Abstract
Objective:
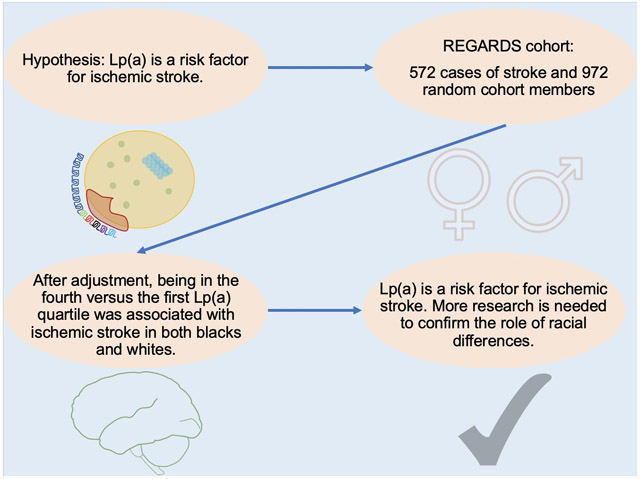
Increased lipoprotein (a) [Lp(a)] is associated with coronary heart disease risk, but links with stroke are less consistent. African-Americans (blacks) have higher Lp(a) levels and stroke incidence than Caucasians (whites) but have been under-represented in studies. We hypothesized that Lp(a) is a risk factor for ischemic stroke and that this risk differs by race.
Approach and Results:
REGARDS recruited 30,239 black and white U.S. adults aged ≥45 in 2003-7 to study regional and racial differences in stroke mortality. We measured Lp(a) by immunonephelometric assay in 572 cases of incident ischemic stroke and a 967-person cohort random sample. The hazard ratio (HR) of stroke by baseline Lp(a) was calculated using Cox proportional hazards models, stratified by race. Lp(a) was modeled in sex- and race-specific quartiles, given known differences in distributions by race and sex. Interactions were tested by including interaction terms in the proportional hazards models, with p <0.10 considered statistically significant.
After adjustment for age, sex, and stroke risk factors, being in the fourth versus the first Lp(a) quartile was weakly associated with ischemic stroke overall, HR 1.45 (95% CI 0.96, 2.19). In blacks the HR was 1.96 (95% CI 1.10, 3.46), while in whites this was 1.14 (95% CI 0.64, 2.04); p interaction 0.12. Lp(a) was lower in men than women, but sex associations were similar.
Conclusions:
We confirm that Lp(a) is a risk factor for ischemic stroke. Further research is needed to confirm the role of racial differences of the Lp(a) risk multiplier in ischemic stroke.
Keywords: Lipoprotein, race and ethnicity, stroke
Subject Codes: Biomarkers, epidemiology, ischemic stroke, race and ethnicity
Graphical Abstract
Introduction
Lipoprotein (a) [Lp(a)] is a cardiovascular risk factor that has been under intense investigation in recent years. Proposed mechanisms for its associated pro-atherosclerotic effects include a role in foam cell formation, promotion of cholesterol deposition into atherosclerotic plaques, and alterations in immunogenic responses.1, 2 Lp(a) levels are largely genetically determined and are thought to be only minimally affected by lifestyle.3 This suggests that there may be an inherent predisposition to cardiovascular disease and stroke in people with elevated Lp(a). Given its aforementioned properties, Lp(a) has been investigated as a risk factor for incident cardiovascular disease and stroke.
It is well established that stroke risk and mortality differs between African-American (blacks) and Caucasian (whites) patients in the United States.4 Alongside the conventional cardiovascular risk factors, such as low density lipoprotein cholesterol (LDL-C), there is emerging interest in identifying biomarkers that can help to explain racial differences in ischemic stroke risk.5, 6 Prior data from other large, population-based cohort studies has been conflicting on stroke, with some studies linking elevated Lp(a) levels to a higher incidence of ischemic stroke,7-10 whereas others have not found an association.11-13 This may be partly attributable to lack of differentiation between the subtypes of incident stroke13 or to racial or other differences in cohort composition. This may also be secondary to lack of adjustment for LDL-C levels, which is an important risk factor for stroke.5, 6
To address these limitations, we examined the association of Lp(a) levels with ischemic stroke in a large biracial population-based cohort study. We specifically investigated whether Lp(a) levels differed by race and gender and the role of Lp(a) as a predictor of future stroke events in blacks compared to whites and in men compared to women.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects
The Reasons for Geographic And Racial Differences in Stroke (REGARDS) cohort is well-described population-based cohort study that is evaluating racial and geographic differences in cardiovascular disease.14 The study participants included 30,239 men and women, age 45 and older that were enrolled over four years across the contiguous United States. Participants from the region known as the ‘stroke belt’ (the states of Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee) were oversampled, as were black participants with a goal of 50% in the stroke belt, 50% black, and 50% women. Computer-assisted telephone interviews were used to elicit verbal informed consent and information on demographics, medical history, and socioeconomic factors. Further data, such as electrocardiography, medication reconciliation, blood samples for laboratory examination, and written informed consent, was obtained via in-home examination at baseline. The Institutional Review Boards of each participating institution reviewed and approved the study methods.
