Abstract
Acetabular labral tears are common in patients presenting with hip or groin pain. When the labrum is irreparable, labral reconstruction procedures are warranted. A circumferentially intact labrum is essential for joint health, whereas labral deficiency may predispose hip joints to osteoarthritis (OA). We aimed to determine whether labral reconstruction provides a benefit in terms of reduced OA development compared with labral resection. We performed labral reconstructions, labral resections, and sham operations in a porcine model. We assessed subsequent OA development by macroscopic observation, scanning electron microscopy, nanoindentation, histology, magnetic resonance imaging of cartilage in the acetabulum and femoral head, and enzyme-linked immunosorbent assays for inflammatory cytokines in the synovial fluid. We subjected the postoperative implants to biomechanical, histological, and mRNA expression analyses. At 24 weeks after surgery, the resected joints displayed apparent degenerative changes, in which the labral defects were filled with tiny amounts of loose, fibrous scar tissue. Compared with the resected joints, the reconstructed joints showed smooth and homogeneous geomorphology of cartilage surfaces; increased cartilage extracellular matrix (type II collagen and proteoglycans) content, elastic modulus, and hardness; and decreased type X collagen content, macroscopic and histological pathology scores, and inflammatory cytokines in the synovial fluid. The postoperative implants had compression, tensile, and histological features similar to those of the native labrum, which may have helped to attenuate OA development following labral reconstruction. Labral reconstruction using autografts greatly reduced OA progression compared with labral resection. The autologous implants used for reconstruction effectively restored the biomechanical and histological features of the labrum, contributing to hip joint homeostasis.
Keywords: Acetabular labral reconstruction, acetabular labral resection, autograft, osteoarthritis, biomechanical features
Introduction
The prevalence of acetabular labral tears in patients presenting with hip or groin pain ranges from 22% in athletes to 55% in patients of unknown etiology [1]. Treatments for acetabular labral damage have evolved continuously over the past decade. Despite the improved outcomes obtained with labral resection, many scholars emphasize the importance of labral tissue preservation, given that an intact labrum is essential for the functionality and longevity of the hip joint [2]. Comparative studies have reported that labral repair procedures result in significantly better pain relief and joint function compared with resection surgery [3]. However, the labrum is not suitable for repair in certain cases, such as those involving severe damage or irreparably degenerative, ossified, or small labral tissue. A novel procedure termed labral reconstruction, which was first reported by Philippon et al. in 2010, has been applied in patients with irreparable labral tears [4]. Several related studies reported that labral reconstruction with autografts or allografts provided sufficient short- and medium-term reduction in pain and increase in function [2,5,6].
Because a circumferentially intact labrum is vital for the functionality of the hip joint, existing labrum defects usually cause degenerative changes [7-9]. In addition to the relief of pain and mechanical symptoms, one of the most important goals of labral reconstruction is to maintain hip joint health, especially by protecting the articular cartilage against degenerative changes. It remains unclear, however, whether osteoarthritis (OA) progression would be induced by labral resection procedures and ameliorated by labral reconstruction.
The structure and biomechanical properties of the extracellular matrix (ECM) are vital to the functionality of the labrum. One previous study in a porcine model revealed that implants used for labral reconstruction could be converted to fibrocartilage rich in type I collagen, type II collagen, and proteoglycans by 24 weeks post-surgery [10]. This might help the implants acquire some of the biomechanical features of the native labrum. It remains unknown, however, whether the implants can provide biomechanical support similar to that of the native labrum.
The recently characterized porcine model is an excellent surrogate with which to study the treatment of human labral tears, because the anatomical structures and functions of the labrum and implant are similar between pigs and humans. We used the porcine model of labral tears to study 1) whether labral resection causes degenerative changes to the hip joint, 2) whether labral reconstruction with autografts attenuates OA development compared with labral resection, and if so, 3) whether the autografts can provide biomechanical support similar to that provided by the native labrum. We hypothesized that labral resection would induce OA in hip joints, whereas labral reconstruction using autografts would not cause OA development. We found that postoperatively, the implants effectively restored the biomechanical features of the labrum, contributing to hip joint homeostasis.
Materials and methods
All of the methods were carried out in accordance with the relevant guidelines. The experimental protocols involving animals were approved by the Animal Care and Use Committee of Peking University Third Hospital.
Surgical procedures
We used 12 male, skeletally mature, healthy experimental miniature pigs (24 weeks old, weighing between 25 kg and 30 kg) in this study. All of the pigs were subjected to bilateral hip joint surgeries. For the right hip joints, we performed labral reconstruction or labral resection (n = 6 for each group) in a randomly assigned manner. Sham procedures were performed on the left hip joints. During surgery, the pigs were anesthetized intravenously using propofol (20 drops/min). After skin disinfection, an S-shaped incision was made. The skin, subcutaneous fascia superficialis, and aponeurotic fascia were then exposed and incised. After bluntly separating the musculus gluteus superficialis, the mesogluteus was approached, and. an appropriate 1.0 cm-long and 0.4 cm-wide piece of mesogluteus tendon tissue was harvested for use as an implant (Figure 1A). After thoroughly removing any residual fresh muscular tissue from the implant, both ends of the implant were secured with non-absorbable 2-0 sutures. The musculus gluteus profundus was then bluntly separated and the acetabular labrum exposed via an incision in the hip joint capsule (Figure 1A). An appropriately 1.0 cm-long, anterior (cranial) dorsal labrum was transected sharply at both ends and removed from its bony origin down to the bleeding acetabular rim (Figure 1A). Care was taken to avoid causing cartilage damage at the acetabulum and femoral head. For the acetabular labral reconstruction procedure, the defect was reconstructed with the tendon implant fixed with two suture anchors (Arthrex, Inc., USA) (Figure 1A). For the acetabular labral resection procedure, no additional measures were performed after the labrum removal. We did not perform labrum removal or reconstruction for the sham procedures. Finally, the hip joint capsule, periarticular soft tissue, and skin were sutured with non-absorbable 3-0 sutures.
