Abstract
Apoptosis evading is a hallmark of cancer. Tumor cells are characterized by having an impaired apoptosis signaling, a fact that deregulates the balance between cell death and survival, leading to tumor development, invasion and resistance to treatment. In general, patients with adrenocortical carcinomas (ACC) have an extremely bad prognosis, which is related to disease progression and significant resistance to treatments. In this report, we performed an integrative review about the disruption of apoptosis in ACC that may underlie the characteristic poor prognosis in these patients. Although the apoptosis has been scarcely studied in ACC, the majority of the deregulation phenomena already described are anti-apoptotic. Most importantly, in a near future, targeting apoptosis modulation in ACC patients may become a promising therapeutic.
Keywords: adrenocortical tumors, adrenocortical carcinomas, apoptosis, molecular deregulations
Adrenocortical tumors
Adrenal cortex tumors (ACT) are common tumors with a reported prevalence above 4% in most populations (1). However, the majority of ACT are benign, non-functioning and incidentally discovered during imaging studies performed for unrelated clinical reasons (1, 2). On the other side, adrenocortical carcinomas (ACC) are rare but usually have an aggressive behavior and a poor prognosis (1, 3, 4, 5, 6). According to the ENSAT classification, the 5-year disease-specific survival rate is approximately 82% for stage I, 61% for stage II, 50% for stage III and 13% for stage IV (4). This dismal clinical outcomes of ACC patients is related to the diagnosis at an advanced clinical stage and because there is no effective adjuvant or neoadjuvant therapy for late-stage diagnosed patients (7). Only few targeted therapies based on the advances in the knowledge of adrenal tumor pathophysiology were developed and up until now they generally failed in clinical settings. One of the main reasons associated to adrenal tumor cells resistance to chemotherapy is compromised apoptosis signaling, a fact that deregulates the balance between cell death and survival, thus facilitating tumor development, invasion and resistance to treatment (8, 9, 10, 11). In the following section, we will briefly review the regulation of apoptosis and then explore the abnormalities in apoptosis in ACC that have been reported to date.
Caspases alterations
There are two main apoptosis activation pathways: the extrinsic one that involves activation of cell death receptors and the intrinsic cascade. The latter is also known as the mitochondrial pathway, as it involves the permeation of the mitochondrial outer membrane followed by the release of apoptotic intervenients (12, 13). Both apoptosis pathways converge into caspase activation and cellular disintegration (12).
Caspases are a family of cysteine proteases that play a crucial role at apoptosis (14, 15). Caspases can be classified as initiators or executioners, according to the point of entry into the apoptosis cascade (Table 1) (15). Initiator caspases such as caspase-8 and caspase-10 are characterized by containing a death effector domain (DED), involved in the extrinsic apoptosis pathway. Alternatively, the caspases involved in the intrinsic apoptosis pathway such as caspase-2 and caspase-9 contain a caspase recruitment domain (CARD). These caspases are able to cleave and activate executioner caspases: caspase-3, caspase-6 and caspase-7. A third group of caspases including caspase-1, caspase-4, caspase-5 and caspase-12 participate in innate immune responses rather than in the apoptotic cascade (16).
Table 1.
Classification of caspases according to the point of entry into the apoptosis cascade.
| Initiator caspases | Executioner caspases | Inflammatory caspases | |
|---|---|---|---|
| Extrinsic pathway | Intrinsic pathway | ||
| Caspase 8 Caspase 10 |
Caspase 2 ↑ Caspase 9 ↓ |
Caspase 3 ↓ Caspase 6 Caspase 7 |
Caspase 1 Caspase 4 Caspase 5 Caspase 12 |
↓ represents the caspases that are underexpressed in adult ACC, ↑ represents the overexpression caspases.
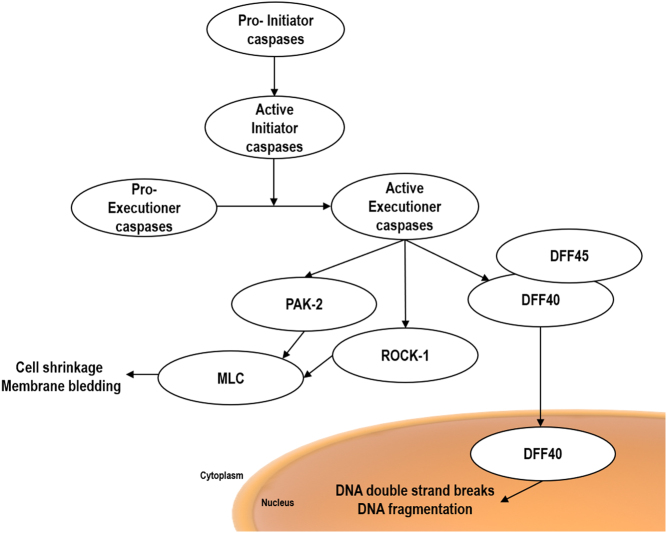
Initiator caspases are produced as inactive monomeric procaspases and are activated by dimerization via the designated ‘induced proximity model’ (16, 17). The executioner caspases are also produced as inactive procaspases and they require cleavage by the initiator caspases in order to be activated (16, 18). Executioner caspases, when activated are able to cleave and activate Rho-associated protein kinase (ROCK-1) and p21-activated kinase 2 (PAK-2), by removing the inhibitory domain. Both, ROCK-1 and PAK-2 induce myosin regulatory light-chain (MLC) phosphorylation that results in cell shrinkage and cell membrane blebbing (19, 20). In addition, executioner caspases cleave DNA fragmentation factors (DFF) in two fragments: the DFF40 and its inhibitor DFF45. This cleavage allows DFF40 translocation into the cell nucleus, which induces DNA double-strand breaks resulting in DNA fragmentation (Fig. 1) (21, 22).
Figure 1.
Schematic representation of apoptosis regulated by caspases. After activation of the initiator caspases, they activate executioner caspases by cleavage. Executioner caspases, when activated cleave and activate ROCK-1, PAK-2 and DFF40/45 leading to cell shrinkage, membrane blebbing and DNA fragmentation.
In ACC occurring in adulthood, the expression of genes that encode initiator caspases (caspase-2 and caspase-9) involved in the intrinsic apoptosis pathway was found to be altered (Table 1) (23). The expression of CASP9 was decreased in ACC while the CASP2 was increased when compared with adrenocortical adenomas (ACAs) (23). The executioner CASP3 expression was found to be decreased in ACC compared with ACAs as well as with normal adrenal glands. On the contrary, in ACT occurring in childhood, the caspases CASP3, CASP8 and CASP9 have been studied and their expression was not found to be different in malignant when compared to benign ACT (24). Concerning prognostic differences in children with ACC, a low CASP3 expression was found to be associated with a lower 5-year event-free survival, while low levels of CASP9 were associated with a higher 5-year event-free survival (24). Also, a significant negative correlation between CASP3 expression and tumor size was found in adults with ACC.
Extrinsic apoptosis signaling pathway alterations
The apoptosis extrinsic pathway is triggered by extracellular ligands that activate the death receptors located in the plasma membrane. These receptors possess a cytosolic death domain (12, 25, 26).
The most well-known ligands and their respective death receptors are fatty acid synthetase ligand (FasL)/fatty acid synthetase receptor (Fas); TNF-related apoptosis-inducing ligand (TRAIL)/death receptor 4 and 5 (DR4 and DR5) and tumor necrosis factor α (TNF-α)/tumor necrosis factor receptor 1 (TNF1-R) (12, 25, 26).
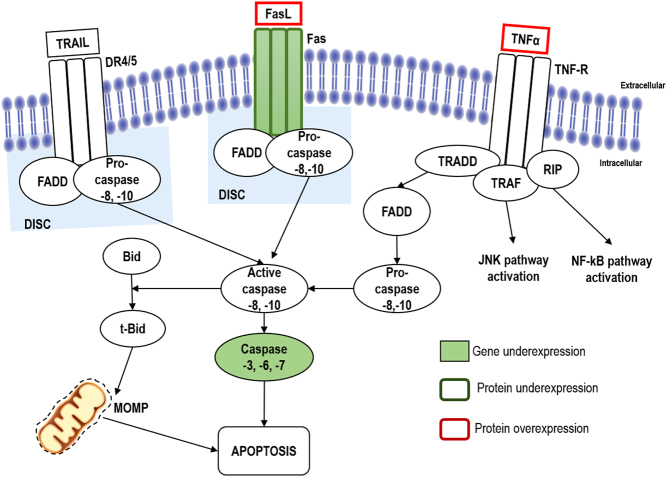
FasL binding to Fas, triggers the aggregation of Fas trimers and the recruitment of the death domain-containing protein (FADD) that binds to Fas death domain (Fig. 2) (12, 27). Besides, FADD presents a DED domain that allows initiator caspases binding, such as pro-caspase 8, creating a death-inducing signaling complex (DISC) (12, 26). DISC formation then causes the cleavage of pro-caspase 8 into active caspase 8. The activation of initiator caspases can then cleave and activate executioner caspases (13, 16). Moreover, caspase 8 also cleaves and activates BH3 interacting domain death agonist (Bid) that releases mitochondrial cytochrome c, which can also activate caspase 3 (28).
Figure 2.
Schematic representation of extrinsic apoptotic pathway. Stimulation of death receptors of the TNF-R, Fas and DR4/5 by their respective ligands, results in receptor aggregation and recruitment of FADD and caspase-8 and caspase-10. These caspases become activated and cleaves the executioner caspases-3, caspase-6 and caspase-7, leading to apoptosis. Abnormalities in mRNA and in protein expression alterations already described in adrenocortical carcinomas are highlighted in solid and open squares, respectively.
The activation of DR4 and DR5 by TRAIL triggers a similar pathway as the one described for Fas-induced apoptosis with the recruitment FADD and pro-caspase 8 to form DISC (Fig. 2) (12, 13).
