Abstract
Background
The presence of deleterious mutations in breast cancer 1 gene (BRCA1) or breast cancer 2 gene (BRCA2) significantly increases the risk of developing some cancers, such as breast and high‐grade serous cancer (HGSC) of ovarian, tubal and peritoneal origin. Risk‐reducing salpingo‐oophorectomy (RRSO) is usually recommended to BRCA1 or BRCA2 carriers after completion of childbearing. Despite prior systematic reviews and meta‐analyses on the role of RRSO in reducing the mortality and incidence of breast, HGSC and other cancers, RRSO is still an area of debate and it is unclear whether RRSO differs in effectiveness by type of mutation carried.
Objectives
To assess the benefits and harms of RRSO in women with BRCA1 or BRCA2 mutations.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 7) in The Cochrane Library, MEDLINE Ovid, Embase Ovid and trial registries, with no language restrictions up to July 2017. We handsearched abstracts of scientific meetings and other relevant publications.
Selection criteria
We included non‐randomised trials (NRS), prospective and retrospective cohort studies, and case series that used statistical adjustment for baseline case mix using multivariable analyses comparing RRSO versus no RRSO in women without a previous or coexisting breast, ovarian or fallopian tube malignancy, in women with or without hysterectomy, and in women with a risk‐reducing mastectomy (RRM) before, with or after RRSO.
Data collection and analysis
We extracted data and performed meta‐analyses of hazard ratios (HR) for time‐to‐event variables and risk ratios (RR) for dichotomous outcomes, with 95% confidence intervals (CI). To assess bias in the studies, we used the ROBINS‐I 'Risk of bias' assessment tool. We quantified inconsistency between studies by estimating the I2 statistic. We used random‐effects models to calculate pooled effect estimates.
Main results
We included 10 cohort studies, comprising 8087 participants (2936 (36%) surgical participants and 5151 (64%) control participants who were BRCA1 or BRCA2 mutation carriers. All the studies compared RRSO with or without RRM versus no RRSO (surveillance). The certainty of evidence by GRADE assessment was very low due to serious risk of bias. Nine studies, including 7927 women, were included in the meta‐analyses. The median follow‐up period ranged from 0.5 to 27.4 years.
Main outcomes: overall survival was longer with RRSO compared with no RRSO (HR 0.32, 95% CI 0.19 to 0.54; P < 0.001; 3 studies, 2548 women; very low‐certainty evidence). HGSC cancer mortality (HR 0.06, 95% CI 0.02 to 0.17; I² = 69%; P < 0.0001; 3 studies, 2534 women; very low‐certainty evidence) and breast cancer mortality (HR 0.58, 95% CI 0.39 to 0.88; I² = 65%; P = 0.009; 7 studies, 7198 women; very low‐certainty evidence) were lower with RRSO compared with no RRSO. None of the studies reported bone fracture incidence. There was a difference in favour of RRSO compared with no RRSO in terms of ovarian cancer risk perception quality of life (MD 15.40, 95% CI 8.76 to 22.04; P < 0.00001; 1 study; very low‐certainty evidence). None of the studies reported adverse events.
Subgroup analyses for main outcomes: meta‐analysis showed an increase in overall survival among women who had RRSO versus women without RRSO who were BRCA1 mutation carriers (HR 0.30, 95% CI 0.17 to 0.52; P < 0001; I² = 23%; 3 studies; very low‐certainty evidence) and BRCA2 mutation carriers (HR 0.44, 95% CI 0.23 to 0.85; P = 0.01; I² = 0%; 2 studies; very low‐certainty evidence). The meta‐analysis showed a decrease in HGSC cancer mortality among women with RRSO versus no RRSO who were BRCA1 mutation carriers (HR 0.10, 95% CI 0.02 to 0.41; I² = 54%; P = 0.001; 2 studies; very low‐certainty evidence), but uncertain for BRCA2 mutation carriers due to low frequency of HGSC cancer deaths in BRCA2 mutation carriers. There was a decrease in breast cancer mortality among women with RRSO versus no RRSO who were BRCA1 mutation carriers (HR 0.45, 95% CI 0.30 to 0.67; I² = 0%; P < 0.0001; 4 studies; very low‐certainty evidence), but not for BRCA2 mutation carriers (HR 0.88, 95% CI 0.42 to 1.87; I² = 63%; P = 0.75; 3 studies; very low‐certainty evidence). One study showed a difference in favour of RRSO versus no RRSO in improving quality of life for ovarian cancer risk perception in women who were BRCA1 mutation carriers (MD 10.70, 95% CI 2.45 to 18.95; P = 0.01; 98 women; very low‐certainty evidence) and BRCA2 mutation carriers (MD 13.00, 95% CI 3.59 to 22.41; P = 0.007; very low‐certainty evidence). Data from one study showed a difference in favour of RRSO and RRM versus no RRSO in increasing overall survival (HR 0.14, 95% CI 0.02 to 0.98; P = 0.0001; I² = 0%; low‐certainty evidence), but no difference for breast cancer mortality (HR 0.78, 95% CI 0.51 to 1.19; P = 0.25; very low‐certainty evidence). The risk estimates for breast cancer mortality according to age at RRSO (50 years of age or less versus more than 50 years) was not protective and did not differ for BRCA1 (HR 0.85, 95% CI 0.64 to 1.11; I² = 16%; P = 0.23; very low‐certainty evidence) and BRCA2 carriers (HR 0.88, 95% CI 0.42 to 1.87; I² = 63%; P = 0.75; very low‐certainty evidence).
Authors' conclusions
There is very low‐certainty evidence that RRSO may increase overall survival and lower HGSC and breast cancer mortality for BRCA1 and BRCA2 carriers. Very low‐certainty evidence suggests that RRSO reduces the risk of death from HGSC and breast cancer in women with BRCA1 mutations. Evidence for the effect of RRSO on HGSC and breast cancer in BRCA2 carriers was very uncertain due to low numbers. These results should be interpreted with caution due to questionable study designs, risk of bias profiles, and very low‐certainty evidence. We cannot draw any conclusions regarding bone fracture incidence, quality of life, or severe adverse events for RRSO, or for effects of RRSO based on type and age at risk‐reducing surgery. Further research on these outcomes is warranted to explore differential effects for BRCA1 or BRCA2 mutations.
Keywords: Adult; Female; Humans; Middle Aged; Genes, BRCA1; Genes, BRCA2; Breast Neoplasms; Breast Neoplasms/mortality; Breast Neoplasms/prevention & control; Heterozygote; Mastectomy; Mastectomy/methods; Mutation; Mutation/genetics; Ovarian Neoplasms; Ovarian Neoplasms/mortality; Ovarian Neoplasms/prevention & control; Quality of Life; Salpingo‐oophorectomy; Salpingo‐oophorectomy/adverse effects; Salpingo‐oophorectomy/mortality; Salpingo‐oophorectomy/statistics & numerical data
Plain language summary
Risk‐reducing surgical removal of fallopian tubes and ovaries in women with mutations in BRCA1 or BRCA2 genes
Background Mutations in the breast cancer 1 gene (BRCA1) or breast cancer 2 gene (BRCA2) increase the risk of developing some cancers including breast, ovarian, tubal and peritoneal cancers. Risk‐reducing salpingo‐oophorectomy (RRSO) (removal of both fallopian tubes and ovaries) is usually offered to women with BRCA1, BRCA2 or both mutations after they have finished their childbearing. However, how much of a reduction in risk of breast and high‐grade serous cancer (HGSC) of fallopian tube, ovarian and primary peritoneal origin RRSO offers, and the effect on other health outcomes, are still uncertain and it is unclear whether RRSO differs in effectiveness by type of mutation.
Review question Does RRSO in women with mutations in BRCA1 or BRCA2 genes reduce the risk of developing breast and HGSC and what effect does this have on risk of death (overall survival) and quality of life?
Study characteristics In this review, we analysed data from 10 non‐randomised (cohort; a study in which a defined group of people (the cohort) is followed over time, to examine associations between different treatments received and subsequent outcomes) studies. All the studies compared RRSO with or without risk‐reducing mastectomy (RRM; surgical removal of breasts) versus no RRSO (surveillance). The evidence is current to July 2017.
Main findings Including data from both BRCA1 and BRCA2 mutation carriers, this analysis found that RRSO may improve overall survival, and reduce deaths from HGSC and breast cancer. When analysed by mutated gene, there was evidence for a reduction in risk of HGSC and breast cancer for women with BRCA1 mutations, but may or may not have been an effect on women with BRCA2 mutations due to low numbers of women with these mutations in the studies. None of the studies reported on bone fracture or severe side effects. Both RRSO and RRM may have improved overall survival, but did not reduce deaths from breast cancer. There was no protection and differences for breast cancer mortality according to age at RRSO (50 years of age or less versus more than 50 years) in BRCA1 or BRCA2 carriers. RRSO may have improved quality of life with regard to ovarian cancer risk perception.
Reliability of the evidence The reliability of the evidence was low to very low due to the small numbers of participants and low methodological quality of included studies.
What are the conclusions? RRSO in women with BRCA1 or BRCA2 mutations may improve overall survival and may reduce the number of HGSC and breast cancer deaths when women with mutations in both genes were combined. RRSO may reduce the risk of death from HGSC and breast cancer in women with BRCA1 mutations, but may or may not reduce the risk for BRCA2 mutation carriers. These results should be interpreted with caution due to low quality of study designs and risk of bias profiles. We cannot make any conclusions regarding number of bone fracture, overall quality of life, severe side effects for RRSO and effects of RRSO based on type of risk‐reducing surgery and age at the time of RRSO. However, we found the reliability of the evidence to be very low, so there is still a need for large, high‐quality studies which should specifically look at these outcomes for differences in BRCA1 or BRCA2 carriers.
Summary of findings
Summary of findings for the main comparison. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers.
| RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers | ||||||
|
Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and USA Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers | |||||
| Overall survival: BRCA1 or BRCA2 Follow‐up: median 0.5–27.4 years | Study population | HR 0.32 (0.19 to 0.54) | 2548 (3 studies) | ⊕⊝⊝⊝ Very lowa | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| HGSC mortality: BRCA1 or BRCA2 Follow‐up: median 0.5–27 years | Study population | HR 0.06 (0.02 to 0.17) | 2534 (3 studies) | ⊕⊝⊝⊝ Very lowa | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: BRCA1 or BRCA Follow‐up: median 0.5–27 years | Study population | HR 0.58 (0.39 to 0.88) | 7198 (7 studies) | ⊕⊝⊝⊝ Very lowa | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Bone fracture incidence Follow‐up: median 0.5–27 years | See comment | See comment | Not estimable | 0 (0) | See comment | No studies reported this outcome. |
| Quality of life (ovarian cancer risk perception): BRCA1 or BRCA2 Follow‐up: mean 1 years | See comment | See comment | Not estimable | 200 (1 study) | ⊕⊝⊝⊝ Very lowa | Unable to perform meta‐analysis as only 1 study reported the outcome. |
| Quality of life (breast cancer risk perception): BRCA1 or BRCA2 Follow‐up: mean 1 years | See comment | See comment | Not estimable | 200 (1 study) | ⊕⊝⊝⊝ Very lowa | Unable to perform meta‐analysis as only 1 study reported the outcome. |
| Severe adverse events Follow‐up: mean 1 years | See comment | See comment | Not estimable | 0 (0) | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; CI: confidence interval; HGSC: high‐grade serous cancer; HR: hazard ratio; RRSO: risk‐reducing salpingo‐oophorectomy. | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies.
Summary of findings 2. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status.
| RRSO vs no RRSO according to BRCA mutation status | ||||||
|
Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and USA Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO according to BRCA mutation status | |||||
| Overall survival: BRCA1 only Follow‐up: median 0.5–27 years | Study population | HR 0.30 (0.17 to 0.52) | 2548 (3 studies) | ⊕⊝⊝⊝ Very lowa | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Overall survival: BRCA2 only Follow‐up: median 0.5–27 years | Study population | HR 0.44 (0.23 to 0.85) | 2122 (2 studies) | ⊕⊝⊝⊝ Very lowa | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| HGSC mortality: BRCA1 only Follow‐up: median 0.5–27 years | Study population | HR 0.1 (0.02 to 0.41) | 1983 (2 studies) | ⊕⊝⊝⊝ Very lowa | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| HGSC mortality: BRCA2 only Follow‐up: median 0.5–27 years | See commentb | See commentb | Not estimable See commentc |
1983 (2 studies) | ⊕⊝⊝⊝ Very lowa |
bAs a result of the way HRs were calculated, assumed and corresponding risks were not estimated. cUnable to perform meta‐analysis as no mortality events were recorded in any study and HRs could not be estimated. |
| Breast cancer mortality: BRCA1 only Follow‐up: median 0.5–27 years | Study population | HR 0.45 (0.30 to 0.67) | 2203 (4 studies) | ⊕⊝⊝⊝ Very lowa | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: BRCA2 only Follow‐up: median 0.5–27 years | Study population | HR 0.88 (0.42 to 1.87) | 5882 (3 studies) | ⊕⊝⊝⊝ Very lowa | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| Quality of life (ovarian cancer risk perception): BRCA1 only Follow‐up: mean 1 years | See comment | See comment | Not estimable | 98 (1 study) | ⊕⊝⊝⊝ Very lowa | Unable to perform meta‐analysis as only 1 study reported the outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; CI: confidence interval; HR: hazard ratio; RRSO: risk‐reducing salpingo‐oophorectomy. | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies.
Summary of findings 3. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery.
| RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery | ||||||
|
Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and USA Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery | |||||
| Overall survival: RRSO alone vs RRSO and RRM Follow‐up: median 0.5–27 years | See comment | See comment | Not estimable | 0 (0) | See comment | No studies reported this outcome. |
| Overall survival: RRSO and RRM vs no RRSO Follow‐up: median 0.5–27 years | Study population | HR 0.14 (0.02 to 0.98) | 261 (1 study) | ⊕⊝⊝⊝ Very lowa | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: RRSO alone vs RRSO and RRM Follow‐up: median 0.5–27 years | See comment | See comment | Not estimable | 0 (0) | See comment | No studies reported this outcome. |
| Breast cancer mortality: RRSO and RRM vs no RRSO Follow‐up: median 0.5–27 years | See comment | See comment | Not estimable | 722 (1 study) | ⊕⊝⊝⊝ Very lowa | As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. |
| Bone fracture incidence | See comment | See comment | Not estimable | 0 (0) | See comment | No studies reported this outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 (0) | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; CI: confidence interval; HR: hazard ratio; RRM: risk‐reducing mastectomy; RRSO: risk‐reducing salpingo‐oophorectomy. | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to confounding and bias due in selection of participants in the study).
Summary of findings 4. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 mutation carriers according to age at RRSO.
| RRSO vs no RRSO in BRCA1 mutation carriers according to age at RRSO | ||||||
|
Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and America Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA1 mutation carriers according to age at RRSO | |||||
| Overall survival | See comment | See comment | Not estimable | 0 (0) | See comment | No studies reported this outcome. |
| HGSC mortality | See comment | See comment | Not estimable | 0 (0) | See comment | No studies reported this outcome. |
| Breast cancer mortality: ≤ 50 years Follow‐up: median 3.1–6.8 years | Study population | HR 0.78 (0.55 to 1.09) | 4566 (3 studies) | ⊕⊝⊝⊝ Very lowa,b |
As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: > 50 years Follow‐up: median 3.1–6.8 years | Study population | HR 1.27 (0.67 to 2.38) | 4566 (3 studies) | ⊕⊝⊝⊝v Very lowa,b |
As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Bone fracture incidence | See comment | See comment | Not estimable | 0 (0) | See comment | No studies reported this outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 (0) | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; CI: confidence interval; HGSC: high‐grade serous cancer; HR: hazard ratio; RRSO: risk‐reducing salpingo‐oophorectomy. | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies.
bDowngraded by one level for serious imprecision: the confidence intervals overlapped 1 and either 0.75 or 1.25 or both (i.e. wide confidence intervals in all included studies, which crossed the line of unity).
Summary of findings 5. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA2 mutation carriers according to age at RRSO.
| RRSO versus no RRSO in BRCA2 mutation carriers according to age at RRSO | ||||||
|
Participants: women with BRCA1 or BRCA2 mutation carriers Settings: hospitals in Europe and America Intervention: RRSO with or without risk‐reducing mastectomy Comparison: no RRSO or surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RRSO vs no RRSO in BRCA2 mutation carriers according to age at RRSO | |||||
| Overall survival | See comment | See comment | Not estimable | 0 (0) | See comment | No studies reported this outcome. |
| HGSC mortality | See comment | See comment | Not estimable | 0 (0) | See comment | No studies reported this outcome. |
| Breast cancer mortality: ≤ 50 years Follow‐up: mean 3.1–6.8 years | Study population | HR 0.49 (0.08 to 2.9) | 444 (2 studies) | ⊕⊝⊝⊝ Very lowa,b |
As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Breast cancer mortality: > 50 years Follow‐up: mean 3.1–6.8 years | Study population | HR 1.36 (0.68 to 2.75) | 444 (2 studies) | ⊕⊝⊝⊝ Very lowa,b |
As a result of the way HRs were calculated, assumed and corresponding risks were not estimated. | |
| See comment | See comment | |||||
| Moderate | ||||||
| — | — | |||||
| Bone fracture incidence | See comment | See comment | Not estimable | 0 (0) | See comment | No studies reported this outcome. |
| Severe adverse events | See comment | See comment | Not estimable | 0 (0) | See comment | No studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; CI: confidence interval; HGSC: high‐grade serous cancer; HR: hazard ratio; RRSO: risk‐reducing salpingo‐oophorectomy. | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded by one level for serious risk of bias: there was overall moderate risk of bias (bias due to selection of participants into the study and bias due to missing data) in all the studies.
bDowngraded by one level for serious imprecision: the confidence intervals overlapped 1 and either 0.75 or 1.25 or both (i.e. wide confidence intervals in all included studies, which cross the line of unity).
Background
Description of the condition
Ovarian cancer is the fifth most common type of cancer, and the fourth most common cause of cancer mortality, in women (ESMO 2013; Gottschau 2016). Globally approximately 204,000 women are diagnosed with ovarian cancer each year, of whom nearly 115,000 die from their disease, with an incidence rate of 6.1/100,000 and a mortality rate of 3.8/100,000 (IARC 2012; Ozols 2006). The estimated lifetime risk for a woman developing ovarian cancer is about 1/54 (ESMO 2013). The incidence of ovarian cancer increases with age and is most prevalent in postmenopausal women, with a median age of 63 years at the time of diagnosis (McGuire 2016; NCCN 2014). Women with early‐stage disease have few or vague symptoms, which may contribute to their late presentation (Ang 2011; NCCN 2014). More than 70% of women present with advanced disease, and less than 40% of women with ovarian cancer in the USA survive more than five years following diagnosis (NCCN 2014), but more than 40% survive in European populations (Gottschau 2016).
Studies have shown that the presence of deleterious mutations in the breast cancer 1 gene (BRCA1) or breast cancer 2 gene (BRCA2) increases the risk of development of various cancers including breast and high‐grade serous cancer (HGSC) (Eccles 2016; Guidozzi 2016; Iavazzo 2016). BRCA1 and BRCA2 are separate genes that map onto two different chromosomes, 17q21 and 13q12.3, respectively (Girolimetti 2014; Staples 2013). They have distinctive primary sequences although interruption of either BRCA gene leads to comparable pathophysiological effects, in addition to similar cancer spectra. BRCA1 and BRCA2 are tumour suppressor genes for DNA repair. In addition to, and as part of, their roles as tumour suppressor genes, BRCA1 and BRCA2 are involved in homologous DNA repair, genomic stability, transcriptional regulation, protein ubiquitination, chromatin remodeling and cell cycle control (Iodice 2010; Tutt 2002; Venkitaraman 2014). Loss of BRCA function results in development of chromosomal instability (Tutt 2002; Venkitaraman 2014).
BRCA gene mutations only account for a small fraction of the overall breast and ovarian cancers. Approximately 1/300 to 1/800 women carry the mutations in the general population (ACOG 2009). In a more recent prospective cohort study involving participants mainly from large national studies in the UK, the Netherlands and France, the breast or ovarian cancer incidences were reported to be 44% in BRCA1 mutation carriers and 17% in BRCA2 mutation carriers (Kuchenbaecker 2017). The overall frequencies of the BRCA1 and BRCA2 mutations were 10.2% in breast cancer Arabic women and 30.7% in ovarian cancer Arabic women (Alhuqail 2018). Two studies found that BRCA1 and BRCA2 mutations represent 10% to 15% of all ovarian cancers (Pal 2005; Risch 2001).
Specific mutations in the BRCA1 or BRCA2 gene occur more frequently in certain populations, including Ashkenazi Jews, French Canadians and Icelanders (Hartge 1999; Lynch 2013). The lifetime risk of ovarian cancer for a woman is 39% to 46% with a BRCA1 mutation and 12% to 20% with a BRCA2 mutation, and the risk of breast cancer for a woman with a BRCA1 or BRCA2 mutation is 65% to 74% (Girolimetti 2014; Meaney‐Delman 2013).
In women with BRCA1 mutations, less than 2% to 3% of carriers develop ovarian cancer by the age of 40 years. This increases to 10% to 21% by the age of 50 years. In women with BRCA2 mutations, less than 3% of carriers develop ovarian cancer by the age of 50 years. However, 26% to 34% of these women appear to develop breast cancer by the age of 50 years (Ford 1998; King 2003; Rebbeck 2002; Satagopan 2002; Struewing 1997). Therefore, recommendations have been made that women with BRCA1/2 mutations should be offered risk‐reducing salpingo‐oophorectomy (RRSO) by the age of 40 years or when childbearing is complete (ACOG 2009). Estimates of the frequency of fallopian tube cancer in BRCA mutation carriers are limited by the lack of precision in the assignment of site of origin for high‐grade, metastatic, serous carcinomas at initial presentation (Lengyel 2013).
BRCA‐positive women with ovarian cancer have a better prognosis than controls (women who are BRCA1 or BRCA2 mutation negative) in terms of overall survival due to greater chemosensitivity of BRCA‐positive tumours (Biglia 2016). The histopathology of ovarian cancers associated with BRCA1 and BRCA2 mutations is predominantly high‐grade serous and endometrioid carcinomas, rather than mucinous and borderline tumours (ACOG 2009). Primary peritoneal cancer is an aggressive malignancy which, due to the absence of a specific screening test, cannot be diagnosed in its early stages (Iavazzo 2016). Studies have suggested that many ovarian and primary peritoneal cancers may be of tubal origin, and therefore part of the spectrum of disease associated with these mutations, collective known as HGCS (Callahan 2007; NCCN 2014). Collaborative efforts to devise international guidelines around BRCA1 and BRCA2 testing in ovarian cancer and other cancers to ensure consistent screening practices are needed (Arts‐de Jong 2016; Karakasis 2016; Lheureux 2016).
Description of the intervention
Prophylactic RRSO refers to the surgical removal of both fallopian tubes and ovaries in women not thought to have cancer before the surgical procedure, but who have a high lifetime risk (Rebbeck 2009; Shu 2016). The specific protocol for RRSO for high‐risk women involves exploring the pelvic organs for any evidence of cancer, performing a peritoneal wash (the pelvis is bathed in saline and fluid collected to look for any cancer cells that may be free in the abdominal cavity), and removal of the ovaries and fallopian tubes in their entirety. The 'Intensive' RRSO protocol includes: bilateral salpingo‐oophorectomy and removal of entire length of the fallopian tubes, cytological examination of peritoneal washings, and random peritoneal and omental biopsies (Powell 2014; Ready 2011). If there are adhesions between the pelvic side wall peritoneum and ovary, care must be taken not to fracture them (Dowdy 2004). It is recommended that the adhesions will be resected along with the ovary with the use of a retroperitoneal approach (Dowdy 2004). This is necessary to prevent ovarian remnant syndrome (Dowdy 2004). More important, any residual ovarian cells have a high likelihood of undergoing malignant transformation.
Microscopic (occult) cancer of the ovary or fallopian tube might be identified following RRSO and proportionally more fallopian tube cancers have been detected than ovarian cancers following prophylactic surgery (Powell 2005). One study in 122 BRCA‐mutation positive women undergoing RRSO detected occult cancers in 6% at the time of surgery; all originating within the fallopian tubes (Callahan 2007). This study suggests that much of the 'ovarian' cancer in BRCA carriers may begin in the fallopian tubes. Therefore, it is important to remove the tubes in BRCA‐mutation carriers and to perform 'serial sectioning' of the fallopian tubes to exclude occult cancers or serous intraepithelial tubal carcinomas (STIC). In the SEE‐FIM protocol (Sectioning and Extensively Examining of the Fimbriated end), the greatest surface area of the tube is histologically examined, based on the suggestion that multiple deeper sections should be examined, if the initial haematoxylin and eosin (H&E) sections are negative. In one study, the single H&E section, compared to the SEE‐FIM approach, detected only 75% (95% confidence interval (CI) 51% to 90%) of STIC (Mahe 2013). The SEE‐FIM protocol should be considered especially in cases of endometrial carcinoma, non‐uterine pelvic serous cancers or serous borderline ovarian tumours (Crum 2007; Koc 2018; Leonhardt 2011).
Laparoscopy is the preferred method for performing a RRSO (Blok 2016), due to a lower morbidity than laparotomy. Although hysterectomy is not a part of risk‐reducing surgery for BRCA1/2 mutations, it could theoretically reduce risk of cancer in the cornual fallopian tube (Karlan 2004). Hysterectomy may be considered for other potential medical indications, or for women taking tamoxifen to reduce risk of endometrial cancer (ACOG 2009). However, most clinicians view the role of synchronous hysterectomy as controversial (Lee 2017a; Saule 2018; Segev 2013; Shu 2016), as the risk of endometrial cancer in women with hereditary breast and ovarian cancer is not significantly elevated (Lee 2017a; Segev 2013), although the authors of Shu 2016 reported an increased risk of serous endometrial carcinoma.