Measurements and Definitions
Age, race, sex, smoking history, and prebaseline stroke were determined by self-report. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic pressure ≥90 mmHg or self-reported hypertension treated with antihypertensive medications. Diabetes mellitus was defined by fasting glucose >126 mg/dL, non-fasting glucose >200 mg/dL, or self-report with the use of antidiabetic medications. Atrial fibrillation was defined as self-report or presence on electrocardiogram (ECG). Prevalent cardiovascular disease was defined as self-reported myocardial infarction, coronary artery bypass, percutaneous coronary intervention, or myocardial infarction on ECG. Left ventricular hypertrophy was determined based on electrocardiogram criteria.
Blood was collected in the morning after an overnight fast and transported to a local laboratory where it was centrifuged and serum or plasma separated. Samples were shipped overnight on ice to the Laboratory for Clinical Biochemistry Research at the University of Vermont where they were re-centrifuged at 30,000xG and either analyzed or stored at −80ºC.15
Stroke Ascertainment
The primary outcome was first ischemic stroke through September 1, 2011. Incident stroke that occurred after study inclusion has been defined previously.16 Stroke occurrence was determined by contacting participants and/or proxies every 6 months via telephone and through review of medical records by two independent stroke expert physicians from the adjudication committee. All ischemic stroke events were adjudicated by an expert committee using pre-defined criteria.14 We classified strokes as ischemic or hemorrhagic then further defined the etiologic sub-types of ischemic stroke as large vessel disease, small vessel disease, cardioembolic, other, or unknown.
Case Cohort Sample
A case-cohort sample with a mean follow-up of 5.4 years was selected from the REGARDS cohort to evaluate the hypotheses. Cases were 572 participants without any baseline stroke who developed ischemic stroke during follow-up. The cohort random sample was selected from the entire cohort, details of which have been previously published.17 Briefly, the cohort random sample for this analysis consisted of 967 participants. They were selected using age (20% 45–54, 20% 55–64, 25% 65–74, 25% 75–84 and 10% ≥85), sex (50% women, 50% men), and race (50% black, 50% white) stratified sampling. Similar to the cases, participants with baseline stroke were excluded for this analysis.
Laboratory Methods
Cholesterol, high-density lipoprotein cholesterol (HDL-C) and triglycerides were measured in baseline serum samples as they arrived at the central laboratory using colorimetric reflectance spectrophotometry on the Ortho Vitros Clinical Chemistry System 950IRC instrument (Johnson & Johnson Clinical Diagnostics, Rochester, NY). Low density lipoprotein cholesterol was calculated by the Friedwald equation for participants with triglycerides < 400 mg/dl. The coefficients of variation for cholesterol, HDL and triglyceride were <2%, 7% and <2%, respectively. Lp(a) was measured in 2012 using baseline plasma retrieved from −80C storage that had been thawed 1-2 times prior to analysis. We used an automated particle enhanced immunonephelometric assay (N Latex Lp(a), Siemens Healthcare Diagnostics, Inc) in which polystyrene particles are coated with monoclonal antibodies to Lp(a) that agglutinate in the presence of antigen to cause an increase in the intensity of scattered light. The increase in scattered light is proportional to the amount of Lp(a) in the sample, as compared to a standard (N Latex Lp(a) Standard SY). The coefficient of variation was 2-5% at different concentrations.
Statistical Methods
Two models were created for proportional hazards regression in order to evaluate the relationship between Lp(a) and stroke. Model 1 included age, race, sex, and an age*race interaction term. Model 2 also included the Framingham stroke risk factors (atrial fibrillation, smoking, systolic blood pressure, prior cardiovascular disease, left ventricular hypertrophy, and use of any antihypertensive medication). For all modeling, gender- and race-specific quartiles were used for Lp(a) values. Hazard ratios for stroke comparing the stroke incidence in the second, third, and fourth quartiles, to incidence in the first quartile were calculated. Interactions were tested by including interaction terms in the proportional hazards models, with p <0.10 considered statistically significant. A type I error rate of 0.10 was chosen a priori for tests of interactions, recognizing that the increased power provided by this comes at the expense of increased risk of false positive findings. This is because we perceived a type II error to be more costly than a type I error in this area.18
Sensitivity Analyses
We conducted two sensitivity analyses. One was to determine the relationship of Lp(a) with ischemic stroke subtypes, with separate proportional hazards models fit for each stroke subtype. The second was to determine if the association of Lp(a) with stroke varied by baseline statin use, which was examined by including an Lp(a) by statin use interaction term in the models and by assessing if addition of statin use to the models attenuated the relationship between Lp(a) and stroke.
Results
Lipoprotein (a) values ranged from 1.0-217.0 milligrams per deciliter (mg/dl) in the cohort sample of 967 study participants. There were 242 black women, 236 black men, 246 white women, and 243 white men, in the cohort sample. Median (interquartile range) values for Lp(a) in the cohort random sample were 32.8 (16.8-55.0) mg/dl in black women, 26.7 (12.6-56.4) mg/dl for black men, 11.0 (3.5-39.5) mg/dl for white women, and 8.8 (3.3-30.6) mg/dl for white men (Table 1). In the highest quartile of Lp(a) concentrations, the mean (range) Lp(a) concentrations were 99.0 (72.4-125.6) mg/dl in black women, 72.2 (60-104.8) mg/dl in black men, 56.7 (49.6-75.3) mg/dl in white women, and 53.2 (39.5-61.7) mg/dl in white men.
