Abstract
Background
Prevention of relapse is a major issue in the management of quiescent Crohn's disease (CD). Current therapies (e.g. methotrexate, biologics, 6‐mercaptopurine and azathioprine) may be effective for maintaining remission in CD, but these drugs may cause significant adverse events. Interventions that are effective and safe for maintenance of remission in CD are desirable.
Objectives
The primary objectives were to evaluate the efficacy and safety of enteral nutrition for the maintenance of remission in CD and to assess the impact of formula composition on effectiveness.
Search methods
We searched MEDLINE, Embase, CENTRAL, the Cochrane IBD Group Specialized Register and clinicaltrials.gov from inception to 27 July 2018. We also searched references of retrieved studies and reviews.
Selection criteria
Randomised controlled trials (RCTs) including participants of any age with quiescent CD were considered for inclusion. Studies that compared enteral nutrition with no intervention, placebo or any other intervention were selected for review.
Data collection and analysis
Two authors independently screened studies for inclusion, extracted data and assessed methodological quality using the Cochrane risk of bias tool. The primary outcome was clinical or endoscopic relapse as defined by the primary studies. Secondary outcomes included anthropometric measures (i.e. height and weight), quality of life (QoL), adverse events, serious adverse events and withdrawal due to adverse events. We calculated the risk ratio and 95% confidence interval (CI) for dichotomous outcomes. For continuous outcomes, we calculated the mean difference and 95% CI. A random‐effects model was used for the statistical analysis. We used the GRADE criteria to assess the overall certainty of the evidence supporting the primary outcome and selected secondary outcomes.
Main results
Four RCTs (262 adult participants) met the inclusion criteria. One study (N = 33) compared an elemental diet to a non‐elemental (polymeric) diet. One study (N = 51) compared a half elemental diet to a regular free diet. Another study (N = 95) compared an elemental diet to 6‐mercaptopurine (6‐MP) or a no treatment control group. One study (N= 83) compared a polymeric diet to mesalamine. Two studies were rated as high risk of bias due to lack of blinding or incomplete outcome data. The other two studies were judged to have an unclear risk of bias. The studies were not pooled due to differences in control interventions and the way outcomes were assessed.
The effect of an elemental diet compared to a polymeric diet on remission rates or withdrawal due to adverse events is uncertain. Fifty‐eight per cent (11/19) of participants in the elemental diet group relapsed at 12 months compared to 57% (8/14) of participants in the polymeric diet group (RR 1.01, 95% CI 0.56 to 1.84; very low certainty evidence). Thirty‐two per cent (6/19) of participants in the elemental diet group were intolerant to the enteral nutritional formula because of taste or smell and were withdrawn from the study in the first 2 weeks compared to zero participants (0/14) in the polymeric diet group (RR 9.75, 95% CI 0.59 to 159.93; low certainty evidence). Anthropometric measures, QoL, adverse events and serious adverse events were not reported as outcomes.
The effect of an elemental diet (half of total daily calorie requirements) compared to a normal free diet on relapse rates is uncertain. Thirty‐five per cent (9/26) of participants in the elemental diet group relapsed at 12 months compared to 64% (16/25) of participants in the free diet group (RR 0.54, 95% CI 0.30 to 0.99; very low certainty evidence). No adverse events were reported. This study reported no differences in weight change between the two diet groups. Height and QoL were not reported as outcomes.
The effect of an elemental diet compared to 6‐MP on relapse rates or adverse events is uncertain. Thirty‐eight per cent (12/32) of participants in the elemental diet group relapsed at 12 months compared to 23% (7/30) of participants in the 6‐MP group (RR 1.61; 95% CI 0.73 to 3.53; very low certainty evidence). Three per cent (1/32) of participants in the elemental diet group had an adverse event compared to 13% (4/30) of participants in the 6‐MP group (RR 0.23, 95% CI 0.03 to 1.98; low certainty evidence). Adverse events in the elemental diet group included surgery due to worsening CD. Adverse events in the 6‐MP group included liver injury (n = 2), hair loss (n = 1) and surgery due to an abscess (n = 1). No serious adverse events or withdrawals due to adverse events were reported. Weight, height and QoL were not reported as outcomes
The effect of a polymeric diet compared to mesalamine on relapse rates and weight is uncertain. Forty‐two per cent (18/43) of participants in the polymeric diet group relapsed at 6 months compared to 55% (22/40) of participants in the mesalamine group (RR 0.76; 95% CI 0.49 to 1.19; low certainty evidence). The mean difference in weight gain over the study period was 1.9 kg higher in the polymeric diet group compared to mesalamine (95% CI ‐4.62 to 8.42; low certainty evidence). Two participants in the polymeric diet group experienced nausea and four had diarrhoea. It is unclear if any participants in the mesalamine group had an adverse event. Height, QoL, serious adverse events and withdrawal due to adverse events were not reported as outcomes.
Authors' conclusions
The results for the outcomes assessed in this review are uncertain and no firm conclusions regarding the efficacy and safety of enteral nutrition in quiescent CD can be drawn. More research is needed to determine the efficacy and safety of using enteral nutrition as maintenance therapy in CD. Currently, there are four ongoing studies (estimated enrolment of 280 participants). This review will be updated when the results of these studies are available.
Plain language summary
Enteral nutrition (liquid feeds) for maintenance of remission in Crohn's disease
What is Crohn's disease?
Crohn's disease is a chronic inflammatory disease of the intestines that frequently occurs in the lower part of the small intestine (ileum). Symptoms include abdominal pain, diarrhoea and weight loss. When people with Crohn's disease are experiencing symptoms the disease is considered 'active'. When the symptoms stop, it is called 'remission'. When people in remission experience symptoms it is called a 'relapse'.
What is enteral nutrition?
Enteral nutrition is a feeding method where a person's daily caloric intake is delivered via a liquid diet using the GI tract. Enteral nutrition can be administered by mouth or by tube feeding, where a tube is inserted through the nose or abdomen into the stomach to deliver the liquid feed. Enteral nutrition is a form of nutritional therapy for Crohn's disease patients. The mechanism by which enteral nutrition may influence inflammation is unknown and is being studied. Enteral nutrition can be classified as elemental and non‐elemental (semi‐elemental and polymeric) diets. Elemental diets are composed of amino‐acids (organic compounds), fats, sugars, vitamins and minerals. Elemental diets are easily absorbed and digested. Non‐elemental diets are based on oligopeptide (organic compounds composed of 2 to 20 amino‐acids) or whole protein sources. Non‐elemental diets are best for people who can digest and absorb nutrients without difficulty.
What is 6‐mercaptopurine?
6‐Mercaptopurine is an immunosuppressive drug that is thought to reduce inflammation in people with Crohn's disease by blocking the immune system.
What is mesalamine?
Mesalamine is a 5‐aminosalicylic acid drug. 5‐Aminosalicylic drugs are thought to treat Crohn's disease by reducing inflammation in the gastrointestinal tract. These drugs are usually taken by mouth.
What did the researchers investigate?
The researchers studied whether enteral nutrition helps to maintain remission in people with Crohn's disease. The researchers also investigated whether one type of enteral nutrition was better than another (e.g. elemental vs.non‐elemental) for maintaining remission in people with Crohn's disease.
What did the researchers find?
Four studies including 262 adult participants with Crohn's disease in remission were included. One study (33 participants) compared an elemental diet to a non‐elemental (polymeric) diet. One study (51 participants) compared an elemental diet to a normal diet (no supplements). One study (95 participants) compared an elemental diet to 6‐mercaptopurine or a no treatment control group. One study (83 participants) compared a non‐elemental polymeric diet to mesalamine. The researchers searched the medical literature extensively up to 27 July 2018.
The study comparing an elemental diet to a polymeric diet found no difference in remission rates at 12 months. Six elemental diet participants were not able to tolerate the enteral nutritional formula because of taste or smell and were withdrawn from the study. Participants who received half of their total daily calorie requirements as elemental diet and the remaining half by normal diet had a lower chance of relapse at 12 months compared to participants who received a free diet. No side effects were reported in this study. The study comparing an elemental diet to 6‐mercaptopurine did not show any difference in relapse rates at 12 months. There was no difference in side effect rates. The only side effect reported in the elemental diet group was surgery due to worsening Crohn's disease. Side effects in the 6‐mercaptopurine group included liver injury in two participants, hair loss in one participant and surgery to treat an abscess in one participant. The study comparing a polymeric diet to mesalamine found no difference in relapse rates at six months. Two participants the polymeric diet group experienced nausea and four had diarrhoea. It is unclear if any participants in the mesalamine group had side effects. No serious side effects were reported in any of the studies.
The results for the outcomes assessed in this review are uncertain and no firm conclusions regarding the effectiveness and safety of enteral nutrition for maintenance of remission in Crohn's disease can be drawn. More research is needed to determine the effectiveness and safety of using enteral nutrition as maintenance therapy in Crohn's disease. Currently, there are four ongoing studies (estimated enrolment of 280 participants). This review will be updated when the results of these studies are available.
