Abstract
Background
There remains uncertainty about the optimum timing of antiretroviral therapy (ART) initiation in HIV‐positive people with cryptococcal meningitis. This uncertainty is the result of conflicting data on the mortality risk and occurrence of immune reconstitution inflammatory syndrome (IRIS) when ART is initiated less than four weeks after cryptococcal meningitis treatment is commenced.
Objectives
To compare the outcomes of early initiation of ART (less than four weeks after starting antifungal treatment) versus delayed initiation of ART (four weeks or more after starting antifungal treatment) in HIV‐positive people with concurrent cryptococcal meningitis.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase for trials published between 1 January 1980 and 7 August 2017. We additionally searched international trial registries, including ClinicalTrials.gov and WHO International Clinical Trials Registry Platform (ICTRP), and conference abstracts from the International AIDS Society (IAS) and the Conference on Retroviruses and Opportunistic Infections (CROI) for ongoing or unpublished studies between 2015 and 2017. We reviewed reference lists of included studies to identify additional studies.
Selection criteria
We included randomized controlled trials (RCTs) that compared early versus delayed ART initiation in HIV‐positive people with cryptococcal meningitis. Children, adults, and adolescents from any setting were eligible for inclusion.
Data collection and analysis
Two review authors independently applied the inclusion criteria and extracted data. We presented dichotomous outcomes as risk ratios (RR) with 95% confidence intervals (CIs). We presented time‐to‐death data as hazard ratios with 95% CIs. We assessed the certainty of the evidence using the GRADE approach.
Main results
Four trials including 294 adult participants met the inclusion criteria of this review. Participants were predominantly from low‐ and middle‐income countries. Two trials treated cryptococcal meningitis with amphotericin B and fluconazole; a third trial used fluconazole monotherapy; and the fourth trial did not specify the antifungal used.
Early ART initiation may increase all‐cause mortality compared to delayed ART initiation (RR 1.42, 95% CI 1.02 to 1.97; 294 participants, 4 trials; low‐certainty evidence). Early ART initiation may reduce relapse of cryptococcal meningitis compared to delayed ART initiation (RR 0.27, 95% CI 0.07 to 1.04; 205 participants, 2 trials, low‐certainty evidence). We are uncertain whether early ART initiation increases or reduces cryptococcal IRIS events compared to delayed ART initiation (RR 3.56, 95% CI 0.51 to 25.02; 205 participants, 2 trials; I2 = 54%; very low‐certainty evidence). We are uncertain if early ART initiation increases or reduces virological suppression at six months compared to delayed ART initiation (RR 0.93, 95% CI 0.72 to 1.22; 205 participants, 2 trials; I2 statistic = 0%; very low‐certainty evidence).
We were unable to pool results related to rate of fungal clearance for the two trials that reported this outcome; individual trial results indicated that there was no difference in cerebrospinal fluid fungal clearance between trial arms. Similarly, we were unable to pool results on adverse events for the trials reporting on this outcome; individual trial results indicated no difference in the occurrence of grade 3 to 5 adverse events between trial arms.
Three of the four included trials had an overall low or unclear risk of bias related to the primary outcome of all‐cause mortality. However, we assessed one trial as at high risk of bias due to selective outcome reporting and other bias. This, in addition to the few clinical events and imprecision of effect estimates, led to downgrading of the evidence to low or very low certainty.
Authors' conclusions
The results of this review are relevant to HIV‐positive adults with cryptococcal meningitis in low‐ and middle‐income countries. These data suggest a higher risk of mortality among people who initiate ART within four weeks of cryptococcal meningitis diagnosis. However, it is unclear if this higher mortality risk is related to cryptococcal meningitis‐IRIS.
11 April 2019
Up to date
All studies incorporated from most recent search
All eligible published studies found in the last search (7 Aug, 2017) were included
Plain language summary
Timing of antiretroviral therapy initiation in HIV‐positive people with cryptococcal meningitis
What is the aim of this review?
The aim of this Cochrane Review was to determine whether initiating antiretroviral therapy (ART) within four weeks of cryptococcal meningitis diagnosis resulted in a higher risk of dying or developing other complications than waiting more than four weeks to initiate ART.
Key messages
Initiating ART within four weeks of cryptococcal meningitis diagnosis may result in more deaths than initiating ART after four weeks. However, initiating ART early may result in a reduction in relapses of cryptococcal meningitis after adequate treatment. There was insufficient evidence to answer questions related to other complications.
What was studied in the review?
Cryptococcal meningitis is a fungal infection of the brain and the membranes covering the brain that occurs most frequently in people with weakened immune systems, such as people who are HIV‐positive. Some studies have shown that HIV‐positive people who start ART soon after initiating cryptococcal meningitis treatment (within four weeks) may deteriorate and die more frequently than those who delay treatment for a longer period (more than four weeks). This higher risk of death in the early ART group has been attributed to the occurrence of a condition called immune reconstitution inflammatory syndrome (IRIS). When ART is initiated, HIV‐positive people with underlying infections such as cryptococcal meningitis may paradoxically develop a deterioration in their condition as their body's immune system attacks the fungus, resulting in worsening symptoms and sometimes death. It has been proposed that IRIS is the cause of more deaths in early ART initiators than in delayed ART initiators. Despite adequate treatment of cryptococcal meningitis with antifungal drugs, a relapse of the disease may occur in some HIV‐positive people with cryptococcal meningitis. To date there have been few trials investigating the effect of ART on mortality, frequency of IRIS, or relapse.
What are the main results of the review?
We found four relevant trials that compared HIV‐positive adults who had cryptococcal meningitis and who initiated ART within four weeks of cryptococcal meningitis diagnosis with those who initiated ART after four weeks.
Pooling the results of these four trials suggested that early ART initiation may increase the frequency of death in HIV‐positive people with cryptococcal meningitis (low‐certainty evidence). Early ART initiators may be less likely to have relapses of cryptococcal meningitis (low‐certainty evidence). We were unable to draw conclusions regarding IRIS frequency as the certainty of the evidence contributing to the IRIS assessment was very low. We are uncertain as to whether or not early ART initiation increases or reduces virological suppression at six months compared to delayed ART initiation (very low‐certainty evidence).
Overall, few trials met the inclusion criteria for this review, which made it hard to draw definite conclusions on the association between ART timing and cryptococcal meningitis in HIV‐positive people.
How up to date is the review?
We searched for studies up to 7 August 2017.
Summary of findings
Summary of findings for the main comparison. Early ART compared to delayed ART initiation in HIV‐positive people with cryptococcal meningitis.
| Early ART compared to delayed ART initiation in HIV‐positive people with cryptococcal meningitis | ||||||
| Patient or population: HIV‐positive people with cryptococcal meningitis Setting: global Intervention: early ART initiation (less than 4 weeks after initiation of cryptococcal meningitis treatment) Comparison: delayed ART initiation (more than 4 weeks after initiation of cryptococcal meningitis treatment) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with delayed ART | Risk with early ART | |||||
| All‐cause mortality at 6 to 12 months | 311 per 1000 | 442 per 1000 (317 to 613) | RR 1.42 (1.02 to 1.97) | 294 (4 RCTs) | ⊕⊕⊝⊝ LOW1,2,3 | Early ART initiation may increase the risk of mortality at 6 to 12 months. |
| Cryptococcal meningitis relapse | 87 per 1000 | 24 per 1000 (6 to 91) | RR 0.27 (0.07 to 1.04) | 205 (2 RCTs) | ⊕⊕⊝⊝ LOW4 | Early ART initiation may reduce relapses of cryptococcal meningitis compared to delayed ART initiation. |
| Cryptococcal IRIS | 87 per 1000 | 311 per 1000 (45 to 1000) | RR 3.56 (0.51 to 25.02) | 205 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW4,5,6 | We are uncertain as to whether or not early ART initiation increases or reduces cryptococcal IRIS events compared to delayed ART initiation. |
| HIV virological suppression at 6 months (viral load < 400 copies/mL) | 534 per 1000 | 497 per 1000 (384 to 651) | RR 0.93 (0.72 to 1.22) | 205 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW7,8 | We are uncertain as to whether or not early ART initiation increases or reduces virological suppression at 6 months compared to delayed ART initiation. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ART: antiretroviral therapy; CI: confidence interval; IRIS: immune reconstitution inflammatory syndrome; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Risk of bias: downgraded by a half point due to high risk of other bias in Makadzange 2010. 2Imprecision: downgraded by 1 for wide CIs including no effect and appreciable harm. In addition, there were few clinical events (< 200). 3Heterogeneity: downgraded by a half point due to qualitative heterogeneity (different drug regimens and study methods). 4Imprecision: downgraded by 2 for wide CIs and very few clinical events. 5Risk of bias: downgraded by 1 as IRIS outcome assessors in Bisson 2013 were unblinded. 6Indirectness: downgraded 1 as despite case definition, diagnosing IRIS can be very subjective/misdiagnosed. 7Risk of bias: downgraded by 2 as not all those who were randomized to early or delayed ART received ART. In addition, not all those who received ART had a viral load done. It cannot be assumed that these results are missing at random. This is explored in the sensitivity analyses. 8Imprecision: downgraded by 1 for few clinical events (< 200).
Background
HIV/AIDS remains one of the world's most significant public health challenges, particularly in low‐ and middle‐income countries. Antiretroviral therapy (ART) has substantially improved HIV prognosis by reducing associated morbidity and mortality. However uncertainty remains as to the optimal time for ART initiation when an HIV‐positive person is co‐infected with an opportunistic infection, particularly cryptococcal meningitis.
Description of the condition
Cryptococcal meningitis is an important opportunistic AIDS‐defining infection and a major contributor to high mortality before and after ART is initiated (Bicanic 2005; WHO 2013). It is caused by the encapsulated yeast Cryptococcus neoformans, which is inhaled as small yeast cells. Patients may present with headache, fever, malaise, and altered mental status; they may also have signs of meningism, papilloedema, and focal neurological deficits. Raised intracranial pressure likely results from poor absorption of cerebrospinal fluid (CSF); this is usually responsible for the central nervous system manifestations. In addition to clinical history and physical examination, laboratory investigations assist accurate and timely diagnosis; recommended laboratory investigations include cryptococcal antigen (CrAg) in CSF or serum, India Ink microscopy of CSF, or culture (Bicanic 2005; WHO 2013).
The case fatality rate in people with cryptococcal meningitis, the most common presentation of HIV‐related cryptococcal disease in adults, remains unacceptably high, particularly in sub‐Saharan Africa, where it is between 35% to 65% compared with 10% to 20% in most high‐income countries (Bicanic 2005; Park 2009; WHO 2016). In people who are immunocompromised, untreated cryptococcal meningitis will uniformly lead to death in a few weeks (Mwaba 2001). Cryptococcal meningitis is a leading cause of death in HIV‐positive people and typically affects those with low cluster of differentiation 4 (CD4) counts (Rajasingham 2017; Williamson 2017). Amphotericin B, flucytosine, and the azoles are the mainstay antifungal treatments for cryptococcal meningitis. Drug toxicity related to amphotericin B has been reported in up to 60% of patients (Bicanic 2015; Meiring 2016). Cryptococcal meningitis is less frequently described in children, although cases do occur (NIH 2017b; Tinashe 2016). The best time to initiate ART in those recently diagnosed with cryptococcal meningitis remains an area of debate.
Description of the intervention
The treatment of cryptococcal meningitis is usually initiated with a combination of antifungal agents (induction phase) followed by a single oral antifungal (consolidation phase) (NIH 2017a; NIH 2017b; WHO 2018). The preferred induction regimen is amphotericin B combined with flucytosine, followed by oral fluconazole for the consolidation phase, and until immune reconstitution on ART. Current guidelines recommend initiating ART within four to six weeks after cryptococcal meningitis treatment has commenced (WHO 2018).
