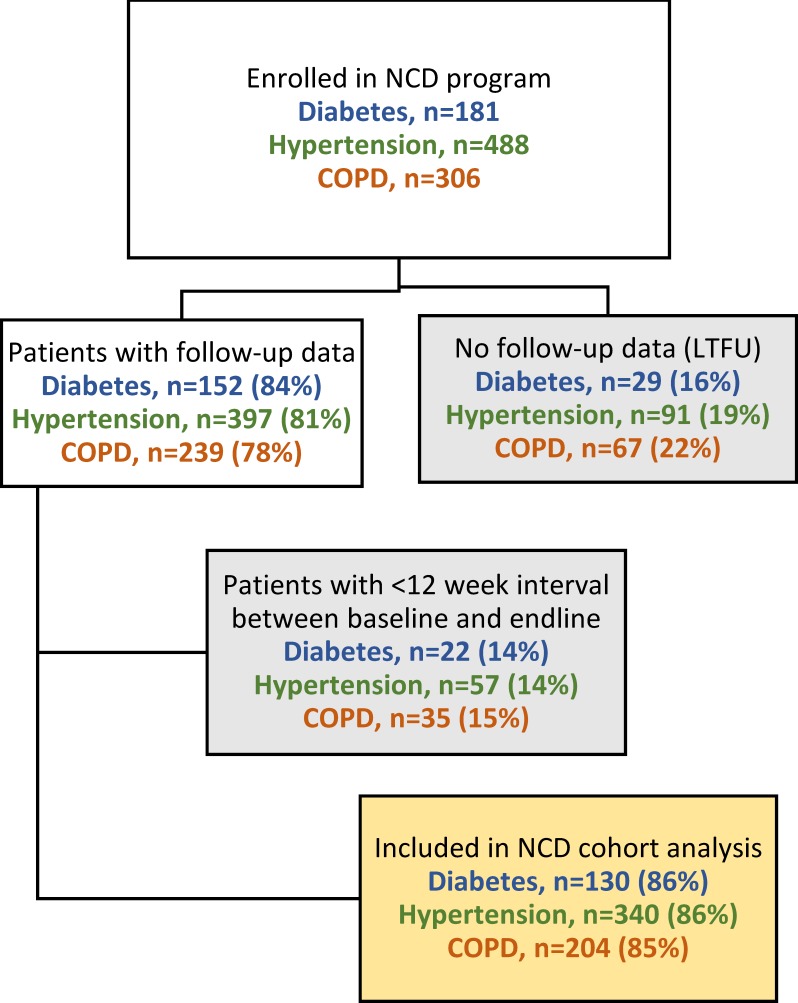
We retrospectively assessed the first 18 months (1 December 2016–31 May 2018) of the intervention for three NCDs: type II diabetes, hypertension and COPD. All patients 18 years of age or older, from the catchment area, and diagnosed with at least one of these conditions, were eligible for enrolment. Among these patients, those who had at least two facility visits during this time, with the relevant condition-specific electronic health record documentation, and at least 12 weeks between baseline and endline visits (to allow sufficient time for intervention effect), were included in the analysis cohort. A condition-specific cohort was created and analysed for each of the three NCDs (figure 1).
Figure 1.
Enrolment of patient cohorts and inclusion within cohort analysis. COPD, chronic obstructive pulmonary disease; LTFU, loss-to-follow-up; NCD, non-communicable disease.
Assessing feasibility of the intervention
Given the remote nature of Achham, many people travel more than 6 hours each way to receive care at Bayalpata Hospital, which poses significant barriers to the continual follow-up needed for optimal NCD care management. All patient visits with MLPs (facilities) and CHWs (communities) were monitored to assess follow-up. Accordingly, a goal of this intervention was to demonstrate the feasibility of continual care, without high loss-to-follow-up (LTFU) rates, in both the communities and facilities, as proxy measures to partially assess the Starfield functions. We defined LTFU as the absence of a facility follow-up visit after enrolment. We calculated the median number of MLP and CHW visits, along with IQRs, and stratified by disease condition. LTFU rates and monthly patient touch-points were then calculated for each cohort.
