Abstract
In recent times, researchers have explored food derived peptides to circumvent the side effects of synthetic drugs. This study therefore examined the amino acid constituents, in vitro antioxidant activities, angiotensin-1-converting enzyme (ACE), α-glucosidase and α-amylase inhibition kinetics of protein hydrolysate obtained from the seed of Luffa cylindrica. The peptide yield by pepsin (16.93 ± 0.28%) and trypsin (13.20 ± 1.02%) were significantly lower than that of Alcalase (34.04 ± 1.96%). Alcalase hydrolysate however displayed the highest ferric reducing antioxidant capacity (FRAC), 1,1-diphenyl-2-picrylhydrazyl (DPPH) and H2O2 scavenging activities (0.63%, 85.88% and 41.69% respectively), while the highest superoxide scavenging activity was shown by peptic hydrolysate (57.89%). The ACE inhibition by the hydrolysates with IC50 of 0.32–0.93 mg/mL, increased as the concentration of the peptic hydrolysate increased with the highest ACE-inhibitory activity (74.99 ± 0.43%) at 1.2 mg/mL of peptic hydrolysate. Tryptic and Alcalase hydrloysates exhibited a strong α-amylase inhibition having 27.96 ± 0.06% and 36.36 ± 0.71% inhibitory capacity respectively with IC50 of 1.02–3.31 mg/mL. Alcalase hydrolysates demonstrated the strongest inhibition (65.81 ± 1.95%), followed by tryptic hydrolysates (54.53 ± 0.52%) in a concentration-dependent inhibition of α-glucosidase (IC50, 0.48–0.80 mg/mL). Kinetic analysis showed that ACE-inhibition by different concentrations of Alcalase, pepsin and trypsin hydrolysates is uncompetitive, mixed-type and non-competitive respectively. α-Amylase was non-competitively inhibited while α-glucosidase was un-competitively inhibited by all the hydrolysates. The total amino acid concentration for Alcalase, trypsin and pepsin hydrolysates was 53.51g/100g, 75.40g/100g and 85.42g/100g of Luffa cylindrica seed protein hydrolysate respectively, with glutamate being the most concentrated essential amino acid in all the three hydrolysates. From these results, it can be deduced that Luffa cylindrica seed Alcalase and tryptic protein hydrolysates may play critical and indispensible role as bio-tools in diabetes and hypertension treatment.
Keyword: Biochemistry
1. Introduction
Amino acids present in food bonded to one another in the primary structure of protein remain inactive; enzymatic hydrolysis can break down this primary structure of protein releasing peptide sequence that can be used as therapeutic agents (Arise et al., 2016a). Of particular importance are those peptides with multifunctional bioactivities. Lifestyle related conditions like hypertension and diabetes have become a major public health issue in developed as well as developing countries, and these conditions are complicated by oxidative stress (Aluko, 2015). Hypertension, a condition characterized by abnormally high blood pressure has affected about 1 billion people and the number is still increasing (WHO, 2013). Renin and ACE are proteins which are enzymatic in nature that play critical roles in the renin-angiotensin system (RAS) which subsequently affects blood pressure (Onuh et al., 2013). Vasoconstrictor angiotensin II is a potent octapeptide that is biosynthesized via renin action on RAS. Bradykinin, a peptide that mediates inflammations and vasodilation is usually degraded by the enzymatic action of ACE. Therefore, the inhibition of ACE is often considered to play critical role in the therapeutic management of hypertension (Arise et al., 2016a). Therapeutic adverse effects that usually arise from ACE inhibitors produced synthetically include parageusia, reflex cough and skin rashes, thus making them undesirable and necessitating the need for more tolerable ACE inhibitors from natural sources (Lahogue et al., 2010; Ghassem et al., 2014). Peptides derived from food sources have shown promising evidence to serve as ACE inhibitors posing no danger of any kind (Girgih et al., 2011).
Diabetes mellitus (DM) is a metabolic disorder which manifest clinically as hyperglycemia (elevated blood glucose concentration). Treatment approach that maintains normal level of glucose in the blood is most effective for DM (Yu et al., 2012). Salivary and pancreatic α-amylases are important glycoside cleaving enzymes that are involved in the digestion of carbohydrate in the mouth to short oligosaccharide, and disaccharide units in the stomach. α-glucosidase in the intestine then mediates the catalytic breakdown of the disaccharides into monosaccharides (glucose) before its assimilation into the blood; which are constituents of mainly plasma and formed elements; blood cells and platelets (Oboh and Aluyor, 2009). Synthetic α-amylase and α-glucosidase inhibitors in the form of commercially available drugs would have been perfect for the management of DM if not for the associated abdominal pain, flatulence, diarrhoea and other side effects with its use. This has led to enormous search for therapeutic alternatives with little or no side effect (Adisakwattana et al., 2012).
Luffa cylindrica often referred to as sponge gourd plant belongs to the Curcubitaceae family (Du et al., 2006). It is a vigorous climbing annual vine with several loped cucumber-like leaves. The fruits with a cucumber-like shape develop at maturity, a network of fibres surrounding numerous flat blackish seeds. Originating from India, it grows ubiquitously as weed in most parts of Nigeria where it is majorly used domestically as sponge. Ogunbanjo et al. (2016) asserted that its seed contain about 23% protein, a substantial quantity for bioactive peptide production. Hence, this study investigated the in vitro potentials of L. cylindrical seed protein hydrolysates as ACE, α-amylase and α-glucosidase inhibitors, as well as kinetics of ACE inhibition, antioxidant and amino acid profiles of the hydrolysates.
2. Materials and methods
2.1. Materials
Luffa cylindrica seeds were obtained from Oja Oba Market, Ilorin, Nigeria and were validated at the Department of Plant Biology Herbarium, University of Ilorin with a voucher number UILH/001/523. The air-dried seeds were peeled and pulverized. Analytically graded chemicals and reagents were used without further purification. Hydrolytic enzymes; trypsin, Alcalase, and pepsin which were obtained from bovine pancreas, Bacillus licheniformis, and porcine gastric mucosa respectively, were purchased from Sigma-Aldrich (USA). Angiotensin-1 converting enzyme, (ACE) from rabbit lung (E.C.3.4.15.1), ACE substrate N-[3-(2-furyl)acryloyl]-L-phenylalanyl-glycyl-glycine (FAPGG), DPPH (1,1-diphenyl-2-picrylhydrazyl), α-glucosidase, p-nitrophenyl-α-D-glucopyranoside, acarbose, p-nitrophenol, α-amylase, maltose and dinitrosalicylic acid were obtained from Sigma-Aldrich (USA). While bovine serum albumin (BSA), starch, ethylenediaminetetraacetic acid (EDTA) and trichloroacetic acid (TCA) were obtained from BDH Chemical Limited (Poole, England).
2.2. Methods
2.2.1. Isolation of Luffa cylindrica seed protein
The defatted Luffa cylindrica seed powder was produced as outlined by Arise et al. (2016b). The seeds were pulverized. Thereafter, flake to solvent ratio of 1:10 (w/v) extraction was carried out using n-hexane solvent to soften the flakes. In addition to these, a continuous stirring using magnetic stirrer was done concomitantly for 1 hour. Plastic containers were used to store the defatted flakes and refrigerated (-10 °C). With slight modifications, the protein constituents of the defatted meal were extracted as reported by Arise et al. (2016b). Fat-free Luffa cylindrica powder got submerged (1:10) in 0.1 M NaOH of pH 12.0 and mixed using a magnetic stirrer for 1hour and subsequently spinned using a centrifuge at 18 °C and 3000 rpm for 10 min. Pooling of supernatants in each case was also carried out following two subsequent extractions. Precipitate was obtained via centrifugation of the supernatant which was previously adjusted to pH 4.0 using 1M HCl. In addition to these, the precipitate was further rinsed with distilled water. 1 M NaOH was subsequently added drop wisely to bring the pH to 7.0. The lyophilized Luffa cylindrica seed protein isolate (LSPI) was stored via refrigeration until required for analysis in relation to this study. The protocol reported by Arise et al. (2015) was used in the determination of protein yield.
