Abstract
Study Objectives:
Sleep deprivation is known to be associated with insulin resistance and diabetes risk. This study investigated whether 2-week sleep extension in chronically sleep-deprived individuals would improve glucose metabolism.
Methods:
A crossover study was conducted in volunteers without diabetes who reported sleeping ≤ 6 h/night. They were randomized to maintain their habitual sleep or extend sleep time for 2 weeks, then crossed over after a washout period. Sleep was monitored by actigraphy. Oral glucose tolerance tests (75 g) with insulin levels was performed at the end of each period. Mixed-effect linear regression analysis, adjusting for sequence and period effects, was applied.
Results:
A total of 21 participants (19 females) with mean (standard deviation) age of 33.1 (6.1) years completed the protocol. Mean sleep duration during habitual sleep was 318.7 (44.3) minutes and the participants extended their sleep by 36.0 (45.2) minutes during sleep extension. The average washout period was 21 (11) days. There were no significant effects of sleep extension on any metabolic parameters. The per-protocol analysis included eight participants who could sleep more than 6 hours during sleep extension (mean sleep duration 396 [25] minutes, extended by 60.1 [28.5] minutes). Among these individuals, sleep extension improved Homeostatic Model Assessment of Insulin Resistance (adjusted mean difference −0.50 [95% confidence interval [CI] −0.89, −0.11, P = .013]), early insulin secretion (insulinogenic index; mean difference 0.39 [95% CI 0.15, 0.63, P = .001]), and β-cell function (disposition index, mean difference 1.07 [95% CI 0.17, 1.97, P = .02]).
Conclusions:
Sleep extension in chronically sleep-deprived individuals improved glucose metabolism in only those who could objectively extend their sleep to more than 6 h/night. Our findings suggest that a critical amount of sleep is needed to benefit metabolic outcomes.
Citation:
So-ngern A, Chirakalwasan N, Saetung S, Chanprasertyothin S, Thakkinstian A, Reutrakul S. Effects of two-week sleep extension on glucose metabolism in chronically sleep-deprived individuals. J Clin Sleep Med. 2019;15(5):711–718.
Keywords: glucose metabolism, insulin resistance, sleep extension
BRIEF SUMMARY
Current Knowledge/Study Rationale: Insufficient sleep has been linked to insulin resistance in experimental sleep restriction studies, and to incident diabetes in epidemiological studies. Scant data exist regarding the benefit of sleep extension in habitual short sleepers.
Study Impact: Sleep extension in habitual short sleepers is feasible, but more effective means are needed to help these individuals reach a physiologically meaningful amount of sleep. Glucose metabolism improved only in those who could sleep more than 6 h/night as measured objectively during sleep extension, suggesting that a critical amount of sleep is needed to benefit metabolic health.
INTRODUCTION
Type 2 diabetes is a major health problem worldwide. In 2015, an estimated 30.3 million in the United States had diabetes,1 with the cost of care estimated at $327 billion in 2017.2 Globally, the prevalence of diabetes is expected to rise 48% by 2045 with an unequal geographic disease burden resulting in one in three adults with diabetes living in the Western Pacific region.3 Although rising obesity contributes significantly to the increasing prevalence of diabetes, sleep disturbances are emerging as novel risk factors for disease development.4
Over the past decades, sleep duration has been declining in the United States, with one-third of adults reporting insufficient sleep.5 Increasing social and work obligations, along with the availability of electronic media, likely contribute to sleep curtailment in modern society. Prospective studies have demonstrated that sleeping ≤ 6 hours was associated with 18% to 48% increase in the risk of the development of diabetes.4 The causal association between insufficient sleep and altered glucose metabolism was confirmed in several well-conducted sleep restriction experiments in healthy volunteers in which restricting sleep to 4 to 5.5 hours in bed decreased insulin sensitivity by 16% to 24%.6–9 Conversely, several experimental studies have found that a few nights of sleep recovery can at least partially reverse the adverse metabolic effects of sleep restriction.6,10,11 These data support the role of sleep duration in glucose metabolism. Furthermore, mounting evidence suggests that insufficient sleep is also linked to obesity, hypertension, and cardiovascular disease as well as mood disturbances.12–15 As a result, in 2015, a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society recommended that adults should sleep 7 or more hours per night on a regular basis to promote optimal health.16
Despite the aforementioned experimental and epidemiological data, as well as clinical practice recommendations, the effects of sleep extension on glucose metabolism in chronically sleep-deprived adults have only been examined in two studies. The first employed a crossover trial in 19 healthy men with chronic, repetitive lifestyle-driven sleep restriction.17 Three nights of catch-up sleep in the laboratory (from 6 to 10 hours) improved insulin sensitivity, as assessed by an oral glucose tolerance test (OGTT).17 The second study was a single-arm home sleep extension study in 16 healthy volunteers who were chronic short sleepers during work days but were able to extend sleep time during non-work days. After 6 weeks, mean sleep duration increased by 44 min/d, with associated improvements in insulin sensitivity that correlated with the increase in sleep duration.18 Although limited, these early data suggested the benefits of sleep extension on glucose metabolism. However, whether a shorter duration of sleep extension in the home environment, with a crossover design, will similarly improve glucose metabolism has not been studied.
