Abstract
Social hierarchies emerge when animals compete for access to resources such as food, mates or physical space. Wild and laboratory male mice have been shown to develop linear hierarchies, however, less is known regarding whether female mice have sufficient intrasexual competition to establish significant social dominance relationships. In this study, we examined whether groups of outbred CD-1 virgin female mice housed in a large vivaria formed social hierarchies. We show that females use fighting, chasing and mounting behaviors to rapidly establish highly directionally consistent social relationships. Notably, these female hierarchies are less linear, steep and despotic compared to male hierarchies. Female estrus state was not found to have a significant effect on aggressive behavior, though dominant females had elongated estrus cycles (due to increased time in estrus) compared to subordinate females. Plasma estradiol levels were equivalent between dominant and subordinate females. Subordinate females had significantly higher levels of basal corticosterone compared to dominant females. Analyses of gene expression in the ventromedial hypothalamus indicated that subordinate females have elevated ERα, ERβ and OTR mRNA compared to dominant females. This study provides a methodological framework for the study of the neuroendocrine basis of female social aggression and dominance in laboratory mice.
Subject terms: Social behaviour, Animal behaviour
Introduction
The contextual and neurobiological factors that influence male intrasexual aggression and social dominance have been well-studied across species1–6. Conversely, female aggression and social dominance have been relatively understudied, with most work focused on maternal aggression expressed by females when they are pregnant or during the early postpartum period where the behavioral focus is on maternal defense of offspring7,8. Few studies have investigated the contextual and neurobiological factors that influence female-female aggression outside of reproduction in rodents9,10.
Social hierarchies are likely to emerge whenever there is competition between individuals for resources such as food, water, territory or access to mates11. The more intense this competition is, the more likely it is that a highly linear social hierarchy will develop. In mammals, male social hierarchies are common as inter-sexual competition is typically dramatically higher in males compared to females, though there are notable exceptions such as hyenas where females have high levels of intra-sexual conflict and form strong female hierarchies11. Female hierarchies have also been observed in other species that have female intrasexual competition for access to resources including degus12, bison13, caribou14, red deer15, vervet monkeys16, and chimpanzees17. Less is known about the formation of social hierarchies in female wild mice, though some population studies suggest that females do generate some form of social hierarchy with dominant aggressive females establishing territories and subordinate females being unable to do so when population sizes increase18,19. Conversely, when population density is very low it appears that female wild mice have relatively little intra-sexual competition and do not form hierarchies20. Female-female aggression also appears to be low if females have social experience with each other prior to the intra-sexual competition21,22. Conversely, small groups of female laboratory mice can establish social ranks based on home cage social interactions23 or their performance in the tube-test24,25.
Previously, we have explored the complex group dynamics and neurobiology of male social hierarchies, demonstrating that male outbred CD-1 mice living in groups of up to 30 individuals will form highly linear social hierarchies when living in a large laboratory-based vivarium5,26,27. As relatively little is known about whether large groups of non-reproductively active female mice will form social hierarchies in the laboratory, we aimed to explore this question by housing groups of 12 virgin outbred CD-1 female mice in large vivaria. One historical reason why female behavior is so vastly understudied in comparison to male behavior in laboratory rodents is due to concerns that female behavior is more variable than males due to fluctuations in steroid hormone levels across the female estrus cycle. Indeed, estrus state has been shown to influence many behavioral states including anxiety-like behavior and exploration28, motivation, addiction29 and fear30. In rodents, some species also show variation in aggressive behavior across the estrous cycle31. Female California deer mice32, rats33,34 and hamsters35–38 are less likely to show aggressive behavior during estrus than diestrus, although other studies have found no effect of estrus state on aggressive behavior39–42. There is also mixed evidence for estrous effects on aggression in female house mice43,44. Given the potential significance of estrous state on female dominance and subordinate behaviors, we examined whether the estrous state of females is associated with the frequency of aggressive behavior within social hierarchies.
The neurobiological basis of female intrasexual aggression among non-reproductive females is receiving increased attention though much less is still known compared to male intrasexual aggression9,10. As in males, brain regions in the social behavior network (medial amygdala (meA), bed nucleus of the stria terminalis (BNST), lateral septum (LS), medial preoptic area (mPOA), anterior hypothalamus (AH), ventromedial hypothalamus (VMH) and periaqueductal grey (PAG)) as well as the mesocorticolimbic dopamine pathway have been found to form the basis of the neural circuit regulating aggression, though there are some important sex differences10. In particular, it is well-established that the VMH is a key modulator of aggression in non-reproductive female rodents9. Further, estradiol, the major estrogen steroid hormone, has been primarily associated with promoting aggressive behaviors in females45–47. Estradiol acts to alter the expression of gene products in the hypothalamus, including progesterone receptors (PR), oxytocin receptors (OTR), opioid receptors, and gonadotropin-releasing hormone (GnRH), all of which are known to regulate female social behaviors including social recognition, memory and aggression48.
The current study used an established paradigm developed in our lab applied to the study of male social hierarchy dynamics to study female social hierarchy behavior and begin to disentangle underlying neurobiological and neuroendocrine mechanisms. We investigated the hierarchical structure of eight groups of twelve females as well as plasma corticosterone and plasma estradiol concentrations for dominant and subordinate mice within these hierarchies. In male mice, social stressors such as social defeat and social stability are known to lead to increases in basal levels of corticosterone but findings from female mice are more variable49,50. We have previously found that subordinate male mice have elevated corticosterone levels compared to dominant male mice but only if the dominant males are highly despotic. We predicted that female subordinate mice may also show higher levels of basal corticosterone compared to dominant females, although previous studies have not found a consistent relationship between social dominance and plasma corticosterone in female mice23,51. We further examined gene expression differences between dominant and subordinate individuals in the VMH and mPOA of the hypothalamus across six genes known to modulate various aspects of social behavior and moderated by the action of estrogen: ERα, ERβ, PR, OTR, OPRM1, and GnRH. The aim of this work was to establish a feasible methodology for the study of female aggression and dominance, as well as their neurobiological and neuroendocrine mechanisms, outside of the reproductive period in laboratory mice.
Results
Hierarchy measures and organization
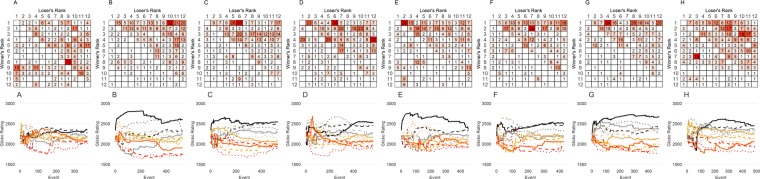
Sociomatrices of win-loss data for all female groups as well as the emergence of individual dominance ranks over time are presented in Fig. 1 and Supplemental Fig. S1. Summary statistics of several aspects of the hierarchical structure of each group are provided in Table 1. We found that seven out of the eight social groups formed a significantly linear and steep social hierarchy as measured by modified Landau’s h′, triangle transitivity and steepness. All eight social groups had significantly high directional consistency of agonistic behavior, indicating that the majority of wins were directed from more dominant to more subordinate individuals. Female groups had relatively low despotism values, indicating that alpha females were not exerting complete dominance over all other females. This interpretation was confirmed by moderate Gini Coefficient values for wins, demonstrating that the number of wins made by dominant females was fairly evenly distributed between the top ranked females. Initial body weight measured at the beginning of group housing was not related to final dominance rank in any group (Spearman Rank correlation tests: all p > 0.12).
Figure 1.
(Row 1) Raw sociomatrices of wins and losses for each cohort (A–H). Each value represents the total number of wins by the individual in each row against the individual in each column. The degree of redness represents the frequency of wins. Individuals are ordered by I & SI rank. (Row 2) The temporal patterning of dominance. The Glicko Rating of dominance (y-axis) against each successive behavioral event (x-axis). Each line represents the dominance rating of one individual in each cohort. The lines are colored from black (eventual most dominant individual) to red (eventual least dominant individual).
Table 1.
Social hierarchy measures for each cohort (A–H).
| Cohort ID | Landau’s h′ | ttri | Steepness | DC | Despotism | GC Wins | GC Losses |
|---|---|---|---|---|---|---|---|
| A | 0.19 | 0.08 | 0.30 | 0.54 | 0.15 | 0.24 | 0.22 |
| B | 0.85 | 0.88 | 0.61 | 0.76 | 0.26 | 0.43 | 0.29 |
| C | 0.71 | 0.82 | 0.55 | 0.70 | 0.23 | 0.45 | 0.27 |
| D | 0.67 | 0.68 | 0.48 | 0.69 | 0.22 | 0.43 | 0.23 |
| E | 0.49 | 0.48 | 0.47 | 0.68 | 0.22 | 0.40 | 0.19 |
| F | 0.46 | 0.36 | 0.46 | 0.72 | 0.25 | 0.46 | 0.21 |
| G | 0.82 | 0.95 | 0.55 | 0.79 | 0.34 | 0.49 | 0.21 |
| H | 0.78 | 0.90 | 0.51 | 0.57 | 0.16 | 0.34 | 0.20 |
Females have significantly linear hierarchies (as measured by Landau’s Modified h′ value and triangle transitivity (ttri)) as well as significantly steep hierarchies (steepness). Aggressive behavior is also significantly directionally consistent (DC). Significant values are bolded. Despotism and the Gini-Coefficient of Wins and Losses measure how evenly distributed aggressive behavior is across ranks. Female hierarchies are not highly despotic.