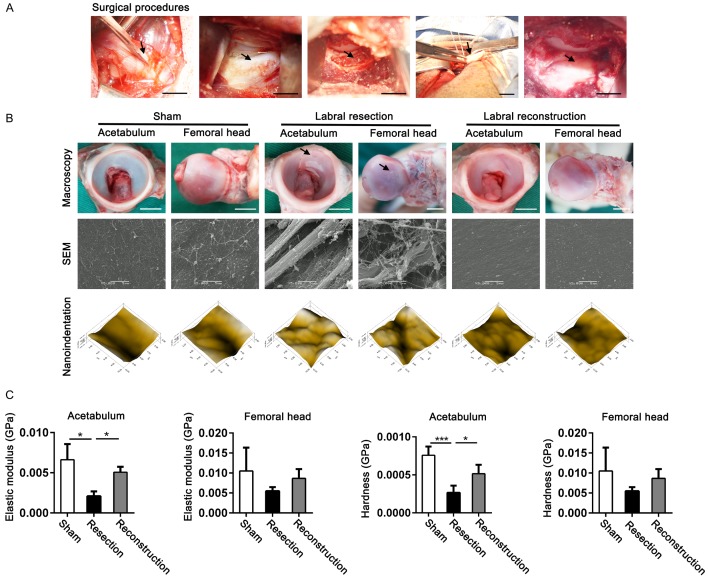
Figure 1.
OA development was halted by labral reconstruction surgery with autograft compared with labral resection, as evaluated by macroscopy and nanoindentation. A. Surgical procedures for sham operation, labral resection, and labral reconstruction with autografts in the porcine model (scale bar, 5 mm; arrows from left to right represent tendon implants, labrum, acetabular rim, tendon implants, and fixed tendon implants). B. Macroscopic and microscopic appearance of the cartilage surface located at the acetabulum and femoral head in the porcine model at 24 weeks postoperatively, as assessed by macroscopy, SEM, and micro-scanning of nanoindentation, respectively (scale bar, 5 mm; arrows represent cartilage lesions). C. Elastic modulus and hardness of cartilage in the acetabulum and femoral head of hip joints were assessed by nanoindentation in the porcine model 24 weeks postoperatively, in which sham, labral resection, and labral reconstruction surgeries were performed (n = 3 for each group). Data are shown as the mean ± SD. *P < 0.05. ***P < 0.001. The elastic modulus of acetabular cartilage was tested using a nonparametric test; others were tested using ANOVA.
Postoperative treatment
Postoperatively, all of the pigs recovered their health without immobilization or weight-bearing restriction. The pigs received prophylactic antibiotics (penicillin, intramuscular injection, 800,000 units, twice per day) for five consecutive days. At 24 weeks after surgery, all animals were sacrificed and assessed using various assays.
Macroscopic and histological examination
The gross morphology of the articular cartilage surface of the hip joint (sham, n = 3; resection and reconstruction, n = 6 each) was photographed and assessed using a semiquantitative scoring system by three researchers who were blinded to the nature of the experiment [11].
All samples were fixed with 10% neutral buffered formalin and decalcified for 3 days. They were then dehydrated in a graded alcohol series and embedded with paraffin. Sections (5 μm-thick) were cut and stained with hematoxylin and eosin (H&E), toluidine blue (TB) to stain positive for proteoglycans, Masson’s stain for type II collagen, and immunohistological stains for type I, II, and X collagen. Histological scores for cartilage in hip joints were graded using the Osteoarthritis Research Society International Cartilage Histopathology Assessment System (OOCHAS) [12] by three researchers who were blinded to the nature of the experiment.
Scanning electron microscopy (SEM)
SEM was performed to evaluate microscopic changes in the cartilaginous surfaces of the acetabulum and femoral head in sham-operated, resected, and reconstructed joints (n = 3 each). Samples were fixed in 25% glutaraldehyde for 3 days and then subjected to critical point drying. A 5-nm layer of gold was sprayed onto the sample surface using a high-vacuum gold spatter coater. Care was taken to avoid damaging the cartilage surface. Finally, the samples were observed using high-resolution SEM (S-2500, Tokyo, Japan).