Therefore, the purpose of this study was to explore the effects of a 2-week sleep extension on glucose metabolism in healthy volunteers with short habitual sleep duration using a crossover design.
METHODS
Participants
Healthy participants age 20 to 55 years were recruited through local advertising. This study was conducted between August 2016 and July 2017. Eligibility criteria included:(1) self-reported sleep duration on weekdays ≤ 6 h/night; (2) desired sleep duration ≥ 7 h/night on weekends; (3) belief that time spent in bed could be increased by at least 1 h/night for 2 weeks; (4) absence of diabetes as assessed by fasting blood glucose < 126 mg/dL within the past 3 years; (5) being at low risk for obstructive sleep apnea as assessed by Berlin questionnaire19,20; (6) reported habitual sleep time before 3:00 am. Exclusion criteria were: (1) presence of pulmonary, cardiovascular, or kidney disease; (2) presence of active psychiatric and/or neurological disorders; (3) presence of insomnia symptoms (ie, reported sleep latency > 30 minutes, wake time after sleep onset > 30 minutes or early awakening > 30 minutes); (4) performing shift work or traveling across time zones; (5) receiving systemic corticosteroids; (6) using sedative drugs or stimulants; (7) cigarette smoking and/or alcohol drinking; and (8) caffeine consumption > 400 mg/d. All participants gave written informed consent. The protocol was approved by the Committee on Human Rights Related to Research Involving Human Subjects, Faculty of Medicine Ramathibodi Hospital and registered at clinicaltrials.gov (NCT02854709).
Screening and Ascertainment of Habitual Sleep Duration and Glycemic Status
After meeting initial eligibility criteria, a general physical examination was performed and medical history was obtained. Work history, including numbers of d/wk and number of h/d, was obtained. The participants then had a 1-week run in period during which habitual sleep duration was measured using actigraphy (described in the following “sleep monitoring” section). They were asked to keep a sleep diary during this time. After 1 week, the participants underwent a 75-g OGTT after an overnight fast to ensure that they did not have diabetes. Those with average sleep duration ≥ 7 h/night and sleep efficiency < 70% or fasting glucose levels ≥ 126 mg/dL or 2-hour glucose levels after an OGTT ≥ 200 mg/dL were excluded from the study.
Study Protocol
The study was conducted using a crossover design. The participants were assigned by alternate sequence to either maintain their habitual sleep for 2 weeks or extend their time in bed by at least 1 h/night for 2 weeks. Modifying bedtime, rather than wake time, was chosen because participants were working, and it was not possible for them to wake up later on work days. Afterward, the participants entered a washout period for at least 2 weeks before being crossed over. General sleep hygiene recommendations were given to all participants at randomization.21
Sleep Extension Intervention
Participants were advised to extend their time in bed by 1 hour. Because all participants worked and could not delay their wake time, they were asked to go to bed earlier by 15 to 20 minutes each day until their bedtime was 1 hour earlier than their habitual bedtime. They then maintained that bedtime throughout the 2-week intervention. We interviewed each participant regarding their evening activities and discussed possible modifications in order to achieve increased time in bed. Specific instructions on scheduled bedtimes and when to turn off electronic devices (1 hour before scheduled bedtime) were individualized to each participant.
