Abstract
Study Design:
Review.
Objectives:
The objectives of this review are to (a) summarize the role of clinical practice guidelines (CPGs), (b) outline the methodology involved in formulating CPGs, (c) provide an illustration of these principles using a CPG developed for degenerative cervical myelopathy, and (d) highlight the importance of knowledge translation.
Methods:
A review of the literature was conducted to summarize current standards in CPG development and implementation.
Results:
CPGs are systematically developed statements intended to affect decisions made by health care providers, policy makers, and patients. The main objectives of CPGs are to synthesize and translate evidence into recommendations, optimize patient outcomes, standardize care, and facilitate shared decision making among physicians, patients, and their caregivers. The main steps involved in the development of CPGs include defining the clinical problem, assembling a multidisciplinary guideline development group and systematic review team, conducting a systematic review of the literature, translating the evidence to recommendations, critically appraising the CPG and updating the document when new studies arise. The final step in developing a CPG is to implement it into clinical practice; this step requires an assessment of the barriers to implementation and the formulation of effective dissemination strategies.
Conclusion:
CPGs are an important component in the teaching and practice of medicine and are available for a wide spectrum of diseases. CPGs, however, can only be used to influence clinical practice if the recommendations are informed by a systematic review of the literature and developed using rigorous methodology. The opportunity to transform clinical management of spinal conditions is an attractive outcome of the application of high-quality CPGs.
Keywords: clinical practice guidelines, guidelines development, knowledge translation, development guidelines
Introduction
Clinical practice guidelines (CPGs) are systematically developed statements intended to affect decisions made by health care providers, policy makers, and patients.1 They are based on a rigorous review of the best available evidence and provide recommendations on how to optimize patient management.2 Today, CPGs are an important component in the teaching and practice of medicine and are available for a wide spectrum of diseases.3 The main objectives of CPGs are to
Synthesize and translate the highest quality of evidence into practice recommendations
Optimize treatment outcomes and reduce the use of any harmful or unnecessary interventions
Establish standards of care and reduce inappropriate practice variations
Facilitate shared decision making among physicians, patients and their caregivers
Inform policy makers in their decisions about the allocation of health care resources.
In contrast, CPGs are not intended to replace clinical judgment or experience, be the sole source for management decisions, influence reimbursement policies, or be used for performance measures, legal precedents, or comprehensive management.2,4,5 Quality health care requires the combination of evidence, clinical expertise, and patient preferences.
Limitations in Previous Guidelines
There have been significant changes to the guideline development process over the past 30 years.6 Traditionally, CPGs consisted of consensus-based statements and recommendations generated by expert physicians. As evidence-based medicine has taken center stage, approaches for developing guidelines have become more rigorous, requiring consideration of past and present research. There can be, however, substantial limitations in the evidence base that can ultimately result in guidelines that are based largely on expert opinion.6-10 Such limitations include (a) a paucity of high-quality studies on the efficacy and effectiveness of various medical interventions, surgical treatments, drugs, and devices; (b) a lack of evidence on rarer conditions or subpopulations such as those with lower socioeconomic status; and (c) an inability to conduct randomized controlled trials due to ethical considerations. Furthermore, even if the results of studies are internally valid (ie, have limited biases related to study design, conduct, and analysis), they may not be generalizable or applicable to other populations (ie, not externally valid), or they may not be feasible to implement due to cost considerations.
Specific issues include (a) formation of a guideline development group (GDG) without attention to intellectual or financial conflicts of interest, (b) a lack of transparency in the derivation of recommendations, (c) poor coordination between the systematic review team and the GDG, (d) inability to control for bias among key stakeholders, and (e) failure to establish a well-balanced multidisciplinary GDG (6). As a result of limitations in the body of evidence along with deficiencies in the guideline development process, there are currently a number of conflicting recommendations for various conditions, and a lack of clarity as to which CPG should be used in practice.6
To address such shortcomings, the Institute of Medicine updated their definition of CPGs to “statements that include recommendations intended to optimize patient care that are informed by a systematic review of the evidence and an assessment of the benefits and harms of alternative care options.”6 Furthermore, in the book Clinical Practice Guidelines We Can Trust, the Institute of Medicine outlines the minimal standards required for CPGs to be trustworthy.6 Specifically,
Recommendations and suggestions must be informed by a rigorous systematic review of the existing evidence
CPGs must be developed by a knowledgeable multidisciplinary group that includes physicians, patients, and policy makers
The process to develop CPGs must be transparent and minimize biases, conflicts of interest, and other distortions
Recommendations must consider alternative treatment options as well as health outcomes
Each statement must be accompanied by a rating of the quality of evidence and the strength of the recommendation
CPGs must be updated regularly and as new evidence arises.
Along with these standards, the Guidelines International Network also recommends that the CPG should specify its objective and scope, be reviewed by external stakeholders before publication and disclose any financial support received for the development of either the systematic review or the recommendations.11
CPGs should also outline reasonable implementation plans, be flexible and adaptable to different settings and consider local resources and feasibility.12 Finally, CPGs must be developed in such a way that they can be easily accessed and understood by patients. Since the Institute of Medicine updated their definitions of CPGs, there was a significant reduction in the number of guidelines published in the National Guideline Clearinghouse from 2619 in 2014 to 1440 in 2018.13 Previously published CPGs that were not informed by a systematic review of the literature were removed from the Clearinghouse.
Funding is another important consideration as there are significant costs associated with the development of a CPG. Externally funded CPGs must explicitly state that the views and interests of the funding body have not influenced the final recommendations.4 Furthermore, appropriate management of conflicts of interest is critical to ensure the CPG has complete editorial independence from the funding source.