Table 1:
Sex-Race Specific Lp(a) Quartile Values in the REGARDS Cohort Random Sample
| Group | Q1 N = 215 |
Q2 N = 241 |
Q3 N = 265 |
Q4 N = 246 |
|---|---|---|---|---|
| Lp(a), black men, mg/dl | <13 | 13-26 | 27-56 | ≥57 |
| Lp(a), black women, mg/dl | <17 | 17-32 | 33-55 | ≥56 |
| Lp(a), white men, mg/dl | <4 | 4-8 | 9-31 | ≥32 |
| Lp(a), white women, mg/dl | <4 | 4-10 | 11-39 | ≥40 |
Legend: N: number and Q: quartile.
Table 2 presents the baseline characteristics of the cohort sample participants based on race-sex specific Lp(a) quartiles, weighted to reflect the entire cohort of 26,242. The only factors that differed by Lp(a) quartile were the prevalence of dyslipidemia and mean low density LDL-C, with higher levels of both dyslipidemia and LDL-C noted with increasing Lp(a) quartiles.
Table 2:
Baseline Characteristics by Lp(a) Quartile in the Cohort Random Sample
| 1 | 2 | 3 | 4 | p value | |
|---|---|---|---|---|---|
| N | 215 | 241 | 265 | 246 | |
| Weighted N | 6108 | 6305 | 7178 | 6651 | |
| Age, mean (SD) | 63.5 (9.3) | 65.1 (9.4) | 65.1 (9.5) | 64.8 (9.0) | 0.06 |
| Atrial Fibrillation, % | 6.5% | 7.2% | 13.3% | 8.4% | 0.09 |
| Body Mass Index, mean (SD) | 29.1 (5.3) | 29.7 (6.5) | 28.8 (5.6) | 29.6 (6.2) | 0.73 |
| Current smoking, % | 20% | 12% | 12% | 11% | 0.09 |
| Diabetes Mellitus, % | 20% | 21% | 22% | 21% | 0.96 |
| Dyslipidemia, % | 56% | 56% | 54% | 69% | 0.01 |
| Stroke Belt Residence | 35.5% | 30.1% | 37.9% | 31.5% | 0.55 |
| HDL-C, mean (SD) mg/dL | 51 (19) | 51 (15) | 52 (16) | 52 (16) | 0.27 |
| Hypertension, % | 53% | 56% | 58% | 59% | 0.60 |
| Left Ventricular Hypertrophy, % | 9.0% | 6.8% | 6.2% | 8.4% | 0.74 |
| LDL-C, mean (SD) mg/dL | 111 (35) | 106 (33) | 113 (30) | 120 (34) | <0.001 |
| Prevalent CVD, % | 13% | 10% | 19% | 24% | 0.001 |
| Statin, % | 28% | 29% | 29% | 45% | 0.002 |
| SBP, mean (SD) mm Hg | 127 (16) | 130 (18) | 126 (17) | 126 (15) | 0.06 |
Legend: CVD: Cardiovascular disease; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; N: Number; SBP: Systolic blood pressure in millimeters of mercury; SD: Standard deviation.
P value represents test of the null hypothesis that the means/percentages are equivalent for the quartiles.
There were 572 incident ischemic stroke cases at median 5.4 years of follow-up (range 1 day to 8.5 years), with 239 strokes in black participants and 333 strokes in white participants. The median Lp(a) for the cases was 24.0 mg/dl (interquartile range 7.0-53 mg/dl). Of note, a total of 20 cohort sample participants had incident stroke during the follow up.
Amongst all cases and controls with ischemic stroke, subjects in the third quartile of Lp(a) quartile had a hazard ratio of 1.43 [95% Confidence Interval (CI): 1.01, 2.03] of having ischemic stroke compared to subjects in the first Lp(a) quartile after adjustment for age, race, and sex (Table 3). There was also a trend towards significance in the fourth quartile. After adjustment for age, sex, and stroke risk factors, being in the fourth versus the first Lp(a) quartile was weakly associated with ischemic stroke overall with a hazard ratio of 1.45 (95% CI: 0.96, 2.19). In the proportional hazards regression model 1, the black participants with Lp(a) in the fourth quartile compared to the first quartile had a hazard ratio of 1.74 (95% CI: 1.03, 2.94) for incident stroke (Table 3). There were no significant association between Lp(a) and incident stroke in white participants, although the interaction between Lp(a) quartiles and race was not statistically significant (p=0.37). After adjustment for the Framingham stroke risk factors in model 2, the association with stroke among black participants in the fourth quartile of Lp(a) was stronger with a hazard ratio of 1.96 (95% CI: 1.10, 3.46) compared to black participants with the Lp(a) levels in the lowest quartile (Table 3). There was no significant association between Lp(a) and incident stroke in white study participants in model 2 (interaction p value=0.12). There was no difference in the association of Lp(a) and incident stroke by sex (Table 3).