Nanoindentation evaluation of cartilage
The biomechanical features of the cartilage, including microscopic geomorphology, elastic modulus, and hardness, were assessed using nanoindentation. Cartilage samples (n = 3 per group) were harvested from the acetabulum and femoral head of animals in each experimental group. Circumfluent phosphate-buffered saline (PBS) solution was used to maintain hydration during the tests. A TriboIndenter (Hysitron Inc., Minneapolis, MN, USA) with a conospherical diamond probe tip with 400-mm radial curvature was applied to evaluate the samples. Each indent site was tested using a trapezoidal load function that included loading (10 s), hold (2 s), and unloading (10 s). The maximum depth of indentation was 500 nm. A micro-scanning apparatus was used to capture the microscopic geomorphology of the indentation zones.
Magnetic resonance imaging (MRI) of cartilage
We evaluated the articular cartilage of the resected and reconstructed joints (n = 3 for each group) on an MRI scanner (Siemens, Magnetom Trio A Tim, 3T, Erlangen, Germany). Five sequences were included in the protocol for morphological analysis. The total acquisition time was about 35 min.
Inflammatory cytokines in the joint synovial fluid
Synovial fluid from hip joints (n = 4) was obtained using 18-gauge needles. After centrifugation at 4000 rpm for 20 min at 4°C, the supernatants of the synovial fluid samples were harvested for testing. Interleukin 6 (IL6) and tumor necrosis factor α (TNFα) in the samples were analyzed using standard enzyme-linked immunosorbent assays (ELISAs, R&D Systems, Minneapolis, MN, USA).
Biomechanical features of the labra and implants
We harvested the labra and implants at 24 weeks post-surgery and tested them immediately after the animals were sacrificed. The compression and tensile properties of the samples were investigated using a universal material testing apparatus, which included the compression elastic modulus, tensile elastic modulus, strain at failure, and tensile stress at failure. All samples were shaped into rectangular forms oriented parallel to the articular surface along the acetabular rim. We measured the length, width, and height using a digital caliper with an accuracy of ± 0.02 mm. The range of the load cell was 0-70 N with a loading rate of 0.5 mm/min in the compression test. Hemostatic forceps were used to fix the specimens on the universal material testing apparatus during the tensile test. The range of the load cell was 0-10 N with a loading rate of 0.5 mm/min in the tensile test. During the tests, the samples were moistened with normal saline.
Real time polymerase chain reaction (RT-PCR)
At 24 weeks postoperatively, animals were euthanized and the native labrum, native tendon tissue, and implants for labral reconstruction (n = 3 for each group) were harvested promptly under sterile conditions and were subsequently used for cell cultures. Total RNA was extracted from the primary cell samples using TRIzol (Invitrogen, Carlsbad, CA, USA) and then reverse-transcribed using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Boston, MA, USA). RT-PCR was performed using the Applied Biosystems StepOnePlus Real-Time PCR System (Foster City, CA, USA). The relative expression levels of extracellular matrix genes related to fibrocartilage, including collagen type I alpha 1 chain (Col1a1) and collagen type II alpha 1 chain (Col2a1), were investigated. All signals were normalized to that of glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and relative gene expression levels were calculated using the 2-ΔΔCT method. Primer sequences are shown in Table 1.
Table 1.
Primer sequences
| Genes | Primers | |
|---|---|---|
| Col1a1 | Forward | GGGCAACAGCAGATTCACCTAC |
| Reverse | GGTCAGCATCACCGATGTCCAA | |
| Col2a1 | Forward | CGGGAAAGACGGCGGAAGAGG |
| Reverse | AGCACCGGGAGGACCAGCGAA | |
| Gapdh | Forward | CTGCCCCTTCTGCTGATGC |
| Reverse | CATCACGCCACAGTTTCCCA | |
Statistical analysis
We used SPSS 18.0 statistical software (IBM Corp, Chicago, IL, USA) for statistical analysis. All data were recorded as the mean ± standard deviation (SD). Unpaired two-tailed Student’s t-tests (two groups) or one-way ANOVA (homogeneity of variance, three groups) were used to analyze normally distributed data. Nonparametric tests were used to analyze non-normally distributed data. A P value < 0.05 was considered as indicating statistical significance.
Results
Prevention of OA development by labral reconstruction using autografts
To clarify whether labral reconstruction with autologous tendon tissue could attenuate OA development compared with labral resection, we evaluated the articular cartilage located at the acetabulum and the femoral head for signs of degeneration. Macroscopic evaluation revealed smooth and continuous cartilage surfaces on the acetabulum and femoral head in the sham-operated joints. In the resected joints, the cartilage surfaces on the acetabulum and femoral head showed fibrillation, crevasse, and delamination, especially in the zone where the anterior (cranial) dorsal labrum was removed, suggesting that degenerative changes were induced by labral deficiency. In the reconstructed joints, the cartilage surfaces were smooth, transparent, and continuous, similar to those in the sham-operated joints (Figure 1B). The macroscopic scores used to evaluate OA severity in the different groups showed that labral resection (6.2 ± 0.8) could significantly induce OA development compared with the sham procedure (0.0 ± 0.0), but labral reconstruction markedly inhibited OA (0.7 ± 0.5) compared with labral resection (Figure S1A).
