Abstract
Objective
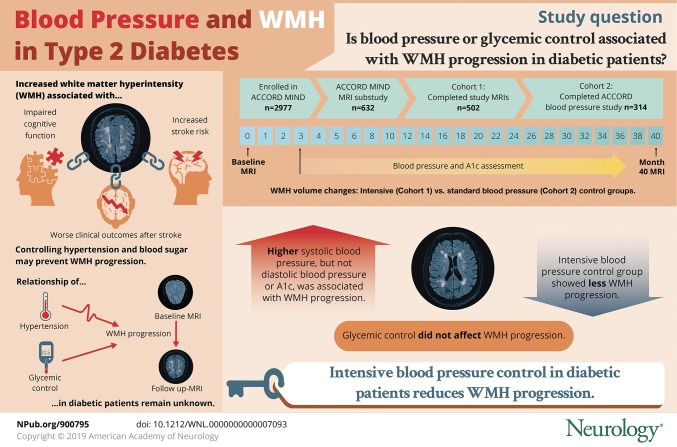
To determine whether higher blood pressure mean (BPM) or hemoglobin A1c is associated with progression of white matter hyperintensity (WMH) on MRI in patients with type 2 diabetes, and whether intensive blood pressure or glycemic control can reduce that progression.
Methods
We performed a secondary analysis of the Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes (ACCORD MIND) research materials. The primary outcome is change in WMH volume (ΔWMH) between a baseline and month-40 MRI, and the primary predictor is BPM and A1c between the MRIs. Additional analyses compared ΔWMH in the intensive vs standard glycemic control randomization arms (n = 502) and intensive vs standard blood pressure control randomization arms (n = 314).
Results
Higher systolic BPM, but not diastolic BPM or A1c, was associated with WMH progression. The ΔWMH in tertiles of increasing systolic BPM (115 ± 4, 127 ± 3, and 139 ± 6 mm Hg) was 0.7, 0.9, and 1.2 cm3 (p < 0.001). ΔWMH was lower in the intensive vs standard blood pressure control randomization arm (ΔWMH = 0.67 ± 0.95 vs 1.16 ± 1.13 cm3, p < 0.001), but there was no difference in the glycemic control arms (p = 0.917).
Conclusion
In ACCORD MIND, higher systolic blood pressure was associated with WMH progression. The intensive blood pressure control intervention reduced this progression. Comorbid diabetes and hypertension has synergistic deleterious properties that increase the risk of micro- and macrovascular complications. These results provide further support for an aggressive approach to blood pressure control in type 2 diabetics.
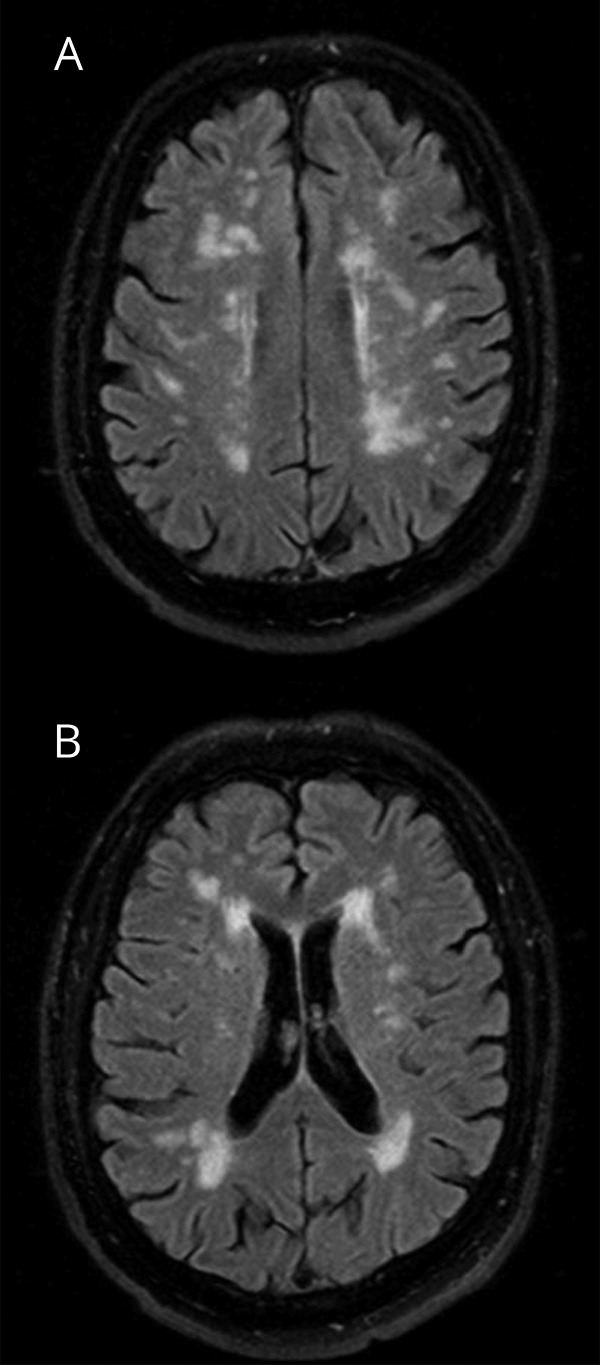
White matter hyperintensity (WMH), also referred to as leukoaraiosis, is a neuroimaging finding detected on a fluid-attenuated inversion recovery MRI sequence and is most often attributed to cerebral small vessel disease (figure 1).1–5 WMH burden can be measured with visual qualitative scales,6 but the preferred methodology is algorithmic quantification of WMH volume.7 The pathophysiology of WMH is not entirely understood, but the best clinical predictors of WMH burden are advanced age and hypertension.1,8–11 A larger WMH burden is independently associated with impaired cognitive function, higher risk of both ischemic and hemorrhagic stroke, and worse clinical outcome after stroke.12–14 Longitudinal observational studies show that uncontrolled hypertension and smoking are associated with the progression of WMH burden,15–17 and effective control of hypertension can prevent WMH progression.18,19
Figure 1. Axial fluid-attenuated inversion recovery images show characteristic scattered subcortical (A) and periventricular MRI hyperintensities (B) in a patient with a moderate burden of white matter hyperintensities.
