Abstract
In April 2017, a workshop sponsored by the National Heart, Lung, and Blood Institute, Division of Blood Diseases and Resources (DBDR) and the Center for Translation Research and Implementation Science (CTRIS) was held to discuss blood availability and transfusion safety in low and middle income countries (LMIC). The purpose of the workshop was to identify research opportunities for implementation science to improve the availability of safe blood and blood components, and transfusion practices in LMIC. Implementation science describes the late stages of the translational research spectrum and studies optimal and sustainable strategies to deliver proven-effective interventions. Regional working groups were formed to focus on opportunities and challenges in East Africa, Central/West Africa, Middle East and North Africa, Latin America and the Caribbean, Southeast Asia, Western Pacific Asia, Eastern Europe and Central Asia. The need for an “adequate supply of safe blood” emerged as the major overriding theme. Among the regional working groups, common cross-cutting themes were evident. The majority of research questions, priorities and strategies fell into the categories of: blood availability, blood transfusion safety, appropriate use of blood, quality systems, health economics and budgeting, training and education in implementation science. The workshop also brought into focus inadequate country-level data that can be used as the basis for implementation science initiatives. A mixed approach of needs assessment and targeted interventions with sufficient evidence base to move toward sustainment is an appropriate next step for blood availability and transfusion safety research in LMIC.
Keywords: Global health, blood donation, transfusion, low and middle income countries
Introduction
Currently, about 80% of the world’s population has access to only 20% of the world’s blood supply. Low and middle income countries (LMIC) often are challenged to meet transfusion needs.1 The approaches to securing a safe and adequate blood supply in high income countries (HIC) are not appropriate, practical, or validated for the broad range of LMIC settings. Inadequate or inconsistent supplies and acute shortages of blood for transfusion in low resources settings, often coupled with questionable quality, pose real risks to the health of patients who need transfusion.2 Such patients may include those in need of transfusion secondary to malaria or other infections, sickle cell disease, thalassemia, obstetric hemorrhage, and trauma.3 For these reasons, country or regional-level approaches focused on the local context may have greater impact than global recommendations.4
At the National Institutes of Health (NIH), research on blood availability and transfusion safety is supported by the National Heart, Lung, and Blood Institute (NHLBI). To identify key research priorities for improving global blood availability and transfusion safety, NHLBI convened a workshop on April 18–19, 2017 in Bethesda, MD, which was jointly organized by the Division of Blood Diseases and Resources (DBDR) and the Center for Translation Research and Implementation Science (CTRIS). DBDR has a major responsibility for research conducted to assure the adequacy and safety of the blood supply and transfusion safety while CTRIS plans, fosters, and supports research to understand optimal and sustainable implementation strategies for evidence-based interventions. The purpose of the workshop was to identify research opportunities for Implementation Science (IS) to improve the availability of safe blood and blood components, and transfusion practices in LMIC. This report which summarizes the workshop’s deliberations and recommendations seeks to place research opportunities in blood availability and transfusion safety in LMIC settings into a broader IS context. The NHLBI workshop should be viewed as complementary to other recent efforts. In 2015, a Workshop on Blood Transfusion Research in sub-Saharan Africa (SSA) to define research priorities was held by T-REC, an international consortium of academics and health practitioners working to strengthen the capacity for blood transfusion research in SSA.5 Contemporaneous with the NHLBI workshop, America’s Blood Centers and Global Healing held a workshop, titled “Workshop on Ensuring Sustainable Access to Blood in Developing Countries: International Blood Safety Forum, March 23, 2017.” (See this Issue of TRANSFUSION).
Approach
Participants
Experts from LMIC and HIC were placed in working groups representing East Africa, Central/West Africa, Middle East and North Africa, Latin America and the Caribbean, Southeast Asia, Western Pacific Asia, Eastern Europe and Central Asia. Each working group included persons with regional expertise and most working groups included at least one expert from the local region, other experts in transfusion medicine and, where possible, IS researchers. Representatives from WHO, the Centers for Disease Control and Prevention (CDC), the International Society for Blood Transfusion (ISBT), AABB (formerly American Association for Blood Banks) and the America’s Blood Centers (ABC) as well as other agencies within and outside of NIH also participated. Overall, a broad range of stakeholders were included from blood centers, to transfusing physicians, public health authorities, regulators, researchers/educators, vendors/manufacturers, and non-government organizations (NGOs).
Process
Three conference calls of participants were held before the workshop to discuss challenges and opportunities in regional blood availability and transfusion safety, and to identify gaps as well as potential key scientific priorities and research strategies to be further discussed at the in-person meeting. Before meeting in-person, working groups also held multiple independent calls. Using a template, research priorities defined by consensus within each working group were developed into synopses of the specific research questions and approaches. The template was made available to all participants and detailed additional research concepts were submitted from the working groups and other contributors.
The two day in-person workshop was structured into five sessions: 1) Overview of Translation Research and Implementation Science, 2) Introduction to Global Blood Safety and Availability – Regional Challenges and Opportunities, 3) Discussion of Regional Challenges and Opportunities and Cross-cutting Themes, 4) Burden Assessment, Emerging Topics, and Research Methods Relevant to Transfusion, and 5) Discussion and Synthesis of Key Scientific Priorities and Research Strategies. Invited experts who were not able to attend in-person participated by teleconference during the workshop.
T4 Translation Research and Implementation Science
The research translation spectrum includes five stages, T0 – T4 (Figure 1).6 The first stage (T0) is fundamental discovery, followed by (T1) the process of elucidating and applying T0 findings to humans, including mechanistic elaboration. The next stages (T2 & T3) are focused on clinical trials and observational studies to establish efficacy and other measures of intervention performance, followed by effectiveness. The final stage (T4) is research on strategies to deliver proven-effective interventions from earlier phases, translated to diverse real world settings. The terms ‘Translation Research’ and ‘Implementation Science’ were to some extent unfamiliar to many of the participants who work in transfusion. Review articles provide more detailed descriptions.6–8 Briefly, IS describes the late stages of the translational research spectrum and studies optimal and sustainable strategies to deliver proven-effective interventions.9 The emphasis is on external validity. T4 translation research studies various intervention strategies for clinics, communities, and health systems where the intent is to obtain broad generalizable new knowledge on delivery of interventions.10 Implementation Science research is broader and can also include policy and program evaluation and quality improvements efforts for service delivery that are specific to these types of efforts, and for which the findings are not generalizable new knowledge that applies in other settings. This research can also consider the resources and processes for sustaining interventions over a longer period of time, i.e. what is required to actively sustain an intervention program and to conduct research addressing questions that seek to achieve this objective.11 Implementation research hinges on building partnerships between communities and researchers.12
Figure 1.
