Abstract
Background
Miscarriage is a common complication encountered during pregnancy. It is defined as spontaneous pregnancy loss before 20 weeks' gestation. Progesterone's physiological role is to prepare the uterus for the implantation of the embryo, enhance uterine quiescence and suppress uterine contractions, hence, it may play a role in preventing rejection of the embryo. Inadequate secretion of progesterone in early pregnancy has been linked to the aetiology of miscarriage and progesterone supplementation has been used as a treatment for threatened miscarriage to prevent spontaneous pregnancy loss. This update of the Cochrane Review first published in 2007, and previously updated in 2011, investigates the evidence base for this practice.
Objectives
To determine the efficacy and the safety of progestogens in the treatment of threatened miscarriage.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (8 August 2017) and reference lists of retrieved trials.
Selection criteria
Randomised, quasi‐randomised or cluster‐randomised controlled trials, that compared progestogen with placebo, no treatment or any other treatment for the treatment of threatened miscarriage in women carrying singleton pregnancy.
Data collection and analysis
At least two review authors assessed the trials for inclusion in the review, assessed trial quality and extracted the data and graded the body of evidence.
Main results
We included seven trials (involving 696 participants) in this update of the review. The included trials were conducted in different countries, covering the full spectrum of the World Bank's economic classification, which enhances the applicability of evidence drawn from this review. Two trials were conducted in Germany and Italy which are high‐income countries, while four trials were conducted in upper‐middle income countries; two in Iran, one in Malaysia and the fourth in Turkey, and the seventh trial was conducted in Jordan, which is a lower‐middle income country. In six trials all the participants met the inclusion criteria and in the seventh study, we included in the meta‐analysis only the subgroup of participants who met the inclusion criteria. We assessed the body of evidence for the main outcomes using the GRADE tool and the quality of the evidence ranged from very low to moderate. Downgrading of evidence was based on the high risk of bias in six of the seven included trials and a small number of events and wide confidence intervals for some outcomes.
Treatment of miscarriage with progestogens compared to placebo or no treatment probably reduces the risk of miscarriage; (risk ratio (RR) 0.64, 95% confidence interval (CI) 0.47 to 0.87; 7 trials; 696 women; moderate‐quality evidence). Treatment with oral progestogen compared to no treatment also probably reduces the miscarriage rate (RR 0.57, 95% CI 0.38 to 0.85; 3 trials; 408 women; moderate‐quality evidence). However treatment with vaginal progesterone compared to placebo, probably has little or no effect in reducing the miscarriage rate (RR 0.75, 95% CI 0.47 to 1.21; 4 trials; 288 women; moderate‐quality evidence). The subgroup interaction test indicated no difference according to route of administration between the oral and vaginal subgroups of progesterone.
Treatment of miscarriage with the use of progestogens compared to placebo or no treatment may have little or no effect in reducing the rate of preterm birth (RR 0.86, 95% CI 0.52 to 1.44; 5 trials; 588 women; low‐quality evidence).
We are uncertain if treatment of threatened miscarriage with progestogens compared to placebo or no treatment has any effect on the rate of congenital abnormalities because the quality of the evidence is very low (RR 0.70, 95% CI 0.10 to 4.82; 2 trials; 337 infants; very‐low quality evidence).
Authors' conclusions
The results of this Cochrane Review suggest that progestogens are probably effective in the treatment of threatened miscarriage but may have little or no effect in the rate of preterm birth. The evidence on congenital abnormalities is uncertain, because the quality of the evidence for this outcome was based on only two small trials with very few events and was found to be of very low quality.
Plain language summary
Progestogen for treating threatened miscarriage
What is the issue?
Spontaneous miscarriage occurs in about 15% to 20% of pregnancies. Threatened miscarriage occurs when a mother might be losing her baby at less than 20 weeks' gestation. The symptoms of threatened miscarriage are vaginal bleeding, with or without abdominal pain, while the cervix of the womb is closed and the baby inside the womb is alive. Progesterone is a hormone that is known to prepare the uterus for implantation of the fertilized egg and suppress uterine contractions until term. Medications that mimic the action of progesterone are known as progestogens. Treatment with progestogens may be effective in reducing the rate of miscarriage in women who have threatened miscarriage. This Cochrane Review examines whether progestogens could reduce miscarriage for women with threatened miscarriage, and also addresses the safety of these medications for mother and baby.
Why is this important?
We were interested to investigate if progestogens are effective and safe in the treatment of threatened miscarriage, which may increase the women's chances of having a successful pregnancy and a live birth.
What evidence did we find?
In this review of the literature, up to August 2017, we identified seven randomised trials involving 696 women that compared the use of progestogens in the treatment of threatened miscarriage with either placebo or no treatment. We found that the use of a progestogen probably reduces the rate of spontaneous miscarriage and this was supported by moderate‐quality evidence. Five trials, involving 588 women, reported on the effectiveness of progestogens given for threatened miscarriage in reducing the rate of preterm delivery and showed little or no effect, with low‐quality evidence. Two trials, involving 337 women, reported on the effect of treatment with progestogens given for threatened miscarriage on the rate of occurrence of congenital abnormalities in the newborns. The evidence on congenital abnormalities is uncertain, because the quality of the evidence for this outcome was based on only two small trials with very few events and was found to be of very low quality.
What does this mean?
The evidence suggests that progesterone probably reduces the rate of spontaneous miscarriage but may make little or no difference to the number of preterm deliveries. The evidence for congenital abnormalities is uncertain because the quality of the evidence for this outcome was based on only two small trials with very few events and was found to be of very low quality.
Summary of findings
Summary of findings for the main comparison. Progesterone compared to placebo or no treatment for treating threatened miscarriage.
| Progesterone compared to placebo or no treatment for treating threatened miscarriage | ||||||
| Patient or population: women with threatened miscarriage Setting: two in high‐income countries (Germany and Italy), four in upper‐middle income countries (two in Iran, one in Malaysia and one in Turkey) and one in lower‐income country (Jordan). All in hospitals (university or women's) or medical centres Intervention: progesterone Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with progesterone | |||||
| Miscarriage | Study population | RR 0.64 (0.47 to 0.87) | 696 (7 RCTs) | ⊕⊕⊕⊝ Moderate1 | ||
| 242 per 1000 | 138 per 1000 (102 to 189) | |||||
| Preterm birth | Study population | RR 0.86 (0.52 to 1.44) | 588 (5 RCTs) | ⊕⊕⊝⊝ Low1, 2 | ||
| 91 per 1000 | 84 per 1000 (49 to 142) | |||||
| Congential abnormalities | Study population | RR 0.70 (0.10 to 4.82) | 337 (2 RCTs) | ⊕⊝⊝⊝ Very low1, 2, 3 | ||
| 13 per 1000 | 9 per 1000 (1 to 62) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Random sequence generation and/or allocation concealment had a high or unclear risk of bias in the majority of the trials (methodological quality of trials) (‐1). 2Wide confidence interval crossing the no effect line (imprecision) (‐1). 3A small number of events (‐1).
Background
Description of the condition
"Miscarriage is pregnancy loss before 20 weeks' gestation based on the first day of the last menstrual period or, if gestation age is unknown, it is the loss of an embryo or a fetus of less than 400 g" (Zegers‐Hochschild 2009). Threatened miscarriage is manifested by vaginal bleeding, with or without abdominal pain, while the cervix is closed and the fetus is viable and inside the uterine cavity (Cunningham 2001a). Once the cervix begins to dilate, miscarriage and pregnancy loss are inevitable. When the fetus is non‐viable and the cervix is closed, this is known as missed miscarriage or missed abortion (Cunningham 2001a). The presence of a short cervix or funnelling of the internal cervical os in the gestation period between 16 and 24 weeks has been found to indicate increased risk or threat to miscarriage (Owen 2004; Rust 2005).
Miscarriage is a common complication of pregnancy occurring in 15% to 20% of all clinically recognised pregnancies (Blohm 2008; Cohain 2017). As many as 50% of the miscarried fetuses and embryos have normal chromosomes (Suzumori 2010; Vorsanova 2005). In many cases, the cause of miscarriage cannot be identified, however, among the recognised risk factors of miscarriage are maternal age over 34 years and paternal age over 40 years (De La Rochebrochard 2002), previous history of two or more miscarriages (Sugiura‐Ogasawara 2009), and maternal autoimmune factors such as phospholipids antibodies (Check 2011). In addition, many modifiable risk factors were recognised including maternal obesity (Hahn 2014), maternal infection such as genital herpes simplex, HIV‐1 and vaginal colonisation of group B streptococci (Nigro 2011; Rocchetti 2011; Temmerman 1992). Maternal endocrine abnormalities such as uncontrolled diabetes mellitus (Galindo 2006), polycystic ovary syndrome (Arredondo 2006) and insufficient production of progesterone are other known risk factors. Progesterone insufficiency is due to corpus luteum (CL) deficiency, which is a group of cells in the ovary that are formed after the release of the ovum (Cunningham 2001b). The main function of the CL is to produce sufficient progesterone to prepare and support the lining of the uterus and facilitate implantation of the ovum (Cunningham 2001b). It is suggested that progesterone deficiency in early pregnancy may be an aetiological factor for miscarriage, hence, treatment with progesterone may prevent miscarriage. However the evidence support for the effectiveness and safety of such treatment is uncertain based on the results of recently published trials (Coomarasamy 2015; Saccone 2017).
Description of the intervention
Progestogens are a group of hormones that bind to the progesterone receptors; they include both the natural female sex hormone progesterone and the synthetic forms. Medications that mimic the action of progesterone are known as progestational agents. Progesterone and progestational agents are known as progestogens. Progesterone is secreted during early pregnancy from the ovary by CL (Cunningham 2001b). It is an essential hormone for the establishment and maintenance of pregnancy. Progestogens can be given to women with threatened miscarriage orally, as intramuscular injection or in the form of vaginal suppositories. Due to the wide range of doses, preparations and types of progestogens used for the treatment of threatened miscarriage (Carp 2012; Mirza 2016), it is uncertain what is the best type, dose and route of administration for the treatment of threatened miscarriage.
How the intervention might work
Progesterone induces secretory changes in the lining of the uterus, which are important for implantation of the fertilised ovum (Jin 2006). In addition, it modulates the immune response of the mother to prevent rejection of the embryo through a protein called progesterone induced blocking factor (PIBF), which is produced by the lymphocytes (white blood cells) of the pregnant woman (Szekeres‐Bartho 2010), and it enhances uterine quiescence and suppresses uterine contractions (Szekeres‐Bartho 2008; Szekeres‐Bartho 2009). Low progesterone levels have been linked to increased risk of first trimester miscarriage (Osmanağaoğlu 2010).
Owing to the documented physiological role of progesterone in maintaining pregnancy, it has been used to treat women with threatened miscarriage and presumed progesterone deficiency to improve expectations for continuity of pregnancy (Palagiano 2004). The therapeutic value of progesterone in preventing or treating threatened miscarriage has not been established (Kalinka 2005), although more recent evidence suggests that it may be effective in preventing miscarriage in women with a history of recurrent miscarriage of unclear etiology (Haas 2018). This might be due to the poor designs of the trials done to evaluate its effectiveness (Kalinka 2005), and the inclusion of women in these trials with different aetiologies for threatened miscarriage.
Why it is important to do this review
Miscarriage is associated with considerable physical and psychological morbidity. Women who had threatened miscarriage were found to have increased rate of antepartum haemorrhage, pre‐labour rupture of the membranes, preterm delivery, and intrauterine growth restriction when compared with women who did not have threatened miscarriage (Saraswat 2010). The emotional response to miscarriage can be profound; it includes depression, sleep disturbance, anger, and marital disturbances (Marcinko 2011). The introduction of ultrasound scans in the management of bleeding in early pregnancy has improved the diagnosis by rapid confirmation of viability and has improved the management by introducing prognostic factors such as fetal bradycardia and discrepancy between gestational age and crown‐to‐rump length (Dede 2010; Makrydimas 2003). This has rationalised the management of threatened miscarriage, as attempts to maintain a pregnancy are likely to be effective only if the fetus is viable and has no chromosomal abnormalities (Lede 2005).
The importance of progesterone on the maintenance of pregnancy was demonstrated by the successful use of progesterone antagonists, such as mifepristone (RU 486) in the elective induction of abortion (Dabash 2015). In a recently published systematic review vaginally administered progesterone was more effective in the prevention of preterm birth compared to the injectable progesterone (Oler 2017). This raised the question about the importance of the route of administration and the type of progestogen used to prevent preterm labour (Di Renzo 2005). These same questions might apply to the use of progestogens in the treatment of threatened miscarriage.
In earlier reports progestogen therapy has been linked to the development of hypospadias (deformity of the penis) in the male fetus (Silver 1999); however, recent reports did not show an increase in rate of hypospadias in infants of mothers who received progestogens in early pregnancy (Källén 2010).
