Abstract
Objective:
The apolipoprotein E (APOE) gene is an established risk factor for sporadic Alzheimer’s disease, with elevated risk for ε4-carriers and reduced risk for ε2-carriers. However, it is unclear whether APOE modifies risk for cognitive decline in normal aging. The objective of this study was to determine whether ε2 and ε4 are associated with rates of normal cognitive aging, and whether associations of ε4 with cognitive decline are modified by sex, education or health behaviors (exercise, alcohol consumption, smoking).
Method:
A community-based sample of 1,393 older adults were genotyped for APOE and underwent cognitive assessment up to seven times over a maximum of period of 27 years.
Results:
ε2-carriers showed slower executive function decline with age relative to ε3 homozygotes or ε4-carriers, whereas ε4-carriers demonstrated more rapid executive function and verbal fluency decline. Accelerated executive function decline was particularly pronounced in ε4-carriers with lower education. After excluding individuals with cognitive impairment, faster executive function decline was still apparent in ε4-carriers, and the effect of ε4 on episodic memory interacted with alcohol consumption, such that only ε4-carriers who did not drink showed more rapid memory decline than ε4 non-carriers. The influence of ε4 on cognitive aging did not differ by sex, nor was it modified by smoking or exercise.
Conclusions:
These findings indicate that the ε2 and ε4 alleles have differential effects on cognitive aging, and that negative effects of ε4 may be partly mitigated by behavioral choices.
Keywords: cognitive aging, apolipoprotein E, executive function, memory, education
Introduction
The apolipoprotein E (APOE) ε4 allele is one of the strongest genetic risk factors for sporadic Alzheimer’s disease, whereas the ε2 allele is protective against Alzheimer’s disease (Roses, 1996). Relative to ε3 homozygotes, risk of Alzheimer’s disease is increased an estimated 3.2 times in ε3/ε4 heterozygotes and 14.9 times in ε4 homozygotes, whereas risk is reduced 1.7 times for carriers of the ε2 allele (Farrer et al., 1997). In contrast to this clear association with Alzheimer’s disease, effects of APOE on cognitive change in normal aging are less well understood.
Cross-sectional studies in non-demented older adults have suggested that APOE-ε4 is associated with impaired memory, executive function, processing speed and global cognitive function (Small, Rosnick, Fratiglioni, & Backman, 2004; Wisdom, Callahan, & Hawkins, 2011). Although there have been fewer longitudinal investigations on cognitive change as a function of APOE genotype, several studies have reported more rapid cognitive decline for ε4-carriers than non-carriers (Bretsky et al., 2003; Caselli et al., 2009; De Jager et al., 2012). Others, however, found no differences in rates of change between ε4-carriers and non-carriers (Bunce et al., 2014). These discrepancies may be due to methodological differences, for instance in sample size, length of follow-up, and neuropsychological assessments used, or to differences in participant characteristics or health. Inclusion of individuals with unrecognized or prodromal dementia may inflate rates of decline for ε4-carriers. Efforts to resolve this concern by excluding cognitively impaired individuals have yielded mixed results; cognitive decline in ε4-carriers remained elevated in some studies (Albrecht et al., 2015; Caselli et al., 2009; Christensen et al., 2008), while others reported no difference between carriers and non-carriers (Batterham, Bunce, Cherbuin, & Christensen, 2013; Praetorius, Thorvaldsson, Hassing, & Johansson, 2013).
Differences between cohorts in demographic and health-related factors that may modify APOE effects could contribute to discrepant reports. For example, effects of APOE on risk for Alzheimer’s disease differ by age, sex and ethnicity (Farrer et al., 1997), as well as by modifiable factors such as education (Ferrari et al., 2013; Wang et al., 2012), physical activity (Kivipelto et al., 2008; Rovio et al., 2005), alcohol consumption (Anttila et al., 2004; Mukamal et al., 2003; Virtaa et al., 2010) and smoking (Kivipelto et al., 2008; Ott et al., 1998). It is unclear whether these factors moderate effects of APOE on normal cognitive aging, as only a handful of studies have evaluated effects of education (Seeman et al., 2005), exercise (Niti, Yap, Kua, Tan, & Ng, 2008; Podewils et al., 2005; Schuit, Feskens, Launer, & Kromhout, 2001), alcohol consumption (Dufouil et al., 2000), or smoking (Bangen et al., 2013; Dufouil et al., 2000; Reitz, Luchsinger, Tang, & Mayeux, 2005) on the association of APOE with cognitive decline. It has been proposed that ε4 is a vulnerability gene, enhancing susceptibility to both the positive and negative effects of environmental or health factors on brain health (Sachs-Ericsson, Sawyer, Corsentino, Collins, & Blazer, 2010). Although findings are mixed, this explanation is supported by reports that stimulating leisure activities, exercise, moderate drinking, and abstaining from smoking are particularly cognitively protective for ε4-carriers (Carmelli, Swan, Reed, Schellenberg, & Christian, 1999; Kivipelto et al., 2008; Niti et al., 2008; Smith, Nielson, Woodard, Seidenberg, & Rao, 2013).
Effects of the ε2 allele on normal cognitive aging have been less frequently studied, perhaps due to its low prevalence (8% in Caucasians (Zannis, Just, & Breslow, 1981)). Findings from the few studies that examined effects of ε2 on cognitive aging are inconclusive. Some have reported that ε2-carriers decline more slowly than non-carriers on tests of memory, verbal ability, executive function or global cognitive function (Davies et al., 2014; Helkala et al., 1996; Wilson, Bienias, Berry-Kravis, Evans, & Bennett, 2002; Yaffe, Cauley, Sands, & Browner, 1997), while others found no effect of ε2 on rates of cognitive change (Batterham et al., 2013; Schiepers et al., 2012). With the exception of the meta-analyses reported by Davies et al. (2014), which identified a nominally significant association of ε2 with slower cognitive decline only among women, these studies had relatively few ε2-carriers or short follow-up periods.
In the present study we examined effects of APOE genotype on trajectories of change in global cognition, episodic memory, executive function and verbal fluency in a large, well-characterized population-based cohort of community-dwelling older adults followed for up to 27 years. We aimed to determine whether ε2 and ε4 are differentially associated with rates of cognitive decline in normal aging, and whether effects of ε4 on cognitive aging are modified by sex, education or health-related behaviors, including exercise, alcohol consumption and smoking. We further examined ε4 effects after excluding individuals with evidence of cognitive impairment to minimize possible inclusion of individuals with prodromal Alzheimer’s disease. We hypothesized that ε2-carriers and ε4-carriers would show slower and more rapid cognitive decline than non-carriers, respectively, that negative effects of ε4 would be mitigated by higher education and healthy behaviors, and that ε4 effects would be apparent even within cognitively intact individuals. Because the ε4 allele is associated with earlier mortality (Hayden et al., 2005) and cognitive decline accelerates in the years before death (Wilson, Beckett, Bienias, Evans, & Bennett, 2003), we used joint models of cognitive decline and time to death (Rizopoulos, 2010) to assess whether differences in rates of cognitive decline by ε4 status arose from differences in mortality.
Materials and methods
Study participants
Participants were southern California residents who were members of the Rancho Bernardo Study (RBS), a longitudinal study of community-dwelling older adults established in 1972. Cognitive function was first assessed at the 1988–1992 visit, considered baseline for the current study. Repeated assessments occurred approximately every four years thereafter, with the most recent assessment in 2014–2016.