Assessing effectiveness of the intervention
In order to assess the effectiveness of the intervention, we developed a set of streamlined, evidence-based, ‘at-goal’ metrics for each NCD32–34 (table 1). Patient’s ‘at-goal’ status was assessed at each MLP visit, in the context of routine care. We assessed the change in proportion of patients ‘at-goal’ from baseline to endline using McNemar’s test for paired categorical variables, excluding LTFU patients. For patients who died during the study period, we conservatively imputed endline status as being ‘not at-goal’ even if their most recent recorded status was ‘at-goal’. While the causes of death were not known for these patients, we felt that the most conservative evaluation methodology, especially given that it was retrospective, was to assume ‘not at-goal’ status. We conducted bivariate analyses stratifying patients’ ‘at-goal’ status at endline to explore associations with characteristics such as sex, caste, age, comorbidity, smoking status and number of follow-up visits. We conducted χ2 tests for bivariate analyses with categorical variables and t-tests for continuous variables. When patients had comorbid study conditions, we analysed the ‘at-goal’ status stratified by condition. All analyses were conducted using SAS V.9.4.
Table 1.
Clinical definitions of ‘at-goal’ status for each intervention condition
| Non-communicable disease | Management metric | ‘At-goal’ definition |
| Type II diabetes mellitus | Haemoglobin A1c OR fasting blood sugar | Haemoglobin A1c <7.5 OR fasting blood sugar <130* |
| Hypertension | Blood pressure | Systolic blood pressure <130 mm Hg or patient-tailored goal per risk stratification† |
| Chronic obstructive pulmonary disease | Exacerbation status | <2/3 Anthonisen criteria‡ |
*Type II diabetes mellitus: The 2018 American Diabetes Association guidelines32 call for a goal A1c <7% for most patients or A1c <8% in ‘patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbid conditions, or long-standing diabetes in whom the goal is difficult to achieve despite diabetes self-management education, appropriate glucose monitoring, and effective doses of multiple glucose-lowering agents including insulin’. For our clinicians, we established 7.5% as our goal to pragmatically accommodate both populations.
†Hypertension: Based on the 2017 update to the Joint National Committee-7 guidelines,33 we established <130 mm Hg as a default treatment goal, with patient-tailored goals for select patients (≥65 years of age, multiple comorbidities, limited life expectancy, clinical judgement, patient preference).
‡Chronic obstructive pulmonary disease (COPD): The 2017 update to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines‡, 53 define COPD exacerbation as an ‘acute worsening of respiratory symptoms that results in additional therapy’. We used the Anthonisen criteria of worsening sputum volume, sputum purulence and increased dyspnoea to define the ‘worsening of respiratory symptoms’ specified in the GOLD guidelines. We established a threshold of no more than one Anthonisen criterion as a pragmatic tool for determining clinical status.
Results of the intervention
During the study period, 181 patients with diabetes, 488 with hypertension and 306 with COPD were enrolled in the intervention. Among these patients, 597 met all the inclusion criteria and had requisite data for cohort analysis, and were thus included in this assessment (figure 1). LTFU rates for diabetes, hypertension and COPD were 16%, 19% and 22%, respectively. Patient demographics are presented in table 2.
Table 2.
Characteristics of patients included in the cohort analysis
| Disease | Patients | Sex | Age | Caste | Comorbidities | ||
| n (% of total) | Female n (%) |
Male n (%) |
Mean±SD | Bahun/Chhetri n (%) |
Non-Bahun/ non-Chhetri n (%) |
n (%) | |
| Diabetes | 130 (22) | 28 (22) | 102 (78) | 55±12 | 78 (60) | 52 (40) | 50 (38) |
| Hypertension | 340 (57) | 166 (49) | 174 (51) | 56±11 | 200 (59) | 140 (41) | 70 (21) |
| COPD | 204 (34) | 138 (68) | 66 (32) | 59±11 | 117 (57) | 87 (43) | 45 (22) |
| Total unique patients with at least one comorbid NCD, n (%) | 88 (15) | ||||||
Total unique patients in NCD analysis cohort: 597.
COPD, chronic obstructive pulmonary disease; NCD, non-communicable disease.
Patient follow up
The 597 patients had 4657 MLP visits and 5664 CHW visits across 18 months. A summary of MLP and CHW follow-up visits, by condition, is presented in table 3. The median number of follow-up visits for patients with diabetes, hypertension and COPD was 6, 6 and 7 facility visits, and 10, 10 and 11 community visits, respectively, with 0.9 monthly touch-points per patient for all three cohorts. The median time between visits was approximately 2 months for all facility visits and approximately 1 month for all community visits.
Table 3.