The potential adverse effects of RRSO are associated surgical morbidity and premature menopause in younger women (Bober 2015). Apart from significant menopausal symptoms, RRSO could lead to increased risk for bone mineral loss (osteopenia and osteoporosis) and cognitive dysfunction (Guidozzi 2016). Risk for cardiovascular disease is also increased, if the procedure is performed in women under 50 years of age (Guidozzi 2016). It is important for women who have undergone surgical menopause, or who are considering RRSO, to discuss menopausal symptoms and management with their healthcare team. Studies have found that short‐term hormone replacement therapy (HRT) use does not negate the protective effect of salpingo‐oophorectomy on subsequent breast cancer risk in BRCA1/2 mutation carriers until the time of expected natural menopause at about age 50 years (Armstrong 2004; Rebbeck 2005).
In women who do not also have risk‐reducing mastectomy, there is growing concern regarding the possible adverse effect on the risk of breast cancer associated with the use of a combination of oestrogen and progesterone, especially among younger women who would use the agents for more than 10 years. Because of the theoretical increased risk of breast cancer associated with combined treatment with oestrogen and progesterone HRT (compared with oestrogen‐only HRT), the Society of Gynecologic Oncology suggests the use of a progestin‐containing intrauterine device to accompany oestrogen replacement and thus avoid the administration of systemic therapy with progestin (Hartmann 2015; Hartmann 2016; Walker 2015). However, performing bilateral risk‐reducing mastectomy (BRRM) may lead to a highly significant risk reduction of breast cancer in BRCA1 and BRCA2 mutation carriers (De Felice 2015). The risk reduction of breast cancer is estimated to be 94% to 95% when BRRM is performed, nearly 89% in women who received BRRM plus RRSO, and 46% when RRSO alone was carried out, suggesting that RRSO alone cannot replace the beneficial impact of BRRM in breast cancer occurrence (De Felice 2015). This information may allow clinicians to discuss all the available options with women in order to design individual management strategies.
How the intervention might work
RRSO may reduce the risk for ovarian and fallopian tube cancers by 85% to 90% and for breast cancer by 40% to 70% in women with known BRCA1/2 mutations (ACOG 2009; Finch 2014). Additionally, risk‐reducing strategies have been shown to have associations with a gain in life expectancy in BRCA1/2 carriers (Salhab 2010). Previously, ovarian cancers were believed to develop from the lining of the ovary, as a result of the constant rupture and repair process during ovulation. More recent studies suggest that many ovarian cancers in BRCA gene mutation carriers originate in the distal fallopian tube (part of the tube closest to the ovary), causing researchers to question whether salpingectomy alone (removal of the fallopian tubes) might reduce ovarian cancer risk. A candidate precursor to tubal intraepithelial carcinoma, entitled the 'p53 signature,' suggests that molecular events associated with serous cancer (p53 mutations) may be detected in benign mucosa (Crum 2007; Leonhardt 2011).
Current expert guidelines recommend that women with BRCA mutations should be offered RRSO between the ages of 35 and 40 years or after childbearing is completed. Ovaries secrete the hormones that control the reproductive cycle. Surgical removal of ovaries will substantially reduce the levels of the hormones oestrogen and progesterone that circulate in the body (Metcalfe 2015; Olivier 2004). Bilateral salpingo‐oophorectomy can halt or slow breast cancers that need these hormones to grow (van Verschuer 2014). Some studies have suggested that the level of breast cancer risk reduction may differ between BRCA1 and BRCA2 carriers who choose RRSO (Powell 2011; Powell 2014; van Verschuer 2014). Kauff 2008 reported from a multicentre study, that women with BRCA2 mutations who had RRSO lowered their risk for breast cancer by 72%. Risk reduction was less (about 29%) for women with BRCA1 mutations. Kauff 2008 suggested that oophorectomy may be more protective for women with BRCA2 mutations, since their breast cancers are more likely to be hormone receptor‐positive, while breast cancers in BRCA1 mutation carriers are usually hormone receptor‐negative (van Verschuer 2014; Veronesi 2005). Overall, their risk of dying from breast cancer is reduced by 56% with BRCA1/2 mutation carriers who had oophorectomy (Domchek 2010). Since breast tumours are largely oestrogen‐driven, it has been suggested that the hormonal blockade by oophorectomy inhibits the development of breast tumours (Narod 2001). Thus, prophylactic oophorectomy may have the advantage of reducing the risk of breast cancer, as well as ovarian cancer (Mitrunen 2003). Breast cancer risk reduction in BRCA‐mutation carriers who undergo RRSO may extend beyond women under 50 years of age (the mean age of menopause), but some studies have suggested a benefit for breast cancer risk reduction in women who underwent RRSO after the menopause. Barlin 2013 reported that 199 postmenopausal BRCA‐mutation carriers who received RRSO postmenopausally had a 57% reduction in breast cancer risk. Barlin 2013 hypothesised that, although the ovaries stop producing oestrogen and progesterone after natural menopause, they continue producing some hormones, including testosterone, which might explain why RRSO after menopause still has protective effects against breast cancer.
Why it is important to do this review
In women at increased risk, due to a family history or confirmed mutation in high penetrance genes, such as BRCA1/2, annual screening with CA125 using a cut‐off and transvaginal ultrasound scan did not detect early‐stage cancers (Hermsen 2007; Stirling 2005). This was reconfirmed by the UK Familial Ovarian Cancer Screening Study (UKFOCSS) (Rosenthal 2013a; Rosenthal 2013b). Similarly, a large randomised trial (the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial) found that screening did not decrease mortality from ovarian cancer (Pinsky 2013). While the results of the phase II study were encouraging, screening at present cannot be considered a safe alternative to RRSO. As surveillance for ovarian, peritoneal and fallopian tube cancer has not been proven to be effective, RRSO has been widely adopted as a key component of breast and gynaecological cancer risk‐reduction in women with BRCA1 or BRCA2 mutations (Girolimetti 2014). The risk of breast cancer can be reduced either with risk‐reducing oophorectomy or mastectomy, or both (Maeshima 2016). Although some authors have shown that fallopian tubes may be the cause of many gynaecological cancers in mutation carriers, researchers caution that there is not enough evidence to suggest that all ovarian cancer cases start in the fallopian tubes (Kramer 2013). Also removing only the fallopian tubes is not likely to lower the risk for breast cancer. More research is needed to completely understand the role of the fallopian tubes in the development of these cancers. Although previous non‐systematic reviews (Calderon‐Margalit 2004; Domchek 2007; Dowdy 2004; Oliver 2015; Olopade 2004; Salhab 2010; Schenberg 2014), systematic reviews (Ludwig 2016; Marchetti 2014; Tschernichovsky 2017), and meta‐analysis (Rebbeck 2009), or both (Li 2016), have been published on the benefit of RRSO in women with BRCA1 or BRCA2 mutations, its role in reducing the incidence of breast, ovarian, fallopian and other cancers, including other health outcomes are uncertain (De Felice 2017; Fakkert 2015; Heemskerk‐Gerritsen 2015a). A Cochrane systematic review is needed to assess the efficacy and adverse effects of RRSO in women with BRCA1 or BRCA2 mutations.
Objectives
To assess the benefits and harms of RRSO in women with BRCA1 or BRCA2 mutations.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs (studies where participant allocation or enrolment is open to systematic bias/errors, as all participants do not have an equal chance of being in one group or the other) were unlikely or not possible due to ethical reasons. Therefore, we examined the following types of studies.
Non‐randomised studies (NRS), prospective and retrospective cohort studies, and case series (all with concurrent comparison groups).
To minimise selection bias, we only included studies that use statistical adjustment for baseline case mix using multivariable analyses.
We excluded case‐control studies and uncontrolled observational studies. We also excluded controlled before‐and‐after studies (a study in which observations are made before and after the implementation of an intervention, both in a group that receives the intervention and in a control group that does not) because there was no concurrent comparison groups.
Types of participants
Women, 18 years or older, with known BRCA1 or BRCA2 mutations. We included women without a previous or coexisting breast, ovarian or fallopian tube malignancy, and women with or without concomitant hysterectomy. We included women with a mastectomy before, concomitant with, or after RRSO, even if mastectomy had been the focus of another Cochrane review (Lostumbo 2010). We excluded women with a previous or coexisting breast malignancy and women with unilateral oophorectomy or salpingectomy or salpingo‐oophorectomy (both). In addition, we excluded women with prophylactic salpingectomy with delayed oophorectomy or ovarian conservation (Harmsen 2015; Harmsen 2016; Tschernichovsky 2017).
Types of interventions
RRSO (surgery to remove both fallopian tubes and ovaries as an option for women with BRCA1 or BRCA2 mutations not thought to have cancer before the surgical procedure, but who had a high lifetime ovarian cancer, fallopian tube cancer or breast cancer risk) versus no RRSO.
Types of outcome measures
Primary outcomes
Overall survival: survival until death from all causes. We assessed survival from the time when women were enrolled in the study.
HGSC (fallopian tube, ovarian and primary peritoneal cancer) mortality.
Breast cancer mortality.
Secondary outcomes
HGSC (fallopian tube, serous tubal intraepithelial carcinoma, ovarian and primary peritoneal cancer) incidence (all cases of serous peritoneal cancer diagnosed after prophylactic salpingo‐oophorectomy were considered primary peritoneal cancer).
Breast cancer incidence.
Bone fracture incidence.
Disease‐free survival: time from surgical procedure to cancer diagnosis.
-
Morbidity:
direct surgical morbidity;
surgically related systemic morbidity (e.g. chest/wound/urine infection, venous thromboembolism, premature menopause, etc.).
Recovery, readmission.
Quality of life, measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication (Roila 2001; Spitzer 1981).
-
Adverse events, we intended to categorise the severity of the following adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE 2010): surgery‐related complications measured as the proportion of women who developed one or more of the items below (according to the study definition) within 12 weeks. We classified complications into intraoperative and postoperative complications:
-
intraoperative complications:
haemorrhage;
ureteric injury;
cardiac or respiratory complications;
anaphylaxis;
-
postoperative complications were classified as either early (before discharge from hospital or within seven days of surgery), late (from seven days to follow‐up: within 12 weeks of surgery), or total (early and late):
wound breakdown;
pulmonary embolism;
deep vein thrombosis;
psychiatric/psychosexual problem.
-
Search methods for identification of studies
We searched for papers in all languages and translated them as necessary.
Electronic searches
We searched the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 7) in The Cochrane Library (Appendix 1).
MEDLINE Ovid (January 1946 to July week 2 2017) (Appendix 2).
Embase (January 1980 to 2017 week 30) (Appendix 3).
We identified all relevant articles on PubMed and using the 'related articles' feature we performed a further search for newly published articles.
Searching other resources
Unpublished and grey literature
We searched the following for ongoing studies:
metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com/rct).
Physicians Data Query (www.nci.nih.gov).
USA National Institutes of Health (clinicaltrials.gov/ct).
USA National Cancer Institute (www.cancer.gov/clinicaltrials).
ISRCTN registry (www.isrctn.com/).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/).
If ongoing studies that have not been published were identified through these searches, we approached the principal investigators, and major co‐operative groups active in this area, to ask for relevant data.
Handsearching
We handsearched the citation lists of included studies, key textbooks and previous systematic reviews and contacted experts in the field to identify further reports of studies. We also handsearched the reports of conferences in the following sources.
Gynecologic Oncology (Annual Meeting of the American Society of Gynecologic Oncologist).
International Journal of Gynecological Cancer (Annual Meeting of the International Gynecologic Cancer Society).
British Journal of Cancer.
British Cancer Research Meeting.
Annual Meeting of European Society of Medical Oncology (ESMO).
Annual Meeting of the American Society of Clinical Oncology (ASCO).
We handsearched the following breast cancer journals:
Breast Cancer Research and Treatment.
Breast Cancer Research.
Clinical Breast Cancer.
Breast Cancer.
Journal of Breast Cancer.
Open Breast Cancer Journal.
Breast Cancer Online.
Advances in Breast Cancer.
Gastric and Breast Cancer .
Current Breast Cancer Reports.
Breast Cancer: Targets and Therapy.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database (EndNote X7), and removed duplicates. Two review authors (GE and IE) examined the remaining references independently. We excluded those studies that clearly did not meet the inclusion criteria and we obtained full‐text copies of potentially relevant references. Two review authors (GE and IE) independently assessed the eligibility of the retrieved reports/publications. We resolved any disagreement through discussion or, if required, we consulted a third review author (AC). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Liberati 2009).
Data extraction and management
For included studies, we extracted the following data.
Author, year of publication and journal citation (including language).
Country.
Setting.
Ethnicity.
Inclusion and exclusion criteria.
Study design, methodology.
-
Study population:
total number enrolled;
participant characteristics (e.g. BRCA1, BRCA2 or both);
age;
comorbidities;
other baseline characteristics.
-
Intervention details:
type of surgery;
occult cancer;
type of screening test;
period of screening test;
type of chemoprevention;
dose of chemoprevention;
course of chemoprevention;
type of histology protocol adopted (e.g. the SEE‐FIM protocol) as documented in Blok 2016 and Mahe 2013;
use of peritoneal washing cytology (Blok 2016);
use of oral contraceptives.
Comparison: we compared the outcomes for women with adnexa‐preserving.
Risk of bias in study (Assessment of risk of bias in included studies).
Duration of follow‐up.
Outcomes: for each outcome, we extracted the outcome definition and unit of measurement (if relevant). For adjusted estimates, we recorded variables adjusted for in analyses.
Results: we extracted the number of participants allocated to each intervention group, the total number analysed for each outcome and the missing participants.
We extracted the results as follows.
For time‐to‐event data (overall survival and disease‐specific survival), we extracted the log of the hazard ratio (log(HR)) and its standard error from trial reports. If these were not reported, we estimated the log(HR) and its standard error using the methods described by Parmar 1998.
For dichotomous outcomes (e.g. adverse events or deaths, if it is not possible to use a HR), we extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at endpoint, in order to estimate a risk ratio (RR).
For continuous outcomes (e.g. quality of life measures), we extracted the final value and standard deviation (SD) of the outcome of interest and the number of participants assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference (MD) between treatment arms and its standard error.
If reported, we extracted both unadjusted and adjusted statistics. Where possible, all data extracted were those relevant to an intention‐to‐treat analysis, in which we analysed participants in groups to which they were assigned.
When possible, we noted the time points at which outcomes were collected and reported.
Two review authors (GE and IE) extracted data independently onto a data abstraction form specially designed for the review. We resolved differences between review authors by discussion or by appeal to a third review author (AE) if necessary. We approached the principal investigators of included studies to ask for any missing relevant unpublished data.
Assessment of risk of bias in included studies
As detailed in Results of the search, we identified no RCTs or quasi‐randomised studies were identified, therefore we assessed the risk of bias for NRS using the ROBINS‐I (Risk Of Bias In Non‐randomised Studies‐of Interventions). A new tool for evaluating risk of bias in estimates of the comparative effectiveness (harm or benefit) of interventions from studies that did not use randomisation to allocate units (individuals or clusters of individuals) to comparison groups (Sterne 2016).
We achieved consensus on seven domains through which bias might be introduced into an NRS:
confounding;
selection of participants into the study;
classification of interventions;
deviations from intended interventions;
missing data;
measurement of outcomes;
selection of the reported result.
The first two domains, covering confounding and selection of participants into the study, addressed issues before the start of the interventions that were compared ('baseline'). The third domain addressed classification of the interventions themselves. The other four domains addressed issues arising after the start of interventions: biases due to deviations from intended interventions, missing data, measurement of outcomes and selection of the reported result (Sterne 2016).
The assessment of each NRSI included in the review involved following the six steps below. Steps 3 to 6 were repeated for each key outcome of interest: 1. specifying the research question through consideration of a target trial; 2. specifying the outcome and result being assessed; 3. for the specified result, examining how the confounders and cointerventions were addressed; 4. answering signalling questions for the seven bias domains; 5. formulating risk of bias judgements for each of the seven bias domains, informed by answers to the signalling questions; 6. formulating an overall judgement on risk of bias for the outcome and result being assessed (Sterne 2016).
Examination of confounders and cointerventions involves determining whether the important confounders and cointerventions were measured or administered in the study at hand, and whether additional confounders and cointerventions were identified (Sterne 2016). The following were the potential confounding variables: coexisting or history of breast or ovarian cancer, type of mutation, race, year of birth, parity, socioeconomic status, breastfeeding, oral contraceptive use, oestrogen therapy, ovarian stimulation and type of surgery (oophorectomy without concomitant salpingectomy or risk‐reducing mastectomy, or both). We assessed whether study authors had employed methods to control for selection bias at the design stage (e.g. matching or restriction to particular subgroups) and in their methods of analysis (e.g. the use of stratification or regression modelling). The focus was on whether a solution to a bias concern in a study was adequate.
The full tool with the signalling questions were addressed within each bias domain. The response options were: 'yes;' 'probably yes;' 'probably no;' 'no;' and 'no information.' Some questions were answered only if the response to a previous question was 'yes' or 'probably yes' (or 'no' or 'probably no'). Responses of 'yes' were intended to have similar implications to responses of 'probably yes' (and similarly for 'no' and 'probably no'), but allowed for a distinction between something that was known and something that was likely to be the case. Free text was used to provide support for each answer, using direct quotations from the text of the study where possible. Responses to signalling questions provided the basis for domain‐level judgements about risk of bias, which then provided the basis for an overall risk of bias judgement for a particular outcome (Sterne 2016).
The categories for risk of bias judgements were 'low risk,' 'moderate risk,' 'serious risk' and 'critical risk' of bias. Importantly, 'low risk' corresponded to the risk of bias in a high‐quality randomised trial (Sterne 2016).
Two review authors (GE and IE) independently applied the new ROBINS‐I 'Risk of bias' assessment tool and resolved any differences in opinion by discussion or by appeal to a third review author (AE). We summarised results in both a 'Risk of bias' table and a 'Risk of bias' summary. We interpreted the results of meta‐analyses in light of the findings with respect to risk of bias.
We listed the individual 'Risk of bias' items that we adapted for our review in Appendix 2. Table 6 depicts the interpretation of domain‐level and overall risk of bias judgements in ROBINS‐I (Sterne 2016).
1. Interpretation of domain levels and overall risk of bias judgement in ROBINS‐I.
| Judgement | Within each domain | Across domains | Criterion |
| Low risk of bias | The study is comparable to a well‐performed randomised trial with regard to this domain. | The study is comparable to a well‐performed randomised trial. | The study is judged to be at low risk of bias for all domains. |
| Moderate risk of bias | The study is sound for a non‐randomised study with regard to this domain but cannot be considered comparable to a well‐performed randomised trial. | The study provides sound evidence for a non‐randomised study but cannot be considered comparable to a well‐performed randomised trial. | The study is judged to be atlow or moderate risk of bias for all domains. |
| Serious risk of bias | The study has some important problems in this domain. | The study has some important problems. | The study is judged to be at serious risk of bias in at least 1 domain, but not at critical risk of bias in any domain. |
| Critical risk of bias | The study is too problematic in this domain to provide any useful evidence on the effects of intervention. | The study is too problematic to provide any useful evidence and should not be included in any synthesis. | The study is judged to be at critical risk of bias in at least 1 domain. |
| No information | No information on which to base a judgement about risk of bias for this domain | No information on which to base a judgement about risk of bias. | There is no clear indication that the study is at serious or critical risk of bias and there is a lack of information in 1 or more key domains of bias (a judgement is required for this). |
ROBINS‐I: Risk Of Bias In Non‐randomised Studies‐of Interventions.
Measures of treatment effect
We used the following measures of the effect of treatment.
For time to event data, we used the HR, if possible.
For dichotomous outcomes, we used the RR.
For continuous outcomes, we used the MD between treatment arms.
Unit of analysis issues
We did not anticipate unit of analysis issues.
Dealing with missing data
We did not impute missing outcome data for the primary or secondary outcomes. If data were missing or the included studies only reported imputed data, we contacted study authors to request data on the outcomes only among participants who were assessed.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots, by estimation of the percentage heterogeneity between studies that could not be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001), and, if possible, by subgroup analyses. If there was evidence of substantial heterogeneity, we investigated and reported the possible reasons for this.
Assessment of reporting biases
When we suspected or there was direct evidence of selective outcome reporting, we asked the study authors for additional information. We examined funnel plots corresponding to meta‐analysis of the primary outcome to assess the potential for small‐study effects, such as publication bias, if we identified a sufficient number of studies.
Data synthesis
If sufficient, clinically similar studies were available, we pooled their results in meta‐analyses using Review Manager 2014 (RevMan 5).
For time‐to‐event data, we pooled HRs using the generic inverse variance facility of RevMan 5 (Review Manager 2014).
For any dichotomous outcomes, we calculated the RRs for each study and we then pool these values.
For continuous outcomes, we pooled the MDs between the treatment arms at the end of follow‐up if all studies measured the outcome on the same scale, otherwise we pooled standardised MD values.
We used the random‐effects model with inverse variance weighting for all meta‐analyses (DerSimonian 1986).
'Summary of findings' table
We assessed the certainty of evidence using the GRADE system, used GRADEpro software and presented the review results in ’Summary of findings’ tables. A 'Summary of findings' table consists of three parts: information about the review, a summary of the statistical results and the grade of the certainty of evidence (Appendix 3). Appendix 3 displays a draft 'Summary of findings' table, which were prepared to summarise the results of the meta‐analysis based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We presented the results of the meta‐analysis for the following outcomes as outlined in the Types of outcome measures section.
Overall survival.
HGSC mortality.
Breast cancer mortality.
Bone fracture incidence.
Quality of life.
Severe adverse events.
We presented the overall certainty of the evidence for each outcome according to the GRADE approach, which took into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity such as directness of results (Langendam 2013). The five factors were used to judge whether the quality of the collected evidence should be decreased if we were dealing with RCTs or increased if we were dealing with observational studies. We created a 'Summary of findings' table based on the methods described by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and using GRADEpro Guideline Development Tool (GDT) (GRADEpro GDT 2014). We used the GRADE checklist and GRADE Working Group certainty of evidence definitions (Meader 2014). We downgraded the evidence from 'high' certainty by one level for serious (or by two for very serious) limitations.
High‐certainty: we were very confident that the true effect lay close to that of the estimate of the effect.
Moderate‐certainty: we were moderately confident in the effect estimate: the true effect was likely to be close to the estimate of the effect, but there was a possibility that it was substantially different.
Low‐certainty: our confidence in the effect estimate was limited: the true effect may have been substantially different from the estimate of the effect.
Very low‐certainty: we had very little confidence in the effect estimate: the true effect was likely to be substantially different from the estimate of effect.
If meta‐analysis was not possible, we could have presented results in a narrative ‘Summary of findings’ table format, such as that used by Chan 2011.
Subgroup analysis and investigation of heterogeneity
We subgrouped by BRCA mutation (BRCA1, BRCA2 or both) and the type of surgery (RRSO alone versus RRSO and mastectomy, or RRSO and mastectomy versus no RRSO). When reported by any of the included studies, we considered factors such as age at RRSO, obesity, race, reproductive history, ovarian stimulation, menstrual history, use of the oral contraceptives, breastfeeding, oestrogens therapy, pelvic inflammatory disease, length of follow‐up and risk of bias status in our interpretation of any heterogeneity. We also considered women who were BRCA mutation carriers receiving bilateral prophylactic risk‐reducing oophorectomy without concomitant breast malignancy, with or without concomitant hysterectomy, and with or without concomitant mastectomy. Where possible, we assessed the difference between subgroups by interaction tests.
Sensitivity analysis
We could not perform sensitivity analyses for each type of effect measure, as there were insufficient numbers of studies as well as the fact that the overall survival and mortality outcomes (which were main outcomes reported) were analysed appropriately using HRs which took into account all points in time and allowed for censoring. Similarly, sensitivity analyses based on the risk of bias assessment, although planned, were not carried out because of the moderate risk of bias in all (except Kramer 2005 which was serious risk of bias) of included studies.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies tables.
Results of the search
The search identified 1395 bibliographic references, 1336 through database searching and 59 through other sources. We excluded 118 duplicates and screened the 1277 remaining references and excluded 1231 records as clearly irrelevant. We obtained full‐text articles of 46 records, and two review authors (GUE and IUE) independently assessed them for eligibility. After careful scrutiny, we excluded 36 references as they did not fulfil the inclusion criteria. We present reasons for exclusion in the Characteristics of excluded studies table. Subsequently, 10 references describing 10 non‐randomised prospective or retrospective cohort studies met the inclusion criteria for this systematic review (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Madalinska 2007; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004), and all but one study were included in the meta‐analysis (Madalinska 2007). We outlined the study selection in the PRISMA flow diagram shown in Figure 1.
1.
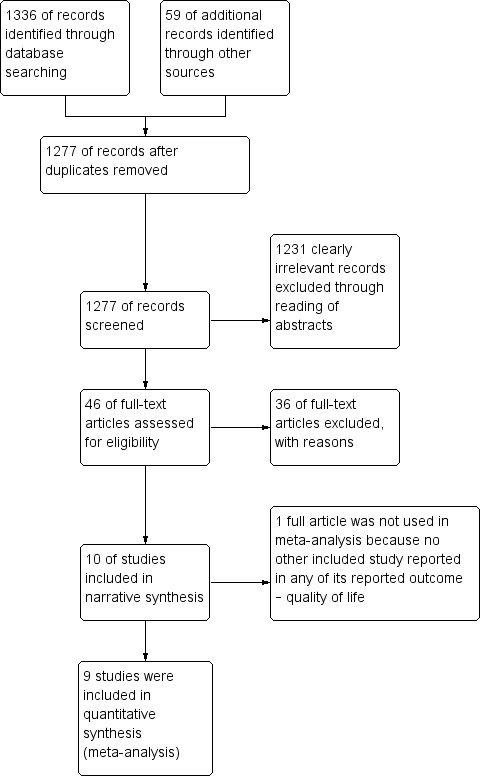
Study flow diagram for searches on risk‐reducing salpingo‐oophorectomy in women with BRCA1 or BRCA2 mutation carriers.
