Abstract
Background:
Tendon injuries are common problems among athletes. Complete recovery of the mechanical structure and function of ruptured tendons is challenging. It has been demonstrated that upregulation of glycolysis and lactate production occurs in wounds, inflammation sites, and cancerous tumors, and these metabolic changes also control growth and differentiation of stem and progenitor cells. Similar metabolic changes have been reported in human healing tendons. In addition, lactate production has increased in progenitors isolated from injured tendons after treatment with IL-1β. It is thought that the metabolic changes play a role in tendon healing after injury.
Hypothesis:
Glucose metabolism is altered during tendon injury and healing, and modulation of this altered metabolism improves tendon repair.
Study Design:
Controlled laboratory study.
Methods:
The authors used the tendon injury model involving a complete incision of Achilles tendon in C57BL/6J female mice and studied alterations of glucose metabolism in injured tendons with [U-13C]glucose and metabolomics analysis 1 and 4 weeks after surgery. They also examined the effects of dichloroacetate (DCA; an indirect lactate synthesis inhibitor) treatment on the recovery of structure and mechanical properties of injured tendons 4 weeks after surgery in the same mouse model.
Results:
Significant changes in glucose metabolism in tendons after injury surgery were detected. 13C enrichment of metabolites and intermediates, flux through glycolysis, and lactate synthesis, as well as tricarboxylic acid cycle activity, were acutely increased 1 week after injury. Increased glycolysis and lactate generation were also found 4 weeks after injury. DCA-treated injured tendons showed decreased cross-sectional area and higher values of modulus, maximum stress, and maximum force when compared with vehicle-treated injured tendons. Improved alignment of the collagen fibers was also observed in the DCA group. Furthermore, DCA treatment reduced mucoid accumulation and ectopic calcification in injured tendons.
Conclusion:
The findings indicate that injured tendons acutely increased glycolysis and lactate synthesis after injury and that the inhibition of lactate synthesis by DCA is beneficial for tendon healing.
Clinical Relevance:
Changing metabolism in injured tendons may be a therapeutic target for tendon repair.
Keywords: Achilles tendon, biology, biomechanics, microscopic pathology, metabolism
Tendon injuries are some of the most common problems among athletes, both young and old who participate in a variety of sports. These injuries can result in significant pain, weakness, mobility restriction, and impairment of limb function. This can result in a considerable effect on quality of life, associated work productivity loss, and substantial health care costs.39,44 The multiple factors that affect tendon healing are not completely understood, and our understanding continues to evolve with emerging scientific data. Existing clinical and preclinical experiments have utilized drug- and cell-based therapies, physical therapy, and other treatment modalities to augment tendon healing.32,39,44 The effects of current treatment strategies on tendon injuries remain moderately effective.14,32,39 Tendon-healing strategies that result in consistently reproducible painless return to full preinjury function remain a challenge for clinicians.
INTRODUCTION
Metabolic disorders such as diabetes mellitus and obesity are frequently associated with chronic tendinopathy and may have a negative effect on tendon repair.1–4,8,35 High plasma glucose levels in diabetes are considered a risk factor for tendinopathy.25 This suggests a possibility that glucose metabolism is closely linked to tendon homeostasis and healing capacity. Indeed, previous studies showed that metabolites of glycolysis and lactate are elevated in healing human Achilles tendons.16 In a case study, glucose uptake increased acutely after Achilles tendon surgery and remained high 3 months after surgery.28 Tendons and neighboring tissues contain tendon stem/progenitor cells that may contribute to injury repair.21,27 These cells were reported in uninjured tendons in rodents,6,36 rabbits,48 horses,26 and humans47 and observed in the repair process of overuse-induced minor tendon tears.46 High glucose levels upregulate proteases in tendon cells and inhibit tenogenic differentiation in tendon-derived stem cells in vitro and in tendon explants.23,24 IL-1β, which is upregulated at the tendon injury site,38 inhibits tenogenic differentiation of these injured-tendon derived progenitors and increases their lactate synthesis, while inhibition of lactate synthesis competes against the IL-1β action on tenogenic differentiation.50
The purpose of this study was to investigate the biological and mechanical relationships between glucose metabolism (glycolysis) and lactate synthesis and tendon health with a murine model. We hypothesized that glycolysis and lactate synthesis increase in response to tendon injury and that the pharmacologic modulation of lactate synthesis is beneficial for tendon repair. We analyzed glucose metabolism with [U-13C]glucose and metabolomics analysis in injured mouse tendons and examined the effects of dichloroacetate (DCA), an indirect inhibitor of lactate production, on recovery of collagen fiber formation, mucoid degeneration, and biomechanical properties.
METHODS
All animal experiment procedures were approved by the Institutional Animal Care and Use Committee of the Children’s Hospital of Philadelphia and University of Maryland, Baltimore.
Tendon Surgery
A total of 62 C57/BL6J female mice, aged 7 to 8 weeks, were used in this study. Animals were randomly selected to receive a surgically induced acute rupture of the Achilles tendon (n = 54 animals) or sham surgery (skin incision, n = 8) (see Appendix Figure A1, available in the online version of this article). Specifically, a complete transverse incision was made at the midpoint of the right Achilles tendon, and the gap was left open before wound closure.5 Animals were returned to cage activity and euthanized at 1 (n = 8), 4 (n = 44), or 10 (n = 10) weeks after surgery. Harvested tendons were subjected to metabolomics, histological, and biomechanical analyses.