Sleep Monitoring
Objective sleep assessment was obtained using Actiwatch 2 (Philips Respironics, Bend, Oregon, United States), which the participants wore for the entire 2 weeks during the habitual and sleep extension periods. These monitors use highly sensitive omnidirectional accelerometers to count the number of wrist movements in 30-second epochs. The software scores each 30-second epoch as sleep or wake based on a threshold of activity counts that is estimated using activity within the epoch being scored as well as the epochs 2 minutes before and after that epoch. Bedtime and wake time are set by the researcher using the event markers, sleep log data, as well as an in-person review of sleep timing with the participants upon return of the watch. Sleep duration was defined as the amount of actual sleep obtained at night. Sleep efficiency was the percentage of time in bed spent sleeping. Both parameters were calculated using Actiware 6.0 software, supplied by the manufacturer. Mid-sleep time was calculated as the midpoint between sleep start and sleep end times.
Primary Outcome of Interest
Primary outcomes of interest were metabolic parameters obtained from a 75-g OGTT. At the end of each sleep period and after an overnight fast, participants were given 75 g of glucose orally. Blood samples were obtained at 0, 30, 60, 90, and 120 minutes for glucose and insulin measurements. Glucose levels were assayed in the clinical laboratory of Ramathibodi Hospital using the hexokinase enzymatic method. Serum insulin was measured by an electrochemiluminescence immunoassay on a Cobase 411 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). The assays have intra-assay and interassay precision of 2.0% and 2.8%, respectively.
The following markers of glucose metabolism were calculated. Area under the curve for glucose and insulin response to glucose challenge was calculated using the trapezoidal rule. Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), an index of fasting insulin resistance, was calculated using the following formula22:
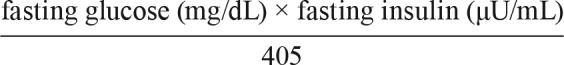 |
The Insulinogenic Index, an estimate of early insulin secretion, was calculated by dividing the increment in insulin during the first 30 minutes by the increment in glucose over the same period (ΔI30/ΔG30).23 The Matsuda index, an index of whole body insulin sensitivity, was calculated from the glucose and insulin levels during the glucose challenge as previously described.24 Finally, the disposition index, an indicator of β-cell function adjusted for insulin sensitivity, was calculated as a product of the insulinogenic index and the Matsuda index.25
Secondary Outcomes of Interest
Secondary outcomes of interest included self-reported sleep assessment, dietary intake, and weight. Self-reported sleep assessments were obtained at the end of each period (habitual sleep and sleep extension). Daytime sleepiness was assessed using a validated Thai version of the Epworth Sleepiness Scale (ESS),26 with higher score reflecting greater daytime sleepiness. Self-reported sleep quality was assessed using a validated Thai version of the Pittsburg Sleep Quality Index (PSQI),27 with higher scores reflecting poorer sleep quality. Self-reported sleep assessments were done at the end of each randomization period.
For dietary assessment, participants were asked to maintain a food diary for 3 days each week (1 weekend day and 2 weekdays), for a total of 6 days during each sleep period. Daily caloric consumption was calculated by a dietician using a Thai food database (INMUCAL-Nutrients V3, Institute of Nutrition, Mahidol University). Weight was obtained in the morning, fasting, in light clothing after each sleep period using a calibrated electronic scale (seca 767; Hamburg, Germany).
Sample Size
The sample size was estimated based on testing two means of HOMA-IR of 0.25, which was a difference in the HOMA-IR after 1 night of sleep restriction.28 A total of 16 participants were required and the probability was 81% that the study will detect a treatment difference at a two-sided .05 significance level. Twenty participants were planned to account for a 20% dropout rate.
Statistical Analysis
Data are expressed as mean (standard deviation) for continuous variables or frequency (%). Mixed-effect linear regression analysis was applied to assess intervention effect by fitting intervention on each outcome variable. In addition, sequence, and period effects are taken into account in the model to account for crossover design effects. Analyses were performed using STATA version 14 (StataCorp LLC, College Station, Texas, United States). A value of P < .05 was considered statistically significant.
RESULTS
Figure 1 illustrates the flow of the study. Of a total of 78 individuals who were initially screened, 35 were found to be eligible and 28 consented to the study and underwent a baseline screening. After completion of the screening, 6 participants were excluded (3 changed their mind, 1 became pregnant, 1 had sleep duration > 6 h/night according to actigraphy and 1 could not tolerate glucose solution during an OGTT), and 22 were then randomized. One participant was subsequently excluded due to the use of an antihistamine during the protocol, resulting in 21 participants with valid data for the analysis.
