Supplemental Digital Content is available in the text
Keywords: CXCL-10, dendritic cells, HIV, immunological nonresponders, interferon-inducible protein-10, monocytes, regulatory T cells
Abstract
Objective:
To explore monocyte and dendritic cell immune responses, and their association with future CD4+ gain in treated HIV patients with suboptimal CD4+ recovery.
Design:
A cross-sectional study of HIV-infected, virally suppressed individuals on antiretroviral therapy for at least 24 months; 41 immunological nonresponders (INRs) (CD4+ cell count <400 cells/μl) and 26 immunological responders (CD4+ cell count >600 cells/μl). Ten HIV-infected antiretroviral therapy-naive and 10 HIV-negative healthy persons served as controls. CD4+ cell counts were registered after median 2.4 and 4.7 years.
Methods:
Monocyte, dendritic-cell and T-cell activation and regulatory T cells (Tregs) were analyzed by flow cytometry. In INR and immunological responder subgroups matched on age and nadir CD4+ cell count, upregulation of interferon-inducible protein-10 (IP-10) and indoleamine 2,3-dioxygenase in monocytes and dendritic cells and cytokines in cell supernatants were measured in vitro in peripheral blood mononuclear cells stimulated with aldrithiol-2-inactivated HIV-1.
Results:
The INR group displayed higher spontaneous activation of both monocytes (HLA-DR+) and myeloid and plasmacytoid dendritic cells (HLA-DR+, CD83+ and CD86+) compared with immunological responders, and this was associated with increased T-cell activation (CD38+HLA-DR+), an effector memory T-cell phenotype and activated Tregs. The IP-10 response in monocytes after in-vitro HIV stimulation was negatively associated with prospective CD4+ gain. IP-10, indoleamine 2,3-dioxygenase and cytokines levels were comparable between the groups, but inversely correlated with activated Tregs in INRs.
Conclusion:
HIV-infected individuals with suboptimal immune recovery demonstrated more activated monocytes and in particular dendritic cells, compared with patients with acceptable CD4+ gain. A high level of HIV-specific IP-10 expression in monocytes may be predictive of future CD4+ recovery.
Introduction
Despite modern antiretroviral therapy (ART) and persistent viral suppression, 15–30% of people living with HIV (PLWH) do not normalize their CD4+ cell count, denoted immunological nonresponders (INRs) [1–4]. Low nadir CD4+ cell count, long duration of HIV-infection before ART, coinfections such as hepatitis C and older age are well known factors associated with an incomplete immune recovery [2,4–6]. INRs have increased chronic immune activation and inflammation, which probably contribute to the higher morbidity and mortality seen in this group [7–11]. Given the substantial proportion of PLWH who still initiates ART in late disease, INRs will continue to be of clinical relevance [4,12].
The underlying immunological causes of incomplete immune recovery are multifactorial. Several studies have shown a more activated and differentiated T-cell phenotype [9,13–16] as well as higher percentages of activated regulatory T cells (aTregs) [10,15,16] in INRs. We have recently published increased levels of plasma interferon-inducible protein-10 (IP-10) in an INR cohort, in which IP-10 and kyurenine/tryptophan ratio, a measure of indoleamine 2,3-dioxygenase (IDO) activity, were negatively associated with the CD4+ cell count after 2 years [16]. IP-10 is mainly produced by monocytes and myeloid dendritic cells (mDCs) in HIV-negative persons after in-vitro HIV-1 stimulation [17] and is also associated with monocyte activation in HIV-infection [18]. Increased activation of monocytes is reported even in ART-treated PLWH and is related to cardiovascular disease and markers of inflammation and coagulation [19–23]. IDO-activity predicts mortality in treated HIV-infection [24] and is expressed in mDCs and plasmacytoid dendritic cells (pDCs) both in simian immunodeficiency virus (SIV)-infected macaques [25] and in HIV-negative humans after in vitro stimulation with inactivated HIV [26,27], or lipopolysaccharide (LPS) in combination with interferon-gamma (IFN-γ) [28].
Dendritic cells serve as a bridge between innate and adaptive immunity, being major drivers of Th1 responses and persistent IFNα secretion which have antiviral functions, but also contribute to chronic immune activation. Furthermore, dendritic cells upregulate IDO and induce Tregs that could both reduce harmful, general inflammation and dampen beneficial HIV-specific immune responses [29–31]. Even virally suppressed PLWH have signs of dendritic cell dysregulation as some demonstrate subnormal dendritic cell counts in blood [32–36], weakened pDC IFNα secretion after exogenous stimuli [36,37] and impaired mDC induction of Th1 responses [38].
To our knowledge, few studies have investigated monocytes and dendritic cells in INRs. Increased proportion of intermediate monocytes [39,40] and lower absolute pDC count with reduced IFNα production have been reported in INRs compared with PLWH with normalized CD4+ cell count [41].
We set out to study activation of monocytes and dendritic cells in INRs compared with immunological responders, ART-naive PLWH and healthy controls, and in-vitro HIV-specific monocyte and dendritic cell responses in INR and immunological responder subgroups matched on age and nadir CD4+ cell count. We hypothesized that INRs had more activated monocytes and dendritic cell subsets and higher in-vitro production of IP-10, IDO and cytokines than the immunological responder group that might contribute to an inadequate future immune reconstitution in INRs.
Methods
Study participants
Forty-one virally suppressed HIV-infected INRs with CD4+ cell count less than 400 cells/μl and 26 immunological responders with CD4+ cell count more than 600 cells/μl were recruited between October 2012 and April 2013 as previously reported [16]. Both groups had received continuous ART for at least 24 months with HIV-RNA 20 copies/ml or less for the last 18 months. CD4+ cell counts were obtained from fresh samples and recorded at baseline and median 2.4 and 4.7 years after inclusion. The last routine CD4+ cell counts that were available prior to data analyses of the previous [16] and the present reports were used. For comparison, 10 ART-naive individuals with duration of HIV-infection at least one year and 10 HIV-negative healthy controls, all age and sex matched, were included. Peripheral blood mononuclear cells (PBMCs) and EDTA plasma were sampled from all participants at inclusion, frozen and stored for later analyses. All participants provided written informed consent. The study was approved by the Regional Ethics Committee (1.2007.83 and 2015/629).
Flow cytometry analyses of ex-vivo monocyte and dendritic cell activation
Flow cytometry analyses were performed on thawed PBMCs. After 2 h rest, one million (viability >85%) PBMCs were incubated with Fc block (BD Biosciences, San Jose, California, USA) prior to staining with surface markers for 15 min in room temperature. The cells were fixated in 1% BD CellFIX (BD Biosciences) before acquisition on BD FACSCanto II (BD Biosciences). The fluorochrome-conjugated antibodies for the monocyte and dendritic cell panels are listed in Table S1. Results were analyzed with the FlowJo software version 10.4.1 (Tree Star Inc, Ashland, Oregon, USA). As staining controls, all antibodies in the other channels were combined with concentration matched isotypes for the activation markers, and fluorescence minus one (FMO) was used for anti-CD83. The gating strategy is shown in Fig. S1. Monocyte subsets were defined as CD45+HLA-DR+Lineage−FixableViability− and either CD14++CD16− (classical), CD14++CD16+ (intermediate) or CD14+CD16++ (nonclassical). Dendritic cells were characterized as CD45+HLA-DR+Lineage−FixableViability− and further subdivided into CD1c+ mDCs, CD141++ mDCs or CD303+ pDCs.