How the intervention might work
There remains uncertainty as to when ART should be initiated in people with cryptococcal meningitis. This is related to reports of higher mortality rates among patients who initiate ART early in cryptococcal meningitis treatment (Njei 2013). Immune reconstitution inflammatory syndrome (IRIS), an exaggerated inflammatory response that can cause a paradoxical clinical deterioration soon after ART initiation, has frequently been described in association with cryptococcal meningitis. Some reports suggest that IRIS may contribute to higher mortality among HIV‐positive people who initiate ART early after a cryptococcal meningitis diagnosis as compared to starting later, however there is no clear evidence of such an association (Bicanic 2009; Müller 2010; Shelburne 2005). Additionally, in many settings where cryptococcal meningitis is prevalent, amphotericin B and flucytosine are unavailable due to resource constraints. In such settings the use of suboptimal cryptococcal meningitis treatment regimens with inadequate fungal clearance may put patients at increased risk of developing relapses and IRIS (Bicanic 2006; Loyse 2013).
Immune reconstitution inflammatory syndrome related to cryptococcal disease has been defined as events occurring within 12 months of ART initiation, reintroduction, or regimen switching after previous failure manifesting clinically as worsening disease with one or more inflammatory manifestations of cryptococcosis (meningitis, lymphadenopathy, intracranial space‐occupying lesion or lesions, multifocal disease, cutaneous or soft‐tissue lesions, pneumonitis, or pulmonary nodules) (Haddow 2010).
Although many recommendations support delaying ART initiation in people with cryptococcal meningitis (NIH 2017a; WHO 2018), delaying ART for long periods may increase the risk of death or the development of other opportunistic infections (Lundgern 2015).
Why it is important to do this review
Studies that have evaluated the impact of timing of ART initiation in cryptococcal meningitis on mortality have, to date, had mixed results. Most cohort studies have shown no association between ART timing and mortality (Crabtree Ramírez 2017; Ingle 2015; Manosuthi 2008). However, these cohort studies were underpowered and limited by the inherent methodological issues with the cohort study design (Table 2). A previous Cochrane Review suggested that there was insufficient evidence in support of either early or delayed initiation of ART (Njei 2013).
1. Cohort studies evaluating time to ART initiation in cryptococcal meningitis.
| Trial ID | Design | Location | Definitions | Study period | Duration of follow‐up | Mortality | Trial conclusions | ||
|
Early (n/N) |
Late (n/N) |
Association | |||||||
| Manosuthi 2008 | Retrospective cohort | Thailand | Early < 1 month; late ≥ 1 month | 2002 to 2006 | 1050 patient years | 9/52 | 46/229 | Adjusted HR 0.833 (95% CI 0.379 to 1.831) | No difference, however underpowered and risks of selection bias and unmeasured confounders |
| Crabtree Ramírez 2017 | Retrospective cohort | USA and Latin America | Early < 2 weeks; late 2 to 8 weeks | 1985 to 2014 | Unknown | 7/24 | 14/53 | Adjusted OR 1.09 (95% CI 0.44 to 2.67) | No difference, however underpowered and risks of selection bias and unmeasured confounders |
| Ingle 2015 | Retrospective cohort (conference abstract) | North America | Early ≤ 14 days; late 14 to 56 days since cryptococcal meningitis diagnosis | 1998 to 2009 | Unknown | 7/62 | 7/67 | Crude HR 1.29 (0.68 to 2.43) and adjusted HR 1.30 (0.66 to 2.55) | No association between timing and mortality, however unmeasured confounders and selection bias an issue. Low power to detect a difference |
Abbreviations: ART: antiretroviral therapy; CI: confidence interval; HR: hazard ratio; OR: odds ratio
Determining when ART should be initiated after a diagnosis of cryptococcal meningitis involves balancing the survival benefit conferred by ART against the risk of IRIS. Conflicting data regarding the relationship between the timing of ART for cryptococcal meningitis and the high mortality pose a therapeutic dilemma, and studies to date have had variable results. We aimed to incorporate recent randomized controlled trials and to update the findings from the earlier review by Njei 2013.
Objectives
To compare the outcomes of early initiation ART (less than four weeks after starting antifungal treatment) versus delayed initiation of ART (four weeks or more after starting antifungal treatment) in HIV‐positive people with concurrent cryptococcal meningitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
HIV‐positive children, adolescents, and adults with cryptococcal meningitis.
Types of interventions
Early initiation of ART (ART initiated within four weeks of starting antifungal treatment) versus delayed initiation of ART (ART initiated at least four weeks after starting antifungal treatment).
Types of outcome measures
Primary outcomes
All‐cause mortality
Secondary outcomes
-
Cryptococcal meningitis relapse
Recurrence of cryptococcal meningitis symptoms in previously laboratory‐confirmed episode of cryptococcal meningitis, with resolution of symptoms for one month after treatment with participants being adherent to antifungal therapy, and now presenting with CSF antigen test or culture positive for C neoformans.
CSF fungal clearance
Mortality hazard ratio (HR)
-
Paradoxical cryptococcal IRIS
Fulfilling the Haddow 2010 case definition
HIV virological suppression at six months
Length of hospital stay
-
Adverse events
Clinical adverse events
Laboratory adverse events
Other adverse events
Search methods for identification of studies
We identified all relevant trials regardless of language or publication status.
Electronic searches
We searched the following databases:
Routine databases
Cochrane Central Register of Controlled Trials (CENTRAL): Issue 7 of 12, July 2017, search date 7 August 2017
MEDLINE; search date 7 August 2017 (Appendix 1)
Embase; search date 7 August 2017 (Appendix 2)
International trial registries
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov), searched on 7 August 2017
WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch), searched on 7 August 2017
Searching other resources
Conference proceedings
International AIDS Society (IAS) conference abstracts (2015 to 2017) (www.iasociety.org)
Conference on Retroviruses and Opportunistic Infections (CROI) (2015 to 2017) (www.croiconference.org/)
Other sources
We checked the reference lists of existing reviews and all trials identified for other potentially relevant trials.
Data collection and analysis
We conducted data collection and analysis following methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions and the Methodological Expectations for the Conduct of Cochrane Intervention Reviews (MECIR) (Higgins 2011; Higgins 2016).
Selection of studies
Two review authors (MPO and IEW) independently screened the titles and abstracts of all citations identified by searches against our inclusion criteria based on types of studies, interventions, participants, and outcomes. Both review authors evaluated the full‐text articles for inclusion. Where there was uncertainty about the inclusion of a trial, we consulted a third review author (TB).
Data extraction and management
We piloted and then finalized a data extraction tool. Two review authors (MPO and IEW) independently completed data extraction for the included trials and discussed discrepancies. In case of disagreement, we consulted a third review author (TB).
For dichotomous outcomes, we extracted the number of participants who experienced the event and the number of participants randomized to each treatment group. For time‐to‐event outcomes, we extracted HRs and confidence intervals (CIs).
Assessment of risk of bias in included studies
Two review authors (MPO and IEW) independently assessed the risk of bias for each trial using the Cochrane ‘Risk of bias’ assessment tool (Higgins 2011). We contacted trial authors for missing information or clarification, but did not receive any responses to these queries. Two review authors independently applied the ‘Risk of bias’ criteria to each trial, resolving any differences in opinion through discussion or by consulting a third review author (MR). We assessed the risk of bias across six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential biases (Higgins 2011). For each domain, we assigned a judgement of either low, high, or unclear risk of bias.
Measures of treatment effect
We presented dichotomous data using risk ratios (RR) with 95% CIs. We used intention‐to‐treat analyses for all dichotomous data. We presented time‐to‐event data using HRs with CIs.
Unit of analysis issues
No cluster‐RCTs or trials with more than one intervention arm met the inclusion criteria of this review.
Dealing with missing data
Where a trial publication provided insufficient data, we contacted the trial authors to request the additional data (Bisson 2013; Makadzange 2010). For analyses with persistent missing data, we used available‐case analysis and examined the robustness of this approach by examining best‐ and worse‐case scenarios. Bisson 2013 provided an HR with 95% CI that was not available in the published manuscript; the method the authors used to generate this HR is uncertain.
Assessment of heterogeneity
We assessed the degree of heterogeneity by a visual inspection of forest plots and by examining the Chi2 test.
We quantified the extent of heterogeneity by calculating an estimation of the I2 statistic. We followed the guidance outlined in Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
Where heterogeneity was present in pooled effect estimates, we explored possible reasons for variability by conducting subgroup analyses. Due to the limited number of trials and participants contributing to each outcome, we were restricted in the number of subgroup analyses we could conduct.
Assessment of reporting biases
We assessed publication bias qualitatively based on the characteristics of the included trials. There were insufficient trials to construct a funnel plot.
Data synthesis
We performed analyses using Review Manager 5 (RevMan 2014). When trials were considered clinically and methodologically comparable, we conducted meta‐analyses using a random‐effects model. We presented narrative results of outcomes only if there was insufficient data for a meta‐analysis. For time‐to‐event data, we used HRs and CIs to generate log HRs and standard errors and used the generic inverse‐variance method to analyse these data.
Certainty of the evidence
We assessed the certainty of the evidence for each outcome using the GRADE approach (Guyatt 2008), which defines the certainty of the evidence for each outcome as “the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest” (Higgins 2011). The GRADE certainty rating has four levels: high, moderate, low, or very low. We initially categorized RCTs as high‐certainty evidence, and downgraded the certainty of the evidence after assessment of five criteria: risk of bias, consistency, directness, imprecision, and publication bias (Guyatt 2011). Two review authors (IEW and MPO) independently performed this assessment, resolving any disagreements by discussion.
We constructed ‘Summary of findings' tables and GRADE evidence tables to present the results of primary and secondary outcomes. We used GRADEpro GDT software to generate the ‘Summary of findings' tables (GRADEpro GDT 2015).
Subgroup analysis and investigation of heterogeneity
We had initially intended to conduct subgroup analyses for the primary outcome to investigate heterogeneity produced by antifungal drug treatment, geographical region, Glasgow coma score at randomization, and CSF white blood cell count. Due to limited data, we only conducted a subgroup analysis by antifungal drug treatment.
Sensitivity analysis
We used sensitivity analyses to assess the robustness of results. Where data were missing, we assessed best‐ and worst‐case scenarios to determine the possible impact of missing data on the overall effect estimates. For the cryptococcal IRIS outcome, those participants who died in the early ART and delayed ART groups may not have been missing completely at random, therefore the assumption in the main analysis (available‐case analysis) may be flawed. In addition, using an intention‐to‐treat analysis would suggest that all those who died would not have developed IRIS. We therefore conducted sensitivity analyses to determine the influence of different risks for IRIS among those who died before they could have received ART or developed IRIS. To evaluate this, we applied a best‐case scenario (8% of those who died in the early ART group would have developed IRIS and 46% of those who died in the delayed ART group would have developed IRIS) and a worst‐case scenario (46% of those who died in the early ART group would have developed IRIS and 8% of those who died in the delayed ART group would have developed IRIS). This was based on the range of reported incidence (8% to 46%) of paradoxical cryptococcal IRIS (Haddow 2010).
Viral load results were only available for a subset of participants in the two trials that reported on this outcome. For our current analysis we assumed these participants to be missing completely at random, however it is possible that participants who died before they received ART or who did not have viral load measurements done despite receiving ART differed from those who had results. We applied best‐ and worst‐case scenarios to these estimates to examine our results. These estimates were based on the range of viral load suppression rates (50% to 89%) reported by Joint United Nations Programme on HIV/AIDS (UNAIDS) for Eastern and sub‐Saharan Africa (UNAIDS 2017).