Figure 1. Flow chart of the study.
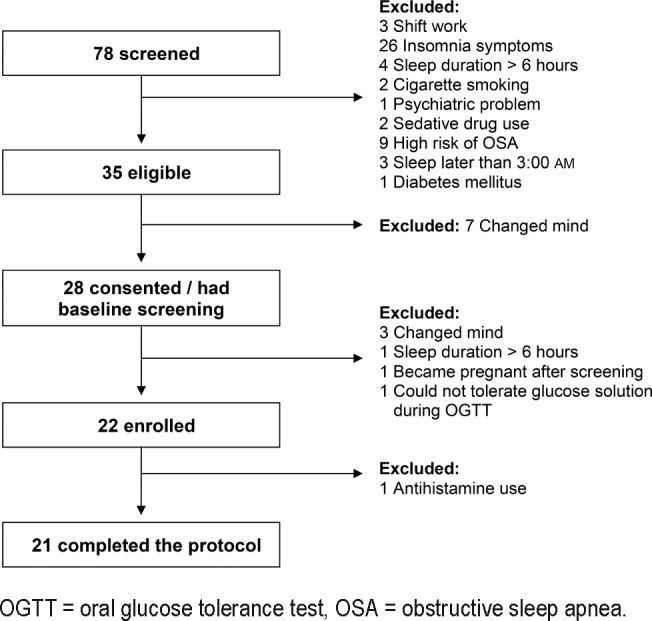
OGTT = oral glucose tolerance test, OSA = obstructive sleep apnea.
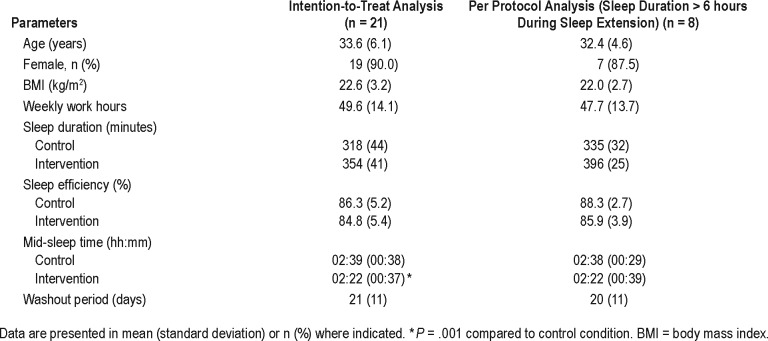
Table 1 describes the participants' characteristics. In this study, we employed two analyses. The intention to treat analysis included all 21 participants who completed the protocol. In addition, a per-protocol analysis was performed, including only those who could sleep more than 6 hours during the sleep extension period (n = 8). Sleep efficiency did not change after sleep extension (P > .05 in both analyses). There was a significant shift in mid-sleep time in the intention to treat analysis of approximately 17 minutes earlier during sleep extension. The change in mid-sleep time was not significant for the per-protocol analysis.
Table 1.
Characteristics of all participants.
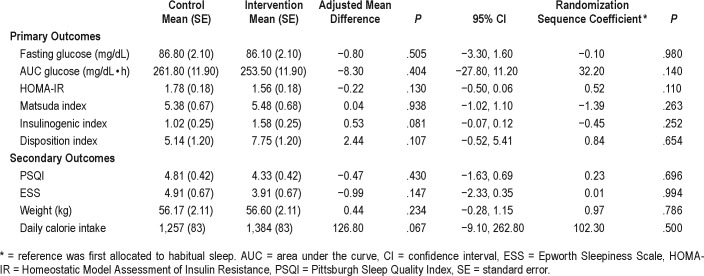
Table 2 demonstrates the primary and secondary outcomes of the intention to treat analysis. The participants extended their sleep by an average (standard deviation) of 36.0 (45.2) minutes, from a mean habitual sleep duration of 318 (44) minutes to 354 (41) minutes during sleep extension. The participants maintained their sleep efficiency throughout the protocol. For primary outcomes of glucose metabolism, there were no significant differences in any glycemic parameters between the two sleep conditions. Self-reported sleep quality, daytime sleepiness, caloric intake, and weight were also not different. Randomization sequence did not affect glycemic outcomes, that is, glycemic parameters did not differ between participants allocated first to sleep extension versus habitual sleep (all P > .05). In addition, sleep duration on sleep extension also did not differ between participants allocated first to sleep extension versus habitual sleep (P = .949).