The relationship between diabetes and WMH burden is controversial,20–23 but the effect of intensive glycemic control on WMH progression is unknown. Furthermore, hypertension is common in diabetics, and when comorbid, diabetes and hypertension have synergistic deleterious properties that increase the risk of both micro- and macrovascular complications.24–26 Prior studies of WMH progression have not evaluated the effect of hypertension or glucose control in patients with diabetes mellitus. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial was a double 2-by-2 factorial, parallel-group, randomized trial of patients aged 45 to 79 years with type 2 diabetes mellitus, elevated hemoglobin A1c concentration (>7.5%), and a high risk of cardiovascular disease events suggested by significant atherosclerosis, albuminuria, left ventricular hypertrophy, or at least 2 additional risk factors.27,28 Patients were first randomized to intensive (target A1c <6.0%) or standard glycemic control (target A1c 7.0%–7.9%) and then, if they qualified, to multiple lipid management or blood pressure control strategies. Patients in the ACCORD Memory in Diabetes (MIND) ancillary trial had an MRI at baseline and again at 40 months from enrollment.29–31
The primary objectives of our study were to determine whether (1) patients in ACCORD MIND with higher systolic blood pressure mean (BPM) or A1c had more WMH progression and (2) patients randomized to the intensive blood pressure or glycemic control randomization arm had less WMH progression. As a secondary objective, we sought to determine whether higher visit-to-visit systolic blood pressure variability (BPV) could also predict WMH progression. In prior research, BPV has been associated with a higher risk of stroke, coronary artery disease, and chronic kidney disease.32–34 However, the reported effects of BPV on WMH progression are controversial.35,36 ACCORD MIND offers unique advantages to examine these hypotheses, including a large sample size, an extended follow-up period between study MRIs, and a standardized MRI protocol with semiautomated quantitative volumetric measurement of WMH.
Methods
Standard protocol approvals, registrations, and patient consents
This is a secondary analysis of the ACCORD research materials, a deidentified publicly available dataset. With a local institutional review board waiver, we obtained the dataset from the National Heart, Lung, and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center.
Study population and outcomes
Our study has 2 cohorts (figure 2). The first cohort (n = 502) consists of patients enrolled in ACCORD MIND who had both study MRIs (baseline and month 40) and were randomized in the glycemic control trial. The second cohort (n = 314) is a subset of the first, limited to patients who were secondarily randomized to intensive blood pressure control (systolic target <120 mm Hg) or standard blood pressure control (systolic target <140 mm Hg). Patients were randomized to the glycemic or blood pressure control study arms prior to recruitment for the ACCORD MIND MRI substudy.