A total of 1,448 RBS participants who completed cognitive testing at the 1988–1992 visit and had complete genetic data were eligible for study inclusion. Of these, 16 were excluded due to missing education information. Because of the opposing effects of the ε2 and ε4 alleles on Alzheimer’s disease risk, participants with the ε2/ε4 genotype (N = 39) were excluded. The final sample included 1,393 participants, aged 44–99 (mean ± SD = 73.2 ± 9.5 years; 90% aged 60 or older; 59% women) at baseline.
Study procedures were approved by the University of California, San Diego Human Research Protections Program Board, and all participants provided informed written consent prior to each visit.
Cognitive assessment
A cognitive test battery was administered by a trained interviewer at each research visit beginning in 1988. The Mini-Mental State Exam (MMSE), Trails Making Test, Part B (Trails B) of the Halstead-Reitan Battery, and category fluency tests were administered at all seven visits; the Buschke-Fuld Selective Reminding test was administered at five visits. The MMSE (Tombaugh, McDowell, Kristjansson, & Hubley, 1996) evaluates orientation, attention, language and memory to yield a measure of global cognition with a maximum score of 30. Trails B (Reitan, 1958) requires participants to connect alternating letters and numbers in sequence, with a maximum completion time of 300 seconds, to provide a measure of psychomotor tracking and executive function. The category fluency test (Borkowsk, Benton, & Spreen, 1967) assesses verbal semantic fluency by having participants name as many unique animals as possible in 60 seconds. The Buschke-Fuld Selective Reminding test (Buschke & Fuld, 1974) measures verbal episodic memory, requiring participants to recall as many words as possible from a list of ten words read by the examiner. Participants are reminded of any forgotten words and asked to recall all ten words again; this process is repeated six times. The number of correctly recalled words over all six trials (total recall) was analyzed.
Participant genetic and lifestyle assessment
Data on participant health behaviors were collected at each clinic visit through standardized questionnaires. Participants reported whether they exercised regularly three or more times per week, how many alcoholic beverages they consumed during an average week, and whether they never, previously or currently smoked. Individuals were classified as drinkers if they reported any weekly alcohol consumption in an average week, and non-drinkers if they reported no alcohol consumption during an average week. Education level was acquired at enrollment and participants were classified as those attended some college or those who attended high school or less. Waist-to-hip ratio was used as an estimate of central obesity. Self-assessed health was reported on a five-point scale (excellent, very good, good, fair, poor). Measures of motor speed and depression were not acquired at the baseline assessment. However, these measures were available on participants who attended at a later visit (1997–1999) in which depressed mood was assessed with the Beck Depression Inventory, and physical function and motor speed were assessed with the Timed Up and Go test, (Dam, von Muhlen, & Barrett-Connor, 2009).
ApoE genotype was determined as previously described (Siest et al., 1995). DNA was extracted by Sequana Therapeutics (La Jolla, CA) using standard techniques (Puregene; Gentra, Minneapolis, MN). Participants were classified as ε4-carriers (ε3/ε4 or ε4/ε4), ε2/ε3 and ε3/ε3; the cohort did not contain any ε2-homozygotes.
Statistical analysis
Demographic and behavioral characteristics at baseline were compared between APOE groups (ε2/ε3, ε3/ε3, ε4-carrier) using univariate analysis of variance (ANOVA) for continuous variables, and chi-squared tests for categorical variables. Comparison of baseline cognitive test scores by APOE genotype were adjusted for age, sex and education.
Mixed-effects regression models were used to investigate effects of APOE on age-related change on each cognitive test. Models included intercept and age as random effects, which allow individual subject baseline levels (intercept) and slopes to vary randomly about the mean trajectory described by the fixed effect terms. Models included linear and quadratic age terms, as well as fixed effects of sex, education (some college/less than college) and APOE status (ε2/ε3, ε3/ε3, and ε4-carriers). Retest effects, coded as 0 for first assessment and 1 for all subsequent assessments, were included for Trails B and MMSE, as we previously found significant practice effects for these tests (Frank, Wiederholt, Kritz-Silverstein, Salmon, & Barrett-Connor, 1996; Reas et al., 2017). Interaction terms were included to assess whether age-related change differed by APOE status, whether APOE interacted with quadratic age effects, and whether the effect of APOE on cognitive change was modified by sex or education. Because interaction terms of APOE × age × sex and of APOE × age2 were not significant, these terms were omitted from the final models. Models were repeated with adjustment for lifestyle factors, including exercise (regular exercise three or more times weekly; yes/no), alcohol consumption (non-drinker/drinker), smoking (never/ever) as time-varying covariates.
To further test our a priori hypotheses that ε4 is associated with more rapid age-related change, and that ε4 increases vulnerability to negative health behaviors, models stratified APOE status as ε4-carrier versus non-carrier. Linear mixed effects models examined rates of change on each test, adjusting for age, education, and re-test effects as described above, and after further adjustment for exercise, alcohol consumption and smoking status. To evaluate whether effects were due to differences in survival, minimally-adjusted joint effects models were constructed, using a survival model including age, sex, education and APOE. Separate mixed-effects models were run examining interactions of the association of ε4 and age-related cognitive change with exercise, alcohol consumption, and smoking. Because preliminary analyses showed no differences between moderate (<1 drinks/day for women, <2 drinks/day for men) and heavy (1+ drinks/day for women, 2+ drinks/day for men) drinkers, alcohol consumption was classified as non-drinker/drinker. These models included age (linear and quadratic) as random effects, and sex, education (years of education), ε4 status, retest (Trails B, MMSE), and the time-varying lifestyle variables as fixed effects, as well as three-way interaction terms (age, ε4, lifestyle variable).
Because ε4 is a risk factor for Alzheimer’s disease, and because individuals with incipient dementia demonstrate accelerated cognitive decline prior to diagnosis (Machulda et al., 2013; Petersen et al., 1999), analyses comparing ε4-carriers to non-carriers were repeated after excluding individuals with evidence of cognitive impairment. We defined cognitive impairment as an MMSE score greater than two standard deviations below sex-, age-, and education-adjusted means at either first or last assessment, based on normative data from the National Alzheimer’s Coordinating Center Uniform Data Set (Shirk et al., 2011).
Rates of cognitive aging may be affected by declines in physical health, depression, or in the case of the Trails B test, motor slowing. Thus, models comparing ε2/ε3, ε3/ε3, and ε4-carriers were repeated with additional adjustment for overall health.
P-values for two-sided tests are shown; p < 0.05 was considered statistically significant. Data were analyzed using SAS v9.3 (SAS Institute, Cary, NC). The JointModel package (Rizopoulos, 2010) in R (R Foundation for Statistical Computing, Vienna, Austria) was used to assess the influence of survival on rates of cognitive decline associated with ε4 status.
Results
Participant characteristics
ε4-carriers comprised 22% of the sample (ε3/ε4 N = 305; ε4/ε4 N = 1); ε2-carriers comprised 14% (ε2/ε3 =196), and the remaining 64% were ε3 homozygotes (N=891). Participants completed an average of 2.8 (± 1.8) cognitive assessments and were followed for an average of 7.4 (± 7.3) years (maximum 27 years). Twenty-one percent of participants attended five or more cognitive assessments; 24% attended three or four, 21% attended two, and 34% attended one. Participant demographic, lifestyle and baseline cognitive data by APOE status are presented in Table 1. Participants did not differ by APOE genotype on any demographic or lifestyle measure or, after adjustment for age, sex and education, on baseline cognitive performance on any test (ps > 0.10). There were no significant interactions between APOE status and sex or education for any cognitive test (ps > 0.05).
