Abstract
Background
Since the 2000s, there has been a trend towards decreasing tidal volumes for positive pressure ventilation during surgery. This an update of a review first published in 2015, trying to determine if lower tidal volumes are beneficial or harmful for patients.
Objectives
To assess the benefit of intraoperative use of low tidal volume ventilation (less than 10 mL/kg of predicted body weight) compared with high tidal volumes (10 mL/kg or greater) to decrease postoperative complications in adults without acute lung injury.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 5), MEDLINE (OvidSP) (from 1946 to 19 May 2017), Embase (OvidSP) (from 1974 to 19 May 2017) and six trial registries. We screened the reference lists of all studies retained and of recent meta‐analysis related to the topic during data extraction. We also screened conference proceedings of anaesthesiology societies, published in two major anaesthesiology journals. The search was rerun 3 January 2018.
Selection criteria
We included all parallel randomized controlled trials (RCTs) that evaluated the effect of low tidal volumes (defined as less than 10 mL/kg) on any of our selected outcomes in adults undergoing any type of surgery. We did not retain studies with participants requiring one‐lung ventilation.
Data collection and analysis
Two authors independently assessed the quality of the retained studies with the Cochrane 'Risk of bias' tool. We analysed data with both fixed‐effect (I2 statistic less than 25%) or random‐effects (I2 statistic greater than 25%) models based on the degree of heterogeneity. When there was an effect, we calculated a number needed to treat for an additional beneficial outcome (NNTB) using the odds ratio. When there was no effect, we calculated the optimum information size.
Main results
We included seven new RCTs (536 participants) in the update.
In total, we included 19 studies in the review (776 participants in the low tidal volume group and 772 in the high volume group). There are four studies awaiting classification and three are ongoing. All included studies were at some risk of bias. Participants were scheduled for abdominal surgery, heart surgery, pulmonary thromboendarterectomy, spinal surgery and knee surgery. Low tidal volumes used in the studies varied from 6 mL/kg to 8.1 mL/kg while high tidal volumes varied from 10 mL/kg to 12 mL/kg.
Based on 12 studies including 1207 participants, the effects of low volume ventilation on 0‐ to 30‐day mortality were uncertain (risk ratio (RR) 0.80, 95% confidence interval (CI) 0.42 to 1.53; I2 = 0%; low‐quality evidence). Based on seven studies including 778 participants, lower tidal volumes probably reduced postoperative pneumonia (RR 0.45, 95% CI 0.25 to 0.82; I2 = 0%; moderate‐quality evidence; NNTB 24, 95% CI 16 to 160), and it probably reduced the need for non‐invasive postoperative ventilatory support based on three studies including 506 participants (RR 0.31, 95% CI 0.15 to 0.64; moderate‐quality evidence; NNTB 13, 95% CI 11 to 24). Based on 11 studies including 957 participants, low tidal volumes during surgery probably decreased the need for postoperative invasive ventilatory support (RR 0.33, 95% CI 0.14 to 0.77; I2 = 0%; NNTB 39, 95% CI 30 to 166; moderate‐quality evidence). Based on five studies including 898 participants, there may be little or no difference in the intensive care unit length of stay (standardized mean difference (SMD) –0.06, 95% CI –0.22 to 0.10; I2 = 33%; low‐quality evidence). Based on 14 studies including 1297 participants, low tidal volumes may have reduced hospital length of stay by about 0.8 days (SMD –0.15, 95% CI –0.29 to 0.00; I2 = 27%; low‐quality evidence). Based on five studies including 708 participants, the effects of low volume ventilation on barotrauma (pneumothorax) were uncertain (RR 1.77, 95% CI 0.52 to 5.99; I2 = 0%; very low‐quality evidence).
Authors' conclusions
We found moderate‐quality evidence that low tidal volumes (defined as less than 10 mL/kg) decreases pneumonia and the need for postoperative ventilatory support (invasive and non‐invasive). We found no difference in the risk of barotrauma (pneumothorax), but the number of participants included does not allow us to make definitive statement on this. The four studies in 'Studies awaiting classification' may alter the conclusions of the review once assessed.
Plain language summary
Use of small volumes of breath insufflation for intraoperative mechanical ventilation during surgery
Background
Inspiration (breathing in) is produced by the shortening (contraction) of various muscles that stretch the lungs to increase their size like rubber balloons. During this phase, oxygen enters the lungs. When these muscles stop their contractions, the lungs go back to their initial size. During this phase, carbon dioxide goes out of the lungs. When people are cared for under general anaesthesia, some of the drugs used will stop the movements of the muscles controlling lung size. Insufflation is the act of mechanically forcing air into a person's respiratory system. A machine is required to replace the effects of the muscles. A mixture of gas containing oxygen is blown into the lungs. It is actually not known whether it is better to blow small volumes of gas at a higher rate or bigger volumes at a lower rate. In this review, we tried to determine whether this volume should be lower or higher than 10 millilitres per kilogram of body weight.
Study characteristics
We searched medical databases up to 19 May 2017. We included 19 studies with 1548 adults of both sexes. The participants had had operations on the abdomen (tummy), heart, blood vessels of the lungs, back, lower limbs or various surgeries. Two studies mentioned financial support from the pharmaceutical industry or from medical equipment manufacturers. We do not think that this had an effect on the results as high or low volumes may be administered with any machine.
Key results
We did not find a difference in 0‐ to 30‐day mortality (death within one month). We found that using a volume lower than 10 millilitres per kilogram of body weight reduced the risk of pneumonia (lung infection) and increased the chances that people would be able to get back to their normal respiratory status immediately after surgery. Low volumes should be used preferentially during surgery. For every 1000 people operated on, 84 would have pneumonia after the operation if high volumes were used during surgery. This number was reduced to 43 if low volumes were used instead. Likewise, the number of people needing additional non‐invasive ventilatory support (through a mask applied to the face) would be reduced from 115 to 36 if volumes lower than 10 millilitres per kilogram of body weight were used during surgery and the need invasive ventilatory support (through a tube inserted in the person's windpipe) would be reduced from 39 to 13. Hospital length of stay may be slightly reduced (equivalent to almost one day). We identified no possible harmful effects of using low volumes.
Reliability of evidence
We judged the reliability of the evidence as moderate for pneumonia and reduced need for ventilatory support (non‐invasive or invasive). Results on these three outcomes may be affected with additional data.
Summary of findings
Summary of findings for the main comparison. Low tidal volume compared to high tidal volume for surgery.
| Low tidal volume compared to high tidal volume for surgery | ||||||
|
Patient or population: adults (aged > 16 years) without acute lung injury needing mechanical positive pressure ventilation during their surgery and undergoing any type of open or laparoscopic surgery, elective or emergency Settings: university hospital (16) or in‐hospital (3). Trials were conducted in China (3), France (1), Germany (1), India (1), Italy (1), Japan (1), Russia (1), South Korea (2), The Netherlands (1), Turkey (1) or USA (6) Intervention: low tidal volume Comparison: high tidal volume | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| High tidal volume | Low tidal volume | |||||
| Mortality Follow‐up: 0 to 30 days after surgery | Study population | RR 0.80 (0.42 to 1.53) | 1207 (12 studies) | ⊕⊕⊝⊝ Lowa | Participants were undergoing abdominal surgery (6 studies), heart surgery (1 study), pulmonary thromboendarterectomy (1 study) or various surgeries (1 study) | |
| 30 per 1000 | 24 per 1000 (13 to 46) | |||||
| Low | ||||||
| 20 per 1000 | 16 per 1000 (8 to 31) | |||||
| High | ||||||
| 80 per 1000 | 64 per 1000 (34 to 122) | |||||
| Pneumonia Follow‐up: 0 to 7 days after surgery | Study population | RR 0.45 (0.25 to 0.82) | 778 (7 studies) | ⊕⊕⊕⊝ Moderateb | Participants were undergoing abdominal surgery (5 studies) or spine surgery (2 studies) | |
| 84 per 1000 | 43 per 1000 (23 to 79) | |||||
| Low | ||||||
| 20 per 1000 | 10 per 1000 (5 to 19) | |||||
| High | ||||||
| 120 per 1000 | 61 per 1000 (32 to 113) | |||||
| Need for postoperative non‐invasive ventilatory support Follow‐up: 0 to 7 days (between discharge from the postoperative care unit and 7 days after the surgery) | Study population | RR 0.31 (0.15 to 0.64) | 506 (3 studies) | ⊕⊕⊕⊝ Moderateb | Participants were undergoing abdominal surgery (1 study), spine surgery (1 study) or knee surgery (1 study) | |
| 115 per 1000 | 36 per 1000 (17 to 73) | |||||
| Low | ||||||
| 20 per 1000 | 6 per 1000 (3 to 13) | |||||
| High | ||||||
| 180 per 1000 | 56 per 1000 (27 to 115) | |||||
| Need for postoperative invasive ventilatory support Follow‐up: 0 to 7 days (between discharge from the postoperative care unit and 7 days after the surgery) | Study population | RR 0.33 (0.14 to 0.77) | 957 (11 studies) | ⊕⊕⊕⊝ Moderateb | Participants were undergoing abdominal surgery (5 studies), heart surgery (3 studies), spine surgery (2 studies) or knee surgery (1 study) | |
| 39 per 1000 | 13 per 1000 (6 to 30) | |||||
| Low | ||||||
| 8 per 1000 | 3 per 1000 (1 to 6) | |||||
| High | ||||||
| 60 per 1000 | 20 per 1000 (8 to 46) | |||||
| Intensive care unit length of stay (days) | The mean intensive care unit length of stay in the intervention groups was 0.06 standard deviations lower (0.22 lower to 0.10 higher) | 898 (5 studies) | ⊕⊕⊝⊝ Lowc | A standard deviation of 0.2 represents a small difference between groups Participants were undergoing abdominal surgery (2 studies), heart surgery (1 study), pulmonary thromboendarterectomy (1 study) or various surgeries (1 study) |
||
| Hospital length of stay (days) | The mean hospital length of stay in the intervention groups was 0.15 standard deviations lower (0.29 lower to ‐0.00 lower) | 1298 (14 studies) | ⊕⊕⊝⊝ Lowc | A standard deviation of 0.2 represents a small difference between groups Participants were undergoing abdominal surgery (7 studies), heart surgery (3 studies), pulmonary thromboendarterectomy (1 study) spine surgery (1 study), knee surgery (1 study) or various surgeries (1 study) The difference was equivalent to 0.8 day |
||
| Barotrauma: pneumothorax Follow‐up: 0 to 7 days | Study population | RR 1.77 (0.52 to 5.99) | 708 (5 studies) | ⊕⊝⊝⊝ Very lowd | Participants were undergoing abdominal surgery (4 studies) or pulmonary thromboendarterectomy (1 study) | |
| 11 per 1000 | 20 per 1000 (6 to 67) | |||||
| Low | ||||||
| 5 per 1000 | 9 per 1000 (3 to 30) | |||||
| High | ||||||
| 30 per 1000 | 53 per 1000 (16 to 180) | |||||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardized mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for risk of bias and by one level for imprecision. bDowngraded one level for imprecision. cDowngraded one level for risk of bias and by one level for heterogeneity.
dDowngraded one level for risk of bias and by two levels for imprecision.
Background
Description of the condition
When a person comes to the operating room for surgery, choices for anaesthesia are local anaesthesia (infiltration of local anaesthetic at the site of the surgery), regional anaesthesia (blockade of nerve conduction at the level of the spine, a plexus or a nerve) and general anaesthesia. For many surgeries under general anaesthesia a neuromuscular blocking agent is administered thus paralysing the respiratory muscles. To ensure appropriate delivery of oxygen and elimination of carbon dioxide artificial (mechanical) ventilation is necessary. In brief, a certain volume of gas will be insufflated into the lungs a certain number of times per minute to match the specific person's needs. Thus, the required amount of gas insufflated each minute can be delivered with high volumes at a low frequency or with small volumes at a higher frequency. In healthy non‐anaesthetized people, the tidal volume (volume of gas delivered at each respiration) is approximately 5 mL/kg to 6 mL/kg of body weight (Aliverti 2011).
The drugs that are used to produce general anaesthesia, insertion of the tracheal tube, inhalation of cold non‐humidified gas and the change from physiological negative pressure ventilation to artificial positive pressure ventilation will induce various physiological changes (Aliverti 2011). One of the many significant outcomes of these interventions (along with the direct effects from surgery) is lung collapse (atelectasis) in the dependent lung zones (Cai 2007). With positive pressure ventilation, gas will go preferentially to the uppermost parts of the lung (zones of lower resistance to lung expansion) while blood flow (following gravity) will go preferentially into the lowermost parts of the lungs, the atelectatic (closed) ones. The difference between non‐dependent and dependent zones is far less pronounced for ventilation than it is for perfusion (Petersson 2010). Thus initiation of positive pressure ventilation in an anaesthetized person will increase the 'mismatch' between the ventilation (going preferentially to the non‐dependent parts of the lungs) and the perfusion (going preferentially to the dependent parts of the lungs). We called this 'shunting'. The shunt is the fraction of the cardiac output not exposed to gas exchange in the pulmonary capillary bed. Although a certain amount of physiological shunting exists (the bronchial circulation, a fraction of the blood returning from the left myocardium and with a possible contribution of dormant arteriovenous intrapulmonary shunts (Eldridge 2004)), in healthy people, when the shunt increases the gas exchange (mainly oxygen entry into the blood vessels) will become suboptimal creating abnormally low levels of blood oxygen concentration (hypoxaemia).
Intraoperative atelectasis is reversible by passive hyperinflation, consisting of maintaining a positive pressure of 20 cmH2O to 25 cmH2O for 10 seconds, therefore Bendixen and colleagues hypothesized that supraphysiological tidal volumes would decrease intraoperative atelectasis and the subsequent increase in intrapulmonary shunt (Bendixen 1963). In their experiment, they included 18 healthy people (aged 24 to 87 years) coming for surgery and requiring muscle paralysis. Participants were divided into two groups, one receiving pure oxygen and halothane 1% while the other group received various proportions of nitrous oxide and oxygen. Pressure‐controlled positive pressure ventilation was provided at a rate between 20 breaths per minute and 25 breaths per minute with a pressure between 15 cmH2O and 20 cmH2O in both groups. Arterial partial pressure of oxygen (PaO2), partial pressure of carbon dioxide (PaCO2) and compliance were measured every 10 to 30 minutes starting after a 10‐second hyperinflation at 20 cmH2O to 25 cmH2O. Compliance (15% decrease) and PaO2 (22% decrease) fell over time. There was a linear relationship between the increase in PaCO2 and the decrease in PaO2, therefore the authors extrapolated that hyperventilation (resulting in lower carbon dioxide tension) would reduce the chances of having atelectasis and hypoxaemia. All their participants were ventilated at approximately the same rate, so they further deduced that the continuous hyperinflation that resulted from larger tidal volumes would be protective against atelectasis and intraoperative intrapulmonary shunt, whereas low tidal volumes would facilitate them. Thus, since the 1960s, clinicians have relied on supraphysiological tidal volumes of 10 mL/kg to 15 mL/kg positive pressure ventilation in the hope of reducing intraoperative atelectasis and hypoxaemia (Bendixen 1963).
Although the time and oxygen inspired fraction dependent formation of atelectasis in healthy people under anaesthesia and ventilated with low tidal volumes (7 mL/kg to 10 mL/kg) (Edmark 2011), and no positive end‐expiratory pressure (PEEP) (Rusca 2003), is now a well established fact (Brismar 1985; Gunnarsson 1991), the strength of the demonstration by Bendixen and colleagues that higher tidal volumes would reduce this phenomenon remains a point of controversy. By favouring oxygen absorption in the capillaries from the areas of low ventilation and perfusion (such as found in the dependent zones of the lungs of healthy anaesthetized people), a high oxygen inspired fraction (FiO2) will hasten the formation of atelectasis (absorption atelectasis) (Edmark 2011). Bendixen and colleagues ventilated half of their participants with 99% oxygen and this confounding factor was not taken into account in the analysis of their results, obtained from samples taken at varying intervals (Bendixen 1963). Furthermore, when PEEP is applied during volume‐controlled mechanical ventilation of people under anaesthesia, a reduction in the formation of atelectasis in the PEEP group is accompanied by a lower PaCO2 despite the use of fixed identical tidal volumes (10 mL/kg at 10 breaths per minute) (Rusca 2003). This suggests that alveolar ventilation may be higher in people with reduced atelectasis, given identical delivered minute‐volume ventilation. Bendixen and colleagues attributed the lower PaCO2 observed in some of their participants to the use of higher tidal volumes during pressure‐controlled ventilation but they did not formally measure the tidal volumes administered (delivered or expired). Thus, a decreased amount of atelectasis (caused by a lower inspired oxygen concentration as an example) in some of the participants in the Bendixen study may have favoured both carbon dioxide elimination and oxygen absorption at the same time. The inverse relationship observed between PaO2 and PaCO2 in their participants may not be a causal relationship but simply two different results of another third factor, hastened formation of atelectasis in people receiving a higher inspired oxygen concentration. Bendixen and colleagues did not therefore produce any clear evidence that higher tidal volumes in anaesthetized people will reduce atelectasis formation.
In 2000, one large randomized trial involving people with acute lung injury reported decreased mortality in people ventilated with 6 mL/kg and a maximal plateau pressure of 30 cmH2O compared to people ventilated with 12 mL/kg and a maximal plateau pressure of 50 cmH2O (31.0% with 6 mL/kg versus 39.8% with 12 mL/kg; P = 0.007) (Acute Respiratory Distress Syndrome Network). Although this trial was prematurely stopped after the enrolment of 861 participants, one subsequent Cochrane Review confirmed that clinical trials on people with acute lung injury showed that a combination of physiological tidal volume (7 mL/kg or less of predicted body weight), sufficient PEEP to prevent alveolar repetitive closing‐opening injury and plateau pressure less than 30 cmH2O improved the outcome with a risk ratio (RR) for mortality at day 28 of 0.74 (95% confidence interval (CI) 0.61 to 0.88) (Petrucci 2013). Based on the findings of the ARMA trial (Acute Respiratory Distress Syndrome Network), clinicians have been inclined to apply these results to people without acute lung injury and have started to use lower tidal volume ventilation intraoperatively. In one large retrospective trial, Levin and colleagues found that in their institution the median tidal volume per kilogram of ideal body weight decreased from 9.0 mL/kg in 2008 to 8.3 mL/kg in 2011 (P < 0.01) (Levin 2014). This newer clinical practice is supported by a multicentre clinical trial performed on people without acute lung injury that included 400 participants and showed that the use of a strategy that included low tidal volumes could reduce the risk of a composite of major pulmonary and extrapulmonary complications occurring within seven days of surgery in people at high or moderate risk of complications and undergoing abdominal surgery lasting two hours or more (Futier 2013). This strategy included tidal volumes of 6 mL/kg to 8 mL/kg of ideal body weight, positive PEEP of 6 cmH2O to 8 cmH2O and recruitment manoeuvres repeated every 30 minutes versus tidal volumes of 10 mL/kg to 12 mL/kg, no PEEP and no recruitment manoeuvres (Futier 2013). A recommendation on the use of lower tidal volume in people without acute lung injury for mechanical ventilation during surgery is, however, still a controversial issue. By using a propensity score analysis on their institutional data for 29,343 participants, Levin and colleagues reported that the use of low intraoperative tidal volume with minimal PEEP (median 4, interquartile range (IQR) 2.2 cmH2O to 5 cmH2O) was associated with an increased risk of 30‐day mortality (Levin 2014). Low tidal volumes of 6 mL/kg to 8 mL/kg of ideal body weight were associated with a significant increase in 30‐day mortality versus tidal volumes of 8 mL/kg to 10 mL/kg of ideal body weight with a hazard ratio of 1.6 (95% CI 1.25 to 2.08; P = 0.0002). In this large retrospective trial, the dose–response curve indicated a threshold tidal volume of 9.7 mL/kg of body weight (Levin 2014). However, it is relevant to note that all their participants were ventilated with a relatively high FiO2. The median FiO2 in the tidal volume 3 mL/kg to 6 mL/kg of body weight group was 0.76 versus 0.73 in the group with a tidal volume of 12 mL/kg to 20 mL/kg (Levin 2014). This is in contrast to Futier and colleague's study where the mean FiO2 used was 0.46 versus 0.47, making the exact contribution of the tidal volume unclear (Futier 2013).
Description of the intervention
We evaluated using low tidal volumes for the intraoperative mechanical ventilation of people without acute lung injury. We defined a low tidal volume as less than 10 mL/kg of predicted body weight per breath (insufflation).
How the intervention might work
Administration of larger tidal volumes requires higher airway positive pressure (Levin 2014). This overpressure distributes preferentially in the more compliant lung zones, therefore alveoli contained in these more compliant lung zones may become overdistended with stretching and sheer forces on the alveolar wall (volutrauma), even possibly leading to disruption of the alveolar wall with air diffusing into the extra‐alveolar tissues (barotrauma). The overall lung damage induced by mechanical ventilation is called ventilator‐induced lung injury (VILI). Increased incidence of pulmonary complications may lead to increased duration of postoperative tracheal intubation, increased rate of infection (ventilator‐associated pneumonia (VAP)) and eventually an increased death rate.
Why it is important to do this review
With improvement of the equipment and the availability of adding intraoperative PEEP adjusted to decrease alveolar closure (Tusman 2014), the relevance of keeping to the clinical practice of using high tidal volumes has been questioned. Conflicting results have, however, been reported with the use of low tidal volumes. Some authors reported that the use of a strategy including low tidal volumes reduced the risk of a composite of major pulmonary and extrapulmonary complications occurring within seven days of surgery in people at high or moderate risk of complications and undergoing abdominal surgery lasting two hours or more (Futier 2013). Others have reported that low tidal volumes with minimal PEEP may increase postoperative mortality (Levin 2014). By summing the evidence from all available trials, it could then perhaps be possible to determine which strategy (low or high tidal volumes) is the most beneficial for everyday clinical practice.
Objectives
To assess the benefit of intraoperative use of low tidal volume ventilation (less than 10 mL/kg of predicted body weight) compared with high tidal volumes (10 mL/kg or greater) to decrease postoperative complications in adults without lung injury.
Methods
Criteria for considering studies for this review
Types of studies
We included all parallel randomized controlled trials (RCTs) that evaluated the effect of low tidal volumes on any of our selected outcomes. We excluded observational studies, quasi‐randomized trials, cross‐over trials and cluster‐randomized trials. We did not exclude any study based on language of publication or publication status.