Intracellular interferon-inducible protein-10 and indoleamine 2,3-dioxygenase detection after in-vitro stimulation with inactivated HIV-1
Owing to the strong association of nadir CD4+ with low CD4+ recovery, 20 INRs and 20 immunological responders with comparable age and nadir CD4+ cell count, and eight age and sex-matched healthy controls were selected for in-vitro HIV-stimulation analyses (Table S2). One million thawed PBMCs (viability >90%), were cultured for 18 h at 37 °C, 5% CO2 in 200 μl RPMI 1640 (Lonza, Verviers, Belgium) containing 10% heat-inactivated fetal calf serum and penicillin/streptomycine/l-glutamine, with either aldrithiol-2-inactivated (AT-2) HIV-1 (dual-tropic; X4 and R5, lot P4311) at a final concentration of 500 ng/ml p24 [42], or microvesicles with equivalent protein concentration (mock) as negative control [43]. Brefeldin A 1 μl/ml (BD Biosciences) was added after 6 h. Following stimulation supernatants were harvested and the cells stained for either monocyte or dendritic cell surface markers as previously described (Table S1), subsequently fixated and permeabilized using BD Cytofix/Cytoperm (BD Biosciences) and stained intracellularly for IP-10 and IDO (Table S1). All IP-10 and IDO responses were calculated with subtraction of the corresponding mock stimulated control. For gating strategy see Fig. S2.
ELISA and multiplex analyses
Soluble (s)CD163 was analyzed in INRs and immunological responders from snap frozen EDTA plasma in duplicate by immunoassay (DY1607; R&D Systems, Minneapolis, Minnesota, USA) according to the manufacturer's instructions. The intraassay and interassay coefficients of variation (CV) were less than 10%.
Concentrations of IL-1β, IL-1 receptor antagonist (IL-1ra), IL-6, IL-10, IL-18, IFNα2, IFNγ, tumor necrosis factor, IP-10 and macrophage inflammatory protein 1beta (MIP-1β)/CCL4 were measured in thawed supernatants (10 000 g/10 min/4 °C) by nine-plex and single-plex (Bio-Rad, Oslo, Norway) assays, respectively. The samples were diluted 1 : 7.5 and 1 : 75 as appropriate, analyzed with a Luminex IS 100 instrument (Bio-Rad, Hercules, California, USA) according to instructions from the manufacturer and run in duplicates. Both intraassay and interassay CV were less than 10%. All AT-2 HIV virus responses were found by subtracting concentrations from the corresponding mock stimulated sample.
Multiplex and ELISA analyses of other soluble markers in plasma and flow cytometry phenotyping of T-cell subsets including Tregs have been previously presented [16], and are only used in correlation analyses in the current article. The methods are described in brief in the Supplementary methods.
Statistical analyses
Statistical analyses and graphical presentations were performed by SPSS statistics 25 (IBM Corp., Armonk, New York, USA) and GraphPad Prism V7.04 software (GraphPad, San Diego, California, USA) using nonparametric statistics. To reduce the percentage of CV (%CV) in the flow cytometry experiments caused by small cell populations, populations consisting of less than 100 cells were excluded for further analyses of percentages of activation markers and when less than 50 cells also for determination of median fluorescence intensity (MFI) [44]. For comparison between more than two groups Kruskal–Wallis test followed by Dunn's post-hoc test with correction for multiple comparisons were applied. Mann–Whitney U test was used when analyzing two groups, Wilcoxon test for paired samples, Fisher's exact test or Pearson chi-squared test for categorical variables and Spearman's rank correlation for correlation between parameters. Factors associated with prospective CD4+ gain were further analyzed with binary logistic regression adjusting for age, nadir CD4+ cell count and duration of ART. The outcome variable was dichotomized on median within the actual patient group. A two-tailed significance level of 0.05 was set.
Results
Characteristics of the study participants
The baseline characteristics for the INR and immunological responder cohorts have previously been described in detail [16]. The ART-naive had median CD4+ cell count 553 (interquartile range 346–667) cells/μl, CD4+/CD8+ ratio comparable with INRs, shorter duration of HIV infection (P = 0.004) and higher nadir CD4+ cell count (P < 0.001) than both INRs and immunological responders (Table 1). In the subgroups matched on age and nadir CD4+, selected for AT-2 HIV stimulation assay, the INR group (n = 20) had lower viral load at initiation of continuous ART (P = 0.02) and shorter duration of both viral suppression (P = 0.01) and ART use (P = 0.01) compared with the immunological responder group (n = 20) (Table S2).
Table 1.
Characteristics of the study cohort at inclusion.
| Total study population | INR, n = 41 | IR, n = 26 | ART-, n = 10 | HC, n = 10 | P value* |
| Age (IQR) | 49.9 (41.6–57.9) | 45 (39.3–53.8) | 50.6 (39.6–59.4) | 47.2 (43.5–58.0) | NS |
| Male sex, n (%) | 35 (85.4) | 18 (69.2) | 7 (70) | 7 (70) | NS |
| Ethnicity, n (%) | |||||
| White | 29 (70.7) | 17 (65.4) | 5 (50) | 10 (100) | NS |
| Risk group, n (%) | |||||
| MSM | 22 (53.7) | 13 (50) | 1 (10)** | 0.04 | |
| Othera | 19 (46.3) | 13 (50) | 9 (90)** | 0.04 | |
| Comorbid diseases, n (%) | |||||
| Cardiovascular | 6 (14.6) | 0 (0) | 2 (20) | NS | |
| Any comorbidityb | 21 (51.2) | 6 (23.1) | 5 (50) | NS | |
| CMV IgG pos | 41 (100) | 25 (96.2) | 9 (90) | NS | |
| HIV characteristics (IQR) | |||||
| Years since HIV diagnosis | 8.6 (6.4–15.2) | 9.2 (7.0–14.1) | 3.6 (2.2–6.8)**,*** | 0.004 | |
| Years of continuous ART | 5.5 (3.1–6.7) | 6.6 (4.4–8.7) | NS | ||
| Viral load at ART initiation (copies/ml) | 67 500 (29 000–110 000) | 100 000 (50 000–330 000) | NS | ||
| Duration of viral suppression (years) | 3.8 (2.0–5.8) | 6.1 (4.0–7.6) | 0.01 | ||
| Viral load at inclusion (copies/ml) | ≤20 | ≤20 | 36 000 (19 750–128 000)**,*** | <0.001 | |
| CD4+ cell count nadir (cells/μl) | 100 (20–157) | 180 (120–220) | 369 (346–512)**,***,**** | <0.001 | |
| CD4+ cell count at inclusion (cells/μl) | 285 (232–348) | 810 (740–864) | 553 (346–677)**,**** | <0.001 | |
| CD8+ cell count at inclusion (cells/μl) | 670 (521–890) | 1005 (820–1587) | 1257 (973–2491)**,**** | <0.001 | |
| CD4+/CD8+ at inclusion | 0.43 (0.32–0.57) | 0.79 (0.63–0.99) | 0.35 (0.25–0.74)***,**** | <0.001 | |
Data are presented as no. (%) of study participants or median IQR values. ART, antiretroviral therapy; ART-, antiretroviral therapy naive HIV-infected; CMV, cytomegalovirus; HC, healthy control; INR, immunological nonresponder; IQR, interquartile range; IR, immunological responder.
aOther. Heterosexual or unknown. There were no intravenous drug abusers.
bOne or more of the following comorbidities; cardiovascular disease, hypertension, diabetes, renal disease, osteoporosis, chronic obstructive pulmonary disease, neurodegenerative disease, previous cancer or Mycobacterium tuberculosis infection.
