Abstract
Background
There has been considerable interest in providing antenatal dietary and lifestyle advice for women with obesity or who are overweight during pregnancy, as a strategy to limit gestational weight gain and improve maternal and infant health. However, such antenatal interventions appear to have a modest effect on gestational weight gain and other clinical pregnancy and birth outcomes and additional strategies are required.
Metformin is an oral insulin‐sensitising medication that acts to decrease blood glucose concentrations. Metformin is commonly used in the treatment of type 2 diabetes mellitus and polycystic ovarian syndrome, and is being used increasingly in the treatment of gestational diabetes, having been shown to result in decreased rates of caesarean birth and neonatal hypoglycaemia. Metformin may be an adjuvant therapy to current antenatal strategies in pregnant women with obesity or who are overweight, acting to reduce glucose production in the liver and improve glucose uptake in smooth muscle cells, and therefore improve the overall metabolic health of women in pregnancy and reduce the risk of known adverse pregnancy outcomes.
Objectives
To evaluate the role of metformin in pregnant women with obesity or who are overweight, on maternal and infant outcomes, including adverse effects of treatment and costs.
Search methods
We searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (11 October 2017), and reference lists of retrieved studies.
Selection criteria
All published and unpublished randomised controlled trials evaluating metformin use (compared with placebo or no metformin) in women with obesity or who are overweight in pregnancy for improving outcomes, alone or in combination with other interventions were eligible for inclusion.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We used the GRADE approach to assess the quality of the evidence.
Main results
We included three studies which randomised women (1099) with a body mass index (BMI) of 30 kg/m2 (1 study) and 35 kg/m2 (2 studies), with outcomes available for 1034 participants. None of the studies assessed women with a BMI between 25 kg/m2and 29.9 kg/m2, therefore we could not assess the use of metformin in women considered overweight. We did not identify studies of metformin in combination with another treatment. Two other studies are ongoing.
All three included studies were randomised controlled trials and compared metformin with placebo, commencing early in the second trimester. Doses ranged from 500 mg twice daily to 3.0 g per day. All three studies (two in the UK, one in Egypt) included women attending hospitals for antenatal care.
Two studies were generally at a low risk of bias across the majority of domains. We assessed the third study as being at an unclear risk of selection bias, performance and detection bias due to insufficient information in the report. We assessed the trial as being at a low risk of attrition bias and other bias; we felt it was at a high risk of reporting bias.
The primary outcome for this review was infant birthweight large‐for‐gestational‐age (> 90th centile for gestational age and infant sex). Women who received metformin or placebo had a similar risk of their baby being born large for his or her gestational age (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.70 to 1.30; 2 studies, 831 infants; high‐quality evidence).
Women who received metformin may have a slightly lower gestational weight gain (mean difference (MD) ‐2.60 kg, 95% CI ‐5.29 to 0.10; 3 studies, 899 women; low‐quality evidence).
Metformin may make little or no difference in the risk of women developing gestational hypertension (average RR 1.02, 95% CI 0.54 to 1.94; 3 studies, 1040 women; low‐quality evidence) or pre‐eclampsia (RR 0.74, 95% CI 0.09 to 6.28; 2 studies, 840 women; low‐quality evidence). Metformin probably makes little or no difference in the risk of women developing gestational diabetes (RR 0.85, 95% CI 0.61 to 1.19; 3 studies, 892 women; moderate‐quality evidence).
One study of 400 women reported women receiving metformin were more likely to experience any adverse effect compared with women receiving placebo (RR 1.63, 95% CI 1.27 to 2.08; 1 study, 400 women). Adverse effects included abdominal pain, diarrhoea, or headache. When considering individual side effects, women receiving metformin were more likely to experience diarrhoea than women receiving placebo (RR 2.34, 95% CI 1.74 to 3.14; 797 women; 2 studies, 797 women; high‐quality evidence). No other important differences were identified between Metformin and placebo for other maternal secondary outcomes, including: caesarean birth, birth before 37 weeks of pregnancy, shoulder dystocia, perineal tear, or postpartum haemorrhage.
In terms of other infant outcomes, there was little or no difference in the infant birthweight (MD 6.39 g, 95% CI ‐81.15 to 93.92; 2 studies, 834 infants; high‐quality evidence). There were no other important differences identified for other infant secondary outcomes in this review: hypoglycaemia (low blood sugar); hyperbilirubinaemia (jaundice); Apgar score less than 7 at five minutes; or stillbirth and neonatal death. Only one study reported admission to the neonatal intensive care unit (NICU), indicating similar rates of admission between women receiving metformin or placebo; no other admission data were reported to assess differences in costs.
Authors' conclusions
There is insufficient evidence to support the use of metformin for women with obesity in pregnancy for improving maternal and infant outcomes. Metformin was, however, associated with increased risk of adverse effects, particularly diarrhoea. The quality of the evidence in this review varied from high to low, with downgrading decisions based on study limitations and inconsistency.
There were only a small number of studies included in this review. Furthermore, none of the included studies included women categorised as 'overweight' and no trials looked at metformin in combination with another treatment.
Future research is required in order to further evaluate the role of metformin therapy in pregnant women with obesity or who are overweight, as a strategy to improve maternal and infant health, alone or as an adjuvant to dietary and lifestyle advice.
Plain language summary
Metformin for women with obesity or who are overweight during pregnancy for improving health for women and their babies
What is the issue?
We examined whether metformin has a role in improving health outcomes for pregnant women with obesity or who are overweight, and their babies. We considered possible benefits, adverse effects and healthcare system costs.
Body mass index (BMI), calculated from a person's height and weight, is used to classify someone as having normal weight (BMI less than 25 kg/m2), being overweight (BMI 24.9 kg/m2 to 30 kg/m2) or having obesity (BMI above 30 kg/m2). Women with obesity or who are overweight are more likely than women of normal weight to experience complications like high blood pressure and gestational diabetes during pregnancy. They are also at increased risk of needing a caesarean or developing infection after birth. Their babies are more likely to experience health problems, requiring admission to the neonatal unit or intensive care, have low blood sugar, or problems breathing immediately after birth.
Women with obesity or who are overweight may have some features of diabetes that may contribute to problems during pregnancy and birth. They may not process dietary carbohydrates and sugars efficiently, and are more likely to be resistant to the hormone insulin, released by the pancreas after eating, helping muscles use blood glucose (sugar) for energy. Glucose circulates in the blood for longer, providing excess energy to the growing baby. There is an increased risk of developing diabetes in pregnancy and women may have low levels of inflammatory hormones and proteins circulating in the body. Improving diet and increasing exercise have had a very small effect on reducing weight gain during pregnancy and no effect on complications.
Metformin, a drug used to treat diabetes, reduces the amount of glucose the liver releases into the blood and makes the body more sensitive to insulin. Metformin may help a woman's body use insulin more effectively and reduce the chance that her baby will grow large‐for‐gestational age.
What evidence did we find?
We searched for evidence (October 2017) and found three randomised controlled studies (1099 pregnant women) comparing metformin tablets with placebo (dummy) tablets taken by mouth from 10 to 20 weeks of pregnancy until birth. The studies involved women with obesity; we therefore could not assess the effect of metformin in women who are overweight.
Women who were given metformin or placebo during pregnancy had a similar risk of a baby being born large‐for‐gestational age (measured in weeks since last period). Metformin probably makes little or no difference in the risk of women developing gestational diabetes. Metformin may also have little or no difference in the risk of women developing gestational hypertension (high blood pressure) or pre‐eclampsia.
Women who were given metformin may gain slightly less weight during pregnancy, but are more likely to experience diarrhoea. There were no other important differences identified for other maternal outcomes including, caesarean birth, giving birth before 37 weeks of pregnancy, shoulder dystocia (a birth complication where the baby’s shoulder gets stuck), perineal trauma (damage to the area between the woman’s vagina and the anus), or heavy bleeding after the baby has been born.
Babies of women who were given metformin had similar birthweight to babies of women who were given placebo. We did not identify any other important differences for other infant outcomes of interest: hypoglycaemia (low blood sugar); hyperbilirubinaemia (jaundice); Apgar score at five minutes (a measure of newborn well‐being); or death of the baby before or after being born. One study reported similar rates of admission to neonatal intensive care between groups.
What does this mean?
There is insufficient evidence to support the use of metformin for women with obesity in pregnancy for improving outcomes for the mother and her baby. Metform was associated with increased risk of adverse effects, particularly diarrhoea.
A small number of studies are included in this review and no study included women categorised as 'overweight', or looked at metformin in combination with another treatment.
More research is needed to evaluate the role of metformin in pregnant women with obesity or who are overweight, as a strategy for improving maternal and infant health, either alone or as an additional intervention.