Table 2.
Results of sleep extension on metabolic parameters, intention-to-treat analysis (n = 21).
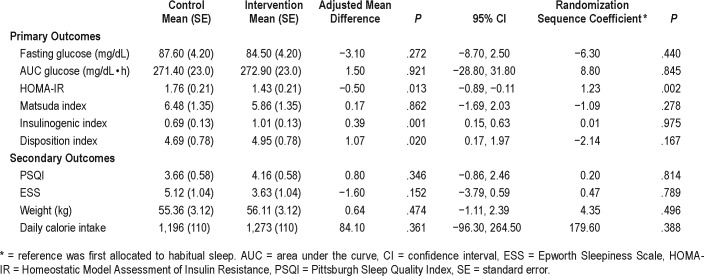
Table 3 demonstrates the results of the per-protocol analysis including only those who could sleep more than 6 hours during sleep extension (n = 8). Participants in this group extended their sleep by 60.2 (28.5) minutes to achieve an average sleep duration of 396 (25) minutes (6.6 [0.4] h/night). This was accompanied by significant improvements in glycemic parameters during sleep extension compared to habitual sleep including reduced fasting insulin resistance (HOMA-IR, adjusted mean difference [MD] −0.50 [95% confidence interval [CI] −0.89, −0.11], P = .013), increased early insulin secretion (insulinogenic index, adjusted MD 0.39 [95% CI 0.15, 0.63], P = .001), and improved β-cell function (disposition index, adjusted MD 1.07 [95% CI 0.17, 1.97], P = .02). Self-reported sleep measures, weight, and caloric consumption were not different between the two conditions. Randomization sequence did not affect glycemic outcome with the exception of HOMAIR, in which participants first allocated to sleep extension had more insulin resistance than those first allocated to habitual sleep. Sleep duration on sleep extension did not differ between participants allocated first to sleep extension versus habitual sleep (P = .311).
Table 3.
Results of sleep extension on metabolic parameters, per-protocol analysis (sleep duration > 6 hours during sleep extension (n = 8).
Further comparisons were performed between those who could sleep more than 6 hours during sleep extension versus those who could not. There were no differences between groups in age, body mass index, ESS, or weekly work hours. However, those who could extend their sleep beyond 6 hours had significantly lower PSQI score (during habitual sleep) than those who slept ≤ 6 hours (3.6 [1.6] versus 5.5 [2.2], P = .048).
Adverse Events
Only one participant had nausea from glucose solution used in an OGTT at baseline assessment. She declined randomization. Otherwise, there were no adverse events during the protocol participation.
DISCUSSION
In this study of healthy volunteers with short habitual sleep duration, 2-week sleep extension did not universally improve glucose metabolism. However, we found that those who could sleep more than 6 h/night measured objectively during sleep extension had significant improvements in their fasting insulin resistance and β-cell function without changes in weight or caloric intake. These results suggest that a critical amount of sleep is needed in order to benefit glucose metabolism, and the improvement was not related to changes in weight or caloric intake. The joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society recommended 7 hours of sleep for optimal health.29 This amount was partly based on epidemiological studies linking self-reported sleep duration and health status. Studies revealed that self-reported sleep duration is generally longer than that from objective measurement (by 20 to 30 min/h of self-reported sleep duration).30,31 Thus, larger future trials should investigate the threshold of sleep duration, both objectively measured and self-reported, that has benefits on metabolic outcomes. The current study supports and further extends the knowledge of the benefits of sleep extension on glucose metabolism in short sleepers.
