Abstract
Background
Nursing home residents with atrial fibrillation are at high risk for ischemic stroke and bleeding events. The most recent national estimate (2004) indicated less than one third of this high‐risk population was anticoagulated. Whether direct‐acting oral anticoagulant (DOAC) use has disseminated into nursing homes and increased anticoagulant use is unknown.
Methods and Results
A repeated cross‐sectional design was used to estimate the point prevalence of oral anticoagulant use on July 1 and December 31 of calendar years 2011 to 2016 among Medicare fee‐for‐service beneficiaries with atrial fibrillation residing in long‐stay nursing homes. Nursing home residence was determined using Minimum Data Set 3.0 records. Medicare Part D claims for apixaban, dabigatran, edoxaban, rivaroxaban, and warfarin were identified and point prevalence was estimated by determining if the supply from the most recent dispensing covered each point prevalence date. A Cochran‐Armitage test was performed for linear trend in prevalence. On December 31, 2011, 42.3% of 33 959 residents (median age: 85; Q1 79, Q3 90) were treated with an oral anticoagulant, of whom 8.6% used DOACs. The proportion receiving treatment increased to 47.8% of 37 787 residents as of December 31, 2016 (P<0.01); 48.2% of 18 054 treated residents received DOACs. Demographic and clinical characteristics of residents using DOACs and warfarin were similar in 2016. Half of the 8734 DOAC users received standard dosages and most were treated with apixaban (54.4%) or rivaroxaban (35.8%) in 2016.
Conclusions
Increases in anticoagulant use among US nursing home residents with atrial fibrillation coincided with declining warfarin use and increasing DOAC use.
Keywords: anticoagulant, atrial fibrillation, nursing home, utilization
Subject Categories: Atrial Fibrillation, Epidemiology, Anticoagulants, Treatment
Clinical Perspective
What Is New?
From 2011 to 2016, the proportion of the US nursing home population with atrial fibrillation that was treated with an oral anticoagulant increased from 42% to 48%.
In 2016, nearly half of treated residents were using direct‐acting oral anticoagulants and more than half of those using direct‐acting oral anticoagulants were using apixaban.
In 2016, 44% of direct‐acting oral anticoagulant users without renal impairment received low dosages and 44% with renal impairment received standard dosages.
What Are the Clinical Implications?
Despite recent increases in oral anticoagulant use, more than half of nursing home residents with atrial fibrillation remain untreated.
Nursing home residents represent a vulnerable population for whom decisions to initiate, alter, or discontinue treatment are complicated by several factors including medical morbidity, cognitive impairment, functional limitations, and abbreviated life expectancy.
The large shift from warfarin to direct‐acting oral anticoagulants among nursing home residents during the period 2011 to 2016 has occurred without comparative effectiveness evidence specific to this population to guide medication and dosage selection.
Introduction
The number of Americans with atrial fibrillation (AF) is projected to double to >12 million between 2010 and 2050, driven by increasing population size, aging, and a rising burden of risk factors such as obesity.1 By 2050, more than half of Americans with AF are expected to be aged >80 years.2 As of 2010, ≈1 in 8 Americans aged >85 years resided in an institutional setting.3
Anticoagulation decisions for older adults with AF at high risk for ischemic stroke are particularly challenging because most are also at high risk of bleeding. This leaves providers uncertain of the net benefit of treatment4, 5 and has contributed to low use of anticoagulants for high‐risk older adults6 despite evidence supporting their safety and effectiveness for older populations.7 Nursing home residents with AF are at particularly high risk for ischemic stroke and bleeding events because of a high prevalence of risk factors, including frailty, advanced age, and comorbidities.8, 9, 10 Yet in the final wave of the National Nursing Home Study conducted in 2004, less than one third of this high‐risk population was anticoagulated.10 Historically, nursing home residents commonly experienced high rates of adverse events related to warfarin therapy, many of which were considered preventable and associated with time spent outside of the therapeutic range.11
Among patients in the United States attending ambulatory care visits, the market entrance of direct‐acting oral anticoagulants (DOACs) was followed by a shift in use from warfarin to DOACs, accompanied by an increase in the fraction of patients with AF receiving anticoagulation.12 This shift extends to high‐risk community dwelling older adults, as the large majority of high‐risk patients initiating anticoagulants during the period 2013 to 2016 were prescribed DOACs.13 Nursing home residents have a high burden of cognitive impairment and shortened life expectancy,14, 15 which demands a complex multi‐stakeholder shared decision‐making process. Furthermore, extensive functional limitations16 diminish residents’ access to specialists outside of the institutional setting who have a greater propensity to prescribe anticoagulants overall and DOACs specifically.17, 18 It is uncertain the extent to which the use of DOACs has disseminated into the nursing home setting and increased anticoagulant use for this high‐risk and vulnerable population.
Methods
Data
Medicare administrative files and the Minimum Data Set (MDS) 3.0 were linked to assemble a near‐comprehensive data source encompassing enrollment and demographic characteristics from the Medicare Beneficiary Summary file, hospital and skilled nursing facility claims (Medicare Part A), prescription claims (Medicare Part D), and clinical and functional assessment data (MDS 3.0). The MDS 3.0 is mandatory for all Medicare and Medicaid certified nursing facilities and the information collected through the MDS 3.0 has been previously validated.19 Medicare administrative files and the MDS 3.0 were used through a data use agreement with the Centers for Medicare and Medicaid Services. Dr Alcusky had full access to all study data and takes responsibility for data integrity and analysis. Because of the sensitive nature of the Centers for Medicare and Medicaid Services research identifiable files, researchers interested in requesting files should consult the information available from the Research Data Assistance Center.20 The University of Massachusetts Medical School Institutional Review Board approved this study (H00015376); informed consent was not required.
Study Design
A repeated cross‐sectional design was used to estimate the point prevalence of oral anticoagulant use overall, by anticoagulant class (ie, warfarin or DOAC), and by specific medication on July 1 and December 31 of calendar years 2011 to 2016. A 12‐month lookback period was used for all cross‐sections with the exception of the first because Medicare and MDS 3.0 data were only available for 6 and 9 months, respectively, before July 1, 2011.
Study Population
For each cross‐section, Medicare fee‐for‐service beneficiaries with diagnosed AF residing in a long‐stay nursing home on the point prevalence date and with at least 6 months of baseline Medicare enrollment were eligible to enter the study population. Included residents had at least one diagnosis of AF or flutter (Table S1) on a Medicare Part A claim and one diagnosis of AF, atrial flutter, or dysrhythmia on an MDS 3.0 assessment in the 12 months preceding the point prevalence date. Excluded residents were aged <65 years, without at least one Part D claim in the preceding 12 months, enrolled in hospice, or in a comatose state.