Summary of findings
Summary of findings for the main comparison. Metformin compared to placebo for women with obesity during pregnancy.
| Metformin compared to placebo for women with obesity during pregnancy | ||||||
| Patient or population: women with obesity during pregnancy Setting: antenatal clinics in the UK (2 trials) and Egypt (1 trial) Intervention: metformin tablets taken orally Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with metformin | |||||
| Large‐for‐gestational‐age infant, defined as birthweight ≥ 90th percentile for gestational age and infant sex | Study population | RR 0.95 (0.70 to 1.30) | 831 (2 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 164 per 1000 | 156 per 1000 (115 to 213) | |||||
| Gestational weight gain (kg) | The mean gestational weight gain across control groups ranged from 6.4 kg to 11.61 kg | MD 2.60 kilograms lower (5.29 lower to 0.10 higher) | ‐ | 899 (3 RCTs) | ⊕⊕⊝⊝ LOW a, b | |
| Gestational hypertension | Study population | Average RR 1.02 (0.54 to 1.94) | 1040 (3 RCTs) | ⊕⊕⊝⊝ LOW a, b | ||
| 64 per 1000 | 65 per 1000 (34 to 124) | |||||
| Pre‐eclampsia | Study population | Average RR 0.74 (0.09 to 6.28) | 840 (2 RCTs) | ⊕⊕⊝⊝ LOW b, c | ||
| 60 per 1000 | 44 per 1000 (5 to 376) | |||||
| Gestational diabetes (as defined by the trial authors) | Study population | RR 0.85 (0.61 to 1.19) | 892 (3 RCTs) | ⊕⊕⊕⊝ MODERATE a | ||
| 143 per 1000 | 121 per 1000 (87 to 170) | |||||
| Adverse effects associated with the treatment: diarrhoea | Study population | RR 2.34 (1.74 to 3.14) | 797 (2 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 126 per 1000 | 295 per 1000 (220 to 396) | |||||
| Birthweight (g) | The mean birthweight across control groups ranged from 3341 g to 3463 g | MD 6.39 grams higher (81.15 lower to 93.92 higher) | ‐ | 834 (2 RCTs) | ⊕⊕⊕⊕ HIGH | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded (‐1) for study limitations. One study of the three studies included has unclear risk of bias for random sequence generation, allocation concealment, performance bias, outcome assessor bias and selective reporting bias and reports a much greater effect than the other two studies. bSubstantial heterogeneity ‐ downgraded for inconsistency (‐1). cDowngraded (‐1) for imprecision ‐ wide confidence intervals crossing the line of no effect.
Background
Description of the condition
Obesity and being overweight affects more than 1.9 billion adults (WHO 2017), 41 million children under the age of five years (WHO 2013), and 270 million children aged five to 17 years (Lobstein 2016), across the globe. 'Overweight' is defined as an individual with body mass index (BMI) between 25.0 kg/m2 and 29.9 kg/m2, while obesity is defined as a BMI above 30.0 kg/m2 (WHO 2000). Obesity and being overweight represents a significant disease burden, contributing to hypertension, cardiovascular disease and diabetes, and their complications (Ezzati 2002). Furthermore, obesity and being overweight have been estimated to contribute to more than 5% of deaths globally each year (WHO 2009).
Obesity and being overweight are associated with significant economic implications, both for the individual and society (Wang 2011), accounting for 36 million disability‐adjusted life years (DALYs). Medical costs for individuals with obesity are estimated to be 30% higher than for individuals with a normal BMI (Withrow 2009), and contribute to increasing health and medical expenditure at a population level (Thompson 2001; Wang 2008).
Obesity and being overweight in pregnancy affects approximately 50% of women across low‐income nations (Chu 2009; Ng 2013; Scheil 2015); and is associated with a range of well recognised maternal and infant health complications. Maternal risks include gestational hypertension, pre‐eclampsia (disorders of blood pressure in pregnancy), and gestational diabetes; women are more likely to have their labour induced, and to birth by caesarean section (Callaway 2006; Dodd 2011). Infants born to women with obesity or who are overweight in pregnancy have a higher risk of being of high birthweight or being large‐for‐gestational age, and of associated complications, including shoulder dystocia (difficulty in the delivery of the infant's shoulders), admission to neonatal intensive care units (NICUs) and the need for treatment of jaundice (yellow pigmentation of infant's skin or eyes due to a build up of bilirubin in the blood) and hypoglycaemia (low blood sugar levels) (Cedergren 2004; Dodd 2011; Ehrenberg 2004; Sebire 2001; Weiss 2004; Yu 2006). It has been estimated that the costs of providing antenatal and postpartum care for women who are overweight are increased by 23% when compared with women of normal BMI, increasing further to 37% for women with obesity (Morgan 2014).
There has been considerable interest in providing antenatal dietary and lifestyle advice for women with obesity or who are overweight during pregnancy, as a strategy to limit gestational weight gain and improve maternal and infant health. However, such antenatal interventions appear to have a modest effect on gestational weight gain and other clinical pregnancy and birth outcomes (i‐WIP Collaborative Group 2017), and additional strategies are required.
Description of the intervention
Metformin is an oral insulin‐sensitising medication that acts to decrease blood glucose concentrations. It inhibits pathways in the liver that stimulate glucose production and also acts to increase glucose uptake into skeletal muscle and fat cells (Bailey 1996; Cusi 1998; Hundal 2000; Inzucchi 1998; Large 1999; Perriello 1994; Wiernsperger 1999). Metformin is commonly used in the treatment of type 2 diabetes mellitus and polycystic ovarian syndrome (Levri 2005), and is being used increasingly in the treatment of gestational diabetes, having been shown to result in decreased rates of neonatal hypoglycaemia and no increased risk of adverse maternal outcomes when compared with insulin (Priya 2018). A Cochrane Review aimed to evaluate antenatal interventions for reducing weight in women with obesity for improving outcomes, however, no randomised controlled trials were identified (Furber 2013).
How the intervention might work
Metformin may be an adjuvant therapy to current antenatal strategies in pregnant women with obesity or who are overweight, acting to reduce glucose production in the liver and improve glucose uptake in smooth muscle cells, and therefore improve the overall metabolic health of women in pregnancy. Metformin also has weight loss effects, attributed to decreased net caloric intake, most likely through appetite suppression (Kirpichnikov 2002). Importantly, adverse effects related to metformin are uncommon, with no reported associations with birth defects (Gilbert 2006; Hawthorne 2006; Lilja 2006), and being increasingly used to treat gestational diabetes (Balsells 2015).
Why it is important to do this review
The role of metformin in pregnancy for women with obesity or who are overweight, and its impact on maternal and infant health outcomes is required. To our knowledge, there are no published systematic reviews of the use of metformin during pregnancy for women with obesity or who are overweight.
Objectives
To evaluate the role of metformin in pregnant women with obesity or who are overweight, on maternal and infant outcomes, including adverse effects of treatment and costs.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials and quasi‐randomised trials that evaluate metformin use (compared with placebo or no metformin) in women with obesity or who are overweight in pregnancy for improving outcomes, alone or in combination with other interventions were eligible for inclusion. Trials published in abstract form were eligible for inclusion.
In future updates, trials using a cluster‐randomised trials as well as any relevant arms of any multi‐armed trials will be eligible for inclusion. Trials using a cross‐over design were not considered for inclusion.
Types of participants
Pregnant women with obesity or who are overweight, defined as women with booking or early pregnancy or pre‐pregnancy body mass index (BMI) ≥ 25.0 kg/m2 and excluding women with pre‐existing diabetes or polycystic ovarian syndrome.
Types of interventions
Metformin versus placebo or no metformin (alone or in combination with other interventions).
Types of outcome measures
Primary outcomes
Large‐for‐gestational‐age infant, defined as birthweight ≥ 90th percentile for gestational age and infant sex
Secondary outcomes
For the woman
Gestational weight gain
Gestational hypertension
Pre‐eclampsia
Gestational diabetes, as defined by the trial authors
Preterm birth, defined as birth before 37 weeks
Preterm birth, defined as birth before 34 weeks
Preterm premature rupture of membranes, defined as rupture of membranes before 37 completed weeks of pregnancy
Induction of labour
Vaginal birth
Caesarean birth
Shoulder dystocia
Third‐ or fourth‐degree perineal tear
Adverse effects associated with treatment, including nausea, vomiting, diarrhoea
Postpartum haemorrhage, as defined by the trial authors
Postpartum infectious morbidity
Venous thromboembolic event
High‐dependency unit admission
Pregnancy‐related maternal death, defined as during pregnancy or within 42 days of conclusion of pregnancy
Quality of life, as defined by the trial authors
For the infant
Birthweight (g)
Birthweight < 2500 g
Birthweight ≥ 4000 g
Hypoglycaemia requiring treatment
Hyperbilirubinaemia requiring treatment
Birth trauma, as defined by the trial authors
Apgar less than 7 at five minutes
Neonatal intensive care unit (NICU) admission (including neonatal unit)
Perinatal death (i. stillbirth, defined as fetal death ≥ 20 weeks' gestation and before birth is completed, that is not due to an induced termination; ii. neonatal death, defined as death of liveborn infant < 28 days old)
Breastfeeding at discharge
Costs to the health services
Antenatal admission to hospital, length of stay
Length of stay in high‐dependency unit or NICU
Search methods for identification of studies
The search methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth's Trials Register by contacting their Information Specialist (11 October 2017).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. For full current search methods used to populate Pregnancy and Childbirth's Trials Register, including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about Cochrane Pregnancy and Childbirth in the Cochrane Library and select the 'Specialized Register' section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth's Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (11 October 2017) (see: Appendix 1 for full search methods used).
Searching other resources
We searched the reference lists of retrieved studies. We did not apply any language or date restrictions.
Data collection and analysis
The methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors (AD and RG) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author (JD).
