Abstract
Background
The relationship between lowering LDL (low‐density lipoprotein) cholesterol with contemporary lipid‐lowering therapies and incident diabetes mellitus (DM) remains uncertain.
Methods and Results
Thirty‐three randomized controlled trials (21 of statins, 12 of PCSK9 [proprotein convertase subtilisin/kexin type 9] inhibitors, and 0 of ezetimibe) were selected using Medline, Embase, and the Cochrane Central Register of Controlled Trials (inception through November 15, 2018). A total of 163 688 nondiabetic patients were randomly assigned to more intensive (83 123 patients) or less intensive (80 565 patients) lipid‐lowering therapy. More intensive lipid‐lowering therapy was defined as the more potent pharmacological strategy (PCSK9 inhibitors, higher intensity statins, or statins), whereas less intensive therapy corresponded to active control group or placebo/usual care of the trial. Metaregression and meta‐analyses were conducted using a random‐effects model. No significant association was noted between 1‐mmol/L reduction in LDL cholesterol and incident DM for more intensive lipid‐lowering therapy (risk ratio: 0.95; 95% CI, 0.87–1.04; P=0.30; R 2=14%) or for statins or PCSK9 inhibitors. More intensive lipid‐lowering therapy was associated with a higher risk of incident DM compared with less intensive therapy (risk ratio: 1.07; 95% CI, 1.03–1.11; P<0.001; I2=0%). These results were driven by higher risk of incident DM with statins (risk ratio: 1.10; 95% CI, 1.05–1.15; P<0.001; I2=0%), whereas PCSK9 inhibitors were not associated with incident DM (risk ratio: 1.00; 95% CI, 0.93–1.07; P=0.96; I2=0%; P=0.02 for interaction).
Conclusions
Among intensive lipid‐lowering therapies, there was no independent association between reduction in LDL cholesterol and incident DM. The risk of incident DM was higher with statins, whereas PCSK9 inhibitors had no association with risk of incident DM.
Keywords: diabetes mellitus, LDL (low‐density lipoprotein) cholesterol, PCSK9 (proprotein convertase subtilisin/kexin type 9), statin
Subject Categories: Meta Analysis, Quality and Outcomes, Complications
Clinical Perspective
What Is New?
Statins and PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors reduce cardiovascular risk by reducing LDL (low‐density lipoprotein) cholesterol.
Statins are known to increase the risk of incident diabetes mellitus (DM), whereas randomized controlled trials have shown numerically higher cases of incident DM with PCSK9 inhibitor therapy.
It is not clearly known whether LDL cholesterol reduction is associated with risk of incident DM and whether this risk might vary across established LDL cholesterol–lowering drugs.
What Are the Clinical Implications?
This meta‐analysis shows that among intensive lipid‐lowering drugs, there was no independent association between LDL cholesterol reduction achieved by these medications and risk of incident DM.
The increased risk of incident DM was associated with statins only; PCSK9 inhibitors did not show any association with DM.
The current study further adds to the safety of LDL cholesterol lowering with regard to the risk of DM.
LDL (low‐density lipoprotein) cholesterol (LDL‐C) is a well‐established modifiable risk factor for clinical atherosclerotic cardiovascular disease.1, 2 Incremental reductions in LDL‐C levels by statins or intensifying statin therapy by adding ezetimibe or PCSK9 (proprotein convertase subtilisin/kexin type 9) have shown correspondingly higher cardiovascular risk reductions.3, 4, 5, 6 In contrast, several studies have shown a significant association between statins and a higher risk of incident diabetes mellitus (DM).7, 8 However, this association is not clear in case of PCSK9 inhibitors. The FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) trial showed nonsignificantly higher numbers of incident DM among participants receiving evolocumab.9 Conversely, the ODYSSEY OUTCOMES (Alirocumab and Cardiovascular Outcomes After Acute Coronary Syndrome) trial showed lesser risk of new onset of DM with alirocumab compared with placebo (9.6% versus 10.1%).10 In a meta‐analysis, exposure to LDL‐C–lowering alleles in or near NPC1L1 (Niemann‐Pick C1‐like 1) or HMGCR (3‐hydroxy‐3‐methylglutaryl‐CoA reductase), PCSK9, ABCG5/G8 (ATP‐binding cassette subfamily G member), and LDLR (LDL receptor), which encode the molecular targets of lipid‐lowering therapies (ie, statins, ezetimibe, and PCSK9 inhibitors) were associated with higher risk of type 2 DM.11
Although the beneficial effects of LDL‐C reduction on cardiovascular outcomes are clearly established, the degree of risk associated with reduction in LDL‐C in terms of new‐onset DM is unclear,7, 8 as is the potential heterogeneity of this effect by LDL‐C–lowering drug class. To assess whether lowering LDL‐C has any association with risk of incident DM and whether this risk varies by different, established LDL‐C–lowering drugs, we performed a meta‐analysis and metaregression analysis.
Methods
Data Availability Statement
The authors declare that all supporting data are available within the article (and its online supplementary files).
Data Sources and Searches
This systematic review and meta‐analysis was conducted according to Cochrane Collaboration guidelines12 and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.13 Two authors (S.U.K. and H.R.) devised a broad search strategy by using relevant keywords (statins, proprotein convertase subtilisin/kexin type 9 inhibitors, PCSK9 inhibitors, ezetimibe, low‐density lipoprotein cholesterol, LDL‐C, diabetes mellitus; Table S1). We searched Medline (PubMed), Embase, and the Cochrane Central Register of Controlled Trials from the inception of the databases to November 15, 2018. Although search restrictions were applied for clinical trials and humans, no restrictions were applied for language, year of publication, or text availability. Additional sources included websites (European Society of Cardiology, https://www.escardio.org/; American College of Cardiology, https://www.acc.org and https://www.cardiosource.org; ClinicalTrialResults.com, http://www.clinicaltrialresults.com/; ClinicalTrials.gov, https://www.clinicaltrials.gov/), proceedings of major cardiology meetings, and references of the relevant articles. The citations were downloaded in Endnote X7 (Thompson ISI Research Soft), and duplicates were identified and removed. Two authors (M.S.K. and H.R.) independently screened the records based on prespecified inclusion criteria. Any disagreements were resolved by mutual consensus or third‐party review (S.U.K.).
Study Selection
The following prespecified inclusion criteria were used. First, randomized controlled trials had to include at least 100 patients receiving the allocated pharmacological lipid‐lowering therapy for a minimum of 12 weeks. Second, consistent with former reports,1, 2, 6 we selected statin and nonstatin therapies in combination with statin that lower LDL‐C levels via mechanisms that ultimately result in upregulation of LDL receptor (R) expression (ezetimibe and PCSK9 inhibitors [alirocumab and evolocumab]) compared with placebo or active controls. Third, studies had to report at least 1 clinical event for incident DM.