Summary of findings
Background
Crohn's disease is a chronic relapsing condition with a high morbidity. There is no cure for Crohn's disease. Treatment is aimed at inducing and maintaining remission of disease activity, correcting malnutrition, addressing complications, and thereby improving the quality of life of patients. In children, a major additional goal is to facilitate normal growth and pubertal development which are frequently impeded. Whilst enteral nutrition may be effective for induction of remission in active Crohn's disease (Akobeng 2000; Gonzalez‐Huix 1993; Heuschkel 2000; Narula 2018; Thomas 1993), rapid return of gastrointestinal symptoms after resumption of normal diet is common (Jones 1985; Wilschanski 1996), and many patients end up receiving corticosteroids (Murphy 2001).
Prevention of relapses is a major issue in the management of Crohn's disease especially as being in remission is associated with improved quality of life (Lichtenstein 2004). Whilst a number of treatment regimens are effective for induction of clinical remission in active Crohn's disease, no treatment is available that can completely prevent relapse of the disorder (Biancone 2003). Corticosteroids (Steinhart 2003), and 5‐ASA preparations (Akobeng 2016), are not effective for the maintenance of remission. Methotrexate (Feagan 2000; Patel 2014), infliximab (Hanauer 2002), adalimumab (Colombel 2007), vedolizumab (Sandborn 2013), and the antimetabolite, 6‐mercaptopurine and its prodrug, azathioprine are effective for maintenance of remission (Chande 2015; Feagan 2003), but these drugs may cause significant adverse events.
In the last three decades, studies have been completed that suggest that long‐term enteral nutritional supplementation, in addition to unrestricted normal food may prolong the period of remission and reduce relapse rates in patients with Crohn's disease (Koga 1993; Verma 2000). Enteral nutrition can be classified as elemental and non‐elemental (semi‐elemental and polymeric) diets. Elemental diets are composed of amino‐acids (organic compounds), fats, sugars, vitamins and minerals. Elemental diets are easily absorbed and digested. Non‐elemental diets are based on oligopeptide (organic compounds composed of 2 to 20 amino‐acids) or whole protein sources. Non‐elemental diets are best for people who can digest and absorb nutrients without difficulty. The exact mechanism by which enteral nutrition may modulate the inflammatory process in Crohn's disease is unknown. Several mechanisms have been proposed (Lewis 1994). These include provision of essential nutrients, reduction of antigenic load, alteration of bowel flora, and improved immune function. Differences in the composition of long‐chain triglycerides and polyunsaturated fatty acids have been proposed as potential contributing factors influencing the efficacy of enteral nutrition (Goh 2003). Enteral nutrition may also inhibit intestinal immune responses by reducing the number of cytokine‐producing cells (Yamamoto 2007).
In a retrospective study, Wilschanski 1996 showed that providing supplementary enteral nutrition (nocturnal nasogastric supplements), without restriction of normal diet, after successful treatment of active Crohn's disease by exclusive enteral nutrition, was associated with prolongation of remission and improved linear growth in children and adolescents with Crohn's disease. Verma 2000 have also examined the role of supplementing normal food with elemental diet in the long term management of adults with Crohn's disease. Verma 2000 enrolled 39 consecutive patients in clinical remission, 21 patients (Group 1) received oral nutritional supplementation taken in addition to a normal diet whilst 18 patients (Group 2) were maintained on a normal unrestricted diet. The patients were followed up for 12 months. A total of 17 patients (81%) tolerated the nutritional supplementation. On an intention to treat basis, 10 patients in Group 1 (10/21, 48%) remained in remission for 12 months, compared to 4/18 (22%) patients in Group 2 (P < 0.0003). In another study, Verma 2001 also demonstrated that long term nutritional supplementation allowed the withdrawal of chronic steroid therapy in patients with steroid‐dependent Crohn's disease. In a controlled cross‐over study, Harries 1983 reported a beneficial effect of oral dietary supplementation (Ensure Plus) in improving nutritional status and decreasing disease activity in adult patients with Crohn's disease. Similar findings have been reported by Koga 1993.
The notion that enteral nutrition may be effective for maintenance of remission is an attractive proposition. If this is the case, then the use of steroids and immunosuppressive agents may be minimized in patients with Crohn's disease, thereby reducing potentially serious adverse events associated with these medications. Long term nutritional support and avoidance of steroids may also lead to improvements in growth in children and adolescents with Crohn's disease. This systematic review was completed over ten years ago and found just two studies (Akobeng 2007), the results suggested a potential role for enteral nutrition for the management of patients with quiescent Crohn's disease. The aim of this updated systematic review was to summarise the available evidence concerning the use of enteral nutrition for the maintenance of remission in Crohn's disease.
Objectives
1. To evaluate the efficacy and safety of long term enteral nutritional supplementation for the maintenance of remission in Crohn's disease. 2. To examine the impact of formula composition on efficacy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials were considered for inclusion.
Types of participants
Patients of any age with Crohn's disease in remission at the time of study entry were considered for inclusion. Remission should have been defined by a recognised Crohn's disease activity index.
Types of interventions
Intervention Studies that assessed enteral nutrition (polymeric, elemental or semi‐elemental) administered by any route (e.g. oral, nasogastric or gastrostomy) were considered for inclusion.
Control Control interventions included no intervention, placebo or other active interventions for maintenance of remission in quiescent Crohn's disease (e.g. a different enteral nutrition formulation or azathioprine or 6‐mercaptopurine).
Types of outcome measures
Primary outcomes
The primary outcome measure was the occurrence of clinical or endoscopic relapse as defined by the primary studies and expressed as a percentage of the number of patients randomised.
Secondary outcomes
Secondary endpoints included: (1) improvements in anthropometric measurements (including weight and height); (2) improvements in quality of life; (3) adverse events; (4) serious adverse events; and (5) withdrawal due to adverse events.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases from inception to 5 July 2017 for relevant studies: 1. MEDLINE; 2. Embase; 3. CENTRAL; 4. The Cochrane IBD Group Specialized Trials Register; and| 5. Clinicaltrials.gov.
The search strategies were not limited by language. The search strategies are reported in Appendix 1.
Searching other resources
The references of all identified studies and relevant systematic reviews were inspected for more trials. There is some evidence that data from abstracts can be inconsistent with data in published articles (Pitkin 1999). Abstract publications were not included in this review.
Data collection and analysis
Selection of studies
Using the above search strategy, papers that appeared to be potentially relevant were independently identified by two authors (DZ and JKM). The authors, after reading the full texts, independently assessed the eligibility of all trials identified based on the inclusion criteria above. Disagreement among authors was discussed and agreement reached by consensus.
Data extraction and management
A data extraction form was developed and used to extract information on relevant features and results of included studies. Two authors (DZ and JKM) independently extracted and recorded data on the predefined checklist. Extracted data included the following items:
a. characteristics of patients: age, sex, disease distribution, disease duration, disease activity index; b. total number of patients originally assigned to each intervention group; c. intervention: type and amount of enteral nutrition; mode of administration; d. control: no intervention, placebo or other interventions; e. concurrent medications; and f. outcomes: time of assessment, length of follow up, type of Crohn's disease activity index used, definitions of remission and relapse, relapse rates, time to relapse, quality of life assessment, adverse events.
Assessment of risk of bias in included studies
Two authors (DZ and JKM) independently assessed study quality using the Cochrane risk of bias tool (Higgins 2011). The following domains were assessed: methods used for randomisation and allocation concealment (selection bias); blinding (performance and detection bias); incomplete outcome data (attrition bias); selective reporting of study outcomes; and other potential sources of bias. Each of the domains was rated as high, low or unclear risk of bias. An item was assessed as unclear risk of bias if insufficient information was available to determine the risk of bias. Any disagreements in the risk of bias assessment were resolved by discussion and consensus.
The overall certainty of the evidence was assessed using the GRADE approach, which allows for overall appraisal of the quality of evidence so that one can determine confidence in how likely the effect estimate reflects the true effect (Guyatt 2008; Schunemann 2011). Randomized trials start as high quality of evidence, but can be downgraded due to risk of bias, indirect evidence, unexplained heterogeneity, imprecision, and publication bias. After consideration of these factors, the overall certainty of the evidence was graded as:
‐ High ‐ further research is unlikely to change confidence in the estimate of effect;
‐ Moderate ‐ further research is likely to have an important impact on confidence in the estimate of effect and may change the effect estimate;
‐ Low ‐ further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the effect estimate; or
‐ Very low ‐ any estimate of effect is very uncertain.