Data extraction and management
We designed a form to extract data (JD). Data extracted also included sources of trial funding, trial dates and trial authors' declarations of interest. For eligible studies, at least two review authors extracted the data (AD and RG) using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author (JD). We entered data into Review Manager 5 software (Review Manager 2014), and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias (AD and RG) for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third review author (JD).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies are at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or could be supplied by the trial authors, we planned to reinclude missing data in the analyses which we undertake.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups with level of missing data < 20%);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation; level of missing data ≥ 20%);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this review we assessed the quality of the evidence using the GRADE approach, as outlined in the GRADE handbook, in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison (metformin versus placebo).
Large‐for‐gestational‐age infant, defined as birthweight ≥ 90th percentile for gestational age and infant sex
Gestational diabetes, as defined by trial authors
Birthweight (g)
Pre‐eclampsia
Gestational hypertension
Gestational weight gain
Diarrhoea
We used GRADEpro Guideline Development Tool to import data from Review Manager 5 in order to create 'Summary of findings' tables (Review Manager 2014). We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CIs).
Continuous data
For continuous data, we used the mean difference (MD) if outcomes are measured in the same way between trials. We used the standardised mean difference (SMD) to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised controlled trials for inclusion in this version of the review. If we identify any for inclusion in subsequent updates, we will include them in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We did not include cross‐over trials in this review.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants will be analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the I² and Chi² statistics and Tau2. We regarded heterogeneity as substantial if I² is greater than 30% and either Tau2 is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (Review Manager 2014). We used a fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies are estimating the same underlying treatment effect, i.e. where trials are examining the same intervention, and the trials' populations and methods were judged to be sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects might differ between trials, or if substantial statistical heterogeneity was detected, we used a random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. We then treated the random‐effects summary as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful we did not combine trials.
Where we used random‐effects analyses, we presented the results as the average treatment effect with 95% CIs, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we had planned to investigate it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses. The currently included studies were similar in BMI inclusion criteria and gestational age at enrolment.
Obesity subclass (BMI 30.0 to 34.9 kg/m2 versus BMI 35.0 to 39.9 kg/m2 versus BMI ≥ 40 kg/m2).
Time of commencing therapy (trimester 1 versus trimester 2 and later).
The following outcome will be used in subgroup analysis.
Large‐for‐gestational age, defined as birthweight > 90 percentile for gestational age and infant sex.
In future updates we plan to assess subgroup differences by interaction tests available within Review Manager (Review Manager 2014). We planned to report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analysis to explore the effects of trial quality assessed by allocation concealment and other risk of bias components, by omitting studies rated as high risk of bias for these components, however there were too few included studies. We will carry out sensitivity analysis to investigate the effect of randomisation unit in future updates of this review if we identify any cluster‐randomised trials. We will restrict sensitivity analysis to the primary outcome if we can include sufficient studies in future updates of this review.
Results
Description of studies
Results of the search
The search retrieved 37 reports in total (see Figure 1). We screened out 24 reports. Three studies (10 reports) were eligible for inclusion. Two studies (three reports) are ongoing (see Characteristics of ongoing studies).
1.
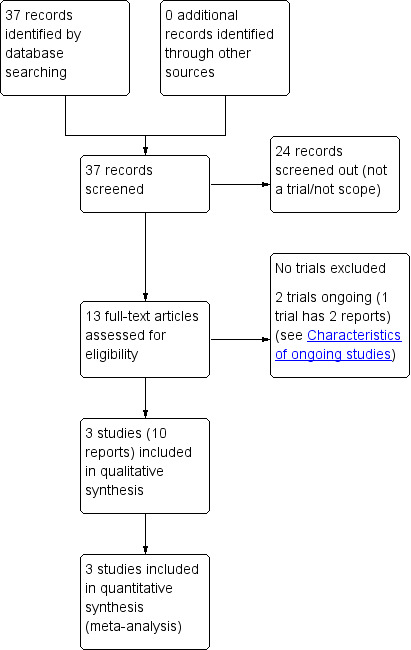
Study flow diagram.
We did not identify any studies reported as abstracts only. We did not identify any studies of metformin in combination with another treatment. We did not identify any cluster‐randomised trials.
Included studies
We included three studies involving 1099 randomised participants, with outcomes available for 1034 participants (Chiswick 2015; PACTR201505001142202; Syngelaki 2016). Chiswick 2015 enrolled women from February 2011 to January 2014, Syngelaki 2016 enrolled women from October 2010 to June 2015. The PACTR201505001142202 trial report did not include the dates of enrolment.
Design
All three included studies were reported to be randomised controlled trials (Chiswick 2015; PACTR201505001142202; Syngelaki 2016).
Sample size
The available sample size ranged from 200 in the PACTR201505001142202 trial to 450 women in the Chiswick 2015 and Syngelaki 2016 trials.
Setting
One trial was conducted in a maternity hospital in Alexandria, Egypt (PACTR201505001142202). The remaining two studies were conducted in National Health System (NHS) hospitals in the UK (Chiswick 2015; Syngelaki 2016).
Participants
The study conducted in Egypt included women with BMI ≥ 35 kg/m2 (PACTR201505001142202). Chiswick and colleagues included pregnant women with BMI ≥ 30.0 kg/m2, and who were identified to have a normal glucose tolerance test; exclusion criteria included women who were non‐white, who had a previous history of gestational diabetes, a small baby, or early pre‐eclampsia (Chiswick 2015). Syngelaki and colleagues included pregnant women with BMI (measured at 12 to 18 weeks of pregnancy) ≥ 35 kg/m2 (Syngelaki 2016). There were no studies that included women who were overweight, classified as a BMI of 25.0 kg/m2 to 29.9 kg/m2.
Interventions and comparisons
All three included studies compared metformin with placebo. Metformin was started in the early second trimester in all studies. Doses ranged from 500 mg twice a day (total 1 g/day) in the PACTR201505001142202 trial to 3 g per day in the Syngelaki 2016 trial. The Chiswick 2015 trial utilised a maximum metformin dose of 2.5 g/day in two to three divided doses, titrated from a starting dose of 500 mg/day.
Outcomes
Outcome reporting across the three included studies varied. The PACTR201505001142202 trial reported gestational hypertension, gestational diabetes, and infant birthweight only. The primary outcome for the Chiswick 2015 trial was birthweight z‐score corresponding to the gestational age, parity, and sex‐standardised birthweight percentile of liveborn babies delivered at 24 or more weeks' gestation. This trial also reported a range of secondary outcomes for women and infants, including biochemical and inflammatory markers such as glucose, insulin, and lipids, and measures of maternal body composition. The Syngelaki 2016 trial utilised a similar primary outcome relating to infant birthweight z‐score, and also reported a range of secondary clinical outcomes.
Funding sources
One trial did not report information on funding sources (PACTR201505001142202). The Chiswick 2015 trial was funded by a grant from the Medical Research Council, National Institute for Health Research (NIHR) and Tommy's, the baby charity. The funding bodies had no role in any part of the study design, conduct or data collection and analysis. The Syngelaki 2016 trial was funded by a grant from the Fetal Medicine Foundation.
Declarations of interest (reported by the trialists)
The authors of the included studies declared having no conflicts of interest.
Excluded studies
There are no excluded studies in this review.
Risk of bias in included studies
For a summary of our risk of bias assessments please see Figure 2 and Figure 3.
2.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
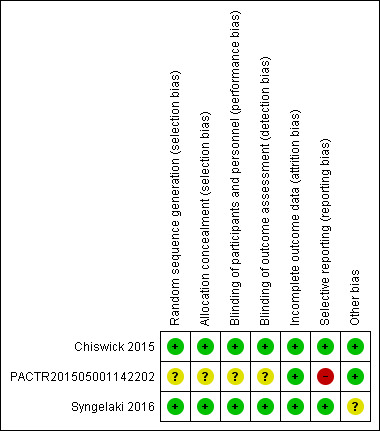
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed the risk of bias as low for allocation concealment in two studies (Chiswick 2015; Syngelaki 2016), and unclear risk in one study (PACTR201505001142202).
Blinding
We assessed two studies as low risk of bias as they described their studies as blinded and used an identically appearing placebo (Chiswick 2015; Syngelaki 2016), with the Chiswick 2015 study stating "Participants, caregivers, and study personnel were masked to treatment assignment". It is unclear if the study by PACTR201505001142202 was blinded.
Incomplete outcome data
We assessed the overall risk of attrition bias as low, with data available on 94% of the women enrolled in the three included studies (Chiswick 2015; PACTR201505001142202; Syngelaki 2016).
Selective reporting
We assessed risk of selective reporting bias as low for two studies with available trial registrations (Chiswick 2015; Syngelaki 2016). We assessed the PACTR201505001142202 study as having a high risk of reporting bias because one of two specified outcomes was not reported.
Other potential sources of bias
We did not detect any other potential sources of bias in two studies (Chiswick 2015; PACTR201505001142202), and decided it was unclear for one study (Syngelaki 2016); this study's trial registration occurred after recruitment commenced.
Effects of interventions
See: Table 1
Metformin versus placebo
Primary outcomes
Large‐for‐gestational‐age infant
Women who received metformin or placebo had a similar risk of their baby being born with a birthweight considered to be large for his or her gestational age (> 90th centile for gestational age and infant sex) (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.70 to 1.30; 2 studies, 831 infants, I2 = 0%; high‐quality evidence; Analysis 1.1).