Overview of Guideline Methodology
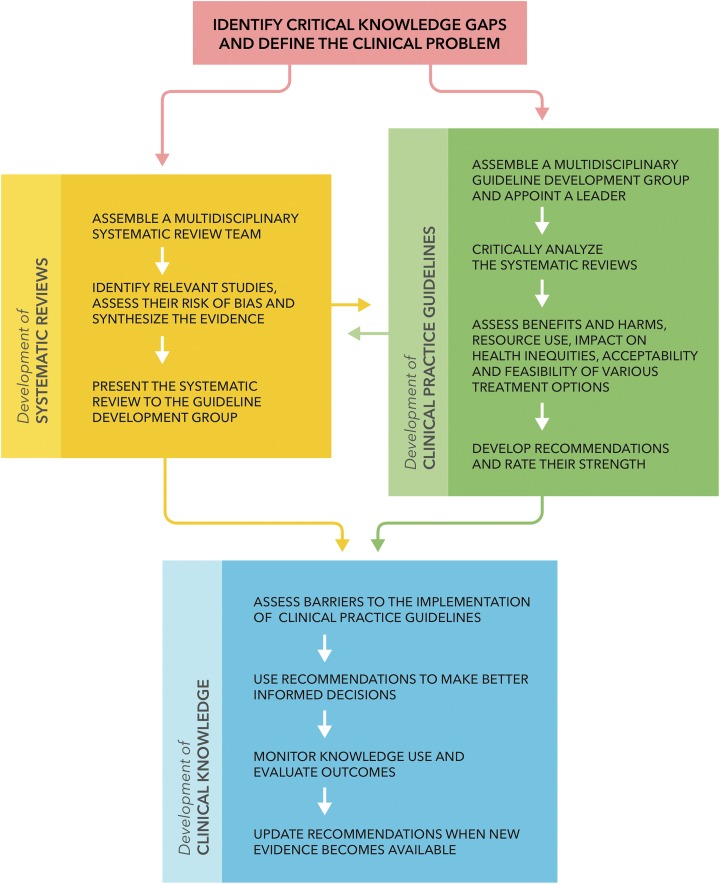
Figure 1 provides an overview of the three critical steps involved in the development of a CPG.
Figure 1.
An overview of the methodology involved in developing clinical practice guidelines.
Define the Clinical Problem
The first step in creating a CPG is to identify critical knowledge gaps and define the clinical problem, the scope and the target audience. Selected topics should be both “high-priority” and feasible.14 The development of a CPG is considered “high-priority” if there is
Significant disease burden and cost of management
Controversy or uncertainty around the topic and supporting evidence
New evidence arising that may impact current recommendations
Potential to improve health outcomes and quality of life, reduce mortality and morbidity, and affect decision making
Public or provider interest
Potential to reduce variations in care and cost.
Furthermore, proposed topics must have clear definitions of the disease or the procedure of interest and there must be an adequate evidence base to inform the recommendations.
The need for a CPG can also be evaluated by surveying key stakeholder groups. For example, Nater et al15 conducted a survey of the AOSpine International community to assess whether members would benefit from a CPG outlining how to manage perioperative neurologic deficit during spine surgery. The survey also aimed to gauge the likelihood that surgeons would use this guideline in their clinical practice. Information from surveys can be used to prioritize topics for CPGs, evaluate current practice standards, and initiate the process of knowledge translation.
Establish a Guideline Development Group and Leadership
A GDG should be multidisciplinary and consist of 10 to 20 members.16 If multiple specialties are represented in the GDG, there is an increased likelihood that all contributory scientific evidence will be identified and analyzed, and that any practical problems in guideline application will be addressed.6 Members of the group should include
Specialists on the topic
Nonspecialists who may either implement or be affected by the CPG
Physical therapists, allied health and nursing professionals
Patient advocates, public representatives, and caregivers
Epidemiologists, statisticians, and experts in decision analysis, informatics, and clinical or social psychology.
All participants are required to complete a disclosure form outlining any financial, personal, or intellectual conflicts of interest.5
The GDG should have a group leader who is qualified and experienced in facilitating discussion among healthcare professionals and consumer representatives. The leader is responsible for appointing a vice-chair, ensuring that key stakeholders are adequately represented in the GDG and managing conflicts of interest. The leader also “needs to allow sufficient time for all members to express their views without feeling intimidated or threatened and should check that all members in the group agree to endorse any recommendation.”6 This individual must remain neutral, encourage positive group processes and discourage minority influence, group polarization, and “groupthink” (ie, when members’ desire for unanimity override objective appraisal of the evidence).17,18