Anticoagulant Use
Oral anticoagulant use including apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin was measured using a daily approach, enabling a precise estimate of current anticoagulant use in a national population of residents who were known to be residing in a nursing home on a specific date. The point prevalence of oral anticoagulant use was estimated on the first day of each half‐year of the study (July 1 and December 31 of calendar years 2011–2016) by summing the number of eligible residents exposed to an anticoagulant on that date and dividing by the number of residents in the population. Exposure was estimated using fill dates and number of days supplied from Part D claims in the 12‐months preceding the point prevalence date. Each day a resident was present in the study population was marked as exposed if the supply from the most recent dispensing was sufficient to cover that day, accounting for medication accumulation and in‐patient/skilled nursing facility stays. Residents with at least one dispensing for an oral anticoagulant who were not exposed on the point prevalence date were considered to have discontinued anticoagulant use. For analyses of switching at the level of the class (warfarin or DOAC), residents that made multiple switches during a cross‐section were grouped according to the most recent switch. Among switchers, the proportion switching from one class to the other and back was also described.
Resident Characteristics
Resident characteristics were operationalized using information from the most recent long‐stay MDS 3.0 assessment preceding the point prevalence date, diagnoses on Part A claims and medication information on Part D claims during the 12‐months preceding the point prevalence date. Resident characteristics included demographics, hospital admissions (including for ischemic stroke,21 extracranial bleeding,22 or intracranial hemorrhage22), CHA2DS2‐VASc risk score,23 Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) risk score,24 select comorbid conditions associated with increased ischemic stroke (ie, components of the CHA2ADS2‐Vasc score) or bleeding risk25 (fall history, chronic renal insufficiency26), select medication classes and total unique medications (as an indicator of polypharmacy) used, functional status, and cognitive impairment. Renal functioning was grouped in 4 categories using a combination of information from Medicare claims and MDS 3.0 items: (1) on dialysis (MDS item O0100J2), (2) end‐stage renal disease (MDS item I1500) and not on dialysis, (3) chronic renal insufficiency (corresponding to an estimated glomerular filtration rate <60 mL/min)26 without end‐stage renal disease or dialysis, and (4) no evidence of chronic renal impairment. The select medication classes described were those associated with increased bleeding risk (non‐steroidal anti‐inflammatory drugs, antiplatelets) and chronic medications (statins, angiotensin‐converting enzyme inhibitors, angiotensin‐receptor blockers) used for the prevention of cardiovascular and cerebrovascular events. Use of selective serotonin reuptake inhibitors was also described because of a possible association with bleeding when combined with anticoagulant use.27, 28 A recent history of ≥1 falls since nursing home admission or the last assessment was ascertained from the MDS 3.0 (item J1800) and operationalized dichotomously. Functional status was operationalized as the 4‐item activities of daily living (ADL) score, which summarizes a resident's ability to perform 4 ADLs (personal hygiene, toileting, locomotion, and eating) and ranges from a score of 0 (independent in all 4 ADLs) to 16 (totally dependent in all 4 ADLs).29 Cognitive impairment was scored using the MDS 3.0 Cognitive Function Scale.14
Statistical Analysis
Characteristics of the study population were described overall and by anticoagulant use for the residents included in the December 31, 2011 and 2016 point prevalence estimates. Among residents using oral anticoagulants as of December 31, 2011 and 2016, resident characteristics were summarized separately for users of DOACs and warfarin. Descriptive statistics included frequencies and percentages for categorical variables and medians with first and third quartiles for continuous variables.
The prevalence of anticoagulant use was plotted overall and by anticoagulant class for the 12 half‐years comprising the study period. For each half‐year, the prevalence of anticoagulant use was also described by specific medication. The prevalence of anticoagulant use overall and by medication class was also described within subgroups defined by renal function, cognition, and functional status for the December 31, 2011 and 2016 cross‐sections. The prevalence of anticoagulant discontinuation and the prevalence of switching between medication classes were each plotted over the course of the study period. A Cochran‐Armitage test with a 2‐sided statistical significance level of <0.05 was performed for linear trend in prevalence of anticoagulant use. Data analyses were performed with SAS Version 9.4 (SAS Institute Inc) and Microsoft Excel 2016 (Microsoft Corporation).
Results
Resident Characteristics
The number of residents included ranged from 17 895 for the July 1, 2011 cross‐section to 37 787 for the December 31, 2016 cross‐section. Resident age remained consistent between 2011 (median: 85; Q1 79, Q3 90) and 2016 (median: 84; Q1 78, Q3 90). In 2016, 34% of residents were men; with 29% men in 2011. The proportion of residents with CHA2DS2‐Vasc scores ≥6 was 36% in 2011 and 30% in 2016; in each time period >99% of residents had scores of ≥2. The fraction with renal impairment (chronic renal insufficiency, end‐stage renal disease, or using dialysis) was 51% in 2016 and 43% in 2011. Residents were substantially limited in ADLs in 2011 and 2016. The prevalence of moderate‐to‐severe cognitive impairment was 39% in 2011 and 34% in 2016. The median number of unique prescriptions among residents in 2011 was 17 and in 2016 was 18.
Table 1 displays characteristics of the resident population in 2011 and 2016 for both treated and untreated residents. In 2011 and 2016, the median CHA2DS2‐Vasc score was 5 (Q1 4, Q3 6) and the median ATRIA risk score was 3 (Q1 3, Q3 6) among both treated and untreated residents. In 2011, moderate‐to‐severe cognitive impairment was present among 44% of untreated and 34% of treated residents, while in 2016 the prevalence was 40% and 30%, respectively. During the 2011 and 2016 cross‐sections, 5% of untreated and 7% of treated residents had been hospitalized for ischemic stroke. Among untreated residents, 23% used antiplatelets in 2011 and 19% used antiplatelets in 2016; <10% of treated residents used antiplatelet medication during either time period. Table 2 displays characteristics of nursing home residents treated with DOACs and warfarin in 2011 and 2016.
Table 1.
