Key Points
Question
What is the association of tumor (T) category for eyelid squamous cell carcinoma according to the AJCC Cancer Staging Manual, eighth edition (AJCC 8), with local recurrence, nodal metastasis, and disease-specific survival?
Findings
In this cohort study including 109 patients with eyelid and periocular SCC, T category differed between the seventh and eighth editions of the AJCC Cancer Staging Manual in 33 patients (30.3%). Clinical stage of T2c or worse in the AJCC 8 was associated with higher risk of nodal metastasis and worse disease-specific survival, and distant metastasis was associated with nodal metastasis at presentation.
Meaning
These results suggest that tumors of clinical stage T2c or worse are associated with risk of nodal metastasis and worse disease-specific survival.
This cohort study analyzes the association of tumor category in AJCC Cancer Staging Manual, eighth edition, with local recurrence, nodal metastasis, distant metastasis, and disease-specific survival for eyelid and periocular squamous cell carcinoma.
Abstract
Importance
To our knowledge, there are no validation studies to date of the prognostic value of the AJCC Cancer Staging Manual, eighth edition (AJCC 8), criteria for eyelid and periocular squamous cell carcinoma.
Objective
To determine the association of tumor (T) category in AJCC 8 with local recurrence, nodal metastasis, distant metastasis, and disease-specific survival (DSS) for eyelid and periocular squamous cell carcinoma.
Design, Setting, and Participants
In this retrospective, single-center cohort study, 109 consecutive patients with eyelid and periocular squamous cell carcinoma treated from January 1999 to April 2018 were included. Patients with secondary involvement of the periocular region were excluded.
Main Outcomes and Measures
Local recurrence, nodal metastasis, distance metastasis, and DSS.
Results
Of the 109 included patients, 81 (74.3%) were male, and the median (range) age was 66 (40-91) years. At presentation, 43 patients (39.4%) had recurrent tumor, 4 (3.7%) had nodal metastasis, and 1 (0.9%) had distant metastasis. The median (range) follow-up was 23 (1-161) months. During follow-up, 11 patients (10.1%) developed local recurrence, 7 (6.4%) developed nodal metastasis, 2 (1.8%) developed distant metastasis, and 9 (8.3%) died of disease. The 5-year DSS rate was 87.7% (95% CI, 79.5-96.9). Chronic immunosuppression (hazard ratio, 47.24; 95% CI, 7.33-304.30; P < .001) and presentation with recurrent squamous cell carcinoma (hazard ratio, 5.22; 95% CI, 1.12-24.31; P = .04) were associated with local recurrence during follow-up. Of the 11 patients with local recurrence during follow-up, 7 (64%) had perineural invasion. T category was associated with nodal metastasis; clinical stage of T2c or worse at presentation was associated with higher risk of nodal metastasis and death of disease but not with a higher risk of local recurrence. Distant metastasis was associated with nodal metastasis at presentation (hazard ratio, 32.50; 95% CI, 1.97-536.40; P = .02) and during follow-up. A total of 33 patients (30.3%) had different T categories depending on whether disease was staged according to the seventh or eighth edition of the AJCC Cancer Staging Manual. Compared with AJCC 7, AJCC 8 showed a better predictive value in terms of local recurrence (T3, 17% vs 14%; T4, 11% vs 16%) and DSS.
Conclusions and Relevance
These findings suggest that T category in AJCC 8 is associated with nodal metastasis and DSS. Immunosuppression and presentation with recurrent disease are associated with increased risk of future local recurrence. Patients with tumors of clinical stage T2c or worse at presentation are at increased risk of nodal metastasis and worse DSS and should undergo surveillance for nodal metastasis. Future studies, ideally prospective in design, could provide greater confidence in these findings.
Introduction
Eyelid and periocular squamous cell carcinoma (SCC) is the second most common periocular malignancy and accounts for 5% to 10% of all eyelid neoplasms.1,2,3 Reported rates range from 6.8% to 36.9% for local recurrence,4,5,6,7 from 1.3% to 24.0% for regional nodal metastasis,7,8,9,10,11 and from 0.8% to 6.2% for distant metastasis.6,7,12 Perineural invasion (PNI) along cranial nerves can cause intraorbital and intracranial tumor spread1,13 and has been reported in 4.3% to 25.0% of patients.4,5,13
Two published studies4,5 evaluated the predictive value of tumor (T) category in the staging system for eyelid and periocular SCC in the AJCC Cancer Staging Manual, seventh edition (AJCC 7). Nasser et al4 showed that a presentation clinical stage of T2b or worse was associated with a higher risk of regional nodal metastasis. Sun et al5 suggested that worse T category at presentation was associated with local recurrence and that PNI in the surgical specimen was associated with both worse T category and recurrent tumor at presentation.
