Abstract
Objective:
Examine the effects of a brief behavioral intervention for insomnia (BBTi) on sleep parameters, mood and cognitive functioning in older adults.
Methods:
Older adults (aged 65+) underwent four weekly sessions of BBTi or self-monitoring control (SMC). Participants completed 14 days of sleep diaries and actigraphy measuring sleep onset latency (SOL), wake after sleep onset (WASO), total sleep time (TST), sleep efficiency (SE), and sleep quality ratings at baseline, post-treatment, and 3 month follow-up. Participants also completed mood scales (Geriatric Depression Scale, GDS; Beck Depression Inventory-II; and State Trait Anxiety Inventory) and neuropsychological testing (measuring global cognition, language, memory, attention and processing speed, and executive function) at the three timepoints.
Results:
Significant condition (BBTi vs. SMC) x time (baseline vs. post-treatment vs. follow-up) interactions revealed that BBTi improved relative to baseline in sleep diary reported SOL, WASO, SE, and sleep quality, and these improvements were maintained at follow-up. SMC showed no change on these measures. A main effect of time showed that actigraphically measured WASO improved from baseline for both BBTi and SMC at post-treatment. A main effect of time revealed that both BBTi and SMC patients endorsed fewer GDS symptoms relative to baseline at post-treatment and follow-up. We observed no change in performance on neuropsychological measures.
Conclusions:
A four week BBTi is an efficacious intervention for reducing insomnia symptoms in older adults. BBTi does not selectively improve mood or cognitive functioning. Future work should examine effects of BBTi on physiological measures of sleep architecture and day to day cognition.
Keywords: insomnia, treatment outcome, elderly, brief behavioral intervention, mood, cognition
1. Introduction
Insomnia is defined as difficulty initiating, maintaining or experiencing non-restorative sleep concurrent with distress or impairment in critical areas of everyday functioning such as increased daytime fatigue, negative mood, and poor concentration (American Psychiatric Association, 2013). Given that the amount of deep sleep (i.e., slow wave and REM) declines with age (Bliwise, 1993), older adults are at greater risk for middle of the night and early morning awakenings compared to younger individuals (Ohayon, Carskadon, Guilleminault, & Vitiello, 2004). In fact, insomnia is the most common sleep disorder in later life and impacts approximately 20-50% of older adults [≥65 years; (D. J. Foley, Monjan, Simonsick, Wallace, & Blazer, 1999; Ohayon, Zulley, Guilleminault, Smirne, & Priest, 2001)]. While pharmacological treatment of insomnia in old age continues to be much more common, there is a robust body of evidence that psychological treatments for insomnia, generally called cognitive behavioral therapy for insomnia (CBT-I) are highly efficacious (C. M. Morin et al., 2006), can result in fewer adverse side effects (Omvik, Pallesen, Havik, Kvale, & Nordhus, 2006) and are preferred (Vincent & Lionberg, 2001) relative to pharmacological treatments. In fact, the Amercian College of Physicians now recommends CBT-I as the first line of treatment for older adults with insomnia (Qaseem, Kansagara, Forciea, Cooke, & Denberg, 2016). The majority of the studies on CBT-I to date have utilized relatively lengthy treatment protocols (weekly one hour individual sessions for 6 to 8 weeks). The current study investigates the impact of brief behavioral treatment for insomnia (BBT-I) on sleep, mood and cognitive outcomes in older adults.
1.1. Primary vs. Comorbid Insomnia in Older Adults
Despite the fact that approximately 80-90 % of patients with insomnia also have a concurrent medical or psychological diagnosis (Taylor et al., 2007), previous research on older adults has focused on insomnia in isolation. This was mainly due to the belief that insomnia symptoms which occurred in the context of another medical or psychological condition were “secondary” to that condition. Thus, the treatment focus was on the “primary” non-sleep condition, and the sleep problem was not typically treated independently (Zorick & Walsh, 2000). However, there has been a significant shift in the clinical and research conceptualization of insomnia based on evidence which was outlined in a 2005 State-of-the-Science Conference regarding chronic insomnia (NIH, 2005). Based on the recommendations from that conference, insomnia is now conceptualized as comorbid, rather than secondary in nature when there are concurrent medical or psychological diagnoses. There is a growing body of empirical research which indicates that insomnia can be independently treated without the need to first resolve the comorbid condition (Jungquist et al., 2010; Morin, Kowatch, & Wade, 1989; Quesnel, Savard, Simard, Ivers, & Morin, 2003; Rybarczyk et al., 2005; Sweetman et al., 2017). However, many of the studies to date among older adults have specifically excluded individuals based on the presence of comorbid medical or psychological conditions (Morin & Azrin, 1988; Lichstein et al., 2001; Riedel et al., 1995; Silversetien et al., 2006). Given that conditions such as chronic pain, depression, and anxiety are particularly common among older individuals with chronic insomnia (D. Foley, Ancoli-Israel, Britz, & Walsh, 2004), a strength of the current study is its inclusion of older individuals with a wide variety of comorbid medical and psychological symptoms. Importantly, results obtained for these individuals are more likely to be representative of the average older adult population with insomnia.
1.2. Efficacy of CBT-I
The efficacy of CBT-I is well established (Irwin, Cole, & Nicassio, 2006; Montgomery & Dennis, 2003), and research has shown it is superior to benzodiazepine and non-benzodiazepine sleeping medications for the long term treatment of insomnia (Mitchell, Gehrman, Perlis, & Umscheid, 2012). However, when delivering a multi-component treatment such as CBT-I, determining the critical elements of the intervention is not possible. A full decomposition study on CBT-I has not been performed to determine the most impactful elements of the treatment, but there is evidence to suggest that more succinct models of psychological treatment for insomnia may be as efficacious as longer treatment schedules among older adults (Irwin et al., 2006; Pallesen et al., 2003). Several techniques including stimulus control, progressive muscle relaxation, and the cognitive technique of paradoxical intention have been judged to meet criteria as well established standalone treatments (Montgomery & Dennis, 2003). In addition, there is some evidence to suggest that cognitive therapy can be efficacious as a standalone treatment (Harvey, Sharpley, Ree, Stinson, & Clark, 2007). Sleep hygiene has not been previously found to be an efficacious standalone treatment (Stepanski & Wyatt, 2003) while relaxation and stimulus control have been found to be efficacious (Lacks, Bertelson, Gans, & Kunkel, 1983; C. M. Morin et al., 2006). Of CBT-I studies in older adults, three to date have utilized either a more brief inperson behavioral intervention of 4 sessions (Lovato, Lack, Wright, & Kennaway, 2014; Pallesen et al., 2003) or a brief mixed in-person and telephone intervention of 4 sessions (Buysse et al., 2011). In the Palleson and colleagues study, a comparison of sleep hygiene and relaxation, sleep hygiene and stimulus control, and wait-list control found no difference in efficacy between the two treatment approaches and both groups showed significant improvement compared to a waitlist group (Pallesen et al., 2003). In Buysse and colleagues’ study, BBTi resulted in significant improvements in subjective and objective sleep measures relative to a control group that read brochures on sleep, aging, and sleep hygiene; however, the two groups were not matched on time spent with a therapist. Similarly, in Lovato and colleagues study evaluating small group-session BBTi, improvements in subjective wake after sleep onset, sleep efficiency, and daytime functioning were observed relative to a waitlist control group at post-treatment relative to baseline. The present study adds to this sparse literature by testing the impact of inperson delivery of a 4 session BBTi protocol on primary subjective and objective sleep outcomes compared to a self-monitoring control that provided equivalent contact with study personnel.
1.3. Insomnia and mood disturbance
Chronic insomnia has been linked to worse mood across the lifespan (Cole & Dendukuri, 2003; Johnson, Roth, & Breslau, 2006; Ohayon, Caulet, & Lemoine, 1998), with nearly 90% of participants with insomnia symptoms in one epidemiological study reporting symptoms of depression or anxiety (Ohayon et al., 1998). While sleep disturbance is included in the diagnostic criteria for numerous mood and anxiety disorders, insomnia often develops an independent course over time as patients adopt compensatory behaviors and thought patterns intended to help improve their sleep, but which often have the opposite effect. Over time, the insomnia is maintained by these factors rather than those that contributed to its onset (McCrae, 2009). Additionally, there is considerable evidence that insomnia is a risk factor for subsequent development of depressive symptoms (Johnson et al., 2006; Riemann & Voderholzer, 2003). Daily patterns of covariation have been noted between disturbed sleep and daytime functioning. For instance, greater subjective sleep disturbance have been shown to predict worse mood (McCrae et al. 2008) and global daytime functioning the next day (Smith, Lack, Lovato, & Wright, 2015). While the underlying mechanisms for this relationship remain to be determined, it is clear that sleep and mood factors are highly related. There is some evidence to suggest that the use of CBT-I in the treatment of insomnia can result in improvements in depression and anxiety symptoms (Belleville, Cousineau, Levrier, & St-Pierre-Delorme, 2011; Manber et al., 2008). However, these findings have not been consistent across the existing research and deserve further investigation to determine whether brief interventions for insomnia can result in improvements in other mental health symptoms.
1.4. Insomnia and cognition
Changes in cognition concurrent with aging have been documented across a number of domains including memory, attention, processing speed, and executive functioning. In addition, there is evidence that self-reported sleep disturbance among older adults is related to cognitive performance impairments in the domains of memory, attention and executive function (Bastien et al., 2003; Hauri, 1997; Schmutte et al., 2007; Vignola et al., 2000). While there is evidence to suggest a relationship between subjective sleep disruption and cognitive functioning (Fortier-Brochu & Morin, 2014), results across numerous studies have not been consistent among crosssectional and longitudinal designs, with many studies finding no meaningful differences between those with and without insomnia. (Fortier-Brochu, Beaulieu-Bonneau, Ivers, & Morin, 2012; Lovato, Lack, Wright, Cant, & Humphreys, 2013; Riedel & Lichstein, 2000). These inconsistent findings may be partially explained by small sample sizes, methodological differences in the assessment of insomnia symptoms, and the different populations under study (Fortier-Brochu et al., 2012; Riedel & Lichstein, 2000). The relationship between sleep disruption and cognitive impairment may be moderated by the relative amount of cognitive reserve/resilience, measured by proxy using educational attainment, such that individuals with greater pre-morbid education display less cognitive impairment in the presence of sleep onset or maintenance difficulties (Zimmerman, Bigal, Katz, Brickman, & Lipton, 2012). In addition, independent of the effects of health problems or use of sleeping medication, chronic insomnia has been found to be associated with an increased risk for cognitive decline among older men compared to those without insomnia but not among older women (Cricco, Simonsick, & Foley, 2001). Results from a secondary analysis in Buysse and colleagues BBTi study supported these suggestions and showed that the intervention did not improve executive function or episodic memory over time or relative to an informational control group (Wilckens, Hall, Nebes, Monk, & Buysse, 2016). However, the authors note that the length of treatment (two in person and two telephone sessions) and/or the follow-up period may not have been long enough to detect cognitive changes (Wilckens et al., 2016). At this time there is not sufficient evidence to conclude that treatment of insomnia symptoms will result in improvements in cognitive functioning (Dzierzewski, Dautovich, & Ravyts, 2018). However, there is a clear need for additional research into the potential effects of sleep disruption on cognitive functioning among older adults.