Fas expression was found to be reduced and FasL expression increased in ACC when compared with ACA and normal adrenal glands (29). Another group reported that Fas gene expression was absent in all analyzed ACC and present in ACA and normal adrenal glands (30). On the other side, soluble Fas antigen (sFas) plasma levels were also observed to be higher in patients with ACT than in healthy blood donors. sFas plasma levels were particularly higher in patients with aldosterone producing adenomas. In addition, sFas plasma levels were positively correlated with the ACC size (31, 32). In childhood ACT, Fas gene expression was lower in ACT when compared with normal adrenal glands, but no differences were observed between ACC and ACA (24).
TNFα binding to the extracellular domain of TNF1-R leads to the binding of the TNF receptor-associated death domain (TRADD) adaptor protein to the intracellular domain of TNF1-R, resulting in the recruitment of the receptor interacting protein (RIP), FADD and TNF-R-associated factor (TRAF). FADD recruitment then leads into the signaling apoptosis pathway, while RIP activates the nuclear factor of κB (NF-κB) through stimulation of the inhibitor of κB-kinase (IKK) and TRAF activates the JNK pathway stimulating the transcription factor, activating protein-1 (AP-1) (Fig. 2) (12, 25, 26).
Although TNF gene expression was not found to be altered in ACC, the tumor necrosis factor alpha-induced protein 3 (TNFAIP3), a ubiquitin-modifying enzyme that negatively regulates TNF response, was found to be overexpressed in ACC when compared to the normal adrenal gland. Moreover, high TNFAIP3 expression was significantly associated with poor overall survival of ACC patients (33). TNFRSF19, a gene that encodes a member of the TNF receptor superfamily, was also found to be overexpressed in ACC and it was associated with poor prognosis in another study (23).
TNFα serum levels were significantly higher in patients with ACT before adrenalectomy when compared to healthy subjects. TNFα serum levels were particularly higher in patients with ACC and aldosteronomas. In contrast, soluble TNF receptors (TNF1-R and TNF2-R) serum levels were similar in ACT patients and in healthy subjects. Following adrenalectomy, TNFα levels decreased in patients with ACC, non-functioning ACA and in patients with aldosteronomas. On the other hand, reduction of TNF1-R and TNF2-R serum levels was observed only in patients with unilateral aldosteronomas (34).
TNF expression was significantly lower in childhood ACC when compared with ACA and associated with a lower 5-year event-free survival (24). In these pediatric patients, a strong and moderate immunoreactivity for TNF-α protein expression was associated with a higher 5-year event-free and overall survival (24).
Tumor necrosis factor receptor-associated factor 4 (TRAF4), a mediator of the TNF-induced signaling pathway, is able to inhibit the Fas-induced apoptosis (35). TRAF4 overexpression was associated with poor prognosis in patients with ACC (23).
Intrinsic apoptosis pathway signaling alterations
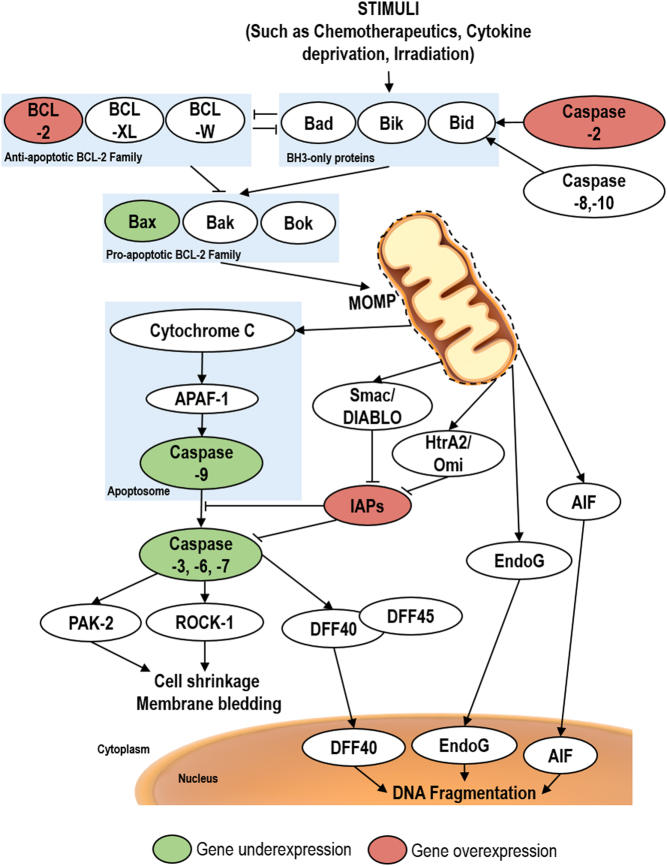
The intrinsic apoptosis pathway is triggered by intracellular stimuli that include DNA damage, absence of growth factors, oxidative stress and endoplasmic reticulum stress (13). These stimuli lead to mitochondrial outer membrane permeation (MOMP) resulting in the release of pro-apoptotic factors such as cytochrome c and other intermembrane space proteins such as Smac/DIABLO, HtrA2/Omi, apoptosis-inducing factor (AIF) and Endonuclease G (EndoG) (Fig. 3). Cytochrome c binds to the apoptotic protease-activating factor-1 (APAF-1) inducing its oligomerization, a conformational change that leads to apoptosome formation. The apoptosome then binds and activates pro-caspase 9 to caspase 9, which is then able to activate the executioner caspases 3, 6 and 7 (12, 36, 37). Smac/DIABLO and HtrA2/Omi potentiate caspase activation, since they are antagonists of the inhibitors of apoptosis proteins (IAPs). IAPs are able to inhibit activated executioner caspases stopping the caspase-dependent apoptosis (26, 38, 39). After being released from mitochondria, caspase-independent cell death effectors AIF and EndoG translocate to the nucleus and trigger nuclear condensation and DNA fragmentation (40, 41).
Figure 3.
Schematic representation of intrinsic apoptotic pathway. Stress signals leads to the pro-apoptotic BCL-2 family proteins activation that induce the mitochondrial outer membrane permeation (MOMP). MOMP allows the release of the Cytochrome C, AIF, EndoG, HtrA2/Omi and Smac/DIABLO. Cytochrome C release leads to the formation of apoptosome complex that triggers caspase-3, -6, -7 activation. They cleave ROCK-1, PAK-2 and DFF40/45 leading to cell shrinkage, membrane blebbing and DNA fragmentation. HtrA2/Omi and Smac/DIABLO inhibit IAPs, avoiding the caspase inhibition by them. EndoG and AIF lead to DNA fragmentation. Abnormalities in the expression of genes involved in the intrinsic apoptotic pathway in adrenocortical carcinomas are highlighted in red or green circles.
MOMP is regulated by the members of the Bcl-2 family (26, 42, 43). There are three types of Bcl-2 family proteins based in their apoptotic function and Bcl-2 homology domains (BH1, 2, 3 and 4) (42): (1) the anti-apoptotic proteins that contain 3 or 4 BH domains, like Bcl-2, Bcl-xL and Bcl-w; (2) the pro-apoptotic proteins that contain 2 or 3 BH domains, like Bax, Bak and Bok (3) the BH3-only proteins that are also pro-apoptotic but have only the BH3 domain, like Bad, Bik, Bid, Noxa and Puma (26, 44, 45, 46, 47, 48, 49, 50, 51). Anti-apoptotic proteins are able to inhibit MOMP by sequestering BH3-only proteins and/or Bax/Bak (43, 52, 53). MOMP only occurs when the anti-apoptotic Bcl-2 family proteins are inhibited and Bax or Bak are activated by the activator BH3-only proteins thus inducing Bax/Bak oligomerization (43, 53).
BCL2 expression levels were found to be higher in ACC when compared with non-functioning ACA, while the Bax gene expression was found to be similar among all the different ACT analyzed by Ando et al. (54). This finding was not confirmed in subsequent studies where BCL2 and BCLXL gene expression was similar among ACC, ACA and normal adrenal glands (30, 55). Bax gene expression was absent in ACC, implying a reduction in apoptosis, whereas it was always present in ACA and normal adrenal glands (55). In another study, the BCL2L12 gene that encodes another anti-apoptotic Bcl-2 family protein was increased in the ACC, while the Bok gene that encodes a pro-apoptotic Bcl-2 family protein were described to be increased in ACC with poor prognosis (23).
In pediatric patients with ACT, BCL2 gene expression was similar in ACC, ACA and normal adrenal glands. However, lower BCL2 gene and protein expression were associated with poor prognosis in these pediatric patients with ACC (24). In contrast, another study in pediatric patients did not find an association between Bcl-2 protein expression and prognosis (56).
In adult patients, Bcl-2 protein expression was found to be similarly increased in ACC and non-functioning ACA when compared with normal adrenal glands in a single study (57). However, these results have not been confirmed in several other studies (58, 59, 60, 61).
The IAPs family have been highly studied in the context of the cancer due to their key role in the regulation of apoptosis mostly through the direct inhibition of executioner caspases (62, 63).
Altieri et al. studied three elements of the IAPs family: MLIAP/livin/BIRC7, survivin/BIRC5 and XIAP/BIRC4. They found that increased livin mRNA and protein levels were promising markers for malignancy diagnosis in ACT. BIRC5 expression was found to be similar between ACC and ACA but higher than that in normal adrenal glands (64). Contrarily, other authors found that BIRC5 expression is increased in the ACC group (23, 65). Survivin expression was found to have a negative impact on overall survival in patients with ACC (64); increased survivin was found in more aggressive tumors (23, 65). Although increased in ACC, no association was observed between livin expression and ACC aggressiveness features (64).