Table 3:
Association of Race- and Sex-Specific Lp(a) Quartiles with Incident Stroke
| First Quartile HR (95% CI) |
Second Quartile HR (95% CI) |
Third Quartile HR (95% CI) |
Fourth Quartile HR (95% CI) |
Interaction P value |
|
|---|---|---|---|---|---|
| All | |||||
| N cases | 99 | 114 | 174 | 160 | |
| N controls | 215 | 241 | 265 | 246 | |
| Model 1 | 1.0 (ref) | 1.05 (0.72, 1.53) | 1.43 (1.01, 2.03) | 1.38 (0.96, 1.98) | |
| Model 2 | 1.0 (ref) | 1.24 (0.81, 1.92) | 1.49 (0.98, 2.25) | 1.45 (0.96, 2.19) | |
| Blacks | |||||
| N Cases | 39 | 43 | 66 | 79 | |
| N Controls | 104 | 124 | 122 | 128 | |
| Model 1 | 1.0 (ref) | 0.90 (0.51, 1.59) | 1.47 (0.87, 2.49) | 1.74 (1.03, 2.94) | |
| Model 2 | 1.0 (ref) | 0.95 (0.50, 1.81) | 1.48 (0.82, 2.67) | 1.96 (1.10, 3.46) | |
| Whites | |||||
| N Cases | 60 | 71 | 108 | 81 | |
| N Controls | 111 | 117 | 143 | 118 | |
| Model 1 | 1.0 (ref) | 1.14 (0.70, 1.86) | 1.36 (0.86, 2.14) | 1.16 (0.72, 1.87) | |
| Model 2 | 1.0 (ref) | 1.59 (0.87, 2.90) | 1.39 (0.79, 2.47) | 1.14 (0.64, 2.04) | |
| Race Interaction, Model 1/2 | 0.37/0.12 | ||||
| Women | |||||
| N Cases | 49 | 57 | 86 | 73 | |
| N Controls | 107 | 117 | 137 | 127 | |
| Model 1 | 1.0 (ref) | 0.97 (0.58, 1.63) | 1.27 (0.78, 2.07) | 1.17 (0.71, 1.94) | |
| Model 2 | 1.0 (ref) | 1.10 (0.61, 2.01) | 1.32 (0.75, 2.31) | 1.23 (0.68, 2.21) | |
| Men | |||||
| N Cases | 50 | 57 | 88 | 87 | |
| N Controls | 108 | 124 | 128 | 119 | |
| Model 1 | 1.0 (ref) | 1.11 (0.66, 1.86) | 1.55 (0.95, 2.52) | 1.63 (0.99, 2.67) | |
| Model 2 | 1.0 (ref) | 1.33 (0.73, 2.42) | 1.61 (0.91, 2.86) | 1.62 (0.90, 2.92) | |
| Sex Interaction, Model 1/2 | 0.88/0.96 | ||||
| Black Women | |||||
| N Cases | 24 | 25 | 29 | 47 | |
| N Controls | 52 | 60 | 62 | 68 | |
| Model 1 | 1.0 (ref) | 0.94 (0.44, 1.99) | 1.12 (0.54, 2.32) | 1.55 (0.77, 3.13) | |
| Model 2 | 1.0 (ref) | 1.00 (0.40, 2.50) | 1.27 (0.56, 2.92) | 1.90 (0.86, 4.17) | |
| Black Men | |||||
| N Cases | 15 | 18 | 37 | 32 | |
| N Controls | 52 | 64 | 60 | 60 | |
| Model 1 | 1.0 (ref) | 0.98 (0.42, 2.28) | 2.21 (1.02, 4.77) | 2.17 (0.98, 4.78) | |
| Model 2 | 1.0 (ref) | 0.66 (0.23, 1.93) | 1.93 (0.81, 4.65) | 2.04 (0.79, 5.32) | |
| White Women | |||||
| N Cases | 25 | 32 | 57 | 26 | |
| N Controls | 55 | 57 | 75 | 59 | |
| Model 1 | 1.0 (ref) | 1.01 (0.49, 2.07) | 1.35 (0.69, 2.64) | 0.84 (0.40, 1.76) | |
| Model 2 | 1.0 (ref) | 1.79 (0.69, 4.67) | 1.31 (0.54, 3.20) | 0.78 (0.30, 2.02) | |
| White Men | |||||
| N Cases | 35 | 39 | 51 | 55 | |
| N Controls | 56 | 60 | 68 | 59 | |
| Model 1 | 1.0 (ref) | 1.22 (0.63, 2.38) | 1.28 (0.68, 2.39) | 1.41 (0.75, 2.65) | |
| Model 2 | 1.0 (ref) | 1.76 (0.75, 4.11) | 1.34 (0.61, 2.96) | 1.53 (0.64, 3.65) |
Hazard ratios are compared to those in the first quartile (reference).
Legend: CI = confidence interval; HR = hazard ratio; N = number; ref = reference.
Model 1. Adjusted for age, race, sex, and age*race. Interaction terms for Lp(a) quartiles, model 1: age=0.82, race=0.37, sex=0.88.
Model 2. Adjusted for age, sex, and Framingham stroke risk factors (atrial fibrillation, smoking, prior cardiovascular disease, left ventricular hypertrophy, and use of any antihypertensive medication). Interaction terms for Lp(a) quartiles: age=0.77, race=0.12, sex=0.96.
The sensitivity analysis by stroke subtype did not reveal a statistically significant association between elevated Lp(a) and specific ischemic stroke subtypes owing to small numbers (Table 4). However, the patterns of association suggested similar associations to overall stroke except that there was no association with small vessel subtype. Furthermore, there was no difference in the association of Lp(a) with stroke risk by statin use (interaction p value=0.37 for model 1 and p=0.29 for model 2). Also, the hazard ratios of stroke with higher Lp(a) were similar after adding statin use to the models (Supplemental Table 1).