Emergence of hierarchies over time
Despite having no previous social experience with each other, 4 of 8 social groups had a significantly linear hierarchy by the end of Day 1 that continued throughout the 14-day observation period (Fig. 2). Two further social groups were significantly linear by the end of Day 2 and thereafter. One cohort did not have a stable linear hierarchy until Day 9, although this hierarchy did show significant triangle transitivity on Days 1 and 4 suggesting some linearity in the first week of co-housing. The social group that did not have a significant hierarchy by the end of the observation period did have some linear organization, having a significantly linear hierarchy on Day 3.
Figure 2.
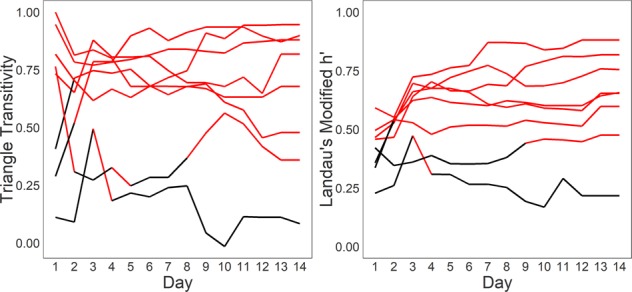
Female dominance hierarchies emerge rapidly. The triangle transitivity and Landau’s Modified h′ value across the fourteen days of the housing period. Each line represents a different female cohort. Red lines indicate significant values.
Sex differences in mouse social hierarchies
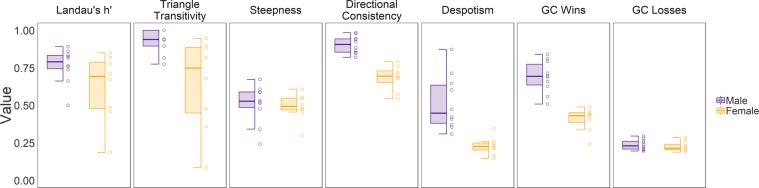
Female social hierarchies were significantly different from male social hierarchies in several aspects of their dominance organization (see Methods about male comparison group). Female social hierarchies were significantly less linear by triangle transitivity (W = 14, p < 0.05) and had lower directional consistency (W = 0, p < 0.001) than male social hierarchies (Fig. 3). The distribution of power was more even in females than in males. Alpha females were significantly less despotic than alpha males (W = 1, p < 0.001), and the Gini coefficient of wins (W = 0, p < 0.001) was significantly lower in female hierarchies than male hierarchies. There was no difference in the steepness of hierarchies (W = 31, p = 0.460), the Gini coefficient of losses (W = 32, p = 0.515.) or Landau’s modified h′ value (W = 23, p = 0.146).
Figure 3.
Comparison between male and female social hierarchies. Boxplots compare modified Landau’s h′, triangle transitivity, steepness, directional consistency, despotism, and Gini coefficient (GC) of wins and losses from 8 female social groups (orange) and 10 male social groups (purple).
Frequency of each agonistic behavior over the group housing period
The hourly rate of each agonistic behavior is shown in Fig. S2. Fighting and chasing were observed at significantly higher rates than mounting. The rate of fighting behavior showed a significant decrease by day over the group housing period (bday = −0.62 [−0.89, −0.36]) while those of chasing and mounting behaviors did not show significant changes by day (chasing: bday = 0.08 [−0.29, 0.44]; mounting: bday = 0.10 [−0.03, 0.23]).
Relationships between mounting, chasing and fighting
To determine if the directionality of fighting, chasing and mounting was consistent between individuals we performed QAP correlation tests on the fighting, chasing and mounting sociomatrices (Fig. S3). Fighting and chasing sociomatrices were highly correlated across all cohorts (data presented as median [IQR] across all eight cohorts: r = 0.79 [0.75, 0.80], all p < 0.001). Chasing and mounting sociomatrices were also significantly correlated across all cohorts (r = 0.44 [0.38, 0.54], all p < 0.025). Fighting and mounting sociomatrices were correlated for 7/8 cohorts (r = 0.37[0.35,0.39], all p < 0.025 for significant correlations). See Supplemental Fig. S3 for individual sociomatrices.
Directional consistency of each agonistic behavior
All groups showed highly significant directionally consistent behavior for all behaviors (all p < 0.001). Notably, the most directionally consistent behavior was mounting behavior (median [IQR] = 0.87 [0.83, 0.93]), which was more consistent than chasing (0.75 [0.70, 0.77]) and significantly more consistent than fighting (0.72 [0.69, 0.74]) (Fig. S4A; Friedman’s test X2 = 7,df = 2, p = 0.03; post-hoc test mounting vs fighting p = 0.033, mounting vs chasing p = 0.112).
Frequency of each agonistic behavior across individuals and ranks
As expected, the Gini coefficient for the total number of attacks and chases made by the females in each group was moderately high, indicating that a few females are responsible for a disproportionate number of these aggressive acts (Fig. S4B,C). Unexpectedly, the Gini coefficient for mounting other females was even higher than for fighting or chasing across cohorts (Friedman Test: X2 = 12, df = 2, p = 0.002; post hoc tests p < 0.01). This analysis demonstrates that in each social group a small number of females are responsible for a very large proportion of all mounting acts. The Gini coefficient for the total number of attacks and chases received by each female in each social group was moderately low. This finding indicates that most females are the recipients of fights and chases and these events are relatively evenly distributed across females in each social group. Again, it was unexpected that the Gini coefficient of mounts received was significantly higher than that for receiving the other two behaviors (Friedman Test: X2 = 9.2, df = 2, p = 0.01; post hoc tests p < 0.05). This finding indicates that being mounted is far more unequally distributed across females in each group than being chased or being attacked: certain females in each social group are the targets for a disproportionately high number of mounts from other females.
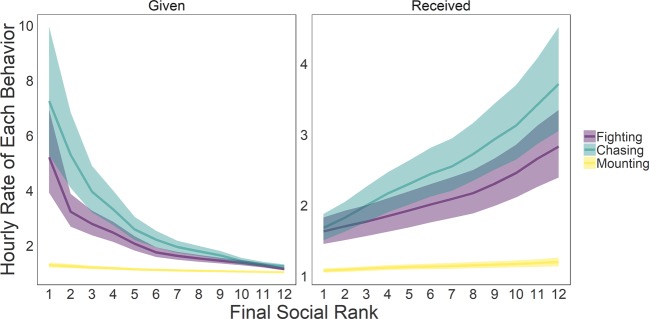
We further examined if there is an effect of social rank on the hourly occurrence rate of each agonistic behavior either given or received. Fighting and chasing, but not mounting, were exhibited at significantly higher hourly rates by more dominant females (Fig. 4, fighting given: brank = −1.11 [−1.44, −0.81]; chasing given: brank = −1.13 [−1.46, −0.85]; mounting given: brank = −0.25 [−0.61, 0.12]). Similarly, there were significant effects of social rank on the hourly rate of receiving fighting and chasing but not mounting, showing more subordinate females received more fighting and chasing behaviors (fighting given: brank = −0.38 [0.19, 0.58]; chasing given: brank = 0.47 [0.25, 0.71]; mounting given: brank = 0.10 [−0.20, 0.42]). Although animals of each rank differed in the absolute frequencies of each agonistic behavior used, there was no effect of rank on the proportion of each behavior used (Fig. S5). That is, all animals use each behavior proportionally equivalently.
Figure 4.
The effect of final social rank on the hourly rate of each agonistic behavior given (left) and received (right). Lines represent the median posterior means of fighting, chasing and mounting events per hour of observation by rank across the fourteen days of observation. The shaded areas show the 95% CI.
Estrus cycle and female behavior
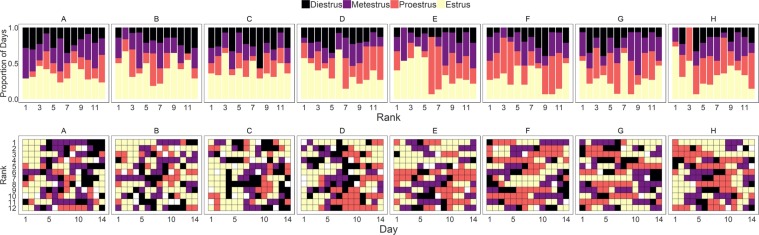
The median (+/−IQR) proportion of time that individuals were in each estrous state were - estrus: 33.3% [25.8%, 46.7%]; proestrus: 21.4% [13.3%, 36.4%], diestrus: 14.3% [0.08%, 23.1%], metestrus: 21.4% [14.3%, 28.6%] (Fig. 5A,B). Female mice with higher social rank were more likely to be in estrus compared to metestrus or proestrus (log-odds of being in estrus vs. metestrus: 0.48 [0.02, 0.95]; estrus vs. proestrus: 0.36 [018, 1.57]). Mice did not significantly differ by social rank in the likelihood to be in estrus compared to diestrus (estrus vs. diestrus: 0.50 [−0.03, 1.06]), or between other states (metestrus vs. proestrus: 0.32 [−0.36, 1.05]; metestrus vs. diestrus: 0.02 [−0.57, 0.58]; proestrus vs. diestrus: −0.22 [−0.78, 0.35]).
Figure 5.
(Row 1) The proportion of the fourteen days of observations in each estrus state by rank across each cohort (diestrus = black; metestrus = purple; proestrus = orange; estrus = yellow). (Row 2) The estrus state assigned by vaginal smear for each rank across cohorts (diestrus = black; metestrus = purple; proestrus = orange; estrus = yellow; unable to determine = white).