1.5. Goals of the Present Study
In sum, the present study investigated the effect of a BBTi in older adults with insomnia on sleep, cognition, and mood outcomes, immediately following treatment and at a three-month follow-up. We hypothesized that compared to self-monitoring controls (SMC), older adults with insomnia who undergo BBTi would exhibit significant improvements in self-reported sleep quality and self-reported and actigraphically measured outcomes related to insomnia [sleep onset latency (SOL), wake after sleep onset (WASO), total sleep time (TST), sleep efficiency (SE)] and those gains would either be maintained or further improved at three-month follow-up. We further hypothesized that compared to SMC, older adults with insomnia who undergo BBTi would exhibit significant improvements in anxiety and depression immediately following treatment, and those gains would either be maintained or further improved at 3 month follow-up. Finally, given lack of prior conclusive findings regarding the relationship between insomnia and cognition, it is unclear whether compared to SMC, older adults with insomnia who undergo BBTi would exhibit significant improvements in cognitive performance across various domains (i.e., global cognition, attention/processing speed, memory, language and executive functioning). Given the large range of sleep (both self-reported and objective), cognition, and mood evaluations, results of the present study will provide insight into the mechanisms underlying efficacy of BBTi and/or help determine whether sleep disruption is an important pathway associated with mood and cognition in older adults.
2. Methods
2.1. Overview
The present trial compared changes in sleep, mood, and cognitive performance in older adults with insomnia immediately and 3 months following 4 weeks of BBTi or SMC. The University of Florida’s Institutional Review Board (IRB-02) approved the trial protocol. All participants gave written informed consent to participate. This trial is registered at www.clinicaltrials.gov (NCT02967185).
2.2. Participants
Participants (N = 62) were recruited from Gainesville and surrounding areas through newspaper and other community advertisements. Sample size was determined by an a priori power analysis in which we estimated moderate effects (ES = .5) for the group by time interactions. For the group comparisons and the group by time comparisons with the control condition, power was expected to exceed .8, with alpha at .05, with 30 participants per group for the sleep and cognition outcomes.
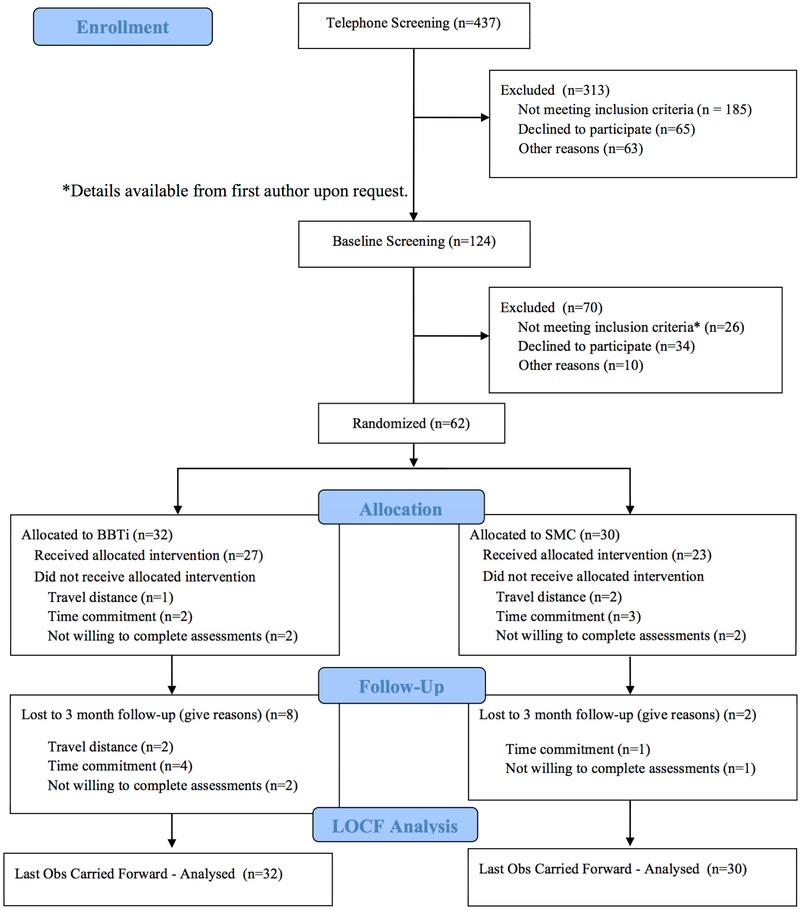
General inclusion criteria were: aged 65 or older, willing to undergo randomization, and able to read and understand English. Chronic insomnia criteria were: insomnia complaints (sleep onset or awake time during night > 30 minutes) at least three nights per week for more than 6 months; sleep diary confirmation of insomnia (sleep onset or awake time during night > 30 minutes) at least six nights during the two week baseline period; daytime dysfunction due to insomnia (mood, cognitive, social or occupational impairment); and no prescribed or over-the-counter sleep medication for at least one month, or stabilized on medication for at least six months. Exclusion criteria were: sleep disorder diagnosis other than insomnia, specifically sleep apnea and periodic limb movements disorder; suspected sleep-disordered breathing based on a single-night ambulatory monitoring (Compass F10; Embla, Broomfield, CO) of blood-oxygen saturation and respiration indicating an apnea-hypopnea index of greater than 15/hour with oxygen saturation below 93%; significant medical (e.g., cancer) or neurological (e.g., dementia) disorder; severe untreated psychiatric comorbidity (e.g., schizophrenia, substance abuse); severe depressive symptomatology [BDI-II (Beck, Steer, Garbin, 1996) score higher than 23 or GDS (Yesavage, 1983) score higher than 12]; cognitive impairment based on Mini-Mental State Examination score below 23 (9th grade education or higher) or below 18 [less than 9th grade education; (Murden, McRae, Kaner, & Bucknam, 1991)]. See Figure 1 for the CONSORT flow diagram for the trial. Once randomized, participants withdrew from the trial for one of three reasons (travel distance, time commitment, not willing to complete assessments). There were no significant harms or unintended effects.
Figure 1.
Participant Recruitment CONSORT-Style Flow Diagram
Participants were compensated $40. Participants received the treatment and parking on the UF campus at no charge. SMC participants were offered the treatment following completion of their follow up assessments.
A physician (R. B. B.) board certified in sleep medicine reviewed all ambulatory monitoring records. Advanced doctoral students in UF’s American Psychological Association (APA) accredited predoctoral programs in clinical and counseling psychology conducted all screening interviews using structured and semi-structured instruments. A licensed clinical psychologist (C. S. M.) certified in behavioral sleep medicine supervised all screening interviews and confirmed final insomnia diagnoses.
2.3. Procedures
2.3.1. Screening
First, the project coordinator conducted a brief, structured telephone interview to address inclusion/exclusion criteria and establish probable insomnia diagnosis. At the lab, study personnel conducted a semi-structured clinical interview, administered questionnaires to assess mood and cognitive status, and administered the neuropsychological assessment battery. Then, participants underwent a single night of ambulatory monitoring of blood-oxygen saturation and respiration in their own homes to rule out suspected sleep disordered breathing. Finally, two weeks of sleep diaries were also collected to confirm the insomnia diagnosis. Screening, with the exception of the telephone and clinical interview, also served as the baseline assessment.
2.3.2. Randomization and masking
Participants were randomly assigned to condition by computer-generated block randomization. They provided informed consent and completed the baseline assessment period prior to being informed of their assignment by the project coordinator. Researchers involved in recruitment and who obtained and assessed outcomes were masked to assignment, as were the statisticians who undertook the analysis. Due to the nature of the treatment, interventionist and participant masking was not possible.
2.3.3. BBTi Intervention
A manualized, 4-week behavioral treatment program for insomnia was individually administered and included instruction on four techniques shown to be effective for treating late life insomnia (Lichstein & Morin, 2000): sleep hygiene, stimulus control, sleep restriction, and relaxation. Session 1 involved sleep education, sleep hygiene, and stimulus control. Sleep education provided a brief overview of sleep and aging, and a general discussion of the impact of behaviors and cognitions on sleep. For sleep hygiene and stimulus control, participants were asked to follow specific instructions when implementing each technique at home. The sleep hygiene instructions included avoiding caffeine after noon and avoiding exercise, nicotine, alcohol, and heavy meals within two hours of bedtime. The stimulus control instructions were as follows: 1) Do not use your bed or bedroom for anything (anytime) but sleep (or sex). 2) If you do not fall asleep within 15–20 minutes, leave the bed and do something non-stimulating in another room. Return to bed only when sleepy. If you do not fall asleep within 15-20 minutes upon returning to bed, repeat this instruction as needed. 3) If you wake up and do not fall back asleep within 20 minutes, follow #2 again. 4) Avoid napping. If a participant was not willing to stop napping altogether, the therapist encouraged them to limit naps to earlier in the day (preferably before noon) for 1 hour or less and to always nap in their bed. For instructions 2 and 3, participants were specifically told not to use the clock to determine when 15-20 minutes had passed. Additionally, the therapist and participant worked together to create a specific plan for where the participant would go after leaving the bedroom and what they would do. Together, they created a written list (in the participant’s workbook) of at least three potential nonstimulating activities that the participant could engage in after leaving the bedroom.
Session 2 introduced sleep restriction which involved tailoring the amount of time spent in bed to the participant’s reported average total sleep time at baseline. Specifically, the therapist calculated an initial time in bed (TIB) prescription based on the participant’s average total sleep time at baseline plus 30 minutes. If this was 5 hours or less, the TIB prescription was set at 5 hours. The therapist and participant worked together to set regular bed- and waketimes to help the participant follow the prescription. The TIB prescription was reviewed and adjusted during sessions 3 and 4 (if needed). To determine if an adjustment was necessary, the therapist calculated the participant’s average TST, TIB and SE for the prior week. The TIB prescription was adjusted if the following criteria were met. If not, the TIB prescription remained the same.
-
1)
If their average SE% was >90% and the participant reported daytime fatigue/sleepiness, 30 minutes were added to their TIB prescription, and necessary adjustments in bed- and waketimes were discussed.
-
2)
If their average SE% was <85%, 30 minutes was subtracted from their TIB prescription, and necessary adjustments in bed- and waketimes were discussed.
Session 3 focused on passive relaxation (10 minutes). The therapist followed a script to lead the participant through the relaxation exercise during the session. This was audiotaped and given to participants for daily practice at home. Specifically, participants were asked to complete the relaxation exercise at least twice each day, once during the day and once at bedtime. They were also instructed to incorporate relaxation into their stimulus control practice during the night. Each time they awoke during the night and were unable to fall back asleep within 15-20 minutes, they were asked to practice the relaxation exercise. If still unable to fall back to sleep, they were to get out of bed, go into another room, and follow the stimulus control instructions as previously described. Importantly, for each nighttime awakening of 15-20 minutes, participants were instructed to use the relaxation exercise only once before proceeding with the stimulus control instructions as usual.
Session 4 involved a review of all techniques taught, discussion of challenges to implementation and how to address those challenges, and plans for continued practice of the techniques to help ensure on-going maintenance of treatment gains. In particular, the therapist and participant developed a plan for gradual relaxation of the TIB prescription/sleep restriction schedule over time. For example, the therapist and participant discussed targets for sleep quality, TIB, and bed- and waketimes and gradually allowing an earlier bed or later rising time, not to exceed 30 minutes a week, until those targets are reached.
Sessions were administered by a therapist for a duration of one hour per week. Each participant was provided with a participant workbook that contained detailed instructions on the sleep techniques for their assigned treatment condition, including the rationale for each. The workbook also contained copies of the sleep diaries and compliance logs. At each session, the therapist carefully reviewed the participants’ compliance logs and sleep diaries and provided the participants with graphs charting their sleep efficiency and total sleep time for the previous week. The treatment manual and workbook is available from C. S. M. upon request.
2.3.4. Control condition
Participants assigned to the SMC condition met for one hour weekly for 4 weeks with a therapist and participated in social conversations with the therapist that were not related to the BBTi intervention details or sleep, equating the duration of contact with study personnel in both groups. Control participants were provided with a workbook containing copies of the sleep diaries. Participants in the SMC group completed the same assessments (i.e., sleep diary, actigraphy) as the BBTi group, equating the self-monitoring of sleep aspect in both groups.