Figure 2. (A) Flowchart of the 2 study cohorts, and (B) study timeline showing both study MRIs (baseline and month 40) and blood pressure ascertainment period between the study MRIs.
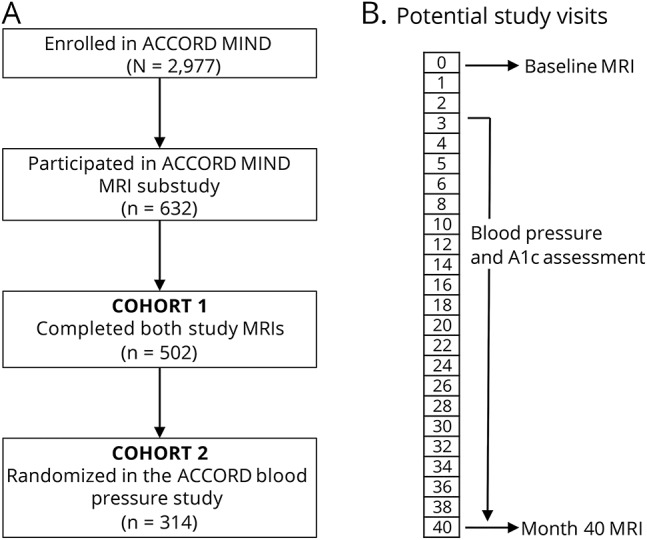
ACCORD = Action to Control Cardiovascular Risk in Diabetes; MIND = Memory in Diabetes.
The primary outcome of our study is progression of WMH volume (ΔWMH) between the ACCORD MIND baseline and month-40 MRI (figure 2). The MRI parameters and quality-control procedures used in ACCORD MIND have been previously described in detail.29,37 The fluid-attenuated inversion recovery sequence parameters were as follows: repetition time 8,000 milliseconds (ms); inversion time 2,000 ms; echo time 100 ms; and voxel size 3.0 × 0.9 × 0.9 mm. Image quality was monitored according to the American College of Radiology's MRI Quality Control Program (acr.org/accreditation/mri.aspx). WMH volume was measured in cubic centimeters using a validated algorithmic approach.38,39 We derived ΔWMH by subtracting the WMH volume on the baseline MRI from WMH volume on the month-40 MRI. A minority of patients had a negative value for ΔWMH. This may have been related to differences in technique between MRI scans and/or regression of WMH burden, which has been previously described.40–42
Statistical methods
To reduce confounding from the initial blood pressure reduction in the trial, only blood pressures collected between the study visit 3 months after randomization and the month-40 visit were used for calculations. BPM was calculated as the mean of all available systolic and diastolic blood pressure readings. BPV was evaluated with standard deviation, coefficient of variation, and average real variability, which we chose based on prior literature advocating multiple approaches for BPV evaluation.43 The formulas for BPV calculation are: SD =  ; CV =
; CV =  ; and ARV =
; and ARV =  . Likewise, A1c values were included from the study visit 3 months after randomization to the month-40 visit to calculate the mean A1c.
. Likewise, A1c values were included from the study visit 3 months after randomization to the month-40 visit to calculate the mean A1c.
For cohort 1, we investigated the clinical predictors of baseline WMH volume at trial enrollment by fitting unadjusted linear regression to baseline WMH volume in cubic centimeters. We evaluated the relationship between the predictors (BPM, randomization arms, and BPV) and ΔWMH using linear regression models. We also examined tertiles of BPM and BPV in association with ΔWMH and used analysis of variance to test for significance. For cohort 2, we compared characteristics between patients randomized to intensive or standard blood pressure control with the χ2 test for categorical variables and Student t test for continuous variables. We fit multivariate linear regression models to ΔWMH. Model 1 was adjusted for covariates chosen with backward selection set to a threshold of p value <0.1 and model 2 was adjusted for the same variables in model 1 as well as variables expected to influence ΔWMH based on prior research. We conducted sensitivity analyses that (1) adjusted ΔWMH for the intrapatient total brain volume, (2) excluded the outliers of ΔWMH data (top and bottom 10%), or (3) was restricted to patients with a baseline WMH volume of ≥1 cm3. Stata 15.1 (StataCorp LLC, College Station, TX) was used for all analyses with statistical significance defined as a 2-sided p value <0.05.
