Abstract
This is a protocol for a Cochrane Review (Intervention). The objectives are as follows:
To assess the effects of staple‐line reinforcement for adults undergoing bariatric surgery with gastric transection.
Background
Description of the condition
Overweight and obesity are major health problems, to the point of being considered an epidemic. Their prevalence has increased dramatically since 1980, affecting about 2.1 billion people in 2013 (Ng 2014; WHO 2014). A high body mass index (BMI) is an important risk factor for death, estimated to cause about 3.4 million deaths a year (Lim 2012). Excessive weight is also strongly associated with other diseases and disorders, such as type 2 diabetes mellitus, cardiovascular diseases, dyslipidaemia, and sleep apnoea.
Bariatric (metabolic) surgery has been proposed as an alternative for the management of obesity and its related comorbidities (Colquitt 2014). Currently, although there are variations between countries, the most frequently performed bariatric operations include gastric bypass (GB) and sleeve gastrectomy (SG), followed by gastric banding (Buchwald 2011). Gastric bypass involves the division of the stomach into two parts, creating a small reservoir or pouch on top. Afterwards, the small intestine is rearranged to connect both parts of the stomach.The 'Roux‐en‐Y' reconstruction is the most frequently performed. The Y configuration starts with the division of the small intestine and the anastomosis of the distal end to the reservoir (gastrojejunostomy). Then, the proximal portion of the divided small intestine is connected to the small intestine further down to allow drainage of gastric, biliary and pancreatic juices (jejunojejunostomy) (Figure 1). In the SG procedure, a gastric sleeve is created by resecting the fundus and the greater curvature of the stomach (Figure 2). For these procedures, the laparoscopic approach has replaced open surgery in most centres (Nguyen 2011).
Figure 1.

Figure 2.
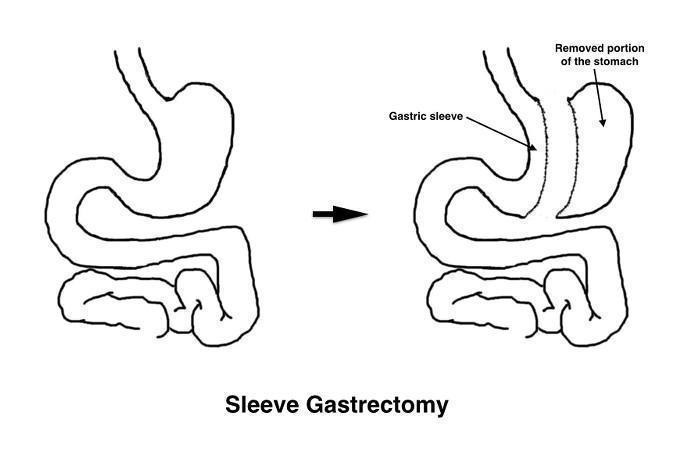
Both the construction of the gastric pouch and the gastric sleeve require the use of linear staplers for sectioning the stomach. Surgical staplers are widely used in surgery to replace sutures when sectioning or joining structures, providing wound closure. Staplers are loaded with disposable cartridges containing rows of staples, generally made of titanium. The staple line can be straight (linear), circular or curved. The configuration of surgical staplers most widely used in laparoscopic surgery is to place two triple rows of staples and cut the tissue between them.
Staple line complications are an important issue to consider in bariatric surgical procedures in which a gastric transection is needed. Haemorrhage and leakage from the staple line are important adverse events that can lead to severe morbidity, reoperations, prolonged hospitalisation both on the ward and in the intensive care unit, higher costs, and even mortality (Bransen 2015). The incidence of staple line leakage in SG procedures is around 2.3%, varying among surgical series between 0% and 5.4% (Fuks 2009; Sánchez‐Santos 2009; Zellmer 2014). Most of these leaks, nearly 80% to 90%, occur in the higher part of the sleeve (Aurora 2012; Burgos 2009). Moreover, leakage rates increase when SG is used as a revision procedure (Foletto 2010; Iannelli 2009). The incidence of leaks after GB is about 1.9%, ranging from 0% to 5.6% across trials (Gonzalez 2004; Zellmer 2014). Leakage after GB can take place at the level of the anastomoses or at the staple lines. Bleeding after bariatric surgery may originate from different sites. Haemorrhage can be intraluminal, from the staple lines or anastomoses; or intraperitoneal, either from the staple lines, vessels sectioned during dissection, or from the abdominal wall incisions. Its incidence is around 3% after GB and 2% after SG (D'Ugo 2013; Zellmer 2014).
Description of the intervention
Different methods have been proposed to reinforce the staple line in an attempt to reduce the incidence of complications. These methods comprise oversewing the staple line, with or without an omental fat patch (omentoplasty) covering the oversewn staple line (Gentileschi 2012), the application of fibrin glue or sealants (Musella 2014; Sapala 2004), and more recently, the use of buttressing materials. Buttressing materials are loaded onto the staple cartridges and get incorporated into the staple line when the stapler is fired. The most commonly used buttressing includes biologic materials, like those derived from the bovine pericardium (Shah 2014; Shikora 2008), and synthetic absorbable polymers, such as synthetic polyester, composed of glycolide, dioxanone, and trimethylene carbonate (Gill 2012), or polyglycolic acid and trimethylene carbonate copolymer (Dapri 2010; Miller 2007). In a recent consensus summit on sleeve gastrectomy, 75% of the expert surgeons surveyed reinforced the staple line; of them, 57% buttressed the staple line and 43% oversewed it, 6% with an omental patch (Gagner 2013).