Measures of treatment effect
The Cochrane Collaboration review manager software (RevMan) was used for the data analyses. Data were analysed according to the intention‐to‐treat principle, using as the denominator, the total number of patients randomised. For dichotomous outcomes we calculated the risk ratio (RR) and corresponding 95% confidence interval (95% CI). For continuous outcomes, we calculated the mean difference (MD) and corresponding 95% CI. We used a random‐effects model for the statistical analysis. We explored heterogeneity using the Chi2 and I2 statistics.
Unit of analysis issues
For studies that reported outcomes at fixed intervals, we planned to pool data at those time points as well. For a parallel group design a single measurement for each outcome per participant was collected. For crossover trials, data from the first part of the trial (prior to crossover) were extracted. For cluster‐randomised trials, we planned to extract data at the level of the individual. When more than one efficacy or safety event was reported per subject, we used the proportion of subjects with at least one event as the outcome. Separate analyses were planned for different active comparators.
Dealing with missing data
For missing dichotomous outcomes, we analysed data according to the intention‐to‐treat principle. Participants with missing data were assumed to be treatment failures. For continuous outcomes we used the number of participants who completed the trial (i.e. available case analysis). We did not impute any data for missing continuous outcomes.
Assessment of heterogeneity
For future updates of this systematic review, we plan to explore heterogeneity using the Chi2 and I2 statistics. We plan to conduct sensitivity analyses as appropriate to explore potential explanations for any heterogeneity that is identified.
Assessment of reporting biases
Potential reporting bias was evaluated by comparing outcomes listed in protocols to published manuscripts. When protocols were not available, we compared outcomes listed in the methods section of published manuscripts to those described in the results section. If a sufficient number of studies are included (i.e. > 10) in the pooled analyses, we planned to investigate potential publication bias using funnel plots (Egger 1997).
Data synthesis
We planned to pool data from individual trials when the interventions, patient groups and outcomes were sufficiently similar (as determined by consensus). We did not pool any studies as none of the included studies had similar interventions or comparators. For future updates of this systematic review, we plan to calculate the pooled RR and 95% CI for dichotomous outcomes and the pooled MD and corresponding 95% CI for continuous outcomes. We plan to calculate the standardized mean difference (SMD) and 95% CI when difference scales are used to measure the same outcome (e.g. different quality of life instruments). We plan to use a random‐effects model for meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We did not pre‐specify any subgroup analyses.
Sensitivity analysis
We did not pre‐specify any sensitivity analyses.
Results
Description of studies
The literature search conducted on 27 July 2018 identified 1365 records. Thirteen additional records were identified through other sources. After duplicates were removed, a total of 933 remained for review of titles and abstracts. Based on a review of titles and abstracts, 35 studies were initially identified as being potentially eligible for inclusion and were selected for full‐text review (See Figure 1). After reviewing the full manuscripts, 27 studies were excluded for not meeting the inclusion criteria. Seven studies were excluded because these studies were not maintenance of remission studies (Canani 2006; Gorard 1993; Harries 1983; Johnson 2006; Knight 2005; Phylactos 2001; Yamamoto 2005). Fourteen studies were not randomised controlled trials (Esaki 2005; Giaffer 1991; Hirakawa 1993; Jones 1987; Koga 1993; Matsueda 1995; Navarro 1982; Nomura 1995; Triantafillidis 2006; Verma 2000; Wilschanski 1996; Yamamoto 2007; Yamamoto 2010; Yamamoto 2015). Four review articles were excluded (Dray 2005; Forbes 2002; Griffiths 2005; Valentini 2002). One further article was excluded because separate results were not reported for participants with Crohn's disease and ulcerative colitis (Iakovlev 2014). One randomised study was published in abstract form only and the full study was never published (Roggero 2003). Four studies (262 participants) were identified that met the inclusion criteria and these studies were included in the review (Hanai 2012; Takagi 2006; Triantafillidis 2010; Verma 2001). Four ongoing studies were also identified (NCT01823042; NCT02201693; NCT02231814; NCT02843100).
1.
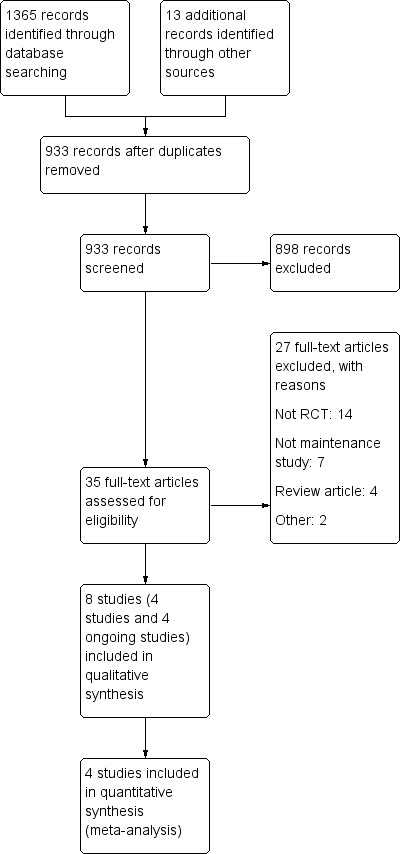
Figure 1
Takagi 2006 This study examined the effectiveness of an elemental diet (ED) as maintenance therapy for Crohn's disease. Fifty one adults with Crohn's disease in remission (defined as a Crohn's Disease Activity Index, CDAI < 150) were recruited from two centres in Japan. Participants were randomised into two groups. The half elemental diet group (n = 26) received half the amount of their daily allowance of calories as Elental (Ajinomoto Pharma Co., Tokyo, Japan) and the remaining half by normal diet. The free diet group (n = 25) received all their daily calories by normal diet. All participants were instructed to take oral mesalazine (2250 to 3000 mg/day) in accordance with local guidelines. Patients already receiving azathioprine were allowed to continue taking the medication throughout the study period. The primary outcome measure was the clinical relapse rate over a two year period. Relapse was defined as either a CDAI score of > 200, or the need for therapy to induce remission. In our judgement, there did not appear to be any clinically relevant differences in baseline characteristics between the two groups at study entry. Two patients in the half elemental diet group and four patients in the free diet group were on azathioprine at entry. Six patients withdrew from the half elemental diet group because they discontinued the supplements, and five withdrew from the free diet group because they started to take some supplements during the study period.
Verma 2001 This study investigated the ability of enteral nutrition to maintain remission and to reduce steroid dependency in Crohn's disease. The study which compared an elemental formula (EO28, Scientific Hospital Supplies Ltd, Liverpool, UK) with a polymeric formula (Fortisip, Nutricia, UK) was conducted in the UK. The researchers evaluated 33 adults with Crohn's disease who were steroid dependent and had inactive disease with no bowel symptoms, a CDAI score of < 150 in the previous 2 weeks and an ESR of < 20 mm/hour. In addition to their normal unrestricted diet, patients were randomised to receive either E028 (n = 19) or Fortisip (n = 14) orally, to provide 35 to 50% of pre‐trial total calorie intake. Concurrent use of azathioprine, 6‐mercaptopurine and 5‐aminosalicylate preparations was permitted. Participants had disease in remission for a mean of 22.1 +/‐ 6.7 months (range 1 to 204) prior to study entry. Prednisolone was withdrawn gradually at a rate of 1 mg every 2 weeks until stopped but azathioprine, 6‐mercaptopurine and 5‐aminosalicylate were continued at the same doses throughout the study. Patients were followed up at 1 month, 2 months and then 3 monthly until 12 months or until a relapse occurred. The main outcome measure was 'treatment success' within 12 months. This was defined as remaining in remission for 12 months (CDAI < 200 and increasing by < 100 from baseline) after complete withdrawal of steroids. In our judgement, the baseline characteristics of the two groups were generally similar but the duration of steroid usage before study entry appeared to be longer in the Fortisip group (61.0 versus 31.6 months in the EO28 group), and the duration of remission before study entry also appeared to be longer in the Fortisip group (28.7 versus 11.6 months in the EO28 group).
Hanai 2012 This study aimed to investigate the use of elemental diet versus 6‐mercaptopurine as maintenance therapy in Crohn's disease. Ninety‐five eligible patients with a Crohn's disease activity index of ≤ 150 were randomly assigned to: 6‐mercaptopurine (0.5 to 1.5 mg/kg/day, n = 30), Elental as an elemental diet (≥900 kcal/day, n = 32), or a no treatment control group (n = 33). Patients who were taking 5‐aminosalicylic acid at entry were allowed to continue taking the drug at doses of 2250 to 3000 mg per day. Patients were followed for two years and the rate of relapse (Crohn's disease activity index ≥200) was monitored.