The staging system for eyelid and periocular SCC in the recently published AJCC Cancer Staging Manual, eighth edition (AJCC 8), includes significant changes in definitions of T and lymph node (N) categories. T category is now based mostly on size of eyelid carcinoma and whether partial-thickness or full-thickness eyelid margin involvement exists. Whereas the T category definitions in AJCC 7 included PNI and subjective terms, such as “not resectable” and “requires enucleation, exenteration,” these criteria were removed from T category definitions in AJCC 8.
To our knowledge, there are no published validation studies to date of AJCC 8 staging criteria for eyelid and periocular SCC. We sought to validate these criteria and to identify risk factors for local recurrence, nodal metastasis, distant metastasis, and disease-specific survival (DSS) in a large cohort of patients with eyelid SCC. We further compared AJCC 8 with AJCC 7 in terms of their prognostic value.
Methods
All consecutive patients diagnosed as having eyelid or periocular SCC treated by the senior author (B.E.) from January 1999 through April 2018 were included. All patients who underwent eye-sparing surgery had surgical excision of the lesion with frozen section control of margins when appropriate (eg, at the eyelid margin), with dedicated en face margins. For larger lesions with deep extension (eg, medial canthal lesions), a wider excision and total surgical resection with frozen section margin control was carried out. Data on age, race/ethnicity, sex, primary tumor size and location, primary vs recurrent SCC at presentation, local recurrence during follow-up, nodal and distant metastasis at presentation and during follow-up, PNI as seen in the surgical specimen (termed histologic PNI), and treatment were collected. Patients with secondary involvement of the periocular region were excluded. The SCCs were staged using both AJCC 8 and AJCC 7. Institutional review board approval was obtained for this retrospective study, and the requirement for informed consent was waived. This work was performed in compliance with the Declaration of Helsinki14 and the Health Insurance Portability and Accountability Act.
Overall survival (OS) and DSS were defined as time from date of definitive surgery to date of death of any cause and to death related to eyelid SCC, respectively. Overall survival was censored at last follow-up date for living patients, and DSS was censored at last follow-up date for living patients or date of death for patients with death unrelated to SCC. The other time-to-event outcomes were defined as time from date of definitive surgery to date of event of interest and were censored at last follow-up date or date of death, whichever occurred first, for patients who did not experience the event. Follow-up time was defined as time from date of definitive surgery to last follow-up date or date of death, whichever occurred first.
Associations of T category and clinical factors with outcomes during follow-up were studied using survival analysis. Survival curves were estimated using the Kaplan-Meier method, and effect sizes of risk factors with confidence intervals and P values were obtained using Cox regression models. When fitting this model was not plausible because of limited sample sizes of subgroups, differences in survival among groups were assessed using 2-sided log-rank tests. Unless otherwise specified, we reported Wald test P values from Cox regression models. Significance was set at a P value less than .05.
Associations of T category and clinical factors with outcomes diagnosed at presentation and by the end of the study were assessed using Fisher exact test. For analyses of outcomes diagnosed by the end of the study, we used the last information available for each patient, regardless of follow-up time. Because of the limited number of events for each outcome, all associations were studied in the univariate setting. Stage distributions according to AJCC 8 and AJCC 7 were compared via a χ2 test of homogeneity. Statistical analyses were conducted in R version 3.4.2 (The R Foundation).
Results
Validation of AJCC 8 Staging System
Patient, Tumor, and Treatment Characteristics
The study included 109 patients, of whom 81 (74.3%) were men and 103 (94.5%) were white. The median (range) age was 66 (40-91) years. Tumor location was the lower eyelid in 35 patients (32.1%), upper eyelid in 17 (15.6%), medial canthus in 14 (12.8%), lateral canthus in 7 (6.4%), brow in 4 (3.7%), and more than 1 anatomic unit in 15 (13.8%); there were 14 patients with lesions with orbital invasion (12.8%) and 3 with lesions with skull base involvement (2.8%). The median (range) follow-up period was 23 (1-161) months.
A total of 66 patients (60.6%) presented with primary SCC and 43 (39.4%) with recurrent SCC. There were 48 patients (44.0%) with history of nonmelanoma skin cancer elsewhere. Eight patients (7.3%) were chronically immunosuppressed at diagnosis, and 51 (46.8%) had history of smoking. A total of 65 patients (59.6%) had tumors on the left side only, 40 (36.7%) had tumors on the right side only (0.62 vs 0.50; 95% CI, 0.52-0.71; P = .02), and 4 (3.7%) had bilateral disease. Forty patients (36.7%) had histologic PNI.