Adverse effects of the intervention
Improper placement or handling of the reinforcement systems can cause stapling failure, increasing the risk of staple‐line leakage and bleeding. Staple cartridges are available in various sizes and heights. Depending on the stomach wall thickness, the surgeon should choose an appropriate staple height. When the closed staple height is too high, tissues can be improperly joined and result in leakage or haemorrhage. As buttressing materials are stapled with the tissue, buttress thickness is also an important issue to consider when choosing staple height. If the staple height selected is too low, it may be insufficient for stapling both the reinforcing material and the stomach, or it can cause excessive strength, potentially leading to ischaemia and leakage (Chekan 2014; Huang 2015). Moreover, reinforcing the staple line may cause an increased inflammatory response and scarring that may lead to other complications such as anastomotic or gastric sleeve stenosis (Efthimiou 2010; Ibele 2014).
How the intervention might work
Theoretically, the various reinforcement mechanisms should improve wound healing and haemostasis at the staple line, promote tissue compression to prevent bleeding, and increase the strength of the staple line to reduce the incidence of leakage. Fibrin glue and other haemostatic sealants derived from human plasma have proven to be effective and safe in a variety of surgical procedures (Emilia 2011). The use of buttress materials or oversewing of the staple line has been shown to increase the burst pressure of the stomach, both in animal models and in human gastric remnants obtained from SG patients (Arnold 2005; López‐Monclova 2013).
Why it is important to do this review
A Cochrane review has been carried out to assess the effects of surgery to treat obesity and its comorbidities. Among the trials included in this review, gastric bypass and sleeve gastrectomy were the most commonly investigated bariatric procedures (Colquitt 2014). Five systematic reviews have been published to study leak rates after sleeve gastrectomy (Aurora 2012; Choi 2012; Gagner 2014; Knapps 2013; Parikh 2013). Three reviews, that included participants from comparative trials and from large series, found no marked differences in the leak rate and overall morbidity between the groups of participants with or without staple line reinforcement. (Aurora 2012; Knapps 2013; Parikh 2013). Another review found a decrease in the leak rate in those participants in which the staple line was reinforced with an absorbable buttressing material (Gagner 2014). Finally, a meta‐analysis of randomised and non‐randomised trials found a decrease in the leak rate and global complications with the use of reinforcement with buttressing, oversewing, or both (Choi 2012). With regard to gastric bypass, a systematic review and meta‐analysis of three randomised controlled trials found a reduction in the rate of leakage in participants with reinforcement of the staple line (Sajid 2011). However, the results of this review should be treated with caution since one of the included trials did not report leaks properly and was responsible for the overall difference between the groups. In summary, there are several published reviews on this topic, but the results are contradictory, so it is unclear whether staple line reinforcement reduces complications such as the incidence of haemorrhage, leakage or both. A systematic review including newly published randomised controlled trials and following Cochrane methodology would be of interest.
Objectives
To assess the effects of staple‐line reinforcement for adults undergoing bariatric surgery with gastric transection.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Types of participants
Adult participants (older than 18 years) undergoing laparoscopic bariatric surgery with gastric transection.
Definition of gastric transection
Gastric transection involves cutting across the stomach to divide or remove a part of it. Two techniques are evaluated in this review.
Gastric bypass
Sleeve gastrectomy
Types of interventions
We plan to investigate the following comparisons of intervention versus control/comparator where the same letters indicate direct comparisons.
Intervention
Reinforcement of the staple line during bariatric surgery.
(a) Buttressing of the staple line.
(b) Oversewing of the staple line.
(c) Use of fibrin glue or other haemostatic sealants.
Comparator
No reinforcement of the staple line compared with (a), (b), or (c)
Studies comparing (a), (b), and (c) with each other
Concomitant interventions will have to be the same in both the intervention and comparator groups to establish fair comparisons.
Exclusion criteria
Trials on bariatric surgery considered obsolete (vertical gastroplasty or vertical banded gastroplasty). Currently these procedures are rarely performed due to insufficient maintenance of weight loss and high incidence of complications.
Trials on bariatric surgery using open approaches.
Types of outcome measures
Primary outcomes
Adverse events.
Reoperations.
All‐cause mortality.
Secondary outcomes
Operative time.
Hospital stay.
Conversion to open surgery.
Weight loss.
Health‐related quality of life.
Socioeconomic effects.
Method and timing of outcome measurement
Adverse events: such as haemorrhage (however measured in the trials (e.g. number of clips used for staple‐line haemostasis, number of participants with postoperative haemorrhage requiring blood transfusion, endoscopic or surgical treatment). We will consider haemorrhage that occurs in the first 30 days after surgery; leakage: detected by clinical or radiological evidence, or both. Leaks can be defined as early (those appearing one to four days after surgery), intermediate (five to nine days after surgery), or late (10 or more days following surgery).
Reoperations: defined as a return to the operating room for a major surgical intervention within the first 30 days of the primary operation.
All‐cause mortality: defined as death from any cause during the first 30 days after the operation.
Operative time: defined as the time from the first skin incision to skin closure; measured at the end of the operation.
Hospital stay: defined as postoperative length of stay; measured at hospital discharge.
Conversion to open surgery: defined as conversion from laparoscopic to open approach during the operation.
Weight loss: however measured in the studies (e.g. percentage excess weight loss, percentage body mass index loss); measured at least one year after surgery.