Loss-to-follow-up and follow-up rates of cohort analysis patients
| NCD cohort | LTFU* | Facility (MLP) | Community (CHW) | Combined | ||
| Follow-up visits | Days between last two visits | Follow-up visits | Days between last two visits | Monthly touch-points per patient | ||
| (%) | Median (Q1,Q3) |
Median (Q1,Q3) |
Median (Q1,Q3) |
Median (Q1,Q3) |
Median (Q1,Q3) |
|
| Diabetes | 16 | 6 (44, 8) | 67 (38, 126) | 10 (44, 13) | 29 (2121, 41) | 0.9 (0.5, 1.2) |
| Hypertension | 19 | 6 (44, 9) | 62 (36, 111) | 10 (55, 13) | 30 (2525, 42) | 0.9 (0.6, 1.2) |
| COPD | 22 | 7 (44, 9) | 56 (34, 98) | 11 (66, 14) | 30 (2626, 39) | 0.9 (0.7, 1.3) |
*A patient was defined as LTFU if they never had a follow-up visit at the facility. These patients were excluded from the analysis cohort.
CHW, community health worker; COPD, chronic obstructive pulmonary disease; LTFU, lost to follow-up; MLP, mid-level practitioner; NCD, non-communicable disease.
Disease control
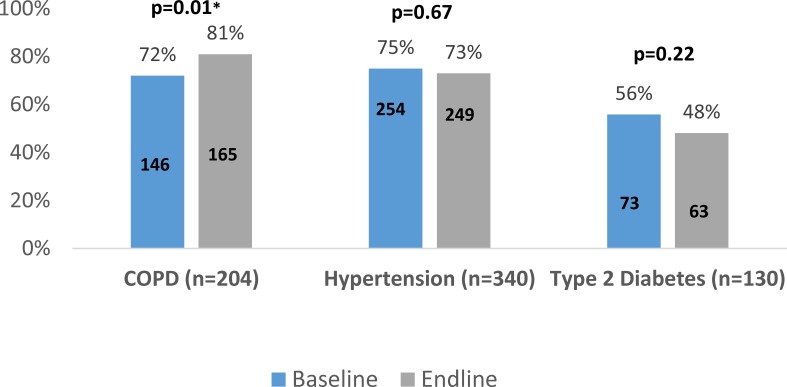
We present results summarising the changes in the cohorts’ ‘at-goal’ status in figure 2. Among patients with COPD, there was a statistically significant improvement in the proportion of ‘at-goal’ patients between baseline (72%) and endline (81%) (p=0.01). No statistically significant changes were observed in the proportion of patients with diabetes and hypertension ‘at-goal’ from baseline to endline. We did not observe any significant association of endline ‘at-goal’ status when stratifying by patient age, sex, caste, comorbid condition, smoking status, or the number of CHW or MLP follow-up visits.
Figure 2.
Change in proportion of cohort patients ‘at-goal’ status from baseline to endline, by condition. COPD, chronic obstructive pulmonary disease. *denote statistical significance.
Lessons learnt
We have presented our experience designing and implementing an integrated NCD management intervention in rural Nepal based on the Starfield 4C principles of primary care. The Nepali government is committed to this type of primary healthcare approach in its NCD care strategy,14 15 22 but in order to address this growing burden, while grappling with the challenges of a healthcare system that has not been historically well equipped to provide high-quality, longitudinal care, there is an acute need for care innovation.
Our intervention addressed two types of problems: (1) insufficient workforce and (2) inappropriate design for optimal NCD management. By recruiting and employing MLPs and CHWs as the primary healthcare providers, we increased workforce availability. We simultaneously developed an integrated network of providers designed to address the Starfield functions. Digital tools have facilitated continuity and coordination of patient visits across settings while supporting algorithmic care using clinical decision support to decrease variability in service delivery, addressing the well-documented ‘know-do gap’ in quality care provision.35 Patient-specific counselling and risk modification addressed both preventative and curative aspects of NCD management, contributing to a comprehensive approach. To our knowledge, this is the first integrated, community-based and facility-based primary care NCD intervention in rural Nepal.
From a feasibility perspective, our intervention was successful in demonstrating high follow-up rates and monthly patient touch-points, with low LTFU rates (table 3). There is scant literature or guidelines to suggest the optimal frequency of follow-up, or acceptable LTFU rates, for an intervention such as ours. Some literature reports 0.2–0.3 monthly patient touch-points36 37 and LTFU rates of 20%–50%.36–40 By comparison, our cohort has had substantially more frequent follow-up and more monthly patient touch-points, with lower LTFU rates. Thus, while imperfect, these can be clinically meaningful proxy measures for the Starfield functions of first-contact access, continuity and care coordination, suggesting that such an intervention may be an important component of the longitudinal patient engagement needed for high-quality NCD care management.