The AJCC 8 TNM designations at presentation were as follows: TisN0M0, 23 patients (21.1%); T1aN0M0, 7 (6.4%); T1bN0M0, 5 (4.6%); T1cN0M0, 1 (0.9%); T2aN0M0, 9 (8.3%); T2bN0M0, 5 (4.6%); T2cN0M0, 7 (6.4%); T3aN0M0, 10 (9.2%); T3bN0M0, 1 (0.9%); T3cN0M0, 2 (1.8%); T3cN1M0, 1 (0.9%); T4aN0M0, 23 (21.1%); T4aN1M0, 3 (2.8%); T4bN0cM0, 1 (0.9%); T4bN0M0, 10 (9.2%); and T4bN0M1, 1 (0.9%). Fifty-nine patients (54.1%) had eye-sparing surgery with frozen section control of margins and immediate reconstruction, and 34 (31.2%) had eye-sparing surgery with frozen section control of margins, immediate reconstruction, and adjuvant radiotherapy (n = 25) or concurrent chemoradiation (n = 9). Thirteen patients (11.9%) had orbital exenteration followed by adjuvant radiotherapy (n = 3) or chemoradiation (n = 10). Thirty-six of 40 patients with PNI (90%) received adjuvant radiotherapy (Table).
Table. Patient, Tumor, and Treatment Characteristics.
| Characteristic | No. (%) |
|---|---|
| Total, No. | 109 |
| Sex | |
| Male | 81 (74.3) |
| Female | 28 (25.7) |
| Race/ethnicity | |
| Asian | 1 (0.9) |
| Hispanic | 5 (4.6) |
| White | 103 (94.5) |
| Age, median (range), y | 66 (40-91) |
| Smoking history | |
| Yes | 51 (46.8) |
| No | 58 (53.2) |
| Sign of ocular area on presentation | |
| Primary | 66 (60.6) |
| Recurrent | 43 (39.4) |
| Nonmelanoma skin cancer elsewhere | |
| Yes | 48 (44.0) |
| No | 61 (56.0) |
| Immunosuppression | |
| Yes | 8 (7.3) |
| No | 101 (92.7) |
| Treatment | |
| Eye-sparing surgery without adjuvant therapy | 59 (54.1) |
| Eye-sparing surgery with radiotherapy | 25 (22.9) |
| Eye-sparing surgery with chemoradiation | 9 (8.3) |
| Exenteration with chemoradiation | 10 (9.2) |
| Exenteration with radiotherapy | 3 (2.8) |
| Eye-sparing surgery, radiotherapy, and anti-PD1 | 1 (0.9) |
| Eye-sparing surgery, chemoradiation, and anti-PD1 | 2 (1.8) |
| FNA | |
| Yes | 30 (27.5) |
| No | 79 (72.5) |
| FNA result, No./total No. (%) | |
| Positive | 11/30 (37) |
| Negative | 19/30 (63) |
| Perineural invasion | |
| Positive | 40 (36.7) |
| Negative | 69 (63.3) |
| Laterality | |
| Bilateral | 4 (3.7) |
| Left | 65 (59.6) |
| Right | 40 (36.7) |
| N category at presentation | |
| N0 | 105 (96.3) |
| N1 | 4 (3.7) |
| M category at presentation | |
| M0 | 108 (99.1) |
| M1 | 1 (0.9) |
Abbreviations: FNA, fine-needle aspiration; M, metastasis; N, lymph node; PD1, programmed cell death protein 1.
Local Recurrence
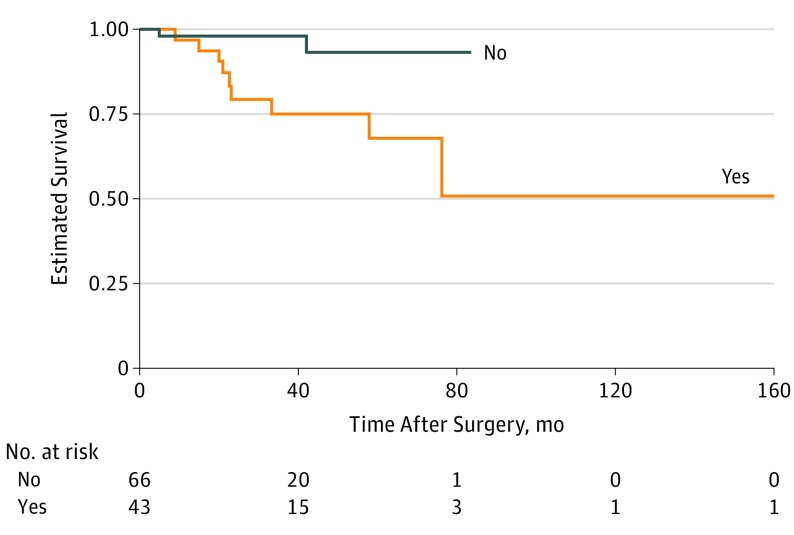
Forty-three patients (39.4%) presented with recurrent eyelid or periocular SCC, and 11 patients (10.1%) developed local recurrence during follow-up, with a median (range) time to observed local recurrence of 22.5 (4.7-76.0) months. Time to local recurrence was longer in patients with primary SCC than in those with recurrent SCC at presentation (hazard ratio [HR], 0.19; 95% CI, 0.04-0.89; P = .04) (Figure 1).