SEM revealed intact cartilage surfaces in the sham group. There was abrasion on the cartilage surface of the acetabulum and femoral head in the resected joints, including discontinuous and delaminated cartilage surface and loose, irregular, broken, or exposed collagen fibers. By contrast, the cartilage surface of the acetabulum and femoral head in the reconstructed joints were smooth and homogeneous, without cracks or delamination. The collagen fibers in the reconstructed joints were arranged tightly without breaks (Figure 1B), which was consistent with the macroscopic observations.
We performed nanoindentation to capture the microscopic geomorphology and test the biomechanical properties of articular cartilage in the joints. Using micro-scanning, we observed that the articular surface in the resected joints was much rougher and more uneven than that in the sham-operated joints. The articular surfaces in the reconstructed joints were smooth and homogeneous, similar to those in the sham-operated joints (Figure 1B).
The elastic modulus and hardness of the cartilage in the acetabulum were markedly decreased in the resected joints compared with those in the sham-operated joints. These properties were effectively restored in the reconstructed joints, illustrating recovery of compression properties after reconstruction. The elastic modulus and hardness of the cartilage obtained from the femoral head showed similar trends to those in the cartilage from the acetabulum, although there were no statistically significant differences in mechanical indices of the femoral cartilage among the sham-operated, resected, and reconstructed joints (Figure 1C).
Histological assessment of OA
We performed histological staining to further determine whether joint health was maintained after labral reconstruction with autografts. H&E staining revealed that the articular cartilage of the hip joint was smooth and intact in the sham-operated joints, with oval and round-shaped chondrocytes apparent within the cartilage tissue. In the resected joints, the cartilaginous surfaces of the acetabulum and the femoral head were uneven and discontinuous, with complex vertical and horizontal cracks that reached down to the subchondral bone. Microfractures and fibrous tissue repair were simultaneously visible, and there was an incomplete tide line and decreased numbers of chondrocytes. In the reconstructed joints, the cartilage surface, ECM structure, and chondrocyte phenotype remained intact (Figure 2A).
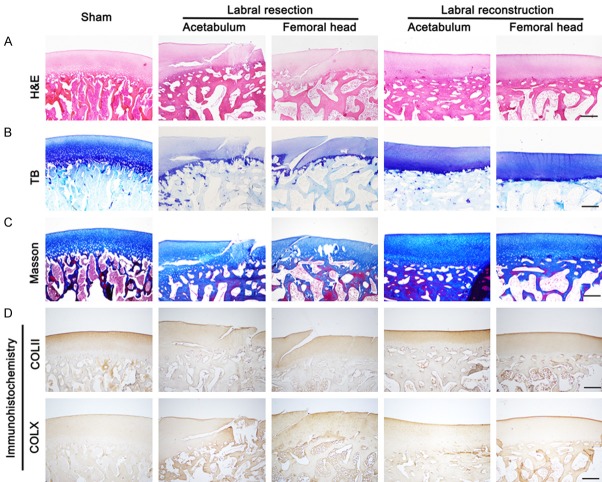
Figure 2.
Histological assessment of articular cartilage of acetabulum and femoral head in sham-operated, resected, and reconstructed joints of porcine models at 24 weeks postoperatively, including H&E, TB, Masson staining, and immunohistochemical staining for type II and X collagen, as shown in (A-D) (sham, n = 3; resection and reconstruction, n = 6). Scale bar, 500 μm.
TB staining of proteoglycans revealed strong positive and uniform coloring in the articular cartilage of the sham-operated joints. By contrast, the TB staining in the articular cartilage was shallower and more uneven in the resected joints, indicating decreased proteoglycan content. The articular cartilage in the reconstructed joints showed strong and uniform TB staining resembling that in the sham-operated joints (Figure 2B).
Type II collagen fibers are the most important ECM component within cartilage tissue. Type II collagen (Masson) staining in the sham-operated joints was strongly positive and uniform, with the collagen fibers arranged neatly. By contrast, type II collagen fiber staining in the resected joints was weak and uneven with a disordered structure, suggesting that type II collagen content was markedly decreased after labral resection, compared with that after the sham operation. Similar to collagen staining in the sham-operated joints, the collagen staining in the reconstructed joints was strong and uniform (Figure 2C, 2D).
Type X collagen is a sign of cartilage hypertrophy, which increases significantly when the cartilage is damaged. As shown in Figure 2D, type X collagen staining in the resected joints was stronger and more uneven than that in the sham-operated joints, indicating increased type X collagen content in the resected joints. Type X collagen staining was significantly weaker in the reconstructed joints compared with that in the resected joints.
We measured the severity of OA using histological scores according to the OOCHAS. The OOCHAS score for the resected joints was 8.7 ± 1.6, which was significantly higher than that for the sham-operated joints. The OOCHAS score for the reconstructed joints (1.2 ± 0.8) was lower than that for the resected joints, suggesting that fewer OA changes occurred after labral reconstruction surgery than after labral resection (Figure S1B).
MRI and ELISA for inflammatory cytokines to determine OA attenuation
We used high-resolution MRI arthrography to evaluate articular cartilage changes following labral resection and labral reconstruction. In the resected joints, the signal density of the articular cartilage was uneven, and there was contrast infiltration into the cartilage tissue, suggesting that the labral resection procedure induced degenerative changes. In the reconstructed joints, the cartilage surface was smooth and intact, indicating that degeneration of the cartilage structure was effectively prevented (Figure 3A).
