Abstract
Background
As part of efforts to prevent childhood overweight and obesity, we need to understand the relationship between total fat intake and body fatness in generally healthy children.
Objectives
To assess the effects and associations of total fat intake on measures of weight and body fatness in children and young people not aiming to lose weight.
Search methods
For this update we revised the previous search strategy and ran it over all years in the Cochrane Library, MEDLINE (Ovid), MEDLINE (PubMed), and Embase (Ovid) (current to 23 May 2017). No language and publication status limits were applied. We searched the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov for ongoing and unpublished studies (5 June 2017).
Selection criteria
We included randomised controlled trials (RCTs) in children aged 24 months to 18 years, with or without risk factors for cardiovascular disease, randomised to a lower fat (30% or less of total energy (TE)) versus usual or moderate‐fat diet (greater than 30%TE), without the intention to reduce weight, and assessed a measure of weight or body fatness after at least six months. We included prospective cohort studies if they related baseline total fat intake to weight or body fatness at least 12 months later.
Data collection and analysis
We extracted data on participants, interventions or exposures, controls and outcomes, and trial or cohort quality characteristics, as well as data on potential effect modifiers, and assessed risk of bias for all included studies. We extracted body weight and blood lipid levels outcomes at six months, six to 12 months, one to two years, two to five years and more than five years for RCTs; and for cohort studies, at baseline to one year, one to two years, two to five years, five to 10 years and more than 10 years. We planned to perform random‐effects meta‐analyses with relevant subgrouping, and sensitivity and funnel plot analyses where data allowed.
Main results
We included 24 studies comprising three parallel‐group RCTs (n = 1054 randomised) and 21 prospective analytical cohort studies (about 25,059 children completed). Twenty‐three studies were conducted in high‐income countries. No meta‐analyses were possible, since only one RCT reported the same outcome at each time point range for all outcomes, and cohort studies were too heterogeneous to combine.
Effects of dietary counselling to reduce total fat intake from RCTs
Two studies recruited children aged between 4 and 11 years and a third recruited children aged 12 to 13 years. Interventions were combinations of individual and group counselling, and education sessions in clinics, schools and homes, delivered by dieticians, nutritionists, behaviourists or trained, supervised teachers. Concerns about imprecision and poor reporting limited our confidence in our findings. In addition, the inclusion of hypercholesteraemic children in two trials raised concerns about applicability.
One study of dietary counselling to lower total fat intake found that the intervention may make little or no difference to weight compared with usual diet at 12 months (mean difference (MD) ‐0.50 kg, 95% confidence interval (CI) ‐1.78 to 0.78; n = 620; low‐quality evidence) and at three years (MD ‐0.60 kg, 95% CI ‐2.39 to 1.19; n = 612; low‐quality evidence). Education delivered as a classroom curriculum probably decreased BMI in children at 17 months (MD ‐1.5 kg/m2, 95% CI ‐2.45 to ‐0.55; 1 RCT; n = 191; moderate‐quality evidence). The effects were smaller at longer term follow‐up (five years: MD 0 kg/m2, 95% CI ‐0.63 to 0.63; n = 541; seven years; MD ‐0.10 kg/m2, 95% CI ‐0.75 to 0.55; n = 576; low‐quality evidence).
Dietary counselling probably slightly reduced total cholesterol at 12 months compared to controls (MD ‐0.15 mmol/L, 95% CI ‐0.24 to ‐0.06; 1 RCT; n = 618; moderate‐quality evidence), but may make little or no difference over longer time periods. Dietary counselling probably slightly decreased low‐density lipoprotein (LDL) cholesterol at 12 months (MD ‐0.12 mmol/L, 95% CI ‐0.20 to ‐0.04; 1 RCT; n = 618, moderate‐quality evidence) and at five years (MD ‐0.09, 95% CI ‐0.17 to ‐0.01; 1 RCT; n = 623; moderate‐quality evidence), compared to controls. Dietary counselling probably made little or no difference to HDL‐C at 12 months (MD ‐0.03 mmol/L, 95% CI ‐0.08 to 0.02; 1 RCT; n = 618; moderate‐quality evidence), and at five years (MD ‐0.01 mmol/L, 95% CI ‐0.06 to 0.04; 1 RCT; n = 522; moderate‐quality evidence). Likewise, counselling probably made little or no difference to triglycerides in children at 12 months (MD ‐0.01 mmol/L, 95% CI ‐0.08 to 0.06; 1 RCT; n = 618; moderate‐quality evidence). Lower versus usual or modified fat intake may make little or no difference to height at seven years (MD ‐0.60 cm, 95% CI ‐2.06 to 0.86; 1 RCT; n = 577; low‐quality evidence).
Associations between total fat intake, weight and body fatness from cohort studies
Over half the cohort analyses that reported on primary outcomes suggested that as total fat intake increases, body fatness measures may move in the same direction. However, heterogeneous methods and reporting across cohort studies, and predominantly very low‐quality evidence, made it difficult to draw firm conclusions and true relationships may be substantially different.
Authors' conclusions
We were unable to reach firm conclusions. Limited evidence from three trials that randomised children to dietary counselling or education to lower total fat intake (30% or less TE) versus usual or modified fat intake, but with no intention to reduce weight, showed small reductions in body mass index, total‐ and LDL‐cholesterol at some time points with lower fat intake compared to controls. There were no consistent effects on weight, high‐density lipoprotein (HDL) cholesterol or height. Associations in cohort studies that related total fat intake to later measures of body fatness in children were inconsistent and the quality of this evidence was mostly very low. Most studies were conducted in high‐income countries, and may not be applicable in low‐ and middle‐income settings. High‐quality, longer‐term studies are needed, that include low‐ and middle‐income settings to look at both possible benefits and harms.
Plain language summary
Effect of cutting down the amount of fat on bodyweight in children
Review question
What is the relationship between the amount of fat a child eats and their weight and body fat?
Background
To try to better prevent people from being overweight and obese, we need to understand what the ideal amount of total fat in our diets should be, and particularly how this is related to bodyweight and fatness. This relationship differs in children compared to adults, because children are still growing and developing.
Study characteristics
This review looked at the effects of eating less fat on bodyweight and fatness in healthy children aged between two and 18 years, who were not aiming to lose weight. We carried out a comprehensive search for studies up to May 2017.
Key results
We found three randomised controlled trials (clinical trials where people are randomly put into one of two or more treatment groups) conducted in 1054 children in high‐income (wealthy) countries. Two studies recruited children aged between 4 and 11 years and one study recruited children aged 12 to 13 years. The studies looked at different types of interventions, including individual and group educational sessions or advice. The sessions were delivered in clinics, schools and homes by dieticians, nutritionists or teachers. The interventions used in the studies were intended to help children to eat less total fat in their diet (30% or less of their total daily energy). These interventions were compared with a usual or modified fat intake (more than 30% of their total daily energy) for between one and seven years. Some of these results showed that a lower fat intake may reduce body mass index (BMI; a measure of body fatness based on height and weight) and the blood levels of different types of cholesterol (a fat carried in the blood) when compared to a higher fat intake. However, these effects varied over time with some results showing that a lower fat intake may make little or no difference. Evidence from one trial suggested that lower fat intake probably had no effect on blood levels of one type of cholesterol (called HDL‐cholesterol) and may have no effect on height compared to higher fat intakes. This evidence cannot necessarily be applied to all healthy children, as two studies were done in children with raised blood cholesterol levels.
We also looked at 21 studies in approximately 25,059 children that observed and measured the children's intake of fat and their weight, BMI, and other body measures over time, but did not seek to directly change what they ate (these are called cohort studies). Over half of these cohort studies that reported on body fatness suggested that as total fat intake increases, body fatness may move in the same direction. However, results varied across all these studies and we could not draw any firm conclusions.
Quality of the evidence
We found no high‐quality evidence with which to answer this question. Evidence from the cohort studies was generally of very low quality so we are uncertain about these results and cannot draw conclusions. For the three randomised controlled trials, the results that we were most interested in were generally of moderate‐ or low‐quality evidence. We could not make any conclusions about children in low‐ and middle‐income countries as 23 of the 24 studies were done in high‐income countries. More high‐quality, long‐term studies are required that also include children from low‐ and middle‐income settings.
Summary of findings
Background
Description and implications of the condition
Childhood obesity is an important global public health problem. The World Health Organization (WHO) defines childhood obesity as the proportion of children with weight‐for‐height z‐score (WHZ) values greater than three standard deviations (SDs) from the WHO growth standard median (de Onis 2007), with slightly different standards being reported by other organisations such as the International Obesity Task Force (IOTF) (Cole 2000). Overweight and obesity levels among infants, children and adolescents are rising globally. The combined prevalence of overweight and obesity in children increased by 47.1% between 1980 and 2013 (Ng 2014). Overweight and obesity affects disadvantaged population groups more, and rising levels are being seen particularly in low‐ and middle‐income countries (LMICs), largely due to the rapid nutrition transition (de Onis 2010; GBD 2017a; WHO 2016). Of all children under five years of age who were overweight in 2016, 49% lived in Asia and 24% in Africa (UNICEF 2017).
Obesity has physical and psychosocial health consequences during childhood that are likely to extend into adulthood. Children who are obese are more at risk of high blood pressure and high cholesterol; impaired glucose tolerance and type 2 diabetes; asthma and musculoskeletal complications (Pollock 2015). It also increases the risk of psychosocial problems such as depression and poor socialisation (Fenner 2016; WHO 2016). Beyond its consequences in children, childhood obesity is an independent risk factor for adult obesity, with the associated health and economic implications for individuals as well as societies (WHO 2016). Overweight and obesity in adulthood are associated with increased risks of many cancers, coronary heart disease and stroke, and were among the top risk factors contributing to disability‐adjusted life years in 2015 (GBD 2017b).
Given the rising global burden of childhood obesity and its far‐reaching consequences, prevention, by addressing modifiable risk factors, is one of the most important actions. Obesity develops from sustained positive energy balance linked to various genetic, biological, behavioural, environmental and socioeconomic factors (Lobstein 2004; WHO 2016). Ethnicity has been linked to risk of obesity, with non‐white ethnicities living in westernised countries being at greater risk. In the USA, the prevalence of overweight among Hispanic and African‐American children rose twice as fast in a 12‐year period compared to white children (Lobstein 2004). Other factors that influence bodyweight measures in children include parental overweight or obesity, due to genetic and lifestyle influences. Lower socioeconomic status is also associated with higher bodyweight (Lobstein 2004; Ng 2014). There are greater absolute numbers of overweight and obese children in LMICs (Ng 2014). In high‐income countries, obesity risk is greater among populations of lower socioeconomic status whereas in developing countries it is more prevalent among wealthier populations (Lobstein 2004; Ng 2014). Rising levels of obesity are also seen among urban populations in developing countries due to westernised diets and the nutrition transition. This association between socioeconomic status and obesity risk is independent of the association between lower education levels and higher bodyweight measures (Lobstein 2004). Markers of maturation, such as age at menarche, stage of puberty or peak height velocity also influence body fatness, with children who mature more rapidly or earlier being at greater risk of obesity (Parsons 1999). Insufficient physical activity and excessive inactivity (e.g. television viewing) are also associated with risk of obesity (LeBlanc 2012; WHO 2004). Dietary risk factors associated with excess weight gain include high intake of sugar‐sweetened drinks or energy‐dense, nutrient‐poor foods (WHO 2004). Among these dietary risk factors is total fat intake, which may have important effects on body fatness measures in children, with international expert panels having debated on the optimal fat intakes (WCRF/AICR 2009), and which is the subject of this review.
Description of the intervention/exposure
The intervention or exposure of interest in this review is a reduced total fat intake in healthy non‐obese children and young people. Reduced fat intake may be achieved through interventions of nutrition education (e.g. counselling), changes in the food environment, peer‐support programmes, food provision or combinations of these.
Importantly, dietary intake is challenging to measure accurately, and any single common method used (such as the 24‐hour dietary recall, dietary record (DR), dietary history, and Food Frequency Questionnaire (FFQ)) provides subjective estimates, with strengths and limitations related to validity (Shim 2014). Although it is well known that the research objective, hypothesis, design, and available resources need to be carefully considered to select the most appropriate dietary assessment method (Shim 2014), the fidelity of application of dietary assessment methods varies widely across research studies, and adherence to nutrition counselling by study participants also varies widely. These factors may introduce a lot of variation into the relationship between estimates of total fat intake and body fatness measures, which is often difficult to quantify accurately and leads to disparate findings and distortion in the estimated measure of association across studies. Additionally, studies usually quantify total fat intake in absolute grams per day, as a percentage of total energy (%TE) intake or both. These different measures are then used in various ways across studies in data analyses, which may add to the heterogeneity in effects and associations being examined. Studies have shown positive associations between proportion of energy intake as fat and bodyweight measures in children, with less clear associations in longitudinal compared to cross‐sectional studies (Johnson 2008; Lobstein 2004; McGloin 2002; Pérez‐Escamilla 2012). A meta‐regression in a systematic review of randomised controlled trials (RCTs) on the effects of step I and II diets of the National Heart, Lung and Blood Institute national cholesterol education programme to reduce the risk of cardiovascular disease in the general population and those at increased cardiovascular risk, respectively, found a strong relation between total fat intake and bodyweight (Yu‐Poth 1999). The German Nutrition Society guidelines state that whereas intervention and cohort studies in adults that have adjusted for energy intake show a probable lack of association between fat intake and risk of obesity, other studies that have not adjusted for energy intake, show a probable association between total fat intake and risk of obesity (Wolfram 2015).
Fat and energy intake can influence body fatness, and fat intake closely correlates with energy intake, which makes it difficult to separate their individual effects on bodyweight (Wolfram 2015). Change in body fatness that occurs with modifying intakes of total fat are mediated via changes in energy intakes. Additionally, differences in total energy intake can result in extraneous variation in nutrient intake because of individual differences in body size, physical activity and metabolic efficiency. Thus, to distinguish the isolated effect of fat intake on bodyweight, the effect of energy intake needs to be adjusted for in analyses (Jakes 2004; Rhee 2014). In observational studies, statistical models that adjust for prognostic variables, such as energy intake, attempt to simulate the comparability of randomised groups in an intervention study (Wolfram 2015). Similarly, in intervention studies where energy intake is ad libitum, it can confound the association between fat intake and weight gain, and isocaloric comparisons can be simulated through statistical modelling, controlling for the effect of energy intake.
Successfully isolating the effect of a single nutrient, such as fat, on weight is challenging given the complex mixture of nutrients and other components that make up our diets, typically characterised by various dietary patterns (different quantities, proportions, variety, and combinations of different foods and beverages) consumed over time. The nutrients provided by dietary patterns also have synergistic, additive or antagonistic effects on health. One review in Asian children on the relationship between dietary patterns as the exposure variable and childhood overweight and obesity as the outcome reported several meaningful, yet inconsistent, associations between dietary patterns and childhood overweight/obesity in children and adolescents, and heterogeneity of studies in terms of measures of dietary patterns and obesity standards (Yang 2012). Thus, carefully considering the way in which diets differ in components other than only total fat is part of better understanding the relationship between fat intake, weight and other health outcomes.
Another factor that can influence observable effects of total fat intake on bodyweight measures is the time‐varying nature of this relationship. Studies have different periods of observation and follow‐up, and different frequencies or intervals of study contacts and measurement. The duration of lower fat intake interventions or the duration of the exposure to lower total fat intake influence potential changes in bodyweight outcomes. It is thus important to consider this factor when examining the relationship between fat intake and weight, particularly in prospective cohort studies and the often secular nature of their data.
Why is it important to do this review?
Existing reviews looking at low‐fat diets included studies where weight loss was a goal of the intervention (Yu‐Poth 1999), which may have overstated any relation because the advice was to lower both fat and energy intake, did not explore the effect of low‐fat diets on weight or other body fatness outcomes (Schwingshackl 2013a), or looked at low‐fat intake as part of a wider health promotion intervention (Ni 2010). Other reviews that assessed body fatness were either limited to the effect of low‐fat dairy versus high‐fat dairy consumption (Benatar 2013), or investigated it as part of looking at overall dietary patterns (Ambrosini 2014), or diet quality (Aljadani 2015).
To examine these issues, a Cochrane Review including RCTs and cohort studies in adults and children was updated in 2015 (Hooper 2015a). With the aim of ensuring all relevant data in children were summarised, the WHO commissioned an expedited update of this systematic review in children only, to aid the understanding of the relation between total fat intake and bodyweight in children, in studies not intending to induce weight loss, with a view to inform the updating of their guidelines on total fat intake. Therefore, the combined review in children and adults (Hooper 2015a) was split into two reviews with the titles, "Effects of total fat intake on bodyweight in adults;" (in preparation) and "Effects of total fat intake on bodyweight in children." The 2015 combined (adults and children) review will be withdrawn with notes to direct readers to the two separate reviews.
Objectives
To assess the effects and associations of total fat intake on measures of weight and body fatness in children and young people not aiming to lose weight.
Methods
Criteria for considering studies for this review
Types of studies
RCTs of children and young people: trials of lower fat intake compared with usual diet or modified fat intake, with no intention to reduce weight (in any groups), continued for at least six months, unconfounded by non‐nutritional interventions and assessing a measure of body fatness at least six months after the intervention was initiated.
We included studies that randomised participants (i.e. parallel‐group design), and cluster randomised trials where at least six groups of children (i.e. clusters) were randomised. We had intended to exclude cross‐over trials (as previous weight gain or weight loss is likely to affect future weight trends) unless the first half of the cross‐over could be used independently, but we did not find any eligible cross‐over trials.
Cohort studies of children and young people: analytical prospective cohort studies that followed participants for at least 12 months after baseline assessment of total fat intake, and related baseline total fat intake to absolute or change in body fatness at least 12 months later. Cohort studies using explanatory models were included, but those that used baseline data to predict later body fatness without empirical data from the later time point (predictive models) were excluded.
Considering the research focus on identifying weight management strategies in overweight and obese children, and the nature of our question that addresses an intervention to prevent overweight and obesity, we anticipated not finding many longer‐term trials (randomised and non‐randomised) in children not intending to manage or reduce weight. We therefore excluded non‐randomised trials and rather included the next best available evidence for the question, which are analytical prospective cohort studies. Additionally, decision‐makers are required to identify and use the best available evidence in formulating recommendations, and this generally translates into evidence that is of the highest quality as assessed by GRADE, for each important outcome. The fact that we did not know a priori what type of evidence (i.e. from RCTs or observational studies) would be of highest quality was a further rationale for including prospective cohort studies.
Types of participants
We included studies in children and young people (aged 24 months to 18 years) with or without risk factors for cardiovascular disease, for example, a family history of cardiovascular disease, raised blood pressure or raised lipid levels. Participants could be of either sex, but we excluded children who were acutely ill, as well as disease‐ or condition‐specific populations, such as children with cystic fibrosis, autism or diabetes. We excluded intervention studies where the selection of the participants was primarily for raised weight or body mass index (BMI) with the intention to reduce weight.
Studies including a subset of eligible participants (e.g. aged 15 to 24 years) were included if results were reported separately for the eligible subset (e.g. 15 to 18 years). If not, such studies were only included if more than 80% of the baseline sample were aged 24 months to 18 years. We intended to exclude data from these studies in sensitivity analyses to test the robustness of the primary meta‐analyses, but we did not pool data. Birth cohorts were only included if baseline total fat intake was related to absolute or change in body fatness at least 12 months later, and both these time points fell within our eligible age range, in which the earlier time point was regarded as the baseline.
Types of interventions
Interventions
We considered all RCTs of interventions stating an intention to reduce total dietary fat intake (by provision of nutrition education in any form, foods or both), when compared with a usual or modified fat intake.
We considered a lower fat intake to be one where fat intake was 30% or less of total energy (30%TE or less), and energy lost was at least partially replaced with carbohydrates (simple or complex), protein, or fruit and vegetables. We considered a 'usual' fat diet to be one with total fat intake greater than 30%TE, and considered a modified fat diet to be one with greater than 30%TE from fats, and that included higher levels of monounsaturated or polyunsaturated fats than a 'usual' fat diet. Interventions consisting of meals or food items lower in fat were included if they were provided with the intention of reducing fat intake over a period, thus targeting total fat intake.
As we were interested in the effects of total fat intake on bodyweight and fatness in everyday dietary intake over time (rather than in those aiming to reduce their bodyweight in weight‐reducing diets), we excluded studies aiming primarily to reduce the weight of some or all participants, as well as those that included only participants who had recently lost weight, or recruited participants primarily according to a raised bodyweight or BMI.
We excluded multifactorial interventions other than diet or supplementation, unless the effects of diet or supplementation could be separated such that the additional intervention was consistent between the intervention and control groups (e.g. studies that reduced fat and encouraged physical activity in one group and compared this with encouraging physical activity in the control group were included; studies that reduced fat and encouraged physical activity in one group and compared this with no interventions in the control group were excluded; studies that reduced fat and encouraged fruit and vegetables in one group and compared this with no intervention in the control group were included). Studies that selected groups based on a possible prognostic variable other than total fat intake, for example, genotype, were excluded.
We excluded Atkins‐type diets aiming to increase protein and fat intake, as well as studies where fat was reduced by means of a fat substitute (such as Olestra). We excluded studies that included enteral and parenteral feeding, as well as nutritional formula‐based weight‐reducing or other weight‐reducing diets.
Thus, we included all trials that intended to reduce dietary fat to 30%TE or less in one group compared to usual or modified fat intake (greater than 30%TE from fat) in another group regardless of the degree of difference between fat intake in the two groups (i.e. 'dose difference'). We intended to explore the effects of the difference in %TE from fat between control and intervention groups, as well as the effects of fat intake in the control groups and adherence to dietary fat goals in the intervention groups in subgroup analyses, but data did not allow us to perform these.
Exposures
For analytical prospective cohort studies, total dietary fat intake, in grams, as a percentage of total dietary energy intake or as one of the defining characteristics of a dietary pattern, had to be assessed at baseline and related to a measure of body fatness, or change in body fatness, at least one year later.
Types of outcome measures
Primary outcomes
Body fatness, including bodyweight (kg), BMI (kg/m2), waist circumference (cm), skinfold thickness (mm) and percentage body fat.
Secondary outcomes
Other routine cardiovascular risk factors, namely circulating total low‐density lipoprotein (LDL) and high‐density lipoprotein (HDL) cholesterol and triglyceride concentrations, and systolic (SBP) and diastolic blood pressure (SBP).
Height (adverse outcome). It is plausible that reducing total fat intake would reduce total energy and nutrient intake in children, possibly increasing the risk for suboptimal statural growth.
Tertiary outcomes (randomised controlled trials only)
Process outcomes, including changes in saturated and total fat intakes, as well as other macronutrients.
This is not a systematic review of the effects of lower fat on these secondary or tertiary outcomes, but we collated the outcomes from included studies to understand whether any effects on weight or body fatness might have been influenced by changes in these outcomes.
Search methods for identification of studies
Electronic searches
For this update in children only, we developed a new search strategy, which was run in the Cochrane library (May 2017, Issue 5) and in MEDLINE (Ovid, 1946 to May 2017), MEDLINE (PubMed, 1946 to May 2017) and Embase (Ovid, 1947 to May 2017) (Appendix 1). We searched comprehensively for all eligible studies, regardless of language and publication status.
Searching other resources
The previous authors (Hooper 2015a) searched the bibliographies of all identified systematic reviews for further trials and cohort studies, including Ajala 2013; Aljadani 2013; Aljadani 2015; Ambrosini 2014; Benatar 2013; Chaput 2014; Gow 2014; Havranek 2011; Hu 2012; Kratz 2013; Ni 2010; Schwingshackl 2013a; Schwingshackl 2013b; and Yang 2013. We searched the bibliographies of all included RCTs in this update. We also searched the tables of included and excluded studies in children in the previous version of this review that included both adults and children (Hooper 2015b).
To identify ongoing and unpublished studies, we searched the WHO International Clinical Trials Registry Platform (inception to 5 June 2017; WHO ICTRP, apps.who.int/trialsearch/) and ClinicalTrials.gov (inception to 5 June 2017; www.clinicaltrials.gov) (5 June 2017) (Appendix 1).
Data collection and analysis
This update was prepared in Review Manager 5 (RevMan 2014).
Selection of studies
One review author (CN) conducted an initial title screen using keywords to remove records that were obviously irrelevant. Keywords used for the title screen included words indicative of animal studies (e.g. 'murine'), ineligible participants (e.g. 'cystic fibrosis,' 'autism,' 'anorexia nervosa') and ineligible interventions (e.g. 'ketogenic,' 'parenteral,' 'olestra'). For quality assurance purposes, a second review author (MV) screened a random selection of 10% of the removed records, yielding a 98% inter‐rater agreement. Thereafter, two review authors independently screened all remaining titles and abstracts using Covidence (Covidence). We obtained the full‐text articles of records identified as potentially eligible, and screened these in duplicate and independently to determine final eligibility. When an abstract could not be rejected with certainty, we obtained the full text of the article for further evaluation. We were careful not to exclude studies based on outcome reporting. We did this by examining the objectives and methods of the study and deciding whether our eligible outcomes were likely to be within the scope of the study (i.e. considering whether one would expect them to be reported in the particular study, or they were measured and results were not reported). We only excluded studies when none of our eligible outcomes were reported and we judged that our eligible outcomes were outside of the scope of the study. We resolved any disagreements through discussion and consultation with two other review authors (CN or AS) when necessary.
Data extraction and management
We extracted data concerning participants, interventions or exposures, controls and outcomes, and trial or cohort quality characteristics onto forms designed and piloted for the review. We extracted data on potential effect modifiers from RCTs (including duration of intervention, control group fat intake, sex, year of first publication, difference in %TE from fat between the intervention and control groups, type of intervention (food or nutrition education provided), the dietary fat goals set for each group, baseline BMI and health at baseline), and from cohort studies (age, sex, energy intake, ethnicity, parental BMI, physical activity (or screen time, or both), pubertal stage and socioeconomic (income and educational) status). Where provided, we collected data on risk factors for cardiovascular disease (secondary and tertiary outcomes). When assessment of fat intake was reported using more than one dietary assessment method for the same outcome in the same participants, we selected the method deemed to be most appropriate and valid (e.g. multiple applications over time were better than a single once‐off application), or most likely to be relevant to answering our question. If different methods were judged to have similar validity, we used multiple food frequencies preferentially, as these were more likely to represent usual dietary intake (Gibson 2005).
We extracted outcome data according to the following time point ranges, when available: RCTs: from baseline to six months, six to 12 months, one to two years, two to five years and more than five years; cohort studies: baseline to one year, one to two years, two to five years, five to 10 years and more than 10 years. When outcome data were reported at more than one point within our time point ranges (e.g. three and five years), we extracted data from the latest point available within each range (five years in this example), unless the data from this time point were judged to be less reliable than the data from the earlier time point, in which case we used the more reliable data with an explanation.
All trial outcomes were continuous and where possible in trials, we extracted change data (change in the outcome from baseline to outcome assessment) with relevant data on variance for intervention and control groups (along with numbers of participants at that time point). Where change data were not available, we extracted data at study end (or other relevant time point) along with the variance and numbers of participants for each group. In the cohort studies, we extracted the most adjusted odds ratio, risk ratio, mean change or mean end values per group, when comparing the most exposed group of participants (highest fat intake) with the least exposed group (lowest fat intake). The most adjusted regression outputs (e.g. beta coefficient and its variance, P value, T value) were extracted when total dietary fat intake was assessed at baseline and related to a measure of body fatness, or change in body fatness, at least one year later. Two review authors extracted all data independently, with discrepancies resolved by another review author.
Assessment of risk of bias in included studies
We carried out 'Risk of bias' assessments independently and in duplicate. We assessed risk of bias in RCTs using the Cochrane tool for assessment of risk of bias (Higgins 2011a). For included RCTs, we also assessed whether trials were free of differences in diet (between intervention and control groups) other than dietary fat intake, as this may also influence differences in weight, body fatness and other related outcomes. We used the category 'other bias' for this assessment, and also to note any further issues of methodological concern.
For cohort studies we assessed the following.
Was adequate outcome data available?
Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome, or were relevant statistical adjustments done?
Did the exposures between groups differ in components other than only total fat?
Could we be confident in the assessment of outcomes?
Could we be confident in the assessment of exposure?
Could we be confident in the assessment of presence or absence of prognostic factors?
Was selection of less‐exposed and more‐exposed groups from the same population? (Cochrane Methods; Guyatt 2011).
Measures of treatment effect
The effect measure of choice for continuous outcomes was the mean difference (MD). Where data allowed, we presented the MD alongside its 95% confidence interval (CI).
Unit of analysis issues
We found no cluster‐randomised or cross‐over trials. Where there was more than one intervention and control group, we selected the most relevant intervention group and most relevant control group for this review. We excluded intervention groups that were not appropriate for this review, or less appropriate than another group.
When primary outcomes were assessed at more than one time point in our time point ranges, we used the data from the latest time point available (in participants in the eligible age range) in general analyses. We also intended to use this data in relevant subgroup analyses, but we could not perform meta‐analyses as the data did not allow this. We were careful not to present the same study sample of participants more than once per outcome and time point range (e.g. Table 2), unless the different analyses were from the same study sample were clearly referenced (e.g. Tables 6 to 15).
Summary of findings 2. Total fat intake and body weight in children (cohort studies)a,b.
|
Total fat intake and bodyweightin children (cohort studies) A comprehensive table including data for all time points for each outcome can be found in Appendix 3 | ||||
|
Patient or population: boys and girls aged 24 months to 18 years Setting: communities, schools, households, healthcare centres in high‐income countries Exposure: total fat intake | ||||
| Outcomes |
No of studies (No of participants) |
Impact | Quality | What happens |
|
Weight (kg) Follow‐up: 2 to 5 years |
4 cohort studies (13,802) |
2 studies that adjusted for TE intake: After 3 years, "Dairy fat was not a stronger predictor of weight gain than other types of fat, and no fat (dairy, vegetable, or other) intake was significantly associated with weight gain after energy adjustment, nor was total fat intake;" no numerical results reported. After 3 years, for every 1% increase in TE intake from total fat of children, weight will decrease by 0.0011 kg. 2 studies that did not adjust for TE intake: After 4 years, weight of children with low‐fat intake (< 30%TE) will increase by 8.1 kg on average, and by 8.9 kg on average in children with high‐fat intake (> 35%TE). After 2 years, children with low‐fat intake (≤ 30%TE) will gain on average 0.2 kg per year more than children with high‐fat intakes (> 30%TE) |
⊕⊝⊝⊝ Very low1,2 | When adjusted for TE, we were uncertain whether fat intake was associated with weight in children over 2 to 5 years. When not adjusted for TE, we were uncertain whether lower fat was associated with weight in children over 2 to 5 years. |
| Follow‐up: 5 to 10 years | 1 cohort study (126) |
1 study that did not adjust for TE intake: After 6 years, weight of children with low‐fat intake (< 30%TE) will increase by 16.8 kg on average, and by 13.9 kg on average in children with high‐fat intake (> 35%TE) |
⊕⊝⊝⊝ Very low3,4,5,6 | We were uncertain whether fat intake was associated with weight over 5 to 10 years (1 study). |
|
BMI (kg/m2, kg/m2 per year, z‐score, percentile) Follow‐up: 2 to 5 years |
7 cohort studies (3143) |
4 studies that adjusted for TE intake: After 3 years, for every 1% increase in energy intake from total fat, BMI will decrease by 0.63 z‐score in boys but increase by 0.07 z‐score in girls. "Dietary factors were not associated with BMI across the three study years." After 3 years, for every 1% increase in energy intake from total fat, BMI will decrease by 0.00008 kg/m2. After 4 years, increase in the total fat intake, will increase BMI by 0.087 z‐score. The model explained 48% of variance in the change of BMI z‐score. 2 studies that did not adjust for TE intake: After 2.08 years, low‐fat intake (≤ 30%TE) will result in a 0.02 kg/m2 per year greater increase in BMI on average, compared to high‐fat intake (> 30%TE). After 3 years, for every 1% increase in energy intake from total fat, BMI will decrease by 0.01 percentile in girls. 1 study where TE adjustment was not applicable, as TE was part of exposure: After 3 years, for every 1 z‐score increase in the energy‐dense, high‐fat and low‐fibre dietary pattern, BMI will increase by 0.03 z‐score in boys and by 0.99 z‐score in girls. After 3 years, the ratio of odds for being overweight/obese was 1.04 greater in boys and 1.02 greater in girls with higher dietary pattern z‐scores, compared to the odds in boys and girls with lower dietary pattern z‐scores. |
⊕⊝⊝⊝ Very low6,7,8 |
We were uncertain whether fat intake was associated with BMI in children over 2 to 10 years. |
| Follow‐up: 5 to 10 years | 4 cohort studies (1158) |
3 studies that adjusted for TE intake: After 6 years, for every 1% increase in energy intake from total fat, BMI will decrease by 0.011 z‐score in boys but increase by 0.005 z‐score in girls. After 9 years, increase in the total fat intake will increase BMI by 0.122 z‐score. After 10 years, for every 1% increase in energy intake from total fat, BMI will increase by 0.029 kg/m2 in white girls and by 0.012 kg/m2 in black girls. 1 study that did not adjust for TE intake: After 6 years, for every 1 g increases in the fat intake, BMI will increase by 0.01 kg/m2 |
⊕⊝⊝⊝ Very low6,9 | |
|
LDL‐C (mmol/L) Follow‐up: 2 to 5 years |
1 cohort study (1163) |
1 study where TE adjustment not applicable, as TE was part of exposure: After 3 years, for every 1 z‐score increase in the energy‐dense, high‐fat and low‐fibre dietary pattern, LDL‐C will increase by 0.001 mmol/L in boys and 0.04 mmol/L in girls |
⊕⊝⊝⊝ Very low4,5,6,11 | We were uncertain whether fat intake was associated with LDL‐C in children over 2 to 5 years (1 study). |
|
HDL‐C (mmol/L) Follow‐up: 2 to 5 years |
2 cohort studies (1393) |
1 study that adjusted for TE intake: After 3 years, for every 1% increase in energy intake from total fat, HDL‐C will decrease by 0.21 mmol/L in girls. 1 study where TE adjustment not applicable, as TE was part of exposure: After 3 years, for every 1 z‐score increase in the energy‐dense, high‐fat and low‐fibre dietary pattern, HDL‐C will decrease by 0.002 mmol/L in boys but increase by 0.02 mmol/L in girls. |
⊕⊕⊝⊝ Low11,12 | When adjusted for TE, fat intake may be inversely associated with HDL‐C in girls over 2 to 5 years (1 study). When not adjusted for TE, fat intake may make little or no difference to HDL‐C in girls over 2 to 5 years (1 study). |
|
Triglycerides (mmol/L) Follow‐up: 2 to 5 years |
1 cohort study (1163) |
1 study where TE adjustment not applicable, as TE was part of exposure: After 3 years, for every 1 z‐score increase in the energy‐dense, high‐fat and low‐fibre dietary pattern, triglycerides will increase by 1% in either boys or girls. |
⊕⊝⊝⊝ Very low4,5,6,11 | We were uncertain whether fat intake was associated with triglycerides in children over 2 to 5 years (1 study). |
|
Height (cm) Follow‐up: 2 to 5 years |
3 cohort studies (973) |
1 study that adjusted for TE intake: After 3 years, for every 1% increase in energy intake from fat, height in children will decrease by 0.0009 cm on average. 2 studies that did not adjust for TE intake: After 2 years, low‐fat intake (≤ 30%TE) will result in a 0.2 cm per year greater increase in height on average compared to high‐fat intake (> 30%TE). After 4 years, on average children in low‐fat intake (< 30%TE) gain 27.9 cm in height, while children in high‐fat intake (> 35%TE) gain 28.3 cm in height. |
⊕⊝⊝⊝ Very low6,10 | We were uncertain whether fat intake was associated with height in children over 2 to 10 years. |
| Follow‐up: 5 to 10 years Age at baseline: 2 years |
1 cohort study (126) |
1 study that did not adjust for TE intake: At 6 years, on average children in low‐fat intake (< 30%TE) gain 44.9 cm in height while children in high‐fat intake (> 35%TE) gain 40.3 cm in height. |
⊕⊝⊝⊝ Very low3,4,5,6 | |
|
BMI: body mass index; HDL‐C: high‐density lipoprotein cholesterol; LDL‐C: low‐density lipoprotein cholesterol; MD: mean difference; TE: total energy. aNotes: Some cohort studies reported more than one eligible analysis for the same outcome (e.g. BMI as continuous or binary outcome) or different measures of exposure (e.g. fat intake as continuous %TE or as binary classification of less‐exposed vs more‐exposed). In these cases, we selected outcomes and exposure measures so as not to use the same study sample of participants more than once per outcome and time point range in the table. For all outcomes, there were too few studies to assess publication bias. | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
1Although, risk of bias was concerning (studies with strong contributions did not adjust for all important prognostic variables), plausible residual confounding would likely reduce the demonstrated effect in the studies that did not adjust for total energy intake; thus we chose not to downgrade for risk of bias.
2Downgraded by 1 for imprecision: in studies reporting variance, the variance included no effect and important benefit or harm.
3Although risk of selection bias (no matching of exposed and non‐exposed groups, or statistical adjustments) and attrition bias (> 50% attrition) was concerning, plausible residual confounding would likely reduce the demonstrated effect as this study did not adjust for total energy; thus we chose not to downgrade for selection bias.
4Only 1 study for this outcome, therefore we could not rate for inconsistency.
5Downgraded by 1 for indirectness: a single study in a high‐income country likely has limited generalisability.
6Imprecision was considered, but we considered a decision would not impact on the rating and thus no judgement was made for imprecision.
7Downgraded by 1 for risk of bias: risk of selection bias: 5 studies did not match exposed and non‐exposed groups or make important statistical adjustments; high risk of detection bias: dietary assessment for 3 studies were not adequately rigorous.
8Downgraded by 1 for inconsistency: some studies reported small to large positive associations between exposure and outcome, while others reported no association or a small to medium inverse association between exposure and outcome.
9Downgraded by 1 for risk of bias: risk of selection bias: 2 studies with strongest contributions, did not adjust for all important prognostic variables; high risk of detection bias: dietary assessment in 1 study was not adequately rigorous.
10Downgraded by 1 for risk of bias: risk of selection bias; no matching of exposed and unexposed groups or adjustment for all important prognostic variables.
11Study was judged to have a lower overall risk of bias; attrition < 50% and satisfactory assessment of exposure.
12Not downgraded for serious imprecision as judged to be precise estimates of no effect in both studies.
Dealing with missing data
Where study authors had not reported all relevant statistics per outcome (e.g. SD of change per group for continuous data), we attempted to calculate or estimate the required data from other statistics reported in the study by using relevant formulas from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). If we could not calculate or estimate these statistics with reasonable confidence, we emailed the study authors. Where we did not receive a timely response, or where we received a response for which we lacked confidence, we did not impute the missing values but instead reported the available results in a table. We indicated in the tables where we made use of unpublished data supplied to us by study authors.
Assessment of heterogeneity
We intended to examine heterogeneity per outcome and time point by visual inspection of the forest plots (i.e. we looked at physical overlap of CIs across the included studies). We intended to assess statistical heterogeneity among the intervention effects across the included studies in the meta‐analyses as follows:
Chi2 test for heterogeneity;
I2 statistic to quantify heterogeneity; and
Tau2 statistic to measure the extent of heterogeneity.
In meta‐analyses, we intended to consider heterogeneity as an I2 value of greater than 30% and either a Chi2 of less than 0.1 or Tau2 greater than 0. We planned to perform subgroup analyses to explore heterogeneity, but data did not allow meta‐analyses (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Where more than 10 included studies addressed a primary outcome, we intended to used funnel plots to assess the possibility of small‐study effects. For future review updates, in the case of asymmetry, we will consider various explanations such as publication bias, poor study design and the effect of study size.
Data synthesis
We sought to combine data by the inverse variance method in random‐effects meta‐analysis to assess MDs between lower and higher fat intake arms, but data did not allow for any meta‐analyses. Where possible, we converted variables to comparable units to allow pooling of data if appropriate. We planned to conduct separate meta‐analyses of data from RCTs and data from cohort studies, and only where data from separate studies were similar enough to be combined (see Assessment of heterogeneity).
We intended not to use end data in meta‐analysis, where the difference between the intervention and control groups at baseline was greater than the change in that measure between baseline and endpoint in both groups. Instead, we intended to use change data in forest plots but without SDs, so the data did not add to the meta‐analyses but instead provided comparative information. However, this was not relevant in this update as we could not meta‐analyse the data.
'Summary of findings' tables
Based on the methods described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011), we prepared two 'Summary of findings' tables to present the results of the RCTs and cohort studies separately. In both 'Summary of findings' tables we included our primary outcome of body fatness (measured by weight‐for‐age z‐score, weight and BMI), cardiovascular risk factors (total cholesterol, LDL, HDL and triglyceride concentrations), and height (in cm or height‐for‐age z‐score). We deemed these outcomes the most important as guided by our question and the primary purpose of the review. Given the large number of time points examined, we selected time points for inclusion in the tables by considering the influence of:
height gain on bodyweight change in children;
intervention fidelity over time in RCTs; and
the challenges with repeated dietary intake measurements over time in cohort studies.
Summary tables for all time points are presented in Appendix 2 (RCTs) and Appendix 3 (cohort studies).
We used the GRADE system to rank the quality of the evidence using GRADEpro GDT software (GRADEpro GDT). As data were reported heterogeneously, and meta‐analyses were not possible, we presented results in a narrative 'Summary of findings' table for cohort studies (drawing on McNeill 2017 as an example).
Subgroup analysis and investigation of heterogeneity
For this update, we classified all dietary interventions and exposures as lower fat versus usual or modified fat. We intended to compare the intervention effects or associations across the following subgroups, but the available data did not allow us to perform any of these:
difference in %TE from fat between lower fat and control groups in RCTs (e.g. up to 5%TE from fat, 5%TE to 10%TE from fat, 10%TE to 15%TE from fat, 15%TE or greater from fat or unknown difference);
type of intervention in RCTs (e.g. nutrition counselling only versus nutrition counselling plus food provided);
adherence to fat intake goals in the intervention group in RCTs (e.g. achieved 30%TE from fat or less versus did not achieve this);
weight status at baseline (e.g. by BMI‐for‐age z‐score);
reported estimated energy reduction in the intervention compared with the control group during the intervention period in RCTs (e.g. estimated energy intake the same or greater in the lower fat group, energy intake 1 kcal/day to 100 kcal/day lower in the lower fat group, 101 kcal/day to 200 kcal/day lower in the lower fat group, greater than 200 kcal/day lower in the lower fat group); and
cohort studies that statistically adjusted for energy intake when relating total fat intake to body fatness versus cohort studies that did not adjust for energy intake.
Sensitivity analysis
Where possible, we carried out sensitivity analyses for primary outcomes, assessing the effect of:
our selected time point ranges by including only the longest follow‐up data per study; and
our selected time point ranges by including only the shortest follow‐up data per study.
We had planned to perform other sensitivity analyses; however, since we only identified three RCTs and did not meta‐analyse cohort studies, we deemed other sensitivity analyses inappropriate. In future updates, it may be feasible to assess the influence of excluding studies with unclear or inadequate allocation concealment in RCTs, performing fixed‐effect meta‐analyses (rather than random‐effects) (Higgins 2011b), excluding studies with only a subset of eligible participants, excluding studies that were not free of systematic differences in care (performance bias) (or where it was unclear) and excluding studies that were not free of dietary differences other than total fat (or where it was unclear).
Results
Description of studies
The flow diagram of search results and study selection for this systematic review update is presented in Figure 1.
1.
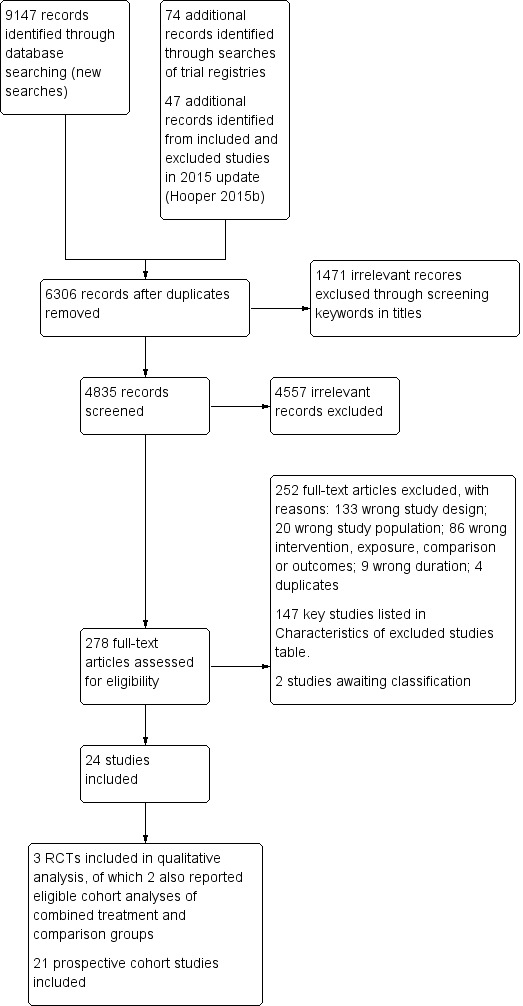
Study flow diagram. RCT: randomised controlled trial.
Results of the search
The search for RCTs and cohort studies in adults and children in a previous version of this review (Hooper 2012) identified 32,220 titles and abstracts from the electronic searches plus 28 further potential studies from other sources. For the previous update (Hooper 2015a), the electronic searches identified 7729 possible titles and abstracts, plus review authors assessed a further 24 potential studies after checking for potentially relevant trials and cohort studies included in other systematic reviews. Of these 7753 potential titles and abstracts, the review authors assessed 218 full‐text articles for eligibility (additional to the 465 assessed for the original review). This review in adults and children in 2015 included one RCT and 11 cohort studies in children (Hooper 2015b). Our flow diagram in Figure 1 does not include the search results from previous versions of this review, as they also included studies in adults and are thus not combinable with the search results for this review update.
Our new search strategy tailored for children (Appendix 1), yielded 9301 records, with 6306 records remaining following duplicate removal. After removing obviously ineligible records using a keyword search, we screened 4835 titles and abstracts, with 278 full‐texts identified as potentially eligible. After excluding 252 studies with reasons and two studies awaiting classification, we included 24 studies comprising three parallel‐group RCTs (reported in 12 records) and 21 prospective cohort studies (92 eligible analyses, reported in 47 records) (Figure 1). Two of the included RCTs (Obarzanek 2001 (RCT); Tershakovec 1998 (RCT)) also reported eligible cohort analyses that we included with the cohort data, and these are presented throughout the review as two 'additional' study references (Obarzanek 1997 (cohort); Tershakovec 1998 (cohort)).
Included studies
See Characteristics of included studies table for detailed characteristics of all included studies.
Randomised controlled trials
Study location, participants and duration
Mihas 2010: conducted in Greece; boys and girls aged 12 to 13 years with no known cardiovascular disease risk factors; follow‐up over 17 months.
Obarzanek 2001 (RCT): conducted in the USA; boys and girls aged seven to 11 years with primary elevated serum LDL‐cholesterol levels; follow‐up over approximately seven years.
Tershakovec 1998 (RCT): conducted in the USA; boys and girls aged four to 11 years who were hypercholesterolaemic; follow‐up over one year.
Interventions
Interventions to reduce total fat intake were delivered as combinations of individual and group counselling and education sessions in clinics, schools and homes, with some involvement of parents in the sessions and one trial also including telephone contacts between sessions. Sessions were delivered by paediatric dieticians, nutritionists, behaviourists or trained and supervised teachers, as classroom curriculum or using other education resources, such as posters, workbooks, audiotape stories and picture books. Detailed descriptions of the interventions in the three RCTs are shown in Table 4.
1. Summary of the intervention details (using TIDieRa items) for each RCT in the systematic review.
| Recipients | Why | What (materials) | What (procedures) | Who provided | How and where | When and how much | Strategies to improve or maintain intervention fidelity; tailoring and modification | Extent of intervention fidelity |
| Tershakovec 1998 (RCT) | ||||||||
| 4‐ to 9‐year‐old children with hypercholesterolaemia (plasma total cholesterol > 4.55 mmol/L, fasting plasma LDL‐C 2.77‐4.24 mmol/L for boys and 2.90‐4.24 mmol/L for girls), at ≥ 85% of ideal body weight. | Limited dietary fat was recommended for children aged > 2 years, but there were concerns that lower fat intake of children may affect their growth. Trial evaluated growth of children with hypercholesterolaemia completing an innovative, physician‐initiated, home‐based nutrition education programme or standard nutrition counselling that aimed to lower dietary fat intake. | Nutrition education programme complied with recommendations of the National Cholesterol Education Program Expert Panel on Blood Cholesterol Levels in Children and Adolescents. | Children and ≥ 1 parent (usually mother) attended 45‐ to 60‐minute counselling session with paediatric dietician. Children and parents in at‐risk control and not‐at‐risk control groups were not provided educational information or materials. | 1) Not described; 2) paediatric registered dieticians. | 1) Audiotape stories and picture books and follow‐up paper/pencil activities for children as well as manual for parents. Story and activities to be completed each week; 2) face‐to‐face individual counselling by a dietician. 1) At home; 2) paediatric practice. |
10 weeks with 1) talking‐book lesson; 2) 45‐60 minutes counselling session each week. | Not described Tailoring and modification of intervention during trial were not described. |
1) 71/88; 2) 77/86 completed intervention programmes and returned for evaluation at 3 months after baseline. |
| Obarzanek 2001 (RCT) | ||||||||
| Prepubertal boys and girls aged 8‐11 years with LDL‐C levels ≥ 80th and < 98th percentiles for age and sex percentiles of the Lipid Research Clinics population. | Aimed to assess feasibility, safety, efficacy and acceptability of lowering dietary intake of total fat, saturated fat and cholesterol to decrease LDL‐C levels. | Intervention group received dietary counselling sessions based on National Cholesterol Education Programme guidelines: 28% of energy from total fat, < 8% from saturated fat, > 9% from polyunsaturated fat, and < 75 mg/1000 kcal of cholesterol per day, not to exceed 150 mg/day. Guidebooks including activities and recipes on diets and food recommendations given to participants and their families. | In first 6 months, 6 weekly and then 5 biweekly group sessions were led by nutritionists and behaviourists, and 2 individual visits were held with nutritionist. Over second 6 months, 4 group and 2 individual sessions were held. During 2nd and 3rd years, group and individual maintenance sessions were held 4‐6 times/year, with monthly telephone contacts between group sessions. During 4th year of follow‐up, 2 group events + 2 individual visits conducted with additional telephone contacts as appropriate. | Nutritionists and behaviourists | 1) Group sessions and 2) individual visits were held, accompanied by telephone contacts in between sessions. 1) At clinics, 2) at home |
6 weekly, 5 biweekly group sessions and 2 individual visits during first 6 months; 4 group and 2 individual sessions during second 6 months; 4‐6 maintenance sessions with telephone contacts between sessions during 2nd and 3rd years; 2 group and 2 individual sessions with telephone contacts as appropriate by 4th year. | By 4th year of follow‐up, individual visits used an individualised approach based on motivational interviewing and stage of change for increasingly busy teenagers. Tailoring and modification of intervention during trial not described. |
295/334 attended the last visit (> 5 years' follow‐up). |
| Mihas 2010 | ||||||||
| Students aged 12‐13 years from an urban area in Greece. | Aimed to evaluate the short‐term (15‐day) and long‐term (12‐month) effects of a 12‐week school‐based health and nutrition interventional programme regarding energy and nutrient intake, dietary changes and BMI. | Teaching material for teachers and workbooks for students on nutrition‐dietary habits and physical activity and health based on Social Learning Theory Model were developed and distributed to teacher and each student. | Multicomponent workbooks covering mainly dietary issues, but also dental health hygiene and consumption attitudes, were produced with each student being supplied a workbook. The class home economics teacher implemented 12‐hour‐classroom curriculum incorporating health and nutrition promotion during 12 weeks. 2 meetings were conducted with parents (given screening results of children; presentations given on dietary habits of children to improve health profile of children and prevent development of chronic diseases in the future). Cues and reinforcing messages in the form of posters and displays were provided in the classroom. | Educational intervention (classroom curriculum) delivered by class home economics teachers who were trained and supervised by health visitor or family doctor. | Classroom curriculum; cues and reinforcing messages in the form of posters and displays provided in classroom; nutrition education meetings for parents in group. At school. |
12 hours of classroom material, 2 meetings for parents during a 12‐week period. | Health visitor or family doctor supervised the programme implementation of class home economics teachers who were given 2 × 3‐hour seminars with aims to familiarise teachers about objectives of intervention and their role therein, and to increase their awareness of significance of incorporating health and nutrition in their curriculum before delivering the intervention. Tailoring and modification of intervention during trial not described. |
107/109 participation rates at 15‐days' follow‐up and 98/109 at 12 months' follow‐up. |
aTIDieR: Template for Intervention Description and Replication, template for this table from Hoffman 2017.
BMI: body mass index; LDL‐C: low‐density lipoprotein cholesterol; RCT: randomised controlled trial.
Funding and authors' declarations of interest
The older of the US trials was funded by the National Heart, Lung, and Blood Institute (HL43880‐03), the Howard Heinz Endowment, and the University of Pennsylvania Research Foundation (Tershakovec 1998 (RCT)), and the other US trial by the National Heart, Lung, and Blood Institute (Obarzanek 2001 (RCT)). There were no authors' declarations of interest reported for these trials in the articles we assessed. The trial in Greece was funded by the Ministry of Education and the National Foundation for the Youth and the authors declared no competing interests (Mihas 2010).
Prospective cohort studies
Study location, participants and duration
In most studies, children or families were recruited conveniently from schools, communities, daycare centres, clinics or hospitals, or were sampled from existing large cohort study samples. Participants in all included cohort analyses were healthy children, except for the two cohort analyses of the RCTs that included children with hypercholesteraemia (Tershakovec 1998 (cohort)) or primary elevated serum LDL‐cholesterol levels (Obarzanek 1997 (cohort)).
Mean age at baseline ranged across studies from two years to 14 years. Five studies followed children from baseline to one year (Bogaert 2003; Butte 2007; Niinikoski 1997a; Schwandt 2011; Tershakovec 1998 (cohort)), five studies for more than one to two years (Davison 2001; Klesges 1995; Lee 2001; Lee 2012; Setayeshgar 2017), seven studies for more than two to five years (Appannah 2015; Berkey 2005; Boreham 1999; Cohen 2014; Jago 2005; Obarzanek 1997 (cohort); Shea 1993), four studies for more than five to 10 years (Ambrosini 2016; Brixval 2009; Morrison 2008; Skinner 2004), and two studies followed children for more than 10 years (Alexy 2004; Magarey 2001).
Of the 21 included prospective cohort studies, one study was conducted in a middle‐income country (Korea; Lee 2012). All the others were conducted in high‐income countries, as follows: 10 in the USA (Berkey 2005; Butte 2007; Cohen 2014; Davison 2001; Jago 2005; Klesges 1995; Lee 2001; Morrison 2008; Shea 1993; Skinner 2004), one in Canada (Setayeshgar 2017), one in the UK (Ambrosini 2016), one in Northern Ireland (Boreham 1999), two in Germany (Alexy 2004; Schwandt 2011), one in Denmark (Brixval 2009), one in Finland (Niinikoski 1997a), and three in Australia (Appannah 2015; Bogaert 2003; Magarey 2001). Most studies included both sexes and all ethnicities, except one study that only included white children (Skinner 2004), one study that only included Hispanic children (Butte 2007), two studies that only included girls (Cohen 2014; Lee 2001), one study that only included white girls (Davison 2001), and one study that only included black and white girls (Morrison 2008).
