Abstract
Objectives:
The emergence of human-unique cognitive abilities has been linked to our species’ extended juvenile period. Comparisons of cognitive development across species can provide new insights into the evolutionary mechanisms shaping cognition. This study examined the development of different components of spatial memory, cognitive mechanisms that support complex foraging, by comparing two species with similar life history that vary in wild ecology: bonobos (Pan paniscus) and chimpanzees (Pan troglodytes).
Materials and Methods:
Spatial memory development was assessed using a cross-sectional experimental design comparing apes ranging from infancy to adulthood. Study 1 tested 73 sanctuary-living apes on a task examining recall of a single location after a one-week delay, compared to an earlier session. Study 2 tested their ability to recall multiple locations within a complex environment. Study 3 examined a subset of individuals from Study 2 on a motivational control task.
Results:
In Study 1, younger bonobos and chimpanzees of all ages exhibited improved performance in the test session compared to their initial learning experience. Older bonobos, in contrast, did not exhibit a memory boost in performance after the delay. In Study 2, older chimpanzees exhibited an improved ability to recall multiple locations, whereas bonobos did not exhibit any age-related differences. In Study 3, both species were similarly motivated to search for food in the absence of memory demands.
Discussion:
These results indicate that closely-related species with similar life history characteristics can exhibit divergent patterns of cognitive development, and suggests a role of socioecological niche in shaping patterns of cognition in Pan.
Keywords: Comparative cognition, spatial cognition, life history, ecology, apes
1. Introduction
Complex cognition is one of the defining traits of the human species, so understanding the origins of the human mind is a pressing issue for human evolution. Why do we possess such complex cognitive abilities, and how are these skills acquired? The flexible behavior that humans exhibit has motivated numerous theories concerning the human mind, examining both ultimate questions about the evolutionary history of human cognition, as well as proximate questions about the mechanisms underpinning behavior (Hare 2017; Hill et al. 2009; Kaplan et al. 2000; MacLean 2016; Tomasello 2014; Whiten and Erdal 2012). An emerging approach to answering these issues is to probe the evolutionary and developmental roots of human cognition in an integrative fashion, by examining patterns of comparative cognitive development across species (Bjorklund and Green 1992; Gomez 2005; Matsuzawa 2007; Matsuzawa et al. 2006; Rosati et al. 2014b). The current work compared patterns of cognitive development in chimpanzees (Pan troglodytes) and bonobos (Pan paniscus), our two closest-living relatives, to illuminate the evolutionary origins of complex cognitive skills across species, including in humans.
Several proposals specifically link human cognitive capacities to our species’ specialized life history characteristics. Humans are marked by an extended juvenile period as well as increased total longevity (Bogin 2010; Bogin and Smith 1996; Leigh 2004; Leigh 2012; Robson and Wood 2008; Schwartz 2012). According to the life history hypothesis, humans’ prolonged juvenile period enables greater behavioral flexibility and extended periods of complex skill acquisition over development, and our increased longevity allows for these skills to be exploited such that they ‘pay off’ over the lifespan (Bjorklund and Green 1992; Bjorklund and Bering 2003; Janson and van Schaik 1993; Kaplan et al. 2000; Schuppli et al. 2012). This view suggests that life history characteristics drive shifts in cognitive development, such that a longer juvenile period can allow for longer periods of cognitive development and skill refinement. This proposal is supported by evidence from human forager populations indicating that individuals may not exhibit adult-like hunting or foraging skills until adulthood (Gurven et al. 2006; Kaplan et al. 2000; Koster et al. 2019). Taking a broader comparative perspective, primates also exhibit a relatively extended juvenile period, which has been proposed to stem from the long time needed to acquire skills to exploit complex diets (Ross and Jones 1999; although note that young chimpanzees already exhibit adult-like dietary breadth; Bray et al., 2017). Other evidence comes from comparisons showing that larger brain size and slower life histories tend to co-vary across species (Anton et al. 2014; Isler and van Schaik 2014; Schuppli et al. 2016; Schwartz 2012). However, this line of work primarily uses brain size as a proxy for cognitive skills. Yet brain size is only a rough index of more specific cognitive abilities (Healy and Rowe 2007; Logan et al. 2018), and there is clear evidence that closely-related species may have similar overall brain size but nonetheless exhibit major differences in cognition and behavior (Hare et al. 2012; Maclean et al. 2014; Rilling et al. 2011; Rosati 2017a). Consequently, comparisons of cognitive development across species is crucial to test whether life history characteristics shape cognitive development.
A second perspective on the relationship between cognitive evolution and development focuses on how differences in ontogenetic trajectories shape an organism’s phenotype in relation to their adaptive niche. In this view, shifts in the timing of development, or heterochrony, is an evolutionary mechanism for producing new adaptive traits across species (Gould 1977; Moczek et al. 2015). A classic example of this ‘evo-devo’ approach concerns variation in beak morphology in Galapagos finch species. This radiation of finches exhibit major differences in the length and breadth of their beaks, thereby allowing different species to occupy different dietary niches (Grant 1986). Mechanistically, these differences in beak structure arise from developmental variation in the expression of genes affecting beak growth (Abzhanov et al. 2006; Abzhanov et al. 2004; Grant et al. 2006). This line of work shows how small tweaks in the timing of developmental processes produce phenotypic variation that can impact a species’ ecological niche. Extending this view to cognitive traits, closely-related species that differ in socioecological characteristics may differ in the pace and patterns of cognitive development (Rosati et al. 2014b; Wobber et al. 2010a). This socioecology hypothesis predicts that patterns of cognitive development will co-vary with socioecological characteristics across species. To date, evidence in support of this view comes primarily from studies of social cognition. For example, gaze-following (or co-orienting with others) allows individuals to apprehend important information that others detect in their environment. While many primates exhibit gaze-following responses similar to humans as adults, comparative developmental studies have revealed that some species may acquire this skill over slower timescales (Ferrari et al. 2008; Ferrari et al. 2000; Rosati et al. 2016; Rosati and Santos 2017; Tomasello et al. 2001; Wobber et al. 2014), suggesting different developmental pathways that require more social experience in species with different social organizations than humans (Ferrari et al. 2008; Ferrari et al. 2000). However, it is unclear whether these findings concerning the development of social cognition can be extended to other cognitive abilities more broadly.
The current work provides a new test of the life history and socioecology hypotheses by comparing patterns of spatial memory development in chimpanzees (Pan troglodytes) and bonobos (Pan paniscus). Chimpanzees and bonobos are our closest-living relatives (Pruefer 2012), and are an important model for the mind and behavior of the last common ancestor of humans and other apes (e.g., McGrew 2010; Muller et al. 2017; Stanford 2012; Wrangham and Pilbeam 2001). These species also provide a targeted test of the life history and socioecological hypotheses, because they show different socioecological niches but share similar life history traits. Importantly, these hypotheses are not mutually exclusive: increased intelligence might emerge from changes in socioecology, life history, or both in tandem. For example, humans are marked by a suite of potentially-linked characteristics including extreme intelligence, long life history, and complex diets (Kaplan et al. 2000; Robson and Wood 2008; Schuppli et al. 2012). Broad comparative analyses similarly suggest strong co-variation across primates between life history and dietary complexity (indexed by frugivory versus folivory; Leigh 1994), as well between brain size, life history and dietary complexity (indexed by food processing behaviors or wide dietary breadth; Schuppli et al. 2016). The current work can help disentangle these pathways for generating complex cognition because the life history and sociological characteristics of these species provide distinct predictions for patterns of cognitive development in Pan.
In terms of life history, apes exhibit relatively slow life histories, with long juvenile periods and extended postnatal brain and cognitive development more like humans (Bianchi et al. 2013; Leigh 2004; Leigh 2012; Matsuzawa 2007; Matsuzawa et al. 2006; Sakai et al. 2011; Teffler et al. 2013). For example, chimpanzees exhibit slower rates of white matter maturation (Sakai et al. 2011) and longer periods of synaptogenesis in childhood (Bianchi et al. 2013) compared to Old World monkeys. While there are not comparable studies of bonobo brain development, studies of physical maturation indicate that chimpanzees and bonobos exhibit largely similar life history milestones, such as age of weaning, sexual maturity, or first birth (Robson and Wood 2008; Walker et al. 2018). Thus, the life history hypothesis predicts that chimpanzees and bonobos will both exhibit similar patterns of cognitive development, given their similar life history patterns.
In terms of socioecology, however, these species exhibit key differences. Chimpanzees exhibit stronger male bonds and more escalated aggression, whereas bonobos exhibit stronger bonds between females and increased socio-sexual behaviors (Gruber and Clay 2016; Hare et al. 2012; Parish 1996; Surbeck and Hohmann 2008; Wrangham and Pilbeam 2001). An influential proposal specifically links these differences in social behavior to core differences in their feeding ecology. Chimpanzees are more dependent on patchy, seasonably-variable fruit resources, exhibit larger day ranges, and engage in effortful and time-consuming food processing or hunting techniques; bonobos, in contrast, have more access to homogenously-distributed terrestrial herbs, rarely hunt, and have not been observed to use tool in the wild (Furuichi et al. 2015; Hare et al. 2012; Kano 1992; Malenky and Wrangham 1993; Rosati 2017a; Stanford 1998; Surbeck and Hohmann 2008; White 1989; White 1998; White and Wrangham 1988; Wrangham 2000; Wrangham and Peterson 1996). These wild observations suggest that chimpanzees typically face more ‘difficult’ foraging problems than bonobos, and several of these differences specifically reflect the fact that chimpanzees exploit foods with more variable distributions across time and space, and forage over larger areas, than do bonobos. Accordingly, the socioecology hypothesis predicts that chimpanzees need and bonobos will exhibit targeted differences in the development of cognitive skills that are relevant to these foraging problems, such as spatial memory.
Spatial memory is a good target for comparative developmental studies for several reasons. First, spatial memory is an important cognitive substrate for foraging behaviors, as wild primates generally face complex spatial problems revolving around locating resources and travelling efficiently through their environment (Gallistel 1990; Janmaat et al. 2013; Janson 1998; Janson 2007; Janson and Byrne 2007; Normand et al. 2009; Normand and Boesch 2009; Shettleworth 1998). Spatial memory likely plays a crucial role in human forager’s daily ranging and central-place foraging patterns (Marlowe 2005), which pose more complex spatial problems than faced by apes, who feed on-the-go within smaller areas. The ability to track and navigate between high-value, patchy resources are therefore crucial components of the human foraging niche. Spatial memory also develops on a fairly well-characterized pathway in humans that provide clear benchmarks for comparative work, as children show shifts in abilities to encode locations through late childhood (Balcomb et al. 2011; Haun et al. 2006b; Hermer and Spelke 1994; Hermer-Vazquez et al. 2001; Levinson et al. 2002; Newcombe et al. 1998; Ribordy et al. 2013; Sluzenski et al. 2004). For example, the emergence of language-based encoding of spatial locations allows humans to solve spatial problems within creasing flexibility and accuracy compare to other species (e.g., Hermer-Vazquez et al. 2001; Hermer-Vazquez et al. 1999). Thus, human spatial memory exhibits the extended developmental trajectory as well as the potentially species-unique features that are central to the life history hypothesis. Finally, studies of spatial memory comprise some of the strongest evidence that socioecology can shape cognition across species (Pravosudov and Roth 2013; Rosati 2017b; Sherry 2006), with links between foraging ecology and spatial cognition documented in birds (Healy et al. 2005; Pravosudov and Roth 2013; Sherry 2006), voles (Gaulin and Fitzgerald 1989; Jacobs et al. 1990), and primates (Platt et al. 1996; Rosati et al. 2014a).
What is currently known about the development of spatial memory in chimpanzees and bonobos? There is clear evidence that apes exhibit several sophisticated abilities for dealing with spatial problems. For example, chimpanzees and bonobos use cognitive spatial maps to follow an optional search path when locating targets in large spaces (Menzel et al. 2002; Menzel 1973), and these kinds of memories may persist over very long time scales (Martin-Ordas et al. 2013; Mendes 2008). Apes can also solve some spatial problems using complex ‘episodic-like’ memory skills (Martin-Ordas et al. 2010) that integrate information about events with their spatial and temporal contexts. However, current comparative data on spatial cognitive development in apes is limited in several regards. First, most work on spatial memory in these species has used mixed-age samples without examining developmental trajectories, likely due to sample size limitations (Albiach-Serrano et al. 2010; Haun et al. 2006a; Haun et al. 2006b; Hribar and Call 2011; Hribar et al. 2011; Martin-Ordas et al. 2010). There are generally few studies of cognitive development in apes, and most to date have typically traced the development of just a few individuals of one species (three or fewer; Bering et al. 2000; Bjorklund et al. 2000; Matsuzawa 2007; Matsuzawa et al. 2006; Rosati 2015; Tomasello and Carpenter 2005).
In terms of comparative development, some work with larger samples indicates that chimpanzees and bonobos exhibit broad similarities in their cognitive abilities across several distinct domains of cognition, including basic object knowledge, numerical knowledge, and social cognition (Herrmann et al. 2010; Wobber et al. 2014). But there also appears to be some relevant differences in their patterns of cognitive development. For example, chimpanzees exhibit faster development of social inhibitory control than bonobos (Wobber et al. 2010b), and chimpanzees also exhibit improvements in their memory for multiple locations as they transition out of infancy, whereas bonobos do not (Rosati and Hare 2012). These findings parallel research contrasting other aspects of development in chimpanzees and bonobos. For example, bonobos exhibit developmental delays in their dental and cranial morphology relative to chimpanzees, retaining more juvenile features in the size-shape relationships of the head (Lieberman et al. 2007; Shea 1983a; Shea 1984; Shea 1983b). Bonobos exhibit more juvenile-like levels of thyroid hormone and testosterone at later ages than do chimpanzees (Behringer et al. 2014; Wobber et al. 2013). In terms of behavioral development, bonobos continue to exhibit more juvenile-like patterns of social behaviors and tolerance as they age (Furuichi and Ihobe 1994; Hohmann and Fruth 1993; Kuroda 1989; Palagi 2006; Wobber et al. 2010b). Overall, these findings suggest that bonobos may exhibit paedomorphism, or developmental delays in acquisition of traits resulting in a juvenilized or under-developed set of adult traits (Hare et al. 2012).
