Abstract
Background
Patients undergoing haemodialysis (HD) through a central venous catheter (CVC) are exposed to several risks, being a catheter‐related infection (CRI) and a CVC lumen thrombosis among the most serious. Standard of care regarding CVCs includes their sealing with heparin lock solutions to prevent catheter lumen thrombosis. Other lock solutions to prevent CRI, such as antimicrobial lock solutions, have proven useful with antibiotics solutions, but not as yet for non–antibiotic antimicrobial solutions. Furthermore, it is uncertain if these solutions have a negative effect on thrombosis incidence.
Objectives
To assess the efficacy and safety of antimicrobial (antibiotic, non‐antibiotic, or both) catheter lock solutions for preventing CRI in participants undergoing HD with a CVC.
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register up to 18 December 2017 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal, and ClinicalTrials.gov.
Selection criteria
We included all randomised or quasi‐randomised control trials (RCTs) comparing antimicrobial (antibiotic and non‐antibiotic) lock solutions to standard lock solutions, in participants using a CVC for HD, without language restriction.
Data collection and analysis
Two authors independently assessed studies for eligibility, and two additional authors assessed for risk of bias and extracted data. We expressed results as rate ratios (RR) per 1000 catheter‐days or 1000 dialysis sessions with 95% confidence intervals (CI). Statistical analyses were performed using the random‐effects model.
Main results
Thirty‐nine studies, enrolling 4216 participants, were included in this review, however only 30 studies, involving 3392 participants, contained enough data to be meta‐analysed. Risk of bias was low or unclear for most domains in the majority of the included studies.
Studies compared antimicrobial lock solutions (antibiotic and non‐antibiotic) to standard sealing solutions (usually heparin) of the CVC for HD. Fifteen studies used antibiotic lock solutions, 21 used non‐antibiotic antimicrobial lock solutions, and 4 used both (antibiotic and non‐antibiotic) lock solutions. Studies reported the incidence of CRI, catheter thrombosis, or both.
Antimicrobial lock solutions probably reduces CRI per 1000 catheter‐days (27 studies: RR 0.38, 95% CI 0.27 to 0.53; I2 = 54%; low certainty evidence), however antimicrobial lock solutions probably makes little or no difference to the risk of thrombosis per 1000 catheter days (14 studies: RR 0.79, 95% CI 0.52 to 1.22; I2 = 83%; very low certainty evidence). Subgroup analysis of antibiotic and the combination of both lock solutions showed that both probably reduced CRI per 1000 catheter‐days (13 studies: RR 0.30, 95% CI: 0.22 to 0.42; I2 = 47%) and risk of thrombosis per 1000 catheter‐days (4 studies: RR 0.26, 95% CI: 0.14 to 0.49; I2 = 0%), respectively. Non‐antibiotic antimicrobial lock solutions probably reduced CRI per 1000 catheter‐days for tunnelled CVC (9 studies: RR 0.60, 95% CI 0.40 to 0.91) but probably made little or no difference with non‐tunnelled CVC (4 studies: RR 0.93, 95% CI 0.48 to 1.81). Subgroup analyses showed that antibiotic (5 studies: RR 0.76, 95% CI 0.42 to 1.38), non‐antibiotic (8 studies: RR 0.85, 95% CI 0.44 to 1.66), and the combination of both lock solutions (3 studies: RR 0.63, 95% CI 0.22 to 1.81) made little or no difference to thrombosis per 1000 catheter‐days compared to control lock solutions.
Authors' conclusions
Antibiotic antimicrobial and combined (antibiotic‐non antibiotic) lock solutions decreased the incidence of CRI compared to control lock solutions, whereas non‐antibiotic lock solutions reduce CRI only for tunnelled CVC. The effect on thrombosis incidence is uncertain for all antimicrobial lock solutions. Our confidence in the evidence is low and very low; therefore, better‐designed studies are needed to confirm the efficacy and safety of antimicrobial lock solutions.
Plain language summary
Antimicrobial lock solutions for preventing infections in patients using a catheter for haemodialysis
What is the issue?
Most of the people presenting end‐stage kidney disease use haemodialysis (HD) to replace kidney function. Frequently, a central venous catheter (CVC) is needed to begin HD. In between HD sessions, the CVC needs a sealing solution to avoid catheter thrombosis (an obstruction due to clots), and this is frequently heparin.
In addition to catheter thrombosis, another frequent complication is catheter‐related infection (CRI). CRI originates in the catheter and then spreads to the blood or other organs.
Heparin prevents clot formation but does not prevent infections. Therefore, instead of heparin, the use of sealing solutions that can reduce CRIs has been proposed. These antimicrobial lock solutions could be divided into antibiotic (e.g. vancomycin) and non‐antibiotic (e.g. citrate) solutions. Antimicrobial lock solutions should fill the catheter lumen and then be locked in the catheter during in‐between HD sessions with or without heparin.
What did we do?
We did a systematic review to assess the question whether antimicrobial (antibiotic or non‐antibiotic) lock solutions were better than heparin to prevent CRIs in patients undergoing HD through a CVC and thrombosis compared to heparin. We searched the literature up until 18 December 2017 and identified 39 studies enrolling 4216 patients that met our inclusion criteria.
What did we find?
We included 39 studies, including 3,945 participants undergoing HD through a CVC. The studies compared CVC sealing solutions with heparin to antimicrobial lock solutions. Fifteen studies used only antibiotic lock solutions, 21 used non‐antibiotic lock solutions, and 4 used both (antibiotic and non‐antibiotic) lock solutions. Studies measured the incidence of CRIs and catheter thrombosis, or both. Overall quality of the studies was low for CRIs and very low for thrombosis. There was no information on funding sources for most of the studies.
In general antimicrobial lock solutions are likely superior to standard solutions in preventing CRIs among patients undergoing HD through a CVC, but non‐antibiotic solutions did not prove to reduce CRI. They are no worse than heparin at preventing thrombosis. Other adverse effects were not reported in most studies. Our confidence in these results is low due to the quality of the studies.
Conclusion
Some antimicrobial (antibiotic and the combination of antibiotic‐non antibiotic) lock solutions decrease the incidence of CRIs compared to heparin. Their effect on CVC permeability remains unclear. The quality of the studies is low and very low, respectively; therefore, more studies are needed to confirm the benefits and harms of antimicrobial lock solutions.
Summary of findings
Summary of findings for the main comparison. Antimicrobial lock solutions vs control for preventing catheter‐related infections in patient undergoing haemodialysis.
| Antimicrobial lock solutions vs control for preventing catheter‐related infections in patient undergoing haemodialysis | ||||||
| Patient or population: CVC‐related infection Setting: haemodialysis therapy Intervention: antimicrobial lock solutions Comparison: heparin and other lock solutions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with heparin and other lock solutions | Risk with antimicrobial lock solutions | |||||
| CVC ‐ related infections assessed with: per 1000 days/catheter | Low | RR 0.38 (0.27 to 0.53) | 2994 (27 RCTs) | ⊕⊕⊝⊝ LOW 1 2 3 4 5 | ||
| 43 per 1.000 | 16 per 1.000 (12 to 23) | |||||
| High | ||||||
| 260 per 1.000 | 99 per 1.000 (70 to 138) | |||||
| Thrombosis assessed with: per 1000 days/catheter | Low | RR 0.79 (0.52 to 1.22) | 2080 (14 RCTs) | ⊕⊝⊝⊝ VERY LOW 6 7 8 9 10 | ||
| 6 per 1.000 | 5 per 1.000 (3 to 7) | |||||
| High | ||||||
| 330 per 1.000 | 261 per 1.000 (172 to 403) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 This judgment is based on the lack of information regarding the following criteria: random sequence generation, allocation concealment and blinding of outcome assesment
2 the statistics do not show serious heterogeneity and confidence intervals overlap.
3 the evidence is direct because the studies are in hemodialysis patients and the same sealing solutions the question of this review are used.
4 no imprecision is observed; because the decision regarding the use of antimicrobial lock solution is sealed better than heparin along the confidence interval
5 It is suspected a degree of publication bias
6 30% of the studies presented insufficient information to assess citerios of:random sequence generation, allocation concealment and blinding of outcome assesment
7 the statistical test showed a high heterogeneity and the confidence intervals do not overlap
8 the evidence is not indirect because the studies are in hemodialysis patients and the same sealing solutions the question of this review are used.
9 The 95% confidence interval of the pooled estimate ranges from 0.41 to 1.39, which is not narrow enough for a confident judgment of the effect size.
10 Publication bias is suspected as the funnel plot for this outcome shows asymmetry
Background
Description of the condition
Currently, arteriovenous fistulas (AVF) are the preferred access for haemodialysis (HD) patients; nevertheless, when AVFs are not available, a central venous catheter (CVC) has to be installed to start HD. The use of CVC for HD varies among different countries and changes over time in the same country (Ethier 2008). In the United States, the use of CVC has decreased from 27% in 1997 to 15% in 2013 (Pisoni 2015). Using a CVC for HD exposes participants to events such as infections and thrombosis. The use of CVC also increases the risk of mortality and treatment costs (Beathard 2008; Bradbury 2007; Foley 2009; Little 2001; Napalkov 2013). Catheters have a high likelihood of providing an adequate environment for bacterial growth, and leucocytes are unable to surround or phagocytise bacteria. Furthermore, proteins and glycocalyx biofilm coatings of catheters may protect bacteria from antibiotics and leucocytes (Ash 2000). A common complication among participants who undergo HD through CVCs is catheter‐related infection (CRI). The incidence of CRI varies according to different settings and different definitions of CRI, but they are reported at rates of 2.5 to 5.5 cases/1000 catheter‐days, or 0.9 to 2 episodes/patient/year (Katneni 2007; Napalkov 2013). CRI increase treatment costs and adversely affect participants' quality of life, since they often require hospitalisation to remove the CVC and to initiate intravenous antibiotic therapy according to blood cultures (Klevens 2008; NKF 2006). Several interventions to prevent CRI have been described, being the most important the decrease in the number of patients using a HD catheter and the use of strict aseptic protocols, if it is impossible to remove the catheter. Other interventions are the different types of medicated or impregnated dressings (Ullman 2015), catheter impregnation or coating (Lai 2016), application of mupirocin ointment (McCann 2010), and the use of an anti‐infective solution in each CVC lumen while not being used (lock solutions) in order to help prevent colonisation of the intraluminal surface by micro‐organisms that can form a biofilm on the inner wall of the CVC (Labriola 2008; Weijmer 2002).
Description of the intervention
Current standard of care for maintenance of HD CVC is to use lock solutions containing high concentrations of heparin to help prevent thrombosis (Ash 2000). However, the use of heparin may cause complications due to its systemic anticoagulant effect. Heparin can also antagonise bactericidal properties of some antibiotics and may promote biofilm formation (Moran 2008; Vanholder 2010). Locking catheter lumens using anti‐infective solutions – either antibiotic lock solutions (e.g. gentamicin, vancomycin, minocycline, cefazolin, cefotaxime) or non‐antibiotic lock solutions (e.g. sodium citrate, taurolidine) – can help prevent CRI (Betjes 2011; Labriola 2008; Yahav 2008). Antimicrobial (either antibiotic or on‐antibiotic) lock solutions may be administered in combination with an anticoagulant (usually heparin) and sometimes with another antibiotic. Citrate is often administered in combination with taurolidine and heparin (Grudzinski 2015; Labriola 2008; Yahav 2008).
How the intervention might work
It has been demonstrated that, compared to standard care, antibiotic lock solutions can reduce the risk of CRI (Yahav 2008). However, antibiotic lock solutions can increase the likelihood of adverse effects such as ototoxicity (associated with gentamicin). Antibiotic lock solutions can also cause potential antimicrobial resistance, which is the main reason this intervention has not been adopted widely (Venditto 2010). In contrast, non‐antibiotic antimicrobial lock solutions do not present these specific side effects and they are cheaper than heparin, however, their capacity to reduce CRI is uncertain (Grudzinski 2015; Yahav 2008). In vitro studies have indicated that high concentrations of trisodium citrate for locking catheters reduce antimicrobial activity (Weijmer 2005).
Why it is important to do this review
Although the effects of antimicrobial lock solutions for CVC used in HD have been assessed for the last decades, results and recommendations are controversial. A systematic review including eight randomised controlled trials (RCTs) (Labriola 2008) concluded that, compared to heparin, antimicrobial lock solutions reduced the risk of infection (RR 0.32, 95% CI 0.10 to 0.42), but the results did not differentiate between antibiotic lock solutions and non‐antibiotic antimicrobial lock solutions or evaluate the safety of the intervention.
Another systematic review (Yahav 2008) including 16 studies concluded that, compared to heparin, antibiotic lock solutions reduced the risk of CRI (RR 0.39, 95% CI 0.31 to 0.50), whereas the results for non‐antibiotic antimicrobial lock solutions were heterogeneous and effective only when associated with other measures to prevent CRI, such as nasal mupirocin or exit site topical iodine/chlorhexidine (RR 0.37, 95% CI 0.30 to 0.47). A more recent systematic review including five RCTs compared citrate to heparin and found no significant difference for bacteraemia, CVC permeability, and bleeding (Grudzinski 2015).
Although evidence for antibiotic lock solutions looks promising, this is not yet a standard of care due to uncertainty on safety issues. Furthermore, current evidence on non‐antibiotic antimicrobial lock solutions is conflicting and insufficient to recommend their use. Therefore, a new systematic review should assess the safety and efficacy of these interventions in order to help prevent CRIs.
Objectives
To assess the efficacy and safety of antimicrobial (antibiotic, non‐antibiotic, or both) catheter lock solutions for preventing CRI in participants undergoing HD with a CVC.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi‐RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods, looking at antimicrobial (antibiotic, non‐antibiotic, or both) catheter lock solutions for preventing CRI and thrombosis in people undergoing HD through a CVC.
Types of participants
Inclusion criteria
Adults or children with acute kidney injury or end‐stage kidney disease undergoing HD using a CVC.
Exclusion criteria
We excluded studies of participants using a CVC for anything other than HD.
Types of interventions
All antimicrobial lock solutions: antibiotic (e.g. gentamicin, vancomycin, cefotaxime, and minocycline), non‐antibiotic (e.g. citrate, taurolidine, and ethanol) or both compared to heparin, tissue plasminogen activator, and other lock solutions with unknown antimicrobial properties
Studies investigating non‐lock solution interventions were excluded.
Studies investigating the treatment of CRI with antimicrobial‐lock solutions were excluded.
Types of outcome measures
Primary outcomes
CRI: defined as the presence of symptoms and signs suggesting systemic infection, such as fever (temperature ≥ 38°C) or hypotension, accompanied by positive blood cultures drawn from the catheter and a peripheral vein. Growth of the same micro‐organism in blood cultures drawn through both the catheter and a peripheral vein, without other bacteraemia sources of infection than the CVC.
Secondary outcomes
CVC‐related thrombosis, defined as a persistent inability to maintain a blood flow of > 250 mL/min or the need of thrombolytic therapy, or CVC removal due to occlusion.