DCA Administration
DCA (sodium DCA; Millipore Sigma) was dissolved in saline and intraperitoneally given to C57/BL6/j mice: daily injections (100 mg/kg, 100 μL/mouse) from 1 day to 4 weeks after surgery. The same volume of saline as DCA solution was given to the control mice.
13C-glucose Labeling Study
[U-13C]glucose (D-Glucose-13C6, 400 mg/kg; Millipore Sigma) was peritoneally injected 1 hour before euthanasia. At the endpoint shown in Appendix Figure A1, part A, the uninjured or injured tendons covering neoforming tissue in midsubstance (n = 4/group) were snap-frozen in liquid nitrogen and subsequently ground to powder for perchloric acid extraction. A neutralized perchloric acid extract was prepared and used for measurement of glycolysis and metabolomics analysis.
GC-MS and LC/MS/MS Methodology
For measurement of the 13C enrichment, samples were prepared as described previously.22,30,31 13C enrichment in glucose, glyceraldehyde-3P (GA3P), and ribose-5P was determined with LC-MS (liquid chromatography–mass spectrometry) in multiple reaction–monitoring mode.22 For glucose, we measured ion pairs 511–175, 512–175, 513–175, 514–175, 515–175, 516–175, and 517–175 for determination of M0, M1, M2, M3, M4, M5, and M6, respectively (containing 1–6 13C atoms above natural abundance). For GA3P, we measured ion pairs at 501–175, 502–175, 503–175, and 504–175 for determination of M0, M1, M2, and M3, respectively (containing 1–3 13C atoms above M0, the natural abundance). For ribose-5P, we measured ion pairs 561–175, 562–175, 563–175, 564–175, 565–175, and 566–175 for determination of M0, M1, M2, M3, M4, and M5, respectively (containing 1–5 13C atoms above natural abundance). We used GC-MS (gas chromatography–mass spectrometry) [AQ6: Please confirm or update highlighted text for definition of GC-MS.] for determination of 13C enrichment in organic or amino acids as described.30,31 Isotopic enrichment for 13C-lactate isotopomers was monitored at m/z 261, 262, 263, and 264 for M0, M1, M2, or M3, respectively (containing 1–3 13C atoms above M0, the natural abundance). Isotopic enrichment in 13C-alanine isotopomers was monitored with ions at m/z 260, 261, 262, and 263 for M0, M1, M2, or M3, respectively (containing 1–3 13C atoms above M0, the natural abundance). Isotopic enrichment in [13C]glutamate isotopomers was monitored with ions at m/z 432, 433, 434, 435, 436, and 437 for M0, M1, M2, M3, M4, or M5, respectively (containing 1–5 13C atoms above M0, the natural abundance). Isotopic enrichment in [13C]aspartate isotopomers was monitored with ions at m/z 418, 419, 420, 421, and 422 for M0, M1, M2, M3, and M4, respectively (containing 1–4 13C atoms above M0, the natural abundance). Isotopic enrichment in 13C malate isotopomers was monitored with ions at m/z 419, 420, 421, 422, and 423 for M0, M1, M2, M3, and M4, respectively (containing 1–4 13C atoms above the natural abundance), and 13C enrichment in [13C]citrate isotopomers was monitored with ions at m/z 459, 460, 461, 462, 463, 464, and 465 for M0, M1, M2, M3, M4, M5, and M6, respectively (containing 1–6 13C atoms above the natural abundance).
13C enrichment in glucose-derived metabolites and intermediates was expressed as molar percentage enrichment (M%E), which is the molar fraction percentage of analyte containing 13C atoms above natural abundance.30,31 The M%E of an individual 13C-labeled mass isotopomer was calculated as reported previously,30,31 and the amount in the tendons of 13C-labeled mass isotopomer was calculated by the product of M%E/100 multiplied by the concentration (nmol/mg of protein) and was expressed as nanomole (nmol) of 13C-labeled metabolite per milligram (mg) of protein.