The current work addresses whether chimpanzees and bonobos show similar divergences in the ontogeny of spatial cognition. The first study examined apes’ memory for a single location after a long delay. Different cognitive and neurobiological processes support short-term memory on the scale of seconds, versus long-term memory over minutes, days, or longer periods (Baddeley 2010; Mankin et al. 2012; Nielson et al. 2015; Norris 2017). Children’s memory for locations over even minutes-long delays exhibits major developmental improvements beginning in toddlerhood: 18-month-olds made almost twice as many errors searching for an object they had seen hidden two minutes before than did 42-month-olds (Sluzenski et al. 2004). Memory retained over more extended periods is likely to be especially crucial for foraging behaviors, as animals in the wild must locate resources that are widely dispersed in time and space (Janson and Byrne 2007). In Study 1, apes were therefore tested for their ability to recall a baited location after a one-week delay. In an initial session, apes learned that one of two possible locations was consistently baited with hidden food; their recall of that location was assessed in a test session one week later. As trial-and-error learning could occur within both sessions, contrasting performance in the test session with performance in the initial learning session can isolate the memory benefit of the earlier experience on recall or re-learning. This basic procedure has previously been used to compare spatial memory across several species of lemurs and found that a highly frugivorous lemur species was the most accurate on their first test trial, and exhibited more relative improvement during the test session, even though a more folivorous species actually exhibited faster initial learning (Rosati et al. 2014a). This basic setup has also been validated for apes as a measure of recall for a specific place in space (also referred to as a ‘cognitive map’ of space; Tolman 1948), rather than a habitual motor memory that depends on representations of the organisms’ egocentric movements centered on their own body (e.g., ‘turn left to find food’; Knowlton et al. 1996; Packard 1996; Packard 2009; Poldrack and Packard 2003). Apes had relatively few trials of experience (as habit-based memory strengthens over extended experience), and prior work shows that apes exhibit a spatial strategy in this particular (Rosati 2015; Rosati unpublished data). Thus, performance in this situation reflects memory for particular spatial locations in apes.
The second study then examined apes’ abilities to recall multiple locations in larger, a more naturalistic environment. The ability to navigate through multiple locations in the environment necessarily requires memories for places (Maguire et al. 1998; Morris et al. 1982; Sluzenski et al. 2004), and prior experimental work in large spaces indicate that chimpanzees and bonobos use cognitive maps of space to optimally navigate between different locations (Menzel et al. 2002; Menzel 1973). Importantly, children’s memory for multiple locations in larger spaces also exhibits developmental improvements beginning in toddlerhood, as older children show more spatially-specific searches and can recall more locations as they move to search for hidden targets (Balcomb et al. 2011; Newcombe and Huttenlocher 2006; Sluzenski et al. 2004). In Study 2, apes initially watched an experimenter hide four pieces of food in a large outdoor enclosure filled with a variety of landmarks such as trees, bushes, rocks, and posts. An additional four control pieces had been previously hidden at a set of matched locations while the ape could not observe. Wild animals can use a variety of additional types of information such as visual cues, olfaction, or even social learning to locate food (Janson and Byrne 2007), so this experiment contrasted abilities to locate test pieces (that apes observed being hidden) and control pieces (which they did not) to specifically assess spatial memory, in the absence of these other sources of information. After a 10-minute delay, apes could enter the enclosure to search for food. Each ape completed one test session, in order to examine their spontaneous ability to recall the location of the hidden food, following the basic procedure used previously with apes (Rosati and Hare 2012) and lemurs (Rosati et al. 2014a). Importantly, the number of hiding locations, delay to search, and size of the space in this study exceeds prior work examining memory in human children (Newcombe and Huttenlocher 2006; Sluzenski et al. 2004), so recalling these locations is likely quite challenging for young apes.
The final study then retested a subset of individuals from Study 2 on control task that equated basic motivational demands but did not require spatial memory. One possibility is that apes’ performance in Study 2 could stem from differences (across species or ages) in motivation to travel through the larger space and search for food. This final study explicitly tested this by assessing how successful apes were at navigating the enclosure to pick up food that was placed on the ground, rather than hidden under grass. Prior work suggests that motivation does not account for differences between chimpanzee and bonobo memory, as both species are fairly successful when the memory-specific demands are reduced (for example, because fewer test pieces are hidden and the delay to search is shortened; Rosati and Hare 2012). Study 3 provides a stronger test of a motivational interpretation, as there is no need to recall specific locations at all to succeed. Importantly, given the large distances and complex natural context, food placed in distal locations in the enclosure were not salient from the apes’ initial observation point. Thus, apes did need to be motivated to traverse the space of the enclosure and to find the food, as in Study 2.
To assess developmental trajectories for spatial memory in chimpanzees and bonobos, this work utilized cross-sectional experimental tests of cognition in apes ranging from infancy to young adulthood. There are two primary methodological approaches to assessing developmental changes in psychology: longitudinal (within-subject) designs that examine developmental sequences within a given individual, or cross-sectional (‘snapshot’) designs comparing individuals of different ages (Baltes et al. 2016). Cross-sectional designs are especially common in studies of human cognitive development because repeated re-testing of the same individuals on a cognitive test can lead to improvements in performance that are due to practice or experience, not age-related change in abilities per se. In fact, most relevant studies of spatial memory development in children have used cross-sectional designs (Balcomb et al. 2011; Hermer-Vazquez et al. 2001; Newcombe and Huttenlocher 2006; Newcombe et al. 1998; Sluzenski et al. 2004), and these methods were adapted in the current work to examine spatial memory development in chimpanzees and bonobos.
This work took an experimental approach because disentangling specific cognitive processes requires controlled experiments that can rule out alternative explanations for observed patterns of results. As any given behavior can be psychologically implemented by many different possible psychological mechanisms, observational research alone is limited in terms of inferences about the cognitive mechanisms causally underpinning behavior. As experimental research is extremely challenging (or impossible) to carry out with wild populations (Zuberbühler 2014), studies in captivity are therefore often necessary to appropriately rule out alternative psychological explanations (see Tomasello and Call 2008). The current studies examined cognition in two populations of apes living in African sanctuaries, where apes semi-free-range in large, naturalistic rainforest enclosures within complex social groups, but controlled experiments are also possible. Apes in these sanctuaries are typically born in the wild, and enter the sanctuary after being confiscated at an early age as a result of the trade in wildlife for pets and bushmeat. Individuals are cared for by a surrogate human parent and then rapidly integrated into a peer group (Cox et al. 2000), an approach that has been shown to produce the most optimal psychological outcomes in direct comparisons of different ape rearing practices (van IJzendoorn et al. 2008). Indeed, African sanctuaries meet or exceed recommended standards for high-quality physical and social environments for captive apes derived from these species’ wild conditions (Pruetz and McGrew 2001). Moreover, empirical observations of the sanctuary populations tested here show that apes at both sites are psychologically healthy relative to other captive populations, and rarely or never show aberrant behaviors seen in zoo or laboratory populations (Rosati et al. 2013; Wobber and Hare 2011). Direct comparisons of orphaned apes with age-matched mother-reared individuals show no major difference in cognition across multiple social and physical cognitive tasks (Wobber et al. 2014); orphans and mother-reared apes also exhibit similar cortisol profiles (Wobber and Hare 2011). Overall, this indicates that the orphaned apes in these populations exhibit typical patterns of cognitive and physiological development. Importantly, sanctuary sites can also better approximate the environments that these different species experience over their individual lifetimes. For example, wild chimpanzees and bonobos might acquire different cognitive skills in direct response to their individual experiences in different habitats. While sanctuary apes semi-free-range, they receive the majority of their food through provisioning, and experience relatively standardized rearing procedures across these sites (Farmer 2002). Thus, experimental comparisons of psychology in these populations can provide new insights into the evolution of cognitive traits.
2. Materials and Methods
2.1. Ethics statement
All behavioral studies were approved by Duke University (IACUC #A078–08-03) and adhered to host country legal requirements in Congo Republic (Ministry of Scientific Research and Technological Innovation, permit 009/MRS/DGRST/DMAST), and the Democratic Republic of Congo (Ministry of Research and the Ministry of Environment, permit MIN.RS/SG/004/2009).
2.2. Subjects and Study Sites
These studies examined chimpanzees living at Tchimpounga Chimpanzee Sanctuary in Pointe Noire, Congo Republic; and bonobos living at Lola ya Bonobo Sanctuary in Kinshasa, Democratic Republic of Congo. Both sanctuaries are accredited members of the Pan-African Sanctuary Alliance (PASA), and animal care practices at both sites complied with PASA standards. Apes at both sites are socially housed, and the majority are semi-free-ranging in large tracts of tropical forest during the day (5–40 hectares across groups). In the evening, all apes spend the night in indoor dormitories (12 m2-160 m2). Apes had ad libitum access to water and were never food deprived for testing. In addition to the food the apes could eat in their forest enclosures, they were provisioned with a variety of fruits, vegetables, and other species-appropriate foods two to four times daily. Data were collected in 2011 by the author and a research assistant, with additional assistance from on-site animal caretakers.
Apes completed the studies depending on their access to appropriate testing locations and willingness to participate (see Electronic Supplementary Materials, Table S1 for subject details). Study 1 included 73 apes: 45 chimpanzees (21 females and 24 males, ranging from 3 to 20 years), and 28 bonobos (11 females and 17 males, 3 to 13 years). This study was conducted inside the apes’ night dormitories; as such, all individuals who had access to an indoor dormitory room of the appropriate configuration for the study and were willing to participate were tested, including all individuals in the sanctuary under 10 years of age at the time of the study. Two individuals stopped participating in their test session during Study 1, and therefore only provided partial data for that study (see Analysis section). Study 2 included 73 apes: 41 chimpanzees (18 females and 23 males, ranging from 2 to 10 years of age), and 32 bonobos (14 females and 18 males, ranging from 2.5 to 13 years). Forty-three of these individuals also completed Study 1; 15 bonobos and 28 chimpanzees participated in a previous memory study using a similar setup more than a year earlier (Rosati and Hare 2012), whereas the rest were naïve. Study 2 was conducted in outdoor enclosures adjacent to the night dormitories; all individuals who had access to an appropriate enclosure and were willing to participate were tested, again including all individuals under age 10 years. Finally, Study 3 was a motivational control where a subset of individuals from Study 2 were re-rested a version of the setup with reduced memory demands. Thirty-two apes participated in Study 3: 16 bonobos (ranging from 2.5 to 12 years) and 16 chimpanzees (ranging from ages 3 to 8 years). Across these studies, no additional individuals were selected for testing and then excluded due to lack of participation.
As exact birth dates for sanctuary apes born in the wild are generally unknown, individual’s ages at these sites are estimated using initial assessments by sanctuary veterinarians at arrival (typically when the infants are between 1 and 3 years old), adjusted based on longitudinal measurements of weight and dental emergence data from those individuals based on known patterns of ape development, and finally validated through checks of individuals born at the sites with known exact birthdates (for more details on the age estimation method, see Rosati and Hare 2012; Wobber et al. 2014; Wobber et al. 2010b). These age estimates have accorded well with results from teeth and weight estimates alone in prior work examining cognitive and physiological development in these populations (Wobber et al. 2013; Wobber et al. 2010b).
2.3. Procedure: Study 1 (Long Delay)
In Study 1, apes first experienced that one location (of two possible) was baited with hidden food in an introductory session, and then then their memory for that location was assessed in a test session one week later (see Figure 1a for a diagram and Videos S1–S2 for example movies). Across both sessions, each ape was tested individually in an indoor dormitory room (the same room for a given individual across both sessions). The two experimenters stood outside the ape’s room and baited the locations or centered the subject though the mesh or bars (see Figure 1a). The hiding locations were two overturned bowls (24.5 cm diameter, 15 cm tall, and green in color) that were placed 2m apart immediately outside the room. Food could therefore be hidden under them, and apes could easily approach and touch them to indicate their choice. There were no additional cues to the correct location; the room was otherwise identical to its typical setup as a sleeping room. All containers were rubbed with fruit rewards prior to the start of the task to ensure that apes used memory (rather than olfactory cues) to locate the hidden reward.
Figure 1: Setup for memory studies.