Characteristics of Residents Treated or Not Treated With an Anticoagulant, by Time Point
| December 31, 2011 | December 31, 2016 | |||
|---|---|---|---|---|
| Untreated (n=19 598) | Treated (n=14 361) | Untreated(n=19 733) | Treated (n=18 054) | |
| Demographics | ||||
| Age in y, median (Q1, Q3) | 86 (80, 91) | 84 (78, 89) | 86 (79, 91) | 83 (77, 89) |
| Women, % | 70.9 | 71.2 | 66.2 | 65.9 |
| Hospital admissions in prior year, % | ||||
| Number of hospitalizations, % | ||||
| 2 to 3 | 38.0 | 38.4 | 36.8 | 37.9 |
| 4+ | 13.7 | 14.1 | 12.1 | 13.1 |
| Ischemic stroke | 5.0 | 7.3 | 4.7 | 7.3 |
| Extracranial bleed | 7.9 | 5.3 | 7.2 | 4.5 |
| Intracranial hemorrhage | 1.0 | 0.4 | 1.2 | 0.5 |
| Unique medications, median (Q1, Q3) | 17 (12, 22) | 19 (14, 24) | 16 (12, 22) | 18 (14, 24) |
| Select medications, %a | ||||
| NSAID | 18.3 | 15.8 | 17.7 | 16.9 |
| Antiplatelet | 22.5 | 9.5 | 18.5 | 8.6 |
| Statin | 42.4 | 51.0 | 51.1 | 60.6 |
| SSRI | 52.3 | 55.9 | 50.0 | 52.0 |
| ACE inhibitor or ARB | 49.8 | 54.0 | 45.3 | 50.1 |
| Select comorbidities, %b | ||||
| Diabetes mellitus | 35.2 | 41.0 | 36.3 | 41.8 |
| Heart failure | 42.1 | 48.7 | 42.0 | 48.1 |
| Hypertension | 82.7 | 84.3 | 85.7 | 87.8 |
| Coronary artery disease | 33.4 | 31.2 | 31.3 | 29.3 |
| Anemia | 38.4 | 32.4 | 39.2 | 33.2 |
| Fall since nursing home admission/last assessment | 22.1 | 18.8 | 21.8 | 18.1 |
| Stroke | 19.6 | 24.3 | 12.5 | 16.2 |
| CHA2DS2‐Vasc Risk Score, % | ||||
| 2 to 3 | 13.6 | 11.1 | 15.7 | 13.0 |
| 4 | 25.0 | 22.3 | 26.9 | 24.3 |
| 5 | 27.1 | 27.3 | 28.1 | 29.7 |
| 6 | 19.4 | 21.7 | 18.7 | 20.3 |
| 7+ | 14.5 | 17.4 | 10.1 | 12.4 |
| ATRIA Bleeding Risk Score, %c | ||||
| Low (0–3) | 54.1 | 60.8 | 50.7 | 55.8 |
| Intermediate (4) | 3.6 | 4.6 | 5.8 | 6.5 |
| High (5–10) | 42.3 | 34.6 | 43.6 | 37.7 |
| Cognitive skills, % | ||||
| Mildly impaired | 25.4 | 26.2 | 26.1 | 26.5 |
| Moderately to severely impaired | 44.4 | 34.2 | 39.6 | 29.4 |
| ADL score (0–16), median (Q1, Q3)d | 9 (6, 12) | 9 (6, 11) | 10 (7, 11) | 9 (7, 11) |
ACE indicates angiotensin‐converting enzyme; ADLs, activities of daily living; ARB, angiotensin‐receptor blocker; ATRIA, Anticoagulation and Risk Factors in Atrial Fibrillation; NSAID, non‐steroidal anti‐inflammatory drugs; SSRI, selective serotonin reuptake inhibitor.
Any Part D claim during the 12‐month period.
Resident characteristics exclude residents with missing values for fall history, heart failure, hypertension, diabetes mellitus, and stroke (n≤10 for all characteristics with missing values in 2011 and 2016).
Percentages may not total 100% because of rounding.
Higher scores indicate greater limitation in ADLs.
Table 2.
Characteristics of Residents Using DOACs or Warfarin, by Time Point
| December 31, 2011 | December 31, 2016 | |||
|---|---|---|---|---|
| Warfarin (n=13 375) | DOAC (n=986) | Warfarin (n=9320) | DOAC (n=8734) | |
| Demographics | ||||
| Age in y, median (Q1, Q3) | 84 (78, 89) | 83 (77, 88) | 84 (77, 89) | 83 (76, 88) |
| Women, % | 71.1 | 71.6 | 64.6 | 67.3 |
| Hospital admissions in prior year, % | ||||
| Number of hospitalizations, % | ||||
| 2 to 3 | 38.2 | 41.0 | 36.8 | 39.1 |
| 4+ | 13.9 | 15.7 | 12.5 | 13.8 |
| Ischemic stroke | 7.1 | 10.0 | 5.9 | 8.9 |
| Extracranial bleed | 5.0 | 6.5 | 5.0 | 4.0 |
| Intracranial hemorrhage | 0.4 | Sup. | 0.5 | 0.5 |
| Medications | ||||
| Unique medications, median (Q1, Q3) | 19 (14, 24) | 20 (15, 26) | 18 (14, 23) | 19 (14, 24) |
| Less than standard anticoagulant dose, % | NA | 36.0 | NA | 50.0 |
| Select medications, %a | ||||
| NSAID | 15.6 | 17.9 | 15.1 | 18.7 |
| Antiplatelet | 9.2 | 13.8 | 7.5 | 9.7 |
| Statin | 50.7 | 54.0 | 60.2 | 61.1 |
| SSRI | 55.5 | 62.0 | 51.0 | 53.1 |
| ACE inhibitor or ARB | 53.7 | 57.6 | 48.8 | 51.6 |
| Select comorbidities, %b | ||||
| Diabetes mellitus | 41.0 | 41.3 | 41.8 | 41.9 |
| Heart failure | 49.0 | 45.0 | 50.3 | 45.7 |
| Hypertension | 84.2 | 85.5 | 87.4 | 88.1 |
| Coronary artery disease | 31.2 | 30.8 | 30.0 | 28.5 |
| Anemia | 32.4 | 32.5 | 33.9 | 32.5 |
| Fall since nursing home admission/last assessment | 18.6 | 20.5 | 17.6 | 18.6 |
| Stroke | 24.2 | 26.8 | 15.7 | 16.7 |
| CHA2DS2‐Vasc Risk Score, % | ||||
| 2 to 3 | 11.2 | 12.6 | 12.7 | 13.9 |
| 4 | 22.6 | 19.5 | 23.8 | 24.8 |
| 5 | 27.3 | 27.0 | 30.2 | 29.9 |
| 6 | 21.7 | 22.4 | 20.9 | 19.7 |
| 7+ | 17.3 | 18.6 | 12.4 | 12.5 |
| ATRIA Bleeding Risk Score, % | ||||
| Low (0–3) | 60.6 | 62.6 | 54.1 | 57.6 |
| Intermediate (4) | 4.6 | 5.4 | 6.3 | 6.6 |
| High (5–10) | 34.8 | 32.0 | 39.6 | 35.8 |
| Cognitive skills, % | ||||
| Mildly impaired | 26.0 | 28.3 | 25.9 | 27.1 |
| Moderately to severely impaired | 34.3 | 33.7 | 28.7 | 30.1 |
| ADL score (0–16), median (Q1, Q3)c | 9 (6, 11) | 9 (6, 11) | 9 (7, 11) | 9 (7, 11) |
ACE indicates angiotensin‐converting enzyme; ADLs, activities of daily living; ARB, angiotensin‐receptor blocker; ATRIA, Anticoagulation and Risk Factors in Atrial Fibrillation; DOAC, direct‐acting oral anticoagulant; NSAID, non‐steroidal anti‐inflammatory drugs; SSRI, selective serotonin reuptake inhibitor; Sup., suppressed.
