Abstract
Background:
Individuals with Avoidant Restrictive Food Intake Disorder (ARFID) experience impairing health consequences from insufficient nutritional variety and/or quantity. Early medical conditions and/or somatic symptoms such as abdominal pain may lead some with ARFID to experience somatic sensations as aversive. As such, food avoidance may be part of a broader behavioral repertoire aimed at suppressing bodily sensations. Avoiding these necessary and informative signals (e.g. growls of hunger) may subvert the emergence of healthy self-awareness and self-regulation. Teaching children with ARFID to engage adaptively with bodily sensations may help decrease aversiveness, increase self-awareness, and increase approach behaviors.
Methods:
Drawing from interventions for panic disorder and irritable bowel syndrome, we developed an acceptance-based interoceptive exposure treatment for young children with ARFID, Feeling and Body Investigators (FBI)-ARFID Division. Using playful cartoons and developmentally sensitive exposures, we teach young children how to map interoceptive sensations onto meanings (e.g., emotions) and actions (e.g. if I feel nervous, I’ll hold someone’s hand).
Results:
We present a case study of a 4-year old child with lifelong poor appetite/food indifference.
Conclusions:
Some individuals with ARFID may avoid food to avoid internal sensations. Developmentally appropriate interoceptive exposures may decrease ARFID symptoms while increasing more general self-regulation skills.
Keywords: ARFID, interoception, exposure, treatment
Introduction
The diagnosis of Avoidant Restrictive Food Intake Disorder (ARFID) is broad by design: intending to capture food avoidance regardless of motivation or age, and meant to aid in the detection of conditions not captured by prior eating or feeding disorder diagnoses. Emerging research suggests that within ARFID there are diverse manifestations (e.g., sensory aversions) indexing a different pathophysiology and requiring distinct treatments (Thomas et al., 2017). Notwithstanding, one process that may be relevant for many individuals with ARFID is the experience of somatic/interoceptive or sensory sensations as aversive and the engagement in behaviors created to suppress these sensations. In support of this hypothesis, painful or unpleasant somatic sensations are common in children with ARFID (Cooney, Lieberman, Guimond, & Katzman, 2018; Sharp et al., 2013). For example, in a pilot treatment study for children with ARFID, 60% had gastroesophogeal reflux, a condition whereby pain follows eating (Sharp et al., 2016). Additionally, some uncomfortable somatic symptoms (e.g., constipation) may be a consequence of level of malnourishment. Sensitivity and aversive reactions to sensations from various parts of the digestive tract could contribute to food avoidance (e.g., oral sensitivity to taste/texture; esophageal sensitivity to feelings of gagging and choking; bloating from constipation contributing to aversive feelings of fullness). Importantly, whether or not the aversive experience of interoceptive and/or exteroceptive sensations contribute to disorder etiology, the presence of these sensations may complicate the course of the disorder, necessitating treatment.
Learning models of pain avoidance may inform ARFID treatment approaches (Vlaeyen & Linton, 2000). Theorists have long proposed that pain specifically, and unpleasant bodily sensations more generally, are salient and informative events (Cannon, 1915). Emotions, such as fear, as motivated response systems, would become associated with and co-occur with aversive sensations to help individuals learn to avoid noxious somatic events such as pain. That is, since pain is a danger signal, it would be adaptive for fear and pain to be linked. This process can become maladaptive, however, through classical and operant conditioning (Pavlov, 1957; Skinner, 1969). For example, if post-ingestive sensations of fullness are experienced as uncomfortable, then behaviors such as the premature termination of a meal prior to the establishment of satiety would be negatively reinforced as these behaviors help to avoid the aversive sensations of fullness - an example of operant conditioning. If sensations of hunger predict aversive sensations of fullness, then the neutral sensation of hunger can be classically conditioned to be experienced as aversive, increasing the chances of avoidance (see also Craske et al., 2011). This learning process is referred to as interoceptive conditioning: the conditioned fear of innocuous interoceptive cues because of their association with distress (Bouton, Mineka, & Barlow, 2001). Comparable learning frameworks can be applied to exteroceptive sensations such as taste and texture, whereby the individual avoids unpleasant oral sensations by restricting food variety.