Figure 1. Kaplan-Meier Survival Curve for Local Recurrence of Periocular Squamous Cell Carcinoma by Primary vs Recurrent Tumor at Presentation.
Tumor category was determined by AJCC Cancer Staging Manual, eighth edition.
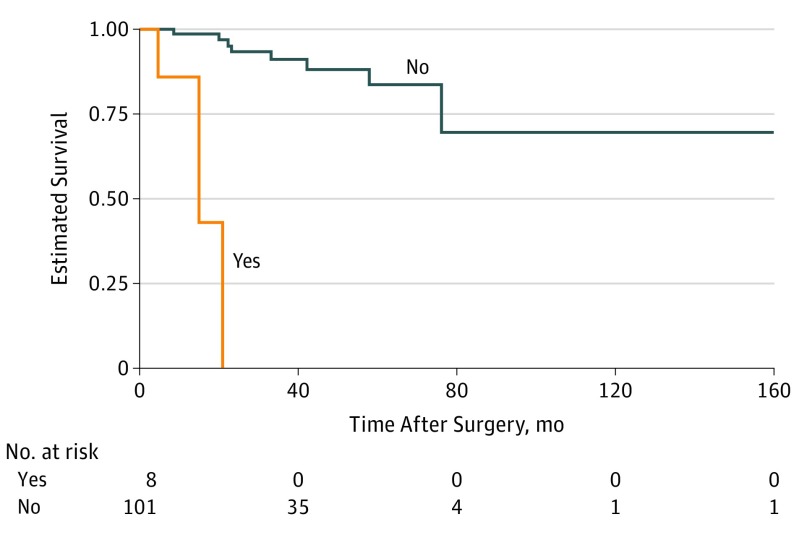
Patients with chronic immunosuppression had a significantly higher risk of local recurrence (HR, 47.24; 95% CI, 7.33-304.30; P < .001) (Figure 2). Seven of 11 patients (64%) with local recurrence during follow-up had histologic PNI. T category was not significantly associated with risk of local recurrence (HR, 2.78; 95% CI, 0.81-9.54; P = .10). Time to local recurrence for patients with T2c, T3a, and T3b or more advanced tumors was similar to time to local recurrence for patients with tumors of lower T category.
Figure 2. Kaplan-Meier Survival Curve for Local Recurrence of Periocular Squamous Cell Carcinoma by Immune Status.
Tumor category was determined by AJCC Cancer Staging Manual, eighth edition.
Nodal Metastasis
Four patients (3.7%) had nodal metastasis at presentation, and 7 patients (6.4%) developed nodal metastasis during follow-up, with a median (range) time to observation of nodal metastasis of 5.2 (0.7-57.9) months. Clinical stage of T2c or worse at presentation was associated with a higher risk of nodal metastasis.
Nodal metastasis observed at any time (from presentation to the end of follow-up) was significantly associated with T category at presentation. Nodal metastasis observed at any time was more common in patients with T2c, T3a, and T3b or more advanced tumors than in patients with tumors of lower T category. Nodal metastasis at any time was not significantly associated with recurrent disease at presentation (odds ratio, 1.97; 95% CI, 0.46-8.76; P = .34) or with PNI (odds ratio, 2.24; 95% CI, 0.53-10.02; P = .21).
Distant Metastasis
One patient presented with T4bN0M1 disease with lung metastasis that had been treated with cisplatin elsewhere; this patient died of other causes 17 months after eye-sparing surgery with frozen section control of margins and immediate reconstruction. Two additional patients, one with T4aN1M0 disease and one with T4bN0M0 disease at presentation, developed lung metastasis during follow-up. Both patients were treated with orbital exenteration and adjuvant concurrent chemoradiation. They developed lung metastasis 27 and 33 months, respectively, after exenteration and were treated with carboplatin plus docetaxel. Their metastases partially responded to chemotherapy, and both patients were alive with disease at last follow-up 86 months and 37 months, respectively, after exenteration.