Exposures
Exposures to total daily fat intake were estimated using different methods including 24‐hour dietary recall, FFQ and DRs. To examine associations with body fatness outcomes over time, total fat intake exposure estimates were expressed in different units, and applied in different ways across studies, as follows:
binary fat intake exposures: lower versus higher percentiles of fat intake, or lower versus higher fat intake groups (based on dietary intake assessments), and using cut‐offs of %TE from fat (e.g. 30%TE or less and greater than 30%TE or less than 30%TE and greater than 35%TE) (Alexy 2004; Ambrosini 2016; Lee 2001; Niinikoski 1997a; Shea 1993; Tershakovec 1998 (cohort);
continuous fat intake exposures: in %TE, absolute number of grams, per 10 grams of intake, by number of servings (Berkey 2005; Bogaert 2003; Boreham 1999; Brixval 2009; Butte 2007; Cohen 2014; Davison 2001; Jago 2005; Klesges 1995; Lee 2012; Morrison 2008; Obarzanek 1997 (cohort); Schwandt 2011; Setayeshgar 2017; Skinner 2004), or as a high‐fat dietary pattern in two studies (Ambrosini 2016; Appannah 2015), with two studies using both binary and continuous fat intake exposures to apply the exposure variables in analyses (Appannah 2015; Magarey 2001).
Figure 2 presents the spread of the different ways in which total fat intake estimates were expressed and applied to examine associations with body fatness in the 81 analyses that reported primary outcomes (weight, BMI, waist circumference, body fat and skinfold thickness) in the five time point ranges. The heterogeneous application of fat intake exposure at different time points for different outcomes across the included studies is evident in Figure 2.
2.
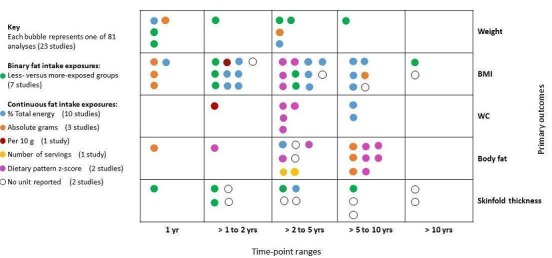
The bubble‐plot presents the spread of the different ways in which total fat intake estimates were expressed and applied to examine associations with body fatness in the 81 analyses, reporting primary outcomes in the five time point ranges. Combining the many various total fat intake exposure estimates reporting on the same outcome in the same time point range was deemed inappropriate. BMI: body mass index; WC: waist circumference; yr: year.
The studies reporting dietary patterns as the exposure used reduced rank regression to identify dietary patterns or combinations of food intake, that attempted to explain the maximum variation in a set of response variables hypothesised to be on the pathway between food intake and obesity (Ambrosini 2016; Appannah 2015). Participants were scored for each dietary pattern at each age using a z‐score that quantified how their reported dietary intake reflected each dietary pattern relative to other respondents in the study sample. The model used calculates dietary z‐scores for each respondent as a linear, weighted combination of all their standardised food group intakes by using weights unique to each dietary pattern. Increasing intakes of foods with positive factor loadings increases the dietary pattern z‐score, and increasing intakes of foods with negative factor loadings decreases the dietary pattern z‐score. The energy‐dense, high‐fat, low‐fibre dietary pattern reflected high intakes of processed meat, chocolate and confectionery, low‐fibre bread, crisps and savoury snacks, and fried and roasted potatoes (high intake of these foods increased the participant's dietary pattern z‐score).
Funding and authors' declarations of interest
Five of the 21 cohort studies had combined public and private funding including from the food industry and financial services industry (Berkey 2005; Bogaert 2003; Lee 2001; Niinikoski 1997a; Skinner 2004). In these studies, no author declarations of interest were reported. Two studies did not report their funding sources (Brixval 2009; Lee 2012), and in these studies, authors declared no conflicts of interests. The remaining 14 cohort studies were publicly funded, with six of these reporting no conflicts of interest by authors (Ambrosini 2016; Appannah 2015; Butte 2007; Cohen 2014; Morrison 2008; Setayeshgar 2017), and the rest containing no author declarations of interest.
Excluded studies
After full‐text screening, we excluded 252 studies. Key studies (n = 147) with their reasons for exclusion are in the Characteristics of excluded studies table. Briefly, 133 studies were excluded for inappropriate study design (98 did not analyse children's baseline to fat intake to body fatness at least 12 months later; 16 cross‐sectional; five reviews; two editorials; three analysed twin‐pairs; six non‐RCTs; one randomised fewer than six clusters; one case‐control; one prediction model used), 20 for unsuitable study population (e.g. adults or overweight children with intention to reduce weight), 58 for inappropriate intervention (e.g. school lunch programme), 14 for inappropriate exposure (e.g. dairy food intake or cereal intake), eight for no eligible outcomes reported and our outcomes deemed to be outside of the scope of the study (e.g. psychological outcomes), six for inappropriate comparison, nine for inappropriate duration (e.g. less than one year for cohort studies) and four duplicates. We excluded the Special Turku Coronary Risk Factor Intervention Project (STRIP) trial (Niinikoski 2014), as the primary intention of the intervention was to reduce saturated fat intake through replacement with unsaturated fat, thus changing the 'quality' of fat intake or composition of fat intake. Our question primarily concerns the quantity of total fat intake.
Studies awaiting classification
We found two published abstracts from the one study awaiting assessment (Khalil 2015) and contacted the authors for additional information, but did not receive a response in time for assessment for inclusion in this review. We also contacted the authors of Twisk 1998, but did not receive the requested information in time.
Ongoing studies
We found no eligible ongoing studies.
Risk of bias in included studies
Figure 3 represents each risk of bias item presented as percentages across all included RCTs and across all included cohort studies. A visual representation of the risk of bias for each domain per included RCT and cohort study is presented in Figure 4. For the two trials that also report eligible cohort analyses (Obarzanek 1997 (cohort); Tershakovec 1998 (cohort)), we reported risk of bias judgements for each study design.
3.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
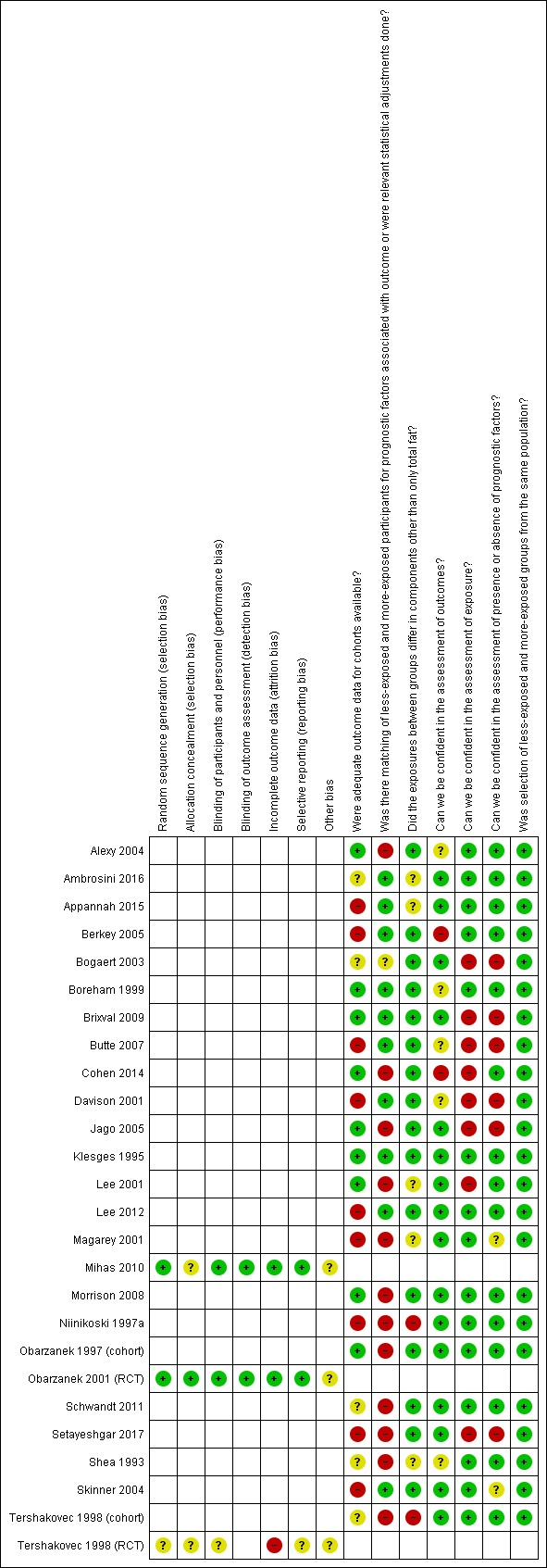
Risk of bias summary: review authors' judgements about each risk of bias item for each included study. RCT: randomised controlled trial.
See the Characteristics of included studies table for details of risk of bias judgements per trial and per cohort study.
Validity of randomised controlled trials
Allocation (selection bias)
We judged two RCTs to have an unclear risk of selection bias because allocation concealment was not reported (Mihas 2010; Tershakovec 1998 (RCT)), and Tershakovec 1998 (cohort) also lacked clarity in the reporting of random sequence generation. Obarzanek 2001 (RCT) was at low risk of selection bias.
Blinding (performance bias and detection bias)
Tershakovec 1998 (RCT) did not report on blinding and we judged this study at unclear risk of performance and detection bias. Obarzanek 2001 (RCT) reported blinding of outcome assessors and not of participants. However, since this was unlikely to have influenced the primary study outcomes, we judged this trial at low risk for performance and detection bias. Similarly, we judged Mihas 2010 at low risk of bias for this domain because although the authors reported blinding was not feasible, it was unlikely that the primary outcome was influenced by a lack of blinding.
Incomplete outcome data (attrition bias)
We assessed those studies that lost more than 10% of participants in total at high risk of attrition bias, unless they adequately report dropout analyses showing no differences in reasons and key characteristics between completers and non‐completers. Attrition rates were greater than 10% over the one‐year follow‐up for Tershakovec 1998 (RCT) and reasons for missing outcome data per group were not provided; thus, it was at high risk of bias. We assessed the other two RCTs at low risk of attrition bias due to reported attrition rates of less than 10% (Mihas 2010; Obarzanek 2001 (RCT)).
Selective reporting (reporting bias)
Tershakovec 1998 (cohort) was at unclear risk of reporting bias because outcomes reported by the authors were not prespecified. We judged the other two RCTs at low risk of reporting bias because they prespecified their outcomes in the methods section and addressed them in the results section (Mihas 2010; Obarzanek 2001 (RCT)). Generating funnel plots was not possible due to the small number of included trials.
Other potential sources of bias
All three RCTs were at unclear risk of 'other bias' because limited information on the control diet prescription made it difficult to judge if the intervention and control diets differed in components other than only total fat.
Validity of cohort studies
Was adequate outcome data available? (attrition bias)
Nine studies were at high risk of attrition bias due to high attrition (greater than 5% attrition per year) and reasons for attrition were not reported or incompletely described (Appannah 2015; Berkey 2005; Butte 2007; Davison 2001; Lee 2012; Magarey 2001; Niinikoski 1997a; Setayeshgar 2017; Skinner 2004). Four studies with high attrition conducted dropout analyses of baseline anthropometric and dietary intake variables: two were at low risk of bias because they adequately reported no difference between completers and non‐completers (Brixval 2009; Klesges 1995); and the other two were at unclear risk of bias because insufficient information was provided to permit judgement (Bogaert 2003; Tershakovec 1998 (cohort)). Attrition bias could not be determined for two studies (judged at unclear risk of bias), as Shea 1993 did not report how many children completed the last follow‐up visit, and Schwandt 2011 reported the dropout analysis inadequately. The remaining seven studies had low risk of attrition bias.
Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome, or were relevant statistical adjustments done? (selection bias)
Eight studies compared outcome data in less‐exposed versus more‐exposed groups and none of these matched their participants for prognostic factors (Alexy 2004; Ambrosini 2016; Appannah 2015; Lee 2001; Magarey 2001; Niinikoski 1997a; Shea 1993; Tershakovec 1998 (cohort)). Twelve studies were at high risk of selection bias due to no or incomplete adjustment for important prognostic variables, namely, age, sex, energy intake, ethnicity, parental BMI, physical activity (and/or screen time), pubertal stage and socioeconomic (income and educational) status (Alexy 2004; Cohen 2014; Jago 2005; Lee 2001; Magarey 2001; Morrison 2008; Niinikoski 1997a; Obarzanek 1997 (cohort); Schwandt 2011; Setayeshgar 2017; Shea 1993; Tershakovec 1998 (cohort)). We judged Bogaert 2003 to have an unclear risk of selection bias because prognostic variables included in the analyses were not described. The remaining 10 studies were at low risk of bias for this domain.
Did the exposures between groups differ in components other than only total fat? (performance bias)
Two trials that reported eligible cohort analyses comparing less‐exposed and more‐exposed groups were at high risk of bias because the exposures in the original trial groupings differed in components other than only total fat (Niinikoski 1997a; Tershakovec 1998 (cohort)). The risk was unclear in the other five studies that reported data on less‐exposed versus more‐exposed groups (Ambrosini 2016; Appannah 2015; Lee 2001; Magarey 2001; Shea 1993), and low risk in one (Alexy 2004). We judged the other 15 studies that only reported single group associations between fat intake and weight over time as low risk of bias for this domain.
Can we be confident in the assessment of outcomes? (detection bias)
Cohen 2014 and Berkey 2005 were at high risk of detection bias because methods for measuring body fat were inconsistent across different time points during the study , and self‐reporting of weight and height was used, respectively. The risk was unclear in five studies that did not provide sufficient detail to make a judgement (Alexy 2004; Boreham 1999; Butte 2007; Davison 2001; Shea 1993), and the remaining studies had a low risk of detection bias.
Can we be confident in the assessment of exposure? (detection bias)
Fifteen of 23 studies were at low risk as they assessed dietary intake repeatedly throughout the duration of the study using recognised or validated methods such as three‐day, four‐day or seven‐day food records, FFQs, and multiple 24‐hour recall questionnaires. Three studies used multiple 24‐hour recall questionnaires (Lee 2012; Obarzanek 1997 (cohort); Tershakovec 1998 (cohort)). Seven studies were at high risk of bias for this domain, since they only assessed dietary intake at baseline (regardless of the methods they used) (Bogaert 2003; Brixval 2009; Butte 2007; Cohen 2014; Davison 2001; Lee 2001; Setayeshgar 2017), and one study used direct observation for dietary assessments, which is likely to introduce the Hawthorne effect (Jago 2005).
Can we be confident in the assessment of presence or absence of prognostic factors? (selection bias)
In this domain, we specifically looked at our most important prognostic factors, such as physical activity, parental BMI, pubertal stage and whether these factors were adequately assessed in the included cohort studies. We judged 15/23 studies at low risk of bias for this domain. Twelve of these studies repeatedly ascertained or measured the prognostic factors using validated methods, which were well described in the reports (Ambrosini 2016; Appannah 2015; Berkey 2005; Boreham 1999; Cohen 2014; Klesges 1995; Lee 2012; Morrison 2008; Niinikoski 1997a; Obarzanek 1997 (cohort); Schwandt 2011; Tershakovec 1998 (cohort)). We allocated low risk of bias to three studies that did not apply, and thus did not measure, prognostic factors in their studies (Alexy 2004; Lee 2001; Shea 1993). We allocated high risk to studies that performed a single assessment (Bogaert 2003; Brixval 2009; Butte 2007; Setayeshgar 2017), multiple assessments but with inconsistent methods (Davison 2001), direct observation of physical activity (Jago 2005), and self‐reported parental weight and height and pubertal stage (Brixval 2009; Butte 2007). The risk of bias was unclear for studies that did not adequately describe measurement methods for physical activity (Skinner 2004) and parental weight and height (Magarey 2001).
Was selection of less‐exposed and more‐exposed groups from the same population? (selection bias)
We considered all included cohort studies at low risk of bias for this domain because they all recruited children from the same cohort sample or study population.
Effects of interventions
Summary of findings for the main comparison. Total fat intake 30% or less of total energy compared to usual fat intake for body weight in children (RCTs)a.
|
Total fat intake ≤ 30% of total energy compared to usual fat intake for bodyweight in children (RCTs) A comprehensive table including data for all time points for each outcome can be found in Appendix 2 | |||||
|
Patient or population: boys and girls aged 24 months to 18 years Setting: paediatric practices, schools and health maintenance organisations in high‐income countries Intervention: lower total fat intake ≤ 30%TE Comparison: usual or modified fat intake | |||||
|
Outcomes (at time point ranges where data were reported) |
No of participants (No of studies) |
Illustrated comparative effect (95% CI) | Quality | What happens | |
| Usual fat intake1 | Effect difference with total fat ≤ 30% of total energy2 | ||||
|
Weight‐for‐age z‐score Follow‐up: range 6 to 12 months |
151 (1 RCT) |
The mean weight‐for‐age z‐score in control group was 0.29 | MD 0.18 lower (0.51 lower to 0.15 higher) | ⊕⊝⊝⊝ Very low3,4,5,6 | We were uncertain whether lower total fat intake (≤ 30%TE) had an effect on weight‐for‐age in children over a 12‐month period (1 study). |
| Weight (kg) Follow‐up: range 6 to 12 months | 620 (1 RCT) |
The mean weight (kg) in control group was 38.2 | MD 0.5 lower (1.78 lower to 0.78 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | Lower total fat intake (≤ 30%TE) may have made little or no difference to weight in children over a 5‐year period (1 study). |
| Follow‐up: range 2 to 5 years | 612 (1 RCT) |
The mean weight (kg) in control group was 49.5 | MD 0.6 lower (2.39 lower to 1.19 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | |
| BMI (kg/m2) Follow‐up: range 6 to 12 months | 620 (1 RCT) |
The mean BMI (kg/m2) in control group was 18.5 | MD 0.3 lower (0.75 lower to 0.15 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | Lower total fat intake (≤ 30%TE) may have made little or no difference to BMI in children over a 1‐year period (1 study). |
| Follow‐up: range 1 to 2 years | 191 (1 RCT) |
The mean BMI (kg/m2) in control group was 24.8 | MD 1.5 lower (2.45 lower to 0.55 lower) | ⊕⊕⊕⊝ Moderate4,9,10 | Lower total fat intake (≤ 30%TE) probably reduced BMI in children over a period of 1 to 2 years (1 study). |
| Follow‐up: range 2 to 5 years | 541 (1 RCT) |
The mean BMI (kg/m2) in control group was 21.7 | MD 0 (0.63 lower to 0.63 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | Lower total fat intake (≤ 30%TE) may have made little or no difference to BMI in children over a 2 to 5‐year period and > 5‐years (1 study). Please see Appendix 2 for Data for > 5 years. |
| Total cholesterol (mmol/L) Follow‐up: range 6 to 12 months | 618 (1 RCT) |
The mean total cholesterol (mmol/L) in control group was 5.1 | MD 0.15 lower (0.24 lower to 0.06 lower) | ⊕⊕⊕⊝ Moderate4,5,7,11 | Total fat intake ≤ 30%TE probably slightly reduced total cholesterol in children over a 12‐month period (1 study). |
| Follow‐up: range 2 to 5 years | 522 (1 RCT) |
The mean total cholesterol (mmol/L) in control group was 4.6 | MD 0.06 lower (0.17 lower to 0.05 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | Lower total fat intake (≤ 30%TE) may have made little or no difference to total cholesterol in children over a 2 to 5‐year period and > 5‐years (1 study). Please see Appendix 2 for Data for > 5 years. |
| LDL‐C (mmol/L) Follow‐up: range 6 to 12 months | 618 (1 RCT) |
The mean LDL‐C (mmol/L) in control group was 3.29 | MD 0.12 lower (0.2 lower to 0.04 lower) | ⊕⊕⊕⊝ Moderate4,5,7,11 | Lower total fat intake (≤ 30%TE) probably reduced LDL‐C in children over a 12‐month period (1 study) and over a 2 to 5‐year period (1 study). Please see Appendix 2 for Data for > 5 years. |
| Follow‐up: range 2 to 5 years | 623 (1 RCT) |
The mean LDL‐C (mmol/L) in control group was 3.07 | MD 0.09 lower (0.17 lower to 0.01 lower) | ⊕⊕⊕⊝ Moderate4,5,7,11 | |
| HDL‐C (mmol/L) Follow‐up: range 6 to 12 months | 618 (1 RCT) |
The mean HDL‐C (mmol/L) in control group was 1.47 | MD 0.03 lower (0.08 lower to 0.02 higher) | ⊕⊕⊕⊝ Moderate4,5,7,12 | Lower total fat intake (≤ 30%TE) probably made little or no difference to HDL‐C in children over a 6 to 12‐month period (1 study) and over a 2 to 5‐year period (1 study). Please see Appendix 2 for Data for > 5 years. |
| Follow‐up: range 2 to 5 years | 522 (1 RCT) |
The mean HDL‐C (mmol/L) in control group was 1.32 | MD 0.01 lower (0.06 lower to 0.04 higher) | ⊕⊕⊕⊝ Moderate4,5,7,12 | |
| Triglycerides (mmol/L) Follow‐up: range 6 to 12 months | 618 (1 RCT) |
The mean triglycerides (mmol/L) in control group was 0.98 | MD 0.01 lower (0.08 lower to 0.06 higher) | ⊕⊕⊕⊝ Moderate4,5,7,12 | Lower total fat intake (≤ 30%TE) probably made little or no difference to triglycerides in children over a 6 to 12‐month period (1 study). Please see Appendix 2 for Data for > 2 years. |
|
Height‐for‐age z‐score Follow‐up: range 6 to 12 months |
151 (1 RCT) |
The mean height‐for‐age z‐score in control group was 0.05 | MD 0.05 lower (0.08 lower to 0.02 lower) | ⊕⊝⊝⊝ Very low3,4,5,13 | We were uncertain whether lower total fat intake (≤ 30%TE) reduced height‐for‐age in children over a 12‐month period (1 study). |
| Height (cm) Follow‐up: range 6 to 12 months | 642 (1 RCT) |
The mean height (cm) in control group was 143.1 | MD 0 (1.11 lower to 1.11 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | Lower total fat intake (≤ 30%TE) may have made little or no difference to height in children over a period > 5 years (1 study). |
| Follow‐up: range 2 to 5 years | 540 (1 RCT) |
The mean height (cm) in control group was 167.4 | MD 0.10 lower (1.54 lower to 1.34 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | |
|
%TE: percentage of total energy; BMI: body mass index; CI: confidence interval; HDL‐C: high‐density lipoprotein cholesterol; LDL‐C: low‐density lipoprotein cholesterol; MD: mean difference; RCT: randomised controlled trial. aNotes: For all outcomes, there were too few studies to assess publication bias. | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Mean change observed between baseline and follow‐up in the control group.
2Difference in intervention group (and its 95% confidence interval) was based on the assumed change in the comparison group (and its 95% confidence interval).
3Downgraded by 1 for risk of bias: unclear risk of bias across all domains.
4Only 1 study for this outcome, therefore we could not rate for inconsistency.
5Downgraded by 1 for indirectness: participants were children with raised blood lipids, thus results may not be directly generalisable to all children.
6Downgraded by 1 for imprecision: small sample size and confidence interval included no effect and important benefit or harm.
7Not downgraded for serious risk of bias; a well‐conducted trial (methods in place to minimise risk of selection, performance, detection, attrition and reporting bias).
8Downgraded by 1 for imprecision: confidence interval included no effect and important benefit or harm.
9Downgraded by 1 for risk of bias: allocation concealment not reported.
10Not downgraded for serious imprecision: both bounds of the confidence interval indicate benefit, and calculated optimal information size met (158 patients are required to have a 80% chance of detecting, as significant at the 5% level, an important decrease in BMI of 1.7 kg/m2 (the average of the change across the 50th to 97th percentiles in 12.5 year‐olds, as per BMI‐for‐age tables, Centers of Disease Control & Prevention, 2000).
11Not downgraded for serious imprecision: both bounds of the confidence interval indicate benefit.
12Not downgraded for serious imprecision: precise estimate of no effect.
13Downgraded by 1 for imprecision: small sample size (optimal information size not met).
Effects of reducing dietary fat to 30% or less of total energy on body fatness in children (as seen in RCTs)
Table 1 presents the effects of a total fat intake of 30% or less in relation to total energy (≤ 30%TE) compared to usual or modified fat intake for bodyweight in children for data from RCTs. The data for all time points are presented in Appendix 2.
Primary outcomes
Weight
We have presented a summary of MDs and 95% CI between intervention and control groups for weight outcomes over time in Table 5 and Analysis 1.1. No pooling of data was possible due to the use of different outcomes by the two trials. We extracted weight‐for‐age z‐scores from Tershakovec 1998 (RCT) at baseline, and end values at six months (MD ‐0.14, 95% CI ‐0.45 to 0.17; n = 149; very low‐quality evidence), and 12 months (MD ‐0.18, 95% CI ‐0.51 to 0.15; n = 151; very‐low quality evidence); we are uncertain whether lower fat intake had an effect on weight‐for‐age z‐scores. Obarzanek 2001 (RCT) reported bodyweight (kg), and we extracted data at baseline and end values at 12 months (MD ‐0.50 kg, 95% CI ‐1.78 to 0.78; n = 620; low‐quality evidence), and three years (MD ‐0.60 kg, 95% CI ‐2.39 to 1.19; n = 612; low‐quality evidence). Fat intake of ≤ 30%TE versus usual or modified total fat intake in children may have made little or no difference to weight (kg) over these follow‐up periods.
2. Mean differences in body fatness outcomes for total fat intake of 30% or less of total energy compared to usual fat intake in children over time (three RCTs).
|
Outcome Study ID |
Follow‐up from baseline | |||||
|
Baseline Mean (SD)a |
6 months MD (95% CI) |
> 6 to 12 months MD (95% CI) |
> 1 to 2 years MD (95% CI) |
> 2 to 5 years MD (95% CI) |
> 5 years MD (95% CI) |
|
| Weight‐for‐age z‐scoreb | ||||||
| Tershakovec 1998 (RCT) | 0.04 (1.02); 0.26 (0.93) | ‐0.14 (‐0.45 to 0.17) |
‐0.18b (‐0.51 to 0.15) |
ND | ND | ND |
| Body weight (kg)b | ||||||
| Obarzanek 2001 (RCT) | 32.7 (6.8); 33.1 (6.9) | ND | ‐0.50b (‐1.78 to 0.78) |
ND | ‐0.60 (‐2.39 to 1.19) |
ND |
| BMI (kg/m2) | ||||||
| Obarzanek 2001 (RCT) | 17.5 (2.3); 17.6 (2.4) | ND | ‐0.30 (‐0.75 to 0.15) |
ND | 0.00 (‐0.63 to 0.63) |
‐0.10 (‐0.75 to 0.55) |
| Mihas 2010 | 24 (3.1); 24.3 (3.3) | ND | ND | ‐1.50 (‐2.45 to ‐0.55) |
ND | ND |
aReduced fat intake group (≤ 30%TE); usual fat intake group.
bWeight‐for‐age z‐score and weight (kg) could not be pooled.
%TE: percentage of total energy; BMI: body mass index; CI: confidence interval; MD: mean difference; ND: no data in this time point range; SD: standard deviation.
1.1. Analysis.
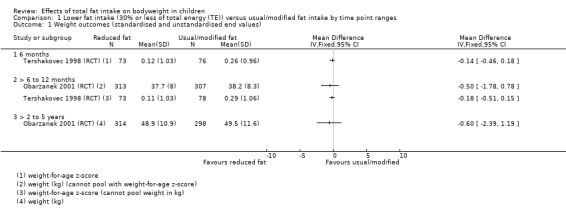
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 1 Weight outcomes (standardised and unstandardised end values).
Body mass index
We have presented a summary of MDs and 95% CI between intervention and control groups for BMI over time in Table 5 and Analysis 1.2. The trials did not report BMI data over similar time ranges and so could not be pooled. For Obarzanek 2001 (RCT) we extracted baseline and end values at 12 months, five years and last visit (approximately six to 10 years). Obarzanek 2001 (RCT) found that fat intake of ≤ 30%TE may make little or no difference to children's BMI (kg/m2) at 12 months (MD ‐0.30 kg/m2, 95% CI ‐0.75 to 0.15; n = 620; low‐quality evidence), five years (MD 0.0 kg/m2, 95% CI ‐0.63 to 0.63; n = 541; low‐quality evidence), or at last visit (MD ‐0.10 kg/m2, 95% CI ‐0.75 to 0.55; n = 576; low‐quality evidence), when compared to usual or modified fat intake. For Mihas 2010, we were able to extract BMI data for baseline and end values 17 months later. This trial reported that after 17 months, a fat intake of ≤ 30%TE compared to usual fat intake probably reduced children's BMI (MD ‐1.5 kg/m2, 95% CI ‐2.45 to ‐0.55; n = 191; moderate‐quality evidence). We conducted sensitivity analyses to exclude possible effects of our selected time ranges, by including BMI data from only the longest follow‐up periods per study (Mihas 2010 at 12 months, and Obarzanek 2001 (RCT) at more than five years; Analysis 1.3). This analysis showed significant heterogeneity, to the extent that we could not pool the data (Chi2 P = 0.02; I2 = 82.5%). Similarly, pooling data from the shortest follow‐up periods per study showed significant heterogeneity (Mihas 2010 at 12 months and Obarzanek 1997 (cohort) at 12 months; Analysis 1.4) (Chi2 P = 0.03; I2 = 80%), and precluded the pooling of these data.
1.2. Analysis.
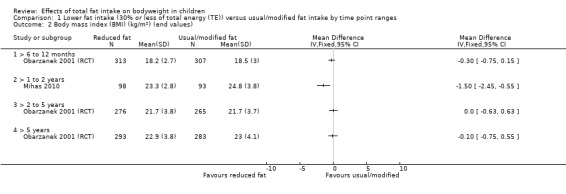
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 2 Body mass index (BMI) (kg/m2) (end values).
1.3. Analysis.
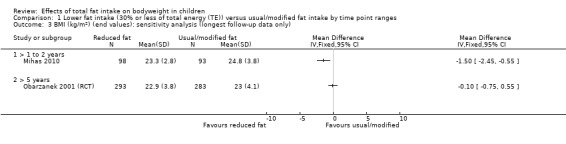
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 3 BMI (kg/m2) (end values): sensitivity analysis (longest follow‐up data only).
1.4. Analysis.
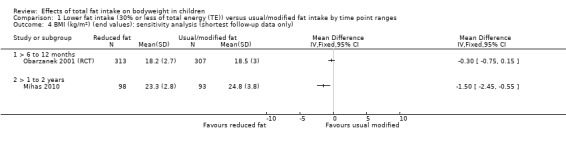
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 4 BMI (kg/m2) (end values): sensitivity analysis (shortest follow‐up data only).
Secondary outcomes
Serum lipids
We have presented a summary of MDs and 95% CI between intervention and control groups for serum lipids over time Table 6 and in Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8.
3. Mean differences in serum lipids and blood pressure for total fat intake 30% or less of total energy compared to usual fat intake in children in over time (one RCT).
|
Obarzanek 2001 (RCT) Outcome |
Follow‐up from baseline | |||||
|
Baseline Mean (SD)a |
6 months MD (95% CI) |
> 6 to 12 months MD (95% CI) |
> 1 to 2 years MD (95% CI) |
> 2 to 5 years MD (95% CI) |
> 5 years MD (95% CI) |
|
| Total cholesterol (mmol/L) | 5.17 (0.38); 5.17 (0.38) | ND | ‐0.15 (‐0.24 to ‐0.06) |
ND | ‐0.06 (‐0.17 to 0.05) |
‐0.02 (‐0.13 to 0.09) |
| LDL‐C (mmol/L) | 3.38 (0.31); 3.38 (0.3) | ND | ‐0.12 (‐0.20 to ‐0.04) |
ND | ‐0.09 (‐0.17 to ‐0.01) |
0.01 (‐0.01 to 0.03) |
| HDL‐C (mmol/L) | 1.48 (0.28); 1.47 (0.29) | ND | ‐0.03 (‐0.08 to 0.02) |
ND | ‐0.01 (‐0.06 to 0.04) |
0.02 (‐0.03 to 0.07) |
| Triglycerides (mmol/L) | 0.9 (0.33); 0.92 (0.32) | ND | ‐0.01 (‐0.08 to 0.06) |
ND | 0.06 (‐0.04 to 0.16) |
0.03 (‐0.06 to 0.12) |
| SBP (mmHg) | 97.31 (9.1); 97.55 (9.4) | ND | ‐0.40 (‐1.70 to 0.90) |
ND | ‐0.40 (‐1.84 to 1.04) |
ND |
| DBP (mmHg) | 61.97 (9.54); 61.67 (10.23) | ND | ‐0.50 (‐2.00 to 1.00) |
ND | ‐0.90 (‐2.30 to 0.50) |
ND |
aReduced fat intake group (≤ 30%TE); usual fat intake group.
%TE: percentage of total energy; CI: confidence interval; DBP: diastolic blood pressure; HDL‐C: high‐density lipoprotein cholesterol; LDL‐C: low‐density lipoprotein cholesterol; MD: mean difference; ND: no data in this time point range; SBP: systolic blood pressure; SD: standard deviation.
1.5. Analysis.
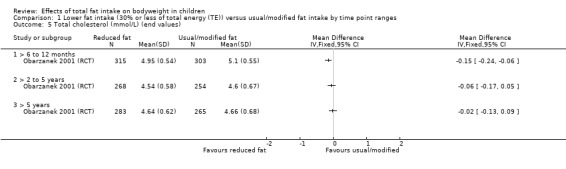
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 5 Total cholesterol (mmol/L) (end values).
1.6. Analysis.
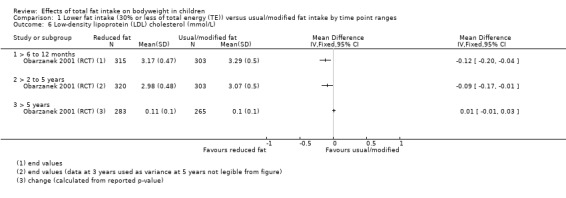
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 6 Low‐density lipoprotein (LDL) cholesterol (mmol/L).
1.7. Analysis.
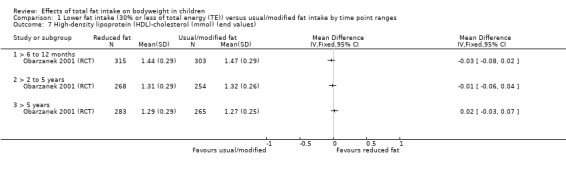
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 7 High‐density lipoprotein (HDL)‐cholesterol (mmol) (end values).
1.8. Analysis.
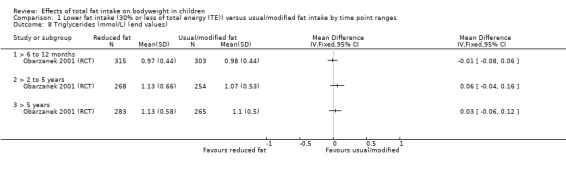
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 8 Triglycerides (mmol/L) (end values).
Only Obarzanek 2001 (RCT) reported serum lipids, and we extracted baseline data and end values at 12‐month follow‐up for total cholesterol, LDL‐cholesterol, HDL‐cholesterol and triglycerides (mmol/L). Additionally, we extracted end values for total cholesterol, HDL‐cholesterol and triglycerides at five years after baseline and at last visit (approximately six to 10 years after baseline). For LDL‐cholesterol, we could not extract variances from the five‐year figures, therefore we used end values and their SDs reported per group at three years. For the last visit, we extracted change scores for LDL‐cholesterol and calculated SDs from a reported P value. This trial found that fat intake of ≤ 30%TE probably reduced total cholesterol slightly over 12 months compared to controls (MD ‐0.15 mmol/L, 95% CI ‐0.24 to ‐0.06; n = 618; moderate‐quality evidence), but may have made little or no difference over longer time periods (at five years MD ‐0.06 mmol/L, 95% CI ‐0.17 to 0.05; n = 522; low‐quality evidence; at last visit MD ‐0.02 mmol/L, 95% CI ‐0.13 to 0.09; n = 548; low‐quality evidence).
Obarzanek 2001 (RCT) also found that fat intake of ≤ 30%TE probably decreased LDL‐cholesterol slightly at 12 months (MD ‐0.12 mmol/L, 95% CI ‐0.20 to ‐0.04; n = 618, moderate‐quality evidence) and for the time period of two to five years (MD ‐0.09, 95% CI ‐0.17 to ‐0.01; n = 623; moderate‐quality evidence) compared to controls, but probably made little or no difference over longer periods. For HDL‐cholesterol fat intake of ≤ 30%TE versus fat intake > 30%TE probably made little or no difference to levels at 12 months (MD ‐0.03 mmol/L, 95% CI ‐0.08 to 0.02; n = 618; moderate‐quality evidence), five years (MD ‐0.01 mmol/L, 95% CI ‐0.06 to 0.04; n = 522; moderate‐quality evidence), or last visit (MD 0.02 mmol/L, 95% CI ‐0.03 to 0.07; n = 548; moderate‐quality evidence) (Obarzanek 2001 (RCT)).
Results for triglycerides in children also showed that fat intake of ≤ 30%TE probably made little or no difference to triglyceride levels at 12 months (MD ‐0.01 mmol/L, 95% CI ‐0.08 to 0.06; n = 618; moderate‐quality evidence), and may have made little or no difference at five years (MD 0.06 mmol/L, 95% CI ‐0.04 to 0.16; n = 522; low‐quality evidence), or last visit (MD 0.03 mmol/L, 95% CI ‐0.06 to 0.12; n = 548; low‐quality evidence) (Obarzanek 2001 (RCT)).
Systolic and diastolic blood pressure
We have presented a summary of MDs and 95% CI between intervention and control groups for SBP and DBP over time in Table 6 and Analysis 1.9 and Analysis 1.10. Only Obarzanek 2001 (RCT) reported blood pressure (mmHg) and we extracted baseline data and end values at 12 months (SBP MD ‐0.40 mmHg, 95% CI ‐1.70 to 0.90; n = 621; DBP MD ‐0.50 mmHg, 95% CI ‐2.00 to 1.00; n = 621), and three years (SBP MD ‐0.40 mmHg, 95%CI ‐1.84 to 1.04; n = 583; DBP MD ‐0.90 mmHg, 95% CI ‐2.30 to 0.50; n = 583).
1.9. Analysis.
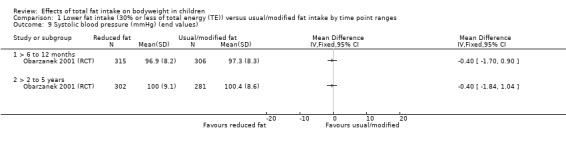
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 9 Systolic blood pressure (mmHg) (end values).
1.10. Analysis.
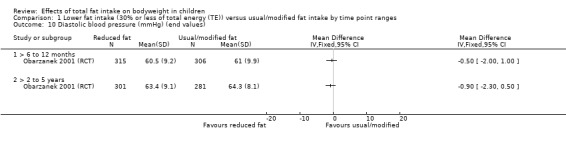
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 10 Diastolic blood pressure (mmHg) (end values).
Height (adverse outcome)
We have presented a summary of MDs and 95% CI between intervention and control groups for height outcomes over time in Table 7 and Analysis 1.11. Pooling of data was not possible due to use of different outcomes by the two trials. We extracted height‐for‐age z‐scores from Tershakovec 1998 (RCT) at baseline, and end values at six months (MD ‐0.02, 95% CI ‐0.06 to 0.02; n = 149; very low‐quality evidence), and 12 months (MD ‐0.05, 95% CI ‐0.08 to 0.02; n = 151; very low‐quality evidence), and, due to the very low quality of the evidence, we are uncertain whether lower fat intake had an effect on height‐for‐age z‐scores.
4. Mean differences in height outcomes for total fat intake 30% or less of total energy compared to usual fat intake in children over time (two RCTs).
|
Outcome Study ID |
Follow‐up from baseline | |||||
|
Baseline Mean (SD)a |
6 months MD (95% CI) |
> 6 to 12 months MD (95% CI) |
> 1 to 2 years MD (95% CI) |
> 2 to 5 years MD (95% CI) |
> 5 years MD (95% CI) |
|
| Height‐for‐age z‐scoreb | ||||||
| Tershakovec 1998 (RCT) | ‐0.12 (1.02); 0.06 (0.93) | ‐0.02 (‐0.06 to 0.02) |
‐0.05b (‐0.08 to‐0.02) |
ND | ND | ND |
| Height (cm)b | ||||||
| Obarzanek 2001 (RCT) | 136.2 (6.8); 136.5 (7) | ND | 0.00b (‐1.11 to 1.11) |
ND | ‐0.10 (‐1.54 to 1.34) |
‐0.06 (‐2.06 to 0.86) |
aReduced fat intake group (≤ 30%TE); usual fat intake group.
bHeight‐for‐age z‐score and height (cm) cannot be pooled.
%TE: percentage of total energy; CI: confidence interval; MD: mean difference; ND: no data in this time point range; RCT: randomised controlled trial; SD: standard deviation.
1.11. Analysis.
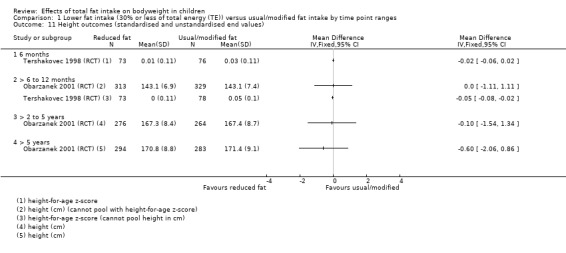
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 11 Height outcomes (standardised and unstandardised end values).
Obarzanek 2001 (RCT) reported height (cm); we extracted data at baseline and end values at 12 months, five years, and approximately six to 10 years from baseline (last visit). Lower versus usual or modified fat intake may have made little or no difference to height over 12 months (MD 0 cm, 95% CI ‐1.11 to 1.11; n = 642; low‐quality evidence), five years (MD ‐0.10 cm, 95% CI ‐ 1.54 to 1.34; n = 540; low‐quality evidence), or more than five years (MD ‐0.60 cm, 95% CI ‐2.06 to 0.86; n = 577; low‐quality evidence) (Obarzanek 2001 (RCT)).
Tertiary outcomes
Dietary intake
We have presented a summary of MDs and 95% CI between intervention and control groups for dietary intake variables over time in Table 8 and in Analysis 1.12; Analysis 1.13; Analysis 1.14; Analysis 1.15; Analysis 1.16. The two trials did not report eligible dietary outcomes for similar time ranges and thus we could not pool the data. End values for energy (kJ), fat, saturated fat, protein and carbohydrate intake (%TE) were reported by Obarzanek 2001 (RCT) at 12 months and three years, and by Mihas 2010 at 17 months. In both trials, the differences in the proportion of TE provided by the macronutrients (fat, protein and carbohydrates) in the diets of children in intervention groups showed lower intake of total fat (range of 95% CI ‐6.91 to ‐3.55% of TE) and greater intake of total protein (range of 95% CI 0.38 to 1.48% of TE) and total carbohydrates (range of 95% CI 1.16 to 4.84% of TE), compared to control groups, at all reported time points, which is in line with the diets being tested.
5. Mean differences in dietary intake for total fat intake 30% or less of total energy compared to usual fat intake in children over time (two RCTs).
|
Outcome Study ID |
Follow‐up from baseline | |||||
|
Baseline Mean (SD)a |
6 months MD (95% CI) |
> 6 to 12 months MD (95% CI) |
> 1 to 2 years MD (95% CI) |
> 2 to 5 years MD (95% CI) |
> 5 years MD (95% CI) |
|
| Energy (kJ) | ||||||
| Obarzanek 2001 (RCT) | 7364 (1832); 7229 (1841) | ND | ‐356.00 (‐655.22 to ‐56.78) |
ND | ‐602.00 (‐943.94 to ‐260.06) |
ND |
| Mihas 2010 | 8503.3 (1419.3); 8583.7 (1522.4) | ND | ND | ‐645.50 (‐1075.66 to ‐215.34) |
ND | ND |
| Fat (%TE) | ||||||
| Obarzanek 2001 (RCT) | 33.4 (5.5); 34 (4.9) | ND | ‐4.60 (‐5.50 to ‐3.70) |
ND | ‐4.40 (‐5.25 to ‐3.55) |
ND |
| Mihas 2010 | 35.4 (4.7); 36.2 (5.2) | ND | ND | ‐5.60 (‐6.91 to ‐4.29) |
ND | ND |
| Saturated fat (%TE) | ||||||
| Obarzanek 2001 (RCT) | 12.5 (2.7); 12.7 (2.5) | ND | ‐2.60 (‐3.02 to ‐2.18) |
ND | ‐2.10 (‐2.49 to ‐1.71) |
ND |
| Mihas 2010 | 12.4 (2.0); 12.8 (2.3) | ND | ND | ‐3.10 (‐3.78 to ‐2.42) | ND | ND |
| Protein (%TE) | ||||||
| Obarzanek 2001 (RCT) | 14.8 (2.8); 14.6 (2.7) | ND | 1.00 (0.52 to 1.48) |
ND | 0.90 (0.38 to 1.42) |
ND |
| Mihas 2010 | 15.3 (1.4); 14.9 (1.8) | ND | ND | 1.30 (0.80 to 1.80) |
ND | ND |
| Carbohydrates (%TE) | ||||||
| Obarzanek 2001 (RCT) | 53.0 (6.7); 52.8 (6.2) | ND | 3.70 (2.63 to 4.77) |
ND | 3.30 (2.25 to 4.35) |
ND |
| Mihas 2010 | 49.7 (6.2); 48.4 (6.8) | ND | ND | 3.00 (1.16 to 4.84) |
ND | ND |
aReduced fat intake group (≤ 30%TE); usual fat intake group.
%TE: percentage of total energy; MD: mean difference; ND: no data in this time point range; RCT: randomised controlled trial; SD: standard deviation.
1.12. Analysis.
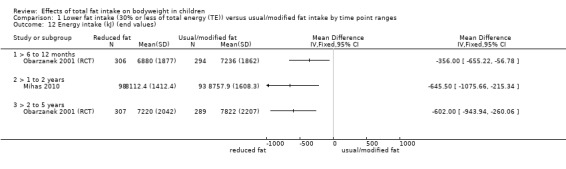
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 12 Energy intake (kJ) (end values).
1.13. Analysis.
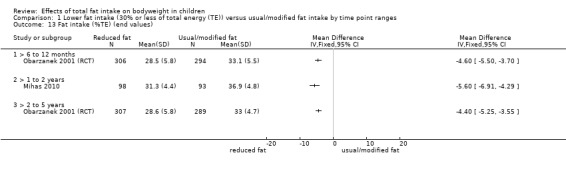
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 13 Fat intake (%TE) (end values).
1.14. Analysis.
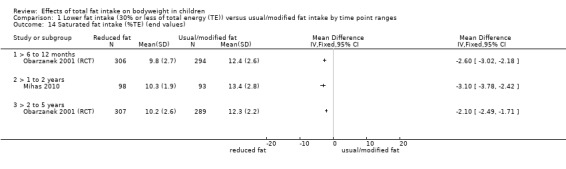
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 14 Saturated fat intake (%TE) (end values).
1.15. Analysis.
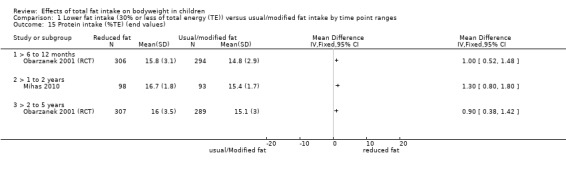
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 15 Protein intake (%TE) (end values).
1.16. Analysis.
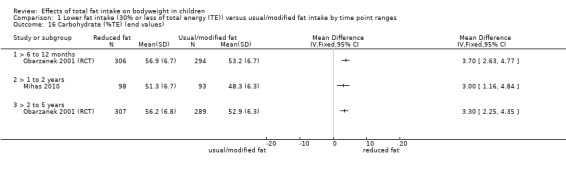
Comparison 1 Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges, Outcome 16 Carbohydrate (%TE) (end values).
Associations between total dietary fat exposure and measures of body fatness in children (as seen in cohort studies)
Table 2 and Appendix 3 present a summary of the association between total dietary fat exposure, weight and body fatness in children for data from prospective cohort studies. Importantly, some cohort studies reported more than one eligible analysis for the same outcome, as a continuous or binary outcome (e.g. BMI in kg/m2 and overweight/obese according to IOTF cut‐offs). Where a single study reported different analyses for the same important outcome in the same participants (e.g. BMI z‐scores versus BMI cut‐offs for overweight and obesity), we selected the most relative continuous analysis for inclusion in the table. Similarly, when a single study reported different measures of the exposure in relation to the same outcome in the same participants (e.g. total fat intake in absolute grams versus as %TE), we selected the most relative continuous exposure for the table. In this way, we were careful not to use the same study sample of participants more than once per outcome and time range in Table 2 nor Appendix 3.
We considered meta‐analyses of cohort studies, but considered that the methodologies, analysis methods, dietary assessments, ages at baseline, applications of total fat intake exposure and eligible outcome measures were so varied across studies in the five time ranges, that combining studies was not appropriate. In addition, important information, such as measures of variation and numerical results, were not reported in many of the studies.
Primary outcomes
Weight
We have summarised the various standardised and unstandardised weight outcomes, total fat exposure variables and results of reported associations, including adjustments made within each time range in Table 9.
6. Results of cohort studies: weight.
|
Study ID; mean age at baseline; analysis |
Outcome | Outcome units | Time point (year) | Exposure | Exposure unit | Results of association (all reported values) |
Direction;a energy intake adjusted (yes/no) |
Matched groups or adjusted for (or both) | |
| Weight at 1 year: 4 cohort studies; 4 analyses (n ˜ 1949) in boys and girls aged 2‐11 years | |||||||||
|
Niinikoski 1997a 2 years old; mean end values per group |
Relative weightb | % | 1 | Total fat intake (single 4‐day dietary record at baseline, 1.5 and 2 years) | LF (27.7‐28.7 %TE; HF (> 28.7 %TE) |
n overall = 740 (LF = 35, HF = 705); mean end values (SD). Baseline: LF = 1 (8); HF = 1 (8). At 1 year: LF = 1 (7); HF = 1 (8); P = 0.81. After 1 year, no difference in relative weight change of children with LF intake compared to children with HF intakes. |
0 No |
No matching reported. No adjustment for prognostic variables. | |
|
Schwandt 2011 6.8 years old; regression |
Weight | kg | 1 | Total fat intake (single 7‐day weighed dietary record at baseline and 1 year) | g | n overall = 411; regression result. B = 0.09, SE 0.019; P < 0.05. After 1 year, for every 1 g increase in total fat intake of children, weight will increase by 0.09 kg. |
+ No |
Adjusted for age, gender and physical activity. | |
|
Butte 2007 11 years old; regression |
Weight | kg/year | 1 | Total fat intake (multiple 24‐hour dietary recalls at baseline) | %TE | n overall = 798; regression result. B = 0.044, SE 0.018; P = 0.014. For every 1% increase in energy intake from total fat in children, weight will increase by 0.04 kg/year. |
+ No |
Adjusted for gender, age, age squared, Tanner stage and BMI. | |
|
Tershakovec 1998 (cohort) 6.2 years old; mean end values per group |
Weight | z‐score | 1 | Total fat intake (multiple 24‐hour dietary recalls at baseline and 1 year) | LF quintile (24 %TE) HF quintile (34%TE) |
n overall = NR (LF = NR, HF = NR); mean end values (SD NR). Baseline: LF = ‐0.21; HF = 0.44. At 1 year: LF = ‐0.14; HF = 0.45. After 1 year, weight‐for‐age of children with LF intake will increase by 0.07 z‐scores on average, and by 0.01 z‐scores in children with HF intake. |
‐ No |
No matching reported. No adjustment for prognostic variables. | |
| Weight at > 1to 2 years: 1 cohort study; 1 analysis (n = 126) in boys and girls aged 2 years | |||||||||
|
Magarey 2001 2 years old; mean end values per group |
Weight | kg | 2 | Total fat intake (single 3‐day weighed dietary records at baseline and 2 years) | LF < 30%TE; HF > 35%TE | n overall = 126 (LF = 14, HF = 112); mean end values (SD). Baseline: LF = 12.6 (1); HF = 12.8 (1.7). At 2 years: LF (n = 20) 18.4 (2.6); HF (n = 76) 17.9 (2.1); P > 0.05. After 2 years, weight of children with LF intake will increase by 5.8 kg on average, and by 5.1 kg on average in children with HF intake. |
‐ No |
No matching reported. No adjustment for prognostic variables. | |
| Weight at > 2to 5 years: 4 cohort studies; 4 analyses (n = 13,802) in boys and girls aged 2‐14 years | |||||||||
|
Shea 1993 4.4 years old; mean change per group |
Weight | kg/year | 2.1 | Total fat intake (multiple FFQs at baseline) | LF ≤ 30%TE; HF > 30%TE | n overall = 215 (LF = 37, HF = 178); mean change (SD). Baseline: NR. LF = 3 (1.3); HF = 2.8 (1.3); P > 0.05 MD 0.2 (95% CI ‐0.26 to 0.66). After 2 years, children with LF intake will gain on average 0.2 kg/year more than children with HF intakes. |
‐ No |
No matching reported. No adjustment for prognostic variables. | |
|
Berkey 2005 9‐14 years‐old; regression |
Weight | kg, 1‐year change | 3 | Total fat intake (single FFQ at baseline, 1, 2 and 3 years) | g | n overall = 12,829; only reported as text. After 3 years, "Dairy fat was not a stronger predictor of weight gain than other types of fat, and no fat (dairy, vegetable, or other) intake was significantly associated with weight gain after energy adjustment, nor was total fat intake." |
0 Yes |
Adjusted for age, ethnicity, pubertal stage, annual height growth, baseline BMI and same‐year physical activity. | |
|
Obarzanek 1997 (cohort) 9.6 years old; regression |
Weight | kg | 3 | Total fat intake (multiple 24‐hour recalls at baseline, 1 and 3 years) | %TE | n overall = 632; regression results. B = ‐0.0011, P = 0.8. After 3 years, for every 1% increase in total energy intake from total fat of children, weight will decrease by 0.0011 kg. |
‐ Yes |
Adjusted for gender, physical activity, treatment, visit number, other sources of energy than fat and interactions: fat intake‐by‐treatment, fat intake‐by‐gender, fat intake‐by‐visit number and visit number‐by‐treatment. | |
|
Magarey 2001 2 years‐old; mean end values per group |
Weight | kg | 4 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years) | LF < 30%TE; HF > 35%TE | n overall = 126 (LF = 14, HF = 112); mean end values (SD). Baseline: LF = 12.6 (1); HF = 12.8 (1.7). At 4 years: LF (n = 14) 20.7 (3.4); HF (n = 88) 21.7 (3); P > 0.05. After 4 years, weight of children with LF intake will increase by 8.1 kg on average, and by 8.9 kg on average in children with HF intake. |
+ No |
No matching reported. No adjustment for prognostic variables. | |
| Weight at > 5to 10 years: 1 cohort study; 1 analysis (n = 126) in boys and girls aged 2 years | |||||||||
|
Magarey 2001 2 yrs‐old; mean end values per group |
Weight | kg | 6 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years; single 4‐day weighed dietary record at 6 years) | LF < 30 %TE; HF > 35 %TE | n overall = 126 (LF = 14, HF = 112); mean end values (SD). Baseline: LF = 12.6 (1); HF = 12.8 (1.7). At 6 years: LF (n = 13) 29.4 (5.9); HF (n = 72) 26.7 (4.3); P > 0.05. After 6 years, weight of children with LF intake will increase by 16.8 kg on average, and by 13.9 kg on average in children with HF intake. |
‐ No |
No matching reported. No adjustment for prognostic variables. | |
aDirection refers to whether there was a positive (+: exposure and outcome moved in the same direction, inverse/negative (‐: exposure and outcome moved in opposite directions) or zero (0: no association) between total fat intake and the outcome.
bRelative weight, deviation in percentages from the mean weight of healthy Finnish children of the same height and gender.