Interoceptive exposure treatments developed for anxiety and gastrointestinal disorders may provide a relevant framework to address the sensation avoidance in ARFID. One hypothesis whereby interoceptive exposure treatments exert their influence is via inhibitory learning (Craske, Treanor, Conway, Zbozinek, & Vervliet, 2014). According to this model, exposures that intentionally provoke interoceptive sensations should be designed to disconfirm underlying expectancies of negative outcomes (e.g., I can’t handle pain; Craske et al., 2014). The degree to which these exposures more closely replicate the avoided experience (e.g., provoking a strong sensation) and create an expectancy violation (i.e., helping the individual to learn that outcomes differ from expectations), the more effective the interoceptive exposure.
In accordance with these models and mechanistic hypotheses, we developed an interoceptive and exteroceptive exposure treatment for young children with ARFID. Given that children with ARFID may exhibit sensitivity to interoceptive and exteroceptive sensations as a trait feature, we provided children with a framework whereby they can perceive these sensations with curiosity rather than fear, in order to learn to tolerate the sensations. Thus, the focus is on experiencing and exploring uncomfortable or aversive sensations rather than terminating them, in an acceptance-based framework. Further, since interoceptive sensations are informative and communicate needs such as hunger or fatigue or signal emotional experience, it was important to give children tools to help decipher their meaning and link these sensations to adaptive actions that meet particular needs.
This is particularly crucial given the age of onset of ARFID, often in early childhood. One of the fundamental tasks of healthy childhood development is learning to decipher and respond to one’s motivational and emotional needs; to map interoceptive states onto meaning and action: gut butterflies = anxiety = hold someone’s hand (Hietanen, Glerean, Hari, & Nummenmaa, 2016). The mapping process is the foundation of healthy self-awareness. The experience of sensations as aversive early in development may disrupt this mapping - preventing the emergence of self-awareness and leading to conditions and symptoms intended to mute bodily sensations (Zucker et al., 2017). Thus, an additional advantage of our approach is that it may aid in the emergence of self-awareness. Combined, our intention was to create a context whereby children could regard interoceptive and exteroceptive sensations with playful curiosity, thereby not allowing these sensations to interfere with ongoing experience while learning to decipher the meaning of sensations to guide adaptive actions.
Our resulting intervention is called Feeling and Body Investigators- ARFID Division, (FBI-ARFID). Children and parents are taught to be “FBI Agents,” body detectives who view body sensations as clues and whose job it is to figure out the message that interoceptive and exteroceptive sensations are trying to communicate. Each session follows a similar format, with modifications depending on motivations for food avoidance (see Table 1). In Session 1, children are provided a framework that they have “sensory superpowers...that make the world a more exciting and joyful place. However, these sensory superpowers also means that children feel things very deeply. These powers are a gift that they can learn to work with. In fact, their gifts of perception may even help others in need.” Children create a body map by having the parent trace their body. New things learned about the body are summarized on this map (Supplemental Materials). Sessions begin with homework review and additions to the body map. Next, parents read aloud workbook pages that teach about body sensations via the use of playful cartoon characters (e.g., Betty the Butterfly, Figure 1). Interoceptive exercises teach more about these characters/sensations (what leads to more butterflies - x or x?). A sample lesson resulting from interoceptive exposures are that bodily sensations prepare the body for action (Table 1). Correspondingly, interoceptive exercises are designed to teach something about the body, often in the form of an expectancy violation (see Case). After interoceptive/exteroceptive exercises, things learned about the body (or food) are added to the body map. A “Body Brainstorms” worksheet designed to facilitate generalization across contexts is provided and allows children to think about other experiences that create the same bodily sensations (e.g. increased heart rate). Finally, a decision tree worksheet helps children link sensations to meanings and actions (Figure 2).
Table 1.
