Abstract
This study inspected whether calcitriol could exert a mineralization-inductive effect comparable to that of vitamin C in cultured human periodontium cells (hPDCs). The mRNA expression of the mineralization-related biomarkers core-binding factor subunit alpha-1 (Cbfa1), collagen 1 α1 (Col-I), alkaline phosphatase (ALP), osteopontin (OPN), bone sialoprotein (BSP), osteocalcin (OCN), vitamin D receptor (VDR), cementum protein 1 (CEMP-1), cementum attachment protein (CAP), interleukin 6 (IL-6), transforming growth factor-β1 (TGF-β1) and osteoprotegerin (OPG) was surveyed after incubation of hPDCs with vitamin C and calcitriol for 2 weeks. Translational expression information from ALP activity and CEMP-1 and CAP immunofluorescence assays was acquired from hPDCs at the second and third weeks. Extracellular calcifications were confirmed by von Kossa staining, Alizarin Red staining and synchrotron transmission X-ray microscopy (TXM) at the fourth and fifth weeks. It was found that both vitamin C and calcitriol not only increased mineralization-related mRNA fold-changes but also enhanced ALP activity, CEMP-1 immunofluorescence, von Kossa and Alizarin Red staining and TXM-associated calcifications. Generally, 10-8 M calcitriol displayed greater mineralization significance than 10-7 M calcitriol in the assays tested. However, vitamin C stimulated lower Cbfa1, Col-1, ALP, OPN, BSP, OCN, VDR, CEMP-1 and IL-6 mRNA fold-changes than 10-8 M calcitriol. Finally, TXM analysis indicated that a 10-8 M calcitriol treatment stimulated greater calcifications than vitamin C treatment. Therefore, the analytical results confirmed the osteo-inductive potential of vitamin C in cultured hPDCs. In contrast, 10-8 M calcitriol could potentially function as a substitute because it stimulates a greater mineralization effect than vitamin C or 10-7 M calcitriol.
Keywords: Cultured human periodontium cells, vitamin C, calcitriol, synchrotron transmission X-ray microscope, mineralization
Introduction
Calcitriol forms in the kidneys, and one of its important functions is to regulate physiological calcium and phosphorus for bone mineralization in the body [1]. In murine bone cell cultures, calcitriol inhibited cell proliferation and induced osteoblast differentiation [2]. Calcitriol application promoted early stages of osteoblastogenesis in adipose-derived mesenchymal stem cells (MSCs) [3]. Calcidiol also promoted osteogenic differentiation in human bone marrow and adipose tissue-derived stromal cells [4,5]. Other studies suggested that calcitriol promoted the expression of early and late stage markers of osteoblast differentiation, including alkaline phosphatase (ALP), trans-forming growth factor-β1 (TGF-β1), osteopontin (OPN), secreted phosphoprotein 1 (SPP1), bone sialoprotein 1 (BSP-1), and osteocalcin (OCN) in human MSC cultures [4,6,7]. Our previous studies not only demonstrated that treatment of human alveolar periosteum-derived MSCs with calcitriol induced osteoinduction by increasing ALP, core-binding factor subunit alpha-1 (Cbfa1), Collagen-1 (Col-1), and OCN mRNA expression [8] but also revealed that both systemic and local calcitriol application accelerated alveolar bone regeneration in Beagle dogs [9,10]. In another study, calcitriol enhanced dexamethasone-induced osteogenic differentiation of human MSCs, elevated ALP activity and increased the level of matrix mineralization [11].
Core-binding factor alpha-1/runt-related transcription factor 2 (Cbfa1/RUNX2) is an indicator of immature osteoprogenitors derived from MSC differentiation and a regulator of bone formation produced by differentiated osteoblasts beyond maturation [12]. Col-1 characterizes mature osteoprogenitors that develop into preosteoblasts. Bone sialoprotein (BSP), a significant noncollagenous protein of the bone extracellular matrix, possibly acts as a crystallization nucleus for apatite crystal formation. BSP mRNA provides evidence of early mature osteoblast development [13].
Stimulated multipotent stromal cells of the periodontal ligament (PDL) could release autocrine growth factors, such as TGF-β1 and interleukin 6 (IL-6), to promote periodontal healing and regeneration by mimicking natural root development [14,15]. Osteoprotegerin (OPG) triggered osteoblast proliferation and inhibited both osteoclastogenesis and osteoclastic function indirectly; both were essential for alveolar bone regeneration [16]. Nevertheless, whether calcitriol supports periodontal regeneration by affecting TGF-β1, OPG, OPN, and IL-6-like enamel matrix derivative (EMD) warrants further investigation [17].
Synchrotron transmission X-ray microscopy (TXM) has been applied to identify the 3D ultramicrostructures of many kinds of dinosaur teeth [18,19]. TXM utilizes a Fresnel zone plate along with ultrahigh-brightness synchrotron hard X-rays and aims to achieve a spatial resolution of up to 30 nm. TXM has been widely employed in industry; however, TXM studies on the extracellular calcifications of cultured human periodontium-derived cells are limited.
Few studies have examined whether calcitriol could induce the mineralization process in human periodontal ligament-derived cells (hPDCs). In this study, we hypothesized that calcitriol might exert a similar osteogenic induction as that of vitamin C. Therefore, the mRNA expression of the mineralization-related biomarkers Cbfa1, Col-I, ALP, OPN, BSP, OCN, vitamin D receptor (VDR), cementum protein 1 (CEMP-1), cementum attachment protein (CAP), IL-6, TGF-β1 and OPG were surveyed after incubation of hPDCs with vitamin C and calcitriol for 2 weeks. Translational expression information from ALP enzyme activity and CEMP-1 and CAP immunofluorescence assays was acquired from hPDCs at the second and third weeks. Extracellular calcifications were confirmed by von Kossa staining, Alizarin Red staining and synchrotron transmission X-ray microscopy (TXM) at the fourth and fifth weeks.
Materials and methods
Isolation and culture of hPDCs
The hPDCs were acquired from healthy individuals who underwent premolar extraction for orthodontic reasons or removal of third molars for malposition extraction [20-22]. This study complied with the Declaration of Helsinki guidelines and was approved by the Medical Ethics Committee of Chang Gung Memorial Hospital. The Institutional Review Board (IRB) number was 103-5011B. All individuals provided written informed consent. The periodontium tissues were harvested and stored in Dulbecco’s phosphate-buffered saline (DPBS; Gibco, Carlsbad, CA, USA) with 300 U/mL penicillin and 300 g/mL streptomycin (Gibco BRL®) and transferred to a laboratory within 4 hours. The obtained fragments were placed on 35-mm culture plates (Corning) containing 1.5 ml of growth medium 1 [Minimum Essential Medium 1m Alpha Modification (HyClone®), 10% fetal bovine serum (FBS, Invitrogen®), 300 U/mL penicillin, and 300 μg/mL streptomycin] and incubated at 37°C under 5% CO2. The culture media were changed after 3 days and twice per week thereafter. When cultured hPDCs reached 80% confluence, the adherent cells were detached using 0.25% trypsin and replanted in growth medium 2 (α-modified Eagle’s medium, 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin). Each subsequent passage was performed after the cells achieved approximately 80-90% confluence through the same protocol. Subcultures with a ratio of 1:15 per well for cells between passages 4 to 6 were commenced in all experiments [8].