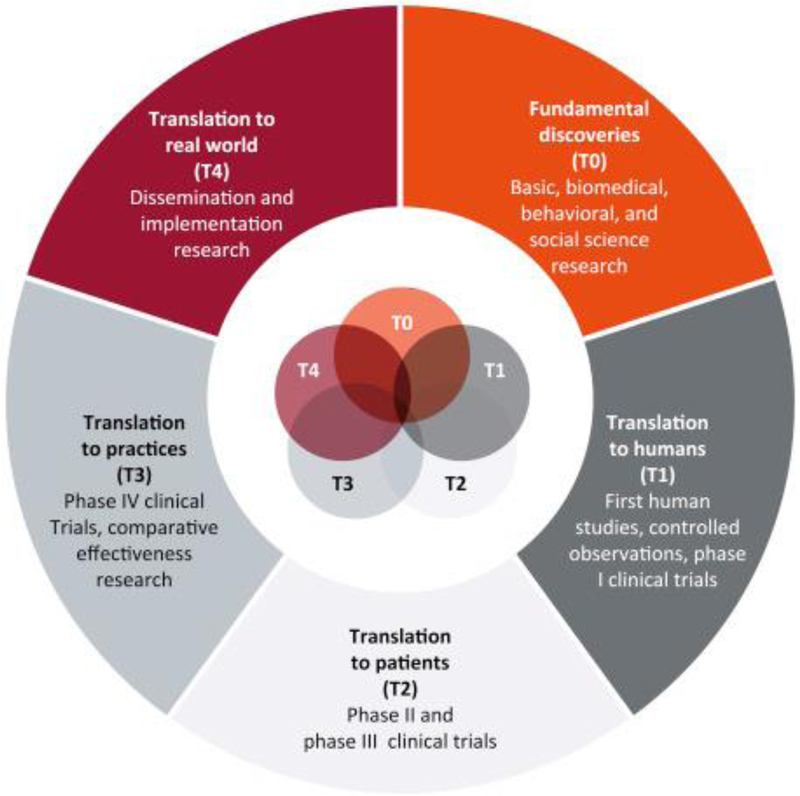
Research cycle in the clinical sciences translation research paradigm: fundamental research discoveries and translational steps toward implementation. The figure depicts the circular process from fundamental discovery to population health impact in real-world settings. The center highlights feedback loops and intersections that indicate progression is nonlinear and that translational steps may be skipped in some lines of research. 6 Used with permission.
Implementation Science “Best Buys” are those defined as effective, affordable interventions for specific real-world settings guided by country-driven health priorities. Examples are available for some heart, lung and blood diseases, including reduction in chronic lung disease in women and children through programs to provide clean cook stoves, lowering blood pressure to reduce cardiovascular events through community-level behavioral modification related to salt intake, and medical education and building research capacity in HIV/AIDS in SSA.9 No examples in blood availability and transfusion safety are readily available. In transfusion medicine and blood banking the closest good examples are the efforts to establish quality programs in laboratory screening in countries participating in President’s Emergency Plan For AIDS Relief (PEPFAR) initiatives. Though at inception PEPFAR was not specifically targeted to blood safety, the program has directly contributed to sustained progress in reducing TTIs and overall strengthening of blood systems.13,14
A common study design that is used in blood availability and transfusion safety research is the before/after study.15 It is one of the most common ways to assess the impact of policy change or intervention adoption, and is therefore often used to accrue evidence in support of effectiveness. This study design stops short of true IS. However, IS methods include ‘hybrid’ study designs that serve as a bridge between effectiveness and implementation (Figure 2), and these study designs have high relevance for blood availability and transfusion safety research in both HIC and LMIC settings. Hybrid studies in IS can be classified into three levels depending on whether effectiveness or implementation is the primary objective.16 Several of the priorities defined by the working groups fit within the hybrid designs classification.
Figure 2.
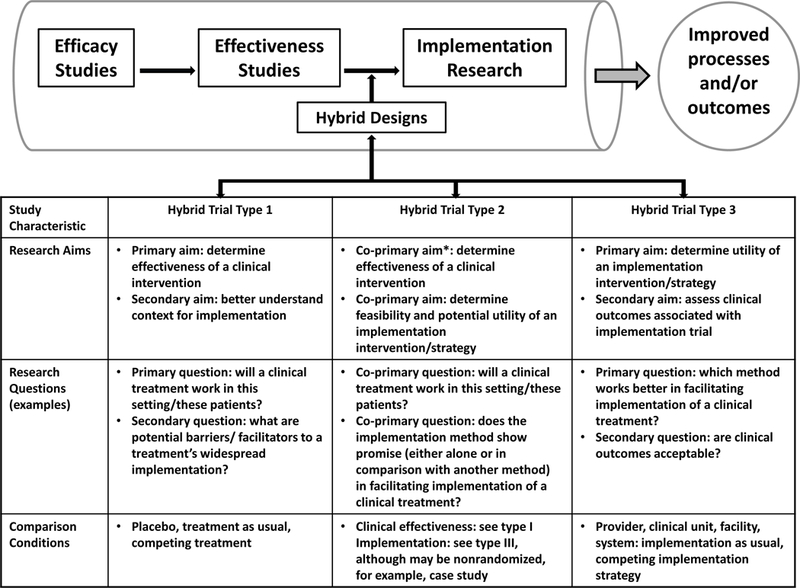
Research pipeline and location of hybrid study designs, including a summary of some characteristics and design features. Further descriptions and information about each hybrid design are available.16 Used and adapted with permission.
Challenges, Opportunities, and Major Research Priorities
The need for an “adequate supply of safe blood” emerged as the major overriding message from the workshop. Significant gaps remain in safe blood availability especially in low resource settings, and in employing appropriate clinical transfusion guidelines. As different regions have different levels of development and infrastructure, with significant interregional variability also, each has specific challenges to the availability and safety of blood. Table 1 provides a summary of the major themes identified by the seven regional working groups. Some common cross-cutting themes were evident. The majority of research questions, priorities and strategies identified by each of the seven working groups can be categorized into the following themes: blood availability, blood transfusion safety, appropriate use of blood, quality systems, health economics and budgeting, training and education in implementation science. Each of these themes is elaborated on in subsequent sections.