Outline of Session Content
| Session # | Workbook | Body Investigation | Lesson | Worksheets/Practice | Decision Point |
|---|---|---|---|---|---|
| Session 1: Introduction to Feeling and Body Investigators (FBI) – ARFID Division | Henry Heartbeat Gassy Gus Samantha Sweat Betty Butterfly | Which of two activities makes our heart beat faster or slower? | My heart changes to meet my needs. | Body Brainstorms (e.g., activities outside that make your heart beat faster or slower, things that make you sweat); Pick 2 sensations and draw what you were doing in your FBI journal. |
|
| Session 2: The Eats a | Harold the Hunger Pain, Georgia the Gut Growler, Solomon Satisfied, Sabrina Stuffed | How many crackers are needed to move from Georgia to Solomon? (Compare parent’s guess with child’s guess.) | My body tells me if I’m hungry when I learn to listen. | Body Brainstorms (e.g., think of last meal you felt satisfied, last time you felt Harold); Learn Step 1 and 2 of Decision-Tree Worksheet (label and contextualize sensations). |
Does child need additional practice with hunger and fullness detection? a If yes, assess energy and refuel after body investigations every session. Energy monitoring added to FBI-ARFID worksheet |
| Session 3: The Noisies a | Gassy Gus, Victor Vomit, Gaggy Greg, Gordon Gotta Go | Can we guess the location of our gag reflex? | My body has a lot of safety strategies to help me. | ||
| Session 4: The Feels a | Betty Butterfly, Nauseous Ned, Julie Jitters, Ricky the Rock, Tommy Thunderbolt | How can we can get the most butterflies (e.g., sitting in the dark and telling scary stories, doing timed math problems)? | Butterflies help you get ready to face challenges. | Body Brainstorms (e.g., what gives you the butterflies? positive jitters?); Body Decision Tree Worksheet (all 5 steps from now on, with Hunger exercises if needed.) Body missions tailored to child’s needs, for example, increasing energy intake. | Does child need additional practice with sensory aversions to food? b If yes, exteroceptive sessions (Sessions 5a – 5d) focusing on Appearance (e.g., Lumpy Lucy); Smell (e.g., Floral Fanny); Taste (e.g., Spicy Sahib); Texture (e.g., Clingy Clara) –, following the same format (education, exposure, lesson summary, generalization, practice) |
| Sample Exteroceptive Session 5a: The Looks ab | Smooth Stephanie, Bright Bea, Flawed Frankie | Sample Food Investigation: Examine effects of changes in appearance on taste. | Flaws can be friendly and fascinating. | Food Brainstorms (e.g., find 10 foods that are bright red); Food Investigations Worksheet | |
| Session 6: The Ouches, Part 1 ab | Polly Pain, Patricia the Poop Pain, Brenda the Brain, Ella the Emotional Pain | How fast can we run with a belt tight around the tummy? (Food exposure as needed.) | Pain is a messenger to help you take care of yourself. | Body Brainstorms (e.g., last time you had poop pain, fun things to do on the toilet); Decision Tree Worksheet (and/or Combined FBI-ARFID Worksheet with Sensory Challenges.) |
Notes. For each session, we provide some examples of the characters that form the psychoeducation for each session. We also provide a sample interoceptive exercise and the new experience/lesson that may correspond with this exercise. For the various motivations for food avoidance in ARFID (e.g., indifference, sensory aversions), there are decision points in which different skills may need to be emphasized (e.g., more training in hunger awareness or repeated sessions with food exposures to address sensory aversions). Each session has interoceptive exposures to at least 2 body sensations.
If monitoring energy/hungry is problematic, checking in on energy and refueling becomes part of subsequent sessions. Monitoring hunger is also added to Decision-Tree worksheets.
If sensory aversions to food is problematic, food exposures are added as one of the exposures in subsequent sessions. However, each food exposure session also includes an interoceptive exposure exercise so the focus is not solely on food.
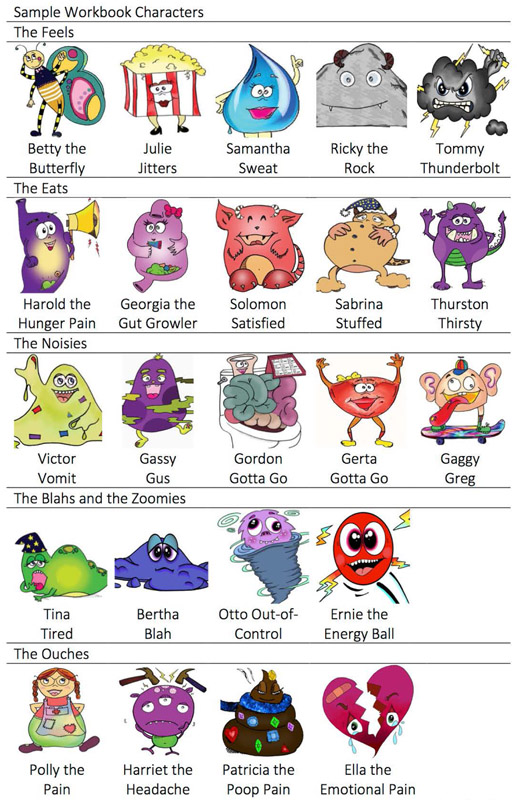
Figure 1. Sample body characters of the FBI-ARFID intervention.