Helath‐related quality of life: measured with a validated generic or obesity specific health‐related quality of life measure; measured at least one year after surgery.
Socioeconomic effects: direct costs, including those related to surgical supplies and those related to hospital stay; measured at 30 days from the operation.
Summary of findings' table
We present 'Summary of finding' tables to report the following outcomes, listed according to priority.
Adverse events.
Reoperations.
Conversion to open surgery.
Hospital stay.
All‐cause mortality.
Health‐related quality of life.
Socioeconomic effects.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception of each database to the specified date and placed no restrictions on the language of publication.
Cochrane Central Register of Controlled Trials (28.10.2015)
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) 1946 to Present (28.10.2015)
Embase 1974 to 2015 October 27 (28.10.2015)
LILACS "Last update: Oct., 23th., 2015" (28.10.2015)
ClinicalTrials.gov (28.10.2015)
International Clinical Trials Registry Platform (ICTRP) search portal (http://apps.who.int/trialsearch/), (28.10.2015)
We continuously applied a MEDLINE (via Ovid) email alert service established by the Cochrane Metabolic and Endocrine Disorders (CMED) Group to identify newly published studies using the same search strategy as described for MEDLINE (for details on search strategies, see Appendix 1). After supplying the final review draft for editorial approval, the CMED Group performed a complete updated search on all databases available at the editorial office and sent the results to the review authors. Should we have identified new studies for inclusion, we evaluated these, incorporated the findings into our review, and resubmitted another review draft (Beller 2013). If we detected additional relevant key words during any of the electronic or other searches, we modified the electronic search strategies to incorporate these terms and document the changes.
Searching other resources
We tried to identify other potentially‐eligible trials or ancillary publications by searching the reference lists of retrieved included trials, (systematic) reviews, meta‐analyses and health technology assessment reports. We also contacted trial authors of included trials to identify any further studies that we may have missed.
Data collection and analysis
Selection of studies
Two review authors (AMR, MC) independently scanned the abstract, title, or both, of every record retrieved, to determine which trials should be assessed further. We investigated the full‐text articles of all potentially relevant references. We resolved discrepancies through consensus or recourse to a third review author (CMS). If we cannot resolve a disagreement, we categorised the trial as a 'study awaiting classification' and contact the trial authors for clarification. We present an adapted Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram to show the process of trial selection (Liberati 2009).
Data extraction and management
For trials that fulfil inclusion criteria, two review authors (MLHB, CRO) independently extracted key participant and intervention characteristics. We reported data on efficacy outcomes and adverse events using a standard data extraction from the CMED Group. We resolved any disagreements by discussion or, if required, by consultation with a third review author (JBME) (for details see Characteristics of included studies; Table 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10).
We provided information about potentially relevant ongoing trials, including trial identifier in the Characteristics of ongoing studies table and in Appendix 5 ('Matrix of trial endpoint (publications and trial documents)'). We tried to find the protocol for each included trial and reported primary, secondary, and other outcomes in comparison with data in publications in Appendix 5.
We emailed all authors of included trials to enquire whether they would be willing to answer questions regarding their trials. Appendix 11 shows the results of this survey. Thereafter, we sought relevant missing information on the trial from the primary author(s) of the article, if required.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary trial, we maximised the information yield by collating all available data and used the most complete dataset aggregated across all known publications.
Assessment of risk of bias in included studies
Two review authors (AMR, MC) independently assessed the risk of bias of each included trial. We resolved any disagreements by consensus, or by consultation with a third review author (CMS).
In cases of disagreement, we consulted the rest of the group and make a judgement based on consensus. If adequate information was not available from trial authors, trial protocols or both we contacted trial authors for missing data on risk of bias items.
We used the Cochrane 'Risk of bias' assessment tool (Higgins 2011a; Higgins 2011b) and judged 'Risk of bias' criteria as either 'low', 'high', or 'unclear' risk and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) where any of the specified criteria for a judgement on 'low', unclear' or 'high' risk of bias justifies the associated categorisation.
Random sequence generation (selection bias due to inadequate generation of a randomised sequence) ‐ assessment at trial level We described for each included trial the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing of lots, tossing a coin, shuffling cards or envelopes, and throwing dice are adequate if performed by an independent person not otherwise involved in the trial. Use of the minimization technique was considered as equivalent to being random.
Unclear risk of bias: insufficient information about the sequence generation process.
High risk of bias: the sequence generation method was non‐random (e.g. sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number; allocation by judgement of the clinician; allocation by preference of the participant; allocation based on the results of a laboratory test or a series of tests; allocation by availability of the intervention).
Allocation concealment (selection bias due to inadequate concealment of allocations prior to assignment) ‐ assessment at trial level We described for each included trial the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
Low risk of bias: central allocation (including telephone, interactive voice‐recorder, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
Unclear risk of bias: insufficient information about the allocation concealment.
High risk of bias: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards; alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
We also evaluated trial baseline data to incorporate assessment of baseline imbalance into the risk of bias judgement for selection bias (Corbett 2014). Chance imbalances might also affect judgements on the risk of attrition bias. In case of unadjusted analyses we distinguished between trials rated as at low risk of bias on the basis of both randomisation methods and baseline similarity, and trials rated as at low risk of bias on the basis of baseline similarity alone (Corbett 2014). We re‐classified judgements of unclear, low or high risk of selection bias as specified in Appendix 15.