Any Part D claim during the 12‐month period.
Resident characteristics exclude residents with missing values for fall history, heart failure, hypertension, diabetes mellitus, and stroke (n≤10 for all characteristics with missing values in 2011 and 2016).
Higher scores indicate greater limitation in ADLs.
Anticoagulant Use
The proportion of residents with AF treated with oral anticoagulants was 42.4% as of July 1, 2011, at which time the majority of treated residents were using warfarin. Anticoagulant use remained stable through the close of 2013, at which time 42.8% of residents were treated, 35.2% with warfarin and 7.7% with DOACs (Figure 1). Beginning in the first half of 2014 the prevalence of anticoagulant use increased during each half‐year through the end of the study (December 31, 2016), at which time 47.8% of residents were treated (P value for 2011–2016 trend <0.001). This period (2014–2016) of increasing anticoagulant use coincided with a decline in warfarin use and a rise in DOAC use such that by the end of 2016, the prevalence of warfarin use (24.7%) was nearly equal to DOAC use (23.1%).
Figure 1.
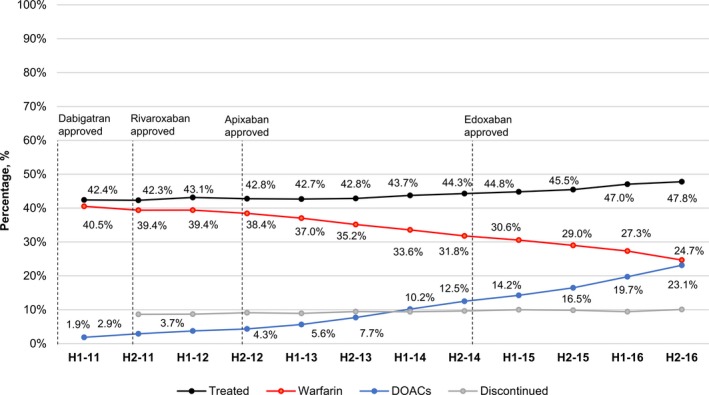
Percentage of US nursing home residents with atrial fibrillation treated with warfarin and direct‐acting oral anticoagulants, 2011 to 2016 by half year. DOAC indicates direct‐acting oral anticoagulants; H, half.
Dabigatran use increased during the early study period and peaked in the first half of 2012 before stabilizing in the range of 2.2% to 3.1% through 2016 (Table 3). In contrast, the prevalence of rivaroxaban and apixaban use continued to rise through the end of the study. Over the 5 full years (2012–2016) after market entry, rivaroxaban use increased from 0.4% to 8.3%. During the 4 full years after market entry (2013–2016), apixaban use grew from 0.1% to 12.6%. In contrast, edoxaban use remained rare after its approval in 2015.
Table 3.
Percentage of Treated Residents by Anticoagulant Class and Medication
| H1‐2011 | H2‐2011 | H1‐2012 | H2‐2012 | H1‐2013 | H2‐2013 | H1‐2014 | H2‐2014 | H1‐2015 | H2‐2015 | H1‐2016 | H2‐2016 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n, total | 17 895 | 33 959 | 33 493 | 33 956 | 35 709 | 37 118 | 36 183 | 36 379 | 36 807 | 37 644 | 37 474 | 37 787 |
| Treated, %a | 42.4 | 42.3 | 43.1 | 42.8 | 42.7 | 42.8 | 43.7 | 44.3 | 44.8 | 45.5 | 47.0 | 47.8 |
| Warf., % | 40.5 | 39.4 | 39.4 | 38.4 | 37.0 | 35.2 | 33.6 | 31.8 | 30.6 | 29.0 | 27.3 | 24.7 |
| DOAC, % | 1.9 | 2.9 | 3.7 | 4.3 | 5.6 | 7.7 | 10.2 | 12.5 | 14.2 | 16.5 | 19.7 | 23.1 |
| Dab., %b | 1.9 | 2.9 | 3.3 | 3.1 | 2.7 | 2.6 | 2.7 | 2.6 | Sup. | Sup. | Sup. | 2.2 |
| Riv., % | 0.0 | 0.0 | 0.4 | 1.2 | 2.9 | 4.6 | 6.2 | 7.1 | 7.1 | 7.3 | 7.8 | 8.3 |
| Apix., % | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.5 | 1.3 | 2.8 | 4.7 | 7.0 | 9.8 | 12.6 |
| Edox., %b | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | Sup. | Sup. | Sup. | 0.1 |
Apix. indicates apixaban; Dab., dabigatran; DOAC, direct‐acting oral anticoagulant; Edox., edoxaban; H, half; Riv., rivaroxaban; Warf., warfarin.
Treated percentage may not equal the sum of warfarin and DOAC percentages because of rounding.
Cell values suppressed to prevent any individual cell size from being <11.