2.2.2. Peptide yield determination
The protocol outlined by Arise et al. (2016a,b) was used to determine the percentage peptide yield which is expressed as-
2.2.3. Preparation of Luffa cylindrica seed protein hydrolysates (LSPH)
Hydrolysis of protein isolate by pepsin, Alcalase, and trypsin was carried out at pH 2.2, 8.0, 8.0 and temperatures 37 °C, 37 °C and 60 °C respectively via the methods outlined by Arise et al. (2016a) with minimal changes. 5% w/v of Luffa cylindrica protein isolate; trypsin or Alcalase was submerged in phosphate buffer at pH 8, while pepsin was dissolved in glycine buffer at pH 2.2. In addition to these, the organic catalyst was introduced at a substrate to enzyme ratio of 100:1. Hydrolysis lasted for 5 hrs using reaction vessel and subsequently submerged in hot water at 100 °C for 900 seconds. The solution was made up to assume pH 4.0, and then spun for 30min to obtain the supernatant at 4000 rpm. This was subsequently analyzed to determine the degree of hydrolysis as outlined by Arise et al. (2016b). In addition to these, the outlined method of Girgih et al. (2011) was used in the determination of percentage peptide yield.
2.2.4. Determination of amino acid profile
The procedure of Benitez (1989) was adopted to determine the amino acid profile in the three hydrolysates. Samples were dried to constant weight, defatted, hydrolyzed, evaporated with a rotary evaporator and loaded into the Applied Biosystems PTH Amino Acid Analyzer.
2.2.5. Evaluation of LSPH inhibition of ACE
LSPH inhibitory property of ACE was evaluated by the outlined protocol of Holmquist et al. (1979) employing N-[3-(2-furyl)acryloyl]-Lphenylalanyl-glycyl-glycine (FAPGG) as substrate.
2.2.6. Kinetic parameters of ACE inhibition determination
Girgih et al. (2011) method with little modification was adopted for kinetic parameters of ACE inhibition determination.
2.2.7. Evaluation of LSPH inhibition of α-amylase
Protocol outlined by Bernfeld (1951) and reported by Oboh and Aluyor (2009) and Arise et al. (2016b) was used for α-amylase inhibition determination.
2.2.8. Kinetic analysis of α-amylase inhibition
The method outlined by Arise et al. (2016b) was used for the kinetic analysis.
2.2.9. Determination of α-glucosidase inhibition
Apostolidis et al. (2006) outlined protocol was used in the determination of α-glucosidase inhibition.
2.2.10. Kinetic analysis of α-glucosidase inhibition
Inhibition of α-glucosidase by LSPH was determined by increasing PNPG concentration following a modified protocol outlined by Gurudeeban et al. (2012).
2.2.11. DPPH free radical scavenging activity determination
LSPH DPPH radical-scavenging activity was determined by the protocol outlined by Arise et al. (2016a).
2.2.12. Ferric reducing antioxidant power determination
The reductive potential of LSPH on iron (III) was assessed according to the protocol laid out by Chen et al. (2009).
2.2.13. Superoxide radical-scavenging activity assessment
The assessment of superoxide scavenging activity was done using the outlined procedure of Pownall et al. (2010).
2.2.14. Hydrogen peroxide scavenging capacity determination
LSPH scavenging activity of hydrogen peroxide was assessed based on the protocol of Ruch et al. (1989) as outlined by Arise et al. (2016a).
2.2.15. Statistical analysis
Values were expressed in three determinations as mean ± standard deviation (SD) followed by analysis of variance and Tukey's multiple range tests using GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA). Differences were considered significant at p < 0.05.
3. Results and discussion
3.1. Proteins isolate yield and degree of hydrolysis
The percentage peptide yield shows a measure of the protein that is obtainable in a protein extraction procedure. 39.53% protein yield (Table 1) was obtained in this study which is higher than 18.91% obtained from Citrullus lanatus seed (Arise et al., 2016b). This may be because Luffa cylindrica seed contains less salt soluble protein fraction (Singh and Matta, 2010). The percentage yield of peptide gotten from this study is lower than the protein yield reported by Arise et al. (2015) of 52% protein yield from South African bambara groundnut landraces. This might be attributed to the extraction method employed since the degree of protein yield depends on the extraction protocol used (Boye et al., 2010).
Table 1.
Protein isolate yield, degree of hydrolysis, and peptide yield
| Parameter/Enzyme | Degree of Hydrolysis (%) | Peptide Yield (%) | |
|---|---|---|---|
| Protein Yield of Isolate (%) | 39.53 | — | — |
| Pepsin | — | 48.57 ± 0.68a | 16.93 ± 0.28a |
| Trypsin | — | 56.20 ± 0.59b | 34.04 ± 1.96c |
| Alcalase | — | 38.40 ± 0.42c | 13.20 ± 1.02b |
Values depict the mean of three replicate determinations ±standard deviation (SD). Values showing different letters are significantly different at p < 0.05.
To establish the level of peptide bonds cleavage, the proteolysis monitoring parameter known as degree of hydrolysis (DH) is often used (Jrad et al., 2014). The higher DH (p < 0.05) by tryptic digestion compared to that of Alcalase shows that trypsin is most effective in hydrolyzing Luffa cylindrica seed protein. Peptic digestion also proved effective in the hydrolysis more than Alcalase but less than tryptic digestion. Therefore, the higher values of tryptic and peptic digestion show that there could be large number of positively charged amino acid residues for which trypsin is specific, as well as acidic residues to which pepsin is highly specific (Naik, 2012).
Malomo et al. (2015) asserted that DH values could be used to predict peptide chain length as higher DH values specify shorter length peptides while lower values specify longer length peptides. This therefore suggests that Alcalase which has the least DH value (38.40%) may contain longer length of peptides whereas peptides obtained by trypsin and pepsin may contain shorter length of bioactive peptides. Peptides produced by Alcalase possess various biological activities e.g. antioxidant activity. Alcalase have been reported to provide higher yields of antioxidative peptides that are unhydrolyzable by digestive enzymes (Kim et al., 2001).
The decreased peptide yield for Alcalase (13.20%) when compared to that of pepsin and trypsin (16.93% and 34.04% respectively) suggests more bioactive peptides may have been produced by trypsin and pepsin than Alcalase during the enzymatic hydrolysis. The higher value for trypsin could be due to its ability to hydrolyze esters and amides of amino acids as well as its specificity for hydrophobic amino acids (Naik, 2012). The 16.93% peptide yield obtained from the peptic digestion of LSPH was significantly lower to that reported for hemp seed peptic hydrolysis by Girgih et al. (2011) with a yield of 65.70%.
3.1.1. Profile of amino acid content in Alcalase hydrolysate
The amino acid composition of LSPH as determined by the PTH amino acid analyzer is summarized in Table 2. The seed protein hydrolysate consisted of 18 amino acids of which 10 are essential amino acids. From the study, the total amino acid concentration for Alcalase hydrolysate was 53.51g/100g of LSPH and the total essential amino acid with histidine concentration was 23.19g/100g of LSPH. This pattern of result correlates with FAO/WHO (1991) requirements of 23.20g/100g for essential amino acids with histidine. Leucine (5.36g/100g) was the most concentrated essential amino acid in contrast to that reported by Aremu et al. (2008). Zhang et al. (2007) posited that the increase in dietary leucine is capable of ameliorating diet-induced obesity, high blood sugar and hypercholesterolemia in human subjects and rodents via multiple mechanisms. The most concentrated non-essential amino acid in Luffa cylindrica seed protein hydrolysate was glutamic acid (8.33g/100g), followed by aspartic acid (5.21g/100g). Rabab (2017) made a similar observation.
Table 2.