Overall there was no large effect of daily estrus state on the hourly rate of giving or receiving aggression. There was small effect that mice showed a higher hourly rate of giving aggression when in metestrus compared to proestrus (bmetestrus-proestrus: 0.19 [0.005, 0.35]). We further examined the effect of daily estrus state on the hourly rate of giving or receiving each agonistic behavior individually (fighting, chasing, mounting). For the rate of giving chases, mice had a higher rate when they are in metestrus compared to diestrus and proestrus (bmetestrus-diestrus: 0.22 [0.03, 0.40]; bmetestrus-proestrus: 0.19 [0.01, 0.36]). Compared to during diestrus, mice received higher rates of mounting when they were in estrus or metestrus (bestrus-diestrus: 0.31 [0.09, 0.53]; bmetestrus-diestrus: 0.33 [0.08, 0.58]). The hourly rates of other behaviors given or received did not differ across different estrus states.
Plasma corticosterone and estradiol levels
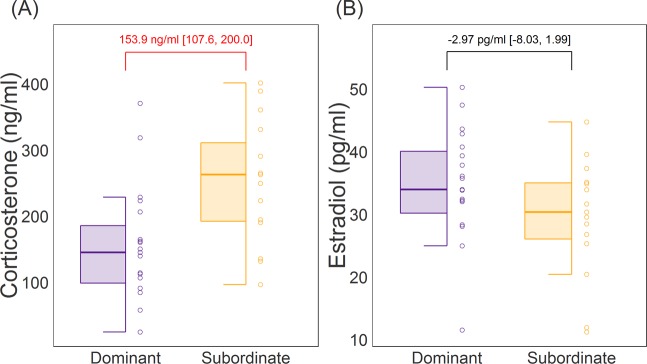
Plasma corticosterone levels were found to be significantly higher for subordinate individuals as compared to dominant individuals (Fig. 6A, bsubordinate-dominant: 153.9 ng/ml [107.6, 200.0]). We did not find an effect of estrus cycle state on corticosterone level. There was no effect of dominant-subordinate status on plasma estradiol levels (Fig. 6B, bsubordinate-dominant: −2.97 pg/ml [−8.03, 1.99]). Mice that were in diestrus state on the day of blood collection had significantly higher estradiol levels compared to those that were in metestrus or proestrus (bdiestrus-metestrus: 6.79 pg/ml [0.29, 13.2]; bdiestrus-proestrus: 10.1 pg/ml [3.07, 17.5]). There was no effect of estrus state on the estradiol levels among other states.
Figure 6.
Plasma Corticosterone and Estradiol levels for dominant and subordinate females. (A) Subordinate females have significantly higher levels of plasma corticosterone than dominant females. (B) There is no difference between dominant and subordinate individuals in plasma estradiol levels. Text in each panel indicates posterior means and 95% CI between dominant and subordinate females (bsubordinate-dominant). Red text indicates significant differences.
Gene expression in the VMH and the mPOA
In the VMH, there was small but significant effect of dominant-subordinate status on the levels of expression in the VMH of ERα, ERβ, and OTR genes (Fig. 7), with subordinate mice showing higher expression than dominants. There were no significant differences in expression levels of OPRM1 and PR genes in the VMH. There were no significant differences between dominant and subordinate individuals in the expression any of the genes examined in the mPOA.
Figure 7.
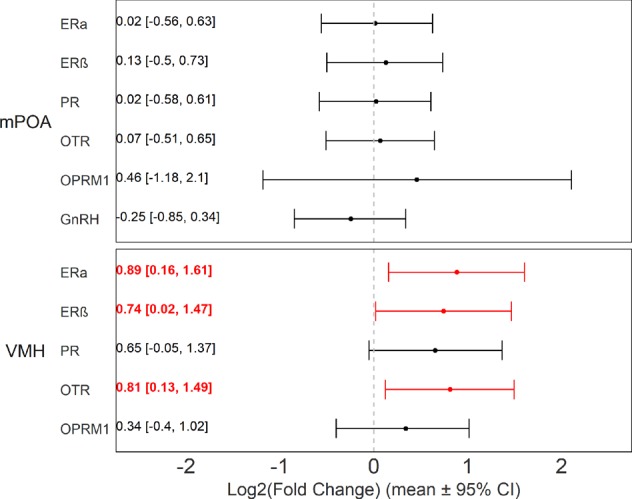
ERα, ERβ, and OTR mRNA levels in the VMH are higher for subordinate compared to dominant females. Solid dots and whiskers indicate posterior means and 95% credible intervals respectively for gene expression differences between dominant and subordinate females in each brain region and gene (bsubordinate-dominant). Text on the left notes posterior means and 95% CI for each comparison. Red text indicates significant differences.
Discussion
Here we show that female mice living in social groups of up to 12 females are capable of forming significantly linear dominance hierarchies that are stable for up to 14 days. Seven out of eight female social groups had significantly linear hierarchies, as measured by Landau’s h-value and triangle transitivity, and significantly steep hierarchies as measured by the relative differences in David’s scores. All eight female social groups had significantly high directional consistency of agonistic behavior demonstrating that dominant individuals more often won competitive interactions against subordinate individuals. Similar to males, female mammals can form stable dominant-subordinate relationships whenever there is intrasexual competition for resources23,24,52–54. In our study of virgin female mice, there is no intrasexual competition for access to mates or food and water, however, female mice may compete for access to preferred areas of the vivaria (such as nestboxes) or bedding. Our results also extend the findings from studies of round-robin tube-tests conducted in groups of 5 or 8 female mice that report that females were capable of forming linear social hierarchies based on wins and losses in that competitive exclusion test24,25. These findings are also consistent with studies of wild mice that have shown that females will form territorially based social hierarchies when living in large environments with relatively high population densities18,19. It appears that there may be a critical population density limit required for there to be sufficient intrasexual competition for females to engage in sufficient aggression to establish hierarchies, as one recent study found that groups of six females living in enclosures of 7 m2 did not form social hierarchies20.
Our results demonstrate that females use fighting, chasing and mounting behaviors to establish and maintain dominance relationships. The highest rates of fighting were observed during the establishment of hierarchies (which typically occurred rapidly within the first two days), and the rate of fighting significantly decreased thereafter. The rates of chasing and mounting did not decrease across the housing period. Unsurprisingly, given the existence of a linear hierarchy, higher ranked individuals exhibited higher rates of fighting and chasing than lower ranks. Similarly, lower-ranked individuals received higher amounts of each of these behaviors. With respect to mounting behavior, we did not observe a significant overall effect of rank on the rate of giving or receiving mounting. Indeed, there has been some controversy as to the proximal function of female-female mounting in rodents and whether it should be considered to be a dominance behavior, a sexual behavior, a masculinized behavior or something else55–57. Our data suggest that mounting is being used by some females as a dominance behavior but not consistently by all individuals. That is, females are more likely to mount females subordinate to themselves but not all females engage in this behavior. We observed that the group sociomatrices for fighting, chasing and mounting behaviors given and received all highly correlated with each other, indicating that the direction and magnitude of these behaviors within each social relationship was consistent. Secondly, we observed that the directional consistency of mounting behavior (0.87) was even stronger than the directional consistency for fighting (0.72) and chasing (0.75). This finding indicates that mounting behavior consistently occurred 87% of the time in the direction of more dominant to more subordinate females. We also noted that females of all ranks, despite having different absolute rates of each behavior, used each of the three behaviors in roughly equal proportions -suggesting that higher- or lower-ranked females do not preferentially utilize mounting behavior over the other two as a dominance behavior. Additionally, the Gini coefficient of behavior given and received was significantly higher for mounting as compared to the other two agonistic behaviors. Thus, in each social group the majority of mounts given and received were by specific individuals, and these interactions occurred in a directionally consistent manner.
Female mouse hierarchies exhibit several differences when compared to male social hierarchies. Although both sexes produce linear hierarchies, those of females are less linear and have lower directional consistency than male hierarchies. Male hierarchies tend to be despotic, with one alpha male exhibiting the vast majority of all aggressive acts5,26,27. We did not observe such despotism in female hierarchies as evidenced by the lower despotism scores and lower Gini coefficient of wins. More dominant females in social groups tend to more equally distribute aggression towards other lower-ranked females. These sex differences may be rooted in the ancestral biology of the house mice. In the wild, there are fundamental differences in the reproductive strategies of males and females. Males have high levels of intersexual competition and have high reproductive skew58. Females generally exhibit higher parental investment in offspring and have reduced conflict over reproductive opportunities54,59,60. Indeed, even when given the opportunity to compete for males, female mice do not necessarily increase their rates of aggression20. Across mammals, female adaptations for intrasexual competition can involve more subtle behaviors such as low-level persistent aggression instead of overt displays of physical aggression54. Our data suggest that in laboratory mice there persists low-level aggression that results in females forming stable social hierarchies even with unlimited access to food, water, space and nesting material and no access to males.
We found that the estrous state of each female did not have a large effect on the likelihood of females giving or receiving aggression. Females in all states were equally likely to bite or be bitten. However, we did observe a very small effect for females in metestrus to have a higher rate of chasing than those females in proestrus or diestrus. Previous work in rodents has suggested that estrus state can influence the propensity of females to engage in intrasexual aggression although these effects are inconsistent and appear to be highly influenced by many other contextual factors. For instance, female mice have been reported to show higher aggression in the resident-intruder test during metestrus and proestrus compared to estrus and distrus43. Similarly, female California mice show their highest aggression during diestrus compared to proestrus and estrus32. Such a decline in aggression during estrus has been suggested to facilitate mating, yet female mice in estrus have been shown to be capable of exhibiting aggression and forming dominant-subordinate relationships61. Conversely, no changes in female-female aggression across the estrous cycle have also been observed in rats and hamsters41,42. Although there was no effect of estrous state on the likelihood to mount other females, we did find that female mice in estrus or metestrus received significantly more mounts than those females in diestrus. This finding is somewhat consistent with evidence from several species including baboons62, squirrel monkeys63, hanuman langurs64, rabbits65 and rats56 that mounted females tend to be subordinates in estrus.