Included studies
Setting
The year of publication for the included studies ranged from 1999 to 2017 and all were published in English (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Madalinska 2007; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004).
The country of origin for the included studies were two USA (Kramer 2005; Rebbeck 1999), two Netherlands (Heemskerk‐Gerritsen 2015a; Madalinska 2007), one UK (Ingham 2013), and five multiple countries (Domchek 2006; Domchek 2010; Kotsopoulos 2017; Rebbeck 2002; Rebbeck 2004).
All 10 included studies had different settings:
Domchek 2006 took place in 13 US and European centres that comprised the PROSE (PRevention and Observation of Surgical Endpoints) consortium.
Domchek 2010 took place in 22 centres who were part of the PROSE consortium.
Heemskerk‐Gerritsen 2015a took place in the Netherlands as part of the Hereditary Breast and Ovarian Cancer in the Netherlands (HEBON) study. Data on participant and tumour characteristics and on preventive strategies were retrospectively as well as prospectively retrieved and updated through medical files and questionnaires, and through linkages to the Netherlands Cancer Registry and the Dutch Pathology Database.
Ingham 2013 study data were from the Genetic Medicine Database (Manchester Regional Genetics Service, UK), patient records and from records at the North West Cancer Intelligence Service (NWCIS), UK.
Kotsopoulos 2017 identified deleterious BRCA1 and BRCA2 mutation carriers from 78 participating centres in 12 countries worldwide.
Kramer 2005 took place at the National Cancer Institute, USA.
Madalinska 2007 was conducted at gynaecology departments of eight hospitals in the Netherlands that had a clinical genetics centre.
Rebbeck 1999 obtained study data from the registry databases of five institutions in USA, while Rebbeck 2002 and Rebbeck 2004 studies identified women from 11 North American and European registries.
Ethnicity
None of the studies reported ethnicity.
Inclusion criteria
Eight studies included women with either BRCA1 or BRCA2 mutations (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Madalinska 2007; Rebbeck 2002; Rebbeck 2004). Two studies included only women with BRCA1 mutations (Kramer 2005; Rebbeck 1999). None of the studies reported or recruited women with both BRCA1 and BRCA2 mutations or only BRCA2 mutations.
Domchek 2006 included women who had undergone RRSO and control participants who were cancer free (i.e. had never had a cancer diagnosis) at enrolment and did not have a cancer diagnosis within six months after enrolment and had not had any previous prophylactic surgery, including mastectomy and oophorectomy.
Heemskerk‐Gerritsen 2015a selected women with BRCA1 or BRCA2 mutation from the HEBON cohort: 1. no history of cancer at the date of DNA test result, 2. both breasts and ovaries in situ at the date of DNA test result and 3. no cancer diagnosis within the first six months of the study.
Ingham 2013 included women if they were alive at the date of family ascertainment (i.e. the date when all incident tumours in a family registry's surveillance population were captured in the registry's database) and did not have a diagnosis of breast or ovarian cancer (this translated to inclusion of a small number of women who had already undergone RRSO).
Kotsopoulos 2017 included women who consented and completed at least one follow‐up questionnaire on family information and personal history of cancer, and reproductive and medical histories, including preventive oophorectomy and mastectomy. However, women with unilateral oophorectomy were included in the no‐oophorectomy group.
Kramer 2005 eligibility criteria were: women; bloodline family member (siblings, parents, grandparents); no history of breast cancer before ascertainment; no history of bilateral mastectomy and under 20 years of age by the study closing date. A diagnosis of malignancy other than breast cancer did not affect eligibility.
Madalinska 2007 included women aged between 30 and 70 years and completed childbearing, hereditary breast/ovarian cancer (HBOC) in the family, and referral to the gynaecology clinic by a clinical geneticist specifically for the purpose of discussing the prevention of ovarian cancer.
Rebbeck 1999 included women if they had undergone bilateral oophorectomy prior to or at the time of enrolment or if they reported having had this procedure during follow‐up by the collaborating institutions. Surgical participants were also included if their surgery was not performed to treat ovarian or related peritoneal cancers. Potential control participants were eligible if they had the BRCA1 mutation, were alive and had both ovaries (i.e. no history of oophorectomy), had no history of breast or ovarian cancer, and had no history of prophylactic mastectomy at or before the time of the surgical participant’s surgery. Control participants were matched to surgical participants on year of birth (within five years) and on the collaborative institution from which they were ascertained.
Rebbeck 2002 selected one or more controls for inclusion if they could be matched to a participant who had undergone prophylactic oophorectomy according to type of mutation (BRCA1 or BRCA2 ), treatment centre and year of birth (within five years). The authors also included women to determine the risk of ovarian cancer only if their surgery was not performed to treat ovarian cancer, and a control participant was eligible if she had BRCA1 or BRCA2 mutation, was alive with both ovaries intact at the time the woman with whom she was matched underwent prophylactic oophorectomy and had no history of ovarian cancer at the time of the matched participant’s prophylactic oophorectomy.
Rebbeck 2004 included a subset of women from the total sample who had undergone bilateral prophylactic mastectomy but had not undergone bilateral prophylactic oophorectomy before this procedure. Control participants were eligible if they had not undergone bilateral prophylactic oophorectomy and were alive and cancer‐free with both breasts intact at the time of the matched participant’s bilateral prophylactic mastectomy. The analysis was performed on the subset of women who had not had bilateral prophylactic mastectomy at the time of their centre ascertainment and controls were excluded if they had a diagnosis of breast or ovarian cancer at or before the time of the matched surgical participant’s bilateral prophylactic mastectomy. Surgical participants and matched control participants were included regardless of their history of bilateral prophylactic oophorectomy and included 57 bilateral prophylactic mastectomy participants and 107 control participants.
Exclusion criteria
Madalinska 2007 excluded women with prior oophorectomy performed as treatment for breast cancer or for any pathology in the ovaries and metastatic cancer or any other severe comorbidity.
Domchek 2006 excluded women with BRCA1 or BRCA2 variants of unknown functional importance, and women who underwent bilateral prophylactic mastectomy – either before enrolment or during follow‐up period.
Domchek 2010 excluded women if they had a cancer diagnosis within the first six months of follow‐up to avoid including cancers that would have been minimally influenced by RRSO or RRM as well as women who had both BRCA1 and BRCA2 mutations, women who underwent RRSO before ascertainment date, or women diagnosed with ovarian cancer before ascertainment date, or women with diagnosis of cancer within the first six months of follow‐up, or incident cases.
Kotsopoulos 2017 excluded women with prior diagnosis of breast cancer, ovarian cancer, other cancers or completion of follow‐up questionnaire prior to receipt of their genetic test results.
Kramer 2005 excluded families with variants of uncertain significance.
Rebbeck 1999 excluded women if they had only unilateral oophorectomies, if they had undergone mastectomy prior to their oophorectomy, or if they had a personal history of breast or ovarian cancer at or before the time of their oophorectomy. Women with BRCA2 mutations carriers were excluded because of relatively small numbers of BRCA2 mutation carriers available in their study population and because the risk of breast and ovarian cancers (and possibly patterns of surgery use) may have differed from BRCA1 mutation carriers.
Rebbeck 2002 excluded women with BRCA1 or BRCA2 variants of unknown functional importance as well as women who had undergone unilateral oophorectomy or had a history of ovarian cancer (including borderline tumours or tumours of low malignant potential) before undergoing prophylactic oophorectomy risk, except that women who had undergone prophylactic oophorectomy were excluded if they had previously undergone mastectomy or had a history of breast cancer (including carcinoma in situ) at the time of the prophylactic oophorectomy. Control women were excluded if they had undergone prophylactic oophorectomy or had a history of breast cancer at the time of the matched participant’s prophylactic oophorectomy.
Rebbeck 2004 excluded women with BRCA1 or BRCA2 variants of unknown functional significance as well as study participants who had prior or concurrent breast cancer at time of surgery.
Heemskerk‐Gerritsen 2015a and Ingham 2013 presented no exclusion criteria.
Study design and methodology
The 10 articles included in this review were very heterogeneous. None of the studies was case series. Three studies included in this review were prospective cohort studies with matching design (Domchek 2006; Rebbeck 1999; Rebbeck 2004); one was a retrospective cohort study with matching design (Rebbeck 2002); five studies were prospective cohort studies with unmatching design (Domchek 2010; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Madalinska 2007), while one study was partly retrospective and partly prospective cohort study with matching design (Heemskerk‐Gerritsen 2015a). Control participants were matched to surgical participants on year of birth (within five years) (Domchek 2006; Rebbeck 1999), and on the collaborative institution from which they were ascertained in Rebbeck 1999 study. Although Kramer 2005 reported women who were both BRCA1‐positive and BRCA1‐negative mutation carriers, we used only the data of women who were BRCA1‐positive mutation carriers among women who had RRSO and no RRSO (surveillance).
Heemskerk‐Gerritsen 2015a replicated the analyses of four previous studies, performed by Domchek 2006; Domchek 2010; Eisen 2005; and Kauff 2008, within a Dutch cohort, first to examine if their study cohort was comparable with the cohorts used in the previous studies and second to estimate the effect of RRSO on breast cancer risk in the Dutch cohort using a specified design and analyses in order to minimise bias.
Nine studies used survival analysis (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004), and one study used a validated scale for quality of life assessment (Madalinska 2007). All the nine studies that used survival analysis reportedly used adjusted or corrected models (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004). Seven studies adjusted for age or date of birth (within five years) (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Rebbeck 2002); four studies adjusted for centre (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Rebbeck 2002); five studies adjusted for type of BRCA mutation (Domchek 2006; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Rebbeck 2002); three adjusted for age at menarche in order to account for duration of endogenous ovarian hormone exposure (Kotsopoulos 2017; Rebbeck 1999; Rebbeck 2004); two adjusted for oral contraceptive use (Domchek 2010; Kotsopoulos 2017); and one adjusted for family history of breast cancer, country of residence, parity, breastfeeding and oestrogen receptor status of the breast cancer (Kotsopoulos 2017).
The analytic techniques employed in the studies included Kaplan‐Meier survival analysis in two studies (Ingham 2013; Kramer 2005), and Cox proportional hazards regression in nine studies (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004). Madalinska 2007 assessed the overall quality of life using the single quality of life item of the EORTC Quality of Life Questionnaire. Time origin for survival analysis was generally the time of DNA testing, except in the case of treatment or surgical cohorts where the time of origin was the beginning of treatment or the date of surgery.
Heemskerk‐Gerritsen 2015a used the Simon and Makuch method for survival analysis (Simon 1984), with chronological age as the time variable. This method takes into account the change in an person’s covariate status over time per 1000 person‐years of observation. Variables that were considered as potential confounders were type of mutation, year of birth and centre but they did not meet the criteria for incorporation in a multivariable Cox model. They used a robust variance‐covariance estimation method to correct for non‐independence of observations in women from the same family. The study also performed sensitivity analyses to estimate the effect of RRSO on breast cancer risk in different settings. The study estimated breast cancer risk reduction after RRSO for participants who never underwent RRM in order to investigate the effect of excluding the breast cancer‐free time before RRM, and the authors explored the effect of RRSO on breast cancer risk when the time before RRSO was excluded from the analysis. The authors also examined the effect on breast cancer risk when RRSO was performed in women under the age of 51 years (mean age of postmenopausal status in the Netherlands), and at the age of 51 years and above.
Study population
Total number enrolled
The review included a total of 8087 women (2936 (36%) surgical participants and 5151 (64%) control participants). The number of women in the included studies were 155 surgical participants and 271 control participants (Domchek 2006), 465 surgical participants and 1092 control participants (Domchek 2010), 146 surgical participants and 576 control participants (Heemskerk‐Gerritsen 2015a), 108 surgical participants (RRSO or bilateral prophylactic mastectomy (or both) participants) and 457 control participants (Ingham 2013), 1552 surgical participants and 2170 control participants (Kotsopoulos 2017), 33 surgical participants and 65 control participants (Kramer 2005), 118 surgical participants and 42 control participants (Madalinska 2007), 43 surgical participants and 79 control participants (Rebbeck 1999), 259 surgical participants and 292 control participants (Rebbeck 2002), 57 surgical participants (bilateral prophylactic mastectomy participants plus RRSO) and 107 control participants (Rebbeck 2004),
Participant characteristics (e.g. BRCA1, BRCA2 or both)
All participants included were either BRCA1 or BRCA2 mutation carriers. Participants with both BRCA1 and BRCA2 mutation status were either not reported or excluded. Domchek 2010 excluded 12 participants because they had both BRCA1 and BRCA2 mutations. Heemskerk‐Gerritsen 2015a reported 46 BRCA1 and 100 BRCA2 mutation carriers in RRSO participants. Ingham 2013 reported 56 BRCA1 and 52 BRCA2 mutation carriers in RRSO participants and 219 BRCA1 and 238 BRCA2 mutation carriers in control participants. Kotsopoulos 2017 reported 1187 BRCA1 and 355 BRCA2 mutation carriers in RRSO participants and 1782 BRCA1 and 370 BRCA2 mutation carriers in control participants. Kramer 2005 reported only 98 BRCA1 mutation carriers while Rebbeck 1999 reported 122 BRCA1 mutation carriers.
Age
Reporting of age varied widely across studies. The mean ages were 39.4 (range: 22 to 63) years in surgical participants and 35.3 (range: 17 to 65) years in control participants (Rebbeck 1999), 44.8 (SD 8.5) years in surgical participants and 42.6 (SD 10.0) years in control participants (Domchek 2006), 43.2 (range: 20.5 to 79.0) years in surgical participants and 36.7 (range: 18.1 to 90.4) years in control participants (Domchek 2010), 44 years in surgical participants and 33 years in control participants (Heemskerk‐Gerritsen 2015a), 46.2 (SD 6.35) (range: 21 to 88) years in surgical participants and 33.4 (SD 5.45) (range: 13 to 85) years in control participants (Kotsopoulos 2017), 48.3 (SD 8.4) years in surgical participants and 45.3 (SD 8.1) years in control participants (Madalinska 2007), 42.0 (range: 21.2 to 74.8) years in surgical participants and 40.9 (range: 19.6 to 79.1) years in control participants (Rebbeck 2002).
Ingham 2013 did not report the mean age of participants in either the surgical and control groups, but they only reported median age of ascertainment of 34.4 (range: 2 to 87) years for BRCA1 mutation carriers and 37.4 (range: 5 to 85) years for BRCA2 mutation carriers. Kramer 2005 did not report the mean or median age of the participants.
Comorbidities
Only Madalinska 2007 reported comorbidities, which were asthma and other chronic respiratory diseases; and cardiovascular, renal, rheumatic diseases, hypertension and diabetes.
Other baseline characteristics
Baseline characteristics of the women were not comparable between the two groups in all studies.
Intervention details
Type of surgery
Three studies performed bilateral oophorectomy (Kotsopoulos 2017; Kramer 2005; Rebbeck 2002), while in other seven studies performed bilateral salpingo‐oophorectomy (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Madalinska 2007; Rebbeck 1999; Rebbeck 2004).
Four trials performed concurrent risk‐reducing mastectomy (Domchek 2010; Ingham 2013; Madalinska 2007; Rebbeck 2004), four studies excluded concurrent risk‐reducing mastectomy (Domchek 2006; Kotsopoulos 2017; Kramer 2005; Rebbeck 2002), and three trials did not report concurrent risk‐reducing mastectomy (Heemskerk‐Gerritsen 2015a; Rebbeck 1999).
None of the trials reported concurrent hysterectomy.
Route of surgery
Only Ingham 2013 described the route of surgery, which used both laparoscopy and open surgical techniques.
Occult cancer
Only two studies reported occult cancers (cancer of unknown primary origin) (Domchek 2010; Ingham 2013). Domchek 2010 reported nine cases of occult cancers (seven cases in BRCA1 mutation carriers and two in BRCA2 mutation carriers), but women were excluded if they were diagnosed with an occult ovarian cancer at RRSO. Ingham 2013 reported six cases of occult cancer (three in BRCA1 and three in BRCA2 mutation carriers).
Type of screening test
All studies confirmed the BRCA1/2 mutation status of all participants by direct mutation or DNA testing.
Period of screening test
None of the studies reported the period of screening, except that the screening for BRCA1 or BRCA2 mutation status was prior to enrolment in all the included studies.
Type of chemoprevention
None of the studies reported on type of chemoprevention.
Dose of chemoprevention
None of the studies stated the dose of chemoprevention except that Ingham 2013 reported that chemoprevention agents such as tamoxifen and raloxifene could not be used in any of the recruited participants because they were not licensed for chemoprevention in the UK and most European countries.
Course of chemoprevention
None of the studies reported the course of chemoprevention.
Type of histology protocol adopted
None of the studies reported the type of histology protocol adopted (e.g. the SEE‐FIM protocol) as documented in Blok 2016 and Mahe 2013.
Use of peritoneal washing cytology
None of the studies reported the use of peritoneal washing cytology.
Use of oral contraceptives
Only Domchek 2006 reported the use of oral contraceptives. Domchek 2006; Kotsopoulos 2017; and Rebbeck 2002 also reported on HRT.
Duration of follow‐up
The reporting follow‐up time varied widely across studies. Postsurgery, follow‐up duration was 3.1 (SD 2.4) years in surgical participants and 2.1 (SD 2.0) years in controls participants (Domchek 2006), 6.8 years in surgical participants and 3.1 years in control participants (Heemskerk‐Gerritsen 2015a), 5.6 years (Kotsopoulos 2017), 35 years (Kramer 2005); 1 year in both surgical and control participants (Madalinska 2007), 9.6 years in surgical participants and 8.1 years in control participants (Rebbeck 1999), 5.5 years in surgical participants and 6.7 years in control participants (Rebbeck 2004).
Domchek 2010 and Ingham 2013 did not report the mean duration of follow‐up. Domchek 2010 reported the median duration was 3.65 (range: 0.52 to 27.4) years in participants who underwent RRSO and 4.29 (range: 0.5 to 27.9) years in control participants who did not undergo surgery. Ingham 2013 reported the median duration of follow‐up (from ascertainment to death or loss to follow‐up) was 13.3 years.
Studies followed up surgical and control participants from the date of the participant's RRSO (Rebbeck 2002), or date of ascertainment (i.e. date of genetic testing or date of baseline questionnaire, whichever was later) (Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017), or time of centre ascertainment to censoring or death due to: any cause, breast or HGSC (Domchek 2006; Ingham 2013; Kramer 2005). Domchek 2010 followed surgical participants from date of RRSO or RRM and non‐surgical participants or controls from date of ascertainment.
Outcome
Three studies assessed and reported overall survival until death from all causes (Domchek 2006; Domchek 2010; Ingham 2013).
Three studies assessed and reported HGSC mortality (Domchek 2006; Domchek 2010; Rebbeck 2002).
Seven studies assessed and reported breast cancer mortality (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017; Kramer 2005; Rebbeck 1999; Rebbeck 2002).
Four studies assessed and reported HGSC incidence (Domchek 2006; Heemskerk‐Gerritsen 2015a; Ingham 2013; Rebbeck 2002). (All cases of serous peritoneal cancer diagnosed after prophylactic salpingo‐oophorectomy were considered primary peritoneal cancer.)
Six studies assessed and reported breast cancer incidence (Domchek 2006; Heemskerk‐Gerritsen 2015a; Kramer 2005; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004).
One study evaluated and reported on quality of life (Madalinska 2007), measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication (Roila 2001; Spitzer 1981).
Excluded studies
We excluded 36 studies (Benshushan 2009; Chang‐Claude 2007; Eisen 2005; Evans 2009; Evans 2013; Finch 2006; Finch 2009; Finch 2011; Finch 2013; Finch 2014; Finkelman 2012; Heemskerk‐Gerritsen 2013; Heemskerk‐Gerritsen 2015b; Hunsinger 2016; Iavazzo 2016; Johansen 2016; Johansen 2017; Kauff 2002; Kauff 2008; Kwon 2013; Laki 2007; Madalinska 2005; Manchanda 2011; Meijers‐Heijboer 2001; Menkiszak 2016; Metcalfe 2014; Miller 2017; Perabo 2014; Powell 2011; Rocca 2006; Rutter 2003; Schmeler 2006; Skytte 2011; Struewing 1995; van Sprundel 2005; Vermeulen 2017).
Seven studies included women with a previous or coexisting breast malignancy (Chang‐Claude 2007; Eisen 2005; Finch 2006; Finkelman 2012; Kauff 2002; Kauff 2008; Schmeler 2006).
Four studies did not report the BRCA1 or BRCA2 mutations status of the participants (study group or controls), even though the participants consisted of two groups either receiving RRSO or no RRSO (Benshushan 2009; Johansen 2016; Johansen 2017; Rocca 2006).
Four studies included some women with unknown BRCA1 or BRCA2 mutation carrier status (Evans 2009; Madalinska 2005; Rutter 2003; Struewing 1995).
Three studies were controlled before‐and‐after studies (Finch 2009; Finch 2011; Finch 2013).
Eight studies were single arm studies without comparison groups (Finch 2014; Hunsinger 2016; Laki 2007; Manchanda 2011; Menkiszak 2016; Miller 2017; Perabo 2014; Powell 2011).
Seven studies included women with or without a family history or personal history of breast cancer who were carriers of BRCA1 and BRCA2 mutations and initially treated with unilateral or bilateral mastectomy, but without bilateral RRSO (Evans 2013; Heemskerk‐Gerritsen 2013; Heemskerk‐Gerritsen 2015b; Meijers‐Heijboer 2001; Metcalfe 2014; Skytte 2011; van Sprundel 2005).
One study had two comparison groups that received surgical interventions (Kwon 2013).
Two studies were review articles (Iavazzo 2016; Vermeulen 2017).
None of the studies was excluded because they used unadjusted analysis.
We did not identify any ongoing studies.
Risk of bias in included studies
Risk of bias of included studies is presented in Table 7. No studies were at low risk for bias, primarily because none of the studies was an RCT.