%TE: percentage of total energy; B: unstandardized beta‐coefficient; BMI: body mass index; CI: confidence interval; FFQ: Food Frequency Questionnaire; LF: low fat; HF: high fat; n: number of participants; NA: not applicable; MD: mean difference; NR: not reported; SD: standard deviation; SE: standard error.
Four cohort studies reported weight outcomes at one‐year follow‐up in four analyses (n = approximately 1949) in boys and girls (mean age at baseline: two to 11 years), and none of these studies adjusted for TE intake in their analyses (Butte 2007; Niinikoski 1997a; Schwandt 2011; Tershakovec 1998 (cohort)). The two studies that examined total fat intake exposure as a continuous variable at one year, reported positive associations: for every 1 g increase in total fat intake of children, weight increased by 0.09 kg (Schwandt 2011), and for every 1% increase in energy intake from total fat in children, weight increased by 0.04 kg/year (Butte 2007). After one year, Niinikoski 1997a analysed total fat intake as a binary variable and reported the same relative mean weight change in low‐fat compared to high‐fat intake children (low‐fat group 1% (SD 7), high‐fat group 1% (SD 8), P = 0.81). Tershakovec 1998 (cohort) used quintiles of fat intake and reported that weight‐for‐age of children with low‐fat intake increased by 0.07 z‐scores on average, and by 0.01 z‐scores in children with high‐fat intake.
Magarey 2001 reported weight at two‐year follow‐up (boys and girls, aged two years at baseline, n = 126) in a single analysis as mean end values in a low‐fat group (< 30%TE) and a high‐fat group (> 30%TE) based on baseline fat intake, and found that weight increased by 5.8 kg on average with low‐fat intake, and by 5.1 kg on average with high‐fat intake.
At 2 to 5 years, Berkey 2005 (n = 12 829) and Obarzanek 1997 (cohort) (n = 632) examined associations (regressions) between weight and continuous exposure to fat (absolute grams and %TE) in nine‐ to 14‐year olds, with adjustments for TE intake, and reported no or negligible associations. Magarey 2001 reported mean end values per group in two‐year olds (cut‐offs of fat intake < 30%TE and > 35%TE), and Shea 1993 reported mean change per group in four‐year olds, with exposure to lower fat versus higher fat intake groups (cut‐offs of fat intake ≤ 30%TE and > 30%TE). With no adjustment for TE intake, Magarey 2001 found that after four years, weight of children with low‐fat intake increased by 8.1 kg on average, and by 8.9 kg on average in children with high‐fat intake (n = 126), and Shea 1993 found that children with low‐fat intake gained on average 0.2 kg/year more than children with high‐fat intake (n = 215).
Magarey 2001 also reported the same analysis at six years (5 to 10 years), and, with no adjustment for TE, found that the weight of children with low‐fat intake increased by 16.8 kg on average, and that of children with high‐fat intake increased by 13.9 kg on average (n = 126).
Body mass index
We have summarised BMI outcomes (standardised and unstandardised), total fat exposure descriptions, and results of reported associations within each time range, including the adjustments made in Table 10.
7. Results of cohort studies: body mass index.
|
Study ID; mean age at baseline; analysis |
Outcome | Outcome units | Time point (year) | Exposure | Exposure unit | Results of association (all reported values) | Direction;a energy intake adjusted? (yes/no) | Matched groups or adjusted for (or both) |
| BMI at 1 year: 3 cohort studies; 4 analyses (n ˜ 11,180) in boys and girls aged 7‐14 years | ||||||||
|
Berkey 2005 9‐14 years; regression |
BMI | kg/m2, 1‐year change |
1 | Total fat intake (single FFQ at baseline and 1 year) | g | n girls = 6149; regression result. B = 0.0008, SE 0.0016, P = 632. After 1 year, for every 1 g increase in total fat intake, BMI will increase by 0.0008 kg/m2 in girls. |
+ Yes |
Adjusted for total energy intake, age, ethnicity, pubertal stage, annual height growth, baseline BMI and physical activity. |
|
Berkey 2005 9‐14 years; regression |
BMI | kg/m2, 1‐year change |
1 | Total fat intake (single FFQ at baseline and 1 year) | g | n boys = 4620; regression result. B = ‐0.0015, SE 0.0017, P = 0.375. After 1 year, for every 1 g increase in the total fat intake, BMI will decrease by 0.0015 kg/m2 in boys. |
‐ Yes |
Adjusted for total energy intake, age, ethnicity, pubertal stage, annual height growth, baseline BMI and physical activity. |
|
Bogaert 2003 8.6 years; regression |
BMI | z‐score | 1 | Total fat intake (single 3‐day record at baseline) | %TE | n overall = NR; regression result = NR. "We are unable to demonstrate a positive relation between dietary fat and BMI z‐score change from baseline to 12 months." |
0 NR |
Prognostic variables were adjusted for, but not specified which one. |
|
Schwandt 2011b 6.8 years; regression |
BMI | kg/m2 | 1 | Total fat intake (single 7‐day weighed record at baseline and 1 year) | g | n overall = 411; regression result. B = 0.08, SE 0.007, P = 0.085. After 1 year, for every 1 g increase in the total intake, BMI will increase by 0.08 kg/m2. |
+ No |
Adjusted for age, sex and physical activity. |
| BMI at > 1to 2 years: 7 cohort studies; 10 analyses (n = 3347) in boys and girls aged 2‐13 years | ||||||||
|
Ambrosini 2016 3.6 years; mean end values per group |
BMI | kg/m2 | 1.5 | Total fat intake (single 3‐day unweighed food record at baseline) | LF quintile (30.4%TE); HF quintile (41.8 %TE) |
n boys, at baseline = 438; At 1.5 years = 383 (LF = NR, HF = NR); mean end values (SD). Baseline: LF = 16.6 (95% CI 16.4 to 16.8); HF = 16.3 (95% CI 16.1 to 16.5). At 1.5 years: LF = 16.1 (95% CI 15.8 to 16.3); HF = 15.7 (95% CI 15.5 to 16.0). After 18 months, average BMI decreased by 0.5 kg/m2 among boys in LF intake (30.4%TE) group and by 0.6 kg/m2 in boys in HF intake (41.8%TE) group. |
‐ No |
No matching reported. No adjustment for prognostic variables. |
|
Ambrosini 2016 3.6 years; mean end values per group |
BMI | kg/m2 | 1.5 | Total fat intake (single 3‐day unweighed food record at baseline) | LF quintile (30.4 %TE); HF quintile (41.8 %TE) |
n girls, at baseline = 351; at 1.5 years = 323) (LF = NR, HF = NR); mean end values (SD). Baseline: LF = 16.6 (95% CI 16.3 to 16.9); HF = 16.4 (95% CI 16.1 to 16.7). At 1.5 years: LF = 16.1 (95% CI 15.7 to 16.4); HF = 16.1 (95% CI 15.8 to 116.4). After 18 months,average BMI decreased by 0.5 kg/m2 among girls in LF intake group (30.4%TE) and by 0.3 kg/m2 in girls in HF intake group (41.8%TE). |
+ No |
No matching reported. No adjustment for prognostic variables. |
|
Davison 2001 5.4 years; regression |
BMI | kg/m2, 2‐years change |
2 | Total fat intake (multiple 24‐hour recalls at baseline) | %TE | n overall = 168; regression result. R2 = 0.26, P entry = 0.01, F‐test = 9.27, df = 6, P change = 0.0001. "Percentage of fat intake, baseline BMI, family risk of overweight, mothers’ BMI, fathers’ enjoyment of activity explained 26% of the variance in the change of BMI." |
+ Yes |
Adjusted for age, baseline BMI, family risk of overweight, mothers' change in BMI and fathers' enjoyment of activity. |
|
Klesges 1995 4.4 years; regression |
BMI | kg/m2, 2‐years change |
2 | Change (year 2 to 3 of follow‐up) in total fat intake (single FFQ at baseline, 1 and 2 years) | %TE | n overall = 146; regression result. B = ‐0.04, P = 0.011, t value = 2.58. After 2 years, for every 1% increase in energy intake from total fat from year 2 to 3 of follow‐up, BMI will decrease by 0.04 kg/m2. |
‐ No |
Adjusted for age, sex, parental BMI and physical activity. |
|
Klesges 1995 4.4 years; regression |
BMI | kg/m2, 2‐years change |
2 | Baseline dietary fat (single FFQ) | %TE | n overall = 146; regression result. B = 0.034, P = 0.0521, t value = 1.96. After 2 years, for every 1% increase in energy intake from baseline total fat, BMI will increase by 0.034 kg/m2. |
+ No |
Adjusted for age, sex, parental BMI and physical activity. |
|
Lee 2001 5 years; mean end values; mean change per groups |
BMI | kg/m2 | 2 | Total fat intake (multiple 24‐hour recalls at baseline) | LF ≤ 30%TE; HF > 30%TE |
n girls = 192 (LF = 84; HF = 108); mean end values (SD); mean change (SD). Baseline: LF = 15.8 (1.83); HF = 16 (2.08). At 2 years: LF = 16.4 (1.83); HF = 16.9 (3.12); change LF = 0.6 (0.92); change HF = 1.0 (2.08); P < 0.05. MD ‐0.4 (95% CI ‐0.84 to 0.04) After 2 years, LF intake (≤ 30%TE) will result in 0.4 kg/m2 smaller increase in BMI on average compared to HF intake (> 0%TE) in girls. |
+ No |
No matching reported. No adjustment for prognostic variables. |
|
Lee 2012 7.3 years; regression |
BMI 1st graders | kg/m2, 2‐years change |
2 | Total fat intake (multiple 24‐hour recalls at baseline, 1 and 2 years) | %TE | n overall = 474; regression result. B = 0.021 (95% CI ‐0.004 to 0.046), P = 0.104. After 2 years, for every 1% increase in energy intake from total fat, BMI will increase by 0.021 kg/m2. |
+ Yes |
Adjusted for age, gender, sexual maturation at 6 years' follow‐up, baseline BMI, exercise frequency, screen time, sleep duration, meal skipping and snacking, parental BMI and SES. |
|
Lee 2012 10 years; regression |
BMI 4th graders | kg/m2, 2‐years change |
2 | Total fat intake (multiple 24‐hour recalls at baseline, 1 and 2 years) | %TE | n overall = 1030; regression result. B = ‐0.007 (95% CI ‐0.024 to 0.012), P = 0.449. After 2 years, for every 1% increase in energy intake from total fat, BMI will decrease by 0.007 kg/m2. |
‐ Yes |
Adjusted for age, gender, sexual maturation at 6 years' follow‐up, baseline BMI, exercise frequency, screen time, sleep duration, meal skipping and snacking, parental BMI and SES. |
|
Magarey 2001 2 years; regression |
BMI | z‐score | 2 | Total fat intake (single 3‐day weighed dietary record at baseline and 2 years) | NR | n overall = 155; regression result. β = 0.079, P > 0.1; R2 = 0.493, P < 0.0001. After 2 years, increase in the total fat intake will increase BMI by 0.079 z‐score. |
+ Yes |
Adjusted for baseline BMI‐z score, gender, mother's BMI and father's BMI. |
|
Setayeshgar 2017 12.5 years; regression |
BMI | z‐score | 2 | Total fat intake (single 24‐hour recall at baseline) | per 10 g | n overall = 330; regression result. β = 0.009 (95% CI ‐0.006 to ‐0.02), P = NS. After 2 years, for every 10 g increase in total fat intake, BMI will increase by 0.009 z‐score. |
+ Yes |
Adjusted for baseline BMI z‐score, moderate to vigorous physical activity, vegetables and fruit, fibre, milk, sodium and added sugar intakes. |
| BMI at > 2to 5 years: 7 cohort studies; 11 analyses (n = 4491) in boys and girls aged 2‐14 years | ||||||||
|
Shea 1993 4.4 years; mean change per group |
BMI | kg/m2 per year | 2.1 | Total fat intake (multiple FFQs at baseline) | LF ≤ 30%TE; HF > 30%TE |
n overall = 215 (LF = 37, HF = 178); mean change (SD). LF = 0.2 (0.81), HF = 0.18 (0.68); P > 0.05. MD 0.02 (95% CI ‐0.26 to 0.30). After 25 months, LF intake (≤ 30%TE) will result in a 0.02 kg/m2 per year greater increase in BMI on average, compared to HF intake (> 30%TE). |
‐ No |
No matching reported. No adjustment for prognostic variables. |
|
Appannah 2015 14 years; regression |
BMI | z‐score | 3 | Energy‐dense, HF and low‐fibre dietary patternc (single FFQ at baseline and 3 years) | z‐score | n girls = 649; regression result. β = 0.99 (95% CI ‐0.05 to 0.05), P = NR. After 3 years, for every 1 z‐score increase in the energy‐dense, HF and low‐fibre dietary pattern z‐score, BMI will increase by 0.99 z‐score in girls. |
+ NA; exposure included energy intake |
Adjusted for age, dietary misreporting, physical fitness, smoking and BMI z‐score. |
|
Appannah 2015 14 years; regression |
BMI | z‐score | 3 | Energy‐dense, HF and low‐fibre dietary patternc (single FFQ at baseline and 3 years) | z‐score | n boys = 699; regression result. β = 0.03 (95% CI ‐0.01 to 0.08), P = NR. After 3 years, for every 1 z‐score increase in the energy‐dense, HF and low‐fibre dietary pattern, BMI will increase by 0.03 z‐score in boys. |
+ NA; exposure included energy intake |
Adjusted for age, dietary misreporting, physical fitness, smoking and BMI z‐score. |
|
Appannah 2015 14 years; regression and OR higher vs lower dietary pattern z‐score |
BMI | Overweight/obese by IOTF;d odds |
3 | Energy‐dense, HF and low‐fibre dietary patternc (single FFQ at baseline and 3 years) | z‐score | n girls = 649; regression result. OR = 1.02 (95% CI 0.87 to 1.19), P = NR. After 3 years, the ratio of odds for being overweight/obese was 1.02 greater in girls with higher dietary pattern z‐scores compared to the odds in girls with lower dietary pattern z‐scores. |
+ NA; exposure included energy intake |
Adjusted for age, dietary misreporting, physical activity and smoking. |
|
Appannah 2015 14 years; regression and OR higher vs lower dietary pattern z‐score |
BMI | Overweight/obese by IOTF;d odds |
3 | Energy‐dense, HF and low‐fibre dietary patternc(single FFQ) at baseline and 3 years) | z‐score | n boys = 699; regression result. OR = 1.04 (95% CI 0.9 to 1.2), P = NR. After 3 years, the ratio of odds for being overweight/obese is 1.04 greater in boys with higher dietary pattern z‐scores compared to the odds in boys with lower dietary pattern z‐scores. |
+ NA; exposure includes energy intake |
Adjusted for age, dietary misreporting, physical activity and smoking. |
|
Brixval 2009 9.7 years; regression |
BMI | z‐score, 3‐years change |
3 | Dietary fat (single 24‐hour recall at baseline) | %TE | n boys = 181; regression result. β = ‐0.63 (95% CI ‐2.07 to 0.82), P = 0.39. After 3 years, for every 1% increase in energy intake from total fat, BMI will decrease by 0.63 z‐score in boys. |
‐ Yes |
Adjusted for age, physical activity level, dietary volume and puberty at baseline. |
|
Brixval 2009 9.7 years; regression |
BMI | z‐score, 3‐years change |
3 | Dietary fat (single 24‐hour recall at baseline) | %TE | n girls = 217; regression result. β = 0.07 (95% CI ‐1.08 to 1.25), P = 0.72. After 3 years, for every 1% increase in energy intake from total fat, BMI will increase by 0.07 z‐score in girls. |
+ Yes |
Adjusted for age, physical activity level, dietary volume and puberty at baseline. |
|
Cohen 2014 13.9 years; regression |
BMI | Percentile, % |
3 | Total fat intake (single FFQ at baseline, 1, 2 and 3 years) | %TE | n girls = 265; regression result. B = ‐0.01, SE = 0.01, P = 0.16. After 3 years, for every 1% increase in energy intake from total fat, BMI will decrease by 0.01 percentile in girls. |
‐ No |
Adjusted for age, ethnicity, protein calories, CHO calories, physical activity, physical inactivity and SES. |
|
Jago 2005 4.4 years; regression |
BMI | kg/m2 | 3 | Total fat intake (observed 4‐day dietary intake at baseline, 1 and 2 years and 3‐day dietary intake at 3 years) | %TE | n overall = 133; regression result. R2 = 0.65, P = NR. "Dietary factors were not associated with BMI across the three study years." |
NR Yes |
Adjusted for ethnicity, gender, baseline BMI, TV viewing, sedentary behaviour, physical activity, dietary behaviours and interaction terms for variables differing by year. |
|
Obarzanek 1997 (cohort) 9.6 years; regression |
BMI | kg/m2 | 3 | Total fat intake (multiple 24‐hour recalls at baseline, 1 and 3 years) | %TE | n overall = 632; regression result. B = ‐0.00008, P = 0.9. After 3 years, for every 1% increase in energy intake from total fat, BMI will decrease by 0.00008 kg/m2. |
‐ Yes |
Adjusted for gender, physical activity, treatment, visit number, other sources of energy than fat, and for interactions: fat intake‐by‐treatment, fat intake‐by‐gender, fat intake‐by‐visit number and visit number‐by‐treatment. |
|
Magarey 2001 2 years; regression |
BMI | z‐score | 4 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years) | NR | n overall = 152; regression result. β = 0.087, P > 0.1; R2 = 0.48, P < 0.0001. After 4 years, increase in the total fat intake, will increase BMI by 0.087 z‐score. The model explained 48% of variance in the change of BMI z‐score. |
+ Yes |
Adjusted for baseline BMI‐z score, gender, mother's BMI and father's BMI. |
| BMI at > 5to 10 years: 4 cohort studies; 6 analyses (n = 1158) in boys and girls aged 2‐10 years | ||||||||
|
Brixval 2009 9.6 years; regression |
BMI | z‐score, 6‐years change |
6 | Dietary fat (single 24‐hour recall at baseline) | %TE | n girls = 177; regression result. β = 0.005, SE 0.008, P = 0.54. After 6 years, for every 1% increase in energy intake from total fat, BMI will increase by 0.005 z‐score in girls. |
+ Yes |
Adjusted for age, puberty status, parent's income level, self‐reported activity, inactivity and number of overweight parents. |
|
Brixval 2009 9.6 years; regression |
BMI | z‐score, 6‐years change |
6 | Dietary fat (single 24‐hour recall at baseline) | %TE | n boys = 147; regression result. β = ‐0.011, SE 0.009, P = 0.2. After 6 years, for every 1% increase in energy intake from total fat, BMI will decrease by 0.011 z‐score in boys. |
‐ Yes |
Adjusted for age, puberty status, parent's income level, self‐reported activity, inactivity and number of overweight parents. |
|
Skinner 2004 2 years; regression |
BMI | kg/m2 | 6 | Longitudinal dietary fat (single 24‐hour dietary recall and 2‐day food record at baseline, every 3 months during 1 year, every 6 months during 2 and 3 years, every year during 4, 5 and 6 years) | g | n overall = 70; regression result. B = 0.01, SE 0.01, P = 0.0039, F‐test = 9; R2 = 0.43, P = 0.0001, F‐test = 17.6. After 6 years, for every 1 g increases in the fat intake, BMI will increase by 0.01 kg/m2. |
‐ No |
Adjusted for baseline BMI, birthweight, cereal introduction age, breastfeeding duration, dietary variety score 42‐84 months, adiposity rebound, picky eater at age 6 years, sedentary activity at ages 6 and 7 years, foods liked at age 8 years, mother's BMI and father's BMI. |
|
Magarey 2001 2 years; regression |
BMI | z‐score | 9 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years, single 4‐day weighed dietary record at 6 and 9 years) | NR | n overall = 243; regression result. β = 0.122, P > 0.1; R2 = 0.38, P < 0.0001. After 9 years, increase in the total fat intake will increase BMI by 0.122 z‐score. |
+ Yes |
Adjusted for baseline BMI‐z score, gender and parental BMI. |
|
Morrison 2008 10.1 years; regression |
BMI | kg/m2, 10‐years change |
10 | Total fat intake (single 3‐day dietary records at 1, 2, 3, 4, 5, 7, 8 and 10 years) × baseline IR | %TE | n white girls = 241; regression result. B = 0.029, SE 0.0028, P < 0.0001, partial R2 = 27. After 10 years, for every 1% increase in energy intake from total fat, BMI will increase by 0.029 kg/m2 in white girls. |
+ Yes |
Adjusted for age, BMI, IR and maturation stage at baseline; change in IR over 10 years' follow‐up; percentage of calories from protein, fat and CHO (mean of interviews) during 10 years' follow‐up; and interaction terms (nutrients × baseline IR). |
|
Morrison 2008 10.1 years; regression |
BMI | kg/m2, 10‐years change |
10 | Total fat intake (single 3‐day dietary records at 1, 2, 3, 4, 5, 7, 8 and 10 years) × baseline IR | %TE | n black girls = 280; regression result. B = 0.012, SE 0.0032, P = 0.0002, partial R2 = 3.6. After 10 years, for every 1% increase in energy intake from total fat, BMI will increase by 0.012 kg/m2 in black girls. |
‐ Yes |
Adjusted for age, BMI, IR and maturation stage at baseline; change in IR over 10 years' follow‐up; percentage of calories from protein, fat and CHO (mean of interviews) during 10 years' follow‐up; and interaction terms (nutrients × baseline IR). |
| BMI at > 10 years: 2 cohort studies; 2 analyses (n = 330) in boys and girls aged 2‐3 years | ||||||||
|
Magarey 2001 2 years; regression |
BMI | z‐score | 13 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years, single 4‐day weighed dietary record at 6, 9, 11 and 13 years) | NR | n overall = 218; regression result. β = 0.16, 0.05 < P ≤ 0.1; R2 = 0.23, P < 0.0001. After 13 years, increase in the total fat intake will increase BMI by 0.16 z‐score. |
+ Yes |
Adjusted for baseline BMI‐z score, gender, mother's BMI and father's BMI. |
|
Alexy 2004 3.2 years; mean end values per group |
BMI | z‐score | 17 | Total fat intake (single 3‐day weighed dietary record at baseline and each year follow‐up) | LF (32%TE); HF (40%TE) |
n overall = 112 (LF = 55; HF = 57); mean end values (SD). Baseline: LF = 0.36 (0.75); HF = 0.07 (0.81). At 17 years: LF = 0.23 (0.9); HF = 0.11 (1.09). After 17 years, on average BMI decrease 0.13 z‐score in the LF (32%TE) group while increase 0.04 z‐score in the HF (40%TE) group. |
+ No |
No matching reported. No adjustments for prognostic variables. |
aDirection refers to whether there was a positive (+: exposure and outcome moved in the same direction), inverse/negative (‐: exposure and outcome moved in opposite directions) or zero (0: no association) between total fat intake and the outcome.
bUnpublished data provided by study authors.
c"Energy dense, high fat, low fibre" dietary pattern reflected high intakes of processed meat, chocolate and confectionery, low‐fibre bread, crisps and savoury snacks, fried and roasted potatoes, the high intake of these foods increase the individual’s dietary pattern z‐score.
dOverweight/obese was defined by IOTF for children aged 14 years (boys, BMI > 22.62 kg/m2; girls, BMI > 23.34 kg/m2), and aged 17 years (boys, BMI > 24.46 kg/m2; girls, BMI > 24.70 kg/m2).
%TE: percentage of total energy; B: unstandardised beta‐coefficient; β: standardised beta‐coefficient; BMI: body mass index; CHO: carbohydrate; CI: confidence interval; df: degrees of freedom; FFQ: Food Frequency Questionnaire; HF: high fat; IR: insulin resistance; IOTF: International Obesity Task Force; LF: low fat; MD: mean difference; n: number of participants; NA: not applicable; NR: not reported; NS: not significant; OR: odds ratio; SD: standard deviation; SE: standard error; SES: socioeconomic status; TV: television.
At one year follow‐up, two studies reported associations of fat intake (continuous) with BMI (kg/m2); Schwandt 2011 in children who were seven years old at baseline (n = 411), while Berkey 2005 reported one‐year change in BMI in children who were nine to 14 years old at baseline (girls n = 6149, boys n = 4620). Bogaert 2003 reported this association using standardised BMI (z‐score) as an outcome in children who were nine years old at baseline (n = not reported). The large Berkey 2005 study, which adjusted for TE and most important prognostic variables, reported no association between total fat intake (in grams) and BMI in boys and girls.
At 1 to 2 years follow‐up, seven studies (10 analyses; n = 3347) reported on BMI in children between two and 13 years of age. Ambrosini 2016 (boys n = 383, girls n = 323) and Lee 2001 (n = 192 girls) reported the relationship between BMI, two‐year BMI change or BMI z‐scores, and lower and higher fat intake groups (lowest and highest quintiles of fat intake and ≤ 30%TE and > 30%TE) in children who were four to five years old at baseline, while Davison 2001; Klesges 1995; Lee 2012; and Setayeshgar 2017 used continuous fat intake (%TE and per 10 g) in their analyses of children who were four to 13 years old at baseline. The four studies that adjusted for TE intake reported the following: "Percentage of fat intake, baseline BMI, family risk of overweight, mothers’ BMI, fathers’ enjoyment of activity explained 26% of the variance in the change of BMI." (Davison 2001; n = 168); for every 1% increase in energy intake from total fat, BMI increased by 0.021 kg/m2 in first graders (mean age: 7.3 years) (n = 474), and for every 1% increase in energy intake from total fat, BMI decreased by 0.007 kg/m2 in fourth graders (mean age: 10 years) (n = 1030) (Lee 2012). Greater total fat intake increased BMI by 0.079 z‐scores (Magarey 2001; n = 155); and for every 10 g increase in total fat intake, BMI increased by 0.009 z‐scores (Setayeshgar 2017; n = 330).
Three studies did not adjust for energy intake, and found the following:
average BMI in boys (n = 383) decreased by 0.5 kg/m2 in the low‐fat group (30.4%TE) and by 0.6 kg/m2 in the high‐fat group (41.8%TE) (Ambrosini 2016);
average BMI in girls (n = 323) decreased by 0.5 kg/m2 in the low‐fat group (30.4%TE) and by 0.3 kg/m2 in the high‐fat group (41.8%TE) (Ambrosini 2016);
for every 1% increase in energy intake from total fat in year two to three of follow‐up, BMI decreased by 0.04 kg/m2;
for every 1% increase in energy intake from baseline total fat, BMI increased by 0.034 kg/m2 (Klesges 1995; n = 146);
low‐fat intake (≤ 30%TE) resulted in 0.4 kg/m2 smaller increase in BMI on average compared to high‐fat intake (> 0%TE) in girls (Lee 2001; n = 192).
Various continuous and binary measures of BMI were related to a high‐fat dietary pattern z‐score (Appannah 2015), and other continuous measures of total fat intake (Brixval 2009; Cohen 2014; Jago 2005; Obarzanek 1997 (cohort)), or lower and higher fat intake groups (Shea 1993), at 2 to 5 years follow‐up in children aged two to 14 years. The four studies that adjusted their analyses for TE intake found the following:
for every 1% increase in energy intake from total fat, BMI decreased by 0.63 z‐scores in boys (n = 181), but increased by 0.07 z‐scores in girls (n = 217) (Brixval 2009);
Jago 2005 (n = 133) reported that "Dietary factors were not associated with BMI across the three study years";
for every 1% increase in energy intake from total fat, BMI decreased by 0.00008 kg/m2 (Obarzanek 1997 (cohort); n = 632);
an increase in total fat intake increased BMI by 0.087 z‐scores (Magarey 2001; n = 152).
The two studies that did not adjust for energy intake reported the following:
low‐fat intake (≤ 30%TE) resulted in a 0.02 kg/m2 per year greater increase in BMI on average compared to high‐fat intake (> 30%TE) (Shea 1993; n = 215);
for every 1% increase in energy intake from total fat, BMI decreased by 0.01 percentile in girls (Cohen 2014; n = 265).
In the Appannah 2015 study, where energy adjustment was not applicable as it was part of the dietary pattern exposure, the authors found that for every 1 z‐score increase in the energy‐dense, high‐fat and low‐fibre dietary pattern, BMI increased by 0.03 z‐scores in boys and by 0.99 z‐scores in girls. In different analyses the odds ratio (OR) for being overweight/obese was 1.04 greater in boys and 1.02 greater in girls with higher dietary pattern z‐scores, compared to boys and girls with lower dietary pattern z‐scores (girls n = 649, boys n = 699).
In studies following children for 5 to 10 years, fat intake (%TE or grams) was related to BMI z‐scores (Magarey 2001), change in BMI (Brixval 2009; Morrison 2008), or absolute BMI (Skinner 2004). The three studies that adjusted for TE intake reported the following:
for every 1% increase in energy intake from total fat, BMI decreased by 0.011 z‐scores in boys (n = 147) (Brixval 2009), but.
for every 1% increase in energy intake from total fat, BMI increased by 0.005 z‐scores in girls (n = 177) (Brixval 2009);
an increase in total fat intake increased BMI by 0.122 z‐scores (Magarey 2001; n = 243);
for every 1% increase in energy intake from total fat, BMI increased by 0.029 kg/m2 in white girls (n = 241) and by 0.012 kg/m2 in black girls (n = 280) (Morrison 2008).
Without adjusting for energy intake, Skinner 2004 (n = 70) reported that for every 1 g increase in the fat intake, BMI increased by 0.01 kg/m2.
After 13 years of follow‐up and with adjustment for energy intake, Alexy 2004 (n = 112) reported that an increase in the total fat intake increased BMI by 0.16 z‐scores. Without adjustment for energy, Magarey 2001 (n = 218) found that after 17 years, on average BMI decreased 0.13 z‐scores in the low‐fat group (32%TE), but increased 0.04 z‐scores in the high‐fat group (40%TE).
Waist circumference
We have summarised standardised and unstandardised waist circumference outcomes, fat intake exposure variables used, and results of reported associations within each time range, including adjustments made, in Table 11.
8. Results of cohort studies: waist circumference.
|
Study ID; mean age at baseline; analysis |
Outcome | Outcome units | Time point (year) | Exposure | Exposure unit | Results of association (all reported values) |
Direction;a energy intake adjusted? (yes/no) |
Matched groups or adjusted for (or both) | |
| Waist circumference at > 1to 2 years: 1 cohort study; 1 analysis (n = 310) in boys and girls aged 13 years | |||||||||
|
Setayeshgar 2017 12.5 years; regression |
WC | cm | 2 | Total fat intake (single 24‐hour recall at baseline) | per 10 g | n overall = 310, regression result. B = 0.31 (95% CI 0.08 to 0.58), P ≤ 0.05. After 2 years, for every 10‐g increase in the total fat intake of children, WC will increase by 0.31 cm. |
+ No |
Age, gender, baseline BMI z‐score, baseline WC, moderate to vigorous physical activity, vegetables and fruit, fibre, milk, sodium and added sugar. | |
| Waist circumference at > 2to 5 years: 1 cohort study; 4 analyses (n = 2680) in boys and girls aged 14 years | |||||||||
|
Appannah 2015 14 years; regression and OR higher vs lower dietary pattern z‐score |
WC | WC ≥ 80 cm, odds | 3 | Energy‐dense, high‐fat and low‐fibre dietary patternb (single FFQ at baseline and 3 years) | z‐score | n boys = 697, regression result. OR = 1 (95% CI 0.82 to 1.22). After 3 years, the ratio of odds that WC is ≥ 80 cm is the same in boys with higher dietary pattern z‐scores compared to the odds in boys with lower dietary pattern z‐scores, during the period from 14 to 17 years of age. |
0 NA; exposure includes energy intake |
Age, dietary misreporting, physical fitness, smoking and BMI z‐score. | |
|
Appannah 2015 14 years; regression and OR higher vs lower dietary pattern z‐score |
WC | WC ≥ 80 cm, odds | 3 | Energy‐dense, high‐fat and low‐fibre dietary patternb (single FFQ at baseline and 3 years) | z‐score | n girls = 643, regression result. OR = 1.28 (95% CI 1.00 to 1.63). After 3 years, the ratio of odds that WC is ≥ 80 cm is 1.28 greater in girls with higher dietary pattern z‐scores compared to the odds in girls with lower dietary pattern z‐scores, during the period from 14 to 17 years of age. |
+ NA; exposure includes energy intake |
Age, dietary misreporting, physical fitness, smoking and BMI z‐score. | |
|
Appannah 2015 14 years; regression |
WC | z‐score | 3 | Energy‐dense, high‐fat and low‐fibre dietary patternb (single FFQ at baseline and 3 years) | z‐score | n boys = 697, regression result. β = 0.003 (95% CI ‐0.02 to 0.03). After 3 years, for every 1 unit increase in z‐score of the energy‐dense, high‐fat and low‐fibre dietary pattern of boys, WC will increase by 0.003 z‐scores. |
+ NA; exposure includes energy intake |
Age, dietary misreporting, physical fitness, smoking and BMI z‐score. | |
|
Appannah 2015 14 years; regression |
WC | z‐score | 3 | Energy‐dense, high‐fat and low‐fibre dietary patternb (single FFQ at baseline and 3 years) | z‐score | n girls = 643, regression result. β = 0.04 (95% CI 0.01 to 0.07). After 3 years, for every 1 unit increase in z‐score of the energy‐dense, high‐fat and low‐fibre dietary pattern of girls, WC will increase by 0.04 z‐scores. |
+ NA; exposure includes energy intake |
Age, dietary misreporting, physical fitness, smoking and BMI z‐score. | |
| Waist circumference at > 5to 10 years: 1 cohort study; 2 analyses (n = 512) in girls aged 10 years | |||||||||
|
Morrison 2008 10.1 years; regression |
WC | cm, 10‐years change |
10 | Total fat intake (single 3‐day dietary records at 1, 2, 3, 4, 5, 7, 8 and 10 years) × baseline IR | %TE | n white girls = 236. B = 0.053, SE 0.0065, P < 0.0001. After 10 years, for every 1% increase in energy intake from total fat in white girls, WC will increase by 0.053 cm. |
+ Yes |
Age, WC, IR, and maturation stage at baseline; change in IR over 10‐years follow‐up; percentage of calories from protein, fat, and CHO (mean of interviews) during 10‐years follow‐up; and interaction terms (nutrients × baseline IR). | |
|
Morrison 2008 10.1 years; regression |
WC | cm, 10‐years change |
10 | Total fat intake (single 3‐day dietary records at 1, 2, 3, 4, 5, 7, 8 and 10 years) × baseline IR | %TE | n black girls = 276. B = 0.028, SE 0.0056, P < 0.0001. After 10 years, for every 1% increase in energy intake from total fat in black girls, WC will increase by 0.028 cm. |
+ Yes |
"Age, waist circumference, IR, and maturation stage at baseline; change in IR over 10‐y follow‐up; percentage of calories from protein, fat, and CHO (mean of interviews) during 10‐y follow‐up; and interaction terms (nutrients baseline IR)." | |
aDirection refers to whether there was a positive (+: exposure and outcome moved in the same direction), inverse/negative (‐: exposure and outcome moved in opposite directions) or zero (0: no association) between total fat intake and the outcome.
b"Energy dense, high fat, low fibre" dietary pattern reflected high intakes of processed meat, chocolate and confectionery, low‐fibre bread, crisps and savoury snacks, fried and roasted potatoes, the high intake of these foods increase the individual’s dietary pattern z‐score.
%TE: percentage of total energy; B: unstandardised beta‐coefficient; β: standardised beta‐coefficient; BMI: body mass index; CHO: carbohydrate; CI: confidence interval; FFQ: Food Frequency Questionnaire; IR: insulin resistance; n: number of participants; NA: not applicable; OR: odds ratio; WC: waist circumference.
Only Setayeshgar 2017 (n = 310) reported waist circumference in relation to total fat intake per 10 g at two years. With no adjustment for total energy intake, they found that for every 10 g increase in total fat intake of children, waist circumference increased by 0.31 cm. Appannah 2015 reported the association between a high‐fat dietary pattern z‐scores and various measures of waist circumference at 2 to 5 years, and found that after three years, for every one unit increase in z‐score of the energy‐dense, high‐fat and low‐fibre dietary pattern, waist circumference in boys (n = 697) increased by 0.003 z‐scores, and waist circumference in girls (n = 643) increased by 0.04 z‐scores. Morrison 2008 related total fat intake (%TE) to 10‐year change in waist circumference (cm), and, with TE intake adjustment, reported that for every 1% increase in energy intake from total fat, waist circumference increased by 0.053 cm in white girls (n = 236), and by 0.028cm in black girls (n = 276).
Body fat and fat mass index
We have summarised the various outcomes, exposures and results of reported associations within each time range, for body fat, in Table 12, and for fat mass index in Table 13, including adjustments made.
9. Results of cohort studies: body fat.
|
Study ID; mean age at baseline; analysis |
Outcome | Outcome units |
Time point (years) |
Exposure | Exposure unit | Results of association (all reported values) |
Direction;a energy intake adjusted (yes/no) |
Matched groups or adjusted for (or both) | |||
| Body fat at 1 year: 1 cohort study; 1 analysis (n = 411) in boys and girls aged 7 years | |||||||||||
|
Schwandt 2011b 6.8 years; regression |
Body fat (skinfold thickness) |
% | 1 | Total fat intake (single 7‐day weighed dietary record at baseline and 1 year) | g | n overall = 411, regression result. B = 0.011, SE 0.017, P < 0.05. After 1 year, for every 1 g increase in the total fat intake of children, body fat will increase by 0.01%. |
+ No |
Adjusted for age, gender and physical activity. | |||
| Body fat at > 1to 2 years: 1 cohort study; 1 analysis (n = 625) in boys and girls aged 5 years | |||||||||||
|
Ambrosini 2016 5.2 years; regression |
Body fat (DEXA) |
kg | 2 | Energy‐dense, high‐fat, low‐fibre dietary patternc (single 3‐day dietary record at baseline and 2 years) | z‐score | n overall = 625, regression result. B = 0.28 (95% CI 0.05 to 0.53), P = 0.02. After 2 years, for every 1 unit increase in the dietary pattern z‐score of children, body fat will increase by 0.28 kg. |
+ NA; exposure includes energy intake |
Adjusted for height at age 9 years, gender, misreporting status, maternal BMI, maternal education (5 categories), overweight status (by BMI) at baseline and television watching at 54 months. | |||
| Body fat at > 2to 5 years: 3 cohort studies; 6 analyses (n = 968) in boys and girls aged 2‐14 years | |||||||||||
|
Cohen 2014 13.9 years; regression |
Body fat (skinfold thickness, BIA) |
% | 3‐5 | Total fat intake (single FFQ at baseline and once during follow‐up period) | %TE | n girls = 265, regression result. B = ‐0.005, SE 0, P = 0.03. After 3‐5 years, for every 1 % increase in energy intake from total fat of girls, body fat will decrease by 0.005%. |
‐ No |
Adjusted for age, ethnicity, protein calories, CHO calories, physical activity, physical inactivity and SES. | |||
|
Ambrosini 2016 5.2 years; regression |
Body fat (DEXA) |
kg | 4 | Energy‐dense, high‐fat, low‐fibre dietary patternc (single 3‐day dietary record at baseline and 2 years) | z‐score | n overall = 483, regression result. B = 0.15 (95 % CI ‐0.15 to 0.45), P = 0.34. After 4 years, for every 1 unit increase in the dietary pattern z‐score of children, body fat will increase by 0.15 kg. |
+ NA; exposure includes energy intake |
Adjusted for height at age 9 years, gender, misreporting status, maternal BMI, maternal education (5 categories), overweight status (by BMI) at baseline and television watching at 54 months. | |||
|
Skinner 2004 2 years; regression |
Body fat (DEXA) |
% | 4 | Longitudinal dietary fat (single 24‐hour dietary recall and 2‐day food record at baseline, every 3 months during 1 year, every 6 months during 2 and 3 years, and yearly at 4 years) | NR | n overall = 53, regression result. B = 0.619, SE 0.261, P = 0.02, F‐test = 5.63, R2 = 0.51, p = 0.0001, F‐test = 7.88. After 4 years, for every 1 unit increase in total fat intake of children, body fat will increase by 0.61%. |
+ No |
Adjusted for baseline BMI, parental BMI, gender, protein, calcium and monounsaturated fat. | |||
|
Skinner 2004 2 years; regression |
Body fat (DEXA) |
g | 4 | Longitudinal dietary fat (single 24‐hour dietary recall and 2‐day food record at baseline, every 3 months during 1 year, every 6 months during 2 and 3 years, and yearly at 4 years) | NR | n overall = 53, regression result. B = 178.65, SE 70.06, P = 0.01, F‐test = 6.5, R2 = 0.51, P = 0.0001, F‐test = 9.84. After 4 years, for every 1 unit increase in total fat intake of children, body fat will increase by 178 g. |
+ No |
Adjusted for baseline BMI, parental BMI, gender, protein, calcium and monounsaturated fat. | |||
|
Skinner 2004 2 years; regression |
Body fat (DEXA) |
% | 4 | Longitudinal dietary fat (single 24‐hour dietary recall and 2‐day food record at baseline, every 3 months during 1 year, every 6 months during 2 and 3 years, and yearly at 4 years). | Number of servings | n overall = 53, regression result. B = 0.465, SE 0.255, P = 0.07, F‐test = 3.34. R2 = 0.47, P = 0.0001, F‐test = 6.93. After 4 years, for every 1 unit increase in the number of fat servings, body fat will increase by 0.47%. |
+ No |
Adjusted for baseline BMI, parental BMI, gender, protein, calcium and monounsaturated fat. | |||
|
Skinner 2004 2 years; regression |
Body fat (DEXA) |
g | 4 | Longitudinal dietary fat (single 24‐hour dietary recall and 2‐day food record at baseline, every 3 months during 1 year, every 6 months during 2 and 3 years, and yearly at 4 years). | Number of servings | n overall = 53, regression result. B = 136.48, SE 69.74, P = 0.06, F‐test = 3.83, R2 = 0.47, p = 0.0001, F‐test = 8.31. After 4 years, for every 1 unit increase in the number of fat servings, body fat will increase by 136 g. |
+ No |
Adjusted for baseline BMI, parental BMI, gender, protein, calcium and monounsaturated fat. | |||
| Body fat at > 5to 10 years: 1 cohort study; 3 analyses (n = 156) in boys and girls aged 2 years | |||||||||||
|
Skinner 2004 2 years; regression |
Body fat (DEXA) | % | 6 | Longitudinal dietary fat (single 24‐hour dietary recall and 2‐day food record at baseline, every 3 months during 1 year, every 6 months during 2 and 3 years, every year during 4, 5 and 6 years). | g | n overall = 52, regression result. B = 0.08, partial R2 = 0.06, P = 0.001, F‐test = 4.66, R2 = 0.336, P = 0.002. After 6 years, for every 1 g increase in total fat intake of children, body fat will increase by 0.08%. |
+ No |
Adjusted for gender, sedentary activity, intakes of calcium and polyunsaturated fat. | |||
|
Skinner 2004 2 years; regression |
Body fat (DEXA) |
% | 6 | Longitudinal dietary fat (single 24‐hour dietary recall and 2‐day dietary record at baseline, every 3 months during 1 year, every 6 months during 2 and 3 years, every year during 4, 5 and 6 years). | g | n overall = 52, regression result. B = 0.09, partial R2 = 0.02, P = 0.001, F‐test = 4.37, R2 = 0.322, P = 0.002. After 6 years, for every 1 g increase in total fat intake, body fat will increase by 0.09%. |
+ No |
Adjusted for gender, sedentary activity, calcium intake, and polyunsaturated fat intake and father's BMI. | |||
|
Skinner 2004 2 years; regression |
Body fat (DEXA) |
kg | 6 | Longitudinal dietary fat (single 24‐hour dietary recall and 2‐day food record at baseline, every 3 months during 1 year, every 6 months during 2 and 3 years, every year during 4, 5 and 6 years) | g | N overall = 52, regression result. B = 0.034, partial R2 = 0.06, P = 0.01, F‐test = 4.19, R2 = 0.26, P = 0.006. After 6 years, for every 1 g increase in total fat intake of children, body fat will increase by 0.03 kg. |
+ No |
Adjusted for sedentary activity, calcium intake and polyunsaturated fat intake. | |||
aDirection refers to whether there was a positive (+: exposure and outcome moved in the same direction, inverse/negative (‐: exposure and outcome moved in opposite directions) or zero (0: no association between total fat intake and the outcome.
bUnpublished data provided by study authors.
c"Energy dense, high fat, low fibre" dietary pattern reflected high intakes of processed meat, chocolate and confectionery, low‐fibre bread, crisps and savoury snacks, fried and roasted potatoes, the high intake of these foods increase the individual's dietary pattern z‐score.
%TE: percentage of total energy; B: unstandardised beta‐coefficient; BIA: bioelectrical impedance, BMI: body mass index; CHO, carbohydrate; CI: confidence interval; DEXA: dual energy X‐ray absorptiometry; FFQ: food frequency questionnaire; n: number of participants; NA: not applicable; NR: not reported; SD: standard deviation; SE: standard error; SES: socioeconomic status.
10. Results of cohort studies: fat mass index.
|
Study ID; mean age at baseline; analysis |
Outcome | Outcome units |
Time point (year) |
Exposure | Exposure unit | Results of association (all reported values) | Direction;a energy intake adjusted (yes/no) | Matched groups or adjusted for (or both) |
| Fat mass index at > 2to 5 years: 1 cohort study; 1 analysis (n = 4002) in boys and girls aged 8 years | ||||||||
|
Ambrosini 2016 7.5 years; regression |
Fat mass indexb | z‐score | 4 | Energy‐dense, high‐fat, low‐fibre dietary patternc (single 3‐day dietary records at baseline and 2 years) | z‐score | n overall = 4002, regression result. β = 0.07 (95% CI 0.05 to 0.10), P ≤ 0.0001. After 4 years, for every 1 z‐score increase in the dietary pattern, the fat mass index will increase by 0.07 z‐scores. |
+ NA; exposure includes energy intake |
Adjusted for gender, age at dietary assessment, dietary misreporting, total physical activity at 11 years, maternal prepregnancy BMI and maternal education. |
| Fat mass index at > 5to 10 years: 1 cohort study; 5 analyses (n = 21,542) in boys and girls aged 8 years | ||||||||
|
Ambrosini 2016 7.5 years; regression |
Fat mass indexb | z‐score | 8 | Energy‐dense, high‐fat, high‐sugar, low‐fibre dietary patternc (single 3‐day dietary record at baseline, 3 and 6 years) | z‐score | n overall = 4729, regression result. β = 0.04 (95% CI 0.01 to 0.08), P = 0.028. After 8 years, for every 1 z‐score increase in the dietary pattern, the fat mass index will increase by 0.04 z‐scores. |
+ NA; exposure includes energy intake |
Adjusted for age, gender, dietary misreporting, physical activity and maternal social class. |
|
Ambrosini 2016 7.5 years; regression |
Fat mass indexb | z‐score | 8 | Non‐energy‐dense, high‐sugar, LF dietary patternd (single 3‐day dietary record at baseline, 3 and 6 years) | z‐score | n overall = 4729, regression result. β = ‐0.03 (95% CI ‐0.07 to 0.02), P = 0.22. After 8 years, for every 1 z‐score increase in the dietary pattern, the fat mass index will decrease by 0.03 z‐scores. |
‐ NA; exposure includes energy intake |
Adjusted for age, gender, dietary misreporting, physical activity and maternal social class. |
|
Ambrosini 2016 7.5 years; regression |
Fat mass indexb | z‐score | 8 | Energy‐dense, high‐fat, low‐fibre dietary patternc (single 3‐day dietary record at baseline, 3 and 6 years) | z‐score | n overall = 2626, regression result. β = 0.06 (95% CI 0.03 to 0.10), P = 0.0004. After 8 years, for every 1 z‐score increase in the dietary pattern, the fat mass index will increase by 0.06 z‐scores. |
+ NA; exposure includes energy intake |
Adjusted for gender, age at dietary assessment, dietary misreporting, total physical activity at 11 years, maternal pre‐pregnancy BMI and maternal education. |
|
Ambrosini 2016 7.5 years; regression |
Fat mass indexb | FMI z‐score > 80th percentile; odds | 8 | Energy‐dense, high‐fat, high‐sugar, low‐fibre dietary patternc (single 3‐day dietary record at baseline, 3 and 6 years) | z‐score | n overall = 4729, regression result. OR 1.11 (95% CI 0.97 to 1.28), P = 0.14. After 8 years, the ratio of odds for having FMI z‐score > 80th percentile is 1.11 greater in children with higher dietary pattern z‐scores compared to the odds in children with lower dietary pattern z‐scores. |
+ NA; exposure includes energy intake |
Adjusted for age, gender, dietary misreporting, physical activity and maternal social class. |
|
Ambrosini 2016 7.5 years; regression |
Fat mass indexb | FMI z‐score > 80th percentile; odds | 8 | Non‐energy‐dense, high‐sugar, LF dietary patternd (single 3‐day dietary record at baseline, 3 and 6 years) | z‐score | n overall = 4729, regression result. OR 0.92 (95% CI 0.78 to 1.09), P = 0.34. After 8 years, the ratio of odds for having FMI z‐score > 80th percentile is 0.92 smaller in children with higher dietary pattern z‐scores compared to the odds in children with lower dietary pattern z‐scores. |
‐ NA; exposure includes energy intake |
Adjusted for age, gender, dietary misreporting, physical activity and maternal social class. |
aDirection refers to whether there was a positive (+: exposure and outcome moved in the same direction), inverse/negative (‐: exposure and outcome moved in opposite directions) or zero (0: no association) between total fat intake and the outcome;
bFMI was calculated by dividing fat mass (measured by dual‐energy X‐ray Absorptiometry) (kg) by height (m) raised to the optimum power (calculated by using log‐log regression analysis) to remove any residual correlation between fat mass and height;
c"Energy‐dense, high‐fat, low‐fibre" dietary pattern reflected high intakes of processed meat, chocolate and confectionery, low‐fibre bread, crisps and savoury snacks, fried and roasted potatoes, the high intake of these foods increase the individual’s dietary pattern z‐score.
dNon‐energy‐dense, high‐sugar, low‐fat dietary pattern reflected higher intakes of sugary foods including sugar‐sweetened beverages, fruit juices, ready‐to‐eat breakfast cereals (low‐fibre breakfast cereals) and low intakes of whole milk, margarines and oils, cheese and crisps.
β: standardised beta‐coefficient; BMI: body mass index; FMI: Fat Mass Index ; n: number of participants; NA: not applicable; OR: odds ratio.
Schwandt 2011 (n = 411) reported the association between body fat (%; calculated from skinfold thickness) and total fat intake (g) at one‐year follow‐up, and found that for every 1 g increase in children's total fat intake, body fat increased by 0.01% (with no energy intake adjustment). Ambrosini 2016 reported the link between body fat (kg; measured by dual energy X‐ray absorptiometry (DEXA)) and high‐fat dietary pattern z‐scores at two years, where for every one unit increase in the dietary pattern z‐score, children's body fat increased by 0.28 kg (n = 625), and at four years where for every one unit increase in the dietary pattern z‐score, body fat increased by 0.15 kg (n = 483). For this later time range, Cohen 2014 and Skinner 2004 related various measures of total fat intake to body fat (% and g). With no energy adjustment, Skinner 2004 found that after four years (n = 53), for every one unit increase in children's total fat intake, body fat increased by 0.61% or 178 g (both measured by DEXA), and after six years (n = 52), for every 1 g increase in total fat intake, body fat increased by 0.09% or 30 g (both measured by DEXA).
Ambrosini 2016 was the only study to report analyses on various measures of fat mass index in relation to a high‐fat dietary pattern at 2 to 5 years (one analysis, n = 4002) and to a high‐fat and a low‐fat dietary pattern at 5 to 10 years (four analyses, n = 2626 to 4729). Fat mass index was calculated by dividing fat mass (kg; measured by DEXA) by height (m) raised to the optimum power (calculated by using log‐log regression analysis) to remove any residual correlation between fat mass and height.
This trial used two dietary patterns, high‐fat and low‐fat. The energy‐dense, low‐fibre high‐fat dietary pattern reflected high intakes of processed meat, chocolate and confectionery, low‐fibre bread, crisps and savoury snacks, fried and roasted potatoes. High intake of these foods increases the individuals' dietary pattern z‐score. The non‐energy‐dense, high‐sugar, low‐fat dietary pattern reflected higher intakes of sugary foods including sugar‐sweetened beverages, fruit juices, ready‐to‐eat breakfast cereals (low‐fibre breakfast cereals) and low intakes of whole milk, margarines and oils, cheese and crisps. After four years, for every one z‐score increase in the high‐fat dietary pattern, the fat mass index increased by 0.07 z‐scores. After eight years, analyses showed that for every one z‐score increase in the high‐fat dietary pattern, the fat mass index increased by 0.06 z‐scores, and in a different analysis that the ratio of odds for having fat mass index z‐score greater than the 80th percentile was 1.11 greater in children with greater high‐fat dietary pattern z‐scores compared to the odds in children with smaller z‐scores. After eight years, for every one z‐score increase in the low‐fat dietary pattern, the fat mass index decreased by 0.03 z‐scores, and in a different analysis, the OR for having a fat mass index z‐score greater than the 80th percentile was 0.92 smaller in children with greater low‐fat dietary pattern z‐scores compared to the odds in children with smaller z‐scores (Ambrosini 2016).
Skinfold thickness
We have summarised sums of multiple skinfold thickness measurements (standardised and unstandardised), fat intake exposure variables and results of reported associations within each time range in Table 14. We have summarised single skinfold thickness measurements (subscapular and triceps) in Table 15, including adjustments made.