Patients and their caregivers reflect the group that will be most deeply affected by a CPG.6 They will undoubtedly bring perspectives to discussions that differ from those of clinicians and ensure that recommendations are presented in a way that is understandable and accessible to patients.6 One of the challenges is that patients may lack the relevant training and scientific literacy required to follow and meaningfully contribute to discussions.6 Moreover, a consumer representative or a patient suffering from the disease of interest may have personal experiences that could interfere with their ability to accurately evaluate the evidence and recommendations.6
Assemble a Multidisciplinary Systematic Review Team
Similar to the GDG, the systematic review team should be multidisciplinary and include methodology experts. There can be complete isolation, limited interaction or complete interaction between the GDG and the systematic review team. The Institute of Medicine highlights the advantages and disadvantages of the varying levels of interaction.6
Conduct a Systematic Review of the Literature
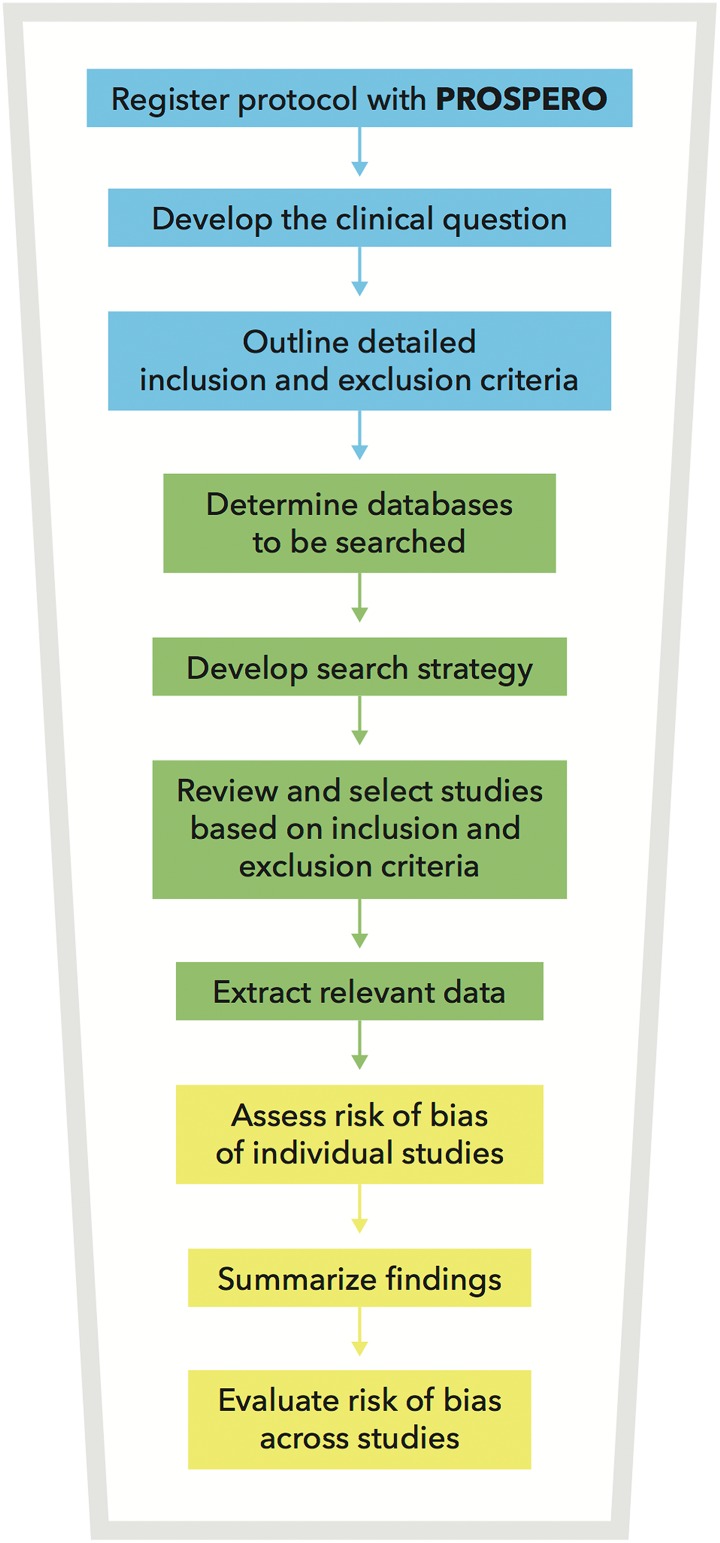
Systematic reviews are required to summarize and synthesize the evidence that will ultimately inform the CPG. The PRISMA statement (Preferred Reporting Items for Systematic reviews and Meta-Analyses) outlines the minimum set of items required for a systematic review or meta-analysis.19 Figure 2 highlights the methodology involved in conducting a systematic review:
Protocol and Registration: It is recommended that the protocol for each systematic review is registered with PROPSERO, an international prospective register of systematic reviews.20
Clinical Question: A clinical question should be developed in the format “should A (versus B) be used to treat/manage patients with C?”
Eligibility Criteria: Detailed inclusion and exclusion criteria should be defined before conducting a database search. The use of a PICO (population, intervention, comparison, outcome) or PPO (population, prognostic factor, outcome) table is recommended.21 Furthermore, any limitations on language, years of publication, and study design should be specified a priori.
Information Sources: EMBASE, MEDLINE, the Cochrane Central Register of Controlled Trials, and other relevant databases should be searched.22
Search: A detailed search strategy should be developed, preferably with the help of a librarian. Searches should be highly sensitive and use both free-text and subject headings.
Study Selection: Two independent reviewers should screen all abstracts (and full texts, where necessary) to determine which studies satisfy the inclusion and exclusion criteria.
Data extraction: Two independent reviewers should also extract the relevant data from each study using data collection forms or other unified methodology. Furthermore, in cases where invaluable information is missing from the study, steps should be taken to acquire this information (eg, contacting the primary investigator). Finally, any assumptions or simplifications applied during data extraction should be noted.
Assess Risk of Bias of Individual Studies: Numerous tools, such as the Newcastle Ottawa Scale and Critical Appraisal Skills Programme tool, are available to evaluate the quality of primary studies.23,24 The main types of bias that should be assessed are selection, performance, detection, information, reporting and attrition bias.
Summarize Findings
Evaluate Risk of Bias Across Studies: The strength of the overall body of evidence should be determined. Methods proposed by the GRADE (Grading of Recommendation, Assessment, Development, and Evaluation) Working Group have been increasingly used to evaluate risk of bias across studies and to determine how confident one can be about the estimate of effect.25,26
Figure 2.
An overview of the methodology required for a systematic review.