Table 1.
Participant characteristics and cognitive test scores at baseline according to APOE status a.
| ε2/ε3 (N = 196) |
ε3/ε3 (N = 891) |
ε4+ (N = 306) |
p-value for APOE effect |
|
|---|---|---|---|---|
| Sex (% women) | 59% | 60% | 56% | 0.42 |
| Age (yrs) [Range] |
72.2 ± 9.4 [44–99] |
73.4 ± 9.6 [44–96] |
73.2 ± 9.1 [48–93] |
0.29 |
| No. cognitive assessments | 2.7 ± 1.8 | 2.9 ± 1.8 | 2.7 ± 1.8 | 0.33 |
| Follow-up period | 7.2 ± 7.4 | 7.6 ± 7.4 | 7.0 ± 7.1 | 0.32 |
| Some college education (%) | 74 | 68 | 69 | 0.29 |
| Exercise (% 3+ times/week) | 75 | 68 | 71 | 0.12 |
| Alcohol (% drinker) | 77 | 78 | 78 | 0.88 |
| Smoking (% ever smoked) | 57 | 56 | 62 | 0.23 |
| Health b | 1.73 | 1.75 | 1.67 | 0.46 |
| % Cognitively Impaired | 24 | 27 | 31 | 0.18 |
| Waist-to-hip ratio | 0.84 ± 0.09 | 0.84 ± 0.09 | 0.85 ± 0.09 | 0.63 |
| Beck Depression Inventory c | 5.3 ± 4.0 | 5.3 ± 4.1 | 6.0 ± 5.1 | 0.29 |
| Timed Up & Go (sec) c | 10.7 ± 2.4 | 11.1 ± 3.0 | 11.3 ± 3.3 | 0.38 |
| MMSE d | 27.4 ± 1.9 | 27.2 ± 2.2 | 27.1 ± 2.0 | 0.48 |
| Trails B d | 127.6 ± 63.2 | 132.6 ± 65.5 | 135.4 ± 67.1 | 0.36 |
| Category Fluency d | 18.1 ± 4.5 | 17.9 ± 5.1 | 17.9 ± 5.5 | 0.75 |
| Buschke Total Recall d | 38.6 ± 9.4 | 38.1 ± 9.7 | 37.9 ± 10.0 | 0.98 |
Values are mean ± SD unless otherwise indicated
Self-assessed health is on a scale from 1 to 5, with higher scores representing worse health
Means are from a subset of participants (Beck, N = 537; Timed Up & Go, N = 522) who attended the 1997–1999 visit.
Means are adjusted for age, sex and education
Effects of APOE on cognitive change with age
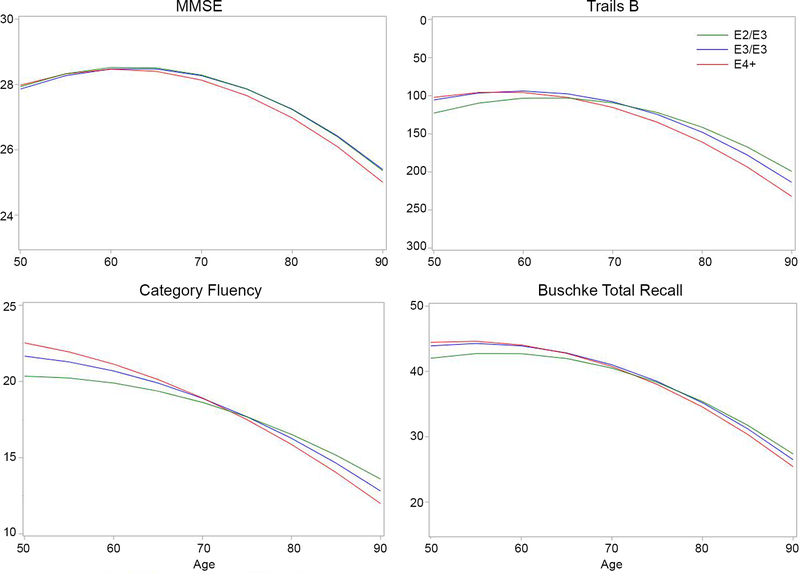
Performance declined significantly as a function of age on all tests. In minimally adjusted linear effects models (Table 2), ε2 and ε4 interacted with age for Trails B, with slower decline for ε2/ε3 (p = 0.02) and more rapid decline for ε4-carriers (p = 0.05) than for ε3/ε3 participants (Figure 1). After further adjustment for exercise, alcohol consumption and smoking, these effects remained significant (ε2, p = 0.02; ε4, p = 0.04). On the category fluency test ε2 was associated with slower decline (p < 0.05), and there was a trend for faster decline in ε4-carriers (p = 0.05). There were no main effects of APOE status on MMSE or total recall scores.
Table 2.
Intercept and slope parameters (β-estimates) for each cognitive test for base mixed-effects models comparing ε2/ε3, ε3/ε3 and ε4-carriers.
| Trails B | Category Fluency | Buschke Total Recall | MMSE | |
|---|---|---|---|---|
| Intercept | 102.42 *** | 19.98 *** | 45.06 *** | 27.40 *** |
| Age | −17.90 *** | −0.32 | 1.20 | 0.97 *** |
| Age Squared | 12.93 *** | −0.42 *** | −1.46 *** | −0.41 *** |
| Retest | −1.60 | - | - | 0.58 *** |
| Sex | −5.71 | 1.87 *** | −3.94 *** | 0.45 * |
| Education | 7.10 | 1.31 * | 0.68 | −0.11 |
| ε2 | 19.11 ** | −1.59 * | −1.96 | 0.12 |
| ε4 | −1.75 | 1.15 | 1.08 | 0.31 |
| Age × Sex | −1.95 | −0.44 * | −0.18 | −0.30 *** |
| Age × Education | −7.91 *** | −0.05 | 0.44 | 0.22 * |
| Age × ε2 | −7.40 * | 0.51 | 0.67 | −0.03 |
| Age × ε4 | 5.35 * | −0.42 | −0.39 | −0.12 |
| Sex × ε2 | −7.84 | 0.75 | 0.25 | −0.10 |
| Sex × ε4 | −3.71 | −0.68 | −1.40 | −0.48 * |
p < 0.05,
p < 0.01,
p < 0.001.
The intercept shows the modeled score of a 50 year old; the age term is the modeled change in score per ten-year increase in age over age 50.
Figure 1. Cognitive trajectories by APOE status for ε2/ε3, ε3/ε3 and ε4-carriers.
Modeled trajectories for scores on each cognitive test as a function of age are shown for ε2/ε3, ε3/ε3, and ε4-carriers. The y-axis for Trails B is inverted because lower scores indicate better performance.