2.4. Therapists
The therapists were three predoctoral students in UF’s American Psychological Association (APA) accredited counseling psychology program. These therapists were trained and supervised by the first author, a licensed clinical psychologist certified in behavioral sleep medicine by the American Board of Sleep Medicine (CSM). Supervision was available during treatment sessions, and 1 hour, weekly group supervision meetings were held with the therapists.
2.5. Enactment/Adherence Logs
2.5.1. Compliance logs
Participants’ adherence to BBTi was monitored with daily logs over the course of treatment and posttreatment, as described below. Following each treatment session, compliance logs were reviewed, and any difficulties with compliance were troubleshooted. The rationale for the intervention was revisited to increase adherence as needed. To explore compliance to instructions, we compared the values of therapy assigned TIB to TIB reported on sleep diaries and here we found no differences (t = .35, p = .729).
2.5.2. Sleep Hygiene Log
For four weeks, following completion of the first session, participants indicated whether they had avoided caffeine after noon and each of the following 4 behaviors/substances within 2 hours of bedtime: alcohol, exercise, nicotine, and eating a heavy meal.
2.5.3. Stimulus Control Log
Subsequent to the first session, participants also filled out a stimulus control log for four weeks indicating whether they had followed each of the following instructions: (1) avoiding any activity in the bedroom other than sleep and sex; (2) leaving the bedroom if sleep does not happen within 15-20 minutes of going to bed, including getting up, going to another room and engaging in a calm activity until they are sleepy enough to return to bed; (3) during the night, leaving the bedroom if awakened for 15-20 minutes, including getting up, going to another room and engaging in a calm activity until they are sleepy enough to return to bed again; and (4) avoiding napping after 3 pm.
2.5.4. Relaxation Log
After the third session, participants were asked to practice relaxation twice a day and to provide the duration of the time spent engaging in relaxation, as well as, pre- and post-pulse (number of heart beats measured at the wrist or neck for 60 seconds) and relaxation ratings. Relaxation rating scores ranged from 1 (very aroused and upset) to 10 (completely and deeply relaxed) with a score of 5 indicating normal and calm.
2.6. Treatment Integrity & Credibility
2.6.1. Treatment Integrity
Several procedures were followed in order to maintain treatment integrity (Lichstein, Riedel, & Grieve, 1994). To ensure the treatment was delivered as intended, all treatment sessions were taped with 50% randomly selected for scoring by one of the authors. To ensure adequate comprehension of treatment, participants were questioned about their experience with home practice of techniques and procedural modifications were adopted as necessary (e.g., extending permissible amount of awake time in bed). Additionally, at the beginning of the 2nd inperson treatment session, participants completed a short quiz (10 questions) to ensure they had mastered the treatment rationale and procedures. To ensure adequate adherence to home practice assignments, the participant workbooks contained written instructions for each sleep technique, and participants completed daily compliance logs (see below) of their home practice sessions.
2.6.2. Treatment Credibility
Participants completed the Opinions about Treatment (see above) measure at the end of the 3rd in-person treatment session. To ensure truth in responding, participants were: (1) provided with an envelope and instructed to place their completed questionnaire in a sealed envelope before returning it, and (2) assured that their therapist would not have access to their responses. The sealed envelopes were delivered to the project coordinator for data entry and scoring. Participants provided confidential ratings (1=strongly disagree, 10=strongly agree) of treatment reasonableness, opinion of therapist, improvement expectations, and willingness to recommend treatment.
2.7. Treatment Outcomes
2.7.1. Sleep Measures.
2.7.1.1. Sleep Diaries
Participants were instructed to complete a sleep diary upon arising each morning throughout the study (10 weeks total = 2 weeks at baseline, posttreatment, and 3 month follow up + 4 weeks of treatment). The sleep diary provided the following variables: (1) sleep onset latency (SOL)-time from initial lights-out until sleep onset; (2) wake time after sleep onset (WASO)-time spent awake after initial sleep onset until last awakening; (3) total wake time (TWT)-computed by adding SOL, WASO, and time from last awakening until getting out of bed for the final time; (4) total sleep time (TST)-computed by subtracting TWT from time spent in bed; (5) sleep efficiency percentage (SE)-ratio of TST to total time spent in bed × 100; and (6) sleep quality rating-scaled from 1 (very poor) to 5 (excellent). A mean was computed for each variable for the 2-week baseline and posttreatment periods. The main outcomes of interest to the present study were SOL, WASO, TST, SE, and sleep quality rating.
2.7.1.2. Actigraphy
Participants wore an Actiwatch-L® (ACTL) with an integral ambient light sensor (Mini Mitter Co., Inc.) on their nondominant wrists continuously throughout the study (10 weeks). The ACTL monitors ambient light exposure and gross motor activity and contains an omnidirectional piezoelectric accelerometer with sensitivity of 0.01λg-force or greater. The sensors of the ACTL are sampled 32 times per second and record peak values for each second. These peak values are then summed into 30-second “activity” counts. These activity counts are downloaded to a personal computer and analyzed using Actiware-Sleep v. 3.3, which uses a validated algorithm to identify each epoch as sleep or wake. Bedtime and time out of bed in the morning were based on sleep diary entries as recommended in the software manual. Actiware-Sleep determined sleep start automatically by searching for the first 10 minutes during which no more than one epoch was scored as wake. Likewise, sleep end was the last 10 minutes during which no more than one epoch was scored as wake. The software provides three default sensitivity settings (high, medium, low). This study utilized medium sensitivity, which sets the threshold at 40 activity counts. If the total activity for an epoch was ≥ 40, it was scored as wake. If the total activity was ≤ 40, the final activity count for the epoch was based on the level of activity in the surrounding 2 minutes.
The following outcomes were computed from actigraphy assessments; SOL, WASO, TST, SE. Data loss was minimal. There were no equipment failures. During each assessment period, a few participants reported leaving their watches off for an entire day (24 hours): 3 at baseline, 2 at post-treatment, and 1 at follow-up. To make up for these lost days, these participants wore their watches and completed their sleep diaries for an additional day to ensure a full 14 days of concurrent sleep diary and actigraphy data at baseline, post-treatment, and follow-up.
2.7.2. Mood and Anxiety Outcomes
2.7.2.1. Geriatric Depression Scale (GDS)
The GDS (Yesavage et al., 1983) is the first depression scale designed specifically for screening for depression in older adults. It differs from other depression measures in that it eliminates questions about sexual and somatic complaints that may not be as salient for older adults. The scale consists of a ‘yes-no’ response format with each items scored as 1 or 0 for a possible total score of 30. Total scores are classified as: non-symptomatic (0-9), mild depression (10-19), and severe depression (20+) (Yesavage et al., 1983). The GDS has a well-established internal consistency with high Cronbach’s alpha reliabilities reported ranging from .87 (Adams, 2001) and .91 (Parmelee, Lawton, & Katz, 1998), to .94 (Yesavage et al., 1983). The GDS has been found to have high concurrent validity against other self-report measures of depression such as the Hamiliton Depression Rating Scale (Feher, Larrabee, & Crook, 1992) and the BDI-II (Jefferson, Powers, & Pope, 2001). The GDS has been shown to have both adequate sensitivity and specificity regarding the detection of depression in the elderly (Burke, Nitcher, Roccaforte, & Wengel, 1992).
2.7.2.2. Beck Depression Inventory-Second Edition (BDI-II)
The BDI-II (Beck, Steer, & Garbin, 1996) is a 21-item self-report questionnaire measuring severity of depressive symptomatology on a three-point scale (0 [absence of symptoms] to 3 [most severe]). Typically, respondents answer for the previous week, but the previous two weeks were used in the present study to match the two-week recording period. Scores range from 0 to 63. Ranges for clinical levels of depression are: under 10 = none or minimal, 10 to 18 = mild to moderate, 19 to 29 = moderate to severe, and over 29 = severe. The BDI-II has demonstrated adequate reliability and validity (Dozois, Dobson, & Ahnberg, 1998; Segal, Coolidge, Cahill, & O’Riley, 2008). Higher scores indicate greater maladjustment.
2.7.2.3. State Trait Anxiety Inventory-Form Y (STAI-Y).
The STAI-Y (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) contains 20 self-descriptive statements rated on a 4-point scale indicating how often the statement is true. Scores range from 20 to 80. The STAI-Y1 demonstrates test-retest reliability exceeding .7 and reliably distinguishes patient and normal groups (Kabacoff, Segal, Hersen, & Van Hasselt, 1997; Spielberger et al., 1983). The STAI-Y1 was also based on the previous two weeks. Higher scores indicate greater maladjustment.
2.7.3. Neuropsychological Battery
Several measures were included in the neuropsychological battery. See Table 1 for further descriptions.
Table 1.