Table 1.
Summary of Research Priorities Identified by Regional Working Groups.
| East Africa | West/ Central Africa | Middle East / North Africa | Latin America | Southeast Asia | Western Pacific | Eastern Europe / Central Asia |
|---|---|---|---|---|---|---|
| Blood Availability and Safety | ||||||
| Factors Influencing blood donation | Role of VNRBD & context specific DHQ | VNRBD and risk of infection (assessment of WNV) |
Most effective interventions to increase repeat voluntary donation | KAP study & Implementa-tion strategy for VNRBD | Role of VNRBD & motivations / inhibitors to donation | Role of VNRBD / Replacement donors; donor motivations |
| Emerging and re-emerging pathogens | Intervention studies to improve selection of donors & communication | TTI marker assessment and selection of appropriate interventions | TTI marker assessment & audits; selection of appropriate tests | |||
| Use of pathogen reduction technologies | Use of pathogen reduction technologies | Minipool solvent detergent plasma preparation (small scale) | Blood component safety during transportation | |||
| Iron status & iron depletion in donors, including cost-effective intervention assessment | Sustained attraction of younger donors | Sustained attraction of younger donors/ iron stores evals. | ||||
| Transfusion Safety and Utilization | ||||||
| Blood needs & utilization reviews (audits) | Blood needs & utilization reviews (audits) | Minipool IVIG system | Blood needs & utilization reviews (audits) | Blood needs & utilization reviews (audits) | Knowledge transfer and best practices | Blood needs & utilization reviews (audits) |
| Cost-effective anti-human globulin testing | Sickle cell disease care and sustainable treatments | Prevention of alloimmuni-zation in chronic transfusion (thalassemia) | Transfusion nurse – clinical training for the bedside | |||
| Low-cost, practical patient identification mechanisms | ||||||
| Hemovigilance | Surveillance /Repositories | |||||
| Cross-cutting Initiatives | ||||||
| Laboratory information systems (cloud based) | Economic models for blood supply and transfusion sustainment | International TTI marker assessment (includes aspect of EQAS) | Quality Systems (EQAS & QAQC) | Quality Systems (EQAS & QAQC) | ||
Abbreviations: VNRBD – voluntary non-remunerated blood donation, DHQ – donor health questionnaire, WNV – West Nile virus, KAP – knowledge, attitudes and practices, TTI – transfusion-transmissible infections, EQAS – external quality assurance system, QAQC – quality assurance quality control.
Common challenges include lack of financial support or a sustainable financial model, lacking or low government/regulatory oversight, lack of quality systems, lack of adequately trained personnel, and lack of suitable donation screening algorithms and tests. To address these issues and improve blood availability and transfusion safety, there is a compelling need for the systematic collection and compilation of data so that situational assessments can be conducted. These findings can be used to develop studies to inform local epidemiology as well as specific IS initiatives. A need for systematic collection of country or regional data was a recurrent theme, starting with formal assessments of safety and availability gaps, needs in behavioral research in motivations and deterrents to donation, and assessments of blood utilization. Whether such efforts could be promoted or reinforced by a global database platform open to blood centers and transfusion services in LMIC to record their donation, blood screening and blood utilization data should be assessed for feasibility. Synthesis of the evidence would serve as the required framework to then be able to select appropriate interventions that could be evaluated using translation research and implementation science methods. Appendix Table 1 lists the specific research priorities areas by region.
Appendix Table 1.
Research priority areas by region.
| Region | Research Question/Topic Area |
|---|---|
| Middle East & North Africa | Mini-Pool Solvent Detergent Virus Inactivation of Plasma and Cryoprecipitate: Will the implementation of this technology, and based on accumulating evidence of safety and efficacy, help to improve access of the patients with IBDs in EMR to safe plasma components in addition to know CFCs? |
| Can Mini-Pool Intravenous Immunoglobulins (MP-IVIG) be implemented in blood transfusion centers using the newly developed medical devices? | |
| In the population of voluntary non-remunerated blood donors within the National Blood Transfusion Services in Egypt, does the demographic distribution influence the prevalence of WNV? | |
| Is it possible to avoid alloimmunization to RBC blood group antigens and minimize hemolytic transfusion reactions in Thalassemia patients who receive regular blood transfusion as long-life treatment? | |
| West & Central Africa | What are the current blood needs in Africa and what are the best methods of estimating blood needs in an African country? |
| Which model for financing blood safety in Africa is sustainable for a given country profile? | |
| What are the motivations and deterrents to blood donation in Africa? | |
| Can we design a more sensitive and specific donor health questionnaire (DHQ) for use in the African setting? | |
| Is adding whole blood pathogen reduction technique in blood safety strategy in Africa affordable and sustainable? Is whole blood pathogen reduction technique efficient and safe for African patient? | |
| Which blood transfusion strategy is more cost-effective in patient blood management of sickle cell disease (SCD) in resource limited African settings? | |
| What is the risk of emerging and re-emerging pathogens (ERP) to blood safety in Africa? | |
| Which strategy is the most appropriate for blood services of resources limited settings to reduce the risk of ERP? | |
| Eastern Africa | Can donated blood be utilized as a biomedical resource through the establishment, maintenance and archiving of blood collected for research use? |
| Does pathogen reduction (PR) of whole blood provide a major leap of blood safety in Africa? | |
| Can a laboratory information system (LIS) located “in the cloud” and serving hundreds of healthcare facilities be developed and sustained in Africa? | |
| Can cost-effective anti-human globulin (AHG) testing be implemented in Africa? | |
| Can a low-cost method for proper identification of transfusion recipients be developed for Africa? | |
| What are the factors that influence blood donation? | |
| Are lysine analogues a safe and effective alternative to prophylactic platelet transfusions for the support of patients with chemotherapy-associated transient thrombocytopenia? | |
| How can health facilities in Africa effectively implement appropriate use of blood and transfusion monitoring (hemovigilance) in recurrent blood recipients? A case study in sickle cell disease and cancer centers. | |
| Will introduction of apheresis and exchange blood transfusion be feasible and effective in Africa? | |
| What is the clinical epidemiology of adverse transfusion reactions (immediate and delayed) in Africa? | |
| What are the factors that influence blood donation? | |
| Eastern Europe & Central Asia | Does ALT testing provide any additional recipient safety? |
| Would establishing a local centralized proficiency testing reference lab in Central Asia contribute to assessing and improving the quality of blood donor screening in Central Asian republics? | |
| What are effective donor recruitment strategies for increasing blood collections in resource limited settings? | |
| Are methods used by blood banks sufficient in identifying TTI? If not, what is the true prevalence of TTI in blood donations? Could implementation of auditing procedures reduce transfusion transmission? | |