CVC colonisation, defined as a positive culture by any methods in participants with or without signs of infection.
Bacteraemia from any sources.
Survival of CVC without thrombosis or infection, defined as the number of days the catheter is permeable and free of infection or thrombosis.
All‐cause mortality.
Adverse effects such as bacterial antibiotic resistance, bleeding episodes, or pulmonary embolism.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register up to 18 December 2017 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from the following sources:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of included studies and previous relevant systematic reviews.
Abstracts from major conferences and meetings for the past seven years between 2010 and 2017.
Data collection and analysis
Selection of studies
The search strategy was used to obtain titles and abstracts of studies that might be relevant to the review. Retrieved titles and abstracts were reviewed by two authors, and studies considered by any of the reviewers as potentially relevant were initially selected. Full text articles of these studies were further reviewed for eligibility by both authors, who had to both agree for including them in the review. Disagreements not resolved by discussion between authors were referred to a third author.
Data extraction and management
Studies reported in languages other than English or Spanish were translated before assessment. When more than one publication of the same study was found, reports were analysed to select the publication with the most complete data to be included in the analyses. Only when relevant outcomes were published in an earlier version, this version was used.
Two authors carried out data extraction, independently using a data extraction form, devised to record details of participant characteristics, interventions and outcome measures for each included study.
Assessment of risk of bias in included studies
Included studies were independently assessed for methodological quality by two authors. We assessed the following items using the risk of bias assessment tool (Higgins 2011) (Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Were reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk for bias?
Measures of treatment effect
Studies expressed the main outcomes either as rates of events per catheter‐days or per dialysis sessions, we used the generic inverse‐variance method to pool the results, reporting the rate ratio (RR) with 95% confidence intervals (95% CI) for each comparison (Higgins 2011).
Unit of analysis issues
We used catheter‐days or dialysis‐sessions for data reporting and analysis.
Dealing with missing data
Missing data was requested from the authors of included studies, by up to three e‐mails to the corresponding author; all relevant information obtained was included in the review.
Assessment of heterogeneity
We explored evidence of statistical heterogeneity across studies was explored using the I2 and the Chi2 test for heterogeneity, P‐value for statistical significance of chi‐square was set at 0.05. I2 of 0% to 25%, 26% to 50% and over 51%; corresponded to low, medium and high levels of heterogeneity respectively (Higgins 2011).
Assessment of reporting biases
Our search strategy aimed to minimize publication bias. We used funnel plots to assess publication bias for CVC‐related infection.
Data synthesis
We pooled data using random‐effects model.
Subgroup analysis and investigation of heterogeneity
Planned a priori subgroup analyses were used to explore possible sources of heterogeneity. Heterogeneity was explored according to the type of lock solutions and the type of catheters (tunnelled or non‐tunnelled).
Sensitivity analysis
Sensitivity analyses were performed on the main outcomes and comparisons excluding studies with high risk of bias and separating randomised from quasi‐randomised studies.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b)). We presented the outcomes 1) CVC‐related infections per 1000 catheter‐days, and 2) thrombosis per 1000 catheter‐days Table 1.
Results
Description of studies
Results of the search
After searching the Register we identified 196 records. Titles and abstracts were screened and we retrieved 88 full‐text articles for further assessment. Of these, 39 studies (58 records) were included and 22 studies (29 records) were excluded. One study was completed prior to publication of this review (CLOCK 2017) and will be assessed in a future update (Figure 1).
1.
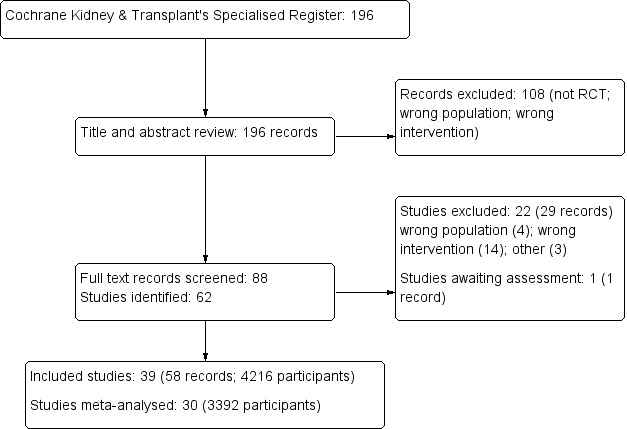
Study selection flow diagram
Included studies
We included 39 studies (4216 participants) in this review (Al‐Hwiesh 2007; AZEPTIC 2011; Betjes 2004; Bleyer 2005; Buturovic 1998; Campos 2011; CHARTS 2008; CITRIM 2017; Corbett 2013; Cooper 1999; Davanipur 2011; D'Avella 2007; Dogra 2002; Geron 2008; Hendrickx 2001; Hermite 2012; Kanaa 2015; Kim 2006a; Kokenge 2010; Lange 2007; Lustig 2011; McIntyre 2004; Moghaddas 2015; Moran 2012; Mortazavi 2011; Nori 2006; Oguzhan 2012; Pervez 2002; Plamandon 2005a; Power 2009; Saxena 2006; Saxena 2012; Shirzad 2013; Sofroniadou 2012; Solomon 2010; Vercaigne 2016a; Weijmer 2005; Zhang 2009c; Zwiech 2016a).
Of these, nine studies either did not provide enough data, or the relevant data could not be extracted, and therefore were not meta‐analysed (Corbett 2013; Kanaa 2015; Kokenge 2010; Lange 2007; Lustig 2011; Plamandon 2005a; Power 2009; Shirzad 2013; Zwiech 2016a).
Study design
Thirty‐seven studies were parallel RCTs and two were quasi‐randomised (CHARTS 2008; Power 2009).
Setting
Twenty‐five studies were undertaken in an outpatient setting, eight in an inpatient setting (Al‐Hwiesh 2007; Betjes 2004; Bleyer 2005; CITRIM 2017; Hermite 2012; Kim 2006a; Moghaddas 2015; Sofroniadou 2012), and six did not describe a specific setting (D'Avella 2007; Geron 2008; Kokenge 2010; Lange 2007; Lustig 2011; Plamandon 2005a).
Participants
All studies included adults with ESKD undergoing HD through a CVC; none of the studies included children. Twenty‐four studies used tunnelled catheters (Al‐Hwiesh 2007; AZEPTIC 2011; CHARTS 2008; Cooper 1999; Corbett 2013; Dogra 2002; Geron 2008; Hendrickx 2001; Kanaa 2015; Kokenge 2010; McIntyre 2004; Moghaddas 2015; Mortazavi 2011; Moran 2012; Nori 2006; Oguzhan 2012; Pervez 2002; Plamandon 2005a; Power 2009; Saxena 2006; Saxena 2012; Solomon 2010; Vercaigne 2016a; Zhang 2009c), six studies used non‐tunnelled catheters (Buturovic 1998; CITRIM 2017; Davanipur 2011; Hermite 2012; Kim 2006a; Sofroniadou 2012), six studies used both types of catheters (Betjes 2004; Bleyer 2005; Campos 2011; Lange 2007; Weijmer 2005; Zwiech 2016a), and three studies did not describe the type of catheter (D'Avella 2007; Lustig 2011; Shirzad 2013).
Interventions
Antibiotic lock solutions versus heparin
Fifteen studies used antibiotic lock solutions.
Minocycline/EDTA and heparin (Nori 2006)
Vancomycin hydrochloride, gentamicin sulphate, and heparin (Al‐Hwiesh 2007)
Minocycline and EDTA (Bleyer 2005; Campos 2011)
Cloxacillin and heparin (Davanipur 2011)
Cefazolin, gentamicin, and heparin (Kim 2006a)
Cefotaxime and heparin (Mortazavi 2011; Saxena 2006; Saxena 2012)
Gentamicin and heparin (McIntyre 2004; Zhang 2009c)
Cefazolin and heparin (Shirzad 2013)
Cotrimoxazole (Moghaddas 2015)
Linezolid and vancomycin (Sofroniadou 2012)
Gentamicin (Cooper 1999).
Non‐antibiotic antimicrobial lock solutions versus heparin and other solutions
Twenty‐one studies used non‐antibiotic antimicrobial lock solutions. Twenty studies compared non‐antibiotic antimicrobial lock solutions versus heparin.
Hypertonic saline solution (≥ 12%) (D'Avella 2007; Oguzhan 2012)
Tauloridine (Geron 2008)
Citrate plus taurolidine (Betjes 2004; Corbett 2013; Solomon 2010; Zwiech 2016a)
Ethanol plus citrate (Vercaigne 2016a)
Trisodium citrate (4%) (Buturovic 1998; CHARTS 2008; Plamandon 2005a)
Trisodium citrate (5%) (Hendrickx 2001)
Citrate solution (30%) (CITRIM 2017; Weijmer 2005)
Citrate solution (46.7%) (Power 2009)
Sodium citrate (7%), methylene blue (0.05%), methylparaben (0.15%), and propylparaben (0.015%) (AZEPTIC 2011)
Sodium citrate (3.13%) (Lange 2007; Kokenge 2010)
Trisodium citrate‐ethanol‐methylene blue (Lustig 2011)
Cathasept (Kanaa 2015).
One study compared 46.7% citrate solution to 0.9% saline solution (Hermite 2012).
Antibiotic lock solutions plus non‐antibiotic antimicrobial lock solutions versus heparin
Four studies used antibiotic lock solutions plus non‐antibiotic antimicrobial lock solutions.
Gentamicin (320 µg/mL) in sodium citrate (4%) (Moran 2012)
Gentamicin (40 mg/mL) and 1 mL citrate (3.13%) (Dogra 2002)
Gentamicin (40 mg/mL) plus tricitrasol (46.7%) (Pervez 2002)
Gentamicin (4 mg/mL) in citrate (3.13%) (Nori 2006).
Outcomes
All included studies reported at least one of our outcomes. Seventeen studies reported only CRI (Al‐Hwiesh 2007; CHARTS 2008; Cooper 1999; Davanipur 2011; D'Avella 2007; Dogra 2002; Geron 2008; Hendrickx 2001; Kim 2006a; Lustig 2011; McIntyre 2004; Mortazavi 2011; Saxena 2012; Shirzad 2013; Sofroniadou 2012; Vercaigne 2016a; Zwiech 2016a); four studies reported only thrombosis (Buturovic 1998; Kokenge 2010; Lange 2007; Plamandon 2005a); 15 studies reported CRI and thrombosis (AZEPTIC 2011; Campos 2011; CITRIM 2017; Corbett 2013; Hermite 2012; Moghaddas 2015; Moran 2012; Nori 2006; Oguzhan 2012; Pervez 2002; Power 2009; Saxena 2006; Solomon 2010; Weijmer 2005; Zhang 2009c); two studies reported CRI and colonisation (Betjes 2004; Bleyer 2005); and one study reported CRI, thrombosis, and colonisation (Kanaa 2015).
The 39 studies included were further divided into three groups according to the type of antimicrobial lock solutions: antibiotic, non‐antibiotic, and the combination of both. One study with three arms (gentamicin/citrate, minocycline/EDTA and heparin), was included in the two comparisons (antibiotic lock solutions plus non‐antibiotic antimicrobial lock solutions and antibiotic lock solutions) (Nori 2006).
Excluded studies
We excluded 22 studies after reviewing the full‐text reports (see Characteristics of excluded studies). Four studies had different populations (Beigi 2010; Khosroshahi 2006b; Khosroshahi 2015; Onder 2008); 13 studies used different interventions (Chen 2014b; Chu 2016, Coli 2010; HEALTHY‐CATH 2009; Hryszko 2013; Hu 2011; Malo 2010; Mohammad 2016; Oran 2008; PreCLOT 2006; Ray 1999; Sishir 2014; Thomson 2011); one study used a different comparison (Meeus 2005); one study reported different outcomes (Bosma 2010); one study was terminated early (NCT01989091); one study has not been verified since 2009 and no results have been published (NCT00862966); and one study protocol could not be located (ISRCTN27307877).
Risk of bias in included studies
Risk of bias was low or unclear for most domains in the majority of studies. Most authors were contacted for additional or missing information regarding their included studies; however, we only obtained response from one author (Hermite 2012).
Allocation
Sequence generation
Two studies were considered at high risk of bias due to the method used for sequence generation (CHARTS 2008; Power 2009). Seventeen studies reported an appropriate method of sequence generation and were judged to be at low risk of bias (AZEPTIC 2011; Betjes 2004; CITRIM 2017; Dogra 2002; Kanaa 2015; Kim 2006a; Moran 2012; Mortazavi 2011; Oguzhan 2012; Pervez 2002; Saxena 2006; Saxena 2012; Sofroniadou 2012; Solomon 2010; Vercaigne 2016a; Weijmer 2005; Zwiech 2016a). Twenty studies reported insufficient information to estimate this risk of bias (Al‐Hwiesh 2007; Bleyer 2005; Buturovic 1998; Campos 2011; Cooper 1999; Corbett 2013; D'Avella 2007; Davanipur 2011; Geron 2008; Hendrickx 2001; Hermite 2012; Kokenge 2010; Lange 2007; Lustig 2011; McIntyre 2004; Moghaddas 2015; Nori 2006; Plamandon 2005a; Shirzad 2013; Zhang 2009c).
Allocation concealment
Seven studies reported adequate allocation concealment, representing a low risk of bias (Betjes 2004; CITRIM 2017; Kanaa 2015; Saxena 2006; Saxena 2012; Vercaigne 2016a; Weijmer 2005). One study was at high risk of bias as they did not use allocation concealment (CHARTS 2008). The remaining 31 studies had insufficient details of the method of allocation concealment and were judged to be at unclear risk of bias (Al‐Hwiesh 2007; AZEPTIC 2011; Betjes 2004; Buturovic 1998; Campos 2011; Cooper 1999; Corbett 2013; D'Avella 2007; Davanipur 2011; Dogra 2002; Geron 2008; Hendrickx 2001; Hermite 2012; Kim 2006a; Kokenge 2010; Lange 2007; Lustig 2011; McIntyre 2004; Moghaddas 2015; Moran 2012; Mortazavi 2011; Nori 2006; Oguzhan 2012; Pervez 2002; Plamandon 2005a; Power 2009; Shirzad 2013; Sofroniadou 2012; Solomon 2010; Zhang 2009c; Zwiech 2016a).
Blinding
Participants and personnel
Nine studies were at low risk of bias (Bleyer 2005; CITRIM 2017; Dogra 2002; Hermite 2012; Saxena 2006; Saxena 2012; Solomon 2010; Weijmer 2005; Zhang 2009c). Seven studies showed a high risk of bias because either study participants or personnel were unblinded (Al‐Hwiesh 2007; AZEPTIC 2011; CHARTS 2008; Kanaa 2015; McIntyre 2004; Moghaddas 2015; Nori 2006). In the remaining 23 studies insufficient information was provided to determine who were blind and therefore were judged unclear risk of bias (Betjes 2004; Buturovic 1998; Campos 2011; Cooper 1999; Corbett 2013; D'Avella 2007; Davanipur 2011; Geron 2008; Hendrickx 2001; Kim 2006a; Kokenge 2010; Lange 2007; Lustig 2011; Moran 2012; Mortazavi 2011; Oguzhan 2012; Pervez 2002; Plamandon 2005a; Power 2009; Shirzad 2013; Sofroniadou 2012; Vercaigne 2016a; Zwiech 2016a).