The aim of this review is to study all the available data on the effectiveness of administration of progestogens for the treatment of threatened miscarriage.
Objectives
To determine the efficacy and the safety of progestogens in the treatment of threatened miscarriage.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, quasi‐randomised or cluster‐randomised controlled trials, that assessed the effectiveness and safety of progestogens in the treatment of threatened miscarriage compared to placebo, no treatment or other intervention, if viability of the embryo or the fetus was confirmed before the commencement of treatment.
Types of participants
We included pregnant women, with threatened miscarriage at or less than 23 weeks, with singleton embryo or fetus, and who had a confirmed viable pregnancy. Fetal viability was to ensure that we excluded from this review trials that included women with bleeding in early pregnancy due to missed miscarriage. We have placed no restriction on the age of the woman or past obstetric history.
Types of interventions
We included any type of progestogens, natural or synthetic, used in the treatment of threatened miscarriage regardless of the dose, duration or route of administration compared with placebo, no treatment or other intervention.
Types of outcome measures
Primary outcomes
Miscarriage
Secondary outcomes
Mother
Pain relief (as defined by the study authors)
Thromboembolism
Preterm birth
Depression (as defined by the study authors)
Any other adverse outcomes that were reported (pregnancy‐induced hypertension; antepartum haemorrhage)
Child
Preterm birth
Stillbirth
Neonatal death
Congenital abnormalities, including genital malformations
Any other adverse neonatal outcomes that were reported (intrauterine growth restriction; respiratory distress syndrome)
Low birthweight (not prespecified)
Birthweight (not prespecified)
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (8 August 2017)
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Two people screen the search results and review the full text of all relevant trial reports identified through the searching activities described above. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (8 August 2017) using the search terms given in Appendix 1.
Searching other resources
We scanned bibliographies of identified papers.
We did not apply any language restrictions.
Data collection and analysis
We have outlined the methods of data collection and analysis that we used to assess Gerhard 1987 and Palagiano 2004 in the previous version of this review, Wahabi 2011.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Three review authors independently assessed for inclusion all the potential trials we identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
For methods used in the previous version of this review, see Wahabi 2011.
For this update, we used the following methods for assessing the 29 reports that the Information Specialist identified as a result of the updated search.
Assessment of risk of bias in included studies
The four review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
For each included study we assessed the method as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that trials were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as being at:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as being at:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed methods as being at:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
We excluded trials where more than 20% of participants were lost to follow‐up.
(5) Selective reporting (checking for reporting bias)
For each included study we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study we described any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether trials were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it was likely to have an impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (Sensitivity analysis).
Assessment of the quality of the evidence using the GRADE approach
For this update of the review, we used the GRADE approach, as outlined in the GRADE handbook (GRADE 2013), to assess the quality of the body of evidence relating to the following outcomes for the main comparisons, progesterone versus placebo or no treatment.
Miscarriage
Preterm birth
Congenital abnormalities
We used the GRADEpro Guideline Development tool (GRADEpro GDT 2015) to import data from Review Manager 5 (RevMan 5) (RevMan 2014) in order to create 'Summary of findings' tables. We produced a summary of the intervention effect and using the GRADE approach, a measure of quality for each of the above outcomes. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as summary risk ratio (RR) with 95% confidence intervals (CIs).
Continuous data
None of the outcomes were reported as continuous data in this update. In future updates, if trials measure outcomes in the same way, we will use the mean difference. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion. If we identify any cluster‐randomised trials in future updates of this review, we will include them in the analyses along with individually randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions, using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population (Higgins 2011c). If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For included trials, we noted levels of attrition. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² (Higgins 2003) and Chi² statistics (Deeks 2011). We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more trials in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it (Sterne 2011).
Data synthesis
We carried out statistical analysis using the RevMan 5 software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that trials were estimating the same underlying treatment effect: that is, where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. In future updates, if there is clinical heterogeneity sufficient to expect that the underlying treatment effects would differ between trials, or if we detect substantial statistical heterogeneity, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. We will treat the random‐effects summary as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful we will not combine trials (Deeks 2011).
If we use random‐effects analyses, we will present the results as the average treatment effect with 95% CIs, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We did not identify substantial heterogeneity. However, we carried out the following subgroup analyses for the primary outcome.
Route of administration
If we identify substantial heterogeneity in future updates of this review, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it. We will include the following subgroups, in addition to route of administration.
Type of progestogen
Progestogen dose
Effect of progestogens in early (no more than 12 weeks) and late (more than 12 weeks) threatened miscarriage
We will restrict subgroup analysis to the primary outcome. We will assess subgroup differences by interaction tests available within RevMan 5 (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value (Deeks 2011).
Sensitivity analysis
We included seven trials in this review. All but one were similar in terms of risk of bias. For future updates of this review, we will carry out sensitivity analysis to explore the effect of risk of bias. This will involve analysis based on our 'Risk of bias' judgements for allocation concealment, performance bias and attrition bias. We will compare the results of those trials assessed as being rated 'low' risk of bias for these domains with those rated as 'high' or 'unclear' risk of bias.
Results
Description of studies
Results of the search
We assessed 29 new trial reports of potential relevance identified by the updated search (see: Figure 1).
1.
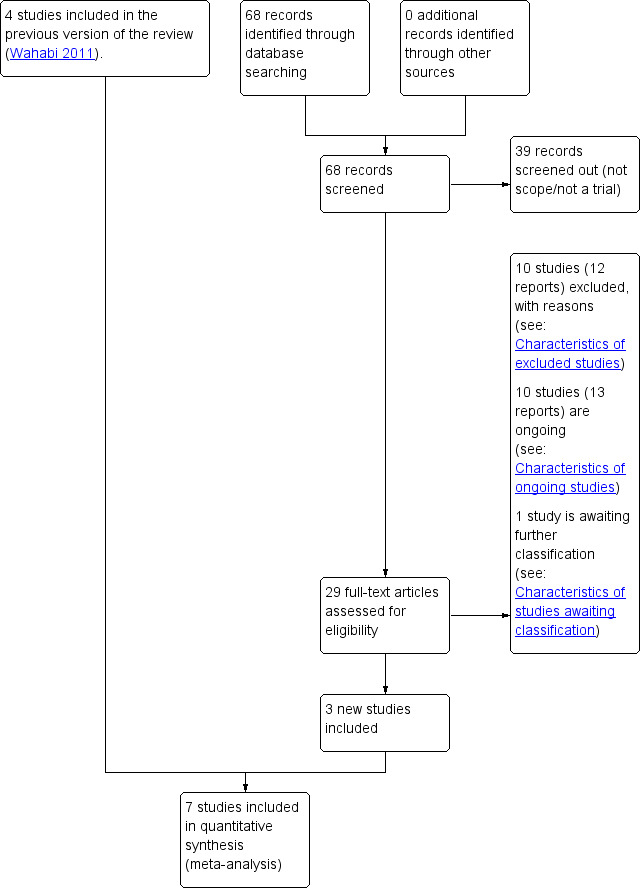
Trial flow diagram
Included studies
Trial location
We included seven trials (involving 696 participants) in this update of the review. The included trials were conducted in different countries, covering the full spectrum of the World Bank's economic classification (World Bank 2017), which may enhance the applicability and generalisation of evidence drawn from this review. Two trials were conducted in Germany and Italy (Gerhard 1987; Palagiano 2004), which are high‐income countries, while four trials were conducted in upper‐middle income countries; two in Iran (Alimohamadi 2013; Yassaee 2014), one in Malaysia (Pandian 2009) and the fourth in Turkey (Turgal 2017). The seventh trial was conducted in Jordan (El‐Zibdeh 2009), which is a lower‐middle income country. All trials were conducted in hospital settings.
Trial design
We included six randomised controlled trials (Alimohamadi 2013; Gerhard 1987; Palagiano 2004; Pandian 2009; Turgal 2017; Yassaee 2014) and one quasi‐randomised controlled trial (El‐Zibdeh 2009).
Sample size
A total of 696 women were included in this review. The largest trial involved 191 women (Pandian 2009) and the smallest involved 35 women (Gerhard 1987).
Participants
We included pregnant women, with threatened miscarriage at or less than 23 weeks and who had a confirmed viable pregnancy. Fetal viability was to ensure that we excluded from this review trials that included women with bleeding in early pregnancy due to missed miscarriage. We placed no restriction on the age of the woman or past obstetric history.
Type of intervention
The seven included trials compared progestogens to either placebo (Alimohamadi 2013; Gerhard 1987; Palagiano 2004; Turgal 2017) or no treatment (El‐Zibdeh 2009; Pandian 2009; Yassaee 2014). Three trials investigated oral progestogen (El‐Zibdeh 2009; Pandian 2009; Turgal 2017) and four investigated vaginal progesterone (Alimohamadi 2013; Gerhard 1987; Palagiano 2004; Yassaee 2014).
Outcome measure
Primary outcome
Miscarriage
All the included trials investigated this primary outcome. Three trials investigated oral progestogen (El‐Zibdeh 2009; Pandian 2009; Turgal 2017), and four trials investigated vaginal progesterone (Alimohamadi 2013; Gerhard 1987; Palagiano 2004; Yassaee 2014).
Secondary outcomes
Pain relief
Two trials (Palagiano 2004; Yassaee 2014) reported pain relief as an outcome of treatment with progesterone.
Preterm birth
Five trials (Alimohamadi 2013; El‐Zibdeh 2009; Gerhard 1987, Pandian 2009; Turgal 2017); with 588 women, reported the effect of oral or vaginal progestogens on preterm birth.
Pregnancy‐induced hypertension
Two trials (El‐Zibdeh 2009; Pandian 2009) reported pregnancy‐induced hypertension as a maternal adverse outcome.
Antepartum haemorrhage
Two trials (El‐Zibdeh 2009; Pandian 2009) reported antepartum haemorrhage as a maternal adverse outcome.
Perinatal death
One trial investigated the effect of progestogens on neonatal death (Alimohamadi 2013) and two trials investigated the effect of progestogens on stillbirth (Pandian 2009; Turgal 2017).
Congenital abnormalities
Two trials (El‐Zibdeh 2009; Pandian 2009) reported congenital abnormalities as an outcome.
Intrauterine growth restriction and low birthweight
Two trials (Turgal 2017, El‐Zibdeh 2009) reported on the frequency of intrauterine growth restriction in the intervention and control groups. Another trial (Alimohamadi 2013) reported on the frequency of low birthweight in the progesterone and the placebo group.
Respiratory distress syndrome
One trial (Alimohamadi 2013) investigated the rate of respiratory distress syndrome in the progesterone and the placebo group.
Birthweight
Only one trial (Turgal 2017) reported the difference in birthweight as an outcome.
Date of trials
Six trials were published between 2004 and 2017 (Alimohamadi 2013; El‐Zibdeh 2009; Palagiano 2004; Pandian 2009; Turgal 2017; Yassaee 2014). One trial was published in 1987 (Gerhard 1987).
Funding source
Solvay Pharmaceuticals funded two trials (El‐Zibdeh 2009; Pandian 2009). The rest of the trials did not mention the source of funding.
Delcaration of interest
None of the study authors reported any conflict of interest.
For further details, see Characteristics of included studies.
Excluded studies
In this update of the review, we excluded 10 (plus two duplicate) full‐text trials, in addition to the 32 excluded from the previous version. The reasons for exclusion of the 42 trials was as follows: 18 trials had a different population to this review' criteria (Brenner 1962; Corrado 2002; El Zibdeh 2000; El Zibdeh 2002; El Zibdeh 2005; Fuchs 1966; Goldzieher 1964; Johnson 1975; Klopper 1965; Le Vine 1964; Nyboe 2002; Porcaro 2015; Prietl 1992; Reijnders 1988; Shearman 1963; Smitz 1992; Swyer 1953; Turner 1966); 14 trials, either compared progesterone to another type of progesterone or used it in combination with another therapy (Beigi 2016; Berle 1977; Check 1995; Chye 2014 (and duplicate); Czajkowski 2007; Famina 2015; Hui 2015; Luz 1988 [pers comm]; Pustotina 2018; Siew 2014; Siew 2015; Song 2007; Vincze 2006; Zhang 2000); seven trials did not use a reliable method to confirm the viability of the fetus (Berle 1980; Crowder 1950; Govaerts‐Videtzky 1965; Moller 1965; Sondergaard 1985; Souka 1980; Tognoni 1980); one study (and duplicate) had a mixed group of participants and we could not obtain separate results for the threatened‐miscarriage group (Costantino 2016); one was not an RCT (Akhtar 2012); and one study had a high rate of attrition, more than 20% (Omar 2005).
For further details, see Characteristics of excluded studies.
Risk of bias in included studies
2.