While our retrospective evaluation was not capable of understanding exactly which components of the intervention contributed to these higher follow-up and lower LTFU rates, we hypothesise that the presence of CHWs and their deep, proactive engagement within their communities likely plays a key role. As described below, we plan to investigate these effects more deeply in a large-scale implementation trial in the future.
From an effectiveness perspective, our intervention demonstrated positive changes in the COPD cohort, improving the percentage of patients with COPD ‘at-goal’ during the 18 months of follow-up. This is an encouraging result; however, there were no statistically significant improvements for patients with hypertension or diabetes. The literature8 41 and clinical experience suggest that 18 months may be sufficient to see improvement in disease-specific control measures. Thus, our mixed effectiveness results underscore that, while this type of integrated intervention may be valuable, it may also still be insufficient to drive population-level outcome improvements. The reasons for this are likely multifactorial, but with the limited retrospective data and cohort size we are unable to further clarify the detailed mechanisms behind this lack of improvement. Below, we comment on specific limitations that may have contributed and we describe plans to conduct a larger prospective trial to further explore how to best optimise this type of intervention.
Limitations
Our intervention and assessment have several limitations. First, it is a single-site intervention, limiting external validity to other settings. Moreover, Achham—and the area surrounding Bayalpata Hospital specifically—has high transient and long-term migration rates,26 potentially contributing to higher rates of patient discontinuity and LTFU than other rural settings. These migration patterns may detract from the intervention, limiting patients’ engagement in care and obfuscating potential impact of the intervention in areas with lower rates of internal and external migration.
Second, our assessment included only 18 months of patient follow-up, which may not be sufficient to see morbidity and mortality improvement for these conditions. A larger, longer term evaluation will be necessary to evaluate such outcomes.
From a data perspective, our patient-level data are limited to a simplified ‘at-goal’ metric, which was developed to be clinically informative, while minimising documentation burden for healthcare workers. The MLPs and CHWs in our intervention have had difficulties with the time-intensive and technical challenges of detailed clinical documentation,42 43 especially given large, daily patient volumes. Accordingly, the digital tools in the intervention were designed to prioritise clinical decision making and longitudinal follow-up, rather than extensive documentation. While this was designed to make the intervention easier for healthcare workers, it limits the level of information in the data. This also prevented us from being able to assess the quality of care, such as medication adherence rates, risk factor modification success rates and provider adherence to best practices.
Future directions
This retrospective analysis has provided insights about our intervention and suggests steps to better understand its potential impact moving forward. We intend to conduct a type 2 hybrid effectiveness-implementation trial44 of this intervention, across two districts, with a total catchment population of 250 000. In this planned study, we will evaluate the effectiveness of the intervention, and use the RE-AIM (Reach, Efficacy, Adoption, Implementation, and Maintenance) evaluation framework45 to assess implementation. This will include attributes of the intervention such as patient-level medication adherence, risk factor modification success rates, the quality of care provided by MLPs and CHWs—as assessed by supervised patient visits and chart audits—and other intervention-level process metrics. Through this, we hope to learn why this current assessment did not show improvements in the hypertension or diabetes cohorts and better understand how to optimise this intervention.
From a preventative perspective, developing the current counselling intervention has been a positive step forward, and we plan to improve it in the future. Specifically, we will augment the traditional model of health education and risk factor counselling with motivational interviewing by training our MLPs in this technique. Motivational interviewing has been well documented in the NCD domain for behaviours such as smoking cessation and nutritional improvement for diabetes and hypertension.46–48 We expect this will be a valuable addition to our intervention and will study its feasibility and effectiveness in the aforementioned trial.
We are also cognisant that, to date, we have not optimised the health literacy attributes or people-centredness of our intervention. From a health literacy perspective, while our current individual-level counselling has been positively received, methods such as the OPtimising HEalth LIterAcy approach, including the Health Literacy Toolkit49 50 will be critical.51 52 Similarly, people-centred care has been identified as a key priority for the future of strong primary healthcare systems.12 To build on our current work, we plan to integrate patient-reported outcomes into our data collection systems and develop feedback loops to inform healthcare workers.