Characters were used to portray different bodily sensations. A few new characters were learned each session. The program started with more basic sensations such as hunger and fullness and progressed on to sensations of emotional experience (for example, Ricky the rock, Betty butterfly, Julie jitters) and different forms of pain (for example, Patricia the poop pain, Harold the hunger pain).
Figure 2. Worksheet for the FBI-ARFID intervention.
Sections of the worksheet were added as the intervention progressed. The goal of the worksheet was to help children learn to understand the messages of bodily experiences and to respond to those messages in an investigative fashion. The intention was to teach children that bodily signals communicate needs and that if children listen to what their bodies’ are communicating, they will learn more about what they need, will get to know themselves better, and will learn to trust themselves and their bodies. Contact the first author for intervention materials (Nancy.Zucker@duke.edu).
FBI was originally developed for children from age 5 to up to age 10 years old with functional abdominal pain in a 10-session outpatient intervention (Zucker et al., 2017). However, as siblings attended and actively participated, we were able to pilot FBI-ARFID in children as young as 4 years old. Similarly, FBI-ARFID has been developed for children from age 4 and up to 10 years old. Thus, FBI-ARFID builds on the foundation of FBI, taking the original 10 sessions of FBI and adding sessions (approximately 5) for sensory food exposures. In addition, hunger/energy awareness is largely focused on in Session 2 of FBI. However, in FBI-ARFID, these skills are practiced every session beginning with Session 2 (see Table 1). Thus, FBI-ARFID contains the original 10 sessions of FBI plus 1–5 additional sessions (11–15 sessions total). Below we present a case with ARFID employing the FBI-ARFID treatment.
Case
Sophie (not the child’s real name) is a four-year-old female child, referred to an outpatient eating disorders program by a psychiatrist to help the child initiate eating independently so that her percutaneous endoscopic gastrostomy (PEG) tube, placed at 14 months of age, could be removed. The parents also identified issues related to their child’s generalized high arousal, including difficulty adjusting to changes in routine, and lack of responsiveness to attempts to soothe by others or self. Sophie had low birth weight (5 lbs. 12 oz.) and was induced at 39 6/7weeks. The pregnancy was classified as high risk at 28 weeks due to intrauterine growth restriction. Sophie was exclusively breast fed, and demonstrated poor appetite and growth since birth. The mother described a pattern in which the child would feed for short periods, fall sleep, and then wake up shortly afterwards, crying and distressed. Repeated doctor visits for weight checks resulted in no diagnosis. Moreover, since a younger sibling later exhibited similar signs of poor appetite and was diagnosed with a cow’s milk allergy, the mother expressed guilt that a cow’s milk allergy was undiagnosed and contributed to early aversive conditioning of feeding. Poor appetite persisted and a (PEG) tube was advised during a visit to a new pediatric gastrointestinal clinic. At time of referral, Sophie had achieved age-typical oral motor control and a healthy weight, but had not progressed otherwise during 3 years of medical treatment to increase food consumption, (e.g., occupational, speech, and pharmaco-therapy). This led to the conclusion that psychological barriers including chronic high emotional arousal, poor appetite sensing, and poor arousal regulation were contributing to food indifference and an ARFID diagnosis. Notably, Mirtazapine was initiated a month prior to the start of behavioral treatment for anxiety management.
An initial session was conducted with Sophie’s parents alone to discuss the treatment rationale. The next session proceeded as described above with a focus on sensations that are relatively easy for children to identify (Table 1). The first task was to see how fast we could get her heart to beat and to guess if running or skipping would be better. We then compared strategies (coloring on a whiteboard or walking very slowly) to see which would slow it down more. We added what we learned to the body map. Notably, Sophie seemed surprised and delighted that she could initiate actions that made her heart beat faster and that she could sense this change in heartbeat (Lesson: My body helps me to prepare for things). Subsequent sessions proceeded in the same format: doing a “Henry Heartbeat” investigation to manipulate arousal up and/or down; reviewing homework; adding things learned to the body map or drawing them; learning new characters; engaging in new investigations; and completion of body brainstorm worksheets and homework assignment. In Session 2, hunger and fullness were taught (e.g., Georgia the Gut Growler). Food was available at all subsequent sessions with children using these snacks to practice the ability sense hunger and current energy level. As an example of an expectancy violation, Sophie guessed how many mini-sandwiches would increase her energy, and then learned by checking in with her energy that it was more than she thought. Sophie measured changing energy by moving her hands further away from each other as bites were taken and energy increased, until she could flex her arm muscles, signaling she was energized. Sophie determined the amount of energy needed– even if a small amount. Food was prepared in a non-threatening way by using small portions. Sandwiches were cut into 1 cm squares with each piece being a “teeny tiny” sandwich that was served with “teeny tiny” glasses of milk, an approach that we employ across ages to attempt to increase self-efficacy regarding food approach.
