Abstract
INTRODUCTION,
we tested the hypothesis that brain arterial dilatation increases the risk of Alzheimer dementia (AD).
METHODS,
we studied dementia-free participants in the Washington Heights-Inwood Columbia Aging Project (WHICAP) who had a brain MRI and post-MRI dementia adjudication. We measured the axial T2-proton density diameters of the intracranial carotids and basilar diameters and used Cox models to obtain AD hazard ratios and 95% intervals.
RESULTS,
Out of 953 participants (mean age 77 ± 7, women 64%, 71% non-white) followed on average for 3 ± 3 years, 76 (8%) developed AD. In a model adjusted for demographics, vascular risks, APOE-ε4, and white matter hyperintensities, larger carotid diameters increased the risk of AD, defined categorically as ≥ 90th percentile (HR 4.34, 1.70-11.11) or continuously (HR 1.44 per SD, 1.07-1.94).
DISCUSSION,
understanding the pathophysiology of the association between AD and brain arterial dilatation may reveal new clues to the vascular contributions to AD.
Keywords: brain arterial dilatation, dolichoectasia, Alzheimer disease, dementia, carotid artery, brain arterial diameters
INTRODUCTION
The prevailing hypothesis of Alzheimer disease (AD) pathogenesis is that amyloid deposition initiates a cascade of biological events that lead to dementia and that minimizing amyloid deposition or removing amyloid may prevent, slow, or arrest AD.(1, 2) However, the amyloid pathway may not be the only target to modify the natural history of AD. In addition to amyloid deposition, individuals with AD often suffer from cerebrovascular disease. For example, individuals with AD have a high prevalence of intracranial large artery atherosclerosis (ILAA) and small vessel disease (SVD) at the time of death.(3) Additional clinical data further support the relationship between vascular disease and AD. Individuals with incident stroke are at higher risk of subsequent dementia,(4) and those with dementia are at higher risk of subsequent stroke.(5) In fact, cognitive decline may precede incident stroke, but incident stroke accelerates the slope of decline thereafter.(6) Even among individuals without clinical cerebrovascular disease, there is substantial evidence that “silent” MRI biomarkers of cerebrovascular disease, such as white matter hyperintensities (WMH), brain infarct, and microhemorrhages, relate to AD.(7–9)
Although the majority of brain large artery studies have focused on ILAA, we believe that ILAA is not the only brain large artery phenotype that relates to AD. Atherosclerosis is typically associated with reduction in luminal diameters, (10, 11) which may limit distal flow causing hypoperfusion.(12, 13) Dolichoectasia, on the other hand, is a form of brain large artery disease that consists of dilatation and/or tortuosity.(14) We previously validated the construct of brain arterial diameters as a normally-distributed spectrum of brain arterial health in which individuals with the smallest and the largest brain arterial diameters have a higher risk of vascular events than individuals with more average arterial diameters.(15, 16) We have also demonstrated in an autopsy sample that non-atherosclerotic brain arterial aging consists of luminal dilatation, elastin loss, internal elastic lamina gaps, and concentric thickening of the intima. A higher prevalence of pathological markers of brain arterial aging is associated with AD independent of brain infarcts, large artery amyloidosis, and ILAA.(17)
It is uncertain whether brain arterial dilatation may predict the risk of AD in living individuals. Understanding the role that brain arterial dilatation plays in cognitive decline and/or AD may uncover new pathways to reduce the risk of dementia. In this study, we tested the hypothesis that brain arterial dilatation is an MRI risk factor for AD.
METHODS
Sample:
The Washington Heights-Inwood Columbia Aging Project (WHICAP) enrolled Northern Manhattan community members in three recruitment waves, beginning in 1992 (n=2125), 1999 (n=2183), and 2010 (n=2124), using a sample of Medicare beneficiaries >65 years old. At baseline (i.e., enrollment visit), trained research staff obtain medical and neurological history and conduct a standardized physical and neurological examination. Each participant receives an assessment of health and function and a neuropsychological battery.(18) Age, ethnicity and sex were self-reported. Participants are followed approximately every 18-24 months and functional and cognitive data are collected at each visit. Hypertension, diabetes and dyslipidemia were defined as self-reported diagnosis or self-reported use of medications to treat these conditions. Smoking was defined as current at the time of MRI and based on self-report.
Magnetic resonance imaging:
Participants who remained free of dementia in their last neuropsychological testing and evaluation were invited to undergo structural MRI beginning in 2004 and in 2011. In 2004, MRI images were obtained on a 1.5T Philips Intera scanner with the following specification: T1-weighted (repetition time=20 ms, echo time=2.1ms, field-of-view 240 cm, 256×160 matrix, 1.3 mm slice thickness), T2-weighted FLAIR (repetition time=11,000ms, echo time=144.0 ms, inversion time=2800, field-of-view 25cm, 2nex, 256×192 matrix with 3 mm slice thickness) and proton density (repetition time=2675 ms, echo time=12 ms, field-of-view 220×165×140, 4 mm slice thickness). In 2011, MRI images were obtained on a 3T Philips scanner with the following specifications: T1-weighted (repetition time=6.6ms, echo time=3.0 ms, field-of-view 256×256×165, 1.0 mm slice thickness), T2-weighted FLAIR (repetition time=8000 ms, echo time=332 ms, field-of-view=240×240×180, 0.43 mm slice thickness) and proton density (repetition time=4000 ms, echo time=38 ms, field-of-view 230×187×148, 4 mm slice thickness). Images were acquired axially in both instances.