Results
Description of studies
Four trials met the inclusion criteria for this review (Bisson 2013; Boulware 2014; Makadzange 2010; Zolopa 2009). See Table 3, the ‘Characteristics of included studies' tables, and the ‘Characteristics of excluded studies' tables for trial details.
2. Summary of included studies.
| Trial ID | Country |
Randomized (N) |
Male (N; %) | Age (median; IQR or mean; SD) | Duration of antifungal therapy prior to randomization | Antifungal regimen | Time to ART initiation after randomization | ART regimen1 | Dropouts (N) | |
| Early | Delayed | |||||||||
| Bisson 2013 | Botswana | 28 | 14; 50 | 35 (32 to 41) | 72 hrs | Amphotericin B and fluconazole | 7 days (range 5 to 10) | 32 days (range 28 to 36) | TDF/FTC/EFV or NVP | 1 |
| Boulware 2014 | Uganda, South Africa | 177 | 93; 53 | 35 (28 to 40) early; 36 (30 to 40) delayed |
7 to 11 days | Amphotericin B and fluconazole | 1 to 2 weeks after diagnosis | 5 weeks after diagnosis | AZT/3TC/ EFV (80%), D4T/3TC/EFV (19%), TDF/3TC/EFV (1%) |
0 |
| Makadzange 2010 | Zimbabwe | 54 | 28; 52 | 37 (SD 8.5) early; 38 (SD 6.9) delayed |
0 days | Fluconazole | 72 hours | 10 weeks | D4T/3TC/ NVP | 6 |
| Zolopa 20092 | USA, Puerto Rico, South Africa | 35 | NR | NR | ≤ 14 days | NR | 48 hours | 6 to 12 weeks | NNRTI or PI + 2 NRTIs (3TC or FTC) | NR |
Abbreviations: IQR: interquartile range; N: number of participants; NR: not reported; SD: standard deviation; AZT: Zidovudine; D4T: Stavudine
1TDF: tenofovir; FTC: emtricitabine; EFV: efavirenz; NVP: nevirapine; 3TC: lamivudine; NNRTI: non‐nucleoside reverse transcriptase inhibitor; NRTI: nucleoside reverse transcriptase inhibitor; PI: protease inhibitor. 2This trial reported results for participants with a variety of opportunistic infections and did not provide descriptive data specifically for those with cryptococcal meningitis.
Results of the search
We searched for trials published between 1 January 1980 and 7 August 2017. The search results are presented in a PRISMA flow diagram (Figure 1). The electronic searches identified 314 titles and abstracts (80 from clinical trial registries, 180 from routine databases, and 54 from conference abstracts). After de‐duplication, 273 records underwent title and abstract screening. We identified 13 records for full‐text eligibility screening. We excluded seven of these records with reasons (two further duplicates, two journal correspondence, three wrong study design). We included six records representing four trials in the qualitative and quantitative synthesis.
1.
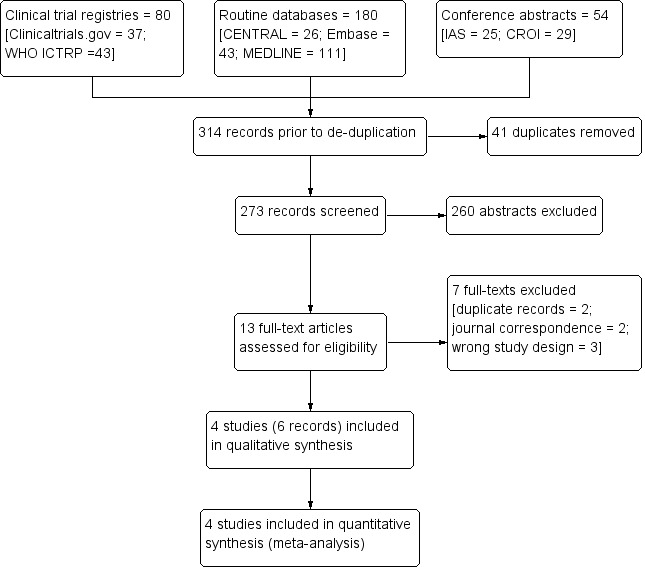
Study flow diagram.
Included studies
We have provided a summary of the included trials in Table 3 and further individual trial details in the ‘Characteristics of included studies' tables.
Participants
The Zolopa 2009 trial included participants with several different types of opportunistic infections and did not disaggregate information for those with cryptococcal meningitis, therefore limited descriptive data could be provided for this trial.
Setting
We included four trials with 294 participants from Botswana, Puerto Rico, South Africa, Uganda, the USA, and Zimbabwe. Trials were conducted between May 2003 and November 2011.
Demographic characteristics
We did not identify any studies conducted in children less than 13 years old. Most trials included only adults aged more than 18 or 21 years, except Zolopa 2009, which also included adolescents aged 13 years and over. Approximately 50% of participants were male in Boulware 2014, Bisson 2013, and Makadzange 2010. The median or mean age ranged from 35 to 38 years.
HIV illness
All trials included participants who were HIV‐positive and had a diagnosis of cryptococcal meningitis or other opportunistic infection (Zolopa 2009).
Exclusion criteria
Previous or current ART use was an exclusion criterion for all trials. All trials except Zolopa 2009 further excluded pregnancy/lactation and concurrent central nervous system infections such as bacterial meningitis.
Intervention and comparison
Antifungal therapy
Antifungal treatment regimens differed markedly between trials. Two trials used a combination of amphotericin B and fluconazole in the induction and consolidation antifungal regimen (Bisson 2013; Boulware 2014); one trial used fluconazole only (Makadzange 2010); and one trial did not report on the antifungal regimen (Zolopa 2009). Bisson 2013 provided amphotericin B (0.7 mg/kg/day) for two weeks, followed by oral fluconazole 400 mg daily for eight weeks. Boulware 2014 administered two weeks of amphotericin B (0.7 to 1.0 mg/kg/day) combined with fluconazole (800 mg/day) followed by 800 mg of fluconazole per day for at least three weeks or until a CSF culture was sterile, followed by 400 mg of fluconazole per day thereafter, for a total consolidation period of at least 12 weeks. Makadzange 2010 used fluconazole 800 mg daily for 10 weeks. These three trials also reported prescribing secondary prophylaxis of 200 mg of fluconazole per day after the induction and consolidation phases of antifungal treatment.
Boulware 2014 reported that participants received between 7 and 11 days of antifungal therapy prior to randomization, and Zolopa 2009 also allowed for ≤ 14 days of antifungal treatment prior to randomization for ART initiation. Bisson 2013 randomized participants within 72 hours of antifungal therapy initiation, and Makadzange 2010 randomized participants at the time of cryptococcal meningitis diagnosis. Only one trial described providing supportive care in the form of electrolyte replacement, fluid management, and routine therapeutic lumbar punctures (Boulware 2014).
Antiretroviral therapy
Three trials reported using a combination of two nucleoside reverse transcriptase inhibitors including tenofovir, emtricitabine, zidovudine, and stavudine, and one non‐nucleoside reverse transcriptase inhibitor, either efavirenz or nevirapine (Bisson 2013; Boulware 2014; Makadzange 2010), or protease inhibitor (Zolopa 2009).
Early ART
For early initiation of ART, all trials reported starting ART within two weeks of randomization (Bisson 2013; Boulware 2014; Makadzange 2010), with one trial initiating ART within 5 to 10 days (Bisson 2013), another within 1 to 2 weeks (Boulware 2014), and two trials initiating ART within 72 and 48 hours, respectively (Makadzange 2010; Zolopa 2009).
Delayed ART
Two trials reported initiating delayed ART around the one‐month mark: Boulware 2014 (five weeks) and Bisson 2013 (32 days). Makadzange 2010 initiated delayed ART at a median of 10 weeks, and the delayed group in Zolopa 2009 initiated ART between 6 and 12 weeks after randomization.
Outcomes
Three trials reported on all‐cause mortality at 6 to 12 months after randomization; we obtained additional data for this outcome directly from the authors of Makadzange 2010. Zolopa 2009 reported 12‐month mortality, while the remaining trials reported six‐month mortality.
Only Bisson 2013 and Boulware 2014 reported cryptococcal meningitis relapse, CSF fungal clearance, cryptococcal IRIS, and HIV virological suppression at six months. Cerebrospinal fluid fungal clearance was reported by treatment arm as the rate of fungal clearance by Bisson 2013 and as the cumulative incidence of CSF culture positivity at 14 days of amphotericin B therapy by Boulware 2014.
Three trials presented mortality time‐to‐event data (Bisson 2013; Boulware 2014; Makadzange 2010). Makadzange 2010 and Boulware 2014 reported unadjusted HRs, while Bisson 2013 presented a Kaplan Meier survival curve from which the HR was derived (see Dealing with missing data).
No trials reported length of hospital stay as a trial outcome; Bisson 2013 reported only on prolonged hospitalizations, however this was not defined.
Varying details on adverse events were present for all trials except Zolopa 2009. Bisson 2013 described at least one grade 3 to 5 Division of AIDS (DAIDS) adverse event, and Boulware 2014 described the cumulative incidence of such events, while Makadzange 2010 gave limited narrative information on adverse events.
Excluded studies
We excluded seven publications after full‐text assessment for the reasons described in the ‘Characteristics of excluded studies' tables (Makadzange 2015a; Makadzange 2015b; Makadzange 2015c; Manosuthi 2008; Sungkanuparph 2009; Sunpath 2012; Torok 2005).
Risk of bias in included studies
We evaluated the risk of bias of included trials for each of the six domains (see the ‘Characteristics of included studies' tables). We did a graphical summary of the ‘Risk of bias' assessment as represented in Figure 2 and Figure 3 .
2.
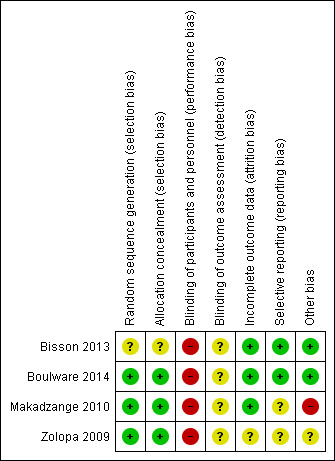
‘Risk of bias' summary: review authors' judgements about each ‘Risk of bias' item for each included trial.
3.
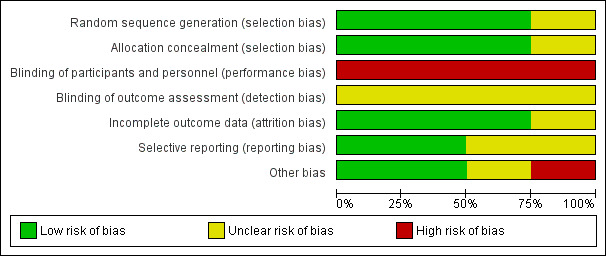
‘Risk of bias' graph: review authors' judgements about each ‘Risk of bias' item presented as percentages across all included trials.
Allocation
Three of the four included trials adequately described random sequence generation and allocation concealment and were considered free of selection bias (Boulware 2014; Makadzange 2010; Zolopa 2009). One trial had an unclear risk of selection bias for both random sequence generation and allocation concealment, which were inadequately described (Bisson 2013).
Blinding
All four trials were open‐label trials, that is participants and personnel were not blinded to treatment allocation group; we assessed these trials as having a high risk for performance bias. Outcome assessors were generally not blinded in the included trials for most outcomes. Two trials blinded IRIS outcome assessors (Boulware 2014; Zolopa 2009). We did not consider blinding an issue for mortality assessment and laboratory results, as these are objective outcomes, however adverse events and IRIS are fairly subjective. As a result, we assessed most trials as having an overall unclear risk of bias for outcome assessment.