Effects of health and lifestyle on cognitive change with age in ε4-carriers
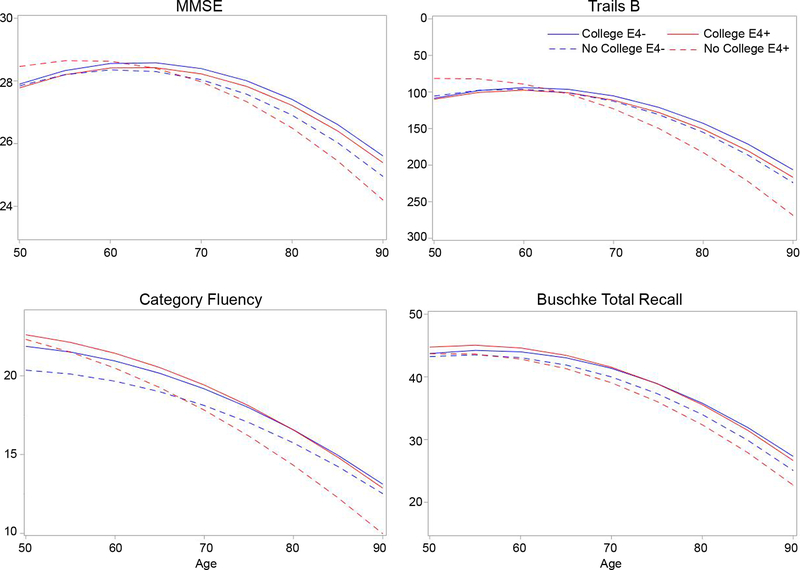
To further explore effects of health and lifestyle on cognitive change in ε4-carriers, ε4-carriers were compared with all non-carriers. ε4-carriers and non-carriers did not differ on any demographic or lifestyle measure or on baseline cognitive performance (ps > 0.05). Parameter estimates from minimally adjusted models comparing ε4-carriers and non-carriers are presented in Table 3. As reported above, ε4 interacted with age on Trails B (p < 0.001) and category fluency (p = 0.005), with faster decline for ε4-carriers than non-carriers. The effect of ε4 on age-related decline differed by education for Trails B (p = 0.008); only ε4-carriers without a college education declined faster than non-carriers (Figure 2). Although a similar pattern of faster decline for ε4-carriers without college education was observed for the other tests, the interactions were not significant.
Table 3.
Intercept and slope parameters (β-estimates) for each cognitive test for base mixed-effects models comparing ε4-carriers and non-carriers.
| Trails B | Category Fluency | Buschke Total Recall | MMSE | |
|---|---|---|---|---|
| Intercept | 109.51 *** | 19.54 *** | 44.81 *** | 27.29 *** |
| Age | −21.62 *** | −0.11 | 1.36 | 1.04 *** |
| Age Squared | 13.02 *** | −0.42 *** | −1.46 *** | −0.41 *** |
| Retest | −1.62 | - | - | 0.58 *** |
| Sex | −6.97 | 2.01 *** | −3.88 *** | 0.41 * |
| Education | 2.95 | 1.51 * | 0.49 | 0.06 |
| ε4 | −23.31 * | 2.33 * | 1.12 | 0.80 |
| Age × Sex | −2.21 | −0.42 * | −0.16 | −0.29 *** |
| Age × Education | −5.13 * | −0.23 | 0.44 | 0.15 |
| Age × ε4 | 17.21 *** | −1.12 ** | −0.70 | −0.34 |
| Sex × ε4 | −1.99 | −0.92 | −1.61 | −0.45 * |
| Education × ε4 | 25.51 * | −1.22 | 0.56 | −0.74 |
| Age × Education × ε4 | −14.97 ** | 0.88 | 0.28 | 0.32 |
p < 0.05,
p < 0.01,
p < 0.001.
The intercept shows the modeled score of a 50 year old; the age term is the modeled change in score per ten-year increase in age over age 50.
Figure 2. Cognitive trajectories by ε4 status and education level.
Modeled trajectories for scores on each cognitive test as a function of age are shown for ε4-carriers and non-carriers, with at least some college education, or with less than a college education. The y-axis for Trails B is inverted because lower scores indicate better performance.
Adjusting for exercise, alcohol consumption and smoking did not affect the interactions between age and ε4 on Trails B (p < 0.001) or category fluency (p = 0.006) performance, nor did it alter the interaction among age, ε4 and education on Trails B performance (p = 0.008). Effects of ε4 on age-related change did not differ by exercise or smoking on any test (ps > 0.10). The interaction of alcohol consumption with ε4 on age-related change approached significance (p = 0.06) for total recall.
Effects of ε4 on cognitive change in cognitively intact participants
Analyses comparing ε4-carriers and non-carriers were repeated after excluding 383 participants with cognitive impairment (MMSE score >2 SD below sex-, age-, and education-adjusted mean at first or last assessment). Relative to cognitively intact participants, impaired participants were older at baseline, included a greater proportion of men, had higher waist-to-hip ratios, participated in fewer cognitive assessments and for a shorter follow-up period, and performed worse on all cognitive tests at baseline (ps < 0.001) (Table 4). Among the cognitively intact cohort (N = 1,010), ε4-carriers and non-carriers did not differ on any demographic measure, or on baseline test performance.
Table 4.
Characteristics and cognitive test scores at baseline for cognitively intact and cognitively impaired participants a.
| Cognitively Intact (N = 1010) |
Cognitively Impaired (N = 383) |
p-value Intact versus Impaired |
|
|---|---|---|---|
| Sex (% women) | 65 | 43 | < 0.001 |
| Age (yrs) [Range] |
71.5 ± 9.5 [44–99] |
77.7 ± 7.9 [53–95] |
< 0.001 |
| ApoE status (% ε4+) | 21 | 25 | 0.09 |
| Some college education (%) | 68 | 72 | 0.20 |
| No. cognitive assessments | 2.9 ± 1.9 | 2.5 ± 1.5 | < 0.001 |
| Follow-up period | 7.9 ± 7.7 | 6.1 ± 6.1 | < 0.001 |
| Exercise (% 3+ times/week) | 71 | 67 | 0.14 |
| Alcohol (% drinker) | 78 | 76 | 0.50 |
| Smoking (% ever smoked) | 58 | 55 | 0.32 |
| Health b | 1.70 | 1.79 | 0.10 |
| Waist-to-hip ratio | 0.83 ± 0.9 | 0.87 ± 0.8 | < 0.001 |
| MMSE c | 27.8 ± 1.2 | 25.7 ± 2.8 | < 0.001 |
| Trails B c | 125.4 ± 57.2 | 151.7 ± 76.4 | < 0.001 |
| Category Fluency c | 18.6 ± 4.9 | 16.3 ± 5.0 | < 0.001 |
| Buschke Total Recall c | 39.5 ± 8.7 | 34.3 ± 9.6 | < 0.001 |
Values are mean ± SD unless otherwise indicated
Self-assessed health is on a scale from 1 to 5, with higher scores representing worse health
Means are adjusted for age, sex and education
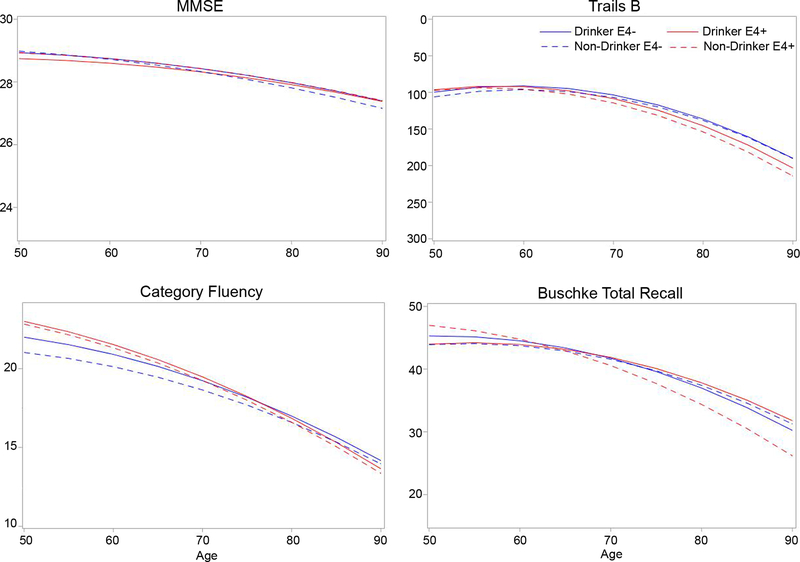
APOE effects in the cognitively intact subgroup were generally consistent with those in the full cohort. ε4-carriers declined more rapidly than non-carriers on Trails B (p = 0.01); but the effect of ε4 on category fluency decline was no longer significant (p = 0.16). The modifying effect of education on decline by APOE status on Trails B performance approached significance (p = 0.09). The interaction of alcohol consumption with ε4 on age-related change in total recall was significant (p = 0.01; Figure 3). Steeper memory decline with age was only observed for ε4 carriers who did not drink. Rates of memory decline did not differ by alcohol consumption for ε4 non-carriers.