Data availability
The data in this study are publically available at biolincc.nhlbi.nih.gov/studies/accord/?q=accord.
Results
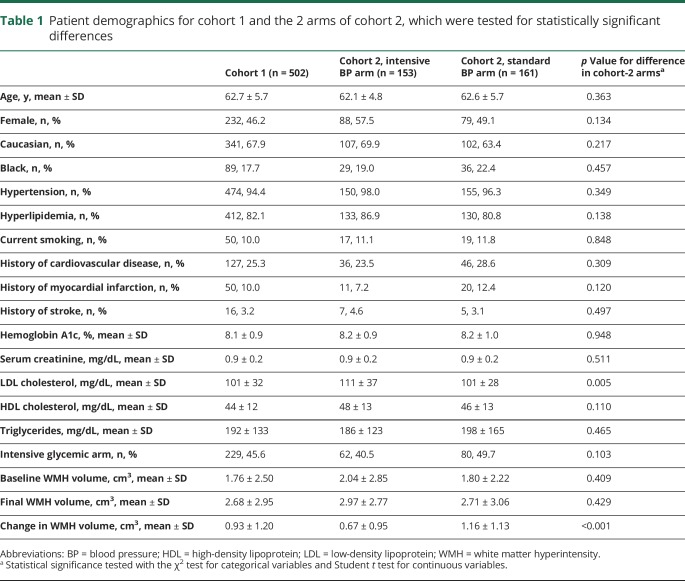
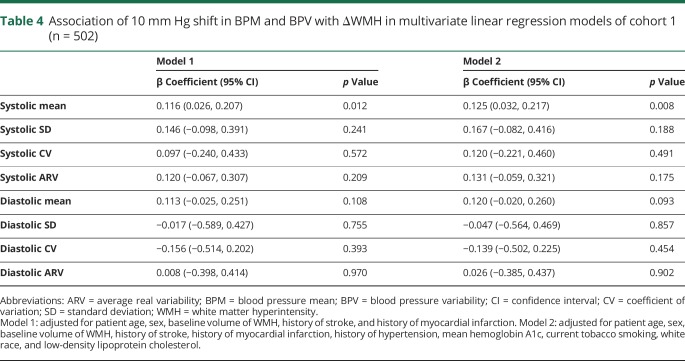
The demographics of the 2 cohorts are shown in table 1. The number of blood pressure readings per patient (mean ± SD) was 13 ± 5 in cohort 1 and 14 ± 5 in cohort 2. The absolute values of BPM and BPV for both cohorts are shown in table 2. For cohort 1 (n = 502), the predictors of baseline WMH volume were patient age, history of hypertension, history of cardiovascular disease, stroke, or myocardial infarction, and serum creatinine level (table 3). Between the baseline and 40-month MRI, 420/502 (84%) had WMH progression, 61/502 (12%) had stable WMH, and 21/502 (4%) had WMH regression. The mean A1c during ACCORD was not associated with ΔWMH (coefficient = −0.04, 95% confidence interval [CI] = −0.15, 0.08, p = 0.540). Randomization to intensive or standard glycemic control was not associated with ΔWMH (standard glycemic control arm ΔWMH = 0.92 ± 1.31 cm3, intensive glycemic control ΔWMH = 0.93 ± 1.06 cm3, p = 0.917), and the interaction term between the blood pressure and glucose control randomization arms was not significant (p = 0.523). Systolic BPM, but not diastolic BPM or any measures of BPV, was associated with ΔWMH (table 4). The mean ΔWMH in the tertiles of increasing systolic BPM was 0.7, 0.9, and 1.2 cm3 (ptrend < 0.001) for corresponding systolic BPMs of 115 ± 4, 127 ± 3, and 139 ± 6 mm Hg.
Table 1.
Patient demographics for cohort 1 and the 2 arms of cohort 2, which were tested for statistically significant differences
Table 2.