*P values for Kruskal–Wallis test or Pearson Chi-squared test for comparison between multiple groups, Mann–Whitney U test for comparison INR vs. IR. Significant values are shown in bold.
**P less than 0.05 for comparison INR vs. ART-. Fisher's exact test or Dunn's post-hoc test.
***P less than 0.05 for comparison IR vs. ART-. Dunn's post-hoc test.
****P less than 0.05 for comparison INR vs. IR. Dunn's post-hoc test.
A median of 4.7 years after inclusion the median CD4+ gain was 55 (−9 to 115) cells/μl among INRs and 37% had reached a CD4+ cell count above 400 cells/μl. However, only three persons achieved CD4+ cell count above 500 cells/μl. The median increase in CD4+ from 2.4 until 4.7 years after inclusion was 18 (−29 to 75) cells/μl and 58% of the INRs increased their CD4+ cell count in this period. Nevertheless, the CD4+ cell counts stabilized the last 12 months of follow-up (Fig. S3). Duration of continuous ART and viral suppression, nadir CD4+ and age were all related to prospective CD4+ cell count and/or CD4+/CD8+ ratio (Table S3).
Activated monocytes in immunological nonresponders correlate with T-cell activation and low CD4+ cell counts
We first analyzed the phenotypes of the various monocyte subsets. In the INR group, the frequency of monocytes was negatively associated with the CD4+ cell count at inclusion (r = −0.39, P = 0.0012), and the monocytes constituted a higher fraction of the CD45+ cells within the INR than in the immunological responder group (Fig. S3). The INRs demonstrated increased MFI of HLA-DR in monocytes compared with immunological responders (P = 0.043), particularly in the CD14++CD16+ subset (P = 0.013) (Fig. 1a). The HLA-DR expression correlated with T-cell activation, an effector memory (EM) T-cell phenotype and negatively with baseline CD4+ cell count and CD4+/CD8+ ratio (r = −0.35, P = 0.004) (Table S4). Overall, the ART-naive had the highest CD16 expression in monocytes (P = 0.002) (Fig. S4). However, we found no differences between the HIV groups neither in the distribution of the various monocyte subsets, nor in the MFI values or frequencies of cells expressing the activation markers CD142 [tissue factor (TF)], CD163 or CD11b on these subsets (Fig. S4 and Fig. S5). Nevertheless, all HIV-positive groups showed higher MFI of CD142 and lower MFI and fractions of CD163+ monocytes than healthy controls (Fig. S5). The expression of CD163 or percentages of CD163+ monocytes were not associated with any inflammation markers, T-cell activation or Tregs. Furthermore, analysis of plasma sCD163 revealed no difference between INRs and immunological responders and no correlation with cellular CD163 expressed as MFI or frequencies of CD163+ monocytes.
Fig. 1.
HLA-DR expression in monocyte and dendritic cell subsets in the different cohorts.
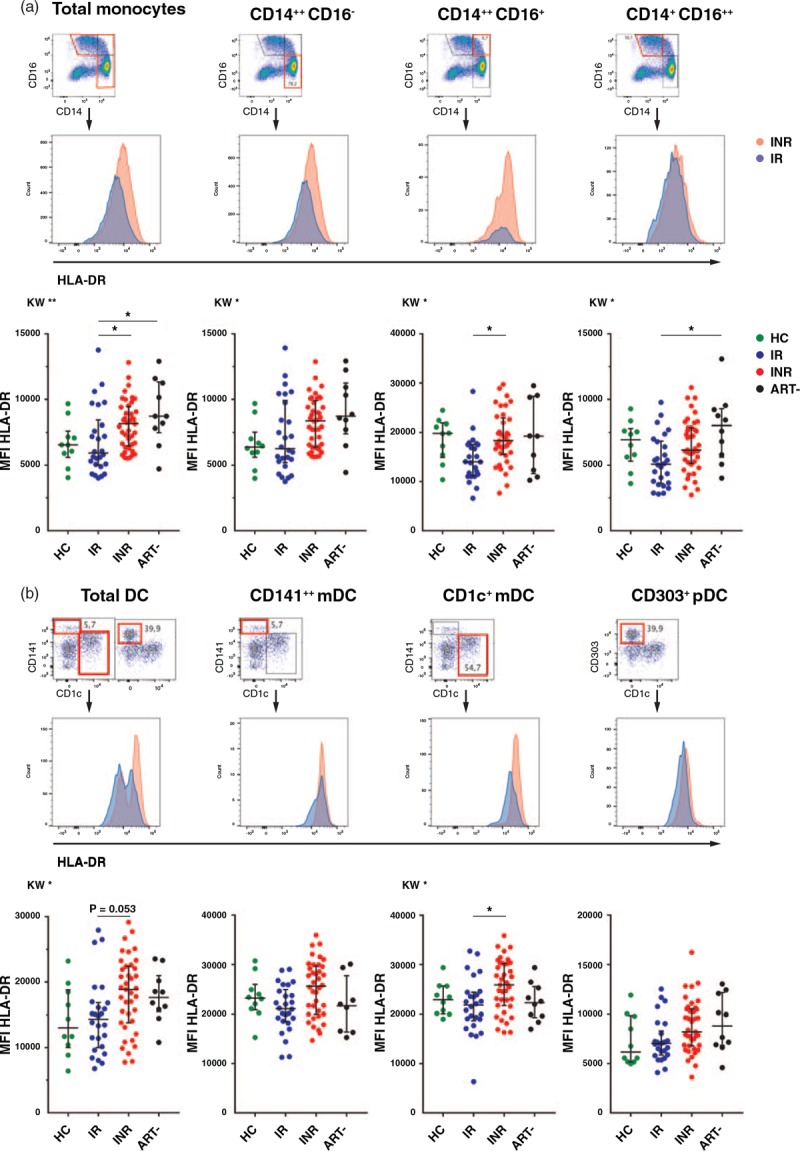
Parts (a) and (b) show overlay plots of median fluorescence intensity of HLA-DR in one immunological nonresponder and one immunological responder for various monocyte and dendritic cell subsets, respectively. The graphs display the differences among healthy control, immunological responder, immunological nonresponder and antiretroviral therapy-naive groups in the HLA-DR expression in the specific monocyte (a) and dendritic cell (b) subsets. Kruskal–Wallis test followed by Dunn's post-hoc test for correction for multiple comparisons. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Lines indicate median and interquartile range. ART-, antiretroviral therapy naive HIV-infected; DC, dendritic cells; HC, healthy control; INR, immunological nonresponder; IR, immunological responder; MFI, median fluorescence intensity.