| Are the donor interview procedures adequate in excluding high risk donors to prevent TTI or other transfusion transmitted adverse events? Are donor selection processes adequate and where relevant would changing from paid donors to a voucher, incentive program or blood credit program be effective? | |
| Blood utilization patterns and potential impact of public versus private blood banks and hospitals. | |
| Can we identify TTI transmission by prospectively following transfusion recipients? Can patients who require therapeutic transfusions for thalassemia or other diseases be monitored for TTI? Can transfusion recipients return for follow up testing 6 months after transfusion? | |
| Southeast Asia | Knowledge, attitudes, and practices (KAP) study and implementation strategy to achieve a VNRBD supply in South Asia. |
| Recruitment of future donors by interventions with high school teachers and introducing chapters in course curriculum for students? | |
| To improve transfusion safety practices in hospital by providing training courses for transfusion nurses. | |
| Audit transfusion practices/blood utilization in South Asian countries and to develop guidelines for rational use of blood. | |
| To study status of temperature monitored and controlled transportation of blood and components in South Asian countries and planning of intervention whenever applicable. | |
| Western Pacific | What are the major motivational and inhibitory factors for VNRBD? |
| What measures can be taken to overcome these inhibitory factors in various countries in the WPR? What are the major enabling factors for the successful transforming from family/replacement/paid donations to voluntary non-remunerated blood donations in the countries in the WPR? | |
| What systems can we develop to encourage young donors to donate blood and assure ourselves of the safety of blood donation, and how can we communicate with and transmit information to them to address their barriers? | |
| How do we measure and assess residual risk for major TTI, and develop the capacity to evaluate and select the appropriate safety measures for implementation? | |
| What are the success factors and strategies adopted by successful blood systems in ensuring blood availability and transfusion safety, including the allocation of resources and effecting governance? | |
| What needs to be done to improve/ strengthen/correct or implement quality systems for blood safety? And how can blood quality be improved with appropriate tools including external quality assessment system (EQAS) and quality control (QC) in developing countries? | |
| Latin America | How are blood transfusions used in Latin America? -Do blood centers and hospitals have established guidelines for transfusion indications and triggers? -How frequently are blood requests not fulfilled by blood banks and for what reasons? -What is the best way to choose and implement Guidelines for Blood Transfusions to avoid unnecessary blood transfusion? |
| What is the rate of iron depletion among Latin America blood donors and what are the best interventions to avoid iron deficiency among repeat blood donors in Latin America? | |
| What is the prevalence of confirmed infectious disease markers in different Latin American countries and subgroups of donors defined by demographics and donation categories? - For example, in Brazil voluntary donations have a higher prevalence and incidence of HIV as compared to replacement donations – is the situation similar in other Latin American countries? |
|
| What are the most effective interventions to increase repeat voluntary donations and still maintain blood safety? |
|
| Other Submitted Research Ideas | |
| West Africa, East Africa, Middle East | Development of tiered Blood Transfusion Officers, including Regional, National and Hospital based experts to establish blood/transfusion monitoring programs |
| Evaluation of a regional roving blood supply for non-urban areas in SSA? | |
| Can cost-effective anti-human globulin (AHG) testing be implemented in sub-Saharan Africa (SSA)? | |
| Assessment of the efficacy and cost-effectiveness of pre-emptive anti-malarial treatment and Pathogen Reduction of Whole blood (WB-PRT) for prevention of transfusion-transmitted malaria (TTM)? | |
| Comparison of whole blood pathogen reduction technique (WB-PRT) in areas where malaria non-immune and semi-immune patients co-exist. | |
| Can a cheap, simple, genomic detection of Plasmodium (sensitivity ~1000 copies/ml) efficiently prevent transfusion-transmitted malaria (TTM)? | |
| Can family blood donors be transformed into repeat volunteer donors? | |
| Designing algorithm for blood safety in peripheral, isolated blood centers in sub-Saharan Africa (SSA) | |
| How to define fresh whole blood and what are its specific uses in sub-Saharan Africa (SSA)? | |
| What is the real cost of manufacturing and providing blood products in sub-Saharan Africa (SSA) and who pays? | |
Blood Availability
Regional working groups identified that blood availability is a priority. Disproportionately low percentages of the population in LMIC donate blood, and rapidly increasing demand due to improved access to healthcare in some LMIC directly contributes to the gap between supply and demand. Furthermore, the gap between blood availability and demand in active conflict areas in LMIC poses even greater challenges with increased need and decreased availability. Within the broad category of blood availability, specific research topics were identification of factors that influence blood donation; effective donor recruitment strategies for increasing blood collections in resource limited settings; context-appropriate sensitive and specific donor health questionnaires (DHQ); and knowledge, attitude, practice (KAP) surveys. Participants emphasized that donor recruitment and donation processes which work well in HIC cannot simply be ‘transferred to’ or ‘adopted by’ LMIC. Motivations and deterrents to blood donation include setting-specific social and cultural factors.17,18 These factors may be poorly understood in LMIC, leading to inadequate design of the necessary research to promote donation, and sub-optimal donor recruitment and retention strategies. Country-level assessments useful for defining future research are beginning to appear in the peer-reviewed literature.19,20
Regional working groups identified efforts to achieve a voluntary non-remunerated blood donation (VNRBD) donor base as very important.21–23 Recommended areas of focus are assessing the most effective interventions to increase and sustain repeat voluntary donation; development of systems to encourage young donors, including educational interventions as part of high school curricula to promote future donation behavior; and defining the major enabling factors for the successful transformation of family/replacement donors to repeat VNRBD.
Donor health concerns, such as donation-induced anemia and iron deficiency, have not been assessed in many LMIC. In some settings pre-donation assessment of hemoglobin/hematocrit may not even be performed. In most settings, where hemoglobin or hematocrit are measured, anemia anemia is the primary reason for deferral of donors24,25 yet medical follow-up or treatment in LMIC may not occur. While literature in HIC has clearly documented this problem,26–30 the rates and clinical consequences of iron depletion among blood donors in LMIC,31,32 as well as the most appropriate interventions to reduce iron deficiency among repeat blood donors are unknown. The safety of blood donation is of critical importance when younger donors (<20 years of age) are targeted for recruitment.