Figure 3. Cognitive trajectories by ε4 status and alcohol consumption, for cognitively intact participants.
Modeled trajectories for scores on each cognitive test as a function of age are shown for ε4-carriers and non-carriers, who did or did not consume alcohol, after excluding cognitive impaired participants. The y-axis for Trails B is inverted because lower scores indicate better performance.
Effects of survival on associations between APOE and cognitive decline
To determine whether survival differences influenced the association of ε4 with cognitive decline, analyses were repeated using joint models. Overall, ε4 was not associated with increased risk of earlier mortality (HR = 1.14; p = 0.11) and results were essentially unchanged in joint models accounting for survival effects: The ε4 by age interactions remained for Trails B (p < 0.001) and category fluency (p = 0.007), and the three-way interaction among ε4, age and education remained significant for Trails B (p < 0.001).
Effects of health, motor slowing and depression
All associations between APOE status and cognitive change remained significant after additional adjustment for self-reported health. On the subset of participants with Beck Depression Inventory scores and Timed Up and Go scores, neither measure differed by APOE genotype (Beck: F(531,2) = 1.24, p = 0.29; Timed Up and Go: F(516,2) = 0.97, p = 0.38, Table 1). Beck scores significantly correlated with self-reported health (r(1063) = 0.42, p < 0.001).
Discussion
APOE genotype is an established risk factor for Alzheimer’s disease; however, its role in normal cognitive aging is unclear. Our results showed that relative to ε3 homozygotes, ε2 carriers showed slower executive function decline with age, whereas ε4 carriers showed faster decline, even after exclusion of individuals with evidence of cognitive impairment. Education and alcohol moderated the negative effects of ε4 on age-related decline. Adjusting for differences in survival did not alter the association of ε4 with cognitive decline.
We observed slower rates of decline on Trails B, a test of executive function and psychomotor processing speed, and category fluency, a test of executive function and semantic processing, for ε2-carriers compared to ε3 homozygotes. Other studies have found reduced decline for ε2-carriers in other cognitive domains, including memory, verbal ability and global cognitive function (Davies et al., 2014; Helkala et al., 1996; Kim et al., 2017; Wilson et al., 2002), whereas most that have examined executive function or processing speed reported no association between ε2 and rates of decline (Helkala et al., 1996; Kim et al., 2017; Wilson et al., 2002). Although Yaffe et al. (1997) reported slower executive function decline for ε2-carriers assessed with the Digit Symbol test, they observed no effect of ε2 on the Trails B test. Our longer follow-up period may have enabled us to detect effects of ε2 on Trails B.
In contrast, the ε4 allele was associated with accelerated age-related decline on Trails B and category fluency. Executive function and processing speed are among the domains most susceptible to decline in normal aging, but are typically less affected than memory in initial stages of Alzheimer’s disease. This vulnerability of executive function to APOE is consistent with other studies that also found an effect of ε4 on age-related change in executive function (Caselli et al., 2011; Wilson et al., 2002), and with neuroimaging studies reporting that ε4 is associated with thinner frontal cortex (Fennema-Notestine et al., 2011) and increased functional activation during performance of executive function tasks (Wishart et al., 2006). Because the Trails B test measures psychomotor processing speed, motor slowing may have contributed to age-related declines in Trails B performance; however, adjustment for physical health did not alter this association and scores on a test of motor speed did not differ by APOE status, suggesting that physical function was unlikely to be driving the observed association between APOE and Trails B decline. APOE effects on Trails B decline remained significant after excluding individuals with cognitive impairment. Our results are consistent with a prior study showing that, after excluding cognitively impaired individuals, ε4-carriers demonstrated deficits on a test of executive function and processing speed while performance on a range of other neuropsychological tests was comparable to non-carriers (Foster et al., 2013). These findings suggest that ε4 may regulate the progression of biological changes associated with aging, possibly independent of pathology related to dementia.
In our study, effects of ε4 on category fluency decline were significant in the full sample, but not after exclusion of individuals with cognitive impairment. This is consistent with prior studies that reported no association between APOE and verbal fluency decline in cognitively healthy older adults (Batterham et al., 2013; Schiepers et al., 2012). Since category fluency is impaired in individuals with mild cognitive impairment and mild Alzheimer’s disease (Monsch et al., 1992; Mueller et al., 2015), accelerated category fluency decline among our full sample may have been due to inclusion of individuals with prodromal or early Alzheimer’s disease.
Memory is the most consistently reported cognitive domain to be affected by ε4 in normal aging (Albrecht et al., 2015; Caselli et al., 2015; Schiepers et al., 2012), but we did not observe a main effect of APOE on memory decline. Given that memory is one of the earliest cognitive functions affected in Alzheimer’s disease, inclusion of individuals with dementia may have contributed to the association of ε4 with memory decline observed in prior studies (Batterham et al., 2013; Dik et al., 2000; Ma, Wang, Miao, Zhao, & Wang, 2011; Packard et al., 2007; Praetorius et al., 2013). However, ε4 may accelerate memory decline even in individuals who remain free of cognitive impairment (Schiepers et al., 2012). One possible reason for our discrepant findings may be that ε4 effects are dose-dependent, with strongest effects among ε4 homozygotes (Packard et al., 2007). Our sample included only one ε4 homozygote, whereas other studies were enriched for ε4 homozygotes (Caselli et al., 2015) or included a larger proportion of ε4 homozygotes than our study (Batterham et al., 2013; Packard et al., 2007; Schiepers et al., 2012).
Another possibility for inconsistent APOE effects on change in memory may relate to variability across samples in moderating factors. We found that alcohol intake interacted with the effect of APOE on memory decline: only ε4-carriers who did not drink declined faster than non-carriers. The RBS cohort has a much smaller proportion of non-drinkers than nationally representative samples (McEvoy, Kritz-Silverstein, Barrett-Connor, Bergstrom, & Laughlin, 2013); thus, ε4 effects on memory decline may be more apparent in samples with larger proportions of non-drinkers.