Outcome assessment
In seven studies the outcome assessment was blinded (AZEPTIC 2011; Dogra 2002; Hermite 2012; Kanaa 2015; Saxena 2006; Saxena 2012; Solomon 2010). In four studies the outcome assessment was not blinded and was judged to be at high risk of bias (CHARTS 2008; Kim 2006a; Moghaddas 2015; Zhang 2009c). Twenty‐eight studies did not provide enough information to be judged, therefore this risk of bias was uncertain (Al‐Hwiesh 2007; Betjes 2004; Bleyer 2005; Buturovic 1998; Campos 2011; CITRIM 2017; Cooper 1999; Corbett 2013; D'Avella 2007; Davanipur 2011; Geron 2008; Hendrickx 2001; Kokenge 2010; Lange 2007; Lustig 2011; McIntyre 2004; Moran 2012; Mortazavi 2011; Nori 2006; Oguzhan 2012; Pervez 2002; Plamandon 2005a; Power 2009; Shirzad 2013; Sofroniadou 2012; Vercaigne 2016a; Weijmer 2005; Zwiech 2016a).
Incomplete outcome data
Twenty‐two studies had a low risk of bias, reporting complete data charts (AZEPTIC 2011; Betjes 2004; Campos 2011; CHARTS 2008; Dogra 2002; Hendrickx 2001; Kim 2006a; McIntyre 2004; Moghaddas 2015; Moran 2012; Mortazavi 2011; Nori 2006; Oguzhan 2012; Pervez 2002; Saxena 2006; Saxena 2012; Sofroniadou 2012; Solomon 2010; Vercaigne 2016a; Weijmer 2005; Zhang 2009c; Zwiech 2016a). One study was rated high risk of bias due to incomplete outcome data (Bleyer 2005). Sixteen studies did not provide sufficient information to determine whether incomplete outcome data were adequately addressed (Al‐Hwiesh 2007; Buturovic 1998; CITRIM 2017; Cooper 1999; Corbett 2013; D'Avella 2007; Davanipur 2011; Geron 2008; Hermite 2012; Kanaa 2015; Kokenge 2010; Lange 2007; Lustig 2011; Plamandon 2005a; Power 2009; Shirzad 2013).
Selective reporting
Twenty‐eight studies were judged to be at low risk of reporting bias (Al‐Hwiesh 2007; AZEPTIC 2011; Betjes 2004; Bleyer 2005; Campos 2011; CHARTS 2008; CITRIM 2017; Davanipur 2011; Dogra 2002; Hendrickx 2001; Hermite 2012; Kanaa 2015; Kim 2006a; McIntyre 2004; Moghaddas 2015; Moran 2012; Mortazavi 2011; Nori 2006; Oguzhan 2012; Pervez 2002; Saxena 2006; Saxena 2012; Sofroniadou 2012; Solomon 2010; Vercaigne 2016a; Weijmer 2005; Zhang 2009c; Zwiech 2016a). One study was judged to be at high risk of bias (Power 2009) and 10 studies were judged to have unclear risk of bias (Buturovic 1998; Cooper 1999; Corbett 2013; D'Avella 2007; Geron 2008; Kokenge 2010; Lange 2007; Lustig 2011; Plamandon 2005a; Shirzad 2013).
Other potential sources of bias
One study was judged as high risk of bias because researchers declared conflict of interest related to research funding (Moran 2012). Three studies were judged as low risk of bias (Al‐Hwiesh 2007; AZEPTIC 2011; Hermite 2012), and the remaining 35 studies reported insufficient information and they were judged to have unclear risk of bias (Betjes 2004; Bleyer 2005; Buturovic 1998; Campos 2011; CHARTS 2008; CITRIM 2017; Cooper 1999; Corbett 2013; D'Avella 2007; Davanipur 2011; Dogra 2002; Geron 2008; Hendrickx 2001; Kanaa 2015; Kim 2006a; Kokenge 2010; Lange 2007; Lustig 2011; McIntyre 2004; Moghaddas 2015; Mortazavi 2011; Nori 2006; Oguzhan 2012; Pervez 2002; Plamandon 2005a; Power 2009; Saxena 2006; Saxena 2012; Shirzad 2013; Sofroniadou 2012; Solomon 2010; Vercaigne 2016a; Weijmer 2005; Zhang 2009c; Zwiech 2016a).
Effects of interventions
See: Table 1
See Table 1.
Although 39 studies met the inclusion criteria, only 30 could be incorporated into the meta‐analyses, as the other nine studies did not present enough data to be included in the meta‐analysed (Corbett 2013; Kanaa 2015; Kokenge 2010; Lange 2007; Lustig 2011; Plamandon 2005a; Power 2009; Shirzad 2013; Zwiech 2016a) so all further descriptions refer to the 30 meta‐analysed studies.
Antimicrobial lock solutions (antibiotic, non‐antibiotic or both) versus control
Catheter‐related infection
Twenty seven studies reported the incidence of CRI per 1000 catheter‐days (AZEPTIC 2011; Betjes 2004; Bleyer 2005; Campos 2011; CHARTS 2008; CITRIM 2017; Cooper 1999; D'Avella 2007; Davanipur 2011; Dogra 2002; Geron 2008; Hermite 2012; Kim 2006a; McIntyre 2004; Moghaddas 2015; Moran 2012; Mortazavi 2011; Nori 2006; Oguzhan 2012; Pervez 2002; Saxena 2006; Saxena 2012; Sofroniadou 2012; Solomon 2010; Vercaigne 2016a; Weijmer 2005; Zhang 2009c). Antimicrobial lock solutions probably reduces the incidence of CRI per 1000 catheter days compared to control (Analysis 1.1: RR 0.38, 95% CI 0.27 to 0.53; I2 = 54%; moderate certainty evidence).
1.1. Analysis.
Comparison 1 All antimicrobial (antibiotic plus non‐antibiotic plus the combination) solutions versus control, Outcome 1 Catheter‐related infection (per 1000 catheter‐days).
Two studies measured the incidence of CRI per 1000 dialysis sessions (Al‐Hwiesh 2007; Hendrickx 2001). Antimicrobial lock solutions may make little or no difference to the reduction of CRI compared with heparin lock solutions (Analysis 1.2: RR 0.47, 95% CI 0.07 to 3.13; I2 = 81%; low certainty evidence). Heterogeneity was high for this analysis.
1.2. Analysis.
Comparison 1 All antimicrobial (antibiotic plus non‐antibiotic plus the combination) solutions versus control, Outcome 2 Catheter‐related infection (per 1000 dialysis sessions).
Thrombosis
Fourteen studies reported the incidence of thrombosis, per 1000 catheter‐days (AZEPTIC 2011; Buturovic 1998; Campos 2011; CITRIM 2017; Hermite 2012; Moghaddas 2015; Moran 2012; Nori 2006; Oguzhan 2012; Pervez 2002; Saxena 2006; Solomon 2010; Weijmer 2005; Zhang 2009c). Antimicrobial lock solutions probably make little or no difference to the risk of thrombosis compared to control (Analysis 1.3: RR 0.79, 95% CI 0.52 to 1.22; I2 = 83%; moderate certainty evidence). Heterogeneity was high for this analysis.
1.3. Analysis.
Comparison 1 All antimicrobial (antibiotic plus non‐antibiotic plus the combination) solutions versus control, Outcome 3 Thrombosis (per 1000 catheter‐days).
Colonisation
Two studies reported the incidence of colonisation (Betjes 2004; Bleyer 2005). Antimicrobial lock solutions may make little or no difference to the reduction in colonisation compared to control (Analysis 1.4: RR 0.37 95% CI 0.04 to 3.36; I2 = 70%; low certainty evidence), with high heterogeneity.
1.4. Analysis.
Comparison 1 All antimicrobial (antibiotic plus non‐antibiotic plus the combination) solutions versus control, Outcome 4 Colonisation.
Subgroup analyses
To explore sources of heterogeneity, subgroup analysis by CVC type was carried out for CRI and lumen thrombosis.
Eighteen studies used only tunnelled catheters (AZEPTIC 2011; CHARTS 2008; Cooper 1999; D'Avella 2007; Dogra 2002; Geron 2008; McIntyre 2004; Moghaddas 2015; Moran 2012; Mortazavi 2011; Nori 2006; Oguzhan 2012; Pervez 2002; Saxena 2006; Saxena 2012; Solomon 2010; Vercaigne 2016a; Zhang 2009c) and five used only non‐tunnelled catheters (CITRIM 2017; Davanipur 2011; Hermite 2012; Kim 2006a; Sofroniadou 2012). Four used both types of catheters (Betjes 2004; Bleyer 2005; Campos 2011; Weijmer 2005) however only three delivered data separating tunnelled and non‐tunnelled catheters (Betjes 2004; Campos 2011; Weijmer 2005). For the purpose of the analysis, the studies including both types were included separately in the tunnelled or non‐tunnelled group.
Due to insufficient data on included studies, it was not possible to explore other sources of heterogeneity, such as subgroups of diabetic participants or co‐interventions to prevent CRI.
Catheter‐related infection (tunnelled and non‐tunnelled catheters)
Twenty‐one studies reported data for tunnelled catheters (AZEPTIC 2011; CHARTS 2008; Cooper 1999; D'Avella 2007; Geron 2008; McIntyre 2004; Moghaddas 2015; Moran 2012; Mortazavi 2011; Nori 2006; Oguzhan 2012; Pervez 2002; Saxena 2006; Saxena 2012; Solomon 2010; Vercaigne 2016a; Zhang 2009c, Betjes 2004; Campos 2011; Dogra 2002; Weijmer 2005). Antimicrobial lock solutions probably reduce the incidence of CRI per 1000 catheter days compared to control in tunnelled catheters (Analysis 1.5: RR 0.40, 95% CI 0.30 to 0.53; I2 = 14%).
1.5. Analysis.
Comparison 1 All antimicrobial (antibiotic plus non‐antibiotic plus the combination) solutions versus control, Outcome 5 Subgroup analysis: catheter‐related infection (per 1000 catheter‐days) in tunnelled catheters.
Eight studies reported data for non‐tunnelled catheters (Betjes 2004; Campos 2011; CITRIM 2017; Davanipur 2011; Hermite 2012; Kim 2006a; Sofroniadou 2012; Weijmer 2005). Antimicrobial lock solutions probably reduce the incidence of CRI per 1000 catheter days compared to control in non‐tunnelled catheters (Analysis 1.6: RR 0.37, 95% CI 0.16 to 0.86; I2 = 67%). Heterogeneity was high.
1.6. Analysis.
Comparison 1 All antimicrobial (antibiotic plus non‐antibiotic plus the combination) solutions versus control, Outcome 6 Subgroup analysis: catheter‐related infection (per 1000 catheter‐days) in non‐tunnelled catheters.
Heterogeneity disappears if only tunnelled catheters are analysed, therefore some heterogeneity in the main analysis might be explained on the basis of type of CVC.
Thrombosis (tunnelled and non‐tunnelled catheters)
Ten studies reported data for tunnelled catheters (AZEPTIC 2011; Moghaddas 2015; Moran 2012; Nori 2006; Oguzhan 2012; Pervez 2002; Saxena 2006; Solomon 2010; Weijmer 2005; Zhang 2009c). Antimicrobial lock solutions probably make little or no difference to the risk of thrombosis in tunnelled catheters (Analysis 1.7: RR 0.83, 95% CI 0.50 to 1.37; I2 = 76%). High heterogeneity was observed.
1.7. Analysis.
Comparison 1 All antimicrobial (antibiotic plus non‐antibiotic plus the combination) solutions versus control, Outcome 7 Subgroup analysis: thrombosis (per 1000 catheter‐days) in tunnelled catheters.
Four studies reported data for non‐tunnelled catheters (Buturovic 1998; CITRIM 2017; Hermite 2012; Weijmer 2005). Antimicrobial lock solutions probably make little or no difference to the risk of thrombosis in non‐tunnelled catheters (Analysis 1.8: RR 0.66, 95% CI 0.25 to 1.72; I2 = 92%). High heterogeneity was observed.
1.8. Analysis.
Comparison 1 All antimicrobial (antibiotic plus non‐antibiotic plus the combination) solutions versus control, Outcome 8 Subgroup analysis: thrombosis (per 1000 catheter‐days) in non‐tunnelled catheters.
For this outcome, the type of catheter does not explain the observed heterogeneity.
Sensitivity analyses
We carried out a sensitivity analyses by excluding the studies deemed as to be at high risk of bias (for any domain), to determine whether the results of the meta‐analysis regarding the main outcomes were robust.
Catheter‐related infection
Eighteen studies with low or unclear risk of bias were analysed (Betjes 2004; Campos 2011; CITRIM 2017; Corbett 2013; D'Avella 2007; Davanipur 2011; Dogra 2002; Geron 2008; Hermite 2012; Mortazavi 2011; Oguzhan 2012; Pervez 2002; Saxena 2006; Saxena 2012; Sofroniadou 2012; Solomon 2010: Weijmer 2005). There was no change to the results; there was less precision and heterogeneity remained the same (Analysis 2.1: (RR 0.47, 95% CI 0.32 to 0.68; I2 = 58%).
2.1. Analysis.
Comparison 2 Sensitivity analysis: all antimicrobial lock solutions versus control excluding studies deemed with high‐risk bias, Outcome 1 Catheter‐related infection (per 1000 catheter‐days).
Thrombosis
Nine studies with low or unclear risk of bias were analysed (Buturovic 1998; Campos 2011; CITRIM 2017; Hermite 2012; Oguzhan 2012; Pervez 2002; Saxena 2006; Solomon 2010; Weijmer 2005). There was no change to the results; either in direction or magnitude of effect (Analysis 2.2: (RR 0.85, 95% CI 0.48 to 1.52; I2 = 88%).
2.2. Analysis.
Comparison 2 Sensitivity analysis: all antimicrobial lock solutions versus control excluding studies deemed with high‐risk bias, Outcome 2 Thrombosis (per 1000 catheter‐days).
Antibiotic lock solutions versus control
Catheter‐related infection
Thirteen studies reported CRI per 1000 catheter‐days (Bleyer 2005; Campos 2011; Cooper 1999; Davanipur 2011; Kim 2006a; McIntyre 2004; Moghaddas 2015; Mortazavi 2011; Nori 2006; Saxena 2006; Saxena 2012; Sofroniadou 2012; Zhang 2009c). Antibiotic lock solutions probably reduce the incidence of CRI per 1000 catheter days compared to control (Analysis 3.1: RR 0.30, 95% CI 0.22 to 0.42; I2 = 18%; moderate certainty evidence). Heterogeneity was low.