Triantafillidis 2010 This study investigated the administration of a special diet (Modulen IBD) rich in transforming growth factor‐β2 compared with administration of mesalamine, for maintaining remission in patients with Crohn’s disease. Patients were randomly assigned to receive either two meals (2 x 50 g) of Modulen IBD plus two regular meals per day (n = 43) or mesalamine at a dose of 800 mg three times a day (n = 40) for six months. No other medications were allowed during the trial period. Patients were assessed at sixth months after initiation of the trial. The primary outcome was relapse was defined as a CDAI of greater than 150 points or an increase of at least 60 points over baseline. Various anthropometric parameters and serum estimations including ESR, CRP, platelets, albumin, vitamin B12 and folic acid were measured at the beginning of the trial and after six months. Of the 83 patients initially included in the study, 76 (36 in the Modulen group and 30 in the mesalamine group) completed the trial.
Risk of bias in included studies
The results of the risk of bias analysis are summarized in Figure 2.
2.
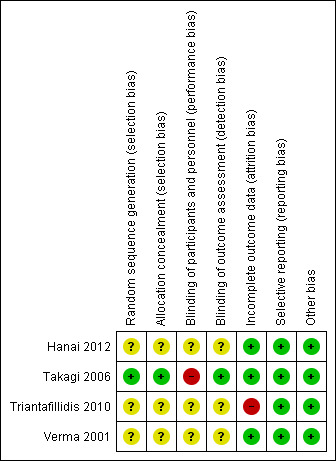
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
One study had a low risk of bias for random sequence generation and allocation concealment (Takagi 2006). The remaining three studies were rated to have an unclear risk of bias due to insufficient information to permit judgement for both of these items (Hanai 2012; Triantafillidis 2010; Verma 2001).
Blinding
One study had a high risk of bias for blinding of participants but low risk of bias for blinding of outcome assessment (Takagi 2006). The remaining three studies were rated as unclear risk of bias due to insufficient information to permit judgment for both of these items (Hanai 2012;Triantafillidis 2010;Verma 2001).
Incomplete outcome data
Three studies were rated as low risk of bias for incomplete outcome data (Hanai 2012;Takagi 2006; Verma 2001), while one study was rated as high risk of bias for incomplete outcome data reporting (Triantafillidis 2010).
Selective reporting
All studies were rated to have low risk of bias in terms of selective reporting bias.
Other potential sources of bias
All studies appeared to be free from other sources of bias and were rated to be low risk of bias for this item.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Elemental formula supplements compared to polymeric formula supplements for maintenance of remission in Crohn's disease.
| Elemental diet compared to non‐elemental (polymeric) diet for maintenance of remission in Crohn's disease | ||||||
| Patient or population: Participants with Crohn's disease in remission Setting: Outpatient Intervention: Elemental formula supplements Comparison: Polymeric formula supplements | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk polymeric formula supplements | Risk with Elemental formula supplements | |||||
|
Relapse Follow‐up: 12 months |
571 per 1,000 | 577 per 1,000 (320 to 1,051) | RR 1.01 (0.56 to 1.84) | 33 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| Change in weight | Not reported | This outcome was not reported | ||||
| Change in height | Not reported | This outcome was not reported | ||||
| Change in quality of life | Not reported | This outcome was not reported | ||||
| Adverse events | Not reported | This outcome was not reported | ||||
| Serious adverse events | Not reported | This outcome was not reported | ||||
|
Withdrawals due to adverse events Follow‐up: 12 months |
0 per 1,000 | 0 per 1,000 (0 to 0) | RR 9.75 (0.59 to 159.93) | 33 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | Six participants withdrew from the elemental diet group for intolerance to the elemental formula due to taste or smell |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to unclear risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel and blinding of outcome assessment
2 Downgraded two levels due to very serious imprecision (19 events)
3 Downgraded two levels due to very serious imprecision (6 events)
Summary of findings 2. Elemental formula supplements compared to no supplementation for maintenance of remission in Crohn's disease.
| Elemental formula supplements compared to no supplementation for maintenance of remission in Crohn's disease | ||||||
| Patient or population: Participants with Crohn's disease in remission Setting: Outpatient Intervention: Elemental formula supplements Comparison: No supplementation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no supplementation | Risk with elemental formula supplements | |||||
|
Relapse Follow‐up: 12 months |
640 per 1,000 | 346 per 1,000 (192 to 634) | RR 0.54 (0.30 to 0.99) | 51 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | |
|
Change in weight (kg) Follow‐up: 12 months |
See comments | This study reported no differences in weight change between the two diet groups (mean weights and standard deviations were not reported) | ||||
| Change in height | Not reported | This outcome was not reported | ||||
| Change in quality of life | Not reported | This outcome was not reported | ||||
|
Adverse events Follow‐up: 12 months |
0 per 1,000 | 0 per 1,000 (0 to 0) | not estimable | 51 (1 RCT) | ‐ | No adverse events were reported |
|
Serious adverse events Follow‐up: 12 months |
0 per 1,000 | 0 per 1,000 (0 to 0) | not estimable | 51 (1 RCT) | ‐ | No adverse events were reported |
|
Withdrawal due to adverse events Follow‐up: 12 months |
0 per 1,000 | 0 per 1,000 (0 to 0) | not estimable | 51 (1 RCT) | ‐ | No adverse events were reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to high risk of bias for blinding of participants and personnel
2 Downgraded two levels due to very serious imprecision (25 events)
Summary of findings 3. Enteral nutrition compared to 6‐MP for maintenance of remission in Crohn's disease.
| Enteral nutrition compared to 6‐MP for maintenance of remission in Crohn's disease | ||||||
| Patient or population: Participants with Crohn's disease in remission Setting: Outpatient Intervention: Enteral nutrition Comparison: 6‐MP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 6‐MP | Risk with enteral nutrition | |||||
|
Relapse Follow‐up: 24 months |
233 per 1,000 | 376 per 1,000 (170 to 824) | RR 1.61 (0.73 to 3.53) | 62 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| Change in weight | Not reported | This outcome was not reported | ||||
| Change in height | Not reported | This outcome was not reported | ||||
| Change in quality of life | Not reported | This outcome was not reported | ||||
|
Adverse events Follow‐up: 24 months |
133 per 1,000 | 31 per 1,000 (4 to 264) | RR 0.23 (0.03 to 1.98) | 62 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | |
|
Serious adverse events Follow‐up: 24 months |
0 per 1,000 | 0 per 1,000 (0 to 0) | not estimable | 62 (1 RCT) | ‐ | The authors reported that no serious adverse events were observed |
| Withdrawal due to adverse events | Not reported | This outcome was not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to unclear risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel and blinding of outcome assessment
2 Downgraded two levels due to very serious imprecision (19 events)
3 Downgraded two levels due to very serious imprecision (5 events)
Summary of findings 4. Enteral nutrition compared to mesalamine for maintenance of remission in Crohn's disease.
| Enteral nutrition compared to mesalamine for maintenance of remission in Crohn's disease | ||||||
| Patient or population: Participants with Crohn's disease in remission Setting: Outpatient Intervention: Enteral nutrition Comparison: Mesalamine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with mesalamine | Risk with enteral nutrition | |||||
|
Relapse Follow‐up: 6 months |
550 per 1,000 | 418 per 1,000 (270 to 655) | RR 0.76 (0.49 to 1.19) | 83 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | 40 events |
|
Change in weight (kg) Follow‐up: 6 months |
The mean weight was 0 | MD 1.9 higher (4.62 lower to 8.42 higher) |
‐ | 66 (1 RCT) | ⊕⊕⊝⊝ LOW 3 4 | Weight measurements were available for 66 participants Weight was 1.9 kg lower in the mesalamine group |
| Change in height | Not reported | This outcome was not reported | ||||
| Change in quality of life | Not reported | This outcome was not reported | ||||
|
Adverse events Follow‐up: 6 months |
Not reported | Two patients in the enteral nutrition group experienced nausea and four had diarrhoea It is unclear if any participants in the mesalamine group had an adverse event |
||||
| Serious adverse events | Not reported | This outcome was not reported | ||||
| Withdrawal due to adverse events | Not reported | This outcome was not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to unclear risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel and blinding of outcome assessment
2 Downgraded one level due to serious imprecision (40 events)
3 Downgraded one level due to serious imprecision (66 participants)
4 Downgraded one level due to high risk of attrition bias
A meta‐analyses was originally planned for this review. However, on closer examination of the results of the included primary studies, it became obvious that pooled statistical analyses were not possible. This is because the control interventions, and the way outcomes were assessed differed greatly across the four studies. The results of the individual studies are therefore presented below.
Elemental diet versus non‐elemental (polymeric) diet The effect of an elemental diet compared to a polymeric diet on remission rates or withdrawal due to adverse events is uncertain. After 12 months, 58% (11/19) of participants in the elemental diet group relapsed compared to 57% (8/14) of participants in the polymeric diet group (RR 1.01, 95% CI 0.56 to 1.84; GRADE very low certainty evidence). Thirty‐two per cent (6/19) of participants in the elemental diet group were intolerant to the enteral nutritional formula because of taste or smell and were withdrawn from the study in the first 2 weeks compared to zero participants (0/14) in the polymeric diet group (RR 9.75, 95% CI 0.59 to 159.93; GRADE very low certainty evidence). Verma 2001 reported that three patients (9%) developed steroid withdrawal symptoms, including muscle aches, depression, feeling generally unwell and mild abdominal pain. The authors did not report which group these patients belonged to. No serious adverse events were reported. Verma 2001 did not report on changes in weight or height in a manner that would allow for a comparison between elemental and polymeric formulas. Verma 2001 did not report on quality of life.