11. Results of cohort studies: sum of multiple skinfold thicknesses.
|
Study ID; mean age at baseline; analysis |
Outcome | Outcome units | Time point (year) | Exposure | Exposure unit | Results of association (all reported values) |
Direction;a energy intake adjusted (yes/no) |
Matched groups or adjusted for (or both) |
| Sum of 4 skinfolds (BC, TC, SC, SI) at 1 year: 1 cohort study; 1 analysis (n = NR) in boys and girls aged 6 years | ||||||||
|
Tershakovec 1998 (cohort) 6.2 years; mean end values per group |
Sum of skinfolds (BC, TC, SS, SI) | mm | 1 | Total fat intake (multiple 24‐hour recalls at baseline, 3 and 6 months and 1 year) | LF quintile (24%TE); HF quintile (34%TE) | n overall = NR (LF = NR, HF = NR), mean end values (95% CI). Baseline: LF = 24.7 (95% CI 23 to 26.5); HF = 28.8 (95% CI 26.1 to 31.8). At 1 year: (reported in the figure without exact values), LF = lower than baseline; HF = greater than baseline. After 1 year, the sum of skinfolds will decrease in children with a low‐fat intake, and increase in children with high‐fat intake |
+ No |
No matching reported. No adjustment for prognostic variables. |
| Sum of 4 skinfolds (BC, TC, SC, SI) at > 1to 2 years: 1 cohort study; 1 analysis (n = 126) in boys and girls aged 2 years | ||||||||
|
Magarey 2001 2 years; mean end values per group |
Sum of skinfolds (TC, BC, SS, SI) | mm | 2 | Total fat intake (single 3‐day weighed dietary record at baseline and 2 years) | LF < 30%TE; HF > 35%TE | n overall = 126 (LF = 14, HF = 112), mean end values (SD). Baseline: LF = 33.4 (6.8); HF = 32.8 (6.3). At 2 years: LF (n = 20) = 31 (9.2); HF (n = 76) = 31.4 (6.3); P > 0.05. After 2 years, the sum of skinfolds of children with LF intakes will decrease by 2.4 mm on average, and by 1.4 mm in children with HF intake. |
+ No |
No matching reported. No adjustment for prognostic variables. |
| Sum of 4 skinfolds at > 2to 5 years: 1 cohort study; 1 analysis (n ˜ 126) in boys and girls aged 2 years | ||||||||
|
Magarey 2001 2 years; mean end values per group |
Sum of skinfolds (TC, BC, SS, SI) | mm | 4 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years) | LF < 30%TE; HF > 35%TE | n overall = 126 (LF = 14, HF = 112), mean end values (SD). Baseline: LF = 33.4 (6.8); HF = 32.8 (6.3); P > 0.05. At 4 years: LF (n = 14) = 27.2 (8); HF (n = 88) = 29.2 (8.9); P > 0.05. After 4 years, the sum of skinfolds of children with LF intakes will decrease by 6.2 mm on average, and by 3.6 mm in children with HF intake |
+ Yes |
No matching reported. No adjustment for prognostic variables. |
| Sum of 4 skinfolds at > 5to 10 years: 1 cohort study; 1 analysis (n = 126) in boys and girls aged 2 years | ||||||||
|
Magarey 2001 2 years; mean end values per group |
Sum of skinfolds (TC, BC, SS, SI) | mm | 6 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years, single 4‐day weighed dietary record at 6 years) | LF < 30%TE; HF > 35%TE | n overall = 126 (LF=14, HF=112), mean end values (SD). Baseline LF = 33.4 (6.8); HF = 32.8 (6.3), P > 0.05. At 6 years: LF (n = 13) = 32.8 (13.3); HF (n = 72) = 31.8 (12.8), P > 0.05. After 6 years, the sum of skinfolds of children with LF intakes will decrease by 0.6 mm on average, and by 1 mm in children with HF intake. |
‐ No |
No matching reported. No adjustment for prognostic variables. |
| Sum of 3 skinfolds at > 2to 5 years: 1 cohort study; 1 analysis (n = NR) in boys and girls aged 10 years | ||||||||
|
Obarzanek 1997 (cohort) 9.6 years; regression |
Sum of skinfolds (TC, SS, SI) | mm | 3 | Total fat intake (multiple 24‐hour recalls at baseline, 1 and 3 years) | %TE | n overall = NR; regression result. B = ‐0.005, P = 0.2. After 3 years, for every 1% increase in energy intake from total fat of children, the sum of skinfolds will decrease by 0.005 mm |
‐ Yes |
Adjusted for gender, physical activity, treatment, visit number, other sources of energy than fat, and for interactions: fat intake‐by‐treatment, fat intake‐by‐sex, fat intake‐by‐visit number and visit number‐by‐treatment. |
| Sum of 2 skinfolds at > 1to 2 years: 1 cohort study; 1 analysis (n = 192) in girls aged 5 years | ||||||||
|
Lee 2001 5 years; mean change per group |
Sum of skinfolds (TC, SS) | mm | 2 | Total fat intake (multiple 24‐hour recall at baseline) | LF ≤ 30%TE, HF > 30%TE | n girls = 192 (LF = 84; HF = 108); mean change (SD). Baseline: NR. LF = 0.9 (3.67), HF = 2.1 (5.2); P < 0.05. MD ‐1.2 (95% CI ‐2.46 to 0.06). After 2 years, the sum of skinfolds of girls with LF intake will increase on average by 1.2 mm less than girls with HF intake. |
+ No |
No matching reported. No adjustment for prognostic variables. |
aDirection refers to whether there was a positive (+: exposure and outcome moved in the same direction, inverse/negative (‐: exposure and outcome moved in opposite directions) or zero (0: no association) between total fat intake and the outcome.
%TE: percentage of total energy; BC: biceps; CI: confidence interval; HF: high fat; LF: low fat; MD: mean difference; n: number of participants; NA: not applicable; NR: not reported; SD: standard deviation; SI: supra‐ileac; SS: subscapular; TC: triceps.
12. Results of cohort studies: subscapular and triceps skinfold thickness.
|
Study ID; mean age at baseline; analysis |
Outcome | Outcome units | Time point (year) | Exposure | Exposure unit | Results of association (all reported values) | Direction;a energy intake adjusted (yes/no) | Matched groups or adjusted for (or both) |
| Subscapular skinfold at > 1to 2 years: 1 cohort study; 1 analysis (n = 155) in boys and girls aged 2 years | ||||||||
|
Magarey 2001 2 years; regression |
Subscapular skinfold | z‐score | 2 | Total fat intake (single 3‐day weighed dietary record at baseline and 2 years) | NR | n overall = 155; regression result. β = 0.081, P > 0.1, R2 = 0.47, P < 0.001. After 2 years, increase in the total fat intake will increase subscapular skinfold by 0.081 z‐score |
+ Yes |
Adjusted for subscapular z‐score at baseline, energy intake, gender, mother' subscapular and father' subscapular. |
| Subscapular skinfold at > 2to 5 years: 1 cohort study; 1 analysis (n = 152) in boys and girls aged 2 years | ||||||||
|
Magarey 2001 2 years; regression |
Subscapular skinfold | z‐score | 4 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years) | NR | n overall = 152; regression result. β = 0.072, P > 0.1, R2 = 0.38, P < 0.001. After 4 years, increase in the total fat intake will increase subscapular skinfold by 0.072 z‐score. |
+ Yes |
Adjusted for subscapular z‐score at baseline, energy intake, gender, mother' subscapular and father' subscapular. |
| Subscapular skinfold at > 5to 10 years: 1 cohort study; 1 analysis (n = 243) in boys and girls aged 2 years | ||||||||
|
Magarey 2001 2 years; regression |
Subscapular skinfold | z‐score | 9 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years, single 4‐day weighed dietary record at 6 and 9 years) | NR | n overall = 243; regression result. β = 0.069, P > 0.1, R2 = 0.26, P < 0.001. After 9 years, increase in the total fat intake will increase subscapular skinfold by 0.069 z‐score. |
+ Yes |
Adjusted for subscapular z‐score at baseline, energy intake, gender, mother' subscapular and father' subscapular. |
| Subscapular skinfold at > 10 years: 1 cohort study; 1 analysis (n = 218) in boys and girls aged 2 years | ||||||||
|
Magarey 2001 2 years; regression |
Subscapular skinfold | z‐score | 13 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years, single 4‐day weighed dietary record at 6, 9, 11 and 13 years) | NR | n overall = 218; regression result. β = 0.233, P ≤ 0.01. After 13 years, increase in the total fat intake will increase subscapular skinfold by 0.233 z‐score. |
+ Yes |
Adjusted for subscapular z‐score at baseline, energy intake, gender, mother' subscapular and father' subscapular. |
| Triceps skinfold at > 1to 2 years: 1 cohort study; 1 analysis (n = 155) in boys and girls aged 2 years | ||||||||
|
Magarey 2001 2 years; regression |
Triceps skinfold | z‐score | 2 | Total fat intake (single 3‐day weighed dietary record at baseline and 2 years) | NR | n overall = 155; regression result. β = 0.038, P > 0.1, R2 = 0.27, P ≤ 0.001. After 2 years, increase in the total fat intake will increase triceps skinfold by 0.038 z‐score. |
+ Yes |
Adjusted for triceps z‐score at baseline, gender, mother's triceps and father's triceps. |
| Triceps skinfold at > 2to 5 years: 1 cohort study; 1 analysis (n = 152) in boys and girls aged 2 years | ||||||||
|
Magarey 2001 2 years; regression |
Triceps skinfold | z‐score | 4 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years) | NR | n overall = 152; regression result. Β = 0.11, P > 0.1, R2 = 0.043, P > 0.01. After 4 years, increase in the total fat intake will increase triceps skinfold by 0.11 z‐score |
+ Yes |
Adjusted for triceps z‐score at baseline, gender, mother's triceps and father's triceps. |
| Triceps skinfold at > 5to 10 years: 1 cohort study; 1 analysis (n = 243) in boys and girls aged 2 years | ||||||||
|
Magarey 2001 2 years; regression |
Triceps skinfold | z‐score | 9 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years, single 4‐day weighed dietary record at 6 and 9 years) | NR | n overall = 243; regression result. β = 0.059, P > 0.1; R2 = 0.12, P ≤ 0.01. After 9 years, increase in the total fat intake will increase triceps skinfold by 0.059 z‐score |
+ Yes |
Adjusted for triceps z‐score at baseline, gender, mother's triceps and father's triceps. |
| Triceps skinfold at > 10 years: 1 cohort study; 1 analysis (n = 218) in boys and girls aged 2 years | ||||||||
|
Magarey 2001 2 years; regression |
Triceps skinfold | z‐score | 13 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years, single 4‐day weighed dietary record at 6, 9, 11 and 13 years) | NR | n overall = 218; regression result. β = 0.164; 0.05 < P ≤ 0.1. After 13 years, increase in the total fat intake will increase triceps skinfold by 0.164 z‐score |
+ Yes |
Adjusted for triceps z‐score at baseline, gender, mother's triceps and father's triceps. |
aDirection refers to whether there was a positive (+: exposure and outcome moved in the same direction), inverse/negative (‐: exposure and outcome moved in opposite directions) or zero (0: no association) between total fat intake and the outcome;
B: unstandardised beta‐coefficient; β: standardised beta‐coefficient; n: number of participants; NR: not reported.
Lee 2001 reported the mean change in the sum of triceps and subscapular skinfolds in lower fat (< 30%TE) versus a higher fat (> 30%TE) intake groups at two years, where the sum of two skinfolds of girls with low‐fat intake increased on average by 1.2 mm less than in girls with high‐fat intake (n = 192). Obarzanek 1997 (cohort) related the sum of triceps, subscapular and supra‐ileac skinfolds to total fat intake (%TE) after three years and showed that for every 1% increase in energy intake from total fat in children, the sum of three skinfolds decreased by 0.005 mm (n = not reported). Magarey 2001 reported the mean change in the sum of biceps, triceps, subscapular and supra‐ileac skinfolds in a lower fat (< 30%TE) versus higher fat (> 35%TE) intake group after two years and saw that the sum of four skinfolds decreased by 2.4 mm on average in children with low‐fat intakes, and by 1.4 mm in children with high‐fat intake (n =126). At four years the sum of four skinfolds of children with low‐fat intakes had decreased by 6.2 mm on average, and by 3.6 mm in children with high‐fat intake (n = 126). At six years the sum of skinfolds of children with low‐fat intakes had decreased by 0.6 mm on average, and by 1 mm in children with high‐fat intake; n = 126). Tershakovec 1998 (cohort) agreed with this finding, showing that after one year the sum of skinfolds decreased in children in the lowest quintile of fat intake and increased in children in the highest quintile of fat intake.
Magarey 2001 also related total fat intake to standardised triceps and standardised subscapular skinfold thicknesses:
at two years when increase in the total fat intake increased triceps skinfold thickness by 0.038 z‐scores (n = 155), and subscapular skinfold thickness by 0.081 z‐scores (n = 155);
at four years when increase in total fat intake increased triceps skinfold thickness by 0.11 z‐scores (n = 152), and subscapular skinfold thickness by 0.072 z‐scores (n = 152);
at nine years when increase in total fat intake increased triceps skinfold thickness by 0.059 z‐scores (n = 243), and subscapular skinfold thickness by 0.069 z‐scores (n = 243); and
at 13 years when increase in total fat intake increased triceps skinfold thickness by 0.164 z‐scores (n = 218); and subscapular skinfold by 0.069 z‐scores (n = 243).
Secondary outcomes
Blood lipids and blood pressure
We have summarised results of reported associations between total fat intake and LDL‐cholesterol, HDL‐cholesterol and triglycerides within each time range, including adjustments made, in Table 16.
13. Results of cohort studies: blood lipids.
|
Study ID; mean age at baseline; analysis |
Outcome | Outcome units | Time point (year) | Exposure | Exposure unit | Results of association (all reported values) | Direction;a energy intake adjusted (yes/no) | Matched groups or adjusted for (or both) |
| LDL‐C at > 2to 5 years: 1 cohort study; 2 analyses (n = 1163) in boys and girls aged 14 years | ||||||||
|
Appannah 2015 14 years; regression |
LDL‐C | mmol/L | 3 | Energy‐dense, high‐fat and low‐fibre dietary patternb (single FFQ at baseline and 3 years) | z‐score | n girls = 558, regression result. B = 0.04 (95% CI ‐0.01 to 0.08). After 3 years, for every 1 z‐score increase in the dietary pattern, LDL‐C will increase by 0.04 mmol/L in girls. |
+ NA; exposure includes energy intake |
Adjusted for age, dietary misreporting, physical fitness, smoking and BMI z‐score. |
|
Appannah 2015 14 years; regression |
LDL‐C | mmol/L | 3 | Energy‐dense, high‐fat and low‐fibre dietary patternb (single FFQ at baseline and 3 years) | z‐score | n boys = 605, regression result. B = 0.001 (95% CI ‐0.04 to 0.03). After 3 years, for every 1 z‐score increase in the dietary pattern, LDL‐C will increase by 0.001 mmol/L in boys. |
+ NA; exposure includes energy intake |
Adjusted for age, dietary misreporting, physical fitness, smoking and BMI z‐score. |
| HDL‐C at > 2to 5 years: 2 cohort studies; 3 analyses (n = 1393) in boys and girls aged 13 and 14 years | ||||||||
|
Appannah 2015 14 years; regression; |
HDL‐C | mmol/L | 3 | Energy‐dense, high‐fat and low‐fibre dietary patternb (single FFQ at baseline and 3 years) | z‐score | n girls = 558, regression result. B = 0.02 (95% CI 0.002 to 0.04). After 3 years, for every 1 z‐score increase in the dietary pattern HDL‐C will increase by 0.02 mmol/L in girls. |
+ NA; exposure includes energy intake |
Adjusted for age, dietary misreporting, physical fitness, smoking and BMI z‐score. |
|
Appannah 2015 14 years; regression; |
HDL‐C | mmol/L | 3 | Energy‐dense, high‐fat and low‐fibre dietary patternb (single FFQ at baseline and 3 years) | z‐score | n boys = 605, regression result. B = ‐0.002 (95% CI ‐0.02 to 0.01). After 3 years, for every 1 z‐score increase in the dietary pattern HDL‐C will decrease by 0.002 mmol/L in boys. |
‐ NA; exposure includes energy intake |
Adjusted for age, dietary misreporting, physical fitness, smoking and BMI z‐score. |
|
Boreham 1999 12.5 years; regression; |
HDL‐C | mmol/L | 3 | Total fat intake (dietary history at baseline and 3 years) | %TE | n girls = 230, regression result. β = ‐0.21, SE 0.1, P = 0.031. After 3 years, for every 1% increase in energy intake from total fat, HDL‐C will decrease by 0.21 mmol/L in girls. |
‐ Yes |
Adjusted for sexual maturation, SES, cholesterol intake, CHO intake, cigarette smoking |
| Triglycerides at > 2to 5 years: 1 cohort study; 2 analyses (n = 1163) in boys and girls aged 14 years | ||||||||
|
Appannah 2015 14 years; regression |
Triglycerides | % | 3 | Energy‐dense, high‐fat and low‐fibre dietary patternb (multiple FFQs at baseline and 3 years) | z‐score | n girls = 558, regression result. B = 1 (95% CI 0 to 3). After 3 years, for every 1 z‐score increase in the dietary pattern, triglycerides will increase by 1% in girls. |
+ NA; exposure includes energy intake |
Adjusted for age, dietary misreporting, physical fitness, smoking and BMI z‐score. |
|
Appannah 2015 14 years; regression |
Triglycerides | % | 3 | Energy‐dense, high‐fat and low‐fibre dietary patternb (multiple FFQs at baseline and 3 years) | z‐score | n boys = 605, regression result. B = 1 (95% CI 0 to 3). After 3 years, for every 1 z‐score increase in the dietary pattern, triglycerides will increase by 1% in boys |
+ NA; exposure includes energy intake |
Adjusted for age, dietary misreporting, physical fitness, smoking and BMI z‐score. |
aDirection refers to whether there was a positive (+: exposure and outcome moved in the same direction), inverse/negative (‐: exposure and outcome moved in opposite directions) or zero (0: no association) between total fat intake and the outcome.
b"Energy dense, high fat, low fibre" dietary pattern was defined as high intakes of processed meat, chocolate and confectionery, low‐fibre bread, crisps and savoury snacks, fried and roasted potatoes, the high intake of these foods increase the individual’s dietary pattern z‐score.
%TE: percentage of total energy; B: unstandardised beta‐coefficient; BMI: body mass index; CHO: carbohydrate; FFQ: food frequency questionnaire; LDL‐C: low‐density lipoprotein cholesterol; HDL‐C: high‐density lipoprotein cholesterol; NA: not applicable; SE: standard error; SES: socioeconomic status.
After three years, Appannah 2015 reported that:
for every 1 z‐score increase in the high‐fat dietary pattern, LDL‐cholesterol increased by 0.04 mmol/L in girls (n = 558); and by 0.001 mmol/L in boys (n = 605);
for every 1 z‐score increase in the high‐fat dietary pattern, HDL‐cholesterol increased by 0.02 mmol/L in girls (n = 558), and by 0.002 in boys (n = 605);
for every 1 z‐score increase in the high‐fat dietary pattern, triglycerides increased by 1% in girls (n = 558), and boys (n = 605).
Boreham 1999 examined the association between total fat intake (%TE) and HDL‐cholesterol in girls (n = 230) after three years, and reported that for every 1% increase in energy intake from total fat, HDL‐cholesterol decreased by 0.21 mmol/L (with adjustment for energy intake).
We have summarised standardised and unstandardised SBP and DBP outcomes, fat intake exposure variables and results of reported associations within each time range, in Table 17, including adjustments made. Two studies related SBP and DBP to total fat intake per 10 g at two years (Setayeshgar 2017; n = 310), and in absolute grams at three years (Obarzanek 1997 (cohort); n = not reported). According to Setayeshgar 2017, with no adjustment for TE intake, for every 10 g increase in total fat intake, SBP increased by 0.03 z‐scores and DBP increased by 0.03 z‐scores. With adjustment for TE intake, for every 1 g increase in total fat intake, SBP increased by 0.4 mmHg and DBP increased by 0.43 mmHg (Obarzanek 1997 (cohort)).
14. Results of cohort studies: blood pressure.
|
Study ID; mean age at baseline; analysis |
Outcome | Outcome units | Time point (year) | Exposure | Exposure unit | Results of association (all reported values) |
Direction;a energy intake adjusted (yes/no) |
Matched groups or adjusted for (or both) |
| SBP at > 1to 2 years: 1 cohort study; 1 analysis (n = 310) in boys and girls aged 13 years | ||||||||
|
Setayeshgar 2017 12.5 years; regression |
SBP | z‐score | 2 | Total fat intake (single 24‐hour recall at baseline) | per 10 g | n overall = 310; regression result. β = 0.03 (95% CI 0.00004 to 0.06), P < 0.05. After 2 years, for every 10 g increase in total fat intake, SBP will increase by 0.03 z‐score |
+ No |
Adjusted for baseline BMI z‐score, baseline SBP and DBP, moderate to vigorous physical activity, vegetables and fruit, fibre, milk, sodium and added sugar. |
| SBP at > 2to 5 years: 1 cohort study; 1 analysis (n = NR) in boys and girls aged 10 years | ||||||||
|
Obarzanek 1997 (cohort) 9.6 years; regression |
SBP | mmHg | 3 | Total fat intake (multiple 24‐hour recalls at baseline, 1 and 3 years) | g | n overall = NR; regression result. B = 0.4, P < 0.1. After 3 years, for every 1 g increase in total fat intake, SBP will increase by 0.4 mmHg |
+ Yes |
Adjusted for height, weight and gender, with all sources of calories in the model. |
| DBP at > 1to 2 years: 1 cohort study; 1 analysis (n = 310) in boys and girls aged 13 years | ||||||||
|
Setayeshgar 2017 12.5 years; regression |
DBP | z‐score | 2 | Total fat intake (single 24‐hour recall at baseline) | per 10 g | n overall = 310. β = 0.03 (95% CI 0.003 to 0.05), P < 0.05. After 2 years, for every 10 g increase in total fat intake, DBP will increase by 0.03 z‐scores |
+ No |
Adjusted for baseline BMI z‐score, baseline SBP and DBP, moderate to vigorous physical activity, vegetables and fruit, fibre, milk, sodium and added sugar. |
| DBP at > 2to 5 years: 1 cohort study; 1 analysis (n = NR) in boys and girls aged 10 years | ||||||||
|
Obarzanek 1997 (cohort) 9.6 years; regression |
DBP | mmHg | 3 | Total fat intake (multiple 24‐hour recalls at baseline, 1 and 3 years) | g | n overall = NR. B = 0.43, 0.01 < P < 0.06. After 3 years, for every 1 g increase in total fat intake, DBP will increase by 0.43 mmHg |
+ Yes |
Adjusted for height, weight and gender, with all sources of calories in the model. |
aDirection refers to whether there was a positive (+: exposure and outcome moved in the same direction), inverse/negative (‐: exposure and outcome moved in opposite directions) or zero (0: no association) between total fat intake and the outcome.
B: unstandardised beta coefficient; β: standardised beta‐coefficient; BMI: body mass index; CI: confidence interval; DBP: diastolic blood pressure; NR: not reported; SBP: systolic blood pressure.
Height
We have summarised the various standardised and unstandardised height outcomes, total fat exposure variables and results of reported associations within each time range, including adjustments made, in Table 18.
15. Results of cohort studies: height.
|
Study ID; mean age at baseline; analysis |
Outcome | Outcome units | Time point (year) | Exposure | Exposure unit | Results of association (all reported values) |
Direction;a energy intake adjusted (yes/no) |
Matched groups or adjusted for (or both) |
| Height at 1 year: 2 cohort studies; 2 analyses (n ˜ 740) in children aged 2‐6 years | ||||||||
|
Niinikoski 1997a 2 years; mean end values per group |
Relative heightb | % | 1 | Total fat intake (single 4‐day dietary record at baseline, 1.5 and 2 years) | LF (27.7‐28.7 %TE); HF (> 28.7 %TE) |
n overall = 740 (LF = 35, HF = 705); mean end values (SD). Baseline: LF = 0.30 (0.9); HF = 0.32 (0.9). At 1 year: LF = 0.18 (1.0); HF = 0.16 (0.9); P = 0.93. After 1 year, on average children with LF intake (27.7‐28.7 %TE) have a relative height change of 0.12% compared to 0.16% for children with HF intake (> 28.7 %TE). |
‐ No |
No matching reported. No adjustment for prognostic variables. |
|
Tershakovec 1998 (cohort) 6.2 years; mean end values per group |
Height | z‐score | 1 | Total fat intake (multiple 24‐hour dietary recalls at baseline and 1 year) | LF quintile (24%TE) HF quintile (34%TE) |
n overall = NR (LF = NR, HF = NR); mean end values (SD NR). Baseline: LF = ‐0.23; HF = 0.17. At 1 year: LF = ‐0.11; HF = 0.22. After 1 year, on average children in LF intake (24%TE) quintile gain 0.12 z‐score in height while children in HF intake (34%TE) quintile gain 0.05 z‐score in height. |
+ No |
No matching reported. No adjustment for prognostic variables. |
| Height at > 1to 2 years: 2 cohort study; 3 analysis (n = 836) in boys and girls aged 2‐4 years | ||||||||
|
Ambrosini 2016 3.6 years; mean end values per group |
Height | cm | 1.5 | Total fat intake (single 3‐day unweighed food record at baseline) | LF quintile (30.4%TE) HF quintile (41.8%TE) |
n boys, at baseline = 439; at 1.5 years = 387 (LF = NR, HF = NR); mean end values (SD). Baseline: LF = 99.9 (95% CI 99.2 to 100.5); HF = 99.3 (95% CI 98.7 to 99.9). At 1.5 years: LF = 110.7 (95% CI 109.9 to 111.5); HF = 109.9 (95% CI 109.1 to 110.7). After 1.5 years, on average boys with LF intake (30.4%TE) quintile gain 10.8 cm in height while boys with HF intake (41.8%TE) quintile gain 10.6 cm in height. |
‐ No |
No matching reported. No adjustment for prognostic variables. |
|
Ambrosini 2016 3.6 years; mean end values per group |
Height | cm | 1.5 | Total fat intake (single 3‐day unweighed food record at baseline) | LF quintile (30.4%TE) HF quintile (41.8%TE) |
n girls, at baseline = 351; at 1.5 years = 323) (LF = NR, HF = NR); mean end values (SD). Baseline: LF = 99.9 (95% CI 98.0 to 99.8). HF = 98.3 (95% CI 97.6 to 99.1). At 1.5 years: LF = 110.0 (95% CI 108.9 to 111.1); HF = 109.3 (95% CI 108.3 to 110.3). After 1.5 years, on average girls in LF intake (30.4%TE) quintile will gain10.1 cm in height while girls in HF intake (41.8%TE) quintile will gain 11 cm in height. |
+ No |
No matching reported. No adjustment for prognostic variables. |
|
Magarey 2001 2 years; mean end values per group |
Height | cm | 2 | Total fat intake (single 3‐day weighed dietary records at baseline and 2 years) | LF < 30%TE HF > 35%TE |
n overall = 126 (LF = 14, HF = 112); mean end values (SD). Baseline: LF = 86.1 (2.6); HF = 87.7 (3.3). At 2 years: LF (n = 20) = 107 (5.5); HF (n = 76) = 106 (3.9); P = NS. After 2 years, on average children with LF intake (< 30%TE) gain 20.9 cm in height, while children with HF intake > 35%TE) gain 18.3 cm in height. |
‐ No |
No matching reported. No adjustment for prognostic variables. |
| Height at > 2to 5 years: 3 cohort studies; 3 analyses (n = 973) in boys and girls aged 2‐10 years | ||||||||
|
Shea 1993 4.4 years; mean change per group |
Height | cm/year | 2.1 | Total fat intake (multiple FFQs at baseline) | LF ≤ 30%TE HF > 30%TE |
n overall = 215 (LF = 37, HF = 178), mean change (SD). Baseline: LF = 6.8 (1.4); HF = 6.4 (0.8); P > 0.05. MD 0.2 (95% CI ‐0.24 to 0.64). After 2 years, LF intake (≤ 30%TE) will result in a 0.2 cm/year greater increase in height on average compared to HF intake (> 30%TE). |
‐ No |
No matching reported. No adjustment for prognostic variables. |
|
Obarzanek 1997 (cohort) 9.6 years regression |
Height | cm | 3 | Total fat intake (multiple 24‐hour recalls at baseline, 1 and 3 years) |
%TE | n overall = 632; regression results. B = ‐0.0009, P = 0.6. After 3 years, for every 1% increase in energy intake from fat, height in children will decrease by 0.0009 cm on average. |
‐ Yes |
Adjusted for gender, physical activity, treatment, visit number, other sources of energy than fat, and for interactions: fat intake‐by‐treatment, fat intake‐by‐gender, fat intake‐by‐visit number and visit number‐by‐treatment. |
|
Magarey 2001 2 years; mean end values per group |
Height | cm | 4 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years) | LF < 30%TE HF > 35%TE | n overall = 126 (LF = 14, HF = 112); mean end values (SD). Baseline: LF = 86.1 (2.6); HF = 87.7 (3.3). At 4 years: LF (n = 14) = 114 (5.5); HF (n = 88) = 116 (4.3); P > 0.05. After 4 years, on average children with LF intake (< 30%TE) gain 27.9 cm in height, while children with HF intake (> 35%TE) gain 28.3 cm in height. |
+ No |
No matching reported. No adjustment for prognostic variables. |
| Height at > 5to 10 years: 1 cohort study; 1 analysis (n = 126) in boys and girls aged 2 years | ||||||||
|
Magarey 2001 2 years; mean end values per group |
Height | cm | 6 | Total fat intake (single 3‐day weighed dietary record at baseline, 2 and 4 years; single 4‐day weighed dietary record at 6 years) | LF < 30%TE HF > 35%TE | n overall = 126 (LF = 14, HF = 112); mean end values (SD). Baseline: LF = 86.1 (2.6); HF = 87.7 (3.3). At 6 years: LF (n = 13) = 131 (7.7); HF (n = 72) = 128 (5.2); P > 0.05. At 6 years, on average children in LF intake (< 30%TE) gain 44.9 cm in height while children in HF intake (> 35%TE) gain 40.3 cm in height. |
‐ No |
No matching reported. No adjustment for prognostic variables. |
aDirection refers to whether there was a positive (+: exposure and outcome moved in the same direction), inverse/negative (‐: exposure and outcome moved in opposite directions) or zero (0: no association) between total fat intake and the outcome.
bRelative height, deviation in percentages from the mean height of healthy Finnish children of the same height and gender.
%TE: percentage of total energy; FFQ: Food Frequency Questionnaire; LF: low fat; HF: high fat; MD: mean difference; NA: not applicable; NR: not reported; SD: standard deviation.
After one year, Niinikoski 1997a (n = 740) reported that on average children with low‐fat intake (27.7% to 28.7%TE) had a relative height change of 0.12% compared to 0.16% for children with high‐fat intake (> 28.7%TE), with no adjustment for energy intake. After one year, Tershakovec 1998 (cohort) (n = not reported) found that on average children in the low‐fat intake (24%TE) quintile gained 0.12 z‐scores in height while children in the high‐fat intake (34%TE) quintile gained 0.05 z‐scores in height.
After 1.5 years, Ambrosini 2016 reported that boys (n = 387) in the low‐fat intake (30.4%TE) quintile gained 10.8 cm in height on average, while boys in the high‐fat intake (41.8%TE) quintile gained 10.6 cm; girls (n = 323) in the low‐fat intake quintile gained 10.1 cm in height on average, while high‐fat intake quintile girls gained 11 cm on average. Magarey 2001 reported mean end values for height in the lower and higher fat intake groups after two years; children with low‐fat intake (< 30%TE) gained 20.9 cm in height on average, while children with high‐fat intake (> 35%TE) gained 18.3 cm in height (n = 126).
Shea 1993 (n = 215) reported that low‐fat intake (≤ 30%TE) resulted in a 0.2 cm/year greater increase in height on average compared to high‐fat intake (> 30%TE) at 25‐month follow‐up. At three years, Obarzanek 1997 (cohort) reported that for every 1% increase in energy intake from fat, the increase in children's height decreased by 0.0009 cm on average; and at four years, Magarey 2001 found that average children with low‐fat intake (< 30%TE) had gained 27.9 cm in height, on average, while children with high‐fat intake (> 35%TE) gained 28.3 cm in height.
Magarey 2001 (n = 126) reported that at six years children with low‐fat intake (< 30%TE) had gained 44.9 cm in height, while children with high‐fat intake (> 35%TE) had gained 40.3 cm in height.
Discussion
Summary of main results
Our review aimed to assess the effects of total fat intake on measures of weight and body fatness in children and young people not aiming to lose weight. We included 24 studies comprising three parallel‐group RCTs (reported in 12 records) and 21 prospective cohort studies (92 eligible analyses, reported in 47 records), with 23 being conducted in high‐income countries.
Randomised controlled trials
Although RCT evidence was limited to one study reporting the same outcome per time point range, and by lack of results for all important outcomes at all time points, the evidence was of low to moderate quality for most outcomes (Table 1; all time points presented in Appendix 2).
Body mass index and weight
Compared to fat intake greater than 30% of total energy (TE), lower total fat intake (30%TE or less) probably decreased BMI in children over a period of one to two years (MD ‐1.5 kg/m2, 95% CI ‐2.45 to ‐0.55; 1 RCT; n = 191; moderate‐quality evidence). This finding was not consistent for comparisons in children at six to 12 months' follow‐up (1 RCT; n = 620; low‐quality evidence) nor over the longer follow‐up periods. Lower total fat intake (30%TE or less) compared to usual/modified fat intake may make little or no difference to weight (kg) in children over a five‐year period (MD ‐0.60 kg, 95% CI ‐2.39 to 1.19; 1 RCT; n = 612; low‐quality evidence), and we are uncertain if it has an effect on weight‐for‐age in children over a 12‐month period (1 RCT; n = 149; very low‐quality evidence). It should be noted that none of the included trials set out to answer the review question whether lower fat compared to higher fat diets are safe and effective for preventing abnormal weight gain over the longer term, in generally healthy children with healthy bodyweights.
Blood lipids
Lower total fat intake (30%TE or less) probably slightly reduced total cholesterol in children over a 12‐month period when compared to fat intake greater than 30%TE (MD ‐0.15, 95% CI ‐0.24 to ‐0.06; 1 RCT; n = 618; moderate‐quality evidence), but may make little or no difference over longer time periods (1 RCT per outcome per time point range, low‐quality evidence). Compared to fat intake greater than 30%TE, lower total fat intake (30%TE or less) probably decreased LDL‐cholesterol in children over a 12‐month period (MD ‐0.12 mmol/L, 95% CI ‐0.20 to ‐0.04; 1 RCT; n = 618, moderate‐quality evidence) and over a two‐ to five‐year period (MD ‐0.09, 95% CI ‐0.17 to ‐0.01; 1 RCT; n = 623; moderate‐quality evidence). Lower total fat intake (30%TE or less) versus fat intake greater than 30%TE probably made little or no difference to HDL‐cholesterol (1 study, moderate‐quality evidence) and triglycerides in children over the various reported time point ranges (1 study, low‐ to moderate‐quality evidence).
Height
When compared to fat intake greater than 30%TE, we were uncertain whether lower total fat intake (30%TE or less) reduced height‐for‐age in children over a 12‐month period (1 study; very low‐quality evidence) and may have made little or no difference to height in children over a longer time point ranges (1 RCT per outcome per time point range, low‐quality evidence).
Dietary intakes
Both RCTs that reported dietary intake data at various time point ranges show that, compared to the group with fat intake greater than 30%TE, children with lower fat intake had lower TE intake from total and saturated fat intake (%TE) and consequently greater proportions of total energy from carbohydrates and protein at all reported time point ranges. This indicates a certain level of adherence to the lower and higher fat diets being compared in these two trials.
Summary of evidence from randomised controlled trials
In summary, limited evidence from three trials in high‐income countries that randomised 1054 children to a lower total fat intake (30%TE or less) versus usual or modified fat intake, but with no intention to reduce weight, showed small reductions in BMI, total‐cholesterol and LDL‐cholesterol at some time points with lower fat intake compared to controls, and no consistent differences in effects on weight and HDL‐cholesterol. There were no adverse effects on height. Inclusion of hypercholesteraemic children in two trials may limit generalisability of these findings.
Cohort studies
We identified more eligible evidence in prospective cohort studies, but heterogeneous reporting and methods across studies, and the judgements of predominantly very low‐quality evidence, made it difficult to draw any firm conclusions. Thus, the true relationships may be substantially different from those reported (Table 2; all time points presented in Appendix 3). Bearing the quality of evidence in mind, and although measures of total fat intake, magnitudes of associations and adjustments for prognostic variables in the included cohort studies varied considerably, over half of the included analyses that reported on primary outcomes suggested that total fat intake and body fatness measures moved in the same direction.
In trying to form a general picture for BMI, as one of the key outcomes, 11/18 analyses that explicitly adjusted for TE intake showed small positive associations, and 6/18 showed small inverse associations, between various measures of total fat intake and BMI across the various time point ranges. In analyses that did not adjust for energy intake (or where this was not applicable as it was included as part of the exposure variable), 9/14 analyses reported positive relationships between various total fat intake measures and BMI across time points.
Most of the included cohort studies were not designed to primarily answer the question whether total fat intake during childhood is a risk factor for abnormal weight gain, but sought to examine the relationship between total fat intake over time in normal weight children and its influence on or relationship with measures of body fatness.
Summary of evidence from cohort studies
In summary, associations in 21 prospective cohort studies (n = approximately 25,059 completed), of which 20 were done in high‐income countries, that related total fat intake to later measures of body fatness in children were inconsistent and the quality of this evidence was mostly very low, meaning that the true effect is likely to be substantially different form the estimate of effect.
Overall completeness and applicability of evidence
We searched and screened carefully to identify all relevant RCTs and cohort studies in children that assessed the relationship between total fat intake and measures of body fatness. We searched for trials that lowered total fat intake to 30%TE or less in one group and not in the other group, regardless of the primary aims or outcomes mentioned in the title or abstracts. The three trials reported the same outcomes but at different time points, which meant that only one study reported the same outcome in the same predefined time point range for all eligible outcomes. Findings from two of the three RCTs were directly applicable only to children with raised blood lipids in high‐income countries, with limited applicability in generally healthy children in all settings.
We may have been limited in how well we were able to assess completeness for cohort studies, where the risk of missing studies was perhaps greater, since relevant analyses may be described and reported in the text and did not appear in the title or abstract. Findings of all except three of the cohorts were directly applicable to generally healthy children in high‐income countries, with limited applicability in LMICs.
Quality of the evidence
The reporting of trials included in this review was generally poor, with very heterogeneous methods and approaches used for outcomes and exposures. We contacted study authors but did not receive timely responses from most authors. Our risk of bias assessment identified the following to be of high concern in the cohort studies: selection bias (related mainly to the lack of matching or adjusting for all key prognostic variables), and attrition bias and detection bias (related to mostly to assessment of exposure).
Our GRADE assessments for RCTs (Table 1; Appendix 2) varied from very low to moderate, and in cohort studies (Table 2; Appendix 3) from very low to low in one outcome, which means that future research is likely to impact on the findings. Therefore, our confidence in the validity of the findings was limited.
We considered the GRADE domain indirectness to be problematic in this body of evidence as two trials only included children with raised blood lipids (Obarzanek 2001 (RCT); Tershakovec 1998 (RCT)). For the GRADE domain imprecision, it was very difficult to come up with specific thresholds for benefit or harm for our outcomes due to the many other factors that influence these outcomes, as is often the case with nutrition outcomes (especially in children). Therefore, we used the following approach to grading imprecision: we downgraded all outcomes with a 95% CI that crossed the null for serious imprecision, the implication being that any outcome where the intervention or exposure may result in a greater risk of a negative outcome, no matter how small, was downgraded. Conversely, when the 95% CI did not cross the null, we did not downgrade for imprecision if the optimal information size criterion was met (calculation of the number of patients required for an adequately powered individual trial), and downgraded if the optimal information size was not met. The exception to downgrading for serious imprecision when a 95% CI crossed the null, was when an outcome had a very narrow 95% CI around the null, such that we were quite confident that the results are reflecting a true null effect.
Potential biases in the review process
The decision to exclude trials that aimed to reduce weight may have led to a lower number of included RCTs. However, this exclusion served to avoid the potential confounding effects of dieting and unconscious energy restriction or other diet changes. Restricting inclusion to studies with a minimum of six months' duration for RCTs or one year' duration for cohorts could have led to missing some potentially eligible studies. However, our question, and the time‐dependant nature of the relationship between fat intake and our primary outcomes made it important for us to examine this relationship over a longer period. Trials with a longer follow‐up period ensure that data are relevant to long‐term changes in fatness, which affects longer‐term health. It could also be argued that our choice of predefined time point ranges may have introduced bias. This could not be explored in full due to the inability to perform all sensitivity analyses. However, the two possible sensitivity analyses showed high heterogeneity, as expected, when we ignored predefined time point ranges, and the same outcomes in two trials were pooled at longest and shortest time point ranges.
We were not able to search the reference lists of all included studies and any systematic reviews identified, due to time constraints. Therefore, there is a possibility that we missed some relevant evidence. However, the authors of the previous update did search the bibliographies of all identified systematic reviews up to 2015 for further trials and cohort studies, reducing the risk that we omitted relevant trials.
Behavioural adherence is one of the key determinants of the effects of dietary interventions and, similarly, components of the diet other than total fat are also likely to influence effects on eligible outcomes. We sought to investigate these effects using subgroup analyses, but the data did not allow this. We sought to assess the causal pathway between restriction of energy from fat and weight using subgroup analyses, but this was not possible. Many of the cohort analyses show that energy intake was important in mediating the effect of lowering fat intake on bodyweight. Fourteen of the included studies were published before 2005. With the rising obesity trend, most recent studies focused on weight reduction and were thus ineligible for this review.
Agreements and disagreements with other studies or reviews
Evidence on the link between dietary fat intake and body fatness in non‐obese children across systematic reviews was sparse. Also, findings were limited by the variety of outcome measurements used, and reliable dietary intake and adherence data was at best challenging to obtain. In the previous version of this review, Hooper 2015a (search date November 2014) concluded that the "effect of reducing total fat was not consistently reflected in cohort studies assessing the relationship between total fat intake and later measures of body fatness or change in body fatness in studies of children, young people or adults." Similarly, Rouhani 2016 examined evidence from observational studies (search date January 2015) and 14/37 included studies were in children aged between two and 18 years. They found that in cohort studies (some of which overlapped with our included cohort studies), a higher dietary energy density (including higher fat intakes) was directly associated with weight gain, adjusted means for BMI, and adiposity risk. However, similar to our review, they reported considerable heterogeneity. Additionally, they did not perform analyses separately in children only.
Authors' conclusions
Implications for practice.
Uncertainty remains on the exact relationship between lower total fat intake for maintaining healthy weights in children. Single randomised controlled trials (RCTs) (moderate‐ to low‐quality evidence) found lower body mass index (BMI) with total fat intake at 30% of total energy (30%TE) or less and beneficial effects on total cholesterol and low‐density lipoprotein (LDL) cholesterol, with no meaningful effects on any of the other outcomes. Cohort studies in children generally found no clear and consistent relationship between total fat intake and measures of body fatness over time. Some cohort studies in children suggested no relationship between total fat intake and later measures of body fatness, others showed that greater fat intake led to greater fatness and others found the inverse. Bearing the quality of evidence in mind, and although measures of total fat intake, magnitudes of associations and adjustments for prognostic variables in the included cohort studies varied considerably, over half of the included analyses that reported on primary outcomes suggested that total fat intake and body fatness measures move in the same direction.
Reducing total fat intake in children may be one of the ways in which total energy intake could be moderated to maintain a healthy weight gain, and prevent overweight and obesity in children, along with other complementary approaches at individual, household, community and population levels.
Implications for research.
High‐quality longer‐term trials and prospective cohort studies, published using recommended reporting guidelines, are needed to investigate the effects of lower fat intake on bodyweight in children in low‐ and middle‐income countries (LMICs), including both possible benefits and risks. There are ethical issues that would need to be considered in such trials and studies in children from LMICs. Diets in LMICs are traditionally cereal or tuber‐based, with a relatively low energy density. Adequate energy density in children's diets is one of the main requirements to support proper growth and development. In most foods, energy density is highly correlated to fat content, and this is one of the main benefits of dietary fat for children in LMICs. Dietary fat also ensures the absorption of essential fatty acids and fat‐soluble vitamins, such as vitamin A, which themselves are often in poor supply in traditional diets. If provided by the diet, low amounts of dietary fat will facilitate adequate absorption of these vitamins (Jayarajan 1980; Ribaya‐Mercado 2007). The heavy burden of infectious and parasitic diseases in young children in poor areas of LMICs is also important to consider, and the growth‐limiting effects of diseases such as diarrhoea, and interactions with diet, are well known. However, the nutrition transition has happened extremely rapidly in many LMICs, with swift departure from traditional diets. These transitions are accompanied by rapidly increasing levels of obesity and its comorbidities (de Onis 2010; GBD 2017a; UNICEF 2017; WHO 2016). This double burden imposes difficult challenges for the design and conduct of nutrition trials and studies in children, and consequently for the development of evidence‐informed dietary recommendations. Guidance from initiatives such as the Standards for Research (StaR) in Child Health may be helpful, as this aims to address the paucity and limitations of paediatric clinical trials in all settings (Van't Hoff 2015).
There is a need for new longer‐term studies that are designed specifically to answer the question of whether lower fat compared to higher fat diets are safe and effective for preventing abnormal weight gain, overweight or obesity in the long term in generally healthy children with healthy bodyweights. Specific elements that would need to be considered in the design of such studies include valid dietary intake methodology, clear definitions of abnormal weight gain, age, maturation status, socioeconomic status, parental weight status, food environments and physical activity. Importantly, total energy intake and dietary components other than total fat are also very important to consider when designing such studies. Examining higher versus lower total fat intakes as part of well‐defined dietary pattern interventions, or considering total fat intake as part of well‐defined dietary pattern exposures, are approaches that could be used to better understand the answer to this question. Importantly, estimates of dietary intakes in longer term studies should not be based on a single dietary assessment at recruitment with the assumption that neither individual dietary habits nor the composition of the food supply will not change during follow‐up periods, as has been assumed in many prospective cohort studies previously. A focus on investigating the effects of total fat intake on abnormal weight gain (relative to linear growth) in school‐aged children may be justified. Additionally, consistency in methods of analyses used and consistency in reporting in these studies should be improved to enable more efficient synthesis of this evidence base to better inform policy and practice.
What's new
| Date | Event | Description |
|---|---|---|
| 27 June 2018 | Amended | Abstract and plain language summary revised to clarify the nature of the intervention delivered. |
| 27 June 2018 | New citation required but conclusions have not changed | The edits made to the abstract do not impact on the interpretation of the results or on the review conclusions. |
History
Review first published: Issue 2, 2018
| Date | Event | Description |
|---|---|---|
| 8 February 2018 | New citation required and conclusions have changed | The previous version of this review (Hooper 2015a) included both children and adults. However, this update is a WHO commission specifically addressing the question of the effects of total fat intake on bodyweight in children only. Therefore the conclusions have changed. |
| 9 November 2017 | New search has been performed | With the aim of ensuring all relevant data in children is summarised, the WHO commissioned an expedited update of this systematic review (Hooper 2015a) in children only. Previously the review included both children and adults. |
| 19 August 2016 | Feedback has been incorporated | Comment and authors' response added. |
| 2 March 2016 | Amended | The description of data included in the main analysis for the WHI study was incorrect, so the entry for the "Characteristics of Included Studies" table now reflects that the weight, BMI and waist circumference data used in the main analyses were 7.5 year follow up data (as is appropriate). The data in the forest plots were already correct. Additionally the main reference for WHI is now indicated as the paper that provides this 7.5 year follow up data. The first paragraph of the text on "Associations between total dietary fat in youth and measures of body fatness in children, young people and adults (as seen in cohorts)" was unclear, so we have tried to clarify these results. Table 2 is helpful to read in understanding this section. |
| 21 July 2015 | New search has been performed | The searches were run on 12 November 2014. |
| 11 July 2015 | New citation required and conclusions have changed | We split a previously published review (Reduced and modified dietary fat for preventing cardiovascular disease, DOI: 10.1002/14651858.CD002137.pub3) into six smaller review updates. The conclusions are therefore now focused on the effects of total fat intake on body weight instead of the effects of reducing or modifying fat intake overall on cardiovascular disease risk. At the request of the World Health Organization (WHO) Nutrition Guidance Expert Advisory Group (NUGAG) group we extended this review to include cohort studies, and studies in children and young people. This split review update includes 32 randomised controlled trials and also 30 sets of analyses of 25 cohorts. |
| 11 June 2010 | New citation required and conclusions have changed | — |
| 9 September 2008 | Amended | — |
| 1 February 2000 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
We thank the following people:
Lee Hooper and coauthors of the previous reviews on total fat intake and bodyweight in both adults and children;
Vittoria Lutje for the search strategy and searches conducted;
Selvan Naidoo (SN) for assistance with screening and data extraction;
Toby Lasserson, Helen Wakeford and Kerry Dwan from the Cochrane Editorial and Methods Department for invaluable assistance throughout the review process;
Authors of included studies who provided requested study information;
WHO for funding.
Appendices
Appendix 1. New search strategies for this review in children: 23 May 2017
Database: Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily, Ovid MEDLINE and Versions(R) <1946 to present>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Dietary Fats/ or Diet, High‐Fat/
2 Diet, Fat‐Restricted/
3 ("dairy fat*" or "dietary fat*" or "fat intake" or "reduced fat*" or "reducing fat*" or "low fat").ti.
4 ("dairy fat*" or "dietary fat*" or "fat intake" or "reduced fat*" or "reducing fat*" or "low fat").ab.
5 ("lower fat" or "lowered fat" or "modified fat" or "modifying fat" or "animal fat*" or "total fat" or "milk fat").ab.
6 ("lower fat" or "lowered fat" or "modified fat" or "modifying fat" or "animal fat*" or "total fat" or "milk fat").ti.
7 ("energy from fat" or "calories from fat" or " kilojoules from fat" or "plasma lipid*" or "serum lipid*").ti. or ("energy from fat" or "calories from fat" or " kilojoules from fat" or "plasma lipid*" or "serum lipid*").ab.
8 cholesterol/ or lipoproteins/ or Hypercholesterolemia/
9 ("blood lipid*" or cholesterol or lipoprotein* or LDL or HDL or LDL HDL or "total HDL" or "apolipoprotein(a) OR apo(a)").ti.
10 ("blood lipid*" or cholesterol or lipoprotein* or LDL or HDL or LDL HDL or "total HDL" or "apolipoprotein(a)" or "apo(a)").ab.
11 1 or 2 or 3 or 4 or 5 or 6 or 7
12 8 or 9 or 10
13 11 and 12
14 triglycerides/ or Hypertriglyceridemia/
15 ("energy from fat" or "calories from fat" or " kilojoules from fat").ab. or ("energy from fat" or "calories from fat" or " kilojoules from fat").ti.
16 1 or 2 or 3 or 4 or 5 or 6 or 15
17 ("plasma lipid*" or "serum lipid*").ab. or ("plasma lipid*" or "serum lipid*").ti.
18 8 or 9 or 10 or 17
19 16 and 18
20 (triglycer* or triacylgly* or "TG HDL" or hypertriglycer*).ab. or (triglycer* or triacylgly* or "TG HDL" or hypertriglycer*).ti.
21 14 or 20
22 16 and 21
23 Birth Weight/
24 exp bodyweight/
25 24 not 23
26 body mass index/ or waist circumference/ or obesity/ or waist‐hip ratio/ or body fat distribution/ or Skinfold thickness/
27 Abdominal Fat/de, gd [Drug Effects, Growth & Development]
28 ("body mass" or weight or weights or BMI or overweight or obesity or obese or "waist circumference*").ab. or ("body mass" or weight or weights or BMI or overweight or obesity or obese or "waist circumference*").ti.
29 ("waist‐hip" or "waist‐to‐hip" or "body fat" or "body fats" or adipos* or "percentage body fat*" or "metabolic syndrome").ab. or ("waist‐hip" or "waist‐to‐hip" or "body fat" or "body fats" or adipos* or "percentage body fat*" or "metabolic syndrome").ti.
30 25 or 26 or 27 or 28 or 29
31 16 and 30
32 blood pressure/ or hypertension/
33 ("blood pressure*" or hypertension or systolic or diastolic).ab. or ("blood pressure*" or hypertension or systolic or diastolic).ti.
34 32 or 33
35 16 and 34
36 exp Diabetes Mellitus, Type 2/
37 (diabetes or MODY or NIDDM).ab. or (diabetes or MODY or NIDDM).ti.
38 36 or 37
39 16 and 38
40 insulin resistance/ or glucose Intolerance/ or blood glucose/ or hyperglycemia/
41 (insulin or glucose or hyperglycem* or hyperinsulin* or "insulin sensitiv*").ab. or (insulin or glucose or hyperglycem* or hyperinsulin* or "insulin sensitiv*").ti.
42 40 or 41
43 16 and 42
44 "Growth and Development"/
45 *Growth/
46 Body height/ or Adolescent Development/ or Child Development/
47 (growth or development).ab. or (growth or development).ti.
48 Body Size/
49 44 or 45 or 46 or 47 or 48
50 16 and 49
51 19 or 22 or 31 or 35 or 39 or 43 or 50
52 child/ or adolescent/ or young adult/
53 (child or children or adolescent or adolescents or adolescence or teen or teens or teenager or teenagers or youth or youths or childhood or "young adult" or "young adults").ab. or (child or children or adolescent or adolescents or adolescence or teen or teens or teenager or teenagers or youth or youths or childhood or "young adult" or "young adults").ti.
54 52 or 53
55 51 and 54
56 Randomized Controlled Trial/
57 Controlled Clinical Trial/
58 controlled clinical trial.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
59 (randomised or placebo).ab. or (randomised or placebo).ti.
60 (trial or groups or randomly).ab.
61 56 or 57 or 58 or 59 or 60
62 drug therapy.fs.
63 61 or 62
64 55 and 63
65 (randomi?ed or double‐blind* or single‐blind*).ab. or (randomi?ed or double‐blind* or single‐blind*).ti.
66 63 or 65
67 55 and 66
68 cohort studies.mp.
69 cohort study/
70 epidemiological studies.mp.
71 ("follow‐up" or longitudinal or cross‐sectional or cohort*).ab. or ("follow‐up" or longitudinal or cross‐sectional or cohort*).ti.
72 68 or 69 or 70 or 71
73 55 and 72
74 67 or 73
Database: Embase 1947‐Present, updated daily
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Dietary Fats/ or Diet, High‐Fat/
2 Diet, Fat‐Restricted/
3 ("dairy fat*" or "dietary fat*" or "fat intake" or "reduced fat*" or "reducing fat*" or "low fat").ti.
4 ("dairy fat*" or "dietary fat*" or "fat intake" or "reduced fat*" or "reducing fat*" or "low fat").ab.
5 ("lower fat" or "lowered fat" or "modified fat" or "modifying fat" or "animal fat*" or "total fat" or "milk fat").ab.
6 ("lower fat" or "lowered fat" or "modified fat" or "modifying fat" or "animal fat*" or "total fat" or "milk fat").ti.
7 ("energy from fat" or "calories from fat" or " kilojoules from fat" or "plasma lipid*" or "serum lipid*").ti. or ("energy from fat" or "calories from fat" or " kilojoules from fat" or "plasma lipid*" or "serum lipid*").ab.
8 cholesterol/ or lipoproteins/ or Hypercholesterolemia/
9 ("blood lipid*" or cholesterol or lipoprotein* or LDL or HDL or LDL HDL or "total HDL" or "apolipoprotein(a) OR apo(a)").ti.
10 ("blood lipid*" or cholesterol or lipoprotein* or LDL or HDL or LDL HDL or "total HDL" or "apolipoprotein(a)" or "apo(a)").ab.
11 1 or 2 or 3 or 4 or 5 or 6 or 7
12 8 or 9 or 10
13 11 and 12
14 triglycerides/ or Hypertriglyceridemia/
15 ("energy from fat" or "calories from fat" or " kilojoules from fat").ab. or ("energy from fat" or "calories from fat" or " kilojoules from fat").ti.
16 1 or 2 or 3 or 4 or 5 or 6 or 15
17 ("plasma lipid*" or "serum lipid*").ab. or ("plasma lipid*" or "serum lipid*").ti.
18 8 or 9 or 10 or 17
19 16 and 18
20 (triglycer* or triacylgly* or "TG HDL" or hypertriglycer*).ab. or (triglycer* or triacylgly* or "TG HDL" or hypertriglycer*).ti.
21 14 or 20
22 16 and 21
23 Birth Weight/
24 exp bodyweight/
25 24 not 23
26 body mass index/ or waist circumference/ or obesity/ or waist‐hip ratio/ or body fat distribution/ or Skinfold thickness/
27 [Abdominal Fat/
28 ("body mass" or weight or weights or BMI or overweight or obesity or obese or "waist circumference*").ab. or ("body mass" or weight or weights or BMI or overweight or obesity or obese or "waist circumference*").ti.
29 ("waist‐hip" or "waist‐to‐hip" or "body fat" or "body fats" or adipos* or "percentage body fat*" or "metabolic syndrome").ab. or ("waist‐hip" or "waist‐to‐hip" or "body fat" or "body fats" or adipos* or "percentage body fat*" or "metabolic syndrome").ti.