After 2 sessions, quantity of food consumed improved. After 6 sessions, spontaneous requesting of snacks increased as did the capacity to manage distress more generally. The parents began gradually reducing the amount of supplemental feedings and stopped supplemental feeds approximately two months after treatment initiation. As a conservative measure, the PEG tube remained in place while the family welcomed a new child. In addition, there was a noted improvement in Sophie’s capacity to manage change, to enjoy and participate in preschool, and to respond to soothing by both self and others. Sessions occurred weekly for 8 sessions and bi-monthly for 4 sessions. At the conclusion, Sophie no longer met criteria for ARFID and was given the option to discharge from treatment. Nonetheless, the parents continued monthly maintenance therapy to learn skills of self-awareness, because they had a difficult time finding the appropriate treatment team, and because Sophie really enjoys treatment.
Formulation and Discussion
In considering diverse food motivations that contribute to ARFID, Sophie is best conceptualized as having indifference to food, lack of awareness of hunger, and fearful reactions to arousal. At four years old, she did not articulate any beliefs about fears of vomiting or other fears related to food. Rather, she has a nervous system seemingly very sensitive to change and one in which intense arousal impeded several areas of functioning: eating, attending preschool, and managing changes in routine. Conceivably, FBI-ARFID division may provide children with a playful framework to organize arousal so that internal sensations are no longer threatening. This can be conceptualized as a form of inhibitory learning: formerly the presence of high arousal and/or the experience of sensations that were unknown or uncertain resulted in immobilization around food and potentially, the threat of discomfort. In treatment, Sophie is having a new learning experience in which uncertain or intense sensations are no longer viewed as signals of impending threat but are viewed as clues to a mystery.
This case raises intriguing questions about the experience and impact of early nociceptive pain on the strength of visceral memories and subsequent aversive conditioning (Fitzgerald, 2015; Kassab, Hamadneh, Nuseir, B, & Hamadneh, 2018). Given the importance of pain and the recognition and avoidance of pain for survival, it is logical that pain may facilitate mechanisms to increase learning and memory (Vlaeyen & Linton, 2000). Thus, it is notable that Sophie experienced repeated events that may have resulted in aversive conditioning to food, to the process of eating, or to the experience of somatic sensations more generally. Such early conditioning stresses the importance of early intervention.
Sophie had been on Mirtazapine for a month prior to treatment. It is possible that the medication helped to facilitate (or was responsible for) treatment gains. There is little evidence for the use of psychopharmacologic agents in the treatment of ARFID, however, a case report supported the use of Mirtazapine to promote weight gain and improve gastric emptying while decreasing nausea and vomiting (Gray, Chen, Menzel, Schwartz, & Kaye, 2018). Additional limitations of the study include our lack of objective assessment of psychological outcomes. The behavioral observations of the parents and successful removal of the PEG tube help to minimize but not eliminate these concerns. Data on the initial FBI treatment established changes in negative affect, distress about somatic sensations, and pain with results of medium to large statistical and clinical effect in 24 children with functional abdominal pain (Zucker et al., 2017), with 12% meeting criteria for ARFID in a retrospective analysis. However, our conceptual model of visceral and oral sensitivity and the development of strategies to manage this sensitivity may not apply to all cases of ARFID.
In summary, we attempted to intervene in the process of aversive interoceptive conditioning by creating a context in which bodily sensations are viewed with interest and curiosity – training young children to be FBI Agents-ARFID division. By helping children to trust and feel safe in their own bodies, we hope to create a foundation that helps in the management of ARFID and related comorbid psychopathology and may help to prevent the emergence of later mental illness. Failing that, the treatment is simply a lot of fun.
Supplementary Material
Acknowledgements
We are grateful for the families who were willing to share their stories so more individuals could learn about ARFID and its treatment.