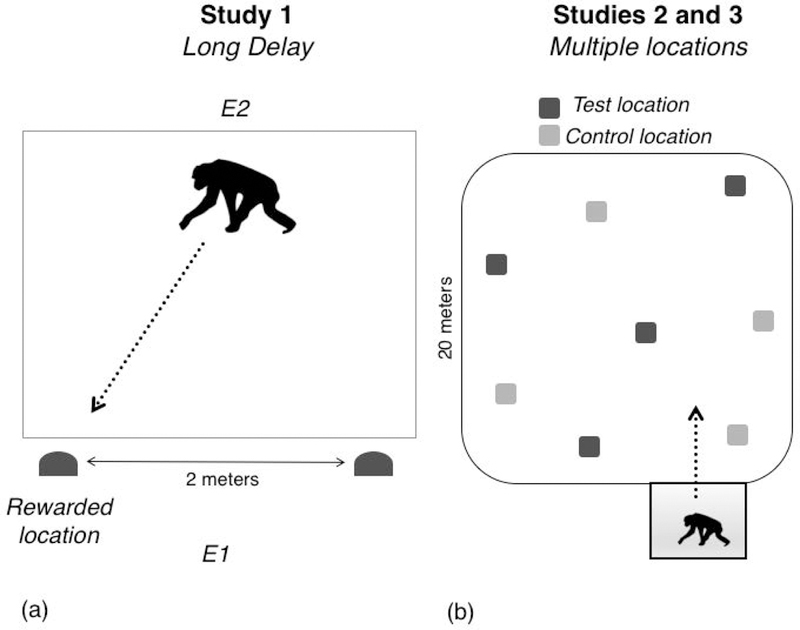
(a) In Study 1 (Long Delay), apes first learned that one location was consistently baited with food in initial learning trials, and then were tested on their memory for that location in test trials after a one-week delay. Experimenter 1 (E1) baited the locations, and Experimenter 2 (E2) centered the ape at the starting position on each trial. (b) In Study 2 (Multiple Locations), apes observed an experimenter hide four test pieces of food in a large enclosure; four control pieces were hidden at matched locations. Apes could then search in the enclosure following a 10-min delay. Study 3 (Motivational Control) had a similar setup, but the food pieces were placed directly on the ground, thus removing the memory demands associated with the task while still requiring motivation to search for the food.
Two experimenters ran the study. Experimenter 1 (E1) baited (or fake-baited, as relevant) the two containers, and then moved away and stood behind the centered camera, so as to not bias the ape towards one side. During this time, Experimenter 2 (E2) centered the ape at the starting position on the opposite side of the room, attracting the subject with a small piece of food. Once E1 had completed the baiting, E2 gave the ape the small piece of food at the centering position, and then walked away so the ape would approach the containers on the other side of the room (see Figure 1a). If the ape failed to approach either cup within 2 minutes (a very rare occurrence), E1 then attracted them to the center position between the containers with a small piece of food. For younger apes, a familiar caretaker sat inside the room at the starting position (to ensure that infants were comfortable participating) but never provided any cues as to the food location. The subsequent trial started after E2 attracted the subject back to the centered starting position.
In the initial introductory session, apes learned which location was consistently baited. First, apes completed two initial exposure trials where E1 baited both containers with hidden food and placed an additional visible food piece of food on top of both containers to attract the apes. These trials introduced the testing setup, and ensured that all apes were willing to touch the containers and had experienced that the containers could contain hidden food. Apes had to retrieve all the food in these trials before proceeding, demonstrating that they were comfortable and familiar with the basic setup before proceeding to the main trials. Note that these trials did not provide any additional information about the baited location. Apes then completed 12 learning trials in which only one location was baited with hidden food out of their sight, thus requiring that they learn about its spatial location through trial-and-error across trials. In particular, E1 baited and fake-baited both locations using the same hand motions in counter-balanced order, to avoid providing any additional cues to the food’s whereabouts. Once the ape approached or touched one container, E1 would then reveal what was under the container and give them the food if they were correct. If apes initially approached the wrong location (e.g., because they did not know which location was baited, especially early in the introductory session), they could self-correct by approaching the alternative location, (following methods from previous studies of animal memory; Packard 1996; Rosati et al. 2014a). Crucially, this procedure ensured that all individuals had equivalent experience with receiving food at the correct location before proceeding to the test session. The side assignment of the baited location was counterbalanced across individuals.
One week later, apes completed a test session with 10 test trials (following the methods in Rosati et al. 2014a). These trials were identical to learning trials and occurred in the same room with the same side baited, except that apes could not self-correct; this lack of self-correction was implemented to limit additional opportunities to learn by trial-and-error within the test session. Here, E1 immediately revealed the contents of the container once the ape indicated their choice. If the ape was incorrect, E1 then immediately removed the food from the correct location so the ape could not obtain it; note that apes were present in the testing room continuously, including when E1 removed the food following incorrect choices. The logic of this design was that performance in the test session, as compared to initial performance in the introductory session, could isolate the benefit of the prior experience on memory recall above and beyond trial-by-trial learning that could occur in both sessions.
2.4. Procedure: Study 2 (Multiple Locations)
In Study 2, each ape was assessed for their ability to recall multiple locations in a familiar outdoor enclosure after watching food being hidden across this space (see Figure 1b for a diagram and Videos S3–S4). Chimpanzees were tested individually in one of four enclosures, and bonobos were tested in one of three, corresponding to the enclosure their social group’s physical location. As the enclosures were not completely identical in size across these different groups, food was hidden in a space approximately 20m x 20m adjacent to the ape’s starting observation position, therefore equalizing the distances individuals had to travel to acquire the food in the enclosure. All food was hidden directly next to landmarks, including natural items (such as trees, rocks, bushes, or grass patches), as well as artificial items (such as fence posts, water spouts, pools, and climbing structures). For each enclosure, there were two sets of locations that were approximately matched in landmark type and distance to the ape’s starting position. To ensure that certain locations were not intrinsically attractive to the apes, the assignment of those two sets as test or control locations was counterbalanced across subjects tested in the same enclosure.
In the demonstration phase, the ape watched an experimenter hide 4 test pieces of food next to various landmarks in the enclosure. Older apes observed from a building or tunnel that they used to access the enclosure, whereas younger apes were held by a familiar human caretaker in the same approximate location. The experimenter first stood approximately 0.5m away from the ape, showed them a red bowl full of a highly-preferred food (large apple slices for bonobos; large banana pieces for chimpanzees; the most preferred fruit in each population in prior comparisons of food preferences; Rosati and Hare 2013). The experimenter held up one piece of food in her hand and then walked in a direct path to the hiding location while calling the ape’s name and visibly waving the food piece. Once the experimenter reached the hiding location she again called the ape’s name until the ape oriented in her direction (to ensure all apes paid attention to the baiting event), and then hid the food while the ape was observing. The food was always hidden under the grass, such that the food was not visible unless the ape actively approached and searched in that location. The experimenter repeated this procedure for all four test pieces; the order in which the experimenter hid the food at different locations was randomized. An additional four control pieces had been hidden in the enclosure prior to the demonstration, while the ape was in a different location and could not observe the enclosure. These pieces therefore accounted for any potential olfactory cues to the food’s location as well as provided an index of differences in motivation to generally search through the enclosure.
After a 10-minute delay following the hiding of the last test piece, the ape was released into the enclosure for the search phase to locate the food. The search phase lasted 10 minutes, a period more than sufficient for an ape to traverse the enclosure and consume the food. Entering these enclosures alone or in small groups for brief periods of time is a fairly standard occurrence at these sites, and all apes were familiar with retrieving food inside the enclosures during normal sanctuary provisioning procedures. Older apes entered the enclosure individually for the search phase. The youngest infants (who were still being cared for in a nursery group by a human surrogate parent) entered the enclosure with their human caretaker to ensure the infant was comfortable during the test. The caretaker sat in the middle of the enclosure never provided any cues about the food’s location. There were not any behavioral signs that apes were uncomfortable when entering the enclosure following these procedures.
Finally, as a check for food motivation, any apes that failed to find any food in the search phase was given food in a motivation check immediately after the search phase ended. Here, apes were directly given food once the search phase had concluded in order to confirm that they were in fact motivated to consume the food at that particular time, and check that they were not failing to locate food due to satiation or dislike of the food. In fact, all tested apes readily ate food in this context immediately after the main test, suggesting they did like the food and were not satiated at that particular point in time that they completed the study.
2.5. Procedure: Study 3 (Motivational Control)
A subset of individuals who participated in Study 2 then completed a follow-up study to further examine if apes were motivated to acquire food in the same context in the absence of memory demands. In this study, individuals who found two or fewer total pieces in Study 2 completed a second search test that was identical to Study 2, except that apes did not need to recall specific locations in space. Highly successful individuals from Study 2 were not tested in the control session since they had already demonstrated that they were motivated to search in the enclosure. The procedure for the control session was largely identical to that in Study 2, except in two respects. First, the four food items were visibly placed next to landmarks in the enclosure during the demonstration phase, rather than hidden in the grass, to remove any memory demands associated with locating the food. Second, the ape could enter the enclosure without a delay immediately after the experimenter completed the demonstration, to further reduce any memory demands associated with the time delay. As in the main study, the apes had 10 minutes to traverse the enclosure to locate the food. For each individual, the four baited locations comprised two from the previous test set and two from the previous control set, to equalize ape’s experience with the various locations (as some of these individuals had located control pieces in the main test). Apes completed Study 3 a few days after Study 2. Crucially, this study required that apes traverse the enclosure and pick up widely distributed food pieces in the same way as in Study 2, as the baited locations were not immediately visible from the apes’ initial observation point in this large space.
2.6. Video coding and reliability
All experimental sessions were videotaped. In Study 1, the room was filmed with a single camera centered outside the testing room, on E1’s side (see Figure 1a). Apes’ choices to approach the different locations were coded live by E1, and a coder blind to the correct side then coded 20% of sessions for reliability; agreement was 100%. In Studies 2 and 3, sessions were videotaped from two camera angles: a static wide angle shot of the entire enclosure that captured how apes traveled through the space, and a second hand-held camera that was zoomed in on the apes’ actions (e.g., to better see if they were actually searching in the grass and picking up food at different locations). The experimenter narrated locations the ape searched, and then afterwards physically checked all eight hiding locations to confirm which pieces had been retrieved. The experimenter then coded (from video) the order in which pieces were found and the latency to find each piece. A reliability coder, who was blind to the specific test and control locations in the enclosure, coded 20% of all sessions for when the ape located food items; this reliability coding was done without sound as the primary experimenter had narrated the apes’ searches live. The coder had high reliability for whether pieces of food were found [Cohen’s Kappa = 0.96 with agreement on 141 of 144 possible pieces], as well as latency to find those pieces: Pearson’s r = 0.99].
2.7. Data analysis
Data for these studies were analyzed using statistical models implemented in R v3.5.0 (R Development Core Team 2018). Trial-by-trial responses coded as a binary outcome were analyzed using generalized linear mixed models (GLMM) implemented with the glmer function from the lme4 software package (Bates 2010). These models used a binomial (logit link) function, with random subject intercepts to account for repeated trials within subjects. Linear mixed models were implemented using the lmer function from the lme4 package; these models were fitted with restricted maximum likelihood for parameter estimation, and refit using maximum likelihood for model comparisons (Zuur et al. 2009). Parameter significance of these models were calculated using the lmerTest package (Kuznetsova et al. 2015). Finally, linear regressions were implemented with the lm function in the lme4 package. Across analyses, the fit of different models were compared using likelihood ratio tests (LRT: Bolker et al. 2008). Post-hoc pairwise comparisons were implemented with the emmeans function in the emmeans package (Lenth 2018) using a Tukey correction. Comparisons of age effects across species (e.g. comparisons of the slope of trend lines for age) were conducted using the emtrends function in the emmeans package. Finally, graphs showing predicted effects and 95% confidence intervals (CIs) from these models were calculated using the effects package in R (Fox 2003). Reported parameter estimates are unstandardized.
While several analyses used age in years as a linear predictor, some analyses split apes into two age cohorts in order to examine interactions between species and age group. In particular, apes were split into infants up to 5 years, and older apes over 5 years (juveniles and adults). This split is based on patterns of life history in these species, and has been used in studies of growth patterns (Hamada et al. 1996) as well as wild behavior (Bray et al. 2017) in Pan; prior work examining ape memory development has also used this cutoff for infancy (Rosati and Hare 2012).
In Study 1, four individuals (two per species) did not complete the entire test session: two individuals stopped participating of their own accord (one per species), and the experimenter made a baiting error (e.g., baiting the wrong side) for two others. These individuals’ introductory and partial test session data (up to when they stopped participating or experienced the error) was included in analyses when possible, as generalized linear mixed models can account for unequal repeats across subjects (Baayen 2008). However, these subjects could not be included in analyses examining difference scores (mean improvement across whole sessions) or comparisons of average overall session performance, so those analyses had a sample size of 69 apes. No other individuals were tested and excluded from these studies. In Study 2, to control for any potential differences in speed of search and eating across individuals, the analyses reported here focused on the first four pieces that apes located (following the approach used in Rosati & Hare, 2012). The logic of this is that an individual with perfect memory should first search at the four test locations, even if they then continued to search elsewhere in the enclosure for any time left in the session.
2.8. Data Availability
All data from these studies will be available in Dryad Digital Repository.
3. Results
3.1. Memory after a long delay (Study 1)
Study 1 examined developmental changes in apes’ memory for a location over a one-week delay. The first set of analyses confirmed that both species did successfully learn the baited location over the course of the introductory session. On their first learning trial, before any experience with the reward location, apes did not show a preference for the baited location: only 53.6% of bonobos [binomial test: 15 of 28 correct, p > 0.85, n.s.] and 44.4% of chimpanzees [20 of 45 correct; p > 0.55, n.s.] chose correctly on their first trial. This confirms that in the absence of experience with the baited location, the apes were (appropriately) at chance and could not directly detect the food’s location through some other means, such as by using olfactory cues. Over the course of the learning trials in the initial introductory session, both species learned to choose the correct location. Over all learning trials, bonobos chose the correct location on M = 61.9 ± SE = 5.1% of learning trials [one-sample t-test: t27 = 2.34, p < 0.05], and chimpanzees did so on 60.9 ± 4.2% of learning trials [t44 = 2.62, p < 0.05]. Indeed, by their final (12th) learning trial, 78.6% of bonobos [binomial test: 22 of 28 correct, p < 0.005] and 66.7% of chimpanzees [binomial test: 30 of 45 correct, p < 0.05] approached the baited location, a significant preference in both species. Thus, both species could successfully learn through trial-and-error which location was rewarded over the course of the introductory session.