Incomplete outcome data
The proportion of participants lost to follow‐up was low (< 15%) in all trials. There was also no evidence of differential loss to follow‐up. Zolopa 2009 only reported attrition for the total number of randomized participants and not specifically for those with cryptococcal meningitis; overall approximately 13% of participants were lost follow‐up in this trial. We assessed all trials as at low risk of attrition bias.
Selective reporting
Two trials had published protocols that were available for assessment (Bisson 2013; Boulware 2014); these trials reported all relevant outcomes and were assessed as at low risk of reporting bias. We classified two trials as at unclear risk of bias for selective outcome reporting: Zolopa 2009 did not have a protocol available for assessment, and the protocol for Makadzange 2010 was only published after the trial was completed, and no prespecified outcomes were included in the published protocol.
Other potential sources of bias
One trial was at high risk of other potential sources of bias (Makadzange 2010). Some reported results were not arithmetically correct, which could have had an impact on effect estimates. In addition, the authors were not consistent with the intention‐to‐treat approach, which could have affected the time‐to‐event analysis. Concerns about the results of this trial are echoed in comments from other trial authors in the same field, as referenced in the Characteristics of included studies tables.
Effects of interventions
See: Table 1
Early (< 4 weeks) versus delayed (≥ 4 weeks) ART initiation in HIV‐positive people with cryptococcal meningitis
Four trials with 294 participants contributed data to this comparison. See also Table 1 for a summary of the overall results.
Primary outcomes
All‐cause mortality at six to 12 months
There was higher mortality at this time point for those receiving early ART compared to those for whom ART was delayed (risk ratio (RR) 1.42, 95% confidence interval (CI) 1.02 to 1.97; 294 participants, 4 trials; I2 = 13%; Analysis 1.1; Figure 4). The certainty of the evidence contributing to this outcome was low due to concerns regarding risk of bias and qualitative heterogeneity in the included trials and imprecision. We conducted a sensitivity analysis including only trials at an overall low risk of bias (Analysis 1.2).
1.1. Analysis.
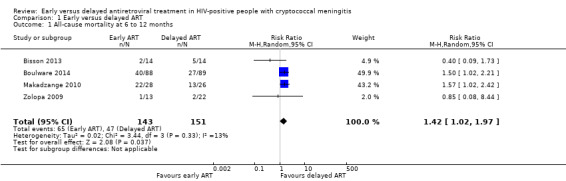
Comparison 1 Early versus delayed ART, Outcome 1 All‐cause mortality at 6 to 12 months.
4.
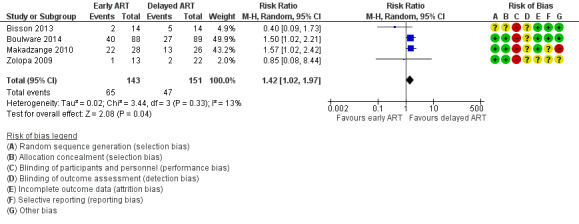
Forest plot of comparison: 1 Early versus delayed ART, outcome: 1.1 All‐cause mortality at 6 to 12 months.
1.2. Analysis.
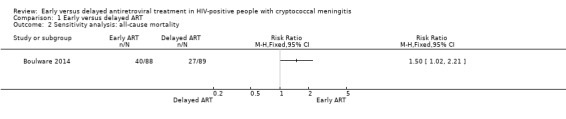
Comparison 1 Early versus delayed ART, Outcome 2 Sensitivity analysis: all‐cause mortality.
Secondary outcomes
Cryptococcal meningitis relapse
There was a trend towards a reduction in cryptococcal meningitis relapse with early ART compared to delayed ART. However, this effect did not reach statistical significance, as the CI included no effect (RR 0.27, 95% CI 0.07 to 1.04; 205 participants, 2 trials; I2 = 0%; Analysis 1.3; Figure 5). The certainty of the evidence contributing to this outcome was low due to imprecision (few clinical events).
1.3. Analysis.
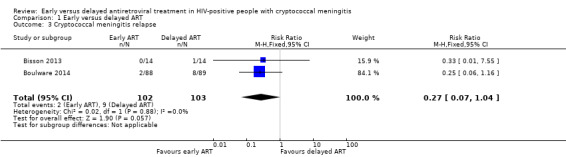
Comparison 1 Early versus delayed ART, Outcome 3 Cryptococcal meningitis relapse.
5.
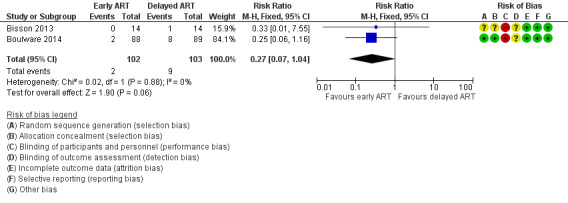
Forest plot of comparison: 1 Early versus delayed ART, outcome: 1.3 Cryptococcal meningitis relapse.
Cerebrospinal fluid fungal clearance
Due to the variability in methods of reporting CSF fungal clearance, we could not pool the results from the two trials that reported on this outcome. We have presented these results narratively in Table 4. Both trials found no difference in the rate of CSF fungal clearance between the two trial arms.
3. Cerebrospinal fluid fungal clearance results.
| Trial ID | Number of participants assessed for this outcome | Trial conclusions | Results | |
| Early ART | Delayed ART | |||
| Bisson 20131 | 28 | No difference between groups | Rate of fungal clearance: ‐0.32 log10 CFUs/mL/day | Rate of fungal clearance: ‐0.52 log10 CFUs/mL/day |
| Boulware 20142 | 166 | No difference between groups | CSF culture positivity at 14 days of amphotericin B therapy: cumulative incidence of 37% (95% CI 26% to 49%) | CSF culture positivity at 14 days of amphotericin B therapy: cumulative incidence of 39% (95% CI 28% to 50%) |
Abbreviations: CFU: colony forming units; CI: confidence interval; CSF: cerebrospinal fluid
1The trial authors reported: "The median numbers of CSF CFU measurements for the control and intervention arms, respectively, were 3 (IQR, 2–4 [range, 1–9]) and 4 (IQR, 2–5 [range, 1–7]) (P = .2, rank‐sum test). The generalized estimating equation regression coefficient for the intervention was 0.20 (95% CI, ‐.85 to 1.25), indicating that intervention subjects had a rate of CSF clearance that tended to be 0.20 log10 CSF CFU/mL/day slower than controls, although this difference was not significant." 2The trial authors reported: "Similar rates of CSF culture positivity at 14 days (37% in the earlier‐ART group and 39% in the deferred‐ART group, P = 0.87). Among 59 participants with positive CSF cultures at 14 days, the median cryptococcal growth was 100 CFU per millilitre (interquartile range, 15 to 500), with no significant difference between treatment groups (P = 0.13); only 5 participants had more than 10,000 CFU per millilitre in CSF."
Mortality hazard ratio
Due to considerable unexplained heterogeneity in the pooled effect estimate from the trials where hazard ratios were provided (237 participants, 3 trials; I2 = 84%; Analysis 1.4), we did not meta‐analyse this outcome.
1.4. Analysis.
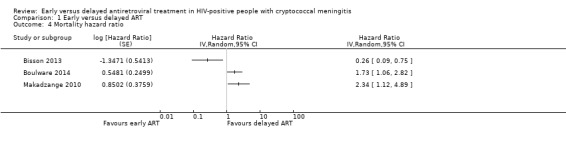
Comparison 1 Early versus delayed ART, Outcome 4 Mortality hazard ratio.
Cryptococcal IRIS
There was a suggestion of an increased risk of cryptococcal IRIS associated with early ART (RR 3.56, 95% CI 0.51 to 25.02; 205 participants, 2 trials; I2 = 54%; Analysis 1.5; Figure 6). However, the CI around this estimate was very wide and included no effect. The certainty of the evidence for this outcome was very low due to marked imprecision of the estimate, risk of bias, and indirectness related to the IRIS assessment. We explored the use of available‐case analysis for this outcome in a sensitivity analysis (Analysis 1.6, Figure 7).
1.5. Analysis.
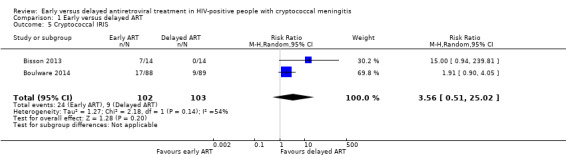
Comparison 1 Early versus delayed ART, Outcome 5 Cryptococcal IRIS.
6.
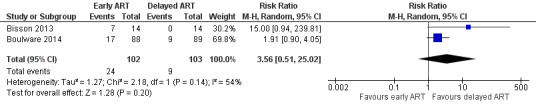
Forest plot of comparison: 1 Early versus delayed ART, outcome: 1.5 Cryptococcal IRIS.
1.6. Analysis.
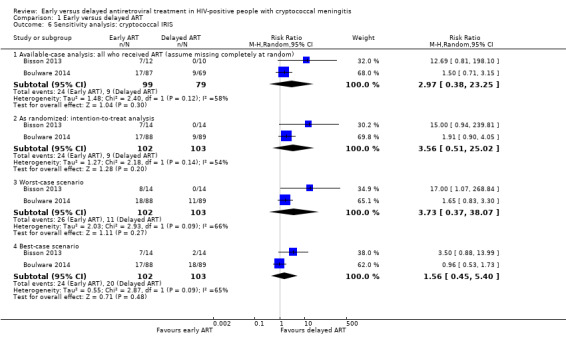
Comparison 1 Early versus delayed ART, Outcome 6 Sensitivity analysis: cryptococcal IRIS.
7.
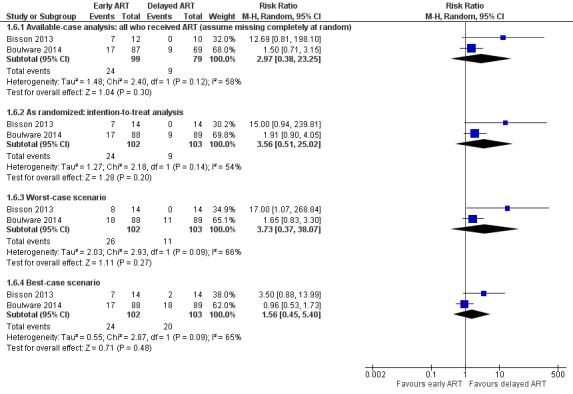
Forest plot of comparison: 1 Early versus delayed ART, outcome: 1.6 Sensitivity analysis: cryptococcal IRIS.
HIV virological suppression
There was little to no difference in virological suppression at 24 weeks with early ART compared to delayed ART (RR 0.93, 95% CI 0.72 to 1.22; 205 participants, 2 trials; I2 = 0%; Analysis 1.7). The certainty of the evidence contributing to this outcome was very low due to high risk of bias and imprecision (few clinical events). We explored the use of available‐case analysis for this outcome in a sensitivity analysis (Analysis 1.8; Figure 8).
1.7. Analysis.
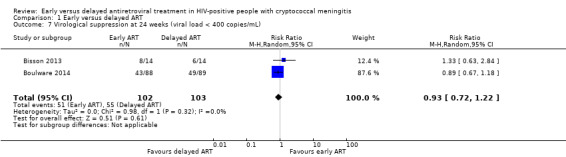
Comparison 1 Early versus delayed ART, Outcome 7 Virological suppression at 24 weeks (viral load < 400 copies/mL).