As of the second half of 2016, 48.3% of white residents, 46.5% of black residents, 42.3% of Hispanic residents, and 36.7% of Asian/Pacific Islander residents were treated with oral anticoagulants (Table S2). No sex‐based differences in anticoagulant use were observed. In 2011, 36% of residents with moderate‐to‐severe cognitive impairment were treated with oral anticoagulants and 46.4% of cognitively intact or mildly impaired residents were treated (Table 4); the percentages treated in 2016 were 40.4% and 51.7%, respectively. The change in the prevalence of oral anticoagulant use between 2011 and 2016 was 5% among residents with and without chronic renal insufficiency (43% to 48%); the change among those with end‐stage renal disease was 8% (39% to 47%) (Table 5). In 2016, use of low DOAC doses was common (44%) among residents without a diagnosis of renal impairment, while standard DOAC doses were commonly used among those with chronic renal insufficiency (47%), end‐stage renal disease (40%), and those on dialysis (31%). Among 2676 apixaban users likely to have an indication for dosage reduction (at least 2 of the following: weight ≤60 kg, renal impairment, age ≥80 years), 26.2% received the standard dose. Among 3122 apixaban users who likely did not have an indication for dose reduction, 36.0% received the low dose.
Table 4.
Percentage of Nursing Home Residents With Atrial Fibrillation Treated With Oral Anticoagulants by Cognitive Status and Functioning in ADLs
| December 31, 2011 | December 31, 2016 | |||
|---|---|---|---|---|
| Cognitively Intact or Mild Impairment | Moderate or Severe Cognitive Impairment | Cognitively Intact or Mild Impairment | Moderate or Severe Cognitive Impairment | |
| n | 20 338 | 13 621 | 24 660 | 13 127 |
| Treated, % | 46.4 | 36.1 | 51.7 | 40.4 |
| ADL Score 0 to 4, na | 4275 | 772 | 4076 | 677 |
| Treated, % | 46.9 | 33.0 | 51.7 | 40.9 |
| ADL Score 5 to 8, na | 6401 | 2470 | 7714 | 2349 |
| Treated, % | 45.5 | 35.7 | 51.5 | 43.1 |
| ADL Score 9 to 12, na | 8195 | 5953 | 11 501 | 6850 |
| Treated, % | 47.1 | 37.0 | 52.3 | 40.3 |
| ADL Score 13 to 16, na | 1467 | 4426 | 1369 | 3251 |
| Treated, % | 45.5 | 35.7 | 48.1 | 38.7 |
ADLs indicates activities of daily living.
Higher scores indicate greater limitation in ADLs.
Table 5.
Anticoagulant Use by Renal Function Among Nursing Home Residents With Atrial Fibrillation
| No Diagnosis of Renal Insufficiency | Chronic Renal Insufficiencya | End‐Stage Renal Diseaseb | On Dialysisc | |
|---|---|---|---|---|
| December 31, 2016 | ||||
| n | 18 606 | 10 956 | 7014 | 1211 |
| Treated, % | 47.8 | 48.5 | 47.1 | 44.2 |
| Warfarin, % | 24.0 | 24.2 | 25.8 | 33.1 |
| Low‐dose DOAC, % | 10.5 | 12.9 | 12.8 | 7.7 |
| Standard‐dose DOAC, % | 13.4 | 11.4 | 8.4 | 3.4 |
| December 31, 2011 | ||||
| n | 19 319 | 9116 | 4713 | 811 |
| Treated, % | 43.1 | 42.8 | 38.5 | 39.7 |
| Warfarin, % | 40.0 | 39.8 | 36.1 | 39.7 |
| Low‐dose DOAC, % | 0.8 | 1.4 | 1.3 | 0.0 |
| Standard‐dose DOAC, % | 2.2 | 1.6 | 1.1 | 0.0 |
DOAC indicates direct‐acting oral anticoagulant.
Identified from in‐patient diagnoses. Corresponds to an estimated glomerular filtration rate <60 mL/min. Residents with evidence of more severe disease (end‐stage renal disease or on dialysis) were assigned to the more severe category.
Identified from the most recent MDS 3.0 assessment (item I1500: end‐stage renal disease). Residents with evidence of more severe disease (on dialysis) were assigned to the more severe category.
Identified from the most recent MDS 3.0 assessment (item O0100J2) which indicates whether the resident has received dialysis within the past 14 days while a resident of the nursing facility.
Anticoagulant Switching and Discontinuation
The proportion of nursing home residents with AF discontinuing oral anticoagulants was in the range of 8.6% to 10.1% for each half‐year of the study period (Figure 1). Among treated residents, the fraction switching from a DOAC to warfarin remained in a narrow range (1.8%–2.4%) from the second half of 2011 through the end of 2016 (Figure 2). Switchers from warfarin to a DOAC comprised 2.4% of treated residents in the first half of 2011 and 7.8% of treated residents in the second half of 2016. Among residents that switched between anticoagulant classes, the percentage of switchers that switched back to their original anticoagulant class ranged from 13% to 21% of all switchers during 2011 and 2012, and from 8% to 11% of all switchers during 2013 to 2016 (≈1% of the total treated population).
Figure 2.
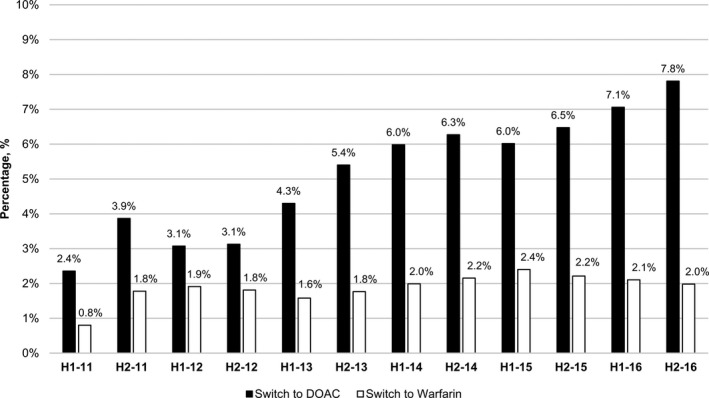
Percentage of treated residents that switched between warfarin and direct‐acting oral anticoagulants,* 2011 to 2016 by half year. *Residents that made multiple switches were grouped according to their most recent switch. DOAC indicates direct‐acting oral anticoagulants; H, half.
Discussion
The proportion of US nursing home residents with AF using oral anticoagulants was stable during the initial 3‐year period following the market release of the DOACs in the United States, but then steadily increased from 2014 to 2016. Underlying this overall trend, the pace of gradual decline in warfarin use mirrored uptake in DOAC use during 2011 to 2013, with DOAC uptake consistently outpacing declines in warfarin use beginning in 2014. Utilization growth peaked for dabigatran in 2012 and slowed for rivaroxaban in 2015. Continued increases in DOAC use and anticoagulation overall were fueled by the rapid uptake of apixaban, which began ≈1 year after its market entrance (2014) and was sustained through the end of 2016. By the end of 2016, approximately equal fractions of residents were treated with DOACs as were treated with warfarin.