Blinding of participants and study personnel (performance bias due to knowledge of the allocated interventions by participants and personnel during the trial) ‐ assessment at outcome level
We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self reported, investigator‐assessed or adjudicated outcome measures (see below).
Low risk of bias: blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken; no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Unclear risk of bias: insufficient information about the blinding of participants and study personnel; the trial did not address this outcome.
High risk of bias: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding; blinding of trial participants and key personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
Blinding of outcome assessment (detection bias due to knowledge of the allocated interventions by outcome assessment) ‐ assessment at outcome level We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self reported, investigator‐assessed or adjudicated outcome measures (see below).
Low risk of bias: blinding of outcome assessment ensured, and unlikely that the blinding could have been broken; no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding.
Unclear risk of bias: insufficient information about the blinding of outcome assessors; the trial did not address this outcome.
High risk of bias: no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data (attrition bias due to amount, nature or handling of incomplete outcome data) ‐ assessment at outcome level We described for each included trial, and for each outcome, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the number included in the analysis at each stage (compared with the number of randomised participants per intervention/comparator groups), if reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We considered the implications of missing outcome data per outcome such as high drop‐out rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between trial arms).
Low risk of bias: no missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; appropriate methods, such as multiple imputation, were used to handle missing data.
Unclear risk of bias: insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias; the trial did not address this outcome.
High risk of bias: reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ or similar analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation.
Selective reporting (reporting bias due to selective outcome reporting) ‐ assessment at trial level We assessed outcome reporting bias by integrating the results of the Appendix 5 'Matrix of trial endpoints (publications and trial documents)' (Boutron 2014; Mathieu 2009), with those of the Appendix 6 'High risk of outcome reporting bias according to ORBIT classification' (Kirkham 2010). This analysis formed the basis for the judgement of selective reporting.
Low risk of bias: the trial protocol is available and all of the trial’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol was not available but it was clear that the published reports included all expected outcomes (ORBIT classification).
Unclear risk of bias: insufficient information about selective reporting.
High risk of bias: not all of the trial’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the trial report fails to include results for a key outcome that would be expected to have been reported for such a trial (ORBIT classification).
Other bias (bias due to problems not covered elsewhere) ‐ assessment at trial level
Low risk of bias: the trial appeared to be free of other sources of bias.
Unclear risk of bias: insufficient information to assess whether an important risk of bias existed; insufficient rationale or evidence that an identified problem introduced bias.
High risk of bias: had a potential source of bias related to the specific trial design used; has been claimed to have been fraudulent; had some other serious problem.
We present a 'Risk of bias' graph and a 'Risk of bias' summary figure.
We distinguished between self‐reported, investigator‐assessed and adjudicated outcome measures.
We defined the following endpoints as self‐reported outcomes.
Adverse events, reported by participants.
Weight loss, as measured by participants.
Health‐related quality of life.
We defined the following endpoints as investigator‐assessed outcomes.
Adverse events, as measured by study personnel.
Reoperations.
All cause mortality.
Operative time.
Hospital stay.
Conversion to open surgery.
Weight loss, as measured by study personnel.
Socioeconomic effects.
Summary assessment of risk of bias
Risk of bias for a trial across outcomes: some risk of bias domains like selection bias (sequence generation and allocation sequence concealment) affect the risk of bias across all outcome measures in a trial. In case of high risk of selection bias, all endpoints investigated in the associated trial were be marked as 'high' risk. Otherwise, we did not performed a summary assessment of the risk of bias across all outcomes for a trial.
Risk of bias for an outcome within a trial and across domains: we assessed the risk of bias for an outcome measure including all of the entries relevant to that outcome, i.e. both trial‐level entries and outcome specific entries. 'Low' risk of bias was defined as low risk of bias for all key domains, 'unclear' risk of bias as unclear risk of bias for one or more key domains and 'high' risk of bias as high risk of bias for one or more key domains.
Risk of bias for an outcome across trials and across domains: these are our main summary assessments that have been incorporated in our judgements about the quality of evidence in the 'Summary of finding' tables. 'Low' risk of bias is defined as most information coming from trials at low risk of bias, 'unclear' risk of bias as most information coming from trials at low or unclear risk of bias and 'high' risk of bias as sufficient proportion of information coming from trials at high risk of bias.
Measures of treatment effect
When at least two trials are available for a comparison and a given outcome, we expressed dichotomous data as odds ratio (OR) or risk ratio (RR) with 95% confidence interval (CI). We expressed continuous data as mean difference (MD) with 95% CI. We expressed time‐to‐event data as hazard ratio (HR) with 95% CI.
Unit of analysis issues
We took into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome.
Dealing with missing data
If possible, we obtained missing data from trial authors and carefully evaluated important numerical data such as screened, randomly assigned participants as well as intention‐to‐treat (ITT), and as‐treated and per‐protocol populations. We investigated attrition rates (e.g. drop‐outs, losses to follow‐up, withdrawals), and we critically appraised issues concerning missing data and imputation methods (e.g. last observation carried forward (LOCF)).
Where means and standard deviations (SDs) for outcomes have not been reported and we have not received the needed information from trial authors, we imputed these values by estimating the mean and variance from the median, range, and the size of the sample (Hozo 2005) or by assuming the SD of the missing outcome to be the average of the SD from those trials in which this information was reported.
We investigated the impact of imputation on meta‐analyses by performing sensitivity analysis.