The second set of analyses then examined whether apes had more accurate performance in the test session relative to the introductory session, the main test of whether spatial information was recalled over the one-week delay (see Figure 2a). Neither species chose the baited location above chance on their first test trial [bonobos: 57.1% (16 of 28) were correct, p > 0.57, n.s.; chimpanzees: 51.1% (23 of 45) were correct; p > 0.51, n.s.], the strongest test of their long-term memory for the baited location. Importantly, trial-by-trial learning could occur in both sessions, so a comparison of overall performance in these sessions can also isolate the memory benefit of the initial experience above and beyond such learning, while also accounting for any individual variation in general performance. Such ‘savings in relearning’ are generally taken as evidence for memory traces, for example in developmental studies of children’s memory (Bauer 2005; Bauer 2006; Bauer et al. 2003). While such memory traces might be weaker than those supporting recall on the first test trial, differences in rate of relearning can provide additional evidence into differences in memory storage across species and age cohort.
Figure 2: Memory after a long delay (Study 1).
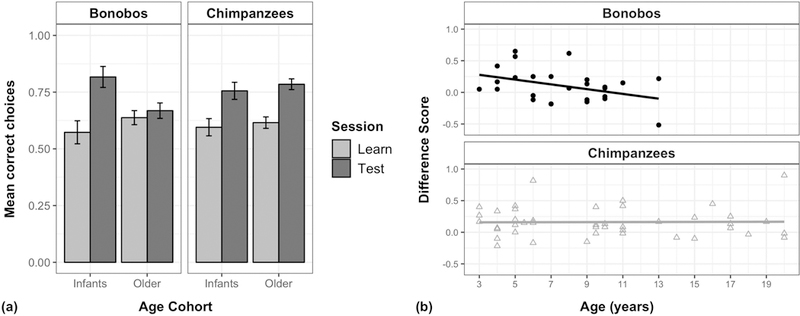
(a) Mean performance in the learning session and test session one week later; error bars indicate SE. (b) Scatter plot of difference scores (indexing each individual’s relative improvement between the introductory and test session), plotted by age for each species (bonobo r2 = 0.15, chimpanzee r2 = 0.00).
In fact, species and age cohorts differed in their degree of relative improvement in the test session. Infant chimpanzees, older chimpanzees, and infant bonobos all showed more accurate performance in their test session compared to their initial learning session performance [infant chimpanzees: 76.2 ± 7.4% correct in test session, 15.9% improvement compared to learning session; paired-samples t-test: t12 = 2.91, p < 0.05; older chimpanzees: 78.7 ± 4.5% correct in test session, 16.2% improvement compared to learning session; t29 = 3.47, p < 0.005; infant bonobos: 82.9% ± 9.2% correct in test session, 30.5% improvement compared to learning session; t6 = 3.32, p < 0.05]. However, older bonobos did not show improvement [67.4 ± 6.4% correct in test session, 2.8% improvement compared to learning session; t18 = 0.53, p > 0.60, n.s.]. This indicates that while younger bonobos and chimpanzees of all ages showed a memory benefit in the test session after the delay, older bonobos showed identical patterns of performance in both sessions, despite their additional experience when they completed the test session.
To further probe this finding, generalized linear mixed models (GLMM) were used to examine improvements in performance across sessions while accounting for trial-by-trial changes in performance due to learning. Here, an initial base model accounted for subject (as a random factor), trial number (1–12), species, and age cohort (infants versus older individuals) as predictors. The inclusion of trial number specifically accounts for any within-session improvements due to trial-by-trial learning. A second model then added session (introductory versus test) as an additional predictor to test whether apes showed overall better performance in test trials compared to initial leaning trials, reflecting a memory benefit in performance. This improved model fit [LRT: χ2 = 58.08, df = 1, p < 0.0001], indicating that apes were overall more accurate in the test session. Finally, a third model then added a three-way interaction between species, cohort, and session to test the key hypothesis that species exhibited different developmental patterns in the strength of this memory benefit. In fact, this interaction further improved model fit [LRT: χ2 = 13.78, df = 4, p < 0.01; see Table 1 for parameters from the full model]. Post-hoc pairwise comparisons revealed that infant chimpanzees, older chimpanzees, and infant bonobos all improved in their test session compared to the introductory session [p < 0.01 for significant pairwise comparisons], aligning with results reported above. That is, younger bonobos and chimpanzees of all ages showed a memory benefit from their initial experience, when they were tested again one week later. However, older bonobos did not show a significant improvement between the introductory session and test session [p > 0.88, n.s.], indicating that they exhibited similar performance across both sessions. This trial-by-trial analysis therefore also supports the conclusion that chimpanzees and bonobos exhibit developmental differences. Additional checks showed that curtailing the chimpanzee age range to match bonobos, as well as removing two outlier chimpanzees, produced the same basic results (see ESM for these details).
Table 1: Factors influencing performance in the long delay task (Study 1).
Predictors from the full (best-fit) generalized linear mixed model examining trial-by-trial binary responses (correct or incorrect) in Study 1. All models included trial number, species, age cohort, and subject (as a random factor); session (learning versus test) and the species X cohort X session interaction were then added to successive models to test their importance as predictors. Reference values for predictors are indicated in the table.
| Factor | Estimate | S.E. | Z | P |
|---|---|---|---|---|
| Trial number (1–12) | 0.154 | 0.020 | 7.653 | < 0.0001 |
| Species (Bonobo reference) | 0.078 | 0.645 | 0.120 | > 0.90 |
| Cohort (Infant reference) | 0.384 | 0.609 | 0.631 | > 0.52 |
| Session (Learning reference) | 1.823 | 0.431 | 4.235 | < 0.0001 |
| Species X Cohort | −0.201 | 0.770 | −0.261 | > 0.79 |
| Species X Session | −0.700 | 0.525 | −1.332 | > 0.18 |
| Cohort X Session | −1.509 | 0.490 | −3.082 | < 0.005 |
| Species X Cohort X Session | 1.619 | 0.612 | 2.647 | < 0.01 |
To further isolate the benefit provided by the initial learning experience for the apes’ subsequent memory in the test session, each individual was then assigned a difference score indexing their relative improvement in the test session after the one-week delay (percent correct in test session minus percent correct in introductory session; see Figure 2b), the same approach as prior work using this task with lemurs (Rosati et al. 2014a). This difference score therefore captures the relative improvement in test trials compared to that individual’s performance in initial learning trials. Here, an initial linear regression model accounted for age (as a continuous predictor) and species; an age by species interaction was then added in a second model to test whether apes exhibited any developmental differences in their relative improvement. In fact, this improved model fit [LRT: χ2 = 4.18, df = 1, p < 0.05]. Post-hoc comparisons of the two species’ age effects revealed that bonobos’ difference score declined with age, significantly different from the pattern seen in chimpanzees [p < 0.05]. Indeed, difference scores were negatively correlated with age in bonobos [rp = −0.39, p < 0.05], but showed no relationship with age in chimpanzees [rp = 0.01, p > 0.94, n.s.]. This age-related change in the difference score provides additional evidence that chimpanzees and bonobos differed in their spatial memory trajectories. The same additional checks on subsets of the data (described above) also produced the same basic results for the difference score analysis (see ESM).
The final set of analyses then examined trial-by-trial learning rates across the species and age cohorts (see Figure 3). This analysis examined whether some animals learned the baited location faster in the test session compared to the learning session, by directly comparing their learning rates across the two sessions. To do this, an initial base model for each species accounted for subject (as a random factor), trial number (within a session), session type (learning or test) and age cohort as predictors. A second model then added the interaction between trial number and session to test whether trial-by-trial learning rates differed across the two sessions. The full model finally included a three-way interaction between trial number, session, and cohort to examine whether the younger and older apes differed in their respective learning patterns. For chimpanzees, this analysis revealed that both trial number [estimate = 0.161, SE = 0.054, z = 2.972, p < 0.005] and session type [estimate = 0.155, SE = 0.618, z = 2.508, p < 0.05] were significant predictors in the full model, whereas cohort was not [estimate = 0.576, SE = 0.636, z = 0.906, [p > 0.36, n.s.]. Importantly the inclusion of the trial number X session interaction term did not improve model fit compared to the base model [LRT: χ2 = 2.21, df = 1, p > 0.13, n.s.], indicating that chimpanzees exhibited the same learning-based rates of improvement across both sessions. Including the cohort X trial number X session interaction [LRT: χ2 = 6.45, df = 4, p > 0.16, n.s.], also did not improve fit, aligning with the prior results that younger and older chimpanzees showed similar patterns of performance. Together, this indicates that chimpanzees in both age cohorts show similar learning rates across both sessions, but were overall better in the test session—with no age-related differences in performance.
Figure 3: Learning about rewarded locations across trials (Study 1).
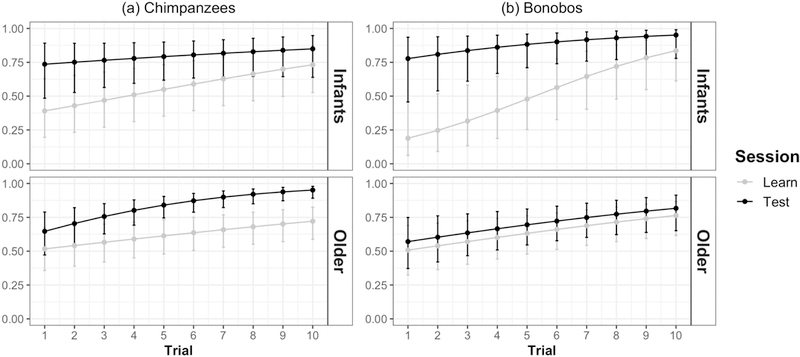
Estimated values for correct responses, split by session (initial learning trials versus test trials after 1-week delay) and age cohort (infants versus older) for both species. Estimates are derived from linear mixed models accounting for trial number, session, and age cohort. Error bars indicate 95% confidence intervals for estimates.
Bonobos showed a different pattern of learning compared to chimpanzees. In the full model, trial number [estimate = 0.343, SE = 0.083, z = 4.144, p < 0.0001], session type [estimate = 2.860, SE = 0.898, z = 3.186, p < 0.005], and cohort [estimate = 1.704, SE = 0.822, z = 2.073, p < 0.05] were significant predictors. As in chimpanzees, the inclusion of the trial number X session interaction terms did not improve model fit [LRT: χ2 = 0.37, df = 1, p > 0.54, n.s.], indicating similar rates of trial-by-trial learning across both sessions for bonobos. However, the inclusion of the three-way interaction between trial number, session, and cohort did improve model fit for bonobos [LRT: χ2 = 16.09, df = 4, p < 0.005]; post-hoc tests further revealed that infants showed more accurate performance in the test session compared to control session [p < 0.001], whereas older bonobos did not [p > 0 .61, n.s.]. That is, bonobos exhibited within-session learning effects much like chimpanzees did, but only younger bonobos exhibited overall better performance in the test session. Older bonobos, in contrast, learned about the location of the reward across trials within each session, but showed no relative gain in performance when they were tested after the week-long delay (see also ESM Figure S1).
Overall, these results show that chimpanzees and bonobos exhibited different patterns of development in this delayed recall context. First, chimpanzees showed a similar memory benefit in their test session regardless of their age: both younger and older chimpanzees improved in the test session compared to the introductory session, and there was no relationship between their difference score indexing their improvement and an individual’s age. This ‘savings in relearning’ (Bauer 2005; Bauer 2006; Bauer et al. 2003) suggests that chimpanzees did retain a memory trace of their experiences in the introductory session, albeit on that may have been weak. Bonobos, however, showed a different developmental pattern. Younger bonobos improved in the test session compared to their initial experience one week before, more like chimpanzees. In contrast, older bonobos did not exhibit any memory benefits or savings in relearning. Finally, the analysis of learning rates showed similar patterns of trial-by-trial learning within sessions across these groups. The main difference rather seems to be that younger bonobos and chimpanzees of all ages had an overall boost in memory performance in the test session, whereas older bonobos effectively re-learned the baited location over the same time-course in their test session as they previously had in their initial learning trials. Notably, neither species selected the baited location above chance on their first test trial, unlike some species of lemurs tested on a similar task (Rosati et al. 2014a). This may be due in part to the fact that the current study provided only minimal cues (e.g., presence of the colored hiding containers) signaling that apes were in this particular test rather than a normal feeding situation inside their night dormitory. This contrasts with the lemur work, where animals searched on a novel maze apparatus that was introduced to the room only during the task.
3.2. Memory for multiple locations (Study 2)
Study 2 examined developmental changes in apes’ memory for multiple locations in a larger space. Overall, apes found M = 1.08 ± SE = 0.15 test pieces, but only 0.16 ± 0.05 control pieces, a significant difference [t72 = 6.48, p < 0.0001]. This indicates that apes did utilize spatial memory to retrieve food when searching in the enclosure, as they found more test pieces (that they had previously seen hidden) than control pieces (which they had not). However, there were also important differences in performance across age cohorts and species. For example, whereas infant chimpanzees found an average of 0.57 ± 0.18 test pieces (modal number of zero test pieces), older chimpanzees found an average of 2.05 ± 0.34 test pieces (with a model number of 3 pieces out of four possible located; see Figure 4a for complete breakdown). The first analysis of this task therefore compared the abilities of chimpanzees and bonobos of different ages to locate test versus control pieces. Linear mixed models were implemented to analyze the number of test versus control pieces that each subject located during the search phase. Here, an initial base included subject (as a random factor accounting for repeated measurements), species, and age cohort. A second model then added location type (test versus control location), which improved model fit [χ2 = 34.12, df = 1, p < 0.0001]. This confirms that apes found more test than control pieces of food overall, even when accounting for variation across ages and species. The full model then added the interaction between species, cohort, and location, the main test of the hypothesis that chimpanzees and bonobos differed in the development of their spatial memory abilities. Including this interaction further improved model fit [χ2 = 27.20, df = 4, p < 0.0001], and post-hoc pairwise comparisons revealed that older chimpanzees found more test pieces than control pieces, as well as more test pieces than any other group found either test or control pieces [p < 0.05 for all significant comparisons]. This analysis further indicated that location type (test versus control) and the three-way interaction between species, cohort, and location were only significant predictors in the full model (see Table 2 for all parameters). This indicates that apes used spatial memory to guide their searches in the enclosure, but also that chimpanzees exhibited great improvements in spatial memory with age than bonobos. This result aligns with prior work showing the same basic pattern using a modified version of the same task (Rosati and Hare 2012).