We excluded trials if (1) nonstatin therapy did not reduce LDL‐C levels primarily via upregulation of LDLR expression (fibrates, niacin, and cholesteryl ester transfer protein inhibitors), (2) interventions showed concomitant effect on DM (bile acid sequestrants, ileal bypass surgery, exercise, and diet),14, 15, 16 (3) findings of the study were reported as abstracts and do not have subsequent full‐text publication (risk of having discrepancies between meeting abstract results and full‐text publication),17, 18 and (4) trials assessing efficacy of bococizumab, which is not a therapeutic option because of immunogenicity.19
Data Extraction and Quality Assessment
Data extraction was performed by 2 independent authors (S.U.K. and H.R.) on a standard data collection form. The data abstraction was based on baseline characteristics of participants, treatment groups, events, total number of patients in each group, diabetic patients in each group, nondiabetic patients in each group (calculated as total patients minus diabetic patients), baseline LDL‐C and reduction in LDL‐C in each group, achieved LDL‐C in each group and difference between the groups, and follow‐up duration of each trial. We extracted data on incident DM using the methodology reported in a former study, namely, if the trial had clearly reported newly diagnosed DM as an adverse event or study participants had commenced antidiabetic drug treatment during the trial or if patients had 2 consecutive fasting blood glucose levels ≥126 mg/dL during the study period.7
The absolute change in LDL‐C was calculated as mean or median difference, whichever was available, averaged over the course of follow‐up between 2 groups. If not reported, then the achieved LDL‐C value at the point closest to 50% of the median follow‐up was used.1 To assess the precision of calculated LDL‐C values, we compared our results with the Cholesterol Treatment Trialists (CTT) collaboration meta‐analysis20 and a meta‐analysis by Silverman et al.1 In older studies, for which LDL‐C was not available, we calculated the LDL‐C from total cholesterol using the following regression equation: LDL‐C=(total cholesterol)×[(total cholesterol)×0.0012+0.3793].1 When available, we extracted data for intention to treat analysis. Any discrepancy related to data was resolved by discussion and referring to the original article. We also reviewed prior systematic reviews and meta‐analyses for any additional information on the included studies in case the authors had reported further data beyond published trials in those meta‐analyses.1, 7, 8 The Cochrane Collaboration tool for bias risk assessment was used by 2 independent reviewers (V.O. and M.S.K.) to assess the quality of each trial (Table S2).21
More intensive lipid‐lowering therapy was defined as a more potent pharmacological strategy, whereas less intensive lipid‐lowering therapy corresponded to placebo/usual care or the active control group of the trial.2, 6 The group allocation was designated as such: (1) for statin versus placebo/usual care trials, statin therapy belonged to the more intensive therapy group and placebo/usual care was allocated to the less intensive therapy arm; (2) for higher intensive versus lower intensity statin trials, higher intensity statin was grouped with more intensive lipid‐lowering therapy and less intensive statin was grouped with less intensive lipid‐lowering therapy; and (3) for PCSK9 inhibitor trials, PCSK9 inhibitor therapy was grouped with more intensive lipid‐lowering therapy and placebo/usual care or active control (ezetimibe) was grouped with less intensive lipid‐lowering therapy.
Data Synthesis and Analysis
To account for potential between‐study variance, estimates were pooled using a DerSimonian and Laird random‐effects model.22 The principal summary statistic was risk ratio (RR), supplemented by risk difference (RD) with 95% CI. Heterogeneity was assessed using Cochrane Q statistics and quantified by I2 with values >25%, 50%, and 75% consistent with low, moderate, and high degrees of heterogeneity, respectively.23 Publication bias was assessed using the funnel plot and Egger regression test.24 Statistical significance was set at 5%.
Metaregression analyses were performed using random‐effects models with the restricted maximum likelihood estimation. The Knapp and Hartung adjustment was applied for calculation of standard errors of the estimated coefficients to calculate summary effect estimates.25 Metaregression analyses were conducted to estimate the associations among absolute amount of reduction in LDL‐C (calculated as the difference in the achieved LDL‐C between the 2 interventions),1 percentage reduction in LDL‐C (each 10%), baseline LDL‐C, and absolute reduction in LDL‐C adjusted for baseline LDL‐C and incident DM. The index R 2 value (defined as the ratio of explained/total variance) was used to determine the proportion of variance accounted for by the change in LDL‐C.
Subgroup analyses were conducted according to weighted between‐group LDL‐C differences observed at follow‐up across the trials for particular lipid‐lowering strategies as suggested by CTT collaboration meta‐analysis20 and interventions: statins, PCSK9 inhibitors, statins versus no statins, and high‐intensity statins (atorvastatin 80 mg, simvastatin 80 mg, or rosuvastatin 40 mg) versus low‐intensity statins (lesser doses of corresponding statin therapy [atorvastatin 10 mg, simvastatin 20–40 mg, and rosuvastatin up to 20 mg]). Additional sensitivity analyses included meta‐analyses by fixed‐effects model, analyses of trials with sample sizes of ≥500 patients that reported outcomes at follow‐up ≥1 year, analyses according to year of publication,6 and trials with the same definition for DM. Analyses were performed using Comprehensive Meta‐Analysis software v3.0 (Biostat) and Metafor package v3.30 (R Project for Statistical Computing).
Results
The initial electronic search yielded 3711 citations, of which 1400 studies were removed as duplicates. Of the remaining 2311 articles, 1970 citations were excluded at title‐ and abstract‐level screening. A total of 341 full‐text articles were considered relevant, of which 308 were excluded based on a priori selection criteria. Ezetimibe data were presented as an abstract at the European Society of Cardiology Congress 2015 in subgroup analysis of IMPROVE IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), which showed no significant association of ezetimibe plus simvastatin versus simvastatin alone on incident DM (hazard ratio: 1.04; P=0.46).26 This study was excluded based on a priori selection criteria, that is, if findings of that study were reported as abstracts and did not have subsequent full‐text publication, the study would be excluded because of risk of discrepancies between abstract results and full‐text publication. Ultimately, 33 trials met the criteria for the final list of studies (Figure 1).
Figure 1.
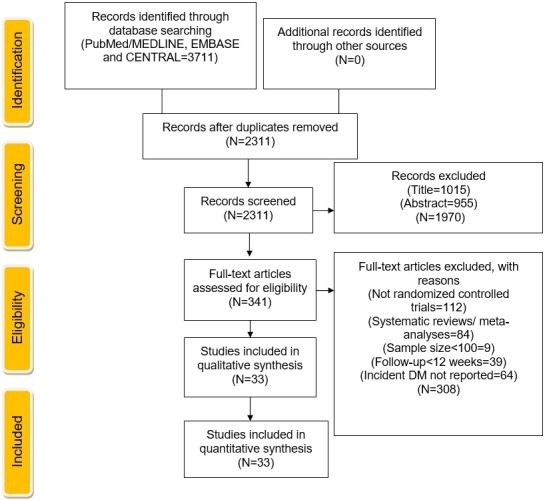
Study selection according to Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. CENTRAL indicates Cochrane Central Register of Controlled Trials; DM, diabetes mellitus.
Twenty‐one trials of statins (124 755 patients) and 12 trials of PCSK9 inhibitors (38 933 patients) reported incident DM (Table 1).9, 10, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 The pooled mean baseline LDL‐C was 3.37±0.71 mmol/L, and mean follow‐up duration was 4.2±1.2 years. A total of 163 688 nondiabetic patients were randomly assigned to more intensive (83 123 patients) or less‐intensive (80 565 patients) lipid‐lowering therapy. A total of 9855 (6.0%) incident DM cases were reported in the total study population. Additional characteristics of included trials are reported in Table S3.
Table 1.