At the strategic level, a coordinated multi-tiered approach to provision of blood within a country or region could be developed and should be assessed. For example, a centralized blood supply system(s) supplemented with various hospital-or organization-based supplies tapering out to the furthest rural or hard to reach parts of a country or region which might be serviced by novel approaches to providing screened blood for transfusion.33 The motivators and inhibitors or deterrents to blood donation in local settings, including the impact of socio-psychological and behavioral factors need to be identified. Once motivators and deterrents are known and potential recruitment and retention strategies identified, IS can be used to evaluate which strategies may best improve sustained recruitment and retention of safe blood donors. While recruiting VNRBD remains an important goal in many countries, research into other feasible approaches to enhance blood availability should be conducted, such as conversion of family/replacement donors to VNRBD. Non-material inducements/incentives for young people to become blood donors could be explored, providing donor health and transfusion safety are safe-guarded. Donor safety in general, including research to evaluate the consequences of and how to mitigate iron depletion, as well as cost-effectiveness assessments of appropriate interventions to protect donor health are under-studied in LMIC.
Blood and Transfusion Safety
Blood and transfusion safety themes were common to all regions, but the priority topics were variable. National reports to the World Health Organization (WHO) Global Database for Blood Safety (GDBS) as well as other published data suggest that poor quality or lack of blood donation screening could be the most important gap in blood safety internationally.34 In most LMIC, blood supplies rely on volunteer as well as family/replacement donors in ratios ranging between 0 and 100% VNRBD. While the number of paid blood donations has been reduced in some settings, with successful conversion to voluntary donation, it is believed paid donation of blood or blood components may still account for a significant proportion of transfusion-transmissible infections globally. Challenges to transfusion safety include insufficient regulatory and professional oversight; lack of legislation, regulations and policies, or their effective implementation; lack of quality systems and safety programs such as those for donor screening and donation testing35,36; and lack of monitoring systems (hemovigilance) to track patient and donor outcomes, including both infectious and non-infectious adverse events. In some LMIC, the high rates of TTIs pose substantial challenges,13 and there is a high likelihood these rates are currently underestimated.37,38 Emerging infections are geographically unique and therefore pose unique challenges.
Access to “leap-frog technologies” holds great potential, such as shared laboratory information systems (LIS) located “in the cloud” to support multiple institutions and countries with insufficient local infrastructure39; low-cost methods for pre-transfusion identity verification of recipients40; hemovigilance systems for TTIs and noninfectious serious transfusion hazards41 (e.g. immediate and delayed hemolysis); prevention of alloimmunization to RBCs antigens in congenital anemia patients; and assessment of the safety, effectiveness, affordability and sustainability of implementing whole blood or component pathogen reduction technology.42 For malaria endemic areas, the neglected topic of transfusion-transmitted malaria needs to be addressed in the context of molecular testing, new preemptive anti-malarial drugs, and pathogen reduction methods.43
Both innovation and IS are needed to develop and evaluate low-cost patient identification mechanisms (to decrease ABO incompatibility errors),3 laboratory information systems (to keep track of donor/donation and patient information), rapid tests and point of care testing with high predictive values,33,44,45 the feasibility of small plasma pool inactivation and fractionation methods to increase the availability of components or factor concentrates,46–48 transportation and delivery systems, and the viability of pathogen reduction techniques appropriate in low resource settings.42 Furthermore, research is required to identify and implement effective, yet locally feasible and sustainable quality systems (including external quality assessment schemes or EQAS) and procedures to optimize the quality of donor selection, donation screening, processing and delivery, and transfusion to recipients. While many challenges exist in low resource settings, the availability of new technologies, such as rapid tests and mobile communications, offer unique, but mostly unexplored opportunities throughout the transfusion chain. In view of the lack of reliable data on prevalence and incidence of major TTIs (HIV, HBV and HCV) as well as associated risk factors in many low-resource settings, epidemiological research is also required to generate baseline data to inform country and local policy formulation and decision making. Solid data on transfusion-transmitted malaria and policies to prevent such transmission, particularly in vulnerable patients such as children and pregnant women, need to be generated.
Appropriate Use of Blood
Unmet needs for transfusion in LMIC were identified. These needs could be due to inappropriate clinical use, deferral of a relatively large proportion of donors because of high infection rates for TTIs or false positive screening results, or wastage and discard due to a lack of appropriate storage, delivery, inventory, and management capacities. In many countries, blood transfusion services are highly fragmented with significant variation in quality and performance based on geography (urban vs. rural settings) or differences in infrastructure development. Even with national systems, disparities within countries remain challenging to address.49 Wastage of blood components is another factor leading to unmet need. In certain countries, large amounts of plasma recovered from whole-blood donations are discarded because of quality concerns or logistical, contractual, or budgetary requirements that prevent the use of this plasma in fractionation.
The relationship between an insufficient supply of blood for transfusion and unnecessary transfusion is not well-defined for LMIC. Inappropriate transfusion of blood products exists in many LMIC, which could be due to limited training in transfusion medicine50 and either a lack of clinical transfusion guidelines or their effective implementation.51 High burdens of HIV infection may influence the appropriate use of blood because of anemia and other HIV-related complications.52 Social and cultural factors could also play a role in adherence to suboptimal transfusion practices. Chronic blood shortage has a major influence on blood product prescription. For instance, changes in transfusion triggers to hemoglobin levels of 4–6 g/dL instead of 7–8 g/dL could enhance transfusion availability53, and the limitation of transfusion requests to one compared to two blood units could directly increase the availability of blood to more patients.54
Regions also identified appropriate use of blood as a priority. Studies are needed to define appropriate indications for blood transfusion relevant to clinical illnesses in LMIC, to assess how blood transfusions are being used, and to understand whether actual use conforms to relevant best clinical practice. Transfusion of fresh whole blood has a prominent place in some LMIC but specifically needs to be examined with respect to meaning of the term in each setting, quality assurance and clinical indications. There is insufficient information on the extent to which blood centers and hospitals have established guidelines for transfusion indications and on the best way to implement guidelines for blood transfusions to avoid either under-transfusion or unnecessary blood transfusion. Research is needed into the role of hospital transfusion committees as well as effective implementation and sustainable uptake of clinical transfusion guidelines and hemovigilance systems. Patient blood management in LMIC may be one path to begin to overcome these challenges.55
Research on disease burden, blood needs, including affordable methods for estimating such needs, and actual blood utilization,56,57 as well as IS to evaluate patient blood management strategies are necessary. Further, related to blood availability are the issues of the appropriate balance between whole blood and blood component transfusion and effective strategies to monitor and minimize wastage.3 Research on feasible and sustainable means, including improved inventory management and use of recovered plasma for fractionation, could not only enhance blood availability for clinical transfusion but may also provide opportunities for improving the supply of fractionated blood products which are often in short supply in LMIC.