Elemental diet versus free diet (no supplementation) The effect of an elemental diet compared to a normal free diet on relapse rates is uncertain. After a mean follow up of 11.9 months, 35% (9/26) of participants in the elemental diet group relapsed compared to 64% (16/25) of participants in the free diet group (RR 0.54, 95% CI 0.30 to 0.99, P = 0.05; GRADE very low certainty evidence). No adverse events or serious adverse events were reported (Takagi 2006). Takagi 2006 reported that there were no significant differences in weight change between the elemental diet and free diet groups throughout the study. Takagi 2006 did not report on changes in height or quality of life.
Elemental diet versus 6‐mercaptopurine The effect of an elemental diet compared to 6‐MP on relapse rates or adverse events is uncertain. Thirty‐eight per cent (12/32) of participants in the elemental diet group relapsed at 12 months compared to 23% (7/30) of participants in the 6‐mercaptopurine group (RR 1.61; 95% CI 0.73 to 3.53; GRADE very low certainty evidence). Three per cent (1/32) of participants in the elemental diet group had an adverse event compared to 13% (4/30) of participants in the 6‐mercaptopurine group (RR 0.23, 95% CI 0.03 to 1.98; GRADE very low certainty evidence). Adverse events in the elemental diet group included surgery due to worsening Crohn's disease (one participant). Adverse events in the 6‐mercaptopurine group included liver injury (two participants), hair loss (one participant) surgery due to an abscess (one participant). No withdrawals due to adverse events or serious adverse events were reported (Hanai 2012). Hanai 2012 did not report on changes in weight, height or quality of life.
Non‐elemental (polymeric) diet versus mesalamine The effect of a polymeric diet compared to mesalamine on relapse rates and weight is uncertain. Forty‐two per cent (18/43) of participants in the polymeric diet group relapsed at six months compared to 55% (22/40) of participants in the mesalamine group (RR 0.76; 95% CI 0.49 to 1.19; GRADE low certainty evidence). Triantafillidis 2010 reported that two patients experienced nausea and four had diarrhoea. These adverse events were attributed to nutritional treatment. It is unclear if any patients in the mesalamine group had an adverse event. No withdrawals due to adverse events or serious adverse events were reported. Although participants in the polymeric group gained more weight over the six month study period than participants in the mesalamine group, this difference does not appear to be clinically meaningful (MD 1.90, 95% CI ‐4.62 to 8.42; GRADE moderate certainty evidence). Triantafillidis 2010 did not report on changes in height or quality of life.
Discussion
This updated systematic review includes four studies (262 adult participants). The trials were wide ranging and have significant clinical and methodological heterogeneity. The sample sizes of the included studies were generally small and the studies may have lacked statistical power. It is therefore difficult to draw any definite conclusions from these data. Very low certainty evidence suggests that a supplementary elemental diet may be superior to a free diet for maintenance of remission in Crohn's disease. Participants who received half of their total daily calorie requirements as elemental diet and the remaining half by normal diet had a lower chance of relapse at 12 months compared to participants who received a free diet (Takagi 2006). No adverse effects were reported in this study. More research is required to confirm this benefit. Very low certainty evidence suggests no difference in efficacy between elemental and polymeric formulas. The Verma 2001 study found no difference between an elemental and a polymeric formula with regard to the promotion of steroid withdrawal, and prevention of the need for surgery or steroid therapy. However, thirty‐two per cent (6/19) of participants in the elemental diet group were not able to tolerate the enteral nutritional formula because of taste or smell and were withdrawn from the study. Tolerance issues were not reported with the polymeric formula. Dropouts in enteral nutrition trials are frequently due to poor acceptance of a nasogastric tube and unpalatable formulations. The development of palatable enteral nutrition formulations that can be delivered without use of a nasogastric tube may lead to increased patient adherence with this therapy. A claim of equal efficacy for elemental and polymeric formulas would require evidence from a formal equivalence trial. More research including adequately powered trials is required to determine the optimal enteral nutrition formulation for use in people with quiescent Crohn's disease.
Very low certainty evidence suggests no difference in efficacy between an elemental diet and 6‐mercaptopurine. The Hanai 2012 study compared elemental diet with 6‐mercaptopurine and found no difference in relapse or adverse event rates. The only adverse event reported in the elemental diet group was surgery due to worsening Crohn's disease. Adverse events in the 6‐mercaptopurine group included liver injury in two participants, hair loss in one participant and surgery to treat an abscess in one participant. No serious adverse events were reported in this study. More research is needed to allow for a firm conclusion regarding the relative efficacy and safety of enteral nutrition and 6‐mercaptopurine for maintenance therapy in Crohn's disease.
Low certainty evidence suggests no difference in efficacy between a polymeric diet and mesalamine. Triantafillidis 2010 compared a polymeric diet to mesalamine and found no difference in relapse rates at six months, although the trend was towards superiority of elemental diet. Two participants the polymeric diet group experienced nausea and four had diarrhoea. It is unclear if any participants in the mesalamine group had any adverse events. No serious side effects were reported in this study. More research is needed to allow for a firm conclusion regarding the relative efficacy and safety of enteral nutrition and mesalamine for maintenance therapy in Crohn's disease.
Long term nutritional support and avoidance of steroids may also lead to improvements in growth in children and adolescents with Crohn's disease (Yamamoto 2007; Verma 2000; Wilschanski 1996). However, none of the included studies assessed the use of enteral nutrition in children with quiescent Crohn's disease. Enteral nutrition may provide a benefit for children with active Crohn's disease (Narula 2018). The use of enteral nutrition in children with quiescent Crohn's diseases should be investigated.
When considering the results of this systematic review in the context of the previously published review (Akobeng 2007), it is disappointing that the size and scope of the evidence base has not increased more. Indeed, whilst a randomised controlled trial is always a significant undertaking, such trials that focus on dietary interventions are in many ways a less daunting and costly proposal than drug interventions and it would have been expected that this, combined with the promising results reported in the previous publication, would have led to many more studies. Future studies should be well‐powered and should also investigate the amount of enteral nutritional supplements that will produce optimal benefits. These studies should also assess cost‐effectiveness and the impact of supplementation on patients' quality of life.
Authors' conclusions
Implications for practice.
The results for the outcomes assessed in this review are uncertain and no firm conclusions regarding the efficacy and safety of enteral nutrition in quiescent CD can be drawn.
Implications for research.
More research is needed to determine the efficacy and safety of using enteral nutrition as maintenance therapy in Crohn's disease. Larger well‐powered randomised controlled trials are needed. Future studies should compare enteral nutrition with interventions such as methotrexate, azathioprine, infliximab and other active drugs that are known to be effective for maintaining remission in Crohn's disease. Large numbers of patients would need to be randomised for these studies. Future studies should also investigate the amount of enteral nutritional supplements that will produce optimal benefits. These studies should also assess cost‐effectiveness and the impact of supplementation on patients' quality of life. Currently, there are four ongoing studies (estimated enrolment of 280 participants). This review will be updated when the results of these studies are available.
What's new
| Date | Event | Description |
|---|---|---|
| 27 July 2018 | New search has been performed | New literature search was run on 27 July 2018. Two new studies were added to the review |
| 27 July 2018 | New citation required and conclusions have changed | Updated review with changes to the conclusions and new authors |
Acknowledgements
Funding for the Cochrane IBD Group (May 1, 2017 ‐ April 30. 2022) has been provided by Crohn's and Colitis Canada (CCC).
Appendices
Appendix 1. Search strategies
MEDLINE on OVID:
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. randomized controlled trial/
14. or/1‐13
15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
16. 14 not 15
17. crohn*.mp.
18. exp Crohn disease/
19. ileitis.mp.
20. enteritis, regional.mp.
21. or/17‐20
22. 16 and 21
23. enteral nutrition.mp. or exp enteral nutrition/
24. food.mp. or exp food/
25. diet.mp. or exp diet/
26. polymeric diet.mp. or exp polymeric diet/
27. elemental diet.mp. or exp elemental diet/
28. or/23‐27
29. 22 and 28
Embase on OVID:
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. crohn*.mp.
22. exp Crohn disease/
23. ileitis.mp.
24. enteritis, regional.mp.