Baseline Characteristics of Studies and Population Meeting Inclusion Criteria
| Studies | Diabetic/Nondiabetic Patients, n (%) | Trial Population | Baseline LDL‐C (mmol/L) | Between‐Group Difference in Achieved LDL‐C (mmol/L) | Diagnostic Criteria for Incident DM | Follow‐up (wk) |
|---|---|---|---|---|---|---|
| Statin | ||||||
| PMSGCRP (1993)30 | 1/1062 (0.1) | Hypercholesterolemia + ≥2 atherosclerotic CVD risk factors | 4.68 | 2.01 | Adverse event reported; medication | 161 |
| 4S (1994)56 | 391/4242 (9.2) | Previous angina or MI | 4.88 | 1.75 | Adverse event reported; medication; 1 FBG ≥126 mg/dL | 281 |
| WOSCOPS (1995)31 | 168/5974 (2.8) | Hypercholesterolemia | 4.96 | 0.98 | Two FBG ≥126 mg/dL | 250 |
| LIPID (1998)32 | 264/6997 (3.8) | Unstable angina or MI within past 3 y | 3.88 | 0.97 | One FBG ≥126 mg/dL; medication | 318 |
| AFCAPS/TexCAPS (1998)33 | 146/6211 (2.4) | Hypercholesterolemia | 3.89 | 1.08 | Adverse event reported; medication; 1 FBG ≥126 mg/dL | 271 |
| GISSI PREV (2000)34 | 201/3460 (5.8) | MI within past 6 mo | 3.92 | 0.35 | Adverse event reported; 1 FBG ≥126 mg/dL | 166 |
| ALLHAT‐LLT (2002)29 | 451/6087 (7.4) | CHD or risk factors for CHD | 3.76 | 0.62 | Adverse event reported; 1 FBG ≥126 mg/dL | 250 |
| GREACE (2002)35 | 54/1287 (4.2) | CHD | 4.64 | 1.86 | Adverse event reported; medication | 156 |
| PROSPER (2002)36 | 292/5181 (5.6) | Elderly patients with CHD or carrying high risk for CHD | 3.79 | 1.03 | One FBG ≥126 mg/dL; medication | 156 |
| HPS (2003)37 | 628/14 573 (4.3) | High risk of cardiovascular events | 3.38 | 1.29 | Adverse event reported; medication | 260 |
| ASCOT‐LLA (2003)38 | 249/5860 (4.2) | Hypertension, risk factors for CHD | 3.44 | 1.20 | WHO 1999 criteria | 172 |
| A to Z (2004)39 | 112/3504 (3.2) | ACS | 2.09 | 0.36 | Adverse event reported; medication; 2 FBG ≥126 mg/dL; medication | 104 |
| PROVE IT (2004)40 | 200/3395 (5.9) | ACS | 2.62 | 0.84 | Adverse event reported; medication; 2 FBG ≥126 mg/dL; medication | 156 |
| IDEAL (2005)41 | 449/7461 (6.0) | History of previous MI | 2.64 | 0.56 | Adverse event reported; medication; 2 FBG ≥126 mg/dL; medication | 250 |
| TNT (2005)42 | 776/7595 (10.2) | CHD | 2.52 | 0.62 | Adverse event reported; medication; 2 FBG ≥126 mg/dL; medication | 255 |
| MEGA (2006)43 | 336/6086 (5.5) | Hypercholesterolemia without history of MI or stroke | 4.05 | 0.59 | Adverse event reported; medication; 2 FBG ≥126 mg/dL | 276 |
| CORONA (2007)44 | 188/3534 (5.3) | Elderly patients with systolic HF | 3.55 | 1.61 | Adverse event reported | 140 |
| GISSI‐HF (2008)45 | 440/3378 (13.0) | Chronic HF | 3.06 | 0.75 | 2 FBG ≥126 mg/dL | 203 |
| JUPITER (2008)46 | 486/17 802 (2.7) | No history of CHD | 2.70 | 1.42 | Adverse event reported; medication, OGTT positive, elevated random glucose with symptoms, 2 FBG ≥126 mg/dL | 260 |
| ASTRONOMER (2010)47 | 1/269 (0.4) | Mild to moderate aortic stenosis | 3.15 | 1.67 | Adverse event reported | 182 |
| SEARCH (2010)27 | 1212/10 797 (11.2) | History of previous MI | 2.50 | 0.35 | Adverse event reported; medication; 2 FBG ≥126 mg/dL; medication | 349 |
| PCSK9 inhibitor | ||||||
| ODYSSEY OPTIONS I (2015)48 | 4/103 (3.9) | High risk for CVD | 2.77 | 0.66 | Adverse event reported; medication | 32 |
| ODYSSEY FH I (2015)49 | 10/429 (2.3) | Heterozygous FH | 3.70 | 1.44 | Adverse event reported; medication | 78 |
| ODYSSEY FH II (2015)49 | 10/239 (4.2) | Heterozygous FH | 3.50 | 1.55 | Adverse event reported; medication | 78 |
| ODYSSEY LONG TERM (2015)50 | 28/1503 (1.9) | Heterozygous FH or CHD or equivalent | 3.16 | 1.83 | Adverse event reported; medication | 78 |
| OSLER (2015)51 | 45/3866 (1.2) | Population from 12 different trials including patients with high risk for CHD; heterozygous FH | 3.10 | 1.86 | Adverse event reported; medication | 56 |
| GLAGOV (2016)28 | 35/766 (4.6) | CHD | 2.39 | 1.46 | Adverse event reported | 78 |
| ODYSSEY OPTIONS II (2016)55 | 4/103 (2.4) | Hypercholesterolemia; high risk for CVD | 2.81 | 0.52 | Adverse event reported | 24 |
| ODYSSEY CHOICE I (2016)52 | 14/586 (2.4) | High risk for CVD | 3.24 | 2.02 | Adverse event reported; diabetes mellitus or microvascular complications using coding system. | 56 |
| ODYSSEY JAPAN (2016)53 | 16/201 (8.0) | Heterozygous FH; high risk for CVD | 3.70 | 2.25 | Adverse event reported; medication | 52 |
| YUKAWA‐2 (2016)54 | 1/207 (0.5) | High risk for CVD | 3.6 | 2.30 | Adverse event reported; medication | 12 |
| FOURIER (2017)9 | 1321/17 451 (7.6) | Atherosclerotic CVD | 2.38 | 1.40 | Adverse event reported; new‐onset DM defined based on ADA and NDIC, ie, 2 FBG ≥126 mg/dL | 115 |
| ODYSSEY OUTCOMES (2018)10 | 1324/13 459 (9.8) | Recent ACS | 2.38 | 1.70 | Adverse event reported | 146 |
Values are reported as mean or median, whichever was available. ADA. American Diabetes Association; ACS indicates acute coronary syndrome; A to Z, Aggrastat to Zocor; AFCAPS/TexCAPS, Air Force/Texas Coronary Atherosclerosis Prevention Study; ALLHAT‐LLT, Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trials; ASCOT‐LLA, Prevention of Coronary and Stroke Events With Atorvastatin in Hypertensive Patients Who Have Average or Lower‐Than‐Average Cholesterol Concentrations in the Anglo‐Scandinavian Cardiac Outcomes Trial–Lipid‐Lowering Arm; ASTRONOMER, Aortic Stenosis Progression Observation: Measuring the Effects of Rosuvastatin; CHD, coronary heart disease; CORONA, Controlled Rosuvastatin in Multinational Trial in Heart Failure; CVD, cardiovascular disease; DM, diabetes mellitus; FBG, fasting blood glucose; FH, familial hypercholesterolemia; 4S, Scandinavian Simvastatin Survival Study; FOURIER, Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk; GISSI PREV, GruppoItaliano per lo Studio dellaSopravvivenzanell'InfartoMiocardico; GISSI HF, The GruppoItaliano per lo Studio dellaSopravvivenzanell'Insufficienza Cardiac–Heart Failure; GLAGOV, Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound; GREACE, Greek Atorvastatin and Coronary‐Heart‐Disease Evaluation; HF, heart failure; HPS, Heart Protection Study; IDEAL, Incremental Decrease in End Points Through Aggressive Lipid Lowering; JUPITER, Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin; LDL‐C, LDL (low‐density lipoprotein) cholesterol; LIPID, Long‐Term Intervention With Pravastatin in Ischemic Disease; MEGA, Management of Elevated Cholesterol in the Primary Prevention Group; MI, myocardial infarction; NDIC, National Diabetes Information Clearinghouse; ODYSSEY CHOICE I, Study to Evaluate the Efficacy and Safety of an Every Four Weeks Treatment Regimen of Alirocumab (REGN727/SAR236553) in Patients With Primary Hypercholesterolemia; ODYSSEY FH I, Efficacy and Safety of Alirocumab (SAR236553/REGN727) Versus Placebo on Top of Lipid‐Modifying Therapy in Patients With Heterozygous Familial Hypercholesterolemia Not Adequately Controlled With Their Lipid‐Modifying Therapy; ODYSSEY FH II, Study of Alirocumab (REGN727/SAR236553) in Patients With heFH (Heterozygous Familial Hypercholesterolemia) Who Are Not Adequately Controlled With Their LMT (Lipid‐Modifying Therapy); ODYSSEY LONG TERM, Long‐Term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients With Hypercholesterolemia Not Adequately Controlled With Their Lipid‐Modifying Therapy; ODYSSEY OPTIONS I, Study of the Efficacy and Safety of Alirocumab (REGN727/SAR236553) in Combination With Other Lipid‐Modifying Treatment; ODYSSEY OPTIONS II, Study of Alirocumab (REGN727/SAR236553) Added‐On to Rosuvastatin Versus Other Lipid Modifying Treatments; ODYSSEY OUTCOMES, Alirocumab and Cardiovascular Outcomes After Acute Coronary Syndrome; OSLER, Open‐Label Study of 12 Early Phase 2–3 Trials; PCSK9, proprotein convertase subtilisin/kexin type 9; PMSGCRP, Pravastatin Multinational Study Group for Cardiac Risk Patients; PROVE IT, Pravastatin or Atorvastatin Evaluation and Infection Therapy; OGTT, Oral Glucose Tolerance Test; PROSPER, Pravastatin in Elderly Individuals at Risk of Vascular Disease; SEARCH, Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine; TNT, Treating to New Targets; WHO, World Health Organization; WOSCOPS, West of Scotland Coronary Prevention Study Group; YUKAWA‐2, Study of LDL‐Cholesterol Reduction Using a Monoclonal PCSK9 Antibody in Japanese Patients With Advanced Cardiovascular Risk.