Experimental design
Four groups contained in culture media were characterized as: the control group [α-MEM (HyClone), 5% FBS (Invitrogen), 10 mM β-glycerophosphate (Sigma), and 10-7 M dexamethasone (Sigma)], vitamin C group (control plus 100 µM vitamin C), 10-8 M calcitriol group (control plus 10-8 M calcitriol, Nang Kuang Pharmaceutical Co., Ltd), and 10-7 M calcitriol group (control plus 10-7 M calcitriol).
Reverse transcription and quantitative real-time polymerase chain reaction
TRIzol® reagent was provided to isolate total RNA after 7 and 14 days of osteoblast differentiation, and 1.0 µg of RNA was reverse-transcribed using avian myeloblastosis virus reverse transcriptase (Roche®). First-strand complementary DNA (cDNA) was synthesized, and quantitative polymerase chain reaction (qPCR) assays were performed using 5 ng/μL cDNA. Quantitative real-time (qRT)-PCR was conducted using primers for Cbfa1/RUNX2, Col-I, ALP, OPN/SPP1/BSP-1, BSP, OCN, VDR, CEMP1, CAP/protein tyrosine phosphatase-like A (PTPLa), IL-6, TGF-β1 and OPG mRNA determination. qPCR was performed using SYBR Green PCR Master Mix and TaqMan Master Mix (Applied Biosystems) following the manufacturer’s instructions. Furthermore, the reactions were performed using a ViiA7 Real-Time PCR system (Applied Biosystems) with TaqMan Master Mix at 50°C for 2 min, followed by 95°C for 10 min, then 40 cycles each at 95°C for 15 s and 60°C for 60 s. For SYBR Green, the PCR consisted of 95°C for 10 min, then 40 cycles each at 95°C for 15 s and 60°C for 60 s, and finally 60°C for 15 min. The Ct values for Cbfa1, Col-I, ALP, OPN, BSP, OCN, VDR, CEMP1, CAP/PTPLa, IL-6, TGF-β1 and OPG messenger RNAs (mRNAs) were normalized to the value of the housekeeping gene GAPDH mRNA to confirm the differentiation of hPDCs into a mineralization-related phenotype at the molecular level. The expression of human mRNAs at the first and second weeks through qRT-PCR using human-specific primers was monitored. The results are presented as the fold change relative to that of the control group results, which was set to a value of 1. The effects of calcitriol and vitamin C on ALP activity were analyzed, and cementogenesis was identified with an anti-CAP antibody, 3G9. Mineralization was confirmed by von Kossa staining. Primer sequences are shown in Table 1.
Table 1.
Primer pairs used for quantitative real-time polymerase chain reaction
| Gene | Sequence |
|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | Hs99999905_m1 |
| Core-binding factor subunit alpha-1 (Cbfa1) | Hs00231692_m1 |
| Osteocalcin (OCN) | Hs01587814_g1 |
| Vitamin D receptor (VDR) | Hs01045846_m1 |
| Collagen 1 α1 (Col-I) | Forward primer 5’ AAAGTGAGAACGGGGAACCT-3’ |
| Reverse primer 5’-GATGCAAAGCCAGAATGGAT-3’ | |
| Bone sialoprotein (BSP) | Hs00173720_m1 |
| Osteopontin (OPN) | Hs00959010_m1 |
| Alkaline phosphatase (ALP) | Hs01029144_m1 |
| Cementum protein 1 (CEMP1) | Hs04185363_s1 |
| Protein tyrosine phosphatase-like A (PTPLa) | Hs00171965_m1 |
| Interleukin 6 (IL-6) | Hs00985639_m1 |
| Transforming growth factor-β1 (TGF-β1) | Forward primer 5’-CCCAGCATCTGCAAAGCTC-3’ |
| Reverse primer 5’-GTCAATGTACAGCTGCCGCA-3’ | |
| Osteoprotegerin (OPG) | Hs00900358_m1 |
ALP activity assay
The hPDCs were treated with osteogenic medium and assessed for ALP activity on the second and third weeks of culture to examine the extent of osteogenesis. The culture cells were lysed with 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 1 mM DTT, 1% Triton X-100 and protease inhibiter (complete tablets Mini, EDTA-free, Roche) and the ALP Activity Colorimetric Assay Kit (BioVision®) to determine ALP activity. As described previously [23,24], total formed p-nitrophenol was converted from p-nitrophenyl phosphate after dephosphorylation by ALP, and the absorbance was determined at a wavelength of 405 nm. The amount of p-nitrophenol was calibrated to the Assay Kit’s standard value to determine ALP activity in one hour. Tested calcitriol concentrations enhanced ALP mRNA fold-changes significantly in hPDC osteoblast differentiation, and ALP activity assays of the control, vitamin C, 10-8 M calcitriol, and 10-7 M calcitriol groups were therefore examined.
Von Kossa staining
The hPDC cells were seeded in 6-well plates at a density of 5,000 cells per well. At 80% confluence, the cultures were changed to media containing 100 μM vitamin C, 10-8 M calcitriol and 10-7 M calcitriol for 4 weeks. Subsequently, the cells were washed with PBS and fixed in 10% formalin for 30 mins. Then, 1 ml of 5% silver nitrate was added to each well and incubated under ultraviolet light for one hour. The plates were washed with 5% sodium thiosulfate for 3 min three times and then washed with distilled water thoroughly twice to terminate the reaction. Macro photographs of mineral deposits were taken [23,24].
Alizarin red staining
The hPDCs designed for Alizarin Red staining were incubated following similar protocols as those applied for von Kossa staining at 4 and 5 weeks. After 4 to 5 weeks of differentiation, 40 mM Alizarin Red staining solution (pH 4.2) (Merck) was used to stain the cultured cells for 12 minutes. Then, the cells were washed with 1x PBS 5 times and rinsed with distilled water twice before air-drying.