We observed that group-housed females had extended estrous cycles with prolonged periods of diestrus and estrus. The mouse estrus cycle is regulated by luteinizing hormone and follicle-stimulating hormone released from the pituitary in response to gonadotrophin releasing hormone released from the hypothalamus, which is in turn under control from estrogen and progesterone. The duration of this cycle is usually 4–6 days for females housed in isolation or in pairs, but can be much longer for group-housed females – an effect known as the Lee-Boot effect66–69. For instance, female mice living in groups of 8 have been shown to have estrous cycles lasting up to 14 days66 and females in groups of 30 have estrus cycles up to 40 days in duration69. These cycles are typically extended due to longer periods of time spent in diestrus, but they may also become disrupted. Following exposure to males, females quickly enter estrus and become sexually receptive69. Our results are largely congruent with these earlier studies in that we do observe extended estrous cycles in group-housed animals. Notably, many females in our study were in estrous for prolonged periods of time, and this effect was larger in dominant females compared to subordinate females. It is possible that this is an adaptive mechanism by which dominant females ensure more or earlier mating opportunities than subordinate females, however it is unclear as to what physiological processes underpin the extended length of estrous cycles we observed.
We found no significant differences between dominant and subordinate mice in plasma estradiol. Peripheral estrogens are known to promote aggression in both males and females10,70,71, and therefore we predicted there would be higher estradiol levels in dominant females. However, our hormone samples were taken at the end of group housing on Day 14 at a time when the social hierarchies had stabilized and aggression was at a low level. Further, estrogens can have many, sometimes opposite, effects, depending on where in the brain and on what receptors they are acting72,73. It is therefore perhaps not surprising that estradiol levels found in plasma at one time point do not differ between dominant and subordinate individuals.
Conversely, we did find that subordinate females had significantly higher levels of plasma corticosterone than dominant females. This effect was moderately large and much larger than the effect that we had previously observed in male hierarchies, where significant differences in plasma corticosterone levels between dominants and subordinates only occur in highly despotic hierarchies27. In females, all hierarchies were considered to be very low on the despotism scale. These results are interesting in the context of the established literature on sex differences in stress responses. Several studies have shown that male mice show a much more robust physiological response to stressors such as social defeat, social instability and chronic stress compared to female mice49,50. However, these results are not always consistent, and sometimes females do show increases in corticosterone depending on the social context of the stressor74–76. It has also been reported that highly aggressive territorial females living in large groups have elevated corticosterone compared to non-aggressive females51, though one other study found results consistent to ours that subordinate female mice had higher corticosterone than dominant females in small groups of up to five albino mice23. Elevated levels of corticosterone in subordinates post-hierarchy formation have been shown to facilitate social memories for being socially subordinate in rats77,78. The functional significance of the elevated corticosterone in subordinate females living in our relatively stable social housing remains to be addressed, but these data suggest the potential for studying female social hierarchies as a model of social stress.
The neurobiology of female intrasexual aggression has been relatively under-studied compared to that of male intrasexual aggression, although this area has started to receive increased attention9,10. Here we found that the expression of ERα, ERβ and OTR was moderately higher in subordinate female mice compared to dominant female mice in the VMH. The VMH is known to be a critical regulator of female aggression. Early studies in rats and hamsters demonstrated that lesions of the VMH lead to increased aggression by females45,79, whereas implants into the VMH with either estradiol or progesterone reduce aggression42,80,81. More recently in mice, estrogen-receptor expressing neuronal populations in the ventrolateral VMH have been found to become active when females bite versus mount other females82. This work builds on previous studies that demonstrated an important role for central estrogen receptors in regulating male and female aggression. ERα knockout male mice show reduced aggression towards other males83, whereas females lacking ERα expression are more aggressive to other females84,85. Loss of ERβ has also been associated with increased aggression in males86,87. Central administration of selective ERα agonists to female mice increases aggressive attacks in a resident-intruder paradigm88, while selective ERβ agonists treatment leads to an increase in non-aggressive social behaviors89. The role of each of these receptors in coordinating female aggressive behavior is clearly complex and context-dependent, but it is possible that our observed increased VMH expression in both ERα and ERβ in subordinate mice may underlie their inhibition of aggression. However, given the multitude of functions of these receptors in this region it is possible that these differences may be unrelated to aggression and associated with other social behaviors such as social recognition, learning and memory90.
Likewise, oxytocin acting on oxytocin receptors has a range of effects on social behavior in females, and its roles in promoting or inhibiting aggression are highly contextually dependent. In non-lactating females oxytocin generally appears to inhibit aggression10. OT knockout mice show reduced aggression to each other91 and central or injections of OT into the MPOA or AH can reduce aggression92,93. These findings may be congruent with our finding that subordinate females have higher VMH OTR expression than dominant females. Notably, we did not observe any association between social status and mRNA levels of OTR or any other gene in the MPOA. Though some lesion studies in other rodents have reported a role for the MPOA in female aggressive behavior94, our results would suggest that plasticity in gene expression in response to an individual’s social status occurs primarily in the VMH.
Conclusion
In the present study, we establish that outbred CD-1 female mice living in groups of 12 individuals are capable of forming significant linear hierarchies. These hierarchies are linear and directionally consistent, but less despotic than male social hierarchies. These hierarchies emerge quickly and are stable over 14 days and are relatively unaffected by the estrous cycle. All group-housed females also show an extended estrous cycle, and dominants spend longer in estrus than subordinate females. Subordinate females have significantly higher levels of plasma corticosterone than dominant females, suggesting that subordinate females are more susceptible to the social stress of group living than male mice. We also find that subordinate females have higher levels of ERα, ERβ, and OTR mRNA than dominant females in the VMH, suggesting that these genes in this region may facilitate in part the reduced aggression displayed by these females. This work furthers our understanding of group female social behavior, begins to explore sex differences between male and female social hierarchy formation and maintenance and provides evidence that the actions of estrogen may play a role in modulating female social hierarchy behavior.
Methods
Subjects and housing
A total of 96 female outbred CD-1 mice were obtained from Charles River Laboratories at 7 weeks of age. Mice were housed in the animal facility in the Department of Psychology at Columbia University, with constant temperature (21–24 °C) and humidity (30–50%). The room was kept on a 12/12 light/dark cycle, with white light (light cycle) on at 2400 hours and red lights (dark cycle) on at 1200 hours. All mice were uniquely marked by dying their fur with a blue, nontoxic animal marker (Stoelting Co.), enabling individuals to be identified throughout the study. These marks remain for up to 12 weeks and only require one application. All experiments and housing mice were approved and conducted in accordance with the Columbia University Institutional Animal Care and Use Committee (IACUC Protocol No. AC-AAAP5405) and National Health Institute (NIH; Bethesda, MD, USA) animal care guidelines.
Social behavior observations
Following arrival at the animal facility, mice were housed in groups of 3 for 2 weeks in standard sized cages. At 9 weeks of age, groups of 12 mice were weighed and placed into large, structurally complex, custom built vivaria (length 150 cm, height 80 cm, width 80 cm; Mid-Atlantic; Fig. S6). The vivaria were constructed as described in Williamson et al.26. Each vivarium consists of an upper level constructed of multiple shelves connected by plastic tubes and covered in pine bedding and a lower level comprised of 5 interconnected standard sized cages filled with pine bedding and connected by a system of plastic tubes. Mice can access all levels of the vivarium at any time through this interconnecting system of ramps and tunnels. Standard chow and water were provided ad libitum on the top level of the vivarium. Social groups were created such that in each group of 12 females, each individual had previous social experience with maximum only one other individual and at least 6 females per group had absolutely no experience with any of the other individuals. Mice were placed in the vivarium at the onset of the dark cycle on Day 1 of the experiment and were observed by trained observers for 2 hours directly following introduction to the group and for 2 hours each day for the next two weeks (Day 1–Day 14). Observations always occurred during the dark cycle at some point during the first 6 hours of lights off (red light). During these live observations, observers used all occurrence sampling to record the winner and loser in all instances of fighting, chasing, mounting, subordinate posture, and induced-flee behaviors (see Table S1 for an ethogram of these behaviors). Winners of each encounter were considered to be those that chased, bit, mounted, or forced another individual to exhibit subordinate behavior. If behaviors between two females co-occurred within 2 seconds of each other they were recorded with the priority fighting, chasing, mounting, subordinate posture, flee. This method has been used previously in our lab to understand the social organization of groups of male mice5,6,26,27,95,96. Vaginal smears were collected from every mouse each evening at the same time of day (six to eight hours post lights-off). To collect the samples, trained lab members removed mice from the vivaria individually and placed them back as soon as the sample was collected. Collecting samples from each social group interrupted the group for less than 5 minutes. Smear samples were analyzed under a microscope by a single trained lab member and double checked by a second lab member to verify accuracy. Mice were weighed, final estrus smears taken, and euthanized via decapitation 2 hours post lights off on Day 15. Trunk blood was collected into heparinized tubes, immediately placed on ice, centrifuged at 4 °C in a refrigerated centrifuge, and plasma separated and frozen at −80 °C until analyzed for corticosterone and estradiol levels via radioimmunoassay. Brains were collected and flash frozen in hexane and stored at −80 °C until dissection. At the end of group housing, the 2 most dominant and 2 most subordinate individuals were determined using the Glicko Rating System26,97 as well as David’s Scores26,98. Plasma hormone and brain mRNA levels were measured for these two most dominant and two most subordinate individuals in each group, except for in two cohorts where it was difficult to distinguish the beta and gamma female so three dominant individuals and two subordinate individuals were used.