Types of participants
We included studies performed on adults (aged over 16 years) needing mechanical positive pressure ventilation during their surgery and undergoing any type of open or laparoscopic surgery, elective or emergency, with the exception of participants undergoing surgery with one‐lung ventilation. We included participants managed with laryngeal mask airways or endotracheal tubes and participants ventilated with or without continuous muscle relaxation (infusion or repeated doses throughout the surgery). We excluded studies performed on participants with acute lung injury.
Types of interventions
We included studies where low tidal volumes, defined as less than 10 mL/kg of predicted body weight, in the treatment group were compared to high tidal volumes, defined as 10 mL/kg or greater of the predicted body weight, in the control group. Provided that the tidal volume was measured, we retained studies whether the ventilation was pressure or volume‐controlled and whether or not any other ventilation modalities were added such as PEEP at any level (Barbosa 2014), recruitment manoeuvres or other.
Types of outcome measures
Primary outcomes
Mortality within 30 days after the surgery.
Secondary outcomes
Pneumonia (authors definition) within seven days after the surgery.
Need for postoperative non‐invasive ventilation between discharge from the postoperative care unit and seven days after the surgery.
Need for postoperative invasive ventilation between discharge from the postoperative care unit and seven days after the surgery.
Intensive care unit (ICU) length of stay in days.
Hospital length of stay in days.
Barotrauma, defined as the clinically (or radiologically) diagnosed presence or absence of pneumothorax, pneumomediastinum or subcutaneous emphysema within seven days after the surgery.
Search methods for identification of studies
Electronic searches
The search strategy was developed in consultation with the Information Specialist. We identified RCTs through literature searching with systematic and sensitive search strategies as outlined in Section 6.4 of the Cochrane Handbook of Systematic reviews of Interventions (Higgins 2011). We applied no restrictions to language or publication status. We searched the following databases for relevant trials: Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 5) (Appendix 1), MEDLINE (OvidSP) (from 1946 to May 2017) (Appendix 2), and Embase (OvidSP) (from 1974 to May 2017) (Appendix 3). We looked at PsycINFO (from inception in May 2017) as source of possible grey literature (Appendix 4).
The search was rerun 3 January 2018 with a different search strategy (Appendix 5).
Searching other resources
We searched trial registries ClinicalTrials.gov (www.clinicaltrials.gov), ISRCTN Registry (isrctn.org), UMIN Clinical Trials Registry (www.umin.ac.jp/ctr/index.htm), Australian New Zealand Clinical Trials Registry (www.anzctr.org.au/), Nederlands Trial Register (www.trialregister.nl/), and European Clinical Trials Database (eudract.ema.europa.eu/) for trials in progress in September 2014 and June 2017. We screened the reference lists of all studies retained and of recent meta‐analysis related to the topic during data extraction. We also screened conference proceedings of anaesthesiology societies, published in two major anaesthesiology journals: British Journal of Anaesthesiology (2012 to 2016), andEuropean Journal of Anaesthesiology (2012 to 2017) and the website of the American Society of Anesthesiologists (2012 to 2016).
Data collection and analysis
Selection of studies
Two authors (JG and SK for this update) independently screened the list of all titles and abstracts identified by the search. We (JG and SK) retrieved and independently read any potential articles to determine their eligibility. We resolved discrepancies by discussion; the input of the third author (EAO) was not required. We listed the reasons for exclusions in the Characteristics of excluded studies table.
Data extraction and management
Two authors (JG and SK) independently selected the studies, extracted data (Assessment of risk of bias in included studies; Types of outcome measures; Assessment of heterogeneity), and entered the data in our data extraction sheet. We first entered the site where the study was performed and the date of data collection (to facilitate exclusion of duplicate publications), then whether the study was kept or the reason for rejection. After agreement, one author (JG) entered data and moderators for exploration of heterogeneity into Comprehensive Meta‐analysis. Also, after agreement, the same author (JG) entered our evaluation of the risk of bias into Review Manager 5 (Review Manager 2014). We resolved any disagreements by discussion and the help of the third author (EAO) was not required. We contacted authors to obtain additional information when required. We then transferred data for analysis into Review Manager 5 in the format required, to include the maximal numbers of studies (events and total number of participants for each group; means, standard deviations and number of participants included in each group; or generic inverse variance if necessary). When possible, we entered the data as an intention‐to‐treat (ITT) analysis.
Assessment of risk of bias in included studies
Two authors (JG and SK) independently assessed the quality of the retained studies with the Cochrane 'Risk of bias' tool (Higgins 2011; Review Manager 2014). We resolved any disagreements by discussion. We considered a trial as having a low risk of bias if we assessed all of the following criteria as adequate and at risk of bias if we assessed one or more of the criteria as inadequate. We assessed the risk of bias based on the information presented in the reports, with no assumptions.
Generation of the allocation sequence of the interventions: we considered randomization adequate if it was generated by a computer or random number table algorithm. We judged other processes, such as tossing of a coin, adequate if the whole sequence was generated prior to the start of the trial. We considered the trial as quasi‐randomized if a non‐random system, such as dates, names or identification numbers, was used.
Concealment of allocation: we considered concealment adequate if the process that was used prevented participant recruiters, investigators and participants from knowing the intervention allocation of the next participant to be enrolled in the study. We considered concealment inadequate if the allocation method allowed the participant recruiters, investigators or participants to know the treatment allocation of the next participant to be enrolled in the study.
Blinding of participants and personnel: we considered blinding adequate if the participant and the personnel taking care of the participant were each blinded to the intervention. We considered blinding inadequate if the participants or the personnel were not each blinded to the intervention.
Blinding of outcome assessment: we considered blinding adequate if the outcome assessor was blinded to the intervention. We considered blinding inadequate if the outcome assessor was not blinded to the intervention.
Incomplete outcome data (attrition bias): we considered the trial adequate if all dropouts or withdrawals were accounted for, the number of dropouts was small (less than 20%), similar for both interventions and the reasons for the dropping out of the participants seemed reasonable. We considered the trial inadequate for this specific item if the reasons for dropping out of the participant were not stated or did not sound reasonable, the number was high (20% or greater) or highly different between the groups.
Selective reporting (reporting bias): we considered the trial as low risk of bias if all the measurements stated in the methods section were included in the results and at high risk if only a part of the results mentioned in the methods section were given in the results section.
Any other risk of bias: any other reason that may have influenced the results. We considered an apparent conflict of interest as a risk of bias.
Measures of treatment effect
We gave results as risk ratio (RR) with a 95% confidence interval (CI) for dichotomous data and mean difference with 95% CI for continuous data as far as was feasible. If some of the continuous data were given on different scales, we reported the results as a standardized mean difference (SMD) and 95% CI. For SMD, we considered 0.2 a small effect, 0.5 a medium effect and 0.8 or greater a large effect (Pace 2011). When there was an effect, we calculated a number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) from the odds ratio (EMB Website). When there was no effect, we calculated the optimum information size in order to make sure that there were enough participants included in the retained studies to justify a conclusion on the absence of effect (Pogue 1998; Rollin Brant). We considered a difference of 1% for the mortality rate and 15% (increase or decrease) for the other outcomes as the minimal clinically relevant difference.
Unit of analysis issues
We included only parallel‐group trials. If a study contained more than two groups, we fused the two groups (by using the appropriate formula for adding the standard deviations when required) when we thought that they were equivalent according to the criteria of our protocol (taking our factors for heterogeneity exploration into account) or separated them and split the control group in half if we thought that they were different.
Dealing with missing data
We did not use medians as equivalent to means. Instead, we used the P value and the numbers of participants included in each group to calculate the effect size. We did not use imputed results. We entered data as ITT as far as was feasible. If not, we noted it in other risks of bias and then entered the data on a per protocol basis. We included P values when means and standard deviations were not provided. Authors were contacted to obtain additional information when we were unable to extract data.
Assessment of heterogeneity
We considered clinical heterogeneity before pooling results and examined statistical heterogeneity before carrying out any meta‐analysis. We quantified statistical heterogeneity using the I2 statistic with data entered in the way (benefit or harm) yielding the lowest amount (switching event and non‐event) (Deeks 2002). We qualified the amount as low (25% or less), moderate (25% to 74%) or high (75% or greater) depending of the value obtained for the I2 statistic (Higgins 2003).
Assessment of reporting biases
We examined publication bias with the Duval and Tweedie's trim and fill technique for each outcome (Duval 2000a; Duval 2000b). Publication bias is the risk of bias introduced by the possibility that medical journals publish studies favouring one treatment more often than studies favouring the other. When there is no publication bias and no small‐study effect, if a graph is constructed with either the standard error or the precision (1/standard error) on the y‐axis and the logarithm of the odds ratio on the x‐axis, then studies should be equally distributed on both sides of a vertical line passing through the effect size found (log odds ratio). The entire graph should have the shape of a reversed funnel. The Duval and Tweedie's trim and fill analysis corrects the asymmetry by removing the extremely small studies from the positive side (recomputing the effect size at each iteration until the funnel plot is symmetric around the new effect size). The algorithm then adds the original studies back into the analysis and imputes a mirror image for each. The latter step does not modify the 'new effect size' but corrects the variance that was falsely reduced by the first step. The Duval and Tweedie's trim and fill analysis gives an estimate of what the effect size would be (odds ratio, RR, etc.) if there was no publication bias (Borenstein 2009).
Data synthesis
We analysed the data with Review Manager 5 (Review Manager 2014) and Comprehensive Meta Analysis Version 2.2.044 (www.meta‐analysis.com), with fixed‐effect models for comparisons with a low amount of heterogeneity as assessed by the I2 statistic (less than 25%) or random‐effects models for comparisons containing a moderate or high amount of heterogeneity (I2 statistic 25% or greater) (Higgins 2003). Fixed‐effect and random‐effects models give the same results in the absence of statistical heterogeneity (I2 = 0%). When there is statistical heterogeneity, random‐effects models will usually widen the CI, thus decreasing the chance of finding an effect when there is none. They may, however, increase the weight of smaller studies. We presented the characteristics of included and excluded studies in tables. We presented the 'Risk of bias' assessment in a 'Risk of bias' graph. We presented results for each comparison as forest plots when appropriate. For comparisons with only one study available, or that still included a moderate or high level of heterogeneity after heterogeneity exploration, we provided the results as a narrative review.
Subgroup analysis and investigation of heterogeneity
We explored any amount of heterogeneity but we focused more specifically on comparisons with significant heterogeneity (I2 greater than 25%) (Higgins 2003). We explored heterogeneity using Egger's regression intercept (to assess the possibility of a small‐study effect; Rucker 2011), visual inspection of the forest plots with studies placed in order according to a specific moderator, subgroupings (categorical moderators) or meta‐regressions (continuous moderators). Factors that we considered in the heterogeneity exploration were: exact tidal volume (or less than 6 mL/kg, 6 mL/kg to less than 8 mL, 8 mL/kg to less than 10 mL/kg, 10 mL/kg to less than 12 mL/kg, 12 mL/kg to less than 15 mL/kg, 15 mL/kg or greater); pressure versus volume‐controlled ventilation; presence or absence of chronic obstructive pulmonary disease or emphysema; peak and plateau inspiratory pressure (maximal measured); PEEP (amount, less than 5 cmH2O versus 5 cmH2O or greater, and technique used to determine the level); recruitment manoeuvres; inspired oxygen concentration; use of nitrous oxide; type and site of surgery (possibility of decreased chest compliance or increased intra‐abdominal pressure (laparoscopic surgery), or both); elective versus emergent surgery; length of surgery; body mass index (BMI); use of cardiopulmonary bypass; use of epidural analgesia; surgical position; tidal volume adjusted for predicted body weight or not; use of neuromuscular blocking agents or not; tracheal tube versus laryngeal mask airway; and age.
Sensitivity analysis
We had planned a sensitivity analysis (based mainly on the 'Risk of bias' assessment: allocation concealment and blinding of the assessor) but we did not perform this as we considered no study to be of completely unacceptable quality and all the statistical heterogeneity could be explained based on clinical differences between the studies.
'Summary of findings' table and GRADE
We judged the quality of the body of evidence according to the system developed by the GRADE working group and presented this in a 'Summary of findings' table (ims.cochrane.org/revman/gradepro), for each outcome: mortality, pneumonia, need for postoperative non‐invasive ventilation, need for postoperative invasive ventilation, ICU length of stay, hospital length of stay and barotrauma. Briefly, the study design comes first: RCTs are moderate‐ or high‐quality evidence (Guyatt 2011). The evidence is lower quality if the risk of bias of included studies is high or very high, there is some heterogeneity (I2 75% or greater without an explanation), the demonstration of effect is indirect, there is imprecision in the results (95% CI around the effect size) or there is a risk of publication bias (classical fail‐safe number or funnel plot). When the quality of the body of evidence is high, further research is very unlikely to change our confidence in the estimate of effect. When the quality is moderate, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. When the quality is low, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. When the quality is very low, any estimate of effect is very uncertain (Guyatt 2008).
Results
Description of studies
Results of the search
The flow diagram of the study selection process is provided in Figure 1.
1.
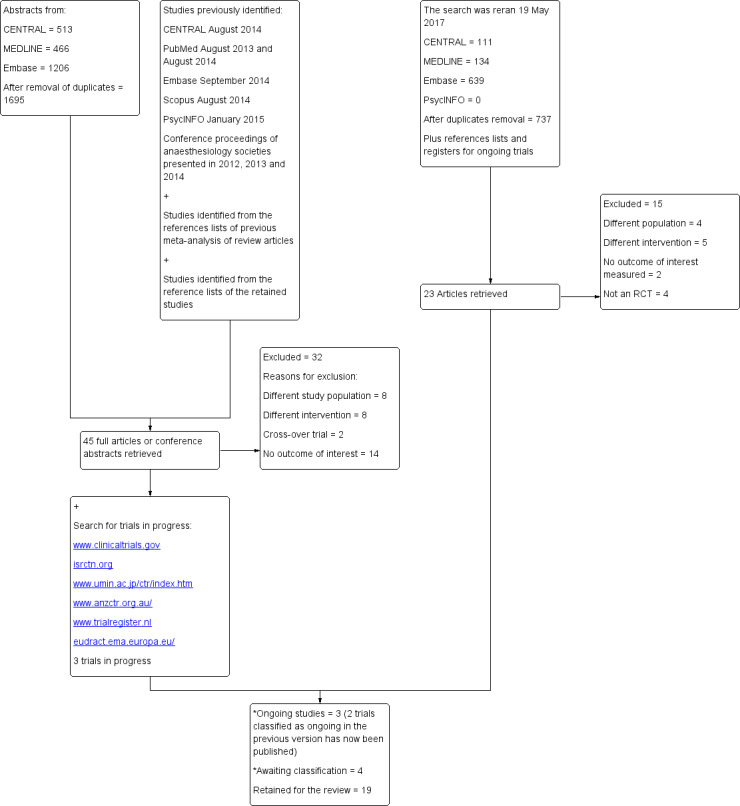
Diagram of the flow search. RCT: randomized controlled trial.
The original search (2014) performed for the first published version (2015) was rerun 19 May 2017.
*A search with a modified strategy was run 8 January 2018: one trial classified as ongoing has been published is now awaiting classification. We found two new trials published between May 2017 and January 2018 and added them to the list of studies awaiting classification.
In the previous version (Guay 2015), we had 12 included trials, 1 trial awaiting classification, 3 ongoing trials and 32 excluded trials.
When we reran the search 19 May 2017, we identified 884 potentials titles (737 after duplicates removal). From titles/abstracts, 23 articles were retrieved for further evaluation of which 15 trials were excluded: different study population (N = 4), different intervention (N = 5), no outcome of interest measured (N = 2) or not a RCT (N = 4). Please see Characteristics of excluded studies for further details.
We now have 19 included trials, 47 excluded trials, 4 trials awaiting classification and 3 ongoing trials.
Included studies
Population and settings
We included 19 studies with 1548 participants equally distributed between the two groups: 776 participants in the low tidal volume groups and 772 in the high volume groups. The mean age of the participants included in the studies varied from 35.5 to 73.0 years. Eight studies scheduled participants for abdominal surgery (Choi 2006; Chugh 2012; Futier 2013; Kuzkov 2016; Park 2016a; Sato 2016; Treschan 2012; Weingarten 2010), eight studies for heart surgery (Chaney 2000; Koner 2004; Sundar 2011; Zupancich 2005), four studies for spinal surgery (Ge 2013; Memtsoudis 2012; Soh 2018; Xiong 2016), and one study for knee surgery (Fernandez‐Bustamante 2014). One study included participants undergoing pulmonary thromboendarterectomy (Bates 2015), and one study included mixed surgeries (Shen 2015). One study performed laparoscopic abdominal surgery for 42.5% of the participants (Futier 2013), and another study for all participants (Park 2016a). One study used epidural anaesthesia/analgesia for 40% of the participants (Futier 2013), one study for 82.2% of participants (Treschan 2012), and three studies for all participants (Choi 2006; Kuzkov 2016; Sato 2016).
Interventions and comparators
Low tidal volumes varied from 6 mL/kg to 8.1 mL/kg while high tidal volumes varied from 10 mL/kg to 12 mL/kg except for one study (Bates 2015), where the mean measured delivered volume of the high volume group was 9.6 mL/kg (target 10 mL/kg). The FiO2 administered during the surgery varied from 0.3 to 1.0. Eleven studies administered PEEP varying from 3 cmH2O to 12 cmH2O in the low tidal groups only (Choi 2006; Chugh 2012; Futier 2013; Ge 2013; Memtsoudis 2012; Park 2016a; Shen 2015; Soh 2018; Weingarten 2010; Xiong 2016; Zupancich 2005), four studies administered PEEP to both groups (Chaney 2000; Fernandez‐Bustamante 2014; Sundar 2011; Treschan 2012), and one study administered PEEP to the low tidal volume group and half of the participants of the high tidal volume group (Koner 2004). For Bates 2015, all participants received PEEP according to the recommendations of the ARDS Network (Acute Respiratory Distress Syndrome Network). Six studies used recruitment manoeuvres in the intervention group (Futier 2013; Ge 2013; Shen 2015; Soh 2018; Weingarten 2010; Xiong 2016), one study in the high tidal volume group (Park 2016a), and three studies in both groups (Bates 2015; Sato 2016; Treschan 2012). Nine studies did not use, or did not mention using, recruitment manoeuvres (Chaney 2000; Choi 2006; Chugh 2012; Fernandez‐Bustamante 2014; Koner 2004; Kuzkov 2016; Memtsoudis 2012; Sundar 2011; Zupancich 2005).
Funding sources
Four studies were supported by charitable funding (Fernandez‐Bustamante 2014; Memtsoudis 2012; Park 2016a; Sato 2016). Three studies received governmental support (Kuzkov 2016; Xiong 2016; Zupancich 2005). Seven studies were supported by institutional/departmental resources only (Chaney 2000; Choi 2006; Shen 2015; Soh 2018; Sundar 2011; Treschan 2012; Weingarten 2010). One study was supported by a pharmaceutical company (Koner 2004). For one study, some authors declared consultant fees or travel expenses, or both, from industry (Futier 2013). Three studies did not mention source of funding (Bates 2015; Chugh 2012; Ge 2013).
Setting
Trials were conducted in China (three; Ge 2013; Shen 2015; Xiong 2016), France (one; Futier 2013), Germany (one; Treschan 2012), India (one; Chugh 2012), Italy (one; Zupancich 2005), Japan (one; Sato 2016), Russia (one; Kuzkov 2016), South Korea (two; Park 2016a; Soh 2018), The Netherlands (one; Choi 2006), Turkey (one; Koner 2004) or the USA (six; Bates 2015; Chaney 2000; Fernandez‐Bustamante 2014; Memtsoudis 2012; Sundar 2011; Weingarten 2010).
Excluded studies
We excluded 47 studies. See the Characteristics of excluded studies table for details of the reasons for exclusion.
Twelve trials studied a different population (Determann 2010; Kang 2014; Kim 2012; Lee 1990; Lin 2008; Mascia 2010; Maslow 2013; Michelet 2006; Pinheiro 2010; Weismann 2010; Wrigge 2005; Yang 2011). Thirteen trials studied a different intervention (Akca 2013; Blum 2013; Ding 2016; Ferrando 2015; Hosten 2017; Jain 2016; Liu 2016; Reis Miranda 2005a; Reis Miranda 2005b; Satoh 2012; Severgnini 2013; Tugrul 1998; Tusman 1999). There were two cross‐over trials (Tweed 1991; Visick 1973). Sixteen trials did not measure any outcome of interest for this review (Arora 2017; Baki 2014; Cai 2007; Clarke 1998; Cui 2015; Ela 2014; Gong 2007; Jiang 2007; Kaisers 2009; Kanaya 2011; Kokulu 2015; Shin 2010; Thornton 1998; Wrigge 2000; Wrigge 2004; Zhan‐fang 2010). Four trials were classified as not randomized (Gajic 2004; Gajic 2005; Lellouche 2012; Wolthuis 2007).
Studies awaiting classification
Four trials are awaiting classification (Studies awaiting classification table). We were unable to access the report of one trial (Moussa 2003). This study, which included 20 participants, could contain data for ICU and hospital lengths of stay and possibly on pulmonary complications. We reran the search 3 January 2018 and identified three potential new trials (Asida 2015; Haliloglu 2017; Tang 2017). One trial was previously included as ongoing trial and has now been published (Asida 2015). Asida 2015 contains data on length of hospital stay. This trial will be evaluated at the next review update. The two other trials were published between May 2017 and January 2018 (Haliloglu 2017; Tang 2017). They do not contain any outcomes of interest to this review. They will also be formally evaluated at the next review update.
Ongoing studies
We found three ongoing RCTs fitting our inclusion criteria (Characteristics of ongoing studies table). One study is collecting data on pulmonary complications (including pneumonia and need for ventilatory support), ICU and hospital lengths of stay, and 30‐day mortality in participants undergoing major abdominal surgery (ACTRN12614000790640). One trial is collecting data on postoperative mortality in participants undergoing surgery of four hours' duration or more (NCT01003730). One trial is collecting data on pneumonia in adults with a high BMI undergoing laparoscopic surgery (NCT03157479).
Risk of bias in included studies
The risk of bias of the included studies is shown in the Characteristics of included studies table and Figure 2. Figure 3 summarizes the percentage of studies for which we judged each item evaluated as at low, high or unclear risk of bias. Futier 2013, the largest trial available, which included 400 participants, and Memtsoudis 2012 were the studies with the lowest risk of bias. For Futier 2013, potential financial conflict of interest was mentioned (consultant fees and travel expenses for some of the authors), however we do not think that this has affected the results because low or high tidal volumes may be administered with any mechanical ventilators currently available on the market. Therefore, we did not judge this potential conflict of interest as a risk of bias.