Several organizations that advocate evidence-based medicine have endorsed the GRADE process, including the World Health Organization, the BMJ Clinical Evidence and the Agency for Health Research and Quality (AHRQ).27
The initial rating of the quality of evidence is based on whether the studies are randomized controlled trials (baseline level = HIGH) or observational studies (baseline level = LOW).26 The quality of evidence may then be upgraded or downgraded based on a number of factors. Criteria for downgrading the quality by 1 or 2 levels include limitations in the study design or execution, inconsistency of results, indirectness of evidence, imprecision, and publication bias.28-32 Alternatively, criteria for upgrading the quality by 1 or 2 levels include a large magnitude of effect, if plausible confounding would reduce the demonstrated effect or increase the effect if no effect was observed, or if there was a dose-response gradient.33 Table 1 summarizes how to determine whether the quality of evidence should be upgraded or downgraded.
Table 1.
Reasons for Upgrading or Downgrading the Quality of the Overall Body of Evidence.a
| Factor | Examples | Consequence |
|---|---|---|
| Factors than can downgrade the quality of evidence | ||
| Limitations in study design or execution (risk of bias) |
|
Downgrade 1 or 2 levels |
| Inconsistency of results |
Unexplained heterogeneity of results across
studies:
|
Downgrade 1 or 2 levels |
| Indirectness of evidenceb |
Sources of indirectness include
|
Downgrade 1 or 2 levels |
| Imprecision |
|
Downgrade 1 or 2 levels |
| Publication bias |
|
Downgrade 1 or 2 levels |
| Factors that can upgrade the quality of evidence | ||
| Large magnitude of effectc |
Very large: RR >5 or
<0.2 Large: RR >2 or <0.5 |
Upgrade 1 or 2 levels |
| All plausible confounding would reduce the demonstrated effect or increase the effect if no effect was observed | N/A | Upgrade 1 level |
| Dose-response gradient | N/A | Upgrade 1 level |
Abbreviations: N/A, not applicable; RCT, randomized controlled trial; RR, relative risk.
a Derived from the GRADE handbook.33
b Direct evidence directly compares the interventions of interest delivered to the populations of interest and measures the outcomes important to the patients.
c Only applied to relative risks or hazard ratios. May not be applicable to odds ratios (ORs); GRADE suggests converting OR to RR and then assessing the magnitude of effect.
Following this process, the quality of evidence can be considered high, moderate, low, or very low (Table 2).26
Table 2.
Interpretation of Quality of Evidence Ratings.a
| Grade | Definition |
|---|---|
| High | High confidence in the effect estimate. The true effect lies close to that of the estimate of effect. |
| Moderate | Moderate confidence in the effect estimate. The true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. |
| Low | Limited confidence in the effect estimate. The true effect may be substantially different from the estimate of effect. |
| Very low | Very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. |
a Derived from the GRADE handbook.33
Development of Clinical Practice Guidelines
The development of CPGs requires the formation of recommendations using evidence summarized in a systematic review. The initial step in any guideline project is to construct a protocol that includes the general focus, purpose, and rationale of the CPG, relevant definitions, aspects of care covered by the guideline, users, and implementation strategies. The next steps consist of examining and critiquing the evidence, formulating evidence based-recommendations, evaluating the overall certainty of the evidence, and defining the strength of recommendations. Several tools can be used to guide the development process, including the Conference on Guideline Standardization (COGS) checklist for reporting CPGs, the AGREE (Appraisal of Guidelines for Research and Evaluation) tool, the ADAPTE framework, the GRADE guideline development tool (GDT), and the evidence-to-recommendation framework.10,34-38
The GRADE “evidence-to-recommendation” framework is increasingly used to guide the development process. This tool consists of several questions aimed to assess the benefits and harms, resource use, the impact on health inequities, the acceptability, and the feasibility of various treatment options (Table 3).39 This framework ensures that (a) all important factors that influence a recommendation are considered, (b) judgments about each factor are informed by the best available evidence, (c) discussions are structured, (d) reasons for disagreement among members of the GDG are identified, and (e) the rationale for each recommendation is transparent to the target audience.
Table 3.
The Evidence-to-Recommendation Framework.a
| Question | Response Options |
|---|---|
| Benefits and harms | |
| What is the overall certainty of the evidence? | No included studies, very low, low, moderate, high |
| Is there important uncertainty about how much people value the main outcomes? | Important uncertainty or variability, possibly important uncertainty or variability, probably no important uncertainty or variability, no important uncertainty or variability, no known undesirable |
| Are the desirable anticipated effects large? | No, probably no, uncertain, probably yes, yes, varies |
| Are the undesirable anticipated effects small? | No, probably no, uncertain, probably yes, yes, varies |
| Are the desirable effects large relative to the undesirable effects? | No, probably no, uncertain, probably yes, yes, varies |
| Resource use | |
| Are the resources required small? | No, probably no, uncertain, probably yes, yes, varies |
| Is the incremental cost small relative to the net benefit? | No, probably no, uncertain, probably yes, yes, varies |
| Equity | |
| What would be the impact on health inequities? | Increased, probably increased, uncertain, probably reduced, reduced, varies |
| Acceptability | |
| Is the option acceptable to key stakeholders? | No, probably no, uncertain, probably yes, yes, varies |
| Feasibility | |
| Is the option feasible to implement? | No, probably no, uncertain, probably yes, yes, varies |
| Balance of the consequences | |
| What is the balance between undesirable and desirable consequences? | Undesirable consequences clearly outweigh desirable consequences, undesirable consequences probably outweigh desirable consequences in most settings, the balance between desirable and undesirable consequences is closely balanced or uncertain, desirable consequences probably outweigh undesirable consequences in most settings, desirable consequences clearly outweigh undesirable consequences in most settings |
| Type of recommendation | |
| What is the strength and direction of the recommendation? | We recommend against offering this option we suggest not offering this option, we suggest offering this option, we recommend offering this option |