25. or/21‐24
26. 20 and 25
27. enteral nutrition.mp. or exp enteral nutrition/
28. food.mp. or exp food/
29. diet.mp. or exp diet/
30. polymeric diet.mp. or exp polymeric diet/
31. elemental diet.mp. or exp elemental diet/
32. or/27‐31
33. 26 and 32
CENTRAL
#1. crohn* #2. enteral nutrition or food or diet or polymeric diet or elemental diet #3. #1 and #2
Cochrane IBD Group Specialized Trials Register
1. Crohn 2. Enteral nutrition 3. 1 and 2
Clinicaltrials.gov
1. Crohn 2. Enteral nutrition 3. 1 and 2
Data and analyses
Comparison 1. Elemental diet versus non‐elemental (polymeric) diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse at 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
1.1. Analysis.
Comparison 1 Elemental diet versus non‐elemental (polymeric) diet, Outcome 1 Relapse at 12 months.
1.2. Analysis.
Comparison 1 Elemental diet versus non‐elemental (polymeric) diet, Outcome 2 Withdrawals due to adverse events.
Comparison 2. Elemental diet versus free diet (no supplementation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse at 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
2.1. Analysis.
Comparison 2 Elemental diet versus free diet (no supplementation), Outcome 1 Relapse at 12 months.
2.2. Analysis.
Comparison 2 Elemental diet versus free diet (no supplementation), Outcome 2 Adverse events.
2.3. Analysis.
Comparison 2 Elemental diet versus free diet (no supplementation), Outcome 3 Serious adverse events.
2.4. Analysis.
Comparison 2 Elemental diet versus free diet (no supplementation), Outcome 4 Withdrawals due to adverse events.
Comparison 3. Elemental diet versus 6‐mercaptopurine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse at 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
3.1. Analysis.
Comparison 3 Elemental diet versus 6‐mercaptopurine, Outcome 1 Relapse at 24 months.
3.2. Analysis.
Comparison 3 Elemental diet versus 6‐mercaptopurine, Outcome 2 Adverse events.
3.3. Analysis.
Comparison 3 Elemental diet versus 6‐mercaptopurine, Outcome 3 Serious adverse events.
3.4. Analysis.
Comparison 3 Elemental diet versus 6‐mercaptopurine, Outcome 4 Withdrawals due to adverse events.
Comparison 4. Non‐elemental (polymeric) diet versus mesalamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Weight | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
4.1. Analysis.
Comparison 4 Non‐elemental (polymeric) diet versus mesalamine, Outcome 1 Relapse at 6 months.
4.2. Analysis.
Comparison 4 Non‐elemental (polymeric) diet versus mesalamine, Outcome 2 Weight.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hanai 2012.
| Methods | Randomised controlled trial | |
| Participants | 95 patients, between the ages of 19 to 48, with a diagnosis of Crohn's disease and in clinical remission as defined by CDAI < 150 | |
| Interventions | 30 patients received 6‐mercaptopurine, 32 patients received enteral nutrition (elemental diet), 33 patients served as the control group and did not receive any additional medications | |
| Outcomes | Primary outcome was rate of relapse over the 2 years follow‐up time, relapse was defined as CDAI > 200 or the need for an additional medication to suppress worsening symptoms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Takagi 2006.
| Methods | Randomised controlled trial | |
| Participants | 51 adults (37 men) with Crohn's disease in remission | |
| Interventions | 26 received half of their daily allowance as elemental diet and half as normal diet. 25 received all their daily allowance as normal diet | |
| Outcomes | Primary outcome: relapse within 2 years. Relapse was defined as a CDAI score of > 200 or the need for therapy to induce remission | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation generated by an independent randomisation centre |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by an independent randomisation centre |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants in the ED group took their ED as powder in solution; participants in the free diet group took all their nutrients via their usual unrestricted meals |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "To maintain the blinding of the principal investigators at each site, the results of the lab tests and the CDAI were reviewed by co‐investigators who had no contact with the patients, and these results were reported in a separate case report form" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Triantafillidis 2010.
| Methods | Randomised controlled trial | |
| Participants | Eighty‐three patients with Crohn's disease in remission, defined as CDAI < 150 | |
| Interventions | 36 patients received enteral nutrition (a non‐elemental polymeric diet ‐ Modulen) and 30 patients received mesalamine | |
| Outcomes | Relapse was defined as CDAI > 150 or at least 60 points over baseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients lost to follow‐up not described |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Verma 2001.
| Methods | Randomised controlled trial | |
| Participants | 33 adults (11 men) with Crohn's disease in remission | |
| Interventions | 19 participants received 35‐50% of their pretrial calorie intake as elemental diet (EO28 Extra, Scientific Hospital Supplies Ltd, Liverpool, UK) 14 participants received 35‐50% of their pretrial calorie intake as polymeric diet (Fortisip, Nutricia, UK) |
|
| Outcomes | Primary outcome: Treatment success within 12 months This was defined as remaining in remission for 12 months (CDAI < 200 and increasing by < 100 from baseline) after complete withdrawal of steroids |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | The study appears to be free from other sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Canani 2006 | Not a maintenance study |
| Dray 2005 | Review article |
| Esaki 2005 | Not an RCT |
| Forbes 2002 | Review article |
| Giaffer 1991 | Not an RCT |
| Gorard 1993 | Not a maintenance study |
| Griffiths 2005 | Review article |
| Harries 1983 | Not a maintenance study |
| Hirakawa 1993 | Not an RCT |
| Iakovlev 2014 | Does not report separate results for Crohn's disease and ulcerative colitis; does not report number of patients randomised to each group |
| Johnson 2006 | Not a maintenance study |
| Jones 1987 | Not an RCT; studied food exclusion diets, not enteral nutrition |
| Knight 2005 | Not a maintenance study |
| Koga 1993 | Not an RCT |
| Matsueda 1995 | Not an RCT |
| Navarro 1982 | Not an RCT |
| Nomura 1995 | Not an RCT |
| Phylactos 2001 | Not a maintenance study |
| Roggero 2003 | Abstract publication of RCT Never published in full |
| Triantafillidis 2006 | Not an RCT |
| Valentini 2002 | Review article |
| Verma 2000 | Not an RCT |
| Wilschanski 1996 | Not an RCT |
| Yamamoto 2005 | Not a maintenance study |
| Yamamoto 2007 | Not an RCT |
| Yamamoto 2010 | Not an RCT |
| Yamamoto 2015 | Not an RCT |
Characteristics of ongoing studies [ordered by study ID]
NCT01823042.
| Trial name or title | A Randomized, Controlled, Open‐label Study to Assess the Efficacy of Enteral Nutrition in Fill of the Treatment Blank Period of the Postoperative Maintain Remission Medication for Crohn's Disease (CD) |
| Methods | Randomized, controlled, open‐label study |
| Participants | All patients, aged 18 to 75, with an established diagnosis of Crohn's disease with a CDAI score < 150 at week 0 |
| Interventions | One group received azathioprine, one group received azathioprine and enteral nutrition |
| Outcomes | Change in CDAI at week 0, week 4, week 8 and week 12 |
| Starting date | October 2012 |
| Contact information | Zhu Weiming, PhD, MD, dr_zhuweiming@126.com |
| Notes | Estimated enrolment: 100 |
NCT02201693.
| Trial name or title | Randomised Trial Comparing 12 Months of Cyclic Enteral Nutrition to Supplementary Enteral Nutrition as Maintenance Therapy for Pediatric Crohn's Disease |
| Methods | Randomised trial |
| Participants | Patients, aged 6 to 18, with confirmed Crohn's disease, who have completed a cycle of induction therapy for at least 6 weeks |
| Interventions | One group received cyclic exclusive enteral nutrition (100% of daily calories) for two weeks every 8 weeks; one group received supplementary enteral nutrition (25% daily calories) |
| Outcomes | The first relapse at 12 months assessed with PCDAI, clinical remission assessed with PCDAI, CDAI and PGA |
| Starting date | December 2014 |
| Contact information | Frank Ruemmele, MD, PhD, frank.ruemmele@nck.aphp.fr |
| Notes | Estimated enrolment: 100 |
NCT02231814.
| Trial name or title | Dietary Therapy Using Partial Enteral Nutrition and the Crohn's Disease Exclusion Diet (CDED) for Induction and Maintenance of Remission in Mild to Moderate Crohn's Disease in Adults‐ A Pilot Study |
| Methods | Prospective, open‐label, randomised controlled pilot trial |
| Participants | Patients, aged 18 to 55, with an established Crohn's disease, with HBI between 5 and 15 |
| Interventions | One group will receive the Crohn's disease exclusion diet and partial enteral nutrition, while another group receives the Crohn's disease exclusion diet alone. The maintenance phase will be assessed from weeks 13 through 24 |
| Outcomes | Steroid free mission assessed at week 6, 12 and 24 |
| Starting date | December 2016 |
| Contact information | Arie Levine, MD, alevine@wolfson.health.gov.il |
| Notes | Estimated enrolment: 40 |
NCT02843100.