Histologic, Histochemical, and Immunohistochemical Analyses
Distal half hind limbs were dissected and fixed with 4% (v/v) paraformaldehyde, decalcified with EDTA solution for 2 to 3 weeks, and embedded in paraffin. Longitudinal sections of the Achilles tendons (n = 4/group) (Appendix Figure A1, part B) were prepared and subjected to histologic staining with hematoxylin and eosin or alcian blue (pH 1.0). Ten to 15 paraffin section slides (3 or 4 sections/slide) covering the middle portion of injured tendons were obtained, and 2 or 3 slides (4 or 5 sections in total) were chosen and analyzed to evenly inspect the entirety of the injured tendons. Glycosaminoglycan accumulation was quantified by measuring alcian blue–positive area with the Hybrid Cell Count program in the BZ-X Analyzer (Keyence). Collagen fiber alignment was quantitatively evaluated by polarized light image analysis with custom Matlab software (Mathworks).11
For detection of lactate dehydrogenase A (LDHA) and lactate transporter (MCT1 and MCT4) expression, the sections were treated with sodium citrate buffer (pH 6.0) for 10 minutes at 95°C and blocked with 10% goat serum in phosphate-buffered solution (PBS). Endogenous avidin/biotin was quenched with the Avidin/Biotin Blocking Kit (Vector Laboratories), followed by overnight incubation with rabbit anti-LDHA antibody (1:250; Cell Signaling Technology) or rabbit anti-MCT1 or anti-MCT4 antibodies (1:1000, generated by N. Philp) at 4°C. After washes with PBS, the sections were incubated with goat anti-rabbit biotinylated secondary antibody (1:200, Vector Laboratories) at room temperature for an hour and with ABC reagent (Vector Laboratories) for an hour, followed by visualization of the antibody with the ImmPACT DAB kit (Vector Laboratories) and counterstaining with hematoxylin. The results of immunostaining (n = 3 or 4/group) were evaluated by semiquantification of the images (score, 0–4; 4 is maximum), captured by the SPOT digital microscope camera with SPOT Imaging Software (Meyer Instruments, Inc).
Mechanical Testing and Collagen Alignment Measurement
The distal hind limbs were dissected from the mice treated with DCA or vehicle (n = 10/group) 4 weeks after surgery (Appendix Figure A1, part C) and stored at –30°C until the analysis. In before mechanical testing, Achilles tendons with calcanei were fine dissected such that all musculature and surrounding soft tissue were removed. Specimens were hydrated in PBS immediately after dissection and throughout the course of mechanical testing. Tendon cross-sectional area was calculated with a custom laser-based device.12 Mechanical testing was performed in an Instron 5542 test frame (Instron), and tendons were held in place with a custom fixture that gripped the calcaneus and tendon ends to a gauge length of 5 mm. Specimens underwent a previously described protocol consisting of preconditioning, stress-relaxation, and ramp to tensile failure at a rate of 0.1%/s.9 Grayscale images were captured during mechanical testing, and fiber alignment was subsequently quantified with a cross-polarized light protocol, according to previously published methods.19
Micro–Computed Tomography Analyses
The distal hind limbs were dissected from the mice treated with DCA or vehicle (n = 5/group) 10 weeks after surgery (Appendix Figure A1, part C), fixed with 10% neutralized formalin at 4°C, and subjected to micro–computed tomography analysis with a CT40 scanner (Scanco USA, Inc) at 55 kV and 70 mA. Data were analyzed at threshold 244 for detection of ectopically mineralized components.
Statistics
Student t tests or 2-way factorial analysis of variance, followed by Bonferroni post hoc multiple comparison tests, was used to identify differences between groups. Significance for tests was set as P < .05 or P < .01.
RESULTS
Injury-Induced Changes in Glucose Metabolism in Tendons
In the first set of experiments, we performed 13C-metabolic analysis to examine alterations in glucose metabolism in tendons in response to injury. [U-13C]glucose was injected 1 hour before collection of the tendons at 1 or 4 weeks after surgery. We determined the relative percentage of [U-13C]glucose in the tendons, as well as the 13C enrichment (M%E) of metabolites in the glycolysis pathway (eg, GA3P) and the pentose phosphate pathway (ribose-5P); we also analyzed lactate synthesis, intermediates of the tricarboxylic acid (TCA) cycle, and amino acids. The isotopomers that we show in Figures 1 and 2 were the direct metabolites from [U-13C]glucose in the glycolysis pathway. There was no difference in 13C-labeled glucose (M%E) in injured versus normal tendons (Figure 1B), suggesting a similar uptake of the injected [U-13C]glucose. However, the M%E of M3 isotopomer of 13C-labeled GA3P (Figure 1C) and M5 ribose-5P (Figure 1D) increased at 1 week after surgery with only a little difference at 4 weeks as compared with the uninjured tendons, suggesting that injured tendons greatly altered glucose metabolism by increasing the relative pentose phosphate pathway and overall glycolysis. The stimulation of glycolysis by the injured tendons is further demonstrated by a significant increased enrichment of M3 isotopomers (M%E) of 13C-labeled lactate (Figure 1E). Similarly, the synthesis of M313C-alanine was significantly higher at 1 and 4 weeks after injury (Figure 1F). 13C-labeled lactate and alanine are both products derived from [U-13C]glucose through pyruvate (Figure 1A). Of interest is the observation that the augmentation of 13C-labeled lactate and alanine production was similar at 1 and 4 weeks after injury, suggesting a persistent stimulation of glycolysis even after 4 weeks of injury. The product concentrations of 13C-labeled lactate and alanine were also calculated. The results are similar to those of 13C enrichment (Appendix Figure A2).
Figure 1.