Assessment of heterogeneity
In the event of substantial clinical, methodological or statistical heterogeneity, we did not reported trial results as the pooled effect estimate in a meta‐analysis.
We identified heterogeneity (inconsistency) by visually inspecting the forest plots and by using a standard Chi² test with a significance level of α = 0.1. In view of the low power of this test, we will also consider the I² statistic, which quantifies inconsistency across trials to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003); where an I² statistic of 75% or more indicates a considerable level of heterogeneity (Higgins 2011a).
Had we found heterogeneity, we attempted to determine possible reasons for it by examining individual trial and subgroup characteristics.
Assessment of reporting biases
If we included 10 trials or more investigating a particular outcome, we used funnel plots to assess small‐trial effects. Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias. We therefore interpreted results carefully (Sterne 2011).
Data synthesis
Unless there was good evidence for homogeneous effects across trials, we summarised primarily low risk of bias data using a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual trial (Riley 2011). In addition, we performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Quality of evidence
We presented the overall quality of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues related not only to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results. Two review authors (NN, NN) independently rated the quality of evidence for each outcome. We present a summary of the evidence in a 'Summary of findings' table. This provided key information about the best estimate of the magnitude of the effect, in relative terms and as absolute differences, for each relevant comparison of alternative management strategies, numbers of participants and trials addressing each important outcome and rating of overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table on the basis of methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented results for the outcomes as described in Types of outcome measures section. If meta‐analysis was not possible, we presented the results in a narrative 'Summary of findings' table .
In addition, we established an appendix 'Checklist to aid consistency and reproducibility of GRADE assessments' (Meader 2014) to help with standardisation of the 'Summary of findings' tables. Should we used the GRADEpro software (www.gradepro.org) we attached evidence profile tables as an appendix.
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and we planned to carry out the following subgroup analyses with investigation of interactions.
Type of bariatric surgery technique (e.g. sleeve gastrectomy, gastric bypass).
Type of reinforcement (e.g. oversewing, buttressing materials, fibrin glue).
Initial body mass index.
Initial comorbidities.
Age.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes by restricting the analysis to:
published trials;
very long or large trials to establish the extent to which they dominate the results;
trials using the following filters: diagnostic criteria, imputation, language of publication, source of funding (industry versus other), or country; and
by taking into account risk of bias, as specified in the 'Assessment of risk of bias in included studies' section.
We also tested the robustness of results by repeating the analysis using different measures of effect size (RR, OR etc) and different statistical models (fixed‐effect and random‐effects models).
Appendices
Appendix 1. Search strategies
| Cochrane Central Register of Controlled Trials (CRSO) |
| 1 MESH DESCRIPTOR Bariatric Surgery 2 MESH DESCRIPTOR Obesity, Morbid WITH QUALIFIERS SU [surgery] 3 MESH DESCRIPTOR Obesity WITH QUALIFIERS SU [surgery] 4 MESH DESCRIPTOR Gastrectomy 5 MESH DESCRIPTOR Gastric Bypass 6 gastrectom*:TI,AB,KY 7 (gastr* ADJ3 (bypass OR sleeve)):TI,AB,KY 8 ((bariatr* OR obes*) ADJ3 surg*):TI,AB,KY 9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 10 MESH DESCRIPTOR Surgical Stapling 11 MESH DESCRIPTOR Fibrin Tissue Adhesive 12 MESH DESCRIPTOR Absorbable Implants 13 MESH DESCRIPTOR Suture Techniques 14 MESH DESCRIPTOR Tissue Adhesives 15 MESH DESCRIPTOR Coated Materials, Biocompatible 16 stapl*:TI,AB,KY 17 reinforc*:TI,AB,KY 18 sutur*:TI,AB,KY 19 buttress*:TI,AB,KY 20 fibrin*:TI,AB,KY 21 (oversew* OR overstich*):TI,AB,KY 22 seal*:TI,AB,KY 23 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 24 #9 AND #23 |
| MEDLINE (Ovid SP) |