Cell cycle and apoptosis regulation
Cell cycle instabilities can lead to apoptosis. Some cell cycle genes are also involved in apoptosis regulation, of which TP53, c-myc and pRB/E2F are the most well-known examples (12, 66, 67).
p53 is involved in both intrinsic and extrinsic apoptotic pathways. However, its main role occurs in the intrinsic pathway (68, 69). After DNA damage, p53 and MDM2 are phosphorylated, which inhibits their interaction resulting in p53 accumulation in the nucleus and activation of transcription of various pro-apoptotic genes including Bax, Noxa, Puma, BID, Fas, APAF-1, DR5. In addition, p53 accumulation in the nucleus represses anti-apoptotic genes transcription, such as BIRC5 that encodes survivin, a member of the IAP family (68, 69, 70, 71, 72, 73). Moreover, p53 can bind directly to the anti-apoptotic proteins, Bcl-2 and Bcl-xL, and also translocate to mitochondria inducing Bax/Bak oligomerization, thus promoting MOMP (74). It has also been described that P53 overexpression increases Fas levels at the cell surface, by promoting its trafficking from Golgi complex. Finally, p53 can also activate DR5 (75, 76).
TP53 mutations usually result in the loss of p53 tumor suppressive activity, mainly due to repression of p53 target genes transactivation (77). TP53 mutations in ACT was demonstrated to be absent in the majority of ACA. In contrast, the prevalence of somatic TP53 mutations in sporadic ACC can varies from 20 to 30% of the cases (78, 79, 80, 81). Besides that, the majority of ACC patients with mutated TP53 were observed to have poor outcomes (78). Aberrant p53 protein expression was also found to be associated with decreased disease-free survival (80). However, in ACC p53 expression was found to be highly variable (5–52%). Thus, p53 expression cannot be considered a reliable molecular marker to identify ACC (58, 61, 82, 83, 84). Still, loss of heterozygosity (LOH) in chromosome 17q13 harboring TP53 was demonstrated to be present in approximately 80% of ACC. Nevertheless, LOH at 17q13 is not always associated with TP53 mutations suggesting that other genes in the same chromosomal region contribute to ACC biology (85, 86, 87).
TP53 gene germline pathogenic variants are typically associated with Li-Fraumeni or Li-Fraumeni-like syndrome, which is associated with an hereditary predisposition to neoplasms, in particular to pediatric ACC (88).
Contrarily to the majority of mutations associated with LFS that are in p53 DNA-binding domain, 80% of pediatric ACC have mutations in p53 oligomerization domain, in particular in the exon 10 of the short arm of chromosome 17 (p.R337H). p.R337H mutation appears to be particularly prevalent in Southeast and Southern Brazilian populations, where the incidence of pediatric ACT is estimated to be 10–15 times higher than worldwide (89, 90, 91). In vivo studies found that this mutation is associated to an increased DNA damage, a mildly decreased apoptosis and cell cycle arrest. These tumor-suppressive activity alterations have been proposed to be sufficient to induce tumorigenesis and to confer tumor growth advantages (92, 93). Otherwise, genomic profiles of ACC with p.R337H mutation were found to be similar to ACC with a different TP53 mutation (94). Pediatric ACT with p.R337H mutation were found to have a significantly lower expression of apoptosis-related genes when compared to non-neoplastic adrenals (24).
The cross-talk between c-Myc and p53 is important for driving cell decision to undergo apoptosis in response to stress (95, 96). c-Myc can also drive apoptosis in a manner that does not require p53 (96, 97). c-Myc is able to suppress anti-apoptotic Bcl-2 family members and to induce the expression or activation of pro-apoptotic Bcl-2 proteins, such as Bax, Bak and Bim and transcriptionally activate Bax (95, 98, 99, 100). Besides that, c-Myc overexpression sensitizes cells to the apoptotic action of TNF and TRAIL death ligands (101). Furthermore, c-Myc also inhibits the activation of anti-apoptotic c-Jun kinases and NF-kB (95, 102). In ACC, c-Myc was found to be underexpressed compared to ACA and to the normal adrenal cortex (23, 103, 104, 105, 106).
Loss of pRB induces apoptosis and the mechanisms behind this phenomena are mainly related to the action of the E2F transcription factors, the well-known targets of pRB (107). The cytochrome c, AIF and SMAC are transcriptionally regulated by E2F1 (108, 109, 110). Death-inducing-protein (DIP) is located in the mitochondria and mediates E2F1-induced apoptosis, in a p53-independent pathway. In E2F1-activated cells, DIP suppression results in a decrease of apoptosis (111, 112). The loss of pRB was suggested to be a marker of poor prognosis as it appears to be a characteristic of the more aggressive ACC (113). A significant overexpression of E2F genes was found in ACC (104, 114). However, contrarily to expected, DIP was found to be underexpressed when compared with ACA (23).
miRNAs and apoptosis
miRNAs are non-coding RNAs that are able to silence their target genes at post-transcriptional level. Functional studies showed that abnormal miRNA expression is a key to cancer development by abnormally regulating several cellular processes, including apoptosis. Studies on ACC showed that deregulated miRNAs expression is able to negative modulate ACC apoptosis (115, 116).
miRNA-483-3p was found to be overexpressed in ACC compared with ACA (116, 117). This miRNA is able to protect the cancer cells from apoptosis through the negative modulation of the pro-apoptotic protein PUMA. A combination of the expression of the miR-483-3p and Smad4, a critical effector in the TGF-β signaling pathway, was demonstrated to have more powerful diagnostic accuracy than the classic pathology Weiss score system alone (117). Another study found that miR-483-3p is an excellent marker for the differential diagnosis between ACC and ACA. Higher miR-483-3p was found in ACC and there was no overlap between ACC and ACA (118).
Wu et al. found that miRNA-205 was significantly decreased in ACC compared with ACA. miRNA-205 was able to activate Bcl-2, in ACC tumor cells, leading to the activation of the intrinsic apoptosis pathway by activating caspase-9 and caspase-3 (119).
miR-195 is also downregulated in ACC compared to ACA (120). Low expression of miR-195 was significantly associated with poor overall survival (120). In the ACC cell line H295R, the restoration of the miR-195 expression led to increased expression of caspase-3, resulting in decreased cell viability. This finding in ACC confirmed the role of miR-195 on promoting apoptosis (116). In line with this finding, in colorectal tumors the miR-195 targeted Bcl-2 and induced apoptosis (121).
Apoptosis as a therapeutic target in ACC
Several in vitro and in vivo studies have attempted to improve the prognosis of patients with cancer by targeting apoptosis pathways either by targeting the overexpressed anti-apoptotic proteins or by stimulating pro-apoptotic molecules expression (9). These studies reported great potential of apoptosis-targeted therapies. Some of these have focused on reducing anti-apoptotic proteins such as the Bcl-2 family proteins, IAPs or c-FLIP (cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein) in order to allow the activation of apoptosis (62, 63, 122, 123). In ACC few studies were performed in order to evaluate the efficacy of those therapies (64, 65, 124, 125).
Gossypol is a polyphenolic compound extracted from cotton plants with the ability of binding to the anti-apoptotic proteins, Bcl-2 and Bcl-xL inhibiting them (126). In 1991, a study found that this compound has multiple inhibitory effects on adrenal steroidogenesis (127). Some years later, a trial of this compound was performed in 18 patients with metastatic ACC and the authors found that three patients that were refractory to chemotherapeutic agents had partial responses and one patient had stable disease. However, the majority of the patients had tumor progression (124). The low therapeutic response of Gossypol was considered similar to the other medical therapies available. Later, some studies found that Gossypol presents two stereoisomers and that the (-) Gossypol stereoisomer is the one with highest affinity for Bcl-2 and Bcl-xL (126, 128, 129). Since the drug used before was a mixture between the two stereoisomers, the use of the (-) Gossypol alone may have better effects compared to what was observed in the ACC patients. The compound (-) Gossypol, actually known as AT-101, has already been tested in clinical trials in patients with prostate cancer, leukemia and lung cancer (130, 131, 132). In ACC, only pre-clinical in vitro and in vivo studies were performed with that drug. Schteingart et al. studied the effects of (-) Gossypol in two ACC cell lines, H295 which is a cell line with low levels of Bcl-xL and RL25, that presents high levels of Bcl-xL. The authors demonstrated that the expression of Bcl-xL influences the effects of the drug, since better results were found in the RL25 cell line with a complete suppression of the tumor growth (125).
Some studies also performed pre-clinical studies in order to evaluate the efficacy of the IAPs in inhibit ACC. Sbiera et al. knocked down survivin expression by siRNA transfection in an ACC cell line and it doubled the apoptosis rates comparing to the non-transfected cells treated with etoposide. The authors also studied if survivin inhibition could be a sensitizing factor to chemotherapeutic drugs. However, the treatment with addition of etoposide to the survivin siRNA-transfected ACC cells showed only a slight increase in the apoptosis rate (65).
Altieri et al. studied the efficacy of another IAP (livin) in ACC cell lines. Using livin transfected H295R cells, they observed the expected decrease in Casp3 levels. However, no differences in the cell viability and proliferation were observed, suggesting that additional mechanisms are possibly involved in the already complex mechanism of the apoptotic cascade in ACC (64).
Evaluation of the apoptosis are highly used to verify the cancer efficiency of drugs but mostly in pre-clinical studies. In ACC cell lines, the efficacy of a given drug in increasing the apoptosis rate has been mainly studied using the Annexin V/Propidium Iodide assay, TUNEL assay and the final apoptotic molecules involved, such as the executioner caspases (133, 134). Unfortunately, it does not give us the full comprehensive mechanistic understanding of tumoral apoptosis inductor. Table 2 summarizes the available studies targeting ACC and their relationship with apoptosis.
Table 2.