a Derived from the Decide Collaboration.39
The final step in the process is to balance the consequences and determine the strength of each recommendation (Table 3). The 4 factors that influence the strength of the recommendation are the balance between desirable and undesirable outcomes, the confidence in the magnitude of the estimate of effect (as gauged by the quality of the evidence), the confidence in values and preferences of key stakeholders and resource use.40,41 The wording of the recommendation signifies whether it is “strong” or “weak.” Specifically, the word “recommend” denotes a stronger recommendation, whereas the word “suggest” indicates a weaker recommendation.5
The strength of the recommendation has different implications for patients, clinicians, and policy makers. A strong recommendation means that (a) most patients would want to receive the recommended course of action and only a small proportion would not, (b) most patients should receive the recommended course of action, and (c) the recommendation can be adapted as policy in most situations and be used as a performance indicator.36,40 Furthermore, formal decision aids are unlikely to be needed to assist patients in making a decision consistent with their values and preferences.40 A weak recommendation means that (a) the majority of individuals would want the suggested course of action but many would not, (b) clinicians must recognize that different choices will be appropriate for different patients and should help a patient arrive at a decision consistent with his or her values or preferences, and (c) policy making will require substantial debates and involvement of many stakeholders.36,40
Guideline Appraisal Tools
It is often the case that multiple CPGs are published with conflicting recommendations on the same topic. These differences may arise due to variations in practice standards across countries or due to discrepancies in opinions related to the anticipated harms, benefits, cost-effectiveness, feasibility, and acceptability of each recommendation.42 In contrast, contradictory CPGs may be a result of conflicts of interest, failure of the GDG to critically appraise the evidence or inadequate representation of important stakeholder groups.42 Clinicians must be able to identify CPGs that have been rigorously developed and are based on a systematic assessment of the evidence.
The Appraisal of Guidelines for Research and Evaluation (AGREE) II is an instrument designed to assess the quality of and provide a framework for the development and reporting of CPGs.35 The AGREE II can be used by (a) health care providers and policy makers to evaluate which CPG should be used in practice or to inform policy decisions, (b) guideline developers to ensure rigorous methodology and guideline validity, and (c) educators to improve critical appraisal skills among health care professionals.
The AGREE II consists of 23 items rated on a 7-point scale (1 = strongly disagree, 7 = strongly agree) in the following domains: scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence.43 There are also 2 global rating items that include the rating of the overall quality of the guideline and whether the guideline should be recommended for use in clinical practice. Table 4 summarizes the key questions included in AGREE II.
Table 4.
The Appraisal of Guidelines for Research and Evaluation (AGREE) II.a
| Domain | Key Items |
|---|---|
| 1. Scope and purpose |
|
| 2. Stakeholder involvement |
|
| 3. Rigor of development |
|
| 4. Clarity of presentation |
|
| 5. Applicability |
|
| 6. Editorial independence |
|
a Derived from the AGREE II User Manual.42
The CPG should be externally appraised by a multidisciplinary group of clinicians, a patient advocate, and other relevant stakeholders.
Update Planning
The Guidelines International Network and the Institute of Medicine have outlined important standards for updating CPGs.6,11 They suggest that the literature should be monitored regularly to identify the emergence of new evidence and to evaluate the ongoing validity of the CPG. Specifically, an update is necessary if there are changes in the evidence related to benefits and harms, outcomes that would be considered critical for decision making, ranking of current outcomes and available interventions and resources.44 Developers should specify a detailed plan for updating a CPG.
An Illustrative Example
In 2017, 6 CPGs were developed to outline how to best manage patients with degenerative cervical myelopathy (DCM) and spinal cord injury (SCI).45-50 Table 5 summarizes the steps involved in guideline development by using the DCM guideline as an example.
Table 5.
An Illustrative Example.
| Step | Action |
|---|---|
| Define the clinical problem | What is the best management strategy for patients with mild, moderate and severe DCM and nonmyelopathic patients with evidence of cord compression? |
| Establish a guideline development group and leadership | The GDG group was multidisciplinary and consisted of neurosurgeons, orthopedic surgeons, neurologists, rheumatologists, rehabilitation specialists, epidemiologists, a physiotherapist, a nurse, and a patient advocate. |
| Assemble a multidisciplinary systematic review team | The systematic review team was also multidisciplinary and contained some, but not all, members from the GDG. |
| Conduct a systematic review of the literature | Systematic reviews were conducted to summarize the natural history, the comparative efficacy of operative versus non-operative management, the expected functional, disability and pain outcomes following surgery and nonoperative treatment and the management of nonmyelopathic patients with cervical spinal cord compression, canal stenosis, and/or OPLL. |
| Development of clinical practice guidelines | The CPG addressed the following questions:
Recommendations were developed based on the systematic reviews, benefits and harms, resource use, impact on health inequities, acceptability and feasibility. The GRADE evidence-to-recommendation framework was used to facilitate discussions and document the process. Finally, the GDG weighed the undesirable and desirable consequences, formulated the recommendations and agreed on the strength of each recommendation. |
| Guideline appraisal | The AGREE II tool was used to appraise the CPG. The CPG was also evaluated externally by a multidisciplinary group of clinicians, a patient advocate, AOSpine North America, AOSpine International, and the Cervical Spine Research Society. |
| Update planning | The CPG will be reviewed by the primary sponsor every 3 to 5 years following publication. |
Abbreviations: AFREE II, Appraisal of Guidelines for Research and Evaluation II; CPG, clinical practice guidelines; DCM, degenerative cervical myelopathy; GDG, guideline development group; GRADE, Grading of Recommendation, Assessment, Development, and Evaluation; OPLL, ossification of the posterior longitudinal ligament.