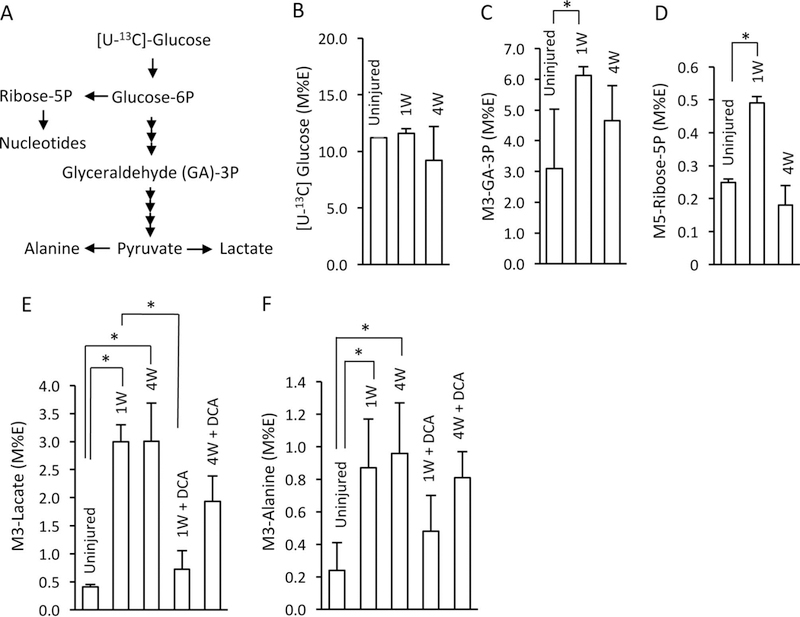
The effect of injured tendons on glycolysis with or without treatment with dichloroacetate (DCA). Injured Achilles tendons were harvested 1 week (1W) or 4 weeks (4W) after the Achilles injury surgery. [U-13C]glucose (D-Glucose-13C6, 400 mg/kg) was peritoneally injected 1 hour before euthanasia without or with DCA treatment (100 mg/kg, daily intraperitoneal injections). The extracts from uninjured or injured tendons (n = 4) were used for measurement of 13C enrichment (M%E/) in (B) 13C-labeled glucose or metabolites: (C) GA-3P, (D) ribose-5P, (E) lactate, or (F) alanine—the metabolism of which is shown in panel A. Values are presented as mean ± 1 SD. *P < .05. M%E, molar percentage enrichment.
Figure 2.

The generation of 13C mass isotopomers of citrate, glutamate, and aspartate. The same tendon extracts described Figure 1 were analyzed for 13C enrichment (M%E) to the total amount of 13C mass isotopomers of (B) citrate, (C) glutamate, or (D) aspartate—the metabolism of which is shown in panel A. Values are presented as mean ± 1 SD. *P < .05. 1W, 1 week; 4W, 4 weeks; M%E, molar percentage enrichment; PC, pyruvate carboxylase; PDH, Pyruvate dehydrogenase; TCA, tricarboxylic acid.
Notwithstanding a high rate of glycolysis during the first 4 weeks after injury, flux through the TCA cycle was also significantly increased after 1 week and returned to normal range after 4 weeks of injury. This conclusion is supported by the generation of M2 13C-labeled citrate (Figure 2B) and M2 13C-labeled glutamate (Figure 2D). M2 glutamate isotopomers, a marker of the flux through the TCA cycle,30,31 is generated from M2 13C-labeled α-ketoglutarate via the glutamate dehydrogenase pathway (Figure 2A). The production of M2 13C-aspartate was significantly increased at 1 and 4 weeks after injury (Figure 2D). While 13C aspartate is generated from 13C-labeled oxaloacetate via transamination, 13C-labeled oxaloacetate may be produced from pyruvate via pyruvate carboxylase and/or from malate via malate dehydrogenase.30,31 The persistent generation of 13C-labeled aspartate in the injured tendon is parallel to generation of 13C-labeled lactate and alanine at 1 and 4 weeks after injury (Figure 1E, F), suggesting that similar to lactate and alanine, the 13C-labeled aspartate is mainly formed from pyruvate via the pyruvate carboxylase pathway. The product concentrations of 13C-labeled citrate, glutamate, and aspartate were also increased in tendons after injury (Appendix Figure A2).
The results of the metabolomics analysis suggest that the injured tendons are high in the lactate synthesis from pyruvate. This was supported by the immunohistochemical examination for LDHA and lactate transporters (Figure 3, Appendix Figure A3). Expression of LDHA (Figure 3F, J) and lactate transporters MCT1 (Figure 3G, K) and MCT4 (Figure 3H, L) were clearly detected at 1 week after surgery in the injured site, which as were rich in fibroblastic cells and vessels (Figure 3I), while the uninjured tendons showed marginal positive staining with these molecules (Figure 3B–D). At 4 weeks after injury, the staining of these molecules became weak but were still detected in the chondroid lesion at the edge of the injured tendons (Figure 3M–P, circled with dotted lines) where ectopic calcification is induced at later stages. Expression of MCT1 and MCT4 was found in the region of the midsubstance in the injured tendons where the cellular density was high (Figure 3S, T, circled).
Figure 3.