Immunofluorescence staining
The hPDC cells employed for immunofluorescence staining were treated the same as those used for von Kossa staining for the first 4 to 5 weeks, except the hPDC cells were placed on a glass slide in the plate. Following 4 and 5 weeks of differentiation, the cells were preserved with 3.5% paraformaldehyde for 30 minutes, blocked with 1% BSA in PBS for 30 minutes and then treated with primary antibodies for one hour. The primary antibodies used were diluted as follows: 1:120 for anti-CEMP1 (ab134231, Abcam) and 1:200 for anti-CAP (SC53947, Santa Cruz Biotechnology). The cells were then treated with Alexa Fluor®594 goat anti-mouse IgG (InvitrogenTM Molecular Probes) for 15 minutes and Alexa Fluor®488 donkey anti-rabbit IgG (InvitrogenTM Molecular Probes) for 1 hour. Cell nuclei were stained with DAPI (4’,6-diamidino-2-phenylindole). Fluorescence results were visualized by a fluorescence microscope (Olympus BX50), and the pictures were recorded.
Synchrotron transmission X-ray microscope (TXM)
Mineralization of the vitamin C-, 10-8 M (10 nM) calcitriol- and 10-9 M (1 nM) calcitriol-treated periodontium cell cultures was quantitatively analyzed using the TXM at BL01B1 beamline. TXM has been demonstrated to provide high-resolution X-ray 2D radiography and 3D tomography for animal fossils of mineralized biospecimens [18]. In this experiment, a monochromatic light source was selected at 8 keV X-ray energy using a Ge (111) double crystal monochromator with 1/1000 energy resolution. The high-resolution absorption-contrast images were projected onto a scintillator using a zone plate as the X-ray objective. Then, a 20X optical objective was used after the scintillator to further magnify the images, which were captured using a charge-coupled device detector with a 15 × 15 µm2 field of view. Each TXM image was collected with 60 s exposure time and a camera binning of 2 (512 × 512 in pixels). The spatial resolution of the TXM system is approximately 60 nm for both two-dimensional radiography and three-dimensional tomography. No other specimen preparation process is needed before the observation. After acquiring a series of 2D projections with the sample rotated stepwise, the Faproma alignment algorithm was used for correcting three-dimensional motion errors of each projection to improve 3D reconstruction quality [19]. The aligned projections were reconstructed by applying a filtered back-projection reconstruction algorithm using 181 sequential projections taken with the azimuth angle rotating from -90° to + 90°. The final 3D tomography was visualized using Amira 3D software.
Statistical analysis
The data are presented as the means ± standard error for all of the quantitative assays. All statistical analyses, using the Wilcoxon signed-rank test, were performed at a significance level of P<0.05.
Results
mRNA expression of mineralization-related genes
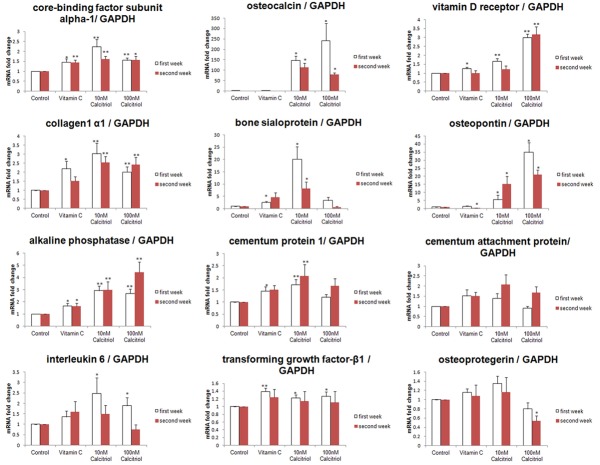
Most tested parameters presented a nonsignificant fold-change between the first and second weeks, except for OPN mRNA expression in the vitamin C and 10-8 M (10 nM) calcitriol groups. Vitamin C functioned as a positive control, upregulated Cbfa-1 and ALP mRNA fold-changes at both tested time-points, and significantly increased Col-1, BSP, VDR, CEMP-1, TGF-β1, and OPG mRNAs at the first week. Vitamin C did not significantly modify OCN, CAP IL-6 or OPG mRNAs. However, it down-regulated OPN mRNA at the second week (Figure 1 and Table S1).
Figure 1.
Messenger RNA expression of mineralization-related genes. The mRNA expression of mineralization-related biomarkers including core-binding factor subunit alpha-1, collagen 1 α1, alkaline phosphatase, interleukin 6, osteocalcin, bone sialoprotein, cementum protein 1, transforming growth factor-β1, vitamin D receptor, osteopontin, cementum attachment protein, and osteoprotegerin was surveyed after treatment of cultured human periodontium cells with vitamin C and calcitriol for 2 weeks. Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase. *P<0.05, **P<0.01.
Compared to the control group, the two tested calcitriol concentrations not only significantly enhanced Cbfa-1, Col-1, ALP, OPN, and OCN mRNA fold-changes at both weeks but also augmented VDR, IL-6 and TGF-β1 mRNAs at the first week. Furthermore, 10-8 M calcitriol significantly increased BSP and CEMP-1 mRNA fold-changes at both time-points. Additionally, 10-7 M (100 nM) calcitriol significantly enhanced the VDR mRNA fold-change in the second week. However, 10-7 M calcitriol significantly reduced the OPG mRNA fold-change compared to that of the control group at the second week. Finally, 10-8 M and 10-7 M calcitriol did not affect CAP mRNA significantly at either time-point.
In contrast to vitamin C, 10-8 M calcitriol enhanced the fold-change of Col-1, OPN, BSP and OCN mRNA at the first and second weeks and of Cbfa-1, ALP, VDR, CEMP-1, and IL-6 mRNA at the first week (P<0.05). Additionally, 10-7 M calcitriol augmented the fold-changes of ALP OPN, OCN and VDR mRNA at the first and second weeks; however, 10-7 M calcitriol exhibited significant down-regulation compared to that of vitamin C for OPG and CAP mRNAs at both time-points and BSP mRNA at the second week.
Corresponding to 10-8 M calcitriol, the fold-change of VDR mRNA at both weeks and OPN mRNA at the first week were upregulated significantly by 10-7 M calcitriol. In contrast, 10-7 M calcitriol showed a significantly lower fold-change of BSP and CAP mRNA at both weeks and of Col-1, CEMP-1, IL-6 and OPG mRNA at first week than that of the control.
ALP activity assay
The time study showed that vitamin C and 10-7 M calcitriol at the third week and 10-8 M calcitriol at both weeks presented a significant ALP activity increase compared to that of the control group. Additionally, 10-8 M calcitriol at the second week presented the most significant upregulation among the groups (Figure 2 and Table S2).
Figure 2.