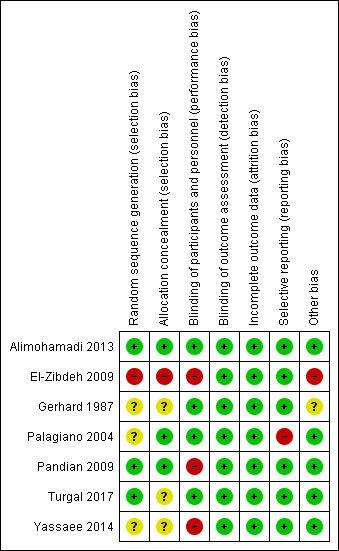
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial
3.
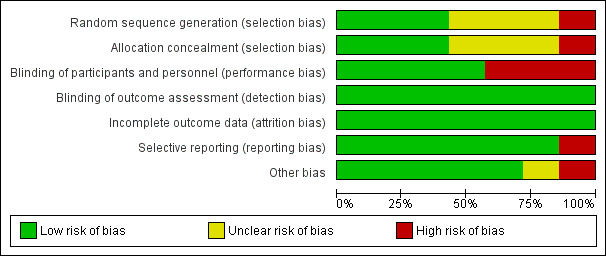
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials
We judged only three of the seven included trials (42%) to be at low risk of bias for random sequence generation and allocation concealment, and four trials (57%) to be at low risk for performance bias. We judged all trials (100%) at low risk of detection bias and attrition bias. We judged six trials at low risk of selective reporting bias (85%), and five trials (71%) at low risk of other biases.
Allocation
In three trials (Alimohamadi 2013; Pandian 2009; Turgal 2017), the investigators described a random component in the sequence‐generation process by using computer random‐number generators, so we judged these trials to have low risk of selection bias. In three trials (Gerhard 1987; Palagiano 2004; Yassaee 2014), there was insufficient information about the sequence‐generation process to permit judgement of low or high risk, so we judged them as unclear risk of selection bias. We judged one study (El‐Zibdeh 2009) as high risk for selection bias, as the investigators described a non‐random component in the sequence‐generation process, which was based on the day of the week that women presented to the clinic.
Participants and investigators enrolling participants could not foresee assignment to intervention or control group in three trials because Alimohamadi 2013 and Palagiano 2004 used sequentially numbered drug containers of identical appearance and Pandian 2009 used sequentially numbered concealed envelopes; we judged these trials at low risk of selection bias. Three trials (Gerhard 1987; Turgal 2017; Yassaee 2014), did not describe the method of concealment sufficiently to allow a definite judgement, so we judged them at unclear risk of selection bias. In El‐Zibdeh 2009, participants and investigators enrolling participants could foresee assignments, as the allocation was performed by the attending physician based on the day of the week the women visited the clinic, and thus the risk of selection bias introduction was high.
Blinding
Four trials (Alimohamadi 2013; Gerhard 1987; Palagiano 2004; Turgal 2017) ensured blinding of participants and key study personnel, and it is unlikely that the blinding could have been broken, so we judged the risk of performance bias to be low. Three trials did not use placebo for the control group. Two of these (El‐Zibdeh 2009; Pandian 2009) did not blind either personnel or women to the treatment received and one (Yassaee 2014) was a single‐blind study, in which the researchers were unaware which woman had received progesterone. Although outcomes such as miscarriage, preterm birth and fetal structural malformations are not likely to be influenced by lack of blinding, lack of blinding of participants or personnel could have introduced performance bias if participants inadvertently took a different type of progestogen than the one in the trial or exaggerated their reports of subjective outcomes, such as pain.
Five of the seven trials blinded outcome assessors to the treatment the women had received (Alimohamadi 2013; El‐Zibdeh 2009; Gerhard 1987; Palagiano 2004; Yassaee 2014). Pandian 2009 did not blind the assessors, and Turgal 2017 did not give any information about blinding. Outcomes such as miscarriage, preterm birth and fetal structural malformations are not likely to be influenced by lack of assessor's blinding, so we judged all seven trials as having low risk of detection bias.
Incomplete outcome data
There was no missing outcome data in five of the seven included trials (Alimohamadi 2013;El‐Zibdeh 2009; Palagiano 2004; Pandian 2009; Yassaee 2014); Gerhard 1987 excluded two women (5.7%) from the study after randomisation, and Turgal 2017 reported incomplete outcome data for 12 women (14.5%), equal in both groups. We judged all seven trials to be at low risk of attrition bias.
Selective reporting
All the included trials, except one (Palagiano 2004), reported all of the expected prespecified (primary and secondary) outcomes, so we assessed these at low risk of reporting bias. In Palagiano 2004, the study authors mentioned in the methods that they would report all the adverse effects, but in the results there was no explicit mention of the absence or presence of any adverse effects, so we judged it to be at high risk of reporting bias.
Other potential sources of bias
We assessed one study at high risk of other biases due to the difference in the number of participants recruited to the experimental and the control groups (86 versus 60; El‐Zibdeh 2009). In another study (Gerhard 1987), the risk of other bias was not clear because we included only participants with evidence of fetal viability, which amounts to only half of the participants.
Effects of interventions
See: Table 1
Seven trials met the inclusion criteria, involving 696 participants. We performed subgroup analysis for the effect of progestogens by route of administration; however, due to paucity of data we could not carry out subgroup analysis for type and dose of progestogen. We undertook a total of six meta‐analyses.
Primary outcome
Miscarriage
The seven included trials, with 696 participants, compared progestogens to either placebo (Alimohamadi 2013; Gerhard 1987; Palagiano 2004: Turgal 2017) or no treatment (El‐Zibdeh 2009; Pandian 2009; Yassaee 2014). Treatment of miscarriage with progestogens compared to placebo or no treatment probably reduces the risk of miscarriage; (risk ratio (RR) 0.64, 95% confidence interval (CI) 0.47 to 0.87; 7 trials; 696 women; moderate‐quality evidence).
Subgroup analysis by route of administration
Three trials investigated treatment with oral progestogen (El‐Zibdeh 2009; Pandian 2009; Turgal 2017), and showed that treatment with oral progestogen compared to no treatment probably reduces the miscarriage rate (RR 0.57, 95% CI 0.38 to 0.85; 3 trials; 408 women; moderate‐quality evidence; Analysis 1.1.1). Four trials investigated treatment with vaginal progesterone compared to placebo (Alimohamadi 2013; Gerhard 1987; Palagiano 2004; Yassaee 2014), which probably has little or no effect in reducing the miscarriage rate (RR 0.75, 95% CI 0.47 to 1.21; 4 trials; 288 women; moderate‐quality evidence; Analysis 1.1.2). However, It should be noted that there were no differences between these two subgroups according to the subgroup interaction test (test for subgroup differences: Chi² = 0.76, df = 1 (P = 0.38), I² = 0%).
1.1. Analysis.
Comparison 1 Progestogens versus placebo or no treatment, Outcome 1 Miscarriage.
Secondary outcomes
Maternal outcomes
Pain relief
Two trials (Palagiano 2004; Yassaee 2014) reported pain relief as an outcome of treatment with progesterone. Palagiano 2004 reported reduction on the mean pain score with the use of progesterone from 2.6 ± 0.9 before treatment to 0.4 ± 0.7 (mean ± standard deviation) after treatment (P < 0.01), while no change in pain score was observed in women who received placebo from 2.5 ± 1.0 before treatment to 2.4 ± 0.8 (mean ± standard deviation) after treatment (P > 0.05). They reported pain using a progressive score from 0 to 4, where a score of 0 indicated 'no pain' and a score of 4 indicated 'extreme pain'. In Yassaee 2014, nine (30%) of the women in the progesterone and seven (23.3%) of the control group, continued to have pain after intervention (P = 0.55). We could not pool the results of these two trials due to the different types of data (dichotomous and continuous).
Preterm birth
Five trials (Alimohamadi 2013; El‐Zibdeh 2009; Gerhard 1987, Pandian 2009; Turgal 2017); reported the effect of progestogens on preterm birth. Treatment of preterm birth with the use of progestogens compared to placebo or no treatment may have little or no effect in reducing the rate of preterm birth (RR 0.86, 95% CI 0.52 to 1.44; 5 trials; 588 women; low‐quality evidence; Analysis 1.2).
1.2. Analysis.
Comparison 1 Progestogens versus placebo or no treatment, Outcome 2 Preterm birth.
Other maternal adverse outcomes
Pregnancy‐induced hypertension
Two trials (El‐Zibdeh 2009; Pandian 2009) reported pregnancy‐induced hypertension as a maternal adverse outcome. There was no difference in the occurrence of pregnancy‐induced hypertension between the progestogen and the control group (RR 1.00, 95% CI 0.54 to 1.88; 2 trials; 337 women; Analysis 1.3).
1.3. Analysis.
Comparison 1 Progestogens versus placebo or no treatment, Outcome 3 Pregnancy‐induced hypertension.
Antepartum haemorrhage
Two trials (El‐Zibdeh 2009; Pandian 2009) reported antepartum haemorrhage as maternal adverse outcome. Progestogens have little or no difference in the occurrence of antepartum haemorrhage (RR 0.76, 95% CI 0.30 to 1.94; 2 trials; 337 women; Analysis 1.4).
1.4. Analysis.
Comparison 1 Progestogens versus placebo or no treatment, Outcome 4 Antepartum haemorrhage.
Child outcomes
Stillbirth
Two trials investigated the effect of progestogens on the stillbirth rate (Pandian 2009; Turgal 2017). Progestogens have little or no difference effects on the rate of stillbirth (RR 1.94, 95% CI 0.18 to 20.49; 2 trials; 262 women; Analysis 1.5).
1.5. Analysis.
Comparison 1 Progestogens versus placebo or no treatment, Outcome 5 Stillbirth.
Congenital abnormalities
Two trials (El‐Zibdeh 2009; Pandian 2009) reported congenital abnormalities as an outcome. We are uncertain if treatment of threatened miscarriage with progestogens compared to placebo or no treatment has any effect on the rate of congenital abnormalities, (RR 0.70, 95% CI 0.10 to 4.82; 2 trials; 337 infants; very low‐quality evidence; Analysis 1.6).
1.6. Analysis.
Comparison 1 Progestogens versus placebo or no treatment, Outcome 6 Congential abnormalities.
Neonatal death
Only one trial reported the effect of progestogens on neonatal death (Alimohamadi 2013). Progestogens have little or no effect in the neonatal death rate (RR 1.35, 95% CI 0.31 to 5.83; 1 trial; 145 women).
Other neonatal adverse outcomes
Intrauterine growth restriction and low birthweight
One trial (Turgal 2017) reported on the frequency of intrauterine growth restriction in the intervention and control group and showed no difference between the two groups; 0 (0%) in the progestogen group, one (3.5%) in the control group. Another trial (Alimohamadi 2013) reported on the frequency of low birthweight in the progesterone and the placebo group and showed no difference between the two groups; five (7%) for the progesterone group and seven (9.8%) for the placebo group.
Birthweight
Only one trial (Turgal 2017) reported the difference in birthweight as an outcome. It showed no difference in the mean birthweight between the progestogen and the control group; 3.1156 ± 0.643 kg for the intervention group and 3.2076 ± 0.500 kg for the control group.
Respiratory distress syndrome
One trial (Alimohamadi 2013) investigated the rate of respiratory distress syndrome in the progesterone and the placebo groups, and showed no difference between the two groups; there were two neonates with respiratory distress syndrome (2.8%) in the progesterone group and one (1.4%) in the placebo group.
Outcomes not reported in the primary trials
Due to paucity of data, we could not evaluate the following secondary outcomes.
Thromboembolism
Depression
In addition we could not perform the following subgroup analyses.
Route of progestogen administration
Type of progestogen
Progestogen dose
Effect of progestogens in early and late miscarriage
Discussion
Summary of main results
We included seven trials (involving 696 participants) in this update of the review. The included trials were conducted in different countries, covering the full spectrum of the World Bank's economic classification (World Bank 2017), which enhances the applicability of evidence drawn from this review. Two trials were conducted in Germany and Italy which are high‐income countries, while four trials were conducted in upper‐middle income countries; two in Iran, one in Malaysia and the fourth in Turkey, and the seventh trial was conducted in Jordon, which is a lower‐middle income country. In six trials all the participants met the inclusion criteria and in the seventh study, we included only the subgroup of participants who met the inclusion criteria in the meta‐analysis. Using the GRADE tool, we assessed the body of evidence for the main outcomes as ranging from very low‐quality to moderate‐quality evidence. Downgrading of evidence was based on the high risk of bias in six of the seven included trials and imprecision for some of the outcomes.
Treatment of miscarriage with progestogens compared to placebo or no treatment probably reduces the risk of miscarriage. Subgroup analysis by route of administration showed that treatment with oral progestogen compared to no treatment probably reduces the miscarriage rate, although treatment with vaginal progesterone compared to placebo, probably has little or no effect in reducing the miscarriage rate. However, the subgroup interaction test indicated no difference according to route of administration between the oral and vaginal subgroups of progesterone. Treatment of preterm birth with the use of progestogens compared to placebo or no treatment may have little or no effect in reducing the rate of preterm birth. We are uncertain if treatment of threatened miscarriage with progestogens compared to placebo or no treatment has any effect on the rate of congenital abnormalities.