3.1. Analysis.
Comparison 3 Antibiotic lock solutions versus control, Outcome 1 Catheter‐related infection (per 1000 catheter‐days).
One study (Al‐Hwiesh 2007) reported antibiotic lock solutions reduced the incidence in CRI per dialysis session compared to control (Analysis 3.2: RR 0.18, 95% CI 0.05 to 0.59).
3.2. Analysis.
Comparison 3 Antibiotic lock solutions versus control, Outcome 2 Catheter‐related infection (per 1000 dialysis sessions).
Thrombosis
Five studies reported thrombosis per 1000 catheter‐days (Campos 2011; Moghaddas 2015; Nori 2006; Saxena 2006; Zhang 2009c). Antibiotic lock solutions probably make little or no difference to the risk of thrombosis per 1000 catheter‐days compared to control (Analysis 3.3; RR 0.76, 95% CI 0.42 to 1.38; I2 = 47%; moderate certainty evidence). Moderate heterogeneity was observed.
3.3. Analysis.
Comparison 3 Antibiotic lock solutions versus control, Outcome 3 Thrombosis (per 1000 catheter‐days).
Colonisation
One study (Bleyer 2005) reported that antibiotic lock solutions reduced the incidence of catheter colonisation compared to heparin (Analysis 3.4: RR 0.10, 95% CI 0.01 to 0.79).
3.4. Analysis.
Comparison 3 Antibiotic lock solutions versus control, Outcome 4 Colonisation.
Subgroup analyses
Catheter‐related infection
Nine studies reported data for tunnelled catheters (Campos 2011; Cooper 1999; McIntyre 2004; Moghaddas 2015; Mortazavi 2011; Nori 2006; Saxena 2006; Saxena 2012; Zhang 2009c). Antibiotic lock solutions probably reduce the incidence of CRI per 1000 catheter‐days compared to control in tunnelled catheters (Analysis 3.5: RR 0.30, 95% CI 0.18 to 0.50; I2 = 35%). Moderate heterogeneity was observed.
3.5. Analysis.
Comparison 3 Antibiotic lock solutions versus control, Outcome 5 Subgroup analysis: catheter‐related infection (per 1000 catheter‐days) in tunnelled catheters.
Four studies reported data for non‐tunnelled catheters (Campos 2011; Davanipur 2011; Kim 2006a; Sofroniadou 2012). Antibiotic lock solutions probably reduce the incidence of CRI per 1000 catheter days compared to control in non‐tunnelled catheters (Analysis 3.6: RR 0.14, 95% CI 0.05 to 0.36; I2 = 0%).
3.6. Analysis.
Comparison 3 Antibiotic lock solutions versus control, Outcome 6 Subgroup analysis: catheter‐related infection (per 1000 catheter‐days) in non‐tunnelled catheters.
Thrombosis
Three studies reported data for tunnelled catheters (Moghaddas 2015; Saxena 2006; Zhang 2009c). Antibiotic lock solutions probably make little or no difference to the risk of thrombosis per 1000 catheter‐days compared to control for tunnelled catheters (Analysis 3.7: RR 0.61, 95% CI 0.31 to 1.23; I2 = 45%).
3.7. Analysis.
Comparison 3 Antibiotic lock solutions versus control, Outcome 7 Subgroup analysis: thrombosis per 1000 catheter‐days in tunnelled catheters.
No analysis was performed for non‐tunnelled catheters as no separate data was reported in the three studies that used both types of catheters.
Sensitivity analyses
Sensitivity analyses excluding studies deemed as to be at high risk of bias (for any domain) were undertaken.
Catheter‐related infection
Seven studies with low or unclear risk of bias were included in this analysis (Campos 2011; Cooper 1999; Davanipur 2011; Mortazavi 2011; Saxena 2006; Saxena 2012; Sofroniadou 2012). There was no change to the results with less heterogeneity and higher precision (Analysis 4.1: RR 0.37, 95% CI 0.28 to 0.48; I2 = 4%).
4.1. Analysis.
Comparison 4 Sensitivity analysis: antibiotic lock solutions versus control excluding studies judged to be at high‐risk bias, Outcome 1 Catheter‐related infection (per 1000 catheter‐days).
Thrombosis
Two studies with low or unclear risk of bias were included in this analysis (Campos 2011; Saxena 2006). There was no change to results, however heterogeneity was higher (Analysis 4.2: RR 0.79, 95% CI 0.24 to 2.63; I2 = 82%).
4.2. Analysis.
Comparison 4 Sensitivity analysis: antibiotic lock solutions versus control excluding studies judged to be at high‐risk bias, Outcome 2 Thrombosis (per 1000 catheter‐days).
Non‐antibiotic antimicrobial lock solutions versus control
Catheter‐related infection
Eleven studies reported CRI per 1000 catheter‐days (AZEPTIC 2011; Betjes 2004; CHARTS 2008; CITRIM 2017; D'Avella 2007; Geron 2008; Hermite 2012; Oguzhan 2012; Solomon 2010; Vercaigne 2016a; Weijmer 2005). Non‐antibiotic antimicrobial lock solutions probably make little or no difference to the incidence of CRI per 1000 catheter days compared to control (Analysis 5.1: RR 0.65, 95% CI 0.41 to 1.05; I2 = 51%). Heterogeneity was moderate.
5.1. Analysis.
Comparison 5 Non‐antibiotic antimicrobial lock solutions versus control, Outcome 1 Catheter‐related infection (per 1000 catheter‐days).
One study (Hendrickx 2001) reported no difference in the incidence of CRI per 1000 dialysis sessions between non‐antibiotic antimicrobial lock solutions and control (Analysis 5.2: RR 1.22, 95% CI 0.38 to 3.88).
5.2. Analysis.
Comparison 5 Non‐antibiotic antimicrobial lock solutions versus control, Outcome 2 Catheter‐related infection (per 1000 dialysis sessions).
Thrombosis
Eight studies reported thrombosis per 1000 catheter‐days (AZEPTIC 2011; Buturovic 1998; CHARTS 2008; CITRIM 2017; Hermite 2012; Oguzhan 2012; Solomon 2010; Weijmer 2005). Seven studies used heparin as control and one study (Hermite 2012) used saline solution 0.9% as control lock solution. Non‐antibiotic antimicrobial lock solutions probably make little or no difference to the risk of thrombosis per 1000 catheter‐days compared to control (Analysis 5.3: RR 0.85, 95% CI 0.44 to 1.66; I2 = 89%). Heterogeneity is high.
5.3. Analysis.
Comparison 5 Non‐antibiotic antimicrobial lock solutions versus control, Outcome 3 Thrombosis (per 1000 catheter‐days).
One study (Hendrickx 2001) reported a decrease in the incidence of thrombosis per 1000 dialysis sessions with non‐antibiotic antimicrobial lock solutions versus heparin (Analysis 5.4 RR 0.11, 95% CI 0.04 to 0.32).
5.4. Analysis.
Comparison 5 Non‐antibiotic antimicrobial lock solutions versus control, Outcome 4 Thrombosis (per 1000 dialysis sessions).
Colonisation
One study (Betjes 2004) reported no difference in the reduction of catheter colonisation between non‐antibiotic antimicrobial lock solutions and control (Analysis 5.5: RR 0.99, 95%CI 0.27 to 3.68).
5.5. Analysis.
Comparison 5 Non‐antibiotic antimicrobial lock solutions versus control, Outcome 5 Colonisation.
Subgroup analyses
Catheter‐related infection
Nine studies reported data for tunnelled catheters (AZEPTIC 2011; Betjes 2004; CHARTS 2008; D'Avella 2007; Geron 2008; Weijmer 2005; Oguzhan 2012; Solomon 2010; Vercaigne 2016a). Non‐antibiotic antimicrobial lock solutions probably reduce the incidence of CRI per 1000 catheter‐days compared to control in tunnelled catheters (Analysis 5.6: RR 0.60, 95% CI 0.40 to 0.91; I2 = 0%).
5.6. Analysis.
Comparison 5 Non‐antibiotic antimicrobial lock solutions versus control, Outcome 6 Subgroup analysis: catheter‐related infection (per 1000 catheter‐days) in tunnelled catheters.
Four studies reported data for non‐tunnelled catheters (Betjes 2004; CITRIM 2017; Hermite 2012; Weijmer 2005). Non‐antibiotic antimicrobial lock solutions probably make little or no difference to the risk of CRI per 1000 catheter‐days compared to control for tunnelled catheters (Analysis 5.7: RR 0.93, 95% CI 0.48 to 1.81; I2 = 41%). Heterogeneity is moderate.
5.7. Analysis.
Comparison 5 Non‐antibiotic antimicrobial lock solutions versus control, Outcome 7 Subgroup analysis: catheter‐related infection (per 1000 catheter‐days) in non‐tunnelled catheters.
Thrombosis
Five studies reported data for tunnelled catheters (AZEPTIC 2011; CHARTS 2008; Oguzhan 2012; Solomon 2010; Weijmer 2005). Non‐antibiotic antimicrobial lock solutions probably make little or no difference to the risk of thrombosis per 1000 catheter‐days compared to control for tunnelled catheters (Analysis 5.8: RR 1.17, 95% CI 0.57 to 2.41; I2 = 72%). Heterogeneity was high.
5.8. Analysis.
Comparison 5 Non‐antibiotic antimicrobial lock solutions versus control, Outcome 8 Subgroup analysis: thrombosis (per 1000 catheter‐days) in tunnelled catheters.
Four studies reported data for non‐tunnelled catheters (Buturovic 1998; CITRIM 2017;Hermite 2012; Weijmer 2005) Non‐antibiotic antimicrobial lock solutions probably make little or no difference to the risk of thrombosis per 1000 catheter‐days compared to control for non‐tunnelled catheters (Analysis 5.9: RR 0.66, 95% CI 0.25 to 1.72; I2 = 92%). Heterogeneity was high.
5.9. Analysis.
Comparison 5 Non‐antibiotic antimicrobial lock solutions versus control, Outcome 9 Subgroup analysis: thrombosis (per 1000 catheter‐days) in non‐tunnelled catheters.
Sensitivity analyses
Sensitivity analyses excluding studies deemed as to be at high risk of bias (for any domain) were undertaken.
Catheter‐related infection
Nine studies with low or unclear risk of bias were included in this analysis (Betjes 2004; CITRIM 2017; Geron 2008; Hermite 2012; Oguzhan 2012; Solomon 2010; Vercaigne 2016a; Weijmer 2005). The analysis showed a similar magnitude of effect but with less precision and higher heterogeneity (Analysis 6.1: RR 0.65, 95% CI 0.38 to 1.12; I2 = 61%).
6.1. Analysis.
Comparison 6 Sensitivity analysis: non‐antibiotic lock solutions versus control excluding studies judged to be at high‐risk bias, Outcome 1 Catheter‐related infection (per 1000 catheter‐days).
Thrombosis
Six studies with low or unclear risk of bias were included in this analysis (Buturovic 1998; CITRIM 2017; Hermite 2012; Oguzhan 2012; Solomon 2010; Weijmer 2005). The analysis showed a similar magnitude of effect but with less precision and same heterogeneity (Analysis 6.2: RR 0.90, 95% CI 0.43 to 1.91; I2 = 92%).
6.2. Analysis.
Comparison 6 Sensitivity analysis: non‐antibiotic lock solutions versus control excluding studies judged to be at high‐risk bias, Outcome 2 Thrombosis (per 1000 catheter‐days).
Combined antibiotic plus non‐antibiotic antimicrobial lock solutions versus control
Catheter‐related infection
Four studies reported CRI per 1000 catheter‐days (Dogra 2002; Moran 2012; Nori 2006; Pervez 2002). Antibiotic plus non‐antibiotic antimicrobial lock solution probably reduce the incidence of CRI per 1000 catheter‐days compared to control (Analysis 7.1: RR 0.26, 95% CI 0.14 to 0.49; I2 = 0%; moderate certainty evidence).
7.1. Analysis.
Comparison 7 Combined antimicrobial lock solutions versus control, Outcome 1 Catheter‐related infection (per 1000 catheter‐days).
Thrombosis
Three studies reported thrombosis per 1000 catheter‐days (Moran 2012; Nori 2006; Pervez 2002). Antibiotic plus non‐antibiotic antimicrobial lock solution probably make little or no difference to the incidence of thrombosis per 1000 catheter‐days compared to control (Analysis 7.2: RR 0.63, 95% CI 0.22 to 1.81; I2 = 0%; moderate certainty evidence).
7.2. Analysis.
Comparison 7 Combined antimicrobial lock solutions versus control, Outcome 2 Thrombosis (per 1000 catheter‐days).
Colonisation
No study reported catheter colonisation.
Subgroup analyses
All four studies used tunnelled catheters.
Sensitivity analyses
Sensitivity analyses excluding studies deemed as to be at high risk of bias (for any domain) were undertaken.
Catheter‐related infection
Two studies with low or unclear risk of bias were included in this analysis (Dogra 2002; Pervez 2002). The result no longer showed a reduction in CRI (Analysis 8.1: RR 0.16, 95% CI 0.02 to 1.03; I2 = 0%).
8.1. Analysis.
Comparison 8 Sensitivity analysis: combined antimicrobial lock solutions versus control excluding studies deemed with high‐risk bias, Outcome 1 Catheter‐related infection (per 1000 catheter‐days).
Thrombosis
One study (Pervez 2002) showed a similar magnitude of effect but with less precision (Analysis 8.2: RR 0.66, 95% CI 0.18 to 2.44).
8.2. Analysis.
Comparison 8 Sensitivity analysis: combined antimicrobial lock solutions versus control excluding studies deemed with high‐risk bias, Outcome 2 Thrombosis (per 1000 catheter‐days).
Other planned outcomes
Survival of the catheter, infection and thrombosis‐free days, were reported in a small number of included studies, however the data was not presented in enough detail to be meta‐analysed.
Mortality and adverse effects were not reported.
Discussion
Summary of main results
Antimicrobial lock solutions reduced CRI in patients using CVC for HD, compared to standard lock solutions, usually heparin (moderate certainty evidence). This beneficial effect was best for antibiotic lock solutions and for combined (antibiotic plus non‐antibiotic antimicrobial) lock solutions. Heterogeneity was low for antibiotic lock solutions and for combined lock solutions (I2 = 18% and 0% respectively) but high for all antimicrobial lock solutions and non‐antibiotic antimicrobial lock solutions (I2 = 54% and 51% respectively). Therefore the type of antimicrobial lock solution might explain some of the heterogeneity in the main analysis. Type of catheter (tunnelled vs non‐ tunnelled) might also influence the effect of antimicrobial lock solutions. For both tunnelled (RR 0.40; 95% CI 0.30 to 0.53; I2 = 14%) and non‐tunnelled catheters, antimicrobial lock solutions (RR 0.37, 95% CI 0.16 to 0.86; I2 = 67%) reduced CRI.