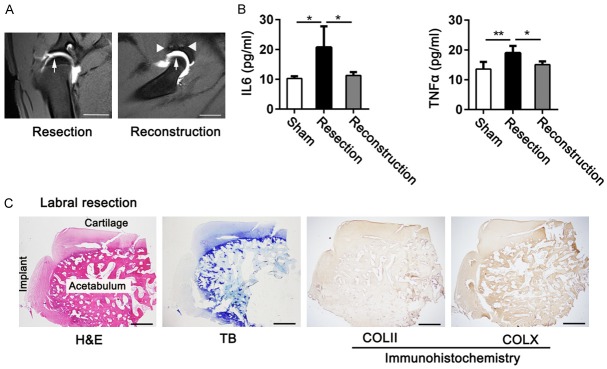
Figure 3.
OA attenuation induced by labral reconstruction procedures was assessed by MRI and ELISAs. Histological features in the site of labral deficiency in resected joints were also evaluated. A. Cartilage features of hip joints in the labral resected and reconstructed joints were observed using MRI at 24 weeks postoperatively (scale bar, 2 cm; arrows represent articular cartilage; triangles represent suture anchors; n = 3 for each group). B. Concentrations of IL6 and TNFα cytokines in hip joint synovial fluid in the sham, labral resection, and labral reconstruction groups were measured by ELISA at 24 weeks postoperatively (n = 4 for each group; IL6, ANOVA; TNFα, nonparametric test). C. Histological assessment of regenerated tissue located at labral defects in the labral resection group using H&E, TB, and immunohistochemical staining at 24 weeks postoperatively (n = 6; scale bar, 1 mm). Data are shown as mean ± SD. *P < 0.05. **P < 0.01. ***P < 0.001.
We used ELISAs to evaluate the levels of IL6 and TNFα, the most important inflammatory cytokines within the hip joint synovial fluid. As shown in Figure 3B, IL6 and TNFα concentrations were significantly elevated in the resected joints compared with those in the sham-operated joints as well as those in the reconstructed joints.
Effective restoration of biomechanical properties of the labrum after labral reconstruction with autografts
To determine the underlying mechanism by which labral reconstruction could effectively attenuate OA development in hip joints, we tested the biomechanical features of the labra and implants, including their compression and tensile properties. Only tiny amounts of loose scar tissue, lacking proteoglycans and type II collagen but positive for type X collagen, appeared in the area of the labral defect after labral resection at 24 weeks after surgery (Figures 1B, 3C). We therefore examined the biomechanical properties of tendon implants in the reconstructed joints and the labra in the sham-operated joints at 24 weeks after surgery to determine whether the implants could restore the biomechanical features of the labra.
For the compression test, we obtained labra and implants of similar sizes (Figure 4A, 4B). The typical compression force-stroke curve of the implants was similar to that of the labra (Figure 4C, 4D). Moreover, the compression elastic modulus of the implants (43.1 ± 4.6 MPa) was partly restored to that of the labra (64.4 ± 9.9 MPa) (Figure 4E).
Figure 4.
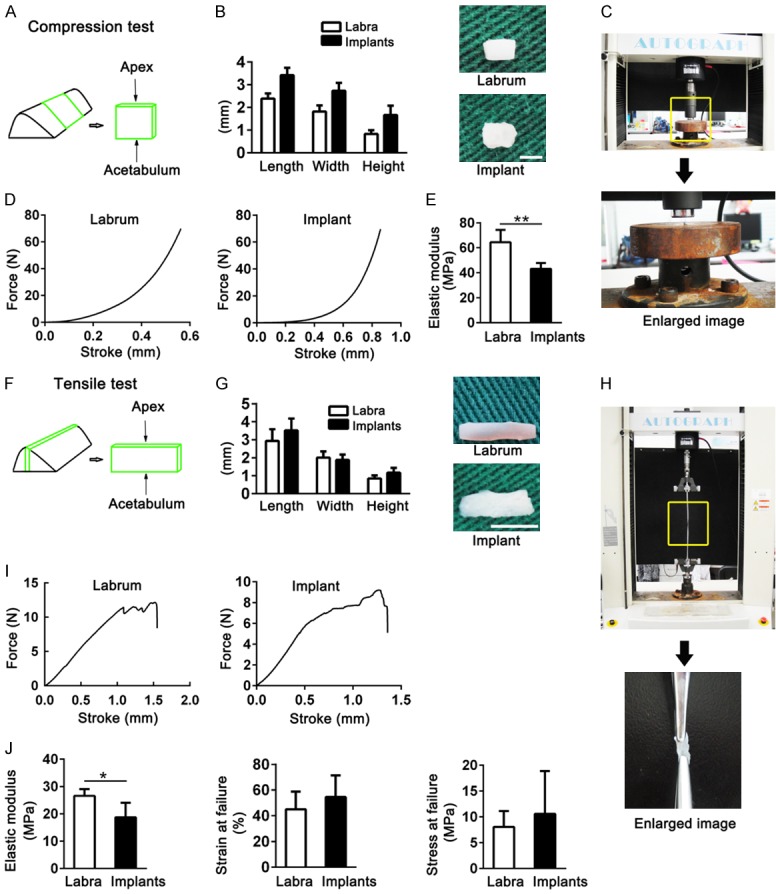
Biomechanical properties of native labra in sham-operated joints and in implants in reconstructed joints were evaluated in the porcine model at 24 weeks postoperatively. (A-E) Showed outcomes from compression tests. (A) Schematic of sample harvesting. (B) The length, width, and height of the samples (scale bar, 1 mm). (C) The test platform with universal material testing apparatus. (D) Force-stroke curve of the labra and implants. (E) Elastic modulus of the labra and implants (unpaired two-tailed Student’s t-tests; n = 5 for each group). (F-J) Showed the tensile tests results. (F) Schematic of sample harvesting. (G) Sample sizes (scale bar, 2 mm). (H) The universal material testing apparatus used for the test. (I) Force-stroke curve of the samples. (J) Biomechanical features of the labra and implants (unpaired two-tailed Student’s t-tests; elastic modulus, n = 5; strain at failure and stress at failure, n = 3 for labra, n = 4 for implants). Data are shown as mean ± SD. *P < 0.05. **P < 0.01.