Neuropsychological Test Battery
| Domain | Measure | Source |
|---|---|---|
| Overall Cognitive Functioning |
Mini-Mental Status Exam (MMSE) The MMSE (Folstein, Folstein, & McHugh, 1975) was used to assess cognitive impairment and tapped the following domains: orientation to time and place, memory, basic arithmetic, language use and comprehension, and basic motor skills. Scores range from 0-30 points with scores lower than 23 (9th grade education or higher) or lower than 18 (less than 9th grade education) indicating impairment. Test-retest reliabilities are acceptable, ranging from .48 - .65 (Tombaugh, 2005), and the MMSE is a valid measure of a breadth of cognitive functioning, particularly verbal learning and memory, in older adults (Mitrushina & Satz, 1991) |
Folstein, Folstein, McHugh, 1975 |
|
Vocabulary, Digit Symbol- Wechsler Adult Intelligence Scale-III Vocabulary. The Vocabulary subtest consists of a list of words measuring verbal expression and comprehension. Participants are shown the written form of the word as it is spoken aloud by the examiner. The participant then provides a verbal definition of the word that is recorded by the examiner. The vocabulary subtest has shown good test-retest reliability [(r = .94) (Dikmen, Heaton, Grant, & Temkin, 1999)] |
Wechsler, 1997 |
|
| Digit Symbol. The Digit Symbol subtest consists of a series of symbols that are paired with numbers. The participant draws each symbol under its corresponding number. There is a 120-second time limit for the task and the total score consists of summing the number of symbols correctly drawn by the participant. The Digit Symbol subtest has a test-retest reliability ranging from .84 to .93 as tested in age groups ranging from age 65 to 89 (Wechsler, 1997b)The Digit Symbol subtest is a valid measure of the constructs of speed processing and working memory within individuals age 65 and older (Joy, Kaplan, & Fein, 2004) | ||
| Attention, Processing Speed |
Trailmaking Test A In the Trailmaking Test A [TMT part A; (Reitan, 1959)] participants were presented with a white sheet of paper on which numbered circles were distributed. Participants were told to connect the circles in order (i.e., 1 to 2 to 3, etc.) as fast as they could. The primary score was the number of seconds needed to finish but the number of errors was also recorded. The errors were immediately pointed out by the examiner, and the participant was required to correct the error. Thereafter, the subject could continue in the proper sequence. Performance time was unlimited. Good test-retest reliability has been shown for TMT parts A (r = .75) (Giovagnoli et al., 1996). TMT A has been shown to be valid measures of attention and visuoperceptual abilities (O’donnell, Macgregor, Dabrowski, Oestreicher, & Romero, 1994; Sanchez-Cubillo et al., 2009) |
Reitan, 1959 |
| Language |
Controlled Oral Word Association (COWA)-Semantic Verbal Fluency Measure of an individual’s ability to make associations to specific categories (animals, fruits, vegetables, and names). There is a one minute time limit for each category (Benton, Hamsher, & Sivan, 1989) The COWA has shown acceptable test-retest reliability (Dikmen et al., 1999) and acceptable criterion validity with tests such as digit span, oral spelling, Stroop, and mental calculations (Lezak, Howieson, & Loring, 2004). |
Benton, Hamsher & Sivan 1989 |
|
Boston Naming Test (BNT) – Second Edition The BNT (Kaplan, Goodglass, & Weintraub, 2001) was used to assess language. The (BNT) is a wide-ranging confrontation naming test consisting of 60 pictures ordered from easiest to most difficult. The participant has 20 seconds to name the picture once presented. If the participant provides a response that is a misperception of the picture, he or she is provided with a stimulus cue (e.g. “It is something to eat”). If the participant fails to recognize or misnames the picture after the stimulus cue, he or she is provided with a phonemic cue (e.g. “Begins with ‘ph’”). The test is scored by summing all of the items the participant correctly named for a total possible score of 60 points. The BNT has an established concurrent validity with the Visual Naming Test of the Multilingual Aphasia Examination (Axelrod, Ricker, & Cherry, 1994). Acceptable test-retest reliability was observed in a sample of older adults (Flanagan & Jackson, 1997). |
Kaplan, Goodglass, Weintraub, 2001 | |
| Memory |
California Verbal Learning Test (CVLT-II) The CVLT-II is a measure of both the recall and recognition of word lists that is conducted over several trials. Participants are presented with a list of 16 words (four words from each of four sementic categories). Participants must then recall the lists over five trials, recall an interference list of words, recall tlhe original list of words, and deliver a category-cued recall of the first list of words. After a 20 minute delay, free recall, category-cued recall, and recognition of the first list are assessed. |
Delis, Kaplan, Kramer, & Ober, 2000 |
| The CVLT shows adequate criterion validity with good correlations with another word list assessment tool, the HVLT (Brandt, 1991). Correlations between the CVLT and the HVLT for the total number of words learned (.74) were good, while there were more moderate associations for discriminability (.46) and false-positive errors (.60) on the recognition testing portion of these tasks (Lacritz & Cullum, 1998). In terms of reliability, according to the test manual, internal consistency reliability ranges from .70 – .92 (Delis, Kramer, Kaplan, & Ober, 1987). Test-retest reliability with a sample of healthy older adults was .64 (Cellucci, Evans, Cattaruzza, & Carter, 2001). | ||
|
Rey-Osterreith Complex Figure Test (Rey-O) The Rey-O (Osterrieth, 1944; Rey, 1941)was used to assess memory. The Rey-O is a measure of short and long-term visuographic memory. This test is administered in three stages: Copy where the participants are asked to copy the Figure, Immediate Recall (IR) where the respondents are asked to reproduce the Figure from memory, and Delay Recall (DR), where the respondent are asked again to reproduce the Figure after a 30-minute delay. The final score, ranging from 0 to 36, is separately scored for each of the three stages (Copy, IR, and DR). The complex visual memory measure was validated with healthy adults, older and/or depressed populations (Elderkin-Thompson et al., 2004). Interrater reliability estimates report correlation coefficient of .98 (Loring, Martin, Meador, & Lee, 1990; Strauss & Spreen, 1990). The Rey-O has been shown to be a valid measure of visuospatial perception and memory in an older adult sample (Berry, Allen, & Schmitt, 1991). |
Osterrieth, 1944; Rey, 1941 |
|
|
Logical Memory from Wechsler Memory Scale 3 (LM-WMS-III) The Logical Memory subtest of the Wechsler Adult Intelligence Scale – III (Wechsler, 1997a)was used to assess memory. The scale consists of a number of immediate and delayed auditory recall tasks. The examiner reads two short stories to the participant and then asks the examinee to recall details of the story immediately after each story is read and after a delay. Additionally, a recognition task is given that consists of questions about both stories requiring a yes or no response. |
Wechsler, 1997 |
|
| The Logical Memory subtest has a test-retest reliability ranging from .76 to .80 as tested in age groups ranging from age 55 to 89 (Wechsler, 1997a). In a sample of individuals age 77 and older, the logical memory total score was found to be significantly correlated with the mini-mental exam score suggesting the logical memory as useful tool for detecting cognitive impairment (Ferrario, Seccia, Massaia, Fonte, & Molaschi, 1998). | ||
| Executive Functioning |
COWA – Phonemic Fluency Measure of an individual’s ability to make associations to specific letters (F, A, and S). There is a time limit for each letter with one minute allotted for ‘F’ and ‘A’ and two minutes allotted for the ‘S’. (Benton et al., 1989) The COWA has shown acceptable test-retest reliability (Dikmen et al., 1999) and acceptable criterion validity with tests such as digit span, oral spelling, Stroop, and mental calculations (Lezak et al., 2004). |
Benton, Hamsher & Sivan 1989 |
|
TMT B In the TMT B (Reitan, 1959) participants were presented with a white sheet of paper on which numbered circles and letters were distributed. Participants were told to connect the circles in order (i.e., 1 to A to 2 to B, etc.) as fast as they could. The primary score was the number of seconds needed to finish but the number of errors was also recorded. The errors were immediately pointed out by the examiner, and the participant was requiredto correct the error. Thereafter, the subject could continue in the proper sequence. Performance time was unlimited. Good test-retest reliability has been shown for TMT Part B (r = .85) (Giovagnoli et al., 1996). TMT B has been shown to be a valid measure of cognitive flexibility, an executive function (Kortte, Horner, & Windham, 2002). |
Reitan, 1959 |
2.8. Statistical Analysis
2.8.1. Baseline Demographics and Clinical Characteristics
Differences between the BBTi vs SMC groups in baseline demographics and other characteristics were analyzed using analyses of variance (ANOVA) for continuous variables (age, number of health conditions, number of prescribed medications, number of over-the-counter medications, BMI, duration of insomnia duration, MMSE) and chi-square analyses for categorical variables (sex, marital status, ethnicity, education).
2.8.2. Treatment Outcomes
All randomized participants were included in analyses, following an intent to treat approach using the last observation carried forward method for missing data due to dropout. Separate univariate 2 (group: BBTi vs. SMC) X 3 (time: baseline vs. post-treatment vs. followup) repeated measures ANCOVAs were conducted for each of the sleep (diary and actigraphy), mood, and cognitive outcomes. Bonferroni correction was applied to control for family-wise error, resulting in the following alpha criteria for group × time interactions and main effects: .01 (.05/5) for sleep diary outcomes, .012 (.05/4) for actigraphy outcomes, .017 (.05/3) for mood outcomes, and .003 (.05/17) for neuropsychological measures. Greenhouse-Geisser correction was applied to control for violations of sphericity. Follow-up analyses of significant univariate interactions used repeated measures ANOVAs to examine within group simple effects of time, with Bonferroni control. Effect sizes were examined using the univariate partial eta squared values (η2p ) provided by the repeated measures ANCOVAs [values of .01, .06, and .14 indicate small, medium, and large effect sizes, respectively (Cohen, 1977)].
2.8.2. Clinical Significance
Clinical significance was evaluated using established cut-offs (Buysse et al., 2011). Participants were classified as responders if there was a 10% or greater increase in SE. They were classified as remitters if they met response criteria plus SE was 85% or greater, and sleep quality was rated as 2.5 or greater. Group differences were analyzed using using chi-square tests.
3. Results
3.1. Treatment Adherence
Of the sixty-two participants who were randomized, eight declined and did not participate in a single weekly session. Chi-square analyses to assess for systematic group differences in this baseline dropout rate were not significant [χ2 (1, N = 62) = 2.61, p = .11]. Of the remaining 54 participants, all completed the first 2 weeks, 3 dropped out after the second session, and 1 dropped out after the third session. The remaining participants completed all sessions (n = 50), and the majority of completers (n = 40) also returned for the 3-month follow-up. Chi-square analyses to assess for systematic group differences in post-treatment [χ2 (1, N = 62) = .59, p = .44] and follow-up [χ2 (1, N = 62) = .76, p = .38] drop-out rates were not significant. Average weekly adherence rates were >90% for Sleep Hygiene and Stimulus Control, and >80% for relaxation. Total adherence rates, which compared participants reported adherence to ‘per protocol’ adherence across the course of treatment, were somewhat lower, but were still >80% for Sleep Hygiene and Stimulus Control, and approached 70% for Relaxation. See Table 3 for details. To address whether participants adhered to sleep restriction guidelines, we explored our TIB data to see whether participants restricted their time in bed during treatment. In the BBTi group, we observed a reduction in TIB relative to baseline during the first half of treatment (p < .001), as well as the second half of treatment (p < .001) and post-treatment (p < .001). At 3 mo. followup, this reduction in TIB was not maintained (p = .99) Therefore, on average, participants were restricting their sleep as per treatment guidelines during the treatment portion of the study.
Table 3.
Adherence Rates (Percentages)a for Sleep Hygiene, Stimulus Control, and Relaxation.
| Sleep Hygiene | Stimulus Control | Relaxation | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Total | 86.19 | 20.13 | 83.01 | 20.21 | 68.83 | 30.43 |
| Week 1 | 95.03 | 9.66 | n/a | n/a | n/a | n/a |
| Week 2 | 95.16 | 10.05 | 90.25 | 17.22 | n/a | n/a |
| Week 3 | 96.19 | 9.45 | 92.56 | 12.98 | 82.14 | 26.17 |
| Week 4 | 93.33 | 10.63 | 90.82 | 11.88 | 81.51 | 23.56 |
Note. n/a – Stimulus Control introduced in Week 2, Relaxation introduced in Week 3.
Percentages based on: Sleep Hygiene (Weeks 1-4) = number of items adhered to/35; Total Sleep Hygiene = number of items adhered to across 4 weeks/140; Stimulus Control (Weeks 1-4) = number of items adhered to/28; Stimulus Control Total = number of items adhered to/84; Relaxation (Weeks 1-4) = number of days practiced/7; Total Relaxation = total number of items adhered to/14.
3.2. Treatment Credibility
Average total scores on the Opinions About Treatment measure indicate BBTi participants found the treatment to be highly credible (M = 8.25, SD = 1.90). Means and standard deviations by item were: reasonableness of treatment (M = 8.38, SD = 1.98); opinion of therapist (M = 8.81, SD = 1.94); improvement expectations (M = 7.92, SD = 1.87); and willingness to recommend treatment (M = 7.88, SD = 2.32). Two of the BBTi drop-outs and 2 participants who completed the protocol did not complete the treatment credibility measure.
3.3. Baseline Comparisons
The only significant baseline group difference was in duration of insomnia. Specifically, participants in the SMC reported a longer duration of insomnia on average than those in the BBTi group (see Table 2 for details). In all treatment outcome analyses reported below, analysis of covariance (ANCOVA) was used to control for this baseline difference in insomnia duration.
Table 2.