Metaregression analysis did not demonstrate significant association between absolute reduction in LDL‐C (for every 1 mmol/L) and incident DM for more intensive lipid‐lowering therapy (RR: 0.95; 95% CI, 0.87–1.04; P=0.30; R 2=14%; RD: −0.002; 95% CI, −0.006 to 0.002; P=0.32; R 2=0; Figure 2), for statins (RR: 1.02; 95% CI, 0.91–1.14; P=0.67; R 2=0; RD: −0.002; 95% CI, −0.007 to 0.003; P=0.44; R 2=0), or for PCSK9 inhibitors (RR: 1.09; 95% CI, 0.60–1.99; P=0.74; R 2=0; RD: 0.009; 95% CI, −0.010 to 0.028; P=0.37; R 2=0). This effect remained consistent for change in baseline LDL‐C values and absolute reduction in LDL‐C adjusted for baseline LDL‐C (Table 2). Similarly, more intensive lipid‐lowering therapy (RR: 0.99; 95% CI, 0.97–1.01; P=0.48; R 2=0; RD: −0.0002; 95% CI, −0.0001 to 0.0001; P=0.74; R 2=0; Figure 3), statins (RR: 1.00; 95% CI, 0.99–1.00; P=0.18; R 2=0; RD: 0.00007; 95% CI, −0.0001 to 0.0003; P=0.52; R 2=0), or PCSK9 inhibitors (RR: 1.04; 95% CI, 0.98–1.11; P=0.12; R 2=0.27; RD: 0.0007; 95% CI, −0.0007 to 0.002; P=0.28; R 2=0.59) showed consistent nonsignificant association with risk of incident DM per 10% reduction in LDL‐C values.
Figure 2.
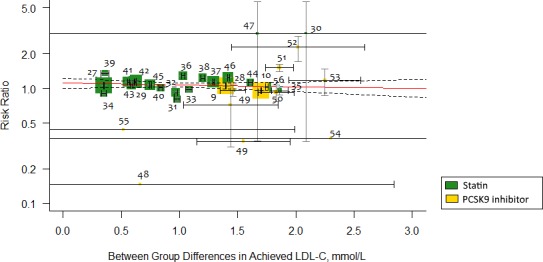
Metaregression showing association of between‐group differences in achieved LDL (low‐density lipoprotein) cholesterol (LDL‐C) levels (mmol/L) and risk ratio of incident diabetes mellitus. Each trial is represented by a data marker, the size of which is proportional to the weight in the metaregression. The metaregression slope (predicted risk for degree of LDL‐C reduction) is represented by a red line, and 95% CIs are presented as dashed lines. The horizontal lines through each square represent ±1 SE for the associated absolute change in LDL‐C, and the vertical line through each square represents the 95% CI for relative risk. For converting millimoles to milligrams, multiply by 38.5. PCSK9 indicates proprotein convertase subtilisin/kexin type 9.
Table 2.
Metaregression Analyses for the Associations of LDL‐C With Incident DM
| Studies | Patients | RR (95% CI) | |||
|---|---|---|---|---|---|
| Reduction of LDL‐C, per 1 mmol/L | Increase in Baseline LDL‐C, per 1 mmol/L | Reduction of LDL‐C Adjusted for Baseline LDL‐C | |||
| Total population | |||||
| More intensive lipid‐lowering therapy | 33 | 163 688 | 0.95 (0.87–1.04) | 0.97 (0.91–1.03) | 0.97 (0.87–1.07) |
| Statins | 21 | 124 755 | 1.02 (0.91–1.14) | 0.94 (0.88–1.01) | 1.11 (0.98–1.28) |
| PCSK9 inhibitors | 12 | 38 933 | 1.09 (0.60–1.99) | 0.95 (0.62–1.43) | 1.69 (0.71–4.05) |
| Trials with sample size of ≥500 patients which reported outcome at follow‐up ≥1 y | |||||
| More intensive lipid‐lowering therapy | 25 | 161 531 | 0.96 (0.88–1.05) | 0.98 (0.91–1.03) | 0.97 (0.87–1.07) |
| Statins | 20 | 124 486 | 1.02 (0.91–1.13) | 0.94 (0.88–1.01) | 1.11 (0.99–1.28) |
| PCSK9 inhibitors | 5 | 37 045 | 0.77 (0.48–1.20) | 1.28 (0.65–2.53) | 0.71 (0.44–1.15) |
DM indicates diabetes mellitus; LDL‐C, LDL (low‐density lipoprotein) cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; RR, risk ratio.
Figure 3.
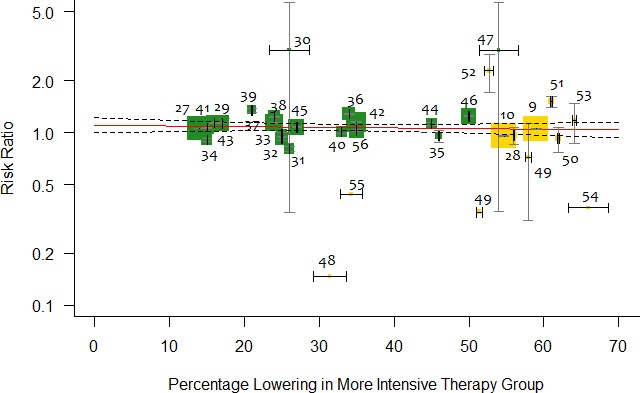
Metaregression showing association between percentage reduction of LDL (low‐density lipoprotein) cholesterol (LDL‐C) in the active arm and relative risk of incident diabetes mellitus. Each trial is represented by a data marker, the size of which is proportional to the weight in the metaregression. The metaregression slope (predicted risk for degree of LDL‐C reduction) is represented by a red line, and 95% CIs are presented as dashed lines. The horizontal lines through each square represent ±1 SE for the associated absolute change in LDL‐C, and the vertical line through each square represents the 95% CI for relative risk.