The mechanisms linking sleep restriction to abnormal glucose tolerance have been elucidated in several experimental studies.32 These include decreased brain glucose utilization,33 increased sympathetic nervous system activity,6,34 activation of the hypothalamic-pituitary-adrenal axis with consequential elevations of afternoon and evening cortisol levels,6,9 alterations in appetite regulating hormones,35 prolonged nocturnal growth hormone secretion,36 elevated inflammatory markers as well as elevated leukocytes and monocytes,37–39 and abnormal adipocyte function.8 These alterations have been directly linked to insulin resistance. In contrast, sleep extension studies have shown benefits of increasing sleep duration on glucose metabolism.17,18 Three nights of catch-up sleep in the laboratory (from 6 hours to 10 hours) in chronically sleep-deprived individuals resulted in an approximately 20% reduction in HOMAIR17 that was similar in magnitude to results reported among our participants. A trend toward improved β-cell function was also observed in the study previously referenced; however, the results did not reach statistical significance.17 Changes in appetite-regulating hormones were explored and revealed that leptin was significantly reduced following 10 hours of catch-up sleep compared to 6 hours, along with a reduction in peptide YY, but no changes were seen in total ghrelin levels.17 Even though leptin was demonstrated to be reduced, no significant change in energy intake was observed, potentially reflecting the complex mechanisms of appetite regulating hormones on metabolism.17 Fasting cortisol also did not differ between the two conditions.17 In a study by Leproult et al., sleep extension for 6 weeks resulted in improved insulin resistance but, similar to our findings, there were no changes in body weight.18 These early studies provided insights into mechanisms linking increase sleep duration and improvement in glucose metabolism.
Importantly, the magnitude of the improvement in HOMAIR in our study is clinically significant. The difference of approximately 17% is similar to the differences found in individuals with and without diabetes.40 This suggests potential benefit of adequate sleep duration in diabetes prevention; however, longer prospective studies must be conducted. Some early evidence, however, has suggested that this could be the case. In the Special Diabetes Program for Indians Diabetes Prevention Demonstration Project, 1,899 individuals with prediabetes underwent 16 sessions of intensive lifestyle modification with diet and exercise.41 Those reporting ≤ 6 h/night of sleep at enrollment lost less weight and had significantly higher rates of incident diabetes compared to those sleeping 7 hours (4.6 versus 3.2 per 100 person-years).41 In another study of 125 individuals with obesity and short sleep duration, spontaneous home sleep extension for a median duration of 81 days was associated with an improvement in insulin resistance and a reduction in the prevalence of abnormal fasting glucose and metabolic syndrome.42 Larger and longer studies exploring the effects of sleep extension on diabetes prevention are needed.
Strengths of the current study include its crossover design and confirmation of the safety of home sleep extension. Importantly, the results also suggested that a critical amount of sleep needs to be achieved before benefits on glucose metabolism are observed. This is likely because our participants were extra-short sleepers, with a mean sleep duration of only 318 minutes at baseline (5.3 hours). This was significantly lower than two other studies in which baseline sleep duration was 6.3 and 6.2 hours.17,18 Although we did not systematically examine the barriers to sleep extension in this study, one potential reason could be the notorious traffic congestion in Bangkok, ranked among the worst in the world43; this may have necessitated early wake times for this working population (average 5:43 am) and also delayed evening arrivals at home after which participants needed to complete house chores. As a result, only 38% of our participants could sleep more than 6 hours during sleep extension which is a limitation of this study. Post hoc analysis showed that those who could sleep more than 6 hours had better sleep quality than those who could not, suggesting that baseline sleep quality may predict the success of sleep extension ability. Barriers in sleep extension should be explored in future studies. Identification of these barriers will aid development of more effective methods to assist short sleepers in extending their sleep.
There are several limitations of this study. Formal polysomnography was not utilized to explore sleep staging, or exclude obstructive sleep apnea. We did, however, exclude participants who were at high risk for obstructive sleep apnea by Berlin questionnaire and our participants were mostly lean. Our intervention involved shifting bedtime earlier, and indeed we did see a significant shift in mid-sleep time earlier by 17 minutes in the intention- to-treat analysis. It is known that altered circadian timing could affect glucose metabolism.44 However, we do not know how this small shift aligned with the participants' internal circadian timing because we did not measure such parameters (eg, dim-light melatonin onset). Future sleep extension studies should assess circadian timing as well. Most of our participants were female; thus, whether sleep extension affects glucose metabolism in men needs to be further explored. Changes in exercise or activities pattern and how this related to glucose metabolism during each intervention period were not systematically examined. Last, we did not specifically study the participants during the same menstrual cycle phase. Approximately half of the participants were studied during the same phase and numerically more were studied during their luteal phase after sleep extension (although the differences were not statistically significant). Because insulin resistance could be higher during the luteal phase,45 we believed that our findings of improved glucose metabolism after sleep extension were independent of the OGTT timing in relation to menstrual cycles. This should be further explored in larger numbers of participants.