We tested the tensile features of labra and implants of similar length, width, and height (Figure 4F-H). The labra and implants had similar tensile force-stroke curves (Figure 4I). The implants could support effective resistance to tensile force, with an elastic modulus of 18.7 ± 5.3 MPa, strain at failure of 54.5 ± 17.0%, and stress at failure of 10.1 ± 8.3 MPa. The labra from the sham-operated joints scored 26.6 ± 2.4 MPa for elastic modulus, 45.0 ± 13.8% for strain at failure, and 8.0 ± 3.1 MPa for stress at failure (Figure 4J). Those outcomes indicated that the implants at 24 weeks after surgery provided effective support to resist the compression and tensile forces produced during hip joint motion, which might have contributed to hip joint homeostasis following the labral reconstruction procedure.
Fibrocartilage tissue was regenerated within implants for labral reconstruction
To evaluate whether tendon implants for labral reconstruction could transform into fibrocartilage tissue, histological staining was performed, including H&E and immunohistochemical staining for type I and II collagen. Fibrocartilage tissue, which was rich in type I and II collagen, was observed within implants harvested 24 weeks postoperatively (Figure S2). The morphologies of tendon, labrum, and implant cells in the labral reconstructed joints were then evaluated microscopically. As shown in Figure 5A, tendon cells appeared long and spindle-shaped, whereas the acetabular labral cells were rounded and spindle-shaped. The cellular phenotype in the implants at 24 weeks after operation was rounded and spindle-shaped, similar to the labral cells.
Figure 5.
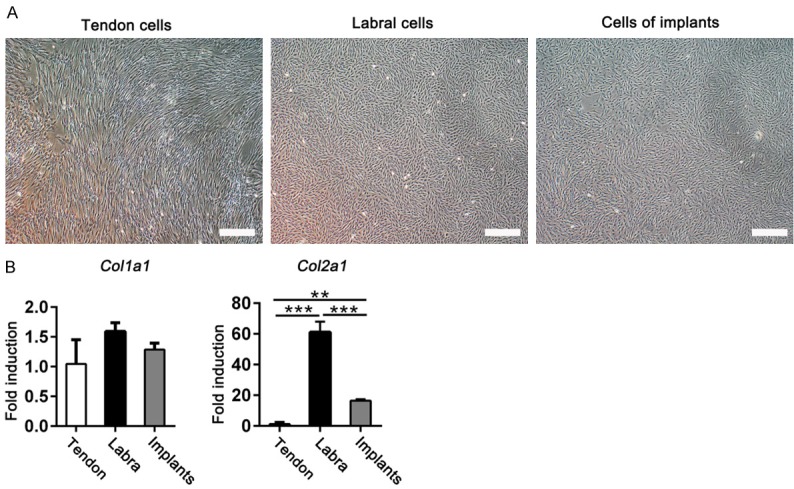
A. Cellular phenotypes in tendons, labra, and implants in labral reconstructed joints at 24 weeks postoperatively were observed under the light microscope. Scale bar, 200 μm. B. Col1a1 and Col2a1 mRNA levels were tested in tendon, labrum, and implant cells in the labral reconstruction group at 24 weeks postoperatively (n = 3 for each group; Col1a1, nonparametric test; Col2a1, ANOVA). Data are shown as the mean ± SD. **P < 0.01. ***P < 0.001.
In addition, the mRNA expression levels of extracellular matrix-related genes were analyzed. Col1a1 expression levels showed no differences between tendon cells, labral cells, and cells from labral reconstruction implants at 24 weeks postoperatively (Figure 5B), whereas Col2a1 mRNA levels were remarkably elevated in implant cells compared to the levels in tendon cells (Figure 5B). These results suggest that implants effectively restored the biomechanical features of the labra through regenerated fibrocartilage tissue.