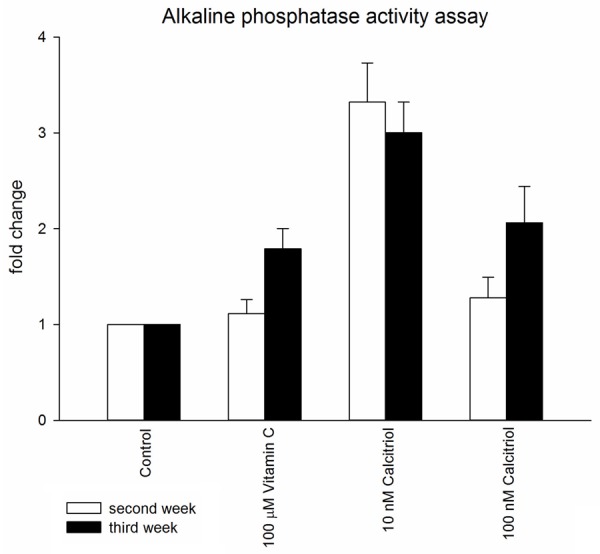
Alkaline phosphatase activity assay. The cultured human periodontium cells were treated with osteogenic medium and assessed for ALP activity on the second and third weeks of culture to examine the extent of osteogenesis. *P<0.05, **P<0.01.
Immunofluorescence staining
Corresponding to the mRNA examination, vitamin C and calcitriol did not reveal positive CAP immunofluorescence staining at the fourth and fifth weeks. However, both the vitamin C and calcitriol groups displayed positive CEMP-1 immunofluorescence staining at both weeks (Figure 3).
Figure 3.
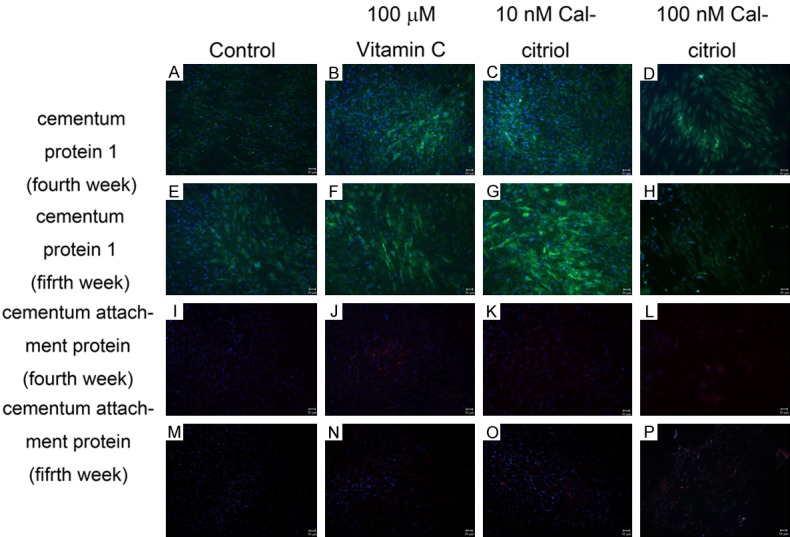
Cementum protein 1 and cementum attachment protein immunofluorescence staining. Both vitamin C and calcitriol groups displayed positive CEMP-1 immunofluorescence staining (green) at the fourth and fifth weeks. Vitamin C and calcitriol showed negative CAP immunofluorescence staining at the fourth and fifth weeks.
Von Kossa staining
Both the vitamin C and 10-8 M calcitriol groups demonstrated significant von Kossa staining at both weeks. Additionally, 10-7 M calcitriol displayed stronger von Kossa expression at the fifth week than at the fourth week. However, the 10-7 M calcitriol group presented a weaker von Kossa manifestation than that for the vitamin C and 10-8 M calcitriol groups at both weeks (Figure 4).
Figure 4.
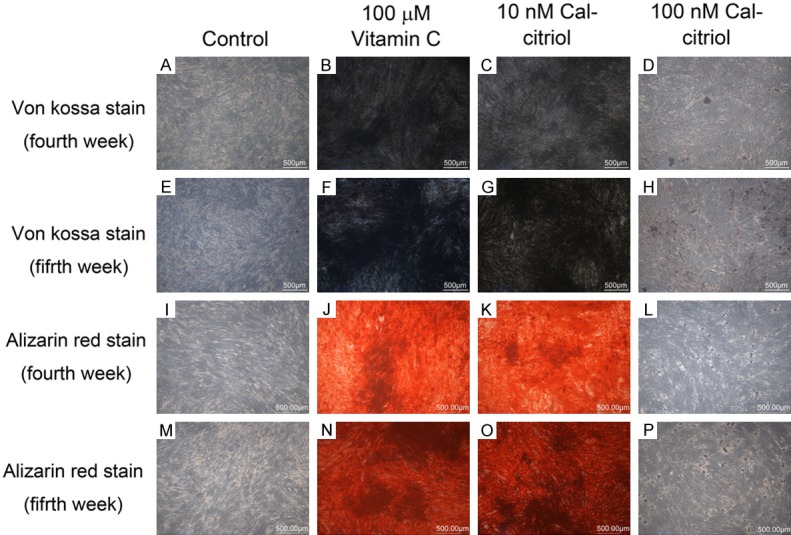
Von Kossa and Alizarin Red staining. Both vitamin C and 10-8 M (10 nM) calcitriol groups showed positive von Kossa (black) and Alizarin Red (red) staining at the fourth and fifth weeks.
Alizarin Red staining
Similar to the results of von Kossa staining, both the vitamin C and 10-8 M calcitriol groups responded significantly to the Alizarin Red test at the fourth and fifth weeks. Additionally, 10-7 M calcitriol illustrated insignificant Alizarin Red expression at both weeks. However, 10-8 M calcitriol displayed a higher substantial reaction than that of vitamin C at both weeks (Figure 4).
Synchrotron TXM analysis
The calcified samples revealed different significant features on high-resolution X-ray radiographs of TXM scans (Figure 5). Vitamin C culture presented a sparse submicrometer calcified structure in 2D and 3D features (Figure 5A and 5D); 10-9 M (1 nM) calcitriol demonstrated a micrometer (µm) calcified characteristic in 2D and 3D images (Figure 5B and 5E); and 10-8 M (10 nM) calcitriol exhibited a relatively dense calcified deposit that was larger than 1 µm in 2D and 3D reconstructions (Figure 5C and 5F). The volumes of calcification were 0.22, 0.27, and 0.64 µm3/µm2 on cultured dishes for vitamin C, 10-9 M calcitriol and 10-8 M calcitriol, respectively. The thicknesses of the calcified layers of the vitamin C, 10-9 M calcitriol and 10-8 M calcitriol groups were approximately 1.5 (Figure 5G), 1.7 (Figure 5H), and 4 µm (Figure 5I), respectively (Supplementary Movies 1-3). The quantitative analysis indicated that 10-8 M calcitriol treatment stimulated more calcification than the 10-9 M calcitriol and vitamin C treatments.