1.1. Analysis.
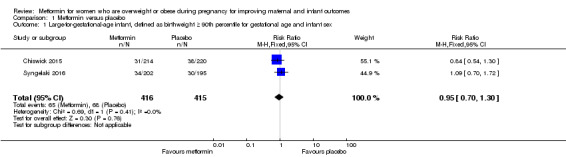
Comparison 1 Metformin versus placebo, Outcome 1 Large‐for‐gestational‐age infant, defined as birthweight ≥ 90th percentile for gestational age and infant sex.
Secondary outcomes (maternal)
Gestational weight gain
Women who received metformin may have a slightly lower gestational weight gain compared with women who received placebo (mean difference (MD) ‐2.60 kg, 95% CI ‐5.29 to 0.10 kg; 3 studies, 899 women, random‐effects, I2 = 96%, Tau2 = 5.41; low‐quality evidence; Analysis 1.2), although there was a high degree of heterogeneity between the two included trials.
1.2. Analysis.
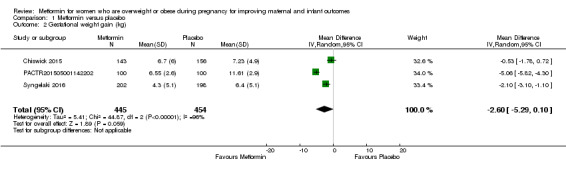
Comparison 1 Metformin versus placebo, Outcome 2 Gestational weight gain (kg).
Gestational hypertension
There may be little or no difference in risk of gestational hypertension between women who received metformin or placebo (average RR 1.02, 95% CI 0.54 to 1.94; 3 studies, 1040 women, I2 = 38%, Tau2 = 0.12; low‐quality evidence; Analysis 1.3). There was a high degree of heterogeneity between the three included trials.
1.3. Analysis.
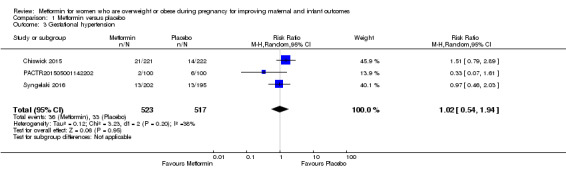
Comparison 1 Metformin versus placebo, Outcome 3 Gestational hypertension.
Pre‐eclampsia
Metformin may make little or no difference in the risk of women developing pre‐eclampsia (average RR 0.74, 95% CI 0.09 to 6.28; 2 studies, 840 women, random‐effects, I2 = 86%, Tau2 = 2.06; low‐quality evidence; Analysis 1.4), although there was a high degree of heterogeneity between the two included trials.
1.4. Analysis.
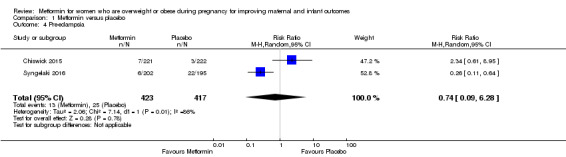
Comparison 1 Metformin versus placebo, Outcome 4 Pre‐eclampsia.
Gestational diabetes, as defined by the trial authors
There is probably little or no difference in the rates of gestational diabetes between groups of women who received metformin or placebo (RR 0.85, 95% CI 0.61 to 1.19; 3 studies, 892 women, I2 = 16%; moderate‐quality evidence; Analysis 1.5).
1.5. Analysis.
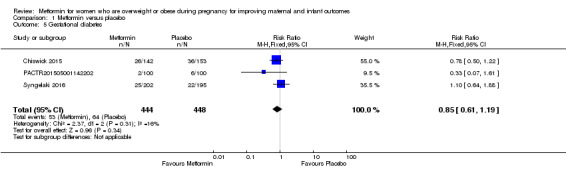
Comparison 1 Metformin versus placebo, Outcome 5 Gestational diabetes.
Preterm birth, defined as birth before 37 weeks
There were no clear differences in the risk of a baby being born before 37 weeks of pregnancy for women who received metformin compared with women who received placebo (average RR 0.89, 95% CI 0.41 to 1.93; 2 studies, 831 women, random‐effects, I2 = 63%, Tau2 = 0.20; Analysis 1.6). We observed a high level of heterogeneity.
1.6. Analysis.
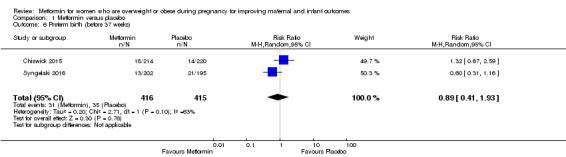
Comparison 1 Metformin versus placebo, Outcome 6 Preterm birth (before 37 weeks).
Preterm birth, defined as birth before 34 weeks
This outcome was not reported in the included studies.
Preterm premature rupture of membranes, defined as rupture of membranes before 37 completed weeks of pregnancy
The rate of preterm premature rupture of membranes was similar for women who received metformin (4/202) and those women who received placebo (6/198) (RR 0.65, 95% CI 0.19 to 2.28; 1 study, 400 women; Analysis 1.7).
1.7. Analysis.
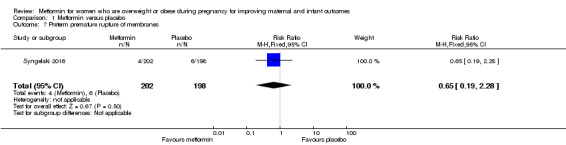
Comparison 1 Metformin versus placebo, Outcome 7 Preterm premature rupture of membranes.
Induction of labour
Women who received metformin (78/214) or placebo (96/221) had similar rates of induction of labour (RR 0.84, 95% CI 0.67 to 1.06; 1 study, 435 women; Analysis 1.8).
1.8. Analysis.
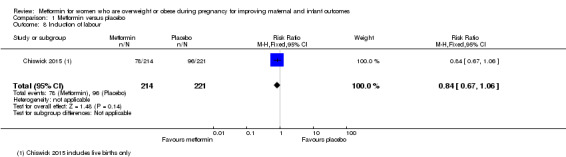
Comparison 1 Metformin versus placebo, Outcome 8 Induction of labour.
Vaginal birth
The rate of vaginal birth was similar for women who received metformin (257/416) and women who received placebo (244/416) (RR 1.06, 95% CI 0.95 to 1.18; 2 studies, 832 women, I2 = 0%; Analysis 1.9).
1.9. Analysis.
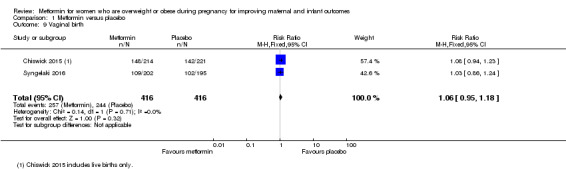
Comparison 1 Metformin versus placebo, Outcome 9 Vaginal birth.
Caesarean birth
The rate of caesarean birth was similar for women who received metformin (145/421) and women who received placebo (158/417) (RR 0.91, 95% CI 0.76 to 1.08; 2 studies, 838 women, I2 = 0%; Analysis 1.10).
1.10. Analysis.
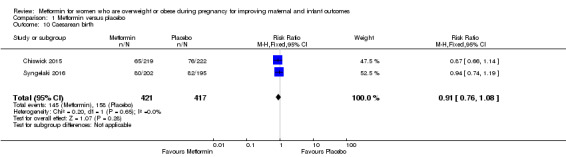
Comparison 1 Metformin versus placebo, Outcome 10 Caesarean birth.
Shoulder dystocia
The risk of shoulder dystocia was similar for women who received metformin (2/418) or placebo (4/418) (RR 0.54, 95% CI 0.12 to 2.53; 2 studies, 846 women, I2 = 0%; Analysis 1.11).
1.11. Analysis.
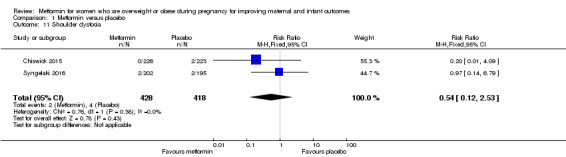
Comparison 1 Metformin versus placebo, Outcome 11 Shoulder dystocia.
Third‐ or fourth‐degree perineal tear
There were similar rates of third‐ or fourth‐degree perineal tear for women who received metformin (2/202) or placebo (1/195) (RR 1.93, 95% CI 0.18 to 21.12; 1 study, 397 women; Analysis 1.12).
1.12. Analysis.
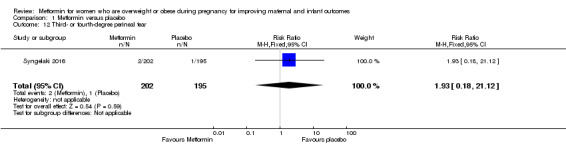
Comparison 1 Metformin versus placebo, Outcome 12 Third‐ or fourth‐degree perineal tear.
Adverse effects associated with treatment, including nausea, vomiting, diarrhoea
Adverse effects (at least one)
Compared to women who received placebo, women in the metformin group were more likely to experience at least one adverse effect (RR 1.63, 95% CI 1.27 to 2.08; 1 study, 400 women; Analysis 1.13).