Profile of amino acid content of the hydrolysates.
| Hydrolysates | |||
|---|---|---|---|
| Amino Acids | Alcalase (g/100g of LSPH) | Trypsin (g/100g of LSPH) | Pepsin (g/100g of LSPH) |
| Leucine | 5.36 | 7.11 | 8.97 |
| Lysine | 3.44 | 4.66 | 6.07 |
| Isoleucine | 2.62 | 3.20 | 4.01 |
| Phenylalanine | 3.02 | 3.99 | 4.54 |
| Norleucine | - | - | - |
| Tryptophan | 0.58 | 0.73 | 0.90 |
| Valine | 2.63 | 4.21 | 3.92 |
| Methionine | 1.34 | 2.62 | 2.30 |
| Proline | 2.23 | 2.84 | 3.65 |
| Arginine | 4.22 | 5.16 | 6.54 |
| Tyrosine | 2.41 | 3.10 | 3.78 |
| Histidine | 1.09 | 1.98 | 2.24 |
| Cystine | 0.36 | 0.60 | 0.97 |
| Alanine | 3.03 | 4.55 | 4.17 |
| Glutamic acid | 8.33 | 15.14 | 13.63 |
| Glycine | 2.42 | 3.09 | 3.42 |
| Threonine | 3.11 | 3.38 | 3.00 |
| Serine | 2.11 | 3.02 | 4.32 |
| Aspartic acid | 5.21 | 6.02 | 8.99 |
3.1.2. Profile of amino acid content in trypsin hydrolysate
The amino acid composition of LSPH is depicted in Table 2. The seed protein hydrolysate consisted of 18 amino acids of which 9 are essential amino acids. From the study, the total amino acid concentration for trypsin hydrolysate was 75.4g/100g of LSPH which was higher than Alcalase hydrolysate. The total essential amino acid concentration with histidine was 31.6g/100g of LSPH. This result correlates with FAO/WHO (1991) requirements (23.20g/100g) for essential amino acids with histidine. Leucine (7.11g/100g) was the most concentrated essential amino acid which is in contrast to what Aremu et al. (2008) reported. It is interesting to note that Zhang et al. (2007) has reported that increase in dietary leucine is capable of ameliorating diet-induced obesity and high blood sugar in human subjects. The most concentrated amino acid in Luffa cylindrica seed hydrolyate was glutamic acid (15.14g/100g), closely followed by aspartic acid (6.02g/100g) as the second most concentrated non-essential amino acid. This is in agreement with the observation of Rabab (2017).
3.1.3. Profile of amino acid content in pepsin hydrolysate
The amino acid composition of LSPH is presented in Table 2. The seed protein consisted of 18 amino acids namely leucine, lysine, iso-leucine, phenylalanine, trytophan, valine, glutamic, threonine, serine, aspartic acid, glycine, alanine, methionine, proline, arginine, tyrosine, histidine and cysteine of which 9 are essential. From the study, the total amino acid concentration for trypsin hydrolysate was 85.4g/100g of LSPH which was higher than that of Alcalase and trypsin hydrolysate. The total essential amino acid concentration with histidine was 35.95g/100g of LSPH, and this was found to meet the FAO/WHO (1991) requirements (23.20g/100g) for essential amino acids with histidine. Leucine (8.97g/100g) was the most concentrated essential amino acid but differs from the findings of Aremu et al. (2008). The most concentrated amino acid in Luffa cylindrica seed hydrolysate was glutamic acid (13.63g/100g) with aspartic acid (8.99g/100g) as the second most concentrated non-essential amino acid. This is not different to the observation made by Rabab (2017).
3.1.4. ACE-inhibitory activity of Luffa cylindrica seed hydrolysate
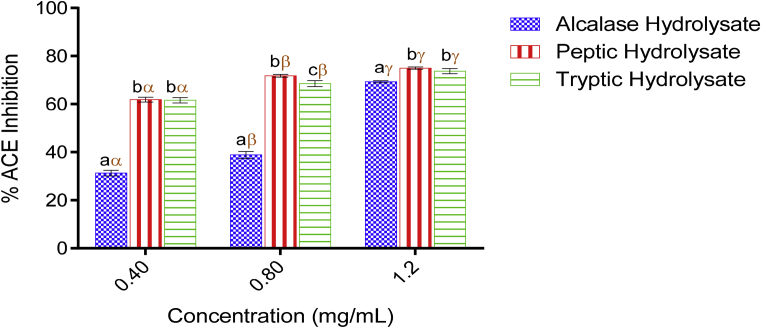
Remarkable ACE-inhibitory activity was displayed by tryptic and peptic hydrolysates in a concentration-dependent manner (Fig. 1). In addition to these, Alcalase hydrolysate also expressed a concentration-dependent inhibition against ACE while peptic hydrolysate expressed the best inhibitory activity.
Fig. 1.
ACE-inhibitory activity of Luffa cylindrica seed protein hydrolysates. Each bar depicts the mean of three replicates determinations ±SD. Bars at the same concentration but with different Latin letters (abc) are significantly different at p < 0.05. Bars at different concentrations belonging to the same sample/enzyme but with different Greek letters (αβγ) shows significant difference at p < 0.05.
The highest ACE inhibitory value obtained for peptic hydrolysate (74.99 ± 0.43%) at 1.20 mg/mL was significantly higher (p < 0.05) compared with all the other values obtained at lower peptic hydrolysate concentrations. This value shows no significant difference (p > 0.05) in the percentage inhibition obtained for tryptic hydrolysate at the same concentration but significantly different from that of Alcalase hydrolysate. Similar trend was also obtained at 0.40 mg/mL. The strong inhibition of ACE by peptic hydrolysate and tryptic hydrolysate therefore may be as a result of residual amino acids found in the peptides which emanated from peptide bond breakage (Table 2) i.e. hydrophobic/aromatic and acidic residues for pepsin and cationic residues for trypsin (Naik, 2012). It is known that ACE has affinity for molecules acting as substrates or inhibitors with hydrophobic (aromatic and branched chain such as Trp, Trp, Tyr, Phe, Pro or Lys) amino acid residues at its C-terminal positions (Wu et al., 2006; Alashi et al., 2014). This also supports why peptic hydrolysate had higher inhibitory activity as many of these residues are targets of its point of peptide bond hydrolysis.
The IC50 values obtained for peptic hydrolysate, tryptic hydrolysate and Alcalase hydrolysate were 0.32, 0.31 and 0.93 mg/mL respectively. The IC50 value obtained for peptic hydrolysate exhibited no significant difference (p > 0.05) when compared to that of tryptic hydrolysate, however both values were significantly different (p < 0.05) from the value obtained for Alcalase hydrolysate, this suggests that both hydrolysates are effective in inhibiting ACE. The tryptic hydrolysate IC50 value obtained in this study was lower than 1.03–1.06 mg/mL and 0.05 mg/mL previously reported for bovine milk tryptic hydrolysate (Haque and Chand, 2008) and flaxseed tryptic protein hydrolysate (Udenigwe et al., 2009) respectively.
3.1.5. Kinetics of ACE inhibition by Luffa cylindrica seed protein hydrolysates
In other to comprehend the efficacy of the catalytic activity-reducing ability of peptides, it is important to evaluate their kinetic parameters. Kinetic expressions also form an approximate of the quantity of peptide (inhibitor) needed to inhibit the enzyme activity, a reflection of the tendency to act on the catalytic site. Ki, the enzyme-inhibitor dissociation constant, describes the inhibitor binding capacity of the enzyme to establish the enzyme-inhibitor (E-I) complex (Barbana and Boye, 2011; Girgih et al., 2015). Inhibition mechanism of ACE by Luffa cylindrica protein hydrolysates derived by pepsin, trypsin and Alcalase was estimated by carrying out kinetic examinations of enzyme activity either when Luffa cylindrica protein hydrolysate samples were present or not (Table 3). Therefore, the pattern of Lineweaver-Burk plots of ACE-catalysed FAPGG-FAP conversion when peptide inhibitors: pepsin, trypsin and Alcalase hydrolysates were present or absent indicates uncompetitive inhibition (for Alcalase hydrolysate) and non-competitive inhibition (for tryptic hydrolysate) and mixed type inhibition (for peptic hydrolysate). The Km value of ACE activity without the inhibitor (Luffa cylindrica seed hydrolysate) obtained in this study is 2.51 mM which is higher than values earlier published- 0.30 mM (Arise et al., 2016a,b), 0.30mM (Holmquist et al., 1979) and 0.31 mM (Udenigwe et al., 2009). The uncompetitive mode of inhibition exhibited by Alcalase hydrolysate as observed by the concentration-dependent reduction in the Km value of the enzyme indicates an increase in the binding-rate of the enzyme (ACE) for its substrate (FAPGG) with an increase in Alcalase hydrolysate concentration (Berg et al., 2002). This means that the peptides present in Alcalase hydrolysate binds only to the enzyme-substrate complex at locations other than the active site and inhibition cannot be reversed by increasing substrate concentration.