In conclusion, sleep extension in chronically sleep-deprived individuals resulted in improved glucose metabolism but only among those who could sleep more than 6 h/night as measured objectively during sleep extension. Our findings suggest that a critical amount of sleep is needed to benefit metabolic outcomes.
DISCLOSURE STATEMENT
Work for this study was performed at Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. Dr. Reutrakul reports grants from Merck Sharp and Dohme, nonfinancial support from ResMed, personal fees from Novo Nordisk, personal fees from Sanofi Aventis, personal fees from Medtronic, outside the submitted work. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
This study was funded by the Endocrine Society of Thailand. We thank all individuals for their participation in the study. Author contributions: A.S. researched and analyzed the data, wrote the manuscript, contributed to the discussion, and reviewed/edited the manuscript; N.C. reviewed and edited the manuscript, S.S. researched data, reviewed and edited the manuscript; A.T. analyzed data, reviewed and edited manuscript; S.R. conceptualized the study, researched and analyzed the data, wrote the manuscript, contributed to the discussion, reviewed/edited the manuscript and is the guarantor of this work and, as such, had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ABBREVIATIONS
- ESS
Epworth Sleepiness Scale
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- OGTT
oral glucose tolerance test
- PSQI
Pittsburgh Sleep Quality Index
- MD
mean difference
REFERENCE
- 1.National Diabetes Statistics Report, 2017: Estimates of Diabetes and Its Burden in the United States [PDF] Centers for Disease Control and Prevention website. [Accessed November 20, 2017]. https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. Published 2017.
- 2.American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–928. doi: 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Diabetes Federation Diabetes Atlas - 8th Edition. International Diabetes Federation website. [Accessed February 8, 2018]. http://www.diabetesatlas.org/resources/2017-atlas.html. Published 2017.
- 4.Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev. 2016;30:11–24. doi: 10.1016/j.smrv.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 5.2009 Sleep in America Poll. National Sleep Foundation website. [Accessed April 5, 2019]. https://www.sleepfoundation.org/professionals/sleep-america-polls/2009-health-and-safety. Published 2009.
- 6.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 7.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94(9):3242–3250. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broussard J, Brady MJ. The impact of sleep disturbances on adipocyte function and lipid metabolism. Best Pract Res Clin Endocrinol Metab. 2010;24(5):763–773. doi: 10.1016/j.beem.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59(9):2126–2133. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broussard JL, Wroblewski K, Kilkus JM, Tasali E. Two nights of recovery sleep reverses the effects of short-term sleep restriction on diabetes risk. Diabetes Care. 2016;39(3):e40–e41. doi: 10.2337/dc15-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Leeuwen WM, Hublin C, Sallinen M, Harma M, Hirvonen A, Porkka-Heiskanen T. Prolonged sleep restriction affects glucose metabolism in healthy young men. Int J Endocrinol. 2010;2010:108641. doi: 10.1155/2010/108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14(4):402–412. doi: 10.1097/MCO.0b013e3283479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calhoun DA, Harding SM. Sleep and hypertension. Chest. 2010;138(2):434–443. doi: 10.1378/chest.09-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagai M, Hoshide S, Kario K. Sleep duration as a risk factor for cardiovascular disease- a review of the recent literature. Curr Cardiol Rev. 2010;6(1):54–61. doi: 10.2174/157340310790231635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20(4):267–277. [PubMed] [Google Scholar]
- 16.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):843–844. doi: 10.5665/sleep.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Killick R, Hoyos CM, Melehan K, Dungan GC, Poh J, Liu PY. The effects of ‘catch-up’ sleep on insulin sensitivity in men with lifestyle driven, chronic, intermittent sleep restriction. Clin Endocrinol. 2015;83(4):498–507. doi: 10.1111/cen.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leproult R, Deliens G, Gilson M, Peigneux P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep. 2015;35(5):707–715. doi: 10.5665/sleep.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 20.Suksakorn S, Rattanaumpawan P, Banhiran W, Cherakul N, Chotinaiwattarakul W. Reliability and validity of a Thai version of the Berlin questionnaire in patients with sleep disordered breathing. J Med Assoc Thai. 2014;97(Suppl 3):S46–S56. [PubMed] [Google Scholar]
- 21.Sleep Hygiene. National Sleep Foundation website. [Accessed April 5, 2019]. https://sleepfoundation.org/sleep-topics/sleep-hygiene. Published 2017.