The difference between both types of catheters could be related to the pathogenesis of infection. In the case of non‐tunnelled catheters the infection could be developed through the either external or the internal surface of the catheter. By contrast, the route for the development of infection of tunnelled catheters corresponds to the intraluminal surface, which is in contact with the antimicrobial lock solutions. However the type of catheter alone, does not explain heterogeneity, probably due to differences in characteristics of patients using one or the other type of catheter. Due to the quality of the evidence, our confidence in the effect of antimicrobial lock solutions on CRI is moderate.
The efficacy of antimicrobial lock solutions in reducing the incidence of CRI is still present in the sensitivity analysis, using the studies classified as having low or unclear risk of bias, however, even though these results are robust, our confidence in the effect on CRI is moderate due to the quality of the evidence.
Regarding the safety of antimicrobial lock solutions, they are no worse than heparin or other lock solutions on the incidence of thrombosis of CVC for HD. These results remain unaltered when analysed according to type of antimicrobial lock solutions, type of CVC, and quality of studies.
It is important to note that there is less evidence for thrombosis, due to a small number of studies reporting this outcome; therefore, these estimates are imprecise. These results are also heterogeneous, which might be due to a study that used saline solution instead of heparin as control lock solution or due to different definitions of thrombosis throughout the studies. Due to the quality of the evidence, our confidence in the effect of antimicrobial lock solutions on lumen thrombosis is low.
It is not possible to draw conclusions regarding CVC colonisation because this outcome was reported in only two studies that used different antimicrobial lock solutions. Survival of the catheter, infection‐ and thrombosis‐free periods, mortality, and adverse effects were not reported.
Overall completeness and applicability of evidence
We included thirty studies in the analyses with a total of 3392 participants. Studies were conducted between 1998 and 2017 in adult participants undergoing HD through a CVC, mainly in an ambulatory setting. The studies compared antimicrobial with other control lock solutions, mainly heparin, in order to prevent CRI without increasing thrombosis.
Our review considered all types of antimicrobial lock solutions together and subsets of antibiotic, non‐antibiotic and combination of both solutions. Other included outcomes were thrombosis and colonisation. Most studies rarely reported other adverse effects, follow‐up were short or not reported so we do not have information regarding long term effects, such as antibiotic resistance.
Our review presents some limitations to its applicability, mainly because adverse effects other than thrombosis were not assessed in studies for all antimicrobial lock solutions.
It is important to remember that some literature shows that sodium citrate is associated with some adverse effects such as hypocalcaemia, ventricular arrhythmias, and few cases of sudden death (Aguinaga 2011), none of which were reported in our included studies. Additionally, antibiotic solutions could cause microbial resistance, especially when used for long periods(Korkor 2009). Antibiotic antimicrobial lock solutions were quite different among studies, so we were unable to determine if any of them was better than the others.
Quality of the evidence
Thirty nine studies were selected for this review including 4216 participants. Thirty‐seven were RCTs and two quasi‐randomised, 17 studies (44%) presented an appropriate sequence of randomizations whereas seven studies (18%) had low risk of bias for allocation concealment. Blinding method was reported in nine studies for participants and health personnel (23%), and seven studies for outcome assessment (18%). Complete data was reported in 22 studies (56%). Reporting bias was low in 28 studies (72%). It was not possible to determine others type of bias in 35 studies (90%), mainly because conflict of interest or funding was not declared (Figure 2; Figure 3)
2.
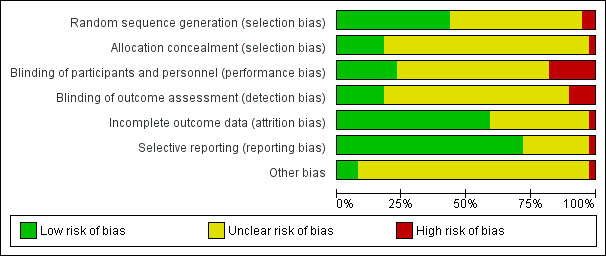
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
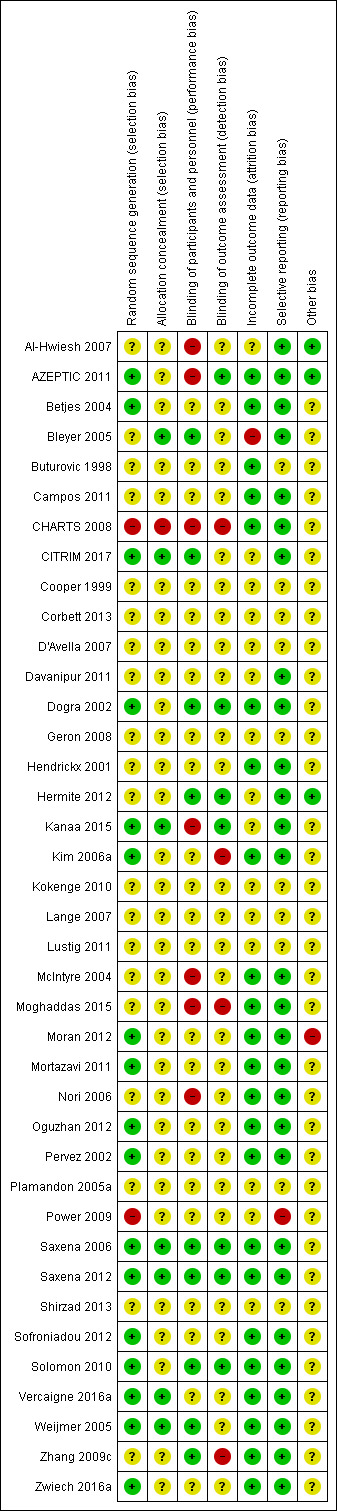
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Publication bias is likely to occur in all comparisons based on the funnel plots (Figure 4; Figure 5; Figure 6).
4.
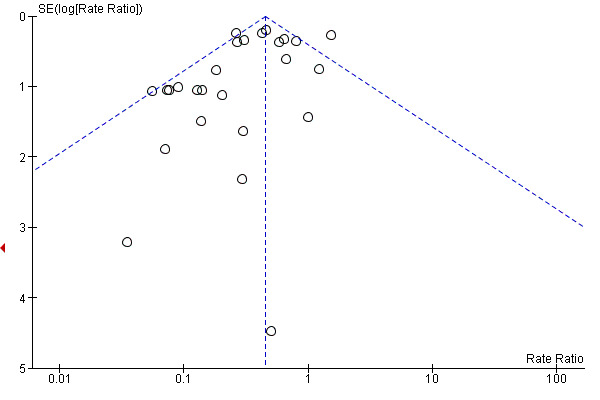
Funnel plot of comparison: 1 Antimicrobial (Antibiotic plus non‐antibiotic) solutions versus control, outcome: 1.1 CVC‐related infection (per 1000 catheter‐days).
5.

Funnel plot of comparison: 1 Antimicrobial (antibiotic plus non‐antibiotic) lock solutions versus control, outcome: 1.3 Thrombosis (per 1000 catheter‐days).
6.
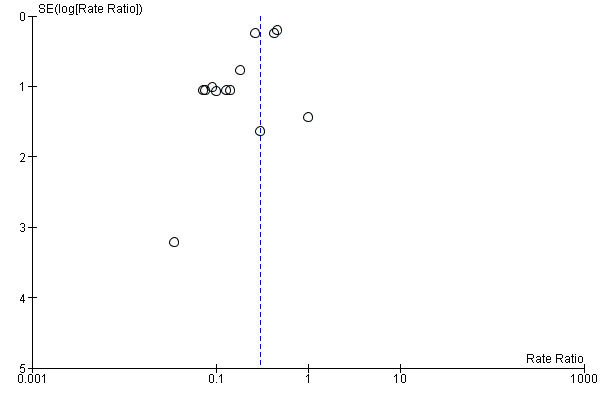
Funnel plot of comparison: 3 Antibiotic lock solutions versus control, outcome: 3.1 Catheter‐related infection (per 1000 catheter‐days).
Potential biases in the review process
A comprehensive search of the Cochrane Kidney and Transplant's Specialised Register was undertaken, thereby decreasing the likelihood of overlooking published studies. However, some studies may have been missed in the process, or may have been presented in some conferences not included in our search. Data was not complete for many studies; we contacted the authors via e‐mail regarding any missing information, however only one responded.
This review was performed independently by two authors in the stages of selection of articles and two others in the extraction of data. A third author participated in both processes when no agreement was reached through consensus. Sensitivity analysis was performed on the main results. As several studies contained insufficient information to make a judgment of quality, we contacted the authors; unfortunately, with no response from them.
Agreements and disagreements with other studies or reviews
Yahav 2008 published a systematic review evaluating antimicrobial lock solutions for the prevention of HD CRI. This review included 16 studies using antibiotic lock solutions or non‐antibiotic lock solutions. We included in our review 9 of the 11 comparing antibiotic lock solutions and 4 of the 5 comparing non‐antibiotic lock solutions. The studies not included in our review did not meet of our inclusion criteria (i.e. inappropriate design or insufficient data reported). Snaterse 2010 published a review analysing the use of catheter locking with antimicrobial, antibiotic and non‐antibiotic solutions for preventing the infection of CVC used for an extended period of time, including multiple indications of prolonged use. All studies that included participants who used catheter for HD were included in the review.
Zhao 2014 evaluated whether citrate is superior to heparin in the permeability of HD CVC and the prevention of infections. Eleven of the 12 studies included in the review are considered in the our systematic review. One study was not included because it was not randomised.
Grudzinski 2015 published a review whose objective was to evaluate the benefits and risks of catheter locks for HD catheters with sodium citrate. The five included studies are included in our review; however, it is not possible to compare these with our review because their aims are the benefits and harms of using citrate versus heparin as a CVC locking solution.
The results of our review are similar regarding the effect of antibiotic lock solutions to those reported by Yahav 2008 and Snaterse 2010, showing that antibiotic lock solutions for HD CVC reduce the incidence of CRI per 1000 catheter‐days. In addition, Zhao 2014 found that citrate in combination with other antimicrobials (non‐antibiotic antimicrobial lock solutions and antibiotic lock solutions) compared with heparin were associated with a lower incidence of CRI; however citrate alone was not shown to be better than heparin. Our review confirmed these findings.
Authors' conclusions
Implications for practice.
Our review shows that antibiotic antimicrobial and combined (antibiotic plus non‐antibiotic) lock solutions decreased the incidence of CRI compared to control lock solutions, usually heparin, but non‐antibiotic lock solutions did not significantly reduce CRI in participants undergoing HD through a CVC. This beneficial effect was also better established for tunnelled CVC. The effect on the incidence of thrombosis is uncertain for all antimicrobial lock solutions due to imprecision and heterogeneity of results. Our confidence on the evidence is low and very low ; better designed studies are needed to confirm the efficacy and safety of antimicrobial lock solutions. In addition, it is necessary to consider our uncertainty in other potential adverse effects such as ototoxicity from the use of gentamycin, risk of antibiotics resistance, among others, that were not assessed in the studies included in this review.
Implications for research.
Although the available data show that some antimicrobial lock solutions are effective in preventing CRI, additional large, well‐designed RCTs are required to evaluate incidence of CRI, thrombosis, and adverse events. These studies should consider a follow‐up of patients sufficiently long to detect microbial resistance in the case of antimicrobial antibiotic solutions and other adverse effects in both the antibiotic and non‐antibiotic antimicrobial lock solutions.
Acknowledgements
We wish to thank the referees for their feedback and comments during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
| Sequence Generation Randomise | Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random) |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention | |
| Unclear: Insufficient information about the sequence generation process to permit judgement | |
|
Allocation Concealed Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes) |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure | |
| Unclear: Randomisation stated but no information on method used is available | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation | |
| Unclear: Insufficient information to permit judgement | |
|
Selective outcome reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon) |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias |
Data and analyses
Comparison 1. All antimicrobial (antibiotic plus non‐antibiotic plus the combination) solutions versus control.
Comparison 2. Sensitivity analysis: all antimicrobial lock solutions versus control excluding studies deemed with high‐risk bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related infection (per 1000 catheter‐days) | 18 | Rate Ratio (Random, 95% CI) | 0.47 [0.32, 0.68] | |
| 2 Thrombosis (per 1000 catheter‐days) | 9 | Rate Ratio (Random, 95% CI) | 0.85 [0.48, 1.52] |
Comparison 3. Antibiotic lock solutions versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related infection (per 1000 catheter‐days) | 13 | Rate Ratio (Random, 95% CI) | 0.30 [0.22, 0.42] | |
| 2 Catheter‐related infection (per 1000 dialysis sessions) | 1 | Rate Ratio (Random, 95% CI) | Totals not selected | |
| 3 Thrombosis (per 1000 catheter‐days) | 5 | Rate Ratio (Random, 95% CI) | 0.76 [0.42, 1.38] | |
| 4 Colonisation | 1 | Rate Ratio (Random, 95% CI) | Totals not selected | |
| 5 Subgroup analysis: catheter‐related infection (per 1000 catheter‐days) in tunnelled catheters | 9 | Rate Ratio (Random, 95% CI) | 0.30 [0.18, 0.50] | |
| 6 Subgroup analysis: catheter‐related infection (per 1000 catheter‐days) in non‐tunnelled catheters | 4 | Rate Ratio (Random, 95% CI) | 0.14 [0.05, 0.36] | |
| 7 Subgroup analysis: thrombosis per 1000 catheter‐days in tunnelled catheters | 3 | Rate Ratio (Random, 95% CI) | 0.61 [0.31, 1.23] |
Comparison 4. Sensitivity analysis: antibiotic lock solutions versus control excluding studies judged to be at high‐risk bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related infection (per 1000 catheter‐days) | 7 | Rate Ratio (Random, 95% CI) | 0.37 [0.28, 0.48] | |
| 2 Thrombosis (per 1000 catheter‐days) | 2 | Rate Ratio (Random, 95% CI) | 0.79 [0.24, 2.63] |
Comparison 5. Non‐antibiotic antimicrobial lock solutions versus control.