Factors associated with baseline WMH burden in cohort 1 (n = 502), determined by linear regression fit to the outcome of baseline WMH volume in cubic centimeters
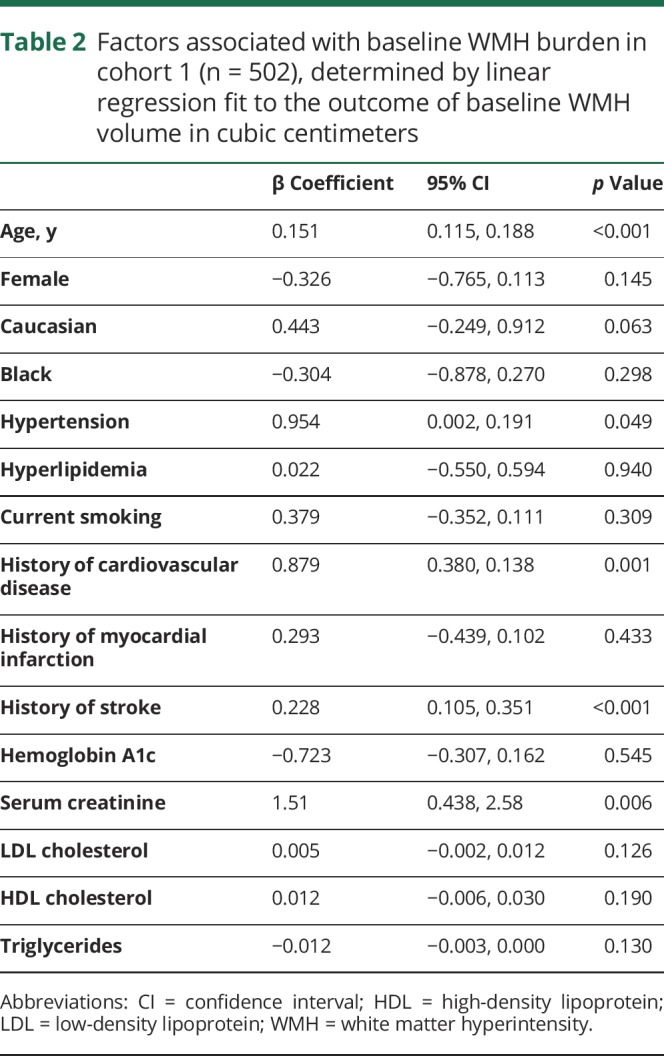
Table 3.
BPM and BPV in both study cohorts for systolic and diastolic blood pressure
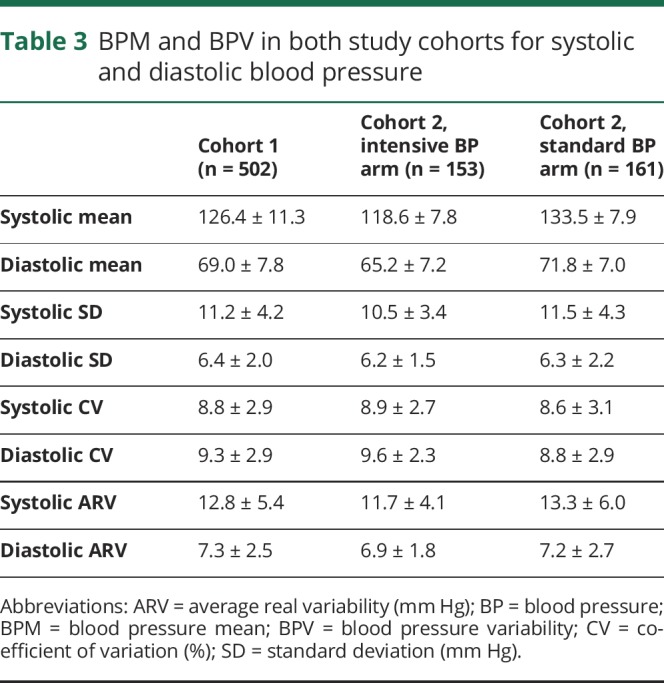
Table 4.
Association of 10 mm Hg shift in BPM and BPV with ΔWMH in multivariate linear regression models of cohort 1 (n = 502)
In cohort 2 (n = 314), 153/314 (49%) were randomized to the intensive blood pressure control arm. There was a 15 mm Hg difference in the systolic BPM between the 2 arms (intensive arm BPM = 118.6 ± 7.8 mm Hg, standard arm BPM = 133.5 ± 7.9 mm Hg, p < 0.001). ΔWMH was lower in the intensive blood pressure control arm (intensive arm ΔWMH = 0.67 ± 0.95 cm3, standard arm ΔWMH = 1.16 ± 1.13 cm3, p < 0.001). There was a strong relationship between ΔWMH and the blood pressure randomization arm (coefficient = −0.50, 95% CI = −0.73, −0.27, p < 0.001), which remained significant after adjusting for patient age, sex, baseline WMH volume, history of stroke, serum creatinine, and the glycemic control randomization arm (coefficient = −0.46, 95% CI = −0.69, −0.23, p < 0.001).