Hormone assays
Plasma corticosterone and plasma estradiol concentrations were measured using commercially available kits (MP Biomedicals) and conducted using the manufacturer’s specifications. For the corticosterone assay, the average inter-assay coefficient of variation was 9.3%, the lowest detectable was 24.78 ng/ml, and the highest detectable was 938.34 ng/ml. For the estradiol assay, the coefficient of variation was 7.2%, the lowest detectable was 8.53 pg/ml, and the highest detectable was 2455.79 pg/ml.
Gene expression
Brains were stored at −80 °C until dissection. Samples of the medial preoptic area (mPOA) and ventromedial hypothalamus (VMH) were collected using a Harris Micro-Punch with reference to the coronal plane from the Mouse Brain Atlas99 and the Allen Brain Atlas100. The mPOA was collected as one 1 mm diameter area along the midline from Bregma + 0.14 mm to −0.7 mm. The VMH was collected as one 1 mm diameter area from each hemisphere from Bregma −1.34 mm to −1.82 mm. RNA was isolated from both brain regions using the AllPrep RNA Micro Kit (Qiagen) and reverse transcribed to cDNA using the SuperScript III First-Strand Synthesis System for RT-PCR applications. Quantitative RT-PCR was performed with 1ul of cDNA using an ABI 7500 Fast Thermal Cycler and the Fast SYBR Green Master Mix reagent (Applied Biosystems). All primer probes (Sigma-Aldrich) were designed to span exon boundaries ensuring amplification of only mRNA. The following validated quantitative PCR primers were used for mRNA analysis: estrogen receptor alpha (ERα – Forward: CGTGTGCAATGACTATGCCTCT; Reverse: TGGTGCATTGGTTTGTAGCTGG), estrogen receptor beta (ERβ – Forward: GTCAGGCACATCAGTAACAAGGG; Reverse: ATTCAGCATCTCCAGCAGCAGGTC), progesterone receptor (PR – Forward: GCGAGAGACAACTGCTTTCAGT; Reverse: CAAACACCATCAGGCTCATCCA), gonadotropin releasing hormone (GnRH – Forward: AGCACTGGTCCTATGGGTTG; Reverse: GGTTCTGCCATTTGATCCAC), oxytocin receptor (OTR – Forward: TTCTTCGTGCAGATGTGGAG; Reverse: CCAAGAGCATGGCAATGATG), opioid receptor µ1 (OPRM1 – Forward: AATGTTCATGGCAACCACAA; Reverse: TTTGAGCAGGTTCTCCCAGT).
Statistical analysis
All statistical analyses were undertaken in R v.3.5.0101.
Group dominance structure and social organization
For each cohort, six measures of dominance structure and organization were calculated: Landau’s modified h′, directional consistency, steepness, triangle transitivity, despotism and Gini’s coefficient of wins and losses. The methods of calculation for these measures are detailed in Williamson et al.26, but briefly: Landau’s modified h′, directional consistency, and steepness are calculated using frequency win/loss sociomatrices, which are created using the total frequency of wins and losses recorded for each individual over the observation period. Landau’s modified h′ evaluates the extent to which individuals in a hierarchy can be linearly ordered98 and ranges from 0–1, with a value of 1 indicating a completely linear hierarchy. Triangle transitivity measures the proportion of relations between all triads that are transitive (i.e. if individual A is dominant to individual B and individual B is dominant to individual C, then individual A is dominant to individual C; a perfect hierarchy would have all transitive triads)102. It is calculated using a binary win/loss sociomatrix, where 1 s are assigned to individuals in rows that won more often against individuals in columns and 0 s are assigned to individuals in rows that lost more often to individuals in columns. Triangle transitivity ranges from 0–1, with 1 indicating that all triads are transitive (i.e. a perfectly linear hierarchy). Steepness measures the unevenness of relative individual dominance within the hierarchy. This is calculated from the relative distribution of David’s Scores, a win proportion measure adjusted for strength of opponents103. It ranges from 0–1 with a score closer to 1 indicating that power is not equitably distributed across the hierarchy, but rather lies in the hands of a few powerful individuals at the top. Directional consistency measures the degree to which all agonistic interactions occur in the direction from the more dominant to more subordinate individual in the pair. It also ranges from 0–1, with 1 indicating that all agonistic interactions occur in the direction of dominant to subordinate. Significance testing for Landau’s modified h′, triangle transitivity, steepness and directional consistency were carried out using appropriate randomization methods26. P-values represent the proportion of times that values were observed in randomized data that were greater than or equal to the observed values from empirical data. Despotism is the proportion of all wins by the dominant male over the total number of aggressive interactions over the observation period. It is a value between 0–1, with 1 indicating that the alpha male performed 100% of all aggression within the group. Gini-coefficient is a measure of equality versus inequality in a distribution. We calculated the Gini-coefficients for the frequency of wins and losses by each animal across cohorts. It ranges from 0–1. Values closer to 1 indicate more inequality meaning that a higher number of wins/losses are associated with relatively few individuals. Values closer to 0 indicate that the frequency of wins/losses are equally distributed across all individuals. We examined the association between initial body weight on Day 1 of group housing and final social rank for each social group using Spearman Rank correlation tests.
Landau’s modified h′, triangle transitivity, directional consistency and despotism were calculated using the R package ‘compete’ v.0.1104. Steepness was calculated using the R package ‘steepness’ v.0.2.2105. Gini coefficients were calculated using the ‘ineq’106 package.
Comparison between fighting, chasing and mounting behaviors
Raw frequency sociomatrices were constructed for each cohort based separately on fighting, chasing and mounting behaviors. To determine how correlated each matrix (fighting, chasing or mounting) was to each other within each cohort we performed a Quadratic Assignment Procedure (QAP) test with 1000 Monte Carlo randomizations of the data using the ‘sna’ package v.2.4107. From these matrices the directional consistency and Gini-coefficient of wins and losses was also calculated for each behavior for each cohort. To test if differences in these values existed between behaviors across cohorts we used Friedman Tests. Significant differences between behaviors were determined using Nemenyi Post-Hoc Tests using the ‘PMCMR’ R package v.4.3108.
Emergence of hierarchies and individual ranks
To determine how quickly each cohort formed a linear hierarchy we calculated Landau’s modified h′ and triangle transitivity as well as running significance tests for the aggregated win-loss data for each cohort up to each day. The emergence of individual ranks across time was identified using Glicko ratings. All individuals begin with a Glicko rating of 2200, and points are added or subtracted based on winning or losing against other individuals. The degree of points won or lost is dependent upon the difference in ratings between the two individuals. If an individual with a high Glicko rating defeats an individual with a low Glicko rating, relatively few points would be added to their total and relatively few points would be subtracted from the defeated individual. If an individual with a low Glicko rating defeats an individual with a high Glicko rating, a larger number of points would be added to their total and subtracted from the loser. Glicko ratings of all individuals in each group are recalculated after every behavioral interaction. We used a constant value of 3 in our calculations of Glicko ratings. See Williamson et al.26 for more information. Glicko ratings were calculated using the ‘PlayerRatings’ package v.1.0 in R109.
Comparison of female social hierarchy behavior to male social hierarchy behavior
To measure differences in social hierarchy structure between male and female social groups, we compared female data from this study with previously published data on male social hierarchies from Williamson et al.26. We recalculated hierarchy measures using the first fourteen days of observation data from the 10 groups of 12 male CD-1 mice who were housed and observed in exactly the same manner as the female groups in the current study. We used Wilcoxon rank sum tests to compare the values of Landau’s modified h′, triangle transitivity, steepness, directional consistency, despotism and Gini-coefficient of wins and losses between male and female groups.
Analysis of hourly occurrence rate of each agonistic behavior
All Bayesian linear and generalized linear regressions were fitted using R package ‘brms’110. For each fighting, chasing, and mounting behavior observed throughout the group housing period for each cohort, we tested whether the day of group housing affects hourly occurrence rate of each behavior by fitting the data with a gaussian distribution. Then we analyzed if the hourly occurrence rate of each behavior differs by individual final social rank using a two-process hurdle-gamma family. In this model, the probability of having zeros as the occurrence rates are modelled with a binomial error distribution with logit link function and non-zero non-integer continuous values are fitted with a gamma error distribution with log link111. We chose to use this model as the hourly occurrence rate data contains a significant number of zeros as subordinate individuals often barely initiate agonistic behaviors and alpha individuals barely receive aggression especially once the hierarchies are established. Further in this model we assume the difference of any effect of individual ranks is not necessarily equidistant between ranks. We therefore treated individual social rank as a monotonic predictor rather than a continuous variable throughout the entire statistical analysis112. The beta coefficients estimated from monotonic models presented in this study indicate the direction and the size (range between the lowest and highest categories of the ordinal fixed factor e.g. rank) of the effects.
Analysis of estrus cycle state
We tested whether a mouse with higher social rank in the hierarchy is more likely to be in one estrus state compared to another by fitting a multinomial logistic mixed effect model with estrus status as the outcome variable, individual social rank as a monotonic predictor and cohort ID and subject ID as random effects. We tested whether an individual is more likely to give (hourly given aggression rate) or receive (hourly received aggression rate) each agonistic behavior when the mouse is in a certain estrus status using a hurdle-gamma model. First, the probability of the hourly occurrence rate being zero was predicted for each individual with daily Glicko rank of each mouse as a monotonic predictor to test whether the observed number of zeros in the data could be explained by rank. After this hurdle, the non-zero values of the hourly occurrence rate were fitted with a predictor of estrus state for each individual across each group housing day. Cohort ID, subject ID and day of group housing were set as random effects for both processes.