Table 4:
Association of Race- and Sex-Specific Lp(a) Quartiles with Incident Ischemic Stroke-Subtypes
| HR Stroke | ||||
|---|---|---|---|---|
| Subtype | Second Quartile HR (95% CI) |
Third Quartile HR (95% CI) |
Fourth Quartile HR (95% CI) |
|
| Cardioembolic | N | |||
| All (107 cases) | ||||
| Model 1 | 1073 | 1.02 (0.50, 2.07) | 1.55 (0.82, 2.95) | 1.76 (0.93, 3.34) |
| Model 2 | 984 | 1.43 (0.61, 3.34) | 1.41 (0.64, 3.14) | 1.63 (0.73, 3.64) |
| Black (36 cases) | ||||
| Model 1 | 514 | 1.20 (0.38, 3.84) | 1.61 (0.53, 4.89) | 2.04 (0.70, 6.00) |
| Model 2 | 472 | 1.71 (0.42, 7.03) | 1.88 (0.49, 7.19) | 3.25 (0.92, 11.52) |
| White (71 cases) | ||||
| Model 1 | 559 | 0.92 (0.38, 2.25) | 1.49 (0.68, 3.25) | 1.65 (0.75, 3.64) |
| Model 2 | 512 | 1.18 (0.36, 3.89) | 0.98 (0.33, 2.87) | 1.02 (0.33, 3.16) |
| Large Vessel Disease | ||||
| All (74 cases) | ||||
| Model 1 | 1040 | 1.42 (0.61, 3.30) | 1.98 (0.90, 4.32) | 2.18 (1.00. 4.77) |
| Model 2 | 948 | 1.55 (0.62, 3.88) | 1.63 (0.69, 3.84) | 2.00 (0.84, 4.75) |
| Black (26 cases) | ||||
| Model 1 | 504 | 1.75 (0.41, 7.40) | 2.74 (0.70, 10.74) | 2.35 (0.57, 9.63) |
| Model 2 | 459 | 1.45 (0.29, 7.25) | 1.90 (0.39, 9.15) | 2.15 (0.44, 10.43) |
| White (48 cases) | ||||
| Model 1 | 536 | 1.29 (0.45, 3.71) | 1.69 (0.65, 4.39) | 2.09 (0.81, 5.39) |
| Model 2 | 489 | 1.81 (0.48, 6.74) | 1.47 (0.44, 4.97) | 2.30 (0.68, 7.75) |
| Small Vessel Disease | ||||
| All (85 cases) | ||||
| Model 1 | 1051 | 1.13 (0.57, 2.24) | 1.55 (0.82, 2.92) | 0.64 (0.30, 1.39) |
| Model 2 | 962 | 1.25 (0.56, 2.77) | 1.99 (0.99, 4.00) | 0.69 (0.30, 1.57) |
| Black (42 cases) | ||||
| Model 1 | 520 | 0.65 (0.23, 1.86) | 1.28 (0.50, 3.26) | 1.15 (0.44, 2.97) |
| Model 2 | 476 | 0.66 (0.18, 2.37) | 1.50 (0.53, 4.23) | 1.16 (0.41, 3.33) |
| White (43 cases) | ||||
| Model 1 | 531 | 1.66 (0.66, 4.18) | 1.64 (0.67, 4.04) | 0.10 (0.01, 0.86) |
| Model 2 | 486 | 2.75 (0.76, 9.95) | 2.58 (0.83, 7.97) | 0.15 (0.02, 1.39) |
| Other | ||||
| All (35 cases) | ||||
| Model 1 | 1001 | 2.18 (0.64, 7.38) | 2.37 (0.72, 7.76) | 2.46 (0.76, 8.02) |
| Model 2 | 913 | 2.30 (0.57, 9.31) | 2.15 (0.60, 7.72) | 1.98 (0.55, 7.07) |
| Black (14 cases)* | ||||
| Model 1 | 492 | -- | -- | -- |
| Model 2 | 450 | -- | -- | -- |
| White (21 cases) | ||||
| Model 1 | 509 | 0.99 (0.23, 4.24) | 1.40 (0.39, 5.10) | 1.30 (0.35, 4.82) |
| Model 2 | 463 | 0.81 (0.14, 4.91) | 1.34 (0.29, 6.19) | 1.11 (0.21, 5.79) |
| Unknown | ||||
| All (243 cases) | ||||
| Model 1 | 1209 | 0.87 (0.54, 1.39) | 1.18 (0.76, 1.83) | 1.27 (0.82, 1.98) |
| Model 2 | 1105 | 0.98 (0.57, 1.67) | 1.30 (0.79, 2.14) | 1.46 (0.88, 2.40) |
| Black (108 cases) | ||||
| Model 1 | 586 | 0.66 (0.32, 1.38) | 1.21 (0.63, 2.34) | 1.68 (0.89, 3.16) |
| Model 2 | 537 | 0.63 (0.27, 1.43) | 1.24 (0.60, 2.55) | 1.98 (1.00, 3.93) |
| White (135 cases) | ||||
| Model 1 | 623 | 1.03 (0.56, 1.90) | 1.12 (0.63, 1.99) | 1.01 (0.55, 1.84) |
| Model 2 | 568 | 1.45 (0.68, 3.11) | 1.33 (0.65, 2.69) | 1.10 (0.53, 2.27) |
Legend: CI = confidence interval; HR = hazard ratio; LVD = small vessel disease; N = number; SVD = small vessel disease.
Note: Other Black: 0 cases in Q1, so hazard ratio undefined.
Discussion
In this population-based cohort, Lp(a) concentration was higher in black participants than white participants and in women compared to men. Elevated Lp(a) was associated with an increased risk of ischemic stroke after adjustment for age, gender, and race. This trend was stronger for black than white participants, although the racial difference was not statistically significant. This relationship was preserved after adjustment for traditional stroke risk factors. Men had significantly lower Lp(a) values compared to women, but the association of Lp(a) with ischemic stroke did not differ between men and women. Finally, baseline statin use did not alter the association between Lp(a) and stroke, and Lp(a) was not differentially related to specific stroke subtypes, with the possible exception of no association with small vessel subtype.