Discussion
Higher mean systolic blood pressure is associated with progression of WMH over 40 months in patients with type 2 diabetes and a high risk of cardiovascular events. Furthermore, patients who were randomized to the intensive blood pressure control arm had WMH progression that was nearly half that of patients randomized to standard blood pressure control (0.67 vs 1.16 cm3). We did not find that glycemic control was associated with WMH progression or that it interacted with blood pressure control. Similar to prior research, the baseline WMH burden was associated with advancing patient age and hypertension, but also a history of cardiovascular disease or stroke and the level of serum creatinine.
These results are consistent with the Three-City (3C)–Dijon Magnetic Resonance Imaging Study and the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy, which found that with more successful blood pressure control, patients had reduced progression of WMH over a 36-month period.18,19 A substudy of the PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes) trial failed to reach this conclusion but had notable weaknesses, including a shorter follow-up period and the study's dichotomization of patients into telmisartan vs placebo cohorts.44 This resulted in a negligible difference (3 mm Hg) in systolic blood pressure between the cohorts, compared to 11 mm Hg for the PROGRESS substudy and 15 mm Hg in our analysis of ACCORD MIND.
The effect size of intensive blood pressure control on WMH progression in this cohort is similar to Systolic Blood Pressure Intervention Trial Memory and Cognition in Decreased Hypertension (SPRINT-MIND), which was recently presented, but has not yet been published.45 In the SPRINT-MIND intensive blood pressure control arm, WMH volume increased by 0.28 vs 0.92 cm3 in the standard blood pressure control arm (p = 0.004). Several differences should be highlighted between our analysis and the SPRINT-MIND data. The first is that the follow-up MRIs were performed at 48 months, instead of the 40-month interval in ACCORD, and despite longer follow-up, the WMH progression in SPRINT-MIND was less than in ACCORD patients. This supports the proposition that hypertension and diabetes have a synergistic negative effect on WMH progression. Although our data did not find that ACCORD's intensive glucose control intervention reduced WMH progression, we do find that the intensive blood pressure control attenuates progression. Thus, our analysis supports the benefit of blood pressure reduction in the higher risk group of diabetic patients.
Unlike several prior studies, we did not find an association between visit-to-visit BPV and WMH progression. Two of the studies that reported this association relied on blood pressure readings from only 3 study visits,46,47 while patients in our analysis had a minimum of 6 visits and a mean of 13 for cohort 1 and 14 for cohort 2. The statistical measurement of variability improves with the number of available data, and these prior studies may have found an erroneous association attributable to undersampling. Indeed, a study of 584 patients with prior stroke that had between 12 and 18 blood pressure measurements per patient failed to find a link between BPV and WMH progression.48 Nonetheless, a prospective randomized trial is necessary to fully disprove the association.
This study has several strengths. The ACCORD and ACCORD MIND trials offer exceptionally high-quality data and a large sample size with a long period of follow-up between study MRIs. The standardized MRI protocol and semiautomated quantitative volumetric measurement of WMH, which is the preferred methodology, are unique advantages that have not been available to prior studies. The ability to focus specifically on patients with diabetes and a high risk of cardiovascular events is novel and should help guide future studies focusing on delaying the progression of WMH in patients at the highest risk of progression. The sensitivity analyses did not change the conclusions of our study (data not shown), thus further reinforcing our findings. The weaknesses of our study include a potential selection bias because patients voluntarily agreed to participate in the ACCORD MIND MRI substudy, residual confounding in our multivariate regression models, a limited number of blood pressure readings to evaluate mean and variability, and blood pressure measurements from office visits only. Future studies should consider ambulatory blood pressure readings, which may be a more accurate predictor of WMH progression.49 High-resolution volumetric MRI sequences could also be used to more precisely evaluate WMH volume progression, accurately determine localization, and define the relationship to changes in perivascular spaces and overall parenchymal volume.
Our finding that higher systolic blood pressure was associated with WMH progression in diabetics, and that progression was attenuated by an intensive blood pressure control intervention, provides further support for an aggressive approach to blood pressure control in type 2 diabetics.