Before DOAC availability, low use of oral anticoagulants among high‐risk older adults with AF was reported in the United States and internationally.6, 30, 31 In 2 large US community‐based AF cohorts (median CHA2DS2‐Vasc: 5), >40% of patients hospitalized for ischemic stroke were discharged without an anticoagulant.4 Estimates of anticoagulant use among US nursing home residents with AF in the 1990s and early 2000s suggested approximately two thirds of residents were not treated with warfarin.9, 32 At that time, reports of high rates of adverse events and labile international normalized ratios for nursing home residents11, 33 were accompanied by physician uncertainty about the relative benefits and risks of warfarin in the long‐term care setting.5 This uncertainty about the net benefit of treatment continues to affect anticoagulant prescribing decisions for high‐risk older adults.4
Low use of anticoagulation was widespread despite evidence supporting clinical benefit. A meta‐analysis of clinical trials comparing warfarin with control reported a 64% risk reduction for stroke and comparable risk for major extracranial hemorrhage in patients with AF.34 Similar findings were reported for warfarin versus aspirin among older adults aged >75 years.7 Prominent reasons clinicians refrain from anticoagulation among high‐risk older adults post‐stroke include perceived fall risk, poor prognosis, and a history of bleeding.4, 5 Considering a history of falls is common among nursing home residents, coupled with a high burden of cognitive impairment14 and short‐life expectancy,15 it is reasonable to expect a lower prevalence of anticoagulation compared with community‐dwelling populations. Interestingly, although the prevalence of bleeding risk factors was directionally consistent with greater provider caution in treating patients with higher bleeding risk, more than half of the untreated population had low bleeding risk (ATRIA <4) and more than three quarters did not have a recent history of falls. This suggests a role for other factors beyond these commonly reported reasons for not prescribing oral anticoagulants. In this respect, our findings were similar to earlier studies in the nursing home setting which reported lower likelihood of preventative treatment for residents with cognitive impairment in addition to atrial fibrillation32 or prior myocardial infarction.35 However, existing functional limitations did not appear to deter anticoagulant use in our study population, as treated fractions were generally consistent across levels of functional limitation.
Even in the presence of bleeding risk factors, cognitive impairment, and/or functional limitations, clinicians should maintain a focus on the overall risk‐benefit profile, while incorporating patient and family input. Patients often place greater weight on the prevention of stroke than the risk of bleeding,36 which may reflect recognition of stroke's long‐term consequences for functioning and cognition.37, 38 However, patient aversion to bleeding risk as well as the need for additional blood testing and clinical evaluation, even with DOACs, may also contribute to lower treatment rates than would be expected if guidelines were strictly followed. Beyond the decision to treat, the selection of dosage may also be affected by resident factors associated with perceived bleeding and stroke risk, potentially leading to dosing that is inconsistent with product labeling. In a large US cohort of privately insured and Medicare Advantage enrollees with atrial fibrillation, 43% of DOAC users received standard doses in the presence of a renal indication for dose reduction while 13% received low doses despite no renal indication.39 In the nursing home population, we estimated 44% of DOAC users without renal impairment (renal insufficiency, end‐stage disease, or on dialysis) received low dosages and 44% with renal impairment received standard dosages. In the community dwelling population, overdosing was associated with a >2‐fold increased risk of bleeding and comparable stroke risk, while under dosing was associated with a >4‐fold higher stroke risk among apixaban (but not rivaroxaban or dabigatran) users.39
After DOACs became available, changes in anticoagulant utilization among US nursing home residents were delayed and smaller in magnitude compared with changes in the broader community‐dwelling population. The prevalence of anticoagulant use among nursing home residents with AF had already increased from 30% in 200410 to 43% at the start of our study (2011). In the early period of DOAC availability, the percentage of residents anticoagulated remained steady before increasing to 48% during 2014 to 2016. This contrasts with the ambulatory care population, where the percentage of office‐based visits for AF with anticoagulant use increased from 52% in 2009 to 67% by the end of 2014.12 The rate of diffusion of DOACs in the community was also faster than in the nursing home, as the number of office visits for AF with DOAC use equaled the number of visits with warfarin use by the close of 2013.12 The proportion of nursing home residents using DOACs did not approach the proportion using warfarin until the end of 2016. However, increases in anticoagulant and DOAC use continued through the end of our study, suggesting these trends may have continued into 2017. The uptake of DOACs among Medicare Supplemental enrollees in the community was slower than the broader community‐dwelling population and more closely resembled uptake among nursing home residents.40
Clinical trial evidence comparing DOACs to warfarin specific to older adults is limited. Meta‐analysis of available trial data has suggested the DOACs have similar or improved efficacy and comparable or lower risk of major bleeding (except for dabigatran) compared with warfarin in adults aged ≥75 years.41, 42 Although time in therapeutic range was below target levels in the DOAC trials (55%–65%),42 similar to studies of real‐world populations,43 inferences on comparative effectiveness among older adults maintained within warfarin's therapeutic range require additional evidence. In the absence of definitive evidence in older frail populations, and in light of highly similar resident characteristics for DOAC and warfarin users in 2016, the increase in anticoagulant use during 2014–2016 may have been driven by several factors. American College of Cardiology/American Heart Association guidelines for AF management published in 2014 listed warfarin and DOACs as class one options for non‐valvular AF.44 Lack of monitoring requirements, fewer drug and dietary interactions, and less frequent need for dose adjustments may have contributed to subgroups of patients receiving treatment with DOACs that historically would not have received warfarin. Furthermore, superiority in safety and effectiveness of apixaban versus warfarin in the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial45 may have tipped the balance of perceived risks and benefits in favor of treatment for certain residents, a possibility supported by the timing and magnitude of increases in apixaban use.
Limitations
In this first national study of anticoagulant use in nursing homes since 2004, we used daily tracking of exposure and repeated point prevalence measurements to understand the evolution of anticoagulant use while accounting for switching and discontinuation. Limitations stem primarily from the use of diagnostic and medication utilization information derived observational data sources. Detailed clinical data on the type of AF, AF disease history, and renal functioning were not available. Use of over‐the‐counter medications such as aspirin was not observed unless there was a Part D claim. Anticoagulant exposure was estimated based on medication fill patterns and actual use may have differed, although non‐adherence is less of a concern because of the nature of medication administration in nursing homes. Finally, this was a population‐based study which used a repeated cross‐sectional design to describe patterns of real‐world medication use over time among US nursing home residents with AF. Although we describe resident characteristics in 2011 and 2016, the contributions of within‐resident correlation and changes in the characteristics of the US nursing home population over time to changes in anticoagulant utilization patterns were not evaluated statistically in the present study.