In sensitivity analysis for trials with ≥500 patients and follow‐up ≥1 year, more intensive lipid‐lowering therapy (RR: 0.96; 95% CI, 0.88–1.05; P=0.41; R 2=16%; RD: −0.002; 95% CI, −0.006 to 0.002; P=0.31; R 2=0), statins (RR: 1.02; 95% CI, 0.91–1.13; P=0.64; R 2=0; RD: −0.001; 95% CI, −0.007 to 0.003; P=0.49; R 2=0), and PCSK9 inhibitors (RR: 0.77; 95% CI, 0.48–1.20; P=0.26; R 2=0; RD: −0.001; 95% CI, −0.022 to 0.020; P=0.93; R 2=0) were not significantly associated with incident DM per 1‐mmol/L decrease in LDL‐C. Meta‐analysis stratified according to between‐group difference LDL‐C achieved across lipid‐lowering strategies did not show significant association (P=0.07 for interaction; Figure 4).
Figure 4.
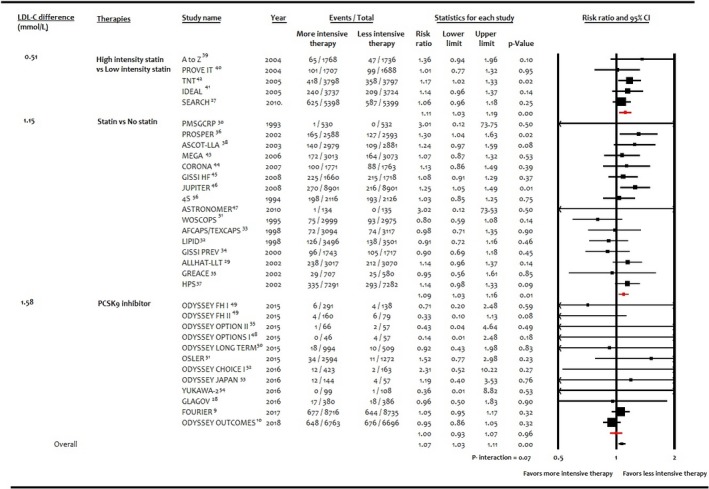
Forest plot showing subgroup analysis according to weighted between‐group difference in LDL (low‐density lipoprotein) cholesterol (LDL‐C) achieved (mmol/L) among interventions and risk of incident diabetes mellitus. PCSK9 indicates proprotein convertase subtilisin/kexin type 9.
Meta‐analysis of the entire population showed that 6.1% (5121/83 123) of patients had incident DM with the more intensive lipid‐lowering therapy versus 5.8% (4734/80 565) with the less intensive lipid‐lowering therapy. More intensive lipid‐lowering therapy was associated with a higher risk of incident DM compared with less intensive therapy (RR: 1.07; 95% CI, 1.03–1.11; P<0.001; I2=0%; RD: 0.003; 95% CI, 0.001–0.006; P=0.002; I2=23%; Figure 5). These results were driven by higher risk of incident DM with statins (RR: 1.10; 95% CI, 1.05–1.15; P<0.001; I2=14%; RD: 0.004; 95% CI, 0.002–0.006; P=0.001; I2=13%), whereas PCSK9 inhibitors were not associated with significant risk of incident DM (RR: 1.00; 95% CI, 0.93–1.07; P=0.96; I2=0%; RD: 0.001; 95% CI, −0.004 to 0.006; P=0.75; I2=11%; P=0.02 for interaction). The higher risk of DM remained consistent when statins were compared with no statins (RR: 1.09; 95% CI, 1.03–1.16; P=0.01; I2=8%; RD: 0.003; 95% CI, 0.001–0.006; P=0.01; I2=0%) or high‐intensity statins versus low‐intensity statins (RR: 1.11; 95% CI, 1.03–1.19; P<0.001; I2=0%; RD: 0.009; 95% CI, 0.003–0.014; P=0.002; I2=16%; P=0.72 for interaction; Figure 6). Sensitivity analysis for trials with ≥500 patients and follow‐up ≥1 year showed consistent results (P=0.03 for interaction; Figure 7). Meta‐analysis according to the fixed‐effects model (Table S4) or sensitivity analyses according to year of publication and definition of DM showed consistent results (Table S5). The Egger regression test did not detect publication bias (Figure S1).
Figure 5.
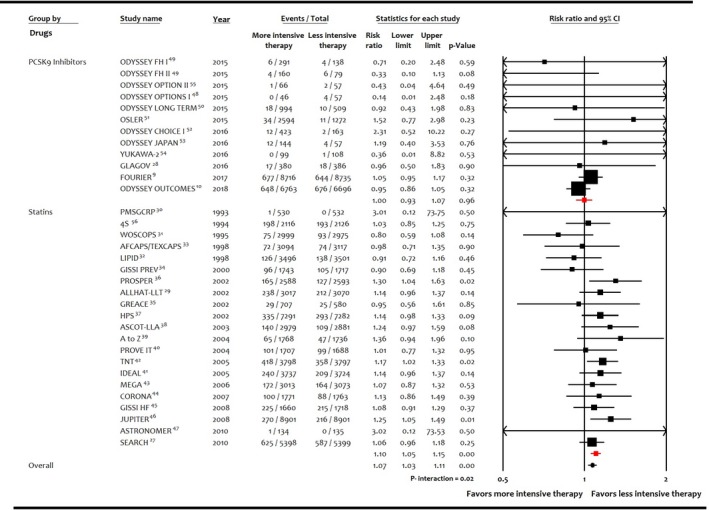
Forest plot comparing risk of incident diabetes mellitus among interventions. PCSK9 indicates proprotein convertase subtilisin/kexin type 9.
Figure 6.
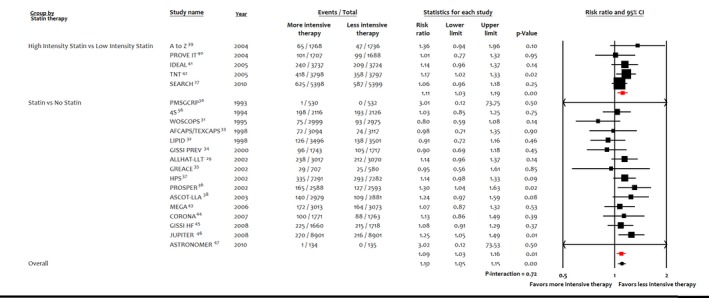
Sensitivity analysis, forest plot showing subgroup analysis of statin therapy on incident diabetes mellitus.
Figure 7.
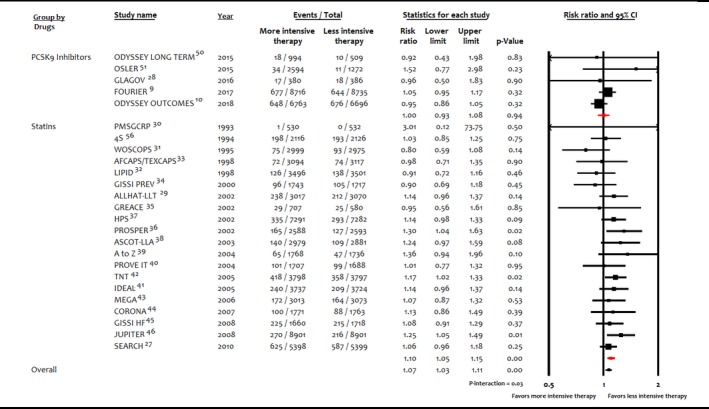
Sensitivity analysis, forest plot comparing risk of incident diabetes mellitus among interventions in trials with sample sizes ≥500 patients and follow‐up ≥1 year. PCSK9 indicates proprotein convertase subtilisin/kexin type 9.