Figure 4: Memory for multiple locations (Study 2).
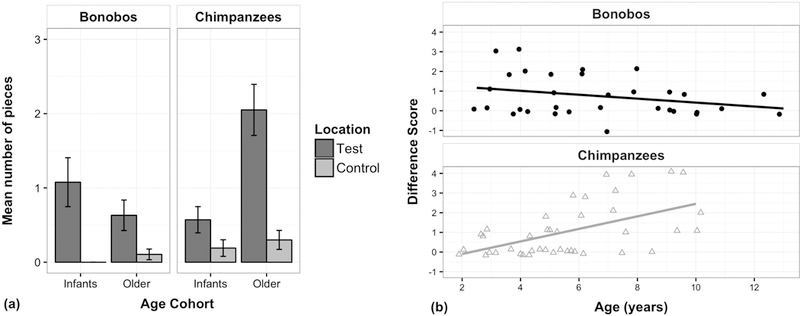
(a) Mean number of test and control pieces found across species and age cohorts; error bars indicate SE. (b) Scatter plot of difference score (indexing each individual’s spatial specificity of searches), plotted by age for each species (bonobos: r2 = 0.08; chimpanzees: r2 = 0.28).
Table 2: Factors influencing performance in the multiple locations task (Study 2).
Predictors from the full (best-fit) linear mixed model examining number of test versus control pieces located in Study 2. All models included species, age cohort, and subject (as a random factor); location (test versus control) and a species X cohort X location interaction were then added to successive models to test their importance as predictors. Reference values for predictors are indicated in the table.
| Factor | Estimate | S.E. | t | P |
|---|---|---|---|---|
| Species (Bonobo reference) | 0.190 | 0.304 | 0.626 | > 0.53 |
| Cohort (Infant reference) | 0.105 | 0.310 | 0.339 | > 0.73 |
| Location Type (Control reference) | 1.077 | 0.303 | 3.554 | < 0.001 |
| Species X Cohort | 0.004 | 0.411 | 0.010 | > 0.99 |
| Species X Location Type | −0.696 | 0.386 | −1.805 | = 0.075 |
| Cohort X Location Type | −0.551 | 0.393 | −1.400 | > 0.16 |
| Species X Cohort X Location Type | 1.920 | 0.521 | 3.686 | < 0.001 |
A more detailed look at the two species’ search patterns further suggests that individuals across both species could successfully recall at least one location, differing primarily in their ability to recall multiple locations. In particular, of the apes that located at least one piece of food, both species first approached a test location above chance [binomial tests: n = 21 of 23 chimpanzees found a test piece first, p < 0.0001; n = 15 of 16 bonobos found a test piece first, p < 0.001]. That is, both chimpanzees and bonobos used their memory to selectively search at a test location when they first entered the enclosure. However, differences emerged in their search patterns for subsequent searches. Chimpanzees continued to preferentially locate test pieces on their second search [15 of 16 who found a second piece of food; p < 0.001], and third search [12 of 15; p < 0.05]. It was only on their final search that chimpanzees did not show a statistical preference for test locations [5 of 9 found a test piece; p > 0.99, n.s.]. In contrast, fewer individual bonobos located additional pieces at all: they showed a trend to target test pieces on their second search [7 of 8; p = 0.07], but only three individuals located a third piece of food (all test pieces), and only one bonobo located a fourth piece (also a test piece). Thus, while both species located an initial test piece above chance, only chimpanzees sustained this pattern in their subsequent searches.
Comparisons of the two species’ search latencies further show that these results could not be explained by differences in the speed at which apes traveled through the enclosure, for example because bonobos searched more slowly than chimpanzees. In fact, there was no difference in how quickly apes located the first test piece: chimpanzees took an average of 50.0 ± 20.6s, whereas bonobos took an average of 36.9 ± 13.1s [t36 = −0.47, p > 0.63, n.s.]. To examine latencies across all test pieces that apes located, a linear base model was fit including random subject intercepts, the order the test piece was found (search 1 through 4), age (as a linear predictor), and species. This revealed that only order was a significant predictor of latency [estimate = 60.04, SE = 9.53, t = 6.40, p < 0.001]. A second model then included the interaction between species and order to check if bonobos simply approached test locations more slowly than chimpanzees. In fact, this interaction did not improve model fit [χ2 = 0.56, df = 1, p > 0.45, n.s.]. This indicates that when bonobos and chimpanzees located a test piece, they found it in a similar timeframe, suggesting they did not differ in the speed at which moved through the enclosure or ate the food.
The final analysis focused on the spatial specificity of the two species’ search patterns. To do so, a difference score was calculated for each individual (test pieces minus control pieces found; see Figure 4b). An ape who entered the enclosure and located many test pieces as well control pieces—that is, who found many pieces of food but did not exhibit targeted searches for test pieces—was therefore assigned a low difference score, similar to an individual who found nothing at all. To compare developmental trajectories across species, an initial linear model included age (as a continuous predictor) and species as predictors for the difference score. A second model then added the interaction between age and species, which improved model fit [χ2 = 15.64, df = 1, p < 0.0001]; post-hoc comparisons of the two species’ age effects revealed that the two species differed in their age trajectories, with chimpanzees exhibiting a more positive relationship than bonobos [p < 0.001]. Indeed, difference scores did not vary with age in bonobos [rp = −0.28, p > 0.11, n.s.], but significantly increased with age in chimpanzees [rp = 0.53, p < 0.001]. This shows that chimpanzees exhibited improvements in the spatial specificity of their searches with age, whereas bonobos did not exhibit developmental change.
Together, these results indicate that chimpanzees exhibited more accurate memory for multiple locations than bonobos, and this difference increased with age. Infants of both species exhibit similar performance, typically finding only one hidden test piece. While older chimpanzees showed significant improvements in their ability to recall the location of multiple test pieces in a large space, older bonobos did not exhibit no age-related improvement compared to younger bonobos. This replicates the basic results from prior work showing that chimpanzees exhibited age-related changes in spatial memory in a slightly different version of this task (involving more hiding locations and a longer delay before apes could search), whereas bonobos did not (Rosati and Hare 2012). The current study further tested a larger sample of bonobos and still found no evidence for developmental change in their memory for multiple locations.
3.3. Motivational control (Study 3)
Finally, Study 3 examined whether performance in Study 2 may have stemmed from motivational constraints, as opposed to memory demands associated with recalling multiple locations in the enclosure. One possibility is that older chimpanzees were increasingly motivated to eat or search for food, which could account for the changes in their performance compared to bonobos. Evidence from Study 2 does not support this alterative interpretation, as the post-test motivational check showed that all individuals would readily eat food they were directly given immediately after their participation in Study 2. Similarly, apes’ latencies to acquire food further suggests that chimpanzees and bonobos approached locations at a similar speed when they did locate test pieces, suggesting similar motivation to acquire food. However, Study 3 provides a more definitive test of motivational differences across chimpanzees and bonobos.
To address whether individuals who did not acquire many test pieces in Study 2 were more successful at acquiring food when the memory demands of the task were removed, the number of food pieces they acquired in Study 3 (where food was placed visibly on the ground) was compared to their performance in Study 2 (see Figure 5). This subset of chimpanzees located an average of 0.44 ± 0.16 test pieces in Study 2, but found 3.56 ± 0.20 pieces in the motivation control, a significant difference [t15 = 14.12, p < 0.0001]. Similarly, this subset of bonobos found 0.75 ± 0.20 pieces in Study 2, but 3.37 ± 0.30 in the motivation control [t15 = 8.72, p < 0.0001]. The modal number of test pieces found in Study 2 across these sample of individuals was zero, whereas the mode was four (the maximum possible amount) in the motivational control. Thus, these individuals were much more successful at acquiring food in a context that was identical to Study 2 in terms of the need for motivation to search in the enclosure and eat the food, but removed the memory-specific demands. This indicates that the different performance of chimpanzees and bonobos in Study 2 was likely due to differences in spatial memory, not a more general lack of motivation to or lack of willingness to travel around the enclosure. In fact, this aligns with past work using a modified version of the control task. A prior study (Rosati and Hare 2012) tested the same populations of apes on slightly different control task, where apes saw the test pieces hidden in the grass (as in Study 2) but then could enter the enclosure to search immediately. As such, this reduced but did not entirely removed the memory demands associated with locating food. In fact, individuals of both species retrieved more test pieces in the control than when they had to wait the full delay. The current results further show that both chimpanzees and bonobos exhibit similar motivation to acquire food in this context when memory demands are entirely removed, in a stronger test of motivation constraints. Overall, this set of findings supports the conclusion that the differential performance of chimpanzees and bonobos stems from differences in their recall of multiple locations, not their motivation to search for food.
Figure 5: Motivation control (Study 3).

A subset of individuals who found few pieces in Study 2 were later tested in a subsequent motivation control session where food was placed visibly on the ground. Graph depicts mean test pieces found in Study 2 versus motivation control session; error bars indicate SE.
4. Discussion
The current results show that bonobos and chimpanzees differ in their developmental patterns for two components of spatial memory. Study 1 found that infant chimpanzees, older chimpanzees, and infant bonobos were more accurate in a test session (after a one-week delay) than in an initial introductory session, without any age-related differences in performance in chimpanzees. These ‘savings in relearning’ (Bauer 2005; Bauer 2006; Bauer et al. 2003) indicate that their performance in the test session built upon a memory trace from their initial experiences in the introductory session. In contrast, older bonobos did not show this memory benefit: they learned about the location of the baited location on a trial-by-trial basis in both sessions, but did not show a memory benefit from the earlier experience in the test session. Study 2 further revealed differences in these species’ developmental trajectories for recall of multiple locations. Here, chimpanzees exhibited robust developmental improvements in spatial searching between infancy and juvenility, much like humans (Balcomb et al. 2011; Sluzenski et al. 2004)—and note that the current task involved more locations, longer delays, and a much larger physical space than those child studies, and therefore likely even imposed more difficult memory demands. Bonobos, in contrast, did not show any change in the same age range, as in prior work using this same basic task (Rosati and Hare 2012). Finally, Study 3 ruled out that this difference in performance was due to motivation: individuals of both species were here very successful at retrieving the food when they did not need to recall any specific locations per se, but did need motivation to travel through the enclosure and search for food. These same individuals did not retrieve much food in the context of Study 2 where memory was needed to acquire the food. Importantly, chimpanzees and bonobos have similar rearing experiences in sanctuaries, as these sites engage in similar standardized care practices as well as extensive food provisioning such that neither species depends primarily on individual foraging for food (Farmer 2002; Wobber and Hare 2011). The similarities in the environments of both populations suggests that these developmental differences are unlikely to have stemmed from different individual experiences with complex foraging problems. Rather, these differences are more likely to reflect species-typical cognitive traits. Overall, these findings support the conclusion that socioecology may shape patterns of cognitive development in Pan, as even closely-related species with similar life history characteristics can exhibit different developmental trajectories for aspects of mature spatial competency.
Prior work examining bonobo morphology, physiology, and behavior have supported the hypothesis that bonobos are paedomorphic relative to chimpanzees, in that they retain more immature or ‘juvenilized’ traits later into development (Behringer et al. 2014; Furuichi and Ihobe 1994; Hohmann and Fruth 1993; Kuroda 1989; Lieberman et al. 2007; Palagi 2006; Shea 1983a; Shea 1984; Shea 1983b; Wobber et al. 2013; Wobber et al. 2010b). The current work similarly indicates that heterochrony, or evolutionary changes in the developmental timing of events, may have played a role in shaping these species’ cognitive abilities (see also Rosati and Hare 2012; Wobber et al. 2010b). In particular, older bonobos exhibited developmental lags in memory for multiple locations relative to chimpanzees, in line with chimpanzees’ greater dependence on patchily-distributed food resources and larger day ranges (Gruber and Clay 2016; Hare et al. 2012; Kano 1992; Malenky and Wrangham 1993). It is important to note that some proposals concerning paedomorphism in bonobos argue that the core selective regime is for reduced aggression, whereas other traits that differ between bonobos and chimpanzees (such as morphology or cognition) are a ‘by-product’ of this selection (Hare et al. 2012). However, even this proposal suggests that selection on reduced aggression stems from differences in dietary ecology, and consequent levels of scramble competition, in these species. An important question for future work is therefore whether changes in spatial memory could actually result from selection on aggression—for example because they are correlated traits or share an underlying biochemical pathway, as is the case for other potential examples of cognitive ‘by-products’ (Hare et al. 2005; Hare et al. 2012).