30 25 or 26 or 27 or 28 or 29
31 16 and 30
32 blood pressure/ or hypertension/
33 ("blood pressure*" or hypertension or systolic or diastolic).ab. or ("blood pressure*" or hypertension or systolic or diastolic).ti.
34 32 or 33
35 16 and 34
36 exp Diabetes Mellitus, Type 2/
37 (diabetes or MODY or NIDDM).ab. or (diabetes or MODY or NIDDM).ti.
38 36 or 37
39 16 and 38
40 insulin resistance/ or glucose Intolerance/ or blood glucose/ or hyperglycemia/
41 (insulin or glucose or hyperglycem* or hyperinsulin* or "insulin sensitiv*").ab. or (insulin or glucose or hyperglycem* or hyperinsulin* or "insulin sensitiv*").ti.
42 40 or 41
43 16 and 42
44 "Growth and Development"/
45 *Growth/
46 Body height/ or Adolescent Development/ or Child Development/
47 (growth or development).ab. or (growth or development).ti.
48 Body Size/
49 44 or 45 or 46 or 47 or 48
50 16 and 49
51 19 or 22 or 31 or 35 or 39 or 43 or 50
52 child/ or adolescent/ or young adult/
53 (child or children or adolescent or adolescents or adolescence or teen or teens or teenager or teenagers or youth or youths or childhood or "young adult" or "young adults").ab. or (child or children or adolescent or adolescents or adolescence or teen or teens or teenager or teenagers or youth or youths or childhood or "young adult" or "young adults").ti.
54 52 or 53
55 51 and 54
56 Randomized Controlled Trial/
57 Controlled Clinical Trial/
58 controlled clinical trial.mp.
59 (randomised or placebo).ab. or (randomised or placebo).ti.
60 (trial or groups or randomly).ab.
61 56 or 57 or 58 or 59 or 60
62 drug therapy.fs.
63 61 or 62
64 55 and 63
65 (randomi?ed or double‐blind* or single‐blind*).ab. or (randomi?ed or double‐blind* or single‐blind*).ti.
66 63 or 65
67 55 and 66
68 cohort studies.mp.
69 cohort study/
70 epidemiological studies.mp.
71 ("follow‐up" or longitudinal or cross‐sectional or cohort*).ab. or ("follow‐up" or longitudinal or cross‐sectional or cohort*).ti.
72 cohort analysis/
73 68 or 69 or 70 or 71 or 72
74 55 and 73
75 67 or 74
Search Name: Cochrane library <inception to present>
ID Search Hits
#1 MeSH descriptor: [Diet, Fat‐Restricted] explode all trees
#2 MeSH descriptor: [Diet, High‐Fat] explode all trees
#3 MeSH descriptor: [Dietary Fats] this term only
#4 "dairy fat*" or "dietary fat*" or "fat intake" or "reduced fat" or "reducing fat" or "low fat" or "lower fat" or "lowered fat" or "modified fat" or "modifying fat" or "animal fat* " or "total fat" or "milk fat"
#5 "energy from fat" or "calories from fat" or "kilojoules from fat"
#6 #1 or #2 or #3 or #4 or #5
#7 MeSH descriptor: [Cholesterol] explode all trees
#8 MeSH descriptor: [Lipoproteins] explode all trees
#9 ("blood lipid*" or "cholesterol" or lipoprotein* or "LDL" or "HDL" or "LDL/HDL" or "total/HDL" or "apolipoprotein" or "apo")
#10 MeSH descriptor: [Hypercholesterolemia] explode all trees
#11 "plasma lipid*" or "serum lipid*"
#12 #7 or #8 or #9 or #10 or #11
#13 #6 and #12
#14 MeSH descriptor: [Triglycerides] explode all trees
#15 MeSH descriptor: [Hypertriglyceridemia] explode all trees
#16 triglycer* or triacylgly* or "TG/HDL" or hypertriglycer*
#17 #14 or #15 or #16
#18 #6 and #17
#19 MeSH descriptor: [bodyweight] explode all trees
#20 MeSH descriptor: [Waist Circumference] explode all trees
#21 MeSH descriptor: [Body Mass Index] explode all trees
#22 MeSH descriptor: [Obesity] explode all trees
#23 MeSH descriptor: [Skinfold Thickness] explode all trees
#24 MeSH descriptor: [Body Fat Distribution] explode all trees
#25 MeSH descriptor: [Abdominal Fat] explode all trees
#26 MeSH descriptor: [Waist‐Hip Ratio] explode all trees
#27 "body mass" or "weight" or "weights" or "BMI" or "overweight" or "obesity" or "obese" or "waist circumference" or "waist circumferences" or "waist‐hip" or "waist‐to‐hip" or "body fat" or "body fats" or adipos* or "percentage body fat" or "metabolic syndrome"
#28 #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27
#29 #6 and #28
#30 MeSH descriptor: [Blood Pressure] explode all trees
#31 MeSH descriptor: [Hypertension] explode all trees
#32 blood pressure* or "hypertension" or "systolic" or "diastolic"
#33 #30 or #31 or #32
#34 #6 and #33
#35 MeSH descriptor: [Diabetes Mellitus, Type 2] explode all trees
#36 "diabetes" or "MODY" or "NIDDM"
#37 #35 or #36
#38 #6 and #37
#39 MeSH descriptor: [Insulin Resistance] explode all trees
#40 MeSH descriptor: [Glucose Intolerance] explode all trees
#41 MeSH descriptor: [Blood Glucose] explode all trees
#42 MeSH descriptor: [Hyperglycemia] explode all trees
#43 "insulin" or "glucose" or hyperglycem* or hyperinsulin* or "insulin sensitiv*"
#44 #39 or #40 or #41 or #42 or #43
#45 #6 and #44
#46 MeSH descriptor: [Growth and Development] this term only
#47 MeSH descriptor: [Growth] this term only
#48 MeSH descriptor: [Body Size] this term only
#49 MeSH descriptor: [Body Height] explode all trees
#50 MeSH descriptor: [Child Development] explode all trees
#51 MeSH descriptor: [Adolescent Development] explode all trees
#52 growth or development:ti (Word variations have been searched)
#53 #46 or #47 or #48 or #49 or #50 or #51 or #52
#54 #6 and #53
#55 #13 or #18 or #29 or #34 or #38 or #45 or #54
#56 MeSH descriptor: [Adolescent] explode all trees
#57 MeSH descriptor: [Child] explode all trees
#58 MeSH descriptor: [Young Adult] explode all trees
#59 "child" or "children" or "adolescent" or "adolescents" or "adolescence" or "teen" or "teens" or "teenager" or "teenagers" or "youth" or "youths" or "childhood" or "young adult" or "young adults"
#60 #56 or #57 or #58 or #59
#61 #55 and #60
Pubmed History
| Search | Query |
| #92 | Search (#91) OR #77 Field: Title/Abstract Sort by: PublicationDate |
| #91 | Search (#90) AND #57 Field: Title/Abstract Sort by: PublicationDate |
| #90 | Search (((#89) OR #88) OR "Cohort Studies"[Mesh]) OR "Epidemiologic Studies"[Mesh:NoExp] Field: Title/Abstract Sort by: PublicationDate |
| #89 | Search "follow‐up" or longitudinal or cross‐sectional Field: Title/Abstract Sort by: PublicationDate |
| #88 | Search "cohort*" Field: Title/Abstract Sort by: PublicationDate |
| #87 | Search "cohort*" Sort by: PublicationDate |
| #86 | Search "Cohort Studies"[Mesh] Sort by: PublicationDate |
| #84 | Search "Epidemiologic Studies"[Mesh:NoExp] Sort by: PublicationDate |
| #81 | Search ((((((double‐blind* or single‐blind*)) AND #57)) OR #77)) NOT #77 Sort by: PublicationDate |
| #80 | Search ((((double‐blind* or single‐blind*)) AND #57)) OR #77 Sort by: PublicationDate |
| #79 | Search ((double‐blind* or single‐blind*)) AND #57 Sort by: PublicationDate |
| #78 | Search double‐blind* or single‐blind* Sort by: PublicationDate |
| #77 | Search (#69) NOT #76 Field: Title/Abstract Sort by: PublicationDate |
| #76 | Search (#72) NOT "Humans"[Mesh] Field: Title/Abstract Sort by: PublicationDate |
| #69 | Search (#68) AND #57 Field: Title/Abstract Sort by: PublicationDate |
| #72 | Search "Animals"[Mesh] Field: Title/Abstract Sort by: PublicationDate |
| #75 | Search "Humans"[Mesh] Sort by: PublicationDate |
| #57 | Search (#51) AND #56 Field: Title/Abstract Sort by: PublicationDate |
| #68 | Search (((((((#67) OR #66) OR #65) OR #64) OR #63) OR #62) OR #61) OR #60 Field: Title/Abstract Sort by: PublicationDate |
| #61 | Search controlled clinical trial [pt] Field: Title/Abstract Sort by: PublicationDate |
| #60 | Search "Randomized Controlled Trial" [Publication Type] Field: Title/Abstract Sort by: PublicationDate |
| #65 | Search trial Field: Title/Abstract Sort by: PublicationDate |
| #64 | Search randomly Field: Title/Abstract Sort by: PublicationDate |
| #63 | Search placebo Field: Title/Abstract Sort by: PublicationDate |
| #62 | Search randomised Field: Title/Abstract Sort by: PublicationDate |
| #66 | Search groups Field: Title/Abstract Sort by: PublicationDate |
| #67 | Search drug therapy [sh] Field: Title/Abstract Sort by: PublicationDate |
| #56 | Search (#52) OR #55 Field: Title/Abstract Sort by: PublicationDate |
| #51 | Search ((((((#17) OR #21) OR #33) OR ((((#35) OR #34)) AND #13)) OR #42) OR #46) OR #50 Field: Title/Abstract Sort by: PublicationDate |
| #55 | Search "child" OR "children" OR "adolescent" OR "adolescents" OR "adolescence" OR "teen" OR "teens" OR "teenager" OR "teenagers" OR "youth" OR "youths" OR "childhood" OR "young adult" OR "young adults" Field: Title/Abstract Sort by: PublicationDate |
| #52 | Search "child"[MeSH] OR "adolescent"[MeSH] OR "young adult"[MeSH] Field: Title/Abstract Sort by: PublicationDate |
| #50 | Search (#49) AND #13 Field: Title/Abstract Sort by: PublicationDate |
| #46 | Search (#45) AND #13 Field: Title/Abstract Sort by: PublicationDate |
| #42 | Search (#41) AND #13 Field: Title/Abstract Sort by: PublicationDate |
| #17 | Search (#16) AND #13 Field: Title/Abstract Sort by: PublicationDate |
| #33 | Search (#32) AND #13 Field: Title/Abstract Sort by: PublicationDate |
| #21 | Search (#20) AND #13 Field: Title/Abstract Sort by: PublicationDate |
| #49 | Search (#48) OR #47 Field: Title/Abstract Sort by: PublicationDate |
| #48 | Search growth or development Field: Title/Abstract Sort by: PublicationDate |
| #47 | Search "Growth and Development" [Mesh:NoExp] OR "Growth" [Mesh:NoExp] OR "Body Size" [Mesh NoExp] OR “Body height” [MeSH] OR "Adolescent Development" [Mesh] OR "Child Development" [Mesh] Field: Title/Abstract Sort by: PublicationDate |
| #45 | Search (#44) OR #43 Field: Title/Abstract Sort by: PublicationDate |
| #43 | Search "insulin resistance" [MeSH] OR "glucose Intolerance" [MeSH] OR "blood glucose" [MeSH] OR "hyperglycemia" [MeSH] Field: Title/Abstract Sort by: PublicationDate |
| #44 | Search "insulin" OR "glucose" OR hyperglycem* OR hyperinsulin* OR "insulin sensitiv*" Field: Title/Abstract Sort by: PublicationDate |
| #41 | Search (#38) OR #40 Field: Title/Abstract Sort by: PublicationDate |
| #40 | Search diabetes OR "MODY" OR "NIDDM" Field: Title/Abstract Sort by: PublicationDate |
| #38 | Search "diabetes mellitus, type 2" [MeSH] Field: Title/Abstract Sort by: PublicationDate |
| #37 | Search (((#35) OR #34)) AND #13 Sort by: PublicationDate |
| #36 | Search (#35) OR #34 Sort by: PublicationDate |
| #34 | Search "blood pressure" [MeSH] OR "hypertension" [MeSH] Field: Title/Abstract Sort by: PublicationDate |
| #35 | Search "blood pressure*" OR "hypertension" OR "systolic" OR "diastolic" Field: Title/Abstract Sort by: PublicationDate |
| #32 | Search ((#31) OR #28) OR #27 Field: Title/Abstract Sort by: PublicationDate |
| #27 | Search “body mass” OR "weight” OR "weights” OR "BMI" OR "overweight" OR "obesity" OR “obese” OR "waist circumference" OR "waist circumferences" OR "waist‐hip" OR "waist‐to‐hip" OR "body fat" OR "body fats" OR adipos* OR “percentage body fat” OR “metabolic syndrome” Field: Title/Abstract Sort by: PublicationDate |
| #28 | Search "body mass index" [MeSH] OR "waist circumference" [MeSH] OR "obesity" [MeSH] OR "waist‐hip ratio" [MeSH] OR "body fat distribution" [MeSH] OR “Skinfold thickness” [MeSH] OR "Abdominal Fat/drug effects"[Mesh] OR "Abdominal Fat/growth and development" [Mesh] Field: Title/Abstract Sort by: PublicationDate |
| #31 | Search (#23) NOT #30 Field: Title/Abstract Sort by: PublicationDate |
| #30 | Search (#29) OR #24 Field: Title/Abstract Sort by: PublicationDate |
| #29 | Search “Fetal weight” [MeSH] OR “Thinness” [MeSH] Field: Title/Abstract Sort by: PublicationDate |
| #26 | Search “Fetal weight” [MeSH] OR “Thinness” [MeSH])) OR "body mass index" [MeSH] OR "waist circumference" [MeSH] OR "obesity" [MeSH] OR "waist‐hip ratio" [MeSH] OR "body fat distribution" [MeSH] OR “Skinfold thickness” [MeSH] OR "Abdominal Fat/drug effects"[Mesh] OR "Abdominal Fat/growth and development" [Mesh] Field: Title/Abstract Sort by: PublicationDate |
| #23 | Search "bodyweight" [MeSH] Field: Title/Abstract Sort by: PublicationDate |
| #25 | Search (#23) NOT #24 Field: Title/Abstract Sort by: PublicationDate |
| #24 | Search "birth weight" [MeSH] Field: Title/Abstract Sort by: PublicationDate |
| #20 | Search (#19) OR ("triglycerides" [MeSH] OR "Hypertriglyceridemia"[Mesh]) Field: Title/Abstract Sort by: PublicationDate |
| #19 | Search triglycer* OR triacylgly* OR “TG/HDL” OR hypertriglycer* Field: Title/Abstract Sort by: PublicationDate |
| #18 | Search "triglycerides" [MeSH] OR "Hypertriglyceridemia"[Mesh] Sort by: PublicationDate |
| #16 | Search (("blood lipid*" OR “cholesterol" OR lipoprotein* OR “LDL” OR “HDL” OR “LDL/HDL” OR “total/HDL” OR “apolipoprotein(a)” OR “apo(a)”)) OR #14 Field: Title/Abstract Sort by: PublicationDate |
| #13 | Search (#12) OR (((("Diet, Fat‐Restricted"[Mesh]) OR "Dietary Fats"[Mesh:NoExp]) OR ("Dietary Fats/administration and dosage"[Mesh])) OR "Diet, High‐Fat"[Mesh]) Field: Title/Abstract Sort by: PublicationDate |
| #14 | Search "cholesterol" [MeSH] OR "lipoproteins" [MeSH] OR "Hypercholesterolemia"[Mesh] Field: Title/Abstract Sort by: PublicationDate |
| #15 | Search "blood lipid*" OR “cholesterol" OR lipoprotein* OR “LDL” OR “HDL” OR “LDL/HDL” OR “total/HDL” OR “apolipoprotein(a)” OR “apo(a)” or "plasma lipid*" or "serum lipid*" Sort by: PublicationDate |
| #12 | Search "dairy fat*" OR "dietary fat*" OR "fat intake" OR “reduced fat” OR "reducing fat" OR “low fat” OR "lower fat" OR "lowered fat" OR "modified fat" OR "modifying fat" OR "animal fat* " OR “total fat” OR “milk fat” Field: Title/Abstract Sort by: PublicationDate |
| #11 | Search "dairy fat*" OR "dietary fat*" OR "fat intake" OR “reduced fat” OR "reducing fat" OR “low fat” OR "lower fat" OR "lowered fat" OR "modified fat" OR "modifying fat" OR "animal fat* " OR “total fat” OR “milk fat” Sort by: PublicationDate |
| #10 | Search ((("Diet, Fat‐Restricted"[Mesh]) OR "Dietary Fats"[Mesh:NoExp]) OR ("Dietary Fats/administration and dosage"[Mesh])) OR "Diet, High‐Fat"[Mesh] Sort by: PublicationDate |
| #9 | Search "Diet, Fat‐Restricted"[Mesh] Sort by: PublicationDate |
| #7 | Search "Dietary Fats"[Mesh:NoExp] Sort by: PublicationDate |
| #6 | Search "Dietary Fats/administration and dosage"[Mesh] Sort by: PublicationDate |
| #3 | Search "Diet, High‐Fat"[Mesh] Sort by: PublicationDate |
| #1 | Search "Diet, High‐Fat"[Mesh] OR "Dietary Fats/administration and dosage" [MeSH] OR “Dietary Fats” [MeSH Terms:noexp] OR “Diet, Fat‐Restricted” [MeSH] Sort by: PublicationDate |
Clinicaltrials.gov <inception to present>
"fat intake" OR "dietary fat" or "low fat " or "high fat " | Child
WHO ICTRP <inception to present>
fat intake OR dietary fat or low fat or high fat (limit to: Clinical trials in children)
Appendix 2. Table of findings 1 (RCTs): Total fat intake 30% or less of total energy compared to usual fat intake for body weight in children
| Total fat intake ≤ 30% of total energy compared to usual fat intake for bodyweight in children (RCTs)a | |||||
|
Patient or population: boys and girls aged 24 months to 18 years Setting: paediatric practices, schools and health maintenance organisations in high‐income countries Intervention: lower total fat intake ≤ 30%TE Comparison: usual or modified fat intake | |||||
|
Outcomes (at time point ranges where data were reported) |
No of participants (No of studies) |
Illustrated comparative effect (95% CI) | Quality | What happens | |
| Usual fat intake1 | Effect difference with total fat ≤ 30% of total energy2 | ||||
| Weight‐for‐age z‐score Follow‐up: 6 months | 149 (1 RCT) |
The mean weight‐for‐age z‐score in control group was 0.26 | MD 0.14 lower (0.46 lower to 0.18 higher) | ⊕⊝⊝⊝ Very low3,4,5,6 | We were uncertain whether lower total fat intake (≤ 30%TE) had an effect on weight‐for‐age in children over a 12‐month period (1 study). |
| Follow‐up: range 6 to 12 months | 151 (1 RCT) |
The mean weight‐for‐age z‐score in control group was 0.29 | MD 0.18 lower (0.51 lower to 0.15 higher) | ⊕⊝⊝⊝ Very low3,4,5,6 | |
| Weight (kg) Follow‐up: range 6 to 12 months | 620 (1 RCT) |
The mean weight (kg) in control group was 38.2 | MD 0.5 lower (1.78 lower to 0.78 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | Lower total fat intake (≤ 30%TE) may have made little or no difference to weight in children over a 5‐year period (1 study). |
| Follow‐up: range 2 to 5 years | 612 (1 RCT) |
The mean weight (kg) in control group was 49.5 | MD 0.6 lower (2.39 lower to 1.19 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | |
| BMI (kg/m2) Follow‐up: range 6 to 12 months | 620 (1 RCT) |
The mean BMI (kg/m2) in control group was 18.5 | MD 0.3 lower (0.75 lower to 0.15 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | Lower total fat intake (≤ 30%TE) may have made little or no difference to BMI in children over a 1‐year period (1 study). |
| Follow‐up: range 1 to 2 years | 191 (1 RCT) |
The mean BMI (kg/m2) in control group was 24.8 | MD 1.5 lower (2.45 lower to 0.55 lower) | ⊕⊕⊕⊝ Moderate4,9,10 | Lower total fat intake (≤ 30%TE) probably reduced BMI in children over a period of 1 to 2 years (1 study). |
| Follow‐up: range 2 to 5 years | 541 (1 RCT) |
The mean BMI (kg/m2) in control group was 21.7 | MD 0 (0.63 lower to 0.63 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | Lower total fat intake (≤ 30%TE) may have made little or no difference to BMI in children over a 2 to 5‐year period (1 study). |
| Follow‐up: > 5 years | 576 (1 RCT) |
The mean BMI (kg/m2) in control group was 23.0 | MD 0.1 lower (0.75 lower to 0.55 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | |
| Total cholesterol (mmol/L) Follow‐up: range 6 to 12 months | 618 (1 RCT) |
The mean total cholesterol (mmol/L) in control group was 5.1 | MD 0.15 lower (0.24 lower to 0.06 lower) | ⊕⊕⊕⊝ Moderate4,5,7,11 | Total fat intake ≤ 30%TE probably slightly reduced total cholesterol in children over a 12‐month period (1 study). |
| Follow‐up: range 2 to 5 years | 522 (1 RCT) |
The mean total cholesterol (mmol/L) in control group was 4.6 | MD 0.06 lower (0.17 lower to 0.05 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | Lower total fat intake (≤ 30%TE) may have made little or no difference to total cholesterol in children over a 2 to 5‐year period and a > 5‐year period (1 study). |
| Follow‐up: > 5 years | 548 (1 RCT) |
The mean total cholesterol (mmol/L) in control group was 4.66 | MD 0.02 lower (0.13 lower to 0.09 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | |
| LDL‐C (mmol/L) Follow‐up: range 6 to 12 months | 618 (1 RCT) |
The mean LDL‐C (mmol/L) in control group was 3.29 | MD 0.12 lower (0.2 lower to 0.04 lower) | ⊕⊕⊕⊝ Moderate4,5,7,11 | Lower total fat intake (≤ 30%TE) probably reduced LDL‐C in children over a 12‐month period (1 study) and over a 2 to 5‐year period (1 study). |
| Follow‐up: range 2 to 5 years | 623 (1 RCT) |
The mean LDL‐C (mmol/L) in control group was 3.07 | MD 0.09 lower (0.17 lower to 0.01 lower) | ⊕⊕⊕⊝ Moderate4,5,7,11 | |
| Follow‐up: > 5 years | 548 (1 RCT) |
The mean LDL‐C (mmol/L) in control group was 3.00 | MD 0.01 higher (0.01 lower to 0.03 higher) | ⊕⊕⊕⊝ Moderate4,5,7,12 | Lower total fat intake (≤ 30%TE) probably made little or no difference to LDL‐C in children over a > 5‐year period (1 study). |
| HDL‐C (mmol/L) Follow‐up: range 6 to 12 months | 618 (1 RCT) |
The mean HDL‐C (mmol/L) in control group was 1.47 | MD 0.03 lower (0.08 lower to 0.02 higher) | ⊕⊕⊕⊝ Moderate4,5,7,12 | Lower total fat intake (≤ 30%TE) probably made little or no difference to HDL‐C in children over a period of up to and > 5 years (1 study). |
| Follow‐up: range 2 to 5 years | 522 (1 RCT) |
The mean HDL‐C (mmol/L) in control group was 1.32 | MD 0.01 lower (0.06 lower to 0.04 higher) | ⊕⊕⊕⊝ Moderate4,5,7,12 | |
| Follow‐up: > 5 years | 548 (1 RCT) |
The mean HDL‐C (mmol/L) in control group was 1.27 | MD 0.02 higher (0.03 lower to 0.07 higher) | ⊕⊕⊕⊝ Moderate4,5,7,12 | |
| Triglycerides (mmol/L) Follow‐up: range 6 to 12 months | 618 (1 RCT) |
The mean triglycerides (mmol/L) in control group was 0.98 | MD 0.01 lower (0.08 lower to 0.06 higher) | ⊕⊕⊕⊝ Moderate4,5,7,12 | Lower total fat intake (≤ 30%TE) probably made little or no difference to triglycerides in children over a period of 6 to 12 months (1 study). Lower total fat intake (≤30%TE) may make little or no difference to triglycerides in children over a period > 2 years (1 study). |
| Follow‐up: range 2 to 5 years | 522 (1 RCT) |
The mean triglycerides (mmol/L) in control group was 1.07 | MD 0.06 higher (0.04 lower to 0.16 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | |
| Follow‐up: > 5 years | 548 (1 RCT) |
The mean triglycerides (mmol/L) in control group was 1.1 | MD 0.03 higher (0.06 lower to 0.12 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | |
| Height‐for‐age z‐score Follow‐up: 6 months | 149 (1 RCT) |
The mean height‐for‐age z‐score in control group was 0.03 | MD 0.02 lower (0.06 lower to 0.02 higher) | ⊕⊝⊝⊝ Very low3,4,5,6 | We were uncertain whether lower total fat intake (≤ 30%TE) reduced height‐for‐age in children over a 12‐month period (1 study). |
| Follow‐up: range 6 to 12 months | 151 (1 RCT) |
The mean height‐for‐age z‐score in control group was 0.05 | MD 0.05 lower (0.08 lower to 0.02 lower) | ⊕⊝⊝⊝ Very low3,4,5,13 | |
| Height (cm) Follow‐up: range 6 to 12 months | 642 (1 RCT) |
The mean height (cm) in control group was 143.1 | MD 0 (1.11 lower to 1.11 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | Lower total fat intake (≤ 30%TE) may have made little or no difference to height in children over a period of 5 years (1 study). |
| Follow‐up: range 2 to 5 years | 540 (1 RCT) |
The mean height (cm) in control group was 167.4 | MD 0.10 lower (1.54 lower to 1.34 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | |
| Follow‐up: > 5 years | 577 (1 RCT) |
The mean height (cm) in control group was 171.4 | MD 0.60 lower (2.06 lower to 0.86 higher) | ⊕⊕⊝⊝ Low4,5,7,8 | |
|
%TE: percentage of total energy; BMI: body mass index; CI: confidence interval; HDL‐C: high‐density lipoprotein cholesterol; LDL‐C: low‐density lipoprotein cholesterol; MD: mean difference; RCT: randomised controlled trial. aNotes: For all outcomes, there were too few studies to assess publication bias. No studies looked at weight‐for‐age at > 12 months, weight at 1 to 2 years and > 5 years, BMI at 6 months, total cholesterol at 6 months and 1 to 2 years, LDL‐C at 6 months and 1 to 2 years, HDL‐C at 6 months and 1 to 2 years, triglycerides at 6 months and 1 to 2 years, height‐for‐age z‐score at > 12 months, and height at 1 to 2 years. | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Footnotes
1Mean change observed between baseline and follow‐up in the control group.
2Difference in intervention group (and its 95% confidence interval) was based on the assumed change in the comparison group (and its 95% confidence interval).
3Downgraded by 1 for risk of bias: unclear risk of bias across all domains.
4Only 1 study for this outcome, therefore we could not rate for inconsistency.
5Downgraded by 1 for indirectness: participants were children with raised blood lipids, thus results may not be directly generalisable to all children.
6Downgraded by 1 for imprecision: small sample size and confidence interval included no effect and important benefit or harm.
7Not downgraded for serious risk of bias; a well‐conducted trial (methods in place to minimise risk of selection, performance, detection, attrition and reporting bias).
8Downgraded by 1 for imprecision: confidence interval included no effect and important benefit or harm.
9Downgraded by 1 for risk of bias: allocation concealment not reported.
10 Not downgraded for serious imprecision: both bounds of the confidence interval indicate benefit, and calculated optimal information size met (158 patients are required to have a 80% chance of detecting, as significant at the 5% level, an important decrease in BMI of 1.7 kg/m2 (the average of the change across the 50th to 97th percentiles in 12.5 year‐olds, as per BMI‐for‐age tables, Centers of Disease Control & Prevention, 2000)).
11 Not downgraded for serious imprecision: both bounds of the confidence interval indicate benefit.
12Not downgraded for serious imprecision: precise estimate of no effect.
13Downgraded by 1 for imprecision: small sample size (optimal information size not met).
Appendix 3. Table of findings 2 (cohort studies) Total fat intake and body weight in children
| Total fat intake and body weight in children (cohort studies)a,b | ||||
|
Patient or population: boys and girls aged 24 months to 18 years Setting: communities, schools, households, healthcare centres in high‐income countries Exposure: total fat intake | ||||
| Outcomes |
No of studies (No of participants) |
Impact | Quality | What happens |
| Weight (kg) Follow‐up: 2 to 5 years | 4 cohort studies (13,802) |
2 studies that adjusted for TE intake: After 3 years, "Dairy fat was not a stronger predictor of weight gain than other types of fat, and no fat (dairy, vegetable, or other) intake was significantly associated with weight gain after energy adjustment, nor was total fat intake;" no numerical results reported. After 3 years, for every 1% increase in TE intake from total fat of children, weight will decrease by 0.0011 kg. 2 studies that did not adjust for TE intake: After 4 years, weight of children with low‐fat intake (< 30%TE) will increase by 8.1 kg on average, and by 8.9 kg on average in children with high‐fat intake (> 35%TE). After 2 years, children with low‐fat intake (≤ 30%TE) will gain on average 0.2 kg per year more than children with high‐fat intakes (> 30%TE) |
⊕⊝⊝⊝ Very low1,2 | When adjusted for TE, we were uncertain whether fat intake was associated with weight in children over 2 to 5 years. When not adjusted for TE, we were uncertain whether lower fat was associated with weight in children over 2 to 5 years. |
| Follow‐up: 5 to 10 years | 1 cohort study(126) |
1 study that did not adjust for TE intake: After 6 years, weight of children with low‐fat intake (< 30%TE) will increase by 16.8 kg on average, and by 13.9 kg on average in children with high‐fat intake (> 35%TE) |
⊕⊝⊝⊝ Very low3,4,5,6 | We were uncertain whether fat intake was associated with weight over 5 to 10 years (1 study). |
|
BMI (kg/m2, kg/m2per year, z‐score, percentile) Follow‐up: 2 to 5 years |
7 cohort studies (3143) |
4 studies that adjusted for TE intake: After 3 years, for every 1% increase in energy intake from total fat, BMI will decrease by 0.63 z‐score in boys but increase by 0.07 z‐score in girls. "Dietary factors were not associated with BMI across the three study years." After 3 years, for every 1% increase in energy intake from total fat, BMI will decrease by 0.00008 kg/m2. After 4 years, increase in the total fat intake, will increase BMI by 0.087 z‐score. The model explained 48% of variance in the change of BMI z‐score. 2 studies that did not adjust for TE intake: After 2.08 years, low‐fat intake (≤ 30%TE) will result in a 0.02 kg/m2 per year greater increase in BMI on average, compared to high‐fat intake (> 30%TE). After 3 years, for every 1% increase in energy intake from total fat, BMI will decrease by 0.01 percentile in girls. 1 study where TE adjustment was not applicable, as TE was part of exposure: After 3 years, for every 1 z‐score increase in the energy‐dense, high‐fat and low‐fibre dietary pattern, BMI will increase by 0.03 z‐score in boys and by 0.99 z‐score in girls. After 3 years, the ratio of odds for being overweight/obese was 1.04 greater in boys and 1.02 greater in girls with higher dietary pattern z‐scores, compared to the odds in boys and girls with lower dietary pattern z‐scores. |
⊕⊝⊝⊝ Very low6,7,8 |
We were uncertain whether fat intake was associated with BMI in children over 2 to 10 years or > 10 years. |
| Follow‐up: 5 to 10 years | 4 cohort studies (1158) |
3 studies that adjusted for TE intake: After 6 years, for every 1% increase in energy intake from total fat, BMI will decrease by 0.011 z‐score in boys but increase by 0.005 z‐score in girls. After 9 years, increase in the total fat intake will increase BMI by 0.122 z‐score. After 10 years, for every 1% increase in energy intake from total fat, BMI will increase by 0.029 kg/m2 in white girls and by 0.012 kg/m2 in black girls. 1 study that did not adjust for TE intake: After 6 years, for every 1 g increases in the fat intake, BMI will increase by 0.01 kg/m2 |
⊕⊝⊝⊝ Very low6,9 | |
| Follow‐up: > 10 years | 2 cohort studies (330) |
1 study that adjusted for TE intake: After 13 years, increase in the total fat intake will increase BMI by 0.16 z‐score. 1 study that did not adjust for TE intake: After 17 years, on average BMI decreased 0.13 z‐score in the low‐fat (32%TE) group while increased 0.04 z‐score in the high‐fat (40%TE) group. |
⊕⊝⊝⊝ Very low6,10 | |
|
LDL‐C (mmol/L) Follow‐up: 2 to 5 years |
1 cohort study (1163) |
1 study where TE adjustment not applicable, as TE was part of exposure: After 3 years, for every 1 z‐score increase in the energy‐dense, high‐fat and low‐fibre dietary pattern, LDL‐C will increase by 0.001 mmol/L in boys and 0.04 mmol/L in girls |
⊕⊝⊝⊝ Very low4,5,6,11 | We were uncertain whether fat intake was associated with LDL‐C in children over 2 to 5 years (1 study). |
|
HDL‐C (mmol/L) Follow‐up: 2 to 5 years |
2 cohort studies (1393) |
1 study that adjusted for TE intake: After 3 years, for every 1% increase in energy intake from total fat, HDL‐C will decrease by 0.21 mmol/L in girls. 1 study where TE adjustment not applicable, as TE was part of exposure: After 3 years, for every 1 z‐score increase in the energy‐dense, high‐fat and low‐fibre dietary pattern, HDL‐C will decrease by 0.002 mmol/L in boys but increase by 0.02 mmol/L in girls. |
⊕⊕⊝⊝ Low11,12 | When adjusted for TE, fat intake may be inversely associated with HDL‐C in girls over 2 to 5 years (1 study). When not adjusted for TE, fat intake may make little or no difference to HDL‐C in girls over 2 to 5 years (1 study). |
|
Triglycerides (mmol/L) Follow‐up: 2 to 5 years |
1 cohort study (1163) |
1 study where TE adjustment not applicable, as TE was part of exposure: After 3 years, for every 1 z‐score increase in the energy‐dense, high‐fat and low‐fibre dietary pattern, triglycerides will increase by 1% in either boys or girls. |
⊕⊝⊝⊝ Very low4,5,6,11 | We were uncertain whether fat intake was associated with triglycerides in children over 2 to 5 years (1 study). |
|
Height (cm) Follow‐up: 2 to 5 years |
3 cohort studies (973) |
1 study that adjusted for TE intake: After 3 years, for every 1% increase in energy intake from fat, height in children will decrease by 0.0009 cm on average. 2 studies that did not adjust for TE intake: After 2 years, low‐fat intake (≤ 30%TE) will result in a 0.2 cm per year greater increase in height on average compared to high‐fat intake (> 30%TE). After 4 years, on average children in low‐fat intake (< 30%TE) gain 27.9 cm in height, while children in high‐fat intake (> 35%TE) gain 28.3 cm in height. |
⊕⊝⊝⊝ Very low6,10 | We were uncertain whether fat intake was associated with height in children over 2 to 10 years. |
| Follow‐up: 5 to 10 years Age at baseline: 2 years |
1 cohort study (126) |
1 study that did not adjust for TE intake: At 6 years, on average children in low‐fat intake (< 30%TE) gain 44.9 cm in height while children in high‐fat intake (> 35%TE) gain 40.3 cm in height. |
⊕⊝⊝⊝ Very low3,4,5,6 | |
|
BMI: body mass index; HDL‐C: high‐density lipoprotein cholesterol; LDL‐C: low‐density lipoprotein cholesterol; MD: mean difference; TE: total energy. aNotes: Some cohort studies reported more than one eligible analysis for the same outcome (e.g. BMI as continuous or binary outcome) or different measures of exposure (e.g. fat intake as continuous %TE or as binary classification of less‐exposed vs more‐exposed). In these cases, we selected outcomes and exposure measures so as not to use the same study sample of participants more than once per outcome and time point range in the table. b No studies looked at weight at > 10 years; LDL‐C, HDL‐C and triglycerides at 12 months, 1 to 2 years and > 5 years, and height at > 10 years. For all outcomes, there were too few studies to assess publication bias. | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
Footnotes
1Although, risk of bias was concerning (studies with strong contributions did not adjust for all important prognostic variables), plausible residual confounding would likely reduce the demonstrated effect in the studies that did not adjust for total energy intake; thus we chose not to downgrade for risk of bias.
2Downgraded by 1 for imprecision: in studies reporting variance, the variance included no effect and important benefit or harm.
3Although risk of selection bias (no matching of exposed and non‐exposed groups, or statistical adjustments) and attrition bias (> 50% attrition) was concerning, plausible residual confounding would likely reduce the demonstrated effect as this study did not adjust for total energy; thus we chose not to downgrade for selection bias.
4Only 1 study for this outcome, therefore we could not rate for inconsistency.
5Downgraded by 1 for indirectness: a single study in a high‐income country likely has limited generalisability.
6Imprecision was considered, but we considered a decision would not impact on the rating and thus no judgement was made for imprecision.
7Downgraded by 1 for risk of bias: risk of selection bias: 5 studies did not match exposed and non‐exposed groups or make important statistical adjustments; high risk of detection bias: dietary assessment for 3 studies were not adequately rigorous.
8Downgraded by 1 for inconsistency: some studies reported small to large positive associations between exposure and outcome, while others reported no association or a small to medium inverse association between exposure and outcome.
9Downgraded by 1 for risk of bias: risk of selection bias: 2 studies with strongest contributions, did not adjust for all important prognostic variables; high risk of detection bias: dietary assessment in 1 study was not adequately rigorous.
10Downgraded by 1 for risk of bias: risk of selection bias; no matching of exposed and unexposed groups or adjustment for all important prognostic variables.
11Study was judged to have a lower overall risk of bias; attrition < 50% and satisfactory assessment of exposure.
12Not downgraded for serious imprecision as judged to be precise estimates of no effect in both studies.
Data and analyses
Comparison 1. Lower fat intake (30% or less of total energy (TE)) versus usual/modified fat intake by time point ranges.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight outcomes (standardised and unstandardised end values) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 > 6 to 12 months | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 > 2 to 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Body mass index (BMI) (kg/m2) (end values) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 > 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 > 1 to 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 > 2 to 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 > 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 BMI (kg/m2) (end values): sensitivity analysis (longest follow‐up data only) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 > 1 to 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 > 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 BMI (kg/m2) (end values): sensitivity analysis (shortest follow‐up data only) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 > 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 > 1 to 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Total cholesterol (mmol/L) (end values) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 > 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 > 2 to 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 > 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Low‐density lipoprotein (LDL) cholesterol (mmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 > 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 > 2 to 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 > 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 High‐density lipoprotein (HDL)‐cholesterol (mmol) (end values) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 > 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 > 2 to 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 > 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Triglycerides (mmol/L) (end values) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 > 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 > 2 to 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 > 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Systolic blood pressure (mmHg) (end values) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 > 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 > 2 to 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Diastolic blood pressure (mmHg) (end values) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 > 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 > 2 to 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Height outcomes (standardised and unstandardised end values) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 > 6 to 12 months | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 > 2 to 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 > 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Energy intake (kJ) (end values) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 > 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 > 1 to 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 > 2 to 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Fat intake (%TE) (end values) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 > 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 > 1 to 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 > 2 to 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Saturated fat intake (%TE) (end values) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 > 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 > 1 to 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 > 2 to 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Protein intake (%TE) (end values) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 > 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 > 1 to 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 > 2 to 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Carbohydrate (%TE) (end values) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 > 6 to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 > 1 to 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 > 2 to 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alexy 2004.
| Methods |
Study design: prospective cohort study. Analysis method for cohort: cluster analysis used to classify children into groups (constant, low‐, medium‐ and high‐fat intake). Non‐parametric Kruskal‐Wallis 1‐way ANOVA used to test differences in SDS‐BMI between groups. How were missing data handled? 55% (274/502) not included in analyses as they had smaller number of DRs due to study abandonment or omitting DRs from study protocol. Baseline characteristics of those excluded not compared to those included in analyses. Number of study contacts: mean (SD) = 12.4 (1.8); median = 12, min = 10, max = 17. Period of follow‐up (total period of observation): 17 years. Periods of recruitment: 1985‐2002. Sample size justification adequately described? No. Sampling method: convenient sampling. Mothers recruited in city of Dortmund and surrounding communities via paediatric practices or personal contacts. Cohorts of about 40‐50 healthy infants enrolled yearly. Study objective: to examine fat intake and other nutrient and food intake of participants with at least 10 dietary measurements from age of 2 up to 18 years. Study population: German children and adolescents aged 2‐18 years. |
|
| Participants |
Baseline characteristics (reported for 2 groups and overall group) Overall (n = 228)
LF intake group (n = 55)
HF intake group (n = 57)
Included criteria: healthy born German children and adolescents participating in the DONALD study, who could provide at least 10 DRs between 2 and 18 years if age within 17 years' follow‐up. The infants had parents with sufficient German language ability and indicated their willingness to participate in a long‐term study. Excluded criteria: NR. Brief description of participants: children and adolescents aged 2‐18 years who were healthy born and had at least 1 parent with sufficient knowledge of the German language. Total number completed in cohort study: 228 (114 boys, 114 girls). Total number enrolled in cohort study: 502. |
|
| Interventions |
Description of exposure for cohorts
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
BMI
|
|
| Identification |
Sponsorship source: Ministry of Education, Science and Research North‐Rhine‐Westphalia, Germany, and German Federal Ministry of Consumer Protection, Food and Agriculture. Country: Germany. Setting: city of Dortmund and surrounding communities. Comments: NA. Author's name: U Alexy. Institution: Research Institute of Child Nutrition (FKE), Heinstueck 11, D‐44225 Dortmund, Germany. Email: alexy@fke‐do.de. Declaration of interests: no. Study ID: Alexy 2004. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | Low risk | Analyses included children with ≥ 10 DRs aged 2‐18 years (45% (228/502) aged > 17 years). Characteristics of children excluded from analyses NR. |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | High risk | No matching reported. No adjustment for parental BMI, physical activity, pubertal stage, SES, e.g. family income. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | Unclear risk | Inadequate description of anthropometric measurement methods. |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | Usual dietary habit assessed using 3‐consecutive‐day weighed DR, which was repeated yearly. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | Physical activity, parental BMI not assessed. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | Children selected for same cohort. |
Ambrosini 2016.
| Methods |
Study design: prospective cohort study. Analyses for cohorts: cohort analysis: mean nutrient intakes across increasing quintiles of DP1a, DP1b and DP2 z‐scores estimated by using linear regression. Then, GEEs applied to investigate longitudinal associations between DP z‐scores and fat mass index (FMI) z‐scores. These models regressed FMI on DP z‐score at the previous time point by using DP z‐scores at 7, 10, and 13 years of age and FMI z‐scores at 11, 13 and 15 years of age. Models adjusted for time‐varying covariates (i.e. age, dietary misreporting, physical activity, Tanner stage) and fixed covariates (sex, maternal social class). CIF subsample analysis: linear regression used to model DP1a and DP2 z‐score at ages 5 and 7 years on FM (kg) at age 9 years. How were missing data handled? Cohort: lost to follow‐up at 7 years (6404/14,536, 44%); at 11 years (7542/14,536, 52%); at 13 years (8554/14,536, 59%) and at 15 years (9192/14,536, 63%). Study website contained details of all participants; reasons for attrition not provided by authors. Data analysis included all available data for the different time points. CIF subsample: complete data on diet and BC available for 521 (36%) children at ages 5 and 9 years and 682 (48%) children at ages 7 years and 9 years. Effect of missing data assessed (no data reported). Number of study contacts: 7 (at age 5, 7, 9, 10, 11, 13 and 15 years). Period of follow‐up (total period of observation): 4 years (CIF subsample from 5 to 9 years); 8 years (whole cohort from 7 to 15 years). Periods of recruitment: 1 April 1991 and 31 December 1992. Sample size justification adequately described? Yes. For a normally distributed quantitative trait (e.g. weight), a sample of 10,000 would be 80% certain to be able to show a difference of 0.19 SD as statistically significant if just 2% of the population had relevant exposure, whereas for a population of 1000, there would be sufficient power to demonstrate a difference of 0.62 SD (Golding 2001) Sampling method: convenience sample. Birth cohort that recruited pregnant women in Avon, UK. Of the 14,472 birth outcomes, 14,062 were live births and 13,988 were alive at 1 year. An additional 713 children whose mothers were initially invited but had not enrolled were recruited later. Total baseline cohort therefore included 14,701 children who were alive at 1 year. Of these, 8297 children attended clinics at age of 7 years. CIF sample: random subsample of 1432 children selected from births in the cohort that occurred in last 6 months of recruitment. Study objective: objective 1 (CIF subsample): to identify a DP that explained DED, FD and % energy from fat and analyse its association with fatness in children aged 5‐9 years. Objective 2 (whole cohort): to examine longitudinal relationships between a DP characterised by DED, % energy from fat and FD and FM in children aged 7‐15 years. Objective 3: to identify DPs characterised by high‐sugar content, HF content, or both, and their longitudinal associations with adiposity in children aged 7‐15 years. Study population: children and adolescents aged 5‐15 years in Avon, UK. |
|
| Participants |
Baseline characteristics (reported for 2 groups: overall cohort and subsample of cohort) Overall cohort
CIF subsample (n = 521)
Included criteria: for cohort analysis, participants of ALSPAC cohort with follow‐up data at ages 7‐15 years were included. For analysis of CIF sample, eligible participants had available data on diet and BC at ages 5, 7 and 9 years. Excluded criteria: NR. Brief description of participants: aged 5‐15 years in ALSPAC cohort, Avon, UK. Total number completed in cohort study: 4729 (at 15 years). Total number enrolled in cohort study: 7285 at age 7 years (CIF subsample: 790 at age 3.6 years). |
|
| Interventions |
Description of exposure for cohorts Overall cohort
CIF subsample
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
Body fat
Height
|
|
| Identification |
Sponsorship source: UK Medical Research Council, Wellcome Trust and the University of Bristol. Country: UK. Setting: community. Comments: ALSPAC. Author's name: Gina L Ambrosini. Institution: School of Population Health, The University of Western Australia, Perth, Australia; Medical Research Council Human Nutrition Research, Cambridge, UK. Email: gina.ambrosini@uwa.edu.au. Declaration of interests: Yes. "no conflicts of interest." Study ID: Ambrosini 2016. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | Unclear risk | Attrition relevant to eligible analyses for FMI was 35% (2556/7285) over 8 years. For eligible analyses for BMI and height in CIF subsample, attrition over 1.5 years was 11% (84/790), and over 4 years for body fat was 7.3% (38/521). Authors reported that children who attended clinics for follow‐up were more likely to come from more affluent or better‐educated families than were children who did not attend clinics (data NR), and that there were no significant differences in dietary and anthropometric variables between children with complete data compared to those who did not (data NR). |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | Low risk | Most prognostic variables adjusted for. Parental BMI not assessed during study period. Data analysis of CIF subsample adjusted for prepregnancy maternal BMI and overweight status. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Unclear risk | NR. |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Standard methods used for measurement of weight, height and body fatness (DEXA). |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | Repeated 3‐day food diaries (non‐consecutive days) completed by parent or child, with parental assistance. Authors assessed dietary misreporting of energy intake. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | Repeated measurements of total physical activity performed using accelerometer. Mean time spent by children watching TV reported by parents at 4.5 years. Pubertal status self‐reported at 11 and 13 years (using validated diagrams). Parental socioeconomic information and prepregnancy heights and weights were self‐reported. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | All participants of the ALSPAC. |
Appannah 2015.
| Methods |
Study design: prospective cohort study. Analysis methods for cohorts: prospective associations between DP z‐scores and cardiometabolic risk factors at 14 and 17 years of age analysed using GEE with an exchangeable correlation structure. Beta coefficients resulting from the regression models for these biomarkers were back‐transformed for interpretation. Logarithmic transformation was applied to insulin, HOMA and TG measurements as they were not normally distributed. How were missing data handled? Out of 2337 adolescents eligible at 14 years, 1857 (79.5%) responded to FFQs and 1286 (55%) attended physical assessments. Number of study contacts: 2 (at 14 and 17 years). Period of follow‐up (total period of observation): 3 years. Periods of recruitment: 1989‐1991 (mothers of participants were recruited). Sample size justification adequately described? No. Sampling method: convenience sample. Present analysis uses data collected at 14 (n = 1857) and 17 (n = 1709) years' follow‐up from Raine cohort study. Original cohort comprised 2900 pregnant women recruited into a trial at King Edward Memorial Hospital (Perth, Western Australia) from 1989 to 1991. At 14 years, 2337 adolescents were eligible for follow‐up. Study objective: to examine associations between an "energy‐dense, high‐fat and low fibre" DP and cardiometabolic risk factors, and the tracking of this DP in adolescence. Study population: Australian adolescents aged 14‐17 years. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: adolescents who participated in the Raine cohort study and had complete dietary and cardiometabolic data at 14 and 17 years. Excluded criteria: NR. Brief description of the participants: adolescents aged 14‐17 years participating in Raine cohort study. Total number completed in cohort study: 1709 (1009 completed FFQ). Total number enrolled in cohort study: 2337. |
|
| Interventions |
Description of exposure for cohorts
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
BMI
WC
LDL‐C
HDL‐C
TGs
|
|
| Identification |
Sponsorship source: Medical Research Council (grant number U105960389) and research grants from the National Health and Medical Research Council of Australia (ID#1022134 (2012‐2014)) and the National Heart Foundation of Australia and Beyond Blue Cardiovascular Disease (grant number G 08P 4036) and Depression Strategic Research Program. Country: Australia. Setting: community in Perth. Comments: Western Australian Pregnancy (Raine) Cohort Study. Author's name: G Appannah. Institution: Department of Nutrition and Dietetics, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Malaysia; Medical Research Council Human Nutrition Research, Cambridge, UK. Email: Gina.Ambrosini@uwa.edu.au Declaration of interests: yes. "Authors have no conflicts of interest to declare." Study ID: Appannah 2015. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | High risk | High lost to follow‐up rate (35‐40% at 14 and 17 years). Authors did not report any comparative analyses between participants lost to follow‐up and participants who completed study. |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | Low risk | Study included mainly white participants, upper income families, stratified for gender. Adjusted for age, dietary misreporting, physical fitness, smoking and BMI‐for‐age z‐score. Not adjusted for parental BMI. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Unclear risk | NR. |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Standard methods performed for measurement of weight, height, WC and fasting blood samples. |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | Repeated assessment using a validated semi‐quantitative FFQ. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | Physical fitness assessed at each session, using validated test (PWC‐170) which was correlated with self‐reported physical activity. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | Mothers of participants selected for 1 cohort. |
Berkey 2005.
| Methods |
Study design: prospective cohort study. Analysis methods for cohorts: linear regression models used to estimate effects of diet and physical activity on annual changes in adiposity with 1‐year change in BMI and weight as the continuous variables. Models adjusted for ethnicity, baseline BMI, annual change in height, menstrual history in girls, pubertal stage and age. How were missing data handled? Number of children who did not return at 1‐year follow‐up (22.8%, 3819/16771) and 3‐year follow‐up (23.5%, 3942/16771). Data on BMI, dietary intake and physical activity compared between children who did not return the questionnaires and children who did. Authors indicated that there did not seem to be bias related to dietary intake or adiposity, but children lost to follow‐up were older and more physically active. Number of study contacts: 2 (baseline, 1 year' follow‐up, Berkey 2000); 4 (baseline, 1, 2 and 3 years' follow‐up, Berkey 2005). Period of follow‐up (total period of observation): 1 year (Berkey 2000); 3 years (Berkey 2005). Period of recruitment: 1996. Sample size justification adequately described? No. Sampling method: convenience sample. Participants were children of mothers who were nurses and participated in Nurses' Health Study II. Letters sent to mothers explaining goals of new study and requesting their consents. Study objective: to examine role of physical activity, inactivity and DPs on annual weight changes among preadolescents and adolescents, taking growth and development into account. Study population: preadolescents and adolescents aged 9‐14 years in the USA. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: children aged in 9‐14 years of Nurses' Health Study II participants with completed questionnaires at baseline. Excluded criteria: children with misreporting data of dietary intake (500 kcal/day or > 5000 kcal/day), physical activity (> 40 hours/week), screen time (> 80 hours/week), height (> 3 SD), BMI (12 kg/m2 or > 3 SD). Brief description of participants: children aged 9‐14 years residing in 50 states of the USA whose mothers were nurses and participated in the Nurses' Health Study II. Total numbers completed in cohort study: 10,769 included in the data analysis out of 12,952 children who returned after 1 year' follow‐up). Number of children included in data analysis at 3 years NR, although 12,829 children returned after 3 years' follow‐up. Total numbers enrolled in cohort study: 16,771. Eligible sample consisted of 26,765 children (of 18,526 mothers in Nurses' Health Study II). |
|
| Interventions |
Description of exposure for cohorts
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
Weight
BMI
|
|
| Identification |
Sponsorship source: grant DK46834 from the National Institutes of Health and, in part, by Kellogg's. Country: USA. Setting: communities in 50 states. Comments: The Growing Up Today Study. Author's name: Catherine S Berky. Institution: Channing Laboratory, Department of Medicine, Brigham Women's Hospital and Harvard Medical School. Email: catherine.berky@channing.harvard.edu. Declaration of interests: no. Study ID: Berkey 2000. Type of record: journal article. |
|
| Notes | We contacted the authors to request relevant numerical outcome data, since they only reported the following sentence about total fat intake and weight in the text: ".... and no fat (dairy, vegetable, or other) intake was significantly associated with weight gain after energy adjustment, nor was total fat intake." We had not received a response by time of publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | High risk | High attrition (35.8% (6002/16771) over 1 year). Data on BMI, dietary intake and physical activity compared between children who did not return the questionnaires and children who did. The authors indicated that there did not seem to be bias related to dietary intake or adiposity, but children lost to follow‐up were older and more physically active. |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | Low risk | Data analyses adjusted for age, gender, ethnicity, pubertal stage while physical activity and total energy intake were included in the model. Parental BMI and SES not adjusted for. Likely that children had similar family income level as their mothers were nurses. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | High risk | Height and weight were self‐reported although specific instructions on how to measure height and weight were given to participants. |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | Repeated self‐administered, semi‐quantitative FFQs used to assess dietary intake. Participants with dietary misreporting were excluded from data analyses. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | Repeated assessments of physical activity, screening time and pubertal stage conducted using validated questionnaires. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | Participants selected for 1 cohort study. |
Bogaert 2003.