Table 3.
Kinetic parameters of ACE-catalysed FAPGG-FAP conversion in the absence and presence of Luffa cylindrica seed protein hydrolysates.
| Catalytic Parameter | No Inhibitor | PepH (mg/mL) |
TrypH (mg/mL) |
AlcH (mg/mL) |
|||
|---|---|---|---|---|---|---|---|
| 0.40 | 0.80 | 0.40 | 0.80 | 0.40 | 0.80 | ||
| Km (mg/mL) | 2.51 | 0.99 | 1.99 | 2.12 | 2.41 | 1.68 | 1.35 |
| Vmax | 0.02 | 0.1322 | 0.06 | 0.19 | 0.09 | 0.02 | 0.01 |
| CE (μmol/mL/min) | 0.01 | 0.13 | 0.03 | 0.09 | 0.04 | 0.01 | 0.01 |
| Ki (mg/mL) | — | 0.31 | 0.14 | 23.82 | |||
Km – Michaelis-Menten constant in the presence and absence of hydrolysate; Vmax –Maximum reaction rate in the presence and absence of hydrolysate; CE – Catalytic efficiency; Ki – Enzyme-inhibitor dissociation constant; PepH, TrpH and AlcH – Peptic, tryptic and Alcalase hydrolysates respectively.
A mixed type inhibition was exhibited by peptic hydrolysate. This is revealed in the dose-determining rise in the Km value of the enzyme thereby indicating a drop in affinity of ACE for FAPGG with an increasing concentration of peptic hydrolysate (Udenigwe et al., 2009). This depicts the possibility of peptides in peptic hydrolysate combination with ACE biomolecule to produce an inert complex irrespective of the fact that the substrate has reacted with the enzyme at the catalytic site. This is close to the report of Girgih et al. (2011) on hemp seed peptic-pancreatic protein hydrolysate. Contrary to peptic hydrolysate, tryptic hydrolysate exhibited a non-competitive inhibition type. The maintenance of constant Km values when the the hydrolysate was present, is evident of its mode of inhibition as this is characteristic of non-competitive inhibition mode. This insinuates that the peptides contained in tryptic hydrolysate bind either to free ACE or the ACE-FAPGG complex allosterically, thus changing the structure of the enzyme resulting in a decrease in catalytic power of the enzyme. The pattern of inhibition expressed in this study is comparable to the work of Barbana and Boye (2011) for lentil tryptic and Alcalase protein hydrolysates.
The effects of Luffa cylindrica seed protein hydrolysates on Vmax expressed a concentration-dependent reduction characteristic of mixed, uncompetitive and non-competitive modes of inhibition. The action on catalytic efficiency (CE) of ACE equally shows a concentration-dependent reduction as well. The inhibition constant (Ki) has been described as the tendency of the inhibitor to bind to the enzyme so as to give rise to a tight enzyme-inhibitor complex. Peptic hydrolysate and tryptic hydrolysate had Ki values of 0.26 and 0.14 respectively. The Similar Ki values of the two hydrolysates corroborate their similar IC50 values. This implies that both hydrolysates have strong affinity for ACE. Conclusion may therefore be drawn that tryptic hydrolysate is a better ACE inhibitor compared to peptic and Alcalase hydrolysates as a result of its lowest Ki value (0.14 mg/mL).
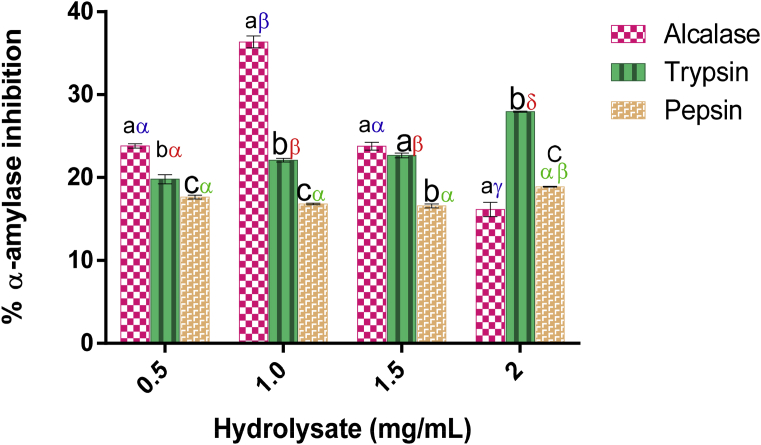
3.1.6. α-Amylase-inhibitory effect of Luffa cylindrica seed protein hydrolysate
Alcalase hydrolysate and tryptic hydrolysate expressed a significant inhibition against α-amylase enzymatic activity (Fig. 2). This was strongly supported by the percentage inhibitory effects attained by both Alcalase hydrolysate (36.36 ± 0.72%) and tryptic hydrolysate (27.96 ± 0.06%). They exhibited a concentration-dependent inhibitory activity while Alcalase hydrolysate elicited its highest inhibition at 1.00 mg/mL. Peptic hydrolysate (18.89 ± 0.11%) expressed very little inhibition against α-amylase in a concentration-dependent manner. This is supported by the weaker response of α-amylase as the concentration of hydrolysate increased compared to Alcalase hydrolysate and tryptic hydrolysate. Nevertheless, Alcalase hydrolysate showed the strongest inhibition against α-amylase activity as supported and reflected in its low IC50 (1.02 ± 0.19 mg/mL).
Fig. 2.
α- Amylase-inhibitory effect of L. cylindrical seed protein hydrolysates. Each bar depicts the mean of three replicates determinations ±SD. Bars at the same concentration but with different Latin letters (abcd) are significantly different at p < 0.05. Bars of the same sample/enzyme at different concentrations with different Greek letters (αβγδ) show significant difference at p < 0.05.
The type of amino acid residues obtained from the cleavage of Luffa cylindrica seed Alcalase and tryptic hydrolysates (Table 2) has been linked to the role they play as strong inhibitors of α-amylase (Naik, 2012). In addition to this, catalytic active sites having Leu, Try, Phe or Trp have been shown to be preferable by Alcalase though in a non-specific manner (Motyan et al., 2013). By speculation, it may be that peptides having branched chain (such as Lys, Phe, Tyr, and Trp) and cationic residues are preferably bound to α-amylase. As revealed in this study, Alcalase hydrolysate has a higher degree of potency compared to tryptic hydrolysate due to its low IC50 value.
3.1.7. Kinetics of α-amylase inhibition by Luffa cylindrica seed protein hydrolysates
The mechanism of enzymatic activity reduction exhibited by Luffa cylindrica seed hydrolysate was determined by assessing the kinetic parameters obtained via Lineweaver-Burk plot of α-amylase inhibition (Table 4). The Km of 1.75 mg/mL obtained when the inhibitor (hydrolysate) was absent agrees with earlier report by Shobana et al. (2009. In this study, non-competitive inhibition is the dormant pattern as strongly supported by the Lineweaver–Burk plot of α-amylase-catalysed in vitro breakdown of starch in the presence and absence of peptide inhibitors. The non-competitive mechanism of inhibition that occurred is comparable to earlier documented observation for pine bark extract (Shobana et al., 2009). This points to the possibility of peptides from Luffa cylindrica seed protein hydrolysates to form enzyme-inhibitor or enzyme-substrate-inhibitor inactive complex (Girgih et al., 2011).
Table 4.
Kinetic parameters of α-amylase-catalyzed degradation of starch in the absence and presence of Luffa cylindrica seed protein hydrolysates.
| Catalytic Parameter | No Inhibitor | PepH (mg/mL) |
TrypH (mg/mL) |
AlcH (mg/mL) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.50 | 1.00 | 1.50 | 0.50 | 1.00 | 1.50 | 0.50 | 1.00 | 1.50 | ||
| Km (mg/mL) | 1.75 | 1.57 | 1.67 | 1.60 | 1.67 | 1.83 | 1.50 | 1.67 | 1.57 | 1.67 |
| Vmax | 2500 | 1429 | 1667 | 2000 | 1250 | 1667 | 1667 | 1667 | 1429 | 1111 |
| CE (μmol/mL/min) | 1429 | 910 | 998 | 1250 | 749 | 911 | 1111 | 998 | 910 | 665 |
| Ki | 4.21 | 3.57 | 1.46 | |||||||
Km – Michaelis constant in the presence and absence of hydrolysate; Vmax – Maximum reaction rate in the presence and absence of hydrolysate; CE – Catalytic efficiency; Ki – Enzyme-inhibitor dissociation constant; PepH, TrypH and AlcH – Peptic, tryptic and Alcalase hydrolysates respectively.