2. ROBINS‐I.
| Study ID | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall bias |
| Domchek 2006 | Low | Low | Low | Moderate |
| Support for judgement | Quote: "Questionnaires were administered at every centre and were self‐administered or completed with the help of clinical‐research staff." All missing data were analysed (intention‐to‐treat). |
Quote: "For our primary analysis, we undertook a matched design that selected controls who had not undergone BPSO at any time during follow‐up, and who were matched within 5 years of age to the corresponding one.” | Quote: "Follow‐up data for BPSO, cancer diagnoses, and deaths were verified by review of medical records, and surgical notes, pathology reports, or both." | At least 1 of the domain was moderate. |
| Domchek 2010 | Moderate | Low | Low | Moderate |
| Support for judgement | Quote: "When missing data were encountered, the individual was dropped from the analysis that involved the missing data point, but the individual was included in other analyses where complete data were available; in fact, because many of the data items were required for enrolment missing data was only applicable to ovarian cancer endpoints, with missing OCP data." | Quote: "For BC endpoints, women were excluded if they underwent RRM prior to ascertainment. Women who had RRM after ascertainment but before RRSO were considered unexposed and were censored at RRM. Women were followed until BC or were censored at OC, RRM, death, or last contact." | Quote: "A robust variance‐covariance estimation method was used to correct for non‐independence of observations among participants from the same family or within centers... Adjustment for year of birth was undertaken in all analyses using Cox regression. Oral contraceptive use was adjusted for when OC was the outcome. Adjustment for center of ascertainment was undertaken by stratifying analyses by center to avoid imposing linear constraints in the model." | At least 1 of the domain was moderate. |
| Heemskerk‐Gerritsen 2015a | Moderate | Low | Low | Moderate |
| Support for judgement | Quote: "Eventually, parity was not considered as a potential confounder because of the large proportion (41.0%) of missing data on this variable." | Quote: "We performed sensitivity analyses to estimate the effect of RRSO on BC risk in different settings. First, to investigate the effect of excluding the BC‐free time before RRM, we estimated BC risk reduction after RRSO for participants who never underwent RRM." | Quote: "We adjusted our analyses for differences in age by using chronological age as the time variable." | At least 1 of the domain was moderate. |
| Ingham 2013 | Low | Moderate | Low | Moderate |
| Support for judgement | Quote: "Women were censored at either date of last follow‐up (date of last contact with the genetics department or other NHS service) or date of death (obtained from NWCIS or death certification).” No evidence of missing data. | Quote: "Date of breast cancer was confirmed in the family files or from records at the North West Cancer Intelligence Service (NWCIS)." Also, possible testing bias of women who developed cancer was made. |
Quote: "The proportional hazards assumption was checked in all analyses by looking at log–log plots and Schoenfeld residuals." | At least 1 of the domain was moderate. |
| Kotsopoulos 2017 | Moderate | Low | Moderate | Moderate |
| Support for judgement | Quote: "Women with both a BRCA1 and BRCA2 mutation were coded as missing." | Quote: "We also performed analyses stratified by BRCA mutation type, estrogen receptor status of the tumor, and excluding women with an oophorectomy at or prior to the baseline questionnaire, as well as analyses censoring at different ages." | Quote: "this finding was based on a post hoc analysis." | At least 1 of the domain was moderate. |
| Kramer 2005 | Low | Moderate | Low | Serious |
| Support for judgement | There was no evidence of missing data. | Quote: "A competing risks model (with death as the competing risk) was then used to estimate the 10‐year cumulative incidence of breast cancer in the two groups of BRCA1 mutation carriers (ie, those with and without ovaries)." | Quote: "To provide estimates of the absolute risk of breast cancer by age in mutation carriers, landmark analyses were performed in which oophorectomy was treated as a time‐fixed covariate, as defined at the beginning of a given age interval. Follow‐up time was divided into 10‐year intervals, with mutation carriers divided into two groups based on oophorectomy status at the beginning of that interval (and conditional on the participant being alive and breast cancer free at that time)." | At least 1 of the domain was serious. |
| Madalinska 2007 | Low | Low | Low | Moderate |
| Support for judgement | Quote: "These records were complete, and in cases where there was any uncertainty, contact was sought with the responsible gynecologist." "Non respondents did not differ significantly from respondents regarding age or choice of preventive measure." | Quote: "All raw scale scores were linearly converted to a 0 to100 scale, with higher scores indicating better perceived health, mental health, and quality of life. The internal consistency reliability of the two Short Form‐36 scales was high (α = 0.81 and 0.85)." | Quote: "Because of restrictions by the medical ethics committees, no other clinical data on the nonrespondents were available (eg, DNA status)." | At least 1 of the domain was moderate. |
| Rebbeck 1999 | Low | Low | Moderate | Moderate |
| Support for judgement | Quote: "However, only BRCA1 mutation carriers were studied here, and no OCCR region has been identified in BRCA1." No evidence of missing data. | Quote: "Because most women were followed only until the time of censoring or until the diagnosis of breast cancer, the incidences reported here do not represent lifetime breast cancer risks in BRCA1 mutation carriers." | Quote: "Furthermore, the inferences from both the robust and nonrobust analyses were identical. Therefore, only the standard model results are presented." | At least 1 of the domain was moderate. |
| Rebbeck 2002 | Moderate | Low | Low | Moderate |
| Support for judgement | Quote: "Bias that arises when later follow‐up is missing for individuals initially included and followed." | Quote: "on vital status and the occurrence of cancer was obtained from medical records, telephone interviews, self‐administered questionnaires, or a combination of these. For women who had died since their entry into the study, we reviewed medical records and family‐history reports to establish the presence or absence of cancer and to verify that they had died." | Quote: "For women who had died since their entry into the study, we reviewed medical records and family‐history reports to establish the presence or absence of cancer and to verify that they had died." | At least 1 of the domain is moderate. |
| Rebbeck 2004 | Moderate | Low | Low | Moderate |
| Support for judgement | Quote: "Percentages calculated using nonmissing data." | Quote: "Survival analyses were adjusted to account for duration of endogenous ovarian hormone exposure as measured by the time from age at menarche to age at bilateral prophylactic oophorectomy or menopause, whichever was sooner." | Quote: "Subjects were censored at the date they developed ovarian cancer, or died, or at the date of last contact. Diagnosis of invasive breast cancer or ductal carcinoma‐in‐situ was considered the primary event of interest." | At least 1 of the domain is moderate. |
BC: Breast Cancer; BPSO: Bilateral prophylactic salpingo‐oophorectomy; BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; NHS: National Health Service; NWCI: North West Cancer Intelligence Service; SOC: Site of Care; OCP: Oral Contraceptive Pill; ROBIS‐I: Risk Of Bias In Non‐randomised Studies‐of Interventions; RRM: risk‐reducing bilateral mastectomy; RRSO: risk‐reducing bilateral salpingo‐oophorectomy.
Bias due to confounding
In all cohort studies included, women in the non‐surgical or surveillance group were drawn from the same population as the surgical cohort. Additionally, all eligible studies containing the population cohort excluded women who had a history of ovarian and breast cancer at the beginning of follow‐up. Seven studies had low risk of bias because the studies excluded women with prior oophorectomy performed as treatment for breast cancer or for any pathology in the ovaries and metastatic cancer or any other severe comorbidity (Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Madalinska 2007; Rebbeck 2002; Rebbeck 2004). Two studies had moderate risk of bias because although potential confounders of BRCA1 versus BRCA2 mutation, age, and centre were adjusted for in a multivariate Cox‐regression model in Domchek 2006, not all analyses were stratified by centre, while Rebbeck 1999 had bias of confounding by indication and familial‐event bias. One study had serious risk of bias due to confounding because the authors stated that the diagnosis of malignancy other than breast cancer did not affect eligibility for their analysis (Kramer 2005) (see Table 7).
Bias in selection of participants into the study
Five studies selected population cohorts matched for age and centre (Domchek 2006; Heemskerk‐Gerritsen 2015a; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004). The remaining five cohort studies constituted women with unmatched design (enrolment of controls without regard to the number or characteristics of the cases) (Domchek 2010; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Madalinska 2007). All studies confirmed mutation status by genetic testing. Five studies had low risk of bias because the cohorts constituted women with matched design (Domchek 2006; Heemskerk‐Gerritsen 2015a; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004), and five studies had moderate risk of bias due to selection of participants because the cohorts constituted women with unmatched design (Domchek 2010; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Madalinska 2007) (Table 7)
Bias in classification of interventions
Eight studies had low risk of bias because the criteria for entry, data collection and follow‐up were undertaken at each collaborating centre without regard to surgical status (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kramer 2005; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004), and two studies had moderate risk of bias due to classification of interventions because the authors stated that women with unilateral oophorectomy were included in the no‐oophorectomy group (Kotsopoulos 2017), or the authors stated that the study was not exclusive of a larger prospective investigation focusing on the psychosocial impact of ovarian cancer prevention (Madalinska 2007) (Table 7).
Bias due to deviations from intended interventions
Eight studies had low risk of bias because the criteria for choosing participants and matched or unmatched controls for the group studied to determine breast‐cancer risk were identical to those for the group studied to determine ovarian‐cancer in those studies (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kramer 2005; Madalinska 2007; Rebbeck 2002; Rebbeck 2004). Two studies had moderate risk of bias due to deviations from intended interventions because in Kotsopoulos 2017 study, bilateral oophorectomy was coded as a time‐dependent variable but If a woman had a bilateral oophorectomy after the completion of the baseline questionnaire (or at any point in the follow‐up), the exposure of interest was changed while in Rebbeck 1999 study, the authors excluded women with BRCA2 mutations because of relatively small numbers BRCA2 available during the study and because the authors perceived that the risk of breast and ovarian cancers (and possibly patterns of surgery use) in BRCA2 might differ from BRCA1 mutation carriers (Table 7).
Bias due to missing data
Five studies had low risk of bias as either there was no evidence of missing data or all missing data were analysed in an intention‐to‐treat basis (Domchek 2006; Ingham 2013; Kramer 2005; Madalinska 2007; Rebbeck 1999). Five studies had moderate risk of bias due to missing data because the authors of these studies reported the existence of bias that arose when later follow‐up is missing for participants initially included and followed up in their studies (Domchek 2010; Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017; Rebbeck 2002; Rebbeck 2004) (Table 7).
Bias in measurement of outcomes
Eight studies had low risk of bias because there was no evidence of bias in the measurement of any of the outcomes assessed in these studies (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017; Madalinska 2007; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004). Two studies had moderate risk of bias in measurement of outcomes because the authors reported that there were possible testing bias of women who developed cancer (Ingham 2013), or a competing risks model (instead of actual report) was used to estimate the 10‐year cumulative incidence of breast cancer in the two groups of BRCA1 mutation carriers (Kramer 2005) (Table 7).
Bias in selection of the reported result
Eight studies had low risk of bias because the medical records and family‐history reports were verified to establish the presence or absence of cancer or deaths or other outcomes (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kramer 2005; Madalinska 2007; Rebbeck 2002; Rebbeck 2004). Two studies had moderate risk of bias in selection of the reported result because the authors either reported some of their findings based on a post hoc analysis (Kotsopoulos 2017), or reported and presented only standard model results (Rebbeck 1999) (Table 7).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
All meta‐analyses pooled data from at least two of these nine studies (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004). Meta‐analyses of survival were based on HRs that were adjusted for prognostic variables.
1. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers
Primary outcomes
Overall survival: survival until death from all causes
We assessed survival from the time when women were enrolled in the study. Meta‐analysis of three studies assessing 2548 participants showed very low‐certainty evidence and found that there may be an increase in the overall survival among women who were BRCA1 or BRCA2 mutation carriers who had RRSO compared to women with no RRSO, after adjustment for important prognostic factors including age and BRCA mutation status (HR 0.32, 95% CI 0.19 to 0.54; P < 0.001; Analysis 1.1; Table 1) (Domchek 2006; Domchek 2010; Ingham 2013). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance was not important (I2 = 0%).
1.1. Analysis.
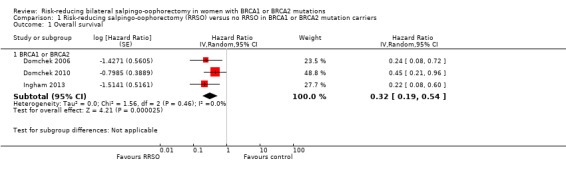
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 1 Overall survival.
High‐grade serous cancer mortality
Meta‐analysis of three studies assessing 2534 participants showed very low‐certainty evidence and found a difference in favour of RRSO versus no RRSO in HGSC (fallopian tube, ovarian and primary peritoneal cancer) mortality, after adjustment for important prognostic factors including age and BRCA mutation status (HR 0.06, 95% CI 0.02 to 0.17; P < 0.0001; Analysis 1.2; Table 1) (Domchek 2006; Domchek 2010; Rebbeck 2002). The percentage of the variability in effect estimates that was due to heterogeneity rather than to chance may have represented substantial heterogeneity (I² = 69%).
1.2. Analysis.
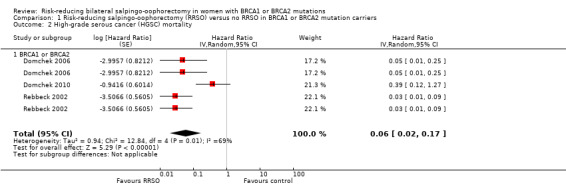
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 2 High‐grade serous cancer (HGSC) mortality.
Breast cancer mortality
Meta‐analysis of seven studies assessing 7198 participants showed very low‐certainty evidence and found a difference in favour of RRSO versus no RRSO in breast cancer mortality, after adjustment for important prognostic factors including age and BRCA mutation status (HR 0.58, 95% CI 0.39 to 0.88; P = 0.009; Analysis 1.3; Table 1) (Domchek 2006; Domchek 2010; Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017; Kramer 2005; Rebbeck 1999; Rebbeck 2002). The percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance) may have represented substantial heterogeneity (I² = 65%).
1.3. Analysis.
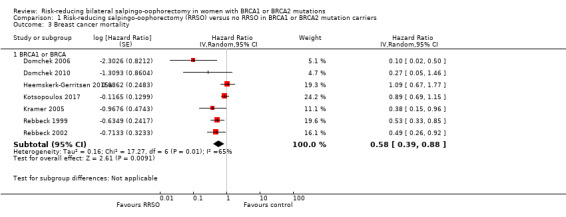
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 3 Breast cancer mortality.
Secondary outcomes
High‐grade serous cancer incidence
Four studies with 1269 participants in the RRSO groups and 2059 participants in the control groups reported HGSC incidence in women who were BRCA1 or BRCA2 mutation carriers (Domchek 2006; Heemskerk‐Gerritsen 2015a; Ingham 2013; Rebbeck 2002). A total of 14/1269 (1%) participants with RRSO versus 194/2059 (9%) participants with no RRSO developed HGSC. The meta‐analysis showed very low‐certainty evidence that RRSO versus no RRSO may have reduced HGSC incidence (RR 0.17, 95% CI 0.04 to 0.75; P = 0.02; Analysis 1.4). The percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) may have represented substantial heterogeneity (I² = 84%).
1.4. Analysis.
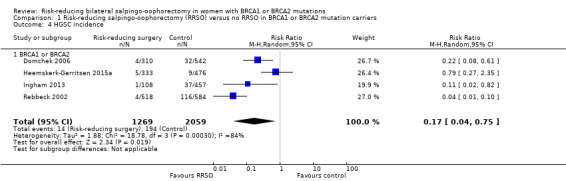
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 4 HGSC incidence.
Breast cancer incidence
Seven studies with 2285 participants in the RRSO groups and 3310 participants in the control groups reported breast cancer incidence in women with BRCA1 or BRCA2 mutations (Domchek 2006; Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017; Kramer 2005; Rebbeck 1999; Rebbeck 2002; Rebbeck 2004). A total of 230/2285 (10%) participants who had had RRSO versus 429/3310 (13%) participants with no RRSO developed breast cancer. The meta‐analysis showed very low‐certainty evidence in favour of RRSO versus no RRSO in reducing breast cancer incidence (RR 0.64, 95% CI 0.43 to 0.96; P = 0.03; Analysis 1.5). The percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) may represent substantial heterogeneity (I² = 75%).
1.5. Analysis.
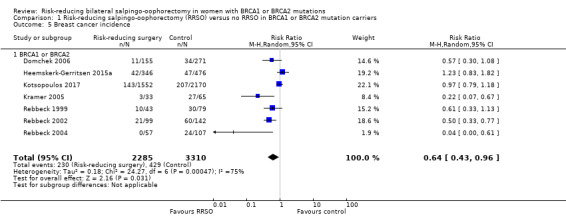
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 5 Breast cancer incidence.
Bone fracture incidence
None of the studies reported bone fracture incidence.
Disease‐free survival (time from surgical procedure to cancer diagnosis)
None of the studies reported disease‐free survival.
Morbidity: direct surgical morbidity and surgically related systemic morbidity
None of the studies reported on direct surgical morbidity or surgically related systemic morbidity (e.g. chest/wound/urine infection, venous thromboembolism, premature menopause, etc.).
Recovery and readmission
None of the studies reported on recovery or readmission.
Quality of life
Quality of life measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication (Roila 2001; Spitzer 1981). Only the Madalinska 2007 study assessed quality of life as an outcome measure and, therefore, no meta‐analysis was performed. Data from one study showed that women who had had RRSO experienced more quality of life disruption, which was higher, when compared to those who did not have RRSO in terms of general health perception (MD (SD): 70.9 (SD 20.5) with RRSO versus 82.0 (SD 13.3) with no RRSO; P < 0.0001; Analysis 1.8), but it was not different in terms of global health status quality of life (MD (SD): 76.0 (SD 20.6) with RRSO versus 79.8 (SD 17.9) with no RRSO; P = 0.26; Analysis 1.7) and mental health quality of life (MD (SD): 70.2 (SD 16.6) with RRSO versus 73.1 (SD 14.5) with no RRSO; P = 0.28; Analysis 1.9). However, there was a difference in favour of RRSO compared with no RRSO in terms of ovarian cancer risk perception quality of life (MD 15.40, 95% CI 8.76 to 22.04; P < 0.00001; very low‐certainty evidence; Analysis 1.6), and breast cancer risk perception quality of life (MD 8.20, 95% CI 0.85 to 15.55; P = 0.03; very low‐certainty evidence; Analysis 1.10).
1.8. Analysis.
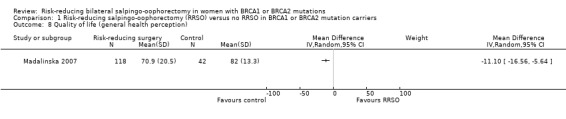
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 8 Quality of life (general health perception).
1.7. Analysis.
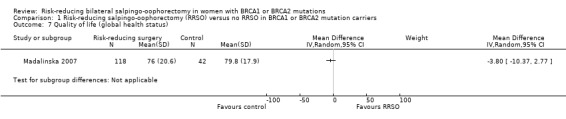
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 7 Quality of life (global health status).
1.9. Analysis.
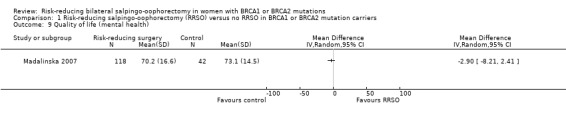
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 9 Quality of life (mental health).
1.6. Analysis.
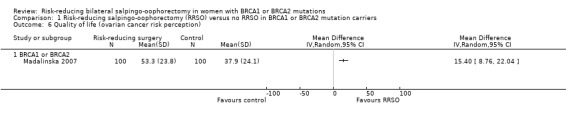
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 6 Quality of life (ovarian cancer risk perception).
1.10. Analysis.
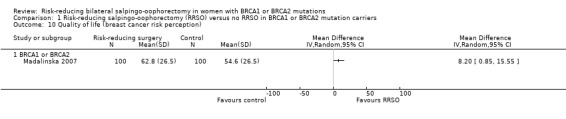
Comparison 1 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers, Outcome 10 Quality of life (breast cancer risk perception).
Severe adverse events, classified according to CTCAE 2010
None of the studies reported on severe adverse events.
2. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status: subgroup analyses
Primary outcomes
Overall survival: survival until death from all causes
Three studies reported overall survival among participants with BRCA1 mutations (Domchek 2006; Domchek 2010; Ingham 2013). Meta‐analysis showed a lower risk of death among women with BRCA1 mutations who had had RRSO than in women who had not had RRSO (HR 0.30, 95% CI 0.17 to 0.52; P < 0001; very low‐certainty evidence; Analysis 2.1; Table 2). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance was not important (I² = 23%).
2.1. Analysis.
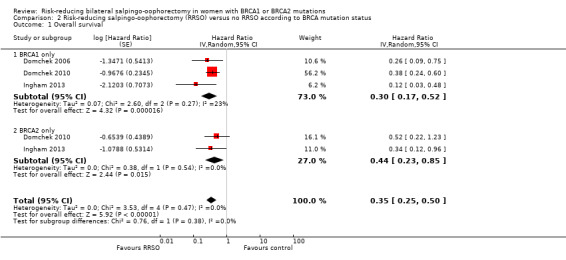
Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 1 Overall survival.
Two studies reported overall survival among participants who were BRCA2 mutation carriers (Domchek 2010; Ingham 2013). Meta‐analysis showed a lower risk of death among women who were BRCA2 mutation carriers who received RRSO than in women with no RRSO (HR 0.44, 95% CI 0.23 to 0.85; P = 0.01; very low‐certainty evidence; Analysis 2.1; Table 2). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance was not important (I² = 0%).
Tests for subgroup differences showed no significant difference in effect between studies assessing RRSO versus no RRSO on overall survival according to the mutation status: BRCA1 and BRCA2 (P = 0.38).
High‐grade serous cancer mortality
Two studies reported HGSC deaths among participants who were BRCA1 mutation carriers (Domchek 2006; Domchek 2010). Meta‐analysis from the two studies showed a difference in favour of RRSO than no RRSO for HGSC deaths among women who were BRCA1 mutation carriers (HR 0.10, 95% CI 0.02 to 0.41; P = 0.001; very low‐certainty evidence; Analysis 2.2; Table 2). The percentage of the variability in effect estimates that was due to heterogeneity rather than to chance may have represented moderate heterogeneity (I² = 54%).
2.2. Analysis.
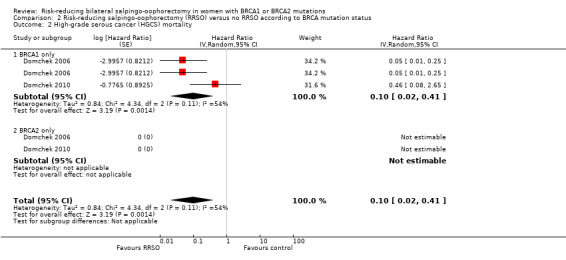
Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 2 High‐grade serous cancer (HGCS) mortality.
Two studies reported HGSC deaths among participants who were BRCA2 mutation carriers (Domchek 2006; Domchek 2010). Data from two studies showed no HGSC deaths among women who were BRCA2 mutation carriers who received RRSO versus no RRSO (HR not applicable; I² = not applicable; Analysis 2.2). No tests for subgroup differences could be performed as HGSC mortality was only reported in women who were BRCA1 only mutation carriers with no reported event in BRCA2 mutation carriers.
Breast cancer mortality
Four studies reported breast cancer mortality among participants who were BRCA1 mutation carriers (Domchek 2006; Domchek 2010; Kramer 2005, Rebbeck 1999). Meta‐analysis from the four studies showed a difference in favour of RRSO compared with no RRSO for breast cancer mortality among women who were BRCA1 only mutation carriers (HR 0.45, 95% CI 0.30 to 0.67; P < 0.0001; very low‐certainty evidence; Analysis 2.3). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance was not important (I² = 0%).
2.3. Analysis.
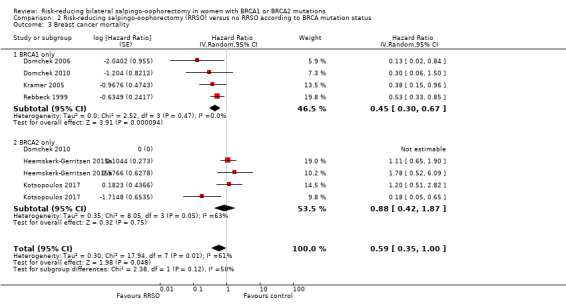
Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 3 Breast cancer mortality.
Three studies reported breast cancer mortality among participants who were BRCA2 mutation carriers (Domchek 2010; Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017). Meta‐analysis from the two studies showed no difference in favour of RRSO compared with no RRSO for breast cancer mortality among women who were BRCA2 mutation carriers (HR 0.88, 95% CI 0.42 to 1.87; P = 0.75; very low‐certainty evidence; Analysis 2.3; Table 2) (Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017). Data from one study showed no breast cancer deaths among women who were BRCA2 mutation carriers who received RRSO versus no RRSO (HR not applicable) (Domchek 2010). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance may represent substantial heterogeneity (I² = 63%).
Tests for subgroup differences showed no difference in effect between studies assessing RRSO versus no RRSO on breast cancer mortality according to the mutation status: BRCA1 and BRCA2 (P = 0.12).
Secondary outcomes
High‐grade serous cancer incidence
None of the studies reported HGSC incidence according to BRCA mutation carrier status: BRCA1 and BRCA2.
Breast cancer incidence
None of the studies reported breast cancer incidence according to BRCA mutation carrier status: BRCA1 and BRCA2.
Bone fracture incidence
None of the studies reported bone fracture incidence according to BRCA mutation carrier status: BRCA1 and BRCA2.
Disease‐free survival (time from surgical procedure to cancer diagnosis)
None of the studies reported disease‐free survival according to BRCA mutation carrier status: BRCA1 and BRCA2.
Morbidity: direct surgical morbidity and surgically related systemic morbidity
None of the studies reported morbidity according to BRCA mutation carrier status: BRCA1 and BRCA2.
Recovery and readmission
None of the studies reported recovery and readmission according to BRCA mutation carrier status: BRCA1 and BRCA2.
Quality of life (ovarian cancer risk perception)
One study with 58 participants in the RRSO groups and 40 participants in the control groups reported quality of life for ovarian cancer risk perception in women who were BRCA1 mutation carriers (Madalinska 2007). Data from one study showed a difference in favour of RRSO compared with no RRSO in improving the quality of life for ovarian cancer risk perception in women who were BRCA1 mutation carriers (MD 10.70, 95% CI 2.45 to 18.95; P = 0.01; very low‐certainty of evidence; Analysis 2.4).
2.4. Analysis.
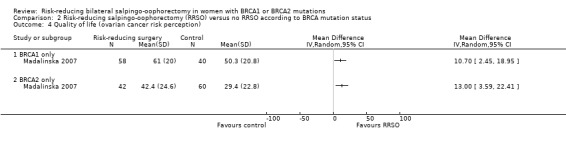
Comparison 2 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status, Outcome 4 Quality of life (ovarian cancer risk perception).
One study with 42 participants in the RRSO groups and 60 participants in the control groups reported quality of life for ovarian cancer risk perception in women who were BRCA2 mutation carriers (Madalinska 2007). Data from one study showed a difference in favour of RRSO compared with no RRSO in improving the quality of life for ovarian cancer risk perception in women who were BRCA2 mutation carriers (MD 13.00, 95% CI 3.59 to 22.41; P = 0.007; very low‐certainty of evidence; Analysis 2.4). Tests for subgroup differences showed no significant difference in effect between studies comparing RRSO versus no RRSO on quality of life for ovarian cancer risk perception according to the mutation status: BRCA1 and BRCA2 (P = 0.72).
Severe adverse events, classified according to CTCAE 2010
None of the studies reported on severe adverse events according to BRCA mutation status.
3. BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery: subgroup analyses
Primary outcomes
Overall survival: survival until death from all causes
RRSO alone versus RRSO and risk‐reducing mastectomy (RRM)
None of the studies reported overall survival among participants who were BRCA1 or BRCA2 mutation carriers who received RRSO alone versus RRSO and RRM.
RRSO and RRM versus no RRSO
One study reported overall survival among participants with BRCA1 or BRCA2 mutations who received RRSO and mastectomy versus no RRSO (Ingham 2013). Data from one study showed an increase in overall survival among women who were BRCA1 or BRCA2 mutation carriers who received RRSO and RRM compared to women with no RRSO (HR 0.14, 95% CI 0.02 to 0.98; P = 0.0001; very low‐certainty evidence; Analysis 3.1; Table 3).
3.1. Analysis.
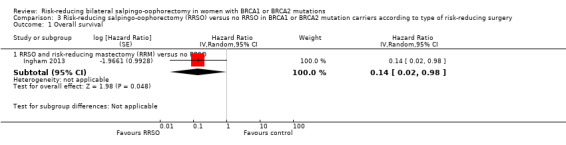
Comparison 3 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery, Outcome 1 Overall survival.