Discussion
Labral tears are the most common cause of intra-articular hip pain and the most common indication for hip arthroscopy [13,14]. Although labral reconstruction with autografts and allografts has shown promising outcomes [2,5,6], its impact on hip joint health remains unclear. We observed degenerative changes in porcine hip joints at 24 weeks after labral resection, characterized by labral defects filled with tiny amounts of loose fibrous scar tissue. We found that acetabular labral reconstruction with autografts markedly attenuated OA development compared with labral resection: the reconstructed joints had increased cartilage ECM content (type II collagen and proteoglycans), decreased OOCHAS scores, and decreased levels of inflammatory cytokines in synovial fluids at 24 weeks after surgery compared with the resected joints. We confirmed that the implants used for labral reconstruction had similar biomechanical and histological properties to the native labrum at 24 weeks after surgery, which may provide the basis for the observed OA attenuation.
The acetabular labrum provides a sealing effect, which creates both hydrostatic pressure to slow cartilage consolidation during hip joint motion and a “suction effect” to resist distraction of the hip joint. Moreover, the labrum provides mechanical support by effectively deepening the acetabular socket [1,7,13,15-17]. Therefore, once the labrum is damaged or deficient, the sealing effect and the mechanical support become compromised, predisposing the hip joint to femoral head instability within the acetabulum. All of these factors contribute to cartilage damage, eventually leading to OA development.
Many studies have explored the causal relationship between labral tears or deficiency and early coxarthrosis. Patients with severely damaged labral tissue often have poor cartilage on the edge of the acetabulum [18]. McCarthy et al. reported that labral lesions contributed to early degenerative hip disease, possibly because of failure of the labral-chondral junction [19]. Furthermore, resected labra displayed decreased intra-articular fluid pressurization and faster cartilage consolidation compared with intact labra [8,9]. A laboratory study using a sheep model reported mild degenerative changes after labral resection, with dense, triangular, fibrous scars that filled the labral defect [20]. Those previous findings support the hypothesis that labral deficiency can cause OA degenerative changes in hip joints. Our research using a porcine model showed that by 24 weeks post-surgery, labral resection greatly disrupted hip joint homeostasis and induced apparent OA development, including decreased content of ECM molecules in the cartilage, uneven cartilage surfaces with cracks, and increased inflammatory cytokines in the synovial fluid. We observed tiny amounts of loose scar tissue in the area of the labral defect following labral resection, which cannot effectively deepen the acetabular volume, possibly explaining why labral resection may lead to OA development in hip joints.
Cadaveric studies reported that implants used for labral reconstruction would allow for various biomechanical benefits similar to those of the native labrum. Philippon et al. found that labral reconstruction effectively restored intra-articular fluid pressurization to levels similar to the intact state via the hip fluid seal, whereas labral resection decreased the intra-articular fluid pressurization [9]. Furthermore, recent cadaveric research revealed that labral reconstruction resulted in improvements in the hip joint contact area and contact pressure, compared with labral resection [21]. Another cadaveric study showed that compared with labral resection, labral reconstruction could significantly improve distractive stability via the suction effect of the hip fluid seal [22]. Accordingly, labral reconstruction with grafts may support joint health maintenance by providing improved biomechanical support in terms of intra-articular fluid pressurization, joint contact area, contact pressure, and distractive strength. We will investigate this hypothesis further in future work.
Conclusions
In conclusion, labral reconstruction using autografts significantly halted OA progression at 24 weeks post-surgery compared with labral resection procedures, along with restoration of histological and biomechanical features in the implants. The OA attenuation benefits presented in our research shed light on the utility of labral reconstruction with autografts for irreparable labral tears. Moreover, it is reasonable to suppose that similar benefits would be realized in human patients.
Acknowledgements
We thank the funding of Natural Science Foundation of Beijing Municipality (7172229). Meanwhile, we thank Jiying Zhang, Xin Fu, and Xiaoning Duan for technical help with the study.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Domb BG, Hartigan DE, Perets I. Decision making for labral treatment in the hip: repair versus debridement versus reconstruction. J Am Acad Orthop Surg. 2017;25:e53–e62. doi: 10.5435/JAAOS-D-16-00144. [DOI] [PubMed] [Google Scholar]