Conclusion
The world—and low-income and middle-income countries especially—needs improved care models to address the growing burden of NCDs. Integrated care delivery systems, based on the Starfield 4C principles of high-quality primary care, offer practical options for low-income and middle-income countries to reorient their healthcare systems in response to this changing epidemiology. We have presented our experience with an integrated NCD management intervention in rural Nepal, demonstrating that this type of intervention is feasible and can achieve high follow-up and low LTFU rates. However, in spite of this feasibility and strong continuity, the intervention has had mixed effectiveness results, indicating that while beneficial in some ways, it may not be sufficient to achieve outcome-level improvements in this population within 18 months. Further study of our work will help to elucidate the beneficial components, and more importantly areas for improvements, to optimise the intervention. We believe that the lessons presented here offer policy and programmatic insights to stakeholders in Nepal and in similar settings globally.
bmjgh-2018-001343supp001.pdf (36.5KB, pdf)
bmjgh-2018-001343supp002.pdf (79.2KB, pdf)
Acknowledgments
We wish to express our appreciation to the Nepal Ministry of Health and Population for their continued efforts to improve the public sector healthcare system in rural Nepal. We wish to give our thanks to Dr Senendra Upreti and colleagues at the Lancet’s Nepal Non-Communicable Diseases and Injuries Poverty Commission, and to our EHR technology partners ThoughtWorks and Dimagi. Lastly, we are indebted to the hospital and community staff whose commitment to serving our patients and dedication to improving the quality of healthcare in rural Nepal continue to inspire us.
Footnotes
Handling editor: Valery Ridde
Contributors: Conceived and developed study design: DS, BA, PA, DC, BD, GD, SD, BG, TG, SH, DJ, SPKa, LK, SMa, SMe, IN, SPa, BP, MP, PR, RS, AT, PT, RT, LW. Intervention implementation and iteration: DS, SD, TG, BG, DJ, LK, MP, PR, AT, PT, RT. Performed the relevant data quality and extraction processes: RM, SPa, AR. Analysed the data: AK, DS, NC. Contributed to writing the manuscript: AK, DS, NC, BA, AA, DC, GD, MD, SH, SKa, BKa, SKi, SMa, SMe, RS, AS, PT, DM. Reviewed and approved the final manuscript: AK, DS, BA, PA, AA, NC, DC, BD, GD, MD, SD, BG, TG, SH, DJ, SKa, BKa, SKi, BKo, LK, RM, SMa, SMe, IN, SPa, BP, MP, SPo, IR, AR, PR, RS, AS, AT, PT, RT, LW, DM. ICMJE criteria for authorship met: AK, DS, BA, PA, AA, NC, DC, BD, GD, MD, SD, BG, TG, SH, DJ, SKa, BKa, SKi, BKo, LK, RM, SMa, SMe, IN, SPa, BP, MP, SPo, IR, AR, PR, RS, AS, AT, PT, RT, LW, DM.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: PA, AA, NC, DC, BD, SD, BG, TG, SH, DJ, SKa, RM, SPa, MP, SPo, IR, AR, PR, AT and RT are employed by and AK, DS, BA, GD, SMa, SMe, RS, LW and DM work in partnership with, a non-profit healthcare company (Nyaya Health Nepal, with support from the USA-based non-profit, Possible) that delivers free healthcare in rural Nepal using funds from the Government of Nepal and other public, philanthropic and private foundation sources. AK is a medical student at and DC, SKi, SMa and DM are faculty members at a private medical school (Icahn School of Medicine at Mount Sinai). DS and RS are employed at an academic medical centre (Brigham and Women’s Hospital) that receives public sector research funding, as well as revenue through private sector fee-for-service medical transactions and private foundation grants. DS and RS are faculty members at a private medical school (Harvard Medical School). DS is employed at an academic medical centre (Beth Israel Deaconess Medical Center) that receives public sector research funding, as well as revenue through private sector fee-for-service medical transactions and private foundation grants. DS is employed at an academic research centre (Ariadne Labs) that is jointly supported by an academic medical centre (Brigham and Women’s Hospital) and a private university (Harvard TH Chan School of Public Health) via public sector research funding and private philanthropy. BA is a faculty member at a public university (University of California, San Francisco). DC is a faculty member at and DC and SH are employed part-time at a public university (University of Washington). GD, BG, SMe, PR and LW are fellows with a bidirectional fellowship programme (HEAL Initiative) that is affiliated with a public university (University of California, San Francisco) that receives funding from public, philanthropic and private foundation sources. GD is employed part-time at a public medical centre (Natividad Medical Center). MD and DJ are employed by the Government of Nepal (Ministry of Health and Population, Nepal Health Research Council and Department of Health Services, respectively). SKa is a graduate student at a private university (Eastern University). BKa and AS are employed at a private university (Kathmandu University). BKa is a faculty member at a public research university (Sun Yat-sen University). SKi is the founding executive director at an advocacy and leadership network (Young Professionals Chronic Disease Network) that receives funding from individual philanthropy. BKo is a faculty member at