Sample Characteristics
| Total Sample N = 62 |
BBTi n = 32 |
SMC n =30 |
||||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years; M (SD) | 69.45 | (7.71) | 67.97 | (5.97) | 71.03 | (9.06) |
| Female (n; %) | 42 | 67.74 | 22 | 68.75 | 20 | 66.67 |
| Marital Status (n; %) | ||||||
| Single | 7 | 11.29 | 1 | 3.13 | 6 | 20.00 |
| Married | 31 | 50.00 | 17 | 53.13 | 14 | 46.67 |
| Cohabitating | 3 | 4.84 | 2 | 6.25 | 1 | 3.33 |
| Widowed | 8 | 12.90 | 5 | 15.63 | 3 | 10.00 |
| Divorced | 10 | 16.13 | 6 | 18.75 | 4 | 13.33 |
| Separated | 2 | 3.23 | 1 | 3.13 | 1 | 3.33 |
| Ethnicity (n; %) | ||||||
| White Non-Hispanic | 51 | 82.26 | 25 | 78.13 | 26 | 86.67 |
| White Hispanic | 4 | 6.45 | 2 | 6.25 | 2 | 6.67 |
| African American | 2 | 3.23 | 2 | 6.25 | 0 | 0 |
| Asian | 2 | 3.23 | 1 | 3.13 | 1 | 3.33 |
| More than 1 reported | 3 | 4.84 | 2 | 6.25 | 1 | 3.33 |
| Education (n; %) | ||||||
| No High School Diploma | 2 | 3.23 | 1 | 3.13 | 1 | 3.33 |
| High School Diploma | 6 | 9.68 | 3 | 9.38 | 3 | 10.00 |
| Some College | 10 | 16.13 | 3 | 9.38 | 7 | 23.33 |
| Associates Degree | 2 | 3.23 | 2 | 6.25 | 0 | 0 |
| Bachelor’s Degree | 12 | 19.35 | 7 | 21.88 | 5 | 16.67 |
| Master’s Degree | 17 | 27.42 | 8 | 25.00 | 9 | 30.00 |
| Doctoral Degree | 8 | 12.90 | 5 | 15.63 | 3 | 10.00 |
| Sleep and Health Characteristics | ||||||
| Conditions1; M (SD) | 2.17 | (1.29) | 1.90 | (1.06) | 2.43 | (1.45) |
| BMI2; M (SD) | 24.77 | (3.94) | 24.24 | (4.00) | 25.35 | (3.88) |
| Duration of insomnia; years; M (SD) | 13.88 | (15.33) | 9.51a | (12.37) | 18.55a | (16.95) |
| Medications | ||||||
| Prescribed3; M (SD) | 3.32 | (2.58) | 2.92 | (1.16) | 3.69 | (3.43) |
| Over-the-counter4; M (SD) | 2.18 | (1.88) | 2.11 | (2.17) | 2.25 | (1.57) |
| Prescribed sleep medication (n; %)5 | 17 | 31.5 | 9 | 30.0 | 8 | 33.3 |
| Overall Cognition | ||||||
| Mini-Mental Status Exam; M (SD) | 28.56 | (1.29) | 28.40 | (1.19) | 28.72 | (1.39) |
Total number of classes of health conditions from the following list: heart problems, cancer, hypertension, neurological disorder, breathing disorder, urinary problems, diabetes, pain, gastrointestinal disorders, and other.
Body Mass Index = (weight/2.2046)/(height/39.37)2.
Total number of prescribed medications participants listed.
Total number of over-the-counter medications participants listed.
Number of participants who reported using medication prescribed for sleep.
Significant difference between groups (p < .01).
3.4. Sleep Diary Outcomes
As shown in Table 4, significant group × time interactions were observed for SOL (p = .01), WASO (p < .01), SE (p < .01), and sleep quality rating (p < .01). The interaction involving TST trended towards significance (p = .08). Within group comparisons with Bonferroni adjustment revealed significant improvements in SOL, WASO, SE, and sleep quality rating from baseline to post-treatment in the BBTi group (ps < .05) and those improvements were maintained at 3 month follow-up (ps > .05). TST did not improve significantly from baseline to posttreatment for BBTi (p = .99), but trended toward improvement relative to baseline at the 3-month follow-up (p = .07). The SMC group did not exhibit improvement relative to baseline in any of the sleep diary variables either at posttreatment or followup (all ps < .05).
Table 4.
Sleep Diary Outcomes - Last Observation Carried Forward Analyses
| Measures | Baseline | Post-Treatment | 3-mo Follow-up | Within Groups |
Between Groups |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to Posta |
Post to Follow-upa |
Base to Follow- upa |
Group × Timeb. c | |||||||||
| M | (SD) | M | (SD) | M | (SD) | Δ (Hedges gav) | Δ (Hedges gav) | Δ (Hedges gav) | F | p | η2p | |
| Sleep Onset Latency (mins.) | 4.96 | .01 | .08 | |||||||||
| BBTi | 51.32 | (37.31) | 27.37 | (25.05) | 29.37 | (24.77) | −23.95 (.75)*** | 2.00 (.08) | −21.95 (.69)*** | |||
| SMC | 41.9 | (41.79) | 34.79 | (32.29) | 37.15 | (56.06) | −7.11 (.19) | 2.36 (.05) | −4.75 (.10) | |||
| Wake After Sleep Onset (mins.) | 8.19 | .00 | 0.12 | |||||||||
| BBTi | 66.95 | (37.24) | 40.84 | 31.87 | 41.64 | (31.08) | −26.11 (.75)*** | 0.80 (.03) | −25.31 (.74)*** | |||
| SMC | 51.66 | (25.02) | 46.01 | 33.6 | 52.15 | (34.18) | −5.65 (.19) | 6.14 (.18)* | 0.49 (.02) | |||
| Total Sleep Time (mins.) | 2.79 | .08 | 0.05 | |||||||||
| BBTi | 349.17 | (68.13) | 355.15 | 68.46 | 371.94 | (71.46) | 5.98 (.09) | 16.79 (.24)* | 22.77 (.33) | |||
| SMC | 373.09 | (91.38) | 390.43 | 77.04 | 377.29 | (85.33) | 17.34 (.21) | −13.14 (.16) | 4.20 (.05) | |||
| Sleep Efficiency (%) | 10.14 | .00 | 0.15 | |||||||||
| BBTi | 69.56 | (12.19) | 80.47 | 12.12 | 80.64 | (12.31) | 10.91 (.90)*** | 0.17 (.01) | 11.08 (.90)*** | |||
| SMC | 75.47 | (12.86) | 78.74 | 12.2 | 77.6 | (14.02) | 3.27 (.26) | −1.14 (.09) | 2.13 (.16) | |||
| Sleep Quality Rating | 6.68 | .00 | 0.1 | |||||||||
| BBTi | 2.69 | (0.55) | 3.07 | 0.63 | 3.11 | (0.59) | 0.38 (.64)*** | 0.04 (.07) | 0.42 (.74)*** | |||
| SMC | 2.96 | (0.52) | 3.05 | 0.62 | 2.99 | (0.56) | 0.09 (.16) | −0.06 (.10) | 0.03 (.06) | |||
Note. BBTi refers to Brief Behavioral Treatment of insomnia group (n = 32); SMC refers to Self-monitoring Controls (n = 30).
Hedges gav = within group (change) effect size. Guidelines for interpreting Hedges gav - 0.20 = small, 0.50 = moderate, 0.80 = large
Greenhouse-Geisser correction was applied to Group × Time interaction.
Guidelines for interpreting η2p: .01 = small, .06 = moderate, .14 = large (Cohen, 1977)
p < .05 (after Bonferroni correction)
p < .01 (after Bonferroni correction)
p < .001 (after Bonferroni correction)
3.5. Actigraphy Outcomes
There were no significant group × time interactions for WASO, SOL, TST, or SE (all ps > .012, see Table 5). Main effects of group and time did not reach the Bonferroni adjusted statistical significance cutoff for SOL, SE, or TST (all ps > .012). However, a main effect of time for WASO was observed, F (1.57, 92.4) = 5.61, p = .009, η2p = .09. Regardless of condition, WASO significantly decreased (p = .01) from baseline (M = 42.3, SD = 16.7) to post-treatment (M = 36.5, SD = 16.2). However, at follow-up (M = 37.2 , SD = 17.7), WASO did not differ from baseline (p = .08) or post-treatment (p = 0.99) values.
Table 5.
Actigraphy Outcomes – Last Observation Carried Forward Analyses
| Measures | Baseline | Post-treatment | 3-mo Follow-up | Group × Timea,b | |||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | η2p | p | |
| Sleep Onset Latency (mins.) | 1.97 | .03 | .15 | ||||||
| BBTi | 19.19 | 18.36 | 13.94 | 12.33 | 20.37 | 21.78 | |||
| SMC | 22.13 | 18.51 | 24.00 | 20.07 | 23.60 | 20.20 | |||
| Wake After Sleep Onset (mins.) | 2.57 | .04 | .09 | ||||||
| BBTi | 42.60 | 18.65 | 32.05 | 17.06 | 33.83 | 17.48 | |||
| SMC | 41.85 | 14.87 | 41.00 | 15.10 | 40.40 | 15.10 | |||
| Total Sleep Time (mins.) | .89 | .02 | .41 | ||||||
| BBTi | 395.63 | 43.46 | 371.79 | 47.69 | 379.71 | 49.41 | |||
| SMC | 385.52 | 66.65 | 375.75 | 63.46 | 375.33 | 55.37 | |||
| Sleep Efficiency (%) | 3.05 | .05 | .07 | ||||||
| BBTi | 84.65 | 7.36 | 86.39 | 7.36 | 85.53 | 7.79 | |||
| SMC | 83.42 | 6.82 | 82.58 | 7.05 | 82.17 | 7.27 | |||
Note. BBTi refers to Brief Behavioral Treatment of insomnia group (n = 32); SMC refers to Self-monitoring control group (n = 30).
Greenhouse-Geisser correction was applied to Group × Time interaction.
Guidelines for interpreting η2p: .01 = small, .06 = moderate, .14 = large (Cohen, 1977)
3.6. Mood and Anxiety Outcomes
There were no significant group × time interactions for BDI-II, GDS, or STAI-Y measures (all ps > .017, see Table 6). However we did observe a main effect of time for GDS, F (1.57, 86.05) = 8.12, p = .002, η2p= .13. Regardless of condition, pairwise comparisons with Bonferroni adjustment showed that all participants reported fewer depressive symptoms (p = .05) at post-treatment (M = 3.05, SD = 3.80) relative to baseline (M = 3.65, SD = 1.86). This pattern persisted, as patients also reported fewer depressive symptoms (p = .006) at follow-up (M = 2.80, SD = 3.83) relative to baseline. There were no main effects of time for BDI-II and STAI-Y (ps > .017). There were also no main effects of condition for any mood outcome (ps > .017)
Table 6.
Mood and Anxiety Outcomes
| Baseline | Post-Treatment | 3-mo Follow-up | Group × Timea,b | |||
|---|---|---|---|---|---|---|
| Measure | Mean (SD) |
Mean (SD) |
Mean (SD) |
F | p | η2p |
| BDI-II (raw score) | ||||||
| BBTi | 9.77 (11.28) | 8.90 (11.12) | 8.42 (11.29) | .74 | .47 | .013 |
| SMC | 10.00 (6.51) | 8.39 (6.45) | 8.71 (6.52) | |||
| GDS (raw score) | ||||||
| BBTi | 3.29 (4.28) | 3.03 (4.35) | 2.71 (4.42) | 2.25 | .11 | .039 |
| SMC | 4.04 (7.95) | 3.11 (3.17) | 2.93 (3.11) | |||
| STAI-Y (raw score) | ||||||
| BBTi | 33.19 (12.84) | 33.45 (12.79) | 33.45 (12.95) | 1.58 | .21 | .027 |
| SMC | 38.96 (10.23) | 37.18 (10.97) | 39.32 (12.34) | |||
Note. BBTi refers to Brief Behavioral Treatment of insomnia group (n = 32); SMC refers to Self-monitoring and Attention Controls (n = 30).
Abbreviations: BDI-II = Beck Depression Inventory-II; GDS = Geriatric Depression Scale; STAI-Y = State Trait Anxiety Inventory Form
Greenhouse-Geisser correction was applied to Group × Time interaction.
Guidelines for interpreting η2p: .01 = small, .06 = moderate, .14 = large (Cohen, 1977)
3.7. Neuropsychological Performance
As shown in Table 7, there were no significant group × time interactions for any of the measured neuropsychological outcomes (all ps > .003). There were also no main effects of time, (all ps > .003) or treatment condition (all ps > .003) for any outcome. Thus, for both BBTi and SMC groups, performance on neuropsychological tests did not change from baseline to post-treatment to follow-up.