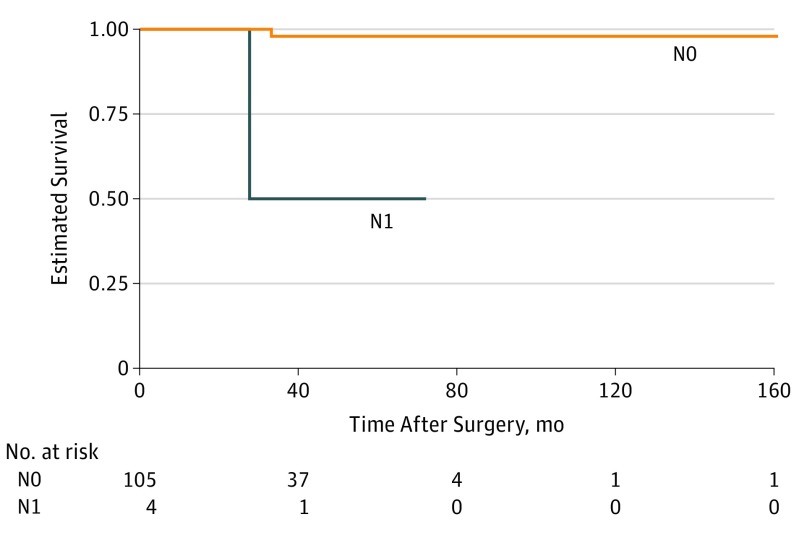
Nodal metastasis at presentation (HR, 32.50; 95% CI, 1.97-536.40; P = .02) and nodal metastasis during follow-up were associated with increased risk of distant metastasis. Time to distant metastasis was shorter for patients with nodal metastasis than for those without (Figure 3).
Figure 3. Kaplan-Meier Survival Curve for Distant Metastasis in Patients With Periocular Squamous Cell Carcinoma by Presence of Nodal Metastasis at Presentation.
Tumor category was determined by AJCC Cancer Staging Manual, eighth edition.
OS and DSS
Twenty-four patients (22.0%) died by last follow-up. The 2-year OS rate was 83.9% (95% CI, 76.4-92.1), and the 5-year OS rate was 67.4% (95% CI, 56.1-80.9). Overall survival did not differ by T category. No significant differences in OS were found between patients with T2c, T3a, and T3b or more advanced tumors and patients with tumors of lower T category.
Of 24 patients (22.0%) who died, 9 died of disease, including 4 who died of T4aN0M0 disease and 5 of T4bN0M0 disease. For patients with T4 disease, the 2-year DSS rate was 92.6% (95% CI, 87.0-98.6), and the 5-year DSS rate was 87.7% (95% CI, 79.5-96.9). Disease-specific survival was significantly worse in patients with T2c, T3a, and T3b or more advanced tumors than in patients with tumors of lower T category. As expected, T4 disease was also associated with worse DSS. Nodal metastasis at presentation or during follow-up was not associated with worse DSS (HR, 3.99; 95% CI, 0.97-16.44; P = .06) (eFigure 1 in the Supplement).
Comparison of AJCC 8 With AJCC 7 Classifications
Patients were stratified into 16 different TNM groups at presentation by AJCC 8 and 13 different TNM groups by AJCC 7. This suggests that patients were more homogeneous within categories of AJCC 8. When patients were grouped into categories by T category (eTable in the Supplement), the distributions differed between patients whose disease was staged per AJCC 8 vs AJCC 7.
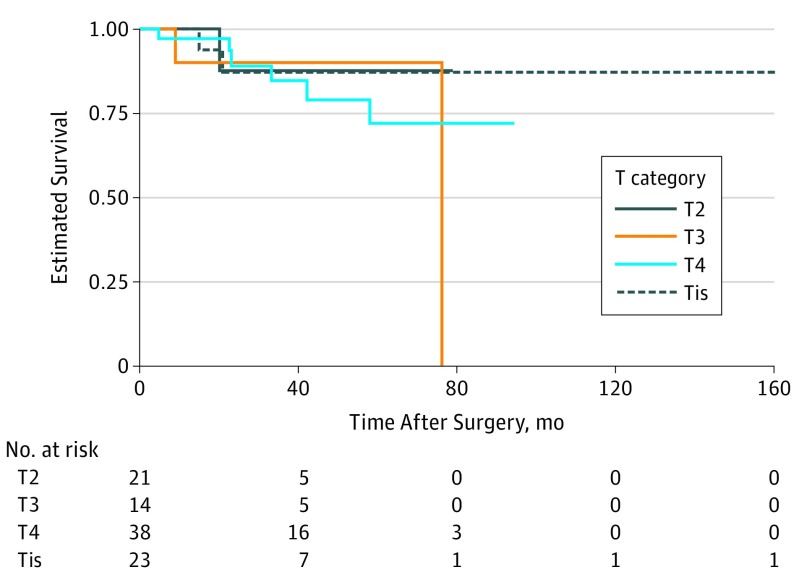
For 33 patients (30.3%), T category differed between AJCC 8 and AJCC 7 (0.30 vs 0; 95% CI, 0.22-0.40; P < .001). T category classifications according to the staging systems in AJCC 8 and AJCC 7 for the entire cohort were significantly different. Twenty patients with T3 disease per AJCC 7 had T4 disease per AJCC 8. Local recurrence–free survival seemed better for patients with T4 than for those with T3 tumors (Figure 4), and the proportion of patients with local recurrence was higher among those with T3 tumors (T3, 17% [7 of 41]; T4, 11% [2 of 18]; P = .71). According to AJCC 8, the proportion of local recurrence comparing T3 vs T4 was similar (T3, 14% [2 of 14]; T4, 16% [6 of 38]; P > .99) (eFigure 2 in the Supplement).