Dendritic cell activation is increased in immunological nonresponders and correlates with T-cell activation and low CD4+ cell counts
We then investigated the distribution of pDCs and mDCs in the various cohorts. The ART-naive had lower fractions of total dendritic cells compared with both INRs and healthy controls (P = 0.008 and P < 0.001), but there were no differences between INRs and immunological responders (Fig. S4). Overall, all HIV groups had more activated dendritic cells than the controls. Still, there was a higher level of dendritic cell activation in the INR compared with the immunological responder group (Figs. 1b and 2b–d) with higher MFI of HLA-DR in the total dendritic cell population, mainly driven by increased expression in CD1+ mDCs (P = 0.021) (Fig. 1b). Furthermore, the expression of the maturation marker CD83 in pDCs, the fraction of CD83+ pDCs and CD83+CD141+ mDCs (Fig. 2b), the expression of the T-cell costimulatory molecule CD86 in both mDC subsets (Fig. 2c) and the fraction of CD83+CD86+CD141+ mDCs (Fig. 2d) were all higher in INRs than immunological responders. Finally, activation of pDCs and mDCs correlated strongly with activated T cells, to a lesser degree with the proportion of aTregs/resting (r)Tregs, and all the activated dendritic cell subsets were negatively associated with the baseline CD4+ cell counts (Table S4). There were no relation between dendritic cell activation and soluble inflammation markers except for a weak correlation between soluble (s)CD14 and HLA-DR expression in CD1c+mDC and CD83 in pDCs (r = 0.30, P = 0.016, and r = 0.32, P = 0.011).
Fig. 2.
Dendritic cell activation markers in the various groups.
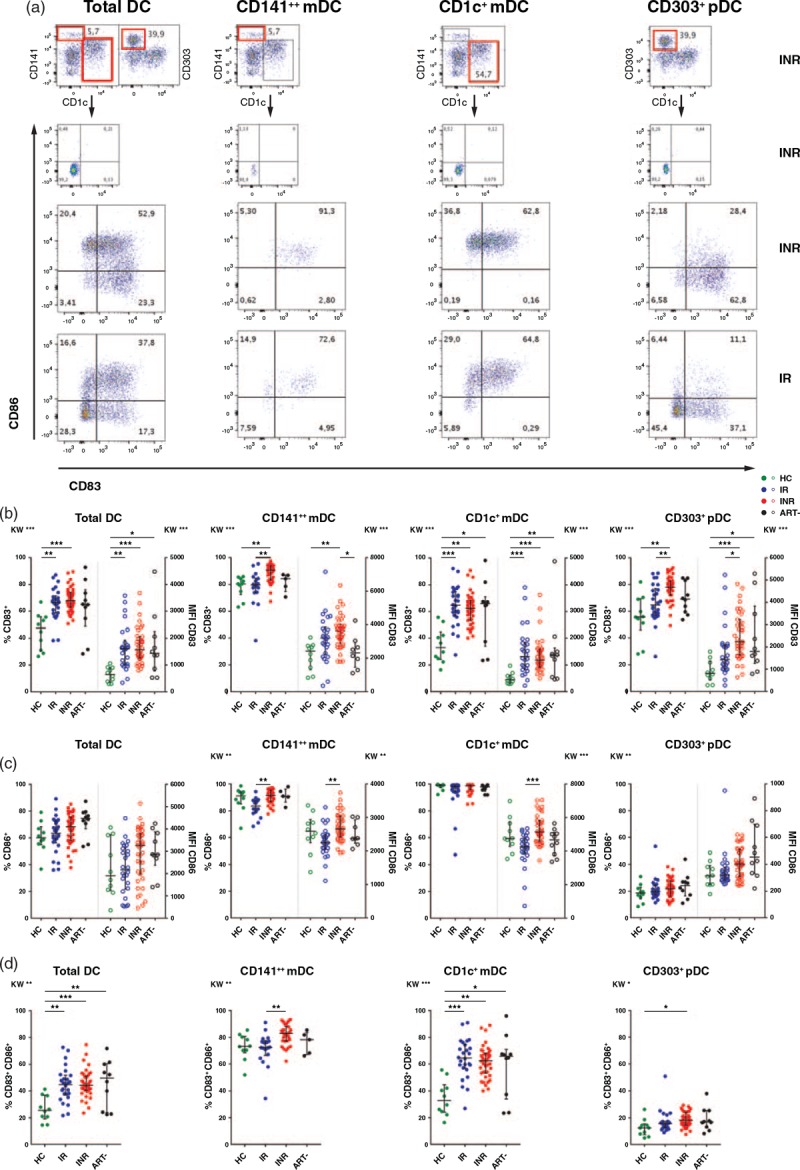
Part (a) illustrates gating strategy of dendritic cell activation markers based on isotype or fluorescence minus one in total dendritic cells and the different dendritic cell subsets. The plots show representative examples from one immunological nonresponder and one immunological responder. In parts (b), (c) and (d), the graphs display both percentage (solid dots) and median fluorescence intensity (open dots) values of the activation markers CD83, CD86 and the coexpression of CD83 and CD86 in the various dendritic cell subsets for each group. Kruskal–Wallis test followed by Dunn's post-hoc test for correction for multiple comparisons. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Lines indicate median and interquartile range. ART-, antiretroviral therapy naive HIV-infected; DC, dendritic cells; HC, healthy control; INR, immunological non-responder; IR, immunological responder; MFI, median fluorescence intensity.
HIV-induced upregulation of interferon-inducible protein-10 and indoleamine 2,3-dioxygenase in monocytes and myeloid dendritic cells in vitro are comparable in immunological nonresponders, immunological responders and controls
Next, we analyzed the effect of exposure to AT-2 HIV in vitro upon the various mDC and monocyte subsets in the INRs, immunological responders and healthy controls. There was a significant upregulation of both MFI of IDO and IP-10 and frequencies of IP-10 positive monocyte and mDC subsets compared with mock-stimulated samples in all cohorts (Fig. 3), with the exception of MFI of IDO in CD141+ mDCs, which decreased. The fraction of IP-10 positive cells increased most in monocytes, whereas IDO expression was highest in mDCs. We found no significant differences between the INR, immunological responder and healthy control groups neither in frequencies of IP-10+ cells, nor in the MFI values of IP-10 or IDO in monocytes and mDCs after AT-2 HIV stimulation (Fig. 3).
Fig. 3.
Interferon-inducible protein-10 and indoleamine 2,3-dioxygenase responses in monocytes and myeloid dendritic cells after exposure to aldrithiol-2 inactivated HIV-1 in vitro.