Figure 5.
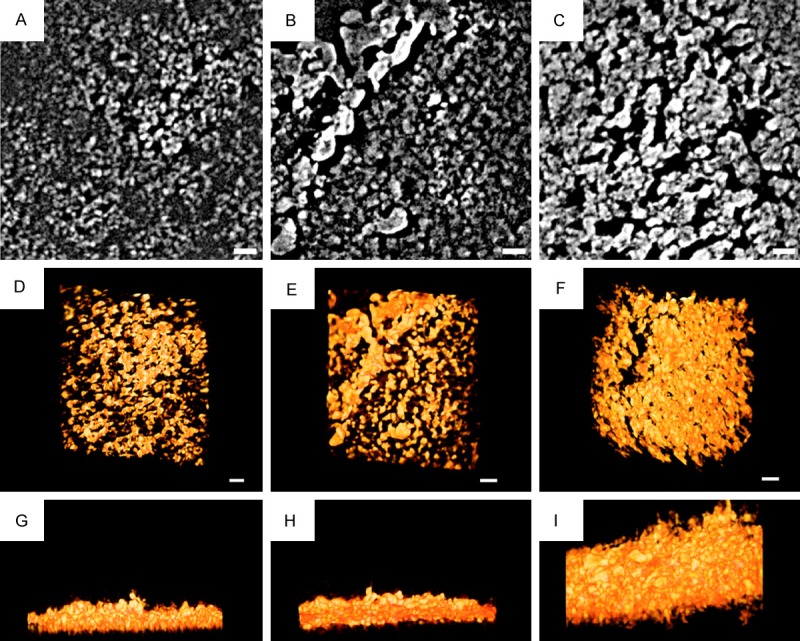
Synchrotron transmission X-ray microscope analysis. The calcified samples revealed different significant features on high-resolution X-ray radiographs of transmission X-ray microscope scans. Vitamin C culture presented a sparse submicrometer calcified structure in 2D and 3D features (A and D); 10-9 M (1 nM) calcitriol demonstrated a one micrometer (µm) calcified characteristic in 2D and 3D images (B and E); and 10-8 M (10 nM) calcitriol exhibited a relatively dense calcified deposit that was larger than 1 µm in 2D and 3D reconstructions (C and F). The volumes of calcification were 0.22, 0.27, and 0.64 µm3/µm2 on cultured dishes for vitamin C, 10-9 M calcitriol and 10-8 M calcitriol, respectively. The thicknesses of the calcified layers of the vitamin C, 10-9 M calcitriol and 10-8 M calcitriol groups were approximately 1.5 (G), 1.7 (H), and 4 µm (I), respectively (Supplementary Movies 1-3). The quantitative analysis indicated that 10-8 M calcitriol treatment stimulated more calcification than the 10-9 M calcitriol and vitamin C groups. Scale bar: 1 µm.
Discussion
Vitamin C induces osteogenic differentiation in mesenchymal stromal cell cultures [25]. It is an essential cofactor for enzymes, such as lysyl oxidase, which hydroxylates proline and lysine in pro-collagen and initiates spontaneous cross-linking. A helical collagen structure is essential for collagen fibrils’ integrity and stabilization [26]. Therefore, the principle role of vitamin C is attributed to Col-1 secretion into the extracellular matrix during osteogenic differentiation; subsequently, many osteoblast-related genes, including Cbfa1, OCN, and OPN, are expressed [27]. Some consistent findings of increased mRNA of Cbfa1 at both tested time-points and Col-1 at the first week with vitamin C treatment were observed in this study. However, vitamin C did not regulate OCN fold-change at either week nor did it significantly increase Col-1 at the second week; furthermore, there was a significant decrease in OPN mRNA at the second week. OPN not only prevented mineral deposition but also actively stimulated its dissolution by physically blocking hydroxyapatite crystal growth in in vitro and in vivo studies [28]. The positive results of enhanced ALP mRNA fold-changes at both time-points, BSP, VDR, TGF-β1, OPG and CEMP-1 mRNAs at the first week, ALP enzyme activity at the third week, CEMP-1 immunofluorescence expression, Alizarin Red and von Kossa staining for hPDCs at the fourth and fifth weeks, and significant 2D/3D TXM mineralization for periodontium-derived cells at the fourth week supported that vitamin C is potentially related to periodontal regeneration and bone osteoinductivity [29].
Optimal 25(OH) vitamin D serum levels diverge across different studies and possibly vary due to factors such as ethnicity and age [30,31]. Previous studies revealed that 25(OH) vitamin D levels ranging from 75 to 100 nmol/l were recommended to avoid negative health effects [30,31]. An Institute of Medicine committee concluded that a serum 25(OH) vitamin D level of 20 ng/ml (50 nmol/l) is desirable for bone and overall health. The average normal serum level of calcitriol is 50-125 pmol/l; however, discussion of optimum calcitriol serum levels associated with bone differentiation, formation and remodeling is limited [26]. We did not accomplish significant findings on calcitriol-related mRNA regulation at the concentrations of 10-10 M and 10-9 M, and cell detachment from the incubation dish was observed when the tested concentration was adjusted to 10-6 M in the human alveolar periosteum test; however, 10-9 M calcitriol presented some calcified results [26]. Therefore, 10-8 M/10-7 M calcitriol applied for most assays and 10-8 M/10-9 M calcitriol used for TXM analysis were selected for this study. Generally, calcitriol responded significantly to all tested assays except CAP mRNA, which is mostly related to cementogenesis. 10-8 M calcitriol promoted more ALP activity than vitamin C for both weeks and 10-7 M calcitriol at the second week. However, vitamin C showed comparable von Kossa staining to 10-8 M calcitriol at the fourth week. The finding is partially rationalized by vitamin C’s impact on extracellular enzymes, such as lysyl oxidase, which stimulates collagen cross-linking and matrix calcification [26,32]. Limited evidence supports calcitriol’s effect on lysyl oxidase modification for extracellular collagen cross-linking and subsequent calcification. However, this report provided some 2D and 3D TXM evidence at the µm level to support the hypothesis that 10-8 M calcitriol induced more significant extracellular mineralization than 10-9 M calcitriol and 100 µM vitamin C in periodontium-derived cells at the fourth week.