| Methods |
Study design: prospective cohort study. Analyses methods for cohorts: multiple regression analyses used to test relation between variables, and partial correlations used to adjust for confounding variables. How were missing data handled? Attrition at 1 year: 31% (reasons not stated). No significant differences in baseline variables observed between children who attended for follow‐up and children who did not. Number of study contacts: 3 (baseline, 6 and 12 months). Period of follow‐up (total period of observation): 1 year. Periods of recruitment: NR. Sample size justification adequately described? No. Sampling method: convenience. Recruitment was done through local advertising. Study objective: to identify, prospectively, whether simply measured indicators of energy intake and expenditure might predict excessive weight gain over time in a cohort of prepubescent children. Study population: prepubertal children aged 6‐9 years in Australia. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: children aged 6‐9 years, who had ≥ 1 biological parent agreeable to participate and the family commitment to continued follow‐up for ≥ 12 months. Excluded criteria: NR. Pretreatment: NA. Brief description of participants: children aged 6‐9 years living in New South Wales, Australia. Total number completed in cohort study: at 12 months: 41 (69%). An attempt was made to follow‐up each participant at each 6‐month interval by letter and telephone. Total number enrolled in cohort study: 59 children (41 mothers, 29 fathers). |
|
| Interventions |
Description of exposure for cohorts
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
BMI
|
|
| Identification |
Sponsorship source: Australian Rotary Health Foundation, Financial Markets Foundation for Children, National Health and Medical Research Council. Country: Australia. Setting: University Teaching Hospital, Western Australia. Comments: NA. Author's name: N Bogaert. Institution: Department of Endocrinology, Royal Prince Alfred Hospital, Camperdown, NSW, Australia. Email: kss@email.cs.nsw.gov.au. Declaration of Interests: no Study ID: Bogaert 2003. Type of record: journal article. |
|
| Notes | We contacted the authors to request relevant numerical outcome data, since they only reported the following in the text: "We were unable to demonstrate a positive relation between dietary fat and BMI z‐score change…" We had not received a response by time of publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | Unclear risk | Attrition at 1 year: 31% (reasons not stated). Authors reported no significant differences in baseline variables observed between children who attended for follow‐up and children who did not (variables were not specified). |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | Unclear risk | Authors stated that partial correlations were used to adjust for confounding variables, but did not specify any variables. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Height and weight measured using standard techniques. BC determined after an overnight fast using BIA. |
| Can we be confident in the assessment of exposure? All outcomes | High risk | Single assessment using a 3‐day DR. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | High risk | Only single 3‐day activity record assessed. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | Participants recruited as part of 1 cohort study. Recruitment undertaken in local area through advertising. |
Boreham 1999.
| Methods |
Study design: prospective cohort study. Analysis methods for cohorts: GEE used to investigate the associations between biological CHD risk factors (BMI, sum of skinfolds, SBP, DBP and serum total cholesterol) and lifestyle predictor variables (habitual physical activity, smoking and dietary intake). How were missing data handled? Complete data sets available for 229 boys and 230 girls (89% follow‐up rate for both sexes). Of children lost to follow‐up, reasons were declined to participate (17%), illness (46%), moving school in the interim (31%) or for other reasons (6%). Number of study contacts: 2 (12 and 15 years). Period of follow‐up (total period of observation): 3 years. Periods of recruitment: 1989‐1990. Sample size justification adequately described? Yes. Sample size calculation for the original cross‐sectional survey: target sample of 250 per age/gender group based on variability of pilot study results and represented a 2% random sample of each population group in Northern Ireland. Sampling method: stratified sample. School children selected from 16 schools in Northern Ireland. Within each school, children were randomly selected. Of all children recruited, overall response rate was 78% (1015 children; 506 boys and girls aged 15 years; 509 boys and girls aged 12 years). Study objective: to examine relationships between the longitudinal development of biological risk factors for CHD in tandem with the development of key risk behaviours in a representative adolescent population drawn from a region with a high prevalence of CHD risk. Study population: school children aged 12 years in Northern Ireland. |
|
| Participants |
Baseline characteristics (reported as 1 overall group)
Included criteria: children aged 12 years attending selected schools in Northern Ireland. Excluded criteria: NR. Brief description of participants: children aged 12 years attending post‐primary education in Northern Ireland. Total number completed in cohort study: 459. Total number enrolled in cohort study: 509 (12‐year old children). |
|
| Interventions |
Description of exposure for cohorts
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
HDL‐C
|
|
| Identification |
Sponsorship source: Northern Ireland Chest, Heart and Stroke Association, British Heart Foundation, Wellcome Trust. Country: Northern Ireland. Setting: post‐primary schools. Comments: Northern Ireland Young Hearts Project. Author's name: C Boreham. Institution: University of Ulster, Jordanstown. Email: NR. Declaration of interests: no. Study ID: Boreham 1999. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | Low risk | Complete data sets available for 229 boys and 230 girls (89% follow‐up rate for both sexes). Of those lost to follow‐up, reasons were: declined to participate (17%); illness (46%), moving school in the interim (31%) or for other reasons (6%). |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | Low risk | Adjusted for physical activity, pubertal stage, SES but not for parental BMI or ethnicity. Regression analysis stratified for gender. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | Unclear risk | Unclear how many skinfold measurements were performed and who performed these. No details provided by authors regarding weight and height measurements. |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | Repeated assessment of dietary intake. Analysis adjusted for misreporting. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | Repeated assessment of physical activity by a 7‐day recall questionnaire. Sexual maturation assessed according to Tanner stage. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | All children were participants of the Northern Ireland Young Hearts cohort study. |
Brixval 2009.
| Methods |
Study design: prospective cohort study. Analyses methods for cohorts: regression analysis in boys and girls related fat intake to a change in BMI‐for‐age z‐score after 3 and 6 years' follow‐up. Adjusted model after 3 years' follow‐up was adjusted for baseline z‐score, physical activity level, pubertal stage at baseline, energy intake and dietary volume. Adjusted model at 6 years' follow‐up also included parent's income level, inactivity and number of overweight parents. How were missing data handled? At 3 years' follow‐up: participants with missing information on any measurement at baseline (n = 41) and incomplete follow‐up (attrition 25.5%; 150/589) excluded from analyses. Dropout analysis revealed baseline characteristics of anthropometrics and dietary information did not differ between participants (n = 308) that did and participants who did not complete follow‐up (all P > 0.05; data not shown). At 6 years' follow‐up: 384 children were re‐examined (attrition 34.8%; 205/589). Possible dropout effects examined indirectly by comparing baseline age, BMI and fat intake of those children participating only at baseline with children participating at both baseline and follow‐up, which showed no difference between groups (no data or statistical tests reported by authors). According to ethical considerations, it was not permitted to contact children who decided not to participate at follow‐up. Sample size justification adequately described? No. Sampling method: state schools in Odense (Denmark) stratified according to school type, location and SES profile. From each stratum, a proportional, 2‐stage sample of children was randomly selected. From the selected schools, 1356 pupils were invited, and 1020 (75.2%) (589 3rd graders and 421 ninth graders) agreed to participate. Periods of recruitment: 1997‐1998. Period of follow‐up (total period of observation): 6 years. Number of study contacts: 3 (baseline, 3 and 6 years). Study objective: objective 1: to examine associations between DED or fibre intake and 3‐year change in BMI‐for‐age z‐score among 8‐ to 10‐year old boys and girls. Objective 2: to investigate the association between fat intake and weight development among a cohort of children aged 9‐10 years at baseline and 15‐16 years at follow‐up, and whether parents' obesity was modifying the association. Study population: children aged 9‐10 years attending schools in Odense, Denmark. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: 9‐ to 10‐year‐old boys and girls attending 3rd grade at selected schools in Odense, Denmark. Excluded criteria: NR. Total number enrolled in cohort study: 589. Total number completed in cohort study: 398 (after 3 years); 384 (after 6 years). Brief description of participants: 9‐ to 10‐year‐old children attending 3rd grade at schools in Odense, Denmark, who participated in the European Youth Heart Study. |
|
| Interventions |
Description of exposure for cohorts
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
BMI
|
|
| Identification |
Sponsorship source: NR. Country: Denmark. Setting: schools in Odense. Comments: Danish component of the European Youth Heart Study. Author's name: Carina S Brixval. Institution: Research Unit for Dietary Studies, Institute of Preventive Medicine, Copenhagen, Denmark. Email: blh@ipm.regionh.dk; SI@ipm.regionh.dk. Declaration of Interests: yes. "The authors declared no conflict of interest." Study ID: Brixval 2009. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | Low risk | Participants with missing information on any measurement at baseline (n = 41) and incomplete follow‐up (attrition 25.5% (150/589) over 3 years) excluded from analyses. Dropout analysis revealed that baseline characteristics of anthropometrics and dietary information did not differ between participants (n = 308) who did and who did not complete the follow‐up (all P > 0.05). At 6 years' follow‐up, 384 children were re‐examined (attrition 34.8% (205/589)). Possible dropout effects examined indirectly by comparing baseline age, BMI and fat intake of those children participating only at baseline with children participating at both baseline and follow‐up, which showed no difference between groups (no data or statistical tests reported by authors). According to ethical considerations, it was not permitted to contact children who decided not to participate at follow‐up. |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | Low risk | Regression model adjusted for most important prognostic variables. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Height (cm) measured to the nearest 0.1 cm with stadiometer. bodyweight (kg) measured to nearest 0.1 kg with calibrated beam‐scale weight. Participants wore underwear or light garments only. |
| Can we be confident in the assessment of exposure? All outcomes | High risk | A single 24‐hour dietary recall was performed at baseline. Although it was validated by an estimated food record (completed by parents for the same 24‐hour period) it was not repeated during follow‐up and therefore not likely to reflect the habitual fat intake of children during the study period. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | High risk | Parental BMIs calculated from self‐reported weights and heights. Presence or absence of regular physical exercise assessed at baseline by self‐report. Children's activity level at baseline measured using accelerometers; however, this variable contained significant missing data (33%). Unclear whether pubertal stage of children was based on an assessment or on self‐report. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | All participants of the European Youth Heart Study in Denmark. |
Butte 2007.
| Methods |
Study design: prospective cohort study. Analyses for cohorts: analyses conducted on subsample of 798 children who gained weight after 1 year. Predictors of weight gain were individually examined using GEE. To account for correlated data within families, a family identification number was used as the cluster variable. Preliminary graphical analysis indicated that weight gain increased non‐linearly with age; thus, a quadratic term was needed. To address potential confounding between BMI status and predictors of weight gain, GEE analyses were repeated and adjusted for BMI status, age, age squared, sex and Tanner stage. How were missing data handled? Lost to follow‐up at 1 year: 14.6% (151/1030) (reasons not stated). Number of study contacts: 3 (2 baseline visits, at 1 year' follow‐up). Period of follow‐up (total period of observation): 1 year. Periods of recruitment: November 2000 to August 2004. Sample size justification adequately described? No. Sampling method: convenience sample. Recruitment conducted through local TV and radio stations and community outreach efforts. Each family was selected from an overweight proband aged 4‐19 years using bivariate ascertainment scheme (i.e. overweight ≥ 95th percentile for BMI and ≥ 85th percentile for FM). In addition, families were required to have ≥ 3 children aged 4‐19 years. Study objective: to test putative sociodemographic, metabolic and behavioural predictors of weight gain: familial characteristics, birth information, child acculturation, dietary intake, eating behaviour, physical activity, energy expenditure and fasting blood biochemistries, while controlling for sex, age and sexual maturation. Study population: children aged 4‐19 years in Hispanic community. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: Hispanic families with ≥ 3 children aged 4‐19 year and ≥ 1 overweight child aged 4‐19 year (overweight was defined as BMI ≥ 95th percentile and FM > 85th percentile). Excluded criteria: NR. Brief description of participants: Hispanic children aged 4‐19 years in the Viva la Familia Study enrolling families with ≥ 1 overweight child. Total number completed in cohort study: 879 (analyses conducted on 798 children). Total number enrolled in cohort study: 1030. |
|
| Interventions |
Description of exposure for cohorts
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
Weight
|
|
| Identification |
Sponsorship source: National Institutes of Health (NIH), US Department of Agriculture. Country: USA. Setting: Hispanic communities, Houston, TX. Comments: Viva la Familia Study. Author's name: Nancy F Butte. Institution: US Department of Agriculture, Agricultural Research Service Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA. Email: nbutte@bcm.edu. Declaration of interests: yes. "None of the authors had a financial conflict of interest in relation to this study." Study ID: Butte 2007. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | High risk | Attrition at 1 year: 14.6% (151/1030). |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | Low risk | The model using dietary fat intake to predict weight gain did not adjust for parental BMI, physical activity, family income or parental education. However, there was no association between physical activity, family income and parental education and weight gain after adjustment for gender, age, pubertal stage and baseline BMI of the child. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | Unclear risk | Insufficient description of outcome measurement methods. |
| Can we be confident in the assessment of exposure? All outcomes | High risk | Dietary intake only assessed once, at baseline. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | High risk | Single assessment of physical activity performed. Pubertal stage self‐reported. Unclear whether parental BMI was self‐reported or measured. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | All children were participants of the Viva la Familia Study. |
Cohen 2014.
| Methods |
Study design: prospective cohort study. Analyses methods for cohorts: linear regression with participant‐level random‐effects model used to examine whether physical activity, diet and environmental exposures were associated prospectively with changes in bodyweight and % body fat. Only variables that were significant were combined into a single multivariate model. How were missing data handled? Only the participants who had valid data for all 3 assessment periods were analysed (n = 265 (87%) compared to n = 303 who were enrolled). Number of study contacts: 3 (baseline in grade 8, 2 follow‐up visits in tenth/eleventh grade or eleventh/twelfth grade). Period of follow‐up (total period of observation): 5 years. Periods of recruitment: 2007, as the follow‐up across grades 10‐12 occurred during 2009‐2011. Sample size justification adequately described? No. Study authors also mentioned that a limitation in the study was the relative small sample size. Sampling method: random sample. Control participants of the TAAG cohort from 2 sites (San Diego, Minneapolis) used (532 eligible girls). For present analysis, 303 girls were randomly selected from 7 different high schools in these sites. Study objective: to study correlates of physical activity and nutrition behaviours and change in BMI percentile and body fat among adolescent girls. Study population: 13‐ to 18‐year‐old girls at high schools in San Diego and Minneapolis. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: 8th grade girls who were control participants enrolled in the TAAG study cohort from 2 sites. Excluded criteria: NR. Brief description of participants: school girls, in grade 8 across 7 high schools from 2 sites in the USA (San Diego and Minneapolis/St Paul). During study period, participants were aged 13‐18 years. Total number completed in cohort study: 265 (87%). Total number enrolled in cohort study: 303. |
|
| Interventions |
Description of exposure for cohorts
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
BMI
Body fat
|
|
| Identification |
Sponsorship source: National Health, Lung and Blood Institute. Country: USA. Setting: high schools, San Diego and Minneapolis. Comments: NA. Author's name: Deborah A Cohen. Institution: RAND Corporation. Email: dcohen@rand.org. Declaration of interests: yes. "None of the authors have any financial relationships relevant to this article or other conflicts of interest to disclose." Study ID: Cohen 2014. Type of record: journal article. Trial ID: TAAG. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | Low risk | Attrition low (13%; 38/303). Children with incomplete data did not differ from children with complete data in terms of ethnicity, mother's education and age (data NR). |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | High risk | Data analysis did not adjust for pubertal stage, parental BMI and total energy intake at baseline. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | High risk | Methods used to measure body fat were inconsistent during the study (skinfold thickness measurements at baseline, BIA during follow‐up). |
| Can we be confident in the assessment of exposure? All outcomes | High risk | No baseline dietary assessment. Unclear whether they received any training or assistance regarding the completion of the FFQ during follow‐up. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | Repeated measurements of physical activity data were performed (accelerometer data for 6 consecutive days). 16.8% of data imputed. Self‐report of variables such as age, ethnicity and mother's education was acceptable at this age. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | All control participants of the TAAG cohort. |
Davison 2001.
| Methods |
Study design: prospective cohort study. Analyses methods for cohorts: hierarchical regression used. Predictor variables hypothesised to be most distal to girls' change in BMI (i.e. parent weight status) were entered 1st into model followed by predictors that were more proximal to girls' change in BMI (i.e. girls' physical activity and dietary intake). How were missing data handled? Only families with complete anthropometric data at both time points were used in analyses, resulting in (85.3%; 168/197). 12 families with outlying BMI values (i.e. > 3 SDs from the mean) were identified and removed from analyses. Characteristics of children with missing data NR. Number of study contacts: 2 (at baseline‐5 years and 2 years' follow‐up). Period of follow‐up (total period of observation): 2 years. Periods of recruitment: NR. Sample size justification adequately described? No. Sampling method: convenience sample. Families recruited using flyers and newspaper advertisements. In addition, families with age‐eligible girls within 5‐county radius received letters inviting them to participate and received follow‐up telephone calls. Study objective: to assess predictors of change in girls' BMI aged 5‐7 years and familial aggregation of risk factors associated with childhood overweight. Study population: 5‐year old white girls in Pennsylvania, USA. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: 5 years; living with both biological parents; absence of severe food allergies or chronic medical problems affecting food intake; absence of dietary restrictions involving animal products. Families were not recruited on weight status. Excluded criteria: NA. Brief description of participants: 5‐year old white girls from central Pennsylvania who were part of a longitudinal study of the health and development of young girls. Total number completed in cohort study: 192 girls (168 included in analysis). Total number enrolled in cohort study: 197 girls. |
|
| Interventions |
Description of exposure for cohorts
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
BMI
|
|
| Identification |
Sponsorship source: National Institutes of Health. Country: USA. Setting: households, Pennsylvania. Comments: NA. Author's name: KK Davison. Institution: Pennsylvania State University. Email: kdavison@psu.edu. Declaration of interests: no. Study ID: Davison 2001. Type of record: journal article. |
|
| Notes | We contacted the authors as they did not report relevant regression coefficients in their regression models. We had not received a response by time of publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | High risk | High attrition (15% (29/197) over 2 years). |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | Low risk | Analyses adjusted for baseline BMI, physical activity, total energy intake of the child and BMI, education and income of parents (SES). |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | Unclear risk | Assessment methods (weight, height) not adequately described. |
| Can we be confident in the assessment of exposure? All outcomes | High risk | Single dietary assessment at baseline (3 × 24‐hour recalls over a 2‐ to 3‐week period during summer). |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | High risk | Methods used to assess physical activity of children at baseline and follow‐up were inconsistent. Only a single assessment of physical activity of parents performed at baseline. Assessment methods for parental weight and height not adequately described. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | Children selected for 1 cohort study. |
Jago 2005.
| Methods |
Study design: prospective cohort study. Analyses methods for cohorts: repeated measures regression analysis with year as a factor and BMI in each year as dependent variable. Behaviours (TV viewing, sedentary behaviour, physical activity and diet variables), demographics (ethnicity and gender), BMI from the beginning of study and interaction terms for variables differing by year (TV viewing, physical activity, sedentary behaviour) included as independent variables. How were missing data handled? Lost to follow‐up at 3 years: 10.7% (16/149), additional information NR. Number of study contacts: 3 (1, 2 and 3 years). Period of follow‐up (total period of observation): 3 years. Period of recruitment: Between summers of 1986 and 1989. Sample size justification adequately described? No. Sampling method: convenience sample. Families recruited using various methods, including newspaper advertisements, fliers and word of mouth. No details provided regarding number of potentially eligible families. Study objective: to examine whether physical activity, TV viewing, other sedentary behaviours and dietary factors predict BMI among a triethnic cohort of 3‐ to 4‐year‐old children followed over 3‐year period. Study population: healthy 3‐ to 4‐year‐old children in the USA. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: 3‐ to 4‐year‐old children with their parents, with only 1 eligible child per family. Excluded criteria: NR. Brief description of participants: healthy 3‐ to 4‐year‐old Anglo‐American, African‐American and Hispanic children in the USA participating in a multicentre study on development of cardiovascular risk factors and associated behaviours. Total number completed in cohort study: 138 (only reported in table). Total number enrolled in cohort study: 149. |
|
| Interventions |
Description of exposure for cohorts
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
BMI
|
|
| Identification |
Sponsorship source: National Heart Lung and Blood Institute, USDA. Country: USA. Setting: NR. Comments: Studies of Child Activity and Nutrition (SCAN) multicentre study. Author's name: R Jago. Institution: Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA. Email: russ.jago@gmail.com. Declaration of interests: no. Study ID: Jago 2005. Type of record: journal article. |
|
| Notes | We contacted the authors to request relevant regression data, since they stated the following in the text: "Dietary factors were not associated with BMI across the three study years." Authors replied that they no longer had the relevant data available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | Low risk | Lost to follow‐up at 3 years: 10.7% (16/149). |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | High risk | No adjustment for total energy intake, parental BMI and SES. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Standardised measurements performed (height, weight). |
| Can we be confident in the assessment of exposure? All outcomes | High risk | Although DRs were done during each study year by direct observation, method may have introduced bias in dietary behaviour of participants. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | High risk | Although assessments of physical activity/inactivity were done during each study year by direct observation using validated methods, direct observation of participants may have introduced bias in their behaviour. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | All participants from 1 cohort study. |
Klesges 1995.
| Methods |
Study design: prospective cohort study. Analyses methods for cohorts: stepwise multiple regression analysis assessed whether baseline % energy from fat, change from baseline to 1 year, 1 year to 2 years, or baseline to 2 years (along with other variables) predicted change in BMI over 2 years. How were missing data handled? Missing data at baseline: 2 fathers were unavailable for baseline assessments (due to multiple scheduling conflicts), 6 families had some missing measures (no reasons given). Lost to follow‐up at 1 year: 35 families were unavailable after 1 year (20.8%); lost to follow‐up at 2 years: 57 (28.1%). Preliminary analyses investigated whether differences due to attrition were significant on baseline variables. 3 groups of families were formed: participants who did not return for the 1‐year follow‐up, participants not returning for the 2‐year follow‐up and participants who completed the study. No significant differences between groups on children's baseline body mass, energy intake, diet composition (percent of kilocalories from fat), physical activity, sex or familial risk of obesity (P > 0.15). Number of study contacts: 3 (baseline, 1 and 2 years). Period of follow‐up (total period of observation): 2 years. Periods of recruitment: NR. Sample size justification adequately described? No. Sampling method: convenience sample of 219 families with 3‐ to 5‐year‐old children recruited through local paediatricians, daycare centres and churches in Memphis, TN, USA. Study objective: to investigate the extent to which largely modifiable and non‐modifiable risk factors simultaneously predicted weight gain and to determine the precise dietary, physical activity and demographic predictors of weight change in preschool children over a 3‐year period. Additionally, changes in largely modifiable risk factors (e.g. increases or decreases in dietary intake) were evaluated to reflect the dynamic nature of body mass change. Study population: preschool children in Memphis, TN. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: natural, biological offspring of his/her parents; no physical handicap or condition that could affect relative weight, dietary intake or physical activity; had parents who were married; had parents without CVD; and had a family who planned to stay in the metropolitan area in the coming year. Excluded criteria: NR. Brief description of participants: preschool children aged 3‐5 years. Total number completed in the cohort study: 146 children completed study; 73 children with some missing data (8 mothers pregnant, 2 fathers not available for baseline assessment, 35 families not available after 1 year, 22 not available at 2 years' follow‐up). Total number enrolled in cohort study: 219 children, including 3 sets of twins of whom only 1 was chosen randomly. |
|
| Interventions |
Description of exposure for cohorts:
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
BMI
|
|
| Identification |
Sponsorship source: National Blood, Heart and Lung Institute. Country: USA. Setting: community. Comments: NA. Author's name: Robert C Klesges. Institution: University Prevention Center, Department of Psychology, The University of Memphis, and the Department of Preventive Medicine, University of Tennessee, Memphis, TN, USA. Email: NR. Declaration of interests: no. Study ID: Klesges 1995. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | Low risk | Although attrition was high (33% over 2 years), authors demonstrated no significant differences (P > 0.05) in baseline BMI, energy intake and diet composition between participants completing the study and participants who did not. |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | Low risk | Child age, sex, baseline BMI, baseline energy intake, physical activity and parental BMI were adjusted using multiple regression analyses. Model was not adjusted for ethnicity or SES; however, authors report that participants were mostly white middle‐class children (data not provided). |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Standard anthropometric methods used. |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | Multiple dietary intake assessments completed by both parents and children using the Willett FFQ (baseline, 1 and 2 years). Questionnaire was validated, and assessed dietary intake over the previous 1‐year period. All questionnaires were checked for completeness while families were still present to correct missing data. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | Child age, sex, baseline BMI, baseline energy intake, physical activity and parental BMI were adjusted using multiple regression analyses. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | All participants in analysis were recruited through local paediatricians, daycare centres as participants of 1 cohort study |
Lee 2001.
| Methods |
Study design: prospective cohort study. Analyses methods for cohorts: girls divided into 2 groups (LF group 20‐30%TE; HF group > 30%TE). The GLM, ANOVA conducted to compare food group intakes, weight status and maternal feeding practices between groups. How were missing data handled? NR. Number of study contacts: baseline (aged 5 years) and after 2 years (aged 7 years) (not clearly reported). Period of follow‐up (total period of observation): 2 years. Period of recruitment: NR. Sample size justification adequately described? No. Sampling method: convenience sample. Girls aged 5‐years and their mothers who were participating in a longitudinal project investigating development of controls of food intake and dieting of girls. Families recruited using flyers and newspaper advertisements. Families with age‐eligible girls (total number NR) within 5‐county radius also received mailings and follow‐up telephone calls. Study objective: to compare girls' diets that had 30% of energy from fat with those meeting the AAP recommendations to maintain dietary fat intake at 30% of energy. Study population: healthy 5‐year‐old girls and their mothers. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: 5‐year old girls living with both biological parents. Excluded criteria: severe food allergies or chronic medical problems affecting food intake, and dietary restrictions involving animal products. Brief description of participants: healthy 5‐ to 7‐year‐old white girls in Pennsylvania, USA. Total number completed in cohort study: 192. Total number enrolled in cohort study: 197. |
|
| Interventions |
Description of exposure for cohorts
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
BMI
Skinfold thickness
|
|
| Identification |
Sponsorship source: National Institutes of Health and the National Dairy Council. Country: USA. Setting: household. Comments: NA. Author's name: Yoonna Lee. Institution: Human Development and Family Studies, Pennsylvania State University. Email: llb15@psu.edu. Declaration of interests: no. Study ID: Lee 2001. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | Low risk | Authors stated that 5 girls (2.5% over 2 years) were excluded because of a dietary misreporting (fat intake < 20%). |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | High risk | Matching NR. Authors did not control for any prognostic factors in analyses. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Unclear risk | NR. |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Standardised methods used at baseline and follow‐up (weight, height, skinfold thickness measurements). |
| Can we be confident in the assessment of exposure? All outcomes | High risk | Single assessment of dietary intake at baseline (3 × 24‐hour recalls during 2‐week period). |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | No data reported in relation to prognostic factors. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | All participants of 1 cohort study. |
Lee 2012.
| Methods |
Study design: prospective cohort study. Analyses methods for cohort: multivariate linear regression modelling for 2 years BMI change of 1st graders and 4th graders. Predictor variables were environmental factors, parental and lifestyle habits. Dependent variables were BMI change between 4 and 6 years' follow‐up. Model adjusted for age, sex, sexual maturation at 6 years' follow‐up (Tanner stage I, II, III, IV, V), baseline BMI, and exercise frequency, screen time, sleep duration, household income, parental BMI, parental education, maternal job, family structure, energy intake, meal skipping and snacking. They only adjusted for the BMI in the 4th survey at 6 years' follow‐up. How were missing data handled? Analytic sample taken of total number of children participating in study. Analytic sample was of children who participated at 4 and 6 years' follow‐up; total of 1504 participants. Original sample was of 893 but new participants were recruited over years (2776 participants at 5 years' follow‐up and 2770 at 6 years' follow‐up). Number of study contacts: 3 (baseline, 1 and 2 years). Period of follow‐up (total period of observation): both 1st graders and 4th graders were followed up for 2 years. Period of recruitment: baseline: 2005. New recruitment in 2008. Sample size justification adequately described? No. Sampling method: in 2005, all 1st graders of 4 elementary schools in Gwacheon city, Seoul were included. In 2008, 1st and 4th graders from 2 elementary schools in Jung‐gu, Seoul and 5 elementary schools in southwestern Gyeonggi province were added to the cohort. Study objective: to assess risk factors associated with children's BMI and their changes over a 2‐year period based on the analysis of the Obesity and Metabolic Disorders Cohort in Childhood registry. Study population: children in elementary school, grades 1 and 4. |
|
| Participants |
Baseline characteristics (reported for 1 overall group) 1st graders (n = 474); 4th graders (n = 1030)
Included criteria: NR. Excluded criteria: NR. Brief description of participants: 474 1st graders (31.5%) and 1030 4th graders (68.5%). Mean ages: 1st graders: 7.3 (SD 0.3) years; 4th graders: 10.0 (SD 0.4) years. Mean BMI of 1st graders 16.0 (SD 2.3) kg/m2 with 12.0% being over 85th percentile of BMI curve, whereas mean BMI of 4th graders was 18.1 (SD 3.0) kg/m2 with 17.3% being over 85th percentile of BMI curve. Total numbers completed in cohort study: analytic sample taken from entire cohort: 1504. Total number enrolled in cohort study: 893 children enrolled in 2005, and another 1847 children enrolled in 2008, thus total 2740. However, in Figure 1 for the 5 years' follow‐up, it showed that there were, at one point, 2776 children enrolled. |
|
| Interventions |
Description of exposure for cohort
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
BMI
|
|
| Identification |
Sponsorship source: NR. Country: Korea. Setting: Elementary schools, Gwacheon city, Seoul. Comments: study name: Obesity and Metabolic Disorders Cohort in Childhood. Author's name: Hyun Hye Lee. Institution: Department of Family Medicine, Inje University College of Medicine, Seoul, Korea. Email: drparkhyunah@gmail.com. Declaration of Interests: Yes. "No potential conflict of interest relevant to this article was reported." Study ID: Lee 2012. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | High risk | Authors used an analytical sample and did not analyse entire cohort, which consisted of 2776 children. Reasons for this not provided. Loss to follow‐up not discussed. |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | Low risk | Adjusted for age, sex, sexual maturation at 6 years' follow‐up, baseline BMI, exercise, screen time, sleep duration, household income, parental BMI and education, maternal job, family structure, energy intake, meal skipping and snacking. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Trained researchers measured height and weight; used sex‐specific 2007 growth charts for Korean children. |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | Authors reported: "Dietary intake was recorded for two weekdays and one day on the weekend by a 24‐hour recall method." Large sample size with multiple assessments to provide usual intake estimation. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | Over the 2‐year follow‐up period physical activity and screen time was assessed at least twice, with detailed definitions for moderate and vigorous activity to guide parents and children with this. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | NA as study did not divide participants into exposed and unexposed groups. All participants were sampled from similar locations. |
Magarey 2001.
| Methods |
Study design: prospective cohort study. Analyses methods for cohort: generalised linear estimating equations evaluated longitudinal relationship between body fatness and macronutrient intake. Regression analysis assessed whether body fatness at a particular age was predicted by intake at any of the previous ages. How were missing data handled? Considerable attrition occurred from 500 selected at birth to 198 at 2 years and 130 at 11 years. Information on participants lost before 8 years not available, but sociodemographic status of children remaining in cohort at 8 years was upwardly skewed compared to original cohort due to cohort attrition. Therefore, new recruitment (n = 113) done at age 11 years with age‐matched and socioeconomic balanced to the cohort (Magarey and Boulton 1994). Number of study contacts: 7 (at 2, 4, 6, 8, 11, 13 and 15 years of age). Period of follow‐up (total period of observation): 13 years. Periods of recruitment: November 1975 to June 1976. Sample size justification adequately described? No. Sampling method: 500 infants randomly selected by birth order from healthy term infants born at Queen Victoria Hospital, Adelaide, South Australia between November 1975 and June 1976. Core sample of approximately 150 children was retained in a longitudinal study of growth and nutrition from birth to 15 years of age. A further 113 children recruited for the 11‐year assessment from an age‐matched cross‐sectional sample of 715 children who had taken part in a family heart disease risk factor precursor study when they were 8 years of age. Study objective: to investigate the longitudinal relationship between macronutrient intake and adiposity at ages 2‐15 years. Study population: healthy born children aged 2‐15 years in Adelaide, South Australia. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: children who participated in the Adelaide Nutrition Study aged 2‐15 years with available follow‐up data. Excluded criteria: NR. Brief description of participants: children who participated in the Adelaide Nutrition Study aged 2‐15 years with 12‐16% of the boys being overweight, 12‐16% of prepubertal girls (aged 2‐8 years) and 17‐22% of adolescent girls (aged 11‐15 years). Total number completed in cohort study: 218 (at 15 years). Total number enrolled in cohort study: 500 (at birth) + 113 (at 11 years). |
|
| Interventions |
Description of exposure for cohort
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
Weight
BMI
Skinfold thickness
Height
|
|
| Identification |
Sponsorship source: National Heart Foundation of Australia, Adelaide Children's Hospital Research Foundation and the National Health and Medical Research Council of Australia. Country: Australia. Setting: community in Adelaide. Comments: Adelaide Nutrition Study (birth cohort). Author's name: AM Magarey. Institution: Department of Public Health, The Flinders University of South Australia. Email: NR. Declaration of interests: no. Study ID: Magarey 2001. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | High risk | High attrition (71.4% over 8 years). No information available on children lost to study between 2 and 8 years. Attrition at 11 years: 74%. Since the children who returned had an upwardly skewed sociodemographic profile, another 115 children were recruited from an age‐matched cross‐sectional sample. |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | High risk | No matching reported. Ethnicity, SES, physical activity and pubertal stage not adjusted for in regression analyses. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Unclear risk | NR. |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Anthropometric measurements done using standard methods by 1 observer. |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | Repeated weighed 3‐day DRs completed by parents and children throughout study. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Unclear risk | Parental anthropometric data were investigator‐measured once when children were 8‐9 years old. Method not described. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | It is likely the 2 groups were from the same population although the original sample were selected from a single hospital (Victoria, Adelaide, Australia) and the additional sample from the same birth cohorts were purposively selected to balance demographic characteristics of the cohorts. |
Mihas 2010.
| Methods |
Study design: RCT. Study grouping: parallel. Allocation ratio in RCTs: 1:1. Analyses methods for RCTs: available‐case analysis; end values. Description of randomisation: from 286 finally eligible students, 218 were assigned randomly using a computerised random number generator to participate in the study in 2 groups of 109 students (intervention group and control group). How were missing data handled? Over 12 months, 11 participants lost in intervention group and 16 in control group. Data analysed based on participants having full data at end of follow‐up (98/109 randomised in intervention group; 93/109 randomised in control group). Number of study contacts: 3. Period of follow‐up (from when duration of active intervention period ended): 14 months. Periods of recruitment: NR. Intervention took place between September 2007 and January 2008. Sample size justification adequately described? Was based on previously reported intervention changes in energy intake among children. To detect standardised differences > 5% in dietary intake (main dependent variable) between study groups before and after intervention, achieving 90% statistical power at a probability level < 0.05, 87 participants should be recruited in each study group. To counter potential low response and dropouts, the authors increased this number by 25% to 109 for each study group. Sampling method: 342 adolescents of 5 high schools located in Vyronas district were initially eligible. 309/342 students voluntarily were interested in participating in study. Study objective: to evaluate short‐term (15‐day) and long‐term (12‐month) effects of a 12‐week school‐based health and nutrition interventional programme regarding energy and nutrient intake, dietary changes and BMI. Study population: students aged 12‐13 years (7th grade). |
|
| Participants |
Baseline characteristics (reported for 2 groups and overall) Lower fat intake (≤ 30%TE)
Usual or modified fat intake
Overall
Included criteria: children aged 12‐13 years at high schools located in Vyronas district, Athens, Greece. Excluded criteria: organic cause for high or low weight, received any medication that might interfere with growth or weight control, or were on specific diets. Pretreatment: no significant differences in age, gender, BMI, overweight/obesity, smoking, screen time, weekly hours of sport activities, weekly hours of playing or walking, and weekly hours of hobbies between groups before the nutrition intervention. Brief description of participants: 12‐ to 13‐year‐old adolescents from Greece; CVD risk: very few children were regular smokers. Total number completed RCT: 98 in intervention group; 93 in control group. Total number randomised: 218. |
|
| Interventions |
Intervention characteristics Lower fat intake (≤ 30%TE)
Usual or modified fat intake
|
|
| Outcomes |
BMI
Energy intake
Fat intake
Saturated fat intake
Protein intake
CHO intake
|
|
| Identification |
Sponsorship source: Ministry of Education and the National Foundation for the Youth. Country: Greece. Setting: high schools, Vyronas district, Athens. Comments: NA. Author's name: Constantinos Mihas. Institution: Department of Internal Medicine, General Hospital of Kimi 'G. Papanikolaou,' Kimi, Evia, 34003 Greece. Email: gas521@yahoo.co.uk. Declaration of interests: yes; conflicts of Interest: none declared. Study ID: Vyronas 2009. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator used; baseline characteristics similar between groups. |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors stated blinding not feasible, but primary outcome not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors stated that blinding was not feasible, but assessment of primary outcome not likely influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar in both groups, paper mentioned loss of 5 participants during trial (due to health problems, lack of interest and move to other schools). Of 109 allocated in each group, 10 in intervention group and 12 in the control group were not analysed (reasons unclear). 10% (22/213) lost over 17 months. |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but prespecified outcomes in methods reported in results section. |
| Other bias | Unclear risk | Limited information on control group diet prescription, unable to judge if prescribed diets being compared differed in components other than total fat. |
Morrison 2008.
| Methods |
Study design: prospective cohort study. Analyses methods for cohort: regression model by stepwise selection from explanatory variables: age, BMI, IR and maturation stage at baseline; change in IR over 10 years' follow‐up; total calorie intake; percentage of calories from protein, fat and CHO (mean of interviews) during 10 years' follow‐up; and interaction terms (nutrients X baseline IR). How were missing data handled? NR. Number of study contacts: 10. Period of follow‐up (total period of observation): 10 years. Periods of recruitment: January 1987 to May 1988. Sample size justification adequately described? Reported for NGHS multicentre study. Primary consideration for sample size was adequate power for comparing change in subscapular skinfold between black and white girls. Sample size was increased to maintain sufficient power should only 65% of children be available for follow‐up measurements. Calculated target sample size was 1150 per group. Sampling method: convenient sampling by 3 clinical centres from public and parochial schools at Berkeley, Cincinnati and Westat (members of a medical program), USA. Study objective: to evaluate the role of preteen IR resistance and insulin in adolescent weight gain and the development of IFG and T2DM. Hypothesised that preteen IR, interacting with dietary factors such as total calories and fat calories, and 10‐year change in IR would positively predict 10‐year increases in BMI and the development of IFG and T2DM. Study population: white and black girls aged 9‐10 years living in Berkeley, Cincinnati and Westat, USA. |
|
| Participants |
Baseline characteristics (reported as 1 overall group and 1 matched subsample) Overall
Subsample (paired matched at enrolment by pubertal stage, FM and insulin)
Included criteria: declared themselves as black or white; aged within 2 weeks of 9 or 10 years at time of 1st clinical visit; parents or guardians who identified themselves as same race as child; parents or guardians completed a household demographic information form and gave consent. Excluded criteria: other ethnic groups. Brief description of participants: 9‐ to 10‐year‐old black and white girls. Total number completed in cohort study: overall n = 639; white n = 280; black n = 359. Total number enrolled in cohort study: overall n = 2379; white n = 1166; black n = 1213. |
|
| Interventions |
Description of exposure for cohort
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
BMI
WC
|
|
| Identification |
Sponsorship source: National Heart, Lung, and Blood Institute and the Lipoprotein Research Fund of the Jewish Hospital of Cincinnati. Country: USA. Setting: clinical centres (Berkeley, Cincinnati and Westat). Comments: NGHS. Author's name: John A Morrison. Institution: Division of Cardiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH (JAM); the Cholesterol Center, Jewish Hospital of Cincinnati, Cincinnati, OH (CJG and PW); the Department of Mathematics, University of Cincinnati, Cincinnati, OH (PSH). Email: john.morrison@cchmc.org; Glueckch@healthall.com. Declaration of interests: yes. "No conflicts of interest for any authors." No honorarium, grant, or other form of payment was given to anyone to produce the manuscript. "None of the authors had a personal or financial conflict of interest." Study ID: Morrison 2008. Type of record: journal article. |
|
| Notes | We contacted authors to request relevant regression data since they did not report the regression coefficients for total dietary fat intake alone as a predictor variable of body fatness in their regression models. We had not received a response by time of publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | Low risk | Out of 639 girls with complete BMI outcome data, only 521 (81.5%) had dietary data. For 10‐year waist changes, 512 girls had complete data. No assessment comparing girls with dietary data compared to girls who did not. |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | High risk | Regression model (n = 521) performed by stepwise selection including age, BMI, IR and pubertal stage, 10‐year change in IR, total TE, percentage of calories from fat, protein, CHO during follow‐up period and interaction terms (nutrients × baseline IR). Physical activity/inactivity, parental BMI or SES not included in regression model. Secondary analyses (n = 172) with pair‐matched for race (black‐white); pubertal stage, BMI and insulin levels at 9‐10 years, adjusted for parental obesity level. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Standard methods used for measurement of height, weight, skinfold and circumference measurements. |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | Dietary intake assessed using repeated 3‐day DRs. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | Data collection methods well described for most variables (e.g. pubertal staging, parental obesity). |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | All participants of the NHLBI growth and health study. |
Niinikoski 1997a.
| Methods |
Study design: prospective cohort study. Analyses methods for cohort: intervention and control children from the STRIP RCT analysed together. Repeated measures unbalanced ANOVA used to compare growth of children who were continuously in lowest fat intake quartile (at 24 months, 27.7%TE and 36 months, 28.7%TE) and children in higher fat intake quartiles. Linear regression model used to predict relative weight on age (children aged between 7 and 30‐36 months with 2 to 2.5 years' follow‐up and who had at least 5 measurements were included in this analysis). How were missing data handled? Children with 5 follow‐up measurements included in analyses while information on children with missing data NR. Number of study contacts: 3 (at 24, 30 and 36 months of age). Period of follow‐up (total period of observation): cohort, 2.5 years; present analyses, 1 year. Periods of recruitment: March 1990 to May 1992. Sample size justification adequately described? Yes, for RCT part of STRIP study. "The required sample size for the trial was predicted to achieve, at a 1% significance with 80% power, a 0.2‐mmol/L true difference in the change of serum cholesterol concentration between the study groups, assuming that the SD of serum cholesterol concentration is 0.9 mmol/L." Sampling method: convenience. Study included 1062 infants of 1054 families (56.5% of eligible families) from the well‐baby clinics of Turku, Finland. Study objective: "to study the fat and energy intakes of children between 7 and 36 months of age with different growth patterns." Study population: 24‐ to 36‐month old toddlers in Turku, Finland. |
|
| Participants |
Baseline characteristics (reported for 2 groups and overall group) Five groups of children representing different extreme growth patterns during the first three years of life were formed (groups: thin, slow‐weight‐gain, normal, rapid‐weight‐gain, and obese ‐ grouped according to relative weight), and their energy and fat intakes analysed. A lower fat (LF) intake group was then formed with children constantly belonging to the lowest relative fat intake quartile, and the rest allocated to other children/higher fat (HF) intake group. Relative weight was defined as deviation of weight in percentages from the mean weight of healthy children of the same height and sex. LF intake
Other children or HF intake
Thin group
Slow weight gain group
Normal group
Rapid weight gain group
Obese group
Overall
Included criteria: families of infants attending routine 5‐month clinic visit. Excluded criteria: NR. Brief description of participants: healthy 24‐ to 36‐month‐old toddlers who participated in the STRIP Baby Trial. Total number completed in cohort study: 848 (children with ≥ 5 measurements between 7 and 36 months included in reported analysis). Total number enrolled in cohort study: 1062. |
|
| Interventions |
Description of exposure for cohort
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
Weight
|
|
| Identification |
Sponsorship source: Mannerheim League for Child Welfare; Finnish Cardiac Research Foundation; Foundation for Pediatric Research, Finland; Academy of Finland; Yrjo ¨ Jahnsson Foundation; Juho Vainio Foundation; Turku University Foundation; City of Turku; Chymos Ltd; Raisio Group; and Van den Bergh Foods Company. Country: Finland. Setting: well‐baby clinics of Turku. Comments: NA. Author's name: Harri Niinikoski. Institution: Cardiorespiratory Research Unit and Department of Pediatrics, University of Turku, Turku, Finland. Email: NR. Declaration of Interests: no. Study ID: Niinikoski 1997. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | High risk | With 30.3% over 1 year lost (740 completed out of 1062 recruited), information on characteristics of children lost to follow‐up NR. |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | High risk | No matching reported. No adjustment of prognostic variables. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | High risk | LF‐intake group likely included toddlers who had been exposed to the nutrition intervention programme. |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Standardised methods for anthropometric measures (weight and height) was performed. |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | Multiple assessments (24, 30 and 36 months) using 4‐day DRs, which included at least 1 weekend day. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | Parental BMI measurement was measured at each visit. Although physical activity was not measured, it is not an important variable at this age |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | All children were recruited from the same Well‐Baby clinics in Turku, Finland. |
Obarzanek 1997 (cohort).
| Methods |
Study design: RCT (cohort analysis). Analyses methods for cohorts: longitudinal linear regression models using data from all 3 time points and taking into account correlation between measurements on same person. How were missing data handled? Attrition at 1 year' follow‐up: 7% (46/663); at 3 years: 5% (31/663). Missing data from children who attended follow‐up visits averaged 3% for dietary measures and 5% for biochemical measures. Number of study contacts: 3 (baseline, follow‐up after 1 and 3 years). Period of follow‐up (total period of observation): 3 years (for this analysis). Periods of recruitment: started 1987. Sampling method: convenience sample of 47,000 children prescreened at schools, prepaid health plans and physician clinics at 6 clinical centres; 5122 children attended 1st screening visit; 1637 children attended 2nd screening visit; 752 attended baseline visits (potentially eligible). Study objective: to assess relationship between energy intake from fat and anthropometric, biochemical, and dietary measures of nutritional adequacy and safety. Study population: school children aged 8‐10 years with moderately elevated LDL‐C levels in USA. |
|
| Participants |
Baseline characteristics (reported as 1 overall group)
Included criteria: boys and girls aged 8‐11 years with primary elevated serum LDL‐C levels (defined as mean of 2 fasting values between 80th and 98th age‐ and sex‐specific percentiles), with no evidence of pubertal development (Tanner stage I) and normal psychosocial and cognitive development. Excluded criteria: major illness; medications that might affect blood lipids or growth (or both); weight‐for‐height < 5th or > 90th percentile, or height 5th percentile for sex‐ and race‐specific growth curves; any household member on a LF or "cholesterol‐lowering" diet; and parental factors such as prior heart disease, extreme obesity or excessive intake of alcohol, which are potential barriers to dietary adherence by the child. Children with serum levels of TGs > 200 mg/dL or of HDL cholesterol 30 mg/dL. Total number completed in cohort study: 632 (at 3 years' follow‐up). Total number enrolled in cohort study: 663. |
|
| Interventions |
Description of exposure for cohorts
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
Weight
BMI
Skinfold thickness
SBP
DBP
Height
|
|
| Identification |
Sponsorship source: NHLBI. Country: USA. Setting: 6 clinical centres. Comments: Dietary Intervention Studies in Children (DISC). Author's name: Eva Obarzanek. Institution: DISC Coordinating Center, Maryland Medical Research Institute. Email: obarzane@nhlbi.nih.gov. Declaration of Interests: no. Study ID: Obarzanek 1997. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | Low risk | Low attrition during follow‐up (7% (46/663) over 1 year; and 6% (40/663) over 3 years). |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | High risk | Analyses adjusted for sex, physical activity and total energy intake. No adjustment for pubertal stage, parental BMI or SES. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Standardised measurements of weight, height and skinfold thickness performed by trained staff. |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | Repeated assessments of dietary intake (baseline, 1 and 3 years' follow‐up) using multiple 24‐hour dietary recalls. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | Repeated assessment of physical activity using validated questionnaire. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | Children selected as participants of 1 RCT. |
Obarzanek 2001 (RCT).
| Methods |
Study design: RCT. Study grouping: parallel group. Allocation ratio in RCTs: 1:1. Analyses methods for RCTs: ITT; end values reported. Description of randomisation: "Computer‐generated randomisation assignments were provided by the coordinating centre to produce within each clinical center approximately equal numbers of participants assigned to the intervention and usual care groups balanced by age and sex;" central allocation; NR who enrolled participants. How were missing data handled? "It was assumed that missing data in both groups would have come from the same distribution as observed data in the usual care group, so missing year 3 LDL‐C data were estimated by drawing values from the usual care group distribution;" "Analyses of secondary outcomes using no imputation for missing values used ANCOVA models for continuous outcomes and Wilcoxon tests for ordered categorical outcomes. Baseline level and sex were included as covariates." Number of study contacts: 8. Period of follow‐up (from when duration of active intervention period ended): approximately 3 years. Period of recruitment: 2.5 years. Sample size justification adequately described? yes: "The sample size of 300 in each treatment group was based on estimates of intervention efficacy. The primary outcomes will be tested at a two‐sided significance level of u=0.05. To test the primary efficacy hypothesis with 90% power, the sample size needed per group is given by n = 2 (1.96 + 1.28)*var/A2, where A is the difference between the average changes in the treatment and control groups, and var is the variance of A. Variance estimates were derived from Bogalusa Heart Study data, using 8‐ to I0‐year‐old children with LDL‐C levels in the 75 to 98th percentile, and calculating baseline and 36‐month follow‐up variances as well as the correlation at these two times." Sampling method: mass mailing used to recruit children from schools, a health maintenance organization and paediatric practices; > 47,000 children were prescreened for potential eligibility; n = 5122 seen for screening 1; n = 1637 for screening 2; n = 752 for baseline visit. Study objective: to assess efficacy and safety of lowering dietary intake of total fat, saturated fat and cholesterol to decrease LDL cholesterol levels in children. Study population: prepubescent boys and girls with primary elevated serum LDL cholesterol levels. |
|
| Participants |
Baseline characteristics (reported for 2 groups and overall group) Lower fat intake (≤ 30%TE)
Usual or modified fat intake
Overall
Included criteria: boys aged 8 years 7 months to 10 years 10 months and girls aged 7 years 10 months to 10 years 1 month, with primary elevated serum LDL‐C levels (defined as mean of 2 fasting values between 80th and 98th age‐ and sex‐specific percentiles), with no evidence of pubertal development (Tanner stage I) and normal psychosocial and cognitive development. Excluded criteria: major illness; medications that might affect blood lipids or growth (or both); weight‐for‐height < 5th or > 90th percentile, or height < 5th percentile for sex‐ and race‐specific growth curves; any household member on a LF or "cholesterol‐lowering" diet; and parental factors such as prior heart disease, extreme obesity or excessive intake of alcohol, which are potential barriers to dietary adherence by the child. Children with serum levels of TGs > 200 mg/dL or of HDL‐C < 30 mg/dL. Pretreatment: NR. Brief description of participants: prepubertal boys (approximately n = 362) and girls (approximately n = 301) aged 7‐11 years with LDL‐C levels ≥ 80th and < 98th percentiles for age and sex percentiles of the Lipid Research Clinics population. Total number completed in RCT: last visit for BMI (> 5 years): intervention group n = 293; control group n = 283. Total number randomised: total n = 663; intervention group n = 334; control group n = 329. |
|
| Interventions |
Intervention characteristics Lower fat intake (≤ 30%TE)
Usual or modified fat intake
|
|
| Outcomes |
Weight
BMI
Total cholesterol
LDL‐C
HDL‐C
TGs
SBP
DBP
Height
Energy intake
Fat intake
Saturated Fat intake
Protein intake
CHO intake
|
|
| Identification |
Sponsorship source: NHLBI. Country: USA. Setting: 6 clinical centres. Comments: NA. Author's name: Eva Obarzanek. Institution: DISC Coordinating Center, Maryland Medical Research Institute, Baltimore, MD, USA. Email: obarzane@nhlbi.nih.gov. Declaration of interests: no. Study ID: DISC 2001. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | In DISC 1995, "computer‐generated randomisation assignments were provided by the coordinating center to produce within each clinical center approximately equal number of participants assigned to the intervention and usual care groups balanced by age and sex." Baseline characteristics similar between groups. |
| Allocation concealment (selection bias) | Low risk | In DISC 1993 authors stated, "eligible children were allocated randomly to intervention and usual‐care groups by the coordinating centre..." thus it appeared that there was a central allocation centre and recruitment at the clinical centres could not have been manipulated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | In DISC 1993, "though it was not possible to have a double blind trial due to the nature of dietary intervention, a single blind was maintained by using data collectors unaware of group assignment." Participants not blinded. However, lack of double blinding was not likely to influence the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers lost to follow‐up: at 3 years: intervention group 14/334 (4.2%) and control group 26/329 (7.9%) (no reasons). At 7 years: intervention group 39/334 (11.7%) and control group 44/329 (13.4%) (no reasons). No differences in age, height, weight, BMI, total and saturated fat intake, serum LDL‐C or serum ferritin, and in distributions of sex, household income and education in those attending final visit vs dropouts. Missing the last visit was not related to treatment assignment. Primary outcomes analysed using ITT, imputation process described; secondary outcomes analysed using per protocol analyses. |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but paper with study design and baseline characteristics available and all the study's prespecified outcomes were reported in the results section. |
| Other bias | Unclear risk | Intervention diet focused only on fat intake changes and encouraged water‐soluble fibre, and control diet AHA publications "Dietary Guidelines for Americans" and "How to Make Your Heart Last a Lifetime" but no detailed nutrition composition detail provided. |
Schwandt 2011.
| Methods |
Study design: prospective cohort study. Analyses methods for cohorts: bivariate and multivariate regression analysis used for age and gender adjustments. As some families had > 1 child in analysis or child pairs with both biological father and mother (or both), GEE used to generate age and gender adjusted odds ratios that accounted for correlation among multiple within‐family observations. How were missing data handled? 575 parents and 411 children (36.1%) completed study at 2 years. Authors did not state how many started study. They only stated that many did not accept the invitation to participate and mentioned incomplete data as a reason for the final numbers of participants. Reported that characteristics of non‐participants and participants were not significantly different (variables not stated). Number of study contacts: 2 (baseline/year 1; year 2). Period of follow‐up (total period of observation): 1 year. Periods of recruitment: NR. Sampling method: convenience sample. 2690 parents and children with complete CVD risk factor profiles and lifestyle data, who participated in a previous PEP substudy. Study objective: to examine whether associations between improved CVD risk profiles and lifestyle changes persist over 1 year in a real‐life setting. Study population: healthy German grade 1 children of elementary schools in Nuremberg, Germany. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: children who did not met exclusion criteria. Excluded criteria: non‐German children; self‐reported cardiovascular, metabolic, endocrine and malignant disorders; extreme physical activity; special nutritional habits and medication. Brief description of participants: healthy German children and parents participating in PEP study. Total number completed in cohort study: 411 (195 boys; 216 girls). 36.1% lost (invited parent‐child pairs), author indicated that characteristics of non‐participants and participants were not significantly different. Total number enrolled in cohort study: 1150 children from 2001 PEP substudy invited. Number enrolled NR. |
|
| Interventions |
Description of exposure for cohorts Time span: 1 year. Dietary assessment method used: weighed DR. Frequency of dietary assessments: single 7‐day weighed DR at baseline and after 1 year' follow‐up. See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
Weight
BMI
Body fat
|
|
| Identification |
Sponsorship source: Foundation for the Prevention of Atherosclerosis, Nuremberg, Germany; Ludwig Maximilian University, Munich, Germany; Bavarian Ministry of Health, Munich; City of Nuremberg. Country: Germany. Setting: community in Nuremberg. Comments: PEP Family Heart Study. Author's name: Peter Schwandt. Institution: Arteriosklerose Präventions Institut and Ludwig Maximilians University, Munich. Email: API.Schwandt.Haas@t‐online.de. Declaration of Interests: no. Study ID: Schwandt 2010. Type of record: journal article. |
|
| Notes | Authors provided separate regression data on children only, since regression data in text referred to both children and adults. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | Unclear risk | Author indicated that characteristics of non‐participants were similar to those who participated in present study but specific variables and analyses NR. Study also had a high non‐response rate as only 36.1% of the invited parent‐child pairs completed follow‐up after 1 year. |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | High risk | Although age, gender and physical activity were adjusted in the data analyses, parental BMI, SES and energy intake were not adjusted for. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Outcome measures undertaken using standardised methods (weight, height, skinfold thickness measurements, BP). |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | 7‐day weighed DRs assessed at baseline and 1 year. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | Data collection done using acceptable methods. Physical activity assessed by validated questionnaires with a 7‐day recall period at baseline and at 1 year. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | All participants from the PEP Healthy Heart study. |
Setayeshgar 2017.