1.13. Analysis.
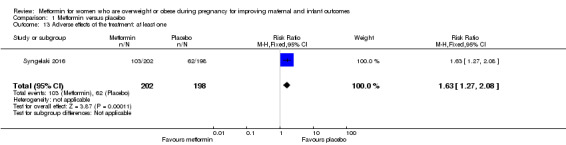
Comparison 1 Metformin versus placebo, Outcome 13 Adverse effects of the treatment: at least one.
Adverse effects ‐ abdominal pain
The number of women who reported abdominal pain during the studies did not differ between women receiving metformin (63/401) or placebo (56/396) (RR 1.12, 95% CI 0.81 to 1.54; 2 studies, 797 women, I2 = 0%; Analysis 1.14).
1.14. Analysis.
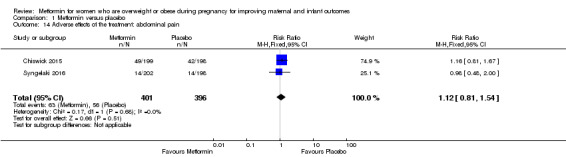
Comparison 1 Metformin versus placebo, Outcome 14 Adverse effects of the treatment: abdominal pain.
Adverse effects ‐ diarrhoea
Women receiving metformin were more likely to report experiencing diarrhoea (118/401) during study participation compared with women receiving placebo (50/396) (RR 2.34, 95% CI 1.74 to 3.14; 2 studies, 797 women, I2 = 0%; high‐quality evidence; Analysis 1.15).
1.15. Analysis.
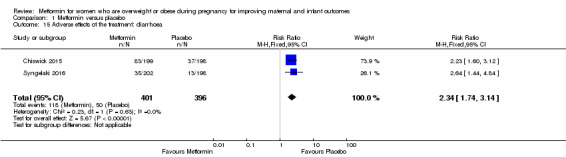
Comparison 1 Metformin versus placebo, Outcome 15 Adverse effects of the treatment: diarrhoea.
Adverse effects ‐ headache
The number of women who reported headache during the studies was similar for women receiving metformin (86/401) or placebo (76/396) (RR 1.32, 95% CI 0.64 to 2.70; 2 studies, 797 women, random‐effects, I2 = 72%, Tau2 = 0.020; Analysis 1.16).
1.16. Analysis.
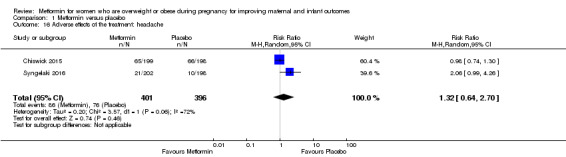
Comparison 1 Metformin versus placebo, Outcome 16 Adverse effects of the treatment: headache.
Postpartum haemorrhage, as defined by the trial authors
There were similar rates of postpartum haemorrhage between the group of women who received metformin (39/414) or placebo (37/411) (RR 1.05, 95% CI 0.68 to 1.61; 2 studies, 825 women, I2 = 0%; Analysis 1.17).
1.17. Analysis.
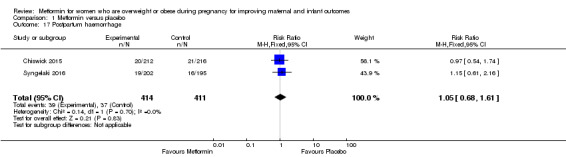
Comparison 1 Metformin versus placebo, Outcome 17 Postpartum haemorrhage.
Postpartum infectious morbidity
This outcome was not reported in the included studies.
Venous thromboembolic event
This outcome was not reported in the included studies.
High‐dependency unit admission
This outcome was not reported in the included studies.
Pregnancy‐related maternal death, defined as during pregnancy or within 42 days of conclusion of pregnancy
This outcome was not reported in the included studies.
Quality of life, as defined by the trial authors
This outcome was not reported in the included studies.
Secondary outcomes (infant)
Birthweight (g)
There was little or no difference in infant birthweight for the babies of women who received metformin or placebo (MD 6.39 g, 95% CI ‐81.15 to 93.92 g; 2 studies, 834 infants; high‐quality evidence; Analysis 1.18).
1.18. Analysis.
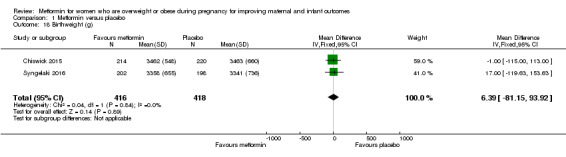
Comparison 1 Metformin versus placebo, Outcome 18 Birthweight (g).
Birthweight < 2500 g
This outcome was not reported in the included studies.
Birthweight ≥ 4000 g
This outcome was not reported in the included studies.
Hypoglycaemia requiring treatment
The rate of neonatal hypoglycaemia was similar for babies of women who received metformin (9/202) or placebo (11/195) (RR 0.79, 95% CI 0.33 to 1.86; 1 study, 397 women; Analysis 1.19).
1.19. Analysis.
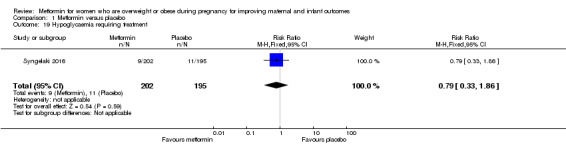
Comparison 1 Metformin versus placebo, Outcome 19 Hypoglycaemia requiring treatment.
Hyperbilirubinaemia requiring treatment
The rate of neonatal hyperbilirubinaemia was similar for babies of women who received metformin (11/202) or placebo (15/195) (RR 0.71, 95% CI 0.33 to 1.50; 1 study, 397 babies; Analysis 1.20).
1.20. Analysis.
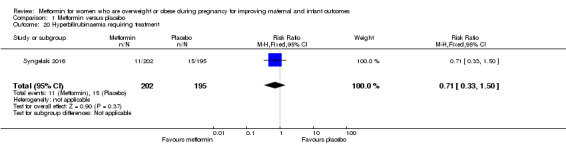
Comparison 1 Metformin versus placebo, Outcome 20 Hyperbilirubinaemia requiring treatment.
Birth trauma, as defined by the trial authors
The number of babies with birth trauma was similar for babies of women who received metformin or placebo (RR 0.97, 95% CI 0.20 to 4.73; 1 study, 397 babies; Analysis 1.21).
1.21. Analysis.
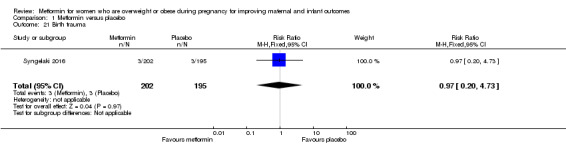
Comparison 1 Metformin versus placebo, Outcome 21 Birth trauma.
Apgar less than 7 at five minutes
There were a similar number of babies with an Apgar score of less than 7 at five minutes in the metformin (1/202) and placebo (3/195) groups (RR 0.32, 95% CI 0.03 to 3.07; 1 study, 397 babies; Analysis 1.22).
1.22. Analysis.
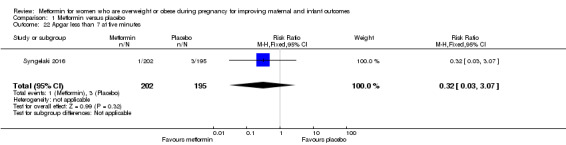
Comparison 1 Metformin versus placebo, Outcome 22 Apgar less than 7 at five minutes.
Neonatal intensive care unit (NICU) admission (including neonatal unit)
There were similar numbers of babies admitted to the NICU or neonatal unit in the metformin (11/202) and placebo groups (14/195) (RR 0.76, 95% CI 0.35 to 1.63; 1 study, 397 babies; Analysis 1.23).
1.23. Analysis.
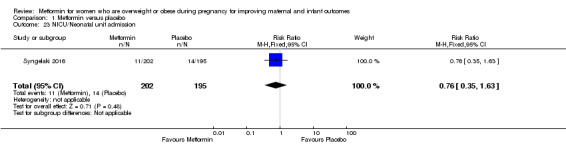
Comparison 1 Metformin versus placebo, Outcome 23 NICU/Neonatal unit admission.
Perinatal death
Stillbirth (fetal death ≥ 20 weeks' gestation and before birth is completed, that is not due to an induced termination)
The risk of stillbirth was similar between the metformin (3/423) and placebo (2/420) groups; (RR 1.39, 95% CI 0.28 to 6.97; 2 studies, 846 babies, I2 = 30%; Analysis 1.24).
1.24. Analysis.
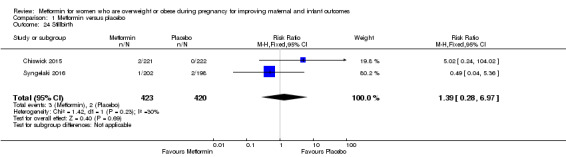
Comparison 1 Metformin versus placebo, Outcome 24 Stillbirth.
Neonatal death (death of liveborn infant < 28 days old)
The risk of an infant dying within their first 28 days (neonatal death) was similar for babies of women who received metformin (1/416) or placebo (3/418) (RR 0.43, 95% CI 0.06 to 2.91; 2 studies, 834 babies, I2 = 0%; Analysis 1.25).