Comparison 6. Sensitivity analysis: non‐antibiotic lock solutions versus control excluding studies judged to be at high‐risk bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related infection (per 1000 catheter‐days) | 9 | Rate Ratio (Random, 95% CI) | 0.65 [0.38, 1.12] | |
| 2 Thrombosis (per 1000 catheter‐days) | 6 | Rate Ratio (Random, 95% CI) | 0.90 [0.43, 1.91] |
Comparison 7. Combined antimicrobial lock solutions versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related infection (per 1000 catheter‐days) | 4 | Rate Ratio (Random, 95% CI) | 0.26 [0.14, 0.49] | |
| 2 Thrombosis (per 1000 catheter‐days) | 3 | Rate Ratio (Random, 95% CI) | 0.63 [0.22, 1.81] |
Comparison 8. Sensitivity analysis: combined antimicrobial lock solutions versus control excluding studies deemed with high‐risk bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related infection (per 1000 catheter‐days) | 2 | Rate Ratio (Random, 95% CI) | 0.16 [0.02, 1.03] | |
| 2 Thrombosis (per 1000 catheter‐days) | 1 | Rate Ratio (Random, 95% CI) | 0.66 [0.18, 2.44] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Hwiesh 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published results include all expected outcomes |
| Other bias | Low risk | Study appears free of other biases |
AZEPTIC 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible consenting participants were randomised 1:1 to one of the two treatment groups from computer‐generated randomizations lists |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent committee assessed the outcome and was blinded to patient treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Low risk | Study appears free of other biases |
Betjes 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐generated table of random numbers. The randomization procedure was done independent of type of catheter or place of insertion" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published results include all expected outcomes |
| Other bias | Unclear risk | Funding sources not reported |
Bleyer 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomised according to a block design, with each block consisting of four patients"; however method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | "Only the pharmacist who prepared and distributed the catheter solutions knew the randomizations code" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study. "Each solution was drawn up into a syringe, and each syringe was wrapped in orange plastic so that differences in color between the two solutions (minocycline–EDTA, orange; heparin, clear) could not be identified." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Three losses in the control group were not considered in the analysis |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Unclear risk | Funding source not reported; 2 authors were patent holders for treatment solution |
Buturovic 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 2
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available but published results include all expected outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Campos 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation was performed by sealed envelope, but did not state if they were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data was reported |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Unclear risk | Funding source not reported |
CHARTS 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomised according to their last name |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was an open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data was reported |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Unclear risk | Funding source not reported |
CITRIM 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐generated list of random numbers in blocks of six." |
| Allocation concealment (selection bias) | Low risk | "...was performed using opaque, sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients and investigators were unaware of the treatment assignments" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Unclear risk | Funding source not reported |
Cooper 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Corbett 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
D'Avella 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Davanipur 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported. "Participants were randomly divided into 2 groups." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Unclear risk | Funding source not reported |
Dogra 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomization using random number tables was performed by Clinical Trials Pharmacists, thereby ensuring allocation concealment" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of microbiology staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to objective |
| Other bias | Unclear risk | Funding source not reported |
Geron 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Hendrickx 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported "...patients were randomly assigned..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Unclear risk | Funding source not reported |
Hermite 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was an open‐label trial; but "as far as possible, the nurses and physicians in the unit in charge of the routine care were blinded to each patient’s treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were evaluated by an independent clinical event committee who were blinded to participants’ treatment group assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Low risk | Study appears free of other biases |
Kanaa 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using a random number table and blocking factor of 10 in a 1:1 ratio" |
| Allocation concealment (selection bias) | Low risk | Was performed using opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of microbiology staff |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Unclear risk | "The sponsors had no role in analysis or interpretation of data, writing the report, or the decision to submit the report for publication." However the study was terminated early |
Kim 2006a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomizations method was made using table of random number |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Researchers assessed the outcomes were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All analyses were performed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Unclear risk | Funding source not reported |
Kokenge 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Lange 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Lustig 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
McIntyre 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was block randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was performed by sealed envelope, but not opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | By the characteristics of the solution used, blinding of intervention was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All analyses were performed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Moghaddas 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was made using cluster randomization among three dialysis units."; method for doing this was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All analyses were performed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Moran 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomly assigned 1:1 to treatment or control groups using a central randomization system with participants randomly assigned within centers in blocks of 2 and 4 to ensure an approximate balance in the number of participants in each group within each centre" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | High risk | This study was entirely funded by Satellite Healthcare, the study sponsor. Most investigators were employees of Satellite Healthcare at the time the study was conducted |
Mortazavi 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used randomisation computerized block protocol |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind study, probably were blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient data reported |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to objective |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Nori 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 1
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Permuted block randomisation was performed at each centre; method not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient data reported |
| Selective reporting (reporting bias) | Low risk | Describes the outcomes according to objectives |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Oguzhan 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomization using random number tables was performed" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient data was reported |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to objective |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Pervez 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use computer‐generated number list |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient data reported |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to objective |
| Other bias | Unclear risk | Was supported by the Dialysis Clinic Inc |
Plamandon 2005a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Power 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Single random‐ numbers method of odd and even numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All patient data reported |
| Selective reporting (reporting bias) | High risk | Outcomes of interest reported incompletely |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Saxena 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use computer‐generated random number list |
| Allocation concealment (selection bias) | Low risk | Numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The patient's and HD staff were blinded of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The microbiologist was blinded of the treatment assigned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to objective |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Saxena 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes, numbered in sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The pharmacy dispensed equal number of identical syringes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Microbiologist was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient outcome data reported |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Shirzad 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Sofroniadou 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Solomon 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use computer‐generated randomised permuted blocks of 10 participants stratified among the 3 main centres |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All study personnel and participants were blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study personnel and participants were blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to objective |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Vercaigne 2016a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomised centrally in permuted blocks of four using computer software (Microsoft Excel 2010 Microsoft Corporation). Randomization was stratified based on whether the catheter was inserted into a new location or changed over a guide wire." |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Ethanol has a distinctive smell |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient data reported |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Unclear risk | Study parted funded by MedXL Inc (medical devices and prefilled syringes) |
Weijmer 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐generated list of random numbers in blocks of six and stratified according to the dialysis centre and to the type of catheter inserted (tunneled cuffed or untunneled)." |
| Allocation concealment (selection bias) | Low risk | "The randomization codes were kept by the central department of pharmacy" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients and investigators were unaware of the treatment assignments" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All analyses were performed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to objective |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Zhang 2009c.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participants and dialysis nurses were blinded of the treatment assignments |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study investigators who assessed outcomes were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient data reported |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to objective |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Zwiech 2016a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use computer‐generated random number list |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Describes the outcome according to protocol |
| Other bias | Unclear risk | Insufficient information to permit judgement |
AKI ‐ acute kidney injury; AV ‐ arteriovenous; CFU ‐ colony‐forming units; CKD ‐ chronic kidney disease; CRI ‐ catheter‐related infection; CRS ‐ catheter‐related sepsis; CVC ‐ central venous catheter; ESKD ‐ end‐stage kidney disease; HD ‐ haemodialysis; ICU ‐ intensive care unit; IV ‐ intravenous; M/F ‐ male/female; RCT ‐ randomised controlled trial; SD ‐ standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beigi 2010 | Wrong population: treating infection rather than preventing |
| Bosma 2010 | Wrong outcome: colonisation in vitro |
| Chen 2014b | Wrong intervention: NaCl at concentrations less than 12% has not proven to be antimicrobial solution |
| Chu 2016 | Wrong intervention: low to high dose heparin (no antimicrobial lock solutions) |
| Coli 2010 | Wrong intervention: compared urokinase lock therapies (no antimicrobial lock solutions) |
| HEALTHY‐CATH 2009 | Wrong intervention: weekly 70% ethanol + heparin lock versus heparin lock (no antimicrobial lock solutions) |
| Hryszko 2013 | Wrong intervention: low versus high dose heparin (no antimicrobial lock solutions) |
| Hu 2011 | Wrong intervention: low versus high dose heparin (no antimicrobial lock solutions) |
| ISRCTN27307877 | Protocol no longer available on the National Research Register website; no full text publication identified |
| Khosroshahi 2006b | Wrong population: treating infection rather than preventing |
| Khosroshahi 2015 | Wrong population: treating infection rather than preventing |
| Malo 2010 | Wrong intervention: low molecular weight heparin versus unfractionated heparin (no antimicrobial lock solutions) |
| Meeus 2005 | Wrong intervention: 5% versus 10% citrate |
| Mohammad 2016 | Wrong intervention: taurolock + heparin versus taurolock + urokinase |
| NCT00862966 | Citrate versus heparin. The recruitment status of this study is unknown; the completion date has passed and the status has not been verified in more than two years. Last verified March 2009 |
| NCT01989091 | B‐lock versus heparin. This study has been terminated (did not meet predetermined primary endpoint) |
| Onder 2008 | Wrong population: treating infection rather than preventing |
| Oran 2008 | Wrong intervention: timing of heparin lock (3 times/week versus 6 times/week) (no antimicrobial lock solutions) |
| PreCLOT 2006 | Wrong intervention: tissue plasminogen activator versus heparin (no antimicrobial lock solutions) |
| Ray 1999 | Wrong intervention: twice‐daily heparin flushes or twice‐daily heparin flushes with once‐weekly urokinase instillation (no antimicrobial lock solutions) |
| Sishir 2014 | Wrong intervention: 70% ethanol versus heparin (no antimicrobial lock solutions) |
| Thomson 2011 | Wrong intervention: low versus high dose heparin (no antimicrobial lock solutions) |
Characteristics of studies awaiting assessment [ordered by study ID]
CLOCK 2017.
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
| Outcomes |
|
| Notes |
|
CKD ‐ chronic kidney disease; CRI ‐ catheter‐related infection; CVC ‐ central venous catheter; HD ‐ haemodialysis; M/F ‐ male/female; RCT ‐ randomised controlled trial; SD ‐ standard deviation
Contributions of authors
Draft the protocol: MCA, MIC, JCC, LML, MC
Develop a search strategy: MCA, MIC
Search for studies: MCA, MIC
Obtain copies of studies: MCA
Study selection: MCA, MIC
Extract data from studies: NR, MR
Enter data into RevMan: MCA, MIC, JCC
Carry out the analysis: JCC, MCA, MIC
Interpret the analysis: MCA, MIC, JCC, LML
Draft the final review: MCA, MIC, JCC, LML
Disagreement resolution: MCA, MIC, JCC, LML
Update the review: MCA, MIC, JCC, LML
Sources of support
Internal sources
New Source of support, Other.
External sources
FONIS: Fondo Nacional de Investigación y Desarrollo en Salud, Chile.
Declarations of interest
All authors were recipients of a clinical research grant from: Fondo Nacional de Investigación y Desarrollo en Salud (FONIS) from the Chilean government.
New
References
References to studies included in this review
Al‐Hwiesh 2007 {published and unpublished data}
- Al Hwiesh AK. Tunneled catheter‐antibiotic lock therapy for prevention of dialysis catheter‐related infections: a single center experience. Saudi Journal of Kidney Diseases & Transplantation 2008;19(4):593‐602. [MEDLINE: ] [PubMed] [Google Scholar]