Figure 4. Kaplan-Meier Curve by Tumor (T) Category for Local Recurrence.
T category was determined by AJCC Cancer Staging Manual, eighth edition.
Six patients with histologic PNI, which is classified as T3a disease in AJCC 7, had T2a or T2b disease when disease was classified by AJCC 8, in which PNI is not used to determine T category. Of these 6 patients, 1 had local recurrence during follow-up; none had nodal metastasis at baseline or during follow-up.
Discussion
In this study, we found that patients with eyelid or periocular SCC who presented with recurrent disease or chronic immunosuppression were at increased risk of local recurrence following definitive treatment. Clinical stage of T2c or worse was associated with an increased risk of nodal metastasis and worse DSS. Patients who presented with nodal metastasis and those who developed nodal metastasis during follow-up had a higher risk of distant metastasis. Other findings included the fact that T category by AJCC 8 and AJCC 7 differed for a proportion of patients (30.3%). AJCC 8 had more precise tumor description than AJCC 7, and patients were more homogeneous within TNM categories by AJCC 8.
Several previous studies have, like our study, suggested an increased risk of recurrence of eyelid SCC in patients with chronic immunosuppression.15,16,17 However, Sun et al5 found that immunosuppression was not associated with increased risk of SCC recurrence. Immunosuppression has been shown to increase the risk of SCC as much as 65-fold, but it increases the risk of basal cell carcinoma by only 10-fold.18 Expression of specific human leukocyte antigen class I proteins on SCC cells may explain the influence of loss of immunosurveillance on SCC pathogenesis.19 Our finding that time to future local recurrence was shorter in patients who presented with recurrent disease agrees with the finding by Sun et al5 that recurrent tumor at presentation was associated with a 4-fold increased risk of further recurrence.
We found histologic PNI in 40 patients (36.7%), of whom 36 (90%) received adjuvant radiotherapy, and there was no association of histologic PNI with higher risk of local recurrence. In contrast, Sun et al5 reported histologic PNI in only 8% of patients and reported that PNI was associated with higher T category at presentation, which is not surprising, as they used AJCC 7 and PNI automatically upstages tumors to T3a. These authors did not report whether patients with PNI received radiotherapy. A 2017 systematic review20 of 12 reports of patients with cutaneous SCC in all anatomic sites included 241 patients with clinical symptoms or radiologic evidence of PNI and 381 with PNI found incidentally in the surgical specimen. The authors found that the risk of local recurrence was 37% in patients with clinical symptoms or radiologic evidence of PNI and 17% in patients with PNI detected incidentally. The authors concluded that both types of PNI may warrant closer surveillance for local recurrence.20
The overall nodal metastasis rate in our cohort, 10%, is similar to the rate in our previous report of a smaller cohort,4 in which nodal metastasis was found in 9% of the 65 patients at a median follow-up time of 27 months. Sun et al5 found that only 2 of 254 patients with eyelid SCC had nodal metastasis at a median follow-up of 40 months. One possible reason for this discrepancy is that all patients in our study had uniform workup for nodal metastasis at presentation, which included ultrasonography, computed tomography, and palpation, and had these tests repeated during follow-up, whereas only 2.0% of the patients in the study by Sun et al5 underwent a workup for nodal metastasis (computed tomography and ultrasonography). Because of our more extensive workup, we believe that the nodal metastasis rate in our study may more reliably reflect the true prevalence of nodal metastasis. Our finding that T category per AJCC 8 was associated with nodal metastasis is in keeping with the findings based on AJCC 7.4
Whereas the overall distant metastasis rate in our series was 3%, in a series of 50 patients with eyelid SCC, Donaldson et al12 reported no cases of distant metastasis at a mean follow-up of 31.1 months. However, no patients in that series had nodal metastasis.
Sun et al5 reported that only 4 (1.6%) of 254 patients died of disease, but their series did not include as many patients with T4 disease as ours did. All 9 patients in our cohort who died of disease had T4 disease, and DSS was significantly worse in patients with tumors of clinical stage T2c or worse. The 2-year and 5-year DSS rates in our series, 92.6% and 87.7%, respectively, are similar to rates previously reported by Nasser et al4 (2-year DSS rate, 90%) and by Petsuksiri et al21 (5-year DSS rate, 86%).