In a non-competitive mode of inhibition, Vmax of an enzyme are brought down in a concentration-dependent pattern. This was exhibited by the effects of LSPH on the Vmax of α-amylase-catalyzed starch hydrolysis. In addition to this, the catalytic efficiency (CE) of α-amylase also expressed a concentration-dependent decrease in activity.
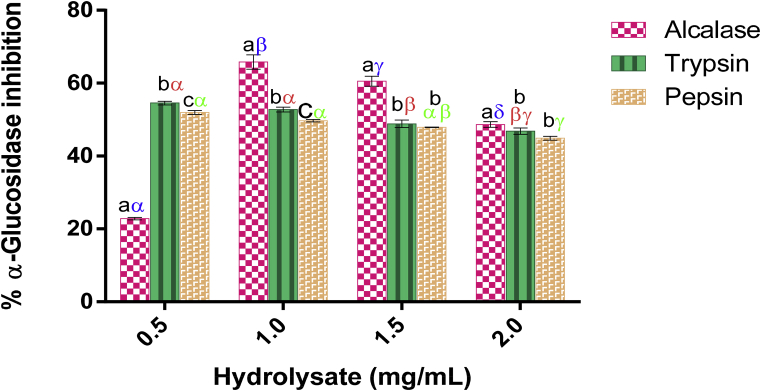
3.1.8. α-Glucosidase-inhibitory effect of LSPH
As an important enzyme in starch catalysis, α-glucosidase hydrolyses starch fragments synthesized by α-amylase into glucose. Therefore, blocking the action of α-glucosidase can effectively reduce the amount of α-D-glucose. The conversion of PnPG to p-nitrophenol catalyzed by α-glucosidase in the presence of Luffa cylindrica seed protein hydrolysate concentrations (0.50–2.00 mg/mL) was measured. From the aforegoing, Alcalase and trypsin hydrolysates showed significant inhibitory activities against α-glucosidase (Fig. 3), the highest percentage inhibition attained by Alcalase hydrolysate was 65.82 ± 1.96% while tryptic hydrolysate was 54.54 ± 0.52%. The inhibition was not concentration dependent as against the report of Arise et al. (2016a,b). Alcalase hydrolysate displayed its highest inhibition at 1.00 mg/mL while tryptic hydrolysate exhibited its highest inhibition at 0.50 mg/mL concentration. Peptic hydrolysate with percentage inhibition of 51.96 ± 0.89%), showed reduced inhibitory effect against α-glucosidase while the percentage inhibition reduced as concentration of Luffa cylindrica seed hydrolysate was increased.
Fig. 3.
α-glucosidase- inhibitory effect of L. cylindrica seed protein hydrolysates. Each bar depicts the mean of three replicates determinations ±SD. Bars at the same concentration but with different Latin letters (abcd) are significantly different at p < 0.05. Bars of the same sample/enzyme at different concentrations with different Greek letters (αβγδ) show significant difference at p < 0.05.
However, the IC50 values of Luffa cylindrica seed protein hydrolysate against α-glucosidase inhibitory activity shows that Alcalase hydrolysate had IC50 value of 0.80 ± 0.1 mg/mL and tryptic hydrolysate had IC50 value of 0.48 ± 0.02 mg/mL. When compared with the IC50 value of Acarbose of 2.15 mg/mL as reported by Sulistiyani et al. (2016), it can be said that Alcalase hydrolysate is a better inhibitor of α-glucosidase.
3.1.9. α-Glucosidase kinetics of inhibition by Luffa cylindrica seed protein hydrolysates
The effects of Alcalase, tryptic and peptic hydrolysates on α-glucosidase in vitro breakdown of starch to simple monosaccharide reducing units are depicted in Table 5. The mechanism of inhibition was ascertained by using the parameters from Lineweaver-Burk Plot of α-glucosidase inhibition by Luffa cylindrica seed protein hydrolysate. From a recent study, the Km value obtained from Acarbose in the absence of inhibitory hydrolysates was 1.24 mg/mL, which is comparable to 6.31 mg/mL obtained in this study for α-glucosidase activity (Al-Dhabaan, 2018). The kinetic plots displayed the mode of inhibition of α-glucosidase by Alcalase, tryptic and peptic hydrolysate to be uncompetitive in the three scenarios. This points to the possibility of Luffa cylindrica seed hydrolysate peptides binding with α-glucosidase enzyme molecule to form inactive enzyme-substrate-inhibitor complex (Girgih et al., 2011). The inhibitory pattern obtained in this study is proportional to early report on Labitatae extracts (Moein, 2017). Both Vmax and catalytic efficiency CE of α-glucosidase-catalyzed glucose hydrolysis expressed inhibitor concentration-dependent reduction in activity.
Table 5.
Kinetic parameters of α-glucosidase-catalyzed degradation of starch in the absence and presence of Luffa cylindrica seed protein hydrolysates.
| Catalytic Parameter | No Inhibitor |
AlcH (mg/mL) |
TrypH (mg/mL) |
PepH (mg/mL) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.50 | 1.00 | 1.50 | 0.50 | 1.00 | 1.50 | 0.50 | 1.00 | 1.50 | ||
| Km (mg/mL) | 6.31 | 1.27 | 1.82 | 3.31 | 0.99 | 1.2 | 2.33 | 1.2 | 1.38 | 2.12 |
| Vmax(x10−2) | 0.33 | 0.05 | 0.07 | 0.15 | 0.05 | 0.07 | 0.12 | 0.61 | 0.07 | 0.12 |
| CE (μmol/mL/min x 10−4) | 5.20 | 3.90 | 3.80 | 4.50 | 5.00 | 5.80 | 5.20 | 5.00 | 5.00 | 5.70 |
| Ki. | 8.41 | 49.83 | 10.51 | |||||||
Km – Michaelis constant in the presence and absence of hydrolysate; Vmax – Maximum reaction rate in the presence and absence of hydrolysate; CE – Catalytic efficiency; Ki – Enzyme-inhibitor dissociation constant; PepH, TrypH and AlcH – Peptic, tryptic and Alcalase hydrolysates respectively.
3.1.10. Antioxidant activity of Luffa cylindrica seed protein hydrolysate
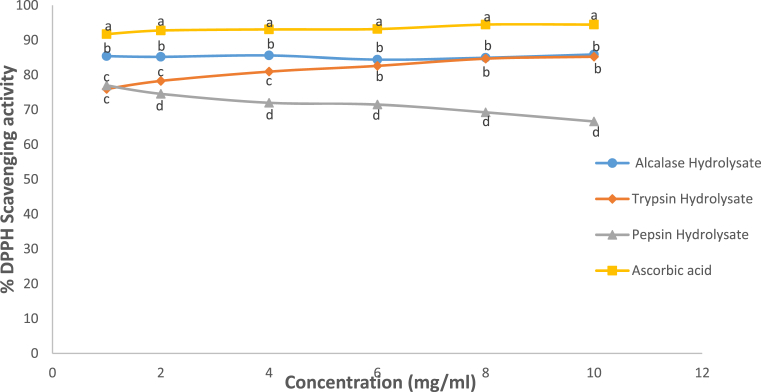
DPPH˙-scavenging activity of the standard antioxidant compound (ascorbate) was significantly higher (p<0.05) compared with the three hydrolysates at the concentrations investigated while that of the hydrolysates were concentration-dependent (Fig. 4). As the concentration of peptic hydrolysate increased, the radical scavenging activity decreased. This may be attributed to the fact that peptic hydrolysate do not have cysteine or tryptophan rich containing peptides which are considered as effective scavengers of DPPH˙ (Liu et al., 2003). This is evident in the low concentration of both amino acids in the peptic hydrolysates (Table 2). The free radical scavenging activity of tryptic hydrolysate increased with increasing hydrolysate concentration because DPPH˙ scavenging activity increased with increasing concentration of antioxidant (Mishra et al., 2012). DPPH. -scavenging activity, widely used to evaluate antioxidant activity shows the ability of antioxidant compound to release electrons or hydrogen (Udenigwe et al., 2009; Chandrasekara and Shahidi, 2011). Compounds with antioxidative property often give out hydrogen atom to free radicals to terminate lipid peroxidation.