Brain Arterial Diameter measurements:
For the axial proton density images, we identified the “black void” (Figure 1) corresponding to the cross-sectional diameters of the ascending portion of the supraclinoid intracranial internal carotid artery (ICA) and the basilar artery (BA) at its most proximal segment to obtain their axial diameters. Each void was measured twice to form an X-shape that could capture variation in arterial angle, and both measurements were averaged to obtain the corresponding arterial diameter for each given void. The diameters of the left and right ICA as well as the BA were normalized and then averaged to obtain a global measure of diameters. We also averaged and normalized the diameter of both ICA to reflect anterior circulation diameters and used the basilar artery diameter alone to reflect the posterior circulation flow. All arterial measurement were obtained by a Vascular Neurologist. To evaluate for reliability, we obtained measures of reliability in 100 scans read by a pregraduate student and a Neurologist. The intraclass correlation coefficient (ICC) between a pregraduate student and Vascular neurologist was 0.75 for the BA, 0.53 for the right ICA and 0.56 for the left ICA and the ICC between a Neurologist and Vascular Neurologist was 0.77 for the BA, 0.74 for the left CA and 0.68 for the right ICA. This suggest a good reliability among more experienced readers. The relatively lower reliability with the carotid arteries is probably due to the varying curvatures and angles using axial T2 black voids as compared with flow-based measurements.
Figure 1. Example of MRI measurements of brain arterial diameters.
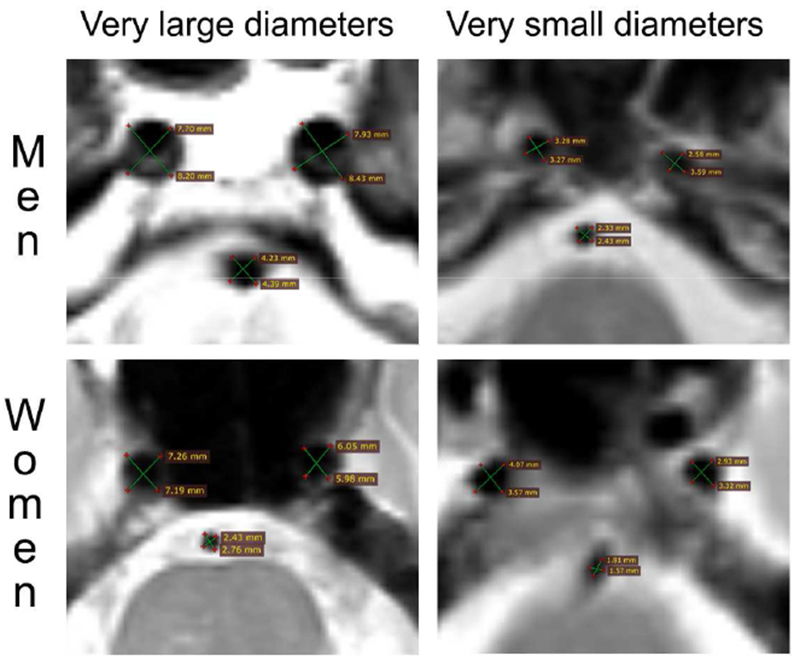
We measured the cross-sectional axial voids in brain MRI T2 sequences. Each artery was measured twice as demonstrated by the green lines intersection each round void. The two measurements in each artery where averaged to account for naturally occurring curvature and circular deformation of brain arteries as they course through the subarachnoid space intracranially.
Other MRI variables.
Whole-brain white matter hyperintensities (WMH) volumes were quantified with in-house developed software from FLAIR images.(19) Briefly, a Gaussian curve was fit to map voxel intensity values after stripping the skull. Voxels ≥1.8 and 2.1 standard deviations above the image mean intensity value for the first and second imaging samples, respectively, were labeled for subsequent quantification. The number of labeled voxels was summed and multiplied by voxel dimensions to yield total WMH volumes in cm3. Brain infarcts (cortical and subcortical) were defined by a pathology-informed algorithm that segregates chronic cavitated brain infarcts from large perivascular spaces.(20) Infarcts were coded if there was a discrete hypointense T1-weighted lesion ≥5mm in axial diameter with a corresponding hyperintense ring in FLAIR. This method has good to excellent reliability.(21)
Neuropsychological evaluation and diagnostic procedures.