Conclusions
Even after a marked increase in anticoagulant use between 2004 and 2016, more than half of nursing home residents with AF remain untreated. The large majority of residents with AF are at high risk for stroke, evidenced by 85% of residents with a CHA2DS2‐Vasc score of ≥4. Recent estimates (2013–2016) of anticoagulant use in the community indicate a large majority (75%) of new‐users are initiating DOACs, including older adults.13 With recent availability of DOAC reversal agents46 and emerging observational evidence reinforcing trial findings in real‐world populations,47, 48 including the frail,49 it is likely the gradual increase in anticoagulation of nursing home residents and ongoing shift from warfarin to DOACs will continue. The early plateau in dabigatran use suggests any further increase in DOAC use among nursing home residents is likely to be driven by the factor Xa inhibitors apixaban, and to a lesser extent, rivaroxaban. Comparative effectiveness research specific to this medically complex older adult population is warranted to determine the clinical implications of these shifts in anticoagulant prescribing.
Sources of Funding
The study was funded by the National Institute on Aging (R21AG060529‐01). Dr. McManus was also supported by R01HL126911, R01HL137734, R01HL137794, and U54HL143541 from the National Heart, Lung, and Blood Institute. Dr. Alcusky was also supported by TL1TR001454 from the National Center for Advancing Translational Sciences.
Disclosures
Dr. McManus has received research grant funding from Bristol‐Myers Squibb, Boeringher‐Ingelheim, Pfizer, Samsung, Philips Healthcare, Biotronik, has received consultant fees from Bristol‐Myers Squibb, Pfizer, Flexcon, Boston Biomedical Associates, and has inventor equity in Mobile Sense Technologies, Inc. The remaining authors have no disclosures to report.
Supporting information
Table S1. ICD‐9 and ICD‐10 Code Based Definitions Applied to Medicare Part A Claims to Identify Specific Conditions
Table S2. Percentage of Residents Treated With Oral Anticoagulants by Race/Ethnicity
(J Am Heart Assoc. 2019;8:e012023 DOI: 10.1161/JAHA.119.012023.)
The abstract for the research described in this manuscript was presented at the American Geriatric Society Annual Meeting, May 2 to 4, 2019, in Portland, OR.
References
- 1. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults. JAMA. 2001;285:2370. [DOI] [PubMed] [Google Scholar]
- 3. Congressional Budget Office . Rising demand for long‐term services and supports for elderly people. 2013. Available at: https://www.cbo.gov/publication/44363. Accessed November 1, 2018.
- 4. McGrath ER, Go AS, Chang Y, Borowsky LH, Fang MC, Reynolds K, Singer DE. Use of oral anticoagulant therapy in older adults with atrial fibrillation after acute ischemic stroke. J Am Geriatr Soc. 2017;65:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Monette J, Gurwitz JH, Rochon PA, Avorn J. Physician attitudes concerning warfarin for stroke prevention in atrial fibrillation: results of a survey of long‐term care practitioners. J Am Geriatr Soc. 1997;45:1060–1065. [DOI] [PubMed] [Google Scholar]
- 6. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645.e4. [DOI] [PubMed] [Google Scholar]
- 7. Mant J, Hobbs FD, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, Murray E; BAFTA Investigators; Midland Research Practices Network (MidReC) . Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation: a randomized controlled trial. Lancet. 2007;370:493–503. [DOI] [PubMed] [Google Scholar]
- 8. Abel Latif AK, Peng X, Messinger‐Rapport BJ. Predictors of anticoagulation prescription in nursing home residents with atrial fibrillation. J Am Med Dir Assoc. 2005;6:128–131. [DOI] [PubMed] [Google Scholar]
- 9. Kojima G. Prevalence of frailty in nursing homes: a systematic review and meta‐analysis. J Am Med Dir Assoc. 2015;16:940–945. [DOI] [PubMed] [Google Scholar]
- 10. Ghaswalla PK, Harpe SE, Slattum PW. Warfarin use in nursing home residents: results from the 2004 national nursing home survey. Am J Geriatr Pharmacother. 2011;10:25–36. [DOI] [PubMed] [Google Scholar]
- 11. Gurwitz JH, Field TS, Radford MJ, Harrold LR, Becker R, Reed G, DeBellis K, Moldoff J, Verzier N. The safety of warfarin therapy in the nursing home setting. Am J Med. 2007;120:539–544. [DOI] [PubMed] [Google Scholar]
- 12. Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300–1305.e2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ, Hylek E, Kowey PR, Mahaffey KW, O'Brien EC, Singer DE, Peterson ED, Piccini JP; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF) Investigators and Patients . Factors associated with non‐vitamin K antagonist oral anticoagulants for stroke prevention in patients with new‐onset atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II (ORBIT‐AF II). Am Heart J. 2017;189:40–47. [DOI] [PubMed] [Google Scholar]
- 14. Thomas KS, Dosa D, Wysocki A, Mor V. The minimum data set 3.0 cognitive function scale. Med Care. 2017;55:e68–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelly A, Conell‐Price J, Covinsky K, Cenzer IS, Chang A, Boscardin WJ, Smith AK. Length of stay for older adults residing in nursing homes at the end of life. J Am Geriatr Soc. 2010;58:1701–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris‐Kojetin L, Sengupta M, Park‐Lee E, Valverde R, Caffrey C, Rome V, Lendon J. Long‐term care providers and services users in the United States: data from the National Study of Long‐Term Care Providers, 2013–2014. National Center for Health Statistics. Vital Health Stat 3. 2016;38:1–105. [PubMed] [Google Scholar]
- 17. Ziakas PD, Kourbeti IS, Poulou LS, Vlachogeorgos GS, Mylonakis E. Medicare part D prescribing for direct oral anticoagulants in the United States: cost, use and the “rubber effect”. PLoS One. 2018;13:e0198674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Neal WT, Sandesara PB, Claxton JS, MacLehose RF, Chen LY, Bengtson LGS, Chamberlain AM, Norby FL, Lutsey PL, Alonso A. Provider specialty, anticoagulation prescription patterns, and stroke risk in atrial fibrillation. J Am Heart Assoc. 2018;7:e007943 DOI: 10.1161/JAHA.117.007943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saliba S, Buchcanan J. Development & validation of a revised nursing home assessment tool: MDS 3.0. Rand Health Corporation; 2008. Centers for Medicare and Medicaid Services Contract No. 500‐00‐0027. [Google Scholar]
- 20. Research Data Assistance Center (ResDAC) . Research identifiable file (RIF) requests. Available at: https://www.resdac.org/research-identifiable-files-rif-requests. Accessed January 2, 2019.