Knowledge Translation
Knowledge translation is defined as “the exchange, synthesis and application of knowledge to accelerate the benefits of research through improved health, more effective services and products, and a strengthened health care system.”51 The first step in any form of knowledge translation is to identify critical knowledge gaps and define the clinical problem.
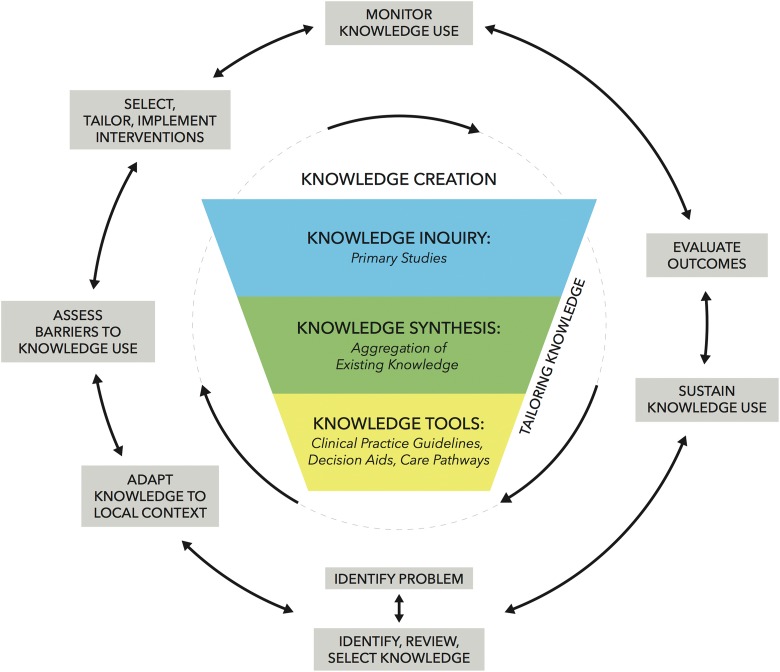
Figure 3 summarizes the knowledge-to-action framework proposed by Graham et al.52 This framework consists of 2 distinct but related components: knowledge creation, represented by the funnel in the center of the figure, and the surrounding action cycle. These 2 components are closely related and can influence each other.
Figure 3.
The knowledge-to-action framework (derived from Graham et al52).
Knowledge creation includes “knowledge inquiry” (ie, the multitude of primary studies available to date), “knowledge synthesis” (ie, the aggregation of existing knowledge through rigorous systematic reviews or meta-analyses), and “knowledge tools” (ie, clinical practice guidelines, decision aids, and care pathways).52 The surrounding action cycle outlines the steps required to effectively translate knowledge creation into practice and sustain knowledge use. These include assessing barriers to knowledge use, devising effective dissemination and implementation strategies, monitoring knowledge use, and evaluating outcomes.
Assessing Barriers to Implementation
The development of a CPG does not necessarily equate to changes in clinical practice. In fact, CPGs are often not applied. As a result, it is estimated that 30% to 40% of patients receive treatment that is not based on scientific evidence and 20% to 25% of patients are managed with either useless or potentially harmful interventions.53
Barriers to the implementation and adherence of CPGs can be categorized into personal, guideline-related, and external factors.54-56 Personal factors include those related to physicians’ knowledge (ie, lack of awareness or lack of familiarity with the CPG and its recommendations), physicians’ attitude (ie, lack of agreement, self-efficacy, skills, motivation, outcome expectancy, and learning culture), and patients’ desires (ie, inability to reconcile patient preferences with the use of knowledge).54-56 Guideline-related factors are those that impact the validity, accessibility, and applicability of the recommendations (eg, lack of evidence, complexity, poor layout, inaccessibility, lack of applicability, and lack of clear intervention goals).55 Finally, external factors impeding the implementation of CPGs include organizational constraints, lack of resources and time, and lack of collaboration.55
Develop Effective Dissemination and Implementation Strategies
Effective dissemination and implementation strategies must address the aforementioned barriers.55 Strategies include
Address physicians’ awareness and familiarity by increasing the dissemination of the guideline, using mass media or educational posters in examination rooms, and organizing continuing medical education activities that focus on specific recommendations.
Address issues with physicians’ attitudes by using opinion leaders, obtaining endorsement from special societies, organizing educational meetings, interactive learning and outreach visits, and providing feedback on individual performance.
Address guideline related factors by employing methods of evidence-based medicine, critically appraising the recommendations, regularly updating the content, and using tablets, smartphones, and mobiles for the provision of guidelines.
Address external barriers by standardizing processes and procedures, improving collaboration among health care professionals, and providing adequate time for the documentation and utilization of the guidelines.
Monitoring Knowledge Use and Evaluating Outcomes
Other important components of the knowledge-to-action framework include monitoring knowledge use and evaluating outcomes. There are three different types of knowledge use: conceptual (ie, changes in level of knowledge, understanding or attitudes), instrumental (ie, changes in behavior or practice), and strategic (ie, manipulation of knowledge to attain specific power or profit goals).52 These can be evaluated by (a) assessing patient attitudes and resource use through surveys and (b) determining the use of care pathways through secondary analysis.
The subsequent phase in the framework is to evaluate the impact of knowledge use on the patient, the physician and the organization. Specifically, the impact on the patient can be measured by improved health status, quality of life and satisfaction, as well as reduced length of hospital stay. The impact on physicians can be assessed using questionnaires and interviews that focus on improved satisfaction with practice and increased time dedicated to new practice. Finally, the impact on organizations can be evaluated through analysis of wait times, length of stay, and costs using administrative or clinical databases.