Using standardized criteria, and through a consensus conference, a multidisciplinary team, including neuropsychologists and neurologists reviewed the cognitive, functional, and medical data collected at each visit and ascertained the diagnosis of AD.(22) MRI measurements were not included in the consensus review, and thus diagnoses were made blind to MRI variables. For this analysis, AD was used as the outcome of interest. We excluded participants with AD diagnosis ascertained prior to, simultaneously to, or within one year after the MRI visit to exclude prevalent AD (N=35).
Statistical analyses:
We constructed Cox proportional models with AD as the main outcome and brain arterial diameters as the main predictors to obtain Hazard ratios and their 95% confidence intervals. Time to event was defined as the time of MRI to the time of AD diagnosis or the date last seen during follow-up. We used separate models for the global, carotids and basilar arterial diameters, expressed continuously or categorized by deciles. We first obtained the crude AD incidence rate per 1,000 person-years using categories of brain arterial diameters ranked by percentiles. Based on prior study relating intracranial arterial diameters to systemic atherosclerosis,(23) we defined arbitrarily these categories as “small brain arterial diameters” if ≤ 10th percentile (n=98), “average brain arterial diameters” if >10th but <90th percentile (n=801), and “large” brain arterial diameter as ≥ 90th percentile (n=99). In each subsequent model, we progressively adjusted for possible confounders and reported the adjusted Hazard Ratios and their 95% confidence intervals. Because normal transformation of anthropometric traits relates better to certain genetic outcome,(24) we explored whether the strength and direction of association between AD and brain arterial diameters (in standard deviations) would differ from models using a normalized score versus the raw diameters (in millimeters). We evaluated nonlinear associations using Restricted Cubic Splines (RCS) macro in SAS,(25) which computes the coefficient of the spline with a predetermined number of knots to test the null hypothesis that a given relationship is linear using a Chi Square distribution.(26) Finally, we investigated whether the association between AD and brain arterial diameters vary by sex and by APOE status. All adjusted model included head size, the most important anthropometric determinant of brain arterial diameters. The statistical analysis was carried out with SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
-Cohort description:
We included 953 WHICAP participants (mean age 77 ± 7, women 64%, non-Hispanic white 29%, non-Hispanic black 34% and Hispanic 37%) with available data for this analysis (Table 1). Participants were followed on average for 4 ± 3 years (range 1-13 years). During follow-up, 76 participants developed AD (8 %).
Table 1:
Characteristics of the sample studied (N=953)
| Non-demented (N=877) | Incident Alzheimer dementia (N=76) | P- value | ||
|---|---|---|---|---|
| Age (mean±SD) | 76.6 ± 6.3 | 80.7 ± 6.0 | <0.001 | |
| Female sex (%) | 63 | 79 | 0.005 | |
| Etinicity (%) | ||||
| Non-Hispanic white | 29 | 16 | <0.001 | |
| Non-Hispanic black | 35 | 26 | ||
| Hispanic | 34 | 58 | ||
| Years of education (mean±SD, median, IQR) | 11 ± 5 | 8 ± 5 | <0.001 | |
| Hypertension (%) | 64 | 49 | 0.006 | |
| Diabetes (%) | 21 | 15 | 0.19 | |
| Dyslipidemia (%) | 35 | 22 | 0.02 | |
| Current smoking (%) | 5 | 7 | 0.52 | |
| Apo E4 (%, homo- or heterozygous) | 27 | 35 | 0.11 | |
| Silent brain infarcts (by MRI, %) | 35 | 38 | 0.61 | |
| White matter hyperintensities volume (mean±SD) | 4.1 ± 6.0 | 4.6 ± 5.6 | 0.50 | |
| Right ICA diameter (in mm, mean±SD) | 4.1 ± 0.7 | 4.1 ± 0.7 | 0.34 | |
| Left ICA diameter (in mm, mean±SD) | 4.2 ± 0.8 | 4.1 ± 0.8 | 0.25 | |
| Basilar artery (in mm, mean±SD) | 2.6 ± 0.7 | 2.4 ± 0.7 | 0.59 | |
Abbreviations: SD, standard deviation; IQR, interquartile range; APO, Apolipoprotein; MRI, magnetic resonance imaging; ICA, right internal carotid artery; mm, millimeter.
-Risk of AD and brain arterial diameters:
Participants with the largest (i.e. ≥ 90th percentile) average carotid diameters had the highest yearly crude rate of incident AD (37 cases per 1000 person-years) compared those with average (23 cases per 1000 person-years) or smaller carotid diameters (17 cases per 1000 person-years) or compared with any other group based on brain arterial diameters (table 2). In a time-based analysis, and adjusting gradually for possible covariates, the rate ratio of AD was higher among participants with larger normalized brain arterial diameters, globally (HR 1.37, 1.02-1.83), but particularly among those with larger normalized carotid arterial diameters (HR 1.44, 1.07-1.94, table 3). The strength of association between larger normalized carotid arterial diameters and AD risk did not change after adjusting for brain infarcts or WMH volume. There was no association between the normalized basilar artery diameter and AD risk. Using raw diameters demonstrated a similar direction of the association as when using normalized diameters but with a smaller effect size. The relationship between brain arterial diameters and AD risk is shown in figure 2.