Quality Systems
The lack of compliance and the ability to comply with TTI testing requirements and an irregular or absent supply of blood donation testing kits with high performance characteristics were highlighted as major issues facing some LMIC.58 The implementation of quality systems was identified as essential for blood and transfusion safety, and without regulatory oversight, is unlikely to be achieved. Some workshop attendees argued that this is the touchstone for all other aspects of blood availability and transfusion safety. Examples of questions that were deemed relevant for LMIC included: the status of temperature monitored and controlled transportation of blood and components; interventions needed to improve, strengthen, correct or implement quality systems for blood safety; regulatory bodies sufficiently developed to promote/require quality systems; improvement of blood quality through quality control of TTI testing, regional centralized proficiency testing reference laboratories, and EQAS.36 Also emphasized was the need for the routine implementation of immunohematology tests using affordable and sensitive techniques to detect antibodies and to prevent or reduce transfusion reactions.
Health Economics and Budgeting
The approach each country takes to funding the blood supply and the provision of transfusion is wide-ranging; some countries have no specific budget allocation or mechanism for funding blood supplies and transfusion, while others may use cost recovery, line items in government budgets, or a mix of the two.34 The way a blood system is funded influences the types of interventions that may be implementable. The need for incorporating aspects of health economics into research for blood availability and transfusion safety was highlighted. Relatively little research has been done to understand how financial structures influence blood availability and transfusion safety. Additionally, the cost of research and assessing intervention priorities is paramount, especially if such activities appear to shift resources away from actual patient care. Relevant areas to investigate include: the success factors and strategies adopted by stable blood systems in ensuring blood availability and transfusion safety, including resource allocation and governance; and sustainable models for financing blood safety in LMIC of different development levels with a focus on low income countries. Equally important is to collect comparative data on blood product production costs, access to transfusion, levels of reimbursement by governments, public or private insurance, and out-of-pocket payment requirements for patients.
Health economics research for sustainability, including policy models of blood system financing such as cost recovery, was recognized as a major gap. Policy-level research in this area may help identify potential options for country-specific blood supplies. Additionally, research that incorporates cost-effectiveness59 within local feasibility and sustainability assessments, by clearly demonstrating the public health benefits of such efforts, may enhance policy support within government. Challenging questions without clear answers are the cost-effectiveness of blood transfusion strategies in LMIC. Examples of topic meriting evaluation include patient blood management in sickle cell disease (SCD), and whether pathogen reduction technologies are cost-effective in LMIC.
Training and Education in Implementation Science
Within blood banking and transfusion medicine gaps are known in LMIC with respect to competent training programs and leadership development.60 The need for better training, education, and developing the leadership potential of blood center professionals in laboratory and clinical practice is reflected as fundamental to many of the priorities stated above. With these needs unmet, research training is even more inadequate. Improved laboratory and clinical training is a platform from which to build local research capacity to develop context-relevant evidence.9 T-REC, focused on SSA, is a model that could be adopted more broadly in other region of the world. The Institut Pasteur also offers a research training course in transfusion medicine oriented toward francophone Africa and its alumni network has carried out several research collaborations.61,62 Additionally, ISBT has developed a program of clinical research design and manuscript preparation (ISBT Training in Research for Young InvesTigators (I TRY IT) geared to LMIC participants throughout the world.63 Partnerships with existing entities may help to parlay available resources to maximum advantage.
There is also a need for training and education in IS to sustain blood availability and transfusion safety in LMIC. There has been limited IS studies conducted by investigators in transfusion medicine and, vice versa, even fewer researchers in IS have considered conducting studies in transfusion medicine. Short of formal university training programs, various venues for advancing IS training should be evaluated. For example, “special training symposia and on the job research training” as performed within programs like the NHLBI Recipient Epidemiology and Donor Evaluation Study – III (REDS-III) program, or training programs supported by the NIH Fogarty International Center in collaboration with relevant NIH institutes and centers, special fellowship programs for physicians to pursue Implementation Science careers, or training provided in combination with programs such as T-REC, Institut Pasteur, and the ISBT I TRY IT program.
Summary
Implementation Science addresses the acceptability, affordability, appropriateness, feasibility, and sustainability of interventions as well as their effectiveness. The workshop identified key research opportunities and priorities that countries and regions can refer to when they develop their research strategies. The workshop also brought into sharp focus the challenge of inadequate country-level data that can be used as the basis to propose Implementation Science initiatives. A mixed approach of needs assessment and targeted interventions with sufficient evidence base to move toward sustainment is an appropriate next step for blood availability and transfusion safety in LMIC. In addition, the workshop delineated which strategies, if adopted, may have the potential to make the greatest advances with blood availability and transfusion safety in LMIC.
Acknowledgments
We would like to thank all of the workshop attendees who participated in person or by teleconference, and we would specifically like to thank the regional working group members, presenters at the workshop, and other participants who submitted detailed research ideas.
Footnotes
Workshop Proceedings
A complete list of all attendees is available by request, and a detailed report of the proceedings is available at https://www.nhlbi.nih.gov/events/2017/research-address-gaps-international-blood-availability-transfusion-safety-challenges. Alternately, please contact Shimian Zou by email at shimian.zou@nih.gov.
Publisher's Disclaimer: Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, National Institutes of Health, or the U.S. Department of Health and Human Services.