Since only subgroup analysis among participants with BRCA1 or BRCA2 mutations who received RRSO and mastectomy versus no RRSO was possible for overall survival, tests for subgroup differences could not be performed for this outcome.
High‐grade serous cancer mortality
None of the studies reported HGSC mortality among participants who were BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery.
Breast cancer mortality
RRSO alone versus RRSO and risk‐reducing mastectomy (RRM)
None of the studies reported breast cancer mortality among participants who were BRCA1 or BRCA2 mutation carriers who received RRSO alone versus RRSO and RRM.
RRSO and RRM versus no RRSO
One study reported cancer mortality among participants who received bilateral salpingo‐oophorectomy and bilateral mastectomy (Heemskerk‐Gerritsen 2015a). There was no difference between bilateral salpingo‐oophorectomy plus bilateral mastectomy and no RRSO for breast cancer mortality among women who received bilateral salpingo‐oophorectomy plus bilateral mastectomy (HR 0.78, 95% CI 0.51 to 1.19; P = 0.25; very low‐certainty evidence; Analysis 3.2; Table 3). Since only one subgroup analysis was possible for breast cancer mortality, tests for subgroup differences could not be performed for this outcome.
3.2. Analysis.
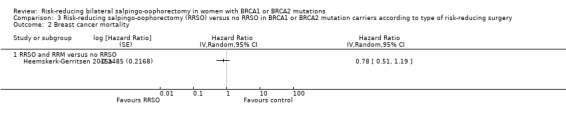
Comparison 3 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery, Outcome 2 Breast cancer mortality.
Secondary outcomes
High‐grade serous cancer incidence
None of the studies reported on HGSC incidence according to type of risk‐reducing surgery.
Breast cancer incidence
None of the studies reported on breast cancer incidence according to type of risk‐reducing surgery.
Bone fracture incidence
None of the studies reported on bone fracture incidence according to type of risk‐reducing surgery.
Disease‐free survival (time from surgical procedure to cancer diagnosis)
None of the studies reported on disease‐free survival according to type of risk‐reducing surgery.
Morbidity: direct surgical morbidity and surgically related systemic morbidity
None of the studies reported on morbidity according to type of risk‐reducing surgery.
Recovery and readmission
None of the studies reported on recovery and readmission according to type of risk‐reducing surgery.
Quality of life
None of the studies reported on quality of life according to type of risk‐reducing surgery.
Severe adverse events, classified according to CTCAE 2010
None of the studies reported on severe adverse events according to type of risk‐reducing surgery.
4. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO for BRCA1 mutation carriers according to age at time of RRSO: subgroup analyses
Primary outcomes
Overall survival: survival until death from all causes
None of the studies reported on overall survival according to age at time of RRSO.
High‐grade serous cancer mortality
None of the studies reported on HGSC mortality according to age at time of RRSO.
Breast cancer mortality
Three studies reported breast cancer mortality among participants who were BRCA1 mutation carriers who received RRSO at 50 years of age or less (Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017; Rebbeck 1999). Meta‐analysis from the three studies showed no difference between RRSO and no RRSO for breast cancer mortality among women who were BRCA1 mutation carriers who received RRSO at 50 years of age or less (HR 0.78, 95% CI 0.55 to 1.09; P = 0.15; very low‐certainty evidence; Analysis 4.1; Table 4). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance was moderate heterogeneity (I² = 42%).
4.1. Analysis.
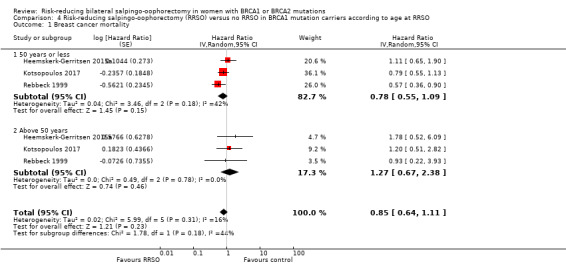
Comparison 4 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 mutation carriers according to age at RRSO, Outcome 1 Breast cancer mortality.
Three studies reported breast cancer mortality among participants who were BRCA1 mutation carriers who received RRSO at more than 50 years of age (Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017; Rebbeck 1999). Meta‐analysis from the three studies showed no difference between RRSO and no RRSO for breast cancer mortality among women who were BRCA1 mutation carriers who received RRSO at more than 50 years of age (HR 1.27, 95% CI 0.67 to 2.38; P = 0.46; very low‐certainty evidence; Analysis 4.1; Table 4). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance was not important (I² = 0%).
Tests for subgroup differences showed no difference in studies that reported breast cancer mortality according to age at surgery: 50 years of age or less and more than 50 years of age (P = 0.18; I² = 43.8%).
Secondary outcomes
High‐grade serous cancer incidence
None of the studies reported on HGSC incidence according to age at time of RRSO.
Breast cancer incidence
None of the studies reported on breast cancer incidence according to age at time of RRSO.
Bone fracture incidence
None of the studies reported on bone fracture incidence according to age at time of RRSO.
Disease‐free survival (time from surgical procedure to cancer diagnosis)
None of the studies reported on disease‐free survival according to age at time of RRSO.
Morbidity: direct surgical morbidity and surgically related systemic morbidity
None of the studies reported on morbidity according to age at time of RRSO.
Recovery and readmission
None of the studies reported on recovery and readmission according to age at time of RRSO.
Quality of life
None of the studies reported on quality of life according to age at time of RRSO.
Severe adverse events, classified according to CTCAE 2010
None of the studies reported on severe adverse events according to age at time of RRSO.
5. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO for BRCA2 mutation carriers according to age at time of RRSO: subgroup analyses
Primary outcomes
Overall survival: survival until death from all causes
None of the studies reported on overall survival according to age at time of RRSO.
High‐grade serous cancer mortality
None of the studies reported on HGSC mortality according to age at time of RRSO.
Breast cancer mortality
Two studies reported breast cancer deaths among participants who were BRCA2 mutation carriers who received RRSO at 50 years of age or less (Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017). Meta‐analysis from the two studies showed no difference between RRSO and no RRSO for breast cancer mortality among women who were BRCA2 mutation carriers who received RRSO at 50 years of age or less (HR 0.49, 95% CI 0.08 to 2.90; P = 0.43; very low‐certainty evidence; Analysis 5.1; Table 5). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance may represent moderate heterogeneity (I² = 85%).
5.1. Analysis.
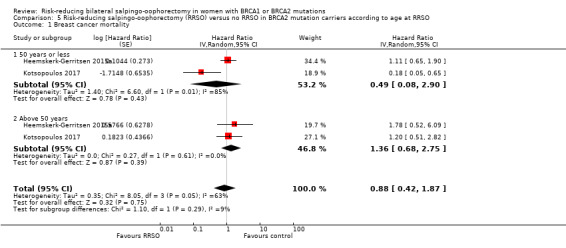
Comparison 5 Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA2 mutation carriers according to age at RRSO, Outcome 1 Breast cancer mortality.
Two studies reported disease‐free survival for breast cancer deaths among participants who were BRCA2 mutation carriers who received RRSO at more than 50 years of age (Heemskerk‐Gerritsen 2015a; Kotsopoulos 2017). Meta‐analysis from the two studies showed no difference between RRSO and no RRSO for breast cancer mortality among women who were BRCA2 mutation carriers who received RRSO at more than 50 years of age (HR 1.36, 95% CI 0.68 to 2.75; P = 0.39; very low‐certainty evidence; Analysis 5.1; Table 5). The percentage of variability in effect estimates that was due to heterogeneity rather than to chance was unimportant (I² = 0%).
Tests for subgroup differences showed no difference in studies that reported breast cancer mortality among participants who were BRCA2 mutation carriers according to age at surgery: 50 years of age or less and more than 50 years of age (P = 0.29; I² = 8.9%).
Secondary outcomes
High‐grade serous cancer incidence
None of the studies reported on HGSC incidence according to age at time of RRSO.
Breast cancer incidence
None of the studies reported on breast cancer incidence according to age at time of RRSO.
Bone fracture incidence
None of the studies reported on bone cancer incidence according to age at time of RRSO.
Disease‐free survival (time from surgical procedure to cancer diagnosis)
None of the studies reported on disease‐free survival according to age at time of RRSO.
Morbidity: direct surgical morbidity and surgically related systemic morbidity
None of the studies reported on morbidity according to age at time of RRSO.
Recovery and readmission
None of the studies reported on recovery and remission according to age at time of RRSO.
Quality of life
None of the studies reported on quality of life according to age at time of RRSO.
Severe adverse events, classified according to CTCAE 2010
None of the studies reported on severe adverse events according to age at time of RRSO.
Discussion
Summary of main results
The limited evidence suggested that RRSO may have increased overall survival and lowered HGSC mortality for BRCA1 and BRCA2 carriers. Additionally, very limited evidence suggested that breast cancer mortality may have been reduced in BRCA1 mutation carriers following RRSO, but may not have reduced breast cancer mortality in women who were BRCA2 mutation carriers. RRSO may also have reduced the risk of death from HGSC and breast cancer in women who were BRCA1 carriers but the evidence for the effect on breast cancer was uncertain in BRCA2 carriers due to low number of reported events. There was no evidence that RRSO affected bone fracture incidence, quality of life or severe adverse events, as well as the effects of RRSO based on type of risk‐reducing surgery and age at the time of RRSO. These results should be viewed with caution, however, as all the studies included in this Cochrane Review were non‐randomised observational studies with the potential of introducing several forms of bias (confounding by indication, detection bias, cancer‐induced testing bias, immortal person‐time bias, ascertainment bias, familial‐events bias and informative censoring bias), all of which had relatively short follow‐up periods of study participants in relation to peak incidence of HGSC. However, the findings should be very important for an increasing number of women with BRCA1/2 mutations and their need to make decisions about surgery. Despite a lack of randomised trials (which are ethically impossible), this Cochrane Review excluded women with a previous or coexisting breast malignancy and some studies excluded women with unilateral oophorectomy or salpingectomy or salpingo‐oophorectomy (Rebbeck 1999; Rebbeck 2002). Additionally, all 10 included studies used statistical adjustment or validated scale for quality of life (quality of life) assessment in their analyses and all adjusted for pertinent variables. These are major strengths to this review. The Table 1 summarises the main outcomes.
Overall completeness and applicability of evidence
We found no studies that assessed adverse events, surgical morbidity and mortality. Although we specified quality of life as an outcome of interest and one study reported this, quality of life after surgical treatment for RRSO is an extremely important outcome, as treatment‐related morbidity very often reduces the quality of life of women who had RRSO for prophylaxis for BRCA1 or BRCA2 mutation carriers. Also, none of the studies reported that they examined biopsied specimens of removed tissues in accordance with the Sectioning and Extensively Examining the Fimbria (SEE/FIM) protocol.
One major issue in the interpretation of this Cochrane Review was the rate of occult malignancy in women who had RRSO as only two studies reported occult carcinoma rates (Domchek 2010; Ingham 2013). Therefore, if a clinically undetected tumour was found in tissue removed during prophylactic surgery, the surgery ultimately did not prevent tumour occurrence, although it may have increased life expectancy. How this possible favourable effect of surgery should be incorporated in the analysis is debatable (Klaren 2003). By counting the event in the surgery group, cancer risk is overestimated and efficacy is underestimated (Klaren 2003), and by excluding the event, efficacy may be overestimated.
The major contribution to the evidence was the inclusion of oophorectomy as a time‐dependent variable in Kotsopoulos 2017. Another major factor is the time of RRSO as women who had surgery at 40 years of age tended to have a higher prevalence of precursor lesions especially in BRCA1 carriers (Lee 2017). Occult carcinoma was seen in 5.4% of asymptomatic BRCA1/2 mutation carriers and 86% of which were tubal in origin (Zakhour 2016). Occult carcinomas were detected in nearly 10% of BRCA1/2 carriers, and 19% of BRCA1/2 carriers over the age of 45 years (Domchek 2007). Unlike in an RCT, which compares disease incidence after the participants are randomly assigned to receive the intervention or no intervention, in a non‐randomised observational clinic‐based cohort study, either retrospective or prospective, the follow‐up time and case ascertainment appropriately begin when the woman is first seen at the clinic. Therefore, diagnoses and person‐time occurring prior to the intervention are considered unexposed while diagnoses and person‐time occurring after the intervention are considered exposed (Wacholder 2004).
Studies also compared RRSO versus no RRSO in reducing risk of breast cancers in the subgroup analysis in premenopausal women aged less than 50 years and 50 years and over. Altered oestrogen receptor expression in mammary gland cells was suggested to play an important role during tumour genesis of breast cancer (Hussein 2008; Leygue 1998). Given the fact that BRCA2‐associated breast cancers are mainly oestrogen‐receptor positive, while the majority of BRCA1‐associated breast cancers are oestrogen‐receptor negative (Loman 1998), breast cancer risk‐reducing effect of RRSO may be expected in BRCA2 mutation carriers but not in BRCA1 mutation carriers. Unfortunately, in the current cohort, the numbers of BRCA2 mutation carriers, and especially the numbers of events in that specific group, were too small to perform conclusive gene‐stratified analyses using the proposed design and analytical method.
However, because the primary goal of Domchek 2010 study was to analyse the impact of RRSO on carriers of BRCA1 and BRCA2 mutations independently, Domchek 2010 excluded 12 participants because they had both BRCA1 and BRCA2 mutations. Interestingly, none of the included participants in this Cochrane systematic review had both BRCA1 and BRCA2 mutation carrier status.
Quality of the evidence
The body of evidence from non‐randomised or observational studies generally begins with a low confidence and then is marked up if they demonstrate characteristics that would increase the reviewers' confidence in the findings from these designs such as a large magnitude of effect, dose–response relationship, or plausible confounding that would have otherwise weakened the effect estimate (Agoritsas 2013). When a methodologically strong observational study yields large or very large and consistent estimates of the magnitude of a treatment or exposure effect, we may be confident about the results. In these situations, the weak study design is unlikely to explain all of the apparent benefit or harm, even though observational studies are likely to provide an overestimate of the true effect. Overall, the certainty of the evidence was derived from non‐randomised studies and was rated as very low according to GRADE methodology for the main comparison, mostly on account of the studies being at moderate risk of bias (Table 1; Table 2; Table 3; Table 4; Table 5). A variety of limitations in the individual studies may have further interfered with the certainty of evidence. Although there were adjustments for meaningful confounding factors, specifically, there were relatively short follow‐up periods of study participants in relation to peak incidence of ovarian cancer or primary peritoneal cancer. Apart from Kramer 2005 which was at serious risk of bias, all other included studies were of moderate risk of bias. Only one out of the 10 included cohort studies excluded women if they had a cancer diagnosis within the first six months of follow‐up in order to avoid including cancers that would have been minimally influenced by RRSO or RRM (Domchek 2010). Heterogeneity among studies might be due to, for example, differences in time of conducting the studies as more recent studies (within five years) appeared to differ widely from older studies in their results. To reflect our concern about heterogeneity, we conducted all analyses using both a fixed‐effect analysis and a random‐effects analysis but results from the two models did not differ, and so we reported a random‐effects analysis. Meta‐analysis of studies reporting on HGSC mortality and breast cancer mortality and in accordance to BRCA2 mutation carriers and age at RRSO in BRCA2 mutation carriers as well as HGSC incidence and breast cancer incidence showed moderate to substantial heterogeneity. However, all the survival analyses and mortality analyses were evaluated using HR, which is the best statistic for summarising differences in risk between two treatment groups over the duration of a study when time to death or disease progression is 'censored' or unknown for some women, as they were still alive (or disease‐free) at the end of the study.
Potential biases in the review process
When evaluating the efficacy and safety of risk‐reducing surgery, the aim is to identify two groups of women who differ in the exposure of interest, namely prophylactic surgery, but who are, or in the analysis can be made, similar with regard to other factors associated with disease outcome. Ideally, this could be accomplished in a randomised clinical trial, but randomisation for prophylactic surgery for BRCA1 or BRCA2 mutation carriers is obviously unethical since we cannot restrict risk‐reducing surgical treatment to women requiring it solely on research. This Cochrane Review was limited to non‐randomised observational studies with the potential of introducing several forms of bias (confounding by indication, detection bias, cancer‐induced testing bias, immortal person‐time bias, ascertainment bias, familial‐events bias and informative censoring bias) (Klaren 2003; Wacholder 2004). Ascertainment bias could result from people at a higher risk for cancer being more likely to seek out and enrol onto studies than those at lower risk for cancer. This effect has been documented in several previous studies, especially those that were based on familial aggregation of cancer in high‐risk families (Begg 2002).
Additionally, the matching factors used in many studies included (i.e. age, centre or mutation type) may favour the selection of relatives into the non‐surgery group. This selection may unintentionally increase bias. For example, there could be a phenomenon of potential dependency between the diagnosis of cancer within the family and individual decisions of relatives undergoing DNA testing, cancer screening, risk‐reducing surgery or a combination of these. If these events are assumed to be independent in studies that include several members of the same family, bias such as confounding by indication and familial‐event bias may arise as was seen in Rebbeck 1999. For instance, one study reported that 59% of the women were related to at least one other study participant, and 32% were related to at least four other study participants (Rebbeck 1999). Despite the acknowledgment of the relationship among study participants, the authors did not consider dependency between events within a family. By selecting the appropriate time period for members of one family to be at risk, bias can be prevented.
To avoid familial‐event bias, the best choice for start of follow‐up is the age at which the control herself was tested or the age of the control at the date of her relative's prophylactic surgery, whichever came last (Klaren 2003; Wacholder 2004). This is because women in the comparison group should be cancer‐free at the point of ascertainment.
This Cochrane Review employed meta‐analyses that used a publication‐based approach and obtaining individual participant data for each study was not possible. Therefore, we adopted clear definitions of exposures and outcome using ROBINS‐I and we adhered to procedures that minimised extraction, recording and retrieval bias, by carefully searching for 'grey' literature. Furthermore, we applied no language restrictions. There was no evidence of publication bias documented as the meta‐analysis did not involve more than 10 studies.
Some studies included in this Cochrane Review employed questionnaires for data extraction on information regarding reproductive history, surgical history (including preventive oophorectomy and mastectomy) and hormone use. Such questionnaire may not distinguish between oophorectomy and salpingo‐oophorectomy, although one recent Swedish study revealed that physicians and genetic counsellors can rely on self‐reported information regarding breast cancer and ovarian cancer in BRCA1 or BRCA2 mutation carriers (Augustinsson 2018).
Altered oestrogen receptor expression in mammary gland cells is suggested to play an important role during tumour genesis of breast cancer (Archey 2017). Given the fact that BRCA2‐associated breast cancers are mainly oestrogen‐receptor positive, while the majority of BRCA1‐associated breast cancers are oestrogen‐receptor negative (Archey 2017; Loman 1998), a breast cancer risk‐reducing effect of RRSO may be expected in BRCA2 mutation carriers than in BRCA1 mutation carriers. Unfortunately, in the current review, the numbers of BRCA2 mutation carriers, and especially the numbers of events in that specific group, were too small to perform conclusive gene‐stratified analyses in the subgroup analyses.
Some studies in this review used controls who were not necessarily undergoing surveillance (Rebbeck 2002), and other studies used controls who were prospectively followed up on an active annual surveillance programme. Also, some studies did not perform direct matching of cases (Domchek 2010; Ingham 2013; Kotsopoulos 2017; Kramer 2005; Madalinska 2007). It is possible that the biological effects of other demographic variables may have been different between the RRSO and the surveillance or control group, such as age at study entry, parity and history of HRT, but might not have been completely corrected by the covariate treatment in the analysis.
More importantly, we excluded women with a previous or coexisting breast malignancy and some studies excluded women with unilateral oophorectomy or salpingectomy or salpingo‐oophorectomy (Rebbeck 1999; Rebbeck 2002). In addition, we excluded women with prophylactic salpingectomy with delayed oophorectomy or ovarian conservation (Harmsen 2015; Harmsen 2016; Nebgen 2018; Tschernichovsky 2017). These are major strengths to this review. If a personal history of breast cancer at the time of study entry had been included, it would have introduced a potential bias into the analysis. For instance, mortality reduction estimates for women with and without a prior history of breast cancer may differ. Therefore, limiting the analyses to participants without a personal history of breast cancer at the time of study entry or RRSO confers more magnitude of protection assessment.
We employed a new ROBINS‐I tool for assessment of risk of bias in observational studies. It includes a structured approach to assessment of risk of bias due to confounding that starts at the review protocol stage and makes it possible for comprehensive risk of bias assessments that are applicable to a wide range of study designs and analyses. The ROBINS‐I tool focuses specifically on bias and does not address problems related to imprecision of results.
The ratio of 1:2 of control participants among all the women who were BRCA1 or BRCA2 mutation carriers enrolled in this Cochrane Review was in line with the recommendations of Klaren 2003 and Wacholder 2004, which were meant to address potential sampling and information biases in studies of risk‐reducing surgeries from multicentre cohorts. The authors of Klaren 2003 and Wacholder 2004 recommended selection of between one and four controls for comparison with every RRSO participant, which was the outcome of this Cochrane review. However, the conclusions were limited by the absence of randomised controlled trials (Finch 2011). The absence of deaths from HGSC in the studies that reported HGSC in BRCA2 mutation carriers may be due to smaller number of samples of BRCA2 mutation carriers compared with BRCA1 mutation carriers in the included studies. In the absence of cancer events, HR cannot be estimated.
Agreements and disagreements with other studies or reviews
Previous systematic reviews (Ludwig 2016; Marchetti 2014; Tschernichovsky 2017), and meta‐analysis (Rebbeck 2009), or both (Li 2016), have been published on the benefit of RRSO in women with BRCA1 or BRCA2 mutations. Some studies included in these previous systematic reviews or meta‐analyses were not included in our present meta‐analysis (Chang‐Claude 2007; Eisen 2005; Evans 2009; Finch 2006; Finch 2014; Finkelman 2012; Kauff 2002; Kauff 2008; Rutter 2003; Schmeler 2006). This was necessary because in a systematic review in which HGSC or breast cancer is the endpoint of interest, studies including women with prior ovarian cancer or breast cancer (or both) should be excluded to avoid biases that would favour either the surgical or non‐surgical group.
The authors of Ludwig 2016 concluded that the reduction in ovarian and breast cancer risks with the use of RRSO improves in survival and clinical management of women at increased risk for breast cancer but requires consideration of risk and quality of life. They excluded non‐English publications. Five of the six included studies were excluded in this Cochrane Review (Evans 2009; Finch 2014; Kauff 2002; Kauff 2008; Schmeler 2006). Evans 2009 was excluded because not all the included women had known BRCA1 or BRCA2 mutation carrier status; Finch 2014 was a single arm study without comparison group; and Kauff 2002, Kauff 2008, and Schmeler 2006 included women with a previous or coexisting breast malignancy.
The authors of Marchetti 2014 concluded that it was justified to recommend RRSO to reduce ovarian cancer risk and all‐causes mortality in women with a BRCA1 or BRCA2 mutation. Two studies out of the three studies included were excluded in this review (Finch 2014; Kauff 2008). Finch 2014 was excluded because it was a single arm study without comparison group and Kauff 2008 included women with a previous or coexisting breast malignancy.
The authors of the Tschernichovsky 2017 systematic review concluded that until more data were made available, RRSO and oral contraceptive pills remain the only recommended preventive measures in carriers of BRCA1 or BRCA2 mutations for substantially reducing the risk of ovarian cancer. Only one study (Domchek 2010) out of the four studies (Domchek 2010; Finch 2014; Marchetti 2014; Rebbeck 2009) included by the authors of Tschernichovsky 2017 systematic review was included in the present review. Finch 2014 was excluded because it was a single arm study without comparison group and Marchetti 2014 and Rebbeck 2009 were review articles.
The Rebbeck 2009 meta‐analysis concluded that RRSO was strongly associated with reductions in the risk of breast cancer and HGSC and should provide guidance to women in planning cancer risk reduction strategies. Six out of 10 studies were excluded in this review (Chang‐Claude 2007; Eisen 2005; Finch 2006; Kauff 2002; Kauff 2008; Rutter 2003). Rutter 2003 was excluded because not all the included women have known BRCA1 or BRCA2 mutation carrier status; Chang‐Claude 2007, Eisen 2005, Finch 2006, Kauff 2002, and Kauff 2008 included women with a previous or coexisting breast malignancy.
Li 2016 concluded that BRCA1 or BRCA2 mutation carriers treated with RRSO have a substantially reduced breast cancer incidence and mortality. Of the 15 studies, 11 studies were excluded in this Cochrane Review (Chang‐Claude 2007; Eisen 2005; Evans 2013; Finkelman 2012; Heemskerk‐Gerritsen 2013; Heemskerk‐Gerritsen 2015b; Kauff 2008; Meijers‐Heijboer 2001; Metcalfe 2014; Skytte 2011; van Sprundel 2005). In addition to what were already stated, we excluded seven studies because they included women with or without a family history or personal history of breast cancer who were carriers of BRCA1 and BRCA2 mutations and initially treated with unilateral RRM or BRRM), but without RRSO (Evans 2013; Heemskerk‐Gerritsen 2013; Heemskerk‐Gerritsen 2015b; Meijers‐Heijboer 2001; Metcalfe 2014; Skytte 2011; van Sprundel 2005). Finkelman 2012 included women with a previous or coexisting breast malignancy.