The current work also highlights some of the difficulties in extending biological concepts like heterochrony to cognitive traits. For example, whereas chimpanzees did not exhibit major changes in their ability to recall a single location over a long delay from infancy to adulthood, older bonobos actually showed less improvements in spatial recall than did younger bonobos or chimpanzee overall. Although this does generally accord with the idea that bonobos exhibit relative delays in their performance with age compared to chimpanzees, this sort of apparent ‘decrease’ in the trait in question is difficult to reconcile with typical applications of the concept of heterochrony, which is grounded in morphological studies of body size and shape. For example, previous support for the claim that bonobos paedomorphic comes from studies showing that adult bonobos have a more juvenilized skull than chimpanzees: the relationship between cranial shape and size seen in chimpanzees is decoupled in bonobos, such that bonobos exhibit a more juvenile shape even when they reach adult size (Lieberman et al. 2007; Shea 1983a; Shea 1984; Shea 1989). Along these lines, most models of paedomorphism focus on differences across species in the rate of developmental processes, differences in the total duration of the developmental process, or differences in the initial starting state of development (see Lieberman et al. 2007). Yet while this heterochrony framework is useful for understanding the emergence of evolutionary variation in cognition across species, directly translating it into psychological terms is not necessarily straightforward (Wobber et al. 2010a). For example, there is no clear morphological parallel for the apparent decrease in a given ‘trait’ with age seen in bonobos’ performance in Study 1: older bonobos actually appear to show less of a memory benefit of the prior experience than do younger bonobos. Yet these kinds of plastic changes across the lifespan, involving both age-related improvements and declines in function, are actually quite common for cognitive traits. For example, many facets of human cognition show declines across the lifespan that parallel the changes seen in the current work. In particular, human memory abilities exhibit relative increases during early development and then decline during aging (Craik and Salthouse 2008; Li et al. 2004; Nyberg et al. 2012; Ronnlund et al. 2005). Similarly, other cognitive skills such as motor inhibitory control show an inverted U-shaped trajectory with age in apes: first increasing through juvenility and then declining after adulthood (Manrique and Call 2015). Thus, reconciling this characteristic of cognitive traits—that they are more flexible and labile across the entire lifespan than are morphological traits, and appear to both ‘increase’ and ‘decrease’ at different points in time—is a key issue for making heterochrony a tractable concept for cognition.
The current work also indicates that different components of spatial memory can be ontogenetically decoupled in chimpanzees and bonobos. In humans, the ability to recall a single location over time and the ability to recall more than one location seem to both emerge around the same time in toddlerhood (Sluzenski et al. 2004). However, in apes these two components of spatial memory appear to have different trajectories. For example, younger and older chimpanzees had similar abilities to recall a single location over a long delay, whereas older chimpanzees outpaced younger chimpanzees in their ability to recall multiple locations. This dissociation has important implications for theories of cognitive evolution. As distinct mental systems can evolve independently across species, whereas functionally linked systems tend to coevolve (Barton 1996; Barton 2006; Striedter 2005), it is important to determine whether different competencies reflect distinct cognitive abilities, versus constituent parts of a common cognitive representation. In fact, there is clear evidence that multiple, functionally distinct systems can support memory (Bird and Burgess 2008; Burgess 2008; Cohen and Squire 1980; Corkin 2002; Iaria et al. 2003; Knowlton et al. 1996; Poldrack et al. 2001; Scoville and Milner 1957; Squire 2004). Even capacities that appear to share a common representation in mature adults can sometimes be dissociated at earlier ages in human development (Jabes and Nelson 2015; Newcombe et al. 1998; Ribordy et al. 2013), possibly because different regions of the hippocampus mature on different timescales (Lavenex and Banta Lavenex 2013; Utsunomiya et al. 1999). The current work with apes further shows that even capacities that appear to be linked in human development can be dissociated in the ontogeny of other species. Although human children exhibit concurrent developmental change in memory for multiple locations in space and recall of a single location over a longer delay (Balcomb et al. 2011; Ribordy et al. 2013; Sluzenski et al. 2004), the current work showed that these are, in principle, dissociable abilities in the development of other species. This finding sets the stage for neurobiological comparisons of brain development. While there have been few direct comparisons of chimpanzee and bonobo neuroanatomy, there are some hints that they exhibit variation in hippocampal structure. For example, sub-adult and adult chimpanzees have a marginally larger, less asymmetrical hippocampus than do age-matched bonobos (Hopkins et al. 2009), and chimpanzee hippocampus also has greater connectivity with other brain regions, as measured through diffusion tensor imaging (Rilling et al. 2011). One important question is therefore how these differences in mature brain structure emerge during ontogeny. Like humans, nonhuman apes experience extended periods of postnatal brain development (Leigh 2004; Sakai et al. 2011). Thus, many of the same maturational changes in regions supporting spatial cognition in humans may also be important in restructuring the brains of nonhuman apes.
A final important question is whether life history and cognitive development are uniquely linked in humans. The current work did not support the conclusion that life history patterns necessarily co-vary with patterns of cognitive development, as chimpanzees and bonobos exhibit similar life history characteristics but divergent spatial memory development. Other work suggests that even species with very different life history patterns can sometimes exhibit similar patterns of cognitive development, such as for social cognition (Rosati et al. 2016; Tomasello et al. 2001). Yet it is important to note that many theoretical views linking life history and cognition in humans have focused on skills such as tool use, hunting, or food processing more generally (Gurven et al. 2006; Kaplan et al. 2000; Schuppli et al. 2016; Schuppli et al. 2012). Thus, although spatial memory is an important component of human foraging behavior, it may be that the life history hypothesis is more relevant for other kinds of cognitive abilities. For example, hunting and tool use involve multi-step sequences of behavior, and also involve some level of cultural learning in humans. Thus, an open question for future work is whether other these other skills are more tightly linked to life history than is spatial memory. Another possibility is that the life history hypothesis may uniquely apply to hominins, rather than reflecting a more general evolutionary principle. In some sense, human cognition and human life history are both such outliers in the animal kingdom that they may require new explanatory frameworks. However, even if this is the case, the life history hypothesis still needs to be refined. The life history view generally posits that humans exhibit slower cognitive development than other primates, in line with our extended juvenile period (Bjorklund and Green 1992; Bjorklund and Bering 2003; Kaplan et al. 2000). Yet recent direct comparisons of cognitive development in humans and apes have revealed that humans may actually develop a given cognitive skill more quickly than other primates (Herrmann et al. 2007; Rosati et al. 2014b; Wobber et al. 2014). Thus, humans may indeed exhibit an exceptionally long developmental period, but that does not necessarily mean that the pace of cognitive development in our species is slower than in other animals with faster life history profiles.
Overall, the current study has several implications for the evolution of human cognitive development. First, these results align with emerging evidence for heterochrony in chimpanzee and bonobo development: bonobos exhibit relative delays in spatial memory compared to chimpanzees, much like the delays they exhibit in morphological, physiological, and behavioral development. Second, this work indicates that distinct systems may support memory for a single location after a long delay and memory for multiple locations. Although these skills appear unitary in human development, they are in fact dissociable in nonhuman ape development. This highlights the critical importance of future comparisons of hippocampal and brain development in general across chimpanzees, bonobos, and humans—which can help tie cognitive change to underlying neurobiological substrates. Finally, differences in the developmental trajectories of chimpanzees and bonobos align with their wild socioecology, providing further evidence that these species do exhibit important differences in their cognition and behavior. Overall, this indicates the importance of integrating data from both species in order to understand human cognitive evolution.
Supplementary Material
Acknowledgements
I thank Chris Krupenye for assistance with data collection; Rosemary Bettle for assistance with coding; Brian Hare for support conducting research at the sanctuary sites; and Felix Warneken for comments on the manuscript. At Tchimpounga Chimpanzee Sanctuary, I thank Rebeca Atencia, Debby Cox, the animal caretakers at Tchimpounga, and the Ministry of Scientific Research and Technological Innovation in the Republic of Congo. At Lola ya Bonobo Sanctuary, I thank Claudine Andre, Dominique Morel, Fanny Mehl, Pierrot Mbonzo, the animal caretakers at Lola ya Bonobo, and the Ministry of Research and the Ministry of Environment in D.R. Congo. This work was supported by a grant from the L.S.B. Leakey Foundation and the National Institute on Aging R01AG04395.
Funding information: This work was supported by an L.S.B. Leakey Foundation, and AGR was supported by NIA R01AG04395.
References
- Abzhanov A, Kuo W, Hartmann C, Grant B, Grant P, and Tabin C. 2006. The calmodulin pathway and evolution of elongated beak morphology in Darwin’s finches. Nature 442:563–567. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Protas M, Grant BR, Grant PR, and Tabin CJ. 2004. Bmp4 and morphological variation of beaks in Darwin’s finches. Science 305:1462–1465. [DOI] [PubMed] [Google Scholar]
- Albiach-Serrano A, Call J, and Barth J. 2010. Great apes track hidden objects after changes in the objects’ position and in subject’s orientation. American Journal of Primatology 72:349–359. [DOI] [PubMed] [Google Scholar]
- Anton S, Potts R, and Aiello L. 2014. Evolution of early Homo: An integrated biological perspective. Science 345:1236828. [DOI] [PubMed] [Google Scholar]
- Baayen RH. 2008. Analyzing linguistic data. A practical introduction to statistics Cambridge, MA: Cambridge University Press. [Google Scholar]
- Baddeley A 2010. Working memory. Current Biology 20:R136–R140. [DOI] [PubMed] [Google Scholar]
- Balcomb F, Newcombe NS, and Ferrara K. 2011. Finding where and saying where: Developmental relationships between place learning and language in the second year. Journal of Cognition and Development 12:315–331. [Google Scholar]
- Baltes PB, Reese HW, and Nesselroade JR. 2016. Life-span developmental psychology: Introduction to research methods: Taylor & Francis. [Google Scholar]
- Barton RA. 1996. Neocortex size and behavioral ecology in primates. Proceedings of the Royal Society B 263:173–177. [DOI] [PubMed] [Google Scholar]
- Barton RA. 2006. Primate brain evolution: Integrating comparative, neurophysiological, and ethological data. Evolutionary Anthropology 15(6):224–236. [Google Scholar]
- Bates D. The LME4 package: linear mixed-effects models using S4 classes. 2010 See http://www.R-project.org. [Google Scholar]
- Bauer PJ. 2005. Developments in declarative memory: Decreasing susceptibility to storage failure in the second year of lif. Psychological Science 16:41–47. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. 2006. Constructing the past in infancy: a neuro-developmental account. Trends in Cognitive Sciences 10:175–181. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Wiebe SA, Carver LJ, Waters JM, and Nelson CA. 2003. Developments in the long-term explicit memory late in the first year of life: Behavioral and electrophysiological indices. Psychological Science 14:629–635. [DOI] [PubMed] [Google Scholar]
- Behringer V, Deschner T, Murtaugh R, Stevens JMG, and Hohmann G. 2014. Age-related changes in thyroid hormone levels of bonobos and chimpanzees indicate heterochrony in development. Journal of Human Evolution 66:83–88. [DOI] [PubMed] [Google Scholar]
- Bering JM, Bjorklund DF, and Ragan P. 2000. Deferred imitation of object-related actions in human-reared juvenile chimpanzees and orangutans. Developmental Psychobiology 36:218–232. [DOI] [PubMed] [Google Scholar]
- Bianchi S, Stimpson CD, Duka T, Larsen MD, Janssen WGM, Collins Z, Bauernfeind AL, Schapiro SJ, Baze WB, McArthur MJ et al. . 2013. Synaptogenesis and development of pyramidal neuron dendritic morphology in the chimpanzee neocortex resembles humans. Proceedings of the National Academy of Sciences 110(Supplement 2):10395–10401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CM, and Burgess N. 2008. The hippocampus and memory: insights from spatial processing. Nature Reviews Neuroscience 9:182. [DOI] [PubMed] [Google Scholar]
- Bjorklund D, and Green B. 1992. The adaptive nature of cognitive immaturity. American Psychologist 47:46–54. [Google Scholar]
- Bjorklund DF, and Bering JM. 2003. Big brains, slow development and social complexity: the developmental and evolutionary origins of social cognition. In: Bruene M, Ribbert H, and Schiefenhoevel W, editors. The social brain: Evolution and pathology Chichester, UK: John Wiley & Sons; p 113–151. [Google Scholar]
- Bjorklund DF, Bering JM, and Ragan P. 2000. A two-year longitudinal study of deferred imitation of object manipulation in a juvenile chimpanzee (Pan troglodytes) and orangutan (Pongo pygmaeus). Developmental Psychobiology 37:229–237. [DOI] [PubMed] [Google Scholar]
- Bogin B 2010. Evolution of human growth. In: Muehlenbein M, editor. Human evolutionary biology Cambridge: Cambridge University Press; p 379–395. [Google Scholar]