1.25. Analysis.
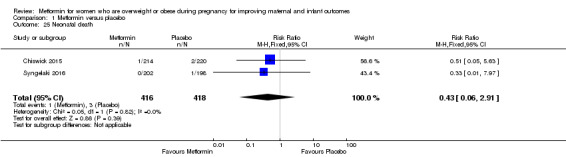
Comparison 1 Metformin versus placebo, Outcome 25 Neonatal death.
Breastfeeding at discharge
This outcome was not reported in the included studies.
Secondary outcomes (costs to the health services)
Antenatal admission to hospital, length of stay
This outcome was not reported in the included studies.
Length of stay in high‐dependency unit or NICU
This outcome was not reported in the included studies.
Discussion
Summary of main results
Three studies met our criteria for inclusion in the review; all of the identified trials contributed data to some of the analyses, these trials had a combined sample size of 1099 women (with outcome data available for 1034 participants). All of the included studies compared oral metformin therapy with placebo in pregnant women with obesity.
Women who received metformin or placebo had a similar risk of their baby being born large for his or her gestational age. Overall, for pregnant women with obesity, the administration of metformin appears to be associated with slightly lower gestational weight gain, but an increased risk of experiencing diarrhoea. However, there were no other clear differences identified in the reporting of metformin‐related side effects
Overall, there were few clear differences between women receiving metformin and women receiving placebo, reflecting in part, the small number of studies contributing data and the resultant insufficient evidence to identify differences between groups.
Long‐term follow‐up of maternal and child participants was lacking in the included trials, and will be necessary to inform any evidence of benefits or harms on outcomes beyond the immediate neonatal period.
Overall completeness and applicability of evidence
The applicability of findings from this systematic review and meta‐analysis in pregnant women with obesity receiving metformin during pregnancy is broadly consistent with the findings reported in the individual included trials. To our knowledge, there have been no other systematic reviews conducted and reported in the literature on this topic involving pregnant women with obesity or who are overweight.
The individual trial characteristics highlight the variation in inclusion criteria relating to maternal body mass index (BMI), the dose of metformin administered and consistency in the definitions of outcomes reported. Invariably, the available information is limited by the characteristics of the included studies. The included studies randomised women with BMI ≥ 30 kg/m2, the findings therefore can only be considered for this group of women and not women who would be classified as overweight (BMI 24 kg/m2 to 29.9 kg/m2 inclusive).
The longer‐term effects of exposure to metformin during pregnancy does not suggest an increased risk of harms (Gilbert 2006; Hawthorne 2006; Lilja 2006), although the publication of trials included in this review are recent, and outcomes beyond the immediate neonatal period are to date unavailable.
Quality of the evidence
Overall, we assessed two of the three included studies as being at low risk of bias overall (Chiswick 2015; Syngelaki 2016), with insufficient information available to reliably assess the third (PACTR201505001142202). Two of the trials were at low risk of bias with adequate sequence generation and allocation concealment, and utilised an adequate placebo, thereby ensuring adequate blinding (Chiswick 2015; Syngelaki 2016). The Syngelaki 2016 study was registered after recruitment of participants had commenced.
The GRADE quality of evidence for the effect of metformin on risk of large‐for‐gestational‐age infants, and maternal side effects of the intervention (diarrhoea) was high. We graded the evidence as moderate for gestational diabetes. In contrast, we graded the evidence for gestational weight gain, gestational hypertension and pre‐eclampsia as low quality. Downgrading decisions were based on study design limitations, imprecision, and inconsistency. See Table 1.
Potential biases in the review process
We acknowledge that there is the potential for bias at all stages of performing a systematic review. We attempted to minimise bias in a number of ways; for example, two review authors independently carried out data extraction and assessed risk of bias.
Agreements and disagreements with other studies or reviews
As highlighted above, we are aware of no other systematic reviews conducted and reported in the literature on this topic involving pregnant women with obesity or who are overweight.
Authors' conclusions
Implications for practice.
Women who received metformin or placebo had a similar risk of their baby being born large for his or her gestational age. Overall, for pregnant women with obesity, the administration of metformin appears to be associated with slightly lower gestational weight gain, but an increased risk of experiencing diarrhoea.
Overall, there is insufficient evidence to support the use of metformin for women with obesity during pregnancy to improve maternal and infant outcomes. The quality of the evidence in this review varied from high to low, with downgrading decisions based on study design limitations, imprecision and inconsistency. There were only a small number of studies included in this review. Furthermore, none of the included studies included women categorised as 'overweight' and no trials looked at metformin in combination with another treatment.
Implications for research.
Future research is required to further evaluate the role of metformin therapy in pregnant women with obesity or who are overweight as a strategy to improve maternal and infant health, alone or as an adjuvant to dietary and lifestyle advice. Future studies could also include women who are classified as overweight (BMI 25 kg/m2 to 29.9 kg/m2).
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team) and the Group's Statistical Adviser.
The National Institute for Health Research (NIHR) provides infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Sevice (NHS) or the Department of Health.
Appendices
Appendix 1. Search methods for ICTRP and ClinicalTrials.gov
ICTRP
Each line was run separately
metformin AND pregnancy AND obes*
metformin AND pregnancy AND overweight
metformin AND pregnancy AND weight
ClinicalTrials.gov
Advanced search, Intervention studies
Intervention = metformin
Condition = obesity, overweight
Other terms = pregnancy
Data and analyses
Comparison 1. Metformin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Large‐for‐gestational‐age infant, defined as birthweight ≥ 90th percentile for gestational age and infant sex | 2 | 831 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.70, 1.30] |
| 2 Gestational weight gain (kg) | 3 | 899 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐5.29, 0.10] |
| 3 Gestational hypertension | 3 | 1040 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.54, 1.94] |
| 4 Pre‐eclampsia | 2 | 840 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.09, 6.28] |
| 5 Gestational diabetes | 3 | 892 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.19] |
| 6 Preterm birth (before 37 weeks) | 2 | 831 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.93] |
| 7 Preterm premature rupture of membranes | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.19, 2.28] |
| 8 Induction of labour | 1 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.67, 1.06] |
| 9 Vaginal birth | 2 | 832 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.18] |
| 10 Caesarean birth | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.08] |
| 11 Shoulder dystocia | 2 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.12, 2.53] |
| 12 Third‐ or fourth‐degree perineal tear | 1 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.18, 21.12] |
| 13 Adverse effects of the treatment: at least one | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.27, 2.08] |
| 14 Adverse effects of the treatment: abdominal pain | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.81, 1.54] |
| 15 Adverse effects of the treatment: diarrhoea | 2 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [1.74, 3.14] |
| 16 Adverse effects of the treatment: headache | 2 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.64, 2.70] |
| 17 Postpartum haemorrhage | 2 | 825 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.68, 1.61] |
| 18 Birthweight (g) | 2 | 834 | Mean Difference (IV, Fixed, 95% CI) | 6.39 [‐81.15, 93.92] |
| 19 Hypoglycaemia requiring treatment | 1 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.33, 1.86] |
| 20 Hyperbilirubinaemia requiring treatment | 1 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.33, 1.50] |
| 21 Birth trauma | 1 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.20, 4.73] |
| 22 Apgar less than 7 at five minutes | 1 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.07] |
| 23 NICU/Neonatal unit admission | 1 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.35, 1.63] |
| 24 Stillbirth | 2 | 843 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.28, 6.97] |
| 25 Neonatal death | 2 | 834 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.06, 2.91] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chiswick 2015.
| Methods | Double‐blind placebo controlled RCT, stratification by study site and BMI category, analysis by intention‐to‐treat; randomisation utilised a web‐based service 15 National Health Service hospitals in the UK enrolled women between February 2011 and January 2014 |
|
| Participants |
Inlcusion criteria: pregnant women with BMI ≥ 30 kg/m2, and normal glucose tolerance test Exclusion criteria: pregnant women who were non‐white, had a history of previous GDM, previous small baby, previous early pre‐eclampsia, and a range of other conditions 449 women were randomised with 97% included in the analysis. 223 were randomised to placebo and 226 to metformin. |
|
| Interventions |
Experimental intervention: metformin maximum dose 2.5 g/day versus placebo, 2 to 3 divided doses, titrated from a starting dose of 500 mg per day Control group: matched placebo tablets |
|
| Outcomes |
Primary outcome
Secondary maternal outcome
Other outcomes
|
|
| Notes | The trial was funded by a grant from the Medical Research Council, National Institute for Health Research (NIHR) and Tommy's, the baby charity. The funding bodies had no role in any part of the study design, conduct or data collection and analysis. The authors declare no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation procedure (block size of 2 to 4) |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups via a web‐based allocation system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants received metformin or matched placebo. Participants, caregivers, and study personnel were masked to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Members of the independent Data Monitoring Committee had access to unmasked data reports, but had no contact with study participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 97% of randomised participants' outcomes available. Data were not available for 3 (1%) women in the control group, and 12 (5%) in the intervention group. |
| Selective reporting (reporting bias) | Low risk | Published protocol available |
| Other bias | Low risk | None identified |
PACTR201505001142202.