1.8. Analysis.
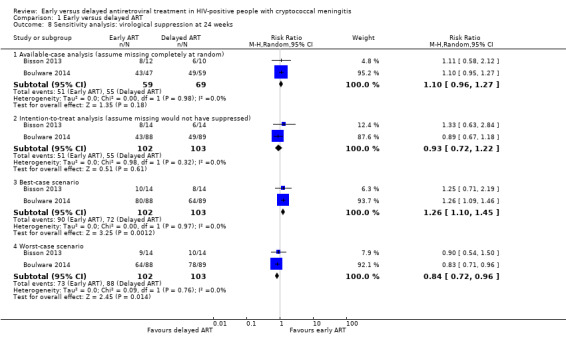
Comparison 1 Early versus delayed ART, Outcome 8 Sensitivity analysis: virological suppression at 24 weeks.
8.
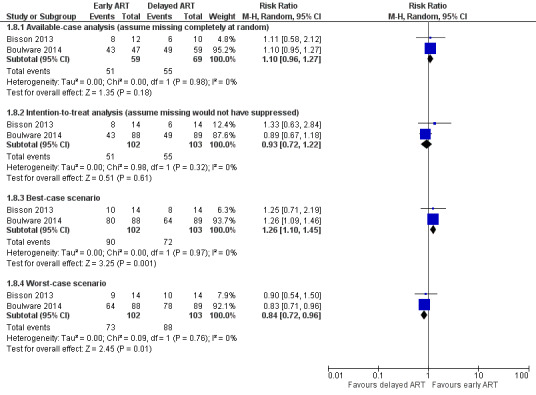
Forest plot of comparison: 1 Early versus delayed ART, outcome: 1.8 Sensitivity analysis: virological suppression at 24 weeks.
Length of hospital stay
No trials reported length of hospital stay as an outcome. Bisson 2013 reported only prolonged hospitalizations as 2 (15%) and 4 (29%) in early and delayed ART arms, respectively.
Adverse events
Adverse event measures were not reported consistently between trials, therefore these data could not be meta‐analysed. Bisson 2013 reported that all 14 participants in the early ART arm and 13/14 participants in the delayed ART arm experienced at least one grade 3 to 5 adverse event. Boulware 2014 reported the cumulative incidence of grade 3 to 5 adverse events as 84% (95% CI 74% to 90%) in the early ART group and 84% (95% CI 75% to 91%) in the delayed ART group. Makadzange 2010 described one episode of desquamating skin rash related to nevirapine and that liver function tests remained normal throughout the trial. Zolopa 2009 did not report adverse events specifically for participants with cryptococcal meningitis.
Subgroup analyses
All‐cause mortality by antifungal therapy
We subgrouped the primary outcome of all‐cause mortality according to type of antifungal therapy used. Among trials where amphotericin B therapy was combined with fluconazole (Bisson 2013; Boulware 2014), there was substantial unexplained heterogeneity between trials (I2 = 66%), and effect estimates were not pooled. A further trial treated cryptococcal meningitis with fluconazole monotherapy (Makadzange 2010); the results of this trial indicated that those who initiated ART early had higher mortality compared to those who initiated ART later (RR 1.57, 95% CI 1.02 to 2.42; 54 participants; Analysis 1.9). Zolopa 2009 did not specify which antifungal therapy was used (RR 0.85, 95% CI 0.08 to 8.44; 35 participants; Analysis 1.9) and showed no overall difference in mortality between the treatment arms.
1.9. Analysis.
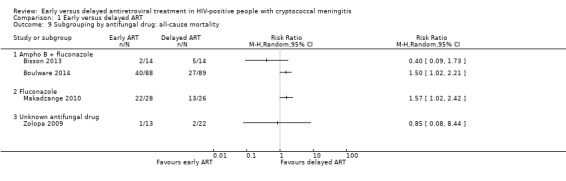
Comparison 1 Early versus delayed ART, Outcome 9 Subgrouping by antifungal drug: all‐cause mortality.
Sensitivity analyses
All‐cause mortality
We explored the effect of the intervention on all‐cause mortality at six months by restricting the analysis to trials with low risk of bias for sequence generation, attrition, and other bias that could influence this outcome.
Only the Cryptococcal Optimal ART Timing (COAT) trial, Boulware 2014, contributed to this analysis. The results from this trial indicated a higher risk of mortality among those who initiated ART early compared to those who delayed ART (RR 1.50, 95% CI 1.02 to 2.21; 177 participants, 1 trial; Analysis 1.2).
Cryptococcal IRIS
Applying an available‐case analysis (RR 2.97, 95% CI 0.38 to 23.25; Analysis 1.6: Figure 7) and applying a low (8%) or high (46%) occurrence of cryptococcal IRIS to the missing cases differentially in the best‐case (RR 1.56, 95% CI 0.45 to 5.40) and worst‐case scenarios (RR 3.73, 95% CI 0.37 to 38.07) only resulted in a lower or higher precision estimate. In all instances, the estimates included no effect, and CIs remained wide and included appreciable benefit and harm. These results are in keeping with the intention‐to treat analysis suggesting that this approach was reasonable for the analysis of cryptococcal IRIS.
Virological suppression on ART
We compared the use of an available‐case analysis and best‐ and worst‐case scenarios to evaluate missing data for this outcome. This resulted in a persistent result of no difference in virological suppression at six months between groups in the available‐case analysis (RR 1.10, 95% CI 0.96 to 1.27; Analysis 1.8; Figure 8). For the best‐case scenario, we assumed 89% virological suppression for those missing viral loads or who did not receive ART in the early ART group and 50% for those missing in the delayed ART group. This resulted in better virological suppression in the early ART group (RR 1.26, 95% CI 1.10 to 1.45). When we applied the worst‐case scenario and assumed that those missing viral loads or who did not receive ART in the early ART group had 50% suppression and in the delayed ART group had 89% suppression, this resulted in better virological suppression in the delayed ART group (RR 0.84, 95% CI 0.72 to 0.96). This suggested that the missing data for virological outcomes could bias results for this outcome and led to a downgrading of this outcome in the GRADE assessment.
Certainty of the evidence
We used GRADE to indicate the level of confidence we have in the results. The certainty of the evidence was either low or very low for most outcomes.
Methodological quality
The overall methodological quality was unclear for most trials that contributed to the analyses, due predominantly to the lack of reporting on several 'Risk of bias' domains. We categorized Makadzange 2010 as at high risk of bias for three domains, and the inclusion of this trial in the mortality analyses resulted in downgrading for risk of bias for these outcomes. Due to the lack of blinding of IRIS outcome assessors in one trial, we downgraded the evidence that contributed to the IRIS analysis. Viral load analysis was not conducted for all participants who were randomized or who received ART; we downgraded the certainty of the evidence for this outcome, as it is possible that randomization was not maintained for the subgroup that was analysed for this outcome.
Consistency
We detected qualitative and quantitative heterogeneity in the analysis of mortality, which led to downgrading the certainty of the evidence for the all‐cause mortality outcome and prevented pooling of the results of the mortality hazard ratio outcome. We explored the use of different antifungal regimens in a subgroup analysis and found substantial heterogeneity between the trials providing amphotericin B, reflecting differences between these trials that could not be explained. This inconsistency could be the result of differences in the trial setting and conduct, however it was not possible to fully account for this in subgroup analyses.
Indirectness
We downgraded the cryptococcal IRIS outcome for indirectness, as we considered IRIS assessment to be quite subjective even when case definitions are applied.
Imprecision
We downgraded all outcomes due to imprecision. There were overall few clinical events contributing to each analysis. In addition, several analyses had wide CIs including no effect and appreciable harm or benefit.
Publication bias and other considerations
It was not possible to formally assess publication bias using funnel plots. However, there was no evidence of publication bias among the included trials, which presented both results with no effect or moderate effects of early ART on mortality.
Discussion
Summary of main results
Four trials including a total of 294 participants evaluated early ART initiation compared to delayed ART initiation (Table 1). The trials were conducted between 2003 and 2011, and all included people from low‐ and middle‐income countries. Two trials used amphotericin B and fluconazole for cryptococcal meningitis induction therapy; one trial used fluconazole monotherapy; and one trial did not report the induction therapy used. All trials were conducted in adults.
Primary outcomes
Overall, when we pooled results from the four included trials we found that early ART initiation may increase all‐cause mortality at six to 12 months among HIV‐positive adults with cryptococcal meningitis, with low‐certainty evidence contributing to this meta‐analysis. A sensitivity analysis restricted to trials at low risk of bias showed higher risk of mortality among those initiating ART early; this analysis included one high‐quality trial (COAT trial), which was terminated early due to the higher mortality risk in the early ART group.
Secondary outcomes
There was low‐certainty evidence suggesting that early ART initiation may reduce the occurrence of cryptococcal meningitis relapse. Two RCTs evaluating the risk of developing IRIS found a tendency towards increased risk of cryptococcal IRIS in the early ART group, however the very low‐certainty evidence contributing to this outcome means that we cannot say whether early ART initiation increases or decreases cryptococcal IRIS events compared to delayed ART initiation. We are uncertain if early ART affects virological suppression at six months, as the evidence contributing to this outcome was of very low certainty. Adverse events were not reported consistently between trials, therefore could not meta‐analyse this outcome. Conclusions from the authors of trials that assessed this outcome suggest no difference in grade 3 to 5 adverse events between the two treatment arms. Similarly, data from the two trials reporting on time to fungal clearance could not be pooled, and the authors of these trials concluded that there was no difference between early and delayed ART arms for this outcome.
Overall completeness and applicability of evidence
We found only a few small trials that evaluated early versus delayed ART initiation in HIV‐positive people with cryptococcal meningitis. The trials were generally conducted in low‐ and middle‐income countries and included only adult participants. The results from this review can therefore be generalized only to these groups. The overall low certainty of the evidence limits the conclusions that can be drawn from these analyses. However, the pooled results do suggest that early ART may increase all‐cause mortality. We were restricted in the number of subgroup and sensitivity analyses that we could perform due to the limited number of included trials and participants. When we restricted analyses to trials at low risk of bias for the primary outcome, the analysis contained one trial (Boulware 2014); these results showed a higher risk of mortality among those who initiated ART early. In addition, two of the included studies with the highest numbers of participants and showing higher mortality with early ART were terminated early due to excessive mortality in the early ART group. One needs to consider whether effect estimates would be higher if these trials had larger samples.
Only two trials reported using amphotericin B‐based induction therapy, which is the standard of care for treatment of people with cryptococcal meningitis. Although fluconazole monotherapy is not routinely recommended, in many low‐ and middle‐income countries this single agent may still be used, and so findings from this trial remain relevant to these settings.
Immune reconstitution inflammatory syndrome has been implicated as the reason for higher mortality in those who initiate ART early. Unfortunately, we could draw no conclusions on the effect of early ART on IRIS occurrence based on the available data.
Certainty of the evidence
Overall, the certainty of the evidence contributing to all outcomes was low or very low. This was due to generally few clinical events and wide CIs around effect estimates. In addition, the poor methodological quality of some trials that contributed to the mortality, cryptococcal meningitis IRIS, and virological suppression analyses led to further downgrading of these outcomes.
Potential biases in the review process
We minimized selection bias by conducting an extensive literature search using a wide range of search terms and databases. Two review authors independently screened the search outputs and evaluated eligibility. In addition, we evaluated the reference lists of included papers and previous systematic reviews.
We have detailed any changes made to the trial protocol after publication and after the review process began in the Differences between protocol and review section.