| Methods |
Study design: prospective cohort study. Analyses methods for cohorts: multivariable mixed‐effect analysis of each dietary component with the outcomes (WC, BMI, SBP and DBP) conducted. The model with WC was adjusted for age, sex and BMI‐for‐age z‐score, WC and physical activity at baseline. The model with BMI was adjusted for baseline BMI‐for‐age z‐score and physical activity. Models with SBP and DBP were adjusted for baseline BMI‐for‐age z‐score, physical activity and SBP or DBP. Model with SBP was also adjusted for year of study. Interaction analysis conducted for each model to identify significant sex‐specific difference in results. How were missing data handled? Authors reported no statistically significant differences in the SBP z‐scores, DBP z‐scores, BMI‐for‐age z‐scores and WC between the 448 students enrolled and 127 (28.3%) students with missing or incomplete information (data not shown). Number of study contacts: 3 (baseline, 1 and 2 years' follow‐up). Period of follow‐up (total period of observation): 2 years (2009‐2010; 2010‐2011). Periods of recruitment: 2007‐2008. Sample size justification adequately described? No. Sampling method: convenience sample of children in grades 5‐10 from 14 secondary schools, Black Gold School District, Alberta. Of approximately 7000 students, 2189 consented to participate in cohort; 774 students completed baseline dietary questionnaire (Forbes 2013). Of these, 448 students had complete data on dietary intake, physical activity and at ≥ 1 cardiometabolic risk factor at baseline and 1 follow‐up visit. Study objective: to investigate whether specific aspects of dietary intake were associated with prospective changes in cardiometabolic risk factors in children and youths. Study population: school children in grades 5‐10, Black Gold School District, Edmonton, Alberta, Canada. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: students with complete data on dietary intake, physical activity and ≥ 1 cardiometabolic risk factor at baseline and ≥ 1 follow‐up. Excluded criteria: energy intake of 500 or ≥ 5000 kcal/day. Brief description of participants: students in grades 5‐10 from rural and urban secondary schools of the Black Gold School District, Edmonton, Alberta, Canada participating in the Healthy Hearts study. Total number completed in cohort study: 321. Total number enrolled in cohort study: 448. |
|
| Interventions |
Description of exposure for cohorts Time span: 2 years. Dietary assessment method used: validated 24‐hour diet recall (Web‐SPAN) to measure week day dietary intake. Frequency: single 24‐hour dietary recall at baseline. See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
BMI
WC
SBP
DBP
|
|
| Identification |
Sponsorship source: Collaborative Research and Innovation Opportunity (CRIO) Team Grant; Alberta Innovates Health Solutions. Country: Canada. Setting: rural and urban schools, Black Gold School District, Alberta. Comments: Healthy Hearts Study. Author's name: Solmaz Setayeshgar. Institution: School of Public Health, population Health Intervention Research Unit, University of Alberta, Canada. Email: paul.veugelers@ualberta.ca. Declaration of Interests: yes. "The authors declare that they have no competing interests." Study ID: Setayeshgar 2017. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | High risk | Proportion of students with incomplete data was high (28.3%). Authors reported no statistically significant differences in outcome variables at baseline between children who were enrolled (n = 448) and children with incomplete information (n = 127) (data not shown). They did not compare children who had incomplete data with children who had complete data (n = 321). |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | High risk | No adjustment for total energy intake, parental BMI, pubertal stage or SES. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Standardised methods used to assess weight, height, WC and BP. |
| Can we be confident in the assessment of exposure? All outcomes | High risk | Single dietary assessment (validated 24‐hour recall) at baseline. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | High risk | Single assessment of physical activity using a validated method (accelerometer) at baseline. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | Children and adolescents were all participants of the Healthy Hearts cohort study. |
Shea 1993.
| Methods |
Study design: prospective cohort study. Analyses methods for cohorts: multiple linear regression analyses done in which change in height, weight and BMI were adjusted for baseline values such as age in months at 1st 24‐hour recall, sex, race/ethnicity and total energy intake. Results did not differ from unadjusted analyses and only unadjusted results were reported. Children categorised based on intake of total fat of < 30% of calories vs ≥ 30%, and groups compared using unpaired 2‐tailed Student's t‐test. How were missing data handled? 215 (90.3%) children followed for ≥ 1 year (no reasons stated for attrition). Number of participants who completed study after 2 years NR. Number of study contacts: mean 8 (range 5‐11). Period of follow‐up (total period of observation): 2.1 (0.31). Periods of recruitment: 1985‐1986. Sample size justification adequately described? No. Sampling method: convenience sample. Participants drawn from children participating in the Columbia University Study of Childhood Activity and Nutrition, a longitudinal observational study. Families recruited mainly through a paediatric practice at The Presbyterian Hospital that served a predominantly Hispanic, densely populated, low‐income neighbourhood in northern Manhattan, New York City. A few families recruited from other community sources. Only 1 child per family was eligible. Study objective: to determine whether a moderately reduced fat diet affected stature or growth of healthy preschool children. Study population: 3‐ to 4‐year‐old children in low‐income neighbourhoods in northern Manhattan, New York City. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: families with a healthy child aged 3‐4 years. Excluded criteria: mother was pregnant or postpartum by < 6 months. Brief description of participants: healthy 3‐4 year old Hispanic children. Total number completed in cohort study: NR. 215 children included in analyses; 23 lost to follow‐up or with incomplete data on either anthropometry or dietary intakes excluded. Total number enrolled in cohort study: 238 children. |
|
| Interventions |
Description of exposure for cohorts
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
Weight
BMI
Height
|
|
| Identification |
Sponsorship source: National Heart, Lung, and Blood Institute and Cancer Research Foundation of America. Country: USA. Setting: clinic, Northern Manhattan, New York City. Comments: Columbia University Study of Childhood Activity and Nutrition. Author's name: Steven Shea. Institution: Division of General Medicine, Department of Medicine, Columbia University, New York, USA. Email: NR. Declaration of Interests: no. Study ID: Shea 1993. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | Unclear risk | 215 (90.3%) children followed for ≥ 1 year (4 follow‐up visits). No reasons stated for attrition. Unclear how many children completed last follow‐up visit after 2 years (mean follow‐up (months) 25 (SD 3.8). |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | High risk | No matching reported. Multiple linear regression analysis performed to adjust for age in months at 1st 24‐hour recall, sex, race/ethnicity and total energy intake, but findings did not differ in any substantive way from bivariate analyses, and only results of bivariate analyses were reported. No adjustment for physical activity, parental BMI or SES. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Unclear risk | NR. |
| Can we be confident in the assessment of outcomes? All outcomes | Unclear risk | Anthropometric measures not adequately described. |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | Multiple assessments of dietary intake by repeated 24‐hour food record and FFQ at baseline. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | Data on parental BMI, SES or physical activity of children not measured. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | Children recruited from 1 cohort study (Columbia University Study of Childhood Activity and Nutrition). |
Skinner 2004.
| Methods |
Study design: prospective cohort study. Analyses methods for cohorts: longitudinal dietary intake based on 9 sets of 3‐day dietary data from children aged 2‐8 years. Changes in energy intake over time and gender differences in energy intake tested with GLM repeated measures ANOVA. How were missing data handled? Lost to follow‐up at 3 years: 23 (reasons: travel time required for interviews); at 3.5 and 8 years: 5 (reasons: n = 4: family moved, discontinued participation; n = 1: consistently incomplete data provided by mother). No analysis performed comparing children who completed study to children who did not. Number of study contacts: 11 (2.0, 2.3, 2.7, 3.0, 3.5, 4.0, 4.5, 5.0, 6.0, 7.0 and 8.0 years). Period of follow‐up (total period of observation): 8 years. Periods of recruitment: May‐September 1992. Sample size justification adequately described? No. Sampling method: purposively selected sample of 98 infants aged 2 months recruited from 2 metropolitan areas in Tennessee. Current analysis based on data from 62 children from original cohort, 2 infants who were selected as replacements prior to 1 year of age for cohort and 6 children aged 2 years who participated in a similar infant study from the same laboratory. Study objective: to identify longitudinal variables related to children's BMI at 8 years. Study population: healthy white children aged 2‐8 years in urban area of Tennessee, USA. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: children who participated in the original birth cohort aged 2‐8 years with available follow‐up data. Excluded criteria: NR. Brief description of participants: children aged 2‐8 years. Total number completed in cohort study: 70 (37 boys, 33 girls). Total number enrolled in cohort study: 98 (+2 prior to 1 year; +6 at age 2 years). |
|
| Interventions |
Description of exposure for cohorts
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
BMI
Body fat
Sum of skinfolds
|
|
| Identification |
Sponsorship source: Gerber Products Company and Tennessee Agricultural Experiment Station. Country: USA. Setting: Urban households, Tennessee. Comments: NA. Author's name: JD Skinner. Institution: Nutrition Department, University of Tennessee, Knoxville, TN, USA. Email: skinner@utk.edu. Declaration of Interests: no. Study ID: Skinner 2004. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | High risk | Relatively high number of dropouts (36.7% over 6 years; 62/98 children recruited for study were analysed). Baseline data between children who completed and children who did not were not compared. |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | Low risk | Age, gender, ethnicity and SES were matched while parental BMI, BMI at baseline, adiposity rebound age and physical inactivity were adjusted in linear regression models. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | Low risk | |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Standard methods performed for measurements of weight, height and DEXA (by trained personnel). |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | Repeated 3‐day DR completed by mothers who were taught to describe and estimate portion sizes of child's food and beverage intake. Dietician reviewed food records with mother. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Unclear risk | Information on physical inactivity self‐reported and data collection method not well described. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | All children selected for 1 cohort study. |
Tershakovec 1998 (cohort).
| Methods |
Study design: RCT (cohort analysis). Analyses methods for cohort: children divided into quintiles by mean caloric intake as fat. Repeated measures analyses of variance and covariance performed to compare changes in height‐for‐age z‐score, weight‐for‐age z‐score, weight‐for‐height median, sum of skinfolds, caloric intake and fat intake over time. Potential influence of age and sex assessed in these analyses. How were missing data handled? Attrition rate 5.8% (20/342). Authors stated that pattern of dropouts over time did not differ with respect to age, sex and ethnicity or study group. Because some children did not have available data for all 4 evaluation points, used BMDP‐5V for repeated measures ANOVA to include all possible participants. Number of study contacts: 4 (baseline, 3, 6 and 12 months). Period of follow‐up (total period of observation): 1 year. Periods of recruitment: 1990‐1992. Sample size justification adequately described? NR. Sampling method: convenience sample. Cholesterol screening programme conducted in 9 suburban paediatric practices to identify "at‐risk" children (plasma total cholesterol > 4.55 mmol/L). If mean LDL‐C was elevated (mean fasting plasma LDL‐C 2.77‐4.24 mmol/L for boys and 2.90‐4.24 mmol/L for girls) and children consented they were randomised into 1 of 2 nutrition education intervention groups or an at‐risk control group. Study objective: to evaluate growth of children with hypercholesterolaemia completing an innovative, physician‐initiated, home‐based nutrition education programme or standard nutrition counselling that aimed to lower dietary fat intake. Study population: children aged 4‐10 years with hypercholesterolaemia from suburban paediatric practices in Philadelphia, USA. |
|
| Participants |
Baseline characteristics (reported for 1 overall group)
Included criteria: children aged 3.9‐9.9 years with elevated plasma total cholesterol > 4.55 mmol/L, fasting plasma LDL‐C 2.77‐4.24 mmol/L for boys and 2.90‐4.24 mmol/L for girls; ≥ 85% of ideal bodyweight. Excluded criteria: secondary causes of hypercholesterolaemia; < 130% of ideal bodyweight. Pretreatment: NR. Brief description of participants: children aged 4‐10 years with hypercholesterolaemia. Total number completed in RCT: intervention group: n = 73/86 and control group: n = 78/87. Total number randomised: n = 271. |
|
| Interventions |
Description of exposure for cohort
See Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18 for details of total fat intake exposure per outcome. |
|
| Outcomes |
Weight:
Skinfold thickness
|
|
| Identification |
Sponsorship source: National Heart, Lung, and Blood Institute (HL43880‐03), the Howard Heinz Endowment, and the University of Pennsylvania Research Foundation. Country: USA. Setting: suburban paediatric practice offices, Philadelphia, PA. Comments: NA. Author's name: Andrew M Tershakovec. Institution: Division of Gastroenterology and Nutrition, Children's Hospital of Philadelphia, PA, USA. Email: NR. Declaration of Interests: no. Study ID: Children's Health Project. Type of record: journal articles. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Were adequate outcome data for cohorts available? All outcomes | Unclear risk | 5.8% (20/342) lost over 1 year. Authors stated that pattern of dropouts over time did not differ with respect to age, sex and ethnicity or study group but no analyses provided. |
| Was there matching of less‐exposed and more‐exposed participants for prognostic factors associated with outcome or were relevant statistical adjustments done? All outcomes | High risk | Data analyses only adjusted for age. |
| Did the exposures between groups differ in components other than only total fat? All outcomes | High risk | Children allocated to intervention groups received various dietary interventions. |
| Can we be confident in the assessment of outcomes? All outcomes | Low risk | Standardised methods used to assess height, weight and skinfold thickness. |
| Can we be confident in the assessment of exposure? All outcomes | Low risk | Repeated dietary assessments done using 3 × 24‐hour dietary recalls per assessment period. |
| Can we be confident in the assessment of presence or absence of prognostic factors? All outcomes | Low risk | Prognostic factors such as physical activity and parental BMI not assessed. |
| Was selection of less‐exposed and more‐exposed groups from the same population? All outcomes | Low risk | All participants of an RCT (Children's Health Project) |
Tershakovec 1998 (RCT).
| Methods |
Study design: RCT. Study grouping: parallel group. Allocation ratio: 1:1. Analyses methods: "Repeated measures analyses of variance and covariance compared group changes in growth over time related to the intervention (analysis 1) or dietary fat intake (analysis 3)." Description of randomisation: "At‐risk children who met the study criteria and agreed to participate were randomised to study groups using a permuted blocks within strata design. Stratifying on age and gender, we employed an adaptive allocation procedure that yielded balance within first order interactions with season and pediatric practice." Allocation concealment NR. NR who enrolled and assigned participants. How were missing data handled? "Because some subjects did not have available data for all four evaluation points, BMDP‐5V was used for the repeated measures analysis of variance to include all possible participants." Number of study contacts: 4. Period of follow‐up (from when duration of active intervention period ended): 9 months. Periods of recruitment: "Subject enrollment began in October 1990 and continued through December 1992." Sample size justification adequately described? NR. Sampling method: cholesterol screening programme conducted in 9 suburban paediatric practices to identify "at‐risk" children (plasma total cholesterol > 4.55 mmol/L). If mean LDL‐C was elevated (mean fasting plasma LDL‐C 2.77‐4.24 mmol/L for boys and 2.90‐4.24 mmol/L for girls) and children consented they were randomised into 1 of 2 nutrition education intervention groups or an at‐risk control group. Study objective: to evaluate the growth of children with hypercholesterolaemia completing an innovative, physician‐initiated, home‐based nutrition education program or standard nutrition counselling that aims to lower dietary fat intake. Study population: children with hypercholesterolaemia aged 4‐10 years from suburban paediatric practices in Philadelphia, PA, USA. |
|
| Participants |
Baseline characteristics (reported for 2 groups and overall group) Lower fat intake (≤ 30%TE)
Usual or modified fat intake
Overall
Included criteria: children aged 3.9‐9.9 years with elevated plasma total cholesterol > 4.55 mmol/L, fasting plasma LDL‐C 2.77‐4.24 mmol/L for boys and 2.90‐4.24 mmol/L for girls; ≥ 85% of ideal bodyweight. Excluded criteria: secondary causes of hypercholesterolaemia; < 130% of ideal bodyweight. Pretreatment: NR. Brief description of participants: children aged 4‐10 years with hypercholesterolaemia. Total number completed in RCT: intervention group: n = 73/86 and control group: n = 78/87. Total number randomised: n = 271. |
|
| Interventions |
Intervention characteristics Lower fat intake (≤ 30%TE)
Usual or modified fat intake
|
|
| Outcomes |
Weight
Height
|
|
| Identification |
Sponsorship source: National Heart, Lung, and Blood Institute (HL43880‐03), the Howard Heinz Endowment, and the University of Pennsylvania Research Foundation. Country: USA. Setting: suburban paediatric practice offices, Philadelphia, PA. Comments: NA. Author's name: Andrew M Tershakovec. Institution: Division of Gastroenterology and Nutrition, Children's Hospital of Philadelphia, PA, USA. Email: NR. Declaration of Interests: no. Study ID: Children's Health Project. Type of record: journal article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Permuted blocks within strata design used with minimisation. Authors reported that at baseline, the 4 groups were balanced, except for race, but no statistical test for differences reported. |
| Allocation concealment (selection bias) | Unclear risk | NR. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NR. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up at 12 months: intervention group: 13/86 (15.1%) and control group: 9/87 (10.3%). Reasons for loss to follow‐up NR, except for withdrawal of consent (intervention group 4 and control group 2). Missing data not imputed but authors reported that BMDP‐5V was used for the repeated measures ANOVA to include all possible participants. |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes not clearly defined in methods. Study protocol not available. |
| Other bias | Unclear risk | Limited information on control diet prescription; unable to judge if prescribed diets being compared differed in components other than total fat. |
%TE: percentage of total energy intake; AAP: American Academy of Pediatrics; AHA: American Heart Association; ALSPAC: Avon Longitudinal Study of Parents and Children; ANOVA: analysis of variance; BC: body composition; BIA: bioelectrical impedance analysis; BMI: body mass index; BMI‐SDS: body mass index‐standard deviation score; BP: blood pressure; CDC: Centers for Disease Control and Prevention; CHD: coronary heart disease; CHO: carbohydrate; CI: confidence interval; CIF: Children in Focus; CVD: cardiovascular disease; DBP: diastolic blood pressure; DED: dietary energy density; DEXA: dual energy X‐ray absorptiometry; DONALD: Dortmund Nutritional and Anthropometric Longitudinally Designed; DP: dietary pattern; DR: dietary record; FD: fibre density; FFQ: Food Frequency Questionnaire; FM: fat mass; FMI: fat mass index; GEE: generalised estimating equation; GLM: general linear model; HDL‐C: high‐density lipoprotein cholesterol; HF: high fat; HOMA: Homeostasis Model Assessment; HOMA‐IR: Homeostasis Model Assessment‐Insulin Resistance; IFG: impaired fasting glucose; IQR: interquartile range; IR: insulin resistance; ITT: intention to treat; LDL‐C: low‐density lipoprotein cholesterol; LF: low fat; LTPA: leisure‐time physical activity; max: maximum; MD: mean difference; MET: metabolic equivalent; min: minimum; MUFA: monounsaturated fatty acid; n: number of participants; NA: not applicable; NCEP: National Centers for Environmental Prediction; NGHS: National Heart, Lung and Blood institute Growth and Health Study; NHLBI: National Heart, Lung and Blood Institute; NR: not reported; NS: not significant; PEP: Prevention Education Program; PUFA: polyunsaturated fatty acid; RCT: randomised controlled trial; SBP: systolic blood pressure; SD: standard deviation; SDS: standard deviation score; SE: standard error; SES: socioeconomic status; SFA: saturated fatty acid; SS‐SDS: subscapular skinfold‐standard deviation score; STRIP: Special Turku Coronary Risk Factor Intervention Project; T2DM: type 2 diabetes mellitus; TAAG: Trial of Activity for Adolescent Girls Cohort; TC‐SDS: triceps skinfold‐standard deviation score; TG: triglyceride; TV: television; WC: waist circumference.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adair 2001 | Wrong exposure. |
| Agostoni 2000 | Wrong intervention. |
| Ahola‐Olli 2014 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Alexy 2002 | Wrong study design; cross‐sectional. |
| Altwaijri 2009 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Alvirde‐Garcia 2013 | Wrong intervention. |
| Arvidsson 2015 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Barton 2005 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Berkey 2009 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Boulton 1995 | No eligible outcomes reported AND our outcomes fell outside scope of study. |
| Brown 2013 | Wrong duration. |
| Brox 2002 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Burke 2001 | Wrong study design; cross‐sectional. |
| Caballero 2003 | Wrong intervention. |
| Cardel 2015 | Wrong duration. |
| Chen 2012 | Wrong duration. |
| Choi 2011 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Coppinger 2010 | Wrong exposure. |
| Couch 2014 | No eligible comparison. |
| Crawford 1995 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Cresanta 1988 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Dalskov 2014 | No eligible comparison. |
| Davies 1997 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Deheeger 1996 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Deheeger 2002 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Dixon 2005 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Donnelly 1996 | Wrong intervention. |
| Dubois 2016 | Wrong study design; analysed twin pairs. |
| Dwyer 2002 | Wrong intervention. |
| Dwyer 2003 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Eck 1992 | Wrong study design; cross‐sectional. |
| Elder 2014 | Wrong intervention. |
| Emmett 2015a | Wrong study design; review. |
| Emmett 2015b | Wrong study design; review. |
| Epstein 2001 | Wrong comparator. |
| Evans 2010 | No eligible outcomes reported AND our outcomes fell outside scope of study. |
| Farris 1984a | Duplicate. |
| Farris 1984b | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Fitzgibbon 2002 | Wrong duration. |
| Fitzgibbon 2005 | Wrong duration. |
| Foster 2008 | Wrong intervention. |
| Frank 1985a | Duplicate. |
| Frank 1985b | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Gillis 2009 | No eligible outcomes reported AND our outcomes fell outside scope of study. |
| Goldberg 1992 | Wrong study design; not RCT. |
| Gortmaker 1999 | Wrong intervention. |
| Harris 2016 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Harris 2017 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Hendrie 2011 | Wrong intervention. |
| Himes 2003 | Wrong intervention. |
| Hollis 1984 | No eligible outcomes reported AND our eligible outcomes fell outside scope of study. |
| Hood 2000 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Jacobson 1998 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Jancey 2014 | Wrong intervention. |
| Jimenez 2003 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Karnehed 2006 | Wrong study design; analysed twin pairs. |
| Khalil 2017 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Kiefte‐de Jong 2013 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Kimm 1999 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Kronsberg 2003 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Kuehl 1993 | Wrong duration. |
| Kuzawa 2003 | Wrong study design; cross‐sectional. |
| Kwiterovich 1997 | Wrong exposure. |
| Kwiterovich 2001 | Wrong exposure. |
| Lagstrom 1997a | Wrong intervention. |
| Lagstrom 1997b | Wrong intervention. |
| Lagstrom 1999 | No eligible comparison. |
| Larsen 2010 | No eligible comparison. |
| Lee 2007 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Lee 2014 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Lee 2017 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Leung 2000a | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Leung 2000b | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Li 2008 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Libuda 2014 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Maclure 1991 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Mamalakis 2001 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Manios 2002 | Wrong intervention. |
| Manios 2006 | Wrong intervention. |
| Marcus 2009 | Wrong intervention. |
| Maresh 1970 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Michels 2015a | Duplicate |
| Michels 2015b | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Michels 2016 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Newby 2003 | Wrong duration. |
| Nicklas 1991 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Nicklas 1992 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Niinikoski 1996 | Wrong intervention. |
| Niinikoski 1997b | Wrong intervention. |
| Niinikoski 2007 | Wrong intervention. |
| Niinikoski 2009 | Wrong intervention. |
| Niinikoski 2009a | Wrong intervention. |
| Niinikoski 2012 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Niinikoski 2014 | Wrong intervention. |
| O'Sullivan 2011 | Wrong study design; cross‐sectional. |
| Obarzanek 1994 | Wrong study design; used baseline data to predict outcomes without including data from the later time point. |
| Ohlund 2011 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Ohrig 2001 | Wrong study design; cross‐sectional. |
| Oranta 2013 | Wrong intervention. |
| Osganian 1996 | Wrong intervention. |
| Paineau 2008 | Wrong intervention. |
| Paineau 2010 | Wrong intervention. |
| Patrick 2006 | Wrong intervention. |
| Pimpin 2016 | Wrong study design; analysed twin pairs. |
| Post 1997 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Proctor 2003 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Raitakari 2005 | Wrong intervention. |
| Rask‐Nissila 2000a | Wrong intervention. |
| Rask‐Nissila 2000b | Wrong intervention. |
| Rask‐Nissila 2002a | Wrong intervention. |
| Rask‐Nissila 2002b | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Rehkopf 2011 | Wrong study population. |
| Robertson 1999 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Ruxton 1995 | Wrong exposure. |
| Sallis 2003 | Wrong intervention. |
| Sanchez‐Bayle 2003 | Wrong study design; not RCT. |
| Sawaya 1998 | Wrong duration. |
| Siega‐Riz 2011 | Wrong intervention. |
| Simell 1999 | Wrong intervention. |
| Spruijt‐Metz 2002 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Spruijt‐Metz 2006 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Stice 2015 | Wrong study population. |
| Stone 1996 | Wrong intervention. |
| Stone 2003 | Wrong intervention. |
| Story 2003 | Wrong intervention. |
| Talvia 2004 | Wrong intervention. |
| Telford 2012 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Telford 2015 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Teufel 1999 | Wrong intervention. |
| Treuth 2003 | Wrong duration. |
| Trevino 2004 | Wrong intervention. |
| Vandongen 1995 | Wrong intervention. |
| Verduci 2007 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Vobecky 1988 | Wrong study design; case‐control. |
| Voortman 2016 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Walker 1992 | Wrong intervention. |
| Walter 1989 | Wrong intervention. |
| Wang 2000 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Wang 2003 | Wrong study population. |
| Wang 2014 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Williams 1998 | Wrong study design; not RCT. |
| Williams 2002 | Wrong study design; not RCT. |
| Williams 2004 | Wrong study design; not RCT. |
| Williams 2008 | Wrong study design; not RCT. |
| Williamson 2010 | Wrong intervention. |
| Wright 2010 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
| Zaqout 2016 | Wrong study design; did not analyse children's baseline total fat intake to body fatness 12 months later. |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Khalil 2015.
| Methods |
Study design: prospective cohort study. Analyses methods for cohorts: NR. How were missing data handled? NR. Number of study contacts? 2 (baseline‐5 years and 9 years). Period of follow‐up: 4 years. Periods of recruitment: NR. Sample size justification adequately described? No. Sampling method: NR. Study objective: to identify the developmental trajectories of BMI during childhood and identify dietary factors associated with trajectory membership. Study population: children aged 5 years in Ireland. |
| Participants |
Baseline characteristics: NR. Included criteria: children from the Lifeways Cross‐Generation birth cohort study with height and weight measurements at 5 and 9 years of age. Excluded criteria: NR. Brief description of participants: children aged 5 years who were participants of the Lifeways Cross‐Generation birth cohort study, Ireland. Total number completed in cohort study: 194 children (at age 9 years). Total number enrolled in cohort study: 194 children (at age 5 years). |
| Interventions |
Description of exposure for cohorts:
|
| Outcomes | NR |
| Notes | We were unable to retrieve a full‐text publication of this study, only 2 conference abstracts. We contacted the authors and requested data analyses reporting the relationship between baseline total fat intake in children and absolute or change in body fatness outcomes after at least 1 year' follow‐up. We had not received a response by time of publication. |
Twisk 1998.
| Methods |
Study design: prospective cohort study. Analyses methods for cohorts: multiple dietary assessments. Analyses: 1st‐order autoregressive model (fatness at each time point related to exposure at previous time point) estimated by GEEs) with the within‐subject correlations taken into account using GEEs. How were missing data handled? 24% (233/307) lost to follow‐up over 1st 4 years of study. Comparisons between dropouts and remaining participants revealed no selective dropout after 1st year in relation to anthropometric variables, nutrition intake and physical activity. Number of study contacts? 4 (baseline‐13 years, 14 years, 15 years, and 16 years). Period of follow‐up: 3 years. Periods of recruitment: 1977. Sample size justification adequately described ‐ yes/no? The AGAHLS study included 698 children from 2 equally large secondary schools in Amsterdam. Schools selected based on location, i.e. 1 of the schools in a rural area, the other in an urban area, as being representative of the Dutch adolescent population of the 1970s. Sampling method: convenience. Healthy pupils from the 1st and 2nd years of 1 secondary school in Amsterdam. Study objective: to analyse longitudinal relationships between BMI/SSF, and biological and lifestyle risk factors for coronary heart disease. Study population: boys and girls aged 13 years in Amsterdam. |
| Participants |
Baseline characteristics (overall)
Included criteria: healthy boys and girls aged 13 years. Excluded criteria: NR. Pretreatment: NA. Brief description of participants: healthy, Dutch school children aged 13 years with above average socioeconomic status who were participants of the Amsterdam Growth Health Longitudinal Study. Total number completed in cohort study: 233 (102 boys, 131 girls) completed 4 annual measurements. Total number enrolled in cohort study: 307 (148 boys, 159 girls). |
| Interventions |
Description of exposure for cohorts Time span: 4 years. Dietary assessment method used: cross‐checked, dietary history interview. Frequency of dietary assessments: 1 assessment at each follow‐up visit (at 14, 15 and 16 years). |
| Outcomes | Regression data reported in a graph. |
| Notes | We contacted the authors about the data at ages 14, 15 and 16 years, but had not received this by time of publication, and thus could not classify this study. |
AGAHLS: Amsterdam Growth and Health Longitudinal Study; BMI: body mass index; FFQ: Food Frequency Questionnaire; GEE: generalised estimating equation; NA: not available; NR: not reported; SD: standard deviation; SSF: sum of skinfolds.
Differences between protocol and review
Differences between review (2015) in adults and children, and this updated review (2018) in children only:
Removed quality of life as an outcome.
Did not exclude studies based on outcome reporting as a criterion, unless none of our eligible outcomes were reported and we judged that our outcomes were outside of the scope of the study (i.e. one would not expect them to be reported in the particular study).
Included only explanatory models and excluded analyses that used baseline data to predict later body fatness without empirical data from the later time point (predictive models).
Added extra domains for assessing risk of bias in cohort studies.
Removal of the following intended subgroup analyses:
year of first publication of results (1960s, 1970s, 1980s, 1990s, 2000s, 2010s);
sex (studies of women only, of men only, of men and women mixed);
by total fat goal in the intervention arm (10% energy to less than 15% energy from fat, 15% energy to less than 20% energy from fat, 20% energy to less than 25% energy from fat, 25% energy to less than 30% energy from fat, 30% energy from fat, and no specific goal stated); and
mean BMI at baseline (less than 25, 25 to less than 30, greater than 30); state of health at baseline (not recruited on the basis of risk factors or disease, recruited on the basis of risk factors such as lipids, hormonal levels, etc., recruited on the basis of having or having had diseases such as diabetes, myocardial infarction, cancer or polyps.
Contributions of authors
The World Health Organization (WHO) Nutrition Guidance Expert Advisory Group (NUGAG) subgroup on diet and health discussed and developed the question for this review. The protocol for the review update in children was drafted by CN and AS, and approved by WHO.
CN and AS developed the search strategy in consultation with the information specialist (VL), who carried out the searches.
CN, AS, MV, KN, SD and SN assessed the eligibility of the studies for inclusion, extracted data and assessed study quality.
CN and AS conducted consensus of trial validity and carried out the GRADE assessment of the trial data.
KN and MV conducted consensus of cohort validity.
CN, AS, MV and KN carried out the GRADE assessments for the cohort studies' data.
CN wrote most sections of the first draft this update, with all other authors contributing drafts of certain sections.
All authors agreed on the final draft of this review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
World Health Organization, Other.
The World Health Organization (WHO) provided funding to Stellenbosch University towards the cost of carrying out the update of this systematic review in children
-
Effective Health Care Research Consortium, UK.
CN, MV, SD and AS are partly supported by the Effective Health Care Research Consortium. This Consortium is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy
Declarations of interest
The World Health Organization (WHO) provided funding to Stellenbosch University towards the cost of carrying out the update of this systematic review. CN, MV, SD and AS are partly supported by the Effective Health Care Research Consortium. This Consortium is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
Edited (no change to conclusions)
References
References to studies included in this review
Alexy 2004 {published data only}
- Alexy U, Schultze‐Pawlitschko V, Sichert‐Hellert W, Kersting M. Cluster analysis of individuals with similar trends of fat intake during childhood and adolescence: a new approach to analyzing dietary data. Nutrition Research 2005;25:251‐60. [Google Scholar]
- Alexy U, Sichert‐Hellert W, Kersting M, Schultze‐Pawlitschko V. Pattern of long‐term fat intake and BMI during childhood and adolescence‐results of the DONALD Study. International Journal of Obesity Related Metabolic Disorders 2004;28(10):1203‐9. [DOI: 10.1038/sj.ijo.0802708] [DOI] [PubMed] [Google Scholar]
Ambrosini 2016 {published data only}
- Ambrosini G, Johns D, Northstone K, Emmett PM, Jebb SA. Free sugars and total fat are important characteristics of a dietary pattern associated with adiposity across childhood and adolescence. Journal of Nutrition 2016;146(4):778‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini G, Johns D, Northstone K, Jebb S. Fat, sugar or both? A prospective analysis of dietary patterns and adiposity in children. FASEB Journal 2015;29:1. [Google Scholar]
- Ambrosini GL, Emmett, PM, Northstone K, Howe LD, Tilling K, Jebb SA. Identification of a dietary pattern prospectively associated with increased adiposity during childhood and adolescence. International Journal of Obesity (2005) 2012;36(10):1299‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J, Pembrey M, Jones R, ALSPAC Study Team. ALSPAC‐The Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatric and Perinatal Epidemiology 2001;15:74‐87. [DOI] [PubMed] [Google Scholar]
- Johnson L, Mander AP, Jones LR, Emmett PM, Jebb SA. A prospective analysis of dietary energy density at age 5 and 7 years and fatness at 9 years among UK children. International Journal of Obesity (2005) 2008;32(4):586‐93. [DOI] [PubMed] [Google Scholar]
- Johnson L, Mander AP, Jones LR, Emmett PM, Jebb SA. Energy‐dense, low‐fiber, high‐fat dietary pattern is associated with increased fatness in childhood. American Journal of Clinical Nutrition 2008;87(4):846‐54. [DOI] [PubMed] [Google Scholar]
- Rogers IS, Emmett PM, Alspac Study Team. Fat content of the diet among preschool children in southwest Britain: II. relationship with growth, blood lipids, and iron status. Pediatrics 2001;108(3):E49. [DOI] [PubMed] [Google Scholar]
Appannah 2015 {published data only}
- Ambrosini GL, Klerk NH, O'Sullivan TA, Beilin LJ, Oddy WH. The reliability of a Food Frequency Questionnaire for use among adolescents. European Journal of Clinical Nutrition 2009;63:1251‐9. [DOI] [PubMed] [Google Scholar]
- Appannah G, Pot GK, Huang RC, Oddy WH, Beilin LJ, Mori TA, et al. Identification of a dietary pattern associated with greater cardiometabolic risk in adolescence. Nutrition, Metabolism, and Cardiovascular Diseases : NMCD 2015;25(7):643‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berkey 2005 {published data only}
- Berkey CS, Rockett H, Willett WC, Colditz GA. Milk, dairy fat, dietary calcium, and weight gain: a longitudinal study of adolescents. Archives of Pediatrics and Adolescent Medicine 2005;159(6):543‐50. [DOI] [PubMed] [Google Scholar]
- Berkey CS, Rockett HR, Field AE, Gillman MW, Frazier AW, Camargo CA Jr, et al. Activity, dietary intake, and weight changes in a longitudinal study of preadolescent and adolescent boys and girls. Pediatrics 2000;105(4):E56. [DOI] [PubMed] [Google Scholar]
Bogaert 2003 {published data only}
- Bogaert N, Steinbeck K, Baur LA, Brock K, Bermingham MA. Food, activity and family ‐ environmental versus biochemical predictors of weight gain in children. European Journal of Clinical Nutrition 2003;57(10):1242‐9. [DOI: 10.1038/sj.ejcn.1601677] [DOI] [PubMed] [Google Scholar]
Boreham 1999 {published data only}
- Boreham C, Savage JM, Primrose D, Cran G, Strain J. Coronary risk factors in schoolchildren. Archives of Disease in Childhood 1993;68(2):182‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boreham C, Twisk J, Mechelen W, Savage M, Strain J, Cran G. Relationships between the development of biological risk factors for coronary heart disease and lifestyle parameters during adolescence: the Northern Ireland Young Hearts Project. Public Health 1999;113(1):7‐12. [DOI] [PubMed] [Google Scholar]
Brixval 2009 {published data only}
- Brixval CS, Andersen LB, Heitmann BL. Fat intake and weight development from 9 to 16 years of age: the European youth heart study ‐ a longitudinal study. Obesity Facts 2009;2(3):166‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring SI, Heitmann BL. Fiber intake, not dietary energy density, is associated with subsequent change in BMI z‐score among sub‐groups of children. Obesity Facts 2008;1(6):331‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedderkopp N, Leboeuf‐Yde C, Andersen LB, Froberg K, Hansen HS. Back pain reporting pattern in a Danish population‐based sample of children and adolescents. Spine 2001;26(17):1879‐83. [DOI] [PubMed] [Google Scholar]
Butte 2007 {published data only}
- Butte N, Cai G, Cole A, Comuzzie AG. Viva la Familia Study: genetic and environmental contributions to childhood obesity and its comorbidities in the Hispanic population. American Journal of Clinical Nutrition 2006;84:646‐54. [DOI] [PubMed] [Google Scholar]
- Butte NF, Cai G, Cole SA, Wilson TA, Fisher JO, Zakeri IF, et al. Metabolic and behavioral predictors of weight gain in Hispanic children: the Viva la Familia Study. American Journal of Clinical Nutrition 2007;85(6):1478‐85. [DOI] [PubMed] [Google Scholar]
Cohen 2014 {published data only}
- Cohen DA, Ghosh‐Dastidar B, Conway TL, Evenson KR, Rodriguez R, Beckman R, et al. Energy balance in adolescent girls: the trial of activity for adolescent girls cohort. Obesity (Silver Spring, Md.) 2014;22(3):772‐80. [DOI: 10.1002/oby.20536] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davison 2001 {published data only}
- Davison K, Birch L. Child and parent characteristics as predictors of change in girls' body mass index. International Journal of Obesity and Related Metabolic Disorders 2001;25(12):1834‐42. [DOI: 10.1038/sj.ijo.0801835] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jago 2005 {published data only}
- Jago R, Baranowski T, Baranowski JC, Thompson D, Greaves KA. BMI from 3‐6y of age is predicted by TV viewing and physical activity, not diet. International Journal of Obesity (2005) 2005;29(6):557‐64. [DOI: ] [DOI] [PubMed] [Google Scholar]
Klesges 1995 {published data only}
- Klesges RC, Klesges LM, Eck LH, Shelton ML. A longitudinal analysis of accelerated weight gain in preschool children. Pediatrics 1995;95(1):126‐30. [PubMed] [Google Scholar]
Lee 2001 {published data only}
- Lee Y, Mitchell DC, Smiciklas‐Wright H, Birch LL. Diet quality, nutrient intake, weight status, and feeding environments of girls meeting or exceeding recommendations for total dietary fat of the American Academy of Pediatrics. Pediatrics 2001;107(6):E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee HH, Park HA, Kang JH, Cho YG, Park JK, Lee R, et al. Factors related to body mass index and body mass index change in Korean children: preliminary results from the obesity and metabolic disorders cohort in childhood. Korean Journal of Family Medicine 2012;33(3):134‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magarey 2001 {published data only}
- Boulton T, Magarey AM. Effects of differences in dietary fat on growth, energy and nutrient intake from infancy to eight years of age. Acta Paediatrica (Oslo, Norway: 1992) 1995;84(2):146‐50. [DOI] [PubMed] [Google Scholar]
- Boulton TJ, Magarey AM, Cockington RA. Serum lipids and apolipoproteins from 1 to 15 years: changes with age and puberty, and relationships with diet, parental cholesterol and family history of ischaemic heart disease. Acta Paediatrica (Oslo, Norway : 1992) 1995;84(10):1113‐8. [DOI] [PubMed] [Google Scholar]
- Magarey AM, Daniels LA, Boulton TJ, Cockington RA. Does fat intake predict adiposity in healthy children and adolescents aged 2‐15 y? A longitudinal analysis. European Journal of Clinical Nutrition 2001;55(6):471‐81. [DOI] [PubMed] [Google Scholar]
Mihas 2010 {published data only}
- Mihas C, Mariolis A, Manios Y, Naska A, Arapaki A, Mariolis‐Sapsakos T, et al. Evaluation of a nutrition intervention in adolescents of an urban area in Greece: short‐ and long‐term effects of the VYRONAS study. Public Health Nutrition 2010;13(5):712‐9. [DOI] [PubMed] [Google Scholar]
Morrison 2008 {published data only}
- Morrison JA, Glueck CJ, Horn PS, Schreiber GB, Wang P. Pre‐teen insulin resistance predicts weight gain, impaired fasting glucose, and type 2 diabetes at age 18‐19 y: a 10‐y prospective study of black and white girls. American Journal of Clinical Nutrition 2008;88(3):778‐88. [DOI] [PubMed] [Google Scholar]
- Morrison JA, Glueck CJ, Wang P. Preteen insulin levels interact with caloric intake to predict increases in obesity at ages 18 to 19 years: a 10‐year prospective study of black and white girls. Metabolism: Clinical and Experimental 2010;59(5):718‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The NHLBI Growth and Health Study Research Group. Obesity and cardiovascular disease risk factors and black and white girls: the NHLBI Growth and Health Study. American Journal of Public Health 1992;82(12):1613‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Niinikoski 1997a {published data only}
- Lapinleimu H, Viikari J, Jokinen E, Salo P, Routi T, Leino A, et al. Prospective randomized trial in 1062 infants of diet low in saturated fat and cholesterol. Lancet 1995;345:471‐6. [DOI] [PubMed] [Google Scholar]
- Niinikoski H, Viikari J, Ronnemaa T, Helenius H, Jokinen E, Lapinleimu H, et al. Regulation of growth of 7‐ to 36‐month‐old children by energy and fat intake in the prospective, randomized STRIP baby trial. Pediatrics 1997;100(5):810‐6. [DOI] [PubMed] [Google Scholar]
- Niinikoski H, Viikari J, Ronnemaa T, Lapinleimu H, Jokinen E, Salo P, et al. Prospective randomized trial of low‐saturated‐fat, low‐cholesterol diet during the first 3 years of life: the STRIP baby project. Circulation 1996;94:1386‐93. [DOI] [PubMed] [Google Scholar]
Obarzanek 1997 (cohort) {published data only}
- Obarzanek E, Hunsberger SA, Horn L, Hartmuller VV, Barton BA, Stevens VJ, et al. Safety of a fat‐reduced diet: the Dietary Intervention Study in Children (DISC). Pediatrics 1997;100(1):51‐9. [DOI] [PubMed] [Google Scholar]
- Simons‐Morton DG, Hunsberger SA, Horn L, Barton BA, Robson AM, McMahon RP, et al. Nutrient intake and blood pressure in the Dietary Intervention Study in Children. Hypertension (Dallas, Tex.: 1979) 1997;29(4):930‐6. [DOI] [PubMed] [Google Scholar]
Obarzanek 2001 (RCT) {published data only}
- Anonymous. Efficacy and safety of lowering dietary intake of fat and cholesterol in children with elevated low‐density lipoprotein cholesterol. The Dietary Intervention Study in Children (DISC). The Writing Group for the DISC Collaborative Research Group. JAMA 1995;273(18):1429‐35. [DOI] [PubMed] [Google Scholar]
- DISC Collaborative Research Group. Dietary Intervention Study in Children (DISC) with elevated low‐density‐lipoprotein cholesterol. Design and baseline characteristics. DISC Collaborative Research Group. Annals of Epidemiology 1993;3(4):393‐402. [DOI] [PubMed] [Google Scholar]
- Kimm SYS, Kwiterovich PO Jr, Santanello NC, Obarzanek E, Lakatos E, Lauer RM, et al. Dietary Intervention Study in Children (DISC) with elevated low‐density‐ lipoprotein cholesterol: design and baseline characteristics. Annals of Epidemiology 1993;3(4):393‐402. [DOI] [PubMed] [Google Scholar]
- Lauer RM, Obarzanek E, Hunsberger SA, Horn L, Hartmuller VW, Barton BA, et al. Efficacy and safety of lowering dietary intake of total fat, saturated fat, and cholesterol in children with elevated LDL cholesterol: the Dietary Intervention Study in Children. American Journal of Clinical Nutrition 2000;72(5 Suppl):1332S‐42S. [DOI] [PubMed] [Google Scholar]
- Lauer RM, Obarzanek E, Kwiterovich PO, Kimm SY, Hunsberger SA, Barton BA, et al. Efficacy and safety of lowering dietary intake of fat and cholesterol in children with elevated low‐density lipoprotein cholesterol: the Dietary Intervention Study in Children (DISC). Journal of the American Medical Association 1995;273(18):1429‐35. [DOI: ] [Google Scholar]
- Obarzanek E, Kimm SY, Barton BA, Horn L, Kwiterovich PO Jr, Simons‐Morton DG, et al. Long‐term safety and efficacy of a cholesterol‐lowering diet in children with elevated low‐density lipoprotein cholesterol: seven‐year results of the Dietary Intervention Study in Children (DISC). Pediatrics 2001;107(2):256‐64. [DOI] [PubMed] [Google Scholar]
- Simons‐Morton DG, Hunsberger SA, Horn L, Barton BA, Robson AM, McMahon RP, et al. Nutrient intake and blood pressure in the Dietary Intervention Study in Children. Hypertension (Dallas, Tex.: 1979) 1997;29(4):930‐6. [DOI] [PubMed] [Google Scholar]
- Horn LV, Stumbo P, Moag‐Stahlberg A, Obarzanek E, Hartmuller VW, Farris RP, et al. The Dietary Intervention Study in Children (DISC): dietary assessment methods for 8‐ to 10‐year‐olds. Journal of the American Dietetic Association 1993;93(12):1396‐403. [DOI] [PubMed] [Google Scholar]
Schwandt 2011 {published data only}
- Schwandt P, Bertsch T, Haas GM. Sustained lifestyle advice and cardiovascular risk factors in 687 biological child‐parent pairs: the PEP Family Heart Study. Atherosclerosis 2011;219(2):937‐45. [DOI: 10.1016/j.atherosclerosis.2011.09.032] [DOI] [PubMed] [Google Scholar]
Setayeshgar 2017 {published data only}
- Setayeshgar S, Ekwaru JP, Maximova K, Majumdar SR, Storey KE, McGavock J, et al. Dietary intake and prospective changes in cardiometabolic risk factors in children and youth. Physiologie Appliquee, Nutrition et Metabolisme [Applied Physiology, Nutrition, and Metabolism] 2017;42(1):39‐45. [DOI] [PubMed] [Google Scholar]
Shea 1993 {published data only}
- Shea S, Basch CE, Stein AD, Contento IR, Irigoyen M, Zybert P. Is there a relationship between dietary fat and stature or growth in children three to five years of age?. Pediatrics 1993;92(4):579‐86. [PubMed] [Google Scholar]
Skinner 2004 {published data only}
- Carruth BR, Skinner JD. The role of dietary calcium and other nutrients in moderating body fat in preschool children. International Journal of Obesity and Related Metabolic Disorders 2001;25(4):559‐66. [DOI] [PubMed] [Google Scholar]
- Skinner JD, Bounds W, Carruth BR, Morris M, Ziegler P. Predictors of children's body mass index: a longitudinal study of diet and growth in children aged 2‐8 years. International Journal of Obesity and Related Metabolic Disorders 2004;28(4):476‐82. [DOI] [PubMed] [Google Scholar]
- Skinner JD, Bounds W, Carruth BR, Ziegler P. Longitudinal calcium intake is negatively related to children's body fat indexes. Journal of the American Dietetic Association 2003;103(12):1626‐31. [DOI] [PubMed] [Google Scholar]
Tershakovec 1998 (cohort) {published data only}
- Tershakovec AM, Jawad AF, Stallings VA, Zemel BS, McKenzie JM, Stolley PD, et al. Growth of hypercholesterolemic children completing physician‐initiated low‐fat dietary intervention. Journal of Pediatrics 1998;133(1):28‐34. [DOI] [PubMed] [Google Scholar]
Tershakovec 1998 (RCT) {published data only}
- Stallings VA, Cortner JA, Shannon BM, Greene GW, Collins SE, Berman MK, et al. Preliminary report of a home‐based education program for dietary treatment of hypercholesterolemia in children. American Journal of Health Promotion 1993;8(2):106‐108. [DOI] [PubMed] [Google Scholar]
- Tershakovec AM, Jawad AF, Stallings VA, Zemel BS, McKenzie JM, Stolley PD, et al. Growth of hypercholesterolemic children completing physician‐initiated low‐fat dietary intervention. Journal of Pediatrics 1998;133(1):28‐34. [DOI] [PubMed] [Google Scholar]
- Tershakovec AM, Shannon BM, Achterberg CL, McKenzie JM, Martel JK, Smiciklas‐Wright H, et al. One‐year follow‐up of nutrition education for hypercholesterolemic children. American Journal of Public Health 1998;88(2):258‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Adair 2001 {published data only}
- Adair LS, Kuzawa CW, Borja J. Maternal energy stores and diet composition during pregnancy program adolescent blood pressure. Circulation 2001;104(9):1034‐9. [DOI] [PubMed] [Google Scholar]
Agostoni 2000 {published data only}
- Agostoni C, Riva E, Scaglioni S, Marangoni F, Radaelli G, Giovannini M. Dietary fats and cholesterol in Italian infants and children. American Journal of Clinical Nutrition 2000;72(5 Suppl):1384S‐91S. [DOI] [PubMed] [Google Scholar]
Ahola‐Olli 2014 {published data only}
- Ahola‐Olli AV, Pitkanen N, Kettunen J, Oikonen MK, Mikkila V, Lehtimaki T, et al. Interactions between genetic variants and dietary lipid composition: effects on circulating LDL cholesterol in children. American Journal of Clinical Nutrition 2014;100(6):1569‐77. [DOI] [PubMed] [Google Scholar]
Alexy 2002 {published data only}
- Alexy U, Sichert‐Hellert W, Kersting M. Fifteen‐year time trends in energy and macronutrient intake in German children and adolescents: results of the DONALD study. British Journal of Nutrition 2002;87(6):595‐604. [DOI] [PubMed] [Google Scholar]
Altwaijri 2009 {published data only}
- Altwaijri YA, Day RS, Harrist RB, Dwyer J, Ausman LM, Labarthe DR. Sexual maturation affects diet‐blood total cholesterol association in children: Project HeartBeat!. American Journal of Preventive Medicine 2009;37(1 Suppl):S65‐70. [DOI] [PubMed] [Google Scholar]
Alvirde‐Garcia 2013 {published data only}
- Alvirde‐Garcia U, Rodriguez‐Guerrero AJ, Henao‐Moran S, Gomez‐Perez FJ, Aguilar‐Salinas CA. Results of a community‐based life style intervention program for children [Resultados de un programa comunitario de intervencion en el estilo de vida en ninos]. Public Health of Mexico 2013;55 Suppl 3:406‐14. [PubMed] [Google Scholar]
Arvidsson 2015 {published data only}
- Arvidsson L, Bogl LH, Eiben G, Hebestreit A, Nagy P, Tornaritis M, et al. Fat, sugar and water intakes among families from the IDEFICS intervention and control groups: first observations from I.Family. Obesity Reviews 2015;16(Suppl 2):127‐37. [DOI: 10.1111/obr.12325] [DOI] [PubMed] [Google Scholar]