Discussion
In this meta‐analysis we report that over a mean follow‐up duration of 4 years, metaregression analysis did not show significant association between reduction in LDL‐C by more intensive lipid‐lowering therapy and risk of incident DM. The 7% RR and 0.3% absolute risk of incident DM across more intensive lipid‐lowering strategy was driven by 10% higher RR and 0.4% absolute risk with statins. Conversely, PCSK9 inhibitors in the setting of background statin therapy were not associated with significant risk of incident DM. These results suggest that among the intensive lipid‐lowering strategies, the modest risk of incident DM may be prominent with statins only.
Statin‐induced DM is a much discussed phenomenon.7, 8, 57 The JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) trial showed a 25% increase in incident DM (physician reported) over a median follow‐up of 1.9 years with rosuvastatin 20 mg compared with placebo.58 This conclusion was also supported by Sattar and colleagues (13 trials, 91 140 patients), showing 9% increased relative risk of incident DM with statins over a mean duration of 4 years,8 and Preiss et al in their comparison of more intensive statin therapy with moderate‐intensity statin therapy.7
The exact mechanism of statin‐induced DM remains unclear, and various mechanisms have been postulated to explain this association. First, statins may derange the glucose metabolism by negative effects on both β‐cell secretion and insulin sensitivity. For example, the METSIM (Metabolic Syndrome in Men) study (9749 patients) showed 46% increased relative risk of type 2 DM, 24% reduction in insulin sensitivity, and 12% reduction in insulin secretion in patients taking statins.59 It is proposed that β‐cell dysfunction might be related to LDLR‐mediated increased levels of intracellular cholesterol. Studies with murine experimental models have shown that the addition of LDL‐C to culture medium of rat islet β cells resulted in cell death.60, 61 To further explore this concept, Besseling et al conducted a study in patients with familial hypercholesteremia (63 320 patients) and showed that prevalence of type 2 DM was significantly lower in familial hypercholesteremia patients than unaffected relatives (1.75% versus 2.93%, P<0.001).62 Hypercholesteremia in familial hypercholesteremia is caused by genetically impaired LDLR‐mediated transcellular cholesterol transport, whereas, conversely, HMGCR inhibition by statins promotes transmembranous cholesterol uptake by increasing expression of LDLR; therefore, the authors proposed that there might be a causal relationship between LDLR‐mediated increased internalization of cholesterol into pancreatic β cells and impaired insulin secretion.62
Second, animal studies have suggested that statin‐induced myopathy occurs because of development of muscle insulin resistance63; using this evidence, Preiss et al hypothesized that the risk might be related to the effect of statins on insulin sensitivity in muscle and liver.7
Third, weight gain may play a causal role in development of DM by increasing insulin resistance. Swerdlow et al studied single‐nucleotide polymorphism in HMGCR genes and used rs17238484 and rs12916 as proxies for HMGCR inhibition by statins.57 This meta‐analysis of 43 genetic studies (223 463 patients) showed that these HMGCR single‐nucleotide polymorphisms were associated with higher body weight, waist circumference, lower LDL‐C, and increased plasma glucose concentration.
Finally, genetic data have shown a potential association between LDL‐C lowering and incident DM. Lotta et al demonstrated that LDL‐C–lowering alleles in or near HMGCR were associated with higher risk of type 2 DM (odds ratio: 1.39; P=0.03).11 Although the possibility of other mechanisms cannot be excluded, the pooled analyses of randomized controlled trials could not strongly demonstrate an association between lowering LDL‐C and incident DM.8, 64
Lotta and colleagues reported that genetic variants in PCSK9 were associated with a 19% (95% CI, 2–38%) higher RR for DM per 1‐mmol/L reduction in LDL‐C.11 On the same note, PCSK9 inhibitor trials also hinted at a potential association of PCSK9 inhibitors with new‐onset DM. In FOURIER, the risk of incident DM was numerically higher with PCSK9 inhibitors (hazard ratio: 1.05; P=0.34).9 However, in a prespecified analysis of the FOURIER trial, evolocumab did not increase the risk of new‐onset DM in nondiabetic patients (hazard ratio: 1.05; 95% CI, 0.94–1.17) or those with prediabetes (hazard ratio: 1.00; 95% CI, 0.89–1.13).65 Similarly, the ODYSSEY OUTCOME trial showed fewer participants with incident DM with PCSK9 inhibitor use compared with placebo.10
We critically compared our results with prior meta‐analyses. Sattar and colleagues showed significantly higher risk of incident DM with statins, but metaregression analysis did not demonstrate an association between change in LDL‐C and risk of incident DM.8 Meta‐analysis by Preiss et al (5 statin trials, 32 752 patients) showed 12% relative risk of incident DM with intensive‐dose statin therapy compared with moderate‐dose statin therapy.7 De Carvalho et al meta‐analyzed 20 randomized controlled trials (68 123 patients) of PCSK9 therapy to investigate its association with incident type 2 DM.66 They reported that during a median follow‐up of 78 weeks, PCSK9 inhibitors increased fasting blood glucose by 1.88 mg/dL and HbA1c by 0.032%; however, this effect did not translate into increased incidence of DM (RR: 1.04; P=0.42). In a metaregression analysis, they showed a 3.8% increase in DM for each 10% lowering of LDL‐C levels; however, this study included the SPIRE trial, which does not reflect contemporary PCSK9 inhibitor therapy. Conversely, findings of Cao et al were consistent with our outcomes.64 Both studies were published before ODYSSEY OUTCOMES and thus lacked this large data set.10 To our knowledge, our current study is the largest updated meta‐analysis that, in addition to systematically evaluating the association of LDL‐C reduction with incident DM, has quantitatively compared the effects of statins and PCSK9 inhibitors to provide a more comprehensive overview of this issue.
The current study is subject to limitations. First, this study is a trial‐level meta‐analysis, and given lack of access to the individual patient data, we could not adjust our analysis for various comorbidities and baseline characteristics such as age, body mass index, baseline fasting blood glucose level, or HbA1c. Therefore, a patient‐level meta‐analysis could provide more valuable information to further evaluate such associations. Second, PCSK9 inhibitors were conducted in the background of statins. Third, it is important to note that the definition of incident DM was not uniform across the trials. Specifically, most trials reported nonadjudicated outcomes of incident DM; however, we tried to compensate for this by performing sensitivity analyses. Fourth, we could not detect publication bias; that said, because of exclusion of a notable number of trials that did not report incident DM, a certain degree of publication bias could not be completely excluded. Finally, like any meta‐analysis, this report is limited by heterogeneity in baseline characteristics, sample sizes, drugs, and durations of studies. Nevertheless, the results had low statistical heterogeneity, and we tried to compensate for variability in sample size and follow‐up duration through sensitivity analysis.
In conclusion, the current study does not demonstrate an association between degree of LDL‐C lowering by contemporary lipid‐lowering therapies and risk of incident DM. Among intense lipid‐lowering therapies, the risk of DM was higher with statins only, whereas PCSK9 inhibitors (in setting of background statin therapy) did not show a significant association with incident DM.