Our finding of more rapid memory decline among ε4-carriers who did not drink is consistent with prior suggestions that moderate drinking protects against memory decline in aging (Richards, Hardy, & Wadsworth, 2005; Stampfer, Kang, Chen, Cherry, & Grodstein, 2005), and with reports that the cognitive benefits of moderate alcohol consumption are stronger for ε4-carriers than non-carriers (Carmelli et al., 1999). Yet other studies have reported beneficial associations of alcohol with cognitive aging among non-carriers only (Downer, Zanjani, & Fardo, 2014; Dufouil et al., 2000), or found no interaction between ε4 status and alcohol intake on cognitive decline (Herring & Paulson, 2017). However, these studies were smaller, had shorter follow-up periods, did not exclude individuals with cognitive impairment, and were characterized by different drinking patterns. Furthermore, considering evidence that heavy drinking is associated with increased risk of dementia particularly among ε4-carriers (Anttila et al., 2004; Mukamal et al., 2003), it is possible that a J-shaped dose-response curve may be accentuated in ε4-carriers, with excessive alcohol consumption being particularly detrimental to cognitive health. Our cohort is characterized by high rates of moderate drinking (<1 drinks/day for women, <2 drinks/day for men) with few excessive drinkers; thus, we cannot rule out the possibility that the moderating effect of alcohol on the association of ε4 with memory decline may differ among heavier drinkers. The association of alcohol intake with slower memory decline that we observed for ε4-carriers was strengthened after excluding cognitively impaired participants, suggesting that it was not driven by a higher likelihood of abstaining ε4-carriers to exhibit memory decline due to incipient dementia.
Education may also affect rates of cognitive aging, and its effects may be strongest in ε4-carriers (Ferrari et al., 2013; Wang et al., 2012). In our sample, ε4-carriers with lower education exhibited faster executive function decline than ε4-carriers with some college. ε4-carriers with a college education did not differ in rates of decline from non-carriers, suggesting that higher education may help counteract genetic risk for cognitive impairment in older age. Our findings align with Seeman and colleagues (2005) who reported more rapid decline for ε4-carriers than non-carriers in those with at least some high school education, a group which largely overlaps with our low education group. The positive association between education and change in executive function for ε4-carriers that we observed was weakened after excluding individuals with cognitive impairment. Thus, high educational attainment may be particularly important for slowing executive function decline in those at greatest risk for dementia.
APOE ε4 is associated with increased risk of early mortality, especially among homozygotes (Hayden et al., 2005), and early mortality could contribute to accelerate cognitive decline in ε4 carriers, due to “terminal decline” (Wilson et al., 2003). However, our results were unchanged in joint models incorporating survival. Furthermore, we observed no difference in self-assessed health by APOE genotype, and results were unaltered by adjustment for health. Though we were unable to directly examine the influence of psychological health on cognitive decline, depression ratings were similar across APOE genotypes and correlated with health ratings, which did not confound our results. Thus, differences in longevity or ailing physical or mental health were unlikely to underlie our findings.
This study included a large, relatively homogeneous sample, which limits our ability to generalize findings to diverse populations, but minimizes confounding due to heterogeneity in socioeconomic status, ethnicity or access to healthcare. RBS has high participation rates and a maximum follow-up period of 27 years, one of the longest follow-up periods in studies of APOE and cognitive aging. We also examined effects of APOE on cognitive decline after excluding individuals with cognitive impairment, minimizing concern that accelerated cognitive decline in ε4-carriers was driven by incipient dementia. However, we cannot exclude the possibility that latent neurodegenerative changes were present in our sample even after screening based on MMSE scores, since Alzheimer’s-related pathology is present prior to onset of cognitive symptoms (Price & Morris, 1999). Since our cohort included only one ε4 homozygote and no ε2 homozygotes, we were unable to examine allele dose-effects or to detect effects related to ε4 or ε2 homozygosity. Finally, because of the relatively small number of ε2-carriers, we were unable to examine effect modification by health or lifestyle factors in ε2-carriers. Given the low prevalence of ε2-carriers in older samples, it may be necessary to combine data across cohorts to replicate our findings and to more fully investigate how ε2 effects interact with environmental exposures to affect cognitive health.
In summary, this study demonstrated differential effects of the APOE ε2 and ε4 genotypes on cognitive decline in normal aging, with slower decline among ε2-carriers and accelerated decline among ε4-carriers. Executive functions, which are most susceptible to aging but are not typically strongly affected in early stages of Alzheimer’s disease, demonstrated the strongest effects of APOE. The association between cognitive aging and ε4 was modified by education and alcohol consumption, suggesting that modifiable lifestyle factors may attenuate genetic risk for accelerated age-related cognitive decline. These findings advance our understanding of the complex interactions between genetics and lifestyle on cognitive aging and may help to guide optimal behaviors to preserve cognitive health into late life.
Public Significance:
As the global elderly population continues to grow, identifying risk factors for aging-related cognitive decline is of mounting concern. Though the APOE gene is known to modify risk for Alzheimer’s disease, its role in normal cognitive aging is undetermined. This study reports that APOE is associated with rates of cognitive decline, even in the absence of dementia, but that lifestyle factors, including education and moderate alcohol consumption, may mitigate genetic risk of cognitive decline associated with APOE.
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (R01 AA021187); the National Institute of Aging (AG028507, AG007181); the National Institute of Diabetes and Digestive and Kidney Diseases (DK031801); and partially supported by the National Institutes of Health (ULRR031980, UL1TR000100). Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the University of California, San Diego. REDCap is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
References
- Albrecht MA, Szoeke C, Maruff P, Savage G, Lautenschlager NT, Ellis KA, … Group, A. R. (2015). Longitudinal cognitive decline in the AIBL cohort: The role of APOE epsilon4 status. Neuropsychologia, 75, 411–419. doi: 10.1016/j.neuropsychologia.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Anttila T, Helkala EL, Viitanen M, Kareholt I, Fratiglioni L, Winblad B, … Kivipelto M (2004). Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ, 329(7465), 539. doi: 10.1136/bmj.38181.418958.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Beiser A, Delano-Wood L, Nation DA, Lamar M, Libon DJ, … Au R (2013). APOE genotype modifies the relationship between midlife vascular risk factors and later cognitive decline. J Stroke Cerebrovasc Dis, 22(8), 1361–1369. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham PJ, Bunce D, Cherbuin N, & Christensen H (2013). Apolipoprotein E epsilon4 and later-life decline in cognitive function and grip strength. Am J Geriatr Psychiatry, 21(10), 1010–1019. doi: 10.1016/j.jagp.2013.01.035 [DOI] [PubMed] [Google Scholar]