Funding source: All phases of the study were supported by the National Institute of Mental Health (R33-MH-097959) and the Duke Institute for Brain Sciences
Abbreviations:
- ARFID
Avoidant Restrictive Food Intake Disorder
- ADHD
Attention Deficit Hyperactivity Disorder
Footnotes
Financial disclosure: The authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of interest: None
Clinical trial registration: N/A
References
- Bouton ME, Mineka S, & Barlow DH (2001). A modern learning theory perspective on the etiology of panic disorder. Psychol Rev 108(1), 4–32. [DOI] [PubMed] [Google Scholar]
- Cannon WB (1915). Bodily changes in pain, hunger, fear and rage. New York: D. Appleton Company. [Google Scholar]
- Cooney M, Lieberman M, Guimond T, & Katzman DK (2018). Clinical and psychological features of children and adolescents diagnosed with avoidant/restrictive food intake disorder in a pediatric tertiary care eating disorder program: a descriptive study. J Eat Disord 6, 7. doi: 10.1186/s40337-018-0193-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Treanor M, Conway CC, Zbozinek T, & Vervliet B (2014). Maximizing exposure therapy: an inhibitory learning approach. Behav Res Ther 58, 10–23. doi: 10.1016/j.brat.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Wolitzky-Taylor KB, Labus J, Wu S, Frese M, Mayer EA, & Naliboff BD (2011). A cognitive-behavioral treatment for irritable bowel syndrome using interoceptive exposure to visceral sensations. Behav Res Ther 49(6–7), 413–421. doi: 10.1016/j.brat.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M (2015). What do we really know about newborn infant pain? Exp Physiol 100(12), 1451–1457. doi: 10.1113/ep085134 [DOI] [PubMed] [Google Scholar]
- Gray E, Chen T, Menzel J, Schwartz T, & Kaye WH (2018). Mirtazapine and Weight Gain in Avoidant and Restrictive Food Intake Disorder. J Am Acad Child Adolesc Psychiatry 57(4), 288–289. doi: 10.1016/j.jaac.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Hietanen JK, Glerean E, Hari R, & Nummenmaa L (2016). Bodily maps of emotions across child development. Dev Sci 19(6), 1111–1118. doi: 10.1111/desc.12389 [DOI] [PubMed] [Google Scholar]
- Kassab M, Hamadneh S, Nuseir K, B AL, & Hamadneh J (2018). Factors Associated With Infant Pain Severity Undergoing Immunization Injections. J Pediatr Nurs. doi: 10.1016/j.pedn.2018.04.002 [DOI] [PubMed] [Google Scholar]
- Pavlov I (1957). The Conditioned Reflex In Experimental Psychology (pp. 245–270): Philosophical Library [Google Scholar]
- Sharp WG, Berry RC, McCracken C, Nuhu NN, Marvel E, Saulnier CA, . . . Jaquess DL (2013). Feeding Problems and Nutrient Intake in Children with Autism Spectrum Disorders: A Meta-analysis and Comprehensive Review of the Literature. Journal of Autism and Developmental Disorders 43(9), 2159–2173. doi: 10.1007/s10803-013-1771-5 [DOI] [PubMed] [Google Scholar]
- Sharp WG, Stubbs KH, Adams H, Wells BM, Lesack RS, Criado KK, . . . Scahill LD (2016). Intensive, Manual-based Intervention for Pediatric Feeding Disorders: Results From a Randomized Pilot Trial. J Pediatr Gastroenterol Nutr 62(4), 658–663. doi: 10.1097/mpg.0000000000001043 [DOI] [PubMed] [Google Scholar]
- Skinner BF (1969). Operant Behavior In Contingencies of Reinforcment: A Theoretical Analysis Meredith Corporation (Reprinted from: B. F. Skinner Foundation Reprint Edition; ). [Google Scholar]
- Thomas JJ, Lawson EA, Micali N, Misra M, Deckersbach T, & Eddy KT (2017). Avoidant/Restrictive Food Intake Disorder: a Three-Dimensional Model of Neurobiology with Implications for Etiology and Treatment. Curr Psychiatry Rep 19(8), 54. doi: 10.1007/s11920-017-0795-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaeyen JW, & Linton SJ (2000). Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain 85(3), 317–332. [DOI] [PubMed] [Google Scholar]
- Zucker N, Mauro C, Craske M, Wagner HR, Datta N, Hopkins H, . . . Egger H(2017). Acceptance-based interoceptive exposure for young children with functional abdominal pain. Behav Res Ther 97, 200–212. doi: 10.1016/j.brat.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.