- Al‐Hwiesh AK, Abdul‐Rahman IS. Successful prevention of tunneled, central catheter infection by antibiotic lock therapy using vancomycin and gentamycin. Saudi Journal of Kidney Diseases & Transplantation 2007;18(2):239‐47. [MEDLINE: ] [PubMed] [Google Scholar]
AZEPTIC 2011 {published data only}
- Ash S, Maki D, Lavin P, Winger R. Multicenter randomized trial of an antimicrobial and antithrombotic lock solution [abstract]. Hemodialysis International 2010;14(1):98. [EMBASE: 70213483] [Google Scholar]
- Maki DG, Ash SR, Winger RK, Lavin P, AZEPTIC Trial Investigators. A novel antimicrobial and antithrombotic lock solution for hemodialysis catheters: a multi‐center, controlled, randomized trial. Critical Care Medicine 2011;39(4):613‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Betjes 2004 {published data only}
- Betjes MG, Agteren M. Prevention of dialysis catheter‐related sepsis with a citrate‐taurolidine‐containing lock solution. Nephrology Dialysis Transplantation 2004;19(6):1546‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bleyer 2005 {published and unpublished data}
- Bleyer AJ, Mason L, Russell G, Raad II, Sherertz RJ. A randomized, controlled trial of a new vascular catheter flush solution (minocycline‐EDTA) in temporary hemodialysis access. Infection Control & Hospital Epidemiology 2005;26(6):520‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Buturovic 1998 {published data only}
- Buturovic J, Ponikvar R, Kandus A, Boh M, Klinkmann J, Ivanovich P. Filling hemodialysis catheters in the interdialytic period: heparin versus citrate versus polygeline: a prospective randomized study. Artificial Organs 1998;22(11):945‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Campos 2011 {published and unpublished data}
- Campos RP, do Nascimento MM, Chula DC, Riella MC. Minocycline‐EDTA lock solution prevents catheter‐related bacteremia in hemodialysis. Journal of the American Society of Nephrology 2011;22(10):1939‐45. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CHARTS 2008 {published and unpublished data}
- MacRae JM, Dojcinovic I, Jung J, Shalansky S, Levin A, Kiaii M. 4% citrate vs heparin and the reduction of thrombosis study (CHARTS) [abstract no: SA‐PO057]. Journal of the American Society of Nephrology 2006;17(Abstracts):588A. [CENTRAL: CN‐00644154] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae JM, Dojcinovic I, Djurdjev O, Jung B, Shalansky S, Levin A, et al. Citrate 4% versus heparin and the reduction of thrombosis study (CHARTS). Clinical Journal of The American Society of Nephrology: CJASN 2008;3(2):369‐74. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CITRIM 2017 {published data only}
- Correa Barcellos F, Pereira Nunes B, Jorge Valle L, Lopes T, Orlando B, Scherer C, et al. Comparative effectiveness of 30 % trisodium citrate and heparin lock solution in preventing infection and dysfunction of hemodialysis catheters: a randomized controlled trial (CITRIM trial). Infection 2017;45(2):139‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cooper 1999 {published data only}
- Cooper RI, Saad TF. Prevention of bacteremia in patients with tunneled cuffed "permanent" hemodialysis catheters (PCS) using gentamicin catheter packing [abstract no: A1032]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):203A. [CENTRAL: CN‐00550653] [Google Scholar]
Corbett 2013 {published data only}
- Corbett R, Ashby D, Edwards C, Prout V, Singh S, Bedi R, et al. A randomised control trial of taurolidine‐heparin‐citrate line locks in prevention of recurrence of catheter related bacteraemia in haemodialysis patients [abstract no: SO063]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i19. [EMBASE: 71075074] [Google Scholar]
D'Avella 2007 {published data only}
- D'Avella JF, Li L, Qamar A, McDaniel A, Robbins K, Cooper B. A randomized trial of hypertonic saline and heparin lock solution in the prevention of hemodialysis catheter related bacteremia [abstract no: SA‐PO547]. Journal of the American Society of Nephrology 2007;18(Abstracts):461A. [Google Scholar]
Davanipur 2011 {published and unpublished data}
- Davanipur M, Pakfetrat M, Roozbeh J. Cloxacillin as an antibiotic lock solution for prevention of catheter‐associated infection. Iranian Journal of Kidney Diseases 2011;5(5):328‐31. [MEDLINE: ] [PubMed] [Google Scholar]
Dogra 2002 {published data only}
- Dogra G, Herson H, Irish AB, Hutchison B, Moody H, Perth Renal Catheter Study Group. Prevention of tunneled haemodialysis catheter(TC) related infections using catheter restricted filling with gentamicin and citrate [abstract no: 54]. Nephrology 2002;7(Suppl 3):A14. [CENTRAL: CN‐00795238] [DOI] [PubMed] [Google Scholar]
- Dogra GK, Herson H, Hutchinson B, Irish AB, Heath CC, Golledge C, et al. Prevention of tunneled hemodialysis catheter related infections using catheter restricted filling with gentamicin and citrate: a randomized controlled study. Journal of the American society of Nephrology 2002;13(8):2133‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Geron 2008 {published data only}
- Geron R, Tanhilevsky O, Tanasijitchouk T, Rubinchik I, Kristal B. Addition of heparin to taurolockTM catheter lock solution (CLS) ‐ does it improve tunnel cuffed catheter (TCC) patency dysfunction? [abstract no: F‐PO1597]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):466A. [Google Scholar]
Hendrickx 2001 {published and unpublished data}
- Hendrickx L, Kuypers D, Evenepoel P, Maes B, Messiaen T, Vanrenterghem Y. A comparative prospective study on the use of low concentrate citrate lock versus heparin lock in permanent dialysis catheters. International Journal of Artificial Organs 2001;24(4):208‐11. [MEDLINE: ] [PubMed] [Google Scholar]
- Kuypers D, Hendrickx I, Evenepoel P, Maes P, Messiaen T, Vanrenterghem Y. A comparative prospective randomized study on the use of low concentrate citrate lock versus heparin lock in permanent dialysis catheters [abstract]. 38th Congress European Renal Association European Dialysis and Transplantation Association; 2001 Jun 24‐27; Vienna, Austria. 2001:232. [CENTRAL: CN‐00831907] [PubMed]
Hermite 2012 {published and unpublished data}
- Hermite L, Quenot JP, Nadji A, Barbar SD, Charles PE, Hamet M, et al. Sodium citrate versus saline catheter locks for non‐tunneled hemodialysis central venous catheters in critically ill adults: a randomized controlled trial. Intensive Care Medicine 2012;38(2):279‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kanaa 2015 {published data only}
- Kanaa M, Wright MJ, Akbani H, Laboi P, Bhandari S, Sandoe JA. Cathasept line lock and microbial colonization of tunneled hemodialysis catheters: a multicenter randomized controlled trial. American Journal of Kidney Diseases 2015;66(6):1015‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2006a {published and unpublished data}
- Kim SH, Song KI, Chang JW, Kim SB, Sung SA, Jo SK, et al. Prevention of uncuffed hemodialysis catheter‐related bacteremia using an antibiotic lock technique: a prospective, randomized clinical trial. Kidney International 2006;69(1):161‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kim SH, Song KI, Cho W. Prevention of catheter‐related bacteremia (CRB) using antibiotic‐lock technique (ALT): a randomized controlled trial [abstract no: SU‐FC075]. Journal of the American Society of Nephrology 2004;15(Oct):59A. [CENTRAL: CN‐00626047] [Google Scholar]
Kokenge 2010 {published data only}
- Kokenge T, Lohofener C, Lange C, Grundemann C, Bergmann K, Januschkewitz K, et al. Efficacy and safety of a low‐dose citrate catheter locking solution ‐ a randomized double blind controlled trial [abstract no: Sa380]. NDT Plus 2010;3(Suppl 3):iii162. [EMBASE: 70483846] [Google Scholar]
Lange 2007 {published data only}
- Lange C, Lohofener C, Hallebach G, Gensel A, Bergmann K, Januschkewitz K, et al. Low‐dose citrate versus standard heparin for dialysis catheter locking ‐ a randomised, controlled, double‐blind clinical trial interim analysis [abstract no: FP414]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi158. [Google Scholar]
Lustig 2011 {published data only}
- Lustig A, Aflalu S. Novel catheter lock solution in prevention of hemodialysis catheter complications [abstract no: 1121942]. Journal of Clinical Pharmacology 2011;51(9):1341. [EMBASE: 70645124] [Google Scholar]
McIntyre 2004 {published and unpublished data}
- McIntyre CW, Hulme LJ, Taal M, Fluck RJ. Locking of tunneled hemodialysis catheters with gentamicin and heparin. Kidney International 2004;66(2):801‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McIntyre CW, Hulme LJ, Taal M, Fluck RJ. Locking of tunnelled haemodialysis catheters with gentamicin and heparin significantly reduces catheter related infection rates and epoetin requirements [abstract no: F‐PO809]. Journal of the American Society of Nephrology 2003;14(Nov):239A. [CENTRAL: CN‐00626118] [Google Scholar]
Moghaddas 2015 {published data only}
- Moghaddas A, Abbasi MR, Gharekhani A, Dashti‐Khavidaki S, Razeghi E, Jafari A, et al. Prevention of hemodialysis catheter‐related blood stream infections using a cotrimoxazole‐lock technique. Future Microbiology 2015;10(2):169‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moran 2012 {published and unpublished data}
- Moran J, Sun S, Khababa I, Pedan A, Doss S, Schiller B. A randomized controlled trial of gentamicin/citrate versus heparin locks for central venous catheters in maintenance hemodialysis [abstract no: LB‐PO3143]. Journal of the American Society of Nephrology 2011;22(Abstracts):2B. [Google Scholar]
- Moran J, Sun S, Khababa I, Pedan A, Doss S, Schiller B. A randomized trial comparing gentamicin/citrate and heparin locks for central venous catheters in maintenance hemodialysis patients. American Journal of Kidney Diseases 2012;59(1):102‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mortazavi 2011 {published and unpublished data}
- Mortazavi M, Alsaeidi S, Sobhani R, Salimi F, Atapour A, Sharif N, et al. Successful prevention of tunneled, central catheter infection by antibiotic lock therapy using cefotaxime. Research Journal of Medical Sciences 2011;16(3):303‐9. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Nori 2006 {published and unpublished data}
- Nori US, Fisher KA, Osman Y, Manoharan A, Besarab A. Efficacy of antibiotic lock solutions in preventing hemodialysis catheter related bacteremia [abstract no: SU‐FC076]. Journal of the American Society of Nephrology 2004;15(Oct):60A. [CENTRAL: CN‐00583540] [Google Scholar]
- Nori US, Manoharan A, Yee J, Besarab A. Comparison of low‐dose gentamicin with minocycline as catheter lock solutions in the prevention of catheter‐related bacteremia. American Journal of Kidney Diseases 2006;48(4):596‐605. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oguzhan 2012 {published and unpublished data}
- Oguzhan N, Pala C, Sipahioglu MH, Cilan H, Durmaz S, Percin D, et al. Locking tunneled hemodialysis catheters with hypertonic saline (26% NaCl) and heparin to prevent catheter‐related infections and thrombosis: a randomized, prospective trial. Renal Failure 2012;34(2):181‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pervez 2002 {published and unpublished data}
- Pervez A, Ahmed M, Ram S, Torres C, Work J. Antibiotic lock technique for prevention of cuffed tunnel catheter associated bacteremia (CAB) [abstract no: A1033]. Journal of the American Society of Nephrology 2000;11(Sept):194A. [Google Scholar]
- Pervez A, Ahmed M, Ram S, Torres C, Work J, Zaman F, et al. Antibiotic lock technique for prevention of cuffed tunnel catheter associated bacteremia. Journal of Vascular Access 2002;3(3):108‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Plamandon 2005a {published data only}
- Plamondon I, Agharazii M, Audy E, Langlois S, Desmeules S. Randomized cross‐over comparison of 4% citrate and heparin for catheter lock of double‐lumen hemodialysis catheters [abstract no: TH‐FC054]. Journal of the American Society of Nephrology 2005;16:12A. [Google Scholar]
Power 2009 {published and unpublished data}
- Duncan N, Singh SK, Amao M, Brown W, Dalby E, Edwards C, et al. A single centre randomized control trial of sodium citrate versus heparin line locks for cuffed central venous catheters [abstract no: F‐PO0539]. Journal of the American Society of Nephrology 2005;16:451A. [CENTRAL: CN‐00676330] [Google Scholar]
- Power A, Duncan N, Singh SK, Brown W, Dalby E, Edwards C, et al. Sodium citrate versus heparin catheter locks for cuffed central venous catheters: a single‐center randomized controlled trial. American Journal of Kidney Diseases 2009;53(6):1034‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saxena 2006 {published and unpublished data}
- Saxena AK, Panhotra BR, Sundaram DS, Al‐Hafiz A, Naguib M, Venkateshappa CK, et al. Tunneled catheters' outcome optimization among diabetics on dialysis through antibiotic‐lock placement. Kidney International 2006;70(9):1629‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Saxena AK, Panhotra BR, Sundaram DS, Morsy MN, Al‐Ghamdi AM. Enhancing the survival of tunneled haemodialysis catheters using an antibiotic lock in the elderly: a randomised, double‐blind clinical trial. Nephrology 2006;11(4):299‐305. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saxena 2012 {published data only}
- Saxena AK, Panhotra B, Al‐Aziz A, Morsey MNF, Venkateshappa CK, Abu‐Oyun B. Optimizing outcomes of tunneled hemodialysis catheters with intraluminal cefotaxime ‐ heparin locks among staphylococcus aureus nasal carriers [abstract no: SA780]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009.
- Saxena AK, Panhotra BR, Al‐hafiz AA, Sundaram DS, Abu‐Oyun B, Al Mulhim K. Cefotaxime‐heparin lock prophylaxis against hemodialysis catheter‐related sepsis among Staphylococcus aureus nasal carriers. Saudi Journal of Kidney Diseases & Transplantation 2012;23(4):743‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shirzad 2013 {published data only}
- Shirzad M, Espahbodi F, Baboli MT, Samakoosh MA, Khalilian A. Effects of heparin lock‐antibiotics to prevent infections in patients undergoing hemodialysis: a clinical trial. Journal of Mazandaran University of Medical Sciences 2012;22(96):99‐104. [EMBASE: 373922908] [Google Scholar]
Sofroniadou 2012 {published data only}
- Sofroniadou S, Revela I, Smirloglou D, Makriniotou I, Zerbala S, Kouloubinis A, et al. Linezolid versus vancomycin antibiotic lock solution for the prevention of nontunneled catheter‐related blood stream infections in hemodialysis patients: a prospective randomized study. Seminars in Dialysis 2012;25(3):344‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Solomon 2010 {published and unpublished data}
- Solomon L, Cheeseborough J, Ebah L, Heap M, Millband N, Waterhouse D, et al. A randomised, double blind, controlled trial of taurolidine‐citrate catheter locks for the prevention of bacteraemia in patients treated with haemodialysis [abstract no: O41]. British Renal Society & Renal Association Conference; 2010 May 17‐20; Manchester, UK. 2010.
- Solomon LR, Cheesbrough JS, Ebah L, Al‐Sayed T, Heap M, Millband N, et al. A randomized double‐blind controlled trial of taurolidine‐citrate catheter locks for the prevention of bacteremia in patients treated with hemodialysis. American Journal of Kidney Diseases 2010;55(6):1060‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vercaigne 2016a {published data only}
- Vercaigne LM, Allan DR, Armstrong SW, Zacharias JM, Miller LM. An ethanol/sodium citrate locking solution compared to heparin to prevent hemodialysis catheter‐related infections: a randomized pilot study. Journal of Vascular Access 2016;17(1):55‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weijmer 2005 {published and unpublished data}
- Weijmer M, Dorpel R, Geelen J, Groeneveld H, Jaarsveld B, Koopmans M, et al. Reduction of bleeding complications in hemodialysis patients with high concentration trisodium citrate for hemodialysis catheter locking: a prospective multicenter double‐blind randomised controlled trial [abstract no: W595]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):737. [Google Scholar]
- Weijmer MC, Dorpel MA, Geelen JA, Groeneveld JO, Jaarsveld VC, Koopmans MG, et al. Reduction of bleeding complications in hemodialysis patients with high concentration trisodium citrate for hemodialysis catheter locking; a prospective multicenter double‐blind randomised controlled trial [abstract no: F‐PO825]. Journal of the American Society of Nephrology 2003;14(Nov):242A. [CENTRAL: CN‐00448317] [Google Scholar]
- Weijmer MC, Dorpel MA, Geelen JA, Groeneveld JO, Jaarsveld VC, Koopmans MG, et al. Substantial reduction of infectious complications in hemodialysis patients with trisodium citrate 30% as catheter locking solution: a prospective multicenter double‐blind randomised controlled trial [abstract no: W604]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):740. [CENTRAL: CN‐00448318] [Google Scholar]
- Weijmer MC, Dorpel MA, Geelen JA, Groeneveld JO, Jaarsveld VC, Koopmans MG, et al. Substantial reduction of infectious complications in hemodialysis patients with trisodium citrate 30% as catheter locking solution; a prospective multicenter double‐blind randomised controlled trial [abstract no: SU‐FC245]. Journal of the American Society of Nephrology 2003;14(Nov):54A. [Google Scholar]
- Weijmer MC, Dorpel MA, Ven PJ, ter Wee PM, Geelen JA, Groeneveld JO, et al. Randomized, clinical trial comparison of trisodium citrate 30% and heparin as catheter‐locking solution in hemodialysis patients. Journal of the American Society of Nephrology 2005;16(9):2769‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhang 2009c {published and unpublished data}