There are many possible mechanistic explanations for our results. Lp(a) appears to accelerate atherogenesis.19 Mounting epidemiologic20, 21 and genetic data22 suggest a causal relationship between Lp(a) and stroke. This explains the stronger relationship between Lp(a) and stroke at higher Lp(a) concentrations that we observed in the analysis of all participants. The basis of racial differences in Lp(a) has been explored previously. There are well-recognized racial variations in the apolipoprotein (a) particle that makes up Lp(a). The size of apolipoprotein (a) size determines the size of Lp(a), which is it itself inversely correlated with overall concentrations of Lp(a).23 In a cohort from New York, black participants had smaller Lp(a) isoforms, a higher overall concentration of Lp(a), and there was a stronger association of Lp(a) with coronary artery disease on coronary angiography compared to white participants.24 Moreover, Lp(a) is increased by conditions like chronic kidney disease.25 This may further explain the racial difference in levels, as blacks have a higher incidence and prevalence of renal disease. However chronic kidney disease was not associated with stroke risk in REGARDS, so we did not consider it as a potential confounder. Additionally, non-whites have an acknowledged higher rate of statin non-adherence.26 Therefore the cardiovascular disease risk that high levels of Lp(a) confer in blacks may be associated with a failure to address other established cardiovascular risk factors due to a disparity in statin usage. We conducted a sensitivity analysis adjusting for baseline statin usage but did not find any evidence for mediation by baseline statin use in the hazards related to Lp(a).
We reiterated prior findings that Lp(a) levels are higher in blacks,1 in participants with cardiovascular disease,27 and in those with higher LDL-C concentrations.28 Prior data from other large, population-based cohort studies has been conflicting on stroke, with some studies linking elevated Lp(a) levels to a higher incidence of ischemic stroke,7-10 whereas others have not found an association.11-13 This may be partly attributable to lack of differentiation between the subtypes of incident stroke13 or to racial or other differences in cohort composition. This may also be secondary to confounding from LDL-C levels, which is an important risk factor for stroke.5, 6 We evaluated the association of Lp(a) concentration with stroke subtypes with inconclusive findings, but no association with the small vessel subtype. A study with a larger number of strokes would be needed to confirm this finding. To the best of our knowledge, most previous studies that have addressed the association of Lp(a) with stroke or subtypes in a study have not included a large number of black participants. Though the statistical testing for racial differences in association did not reach statistical significance in our investigation, we consider our findings to be of clinical significance. The Atherosclerosis Risk in Communities (ARIC) study cohort investigators have previously attempted to explore the relationship between Lp(a) and ischemic stroke. Ohira et al reported an association with ischemic stroke in black participants and white women but not white men.10 Our findings, which included 76 more stroke cases compared to ARIC, are consistent with Ohira et al concerning the relationship in black participants. Additionally, REGARDS had a wider geographic catchment area than the ARIC cohort did. Virani et al later reported 20-year follow up results on association of Lp(a) levels with the risk of both coronary heart disease events and ischemic stroke in the ARIC cohort.27 Virani et al confirmed the previous findings from the ARIC cohort that Lp(a) was associated with incident ischemic stroke in black participants.27 In this larger ARIC study with 663 ischemic stroke events and longer follow-up, the investigators did not find any differences by sex in association of Lp(a) with ischemic stroke. This is concordant with our results from REGARDS.
Our results have important clinical implications. We add to the growing body of literature from large, prospective cohort studies noting the correlation between Lp(a) and ischemic stroke. It is estimated that 1.5 billion people have Lp(a) greater than 50 mg/dl.29 Additionally, the effect of lifestyle and dietary changes on Lp(a) levels remain somewhat unclear.3 This suggests that Lp(a) warrants serious consideration as a cardiovascular risk factor and provides justification for study of direct targeting of levels. Its current use in prognostication of cardiovascular disease remains largely limited to specialist centers and clinical trials. Lipoprotein(a) has also gained a greater clinical presence with the publication of the 2018 multisociety cholesterol management guidelines, where Lp(a) levels greater than or equal to 50 mg/dL or 125 nmol/L were highlighted as an important risk modifier for patients with a history of atherosclerotic cardiovascular disease that is unexplained by major risk factors or a family history of atherosclerotic cardiovascular disease.30 This is pertinent to our investigation, as over 25% of subjects (particularly black subjects), had Lp(a) levels higher than this threshold. Along with this, the concomitant approval of two International Classification of Diseases, 10th edition diagnostic codes by the Centers of Disease Control and Surveillance for elevated Lp(a) may increase the usage of lipoprotein(a) testing in clinical practice.31 There is also a recognized heterogeneity in the measurement methods for Lp(a), with little consensus on a gold standard.19, 32 In terms of pharmacologic interventions, proprotein convertase subtilisin/kexin 9 inhibitors reduce Lp(a) to unprecedented levels.33 This therapeutic class offers an exciting option to address this stroke risk factor that is largely unaffected by statin therapy. Mipomersen and lopitamide are also potential therapeutic options targeting Lp(a).34 Finally, there is an antisense oligonucleotide in development that targets Lp(a).35
We acknowledge that our investigation has limitations. Cohort studies can be hindered by loss to follow-up. Cohort retention in REGARDS was excellent at 97.1% annually in whites and 96.2% annually in blacks, similar to other population-based cohort studies. The REGARDS cohort also did not include participants who were not white or black. However, the purpose of this cohort study was to examine the starkest racial disparity in stroke -- affecting blacks in the U.S. Although we observed differences by race in the relationship between Lp(a) and ischemic stroke, the interaction was not statistically significant.2 These findings should be confirmed in other studies or with longer follow up in REGARDS. The association between Lp(a) and ischemic stroke in other racial groups warrants further investigation. We acknowledge the wide variety of assays available for measuring Lp(a) (Supplemental Table 2). This may make it difficult to interpret study findings from studies using different methodology, and has been identified as an area that needs to be addressed further.19 A strength of the current study is use of a single automated assay with a low analytical coefficient of variation, which reduces imprecision in measurement. However, bias due to sample matrix effects in diluted samples cannot be ruled out. We also note that the hazard ratio for the cardioembolic stroke sub-type trended towards significance. Prior data suggests that Lp(a) may be associated with upregulation of the coagulation pathways and impaired fibrinolysis,36 which could be a predisposing factor for the formation of cardiac thrombi that may lead to cardioembolic stroke. Unfortunately, this was secondary analyses and our study power was limited to evaluate this association more fully, but further confirmation and investigation is warranted. It is important to recognize the different distribution of Lp(a) amongst whites and blacks. In whites, the distribution is highly skewed, whereas the Lp(a) distribution follows a more normal distribution in blacks.2 In this analysis we used race-specific quartile definitions so we could accurately determine the association of Lp(a) with stroke in blacks and whites separately.