- 2.White BJ, Stapleford AB, Hawkes TK, Finger MJ, Herzog MM. Allograft use in arthroscopic labral reconstruction of the hip with front-to-back fixation technique: minimum 2-year follow-up. Arthroscopy. 2016;32:26–32. doi: 10.1016/j.arthro.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Kelly BT, Weiland DE, Schenker ML, Philippon MJ. Arthroscopic labral repair in the hip: surgical technique and review of the literature. Arthroscopy. 2005;21:1496–1504. doi: 10.1016/j.arthro.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Philippon MJ, Briggs KK, Hay CJ, Kuppersmith DA, Dewing CB, Huang MJ. Arthroscopic labral reconstruction in the hip using iliotibial band autograft: technique and early outcomes. Arthroscopy. 2010;26:750–756. doi: 10.1016/j.arthro.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Geyer MR, Philippon MJ, Fagrelius TS, Briggs KK. Acetabular labral reconstruction with an iliotibial band autograft: outcome and survivorship analysis at minimum 3-year follow-up. Am J Sports Med. 2013;41:1750–1756. doi: 10.1177/0363546513487311. [DOI] [PubMed] [Google Scholar]
- 6.Domb BG, El BY, Stake CE, Trenga AP, Jackson TJ, Lindner D. Arthroscopic labral reconstruction is superior to segmental resection for irreparable labral tears in the hip: a matched-pair controlled study with minimum 2-year follow-up. Am J Sports Med. 2014;42:122–130. doi: 10.1177/0363546513508256. [DOI] [PubMed] [Google Scholar]
- 7.Bsat S, Frei H, Beaule PE. The acetabular labrum: a review of its function. Bone Joint J. 2016;98-B:730–735. doi: 10.1302/0301-620X.98B6.37099. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson SJ, Bryant JT, Ganz R, Ito K. The influence of the acetabular labrum on hip joint cartilage consolidation: a poroelastic finite element model. J Biomech. 2000;33:953–960. doi: 10.1016/s0021-9290(00)00042-7. [DOI] [PubMed] [Google Scholar]
- 9.Philippon MJ, Nepple JJ, Campbell KJ, Dornan GJ, Jansson KS, LaPrade RF, Wijdicks CA. The hip fluid seal--part i: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22:722–729. doi: 10.1007/s00167-014-2874-z. [DOI] [PubMed] [Google Scholar]
- 10.Shi YY, Chen LX, Xu Y, Hu XQ, Ao YF, Wang JQ. Acetabular labral reconstruction with autologous tendon tissue in a porcine model: in vivo histological assessment and gene expression analysis of the healing tissue. Am J Sports Med. 2016;44:1031–1039. doi: 10.1177/0363546515623784. [DOI] [PubMed] [Google Scholar]
- 11.Laverty S, Girard CA, Williams JM, Hunziker EB, Pritzker KP. The oarsi histopathology initiative-recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthritis Cartilage. 2010;18(Suppl 3):S53–S65. doi: 10.1016/j.joca.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Custers RJ, Creemers LB, Verbout AJ, van Rijen MH, Dhert WJ, Saris DB. Reliability, reproducibility and variability of the traditional histologic/histochemical grading system vs the new oarsi osteoarthritis cartilage histopathology assessment system. Osteoarthritis Cartilage. 2007;15:1241–1248. doi: 10.1016/j.joca.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Lertwanich P, Plakseychuk A, Kramer S, Linde-Rosen M, Maeyama A, Fu FH, Smolinski P. Biomechanical evaluation contribution of the acetabular labrum to hip stability. Knee Surg Sports Traumatol Arthrosc. 2016;24:2338–2345. doi: 10.1007/s00167-015-3555-2. [DOI] [PubMed] [Google Scholar]
- 14.Hapa O, Barber FA, Basci O, Horoz L, Ertem F, Karakasli A, Havitcioglu H. Biomechanical performance of hip labral repair techniques. Arthroscopy. 2016;32:1010–1016. doi: 10.1016/j.arthro.2015.12.044. [DOI] [PubMed] [Google Scholar]
- 15.Signorelli C, Bonanzinga T, Lopomo N, Zaffagnini S, Marcacci M, Safran M. Evaluation of the sealing function of the acetabular labrum: an in vitro biomechanical study. Knee Surg Sports Traumatol Arthrosc. 2017;25:62–71. doi: 10.1007/s00167-015-3851-x. [DOI] [PubMed] [Google Scholar]
- 16.Myers CA, Register BC, Lertwanich P, Ejnisman L, Pennington WW, Giphart JE, LaPrade RF, Philippon MJ. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(Suppl):85S–91S. doi: 10.1177/0363546511412161. [DOI] [PubMed] [Google Scholar]
- 17.Chahla J, Soares E, Bhatia S, Mitchell JJ, Philippon MJ. Arthroscopic technique for acetabular labral reconstruction using iliotibial band autograft. Arthrosc Tech. 2016;5:e671–e677. doi: 10.1016/j.eats.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philippon MJ, Briggs KK, Yen YM, Kuppersmith DA. Outcomes following hip arthroscopy for femoroacetabular impingement with associated chondrolabral dysfunction: minimum two-year follow-up. J Bone Joint Surg Br. 2009;91:16–23. doi: 10.1302/0301-620X.91B1.21329. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy JC, Noble PC, Schuck MR, Wright J, Lee J. The Otto E. Aufranc Award: the role of labral lesions to development of early degenerative hip disease. Clin Orthop Relat Res. 2001:25–37. doi: 10.1097/00003086-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Miozzari HH, Clark JM, Jacob HA, von Rechenberg B, Notzli HP. Effects of removal of the acetabular labrum in a sheep hip model. Osteoarthritis Cartilage. 2004;12:419–430. doi: 10.1016/j.joca.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Wuerz TH, Shewman E, McCormick FM, Salata MJ, Philippon MJ, Nho SJ. Labral reconstruction with iliotibial band autografts and semitendinosus allografts improves hip joint contact area and contact pressure: an in vitro analysis. Am J Sports Med. 2015;43:98–104. doi: 10.1177/0363546514553089. [DOI] [PubMed] [Google Scholar]
- 22.Nepple JJ, Philippon MJ, Campbell KJ, Dornan GJ, Jansson KS, LaPrade RF, Wijdicks CA. The hip fluid seal-part ii: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc. 2014;22:730–736. doi: 10.1007/s00167-014-2875-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.