Overall completeness and applicability of evidence
Threatened miscarriage is a common health problem. In a longitudinal study by Blohm 2008, one in four multiparous women had experienced a miscarriage and more than 7% of the studied population had experienced three or more miscarriages. In a large population‐based study by Cohain 2017, more than 40% of parous women reported having experienced one or more first trimester spontaneous miscarriages.
This update of the review includes seven trials, with a relatively small number of women (696), from six countries. All trials were conducted in hospital settings. Two trials were conducted in high‐income countries (Gerhard 1987; Palagiano 2004), four in upper‐middle income countries (Alimohamadi 2013; Pandian 2009; Turgal 2017; Yassaee 2014) and one in lower‐middle income country (El‐Zibdeh 2009). Although no trial was conducted in a low‐income country, the participants in the trials of the review have diverse ethnic and economic backgrounds (World Bank 2017). This may make the evidence in this review applicable to a large sector of women with threatened miscarriage.
Quality of the evidence
In this updated review we assessed the body of evidence for miscarriage as moderate quality, mainly due to limitations in study design of the included trials. From the seven included trials we judged only three (42%) to be at low risk of bias for random sequence generation and allocation concealment. Four trials (57%) were at low risk for performance bias, and all were at low risk of detection bias. We assessed all trials at low risk for attrition bias and six were at low risk of selective reporting bias. Only five trials (71%) trials were at low risk for other biases (Table 1).
For preterm birth we assessed the body of evidence as low quality due to imprecision (wide confidence intervals crossing the no‐effect line) and limitations with study design of the included trials. From the five included trials, three (60%) were at low risk of bias for random sequence generation, while three (60%) were at low risk of performance bias and all trials were at low risk of detection bias. In addition, one study randomised different numbers of participants to the two arms of the study, which we considered as additional bias and in one study we included the results of only 50% of the participants who met the inclusion criteria.
For the outcome congenital abnormality, we assessed the body of evidence as very low due to imprecision (wide confidence intervals crossing the no‐effect line), small number of trials (two trials) and participants (337 women) and limitations with study design. We assessed one of the two included trials to be at high risk of selection bias and performance bias and the other study at high risk of performance bias.
Potential biases in the review process
In this review, we took steps to minimise bias, although we are aware that bias may be present in our review: two review authors independently assessed trials for eligibility and extracted the data as necessary. We resolved discrepancies through discussion or, if required, we consulted a third review author; two review authors also performed GRADE assessments independently and resolved discrepancies though discussion; and we conducted a comprehensive search in order to identify all relevant published and unpublished literature.
Agreements and disagreements with other studies or reviews
Our assessment of the body of evidence in this update of the review did not differ much from the previous version of the review (Wahabi 2011). For the main comparison, we have included three additional trials, however, neither the magnitude nor the direction of evidence changed markedly from the earlier version of the review. We have used the recent methodology introduced for Cochrane Reviews in 2008, which assesses risk of bias in the individual trials more carefully than in the past (Higgins 2011b). In addition, we assessed the quality of the evidence using the GRADE approach as outlined in the GRADE Handbook (GRADE 2013).
The results of this review agree with a recently published systematic review of randomised and non‐randomised controlled trials of dydrogesterone (oral progestogen) for the treatment of threatened miscarriage (Carp 2012) and with non‐randomised controlled trials (Akhtar 2012), which investigated the effectiveness of oral progestogens in the treatment of threatened miscarriage. The results of this trial showed that a larger proportion of women who received progestogen continued to be pregnant compared to the control group, however, the difference was not significant.
Recently published trials suggested that the route of administration and the type of progestogen influence its effectiveness in the prevention of miscarriage. In a multicenter, randomised, placebo‐controlled trial on women with unexplained recurrent miscarriages, vaginal progesterone was found not to be significantly different from placebo in the prevention of miscarriage (Coomarasamy 2015). However, the results of a review of randomised trials on the use of progestogen in the prevention of recurrent miscarriage, concluded that synthetic progestogens, but not natural progesterone, were associated with a lower risk of recurrent miscarriage, nevertheless, the conclusion was limited by the low quality of evidence (Saccone 2017).
Our results suggested that progestogen may not be as effective in the prevention of preterm birth (Analysis 1.2). There are conflicting published reports about the effectiveness of progestational agents in the prevention of preterm birth with apparent influence of type of progestogen and route of administration, as well as different subgroups of women, on the effectiveness of treatment (Facchinetti 2017; Heyborne 2016; O'Brien 2016).
Our results relating to the safety of progestogen use for the mother and infant are limited by the small number of trials and participants in addition to the low quality of the evidence. However, our systematic review is in agreement with other systematic reviews, which also suggest that there are no increased risks of adverse outcomes to the mother or the infant from the use of progestogen compared to the control group (Haas 2018).
Authors' conclusions
Implications for practice.
The results of this review suggest that progestogens are probably effective in the treatment of threatened miscarriage but may have little or no effect in the rate of preterm birth. The evidence on congenital abnormalities is uncertain, because the quality of the evidence for this outcome was based on only two small trials with very few events and was found to be of very low quality.
Implications for research.
Further research could investigate the effectiveness and safety of progestogens in the treatment of threatened miscarriage by conducting methodologically sound, randomised trials, with special focus on the type of progestogen and the route of administration.
What's new
| Date | Event | Description |
|---|---|---|
| 27 September 2018 | Amended | Corrected typo in result in Abstract for the outcome 'preterm birth'. |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 8 August 2018 | Amended | Corrected affiliation for Dr Amel Fayed. |
| 8 August 2017 | New citation required but conclusions have not changed | With the addition of the data from the new included trials, analysis suggested that progestogens are still probably effective in the treatment of threatened miscarriage but may have little or no effect in the rate of preterm birth. The evidence on congenital abnormalities is uncertain, because the quality of the evidence for this outcome was based on only two small trials with very few events and was found to be of very low quality. |
| 8 August 2017 | New search has been performed | Search updated, 29 new trial reports identified, from which three trials were included in this update of the review (Alimohamadi 2013; Turgal 2017; Yassaee 2014), one is awaiting classification (Yadav 2015),10 (in addition to three duplicate), are ongoing and 10 (in addition to two duplicate) were excluded. We updated the risk of bias for all included trials. We have assessed the quality of evidence and included a 'Summary of findings' table in this update. |
| 30 September 2011 | New citation required and conclusions have changed | With the addition of the data from the new included studies, meta‐analysis suggested that oral progestogen is effective in treating threatened miscarriage. Data analysis also suggested that treatment of women with threatened miscarriage by progestogens did not increase the risk of congenital abnormalities, pregnancy induced hypertension nor antepartum haemorrhage. However these results should be approached with caution due to the small sample size and the poor methodological quality of the included studies. |
| 30 September 2011 | New search has been performed | Search updated. We have identified two new studies (El‐Zibdeh 2009; Pandian 2009) and included both. Two new authors (Amel A Fayed and Samia A Esmaeil) helped prepare this update. We have updated the methods to reflect the latest Cochrane Handbook (Higgins 2011a). |
| 14 February 2011 | New citation required but conclusions have not changed | New author helped prepare the latest update. |
| 1 December 2009 | New search has been performed | Search updated. Three new reports identified and excluded (Czajkowski 2007; Song 2007; Vincze 2006). One trial previously awaiting classification has been excluded (Zhang 2000). |
| 20 September 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors extend their gratitude to King Saud University, Deanship of Scientific Research, Research Chairs, for funding this update of the review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms for ICTRP and ClinicalTrials.gov
progest* AND miscarriage
progest* AND abortion
dydrogesterone AND miscarriage
dydrogesterone AND abortion
Data and analyses
Comparison 1. Progestogens versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Miscarriage | 7 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.47, 0.87] |
| 1.1 Oral progestogen versus no treatment | 3 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.38, 0.85] |
| 1.2 Vaginal progesterone versus placebo | 4 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.47, 1.21] |
| 2 Preterm birth | 5 | 588 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.52, 1.44] |
| 3 Pregnancy‐induced hypertension | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.54, 1.88] |
| 4 Antepartum haemorrhage | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.30, 1.94] |
| 5 Stillbirth | 2 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.18, 20.49] |
| 6 Congential abnormalities | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.10, 4.82] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alimohamadi 2013.
| Methods |
Design: RCT, double‐blind, placebo‐controlled, parallel‐group Recruitment method: unclear Method of randomisation: computer‐generated block randomisation Setting: Obstetrics and Gynaecology ward of Vali‐e‐Asr teaching hospital in Tehran |
|
| Participants |
Inclusion: pregnant women with clinical symptoms of threatened abortion before 20th week of pregnancy. Exclusion: women with systemic diseases, including maternal hypertension before or during pregnancy, cardiac disease, renal disease, genital tract anomalies of the mother or/and diabetes. Women with uterine tenderness, congenital defects of the fetus, and those who had used a progestational drug during pregnancy, prior to being recruited into the study Particpants randomised: 160 |
|
| Interventions |
Intervention group: (80 women) vaginal progesterone, 200 mg twice daily for 1 week Control group: (80 women) placebo with the same description and duration of treatment as experimental group |
|
| Outcomes | Endocervical concentrations of different cytokines Miscarriage Preterm delivery Neonatal death Low birth weight Respiratory distress syndrome |
|
| Notes |
Journal: Journal of Reproductive Immunology Year of publication: 2013 Country: Iran Income status of the country: upper‐middle‐income Source of funding: not mentioned Conflict of interest: no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation |
| Allocation concealment (selection bias) | Low risk | Pharmacy prepared |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinicians were unaware of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data of 15 participants (9.4%); 8 in intervention and 7 in control groups (balanced attrition) |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other risk of biases |
El‐Zibdeh 2009.
| Methods |
Design: quasi‐experimental Recruitment method: pregnant women consecutively presented to the clinic during study period Method of randomisation: according to the day of the week the women attended the clinic; women attending on Saturday, Monday, and Wednesday were assigned to intervention group and those attending on Sunday, Tuesday, and Thursday were assigned to the control group Setting: Amman Islamic Hospital clinic |
|
| Participants |
Inclusion: women with threatened miscarriage (mild or moderate vaginal bleeding during the first trimester of pregnancy) Exclusion: presence of a systemic illness or fever, the suspected passage of any fetal or pregnancy materials, and the absence of a normal gestational sac at 5 weeks gestational age, a yolk sac at 5.5–6 weeks gestational age, an embryo at 6–6.5 weeks gestational age or cardiac activity at 7 weeks gestational age Particpants randomised: 146 |
|
| Interventions |
Intervention group: (86 women) synthetic progesterone, dydrogesterone, oral 10 mg twice daily. Until 1 week after the bleeding stopped. Standard supportive care, including iron, folic acid and multivitamin supplements and bed rest Control group: (60 women) no treatment. Standard supportive care, including iron, folic acid and multivitamin supplements and bed rest |
|
| Outcomes | Miscarriage Preterm delivery Fetal structural malformations, including genital malformations Maternal hypertension Intra‐uterine growth restriction APH |
|
| Notes |
Journal: Maturitas Year of publication: 2009 Country: Jordan Income status of the country: lower‐middle‐income Source of funding: Solvay Pharmaceuticals Conflict of interest: no actual or potential conflict |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | According to the day of the week the women presented to the clinic. Women attending the clinic on Saturday, Monday or Wednesday were allocated to the intervention group and those attending on Sunday, Tuesday or Thursday were allocated to the control group. |
| Allocation concealment (selection bias) | High risk | The randomisation was performed by the attending physician, who also gave the treatment to the women. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of the participants and study personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was ensured for the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were addressed. |
| Other bias | High risk | The difference in the number of participants recruited to experimental and the control groups (86 versus 60) might have introduced bias. |
Gerhard 1987.
| Methods |
Design: RCT, double‐blind Recruitment method: women with vaginal bleeding were referred to the hospital Method of randomisation: unclear Setting: Women's Hospital, University of Heidelberg |
|
| Participants |
Inclusion: vaginal bleeding and the internal cervical os being closed Exclusion: none Particpants randomised: 35 |
|
| Interventions |
Intervention group: (17 women) 1 vaginal suppository twice daily, containing 25 mg progesterone, and bed rest Control group: (18 women) 1 vaginal suppository twice daily, containing polyethylene glycol, and bed rest |
|
| Outcomes | Miscarriage 14 days of being symptom free Preterm delivery |
|
| Notes |
Journal: Biological Research in Pregnancy Year of publication: 1987 Country: Germany Income status of the country: high‐income Source of funding: not mentioned Conflict of interest: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not mentioned |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and study personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of the outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women (5.7%) were omitted from the study after randomisation |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were addressed |
| Other bias | Unclear risk | As we included only participants with evidence of fetal viability, which amounted to only half the participants, we are unclear how much bias was introduced. |
Palagiano 2004.