Analysis of plasma corticosterone and estradiol levels
Using leave-one-out cross-validation information criteria (LOOIC)113, we compared four models with different combination of fixed effects: (i) dominant-subordinate status, (ii) last measured estrus cycle before blood collection, (iii) dominant-subordinate status and last measured estrus state, (iv) the interaction of social status and estrus state. All models were fitted with cohort ID as a random effect. For corticosterone data, a model with dominant-subordinate status only resulted in the best fit. A model with the status and the estrus state resulted in the best fit for estradiol data.
Analysis of gene expression levels
Using the R package ‘MCMC.qpcr’114, we analyzed the differences in gene expression between dominant and subordinate mice by fitting a generalized mixed effect model with Poisson-lognormal distribution and a Bayesian Markov Chain Monte Carlo sampling approach. This approach provides advantages compared to standard delta-CT analysis as it accounts for random variation between duplicates, increases power by analyzing data for all target genes in one model, and does not require control genes115. Briefly, the raw threshold cycle (CT) values were converted into molecule count data with consideration of the amplification efficiency of each gene. We fitted the model with dominant-subordinate status category as a fixed factor and cohort ID and subject ID as random factors. We confirmed linearity of the model by inspecting diagnostic plots.
Supplementary information
Acknowledgements
We thank Dr. Frances Champagne for advice and suggestions in writing the manuscript and Curley Lab students for help with behavioral observations.
Author Contributions
C.M.W., W.L., A.R.D. and J.P.C. conceived and planned the experiments. C.M.W., W.L., A.R.D., R.D.R. and A.L. carried out the behavioral work and husbandry. R.D.R. planned and carried out the hormone analysis. C.M.W. and A.R.D. carried out the gene expression experiment. C.M.W., W.L. and J.P.C. analyzed the data. C.M.W., W.L. & J.P.C. wrote the paper. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Data Availability
All raw data and code used in this paper are publicly available at GitHub https://github.com/jalapic/females.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43747-w.
References
- 1.Lenkov K, Lee MH, Lenkov OD, Swafford A, Fernald RD. Epigenetic DNA Methylation Linked to Social Dominance. Plos one. 2015;10:e0144750. doi: 10.1371/journal.pone.0144750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maruska KP, Fernald RD. Social Regulation of Gene Expression in the Hypothalamic-Pituitary-Gonadal Axis. Physiology. 2011;26:412–423. doi: 10.1152/physiol.00032.2011. [DOI] [PubMed] [Google Scholar]
- 3.Rosvold H, Enger H, Mirsky AF, Pribram KH. Influence of amygdalectomy on social behavior in monkeys. J. Comp. Physiol. Psychol. 1954;47:173–178. doi: 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- 4.Wang F, et al. Bidirectional Control of Social Hierarchy by Synaptic Efficacy in Medial Prefrontal Cortex. Science. 2011;334:693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- 5.Williamson, C. M., Franks, B. & Curley, J. P. Mouse Social Network Dynamics and Community Structure are Associated with Plasticity-Related Brain Gene Expression. Front. Behav. Neurosci. 10 (2016). [DOI] [PMC free article] [PubMed]
- 6.Williamson CM, Romeo RD, Curley JP. Dynamic changes in social dominance and mPOA GnRH expression in male mice following social opportunity. Horm. Behav. 2017;87:80–88. doi: 10.1016/j.yhbeh.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Erskine MS, Barfield RJ, Goldman BD. Intraspecific fighting during late pregnancy and lactation in rats and effects of litter removal. Behav. Biol. 1978;23:206–218. doi: 10.1016/S0091-6773(78)91814-X. [DOI] [PubMed] [Google Scholar]
- 8.Haney M, Debold JF, Miczek KA. Maternal aggression in mice and rats towards male and female conspecifics. Aggress. Behav. 1989;15:443–453. doi: 10.1002/1098-2337(1989)15:6<443::AID-AB2480150605>3.0.CO;2-U. [DOI] [Google Scholar]
- 9.Been, L. E., Gibbons, A. B. & Meisel, R. L. Towards a neurobiology of female aggression. Neuropharmacology, 10.1016/j.neuropharm.2018.11.039 (2018). [DOI] [PMC free article] [PubMed]
- 10.Duque-Wilckens, N. & Trainor, B. C. Behavioral Neuroendocrinology of Female Aggression. Oxf. Res. Encycl. Neurosci, 10.1093/acrefore/9780190264086.013.11 (2017).
- 11.Holekamp KE, Strauss ED. Aggression and dominance: an interdisciplinary overview. Curr. Opin. Behav. Sci. 2016;12:44–51. doi: 10.1016/j.cobeha.2016.08.005. [DOI] [Google Scholar]
- 12.Correa LA, Frugone MJ, Soto-Gamboa M. Social dominance and behavioral consequences of intrauterine position in female groups of the social rodent Octodon degus. Physiol. Behav. 2013;119:161–167. doi: 10.1016/j.physbeh.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Rutberg AT. Dominance and Its Fitness Consequences in American Bison Cows. Behaviour. 1986;96:62–91. doi: 10.1163/156853986X00225. [DOI] [Google Scholar]
- 14.Barrette C, Vandal D. Social Rank, Dominance, Antler Size, and Access To Food in Snow-Bound Wild Woodland Caribou. Behaviour. 1986;97:118–145. doi: 10.1163/156853986X00342. [DOI] [Google Scholar]
- 15.Ceacero F, et al. Benefits for Dominant Red Deer Hinds under a Competitive Feeding System: Food Access Behavior, Diet and Nutrient Selection. Plos one. 2012;7:e32780. doi: 10.1371/journal.pone.0032780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitten PL. Diet and dominance among female vervet monkeys (Cercopithecus aethiops) Am. J. Primatol. 1983;5:139–159. doi: 10.1002/ajp.1350050205. [DOI] [PubMed] [Google Scholar]
- 17.Murray CM, Eberly LE, Pusey AE. Foraging strategies as a function of season and rank among wild female chimpanzees (Pan troglodytes) Behav. Ecol. 2006;17:1020–1028. doi: 10.1093/beheco/arl042. [DOI] [Google Scholar]
- 18.Chovnick A, Yasukawa NJ, Monder H, Christian JJ. Female behavior in populations of mice in the presence and absence of male hierarchy. Aggress. Behav. 1987;13:367–375. doi: 10.1002/1098-2337(1987)13:6<367::AID-AB2480130605>3.0.CO;2-X. [DOI] [Google Scholar]
- 19.Yasukawa NJ, Monder H, Leff FR, Christian JJ. Role of female behavior in controlling population growth in mice. Aggress. Behav. 1985;11:49–64. doi: 10.1002/1098-2337(1985)11:1<49::AID-AB2480110107>3.0.CO;2-A. [DOI] [Google Scholar]
- 20.Weidt A, Gygax L, Palme R, Touma C, König B. Impact of male presence on female sociality and stress endocrinology in wild house mice (Mus musculus domesticus) Physiol. Behav. 2018;189:1–9. doi: 10.1016/j.physbeh.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 21.Palanza P, Della Seta D, Ferrari PF, Parmigiani S. Female competition in wild house mice depends upon timing of female/male settlement and kinship between females. Anim. Behav. 2005;69:1259–1271. doi: 10.1016/j.anbehav.2004.09.014. [DOI] [Google Scholar]
- 22.Rusu AS, Krackow S. Kin-preferential cooperation, dominance-dependent reproductive skew, and competition for mates in communally nesting female house mice. Behav. Ecol. Sociobiol. 2004;56:298–305. doi: 10.1007/s00265-004-0787-4. [DOI] [Google Scholar]
- 23.Schuhr B. Social structure and plasma corticosterone level in female albino mice. Physiol. Behav. 1987;40:689–693. doi: 10.1016/0031-9384(87)90269-1. [DOI] [PubMed] [Google Scholar]
- 24.van den Berg WE, Lamballais S, Kushner SA. Sex-Specific Mechanism of Social Hierarchy in Mice. Neuropsychopharmacology. 2015;40:1364–1372. doi: 10.1038/npp.2014.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varholick JA, Bailoo JD, Palme R, Würbel H. Phenotypic variability between Social Dominance Ranks in laboratory mice. Sci. Rep. 2018;8:6593. doi: 10.1038/s41598-018-24624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williamson CM, Lee W, Curley JP. Temporal dynamics of social hierarchy formation and maintenance in male mice. Anim. Behav. 2016;115:259–272. doi: 10.1016/j.anbehav.2016.03.004. [DOI] [Google Scholar]
- 27.Williamson CM, Lee W, Romeo RD, Curley JP. Social context-dependent relationships between mouse dominance rank and plasma hormone levels. Physiol. Behav. 2017;171:110–119. doi: 10.1016/j.physbeh.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 28.Palanza P, Gioiosa L, Parmigiani S. Social stress in mice: Gender differences and effects of estrous cycle and social dominance. Physiol. Behav. 2001;73:411–420. doi: 10.1016/S0031-9384(01)00494-2. [DOI] [PubMed] [Google Scholar]
- 29.Roberts DCS, Bennett SaL, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl.) 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- 30.Gray P. Effect of the estrous cycle on conditioned avoidance in mice. Horm. Behav. 1977;8:235–241. doi: 10.1016/0018-506X(77)90040-X. [DOI] [PubMed] [Google Scholar]
- 31.Floody, O. R. Hormones and Aggression in Female Mammals. In Hormones and Aggressive Behavior 39–89 (Springer, Boston, M. A.), 10.1007/978-1-4613-3521-4_3 (1983).