Table 2:
Crude Alzheimer Disease Incidence Rate Per 1000 Person-Year by Brain Arterial Diameters Category
| Average carotid and basilar diameters | Average carotid artery diameters | Basilar artery | |
|---|---|---|---|
| Incidence rate (95% confidence intervals) | Incidence rate (95% confidence intervals) | Incidence rate (95% confidence intervals) | |
| Diameters < 10th percentile | 19.6 (9.8-31.1) | 17.3 (8.2-36.3) | 24.9 (11.9-52.3) |
| Diameters between the 10th to 90th percentile | 27.6 (9.8-39.2) | 26.6 (20.7-34.2) | 26.2 (20.4-33.6) |
| Diameters > 90th percentile | 29.1 (13.1-64.9) | 48.7 (24.4-97.4) | 31.8 (15.2-66.8) |
Table 3:
Risk of Alzheimer Disease and brain arterial diameters
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Hazard ratio, 95% CI | Hazard ratio, 95% CI | Hazard ratio, 95% CI | Hazard ratio, 95% CI | |
| Normalized carotid and basilar arteries average diameter (per SD) | 1.31, 1.02-1.67 | 1.36, 1.04-1.77 | 1.35, 1.03-1.76 | 1.39, 1.06-1.83 |
| Normalized carotid arteries average diameter (per SD) | 1.37, 1.06-1.77 | 1.46, 1.11-1.1.93 | 1.47, 1.11-1.94 | 1.49, 1.12-1.97 |
| Normalized basilar artery diameter (per SD) | 1.07, 0.82-1.37 | 1.04, 0.79-1.36 | 1.01, 0.77-1.33 | 1.04, 0.79-1.39 |
| Raw carotid and basilar arteries average diameter (per mm) | 1.17, 1.01-1.36 | 1.20, 1.03-1.40 | 1.20, 1.02-1.40 | 1.22, 1.03-1.43 |
| Raw carotid arteries average diameter (per mm) | 1.25, 1.04-1.51 | 1.31, 1.08-1.60 | 1.32, 1.08-1.61 | 1.33, 1.08-1.63 |
| Raw basilar artery diameter (per mm) | 1.09, 0.78-1.56 | 1.06, 0.72-1.54 | 1.02, 0.70-1.50 | 1.08, 0.72-1.60 |
Analytic notes:
Model 1: Adjusted for head size and brain MRI strength
Model 2: Model 1 plus age (at the time of MRI), sex, ethnicity, and years of education
Model 3: Model 2 plus hypertension, diabetes, dyslipidemia and smoking
Model 4: Model 3 plus silent brain infarcts and white matter hyperintensities volume
Abbreviations: CI, confidence interval; SD, standard deviation; mm, millimeter.
Figure 2. Risk of Alzheimer disease and brain arterial diameters.
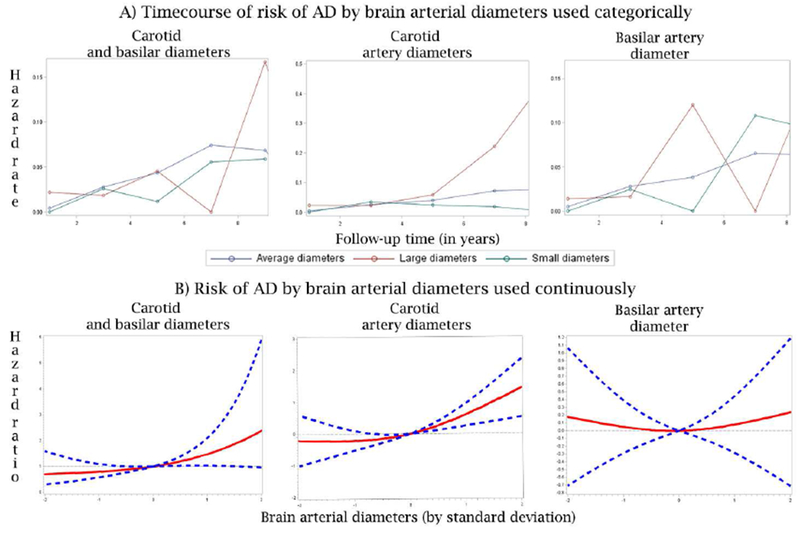
The top row described the risk of AD by time. It is apparent that the risk of AD increases after year 5 of follow up. Among participants with large carotid arteries, the risk increases substantially compared to those with small or more average carotid artery diameters. There appeared to be u-shaped relationship between basilar artery diameters and risk of AD, but the association was not statistically significant.
Plotting the risk of AD over time by brain arterial diameter evidence that the risk of AD for those with large average carotid arteries rose after the 4th year of follow-up whereas the trajectories of risk were equivocal for the other groups (Figure 2, column 2).