| Methods | A prospective study (in the trial registration it is stated to be a RCT) El Shatby Hospital, Alexandria Egypt. Dates of enrolment not reported |
|
| Participants | 200 participants with BMI ≥ 35 kg/m2 and normal blood glucose, HbA1C and plasma insulin, in the early second trimester | |
| Interventions |
Experimental intervention: metformin 500 mg twice a day versus placebo. 100 women enrolled Control intervention: placebo. 100 women enrolled |
|
| Outcomes |
|
|
| Notes | Minimal clinical outcomes No funding sources reported. Authors report no conflicts of interest. More information requested about all criteria for 'Risk of bias' assessment ‐ 15/03/2018 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were divided into two groups. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants received "placebo", although it was not stated if the placebo had an identical appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported on all women enrolled in the study. |
| Selective reporting (reporting bias) | High risk | 1 of 2 specified outcomes was not reported. |
| Other bias | Low risk | None identified |
Syngelaki 2016.
| Methods | Double‐blind placebo controlled trial 3 National Health Service hospitals in London, UK enrolled women from October 2010 to June 2015 |
|
| Participants |
Inclusion criteria: pregnant women with BMI ≥ 35 (at 12 to 18 weeks) Exclusion criteria: a maternal age of < 18 years; a major fetal defect observed on the scan performed at 11 to 13 weeks of gestation; a history of GDM; kidney, liver, or heart failure; a serious medical condition; hyperemesis gravidarum; treatment with metformin at the time of screening; known sensitivity to metformin; and miscarriage before randomisation |
|
| Interventions |
Experimental intervention: metformin 500 mg tablets up to 3 g/per day. 225 women enrolled Control group: matched placebo. 225 women enrolled |
|
| Outcomes |
Primary outcome
Secondary outcomes (maternal)
Secondary outcomes (fetus or neonate)
|
|
| Notes | This trial was supported by a grant from the Fetal Medicine Foundation. The authors report no conflict of interest relevant to this article. Birthweight and gestational weight gain reported as median and interquartile range (IQR); author emailed on 29/03/2018 for means and SDs (AD). Author responded with requested information on 29/03/2018. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups via a web‐based allocation system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants received metformin or placebo identical in taste and appearance. States study is double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The Consultant and his healthcare team would record the clinical data of the participants at each antenatal visit. Delivery notes and neonatal outcomes for each participant would be recorded by the healthcare team. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 23 (10%) of women in the metformin group and 27 (12%) withdrew consent after randomisation. |
| Selective reporting (reporting bias) | Low risk | Stated outcomes have been reported. |
| Other bias | Unclear risk | Trial registration occurred after commencing enrolment of participants. |
BMI: body mass index GDM: gestational diabetes mellitus HDL: high‐density lipoprotein LDL: low‐density lipoprotein RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Dodd 2016.
| Trial name or title | Metformin and dietary advice to improve insulin sensitivity and promote gestational restriction of weight among pregnant women who are overweight or obese: the GRoW Randomised Trial |
| Methods | Randomised controlled trial across 3 metropolitan hospitals in Adelaide, South Australia. Women were enrolled from 2013 until 2016 |
| Participants |
Included: women with BMI ≥ 25 kg/m2, singleton pregnancy, between 10 + 0 and 20 weeks of gestation, without type 1, 2 or gestational diabetes Excluded: women with a contraindication to taking metformin, congenital fetal anomaly or planning to birth at a hospital not involved with the study |
| Interventions |
Intervention: women receive up to 2 g of metformin (titrated from 500 mg over 2 weeks) in divided doses morning and night, from trial entry until birth Control: women receive up to 4 tablets daily, identical in taste and appearance to the metformin tablets, (titrated from 1 tablet over 4 weeks), from trial entry until birth All women received a comprehensive dietary and lifestyle intervention across the duration of pregnancy. |
| Outcomes |
Maternal clinical outcomes
Infant clinical outcomes
|
| Starting date | May 2013 |
| Contact information | Jodie Dodd (jodie.dodd@adelaide.edu.au) |
| Notes |
RBR‐9rpqdn.
| Trial name or title | Use of metformin for the prevention of gestational diabetes mellitus in obese pregnant women |
| Methods | Randomised controlled trial, Joinville, Brazil |
| Participants |
Included: women aged 18 years or more with BMI ≥ 30 kg/m2, singleton pregnancy, between 10 + 0 and 20 weeks of gestation, without gestational diabetes (screened in early pregnancy); or pathology that interferes with glucose metabolism Excluded: women with a contraindication to taking metformin; history or presence of liver disease; gastrointestinal disease or other conditions that interfere with the absorption, distribution, excretion or metabolism of the drug |
| Interventions |
Intervention: women will receive 500 mg metformin orally after breakfast and dinner from 20 weeks of pregnancy until birth; in addition standard monitoring with nutritionist, nurses physiotherapists and obstetricians Control: women will receive standard monitoring with nutritionist, nurses physiotherapists and obstetricians from 20 weeks of pregnancy until birth |
| Outcomes |
Primary outcome: incidence of gestational diabetes Secondary outcome: neonatal hypoglycaemia |
| Starting date | October 2014 |
| Contact information | William Barbosa Sales; Universidade da Região de Joinville. Phone +55 (41) 8859 9064; email: sallesbio@hotmail.com |
| Notes |
BMI: body mass index
Differences between protocol and review
There are some differences between the published protocol (Eames 2013), and this full review. These are explained below.
We have changed the title and scope of the review to include women who are overweight (body mass index (BMI) ≥ 25 kg/m2).
Jodie Dodd has taken over the role of contact person and guarantor for this review.
We have updated the Background sections.
Our search methods now include the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), and ClinicalTrials.gov, as required by Cochrane standards.
The review now includes women who are overweight (BMI ≥ 25 kg/m2). We have added the classification for overweight. We have amended the Background, Objectives, and Methods, including types of participants, to include women who are overweight.
For clarity, we have changed one secondary outcome from 'admission to high‐dependency unit or NICU and length of stay' to 'Length of stay in high‐dependency unit or NICU'.
We have prepared the review to current Cochrane MECIR standards and the standard methods of Cochrane Pregnancy and Childbirth, including the use of GRADE and inclusion of Table 1.
Contributions of authors
Jodie Dodd provided general and methodological advice on the protocol, designed the data extraction template, was consulted over discrepancies with study selection and data extraction, completed the GRADE assessment, and was involved with data analysis and interpretation.
Rosalie Grivell provided advice on clinical and methodological aspects of the protocol, screened studies for inclusion, extracted data and contributed to data analysis and interpretation.
Andrea Deussen provided general advice on the protocol, screened studies for inclusion, extracted data, wrote up the review, completed the GRADE assessment and contributed to data analysis.
William Hague provided general advice on the protocol and interpreted results.
Declarations of interest
Jodie Dodd is chief investigator of an ongoing randomised trial evaluating the role of metformin in pregnancy for women with obesity or who are overweight (Dodd 2016). This trial is potentially eligible for inclusion in future updates of this review when the trial is complete. Decisions relating to this trial will be made by third parties not directly involved in the trial.
Rosalie Grivell is an investigator of an ongoing randomised trial evaluating the role of metformin in pregnancy for women with obesity or who are overweight (Dodd 2016). This trial is potentially eligible for inclusion in future updates of this review when the trial is complete. Decisions relating to this trial will be made by third parties not directly involved in the trial.
William Hague is an investigator of an ongoing randomised trial evaluating the role of metformin in pregnancy women with obesity or who are overweight (Dodd 2016). This trial is potentially eligible for inclusion in future updates of this review when the trial is complete. Decisions relating to this trial will be made by third parties not directly involved in the trial. He has received NHMRD MRFF (rare disease) funding for an RCT comparing UDCA with rifampicin in severe early onset Intrahepatic Cholestasis of Pregnancy (Hague et al 2018‐2022) and is an investigator on the MAGDA study on the follow‐up of women with previous GDM, funded by a Partnership grant from NHMRC.
Andrea Deussen is the trial coordinator of a study of a randomised trial evaluating the role of metformin in pregnancy for women who are obese (Dodd 2016). This trial is potentially eligible for inclusion in future updates of this review when the trial is complete. Decisions relating to this trial will be made by third parties not directly involved in the trial.
New
References
References to studies included in this review
Chiswick 2015 {published data only}
- Chiswick C, Reynolds RM, Denison F, Drake AJ, Forbes S, Newby DE, et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double‐blind, placebo‐controlled trial. Lancet. Diabetes & Endocrinology 2015;3(10):778‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiswick CA, Reynolds RM, Denison FC, Drake AJ, Forbes S, Newby DE, et al. Does metformin reduce excess birthweight in offspring of obese pregnant women? A randomised controlled trial of efficacy, exploration of mechanisms and evaluation of other pregnancy complications. Efficacy and Mechanism Evaluation 2016; Vol. 3, issue 7. [PubMed]
- Chiswick CA, Reynolds RM, Denison FC, Whyte SA, Drake AJ, Newby DE, et al. Efficacy of metformin in pregnant obese women: a randomised controlled trial. BMJ Open 2015;5(1):e006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCTR2009‐017134‐47‐GB. Efficacy of metformin in pregnant obese women, a randomised controlled trial (EMPOWaR) [Does Metformin reduce the future risk of overweight babies?]. clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2009‐017134‐47 (first received 17 March 2010).