Expression of lactate dehydrogenase A (LDHA) and lactate transporters (MCT1 and MCT4) in injured tendons. The longitudinal sections of injured tendons (1 and 4 weeks [1w and 4w] after surgery) and uninjured tendons were subjected to (A, E, I, M, Q) hematoxylin and eosin (HE) staining and immunostaining for (B, F, J, N, R) LDHA, (C, G, K, O, S) MCT1, and (D, H, L, P, T) MCT4. The images were taken at the edges (stub) and midsubstance of tendons. Strong immunoreactivity of LDHA, MCT1, and MCT4 in (A-L) 1 week–injured tendons remained in (M-P, circles) chondroid lesions at edges and (Q-T, circles) the region with high cell density at midsubstance 4 weeks after injury. The scale bar, 100 μm.
Effects of DCA Treatment on Tendon Healing
To examine the effect of augmented lactate synthesis on the healing process of the injured tendon, we treated the mice with DCA after tendon injury surgery. The data showed that the generation of 13C-lactate were significantly reduced by DCA treatment at 1 and 4 weeks after surgery (Figure 1E, F), while the generation of 13C-citrate was increased (Figure 2B, C), indicating that DCA treatment inhibited lactate formation. However, DCA had little or no effect on the flux through the TCA cycle or the generation of 13C-aspartate or glutamate (Figure 2B–G). The body weight and body length were not significantly affected by DCA treatment (Appendix Figure A4).
Mechanical testing assays yielded significant differences between groups at the 4-week time point. The DCA-treated samples had thinner widths (confirmed with histology) and smaller cross-sectional areas (Figure 4A, B) as compared with the control group. Modulus, maximum strength, and maximum force were significantly higher in the DCA-treated tendons than the vehicle-treated tendons (Figure 4C–E). Collagen fiber alignment was improved in the DCA-treated tendons, as they had lower values of circular standard deviation than the control tendon when measured during mechanical testing (Figure 5A) and with polarized light microscopy (Figure 5B).
Figure 4.
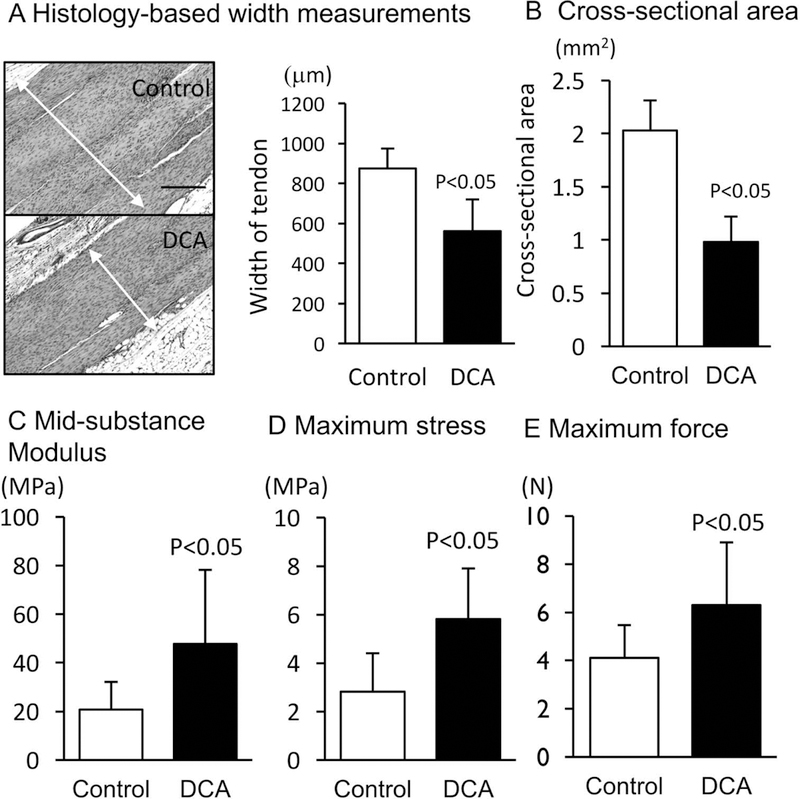
The results from mechanical testing assays. (A) The width of the injured tendons (n = 10) was measured with histology and ImageJ, and (B) the cross-sectional area was determined with a laser-based system; there were significant decreases in the dichloroacetate (DCA) group for both. The DCA group also exhibited significant increases in (C) modulus, (D) maximum stress, and (E) maximum force. Scale bar is 250 mm for panel A. Values are presented as mean ± 1 SD.
Figure 5.

The effects of dichloroacetate (DCA) treatment on alignment of collagen, mucoid accumulation, and ectopic mineralization within the tendon. The DCA group had significant improvement in alignment, as shown by decreases in circular standard deviation during (A) mechanical testing (n = 10) and (B) polarized light microscopy (n = 4). The DCA group had less glycosaminoglycans and less volume of mineralized materials, as evaluated by (C, D) alcian blue (AB) staining (n = 4) and (E, F) micro–computed tomography (μ-CT) analysis (n = 5). Scale bar is 125 mm for panel B. Values are presented as mean ± 1 SD.
In this Achilles tendon surgery model, the injured tendons induced chondroid degeneration and ectopic calcification.5,49 The DCA treatment reduced accumulation of mucopolysaccharides visualized by alcian blue staining (Figure 5C, D) and the volume of mineralized masses (Figure 5E, F).