| Methods |
Design: RCT Recruitment method: unclear Method of randomisation: unclear Setting: a medical centre |
|
| Participants |
Inclusion: women with threatened abortion; viable baby; amenorrhoea 6‐12 weeks; closed uterine cervix Exclusion: women with previous adequate luteal phase; women who were using hormonal treatment or other drugs affecting uterine contractility; women with vaginal infection; absence of embryo’s heartbeat; open cervix (> 2 cm measured by U/S); embryo’s size 1 week more than the corresponding amenorrhoea. Particpants randomised: 50 |
|
| Interventions |
Intervention group: (25 women) 90 mg vaginal progesterone once daily for 5 days Control group: (25 women) placebo |
|
| Outcomes | Pain score Uterine contractility Abortion rate (Followed for 60 days for the occurrence of miscarriage and for 5 days for the other outcomes) |
|
| Notes |
Journal: Annals New York Academy of Sciences Year of publication: 2004 Country: Italy Income status of the country: high‐income Source of funding: not mentioned Conflict of interest: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This is a randomised trial but the method of randomisation wan not mentioned |
| Allocation concealment (selection bias) | Low risk | Code numbers were attached to each container of the gel. The researcher recorded the progressive number of the container given to the patients enrolled, who received a correspondent code. Only 3 months after the end of the database recording, the codes were open and showed to the Data Monitoring Board (DMB) to be evaluated by a statistician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and study personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The effect on pain symptom amelioration was evaluated by a 5‐score intensity grading, whereas uterine contractions were evaluated by standard transvaginal ultrasound, the code was broken only after the analysis by the statistician. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | High risk | In the methods study authors mentioned they will report all adverse effects, but there was no explicit mention of the absence or presence of any adverse effects in the results section. |
| Other bias | Low risk | No risk of other biases |
Pandian 2009.
| Methods |
Design: RCT Recruitment method: all women presenting with vaginal bleeding up to 16 weeks of pregnancy were assessed for inclusion Method of randomisation: sealed envelopes picked by the participants Setting: Department of Obstetrics and Gynaecology, Seberang Jaya Hospital |
|
| Participants |
Inclusion: women with threatened abortion. Viability of fetus confirmed by U/S. no systematic illness or fever and no loss of conception tissue Exclusion: women with recurrent miscarriage (> 3), heavy bleeding, cervical polyp, multiple gestation, empty sac > 26 mm Particpants randomised: 191 women |
|
| Interventions |
Intervention group: (96 women) 40 mg followed by 10 mg twice daily Control group: (95 women) conservative treatment with bed rest only |
|
| Outcomes | Miscarriage rate Placenta previa Preterm delivery Congenital anomalies APH Caesarean section Low birth weight Preganancy induced hypertension Perinatal death |
|
| Notes |
Journal: Maturitas Year of publication: 2009 Country: Malaysia Income status of the country: upper middle‐income Source of funding: Solvay Pharmaceutical Company Conflict of interest: no conflicts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation of each women using sealed envelope |
| Allocation concealment (selection bias) | Low risk | Concealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and the study personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only the statistician was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss of follow‐up |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were addressed |
| Other bias | Low risk | No apparent other sources of bias |
Turgal 2017.
| Methods |
Design: RCT Recruitment method: through assessment of women presented to the hospital Method of randomisation: computerised random number generator program Setting: a university hospital |
|
| Participants |
Inclusion: women with threatened abortion and with presence singleton pregnancy and live embryo, before 9 weeks of gestation Exclusion: non viable fetus, twin pregnancy, presence of subchorionic haematoma and history of hypertension, diabetes mellitus, severe hepatic disorders, uterine leiomyoma, congential uterine anomaly and recurrent pregnancy loss Particpants randomised: 83 |
|
| Interventions |
Intervention group: (42 women) oral micronised progesterone, 400 mg/d for 4 weeks Control group: (41 women) placebo |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes |
Journal: Journal of clinical Utrasound Year of publication: 2017 Country: Turkey Income status of the country: upper middle‐income Source of funding: not mentioned Conflict of interest: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number by using generator program |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome is not affected by blinding of outcomes assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data in 12 women (14.5%), equal in both groups |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were addressed |
| Other bias | Low risk | No other biases |
Yassaee 2014.
| Methods |
Design: RCT Recruitment method: through assessment of women presented to the hospital Method of randomisation: unclear Setting: Taleghani Hospital affiliated to Shahid Beheshti University of Medical Sciences |
|
| Participants |
Inclusion: pregnant women with threatened abortion. The presence of singleton pregnancy and detection of fetal heart activity, besides gestational age of < 20 weeks was verified by U/S Exclusion: women were excluded if they had reaction to Cyclogest, repeated abortions, multiple gestation, absence of fetus or fetal heart tone, uterine anomaly or fetal anomaly Particpants randomised: 60 women |
|
| Interventions |
Intervention group: 400 mg of vaginal progesterone suppository (Cyclogest) each day until their bleeding stopped in < 1 week Control group: no treatment |
|
| Outcomes | Successful delivery Miscarriage rates Pain relief |
|
| Notes |
Journal: Journal of Reproduction and Infertility Year of publication: 2014 Country: Iran Income status of the country: upper middle‐income Source of funding: not mentioned Conflict of interest: no conflicts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on random allocation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no placebo, so participants were not blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of participants to follow‐up |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were addressed |
| Other bias | Low risk | No apparent other risk of bias |
APH: antepartum haemorrhage; LBW: low birthweight; RCT: randomised controlled trial; U/S: ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akhtar 2012 | Not RCT |
| Beigi 2016 | Comparing 2 types of progestogens |
| Berle 1977 | Combination therapy of progesterone and oestrogen was used in this study |
| Berle 1980 | Viability of the fetus was not confirmed before commencement of progesterone treatment |
| Brenner 1962 | Different population |
| Check 1995 | Combination therapy of progestogen and immunotherapy |
| Chye 2014 | Compares 2 types of progestogens |
| Corrado 2002 | Different population |
| Costantino 2016 | Participants were mixed group of women with threatened abortion and subchorionic haematoma, results of intervention cannot be obtained separately |
| Crowder 1950 | Viability was not confirmed before the commencement of the study |
| Czajkowski 2007 | The study compared 2 types of progestogen |
| El Zibdeh 2000 | Different population. Population with recurrent miscarriage rather than threatened miscarriage |
| El Zibdeh 2002 | Different population. Population with recurrent miscarriage rather than threatened miscarriage |
| El Zibdeh 2005 | Different population. Population with recurrent miscarriage rather than threatened miscarriage |
| Famina 2015 | Comparing 2 types of progestogens |
| Fuchs 1966 | Different population. Patients with recurrent miscarriage not threatened miscarriage |
| Goldzieher 1964 | Different population. Population with recurrent miscarriage rather than threatened miscarriage |
| Govaerts‐Videtzky 1965 | Viability was not confirmed before the commencement of the study |
| Hui 2015 | Comparing 2 types of progestogens |
| Johnson 1975 | Different population. Progestogen was used for the prevention of miscarriage in women with recurrent miscarriage or preterm delivery not threatened miscarriage |
| Klopper 1965 | Different population. Progestogen was used for the prevention of miscarriage in women with recurrent miscarriage not threatened miscarriage |
| Le Vine 1964 | Different population. Progestogen was used for the prevention of miscarriage in women with recurrent miscarriage not threatened miscarriage |
| Luz 1988 [pers comm] | Combination therapy of progestogen and oestrogen was used in this study |
| Moller 1965 | Viability was not confirmed before the commencement of the study |
| Nyboe 2002 | Different population. Progestogen was used for the prevention of miscarriage rather than the treatment of threatened miscarriage |
| Omar 2005 | Loss to follow‐up was more than 20% |
| Porcaro 2015 | Different population |
| Prietl 1992 | Different population. Progesterone was used for the prevention of miscarriage rather than the treatment of threatened miscarriage in IVF patients |
| Pustotina 2018 | Comparing 2 types of progestogens |
| Reijnders 1988 | Different population. Progestogen was used for the prevention not the treatment of miscarriage |
| Shearman 1963 | Different population. Progestogen was used for population with recurrent miscarriage not threatened miscarriage |
| Siew 2014 | Comparing 2 types of progestogen. |
| Siew 2015 | Comparing 2 types of progestogens |
| Smitz 1992 | Different population. Progestogen was used for the prevention of miscarriage rather than the treatment of threatened miscarriage in IVF patients |
| Sondergaard 1985 | Viability was not confirmed before the commencement of the study. |
| Song 2007 | Combination progestogen with vitamin E |
| Souka 1980 | Viability was not confirmed before the commencement of the study |
| Swyer 1953 | Different population. Progestogen was used for population with recurrent miscarriage not threatened miscarriage |
| Tognoni 1980 | Viability was not confirmed before the commencement of the study |
| Turner 1966 | Different population. The study participants do not have threatened miscarriage |
| Vincze 2006 | Comparing 2 types of progesterone |
| Zhang 2000 | Combination progesterone with vitamin E |
IVF: in vitro fertilisation; RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Yadav 2015.
| Methods | RCT |
| Participants | 100 |
| Interventions | Dydrogesterone, 30 mg/d |
| Outcomes | Serum levels of IL‐6,TNF‐alpha and IL‐10 were measured at the time of admission, 10 days, 14 weeks Abortion rate |
| Notes | Only abstract is available, waiting response from trial authors |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12611000405910.
| Trial name or title | Does using progesterone in threatened miscarriage increase the live birth rate? Supporting threatened outcomes with progesterone |
| Methods | RCT intervention model: parallel assignment. Blinding (masking use) |
| Participants | 386 women age: ≥ 18 Inclusion criteria; threatened miscarriage, live intrauterine pregnancy; gestation < 10 weeks + 0 days |
| Interventions |
Intervention group: progesterone pessary 400 mg nightly, until 12 weeks + 0 days Control group: placebo |
| Outcomes | Primary outcome:
|
| Starting date | Jan 2012 |
| Contact information | Dr Luke McLindon address Mater Mothers' Hospital, Raymond Terrace, South Brisbane, Queensland, 4101 |
| Notes |
IRCT201012035294N.
| Trial name or title | Clinical trial comparison of progesterone suppository and placebo on the concentration of cervical inflammatory cytokines in patients at risk of miscarriage |
| Methods | Randomised, parallel, double‐blinded study |
| Participants | All women who are 20 weeks pregnant and who have been referred for pain or increased cervical secretion and spotting are included in the study. |
| Interventions |
Intervention group: progesterone suppository 400 mg twice/d for 1 week Control group: placebo |
| Outcomes | Primary
Secondary
|
| Starting date | June 2006 |
| Contact information | Dr Sedigheh Hanustah Zadeh Valiasr Hospital, Tehran University of Medical Sciences Iran, Tehran 00982166581617 hantoushzadeh@tums.ac.ir |
| Notes |
IRCT2015020217035N2.
| Trial name or title | Effect of oral progesterone (dydrogesterone) on incidence of glucose intolerance in pregnant females with threatened miscarriage |
| Methods |
Allocation: randomised double blind placebo controlled trial. Intervention model: parallel assignment. Masking: triple (participant, care provider, investigator). Primary purpose: treatment. |
| Participants | A total of 100 women who are 14 weeks pregnant and presented with threatened miscarriage will be recruited. Participants will be tested to exclude abnormal blood glucose level. Women will be randomised to have either oral Dydrogesterone 10 mg twice daily or placebo for the control group. Inclusion criteria: singleton pregnancy, the absence of any systemic disease, age < 30 years, BMI < 25, no history of stillbirth, no family history of diabetes, non‐smoking, Exclusion criteria: history of diabetes, gestational diabetes and other systemic diseases, any fetal abnormalities on ultrasound examination, multiple pregnancy, and previous macrosomia. Interventions: oral administration of dydrogesterone 10 mg twice daily or placebo. |
| Interventions |
Intervention group: oral dydrogesterone 10 mg twice daily Control group: placebo |
| Outcomes | Treatment of threatened miscarriage and preterm delivery To study the effect of oral progesterone (dydrogesterone) on incidence of glucose intolerance in pregnant women with threatened miscarriage |
| Starting date | March 2015 |
| Contact information | Hafez Hospital Shiraz Fars Iran, Islamic Republic Of 009871361222220 00989173138847 maryamkasraeian@gmail.com |
| Notes |
IRCT2016040627248N1.