-Risk of AD and brain arterial diameters by APOE-ε4 status and sex:
In a stratified analysis by APOE-ε4, the point estimate of risk was similar for carotid diameters in APOE-ε4 negative versus positive participants (HR 1.47 vs. 1.79, P=0.76 for the interaction, table 4). The point estimate of risk attributable to basilar artery diameters was higher, but not significantly different, for APOE-ε4 negative versus APOE-ε4 positive participants (HR 1.16 vs. 0.67, P=0.27 for the interaction, table 4). There was no statistical interaction between brain arterial diameters with sex (P=0.47 for the interaction, table 4).
Table 4:
Risk of Alzheimer Disease and brain arterial diameters by APOE 4 status and sex
| Hazard ratio, 95% CI | P value for the interaction | ||
|---|---|---|---|
| APOE4 negative | APOE4 positive | ||
| Normalized carotid and basilar arteries average diameter (per SD) | 1.41, 0.99-2.01 | 1.42, 0.86-2.36 | 0.95 |
| Normalized carotid arteries average diameter (per SD) | 1.45, 1.00-2.11 | 1.71, 1.03-2.84 | 0.79 |
| Normalized basilar artery diameter (per SD) | 1.12, 0.78-1.61 | 0.81, 0.48-1.36 | 0.53 |
| Men | Women | ||
| Normalized carotid and basilar arteries average diameter (per SD) | 2.04, 1.10-3.79 | 1.29, 0.94-1.76 | 0.51 |
| Normalized carotid arteries average diameter (per SD) | 2.27, 1.13-4.59 | 1.38, 1.01-1.89 | 0.47 |
| Normalized basilar artery diameter (per SD) | 1.39, 0.72-2.67 | 0.97, 0.70-1.36 | 0.54 |
Analytic notes:
Model 1: Adjusted for head size and brain MRI strength
Model 2: Model 1 plus age (at the time of MRI), sex, ethnicity, and years of education
Model 3: Model 2 plus hypertension, diabetes, dyslipidemia and smoking
Model 4: Model 3 plus silent brain infarcts and white matter hyperintensities volume
Abbreviations: CI, confidence interval; SD, standard deviation; mm, millimeter.
DISCUSSION
Brain arterial diameters are imaging biomarkers of vascular health: very small or very large brain arterial diameters are pathological, and predict higher vascular risks.(15) We tested the hypothesis that brain arterial diameters may relate to AD risk. We present evidence that in individuals from a multi-ethnic urban cohort, those with larger carotid arteries are at a higher risk of AD independent of vascular risk factors and of MRI evidence of cerebrovascular disease. Although our study is underpowered to definitively confirm effect modification by sex or APOE-ε4 status, our data suggest that the risk may be higher among women. These results confirm recent observations that larger brain arterial diameters (measured by MRA) relate to poorer cognitive performance and greater cognitive decline over time(16) as well as to pathological evidence relating brain arterial dilatation occurring as part of aging with AD independent of brain infarcts or ILAA.(17) The association between dilated arteries and AD may imply a role in the pathogenesis but further work would be needed to establish such a causal inference.
In the absence of repeated measures of brain arterial diameters in the sample or mechanistic data, it is worth discussing plausible pathophysiological models that may help in contextualizing the reported associations. The first argument pertains to the coupling between brain metabolic demand and brain arterial diameters. In addition to the expected difference in brain arterial diameters dictated by the metabolic demands of the supplied organ, there are acquired and inherited factors that may contribute to brain arterial remodeling.(27) In principle, the greater the brain metabolic demand the higher the need for blood supply. This is demonstrated by the positive linear relationship between the middle cerebral artery diameter with the percentage of the hemisphere supplied by this artery,(28) and the strong positive correlation between head size (a surrogate of brain volume) with brain arterial diameters.(29) This biological principle, i.e. the supply matches the demand, is intuitive and it is not restricted to the brain and its arteries, but also applies to aortic (matching the body surface area)(30) and coronary diameters (matching heart weight).(31) Alzheimer disease consists of neuronal loss (32, 33) and decreased brain metabolic activity.(34) Together, these changes will most likely lead to decrease and not increase blood flow demand. Arteries remodel outward with increased and not decreased blood flow.(35, 36) Consequently, the notion that neurodegeneration of the brain parenchyma precedes or is causally related to dilatation of the carotid arteries, which supply more than 75% of the total brain flow,(28, 37) is counterintuitive, and consequently it may not be the underlying explanation to the findings reported here.
The association between larger carotid diameters and AD risk may also be explained by shared risk factors. For example, traditional risk factors such as hypertension and smoking have been associated with AD risk(38, 39) as well as with dilatation of the brain arteries.(14) Confounding effects by traditional vascular risk factors as an explanation to our findings is less likely given that the point estimate for AD risk attributable to carotid artery dilatation changed little after adjusting for traditional vascular risk factors. It is also possible that carotid artery dilatation and AD may share common genes, or that genes relating to AD risk or carotid artery dilatation are in linkage disequilibrium with other causative genes. There is data that partially support this hypothesis. For example, in a large meta-analysis of > 30,000 individuals with GWAS, some single nucleotide polymorphisms (SNP) that correlated with head size also correlated with height. In this same sample, there was a high genetic correlation between intracranial volume with adult cognitive function and Parkinson disease.(40) Therefore, it is possible that genes related with morphometric traits, such as brain arterial diameters, will also relate to cognition. We have previously reported that the single most important anthropometric trait associated with brain arterial diameter is intracranial volume, and in the absence of intracranial volume, height is the second best predictor.(29) It is possible that some SNPs determining height may determine brain arterial diameters and may correlate with adult cognition. Interestingly, there was no genetic correlation between AD and intracranial volume, but the study did not consider brain arterial diameter (a more direct trait to brain blood supply) as a possible confounder. The potential confounding role for shared genes should be further explored.