- ISRCTN51279843. A multicentre randomised placebo controlled clinical trial of metformin versus placebo in pregnant women to reduce the risk of obesity and metabolic syndrome in their babies. isrctn.com/ISRCTN51279843 (first received 30 April 2010).
- Norman J. Does metformin reduce excess birthweight in offspring of obese pregnant women? A randomised controlled trial of efficacy, exploration of mechanisms and evaluation of other pregnancy complications. www.eme.ac.uk/projectfiles/0824609protocol.pdf (accessed 12 October 2011). [PubMed]
PACTR201505001142202 {published data only}
- PACTR201505001142202. Can metformin limit weight gain in the obese with pregnancy?. pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201505001142202 (first received 15 May 2015).
Syngelaki 2016 {published and unpublished data}
- EUCTR2008‐005892‐83‐GB. Metformin in obese non‐diabetic pregnant women (MOP Trial). clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2008‐005892‐83 (first received 28 April 2009).
- Stanford FC, Alfaris N, Misra M. Metformin versus placebo in obese pregnant women without diabetes (letter). New England Journal of Medicine 2016;374(25):2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syngelaki A, Nicolaides KH, Balani J, Hyer S, Akolekar R, Kotecha R, et al. Metformin versus placebo in obese pregnant women without diabetes mellitus. New England Journal of Medicine 2016;374(5):434‐43. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Dodd 2016 {published data only}
- ACTRN12612001277831. Metformin and dietary advice to improve insulin sensitivity and promote gestational restriction of weight in pregnant women who are obese: the GroW Randomised trial. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612001277831 (first received 17 November 2012).
- Dodd JM, Grivell RM, Deussen AR, Dekker G, Hague W. Metformin and dietary advice to improve insulin sensitivity and promote gestational restriction of weight among pregnant women who are overweight or obese: the GRoW Randomised Trial. BMC Pregnancy and Childbirth 2016;16(1):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
RBR‐9rpqdn {published data only}
- RBR‐9rpqdn. Use of metformin for the prevention of gestational diabetes mellitus in obese pregnant women. ensaiosclinicos.gov.br/rg/RBR‐9rpqdn/ (first received 7 October 2014).
Additional references
Bailey 1996
- Bailey CJ, Turner RC. Metformin. New England Journal of Medicine 1996;334:574‐9. [DOI] [PubMed] [Google Scholar]
Balsells 2015
- Balsells M, García‐Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta‐analysis. BMJ 2015;350:h102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Callaway 2006
- Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Medical Journal of Australia 2006;184(2):56‐9. [DOI] [PubMed] [Google Scholar]
Cedergren 2004
- Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstetrics and Gynecology 2004;103:219‐24. [DOI] [PubMed] [Google Scholar]
Chu 2009
- Chu SY, Kim SY, Bish CL. Prepregnancy obesity prevalence in the United States, 2004‐2005. Maternal and Child Health Journal 2009;13(5):614‐20. [DOI] [PubMed] [Google Scholar]
Cusi 1998
- Cusi K, DeFronzo RA. Metformin: a review of its metabolic effects. Diabetes Review 1998;6:89‐131. [Google Scholar]
Dodd 2011
- Dodd JM, Grivell RM, Nguyen A‐M, Chan A, Robinson JS. Maternal and perinatal health outcomes by body mass index category. Australian and New Zealand Journal of Obstetrics and Gynaecology 2011;51(2):136‐40. [DOI] [PubMed] [Google Scholar]
Ehrenberg 2004
- Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. American Journal of Obstetrics and Gynecology 2004;191(3):964‐8. [DOI] [PubMed] [Google Scholar]
Ezzati 2002
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJL. Selected major risk factors and burden of disease. Lancet 2002;360:1347‐60. [DOI] [PubMed] [Google Scholar]
Furber 2013
- Furber C, McGowan L, Bower P, Kontopantelis E, Quenby S, Lavender T. Antenatal interventions for reducing weight in obese women for improving pregnancy outcome. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD009334] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbert 2006
- Gilbert C, Valois M, Koren G. Pregnancy outcome after first trimester exposure to metformin: a meta‐analysis. Fertility & Sterility 2006;86:658‐63. [DOI] [PubMed] [Google Scholar]
Hawthorne 2006
- Hawthorne G. Metformin use and diabetic pregnancy. Diabetic Medicine 2006;23(3):223‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hundal 2000
- Hundal RS, Krssak M, Dufour S, Laurent D, Lebon, Chandramouli V, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000;49:2063‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
i‐WIP Collaborative Group 2017
- International Weight Management in Pregnancy (i‐WIP) Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta‐analysis of individual participant data from randomised trials. BMJ 2017;358:j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inzucchi 1998
- Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, et al. Efficacy and metabolic effects of metformin and troglitazone in type 2 diabetes mellitus. New England Journal of Medicine 1998;338:867‐72. [DOI] [PubMed] [Google Scholar]
Kirpichnikov 2002
- Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Annals of Internal Medicine 2002;137:25‐33. [DOI] [PubMed] [Google Scholar]
Large 1999
- Large V, Beylot M. Modifications of citric acid cycle activity and gluconeogenesis in streptozotocin‐induced diabetes and effects of metformin. Diabetes 1999;48(6):1251‐7. [DOI] [PubMed] [Google Scholar]
Levri 2005
- Levri KM, Slaymaker E, Last A, Yeh J, Ference J, D'Amico F, et al. Metformin as treatment for overweight and obese adults: a systematic review. Annals of Family Medicine 2005;3(5):457‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lilja 2006
- Lilja AE, Mathieson ER. Polycystic ovary syndrome and metformin in pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2006;85:861‐8. [DOI] [PubMed] [Google Scholar]
Lobstein 2016
- Lobstein T, Jackson‐Leach R. Planning for the worst: estimates of obesity and comorbidities in school‐age children in 2025. Pediatric Obesity 2016;11(5):321‐5. [DOI] [PubMed] [Google Scholar]
Morgan 2014
- Morgan KL, Rahman MA, Macey S, et al. Obesity in pregnancy: a retrospective prevalence‐based study on health service utilisation and costs on the NHS. BMJ Open 2014;4(2):e003983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2013
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study. Lancet 2013;384(9945):766‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perriello 1994
- Perriello G, Misericordia P, Volpi E, Santucci A, Santucci C, Ferrannini E, et al. Acute antihyperglycemic mechanisms of metformin in NIDDM. Evidence for suppression of lipid oxidation and hepatic glucose production. Diabetes 1994;43:920‐8. [DOI] [PubMed] [Google Scholar]
Priya 2018
- Prya G, Kalra S. Metformin in the management of diabetes during pregnancy and lactation. Drugs in Context 2018;7:212523. [DOI: 10.7573/dic.212523] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scheil 2015
- Scheil W, Scott J, Catcheside B, Sage L, Kennare R. Pregnancy Outcome in South Australia 2013. Adelaide: Pregnancy Outcome Unit, SA Health, Government of South Australia, 2015. [Google Scholar]
Sebire 2001
- Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. International Journal of Obesity and Related Metabolic Disorders 2001;25:1175‐82. [DOI] [PubMed] [Google Scholar]
Thompson 2001
- Thompson D, Wolf AM. The medical‐care cost burden of obesity. Obesity Reviews 2001;2(3):189‐97. [DOI] [PubMed] [Google Scholar]
Wang 2008
- Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese?. Obesity 2008;16(10):2323‐30. [DOI] [PubMed] [Google Scholar]
Wang 2011
- Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011;378(9805):1778. [DOI] [PubMed] [Google Scholar]
Weiss 2004
- Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH. Obesity, obstetric complications and caesarean delivery rate ‐ a population‐based screening study. American Journal of Obstetrics and Gynecology 2004;190:1091‐7. [DOI] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. Obesity: preventing and managing the global epidemic. WHO Technical Report Series. Number 894. Geneva (Switzerland): WHO, 2000. [PubMed] [Google Scholar]
WHO 2009
- Mathers C, Stevens G, Mascarenhas M, World Health Organization. Global Health Risks. Mortality and burden of disease attributable to selected major risks. Geneva (Switzerland): WHO Press, 2009. [Google Scholar]
WHO 2013
- World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013‐2020. www.who.int/nmh/events/ncd_action_plan/en/ 2013.
WHO 2017
- World Health Organization. Obesity and overweight. www.who.int/news‐room/fact‐sheets/detail/obesity‐and‐overweight 2017.
Wiernsperger 1999
- Wiernsperger NF, Bailey CJ. The antihyperglycaemic effect of metformin: therapeutic and cellular mechanisms. Drugs 1999;58(Suppl 1):31‐9. [DOI] [PubMed] [Google Scholar]
Withrow 2009
- Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obesity Reviews 2011;12:131‐41. [DOI] [PubMed] [Google Scholar]
Yu 2006
- Yu CKH, Teoh TG, Robinson S. Obesity in pregnancy. BJOG: an international journal of obstetrics and gynaecology 2006;113:1117‐25. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Eames 2013
- Eames AJ, Grivell RM, Deussen AR, Hague W, Dodd JM. Metformin for women who are obese during pregnancy for improving maternal and infant outcomes. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010564] [DOI] [PMC free article] [PubMed] [Google Scholar]