Drugs that positively increased the ACC apoptosis rate in in vitro and in vivo studies.
| Anti-ACC drugs | Drug type | Method to evaluate apoptosis | Samples type | Ref. |
|---|---|---|---|---|
| AMG 900 | Aurora kinase inhibitor | Annexin V-FITC apoptosis detection assay | ACC cell line NCI-H295 | (141) |
| ATR-101 (PD132301-02) | Adrenalytic activity inhibitor | TUNEL assay | NCI-H295R xenographs | (142) |
| Annexin V-FITC apoptosis detection assay | ACC cell line NCI-H295R | |||
| Cholesterol free sHDL nanoparticles in combination with cisplatin, etoposide or mitotane | efflux cholesterol inducer | Annexin V-FITC apoptosis detection assay | ACC cell lines: SW-13 and NCI-H295R | (143) |
| Docosahexenoic acid | n-3 polyunsaturated fatty acid/mTOR complex 1/2 inhibitor | Annexin V-FITC Apoptosis Detection assay | ACC cell lines: SW-13 and NCI-H295R | (144) |
| TUNEL assay | SW-13 xenographs | |||
| DZNep | EZH2 (histone modifier) inhibitor | BCL2, BCL-XL and BIRC5 expression/Caspase 3 activity assay/TUNEL assay | ACC cell line NCI-H295R | (145) |
| Erlotinib and NVP-AEW541 combination | EGFR and IGF1R inhibitor | Annexin V-FITC apoptosis detection assay | ACC cell lines: SW-13 and NCI-H295R | (134) |
| Fingolimod (FTY720) | Sphingosine kinase 1 antagonist | Annexin V-FITC apoptosis detection assay | ACC cell lines: SW-13 and NCI-H295R | (146) |
| G-1 | Non-steroidal G protein-coupled estrogen receptor agonist | Annexin V-FITC Apoptosis Detection assay/Caspase 9 and 3/7 activity assay/TUNEL assay | ACC cell line NCI-H295R | (147) |
| Metformin | Biguanide cationic agent | Annexin V Apoptosis Detection assay/Bcl-xl, Bcl-2, Bcl-w and Cleaved Caspase 3 expression | ACC cell line NCI-H295R | (148) |
| Mitotane (drug used in ACC patients) | Steroidogenesis inhibitor and cytostatic antineoplastic | Annexin V Apoptosis Detection assay/Bax, Bak and Bcl-2 expression | ACC cell line NCI-H295 | (149, 150) |
| Caspase 3/7 activity assay | ACC cell line NCI-H295R | (151) | ||
| Niclosamide | Anti-helminthic agent | Caspase 3/7 activity assay | ACC cell lines: BD140A, SW-13 and NCI-H295R | (152) |
| Rapamycin | mTOR complex 1 inhibitor | Cleaved Caspase 3 expression | Adrenocortical tumors from AdTAg mice | (153) |
| Rottlerin | Anti-helminthic or fertilization antagonist | TUNEL assay | SW-13 xenographs | (133) |
| Annexin V-FITC Apoptosis Detection assay | ACC cell lines: SW-13 and NCI-H295R |
ACC, adrenocortical carcinomas; AMG, urora kinases inhibitor; Bak, B-cell lymphoma 2 homologous antagonist/killer; Bax, B-cell lymphoma 2-associated X protein; BCL-2, B-cell lymphoma 2; BCL-W, B-cell lymphoma-like 2; BCL-XL, B-cell lymphoma-extra-large; BIRC5, Baculoviral IAP repeat containing 5; DZNep, Deazaneplanocin A; EGFR, epidermal growth factor receptor; EZH2, enhancer of zeste homolog 2; FITC, fluorescein isothiocyanate; IGFR, insulin growth factor receptor; mTOR, mammalian target of rapamycin; NCI, National Cancer Institute; sHDL, synthetic high-density lipoprotein; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Clinical implications of apoptosis alterations in ACC
The balance between cell death and survival becomes compromised when apoptosis signaling is deregulated, thus conferring advantages for tumorigenesis and tumor growth (8, 9, 10, 11). The detailed knowledge on the status of apoptosis regulation in ACC can result in pivotal clues that could be translated into the clinical practice, by modifying current therapeutic interventions. Although few alterations in apoptosis regulation were reported in ACC, some of these were shown to be promising.
miR-483-3p, a negative modulator of pro-apoptotic proteins, is overexpressed in ACC depicting a poor prognosis. So miR-483-3p was appointed not only to be an important mechanistic alteration for ACC tumorigenesis and tumor progression, but also demonstrated its diagnostic utility by depicting a more powerful diagnostic accuracy to assess ACC tumor grade than the Weiss score that is currently used in routine clinical practice (116, 117, 118).
TP53 was described as one of ACC driver genes in several studies, including the most recent pan-genomic studies (81, 135). LOH in the chromosome were TP53 is harbored and TP53 mutations are the most frequent alterations observed in ACC, leading to abnormal cell cycle progression and apoptosis inhibition (78, 79, 80, 81, 85, 86, 87). Targeted therapies with the goal of recovering or reactivating p53 function in TP53 mutants were already developed and are currently being tested for the treatment of several tumors, other than ACC (e.g. PRIMA-1MET and MDM2 antagonists) (136). The high prevalence of alterations related to TP53 in ACC makes these tumors good candidates for TP53 modulators therapies.
Survivin, a member of the apoptosis inhibitor protein family, was found to have a negative impact in ACC patients’ survival and was pointed as a promising ACC drug target (23, 64, 65). Although the development of effective survivin inhibitors was hampered by a few reported challenges, several potent survivin inhibitors were already tested in clinical trials for some cancers (137, 138), other than ACC.
Finally, some authors demonstrated that anti-apoptotic molecules expression, such as members of the Bcl-2 family, are able to influence the effectiveness of some drugs, providing an additional rationale for the outcomes variability observed in clinical trials (139). So, when possible, profiling ACC for anti-apoptosis genes expression could be recommended in order to improve the predictability of outcomes achieved and even enable a more personalized treatment approach for ACC patients. (Reviewer #1, Comment #1)
Conclusions
Apoptosis evading is one of well-known process leading to cancer cells proliferation and expansion. It is also a very important hallmark of cancer (140). Tumor cells are not only characterized by genetic alterations leading to increased proliferation but also by a compromised apoptosis signaling, a fact that adds to the imbalance between cell death and survival, leading to tumor development, invasion and resistance to treatment (8, 9, 10, 11). Decreased apoptosis in ACC associated with their aggressiveness and the resistance to treatment was observed in ACC patients. In ACC, pro-apoptotic factors are generally inhibited and anti-apoptotic ones are generally increased. However, although promising, the attempts to revert these alterations in ACC cell lines and in xenograft models have led to insufficient results to date. More studies are needed in order to integrate the key players involved in the apoptosis in ACC and to translate this information into the clinical practice. Unraveling all defects in apoptosis in ACC may have a significant importance either as therapeutic targets or molecular markers for ACC diagnosis or prognosis. So far, none of these expectations has been accomplished.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
The work was supported by the Foundation for Science and Technology (FCT) (PTDC/MEC-ONC/31384/2017). Unit for Multidisciplinary Research in Biomedicine (UMIB) is funded by grants from FCT (UID/Multi/00215/2013).