| Trial name or title | Modified Exclusive Enteral Nutrition With the Crohn's Disease Exclusion Diet for Induction and Maintenance of Remission and Re‐biosis |
| Methods | Randomized, controlled, pilot trial |
| Participants | Patients aged 8 to 18 with mild to severe Cron's disease, with PCDAI between 15 and 47.5 |
| Interventions | One group will receive exclusive enteral nutrition for two weeks followed by twelve weeks of partial enteral nutrition and Crohn's disease exclusion diet; one group will receive standard exclusive enteral nutrition for eight weeks, followed by gradual reduction of enteral nutrition by week 12 |
| Outcomes | Steroid free remission defined as PCDAI < 10 at week 24, biologic therapy free remission defined as PCDAI < 10 at week 24 |
| Starting date | January 2017 |
| Contact information | Arie Levine, MD, alevine@wolfson.health.gov.il |
| Notes | Estimated enrolment: 40 |
Differences between protocol and review
This review update includes a PRISMA flow diagram, a risk of bias assessment of included studies (to replace the Jadad scale), a GRADE analysis and Summary of Findings tables, a per‐protocol analysis for the Triantafillidis 2010 study (drop‐outs were not described) and the inclusion of adverse event outcomes. For the original published version of this review, we contacted leaders in the field and drug companies to identify other potentially eligible studies (Akobeng 2007). This did not identify any studies and we did not do this for the updated version of this review.
Declarations of interest
Anthony K Akobeng: None known
Dongni Zhang: None known
Morris Gordon has received travel fees to attend international scientific and training meeting such as DDW, Advances in IBD, ESPGHAN, BSPGHAN and Cochrane focused international events from companies including: Abbott, Nutricia, Biogaia, Ferring, Allergan, Tillotts. None of these companies have had any involvement in any works completed by me and I have never had any payments for any other activates from these companies.
John K MacDonald: None known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Hanai 2012 {published data only}
- Hanai H, Iida T, Takeuchi K, Arai H, Arai O, Abe J, et al. Nutritional therapy versus 6‐mercaptopurine as maintenance therapy in patients with Crohn's disease. Digestive and Liver Disease 2012;44(8):649‐54. [DOI] [PubMed] [Google Scholar]
Takagi 2006 {published data only}
- Takagi S, Utsunomiya K, Kuriyama S, Yokoyama H, Takahashi S, Iwabuchi M, et al. Effectiveness of an 'half elemental diet' as maintenance therapy for Crohn's disease: A randomized‐controlled trial. Alimentary Pharmacology & Therapeutics 2006;24(9):1333‐40. [DOI] [PubMed] [Google Scholar]
Triantafillidis 2010 {published data only}
- Triantafillidis JK, Stamataki A, Karagianni V, Gikas A, Malgarinos G. Maintenance treatment of Crohn's disease with a polymeric feed rich in TGF‐beta. Annals of Gastroenterology 2010;23(2):113‐8. [Google Scholar]
Verma 2001 {published data only}
- Verma S, Holdsworth CD, Giaffer MH. Does adjuvant nutritional support diminish steroid dependency in Crohn disease?. Scandinavian Journal of Gastroenterology 2001;36(4):383‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Canani 2006 {published data only}
- Canani RB, Terrin G, Borrelli O, Romano MT, Manguso F, Coruzzo A, et al. Short‐ and long‐term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn's disease. Digestive and Liver Disease 2006;38(6):381‐7. [DOI] [PubMed] [Google Scholar]
Dray 2005 {published data only}
- Dray X, Marteau P. The use of enteral nutrition in the management of Crohn's disease in adults. JPEN. Journal of Parenteral and Enteral Nutrition 2005;29(4 Suppl):S166‐72. [DOI] [PubMed] [Google Scholar]
Esaki 2005 {published data only}
- Esaki M, Matsumoto T, Hizawa K, Nakamura S, Jo Y, Mibu R, et al. Preventive effect of nutritional therapy against postoperative recurrence of Crohn disease, with reference to findings determined by intra‐operative enteroscopy. Scandinavian Journal of Gastroenterology 2005;40(12):1431‐7. [DOI] [PubMed] [Google Scholar]
Forbes 2002 {published data only}
- Forbes A. Review article: Crohn's disease‐‐the role of nutritional therapy. Alimentary Pharmacology & Therapeutics 2002;16(Suppl 4):48‐52. [DOI] [PubMed] [Google Scholar]
Giaffer 1991 {published data only}
- Giaffer MH, Cann P, Holdsworth CD. Long‐term effects of elemental and exclusion diets for Crohn's disease. Alimentary Pharmacology & Therapeutics 1991;5(2):115‐25. [DOI] [PubMed] [Google Scholar]
Gorard 1993 {published data only}
- Gorard DA, Hunt JB, Payne‐James JJ, Palmer KR, Rees RG, Clark ML, et al. Initial response and subsequent course of Crohn's disease treated with elemental diet or prednisolone. Gut 1993;34(9):1198‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffiths 2005 {published data only}
- Griffiths AM. Enteral nutrition in the management of Crohn's disease. JPEN. Journal of Parenteral and Enteral Nutrition 2005;29(4 Suppl):S108‐17. [DOI] [PubMed] [Google Scholar]
Harries 1983 {published data only}
- Harries AD, Jones LA, Danis V, Fifield R, Heatley RV, Newcombe RG, et al. Controlled trial of supplemented oral nutrition in Crohn's disease. Lancet 1983;1(8330):887‐90. [DOI] [PubMed] [Google Scholar]
Hirakawa 1993 {published data only}
- Hirakawa H, Fukuda Y, Tanida N, Hosomi M, Shimoyama T. Home elemental enteral hyperalimentation (HEEH) for the maintenance of remission in patients with Crohn's disease. Gastroenterologia Japonica 1993;28(3):379‐84. [DOI] [PubMed] [Google Scholar]
Iakovlev 2014 {published data only}
- Iakovlev AA, Kazaryan TS. Optimization of diagnosis and treatment of nutritional insufficiency in patients with inflammatory bowel disease. United European Gastroenterology Journal 2014;1:A286. [Google Scholar]
Johnson 2006 {published data only}
- Johnson T, Macdonald S, Hill SM, Thomas A, Murphy MS. Treatment of active Crohn's disease in children using partial enteral nutrition with liquid formula: a randomised controlled trial. Gut 2006;55(3):356‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1987 {published data only}
- Jones VA. Comparison of total parenteral nutrition and elemental diet in induction of remission of Crohn's disease. Long‐term maintenance of remission by personalized food exclusion diets. Digestive Diseases and Sciences 1987;32(12 Supplement):100S‐7S. [DOI] [PubMed] [Google Scholar]
Knight 2005 {published data only}
- Knight C, El‐Matary W, Spray C, Sandhu BK. Long‐term outcome of nutritional therapy in paediatric Crohn's disease. Clinical Nutrition (Edinburgh, Scotland) 2005;24(5):775‐9. [DOI] [PubMed] [Google Scholar]
Koga 1993 {published data only}
- Koga H, Iida M, Aoyagi K, Matsui T, Fujishima M. Long‐term efficacy of low residue diet for the maintenance of remission in patients with Crohn's disease. Nippon Shokakibyo Gakkai Zasshi 1993;90(11):2882‐8. [PubMed] [Google Scholar]
Matsueda 1995 {published data only}
- Matsueda K, Shoda R, Takazoe M, Hiwatashi N, Bamba T, Kobayashi K, et al. Therapeutic efficacy of cyclic home elemental enteral alimentation in Crohn's disease: Japanese cooperative Crohn's disease study. Journal of Gastroenterology 1995;30(Suppl 8):91‐4. [PubMed] [Google Scholar]
Navarro 1982 {published data only}
- Navarro J, Vargas J, Cezard JP, Charritat JL, Polonovski C. Prolonged constant rate elemental enteral nutrition in Crohn's disease. Journal of Pediatric Gastroenterology and Nutrition 1982;1(4):541‐6. [DOI] [PubMed] [Google Scholar]
Nomura 1995 {published data only}
- Nomura M, Taruishi M, Ashida T, Ayabe T, Einami K, Saitoh Y, et al. Home enteral nutrition for the maintenance of remission in patients with Crohn's disease‐‐including comparison between Elental and Enterued. Nippon Shokakibyo Gakkai Zasshi 1995;92(1):32‐40. [PubMed] [Google Scholar]
Phylactos 2001 {published data only}
- Phylactos AC, Fasoula IN, Arnaud‐Battandier F, Walker‐Smith JA, Fell JM. Effect of enteral nutrition on antioxidant enzyme systems and inflammation in paediatric Crohn's disease. Acta Paediatrica 2001;90(8):883‐8. [PubMed] [Google Scholar]
Roggero 2003 {published data only}
- Roggero P, Santus F, Barabino A, Canani RB, Cucchiara S, Guariso G, et al. A prospective pediatric multicenter trial of enteral nutrition and azathioprine in preventing relapses of Crohn disease: preliminary results. Journal of Pediatric Gastroenterology and Nutrition 2003;36(4):543. [Google Scholar]