Adapting Guidelines to Different Settings
CPGs developed in one country can be adapted and used in another country. The benefits of transferring guidelines across institutions include avoiding duplication of effort, reducing unnecessary use of resources, and enhancing efficiency.57 The ADAPTE collaboration group was developed to define a process for adapting existing CPGs to different settings.37 Important tenets of this process include
Acknowledge evidence-based principles in guidelines
Use reliable methodology that promotes the quality of adapted guideline
Encourage participation of key stakeholders to ensure acceptance of the guideline and to promote its use
Evaluate the feasibility of implementing the guideline and assess its relevance in local practice and policy
Report the recommendations with transparency
Provide a flexible format of the guideline to accommodate specific needs and circumstances.
Based on these principles, a comprehensive framework was developed that consisted of 3 main phases with 24 steps: (a) set-up (prepare for adapt framework), (b) adaptation (search and screen guidelines, assess guidelines, decide and select, and draft guideline report), and (c) finalization (external review, plan for future review and update, produce final guideline).37
Acknowledgments
MGF would like to acknowledge support from the Gerry and Tootsie Halbert Chair in Neural Repair and Regeneration as well as the DeZwirek Family Foundation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was organized and funded by AOSpine International through the AOSpine Knowledge Forum Spinal Cord Injury, a focused group of international spine experts acting on behalf of AOSpine. Study support was provided directly through the AOSpine Research Department.
ORCID iD: Michael G. Fehlings  https://orcid.org/0000-0002-5722-6364
https://orcid.org/0000-0002-5722-6364
References
- 1. Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318:527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parfrey PS, Barrett B. Clinical Epidemiology: Practice and Methods. 2nd ed New York, NY: Humana Press; 2015. [Google Scholar]
- 3. Berg AO, Atkins D, Tierney W. Clinical practice guidelines in practice and education. J Gen Intern Med. 1997;12(suppl 2):S25–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenfeld RM, Shiffman RN, Robertson P; Department of Otolaryngology State University of New York Downstate. Clinical Practice Guideline Development Manual, Third Edition: a quality-driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 2013;148(1 suppl):S1–S55. [DOI] [PubMed] [Google Scholar]
- 5. Tetreault LA, Skelly AC, Dettori JR, Wilson JR, Martin AR, Fehlings MG. Guidelines for the management of degenerative cervical myelopathy and acute spinal cord injury: development process and methodology. Global Spine J. 2017;7(3 suppl):8S–20S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines; Graham R, Mancher M, Wolman DM, Greenfield S, Steinberg E. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 7. Shaneyfelt TM, Centor RM. Reassessment of clinical practice guidelines: go gently into that good night. JAMA. 2009;301:868–869. [DOI] [PubMed] [Google Scholar]
- 8. US Institute of Medicine. Knowing What Works in Health Care: A Roadmap for the Nation. Washington, DC: National Academies Press; 2008. [Google Scholar]
- 9. Lugtenberg M, Burgers JS, Clancy C, Westert GP, Schneider EC. Current guidelines have limited applicability to patients with comorbid conditions: a systematic analysis of evidence-based guidelines. PLoS One. 2011;6:e25987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shiffman RN, Shekelle P, Overhage JM, Slutsky J, Grimshaw J, Deshpande AM. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med. 2003;139:493–498. [DOI] [PubMed] [Google Scholar]
- 11. Qaseem A, Forland F, Macbeth F, Ollenschlager G, Phillips S, van der Wees P; Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–531. [DOI] [PubMed] [Google Scholar]
- 12. Parfrey PS, Barrett BJ. Clinical Epidemiology: Practice and Methods. New York, NY: Humana Press; 2009. [Google Scholar]
- 13. Shekelle PG. Clinical practice guidelines: what’s next? JAMA. 2018;320:757–758. [DOI] [PubMed] [Google Scholar]
- 14. Institute of Medicine (US) Committee on Methods for Setting Priorities for Guidelines Development; Field MJ. Setting Priorities for Clinical Practice Guidelines. Washington, DC: National Academies Press; 1995. [PubMed] [Google Scholar]
- 15. Nater A, Murray JC, Martin AR, Nouri A, Tetreault L, Fehlings MG. The need for clinical practice guidelines in assessing and managing perioperative neurologic deficit: results from a Survey of the AOSpine International Community. World Neurosurg. 2017;105:720–727. [DOI] [PubMed] [Google Scholar]
- 16. Burgers JS, Grol RP, Zaat JO, Spies TH, van der Bij AK, Mokkink HG. Characteristics of effective clinical guidelines for general practice. Br J Gen Pract. 2003;53:15–19. [PMC free article] [PubMed] [Google Scholar]
- 17. Pagliari C, Grimshaw J. Impact of group structure and process on multidisciplinary evidence-based guideline development: an observational study. J Eval Clin Pract. 2002;8:145–153. [DOI] [PubMed] [Google Scholar]
- 18. Pagliari C, Grimshaw J, Eccles M. The potential influence of small group processes on guideline development. J Eval Clin Pract. 2001;7:165–173. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 20. Booth A, Clarke M, Dooley G, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. 2012;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skelly AC. Credibility matters: mind the gap. Evid Based Spine Care J. 2014;5:2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Booth A. Chapter 3: Searching for studies In: Noyes J, Booth A, Hannes K, et al., eds. Supplementary Guidance for Inclusion of Qualitative Research in Cochrane Systematic Reviews of Interventions. Version 1 London, England: Cochrane Collaboration Qualitative Methods Group; 2011. https://methods.cochrane.org/qi/supplemental-handbook-guidance. [Google Scholar]
- 23. Noyes J, Booth A, Flemming K, et al. Cochrane Qualitative and Implementation Methods Group guidance series—paper 3: methods for assessing methodological limitations, data extraction and synthesis, and confidence in synthesized qualitative findings. J Clin Epidemiol. 2018;97:49–58. [DOI] [PubMed] [Google Scholar]
- 24. Wells GA, Shes BJ, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed February 1, 2019.