Acknowledgment
This article was prepared using ACCORD Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of ACCORD or the NHLBI.
Glossary
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- BPM
blood pressure mean
- BPV
blood pressure variability
- CI
confidence interval
- MIND
Memory in Diabetes
- NHLBI
National Heart, Lung, and Blood Institute
- PROGRESS
Perindopril Protection Against Recurrent Stroke Study
- SPRINT
Systolic Blood Pressure Intervention Trial
- WMH
white matter hyperintensity
Author contributions
A.d.H and N.S.R. were responsible for study design, manuscript drafting, and manuscript editing. Ad.H. and G.S. were responsible for statistical analysis. D.L.T., J.J.M., and J.S.M. were responsible for manuscript drafting and editing.
Study funding
Research reported in this publication was supported by an American Heart Association Scientist Development Grant 17SDG33670114 (Dr. de Havenon). The content is solely the responsibility of the authors and does not represent the official views of the American Heart Association. Dr. Rost is in part supported by NIH-NINDS grants R01NS082285 and R01NS086905.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Basile AM, Pantoni L, Pracucci G, et al. Age, hypertension, and lacunar stroke are the major determinants of the severity of age-related white matter changes. Cerebrovasc Dis 2006;21:315–322. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung HP. MRI white matter hyperintensities: three-year follow-up of the Austrian Stroke Prevention Study. Neurology 1999;53:132–139. [DOI] [PubMed] [Google Scholar]
- 3.Liao D, Cooper L, Cai J, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control: the ARIC study. Stroke 1996;27:2262–2270. [DOI] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Hernández MCV, Muñoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc 2015;4:e001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etherton MR, Wu O, Rost NS. Recent advances in leukoaraiosis: white matter structural integrity and functional outcomes after acute ischemic stroke. Curr Cardiol Rep 2016;18:123. [DOI] [PubMed] [Google Scholar]
- 6.Fazekas F, Barkhof F, Wahlund LO, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis 2002;13(suppl 2):31–36. [DOI] [PubMed] [Google Scholar]
- 7.Rost NS, Sadaghiani S, Biffi A, et al. Setting a gold standard for quantification of leukoaraiosis burden in patients with ischemic stroke: the Atherosclerosis Risk in Communities Study. J Neurosci Methods 2014;221:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habes M, Erus G, Toledo JB, et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 2016;139:1164–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Leeuw FE, de Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain J Neurol 2002;125:765–772. [DOI] [PubMed] [Google Scholar]
- 10.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001;70:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henskens LH, Kroon AA, van Oostenbrugge RJ, et al. Associations of ambulatory blood pressure levels with white matter hyperintensity volumes in hypertensive patients. J Hypertens 2009;27:1446–1452. [DOI] [PubMed] [Google Scholar]
- 12.Au R, Massaro JM, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol 2006;63:246–250. [DOI] [PubMed] [Google Scholar]
- 13.Rost NS, Rahman RM, Biffi A, et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology 2010;75:1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuller LH, Longstreth WT, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke 2004;35:1821–1825. [DOI] [PubMed] [Google Scholar]
- 15.Power MC, Deal JA, Sharrett AR, et al. Smoking and white matter hyperintensity progression: the ARIC-MRI Study. Neurology 2015;84:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottesman RF, Coresh J, Catellier DJ, et al. Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke 2010;41:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longstreth WT, Arnold AM, Beauchamp NJ, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 2005;36:56–61. [DOI] [PubMed] [Google Scholar]
- 18.Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) magnetic resonance imaging substudy. Circulation 2005;112:1644–1650. [DOI] [PubMed] [Google Scholar]
- 19.Godin O, Tzourio C, Maillard P, Mazoyer B, Dufouil C. Antihypertensive treatment and change in blood pressure are associated with the progression of white matter lesion volumes: the Three-City (3C)-Dijon Magnetic Resonance Imaging Study. Circulation 2011;123:266–273. [DOI] [PubMed] [Google Scholar]
- 20.van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes: a systematic review. Diabetes Care 2006;29:2539–2548. [DOI] [PubMed] [Google Scholar]
- 21.Longstreth WT, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke 1996;27:1274–1282. [DOI] [PubMed] [Google Scholar]