Table 7.
Neuropsychological Performance Outcomes
| Baseline | Post-treatment | 3-mo Follow-up | Group × Timea,b | |||
|---|---|---|---|---|---|---|
| Cognitive Measure | Mean (SD) |
Mean (SD) |
Mean (SD) |
F | p | η2p |
| Overall Cognitive Functioning | ||||||
| MMSE (raw score) | ||||||
| BBTi | 28.51 (1.20) | 29.00 (1.52) | 28.81 (1.44) | .561 | .563 | .011 |
| SMC | 28.66 (1.36) | 28.86 (1.58) | 29.00 (1.19) | |||
| Vocabulary (raw score) | ||||||
| BBTi | 52.41 (7.22) | 52.89 (7.54) | 53.78 (7.94) | .908 | .410 | .034 |
| SMC | 53.59 (7.95) | 55.48 (6.99) | 54.30 (9.58) | |||
| Digit Symbol (raw score) | ||||||
| BBTi | 66.85 (12.22) | 70.65 (12.93) | 68.18 (13.34) | .645 | .529 | .024 |
| SMC | 66.83 (13.37) | 69.26 (12.80) | 68.41 (12.18) | |||
| Attention, Processing Speed | ||||||
| Trailmaking Test Part A (seconds) | ||||||
| BBTi | 34.31 (13.59) | 33.98 (9.22) | 33.36 (9.46) | 1.477 | .238 | .055 |
| SMC | 36.98 (14.09) | 32.77 (13.51) | 24.51 (12.71) | |||
| Language | ||||||
| COWA-Semantic Verbal Fluency Score | ||||||
| BBTi | 60.81 (9.97) | 62.30 (10.26) | 62.84 (9.94) | 1.785 | .178 | .067 |
| SMC | 60.45 (13.39) | 64.82 (16.21) | 62.84 (9.94) | |||
| Boston Naming Test (raw sore) | ||||||
| BBTi | 55.06 (7.18) | 56.35 (5.83) | 56.39 (8.23) | .229 | .796 | .009 |
| SMC | 57.79 (2.03) | 57.67 (5.79) | 58.67 (1.90) | |||
| Memory | ||||||
| CVLT-II – Immediate Recall (raw score) | ||||||
| BBTi | 44.45 (10.19) | 47.79 (13.25) | 46.93 (13.02) | 1.472 | .234 | .025 |
| SMC | 44.96 (9.24) | 49.19 (11.19) | 51.42 (12.01) | |||
| CVLT-II - Short Delay Recall | ||||||
| BBTi | 8.92 (4.03) | 10.57 (4.27) | 11.04 (4.28) | .057 | .945 | .001 |
| SMC | 8.60 (3.50) | 10.06 (3.85) | 10.74 (3.31) | |||
| CVLT-II - Long Delay Recall (raw score) | ||||||
| BBTi | 9.29 (3.84) | 10.86 (3.75) | 11.46 (3.50) | .013 | .979 | .000 |
| SMAC | 8.77 (3.82) | 10.26 (4.06) | 10.97 (3.81) | |||
| Rey-O – Immediate Recall (raw score) | ||||||
| BBTi | 16.19 (6.00) | 19.08 (6.15) | 20.16 (7.13) | .213 | .809 | .008 |
| SMAC | 15.49 (5.20) | 19.13 (5.96) | 20.22 (6.24) | |||
| Rey-O – Delayed Recall (raw score) | ||||||
| BBTi | 15.37 (5.69) | 18.22 (5.98) | 18.98 (7.04) | .155 | .857 | .006 |
| SMC | 18.01 (16.15) | 19.15 (5.94) | 19.70 (6.58) | |||
| LM-WMS- Story A Immediate Recall unit score | ||||||
| BBTi | 12.71 (3.65) | 14.40 (3.94) | 15.77 (4.01) | .706 | .499 | .027 |
| SMC | 11.90 (3.86) | 14.29 (4.22) | 14.86 (4.27) | |||
| LM-WMS-Story B Immediate Recall unit score | ||||||
| BBTi | 9.68 (3.44) | 12.47 (4.00) | 13.31 (4.35) | .584 | .561 | .022 |
| SMC | 10.87 (3.24 | 13.18 (3.62) | 13.46 (3.83) | |||
| LM-WMS-Story A Delayed Recall unit score | ||||||
| BBTi | 9.61 (5.21) | 12.07 (5.14) | 14.23 (5.55) | 1.403 | .255 | .052 |
| SMC | 8.97 (4.38) | 12.36 (5.02) | 13.29 (5.29) | |||
| LM-WMS-Story B Delayed Recall unit score | ||||||
| BBTi | 12.47 (4.25) | 14.58 (4.11) | 15.77 (4.60) | .559 | .575 | .021 |
| SMC | 11.72 (3.73) | 14.82 (4.85) | 15.89 (4.68) | |||
| Executive Functioning | ||||||
| COWA – FAS score | ||||||
| BBTi | 50.74 (12.70) | 52.15 (14.63) | 53.04 (16.77) | 1.398 | .257 | .053 |
| SMC | 50.78 (12.82) | 56.64 *16.04) | 53.82 (16.55) | |||
| Trailmaking Test Part B (seconds) | ||||||
| BBTi | 77.67 (25.46) | 77.83 (22.83) | 381.75 (42.50) | .293 | .747 | .011 |
| SMAC | 89.47 (32.54) | 84.18 (57.31) | 381.75 (42.50) | |||
Note. BBTi refers to Brief Behavioral Treatment of insomnia group (n = 32); SMC refers to Self-monitoring Controls (n = 30). Abbreviations: MMSE = Mini-Mental Status Examination; COWA = Controlled Oral Word Association; CVLT-II = California Verbal Learning Test 2; Rey-O = Rey-Osterreith Complex Figure Test; LM-WMS-III = Logical Memory from Wechsler Memory Scale 3.
Greenhouse-Geisser correction was applied to Group × Time interaction.
Guidelines for interpreting η2p: .01 = small, .06 = moderate, .14 = large (Cohen, 1977)
3.8. Clinical Significance
3.8.1. Responders
At post-treatment, the BBTi group (n = 17, 53%) had significantly more responders that the SMC group (n = 4, 13%), χ2 (1, N = 62) = 10.95, p = .001. Similarly, at follow-up, the BBTi group continued to have significantly more responders (n = 17, 53%) than the SMC group (n = 4, 13%), η2(1, N = 62) = 10.95, p = .001.
3.8.2. Remitters
At post-treatment, the BBTi group (n = 9, 28%) had significantly more remitters than SMC (n = 2, 7%), χ2(1, N = 62) = 4.89, p = .03. Similarly, at follow-up, the BBTi group (n = 10, 31%) continued to have more remitters than the SMC group (n = 3, 10%), η2(1, N = 62) = 4.22, p = .04.
4. Discussion
The present randomized clinical trial examined the effectiveness of a 4-week BBTi relative to a SMC group on improving (at post-treatment) and maintaining (at 3-month followup) sleep diary, actigraphy, mood, and neuropsychological outcomes in older adults with chronic insomnia.
4.1. Sleep Diary Outcomes
Consistent with our prediction, we observed reductions in sleep diary measures of SOL and WASO, as well as improvements in SE and sleep quality immediately following BBTi. Improvements across insomnia measures can be classified as moderate to large effects. These results agree with previous favorable findings and similar (Buysse et al., 2011; Pallesen et al., 2003) or larger (Lovato et al., 2014) effect sizes showing improvement in these self-reported variables in older adults following BBTi, but importantly, unlike these prior studies, show that these effects are specific to BBTi treatment and not observed in a control group actively engaged in self-monitoring and matched on therapist attention. Given that the effect sizes observed in the present study were generally moderate, and those observed by Lovato and colleagues across similar self-reported indices were large (Lovato et al., 2014), we offer several potential explanations for these differences. First, Lovato and colleagues included a cognitive restructuring therapy in addition to similar behavioral components employed in the present study. Thus, it is possible that the cognitive components of therapy lead to larger treatment effects on self-reported sleep parameters. However, unlike Lovato and colleagues’ findings, our observed moderate improvements in sleep diary outcomes were maintained at 3-month follow-up. Thus, it is likely that while behavioral plus cognitive therapy prompts larger immediate effects, behavioral therapy alone leads to more durable treatment effects. Second, it should be noted that the large effect sizes in Lovato’s study were observed after group-based delivery of BBTi, without comparison to an attention matched control. Thus, effect sizes in Lovato’s study may be inflated due to non-therapeutic factors, such as social contact or support.
Our findings complement a small group of studies showing that a brief 4-week behavioral intervention for insomnia provide substantial and potentially long lasting effects in older adults. Moreover, our evaluation of clinical significance that showed a higher percentage of BBTi response and insomnia remission relative to SMC further supports the clinical utility of this brief intervention. Given the potential acute and adverse effects of pharmacological treatment for insomnia (Omvik et al., 2006), particularly in this age group where complications due to polypharmacy are a concern (Bertisch, Herzig, Winkelman, & Buettner, 2014), BBTi should be considered as a primary treatment option for older adults with chronic insomnia.
4.2. Actigraphy Outcomes
Contrary to our prediction, but consistent with a previous CBT-I study on older adults (Rybarczyk, Lopez, Benson, Alsten, & Stepanski, 2002), we did not observe improvement in actigraphy based SOL, TST or SE following BBTi in our older adult sample. While we did observe improvement in WASO at post-treament, this was also observed in the SMC group, suggesting that the components common to both conditions - daily self-monitoring and 4 weekly, one hour sessions with a therapist - may be associated with reductions in sleep disruption as measured by actigraphy. Our findings generally disagree with a previous study showing improvement in actigraphy based WASO, SOL, and SE following a 4 week BBTi intervention in older adults with comorbid insomnia (Buysse et al., 2011), however, our results are consistent with a study that showed no improvement in actigraphy outcomes following group-based delivery of BBTi (Lovato et al., 2014). We propose several possible explanations for the discrepancy between diary and actigraphy treatment effects. First, the lack of a group by time interaction in our study could be due to our use of a more rigorous control group. Although Buysse and colleagues found that both diary and actigraphy measures improved for BBTi relative to controls, they noted that they did not match their control group by time spent with a therapist, a feature that was implemented in the present study. Thus, it is possible that our results are more representative of the effectiveness of BBTi in older adults. Second, discrepancies in actigraphy and sleep diary assessments have been observed in older adults with insomnia (Van Den Berg et al., 2008), suggesting the two methods may represent different underlying mechanisms. For instance, is possible that the BBTi treatment targeted sleep quality in particular, and the diary estimates would presumably be more reflective of this index than actigraphic estimates. Thus, the improvements in sleep quality may have influenced the other self-reported estimates (SOL, WASO). Future studies should examine through polysomnographic testing wheter BBTi leads to changes in sleep staging, particularly an increase in restorative slow wave or REM, potential physiological indicators of sleep quality change.
4.3. Mood and Anxiety Outcomes
Contrary to our prediction, we did not observe treatment specific improvement in mood and anxiety outcomes following BBTi. Therefore, unlike previous findings of improvements in mood and anxiety following longer CBT-I protocols (Belleville et al., 2011; Manber et al., 2008), it is possible that shorter BBTi programs are not long enough to elicit changes in these measures in older adults. Given that we observed an improvement in depressive symptoms (i.e., lower scores on the GDS scale) over time, regardless of treatment condition, it is also possible that mood changes are not specific to BBTi per se. That is, perhaps the very act of selfmonitoring sleep related outcomes and spending time one-on-one time with a therapist for an hour each week for 4 weeks, experienced in the present study by both the BBTi and SMC groups, may be associated with endorsing fewer depressive symptoms in older adults. Finally, given that in the present study the average baseline scores on the BDI-II, GDS and STAI-Y can generally be considered as representative of mild depression and anxiety (Beck et al., 1996; Kvaal, Ulstein, Nordhus, & Engedal, 2005; Shiekh, 1986), it is possible that our results do not generalize to patients endorsing more severe symptoms. Therefore, future work should examine whether BBTi improves anxiety and depression in patients with higher baseline symptom scores.