a public university (Tribhuvan University, Institute of Medicine). LK is a fellow at a public university (Virginia Commonwealth University) and is supported by a Hubert H Humphrey Fellowship from the US Department of State. SMa is a voting member on the Board of Directors with Group Care Global, a position for which she receives no compensation. SMe works in partnership with a public medical center on the border of a Native American reservation (Gallup Indian Medical Center) that is managed using public sector funding through the Indian Health Services. IN is a graduate student at and AS is a postdoctoral fellow at a private university (Harvard T H Chan School of Public Health). IN is a voting member on the Board of Directors with Possible, (a position which she joined after the conclusion of the research described in this manuscript), BP is a member on the Board of Advisors with Nyaya Health Nepal, and DM is a non-voting member on the Board of Directors with Possible, positions for which they receive no compensation. BP is employed at a private medical centre (Hospital for Advanced Medicine and Surgery) that receives revenue from fee-for-service transactions. RS is employed at an academic medical centre (Massachusetts General Hospital) that receives public sector research funding, as well as revenue through private sector fee-for-service medical transactions and private foundation grants. PT is a graduate student at a public university (University of New South Wales). LW works in partnership with a medical center (Gallup Indian Medical Center) that receives revenue through fee-for-service medical transactions and private sector grants. All authors have read and understood BMJ Global Health’s policy on declaration of interests and declare that they have no competing financial interests. The authors do, however, believe strongly that healthcare is a public good, not a private commodity.
Patient consent for publication: Not required.
Ethics approval: Approval was obtained from the Nepal Health Research Council (177/2018). All data were routinely collected, de-identified electronic health record data.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data generated and analysed during this study are available from the corresponding author on request and will be deposited in a public data repository.
References
- 1. Bloom DE, Khoury A, Subbaraman R. The promise and peril of universal health care. Science 2018;361 10.1126/science.aat9644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the global burden of Disease Study 2013. Lancet 2014. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization Global status report on noncommunicable diseases 2014. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
- 4. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of Disease Study 2010. The Lancet 2012;380:2163–96. 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Remais JV, Zeng G, Li G, et al. Convergence of non-communicable and infectious diseases in low- and middle-income countries. Int J Epidemiol 2013;42:221–7. 10.1093/ije/dys135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maher D, Ford N, Unwin N. Priorities for developing countries in the global response to non-communicable diseases. Global Health 2012;8 10.1186/1744-8603-8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allotey P, Reidpath DD, Yasin S, et al. Rethinking health-care systems: a focus on chronicity. The Lancet 2011;377:450–1. 10.1016/S0140-6736(10)61856-9 [DOI] [PubMed] [Google Scholar]
- 8. Ogedegbe G, Gyamfi J, Plange-Rhule J, et al. Task shifting interventions for cardiovascular risk reduction in low-income and middle-income countries: a systematic review of randomised controlled trials. BMJ Open 2014;4:e005983 10.1136/bmjopen-2014-005983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hajat C, Kishore SP. The case for a global focus on multiple chronic conditions. BMJ Glob Health 2018;3:e000874 10.1136/bmjgh-2018-000874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Starfield B. Is primary care essential? The Lancet 1994;344:1129–33. 10.1016/S0140-6736(94)90634-3 [DOI] [PubMed] [Google Scholar]
- 11. Bitton A, Ratcliffe HL, Veillard JH, et al. Primary health care as a foundation for strengthening health systems in low- and middle-income countries. J Gen Intern Med 2017;32:566–71. 10.1007/s11606-016-3898-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization WHO global strategy on people-centred and integrated health services: interim report. Geneva, Switzerland: World Health Organization, 2015. [Google Scholar]
- 13. World Health Organization and UNICEF Primary health care: transforming vision into action, OPERATIONAL FRAMEWORK - Draft for Consultation. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
- 14. Yadav U. Ministry of Health and Population . Statement to be delivered by Honorable Deputy Prime Minister and Minister of Health & Population, Nepal, on the occasion of Global Conference on Primary Health Care Astana 25-26 Oct 2018. Astana, Kazakhstan: Ministry of Health and Population, 2018. [Google Scholar]
- 15. Nepal vows to achieve universal health coverage as un member. Available: http://kathmandupost.ekantipur.com/news/2018-10-27/nepal-vows-to-achieve-universal-health-coverage-as-un-member.html [Accessed 6 Mar 2019].