2.
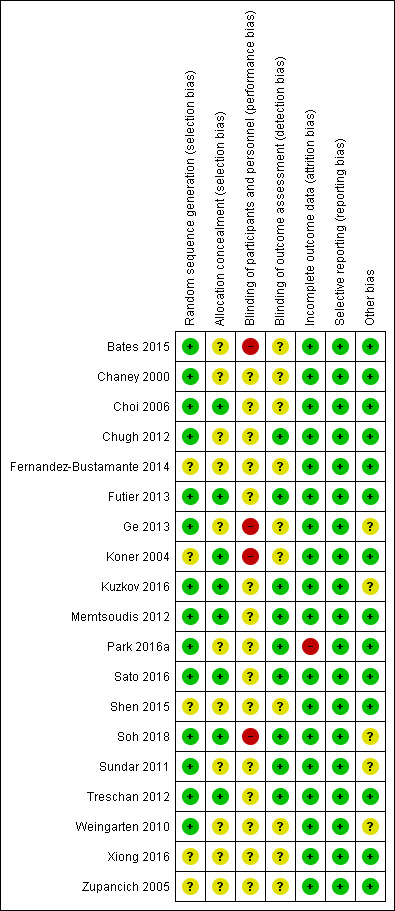
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
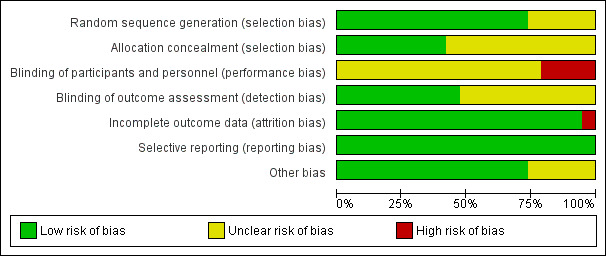
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We judged allocation concealment as unclear for more than 50% of the studies (Figure 3).
Blinding
For more than 50% of the studies, there was uncertainty regarding blinding of the outcome assessor to the treatment group allocation (Figure 3). We judged absence of blinding of participants, personnel and outcomes assessors as a high risk or unclear for all studies.
Incomplete outcome data
We judged most studies as adequate for possible attrition bias (Figure 3).
Selective reporting
All results mentioned in the methods sections were provided (low risk of reporting bias).
Other potential sources of bias
We judged more than 50% of the studies as free of other possible bias (Figure 3). We judged four studies as unclear for this item because of an imbalance in the characteristics of the groups. For Kuzkov 2016, smoking was significantly lower in the high tidal volume group (P = 0.26). For Soh 2018, participants in the low tidal volume group were significantly older than those in the high tidal volume group. For Sundar 2011, the incidence of postoperative complete heart block was higher in the high volume group. For Weingarten 2010, there was a higher proportion of participants with coronary artery disease in the low volume group. Ge 2013 was also judged as unclear for other bias by the the two Chinese reviewers who helped us with this trial. Some outcome definitions were unclear for Ge 2013 (pneumonia).
Overall, we considered the quality of the included trials sufficient to allow us to draw valid conclusions.
Effects of interventions
See: Table 1
Primary outcomes
Mortality
Twelve studies including 1207 participants reported data for mortality within seven days (Koner 2004), during hospital stay (Bates 2015; Chaney 2000; Choi 2006; Chugh 2012; Treschan 2012; Weingarten 2010; Zupancich 2005), within 28 days (Kuzkov 2016; Sundar 2011), or within 30 days (Futier 2013; Shen 2015). We did not find a difference in mortality between low and high tidal volume groups (RR 0.80, 95% CI 0.42 to 1.53; I2 = 0%; Analysis 1.1). Egger's regression intercept showed no evidence of small‐study effect. The impact of asymmetry in the funnel plot led to a trim and fill estimate of RR 0.81 (95% CI 0.42 to 1.57; Figure 4). Based on a basal rate of mortality of 3.0%, such as found in the control groups of the studies included here (Bates 2015; Chaney 2000; Choi 2006; Chugh 2012; Futier 2013; Koner 2004; Kuzkov 2016; Shen 2015; Sundar 2011; Treschan 2012; Weingarten 2010; Zupancich 2005), 32,948 participants (16,474 per group) would be required in a large trial to eliminate a 15% difference (α = 0.05; β = 0.2; 1‐sided test).
1.1. Analysis.
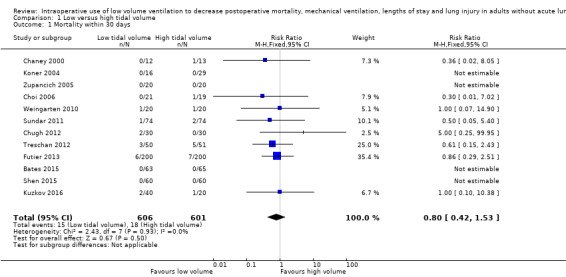
Comparison 1 Low versus high tidal volume, Outcome 1 Mortality within 30 days.
4.
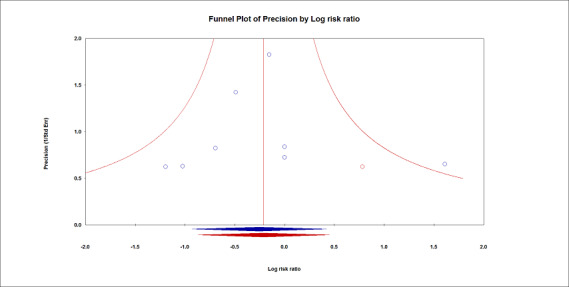
Mortality.
Duval and Tweedie's trim and fill analysis.
Actual results displayed in blue. Results corrected for the possibility of a publication bias displayed in red.
The impact of asymmetry in the funnel plot led to a trim and fill estimate that would not change the conclusion, that is, there would still not be a difference for mortality between the two interventions (red lozenge).
For mortality, we downgraded the level of evidence by one level due to the risk of bias because there was uncertainty around allocation concealment in more than 50% of the studies. There was no heterogeneity (I2 = 0%). We used direct comparisons only and this is not a surrogate marker. We downgraded by one level for imprecision because the optimum information size was not achieved. Correcting for the possibility of publication bias would not change the conclusion (Figure 4). We rated the quality of the evidence as low (Table 1).
Secondary outcomes
Pneumonia
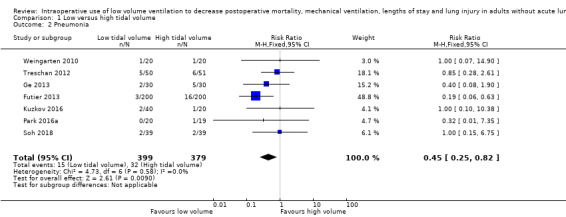
Based on seven studies, which included 778 participants undergoing abdominal surgery (Futier 2013; Kuzkov 2016; Park 2016a; Treschan 2012; Weingarten 2010), or spinal surgery (Ge 2013; Soh 2018), we found a difference in the risk of postoperative pneumonia between the two groups in favour of low tidal volume (RR 0.45, 95% CI 0.25 to 0.82; I2 = 0%; Analysis 1.2). Only two trials provided a clear definition (Table 2) (Futier 2013; Shen 2015). Egger's regression intercept showed no evidence of a small‐study effect. The impact of asymmetry in the funnel plot leads to a trim and fill estimate of RR 0.47 (95% CI 0.26 to 0.85; Figure 5). Based on a basal rate of 8.5%, the NNTB to obtain one more beneficial effect was 24 (95% CI 16 to 160). In a large trial, 3866 (1933 per group) participants would be required to eliminate a 25% difference (α = 0.05; β = 0.2; 1‐sided test).
1.2. Analysis.
Comparison 1 Low versus high tidal volume, Outcome 2 Pneumonia.
1. Diagnostic criteria for pneumonia.
| Study | Definition | Number of participants |
| Futier 2013 | Defined according to CDC criteria Pneumonia was suspected upon the presence of new or progressive (or both) pulmonary infiltrates on chest X‐ray plus ≥ 2 of the following criteria:
|
400 |
| Ge 2013 | Bronchitis | 60 |
| Kuzkov 2016 | Plain chest X‐ray performed as a standard procedure at 24 hours of the postoperative period in the semi‐recumbent position; the films were interpreted by an independent specialist. In cases when postoperative pulmonary complications (e.g. atelectasis, pleuritis, nosocomial pneumonia, etc.) were suspected, chest X‐ray or computed tomography was performed within the period of observation up to day 28 on request either in the intensive care unit or in the radiology department. | 60 |
| Park 2016a | Postoperative chest images were compared to the preoperative ones and interpreted by the blinded radiologist at immediately after operation, 1 and 2 days after surgery. | 40 |
| Shen 2015 | Pulmonary infection defined as new or progressive exudation on chest X‐ray combined with ≥ 2 of the following criteria: body temperature ≥ 38.5 °C or < 36 °C; WBC count ≥ 12,000/mm3 or < 4000/mm3; purulent sputum, coughing or difficult breathing | 120 |
| Soh 2018 | Chest X‐ray; complete blood count; symptoms including dyspnoea, cough, and the presence of secretions; and modified clinical pulmonary infection score were assessed on the day of surgery, postoperative day 1 and 3, as clinically needed, or a combination of these Pneumonia defined as Futier 2013 |
78 |
| Treschan 2012 | Pneumonia | 101 |
| Weingarten 2010 | Pneumonia | 40 |
CDC: Centers for Disease Control and Prevention; mm3: cubic millilitre; WBC: white blood cells.
5.
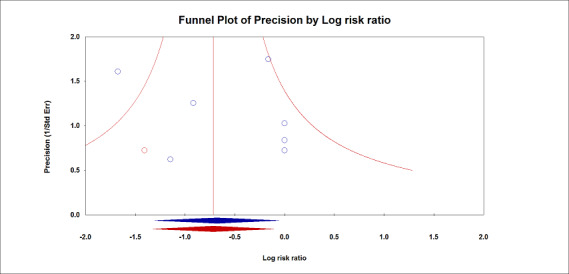
Pneumonia.
Duval and Tweedie's trim and fill analysis.
Actual results displayed in blue. Results corrected for the possibility of a publication bias displayed in red.
The impact of asymmetry in the funnel plot led to a trim and fill estimate that would not change the conclusion, that is, low tidal volumes would still decrease the incidence of pneumonia (red lozenge).
For pneumonia, we did not downgrade for risk of bias because allocation concealment and blinding of the outcome assessor were adequate for more than 50% of the studies. There was no heterogeneity (I2 = 0%). We downgraded by one level for imprecision because the optimum information size was not achieved. Correcting for the possibility of publication bias would not change the conclusion (Figure 5). We rated the quality of the evidence as moderate (Table 1).
Need for postoperative non‐invasive ventilation
Three studies including 506 participants reported need for postoperative non‐invasive ventilation between discharge from the postoperative care unit and seven days after the surgery (Fernandez‐Bustamante 2014; Futier 2013; Soh 2018). The RR was 0.31 (95% CI 0.15 to 0.64; Analysis 1.3). Criteria for the use of non‐invasive ventilation were not defined (Table 3). Based on a basal rate of 11.4%, the NNTB to obtain one more beneficial effect was 13 (95% CI 11 to 24). In a large trial, 2734 (1367 per group) participants would be required to eliminate a 25% difference (α = 0.05; β = 0.2; 1‐sided test).
1.3. Analysis.
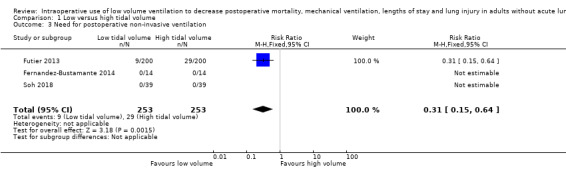
Comparison 1 Low versus high tidal volume, Outcome 3 Need for postoperative non‐invasive ventilation.
2. Additional information on the included studies.
| Study | Criteria for extubation | ICU ventilation | Criteria for non‐invasive ventilation | Criteria for invasive ventilation |
| Bates 2015 | Once participants were titrated down to an FiO2 ≤ 0.5 and PEEP 5 cmH2O, they were assessed twice daily with spontaneous breathing trials. Participants were considered ready for extubation from a pulmonary mechanics standpoint if their rapid shallow breathing index was < 105 for 30 minutes. |
Same parameters for the first 3 after surgery if required | Unspecified | All participants admitted to ICU and kept on mechanical ventilation through an endotracheal tube after surgery |
| Chaney 2000 | Normal ICU protocol: appropriate sensorium, normothermia, haemodynamic stability, adequate pulmonary function (PaO2 > 60 mmHg with FiO2 0.4), adequate urine output and minimal chest tube output | Same mode of ventilation for the first hour after surgery, then tidal volume 8 mL/kg, rate 10/minute, FiO2 1.0 and PEEP 5 cmH2O for all participants | Unspecified | Unspecified |
| Choi 2006 | Unspecified | If the surgical procedure exceeded 5 hours, anaesthesiologists were allowed to change the ventilation strategy thereafter | Unspecified | Unspecified |
| Chugh 2012 | Unspecified | Unspecified | Unspecified | Unspecified |
| Fernandez‐Bustamante 2014 | Unspecified | Unspecified | Unspecified | Unspecified |
| Futier 2013 | Recovery of a spontaneous ventilation with an expired tidal volume 5–8 mL/kg, respiratory rate 12–25 breaths/min, absence of residual neuromuscular blockade (assessed by a T4/T1 ratio ≥ 90%), peripheral oxygen saturation ≥ 95%, stable haemodynamics and body temperature ≥ 36°C | Unspecified | Unspecified | Unspecified |
| Ge 2013 | Unspecified | Unspecified | Unspecified | Unspecified |
| Koner 2004 | Unspecified | Unspecified | Unspecified | Unspecified |
| Kuzkov 2016 | The criteria for discontinuation of respiratory support were as follows: the ability to tolerate 30 minutes of spontaneous breathing trial via the pressure support ventilation with pressure support level of 6–8 cmH2O, PaO2/FiO2 > 200 mmHg, spontaneous minute volume < 10 L/min, and respiratory rate < 30/minute (frequency/tidal volume < 65 1/L and tidal volume > 6 mL/kg predicted body weight) as well as normal body temperature, no obvious bleeding or anaemia, haemodynamic stability and adequate analgesia | Unspecified | Unspecified | Unspecified |
| Memtsoudis 2012 | Unspecified | Unspecified | Unspecified | Unspecified |
| Park 2016a | Unspecified | None of the participants required postoperative ventilatory assistance | Unspecified | Unspecified |
| Sato 2016 | Unspecified | All participants were extubated in the operating room and were spontaneously breathing when they arrived at the postanaesthesia care unit | Unspecified | Unspecified |
| Shen 2015 | Unspecified | Unspecified | Unspecified | Unspecified |
| Soh 2018 | Unspecified | Unspecified | Unspecified | Unspecified |
| Sundar 2011 | Awake status (Riker Sedation‐Agitation Scale score of 3 or 4), haemodynamic stability (minimal doses of nitroglycerine or phenylephrine), and adequate gas exchange (PaCO2 100 mmHg, FiO2 0.4, PEEP 5 cmH2O) Participants were then placed in protocol sequence; they were placed on pressure support ventilation, assessed using the rapid shallow breathing index on PEEP, receiving pressure support levels of 5 cmH2O, followed by a spontaneous breathing trial of 30 min. Participants who passed this sequence were then extubated | Study ventilator settings were applied immediately after induction of general anaesthesia and continued throughout surgery and the subsequent ICU stay | Unspecified | Unspecified |
| Treschan 2012 | Unspecified | Mechanical ventilation of participants who were transferred intubated to the ICU was continued according to group assignment under the discretion of the intensivist in charge | Unspecified | Unspecified |
| Weingarten 2010 | Unspecified | Unspecified | Unspecified | Unspecified |
| Xiong 2016 | Unspecified | Unspecified | Unspecified | Unspecified |
| Zupancich 2005 | Participants were extubated when haemodynamically stable, fully rewarmed, awake, without surgical bleeding and with optimal blood gases | After chest closure, participants were transferred to the ICU and ventilated, with the ventilatory pattern selected randomly | Unspecified | Unspecified |
Prophylactic use of non‐invasive ventilatory support was not mentioned in any of the studies.
cmH2O: centimetres of water; FiO2: inspired fraction of oxygen; ICU: intensive care unit; min: minute; mL/kg: millilitre per kilogram of body weight; mmHg: millilitre of mercury; n: number of participants; PaCO2: arterial partial pressure in carbon dioxide; PaO2: arterial partial pressure in oxygen; PEEP: positive end‐expiratory pressure.
For non‐invasive ventilatory support, we did not downgrade for risk of bias because allocation concealment and blinding of the outcome assessor were adequate for more than 50% of the studies. Heterogeneity and publication bias could not be assessed. We also downgraded the quality of evidence by one level for imprecision based since the optimum information size was not achieved. We rated the quality of the evidence as moderate (Table 1).
Need for postoperative invasive ventilation
Eleven studies, including 957 participants, found low tidal volumes during surgery decreased the need for postoperative invasive ventilatory support (RR 0.33, 95% CI 0.14 to 0.77; I2 = 0%; Analysis 1.4) (Chaney 2000; Choi 2006; Fernandez‐Bustamante 2014; Futier 2013; Koner 2004; Memtsoudis 2012; Park 2016a; Sato 2016; Soh 2018; Sundar 2011; Treschan 2012). Egger's regression intercept showed no evidence of small‐study effect. Duval and Tweedie's trim and fill analysis showed no evidence of publication bias. Based on a basal rate of 3.9%, the NNTB would be 39 (95% CI 30 to 166). In a large trial, 8574 (4287 per group) participants would be required to eliminate a 25% difference (α = 0.05; β = 0.2; 1‐sided test).
1.4. Analysis.
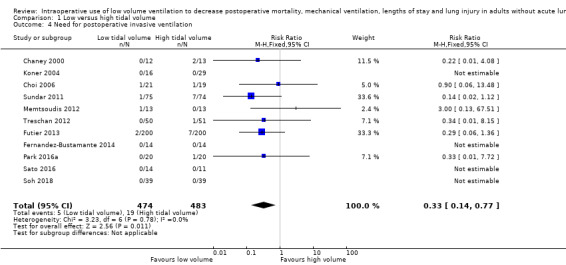
Comparison 1 Low versus high tidal volume, Outcome 4 Need for postoperative invasive ventilation.
For invasive ventilatory support, we did not downgrade for risk of bias because allocation concealment and blinding of the outcome assessor were adequate for more than 50% of the studies. There was no heterogeneity (I2 = 0%) or evidence of publication bias. We downgraded the quality of evidence by one level for imprecision since optimum information size was not achieved. We rated the quality of the evidence as moderate (Table 1).
Intensive care unit length of stay
Based on five studies, which included 898 participants, we found no difference in the ICU length of stay (SMD –0.06 days, 95% CI –0.22 to 0.10; I2 = 33%; random‐effects model; Analysis 1.5) (Bates 2015; Futier 2013; Shen 2015; Sundar 2011; Treschan 2012). Egger's regression intercept showed no evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis showed no evidence of publication bias. The effect size was inversely correlated with the volume administered in the low volume groups (Figure 6; P = 0.02; i.e. the lower the tidal volume, the greater the benefit). Values entered for the meta‐regression were: volume of 6.500 mL/kg (actual volume delivered) and SMD –0.015 days (95% CI –0.362 to 0.331) for Bates 2015, volume of 6.400 mL/kg (actual volume delivered) and SMD –0.055 days (95% CI –0.251 to 0.141) for Futier 2013, volume of 6.020 mL/kg (actual volume delivered 364.2 mL and mean weight predicted body weight of the low volume group 60.5 kg) and SMD –0.344 days (95% CI –0.704 to 0.017) for Shen 2015, volume 6.200 mL/kg (postintubation) and SMD –0.154 days ( 95% CI –0.475 to 0.168) for Sundar 2011, and volume 6.700 mL/kg (actual volume delivered) and SMD 0.302 days (95% CI –0.090 to 0.694) for Treschan 2012.
1.5. Analysis.
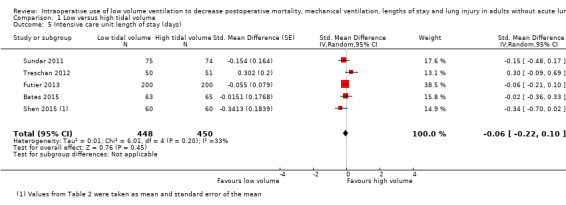
Comparison 1 Low versus high tidal volume, Outcome 5 Intensive care unit length of stay (days).
6.
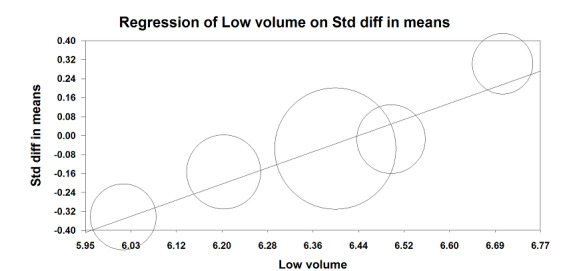
Meta‐regression.
Intensive care unit length of stay versus low tidal volume values.
The effect size was inversely correlated with the tidal volume administered in the low tidal volume groups. P = 0.02
For ICU length of stay, we downgraded the level of evidence by one level because allocation concealment was uncertain in more than 50% of the studies. We also downgraded by one level for heterogeneity (I2 = 33%). We used direct comparisons only and this is not a surrogate marker. We found no evidence of imprecision or publication bias. We also found no evidence for a large effect size or confounding factors justifying upgrading. We did not upgrade for dose–response effect as our meta‐regression (Figure 6), contradicts an absence of effect. An effect might have been seen if the limit for qualifying as a low tidal volume had been fixed at a lower value. We rated the quality of the evidence as low (Table 1).