Fig. 4.
DPPH radical-scavenging activity of Luffa cylindrica seed protein hydrolysates. Each dot depicts the mean of three replicate determinations ±SD. Dots belonging to different enzyme hydrolysates/samples at the same concentration but with different letters show significant difference at p < 0.05.
Oxidative stress results when a non-radical oxidizing species like hydrogen peroxide is transformed to hydroxyl radicals in vivo in the presence of metal ions or when it is synthesized from other radical systems (Rahman et al., 2012). The mop up of hydroxyl radicals is an efficient defense strategy against various diseases caused or propagated by the reactive oxygen species.
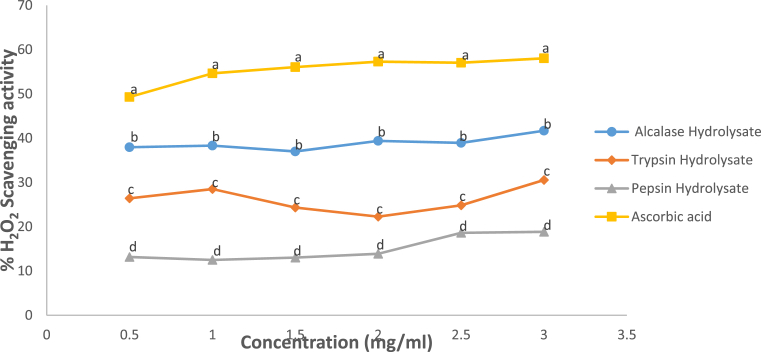
The H2O2-scavenging activity of Luffa cylindrica seed protein hydrolysates shown in Fig. 5 displayed an increasing trend. The Alcalase hydrolysate displayed the strongest H2O2-scavenging activity with 41.69% whereas peptic hydrolysate had the least (18.86%). The result obtained may be due to the presence of certain amino acid residues that are present in Alcalase hydrolysate which could have scavenging effect against H2O2, otherwise not exhibited against other oxidants. Despite the low superoxide-scavenging activity of Alcalase hydrolysate, it however presented a significantly higher H2O2-scavenging power than peptic and tryptic hydrolysate. Alcalase is widely known to have low endopeptidase specificity, thus it can hydrolyze protein at non-specific sites. Some reports however gave an account on some amino acid residues (e.g Trp, Tyr, Phe and Leu) which usually serve as the sites of Alcalase degradation (Motyan et al., 2013) and some of these amino acids were found in substantial amounts in the Alcalase hydrolysate (Table 2). The percentage H2O2-scavenging power of ascorbic acid obtained in the study (49.29 ± 0.50% at 1 mg/mL) correlates with 49.12 ± 0.50% at 0.80 mg/mL obtained previously by Arise et al. (2016a,b), this is expected, as ascorbic acid is a standard antioxidant.
Fig. 5.
H2O2-scavenging capacity of Luffa cylindrica seed protein hydrolysates. Each dot depicts the mean of three replicate determinations ±SD. Dots belonging to different enzyme/sample at the same concentration but with different letters show significant difference at p < 0.05.
Reducing power, considered as a yardstick measure of total antioxidant activity, portrays the electron donating capacity of an antioxidant. Amino acid with dense aromatic rings e.g. phenylalanine, tyrosine and tryptophan can give out protons to electrons deficient radicals. The compounds with reducing power have the capacity to reduce the oxidized intermediates of peroxidation (Ambigaipalan et al., 2015), and thus act as an antioxidant. Potassium ferricyanide, as documented, is commonly used to estimate the reducing power of hydrolysates for which higher absorbance indicates superior ferrous ion formation as a result of the reduction of Fe3+ to Fe2+, indicating a superior reducing ability (Razali et al., 2015).
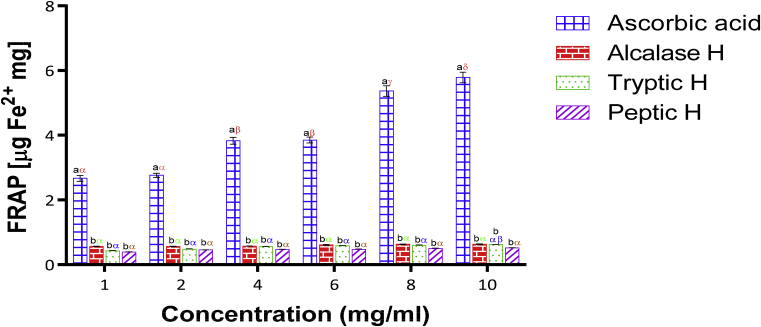
The ferric reducing power of Luffa cylindrica seed hydrolysate was compared with a standard antioxidant (ascorbate) as shown in Fig. 6. The significant drop (p<0.05) in the ferric reducing power of Luffa cylindrica seed hydrolysate at all concentrations in comparison to ascorbic acid indicates the non-comparison of ascorbic acid reducing power to LSPH. The results showed that at the highest concentration (10 mg/mL), Alcalase hydrolysate possesses the highest Fe3+- reducing power (0.63 ± 0.01) followed by tryptic hydrolysate (0.62 ± 0.01) and peptic hydrolysate (0.51 ± 0.01). The higher ferric reducing power of Alcalase hydrolysate may be due to the exposure of more amino acids R-groups during hydrolysis (Matoba, 2002). Peptides from alfalfa leaf protein hydrolyzed with Alcalase showed a higher ferric reducing power (1.43μg Fe2+/mg) than the one obtained from this study (Xie et al., 2008). Similarly, the ferric reducing ability of peptic hydrolysate of C. lanatus seed (0.63 μg Fe2+/mg at 0.80 mg/mL) obtained previously (Arise et al., 2016a,b) was higher than 0.39 μg Fe2+/mg at 1 mg/mL obtained in this study. The increase or decrease in ferric reducing power for protein hydrolysates may not be unconnected to the exposure of electron-dense amino acid side chain groups such as polar or charged moieties during hydrolysis (Liu et al., 2010).
Fig. 6.
Ferric-reducing antioxidant power of ascorbic acid and Luffa cylindrica seed protein hydrolysate. Each bar depicts the mean of three replicate determinations ±SD. Bars at the same concentration but with different Latin letters (abcd) show significant difference at p < 0.05. Bars of the same sample/enzyme at different concentrations with different Greek letters (αβγδ) show significant difference at p < 0.05.
The commonest in vivo free radicals biosynthesized via autoxidation or enzymatic activity are the superoxide anion radicals that play critical role as an antioxidant (Lee et al., 2012). It has been reported to be a potential precursor of highly reactive species although they cannot directly trigger lipid oxidation like hydroxyl radical (Li et al., 2008).
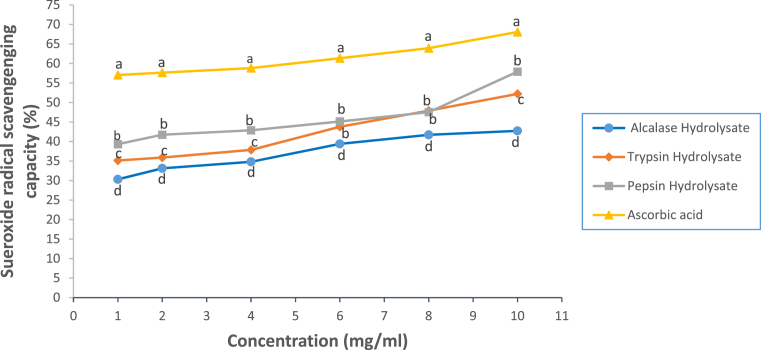
There was a significant difference (p<0.05) in the percentage superoxide ion scavenging activity of the ascorbate and the hydrolysates as most of them had lower superoxide ion scavenging activity compared with the standard antioxidant (Fig. 7). The higher (p < 0.05) ˙O2ˉ-scavenging capacity of peptic hydrolysate with respect to other hydrolysates implies that peptic hydrolysate contained peptides that are able to inhibit the autoxidation of pyrogallol and subsequently prevented the production of ˙O2ˉ. Acidic residue generated from the peptic cleavage of peptides has also been attributed to the probable scavenging power of peptic hydrolysate. These buttress specific binding of acidic and hydrophobic amino acids residues to pepsin; an endopeptidase (Kageyama, 2002; Naik, 2012). Both acidic and hydrophobic amino acids has been reported to enhance free radical scavenging properties of peptides by donating H atom to O2ˉ to form a stable water molecule and the enhancement properties of the hydrophobic residues at terminal ends of peptides (Sarmadi and Ismail, 2010). Each of the hydrolysates demonstrated radical-scavenging capacity in a concentration-dependent fashion.