Agreements and disagreements with other studies or reviews
A previous Cochrane Review conducted by Njei 2013 included two of the four trials included in this review (Makadzange 2010; Zolopa 2009). They concluded that there was insufficient evidence to determine whether early ART had an effect on mortality and suggested that there was a higher risk of IRIS among those who initiated ART early. Although our review did show some evidence of higher risk of IRIS in the early ART group, the certainty of the evidence contributing to this outcome was very low. The additional trials that contributed to this updated review, and rigorous application of the GRADE tool, resulted in low‐certainty evidence of higher mortality among those who initiated ART early.
These findings contrast with cohort studies, which suggest no difference in mortality risk when comparing early ART to delayed ART (Table 2). This may reflect some level of selection bias for this outcome in cohort studies.
Authors' conclusions
Implications for practice.
Despite the low certainty of the evidence, it appears that initiating antiretroviral therapy (ART) within four weeks of cryptococcal meningitis diagnosis increases the risk of mortality compared to delaying ART beyond four weeks. Clinicians and guideline developers need to seriously consider the severe nature of the potential harms of initiating ART early in HIV‐positive people with cryptococcal meningitis. Findings from this Cochrane Review contributed to the formulation of the current World Health Organization (WHO) guidelines for the diagnosis, prevention, and management of cryptococcal disease in HIV‐positive adults, adolescents, and children (WHO 2018).
Implications for research.
With this suggestion of a higher risk of mortality in HIV‐positive people with cryptococcal meningitis who initiate ART early, it is unlikely that more randomized controlled trials will be conducted that evaluate early versus delayed ART as an intervention in people with cryptococcal meningitis. Questions regarding how to best manage patients who develop cryptococcal meningitis soon after ART initiation were not addressed in this Cochrane Review and will become more relevant as rapid ART initiation becomes widespread.
What's new
| Date | Event | Description |
|---|---|---|
| 23 July 2018 | New search has been performed | This is an update of a review last published in 2013 (Njei 2013). The review author team updated the protocol extensively, and differences are highlighted in the ‘Differences between protocol and review' section. |
| 23 July 2018 | New citation required and conclusions have changed | The previous Cochrane Review conducted by Njei 2013 concluded that there was insufficient evidence to determine whether early ART had an effect on mortality and suggested that there was a higher risk of IRIS among those who initiated ART early. This review update did show some evidence of higher risk of IRIS in the early ART group, but the certainty of the evidence contributing to this outcome was very low. The additional trials that contributed to this updated review, and rigorous application of the GRADE tool, resulted in low‐certainty evidence of higher mortality among those who initiated ART early. |
Acknowledgements
The Academic Editor on this review update was Professor George Rutherford.
OPM, IEW, and MR are supported by the Effective Health Care Research Consortium. This Consortium and the CIDG editorial base are funded by UK aid from the UK Government for the benefit of low‐ and middle‐income countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
The Centre for Evidence Based Health Care (CEBHC) at the Faculty of Health Sciences, Stellenbosch University provided support through a fellowship award to OPM. The Centre for Development of Best Practices for Health (CDBPS) Yaounde for the support for protected work time and space at the Centre in Yaounde, Cameroon.
Appendices
Appendix 1. MEDLINE search strategy
| Search | Query |
| 8 | Search (((#5 AND #6))) AND ("1980/01/01"[Date ‐ Publication] : "2017/08/07"[Date ‐ Publication]) |
| 7 | Search (#5 AND #6) |
| 6 | Search (time[tiab] OR timing[tiab] OR early[tiab] OR earlier[tiab] OR delay*[tiab] OR defer*[tiab] OR late[tiab]) AND (initiat*[tiab] OR administrat*[tiab] OR treatment[tiab] OR therapy[tiab] OR ART[tiab] OR antiretroviral*[tiab])) |
| 5 | Search (#1 AND #2 AND #3 AND #4) |
| 4 | Search (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) |
| 3 | Search ("Meningitis, Cryptococcal"[Mesh] OR cryptococcal meningitis[tiab] OR cryptococal meningitis[tiab] OR cryptococcal meningitides[tiab] OR cerebral cryptococcosis[tiab] OR cerebral cryptococcoses[tiab] OR toruloma*[tiab] OR cryptococcus neoforman[mh] OR cryptococcus neoforman[tiab] OR ((cryptococcal[tiab] OR cryptococal[tiab] OR cyptococcosis[tiab] OR cryptococcoses[tiab] OR Cryptococcus[tiab]) AND (meningitis[tiab])) |
| 2 | Search (antiretroviral therapy, highly active[MeSH] OR anti‐retroviral agents[MeSH] OR antiviral agents[MeSH:NoExp] OR ((anti[tiab]) AND (hiv[tiab])) OR antiretroviral*[tiab] OR ((anti[tiab]) AND (retroviral*[tiab])) OR HAART[tiab] OR ((anti[tiab]) AND (acquired immunodeficiency[tiab])) OR ((anti[tiab]) AND (acquired immuno‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immune‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immun*[tiab]) AND (deficiency[tiab])) |
| 1 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) |
Appendix 2. Embase search strategy
| Search | Query | |
| 13 | #10 AND #11 AND [1980‐2017]/py | |
| 12 | #10 AND #11 | |
| 11 | (time:ab,ti OR timing:ab,ti OR early:ab,ti OR earlier:ab,ti OR delay*:ab,ti OR defer:ab,ti OR late:ab,ti) AND (initiat*:ab,ti OR administrat*:ab,ti OR treatment:ab,ti OR therapy:ab,ti OR art:ab,ti OR antiretroviral*:ab,ti) | |
| 10 | #1 AND #2 AND #8 AND #9 | |
| 9 | 'cryptococcal meningitis'/de OR 'cryptococcal meningitis':ab,ti OR 'cryptococcus meningitis':ab,ti OR 'cryptococal meningitis':ab,ti OR 'cryptococcal meningitides':ab,ti OR 'cerebral cryptococcosis':ab,ti OR 'cerebral cryptococcoses':ab,ti OR toruloma*:ab,ti OR 'cryptococcus neoforman':ab,ti OR ((cryptococcal:ab,ti OR cryptococal:ab,ti OR cryptococcosis:ab,ti OR cryptococcoses:ab,ti) AND meningitis:ab,ti) | |
| 8 | #3 NOT #7 | |
| 7 | #4 NOT #6 | |
| 6 | #4 AND #5 | |
| 5 | 'human'/de OR 'normal human'/de OR 'human cell'/de | |
| 4 | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de | |
| 3 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR ((doubl* NEAR/3 blind*):ab,ti) OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR ((cross NEXT/1 over*):ab,ti) | |
| 2 | 'human immunodeficiency virus vaccine'/exp OR 'human immunodeficiency virus vaccine' OR 'human immunodeficiency virus vaccine':ab,ti OR 'anti human immunedeficiency':ab,ti OR 'anti human immunodeficiency':ab,ti OR 'anti human immuno‐deficiency':ab,ti OR 'anti human immune‐deficiency':ab,ti OR 'anti acquired immune‐deficiency':ab,ti OR 'anti acquired immunedeficiency':ab,ti OR 'anti acquired immunodeficiency':ab,ti OR 'anti acquired immuno‐deficiency':ab,ti OR 'anti hiv':ab,ti OR antiretrovir*:ab,ti OR 'anti retroviral':ab,ti OR 'anti retrovirals':ab,ti OR 'anti retrovirus':ab,ti OR haart:ab,ti OR 'aids vaccine':ab,ti OR 'aids vaccines':ab,ti OR 'anti human immunodeficiency virus agent'/exp OR 'anti human immunodeficiency virus agent' OR 'anti human immunodeficiency virus agent':ab,ti OR 'antiretrovirus agent'/exp OR 'antiretrovirus agent' OR 'antiretrovirus agent':ab,ti OR 'highly active antiretroviral therapy'/exp OR 'highly active antiretroviral therapy' OR 'highly active antiretroviral therapy':ab,ti | |
| 1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus' OR 'human immunodeficiency virus':ab,ti OR 'human immuno+deficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune+deficiency virus':ab,ti OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno+deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti OR 'acquired immune+deficiency syndrome':ab,ti |
Data and analyses
Comparison 1. Early versus delayed ART.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality at 6 to 12 months | 4 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.02, 1.97] |
| 2 Sensitivity analysis: all‐cause mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Cryptococcal meningitis relapse | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.07, 1.04] |
| 4 Mortality hazard ratio | 3 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 5 Cryptococcal IRIS | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 3.56 [0.51, 25.02] |
| 6 Sensitivity analysis: cryptococcal IRIS | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Available‐case analysis: all who received ART (assume missing completely at random) | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.38, 23.25] |
| 6.2 As randomized: intention‐to‐treat analysis | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 3.56 [0.51, 25.02] |
| 6.3 Worst‐case scenario | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 3.73 [0.37, 38.07] |
| 6.4 Best‐case scenario | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.45, 5.40] |
| 7 Virological suppression at 24 weeks (viral load < 400 copies/mL) | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.72, 1.22] |
| 8 Sensitivity analysis: virological suppression at 24 weeks | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Available‐case analysis (assume missing completely at random) | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.96, 1.27] |
| 8.2 Intention‐to‐treat analysis (assume missing would not have suppressed) | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.72, 1.22] |
| 8.3 Best‐case scenario | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.10, 1.45] |
| 8.4 Worst‐case scenario | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.72, 0.96] |
| 9 Subgrouping by antifungal drug: all‐cause mortality | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Ampho B + fluconazole | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Fluconazole | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Unknown antifungal drug | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bisson 2013.
| Methods | Study design: open‐label RCT | |
| Participants |
Inclusion criteria: adults ≥ 21 years of age: HIV‐positive, (positive enzyme‐linked immunosorbent assay and/or a detectable (i.e. > 400 copies/mL) plasma viral load); India ink–positive cryptococcal meningitis; ART‐naive, no past use of ART besides for prevention of mother‐to‐child transmission ≥ 6 months previously; could provide written informed consent; to initiate or had initiated amphotericin B ≤ 72 hours prior to enrolment; no antifungal use within the prior 14 days; not pregnant, as determined by a negative urine β–human chorionic gonadotropin test, or were breastfeeding; not initiated antitubercular therapy ≤ 2 weeks prior to assessment; not have bacterial meningitis; unlikely to initiate immunomodulatory therapy (e.g. cancer chemotherapy) prior to the week 4 study visit; not prisoners; available CSF for determination of baseline CFUs; and would obtain outpatient care within the logistical reach of the study team. Informed consent. Exclusion criteria: patients not meeting the inclusion criteria Number randomized: 28 Descriptive baseline data:
Duration of antifungal therapy prior to randomization: 72 hrs Dropouts during study period: 1 |
|
| Interventions |
Antifungal therapy provided: amphotericin B 0.7 mg/kg × 14 days, followed by oral fluconazole 400 mg daily × 8 weeks, followed by oral fluconazole 200 mg daily until the CD4 count is > 200 cells/μL for 6 months Supportive care: not described CSF pressure management: not described ART regimen provided: 18 (82%) participants initiated combination TDF/FTC/EFV, whereas the remaining 4 participants initiated combination zidovudine/lamivudine plus NVP or EFV (2 participants) or TDF/FTC and NVP (2 participants). Early ART: 12 of 13 (92%) participants initiated ART at a median of 7 days (IQR 5 to 10) after randomization. Delayed ART: 10 of 14 (71%) participants in the control arm initiated ART at a median of 32 days (IQR 28 to 36) after randomization. Adherence: not reported |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Timing of outcome measurement Study‐specific visits, performed at entry and at days 7 and 14, week 4, and monthly thereafter, included medical history and physical examination. HIV load, CD4 count, and complete blood count were performed at randomization, at week 4, and then at weeks 12 and 24 after the planned date of ART initiation. Serum chemistries were performed as above and at days 7 and 14. Study‐specific lumbar puncture performed 4 weeks after randomization. |
|
| Notes |
Country: Botswana Setting: hospital setting Dates: September 2009 and November 2011 Funding: Doris Duke Charitable Foundation via a Doris Duke Clinical Scientist Development Award (to GPB) and by the Penn Center for AIDS Research International Core Other: early study termination due to slow recruitment and funding (planned sample size = 25 per study arm) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors mention randomization, but do not describe how this was done. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors do not describe the allocation concealment process. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment was not reported as blinded. Immune reconstitution inflammatory syndrome assessment was unblinded. This is unlikely to bias results for mortality and laboratory tests, however bias could be introduced for adverse events and IRIS. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant dropped out after randomization. |
| Selective reporting (reporting bias) | Low risk | This trial was registered on ClinicalTrials.gov in 2009: clinicaltrials.gov/ct2/archive/NCT00976040 clinicaltrials.gov/ct2/show/NCT00976040 2 proposed outcomes were not reported:
We do not see these as introducing bias, as all main relevant outcomes were reported. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
Boulware 2014.