Our finding that 30.3% of patients had different T categories depending on whether disease was staged according to AJCC 8 or AJCC 7 is in keeping with a previous report from our center on all eyelid carcinomas, which reported a 75% rate of difference in T category between the 2 classifications.22 Our finding of differences in the distributions of T categories assigned using AJCC 8 or AJCC 7 classifications was not surprising given that PNI implies T3 disease in AJCC 7 but is not a determinant of T category in AJCC 8.
Application of AJCC 8 staging criteria resulted in better prediction of DSS. Twenty patients with disease classified as T3 per AJCC 7 had disease classified as T4 per AJCC 8. Seven patients were downstaged from T3a to T2, 6 of whom were positive for histologic PNI. With AJCC 8, T4 tumors were associated with a higher risk of local recurrence than T3 tumors; with AJCC 7, the opposite pattern was observed. All patients who died of disease had T4 disease per AJCC 8. Per AJCC 7, only 6 had T4 disease; the rest had T3 disease.
There was a predominance of left-sided over right-sided lesions. Two previous studies from Australia found a preponderance of right-sided lesions, which was explained as a result of increased sun exposure during driving.12,23
Limitations
Our study had limitations. The study was retrospective, and the various univariate factors could be associated with one another. Because of the relatively small number of events in each category, conducting a multivariate analysis was not possible.
Conclusions
To our knowledge, the current cohort is one of the largest reported cohorts of patients with periocular SCC treated at a single center in the United States, with reliable and fairly standard staging work-up and documentation of nodal and distant metastasis and death of disease done by the same practitioner. Our findings indicate that immunosuppression and recurrent tumor at presentation are associated with an increased risk of local recurrence after surgery. Clinical stage of T2c or worse is associated with higher risk of nodal metastasis and higher risk of death of disease, and nodal metastasis at presentation or during follow-up is associated with higher risk of distant metastasis. In our cohort, T category distribution per AJCC 7 differed significantly from T category distribution per AJCC 8. AJCC 8 allows for a more precise designation of T category and a more homogenous distribution of eyelid SCCs across the T categories.
eTable. Baseline staging (AJCC 7 and AJCC 8) by tumor size.
eFigure 1. Kaplan-Meier survival curve for disease-specific survival of patients with periocular squamous cell carcinoma by nodal metastasis status at last follow-up (n = 109).
eFigure 2. Kaplan-Meier curve by T category for local recurrence (AJCC 7 definition).
References
- 1.Limawararut V, Leibovitch I, Sullivan T, Selva D. Periocular squamous cell carcinoma. Clin Exp Ophthalmol. 2007;35(2):174-185. doi: 10.1111/j.1442-9071.2006.01411.x [DOI] [PubMed] [Google Scholar]
- 2.Deprez M, Uffer S. Clinicopathological features of eyelid skin tumors: a retrospective study of 5504 cases and review of literature. Am J Dermatopathol. 2009;31(3):256-262. [DOI] [PubMed] [Google Scholar]
- 3.Wawrzynski J, Tudge I, Fitzgerald E, et al. . Report on the incidence of squamous cell carcinomas affecting the eyelids in England over a 15-year period (2000-2014). Br J Ophthalmol. 2018;102(10):1358-1361. doi: 10.1136/bjophthalmol-2017-310956 [DOI] [PubMed] [Google Scholar]
- 4.Nasser QJ, Roth KG, Warneke CL, Yin VT, El Sawy T, Esmaeli B. Impact of AJCC ‘T’ designation on risk of regional lymph node metastasis in patients with squamous carcinoma of the eyelid. Br J Ophthalmol. 2014;98(4):498-501. doi: 10.1136/bjophthalmol-2013-304434 [DOI] [PubMed] [Google Scholar]
- 5.Sun MT, Andrew NH, O’Donnell B, McNab A, Huilgol SC, Selva D. Periocular squamous cell carcinoma: TNM staging and recurrence. Ophthalmology. 2015;122(7):1512-1516. doi: 10.1016/j.ophtha.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 6.Ford J, Thakar S, Thuro B, Esmaeli B. Prognostic value of the staging system for eyelid tumors in the 7th edition of the American Joint Committee on Cancer Staging Manual. Ophthalmic Plast Reconstr Surg. 2017;33(5):317-324. doi: 10.1097/IOP.0000000000000901 [DOI] [PubMed] [Google Scholar]