4.4. Cognitive Outcomes
Our findings of no improvement over time in cognitive performance between older adults with insomnia who underwent CBT-I or BBTi agree with previous reports (Fortier-Brochu et al., 2012; Riedel & Lichstein, 2000; Wilckens et al., 2016). It is possible that behavioral interventions for insomnia do not improve cognition. However, we propose several caveats to drawing any definitive conclusions regarding insomnia and cognition. First, we note that in the present study, cognitive performance was evaluated at single timepoints. Therefore, participants may have been able to recruit the necessary cognitive resources to perform at a high level during our ‘single shot’ assessments that were conducted months apart. To examine this possibility, future work may wish to assess the level of effort during cognitive task performance [e.g., subjective ratings of mental effort; Haji, Rojas, Childs, Ribaupierre, and Dubrowski (2015)]. It is also possible that day to day fluctuations in insomnia symptoms are associated with daily cognitive changes. Daily assessment of cognition may be more sensitive to insomnia related effects as participants may be less likely to be able to maintain on-going recruitment of the cognitive resources needed to perform at a high level. This should be examined in future work. Second, it is also possible that the specific neuropsychological measures used in the present study were not sensitive enough to detect subtle changes in cognitive abilities associated with improved sleep. In fact, previous work has shown that an eight week CBT-I program resulted in improvements in performance on a computerized task switching paradigm, presumably reflecting improvements in executive processing (Wilckens et al., 2017). To that end, future work may wish to examine whether BBTi is associated with improvement in additional cognitive outcomes, such as performance on computerized testing paradigms (e.g., n-back tests, choice reaction time tasks, etc.). Finally, given previous findings showing that changes in sleep architecture, namely increases in the amount of slow wave non-REM sleep, are associated with improvements in executive cognitive functioning (Wilckens et al., 2016), the intervention employed in the present study may not have targeted this outcome and thus no gains in cognitive performance were attained.
4.5. Limitations
One potential limitation of the present study concerns the unusually high education level of the older adult participants. Given that the majority (60%) of the participants had at least 16 years of education or higher (i.e., a Bachelor’s degree or higher), they may not represent the average older adult with chronic insomnia. This may also be one reason why we did not observe any changes in cognitive performance following BBTi, as these patients may have already been performing at a high level, and there was little room for improvement. It is also possible that the length of intervention and/or follow-up were not long enough for cognitive improvements to manifest in this older adult sample. To that end, future research should examine whether booster sessions (i.e., short reminder sessions that can take place over the telephone) or longer follow-up after treatment (e.g., 6-months) are associated with changes in neuropsychological function. Additionally, participants were screened for sleep apnea based a two channel monitor that measured oxygen saturation and respiraton, but polysomnography was not used for screening or outcome measurement. Finally, given that the SMC group were told that that would receive BBTi treatment upon study completition, participants in this group likely lacked expectancy for improvement. Thus, future studies may wish to employ a control group that is more closely associated with expectations for improvement in order to examine whether treatment credibility plays a role in the observed outcomes.
4.6. Conclusion
The present clinical trial found that a brief 4-week behavioral therapy for insomnia produced improvements in self-reported sleep measures that were maintained over a 3-month follow-up. Importantly, these improvements were not observed in a self-monitoring control group. We observed no changes in actigraphic sleep measures and did not measure polysomnographic outcomes. BBTi does not appear to selectively improve mood or anxiety, or neuropsychological performance in older adults. Future studies should explore whether BBTi impacts day to day cognitive fluctuations, or investigate potential changes in more sensitive cognitive measures such as computerized testing. Future research using more sensitive cognitive measures should also include polysomnographic outcomes to examine the possibility that BBTi might prompt sleep architecture changes that prior research suggests are associated with improvements in cognitive performance. Despite the lack of evidence showing widespread selective improvement following BBTi on cognitive and mood outcomes, our findings of improved and sustained sleep outcomes in older adults are important for clinical care. As current medications used to treat insomnia have many adverse effects, particularly in older adults (Bertisch et al., 2014; Omvik et al., 2006), 4-session BBTi should be considered an efficacious first line treatment option for chronic insomnia.
Highlights.
Research on efficacy of brief behavioral treatment for insomnia (BBTi) is limited
Older adults completed four sessions of BBTi or self-monitoring control
BBTi improved sleep onset, wake after sleep onset, sleep efficiency and sleep quality
BBTi did not improve cognition, and reduced depression to same degree as controls
BBTi is an efficacious intervention for reducing insomnia symptoms in older adults
Acknowledgements
This research was supported by funds awarded to Christina McCrae from the National Institute on Aging (AG024459-01). Additional support was provided by an Institutional Training Grant from the National Institute on Aging directed by Michael Marsiske (T32-AG-020499). Joseph Dzierzewski was supported by a grant from the National Institute on Aging (K23AG049955).
Support: The project described was supported by Award Number AG024459 (Christina S. McCrae, Ph.D., PI) from the National Institute on Aging (NIA). Additional support was provided by an Institutional Training Grant Award Number AG020499 (Michael Marsiske, PhD, Director) and Award Number K23AG049955 (Joseph Dzierzewski, PhD, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Identifer: NCT02967185
References
- Adams KB (2001). Depressive symptoms, depletion, or developmental change? Withdrawal, apathy, and lack of vigor in the Geriatric Depression Scale. The gerontologist, 41(6), 768–777. [DOI] [PubMed] [Google Scholar]
- Axelrod BN, Ricker JH, & Cherry SA (1994). Concurrent validity of the MAE visual naming test. Archives of Clinical Neuropsychology, 9(4), 317–321. [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Garbin MG (1996). Beck Depression Inventory-Second Edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Belleville G, Cousineau H, Levrier K, & St-Pierre-Delorme ME (2011). Meta-analytic review of the impact of cognitive-behavior therapy for insomnia on concomitant anxiety. Clin Psychol Rev, 31(4), 638–652. doi: 10.1016/j.cpr.2011.02.004 [DOI] [PubMed] [Google Scholar]
- Benton A, Hamsher K. d., & Sivan A (1989). Multilingual Aphasia Examination Iowa City, IA: AJA Associates; NEUROPSYCHOLOGY, BLOCKING, SCHIZOPHRENIA, 59. [Google Scholar]
- Berry DT, Allen RS, & Schmitt FA (1991). Rey-Osterrieth Complex Figure: Psychometric characteristics in a geriatric sample. The Clinical Neuropsychologist, 5(2), 143–153. [Google Scholar]
- Bertisch SM, Herzig SJ, Winkelman JW, & Buettner C (2014). National use of prescription medications for insomnia: NHANES 1999–2010. Sleep, 37(2), 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL (1993). Sleep in normal aging and dementia. Sleep: Journal of Sleep Research & Sleep Medicine. [DOI] [PubMed] [Google Scholar]
- Brandt J (1991). The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. The Clinical Neuropsychologist, 5(2), 125–142. [Google Scholar]
- Burke WJ, Nitcher RL, Roccaforte WH, & Wengel SP (1992). A prospective evaluation of the Geriatric Depression Scale in an outpatient geriatric assessment center. Journal of the American Geriatrics Society, 40(12), 1227–1230. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Moul DE, Franzen PL, Brar LK, Fletcher ME, . . . Reynolds CF (2011). Efficacy of brief behavioral treatment for chronic insomnia in older adults. Archives of Internal Medicine, 171(10), 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellucci T, Evans WJ, Cattaruzza C, & Carter S (2001). Stability and correlates of the California Verbal Learning Test for a sample of normal elderly persons. Psychological Reports, 88(1), 171–174. [DOI] [PubMed] [Google Scholar]
- Cohen J (1977). Statistical power analysis for the behavioral sciences (revised ed.): New York: Academic Press. [Google Scholar]
- Cole MG, & Dendukuri N (2003). Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. American Journal of Psychiatry, 160(6), 1147–1156. [DOI] [PubMed] [Google Scholar]
- Cricco M, Simonsick EM, & Foley DJ (2001). The impact of insomnia on cognitive functioning in older adults. Journal of the American Geriatrics Society, 49(9), 1185–1189. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, & Ober B (1987). California verbal learning test (CVLT). San Antonio: The Psychological CorporationO: Harcourt Brace & Company. [Google Scholar]
- Dikmen SS, Heaton RK, Grant I, & Temkin NR (1999). Test–retest reliability and practice effects of expanded Halstead–Reitan Neuropsychological Test Battery. Journal of the International Neuropsychological Society, 5(4), 346–356. [PubMed] [Google Scholar]
- Dozois DJ, Dobson KS, & Ahnberg JL (1998). A psychometric evaluation of the Beck Depression Inventory-II. Psychological Assessment, 10(2), 83. [Google Scholar]
- Dzierzewski JM, Dautovich N, & Ravyts S (2018). Sleep and cognition in older adults. Sleep Medicine Clinics, 13(1), 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Kumar A, Mintz J, Boone K, Bahng E, & Lavretsky H (2004). Executive dysfunction and visuospatial ability among depressed elders in a community setting. Archives of Clinical Neuropsychology, 19(5), 597–611. [DOI] [PubMed] [Google Scholar]
- Feher EP, Larrabee GJ, & Crook TH (1992). Factors attenuating the validity of the Geriatric Depression Scale in a dementia population. Journal of the American Geriatrics Society, 40(9), 906–909. [DOI] [PubMed] [Google Scholar]
- Ferrario E, Seccia L, Massaia M, Fonte G, & Molaschi M (1998). Mini-Mental State Examination and Wechsler Memory Scale subtest of logical memory: correlation in an over 70-year-old population. Archives of Gerontology and Geriatrics, 26, 175–180. [Google Scholar]
- Foley D, Ancoli-Israel S, Britz P, & Walsh J (2004). Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. Journal of Psychosomatic Research, 56(5), 497–502. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan A, Simonsick EM, Wallace RB, & Blazer DG (1999). Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep, 22 Suppl 2, S366–372. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Fortier-Brochu É, Beaulieu-Bonneau S, Ivers H, & Morin CM (2012). Insomnia and daytime cognitive performance: a meta-analysis. Sleep Medicine Reviews, 16(1), 83–94. [DOI] [PubMed] [Google Scholar]
- Fortier-Brochu E, & Morin CM (2014). Cognitive impairment in individuals with insomnia: clinical significance and correlates. Sleep, 37(11), 1787–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, & Capitani E (1996). Trail making test: normative values from 287 normal adult controls. The Italian journal of neurological sciences, 17(4), 305–309. [DOI] [PubMed] [Google Scholar]