In conclusion, elevated Lp(a) is an emerging risk factor for ischemic stroke. Elevated Lp(a) may also be associated with the racial disparity in stroke affecting blacks in the US, and thus may be a race-specific risk factor for ischemic stroke. More research is needed to confirm the racial differences in Lp(a) and to evaluate apo(a) isoforms in order to identify the role of Lp(a) in the prognostication and prevention of ischemic stroke.
Supplementary Material
Highlights.
In this population-based cohort study, lipoprotein (a) concentrations were higher in black participants than white participants.
Elevated lipoprotein (a) was associated with increased risk of ischemic stroke amongst all participants and this relationship may be stronger for black than white participants. Further research is needed to define racial differences in stroke propensity based on lipoprotein (a) levels.
Baseline statin usage did not alter the association between lipoprotein (a) levels and stroke.
Acknowledgements
We thank investigators, staff, and participants of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study for their valuable contributions. A full list of investigators and institutions can be found at http://www.regardsstudy.org.
Sources of Funding
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. KA was supported by National Heart, Lung, and Blood Institute (NHLBI) T32HL007594-27.
Abbreviations List
- CI
Confidence Interval
- Lp(a)
Lipoprotein (a)
- Mg/dl
Milligrams per Deciliter
- Nmol/L
Nanomoles per Liter
Footnotes
Disclosures and Conflicts of Interest
None
Presented in part at the Scientific Sessions of the American Heart Association, Orlando, FL, November 7-11, 2015.
References
- 1.Dube JB, Boffa MB, Hegele RA and Koschinsky ML. Lipoprotein(a): more interesting than ever after 50 years. Curr Opin Lipidol. 2012;23:133–40. [DOI] [PubMed] [Google Scholar]
- 2.Tsimikas S A Test in Context: Lipoprotein(a). Diagnosis, Prognosis, Controversies, and Emerging Therapies. J Am Coll Cardiol. 2017;69:692–711. [DOI] [PubMed] [Google Scholar]
- 3.Milionis HJ, Winder AF and Mikhailidis DP. Lipoprotein (a) and stroke. J Clin Pathol. 2000;53:487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, Goldstein LB, Gorelick PB, Howard G, Kittner SJ, Manolio TA, Whisnant JP and Wolf PA. American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Stroke. Risk factors. Stroke. 1997;28:1507–17. [DOI] [PubMed] [Google Scholar]
- 5.Anderson TJ, Gregoire J, Hegele RA, Couture P, Mancini GB, McPherson R, Francis GA, Poirier P, Lau DC, Grover S, Genest J Jr., Carpentier AC, Dufour R, Gupta M, Ward R, Leiter LA, Lonn E, Ng DS, Pearson GJ, Yates GM, Stone JA and Ur E. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29:151–67. [DOI] [PubMed] [Google Scholar]
- 6.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV and Tirschwell DL. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 7.Bostom AG, Gagnon DR, Cupples LA, Wilson PW, Jenner JL, Ordovas JM, Schaefer EJ and Castelli WP. A prospective investigation of elevated lipoprotein (a) detected by electrophoresis and cardiovascular disease in women. The Framingham Heart Study. Circulation. 1994;90:1688–95. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen TT, Ellefson RD, Hodge DO, Bailey KR, Kottke TE and Abu-Lebdeh HS. Predictive value of electrophoretically detected lipoprotein(a) for coronary heart disease and cerebrovascular disease in a community-based cohort of 9936 men and women. Circulation. 1997;96:1390–7. [DOI] [PubMed] [Google Scholar]
- 9.Ariyo AA, Thach C and Tracy R. Lp(a) lipoprotein, vascular disease, and mortality in the elderly. N Engl J Med. 2003;349:2108–15. [DOI] [PubMed] [Google Scholar]
- 10.Ohira T, Schreiner PJ, Morrisett JD, Chambless LE, Rosamond WD and Folsom AR. Lipoprotein(a) and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2006;37:1407–12. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Stampfer MJ and Hennekens CH. Plasma concentration of lipoprotein(a) and the risk of future stroke. Jama. 1995;273:1269–73. [PubMed] [Google Scholar]
- 12.Glader CA, Stegmayr B, Boman J, Stenlund H, Weinehall L, Hallmans G and Dahlen GH. Chlamydia pneumoniae antibodies and high lipoprotein(a) levels do not predict ischemic cerebral infarctions. Results from a nested case-control study in Northern Sweden. Stroke. 1999;30:2013–8. [DOI] [PubMed] [Google Scholar]
- 13.Price JF, Lee AJ, Rumley A, Lowe GD and Fowkes FG. Lipoprotein (a) and development of intermittent claudication and major cardiovascular events in men and women: the Edinburgh Artery Study. Atherosclerosis. 2001;157:241–9. [DOI] [PubMed] [Google Scholar]