- 22.Moran C, Phan TG, Chen J, et al. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 2013;36:4036–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao D, Cooper L, Cai J, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology 1997;16:149–162. [DOI] [PubMed] [Google Scholar]
- 24.Song Y, Liu X, Zhu X, et al. Increasing trend of diabetes combined with hypertension or hypercholesterolemia: NHANES data analysis 1999–2012. Sci Rep 2016;6:36093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell DS. Hypertension and diabetes: a toxic combination. Endocr Pract 2008;14:1031–1039. [DOI] [PubMed] [Google Scholar]
- 26.Giunti S, Cooper M. Management strategies for patients with hypertension and diabetes: why combination therapy is critical. J Clin Hypertens 2006;8:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Launer LJ, Miller ME, Williamson JD, et al. Effects of randomization to intensive glucose lowering on brain structure and function in type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol 2011;10:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray AM, Hsu FC, Williamson JD, et al. ACCORDION MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia 2017;60:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson JD, Launer LJ, Bryan RN, et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med 2014;174:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancia G. Visit-to-visit blood pressure variability. Hypertension 2016;68:32–33. [DOI] [PubMed] [Google Scholar]
- 33.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010;375:895–905. [DOI] [PubMed] [Google Scholar]
- 34.Muntner P, Whittle J, Lynch AI, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure and mortality: a cohort study. Ann Intern Med 2015;163:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagai M, Kario K. Visit-to-visit blood pressure variability, silent cerebral injury, and risk of stroke. Am J Hypertens 2013;26:1369–1376. [DOI] [PubMed] [Google Scholar]
- 36.Gunstad J, Cohen RA, Tate DF, et al. Blood pressure variability and white matter hyperintensities in older adults with cardiovascular disease. Blood Press 2005;14:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williamson JD, Miller ME, Bryan RN, et al. The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study (ACCORD-MIND): rationale, design, and methods. Am J Cardiol 2007;99:112i–122i. [DOI] [PubMed] [Google Scholar]
- 38.Lao Z, Shen D, Liu D, et al. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol 2008;15:300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldszal AF, Davatzikos C, Pham DL, Yan MX, Bryan RN, Resnick SM. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr 1998;22:827–837. [DOI] [PubMed] [Google Scholar]
- 40.Adachi M, Sato T. Characterization of the growth of deep and subcortical white matter hyperintensity on MR imaging: a retrospective cohort study. Magn Reson Med Sci 2017;16:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wardlaw JM, Chappell FM, Valdés Hernández MC, et al. White matter hyperintensity reduction and outcomes after minor stroke. Neurology 2017;89:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho AH, Kim HR, Kim W, Yang DW. White matter hyperintensity in ischemic stroke patients: it may regress over time. J Stroke 2015;17:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manning LS, Rothwell PM, Potter JF, Robinson TG. Prognostic significance of short-term blood pressure variability in acute stroke systematic review. Stroke 2015;46:2482–2490. [DOI] [PubMed] [Google Scholar]
- 44.Weber R, Weimar C, Blatchford J, et al. Telmisartan on top of antihypertensive treatment does not prevent progression of cerebral white matter lesions in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) MRI substudy. Stroke 2012;43:2336–2342. [DOI] [PubMed] [Google Scholar]
- 45.National Institutes of Health. NIH-funded researchers present preliminary clinical trial results suggesting aggressive blood pressure control may lower risk of cognitive impairment [online]. 2018. Available at: nih.gov/news-events/news-releases/nih-funded-researchers-present-preliminary-clinical-trial-results-suggesting-aggressive-blood-pressure-control-may-lower-risk-cognitive-impairment. Accessed August 16, 2018.
- 46.Brickman AM, Reitz C, Luchsinger JA, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol 2010;67:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging Study. Stroke 2002;33:26–30. [DOI] [PubMed] [Google Scholar]
- 48.Liu W, Liu R, Sun W, et al. Different impacts of blood pressure variability on the progression of cerebral microbleeds and white matter lesions. Stroke 2012;43:2916–2922. [DOI] [PubMed] [Google Scholar]
- 49.White WB, Wolfson L, Wakefield DB, et al. Average daily blood pressure, not office blood pressure, is associated with progression of cerebrovascular disease and cognitive decline in older people. Circulation 2011;124:2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this study are publically available at biolincc.nhlbi.nih.gov/studies/accord/?q=accord.