DISCUSSION
The results indicate that tendons acutely change glucose metabolism with an increase in glycolysis and lactate synthesis in response to injury. We also demonstrated that treatment with an inhibitor of lactate synthesis stimulated recovery of biomechanical properties in injured tendons, improved collagen fiber alignment, and inhibited mucoid accumulation and ectopic calcification. The findings indicate that tendons alter glucose metabolism in response to injury and that the metabolic change can be targeted by metabolic modifiers for improvement of tendon healing. Our conclusion should be limited to the early phase of tendon healing in mice. The efficacy of DCA needs to be verified for the late phase of the tendon-healing process in mice and for other animal models. The effectiveness of DCA on human tendons remains to be clarified. However, the results in this study are consistent with and complemented by studies of human tendons showing that early-healing Achilles tendons contain higher levels of pyruvate and lactate, metabolites of glycolysis.16
Altered glucose metabolism has been described under various pathologic conditions. In tumors, increases in glycolysis and lactate production under the aerobic condition, called the Warburg effect, have been found and extensively studied to understand the underlying cellular and molecular mechanisms and develop pharmacologic therapies for this condition.10,13 Upregulation of glycolysis and lactate synthesis has also been observed in the milieu of wounds and inflammation.7,15,40 It is also critical for growth and survival of cancerous tumors and polarizing of macrophages.10,17,34 Substantial evidence demonstrates that these metabolic changes control growth and differentiation of stem and progenitor cells.29,42 Greve et al16 examined metabolic activity of human Achilles tendons at 2 weeks after surgery by measuring glucose and metabolites. The results demonstrate elevation of the contents of lactate, pyruvate, and glutamate in the healing tendons and further enhancement of their production by adjuvant intermittent pneumatic compression.16
Consistent with this fundamental study, our metabolomics results revealed that glycolysis and lactate synthesis are stimulated in injured mouse tendons, as characterized by increases in 13C enrichment of metabolites and intermediates, flux through glycolysis, and lactate synthesis in the metabolomics analysis with [U-13C]glucose. These changes were detected not only at the inflammation phase at 1 week after surgery but also at the early repair phase 4 weeks after surgery, indicating that cells responsible for these alterations are not only in inflammatory cells and vessels but also in the connective tissue cells in injured tendons. These connective tissue cells likely include tendon cells, tendon and mesenchymal progenitors, and other fibrous and chondrocytic cells. The results from our immunostaining examination (Figure 2) support this idea. Staining of LDHA and MCT1/MCT4 (lactate transporter) suggests that the lactate metabolic activity was very high in the entirety of injured tendons at the initial phase and became lower but still high in the chondroid lesion and the region with a high density of fibrous cells.
DCA is an inhibitor of pyruvate dehydrogenase kinase that inhibits pyruvate dehydrogenase.43 Thus, DCA is thought to stimulate formation of pyruvate to acetyl-CoA and, thereby, the TCA cycle pathway and indirectly inhibit lactate synthesis. The results of the metabolomics analysis confirmed that DCA treatment reduced lactate formation at 1 and 4 weeks after surgery. However, DCA treatment hardly affected the flux through the TCA cycle or the generation of 13C-aspartate or glutamate. Although decarboxylation of pyruvate was enhanced and, thereby, lactate synthesis inhibited, the flux of intermediates and amino acids via the TCA cycle was not affected. The concentration of DCA that we used in this study could be not enough high to stimulate the TCA cycle activity. Further studies are required for understanding the pharmacologic action of DCA on inhibition of lactate production.
DCA treatment gave a strong beneficial effect on recovery of structure and biomechanical properties of injured tendon at least in female mice. The results indicate that lactate production is critical for tendon healing. In wounds, lactate accumulates regardless of oxygen concentration and stimulates VEGF in macrophages and collagen synthesis in fibroblasts.40 Therefore, lactate may mediate angiogenesis and fibrous tissue formation (scar) in injured tendons. Hyperglycemia and high glucose affect matrix metabolism, growth factor production, and glycation end products in tendon cells and progenitors,23,24 suggesting that control of glucose utilization, such as control of glucose production/absorption and insulin sensitivity, is important in maintenance or stimulation of tendon cell function and may be a therapeutic target for tendinopathy.1,35 Lactate synthesis can be a regulatory point in this scenario.
Mucoid degeneration, accumulation of glycosaminoglycan, is one of the pathologic features of tendinopathy.18 In this study, DCA treatment strongly reduced accumulation of mucoid materials and inhibited ectopic calcification. Studies demonstrated that ectopic calcification in rodent Achilles tendon rupture models is caused by ectopic chondrogenesis-mediated endochondral ossification.5,45 Hypoxia enhances or is associated with chondrogenesis,20,37 and an increase in lactate production was observed during chondrogenesis in human mesenchymal stem cell cultures.33 Thus, inhibition of lactate formation by DCA might suppress initiation or progression of chondrogenesis. It was reported that forced expression of PDK2 (pyruvate dehydrogenase kinase 2), an inhibitor of pyruvate dehydrogenase, induced chondrogenesis in human mesenchymal cells in culture via Sox6 and activation of the JNK/MAPK/ERK pathway.41 Since DCA is an inhibitor of PDKs,43 our finding is consistent with this report. As an increase in ectopic calcification is associated with decreased mechanical strengths,49 inhibition of accumulation of glycosaminoglycan and ectopic calcification is likely to be favorable for tendon repair, although we should further define whether such effects directly lead to improvement of mechanical properties in injured tendons.