| Trial name or title | The efficacy of a special food (fried egg with grape molasses) on threatened miscarriage: a randomised controlled trial |
| Methods | RCT, single‐blind, no placebo |
| Participants | 90 women Inclusion criteria: pregnant women, (up to 12 weeks), presenting with mild‐moderate vaginal bleeding with normal U/S and closed cervix in vaginal examination; singleton pregnancy; and who consent to participate in the study and are ready to consume the special food. |
| Interventions | Intervention group: in addition to intravaginal Cyclogest, the food recipe of fried eggs with butter and grape molasses will be given to women. The other conditions of intervention group, including the duration of eating this food, are similar to the control group. |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | July 2016 |
| Contact information | Dr Fatemeh Moradi School of Traditional medicine, Tehran University of Medical Sciences, SarParast St., Taleghani St., ValiAsr Blvd. 1449614353 Tehran Iran, Islamic Republic Of Tel:00982166976527 e‐mail:dfmoradi@gmail.com |
| Notes |
NCT015018902011.
| Trial name or title | The impact of progesterone treatment on obstetrical outcome among women with first trimester vaginal bleeding |
| Methods |
Allocation: randomised Intervention model: parallel assignment Masking: triple (participant, care provider, investigator) Primary purpose: prevention |
| Participants | Women attending the Gynecological Emergency Unit due to first trimester vaginal bleeding, with a viable singleton pregnancy at a gestational age of 6‐13 completed weeks of gestation |
| Interventions |
Intervention group: progesterone Control group: placebo |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | January 2012 |
| Contact information | Ralika Hershkovitch, MD Soroka University Medical Center Beer Sheva, Israel |
| Notes |
NCT02128685.
| Trial name or title | A randomised double‐blind controlled trial of the use of dydrogesterone in women with threatened miscarriage in the first trimester: study protocol for a randomised controlled trial |
| Methods | Double‐blind, RCT |
| Participants | A total of 400 women presenting with first‐trimester threatened miscarriage will be enrolled |
| Interventions |
Intervention group: Dydrogesterone 40 mg orally, followed by 10 mg orally 3 times/d Control group: placebo |
| Outcomes |
|
| Starting date | Registered on 29 April 2014 |
| Contact information | Department of Obstetrics and Gynaecology, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong Special Administrative Region, China. dcmanka@gmail.com |
| Notes |
NCT02145767.
| Trial name or title | Progesterone for the prevention of miscarriage and preterm delivery in women with first trimester bleeding: PREEMPT trial |
| Methods |
Allocation: randomised Intervention model: parallel assignment Masking: quadruple (participant, care provider, investigator, outcomes assessor) Primary purpose: prevention |
| Participants |
Ages eligible for study: 18 years‐45 years Accepts healthy volunteers: yes Live intrauterine singleton pregnancy of < 14 weeks by crown‐rump length on U/S with documented fetal cardiac activity presence of progestational (subchorionic) hematoma on U/S |
| Interventions |
Intervention group: progesterone 200 mg suppository administered vaginally at bedtime until 34 completed weeks of pregnancy Control group: placebo similar appearing suppository containing vehicle alone administered vaginally at bedtime until 34 completed weeks of pregnancy |
| Outcomes | Primary outcomes
Secondary outcomes:
|
| Starting date | December 2014 |
| Contact information | Contact: Andrea Spence 514‐418‐0875 preempttrial@gmail.com |
| Notes |
NCT02633878.
| Trial name or title | Chinese herbal medicine and micronized progesterone for threatened miscarriage |
| Methods | RCT intervention model: parallel assignment Masking: quadruple (participant, care provider, investigator, outcomes assessor) |
| Participants | 1656 women age:18‐37 years. Inclusion criteria: pregnant (as confirmed by positive urinary pregnancy tests), vaginal bleeding with or without abdominal pain, while the cervix is closed by visual exam and the fetus is viable inside the uterine cavity during early pregnancy (5‐10 weeks' gestation), No previous treatment for miscarriage, ability and willingness to give informed consent and willingness to be randomised and to take daily study medications for up to several months |
| Interventions | Chinese herbal medicine versus progesterone Chinese herbal medicine versus progesterone placebo Chinese herbal medicine placebo versus progesterone Chinese herbal medicine placebo + progesterone placebo |
| Outcomes | Primary outcome:
Secondary outcome measures:
|
| Starting date | Dec 2015 |
| Contact information | No contacts or locations provided |
| Notes |
NCT02690129.
| Trial name or title | Vaginal progesterone for treatment of threatened miscarriage; randomised clinical trial |
| Methods | RCT intervention model: parallel assignment Masking: single (participant) |
| Participants | 290 women age: 20 years‐35 years Inclusion criteria: pregnant < 24 weeks pregnant presented with bleeding with or without pain, with single viable fetus (confirmed by U/S examination) and accepting to participate in the trial. Exclusion criteria: currently under medication for any chronic diseases, hypersensitivity to progesterone, congenital fetal anomaly, on hormonal treatment in the current pregnancy and women conceived via assisted reproduction technique |
| Interventions |
Intervention group: (progesterone group): complete bed rest for first 48‐72 hours. Single daily dose of natural micronised progesterone (Prontogest ® 200 mg) at bedtime for 15 days. If needed, a pain killer as Indomethacin 50 mg/rectally twice daily up to control of uterine pain. Complete abstaining from sexual activity or strenuous effort. Rh‐ve women will be given a shot of anti‐D immunoglobulin 300 uG/IM if they continue to bleed; after 12 weeks' gestation or if undergo surgical evacuation. Control group: (placebo): will follow the same plan of management without progesterone support. |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | Feb 2016 |
| Contact information | Omar M Shaaban, MD Address: Faculty of Medicine Assiut, Egypt Tel:+201223971457 Email:omshaaban2000@yahoo.com |
| Notes |
NCT02950935.
| Trial name or title | Prospective, double‐blind, randomised, placebo controlled, phase iii clinical study assessing the efficacy of natural progesterone 25 mg/bid administered subcutaneously in the maintenance of early pregnancy in women with symptoms of threatened miscarriage |
| Methods | RCT: parallel assignment Masking: quadruple (participant, care provider, investigator, outcomes assessor) |
| Participants | 268 women age: 18‐37 years, BMI: 18‐28 kg/m2 Inclusion criteria: pregnant women attending the study sites with the following characteristics: accept to sign the informed consent form and adhere to the study visit schedule; symptoms of threatened miscarriage including vaginal bleeding and pelvic pain); U/S proof of viable singleton intrauterine pregnancy (positive fetal heart beat); gestation week ≥ 6 weeks (5 week + 1 day) and < 12 weeks (11 week + 1 day) according to U/S dating (CRL); closed uterine cervix; subchorionic hematoma, if detected, with < 50% placental detachment |
| Interventions |
Intervention group: subcutaneous injection of progesterone solution will be performed twice a day from onset of threatened miscarriage symptoms until week 12 of pregnancy Control group: placebo; subcutaneous injection of placebo solution will be performed twice a day from onset of threatened miscarriage symptoms until week 12 of pregnancy |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | April 2017 |
| Contact information | Barbara PS Cometti, PHD. Tel:41583601000. Email:barbara.cometti@ibsa.ch |
| Notes |
APH: antepartum haemorrhage; IUGR: intrauterine growth restriction; PPH: postpartum haemorrhage; PPROM: preterm pre‐labour rupture of membranes; U/S: ultrasound
Differences between protocol and review
The primary outcome 'Miscarriage', was previously listed as 'Early miscarriage up to 12 weeks' and 'Miscarriage later than 12 weeks and less than 23 weeks'. We grouped both outcomes together because the protocol stated that a subgroup analysis for early and late miscarriage would be carried out when data were available
-
The following outcomes are included in this update.
-
For the mother:
pregnancy‐induced hypertension
antepartum haemorrhage
-
For the child:
intrauterine growth restriction
low birthweight
birthweight
respiratory distress syndrome
-
The methods have been updated to reflect the latest Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
For this update, we assessed trial quality for seven selected outcomes using the GRADE approach (see Table 1).
Contributions of authors
For the 2017 update, Dr Hayfaa Wahabi participated in the selection of eligible trials, assessment of the trials for inclusion, data extraction and analysis and grading of evidence. She participated in writing both the initial and final version of the review.
Dr Amel Fayed participated in the selection of eligible trials, assessment of the trials for inclusion, data extraction and analysis.
Dr. Khawater Bahkali participated in the selection of eligible trials, assessment of the trials for inclusion, data extraction and analysis and grading of evidence. She participated in writing the draft of the review.
Dr Samia Ahmed participated in the selection of eligible trials, assessment of the trials for inclusion, and data extraction. She participated in writing the draft of the review.
Sources of support
Internal sources
Cochrane Review Initiative Project, Saudi Arabia.
External sources
No sources of support supplied
Declarations of interest
Hayfaa A Wahabi: none known Amel A Fayed: none known Samia A Esmaeil: none known Khawater Hassan Bahkali: none known
Edited (no change to conclusions)
References
References to studies included in this review
Alimohamadi 2013 {published data only}
- Alimohamadi S, Javadian P, Gharedaghi MH, Javadian N, Alinia H, Khazardoust S, et al. Progesterone and threatened abortion: a randomized clinical trial on endocervical cytokine concentrations. Journal of Reproductive Immunology 2013;98(1‐2):52‐60. [DOI] [PubMed] [Google Scholar]
El‐Zibdeh 2009 {published data only}
- El‐Zibdeh MY, Yousef LT. Dydrogesterone support in threatened miscarriage. Maturitas 2009;65 Suppl 1:S43‐S46. [DOI] [PubMed] [Google Scholar]
Gerhard 1987 {published data only}
- Gerhard I, Gwinner B, Eggert‐Kruse W, Runnebaum B. Double‐blind controlled trial of progesterone substitution in threatened abortion. Biological Research in Pregnancy and Perinatology 1987;8:26‐34. [PubMed] [Google Scholar]
Palagiano 2004 {published data only}
- Palagiano A, Bulletti C, Pace MC, Ziegler D, Cicinelli E, Izzo A. Effects of vaginal progesterone on pain and uterine contractility in patients with threatened abortion before twelve weeks of pregnancy. Annals of the New York Academy of Sciences 2004;1034:200‐10. [DOI] [PubMed] [Google Scholar]
Pandian 2009 {published data only}
- Pandian RU. Dydrogesterone in threatened miscarriage: a Malaysian experience. Maturitas 2009;65 Suppl 1:S47‐S50. [DOI] [PubMed] [Google Scholar]
Turgal 2017 {published data only}
- Turgal M, Aydin E, Ozyuncu O. Effect of micronized progesterone on fetal‐placental volume in first‐trimester threatened abortion. Journal of Clinical Ultrasound 2017;45(1):14‐9. [DOI] [PubMed] [Google Scholar]
Yassaee 2014 {published data only}
- Yassaee F, Shekarriz‐Foumani R, Afsari S, Fallahian M. The effect of progesterone suppositories on threatened abortion: a randomized clinical trial. Journal of Reproduction & Infertility 2014;15(3):147‐51. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Akhtar 2012 {published data only}
- Akhtar M, Fatima N, Jabeen S, Akram M. Role of progesterone in the management of threatened miscarriage. Pakistan Journal of Medical and Health Sciences 2012;6(1):261‐4. [Google Scholar]
Beigi 2016 {published data only}
- Beigi A, Esmailzadeh A, Pirjani R. Comparison of risk of preterm labor between vaginal progesterone and 17‐alpha‐hydroxy‐progesterone caproate in women with threatened abortion: a randomized clinical trial. International Journal of Fertility & Sterility 2016;10(2):162‐8. [IRCT2014123120504N1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berle 1977 {published data only}
- Berle P, Behnke K. The treatment of threatened abortion. Geburtshilfe und Frauenheilkunde 1977;37:139‐42. [PubMed] [Google Scholar]
Berle 1980 {published data only}
- Berle P, Budenz M, Michaelis J. Is hormonal therapy still justified in imminent abortion. Zeitschrift fur Geburtshilfe und Perinatologie 1980;184:353‐8. [PubMed] [Google Scholar]
Brenner 1962 {published data only}
- Brenner WE, Hendricks CH. Effect of medroxyprogesterone acetate upon the duration and characteristics of human gestation and labor. American Journal of Obstetrics and Gynecology 1962;83:1094‐8. [DOI] [PubMed] [Google Scholar]
Check 1995 {published data only}
- Check JH, Tarquini P, Gandy P, Lauer C. A randomized study comparing the efficacy of reducing the spontaneous abortion rate following lymphocyte immunotherapy and progesterone treatment vs progesterone alone in primary habitual aborters. Gynecologic and Obstetric Investigation 1995;39:257‐61. [DOI] [PubMed] [Google Scholar]
Chye 2014 {published data only}
- Chye TT, ChiCTR‐IOR‐17011593. The randomised controlled trial of micronised progesterone and dydrogesterone (TRoMaD) for threatened miscarriage. chictr.org.cn/showproj.aspx?proj=19668 (first received 6 July 2017).
- Chye TT, ChiCTR‐IPR‐14005251. Micronised progesterone versus dydrogesterone: a randomised controlled trial. chictr.org.cn/showproj.aspx?proj=9539http://www.chictr.org.cn/showproj.aspx?proj=9539 (first received 21 September 2014).