One non‐Cochrane systematic review described implications of premenopausal RRSO on quality of life, endocrine symptoms, sexual function, osteoporosis, cardiovascular health, metabolic syndrome, cognitive impairment and safety of HRT (Vermeulen 2017). The results of the review revealed that surgical menopause leads to more menopausal complaints and sexual dysfunction than natural menopause but overall quality of life is not affected by surgery. The authors found no evidence that RRSO leads to more osteopenia in comparison with natural menopause at a young age but revealed that cohort studies showed a slight impaired cardiovascular health and cognitive function decreases later in life in premenopausal oophorectomised women. The authors also concluded that short‐term HRT seemed to decrease postmenopausal complaints and did not seem to increase the risk for breast carcinoma in mutation carriers without a personal history of breast carcinoma (Vermeulen 2017).
Another non‐Cochrane review determined the impact of RRSO on quality of life and health in women who carry a BRCA mutation revealed that preliminary studies focused on the short‐term effects, such as overall quality of life which was similar before and after surgery (Finch 2011). However, vasomotor symptoms related to surgical menopause and changes in sexual functioning were common. HRT appeared to mitigate some but not all of these symptoms. Therefore, a short course of HRT may not be contraindicated for BRCA1 mutation carriers who have undergone menopause and who have no personal history of cancer (Kotsopoulos 2016). Women reported high levels of satisfaction with their decision to have the surgery despite the impact of RRSO.
Authors' conclusions
Implications for practice.
The available data were of very‐low certainty and at overall moderate risk of bias. From this review of non‐randomised data, we conclude that risk‐reducing salpingo‐oophorectomy (RRSO) compared to no RRSO in breast cancer 1 gene (BRCA1) and breast cancer 2 gene (BRCA2) mutation carriers, analysed together, showed an increase in overall survival. Although, when analysed separately, there was a decrease in both high‐grade serous cancer (HGSC) and breast cancer mortalities in BRCA1 mutation carriers, but not in BRCA2 mutation carriers. Data analysis from BRCA1 and BRCA2 mutation carriers together found no effect of RRSO together with risk‐reducing mastectomy (RRM) on breast cancer mortality. These data should not prevent women from seeking risk‐reducing surgical treatment, but they should be aware of their underlying non‐protective effects in BRCA2 mutation carriers or in performing RRSO with or without mastectomy without consideration of the mutation status and the possible increased risk of the effects of hormonal loss or impact on the quality of life. Some caution may also be warranted in counselling women on the definite reduction in the risk of breast cancer following salpingo‐oophorectomy that is performed before menopause, contrary to what has previously been published in non‐Cochrane systematic reviews or meta‐analyses (Li 2016; Ludwig 2016; Rebbeck 2009).
Implications for research.
Follow‐up periods in these studies were relatively short and likely to underestimate the longer‐term influence of RRSO in BRCA carriers. Future studies should aim to significantly increase follow‐up periods to more fully inform women of longer‐term risks and benefits of RRSO.
The data search revealed a paucity of cases reported in BRCA2 compared to BRCA1 mutation carriers and there are problems with publications of mixed data from BRCA1 and BRCA2 mutation carriers as well as inclusion of unconfirmed mutation carriers in either the test or the control participants in some of the searched studies. Future research is needed to address these methodological problems. Such future studies should also address the need to exclude or differentiate (or both) participants who have had previous RRM or breast cancer for ovarian cancer or breast cancer analysis. These future studies should also differentiate and report other types of gynaecological cancers, including not only HGCS but also endometrial cancers, their histiotypes and precursor lesions as well as concurrent report of these factors by type of risk‐reducing surgery and age groups. Such studies should also report bone fracture incidence, adverse events, morbidities, recovery/readmission, cost effectiveness of interventions, associated comorbidities and quality of life.
Although cumulative evidence from a previous non‐Cochrane systematic review suggests that short‐term HRT use following RRSO improves quality of life (Siyam 2017), studies of the long‐term health outcomes and quality of life after RRSO in women who carry a BRCA mutation have not yet been published and so such research should be a priority. Additionally, none of the studies reported on RRSO with concurrent hysterectomy, therefore, future studies on RRSO with concurrent hysterectomy are also a priority since young women who undergo RRSO without HRT may face severe vasomotor symptoms along with elevated risks for osteoporosis, cardiovascular disease and cognitive decline (Guidozzi 2016). Women who had RRSO may be reluctant to use menopausal HRT following RRSO due to concerns that HRT might elevate breast cancer risk. However, some researchers have suggested that given the substantial reduction in breast cancer risk associated with oestrogen‐only therapy, in BRCA1 carriers with intact breasts who have completed childbearing, hysterectomy (which eliminates the need for progestogen therapy) should be incorporated into risk‐reducing gynaecological surgery (Kotsopoulos 2018). This is because an international cohort study which prospectively followed women with BRCA1 mutations, intact breasts, and no history of breast cancer following RRSO up to 10 years, revealed that the cumulative incidence of breast cancer among women who used oestrogen‐only HRT was 12% compared with 22% among women who used oestrogen plus progesterone HRT (absolute difference 10%; log rank P = 0.04) (Kotsopoulos 2018). Studies comparing use of intrauterine progestin (Mirena coil) versus hysterectomy would help to inform this debate.
Acknowledgements
We thank Jo Morrison for clinical and editorial advice; Jo Platt designing and running the searches; and Gail Quinn, Clare Jess and Tracey Harrison for their contribution to the editorial process.
George Eleje and Emmanuel Ugwu received a grant through National Research Foundation (NRF) bursaries to attend Global Evidence Summit in Capetown South Africa in September 2017, where they obtained some training by Jonathan Sterne in ROBINS‐I used in the risk of bias assessment in this systematic review. George Eleje was awarded a writing retreat for completion of the review through the "DFID RPC grant and the Cochrane CAN grant" by Cochrane Nigeria. Many thanks also go to Ekpereonne Esu, Emmanuel Effa, Martin Meremikwu, Olabisi Oduwole and Moriam Chibuzor of Cochrane Nigeria for the training they gave us.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1. MeSH descriptor: [Ovarian Neoplasms] explode all trees #2. MeSH descriptor: [Fallopian Tube Neoplasms] this term only #3. MeSH descriptor: [Peritoneal Neoplasms] this term only #4. MeSH descriptor: [Breast Neoplasms] explode all trees #5. BRCA1 or BRCA2 #6. ((ovar* or fallopian* or peritone* or breast or mammary) near5 (cancer* or neoplasm* or tumor* or tumour* or malignan* or carcinoma* or adenocarcinoma*)) #7. #1 or #2 or #3 or #4 or #5 or #6 #8. MeSH descriptor: [Salpingectomy] this term only #9. MeSH descriptor: [Ovariectomy] this term only #10. oophorectom* or salping* or ovariectom* or RRSO* #11. #8 or #9 or #10 #12. #7 and #11
Appendix 2. MEDLINE search strategy
1 exp Ovarian Neoplasms/ 2 Fallopian Tube Neoplasms/ 3 Peritoneal Neoplasms/ 4 exp Breast Neoplasms/ 5 (BRCA1 or BRCA2).mp. 6 ((ovar* or fallopian* or peritone* or breast or mammary) adj5 (cancer* or neoplasm* or tumor* or tumour* or malignan* or carcinoma* or adenocarcinoma*)).mp. 7 1 or 2 or 3 or 4 or 5 or 6 8 Salpingectomy/ 9 Ovariectomy/ 10 (oophorectom* or salping* or ovariectom* or RRSO*).mp. 11 8 or 9 or 10 12 7 and 11 13 randomized controlled trial.pt. 14 controlled clinical trial.pt. 15 randomized.ab. 16 placebo.ab. 17 clinical trials as topic.sh. 18 randomly.ab. 19 trial.ti. 20 exp cohort studies/ 21 (cohort* or prospective* or retrospective*).mp. 22 (case* and series).mp. 23 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 24 12 and 23 25 exp animals/ not humans.sh. 26 24 not 25
key:
mP = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier pt = publication type ab = abstract ti = title sh = subject heading
Appendix 3. Embase search strategy
1. exp ovary tumor/ 2. uterine tube tumor/ 3. peritoneum tumor/ 4. exp breast tumor/˜ 5. (BRCA1 or BRCA2).mp.˜ 6. ((ovar* or fallopian* or peritone* or breast or mammary) adj5 (cancer* or neoplasm* or tumor* or tumour* or malignan* or carcinoma* or adenocarcinoma*)).mp. 7. 1 or 2 or 3 or 4 or 5 or 6 8. salpingectomy/ 9. ovariectomy/ 10. (oophorectom* or salping* or ovariectom* or RRSO*).mp. 11. 8 or 9 or 10 12. 7 and 11 13. Crossover procedure/ 14. Double‐blind procedure/ 15. Randomized controlled trial/ 16. Single‐blind procedure/ 17. Random*.mp. 18. Factorial*.mp. 19. (crossover* or cross over* or cross‐over*).mp. 20. Placebo*.mp. 21. (double* adj blind*).mp. 22. (singl* adj blind*).mp. 23. Assign*.mp. 24. Allocate*.mp. 25. Volunteer*.mp. 26. exp cohort analysis/ 27. exp prospective study/ 28. (cohort* or prospective* or retrospective*).mp. 29. (case* and series).mp. 30. 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 31. 12 and 30
key: mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier pt = publication type ab = abstract ti = title sh = subject heading
Data and analyses
Comparison 1. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 BRCA1 or BRCA2 | 3 | Hazard Ratio (Random, 95% CI) | 0.32 [0.19, 0.54] | |
| 2 High‐grade serous cancer (HGSC) mortality | 3 | Hazard Ratio (Random, 95% CI) | 0.06 [0.02, 0.17] | |
| 2.1 BRCA1 or BRCA2 | 3 | Hazard Ratio (Random, 95% CI) | 0.06 [0.02, 0.17] | |
| 3 Breast cancer mortality | 7 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 BRCA1 or BRCA | 7 | Hazard Ratio (Random, 95% CI) | 0.58 [0.39, 0.88] | |
| 4 HGSC incidence | 4 | 3328 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.04, 0.75] |
| 4.1 BRCA1 or BRCA2 | 4 | 3328 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.04, 0.75] |
| 5 Breast cancer incidence | 7 | 5595 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.96] |
| 5.1 BRCA1 or BRCA2 | 7 | 5595 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.96] |
| 6 Quality of life (ovarian cancer risk perception) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 BRCA1 or BRCA2 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Quality of life (global health status) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8 Quality of life (general health perception) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9 Quality of life (mental health) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Quality of life (breast cancer risk perception) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 BRCA1 or BRCA2 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO according to BRCA mutation status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | Hazard Ratio (Random, 95% CI) | 0.35 [0.25, 0.50] | |
| 1.1 BRCA1 only | 3 | Hazard Ratio (Random, 95% CI) | 0.30 [0.17, 0.52] | |
| 1.2 BRCA2 only | 2 | Hazard Ratio (Random, 95% CI) | 0.44 [0.23, 0.85] | |
| 2 High‐grade serous cancer (HGCS) mortality | 2 | Hazard Ratio (Random, 95% CI) | 0.10 [0.02, 0.41] | |
| 2.1 BRCA1 only | 2 | Hazard Ratio (Random, 95% CI) | 0.10 [0.02, 0.41] | |
| 2.2 BRCA2 only | 2 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Breast cancer mortality | 6 | Hazard Ratio (Random, 95% CI) | 0.59 [0.35, 1.00] | |
| 3.1 BRCA1 only | 4 | Hazard Ratio (Random, 95% CI) | 0.45 [0.30, 0.67] | |
| 3.2 BRCA2 only | 3 | Hazard Ratio (Random, 95% CI) | 0.88 [0.42, 1.87] | |
| 4 Quality of life (ovarian cancer risk perception) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 BRCA1 only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 BRCA2 only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 or BRCA2 mutation carriers according to type of risk‐reducing surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 RRSO and risk‐reducing mastectomy (RRM) versus no RRSO | 1 | Hazard Ratio (Random, 95% CI) | 0.14 [0.02, 0.98] | |
| 2 Breast cancer mortality | 1 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 2.1 RRSO and RRM versus no RRSO | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA1 mutation carriers according to age at RRSO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breast cancer mortality | 3 | Hazard Ratio (Random, 95% CI) | 0.85 [0.64, 1.11] | |
| 1.1 50 years or less | 3 | Hazard Ratio (Random, 95% CI) | 0.78 [0.55, 1.09] | |
| 1.2 Above 50 years | 3 | Hazard Ratio (Random, 95% CI) | 1.27 [0.67, 2.38] |
Comparison 5. Risk‐reducing salpingo‐oophorectomy (RRSO) versus no RRSO in BRCA2 mutation carriers according to age at RRSO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breast cancer mortality | 2 | Hazard Ratio (Random, 95% CI) | 0.88 [0.42, 1.87] | |
| 1.1 50 years or less | 2 | Hazard Ratio (Random, 95% CI) | 0.49 [0.08, 2.90] | |
| 1.2 Above 50 years | 2 | Hazard Ratio (Random, 95% CI) | 1.36 [0.68, 2.75] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Domchek 2006.
| Methods | Prospective cohort study, matching design | |
| Participants | Country: multicountry: University of Vienna, Austria; Creighton University, Omaha, NE, USA; Dana‐Farber Cancer Institute, Boston, MA, USA; Fox Chase Cancer Center, Philadelphia, PA, USA; Georgetown University, Washington, DC, USA; University of Chicago, Chicago, IL, USA; University of Pennsylvania, Philadelphia, PA, USA; University of Utah, Salt Lake City, UT, USA; Netherlands Cancer Institute, Amsterdam, Netherlands; Royal Marsden Hospital, Sutton, UK; St Mary’s Hospital, Manchester, UK; University of Texas‐Southwestern, Dallas, TX, USA and Yale University, New Haven, CT, USA Enrolled: 155 surgical participants and 271 control participants Women with BRCA1 or BRCA2 mutation carriers |
|
| Interventions | Arm A: RRSO Arm B: general surveillance or non‐RRSO |
|
| Outcomes | Overall survival Ovarian cancer mortality Primary peritoneal cancer mortality Breast cancer mortality Ovarian cancer incidence Breast cancer incidence Primary peritoneal cancer incidence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 7 |
Domchek 2010.
| Methods | Prospective cohort study, non‐matching design | |
| Participants | Country: multicountry: University of Vienna, Austria; Beth Israel, Boston, MA; Baylor‐Charles A. Sammons Cancer Center; City of Hope, Duarte, CA; Creighton University, Omaha, NE; Dana‐Farber Cancer Institute, Boston, MA; Duke University, Durham, NC; NorthShore University HealthSystem, Evanston, IL; Fox Chase Cancer Center, Philadelphia, PA; Guy's Hospital and St. Thomas Foundation Trust, London, UK; Georgetown University, Washington, DC; University of California, Los Angeles; Mayo Clinic College of Medicine, Rochester, MN; Netherlands Cancer Institute, Amsterdam, Netherlands; The Institute of Cancer Research & Royal Marsden NHS Foundation Trust, London & Sutton; St. Mary's Hospital, Manchester, UK; University of Texas‐Southwestern, Dallas; University of Chicago, Chicago, IL; University of Pennsylvania, Philadelphia, PA; University of Utah, Salt Lake City, UT and University of California, Irvine; Women's College Hospital, Toronto, CA and Yale University, New Haven, CT Enrolled: 465 surgical participants and 1092 control participants Women with BRCA1 or BRCA2 mutation carriers |
|
| Interventions | Arm A: RRSO and RRM Arm B: general surveillance or non‐RRSO |
|
| Outcomes | Overall survival Ovarian cancer mortality Breast cancer mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 7 |
Heemskerk‐Gerritsen 2015a.
| Methods | Partly retrospective and prospective cohort study, matching design | |
| Participants | Country: Netherlands Enrolled: 146 surgical participants and 576 control participants Women with BRCA1 or BRCA2 mutation carriers |
|
| Interventions | Arm A: RRSO Arm B: general surveillance or non‐RRSO |
|
| Outcomes | Ovarian cancer mortality Breast cancer mortality Ovarian cancer incidence Breast cancer incidence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 7 |
Ingham 2013.
| Methods | Prospective cohort study, non‐matching design | |
| Participants | Country: UK Enrolled: 108 surgical participants and 457 control participants Women with BRCA1 or BRCA2 mutation carriers |
|
| Interventions | Arm A: RRSO and RRM Arm B: general surveillance or non‐RRSO |
|
| Outcomes | Overall survival Ovarian cancer mortality Breast cancer mortality Ovarian cancer incidence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 7 |
Kotsopoulos 2017.
| Methods | Prospective cohort study, non‐matching design | |
| Participants | Country: multicountry Enrolled: 1552 surgical participants and 2170 control participants Women with BRCA1 or BRCA2 mutation carriers |
|
| Interventions | Arm A: RRO Arm B: general surveillance or non‐RRSO |
|
| Outcomes | Breast cancer incidence Breast cancer mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 7 |
Kramer 2005.
| Methods | Prospective cohort study (non‐matched) | |
| Participants | Country: USA Enrolled: 33 surgical participants and 65 control participants Women with BRCA1 mutation carriers |
|
| Interventions | Arm A: RRO Arm B: general surveillance or non‐RRSO |
|
| Outcomes | Breast cancer incidence Breast cancer mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 7 |
Madalinska 2007.
| Methods | Prospective cohort study, non‐matching design | |
| Participants | Country: Netherlands Enrolled: 118 surgical participants and 42 control participants Women with BRCA1 or BRCA2 mutation carriers |
|
| Interventions | Arm A: RRSO and RRM Arm B: general surveillance or non‐RRSO |
|
| Outcomes | Quality of life (ovarian cancer risk perception) Quality of life (breast cancer risk perception) Quality of life (global health status) Quality of life (general health perception) Quality of life (mental health) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 7 |
Rebbeck 1999.
| Methods | Prospective cohort study, matching design | |
| Participants | Country: USA: Creighton University, Omaha, NE; Dana‐Farber Cancer Institute, Boston, MA; Fox Chase Cancer Center, Philadelphia, PA; University of Pennsylvania, Philadelphia, PA and University of Utah, Salt Lake City, UT). Enrolled: 43 surgical participants and 79 control participants Women with BRCA1 mutation carriers |
|
| Interventions | Arm A: RRSO Arm B: general surveillance or non‐RRSO |
|
| Outcomes | Breast cancer incidence Breast cancer mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 7 |
Rebbeck 2002.
| Methods | Retrospective cohort study, matching design | |
| Participants | Country: multicountry: Creighton University, Dana–Farber Cancer Institute, Fox Chase Cancer Center, Georgetown University, University of Chicago, University of Pennsylvania, University of Utah, Netherlands Cancer Institute, St. Mary’s Hospital, Women’s College Hospital and Yale University Enrolled: 259 surgical participants and 292 control participants Women with BRCA1 or BRCA2 mutation carriers |
|
| Interventions | Arm A: RRSO Arm B: general surveillance or non‐RRSO |
|
| Outcomes | Ovarian cancer incidence Breast cancer incidence Primary peritoneal cancer incidence Primary peritoneal cancer mortality Breast cancer mortality Ovarian cancer mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 7 |
Rebbeck 2004.
| Methods | Prospective cohort study, matching design | |
| Participants | Country: multicountry: Creighton University, Dana–Farber Cancer Institute, Fox Chase Cancer Center, Georgetown University, University of Chicago, University of Pennsylvania, University of Utah, Netherlands Cancer Institute, St. Mary’s Hospital, Women’s College Hospital and Yale University Enrolled: 57 surgical participants and 107 control participants Women with BRCA1 or BRCA2 mutation carriers |
|
| Interventions | Arm A: RRSO and RRM Arm B: general surveillance or non‐RRSO |
|
| Outcomes | Breast cancer incidence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Table 7 |
BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; RRM: risk‐reducing mastectomy; RRO: risk‐reducing oophorectomy; RRSO: risk‐reducing salpingo‐oophorectomy.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Benshushan 2009 | Participants in the control group did not have BRCA1/BRCA2 mutation carriers. |
| Chang‐Claude 2007 | Cohort study that assessed breast cancer risk in a large series of 1187 BRCA1 and 414 BRCA2 carriers from the International BRCA1/2 Carrier Cohort Study but included women (1 arm) with a previous or coexisting breast malignancy. |
| Eisen 2005 | Case‐control study that involved 4569 eligible women, of which 2283 women with a BRCA1/2 mutation carriers but included women (1 arm) with a previous or coexisting breast malignancy. |
| Evans 2009 | Although the study was a cohort study that compared the frequency of peritoneal cancers among women receiving risk‐reducing surgery for ovarian cancer, not all the women enrolled were BRCA1 and BRCA2 mutation carriers and none of the included data were complete for extraction in enrolled women with known BRCA1 or BRCA2 mutation status. |
| Evans 2013 | Cohort study that evaluated the incidence of breast cancer after RRM in healthy BRCA mutation carriers, without risk‐reducing BSO. |
| Finch 2006 | Included women with prior history of breast cancer |
| Finch 2009 | Controlled before‐and‐after study with no concurrent comparison groups |
| Finch 2011 | Controlled before‐and‐after study with no concurrent comparison groups |
| Finch 2013 | Controlled before‐and‐after study with no concurrent comparison groups |
| Finch 2014 | Single‐arm cohort study (without comparison group) aimed at estimating the reduction in risk of ovarian, fallopian tube or peritoneal cancer in women with a BRCA1 or BRCA2 mutation after oophorectomy, by age of oophorectomy; to estimate the impact of prophylactic oophorectomy on all‐cause mortality; and to estimate 5‐year survival associated with clinically detected ovarian, occult and peritoneal cancers diagnosed in the cohort. |
| Finkelman 2012 | Included women with prior history of breast cancer or ovarian cancer |
| Heemskerk‐Gerritsen 2013 | Cohort study that evaluated the incidence of breast cancer after RRM in healthy BRCA mutation carriers, without risk‐reducing BSO. |
| Heemskerk‐Gerritsen 2015b | Cohort study that evaluated the incidence of breast cancer after RRM in healthy BRCA mutation carriers, without risk‐reducing BSO. |
| Hunsinger 2016 | Although all the 8 women included in the study were BRCA mutation positive and received prophylactic mastectomy with BSO, there was no control or comparison group. So all women received surgical interventions. |
| Iavazzo 2016 | Review of cases with peritoneal cancer after PBSO and the possible aetiology of the disease as well as the possible changes in the management of such women. |
| Johansen 2016 | Retrospective cohort study of 294 women who underwent RRSO and 1228 women from the normal group aimed at evaluating the sexual pleasure and discomfort scores and frequency of sexual activity using the Sexual Activity Questionnaire. The BRCA1 or BRCA2 mutations status were not specified in any of the women included in the study. |
| Johansen 2017 | Although participants included 324 women after RRSO and 11,160 postmenopausal controls, a subsample of 950 controls had undergone BSO, whose indication for the BSO was not known and the BRCA1 or BRCA2 mutations status of the participants (study group or controls) too, were not reported. |
| Kauff 2002 | Prospective cohort study that compared the effect of RRSO with that of surveillance for ovarian cancer on the incidence of subsequent breast cancer and BRCA related gynaecological cancers in women with BRCA mutations but included women with prior history of breast cancer, with 70% of the salpingo‐oophorectomy group and 62% of the surveillance group having prior history of breast cancer. |
| Kauff 2008 | Prospective cohort study that compared the effect of RRSO with that of surveillance for ovarian cancer on the incidence of subsequent breast cancer and BRCA related gynaecological cancers in women with BRCA mutations but the study included women with prior history of breast cancer in both the RRSO group and the surveillance group. |
| Kwon 2013 | The 2 different comparison groups received bilateral salpingectomy alone or bilateral salpingectomy with delayed oophorectomy. It did not include a control or comparison group of women who carry BRCA1 or BRCA2 mutations and did not receive prophylactic salpingo‐oophorectomy. So all groups received surgical interventions. |
| Laki 2007 | Retrospective study of 89 BRCA1/BRCA2 mutation carriers who underwent BSO. It did not include a control or comparison group of women who carried BRCA1 or BRCA2 mutations and did not receive prophylactic salpingo‐oophorectomy. So all groups received surgical interventions. |
| Madalinska 2005 | Although the study determined the quality of life effects of PBSO versus gynaecologic screening, only 368/846 included women had known BRCA1/2 mutation carriers (265 (72%) women opted for PBSO, and 103 (28%) women, opted for gynaecological screening. Analysis was not based on BRCA1/2 status. |
| Manchanda 2011 | Prospective cohort single‐arm study (without comparison or surveillance group) of women from high‐risk families whose mutation status was unknown, in addition to women who were confirmed BRCA1 or BRCA2 mutation carriers. |
| Meijers‐Heijboer 2001 | Cohort study that evaluated the incidence of breast cancer after RRM in healthy BRCA mutation carriers, without RRSO. |
| Menkiszak 2016 | Cohort study of 195 women who were carriers of 1 of 3 mutations in BRCA1 gene most commonly occurring in the Polish population (5382insC, 4153delA and C61G) subjected to prophylactic salpingo‐oophorectomy. All women underwent prophylactic surgery and there was no comparison group. So all women received surgical interventions. |
| Metcalfe 2014 | Cohort study included 390 women with a family history of stage I or II breast cancer who were carriers of BRCA1 and BRCA2 mutations and initially treated with unilateral or bilateral mastectomy, without bilateral RRSO. |
| Miller 2017 | Retrospective observational cohort study of 70 women that assessed the potential role of peritoneal and omental biopsies in women undergoing RRSO for prophylactic management of hereditary breast/ovarian cancer syndromes. There is a single arm study without a comparison group. |
| Perabo 2014 | Although all 6 women included in the study received prophylactic mastectomy with BSO (4 women had BRCA‐1 mutations, 1 woman had a BRCA‐2 mutation and 1 woman had a family inheritance pattern with no mutations), there was no control or comparison group. So all groups received surgical interventions. |
| Powell 2011 | Single arm study (without comparison group) of 111 women who were carriers of BRCA mutations and had RRSO in order to identify risk factors associated with finding an occult malignancy at RRSO using a rigorous surgical‐pathological protocol. |
| Rocca 2006 | Matched population‐based cohort study that investigated the survival patterns of 2390 women who had received an oophorectomy compared with 2390 women who had not received an oophorectomy but the BRCA1 or BRCA2 mutation status of the participants were not reported. |
| Rutter 2003 | Cohort study that assessed the level and persistence of reduction of ovarian (including peritoneal) cancer risk after gynaecological surgeries for women who carried BRCA1/2 mutations but were not selected from high‐risk clinics but not all women enrolled in the study have known BRCA1/2 mutation status. |
| Schmeler 2006 | Included women with a personal history of breast cancer. |
| Skytte 2011 | Cohort study that evaluated the incidence of breast cancer after RRM in healthy BRCA mutation carriers, without risk‐reducing BSO. |
| Struewing 1995 | Prospective multicentre cohort study that determined the incidence of postoophorectomy carcinomatosis and quantified the effectiveness of preventive surgery, none of the enrolled women had known BRCA1 or BRCA2 mutation status. |
| van Sprundel 2005 | Cohort study included women with a family history or personal history of breast cancer who were carriers of BRCA1 and BRCA2 mutations and initially treated with unilateral or bilateral mastectomy, but without bilateral RRSO. |
| Vermeulen 2017 | Systematic review of implications of premenopausal RRSO on quality of life, endocrine symptoms, sexual function, osteoporosis, cardiovascular health, metabolic syndrome, cognitive impairment and safety of hormone replacement therapy. |
BRCA1: breast cancer 1 gene; BRCA2: breast cancer 2 gene; BSO: bilateral salpingo‐oophorectomy; PBSO: prophylactic bilateral salpingo‐oophorectomy; RRM: risk‐reducing mastectomy; RRSO: risk‐reducing salpingo‐oophorectomy.