- 16. Global Health Workforce Alliance. World Health Organization Mid-level health workers for delivery of essential health services: A global systematic review and country experiences. Geneva, Switzerland: World Health Organization, 2013. [Google Scholar]
- 17. Hyon CS, Nam KY, Sun HC, et al. Package of essential noncommunicable disease (Pen) interventions in primary health-care settings in the Democratic people's Republic of Korea: a feasibility study. WHO South East Asia J Public Health 2017;6:69–73. [DOI] [PubMed] [Google Scholar]
- 18. Wangchuk D, Virdi NK, Garg R, et al. Package of essential noncommunicable disease (Pen) interventions in primary health-care settings of Bhutan: a performance assessment study. WHO South East Asia J Public Health 2014;3:154–60. 10.4103/2224-3151.206731 [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization Implementation tools: package of essential noncommunicable (Pen) disease interventions for primary health care in low-resource settings. Luxembourg: World Health Organization, 2013. [Google Scholar]
- 20. Scott K, Beckham SW, Gross M, et al. What do we know about community-based health worker programs? A systematic review of existing reviews on community health workers. Hum Resour Health 2018;16 10.1186/s12960-018-0304-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neupane D, McLachlan CS, Mishra SR, et al. Effectiveness of a lifestyle intervention led by female community health volunteers versus usual care in blood pressure reduction (COBIN): an open-label, cluster-randomised trial. The Lancet Global Health 2018;6:e66–73. 10.1016/S2214-109X(17)30411-4 [DOI] [PubMed] [Google Scholar]
- 22. The Lancet NCDI Poverty Commission An Equity Initiative to Address Noncommunicable Diseases and Injuries: National Report 2018. Kathmandu, Nepal: The Nepal NCDI Poverty Commission, 2018. [PubMed] [Google Scholar]
- 23. Government of Nepal, World Health Organization Country Office for Nepal . Multisectoral action Plan for the prevention and control of non communicable diseases (2014-2020). Kathmandu, Nepal: Government of Nepal, 2014. [Google Scholar]
- 24. Acharya B, Hirachan S, Mandel JS, et al. The mental health education gap among primary care providers in rural Nepal. Acad Psychiatry 2016;40:667–71. 10.1007/s40596-016-0572-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Acharya B, Ekstrand M, Rimal P, et al. Collaborative care for mental health in low- and middle-income countries: a WHO health systems framework assessment of three programs. Psychiatric Services 2017;68:870–2. 10.1176/appi.ps.201700232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vaidya NK, Wu J. HIV epidemic in far-western Nepal: effect of seasonal labor migration to India. BMC Public Health 2011;11 10.1186/1471-2458-11-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thapa D, Ghimire I. Labour Migration for Employment: A Status Report for Nepal: 2014/2015 : Kathmandu, ed Ministry of labour and employment. Nepal: Government of Nepal, 2016. [Google Scholar]
- 28. Ministry of Health and Population, New ERA, ICF International Inc Ministry of Health and Population, New ERA, ICF International Inc.,. Nepal Demographic and Health Survey 2016. Kathmandu, Nepal: Ministry of Health and Population, 2017. [Google Scholar]
- 29. Government of Nepal National Planning Commission, United Nations Development Programme . Nepal human development report 2014: beyond geography, unlocking human potential. Kathmandu, Nepal: Government of Nepal National Planning Commission, United Nations Development Programme, 2014. [Google Scholar]
- 30. National Planning Commission Secretariat, Central Bureau of Statistics, Bista B. Population Monograph of Nepal: Volume II (Social Demography). Ramshah Path, Kathmandu, Nepal: National Planning Commission Secretariat, Central Bureau of Statistics, 2014. [Google Scholar]
- 31. Citrin D, Thapa P, Nirola I, et al. Developing and deploying a community Healthcare worker-driven, digitally- enabled integrated care system for municipalities in rural Nepal. Healthc 2018;6:197–204. 10.1016/j.hjdsi.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. American Diabetes Association 6. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41(Suppl 1):S55–S64. 10.2337/dc18-S006 [DOI] [PubMed] [Google Scholar]