- Borkowsk JG, Benton AL, & Spreen O (1967). Word Fluency and Brain Damage. Neuropsychologia, 5(2), 135–140. doi: 10.1016/0028-3932(67)90015-2 [DOI] [Google Scholar]
- Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE, & MacArthur Studies of Successful, A. (2003). The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology, 60(7), 1077–1081. [DOI] [PubMed] [Google Scholar]
- Bunce D, Bielak AA, Anstey KJ, Cherbuin N, Batterham PJ, & Easteal S (2014). APOE genotype and cognitive change in young, middle-aged, and older adults living in the community. J Gerontol A Biol Sci Med Sci, 69(4), 379–386. doi: 10.1093/gerona/glt103 [DOI] [PubMed] [Google Scholar]
- Buschke H, & Fuld PA (1974). Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology, 24(11), 1019–1025. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Reed T, Schellenberg GD, & Christian JC (1999). The effect of apolipoprotein E epsilon4 in the relationships of smoking and drinking to cognitive function. Neuroepidemiology, 18(3), 125–133. doi:26204 [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Locke DE, Baxter LC, Woodruff BK, & Geda YE (2015). Sex-based memory advantages and cognitive aging: a challenge to the cognitive reserve construct? J Int Neuropsychol Soc, 21(2), 95–104. doi: 10.1017/S1355617715000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Locke DE, Hoffman-Snyder CR, Woodruff BK, Rapcsak SZ, & Reiman EM (2011). Longitudinal modeling of frontal cognition in APOE epsilon4 homozygotes, heterozygotes, and noncarriers. Neurology, 76(16), 1383–1388. doi: 10.1212/WNL.0b013e3182167147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, … Reiman EM (2009). Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med, 361(3), 255–263. doi: 10.1056/NEJMoa0809437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H, Batterham PJ, Mackinnon AJ, Jorm AF, Mack HA, Mather KA, … Easteal S (2008). The association of APOE genotype and cognitive decline in interaction with risk factors in a 65–69 year old community sample. BMC Geriatr, 8, 14. doi: 10.1186/1471-2318-8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam TT, von Muhlen D, & Barrett-Connor EL (2009). Sex-specific association of serum vitamin D levels with physical function in older adults. Osteoporos Int, 20(5), 751–760. doi: 10.1007/s00198-008-0749-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G, Harris SE, Reynolds CA, Payton A, Knight HM, Liewald DC, … Deary IJ (2014). A genome-wide association study implicates the APOE locus in nonpathological cognitive ageing. Mol Psychiatry, 19(1), 76–87. doi: 10.1038/mp.2012.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager PL, Shulman JM, Chibnik LB, Keenan BT, Raj T, Wilson RS, … Evans DA (2012). A genome-wide scan for common variants affecting the rate of age-related cognitive decline. Neurobiol Aging, 33(5), 1017 e1011–1015. doi: 10.1016/j.neurobiolaging.2011.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Bouter LM, Geerlings MI, van Kamp GJ, & Deeg DJ (2000). APOE-epsilon4 is associated with memory decline in cognitively impaired elderly. Neurology, 54(7), 1492–1497. [DOI] [PubMed] [Google Scholar]
- Downer B, Zanjani F, & Fardo DW (2014). The relationship between midlife and late life alcohol consumption, APOE e4 and the decline in learning and memory among older adults. Alcohol Alcohol, 49(1), 17–22. doi: 10.1093/alcalc/agt144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufouil C, Tzourio C, Brayne C, Berr C, Amouyel P, & Alperovitch A (2000). Influence of apolipoprotein E genotype on the risk of cognitive deterioration in moderate drinkers and smokers. Epidemiology, 11(3), 280–284. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, … van Duijn CM (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA, 278(16), 1349–1356. [PubMed] [Google Scholar]
- Fennema-Notestine C, Panizzon MS, Thompson WR, Chen CH, Eyler LT, Fischl B, … Kremen WS (2011). Presence of ApoE epsilon4 allele associated with thinner frontal cortex in middle age. J Alzheimers Dis, 26 Suppl 3, 49–60. doi: 10.3233/JAD-2011-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari C, Xu WL, Wang HX, Winblad B, Sorbi S, Qiu C, & Fratiglioni L (2013). How can elderly apolipoprotein E epsilon4 carriers remain free from dementia? Neurobiol Aging, 34(1), 13–21. doi: 10.1016/j.neurobiolaging.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Foster JK, Albrecht MA, Savage G, Lautenschlager NT, Ellis KA, Maruff P, … Group, A. R. (2013). Lack of reliable evidence for a distinctive epsilon4-related cognitive phenotype that is independent from clinical diagnostic status: findings from the Australian Imaging, Biomarkers and Lifestyle Study. Brain, 136(Pt 7), 2201–2216. doi: 10.1093/brain/awt127 [DOI] [PubMed] [Google Scholar]
- Frank R, Wiederholt WC, Kritz-Silverstein DK, Salmon DP, & Barrett-Connor E (1996). Effects of sequential neuropsychological testing of an elderly community-based sample. Neuroepidemiology, 15(5), 257–268. [DOI] [PubMed] [Google Scholar]
- Hayden KM, Zandi PP, Lyketsos CG, Tschanz JT, Norton MC, Khachaturian AS, … Breitner J (2005). Apolipoprotein E genotype and mortality: findings from the Cache County Study. J Am Geriatr Soc, 53(6), 935–942. [DOI] [PubMed] [Google Scholar]
- Helkala EL, Koivisto K, Hanninen T, Vanhanen M, Kervinen K, Kuusisto J, … Riekkinen P Sr. (1996). Memory functions in human subjects with different apolipoprotein E phenotypes during a 3-year population-based follow-up study. Neurosci Lett, 204(3), 177–180. [DOI] [PubMed] [Google Scholar]
- Herring D, & Paulson D (2017). Moderate alcohol use and apolipoprotein E-4 (ApoE-4): Independent effects on cognitive outcomes in later life. J Clin Exp Neuropsychol, 1–12. doi: 10.1080/13803395.2017.1343803 [DOI] [PubMed] [Google Scholar]
- Kim YJ, Seo SW, Park SB, Yang JJ, Lee JS, Lee J, … Kim HJ (2017). Protective effects of APOE e2 against disease progression in subcortical vascular mild cognitive impairment patients: A three-year longitudinal study. Sci Rep, 7(1), 1910. doi: 10.1038/s41598-017-02046-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Rovio S, Ngandu T, Kareholt I, Eskelinen M, Winblad B, … Nissinen A (2008). Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med, 12(6B), 2762–2771. doi: 10.1111/j.1582-4934.2008.00296.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Wang J, Miao R, Zhao W, & Wang Q (2011). Association between apolipoprotein E epsilon4 and longitudinal cognitive decline: nested case-control study among chinese community-dwelling elders. Neuropsychobiology, 64(2), 102–109. doi: 10.1159/000324991 [DOI] [PubMed] [Google Scholar]
- Machulda MM, Pankratz VS, Christianson TJ, Ivnik RJ, Mielke MM, Roberts RO, … Petersen RC (2013). Practice effects and longitudinal cognitive change in normal aging vs. incident mild cognitive impairment and dementia in the Mayo Clinic Study of Aging. Clin Neuropsychol, 27(8), 1247–1264. doi: 10.1080/13854046.2013.836567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy LK, Kritz-Silverstein D, Barrett-Connor E, Bergstrom J, & Laughlin GA (2013). Changes in alcohol intake and their relationship with health status over a 24-year follow-up period in community-dwelling older adults. J Am Geriatr Soc, 61(8), 1303–1308. doi: 10.1111/jgs.12366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, & Thal LJ (1992). Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol, 49(12), 1253–1258. [DOI] [PubMed] [Google Scholar]