Hospital length of stay
Based on 14 studies, which included 1297 participants, hospital length of stay might have been slightly reduced in the low tidal volume groups (SMD –0.15 days (95% CI –0.29 to 0.00; I2 = 27%; random‐effects model; Analysis 1.6) (Bates 2015; Chaney 2000; Chugh 2012; Fernandez‐Bustamante 2014; Futier 2013; Koner 2004; Kuzkov 2016; Park 2016a; Sato 2016; Soh 2018; Shen 2015; Sundar 2011; Treschan 2012; Weingarten 2010). Egger's intercept showed no evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis showed no evidence of publication bias. Subgrouping showed that hospital length of stay was reduced when PEEP was used for participants of the low tidal group only (SMD –0.23 days, 95% CI –0.38 to –0.08; I2 = 0%), but not if PEEP was used for all participants (SMD –0.08 days, 95% CI –0.33 to 0.18; I2 = 46%; Analysis 1.6). Low tidal volumes also reduced hospital length of stay when recruitment manoeuvres were used for participants of the low tidal group only (SMD –0.25 days, 95% CI –0.41 to –0.09; I2 = 0%) or were not used (SMD –0.26 days, 95% CI –0.50 to –0.02; I2 = 14%), but not when recruitment manoeuvres were used for all participants (SMD 0.16 days, 95% CI –0.09 to 0.40; I2 = 0%; Analysis 1.7). Based on a trial with a typical standard deviation and low risk of bias (SD in the control group 5.6 days), the difference would be equivalent to 0.8 days or 19.2 hours (Sato 2016).
1.6. Analysis.
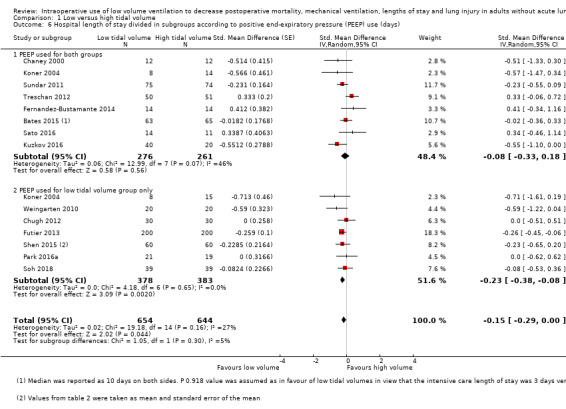
Comparison 1 Low versus high tidal volume, Outcome 6 Hospital length of stay divided in subgroups according to positive end‐expiratory pressure (PEEP) use (days).
1.7. Analysis.
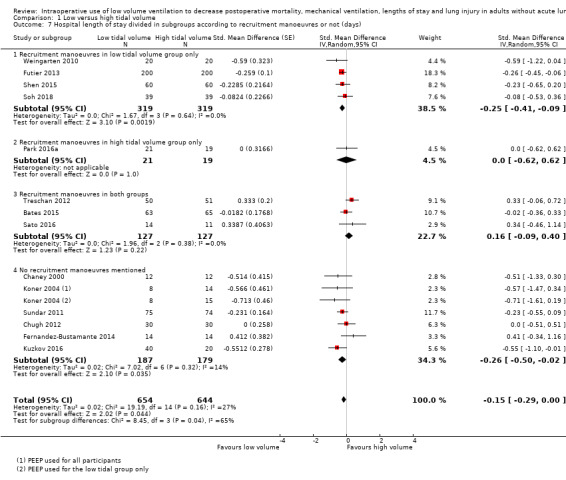
Comparison 1 Low versus high tidal volume, Outcome 7 Hospital length of stay divided in subgroups according to recruitment manoeuvres or not (days).
For hospital length of stay, we downgraded the level of evidence for risk of bias by one level due to uncertainty about allocation concealment for more than 50% of the included studies. We also downgraded on the basis of heterogeneity (I2 = 27%). We did not downgrade for indirectness, imprecision or publication bias. We rated the quality of the evidence as low (Table 1).
Barotrauma
Based on five studies, which included 708 participants (Bates 2015; Futier 2013; Park 2016a; Treschan 2012; Weingarten 2010), two of which contain no events (Park 2016a; Weingarten 2010), we found no difference in the risk of pneumothorax (RR 1.77, 95% CI 0.52 to 5.99; I2 = 0%; Analysis 1.8). Egger's regression intercept showed no evidence of small‐study effect. Duval and Tweedie's trim and fill analysis showed no evidence of publication bias. From a basal rate of 1.1%, 29,606 participants (14,803 per group) would be required to eliminate a difference of 25% (α = 0.05; β = 0.2; 1‐sided test).
1.8. Analysis.
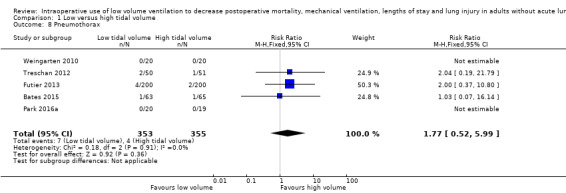
Comparison 1 Low versus high tidal volume, Outcome 8 Pneumothorax.
We downgraded the level of evidence due to the risk of bias by one level on the basis of uncertainty about allocation concealment for more than 50% of the studies. There was no heterogeneity (I2 = 0%), no evidence of publication bias and this is not a surrogate marker. We downgraded the quality of evidence by two levels for imprecision on the basis of a very wide CIs (95% CI 0.52 to 5.99). We judged the quality of the evidence for this outcome as very low (Table 1).
We found no other mention of any other type of barotrauma found in any of the 19 included studies (Bates 2015; Chaney 2000; Choi 2006; Chugh 2012; Fernandez‐Bustamante 2014; Futier 2013; Ge 2013; Koner 2004; Kuzkov 2016; Memtsoudis 2012; Park 2016a; Sato 2016; Shen 2015; Soh 2018; Sundar 2011; Treschan 2012; Weingarten 2010; Xiong 2016; Zupancich 2005).
Discussion
Summary of main results
We did not find a difference in mortality within 30 days after surgery (low‐quality evidence); however, the number of participants included in the present meta‐analysis represented less than 10% of the optimum information size for this outcome. From the data available, a 20% reduction could not be excluded. Therefore, more data will be required before a definitive conclusion can be drawn on the effect of low tidal volume on perioperative death. We found a difference for the risk of pneumonia at zero to seven days after surgery (moderate‐quality evidence). Although a clear definition was not provided for all trials, reducing the risk of pneumonia with low tidal volumes make sense as this modality would reduce the need for ventilatory assistance and therefore promote better mobility and reduce VILI. However, this conclusion may change with additional trials.
Low tidal volumes reduce the need for non‐invasive and invasive ventilatory support (moderate‐quality evidence for both outcomes). However, there were no clear criteria used to apply either of those two treatments (Table 3). Furthermore, no studies used prophylactic non‐invasive ventilatory support to help prevent tracheal reintubation and invasive ventilatory support after surgery. Prophylactic non‐invasive ventilatory support may help decrease the need for invasive ventilatory support, at least in people at high risk of developing postoperative pulmonary complications after cardiothoracic surgery (Olper 2013).
We did not find a difference for ICU length of stay (low‐quality evidence); however, this result contained a moderate amount of heterogeneity. Our data suggested that the effect might have been higher by further reducing the volume of gas insufflated (Figure 6). When we wrote the protocol for this review, the cut‐off limit between high and low tidal volume was fixed at 10 mL/kg of ideal body weight (Nguyen 2014). This was based on the findings of Levin 2014, a large retrospective trial where low tidal volumes increased mortality rate. In Levin 2014, a tidal volume of 9.7 mL/kg of body weight was found as the cut‐off point differentiating between low tidal volumes (increasing 30‐day mortality rate) and high tidal volumes. Our data seemed to indicate that reducing the tidal volume to 6 mL/kg may further increase the benefit of using lower tidal volume (Figure 6). This may have to be further evaluated. We found two studies comparing two different values of "low tidal volumes" (Ding 2016; Severgnini 2013), and evaluating our clinical outcomes after the surgery. Ding 2016 compared a protective strategy including tidal volumes of 6 mL/kg coupled with PEEP at 5 cmH2O and recruitment manoeuvres with tidal volumes of 8 mL/kg, no PEEP and no recruitment manoeuvres in 60 elderly ASA I or II participants undergoing elective digestive tract surgery of two hours or more. They found that the protective strategy group had lower C‐reactive protein and clinical pulmonary infection score at 24 hours after surgery. Severgnini 2013 compared a protective strategy including tidal volumes of 7 mL/kg, PEEP 10 cmH2O and recruitment manoeuvres with tidal volumes of 9 mL/kg, no PEEP and no recruitment manoeuvres in 56 participants scheduled to undergo elective open abdominal surgery lasting more than two hours. Participants ventilated protectively showed better pulmonary functional tests up to day five; fewer alterations on chest X‐ray up to day three; higher arterial oxygenation in air at days one, three and five days after surgery; and lower modified clinical pulmonary infection score at days one and three after surgery. The percentage of participants in hospital at day 28 after surgery was not statistically different between groups (7 with low tidal volume versus 15% with high tidal volume; P = 0.42).
We found a small difference in hospital length of stay (SMD –0.15, 95% CI –0.29 to 0.00; I2 = 27%; equivalent to 0.8 days). However, heterogeneity exploration revealed that the difference was present only when PEEP or recruitment manoeuvres were used for participants of the low tidal volume groups only (PEEP: SMD –0.23, 95% CI –0.38 to –0.08; I2 = 0%; Analysis 1.6; recruitment manoeuvres: SMD –0.25, 95% CI –0.41 to –0.09; I2 = 0%; Analysis 1.7), and not when they were used for all participants. This may indicate that low tidal volume should be used concomitantly with PEEP or recruitment manoeuvres (or both) to obtain a reduction in hospital length of stay or that only the two latter modalities (PEEP and recruitment manoeuvres) have an effect on hospital length of stay or that the effect of PEEP and recruitment manoeuvres on hospital length of stay are much higher than those of low tidal volumes and sufficient to obliterate any further effect of low tidal volumes.
We did not find a difference in the risk of pneumothorax (very low‐quality evidence) and this was the only type of barotrauma found by study authors. The number of participants included in the present meta‐analysis for this outcome represents less than 1% of the optimum information size. Therefore, more data will be required for this outcome.
Overall completeness and applicability of evidence
We found that low tidal volumes during surgery, defined as lower than 10 mL/kg, reduced the risk of pneumonia and the need for non‐invasive and invasive ventilatory support after surgery. A small reduction in hospital length of stay may also have been seen, especially when low tidal volumes were coupled with PEEP and recruitment manoeuvres. We found no deleterious effect of low tidal volumes. Therefore, the results of the present meta‐analysis suggested that tidal volumes lower than 10 mL/kg should be used preferentially during surgery. The exact tidal volume offering maximal protection may need to be further defined (i.e. decreasing to 6 mL/kg may offer better protection). We did not include trials performed on participants undergoing surgery with one‐lung ventilation, therefore our conclusion applied only to people undergoing surgery with two‐lung mechanical ventilation. The number of participants included in our meta‐analysis was too small to eliminate a deleterious effect of small tidal volumes on barotrauma.
Quality of the evidence
We judged the quality of the evidence as moderate for a reduced risk of pneumonia and for decreased requirement of non‐invasive and invasive mechanical ventilation after surgery (Table 1). We rated the quality of the body of evidence as low for no change in the risk of death, no change in ICU length of stay and a possible small reduction in hospital length of stay. The quality of evidence for absence in the risk of pneumothorax was very low.
Potential biases in the review process
We considered our search sufficiently extensive and think that we have identified all available relevant studies. Furthermore, by evaluating publication bias with the Duval and Tweedie's trim and fill analysis, we are confident that our estimates for pneumonia, and non‐invasive and invasive postoperative ventilatory support requirements are accurate. Also, using the exact P value rather than an estimate of the mean and standard deviation when those values were not available may have prevented us from entering possibly wrong data. The amount of data available was, however, insufficient to eliminate an increase in the risk of barotrauma with the use of small tidal volumes. One small published trial (Moussa 2003; report unavailable), and three ongoing trials (ACTRN12614000790640; NCT01003730; NCT03157479), may shed additional light on outcomes for which it was not possible to obtain a high level of evidence as they will be incorporated in futures updates of this review if their results become available.
Agreements and disagreements with other studies or reviews
In their retrospective study, Levin and colleagues found that low tidal volumes could increase postoperative mortality (Levin 2014). Although the relatively small number of participants included in the present meta‐analysis precluded us for drawing firm conclusions on this, we found no trend towards an increased mortality rate associated with tidal volumes lower than 10 mL/kg (Analysis 1.1). It is possible that the results of Levin and colleagues may be attributed to biases introduced by the design of their study (retrospective) or to the use of a relatively high FiO2 (greater than 0.7).
While including four RCTs performed in participants undergoing abdominal surgery, Tao colleagues reported that low tidal volumes (defined as 6 mL/kg to 8 mL/kg) would decrease in the incidence of atelectasis (odds ratio (OR) 0.36, 95% CI 0.22 to 0.60; P < 0.0001; I2 = 0%) and pulmonary infections (OR 0.30, 95% CI 0.14 to 0.68; P = 0.004; I2 = 20%) (Tao 2014). Other meta‐analyses evaluating the effects of low tidal volumes have included non‐RCTs (Serpa Neto 2012; Serpa Neto 2015a), or participants with one‐lung ventilation (Gu 2015; Park 2016b; Serpa Neto 2015b).
Authors' conclusions
Implications for practice.
There is moderate‐quality evidence that low tidal volumes during surgery decrease the risk of pneumonia, and the need for postoperative non‐invasive and invasive ventilatory support. We found no evidence for a detrimental effect of routine use of low intraoperative volumes but number of participants included was insufficient to allow us to draw definitive conclusion on possible detrimental effects of using low tidal volumes. The four studies in the Studies awaiting classification table may alter the conclusions of the review once assessed.
Implications for research.
More data are required on a possible increased risk of barotrauma (pneumothorax) with the use of low tidal volumes. Because this outcome requires a very high number of participants, something that would very difficult to obtain from randomized controlled trials, large retrospective well conducted studies may prove useful for this purpose. Further research on the exact tidal volume offering maximal protection may also prove useful.
What's new
| Date | Event | Description |
|---|---|---|
| 4 October 2018 | Amended | Acknowledgement section amended to include Sign‐off Editor |
History
Protocol first published: Issue 6, 2014 Review first published: Issue 12, 2015
| Date | Event | Description |
|---|---|---|
| 19 May 2017 | New search has been performed | The previous version contained 48 trials (12 included, 32 excluded, three ongoing, one awaiting classification) (Guay 2015). We reran the search 19 May 2017. We found 15 new trials. We excluded seven of the new trials (Arora 2017; Cui 2015; Ding 2016; Ferrando 2015; Hosten 2017; Jain 2016; Liu 2016). Two of the new trials are ongoing (NCT01003730; NCT03157479). We included six new trials in the review (Kuzkov 2016; Park 2016a; Sato 2016; Shen 2015; Soh 2018; Xiong 2016). One trial that we classified as ongoing (NCT00747045) in the previous version of this review (Guay 2015), is now included (Bates 2015). We reran the search again 3 January 2018. Two additional trials published between May 2017 and January 2018 were also added to the list of studies awaiting classification for formal evaluation at the next update. However these two last trials do not mention any outcome of interest measured in their reports. This update contains 63 trials (19 included, 47 excluded, four awaiting classification, three ongoing) |
| 19 May 2017 | New citation required but conclusions have not changed | Conclusions unchanged by inclusion of seven additional trials (six new trials and publication of one of the ongoing trials of the previous version). Since the last publication, one author (SK) joined the review and no authors left the review. Methodology unchanged. |
Acknowledgements
We would like to thank Javier Eslava‐Schmalbach (Content Editor); Cathal Walsh (Statistical Editor); Rajesh M Shetty, Rodrigo Cavallazzi and Martin R Lessard (Peer Reviewers); Patricia Tong (Consumer Referee); and Harald Herkner (Sign‐off Editor); for their help and editorial advice during the preparation of this updated systematic review.
We thank Karen Hovhannisyan who designed the search strategy, and Janne Vendt who updated the search for us. We also thank the University of Montreal and University of Quebec in Abitibi‐Temiscamingue, University of Sherbrooke, Laval University and University of Pennsylvania for granting access to electronic databases and medical journals.
We would also like to thank Jane Cracknell (Managing Editor), Rodrigo Cavallazzi (Content Editor), Marialena Trivella (Statistical Editor) and Samir Jaber and Martin R Lessard (Peer Reviewers) for their help and editorial advice during the preparation of the protocol (Nguyen 2014).
We would like to thank Jane Cracknell (Managing Editor), Rodrigo Cavallazzi (Content Editor), Cathal Walsh (Statistical Editor), Andrew T Jones and Samir Jaber (Peer Reviewers), Patricia Tong (Consumer Referee) and Jenny Bellorini (Copy Editor) for their help and editorial advice during the preparation of the first version of this systematic review (Guay 2015).
We are also in to debt to Mina Nishimori, Jiang Jia, Yuhua‐Gao, Min Su, Liu Yidan, Guo Hai, Shuming Pan and Xueyin Shi for handling the Japanese and Chinese studies and to Jiang Jia for translation of two Chinese studies (Shen 2015; Xiong 2016).
We thank Dat Nhut Nguyen who participated in the review up to 28 May 2015.
Appendices
Appendix 1. CENTRAL (the Cochrane Library) search strategy
#1 MeSH descriptor: [Respiration, Artificial] explode all trees #2 MeSH descriptor: [Tidal Volume] explode all trees #3 ((high and low) near (tidal or volume vetilat*)) or (tidal near (modalit* or ventilat* or recruitment or expiratory or peep)) #4 #1 or #2 or #3 #5 MeSH descriptor: [Postoperative Complications] explode all trees #6 MeSH descriptor: [Intraoperative Period] explode all trees #7 intraoperative:ti,ab or (complicat* near (pulmonary or extra?pulmonary)) #8 #5 or #6 or #7 #9 #4 and #8
Appendix 2. MEDLINE (OvidSP) search strategy
1. exp Respiration, Artificial/ or exp Tidal Volume/ or ((high and low) adj3 (tidal or volume vetilat*)).af. or (tidal adj3 (modalit* or ventilat* or recruitment or expiratory or peep)).mp. 2. exp Postoperative Complications/ or exp Intraoperative Period/ or intraoperative.ti,ab. or (complicat* adj3 (pulmonary or extra?pulmonary)).mp. 3. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 3. Embase (OvidSP) search strategy
1. artificial ventilation/ or tidal volume/ or ((high and low) adj3 (tidal or volume vetilat*)).ti,ab. or (tidal adj3 (modalit* or ventilat* or recruitment or expiratory or peep)).ti,ab. (94288) 2. postoperative complication/ or intraoperative period/ or intraoperative.ti,ab. or (complicat* adj3 (pulmonary or extra?pulmonary)).ti,ab. 3. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 4. PsycINFO
1. tidal volume
2. low
3. surgery
4. 1 AND 2 AND 3
Appendix 5. Search strategy and results 3 January 2018
Database: Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
1 exp Respiration, Artificial/ or exp Tidal Volume/ or ((high and low) adj3 (tidal or volume ventilat*)).af. or (tidal adj3 (modalit* or ventilat* or recruitment or expiratory or peep)).mp. (83841)
2 exp Postoperative Complications/ or exp Intraoperative Period/ or intraoperative.ti,ab. or (complicat* adj5 (pulmonary or extra?pulmonary or postoperative or post operative)).mp. (689456)
3 ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. (1162648)
4 1 and 2 and 3 (715)
Database: Embase <1974 to 2018 Week 01>
1 artificial ventilation/ or tidal volume/ or ((high and low) adj3 (tidal or volume ventilat*)).ti,ab. or (tidal adj3 (modalit* or ventilat* or recruitment or expiratory or peep)).ti,ab. (126624)
2 postoperative complication/ or intraoperative period/ or intraoperative.ti,ab. or (complicat* adj5 (pulmonary or extra?pulmonary or postoperative or post operative)).ti,ab. (498406)
3 (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. (7166088)
4 1 and 2 and 3 (2210)
Central
#1 MeSH descriptor: [Respiration, Artificial] explode all trees 5841
#2 MeSH descriptor: [Tidal Volume] explode all trees 790
#3 ((high and low) near (tidal or volume ventilat*)) or (tidal near (modalit* or ventilat* or recruitment or expiratory or peep)) 1421
#4 #1 or #2 or #3 7037
#5 MeSH descriptor: [Postoperative Complications] explode all trees 35099
#6 MeSH descriptor: [Intraoperative Period] explode all trees 2194
#7 intraoperative:ti,ab or (complicat* near (pulmonary or extra?pulmonary or postoperative or post operative)) 42995
#8 #5 or #6 or #7 58434
#9 #4 and #8 775
#10 #9 in Trials 685
Data and analyses
Comparison 1. Low versus high tidal volume.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality within 30 days | 12 | 1207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.42, 1.53] |
| 2 Pneumonia | 7 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.25, 0.82] |
| 3 Need for postoperative non‐invasive ventilation | 3 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.15, 0.64] |
| 4 Need for postoperative invasive ventilation | 11 | 957 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.14, 0.77] |
| 5 Intensive care unit length of stay (days) | 5 | 898 | Std. Mean Difference (Random, 95% CI) | ‐0.06 [‐0.22, 0.10] |
| 6 Hospital length of stay divided in subgroups according to positive end‐expiratory pressure (PEEP) use (days) | 14 | 1298 | Std. Mean Difference (Random, 95% CI) | ‐0.15 [‐0.29, ‐0.00] |
| 6.1 PEEP used for both groups | 8 | 537 | Std. Mean Difference (Random, 95% CI) | ‐0.08 [‐0.33, 0.18] |
| 6.2 PEEP used for low tidal volume group only | 7 | 761 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.38, ‐0.08] |
| 7 Hospital length of stay divided in subgroups according to recruitment manoeuvres or not (days) | 14 | 1298 | Std. Mean Difference (Random, 95% CI) | ‐0.15 [‐0.29, ‐0.00] |
| 7.1 Recruitment manoeuvres in low tidal volume group only | 4 | 638 | Std. Mean Difference (Random, 95% CI) | ‐0.25 [‐0.41, ‐0.09] |
| 7.2 Recruitment manoeuvres in high tidal volume group only | 1 | 40 | Std. Mean Difference (Random, 95% CI) | 0.0 [‐0.62, 0.62] |
| 7.3 Recruitment manoeuvres in both groups | 3 | 254 | Std. Mean Difference (Random, 95% CI) | 0.16 [‐0.09, 0.40] |
| 7.4 No recruitment manoeuvres mentioned | 6 | 366 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.50, ‐0.02] |
| 8 Pneumothorax | 5 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.52, 5.99] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bates 2015.