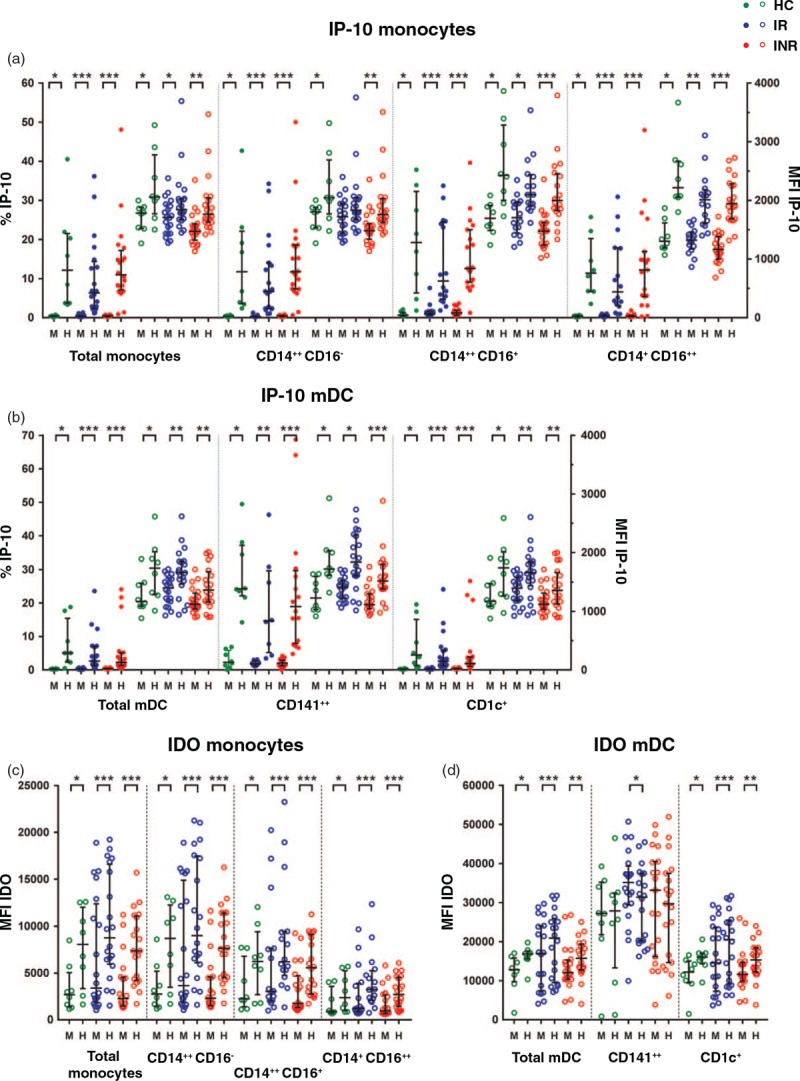
Percentages (solid dots) and median fluorescence intensity (open dots) of interferon-inducible protein-10 in monocytes (a) and myeloid dendritic cells (b) after in-vitro stimulation of peripheral blood mononuclear cells with either mock (M) or aldrithiol-2 inactivated HIV-1 (H) in healthy controls, immunological responders and immunological nonresponders. Parts (c) and (d) display indoleamine 2,3-dioxygenase responses in monocytes and myeloid dendritic cells, respectively. Wilcoxon test for pairwise comparisons between mock and aldrithiol-2 inactivated HIV-1 stimulation.∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Lines indicate median and interquartile range. HC, healthy control; INR, immunological nonresponder; IR, immunological responder; mDC, myeloid dendritic cell; MFI, median fluorescence intensity.
High fractions of activated regulatory T cells are associated with reduced in-vitro HIV-specific responses in immunological nonresponders
We observed clear cytokine responses after AT-2 HIV stimulation, although we did not detect any significant differences between the INRs, immunological responders and healthy controls (Fig. S6). Changes in soluble IP-10 levels correlated strongly with IP-10 measured by flow cytometry in both monocytes (ΔMFI r = 0.55, P < 0.001, Δ% r = 0.63, P = 0.001) and mDCs (ΔMFI r = 0.47, P = 0.002, Δ% r = 0.32, P = 0.044), suggesting that the IP-10 in the supernatants could originate from both monocytes and mDCs. In the INR subgroup the IP-10 expression in monocytes and/or dendritic cells analyzed by flow cytometry corresponded to changes in soluble IFNα2, IL-1ra, IL-18, IFNγ and MIP-1β (Table S5).
Furthermore, in INRs, the upregulation of both IP-10 and IDO in monocytes and mDCs, as well as the IFNα2, IL-18 and IFNγ responses after exposure to AT-2 HIV, were strongly inversely correlated with the proportion of aTregs (Fig. 4a–c). In immunological responders, there were no such associations.
Fig. 4.
Associations between fraction of activated regulatory T cells and interferon-inducible protein-10, indoleamine 2,3-dioxygenase and cytokine upregulation after exposure to aldrithiol-2 inactivated HIV-1 in vitro and correlation between interferon-inducible protein-10 responses in monocytes and CD4+ recovery after 2 and 4 years.
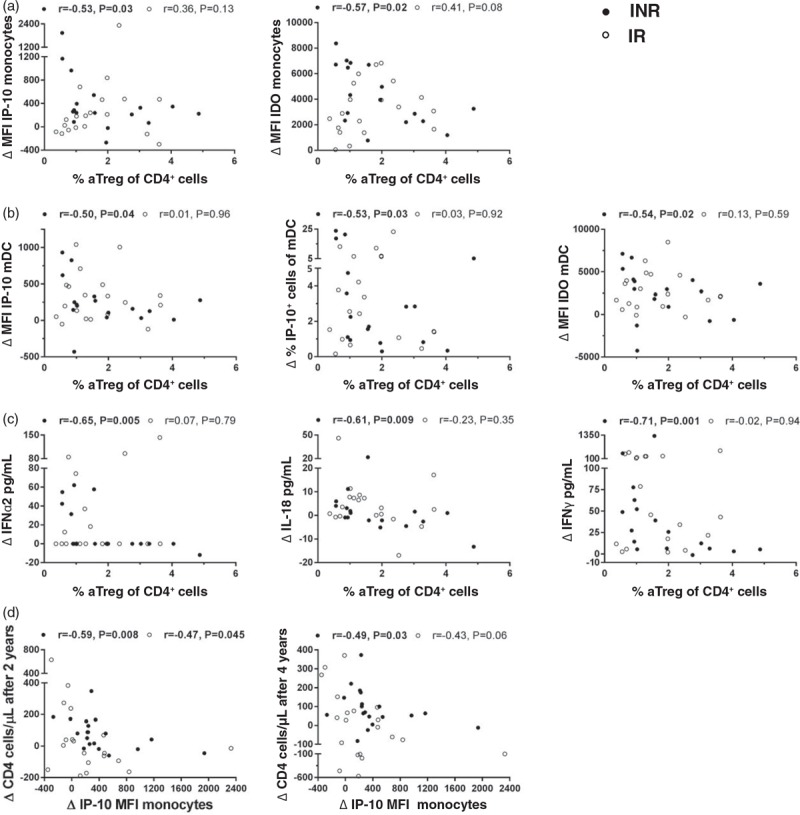
Correlation between percentages of activated regulatory T cells and indoleamine 2,3-dioxygenase and interferon-inducible protein-10 responses in monocytes (a) and myeloid dendritic cells (b) and cytokine responses in supernatants (c) after in-vitro stimulation with aldrithiol-2 inactivated HIV-1. (d) Associations with interferon-inducible protein-10 increase in monocytes after exposure to HIV in vitro and prospective CD4+ gain after 2 and 4 years. Spearman rank order correlation. The aldrithiol-2 inactivated HIV-1 responses are calculated with subtraction of the mock stimulated control. •INR, immunological nonresponder; ∘IR, immunological responder; aTregs, activated regulatory T cells (%CD147++CD25++ of CD4+); mDC, myeloid dendritic cell; MFI, median fluorescence intensity.