The stages of osteoblast differentiation and bone formation include mesenchymal stem cells, immature osteoprogenitors, mature osteoprogenitors, preosteoblasts, mature osteoblasts and osteocyte evolution. These stages have been recognized using verified markers. Cafa1 is present from the mesenchymal stem cell to osteocyte stages [23]; Col-1 and ALP are present from mature osteoprogenitors to mature osteoblasts; OPN from earlier preosteoblasts to osteocytes; BSP from preosteoblasts to osteocytes; and OCN from mature osteoblasts to osteocytes [23]. Calcitriol not only significantly upregulated the represented markers in all developing phases but also increased the related transcription factor VDR, which regulates transcriptional responses and microRNA-directed post transcriptional regulations [33]. Calcitriol enhanced TGF-β1 mRNA fold-change in hPDCs, which was found to increase Cbfa1 mRNA levels in normal primary human osteoblasts [34]. These findings substantiate that calcitriol regulated gene expression at different stages of osteoblastic maturation and indirectly adjusted transcriptional expression posttranslationally.
Cbfa1 mRNA upregulation stimulated by calcitriol in this hPDC test is similar to that found in a human osteoblast study [35]; however, these upregulations counter the finding of Cbfa1 expression downregulation in primary rat calvarial osteoblasts and the mouse MC3T3-E1 osteoblast cell line by calcitriol [36]. Cbfa1 is the earliest molecular marker of osteogenesis and is critical in skeletal mineralization during embryogenesis. It also regulates osteoblast maturation and differentiation. Adding vitamin C and calcitriol significantly increased the expression of this transcription activator during the differentiation of osteoblasts and cementoblasts [37,38].
Out of all tested biomarkers, only OPN exhibited a significant difference between the first and second weeks. A time study showed OPN upregulation by 10-8 M calcitriol and significant downregulation by vitamin C from the first to the second week. OPN was found to be a natural inhibitor of ectopic calcification in vivo, possibly by initiating the process by which osteoclasts develop their ruffled borders to begin bone resorption [28]. During osteoblast differentiation, OPN synthesis was promoted by calcitriol (explained by the increase in osteoblasts) and inhibited by vitamin C (contrary to other studies). Increased calcitriol-regulated OPN mRNA might partially explain the delayed von Kossa staining of the 10-8 M calcitriol group at the fourth week and weak von Kossa staining of the 10-7 M calcitriol group at both weeks.
Calcitriol promoted osteogenic activity and reduced IL-6 expression in a 4-48 hour period in hPDC cultures, but it did not increase VDR mRNA expression [39]. At similar concentrations and longer incubation durations, our results demonstrated that calcitriol not only upregulated VDR, OCN and OPN mRNA expression but also enhanced IL-6 mRNA fold-changes. Greater VDR mRNA fold-changes with longer calcitriol incubation periods implied that VDR mRNA could function as an indicator for mineralizing hPDCs.
CAP was referred from a human cementifying fibroma cDNA library; its monoclonal antibody localizes CAP on cementum only [40], suggesting that CAP might localize exclusively in cementum. However, one animal study failed to identify CAP mRNA in guinea pigs and concluded that CAP was not expressed in teeth [41]. In this hPDC examination, neither vitamin C nor calcitriol had significant effects on CAP regulation, although vitamin C exhibited greater CAP regulation than did 10-7 M calcitriol at both weeks. Species specificity and CAP mRNA presented at different culture stages might partially explain the findings. More studies are required to support the theories.
PDGF, insulin-like growth factors, TGF-β1, basic fibroblast growth factor, dexamethasone and bone morphogenetic proteins are believed to contribute to cementogenesis in periodontal regeneration. However, the nonspecific activity on different cell lineages and the short-lasting effects topically limited the application of these factors for periodontal regeneration [42]. The major organic component of cementum is type I collagen (90%) and serves as a reservoir for hydroxyapatite nucleation during mineralization. Cementum contains many noncollagenous proteins, including OPN and BSP, which are essential for the initiation of Col-1 fibrils’ crystal formation. OCN is a major Gla-protein associated with calcified hard tissues; its expression is localized to cellular and acellular cementum. High levels of OCN mRNA were expressed by cementoblasts lining the root surface during root development in mice; however, OCN mRNA was not expressed in PDL [43,44]. Changes in the level of tissue ALP protein have a significant effect on osteoblast function and subsequent matrix mineralization, indicating that ALP plays a crucial role in the mineralization of bone and cementum [43]. Almost 95% of the cementoblastoma-derived cells responded positively to CEMP-1, while only 6% of periodontal ligament cells were positive for CEMP-1 staining, suggesting that CEMP-1 could be a marker for the cementoblast lineage. In transfected human gingival fibroblasts, CEMP-1 enhanced not only cell proliferation, ALP activity and mineralized nodule formation but also de novo expression of OCN, OPN, BSP, Cbfa-1 and CAP mRNA and protein [45]. The study strongly substantiated that CEMP-1 enabled the differentiation of nonmineralizing gingival fibroblast phenotypes to mineralizing osteoblasts/cementoblasts by regulating gene expression. Additionally, the study suggested that CEMP-1 induced mineralization of extracellular matrix production resembling cementum. Inconsistent with the study that reported vitamin C upregulated cementogenic markers of CAP and CEMP-1, this study could only confirm the 10-8 M calcitriol’s and vitamin C’s enhancement of mRNA and immunofluorescence staining of CEMP-1 on hPDC culture [26].
Species specificity, individual variation, dose of tested samples, incubation duration, and cultured cells at various differentiated osteogenic stages partially explained the inconsistent findings with other studies of calcitriol effects on regulating mineralization-related mRNA.
Our results supported the hypothesis that both calcitriol and vitamin C stimulated the regulation of mineralization in cultured hPDC cells. Although our previous studies [9,10] confirmed a favorable effect of calcitriol in guided bone regeneration in dogs, further studies to confirm the beneficial effect of vitamin C and calcitriol on periodontal regeneration and guided bone regeneration in humans are required.
Conclusions
Both 10-8 M calcitriol and vitamin C exerted mineralization-inductive effects in the cultured hPDCs. Vitamin C increased mineralization-related mRNA fold-changes, ALP enzyme activity, CEMP-1 immunofluorescence staining, Alizarin Red staining and von Kossa staining. Calcitriol not only enhanced mineralization-related mRNAs tested but also stimulated greater Cbfa1, Col-1, ALP, OPN, BSP, OCN, VDR, CEMP-1 and IL-6 mRNA fold-changes and Alizarin Red staining than vitamin C. Finally, the TXM analysis supported that both vitamin C and calcitriol enhanced the formation of extracellular calcifications. Therefore, calcitriol can potentially function as a substitute because it stimulates a greater mineralization effect than vitamin C. Further studies are warranted.