| Methods | RCT with parallel groups Approved by the local ethics committee and informed consents obtained Site: University of California, San Diego Medical Center, San Diego, CA, USA Setting: university hospital Dates of data collection: September 2008 to March 2011 Clinicaltrials.gov: NCT00747045 |
|
| Participants | 128 participants undergoing pulmonary thromboendarterectomy Exclusion criteria: aged < 18 years, planned concurrent procedure (coronary artery bypass graft, valve replacement or lung biopsy), morbid obesity (BMI 40 kg/m2), history of ARDS and inability or refusal to give informed consent |
|
| Interventions |
Treatment group: low tidal volume 6 mL/kg predicted body weight intra‐ and postoperatively for 3 days after surgery (n = 63) Control group: usual care with tidal volume 10 mL/kg (n = 65) All participants received ventilation via a pressure‐regulated volume control mode and underwent a recruitment manoeuvre on arrival to the ICU Thereafter, titration of FiO2, PEEP and respiratory rate for both groups were guided by a modified ARDS Network protocol In the event that it was not possible to meet the goals despite adherence to the aforementioned protocol, then an increase in tidal volume was permitted. Participants were considered ready for extubation from a pulmonary mechanics standpoint if their rapid shallow breathing index was < 105 for 30 min |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: unspecified Declaration of interest: Dr Auger reported grants from Bayer, non‐financial support from Bayer, outside the submitted work; Dr Banks had nothing to disclose; Dr Bates had nothing to disclose; Dr Duwe had nothing to disclose; Dr Fedullo reported personal fees from Actelion Pharmaceuticals, outside the submitted work; Dr Fernandes reported grants from Actelion, personal fees from Bayer, outside the submitted work; Dr Jamieson had nothing to disclose; Dr Kerr reported grants and clinical trial support from Bayer, clinical trial support from Actelion and consulting fees from Bayer, outside the submitted work; Dr Kim had nothing to disclose; Dr King had nothing to disclose; Dr Madani reported personal fees from Bayer, personal fees from Actelion, outside the submitted work; no other relationships/conditions/circumstances that presented a potential conflict of interest. All participants were routinely kept on invasive ventilation after surgery, therefore, this outcome was not entered in the analysis. Median duration of mechanical ventilation: 1 day for each group (P = 0.665) Volume per body weight: predicted body weight calculated based on the equation used by the ARDS Network in their clinical trials: predicted body weight of men: equal to 50 + 0.91 (centimetres of height – 152.4); women equal to 45.5 + 0.91(centimetres of height – 152.4) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants underwent simple 1:1 randomization to either the treatment or control group. Randomization was determined by the Investigational Drug Pharmacy, which used a computer program designed to generate a random allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | The randomization list was kept in the study file, and each participant was assigned a study group based on the sequential study identification number given by the research co‐ordinator on enrolment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The primary clinical team was not blinded to the participant's assigned group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The presence of reperfusion lung injury was determined by 2 pulmonologists with extensive experience in the care of people undergoing pulmonary thromboendarterectomy, blinded to the identity of the participant and study group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After enrolment and randomization, 6 participants were excluded from analysis: 4 had a diagnosis other than chronic thromboembolic pulmonary hypertension made at the time of surgery, 1 had anaphylaxis during induction of anaesthesia and 1 underwent simultaneous coronary artery bypass graft in addition to pulmonary thromboendarterectomy. |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Analysed using intention‐to‐treat method There were no significant differences in age, sex, race, actual body weight, predicted body weight and New York Heart Association functional class between the 2 groups. |
Chaney 2000.
| Methods | RCT with parallel groups Institutional review board approval and informed consents obtained Site: Loyola University Medical Center Setting: university hospital Dates of data collection: unspecified |
|
| Participants | 25 participants scheduled for elective coronary artery bypass graft surgery and early tracheal extubation Exclusion criteria: previous lung surgery or who required preoperative mechanical ventilation |
|
| Interventions |
Treatment group: tidal volume 6 mL/kg; FiO2 1.0; respiratory rate 16 breaths/min; and
PEEP 5, after tracheal intubation (n = 12) Control group: tidal volume 12 mL/kg; respiratory rate 8 breaths/min; FiO2 1.0; and PEEP 5 cmH2O, after tracheal intubation (n = 13) In both groups, the inspiratory/expiratory ratio was 1:3, and the inspiratory flow was adjusted so that the calculated tidal volume was delivered during the entire inspiratory cycle (creating the lowest peak airway pressure). Each mode of ventilation (conventional or protective) was used during the entire intraoperative period and during the first hour after arrival in the ICU. After 1 hour following ICU arrival (and after last data collection time), all participants received mechanical ventilation parameters of respiratory rate 10 breaths/min; tidal volume 8 mL/kg; FiO2 1.0 and PEEP 15, and were weaned from mechanical ventilation according to the normal ICU protocol. Criteria for extubation in the ICU at this institution included an appropriate sensorium, normothermia, haemodynamic stability, adequate pulmonary function (PaO2 > 60 mmHg with a FiO2 0.4), adequate urine output and minimal chest tube output. |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: supported by Loyola University Medical Center, Department of Anesthesiology, Research Fund Declaration of interest: none mentioned Postoperative complications and treatments were recorded daily until hospital discharge Volume per body weight: method used to determine body weight unspecified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "each patient was randomized to one of two groups by a random numbers table." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "before arriving in the operating room"; no other details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Choi 2006.
| Methods | RCT with parallel groups Approved by the Medical Ethics Committee of the University of Amsterdam (Amsterdam, The Netherlands), and informed consents obtained from all participants Site: Academic Medical Center, University of Amsterdam, The Netherlands Setting: university hospital Dates of data collection: December 2003 to March 2005 |
|
| Participants | 46 adults scheduled to undergo a surgical procedure of ≥ 5 hours Exclusion criteria: history of any lung disease, use of immunosuppressive medication, recent infections, previous thromboembolic disease or recent admission to ICU for ventilatory support |
|
| Interventions |
Treatment group: tidal volume 6 mL/kg (IBW) and PEEP 10 cmH2O (n = 24 randomized; n = 21 analysed) Control group: tidal volume 12 mL/kg (IBW) and no PEEP (n = 22 randomized; and n = 19 analysed) The ventilatory protocol consisted of volume‐controlled mechanical ventilation at an FiO2 of 0.4, inspiratory/expiratory ratio of 1:2 and a respiratory rate adjusted to normocapnia. If the surgical procedure exceeded 5 hours, anaesthesiologists were allowed to change the ventilation strategy |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: support provided solely from institutional or departmental sources (or both) Declaration of interest: none mentioned Participants were followed up until hospital discharge or death Volume per body weight: predicted body weight calculated based on the equation used by the ARDS Network in their clinical trials: predicted body weight of men: equal to 50 + 0.91 (centimetres of height – 152.4); women: equal to 45.5 + 0.91 (centimetres of height – 152.4) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed by drawing a presealed envelope." |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was performed by drawing a presealed envelope." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for and low rate of dropout |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced Not intention‐to‐treat: quote: "five patients were randomized but excluded from final analysis, because the initial surgical procedure was converted by the surgeon into another shorter operation (< 3 hours). One patient was randomized, but no lavages were performed upon the surgeon's request after induction of anaesthesia." |
Chugh 2012.
| Methods | RCT with parallel groups Approved by the institutional ethical committee and written informed consents obtained from all participants Site: University College of Medical Sciences, Delhi, India Setting: university hospital Dates of data collection: unspecified |
|
| Participants | 60 adults with intestinal perforation peritonitis‐induced sepsis scheduled for emergency laparotomy | |
| Interventions |
Treatment group: tidal volume 6 mL/kg (IBW) and PEEP 10 cmH2O (n = 30) Control group: tidal volume 10 mL/kg (IBW) and no PEEP (n = 30) In both groups, respiratory rate was varied to maintain eucapnia |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: unspecified Declaration of interest: none mentioned Conference abstract Volume per body weight: IBW, no further details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random number table" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind." Details of blinding not provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind." Details of blinding not provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Baseline characteristics were similar in both groups. |
Fernandez‐Bustamante 2014.
| Methods | RCT with parallel groups The experimental protocol was approved by the University of Colorado Multiple Institutional Review Board (Aurora, Colorado) before performing the study and informed consents were obtained. Site: Webb‐Waring Center, University of Colorado School of Medicine, Aurora, Colorado Setting: university hospital Dates of data collection: unspecified |
|
| Participants | 30 participants scheduled to receive elective orthopaedic surgery for total knee replacement under general anaesthesia Exclusion criteria: ASA class IV; aged ≥ 70 years; emergency procedure; status post pneumonectomy; diagnosed with chronic obstructive pulmonary disease, emphysema, asthma, pulmonary hypertension, sleep apnoea or any other respiratory disease; oxygen therapy during last month; tobacco use in the last 5 years; severe obesity (BMI ≥ 35 kg/m2); immunosuppression within 3 months before the procedure; diagnosed infection or shock |
|
| Interventions |
Treatment group: tidal volume 6 mL/kg (IBW) (n = 14) Control group: tidal volume 10 mL/kg (IBW) (n = 14) The respiratory rate was titrated for eucapnia (end‐tidal carbon dioxide partial pressure 30–40 mmHg), and all participants received the same following ventilatory settings: inspiratory/expiratory ratio, 1:2; inspiratory pause 5%; and fresh gas flow 2 L/min, FiO2 0.5, PEEP 5 cmH2O |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: supported by the Department of Anesthesiology, University of Colorado School of Medicine (Aurora, Colorado) Seed Grant and 2012 Foundation for Anesthesia Education and Research (Rochester, MN) Clinical/Translational‐Mentored Research Training Grant (to Dr Fernandez‐Bustamante); the National Institutes of Health (Bethesda, MD) HL60917 award; the Department of Pathobiology and Lerner Research Institute (NC22), the Cleveland Clinic Foundation (Cleveland, OH), and Case Western Reserve University School of Medicine (Cleveland, OH) (to Dr Erzurum and Ms Janocha); Foundation for Anesthesia Education and Research fellowship award (Rochester, MN) (to C Shah); and institutional support from University of Colorado School of Medicine (Aurora, CO), Departments of Anesthesiology (to Dr Klawitter, Ms Agazio, Dr Christians and Dr Seres), Medicine (to Drs Repine, Moss and Douglas) and Biostatistics and Informatics (to Dr Tran) Declaration of interest: the authors declared no competing interests Volume per body weight: predicted body weight: men: 50 + 0.91 (height in cm – 152.4); women: 45.5 + 0.91 (height in cm – 152.4) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized"; no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30 included, 28 analysed |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Quote: "no significant differences were found between the two groups in terms of age, sex, comorbidities, ASA classification, height, weight, body mass index and IBW." Not intention‐to‐treat: quote: "one patient from each VT group was removed from the study because of a non disclosed steroid course within 10 days before surgery and previously undiagnosed sleep apnoea symptoms. Only the remaining 28 participants (14 per group) were included in the final analyses." |
Futier 2013.
| Methods | RCT with parallel groups Approved by a central ethics committee (Comité de Protection des Personnes Sud‐Est I, Saint‐Etienne, France) according to French law. Written informed consent was obtained before randomization from each participant, on the day before surgery Site: multicentre at 7 French university teaching hospitals Setting: university hospital Dates of data collection: 31 January 2011 to 10 August 2012 ClinicalTrials.gov: NCT01282996 |
|
| Participants | 400 adults aged > 40 years, scheduled to undergo laparoscopic or non‐laparoscopic elective major abdominal surgery with an expected duration ≥ 2 hours, and a preoperative risk index for pulmonary complications > 2 (possible scores from 1 to 5 and a high score indicates a higher risk) Exclusion criteria: received mechanical ventilation within the 2 weeks preceding surgery, BMI ≥ 35 kg/m2, history of respiratory failure or sepsis within 2 weeks preceding surgery, requirement for intrathoracic or emergency surgery, or had a progressive neuromuscular illness |
|
| Interventions |
Treatment group: tidal volume 6–8 mL/kg (IBW), PEEP 6–8 cmH2O and recruitment manoeuvres repeated every 30 min after tracheal intubation (30 cmH2O for 30 seconds) (n = 200) Control group: tidal volume 10–12 mL/kg (IBW), no PEEP and no recruitment manoeuvres (n = 200) During anaesthesia, a plateau pressure ≤ 30 cmH2O was targeted in each group. For episodes of arterial desaturation (defined as a peripheral oxygen saturation ≤ 92%), a transient increase in FiO2 to 100% was permitted, and in participants assigned to the control group, the use of PEEP, recruitment manoeuvres or both was allowed, if required. |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: some authors declared consultant fees or travel expenses, or both, from industry Declaration of interest: Dr Futier reported receiving consulting fees from General Electric Medical Systems, lecture fees from Fresenius Kabi, and reimbursement of travel expenses from Fisher and Paykel Healthcare. Dr Constantin reported receiving consulting fees from Baxter, Fresenius Kabi, Dräger and General Electric Medical Systems; payment for expert testimony from Baxter, Dräger and Fresenius Kabi; lecture fees from General Electric Medical Systems, Dräger, Fresenius Kabi, Baxter, Hospal, Merck Sharp & Dohme and LFB Biomedicaments; payment for the development of educational presentations from Dräger, General Electric Medical Systems, Baxter and Fresenius Kabi; and reimbursement of travel expenses from Bird, Astute Medical, Astellas, Fresenius Kabi, Baxter and Hospal. Dr Paugam‐Burtz reported receiving consulting fees from Fresenius Kabi, lecture fees and reimbursement of travel expenses from Astellas, and payment for the development of educational presentations from LFB Biomedicaments and Merck Sharp & Dohme. Dr Allaouchiche reported receiving consulting fees from Fresenius Kabi and lecture fees from Novartis and Astellas. Dr Leone reported receiving consulting fees from LFB Biomedicaments and lecture fees from Fresenius Kabi and Novartis. Dr Jaber reported receiving consulting fees from Dräger France and Maquet France; lecture fees from Fisher and Paykel Healthcare, Abbott and Philips; and reimbursement of travel expenses from Pfizer. No other potential conflict of interest relevant to this article was reported. 30‐day follow‐up period Volume per body weight: predicted body weight calculated based on the equation used by the ARDS Network in their clinical trials: predicted body weight: men: 50 + 0.91 (height in cm – 152.4); women: 45.5 + 0.91 (height in cm – 152.4) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed with the use of a computer‐generated assignment sequence and a centralized telephone system." Quote: "randomization was stratified according to study site and the planned use or nonuse of postoperative epidural analgesia." |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was performed with the use of a computer‐generated assignment sequence and a centralized telephone system." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "treatment assignments were concealed from patients, research staff, the statistician, and the data and safety monitoring committee. Staff members who collected data during surgery were aware of the group assignments." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "outcome assessors were unaware of these assignments throughout the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were excluded after randomization; surgery was stopped prematurely in 2 of the 3 participants because of extensive illness (duration of surgery, < 2 hours), and 1 had undergone randomization in error (violation of exclusion criteria). An additional 3 participants were thus randomly assigned to a study group to obtain the full sample. |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced Quote: "there was no industry support or involvement in the trial." |
Ge 2013.
| Methods | RCT with parallel groups Approved by the ethics committee and informed consent obtained Site: Ningbo 6th Hospital, Ningbo Zhjiang, China Setting: hospital Dates of data collection: July 2011 to February 2012 |
|
| Participants | 60 participants aged 70–85 years, ASA class II or III, undergoing spinal fusion | |
| Interventions |
Treatment group: tidal volume 6 mL/kg, rate 12˜18/min, inspiratory/expiratory ratio 1:2, PEEP 10 cmH2O mechanical ventilation and alveolar recruitment performed once every 15 min (n = 30) Control group: tidal volume 12 mL/kg, rate 12/min and inspiratory/expiratory ratio 1:2 (n = 30) |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: unspecified Declaration of interest: not mentioned Volume per body weight: predicted body weight: men: 50 + 0.91 × height – 152.4; women: 45.5 + 0.91 × height – 152.4 Information for this trial was extracted by Shumming Pan and Yuhua‐Gao |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned, from Shumming Pan and judged as "randomized controlled trial:" by Yuhua‐Gao from page 82. |
| Allocation concealment (selection bias) | Unclear risk | Unclear The allocation method allowed the record staff to know the treatment allocation of the next participant to be enrolled in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The allocation method allowed the record staff to know the treatment allocation of the next participant to be enrolled in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This study did not describe whether the outcome assessor was blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant selected for this study dropped out |
| Selective reporting (reporting bias) | Low risk | All the measurements stated in the methods section were included in the results. |
| Other bias | Unclear risk | No clear definition for pneumonia (possible inclusion of bronchitis or other pulmonary complications) |
Koner 2004.
| Methods | RCT with parallel groups Approved by the ethics committee and written informed consents obtained Site: Cardiology Institute, Istanbul University, Haseki caddesi, Aksaray‐Istanbul, Turkey Setting: university hospital Dates of data collection: November 2001 to August 2002 |
|
| Participants | 44 adults undergoing coronary artery bypass graft Exclusion criteria: acute infections, pre‐existing pulmonary disease, left ventricular ejection fraction < 40%, myocardial infarction within 1 month, reoperation, coagulopathy, unstable angina pectoris and renal failure |
|
| Interventions |
Treatment group: tidal volume 6 mL/kg, respiratory rate: 15 breaths/min and PEEP 5 cmH2O (n = 15) Control groups:
Before discontinuation of CPB, the lungs were inflated manually up to 40 cmH2O peak airway pressure for 20 seconds and the ventilation was started with a FiO2 of 0.6 then reduced to 0.5. Corticosteroids, antifibrinolytic agents or aprotinin were not used and no ultrafiltration technique was employed throughout the study. |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: partially supported by Fresenius‐Kabi and Aventis Pharma Declaration of interest: partially supported by Fresenius‐Kabi and Aventis Pharma The treatment group was split in half to compare with each control group. As no other recruitment manoeuvre was mentioned apart from the 1 single reinflation manoeuvre just before ending CPB, for heterogeneity exploration, participants were considered as not having received recruitment manoeuvres during surgery. Volume per body weight: IBW, no further details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized;" no details |
| Allocation concealment (selection bias) | Low risk | Quote: "following the anaesthesia induction, patients were randomized." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "anaesthesia and intensive care unit teams were not blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Kuzkov 2016.
| Methods | RCT with parallel groups Approved by the ethics committee Written informed consents obtained Site: Northern State Medical University, Arkhangelsk, Russian Federation Setting: University hospital Dates of data collection: 2014–2016 |
|
| Participants | 60 adults scheduled for elective pancreatoduodenal surgery with duration > 2 hours | |
| Interventions |
Treatment group: tidal volume 6 mL/kg of predicted body weight with (PaCO2 45–60 mmHg; n = 20) or without (PaCO2 32–48 mmHg; n = 20) moderate hypercapnia Control group: tidal volume 10 mL/kg of predicted body weight (n = 20) In all the groups, PEEP 4 cmH2O was set. |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: supported, in part, by the Grant of the President of Russian Federation (grant number MD‐4984.2015.7) Declaration of interest: none mentioned All participants were routinely kept on invasive ventilation after surgery, therefore this outcome was not entered in the analysis. Email send to authors to obtain results for ICU length of stay on 10 June 2017. No reply received Volume per body weight: predicted body weight, no further details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Before anaesthesia and start of mechanical ventilation, participants were randomized using the envelope method |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Tracheal extubation was performed in the ICU by an independent ICU physician on predetermined criteria. Chest X‐rays were interpreted by an independent specialist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced except for smoking that was significantly lower in the high tidal volume group (P = 0.03) |
Memtsoudis 2012.
| Methods | RCT with parallel groups Approved by the Hospital for Special Surgery Institutional Review Board (Protocol no. 28117) and written, informed consents obtained Site: Hospital for Special Surgery, New York, NY, USA Setting: hospital Dates of data collection: February 2009 to September 2010 |
|
| Participants | 26 participants scheduled for elective, primary lumbar decompression and fusion of ≤ 4 spinal levels Exclusion criteria: known previous lung pathology, use of immunosuppressants, renal failure with creatinine > 1.5 mg/dL, recent exposure to a ventilator or surgery during general anaesthesia (< 1 year), and ASA physical status ≥ III |
|
| Interventions |
Treatment group: tidal volume 6 mL/kg (IBW) and PEEP 8 cmH2O (n = 13) Control group: tidal volume 12 mL/kg (IBW) and no PEEP (n = 13) |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: Department of Anesthesiology, Hospital for Special Surgery (Stavros G. Memtsoudis), and Clinical Translational Science Center (CTSC) grant: NIH UL1‐RR024996 (Yan Ma) Declaration of interest: none mentioned Volume per body weight: IBW: men: 50 + 0.91 (height in cm – 152.4); women 45.5 + 0.91 (height in cm – 152.4) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned by a computer generated list of random numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocation sequence was concealed from the research assistant in sequentially numbered, opaque, sealed, and stapled envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "patients, surgeons, and research assistants who were responsible for subsequent data collection were blinded to the randomization." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "one patient in the low tidal volume group withdrew consent for blood draws after randomization and surgery." |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Quote: "despite randomization, patients in the low volume group were older (P value = 0.01)." |
Park 2016a.
| Methods | RCT with parallel groups Approved by the ethics committee Written informed consents obtained Site: Seoul National University College of Medicine, South Korea Setting: university hospital Dates: November 2012 to June 2014 Clinical Research Information Service: KCT0001034 |
|
| Participants | 62 participants undergoing laparoscopic hepatobiliary surgery Exclusion criteria: cardiopulmonary or hepatorenal disease, recent infections, recent ventilator support, previous thromboembolic disease or denial of informed consent |
|
| Interventions |
Treatment group: tidal volume 6 mL/kg (IBW) and PEEP of 5 cmH20 (n = 31) Control group: tidal volume 10 mL/kg (IBW) and recruitment manoeuvres at 40 cmH20 for 30 seconds (n = 31) All groups used volume‐controlled ventilation, inspiration/expiration ratio 1:2 and FiO2 0.5 in medical air |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: supported by Grant No. 02‐2013‐072 from Seoul National University Bundang Hospital Research Fund Declaration of interest: Drs Park, Ryu, Kim, Oh, Han (S.H) and Han (H.S) had no conflicts of interest or financial ties to disclose Volume per body weight: IBW, no further details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization performed before induction of anaesthesia by an anaesthesiologist not otherwise involved in study. Used computer‐generated random number table (Random Allocation Software, version 1.0, Isfahan University of Medical Sciences, Isfahan, Iran) with block size 4. |
| Allocation concealment (selection bias) | Unclear risk | From a table of random numbers, participants were allocated to conventional ventilation with alveolar recruitment manoeuvres (n = 31) or protective lung ventilation strategy group (n = 31). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and outcome assessors blinded to group assignment. However, anaesthesiologist responsible for ventilator setting and the care of participants during surgery was not blinded to assigned group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | After randomization, 12 participants in control group and 10 participants in treament group were excluded due to conversion to open surgery and ICU admission without extubation. For this review, the participant who required postoperative invasive ventilation was included for the outcome need of postoperative invasive ventilation according to the intention‐to‐treat principle. 39 participants (19 participants in alveolar recruitment manoeuvre group and 20 participants in protective lung ventilation strategy group) completed the study and were analysed. |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Sato 2016.