Disclosures
Blaha is on advisory boards for Amgen, Sanofi, Regeneron, Novartis, MedImmune, Medicure and receives grants from Amgen Foundation. The remaining authors have no disclosures to report.
Supporting information
Table S1. Search Strategy
Table S2. Cochrane Quality Risk Assessment
Table S3. Baseline Characteristics of the Entire Study Population for Each Trial
Table S4. Analyses According to Fixed‐Effects Model
Table S5. Sensitivity Analyses According to Year of Publication and Definition of Diabetes Mellitus
Figure S1. Funnel plot for publication bias assessment.
(J Am Heart Assoc. 2019;8:e011581 DOI: 10.1161/JAHA.118.011581.)
References
- 1. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association between lowering LDL‐C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta‐analysis. JAMA. 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 2. Koskinas KC, Siontis GCM, Piccolo R, Mavridis D, Raber L, Mach F, Windecker S. Effect of statins and non‐statin LDL‐lowering medications on cardiovascular outcomes in secondary prevention: a meta‐analysis of randomized trials. Eur Heart J. 2018;39:1172–1180. [DOI] [PubMed] [Google Scholar]
- 3. Khan SU, Talluri S, Riaz H, Rahman H, Nasir F, Bin Riaz I, Sattur S, Ahmed H, Kaluski E, Krasuski R. A Bayesian network meta‐analysis of PCSK9 inhibitors, statins and ezetimibe with or without statins for cardiovascular outcomes. Eur J Prev Cardiol. 2018;25:844–853. DOI: 10.1177/2047487318766612. [DOI] [PubMed] [Google Scholar]
- 4. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 5. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 6. Navarese EP, Robinson JG, Kowalewski M, Kolodziejczak M, Andreotti F, Bliden K, Tantry U, Kubica J, Raggi P, Gurbel PA. Association between baseline LDL‐C level and total and cardiovascular mortality after LDL‐C lowering: a systematic review and meta‐analysis. JAMA. 2018;319:1566–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive‐dose compared with moderate‐dose statin therapy: a meta‐analysis. JAMA. 2011;305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 8. Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet. 2010;375:735–742. [DOI] [PubMed] [Google Scholar]
- 9. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 10. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 11. Lotta LA, Sharp SJ, Burgess S, Perry JRB, Stewart IS, Willems SM, Luan J, Ardanaz E, Arriola L, Balkau B, Boeing H, Deloukas P, Forouhi NG, Franks PW, Grioni S, Kaaks R, Key TJ, Navarro C, Nilsson PM, Overvad K, Palli D, Panico S, Quirós J, Riboli E, Rolandsson O, Sacerdote C, Salamanca‐Fernandez E, Slimani N, Spijkerman AMW, Tjonneland A, Tumino R, van der A DL, van der Schouw YT, McCarthy M, Barroso I, O'Rahilly S, Savage D, Sattar N, Langenberg C, Scott RA, Wareham N. Association between low‐density lipoprotein cholesterol–lowering genetic variants and risk of type 2 diabetes: a meta‐analysis. JAMA. 2016;316:1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 Hoboken, NJ: The Cochrane Collaboration; John Wiley & sons; 2011. [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG. Group P. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. W64. [DOI] [PubMed] [Google Scholar]
- 14. Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan‐Taber L, Albright AL, Braun B. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33:e147–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kondo K, Kadowaki T. Colestilan monotherapy significantly improves glycaemic control and LDL cholesterol levels in patients with type 2 diabetes: a randomized double‐blind placebo‐controlled study. Diabetes Obes Metab. 2010;12:246–251. [DOI] [PubMed] [Google Scholar]
- 16. Zieve FJ, Kalin MF, Schwartz SL, Jones MR, Bailey WL. Results of the glucose‐lowering effect of WelChol study (GLOWS): a randomized, double‐blind, placebo‐controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin Ther. 2007;29:74–83. [DOI] [PubMed] [Google Scholar]
- 17. Toma M, McAlister FA, Bialy L, Adams D, Vandermeer B, Armstrong PW. Transition from meeting abstract to full‐length journal article for randomized controlled trials. JAMA. 2006;295:1281–1287. [DOI] [PubMed] [Google Scholar]
- 18. Yoon U, Knobloch K. Assessment of reporting quality of conference abstracts in sports injury prevention according to CONSORT and STROBE criteria and their subsequent publication rate as full papers. BMC Med Res Methodol. 2012;12:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F, Flather M, Glynn RJ, Gregoire J, Jukema JW, Karpov Y, Kastelein JJP, Koenig W, Lorenzatti A, Manga P, Masiukiewicz U, Miller M, Mosterd A, Murin J, Nicolau JC, Nissen S, Ponikowski P, Santos RD, Schwartz PF, Soran H, White H, Wright RS, Vrablik M, Yunis C, Shear CL, Tardif JC. Cardiovascular efficacy and safety of bococizumab in high‐risk patients. N Engl J Med. 2017;376:1527–1539. [DOI] [PubMed] [Google Scholar]
- 20. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DerSimonian R, Kacker R. Random‐effects model for meta‐analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. [DOI] [PubMed] [Google Scholar]
- 23. Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol. 2012;41:818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Knapp G, Hartung J. Improved tests for a random effects meta‐regression with a single covariate. Stat Med. 2003;22:2693–2710. [DOI] [PubMed] [Google Scholar]
- 26. Blazing MA; IMPROVE‐IT Investigators . Incidence of new onset diabetes in the improve‐it trial: does adding ezetimibe to simvastatin increase risk compared to simvastatin alone? European Society of Cardiology Congress 2015.