HIV-specific monocyte interferon-inducible protein-10 responses are negatively associated with future CD4+ gain
Finally, we aimed to explore markers associated with future CD4+ gain in the INR group. The increase of IP-10 expression in monocytes observed after exposure to AT-2 HIV in vitro, was negatively correlated with the CD4+ gain seen 2.4 and 4.7 years later (Fig. 4d). In INR with CD4+ cell count 300 cells/μl or less at baseline this association was even stronger (r = −0.86, P = 0.007) and there was also a negative correlation between the MFI IP-10 response and the prospective CD4+/CD8+ ratio (r = −0.71, P = 0.047). When adjusting for age and nadir CD4+, an increase in MFI of IP-10 in monocytes above median, could still predict a lower CD4+ gain in INRs after both 2.4 and 4.7 years [2.4 years; odds ratio (OR) = 19.2 (95% confidence interval (CI): 1.1–350.2), P = 0.046 and 4.7 years; OR = 21.2 (95% CI: 1.1–399.6), P = 0.041]. Nevertheless, when including duration of continuous ART in the model, none of the parameters remained significant.
Discussion
We have explored innate immunity in an INR cohort compared with ART-treated PLWH with satisfactory CD4+ recovery, by characterizing monocyte and dendritic cell subsets and responsiveness to in-vitro HIV stimulation and their associations with T-cell phenotypes. To our knowledge, this is the first study that presents such thorough characterization of phenotypes and functionality in innate and adaptive immunity in a group of INRs. Our main findings can be summarized as follows: first, INRs demonstrated increased activation of dendritic cells and monocytes compared with immunological responders. Second, these changes were associated with T-cell activation, a shift towards an EM T-cell phenotype and higher frequency of aTregs. Third, in INRs, the presence of aTregs was correlated with reduced in-vitro HIV-specific IDO and cytokine responses, and fourth, a high HIV-specific increase in IP-10 expression in monocytes was associated with lower CD4+ recovery after 2 and 4 years.
mDCs are potent antigen-presenting cells and recognize diverse pathogens due to their broad expression of Toll-like receptors (TLRs) [30]. Following TLRs engagement, mDCs upregulate major histocompatibility complex class II, the costimulatory molecules CD80 and CD86, and produce IL-12 which induces Th1 cell responses [30,45]. In our cohorts, INRs showed increased CD86 and HLA-DR expression in mDCs compared with the immunological responder group. Activated pDCs are the most potent producers of IFNα [46] which stimulate a wide range of immune cells. The maturation marker CD83 was higher expressed in pDCs in the INR cohort than in immunological responders and HIV-negative controls. Furthermore, we found that the activated dendritic cells were associated with T-cell activation, an EM T-cell phenotype, as well as the fraction of aTregs/rTregs, and inversely with the CD4+ cell count. Our results thereby suggest that activation of dendritic cells is connected to well known disturbances in the T-cell compartment in both immune-stimulatory and immune-suppressive directions.
HIV and other viruses can activate pDCs directly [34,45,47], whereas mDCs seem to be dependent of exposure to pDC-derived cytokines to mature after exposure to HIV [34,45]. In HIV-infected individuals also LPS, other pathogen-derived factors and unmethylated DNA contribute to activation of mDCs and pDCs, respectively [30,48,49]. We found no correlations between dendritic cell activation and soluble inflammation markers in plasma except for a weak correlation with sCD14. Despite lack of association with LPS, dendritic cell activation could still be driven by other microbial products [47,49,50]. Of note, none of the patients had acute infections, hepatitis C or B viremia, but with one exception, all were cytomegalovirus (CMV)-positive and differences in CMV replication and low-level HIV viremia might be partly responsible for the activation of dendritic cells [51].
Contrary to a recent report [39], we discovered higher HLA-DR expression in monocytes in INRs compared with immunological responders. Bandera et al. studied frequencies of HLA-DR+ CD14+ cells and as most monocytes express HLA-DR, such differences could be more difficult to detect. Variations in definition and gating strategies of monocytes and different patient populations can also be a relevant explanation of our diverging results. As seen for dendritic cells, activated monocytes correlated inversely with the CD4+ cell count and were associated with T-cell activation, but not with soluble inflammation markers linked to non-AIDS morbidity in ART-treated PLWH. Nevertheless, in line with two recently published studies, all our HIV groups showed higher expression of TF than controls, which are related to coagulation and cardiovascular disease [22,23].
Despite the increased spontaneous ex-vivo activation of monocytes and dendritic cells seen in INRs, there were no differences between INRs, immunological responders and controls in the in-vitro cell upregulation of IP-10 and IDO or cytokine levels in supernatants. This implies that innate immunity in INRs preserve their capacity to respond to the HIV virus. Our data support other studies reporting similar cytokine production in supernatants in INRs and immunological responders after exposure to single-stranded RNA [52], TLR7 or TLR9 [41], yet IFNα synthesis seemed to be reduced in INRs [41]. Few had a measurable raise in IFNα2 in our study and the numbers are thus too low to draw any conclusions. In line with Simmons et al.[17], we found that monocytes had the highest fractions of IP-10 positive cells after AT-2 HIV stimulation. However, in contrast to Boasso et al.[26], we saw an increase in IDO expression in both monocytes and mDCs indicating that also monocytes could be an important source for IDO activity in HIV infection.
Although the increases in cytokines in supernatants and cellular IP-10 and IDO were similar, the inflammatory responses seemed to be more closely correlated in INRs compared with immunological responders. Only in INRs, the upregulation of IP-10 in monocytes and mDCs were related to the increase in several supernatant cytokines. This could imply that IP-10 responses are accompanied by a more general and possible harmful immune activation in patients with incomplete immune recovery. Moreover, we identified a negative association between the frequency of aTregs and the upregulation of several cytokines and cellular IDO and IP-10 in INRs, suggesting that presence of aTregs may suppress HIV-specific responses in patients with incomplete CD4+ gain.
We recently reported that the level of IP-10 in plasma was negatively associated with the CD4+ cell count after 2.4 years in the individuals with profoundly impaired immune reconstitution at inclusion [16]. In the current study, we found more precisely that the IP-10 response in monocytes after exposure to HIV in vitro was negatively correlated with the CD4+ gain after median 2.4 and 4.7 years both for the INR and immunological responder groups, with the strongest association in individuals with baseline CD4+ cell count 300 cells/μl less. Type I interferons seem to be important for IP-10 expression in monocytes after HIV exposure [17], suggesting that pDCs could play a role in the IP-10 increase. IP-10 stimulates HIV-replication in vitro[53]. Moreover, in primary HIV-infection, IP-10 levels are strongly correlated with HIV RNA and cell-associated DNA and reported to be a better predictor of disease progression than the level of viremia [54,55]. In addition, IP-10 attracts CXCR3+CD4+ T cells which are major target cells for HIV and also contain the highest amount of integrated DNA in treated HIV infection [56]. Hence, monocytes which respond to HIV with a high IP-10 production could be important in HIV pathogenesis and potentially unfavorable in the long term also in treated HIV infection.
Our study has some limitations. We have evaluated HIV-specific monocyte and dendritic cell responses, but inclusion of other stimuli such as LPS, flagellin, CpG-containing DNA and CMV, would have given a more comprehensive knowledge of factors contributing to monocyte and dendritic cell activation in INRs. Furthermore, dendritic cell subsets are rare cell populations and are sometimes too small for phenotyping and evaluation of IP-10 and IDO responses. However, studying PBMCs has an advantage over isolated cell populations as cytokine responses and in particular IP-10 is dependent of cross-talk between different immune cells [57]. Finally, about one third of the INRs experienced an increase in their CD4+ cell count to more than 400 cells/μl after 4 years, in line with other studies [58]. Some of these patients were found in the groups selected for in-vitro AT-2 HIV assays. Although only INRs and immunological responders with similar age and nadir CD4+ cell count were selected for this in-vitro assay, diverse potential for prospective CD4+ recovery in the INR group could have contributed to the observed lack of differences in in-vitro responses between INRs and immunological responders. Thus, for future studies inclusion criteria with even longer duration of ART could be preferable.