Corrado 2002 {published data only}
- Corrado F, Dugo C, Cannata ML, Bartolo M, Scilipoti A, Stella NC. A randomised trial of progesterone prophylaxis after midtrimester amniocentesis. European Journal of Obstetrics & Gynecology and Reproductive Biology 2002;100:196‐8. [DOI] [PubMed] [Google Scholar]
Costantino 2016 {published data only}
- Costantino D, NCT02601898. Vaginal administration of alpha lipoic acid vs progesterone for the subchorionic hematoma treatment. clinicaltrials.gov/show/NCT02601898 (first received 6 November 2015).
- Costantino M, Guaraldi C, Costantino D. Resolution of subchorionic hematoma and symptoms of threatened miscarriage using vaginal alpha lipoic acid or progesterone: clinical evidences. European Review for Medical and Pharmacological Sciences 2016;20(8):1656‐63. [PubMed] [Google Scholar]
Crowder 1950 {published data only}
- Crowder RE, Bills ES, Broadbent JS. The management of threatened abortion: a study of 100 cases. American Journal of Obstetrics and Gynecology 1950;60:896‐9. [DOI] [PubMed] [Google Scholar]
Czajkowski 2007 {published data only}
- Czajkowski K, Sienko J, Mogilinski M, Bros M, Szczecina R, Czajkowska A. Uteroplacental circulation in early pregnancy complicated by threatened abortion supplemented with vaginal micronized progesterone or oral dydrogesterone. Fertility and Sterility 2007;87(3):613‐8. [DOI] [PubMed] [Google Scholar]
El Zibdeh 2000 {published data only}
- Zibdeh MY. Randomized study comparing the efficacy of reducing spontaneous abortion following treatment with progesterone and human chorionic gonadotropin (hCG). Fertility and Sterility 2000;70(3 Suppl 1):S77‐S78. [Google Scholar]
El Zibdeh 2002 {published data only}
- Zibdeh M. Randomized clinical trial comparing the efficacy of dydrogesterone and human chorionic gonadotropin [abstract]. 10th World Congress on Menopause; 2002 July 10‐14; Berlin, Germany. 2002:136.
El Zibdeh 2005 {published data only}
- El‐Zibdeh MY. Dydrogesterone in the reduction of recurrent spontaneous abortion. Journal of Steroid Biochemistry & Molecular Biology 2005;97(5):431‐4. [DOI] [PubMed] [Google Scholar]
Famina 2015 {published data only}
- Famina M. The effect of dydrogesterone on placental angiogenesis and pregnancy outcomes in women with threatened miscarriages. Giornale Italiano di Ostetricia e Ginecologia 2015;37(4):155‐8. [Google Scholar]
Fuchs 1966 {published data only}
- Fuchs F, Olsen P. An attempted double‐blind controlled trial of progesterone therapy in habitual abortion. Ugeskrift for Laeger 1966;128:1461‐2. [PubMed] [Google Scholar]
Goldzieher 1964 {published data only}
- Goldzieher JW. Double‐blind trial of a progestin in habitual abortion. JAMA 1964;188(7):651‐4. [DOI] [PubMed] [Google Scholar]
Govaerts‐Videtzky 1965 {published data only}
- Govaerts‐Videtzky M, Martin L, Hubinont PO. A double‐blind study of progestogen treatment in spontaneous abortion (preliminary report). Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;72:1034. [DOI] [PubMed] [Google Scholar]
Hui 2015 {published data only}
- Hui CYY, Siew SJY, Tan TC. Biochemical and clinical outcomes following the use of micronised progesterone and dydrogesterone for threatened miscarriage ‐ a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2015;122(Suppl S1):276. [Google Scholar]
Johnson 1975 {published data only}
- Johnson JWC, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha‐hydroxyprogesterone caproate in the prevention of premature labor. New England Journal of Medicine 1975;293:675‐80. [DOI] [PubMed] [Google Scholar]
Klopper 1965 {published data only}
- Klopper AI, MacNaughton MC. Hormones in recurrent abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;72:1022‐8. [Google Scholar]
Le Vine 1964 {published data only}
- Vine L. Habitual abortion. A controlled clinical study of progestational therapy. Western Journal of Surgery 1964;72:30‐6. [PubMed] [Google Scholar]
Luz 1988 [pers comm] {published data only}
- Luz NP. Hormones in the treatment of patients with threatened early pregnancy loss. Letter to: Oxford Database of Perinatal Trials, John Radcliffe Infirmary, Oxford 22 October 1988.
Moller 1965 {published data only}
- Moller KJA, Fuchs F. Double blind controlled trial of 6‐methyl‐17‐acetoxyprogesterone in threatened abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;72:1042‐4. [Google Scholar]
Nyboe 2002 {published data only}
- Nyboe Andersen A, Popovic‐Todorovic B, Schmidt KT, Loft A, Lindhard A, Hojgaard A, et al. Progesterone supplementation during early gestations after IVF or ICSI has no effect on the delivery rates: a randomized controlled trial. Human Reproduction 2002;17(2):357‐61. [DOI] [PubMed] [Google Scholar]
Omar 2005 {published data only}
- Omar MH, Mashita MK, Lim PS, Jamil MA. Dydrogesterone in threatened abortion: pregnancy outcome. Journal of Steroid Biochemistry & Molecular Biology 2005;97(5):421‐5. [DOI] [PubMed] [Google Scholar]
Porcaro 2015 {published data only}
- Porcaro G, Brillo E, Giardina I, Iorio R. Alpha lipoic acid (ALA) effects on subchorionic hematoma: preliminary clinical results. European Review for Medical and Pharmacological Sciences 2015;19(18):3426‐32. [PubMed] [Google Scholar]
Prietl 1992 {published data only}
- Prietl G, Diedrich K, Ven HH, Luckhaus J, Krebs D. The effect of 17alpha‐hydroxyprogesterone caproate/oestradiol valerate on the development and outcome of early pregnancies following in vitro fertilization and embryo transfer: a prospective and randomized controlled trial. Human Reproduction 1992;7(1):1‐5. [DOI] [PubMed] [Google Scholar]
Pustotina 2018 {published data only}
- Pustotina OA. Effectiveness of dydrogesterone, 17‐OH progesterone and micronized progesterone in prevention of preterm birth in women with a short cervix. Journal of Maternal‐Fetal & Neonatal Medicine 2018;31(14):1830‐8. [DOI] [PubMed] [Google Scholar]
Reijnders 1988 {published data only}
- Reijnders FJL, Thomas CMG, Doesburg WH, Rolland R, Eskes TKAB. Endocrine effects of 17 alpha‐hydroxyprogesterone caproate during early pregnancy: a double‐blind clinical trial. British Journal of Obstetrics and Gynaecology 1988;95:462‐8. [DOI] [PubMed] [Google Scholar]
Shearman 1963 {published data only}
- Shearman RP, Garrett WJ. Double‐blind study of effect of 17‐hydroxyprogesterone caproate on abortion rate. BMJ 1963;1:292‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siew 2014 {published data only}
- Siew JYS, Allen JC, Malhotra R, Ostbye T, He S, Tan TC. Progesterone for threatened miscarriage: a randomised controlled trial comparing micronised progesterone and dydrogesterone. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(Suppl 1):243. [Google Scholar]
Siew 2015 {published data only}
- Siew S, Hui CYY, Tan TC, Allen JC, Malhotra R. Micronized progesterone compared with dydrogesterone for threatened miscarriage: a randomized controlled trial. Obstetrics & Gynecology 2015;125(5 Suppl):104S. [Google Scholar]
Smitz 1992 {published data only}
- Smitz J, Devroey P, Faguer B, Bourgain C, Camus M, Steirteghem AC. Randomized prospective trial comparing supplementation of the luteal phase and of early pregnancy by natural progesterone given by intramuscular or vaginal administration. Revue Francaise de Gynecologie et d Obstetrique 1992;87:507‐16. [PubMed] [Google Scholar]
Sondergaard 1985 {published data only}
- Sondergaard F, Ottesen B, Detlefsen GU, Schierup L, Pederson SC, Lebech PE. Progesterone treatment of cases of threatened pre‐term delivery in women with a low level of plasma progesterone. Contraception Fertilite Sexualite 1985;13:1227‐31. [Google Scholar]
Song 2007 {published data only}
- Song YL, Zhu LP. The fetus protection effects of Zhixue Baotai Decoction on women of early threatened abortion with dark area surrounding pregnancy sac. Chinese Journal of Integrated Traditional and Western Medicine 2007;27(11):1025‐8. [PubMed] [Google Scholar]
Souka 1980 {published data only}
- Souka AR, Osman M, Sibaie F, Einen MA. Therapeutic value of indomethacin in threatened abortion. Prostaglandins 1980;19:457‐60. [DOI] [PubMed] [Google Scholar]
Swyer 1953 {published data only}
- Swyer GIM, Daley D. Progesterone implantation in habitual abortion. British Medical Journal 1953;1:1073‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tognoni 1980 {published data only}
- Tognoni G, Ferrario L, Inzalaco M, Crosignani PG. Progestagens in threatened abortion. Lancet 1980;2:1242‐3. [DOI] [PubMed] [Google Scholar]
Turner 1966 {published data only}
- Turner SJ, Mizock GB, Feldman GL. Prolonged gynecologic and endocrine manifestations subsequent to administration of medroxyprogesterone acetate during pregnancy. American Journal of Obstetrics and Gynecology 1966;95:222‐7. [DOI] [PubMed] [Google Scholar]
Vincze 2006 {published data only}
- Vincze E, Molnar GB, Foldesi I, Pal A. Treatment possibilities using progestagens and progesteron‐like preparations in threatened abortion [A fenyegeto veteles kezelesenek lehetosegei progeszteron es progeszteron‐szeru keszitmenyekkel]. Magyar Noorvosok Lapja 2006;69(4):281‐4. [Google Scholar]
Zhang 2000 {published data only}
- Zhang J, Zhang Y, Liu G. Clinical and experimental study on yun'an granule in treating threatened abortion. Chung‐Kuo Chung Hsi i Chieh Ho Tsa Chih 2000;20(4):251‐4. [PubMed] [Google Scholar]
References to studies awaiting assessment
Yadav 2015 {published data only}
- Yadav R, Yadav S, Jain A. To study the impact of progesterone (dydrogesterone) on proinflammatory (IL‐6 and TNF‐alpha) and anti‐inflammatory (IL‐10) cytokines in threatened abortion. International Journal of Gynecology and Obstetrics 2015;131(Suppl 5):E196. [Google Scholar]
References to ongoing studies
ACTRN12611000405910 {published data only}
- ACTRN12611000405910. Does using progesterone in threatened miscarriage increase the live birth rate? Supporting Threatened Outcomes with Progesterone (STOP trial). anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000405910 (first received 19 April 2011).
- McLindon L, Beckmann M. Supporting threatened outcomes with progesterone ‐ the stop trial; a protocol. BJOG: an international journal of obstetrics and gynaecology 2015;122(Suppl S1):23. [Google Scholar]
IRCT201012035294N {published data only}
- IRCT201012035294N1. To investigate the possible effect of Progesterone Supp on cytokines endocervical concentration of women at risk of abortion. en.search.irct.ir/view/4741 (first received 17 March 2012).
IRCT2015020217035N2 {published data only}
- IRCT2015020217035N2. Effect of oral progesterone (dydrogestron) on incidence of glucose intolerance in pregnant females with threatened abortion. en.search.irct.ir/view/22059 (first received 19 September 2016).
IRCT2016040627248N1 {published data only}
- IRCT2016040627248N1. The efficacy of a special food (fried egg with grape molasses) on threatened abortion: a randomized controlled trial. en.search.irct.ir/view/29438 (first received 9 July 2016).
NCT015018902011 {published data only}
- NCT01501890. The impact of progesterone treatment on obstetrical outcome among women with first trimester vaginal bleeding. clinicaltrials.gov/show/NCT01501890 (first received 9 September 2011).
NCT02128685 {published data only}
- Chan DM, Cheung KW, Yung SS, Lee VC, Li RH, Ng EH. A randomized double‐blind controlled trial of the use of dydrogesterone in women with threatened miscarriage in the first trimester: study protocol for a randomized controlled trial. Trials 2016;17(1):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02128685. A randomized double‐blind controlled trial of use of dydrogesterone in women with threatened miscarriage in the first trimester. clinicaltrials.gov/show/NCT02128685 (first received 29 April 2014).
NCT02145767 {published data only}
- NCT02145767. Progesterone for the prevention of miscarriage and preterm birth in women with first trimester bleeding: PREEMPT trial. clinicaltrials.gov/show/NCT02145767 (first received 14 May 2014).