| Methods | RCT with parallel groups Approved by the ethics committee Written informed consents obtained Site: Yokohama City University Hospital Setting: university hospital Dates of data collection: October 2008 to September 2009 UMIN‐CTR: UMIN000021371 (3 July 2016); retrospectively registered |
|
| Participants | 28 participants aged 20‐85 years undergoing hepatectomy Exclusion criteria: ASA physical status ≥ III, pre‐existing lung disease, tumour in the portal vein or inferior vena cava, requirement of bile duct or gastrointestinal tract repair, or requirement of additional surgical procedures other than hepatectomy |
|
| Interventions |
Treatment group: tidal volume 6 mL/ kg (predicted body weight) (n = 14) Control group: tidal volume 12 mL/ kg (predicted body weight) (n = 14) |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: supported by the Center for Advanced Medical Promotion of the Yokohama City University Graduate School of Medicine (grant number: 07‐021) and the Grants‐in‐Aid for Scientific Research from the Japan Society for the Promotion of Science (grant number: 26462368) Declaration of interest: the authors declared that they had no competing interests. Volume per body weight: predicted body weight, no further details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment performed using a random number table by an investigator who was not involved in data collection and was notified to anaesthesiologists who were not involved in study using an envelope method. |
| Allocation concealment (selection bias) | Low risk | Assignment performed using a random number table by an investigator who was not involved in data collection and was notified to anaesthesiologists who were not involved in study using an envelope method. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators who collected the data and samples were blinded to the ventilation settings at any time of the experiment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants in the high tidal volume group were excluded because the operation was terminated before the completion of the study due to dissemination of tumour to the peritoneum. |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Shen 2015.
| Methods | RCT with parallel groups Written informed consents obtained Site: Ruijin Hospital North of Shanghai Jiatong University, China Setting: university hospital Dates of data collection: January 2013 to December 2014 |
|
| Participants | 120 participants at high risk of postoperative pulmonary complications: 2 hours' duration, need for postoperative ICU admission, postoperative pulmonary complication risk score 25 | |
| Interventions |
Treatment group: tidal volume 6 mL/kg, PEEP 6 cmH2O and 1 recruitment manoeuvre per hour (30 cmH20 for 30 seconds) (n = 60) Control group: tidal volume 10 mL/kg, PEEP 0 cmH2O (n = 60) |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: departmental resources Declaration of interest: none mentioned Email sent to authors 11 June 2017 to request results for pneumonia: invalid email address. Letter sent 19 June 2017. No reply received Volume per body weight: predicted body weight: men: height in cm – 105; women: height in cm – 110 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned," no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Soh 2018.
| Methods | RCT with parallel groups Approved by the local ethics committee Informed consents obtained Site: Severance Cardiovascular Hospital, Yonsei University College of Medicine, 50 Yonsei‐ro, Seodaemun‐gu, Seoul, Republic of Korea Setting: university hospital Dates of data collection: January 2015 to January 2016 Clinicaltrials.gov: NCT02373475 |
|
| Participants | 78 participants at potential risk of postoperative pulmonary complications undergoing major lumbar spinal surgery in the prone position under general anaesthesia for > 2 hours, and preoperative risk index for postoperative pulmonary complications ≥ 2 Exclusion criteria: increased intracranial pressure or altered mental status before surgery; any neuromuscular disease; BMI > 35 kg/m2; previous lung surgery; repeated treatment for acute exacerbation of asthma or chronic obstructive pulmonary disease; congestive heart failure; use of mechanical ventilation within 2 weeks before surgery; sepsis; and pregnancy |
|
| Interventions |
Treatment group: tidal volume 6 mL/kg of predicted body weight, PEEP 6 cmH2O and recruitment manoeuvres (n = 39) Control group: tidal volume 10 mL/kg, PEEP 0 cmH2O (n = 39) |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: departmental resources Declaration of interest: authors had no funding or conflicts of interest to disclose Risk factors and points: age: ≥ 80 years = 17; 70–79 years = 13; 60–69 years = 9; 50–59 years = 4; functional status totally dependent = 10, partially dependent = 6; weight loss > 10% in past 6 month = 7; history of chronic obstructive pulmonary disease = 5; general anaesthesia = 4; impaired sensorium = 4; history of cerebrovascular accident = 4; blood urea nitrogen level < 2.86 mmol/L (< 8 mg/dL) = 4; 7.85–10.7 mmol/L (22–30 mg/dL) = 2; ≥ 10.7 mmol/L (≥ 30 mg/dL) = 3; transfusion > 4 units = 3; emergency surgery = 3; steroid use for chronic condition = 3; current smoker within 1 year = 3; alcohol intake > 2 drinks/day in past 2 weeks = 3 Class 1 = 0–15 points; Class 2 = 16–25 points; Class 3 = 26–40 points; Class 3 = 41–55 points; Class 5 ≥ 55 points Volume per body weight: predicted body weight: men: 50 + 0.91 (height in cm – 152.4); women: 45.5 + 0.91 (height in cm – 52.4) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list using a permuted 2‐block strategy |
| Allocation concealment (selection bias) | Low risk | Concealment of the group allocation from primary physicians, nurses, participants and investigators was ensured by using non‐transparent envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Envelopes opened on morning of surgery by an anaesthesiologist who was not involved in the investigation but was responsible for the intraoperative participant care and the recording of intraoperative variables |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "single‐blinded clinical trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Unclear risk | Participants characteristics similar between the groups except that participants in the protective group were significantly older than those in the conventional group |
Sundar 2011.
| Methods | RCT with parallel groups Approved by the institutional review board and informed consents obtained from participants or from their nearest relatives Site: Beth Israel Deaconess Medical Center, Boston, MA, USA Setting: hospital Dates of data collection: September 2007 to July 2009 Clinicaltrials.gov: NCT00538161 |
|
| Participants | 149 participants undergoing elective cardiac surgery Exclusion criteria: emergent, non‐scheduled surgery, cardiogenic shock (preoperative inotropic or intra‐aortic balloon support), pre‐existing pulmonary disease (significant obstructive or restrictive lung disease), active infection (treated with antibiotics) and need for single lung ventilation during the procedure |
|
| Interventions |
Treatment group: tidal volume 6 mL/kg (n = 75) Control group: tidal volume 10 mL/kg (n = 74) Study ventilator settings (IBW) applied immediately after induction of general anaesthesia and continued throughout surgery and subsequent ICU stay. PEEP and FiO2 levels were set according to a sliding scale as described by Acute Respiratory Distress Syndrome Network investigators according to protocol but FiO2 1.0 in results tables. Respiratory rates adjusted to maintain PaCO2 40–55 mmHg and pH > 7.25. As no other recruitment manoeuvre was mentioned apart from the 1 single reinflation manoeuvre just before ending CPB, for heterogeneity exploration, participants were considered as not having received recruitment manoeuvres during surgery. Readiness criteria for extubation included awake status (Riker Sedation‐Agitation Scale score 3 or 4), haemodynamic stability (minimal doses of nitroglycerine or phenylephrine) and adequate gas exchange (PaO2 100 mmHg, FiO2 0.4, PEEP 5 cmH2O) |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: support provided solely from institutional or departmental (or both) sources Declaration of interest: none mentioned Volume per body weight: predicted body weight: men: 50 + 2.3 (height in cm – 60); women: 45.5 + 2.3 (height in cm – 60) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a block randomization scheme was used to allocate patients to one of two experimental groups." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collected on ventilator settings, variables of gas exchange, lung mechanics and secondary outcome variables (hospital mortality, hospital length of stay, duration of mechanical ventilation) by observers blinded to participant allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | No commercial entities providing equipment or devices had a role in any aspect of this study Groups well balanced except for a higher incidence of postoperative complete heart block after surgery in the high volume group (7 vs 0) Not intention‐to‐treat. Quote: "difficult intubation off study protocol (n = 1)" |
Treschan 2012.
| Methods | RCT with parallel groups Approved by the local ethics committee (Ethics Committee of the Medical Faculty, Heinrich‐Heine‐University Dűsseldorf, Germany, study number 2974 and informed consents obtained Site: Dűsseldorf University Hospital, Dűsseldorf, Germany Setting: university hospital Dates of data collection: over 2‐year period ClinicalTrials.gov: NCT00795964 |
|
| Participants | 101 participants aged ≥ 50 years, ASA ≥ II, scheduled for an upper abdominal surgery duration of surgery ≥ 3 hours | |
| Interventions |
Treatment group: tidal volume 6 mL/kg (IBW) (n = 50) Control group: tidal volume 12 mL/kg (IBW) (n = 51) Initial breathing rate 14/min (low tidal volume) or 7/min (high tidal volume) was subsequently adjusted to maintain end‐tidal PCO2 4.6–5.4 kPa (35–40 mmHg). Other ventilator settings identical in both groups, including an initial fresh gas flow of 10 L/min with a FiO2 1.0, PEEP 5 cmH2O and inspiratory/expiratory ratio 1:2. The FiO2 was reduced to 0.5 shortly after intubation. If deemed necessary by the attending physician, the FiO2 or PEEP was increased to maintain PaO2 within 20% of preoperative values or SaO2 ≥ 95%. All participants received lung expansion manoeuvre consisting of 3 manual bag ventilations with a maximum pressure of 40 cmH2O shortly before extubation. Epidural analgesia. |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: supported by institutional support, Department of Anaesthesiology, Dűsseldorf University Hospital Declaration of interest: one author (TAT) had received a postgraduate stipend from Novartis‐Stiftung fűr therapeutische Forschung. Volume per body weight: predicted body weight: men: 50.0 + 0.91 × (height in cm – 152.4); women: 45.5 + 0.91 × (height in cm – 152.4) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization codes (permuted blocks of 10, allocation ratio 1:1)" |
| Allocation concealment (selection bias) | Low risk | Quote: "... randomization codes (permuted blocks of 10, allocation ratio 1:1) were kept in sequentially numbered sealed opaque envelopes until shortly before induction of general anaesthesia." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "patients and postoperative investigators were blinded to intraoperative group assignment; thus, all postoperative data were collected in a double‐blinded fashion." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "patients and postoperative investigators were blinded to intraoperative group assignment; thus, all postoperative data were collected in a double‐blinded fashion." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Weingarten 2010.
| Methods | RCT with parallel groups Mayo Clinic Institutional Review Board approved the investigation, and each participant gave written informed consent Site: Saint Mary's Hospital, Rochester, MN, USA Setting: university hospital Dates of data collection: unspecified |
|
| Participants | 40 participants aged > 65 years undergoing major open abdominal surgery Exclusion criteria: significant pulmonary disease with abnormalities in spirometry consistent with either obstructive or restrictive pulmonary disease, active asthma (requiring chronic bronchodilator therapy), previous lung surgery, home oxygen therapy, significant cardiac dysfunction (left ventricular ejection fraction < 40%), BMI > 35 kg/m2 |
|
| Interventions |
Treatment group: tidal volume 6 mL/kg (IBW), PEEP 12 cmH2O and recruitment manoeuvres (after tracheal intubation, repeated at 30 and 60 min after the first recruitment and hourly thereafter) (n = 20) Control group: tidal volume 10 mL/kg (IBW), 0 PEEP (actual PEEP 2.5 cmH2O due to the intrinsic PEEP of the mechanical ventilator) and no recruitment manoeuvres (n = 20) In both groups, inspiratory/expiratory time ratio 1:2 and FiO2 0.5 (balance nitrogen) |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: entirely supported by the Department of Anesthesiology, Mayo Clinic, Rochester, MN, USA Declaration of interest: none mentioned Volume per body weight: predicted (abstract) IBW (methods section), no further details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomized to 1 of 2 ventilatory management strategies using a randomization schedule provided by the Division of Biostatistics |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Unclear risk | Quote: "patient characteristics and preoperative comorbidities were similar between the groups, with the exception that more patients in the low tidal volume group had documented coronary artery disease (P value = 0.044)." |
Xiong 2016.
| Methods | RCT with parallel groups Approved by the ethics committee Informed consents obtained Site: First Affiliated Hospital of Chongqing Medical University, China Setting: university hospital Dates of data collection: unspecified |
|
| Participants | 60 ASA I or II participants aged ≥ 40 years undergoing elective spine surgery in the prone position for ≥ 3 hours and with a risk of complications score ≥ 26 Exclusion criteria: receiving mechanical ventilation 2 weeks before surgery; BMI ≥ 35 kg/m2; acute infection or sepsis 2 weeks before surgery; history of chronic obstructive pulmonary disease, thoracic or emergency surgery, progressive neuromuscular disease, acute lung injury or ARDS; need prolonged mechanical ventilation after surgery; poorly controlled hypertension; drug allergy; nausea and vomiting; history of psychological or neurological disease; or receiving chemotherapy or radiotherapy |
|
| Interventions |
Treatment group: tidal volume 6 mL/kg, PEEP 5 cmH2O and recruitment manoeuvres every 30 min (n = 30) Control group: tidal volume 10 mL/kg (n = 30) |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: Ministry of Health, the National Key Laboratory of Clinical Specialties (Finance and Social Sciences (2011) No. 170); Chongqing Municipal Medical Key Discipline Project (Chongqing Wei Science and Education (2007) 2); Chongqing Municipal Health Bureau of Medical Science Key Project (2012‐018) Health Bureau Medical Research Project (2012‐1‐018) Declaration of interest: none mentioned Email sent to authors to request the number of participants who were diagnosed as having a pneumonia after surgery 17 June 2017. No reply received Volume per body weight: predicted body weight; men: 50 + 0.91 × (height in cm – 152.4); women: 45.5 + 0.91 × (height in cm – 152.4) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Zupancich 2005.
| Methods | RCT with parallel groups Approved by the institutional review board and informed consents obtained Site: Università di Torino, Ospedale S. Giovanni Battista, Turin, Italy Setting: university hospital Dates of data collection: not reported |
|
| Participants | 40 participants undergoing elective coronary artery bypass graft Exclusion criteria: presence of cardiogenic pulmonary oedema, emergency or urgent cases, pre‐existing chronic obstructive pulmonary disease, smoking history, major chest wall abnormalities and enrolment in other studies |
|
| Interventions |
Treatment group: tidal volume 8 mL/kg and PEEP 10 cmH2O after CPB disconnection (n = 20) Control group: tidal volume 10–12 mL/kg and PEEP 2–3 cmH2O after CPB disconnection (n = 20) In both ventilatory strategies, FiO2 0.5, inspiratory/expiratory ratio 1:2 and respiratory rate 12–15 breaths/min |
|
| Outcomes |
Relevant to this review
Others
|
|
| Notes | Funding: supported by a grant from the Italian Minister of University and Research (02‐02548) and from the National Research Council (99‐9854) Declaration of interest: none mentioned Volume per body weight: measured body weight |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "protocol withdrawal occurred with any of the following a priori conditions: need for levels of dobutamine or dopamine of greater than 5 mcg/kg/min, haemodynamically unstable condition with positive end‐expiratory pressure, or major cardiac arrhythmia." but "no patients had to be excluded from the study." |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
ARDS: adult respiratory distress syndrome; ASA: American Anesthesiologists Society physical status classification; BMI: body mass index; cm: centimetre; CPB: cardiopulmonary bypass; cmH2O = centimetres of water; dL: decilitre; FiO2: inspired fraction of oxygen; IBW: ideal body weight; ICU: intensive care unit; kg/m2: kilogram per square metre of body surface area; kPa: kiloPascal; min: minute; mg: milligram; mL/kg: millilitres per kilogram of body weight; mmHg: millimetres of mercury; mmol: millimoles; n: number of participants; PaO2: arterial oxygen partial pressure; PEEP: positive end‐expiratory pressure; PaCO2: arterial carbon dioxide partial pressure; pH: hydrogen ion concentration; RCT: randomized controlled trial; SaO2: oxygen saturation measured by pulse oximetry; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akca 2013 | Different intervention; all participants ventilated with tidal volumes of 8–10 mL/kg of body weight |
| Arora 2017 | No outcome of interest measured |
| Baki 2014 | No outcome of interest measured |
| Blum 2013 | Different intervention; intervention group received tidal volumes of ideal body weight of 7.19 mL/kg vs 7.97 mL/kg of body weight |
| Cai 2007 | No outcome of interest measured |
| Clarke 1998 | No outcome of interest measured |
| Cui 2015 | No outcome of interest measured |
| Determann 2010 | Different population: people from a critical care unit |
| Ding 2016 | Different intervention; both groups ventilated with low tidal volumes, i.e. 8 mL/kg vs 6 mL/kg ideal body weight |
| Ela 2014 | No outcome of interest measured |
| Ferrando 2015 | Different intervention; all participants ventilated with tidal volume ≤ 8 mL/kg |
| Gajic 2004 | Not an RCT: retrospective |
| Gajic 2005 | Not an RCT: retrospective |
| Gong 2007 | No outcome of interest measured |
| Hosten 2017 | Different intervention; pressure control vs volume control mechanical ventilation and all participants initially ventilated with tidal volume 8 mL/kg of body weight |
| Jain 2016 | Different intervention: prospective cohort of 60 morbidly obese participants (body mass index > 35 kg/m2), randomized into 2 groups, received positive pressure ventilation determined by ideal body weight and abdominal obesity‐based tidal volume calculation. In abdominal obesity group, tidal volume calculated by formula weight circumference in cm × 6.0 mL and in ideal body weight group by equation ideal body weight (kilogram) × 13 mL |
| Jiang 2007 | No outcome of interest measured |
| Kaisers 2009 | No outcome of interest measured |
| Kanaya 2011 | No outcome of interest measured |
| Kang 2014 | Different study population; 1‐lung ventilation |
| Kim 2012 | Different study population; 1‐lung ventilation |
| Kokulu 2015 | No outcome of interest measured |
| Lee 1990 | Different study population: intubated people in the surgical intensive care unit randomly assigned to group 1 (tidal volume 12 mL/kg, n = 56) or group 2 (tidal volume 6 mL/kg, n = 47) |
| Lellouche 2012 | Not an RCT |
| Lin 2008 | Different study population: 1‐lung ventilation |
| Liu 2016 | Different intervention; all participants received tidal volume 6 mL/kg, 8 mL/kg or 10 mL/kg (predicted body weight) with 50% oxygen with air without PEEP in a random order and successively for 3 minutes in each setting |
| Mascia 2010 | Different study population; organ donors |
| Maslow 2013 | Different study population; 1‐lung ventilation |
| Michelet 2006 | Different study population; 1‐lung ventilation |
| Pinheiro 2010 | Different study population: people from critical care units |
| Reis Miranda 2005a | Different intervention; recruitment manoeuvres |
| Reis Miranda 2005b | Different intervention; recruitment manoeuvres |
| Satoh 2012 | Different intervention; various levels of PEEP in participants receiving low tidal volumes |
| Severgnini 2013 | Different intervention; comparison between 7 mL/kg and 9 mL/kg of ideal body weight |
| Shin 2010 | No outcome of interest measured |
| Thornton 1998 | No outcome of interest measured |
| Tugrul 1998 | Different intervention; all volumes studied were < 10 mL/kg of body weight |
| Tusman 1999 | Different intervention; high tidal volumes of 18 mL/kg of body weight maintained for 10 breaths only |
| Tweed 1991 | Quasi‐randomized and cross‐over trial |
| Visick 1973 | Cross‐over trial |
| Weismann 2010 | Different population |
| Wolthuis 2007 | Not an RCT: before and after an intervention to improve compliance to the new technique |
| Wrigge 2000 | No outcome of interest measured |
| Wrigge 2004 | No outcome of interest measured |
| Wrigge 2005 | Different study population; intervention applied at arrival in intensive care unit after surgery and not intraoperatively |
| Yang 2011 | Different study population; 1‐lung ventilation |
| Zhan‐fang 2010 | Not an RCT and no outcome of interest |
n: number of participants; PEEP: positive end‐expiratory pressure; RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Asida 2015.
| Methods | Randomized, open‐label controlled trial Ethics committee: approved by the Ethics Committee of Qena Faculty of Medicine Informed consent: written informed consents obtained Site: Qena University Hospital, South Valley University Setting: university hospital Dates of data collection: 2013 Funding: university support Registration: ACTRN12614000100695 |
| Participants | 104 ASA I or II participants aged 18–65 years scheduled for elective open urological operations done in the right or left lateral position expected to last > 2 hours Exclusion criteria: body mass index > 30 kg/m2, history of chronic obstructive lung disease, asthma or sleep disorders, heavy smokers (> 2 packs/day), previous lung surgery or acute lung injury, history of neuromuscular diseases or on medications affecting the respiratory system |
| Interventions |
Treatment group: tidal volume 5–7 mL/kg, PEEP 10 cmH2O and recruitment manoeuvres (n = 52) Control group: tidal volume 10–12 mL/kg, 0 PEEP and no recruitment manoeuvres (n = 52) |
| Outcomes |
Relevant to this review
Others
|
| Notes | Contact information: salasida59@gmail.com (S.M. Asida), mohamad_badawy@yahoo.com (M.Sh. Badawy) Conflict of interest: the authors declared no conflict of interest to this study DOI: 10.1016/j.egja.2015.02.001 Volume per body weight: men: weight in kg = 50 + 0.91 × (height in cm – 152.4); women: weight in kg = 45.5 + 0.91 × (height in cm – 152.4) |
Haliloglu 2017.