A third possible explanation is that carotid artery dilatation may precede or cause neuronal degeneration. For example, there is evidence that arterial dilatation is accompanied by elastin loss and fragmentation of the internal elastic lamina, features that most likely increase brain arterial stiffness.(17, 41) There is also evidence that arterial stiffness begins in the aorta early in life, and with aging, spreads to peripheral branches, and eventually the brain.(41–43) It is rare to have brain arterial disease in the absence of aortic or cervical carotid disease, and thus a centrifugal spreading of arterial disease is a model better supported by the current literature.(44, 45) In this context, brain arterial dilatation may be considered a biomarker of generalized arterial stiffness. The relationship between arterial stiffness and AD risk is supported by the fact that central measures of aortic stiffness are related to poorer cognition (46, 47) and to brain amyloid deposition.(48) If the link between systemic arterial stiffness and AD risk is further confirmed, it would offer a plausible and novel pathophysiological model for explaining the abundant data relating vascular risk factor and vascular disease to AD risk.(49)
The results presented here may be generalizable to similar multi-ethnic cohorts. The role of brain arterial dilatation in middle-age adults or in other ethnicities is less certain. The measurement of brain arterial diameters using axial T2 images is not unprecedented, (50) but using a more proper imaging modality to assess the lumen (i.e. MRA) would decrease error and may produce narrower confidence intervals. Furthermore, we do not have data regarding the collateral status through the circle of Willis nor the prevalence of intracranial stenosis, which is a limitation. This limitation is especially important because we have reported that the combined diameter of the left posterior cerebral artery and the left posterior communication artery may relate to episodic memory and is associated with greater cognitive decline.(51) Consequently, we cannot rule out a contribution of the posterior circulation to the risk of dementia. Also, we do not believe that the lack of intracranial stenosis data may negate the reported association between carotid arterial dilation and AD given that intracranial stenosis relates to atherosclerosis, and atherosclerosis is not often found pathologically in dilated brain arteries.(52, 53) The persistent association between AD risk and brain arterial diameters expressed as millimeters or as standard deviations suggest that raw diameters may be useful and easier to extrapolate to clinical populations.
In summary, and with these limitations in mind, we present data showing that carotid artery dilation is associated with a higher risk of AD. Understanding the mechanism by which carotid artery dilatation increases the risk of AD may offer a novel insight into the pathophysiology of AD and thus lead to explore new pathways to curb the societal burden of AD.
RESEARCH IN CONTEXT.
Imaging biomarkers of cerebrovascular disease, such as brain large artery atherosclerosis, brain infarct or white matter hyperintensities, has been associated with an increased risk of Alzheimer Disease (AD). Brain arterial dilatation has been associated with an increased risk of vascular events and cognitive decline, but whether individuals with brain arterial dilatation are at a higher risk of AD remains unexplored. In this work, we have confirmed the hypothesis that individuals with brain arterial dilatation are at a higher risk of AD independent of demographics, vascular risk factor, education, brain infarcts and white matter hyperintensities. There was no evidence of effect interaction by sex or APOE-ε4. If these results are confirmed mechanistically, they may point a new direction in the understanding of the vascular contribution to AD.