- Zhang P, Yuan J, Tan H, Lv R, Chen J. Successful prevention of cuffed hemodialysis catheter‐related infection using an antibiotic lock technique by strictly catheter‐restricted antibiotic lock solution method. Blood Purification 2009;27(2):206‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhang W, He Q, Yuan J, Xie W, Jiang H, et al. A randomized controlled study on prevention of cuff‐tunneled catheter related bacteremia with gentamicin‐heparin lock solution ‐ the Metaphase result [abstract no: SA‐PO073]. Journal of the American Society of Nephrology 2006;17(Abstracts):592A. [Google Scholar]
Zwiech 2016a {published data only}
- Zwiech R, Adelt M, Chrul S. A taurolidine‐citrate‐heparin lock solution effectively eradicates pathogens from the catheter biofilm in hemodialysis patients. American Journal of Therapeutics 2016;23(2):e363‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beigi 2010 {published data only}
- Beigi AA, Khansoltani S, Masoudpour H, Atapour AA, Eshaghian A, Khademi EF. Influence of intralumenal and antibiotic‐lock of vancomycin on the rate of catheter removal in the patients with permanent hemodialysis catheters. Saudi Journal of Kidney Diseases & Transplantation 2010;21(1):54‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Bosma 2010 {published data only}
- Bosma JW, Siegert CE, Peerbooms PG, Weijmer MC. Reduction of biofilm formation with trisodium citrate in haemodialysis catheters: a randomized controlled trial. Nephrology Dialysis Transplantation 2010;25(4):1213‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2014b {published data only}
- Chen FK, Li JJ, Song Y, Zhang YY, Chen P, Zhao CZ, et al. Concentrated sodium chloride catheter lock solution‐‐a new effective alternative method for hemodialysis patients with high bleeding risk. Renal Failure 2014;36(1):17‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chu 2016 {published data only}
- Chu G, Fogarty GM, Avis LF, Bergin S, McElduff P, Gillies AH, et al. Low dose heparin lock (1000 U/mL) maintains tunnelled hemodialysis catheter patency when compared with high dose heparin (5000 U/mL): a randomised controlled trial. Hemodialysis International 2016;20(3):385‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coli 2010 {published data only}
- Coli L, Donati G, Cianciolo F, Gozzetti A, Ferri V, Cuna S, et al. Optimal urokinase lock therapy for hemodialysis (HD) tunneled cuffed catheter (TCC) thrombosis [abstract]. International Journal of Artificial Organs 2010;33(7):424. [EMBASE: 70869551] [Google Scholar]
HEALTHY‐CATH 2009 {published data only}
- Broom JK, Krishnasamy R, Hawley CM, Playford EG, Johnson DW. A randomised controlled trial of Heparin versus EthAnol Lock THerapY for the prevention of Catheter Associated infecTion in Haemodialysis patients‐‐the HEALTHY‐CATH trial. BMC Nephrology 2012;13:146. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom JK, O'Shea S, Govindarajulu S, Playford EG, Hawley CM, Isbel NM, et al. Rationale and design of the HEALTHY‐CATH trial: a randomised controlled trial of Heparin versus EthAnol Lock THerapY for the prevention of Catheter Associated infecTion in Haemodialysis patients. BMC Nephrology 2009;10:23. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hryszko 2013 {published data only}
- Hryszko T, Brzosko S, Mysliwiec M. Low concentration of heparin used for permanent catheters canal locking is effective and diminishes the risk of bleeding. International Urology & Nephrology 2013;45(3):825‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2011 {published data only}
- Hu HH, Hsu CY, Fang HC, Lee PT, Chen CL, Chang TY, et al. Low‐dose heparin retention in temporary hemodialysis double‐lumen catheter does not increase catheter occlusion and might reduce risk of bleeding. Blood Purification 2011;32(3):232‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ISRCTN27307877 {published data only}
- Abdalia M. Trisodium citrate versus heparin for locking tunnelled haemodialysis catheters: a randomised controlled trial. National Research Register 2005. [CENTRAL: CN‐00583376]
Khosroshahi 2006b {published data only}
- Khosroshahi HT, Aghazadeh MM, Oskuii SF, Soja MM, Khalilzadeh M. Comparisonal management of hemodialysis catheter related infection with and without an adjunctive antibiotic lock solution [abstract no: SP657]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv237. [Google Scholar]
Khosroshahi 2015 {published data only}
- Khosroshahi HT, Mahdipur H, Parkhideh S, Basmenji S, Khalilzadeh M, Tozihi M. The effectiveness of systemic antibiotic therapy with and without ethanol‐locked solution in the treatment of hemodialysis‐related catheter infection. Saudi Journal of Kidney Diseases & Transplantation 2015;26(3):477‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Malo 2010 {published data only}
- Malo J, Jolicoeur C, Theriault F, Lachaine J, Senecal L. Comparison between standard heparin and tinzaparin for haemodialysis catheter lock. ASAIO Journal 2010;56(1):42‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Malo J, Jolicoeur C, Theriault F, Savoie M, Chouinard P, Lachaine J, et al. Comparison between standard heparin and tinzaparin for haemodialysis catheter lock [abstract no: SP592]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv214. [CENTRAL: CN‐00740610] [Google Scholar]
Meeus 2005 {published data only}
- Meeus G, Kuypers DR, Claes K, Evenepoel P, Maes B, Vanrenterghem Y. A prospective, randomized, double‐blind crossover study on the use of 5% citrate lock versus 10% citrate lock in permanent hemodialysis catheters. Blood Purification 2005;23(2):101‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mohammad 2016 {published data only}
- Alali F, Fawzy A, Elsayed M, Hamdy A, Mohammad Z, Ibrahim R, et al. A randomised controlled trial of taurolidine citrate versus taurolidine urokinase lock to prevent tunnelled catheter thrombosis in hemodialysis patients [abstract no: MO034]. Nephrology Dialysis Transplantation 2016;31(Suppl 1):i42. [EMBASE: 72326016] [Google Scholar]
- Hamad A, Fawzy A, Elsayed M, Ibrahim R, Mohammad Z, Hamdy A, et al. A randomized controlled trial of taurolidine citrate versus taurolidine urokinase lock to prevent catheter related infections in hemodialysis patients [abstract no: SP524]. Nephrology Dialysis Transplantation 2016;31(Suppl 1):i268. [EMBASE: 72326627] [Google Scholar]
- Mohammad Z, Hamad A, Hamdy A, Chandra P, Abdul‐Latif T, Ibrahim R, et al. A randomized controlled trial of taurolidine heparin versus taurolidine urokinase lock to improve catheter function [abstract no: 226]. American Journal of Kidney Diseases 2016;67(5):A75. [EMBASE: 72313529] [Google Scholar]
NCT00862966 {published data only}
- Randomized control trial on citrate as the central venous catheter lock solution. www.clinicaltrials.gov/ct2/show/NCT00862966 (first received 13 March 2009).
NCT01989091 {published data only}
- Multi‐center, prospective, randomized, open‐label, sponsor‐blinded, active‐control (heparin) clinical investigation to evaluate the safety and effectiveness of B‐lock™ as an antimicrobial catheter lock solution in dialysis patients with a central venous catheter. www.clinicaltrials.gov/ct2/show/NCT01989091 (first received 30 October 2013).
Onder 2008 {published data only}
- Onder AM, Chandar J, Simon N, Diaz R, Nwobi O, Abitbol CL, et al. Comparison of tissue plasminogen activator‐antibiotic locks with heparin‐antibiotic locks in children with catheter‐related bacteraemia. Nephrology Dialysis Transplantation 2008;23(8):2604‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oran 2008 {published data only}
- Oran NT, Eser I. Impact of heparin locking frequency on preventing temporary dialysis catheter dysfunction in haemodialysis patients. Journal of Renal Care 2008;34(4):199‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PreCLOT 2006 {published data only}
- Hemmelgarn BR, Moist L, Pilkey RM, Lok C, Dorval M, Tam PY, et al. Prevention of catheter lumen occlusion with rT‐PA versus heparin (Pre‐CLOT): study protocol of a randomized trial [ISRCTN35253449]. BMC Nephrology 2006;7:8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmelgarn BR, Moist LM, Lok CE, Tonelli M, Manns BJ, Holden RM, et al. Prevention of dialysis catheter malfunction with recombinant tissue plasminogen activator. New England Journal of Medicine 2011;364(4):303‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Manns BJ, Scott‐Douglas N, Tonelli M, Ravani P, LeBlanc M, Dorval M, et al. An economic evaluation of rt‐PA locking solution in dialysis catheters. Journal of the American Society of Nephrology 2014;25(12):2887‐95. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DR, Moist LM, MacRae JM, Scott‐Douglas N, Zhang J, Tonelli M, et al. Risk factors associated with hemodialysis central venous catheter malfunction; a retrospective analysis of a randomized controlled trial. Canadian Journal of Kidney Health & Disease 2014;1:15. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ray 1999 {published data only}
- Ray CE Jr, Shenoy SS, McCarthy PL, Broderick KA, Kaufman JA. Weekly prophylactic urokinase instillation in tunneled central venous access devices. Journal of Vascular & Interventional Radiology 1999;10(10):1330‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sishir 2014 {published and unpublished data}
- Sishir G, Bhupeshkumar K, Umapati H, Kalpesh G, Mohan R. To study the efficacy of intraluminal 70% ethanol vs heparin lock prior to hemodialysis in prevention of infections in non‐tunneled hemodialysis catheters: a randomised study [abstract no: PS3‐069]. Nephrology 2014;19(Suppl 2):181‐2. [EMBASE: 71617247] [Google Scholar]
Thomson 2011 {published data only}
- Thomson PC, Morris ST, Mactier RA. The effect of heparinized catheter lock solutions on systemic anticoagulation in hemodialysis patients. Clinical Nephrology 2011;75(3):212‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
CLOCK 2017 {published data only}
- Luiz MV, Scavone C, Tzanno C. The CLOCK trial, a double‐blinded randomized controlled trial: Trisodium citrate 30% and minocycline 3 mg/mL plus EDTA 30 mg/mL are effective and safe for catheter patency maintenance among CKD 5D patients on hemodialysis. Hemodialysis International 2017;21(2):294‐304. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Aguinaga 2011
- Aguinaga A, Pozo JL. Catheter‐related infection in hemodialysis: diagnosis, treatment and prevention [Infección asociada a catéter en hemodiálisis: diagnóstico, tratamiento y prevención]. NefroPlus 2011;4(2):1‐10. [DOI: 10.3265/NefroPlus.pre2011.Jun.11016] [DOI] [Google Scholar]
Ash 2000
- Ash SR, Mankus RA, Sutton JM, Criswell RE, Crull CC, Velasquez KA, et al. Concentrated sodium citrate (23%) for catheter lock. Hemodialysis International 2000;4(1):22‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Beathard 2008
- Beathard GA, Urbanes A. Infection associated with tunneled hemodialysis catheters. Seminars in Nephrology 2008;21(6):528‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Betjes 2011
- Betjes MG. Prevention of catheter related infection in patients on hemodialysis. Nature Reviews Nephrology 2011;7(5):257‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bradbury 2007
- Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, et al. Predictors of early mortality among incident US hemodialysis patients in the dialysis outcomes and practice patterns study (DOPPS). Clinical Journal of The American Society of Nephrology: CJASN 2007;2(1):89‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ethier 2008
- Ethier J, Mendelssohn DC, Elder SJ, Hasegawa T, Akizawa T, Akiba T, et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study.[Erratum appears in Nephrol Dial Transplant. 2008 Dec;23(12):4088]. Nephrology Dialysis Transplantation 2008;23(10):3219‐26. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foley 2009
- Foley RN, Chen SC, Collins AJ. Hemodialysis access at initiation in the United States, 2005 to 2007: Still “catheter first”. Hemodialysis International 2009;13(4):533‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grudzinski 2015
- Grudzinski A, Agarwal A, Bhatnagar N, Nesrallah G. Benefits and harms of citrate locking solutions for hemodialysis catheters: a systematic review and meta‐analysis. Canadian Journal of Kidney Health & Disease 2015;2:13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Katneni 2007
- Katneni R, Hedayati SS. Central venous catheter‐related bacteremia in chronic hemodialysis patients: epidemiology and evidence‐based management. Nature Clinical Practice Nephrology 2007;3(5):256‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Klevens 2008
- Klevens RM, Edwards JR, Andrus ML, Peterson KD, Dudeck MA, Horan TC, et al. Dialysis Surveillance Report: National Healthcare Safety Network (NHSN)‐data summary for 2006. Seminars in Nephrology 2008;21(1):24‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Korkor 2009
- Korkor AB, Bretzmann CM. Prevention of hemodialysis catheter‐related infection: an unmet need. Dialysis & Transplantation 2009;38(9):377‐9. [EMBASE: 355523132] [Google Scholar]
Labriola 2008
- Labriola L, Crott R, Jadoul M. Preventing haemodialysis catheter‐related bacteraemia with an antimicrobial lock solution: a meta‐analysis of prospective randomized trials. Nephrology Dialysis Transplantation 2008;23(5):1666‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lai 2016
- Lai NM, Chaiyakunapruk N, Lai NA, O'Riordan E, Pau WS, Saint S. Catheter impregnation, coating or bonding for reducing central venous catheter‐related infections in adults. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD007878.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2001
- Little MA, O’Riordan A, Lucey B, Farrell M, Lee M, Conlon PJ, et al. A prospective study of complications associated with cuffed, tunnelled haemodialysis catheters. Nephrology Dialysis Transplantation 2001;16(11):2194‐200. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McCann 2010
- McCann M, Moore ZE. Interventions for preventing infectious complications in haemodialysis patients with central venous catheters. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006894.pub2] [DOI] [PubMed] [Google Scholar]
Moran 2008
- Moran JE, Ash SR, ASDIN Clinical Practice Committee. Locking solutions for hemodialysis catheters; heparin and citrate‐‐a position paper by ASDIN. Seminars in Dialysis 2008;21(5):490‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Napalkov 2013
- Napalkov P, Felici DM, Chu LK, Jacobs JR, Begelman SM. Incidence of catheter‐related complications in patients with central venous or hemodialysis catheters: a health care claims database analysis. BMC Cardiovascular Disorders 2013;13:86. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NKF 2006
- National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for 2006 Updates: Hemodialysis Adequacy, Peritoneal Dialysis Adequacy and Vascular Access. Guideline 7: Prevention and treatment of catheter and port complications. www2.kidney.org/professionals/KDOQI/guideline_upHD_PD_VA/ (accessed July 2017).
Pisoni 2015
- Pisoni RL, Zepel L, Port FK, Robinson BM. Trends in US vascular access use, patient preferences, and related practices: an update from the US DOPPS practice monitor with international comparisons. American Journal of Kidney Diseases 2015;65(6):905‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Snaterse 2010
- Snaterse M, Ruger W, Scholte Op Reimer WJ, Lucas C. Antibiotic‐ based catheter lock solutions for prevention of catheter‐ related infection: a systematic review of randomised controlled trials. Journal of Hospital Infection 2010;75(1):1‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ullman 2015
- Ullman AJ, Cooke ML, Mitchell M, Lin F, New K, Long DA, et al. Dressings and securement devices for central venous catheters (CVC). Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD010367.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vanholder 2010
- Vanholder R, Canaud B, Fluck R, Jadoul M, Labriola L, Mart‐Monros A, et al. Catheter related blood stream infections (CRBSI): a European view. Nephrology Dialysis Transplantation 2010;25(6):1753‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Venditto 2010
- Venditto M, du Montcel ST, Robert J, Trystam D, Dighiero J, Hue D, et al. Effect of catheter ‐lock solutions on catheter‐related infection and inflammatory syndrome in hemodialysis patients: heparin versus citrate 46% versus heparin/gentamicin. Blood Purification 2010;29(3):268‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weijmer 2002
- Weijmer MC, Debets‐Ossenkopp YJ, Vondervoot FJ, ter Wee P. Superior antimicrobial activity of trisodium citrate over heparin for catheter locking. Nephrology Dialysis Transplantation 2002;17(12):2189‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yahav 2008
- Yahav D, Rozen‐Zvi B, Gafter‐Gvili A, Leibovici L, Gafter U, Paul M. Antimicrobial lock solutions for the prevention of infections associated with intravascular catheters in patients undergoing hemodialysis: systematic review and meta‐analysis of randomized, controlled trials. Clinical Infectious Diseases 2008;47(1):83‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhao 2014
- Zhao Y, Li Z, Zhang L, Yang Y, Tang Y, Fu P. Citrate versus heparin lock for hemodialysis catheters: a systematic review and meta‐analysis of randomized controlled trials. American Journal of Kidney Diseases 2014;63(3):479‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Arechabala 2013
- Arechabala MC, Catoni MI, Claro JC, Rojas NP, Rubio ME, Calvo MA, et al. Antimicrobial lock solutions for preventing catheter‐related infections in haemodialysis. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010597] [DOI] [PMC free article] [PubMed] [Google Scholar]