- 25. Atkins D, Best D, Briss PA, et al. ; GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. [DOI] [PubMed] [Google Scholar]
- 27. Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64:1283–1293. [DOI] [PubMed] [Google Scholar]
- 29. Guyatt GH, Oxman AD, Kunz R, et al. ; GRADE Working Group. GRADE guidelines: 8. Rating the quality of evidence—indirectness J Clin Epidemiol. 2011;64:1303–1310. [DOI] [PubMed] [Google Scholar]
- 30. Guyatt GH, Oxman AD, Kunz R, et al. ; GRADE Working Group. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–1302. [DOI] [PubMed] [Google Scholar]
- 31. Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64:1277–1282. [DOI] [PubMed] [Google Scholar]
- 32. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64:407–415. [DOI] [PubMed] [Google Scholar]
- 33. Guyatt GH, Oxman AD, Sultan S, et al. ; GRADE Working Group. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311–1316. [DOI] [PubMed] [Google Scholar]
- 34. Shiffman RN, Michel G. Toward improved guideline quality: using the COGS statement with GEM. Stud Health Technol Inform. 2004;107(pt 1):159–163. [PubMed] [Google Scholar]
- 35. Brouwers MC, Kho ME, Browman GP, et al. ; AGREE Next Steps Consortium. Development of the AGREE II, part 2: assessment of validity of items and tools to support application. CMAJ. 2010;182:E472–E478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66:719–725. [DOI] [PubMed] [Google Scholar]
- 37. Fervers B, Burgers JS, Voellinger R, et al. ; ADAPTE Collaboration. Guideline adaptation: an approach to enhance efficiency in guideline development and improve utilisation. BMJ Qual Saf. 2011;20:228–236. [DOI] [PubMed] [Google Scholar]
- 38. The ADAPTE Collaboration. The ADAPTE Process: Resource Toolkit for Guideline Adaptation. Version 2.0 http://www.g-i-n.net. Published 2009. Accessed February 1, 2019.
- 39. Decide Grade Group. Evidence to decision (EtD) framework 2015. https://www.decide-collaboration.eu/evidence-decision-etd-framework. Accessed February 1, 2019.
- 40. Guyatt GH, Oxman AD, Kunz R, et al. ; GRADE Working Group. Going from evidence to recommendations. BMJ. 2008;336:1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Andrews JC, Schünemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence to recommendation—determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013;66:726–735. [DOI] [PubMed] [Google Scholar]
- 42. Oxman AD, Glasziou P, Williams JW., Jr What should clinicians do when faced with conflicting recommendations? BMJ. 2008;337:a2530. [DOI] [PubMed] [Google Scholar]
- 43. Brouwers M, Kho ME, Browman GP; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in healthcare. CMAJ. 2010;182:E839–E842. https://www.agreetrust.org/wp-content/uploads/2013/10/AGREE-II-Users-Manual-and-23-item-Instrument_2009_UPDATE_2013.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shekelle P, Woolf S, Grimshaw JM, Schunemann HJ, Eccles MP. Developing clinical practice guidelines: reviewing, reporting, and publishing guidelines; updating guidelines; and the emerging issues of enhancing guideline implementability and accounting for comorbid conditions in guideline development. Implement Sci. 2012;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fehlings MG, Martin AR, Tetreault LA, et al. A clinical practice guideline for the management of patients with acute spinal cord injury: recommendations on the role of baseline magnetic resonance imaging in clinical decision making and outcome prediction. Global Spine J. 2017;7(3 suppl):221S–230S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fehlings MG, Tetreault LA, Aarabi B, et al. A clinical practice guideline for the management of patients with acute spinal cord injury: recommendations on the type and timing of anticoagulant thromboprophylaxis. Global Spine J. 2017;7(3 suppl):212S–220S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fehlings MG, Tetreault LA, Aarabi B, et al. A clinical practice guideline for the management of patients with acute spinal cord injury: recommendations on the type and timing of rehabilitation. Global Spine J. 2017;7(3 suppl):231S–238S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fehlings MG, Tetreault LA, Riew KD, et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Global Spine J. 2017;7(3 suppl):70S–83S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fehlings MG, Tetreault LA, Wilson JR, et al. A clinical practice guideline for the management of patients with acute spinal cord injury and central cord syndrome: recommendations on the timing (≤24 hours versus >24 hours) of decompressive surgery. Global Spine J. 2017;7(3 suppl):195S–202S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fehlings MG, Wilson JR, Tetreault LA, et al. A clinical practice guideline for the management of patients with acute spinal cord injury: recommendations on the use of methylprednisolone sodium succinate. Global Spine J. 2017;7(3 suppl):203S–211S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Canadian Institutes of Health Research. Knowledge translation strategy 2004-20092004. https://www.cihr-irsc.gc.ca/e/26574.html. Accessed February 1, 2019.
- 52. Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26:13–24. [DOI] [PubMed] [Google Scholar]
- 53. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39(8 suppl 2):II46–II54. [DOI] [PubMed] [Google Scholar]
- 54. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. [DOI] [PubMed] [Google Scholar]
- 55. Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and strategies in guideline implementation—a scoping review. Healthcare (Basel). 2016;4:E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Casey DE., Jr Why don’t physicians (and patients) consistently follow clinical practice guidelines? JAMA Intern Med. 2013;173:1581–1583. [DOI] [PubMed] [Google Scholar]
- 57. Kredo T, Bernhardsson S, Machingaidze S, et al. Guide to clinical practice guidelines: the current state of play. Int J Qual Health Care. 2016;28:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]