Barton 2005 {published data only}
- Barton BA, Eldridge AL, Thompson D, Affenito SG, Striegel‐Moore RH, Franko DL, et al. The relationship of breakfast and cereal consumption to nutrient intake and body mass index: the National Heart, Lung, and Blood Institute Growth and Health Study. Journal of the American Dietetic Association 2005;105(9):1383‐9. [DOI] [PubMed] [Google Scholar]
Berkey 2009 {published data only}
- Berkey CS, Colditz GA, Rockett HRH, Frazier AL, Willett WC. Dairy consumption and female height growth: prospective cohort study. Cancer Epidemiology, Biomarkers & Prevention 2009;18(6):1881‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulton 1995 {published data only}
- Boulton TJ, Magarey AM, Cockington RA. Tracking of serum lipids and dietary energy, fat and calcium intake from 1 to 15 years. Acta Paediatrica (Oslo, Norway : 1992) 1995;84(9):1050‐5. [DOI] [PubMed] [Google Scholar]
Brown 2013 {published data only}
- Brown B, Noonan C, Harris KJ, Parker M, Gaskill S, Ricci C, et al. Developing and piloting the Journey to Native Youth Health program in Northern Plains Indian communities. Diabetes Educator 2013;39(1):109‐18. [DOI] [PubMed] [Google Scholar]
Brox 2002 {published data only}
- Brox J, Bjornstad E, Olaussen K, Osterud B, Almdahl S, Lochen ML. Blood lipids, fatty acids, diet and lifestyle parameters in adolescents from a region in northern Norway with a high mortality from coronary heart disease. European Journal of Clinical Nutrition 2002;56 (7):694‐700. [DOI: ] [DOI] [PubMed] [Google Scholar]
Burke 2001 {published data only}
- Burke V, Beilin LJ, Dunbar D. Family lifestyle and parental body mass index as predictors of body mass index in Australian children: a longitudinal study. International Journal of Obesity and Related Metabolic Disorders 2001;25(2):147‐57. [DOI] [PubMed] [Google Scholar]
Caballero 2003 {published data only}
- Caballero B, Clay T, Davis SM, Ethelbah B, Rock BH, Lohman T, et al. Pathways: a school‐based, randomized controlled trial for the prevention of obesity in American Indian schoolchildren. American Journal of Clinical Nutrition 2003;78(5):1030‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cardel 2015 {published data only}
- Cardel M, Lemas DJ, Jackson KH, Friedman JE, Fernandez JR. Higher intake of PUFAs is associated with lower total and visceral adiposity and higher lean mass in a racially diverse sample of children. Journal of Nutrition 2015;145(9):2146‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen JL, Weiss S, Heyman MB, Lustig RH. Efficacy of a child‐centred and family‐based program in promoting healthy weight and healthy behaviors in Chinese American children: a randomized controlled study. Journal of Public Health (Oxford, England) 2012;32(2):219‐29. [DOI: 10.1093/pubmed/fdp105] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2011 {published data only}
- Choi H‐J, Joung H, Lee H‐J, Jang HB, Kang J‐H, Song J. The influence of dietary patterns on the nutritional profile in a Korean child cohort study. Osong Public Health and Research Perspectives 2011;2(1):59‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coppinger 2010 {published data only}
- Coppinger T, Jeanes YM, Dabinett J, Vogele C, Reeves S. Physical activity and dietary intake of children aged 9‐11 years and the influence of peers on these behaviours: a 1‐year follow‐up. European Journal of Clinical Nutrition 2010;64(8):776‐81. [DOI] [PubMed] [Google Scholar]
Couch 2014 {published data only}
- Couch SC, Saelens BE, Hinn K, Dart KB, Khoury P, Mitsnefes M, et al. Effects of a clinic‐initiated behavioral nutrition intervention emphasizing the dash diet on blood pressure control in adolescents with elevated blood pressure. Journal of the American Society of Hypertension 2014;8(1):e116. [DOI: ] [Google Scholar]
Crawford 1995 {published data only}
- Crawford PB, Obarzanek E, Schreiber GB, Barrier P, Goldman S, Frederick MM, et al. The effects of race, household income, and parental education on nutrient intakes of 9‐ and 10‐year‐old girls. NHLBI Growth and Health Study. Annals of Epidemiology 1995;5(5):360‐8. [DOI] [PubMed] [Google Scholar]
Cresanta 1988 {published data only}
- Cresanta JL, Farris RP, Croft JB, Webber LS, Frank GC, Berenson GS. Trends in fatty acid intakes of 10‐year‐old children, 1973 to 1982. Journal of the American Dietetic Association 1988;88(2):178‐84. [PubMed] [Google Scholar]
Dalskov 2014 {published data only}
- Dalskov S‐M, Muller M, Ritz C, Damsgaard CT, Papadaki A, Saris WHM, et al. Effects of dietary protein and glycaemic index on biomarkers of bone turnover in children. British Journal of Nutrition 2014;111(7):1253‐62. [DOI] [PubMed] [Google Scholar]
Davies 1997 {published data only}
- Davies PSW. Diet composition and body mass index in pre‐school children. European Journal of Clinical Nutrition 1997;51(7):443‐8. [DOI] [PubMed] [Google Scholar]
Deheeger 1996 {published data only}
- Deheeger M, Akrout M, Bellisle F, Rossignol C, Rolland‐Cachera MF. Individual patterns of food intake development in children: a 10 months to 8 years of age follow‐up study of nutrition and growth. Physiology & Behavior 1996;59(3):403‐7. [DOI] [PubMed] [Google Scholar]
Deheeger 2002 {published data only}
- Deheeger M, Bellisle F, Rolland‐Cachera MF. The French longitudinal study of growth and nutrition: data in adolescent males and females. Journal of Human Nutrition and Dietetics 2002;15(6):429‐38. [DOI: ] [DOI] [PubMed] [Google Scholar]
Dixon 2005 {published data only}
- Dixon LB, Pellizzon MA, Jawad Abbas F, Tershakovec AM. Calcium and dairy intake and measures of obesity in hyper‐ and normocholesterolemic children. Obesity Research 2005;13(10):1727‐38. [DOI] [PubMed] [Google Scholar]
Donnelly 1996 {published data only}
- Donnelly JE, Jacobsen DJ, Whatley JE, Hill JO, Swift LL, Cherrington A, et al. Nutrition and physical activity program to attenuate obesity and promote physical and metabolic fitness in elementary school children. Obesity Research 1996;4(3):229‐43. [DOI] [PubMed] [Google Scholar]
Dubois 2016 {published data only}
- Dubois L, Diasparra M, Bogl LH, Fontaine‐Bisson B, Bedard B, Tremblay RE, et al. Dietary intake at 9 years and subsequent body mass index in adolescent boys and girls: a study of monozygotic twin pairs. Twin Research and Human Genetics 2016;19(1):47‐59. [DOI: 10.1017/thg.2015.97] [DOI] [PubMed] [Google Scholar]
Dwyer 2002 {published data only}
- Dwyer JT, Feldman HA, Yang M, Webber LS, Must A, Perry CL, et al. Maintenance of lightweight correlates with decreased cardiovascular risk factors in early adolescence. Journal of Adolescent Health 2002;31(2):117‐24. [DOI] [PubMed] [Google Scholar]
Dwyer 2003 {published data only}
- Dwyer JT, Michell P, Cosentino C, Webber L, Seed JM, Hoelscher D, et al. Fat‐sugar see‐saw in school lunches: impact of a low fat intervention. Journal of Adolescent Health 2003;32(6):428‐35. [DOI] [PubMed] [Google Scholar]
Eck 1992 {published data only}
- Eck LH, Klesges RC, Hanson CL, Slawson D. Children at familial risk for obesity: an examination of dietary intake, physical activity and weight status. International Journal of Obesity and Related Metabolic Disorders 1992;16(2):71‐8. [PubMed] [Google Scholar]
Elder 2014 {published data only}
- Elder JP, Crespo NC, Corder K, Ayala GX, Slymen DJ, Lopez NV, et al. Childhood obesity prevention and control in city recreation centres and family homes: the MOVE/me Muevo Project. Pediatric Obesity 2014;9(3):218‐31. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Emmett 2015a {published data only}
- Emmett PM, Jones LR. Diet, growth, and obesity development throughout childhood in the Avon Longitudinal Study of Parents and Children. Nutrition Reviews 2015;73:175‐206. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Emmett 2015b {published data only}
- Emmett PM, Jones LR, Northstone K. Dietary patterns in the Avon Longitudinal Study of Parents and Children. Nutrition Reviews 2015;73(Suppl 3):207‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Epstein 2001 {published data only}
- Epstein LH, Gordy CC, Raynor HA, Beddome M, Kilanowski CK, Paluch R. Increasing fruit and vegetable intake and decreasing fat and sugar intake in families at risk for childhood obesity. Obesity Research 2001;9(3):171‐8. [DOI] [PubMed] [Google Scholar]
Evans 2010 {published data only}
- Evans CEL, Greenwood DC, Thomas JD, Cleghorn CL, Kitchen MS, et al. SMART lunch box intervention to improve the food and nutrient content of children's packed lunches: UK wide cluster randomised controlled trial. Journal of Epidemiology and Community Health 2010;64(11):970‐6. [DOI] [PubMed] [Google Scholar]
Farris 1984a {published data only}
- Farris RP, Cresanta JL, Frank GC, Webber LS, Berenson GS. Dietary studies of children from a biracial population: intakes of fat and fatty acids in 10‐ and 13‐year‐olds. American Journal of Clinical Nutrition 1984;39(1):114‐28. [DOI] [PubMed] [Google Scholar]
Farris 1984b {published data only}
- Farris RP, Cresanta JL, Frank GC, Webber LS, Berenson GS. Dietary studies of children from a biracial population: intakes of fat and fatty acids in 10‐ and 13‐year olds. American Journal of Clinical Nutrition 1984;39(1):114‐28. [DOI] [PubMed] [Google Scholar]
Fitzgibbon 2002 {published data only}
- Fitzgibbon ML, Stolley MR, Dyer AR, Horn L, Kaufer CK. A community‐based obesity prevention program for minority children: rationale and study design for Hip‐Hop to Health Jr. Preventive Medicine 2002;34(2):289‐97. [DOI] [PubMed] [Google Scholar]
Fitzgibbon 2005 {published data only}
- Fitzgibbon ML, Stolley MR, Schiffer L, Horn L, Kauferchristoffel K, Dyer A. Two‐year follow‐up results for Hip‐Hop to Health Jr.: a randomized controlled trial for overweight prevention in preschool minority children. Journal of Pediatrics 2005;146(5):618‐25. [DOI: ] [DOI] [PubMed] [Google Scholar]
Foster 2008 {published data only}
- Foster GD, Sherman S, Borradaile KE, Grundy KM, Vander Veur SS, Nachmani J, et al. A policy‐based school intervention to prevent overweight and obesity. Pediatrics 2008;121(4):e794‐802. [DOI] [PubMed] [Google Scholar]
Frank 1985a {published data only}
- Frank GC, Farris RP, Cresanta JL. Dietary trends of 10‐ and 13‐year‐old children in a biracial community ‐ the Bogalusa heart study. Preventive Medicine 1985;14(1):123‐39. [DOI: ] [DOI] [PubMed] [Google Scholar]
Frank 1985b {published data only}
- Frank GC, Farris RP, Cresanta JL, Webber LS, Berenson GS. Dietary trends of 10‐ and 13‐year‐old children in a biracial community ‐ the Bogalusa Heart Study. Preventive Medicine 1985;14(1):123‐39. [DOI] [PubMed] [Google Scholar]
Gillis 2009 {published data only}
- Gillis B, Mobley C, Stadler DD, Hartstein J, Virus A, Volpe SL, et al. Rationale, design and methods of the HEALTHY study nutrition intervention component. International Journal of Obesity (2005) 2009;33 Suppl 4:S29‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 1992 {published data only}
- Goldberg RJ, Ellison RC, Hosmer DW Jr, Capper AL, Puleo E, Gamble WJ, et al. Effects of alterations in fatty acid intake on the blood pressure of adolescents: the Exeter‐Andover Project. American Journal of Clinical Nutrition 1992;56(1):71‐6. [DOI] [PubMed] [Google Scholar]
Gortmaker 1999 {published data only}
- Gortmaker SL, Peterson K, Wiecha J, Sobol AM, Dixit S, Fox MK, et al. Reducing obesity via a school‐based interdisciplinary intervention among youth: Planet Health. Archives of Pediatrics & Adolescent Medicine 1999;153(4):409‐18. [DOI] [PubMed] [Google Scholar]
Harris 2016 {published data only}
- Harris C, Buyken A, Koletzko S, Berg A, Berdel D, Schikowski T, et al. Associations of dietary fatty acids with serum lipids from childhood to adolescence: results from the GINIplus and LISAplus studies. European Journal of Epidemiology 2016;31:S75. [DOI: ] [Google Scholar]
Harris 2017 {published data only}
- Harris C, Buyken A, Koletzko S, Berg A, Berdel D, Schikowski T, et al. Dietary fatty acids and changes in blood lipids during adolescence: the role of substituting nutrient intakes. Nutrients 2017;9(2):E127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hendrie 2011 {published data only}
- Hendrie GA, Golley RK. Changing from regular‐fat to low‐fat dairy foods reduces saturated fat intake but not energy intake in 4‐13‐y‐old children. American Journal of Clinical Nutrition 2011;93(5):1117‐27. [DOI] [PubMed] [Google Scholar]
Himes 2003 {published data only}
- Himes JH, Ring K, Gittelsohn J, Cunningham‐Sabo L, Weber J, Thompson J, et al. Impact of the pathways intervention on dietary intakes of American Indian schoolchildren. Preventive Medicine 2003;37(6 Pt 2):S55‐61. [DOI] [PubMed] [Google Scholar]
Hollis 1984 {published data only}
- Hollis JF, Sexton G, Connor SL, Calvin L, Pereira C, Matarazzo JD. The family heart dietary intervention program: community response and characteristics of joining and nonjoining families. Preventive Medicine 1984;13(3):276‐85. [DOI] [PubMed] [Google Scholar]
Hood 2000 {published data only}
- Hood MY, Moore LL, Sundarajan‐Ramamurti A, Singer M, Cupples LA, Ellison RC. Parental eating attitudes and the development of obesity in children. The Framingham Children's Study. International Journal of Obesity and Related Metabolic Disorders 2000;24(10):1319‐25. [DOI] [PubMed] [Google Scholar]
Jacobson 1998 {published data only}
- Jacobson MS, Tomopoulos S, Williams CL, Arden MR, Deckelbaum RJ, Starc TJ. Normal growth in high‐risk hyperlipidemic children and adolescents with dietary intervention. Preventive Medicine 1998;27(6):775‐80. [DOI] [PubMed] [Google Scholar]
Jancey 2014 {published data only}
- Jancey JM, Dos Remedios Monteiro SM, Dhaliwal SS, Howat PA, Burns S, Hills AP, et al. Dietary outcomes of a community based intervention for mothers of young children: a randomised controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2014;11:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jimenez 2003 {published data only}
- Jimenez MM, Receveur O, Trifonopoulos M, Kuhnlein H, Paradis G, Macaulay AC. Comparison of the dietary intakes of two different groups of children (grades 4 to 6) before and after the Kahnawake Schools Diabetes Prevention Project. Journal of the American Dietetic Association 2003;103(9):1191‐4. [DOI: 10.1053/jada.2003.50573] [DOI] [PubMed] [Google Scholar]
Karnehed 2006 {published data only}
- Karnehed N, Tynelius P, Heitmann BL, Rasmussen F. Physical activity, diet and gene‐environment interactions in relation to body mass index and waist circumference: the Swedish Young Male Twins Study. Public Health Nutrition 2006;9 (7):851‐8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Khalil 2017 {published data only}
- Khalil H, Murrin C, O'Reilly M, Viljoen K, Segurado R, O'Brien J, et al. Total HDL cholesterol efflux capacity in healthy children ‐ associations with adiposity and dietary intakes of mother and child. Nutrition, Metabolism, and Cardiovascular Diseases : NMCD 2017;27(1):70‐7. [DOI] [PubMed] [Google Scholar]
Kiefte‐de Jong 2013 {published data only}
- Kiefte‐de Jong JC, Vries JH, Escher JC, Jaddoe VWV, Hofman A, Raat H, et al. Role of dietary patterns, sedentary behaviour and overweight on the longitudinal development of childhood constipation: the Generation R study. Maternal and Child Nutrition 2013;9(4):511‐23. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimm 1999 {published data only}
- Kimm SY, Pasagian‐Macaulay A, Aston CE, McAllister AE, Glynn NW, Kamboh MI, et al. Correlates of lipoprotein(a) levels in a biracial cohort of young girls: the NHLBI Growth and Health Study. Journal of Pediatrics 1999;135(2 Pt 1):169‐76. [DOI] [PubMed] [Google Scholar]
Kronsberg 2003 {published data only}
- Kronsberg SS, Obarzanek E, Affenito SG, Crawford PB, Sabry ZI, Schmidt M, et al. Macronutrient intake of black and white adolescent girls over 10 years: the NHLBI Growth and Health Study. Journal of the American Dietetic Association 2003;103(7):852‐60. [DOI] [PubMed] [Google Scholar]
Kuehl 1993 {published data only}
- Kuehl KS, Cockerham JT, Hitchings M, Slater D, Nixon G, Rifai N. Effective control of hypercholesterolemia in children with dietary interventions based in pediatric practice. Preventive Medicine 1993;22(2):154‐66. [DOI] [PubMed] [Google Scholar]
Kuzawa 2003 {published data only}
- Kuzawa CW, Adair LS, Avila JL, Cadungog JHC, Le N‐A. Atherogenic lipid profiles in Filipino adolescents with low body mass index and low dietary fat intake. American Journal of Human Biology 2003;15(5):688‐96. [DOI] [PubMed] [Google Scholar]
Kwiterovich 1997 {published data only}
- Kwiterovich PO Jr, Barton BA, McMahon RP, Obarzanek E, Hunsberger S, Simons‐Morton D, et al. Effects of diet and sexual maturation on low‐density lipoprotein cholesterol during puberty: the Dietary Intervention Study in Children (DISC). Circulation 1997;96(8):2526‐33. [DOI] [PubMed] [Google Scholar]
Kwiterovich 2001 {published data only}
- Kwiterovich PO Jr. Safety and efficacy of treatment of children and adolescents with elevated low density lipoprotein levels with a step two diet or with lovastatin. Nutrition, Metabolism, and Cardiovascular Diseases : NMCD 2001;11 Suppl 5:30‐4. [PubMed] [Google Scholar]
Lagstrom 1997a {published data only}
- Lagstrom H, Jokinen E, Seppanen R, Ronnemaa T, Viikari J, Valimaki I, et al. Nutrient intakes by young children in a prospective randomized trial of a low‐saturated fat, low‐cholesterol diet. The STRIP Baby Project. Special Turku Coronary Risk Factor Intervention Project for Babies. Archives of Pediatrics & Adolescent Medicine 1997;151(2):181‐8. [DOI] [PubMed] [Google Scholar]
Lagstrom 1997b {published data only}
- Lagstrom H, Jokinen E, Seppanen R, Ronnemaa T, Viikari J, Valimaki I, et al. Nutrient intakes by young children in a prospective randomized trial of a low‐saturated fat, low‐cholesterol diet: the STRIP baby project. Archives of Pediatrics and Adolescent Medicine 1997;151(2):181‐8. [DOI] [PubMed] [Google Scholar]
Lagstrom 1999 {published data only}
- Lagstrom H, Seppanen R, Jokinen E, Niinikoski H, Ronnemaa T, Viikari J, et al. Influence of dietary fat on the nutrient intake and growth of children from 1 to 5 y of age: the Special Turku Coronary Risk Factor Intervention Project. American Journal of Clinical Nutrition 1999;69(3):516‐23. [DOI] [PubMed] [Google Scholar]
Larsen 2010 {published data only}
- Larsen TM, Dalskov S, Baak M, Jebb S, Kafatos A, Pfeiffer A, et al. The Diet, Obesity and Genes (Diogenes) Dietary Study in eight European countries ‐ a comprehensive design for long‐term intervention. Obesity Reviews 2010;11(1):76‐91. [DOI] [PubMed] [Google Scholar]
Lee 2007 {published data only}
- Lee SK, Novotny R, Daida YG, Vijayadeva V, Gittelsohn J. Dietary patterns of adolescent girls in Hawaii over a 2‐year period. Journal of the American Dietetic Association 2007;107(6):956‐61. [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee A, Chowdhury R, Welsh J. Increased intake of non‐dairy sugars in foods and beverages is positively associated with annual increases in waist circumference among overweight and obese adolescent females. Circulation 2014;129:AMP52. [Google Scholar]
Lee 2017 {published data only}
- Lee HA, Hwang HJ, Oh SY, Park EA, Cho SJ, Kim HS, et al. The differential effects of changes in individual macronutrient intake on changes in lipid concentrations during childhood: from the Ewha Birth & Growth Cohort. Clinical Nutrition (Edinburgh, Scotland) 2017;17:30156‐5. [DOI] [PubMed] [Google Scholar]
Leung 2000a {published data only}
- Leung SS, Chan SM, Lui S, Lee WT, Davies DP. Growth and nutrition of Hong Kong children aged 0‐7 years. Journal of Paediatrics and Child Health 2000;36(1):56‐65. [DOI] [PubMed] [Google Scholar]
Leung 2000b {published data only}
- Leung SS, Lee WT, Lui SS, Ng MY, Peng XH, Luo HY, et al. Fat intake in Hong Kong Chinese children. American Journal of Clinical Nutrition 2000;72(5 Suppl):1373S‐8S. [DOI] [PubMed] [Google Scholar]
Li 2008 {published data only}
- Li Ji, Wang Y. Tracking of dietary intake patterns is associated with baseline characteristics of urban low‐income African‐American adolescents. Journal of Nutrition 2008;138(1):94‐100. [DOI] [PubMed] [Google Scholar]
Libuda 2014 {published data only}
- Libuda L, Alexy U, Kersting M. Time trends in dietary fat intake in a sample of German children and adolescents between 2000 and 2010: Not quantity, but quality is the issue. British Journal of Nutrition 2014;111(1):141‐50. [DOI: ] [DOI] [PubMed] [Google Scholar]
Maclure 1991 {published data only}
- Maclure M, Travis LB, Willett W, MacMahon B. A prospective cohort study of nutrient intake and age at menarche. American Journal of Clinical Nutrition 1991;54(4):649‐56. [DOI] [PubMed] [Google Scholar]
Mamalakis 2001 {published data only}
- Mamalakis G, Kafatos A, Manios Y, Kalogeropoulos N, Andrikopoulos N. Adipose fat quality vs. quantity: relationships with children's serum lipid levels. Preventive Medicine 2001;33(6):525‐35. [DOI] [PubMed] [Google Scholar]
Manios 2002 {published data only}
- Manios Y, Moschandreas J, Hatzis C, Kafatos A. Health and nutrition education in primary schools of Crete: changes in chronic disease risk factors following a 6‐year intervention programme. British Journal of Nutrition 2002;88(3):315‐24. [DOI] [PubMed] [Google Scholar]
Manios 2006 {published data only}
- Manios Y, Kafatos A, Preventive, Medicine, Nutrition Clinic University of Crete Research Team. Health and nutrition education in primary schools in Crete: 10 years follow‐up of serum lipids, physical activity and macronutrient intake. British Journal of Nutrition 2006;95(3):568‐75. [DOI] [PubMed] [Google Scholar]
Marcus 2009 {published data only}
- Marcus C, Nyberg G, Nordenfelt A, Karpmyr M, Kowalski J, Ekelund U. A 4‐year, cluster‐randomized, controlled childhood obesity prevention study: STOPP. International Journal of Obesity (2005) 2009;33(4):408‐17. [DOI] [PubMed] [Google Scholar]
Maresh 1970 {published data only}
- Maresh MM, Beal VA. A longitudinal survey of nutrition intake, body size, and tissue measurements in healthy subjects during growth. Monographs of the Society for Research in Child Development 1970;35(7):33‐9. [PubMed] [Google Scholar]
Michels 2015a {published data only}
- Michels N, Kriemler S, Marques‐Vidal PM, Nydegger A, Puder J. Psychosocial quality of life, lifestyle and adiposity: a longitudinal study in preschoolers. Psychosomatic Medicine 2015;77(3):A29‐A30. [DOI] [PubMed] [Google Scholar]
Michels 2015b {published data only}
- Michels N, Kriemler S, Marques‐Vidal PM, Nydegger A, Puder JJ. Psychosocial quality‐of‐life, lifestyle and adiposity: a longitudinal study in preschoolers. Obesity Facts 2015;8:187‐8. [DOI: ] [Google Scholar]
Michels 2016 {published data only}
- Michels N, Susi K, Marques‐Vidal PM, Nydegger A, Puder JJ. Psychosocial quality‐of‐life, lifestyle and adiposity: a longitudinal study in pre‐schoolers (Ballabeina Study). International Journal of Behavioral Medicine 2016;23(3):383‐92. [DOI] [PubMed] [Google Scholar]
Newby 2003 {published data only}
- Newby PK, Peterson KE, Berkey CS, Leppert J, Willett WC, Colditz GA. Dietary composition and weight change among low‐income preschool children. Archives of Pediatrics & Adolescent Medicine 2003;157(8):759‐64. [DOI] [PubMed] [Google Scholar]
Nicklas 1991 {published data only}
- Nicklas TA, Webber LS, Berenson GS. Studies of consistency of dietary intake during the first four years of life in a prospective analysis: Bogalusa Heart Study. Journal of the American College of Nutrition 1991;10(3):234‐41. [DOI] [PubMed] [Google Scholar]
Nicklas 1992 {published data only}
- Nicklas TA, Webber LS, Koschak M, Berenson GS. Nutrient adequacy of low fat intakes for children: the Bogalusa Heart Study. Pediatrics 1992;89(2):221‐8. [PubMed] [Google Scholar]
Niinikoski 1996 {published data only}
- Niinikoski H, Viikari J, Ronnemaa T, Lapinleimu H, Jokinen E, Salo P, et al. Prospective randomized trial of low‐saturated‐fat, low‐cholesterol diet during the first 3 years of life. The STRIP baby project. Circulation 1996;94(6):1386‐93. [DOI] [PubMed] [Google Scholar]
Niinikoski 1997b {published data only}
- Niinikoski H, Lapinleimu H, Viikari J, Ronnemaa T, Jokinen E, Seppanen R, et al. Growth until 3 years of age in a prospective, randomized trial of a diet with reduced saturated fat and cholesterol. Pediatrics 1997;99(5):687‐94. [DOI] [PubMed] [Google Scholar]
Niinikoski 2007 {published data only}
- Niinikoski H, Lagstrom H, Jokinen E, Siltala M, Ronnemaa T, Viikari J, et al. Impact of repeated dietary counseling between infancy and 14 years of age on dietary intakes and serum lipids and lipoproteins: the STRIP study. Circulation 2007;116(9):1032‐40. [DOI] [PubMed] [Google Scholar]
Niinikoski 2009 {published data only}
- Niinikoski H, Jula A, Viikari J, Ronnemaa T, Heino P, Lagstrom H, et al. Blood pressure is lower in children and adolescents with a low‐saturated‐fat diet since infancy: the Special Turku Coronary Risk Factor Intervention project. Hypertension (Dallas, Tex.: 1979) 2009;53(6):918‐24. [DOI] [PubMed] [Google Scholar]
Niinikoski 2009a {published data only}
- Niinikoski H, Jula A, Viikari J, Ronnemaa T, Heino P, Lagstrom H, et al. Blood pressure is lower in children and adolescents with a low‐saturated‐fat diet since infancy the Special Turku Coronary Risk Factor Intervention project. Hypertension 2009;53(6):918‐24. [DOI: ] [DOI] [PubMed] [Google Scholar]
Niinikoski 2012 {published data only}
- Niinikoski H, Ruottinen S. Is carbohydrate intake in the first years of life related to future risk of NCDs?. Nutrition, Metabolism, and Cardiovascular Diseases : NMCD 2012;22(10):770‐4. [DOI] [PubMed] [Google Scholar]
Niinikoski 2014 {published data only}
- Niinikoski H, Pahkala K, Viikari J, Ronnemaa T, Jula A, Lagstrom H, et al. The STRIP study: long‐term impact of a low saturated fat/low cholesterol diet. Current Cardiovascular Risk Reports 2014;8(11):1‐7. [DOI: ] [Google Scholar]
O'Sullivan 2011 {published data only}
- O'Sullivan TA, Ambrosini G, Beilin LJ, Mori TA, Oddy WH. Dietary intake and food sources of fatty acids in Australian adolescents. Nutrition (Burbank, Los Angeles County, Calif.) 2011;27(2):153‐9. [DOI] [PubMed] [Google Scholar]
Obarzanek 1994 {published data only}
- Obarzanek E, Schreiber GB, Crawford PB, Goldman SR, Barrier PM, Frederick MM, et al. Energy intake and physical activity in relation to indexes of body fat: the National Heart, Lung, and Blood Institute Growth and Health Study. American Journal of Clinical Nutrition 1994;60(1):15‐22. [DOI] [PubMed] [Google Scholar]
Ohlund 2011 {published data only}
- Ohlund I, Hernell O, Hornell A, Lind T. Serum lipid and apolipoprotein levels in 4‐year‐old children are associated with parental levels and track over time. European Journal of Clinical Nutrition 2011;65(4):463‐9. [DOI: 10.1038/ejcn.2011.14] [DOI] [PubMed] [Google Scholar]
Ohrig 2001 {published data only}
- Ohrig E, Geiss HC, Haas GM, Schwandt P. The Prevention Education Program (PEP) Nuremberg: design and baseline data of a family oriented intervention study. International Journal of Obesity and Related Metabolic Disorders 2001;25 Suppl 1:S89‐92. [DOI] [PubMed] [Google Scholar]
Oranta 2013 {published data only}
- Oranta O, Pahkala K, Ruottinen S, Niinikoski H, Lagstrom H, Viikari JSA, et al. Infancy‐onset dietary counseling of low‐saturated‐fat diet improves insulin sensitivity in healthy adolescents 15‐20 years of age: the Special Turku Coronary Risk Factor Intervention Project (STRIP) study. Diabetes Care 2013;36(10):2952‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osganian 1996 {published data only}
- Osganian SK, Ebzery MK, Montgomery DH, Nicklas TA, Evans MA, Mitchell PD, et al. Changes in the nutrient content of school lunches: results from the CATCH Eat Smart Food service Intervention. Preventive Medicine 1996;25(4):400‐12. [DOI] [PubMed] [Google Scholar]
Paineau 2008 {published data only}
- Paineau DL, Beaufils F, Boulier A, Cassuto D‐A, Chwalow J, Combris P, et al. Family dietary coaching to improve nutritional intakes and body weight control: a randomized controlled trial. Archives of Pediatrics & Adolescent Medicine 2008;162(1):34‐43. [DOI] [PubMed] [Google Scholar]
Paineau 2010 {published data only}
- Paineau D, Beaufils F, Boulier A, Cassuto DA, Chwalow J, Combris P, et al. The cumulative effect of small dietary changes may significantly improve nutritional intakes in free‐living children and adults. European Journal of Clinical Nutrition 2010;64(8):782‐91. [DOI: ] [DOI] [PubMed] [Google Scholar]
Patrick 2006 {published data only}
- Patrick K, Calfas KJ, Norman GJ, Zabinski MF, Sallis JF, Rupp J, et al. Randomized controlled trial of a primary care and home‐based intervention for physical activity and nutrition behaviors: PACE+ for adolescents. Archives of Pediatrics & Adolescent Medicine 2006;160(2):128‐36. [DOI] [PubMed] [Google Scholar]
Pimpin 2016 {published data only}
- Pimpin L, Jebb S, Johnson L, Wardle J, Ambrosini GL. Dietary protein intake is associated with body mass index and weight up to 5 y of age in a prospective cohort of twins. American Journal of Clinical Nutrition 2016;103(2):389‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Post 1997 {published data only}
- Post GB, Kemper HCG, Twisk J, Mechelen W. The association between dietary patterns and cardio vascular disease risk indicators in healthy youngsters: Results covering fifteen years of longitudinal development. European Journal of Clinical Nutrition 1997;51(6):387‐93. [DOI] [PubMed] [Google Scholar]
Proctor 2003 {published data only}
- Proctor MH, Moore LL, Gao D, Cupples LA, Bradlee ML, Hood MY, et al. Television viewing and change in body fat from preschool to early adolescence: the Framingham Children's Study. International Journal of Obesity and Related Metabolic Disorders 2003;27(7):827‐33. [DOI] [PubMed] [Google Scholar]
Raitakari 2005 {published data only}
- Raitakari OT, Ronnemaa T, Jarvisalo MJ, Kaitosaari T, Volanen I, Kallio K, et al. Endothelial function in healthy 11‐year‐old children after dietary intervention with onset in infancy: the Special Turku Coronary Risk Factor Intervention Project for children (STRIP). Circulation 2005;112(24):3786‐94. [DOI] [PubMed] [Google Scholar]
Rask‐Nissila 2000a {published data only}
- Rask‐Nissila L, Jokinen E, Terho P, Tammi A, Lapinleimu H, Ronnemaa T, et al. Neurological development of 5‐year‐old children receiving a low‐saturated fat, low‐cholesterol diet since infancy: a randomized controlled trial. JAMA 2000;284(8):993‐1000. [DOI] [PubMed] [Google Scholar]
Rask‐Nissila 2000b {published data only}
- Rask‐Nissila L, Jokinen E, Ronnemaa T, Viikari J, Tammi A, Niinikoski H, et al. Prospective, randomized, infancy‐onset trial of the effects of a low‐saturated‐fat, low‐cholesterol diet on serum lipids and lipoproteins before school age: The Special Turku Coronary Risk Factor Intervention Project (STRIP). Circulation 2000;102(13):1477‐83. [DOI] [PubMed] [Google Scholar]
Rask‐Nissila 2002a {published data only}
- Rask‐Nissila L, Jokinen E, Viikari J, Tammi A, Ronnemaa T, Marniemi J, et al. Impact of dietary intervention, sex, and apolipoprotein E phenotype on tracking of serum lipids and apolipoproteins in 1‐ to 5‐year‐old children: the Special Turku Coronary Risk Factor Intervention Project (STRIP). Arteriosclerosis, Thrombosis, and Vascular Biology 2002;22(3):492‐8. [DOI] [PubMed] [Google Scholar]
Rask‐Nissila 2002b {published data only}
- Rask‐Nissila L, Jokinen E, Terho P, Tammi A, Hakanen M, Ronnemaa T, et al. Effects of diet on the neurologic development of children at 5 years of age: the STRIP project. Journal of Pediatrics 2002;140(3):328‐33. [DOI] [PubMed] [Google Scholar]
Rehkopf 2011 {published data only}
- Rehkopf DH, Laraia BA, Segal M, Braithwaite D, Epel E. The relative importance of predictors of body mass index change, overweight and obesity in adolescent girls. International Journal of Pediatric Obesity 2011;6(2‐2):e233‐42. [DOI: ] [DOI] [PubMed] [Google Scholar]
Robertson 1999 {published data only}
- Robertson SM, Cullen KW, Baranowski J, Baranowski T, Hu S, Moor C. Factors related to adiposity among children aged 3 to 7 years. Journal of the American Dietetic Association 1999;99(8):938‐43. [DOI] [PubMed] [Google Scholar]
Ruxton 1995 {published data only}
- Ruxton CH, Kirk TR, Holmes MA, Belton NR. No adverse effects on growth seen in Scottish school children consuming either low fat diets or diets relatively high in non‐starch polysaccharide. Health Bulletin 1995;53(6):398‐401. [PubMed] [Google Scholar]
Sallis 2003 {published data only}
- Sallis JF, McKenzie TL, Conway TL, Elder JP, Prochaska JJ, Brown M, et al. Environmental interventions for eating and physical activity: a randomized controlled trial in middle schools. American Journal of Preventive Medicine 2003;24(3):209‐17. [DOI] [PubMed] [Google Scholar]
Sanchez‐Bayle 2003 {published data only}
- Sanchez‐Bayle M, Soriano‐Guillen L. Influence of dietary intervention on growth in children with hypercholesterolaemia. Acta Paediatrica (Oslo, Norway : 1992) 2003;92(9):1043‐6. [DOI] [PubMed] [Google Scholar]
Sawaya 1998 {published data only}
- Sawaya AL, Grillo LP, Verreschi I, Silva AC, Roberts SB. Mild stunting is associated with higher susceptibility to the effects of high fat diets: studies in a shantytown population in Sao Paulo, Brazil. Journal of Nutrition 1998;128(2 Suppl):415S‐20S. [DOI] [PubMed] [Google Scholar]
Siega‐Riz 2011 {published data only}
- Siega‐Riz AM, Ghormli L, Mobley C, Gillis B, Stadler D, Hartstein J, et al. The effects of the HEALTHY study intervention on middle school student dietary intakes. International Journal of Behavioral Nutrition and Physical Activity 2011;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simell 1999 {published data only}
- Simell O, Niinikoski H, Viikari J, Rask‐Nissila L, Tammi A, Ronnemaa T. Cardiovascular disease risk factors in young children in the STRIP baby project. Special Turku coronary Risk factor Intervention Project for children. Annals of Medicine 1999;31 Suppl 1:55‐61. [PubMed] [Google Scholar]
Spruijt‐Metz 2002 {published data only}
- Spruijt‐Metz D, Lindquist CH, Birch LL, Fisher JO, Goran MI. Relation between mothers' child‐feeding practices and children's adiposity. American Journal of Clinical Nutrition 2002;75(3):581‐6. [DOI] [PubMed] [Google Scholar]
Spruijt‐Metz 2006 {published data only}
- Spruijt‐Metz D, Li C, Cohen E, Birch L, Goran M. Longitudinal influence of mother's child‐feeding practices on adiposity in children. Journal of Pediatrics 2006;148(3):314‐20. [DOI] [PubMed] [Google Scholar]
Stice 2015 {published data only}
- Stice E, Yokum S, Burger K, Rohde P, Shaw H, Gau JM. A pilot randomized trial of a cognitive reappraisal obesity prevention program. Physiology and Behavior 2015;138:124‐32. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 1996 {published data only}
- Stone EJ, Osganian SK, McKinlay SM, Wu MC, Webber LS, Luepker RV, et al. Operational design and quality control in the CATCH multicenter Trial. Preventive Medicine 1996;25(4):384‐99. [DOI] [PubMed] [Google Scholar]
Stone 2003 {published data only}
- Stone EJ, Norman JE, Davis SM, Stewart D, Clay TE, Caballero B, et al. Design, implementation, and quality control in the Pathways American‐Indian multicenter trial. Preventive Medicine 2003;37(6 Pt 2):S13‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Story 2003 {published data only}
- Story M, Snyder MP, Anliker J, Weber JL, Cunningham‐Sabo L, Stone EJ, et al. Changes in the nutrient content of school lunches: results from the Pathways study. Preventive Medicine 2003;37(6 Pt 2):S35‐45. [DOI] [PubMed] [Google Scholar]
Talvia 2004 {published data only}
- Talvia S, Lagström H, Räsänen M, Salminen M, Räsänen L, Salo P, et al. A randomized intervention since infancy to reduce intake of saturated fat: calorie (energy) and nutrient intakes up to the age of 10 years in the Special Turku Coronary Risk Factor Intervention Project. Archives of Pediatrics & Adolescent Medicine 2004;158(1):41‐7. [DOI: 10.1001/archpedi.158.1.41] [DOI] [PubMed] [Google Scholar]
Telford 2012 {published data only}
- Telford R, Cunningham R, Telford R, Potter J, Hickman P, Kerrigan J, et al. Do changes in physical activity, fitness, adiposity or diet influence insulin resistance in boys and girls? The LOOK study. Journal of Science and Medicine in Sport 2012;15:S75. [DOI: ] [Google Scholar]
Telford 2015 {published data only}
- Telford R, Cunningham RB, Waring P, Telford RM, Potter JM, Hickman PE, et al. Sensitivity of blood lipids to changes in adiposity, exercise, and diet in children. Medicine and Science in Sports and Exercise 2015;47(5):974‐82. [DOI] [PubMed] [Google Scholar]
Teufel 1999 {published data only}
- Teufel NI, Perry CL, Story M, Flint‐Wagner HG, Levin S, Clay TE, et al. Pathways family intervention for third‐grade American Indian children. American Journal of Clinical Nutrition 1999;69(4 Suppl):803S‐9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Treuth 2003 {published data only}
- Treuth MS, Sunehag AL, Trautwein LM, Bier DM, Haymond MW, Butte NF. Metabolic adaptation to high‐fat and high‐carbohydrate diets in children and adolescents. American Journal of Clinical Nutrition 2003;77(2):479‐89. [DOI] [PubMed] [Google Scholar]
Trevino 2004 {published data only}
- Trevino RP, Yin Z, Hernandez A, Hale DE, Garcia O, Mobley C. Impact of the Bienestar school‐based diabetes mellitus prevention program on fasting capillary glucose levels: a randomized controlled trial. Archives of Pediatrics & Adolescent Medicine 2004;158(9):911‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vandongen 1995 {published data only}
- Vandongen R, Jenner DA, Thompson C, Taggart AC, Spickett EE, Burke V, et al. A controlled evaluation of a fitness and nutrition intervention program on cardiovascular health in 10‐ to 12‐year‐old children. Preventive Medicine 1995;24(1):9‐22. [DOI] [PubMed] [Google Scholar]
Verduci 2007 {published data only}
- Verduci E, Radaelli G, Stival G, Salvioni M, Giovannini M, Scaglioni S. Dietary macronutrient intake during the first 10 years of life in a cohort of Italian children. Journal of Pediatric Gastroenterology and Nutrition 2007;45(1):90‐5. [DOI] [PubMed] [Google Scholar]
Vobecky 1988 {published data only}
- Vobecky JS, David P, Vobecky J. Identification of risk factors of hypercholesterolemia in children ‐ 9‐year follow‐up [Identification des facteurs de risque de l'hypercholesterolemie chez les enfants ‐ suivi apres 9 ans]. Revue d'Epidémiologie et de Santé Publique 1988;36(6):409‐20. [PubMed] [Google Scholar]
Voortman 2016 {published data only}
- Voortman T, Hooven EH, Tielemans MJ, Hofman A, Kiefte‐de Jong JC, Jaddoe VWV, et al. Protein intake in early childhood and cardiometabolic health at school age: the Generation R Study. European Journal of Nutrition 2016;55(6):2117‐27. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 1992 {published data only}
- Walker R, Heller R, Redman S, O'Connell D, Boulton J. Reduction of ischemic heart disease risk markers in the teenage children of heart attack patients. Preventive Medicine 1992;21(5):616‐29. [DOI] [PubMed] [Google Scholar]
Walter 1989 {published data only}
- Walter HJ. Primary prevention of chronic disease among children: the school‐based "Know Your Body" intervention trials. Health Education Quarterly 1989;16(2):201‐14. [DOI] [PubMed] [Google Scholar]
Wang 2000 {published data only}
- Wang Y, Ge K, Popkin BM. Tracking of body mass index from childhood to adolescence: a 6‐y follow‐up study in China. American Journal of Clinical Nutrition 2000;72(4):1018‐24. [DOI] [PubMed] [Google Scholar]
Wang 2003 {published data only}
- Wang Y, Ge K, Popkin BM. Why do some overweight children remain overweight, whereas others do not?. Public Health Nutrition 2003;6(6):549‐58. [DOI] [PubMed] [Google Scholar]
Wang 2014 {published data only}
- Wang J, Light K, Henderson M, O'Loughlin J, Mathieu M, Paradis G, et al. Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. Journal of Nutrition 2014;144(1):81‐6. [DOI] [PubMed] [Google Scholar]
Williams 1998 {published data only}
- Williams CL, Squillace MM, Bollella MC, Brotanek J, Campanaro L, D'Agostino, et al. Healthy Start: a comprehensive health education program for preschool children. Preventive Medicine 1998;27(2):216‐23. [DOI] [PubMed] [Google Scholar]
Williams 2002 {published data only}
- Williams CL, Bollella MC, Strobino BA, Spark A, Nicklas TA, Tolosi LB, et al. "Healthy‐Start": outcome of an intervention to promote a heart healthy diet in preschool children. Journal of the American College of Nutrition 2002;21(1):62‐71. [DOI] [PubMed] [Google Scholar]
Williams 2004 {published data only}
- Williams CL, Strobino BA, Bollella M, Brotanek J. Cardiovascular risk reduction in preschool children: the "Healthy Start" project. Journal of the American College of Nutrition 2004;23(2):117‐23. [DOI] [PubMed] [Google Scholar]
Williams 2008 {published data only}
- Williams Cl, Strobino BA. Childhood diet, overweight, and CVD risk factors: the Healthy Start Project. Preventive Cardiology 2008;11(1):11‐20. [DOI: ] [DOI] [PubMed] [Google Scholar]
Williamson 2010 {published data only}
- Williamson DA, Champagne CM, Harsha D, Han H, Martin CK, Newton R, et al. Efficacy of two obesity prevention programs in rural schools: Primary outcomes for the Louisiana (LA) health study. Obesity Reviews 2010;11:59. [DOI: ] [Google Scholar]
Wright 2010 {published data only}
- Wright CM, Emmett PM, Ness AR, Reilly JJ, Sherriff A. Tracking of obesity and body fatness through mid‐childhood. Archives of Disease in Childhood 2010;95(8):612‐7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Zaqout 2016 {published data only}
- Zaqout M, Michels N, Bammann K, Ahrens W, Sprengeler O, Molnar D, et al. Influence of physical fitness on cardio‐metabolic risk factors in European children. The IDEFICS study. International Journal of Obesity (2005) 2016;40(7):1119‐25. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Khalil 2015 {published data only}
- Khalil H, Murrin C, Viljoen K, O'Brien J, Segurado R, Kelleher C. Developmental trajectories of body mass index (BMI) from birth to late childhood and their relation with paternal and child nutrients intake. Obesity Facts 2014;7:145. [DOI: ] [Google Scholar]
- Khalil H, Murrin C, Viljoen K, Segurado R, Somerville R, O'Brien J, et al. Metabolic syndrome risk in Irish children is associated with maternal diet: prospective findings from the lifeways crossgeneration cohort study 2001‐2014. Atherosclerosis 2015;241(1):e171. [Google Scholar]
Twisk 1998 {published data only}
- Koppes LJ, Boon N, Nooyens ACJ, Mechelen W, Saris WHM. Macronutrient distribution over a period of 23 years in relation to energy intake and body fatness. British Journal of Nutrition 2009;101(1):108‐15. [DOI] [PubMed] [Google Scholar]
- Twisk JW, Kemper HC, Mechelen W, Post GB, Lenthe FJ. Body fatness: longitudinal relationship of body mass index and the sum of skinfolds with other risk factors for coronary heart disease. International Journal of Obesity and Related Metabolic Disorders 1998;22(9):915‐22. [DOI] [PubMed] [Google Scholar]
Additional references
Ajala 2013
- Ajala O, English P, Pinkney J. Systematic review and meta‐analysis of different dietary approaches to the management of type 2 diabetes. American Journal of Clinical Nutrition 2013;97:505‐16. [DOI] [PubMed] [Google Scholar]
Aljadani 2013
- Aljadani H, Patterson A, Sibbritt D, Collins C. The association between diet quality and weight change in adults over time: a systematic review of prospective cohort studies. Diet Quality: An Evidence Based Approach. 2. New York (NY): Springer, 2013:3‐27. [DOI: 10.1007/978-1-4614-7315-2_1] [DOI] [Google Scholar]
Aljadani 2015
- Aljadani H, Patterson A, Sibbritt D, Collins CE. Diet quality and weight change in adults over time: a systematic review of cohort studies. Current Nutrition Reports 2015;4:88‐101. [Google Scholar]
Ambrosini 2014
- Ambrosini GL. Childhood dietary patterns and later obesity: a review of the evidence. Proceedings of the Nutrition Society 2014;73:137‐46. [DOI] [PubMed] [Google Scholar]
Benatar 2013
- Benatar JR, Sidhu K, Stewart RA, Benatar JR, Sidhu K, Stewart RAH. Effects of high and low fat dairy food on cardio‐metabolic risk factors: a meta‐analysis of randomized studies. PloS One 2013;8:e76480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaput 2014
- Chaput JP. Findings from the Quebec Family Study on the Etiology of Obesity: genetics and environmental highlights. Current Obesity Reports 2014;3:54‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane Methods
- Cochrane Methods. Tool to assess risk of bias in cohort studies. methods.cochrane.org/sites/methods.cochrane.org.bias/files/public/uploads/Tool%20to%20Assess%20Risk%20of%20Bias%20in%20Cohort%20Studies.pdf (accessed prior to 3 January 2018).
Cole 2000
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence systematic review software. Melbourne (Australia): Veritas Health Innovation, 2017.
de Onis 2007
- Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school‐aged children and adolescents. Bulletin of the World Health Organization 2007;85:660‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Onis 2010
- Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. American Journal of Clinical Nutrition 2010;92:1257‐64. [DOI] [PubMed] [Google Scholar]
Fenner 2016
- Fenner AA, Howie EK, Davis MC, Straker LM. Relationships between psychosocial outcomes in adolescents who are obese and their parents during a multi‐disciplinary family‐based healthy lifestyle intervention: one‐year follow‐up of a waitlist controlled trial (Curtin University’s Activity, Food and Attitudes Program). Health and Quality of Life Outcomes 2016;14(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
GBD 2017a
- GBD 2016 Risk Factors Collaborators. Worldwide trends in body‐mass index, underweight,overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population‐based measurement studies in128·9 million children, adolescents, and adults. Lancet 2017;S0140‐6736(17):32129‐3. [DOI: 10.1016/S0140-6736(17)32479-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
GBD 2017b
- GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990‐2016: a systematic analysis for the Global Burden of Disease Study. Lancet 2017;390:1345‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibson 2005
- Gibson RS. Measuring food consumption of individuals. In: Gibson RS editor(s). Principles of Nutritional Assessment. 2nd Edition. New York (NY): Oxford University Press, 2005:27‐64. [Google Scholar]
Golding 2001
- Golding J, Pembrey M, Jones R, ALSPAC Study Team. ALSPAC‐The Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatric and Perinatal Epidemiology 2001;15:74‐87. [DOI] [PubMed] [Google Scholar]
Gow 2014
- Gow ML, Ho M, Burrows TL, Baur LA, Stewart L, Hutchesson MJ, et al. Impact of dietary macronutrient distribution on BMI and cardiometabolic outcomes in overweight and obese children and adolescents: a systematic review. Nutrition Reviews 2014;72:453‐70. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group. GRADEpro GDT. GRADE Working Group and Evidence Prime, 2017.
Guyatt 2011
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64:407‐15. [DOI] [PubMed] [Google Scholar]
Havranek 2011
- Havranek EP. A Mediterranean diet reduces cardiovascular risk factors in overweight patients compared with a low‐fat diet. ACP Journal Club 2011;155(12):JC6‐3. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffman 2017
- Hoffmann TC, Oxman AD, Ioannidis JP, Moher D, Lasserson TJ, Tovey DI, et al. Enhancing the usability of systematic reviews by improving the consideration and description of interventions. BMJ 2017;358:j2998. [DOI] [PubMed] [Google Scholar]
Hooper 2015b
- Hooper L, Martin N, Abdelhamid A, Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011737] [DOI] [PubMed] [Google Scholar]
Hu 2012
- Hu T, Mills KT, Yao L, Demanelis K, Eloustaz M, Yancy WS Jr, et al. Effects of low‐carbohydrate diets versus low‐fat diets on metabolic risk factors: a meta‐analysis of randomized controlled clinical trials. American Journal of Epidemiology 2012;176 Suppl 7:S44‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakes 2004
- Jakes RW, Day NE, Luben R, Welch A, Bingham S, Mitchell J, et al. Adjusting for energy intake ‐ what measure to use in nutritional epidemiological studies?. International Journal of Epidemiology 2004;33:1382‐6. [DOI] [PubMed] [Google Scholar]
Jayarajan 1980
- Jayarajan P, Reddy V, Mohanran M. Effect of dietary fat on absorption of beta‐carotene from green leafy vegetables in children. Indian Journal of Medical Research 1980;71:53‐6. [PubMed] [Google Scholar]
Johnson 2008
- Johnson L, Mander AP, Jones LR, Emmett PM, Jebb SA. Energy‐dense, low‐fiber, high‐fat dietary pattern is associated with increased fatness in childhood. American Journal of Clinical Nutrition 2008;87(4):846‐54. [DOI] [PubMed] [Google Scholar]
Kratz 2013
- Kratz MB. The relationship between high‐fat dairy consumption and obesity, cardiovascular, and metabolic disease. European Journal of Nutrition 2013;52:1‐24. [DOI] [PubMed] [Google Scholar]
LeBlanc 2012
- LeBlanc AG, Spence JC, Carson V, Gorber SC, Dillman C, Janssen I, et al. Systematic review of sedentary behaviour and health indicators in the early years (aged 0‐4 years). Applied Physiology, Nutrition, and Metabolism 2012;37:753‐72. [DOI] [PubMed] [Google Scholar]
Lobstein 2004
- Lobstein T, Baur L, Uauy R, IASO International Obesity Task Force. Obesity in children and young people: a crisis in public health. Obesity Reviews 2004;5(Suppl 1):4‐104. [DOI] [PubMed] [Google Scholar]
McGloin 2002
- McGloin AF, Livingstone MBE, Greene LC, Webb SE, Gibson JMA, Jebb SA, et al. Energy and fat intake in obese and lean children at varying risk of obesity. International Journal of Obesity (2005) 2002;26:200‐7. [DOI] [PubMed] [Google Scholar]
McNeill 2017
- McNeill A, Gravely S, Hitchman SC, Bauld L, Hammond D, Hartmann‐Boyce J. Tobacco packaging design for reducing tobacco use. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD011244.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2014
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ni 2010
- Ni MC, Aston LM, Jebb SA. Effects of worksite health promotion interventions on employee diets: a systematic review. BMC Public Health 2010;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parsons 1999
- Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. International Journal of Obesity 1999;23(Suppl 8):S1‐S107. [PubMed] [Google Scholar]
Pollock 2015
- Pollock NK. Childhood obesity, bone development, and cardiometabolic risk factors. Molecular and Cellular Endocrinology 2015;410:52‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pérez‐Escamilla 2012
- Pérez‐Escamilla R, Obbagy JE, Altman JM, Essery EV, McGrane MM, Wong YP, et al. Dietary energy density and body weight in adults and children: a systematic review. Journal of the Academy of Nutrition and Dietetics 2012;112(5):671‐84. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhee 2014
- Rhee JJ, Cho E, Willett WC. Energy‐adjustment of nutrient intakes is preferable to adjustment using body weight and physical activity in epidemiologic analyses. Public Health Nutrition 2014;17(5):1054‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ribaya‐Mercado 2007
- Ribaya‐Mercado JD, Maramag CC, Tengco LW, Dolnikowski GG, Blumberg JB, Solon FS. Carotene‐rich plant foods ingested with minimal dietary fat enhance the total‐body vitamin A pool size in Filipino schoolchildren as assessed by stable‐isotope‐dilution methodology. American Journal of Clinical Nutrition 2007;85(4):1041‐9. [DOI] [PubMed] [Google Scholar]
Rouhani 2016
- Rouhani MH, Haghighatdoost F, Surkan PJ, Azadbakht L. Associations between dietary energy density and obesity: a systematic review and meta‐analysis of observational studies. Nutrition 2016;32(10):1037‐47. [DOI] [PubMed] [Google Scholar]
Schwingshackl 2013a
- Schwingshackl L, Hoffmann G. Comparison of effects of long‐term low‐fat vs high‐fat diets on blood lipid levels in overweight or obese patients: a systematic review and meta‐analysis. Journal of the Academy of Nutrition & Dietetics 2013;113:1640‐61. [DOI] [PubMed] [Google Scholar]
Schwingshackl 2013b
- Schwingshackl L, Hoffmann G. Long‐term effects of low‐fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta‐analysis. Nutrition Journal 2013;12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Gunn E, Vist GE, Paul Glasziou P, et al. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shim 2014
- Shim J, Oh K, Chang Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiology and Health 2014;36:e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
UNICEF 2017
- UNICEF, WHO, World Bank. Levels and Trends in Child Malnutrition: UNICEF‐WHO‐World Bank Joint Child Malnutrition Estimates. UNICEF, New York: UNICEF, 2017. [Google Scholar]
Van't Hoff 2015
- Van't Hoff W, Offringa M, Star Child Health group. StaR Child Health: developing evidence‐based guidance for the design, conduct and reporting of paediatric trials. Archives of Disease in Childhood 2015;100(2):189‐92. [DOI] [PubMed] [Google Scholar]
WCRF/AICR 2009
- World Cancer Research Fund/American Institute for Cancer Research. Preventability of Cancer by Food, Nutrition, and Physical Activity: Appendix A. Policy and Action for Cancer Prevention. Food, Nutrition, and Physical Activity: a Global Perspective. Washington (DC): AICR, 2009. [Google Scholar]
WHO 2004
- World Health Organization. Global Strategy on Diet, Physical Activity and Health. World Health Assembly Resolution 57.17. Geneva (Switzerland): World Health Organization, 2004. [Google Scholar]
WHO 2016
- World Health Organization. Report of the commission on ending childhood obesity 2016. apps.who.int/iris/bitstream/10665/204176/1/9789241510066_eng.pdf?ua=1&ua=1 (accessed 6 November 2017).
Wolfram 2015
- Wolfram G, Bechthold A, Boeing H, Ellinger S, Hauner H, Kroke A, et al. Evidence‐based guideline of the German Nutrition Society: fat intake and prevention of selected nutrition‐related diseases. Annals of Nutrition and Metabolism 2015;67:141‐204. [DOI] [PubMed] [Google Scholar]
Yang 2012
- Yang WY, Williams LT, Collins C, Siew Swee CW. The relationship between dietary patterns and overweight and obesity in children of Asian developing countries: a systematic review. JBI Library of Systematic Reviews 2012;10(58):4568‐99. [DOI] [PubMed] [Google Scholar]
Yang 2013
- Yang Z, Huffman SL. Nutrition in pregnancy and early childhood and associations with obesity in developing countries. Maternal & Child Nutrition 2013;9(Suppl 1):105‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu‐Poth 1999
- Yu‐Poth S, Zhao G, Etherton T, Naglak M, Jonnalagadda S, Kris‐Etherton PM. Effects of the National Cholesterol Education Program's Step I and Step II dietary intervention programs on cardiovascular disease risk factors: a meta‐analysis. American Journal of Clinical Nutrition 1999;69:632‐46. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hooper 2000
- Hooper L, Summerbell CD, Higgins JPT, Thompson RL, Clements G, Capps N, et al. Reduced or modified dietary fat for prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002137] [DOI] [PubMed] [Google Scholar]
Hooper 2001
- Hooper L, Summerbell CD, Higgins JPT, Thompson RL, Capps N, Davey Smith G, et al. Dietary fat intake and prevention of cardiovascular disease: systematic review. BMJ 2001;322:757‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hooper 2012
- Hooper L, Abdelhamid A, Moore HJ, Douthwaite W, Skeaff CM, Summerbell CD. Effect of reducing total fat intake on body weight: systematic review and meta‐analysis of randomised controlled trials and cohort studies. BMJ 2012;345:e7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hooper 2015a
- Hooper L, Abdelhamid A, Bunn D, Brown T, Summerbell CD, Skeaff CM. Effects of total fat intake on body weight. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011834] [DOI] [PMC free article] [PubMed] [Google Scholar]