- Haji FA, Rojas D, Childs R, Ribaupierre S, & Dubrowski A (2015). Measuring cognitive load: performance, mental effort and simulation task complexity. Medical Education, 49(8), 815–827. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Sharpley AL, Ree MJ, Stinson K, & Clark DM (2007). An open trial of cognitive therapy for chronic insomnia. Behav Res Ther, 45(10), 2491–2501. doi: 10.1016/j.brat.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole JC, & Nicassio PM (2006). Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychology, 25(1), 3–14. doi: 10.1037/0278-6133.25.1.3 [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Powers DV, & Pope M (2001). Beck depression inventory-II (BDI-II) and the geriatric depression scale (GDS) in older women. Clinical Gerontologist, 22(3–4), 3–12. [Google Scholar]
- Johnson EO, Roth T, & Breslau N (2006). The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res, 40(8), 700–708. doi: 10.1016/j.jpsychires.2006.07.008 [DOI] [PubMed] [Google Scholar]
- Joy S, Kaplan E, & Fein D (2004). Speed and memory in the WAIS-III Digit Symbol—Coding subtest across the adult lifespan. Archives of Clinical Neuropsychology, 19(6), 759–767. [DOI] [PubMed] [Google Scholar]
- Jungquist CR, O’Brien C, Matteson-Rusby S, Smith MT, Pigeon WR, Xia Y, . . . Perlis ML (2010). The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Medicine, 11(3), 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabacoff RI, Segal DL, Hersen M, & Van Hasselt VB (1997). Psychometric properties and diagnostic utility of the Beck Anxiety Inventory and the State-Trait Anxiety Inventory with older adult psychiatric outpatients. Journal of Anxiety Disorders, 11(1), 33–47. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S (2001). Boston naming test: Pro-ed. [Google Scholar]
- Kortte KB, Horner MD, & Windham WK (2002). The trail making test, part B: cognitive flexibility or ability to maintain set? Applied Neuropsychology, 9(2), 106–109. [DOI] [PubMed] [Google Scholar]
- Kvaal K, Ulstein I, Nordhus IH, & Engedal K (2005). The Spielberger state-trait anxiety inventory (STAI): the state scale in detecting mental disorders in geriatric patients. International Journal of Geriatric Psychiatry, 20(7), 629–634. [DOI] [PubMed] [Google Scholar]
- Lacks P, Bertelson AD, Gans L, & Kunkel J (1983). The effectiveness of three behavioral treatments for different degrees of sleep onset insomnia. Behavior Therapy, 14(5), 593–605. [Google Scholar]
- Lacritz LH, & Cullum CM (1998). The hopkins verbal learning test and CVLT: a preliminary comparison. Archives of Clinical Neuropsychology, 13(7), 623–628. [PubMed] [Google Scholar]
- Lezak M, Howieson D, & Loring D (2004). A compendium of tests and assessment techniques. Neuropsychological assessment, 4. [Google Scholar]
- Lichstein KL, & Morin CM (2000). Treatment of late-life insomnia: Sage. [Google Scholar]
- Lichstein KL, Riedel BW, & Grieve R (1994). Fair tests of clinical trials: A treatment implementation model. Advances in Behaviour Research and Therapy, 16(1), 1–29. [Google Scholar]
- Loring DW, Martin RC, Meador KJ, & Lee GP (1990). Psychometric construction of the Rey-Osterrieth complex figure: Methodological considerations and interrater reliability. Archives of Clinical Neuropsychology, 5(1), 1–14. [PubMed] [Google Scholar]
- Lovato N, Lack L, Wright H, Cant M, & Humphreys J (2013). Working memory performance of older adults with insomnia. Journal of Sleep Research, 22(3), 251–257. [DOI] [PubMed] [Google Scholar]
- Lovato N, Lack L, Wright H, & Kennaway DJ (2014). Evaluation of a brief treatment program of cognitive behavior therapy for insomnia in older adults. Sleep, 37(1), 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, & Kalista T (2008). Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep, 31(4), 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae CS (2009). Late-life comorbid insomnia: diagnosis and treatment. Am J Manag Care, 15 Suppl, S14–23. doi:11074 [pii] [PubMed] [Google Scholar]
- Mccrae CS, McNamara JPH, Rowe MA, Dzierzewski JM, Dirk J, Marsiske M, & Craggs JG (2008). Sleep and affect in older adults: using multilevel modeling to examine daily associations. Journal of Sleep Research, 17(1), 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MD, Gehrman P, Perlis M, & Umscheid CA (2012). Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract, 13, 40. doi: 10.1186/1471-2296-13-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrushina M, & Satz P (1991). Reliability and validity of the Mini-Mental State Exam in neurologically intact elderly. Journal of Clinical Psychology, 47(4), 537–543. [DOI] [PubMed] [Google Scholar]
- Montgomery P, & Dennis J (2003). Cognitive behavioural interventions for sleep problems in adults aged 60+. Cochrane Database Syst Rev(1), CD003161. doi: 10.1002/14651858.CD003161 [DOI] [PubMed] [Google Scholar]
- Morin Kowatch, R. A., & Wade JB (1989). Behavioral management of sleep disturbances secondary to chronic pain. Journal of Behavior Therapy and Experimental Psychiatry, 20(4), 295–302. [DOI] [PubMed] [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, & Lichstein KL (2006). Psychological and behavioral treatment of insomnia:update of the recent evidence (1998–2004). Sleep, 29(11), 1398–1414. [DOI] [PubMed] [Google Scholar]
- Murden RA, McRae TD, Kaner S, & Bucknam ME (1991). Mini-Mental State Exam Scores Vary with Education in Blacks and Whites. Journal of the American Geriatrics Society, 39(2), 149–155. [DOI] [PubMed] [Google Scholar]
- NIH. (2005). State of the Science conference statement on manifestations and management of chronic insomnia in adults, June 13–15, 2005. Sleep, 28, 1049–1057. [DOI] [PubMed] [Google Scholar]
- O’donnell JP, Macgregor LA, Dabrowski JJ, Oestreicher JM, & Romero JJ (1994). Construct validity of neuropsychological tests of conceptual and attentional abilities. Journal of Clinical Psychology, 50(4), 596–600. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, & Vitiello MV (2004). Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep, 27(7), 1255–1273. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Caulet M, & Lemoine P (1998). Comorbidity of mental and insomnia disorders in the general population. Compr Psychiatry, 39(4), 185–197. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Zulley J, Guilleminault C, Smirne S, & Priest RG (2001). How age and daytime activities are related to insomnia in the general population: consequences for older people. J Am Geriatr Soc, 49(4), 360–366. [DOI] [PubMed] [Google Scholar]
- Omvik S, Pallesen S, Havik OE, Kvale G, & Nordhus IH (2006). Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA, 295(24), 2851–2858. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA (1944). Le test de copie d’une figure complexe. Arch Psychol, 30, 206–356. [Google Scholar]
- Pallesen S, Nordhus IH, Kvale G, Nielsen GH, Havik OE, Johnsen BH, & Skjotskift S (2003). Behavioral treatment of insomnia in older adults: an open clinical trial comparing two interventions. Behav Res Ther, 41(1), 31–48. doi:S000579670100122X [pii] [DOI] [PubMed] [Google Scholar]
- Parmelee PA, Lawton MP, & Katz IR (1998). The structure of depression among elderly institution residents: affective and somatic correlates of physical frailty. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 53(2), M155–M162. [DOI] [PubMed] [Google Scholar]
- Qaseem A, Kansagara D, Forciea MA, Cooke M, & Denberg TD (2016). Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Annals of Internal Medicine, 165(2), 125–133. [DOI] [PubMed] [Google Scholar]
- Quesnel C, Savard J, Simard S, Ivers H, & Morin CM (2003). Efficacy of cognitive- behavioral therapy for insomnia in women treated for nonmetastic breast cancer. Journal of Consulting and Clinical Psychology, 71(1), 189. [PubMed] [Google Scholar]
- Reitan R (1959). A manual for the administration and scoring of the Trail Making Test. Indiana University. [Google Scholar]
- Rey A (1941). L’examen psychologique dans les cas d’encéphalopathie traumatique.(Les problems.) Archives de Psychologie. [Google Scholar]
- Riedel BW, & Lichstein KL (2000). Insomnia and daytime functioning. Sleep Medicine Reviews, 4(3), 277–298. [DOI] [PubMed] [Google Scholar]
- Riemann D, & Voderholzer U (2003). Primary insomnia: a risk factor to develop depression? Journal of Affective Disorders, 76(1–3), 255–259. [DOI] [PubMed] [Google Scholar]
- Rybarczyk B, Lopez M, Benson R, Alsten C, & Stepanski E (2002). Efficacy of two behavioral treatment programs for comorbid geriatric insomnia. Psychology and Aging, 17(2), 288. [PubMed] [Google Scholar]
- Rybarczyk B, Stepanski E, Fogg L, Lopez M, Barry P, & Davis A (2005). A placebo- controlled test of cognitive-behavioral therapy for comorbid insomnia in older adults. Journal of Consulting and Clinical Psychology, 73(6), 1164. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cubillo I, Perianez J, Adrover-Roig D, Rodriguez-Sanchez J, Rios-Lago M, Tirapu J, & Barcelo F (2009). Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsychological Society, 15(3), 438–450. [DOI] [PubMed] [Google Scholar]
- Segal DL, Coolidge FL, Cahill BS, & O’Riley AA (2008). Psychometric properties of the Beck Depression Inventory—II (BDI-II) among community-dwelling older adults. Behavior Modification, 32(1), 3–20. [DOI] [PubMed] [Google Scholar]
- Shiekh J (1986). Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clinical gerontology: a guide to assessment and intervention, 165–173. [Google Scholar]
- Smith RA, Lack LC, Lovato N, & Wright H (2015). The relationship between a night’s sleep and subsequent daytime functioning in older poor and good sleepers. Journal of Sleep Research, 24(1), 40–46. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1983). State-Trait Anxiety Inventory, Form Y. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stepanski EJ, & Wyatt JK (2003). Use of sleep hygiene in the treatment of insomnia. Sleep Medicine Reviews, 7(3), 215–225. [DOI] [PubMed] [Google Scholar]
- Strauss E, & Spreen O (1990). A comparison of the Rey and Taylor figures. Archives of Clinical Neuropsychology, 5(4), 417–420. [PubMed] [Google Scholar]
- Sweetman AM, Lack LC, Catcheside PG, Antic NA, Chai-Coetzer CL, Smith SS, . . . McEvoy RD (2017). Developing a successful treatment for co-morbid insomnia and sleep apnoea. Sleep Medicine Reviews, 33, 28–38. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, & Bush AJ (2007). Comorbidity of chronic insomnia with medical problems. Sleep, 30(2), 213–218. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN (2005). Test-retest reliable coefficients and 5-year change scores for the MMSE and 3MS. Archives of Clinical Neuropsychology, 20(4), 485–503. [DOI] [PubMed] [Google Scholar]
- Van Den Berg JF, Van Rooij FJ, Vos H, Tulen JH, Hofman A, Miedema HM, . . . Tiemeier H (2008). Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. Journal of Sleep Research, 17(3), 295–302. [DOI] [PubMed] [Google Scholar]
- Vincent N, & Lionberg C (2001). Treatment preference and patient satisfaction in chronic insomnia. Sleep, 24(4), 411–417. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997a). WAIS-3., WMS-3: Wechsler Adult Intelligence Scale, Wechsler Memory Scale: Technical Manual: psychological corporation. [Google Scholar]
- Wechsler D (1997b). WAIS-III: Wechsler adult intelligence scale: Psychological Corporation. [Google Scholar]
- Wilckens KA, Hall MH, Erickson KI, Germain A, Nimgaonkar VL, Monk TH, & Buysse DJ (2017). Task switching in older adults with and without insomnia. Sleep Medicine, 30, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens KA, Hall MH, Nebes RD, Monk TH, & Buysse DJ (2016). Changes in cognitive performance are associated with changes in sleep in older adults with insomnia. Behavioral Sleep Medicine, 14(3), 295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, & Leirer VO (1983). Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research, 17(1), 37–49. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Bigal ME, Katz MJ, Brickman AM, & Lipton RB (2012). Sleep onset/maintenance difficulties and cognitive function in nondemented older adults: the role of cognitive reserve. Journal of the International Neuropsychological Society, 18(3), 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorick F, & Walsh J (2000). Evaluation and management of insomnia: An overview Principles and Practice of Sleep Medicine (3rd ed). Philadelphia: Saunders, 615–623. [Google Scholar]