- 33. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 2018;71:e127–248. 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 34. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: gold executive summary. Am J Respir Crit Care Med 2013;187:347–65. 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 35. Das J, Woskie L, Rajbhandari R, et al. Rethinking assumptions about delivery of healthcare: implications for universal health coverage. BMJ 2018;361 10.1136/bmj.k1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jafar TH, Hatcher J, Poulter N, et al. Community-based interventions to promote blood pressure control in a developing country. Ann Intern Med 2009;151:593–601. 10.7326/0003-4819-151-9-200911030-00004 [DOI] [PubMed] [Google Scholar]
- 37. Kengne AP, Awah PK, Fezeu LL, et al. Primary health care for hypertension by nurses in rural and urban sub-Saharan Africa. J Clin Hypertens 2009;11:564–72. 10.1111/j.1751-7176.2009.00165.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sobry A, Kizito W, Van den Bergh R, et al. Caseload, management and treatment outcomes of patients with hypertension and/or diabetes mellitus in a primary health care programme in an informal setting. Trop Med Int Health 2014;19:47–57. 10.1111/tmi.12210 [DOI] [PubMed] [Google Scholar]
- 39. Khosravi A, Pourheidar B, Roohafza H, et al. Evaluating factors associated with uncontrolled hypertension: Isfahan cohort study, Iran. ARYA Atheroscler 2014;10. [PMC free article] [PubMed] [Google Scholar]
- 40. Labhardt ND, Balo J-R, Ndam M, et al. Improved retention rates with low-cost interventions in hypertension and diabetes management in a rural African environment of nurse-led care: a cluster-randomised trial. Trop Med Int Health 2011;16:1276–84. 10.1111/j.1365-3156.2011.02827.x [DOI] [PubMed] [Google Scholar]
- 41. He J, Irazola V, Mills KT, et al. Effect of a community health Worker-Led multicomponent intervention on blood pressure control in low-income patients in Argentina: a randomized clinical trial. JAMA 2017;318:1016–25. 10.1001/jama.2017.11358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raut A, Thapa P, Citrin D, et al. Design and implementation of a patient navigation system in rural Nepal: improving patient experience in resource-constrained settings. Healthc 2015;3:251–7. 10.1016/j.hjdsi.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 43. Raut A, Yarbrough C, Singh V, et al. Design and implementation of an affordable, public sector electronic medical record in rural Nepal. Jhi 2017;24:186–95. 10.14236/jhi.v24i2.862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bernet AC, Willens DE, Bauer MS. Effectiveness-implementation hybrid designs: implications for quality improvement science. Implementation Science 2013;8(Suppl 1). 10.1186/1748-5908-8-S1-S2 [DOI] [Google Scholar]
- 45. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999;89:1322–7. 10.2105/AJPH.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rubak S, Sandbaek A, Lauritzen T, et al. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract 2005;55:305–12. [PMC free article] [PubMed] [Google Scholar]
- 47. Heckman CJ, Egleston BL, Hofmann MT. Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis. Tob Control 2010;19:410–6. 10.1136/tc.2009.033175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ren Y, Yang H, Browning C, et al. Therapeutic effects of motivational interviewing on blood pressure control: a meta-analysis of randomized controlled trials. Int J Cardiol 2014;172:509–11. 10.1016/j.ijcard.2014.01.051 [DOI] [PubMed] [Google Scholar]
- 49. Batterham RW, Buchbinder R, Beauchamp A, et al. The optimising health literacy (Ophelia) process: study protocol for using health literacy profiling and community engagement to create and implement health reform. BMC Public Health 2014;14 10.1186/1471-2458-14-694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dodson S, Good S, et al. , Regional Office for South-East Asia . Health literacy toolkit for low and middle-income countries: a series of information sheets to empower communities and strengthen health systems. New Delhi, India: World Health Organization, Regional Office for South-East Asia, 2015. [Google Scholar]
- 51. Budhathoki SS, Pokharel PK, Good S, et al. The potential of health literacy to address the health related un sustainable development goal 3 (SDG3) in Nepal: a rapid review. BMC Health Serv Res 2017;17 10.1186/s12913-017-2183-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Batterham RW, Hawkins M, Collins PA, et al. Health literacy: applying current concepts to improve health services and reduce health inequalities. Public Health 2016;132:3–12. 10.1016/j.puhe.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 53. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Gold executive summary. Am J Respir Crit Care Med 2017;195:557–82. 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2018-001343supp001.pdf (36.5KB, pdf)
bmjgh-2018-001343supp002.pdf (79.2KB, pdf)