In conclusion, ART-treated PLWH with an inadequate immune recovery had a more activated phenotype of monocytes and in particular dendritic cells, changes that were related to activation and regulation in the T-cell compartment. Of note, a high in-vitro HIV-induced IP-10 expression in monocytes was related to a lower future CD4+ recovery. As type I interferons are important for IP-10 expression in monocytes after HIV exposure, an interplay between viral stimulation of pDCs, activation of monocytes and subsequent IP-10 expression could be a relevant pathway involved in inflammation and poor immune recovery during HIV infection.
Acknowledgements
We thank all the study participants. Thanks to Linda Gail Skeie, Kjersti Saelleg, Helene Gjelsaas and Mette Sannes at Oslo University Hospital for invaluable assistance in data collection or laboratory analyses. These experiments utilized regents provided by the AIDS and Cancer Virus Program, Leidos Biomedical Research, Inc./Frederick National Laboratory for Cancer Research supported with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E.
The study was funded by grants from the Research Council of Norway, the Regional Health Authority for South Eastern Norway, the K.G. Jebsen Foundation, Yngvar Saastads Scholarship, Oslo University Hospital and University of Oslo, Norway.
Authors’ contributions: Conceived and designed the study: A.M.D.-R., B.S. Recruited the patients and collected clinical data: B.S. Performed the flow cytometry experiments: B.S., K.B.L. Performed the multiplex analyses: H.C.D.A. Performed the sCD163 ELISA analyses: T.U. Performed the statistical analyses: B.S. Analyzed the data: B.S., M.T., H.C.D.A., A.M.D.-R. Contributed reagents and analyses tools: B.S., A.M.D.-R., H.C.D.A., K.B.L., T.U. Drafted the article: B.S., A.M.D.-R. All authors have critically revised the article.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
References
- 1.Kaufmann GR, Perrin L, Pantaleo G, Opravil M, Furrer H, Telenti A, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med 2003; 163:2187–2195. [DOI] [PubMed] [Google Scholar]
- 2.Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata M, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 2009; 48:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engsig FN, Zangerle R, Katsarou O, Dabis F, Reiss P, Gill J, et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis 2014; 58:1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mutoh Y, Nishijima T, Inaba Y, Tanaka N, Kikuchi Y, Gatanaga H, et al. Incomplete recovery of CD4 count, CD4 percentage, and CD4/CD8 ratio in HIV-infected patients on long-term antiretroviral therapy with suppressed viremia. Clin Infect Dis 2018; 67:927–933. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann GR, Furrer H, Ledergerber B, Perrin L, Opravil M, Vernazza P, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis 2005; 41:361–372. [DOI] [PubMed] [Google Scholar]
- 6.Miller MF, Haley C, Koziel MJ, Rowley CF. Impact of hepatitis C virus on immune restoration in HIV-infected patients who start highly active antiretroviral therapy: a meta-analysis. Clin Infect Dis 2005; 41:713–720. [DOI] [PubMed] [Google Scholar]
- 7.Marchetti G, Bellistri GM, Borghi E, Tincati C, Ferramosca S, La Francesca M, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS 2008; 22:2035–2038. [DOI] [PubMed] [Google Scholar]
- 8.Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Buckner K, Tedaldi EM, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis 2010; 51:435–447. [DOI] [PubMed] [Google Scholar]
- 9.Massanella M, Negredo E, Perez-Alvarez N, Puig J, Ruiz-Hernandez R, Bofill M, et al. CD4 T-cell hyperactivation and susceptibility to cell death determine poor CD4 T-cell recovery during suppressive HAART. AIDS 2010; 24:959–968. [DOI] [PubMed] [Google Scholar]
- 10.Piconi S, Trabattoni D, Gori A, Parisotto S, Magni C, Meraviglia P, et al. Immune activation, apoptosis, and Treg activity are associated with persistently reduced CD4+ T-cell counts during antiretroviral therapy. AIDS 2010; 24:1991–2000. [DOI] [PubMed] [Google Scholar]
- 11.Lapadula G, Cozzi-Lepri A, Marchetti G, Antinori A, Chiodera A, Nicastri E, et al. Risk of clinical progression among patients with immunological nonresponse despite virological suppression after combination antiretroviral treatment. AIDS 2013; 27:769–779. [DOI] [PubMed] [Google Scholar]
- 12.Mocroft A, Lundgren J, Antinori A, Monforte A, Brannstrom J, Bonnet F, et al. Late Presenters Working Group in COHERE in EuroCoord. Late presentation for HIV care across Europe: update from the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study, 2010 to 2013. Euro Surveill 2015; 20:pii=30070. [DOI] [PubMed] [Google Scholar]
- 13.Marziali M, De Santis W, Carello R, Leti W, Esposito A, Isgro A, et al. T-cell homeostasis alteration in HIV-1 infected subjects with low CD4 T-cell count despite undetectable virus load during HAART. AIDS 2006; 20:2033–2041. [DOI] [PubMed] [Google Scholar]
- 14.Marchetti G, Gori A, Casabianca A, Magnani M, Franzetti F, Clerici M, et al. Comparative analysis of T-cell turnover and homeostatic parameters in HIV-infected patients with discordant immune-virological responses to HAART. AIDS 2006; 20:1727–1736. [DOI] [PubMed] [Google Scholar]
- 15.Gaardbo JC, Hartling HJ, Ronit A, Springborg K, Gjerdrum LM, Ralfkiaer E, et al. Regulatory T cells in HIV-infected immunological nonresponders are increased in blood but depleted in lymphoid tissue and predict immunological reconstitution. J Acquir Immune Defic Syndr 2014; 66:349–357. [DOI] [PubMed] [Google Scholar]
- 16.Stiksrud B, Lorvik KB, Kvale D, Mollnes TE, Ueland PM, Troseid M, et al. Plasma IP-10 is increased in immunological nonresponders and associated with activated regulatory T cells and persisting low CD4 counts. J Acquir Immune Defic Syndr 2016; 73:138–148. [DOI] [PubMed] [Google Scholar]
- 17.Simmons RP, Scully EP, Groden EE, Arnold KB, Chang JJ, Lane K, et al. HIV-1 infection induces strong production of IP-10 through TLR7/9-dependent pathways. AIDS 2013; 27:2505–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hearps AC, Maisa A, Cheng WJ, Angelovich TA, Lichtfuss GF, Palmer CS, et al. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS 2012; 26:843–853. [DOI] [PubMed] [Google Scholar]
- 19.De Pablo-Bernal RS, Ramos R, Genebat M, Canizares J, Rafii-El-Idrissi Benhnia M, Munoz-Fernandez MA, et al. Phenotype and polyfunctional deregulation involving interleukin 6 (IL-6)- and IL-10-producing monocytes in HIV-infected patients receiving combination antiretroviral therapy differ from those in healthy older individuals. J Infect Dis 2015; 213:999–1007. [DOI] [PubMed] [Google Scholar]