- Mueller KD, Koscik RL, LaRue A, Clark LR, Hermann B, Johnson SC, & Sager MA (2015). Verbal Fluency and Early Memory Decline: Results from the Wisconsin Registry for Alzheimer’s Prevention. Arch Clin Neuropsychol, 30(5), 448–457. doi: 10.1093/arclin/acv030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT Jr., Mittleman MA, & Siscovick DS (2003). Prospective study of alcohol consumption and risk of dementia in older adults. JAMA, 289(11), 1405–1413. [DOI] [PubMed] [Google Scholar]
- Niti M, Yap KB, Kua EH, Tan CH, & Ng TP (2008). Physical, social and productive leisure activities, cognitive decline and interaction with APOE-epsilon 4 genotype in Chinese older adults. Int Psychogeriatr, 20(2), 237–251. doi: 10.1017/S1041610207006655 [DOI] [PubMed] [Google Scholar]
- Ott A, Slooter AJ, Hofman A, van Harskamp F, Witteman JC, Van Broeckhoven C, … Breteler MM (1998). Smoking and risk of dementia and Alzheimer’s disease in a population-based cohort study: the Rotterdam Study. Lancet, 351(9119), 1840–1843. [DOI] [PubMed] [Google Scholar]
- Packard CJ, Westendorp RG, Stott DJ, Caslake MJ, Murray HM, Shepherd J, … Prospective Study of Pravastatin in the Elderly at Risk, G. (2007). Association between apolipoprotein E4 and cognitive decline in elderly adults. J Am Geriatr Soc, 55(11), 1777–1785. doi: 10.1111/j.1532-5415.2007.01415.x [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, & Kokmen E (1999). Mild cognitive impairment: clinical characterization and outcome. Arch Neurol, 56(3), 303–308. [DOI] [PubMed] [Google Scholar]
- Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, & Lyketsos CG (2005). Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol, 161(7), 639–651. doi: 10.1093/aje/kwi092 [DOI] [PubMed] [Google Scholar]
- Praetorius M, Thorvaldsson V, Hassing LB, & Johansson B (2013). Substantial effects of apolipoprotein E epsilon4 on memory decline in very old age: longitudinal findings from a population-based sample. Neurobiol Aging, 34(12), 2734–2739. doi: 10.1016/j.neurobiolaging.2013.06.002 [DOI] [PubMed] [Google Scholar]
- Price JL, & Morris JC (1999). Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol, 45(3), 358–368. [DOI] [PubMed] [Google Scholar]
- Reas ET, Laughlin GA, Bergstrom J, Kritz-Silverstein D, Barrett-Connor E, & McEvoy LK (2017). Effects of Sex and Education on Cognitive Change Over a 27-Year Period in Older Adults: the Rancho Bernardo Study. The American Journal of Geriatric Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM (1958). Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills, 8, 271–276. [Google Scholar]
- Reitz C, Luchsinger J, Tang MX, & Mayeux R (2005). Effect of smoking and time on cognitive function in the elderly without dementia. Neurology, 65(6), 870–875. doi: 10.1212/01.wnl.0000176057.22827.b7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M, Hardy R, & Wadsworth ME (2005). Alcohol consumption and midlife cognitive change in the British 1946 birth cohort study. Alcohol Alcohol, 40(2), 112–117. doi: 10.1093/alcalc/agh126 [DOI] [PubMed] [Google Scholar]
- Rizopoulos D (2010). JM: An R package for the joint modelling of longitudinal and time-to-event data. Journal of Statistical Software (Online), 35(9), 1–33. [Google Scholar]
- Roses AD (1996). Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annu Rev Med, 47, 387–400. doi: 10.1146/annurev.med.47.1.387 [DOI] [PubMed] [Google Scholar]
- Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, … Kivipelto M (2005). Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurology, 4(11), 705–711. doi: 10.1016/S1474-4422(05)70198-8 [DOI] [PubMed] [Google Scholar]
- Sachs-Ericsson NJ, Sawyer KA, Corsentino EA, Collins NA, & Blazer DG (2010). APOE epsilon4 allele carriers: Biological, psychological, and social variables associated with cognitive impairment. Aging Ment Health, 14(6), 679–691. doi: 10.1080/13607860903292594 [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Harris SE, Gow AJ, Pattie A, Brett CE, Starr JM, & Deary IJ (2012). APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Mol Psychiatry, 17(3), 315–324. doi: 10.1038/mp.2010.137 [DOI] [PubMed] [Google Scholar]
- Schuit AJ, Feskens EJ, Launer LJ, & Kromhout D (2001). Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc, 33(5), 772–777. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Huang MH, Bretsky P, Crimmins E, Launer L, & Guralnik JM (2005). Education and APOE-e4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. J Gerontol B Psychol Sci Soc Sci, 60(2), P74–83. [DOI] [PubMed] [Google Scholar]
- Shirk SD, Mitchell MB, Shaughnessy LW, Sherman JC, Locascio JJ, Weintraub S, & Atri A (2011). A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimers Res Ther, 3(6), 32. doi: 10.1186/alzrt94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siest G, Pillot T, Regis-Bailly A, Leininger-Muller B, Steinmetz J, Galteau MM, & Visvikis S (1995). Apolipoprotein E: an important gene and protein to follow in laboratory medicine. Clin Chem, 41(8 Pt 1), 1068–1086. [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, & Backman L (2004). Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging, 19(4), 592–600. doi: 10.1037/0882-7974.19.4.592 [DOI] [PubMed] [Google Scholar]
- Smith JC, Nielson KA, Woodard JL, Seidenberg M, & Rao SM (2013). Physical activity and brain function in older adults at increased risk for Alzheimer’s disease. Brain Sci, 3(1), 54–83. doi: 10.3390/brainsci3010054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer MJ, Kang JH, Chen J, Cherry R, & Grodstein F (2005). Effects of moderate alcohol consumption on cognitive function in women. N Engl J Med, 352(3), 245–253. doi: 10.1056/NEJMoa041152 [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McDowell I, Kristjansson B, & Hubley AM (1996). Mini-Mental State Examination (MMSE) and the Modified MMSE (3MS): A psychometric comparison and normative data. Psychological Assessmen, 8(1), 48–59. [Google Scholar]
- Virtaa JJ, Jarvenpaa T, Heikkila K, Perola M, Koskenvuo M, Raiha I, … Kaprio J (2010). Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis, 22(3), 939–948. doi: 10.3233/JAD-2010-100870 [DOI] [PubMed] [Google Scholar]
- Wang HX, Gustafson DR, Kivipelto M, Pedersen NL, Skoog I, Windblad B, & Fratiglioni L (2012). Education halves the risk of dementia due to apolipoprotein epsilon4 allele: a collaborative study from the Swedish brain power initiative. Neurobiol Aging, 33(5), 1007 e1001–1007. doi: 10.1016/j.neurobiolaging.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Bienias JL, Evans DA, & Bennett DA (2003). Terminal decline in cognitive function. Neurology, 60(11), 1782–1787. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bienias JL, Berry-Kravis E, Evans DA, & Bennett DA (2002). The apolipoprotein E epsilon 2 allele and decline in episodic memory. J Neurol Neurosurg Psychiatry, 73(6), 672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom NM, Callahan JL, & Hawkins KA (2011). The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging, 32(1), 63–74. doi: 10.1016/j.neurobiolaging.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Flashman LA, Guerin SJ, … McAllister TW (2006). Increased brain activation during working memory in cognitively intact adults with the APOE epsilon4 allele. Am J Psychiatry, 163(9), 1603–1610. doi: 10.1176/ajp.2006.163.9.1603 [DOI] [PubMed] [Google Scholar]
- Yaffe K, Cauley J, Sands L, & Browner W (1997). Apolipoprotein E phenotype and cognitive decline in a prospective study of elderly community women. Arch Neurol, 54(9), 1110–1114. [DOI] [PubMed] [Google Scholar]
- Zannis VI, Just PW, & Breslow JL (1981). Human apolipoprotein E isoprotein subclasses are genetically determined. Am J Hum Genet, 33(1), 11–24. [PMC free article] [PubMed] [Google Scholar]