Triantafillidis 2006 {published data only}
- Triantafillidis JK, Stamataki A, Gikas A, Sklavaina M, Mylonaki M, Georgopoulos F, et al. Beneficial effect of a polymeric feed, rich in TGF‐beta, on adult patients with active Crohn's disease: A pilot study. Annals of Gastroenterology 2006;19(1):66‐71. [Google Scholar]
Valentini 2002 {published data only}
- Valentini G, Capristo E, Scarfone A, Mingrone G, Greco AV, Gasbarrini G. Enteral diet in Crohn's disease. Nutritional and therapeutic implications in patient's management. Minerva Gastroenterologica e Dietologica 2002;48(1):13‐24. [PubMed] [Google Scholar]
Verma 2000 {published data only}
- Verma S, Kirkwood B, Brown S, Giaffer MH. Oral nutritional supplementation is effective in the maintenance of remission in Crohn's disease. Digestive and Liver Disease 2000;32(9):769‐74. [DOI] [PubMed] [Google Scholar]
Wilschanski 1996 {published data only}
- Wilschanski M, Sherman P, Pencharz P, Davis L, Corey M, Griffiths A. Supplementary enteral nutrition maintains remission in paediatric Crohn's disease. Gut 1996;38(4):543‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamamoto 2005 {published data only}
- Yamamoto T, Nakahigashi M, Umegae S, Kitagawa T, Matsumoto K. Impact of elemental diet on mucosal inflammation in patients with active Crohn's disease: cytokine production and endoscopic and histological findings. Inflammatory Bowel Diseases 2005;11(6):580‐8. [DOI] [PubMed] [Google Scholar]
Yamamoto 2007 {published data only}
- Yamamoto T, Nakahigashi M, Umegae S, Kitagawa T, Matsumoto K. Impact of long‐term enteral nutrition on clinical and endoscopic recurrence after resection for Crohn's disease: A prospective, non‐randomized, parallel, controlled study. Alimentary Pharmacology & Therapeutics 2007;25(1):67‐72. [DOI] [PubMed] [Google Scholar]
Yamamoto 2010 {published data only}
- Yamamoto T, Nakahigashi M, Umegae S, Matsumoto K. Prospective clinical trial: enteral nutrition during maintenance infliximab in Crohn's disease. Journal of Gastroenterology 2010;45(1):24‐29. [DOI] [PubMed] [Google Scholar]
Yamamoto 2015 {published data only}
- Yamamoto T, Nakahigashi M, Shimoyama T, Matsumoto K. The long‐term efficacy of concomitant enteral nutritional therapy during maintenance infliximab in patients with Crohn's disease: A prospective observational trial. Journal of Crohn's and Colitis 2015;9:S364. [Google Scholar]
References to ongoing studies
NCT01823042 {published data only}
- NCT01823042. A Randomized, Controlled, Open‐label Study to Assess the Efficacy of Enteral Nutrition in Fill of the Treatment Blank Period of the Postoperative Maintain Remission Medication for Crohn's Disease (CD). clinicaltrials.gov/show/NCT01823042 (accessed 3 August 2017).
NCT02201693 {published data only}
- NCT02201693. Randomised Trial Comparing 12 Months of Cyclic Enteral Nutrition to Supplementary Enteral Nutrition as Maintenance Therapy for Pediatric Crohn's Disease. clinicaltrials.gov/show/NCT02201693 (accessed 3 August 2017).
NCT02231814 {published data only}
- NCT02231814. Dietary Therapy Using Partial Enteral Nutrition and the Crohn's Disease Exclusion Diet (CDED) for Induction and Maintenance of Remission in Mild to Moderate Crohn's Disease in Adults‐ A Pilot Study. clinicaltrials.gov/show/NCT02231814 (accessed 3 August 2017).
NCT02843100 {published data only}
- NCT02843100. Modified Exclusive Enteral Nutrition With the Crohn's Disease Exclusion Diet for Induction and Maintenance of Remission and Re‐biosis. clinicaltrials.gov/show/NCT02843100 (accessed 3 August 2017).
Additional references
Akobeng 2000
- Akobeng AK, Miller V, Stanton J, Elbadri AM, Thomas AG. Double‐blind randomized controlled trial of glutamine‐enriched polymeric diet in the treatment of active Crohn's disease. Journal of Pediatric Gastroenterology and Nutrition 2000;30(1):78‐84. [DOI] [PubMed] [Google Scholar]
Akobeng 2016
- Akobeng AK, Zhang D, Gordon M, MacDonald JK. Oral 5‐aminosalicylic acid for maintenance of medically‐induced remission in Crohn's disease. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD003715.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Biancone 2003
- Biancone L, Tosti C, Fina D, Fantini M, Nigris F, Geremia A, et al. Review article: maintenance treatment of Crohn's disease. Alimentary Pharmacology & Therapeutics 2003;17(Suppl 2):31‐7. [DOI] [PubMed] [Google Scholar]
Chande 2015
- Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6‐mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD000067.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colombel 2007
- Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology 2007;132(1):52‐65. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feagan 2000
- Feagan BG, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. North American Crohn's Study Group Investigators. New England Journal of Medicine 2000;342(22):1627‐32. [DOI] [PubMed] [Google Scholar]
Feagan 2003
- Feagan BG. Maintenance therapy for inflammatory bowel disease. American Journal of Gastroenterology 2003;98 (12 Suppl):S6‐S17. [DOI] [PubMed] [Google Scholar]
Goh 2003
- Goh J, O'Morain CA. Review article: nutrition and adult inflammatory bowel disease. Alimentary Pharmacology & Therapeutics 2003;17(3):307‐20. [DOI] [PubMed] [Google Scholar]
Gonzalez‐Huix 1993
- Gonzalez‐Huix F, Leon R, Fernandez‐Banares F, Esteve M, Cabre E, Acero D, et al. Polymeric enteral diets as primary treatment of active Crohn's disease: a prospective steroid controlled trial. Gut 1993;34(6):778‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman, AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanauer 2002
- Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002;359(9317):1541‐9. [DOI] [PubMed] [Google Scholar]
Heuschkel 2000
- Heuschkel RB, Menache CC, Megerian JT, Baird AE. Enteral nutrition and corticosteroids in the treatment of acute Crohn's disease in children. Journal of Pediatric Gastroenterology and Nutrition 2000;31(1):8‐15. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Jones 1985
- Jones VA, Dickinson RJ, Workman E, Wilson AJ, Freeman AH, Hunter JO. Crohn's disease: maintenance of remission by diet. Lancet 1985;2(8448):177‐80. [DOI] [PubMed] [Google Scholar]
Lewis 1994
- Lewis JD, Fisher RL. Nutrition support in inflammatory bowel disease. Medical Clinics of North America 1994;78(6):1443‐56. [DOI] [PubMed] [Google Scholar]
Lichtenstein 2004
- Lichtenstein GR, Yan S, Bala M, Hanauer S. Remission in patients with Crohn's disease is associated with improvement in employment and quality of life and a decrease in hospitalizations and surgeries. American Journal of Gastroenterology 2004;99(1):91‐6. [DOI] [PubMed] [Google Scholar]
Murphy 2001
- Murphy MS, Randell T. Evaluation of elemental diet therapy as a long term strategy for managing Crohn's disease (abstract). Journal of Pediatric Gastroenterology and Nutrition 2001;32:366. [Google Scholar]
Narula 2018
- Narula N, Dhillon A, Zhang D, Sherlock ME, Tondeur M, Zachos M. Enteral nutritional therapy for induction of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2018, Issue 4. [DOI: 10.1002/14651858.CD000542.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2014
- Patel V, Wang Y, MacDonald JK, McDonald JW, Chande N. Methotrexate for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD006884.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pitkin 1999
- Pitkin RM, Branagan MA, Burmeister LF. Accuracy of data in abstracts of published research articles. JAMA 1999;281(12):1110‐1. [DOI] [PubMed] [Google Scholar]
Sandborn 2013
- Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. New England Journal of Medicine 2013;369(8):711‐21. [DOI] [PubMed] [Google Scholar]
Schunemann 2011
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Steinhart 2003
- Steinhart AH, Ewe K, Griffiths AM, Modigliani R, Thomsen OO. Corticosteroids for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000301] [DOI] [PubMed] [Google Scholar]
Thomas 1993
- Thomas AG, Taylor F, Miller V. Dietary intake and nutritional treatment in childhood Crohn's disease. Journal of Pediatric Gastroenterology and Nutrition 1993;17(1):75‐81. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Akobeng 2007
- Akobeng AK, Thomas AG. Enteral nutrition for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD005984.pub2] [DOI] [PubMed] [Google Scholar]