- Bogin B, and Smith BH. 1996. Evolution of the human life cycle. American Journal of Human Biology 8(6):703–716. [DOI] [PubMed] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, and White JSS. 2008. Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology and Evolution 24:127–135. [DOI] [PubMed] [Google Scholar]
- Bray J, Emery Thompson M, Muller MN, Wrangham RW, and Machanda ZP. 2017. The development of feeding behavior in wild chimpanzees (Pan troglodytes schweinfurthii). American Journal of Physical Anthropology [DOI] [PMC free article] [PubMed]
- Burgess N 2008. Spatial cognition and the brain. Annals of the New York Academy of Sciences 1124:77–97. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, and Squire LR. 1980. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science 210:207–210. [DOI] [PubMed] [Google Scholar]
- Corkin S 2002. What’s new with the amnesic patient H.M.? Nature Reviews Neuroscience 3:153–160. [DOI] [PubMed] [Google Scholar]
- Cox D, Rosen N, Montgomery C, Seal US, and SSC/IUCN CBSG. 2000. Chimpanzee sanctuary guidelines and management workshop: Report Apple Valley, MN: CBSG. [Google Scholar]
- Craik FIM, and Salthouse TA, editors. 2008. The handbook of aging and cognition New York: Psychology Press. [Google Scholar]
- Farmer KH. 2002. Pan-African Sanctuary Alliance: status and range of activities for great ape conservation. American Journal of Primatology 58:117–132. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Coude G, Gallese V, and Fogassi L. 2008. Having access to others’ mind through gaze: The role of ontogenetic and learning processes in gaze-following behavior of macaques. Social Neuroscience 3:239–249. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Kohler E, Fogassi L, and Gallese V. 2000. The ability to follow eye gaze and its emergence during development in macaque monkeys. Proceedings of the National Academy of Sciences 97:3997–14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J 2003. Effect displays in R for generalised linear models. Journal of Statistical Software 8:1–27. [Google Scholar]
- Furuichi T. Contribution of female bonobos to the peaceful nature of the society Evolutionary Anthropology. in press. [DOI] [PubMed]
- Furuichi T, and Ihobe H. 1994. Variation in male relationships in bonobos and chimpanzees. Behaviour 130:211–228. [Google Scholar]
- Furuichi T, Sanz C, Koops K, Sakamaki T, Ryu H, Tokuyama N, and Morgan D. 2015. Why do wild bonobos not use tools like chimpanzees do? . Behaviour 152:425–460. [Google Scholar]
- Gallistel CR. 1990. The organization of learning Cambridge: Bradford Books/ MIT Press. [Google Scholar]
- Gaulin SJC, and Fitzgerald RW. 1989. Sexual selection for spatial-learning ability. Animal Behaviour 37:322–331. [Google Scholar]
- Gomez JC. 2005. Species comparative studies and cognitive development. Trends in Cognitive Sciences 9:118–125. [DOI] [PubMed] [Google Scholar]
- Gould S 1977. Ontogeny and Phylogeny Cambridge: Harvard University Press. [Google Scholar]
- Grant PR. 1986. Ecology and evolution of Darwin’s finches Princeton, NJ: Princeton University Press. [Google Scholar]
- Grant PR, Grant BR, and Abzhanov A. 2006. A developing paradigm for the development of bird beaks. Biological Jorunal of the Linnean Society 88:17–22. [Google Scholar]
- Gruber T, and Clay Z. 2016. A comparison between bonobos and chimpanzees: A review and update. Evolutionary Anthropology 25:239–252. [DOI] [PubMed] [Google Scholar]
- Gurven M, Kaplan HS, and Gutierrez M. 2006. How long does it take to become a proficient hunter? Implications for the evolution of extended development and long life span. Journal of Human Evolution 51:454–470. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Udono T, Teramoto M, and Sugawara T. 1996. The growth pattern of chimpanzees: Somatic growth and reproductive maturation inPan troglodytes. Primates 37:279–295. [Google Scholar]
- Hare B 2017. Survival of the friendliest: Homo sapiens evolved via selection for prosocility. Annual Review of Psychology 68:155–186. [DOI] [PubMed] [Google Scholar]
- Hare B, Plyusnina I, Ignacio N, Schepina A, Wrangham R, and Trut L. 2005. Social cognitive evolution in captive foxes Is a correlated by-product of experimental domestication Current Biology 3:226–230. [DOI] [PubMed] [Google Scholar]
- Hare B, Wobber V, and Wrangam R. 2012. The self-domestication hypothesis: evolution of bonobo psychology is due to selection against aggression. Animal Behaviour 83:573–585. [Google Scholar]
- Haun DBM, Call J, Janzen G, and Levinson SC. 2006. a. Evolutionary psychology of spatial representations in the Hominidae. Current Biology 16:1736–1740. [DOI] [PubMed] [Google Scholar]
- Haun DBM, Rapold CJ, Call J, Janzen G, and Levinson SC. 2006. b. Cognitive cladistics and cultural override in Hominid spatial cognition. Proceedings of the National Academy of Sciences 103:17568–17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy AD, and Rowe C. 2007. A critique of comparative studies of brain size. Proceedings of the Royal Society B 274:453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy SD, de Kort SR, and Clayton NS. 2005. The hippocampus, spatial memory, and food hoarding: a puzzle revisited. Trends Ecol Evol 20:17–22. [DOI] [PubMed] [Google Scholar]
- Hermer L, and Spelke E. 1994. A geometric process for spatial reorientation in young children. Nature 370:57–59. [DOI] [PubMed] [Google Scholar]
- Hermer-Vazquez L, Moffet A, and Munkholm P. 2001. Language, space, and the development of cognitive flexibility in humans: the case of two spatial memory tasks. Cognition 79:263–299. [DOI] [PubMed] [Google Scholar]
- Hermer-Vazquez L, Spelke E, and Katnelson AS. 1999. Sources of flexibility in human cognition: Dual-task studies of space and language. Cognitive Psychology 39:3–36. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Call J, Hernadez-Lloreda MV, Hare B, and Tomasello M. 2007. Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science 317:1360–1366. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Hare B, Call J, and Tomasello M. 2010. Differences in the cognitive skills of bonobos and chimpanzees. PLoS One 5:e12438–e12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Barton M, and Hurtado M. 2009. The emergence of human uniqueness: Characters underlying behavioral modernity. Evolutionary Anthropology 18:187–200. [Google Scholar]
- Hohmann G, and Fruth B. 1993. Field observations on meat sharing among bonobos (Pan paniscus). Folia Primatologica 60:225–229. [Google Scholar]
- Hopkins WD, Lyn H, and Cantalupo C. 2009. Volumetric and lateralized differences in selected brain regions of chimpanzees (Pan troglodytes) and bonobos (Pan paniscus). American Journal of Primatology 71:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hribar A, and Call J. 2011. Great apes use landmark cues over spatial relations to find hidden food. Animal Cognition 14:623–635. [DOI] [PubMed] [Google Scholar]
- Hribar A, Haun D, and Call J. 2011. Great apes’ strategies to map spatial relations. Animal Cognition 14:511–523. [DOI] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, and Bohbot VD. 2003. Cognitive strategies dependent on the hippocampus and caudate neucleaus in human navigation: Variability and change with practice. The Journal of Neuroscience 23:5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isler K, and van Schaik CP. 2014. How humans evolved large brains: Comparative evidence. Evolutionary Anthropology 23:65–75. [DOI] [PubMed] [Google Scholar]
- Jabes A, and Nelson CA. 2015. 20 years after ‘‘The ontogeny of human memory: A cognitive neuroscience perspective,’’ where are we? International Journal of Behavioral Development 39:293–303. [Google Scholar]
- Jacobs LF, Gaulin SJC, Sherry DF, and Hoffman GE. 1990. Evolution of spatial cognition: Sex-specific patterns of spatial behavior predict hippocampal size. Proc Natl Acad Sci U S A 87:6349–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmaat K, Ban SD, and Boesch C. 2013. Chimpanzees use long-term spatial memory to monitor large fruit trees and remember feeding experiences across seasons. Animal Behaviour 86:1183–1205. [Google Scholar]
- Janson CH. 1998. Experimental evidence for spatial memory in wild brown capuchin monkeys (Cebus apella). Animal Behaviour 55:1229–1243. [DOI] [PubMed] [Google Scholar]
- Janson CH. 2007. Experimental evidence for route integration and strategic planning in wild capuchin monkeys. Animal Cognition 10:341–356. [DOI] [PubMed] [Google Scholar]
- Janson CH, and Byrne R. 2007. What wild primates know about resources: opening up the black box. Animal Cognition 10:357–367. [DOI] [PubMed] [Google Scholar]
- Janson CH, and van Schaik CP. 1993. Ecological risk aversion in juvenile primates: slow and steady wins the race. In: Pereira ME, Fairbanks LA, editor. Juvenile primates: Life history, development, and behavior Chicago: The University of Chicago Press; p 57–76. [Google Scholar]
- Kano T 1992. The last ape: Pygmy chimpanzee behavior and ecology Stanford: Stanford University Press. [Google Scholar]
- Kaplan H, Hill K, Lancaster J, and Hurtado M. 2000. A theory of human life history evolution: Diet, intelligence, and longevity. Evolutionary Anthropology 9:156–185. [Google Scholar]
- Knowlton BJ, Mangels JA, and Squire LR. 1996. A neostriatal habit learning system in humans. Science 273:1399–1402. [DOI] [PubMed] [Google Scholar]
- Koster J, Mcelreath R, Hill K, Yu D, Shepard G, Vliet NV, Gurven M, Kaplan H, Trumble B, Bird RB et al. . 2019. The life history of human foraging: Cross-cultural and individual variation bioRxiv:574483. [DOI] [PMC free article] [PubMed]
- Kuroda S 1989. Developmental retardation and behavioral characteristics of pygmy chimpanzees. In: Heltne P, and Marquardt L, editors. Understanding chimpanzees Cambridge: Harvard University press; p 184–193. [Google Scholar]
- Kuznetsova A, Brockhoff PB, and Christensen RHB. 2015. lmerTest: Tests in Linear Mixed Effects Models. Package ‘lmerTest’. R package version 2.0–33
- Lavenex P, and Banta Lavenex P. 2013. Building hippocampal circuits to learn and remeber: insights into the development of human memory. Behavioural Brain Research 254:8–21. [DOI] [PubMed] [Google Scholar]
- Leigh SR. 1994. Ontoaenetic correlates of diet in anthropoid primates. American Journal of Physical Anthropology 94:499–522. [DOI] [PubMed] [Google Scholar]
- Leigh SR. 2004. Brain growth, life history, and cognition in primate and human evolution. American Journal of Primatology 62:139–164. [DOI] [PubMed] [Google Scholar]
- Leigh SR. 2012. Brain size growth and life history in human evolution. Evolutionary Biology 39:587–599. [Google Scholar]
- Lenth R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.2.3. 2018. https://CRAN.R-project.org/package=emmeans.
- Levinson SC, Kita S, Haun DBM, and Rasch BH. 2002. Returning the tables: language affects spatial reasoning. Cognition 84:144–188. [DOI] [PubMed] [Google Scholar]
- Li S, Lindenberger U, Hommel B, Aschersleben G, Prinz W, and Baltes PB. 2004. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science 15:155–163. [DOI] [PubMed] [Google Scholar]
- Lieberman DA, Carlo J, de Leon MP, and Zollikofer CPE. 2007. A geometric morphometric analysis of heterochrony in the cranium of chimpanzees and bonobos. Journal of Human Evolution 52(647–662.). [DOI] [PubMed] [Google Scholar]
- Logan CJ, Avin S, Boogert N, Buskell A, Cross FR, Currie A, Jelbert S, Lukas D, Mares R, Navarrete AF et al. . 2018. Beyond brain size: Uncovering the neural correlates of behavioral and cognitive specialization. Comparatice Cognition and Behavior Rewviews 13:55–89. [Google Scholar]
- MacLean EL. 2016. Unraveling the evolution of uniquely human cognition. Proceedings of the National Academy of Sciences 113:6348–6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean EL, Hare B, Nunn CL, Addessi E, Amici F, Anderson RC, Aureli F, Baker JM, Bania AE, Barnard AM et al. . 2014. The evolution of self-control. Proceedings from the National Academy of Sciences
- Maguire EA, Burgess N, Donnett JG, Frackowiak RSJ, Frith CD, and O’Keefe J. 1998. Knowning where and getting there: A human navigation network. Science 280:921–923. [DOI] [PubMed] [Google Scholar]
- Malenky RK, and Wrangham RW. 1993. A quantitative comparison of terrestrial herbaceous food consumption by Pan paniscus in the Lomako Forest, Zaire, and Pan troglodytes in the Kibale Forest, Uganda. American Journal of Primatology 32:1–12. [DOI] [PubMed] [Google Scholar]
- Mankin EA, Sparks FT, Slayyeh B, Sutherland RJ, Leutgeb S, and Leutgeb JK. 2012. Neuronal code for extended time in the hippocampus [DOI] [PMC free article] [PubMed]
- Manrique HM, and Call J. 2015. Age-dependent cognitive inflexibility in great apes. Animal Behaviour 102:1–6. [Google Scholar]
- Marlowe FW. 2005. Hunter-gatherers and human evolution. Evolutionary Anthropology 14:54–67. [Google Scholar]
- Martin-Ordas G, Berntsen D, and Call J. 2013. Memory fo distance past events in chimpanzees and orangutans. Current Biology 15:1438–1441. [DOI] [PubMed] [Google Scholar]
- Martin-Ordas G, Haun D, Colmenares F, and Call J. 2010. Keeping track of time: evidence for episodic-like memory in great apes. Animal Cognition 13:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa T 2007. Comparative cognitive development. Developmental Science 10:97–103. [DOI] [PubMed] [Google Scholar]
- Matsuzawa T, Tomonaga M, and Tanaka M, editors. 2006. Cognitive development in chimpanzees Tokyo: Springer-Verlag. [Google Scholar]
- McGrew WC. 2010. In search of the last common ancestor: new findings on wild chimpanzees. Philosophical Transactions of the Royal Society B 365:3267–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes N 2008. Spatial memory in chimpanzees: Single-trial learning and 24 hour and 3 month long-term memory. Folia Primatologica 79:283–304. [Google Scholar]
- Menzel CR, Savage-Rumbaugh ES, and Menzel EW. 2002. Bonobo (Pan paniscus) spatial memory and communication in a 20-hectare forest International Journal of Primatology 23:601–619. [Google Scholar]
- Menzel EW. 1973. Chimpanzee spatial memory organization. Science 182:943–945. [DOI] [PubMed] [Google Scholar]
- Moczek AP, Sears KE, Stollewerk A, Wittkopp PJ, Diggle P, Dworkin I, Ledon-Rettig C, Matus DQ, Roth S, Abouheif E et al. . 2015. The significance and scope of evolutionary developmental biology: a vision for the 21st century. Evolution & Development 17:198–219. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, and O’Keefe J. 1982. Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683. [DOI] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW, and Pilbeam DR, editors. 2017. Chimpanzees and human evolution Cambridge: Belknap Press. [Google Scholar]
- Newcombe NS, and Huttenlocher J. 2006. Development of spatial cognition. Handbook of Child Psychology p 734–776.