- 32.Davis ES, Marler CA. C-FOS changes following an aggressive encounter in female California mice: A synthesis of behavior, hormone changes and neural activity. Neuroscience. 2004;127:611–624. doi: 10.1016/j.neuroscience.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 33.Ho H-P, et al. The Serotonin Reuptake Inhibitor Fluoxetine Reduces Sex Steroid-Related Aggression in Female Rats: An Animal Model of Premenstrual Irritability? Neuropsychopharmacology. 2001;24:502–510. doi: 10.1016/S0893-133X(00)00219-0. [DOI] [PubMed] [Google Scholar]
- 34.Seward JP. Aggressive behavior in the rat. I. General characteristics; age and sex differences. J. Comp. Psychol. 1945;38:175–197. doi: 10.1037/h0062251. [DOI] [Google Scholar]
- 35.Ciaccio LA, Lisk RD, Reuter LA. Prelordotic behavior in the hamster: A hormonally modulated transition from aggression to sexual receptivity. J. Comp. Physiol. Psychol. 1979;93:771–780. doi: 10.1037/h0077604. [DOI] [PubMed] [Google Scholar]
- 36.Floody OR, Pfaff DW. Aggressive behavior in female hamsters: The hormonal basis for fluctuations in female aggressiveness correlated with estrous state. J. Comp. Physiol. Psychol. 1977;91:443–464. doi: 10.1037/h0077341. [DOI] [PubMed] [Google Scholar]
- 37.Ward Kislak J, Beach FA. Inhibition of Aggressiveness by ovarian hormones. Endocrinology. 1955;56:684–692. doi: 10.1210/endo-56-6-684. [DOI] [PubMed] [Google Scholar]
- 38.Wise DA. Aggression in the female golden hamster: Effects of reproductive state and social isolation. Horm. Behav. 1974;5:235–250. doi: 10.1016/0018-506X(74)90032-4. [DOI] [PubMed] [Google Scholar]
- 39.Barr GA, Gibbons JL, Moyer KE. Male-female differences and the influence of neonatal and adult testosterone on intraspecies aggression in rats. J. Comp. Physiol. Psychol. 1976;90:1169–1183. doi: 10.1037/h0077291. [DOI] [PubMed] [Google Scholar]
- 40.Cordero MI, Ansermet F, Sandi C. Long-term programming of enhanced aggression by peripuberty stress in female rats. Psychoneuroendocrinology. 2013;38:2758–2769. doi: 10.1016/j.psyneuen.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Jong TRde, Beiderbeck DI, Neumann ID. Measuring Virgin Female Aggression in the Female Intruder Test (FIT): Effects of Oxytocin, Estrous Cycle, and Anxiety. Plos one. 2014;9:e91701. doi: 10.1371/journal.pone.0091701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi LK, Lisk RD. Intrasexual interactions among female golden hamsters (Mesocricetus auratus) over the estrous cycle. J. Comp. Psychol. Wash. DC 1983. 1984;98:267–275. [PubMed] [Google Scholar]
- 43.Hyde JS, Sawyer TF. Estrous cycle fluctuations in aggressiveness of house mice. Horm. Behav. 1977;9:290–295. doi: 10.1016/0018-506X(77)90064-2. [DOI] [PubMed] [Google Scholar]
- 44.Koonce CJ, Frye CA. Female mice with deletion of Type One 5α-reductase have reduced reproductive responding during proestrus and after hormone-priming. Pharmacol. Biochem. Behav. 2014;122:20–29. doi: 10.1016/j.pbb.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albert DJ, Petrovic DM, Walsh ML. Ovariectomy attenuates aggression by female rats cohabitating with sexually active sterile males. Physiol. Behav. 1989;45:225–228. doi: 10.1016/0031-9384(89)90122-4. [DOI] [PubMed] [Google Scholar]
- 46.Rosvall, K. A. et al. Neural sensitivity to sex steroids predicts individual differences in aggression: implications for behavioural evolution. Proc. R. Soc. Lond. B Biol. Sci. rspb20120442, 10.1098/rspb.2012.0442 (2012). [DOI] [PMC free article] [PubMed]
- 47.Rubenstein DR, Wikelski M. Steroid hormones and aggression in female Galápagos marine iguanas. Horm. Behav. 2005;48:329–341. doi: 10.1016/j.yhbeh.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Pfaff DW, et al. Estrogens, brain and behavior: studies in fundamental neurobiology and observations related to women’s health. J. Steroid Biochem. Mol. Biol. 2000;74:365–373. doi: 10.1016/S0960-0760(00)00114-X. [DOI] [PubMed] [Google Scholar]
- 49.Dadomo H, et al. What is stressful for females? Differential effects of unpredictable environmental or social stress in CD1 female mice. Horm. Behav. 2018;98:22–32. doi: 10.1016/j.yhbeh.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 50.Jacobson-Pick S, Audet M-C, McQuaid RJ, Kalvapalle R, Anisman H. Social agonistic distress in male and female mice: changes of behavior and brain monoamine functioning in relation to acute and chronic challenges. PloS One. 2013;8:e60133. doi: 10.1371/journal.pone.0060133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chapman JC, Christian JJ, Pawlikowski MA, Michael SD. Analysis of steroid hormone levels in female mice at high population density. Physiol. Behav. 1998;64:529–533. doi: 10.1016/S0031-9384(98)00115-2. [DOI] [PubMed] [Google Scholar]
- 52.Kaufmann JH. On the Definitions and Functions of Dominance and Territoriality. Biol. Rev. 1983;58:1–20. doi: 10.1111/j.1469-185X.1983.tb00379.x. [DOI] [Google Scholar]
- 53.Rowell TE. The concept of social dominance. Behav. Biol. 1974;11:131–154. doi: 10.1016/S0091-6773(74)90289-2. [DOI] [PubMed] [Google Scholar]
- 54.Stockley P, Bro-Jørgensen J. Female competition and its evolutionary consequences in mammals. Biol. Rev. 2011;86:341–366. doi: 10.1111/j.1469-185X.2010.00149.x. [DOI] [PubMed] [Google Scholar]
- 55.Dagg AI. Homosexual behaviour and female-male mounting in mammals—a first survey. Mammal Rev. 1984;14:155–185. doi: 10.1111/j.1365-2907.1984.tb00344.x. [DOI] [Google Scholar]
- 56.Fang J, Clemens LG. Contextual determinants of female–female mounting in laboratory rats. Anim. Behav. 1999;57:545–555. doi: 10.1006/anbe.1998.1025. [DOI] [PubMed] [Google Scholar]
- 57.Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- 58.Dean MD, Ardlie KG, Nachman MW. The frequency of multiple paternity suggests that sperm competition is common in house mice (Mus domesticus) Mol. Ecol. 2006;15:4141–4151. doi: 10.1111/j.1365-294X.2006.03068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bronson FH. The Reproductive Ecology of the House Mouse. Q. Rev. Biol. 1979;54:265–299. doi: 10.1086/411295. [DOI] [PubMed] [Google Scholar]
- 60.Bronson FH. Mammalian Reproduction: An Ecological Perspective. Biol. Reprod. 1985;32:1–26. doi: 10.1095/biolreprod32.1.1. [DOI] [PubMed] [Google Scholar]
- 61.Brain, P. F., Haug, M. & Parmigiani, S. The aggressive female rodent: Redressing a “scientific” bias. In of Mice and Women: Aspects of Female Aggression 27–35 (Academic Press, San Diego, 1992).