| 1 Bariatric Surgery/ 2 Obesity, Morbid/su [Surgery] 3 Obesity/su [Surgery] 4 Gastrectomy/ 5 Gastric Bypass/ 6 gastrectom*.tw. 7 (gastr* adj3 (bypass or sleeve)).tw. 8 ((bariatr* or obes*) adj3 surg*).tw. 9 or/1‐8 10 Surgical Stapling/ 11 Fibrin Tissue Adhesive/ 12 Absorbable Implants/ 13 Suture Techniques/ 14 Tissue Adhesives/ 15 Coated Materials, Biocompatible/ 16 stapl*.tw. 17 reinforc*.tw. 18 sutur*.tw. 19 buttress*.tw. 20 fibrin*.tw. 21 (oversew* or overstich*).tw. 22 seal*.tw. 23 or/10‐22 24 9 and 23 [25‐34: Cochrane Handbook 2008 RCT filter ‐ sensitivity max. version – without "drug therapy.fs"] 25 randomized controlled trial.pt. 26 controlled clinical trial.pt. 27 randomi?ed.ab. 28 placebo.ab. 29 randomly.ab. 30 trial.ab. 31 groups.ab. 32 or/25‐31 33 exp animals/ not humans/ 34 32 not 33 35 24 and 34 [36‐39: Wong 2006b – systematic reviews filter – SensSpec version] 36 meta analysis.mp,pt. 37 review.pt. 38 search*.tw. 39 or/36‐38 40 24 and 39 41 35 or 40 |
| EMBASE (Ovid SP) |
| 1 bariatric surgery/ 2 sleeve gastrectomy/ 3 stomach bypass/ 4 gastrectom*.tw. 5 (gastr* adj3 (bypass or sleeve)).tw. 6 ((bariatr* or obes*) adj3 surg*).tw. 7 or/1‐6 8 surgical stapling/ 9 fibrin glue/ 10 biodegradable implant/ 11 suturing method/ 12 tissue adhesive/ 13 biomaterial/ 14 stapl*.tw. 15 reinforc*.tw. 16 sutur*.tw. 17 buttress*.tw. 18 fibrin*.tw. 19 (oversew* or overstich*).tw. 20 seal*.tw. 21 or/8‐20 22 7 and 21 [23: Wong 2006a "sound treatment studies" filter ‐ SDSSGS version] 23 random*.tw. or clinical trial*.mp. or exp treatment outcome/ 24 22 and 23 25 limit 24 to embase |
| LILACS (iAHx) |
| (MH:"Bariatric Surgery" OR MH:"Obesity, Morbid/su" OR MH:"Obesity/su" OR MH:"Gastrectomy" OR MH:"Gastric Bypass" OR gastrectom$ OR (gastr$ AND (bypass OR sleeve OR manga OR tubul$)) OR ((bariatr$ OR obes$) AND (surg$ OR ciru$))) AND (MH:"Surgical Stapling" OR MH:"Fibrin Tissue Adhesive" OR MH:"Absorbable Implants" OR MH:"Suture Techniques" OR MH:"Tissue Adhesives" OR MH:"Coated Materials, Biocompatible" OR stapl$ OR reinforc$ OR reforc$ OR reforz$ OR sutur$ OR buttress$ OR fibrin$ OR oversew$ OR seal$ OR sella$ OR sello$ OR sela$ OR selo$ OR adhesiv$ OR adesiv$ OR grampea$ OR engrapa$ OR grapa$) + Filter "Controlled Clinical Trial" |
| ICTRP Search Portal (Standard search) |
| bariatric AND stapl* OR bariatric AND fibrin* OR bariatric AND adhesiv* OR bariatric AND sutur* OR bariatric AND reinforc* OR bariatric AND buttress* OR bariatric AND oversew* OR bariatric AND overstich* OR bariatric AND sealant* OR bariatric AND tachosil* OR bariatric AND seamguard* OR bariatric AND absorbable* OR bariatric AND bioabsorbable* OR gastr* AND stapl* OR gastr* AND fibrin* OR gastr* AND adhesiv* OR gastr* AND sutur* OR gastr* AND reinforc* OR gastr* AND buttress* OR gastr* AND oversew* OR gastr* AND overstich* OR gastr* AND sealant* OR gastr* AND tachosil* OR gastr* AND seamguard* OR gastr* AND absorbable* OR gastr* AND bioabsorbable* |
| ClinicalTrials.gov (Advanced search) |
| ( bariatric OR gastrectomy OR "gastric bypass" OR "gastric sleeve" OR "obesity surgery" ) AND ( stapling OR staple OR stapler OR fibrin OR "tissue adhesive" OR "tissue adhesives" OR absorbable OR bioabsorbable OR suture OR suturing OR reinforcing OR reinforcement OR buttress OR buttressing OR oversew OR oversewing OR overstich OR sealant OR tachosil OR seamguard) AND INFLECT EXACT ( "Adult" OR "Senior" ) [AGE‐GROUP] |
What's new
| Date | Event | Description |
|---|---|---|
| 24 July 2018 | Amended | This protocol was withdrawn by the Editorial Office of the Cochrane Metabolic and Endocrine Disorders Group because finishing the project within adequate deadlines could not be achieved. |
Contributions of authors
Antonio Morandeira‐Rivas (AMR): protocol draft, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, and future review updates.
Carlos Moreno‐Sanz (CMS): protocol draft, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, and future review updates.
Michael Clerveus (MC): protocol draft, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, and future review updates.
Juan Bautista Muñoz de la Espada‐Merlo Córdoba (JBME) protocol draft, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, and future review updates.
Mari Luz Herrero‐Bogajo (MLHB): protocol draft, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, and future review updates.
Carmen Román‐Ortiz (CRO): protocol draft, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, and future review updates.
Declarations of interest
AMR: none known.
CMS: none known.
MC: none known.
JBME: none known.
MLHB: none known.
CRO: none known.
Notes
This protocol was withdrawn by the Editorial Office of the Cochrane Metabolic and Endocrine Disorders Group because finishing the project within adequate deadlines could not be achieved.