References
- 1.Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocrine Reviews 2014. 35 282–326. ( 10.1210/er.2013-1029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pignatelli D. Adrenal cortex tumors and hyperplasias. In Contemporary Aspects of Endocrinology. London, UK: IntechOpen; ( 10.5772/19108) [DOI] [Google Scholar]
- 3.Chagpar R, Siperstein AE, Berber E. Adrenocortical cancer update. Surgical Clinics of North America 2014. 94 669–687. ( 10.1016/j.suc.2014.02.009) [DOI] [PubMed] [Google Scholar]
- 4.Roman S. Adrenocortical carcinoma. Current Opinion in Oncology 2006. 18 36–42. ( 10.1097/01.cco.0000198976.43992.14) [DOI] [PubMed] [Google Scholar]
- 5.Libe R, Borget I, Ronchi CL, Zaggia B, Kroiss M, Kerkhofs T, Bertherat J, Volante M, Quinkler M, Chabre O, et al. Prognostic factors in stage III–IV adrenocortical carcinomas (ACC): an European Network for the Study of Adrenal Tumor (ENSAT) study. Annals of Oncology 2015. 26 2119–2125. ( 10.1093/annonc/mdv329) [DOI] [PubMed] [Google Scholar]
- 6.Fassnacht M, Libe R, Kroiss M, Allolio B. Adrenocortical carcinoma: a clinician’s update. Nature Reviews: Endocrinology 2011. 7 323–335. ( 10.1038/nrendo.2010.235) [DOI] [PubMed] [Google Scholar]
- 7.Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, Terzolo M, Mueller HH, Hahner S, Allolio B, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a revised TNM classification. Cancer 2009. 115 243–250. ( 10.1002/cncr.24030) [DOI] [PubMed] [Google Scholar]
- 8.Fulda S. Evasion of apoptosis as a cellular stress response in cancer. International Journal of Cell Biology 2010. 2010 370835 ( 10.1155/2010/370835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. BioMed Research International 2014. 2014 1–23. ( 10.1155/2014/150845) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Plati J, Bucur O, Khosravi-Far R. Dysregulation of apoptotic signaling in cancer: molecular mechanisms and therapeutic opportunities. Journal of Cellular Biochemistry 2008. 104 1124–1149. ( 10.1002/jcb.21707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernald K, Kurokawa M. Evading apoptosis in cancer. Trends in Cell Biology 2013. 23 620–633. ( 10.1016/j.tcb.2013.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawen A. Apoptosis-an introduction. BioEssays 2003. 25 888–896. ( 10.1002/bies.10329) [DOI] [PubMed] [Google Scholar]
- 13.Elmore S. Apoptosis: a review of programmed cell death. Toxicologic Pathology 2007. 35 495–516. ( 10.1080/01926230701320337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grutter MG. Caspases: key players in programmed cell death. Current Opinion in Structural Biology 2000. 10 649–655. ( 10.1016/S0959-440X(00)00146-9) [DOI] [PubMed] [Google Scholar]
- 15.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Current Opinion in Cell Biology 2003. 15 725–731. ( 10.1016/j.ceb.2003.10.009) [DOI] [PubMed] [Google Scholar]
- 16.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harbor Perspectives in Biology 2015. 7 a026716 ( 10.1101/cshperspect.a008656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, et al. A unified model for apical caspase activation. Molecular Cell 2003. 11 529–541. ( 10.1016/S1097-2765(03)00051-0) [DOI] [PubMed] [Google Scholar]
- 18.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nature Reviews: Molecular Cell Biology 2004. 5 897–907. ( 10.1038/nrm1496) [DOI] [PubMed] [Google Scholar]
- 19.Radu M, Semenova G, Kosoff R, Chernoff J. PAK signalling during the development and progression of cancer. Nature Reviews: Cancer 2014. 14 13–25. ( 10.1038/nrc3645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nature Reviews: Molecular Cell Biology 2003. 4 446–456. ( 10.1038/nrm1128) [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 1997. 89 175–184. ( 10.1016/S0092-8674(00)80197-X) [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Zou H, Widlak P, Garrard W, Wang X. Activation of the apoptotic endonuclease DFF40 (caspase-activated DNase or nuclease). Oligomerization and direct interaction with histone H1. Journal of Biological Chemistry 1999. 274 13836–13840. ( 10.1074/jbc.274.20.13836) [DOI] [PubMed] [Google Scholar]
- 23.de Reynies A, Assie G, Rickman DS, Tissier F, Groussin L, Rene-Corail F, Dousset B, Bertagna X, Clauser E, Bertherat J. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. Journal of Clinical Oncology 2009. 27 1108–1115. ( 10.1200/JCO.2008.18.5678) [DOI] [PubMed] [Google Scholar]
- 24.Lorea CF, Moreno DA, Borges KS, Martinelli CE, Jr, Antonini SR, de Castro M, Tucci S, Jr, Neder L, Ramalho LN, Cardinalli I, et al. Expression profile of apoptosis-related genes in childhood adrenocortical tumors: low level of expression of BCL2 and TNF genes suggests a poor prognosis. European Journal of Endocrinology 2012. 167 199–208. ( 10.1530/EJE-12-0183) [DOI] [PubMed] [Google Scholar]
- 25.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nature Reviews Cancer 2002. 2 420–430. ( 10.1038/nrc821) [DOI] [PubMed] [Google Scholar]
- 26.Xu G, Shi Y. Apoptosis signaling pathways and lymphocyte homeostasis. Cell Research 2007. 17 759–771. ( 10.1038/cr.2007.52) [DOI] [PubMed] [Google Scholar]
- 27.Nagata S. Fas ligand-induced apoptosis. Annual Review of Genetics 1999. 33 29–55. ( 10.1146/annurev.genet.33.1.29) [DOI] [PubMed] [Google Scholar]
- 28.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998. 94 491–501. ( 10.1016/S0092-8674(00)81590-1) [DOI] [PubMed] [Google Scholar]
- 29.Wolkersdorfer GW, Marx C, Brown J, Schroder S, Fussel M, Rieber EP, Kuhlisch E, Ehninger G, Bornstein SR. Prevalence of HLA-DRB1 genotype and altered Fas/Fas ligand expression in adrenocortical carcinoma. Journal of Clinical Endocrinology and Metabolism 2005. 90 1768–1774. ( 10.1210/jc.2004-1406) [DOI] [PubMed] [Google Scholar]
- 30.Kanauchi H, Wada N, Ginzinger DG, Yu M, Wong MG, Clark OH, Duh QY. Diagnostic and prognostic value of fas and telomeric-repeat binding factor-1 genes in adrenal tumors. Journal of Clinical Endocrinology and Metabolism 2003. 88 3690–3693. ( 10.1210/jc.2002-020965) [DOI] [PubMed] [Google Scholar]
- 31.Kushlinskii NE, Britvin TA, Polyakova GA, Abbasova SG, Baronin AA, Bogatyrev OP, Kalinin AP, Lipkin VM. Soluble Fas antigen (sFAS) in the serum from patients with adrenal tumors. Bulletin of Experimental Biology and Medicine 2001. 132 990–992. ( 10.1023/A:1013683731516) [DOI] [PubMed] [Google Scholar]
- 32.Kushlinskii NE, Britvin TA, Polyakova GA, Abbasova SG, Baronini AA, Tishenina RS, Molchanova GS, Sel’chuk VY, Pirogov DA, Bogatyrev OP, et al. Plasma content of soluble fas antigen in patients with adrenal tumors and tumor-like pathologies. Bulletin of Experimental Biology and Medicine 2002. 134 171–174. ( 10.1023/A:1021196517353) [DOI] [PubMed] [Google Scholar]
- 33.Hantel C, Ozimek A, Lira R, Ragazzon B, Jackel C, Frantsev R, Reincke M, Bertherat J, Mussack T, Beuschlein F. TNF alpha signaling is associated with therapeutic responsiveness to vascular disrupting agents in endocrine tumors. Molecular and Cellular Endocrinology 2016. 423 87–95. ( 10.1016/j.mce.2015.12.009) [DOI] [PubMed] [Google Scholar]
- 34.Komorowski J, Jurczynska J, Stepien T, Kolomecki K, Kuzdak K, Stepien H. Serum concentrations of TNF alpha and its soluble receptors in patients with adrenal tumors treated by surgery. International Journal of Molecular Sciences 2010. 11 2281–2290. ( 10.3390/ijms11062281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleckenstein DS, Dirks WG, Drexler HG, Quentmeier H. Tumor necrosis factor receptor-associated factor (TRAF) 4 is a new binding partner for the p70S6 serine/threonine kinase. Leukemia Research 2003. 27 687–694. ( 10.1016/S0145-2126(02)00325-9) [DOI] [PubMed] [Google Scholar]
- 36.Landes T, Martinou JC. Mitochondrial outer membrane permeabilization during apoptosis: the role of mitochondrial fission. Biochimica and Biophysica Acta 2011. 1813 540–545. ( 10.1016/j.bbamcr.2011.01.021) [DOI] [PubMed] [Google Scholar]
- 37.Parsons MJ, Green DR. Mitochondria in cell death. Essays in Biochemistry 2010. 47 99–114. ( 10.1042/bse0470099) [DOI] [PubMed] [Google Scholar]
- 38.Berthelet J, Dubrez L. Regulation of apoptosis by inhibitors of apoptosis (IAPs). Cells 2013. 2 163–187. ( 10.3390/cells2010163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang QH, Church-Hajduk R, Ren J, Newton ML, Du C. Omi/HtrA2 catalytic cleavage of inhibitor of apoptosis (IAP) irreversibly inactivates IAPs and facilitates caspase activity in apoptosis. Genes and Development 2003. 17 1487–1496. ( 10.1101/gad.1097903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 2001. 412 95–99. ( 10.1038/35083620) [DOI] [PubMed] [Google Scholar]
- 41.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999. 397 441–446. ( 10.1038/17135) [DOI] [PubMed] [Google Scholar]
- 42.Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Current Opinion in Cell Biology 2003. 15 691–699. ( 10.1016/j.ceb.2003.10.004) [DOI] [PubMed] [Google Scholar]
- 43.Gillies LA, Kuwana T. Apoptosis regulation at the mitochondrial outer membrane. Journal of Cellular Biochemistry 2014. 115 632–640. ( 10.1002/jcb.24709) [DOI] [PubMed] [Google Scholar]