Fig. 7.
Superoxide anion radical-scavenging capacity of Luffa cylindrica seed protein hydrolysates. Each dot depicts the mean of three replicate determinations ±SD. Dots belonging to different enzyme/sample and at the same concentration but with different letters show significant difference at p < 0.05.
The EC50 value determines the concentration of hydrolysates required to inhibit 50% of the oxidant, thus widely used to measure antioxidant, antiradical and reduction efficiencies (Razali et al., 2015). Low EC50 value is desirable as lower values indicate higher effectiveness. For the DPPH radical and superoxide scavenging activities, Alcalase hydrolysate has the lowest EC50 (0.63 ± 0.06 and 1.60 ± 0.10 mg/mL respectively). Tryptic hydrolysate gave the lowest EC50 for hydrogen peroxide scavenging activity (1.00 ± 0.10 mg/ml). All these EC50 values were higher than those obtained for the standard antioxidant (Table 6).
Table 6.
EC50 values of H2O2 and DPPH/superoxide radical scanvenging activities of different hydrolysates of L. cylindrical seed protein.
| Hydrolysates | H2O2(EC50, mg/mL) | DPPH(EC50, mg/mL) | Superoxide (EC50, mg/mL) |
|---|---|---|---|
| Alcalase | 1.12 ± 0.10a | 0.63 ± 0.06a | 1.60 ± 0.10a |
| Tryptic | 1.00 ± 0.10a | 0.65 ± 0.06a | 8.20 ± 0.61b |
| Peptic | 2.10 ± 0.11b | 0.70 ± 0.06b | 8.10 ± 0.60b |
| Ascorbic acid | 0.65 ± 0.04c | 0.51 ± 0.04c | 0.90 ± 0.05c |
Values depict the mean of three replicate determinations ±standard deviation (SD). Values in each column showing different letters are significantly different at p < 0.05.
4. Conclusion
This in-vitro study has shown that L. cylindrical seed protein hydrolysates have antioxidant and ACE-inhibitory potentials. Pepsin hydrolysate displayed the highest ACE inhibitory activity but trypsin hydrolysate with the least IC50 may be more effective in inhibiting ACE. All the hydrolysates possess α-amylase and α-glucosidase inhibitory activities. Alcalase hydrolysates demonstrated the strongest α-amylase and α-glucosidase inhibitory properties. The hydrolysates contain 18 amino acids each, of which 9 are essential amino acids. Therefore, L. cylindrical seed protein hydrolysate has bioactivities that could be used in the development of affordable, effective and safer nutraceuticals.
Declarations
Author contribution statement
Rotimi Olusanya Arise: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jalil James Idi, Iseoluwa Maureen Mic-Braimoh, Emmanuel Korode, Ayodeji Idowu: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Risikat Nike Ahmed: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Omorefosa Osemwegie: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Rotimi Olusanya Arise and Risikat Nike Ahmed were supported by the Institutional Based Tertiary Education Trust Fund (TETFund) of N1,200,000 which has been used for the laboratory and data analysis.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adisakwattana S., Ruengsamran T., Kampa P., Sompong W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complement Altern. Med. 2012;12:1–8. doi: 10.1186/1472-6882-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alashi A.M., Blanchard C.L., Mailer R.J., Agboola S.O., Mawson A.J., He R., Malomo S.A., Girgih A.T., Aluko R.E. Blood pressure lowering effects of Australian canola protein hydrolysates in spontaneously hypertensive rats. Food Res. Int. 2014;55:281–287. [Google Scholar]
- Al-Dhabaan F. Kinetics of hypoglycemic α-glucosidase inhibitory protein. J. Pure Appl. Microbiol. 2018;12(1):119–126. [Google Scholar]
- Aluko R. Antihypertensive peptides from food proteins. Annu. Rev. Food Technol. (Mysore) 2015;6:235–262. doi: 10.1146/annurev-food-022814-015520. [DOI] [PubMed] [Google Scholar]
- Ambigaipalan P., Al-Khalifa A.S., Shahidi F. Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysates prepared using Alcalase, Flavourzyme and Thermolysin. Journal of Functional Foods. 2015;18:1125–1137. [Google Scholar]
- Aremu M.O., Olaofe O., Okribiti B.Y. Chemical evaluation of the nutritive value of smooth luffa seeds kernel. Electron. J. Environ. Agric. Food Chem. 2008;7(10) 3444-342. [Google Scholar]
- Apostolidis E., Kwon Y.I.I., Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Technol. 2006;8(1):46–54. [Google Scholar]
- Arise A.K., Amonsou E.O., Ijabadeniyi O.A. Influence of extraction methods on functional properties of protein concentrates prepared from South African Bambara groundnut landraces. Int. J. Food Sci. Technol. 2015;50(5):1095–1101. [Google Scholar]
- Arise R.O., Yekeen A.A., Ekun O.E., Olatomiwa O.J. Protein hydrolysates from Citrullus lanatus seed: antiradical and hydrogen peroxide-scavenging properties and kinetics of angiotensin-iconverting enzyme inhibition. Ceylon J. Sci. 2016;45(2):39–52. [Google Scholar]
- Arise R.O., Yekeen A.A., Ekun O.E. In vitro Antioxidant and α-Amylase inhibitory properties of watermelon seed protein hydrolysates. Environ. Exp. Biol. 2016;14:163–172. [Google Scholar]
- Barbana C., Boye J.I. Angiotensin I-converting enzyme inhibitory properties of lentil protein hydrolysates: determination of the kinetics of inhibition. Food Chem. 2011;127(1):94–101. [Google Scholar]
- Berg J.M., Tymoczko J.L., Stryer L. W. H. Freeman and Company; New York, USA: 2002. Biochemistry; pp. 301–350. [Google Scholar]
- Bernfeld P. Enzymes of starch degradation and synthesis. Adv. Enzymol. Relat. Sub. Biochem. 1951;12:379–428. doi: 10.1002/9780470122570.ch7. [DOI] [PubMed] [Google Scholar]
- Boye J.I., Aksay S., Roufik S., Ribéreau S., Mondor M., Farnworth E.R., Rajamohamed S.H. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010;43(2):537–546. [Google Scholar]
- Chandrasekara A., Shahidi F. Inhibitory activities of soluble and bound millet seed phenolics on free radicals and reactive oxygen species. J. Agric. Food Chem. 2011;59:428–436. doi: 10.1021/jf103896z. [DOI] [PubMed] [Google Scholar]
- Chen F., Shi Z., Neoh K.G., Kang E.T. Antioxidant and antibacterial activities of eugenol and carvacrol-grafted chitosan nanoparticles. Biotechnol. Bioeng. 2009;104(1):30–39. doi: 10.1002/bit.22363. [DOI] [PubMed] [Google Scholar]
- Du Q., Xu Y., Li L., Zhao Y., Jerz G., Winterhalter P. Antioxidant constituents in the fruits of Luffa cylindrica (L.) Roem. J. Agric. Food Chem. 2006;54(12):4186–4190. doi: 10.1021/jf0604790. [DOI] [PubMed] [Google Scholar]
- FAO/WHO Protein quality evaluation report of joint FAO/WHO expert consultative. Food Nutr. 1991 [Google Scholar]
- Ghassem M., Babji A., Said M., Mahmoodani F., Arihara K. Angiotensin-1- converting enzyme inhibitory peptides from snakehead fish sarcoplasmic protein hydrolysate. J. Food Biochem. 2014;38:140–149. [Google Scholar]
- Girgih A.T., He R., Hasan F.M., Udenigwe C.C., Gill T.A., Aluko R.E. Evaluation of the in vitro antioxidant properties of a cod (Gadusmorhua) protein hydrolysate and peptide fractions. Food Chem. 2015;173:652–659. doi: 10.1016/j.foodchem.2014.10.079. [DOI] [PubMed] [Google Scholar]