Withdrawn from publication for reasons stated in the review
References
Additional references
- Arnold W, Shikora SA. A comparison of burst pressure between buttressed versus non‐buttressed staple‐lines in an animal model. Obesity Surgery 2005;15(2):164‐71. [DOI: 10.1381/0960892053268309] [DOI] [PubMed] [Google Scholar]
- Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surgical Endoscopy 2012;26(6):1509‐15. [DOI: 10.1007/s00464-011-2085-3] [DOI] [PubMed] [Google Scholar]
- Beller EM, Chen JK, Wang UL, Glasziou PP. Are systematic reviews up‐to‐date at the time of publication?. Systematic Reviews 2013;2(1):36. [2046‐4053: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutron I, Altman DG, Hopewell S, Vera‐Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32:4120‐6. [DOI] [PubMed] [Google Scholar]
- Bransen J, Gilissen LP, Rutte PW, Nienhuijs SW. Costs of leaks and bleeding after sleeve gastrectomies. Obesity Surgery 2015 Feb 1 [Epub ahead of print]. [DOI: 10.1007/s11695-015-1584-z] [DOI] [PubMed]
- Buchwald H, Oien Dm. Metabolic/bariatric surgery worldwide 2011. Obesity Surgery 2013;23(4):427‐36. [DOI: 10.1007/s11695-012-0864-0] [DOI] [PubMed] [Google Scholar]
- Burgos AM, Braghetto I, Csendes A, Maluenda F, Korn O, Yarmuch J, et al. Gastric leak after laparoscopic‐ sleeve gastrectomy for obesity. Obesity Surgery 2009;19(12):1672‐7. [DOI: 10.1007/s11695-009-9884-9] [DOI] [PubMed] [Google Scholar]
- Chekan E, Whelan RL. Surgical stapling‐device‐tissue interactions: what surgeons need to know to improve patients outcomes. Medical Devices (Auckland, N.Z.) 2014;12(7):305‐18. [DOI: 10.2147/MDER.S67338] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YY, Bae J, Hur KY, Choi D, Kim YJ. Reinforcing the staple line during laparoscopic sleeve gastrectomy: does it have advantages? A meta‐analysis. Obesity Surgery 2012;22(8):1206‐13. [DOI: 10.1007/s11695-012-0674-4] [DOI] [PubMed] [Google Scholar]
- Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD003641.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5:79‐85. [DOI] [PubMed] [Google Scholar]
- D’Ugo S, Gentileschi P, Benavoli D, Cerci M, Gaspari A, Berta RD, et al. Comparative use of different techniques for leak and bleeding prevention during laparoscopic sleeve gastrectomy: a multicenter study. Surgery for Obesity and Related Diseases 2014;10(3):450‐4. [DOI: 10.1016/j.soard.2013.10.018] [DOI] [PubMed] [Google Scholar]
- Dapri G, Cadiere GB, Himpens J. Reinforcing the staple line during laparoscopic sleeve gastrectomy: prospective randomized clinical study comparing three different techniques. Obesity Surgery 2010;20(4):462‐7. [DOI: 10.1007/s11695-009-0047-9] [DOI] [PubMed] [Google Scholar]
- Efthimiou E, Al‐Sabah S, Sampalis JS, Christou NV. Fibrin sealant associated with increased body temperature and leukocytosis after laparoscopic gastric bypass. Surgery for Obesity and Related Diseases 2010;6(1):46‐9. [DOI: 10.1016/j.soard.2009.03.002] [DOI] [PubMed] [Google Scholar]
- Emilia M, Luca S, Francesca B, Luca B, Paolo S, Giuseppe F, et al. Topical hemostatic agents in surgical practice. Transfusion and Apheresis Science 2011;45(3):305‐11. [DOI] [PubMed] [Google Scholar]
- Foletto M, Prevedello L, Bernante P, Luca B, Vettor R, Francini‐Pesenti F, et al. Sleeve gastrectomy as revisional procedure for failed gastric banding or gastroplasty. Surgery for Obesity and Related Diseases 2010;6(2):146‐51. [DOI: 10.1016/j.soard.2009.09.003] [DOI] [PubMed] [Google Scholar]
- Fuks D, Verhaeghe P, Brehant O, Sabbagh C, Dumont F, Riboulot M, et al. Results of laparoscopic sleeve gastrectomy: a prospective study in 135 patients with morbid obesity. Surgery 2009;145(1):106‐13. [DOI: 10.1016/j.surg.2008.07.013] [DOI] [PubMed] [Google Scholar]
- Gagner M, Deitel M, Erickson AL, Crosby RD. Survey on laparoscopic sleeve gastrectomy (LSG) at the Fourth International Consensus Summit on Sleeve Gastrectomy. Obesity Surgery 2013;23(12):2013‐7. [DOI: 10.1007/s11695-013-1040-x] [DOI] [PubMed] [Google Scholar]
- Gagner M, Buchwald JN. Comparison of laparoscopic sleeve gastrectomy leak rates in four staple‐line reinforcement options: a systematic review. Surgery for Obesity and Related Diseases 2014;10(4):713‐23. [DOI: 10.1016/j.soard.2014.01.016] [DOI] [PubMed] [Google Scholar]
- Gentileschi P, Camperchioli I, D'Ugo S, Benavoli D, Gaspari AL. Staple‐line reinforcement during laparoscopic sleeve gastrectomy using three different techniques: a randomized trial. Surgical Endoscopy 2012;26(9):2623‐9. [DOI: 10.1007/s00464-012-2243-2] [DOI] [PubMed] [Google Scholar]