- 62.Anthoney TR. The Ontogeny of Greeting, Grooming, and Sexual Motor Patterns in Captive Baboons (Superspecies Papio Cynocephalus) Behaviour. 1968;31:358–372. doi: 10.1163/156853968X00333. [DOI] [PubMed] [Google Scholar]
- 63.Talmage-Riggs G, Anschel S. Homosexual Behavior and Dominance Hierarchy in a Group of Captive Female Squirrel Monkeys (Saimiri sciureus) Folia Primatol. (Basel) 1973;19:61–72. doi: 10.1159/000155519. [DOI] [PubMed] [Google Scholar]
- 64.Borries C, Sommer V, Srivastava A. Dominance, age, and reproductive success in free-ranging female hanuman langurs (Presbytis entellus) Int. J. Primatol. 1991;12:231–257. doi: 10.1007/BF02547586. [DOI] [Google Scholar]
- 65.Albonetti ME, Dessi-Fulgheri F. Female-female mounting in the European rabbit. Z Saugetierkunde. 1990;55:128–138. [Google Scholar]
- 66.Champlin AK. Suppression of Oestrus in Grouped Mice: the Effects of Various Densities and the Possible Nature of the Stimulus. Reproduction. 1971;27:233–241. doi: 10.1530/jrf.0.0270233. [DOI] [PubMed] [Google Scholar]
- 67.Ma W, Miao Z, Novotny MV. Role of the Adrenal Gland and Adrenal-Mediated Chemosignals in Suppression of Estrus in the House Mouse: The Lee-Boot Effect Revisited. Biol. Reprod. 1998;59:1317–1320. doi: 10.1095/biolreprod59.6.1317. [DOI] [PubMed] [Google Scholar]
- 68.Van Der Lee S, Boot LM. Spontaneous pseudopregnancy in mice. Acta Physiol. Pharmacol. Neerl. 1955;4:442–444. [PubMed] [Google Scholar]
- 69.Whitten WK. Occurrence of anoestrus in mice caged in groups. J. Endocrinol. 1959;18:102–107. doi: 10.1677/joe.0.0180102. [DOI] [PubMed] [Google Scholar]
- 70.Lenz, K. M., Nugent, B. M. & McCarthy, M. M. Sexual Differentiation of the Rodent Brain: Dogma and Beyond. Front. Neurosci. 6 (2012). [DOI] [PMC free article] [PubMed]
- 71.Wu MV, et al. Estrogen Masculinizes Neural Pathways and Sex-Specific Behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ervin KSJ, et al. Estrogen involvement in social behavior in rodents: Rapid and long-term actions. Horm. Behav. 2015;74:53–76. doi: 10.1016/j.yhbeh.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 73.Schulz KM, Sisk CL. The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neurosci. Biobehav. Rev. 2016;70:148–158. doi: 10.1016/j.neubiorev.2016.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harris AZ, et al. A Novel Method for Chronic Social Defeat Stress in Female Mice. Neuropsychopharmacology. 2018;43:1276–1283. doi: 10.1038/npp.2017.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jarcho MR, Massner KJ, Eggert AR, Wichelt EL. Behavioral and physiological response to onset and termination of social instability in female mice. Horm. Behav. 2016;78:135–140. doi: 10.1016/j.yhbeh.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt MV, et al. A novel chronic social stress paradigm in female mice. Horm. Behav. 2010;57:415–420. doi: 10.1016/j.yhbeh.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 77.Timmer M, Sandi C. A role for glucocorticoids in the long-term establishment of a social hierarchy. Psychoneuroendocrinology. 2010;35:1543–1552. doi: 10.1016/j.psyneuen.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 78.Weger M, et al. Increased brain glucocorticoid actions following social defeat in rats facilitates the long-term establishment of social subordination. Physiol. Behav. 2018;186:31–36. doi: 10.1016/j.physbeh.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 79.Malsbury CW, Strull D, Daood J. Half-cylinder cuts antero-lateral to the ventromedial nucleus reduce sexual receptivity in female golden hamsters. Physiol. Behav. 1978;21:79–87. doi: 10.1016/0031-9384(78)90280-9. [DOI] [PubMed] [Google Scholar]
- 80.Meisel RL, Sterner MR, Diekman MA. Differential hormonal control of aggression and sexual behavior in female Syrian hamsters. Horm. Behav. 1988;22:453–466. doi: 10.1016/0018-506X(88)90050-5. [DOI] [PubMed] [Google Scholar]
- 81.Takahashi LK, Lisk RD, Burnett AL., II Dual estradiol action in diencephalon and the regulation of sociosexual behavior in female golden hamsters. Brain Res. 1985;359:194–207. doi: 10.1016/0006-8993(85)91429-5. [DOI] [PubMed] [Google Scholar]
- 82.Hashikawa, K. et al. Esr1+ cells in the ventromedial hypothalamus control female aggression. Nat. Neurosci. advance online publication (2017). [DOI] [PMC free article] [PubMed]
- 83.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc. Natl. Acad. Sci. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ogawa S, Taylor JA, Lubahn DB, Korach KS, Pfaff DW. Reversal of Sex Roles in Genetic Female Mice by Disruption of Estrogen Receptor Gene. Neuroendocrinology. 1996;64:467–470. doi: 10.1159/000127154. [DOI] [PubMed] [Google Scholar]
- 85.Ogawa S, et al. Roles of Estrogen Receptor-α Gene Expression in Reproduction-Related Behaviors in Female Mice*This work was supported by the Harry Frank Guggenheim Foundation (to S.O.), the University of Missouri-Columbia molecular biology program (to D.B.L.), and NIH Grant HD-05751 (to D.W.P.) Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 86.Nomura M, et al. Genotype/Age Interactions on Aggressive Behavior in Gonadally Intact Estrogen Receptor β Knockout (βERKO) Male Mice. Horm. Behav. 2002;41:288–296. doi: 10.1006/hbeh.2002.1773. [DOI] [PubMed] [Google Scholar]
- 87.Ogawa S, et al. Survival of reproductive behaviors in estrogen receptor β gene-deficient (βERKO) male and female mice. Proc. Natl. Acad. Sci. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clipperton-Allen AE, Almey A, Melichercik A, Allen CP, Choleris E. Effects of an estrogen receptor alpha agonist on agonistic behaviour in intact and gonadectomized male and female mice. Psychoneuroendocrinology. 2011;36:981–995. doi: 10.1016/j.psyneuen.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 89.Clipperton-Allen AE, Cragg CL, Wood AJ, Pfaff DW, Choleris E. Agonistic behavior in males and females: Effects of an estrogen receptor beta agonist in gonadectomized and gonadally intact mice. Psychoneuroendocrinology. 2010;35:1008–1022. doi: 10.1016/j.psyneuen.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ogawa, S., Tsukahara, S., Choleris, E. & Vasudevan, N. Estrogenic regulation of social behavior and sexually dimorphic brain formation. Neurosci. Biobehav. Rev, 10.1016/j.neubiorev.2018.10.012 (2018). [DOI] [PubMed]
- 91.Ragnauth AK, et al. Female oxytocin gene‐knockout mice, in a semi‐natural environment, display exaggerated aggressive behavior. Genes Brain Behav. 2005;4:229–239. doi: 10.1111/j.1601-183X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 92.Harmon AC, Huhman KL, Moore TO, Albers HE. Oxytocin Inhibits Aggression in Female Syrian Hamsters. J. Neuroendocrinol. 2002;14:963–969. doi: 10.1046/j.1365-2826.2002.00863.x. [DOI] [PubMed] [Google Scholar]
- 93.McCarthy MM. Oxytocin inhibits infanticide in female house mice (Mus domesticus) Horm. Behav. 1990;24:365–375. doi: 10.1016/0018-506X(90)90015-P. [DOI] [PubMed] [Google Scholar]
- 94.Hammond MA, Rowe FA. Medial preoptic and anterior hypothalamic lesions: Influences on aggressive behavior in female hamsters. Physiol. Behav. 1976;17:507–513. doi: 10.1016/0031-9384(76)90115-3. [DOI] [PubMed] [Google Scholar]
- 95.Curley JP. Temporal pairwise-correlation analysis provides empirical support for attention hierarchies in mice. Biol. Lett. 2016;12:20160192. doi: 10.1098/rsbl.2016.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee W, Khan A, Curley JP. Major urinary protein levels are associated with social status and context in mouse social hierarchies. Proc R Soc B. 2017;284:20171570. doi: 10.1098/rspb.2017.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Glickman ME. Parameter estimation in large dynamic paired comparison experiments. J. R. Stat. Soc. Ser. C Appl. Stat. 1999;48:377–394. doi: 10.1111/1467-9876.00159. [DOI] [Google Scholar]
- 98.De Vries H. An improved test of linearity in dominance hierarchies containing unknown or tied relationships. Anim. Behav. 1995;50:1375–1389. doi: 10.1016/0003-3472(95)80053-0. [DOI] [Google Scholar]
- 99.Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates. (Gulf Professional Publishing, 2004).
- 100.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 101.R Core Team. R: A language and environment for statistical computing (2017).
- 102.Shizuka D, McDonald DB. A social network perspective on measurements of dominance hierarchies. Anim. Behav. 2012;83:925–934. doi: 10.1016/j.anbehav.2012.01.011. [DOI] [Google Scholar]
- 103.Gammell MP, Vries H, de, Jennings DJ, Carlin CM, Hayden TJ. David’s score: a more appropriate dominance ranking method than Clutton-Brock et al.’s index. Anim. Behav. 2003;66:601–605. doi: 10.1006/anbe.2003.2226. [DOI] [Google Scholar]
- 104.Curley, J. P. Compete: Organizing and Analyzing Social Dominance Hierarchy Data (2016).
- 105.Leiva, D. & de Vries, H. Steepness: Testing steepness of dominance hierarchies (2014).
- 106.Zeileis, A. Ineq: Measuring inequality, concentration, and poverty (2014).
- 107.Butts, C. T. Sna: Tools for Social Network Analysis (2016).
- 108.Pohlert, T. PMCMR: Calculate Pairwise Multiple Comparisons of Mean Rank Sums (2018).
- 109.Stephenson, A. & Sonas, J. PlayerRatings: Dynamic updating methods for player ratings estimation (2012).
- 110.Bürkner P-C. Brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 2017;1:1. [Google Scholar]
- 111.Pohlmeier W, Ulrich V. An Econometric Model of the Two-Part Decisionmaking Process in the Demand for Health Care. J. Hum. Resour. 1995;30:339–361. doi: 10.2307/146123. [DOI] [Google Scholar]
- 112.Bürkner, P. -C. & Charpentier, E. Monotonic Effects: A Principled Approach for Including Ordinal Predictors in Regression Models, 10.31234/osf.io/9qkhj (2018).
- 113.Gelman A, Hwang J, Vehtari A. Understanding predictive information criteria for Bayesian models. Stat. Comput. 2014;24:997–1016. doi: 10.1007/s11222-013-9416-2. [DOI] [Google Scholar]
- 114.Matz, M. V. MCMC.qpcr: Bayesian Analysis of qRT-PCR Data (2016). [DOI] [PMC free article] [PubMed]
- 115.Matz MV, Wright RM, Scott JG. No Control Genes Required: Bayesian Analysis of qRT-PCR Data. Plos one. 2013;8:e71448. doi: 10.1371/journal.pone.0071448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data and code used in this paper are publicly available at GitHub https://github.com/jalapic/females.