Acknowledgements
This study was supported by research grants from Chang Gung Memorial Hospital (CMRPG3E0311, CMRPG3E0312) and Ministry of Science and Technology, Taiwan (MOST 105-2112-M-213-001).
Disclosure of conflict of interest
None.
Table S1
Table S2
Supplementary Movies 1-3
References
- 1.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491S–499S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 2.Kawase T, Oguro A. Granulocyte colony-stimulating factor synergistically augments 1,25-dihydroxyvitamin D3-induced monocytic differentiation in murine bone marrow cell cultures. Horm Metab Res. 2004;36:445–452. doi: 10.1055/s-2004-825728. [DOI] [PubMed] [Google Scholar]
- 3.Malladi P, Xu Y, Yang GP, Longaker MT. Functions of vitamin D, retinoic acid, and dexamethasone in mouse adipose-derived mesenchymal cells. Tissue Eng. 2006;12:2031–2040. doi: 10.1089/ten.2006.12.2031. [DOI] [PubMed] [Google Scholar]
- 4.Liu P, Oyajobi BO, Russell RG, Scutt A. Regulation of osteogenic differentiation of human bone marrow stromal cells: interaction between transforming growth factor-beta and 1,25(OH)(2) vitamin D(3) in vitro. Calcif Tissue Int. 1999;65:173–180. doi: 10.1007/s002239900678. [DOI] [PubMed] [Google Scholar]
- 5.Halvorsen YD, Franklin D, Bond AL, Hitt DC, Auchter C, Boskey AL, Paschalis EP, Wilkison WO, Gimble JM. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001;7:729–741. doi: 10.1089/107632701753337681. [DOI] [PubMed] [Google Scholar]
- 6.Beresford JN, Joyner CJ, Devlin C, Triffitt JT. The effects of dexamethasone and 1,25-dihydroxyvitamin D3 on osteogenic differentiation of human marrow stromal cells in vitro. Arch Oral Biol. 1994;39:941–947. doi: 10.1016/0003-9969(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen NR, Henriksen Z, Sorensen OH, Civitelli R. Dexamethasone, BMP-2, and 1,25-dihydroxyvitamin D enhance a more differentiated osteoblast phenotype: validation of an in vitro model for human bone marrow-derived primary osteoblasts. Steroids. 2004;69:219–226. doi: 10.1016/j.steroids.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Hong HH, Hong A, Yen TH, Wang YL. Potential osteoinductive effects of calcitriol on the m-RNA of mesenchymal stem cells derived from human alveolar periosteum. Biomed Res Int. 2016;2016:3529561. doi: 10.1155/2016/3529561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong HH, Yen TH, Hong A, Chou TA. Association of vitamin D3 with alveolar bone regeneration in dogs. J Cell Mol Med. 2015;19:1208–1217. doi: 10.1111/jcmm.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong HH, Chou TA, Yang JC, Chang CJ. The potential effects of cholecalciferol on bone regeneration in dogs. Clin Oral Implants Res. 2012;23:1187–1192. doi: 10.1111/j.1600-0501.2011.02284.x. [DOI] [PubMed] [Google Scholar]
- 11.van Driel M, Koedam M, Buurman CJ, Roelse M, Weyts F, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. Evidence that both 1alpha,25-dihydroxyvitamin D3 and 24-hydroxylated D3 enhance human osteoblast differentiation and mineralization. J Cell Biochem. 2006;99:922–935. doi: 10.1002/jcb.20875. [DOI] [PubMed] [Google Scholar]
- 12.Komori T, Kishimoto T. Cbfa1 in bone development. Curr Opin Genet Dev. 1998;8:494–499. doi: 10.1016/s0959-437x(98)80123-8. [DOI] [PubMed] [Google Scholar]
- 13.Sodek J, McKee MD. Molecular and cellular biology of alveolar bone. Periodontol 2000. 2000;24:99–126. doi: 10.1034/j.1600-0757.2000.2240106.x. [DOI] [PubMed] [Google Scholar]
- 14.Sculean A, Schwarz F, Becker J, Brecx M. The application of an enamel matrix protein derivative (Emdogain) in regenerative periodontal therapy: a review. Med Princ Pract. 2007;16:167–180. doi: 10.1159/000100386. [DOI] [PubMed] [Google Scholar]
- 15.Lyngstadaas SP, Lundberg E, Ekdahl H, Andersson C, Gestrelius S. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J Clin Periodontol. 2001;28:181–188. doi: 10.1034/j.1600-051x.2001.028002181.x. [DOI] [PubMed] [Google Scholar]
- 16.He J, Jiang J, Safavi KE, Spangberg LS, Zhu Q. Emdogain promotes osteoblast proliferation and differentiation and stimulates osteoprotegerin expression. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:239–245. doi: 10.1016/j.tripleo.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier P, Yu Z, Tran QT, Bhatti FU, Zhu X, Huang GT. Cementogenic genes in human periodontal ligament stem cells are downregulated in response to osteogenic stimulation while upregulated by vitamin C treatment. Cell Tissue Res. 2017;368:79–92. doi: 10.1007/s00441-016-2513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang CC, Song YF, Song SR, Ji Q, Chiang CC, Meng Q, Li H, Hsiao K, Lu YC, Shew BY, Huang T, Reisz RR. Evolution and function of dinosaur teeth at ultramicrostructural level revealed using synchrotron transmission X-ray microscopy. Sci Rep. 2015;5:15202. doi: 10.1038/srep15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CC, Chiang CC, Liang B, Yin GC, Weng YT, Wang LC. Fast projection matching for X-ray tomography. Sci Rep. 2017;7:3691. doi: 10.1038/s41598-017-04020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakaya H, Osawa G, Iwasaki N, Cochran DL, Kamoi K, Oates TW. Effects of bisphosphonate on matrix metalloproteinase enzymes in human periodontal ligament cells. J Periodontol. 2000;71:1158–1166. doi: 10.1902/jop.2000.71.7.1158. [DOI] [PubMed] [Google Scholar]
- 21.Somerman MJ, Foster RA, Imm GM, Sauk JJ, Archer SY. Periodontal ligament cells and gingival fibroblasts respond differently to attachment factors in vitro. J Periodontol. 1989;60:73–77. doi: 10.1902/jop.1989.60.2.73. [DOI] [PubMed] [Google Scholar]
- 22.Somerman MJ, Young MF, Foster RA, Moehring JM, Imm G, Sauk JJ. Characteristics of human periodontal ligament cells in vitro. Arch Oral Biol. 1990;35:241–247. doi: 10.1016/0003-9969(90)90062-f. [DOI] [PubMed] [Google Scholar]