- 44.Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993. 74 597–608. ( 10.1016/0092-8674(93)90508-N) [DOI] [PubMed] [Google Scholar]
- 45.Gibson L, Holmgreen SP, Huang DC, Bernard O, Copeland NG, Jenkins NA, Sutherland GR, Baker E, Adams JM, Cory S. bcl-w, a novel member of the bcl-2 family, promotes cell survival. Oncogene 1996. 13 665–675. [PubMed] [Google Scholar]
- 46.Chittenden T, Flemington C, Houghton AB, Ebb RG, Gallo GJ, Elangovan B, Chinnadurai G, Lutz RJ. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO Journal 1995. 14 5589–5596. ( 10.1002/j.1460-2075.1995.tb00246.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993. 74 609–619. ( 10.1016/0092-8674(93)90509-O) [DOI] [PubMed] [Google Scholar]
- 48.Hsu SY, Hsueh AJ. A splicing variant of the Bcl-2 member Bok with a truncated BH3 domain induces apoptosis but does not dimerize with antiapoptotic Bcl-2 proteins in vitro. Journal of Biological Chemistry 1998. 273 30139–30146. ( 10.1074/jbc.273.46.30139) [DOI] [PubMed] [Google Scholar]
- 49.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 1995. 80 285–291. ( 10.1016/0092-8674(95)90411-5) [DOI] [PubMed] [Google Scholar]
- 50.Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes and Development 1996. 10 2859–2869. ( 10.1101/gad.10.22.2859) [DOI] [PubMed] [Google Scholar]
- 51.Boyd JM, Gallo GJ, Elangovan B, Houghton AB, Malstrom S, Avery BJ, Ebb RG, Subramanian T, Chittenden T, Lutz RJ. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene 1995. 11 1921–1928. [PubMed] [Google Scholar]
- 52.Billen LP, Kokoski CL, Lovell JF, Leber B, Andrews DW. Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS Biology 2008. 6 e147 ( 10.1371/journal.pbio.0060147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Du H, Wolf J, Schafer B, Moldoveanu T, Chipuk JE, Kuwana T. BH3 domains other than Bim and Bid can directly activate Bax/Bak. Journal of Biological Chemistry 2011. 286 491–501. ( 10.1074/jbc.M110.167148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ando T, Shibata H, Suzuki T, Kurihara I, Hayashi K, Hayashi M, Saito I, Kawabe H, Tsujioka M, Saruta T. The possible role of apoptosis-suppressing genes, bcl-2 and mcl-1/EAT in human adrenal tumors. Endocrine Research 1998. 24 955–960. ( 10.3109/07435809809032715) [DOI] [PubMed] [Google Scholar]
- 55.Kanauchi H, Wada N, Clark OH, Duh QY. Apoptosis regulating genes, bcl-2 and bax, and human telomerase reverse transcriptase messenger RNA expression in adrenal tumors: possible diagnostic and prognostic importance. Surgery 2002. 132 1021–1026; discussion 1026–1027. ( 10.1067/msy.2002.128616) [DOI] [PubMed] [Google Scholar]
- 56.Sbragia L, Oliveira-Filho AG, Vassallo J, Pinto GA, Guerra-Junior G, Bustorff-Silva J. Adrenocortical tumors in Brazilian children: immunohistochemical markers and prognostic factors. Archives of Pathology and Laboratory Medicine 2005. 129 1127–1131. ( 10.1043/1543-2165(2005)129[1127:ATIBCI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 57.Bernini GP, Moretti A, Viacava P, Bonadio AG, Iacconi P, Miccoli P, Salvetti A. Apoptosis control and proliferation marker in human normal and neoplastic adrenocortical tissues. British Journal of Cancer 2002. 86 1561–1565. ( 10.1038/sj.bjc.6600287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stojadinovic A, Brennan MF, Hoos A, Omeroglu A, Leung DH, Dudas ME, Nissan A, Cordon-Cardo C, Ghossein RA. Adrenocortical adenoma and carcinoma: histopathological and molecular comparative analysis. Modern Pathology 2003. 16 742–751. ( 10.1097/01.MP.0000081730.72305.81) [DOI] [PubMed] [Google Scholar]
- 59.Stojadinovic A, Ghossein RA, Hoos A, Nissan A, Marshall D, Dudas M, Cordon-Cardo C, Jaques DP, Brennan MF. Adrenocortical carcinoma: clinical, morphologic, and molecular characterization. Journal of Clinical Oncology 2002. 20 941–950. ( 10.1200/JCO.2002.20.4.941) [DOI] [PubMed] [Google Scholar]
- 60.Zhang PJ, Genega EM, Tomaszewski JE, Pasha TL, LiVolsi VA. The role of calretinin, inhibin, melan-A, BCL-2, and C-kit in differentiating adrenal cortical and medullary tumors: an immunohistochemical study. Modern Pathology 2003. 16 591–597. ( 10.1097/01.MP.0000073134.60541.E8) [DOI] [PubMed] [Google Scholar]
- 61.McNicol AM, Struthers AL, Nolan CE, Hermans J, Haak HR. Proliferation in adrenocortical tumors: correlation with clinical outcome and p53 status. Endocrine Pathology 1997. 8 29–36. ( 10.1007/BF02739705) [DOI] [PubMed] [Google Scholar]
- 62.Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis 2007. 12 1543–1568. ( 10.1007/s10495-007-0087-3) [DOI] [PubMed] [Google Scholar]
- 63.Saleem M, Qadir MI, Perveen N, Ahmad B, Saleem U, Irshad T, Ahmad B. Inhibitors of apoptotic proteins: new targets for anticancer therapy. Chemical Biology and Drug Design 2013. 82 243–251. ( 10.1111/cbdd.12176) [DOI] [PubMed] [Google Scholar]
- 64.Altieri B, Sbiera S, Della Casa S, Weigand I, Wild V, Steinhauer S, Fadda G, Kocot A, Bekteshi M, Mambretti EM, et al. Livin/BIRC7 expression as malignancy marker in adrenocortical tumors. Oncotarget 2017. 8 9323–9338. ( 10.18632/oncotarget.14067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sbiera S, Kroiss M, Thamm T, Beyer M, Majidi F, Kuehner D, Wobser M, Becker JC, Adam P, Ronchi C, et al. Survivin in adrenocortical tumors – pathophysiological implications and therapeutic potential. Hormone and Metabolic Research 2013. 45 137–146. ( 10.1055/s-0032-1327750) [DOI] [PubMed] [Google Scholar]
- 66.Pucci B, Kasten M, Giordano A. Cell cycle and apoptosis. Neoplasia 2000. 2 291–299. ( 10.1038/sj.neo.7900101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pereira SS, Monteiro MP, Bourdeau I, Lacroix A, Pignatelli D. MECHANISMS of ENDOCRINOLOGY: Cell cycle regulation in adrenocortical carcinoma. European Journal of Endocrinology 2018. 179 R95–R110. ( 10.1530/EJE-17-0976) [DOI] [PubMed] [Google Scholar]
- 68.Amaral JD, Xavier JM, Steer CJ, Rodrigues CM. The role of p53 in apoptosis. Discovery Medicine 2010. 9 145–152. [PubMed] [Google Scholar]
- 69.Chipuk JE, Green DR. Dissecting p53-dependent apoptosis. Cell Death and Differentiation 2006. 13 994–1002. ( 10.1038/sj.cdd.4401908) [DOI] [PubMed] [Google Scholar]
- 70.Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. Journal of Biological Chemistry 2002. 277 3247–3257. ( 10.1074/jbc.M106643200) [DOI] [PubMed] [Google Scholar]
- 71.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995. 80 293–299. ( 10.1016/0092-8674(95)90412-3) [DOI] [PubMed] [Google Scholar]
- 72.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Molecular Cell 2001. 7 683–694. ( 10.1016/S1097-2765(01)00214-3) [DOI] [PubMed] [Google Scholar]
- 73.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 2000. 288 1053–1058. ( 10.1126/science.288.5468.1053) [DOI] [PubMed] [Google Scholar]
- 74.Schuler M, Green DR. Transcription, apoptosis and p53: catch-22. Trends in Genetics 2005. 21 182–187. ( 10.1016/j.tig.2005.01.001) [DOI] [PubMed] [Google Scholar]
- 75.Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science 1998. 282 290–293. ( 10.1126/science.282.5387.290) [DOI] [PubMed] [Google Scholar]
- 76.Wu GS, Burns TF, McDonald ER, 3rd, Jiang W, Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nature Genetics 1997. 17 141–143. ( 10.1038/ng1097-141) [DOI] [PubMed] [Google Scholar]
- 77.Robles AI, Harris CC. Clinical outcomes and correlates of TP53 mutations and cancer. Cold Spring Harbor Perspectives in Biology 2010. 2 a001016 ( 10.1101/cshperspect.a001016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ragazzon B, Libe R, Gaujoux S, Assie G, Fratticci A, Launay P, Clauser E, Bertagna X, Tissier F, de Reynies A, et al. Transcriptome analysis reveals that p53 and {beta}-catenin alterations occur in a group of aggressive adrenocortical cancers. Cancer Research 2010. 70 8276–8281. ( 10.1158/0008-5472.CAN-10-2014) [DOI] [PubMed] [Google Scholar]
- 79.Barzon L, Chilosi M, Fallo F, Martignoni G, Montagna L, Palu G, Boscaro M. Molecular analysis of CDKN1C and TP53 in sporadic adrenal tumors. European Journal of Endocrinology 2001. 145 207–212. ( 10.1530/eje.0.1450207) [DOI] [PubMed] [Google Scholar]
- 80.Waldmann J, Patsalis N, Fendrich V, Langer P, Saeger W, Chaloupka B, Ramaswamy A, Fassnacht M, Bartsch DK, Slater EP. Clinical impact of TP53 alterations in adrenocortical carcinomas. Langenbeck’s Archives of Surgery 2012. 397 209–216. ( 10.1007/s00423-011-0868-6) [DOI] [PubMed] [Google Scholar]
- 81.Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, Lerario AM, Else T, Knijnenburg TA, Ciriello G, et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell 2016. 29 723–736. ( 10.1016/j.ccell.2016.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arola J, Salmenkivi K, Liu J, Kahri AI, Heikkila P. p53 and Ki67 in adrenocortical tumors. Endocrine Research 2000. 26 861–865. ( 10.3109/07435800009048609) [DOI] [PubMed] [Google Scholar]