ACKNOWLEDGEMENT:
Data collection and sharing for this project was supported by the Washington Heights-Inwood Columbia Aging Project (WHICAP, PO1AG07232, R01AG037212, RF1AG054023, R01AG057709) funded by the National Institute on Aging (NIA) and by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873. This manuscript has been reviewed by WHICAP investigators for scientific content and consistency of data interpretation with previous WHICAP Study publications. We acknowledge the WHICAP study participants and the WHICAP research and support staff for their contributions to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Siemers ER, Dean RA, Friedrich S, Ferguson-Sells L, Gonzales C, Farlow MR, et al. Safety, tolerability, and effects on plasma and cerebrospinal fluid amyloid-beta after inhibition of gamma-secretase. Clin Neuropharmacol. 2007;30(6):317–25. [DOI] [PubMed] [Google Scholar]
- 2.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):311–21. [DOI] [PubMed] [Google Scholar]
- 3.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a crosssectional study. Lancet Neurol. 2016;15(9):934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tatemichi TK, Paik M, Bagiella E, Desmond DW, Stern Y, Sano M, et al. Risk of dementia after stroke in a hospitalized cohort: results of a longitudinal study. Neurology. 1994;44(10):1885–91. [DOI] [PubMed] [Google Scholar]
- 5.Chi NF, Chien LN, Ku HL, Hu CJ, Chiou HY. Alzheimer disease and risk of stroke: a population-based cohort study. Neurology. 2013;80(8):705–11. [DOI] [PubMed] [Google Scholar]
- 6.Rajan KB, Aggarwal NT, Wilson RS, Everson-Rose SA, Evans DA. Association of cognitive functioning, incident stroke, and mortality in older adults. Stroke. 2014;45(9):2563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodge HH, Zhu J, Woltjer R, Nelson PT, Bennett DA, Cairns NJ, et al. Risk of incident clinical diagnosis of Alzheimer’s disease-type dementia attributable to pathology-confirmed vascular disease. Alzheimers Dement. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tosto G, Zimmerman ME, Hamilton JL, Carmichael OT, Brickman AM. The effect of white matter hyperintensities on neurodegeneration in mild cognitive impairment. Alzheimers Dement. 2015;11(12):1510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vidoni ED, Yeh HW, Morris JK, Newell KL, Alqahtani A, Burns NC, et al. Cerebral beta-Amyloid Angiopathy Is Associated with Earlier Dementia Onset in Alzheimer’s Disease. Neurodegener Dis. 2016;16(3-4):218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352(13):1305–16. [DOI] [PubMed] [Google Scholar]
- 11.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365(11):993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caplan LR. Brain embolism, revisited. Neurology. 1993;43(7):1281–7. [DOI] [PubMed] [Google Scholar]
- 13.Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, et al. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler Thromb Vasc Biol. 2003;23(11):2055–62. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez J, Sacco RL, Wright CB. Dolichoectasia-an evolving arterial disease. Nat Rev Neurol. 2011;7(1):41–50. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez J, Cheung K, Bagci A, Rundek T, Alperin N, Sacco RL, et al. Brain Arterial Diameters as a Risk Factor for Vascular Events. J Am Heart Assoc. 2015;4(8):e002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez J, kulick E, Moon Y Park, Dong C, Cheung CH, Bagci A, et al. Brain arterial diameters and cognitive performance: the Northern Manhattan Study. J Int Neuropsychol Soc. 2017(22):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutierrez J, Honig L, Elkind MS, Mohr JP, Goldman J, Dwork AJ, et al. Brain arterial aging and its relationship to Alzheimer dementia. Neurology. 2016;86(16):1507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stern Y, Andrews H, Pittman J, Sano M, Tatemichi T, Lantigua R, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49(5):453–60. [DOI] [PubMed] [Google Scholar]
- 19.Brickman AM, Zahodne LB, Guzman VA, Narkhede A, Meier IB, Griffith EY, et al. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer’s disease incidence. Neurobiol Aging. 2015;36(1):27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutierrez J, Elkind MSV, Dong C, Di Tullio M, Rundek T, Sacco RL, et al. Brain Perivascular Spaces as Biomarkers of Vascular Risk: Results from the Northern Manhattan Study. AJNR Am J Neuroradiol. 2017;38(5):862–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez J, Elkind MS, Cheung K, Rundek T, Sacco RL, Wright CB. Pulsatile and steady components of blood pressure and subclinical cerebrovascular disease: the Northern Manhattan Study. J Hypertens. 2015;33(10):2115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez J, Rundek T, Cheung K, Bagci A, Alperin N, Sacco RL, et al. Systemic Atherosclerosis Relate to Brain Arterial Diameters: The Northern Manhattan Study. Cerebrovasc Dis. 2017;43(3-4):124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Yuan A, Han G, Gao G, Li Q. Rank-based tests for identifying multiple genetic variants associated with quantitative traits. Ann Hum Genet. 2014;78(4):306–10. [DOI] [PubMed] [Google Scholar]
- 25.Fang J, Austin PC, Tu JV. Test for linearity between continuous confounder and binary outcome first, run a multivariate regression analysis second. SAS Global Forum: Citeseer; 2009. [Google Scholar]