| Methods | Study design: open‐label RCT | |
| Participants |
Inclusion criteria: 18 years or older, diagnosed with HIV infection, no previous receipt of ART, a diagnosis of cryptococcal meningitis based on CSF culture or CSF cryptococcal antigen assay, and treatment with amphotericin‐based therapy Exclusion criteria: inability to undergo follow‐up, contraindication for or refusal to undergo lumbar punctures, multiple concurrent CNS infections, previous cryptococcosis, receipt of chemotherapy or immunosuppressive agents, pregnancy, breastfeeding, and serious coexisting conditions that precluded random assignment to earlier or deferred ART. Number randomized: 177 Descriptive baseline data
Dropouts during study period: 1 |
|
| Interventions |
Duration of antifungal therapy prior to randomization: 7 to 11 days Antifungal therapy provided: induction therapy: 2 weeks amphotericin B (0.7 to 1.0 mg/kg/day) combined with fluconazole (800 mg/day) Followed by 800 mg of fluconazole per day for at least 3 weeks or until a CSF culture was sterile, followed by 400 mg of fluconazole per day thereafter, for a total consolidation period of at least 12 weeks. Secondary prophylaxis with fluconazole (200 mg per day) was then continued for at least 1 year. Supportive care: intravenous fluids (≥ 2 L per day) and electrolyte management CSF pressure management: additional LPs were conducted on days 7 and 14, and for control of intracranial pressure ART regimen provided: AZT, 3TC, EFV (80%), D4t, 3TC, EFV (19%), TDF, 3TC, EFV (1%) Early ART initiation: 86 (98%) received ART within 48 hrs. Median of 9 days (IQR 8 to 9) after cryptococcal meningitis diagnosis Delayed ART initiation: 62 (70%) received ART within 42‐day window. Median of 36 days (IQR 34 to 38) after cryptococcal meningitis diagnosis Adherence: not reported |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Timing of outcome measurement: participants were followed daily while hospitalized, then every 2 weeks for 12 weeks and monthly thereafter through 46 weeks. Lumbar punctures were performed at diagnosis and on days 7 and 14 of amphotericin therapy and as needed for the control of intracranial pressure. |
|
| Notes |
Country: Uganda and South Africa Setting: 3 hospitals Dates: November 2010 to April 2011 (recruitment) Funding: funded by the National Institute of Allergy and Infectious Diseases; President's Emergency Plan for AIDS Relief (PEPFAR) for ART, Merck Sharp & Dohme for EFV Others: among eligible participants with cryptococcal meningitis, 29 died after diagnosis but before randomization. Data safety monitoring committee stopped trial early due to excess mortality in early ART group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors used a computer‐generated, permuted‐block randomization algorithm with blocks of different sizes in a 1:1 ratio, stratified according to site and the presence or absence of altered mental status at the time that informed consent was obtained. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes stored in a lockbox contained the randomization assignments for enrolled participants. Envelopes were opened after written informed consent had been obtained. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | While clinical assessors were blinded for IRIS and mortality, assessment of adverse events was unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no cases of loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all outcomes of interest and all protocol outcomes. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
Makadzange 2010.
| Methods | Study design: RCT | |
| Participants |
Inclusion criteria: eligible participants were aged 18 years and HIV‐positive. All participants had cryptococcal meningitis confirmed by positive results of India ink identification of Cryptococcus neoformans in the CSF or a CSF cryptococcal polysaccharide antigen (CrAg) test (CALAS; Meridian Diagnostics), or both. Participants residing in a 50‐kilometre radius of Harare. Informed consent Exclusion criteria: previous diagnosis of or treatment for cryptococcal meningitis, currently receiving ART, receiving medications that affect the metabolism of fluconazole (especially rifampicin), pregnant or lactating, or a history of hepatic or renal dysfunction Number randomized: 54 Descriptive baseline data
Dropouts during study period: 8 (some numerical discrepancy in flow diagram, which suggests 6) |
|
| Interventions |
Duration of antifungal therapy prior to randomization: 0 days ‐ randomized at time of diagnosis and treatment initiation Antifungal therapy provided: fluconazole (800 mg once per day; Diflucan (Pfizer)), after 10 weeks reduced to a prophylactic dosage of 200 mg once per day. Where treatment failure was suspected (positive culture, positive India ink or persistently elevated CrAg titres), dosage was increased once again to 800 mg daily until CSF clear. Supportive care: not described CSF pressure management: CSF hypertension reduced if clinically indicated or CSF pressure high at study visits where LPs were conducted. ART regimen: fixed‐dose combination of stavudine (30 mg twice per day) and lamivudine (150 mg twice per day), and nevirapine (200 mg twice per day, with a 200 mg once‐daily 2‐week lead‐in dose) Early ART: started within 72 hours of randomization Delayed ART: started after 10 weeks of antifungal therapy Adherence: adherence to fluconazole and ART: self reports and pill counts at each visit (not reported in outcomes) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Timing of outcome assessment: observed at outpatient clinic at 2, 4, 8, and 10 weeks, then monthly. Liver function tests conducted 6 monthly up to 2 years. Cerebrospinal fluid sampled at weeks 2, 4, and 10. |
|
| Notes |
Country: Zimbabwe Setting: a tertiary referral teaching hospital in Harare Dates: October 2006 through April 2008 Funding: The AIDS Care Research in Africa (ACRiA) programme and the small grants funding programme from the Infectious Diseases Society of America Other:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomization schedule was used to assign participants to the early ART and delayed ART arms of the trial. |
| Allocation concealment (selection bias) | Low risk | The randomization sequence was concealed to the trial nurse who was responsible for participant enrolment using sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment was not reported as blinded. Trial did not report on IRIS. This is unlikely to bias results for mortality and laboratory tests, however bias could be introduced for adverse events. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up low and similar in both arms (3 out of 28 and 3/5 out of 26 in early ART and delayed ART arms) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available for review; outcomes not listed on ClinicalTrials.gov (protocol registered after trial was completed) clinicaltrials.gov/ct2/show/NCT00830856 |
| Other bias | High risk | Some reported results were not arithmetically correct, which could have had an impact on effect estimates. In addition, the authors were not consistent with the intention‐to‐treat approach, which could have affected the time‐to‐event analysis. Concerns about the results of this trial are echoed in comments from other trial authors in the same field (Boulware 2010; Bicanic 2010; Grant 2010). |
Zolopa 2009.
| Methods | Study design: open‐label RCT | |
| Participants |
Inclusion criteria: eligible participants were HIV‐positive men or women 13 years of age or older, presenting with an AIDS‐defining opportunistic infection or serious bacterial infection for which effective antimicrobial therapy was available and prescribed. To reflect clinical practice, the trial allowed presumptive and confirmed diagnoses as long as appropriate treatment for the opportunistic infection/bacterial infection had been initiated (cryptococcal disease was required to be confirmed). Participants in whom tuberculosis was diagnosed after randomization remained in the trial. Exclusion criteria: people with or on treatment for tuberculosis were excluded. People were ineligible if they had received ART within 8 weeks prior to study entry, more than 31 days of any ART within 6 months prior to study entry, or more than 1 ART regimen on which they experienced treatment failure. Number randomized: 35 Descriptive baseline data
Dropouts during study period: not reported for cryptococcal meningitis group |
|
| Interventions |
Duration of antifungal therapy prior to randomization: <= 14 days Antifungal therapy provided: not reported Supportive care: not reported CSF pressure management: not reported ART regimen: choice of ART was left to the judgement of the clinician to better reflect common clinical practice. Early ART: 48 hours Delayed ART: 6 to 12 weeks Adherence: monitored by self reporting at 8, 16, 32, and 48 weeks |
|
| Outcomes |
Primary outcome
Timing of outcome assessment: participants were seen at weeks 4, 8, 12, and 16 and every 8 weeks thereafter through week 48 for clinical assessments and routine laboratory monitoring. Participants in the deferred arm shifted to follow‐up at weeks 4, 8, 12, and 16 after initiation of ART and every 8 weeks thereafter until week 48. |
|
| Notes |
Country: USA and South Africa Setting: 39 AIDS Clinical Trials Units in the USA (including Puerto Rico) and Johannesburg, South Africa (which was limited to enrolling 20 participants by the trial sponsor) Dates: May 2003 to August 2007 Funding: AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases Other: how cryptococcal meningitis was confirmed was not specified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After eligibility checklist was completed, randomized treatment assignment was generated by central computer using permuted blocks within strata. |
| Allocation concealment (selection bias) | Low risk | Neither the size of the blocks nor treatment assignments to other sites were public, which prevented individual investigators from deducing the assignment pattern. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessment of adverse events was not blinded, which may have introduced bias for this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants lost to follow‐up was not reported exactly, however the trial authors state: “Eighty‐seven percent of subjects, 123 in each arm, were evaluable for the primary endpoint”, suggesting that loss to follow‐up was 13% or less, which is acceptable. It is difficult to comment specifically on participants with cryptococcal meningitis, as these results were not disaggregated. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was unavailable for evaluation. |
| Other bias | Unclear risk | As the trial had so little information on the participants in our treatment group of interest, it is difficult to comment on bias related to our trial population. |
Abbreviations: ART: antiretroviral therapy; CD4: cluster of differentiation 4; CFU: colony forming units; CNS: central nervous system; CrAg: cryptococcal antigen; CSF: cerebrospinal fluid; DAIDS: Division of AIDS; GCS: Glasgow coma score; HR: hazard ratio; IQR: interquartile range; IRIS: immune reconstitution inflammatory syndrome; LP: lumbar puncture; RCT: randomized controlled trial; SD: standard deviation; TDF: Tenofovir; FTC: Emtricitabine; EFV: Efavirenz; NVP: Nevirapine; AZT: Zidovudine; LFT: Liver function test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Makadzange 2015a | Journal correspondence |
| Makadzange 2015b | Duplicate of Makadzange 2015a |
| Makadzange 2015c | Duplicate of Makadzange 2015a |
| Manosuthi 2008 | Wrong study design: cohort study |
| Sungkanuparph 2009 | Wrong study design: participants were not randomized to early or late ART. This is a substudy of a trial that randomized participants to different cryptococcal treatment strategies. |
| Sunpath 2012 | Wrong study design: cohort study |
| Torok 2005 | Journal correspondence |
Abbreviations: ART: antiretroviral therapy
Differences between protocol and review
This is an update of a previous Cochrane review (Njei 2013). The new review author team extensively revised the protocol, which is available on the CIDG website at cidg.cochrane.org/our‐reviews under the subheading ‘Related content'.