Differences between protocol and review
In order to attune to the current recommended risk of bias assessment for non‐randomised studies, we replaced the previously planned Cochrane 'Risk of bias' assessment tool (Higgins 2011) by ROBINS‐I. The previously planned assessment included the following.
Selection bias: random sequence generation and allocation concealment.
Performance bias: blinding of participants and personnel (participants and treatment providers).
Detection bias: blinding of outcome assessment.
Attrition bias: incomplete outcome data.
Reporting bias: selective reporting of outcomes.
Other possible sources of bias.
Since none of the studies had more than two groups. We omitted the short paragraph about dealing with multiple treatment groups. The previously planned assessment under 'data synthesis' included the following.
If any studies had multiple treatment groups, we divided the 'shared' comparison group into the number of treatment groups and comparisons between each treatment group and treated the split comparison group as independent comparisons.
Since it would be ethically challenging to restrict RRSO treatment to women requiring it. We have now modified the section and appended the following: Types of studies:
Randomised controlled trials (RCTs) and quasi‐randomised trials (studies where participant allocation or enrolment is open to systematic bias/errors, as all participants do not have an equal chance of being in one group or the other) were unlikely or not possible due to ethical reasons. Both type of study design, rather than only RCTs, are ethically impossible. Therefore we examined the following types of studies.
Non‐randomised trials, prospective and retrospective cohort studies, and case series (all with concurrent comparison groups).
The previously planned assessment under 'types of studies' included the following.
Quasi‐randomised trials (studies where participant allocation or enrolment is open to systematic bias/errors, as all participants do not have an equal chance of being in one group or the other).
Non‐randomised trials, prospective and retrospective cohort studies, and case series (all with concurrent comparison groups).
Since there are many adverse events, we have now limited one of the main outcome measure to 'sever adverse events.'
Because of the relevance of breast cancer in the topic of the review, we handsearched the following breast cancer journals.
Breast Cancer Research and Treatment.
Breast Cancer Research.
Clinical Breast Cancer.
Breast Cancer.
Journal of Breast Cancer.
Open Breast Cancer Journal.
Breast Cancer Online.
Advances in Breast Cancer.
Gastric and Breast Cancer.
Current Breast Cancer Reports.
Breast Cancer: Targets and Therapy.
Since ovarian cancer may originate from the tubes in some women, we have combined some of the outcomes to HGSC (from fallopian tube, ovarian, and primary peritoneal cancer) mortality as a primary outcome measure or HGSC (from fallopian tube, serous tubal intraepithelial carcinoma, ovarian, and primary peritoneal cancer) incidence.
Recovery, readmission was changed to Recovery/ readmission incidence while morbidity was changed to morbidity incidence so as to identify the unit of measurement.
We reduced the adverse events into the severe adverse events for ease of comparison and removed:
(intraoperative complications:) bladder injury;
gastrointestinal tract injury ‐ small or large bowel;
vascular injury;
(postoperative complications) infection; abscess/haematoma; bowel obstruction/ileus; bowel perforation; primary haemorrhage; secondary haemorrhage; ureteric obstruction; cardiac or respiratory complications; neurological.
Contributions of authors
Study conception and design: all review authors. Acquisition of data: GE, AE and IE. Analysis and interpretation: GE, AE, IE, JI, EU and OO. Drafting of manuscript: GE, AE, IE, JI and EU. Overall check: all review authors. The review update will be undertaken by GE.
Sources of support
Internal sources
None, Other.
External sources
National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group, UK.
Declarations of interest
GE: none known. AE: none known. IE: none known. JI: none known. EU: none known. OO: none known.
New
References
References to studies included in this review
Domchek 2006 {published data only}
- Domchek SM, Friebel TM, Neuhausen SL, Wagner T, Evans G, Isaacs C, et al. Mortality after bilateral salpingo‐oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncology 2006;7(3):223‐9. [PUBMED: 16510331] [DOI] [PubMed] [Google Scholar]
Domchek 2010 {published data only}
- Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, et al. Association of risk‐reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010;304(9):967‐75. [PUBMED: 20810374] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heemskerk‐Gerritsen 2015a {published data only}
- Heemskerk‐Gerritsen BA, Seynaeve C, Asperen CJ, Ausems MG, Collée JM, Doorn HC, et al. Breast cancer risk after salpingo‐oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. Journal of the National Cancer Institute 2015;107(5):pii: djv033. [PUBMED: 25788320] [DOI] [PubMed] [Google Scholar]
Ingham 2013 {published data only}
- Ingham SL, Sperrin M, Baildam A, Ross GL, Clayton R, Lalloo F, et al. Risk‐reducing surgery increases survival in BRCA1/2 mutation carriers unaffected at time of family referral. Breast Cancer Research and Treatment 2013;142(3):611‐8. [PUBMED: 24249359] [DOI] [PubMed] [Google Scholar]
Kotsopoulos 2017 {published data only}
- Kotsopoulos J, Huzarski T, Gronwald J, Singer CF, Moller P, Lynch HT, et al. Bilateral oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers. Journal of the National Cancer Institute 2017;109(1):pii: djw177. [PUBMED: 27601060] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 2005 {published data only}
- Kramer JL, Velazquez IA, Chen BE, Rosenberg PS, Struewing JP, Greene MH. Prophylactic oophorectomy reduces breast cancer penetrance during prospective, long‐term follow‐up of BRCA1 mutation carriers. Journal of Clinical Oncology 2005;23(34):8629‐35. [PUBMED: 16314625] [DOI] [PubMed] [Google Scholar]
Madalinska 2007 {published data only}
- Madalinska JB, Beurden M, Bleiker EM, Valdimarsdottir HB, Lubsen‐Brandsma L, Massuger LF, et al. Predictors of prophylactic bilateral salpingo‐oophorectomy compared with gynecologic screening use in BRCA1/2 mutation carriers. Journal of Clinical Oncology 2007;25(3):301‐7. [PUBMED: 17235045] [DOI] [PubMed] [Google Scholar]
Rebbeck 1999 {published data only}
- Rebbeck TR, Levin AM, Eisen A, Snyder C, Watson P, Cannon‐Albright L, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. Journal of the National Cancer Institute 1999;91(17):1475‐9. [DOI] [PubMed] [Google Scholar]
Rebbeck 2002 {published data only}
- Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van't Veer L, Garber JE, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. New England Journal of Medicine 2002;346(21):1616‐22. [DOI] [PubMed] [Google Scholar]
Rebbeck 2004 {published data only}
- Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van't Veer L, Garber JE, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. Journal of Clinical Oncology 2004;22(6):1055‐62. [PUBMED: 14981104] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Benshushan 2009 {published data only}
- Benshushan A, Rojansky N, Chaviv M, Arbel‐Alon S, Benmeir A, Imbar T, et al. Climacteric symptoms in women undergoing risk‐reducing bilateral salpingo‐oophorectomy. Climacteric 2009;12(5):404‐9. [PUBMED: 19479488] [DOI] [PubMed] [Google Scholar]
Chang‐Claude 2007 {published data only}
- Chang‐Claude J, Andrieu N, Rookus M, Brohet R, Antoniou AC, Peock S, et al. Age at menarche and menopause and breast cancer risk in the International BRCA1/2 Carrier Cohort Study. Cancer Epidemiology, Biomarkers & Prevention 2007;16(4):740‐6. [PUBMED: 17416765] [DOI] [PubMed] [Google Scholar]
Eisen 2005 {published data only}
- Eisen A, Lubinski J, Klijn J, Moller P, Lynch HT, Offit K, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case‐control study. Journal of Clinical Oncology 2005;23(30):7491‐6. [PUBMED: 16234515] [DOI] [PubMed] [Google Scholar]
Evans 2009 {published data only}
- Evans DG, Clayton R, Donnai P, Shenton A, Lalloo F. Risk‐reducing surgery for ovarian cancer: outcomes in 300 surgeries suggest a low peritoneal primary risk. European Journal of Human Genetics 2009;17(11):1381‐5. [PUBMED: 19367322] [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2013 {published data only}
- Evans DG, Ingham SL, Baildam A, Ross GL, Lalloo F, Buchan I, et al. Contralateral mastectomy improves survival in women with BRCA1/2‐associated breast cancer. Breast Cancer Research and Treatment 2013;140(1):135‐42. [PUBMED: 23784379] [DOI] [PubMed] [Google Scholar]
Finch 2006 {published data only}
- Finch A, Beiner M, Lubinski J, Lynch HT, Moller P, Rosen B, et al. Salpingo‐oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 mutation. JAMA 2006;296(2):185‐92. [PUBMED: 16835424] [DOI] [PubMed] [Google Scholar]
Finch 2009 {published data only}
- Finch A, Metcalfe K, Lui J, Springate C, Demsky R, Armel S, et al. Breast and ovarian cancer risk perception after prophylactic salpingo‐oophorectomy due to an inherited mutation in the BRCA1 or BRCA2 gene. Clinical Genetics 2009;75(3):220‐4. [PUBMED: 19263514] [DOI] [PubMed] [Google Scholar]
Finch 2011 {published data only}
- Finch A, Metcalfe KA, Chiang JK, Elit L, McLaughlin J, Springate C, et al. The impact of prophylactic salpingo‐oophorectomy on menopausal symptoms and sexual function in women who carry a BRCA mutation. Gynecologic Oncology 2011;121(1):163‐8. [PUBMED: 21216453] [DOI] [PubMed] [Google Scholar]
Finch 2013 {published data only}
- Finch A, Metcalfe KA, Chiang J, Elit L, McLaughlin J, Springate C, et al. The impact of prophylactic salpingo‐oophorectomy on quality of life and psychological distress in women with a BRCA mutation. Psycho‐oncology 2013;22(1):212‐9. [PUBMED: 21913283] [DOI] [PubMed] [Google Scholar]
Finch 2014 {published data only}
- Finch AP, Lubinski J, Møller P, Singer CF, Karlan B, Senter L, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. Journal of Clinical Oncology 2014;32(15):1547‐53. [PUBMED: 24567435] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finkelman 2012 {published data only}
- Finkelman BS, Rubinstein WS, Friedman S, Friebel TM, Dubitsky S, Schonberger NS, et al. Breast and ovarian cancer risk and risk reduction in Jewish BRCA1/2 mutation carriers. Journal of Clinical Oncology 2012;30(12):1321‐8. [PUBMED: 22430266] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heemskerk‐Gerritsen 2013 {published data only}
- Heemskerk‐Gerritsen BA, Menke‐Pluijmers MB, Jager A, Tilanus‐Linthorst MM, Koppert LB, Obdeijn IM, et al. Substantial breast cancer risk reduction and potential survival benefit after bilateral mastectomy when compared with surveillance in healthy BRCA1 and BRCA2 mutation carriers: a prospective analysis. Journal of Oncology 2013;24(8):2029‐35. [PUBMED: 23576707] [DOI] [PubMed] [Google Scholar]
Heemskerk‐Gerritsen 2015b {published data only}
- Heemskerk‐Gerritsen BA, Rookus MA, Aalfs CM, Ausems MG, Collee JM, Jansen L, et al. Improved overall survival after contralateral risk‐reducing mastectomy in BRCA1/2 mutation carriers with a history of unilateral breast cancer: a prospective analysis. International Journal of Cancer 2015;136(3):668‐77. [PUBMED: 24947112] [DOI] [PubMed] [Google Scholar]
Hunsinger 2016 {published data only}
- Hunsinger V, Marchac AC, Derder M, Hivelin M, Lecuru F, Bats AS, et al. A new strategy for prophylactic surgery in BRCA women: combined mastectomy and laparoscopic salpingo‐oophorectomy with immediate reconstruction by double DIEP flap. Annales de Chirurgie Plastique et Esthetique 2016;61(3):177‐82. [PUBMED: 26946931] [DOI] [PubMed] [Google Scholar]
Iavazzo 2016 {published data only}
- Iavazzo C, Gkegkes ID, Vrachnis N. Primary peritoneal cancer in BRCA carriers after prophylactic bilateral salpingo‐oophorectomy. Journal of the Turkish German Gynecological Association 2016;17(2):73‐6. [PUBMED: 27403072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johansen 2016 {published data only}
- Johansen N, Liavaag AH, Tanbo TG, Dahl AA, Pripp AH, Michelsen TM. Sexual activity and functioning after risk‐reducing salpingo‐oophorectomy: impact of hormone replacement therapy. Gynecologic Oncology 2016;140(1):101‐6. [PUBMED: 26597462] [DOI] [PubMed] [Google Scholar]
Johansen 2017 {published data only}
- Johansen N, Liavaag AH, Iversen OE, Dorum A, Braaten T, Michelsen TM. Use of hormone replacement therapy after risk‐reducing salpingo‐oophorectomy. Acta Obstetricia et Gynecologica Scandinavica 2017;96(5):547‐55. [PUBMED: 28236297] [DOI] [PubMed] [Google Scholar]
Kauff 2002 {published data only}
- Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, et al. Risk‐reducing salpingo‐oophorectomy in women with a BRCA1 or BRCA2 mutation. New England Journal of Medicine 2002;346(21):1609‐15. [PUBMED: 12023992] [DOI] [PubMed] [Google Scholar]
Kauff 2008 {published data only}
- Kauff ND, Domchek SM, Friebel TM, Robson ME, Lee J, Garber JE, et al. Risk‐reducing salpingo‐oophorectomy for the prevention of BRCA1‐ and BRCA2‐associated breast and gynecologic cancer: a multicenter, prospective study. Journal of Clinical Oncology 2008;26(8):1331‐7. [PUBMED: 18268356] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwon 2013 {published data only}
- Kwon JS, Tinker A, Pansegrau G, McAlpine J, Housty M, McCullum M, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstetrics and Gynecology 2013;121(1):14‐24. [PUBMED: 23232752] [DOI] [PubMed] [Google Scholar]
Laki 2007 {published data only}
- Laki F, Kirova YM, This P, Plancher C, Asselain B, Sastre X, et al. Prophylactic salpingo‐oophorectomy in a series of 89 women carrying a BRCA1 or a BRCA2 mutation. Cancer 2007;109(9):1784‐90. [PUBMED: 17351952] [DOI] [PubMed] [Google Scholar]
Madalinska 2005 {published data only}
- Madalinska JB, Hollenstein J, Bleiker E, Beurden M, Valdimarsdottir HB, Massuger LF, et al. Quality‐of‐life effects of prophylactic salpingo‐oophorectomy versus gynecologic screening among women at increased risk of hereditary ovarian cancer. Journal of Clinical Oncology 2005;23(28):6890‐8. [PUBMED: 16129845] [DOI] [PubMed] [Google Scholar]
Manchanda 2011 {published data only}
- Manchanda R, Abdelraheim A, Johnson M, Rosenthal AN, Benjamin E, Brunell C, et al. Outcome of risk‐reducing salpingo‐oophorectomy in BRCA carriers and women of unknown mutation status. International Journal of Obstetrics and Gynaecology 2011;118(7):814‐24. [PUBMED: 21392246] [DOI] [PubMed] [Google Scholar]
Meijers‐Heijboer 2001 {published data only}
- Meijers‐Heijboer H, Geel B, Putten WL, Henzen‐Logmans SC, Seynaeve C, Menke‐Pluymers MB, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. New England Journal of Medicine 2001;345(3):159‐64. [PUBMED: 11463009] [DOI] [PubMed] [Google Scholar]
Menkiszak 2016 {published data only}
- Menkiszak J, Chudecka‐Glaz A, Gronwald J, Cymbaluk‐Ploska A, Celewicz A, Swiniarska M, et al. Prophylactic salpingo‐oophorectomy in BRCA1 mutation carriers and postoperative incidence of peritoneal and breast cancers. Journal of Ovarian Research 2016;9:11. [PUBMED: 26928677] [DOI] [PMC free article] [PubMed] [Google Scholar]
Metcalfe 2014 {published data only}
- Metcalfe K, Gershman S, Ghadirian P, Lynch HT, Snyder C, Tung N, et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ 2014;348:g226. [PUBMED: 24519767] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2017 {published data only}
- Miller H, Pipkin LS, Tung C, Hall TR, Masand RP, Anderson ML. The role of routine peritoneal and omental biopsies at risk reducing salpingo‐oophorectomy. Journal of Minimally Invasive Gynecology 2017;24(5):772‐6. [PUBMED: 28285055] [DOI] [PubMed] [Google Scholar]
Perabo 2014 {published data only}
- Perabo M, Fink V, Gunthner‐Biller M, Bodungen V, Friese K, Dian D. Prophylactic mastectomy with immediate reconstruction combined with simultaneous laparoscopic salpingo‐oophorectomy via a transmammary route: a novel surgical approach to female BRCA‐mutation carriers. Archives of Gynecology and Obstetrics 2014;289(6):1325‐30. [PUBMED: 24389920] [DOI] [PubMed] [Google Scholar]
Powell 2011 {published data only}
- Powell CB, Chen LM, McLennan J, Crawford B, Zaloudek C, Rabban JT, et al. Risk‐reducing salpingo‐oophorectomy (RRSO) in BRCA mutation carriers: experience with a consecutive series of 111 patients using a standardized surgical‐pathological protocol. International Journal of Gynecological Cancer 2011;21(5):846‐51. [PUBMED: 21670699] [DOI] [PubMed] [Google Scholar]
Rocca 2006 {published data only}
- Rocca WA, Grossardt BR, Andrade M, Malkasian GD, Melton LJ 3rd. Survival patterns after oophorectomy in premenopausal women: a population‐based cohort study. Lancet Oncology 2006;7(10):821‐8. [PUBMED: 17012044] [DOI] [PubMed] [Google Scholar]
Rutter 2003 {published data only}
- Rutter JL, Wacholder S, Chetrit A, Lubin F, Menczer J, Ebbers S, et al. Gynecologic surgeries and risk of ovarian cancer in women with BRCA1 and BRCA2 Ashkenazi founder mutations: an Israeli population‐based case‐control study. Journal of the National Cancer Institute 2003;95(14):1072‐8. [PUBMED: 12865453] [DOI] [PubMed] [Google Scholar]
Schmeler 2006 {published data only}
- Schmeler KM, Sun CC, Bodurka DC, White KG, Soliman PT, Uyei AR, et al. Prophylactic bilateral salpingo‐oophorectomy compared with surveillance in women with BRCA mutations. Obstetrics and Gynecology 2006;108(3 Pt 1):515‐20. [PUBMED: 16946209] [DOI] [PubMed] [Google Scholar]
Skytte 2011 {published data only}
- Skytte AB, Cruger D, Gerster M, Laenkholm AV, Lang C, Brondum‐Nielsen K, et al. Breast cancer after bilateral risk‐reducing mastectomy. Clinical Genetics 2011;79(5):431‐7. [PUBMED: 21199491] [DOI] [PubMed] [Google Scholar]
Struewing 1995 {published data only}
- Struewing JP, Watson P, Easton DF, Ponder BA, Lynch HT, Tucker MA. Prophylactic oophorectomy in inherited breast/ovarian cancer families. Journal of the National Cancer Institute. Monographs 1995;17:33‐5. [PUBMED: 8573450] [PubMed] [Google Scholar]
van Sprundel 2005 {published data only}
- Sprundel TC, Schmidt MK, Rookus MA, Brohet R, Asperen CJ, Rutgers EJ, et al. Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. British Journal of Cancer 2005;93(3):287‐92. [PUBMED: 16052221] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vermeulen 2017 {published data only}
- Vermeulen RFM, Beurden MV, Korse CM, Kenter GG. Impact of risk‐reducing salpingo‐oophorectomy in premenopausal women. Climacteric 2017;20(3):212‐21. [PUBMED: 28509627] [DOI] [PubMed] [Google Scholar]
Additional references
ACOG 2009
- American College of Obstetricians and Gynecologists, ACOG Committee on Practice Bulletins‐Gynecology, ACOG Committee on Genetics, Society of Gynecologic Oncologists. ACOG Practice Bulletin No. 103: hereditary breast and ovarian cancer syndrome. Obstetrics and Gynecology 2009;113(4):957‐66. [DOI] [PubMed] [Google Scholar]
Agoritsas 2013
Alhuqail 2018
- Alhuqail AJ, Alzahrani A, Almubarak H, Al‐Qadheeb S, Alghofaili L, Almoghrabi N, et al. High prevalence of deleterious BRCA1 and BRCA2 germline mutations in Arab breast and ovarian cancer patients. Breast Cancer Research and Treatment 2018;168(3):695‐702. [PUBMED: 29297111] [DOI] [PubMed] [Google Scholar]
Ang 2011
- Ang C, Chan KK, Bryant A, Naik R, Dickinson HO. Ultra‐radical (extensive) surgery versus standard surgery for the primary cytoreduction of advanced epithelial ovarian cancer. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD007697.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Archey 2017
- Archey WB, Arrick BA. Transactivation of the estrogen receptor promoter by BRCA1. Cancer Cell International 2017;17:33. [PUBMED: 28270739] [DOI] [PMC free article] [PubMed] [Google Scholar]
Armstrong 2004
- Armstrong K, Schwartz JS, Randall T, Rubin SC, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. Journal of Clinical Oncology 2004;22(6):1045‐54. [DOI] [PubMed] [Google Scholar]
Arts‐de Jong 2016
- Arts‐de Jong M, Bock GH, Asperen CJ, Mourits MJ, Hullu JA, Kets CM. Germline BRCA1/2 mutation testing is indicated in every patient with epithelial ovarian cancer: a systematic review. European Journal of Cancer 2016;61:137‐45. [PUBMED: 27209246] [DOI] [PubMed] [Google Scholar]
Augustinsson 2018
- Augustinsson A, Ellberg C, Kristoffersson U, Borg A, Olsson H. Accuracy of self‐reported family history of cancer, mutation status and tumor characteristics in patients with early onset breast cancer. Acta Oncologica 2018;57(5):593‐603. [PUBMED: 29164969] [DOI] [PubMed] [Google Scholar]
Barlin 2013
- Barlin J, Pike M, Otegbeye E, Arnold A, Stadler Z, Robson M, et al. Does postmenopausal risk‐reducing salpingo‐oophorectomy reduce the risk of BRCA‐associated breast cancer? Proceedings of the 2013 Society of Gynecologic Oncology (SGO) Annual Meeting on Women’s Cancer; 2013 March 9‐12; Los Angeles (CA). 2013:Abstract 245.
Begg 2002
- Begg CB. On the use of familial aggregation in population‐based case probands for calculating penetrance. Journal of the National Cancer Institute 2002;94(16):1221‐6. [PUBMED: 12189225] [DOI] [PubMed] [Google Scholar]
Biglia 2016
- Biglia N, Sgandurra P, Bounous VE, Maggiorotto F, Piva E, Pivetta E, et al. Ovarian cancer in BRCA1 and BRCA2 gene mutation carriers: analysis of prognostic factors and survival. Ecancermedicalscience 2016;10:639. [PUBMED: 27350785] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blok 2016
- Blok F, Roes EM, Leenders GJ, Beekhuizen HJ. The lack of clinical value of peritoneal washing cytology in high risk patients undergoing risk‐reducing salpingo‐oophorectomy: a retrospective study and review. BMC Cancer 2016;16:18. [PUBMED: 26768420] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bober 2015
- Bober SL, Recklitis CJ, Bakan J, Garber JE, Patenaude AF. Addressing sexual dysfunction after risk‐reducing salpingo‐oophorectomy: effects of a brief, psychosexual intervention. Journal of Sexual Medicine 2015;12(1):189‐97. [PUBMED: 25311333] [DOI] [PMC free article] [PubMed] [Google Scholar]
Calderon‐Margalit 2004
- Calderon‐Margalit R, Paltiel O. Prevention of breast cancer in women who carry BRCA1 or BRCA2 mutations: a critical review of the literature. International Journal of Cancer 2004;112(3):357‐64. [PUBMED: 15382059] [DOI] [PubMed] [Google Scholar]
Callahan 2007
- Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, et al. Primary fallopian tube malignancies in BRCA‐positive women undergoing surgery for ovarian cancer risk reduction. Journal of Clinical Oncology 2007;25(25):3985‐90. [PUBMED: 17761984] [DOI] [PubMed] [Google Scholar]
Chan 2011
- Chan RJ, Webster J, Marquart L. Information interventions for orienting patients and their carers to cancer care facilities. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008273.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crum 2007
- Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Current Opinion in Obstetrics & Gynecology 2007;19(1):3‐9. [PUBMED: 17218844] [DOI] [PubMed] [Google Scholar]
CTCAE 2010
- National Institutes of Health ‐ National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010‐06‐14_QuickReference_8.5x11.pdf (accessed 5 December 2016).