Our findings suggest that reprogramming of metabolism may be a therapeutic target for tendon repair. Metabolic modifiers for glucose metabolism and lactate transport have been studied in clinical trials for cancer therapy. A similar approach might be applied for improvement of tendon healing.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Y. Daikhin, O. Horyn, and Ilana Nissim for assistance with the isotopomer enrichment analysis in the Metabolomic Core facility, Children’s Hospital of Philadelphia. They also thank the Biomechanics Core of the Penn Center for Musculoskeletal Disorders and L. Cantley, A. T. Gunawardena, N. Francois, and W. Kimberly for technical assistance.
source of funding: This study was supported by the Penn Center for Musculoskeletal Disorders Pilot and Feasibility Grant (National Institutes of Health / National Institute of Arthritis and Musculoskeletal and Skin Diseases P30AR069619) and the National Institutes of of Arthritis and Musculoskeletal and Skin Diseases (R01AR070099) and partially by National Institute of Children Health and Human Development (U54HD086984), the National Natural Science Foundation of China (81601900), and the Science and Technology Project of Guangdong Province (2016A020214010). M.W.H. has received research support with DePuy Synthesis and Integra.
Footnotes
One or more of the authors has declared the following potential conflict of interest
Investigation performed at the Children’s Hospital of Philadelphia, University of Pennsylvania, Philadelphia, Pennsylvania, USA, and University of Maryland, Baltimore, Maryland, USA
REFERENCES
- 1.Abate M, Schiavone C, Salini V, Andia I. Occurrence of tendon pathologies in metabolic disorders. Rheumatology (Oxford). 2013;52(4):599–608. [DOI] [PubMed] [Google Scholar]
- 2.Ackerman JE, Geary MB, Orner CA, Bawany F, Loiselle AE. Obesity/type II diabetes alters macrophage polarization resulting in a fibrotic tendon healing response. PLoS One. 2017;12(7):e0181127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann PW, Hart DA. General overview and summary of concepts regarding tendon disease topics addressed related to metabolic disorders. Adv Exp Med Biol. 2016;920:293–298. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed AS, Schizas N, Li J, et al. Type 2 diabetes impairs tendon repair after injury in a rat model. J Appl Physiol (1985). 2012;11`3(11):1784–1791. [DOI] [PubMed] [Google Scholar]
- 5.Asai S, Otsuru S, Candela ME, et al. Tendon progenitor cells in injured tendons have strong chondrogenic potential: the CD105-negative subpopulation induces chondrogenic degeneration. Stem Cells. 2014;32(12):3266–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219–1227. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter KL, Jalloh I, Hutchinson PJ. Glycolysis and the significance of lactate in traumatic brain injury. Front Neurosci. 2015;9:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connizzo BK, Bhatt PR, Liechty KW, Soslowsky LJ. Diabetes alters mechanical properties and collagen fiber re-alignment in multiple mouse tendons. Ann Biomed Eng. 2014;42(9):1880–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connizzo BK, Sarver JJ, Birk DE, Soslowsky LJ, Iozzo RV. Effect of age and proteoglycan deficiency on collagen fiber re-alignment and mechanical properties in mouse supraspinatus tendon. J Biomech Eng. 2013;135(2):021019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123(9):3685–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunkman AA, Buckley MR, Mienaltowski MJ, et al. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013;32(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favatia M Scarless Healing in the Fetus: Implications and Strategies for Postnatal Tendon Repair [dissertation]. Philadelphia, PA: University of Pennsylvania; 2006. ProQuest AAI3246156 [Google Scholar]
- 13.Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813–57825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galatz LM, Gerstenfeld L, Heber-Katz E, Rodeo SA. Tendon regeneration and scar formation: the concept of scarless healing. J Orthop Res. 2015;33(6):823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghani QP, Wagner S, Becker HD, Hunt TK, Hussain MZ. Regulatory role of lactate in wound repair. Methods Enzymol. 2004;381:565–575. [DOI] [PubMed] [Google Scholar]
- 16.Greve K, Domeij-Arverud E, Labruto F, et al. Metabolic activity in early tendon repair can be enhanced by intermittent pneumatic compression. Scand J Med Sci Sports. 2012;22(4):e55–e63. [DOI] [PubMed] [Google Scholar]
- 17.Haschemi A, Kosma P, Gille L, et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15(6):813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon: a controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507–1525. [PubMed] [Google Scholar]
- 19.Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. J Orthop Res. 2009;27(12):1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leijten J, Georgi N, Moreira Teixeira L, et al. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc Natl Acad Sci U S A. 2014;111(38):13954–13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong DJ, Sun HB. Mesenchymal stem cells in tendon repair and regeneration: basic understanding and translational challenges. Ann N Y Acad Sci. 2016;1383(1):88–96. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Qiu B, Lee DS, et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513(7517):251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YC, Li YJ, Rui YF, et al. The effects of high glucose on tendon-derived stem cells: implications of the pathogenesis of diabetic tendon disorders. Oncotarget. 2017;8(11):17518–17528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little C Effects of high glucose and metformin on tendon explants: a role for fibrosis in tendinopathy. Br J Sports Med. 2014;48(suppl 2):A61–A62. [Google Scholar]