- 27. Armitage J, Bowman L, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Peto R, Collins R. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double‐blind randomised trial. Lancet. 2010;376:1658–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman SM, Scott R, Ungi I, Podolec J, Ophuis AO, Cornel JH, Borgman M, Brennan DM, Nissen SE. Effect of evolocumab on progression of coronary disease in statin‐treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373–2384. [DOI] [PubMed] [Google Scholar]
- 29. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT‐LLT). JAMA. 2002;288:2998–3007. [DOI] [PubMed] [Google Scholar]
- 30. Effects of pravastatin in patients with serum total cholesterol levels from 5.2 to 7.8 mmol/liter (200 to 300 mg/dl) plus two additional atherosclerotic risk factors. The Pravastatin Multinational Study Group for Cardiac Risk Patients. Am J Cardiol. 1993;72:1031–1037. [DOI] [PubMed] [Google Scholar]
- 31. Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. [DOI] [PubMed] [Google Scholar]
- 32. Group TL‐TIwPiIDS . Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. [DOI] [PubMed] [Google Scholar]
- 33. Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. [DOI] [PubMed] [Google Scholar]
- 34. Results of the low‐dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge? GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico). Ital Heart J. 2000;1:810–820. [PubMed] [Google Scholar]
- 35. Athyros VG, Papageorgiou AA, Mercouris BR, Athyrou VV, Symeonidis AN, Basayannis EO, Demitriadis DS, Kontopoulos AG. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary‐heart‐disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18:220–228. [DOI] [PubMed] [Google Scholar]
- 36. Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. [DOI] [PubMed] [Google Scholar]
- 37. Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomised placebo‐controlled trial. Lancet. 2003;361:2005–2016. [DOI] [PubMed] [Google Scholar]
- 38. Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations, in the Anglo‐Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT‐LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. [DOI] [PubMed] [Google Scholar]
- 39. de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH, Mukherjee R, Ramsey KE, Palmisano J, Bilheimer DW, Pfeffer MA, Califf RM, Braunwald E. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. [DOI] [PubMed] [Google Scholar]
- 40. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 41. Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, Larsen ML, Bendiksen FS, Lindahl C, Szarek M, Tsai J. High‐dose atorvastatin vs usual‐dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. [DOI] [PubMed] [Google Scholar]
- 42. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 43. Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. [DOI] [PubMed] [Google Scholar]
- 44. Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JGF, Cornel JH, Dunselma P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson Å, Hradec J, Jánosi A, Kamenský G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJV, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. [DOI] [PubMed] [Google Scholar]
- 45. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of rosuvastatin in patients with chronic heart failure (the GISSI‐HF trial): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2008;372:1231–1239. [DOI] [PubMed] [Google Scholar]
- 46. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ; Group JS . Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 47. Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. [DOI] [PubMed] [Google Scholar]
- 48. Bays H, Gaudet D, Weiss R, Ruiz JL, Watts GF, Gouni‐Berthold I, Robinson J, Zhao J, Hanotin C, Donahue S. Alirocumab as add‐on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab. 2015;100:3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kastelein JJ, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R, Blom D, Civeira F, Krempf M, Lorenzato C, Zhao J, Pordy R, Baccara‐Dinet MT, Gipe DA, Geiger MJ, Farnier M. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36:2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 51. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. [DOI] [PubMed] [Google Scholar]
- 52. Roth EM, Moriarty PM, Bergeron J, Langslet G, Manvelian G, Zhao J, Baccara‐Dinet MT, Rader DJ. A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add‐on to statin: ODYSSEY CHOICE I. Atherosclerosis. 2016;254:254–262. [DOI] [PubMed] [Google Scholar]
- 53. Teramoto T, Kobayashi M, Tasaki H, Yagyu H, Higashikata T, Takagi Y, Uno K, Baccara‐Dinet MT, Nohara A. Efficacy and safety of alirocumab in Japanese patients with heterozygous familial hypercholesterolemia or at high cardiovascular risk with hypercholesterolemia not adequately controlled with statins‐ODYSSEY JAPAN randomized controlled trial. Circ J. 2016;80:1980–1987. [DOI] [PubMed] [Google Scholar]
- 54. Kiyosue A, Honarpour N, Kurtz C, Xue A, Wasserman SM, Hirayama A. A phase 3 study of evolocumab (AMG 145) in statin‐treated Japanese patients at high cardiovascular risk. Am J Cardiol. 2016;117:40–47. [DOI] [PubMed] [Google Scholar]
- 55. Farnier M, Jones P, Severance R, Averna M, Steinhagen‐Thiessen E, Colhoun HM, Du Y, Hanotin C, Donahue S. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular‐risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016;244:138–146. [DOI] [PubMed] [Google Scholar]
- 56. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the SCANDINAVIAN Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 57. Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engmann JE, Shah T, Sofat R, Stender S, Johnson PC, Scott RA, Leusink M, Verweij N, Sharp SJ, Guo Y, Giambartolomei C, Chung C, Peasey A, Amuzu A, Li K, Palmen J, Howard P, Cooper JA, Drenos F, Li YR, Lowe G, Gallacher J, Stewart MC, Tzoulaki I, Buxbaum SG, van der A DL, Forouhi NG, Onland‐Moret NC, van der Schouw YT, Schnabel RB, Hubacek JA, Kubinova R, Baceviciene M, Tamosiunas A, Pajak A, Topor‐Madry R, Stepaniak U, Malyutina S, Baldassarre D, Sennblad B, Tremoli E, de Faire U, Veglia F, Ford I, Jukema JW, Westendorp RG, de Borst GJ, de Jong PA, Algra A, Spiering W, Maitland‐van der Zee AH, Klungel OH, de Boer A, Doevendans PA, Eaton CB, Robinson JG, Duggan D, Kjekshus J, Downs JR, Gotto AM, Keech AC, Marchioli R, Tognoni G, Sever PS, Poulter NR, Waters DD, Pedersen TR, Amarenco P, Nakamura H, McMurray JJ, Lewsey JD, Chasman DI, Ridker PM, Maggioni AP, Tavazzi L, Ray KK, Seshasai SR, Manson JE, Price JF, Whincup PH, Morris RW, Lawlor DA, Smith GD, Ben‐Shlomo Y, Schreiner PJ, Fornage M, Siscovick DS, Cushman M, Kumari M, Wareham NJ, Verschuren WM, Redline S, Patel SR, Whittaker JC, Hamsten A, Delaney JA, Dale C, Gaunt TR, Wong A, Kuh D, Hardy R, Kathiresan S, Castillo BA, van der Harst P, Brunner EJ, Tybjaerg‐Hansen A, Marmot MG, Krauss RM, Tsai M, Coresh J, Hoogeveen RC, Psaty BM, Lange LA, Hakonarson H, Dudbridge F, Humphries SE, Talmud PJ, Kivimaki M, Timpson NJ, Langenberg C, Asselbergs FW, Voevoda M, Bobak M, Pikhart H, Wilson JG, Reiner AP, Keating BJ, Hingorani AD, Sattar N. HMG‐coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cederberg H, Stancakova A, Yaluri N, Modi S, Kuusisto J, Laakso M. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: a 6 year follow‐up study of the METSIM cohort. Diabetologia. 2015;58:1109–1117. [DOI] [PubMed] [Google Scholar]
- 60. Cnop M, Hannaert JC, Grupping AY, Pipeleers DG. Low density lipoprotein can cause death of islet beta‐cells by its cellular uptake and oxidative modification. Endocrinology. 2002;143:3449–3453. [DOI] [PubMed] [Google Scholar]
- 61. Roehrich ME, Mooser V, Lenain V, Herz J, Nimpf J, Azhar S, Bideau M, Capponi A, Nicod P, Haefliger JA, Waeber G. Insulin‐secreting beta‐cell dysfunction induced by human lipoproteins. J Biol Chem. 2003;278:18368–18375. [DOI] [PubMed] [Google Scholar]
- 62. Besseling J, Kastelein JJ, Defesche JC, Hutten BA, Hovingh GK. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA. 2015;313:1029–1036. [DOI] [PubMed] [Google Scholar]
- 63. Mallinson JE, Constantin‐Teodosiu D, Sidaway J, Westwood FR, Greenhaff PL. Blunted Akt/FOXO signalling and activation of genes controlling atrophy and fuel use in statin myopathy. J Physiol. 2009;587:219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cao YX, Liu HH, Dong QT, Li S, Li JJ. Effect of proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies on new‐onset diabetes mellitus and glucose metabolism: a systematic review and meta‐analysis. Diabetes Obes Metab. 2018;20:1391–1398. [DOI] [PubMed] [Google Scholar]
- 65. Sabatine MS, Leiter LA, Wiviott SD, Giugliano RP, Deedwania P, De Ferrari GM, Murphy SA, Kuder JF, Gouni‐Berthold I, Lewis BS, Handelsman Y, Pineda AL, Honarpour N, Keech AC, Sever PS, Pedersen TR. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new‐onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941–950. [DOI] [PubMed] [Google Scholar]
- 66. de Carvalho LSF, Campos AM, Sposito AC. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and incident type 2 diabetes: a systematic review and meta‐analysis with over 96,000 patient‐years. Diabetes Care. 2018;41:364–367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search Strategy
Table S2. Cochrane Quality Risk Assessment
Table S3. Baseline Characteristics of the Entire Study Population for Each Trial
Table S4. Analyses According to Fixed‐Effects Model
Table S5. Sensitivity Analyses According to Year of Publication and Definition of Diabetes Mellitus
Figure S1. Funnel plot for publication bias assessment.
Data Availability Statement
The authors declare that all supporting data are available within the article (and its online supplementary files).