- 20.Wilson EM, Singh A, Hullsiek KH, Gibson D, Henry WK, Lichtenstein K, et al. Monocyte-activation phenotypes are associated with biomarkers of inflammation and coagulation in chronic HIV infection. J Infect Dis 2014; 210:1396–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker JV, Hullsiek KH, Singh A, Wilson E, Henry K, Lichtenstein K, et al. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS 2014; 28:831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schechter ME, Andrade BB, He T, Richter GH, Tosh KW, Policicchio BB, et al. Inflammatory monocytes expressing tissue factor drive SIV and HIV coagulopathy. Sci Transl Med 2017; 9:eaam5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 2012; 120:4599–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwa S, Kannanganat S, Nigam P, Siddiqui M, Shetty RD, Armstrong W, et al. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood 2011; 118:2763–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood 2007; 109:3351–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manches O, Munn D, Fallahi A, Lifson J, Chaperot L, Plumas J, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest 2008; 118:3431–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med 2010; 2:32ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol 2011; 11:176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller E, Bhardwaj N. Dendritic cell dysregulation during HIV-1 infection. Immunol Rev 2013; 254:170–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aiello A, Giannessi F, Percario ZA, Affabris E. The involvement of plasmacytoid cells in HIV infection and pathogenesis. Cytokine Growth Factor Rev 2018; 40:77–89. [DOI] [PubMed] [Google Scholar]
- 32.Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis 2003; 187:26–37. [DOI] [PubMed] [Google Scholar]
- 33.Killian MS, Fujimura SH, Hecht FM, Levy JA. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. AIDS 2006; 20:1247–1252. [DOI] [PubMed] [Google Scholar]
- 34.Sabado RL, O’Brien M, Subedi A, Qin L, Hu N, Taylor E, et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood 2010; 116:3839–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang M, Zhang H, Zhang T, Ji Y, Jiao Y, Wu H. Longitudinal changes of peripheral blood DC subsets and regulatory T cells in Chinese chronic HIV-1-infected patients during antiretroviral therapy. PLoS One 2012; 7:e37966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chehimi J, Azzoni L, Farabaugh M, Creer SA, Tomescu C, Hancock A, et al. Baseline viral load and immune activation determine the extent of reconstitution of innate immune effectors in HIV-1-infected subjects undergoing antiretroviral treatment. J Immunol 2007; 179:2642–2650. [DOI] [PubMed] [Google Scholar]
- 37.Martinson JA, Roman-Gonzalez A, Tenorio AR, Montoya CJ, Gichinga CN, Rugeles MT, et al. Dendritic cells from HIV-1 infected individuals are less responsive to Toll-like receptor (TLR) ligands. Cell Immunol 2007; 250:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buisson S, Benlahrech A, Gazzard B, Gotch F, Kelleher P, Patterson S. Monocyte-derived dendritic cells from HIV type 1-infected individuals show reduced ability to stimulate T cells and have altered production of interleukin (IL)-12 and IL-10. J Infect Dis 2009; 199:1862–1871. [DOI] [PubMed] [Google Scholar]
- 39.Bandera A, Mangioni D, Incontri A, Perseghin P, Gori A. Characterization of immune failure by monocyte activation phenotypes in HIV-infected patients receiving antiretroviral therapy. J Infect Dis 2015; 212:839–841. [DOI] [PubMed] [Google Scholar]
- 40.Wilson EM, Hullsiek KH, Sereti I, Baker JV. Response to Bandera et al. J Infect Dis 2015; 212:841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sachdeva N, Asthana V, Brewer TH, Garcia D, Asthana D. Impaired restoration of plasmacytoid dendritic cells in HIV-1-infected patients with poor CD4 T cell reconstitution is associated with decrease in capacity to produce IFN-alpha but not proinflammatory cytokines. J Immunol 2008; 181:2887–2897. [DOI] [PubMed] [Google Scholar]
- 42.Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW, Jr, Vasquez GM, et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol 1998; 72:7992–8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bess JW, Jr, Gorelick RJ, Bosche WJ, Henderson LE, Arthur LO. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology 1997; 230:134–144. [DOI] [PubMed] [Google Scholar]
- 44.Allan AL, Keeney M. Circulating tumor cell analysis: technical and statistical considerations for application to the clinic. J Oncol 2010; 2010:426218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fonteneau JF, Larsson M, Beignon AS, McKenna K, Dasilva I, Amara A, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol 2004; 78:5223–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macal M, Jo Y, Dallari S, Chang AY, Dai J, Swaminathan S, et al. Self-renewal and Toll-like receptor signaling sustain exhausted plasmacytoid dendritic cells during chronic viral infection. Immunity 2018; 48:730–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol Rev 2007; 220:214–224. [DOI] [PubMed] [Google Scholar]
- 48.Dillon SM, Lee EJ, Kotter CV, Austin GL, Gianella S, Siewe B, et al. Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T-cell activation in untreated HIV-1 infection. Mucosal Immunol 2016; 9:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–1371. [DOI] [PubMed] [Google Scholar]
- 50.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol 2012; 10:655–666. [DOI] [PubMed] [Google Scholar]
- 51.Rolle A, Olweus J. Dendritic cells in cytomegalovirus infection: viral evasion and host countermeasures. APMIS 2009; 117:413–426. [DOI] [PubMed] [Google Scholar]
- 52.Merlini E, Tincati C, Biasin M, Saulle I, Cazzaniga FA, d’Arminio Monforte A, et al. Stimulation of PBMC and monocyte-derived macrophages via Toll-like receptor activates innate immune pathways in HIV-infected patients on virally suppressive combination antiretroviral therapy. Front Immunol 2016; 7:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lane BR, King SR, Bock PJ, Strieter RM, Coffey MJ, Markovitz DM. The C-X-C chemokine IP-10 stimulates HIV-1 replication. Virology 2003; 307:122–134. [DOI] [PubMed] [Google Scholar]
- 54.Liovat AS, Rey-Cuille MA, Lecuroux C, Jacquelin B, Girault I, Petitjean G, et al. Acute plasma biomarkers of T cell activation set-point levels and of disease progression in HIV-1 infection. PLoS One 2012; 7:e46143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ploquin MJ, Madec Y, Casrouge A, Huot N, Passaes C, Lecuroux C, et al. Elevated basal preinfection CXCL10 in plasma and in the small intestine after infection are associated with more rapid HIV/SIV disease onset. PLoS Pathog 2016; 12:e1005774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khoury G, Anderson JL, Fromentin R, Hartogensis W, Smith MZ, Bacchetti P, et al. Persistence of integrated HIV DNA in CXCR3 + CCR6 + memory CD4+ T-cells in HIV-infected individuals on antiretroviral therapy. AIDS 2016; 30:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schrier SB, Hill AS, Plana D, Lauffenburger DA. Synergistic communication between CD4+ T cells and monocytes impacts the cytokine environment. Sci Rep 2016; 6:34942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perez-Santiago J, Ouchi D, Urrea V, Carrillo J, Cabrera C, Villa-Freixa J, et al. ART-suppressed subjects with low CD4 T-cell counts segregate according to opposite immunological phenotypes. AIDS 2016; 30:2275–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