- 25.Longo UG, Franceschi F, Ruzzini L, et al. Higher fasting plasma glucose levels within the normoglycaemic range and rotator cuff tears. Br J Sports Med. 2009;43(4):284–287. [DOI] [PubMed] [Google Scholar]
- 26.Lovati AB, Corradetti B, Lange Consiglio A, et al. Characterization and differentiation of equine tendon-derived progenitor cells. J Biol Regul Homeost Agents. 2011;25(2):S75–S84. [PubMed] [Google Scholar]
- 27.Lui PP, Chan KM. Tendon-derived stem cells (TDSCs): from basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev. 2011;7(4):883–897. [DOI] [PubMed] [Google Scholar]
- 28.Masood T, Kalliokoski K, Bojsen-Moller J, Finni T. Muscle-tendon glucose uptake in Achilles tendon rupture and tendinopathy before and after eccentric rehabilitation: comparative case reports. Phys Ther Sport. 2016;21:14–19. [DOI] [PubMed] [Google Scholar]
- 29.Moussaieff A, Rouleau M, Kitsberg D, et al. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab. 2015;21(3):392–402. [DOI] [PubMed] [Google Scholar]
- 30.Nissim I, Horyn O, Daikhin Y, et al. The molecular and metabolic influence of long term agmatine consumption. J Biol Chem. 2014;289(14):9710–9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nissim I, Horyn O, Nissim I, et al. Effects of a glucokinase activator on hepatic intermediary metabolism: study with 13C-isotopomer-based metabolomics. Biochem J. 2012;444(3):537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nourissat G, Berenbaum F, Duprez D. Tendon injury: from biology to tendon repair. Nat Rev Rheumatol. 2015;11(4):223–233. [DOI] [PubMed] [Google Scholar]
- 33.Pattappa G, Heywood HK, de Bruijn JD, Lee DA. The metabolism of human mesenchymal stem cells during proliferation and differentiation. J Cell Physiol. 2011;226(10):2562–2570. [DOI] [PubMed] [Google Scholar]
- 34.Philp A, Macdonald AL, Watt PW. Lactate—a signal coordinating cell and systemic function. J Exp Biol. 2005;208(pt 24):4561–4575. [DOI] [PubMed] [Google Scholar]
- 35.Ranger TA, Wong AM, Cook JL, Gaida JE. Is there an association between tendinopathy and diabetes mellitus? A systematic review with meta-analysis. Br J Sports Med. 2016;50(16):982–989. [DOI] [PubMed] [Google Scholar]
- 36.Rui YF, Lui PP, Li G, et al. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16(5):1549–1558. [DOI] [PubMed] [Google Scholar]
- 37.Schipani E Posttranslational modifications of collagens as targets of hypoxia and Hif-1alpha in endochondral bone development. Ann N Y Acad Sci. 2010;1192:317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugg KB, Lubardic J, Gumucio JP, Mendias CL. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J Orthop Res. 2014;32(7):944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33(6):832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trabold O, Wagner S, Wicke C, et al. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair Regen. 2003;11(6):504–509. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Shan XB, Qiao YJ. PDK2 promotes chondrogenic differentiation of mesenchymal stem cells by upregulation of Sox6 and activation of JNK/MAPK/ERK pathway. Braz J Med Biol Res. 2017;50(2):e5988. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Wang YH, Israelsen WJ, Lee D, et al. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell. 2014;158(6):1309–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitehouse S, Cooper RH, Randle PJ. Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochem J. 1974;141(3):761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu F, Nerlich M, Docheva D. Tendon injuries: basic science and new repair proposals. EFORT Open Rev. 2017;2(7):332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yee Lui PP, Wong YM, Rui YF, et al. Expression of chondro-osteogenic BMPs in ossified failed tendon healing model of tendinopathy. J Orthop Res. 2011;29(6):816–821. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Pan T, Liu Y, Wang JH. Mouse treadmill running enhances tendons by expanding the pool of tendon stem cells (TSCs) and TSC-related cellular production of collagen. J Orthop Res. 2010;28(9):1178–1183. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Wang JH. BMP-2 mediates PGE(2) -induced reduction of proliferation and osteogenic differentiation of human tendon stem cells. J Orthop Res. 2011;30(1):47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang K, Asai S, Hast MW, et al. Tendon mineralization is progressive and associated with deterioration of tendon biomechanical properties, and requires BMP-Smad signaling in the mouse Achilles tendon injury model. Matrix Biol. 2016;52–54:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang K, Asai S, Yu B, Enomoto-Iwamoto M. IL-1beta irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem Biophys Res Commun. 2015;463(4):667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