- 26.Heinzl H, Kaider A. Manual for the SAS-Macro RCS (Version 2. 0). Technical Report KB 1-96 Universitat Wien, Institut fur Medizinische Computerwissenschaften Abteilung fur Klinische Biometrie; 1996. [Google Scholar]
- 27.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330(20):1431–8. [DOI] [PubMed] [Google Scholar]
- 28.van der Zwan A, Hillen B, Tulleken CA, Dujovny M. A quantitative investigation of the variability of the major cerebral arterial territories. Stroke. 1993;24(12):1951–9. [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez J, Elkind MS, Gomez-Schneider M, DeRosa JT, Cheung K, Bagci A, et al. Compensatory intracranial arterial dilatation in extracranial carotid atherosclerosis: The Northern Manhattan Study. Int J Stroke. 2015;10(6):843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearce WH, Slaughter MS, LeMaire S, Salyapongse AN, Feinglass J, McCarthy WJ, et al. Aortic diameter as a function of age, gender, and body surface area. Surgery. 1993;114(4):691–7. [PubMed] [Google Scholar]
- 31.Hutchins GM, Bulkley BH, Miner MM, Boitnott JK. Correlation of age and heart weight with tortuosity and caliber of normal human coronary arteries. Am Heart J. 1977;94(2):196–202. [DOI] [PubMed] [Google Scholar]
- 32.Davies DC, Horwood N, Isaacs SL, Mann DMA. The effect of age and Alzheimer’s disease on pyramidal neuron density in the individual fields of the hippocampal formation. Acta Neuropathologica. 1992;83(5):510–7. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Pinilla E, Ordóñez C, del Valle E, Navarro A, Tolivia J . Regional and Gender Study of Neuronal Density in Brain during Aging and in Alzheimer’s Disease. Frontiers in Aging Neuroscience. 2016;8:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi S, Meguro K, Itoh M, Hayasaka C, Shimada M, Yamazaki H, et al. Decreased cortical glucose metabolism correlates with hippocampal atrophy in Alzheimer’s disease as shown by MRI and PET. J Neurol Neurosurg Psychiatry. 1997;62(6):596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas TL, Doyle JL, Distasi MR, Norton LE, Sheridan KM, Unthank JL. Involvement of MMPs in the outward remodeling of collateral mesenteric arteries. Am J Physiol Heart Circ Physiol. 2007;293(4):H2429–37. [DOI] [PubMed] [Google Scholar]
- 36.Tarhouni K, Guihot AL, Vessieres E, Toutain B, Procaccio V, Grimaud L, et al. Determinants of flow-mediated outward remodeling in female rodents: respective roles of age, estrogens, and timing. Arterioscler Thromb Vasc Biol. 2014;34(6):1281–9. [DOI] [PubMed] [Google Scholar]
- 37.Boyajian RA, Schwend RB, Wolfe MM, Bickerton RE, Otis SM. Measurement of anterior and posterior circulation flow contributions to cerebral blood flow. An ultrasound-derived volumetric flow analysis. J Neuroimaging. 1995;5(1):1–3. [DOI] [PubMed] [Google Scholar]
- 38.Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347(9009):1141–5. [DOI] [PubMed] [Google Scholar]
- 39.Corrada MM, Hayden KM, Paganini-Hill A, Bullain SS, DeMoss J, Aguirre C, et al. Age of onset of hypertension and risk of dementia in the oldest-old: The 90+ Study. Alzheimers Dement. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams HH, Hibar DP, Chouraki V, Stein JL, Nyquist PA, Renteria ME, et al. Novel genetic loci underlying human intracranial volume identified through genome-wide association. Nat Neurosci. 2016;19(12):1569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977;39(1):13–20. [DOI] [PubMed] [Google Scholar]
- 42.Bouissou H, Emery M, Sorbara R. Age related changes of the middle cerebral artery and a comparison with the radial and coronary artery. Angiology. 1975;26(3):257–68. [DOI] [PubMed] [Google Scholar]
- 43.Sorbara R Étude comparative du vieillissement des arteres du polygone de Willis. [Toulouse: ]: Université Paul-Sabatier; 1972. [Google Scholar]
- 44.Fisher CM, Gore I, Okabe N, White PD. Atherosclerosis of the carotid and vertebral arteries-extracranial and intracranial. Journal of Neuropathology & Experimental Neurology. 1965;24(3):455–76. [Google Scholar]
- 45.Sollberg LA, McGarry PA, Moossy J, Strong JP, Tejada C, Loken AC. Severity of atherosclerosis in cerebral arteries, coronary arteries, and aortas. Ann N Y Acad Sci. 1968;149(2):956–73. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain. 2011;134(Pt 11):3398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsao CW, Seshadri S, Beiser AS, Westwood AJ, Decarli C, Au R, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81(11):984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes TM, Kuller LH, Barinas-Mitchell EJ, Mackey RH, McDade EM, Klunk WE, et al. Pulse wave velocity is associated with beta-amyloid deposition in the brains of very elderly adults. Neurology. 2013;81(19):1711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pico F, Labreuche J, Touboul PJ, Amarenco P. Intracranial arterial dolichoectasia and its relation with atherosclerosis and stroke subtype. Neurology. 2003;61(12):1736–42. [DOI] [PubMed] [Google Scholar]
- 51.Gutierrez J, Kulick E, Moon Y Park, Dong C, Cheung K, Ahmet B, et al. Brain Arterial Diameters and Cognitive Performance: The Northern Manhattan Study. J Int Neuropsychol Soc. 2017:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gutierrez J, Goldman J, Dwork AJ, Elkind MS, Marshall RS, Morgello S. Brain arterial remodeling contribution to nonembolic brain infarcts in patients with HIV. Neurology. 2015;85(13):1139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lou M, Caplan LR. Vertebrobasilar dilatative arteriopathy (dolichoectasia). Ann N Y Acad Sci. 2010;1184:121–33. [DOI] [PubMed] [Google Scholar]