- Girgih A.T., Udenigwe C.C., Li H., Adebiyi A.P., Aluko R.E. Kinetics of enzyme inhibition and antihypertensive effects of hemp seed (Cannabis sativa L.) protein hydrolysates. J. Am. Oil Chem. Soc. 2011;88:1767–1774. [Google Scholar]
- Gurudeeban S., Satyavani K., Ramanathan T. Alpha-glucosidase inhibitory effect and enzyme kinetics of coastal medicinal plants. Bangladesh J. Pharmacol. 2012;7(3):186–191. [Google Scholar]
- Haque E., Chand R. Antihypertensive and antimicrobial bioactive peptides from milk proteins. Eur. Food Res. Technol. 2008;277:7–15. [Google Scholar]
- Holmquist B., Bunning P., Riordan J.F. A continuous spectrophotometric assay for angiotensin converting enzyme. Anal. Biochem. 1979;95:540–548. doi: 10.1016/0003-2697(79)90769-3. PMID: 222167. [DOI] [PubMed] [Google Scholar]
- Jrad Z., Girardet J., Adt I., Oulahal N., Degraeve P., Khorchani T., El Hatmi H. Antioxidant activity of camel milk casein before and after in vitro simulated enzymatic digestion. Mljekarstvo. 2014:287–294. [Google Scholar]
- Kageyama T. Pepsinogens, progastricsins, and prochymosins: structure, function, evolution, and development. Cell. Mol. Life Sci. 2002;59(2):288–306. doi: 10.1007/s00018-002-8423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.G., Lee J.H., Jung H.J., Han Y.K., Park K.M., Han I.K. Effect of partial replacement of soybean meal with palm kernel meal (PKM) and corpra meal (CM) on growth performance, nutrient digestibility and carcass characteristics of finishing pigs. Asian-Australas. J. Anim. Sci. 2001;14(6):821–830. [Google Scholar]
- Lahogue V., Réhel K., Taupin L., Haras D., Allaume P. A HPLC-UV method for the determination of angiotensin I-converting enzyme (ACE) inhibitory activity. Food Chem. 2010;118(3):870–875. [Google Scholar]
- Lee S.L., Kim K.H., Kim Y.S., Sung J., Jang Y., Shim J., Han S., Choi Y. Biological activity from the gelatin hydrolysates of duck skin by-products. Process Biochem. 2012;47:1150–1154. [Google Scholar]
- Li Y., Jiang B., Zhang T., Mu W., Lui J. Antioxidant and free radical- Scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106(2):444–450. [Google Scholar]
- Liu Q., Kong B., Xiong Y.L., Xia X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010;118(2):403–410. [Google Scholar]
- Liu Y.W., Han C.H., Lee M.H., Hsu F.L., Hou W.C. Patatin, the tuber storage protein of potato (Solanumtuberosum L.), exhibits antioxidant activity in vitro. J. Agric. Food Chem. 2003;2003(51):4389–4393. doi: 10.1021/jf030016j. [DOI] [PubMed] [Google Scholar]
- Malomo S., Onuh J., Girgih A., Aluko R. Structural and antihypertensive properties of enzymatic hemp seed protein hydrolysates. Nutrients. 2015;7(9):7616–7632. doi: 10.3390/nu7095358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba T. How does the radical-scavenging activity of soy protein food change during heating. DaizuTanpakushitsukenkyu. 2002;5:47–50. [Google Scholar]
- Mishra K., Ojiha H., Chaudhury K.N. Estimation of antiradical properties of antioxidants using DPPH∗ Assay: a critical review and results. Journal Food Chemstry. 2012;130:1036–1043. [Google Scholar]
- Moein S., Pimoradloo E., Moein M., Vessal M. Evaluation of antioxidant potentials and α-amylase inhibition of different fractions of labiatae plants extracts: as a model of antidiabetic compounds properties. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/7319504. 8 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyan J., Toth F., Tozser J. Research applications of proteolytic enzymes in molecular biology. Biomolecules. 2013;3(4):923–942. doi: 10.3390/biom3040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik P. Essentials of Biochemistry. Jaypee Brothers Medical Publishers Ltd; 2012. Protein metabolism; pp. 226–257. [Google Scholar]
- Oboh I.O., Aluyor E.O. Luffacylindrica-an emerging cash crop. Afr. J. Agric. Res. 2009;4(8):684–688. [Google Scholar]
- Ogunbanjo O.R., Awotoye O.O., Jayeoba F.M., Jeminiwa S.W. Nutritional analysis of selected Cucurbitaceae species. Uni. J. Plant Sci. 2016;4(1):1–3. [Google Scholar]
- Onuh J.O., Girgih A.T., Aluko R.E., Aliani M. Inhibition of renin and angiotensin converting enzyme activities by enzymatic chicken skin protein hydrolysates. Food Res. Int. 2013;53:260–267. [Google Scholar]
- Pownall T.L., Udenigwe C.C., Aluko R.E. Amino acid composition and antioxidant properties of pea seed (Pisumsativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem. 2010;58(8):4712–4718. doi: 10.1021/jf904456r. [DOI] [PubMed] [Google Scholar]
- Rabab H.S. Functional characterization of Luffa (Luffacylindrica) seeds powder and their utilization to improve stabilized emulsions. J. Appl. Sci. 2017;7(3):613–625. [Google Scholar]
- Rahman T., Hosen I., Towhidul-Islam M.M., Shekhar H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012;3(7):997–1019. [Google Scholar]
- Razali A.N., Amin A.M., Sarbon N.M. Antioxidant activity and functional properties of fractionated cobia skin gelatin hydrolysate at different molecular weight. Int. Food Res. J. 2015;22(2):651–660. http://www.ifrj.upm.edu.my/.../(29).pdf [Google Scholar]
- Ruch R.J., Cheng S.J., Klaunig J.E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- Sarmadi B.H., Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31(10):1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Shobana S., Sreerama Y.N., Malleshi N.G. Composition and enzyme inhibitory properties of finger millet (EleusinecoracanaL.) seed coat phenolics: mode of Inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009;115:1268–1273. [Google Scholar]
- Singh N.P., Matta N.K. Levels of seed proteins in Citrullus and praecitrullus accessions. Plant Systemat. Evol. 2010;290:47–56. [Google Scholar]
- Sulistiyani M., Safithri Y., Puspita S. Inhibition of α-glucosidase activity by ethanolic extract of Meliaazedarach L.Leaves. IOP Conf. Ser. Earth Environ. Sci. 2016;31 [Google Scholar]
- Udenigwe C.C., Lin Y., Hou W., Aluko R.E. Kinetics of the inhibition of renin and angiotensin I-converting enzyme by flaxseed protein hydrolysate fractions. J. Funct. Foods. 2009:199–207. [Google Scholar]
- World Health Organization . World Health Organisation; 2013. A Global Brief on Hypertension.http://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en Retrieved 2016 March 23 from. [Google Scholar]
- Wu J., Aluko R.E., Nakai S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: quantitative structure-and-activity relationship study of di- and tri-peptides. J. Agric. Food Chem. 2006;54:732–738. doi: 10.1021/jf051263l. [DOI] [PubMed] [Google Scholar]
- Xie Z., Huang J., Xu X., Jin Z. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem. 2008;111:370–376. doi: 10.1016/j.foodchem.2008.03.078. [DOI] [PubMed] [Google Scholar]
- Yu Z., Liu B., Zhao W., Yin Y., Liu J., Chen F. Primary and secondary structure of novel ACE-inhibitory peptides from egg white protein. Food Chem. 2012;133:315–322. doi: 10.1016/j.foodchem.2012.01.032. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Guo K., LeBlanc R.E., Loh D., Schwartz G.J., Yu Y.H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56(6):1647–1654. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]