- Gill RS, Switzer N, Driedger M, Shi X, Vizhul A, Sharma AM, et al. Laparoscopic sleeve gastrectomy with staple line buttress reinforcement in 116 consecutive morbidly obese patients. Obesity Surgery 2012;22(4):560‐4. [DOI: 10.1007/s11695-012-0598-z] [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Nelson LG, Gallagher SF, Murr MM. Anastomotic leaks after laparoscopic gastric bypass. Obesity Surgery 2004;14(10):1299‐307. [DOI: 10.1381/0960892042583978] [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI: 10.1186/1471-2288-5-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Gagner M. A thickness calibration device is needed to determine staple height and avoid leaks in laparoscopic sleeve gastrectomy. Obesity Surgery 2015 May 30 [Epub ahead of print]. [DOI: 10.1007/s11695-015-1705-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannelli A, Schneck AS, Ragot E, Liagre A, Anduze Y, Msika S, et al. Laparoscopic sleeve gastrectomy as revisional procedure for failed gastric banding and vertical banded gastroplasty. Obesity Surgery 2009;19(9):1216‐20. [DOI: 10.1007/s11695-009-9903-x] [DOI] [PubMed] [Google Scholar]
- Ibele AR, Bendewald FP, Mattar SG, McKenna DT. Incidence of gastrojejunostomy stricture in laparoscopic Roux‐en‐Y gastric bypass using an autologous fibrin sealant. Obesity Surgery 2014;24(7):1052‐6. [DOI: 10.1007/s11695-014-1204-3] [DOI] [PubMed] [Google Scholar]
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
- Knapps J, Ghanem M, Clements J, Merchant AM. A systematic review of staple‐line reinforcement in laparoscopic sleeve gastrectomy. Journal of the Society of Laparoendoscopic Surgeons 2013;17(3):390‐9. [DOI: 10.4293/108680813X13654754534639] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair‐Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990—2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2224‐60. [DOI: 10.1016/S0140-6736(12)61766-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Monclova J, Targarona‐Soler E, Balague Ponz C, Vilallonga R, Rodríguez‐Gómez K, Baeza‐Vitolas M. Pilot study comparing the leak pressure of the sleeved stomach with and without reinforcement. Surgical Endoscopy 2013;27(12):4721‐30. [DOI: 10.1007/s00464-013-3123-0] [DOI] [PubMed] [Google Scholar]
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977‐84. [DOI] [PubMed] [Google Scholar]
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KA, Pump A. Use of bioabsorbable staple reinforcement material in gastric bypass: a prospective randomized clinical trial. Surgery for Obesity and Related Diseases 2007;3(4):417‐21. [DOI: 10.1016/j.soard.2007.03.244] [DOI] [PubMed] [Google Scholar]
- Musella M, Milone M, Maietta P, Bianco P, Pisapia A, Gaudioso D. Laparoscopic sleeve gastrectomy: efficacy of fibrin sealant in reducing postoperative bleeding. A randomized controlled trial. Updates in Surgery 2014;66(3):197‐201. [DOI: 10.1007/s13304-014-0257-0] [DOI] [PubMed] [Google Scholar]
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384(9945):766‐81. [DOI: 10.1016/S0140-6736(14)60460-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NT, Masoomi H, Magno CP, Nguyen XM, Laugenour K, Lane J. Trends in use of bariatric surgery, 2003‐2008. Journal of the American College of Surgeons 2011;213(2):261‐6. [DOI: 10.1016/j.jamcollsurg.2011.04.030] [DOI] [PubMed] [Google Scholar]
- Parikh M, Issa R, McCrillis A, Saunders JK, Ude‐Welcome A, Gagner M. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: a systematic review and meta‐analysis of 9991 cases. Annals of Surgery 2013;257(2):231‐7. [DOI: 10.1097/SLA.0b013e31826cc714] [DOI] [PubMed] [Google Scholar]
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- Sajid MS, Khatri K, Singh K, Sayegh M. Use of staple‐line reinforcement in laparoscopic gastric bypass surgery: a meta‐analysis. Surgical Endoscopy 2011;25(9):2884‐91. [DOI: 10.1007/s00464-011-1637-x] [DOI] [PubMed] [Google Scholar]
- Sapala JA, Wood MH, Schuhknecht MP. Anastomotic leak prophylaxis using a vapor‐heated fibrin sealant: report on 738 gastric bypass patients. Obesity Surgery 2004;14(1):35‐42. [DOI: 10.1381/096089204772787266] [DOI] [PubMed] [Google Scholar]
- Shah SS, Todkar JS, Shah PS. Buttressing the staple line: a randomized comparison between staple‐line reinforcement versus no reinforcement during sleeve gastrectomy. Obesity Surgery 2014;24(12):2014‐20. [DOI: 10.1007/s11695-014-1374-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikora SA, Kim JJ, Tarnoff ME. Comparison of permanent and nonpermanent staple line buttressing materials for linear gastric staple lines during laparoscopic Roux‐en‐Y gastric bypass. Surgery for Obesity and Related Diseases 2008;4(6):729‐34. [DOI: 10.1016/j.soard.2008.02.001] [DOI] [PubMed] [Google Scholar]
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Santos R, Masdevall C, Baltasar A, Martínez‐Blázquez C, García Ruiz de Gordejuela A, Ponsi E, et al. Short‐ and mid‐ term outcomes of sleeve gastrectomy for morbid obesity: the experience of the Spanish National Registry. Obesity Surgery 2009;19(9):1203‐10. [DOI: 10.1007/s11695-009-9892-9] [DOI] [PubMed] [Google Scholar]
- World Health Organization. Overweight and Obesity. Factsheet no.311. http://www.who.int/mediacentre/factsheets/fs311/enAugust 2014.
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellmer JD, Mathiason MA, Kallies KJ, Kothari SN. Is laparoscopic sleeve gastrectomy a lower risk bariatric procedure compared with laparoscopic Roux‐en‐Y gastric bypass? A meta‐analysis. The American Journal of Surgery 2014;208(6):903‐10. [DOI: 10.1016/j.amjsurg.2014.08.002] [DOI] [PubMed] [Google Scholar]