- 7.Faustina M, Diba R, Ahmadi MA, Esmaeli B. Patterns of regional and distant metastasis in patients with eyelid and periocular squamous cell carcinoma [published correction appears in Ophthalmology. 2005;112(3):446]. Ophthalmology. 2004;111(10):1930-1932. doi: 10.1016/j.ophtha.2004.02.009 [DOI] [PubMed] [Google Scholar]
- 8.Sullivan TJ. Squamous cell carcinoma of eyelid, periocular, and periorbital skin. Int Ophthalmol Clin. 2009;49(4):17-24. doi: 10.1097/IIO.0b013e3181b7ecd1 [DOI] [PubMed] [Google Scholar]
- 9.Cook BE Jr, Bartley GB. Treatment options and future prospects for the management of eyelid malignancies: an evidence-based update. Ophthalmology. 2001;108(11):2088-2098, 2099-2100, 2121. doi: 10.1016/S0161-6420(01)00796-5 [DOI] [PubMed] [Google Scholar]
- 10.McCord CD Jr, Cavanagh HD. Microscopic features and biologic behavior of eyelid tumors. Ophthalmic Surg. 1980;11(10):671-681. [PubMed] [Google Scholar]
- 11.Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976-990. doi: 10.1016/0190-9622(92)70144-5 [DOI] [PubMed] [Google Scholar]
- 12.Donaldson MJ, Sullivan TJ, Whitehead KJ, Williamson RM. Squamous cell carcinoma of the eyelids. Br J Ophthalmol. 2002;86(10):1161-1165. doi: 10.1136/bjo.86.10.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malhotra R, Huilgol SC, Huynh NT, Selva D. The Australian Mohs database: periocular squamous cell carcinoma. Ophthalmology. 2004;111(4):617-623. doi: 10.1016/j.ophtha.2003.07.020 [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 15.Czarnecki D, Mar A, Staples M, Giles G, Meehan C. The development of non-melanocytic skin cancers in people with a history of skin cancer. Dermatology. 1994;189(4):364-367. doi: 10.1159/000246880 [DOI] [PubMed] [Google Scholar]
- 16.Petter G, Haustein UF. Histologic subtyping and malignancy assessment of cutaneous squamous cell carcinoma. Dermatol Surg. 2000;26(6):521-530. doi: 10.1046/j.1524-4725.2000.99181.x [DOI] [PubMed] [Google Scholar]
- 17.Tingle AJ, Pot KH, Yong FP, Puterman ML, Hancock EJ. Kinetics of isotype-specific humoral immunity in rubella vaccine-associated arthropathy. Clin Immunol Immunopathol. 1989;53(2, pt 2):S99-S106. doi: 10.1016/0090-1229(89)90075-5 [DOI] [PubMed] [Google Scholar]
- 18.Didona D, Paolino G, Bottoni U, Cantisani C. Non melanoma skin cancer pathogenesis overview. Biomedicines. 2018;6(1):E6. doi: 10.3390/biomedicines6010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter A, Barysch MJ, Behnke S, et al. . Cancer-testis antigens and immunosurveillance in human cutaneous squamous cell and basal cell carcinomas. Clin Cancer Res. 2010;16(14):3562-3570. doi: 10.1158/1078-0432.CCR-09-3136 [DOI] [PubMed] [Google Scholar]
- 20.Karia PS, Morgan FC, Ruiz ES, Schmults CD. Clinical and incidental perineural invasion of cutaneous squamous cell carcinoma: a systematic review and pooled analysis of outcomes data. JAMA Dermatol. 2017;153(8):781-788. doi: 10.1001/jamadermatol.2017.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petsuksiri J, Frank SJ, Garden AS, et al. . Outcomes after radiotherapy for squamous cell carcinoma of the eyelid. Cancer. 2008;112(1):111-118. doi: 10.1002/cncr.23143 [DOI] [PubMed] [Google Scholar]
- 22.Ding S, Sagiv O, Guo Y, Kandl TJ, Thakar SD, Esmaeli B. Change in eyelid carcinoma T category with use of the 8th versus 7th edition of the American Joint Committee on Cancer: Cancer Staging Manual. Ophthalmic Plast Reconstr Surg. 2019;35(1):38-41. [DOI] [PubMed] [Google Scholar]
- 23.Wong VA, Marshall JA, Whitehead KJ, Williamson RM, Sullivan TJ. Management of periocular basal cell carcinoma with modified en face frozen section controlled excision. Ophthalmic Plast Reconstr Surg. 2002;18(6):430-435. doi: 10.1097/00002341-200211000-00008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Baseline staging (AJCC 7 and AJCC 8) by tumor size.
eFigure 1. Kaplan-Meier survival curve for disease-specific survival of patients with periocular squamous cell carcinoma by nodal metastasis status at last follow-up (n = 109).
eFigure 2. Kaplan-Meier curve by T category for local recurrence (AJCC 7 definition).