- 83.Reincke M, Karl M, Travis WH, Mastorakos G, Allolio B, Linehan HM, Chrousos GP. p53 mutations in human adrenocortical neoplasms: immunohistochemical and molecular studies. Journal of Clinical Endocrinology and Metabolism 1994. 78 790–794. ( 10.1210/jcem.78.3.8126158) [DOI] [PubMed] [Google Scholar]
- 84.Pereira SS, Morais T, Costa MM, Monteiro MP, Pignatelli D. The emerging role of the molecular marker p27 in the differential diagnosis of adrenocortical tumors. Endocrine Connections 2013. 2 137–145. ( 10.1530/EC-13-0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Libe R, Groussin L, Tissier F, Elie C, Rene-Corail F, Fratticci A, Jullian E, Beck-Peccoz P, Bertagna X, Gicquel C, et al. Somatic TP53 mutations are relatively rare among adrenocortical cancers with the frequent 17p13 loss of heterozygosity. Clinical Cancer Research 2007. 13 844–850. ( 10.1158/1078-0432.CCR-06-2085) [DOI] [PubMed] [Google Scholar]
- 86.Gicquel C, Bertagna X, Gaston V, Coste J, Louvel A, Baudin E, Bertherat J, Chapuis Y, Duclos JM, Schlumberger M, et al. Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer Research 2001. 61 6762–6767. [PubMed] [Google Scholar]
- 87.Juhlin CC, Goh G, Healy JM, Fonseca AL, Scholl UI, Stenman A, Kunstman JW, Brown TC, Overton JD, Mane SM, et al. Whole-exome sequencing characterizes the landscape of somatic mutations and copy number alterations in adrenocortical carcinoma. Journal of Clinical Endocrinology and Metabolism 2015. 100 E493–E502. ( 10.1210/jc.2014-3282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Human Mutation 2003. 21 313–320. ( 10.1002/humu.10185) [DOI] [PubMed] [Google Scholar]
- 89.Ribeiro RC, Sandrini F, Figueiredo B, Zambetti GP, Michalkiewicz E, Lafferty AR, DeLacerda L, Rabin M, Cadwell C, Sampaio G, et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. PNAS 2001. 98 9330–9335. ( 10.1073/pnas.161479898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sandrini F, Villani DP, Tucci S, Moreira AC, de Castro M, Elias LL. Inheritance of R337H p53 gene mutation in children with sporadic adrenocortical tumor. Hormone and Metabolic Research 2005. 37 231–235. ( 10.1055/s-2005-861373) [DOI] [PubMed] [Google Scholar]
- 91.Almeida MQ, Latronico AC. The molecular pathogenesis of childhood adrenocortical tumors. Hormone and Metabolic Research 2007. 39 461–466. ( 10.1055/s-2007-981476) [DOI] [PubMed] [Google Scholar]
- 92.Park JH, Li J, Starost MF, Liu C, Zhuang J, Chen J, Achatz MI, Kang JG, Wang PY, Savage SA, et al. Mouse homolog of the human TP53 R337H mutation reveals its role in tumorigenesis. Cancer Research 2018. 78 5375–5383. ( 10.1158/0008-5472.CAN-18-0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Borges LM, Ayres FM. R337H mutation of the TP53 gene as a clinical marker in cancer patients: a systematic review of literature. Genetics and Molecular Research 2015. 14 17034–17043. ( 10.4238/2015.December.16.4) [DOI] [PubMed] [Google Scholar]
- 94.Lalli E, Figueiredo BC. Pediatric adrenocortical tumors: what they can tell us on adrenal development and comparison with adult adrenal tumors. Frontiers in Endocrinology 2015. 6 23 ( 10.3389/fendo.2015.00023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoffman B, Liebermann DA. Apoptotic signaling by c-MYC. Oncogene 2008. 27 6462–6472. ( 10.1038/onc.2008.312) [DOI] [PubMed] [Google Scholar]
- 96.Prendergast GC. Mechanisms of apoptosis by c-Myc. Oncogene 1999. 18 2967–2987. ( 10.1038/sj.onc.1202727) [DOI] [PubMed] [Google Scholar]
- 97.McMahon SB. MYC and the control of apoptosis. Cold Spring Harbor Perspectives in Medicine 2014. 4 a014407–a014407. ( 10.1101/cshperspect.a014407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitchell KO, Ricci MS, Miyashita T, Dicker DT, Jin Z, Reed JC, El-Deiry WS. Bax is a transcriptional target and mediator of c-myc-induced apoptosis. Cancer Research 2000. 60 6318–6325. [PubMed] [Google Scholar]
- 99.Maclean KH, Keller UB, Rodriguez-Galindo C, Nilsson JA, Cleveland JL. c-Myc augments gamma irradiation-induced apoptosis by suppressing Bcl-XL. Molecular and Cellular Biology 2003. 23 7256–7270. ( 10.1128/MCB.23.20.7256-7270.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Luscher B. Function and regulation of the transcription factors of the Myc/Max/Mad network. Gene 2001. 277 1–14. ( 10.1016/S0378-1119(01)00697-7) [DOI] [PubMed] [Google Scholar]
- 101.Nieminen AI, Partanen JI, Hau A, Klefstrom J. c-Myc primed mitochondria determine cellular sensitivity to TRAIL-induced apoptosis. EMBO Journal 2007. 26 1055–1067. ( 10.1038/sj.emboj.7601551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Klefstrom J, Arighi E, Littlewood T, Jaattela M, Saksela E, Evan GI, Alitalo K. Induction of TNF-sensitive cellular phenotype by c-Myc involves p53 and impaired NF-kappaB activation. EMBO Journal 1997. 16 7382–7392. ( 10.1093/emboj/16.24.7382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, Sanders D, Thomas DG, Doherty G, Hammer G. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clinical Cancer Research 2009. 15 668–676. ( 10.1158/1078-0432.CCR-08-1067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tombol Z, Szabo PM, Molnar V, Wiener Z, Tolgyesi G, Horanyi J, Riesz P, Reismann P, Patocs A, Liko I, et al. Integrative molecular bioinformatics study of human adrenocortical tumors: microRNA, tissue-specific target prediction, and pathway analysis. Endocrine-Related Cancer 2009. 16 895–906. ( 10.1677/ERC-09-0096) [DOI] [PubMed] [Google Scholar]
- 105.Szabo PM, Racz K, Igaz P. Underexpression of C-myc in adrenocortical cancer: a major pathogenic event? Hormone and Metabolic Research 2011. 43 297–299. ( 10.1055/s-0031-1273762) [DOI] [PubMed] [Google Scholar]
- 106.Liu J, Voutilainen R, Kahri AI, Heikkila P. Expression patterns of the c-myc gene in adrenocortical tumors and pheochromocytomas. Journal of Endocrinology 1997. 152 175–181. ( 10.1677/joe.0.1520175) [DOI] [PubMed] [Google Scholar]
- 107.Hickman ES, Moroni MC, Helin K. The role of p53 and pRB in apoptosis and cancer. Current Opinion in Genetics and Development 2002. 12 60–66. ( 10.1016/S0959-437X(01)00265-9) [DOI] [PubMed] [Google Scholar]
- 108.Luciakova K, Barath P, Li R, Zaid A, Nelson BD. Activity of the human cytochrome c1 promoter is modulated by E2F. Biochemical Journal 2000. 351 251–256. ( 10.1042/0264-6021:3510251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vorburger SA, Pataer A, Yoshida K, Liu Y, Lu X, Swisher SG, Hunt KK. The mitochondrial apoptosis-inducing factor plays a role in E2F-1-induced apoptosis in human colon cancer cells. Annals of Surgical Oncology 2003. 10 314–322. ( 10.1245/ASO.2003.05.021) [DOI] [PubMed] [Google Scholar]
- 110.Xie W, Jiang P, Miao L, Zhao Y, Zhimin Z, Qing L, Zhu WG, Wu M. Novel link between E2F1 and Smac/Diablo: proapoptotic Smac/Diablo is transcriptionally upregulated by E2F1. Nucleic Acids Research 2006. 34 2046–2055. ( 10.1093/nar/gkl150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stanelle J, Putzer BM. E2F1-induced apoptosis: turning killers into therapeutics. Trends in Molecular Medicine 2006. 12 177–185. ( 10.1016/j.molmed.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 112.Stanelle J, Tu-Rapp H, Putzer BM. A novel mitochondrial protein DIP mediates E2F1-induced apoptosis independently of p53. Cell Death and Differentiation 2005. 12 347–357. ( 10.1038/sj.cdd.4401532) [DOI] [PubMed] [Google Scholar]
- 113.Ragazzon B, Libe R, Assie G, Tissier F, Barreau O, Houdayer C, Perlemoine K, Audebourg A, Clauser E, Rene-Corail F, et al. Mass-array screening of frequent mutations in cancers reveals RB1 alterations in aggressive adrenocortical carcinomas. European Journal of Endocrinology 2014. 170 385–391. ( 10.1530/EJE-13-0778) [DOI] [PubMed] [Google Scholar]
- 114.Szabo PM, Tamasi V, Molnar V, Andrasfalvy M, Tombol Z, Farkas R, Kovesdi K, Patocs A, Toth M, Szalai C, et al. Meta-analysis of adrenocortical tumour genomics data: novel pathogenic pathways revealed. Oncogene 2010. 29 3163–3172. ( 10.1038/onc.2010.80) [DOI] [PubMed] [Google Scholar]
- 115.Cherradi N. MicroRNAs as potential biomarkers in adrenocortical cancer: progress and challenges. Frontiers in Endocrinology 2015. 6 195 ( 10.3389/fendo.2015.00195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ozata DM, Caramuta S, Velazquez-Fernandez D, Akcakaya P, Xie H, Hoog A, Zedenius J, Backdahl M, Larsson C, Lui WO. The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocrine-Related Cancer 2011. 18 643–655. ( 10.1530/ERC-11-0082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang C, Sun Y, Wu H, Zhao D, Chen J. Distinguishing adrenal cortical carcinomas and adenomas: a study of clinicopathological features and biomarkers. Histopathology 2014. 64 567–576. ( 10.1111/his.12283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koperski Ł, Kotlarek M, Swierniak M, Kolanowska M, Kubiak A, Gornicka B, Jazdzewski K, Wojcicka A. Next-generation sequencing reveals microRNA markers of adrenocortical tumors malignancy. Oncotarget 2017. 8 49191–49200. ( 10.18632/oncotarget.16788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu Y, Wang W, Hu W, Xu W, Xiao G, Nie Q, Ouyang K, Chen S. MicroRNA-205 suppresses the growth of adrenocortical carcinoma SW-13 cells via targeting Bcl-2. Oncology Reports 2015. 34 3104–3110. ( 10.3892/or.2015.4295) [DOI] [PubMed] [Google Scholar]