Conflict of Interest Disclosure: None
References
- 1.Roberts DJ, Field S, Delaney M, et al. Problems and Approaches for Blood Transfusion in the Developing Countries. Hematol Oncol Clin North Am 2016;30: 477–95. [DOI] [PubMed] [Google Scholar]
- 2.Kralievits KE, Raykar NP, Greenberg SL, et al. The global blood supply: a literature review. Lancet 2015;385 Suppl 2: S28. [DOI] [PubMed] [Google Scholar]
- 3.Dzik WS, Kyeyune D, Otekat G, et al. Transfusion Medicine in Sub-Saharan Africa: Conference Summary. Transfus Med Rev 2015;29: 195–204. [DOI] [PubMed] [Google Scholar]
- 4.Ifland L. Promoting national blood systems in developing countries. Curr Opin Hematol 2014;21: 497–502. [DOI] [PubMed] [Google Scholar]
- 5.Bates E. Workshop on blood transfusion research in sub-Saharan Africa Pretoria, South Africa, 2015. Available at http://www.t-rec.eu/ Access date: 15 Dec 2017. [Google Scholar]
- 6.Sampson UK, Chambers D, Riley W, et al. Implementation Research: The Fourth Movement of the Unfinished Translation Research Symphony. Glob Heart 2016;11: 153–8. [DOI] [PubMed] [Google Scholar]
- 7.Peters DH, Adam T, Alonge O, et al. Implementation research: what it is and how to do it. BMJ 2013;347: f6753. [DOI] [PubMed] [Google Scholar]
- 8.Brown CH, Curran G, Palinkas LA, et al. An Overview of Research and Evaluation Designs for Dissemination and Implementation. Annu Rev Public Health 2017;38: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelgau MM, Peprah E, Sampson UK, et al. Perspectives from NHLBI Global Health Think Tank Meeting for Late Stage (T4) Translation Research. Glob Heart 2016. July 21 pii: S2211–8160(16)30640–8. doi: 10.1016/j.gheart.2016.03.640. [DOI] [PubMed] [Google Scholar]
- 10.Westfall JM, Mensah GA. T4 Translational Moonshot: Making Cardiovascular Discoveries Work for Everyone. Circ Res 2018;122: 210–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palinkas LA, Spear SE, Mendon SJ, et al. Measuring sustainment of prevention programs and initiatives: a study protocol. Implement Sci 2016;11: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown CH, Kellam SG, Kaupert S, et al. Partnerships for the design, conduct, and analysis of effectiveness, and implementation research: experiences of the prevention science and methodology group. Adm Policy Ment Health 2012;39: 301–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apata IW, Averhoff F, Pitman J, et al. Progress toward prevention of transfusion-transmitted hepatitis B and hepatitis C infection--sub-Saharan Africa, 2000–2011. MMWR Morb Mortal Wkly Rep 2014;63: 613–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Chevalier MS, Kuehnert M, Basavaraju SV, et al. Progress Toward Strengthening National Blood Transfusion Services −14 Countries, 2011–2014. MMWR Morb Mortal Wkly Rep 2016;65: 115–9. [DOI] [PubMed] [Google Scholar]
- 15.Fergusson D, Hebert P, Shapiro S. The before/after study design in transfusion medicine: methodologic considerations. Transfus Med Rev 2002;16: 296–303. [DOI] [PubMed] [Google Scholar]
- 16.Curran GM, Bauer M, Mittman B, et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50: 217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tagny CT, Kouao MD, Toure H, et al. Transfusion safety in francophone African countries: an analysis of strategies for the medical selection of blood donors. Transfusion 2012;52: 134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tagny CT, Nguefack-Tsague G, Fopa D, et al. Risk factors for human immunodeficiency virus among blood donors in Cameroon: evidence for the design of an Africa-specific donor history questionnaire. Transfusion 2017;57: 1912–21. [DOI] [PubMed] [Google Scholar]
- 19.Riley WJ, McCullough TK, Rhamani AM, et al. Progress in the blood supply of Afghanistan. Transfusion 2017;57: 1665–73. [DOI] [PubMed] [Google Scholar]
- 20.Pitman JP, Wilkinson R, Liu Y, et al. Blood component use in a sub-Saharan African country: results of a 4-year evaluation of diagnoses associated with transfusion orders in Namibia. Transfus Med Rev 2015;29: 45–51. [DOI] [PubMed] [Google Scholar]
- 21.Monsellier M. [Voluntary and non-remunerated blood donation; current situation and perspectives]. Transfus Clin Biol 2017;24: 196–9. [DOI] [PubMed] [Google Scholar]
- 22.Garraud O, Lefrere JJ. Voluntary non-remunerated blood donation and reasons for donating: is there room for philosophy? Blood Transfus 2014;12 Suppl 1: s404–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi F, Perry R, de Wit J, et al. How expanding voluntary non-remunerated blood donations would benefit patients, donors and healthcare systems? Vox Sang 2011;101: 176–7. [DOI] [PubMed] [Google Scholar]
- 24.Kouao MD, Dembele B, N’Goran LK, et al. Reasons for blood donation deferral in sub-Saharan Africa: experience in Ivory Coast. Transfusion 2012;52: 1602–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stern M, O’Meara A, Infanti L, et al. Prognostic value of red blood cell parameters and ferritin in predicting deferral due to low hemoglobin in whole blood donors. Ann Hematol 2012;91: 775–80. [DOI] [PubMed] [Google Scholar]
- 26.Gorlin J, Katz L, Elsmore D, et al. Prevalence of blood donor iron deficiency and feasibility ferritin-based iron replacement: a blood collection agency-based study. Vox Sang 2016;111: 206–8. [DOI] [PubMed] [Google Scholar]
- 27.Badami KG, Taylor K. Iron status and risk-profiling for deficiency in New Zealand blood donors. N Z Med J 2008;121: 50–60. [PubMed] [Google Scholar]
- 28.Birgegard G, Hogman C, Killander A, et al. Serum ferritin levels in male blood donors: relation to number of phlebotomies and iron supplementation. Vox Sang 1978;34: 65–70. [DOI] [PubMed] [Google Scholar]
- 29.Mast AE, Steele WR, Johnson B, et al. Population-based screening for anemia using first-time blood donors. Am J Hematol 2012;87: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion 2012;52: 702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulahriss M, Benchemsi N. Iron deficiency in frequent and first time female blood donors. East Afr J Public Health 2008;5: 157–9. [DOI] [PubMed] [Google Scholar]
- 32.Usanga EA. Iron stores of Nigerian blood donors as assessed by serum ferritin concentration. Cent Afr J Med 1990;36: 170–3. [PubMed] [Google Scholar]
- 33.Barnes L, Delaney M, Petrovsky S. Bloodpak [Monograph on the internet] Bloodworks Northwest; 2017. Available from: https://bloodpak.org/ Access date: 13 Nov 2017. [Google Scholar]