| Methods | Randomized controlled trial Ethics committee: approved by the Institutional Review Board Informed consent: written informed consents obtained Site: Umraniye Education and Research Hospital, Istanbul, Turkey Setting: university hospital Dates of data collection: January to November 2011 Registration: unspecified |
| Participants | 44 ASA I or II participants undergoing robot‐assisted laparoscopic radical prostatectomy Exclusion criteria: cardiovascular and respiratory diseases (forced expiratory volume in 1 second < 50% of the predicted value and forced vital capacity < 50% of the predicted value), acute asthma exacerbation, obesity (body mass index > 40 kg/m2) and home oxygen therapy |
| Interventions |
Treatment group: tidal volume 6 mL/kg, PEEP 8 cmH2O (n = 24) Control group: tidal volume 10 mL/kg, PEEP 0 cmH2O (n = 20) |
| Outcomes |
Relevant to this review
Others
|
| Notes | Contact information: Beliz Bilgili, Fevzi Cakmak Mah, Mimar Sinan Cad. No. 41 34000 Ust Kaynarca, TR‐34899 Istanbul (Turkey) Email belizbilgili@gmail.com Conflict of interest: none DOI: 10.1159/000484693 Volume per body weight: ideal body weight, no further details |
Moussa 2003.
| Methods | Randomized controlled trial |
| Participants | 20 adults undergoing an elective coronary artery bypass procedure |
| Interventions |
Treatment group: tidal volume 6 mL/kg, FiO2 1.0 and PEEP 5 cmH2O Control group: tidal volume 12 mL/kg, FiO2 1.0 and PEEP 5 cmH2O |
| Outcomes |
|
| Notes | The abstract did not contain enough information We were unable to access the article despite the following steps:
|
Tang 2017.
| Methods | Randomized controlled trial Ethics committee: approved by the local Clinical Research Ethics Committees (2014) Informed consent: written informed consents obtained Site: Renmin Hospital of Wuhan University, Wuhan, China and Anhui Provincial Hospital of Anhui Medical University, Hefei, China Setting: university hospital Dates of data collection: March to December 2016 Funding: supported by grants from the National Natural Science Foundation of China (Grant no. 81671891) and Natural Science Foundation of Hubei Province of China (Grant no. 2016CFB167) Registration: ChiCTR‐IPR‐16008029 |
| Participants | 60 ASA I or II participants aged 18–70 years and with normal pulmonary function undergoing craniotomy Exclusion criteria: bronchial infection, obstructive or restrictive lung disease, asthma, sleep apnoea syndrome, severe hypertension, cardiovascular diseases, liver or kidney dysfunction, history of second‐ or third‐degree heart block or ischaemic heart diseases, and body mass index > 35 kg/m2 |
| Interventions |
Treatment group: tidal volume 6 mL/kg and PEEP 10 cmH2O Control group: tidal volume 12 mL/kg and 0 PEEP |
| Outcomes |
Relevant to this review
Others
|
| Notes | Contact information: Beliz Bilgili, Fevzi Cakmak Mah, Mimar Sinan Cad. No. 41 34000 Ust Kaynarca, TR‐34899 Istanbul (Turkey)
Correspondence: Zhongyuan Xia; xiazhongyuan2005@aliyun.com Conflict of interest: authors declared that there was no conflict of interest regarding the publication of this article DOI: 10.1159/000484693 Volume per body weight: unspecified method |
mL/kg: millilitres per kilogram of body weight; ASA: American Society of Anesthesiologists physical status; cmH2O: centimetres of water; PEEP: positive end‐expiratory pressure; RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12614000790640.
| Trial name or title | A prospective randomized trial of two tidal volume ventilator strategies in patients undergoing major surgery |
| Methods | RCT |
| Participants | Adults undergoing major surgery |
| Interventions |
Treatment group: tidal volume 6 mL/kg (ideal body weight) and PEEP 5 cmH2O Control group: tidal volume 10 mL/kg (Ideal body weight) and PEEP 5 cmH2O |
| Outcomes |
|
| Starting date | 1 August 2014 |
| Contact information | Dr Laurence Weinberg: laurence.weinberg@austin.org.au |
| Notes | Recruiting (accessed 8 January 2018) |
NCT01003730.
| Trial name or title | The effects of different ventilator strategies on inflammation and injury in normal lungs |
| Methods | RCT Site: University of Medicine and Dentistry of New Jersey, Newark, NJ, USA |
| Participants | Adults aged 18–65 years undergoing surgery of ≥ 4 hours in supine position |
| Interventions |
Treatment group: tidal volume 6 mL/kg of predicted body weight and PEEP 3 cmH2O or 10 cmH2O Control group: tidal volume 15 mL/kg of predicted body weight and PEEP 3 cmH2O |
| Outcomes |
|
| Starting date | March 2009 |
| Contact information | Delphin E; Rutgers, The State University of New Jersey, New Brunswick, NJ |
| Notes | Terminated but with the possibility of being resumed later (accessed 8 January 2018) |
NCT03157479.
| Trial name or title | Intraoperative protective ventilation for obese patients undergoing gynaecological laparoscopic surgery (Inprove4large) |
| Methods | RCT Site: Catholic University of the Sacred Heart, Rome, Italy |
| Participants | Adult obese (body mass index > 35 kg/m2) women undergoing laparoscopic surgery |
| Interventions |
Treatment group: tidal volume 6–7 mL/kg of predicted body weight and recruitment manoeuvres Control group: tidal volume 10 mL/kg of predicted body weight and PEEP 5 cmH2O |
| Outcomes |
|
| Starting date | 1 May 2017 |
| Contact information | Domenico Luca Grieco, MD; Email: dlgrieco@ymail.com |
| Notes | Recruiting, last update posted 16 June 2017 (accessed 8 January 2018) |
cmH2O: centimetres of water; ICU: intensive care unit; kg/m2: kilogram per square metre of body surface area; mL/kg: millilitres per kilogram of body weight; PEEP: positive end‐expiratory pressure; RCT: randomized controlled trial.
Differences between protocol and review
2017
One author (SK) joined the review.
Differences between the protocol (Nguyen 2014), and the review (Guay 2015)
The protocol stated: mortality within seven days and within 30 days after surgery. However, only one study with no events provided results specifically within seven days after surgery. Therefore, we decided to include all the relevant available information (in hospital or within 30 days) and considered it as within 30 days after surgery.
The protocol stated that we would look inAnesthesiology for abstracts of the American Society of Anesthesiologists but these abstracts are archived on the website of the society. Therefore, we looked on the website of the American Society of Anesthesiologists instead.
The protocol stated that publication bias would be examined with the classical fail‐safe number (fewer than 10 studies) or a funnel plot followed by the Duval and Tweedie's trim and fill technique for each outcome (10 studies or more). However, the classical fail‐safe number will always be zero when there is no effect and the software (Comprehensive Meta‐analysis) will provide an adjusted effect of estimate for any result with three studies or more. Therefore, we decided to use the Duval and Tweedie's trim and fill technique only for publication bias assessment.
Dat Nhut Nguyen was coauthor for the protocol of this review (Nguyen 2014).
Contributions of authors
Updating the review: JG, EO, SK.
Co‐ordinating the review: JG.
Screening search results: JG, SK.
Organizing retrieval of papers: JG.
Screening retrieved papers against inclusion criteria: JG, SK.
Appraising quality of papers: JG, SK.
Abstracting data from papers: JG, SK.
Writing to authors of papers for additional information: JG.
Data management for the review: JG.
Entering data into Review Manager 5 (Review Manager 2014): JG.
Review Manager 5 statistical data: JG.
Other statistical analysis not using Review Manager 5: JG.
Interpretation of data: JG, EO, SK.
Statistical inferences: JG.
Writing the review: JG, EO, SK.
Securing funding for the review: departmental resources only.
Performing previous work that was the foundation of the present update: JG, EO, SK.
Guarantor for the review (one author): JG.
Person responsible for reading and checking review before submission: JG, EO, SK.
Sources of support
Internal sources
-
University of Montreal, Canada.
University of Montreal granted access to electronic databases and to major medical journals
-
University of Quebec in Abiti‐Temiscamingue, Canada.
University of Quebec in Abiti‐Temiscamingue provided access to electronic databases and medical journals.
-
Cochrane Anaesthesia Review Group, Denmark.
The authors wish to thank Karen Hovhannisyan who designed the search strategy and Janne Vendt who updated the search.
-
University of Sherbrooke, Canada.
University of Sherbrooke granted access to electronic databases and to major medical journals.
-
University of Pennsylvania, USA.
University of Pennsylvania granted access to electronic databases and to major medical journals.
-
Laval University, Canada.
Laval University granted access to electronic databases and to major medical journals
External sources
No sources of support supplied
Declarations of interest
JG: I receive fees as an Associate Professor for a course on airway management at the University of Quebec in Abitibi‐Temiscamingue.
EO: I have had no direct relationship with any equipment manufacturer or pharmaceutical company since 2007. I do not hold any stocks or shares other than mutual funds. I have participated as an expert witness in medical legal cases and patent cases.
Sandra Kopp: no conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Bates 2015 {published data only}
- Bates DM, Fernandes TM, Duwe BV, King BO, Banks DA, Test VJ, et al. Efficacy of a low‐tidal volume ventilation strategy to prevent reperfusion lung injury after pulmonary thromboendarterectomy. Annals of the American Thoracic Society 2015;12(10):1520‐7. [PUBMED: 26241077] [DOI] [PubMed] [Google Scholar]
Chaney 2000 {published data only}
- Chaney MA, Nikolov MP, Blakeman BP, Bakhos M. Protective ventilation attenuates postoperative pulmonary dysfunction in patients undergoing cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia 2000;14(5):514‐8. [PUBMED: 11052430] [DOI] [PubMed] [Google Scholar]
Choi 2006 {published data only}
- Choi G, Wolthuis EK, Bresser P, Levi M, Poll T, Dzoljic M, et al. Mechanical ventilation with lower tidal volumes and positive end‐expiratory pressure prevents alveolar coagulation in patients without lung injury. Anesthesiology 2006;105(4):689‐95. [PUBMED: 17006066] [DOI] [PubMed] [Google Scholar]
- Determann RM, Wolthuis EK, Choi G, Bresser P, Bernard A, Lutter R, et al. Lung epithelial injury markers are not influenced by use of lower tidal volumes during elective surgery in patients without preexisting lung injury. American Journal of Physiology. Lung Cellular and Molecular Physiology 2008;294(2):344‐50. [PUBMED: 18083770] [DOI] [PubMed] [Google Scholar]
- Wolthuis EK, Choi G, Dessing MC, Bresser P, Lutter R, Dzoljic M, et al. Mechanical ventilation with lower tidal volumes and positive end‐expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anaesthesiology 2008;108(1):46‐54. [PUBMED: 18156881] [DOI] [PubMed] [Google Scholar]
Chugh 2012 {published data only}
- Chugh V, Tyagi A, Das S, Sethi AK. Intraoperative ventilation with lowered tidal volumes in patients of perforation peritonitis: effect on postoperative organ dysfunction and systemic inflammatory mediators. www.asaabstracts.com 2012; Vol. Respiration:A1138. [A1138]
Fernandez‐Bustamante 2014 {published data only}
- Fernandez‐Bustamante A, Klawitter J, Repine JE, Agazio A, Janocha AJ, Shah C, et al. Early effect of tidal volume on lung injury biomarkers in surgical patients with healthy lungs. Anesthesiology 2014;121(3):469‐81. [PUBMED: 24809976] [DOI] [PMC free article] [PubMed] [Google Scholar]
Futier 2013 {published data only}
- Futier E, Constantin JM, Paugam‐Burtz C, Pascal J, Eurin M, Neuschwander A, et al. A trial of intraoperative low‐tidal‐volume ventilation in abdominal surgery. New England Journal of Medicine 2013;369(5):428‐37. [PUBMED: 23902482] [DOI] [PubMed] [Google Scholar]
- Jabaudon M, Blondonnet R, Roszyk L, Bazin JE, Pereira B, Sapin V, et al. Influence of intraoperative lung‐protective ventilation on lung epithelial marker Srage, the soluble form of the receptor for advanced glycation end‐products, in patients without preexisting lung injury. American Journal of Respiratory and Critical Care Medicine. 2015; Vol. 191:A1194.
- Jabaudon M, Futier E, Roszyk L, Sapin V, Pereira B, Constantin JM. Association between intraoperative ventilator settings and plasma levels of soluble receptor for advanced glycation end‐products in patients without pre‐existing lung injury. Respirology 2015;20(7):1131‐8. [PUBMED: 26122046] [DOI] [PubMed] [Google Scholar]
- Neuschwander A, Futier E, Jaber S, Pereira B, Eurin M, Marret E, et al. The effects of intraoperative lung protective ventilation with positive end‐expiratory pressure on blood loss during hepatic resection surgery: a secondary analysis of data from a published randomised control trial (IMPROVE). European Journal of Anaesthesiology 2016;33(4):292‐8. [PUBMED: 26716865 ] [DOI] [PubMed] [Google Scholar]
Ge 2013 {published data only}
- Ge Y, Yuan L, Jiang X, Wang X, Xu R, Ma W. Effect of lung protection mechanical ventilation on respiratory function in the elderly undergoing spinal fusion. Zhong Nan da Xue Xue Bao. Yi Xue Ban [Journal of Central South University. Medical Sciences] 2013;38(1):81‐5. [PUBMED: 23406861] [DOI] [PubMed] [Google Scholar]
Koner 2004 {published data only}
- Koner O, Celebi S, Balci H, Cetin G, Karaoglu K, Cakar N. Effects of protective and conventional mechanical ventilation on pulmonary function and systemic cytokine release after cardiopulmonary bypass. Intensive Care Medicine 2004;30(4):620‐6. [PUBMED: 14722635] [DOI] [PubMed] [Google Scholar]
Kuzkov 2016 {published data only}
- Kuzkov VV, Rodionova LN, Ilyina YY, Ushakov AA, Sokolova MM, Fot EV, et al. Protective ventilation improves gas exchange, reduces incidence of atelectases, and affects metabolic response in major pancreatoduodenal surgery. Frontiers in Medicine 2016;3:66. [PUBMED: 27999775] [DOI] [PMC free article] [PubMed] [Google Scholar]
Memtsoudis 2012 {published data only}
- Memtsoudis SG, Bombardieri AM, Ma Y, Girardi FP. The effect of low versus high tidal volume ventilation on inflammatory markers in healthy individuals undergoing posterior spine fusion in the prone position: a randomized controlled trial. Journal of Clinical Anaesthesia 2012;24(4):263‐9. [PUBMED: 22001758] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2016a {published data only}
- Park SJ, Kim BG, Oh AH, Han SH, Han HS, Ryu JH. Effects of intraoperative protective lung ventilation on postoperative pulmonary complications in patients with laparoscopic surgery: prospective, randomized and controlled trial. Surgical Endoscopy 2016;30(10):4598‐606. [PUBMED: 26895920] [DOI] [PubMed] [Google Scholar]
Sato 2016 {published data only}
- Sato H, Nakamura K, Baba Y, Terada S, Goto T, Kurahashi K. Low tidal volume ventilation with low PEEP during surgery may induce lung inflammation. BioMed Central Anesthesiology 2016;16(1):47. [PUBMED: 27473050] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shen 2015 {published data only}
- Shen ZJ, Dong R. Value of protective mechanical ventilation for alleviating symptoms of general anesthesia patients with high risk of postoperative pulmonary complications. Journal of Shanghai Jiaotong University (Medical Science) 2015;35(11):1632‐5. [Google Scholar]
Soh 2018 {published data only}
- Soh S, Shim JK, Ha Y, Kim YS, Lee H, Kwak YL. Ventilation with high or low tidal volume with PEEP does not influence lung function after spinal surgery in prone position: a randomized controlled trial. Journal of Neurosurgical Anesthesiology 2018;30(3):237‐45. [PUBMED: 28338504] [DOI] [PubMed] [Google Scholar]
Sundar 2011 {published data only}
- Sundar S, Novack V, Jervis K, Bender SP, Lerner A, Panzica P, et al. Influence of low tidal volume ventilation on time to extubation in cardiac surgical patients. Anesthesiology 2011;114(5):1102‐10. [PUBMED: 21430518] [DOI] [PMC free article] [PubMed] [Google Scholar]
Treschan 2012 {published data only}
- Treschan TA, Kaisers W, Schaefer MS, Bastin B, Schmalz U, Wania V, et al. Ventilation with low tidal volumes during upper abdominal surgery does not improve postoperative lung function. British Journal of Anaesthesia 2012;109(2):263‐71. [PUBMED: 22661750] [DOI] [PubMed] [Google Scholar]
Weingarten 2010 {published data only}
- Weingarten TN, Whalen FX, Warner DO, Gajic O, Schears GJ, Snyder MR, et al. Comparison of two ventilatory strategies in elderly patients undergoing major abdominal surgery. British Journal of Anaesthesia 2010;104(1):16‐22. [PUBMED: 19933173] [DOI] [PubMed] [Google Scholar]
Xiong 2016 {published data only}
- Xiong W, Chen P, Gao J, Yuan R. Lung protective ventilation in elderly patients undergoing spinal operation in the prone position: a randomized controlled trial. Nan Fang Yi Ke da Xue Xue Bao [Journal of Southern Medical University] 2016;36(2):215‐9. [PUBMED: 26922019] [PubMed] [Google Scholar]
Zupancich 2005 {published data only}
- Zupancich E, Paparella D, Turani F, Munch C, Rossi A, Massaccesi S, et al. Mechanical ventilation affects inflammatory mediators in patients undergoing cardiopulmonary bypass for cardiac surgery: a randomized clinical trial. Journal of Thoracic and Cardiovascular Surgery 2005;130(2):378‐83. [PUBMED: 16077402] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akca 2013 {published data only}
- Akca O, Kurz A, Fleischmann E, Buggy D, Herbst F, Stocchi L, et al. Hypercapnia and surgical site infection: a randomized trial. British Journal of Anaesthesia 2013;111(5):759‐67. [PUBMED: 23887247] [DOI] [PubMed] [Google Scholar]
Arora 2017 {published data only}
- Arora V, Tyagi A, Kumar S, Kakkar A, Das S. Intraoperative low tidal volume ventilation strategy has no benefits during laparoscopic cholecystectomy. Journal of Anaesthesiology, Clinical Pharmacology 2017;33(1):57‐63. [PUBMED: 28413273] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baki 2014 {published data only}
- Baki ED, Kokulu S, Bal A, Ela Y, Sivaci RG, Yoldas M, et al. Evaluation of low tidal volume with positive end‐expiratory pressure application effects on arterial blood gases during laparoscopic surgery. Journal of the Chinese Medical Association 2014;77(7):374‐8. [PUBMED: 24950920] [DOI] [PubMed] [Google Scholar]
Blum 2013 {published data only}
- Blum JM, Stentz MJ, Maile MD, Jewell E, Raghavendran K, Engoren M, et al. Automated alerting and recommendations for the management of patients with preexisting hypoxia and potential acute lung injury: a pilot study. Anesthesiology 2013;119(2):295‐302. [PUBMED: 23681144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cai 2007 {published data only}
- Cai H, Gong H, Zhang L, Wang Y, Tian Y. Effect of low tidal volume ventilation on atelectasis in patients during general anaesthesia: a computed tomographic scan. Journal of Clinical Anaesthesia 2007;19(2):125‐9. [PUBMED: 17379125] [DOI] [PubMed] [Google Scholar]
Clarke 1998 {published data only}
- Clarke JP, Schuitemaker MN, Sleigh JW. The effect of intraoperative ventilation strategies on perioperative atelectasis. Anaesthesia and Intensive Care 1998;26(3):262‐6. [PUBMED: 9619219] [DOI] [PubMed] [Google Scholar]
Cui 2015 {published data only}
- Cui Y, Pi X, Wang C, Liu S, Gong Y, Wang Y, et al. Effects of different ventilation strategies on exhaled nitric oxide in geriatric abdominal surgery. Journal of Breath Research 2015;9(1):016006. [PUBMED: 25719610] [DOI] [PubMed] [Google Scholar]
Determann 2010 {published data only}
- Determann RM, Royakkers A, Wolthuis EK, Vlaar AP, Choi G, Paulus F, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Critical Care (London, England) 2010;14(1):R1. [DOI: 10.1152/ajplung.00268.2007; PUBMED: 20055989] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ding 2016 {published data only}
- Ding W, Shen L, Xiang MJ, Yu C, Lu ZJ. Effects of low tidal volume mechanical ventilation and lung recruitment maneuver on early pulmonary infection after abdominal operations in elderly patients. Journal of Shanghai Jiao Tong University (Medical Science) 2016;36(11):1617‐21. [Google Scholar]
Ela 2014 {published data only}
- Ela Y, Baki ED, Ates M, Kokulu S, Keles I, Karalar M, et al. Exploring for the safer ventilation method in laparoscopic urologic patients? Conventional or low tidal?. Journal of Laparoendoscopic & Advanced Surgical Techniques. Part a 2014;24(11):786‐90. [PUBMED: 24918629] [DOI] [PubMed] [Google Scholar]
Ferrando 2015 {published data only}
- Ferrando C, Soro M, Canet J, Unzueta MC, Suarez F, Librero J, et al. Rationale and study design for an individualized perioperative open lung ventilatory strategy (iPROVE): study protocol for a randomized controlled trial. Trials 2015;16:193. [PUBMED: 25927183] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gajic 2004 {published data only}
- Gajic O, Dara SI, Mendez JL, Adesanya AO, Festic E, Caples SM, et al. Ventilator‐associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Critical Care Medicine 2004;32(9):1817‐24. [PUBMED: 15343007] [DOI] [PubMed] [Google Scholar]
Gajic 2005 {published data only}
- Gajic O, Frutos‐Vivar F, Esteban A, Hubmayr RD, Anzueto A. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Medicine 2005;31(7):922‐6. [PUBMED: 15856172] [DOI] [PubMed] [Google Scholar]
Gong 2007 {published data only}