Contributions of authors
Mbah P Okwen, Tihana Bicanic, Marty Richardson, and Ingrid Eshun‐Wilson developed the protocol. Mbah P Okwen and Ingrid Eshun‐Wilson screened and appraised studies, consulting either Tihana Bicanic or Marty Richardson in case of disagreement. Marty Richardson provided expert contributions on statistics, and Tihana Bicanic provided expert inputs on cryptococcal meningitis content. Ingrid Eshun‐Wilson and Marty Richardson conducted the analyses. Ingrid Eshun‐Wilson and Mbah P Okwen drafted the final review, which the remaining author team reviewed.
Sources of support
Internal sources
-
Centre for Evidence Based Health Care, Division of Epidemiology and Biostatistics, Department of Global Health, Stellenbosch University, South Africa.
Fellowship award for Patrick Okwen
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development, UK.
Grant: 5242
Declarations of interest
Ingrid Eshun‐Wilson has no known conflicts of interest. Mbah P Okwen has no known conflicts of interest. Marty Richardson has no known conflicts of interest. Tihana Bicanic has received payment of fees and a grant from Gilead and Basilea for advisory board, speaker fees, and grants outside the submitted work.
Unchanged
References
References to studies included in this review
Bisson 2013 {published data only}
- Bisson GP, Molefi M, Bellamy S, Thakur R, Steenhoff A, Tamuhla N, et al. Early versus delayed antiretroviral therapy and cerebrospinal fluid fungal clearance in adults with HIV and cryptococcal meningitis. Clinical Infectious Diseases 2013;56(8):1165‐73. [DOI: 10.1093/cid/cit019; CN‐00861211] [DOI] [PubMed] [Google Scholar]
Boulware 2014 {published data only}
- Boulware DR, Meya DB, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. New England Journal of Medicine 2014;370(26):2487‐98. [DOI: 10.1056/NEJMoa1312884; CN‐00994166] [DOI] [PMC free article] [PubMed] [Google Scholar]
Makadzange 2010 {published and unpublished data}
- Makadzange AT, Ndhlovu CE, Takarinda K, Reid M, Kurangwa M, Gona P, et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub‐Saharan Africa. Clinical Infectious Diseases 2010;50(11):1532‐8. [DOI: 10.1086/652652; CN‐00748368] [DOI] [PubMed] [Google Scholar]
Zolopa 2009 {published data only}
- Zolopa A, Andersen J, Powderly W, Sanchez A, Sanne I, Suckow C, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS ONE 2009;4(5):e5575. [DOI: 10.1371/journal.pone.0005575; CN‐00702077] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Makadzange 2015a {published data only}
- Makadzange AT, Mothobi N. Randomised controlled trial: delaying initiation of ART for 5 weeks improves survival in patients with HIV infection with cryptococcal meningitis. Evidence‐Based Medicine 2015;20(1):15. [DOI] [PubMed] [Google Scholar]
Makadzange 2015b {published data only}
- Makadzange AT, Mothobi N. Delaying initiation of ART for 5 weeks improves survival in patients with HIV infection with cryptococcal meningitis. Evidence‐Based Medicine 2015;20(1):15. [DOI] [PubMed] [Google Scholar]
Makadzange 2015c {published data only}
- Makadzange AT, Mothobi N, Isaac ML, Larson EB, Boulware DR, Meya DB, et al. Delaying initiation of ART for 5 weeks improves survival in patients with HIV infection with cryptococcal meningitis. Evidence‐Based Medicine 2015;20(1):15. [DOI] [PubMed] [Google Scholar]
Manosuthi 2008 {published data only}
- Manosuthi W, Chottanapund S, Sungkanuparph S. Mortality rate of early versus deferred initiation of antiretroviral therapy in HIV‐1‐infected patients with cryptococcal meningitis. Journal of Acquired Immune Deficiency Syndromes 2008;48(4):508‐9. [DOI] [PubMed] [Google Scholar]
Sungkanuparph 2009 {published data only}
- Sungkanuparph S, Filler SG, Chetchotisakd P, Pappas PG, Nolen TL, Manosuthi W, et al. Cryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in AIDS patients with cryptococcal meningitis: a prospective multicenter study. Clinical Infectious Diseases 2009;49(6):931‐4. [DOI] [PubMed] [Google Scholar]
Sunpath 2012 {published data only}
- Sunpath H, Edwin C, Chelin N, Nadesan S, Maharaj R, Moosa Y, et al. Operationalizing early antiretroviral therapy in HIV‐infected in‐patients with opportunistic infections including tuberculosis. International Journal of Tuberculosis and Lung Disease 2012;16(7):917‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Torok 2005 {published data only}
- Torok ME, Day JN, Hien TT, Farrar JJ. Immediate or deferred antiretroviral therapy for central nervous system opportunistic infections?. AIDS 2005;19(5):535‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Bicanic 2005
- Bicanic T, Harrison TS. Cryptococcal meningitis. British Medical Bulletin 2005;72:99‐118. [DOI] [PubMed] [Google Scholar]
Bicanic 2006
- Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. Symptomatic relapse of HIV‐associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clinical Infectious Diseases 2006;43(8):1069‐73. [DOI] [PubMed] [Google Scholar]
Bicanic 2009
- Bicanic T, Meintjes G, Rebe K, Williams A, Loyse A, Wood R, et al. Immune reconstitution inflammatory syndrome in HIV‐associated cryptococcal meningitis: a prospective study. Journal of Acquired Immune Deficiency Syndromes 2009;51(2):130‐4. [DOI] [PubMed] [Google Scholar]
Bicanic 2010
- Bicanic T, Jarvis JN, Muzoora C, Harrison TS. Should antiretroviral therapy be delayed for 10 weeks for patients treated with fluconazole for cryptococcal meningitis?. Clinical Infectious Diseases 2010;51(8):986‐7. [DOI] [PubMed] [Google Scholar]
Bicanic 2015
- Bicanic T, Bottomley C, Loyse A, Brouwer AE, Muzoora C, Taseera K, et al. Toxicity of amphotericin B deoxycholate‐based induction therapy in patients with HIV‐associated cryptococcal meningitis. Antimicrobial Agents and Chemotherapy 2015;59(12):7224‐31. [DOI: 10.1128/AAC.01698-15] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulware 2010
- Boulware DR. Safety, censoring, and intent‐to‐treat analysis: dangers to generalizability. Clinical Infectious Diseases 2010;51(8):985‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crabtree Ramírez 2017
- Crabtree Ramírez B, Caro Vega Y, Sheperd BE, Le C, Turner M, Frola C, et al. Outcomes of HIV‐positive patients with cryptococcal meningitis in the Americas. International Journal of Infectious Diseases 2017;63:57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT. Version accessed 16 July 2017. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
Grant 2010
- Grant PM, Aberg JA, Zolopa AR. Concerns regarding a randomized study of the timing of antiretroviral therapy in Zimbabweans with AIDS and acute cryptococcal meningitis. Clinical Infectious Diseases 2010;51(8):984‐5. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Haddow 2010
- Haddow LJ, Colebunders R, Meintjes G, Lawn SD, Elliott JH, Manabe YC, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV‐1‐infected individuals: proposed clinical case definitions. Lancet Infectious Diseases 2010;10(11):791‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Available from handbook.cochrane.org: The Cochrane Collaboration.
Higgins 2016
- Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R. Standards for the conduct of new Cochrane Intervention Reviews. In: Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological Expectations of Cochrane Intervention Reviews. London: The Cochrane Library, 2016. [Google Scholar]
Ingle 2015
- Ingle SM, Miro JM, Furrer H, Justice A, Saag MS, Manzardo C, et al. Impact of ART on mortality in cryptococcal meningitis patients: high‐income settings. Conference on Retroviruses and Opportunistic Infections (CROI); 2015 Feb 23‐25; Seattle (WA). San Francisco: CROI Foundation/IAS‐USA, 2015:Poster 837.
Loyse 2013
- Loyse A, Thangaraj H, Easterbrook P, Ford N, Roy M, Chiller T, et al. Cryptococcal meningitis: improving access to essential antifungal medicines in resource‐poor countries. Lancet Infectious Diseases 2013;13(7):629‐37. [DOI: 10.1016/S1473-3099(13)70078-1] [DOI] [PubMed] [Google Scholar]
Lundgern 2015
- Lundgern JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. New England Journal of Medicine 2015;373(9):795‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meiring 2016
- Meiring S, Fortuin‐de Smidt M, Kularatne R, Dawood H, Govender NP, GERMS‐SA. Prevalence and hospital management of amphotericin B deoxycholate‐related toxicities during treatment of HIV‐associated cryptococcal meningitis in South Africa. PLoS Neglected Tropical Diseases 2016;10(7):e0004865. [DOI: 10.1371/journal.pntd.0004865] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mwaba 2001
- Mwaba P, Mwansa J, Chintu C, Pobee J, Scarborough M, Portsmouth S, et al. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgraduate Medical Journal 2001;77(914):769–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Müller 2010
- Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M, IeDEA Southern, Central Africa. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta‐analysis. Lancet Infectious Diseases 2010;10(4):251‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
NIH 2017a
- AIDSinfo. Guidelines for the prevention and treatment of opportunistic infections in HIV‐infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf (accessed prior to 13 September 2017).
NIH 2017b
- AIDSinfo. Guidelines for the prevention and treatment of opportunistic infections among HIV‐exposed and HIV‐infected children. https://aidsinfo.nih.gov/guidelines/html/4/adult‐and‐adolescent‐opportunistic‐infection/0 (accessed prior to 29 September 2017).
Park 2009
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009;23(4):525‐30. [DOI] [PubMed] [Google Scholar]
Rajasingham 2017
- Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV‐associated cryptococcal meningitis: an updated analysis. Lancet Infectious Diseases 2017;17(8):873‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shelburne 2005
- Shelburne SA 3rd, Darcourt J, White AC Jr, Greenberg SB, Hamill RJ, Atmar RL, et al. The role of immune reconstitution inflammatory syndrome in AIDS‐related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clinical Infectious Diseases 2005;40(7):1049‐52. [DOI] [PubMed] [Google Scholar]
Tinashe 2016
- Tinashe KN, Masanganise F, Hagen F, Bwakura‐Dangarembizi MF, Ticklay IMH, Roberston VJ. Cryptococcal meningitis presenting as a complication in HIV‐infected children: a case series from sub‐Saharan Africa. Pediatric Infectious Disease Journal 2016;35(9):979‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
UNAIDS 2017
- Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS global AIDS update. www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf (accessed prior to 1 September 2017).
WHO 2013
- World Health Organization. HIV/AIDS: prevention screening and management of common coinfections. Consolidated ARV guidelines, June 2013. www.who.int/hiv/pub/guidelines/arv2013/coinfection/prevcoinfection/en/index5.html (accessed prior to 31 June 2017).
WHO 2016
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Second edition. www.who.int/hiv/pub/arv/arv‐2016/en/ (accessed prior to 13 September 2017).
WHO 2018
- World Health Organization. Guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV‐infected adults, adolescents and children. Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. www.who.int/hiv/pub/guidelines/cryptococcal‐disease/en/ (accessed prior to 25 April 2018). [PubMed]
Williamson 2017
- Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nature Reviews Neurology 2017;13(1):13‐24. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Njei 2011
- Njei B, Kongnyuy EJ, Kibot L. Optimal timing for antiretroviral therapy initiation in patients with HIV infection and concurrent cryptococcal meningitis. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD009012] [DOI] [PubMed] [Google Scholar]
Njei 2013
- Njei B, Kongnyuy EJ, Kumar S, Okwen MP, Sankar MJ, Mbuagbaw L. Optimal timing for antiretroviral therapy initiation in patients with HIV infection and concurrent cryptococcal meningitis. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD009012.pub2] [DOI] [PubMed] [Google Scholar]