- 14.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS and Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 15.Gillett SR, Boyle RH, Zakai NA, McClure LA, Jenny NS and Cushman M. Validating laboratory results in a national observational cohort study without field centers: the Reasons for Geographic and Racial Differences in Stroke cohort. Clin Biochem. 2014;47:243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kissela BM and Howard G. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zakai NA, Judd SE, Alexander K, McClure LA, Kissela BM, Howard G and Cushman M. ABO blood type and stroke risk: the REasons for Geographic And Racial Differences in Stroke Study. J Thromb Haemost. 2014;12:564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Medical Research Methodology. 2002;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen MR, Tokgozoglu L and Tybjaerg-Hansen A. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smolders B, Lemmens R and Thijs V. Lipoprotein (a) and stroke: a meta-analysis of observational studies. Stroke. 2007;38:1959–66. [DOI] [PubMed] [Google Scholar]
- 21.Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG and Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. Jama. 2009;302:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SR, Prasad A, Choi YS, Xing C, Clopton P, Witztum JL and Tsimikas S. LPA Gene, Ethnicity, and Cardiovascular Events. Circulation. 2017;135:251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavish D, Azrolan N and Breslow JL. Plasma Ip(a) concentration is inversely correlated with the ratio of Kringle IV/Kringle V encoding domains in the apo(a) gene. J Clin Invest. 1989;84:2021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paultre F, Pearson TA, Weil HFC, Tuck CH, Myerson M, Rubin J, Francis CK, Marx HF, Philbin EF, Reed RG and Berglund L. High Levels of Lp(a) With a Small Apo(a) Isoform Are Associated With Coronary Artery Disease in African American and White Men. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:2619–2624. [DOI] [PubMed] [Google Scholar]
- 25.Kronenberg F, Konig P, Neyer U, Auinger M, Pribasnig A, Lang U, Reitinger J, Pinter G, Utermann G and Dieplinger H. Multicenter study of lipoprotein(a) and apolipoprotein(a) phenotypes in patients with end-stage renal disease treated by hemodialysis or continuous ambulatory peritoneal dialysis. J Am Soc Nephrol. 1995;6:110–20. [DOI] [PubMed] [Google Scholar]
- 26.Lewey J, Shrank WH, Bowry AD, Kilabuk E, Brennan TA and Choudhry NK. Gender and racial disparities in adherence to statin therapy: a meta-analysis. Am Heart J. 2013;165:665–78, 678.e1. [DOI] [PubMed] [Google Scholar]
- 27.Virani SS, Brautbar A, Davis BC, Nambi V, Hoogeveen RC, Sharrett AR, Coresh J, Mosley TH, Morrisett JD, Catellier DJ, Folsom AR, Boerwinkle E and Ballantyne CM. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson TA. Lipoprotein(a), cardiovascular disease, and contemporary management. Mayo Clin Proc. 2013;88:1294–311. [DOI] [PubMed] [Google Scholar]
- 29.Saeedi R and Frohlich J. Lipoprotein (a), an independent cardiovascular risk marker. Clinical Diabetes and Endocrinology. 2016;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Sperling L, Virani SS and Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. 2018. [Google Scholar]
- 31.Tremulis S CDC grants ICD-10 codes of elevated lipoprotein(a). 2018; https://www.lipoproteinafoundation.org/news/407398/cdc-grants-icd-10-codes-of-elevated-lipoproteina.htm. Accessed November 20, 2018.
- 32.Tsimikas S and Hall JL. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: a rationale for increased efforts to understand its pathophysiology and develop targeted therapies. J Am Coll Cardiol. 2012;60:716–21. [DOI] [PubMed] [Google Scholar]
- 33.Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ and Stein EA. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–9. [DOI] [PubMed] [Google Scholar]
- 34.Man LC, Kelly E and Duffy D. Targeting lipoprotein (a): an evolving therapeutic landscape. Curr Atheroscler Rep. 2015;17:502. [DOI] [PubMed] [Google Scholar]
- 35.Graham MJ, Viney N, Crooke RM and Tsimikas S. Antisense inhibition of apolipoprotein (a) to lower plasma lipoprotein (a) levels in humans. J Lipid Res. 2016;57:340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dahl T, Kontny F, Slagsvold CE, Christophersen B, Abildgaard U, Ödegaard OR, Mørkrid L and Dale J. Lipoprotein(a), Other Lipoproteins and Hemostatic Profiles in Patients with Ischemic Stroke: The Relation to Cardiogenic Embolism. Cerebrovascular Diseases. 2000;10:110–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