- Newcombe NS, Huttenlocher J, Drummey A, and Wiley JG. 1998. The development of spatial location coding: Place learning and dead reckoning in the second and third years. Cogntitive Development 13:185–200. [Google Scholar]
- Nielson DM, Smith TA, Sreekumar V, Dennis S, and Sederberg PB. 2015. Human hippocampus represents space and time during retrieval of real-world memories. Proceedings of the National Academy of Sciences 112:11078–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normand E, Ban SD, and Boesch C. 2009. Forest chimpanzees (Pan troglodytes verus) remember the location of numerous fruit trees. Animal Cognition 12:797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normand E, and Boesch C. 2009. Sophisticated Euclidean maps in forest chimpanzees. Animal Behaviour 77:1976–1201. [Google Scholar]
- Norris D 2017. Short-term memory and long-term memory are still different. Psychological Bulletin 143:992–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Lovden M, Riklund K, Lindenberger U, and Backan L. 2012. Memory aging and brain maintenance. Trends in Cognitive Sciences 16:292–305. [DOI] [PubMed] [Google Scholar]
- Packard MG. 1996. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of Learning and Memory 65:65–72. [DOI] [PubMed] [Google Scholar]
- Packard MG. 2009. Exhumed from thought: Basal ganglia and response learning in the plus-maze. Behavioral Brain Research 199:24–31. [DOI] [PubMed] [Google Scholar]
- Palagi E 2006. Social play in bonobos (Pan paniscus) and chimpanzees (Pan troglodytes): Implications for natural social systems and interindividual relationships. American Journal of Physical Anthropology 129(418–436). [DOI] [PubMed] [Google Scholar]
- Parish AR. 1996. Female relationships in bonobos (Pan paniscus): Evidence for bonding, cooperation, and female dominance in a male-philopatric species. Human Nature 7(1):61–96. [DOI] [PubMed] [Google Scholar]
- Platt ML, Brannon EM, Briese TL, and French JA. 1996. Differences in feeding ecology predict differences in performance between golden lion tamarins (Leontopithecus rosalia) and Wied’s marmosets (Callithrix kuhli) on spatial and visual memory tasks. Animal Learning and Behavior 24:384–393. [Google Scholar]
- Poldrack RA, Clark MA, Pare-Blagoev EJ, Shohamy D, Creso Moyan J, Myers C, and Gluck MA. 2001. Interactive memory systems in the human brain. Nature 414:546. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, and Packard MG. 2003. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia 41:245–251. [DOI] [PubMed] [Google Scholar]
- Pravosudov VV, and Roth TC. 2013. Cognitve ecology of food hoarding: The evolution of spatial memory and the hippocampus. Annual Review of Ecology, Evolution, and Systematics 44:173–193. [Google Scholar]
- Kea Pruefer. 2012. The bonobo genome compared to the chimpanzee and human genomes. Nature [DOI] [PMC free article] [PubMed]
- Pruetz JD, and McGrew WC. 2001. What does a chimpanzee need? Using natural behavior to guide the care and management of captive populations. In: Brent L, editor. Care and Management of Captive Chimpanzees San Antonio: American Society of Primatologists; p 17–37. [Google Scholar]
- R Development Core Team. 2018. A language and environment for statistical computing Vienna, Austira. [Google Scholar]
- Ribordy F, Jabes A, Banta Lavenex P, and Lavenex P. 2013. Development of allocentric spatial memory abilities in children from 18 months to 5 years of age. Cognitive Psychology 66:1–29. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Scholz J, Preuss TM, Glasser MF, Erangi BK, and Behrens TE. 2011. Differences between chimpanzees and bonobos in neural systems supporting social cognition. Social Cognitive and Affective Neuroscience 7:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson SL, and Wood B. 2008. Hominin life history: reconstruction and evolution. Journal of Anatomy 212:394–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnlund M, Nyberb L, and Backman L. 2005. Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychology and Aging 20:3–18. [DOI] [PubMed] [Google Scholar]
- Rosati AG. 2015. Context influences spatial frames of reference in bonobos (Pan paniscus). Behaviour 152:375–406. [Google Scholar]
- Rosati AG. 2017a. Ecological variation in cognition: Insights from bonobos and chimpanzees. In: Yamamoto BHS, editor. Bonobos: Unique in mind, brain and behavior Oxford: Oxford University Press; p 157–170. [Google Scholar]
- Rosati AG. 2017b. Foraging cognition: Reviving the ecological intelligence hypothesis. Trends in Cognitive Sciences 21:691–702. [DOI] [PubMed] [Google Scholar]
- Rosati AG, Arre AM, Platt ML, and Santos LR. 2016. Rhesus monkeys show human-like changes in gaze following across the lifespan. Proceedings of the Royal Society B 283:20160376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, and Hare B. 2012. Chimpanzees and bonobos exhibit divergent spatial memory development. Developmental Science 15:840–853. [DOI] [PubMed] [Google Scholar]
- Rosati AG, and Hare B. 2013. Chimpanzees and bonobos exhibit emotional respones to decision outcomes. PLoS One 8:e63058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, Herrmann E, Kaminski J, Krupenye C, Melis AP, Schroepfer K, Tan J, Warneken F, Wobber V, and Hare B. 2013. Assessing the psychological health of captive and wild apes: A response to Ferdowsian et al. (2011). Journal of Comparative Psychology 127:329–336. [DOI] [PubMed] [Google Scholar]
- Rosati AG, Rodriguez K, and Hare B. 2014a. The ecology of spatial memory in four lemur species. Animal Cognition 17:947–961. [DOI] [PubMed] [Google Scholar]
- Rosati AG, and Santos LR. 2017. Tolerant Barbary macaques maintain juvenile levels of social attention in old age, but despotic rhesus macaques do not. Animal Behaviour 130:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, Wobber V, Hughes K, and Santos LR. 2014b. Comparative developmental psychology: How is human cognitive development unique? Evolutionary Psychology 12:448–473. [PubMed] [Google Scholar]
- Ross C, and Jones KE. 1999. Socioecology and the evolution of primate reproductive rates. In: Lee P, editor. Comparative primate socioecology New York: Cambridge University Press; p 73–110. [Google Scholar]
- Sakai T, Mikami A, Tomonaga M, Matsui M, Suzuki J, Hamada Y, Tanaka M, Miyabe-Nishiwaki T, Makishima H, Nakatsukasa M et al. . 2011. Differential prefrontal white matter development in chimpanzees and humans. Current Biology 1397–1402. [DOI] [PubMed]
- Schuppli C, Grabre SM, Isler K, and van Schaik CP. 2016. Life history, cognition and the evolution of complex foraging niches. Journal of Human Evolution 92:91–100. [DOI] [PubMed] [Google Scholar]
- Schuppli C, Isler K, and van Schaik CP. 2012. How to explain the unusually late age at skill competence among humans. Journal of Human Evolution 63:843–850. [DOI] [PubMed] [Google Scholar]
- Schwartz G 2012. Growth, development, and life history throughout the evolution of Homo. Current Anthropology 53:S395–S408. [Google Scholar]
- Scoville WB, and Milner B. 1957. Loss of recent memory after bilateral hippocampal lesions. ournal of Neurology Neurosurgery and Psychiatry 20:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea B 1983a. Paedomorphosis and neoteny in the pygmy chimpanzee. Science 222:521–522. [DOI] [PubMed] [Google Scholar]
- Shea B 1984. An allometric perspective on the morphological and evolutionary relationships between pygmy (Pan paniscus) and common (Pan troglodytes) chimpanzees. In: Susman RL, editor. The Pygmy Chimpanzee: Evolutionary Biology and Behavior New York: Plenum Press; p 89–130. [Google Scholar]
- Shea B 1989. Heterochrony in human evolution: the case for neoteny reconsidered. Yearbook of Physical Anthropology 32:69–104. [Google Scholar]
- Shea BT. 1983b. Allometry and heterochrony in the African apes. American Journal of Physical Anthropology 62:275–289. [DOI] [PubMed] [Google Scholar]
- Sherry DF. 2006. Neuroecology. Annual Review of Psychology 57:167–197. [DOI] [PubMed] [Google Scholar]
- Shettleworth SJ. 1998. Cognition, evolution, and behavior. New York: Oxford University Press. [Google Scholar]
- Sluzenski J, Newcombe NS, and Satlow E. 2004. Knowing where things are in the second year of life: Implications for hippocampal development. Journal of Cognitive Neuroscience 16:1443–1451. [DOI] [PubMed] [Google Scholar]
- Squire LR. 2004. Memory systems of the brain: a brief history and current perspective. Neurobiology of Learning and Memory 82:171–177. [DOI] [PubMed] [Google Scholar]
- Stanford CB. 1998. The social behavior of chimpanzees and bonobos - empirical evidence and shifting assumptions. Current Anthropology 39(4):399–420. [Google Scholar]
- Stanford CB. 2012. Chimpanzees and the behavior of Ardipithecus ramidus. Annual Review of Anthropology 41:139–149. [Google Scholar]
- Striedter GF. 2005. Principles of brain evolution Sunderland, MA: Sinauer Associates. [Google Scholar]
- Surbeck M, and Hohmann G. 2008. Primate hunting by bonobos at LuiKotale, Salonga National Park. Current Biology 18:R906–R907. [DOI] [PubMed] [Google Scholar]
- Teffler K, Buxhoeveden DP, Stimpson CD, Fobbs AJ, Schapiro SJ, Baze WB, McArthur MJ, Hopkins WD, Hof PR, Sherwood CC et al. . 2013. Developmental changes in the spatial organization of neurons in the neocortex of humans and common chimpanzees. The Journal of Comparative Neurology 521:4249–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman EC. 1948. Cognitive maps in rats and men. Psychological Review 55:189–208. [DOI] [PubMed] [Google Scholar]
- Tomasello M 2014. A natural history of human thinking Cambridge: Harvard University Press. [Google Scholar]
- Tomasello M, and Call J. 2008. Assessing the validity of ape-human comparisons: A reply to Boesch (2007). Journal of Comparative Psychology 122:449–452. [DOI] [PubMed] [Google Scholar]
- Tomasello M, and Carpenter M. 2005. The emergence of social cognition in three young chimpanzees. Monographs of the Society for Research in Child Development 70 [DOI] [PubMed]
- Tomasello M, Hare B, and Fogleman T. 2001. The ontogeny of gaze following in chimpanzee, Pan troglodytes, and rhesus macaques, Macaca mulatta. Animal Behaviour 61:335–343. [Google Scholar]
- Utsunomiya H, Takano K, Okazaki M, and Mitsudome A. 1999. Development of the temporal lobe in infants and children: analysisby MR-based volumetry. American Journal of Neuroradiology 20:717–720. [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn MH, Bard KA, Bakersmans-Kranenburg MJ, and Ivan K. 2008. Enhancement of attachment and cognitive development of young nursery‐reared chimpanzees in responsive versus standard care. Developmental Psychobiology 51:173–185. [DOI] [PubMed] [Google Scholar]
- Walker KK, Walker CS, Goodall J, and Pusey AE. 2018. Maturation is prolonged and variable in female chimpanzees. Journal of Human Evolution 114:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ. 1989. Ecological correlates of pygmy chimpanzee social structure. In: Standen V, and Foley RA, editors. Comparitive Socioecology: The Behavioral Ecology of Human and Other Mammals Oxford: Blackwell Scientific Publications. [Google Scholar]
- White FJ. 1998. Seasonality and socioecology: The importance of variation in fruit abundance to bonobo sociality. International Journal of Primatology 19:1013–1027. [Google Scholar]
- White FJ, and Wrangham RW. 1988. Feeding competition and patch size in the chimpanzee species Pan paniscus and Pan troglodytes. Behaviour 105. [Google Scholar]
- Whiten A, and Erdal D. 2012. The human socio-cognitive niche and its evolutionary origins. Philosophical Transactions of the Royal Society B 367:2119–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobber V, and Hare B. 2011. Psychological health of orphan bonobos and chimpanzees in African sanctuaries. PLoS One 6:e17147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobber V, Hare B, Lipson S, Wrangham R, and Ellison P. 2013. Different ontogenetic patterns of testosterone production reflect divergent male reproductive strategies in chimpanzees and bonobos. Physiology & Behavior 116–117:44–53. [DOI] [PubMed] [Google Scholar]
- Wobber V, Herrmann E, Hare B, Wrangham R, and Tomasello M. 2014. Differences in the early cognitive development of children and great apes. Developmental Psychobiology 56:547–573. [DOI] [PubMed] [Google Scholar]
- Wobber V, Wrangham R, and Hare B. 2010a. Application of the heterochrony framework to the study of behavior and cognition. Communicative & Integrative Biology 3:337–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobber V, Wrangham R, and Hare B. 2010b. Bonobos exhibit delayed development of social behavior and cognition relative to chimpanzees. Current Biology 20:226–230. [DOI] [PubMed] [Google Scholar]
- Wrangham R 2000. Why are male chimpanzees more gregarious than mothers? A scramble competition hypothesis. In: Kappeler P, editor. Primate Males Causes and Consequences of Variation in Group Composition Cambridge: Cambridge University Press; p 248–258. [Google Scholar]
- Wrangham R, and Peterson D. 1996. Demonic males: Apes and the origins of human violence
- Wrangham R, and Pilbeam D. 2001. African apes as time machines. In: Galdikas E, Briggs, Sheeran, Shapiro & Goodall, editor. All apes great and small Volume I: African apes: Kluwer Academic / Plenum Publishers. [Google Scholar]
- Zuberbühler K 2014. Experimental field studies with non-human primates. Current Opinion in Neurobiology 28:150–156. [DOI] [PubMed] [Google Scholar]
- Zuur A, Ieno EN, Walker N, Saveliev AA, and Smith GM. 2009. Mixed effect models and extensions in ecology New York: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data from these studies will be available in Dryad Digital Repository.