- 21. Kumamaru H, Judd SE, Curtis JR, Ramachandran R, Hardy NC, Rhodes JD, Safford MM, Kissela BM, Howard G, Jalbert JJ, Brott TG, Setoguchi S. Validity of claims‐based stroke algorithms in contemporary Medicare data: reasons for geographic and racial differences in stroke (REGARDS) study linked with Medicare claims. Circ Cardiovasc Qual Outcomes. 2014;7:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 24. Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. A new risk scheme to predict warfarin‐associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pisters R, Lane DA, Nieuwlaat R, De Vos CB, Crijns HJGM, Lip GYH. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 26. Winkelmayer WC, Schneeweiss S, Mogun H, Patrick AR, Avorn J, Solomon DH. Identification of individuals with CKD from Medicare claims data: a validation study. Am J Kidney Dis. 2005;46:225–232. [DOI] [PubMed] [Google Scholar]
- 27. Quinn GR, Hellkamp AS, Hankey GJ, Becker RC, Berkowitz SD, Breithardt G, Fava M, Fox KAA, Halperin JL, Mahaffey KW, Nessel CC, Patel MR, Piccini JP, Singer DE. Selective serotonin reuptake inhibitors and bleeding risk in anticoagulated patients with atrial fibrillation: an analysis from the ROCKET AF trial. J Am Heart Assoc. 2018;7:e008755 DOI: 10.1161/JAHA.118.008755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quinn GR, Singer DE, Chang Y, Go AS, Borowsky LH, Udaltsova N, Fang MC. Effect of selective serotonin reuptake inhibitors on bleeding risk in patients with atrial fibrillation taking warfarin. Am J Cardiol. 2014;114:583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54:M546–M553. [DOI] [PubMed] [Google Scholar]
- 30. Appelros P, Farahmand B, Terént A, Asberg S. To treat or not to treat: anticoagulants as secondary preventives to the oldest old with atrial fibrillation. Stroke. 2017;48:1617–1623. [DOI] [PubMed] [Google Scholar]
- 31. Averlant L, Ficheur G, Ferret L, Boulé S, Puisieux F, Luyckx M, Soula J, Georges A, Beuscart R, Chazard E, Beuscart JB. Underuse of oral anticoagulants and inappropriate prescription of antiplatelet therapy in older inpatients with atrial fibrillation. Drugs Aging. 2017;34:701–710. [DOI] [PubMed] [Google Scholar]
- 32. Gurwitz JH, Monette J, Rochon PA, Eckler MA, Avorn J. Atrial fibrillation and stroke prevention with warfarin in the long‐term care setting. Arch Intern Med. 1997;157:978–984. [PubMed] [Google Scholar]
- 33. McCormick D, Gurwitz JH, Goldberg RJ. Prevalence and quality of warfarin use for patients with atrial fibrillation in the long‐term care setting. Arch Intern Med. 2001;161:2458–2463. [DOI] [PubMed] [Google Scholar]
- 34. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 35. Zullo AR, Sharmin S, Lee Y, Daiello LA, Shah NR, John Boscardin W, Dore DD, Lee SJ, Steinman MA. Secondary prevention medication use after myocardial infarction in U.S. nursing home residents. J Am Geriatr Soc. 2017;65:2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilke T, Bauer S, Mueller S, Kohlmann T, Bauersachs R. Patient preferences for oral anticoagulation therapy in atrial fibrillation: a systematic literature review. Patient. 2017;10:17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, Wadley VG. Trajectory of cognitive decline after incident stroke. JAMA. 2015;314:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dhamoon MS, Moon YP, Paik MC, Boden‐Albala B, Rundek T, Sacco RL, Elkind MS. Long‐term functional recovery after first ischemic stroke: the Northern Manhattan Study. Stroke. 2009;40:2805–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non‐vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017;69:2779–2790. [DOI] [PubMed] [Google Scholar]
- 40. Alalwan AA, Voils SA, Hartzema AG. Trends in utilization of warfarin and direct oral anticoagulants in older adult patients with atrial fibrillation. Am J Health Syst Pharm. 2017;74:1237–1244. [DOI] [PubMed] [Google Scholar]
- 41. Makam RCP, Hoaglin DC, McManus DD, Wang V, Gore JM, Spencer FA, Pradhan R, Tran H, Yu H, Goldberg RJ. Efficacy and safety of direct oral anticoagulants approved for cardiovascular indications: systematic review and meta‐analysis. PLoS One. 2018;13:e0197583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sharma M, Cornelius VR, Patel JP, Davies JG, Molokhia M. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: systematic review and meta‐analysis. Circulation. 2015;132:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wan Y, Heneghan C, Perera R, Roberts N, Hollowell J, Glasziou P, Bankhead C, Xu Y. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;1:84–91. [DOI] [PubMed] [Google Scholar]
- 44. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators . Apixaban versus warfarin. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 46. Rogers KC, Finks SW. A new option for reversing the anticoagulant effect of factor Xa inhibitors: Andexanet Alfa (ANDEXXA). Am J Med. 2019;132:38–41. [DOI] [PubMed] [Google Scholar]
- 47. Coleman CI, Peacock WF, Bunz TJ, Alberts MJ. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and previous stroke or transient ischemic attack. Stroke. 2017;48:2142–2149. [DOI] [PubMed] [Google Scholar]
- 48. Lip GYH, Keshishian A, Li X, Hamilton M, Masseria C, Gupta K, Luo X, Mardekian J, Friend K, Nadkarni A, Pan X, Baser O, Deitelzweig S. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients: the ARISTOPHANES study. Stroke. 2018;49:2933–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Martinez BK, Sood NA, Bunz TJ, Coleman CI. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in frail patients with nonvalvular atrial fibrillation. J Am Heart Assoc. 2018;7:e008643 DOI: 10.1161/JAHA.118.008643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ICD‐9 and ICD‐10 Code Based Definitions Applied to Medicare Part A Claims to Identify Specific Conditions
Table S2. Percentage of Residents Treated With Oral Anticoagulants by Race/Ethnicity