- Gong H, Zhang LN, Cai HW, Wang YJ, Hou YH. Effect of different tidal volume ventilations on atelectasis in patients during general anaesthesia by CT scan. Zhong Nan da Xue Xue Bao. Yi Xue Ban [Journal of Central South University. Medical Sciences] 2007;32(5):850‐4. [PUBMED: 18007083] [PubMed] [Google Scholar]
Hosten 2017 {published data only}
- Hosten T, Kus A, Gumus E, Yavuz S, Irkil S, Solak M. Comparison of intraoperative volume and pressure‐controlled ventilation modes in patients who undergo open heart surgery. Journal of Clinical Monitoring and Computing 2017;31(1):75‐84. [PUBMED: 26992377] [DOI] [PubMed] [Google Scholar]
Jain 2016 {published data only}
- Jain AK. A ventilatory strategy for morbidly obese undergoing laparoscopic surgery ‐ abdominal obesity based approach. Anesthesia and Analgesia 2016;123 (3 Suppl 2):812. [EMBASE: 612648492] [Google Scholar]
Jiang 2007 {published data only}
- Jiang JJ, Yuan HB, Sun PL. Effect of low tidal volume ventilation plus positive end‐expiratory pressure on pulmonary oxygenation in septic patients during operation. Academic Journal of Second Military Medical University 2007;28(5):570‐2. [DOI: 10.3724/SP.J.1008.2007.00570] [DOI] [Google Scholar]
Kaisers 2009 {published data only}
- Kaisers W, Wania V, Eisenberger C, Schmitz A, Treschan T. Influence of high versus low tidal volumes on airway pressure in patients with healthy lungs [Abstract]. European Journal of Anaesthesiology 2009;26(Suppl 45):81. [Google Scholar]
Kanaya 2011 {published data only}
- Kanaya A, Satoh D, Kurosawa S. Influence of tidal volume on functional residual capacity during general anaesthesia. Masui. The Japanese Journal of Anesthesiology 2011;60(10):1149‐52. [PUBMED: 22111353] [PubMed] [Google Scholar]
Kang 2014 {published data only}
- Kang WS, Kim SH, Woo Chung J. Comparison of pulmonary gas exchange according to intraoperative ventilation modes for mitral valve repair surgery via thoracotomy with one‐lung ventilation: a randomized controlled trial. Journal of Cardiothoracic and Vascular Anaesthesia 2014;28(4):920‐5. [PUBMED: 24480179] [DOI] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim SH, Jung KT, An TH. Effects of tidal volume and PEEP on arterial blood gases and pulmonary mechanics during one‐lung ventilation. Journal of Anaesthesia 2012;26(4):568‐73. [PUBMED: 22349751] [DOI] [PubMed] [Google Scholar]
Kokulu 2015 {published data only}
- Kokulu S, Gunay E, Baki ED, Ulasli SS, Yilmazer M, Koca B, et al. Impact of a lung‐protective ventilatory strategy on systemic and pulmonary inflammatory responses during laparoscopic surgery: is it really helpful?. Inflammation 2015;38(1):361‐7. [PUBMED: 25280837] [DOI] [PubMed] [Google Scholar]
Lee 1990 {published data only}
- Lee PC, Helsmoortel CM, Cohn SM, Fink MP. Are low tidal volumes safe?. Chest 1990;97(2):430‐4. [PUBMED: 2288551] [DOI] [PubMed] [Google Scholar]
Lellouche 2012 {published data only}
- Lellouche F, Dionne S, Simard S, Bussieres J, Dagenais F. High tidal volumes in mechanically ventilated patients increase organ dysfunction after cardiac surgery. Anesthesiology 2012;116(5):1072‐82. [DOI: 10.1097/ALN.0b013e3182522df5; PUBMED: 22450472] [DOI] [PubMed] [Google Scholar]
Lin 2008 {published data only}
- Lin WQ, Lu XY, Cao LH, Wen LL, Bai XH, Zhong ZJ. Effects of the lung protective ventilatory strategy on proinflammatory cytokine release during one‐lung ventilation. Ai Zheng [Chinese Journal of Cancer] 2008;27(8):870‐3. [PUBMED: 18710624] [PubMed] [Google Scholar]
Liu 2016 {published data only}
- Liu Y, Lou JS, Mi WD, Yuan WX, Fu Q, Wang M, et al. Pulse pressure variation shows a direct linear correlation with tidal volume in anesthetized healthy patients. BioMed Central Anesthesiology 2016;16:75. [PUBMED: 27609188] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mascia 2010 {published data only}
- Mascia L, Pasero D, Slutsky AS, Arguis MJ, Berardino M, Grasso S, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA 2010;304(23):2620‐7. [PUBMED: 21156950] [DOI] [PubMed] [Google Scholar]
Maslow 2013 {published data only}
- Maslow AD, Stafford TS, Davignon KR, Ng T. A randomized comparison of different ventilator strategies during thoracotomy for pulmonary resection. Journal of Thoracic and Cardiovascular Surgery 2013;146(1):38‐44. [PUBMED: 23380515] [DOI] [PubMed] [Google Scholar]
Michelet 2006 {published data only}
- Michelet P, D'Journo XB, Roch A, Doddoli C, Marin V, Papazian L, et al. Protective ventilation influences systemic inflammation after esophagectomy: a randomized controlled study. Anesthesiology 2006;105(5):911‐9. [PUBMED: 17065884] [DOI] [PubMed] [Google Scholar]
Pinheiro 2010 {published data only}
- Pinheiro de Oliveira R, Hetzel MP, dos Anjos Silva M, Dallegrave D, Friedman G. Mechanical ventilation with high tidal volume induces inflammation in patients without lung disease. Critical Care (London, England) 2010;14(2):R39. [DOI: 10.1186/cc8919; PUBMED: 20236550] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reis Miranda 2005a {published data only}
- Reis Miranda D, Struijs A, Koetsier P, Thiel R, Schepp R, Hop W, et al. Open lung ventilation improves functional residual capacity after extubation in cardiac surgery. Critical Care Medicine 2005;33(10):2253‐8. [PUBMED: 16215379] [DOI] [PubMed] [Google Scholar]
Reis Miranda 2005b {published data only}
- Reis Miranda D, Gommers D, Struijs A, Dekker R, Mekel J, Feelders R, et al. Ventilation according to the open lung concept attenuates pulmonary inflammatory response in cardiac surgery. European Journal of Cardio‐thoracic Surgery 2005;28(6):889‐95. [PUBMED: 16271479] [DOI] [PubMed] [Google Scholar]
Satoh 2012 {published data only}
- Satoh D, Kurosawa S, Kirino W, Wagatsuma T, Ejima Y, Yoshida A, et al. Impact of changes of positive end‐expiratory pressure on functional residual capacity at low tidal volume ventilation during general anaesthesia. Journal of Anaesthesia 2012;26(5):664‐9. [PUBMED: 22584817] [DOI] [PMC free article] [PubMed] [Google Scholar]
Severgnini 2013 {published data only}
- Severgnini P, Selmo G, Lanza C, Chiesa A, Frigerio A, Bacuzzi A, et al. Protective mechanical ventilation during general anaesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 2013;118(6):1307‐21. [PUBMED: 23542800] [DOI] [PubMed] [Google Scholar]
Shin 2010 {published data only}
- Shin HY, Kim SH, Lee YJ, Kim DK. The effect of mechanical ventilation tidal volume during pneumoperitoneum on shoulder pain after a laparoscopic appendectomy. Surgical Endoscopy 2010;24(8):2002‐7. [PUBMED: 20135168] [DOI] [PubMed] [Google Scholar]
Thornton 1998 {published data only}
- Thornton S, Fletcher R, Foster P. Little effect of ventilatory rate and tidal volume on end‐expiratory pressure after cardiac surgery. British Journal of Anaesthesia 1998;80(4):553‐4. [EMBASE: 28171415] [Google Scholar]
Tugrul 1998 {published data only}
- Tugrul M, Camci E, Yavru A, Turnaoglu S, Karakoca S, Ozkan T, et al. Reducing tidal volume does not affect oxygenation in ASA I‐II patients during anaesthesia. Applied Cardiopulmonary Pathophysiology 1998;8(1):29‐35. [EMBASE: 29462282] [Google Scholar]
Tusman 1999 {published data only}
- Tusman G, Bohm SH, Vazquez de Anda GF, do Campo JL, Lachmann B. 'Alveolar recruitment strategy' improves arterial oxygenation during general anaesthesia. British Journal of Anaesthesia 1999;82(1):8‐13. [PUBMED: 10325828] [DOI] [PubMed] [Google Scholar]
Tweed 1991 {published data only}
- Tweed WA, Phua WT, Chong KY, Lim E, Lee TL. Large tidal volume ventilation improves pulmonary gas exchange during lower abdominal surgery in Trendelenburg's position. Canadian Journal of Anaesthesia 1991;38(8):989‐95. [PUBMED: 1752022] [DOI] [PubMed] [Google Scholar]
Visick 1973 {published data only}
- Visick WD, Fairley HB, Hickey RF. The effects of tidal volume and end‐expiratory pressure on pulmonary gas exchange during anaesthesia. Anesthesiology 1973;39(3):285‐90. [PUBMED: 4580631] [DOI] [PubMed] [Google Scholar]
Weismann 2010 {published data only}
- Weismann D, Maier SKG. Prevention of a breathing trauma with low tidal volume ventilation: results of a randomized, controlled prevention study [Prävention eines beatmungstraumas mit niedrigen tidalvolumina. Ergebnisse einer randomisierten, kontrollierten präventionsstudie]. Medizinische Klinik 2010;105(3):178‐9. [EMBASE: 358748244] [Google Scholar]
Wolthuis 2007 {published data only}
- Wolthuis EK, Korevaar JC, Spronk P, Kuiper MA, Dzoljic M, Vroom MB, et al. Feedback and education improve physician compliance in use of lung‐protective mechanical ventilation. Intensive Care Medicine 2005;31(4):540‐6. [DOI: 10.1007/s00134-005-2581-9; PUBMED: 15731892] [DOI] [PubMed] [Google Scholar]
- Wolthuis EK, Veelo DP, Choi G, Determann RM, Korevaar JC, Spronk PE, et al. Mechanical ventilation with lower tidal volumes does not influence the prescription of opioids or sedatives. Critical Care (London, England) 2007;11(4):R77. [DOI: 10.1186/cc5969; PUBMED: 17629900] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wrigge 2000 {published data only}
- Wrigge H, Zinserling J, Stuber F, Spiegel T, Hering R, Wetegrove S, et al. Effects of mechanical ventilation on release of cytokines into systemic circulation in patients with normal pulmonary function. Anesthesiology 2000;93(6):1413‐7. [PUBMED: 11149435] [DOI] [PubMed] [Google Scholar]
Wrigge 2004 {published data only}
- Wrigge H, Uhlig U, Zinserling J, Behrends‐Callsen E, Ottersbach G, Fischer M, et al. The effects of different ventilatory settings on pulmonary and systemic inflammatory responses during major surgery. Anaesthesia and Analgesia 2004;98(3):775‐81. [DOI: 10.1213/01.ANE.0000100663.11852.BF; PUBMED: 14980936] [DOI] [PubMed] [Google Scholar]
Wrigge 2005 {published data only}
- Wrigge H, Uhlig U, Baumgarten G, Menzenbach J, Zinserling J, Ernst M, et al. Mechanical ventilation strategies and inflammatory responses to cardiac surgery: a prospective randomized clinical trial. Intensive Care Medicine 2005;31(10):1379‐87. [PUBMED: 16132888] [DOI] [PubMed] [Google Scholar]
Yang 2011 {published data only}
- Yang M, Ahn HJ, Kim K, Kim JA, Yi CA, Kim MJ, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: a randomized controlled trial. Chest 2011;139(3):530‐7. [PUBMED: 20829341] [DOI] [PubMed] [Google Scholar]
Zhan‐fang 2010 {published data only}
- Zhan‐fang LI, Min ZJ, Qing‐hua LI, Tian ZY, Cheng LU, Jiang B, et al. The optimal combination of mechanical ventilatory parameters under general anaesthesia in obese patients undergoing laparoscopic surgery. Fudan University Journal of Medical Sciences 2010;37(1):11‐5. [Google Scholar]
References to studies awaiting assessment
Asida 2015 {published data only}
- ACTRN12614000100695. Effect of low tidal volume during general anaesthesia for urological procedures on lung functions. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12614000100695 Date first received: 28 January 2014.
- Asida S, Badawy M. Effect of low tidal volume during general anesthesia for urological procedures on lung functions. Egyptian Journal of Anaesthesia 2015;31:127‐34. [CENTRAL: 01070550; DOI: 10.1016/j.egja.2015.02.001; EMBASE: 603507596] [DOI] [Google Scholar]
- Badawy M, Asida S. Effect of low tidal volume during general anesthesia for urological procedures on lung functions. Chest 2015;148:1015A. [CENTRAL: 01163112; DOI: 10.1378/chest.2275080; EMBASE: 72132873] [DOI] [Google Scholar]
- Badawy M, Asida S. Effect of low tidal volume during general anesthesia for urological procedures on lung functions. European Respiratory Journal 2015;46:1824. [CENTRAL: 01162393; DOI: 10.1183/13993003; EMBASE: 72105867] [DOI] [Google Scholar]
Haliloglu 2017 {published data only}
- Haliloglu M, Bilgili B, Ozdemir M, Umuroglu T, Bakan N. Low tidal volume‐positive end‐expiratory pressure versus high tidal volume‐zero positive end‐expiratory pressure and postoperative pulmonary functions in robot‐assisted laparoscopic radical prostatectomy. Medical Principles and Practice 2017;31:31. [DOI: 10.1159/000484693; PUBMED: 29131002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moussa 2003 {published data only}
- Moussa SF, Khlousy M. Clinical and laboratory assessment of protective ventilation versus conventional ventilation in patients undergoing coronary artery bypass surgery. Egyptian Journal of Anaesthesia 2003;19(4):337‐43. [CENTRAL: 00474837] [Google Scholar]
Tang 2017 {published data only}
- Tang C, Li J, Lei S, Zhao B, Zhang Z, Huang W, et al. Lung‐protective ventilation strategies for relief from ventilator‐associated lung injury in patients undergoing craniotomy: a bicenter randomized, parallel, and controlled trial. Oxidative Medicine and Cellular Longevity 2017;2017:1‐12. [CENTRAL: 01394981; DOI: 10.1155/2017/6501248; EMBASE: 617361026; PUBMED: 28757912] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12614000790640 {published data only}
- ACTRN12614000790640. A prospective randomised trial of two tidal volume ventilator strategies in patients undergoing major surgery. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366752&isReview=true Date first received: 24 July 2014.
NCT01003730 {published data only}
- NCT01003730. The effects of different ventilator strategies on inflammation and injury in normal lungs. clinicaltrials.gov/ct2/show/NCT01003730 Date first received: 29 October 2009. [NCT01003730]
NCT03157479 {published data only}
- NCT03157479. Intraoperative protective ventilation for obese patients undergoing gynaecological laparoscopic surgery (Inprove4large). clinicaltrials.gov/ct2/show/NCT03157479 Date first received: 17 May 2017. [NCT03157479]
Additional references
Acute Respiratory Distress Syndrome Network
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine 2000;342(18):1301‐8. [PUBMED: 10793162] [DOI] [PubMed] [Google Scholar]
Aliverti 2011
- Aliverti A, Kostic P, Lo Mauro A, Andersson‐Olerud M, Quaranta M, Pedotti A, et al. Effects of propofol anaesthesia on thoraco‐abdominal volume variations during spontaneous breathing and mechanical ventilation. Acta Anaesthesiologica Scandinavica 2011;55(5):588‐96. [PUBMED: 21385159] [DOI] [PubMed] [Google Scholar]
Barbosa 2014
- Barbosa FT, Castro AA, Sousa‐Rodrigues CF. Positive end‐expiratory pressure (PEEP) during anaesthesia for prevention of mortality and postoperative pulmonary complications. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD007922.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bendixen 1963
- Bendixen HH, Hedley‐Whyte J, Laver MB. Impaired oxygenation in surgical patients during general anaesthesia with controlled ventilation. A concept of atelectasis. New England Journal of Medicine 1963;269:991‐6. [PUBMED: 14059732] [DOI] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins PT, Rothstein HR. Publication bias. In: Borenstein M, Hedges LV, Higgins JP, Rothstein HR editor(s). Introduction to Meta‐Analysis. 1st Edition. Chichester, UK: Wiley, 2009:277‐92. [Google Scholar]
Brismar 1985
- Brismar B, Hedenstierna G, Lundquist H, Strandberg A, Svensson L, Tokics L. Pulmonary densities during anaesthesia with muscular relaxation ‐ a proposal of atelectasis. Anesthesiology 1985;62(4):422‐8. [PUBMED: 3885791] [DOI] [PubMed] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21(11):1575‐600. [PUBMED: 12111921] [DOI] [PubMed] [Google Scholar]
Duval 2000a
- Duval S, Tweedie R. A simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56(2):455‐63. [PUBMED: 10877304] [DOI] [PubMed] [Google Scholar]
Duval 2000b
- Duval S, Tweedie R. A nonparametric "trim and fill" method accounting for publication bias in meta‐analysis. Journal of the American Statistical Association 2000;95(449):89‐98. [Google Scholar]
Edmark 2011
- Edmark L, Auner U, Enlund M, Ostberg E, Hedenstierna G. Oxygen concentration and characteristics of progressive atelectasis formation during anaesthesia. Acta Anaesthesiologica Scandinavica 2011;55(1):75‐81. [PUBMED: 21039356] [DOI] [PubMed] [Google Scholar]
Eldridge 2004
- Eldridge MW, Dempsey JA, Haverkamp HC, Lovering AT, Hokanson JS. Exercise‐induced intrapulmonary arteriovenous shunting in healthy humans. Journal of Applied Physiology 2004;97(3):797‐805. [PUBMED: 15107409] [DOI] [PubMed] [Google Scholar]
Gu 2015
- Gu WJ, Wang F, Liu JC. Effect of lung‐protective ventilation with lower tidal volumes on clinical outcomes among patients undergoing surgery: a meta‐analysis of randomized controlled trials. Canadian Medical Association Journal 2015;187(3):E101‐9. [DOI: 10.1503/cmaj.141005; PUBMED: 25512653] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunnarsson 1991
- Gunnarsson L, Tokics L, Gustavsson H, Hedenstierna G. Influence of age on atelectasis formation and gas exchange impairment during general anaesthesia. British Journal of Anaesthesia 1991;66(4):423‐32. [PUBMED: 2025468] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research Ed.) 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Levin 2014
- Levin MA, McCormick PJ, Lin HM, Hosseinian L, Fischer GW. Low intraoperative tidal volume ventilation with minimal PEEP is associated with increased mortality. British Journal of Anaesthesia 2014;113(1):97‐108. [PUBMED: 24623057] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olper 2013
- Olper L, Corbetta D, Cabrini L, Landoni G, Zangrillo A. Effects of non‐invasive ventilation on reintubation rate: a systematic review and meta‐analysis of randomised studies of patients undergoing cardiothoracic surgery. Critical Care and Resuscitation 2013;15(3):220‐7. [PUBMED: 23944209] [PubMed] [Google Scholar]
Pace 2011
- Pace NL. Research methods for meta‐analyses. Best Practice & Research. Clinical Anaesthesiology 2011;25(4):523‐33. [PUBMED: 22099918] [DOI] [PubMed] [Google Scholar]
Park 2016b
- Park SH. Perioperative lung‐protective ventilation strategy reduces postoperative pulmonary complications in patients undergoing thoracic and major abdominal surgery. Korean Journal of Anesthesiology 2016;69(1):3‐7. [DOI: 10.4097/kjae.2016.69.1.3; PUBMED: 26885294] [DOI] [PMC free article] [PubMed] [Google Scholar]
Petersson 2010
- Petersson J, Ax M, Frey J, Sanchez‐Crespo A, Lindahl SG, Mure M. Positive end‐expiratory pressure redistributes regional blood flow and ventilation differently in supine and prone humans. Anesthesiology 2010;113(6):1361‐9. [PUBMED: 21068656] [DOI] [PubMed] [Google Scholar]
Petrucci 2013
- Petrucci N, Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD003844.pub4; PUBMED: 23450544] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pogue 1998
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomised controlled trials. Lancet 1998;351(9095):47‐52. [PUBMED: 9433436] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rucker 2011
- Rucker G, Schwarzer G, Carpenter JR, Binder H, Schumacher M. Treatment‐effect estimates adjusted for small‐study effects via a limit meta‐analysis. Biostatistics 2011;12(1):122‐42. [PUBMED: 20656692] [DOI] [PubMed] [Google Scholar]
Rusca 2003
- Rusca M, Proietti S, Schnyder P, Frascarolo P, Hedenstierna G, Spahn DR, et al. Prevention of atelectasis formation during induction of general anaesthesia. Anesthesia and Analgesia 2003;97(6):1835‐9. [PUBMED: 14633570] [DOI] [PubMed] [Google Scholar]
Serpa Neto 2012
- Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, Pasqualucci Mde O, et al. Association between use of lung‐protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta‐analysis. JAMA 2012;308(16):1651‐9. [DOI: 10.1001/jama.2012.13730; PUBMED: 23093163] [DOI] [PubMed] [Google Scholar]
Serpa Neto 2015a
- Serpa Neto A, Simonis FD, Barbas CS, Biehl M, Determann RM, Elmer J, et al. Lung‐protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: a systematic review and individual patient data analysis. Critical Care Medicine 2015;43(10):2155‐63. [DOI: 10.1097/CCM.0000000000001189; PUBMED: 26181219] [DOI] [PubMed] [Google Scholar]
Serpa Neto 2015b
- Serpa Neto A, Hemmes SN, Barbas CS, Beiderlinden M, Biehl M, Binnekade JM, et al. Protective versus conventional ventilation for surgery: a systematic review and individual patient data meta‐analysis. Anesthesiology 2015;123(1):66‐78. [DOI: 10.1097/ALN.0000000000000706; PUBMED: 25978326] [DOI] [PubMed] [Google Scholar]
Tao 2014
- Tao T, Bo L, Chen F, Xie Q, Zou Y, Hu B, et al. Effect of protective ventilation on postoperative pulmonary complications in patients undergoing general anaesthesia: a meta‐analysis of randomised controlled trials. BMJ Open 2014;4(6):e005208. [DOI: 10.1136/bmjopen-2014-005208; PUBMED: 24961718] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tusman 2014
- Tusman G, Groisman I, Fiolo FE, Scandurra A, Arca JM, Krumrick G, et al. Noninvasive monitoring of lung recruitment maneuvers in morbidly obese patients: the role of pulse oximetry and volumetric capnography. Anaesthesia and Analgesia 2014;118(1):137‐44. [PUBMED: 24356163] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Guay 2015
- Guay J, Ochroch EA. Intraoperative use of low volume ventilation to decrease postoperative mortality, mechanical ventilation, lengths of stay and lung injury in patients without acute lung injury. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD011151.pub2] [DOI] [PubMed] [Google Scholar]
Nguyen 2014
- Nguyen D‐N, Guay J, Ochroch EA. Intraoperative use of low volume ventilation to decrease postoperative mortality, mechanical ventilation, lengths of stay and lung injury in patients without acute lung injury. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD011151] [DOI] [PubMed] [Google Scholar]