- 23.D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 24.Stucki U, Schmid J, Hammerle CF, Lang NP. Temporal and local appearance of alkaline phosphatase activity in early stages of guided bone regeneration. A descriptive histochemical study in humans. Clin Oral Implants Res. 2001;12:121–127. doi: 10.1034/j.1600-0501.2001.012002121.x. [DOI] [PubMed] [Google Scholar]
- 25.Vater C, Kasten P, Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7:463–477. doi: 10.1016/j.actbio.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 26.Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7:235–246. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- 27.Langenbach F, Handschel J. Effects of dexamethasone, ascorbic acid and beta-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther. 2013;4:117. doi: 10.1186/scrt328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161:2035–2046. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, He Y, Shan C, Pan Q, Li M, Xia D. Topical combined application of dexamethasone, vitamin C, and beta-sodium glycerophosphate for healing the extraction socket in rabbits. Int J Oral Maxillofac Surg. 2015;44:1317–1323. doi: 10.1016/j.ijom.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol. 2014;810:500–525. doi: 10.1007/978-1-4939-0437-2_28. [DOI] [PubMed] [Google Scholar]
- 31.Dahlquist DT, Dieter BP, Koehle MS. Plausible ergogenic effects of vitamin D on athletic performance and recovery. J Int Soc Sports Nutr. 2015;12:33. doi: 10.1186/s12970-015-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong HH, Pischon N, Santana RB, Palamakumbura AH, Chase HB, Gantz D, Guo Y, Uzel MI, Ma D, Trackman PC. A role for lysyl oxidase regulation in the control of normal collagen deposition in differentiating osteoblast cultures. J Cell Physiol. 2004;200:53–62. doi: 10.1002/jcp.10476. [DOI] [PubMed] [Google Scholar]
- 33.Lisse TS, Chun RF, Rieger S, Adams JS, Hewison M. Vitamin D activation of functionally distinct regulatory miRNAs in primary human osteoblasts. J Bone Miner Res. 2013;28:1478–1488. doi: 10.1002/jbmr.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viereck V, Siggelkow H, Tauber S, Raddatz D, Schutze N, Hufner M. Differential regulation of Cbfa1/Runx2 and osteocalcin gene expression by vitamin-D3, dexamethasone, and local growth factors in primary human osteoblasts. J Cell Biochem. 2002;86:348–356. doi: 10.1002/jcb.10220. [DOI] [PubMed] [Google Scholar]
- 35.Prince M, Banerjee C, Javed A, Green J, Lian JB, Stein GS, Bodine PV, Komm BS. Expression and regulation of Runx2/Cbfa1 and osteoblast phenotypic markers during the growth and differentiation of human osteoblasts. J Cell Biochem. 2001;80:424–440. doi: 10.1002/1097-4644(20010301)80:3<424::aid-jcb160>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Drissi H, Pouliot A, Koolloos C, Stein JL, Lian JB, Stein GS, van Wijnen AJ. 1,25-(OH)2-vitamin D3 suppresses the bone-related Runx2/Cbfa1 gene promoter. Exp Cell Res. 2002;274:323–333. doi: 10.1006/excr.2002.5474. [DOI] [PubMed] [Google Scholar]
- 37.Saito MT, Salmon CR, Amorim BR, Ambrosano GM, Casati MZ, Sallum EA, Nociti FH, Silverio KG. Characterization of highly osteoblast/cementoblast cell clones from a CD105-enriched periodontal ligament progenitor cell population. J Periodontol. 2014;85:e205–211. doi: 10.1902/jop.2014.130461. [DOI] [PubMed] [Google Scholar]
- 38.Inubushi T, Tanaka E, Rego EB, Kitagawa M, Kawazoe A, Ohta A, Okada H, Koolstra JH, Miyauchi M, Takata T, Tanne K. Effects of ultrasound on the proliferation and differentiation of cementoblast lineage cells. J Periodontol. 2008;79:1984–1990. doi: 10.1902/jop.2008.080081. [DOI] [PubMed] [Google Scholar]
- 39.Nebel D, Svensson D, Arosenius K, Larsson E, Jonsson D, Nilsson BO. 1α,25-dihydroxyvitamin D3 promotes osteogenic activity and downregulates proinflammatory cytokine expression in human periodontal ligament cells. J Periodontal Res. 2015;50:666–673. doi: 10.1111/jre.12249. [DOI] [PubMed] [Google Scholar]
- 40.Arzate H, Olson SW, Page RC, Gown AM, Narayanan AS. Production of a monoclonal antibody to an attachment protein derived from human cementum. FASEB J. 1992;6:2990–2995. doi: 10.1096/fasebj.6.11.1644261. [DOI] [PubMed] [Google Scholar]
- 41.Schild C, Beyeler M, Lang NP, Trueb B. Cementum attachment protein/protein-tyrosine phosphotase-like member A is not expressed in teeth. Int J Mol Med. 2009;23:293–296. [PubMed] [Google Scholar]
- 42.Arzate H, Zeichner-David M, Mercado-Celis G. Cementum proteins: role in cementogenesis, biomineralization, periodontium formation and regeneration. Periodontol 2000. 2015;67:211–233. doi: 10.1111/prd.12062. [DOI] [PubMed] [Google Scholar]
- 43.D’Errico JA, MacNeil RL, Takata T, Berry J, Strayhorn C, Somerman MJ. Expression of bone associated markers by tooth root lining cells, in situ and in vitro. Bone. 1997;20:117–126. doi: 10.1016/s8756-3282(96)00348-1. [DOI] [PubMed] [Google Scholar]
- 44.Hough TA, Polewski M, Johnson K, Cheeseman M, Nolan PM, Vizor L, Rastan S, Boyde A, Pritzker K, Hunter AJ, Fisher EM, Terkeltaub R, Brown SD. Novel mouse model of autosomal semidominant adult hypophosphatasia has a splice site mutation in the tissue nonspecific alkaline phosphatase gene Akp2. J Bone Miner Res. 2007;22:1397–1407. doi: 10.1359/jbmr.070515. [DOI] [PubMed] [Google Scholar]
- 45.Carmona-Rodriguez B, Alvarez-Perez MA, Narayanan AS, Zeichner-David M, Reyes-Gasga J, Molina-Guarneros J, Garcia-Hernandez AL, Suarez-Franco JL, Chavarria IG, Villarreal-Ramirez E, Arzate H. Human Cementum Protein 1 induces expression of bone and cementum proteins by human gingival fibroblasts. Biochem Biophys Res Commun. 2007;358:763–769. doi: 10.1016/j.bbrc.2007.04.204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.