- 34.Blood Transfusion Safety, Service Delivery and Safety, Health Systems and Innovation, WHO Global Status Report on Blood Safety and Availability 2016 Geneva, Switzerland; 2017. http://apps.who.int/iris/bitstream/10665/254987/1/9789241565431-eng.pdf?ua=1 Access date: 10 Nov 2017. [Google Scholar]
- 35.Laperche S, Boukatou G, Kouegnigan L, et al. Transfusion safety on the African continent: an international quality control of virus testing in blood banks. Transfusion 2009;49: 1600–8. [DOI] [PubMed] [Google Scholar]
- 36.Bloch EM, Shah A, Kaidarova Z, et al. A pilot external quality assurance study of transfusion screening for HIV, HCV and HBsAG in 12 African countries. Vox Sang 2014;107: 333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Ekiaby M, Lelie N, Allain JP. Nucleic acid testing (NAT) in high prevalence-low resource settings. Biologicals 2010;38: 59–64. [DOI] [PubMed] [Google Scholar]
- 38.Bloch EM, Vermeulen M, Murphy E. Blood transfusion safety in Africa: a literature review of infectious disease and organizational challenges. Transfus Med Rev 2012;26: 164–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piette JD, Lun KC, Moura LA Jr., et al. Impacts of e-health on the outcomes of care in low-and middle-income countries: where do we go from here? Bull World Health Organ 2012;90: 365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Etchells E, Koo M, Daneman N, et al. Comparative economic analyses of patient safety improvement strategies in acute care: a systematic review. BMJ Qual Saf 2012;21: 448–56. [DOI] [PubMed] [Google Scholar]
- 41.Heddle NM, Fung M, Hervig T, et al. Challenges and opportunities to prevent transfusion errors: a Qualitative Evaluation for Safer Transfusion (QUEST). Transfusion 2012;52: 1687–95. [DOI] [PubMed] [Google Scholar]
- 42.Allain JP, Goodrich R. Pathogen reduction of whole blood: utility and feasibility. Transfus Med 2017;27 Suppl 5: 320–6. [DOI] [PubMed] [Google Scholar]
- 43.Prentice AM, Cox SE. Iron and malaria interactions: research needs from basic science to global policy. Adv Nutr 2012;3: 583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ritchie AV, Ushiro-Lumb I, Edemaga D, et al. SAMBA HIV semiquantitative test, a new point-of-care viral-load-monitoring assay for resource-limited settings. J Clin Microbiol 2014;52: 3377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tagny CT, Mbanya D, Murphy EL, et al. Screening for hepatitis C virus infection in a high prevalence country by an antigen/antibody combination assay versus a rapid test. J Virol Methods 2014;199: 119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Ekiaby M, Vargas M, Sayed M, et al. Minipool caprylic acid fractionation of plasma using disposable equipment: a practical method to enhance immunoglobulin supply in developing countries. PLoS Negl Trop Dis 2015;9: e0003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Ekiaby M, Sayed MA, Caron C, et al. Solvent-detergent filtered (S/D-F) fresh frozen plasma and cryoprecipitate minipools prepared in a newly designed integral disposable processing bag system. Transfus Med 2010;20: 48–61. [DOI] [PubMed] [Google Scholar]
- 48.Burnouf T, Goubran HA, Radosevich M, et al. A minipool process for solvent-detergent treatment of cryoprecipitate at blood centres using a disposable bag system. Vox Sang 2006;91: 56–62. [DOI] [PubMed] [Google Scholar]
- 49.Pitman JP, Wilkinson R, Basavaraju SV, et al. Investments in blood safety improve the availability of blood to underserved areas in a sub-Saharan African country. ISBT Sci Ser 2014;9: 325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Makani J, Lyimo M, Magesa P, et al. Strengthening medical education in haematology and blood transfusion: postgraduate programmes in Tanzania. Br J Haematol 2017;177: 838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kesinger MR, Nagy LR, Sequeira DJ, et al. A standardized trauma care protocol decreased in-hospital mortality of patients with severe traumatic brain injury at a teaching hospital in a middle-income country. Injury 2014;45: 1350–4. [DOI] [PubMed] [Google Scholar]
- 52.van den Berg K, Murphy EL, Pretorius L, et al. The impact of HIV-associated anaemia on the incidence of red blood cell transfusion: implications for blood services in HIV-endemic countries. Transfus Apher Sci 2014;51: 10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hill SR, Carless PA, Henry DA, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion 2010. October 6;(10):CD002042. doi: 10.1002/14651858.CD002042.pub2. [DOI] [PubMed] [Google Scholar]
- 54.Yang WW, Thakkar RN, Gehrie EA, et al. Single-unit transfusions and hemoglobin trigger: relative impact on red cell utilization. Transfusion 2017;57: 1163–70. [DOI] [PubMed] [Google Scholar]
- 55.Eichbaum Q, Murphy M, Liu Y, et al. Patient Blood Management: An International Perspective. Anesth Analg 2016;123: 1574–81. [DOI] [PubMed] [Google Scholar]
- 56.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388: 1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388: 1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarkodie F, Hassall O, Owusu-Dabo E, et al. Improving the screening of blood donors with syphilis rapid diagnostic test (RDT) and rapid plasma reagin (RPR) in low-and middle-income countries (LMIC). Transfus Med 2017;27: 52–9. [DOI] [PubMed] [Google Scholar]
- 59.Custer B, Janssen MP, Alliance of Blood Operators Risk-Based Decision-Making I. Health economics and outcomes methods in risk-based decision-making for blood safety. Transfusion 2015;55: 2039–47. [DOI] [PubMed] [Google Scholar]
- 60.Eichbaum Q, Shan H, Goncalez TT, et al. Global health and transfusion medicine: education and training in developing countries. Transfusion 2014;54: 1893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murphy EL, McFarland W, Lefrere JJ. Teaching transfusion medicine research methods in the developing world. Transfusion 2009;49: 1532–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tagny CT, Murphy EL, Lefrere JJ, et al. The Francophone Africa Blood Transfusion Research Network: a five-year report (2007–2012). Transfus Med 2013;23: 442–4. [DOI] [PubMed] [Google Scholar]
- 63.ISBT Academy. ISBT Training in Research for Young InvesTigators (I TRY IT) [monograph on the internet] Amsterdam, the Netherlands: ISBT Central Office; 2018. Available from: http://www.isbtweb.org/fileadmin/user_upload/TTID_WP_YI.pdf. Access date: 22 Jan 2018. [Google Scholar]