De Felice 2015
- Felice F, Marchetti C, Musella A, Palaia I, Perniola G, Musio D, et al. Bilateral risk‐reduction mastectomy in BRCA1 and BRCA2 mutation carriers: a meta‐analysis. Annals of Surgical Oncology 2015;22(9):2876‐80. [PUBMED: 25808098] [DOI] [PubMed] [Google Scholar]
De Felice 2017
- Felice F, Marchetti C, Boccia SM, Romito A, Sassu CM, Porpora MG, et al. Risk‐reducing salpingo‐oophorectomy in BRCA1 and BRCA2 mutated patients: an evidence‐based approach on what women should know. Cancer Treatment Reviews 2017;61:1‐5. [PUBMED: 29028552] [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical Methods for Examining Heterogeneity and Combining Results from Several Studies in Meta‐analysis. Systematic Reviews in Health Care: Meta‐Analysis in Context. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Domchek 2007
- Domchek SM, Rebbeck TR. Prophylactic oophorectomy in women at increased cancer risk. Current Opinion in Obstetrics & Gynecology 2007;19(1):27‐30. [PUBMED: 17218848] [DOI] [PubMed] [Google Scholar]
Dowdy 2004
- Dowdy SC, Stefanek M, Hartmann LC. Surgical risk reduction: prophylactic salpingo‐oophorectomy and prophylactic mastectomy. American Journal of Obstetrics and Gynecology 2004;191(4):1113‐23. [PUBMED: 15507929] [DOI] [PubMed] [Google Scholar]
Eccles 2016
- Eccles DM, Balmaña J, Clune J, Ehlken B, Gohlke A, Hirst C, et al. Selecting patients with ovarian cancer for germline BRCA mutation testing: findings from guidelines and a systematic literature review. Advances in Therapy 2016;33(2):129‐50. [PUBMED: 26809252] [DOI] [PubMed] [Google Scholar]
ESMO 2013
- Ledermann JA, Raja FA, Fotopoulou C, Gonzalez‐Martin A, Colombo N, Sessa C: ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2013;24(S6):vi24‐32. [DOI] [PubMed] [Google Scholar]
Fakkert 2015
- Fakkert IE, Abma EM, Westrik IG, Lefrandt JD, Wolffenbuttel BH, Oosterwijk JC, et al. Bone mineral density and fractures after risk‐reducing salpingo‐oophorectomy in women at increased risk for breast and ovarian cancer. European Journal of Cancer 2015;51(3):400‐8. [PUBMED: 25532426] [DOI] [PubMed] [Google Scholar]
Ford 1998
- Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. American Journal of Human Genetics 1998;62(3):676‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Girolimetti 2014
- Girolimetti G, Perrone AM, Santini D, Barbieri E, Guerra F, Ferrari S, et al. BRCA‐associated ovarian cancer: from molecular genetics to risk management. BioMed Research International 2014;2014:787143. [PUBMED: 25136623] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gottschau 2016
- Gottschau M, Mellemkjaer L, Hannibal CG, Kjaer SK. Ovarian and tubal cancer in Denmark: an update on incidence and survival. Acta Obstetricia et Gynecologica Scandinavica 2016;95(10):1181‐9. [PUBMED: 27454324] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro Guideline Development Tool (GDT). Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guidozzi 2016
- Guidozzi F. Hormone therapy after prophylactic risk‐reducing bilateral salpingo‐oophorectomy in women who have BRCA gene mutation. Climacteric 2016;19(5):419‐22. [PUBMED: 27426853] [DOI] [PubMed] [Google Scholar]
Harmsen 2015
- Harmsen MG, Arts‐de Jong M, Hoogerbrugge N, Maas AH, Prins JB, Bulten J, et al. Early salpingectomy (TUbectomy) with delayed oophorectomy to improve quality of life as alternative for risk‐reducing salpingo‐oophorectomy in BRCA1/2 mutation carriers (TUBA study): a prospective non‐randomised multicentre study. BMC Cancer 2015;15:593. [PUBMED: 26286255] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harmsen 2016
- Harmsen MG, IntHout J, Arts‐de Jong M, Hoogerbrugge N, Massuger LF, Hermens RP, et al. Salpingectomy with delayed oophorectomy in BRCA1/2 mutation carriers: estimating ovarian cancer risk. Obstetrics and Gynecology 2016;127(6):1054‐63. [PUBMED: 27159752] [DOI] [PubMed] [Google Scholar]
Hartge 1999
- Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA. The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. American Journal of Human Genetics 1999;64(4):963‐70. [PUBMED: 10090881] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann 2015
- Hartmann LC, Degnim AC, Dupont WD. Atypical hyperplasia of the breast. New England Journal of Medicine 2015;372(13):1271‐2. [PUBMED: 25806929] [DOI] [PubMed] [Google Scholar]
Hartmann 2016
- Hartmann LC, Lindor NM. Risk‐reducing surgery in hereditary breast and ovarian cancer. New England Journal of Medicine 2016;374(24):2404. [PUBMED: 27305204] [DOI] [PubMed] [Google Scholar]
Hermsen 2007
- Hermsen BB, Olivier RI, Verheijen RH, Beurden M, Hullu JA, Massuger LF, et al. No efficacy of annual gynaecological screening in BRCA1/2 mutation carriers; an observational follow‐up study. British Journal of Cancer 2007;96(9):1335‐42. [PUBMED: 17426707] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hussein 2008
- Hussein MR, Abd‐Elwahed SR, Abdulwahed AR. Alterations of estrogen receptors, progesterone receptors and c‐erbB2 oncogene protein expression in ductal carcinomas of the breast. Cell Biology International 2008;32(6):698‐707. [PUBMED: 18296077] [DOI] [PubMed] [Google Scholar]
IARC 2012
- International Agency for Research on Cancer, World Health Organization. GLOBOCAN 2012: estimated cancer incidence, and mortality and prevalence worldwide in 2012. globocan.iarc.fr/Pages/fact_sheets_population.aspx (accessed 5 December 2016).
Iodice 2010
- Iodice S, Barile M, Rotmensz N, Feroce I, Bonanni B, Radice P, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta‐analysis. European Journal of Cancer 2010;46(12):2275‐84. [PUBMED: 20537530] [DOI] [PubMed] [Google Scholar]
Karakasis 2016
- Karakasis K, Burnier JV, Bowering V, Oza AM, Lheureux S. Ovarian cancer and BRCA1/2 testing: opportunities to improve clinical care and disease prevention. Frontiers in Oncology 2016;6:119. [PUBMED: 27242959] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karlan 2004
- Karlan BY. Defining cancer risks for BRCA germline mutation carriers: implications for surgical prophylaxis. Gynecologic Oncology 2004;92(2):519‐20. [DOI] [PubMed] [Google Scholar]
King 2003
- King MC, Marks JH, Mandell JB, New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003;302(5645):643‐6. [DOI] [PubMed] [Google Scholar]
Klaren 2003
- Klaren HM, van't Veer LJ, Leeuwen FE, Rookus MA. Potential for bias in studies on efficacy of prophylactic surgery for BRCA1 and BRCA2 mutation. Journal of the National Cancer Institute 2003;95(13):941‐7. [PUBMED: 12837830] [DOI] [PubMed] [Google Scholar]
Koc 2018
- Koc N, Ayas S, Arinkan SA. Comparison of the classical method and SEE‐FIM protocol in detecting microscopic lesions in fallopian tubes with gynecological lesions. Journal of Pathology and Translational Medicine 2018; Vol. 52, issue 1:21‐7. [DOI: 10.4132/jptm.2016.06.17; PUBMED: 27539290] [DOI] [PMC free article] [PubMed]
Kotsopoulos 2016
- Kotsopoulos J, Huzarski T, Gronwald J, Moller P, Lynch HT, Neuhausen SL, et al. Hormone replacement therapy after menopause and risk of breast cancer in BRCA1 mutation carriers: a case‐control study. Breast Cancer Research and Treatment 2016;155(2):365‐73. [PUBMED: 26780555] [DOI] [PubMed] [Google Scholar]
Kotsopoulos 2018
- Kotsopoulos J, Gronwald J, Karlan BY, Huzarski T, Tung N, Moller P, et al. Hormone replacement therapy after oophorectomy and breast cancer risk among BRCA1 mutation carriers. JAMA oncology 2018, Apr 19;[Epub ahead of print]. [DOI: 10.1001/jamaoncol.2018.0211; PUBMED: 29710224] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 2013
- Kramer L. Mixed reviews on removing fallopian tubes to prevent ovarian cancer. Canadian Medical Association Journal 2013;185(9):E391‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuchenbaecker 2017
- Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos‐Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017;317(23):2402‐16. [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2017
- Lee YJ, Lee SW, Kim KR, Jung KH, Lee JW, Kim YM. Pathologic findings at risk‐reducing salpingo‐oophorectomy (RRSO) in germline BRCA mutation carriers with breast cancer: significance of bilateral RRSO at the optimal age in germline BRCA mutation carriers. Journal of Gynecologic Oncology 2017;28(1):e3. [PUBMED: 27670257] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2017a
- Lee YC, Milne RL, Lheureux S, Friedlander M, McLachlan SA, Martin KL, et al. Risk of uterine cancer for BRCA1 and BRCA2 mutation carriers. European Journal of Cancer 2017;84:114‐20. [PUBMED: 28802188] [DOI] [PubMed] [Google Scholar]
Lengyel 2013
- Lengyel E, Fleming S, McEwen KA, Montag A, Temkin SM. Serial sectioning of the fallopian tube allows for improved identification of primary fallopian tube carcinoma. Gynecologic Oncology 2013;129(1):120‐3. [PUBMED: 23237768] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leonhardt 2011
- Leonhardt K, Einenkel J, Sohr S, Engeland K, Horn LC. p53 signature and serous tubal in‐situ carcinoma in cases of primary tubal and peritoneal carcinomas and serous borderline tumors of the ovary. International Journal of Gynecological Pathology 2011;30(5):417‐24. [PUBMED: 21804388] [DOI] [PubMed] [Google Scholar]
Leygue 1998
- Leygue E, Dotzlaw H, Watson PH, Murphy LC. Altered estrogen receptor alpha and beta messenger RNA expression during human breast tumorigenesis. Cancer Research 1998;58(15):3197‐201. [PUBMED: 9699641] [PubMed] [Google Scholar]
Lheureux 2016
- Lheureux S, Karakasis K, Harter P, Scott C, Bacon M, Bryce J, et al. Germline BRCA1/2 testing practices in ovarian cancer: current state and opportunities for new directions. Gynecologic Oncology 2016;140(1):90‐4. [PUBMED: 26475959] [DOI] [PubMed] [Google Scholar]
Li 2016
- Li X, You R, Wang X, Liu C, Xu Z, Zhou J, et al. Effectiveness of prophylactic surgeries in BRCA1 or BRCA2 mutation carriers: a meta‐analysis and systematic review. Clinical Cancer Research 2016;22(15):3971‐81. [PUBMED: 26979395] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loman 1998
- Loman N, Johannsson O, Bendahl PO, Borg A, Ferno M, Olsson H. Steroid receptors in hereditary breast carcinomas associated with BRCA1 or BRCA2 mutations or unknown susceptibility genes. Cancer 1998;83(2):310‐9. [PUBMED: 9669814] [PubMed] [Google Scholar]
Lostumbo 2010
- Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD002748.pub3] [DOI] [PubMed] [Google Scholar]
Ludwig 2016
- Ludwig KK, Neuner J, Butler A, Geurts JL, Kong AL. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. American Journal of Surgery 2016;212(4):660‐9. [PUBMED: 27649974] [DOI] [PubMed] [Google Scholar]
Lynch 2013
- Lynch HT, Snyder C, Casey MJ. Hereditary ovarian and breast cancer: what have we learned?. Annals of Oncology 2013;24(Suppl 8):viii83‐95. [DOI] [PubMed] [Google Scholar]
Maeshima 2016
- Maeshima Y, Oseto K, Katsuragi R, Yoshimoto Y, Takahara S, Yamauchi A. Experience with bilateral risk‐reducing mastectomy for an unaffected BRCA mutation carrier. Journal of Breast Cancer 2016;19(2):218‐21. [PUBMED: 27382401] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahe 2013
- Mahe E, Tang S, Deb P, Sur M, Lytwyn A, Daya D. Do deeper sections increase the frequency of detection of serous tubal intraepithelial carcinoma (STIC) in the "sectioning and extensively examining the FIMbriated end" (SEE‐FIM) protocol?. International Journal of Gynecological Pathology 2013;32(4):353‐7. [PUBMED: 23722507] [DOI] [PubMed] [Google Scholar]
Marchetti 2014
- Marchetti C, Felice F, Palaia I, Perniola G, Musella A, Musio D, et al. Risk‐reducing salpingo‐oophorectomy: a meta‐analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Women's Health 2014;14:150. [PUBMED: 25494812] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGuire 2016
- McGuire V, Hartge P, Liao LM, Sinha R, Bernstein L, Canchola AJ, et al. Parity and oral contraceptive use in relation to ovarian cancer risk in older women. Cancer Epidemiology, Biomarkers & Prevention 2016;25(7):1059‐63. [PUBMED: 27197274] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meaney‐Delman 2013
- Meaney‐Delman D, Bellcross CA. Hereditary breast/ovarian cancer syndrome: a primer for obstetricians/gynaecologists. Obstetrics and Gynecology Clinics of North America 2013;40(3):475‐512. [DOI] [PubMed] [Google Scholar]
Metcalfe 2015
- Metcalfe K, Lynch HT, Foulkes WD, Tung N, Kim‐Sing C, Olopade OI, et al. Effect of oophorectomy on survival after breast cancer in BRCA1 and BRCA2 mutation carriers. JAMA Oncology 2015;1(3):306‐13. [PUBMED: 26181175] [DOI] [PubMed] [Google Scholar]
Mitrunen 2003
- Mitrunen K, Hirvonen A. Molecular epidemiology of sporadic breast cancer. The role of polymorphic genes involved in oestrogen biosynthesis and metabolism. Mutation Research 2003;544(1):9‐41. [PUBMED: 12888106] [DOI] [PubMed] [Google Scholar]
Narod 2001
- Narod SA. Hormonal prevention of hereditary breast cancer. Annals of the New York Academy of Sciences 2001;952:36‐43. [PUBMED: 11795442] [DOI] [PubMed] [Google Scholar]
NCCN 2014
- National Comprehensive Cancer Network. Epithelial ovarian cancer (including fallopian tube cancer and primary peritoneal cancer) Version 3.2014. NCCN Clinical Practice Guidelines in Oncology. www.nccn.org/professionals/physician_gls/f_guidelines.asp#site (accessed 5 December 2016).
Nebgen 2018
- Nebgen DR, Hurteau J, Holman LL, Bradford A, Munsell MF, Soletsky BR, et al. Bilateral salpingectomy with delayed oophorectomy for ovarian cancer risk reduction: a pilot study in women with BRCA1/2 mutations. Gynecologic Oncology 2018;150(1):79‐84. [PUBMED: 29735278] [DOI] [PubMed] [Google Scholar]
Oliver 2015
- Oliver Perez MR, Magrina J, Garcia AT, Jimenez Lopez JS. Prophylactic salpingectomy and prophylactic salpingoophorectomy for adnexal high‐grade serous epithelial carcinoma: a reappraisal. Surgical Oncology 2015;24(4):335‐44. [PUBMED: 26690823] [DOI] [PubMed] [Google Scholar]
Olivier 2004
- Olivier RI, Beurden M, Lubsen MA, Rookus MA, Mooij TM, Vijver MJ, et al. Clinical outcome of prophylactic oophorectomy in BRCA1/BRCA2 mutation carriers and events during follow‐up. British Journal of Cancer 2004;90(8):1492‐7. [PUBMED: 15083174] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olopade 2004
- Olopade OI, Artioli G. Efficacy of risk‐reducing salpingo‐oophorectomy in women with BRCA‐1 and BRCA‐2 mutations. Breast Journal 2004;10(Suppl 1):S5‐9. [PUBMED: 14984481] [DOI] [PubMed] [Google Scholar]
Ozols 2006
- Ozols RF. Challenges for chemotherapy in ovarian cancer. Annals of Oncology 2006;17(Suppl 5):v181‐7. [DOI] [PubMed] [Google Scholar]
Pal 2005
- Pal T, Permuth‐Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer 2005;104(12):2807‐16. [PUBMED: 16284991] [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pinsky 2013
- Pinsky PF, Zhu C, Skates SJ, Black A, Partridge E, Buys SS, et al. Potential effect of the risk of ovarian cancer algorithm (ROCA) on the mortality outcome of the Prostate, Lung, Colorectal and Ovarian (PLCO) trial. International Journal of Cancer 2013;132(9):2127‐33. [DOI] [PubMed] [Google Scholar]
Powell 2005
- Powell CB, Kenley E, Chen LM, Crawford B, McLennan J, Zaloudek C, et al. Risk‐reducing salpingo‐oophorectomy in BRCA mutation carriers: role of serial sectioning in the detection of occult malignancy. Journal of Clinical Oncology 2005;23(1):127‐32. [DOI] [PubMed] [Google Scholar]
Powell 2014
- Powell CB. Risk reducing salpingo‐oophorectomy for BRCA mutation carriers: twenty years later. Gynecologic Oncology 2014;132(2):261‐3. [PUBMED: 24528542] [DOI] [PubMed] [Google Scholar]
Ready 2011
- Ready K, Gutierrez‐Barrera AM, Amos C, Meric‐Bernstam F, Lu K, Hortobagyi G, et al. Cancer risk management decisions of women with BRCA1 or BRCA2 variants of uncertain significance. Breast Journal 2011;17(2):210‐2. [PUBMED: 21294809] [DOI] [PubMed] [Google Scholar]
Rebbeck 2005
- Rebbeck TR, Friebel T, Wagner T, Lynch HT, Garber JE, Daly MB, et al: PROSE Study Group. Effect of short‐term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. Journal of Clinical Oncology 2005;23(31):7804‐10. [DOI] [PubMed] [Google Scholar]
Rebbeck 2009
- Rebbeck TR, Kauff ND, Domchek SM. Meta‐analysis of risk reduction estimates associated with risk‐reducing salpingo‐oophorectomy in BRCA1 or BRCA2 mutation carriers. Journal of the National Cancer Institute 2009;101(2):80‐7. [PUBMED: 19141781] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Risch 2001
- Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Kwan E, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. American Journal of Human Genetics 2001;68(3):700‐10. [PUBMED: 11179017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roila 2001
- Roila F, Cortesi E. Quality of life as a primary end point in oncology. Annals of Oncology 2001;12 Suppl 3:S3‐6. [PUBMED: 11804381] [DOI] [PubMed] [Google Scholar]
Rosenthal 2013a
- Rosenthal AN, Fraser L, Manchanda R, Badman P, Philpott S, Mozersky J, et al. Results of annual screening in phase I of the United Kingdom familial ovarian cancer screening study highlight the need for strict adherence to screening schedule. Journal of Clinical Oncology 2013;31(1):49‐57. [PUBMED: 23213100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenthal 2013b
- Rosenthal AN, Fraser L, Philpott S, Manchanda R, Badman P, Hadwin R, et al. Final results of 4‐monthly screening in the UK Familial Ovarian Cancer Screening Study (UKFOCSS Phase 2). 2013 ASCO annual meeting. Journal of Clinical Oncology 2013;(Suppl):Abstract 5507. [Google Scholar]
Salhab 2010
- Salhab M, Bismohun S, Mokbel K. Risk‐reducing strategies for women carrying BRCA1/2 mutations with a focus on prophylactic surgery. BMC Women's Health 2010;10:28. [PUBMED: 20961453] [DOI] [PMC free article] [PubMed] [Google Scholar]
Satagopan 2002
- Satagopan JM, Boyd J, Kauff ND, Robson M, Scheuer L, Narod S, et al. Ovarian cancer risk in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Clinical Cancer Research 2002;8(12):3776‐81. [PubMed] [Google Scholar]
Saule 2018
- Saule C, Mouret‐Fourme E, Briaux A, Becette V, Rouzier R, Houdayer C, et al. Risk of serous endometrial carcinoma in women with pathogenic BRCA1/2 variant after risk‐reducing salpingo‐oophorectomy. Journal of the National Cancer Institute 2018;110(2):213‐5. [PUBMED: 28954295] [DOI] [PubMed] [Google Scholar]
Schenberg 2014
- Schenberg T, Mitchell G. Prophylactic bilateral salpingectomy as a prevention strategy in women at high‐risk of ovarian cancer: a mini‐review. Frontiers in Oncology 2014;4:21. [PUBMED: 24575389] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Segev 2013
- Segev Y, Iqbal J, Lubinski J, Gronwald J, Lynch HT, Moller P, et al. The incidence of endometrial cancer in women with BRCA1 and BRCA2 mutations: an international prospective cohort study. Gynecologic Oncology 2013;130(1):127‐31. [PUBMED: 23562522] [DOI] [PubMed] [Google Scholar]
Shu 2016
- Shu CA, Pike MC, Jotwani AR, Friebel TM, Soslow RA, Levine DA, et al. Uterine cancer after risk‐reducing salpingo‐oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncology 2016;2(11):1434‐40. [PUBMED: 27367496] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 1984
- Simon R, Makuch RW. A non‐parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non‐responder bias. Statistics in Medicine 1984;3(1):35‐44. [PUBMED: 6729287] [DOI] [PubMed] [Google Scholar]
Siyam 2017
- Siyam T, Ross S, Campbell S, Eurich DT, Yuksel N. The effect of hormone therapy on quality of life and breast cancer risk after risk‐reducing salpingo‐oophorectomy: a systematic review. BMC Women's Health 2017;17(1):22. [PUBMED: 28320467] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spitzer 1981
- Spitzer WO, Dobson AJ, Hall J, Chesterman E, Levi J, Shepherd R, et al. Measuring the quality of life of cancer patients: a concise QL‐index for use by physicians. Journal of Chronic Diseases 1981;34(12):585‐97. [PUBMED: 7309824] [DOI] [PubMed] [Google Scholar]
Staples 2013
- Staples J, Goodman A. Chapter 14: PARP inhibitors in ovarian cancer. In: Díaz‐Padilla I editor(s). Ovarian Cancer ‐ a Clinical and Translational Update. London: InTech, 2013. [Google Scholar]
Sterne 2016
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ 2016;355:i4919. [PUBMED: 27733354] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stirling 2005
- Stirling D, Evans DG, Pichert G, Shenton A, Kirk EN, Rimmer S, et al. Screening for familial ovarian cancer: failure of current protocols to detect ovarian cancer at an early stage according to the international Federation of gynecology and obstetrics system. Journal of Clinical Oncology 2005;23(24):5588‐96. [PUBMED: 16110018] [DOI] [PubMed] [Google Scholar]
Struewing 1997
- Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. New England Journal of Medicine 1997;336(20):1401‐8. [DOI] [PubMed] [Google Scholar]
Tschernichovsky 2017
- Tschernichovsky R, Goodman A. Risk‐reducing strategies for ovarian cancer in BRCA mutation carriers: a balancing act. Oncologist 2017;22(4):450‐9. [PUBMED: 28314837] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tutt 2002
- Tutt A, Ashworth A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends in Molecular Medicine 2002;8(12):571‐6. [PUBMED: 12470990] [DOI] [PubMed] [Google Scholar]
van Verschuer 2014
- Verschuer VM, Heemskerk‐Gerritsen BA, Deurzen CH, Obdeijn IM, Tilanus‐Linthorst MM, Verhoef C, et al. Lower mitotic activity in BRCA1/2‐associated primary breast cancers occurring after risk‐reducing salpingo‐oophorectomy. Cancer Biology & Therapy 2014;15(4):371‐9. [PUBMED: 24423863] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venkitaraman 2014
- Venkitaraman AR. Cancer suppression by the chromosome custodians, BRCA1 and BRCA2. Science 2014;343(6178):1470‐5. [PUBMED: 24675954] [DOI] [PubMed] [Google Scholar]
Veronesi 2005
- Veronesi A, Giacomi C, Magri MD, Lombardi D, Zanetti M, Scuderi C, et al. Familial breast cancer: characteristics and outcome of BRCA 1‐2 positive and negative cases. BMC Cancer 2005;5:70. [PUBMED: 15996267] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wacholder 2004
- Wacholder S. Bias in intervention studies that enrol patients from high‐risk clinics. Journal of the National Cancer Institute 2004;96(16):1204‐7. [PUBMED: 15316055] [DOI] [PubMed] [Google Scholar]
Walker 2015
- Walker JL, Powell CB, Chen LM, Carter J, Bae Jump VL, Parker LP, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer 2015;121(13):2108‐20. [PUBMED: 25820366] [DOI] [PubMed] [Google Scholar]
Zakhour 2016
- Zakhour M, Danovitch Y, Lester J, Rimel BJ, Walsh CS, Li AJ, et al. Occult and subsequent cancer incidence following risk‐reducing surgery in BRCA mutation carriers. Gynecologic Oncology 2016;143(2):231‐5. [PUBMED: 27623252] [DOI] [PubMed] [Google Scholar]