NCT02633878 {published data only}
- ChiCTR‐IOR‐15007526. Chinese herbal medicine and micronized progesterone for threatened miscarriage: an international cooperation multicenter randomized controlled trial. chictr.org.cn/showproj.aspx?proj=12677 (first received 12 March 2015).
- NCT02633878. Chinese herbal medicine and micronized progesterone for threatened miscarriage RCT. clinicaltrials.gov/ct2/show/record/NCT02633878 (first received 7 December 2015).
NCT02690129 {published data only}
- NCT02690129. Vaginal progesterone for treatment of threatened miscarriage; randomized clinical trial. clinicaltrials.gov/ct2/show/NCT02690129 (first received 19 February 2016).
NCT02950935 {published data only}
- NCT02950935. Prospective, double‐blind, randomised, placebo controlled, phase iii clinical study assessing the efficacy of natural progesterone 25 mg/bid administered subcutaneously in the maintenance of early pregnancy in women with symptoms of threatened abortion. clinicaltrials.gov/show/NCT02950935 (first received 27 October 2016).
Additional references
Arredondo 2006
- Arredondo F, Noble LS. Endocrinology of recurrent pregnancy loss. Seminars in Reproductive Medicine 2006;24:33‐9. [DOI] [PubMed] [Google Scholar]
Blohm 2008
- Blohm F, Friden B, Milsom I. A prospective longitudinal population‐based study of clinical miscarriage in an urban Swedish population. BJOG: an international journal of obstetrics and gynaecology 2008;115:176‐82. [DOI] [PubMed] [Google Scholar]
Carp 2012
- Carp H. A systematic review of dydrogesterone for the treatment of threatened miscarriage. Gynecological Endocrinology 2012;28(12):983‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Check 2011
- Check JH. A practical approach to the prevention of miscarriage: part 5‐‐antiphospholipid syndrome as a cause of spontaneous abortion. Clinical and Experimental Obstetrics and Gynecology 2011;38:5‐9. [PubMed] [Google Scholar]
Cohain 2017
- Cohain JS, Buxbaum RE, Mankuta D. Spontaneous first trimester miscarriage rates per woman among parous women with 1 or more pregnancies of 24 weeks or more. BMC Pregnancy and Childbirth 2017;17(1):437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coomarasamy 2015
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, et al. A randomized trial of progesterone in women with recurrent miscarriages. New England Journal of Medicine 2015;373(22):2141‐8. [DOI] [PubMed] [Google Scholar]
Cunningham 2001a
- Cunningham FG. Section IX. Reproductive success and failure. Williams Obstetrics. 21st Edition. New York: McGraw‐Hill, 2001. [Google Scholar]
Cunningham 2001b
- Cunningham FG, editor(s). Physiology of pregnancy. Williams Obstetrics. 21st Edition. New York: McGraw‐Hill, 2001. [Google Scholar]
Dabash 2015
- Dabash R, Chelli H, Hajri S, Shochet T, Raghavan S, Winikoff B. A double‐blind randomized controlled trial of mifepristone or placebo before buccal misoprostol for abortion at 14‐21 weeks of pregnancy. International Journal of Gynecology & Obstetrics 2015;130(1):40‐4. [DOI] [PubMed] [Google Scholar]
De La Rochebrochard 2002
- Rochebrochard E, Thonneau P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Human Reproduction 2002;17:1649‐56. [DOI] [PubMed] [Google Scholar]
Dede 2010
- Dede FS, Ulucay U, Kose MF, Dede H, Dilbaz S. Fetal loss in threatened abortion after demonstration of fetal cardiac activity in a low socioeconomic population. Journal of Obstetrics and Gynaecology 2010;30:622‐5. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Di Renzo 2005
- Renzo GC, Rosati A, Mattei A, Gojinic M, Gerli S. The changing role of progesterone in preterm labour. BJOG: an international journal of obstetrics and gynaecology 2005;112 Suppl 1:57‐60. [DOI] [PubMed] [Google Scholar]
Facchinetti 2017
- Facchinetti F, Vergani P, Tommaso M, Marozio L, Acaia B, Vicini R, et al. Progestogens for maintenance tocolysis in women with a short cervix: a randomized controlled trial. Obstetrics and Gynecology 2017;130(1):64‐70. [DOI] [PubMed] [Google Scholar]
Galindo 2006
- Galindo A, Burguillo AG, Azriel S, Fuente P de L. Outcome of fetuses in women with pregestational diabetes mellitus. Journal of Perinatal Medicine 2006;34(4):323‐31. [DOI] [PubMed] [Google Scholar]
GRADE 2013
- Editors: Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADE pro GDT. Version 'date accessed' 12/09/17. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Haas 2018
- Haas DM, Hathaway TJ, Ramsey PS. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database of Systematic Reviews 2018, Issue [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hahn 2014
- Hahn KA, Hatch EE, Rothman KJ, Mikkelsen EM, Brogly SB, Sørensen HT, et al. Body size and risk of spontaneous abortion among Danish pregnancy planners. Paediatric and Perinatal Epidemiology 2014;28(5):412‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heyborne 2016
- Heyborne KD. 17‐α hydroxyprogesterone caproate for the prevention of recurrent preterm birth: one size may not fit all. Obstetrics & Gynecology 2016;128(4):899‐90. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jin 2006
- Jin S, Li SW, Long J, Li L, Tan ZJ. The role of progesterone in human early pregnancy is mediated by insulin‐like growth factors binding protein1‐3. Sichuan Da Xue Xue Bao Yi Xue Ban 2006;37:399‐403. [PubMed] [Google Scholar]
Kalinka 2005
- Kalinka J, Szekeres‐Bartho J. The impact of dydrogesterone supplementation on hormonal profile and progesterone‐induced blocking factor concentrations in women with threatened abortion. American Journal of Reproductive Immunology 2005;53:166‐71. [DOI] [PubMed] [Google Scholar]
Källén 2010
- Källén B, Finnström O, Lindam A, Nilsson E, Nygren KG, Otterblad PO. Congenital malformations in infants born after in vitro fertilization in Sweden. Birth Defects Research. Part A Clinical and Molecular Teratology 2010;88:137‐43. [DOI] [PubMed] [Google Scholar]
Lede 2005
- Lede R, Duley L. Uterine muscle relaxant drugs for threatened miscarriage. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD002857.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Makrydimas 2003
- Makrydimas G, Sebire NJ, Lolis D, Vlassis N, Nicolaides KH. Fetal loss following ultrasound diagnosis of a live fetus at 6‐10 weeks of gestation. Ultrasound in Obstetrics and Gynecology 2003;22:368‐72. [DOI] [PubMed] [Google Scholar]
Marcinko 2011
- Marcinko VM, Marcinko D, Dordevic V, Oreskovic S. Anxiety and depression in pregnant women with previous history of spontaneous abortion. Collegium Antropologicum 2011;35 Suppl 1:225‐8. [PubMed] [Google Scholar]
Mirza 2016
- Mirza FG, Patki A, Pexman‐Fieth C. Dydrogesterone use in early pregnancy. Gynecological Endocrinology 2016;32(2):97‐106. [DOI] [PubMed] [Google Scholar]
Nigro 2011
- Nigro G, Mazzocco M, Mattia E, Renzo GC, Carta G, Anceschi MM. Role of the infections in recurrent spontaneous abortion. Journal of Maternal Fetal Neonatal Medicine 2011;24:983‐9. [DOI] [PubMed] [Google Scholar]
O'Brien 2016
- O'Brien JM, Lewis DF. Prevention of preterm birth with vaginal progesterone or 17‐alpha‐hydroxyprogesterone caproate: a critical examination of efficacy and safety. American Journal of Obstetrics and Gynecology 2016;214(1):45‐56. [DOI] [PubMed] [Google Scholar]
Oler 2017
- Oler E, Eke AC, Hesson A. Meta‐analysis of randomized controlled trials comparing 17α‐hydroxyprogesterone caproate and vaginal progesterone for the prevention of recurrent spontaneous preterm delivery. International Journal of Gynecology & Obstetrics 2017;138(1):12‐6. [DOI] [PubMed] [Google Scholar]
Osmanağaoğlu 2010
- Osmanağaoğlu MA, Erdoğan I, Eminağaoğlu S, Karahan SC, Ozgün S, Can G, et al. The diagnostic value of beta‐human chorionic gonadotropin, progesterone, CA125 in the prediction of abortions. Journal of Obstetrics and Gynaecology 2010;30:288‐93. [DOI] [PubMed] [Google Scholar]
Owen 2004
- Owen J, Yost N, Berghella V, MacPherson C, Swain M, Dildy GA 3rd, et al. Can shortened midtrimester cervical length predict very early spontaneous preterm birth?. American Journal of Obstetrics and Gynecology 2004;191(1):298‐303. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rocchetti 2011
- Rocchetti TT, Marconi C, Rall VL, Borges VT, Corrente JE, Silva MG. Group B streptococci colonization in pregnant women: risk factors and evaluation of the vaginal flora. Archives of Gynecology and Obstetrics 2011;283:717‐21. [DOI] [PubMed] [Google Scholar]
Rust 2005
- Rust OA, Atlas RO, Kimmel S, Roberts WE, Hess LW. Does the presence of a funnel increase the risk of adverse perinatal outcome in a patient with a short cervix?. American Journal of Obstetrics and Gynecology 2005;192(4):1060‐6. [DOI] [PubMed] [Google Scholar]
Saccone 2017
- Saccone G, Schoen C, Franasiak JM, Scott RT Jr, Berghella V. Supplementation with progestogens in the first trimester of pregnancy to prevent miscarriage in women with unexplained recurrent miscarriage: a systematic review and meta‐analysis of randomized, controlled trials. Fertility and Sterility 2017;107(2):430‐8. [DOI] [PubMed] [Google Scholar]
Saraswat 2010
- Saraswat L, Bhattacharya S, Maheshwari A, Bhattacharya S. Maternal and perinatal outcome in women with threatened miscarriage in the first trimester: a systematic review. BJOG: an international journal of obstetrics and gynaecology 2010;117:245‐57. [DOI] [PubMed] [Google Scholar]
Silver 1999
- Silver RI, Rodriguez R, Chang TS, Gearhart JP. In vitro fertilization is associated with an increased risk of hypospadias. Journal of Urology 1999;161(6):1954‐7. [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sugiura‐Ogasawara 2009
- Sugiura‐Ogasawara M, Ozaki Y, Kitaori T, Suzumori N, Obayashi S, Suzuki S. Live birth rate according to maternal age and previous number of recurrent miscarriages. American Journal of Reproductive Immunology 2009;62:314‐9. [DOI] [PubMed] [Google Scholar]
Suzumori 2010
- Suzumori N, Sugiura‐Ogasawara M. Genetic factors as a cause of miscarriage. Current Medicinal Chemistry 2010;17:3431‐7. [DOI] [PubMed] [Google Scholar]
Szekeres‐Bartho 2008
- Szekeres‐Bartho J, Wilczynski JR, Basta P, Kalinka J. Role of progesterone and progestin therapy in threatened abortion and preterm labour. Frontiers in Bioscience 2008;13:1981‐90. [DOI] [PubMed] [Google Scholar]
Szekeres‐Bartho 2009
- Szekeres‐Bartho J. Progesterone‐mediated immunomodulation in pregnancy: its relevance to leukocyte immunotherapy of recurrent miscarriage. Immunotherapy 2009;1:873‐82. [DOI] [PubMed] [Google Scholar]
Szekeres‐Bartho 2010
- Szekeres‐Bartho J, Polgar B. PIBF: the double edged sword. Pregnancy and tumor. American Journal of Reproductive Immunology 2010;64(2):77‐86. [DOI] [PubMed] [Google Scholar]
Temmerman 1992
- Temmerman M, Lopita MI, Sanghvi HC, Sinei SK, Plummer FA, Piot P. The role of maternal syphilis, gonorrhoea and HIV‐1 infection in spontaneous abortion. International Journal of Sexually Transmitted Diseases and AIDS 1992;3:418‐22. [DOI] [PubMed] [Google Scholar]
Vorsanova 2005
- Vorsanova SG, Kolotii AD, Iourov IY, Monakhov VV, Kirillova EA, Soloviev IV, et al. Evidence for high frequency of chromosomal mosaicism in spontaneous abortions revealed by interphase FISH analysis. Journal of Histochemistry and Cytochemistry 2005;53:375‐80. [DOI] [PubMed] [Google Scholar]
World Bank 2017
- World Bank. World Bank list of economies. iccmoot.com/wp‐content/uploads/2017/07/World‐Bank‐List‐of‐Economies.pdf.
Zegers‐Hochschild 2009
- Zegers‐Hochschild F, Adamson GD, De MJ, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology. Fertility and Sterility 2009;92:1520‐4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wahabi 2011
- Wahabi HA, Fayed AA, Esmaeil SA, Al Zeidan RA. Progestogen for treating threatened miscarriage. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD005943.pub4] [DOI] [PubMed] [Google Scholar]