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Seltzer HS, Allen EW, Herron AL, Jr, Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1967;46(3):323–335. doi: 10.1172/JCI105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 25.Weiss R, Cali AM, Dziura J, Burgert TS, Tamborlane WV, Caprio S. Degree of obesity and glucose allostasis are major effectors of glucose tolerance dynamics in obese youth. Diabetes Care. 2007;30(7):1845–1850. doi: 10.2337/dc07-0325. [DOI] [PubMed] [Google Scholar]
- 26.Banhiran W, Assanasen P, Nopmaneejumruslers C, Metheetrairut C. Epworth sleepiness scale in obstructive sleep disordered breathing: the reliability and validity of the Thai version. Sleep Breath. 2011;15(3):571–577. doi: 10.1007/s11325-010-0405-9. [DOI] [PubMed] [Google Scholar]
- 27.Sitasuwan T, Bussaratid S, Ruttanaumpawan P, Chotinaiwattarakul W. Reliability and validity of the Thai version of the Pittsburgh Sleep Quality Index. J Med Assoc Thai. 2014;97(Suppl 3):S57–S67. [PubMed] [Google Scholar]
- 28.Cedernaes J, Lampola L, Axelsson EK, et al. A single night of partial sleep loss impairs fasting insulin sensitivity but does not affect cephalic phase insulin release in young men. J Sleep Res. 2016;25(1):5–10. doi: 10.1111/jsr.12340. [DOI] [PubMed] [Google Scholar]
- 29.Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. 2015;38(8):1161–1183. doi: 10.5665/sleep.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cespedes EM, Hu FB, Redline S, et al. Comparison of self-reported sleep duration with actigraphy: results from the Hispanic Community Health Study/ Study of Latinos Sueño Ancillary Study. Am J Epidemiol. 2016;183(6):561–573. doi: 10.1093/aje/kwv251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. 2018;84:56–66. doi: 10.1016/j.metabol.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 34.Rao MN, Neylan TC, Grunfeld C, Mulligan K, Schambelan M, Schwarz JM. Subchronic sleep restriction causes tissue-specific insulin resistance. J Clin Endocrinol Metab. 2015;100(4):1664–1671. doi: 10.1210/jc.2014-3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broussard JL, Kilkus JM, Delebecque F, et al. Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity (Silver Spring) 2016;24(1):132–138. doi: 10.1002/oby.21321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broussard JL, Chapotot F, Abraham V, et al. Sleep restriction increases free fatty acids in healthy men. Diabetologia. 2015;58(4):791–798. doi: 10.1007/s00125-015-3500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Leeuwen WM, Lehto M, Karisola P, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4(2):e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 39.Boyum A, Wiik P, Gustavsson E, et al. The effect of strenuous exercise, calorie deficiency and sleep deprivation on white blood cells, plasma immunoglobulins and cytokines. Scand J Immunol. 1996;43(2):228–235. doi: 10.1046/j.1365-3083.1996.d01-32.x. [DOI] [PubMed] [Google Scholar]
- 40.Sung KC, Reaven GM, Kim SH. Utility of homeostasis model assessment of beta-cell function in predicting diabetes in 12,924 healthy Koreans. Diabetes Care. 2010;33(1):200–202. doi: 10.2337/dc09-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nuyujukian DS, Beals J, Huang H, et al. Sleep duration and diabetes risk in American Indian and Alaska native participants of a lifestyle intervention project. Sleep. 2016;39(11):1919–1926. doi: 10.5665/sleep.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cizza G, Piaggi P, Rother KI, Csako G. Hawthorne effect with transient behavioral and biochemical changes in a randomized controlled sleep extension trial of chronically short-sleeping obese adults: implications for the design and interpretation of clinical studies. PLoS One. 2014;9(8):e104176. doi: 10.1371/journal.pone.0104176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernquest J. Bangkok traffic jams among world's worst. Bangkok Post website. [Accessed July 10, 2018]. https://www.bangkokpost.com/learning/advanced/1201724/bangkok-traffic-jams-among-worlds-worst. Published February 20, 2017.
- 44.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Escalante Pulido JM, Alpizar Salazar M. Changes in insulin sensitivity, secretion and glucose effectiveness during menstrual cycle. Arch Med Res. 1999;30(1):19–22. doi: 10.1016/s0188-0128(98)00008-6. [DOI] [PubMed] [Google Scholar]