- 120.Soon PS, Tacon LJ, Gill AJ, Bambach CP, Sywak MS, Campbell PR, Yeh MW, Wong SG, Clifton-Bligh RJ, Robinson BG, et al. miR-195 and miR-483-5p identified as predictors of poor prognosis in adrenocortical cancer. Clinical Cancer Research 2009. 15 7684–7692. ( 10.1158/1078-0432.CCR-09-1587) [DOI] [PubMed] [Google Scholar]
- 121.Liu L, Chen L, Xu Y, Li R, Du X. microRNA-195 promotes apoptosis and suppresses tumorigenicity of human colorectal cancer cells. Biochemical and Biophysical Research Communications 2010. 400 236–240. ( 10.1016/j.bbrc.2010.08.046) [DOI] [PubMed] [Google Scholar]
- 122.Patel MP, Masood A, Patel PS, Chanan-Khan AA. Targeting the Bcl-2. Current Opinion in Oncology 2009. 21 516–523. ( 10.1097/CCO.0b013e328331a7a4) [DOI] [PubMed] [Google Scholar]
- 123.Day TW, Safa AR. RNA interference in cancer: targeting the anti-apoptotic protein C-FLIP for drug discovery. Mini Reviews in Medicinal Chemistry 2009. 9 741–748. ( 10.2174/138955709788452748) [DOI] [PubMed] [Google Scholar]
- 124.Flack MR, Pyle RG, Mullen NM, Lorenzo B, Wu YW, Knazek RA, Nisula BC, Reidenberg MM. Oral gossypol in the treatment of metastatic adrenal cancer. Journal of Clinical Endocrinology and Metabolism 1993. 76 1019–1024. ( 10.1210/jc.76.4.1019) [DOI] [PubMed] [Google Scholar]
- 125.Schteingart DE, Benitez R, Bradford C, Narayan A, Wang S. Expression of anti-apoptosis genes determines the response of adrenal cancer to apoptosis-inducing chemotherapy. Anticancer Research 2010. 30 4805–4809. [PubMed] [Google Scholar]
- 126.Oliver CL, Miranda MB, Shangary S, Land S, Wang S, Johnson DE. (−)-Gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-X(L)-mediated apoptosis resistance. Molecular Cancer Therapeutics 2005. 4 23–31. [PubMed] [Google Scholar]
- 127.Wu YW, Chik CL, Albertson BD, Linehan WM, Knazek RA. Inhibitory effects of gossypol on adrenal function. Acta Endocrinologica 1991. 124 672–678. [DOI] [PubMed] [Google Scholar]
- 128.Liu S, Kulp SK, Sugimoto Y, Jiang J, Chang HL, Dowd MK, Wan P, Lin YC. The (−)-enantiomer of gossypol possesses higher anticancer potency than racemic gossypol in human breast cancer. Anticancer Research 2002. 22 33–38. [PubMed] [Google Scholar]
- 129.Huang YW, Wang LS, Chang HL, Ye W, Dowd MK, Wan PJ, Lin YC. Molecular mechanisms of (−)-gossypol-induced apoptosis in human prostate cancer cells. Anticancer Research 2006. 26 1925–1933. [PubMed] [Google Scholar]
- 130.Stein MN, Hussain M, Stadler WM, Liu G, Tereshchenko IV, Goodin S, Jeyamohan C, Kaufman HL, Mehnert J, DiPaola RS. A Phase II study of AT-101 to overcome Bcl-2-mediated resistance to androgen deprivation therapy in patients With newly diagnosed castration-sensitive metastatic prostate cancer. Clinical Genitourinary Cancer 2016. 14 22–27. ( 10.1016/j.clgc.2015.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anderson MA, Huang D, Roberts A. Targeting BCL2 for the treatment of lymphoid malignancies. Seminars in Hematology 2014. 51 219–227. ( 10.1053/j.seminhematol.2014.05.008) [DOI] [PubMed] [Google Scholar]
- 132.Baggstrom MQ, Qi Y, Koczywas M, Argiris A, Johnson EA, Millward MJ, Murphy SC, Erlichman C, Rudin CM, Govindan R, et al. A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. Journal of Thoracic Oncology 2011. 6 1757–1760. ( 10.1097/JTO.0b013e31822e2941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhu Y, Wang M, Zhao X, Zhang L, Wu Y, Wang B, Hu W. Rottlerin as a novel chemotherapy agent for adrenocortical carcinoma. Oncotarget 2017. 8 22825–22834. ( 10.18632/oncotarget.15221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xu L, Qi Y, Xu Y, Lian J, Wang X, Ning G, Wang W, Zhu Y. Co-inhibition of EGFR and IGF1R synergistically impacts therapeutically on adrenocortical carcinoma. Oncotarget 2016. 7 36235–36246. ( 10.18632/oncotarget.8827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Assie G, Letouze E, Fassnacht M, Jouinot A, Luscap W, Barreau O, Omeiri H, Rodriguez S, Perlemoine K, Rene-Corail F, et al. Integrated genomic characterization of adrenocortical carcinoma. Nature Genetics 2014. 46 607–612. ( 10.1038/ng.2953) [DOI] [PubMed] [Google Scholar]
- 136.Khoo KH, Verma CS, Lane DP. Drugging the p53 pathway: understanding the route to clinical efficacy. Nature Reviews: Drug Discovery 2014. 13 217–236. ( 10.1038/nrd4236) [DOI] [PubMed] [Google Scholar]
- 137.Xiao M, Li W. Recent advances on small-molecule survivin inhibitors. Current Medicinal Chemistry 2015. 22 1136 – 1146. ( 10.2174/0929867322666150114102146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Garg H, Suri P, Gupta JC, Talwar GP, Dubey S. Survivin: a unique target for tumor therapy. Cancer Cell International 2016. 16 49–49. ( 10.1186/s12935-016-0326-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. BioMed Research International 2014. 2014 1–23. ( 10.1155/2014/150845) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 140.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000. 100 57–70. ( 10.1016/S0092-8674(00)81683-9) [DOI] [PubMed] [Google Scholar]
- 141.Borges KS, Andrade AF, Silveira VS, Marco Antonio DS, Vasconcelos EJR, Antonini SRR, Tone LG, Scrideli CA. The aurora kinase inhibitor AMG 900 increases apoptosis and induces chemosensitivity to anticancer drugs in the NCI-H295 adrenocortical carcinoma cell line. Anti-Cancer Drugs 2017. 28 634–644. ( 10.1097/CAD.0000000000000504) [DOI] [PubMed] [Google Scholar]
- 142.Cheng Y, Kerppola RE, Kerppola TK. ATR-101 disrupts mitochondrial functions in adrenocortical carcinoma cells and in vivo. Endocrine-Related Cancer 2016. 23 1–19. ( 10.1530/ERC-15-0527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Subramanian C, Kuai R, Zhu Q, White P, Moon JJ, Schwendeman A, Cohen MS. Synthetic high-density lipoprotein nanoparticles: a novel therapeutic strategy for adrenocortical carcinomas. Surgery 2016. 159 284–294. ( 10.1016/j.surg.2015.08.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu J, Xu M, Zhao Y, Ao C, Wu Y, Chen Z, Wang B, Bai X, Li M, Hu W. n-3 polyunsaturated fatty acids abrogate mTORC1/2 signaling and inhibit adrenocortical carcinoma growth in vitro and in vivo. Oncology Reports 2016. 35 3514–3522. ( 10.3892/or.2016.4720) [DOI] [PubMed] [Google Scholar]
- 145.Drelon C, Berthon A, Mathieu M, Ragazzon B, Kuick R, Tabbal H, Septier A, Rodriguez S, Batisse-Lignier M, Sahut-Barnola I, et al. EZH2 is overexpressed in adrenocortical carcinoma and is associated with disease progression. Human Molecular Genetics 2016. 25 2789–2800. ( 10.1093/hmg/ddw136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu Y, Dong B, Huang J, Kong W, Xue W, Zhu Y, Zhang J, Huang Y. Sphingosine kinase 1 is overexpressed and promotes adrenocortical carcinoma progression. Oncotarget 2016. 7 3233–3244. ( 10.18632/oncotarget.6564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chimento A, Sirianni R, Casaburi I, Zolea F, Rizza P, Avena P, Malivindi R, De Luca A, Campana C, Martire E, et al. GPER agonist G-1 decreases adrenocortical carcinoma (ACC) cell growth in vitro and in vivo. Oncotarget 2015. 6 19190–19203. ( 10.18632/oncotarget.4241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Poli G, Cantini G, Armignacco R, Fucci R, Santi R, Canu L, Nesi G, Mannelli M, Luconi M. Metformin as a new anti-cancer drug in adrenocortical carcinoma. Oncotarget 2016. 7 49636–49648. ( 10.18632/oncotarget.10421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sbiera S, Leich E, Liebisch G, Sbiera I, Schirbel A, Wiemer L, Matysik S, Eckhardt C, Gardill F, Gehl A, et al. Mitotane inhibits sterol-O-acyl transferase 1 triggering lipid-mediated endoplasmic reticulum stress and apoptosis in adrenocortical carcinoma cells. Endocrinology 2015. 156 3895–3908. ( 10.1210/en.2015-1367) [DOI] [PubMed] [Google Scholar]
- 150.Waszut U, Szyszka P, Dworakowska D. Understanding mitotane mode of action. Journal of Physiology and Pharmacology 2017. 68 13–26. [PubMed] [Google Scholar]
- 151.Hescot S, Amazit L, Lhomme M, Travers S, DuBow A, Battini S, Boulate G, Namer IJ, Lombes A, Kontush A, et al. Identifying mitotane-induced mitochondria-associated membranes dysfunctions: metabolomic and lipidomic approaches. Oncotarget 2017. 8 109924–109940. ( 10.18632/oncotarget.18968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Satoh K, Zhang L, Zhang Y, Chelluri R, Boufraqech M, Nilubol N, Patel D, Shen M, Kebebew E. Identification of niclosamide as a novel anticancer agent for adrenocortical carcinoma. Clinical Cancer Research 2016. 22 3458–3466. ( 10.1158/1078-0432.CCR-15-2256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Batisse-Lignier M, Sahut-Barnola I, Tissier F, Dumontet T, Mathieu M, Drelon C, Pointud JC, Damon-Soubeyrand C, Marceau G, Kemeny JL, et al. P53/Rb inhibition induces metastatic adrenocortical carcinomas in a preclinical transgenic model. Oncogene 2017. 36 4445–4456. ( 10.1038/onc.2017.54) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a